Abstract
Background
Cardiovascular (CV) disease is the leading cause of death in dialysis patients, and strongly associated with fluid overload and hypertension. It is plausible that low dialysate [Na+] may decrease total body sodium content, thereby reducing fluid overload and hypertension, and ultimately reducing CV morbidity and mortality.
Objectives
This review evaluated harms and benefits of using a low (< 138 mM) dialysate [Na+] for maintenance haemodialysis (HD) patients.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 7 August 2018 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials (RCTs), both parallel and cross‐over, of low (< 138 mM) versus neutral (138 to 140 mM) or high (> 140 mM) dialysate [Na+] for maintenance HD patients were included.
Data collection and analysis
Two investigators independently screened studies for inclusion and extracted data. Statistical analyses were performed using random effects models, and results expressed as risk ratios (RR) for dichotomous outcomes, and mean differences (MD) or standardised MD (SMD) for continuous outcomes, with 95% confidence intervals (CI). Confidence in the evidence was assessed using GRADE.
Main results
We included 12 studies randomising 310 patients, with data available for 266 patients after dropout. All but one study evaluated a fixed concentration of low dialysate [Na+], and one profiled dialysate [Na+]. Three studies were parallel group, and the remaining nine cross‐over. Of the latter, only two used a washout between intervention and control periods. Most studies were short‐term with a median (interquartile range) follow‐up of 3 (3, 8.5) weeks. Two were of a single HD session, and two of a single week's HD. Half of the studies were conducted prior to 2000, and five reported use of obsolete HD practices. Risks of bias in the included studies were often high or unclear, lowering confidence in the results.
Compared to neutral or high dialysate [Na+], low dialysate [Na+] had the following effects on "efficacy" endpoints: reduced interdialytic weight gain (10 studies: MD ‐0.35 kg, 95% CI ‐0.18 to ‐0.51; high certainty evidence); probably reduced predialysis mean arterial blood pressure (BP) (4 studies: MD ‐3.58 mmHg, 95% CI ‐5.46 to ‐1.69; moderate certainty evidence); probably reduced postdialysis mean arterial BP (MAP) (4 studies: MD ‐3.26 mmHg, 95% CI ‐1.70 to ‐4.82; moderate certainty evidence); probably reduced predialysis serum [Na+] (7 studies: MD ‐1.69 mM, 95% CI ‐2.36 to ‐1.02; moderate certainty evidence); may have reduced antihypertensive medication (2 studies: SMD ‐0.67 SD, 95% CI ‐1.07 to ‐0.28; low certainty evidence). Compared to neutral or high dialysate [Na+], low dialysate [Na+] had the following effects on "safety" endpoints: probably increased intradialytic hypotension events (9 studies: RR 1.56, 95% 1.17 to 2.07; moderate certainty evidence); probably increased intradialytic cramps (6 studies: RR 1.77, 95% 1.15 to 2.73; moderate certainty evidence).
Compared to neutral or high dialysate [Na+], low dialysate [Na+] may make little or no difference to: intradialytic BP (2 studies: MD for systolic BP ‐3.99 mmHg, 95% CI ‐17.96 to 9.99; diastolic BP 1.33 mmHg, 95% CI ‐6.29 to 8.95; low certainty evidence); interdialytic BP (2 studies:, MD for systolic BP 0.17 mmHg, 95% CI ‐5.42 to 5.08; diastolic BP ‐2.00 mmHg, 95% CI ‐4.84 to 0.84; low certainty evidence); dietary salt intake (2 studies: MD ‐0.21g/d, 95% CI ‐0.48 to 0.06; low certainty evidence).
Due to very low quality of evidence, it is uncertain whether low dialysate [Na+] changed extracellular fluid status, venous tone, arterial vascular resistance, left ventricular mass or volumes, thirst or fatigue. Studies did not examine cardiovascular or all‐cause mortality, cardiovascular events, or hospitalisation.
Authors' conclusions
It is likely that low dialysate [Na+] reduces intradialytic weight gain and BP, which are effects directionally associated with improved outcomes. However, the intervention probably also increases intradialytic hypotension and reduces serum [Na+], effects that are associated with increased mortality risk. The effect of the intervention on overall patient health and well‐being is unknown. Further evidence is needed in the form of longer‐term studies in contemporary settings, evaluating end‐organ effects in small‐scale mechanistic studies using optimal methods, and clinical outcomes in large‐scale multicentre RCTs.
Plain language summary
Dialysate sodium levels for chronic haemodialysis
What is the issue?
Kidneys control salt and water balance in the body by the regulation of urine production. When kidneys no longer work, urine production ceases or becomes insufficient and salt and water balance must be managed using dialysis. Doctors looking after haemodialysis patients must select an appropriate amount of sodium to use in the dialysis fluids that are used to wash the patient's blood. If the sodium level in these fluids is too high, this can lead to the patient feeling thirsty after treatment, drinking too much more water and becoming fluid overloaded by the time the next treatment is due, which can cause heart damage. On the other hand, if the sodium level is too low in dialysis fluids, this will cause the patient to have cramps and drops in blood pressure, which is uncomfortable and potentially also a cause heart damage. The "right" sodium level for dialysis fluid is unknown.
What did we do?
We combined all studies of people treated with haemodialysis where outcomes were compared between people receiving low sodium in their dialysis fluid and those receiving higher levels.
What did we find?
We found 12 studies comparing low sodium levels in dialysis fluid with neutral or high sodium levels. Many studies were performed prior to 2000, studying technology and patients that are not always relevant today. Most were short‐term studies, only lasting a few weeks. Our main findings in these studies were; that low sodium in dialysis fluid improves blood pressure and reduces gain of salt and water in between dialysis treatments, which are probably good things, but increases the number of cramps and low blood pressure events experienced by patients during dialysis, which are definitely bad things. The studies did not provide enough information about the participating patients for us to know which patients might benefit from low sodium dialysis fluid, and which patients might instead be harmed. The studies did not provide definitive information on the effect of low sodium dialysis fluids on heart structure and function, or patient quality of life and survival.
Conclusions
We are uncertain about whether low sodium in dialysis fluid improves overall health and well‐being for people on haemodialysis, since there are a mixture of probably good and bad effects, and available research studies were not designed (or designed well‐enough) to learn about effects of the intervention on the heart or on overall patient health and well‐being. Larger and up‐to‐date definitive studies are needed to evaluate the medium to long‐term effects of low sodium levels in dialysis fluid, and better inform clinical practice.
