Abstract
Introduction
The main symptom of fibromyalgia (FM) is diffuse pain. There is currently no aetiological treatment for FM. However, all pain associations and best practice guidelines strongly advocate the practice of aerobic physical activity to improve the symptoms of FM subjects. The mechanisms of dysfunctional pain are mostly central and related to stress axis dysfunction (autonomic nervous system and corticotropic axis). Our main objective is to assess the efficacy of a specific training programme on endogenous pain control mechanisms in female patients with FM. Further aims include rebalancing the autonomic neurovegetative system, improving quality of life and sleep quality, and reintegrating patients into society and work.
Methods and analysis
110 female patients with FM diagnosed on American College of Rheumatology 2010 criteria, aged 18–65 years and meeting inclusion conditions will be recruited and randomised into two groups (active and semiactive). The training programme will consist of three 45 min sessions per week of supervised, individualised physical activity over 2 years. Only the intensity of the exercises will differ between the two groups (moderate intensity vs low intensity).
All outcome measures will be conducted at baseline (T0), after 6–9 months of training (T6–9) and after 24 months of training (T24). The primary endpoint will be an improvement of pain modulation (activation of diffuse noxious inhibitory control) evaluated by the stimulation test. The secondary endpoint will be relief of pain, anxiety, depression, stress, sleep disorders, pain impact on life quality, and improved heart rate, blood pressure and salivary cortisol.
Ethics and dissemination
This study is approved by the Committee for the Protection of Persons West VI. The results will be published in specialised scientific journals and will be presented at scientific meetings on pain and/or physical activity.
Trial registration number
NCT02486965; Pre-results.
Keywords: pain management, rehabilitation medicine, fibromyalgia
Strengths and limitations of this study.
First randomised controlled double-blind trial to assess the effects of a long-term training programme (24 months) on pain control in fibromyalgia.
The protocol of the training programme is designed to rebalance the neurovegetative system and thereby treat fibromyalgia.
Physical activity intensity will be assessed objectively using a heart rate monitor.
The drop-out rate in patients may be high.
Due to the nature of the intervention, the coaching staff cannot be blinded.
Introduction
Fibromyalgia (FM) affects 1.4%–2.2% of the general population, predominately women (more than 80% of subjects). This syndrome is characterised by extensive diffuse pain, mainly muscular and articular, impairing the functional abilities of the subjects. The symptoms most frequently described by patients with FM are chronic fatigue, sleep disorders, cognitive disorders and emotional disturbances.1 2 They lead to a severe deterioration in the quality of life, sometimes with physical disability leading to social isolation and difficulties staying in employment (recurrent sick leave).
Diagnosis is based on the symptoms and their severity as described by the patients.3–6 There is currently no aetiological treatment for FM syndrome. Treatments are therefore only symptomatic.
Physiopathology of FM
The mechanisms of dysfunctional pain, with no identifiable organic lesions, are mostly central7 and related to dysfunction of the stress axis (autonomic nervous system and corticotropic axis).8 9
At rest, patients with FM show an increased sympathetic response and decreased parasympathetic tone.10 11 This neurovegetative dystonia is a marker of dysfunction of the stress axis.12
Malfunctions of the corticotropic axis in FM have often been described, also marking the dysfunction of the stress axis. However, the form taken by this dysfunction differs according to the study.13–17 The variability of salivary cortisol in response to stress reflects that of plasma free cortisol. Salivary cortisol is, therefore, a method of choice in experimental stress studies.18 Whatever their form, these dysfunctions all compromise the body’s adaptation to daily stressful stimuli.
Studies show that this deficit of the stress axis (neurovegetative dystonia and dysfunctional corticotropic axis) is concurrent with FM19 and associated with altered pain control.8 9 The pain control system and the stress axis have close anatomical and functional links. Nociceptive, neurovegetative and corticotropic systems interact with the central nervous system. The central neuromediators involved in the regulation of the stress axis are mostly common with those of pain neuromodulation (endogenous opioids, norepinephrine, serotonin, etc).
