Abstract
The direct union of primary, secondary, and tertiary carboxylic acids with a chiral glyoxylate-derived sulfinimine provides rapid access into a variety of enantiomerically pure α-amino acids (>85 examples). Characterized by operational simplicity, this radical-based reaction enables the modular assembly of exotic α-amino acids, including both unprecedented structures and those of established industrial value. The described method performs well in high-throughput library synthesis, and has already been implemented in three distinct medicinal chemistry campaigns.
Keywords: amino acid synthesis, decarboxylative radical addition, library synthesis, redox-active esters, synthetic methods
The enduring challenge of efficient a-amino acid (AA) synthesis was identified as early as 1850 with the first report of the Strecker condensation (Figure 1A).[1] Though initially racemic, the evolution of chiral mediators has significantly expanded the utility of this reaction, thereby securing its place as the current choice method for general AA construction.[2] More than a century later, homogeneous asymmetric hydrogenation,[3] a discovery that would earn William Knowles a shared Nobel Prize in 2001,[4] revealed itself as an invaluable route to process-scale enantiopure AAs (Figure 1A).[5] Even so, the reaction is not without its deficiencies and use in discovery settings has waned in the face of more appealing options. Finally, advances in phase-transfer catalysis represented a decisive move toward safety and simplicity, though wide-spread use is hampered by a high substrate dependence and the need for prudent selection of complex catalysts (Figure 1A).[6] While conceptually distinct, these stalwart techniques share a common thread: each relies on a polar bond disconnection, and with that, carry inherent limitations. Notably, access to AAs bearing adjacent quaternary centers is restricted to Strecker conditions,[7] though the catalysts demand multistep protocols with extended reaction times at cryogenic temperatures. The Ellman modification has done much in the way of practical improvements, but still requires preparation of the requisite aldehyde, non-trivial nitrile hydrolysis, and, of course, toxic reagents.[2,8] Given the apparent challenges, a new approach to enantiopure AAs,[9] predicated on a one-electron disconnection, is disclosed. This method benefits principally from the ability of the intermediate radical to mobilize even the most obstinate alkyl (including tertiary) donors, a trait unique to one-electron transformations. With a simple reaction protocol and alkyl carboxylic acid starting materials, the reaction features an unprecedented generality and allows rapid access to otherwise wayward AAs.[10] This is showcased by more than 85 examples and applications in high-throughput parallel AA synthesis.
Figure 1.
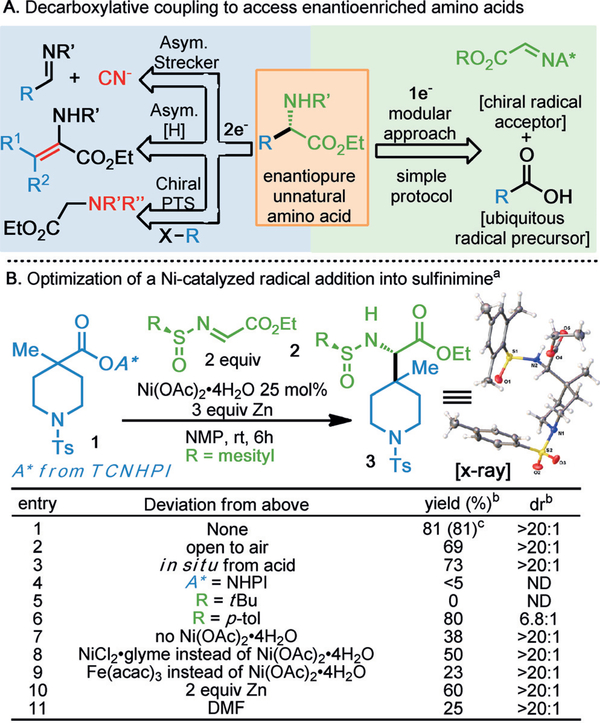
