Abstract
Objectives
Hepatocellular carcinoma (HCC) is a common cancer with high rate of recurrence and mortality. Diverse aetiological agents and wide heterogeneity in individual tumours impede effective and personalised treatment. Tonicity-responsive enhancer-binding protein (TonEBP) is a transcriptional cofactor for the expression of proinflammatory genes. Although inflammation is intimately associated with the pathogenesis of HCC, the role of TonEBP is unknown. We aimed to identify function of TonEBP in HCC.
Design
Tumours with surrounding hepatic tissues were obtained from 296 patients with HCC who received completion resection. TonEBP expression was analysed by quantitative reverse transcription–quantitative real-time PCR (RT-PCR) and immunohfistochemical analyses of tissue microarrays. Mice with TonEBP haplodeficiency, and hepatocyte-specific and myeloid-specific TonEBP deletion were used along with HCC and hepatocyte cell lines.
Results
TonEBP expression is higher in tumours than in adjacent non-tumour tissues in 92.6% of patients with HCC regardless of aetiology associated. The TonEBP expression in tumours and adjacent non-tumour tissues predicts recurrence, metastasis and death in multivariate analyses. TonEBP drives the expression of cyclo-oxygenase-2 (COX-2) by stimulating the promoter. In mouse models of HCC, three common sites of TonEBP action in response to diverse aetiological agents leading to tumourigenesis and tumour growth were found: cell injury and inflammation, induction by oxidative stress and stimulation of the COX-2 promoter.
Conclusions
TonEBP is a key component of the common pathway in tumourigenesis and tumour progression of HCC in response to diverse aetiological insults. TonEBP is involved in multiple steps along the pathway, rendering it an attractive therapeutic target as well as a prognostic biomarker.
Keywords: TonEBP (NFAT5), hepatocellular carcinoma, poor prognosis, inflammation, COX-2
Significance of this study.
What is already known on this subject?
Hepatocellular carcinoma (HCC) is a common cancer with high rate of recurrence and mortality. HCC is widely heterogeneous and diverse imposing formidable challenges to effective treatment and personalised therapy.
Tonicity-responsive enhancer-binding protein (TonEBP) is a critical regulator in many inflammatory diseases such as rheumatoid arthritis and atherosclerosis. While inflammation is intimately implicated in the pathogenesis of HCC, the role of TonEBP is unknown.
What are the new findings?
TonEBP is involved at multiple steps of the common pathway of HCC development and tumour progression: cell injury, induction by oxidative stress and inflammation.
TonEBP stimulated hepatic inflammation including prostaglandin E2 production which contributes to tumour growth and progression.
Expression of TonEBP is elevated in tumours in more than 90% of patients with HCC regardless of aetiology associated.
In patients with HCC who received resection, higher hepatic TonEBP expression is associated with recurrence, metastasis and survival in multivariate analyses.
How might it impact on clinical practice in the foreseeable future?
Examination of TonEBP expression in hepatic tissues from patients who received resection of HCC would provide the patients with valuable prognostic information regarding recurrence and metastasis.
TonEBP is an attractive target for therapeutic agents to prevent recurrence and metastasis as well as tumourigenesis.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the second most common cause of cancer-related death worldwide with steadily increasing incidence.1 The characteristic high mortality of HCC is due to a combination of multiple factors: difficulty in early detection, lack of effective treatments and extremely high rates of recurrence and metastasis.2 The major aetiological agents are HBV, HCV and alcoholic or non-alcoholic fatty liver. Genome sequencing, gene expression profiling and histological analyses have shown that HCC is widely heterogeneous and diverse imposing formidable challenges to effective treatment and personalised therapy.3 4
While molecular pathogenesis of HCC is multifaceted, two sequential mechanisms predominate.5 First is chronic inflammation followed by cirrhosis after tissue damage caused by viral infection, alcohol or metabolic influences. Next come mutations in oncogenes and tumour suppressor genes. Thus, a variety of aetiological insults incite common cellular reactions creating a microenvironment in which sequential mutations and genetic alterations drive formation of dysplastic nodules followed by early HCC and ultimately, metastasis. Investigating the microenvironment might uncover a common molecular pathway useful for a prognostic biomarker and therapeutic target.
Tonicity-responsive enhancer-binding protein (TonEBP), also known as NFAT5, is a central component of the inflammatory enhanceosome in which TonEBP bridges activated transcription factors to histone acetyltransferase p300 on gene promoters.6 TonEBP expression is stimulated by inflammation leading to elevated expression of proinflammatory genes in rheumatoid arthritis7 8 and atherosclerosis.9 Although several studies have shown that TonEBP is involved in tumour cell migration,10 TonEBP’s role in tumour development and progression is unknown. In this study, we examined TonEBP in hepatic tissues obtained from patients with HCC. We found TonEBP expression to be dramatically elevated in tumours than surrounding areas regardless of aetiology in over 90% of patients with HCC confirming the general importance of inflammation. Experiments in animals revealed that TonEBP mediates the initial hepatic injury in response to environmental insults leading to local inflammation, which promotes the development and progression of HCC.
Materials and methods
Human HCC samples and clinical information
A total of 296 patients who underwent hepatic resection for HCC from January 2008 to December 2015 at the Ulsan University Hospital, University of Ulsan, College of Medicine, Ulsan, Korea, were included in the study. All patients were HCC treatment naïve before surgery. All 296 patients who underwent hepatic resection had a grossly complete resection. The patients were predominantly male (84.1%), with average age of 56.6 years. The median follow-up period was 31 months (range=1–105 months). Postoperative recurrence was observed in 144 cases (48.7%). The 2-year and 5-year HCC recurrence rates were 43.6% and 61.1%, respectively. During postoperative follow-up period, metastasis and death were observed in 61 (20.6%) and 84 (28.4%) of cases, respectively. Data were expressed as mean±SD or median (range). For statistical significance, Student’s t-test and χ2 test were used for comparisons of variables between groups. The cumulative relapse and survival rates were evaluated by the Kaplan-Meier method, and differences were determined by the log-rank test. A multivariate analysis was carried out to identify the independent predictor for recurrence and survival using the Cox regression hazard model. All data were analysed using the statistical package SPSS for Windows V.21.0. In all cases, a two-tailed P value less than 0.05 was considered statistically significant. Additional protocols and procedures are described in online supplementary methods.
gutjnl-2017-315348supp001.pdf (1.7MB, pdf)
Mice
All the methods involving live mice were carried out in accordance with the approved guidelines. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Ulsan National Institute of Science and Technology (UNISTACUC-12-15-A).
All experiments were performed in male C57BL/6 mice. To induce HCC, we administrated a single intraperitoneal injection of 25 mg/kg diethylnitrosamine (DEN) (N0756; Sigma) to 2-week-old mice and euthanised them at 9 months of age. In the obesity-amplified DEN-induced HCC model, DEN (25 mg/kg) was injected intraperitoneally into 2-week-old mice. After 4 weeks, mice were separated into two dietary groups and fed either normal diet (10% fat as kilocalories, Research Diets, New Jersey, USA) or high-fat diet (HFD; 60% fat as kilocalories, Research Diets) and euthanised for 36 weeks dietary period. Tumour incidence and size were blindly calculated.
C57BL/6 TonEBPf/f mice11 were bred with Alb-Cre mice or LysM-Cre to generate TonEBPf/f:Alb-Cre or TonEBPf/f:LysM-Cre, respectively. To induce liver injury, TonEBPf/f:Alb-Cre or TonEBPf/f:LysM-Cre mice and their wild type (WT) littermates were intraperitoneally injected single DEN (100 mg/kg) and euthanised after 48 hours. For lipopolysaccharide (LPS)-induced liver injury, TonEBPf/f:Alb-Cre mice were intraperitoneally coinjected with LPS (5 g/kg of body weight) and D-galactosamine (400 mg/kg of body weight) and euthanised after 4 hours.