Elite athlete’s overtraining syndrome: a model of stress axis dysfunction
An overtraining elite athlete may be considered as a model of dysfunction of the stress axis associated with neurovegetative dystonia. The physical and psychological effort of training is known to induce stress. High-level athletes can present an overtraining syndrome when the adaptation limits of the stress axis are reached. This stress-induced phenomenon corresponds to an imbalance between training quantity and recovery. Overtrained athletes present a deconditioning syndrome close to FM symptoms (chronic pain, sleep disorders, neurovegetative dystonia, intense fatigue, etc).20–24
Physical activity and FM
Most studies have shown that physical activity is more efficacious on FM symptoms than pharmacological treatments.25 26 Literature reviews and meta-analyses strongly support the benefits of physical training in patients with FM (decreased pain and depression and improvement in overall health and physical abilities).27 The practice of aerobic exercise in patient with FM is strongly recommended by The American Pain Society,28 the Association of Medical Scientific Societies in Germany,29 the Canadian Rheumatology Association3 and the European League Against Rheumatism.30 Physical exercise is the first-line treatment recommended in FM, but there is still no consensus on the modalities of such training (frequency, duration and intensity). The mechanisms underlying these specific training effects remain to be determined.
Steady physical activity rebalancing the autonomic system is associated with cardiovascular benefits. Physical activity increases parasympathetic tone and decreases sympathetic response.31–34 Mechanisms and structures involved in the activation and regulation of the neurovegetative system may interact with the central nervous system. Central relationships between the neurovegetative system and the motor cortex, the limbic system, the hypothalamus, the pituitary gland and the basal ganglia result in the release of analgesic neurotransmitters such as noradrenalin, serotonin and endogenous opioids.35 36 This release of neurotransmitters due to exercise leads to increased endogenous inhibition and so decreases diffuse pain in FM.35 Central nervous system plasticity induced by physical training can regulate both cardiovascular adaptations34 and endogenous pain control mechanisms.37 38 Thus, strategies to rebalance the autonomic system are the most promising therapies for FM.11 33 34
In this study, we set out to validate a therapeutic alternative that aims to treat FM by rebalancing the stress axis. This treatment consists of a specific, supervised, individualised training programme lasting 2 years. This training protocol is individually adjusted to rebalance the neurovegetative system (parasympathetic and sympathetic). Central neuroplasticity induced by training should regulate endogenous pain control mechanisms. This specific protocol will be associated with psychotherapeutic approaches. In addition to the training programme, multidisciplinary biopsychosocial care will be given at the pain centre of the University Hospital of Brest.3
Objectives
Our main objective is to assess the efficacy of a specific training programme on endogenous pain control in patients with FM. Our secondary objectives are to rebalance the autonomic nervous system and the corticotropic axis, improve life and sleep quality and reintegrate patients into society and work.
Methods and analysis
Design and setting
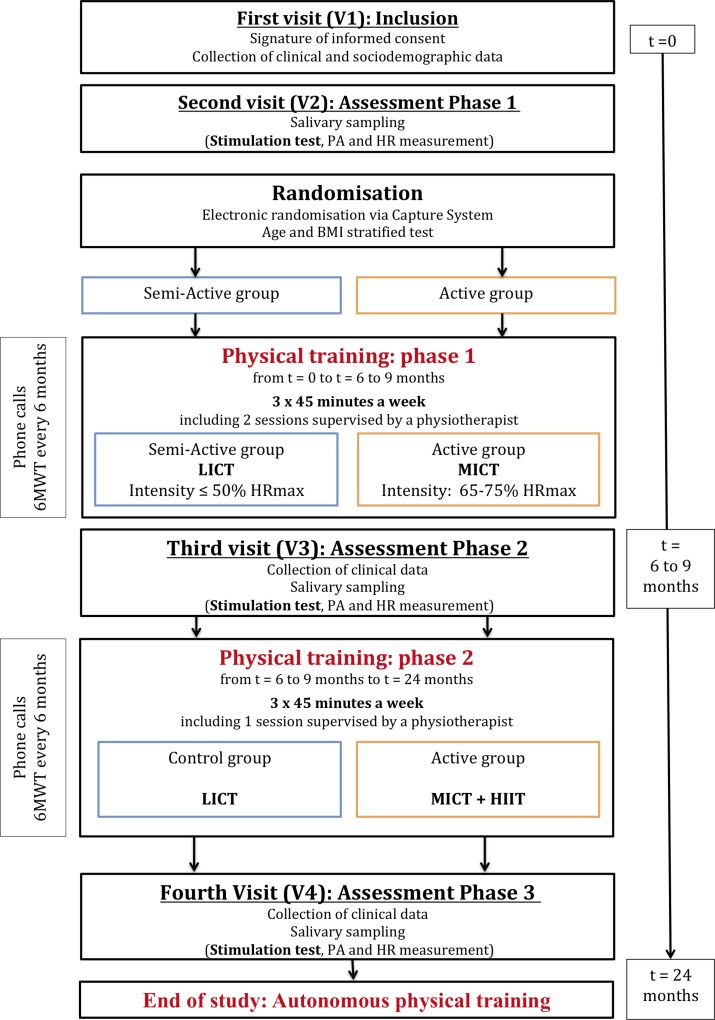
This randomised, double-blind trial will compare an ‘active’ programme to a ‘semiactive’ programme in patients with FM. Patients will be recruited at the pain centre of the University Hospital of Brest on the basis of general criteria. Patients are to follow a re-exercise programme for 24 months. The assessments will take place (1) before, (2) between 6 and 9 months (depending the training level) and (3) at the end of training (24 months), in the neurological functional explorations department of the University Hospital of Brest (figure 1).