A) Decarboxylative coupling to access enantioenriched amino acids. B) Optimization of a Ni-promoted radical addition into chiral sulfinimine. Ts=tosyl; TCNHPI=N-hydroxytetrachlorophthalimide; NHPI=N-hydroxyphthalimide.[27]
Per accepted retrosynthetic logic,[11] the most convergent disconnection of an AA sits at the amine- and acid-bearing carbon center and the sidechain R group. Broad reconstitution of this strategy suggested that a radical approach would best resist the steric and electronic vagaries characteristic of exotic R groups.[12] As perhaps the most abundant and stable radical precursor, attention quickly turned to the ubiquitous alkyl carboxylic acid; specifically, decarboxylative addition to a chiral radical acceptor (Figure 1A).[13] An abbreviated outline of reaction optimization is depicted in Figure 1B. The transformation of redox-active ester (RAE) piperidine 1 to the derivative 3 was found to proceed in 81% yield (>20:1 dr) using the glyoxylate-derived chiral acceptor 2 in the presence of Zn (3 equiv) and an inexpensive hydrated Ni mediator [Ni(OAc)2·4H2O]; no efforts toward moisture exclusion were made. The reaction saw only a slight diminishment in yield when conducted open to air (entry 2), and was similarly facile with in situ acid-to-RAE conversion (entry 3). Importantly, the identity of the RAE proved critical (entry 4), as did the presence of a Ni promoter (entry 7, 9). Alternative Ni sources resulted in a modest drop in yield (entry 8). The sulfinimine mesityl group was selected for its stability to reductive conditions (entry 5) and stereoselectivity (entry 6), while reaction efficiency dictated the Zn loading (entry 10). Finally, N-methyl-2-pyrrolidine (NMP) provided the best reaction media across a range of substrates, though other polar aprotic solvents were competent as well (entry 11).[14]
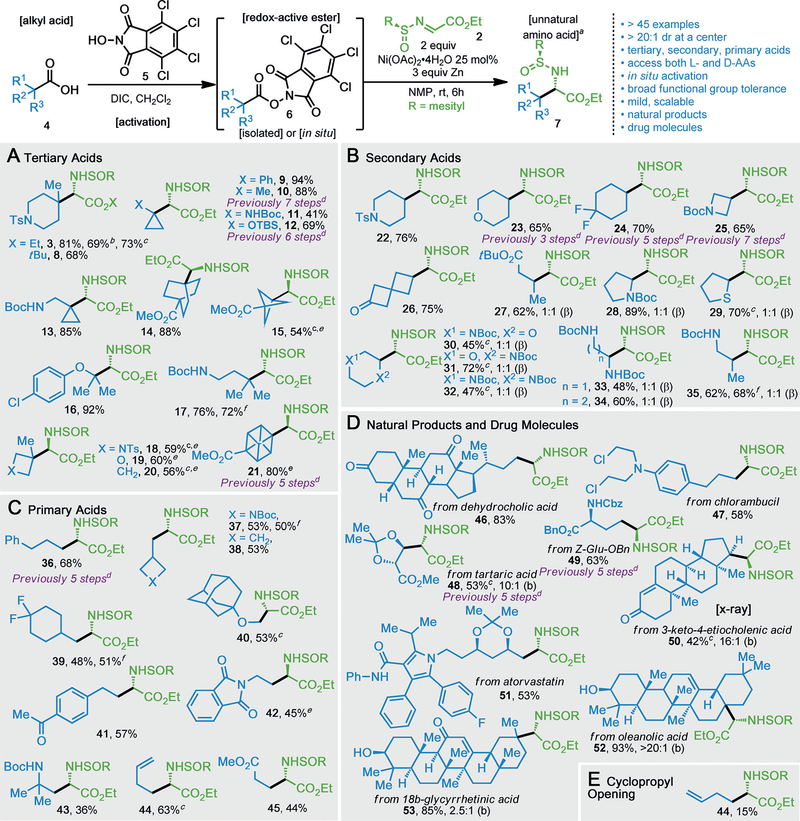
With an optimized procedure in hand, the scope of this asymmetric AA synthesis was evaluated (Scheme 1). The gem-β-disubstituted AAs are in high demand for their role as protein stabilizers in various biological studies,[15] but the difficulty in accessing them using prior methods made them an ideal starting point. Under the established conditions, a variety of tertiary and bridged-tertiary RAEs 4 were efficiently converted into their respective amino acid derivatives (3, 8–21, 41–94%, Scheme 1A). Impressively, tert-butyl α-iminoester was also compatible with this coupling (8) and can be readily hydrolyzed to the free amino acid on acid quench. Beyond esoteric interest, AAs containing phenyl bioisosteres are well known to modulate pharmacokinetic properties.[16] Existing synthetic strategies to access bicyclo[2.2.2]octane,[17] cubane,[18] and [1.1.1]propellane[19] AAs require multiple functional group interconversions followed by an asymmetric Strecker reaction that typically proceeds with moderate diastereoselectivity.[2] In contrast, the current protocol provides these valuable compounds (14, 15, and 21) in one step with excellent stereochemical control.