Summary of findings
Background
Description of the condition
The kidneys control salt and water balance in the body principally by the regulation of urine production. When kidneys no longer function, urine production ceases or becomes insufficient and salt and water balance is managed through renal replacement therapy (RRT) with dialysis or kidney transplantation. Dialysis is the most common modality of RRT. At the end of 2013, there were approximately 3.2 million patients being treated for end‐stage kidney disease (ESKD) worldwide. This number increases by approximately 6% each year, which is significantly higher than the population growth rate. Out of those 3.2 million patients, about 2.5 million were undergoing dialysis treatment (either haemodialysis (HD) or peritoneal dialysis), and about 678,000 were living with kidney transplants.
Patient survival is relatively poor on dialysis, with a prognosis that is similar to or worse than many common cancers. Cardiovascular (CV) disease accounts for the majority of deaths in dialysis patients, and develops de‐novo in most patients who start dialysis without it (Marshall 2017). The precise causal pathways responsible for this accelerated CV disease are not known. However, elevated blood pressure (BP) and salt and water overload are common conditions in dialysis patients and moreover two of the strongest risk factors for CV death, and thought to originate from excessive body sodium content driving increased thirst and water intake (Charra 2004; Davenport 2008; Fischbach 1988; Kimura 1984; Matsuoka 1990; Shepherd 1987; Stiller 2001; Van Stone 1982). In addition, elevation in serum sodium concentration ([Na+]) (above 135 mM) has been observed to directly increase BP by stiffening blood vessel walls (Oberleithner 2007). Ultimately, this combination of features is thought to culminate in left ventricular (LV) hypertrophy, congestive heart failure and premature CV death (Charra 2004).
Excessive body sodium content in dialysis patients derives from either excessive dietary consumption or via the treatment itself. For those on HD, sodium loading during treatment can arise from two main sources: from higher fixed or profiled concentrations of [Na+] in dialysate, and from the saline used to treat intradialytic hypotension and to prime or wash‐back the extracorporeal blood circuit (Dunlop 2012). Of these two sources, the most important one is the dialysate. Dialysate [Na+] that is higher than the patient's will result in transfer of sodium to the patient, and [Na+] that is lower will result in transfer out of the patient. The most common dialysate [Na+] is 140 mM (Hecking 2011; Mc Causland 2012; Peixoto 2011), which is generally higher than that in patients' plasma. This is likely to be contributing factor to the fact that most people on conventional HD regimens (˜four hours, three times/week) have chronic salt and water overload, and hypertension that is inadequately controlled by antihypertensives (Agarwal 2003; Rahman 1999; Zazgornik 1997).
Improving CV morbidity and death is a leading priority in the care of dialysis patients, and a prime opportunity for doing so is through control of excess body sodium content.
Description of the intervention
HD removes fluid and solutes through convection and diffusion. Convective losses of sodium during HD are dependent on ultrafiltration, and are brought about by solvent drag. Diffusive transfer of sodium depends on the direction and extent of the concentration gradient between the dialysate and the patient's plasma, and is brought about by Brownian motion. Plasma contains negatively charged proteins that may complex with sodium ions, reducing their availability to move across the dialyser membrane. The difference between total and diffusable sodium in plasma arises from the Gibbs‐Donan effect (Locatelli 1984). Dialysate contains no proteins; therefore all ionised sodium is ionically active and able to move across the membrane. The difference in available diffusible sodium (the sodium ionic activity) between dialysate and plasma thus drives diffusive transfer (Flanigan 1998). In practical terms, without measuring sodium ionic activity and approximating the Gibbs‐Donan effect, the sodium gradient can be considered neutral if the dialysate [Na+] is set approximately 2 mM below the plasma sodium concentration (Flanigan 2008; Lomonte 2011).
The intervention under consideration in this review is low dialysate [Na+]. Dialysate [Na+] is considered to be low when it is below neutral. There are very few studies of sodium balance in dialysis patients characterising the ionic activity of sodium in plasma water, or calculating the gradient with respect to dialysate. Therefore, for this review, we will consider dialysate [Na+] levels below 138 mM to be "low"; 138 mM to 140 mM as "neutral"; and more than 140 mM as "high". Dialysate [Na+] can be estimated in three ways: 1) from dialysate conductivity multiplied by 10 (Gotch 1990; Ragon 1985); 2) from [Na+] measurements in dialysate using flame photometry or potentiometry with ion specific electrodes (calibrated against aqueous solutions as opposed to plasma); and 3) from the HD machine itself, which uses conductivity measurement in conjunction with proprietary models (specific to the manufacturer) that account for the presence of other ions in the dialysis fluid (Polaschegg 1985). In the routine clinical practice of the approximately 300 million HD treatments performed per year, all are prescribed and monitored by the latter method. The former methods are only performed in research or audit settings.
How the intervention might work
HD patients do not exist in a steady state. They accumulate exogenous and endogenous solutes and water between dialysis treatments, which are then removed by the dialysis procedure to return the patient to a baseline state. For sodium, accumulation between treatments is from dietary intake and removal during HD most obviously by convection: the patient is ultrafiltrated from their observed weight to a target weight, with removal of a proportion of excess sodium body content that is contained in the ultrafiltrated volume.
It has been shown that low dialysate [Na+] leads to greater diffusive sodium removal during dialysis (Manlucu 2010) and therefore lower total body sodium content by the end of treatment, which might therefore lessen thirst and subsequent water intake in the interdialytic period. This in turn might reduce extracellular fluid overload, hypertension, and ultimately, left ventricular hypertrophy and CV death. The clinical value proposition of dialysate [Na+] therefore pertains to a lower risk of excessive body sodium content through both greater sodium diffusion on dialysis, with reduced water intake in the interdialytic period.