Figure 1.
Flow chart of DouFiSport. BMI, body mass index; HIIT, high-intensity interval training; HR, heart rate; HRmax, maximum heart rate; LICT, low-intensity continuous training; MICT, moderate-intensity continuous training; 6MWT, 6 min walk test; PA, physical activity.
Patient involvement
The specific training programme of this study was developed based on the results of a pilot study,39 data from literature and the experiences of patients with FM recorded at the pain centre of the University Hospital of Brest. These patients reported the benefits, constraints, difficulties and effects of their training programme on their symptoms. This information has allowed adjustments to be made to the specific training programme. Patients are not involved in the recruitment and conduct of the study. At the last assessment visit, patients will be asked to assess the burden of the programme. On request, a report outlining the study findings will be given to study participants.
Study population
One hundred and ten patients with FM will be included. The inclusion criteria are: female; aged 18–65 years; diagnosis of FM clearly established according to the criteria of the American College of Rheumatology 2010; body mass index (BMI) 18.5–29.9 kg/m²; spontaneous pain intensity higher than 3/10 on a Visual Analogue Scale (VAS); pain experienced at least 3 days a week; pain caused by palpation greater than or equal to 4/10 on a VAS.
The non-inclusion criteria are: systemic disease (treated or not) generating pain of the musculoskeletal system; pain other than FM; contraindication to physical activity; any active health disorder; change in the last 2 months in any pharmacological treatment; psychiatric diagnosis; taking drugs that affect cortisol secretion (decrease or increase); non-cooperating.
Sample size
Population size is calculated on an expected difference of 20 points (stimulation test)7 between the two groups, for a quantitative primary endpoint (delta VAS) of SD equal to 35, and a power set at 80%. At least 48 subjects per group are therefore required. To take into account lost to follow-up, a sample of 110 subjects, that is, 55 per group, will be recruited.
Randomisation
Patients will be randomised at the end of the first stimulation test, just before the initiation of the training. Randomisation will be conducted by the Centre for Clinical Investigation at the University Hospital of Brest (electronic randomisation via Capture System). The test will be stratified by age and BMI. The cut-off will be set at 50 years for age and 25 kg/m2 for BMI (two strata (18–25) and (25–30)).
Training programme
The training programme is planned over 2 years (24 months) for both groups (active/semiactive). A minimum of 4–6 weeks is needed to observe a decrease in symptoms.39 This 2-year duration is the minimum average training time (depending on the individual progress of each patient) necessary to regain a central neuroplasticity sufficient to restore diffuse noxious inhibitory controls (DNICs) and the neurovegetative system.40
The frequency, intensity and duration of these training sessions are based on both data from the literature39 41 and the results of a preliminary study. The pain was significantly reduced and symptoms such as quality of life, sleep quality and anxiety were also strongly improved in subjects who had undergone this specific training after 5 years.40
The American Pain Society recommends an intensity of 60%–70% of the age-adjusted maximum heart rate (HRmax). At the early stage, the intensity and duration of the training sessions will be adapted to the physical condition of each subject. The intensity exercise will be 3 on the Borg CR10 scale.39 To promote adherence of our patients and to limit pain exacerbation, exercise intensity will start very low and then increase very gradually to reach the neurovegetative goal.28 42
The ideal frequency is three training sessions per week each lasting 45 min.39 40
Active training group
First 6–9 months
Subjects will perform three sessions per week of 45 min of moderate-intensity continuous training (MICT) (65%–75% HRmax), including two sessions supervised by a physiotherapist specially trained and one independent session.