Scheme 1.
Scope of the Ni-promoted asymmetric amino acids synthesis with TCNHPI redox-active esters. Yields of isolated products are indicated in each case. The diastereomeric ratio for α center is greater than 20:1. Reaction conditions: a) RAE (1 equiv), sulfinimine (2 equiv), Ni(OAc)2·4H2O (25 mol%), Zn (3 equiv), NMP, RT, 6 h. b) The reaction was performed open to air. c) In situ reaction with TCNHPI (1 equiv) and DIC (1.1 equiv). d) For previous synthetic approaches to analogous amino acids. e) Using ent-2 as radical acceptor. f) 1 mmol scale, see the Supporting Information for details. DIC=N,N’-diisopropylcarbodiimide; Boc=tert-butyloxycarbonyl; TBS=tert-butyldimethylsilyl; Cbz=carbox- ybenzyl.
This study was further expanded to include secondary (22–35, Scheme 1B) and primary carboxylic acids (36–45, Scheme 1C) as alkyl radical precursors; diastereomeric ratios greater than 20:1 were obtained in all cases. Of note, β-amino-substituted analogues (28, 31–34) were prepared directly from feedstock AAs, albeit with no observed selectivity at the β position, even on additional optimization.
This mild, radical-based method exhibits broad functional group tolerance, as shown in the context of complex natural products and drug molecules (Scheme 1D). Steroid acids (46), terpenoid acids (50, 52 and 53), tartaric acids (48), AA side chains (49), atorvastatin (51), and chlorambucil (47) were all readily converted into chiral amino acids. Additionally, functional groups including esters (14, 15, 21, 27, 45 and 48), carbamates (11, 13, 17, 25, 28, 30–35, 37, 43 and 49), ketones (26, 41 and 46), ethers (16, 19, 23, 30, 31 and 40), acetonides (48 and 51), enones (50 and 53), olefins (44 and 52), and chlorides (16 and 47) remained intact under the mild reaction conditions. Though not the first reported synthesis in many cases (10, 12, 21, 23–25, 36, 48 and 49),[19] this method marks a significant improvement, as previous routes relied on laborious sequences (3–7 steps) that resulted in poor stereo-control.
To illustrate the ease of reaction protocol, β-dimethyl substituted ornithine 17, β-substituted azetidine amino acid 37, and fluoro-substituted cyclohexyl alanine 39 were all synthesized on a 1 mmol scale. Moreover, alkyl carboxylic acids could be activated in situ, thereby delivering the desired AA in a single step. This one-pot procedure provided an alternative avenue for difficult purifications, bypassing isolation of silica gel-labile RAEs (29–32, 40, 48 and 50).