Ethanol feeding-induced liver injury and fat accumulation was demonstrated as described.12 Briefly, mice were acclimated liquid diet feeding with the control Lieber-DeCarli diet (Bio-Serv) ad libitum for 5 days, followed by ethanol Lieber-DeCarli diet (Bio-Serv) containing 5% (vol/vol) ethanol supplemented with maltose dextrin or isocaloric control diet for 10 days. To synergistically induce liver injury and inflammation, on day 11, mice were gavaged with a single dose of ethanol (5 g/kg) or isocaloric dextrin-maltose at 08:00 and sacrificed 9 hours later.
Cell line
HCC cell line, HepG2, from American Type Culture Collection was maintained in modified Eagle’s medium (MEM; Hyclone) supplemented with 10% fetal bovine serum (FBS; Thermo) with penicillin–streptomycin (Hyclone). Cells were transfected with TonEBP small interfering (si)RNA or control scrambled siRNA using Lipofectamine RNAiMAX (Invitrogen) following the manufacturer’s instructions. Cells were transfected with micro-RNA (miRNA)-223 or control miRNA using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. Mouse embryo fibroblasts (MEFs) and HEK293 cells were cultured in Dulbecco’s MEM supplemented with 10% FBS (Thermo) with penicillin–streptomycin (Hyclone). MEFs were established from the TonEBPΔ/Δ mouse. For binding assays, HEK293 cells were transfected with plasmids using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. For hypoxia challenge, HepG2 cells were incubated in hypoxic chamber in 2% O2. Additional protocols and procedures are described in online supplementary methods.
Statistical analysis
Data are presented as means+SD or means+SEM as indicated. Differences between groups were analysed by Student’s t-test, and statistical significance was considered at *P<0.05.
Results
Hepatic TonEBP predicts postoperative prognosis in patients with HCC
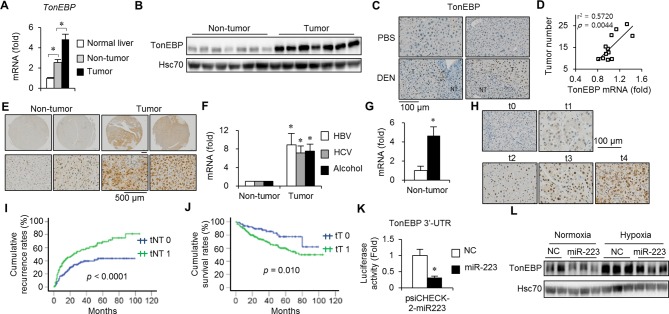
In macrophages, TonEBP expression is markedly stimulated by inflammatory signals.6 Since inflammation is an essential feature of HCC,13 14 we investigated hepatic TonEBP expression in DEN-induced mouse HCC. Expression of TonEBP messenger RNA (mRNA) in non-tumour regions surrounding tumours (non-tumour TonEBP mRNA expression) was higher compared with normal hepatic tissues while lower compared with adjacent tumour (figure 1A). TonEBP expression was higher in tumours compared with non-tumour regions and localised to the nuclei of hepatocytes (figure 1B,C and online supplementary figure 1A). Interestingly, non-tumour TonEBP mRNA expression correlated significantly with tumour numbers (figure 1D) consistent with the importance of inflammation in HCC.
Figure 1.
Hepatic TonEBP expression is elevated in HCC and associated with postoperative recurrence and death in patients with HCC. (A–D) Hepatic TonEBP expression in a mouse model of HCC. (A) TonEBP mRNA levels in tumour-free normal tissues from PBS-injected animals (n=8) and non-tumour and tumours from DEN-injected animals (n=18). Mean+SEM, *P<0.05. (B) Immunoblots of non-tumour and tumours from seven animals. (C) Immunohistochemical images of TonEBP (brown) in hepatic tissues from PBS-injected or DEN-injected animals. Nuclei were counterstained with haematoxylin (blue). (D) Tumour number and TonEBP mRNA in non-tumour in individual animals were plotted (n=12). (E–G) TonEBP expression in hepatic tissues of patients with HCC. (E) Representative immunohistochemical images of TonEBP in hepatic biopsies from patients with HCC. (F) TonEBP mRNA in non-tumour and tumour was measured from patients with HBV- (n=23), HCV- (n=7) and alcohol-associated HCC (n=8). Tumour TonEBP expression of each patient was normalised to its non-tumour region TonEBP expression. Mean+SEM, *P<0.05 compared with corresponding non-tumour. (G) Non-tumour TonEBP mRNA was measured from patients who had recurrence within 2 years of resection (solid bar, n=16) and those who did not (open bar, n=21). Mean+SEM, *P<0.05 compared with the open bar. (H) Representative images of TonEBP immunohistochemical staining of non-tumour from tissue arrays processed simultaneously. Staining intensity was assigned to five grades as shown (t0–t4). (I) Kaplan-Meier plot of postoperative recurrence in two layers of patients: tNT0 (t0, n=130) versus tNT1 (t1–t4, n=166). (J) Kaplan-Meier plot of postoperative survival in two layers of patients tT0 (t0–1, n=80) versus tT1 (t2–4, n=216). (K) HEK293 cells were transfected with microRNA (miR)-223 or NC, followed by transfection of a luciferase reporter construct containing 3′-UTR of TonEBP with a putative miR-223-binding site. Luciferase activity is shown in mean+SD (n=3). *P<0.05 compared with NC. (L) HepG2 cells were transfected with miR-223 or NC followed by a 12-hour hypoxia or normoxia. TonEBP and Hsc70 immunoblotting was performed. DEN, diethylnitrosamine; HCC, hepatocellular carcinoma; mRNA, messenger RNA; NC, non-specific control RNA; NT, non-tumour; PBS, phosphate-buffered saline; TonEBP, tonicity-responsive enhancer-binding protein; T, tumour; UTR, untranslated region.
We examined hepatic tissues obtained from 296 patients with HCC (online supplementary table 1). As in the animals, TonEBP mRNA expression was higher in tumours compared with non-tumour regions (online supplementary figure 1B). Immunohistochemical analyses (figure 1E and online supplementary figure 1C) revealed the same pattern of changes in 92.6% of the patients (274/296). This elevation was observed regardless of aetiology (figure 1F). Interestingly, non-tumour TonEBP mRNA expression was higher in patients who had early recurrence compared with patients who did not (figure 1G). These observations suggest importance of TonEBP in tumourigenesis, and that non-tumour TonEBP promotes postoperative recurrence.
Given the importance of TonEBP, we investigated the role of TonEBP further by stratification of the patients according to their TonEBP expression (figure 1H). Univariate analysis of two layers of patients showed that higher non-tumour TonEBP expression was significantly associated with bigger tumour, advanced tumour grade, recurrence, metastasis (ie, extrahepatic metastasis) and protein induced by higher vitamin K absence or antagonist II (PIVKA II) and HBV DNA level (online supplementary table 2). Kaplan-Meier plot confirmed the higher recurrence (figure 1I) and metastasis (online supplementary figure 2A) in patients with higher non-tumour TonEBP expression. Likewise, higher tumour TonEBP expression was associated with advanced tumour grade, microvascular invasion, recurrence, metastasis, death and higher PIVKA II level (online supplementary table 3). Again, Kaplan-Meier plot confirmed the lower survival (figure 1J) and higher metastasis and recurrence (online supplementary figure 2C,D) in patients with higher tumour TonEBP expression. These observations suggest that TonEBP promotes various aspects of tumourigenesis and progression.
We further investigated TonEBP’s role in postoperative prognosis by multivariate analyses. As for recurrence, tumour size, microvascular and lymphovascular invasion along with non-tumour TonEBP expression displayed strong association (table 1). Male sex, alanine transaminase levels, albumin levels, tumour size, tumour grade, microvascular invasion and non-tumour TonEBP expression showed robust association with metastasis. Finally, microvascular invasion, albumin levels and TonEBP expression in tumour were significantly associated with overall survival. We conclude that hepatic TonEBP expression predicts postoperative recurrence, metastasis and death in patients with HCC.
Table 1.
