Non-motor disturbances in Parkinson’s disease: Globally, population aged 60 or over is growing and considering the World Bank predictions for the next 20 years it is expected that the number of Parkinson’s disease (PD) cases will double at the end of this period thus, reaching an impressive 13–39 million patients worldwide. This scenario is potentially associated with a significant global negative impact on public health systems particularly in countries with increased ageing populations such as the European countries, Asia and Americas. The pathophysiology of PD involves the progressive degeneration of dopaminergic neurons of the substantia nigra pars compacta (SNpc) that triggers denervation of the nigrostriatal pathway and consequent significant reduction of dopamine in the dorsal striatum. Such process leads to a critical motor impairment scenario characterized by bradykinesia, rigidity, resting tremor, and postural instability (Emamzadeh and Surguchov, 2018). However, several other non-motor disturbances develop earlier, thus, being considered as prodromal signs of the neurodegeneration. In this sense, the literature is increasingly emphasizing the importance of investigating mood and olfactory disruptions as high sensitive benchmarks of the early-phase disease. In fact, the need for innovative early-phase diagnostic tools, as well as the elucidation of the pathophysiological mechanisms of such disturbances, is essential priority in the field of investigation of PD. Remarkably, in the year of 2015, a woman was known for an intriguing ability of “smell PD”. Her husband had lived with the disease for twenty years and during this process she noticed that his odor slowly changed to a musky smell. Interestingly, this woman could associate this particular smell with PD after meeting different people with this distinct odor in a charity for PD patients. In fact, this clever observation is aligned with studies showing that hyposmia is found decades before the motor onset. Another type of anecdotal story, frequently reported by the patients, is the often cases of burnt food during cooking. They just fail to sense the burnt smell generating a very frustrating feeling of incapacity.
The notion of that is reinforced by the occurrence of hyposmia in more than 90% of the patients as a result of an increase in the number of periglomerular neurons within the glomerular layer of the olfactory bulb. Likewise, depression is highly related with PD, affecting 30-50% of the patients, although, it is extensively reported a close relationship between depression and olfactory impairment in a non-Parkinsonism context. Depression affects granular and periglomerular interneuron activity, leading to an important reduction of olfactory sensitivity. Also, a previous study from our group have shown that a bilateral olfactory bulbectomy is considered a reliable experimental model of depression in rats, since surgical removal results in hypothalamic and limbic alterations leading to depressive-like behaviors and reduced nigral brain-derived neurotrophic factor levels (Maturana et al., 2014). In fact, this neurochemical outcome impacts dopamine and serotonin levels in the striatum and hippocampus, structures which are closely related to the nucleus accumbens that collectively define the mood circuitry. Interestingly, these areas are part of the brain reward pathway. Thus, the mutual association between neurochemical dysfunctions of this neural mood circuitry and the pathophysiological mechanisms of hyposmia may contribute to increase anhedonia in PD. This raises the central question of how hyposmia could potentially worsen the symptoms of depression. This field is actually very recent and lacks substantial background about the role of different neurons within the olfactory bulb and their interactions with other circuitries classically affected during PD.
Hyposmia and depression in PD, where do we stand: Within the glomerular layer of the olfactory bulb resides a group of dopaminergic interneurons, so-called periglomerular neurons, that plays an inhibitory role (due to dopamine D2 receptor via Gi/o-coupled activation) on olfactory receptor cells and mitral/tufted neurons (Huisman et al., 2004; Mundinano et al., 2011), thus, modulating the transmission of the olfactory stimulus. It is described that during PD degeneration the topographical pattern of the lesions first develop at the olfactory bulb together with related portions of the anterior olfactory nucleus (Braak et al., 2004). Also, remarkably, there is a significant increase in the number of the periglomerular neurons in humans and in rats (Huisman et al., 2004; Mundinano et al., 2011; Rodrigues et al., 2014) raising the hypothesis that this increase is a compensatory response to the loss of dopaminergic neurons in the SNpc (Doty, 2012). This hypothesis is also supported by our recent study that demonstrates an olfaction restoration, observed in a rat model of PD, as a result of a selective dopaminergic lesion (due to 6-hydroxydopamine - 6-hydroxydopamine (6-OHDA) - infusion), within the glomerular layer (Ilkiw et al., 2018). Such interneurons are generated in the subventricular zone (SVZ), a structure associated with the third ventricle and, together with the subgranular zone of the hippocampus, is responsible for adult cell proliferation, present in both rodents and humans. Physiologically, SVZ is responsible for the differentiation of stem cells in neuroblasts that migrate through the rostral migratory pathway to the granular and glomerular layers of the olfactory bulb where they differ in the dopaminergic interneurons (Hoglinger et al., 2004). However, in situations of massive degeneration, those neurons may intensely migrate to the affected regions (Figure 1) apparently in an attempt to regenerate the region and reestablish connections, as demonstrated by the increase of 5-bromo-2-deoxyuridine (BrdU+) neurons in the dorsal striatum in a model of ischemic stroke (Hoehn et al., 2005), as well as in 6-hydroxydopamine-induced PD model (Worlitzer et al., 2012). According to our data, periglomerular neurons appear to have a key role in olfaction because selective lesions of those cells promote a negative impact on this sensory processing, probably eliciting migration of newborn neurons from the SVZ. Complementarily, an acute lesion of the periglomerular layer is able to counteract the olfactory impairment provoked by the SNpc dopaminergic lesion, reinforcing this protagonism.