From 6–9 months (according to pace, abilities and limits) to 24 months
Patients will begin the second stage of training: three sessions per week of at least 45 min each (MICT and high-intensity interval training, HIIT) with one supervised session and two independent sessions. When the patient reaches the initial HR goal, continuous training will be associated with interval training. HIIT will consist of five stages of 1–4 min at 85%–90% HRmax, interspersed by 1–4 min of active recovery at 65%–75% HRmax. Intensity will be assessed objectively using an HR monitor (FT2, Polar). At baseline, Tanaka’s age-based prediction equation (208−0.7×age) will calculate HRmax. After 6–9 months of training, a maximal-effort graded exercise test will determine HRmax and maximum oxygen consumption for each patient.
Semiactive training group
Patients will perform the same infra-active training (low-intensity continuous training: <50% HRmax) for 2 years. Supervision, monitoring and frequency of sessions (3×45 min per week) in both groups will be equivalent.
Training follow-up (for both groups)
Patients will be contacted to record progress, difficulties and if necessary to encourage them to adhere to their programme. These calls will improve compliance and limit patients lost to follow-up.43 44 Subjects will note the characteristics (frequency, duration, intensity, type of activity and supervision) of each training session (both supervised and independent) in a specific training logbook. The physiotherapist will frequently ask patients about their independent training session to provide advice and motivate them. The follow-up at the pain centre will assess compliance with the training protocol.
Patients will perform a 6 min walk test every 6 months (with a physiotherapist). If a patient cannot achieve the specific training requested after 9 months of study, then she will not complete the second phase of training, but will nevertheless attend all assessment visits. The main analysis will be performed on an intention-to-treat basis.
Clinical data, measurements and assessments
Sociodemographic and clinical data
At baseline, data on age, sex, marital status, education level and occupation will be collected. Height and weight will be recorded. Medical background and pain characteristics will be noted. All current drug and non-drug therapies (including tried and stopped) will also be collected, together with their effectiveness on pain.
Questionnaires and pain assessments
Measurements and questionnaires will be carried out (1) at baseline, (2) between 6 and 9 months and (3) at the end of the 24 months of training.
The assessment of pain will be performed by a simple verbal scale and using a VAS. The Saint Antoine Pain Questionnaire will also assess pain. A pain quantitative assessment will be performed with a pressure algometer (pressure pain threshold: PPT).
The Hospital Anxiety Depression Scale (HADS) will assess the patient’s anxiodepressive state.45
The Fibromyalgia Impact Questionnaire will assess the impact of FM on daily life.46
The Pittsburgh Sleep Quality Index will assess sleep quality and quantity.47 48
The International Physical Activity Questionnaire will record the level of physical activity and sedentary lifestyle. The French long telephone questionnaire will be used.49
The Perceived Stress Scale will assess the antecedents of perceived stress.50
Stimulation test
To assess endogenous pain mechanisms, such as DNICs, temporal summation (TS) and perception of pain, we will use an experimental method developed by Tousignant-Laflamme et al and Marchand.7 51 According to this well-characterised paradigm, we will induce in single session two tonic heat pain stimulations separated by a cold pressor test.7 51–53
Thermode test or TS test (P1): a tonic heat pain will be administered for 2 min on the patient’s right arm, using a thermode (CE marking No. 226). The starting temperature is 32°C (skin temperature under normal conditions in a temperate room (20°C–22°C))54 and will quickly reach a fixed value. The experimental temperature will be individually determined to induce 50/100 on a VAS and will remain constant during the test period (2 min). Throughout this period, the patient will evaluate her pain intensity using a Computerised Visual Analogue Scale (CoVAS).
Cold pressor test (P2): to elicit a prolonged pain sensation to trigger DNIC,55 the patient’s right arm will be immersed for 2 min in a cold water bath maintained at 12°C. The patient will continuously evaluate her pain intensity using a CoVAS.