Univariate and multivariate analyses of postoperative recurrence, metastasis and overall survival in patients with HCC (n=296)
| Clinical variables | Recurrence | Metastasis | Overall survival | ||||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||
| Rec (+) n=144 | Rec (−) n=152 | P value | HR (95% CI) |
P value | Metastasis (+) n=61 | Metastasis (−) n=235 | P value | HR (95% CI) |
P value | Death n=84 | Alive n=212 | P value | HR (95% CI) |
P value | |
| Age (years) | 57.6±9.9 | 55.7±9.7 | 0.094 | 58.0±9.9 | 56.3±9.7 | 0.224 | 57.0±11.0 | 56.5±9.4 | 0.715 | ||||||
| Male sex | 118 (81.9%) | 131 (86.2%) | 0.318 | 46 (75.5%) | 203 (86.4%) | 0.037 | 0.506 (0.271 to 0.945) |
0.032 | 69 (82.1%) | 180 (84.9%) | 0.558 | ||||
| Cause of HCC | 0.526 | 0.974 | 0.254 | ||||||||||||
| Alcohol | 14 | 13 | 5 | 21 | 8 | 18 | |||||||||
| HBV | 109 | 125 | 50 | 184 | 63 | 171 | |||||||||
| HCV | 16 | 9 | 4 | 21 | 10 | 15 | |||||||||
| NAFLD | 2 | 3 | 1 | 4 | 0 | 5 | |||||||||
| Undetermined | 3 | 3 | 1 | 5 | 3 | 3 | |||||||||
| AFP (ng/mL) | 3290±19 017 | 3011±15 882 | 0.891 | 8684±31 684 | 1710±10 786 | 0.005 | 5930±25 840 | 2043±12 597 | 0.084 | ||||||
| PIVKA II (mAU/mL) | 1580±8915 | 2299±10 634 | 0.549 | 4150±15 009 | 1365±7843 | 0.057 | 3067±12 679 | 1500±8404 | 0.236 | ||||||
| PT (%) | 98.3±13.8 | 98.4±19.2 | 0.967 | 96.1±14.5 | 99.0±17.3 | 0.231 | 94.3±12.9 | 100.0±17.9 | 0.008 | ||||||
| PLT (103/mm3) | 159±71 | 149±73 | 0.233 | 165±76 | 150±71 | 0.152 | 145±69.7 | 157±73.3 | 0.224 | ||||||
| ALT (IU/L) | 46.0±33.3 | 36.6±19.9 | 0.003 | 35.7±19.0 | 42.6±29.3 | 0.083 | 0.982 (0.968 to 0.996) |
0.014 | 41.5±25.1 | 41.0±26.5 | 0.887 | ||||
| Albumin (g/dL) | 4.15±0.42 | 4.15±0.55 | 0.843 | 4.06±0.43 | 4.18±0.50 | 0.111 | 0.515 (0.302 to 0.879) |
0.015 | 3.9±0.4 | 4.3±0.5 | <0.001 | 0.517 (0.355 to 0.752) |
0.001 | ||
| Total bilirubin (mg/dL) | 0.8±0.5 | 1.3±3.7 | 0.132 | 0.9±0.6 | 1.1±3.0 | 0.545 | 0.9±0.5 | 1.2±3.1 | 0.437 | ||||||
| MELD score | 5.3±3.2 | 5.3±4.5 | 0.915 | 4.8±3.3 | 5.5±4.1 | 0.160 | 5.6±3.1 | 5.2±4.2 | 0.41 | ||||||
| CTP (A/B) | 140/4 | 140/12 | 0.052 | 58/3 | 222/13 | 0.850 | 79/5 | 201/11 | 0.780 | ||||||
| Underlying liver disease (CH/LC) | 27/117 | 36/116 | 0.300 | 27/117 | 36/116 | 0.300 | 13/71 | 50/162 | 0.124 | ||||||
| Tumour size (cm) | 5.1±3.0 | 4.0±3.6 | <0.001 | 0.587 (0.394 to 0.875) |
0.009 | 6.3±3.9 | 4.0±3.0 | <0.001 | 1.114 (1.058 to 1.173) |
<0.001 | 5.2±3.3 | 4.2±3.3 | 0.019 | ||
| <3 cm/>3 cm | 38/106 | 82/70 | <0.001 | 13/48 | 107/128 | 0.001 | 25/59 | 95/117 | 0.017 | ||||||
| Tumour number (single/multiple) | 123/11 | 131/21 | 0.850 | 54/7 | 200/35 | 0.495 | 72/12 | 182/30 | 0.976 | ||||||
| E-S grade (I–II/III–IV) | 59/95 | 85/67 | <0.001 | 14/47 | 120/115 | <0.001 | 0.499 (0.265 to 0.940) |
0.031 | 29/55 | 105/107 | 0.019 | ||||
| Microvascular invasion | 58/86 | 31/121 | <0.001 | 2.320 (1.618 to 3.327) |
<0.001 | 26/35 | 63/172 | 0.016 | 2.123 (1.189 to 3.790) |
0.011 | 40/44 | 105/107 | <0.001 | 2.931 (1.886 to 4.554) |
<0.001 |
| Lymphovascular invasion | 37/107 | 12/140 | <0.001 | 1.856 (1.245 to 2.767) |
0.002 | 18/43 | 31/204 | 0.002 | 17/67 | 32/180 | 0.286 | ||||
| Tumour satellites | 13/121 | 16/136 | 0.665 | 10/51 | 19/216 | 0.052 | 7/77 | 22/190 | 0.594 | ||||||
| Resection margin | 4/140 | 0/152 | 0.055 | 0/61 | 4/231 | 0.584 | 2/82 | 2/210 | 0.319 | ||||||
| Tumour capsule | 37/107 | 32/120 | 0.345 | 18/43 | 51/184 | 0.199 | 21/63 | 48/164 | 0.665 | ||||||
| Tumour septum | 48/96 | 56/96 | 0.527 | 24/37 | 80/155 | 0.440 | 31/53 | 73/139 | 0.668 | ||||||
| TonEBP expression | |||||||||||||||
| TonEBP in non-tumour (tNT 0/1) |
48/96 | 82/70 | <0.001 | 0.566 (0.397 to 0.807) |
0.002 | 20/41 | 110/125 | 0.049 | 0.513 (0.288 to 0.914) |
0.024 | 32/52 | 98/114 | 0.204 | ||
| TonEBP in tumour (tT 0/1) |
29/115 | 51/101 | 0.009 | 10/51 | 70/165 | 0.036 | 15/69 | 65/147 | 0.025 | 0.547 (0.311 to 0.754) |
0.036 | ||||
tNT0 and tNT1 are defined in figure 1I; tT0 and tT1 are defined in figure 1J. A multivariate analysis was carried out to identify the independent predictor for recurrence and survival using the Cox regression hazard model.
AFP, alpha-fetoprotein; ALT, alanine transaminase; CH, chronic hepatitis; CTP, Child-Turcotte-Pugh class; E-S grade, Edmondson-Steiner grade; HCC, hepatocellular carcinoma; LC, liver cirrhosis; MELD score, model for end-stage liver disease score; NAFLD, non-alcoholic fatty liver disease; PIVKA II, protein induced by vitamin K absence or antagonist II; PLT, platelet; PT, prothrombin time; Rec (+), recurrence; Rec (−), non-recurrence; TonEBP, TonEBP, tonicity-responsive enhancer-binding protein.
Hepatic induction of TonEBP is mediated by a fall in the miR-223 abundance
Elevated TonEBP expression is critical for the HCC development and progression. Previous studies showed that miR-223 expression was dramatically suppressed in HCC regardless of aetiology,15 likely due to local hypoxia (online supplementary figure 3A).16 17 As expected, miR-223 abundance was reduced in tumour regions compared with non-tumour regions in patients with HCC (online supplementary figure 3B) and DEN-induced HCC (online supplementary figure 3C). Since TonEBP gene is a target of miR-223 in mouse macrophages,18 we asked whether the hypoxia-mediated fall in miR-223 abundance was responsible for the elevated TonEBP expression. miR-223 interacted with the 3′-untranslated region of human TonEBP mRNA (figure 1K) and lowered the abundance of TonEBP (online supplementary figure 3D,E) both in normoxia and hypoxia (figure 1L). Thus, suppression of miR-223 contributes to the elevated expression of TonEBP in HCC.