Intrigued by the apparent breadth of scope, a preliminary mechanistic inquiry was conducted (Scheme 1E). Radical clock experiments–cyclopropyl ring-opening (44) and the ablation of the stereocenter of enantiopure proline RAE 28 suggest the presence of alkyl radicals. This observation is consistent with previously reported RAE decarboxylative coupling studies.[20]
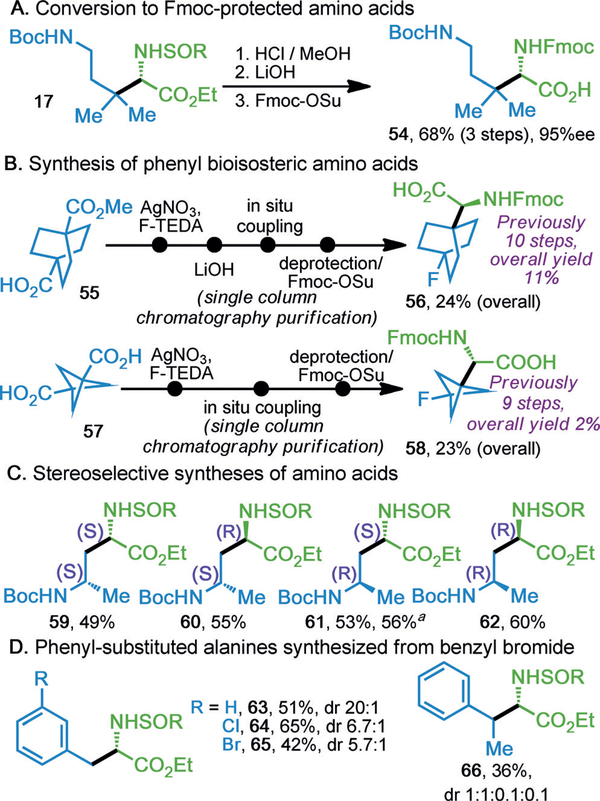
Solid-phase peptide synthesis (SPPS), among the most useful laboratory applications of chiral AAs, requires orthogonal protection of the α-amine and sidechain for successful introduction of an AA into a growing peptide. Indeed, the multistep preparation of those suited for the Fmoc approach renders peculiar variants prohibitively time-consuming and expensive. The described method enables rapid construction of SPPS-compatible AAs (54, Scheme 2A), including the synthesis of particularly challenging bicyclic substrates which serve as phenyl bioisosteres and can be used in proteins
Scheme 2.
A) Conversion into Fmoc-protected amino acids. B) Synthesis of phenyl bioisosteric amino acids. C) Stereoselective synthesis of amino acids. D) Benzyl-substituted amino acids from benzyl bromide. a. 1 mmol scale. See Supporting Information for details. Fmoc=fluor-enylmethyloxycarbonyl; F-TEDA=Selectfluor.
Additionally, F-labeling studies were successfully performed giving 56 and 58 (Scheme 2B).[17–19,21] Optically pure β-AAs afforded diastereomerically-pure α-AAs (59–62, Scheme 2C), with no evidence to suggest erosion of the now-γ chiral center, regardless of auxiliary configuration. The reaction does suffer some limitations, most notably, recalcitrance towards benzyl addition. However, α-benzylated products (63–66) can still be accessed from the corresponding benzyl bromide (Scheme 2D), though under a slightly modified set of conditions; note that in contrast to all other reported substrates, this palliative approach proceeds through a two-electron mechani sm.[22] Though a generally adequate workaround, a decline in dr was observed in the case of meta-substituted benzyl substrates. Additionally, β-ketoacids were found to be entirely ineffectual in the given reaction. The most glaring restriction, perhaps, is the cost of the enantiopure mesitylsulfinamide. On scale up, cost may be mitigated by chiral resolution of racemic mesitylsulfinamide (see Supporting Information for details).[23]
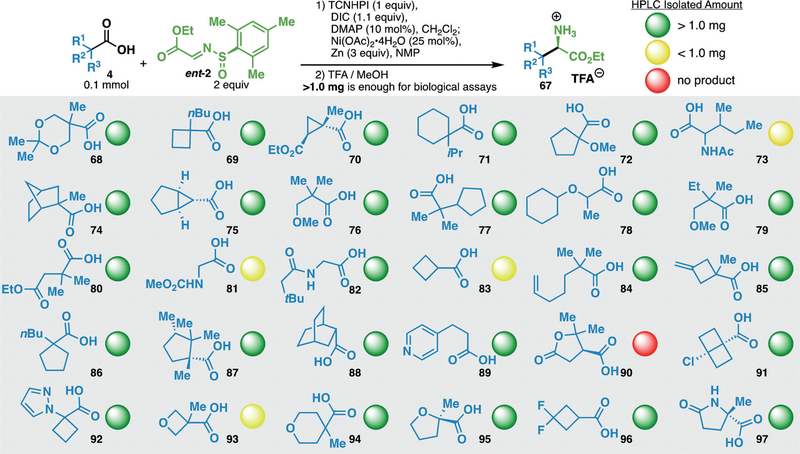
To assess the applicability of this method to medicinal chemistry, 30 reactions were conducted in parallel library synthesis (Scheme 3).[24] Judicious selection of the carboxylic acid radical surrogates focused largely on structural diversity, with an emphasis on those unavailable as the corresponding alkyl bromides or aldehydes required for conventional amino ester synthesis. Among the collection were aromatic and aliphatic heterocycles, including pyridines (89), pyrazoles (92), pyrrolidones (97), oxetanes (93), and tetrahydropyrans (94);all of which are considered privileged medicinal scaffolds.[25] The parallel sequence was conducted on a 100 μmol scale as a two-pot procedure: in situ generation of the TCNHPI-ester and imine addition followed by sulfinamide cleavage provided the unprotected α-amino esters. All reagents, with the exception of zinc, were added as stock solutions to facilitate parallel execution. HPLC analysis indicated that 29 of the 30 substrates successfully underwent the two-pot sequence, while 25 provided sufficient material after isolation (>1.0 mg) for submission to standard bioactivity assays. The molecular weights (<250 Da), hydrogen-bond-donor count (<3), and partition coefficients (cLogP< 3.1) of the product α-amino esters are particularly well-suited for fragment-based drug discovery (FBDD),[26] as are the amine and ester functional handles.