Following this cold pressor test, the thermode test will be performed again (P3).
Pain difference between the two (P3/P1) tonic heat pain stimulations will measure DNIC activation and represents pain modulation.
Measurement of salivary cortisol and salivary flow
Corticotropic axis will be assessed using a measurement of salivary cortisol. Cortisol release is pulsatile (10–20 peaks per day) and follows a nychthemeral cycle. Cortisol level peaks in the early morning. In the morning of each consultation (at baseline, in the middle and at the end of training), patients will collect a salivary sample (1) for 2 min, when they wake up and (2) for 2 min, 30 min later. The salivary cortisol level is measured in nmol/L. The flow rate is calculated in mL/min. Samples will be frozen at −20°C. As salivary cortisol is stable, samples can be stored for many weeks in a freezer.56 After completion of all assessment sessions, sample analysis will be completed. To avoid interlaboratory variation, the same laboratory will assay the samples.
Recording of blood pressure and HR
After 10 min at rest, lying down, blood pressure (BP) and HR will be recorded. BP and HR will then be measured when the patient stands up and once per minute for 4 min while standing.
Blinding strategy
Patients will not be informed of their group (active/semiactive). The investigators will not know the patient’s group. Due to the nature of the intervention (physical activity protocol), the coaching staff will not be blinded.
Statistical analysis
Primary endpoint analysis: The VAS improvements (stimulation test) obtained in the two groups will be compared using Student’s t-test. If the required normality assumption is not sustainable, a non-parametric Wilcoxon signed-rank test will be used. An alpha risk of 0.05 will be set as the limit of statistical significance. The main analysis will be performed on an intention-to-treat basis. A complementary analysis using a linear model with adjustment for age and BMI factors will be completed.
The secondary endpoints (quantitative data: salivary cortisol, BP, PPT quantified by pain threshold pressure, questionnaire assessment) will be analysed in a similar way by comparing the improvements obtained between the two groups.
Methodological limitations
The methodology of this protocol is consistent with the recommendations of the Standard Protocol Items: Recommendations for Interventional Trials. However, because of the nature of the intervention, the coaching staff cannot be blinded. Patients and investigators will be blinded.
Given the study duration (2 years), potential participant drop-out and lost to follow-up may be high. These risks were taken into account in setting sample size. To limit drop-out, patients will be called to encourage them and to discuss any difficulties. In the second stage of training and to limit any long-term monotony effect, physical activity type can be diversified in both groups. The training session will be adapted in the active group (MICT will be associated with HIIT) to reduce sympathetic hyperactivity. To improve compliance and long-term achievement of training, the patient may choose the physical activity type performed without supervision.
Ethics and dissemination
Consent to participate
Patients will be informed of the objectives, constraints, risks and benefits of the study. To be included, patients must sign informed written consent. Data will be collected anonymously. The investigators will take all necessary precautions to ensure the confidentiality of the information, in particular with regard to patient identity.
Dissemination plan
The results of this study will be published in specialised scientific journals. These results will also be presented in scientific meetings on pain and/or physical activity. In addition, a doctoral thesis will be written on this project.
Supplementary Material
Acknowledgments
The authors thank Julie Lelièvre, Marie Le Bellego and Youenn-Thor Bodéré for designing and implementing this protocol. The authors thank Richard Ryan for English language editing.
Footnotes
Patient consent for publication: Not required.
Contributors: CB initiated the idea for the project. CB and ALFB developed the study design. MC, GL, BQ, AK, AW, SM, M-AG-M, FR, LM and AD provided advice for the study design. GL and CB were responsible for supervision of project. CB will conduct the recruitment. AK will conduct the training programme. CB, ALFB and MC will conduct the outcomes assessments and will contribute to the analysis and interpretation of the data. Both authors will contribute to the analyses and interpretation of the data. ALFB, CB and MC wrote early drafts of the manuscript. All authors approved the final version of this protocol.
Funding: This work is supported by the French Ministry of Health (Programme Hospitalier de Recherche Clinique Interrégional 2014, project No. 2014-A00743-44) PHRCi 13-100.
Competing interests: None declared.