TonEBP promotes HCC initiation and growth via oxidative stress and inflammation
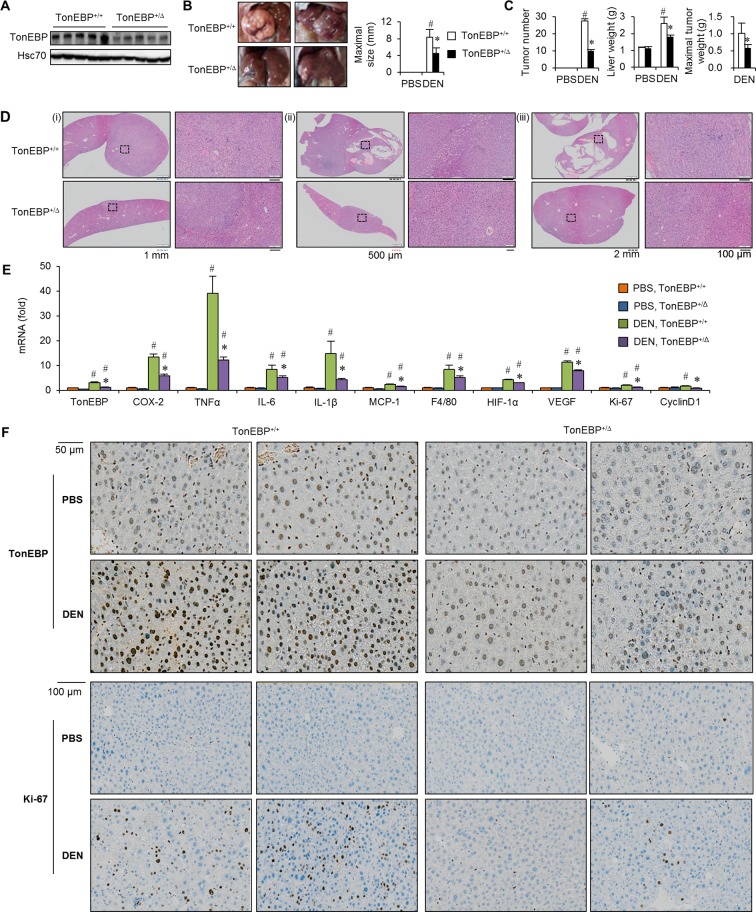
Since hepatic TonEBP expression was closely associated with the number of tumours in a mouse model of HCC (figure 1D), we next investigated the role of TonEBP in HCC using a line of mice with TonEBP haplodeficiency—TonEBP+/Δ (figure 2A).19 Number of tumours and maximal tumour size were both significantly smaller in the TonEBP+/Δ animals than their TonEBP+/+ littermates (figure 2B,C) in association with reduced liver injury (online supplementary table 4). Histological examinations typically revealed smaller tumours (figure 2D(i)), smaller dysplastic nodules (figure 2D(ii)) and often absence of tumours (figure 2D(iii)) in the TonEBP+/Δ animals. Since chronic inflammation leads to cirrhosis followed by HCC,20 we examined hepatic inflammation. Expression of proinflammatory and angiogenic genes was strikingly elevated in HCC mice along with TonEBP (figure 2E), indicating the association of elevated TonEBP expression with inflammation. As expected, expression of these genes was significantly lower in the TonEBP+/Δ animals indicating that TonEBP promoted tumour initiation and growth in association with inflammation and liver injury. In patients with HCC, the association between TonEBP and inflammation was confirmed from analysis of the RNA-seq dataset from TCGA (online supplementary figure 4).
Figure 2.
TonEBP haplodeficiency is resistant to DEN-induced hepatocarcinogenesis. TonEBP+/Δ mice and their TonEBP+/+ littermates were treated with PBS or DEN as in figure 1. (A) Non-tumour regions adjacent to tumours were immunoblotted for TonEBP and Hsc70 from DEN-injected animals. (B) Representative liver images from DEN-injected animals. (C) Tumour number, maximal size of tumours and liver weight from PBS-injected (n=8 for each genotype) or DEN-injected mice (n=18 for each genotype). Mean+SEM. #P<0.05 compared with corresponding PBS-injected animals. *P<0.05 compared with DEN-injected TonEBP+/Δ animals. (D) Representative H&E images of hepatic tissues from DEN-injected animals. Magnified images on the right are from small boxes on the left. Boxes are in tumours (top and bottom of (i), top of (ii) and (iii)), dysplastic nodule (bottom of (ii)) or tumour-free area (bottom of (iii)). (E) real time polymerase chain reaction (RT-qPCR) analyses of TonEBP, inflammatory genes (COX-2, TNFα, IL-1β, IL-6, MCP-1), macrophage marker (F4/80), proliferation markers (Ki-67, cyclin D1) and angiogenic factors (HIF-1α, VEGF) in non-tumour regions adjacent to tumours in animals from (C). (F) Representative immunohistochemical images of TonEBP and Ki-67. COX-2, cyclo-oxygenase-2; DEN, diethylnitrosamine; HIF-1α, hypoxia-inducible factor 1 alpha; IL, interleukin; MCP-1, monocyte chemoattractant protein 1; PBS, phosphate-buffered saline; TNFα, tumour necrosis factor alpha; TonEBP, tonicity-responsive enhancer-binding protein; VEGF, vascular endothelial growth factor.
We noted that proliferation markers were elevated in the mouse model of HCC (figure 2E,F). Inflammatory cytokines are known to promote tumour growth. Interestingly, manipulation of TonEBP expression led to parallel changes in proliferation of HepG2 cells (online supplementary figure 6B,C). Overexpression of TonEBP stimulated proliferation of neighbouring naïve cells (online supplementary figure 6D,E), suggesting that paracrine factors contributed to the TonEBP-dependent stimulation of proliferation. These results provide mechanistic basis for the role of TonEBP on the tumour growth. We conclude that TonEBP promotes tumour initiation and growth via oxidative stress-induced cell injury and inflammation.
DEN causes oxidative stress21 leading to hepatic cell death which, in turn, evokes local inflammation.22 23 TonEBP was induced by hypoxia (figure 1M) and H2O2 (online supplementary figure 5A). Knockdown of TonEBP reduced cell injury (online supplementary figure 5B,C) and inflammation (online supplementary figure 5D) in response to H2O2. Thus, TonEBP mediates cell injury and inflammation in response to oxidative stress.
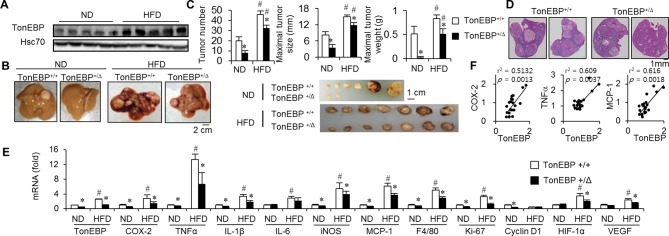
TonEBP is involved in obesity-induced tumour initiation and growth
Epidemiological studies have shown that obesity increases cancer risk.24–26 Obesity-induced chronic inflammation is a major factor contributing to the increased HCC risk.27 Because the data discussed above showed that TonEBP promoted tumour initiation and growth via inflammation, we examined obesity-induced HCC (online supplementary figure 7A). TonEBP+/Δ animals were lighter than their WT littermates both in DEN-treated and PBS-treated groups up to the 12th week of HFD feeding (online supplementary figure 7B). In the DEN-treated group, distinct change took place. After the 26th week, there was a reversion where the TonEBP+/Δ became significantly heavier than their WT littermates because of smaller weight loss. During the reversal of body weight, high blood glucose levels in the WT littermates disappeared (online supplementary figure 7C,D). These changes are likely due to differences in tumour burden.
HFD feeding increased hepatic non-tumour TonEBP expression (figure 3A) along with more and bigger tumours (figure 3B–D), elevated expression of proinflammatory genes, proliferation markers (figure 3E) and liver injury (online supplementary table 5). TonEBP haplodeficiency was associated with fewer and smaller tumours, reduced expression of proinflammatory genes (which correlated with expression of TonEBP (figure 3F)) and proliferation markers, and milder liver injury expression providing strong evidence that TonEBP is involved in the obesity-induced inflammation and hepatocellular carcinogenesis.