Scheme 3.
A 30 compound array asymmetric synthesis of unnatural amino acids. DMAP=4-dimethylaminopyridine.
Unique among the methods available for α-AA synthesis, the modular approach described herein represents a significant departure from the two-electron mechanisms so often prescribed in the literature. This radical-based strategy relies instead on the convergent unification of feedstock carboxylic acids and a chiral glyoxylate-derived fragment, which could vastly expand AA chemical space accessible by library synthesis. Customizable by design, the simplicity and efficiency of this procedure should resonate with medicinal chemists requiring rapid access to complex, enantiopure α-AAs.
Supplementary Material
Acknowledgements
Financial support for this work was provided by NIH (grant number GM-118176), Pfizer, Bristol–Myers Squibb, and LEO Pharma. We gratefully thank China Scholarship Council (fellowship to S.N., Nanjing University, China), Universidad Autlnoma de Madrid (fellowship to A.F.G.-C.), Vividion Therapeutics (predoctoral fellowship to R.R.M), NSF GRFP (fellowship to J.N.D.). We also thank D.-H. Huang and L. Pasternack (Scripps Research) for assistance with NMR spectroscopy; J. S. Chen (Scripps Automated Synthesis Facility); A. Rheigold, C. E. Moore, and M. A. Galella (UCSD) for X-ray crystallographic analysis; D. Sun and D-.R. Wu (BMS) for SFC separation of the racemic mesitylsulfinamide and William R. Ewing for helpful discussions.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Shengyang Ni, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037 (USA).
Alberto F. Garrido-Castro, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037 (USA)
Rohan R. Merchant, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037 (USA)
Justine N. deGruyter, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037 (USA)
Dr. Daniel C. Schmitt, Pfizer Medicinal Sciences, Eastern Point Road, Groton, CT 06340 (USA)
Dr. James J. Mousseau, Pfizer Medicinal Sciences, Eastern Point Road, Groton, CT 06340 (USA)
Dr. Gary M. Gallego, Department of Chemistry, La Jolla Laboratories, Pfizer, 10770 Science Center Drive, San Diego, CA 92121 (USA)
Dr. Shouliang Yang, Department of Chemistry, La Jolla Laboratories, Pfizer, 10770 Science Center Drive, San Diego, CA 92121 (USA)
Dr. Michael R. Collins, Department of Chemistry, La Jolla Laboratories, Pfizer, 10770 Science Center Drive, San Diego, CA 92121 (USA)
Dr. Jennifer X. Qiao, Department of Discovery Chemistry, Bristol-Myers Squibb Company, Research and Development, P.O. Box 4000, Princeton, NJ 08543 (USA)
Dr. Kap-Sun Yeung, Department of Discovery Chemistry, Bristol-Myers Squibb Research, and Development, 5 Research Parkway, Wallingford, CT 06492 (USA)
Dr. David R. Langley, Department of Discovery Chemistry, Bristol-Myers Squibb Research, and Development, 5 Research Parkway, Wallingford, CT 06492 (USA)
Dr. Michael A. Poss, Department of Discovery Chemistry, Bristol-Myers Squibb Company, Research and Development, P.O. Box 4000, Princeton, NJ 08543 (USA)
Dr. Paul M. Scola, Department of Discovery Chemistry, Bristol-Myers Squibb Research, and Development, 5 Research Parkway, Wallingford, CT 06492 (USA)
Dr. Tian Qin, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037 (USA)
Phil S. Baran, Scripps Research, North Torrey Pines Road, La Jolla, CA 92037 (USA)
References
- [1].Strecker A, Ann. Chem. Pharm 1850, 75, 27. [Google Scholar]
- [2].For a recent review on asymmetric Strecker reactions, see: Wang J, Liu X, Feng X, Chem. Rev 2011, 111, 6947.