Why it is important to do this review
From the above arguments, it may seem self‐evident that a lower rather than higher dialysate [Na+] would be more commonly applied during HD. Currently, however, the most common dialysate [Na+] setting around the world is approximately 140 mM, which is not low (Hecking 2012; Mc Causland 2012). This customary practice has been adopted by practitioners to make dialysis more comfortable for patients, guided by cumulative clinical experience over half a century of less intradialytic hypotension and associated symptoms with higher, as opposed to lower, dialysate [Na+] (Cybulsky 1985; Port 1973; Stewart 1972; Wilkinson 1977). The dialysate [Na+] of 140 mM provides these benefits by enhancing salt and water transfer from the interstitial compartment into the blood during HD, thereby maintaining blood volume during ultrafiltration, and reducing the drops in BP during the procedure. Although this is helpful to patients, the relatively high dialysate [Na+] of 140 mM also provides a considerable sodium load to patients (Munoz Mendoza 2011; Peixoto 2011; Raimann 2009; Thein 2007), with potential to drive or at least exacerbate the excess CV morbidity and death in this population. Lower dialysate [Na+] has therefore been suggested as an intervention to avoid this situation, thereby improving CV outcomes. Prima facie, the intervention of low dialysate [Na+] seems attractive, since it addresses an important gap in outcomes, and is simple, universally accessible, and cost‐free to apply. However, at this time there is no definitive study of the intervention, and a review is needed to synthesise evidence of efficacy from existing studies.
Another issue concerns the safety of lower dialysate [Na+], which has been associated with higher death rates in some HD populations (Hecking 2012; Mc Causland 2012). Importantly, these data are not experimental, and a causal pathway has not been proven. Nonetheless, there is a suggestion that low dialysate [Na+] might not be harmless. The first and foremost source of risk arises from the potential loss of increased intradialytic haemodynamic stability afforded by higher dialysate [Na+], especially when large ultrafiltration rates/volumes are required (Peixoto 2011; Santos 2008): lower dialysate [Na+] may increase the occurrence of intradialytic hypotension. The assessment of this endpoint is made difficult by the lack of an agreed definition for the syndrome. The original definition was a decrease in systolic BP by ≥ 20 mmHg, or mean arterial BP (MAP) by ≥ 10 mmHg, as well as associated symptoms (K/DOQI Workgroup 2005). Since, others have defined intradialytic hypotension as fall in BP requiring an intervention such as saline bolus, UF reduction, or blood flow reduction (Eknoyan 2002). Yet others use a definition based on a fall in BP alone, since symptoms and intervention data are often unavailable in large databases (Dheenan 2001; Dubin 2011; Kyriazis 2002; Oliver 2001b; Zhou 2014). Irrespective of definition, the syndrome of intradialytic hypotension is not harmless. Many case and cohort studies have reported end‐organ damage with intradialytic hypotension, such as retinal ischaemia, brain ischaemia, gut ischaemia, loss of residual kidney function, and vascular access thrombosis (Basri 2002; Chang 2011; Eldehni 2012; Eldehni 2015; McIntyre 2011; Taratufolo 2001; Wells 2004). Intradialytic hypotension also reduces patient satisfaction with care (Amro 2014; Caplin 2011; Schipper 2011; Davies 2016 [pers comm]), leading to additional sodium loading through the use of saline boluses to alleviate symptoms, or to early abandonment of HD or even overt non‐adherence lessening treatment effectiveness (Schreiber 2001). Perhaps most importantly, intradialytic hypotension is associated with temporary reduction in heart contractility during HD itself (Boon 2004; Bos 2000; McIntyre 2008), and repeated episodes are associated with the development of regional wall motion abnormalities and evolution of congestive cardiac failure (Burton 2009; Selby 2007a; Sherman 2011). In epidemiological studies, intradialytic hypotension is associated with increased hospitalisation, major CV events, and death (Flythe 2015; Sands 2014; Shoji 2004; Stefansson 2014; Tisler 2003). A review is needed to synthesise evidence of safety of low dialysate [Na+] from existing studies, with particular reference to the important adverse event of intradialytic hypotension and associated symptoms.
The second and more controversial source of risk with low dialysate [Na+] arises from a potential effect on serum [Na+]. There is a well‐established inverse association between serum [Na+] and death in HD patients (Hecking 2012; Mc Causland 2012; Waikar 2011), which predicts an increase in death even when serum [Na+] is at the lower end of the normal range (as it does for the general population (Waikar 2009)). Once again, these data are not experimental, and a causal pathway has not been proven. A variety of causal pathways have been proposed, including; increased inflammation and/or cachexia (Poulikakos 2014; Sukhanov 2011; Swart 2011; Rodrigues Telini 2013), lower resistance to infection (Mandai 2013); changes in vascular responsiveness and therefore resistance to intradialytic hypotension (Grassi 2002); and direct toxicity from osmolar fluctuations on HD (Waikar 2011). However, low serum [Na+] in these studies may also be simply a marker for the severity of co‐morbid disease in HD patients, especially those co‐morbidities that predispose to thirst (Hoorn 2013). In support of this contention are data from Hecking 2012, who identified a frail phenotype who was more likely to have low serum [Na+] in their studies, with diseases such as diabetes mellitus, coronary artery disease, congestive heart failure, lung disease and/or cancer. Concerningly however, a reduction in serum [Na+] has been associated with use of lower dialysate [Na+] in several non‐randomised interventional studies, presumably reflecting a progressive reduction in sodium loading and total body sodium content (Song 2002; Song 2005; Thein 2007). Notwithstanding the controversies in this area, these data must be considered a signal of potential harm. A review of the safety of low dialysate [Na+] should also synthesise data on the outcome of serum [Na+].
At present, there is no agreement as to what [Na+] should be in dialysate, with equipoise arising from a lack of definitive evidence around safety and efficacy. In the Dialysis Outcomes and Practice Patterns Study (DOPPS), the mean dialysate [Na+] across countries varied from 138.3 mM in the UK to 140.8 mM in Italy, with no correlation between dialysate [Na+] and patient characteristics such as serum [Na+] (Hecking 2012; Mc Causland 2012). In the absence of any evidence‐based guidance to clinicians, this review quantifies the relative benefits and harms of low dialysate [Na+] from existing literature, to inform further research in the area and enable appropriate shared decisions between consumers and health care providers / professionals.
Objectives
This review evaluated harms and benefits of using a low (< 138 mM) dialysate [Na+] for maintenance HD patients.
Methods
Criteria for considering studies for this review
Types of studies
We reviewed all randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) that evaluated the effects of using low dialysate [Na+] in maintenance HD patients. We included both parallel group and cross‐over studies.
Types of participants
All patients undergoing HD for ESKD. No age, sex or comorbid inclusion or exclusion criteria were applied.