Figure 3.
Promotion of hepatocarcinogenesis by HFD is tempered by TonEBP haplodeficiency. Animals were injected with DEN as in figure 2, followed by feeding with ND or HFD for 30 weeks (online supplementary figure S7). (A) Non-tumour regions adjacent to tumours were immunoblotted for TonEBP and Hsc70 in TonEBP+/+ animals. (B) Representative liver images. (C) Top: tumour number, maximal tumour size and maximal tumour weight from individual animals fed with ND (n=12 for each genotype) or HFD (n=15 for each genotype). Mean+SEM, *P<0.05 compared with corresponding TonEBP+/+. #P<0.05 compared with corresponding ND. Bottom: tumours larger than 3 mm in diameter from representative individual animals are shown. (D) Representative images of H&E stained liver tissues from animals fed with HFD. (E) RT-qPCR analyses as in figure 2E. (F) Correlation of TonEBP mRNA expression with mRNA expression of COX-2, TNFα and MCP-1 in non-tumour areas of hepatic tissues from TonEBP+/+ animals fed with ND (n=10) and HFD (n=10). COX-2, cyclo-oxygenase-2; DEN, diethylnitrosamine; HFD, high-fat diet; MCP-1, monocyte chemoattractant protein 1; mRNA, messenger RNA; ND, normal diet; TNFα, tumour necrosis factor alpha; TonEBP, tonicity-responsive enhancer-binding protein.
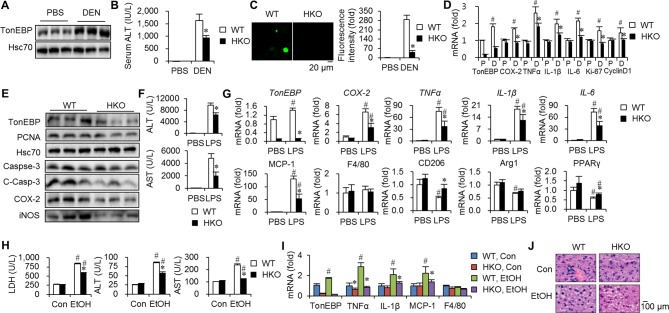
TonEBP in hepatocytes and macrophages mediates DEN-induced cellular injury and inflammation
The data discussed above show that TonEBP promotes inflammation critical in tumour initiation and growth. Since DEN results in cellular injury leading to inflammation and eventually HCC,21 we examined TonEBP’s role in hepatic injury using a line of mice with hepatocyte-specific TonEBP knockout (HKO).11 Hepatic TonEBP expression, especially TonEBP immunohistochemical signal in hepatocytes, was dramatically reduced in these animals (online supplementary figure 8A,B). In WT mice, hepatic TonEBP is elevated 48 hours after DEN treatment (figure 4A) consistent with oxidative injury. DEN-induced hepatic injury was reduced in the HKO animals compared with WT littermates (figure 4B), manifested by lower terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) signal (figure 4C) and caspase-3 activity (figure 4E). Expression of Ki-67 and proliferating cell nuclear antigen (PCNA) was significantly lower in the HKO after DEN treatment along with proinflammatory genes (figure 4D,E). Phosphorylation of mitogen activated-protein kinase (MAPKs) was not affected (online supplementary figure 8C), indicating that upstream signalling was not affected in the HKO. Taken together, hepatocyte TonEBP mediates DEN-induced hepatic injury and inflammation.
Figure 4.
Liver injury and inflammation are tempered by hepatocyte-specific TonEBP deficiency. (A–D) TonEBPfl/fl; Albumin cre+/− mice (HKO) and their TonEBPfl/fl; Albumin cre−/− (WT) littermates were injected with DEN or PBS. Livers and serum samples were analysed 48 hours later. (A) Livers from the WT animals were immunoblotted for TonEBP and Hsc70. (B) Serum ALT levels in PBS-treated (n=5) and DEN-treated animals (n=8). Mean+SEM. *P<0.05 compared with WT. (C) TUNEL signal in hepatic tissues. (D) RT-qPCR analyses of TonEBP, inflammatory genes (COX-2, TNFα, IL-1β, IL-6) and proliferation markers (Ki-67, cyclin D1) in livers of DEN-treated animals. mRNA abundance was expressed relative to WT group. P, PBS; D, DEN. Mean+SEM, #P<0.05 compared with corresponding P. *P<0.05 compared with WT. (E) Immunoblotting of liver samples, three from each genotype, for TonEBP, PCNA, Hsc70, caspase-3, C-Casp-3, COX-2 and iNOS. (F,G) HKO and WT animals were injected with PBS or LPS (5 µg/kg) in combination with D-galactosamine (400 mg/kg) (LPS). The animals were analysed 4 hour later. (F) Serum levels of ALT and AST. (G) RT-qPCR analysis mRNA levels. mRNA abundance was expressed relative to WT, PBS group. Mean+SEM, *P<0.05 compared with WT. #P<0.05 compared with PBS. (H–J) The animals were fed with Con or EtOH for 10 days. (H) Serum ALT, AST and LDH levels. *P<0.05 compared with WT. #P<0.05 compared with corresponding Con-fed animals. (I) RT-qPCR analyses of TonEBP and inflammatory genes in livers. (J) Representative images of H&E of hepatic tissues from animals from (H). ALT, alanine transaminase; AST, aspartate aminotransferase; C-Casp-3, cleaved caspase-3; iNOS, inducible nitric oxide synthase; Con, control diet; COX-2, cyclo-oxygenase-2; DEN, diethylnitrosamine; EtOH, ethanol diet; HKO, hepatocyte-specific TonEBP knockout; IL, interleukin; LDH, lactate dehydrogenase; LPS, lipopolysaccharide; mRNA, messenger RNA; PBS, phosphate-buffered saline; TNFα, tumour necrosis factor alpha; TonEBP, tonicity-responsive enhancer-binding protein; WT, wild type.
As TonEBP is a critical regulator of the proinflammatory activation of macrophages,6 28 we examined the role of macrophage TonEBP using a line of mouse with myeloid-specific TonEBP knockout (MKO).6 These animals displayed normal TonEBP expression in hepatocytes (online supplementary figure 8D). DEN-induced hepatic injury, inflammation and proliferation were reduced in the MKO compared with WT littermates (online supplementary figure 8E,F). These data demonstrate that macrophage TonEBP also contributes to the DEN-induced hepatic injury and inflammation.
TonEBP mediates LPS-induced and alcohol-induced hepatic inflammation
We asked whether TonEBP mediates inflammation induced by agents other than DEN. First, we examined LPS which leads to hepatocyte death and inflammation.29 LPS-responsive hepatic injury (figure 4F) and inflammation (figure 4G) were tempered in the HKO. The effect of TonEBP on LPS-induced inflammation was confirmed in HepG2 cells (online supplementary figure 9A).
Alcohol causes leakage of LPS from gut into circulation leading to hepatic inflammation.30 Since alcoholic hepatitis is a risk factor for HCC31 and TonEBP mediates LPS-induced hepatocyte inflammation as discussed above, the role of TonEBP in alcohol-induced hepatic injury was examined. The chronic and binge alcohol feeding resulted in hepatic damage, inflammation and fat accumulation (figure 4H–J). Hepatic inflammation and injury were reduced in the HKO animals (figure 4H,I) without significant changes in fat accumulation (figure 4J). These data demonstrate that hepatocyte TonEBP mediates alcohol-induced hepatic injury and inflammation to which the TonEBP-mediated LPS-responsive hepatic inflammation described above contributes.
To understand the role of TonEBP in hepatic inflammation in general, we analysed inflammatory stimuli-responsive inflammation. Interleukin (IL)-1β- or tumour necrosis factor alpha-induced inflammation was attenuated in TonEBP-deficient HepG2 cells (online supplementary figure 9B,C) and non-cancerous alpha mouse liver (AML)-12 cells (online supplementary figure 9D). Taken together, these data demonstrate that TonEBP is a general mediator of hepatic inflammation induced by diverse agents, consistent with elevated TonEBP expression regardless the cause of HCC (figure 1G).