- [3].Knowles WS, Sabacky MJ, Chem. Commun 1968, 1445. [Google Scholar]
- [4].a) Knowles WS—Nobel Lecture: Asymmetric Hydrogenations, Nobelprize.org, Nobel Media AB; 2014; [PubMed] [Google Scholar]; b) Knowles WS, Angew. Chem. Int. Ed 2002, 41, 1998; Angew. Chem. 2002, 114, 2096. [PubMed] [Google Scholar]
- [5].For additional asymmetric hydrogenations leading to enantiopure AAs, see: Miyashita A, Yasuda A, Takaya H, Toriumi K, Ito T, Souchi T, Noyori R, J. Am. Chem. Soc 1980, 102, 7932;Burk MJ, Feaster JE, Nugent WA, Harlow RL, J. Am. Chem. Soc 1993, 115, 10125;Burk MJ, Gross MF, Martinez JP, J. Am. Chem. Soc 1995, 117, 9375.
- [6].For reviews on phase-transfer catalysis in AA synthesis, see: Maruoka K, Ooi T, Chem. Rev 2003, 103, 3013; O’Donnell MJ, Acc. Chem. Res 2004, 37, 506.
- [7].For an elegant example to access α-amino acids through the asymmetric Strecker reaction, see: Zuend SJ, Coughlin MP, Ladonde MP, Jacobsen EN, Nature 2009, 461, 968.
- [8].a) For review on the role of EllmanQs sulfinamide in AA synthesis, see section 9 in: Robak MT, Herbage MA, Ellman JA, Chem. Rev 2010, 110, 3600; [DOI] [PubMed] [Google Scholar]; b) Davis FA, Portonovo PS, Reddy RE, Chiu Y, J. Org. Chem 1996, 61, 440; [DOI] [PubMed] [Google Scholar]; c) Davis FA, McCoull W, J. Org. Chem 1999, 64, 3396; [DOI] [PubMed] [Google Scholar]; d) Davis FA, Lee S, Zhang H, Fanelli DL, J. Org. Chem 2000, 65, 8704; [DOI] [PubMed] [Google Scholar]; e) Mabic S, Cordi AA, Tetrahedron 2001, 57, 8861; [Google Scholar]; f) Borg G, Chino M, Ellman JA, Tetrahedron Lett. 2001, 42, 1433; [Google Scholar]; g) Beenen MA, Weix DJ, Ellman JA, J. Am. Chem. Soc 2006, 128, 6304; [DOI] [PubMed] [Google Scholar]; h) Wang H, Zhao X, Li Y, Lu L, Org. Lett 2006, 8, 1379. [DOI] [PubMed] [Google Scholar]
- [9].For a review on the use of radical additions in chiral amine synthesis, see: Friestad GK, Top. Curr. Chem 2014, 343, 1.
- [10].For reviews on the importance of unnatural AAs, see: Walsh CT, O’Brien RV, Khosla C, Angew. Chem. Int. Ed 2013, 52, 7098; Angew. Chem. 2013, 125, 7238; Lang K, Chin JW, Chem. Rev 2014, 114, 4764; Blaskovich MA, J. Med. Chem 2016, 59, 10807.