Types of interventions
We included comparisons between low and higher dialysate [Na+]. For sodium profiled dialysis, we analysed dialysate [Na+] as the time‐averaged concentration. We made the following comparisons:
Low (< 138 mM) dialysate [Na+] versus high (> 140 mM) OR neutral (138 to 140 mM) dialysate [Na+]
Low (< 138 mM) dialysate [Na+] versus neutral (138 to 140 mM) dialysate [Na+]
Low (< 138 mM) dialysate [Na+] versus high (> 140 mM) dialysate [Na+].
We excluded the following interventions.
Low dialysate [Na+] interventions that were combined with other dialysis co‐interventions not present identically in the intervention and comparison groups
Low dialysate [Na+] interventions that were < 3 mM different from the comparison dialysate [Na+]
Sodium profiled dialysis where the profile was not sufficiently described to calculate a time‐averaged dialysate [Na+]
Patients undergoing HD for acute kidney injury
Patient on HD schedules of greater or less than three times/week.
Types of outcome measures
We evaluated the effects of the intervention on the following outcome measures:
Primary outcomes
Interdialytic weight gain (IDWG) (efficacy)
Intradialytic hypotension, as defined by study investigators (safety)
Secondary outcomes
BP: predialysis, postdialysis, intradialytic and interdialytic time points; systolic, diastolic, MAP
Antihypertensive medication burden, as defined by study investigators
Fluid overload (extracellular fluid volume by bioimpedance analysis)
Serum [Na+]: predialysis, postdialysis, intradialytic and interdialytic time points
Thirst
Dietary sodium intake, as defined by study investigators
Cramp during HD treatment sessions
Left ventricular volume (magnetic resonance imaging, echocardiography)
Left ventricular mass (magnetic resonance imaging, echocardiography)
Arterial stiffness (carotid‐femoral pulse wave velocity measurement)
Hospitalisation
Myocardial infarction
Stroke
CV death
Death (all causes)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 7 August 2018 through contact with the Information Specialists using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Handsearching reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
We used the search strategy as described to obtain titles and abstracts of studies that might have been relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were clearly inapplicable or ineligible. However, studies and reviews that might have included relevant data or information were retained in the initial stages. The two authors then independently assessed the retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfy the inclusion criteria.
Data extraction and management
Two authors independently assessed retrieved abstracts and, if necessary the full text of these studies to determine which studies satisfied the inclusion criteria. Any uncertainties about study eligibility were discussed between authors. Data extraction was carried out independently by the two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data used in the analyses. Where relevant outcomes were only published in earlier publications of the study, these data were used. Any discrepancy between published versions were evaluated and highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias? These were pre‐specified as: baseline imbalance, interim reporting, deviation from study protocol in a way that does not reflect clinical practice, pre‐randomisation administration of an intervention that could enhance or diminish the effects of a subsequent randomised intervention, contamination, occurrence of 'null bias' due to interventions being insufficiently well delivered or overly wide inclusion criteria, selective reporting of subgroups, reporting of trial registration, reporting of funding source(s), publication as full journal report, and fraud.
Measures of treatment effect
Extraction of data measurements was performed in adherence of standard operating procedures in the Cochrane Handbook for Interventions in Systematic Reviews (Higgins 2011). For dichotomous outcomes such as death, results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. BP, thirst scores, IDWG, left ventricular hypertrophy), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales were used. A SMD of 0.2 indicates a small difference, 0.5 a moderate difference and 0.8 a large difference. We evaluated mean end of treatment values for continuous outcomes together with the reported standard deviation. Studies that did not report change from baseline (or raw treatment data to calculate change from baseline) were excluded from the meta‐analyses. If subgroups within each outcomes were not independent, with participants that contributed to more than one subgroup, we did not perform meta‐analysis for the entire group, or formally compare effect estimates between sub‐groups.
We compared effect size estimates between the following groups of studies to determine if there was a "dose effect" with respect to the intervention.
Low (< 138 mM) dialysate [Na+] versus neutral (138 to 140 mM) dialysate [Na+]
Low (< 138 mM) dialysate [Na+] versus high (> 140 mM) dialysate [Na+]
To explore any "dose effect", we compared effect size of the intervention on a few key outcomes between the two comparison groups above.
IDWG
Predialysis MAP
Postdialysis MAP
Predialysis serum [Na+]
Postdialysis serum [Na+]
Intradialytic cramps
Intradialytic hypotension
To quantify differences in MD and SMD between subgroups and between comparisons, standard deviation (SD) of effect size point estimates were calculated from 95% CI, and comparisons made using the Student t‐test, in adherence of standard operating procedures in the Cochrane Handbook for Interventions in Systematic Reviews (Higgins 2011), with the addition of a Bonferroni correction. Comparisons were not performed with samples that were not independent, if participants contributed to both subgroups or comparisons.
To quantify corresponding differences in RR between comparisons, we computed standard error (SE) of the log from confidence intervals [95% CI = exp(log(RR)±1.96xSE(log(RR))], and performing a z‐test on the difference of the logs, defining the z‐statistic being their ratio [(log(RR1‐hat)‐log(RR2‐hat)) in the numerator, and sqrt(SE(log(RR1‐hat))^2+SE(log(RR2‐hat))^2) in the denominator], with a null hypothesis that it has mean of zero (Altman 2003). Similarly to above, comparisons were not performed with samples that were not independent, and therefore omitted if participants contributed to both subgroups or comparisons.
Unit of analysis issues
Studies with more than two interventions were evaluated in this review. We used recommended methods for data extraction and analysis described in the Cochrane Handbook for Interventions in Systematic Reviews (Higgins 2011). In these cases, provided there were adequate data from the study, then treatment arms relevant to the treatment comparisons of interest were included in applicable meta‐analyses.
For cross‐over studies, data from the end of the first phase of cross‐over studies was included in applicable meta‐analysis if possible, using an approximation of a paired analysis.
There were no cluster randomised studies included in this meta‐analysis.
Dealing with missing data
Any further information was requested from the original authors by email when appropriate, and any relevant information obtained in this manner included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population were carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) was critically appraised (Higgins 2011).
Assessment of heterogeneity
Statistical heterogeneity in treatment effects among studies was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
There were insufficient data to generate funnel plots to assess for the potential existence of small study bias for the primary outcomes.
Data synthesis
Treatment estimates were summarised using random‐effects meta‐analysis.