Ethics approval: The Committee for the Protection of Persons West VI approved this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Wolfe F, Smythe HA, Yunus MB, et al. . The american college of rheumatology 1990 criteria for the classification of fibromyalgia. report of the multicenter criteria committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]
- 2. Cánovas R, León I, Roldán MD, et al. . Virtual reality tasks disclose spatial memory alterations in fibromyalgia. Rheumatology 2009;48:1273–8. 10.1093/rheumatology/kep218 [DOI] [PubMed] [Google Scholar]
- 3. Fitzcharles MA, Shir Y, Ablin JN, et al. . Classification and clinical diagnosis of fibromyalgia syndrome: recommendations of recent evidence-based interdisciplinary guidelines. Evid Based Complement Alternat Med 2013;2013:1–9. 10.1155/2013/528952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Egloff N, von Känel R, Müller V, et al. . Implications of proposed fibromyalgia criteria across other functional pain syndromes. Scand J Rheumatol 2015;44:416–24. 10.3109/03009742.2015.1010103 [DOI] [PubMed] [Google Scholar]
- 5. Moyano S, Kilstein JG, Alegre de Miguel C. New diagnostic criteria for fibromyalgia: Here to stay? Reumatol Clin 2015;11:210–4. 10.1016/j.reuma.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 6. Wolfe F, Fitzcharles M-A, Goldenberg DL, et al. . Comparison of physician-based and patient-based criteria for the diagnosis of fibromyalgia. Arthritis Care Res 2016;68:652–9. 10.1002/acr.22742 [DOI] [PubMed] [Google Scholar]
- 7. Tousignant-Laflamme Y, Pagé S, Goffaux P, et al. . An experimental model to measure excitatory and inhibitory pain mechanisms in humans. Brain Res 2008;1230:73–9. 10.1016/j.brainres.2008.06.120 [DOI] [PubMed] [Google Scholar]
- 8. Woda A, Dao T, Gremeau-Richard C. Steroid dysregulation and stomatodynia (burning mouth syndrome). J Orofac Pain 2009;23:202–10. [PubMed] [Google Scholar]
- 9. Woda A, L’heveder G, Ouchchane L, et al. . Effect of experimental stress in 2 different pain conditions affecting the facial muscles. J Pain 2013;14:455–66. 10.1016/j.jpain.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 10. Cohen H, Neumann L, Shore M, et al. . Autonomic dysfunction in patients with fibromyalgia: application of power spectral analysis of heart rate variability. Semin Arthritis Rheum 2000;29:217–27. 10.1016/S0049-0172(00)80010-4 [DOI] [PubMed] [Google Scholar]
- 11. da Cunha Ribeiro RP, Roschel H, Artioli GG, et al. . Cardiac autonomic impairment and chronotropic incompetence in fibromyalgia. Arthritis Res Ther 2011;13:R190 10.1186/ar3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinez-Lavin M. Fibromyalgia as a sympathetically maintained pain syndrome. Curr Pain Headache Rep 2004;8:385–9. 10.1007/s11916-996-0012-4 [DOI] [PubMed] [Google Scholar]
- 13. Kadetoff D, Kosek E. Evidence of reduced sympatho-adrenal and hypothalamic-pituitary activity during static muscular work in patients with fibromyalgia. J Rehabil Med 2010;42:765–72. 10.2340/16501977-0597 [DOI] [PubMed] [Google Scholar]
- 14. Tak LM, Cleare AJ, Ormel J, et al. . Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biol Psychol 2011;87:183–94. 10.1016/j.biopsycho.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 15. Riva R, Mork PJ, Westgaard RH, et al. . Comparison of the cortisol awakening response in women with shoulder and neck pain and women with fibromyalgia. Psychoneuroendocrinology 2012;37:299–306. 10.1016/j.psyneuen.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 16. Geiss A, Rohleder N, Anton F. Evidence for an association between an enhanced reactivity of interleukin-6 levels and reduced glucocorticoid sensitivity in patients with fibromyalgia. Psychoneuroendocrinology 2012;37:671–84. 10.1016/j.psyneuen.2011.07.021 [DOI] [PubMed] [Google Scholar]
- 17. Bote ME, García JJ, Hinchado MD, et al. . Inflammatory/stress feedback dysregulation in women with fibromyalgia. Neuroimmunomodulation 2012;19:343–51. 10.1159/000341664 [DOI] [PubMed] [Google Scholar]