- [11].Corey EJ, Cheng XM, The Logic of Chemical Synthesis, Wiley, New York, 1989. [Google Scholar]
- [12].For reviews on radical-based strategies in synthesis, see: Yan M, Lo JC, Edwards JT, Baran PS, J. Am. Chem. Soc 2016, 138, 12692; Smith JM, Harwood SJ, Baran PS, Acc. Chem. Res 2018, 10.1021/acs.accounts.8b00209.
- [13].For asymmetric radical additions to imines, see: Zhong Y-W, Xu M-H, Lin G-Q, Org. Lett 2004, 6, 3953; Zhong Y-W, Izumi K, Xu M-H, Lin G-Q, Org. Lett 2004, 6, 4747; Zhong Y-W, Dong Y-Z, Fang K, Izumi K, Xu M-H, Lin G-Q, J. Am. Chem. Soc 2005, 127, 11956; Akindele T, Yamamoto Y, Maekawa M, Umeki H, Yamada K-I, Tomioka K, Org. Lett 2006, 8, 5729; Fernández-Salas JA, Maestro MC, Rodríguez-Fernández MM, García-Ruano JL, Alonso I, Org. Lett 2013, 15, 1658; Fernández-Salas JA, Rodríguez-Fernández MM, Maestro MC, García-Ruano JL, Eur. J. Org. Chem 2014, 5265; Uraguchi D, Kinoshita N, Kizu T, Ooi T, J. Am. Chem. Soc 2015, 137, 13768; Kizu T, Uraguchi D, Ooi T, J. Org. Chem 2016, 81, 6953; Garrido-Castro AF, Choubane H, Daaou M, Maestro MC, Alemán J, Chem. Commun 2017, 53, 7764.
- [14]. See the Supporting Information for details.
- [15].a) Abele S, Seebach D, Eur. J. Org. Chem 2000, 1; [Google Scholar]; b) Heard KR, Wu W, Li Y, Zhao P, Woznica I, Lai JH, Beinborn M, Sanford DG, Dimare MT, Chiluwal AK, Peters DE, Whicher D, Sudmeier JL, Bachovchin WW, J. Med. Chem 2013, 56, 8339. [DOI] [PubMed] [Google Scholar]
- [16].Myers MR, He W, Hanney B, Setzer N, Maguire MP, Zulli A, Bilder G, Galzcinski H, Amin D, Needle S, Spada AP, Bioorg. Med. Chem. Lett 2003, 13, 3091. [DOI] [PubMed] [Google Scholar]
- [17].Bandak D, Babii O, Vasiuta R, Komarov IV, Mykhailiuk PK, Org. Lett 2015, 17, 226. [DOI] [PubMed] [Google Scholar]
- [18].Wlochal J, Davies RDM, Burton J, Synlett 2016, 27, 919. [Google Scholar]
- [19].a) Pellicciari R, Raimondo M, Marinozzi M, Natalini B, Costantino G, Thomsen C, J. Med. Chem 1996, 39, 2874; [DOI] [PubMed] [Google Scholar]; b) Costantino G, Maltoni K, Marinozzi M, Camaioni E, Prezeau L, Pin J-P, Pellicciari R, Bioorg. Med. Chem 2001, 9, 221; [DOI] [PubMed] [Google Scholar]; c) Mikhailiuk PK, Afonin S, Chernega AN, Rusanov EB, Platonov MO, Dubinina GG, Berditsch M, Ulrich AS, Komarov IV, Angew. Chem. Int. Ed 2006, 45, 5659; Angew. Chem. 2006, 118, 5787; [DOI] [PubMed] [Google Scholar]; d) Filosa R, Marinozzi M, Costantino G, Hermit MB, Thomsen C, Pellicciari R, Bioorg. Med. Chem 2006, 14, 3811; [DOI] [PubMed] [Google Scholar]; e) Pritz S, Pätzel M, Szeimies G, Dathe M, Bienert M, Org. Biomol. Chem 2007, 5, 1789; [DOI] [PubMed] [Google Scholar]; f) Filosa R, Fulco MC, Marinozzi M, Giacchè N, Macchiarulo A, Peduto A, Massa A, de Caprariis P, Thomsen C, Christoffersen CT, Pellicciari R, Bioorg. Med. Chem 2009, 17, 242; [DOI] [PubMed] [Google Scholar]; g) Mykhailiuk PK, Voievoda NM, Afonin S, Ulrich AS, Komarov IV, Fluorine Chem J. 2010, 131, 217; [Google Scholar]; h) Kokhan SO, Tymtsunik AV, Grage SL, Afonin S, Babii O, Berditsch M, Strizhak AV, Bandak D, Platonov MO, Komarov IV, Ulrich AS, Mykhailiuk PK, Angew. Chem. Int. Ed 2016, 55, 14788; Angew. Chem. 2016, 128, 15008. [DOI] [PubMed] [Google Scholar]