Subgroup analysis and investigation of heterogeneity
There were insufficient extractable data to conduct subgroup and univariate meta‐regression analysis to explore the following variables as possible sources of heterogeneity: age, time on dialysis, era in which they were receiving dialysis, residual kidney function, whether they were on conventional or extended hours dialysis regimes, predialysis serum [Na+], presence of diabetes or ischaemic heart disease or other co morbidities, duration of intervention, magnitude of difference between dialysate and serum [Na+], whether [Na+] were individualised or changed on a group level, sample size, whether participants were randomised or crossed over, and how BP outcomes were measured.
Sensitivity analysis
There were insufficient extractable data to perform the following sensitivity analyses in order to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country
Repeating the analysis excluding any cross‐over studies with a washout period of less than one week
Repeating the analysis excluding any studies with single treatment interventions
Repeating the analysis excluding any studies with interventions using sodium profiled dialysis.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for selected outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). The outcomes presented in the 'Summary of findings' tables include (in order of importance).
IDWG
Intradialytic hypotension
Predialysis MAP
Postdialysis MAP
Antihypertensive medication burden, as defined by study investigators
Predialysis serum [Na+]
Intradialytic cramps
Results
Description of studies
Results of the search
The electronic search strategy identified 516 unique records (Figure 1). After duplicates were removed and titles and abstracts screened we retrieved 59 full‐text articles for further assessment.. Of these, 12 studies (15 records) were included and 29 studies (36 records) were excluded. Four ongoing studies were identified (7 records), and one study is awaiting assessment (1), these will be assessed in a future update of this review (Figure 1).
1.
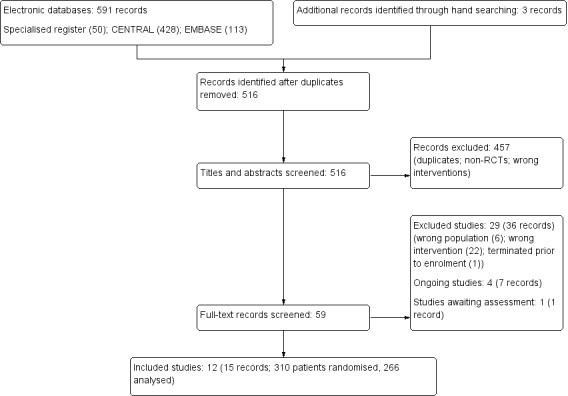
Flow chart illustrating the process of literature searching up to the identification of studies to be included in the systematic review.
Included studies
See: Characteristics of included studies and Table 4 and Table 5.
1. Summary of included studies.
| Study ID | Year of studya | Dialysate [Na+] in mM (control/ intervention) | Study design | ITT population (analysed population) | No. analysed in low dialysate [Na+] arm | No. analysed in higher dialysate [Na+] arm | Mean follow‐up (weeks) |
| Akdag 2015 | 2013 | 137/140 | P | 50 (46) | 22 | 24 | 26 |
| Beduschi 2013 | 2013 | 135/138 | P | 52 (38) | 20 | 18 | 16 |
| Boquin 1977 | 1977 | 130/140 | X | 51 (37) | 37 | 37 | 4 |
| Chambers 2002 | 2002 | 136/140b | X | 16 (16) | 16 | 16 | 1 |
| Daugirdas 1985 | 1985 | 135/143 | X | 10 (7) | 7 | 7 | 4 |
| Henrich 1982 | 1982 | 132/144 | X | 10 (10) | 10 | 10 | 6 |
| Liu 2016 | 2016 | 136/138 | P | 64 (57) | 28 | 29 | 52 |
| MATCH‐NA 2015 | 2012 | 134.3/142.9 | X | 18 (16) | 15 | 16 | 1 |
| Ogden 1978 | 1978 | 131/146 | X | 12 (12) | 12 | 12 | 1 |
| Quereda 1988 | 1988 | 133/139 | X | 8 (8) | 8 | 8 | 2 |
| Suckling 2013 | 2013 | 135/145 | X | 10 (10) | 10 | 10 | 1c |
| Van Kuijk 1996 | 1996 | 134/144 | X | 9 (9) | 9 | 9 | 1c |
Abbreviations: X ‐ cross‐over study; P ‐ parallel group study; ITT ‐ intention to treat
a year of patient accrual if available, year of study publication otherwise
b profiled dialysate [Na+]
c one session intervention and follow‐up
2. Summary of included patients.
| Study ID | Year of studya | Mean age, years (SD) | Mean time on dialysis, months (SD) | % male | % with diabetic kidney disease |
| Akdag 2015 | 2013 | 44.4 (2.6) | 53.9 (35.1) | 42.3 | 28.3 |
| Beduschi 2013 | 2013 | 62.5 (14) | 40.7 (44.6) | 46.8 | 34.2 |
| Boquin 1977 | 1977 | Not reported | Not reported | Not reported | Not reported |
| Chambers 2002 | 2002 | 75.8 (not reported) | Not reported | Not reported | Not reported |
| Daugirdas 1985 | 1985 | Not reported | Not reported | Not reported | Not reported |
| Henrich 1982 | 1982 | 57.2 (24.7) | 31.5 (25.3) | Not reported | 30 |
| Liu 2016 | 2016 | 58 (10.5) | 64.6 (55.4) | 54.4 | 19 |
| MATCH‐NA 2015 | 2012 | 58.8 (9.5) | Not reported | 93.8 | 69 |
| Ogden 1978 | 1978 | Not reported | Not reported | Not reported | Not reported |
| Quereda 1988 | 1988 | 58 (9) | 27 (22) | 25 | 0 |
| Suckling 2013 | 2013 | 60.9 (5.1) | Not reported | 50 | 40 |
| Van Kuijk 1996 | 1996 | 46 (not reported) | 46 (not reported) | 88.9 | 0 |
a year of patient accrual if available, year of study publication otherwise
The 12 studies included randomised 310 patients, but only 266 patients could be analysed after attrition due to dropout. Most studies were cross‐over studies with only three being parallel group studies (Akdag 2015; Beduschi 2013; Liu 2016). Given the predominance of studies with cross‐over designs, of these 266 patients 194 contributed to low dialysate [Na+] arm, and 196 to the higher dialysate [Na+] arm.
The median (interquartile range) for follow‐up was low at 4 (3, 8.5) weeks. Two studies were of only one HD session per intervention (Suckling 2013; Van Kuijk 1996), and three others were of only one week's HD per intervention (Chambers 2002; MATCH‐NA 2015; Ogden 1978).