Figure 1.
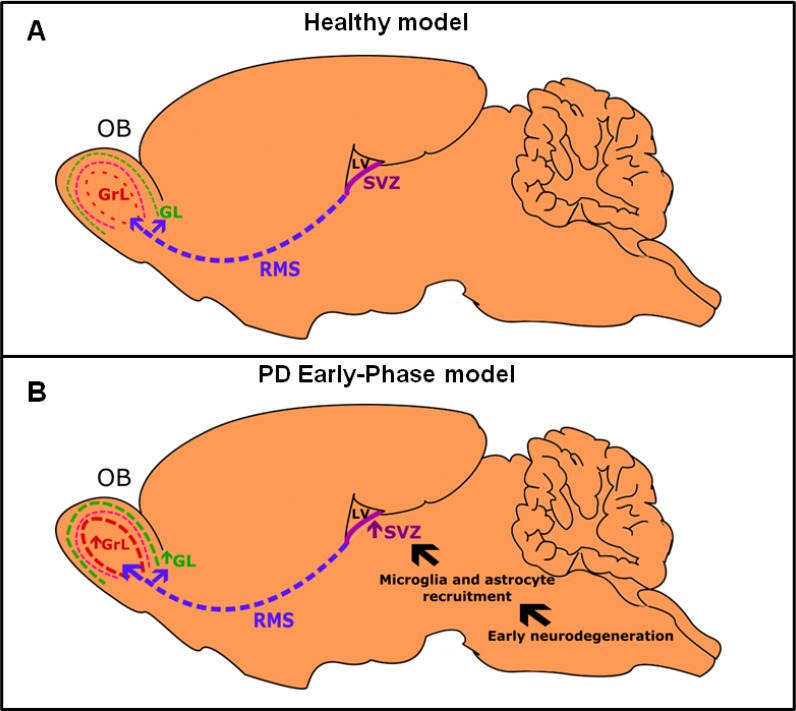
Pattern of interneuron migration towards the olfactory bulb.
In healthy subjects (A), the subventricular zone (SVZ) is responsible for generating newborn neurons. Once they are born, these neurons migrate through rostral migratory stream (RMS) until they reach the olfactory bulb (OB) where they differentiate in granular interneurons at the granular layer (GrL) and periglomerular interneurons at glomerular layer (GL). According to Braak staging, Parkinson’s disease (PD) alterations begin in both OB and brainstem, possibly causing the non-motor disturbances. (B) The early neurodegeneration elicits an important recruitment of neuroinflammatory cells, especially microglia and astrocytes signaling the SVZ to intensify the process of neurogenesis and respective migration towards the OB. Such mechanism is supposedly responsible for creating a circuitry able to promote a potent inhibition of mitral/tufted neurons leading to preclinical olfactory impairment in PD. LV: Lateral ventricle.
Neurons from the glomerular layer of the olfactory bulb are heavily innervated by serotonergic neurons, originating in the raphe nuclei. Deafferentation of those serotonergic fibers generates anosmia and olfactory bulb atrophy in mice (Moriizumi et al., 1994). In order to understand whether serotonin improves or worsens the olfactory function, some studies investigated PD patients, at early stages, observing an upregulation of raphe nuclei serotonin transporter (SERT). However, reductions of SERT were demonstrated in advanced PD patients (Pagano et al., 2017), and were associated with the onset of depression and resting tremor. Nevertheless, no correlation is found between olfactory impairment and SERT binding in several brain structures of those patients, suggesting that SERT decreased levels have no major role in olfactory dysfunction. Conversely, in animal models with mutations in PINK1, SNCA, LRRK2, and Parkin genes, reductions in serotonergic fibers, serotonin levels and SERT binding, within the glomerular layer, are potentially associated with olfactory impairments. To date, certainly there is no consensus about how and when serotonergic neurotransmission impacts olfaction in PD.