- [20].a) Cornella J, Edwards JT, Qin T, Kawamura S, Wang J, Pan CM, Gianatassio R, Schmidt M, Eastgate MD, Baran PS, J. Am. Chem. Soc 2016, 138, 2174; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Qin T, Cornella J, Li C, Malins LR, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, Baran PS, Science 2016, 352, 801; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang J, Qin T, Chen T-G, Wimmer L, Edwards JT, Cornella J, Vokits B, Shaw SA, Baran PS, Angew. Chem. Int. Ed 2016, 55, 9676; Angew. Chem. 2016, 128, 9828; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Toriyama F, Cornella J, Wimmer L, Chen T-G, Dixon DD, Creech G, Baran PS, J. Am. Chem. Soc 2016, 138, 11132; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Qin T, Malins LR, Edwards JT, Merchant RR, Novak AJE, Zhong JZ, Mills RB, Yan M, Yuan C, Eastgate MD, Baran PS, Angew. Chem. Int. Ed 2017, 56, 260; Angew. Chem. 2017, 129, 266; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Sandfort F, O’Neill MJ, Cornella J, Wimmer L, Baran PS, Angew. Chem. Int. Ed 2017, 56, 3319; Angew. Chem. 2017, 129, 3367; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Edwards JT, Merchant RR, McClymont KS, Knouse KW, Qin T, Malins LR, Vokits B, Shaw SA, Bao D-H, Wei F-L, Zhou T, Eastgate MD, Baran PS, Nature 2017, 545, 213; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Smith J, Qin T, Merchant RR, Edwards JT, Malins LR, Liu Z, Che G, Shen Z, Shaw SA, Eastgate MD, Baran PS, Angew. Chem. Int. Ed 2017, 56, 11906; Angew. Chem. 2017, 129, 12068. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) For other example of using RAE cross-coupling, see: Huihui KMM, Caputo JA, Melchor Z, Olivares AM, Spiewak AM, Johnson KA, DiBenedetto TA, Kim S, Ackerman LKG, Weix DJ, J. Am. Chem. Soc 2016, 138, 5016. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) For a seminal use of RAEs of the phthalimide-type, see: Okada K, Okamoto K, Oda M, J. Am. Chem. Soc 1988, 110, 8736. [Google Scholar]
- [21].Qiu X-L, Qing F-L, Eur. J. Org. Chem 2011, 3261. [Google Scholar]
- [22].a) Buesking AW, Baguley TD, Ellman JA, Org. Lett 2011, 13, 964; [DOI] [PubMed] [Google Scholar]; b) See the Supporting Information for control studies (compound 63a). [Google Scholar]
- [23].a) Backes BJ, Dragoli DR, Ellman JA, J. Org. Chem 1999, 64, 5472. [DOI] [PubMed] [Google Scholar]; b) Enantiopure mesitylsulfinamide synthesis, see: Ramachandar T, Wu Y, Zhang J, Davis FA, Org. Synth 2006, 83, 131. [Google Scholar]
- [24].Examples of parallel synthesis of homochiral β-amino acids, see: Davies SG, Mulvaney AW, Russell AJ, Smith AD, Tetrahedron: Asymmetry 2007, 18, 1554.
- [25].Priviledged scaffolds in medicinal chemistry:design, synthesis, evaluation (Ed.: Bräse S), RSC, London, 2015. [Google Scholar]
- [26].Murray CW, Rees DC, Nat. Chem 2009, 1, 187. [DOI] [PubMed] [Google Scholar]
- [27]. CCDC 1861500and 1861501 (3 and 50) contains the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.