Studies were conducted in Brazil (Beduschi 2013), China (Liu 2016), the Netherlands (Van Kuijk 1996), Spain (Quereda 1988), Turkey (Akdag 2015), UK (Chambers 2002; Suckling 2013), and the USA (Boquin 1977; Daugirdas 1985; Henrich 1982; MATCH‐NA 2015; Ogden 1978),
Six studies that reported funding received funding from government or healthcare organisations, one from industry, and five studies did not report their funding source.
The intervention was profiled dialysate [Na+] in only one study (Chambers 2002), with the remainder using fixed concentrations of dialysate [Na+] in the intervention and control arms. In the 12 studies, the mean dialysate [Na+] in the intervention arms was 134 mM, and in the control arms 144 mM.
Excluded studies
See: Characteristics of excluded studies
We excluded 29 studies (36 reports). The most common reasons for exclusion were: the study did not evaluate low dialysate [Na+] as an intervention; the population was in haemodiafiltration rather than HD; or the study evaluated more than one intervention at the same time.
Risk of bias in included studies
Risk of bias in the included studies is described in Characteristics of included studies, Figure 2; Figure 3.
2.
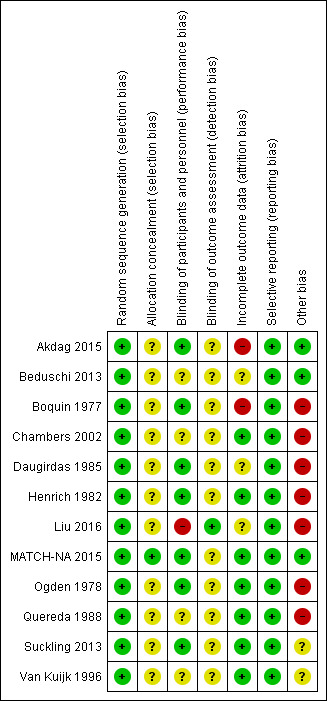
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
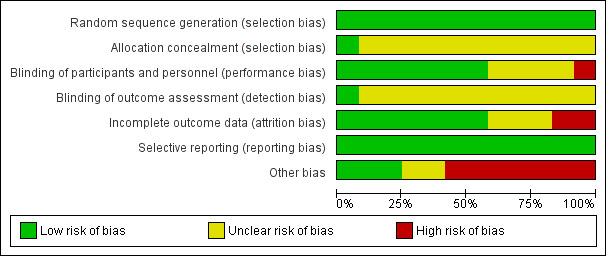
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
In most studies, conduct and reporting of most studies were not guided by the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) initiative (CONSORT home page) because of their era. Of the four studies published since 2010, only one (MATCH‐NA 2015) was reported in a manner compliant with CONSORT standards), and only two were registered with a Clinical Trials Registry (Akdag 2015; MATCH‐NA 2015). Where reporting of methodology was incomplete, authors were contacted directly to seek clarification, particularly about whether the intervention allocation was randomised or not.
Allocation
Random sequence generation
Following updated information from authors, we felt that all the included studies had adequate (low risk) random sequence generation.
Allocation concealment
Only MATCH‐NA 2015 reported allocation concealment. Risks from allocation concealment were unclear in the remaining studies.
Blinding
Performance bias
Only Liu 2016 was open‐label and at high risk of performance bias, while most studies were blinded.
Double‐blind: Akdag 2015, Daugirdas 1985, Henrich 1982, Ogden 1978
Single‐blind: Boquin 1977, MATCH‐NA 2015, Suckling 2013
Risks from blinding were unclear in the remaining four studies (Beduschi 2013; Chambers 2002; Quereda 1988; Van Kuijk 1996
Detection bias
Only Liu 2016 reported assessor blinding and was at low risk of detection bias. Risks from detection bias were unclear in the remaining studies. Of note, most of the outcomes reported in the studies were automated and objective, and not easily amenable observer bias.
Incomplete outcome data
In all studies, there has not been any imputation of missing data, or analyse data using the intention to treat framework rather than a complete/as treated/safety set framework. Risk from attrition bias was low in most studies (Chambers 2002; Henrich 1982; MATCH‐NA 2015; Ogden 1978; Quereda 1988; Suckling 2013; Van Kuijk 1996), either because they had no dropouts, or minimal dropouts that were clearly for reasons unrelated to the intervention. Risk was unclear in three studies (Beduschi 2013; Daugirdas 1985; Liu 2016) because of considerable dropout, albeit for reasons that were reportedly unlikely to be related to the intervention. In this setting the risk of attrition bias in related only to the development of unmeasured confounder imbalance between arms post‐randomisation, and could not be assessed. The risk of attrition bias was assessed as high in two others (Akdag 2015; Boquin 1977). Where risk was high, there had been considerable dropout, for reasons including cramp or intradialytic hypotension in the intervention arm (3 out of 20 in Akdag 2015, and 7 out of 51 in Boquin 1977) and without any attempt to impute missing data in that arm.
Selective reporting
Within the limitations of what was reported in the included study articles, no obvious errors of omission were detected.
Other potential sources of bias
Indirectness was judged to be present in two studies (Suckling 2013; Van Kuijk 1996) due to these being studies of single dialysis treatments, performed with fixed ultrafiltration rates and with interventions being truncated at two hours. Although these studies met the requirements for inclusion, the intervention that was studied was likely to be less effective given the way it was applied, compared to how the intervention would be applied in most routine clinical settings.
Indirectness was also assessed as being present in a number of older studies, since the studies report now‐obsolete HD practices such as the use of cellulosic dialysers (Boquin 1977; Henrich 1982; Ogden 1978; Quereda 1988), acetate‐buffered dialysate (Boquin 1977; Daugirdas 1985; Henrich 1982; Ogden 1978; Quereda 1988), and parallel plate dialysers (Daugirdas 1985; Quereda 1988; Ogden 1978). The age of these studies means that the studies were undertaken in an era of non‐computerised HD monitors and manual ultrafiltration systems. As such, the intervention that was studied may have had a different effect given the way it was applied, compared to the intervention as it would be applied in most modern clinical settings.