TonEBP promotes COX-2 expression in a YY1-dependent manner
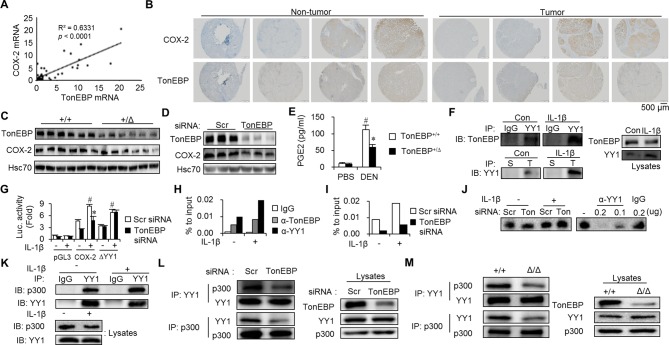
Transcriptional targets of the inflammatory enhanceosome, in which TonEBP is a limiting component, include cyclo-oxygenase-2 (COX-2).6 We investigated COX-2 because it promotes tumourigenesis,32–34 and COX-2 expression in patients with HCC correlates with postoperative recurrence35 like TonEBP. In patients with HCC, COX-2 expression was elevated in tumour regions compared with non-tumour regions (online supplementary figure 10A). TonEBP expression correlated positively with COX-2 expression (figure 5A,B and online supplementary table 6). TonEBP deficiency resulted in reduced COX-2 expression (figure 5C,D and online supplementary figure 10B,C) leading to reduced production of prostaglandin E2 (PGE2) in various animal models (figure 5E and online supplementary figure 10D–F) and HepG2 cells (online supplementary figure 10G). Altered TonEBP expression by hypoxia and miR-223 was accompanied by parallel changes in the COX-2 expression (online supplementary figure 10H). In addition, the TonEBP-dependent stimulation of proliferation (online supplementary figure 6D,E) was dependent on COX-2 (online supplementary figure 10I). These data demonstrate that TonEBP promotes tumourigenesis and recurrence by, at least in part, the stimulation of COX-2 expression.
Figure 5.
TonEBP-dependent stimulation of COX-2 requires transcription factor YY1. (A) TonEBP versus COX-2 mRNA abundance in tumours from 25 patients with HCC. (B) Immunohistochemical images of COX-2 and TonEBP in consecutive hepatic sections from two representative patients with HCC. (C) Immunoblots of livers from TonEBP+/Δ (+/Δ) and their TonEBP+/+ littermates (+/+) from figure 2A. (D) Immunoblots of HepG2 cells transfected with siRNA followed by treatment with IL-1β. (E) Serum PGE2 levels in PBS-treated (n=5) or DEN-treated mice (n=8 for each genotype) from figure 2. Mean+SEM. *P<0.05 compared with TonEBP +/+. #P<0.05 compared with PBS-injected mice. Serum PGE2 levels in mean+SEM. *P<0.05 compared with DEN-injected TonEBP+/Δ animals. (F) Lysates of HepG2 cells treated with IL-1β or not (con) as indicated were immunoprecipitated (IP) with normal IgG, anti-YY1 IgG (YY1), normal serum (S) or anti-TonEBP serum (T). The immunoprecipitates and cell lysates were immunoblotted as indicated. (G) HepG2 cells were transfected with siRNA indicated followed by transfection with pGL3 (vector), COX-2 (COX-2 promoter reporter in pGL3) or ΔYY1 (COX-2 whose YY1 binding site was disabled by mutagenesis) (n=3). (H) HepG2 cells were treated with IL-1β as indicated. ChIP was performed using anti-TonEBP IgG, anit-YY1 IgG or normal IgG. YY1 binding region in the COX-2 promoter was quantified using RT-qPCR. Means of two independent experiments are shown. (I) HepG2 cells were transfected with siRNA and treated with IL-1β. ChIP was performed with anti-YY1 IgG. Means of two independent experiments are shown. (J) Nuclear extracts were prepared from HepG2 transfected with siRNA followed by treatment with IL-1β as indicated. Electrophoretic mobility shift assay was performed (see supplementary material and methods). Anti-YY1 IgG or normal IgG was used to supershift the YY1–DNA complex (right). (K) Lysates of HepG2 cells treated with IL-1β were immunoprecipitated. Lysates and immunoprecipitates were immunoblotted for p300 and YY1. (L) Cells transfected with siRNA were immunoprecipitated as above. (M) MEFs from TonEBP+/+ (+/+) and TonEBPΔ/Δ mouse (Δ/Δ) were immunoprecipitated. ChIP, chromatin immunoprecipitation; COX-2, cyclo-oxygenase-2; DEN, diethylnitrosamine; HCC, hepatocellular carcinoma; IL, interleukin; MEF, mouse embryo fibroblast; mRNA, messenger RNA; PBS, phosphate-buffered saline; PGE2, prostaglandin E2; siRNA, small interfering RNA; TonEBP, tonicity-responsive enhancer-binding protein.
To understand the molecular mechanism underlying the regulation of COX-2, promoter reporters were constructed using human sequence. Initial analyses showed that TonEBP stimulated the COX-2 promoter without DNA binding to its cognate sites (online supplementary figure 11A,B). We hypothesised that TonEBP might be a transcriptional cofactor of a DNA-binding protein within 1 kb from the transcription start site. To identify such a protein, TonEBP-interacting proteins were identified using liquid chromatography–tandem mass spectrometry. One of the proteins picked by the procedure was the transcription factor YY1 (data not shown). We confirmed that YY1 and TonEBP were mutually coimmunoprecipitated; plus the interactions were stimulated by IL-1β without changes in their expression (figure 5F). Knockdown of TonEBP or YY1 blunted the induction of COX-2 mRNA by IL-1β to the same extent, but the effect of double knockdown was not additive (online supplementary figure 11C). TonEBP-dependent COX-2 promoter activity was dependent on the YY1 binding site (figure 5G) demonstrating that TonEBP action required DNA binding of YY1.
TonEBP promotes YY1 recruitment to the COX-2 promoter in response to inflammation
Chromatin immunoprecipitation (ChIP) was performed to investigate the interaction of YY1 and TonEBP at the COX-2 promoter in situ. Interestingly, YY1 bound to its putative binding site of the COX-2 promoter in an IL-1β-dependent manner along with TonEBP (figure 5H) consistent with their interactions. Knockdown of TonEBP dramatically reduced of YY1 binding to the promoter (figure 5I), whereas YY1 expression (online supplementary figure 11D), nuclear translocation (online supplementary figure 11E) and binding to its promoter site (figure 5I) were not affected. Thus, YY1 binding to its cognate site in the COX-2 promoter requires TonEBP.
TonEBP is responsible for the recruitment of the histone acetyltransferase p300, a critical regulator of COX-2 transcription,36 to the inflammatory enhanceosome.6 Here, we found that YY1 interacted with p300 in HepG2 cells and the interaction showed an IL-1β responsiveness (figure 5K) as well as TonEBP dependence (figure 5L), indicating that YY1 was incorporated into the inflammatory enhanceosome on the COX-2 promoter. This observation was supported by reduced interaction between YY1 and p300 in TonEBPΔ/Δ MEF cells where the protein product of the TonEBPΔ allele does not interact with p300.6
To characterise the interaction between TonEBP and YY1, we performed coimmunoprecipitation experiments. Overexpressed TonEBP and YY1 were mutually pulled down by each other (figure 6B). Coimmunoprecipitation with overexpressing various recombinant YY1 proteins (figure 6A) revealed that the spacer domain of YY1 was critical for the interaction with TonEBP (figure 6C). Next, Yc1, an N-terminal-truncated TonEBP with intact Rel-homology domain (RHD) (figure 6A) interacted with YY1 in a spacer-dependent manner (figure 6D), indicating that the C-terminal two-thirds of TonEBP was dispensable for the interaction. Deletion of RHD domain abolished the interaction with YY1 (figure 6E), indicating the importance of RHD. Thus, the interaction between TonEBP and YY1 is mediated by RHD and spacer domain.
Figure 6.