- 18. Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009;34:163–71. 10.1016/j.psyneuen.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 19. Clauw D, Ablin J. The relationship between « stress » and pain: lessons learned from fibromyalgia and related conditions Castro L, Current topics in pain. Seattle: XIIth World Congress on PainIASP press, 2009:245–70. [Google Scholar]
- 20. McKenzie DC. Markers of excessive exercise. Can J Appl Physiol 1999;24:66–73. 10.1139/h99-007 [DOI] [PubMed] [Google Scholar]
- 21. MacKinnon LT. Special feature for the Olympics: effects of exercise on the immune system: overtraining effects on immunity and performance in athletes. Immunol Cell Biol 2000;78:502–9. 10.1111/j.1440-1711.2000.t01-7-.x [DOI] [PubMed] [Google Scholar]
- 22. Iellamo F, Pigozzi F, Parisi A, et al. . The stress of competition dissociates neural and cortisol homeostasis in elite athletes. J Sports Med Phys Fitness 2003;43:539–45. [PubMed] [Google Scholar]
- 23. Baumert M, Brechtel L, Lock J, et al. . Heart rate variability, blood pressure variability, and baroreflex sensitivity in overtrained athletes. Clin J Sport Med 2006;16:412–7. 10.1097/01.jsm.0000244610.34594.07 [DOI] [PubMed] [Google Scholar]
- 24. Kajaia T, Maskhulia L, Chelidze K, et al. . The effects of non-functional overreaching and overtraining on autonomic nervous system function in highly trained athletes. Georgian Med News 2017;264:97–103. [PubMed] [Google Scholar]
- 25. Fontenele JB, Felix FH. Exercise for fibromyalgia: evidence for an integrated modulation of autonomic and nociception neural regulation. Rheumatol Int 2012;32:4075–6. 10.1007/s00296-011-2255-6 [DOI] [PubMed] [Google Scholar]
- 26. Rossy LA, Buckelew SP, Dorr N, et al. . A meta-analysis of fibromyalgia treatment interventions. Ann Behav Med 1999;21:180–91. 10.1007/BF02908299 [DOI] [PubMed] [Google Scholar]
- 27. Busch AJ, Webber SC, Brachaniec M, et al. . Exercise therapy for fibromyalgia. Curr Pain Headache Rep 2011;15:358–67. 10.1007/s11916-011-0214-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Society AP éditeur. Guideline for the management of fibromyalgia syndrome pain in adults and children. American Pain Society 2005:109. [Google Scholar]
- 29. Häuser W, Arnold B, Eich W, et al. . Management of fibromyalgia syndrome–an interdisciplinary evidence-based guideline. Ger Med Sci 2008;6:Doc14. [PMC free article] [PubMed] [Google Scholar]
- 30. Macfarlane GJ, Kronisch C, Atzeni F, et al. . EULAR recommendations for management of fibromyalgia. Ann Rheum Dis 2017;76:e54 10.1136/annrheumdis-2017-211587 [DOI] [PubMed] [Google Scholar]
- 31. Melanson EL, Freedson PS. The effect of endurance training on resting heart rate variability in sedentary adult males. Eur J Appl Physiol 2001;85:442–9. 10.1007/s004210100479 [DOI] [PubMed] [Google Scholar]
- 32. Reland S, Ville NS, Wong S, et al. . Exercise heart rate variability of older women in relation to level of physical activity. J Gerontol A Biol Sci Med Sci 2003;58:B585–B591. 10.1093/gerona/58.7.B585 [DOI] [PubMed] [Google Scholar]
- 33. de Abreu SB, Lenhard A, Mehanna A, et al. . Role of paraventricular nucleus in exercise training-induced autonomic modulation in conscious rats. Autonomic Neuroscience 2009;148(1-2):28–35. 10.1016/j.autneu.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 34. Martins-Pinge MC. Cardiovascular and autonomic modulation by the central nervous system after aerobic exercise training. Braz J Med Biol Res 2011;44:848–54. 10.1590/S0100-879X2011007500102 [DOI] [PubMed] [Google Scholar]
- 35. Brito RG, Rasmussen LA, Sluka KA. Regular physical activity prevents development of chronic muscle pain through modulation of supraspinal opioid and serotonergic mechanisms. Pain Rep 2017;2:e618 10.1097/PR9.0000000000000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Da Silva Santos R, Galdino G. Endogenous systems involved in exercise-induced analgesia. J Physiol Pharmacol 2018;69:3–13. 10.26402/jpp.2018.1.01 [DOI] [PubMed] [Google Scholar]