Most studies were cross‐over studies, with only three being parallel group studies (Akdag 2015; Beduschi 2013; Liu 2016). Cross‐over studies frequently have a carry‐over effect, and very few studies that we reviewed reported a “washout” period to separate the two treatment periods that would minimise such “carry‐over” effects. Only MATCH‐NA 2015) and Suckling 2013 specified a washout period (one week), with Van Kuijk 1996 being unclear, and the remaining having no washout between intervention and control treatments (Boquin 1977; Chambers 2002; Henrich 1982; Ogden 1978; Quereda 1988). For these studies, the effect of lower dialysate [Na+] may have been under‐estimated.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM) for chronic haemodialysis.
| Low dialysate [Na+](< 138 mM) versus neutral dialysate [Na+](138 to 140 mM) or high dialysate [Na+](> 140 mM) for chronic haemodialysis (HD) | |||||
| Patient or population: chronic HD Setting: dialysis units Intervention: Low dialysate [Na+] (< 138 mM) Comparison: neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM) | Risk with Low dialysate [Na+] (< 138 mM) | ||||
| IDWG | The mean IDWG was 2.55 kg | MD 0.35 kg lower (0.51 lower to 0.18 lower) | ‐ | 352 (10) | ⊕⊕⊕⊕ HIGH |
| Intradialytic hypotension | 110 per 1,000 | 167 per 1,000 (125 to 222) | RR 1.52 (1.14 to 2.02) | 12,570 (7) | ⊕⊕⊕⊝ MODERATE 1 |
| Predialysis MAP | The mean predialysis MAP was 104.6 mmHg | MD 3.58 mmHg lower (5.46 lower to 1.69 lower) | ‐ | 156 (4) | ⊕⊕⊕⊝ MODERATE 2 |
| Postdialysis MAP | The mean postdialysis MAP was 101.0 mmHg | MD 3.26 lower (4.82 lower to 1.7 lower) | ‐ | 150 (4) | ⊕⊕⊕⊝ MODERATE 2 |
| Antihypertensive medication | The mean number of antihypertensive medications was 3.1 | SMD 0.67 SD lower (1.07 lower to 0.28 lower) | ‐ | 103 (2) | ⊕⊕⊝⊝ LOW 3 |
| Predialysis serum [Na+] | The mean predialysis serum [Na+] was 138.3 mM | MD 1.69 lower (2.36 lower to 1.02 lower) | ‐ | 258 (7) | ⊕⊕⊕⊝ MODERATE 4 |
| Intradialytic cramps | 74 per 1,000 | 130 per 1,000 (85 to 201) | RR 1.77 (1.15 to 2.73) | 12,186 (6) | ⊕⊕⊕⊝ MODERATE 5 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio; MD: mean difference; SMD: standardised mean difference IDWG: interdialytic weigh gain; MAP: mean arterial pressure | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Down‐graded because of an era effect in some included studies (indirectness), affecting the applicability to modern settings ‐ see section Overall completeness and applicability of evidence
2 Down‐graded because of only moderate number of studies and patients assessed (imprecision), and the lack of concurrent reporting on antihypertensive medication burden in any study in this analysis
3 Down‐graded because of low number of studies and patients assessed (imprecision)
4 Down‐graded because of heterogeneity of [Na+] measurements between studies
5 Down‐graded because of inconsistency between studies, albeit contributed by only one study (Liu 2016)
Summary of findings 2. Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) for chronic haemodialysis.
| Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+](138 to 140 mM) for chronic haemodialysis (HD) | |||||
| Patient or population: chronic HD Setting: dialysis units Intervention: Low dialysate [Na+] (< 138 mM) Comparison: neutral dialysate [Na+] (138 to 140 mM) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with neutral dialysate [Na+] (138 to 140 mM) | Risk with low dialysate [Na+] (< 138 mM) | ||||
| IDWG | The mean IDWG was 2.55 kg | MD 0.33 kg lower (0.51 lower to 0.14 lower) | ‐ | 263 (6 RCTs) | ⊕⊕⊕⊕ HIGH |
| Intradialytic hypotension | 111 per 1,000 | 165 per 1,000 (121 to 225) | RR 1.49 (1.09 to 2.03) | 12084 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 1 |
| Predialysis MAP | The mean predialysis MAP was 107.1 mmHg | MD 3.52 mmHg lower (5.46 lower to 1.57 lower) | ‐ | 112 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 |
| Postdialysis MAP | The mean postdialysis MAP was 100.82 mmHg | MD 3.01 lower (4.69 lower to 1.34 lower) | ‐ | 112 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 |
| Antihypertensive medication | The mean number of antihypertensive medications was 3.1 | SMD 0.67 SD lower (1.07 lower to 0.28 lower) | ‐ | 103 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 |
| Predialysis serum [Na+] | The mean predialysis serum [Na+] was 138.3 mM | MD 1.59 mM lower (2.4 lower to 0.78 lower) | ‐ | 169 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 4 |
| Intradialytic cramps | 66 per 1,000 | 110 per 1,000 (61 to 197) | RR 1.66 (0.92 to 2.98) | 11700 (4 RCTs) | ⊕⊕⊝⊝ LOW 5 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio; MD: mean difference; SMD: standardised mean difference IDWG: interdialytic weigh gain; MAP: mean arterial pressure | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Down‐graded because of an era effect in some included studies (indirectness), affecting the applicability to modern settings ‐ see section Overall completeness and applicability of evidence
2 Down‐graded because of low number of studies and patients assessed (imprecision), and the lack of concurrent reporting on antihypertensive medication burden in any study in this analysis
3 Down‐graded because of low number of studies and patients assessed (imprecision)
4 Down‐graded because of only moderate number of studies and patients assessed (imprecision)
5 Down‐graded because of inconsistency between studies, albeit contributed by only one study (Liu 2016), and overall imprecision of effect
Summary of findings 3. Low dialysate [Na+] (< 138 mM) versus high dialysate [Na+] (> 140 mM) for chronic haemodialysis.