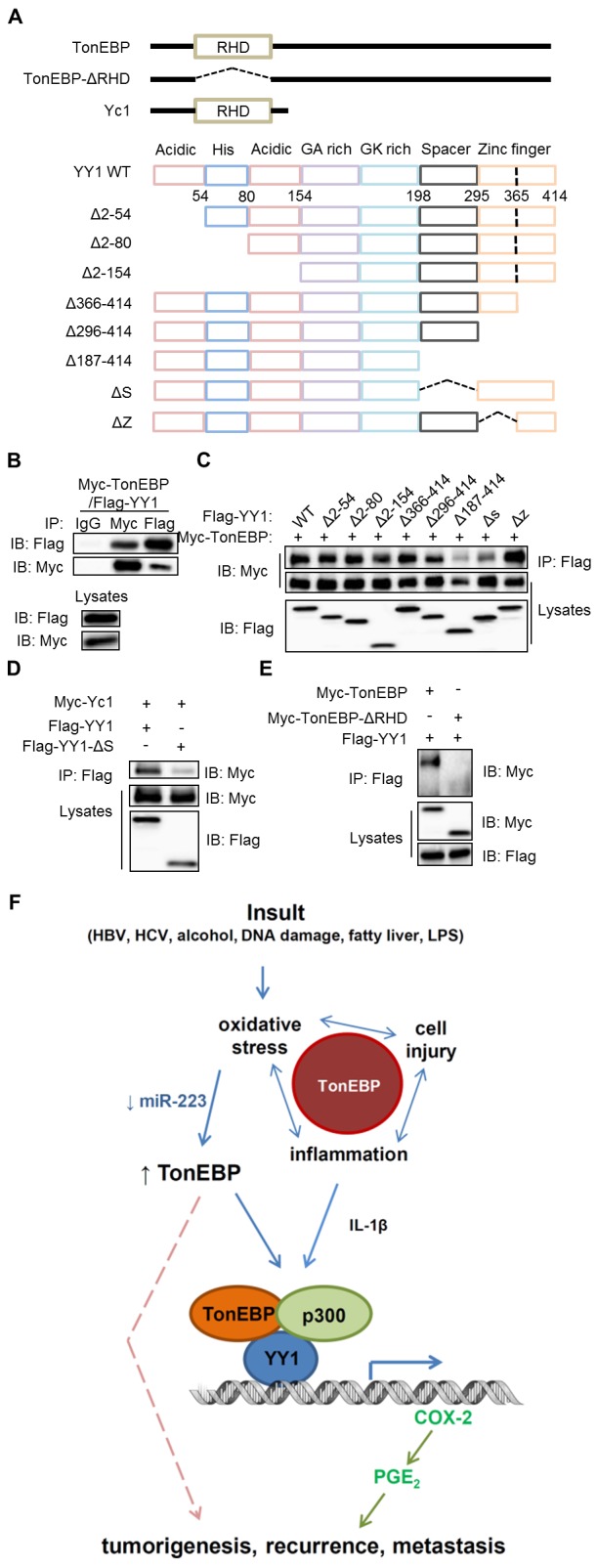
TonEBP and YY1 interact via RHD and spacer. (A) Diagrams of TonEBP and YY1 and their deletion constructs. (B–E) Lysates from HEK293 cells transfected with Myc-TonEBP plus Flag-YY1 (B) or various recombinant fragment of Flag-YY1 (C), Myc-Yc1 plus or Flag-YY1 or Flag-YY1-ΔS (D) or Flag-YY1 plus Myc-TonEBP or Myc-TonEBP-ΔRHD (E) were immunoprecipitated with normal IgG, anti-Flag IgG or anti-Myc IgG as indicated. The immunoprecipitates (upper) and lysates (bottom) were immunoblotted as indicated. (F) Model of TonEBP actions in the pathogenesis of HCC. First, TonEBP mediates oxidative stress-induced cell injury and local inflammation. Second, TonEBP expression is enhanced by oxidative stress. On the promoter of the COX-2 gene, the elevated TonEBP recruits the acetyltransferase p300 to YY1 in a manner dependent on IL-1β. TonEBP promotes tumourigenesis, recurrence and metastasis via PGE2 and possibly other pathways (broken line). See text for details. COX-2, cyclo-oxygenase-2; Flag, anti-Flag IgG; GA rich, glycine-alanin rich; GK rich, glycine-lysine rich; HCC, hepatocellular carcinoma; IB, immunoblot; IgG, normal IgG, Myc, anti-Myc IgG; PGE2, prostaglandin E2; IL, interleukin; IP, immunoprecipitated; LPS, lipopolysaccharide; RHD, Rel-homology domain; TonEBP, tonicity-responsive enhancer-binding protein.
In summary, local inflammation stimulates the assembly of an enhanceosome on the COX-2 promoter (figure 6F, bottom). This assembly is dependent on YY1 binding to its cognate site on DNA. Of interest, TonEBP is required for the recruitment of both YY1 and p300 to the promoter in situ. The critical role of TonEBP in PGE2 production provides a mechanistic basis for the strong association of hepatic TonEBP with postoperative recurrence, metastasis and death in patients with HCC.
Discussion
Given that HCC is a heterogeneous disease with diversity in aetiological agents, tumour architectures, histological characteristics, repertoire of oncogenic mutations and gene expression profiles, it is remarkable that elevated TonEBP expression in tumours over non-tumour regions is observed in more than 90% of patients with HCC. TonEBP is more prevalent than any other immunohistochemical biomarker of HCC and the first one associated with inflammation.37 38 The widespread elevation in tumour TonEBP levels demonstrates that inflammation is the common feature of heterogeneous HCC. In the cohort of patients with HCC studied here, higher tumour TonEBP expression predicts death and is associated with tumour size, grade, recurrence and metastasis. COX-2 expression is driven by the elevated TonEBP leading to the production of PGE2 which promotes tumourigenesis and progression,32–34 providing a mechanistic basis for the TonEBP as a part of the common pathway activated by the diverse aetiological agents (figure 6F).
Hepatic resection has been the treatment of choice for early HCC. However, resection is associated with a 70% recurrence rate in the remaining hepatic tissue.39 Postoperative recurrence is classified as early or late depending on whether the recurrence occurs within 2 years of resection or not. Remarkably, TonEBP mRNA expression was higher in the non-tumour regions of patients who had early recurrence compared with those who did not. In the cohort of patients with HCC, higher non-tumour TonEBP expression predicts recurrence and metastasis and is associated with tumour size and grade. We observe COX-2 expression is also driven by TonEBP in this region. PGE2 is known to educate inflammatory tumour microenvironment to promote tumour progression by orchestrating crosstalk between tumour cells and their microenvironment.32–34 Thus, the TonEBP–COX-2–PGE2 pathway contributes to recurrence. Given the complexity of cellular mechanisms involved in recurrence,39 we suspect that there might be other pathways of TonEBP such as cell migration10 that contribute to recurrence (figure 6F).
Studies in various mouse models of HCC and hepatitis reveal additional TonEBP actions upstream of inflammation. The diverse insults that cause HCC impose oxidative stress40 leading to cell injury and inflammation. Experiments with TonEBP-deficient animals and cultured hepatocytes demonstrate that TonEBP promotes the cell injury and inflammation in response to oxidative stress (figure 6F). In addition, TonEBP expression is dramatically elevated in response to oxidative stress providing a positive reinforcement. Thus, TonEBP is intrinsically involved in multiple steps in the cellular pathways stimulated by the various insults that cause HCC. This explains the strong association of hepatic TonEBP and poor prognosis in patients with HCC. On the other hand, since our patient cohort is heavily biased to HBV-driven HCC, the association needs more robust verification in patients with other aetiologies.
The data presented here suggest that targeting TonEBP is an attractive strategy to prevent recurrence as well as hepatocarcinogenesis and metastasis. In this regard, three distinct action sites of TonEBP can be considered (figure 6F): (1) cell injury and inflammation; (2) induction of TonEBP by oxidative stress and downregulation of miR-223 and (3) transcriptional stimulation of COX-2 and other proinflammatory genes. We reported that a small compound cerulenin inhibited the transcriptional stimulation of proinflammatory genes including COX-2 by blocking the interaction of TonEBP and p300.6 We find that cerulenin blocks the IL-1β-induced transcription of COX-2 in hepatocytes (data not shown). Cerulenin and other agents that target any of the three sites of TonEBP action might be proved efficacious in preventing recurrence and metastasis for patients with HCC whose hepatic TonEBP expression is high.