- 37. Naugle KM, Naugle KE, Fillingim RB, et al. . Intensity thresholds for aerobic exercise-induced hypoalgesia. Medicine & Science in Sports & Exercise 2014;46:817–25. 10.1249/MSS.0000000000000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Law LF, Sluka KA. How does physical activity modulate pain? Pain 2017;158:369–70. 10.1097/j.pain.0000000000000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Häuser W, Klose P, Langhorst J, et al. . Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther 2010;12:R79 10.1186/ar3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bodéré C, Bodéré YT, Quinio B, et al. . Specific training program for the treatment of fibromyalgia: a prospective controlled study 14th world congress on pain - TF505. MilanItaly: IASP, 2012. [Google Scholar]
- 41. Bidonde J, Busch AJ, Bath B, et al. . Exercise for adults with fibromyalgia: an umbrella systematic review with synthesis of best evidence. Curr Rheumatol Rev 2014;10:45–79. 10.2174/1573403X10666140914155304 [DOI] [PubMed] [Google Scholar]
- 42. Lannersten L, Kosek E. Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain 2010;151:77–86. 10.1016/j.pain.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 43. Franke KJ, Domanski U, Schroeder M, et al. . Telemonitoring of home exercise cycle training in patients with COPD. Int J Chron Obstruct Pulmon Dis 2016;11:2821–9. 10.2147/COPD.S114181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yasmin F, Banu B, Zakir SM, et al. . Positive influence of short message service and voice call interventions on adherence and health outcomes in case of chronic disease care: a systematic review. BMC Med Inform Decis Mak 2016;16:46 22 avr 10.1186/s12911-016-0286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krag NJ, Nørregaard J, Larsen JK, et al. . A blinded, controlled evaluation of anxiety and depressive symptoms in patients with fibromyalgia, as measured by standardized psychometric interview scales. Acta Psychiatr Scand 1994;89:370–5. 10.1111/j.1600-0447.1994.tb01531.x [DOI] [PubMed] [Google Scholar]
- 46. Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol 1991;18:728–33. [PubMed] [Google Scholar]
- 47. Osorio CD, Gallinaro AL, Lorenzi-Filho G, et al. . Sleep quality in patients with fibromyalgia using the pittsburgh sleep quality index. J Rheumatol 2006;33:1863–5. [PubMed] [Google Scholar]
- 48. Omachi TA. Measures of sleep in rheumatologic diseases: Epworth Sleepiness Scale (ESS), Functional Outcome of Sleep Questionnaire (FOSQ), Insomnia Severity Index (ISI), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Care Res 2011;63(S11):S287–S296. 10.1002/acr.20544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crinière L, Lhommet C, Caille A, et al. . Reproducibility and validity of the french version of the long international physical activity questionnaire in patients with type 2 diabetes. Journal of Physical Activity and Health 2011;8:858–65. 10.1123/jpah.8.6.858 [DOI] [PubMed] [Google Scholar]
- 50. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 51. Marchand S, Arsenault P. Spatial summation for pain perception: interaction of inhibitory and excitatory mechanisms. Pain 2002;95:201–6. 10.1016/S0304-3959(01)00399-2 [DOI] [PubMed] [Google Scholar]
- 52. Julien N, Goffaux P, Arsenault P, et al. . Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 2005;114(1-2):295–302. 10.1016/j.pain.2004.12.032 [DOI] [PubMed] [Google Scholar]
- 53. Granot M, Granovsky Y, Sprecher E, et al. . Contact heat-evoked temporal summation: tonic versus repetitive-phasic stimulation. Pain 2006;122:295–305. 10.1016/j.pain.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 54. Barrett KE, Barman SM, Boitano S, et al. . Ganong’s Review of Medical Physiology. 24 edn New York: McGraw-Hill Education / Medical, 2012:768. [Google Scholar]
- 55. Potvin S, Paul-Savoie E, Morin M, et al. . Temporal summation of pain is not amplified in a large proportion of fibromyalgia patients. Pain Res Treat 2012;2012:1–6. 10.1155/2012/938595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 1994;19:313–33. 10.1016/0306-4530(94)90013-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.