Indeed, our recent study showed an interesting difference on depressive-like behaviors after a single dopaminergic lesion of the glomerular layer, compared to a double dopaminergic lesion paradigm (glomerular layer plus SNpc). In the former it was only produced behavior despair, without anhedonia. However, in the latter, it was detected decreased swimming, increased immobility, and anhedonic-like behavior. Hence, we believe that such result support the rationale of a maturation process of a retrograde lesion towards the SNpc. Together with the fact that depression causes reductions in olfactory threshold, identification and discrimination abilities in humans, and patients with congenital anosmia are more expected to exhibit signs of depression, it is plausible to suggest a bidirectional relation between hyposmia and depression. Such bidirectional relation appears to be very pronounced in PD and, in some level, recapitulates our central question, previously raised.
Translational limitations of data from animal models of PD: Despite the highly conserved structural and functional features of the main olfactory system along mammals it is worth mentioning that studies from animal models present important limitations, particularly regarding the biological significance of this chemical sense. In natural environments, animals face more complex tasks such as identify odor concentration and its background, odor recognition and odor source localization. Altogether, these suggest that for rodents, odors can represent a detailed spatial map implying that olfaction is more crucial for rodents than for humans. In addition, the investigation of depressive-like behaviors is also a good example of how challenging is to translate the therapeutic benefits observed in preclinical tests to a clinical perspective. Combined, the investigation of how olfaction is influenced by depressive-like behaviors, or vice versa, can be considered an import and very puzzling step for the field of PD.
Conclusions and future perspectives: Our study (Ilkiw et al., 2018) originally demonstrated that a dopaminergic lesion of the glomerular layer is able to produce olfactory deficits and depressive-like behaviors. Remarkably, the acute lesion of the glomerular layer counteracted the olfactory impairment caused by a previous SNpc injury. We demonstrated that this effect is due to a compensatory increment in the number of periglomerular neurons, occurred after the nigral lesion, being strongly associated with the olfactory impairment. Once this neuronal increment is reversed, depressive-like behaviors emerge. This reinforces the idea of a bidirectional relation between hyposmia and depression in PD.
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Araucária (Programa de Apoio a Núcleos de Excelência - PRONEX), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant No. 431279/2016-0) Brazil. MMSL is recipient of CNPq fellowship (grant No. 305986/2016-3).
Additional file: Open peer review report 1 (146.8KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Andrei Surguchov, Kansas Uniiversity Medical Center, USA.
References
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8:329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- Emamzadeh FN, Surguchov A. Parkinson’s disease: biomarkers, treatment, and risk factors. Front Neurosci. 2018;12:612. doi: 10.3389/fnins.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Huisman E, Uylings HB, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov Disord. 2004;19:687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- Ilkiw JL, Kmita LC, Targa ADS, Noseda ACD, Rodrigues LS, Dorieux FWC, Fagotti J, Dos Santos P, Lima MMS. Dopaminergic lesion in the olfactory bulb restores olfaction and induces depressive-like behaviors in a 6-OHDA model of Parkinson’s disease. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-1134-5. doi:10.1007/s12035-018-1134-5. [DOI] [PubMed] [Google Scholar]
- Maturana MJ, Pudell C, Targa AD, Rodrigues LS, Noseda AC, Fortes MH, Dos Santos P, Da Cunha C, Zanata SM, Ferraz AC, Lima MM. REM sleep deprivation reverses neurochemical and other depressive-like alterations induced by olfactory bulbectomy. Mol Neurobiol. 2014;51:349–360. doi: 10.1007/s12035-014-8721-x. [DOI] [PubMed] [Google Scholar]
- Moriizumi T, Tsukatani T, Sakashita H, Miwa T. Olfactory disturbance induced by deafferentation of serotonergic fibers in the olfactory bulb. Neuroscience. 1994;61:733–738. doi: 10.1016/0306-4522(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Mundinano IC, Caballero MC, Ordonez C, Hernandez M, DiCaudo C, Marcilla I, Erro ME, Tunon MT, Luquin MR. Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol. 2011;122:61–74. doi: 10.1007/s00401-011-0830-2. [DOI] [PubMed] [Google Scholar]
- Pagano G, Niccolini F, Fusar-Poli P, Politis M. Serotonin transporter in Parkinson’s disease: A meta-analysis of positron emission tomography studies. Ann Neurol. 2017;81:171–180. doi: 10.1002/ana.24859. [DOI] [PubMed] [Google Scholar]
- Rodrigues LS, Targa AD, Noseda AC, Aurich MF, Da Cunha C, Lima MM. Olfactory impairment in the rotenone model of Parkinson’s disease is associated with bulbar dopaminergic D2 activity after REM sleep deprivation. Front Cell Neurosci. 2014;8:383. doi: 10.3389/fncel.2014.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worlitzer MM, Bunk EC, Hemmer K, Schwamborn JC. Anti-inflammatory treatment induced regenerative oligodendrogenesis in parkinsonian mice. Stem Cell Res Ther. 2012;3:33. doi: 10.1186/scrt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