Acknowledgments
We thank the patients who generously provided tissues in our studies. Dr Neuhofer provided TonEBP floxed mice. Jin Hoi Hur assisted in the microscope-associated work.
Footnotes
Contributors: JHL, NHP and HMK conceived, directed and interpreted results and wrote the manuscript. JHL designed, performed and analysed most of the experiments. SYC helped to design and to interpret results and wrote the manuscript. JHS performed histological analysis and performed and analysed tissue array and IHC. NHP and JHS provided human HCC samples. HJK, HHL, BJY, SWJ and CJK contributed to the performance experiments. GRL provided YY1 constructs. KM, WL-K and JP provided intellectual input. NHP and HMK supervised experiments.
Funding: This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government (Ministry of Science and ICT) (NRF-2011-0020163, NRF-2010-0029621, NRF-2016R1D1A1B03932335, NRF-2012R1A1A2043693, NRF-2017R1E1A1A01074673), Health Technology R&D Project grant of Korea (HI16C1837) and the Institute for Basic Science (IBS-R022-D1-2016) of Korea. This work was also supported by UNIST fund (1.170068.01). JHL was supported by Global PhD Fellowship of Korea (NRF-2014H1A2A1019656).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: This research was approved by the Institutional Review Board at the Ulsan University Hospital (UUH 2015-12-018).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Forner A, Bruix J. Biomarkers for early diagnosis of hepatocellular carcinoma. Lancet Oncol 2012;13:750–1. 10.1016/S1470-2045(12)70271-1 [DOI] [PubMed] [Google Scholar]
- 2. El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–27. 10.1056/NEJMra1001683 [DOI] [PubMed] [Google Scholar]
- 3. Marquardt JU, Andersen JB, Thorgeirsson SS. Functional and genetic deconstruction of the cellular origin in liver cancer. Nat Rev Cancer 2015;15:653–67. 10.1038/nrc4017 [DOI] [PubMed] [Google Scholar]
- 4. Calderaro J, Couchy G, Imbeaud S, et al. . Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol 2017;67:727–38. 10.1016/j.jhep.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 5. Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 2010;29:4989–5005. 10.1038/onc.2010.236 [DOI] [PubMed] [Google Scholar]
- 6. Lee HH, Sanada S, An SM, Sm A, et al. . LPS-induced NFκB enhanceosome requires TonEBP/NFAT5 without DNA binding. Sci Rep 2016;6:24921 10.1038/srep24921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoon HJ, You S, Yoo SA, et al. . NF-AT5 is a critical regulator of inflammatory arthritis. Arthritis Rheum 2011;63:1843–52. 10.1002/art.30229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi S, You S, Kim D, et al. . Transcription factor NFAT5 promotes macrophage survival in rheumatoid arthritis. J Clin Invest 2017;127:954–69. 10.1172/JCI87880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halterman JA, Kwon HM, Leitinger N, et al. . NFAT5 expression in bone marrow-derived cells enhances atherosclerosis and drives macrophage migration. Front Physiol 2012;3:313 10.3389/fphys.2012.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jauliac S, López-Rodriguez C, Shaw LM, et al. . The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol 2002;4:540–4. 10.1038/ncb816 [DOI] [PubMed] [Google Scholar]
- 11. Küper C, Beck FX, Neuhofer W. Generation of a conditional knockout allele for the NFAT5 gene in mice. Front Physiol 2014;5:507 10.3389/fphys.2014.00507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bertola A, Mathews S, Ki SH, et al. . Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 2013;8:627–37. 10.1038/nprot.2013.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elinav E, Nowarski R, Thaiss CA, et al. . Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013;13:759–71. 10.1038/nrc3611 [DOI] [PubMed] [Google Scholar]
- 15. Wong QW, Lung RW, Law PT, et al. . MicroRNA-223 is commonly repressed in hepatocellular carcinoma and potentiates expression of Stathmin1. Gastroenterology 2008;135:257–69. 10.1053/j.gastro.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 16. Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med 2011;364:656–65. 10.1056/NEJMra0910283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology 2012;55:622–33. 10.1002/hep.25497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ying W, Tseng A, Chang RC, et al. . MicroRNA-223 is a crucial mediator of PPARγ-regulated alternative macrophage activation. J Clin Invest 2015;125:4149–59. 10.1172/JCI81656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Go WY, Liu X, Roti MA, et al. . NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci U S A 2004;101:10673–8. 10.1073/pnas.0403139101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alderton GK. Inflammation: the gut takes a toll on liver cancer. Nat Rev Cancer 2012;12:379–79. 10.1038/nrc3283 [DOI] [PubMed] [Google Scholar]
- 21. Paula Santos N, Colaço A, Gil da Costa RM, et al. . N-diethylnitrosamine mouse hepatotoxicity: time-related effects on histology and oxidative stress. Exp Toxicol Pathol 2014;66:429–36. 10.1016/j.etp.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 22. Fan X, He C, Jing W, et al. . Intracellular osteopontin inhibits toll-like receptor signaling and impedes liver carcinogenesis. Cancer Res 2015;75:86–97. 10.1158/0008-5472.CAN-14-0615 [DOI] [PubMed] [Google Scholar]
- 23. Wu J, Li J, Salcedo R, et al. . The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res 2012;72:3977–86. 10.1158/0008-5472.CAN-12-0938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol 2002;3:565–74. 10.1016/S1470-2045(02)00849-5 [DOI] [PubMed] [Google Scholar]
- 25. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579–91. 10.1038/nrc1408 [DOI] [PubMed] [Google Scholar]
- 26. Calle EE, Rodriguez C, Walker-Thurmond K, et al. . Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med Overseas Ed 2003;348:1625–38. 10.1056/NEJMoa021423 [DOI] [PubMed] [Google Scholar]
- 27. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010;51:1820–32. 10.1002/hep.23594 [DOI] [PubMed] [Google Scholar]
- 28. Buxadé M, Lunazzi G, Minguillón J, et al. . Gene expression induced by Toll-like receptors in macrophages requires the transcription factor NFAT5. J Exp Med 2012;209:379–93. 10.1084/jem.20111569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Su GL, Gl S. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol 2002;283:G256–G65. 10.1152/ajpgi.00550.2001 [DOI] [PubMed] [Google Scholar]
- 30. Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol 2010;16:1304–13. 10.3748/wjg.v16.i11.1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist 2010;15(Suppl 4):14–22. 10.1634/theoncologist.2010-S4-14 [DOI] [PubMed] [Google Scholar]
- 32. Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer 2010;10:181–93. 10.1038/nrc2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer 2001;1:11–21. 10.1038/35094017 [DOI] [PubMed] [Google Scholar]
- 34. Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003;3:276–85. 10.1038/nrc1046 [DOI] [PubMed] [Google Scholar]
- 35. He YF, Jin J, Wei W, et al. . Overexpression of cyclooxygenase-2 in noncancerous liver tissue increases the postoperative recurrence of hepatocellular carcinoma in patients with hepatitis B virus-related cirrhosis. Can J Gastroenterol 2010;24:435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deng WG, Zhu Y, Wu KK. Role of p300 and PCAF in regulating cyclooxygenase-2 promoter activation by inflammatory mediators. Blood 2004;103:2135–42. 10.1182/blood-2003-09-3131 [DOI] [PubMed] [Google Scholar]
- 37. Tsuchiya N, Sawada Y, Endo I, et al. . Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol 2015;21:10573–83. 10.3748/wjg.v21.i37.10573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao YJ, Ju Q, Li GC. Tumor markers for hepatocellular carcinoma. Mol Clin Oncol 2013;1:593–8. 10.3892/mco.2013.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sherman M. Recurrence of hepatocellular carcinoma: Mass Medical Soc, 2008. [DOI] [PubMed] [Google Scholar]
- 40. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 2006;6:674–87. 10.1038/nrc1934 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2017-315348supp001.pdf (1.7MB, pdf)