| Low dialysate [Na+](< 138 mmol/L) versus high dialysate [Na+] (> 140 mmol/L) for chronic haemodialysis (HD) | ||||||
| Patient or population: chronic HD Setting: dialysis units Intervention: Low dialysate [Na+] (< 138 mmol/L) Comparison: high dialysate [Na+] (> 140 mmol/L) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with high dialysate [Na+] (> 140 mmol/L) | Risk with Low dialysate [Na+] (< 138 mmol/L) | |||||
| IDWG | The mean IDWG was 2.55 kg | MD 0.42 kg lower (0.8 lower to 0.05 lower) | ‐ | 89 (4 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Intradialytic hypotension | 86 per 1,000 | 148 per 1,000 (49 to 438) | RR 1.71 (0.57 to 5.07) | 486 (2 RCTs) | ⊕⊕⊝⊝ LOW2 | |
| Predialysis MAP | The mean predialysis MAP was 98.44 mmHg | MD 4.48 mmHg lower (12.07 lower to 3.1 higher) | ‐ | 44 (2 RCTs) | ⊕⊕⊝⊝ LOW3 | |
| Postdialysis MAP | The mean postdialysis MAP was 101 mmHg | MD 4.85 mmHg lower (9.1 lower to 0.6 lower) | ‐ | 38 (2 RCTs) | ⊕⊕⊝⊝ LOW3 | |
| Antihypertensive medication | see comment | see comment | ‐ | ‐ | ‐ | Not reported |
| Predialysis serum [Na+] | The mean predialysis serum [Na+] was 138.3 mM | MD 1.92 mM lower (3.15 lower to 0.7 lower) | ‐ | 89 (4 RCTs) | ⊕⊕⊕⊝ MODERATE1 | |
| Intradialytic cramps | 255 per 1,000 | 495 per 1,000 (393 to 623) | RR 1.94 (1.54 to 2.44) | 486 (2 RCTs) | ⊕⊕⊝⊝ LOW4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: mean difference IDWG: interdialytic weigh gain; MAP: mean arterial pressure | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Down‐graded because of only moderate number of studies and patients assessed (imprecision)
2 Down‐graded because of low number of studies and patients assessed (imprecision), and an era effect in some included studies (indirectness), affecting the applicability to modern settings
3 Down‐graded because of low number of studies and patients assessed (imprecision), and the lack of concurrent reporting on antihypertensive medication burden in any study
4 Down‐graded because of low number of studies and patients assessed (imprecision)
The following treatment comparisons were made.
Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM)
Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM)
Low dialysate [Na+] (< 138 mM) versus high dialysate [Na+] (> 140 mM).
Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM)
See Table 1.
Interdialytic weight gain
Low dialysate [Na+] reduced IDWG compared to neutral or high dialysate [Na+] (Analysis 1.1 (10 studies, 352 participants): MD ‐0.35 kg, 95% CI ‐0.51 to ‐0.18; I2 = 0%; high certainty evidence), reflecting an effect that can be regarded as being clinically small in size (Wong 2017). Of note, Suckling 2013) and Van Kuijk 1996 performed single HD session studies, and IDWG data from these studies were not included in this analysis as they were not subject to the study intervention.
1.1. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 1 Interdialytic weight gain.
Predialysis blood pressure
Low dialysate [Na+] probably reduced predialysis MAP compared to neutral or high dialysate [Na+] (Analysis 1.2.1 (4 studies, 156 participants): MD of ‐3.58 mmHg, 95% CI ‐5.46 to ‐1.69; I2 = 0%; moderate certainty evidence), reflecting an effect that can be regarded as being clinically moderate in size (Heerspink 2009).
1.2. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 2 Predialysis BP.
Low dialysate [Na+] may have reduced predialysis systolic BP (Analysis 1.2.2 (3 studies, 83 participants): MD ‐7.56 mmHg, 95% CI ‐15.92 to 0.80; I2 = 0%; low certainty evidence). Low dialysate [Na+] may have made little or no difference to predialysis diastolic BP (Analysis 1.2.3 (2 studies, 52 participants): MD ‐3.13 mmHg, 95% CI ‐11.79 to 5.54); I2 = 0% low certainty evidence).
Of note, Boquin 1977 presented mean predialysis systolic and diastolic BP for the intervention and control, but no measures of distribution or P values relating to them ‐ this study was therefore excluded from the analyses of systolic and diastolic BP. Suckling 2013 and Van Kuijk 1996 performed single HD session studies, and predialysis BP data from these studies were not included in this analysis as they were not subject to the study intervention.
Intradialytic blood pressure
It is uncertain whether low dialysate [Na+] changed intradialytic MAP because the certainty of this evidence is very low (Analysis 1.3.1 (1 study, 20 participants):MD ‐4.00 mmHg, 95% CI ‐18.52 to 10.52; low certainty evidence). Low dialysate [Na+] may have made little or no difference to intradialytic systolic BP (Analysis 1.3.2 (2 studies, 34 participants): MD ‐3.99 mmHg, 95% CI ‐17.96 to 9.99); I2 = 0%; low certainty evidence). Low dialysate [Na+] also may have made little or no difference to intradialytic diastolic BP (Analysis 1.3.3 (2 studies, 34 participants): MD 1.33 mmHg; 95% CI ‐6.29 to 8.95; I2 = 0%; low certainty evidence).
1.3. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 3 Intradialytic BP.
Postdialysis blood pressure
Low dialysate [Na+] probably reduced postdialysis MAP compared to neutral or high dialysate [Na+] (Analysis 1.4,1 (4 studies, 150 participants): ‐3.26 mmHg; 95% CI ‐4.82 to ‐1.70; I2 = 0%; moderated certainty evidence), reflecting an effect that can be regarded as being clinically moderate in size (Heerspink 2009). It is uncertain whether low dialysate [Na+] changes postdialysis systolic BP (Analysis 1.4.2 (1 study, 18 participants): MD ‐5.00 mmHg, 95% CI ‐31.86 to 21.86; very low certainty evidence). It is also uncertain whether low dialysate [Na+] changes postdialysis diastolic BP (Analysis 1.4.3 (1 study, 18 participants): MD 0.00 mmHg, 95% CI ‐13.98 to 13.98; very low certainty evidence).
1.4. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 4 Postdialysis BP.
Of note, Boquin 1977 presented mean postdialysis systolic and diastolic BP, but no measures of distribution or P values relating to them, and this study was therefore excluded from the analyses of systolic and diastolic BP. Henrich 1982 and Beduschi 2013 did not present systolic and diastolic BP, only MAP.
Interdialytic blood pressure
Low dialysate [Na+] may have made little or no difference to Interdialytic systolic BP compared to neutral or high dialysate [Na+] (Analysis 1.5.2 (2 studies, 103 participants): MD ‐0.17 mmHg, 95% CI ‐5.42 to 5.08; I2 = 0%; low certainty evidence). Compared to neutral or high dialysate [Na+], low dialysate [Na+] may have reduced Interdialytic diastolic BP (Analysis 1.5.3 (2 studies, 103 participants):MD ‐2.00 mmHg, 95% CI ‐4.88 to 0.84; I2 = 0%; low certainty evidence).
1.5. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 5 Interdialytic BP.
Of note, both Akdag 2015 and Liu 2016 presented postdialysis systolic and diastolic BP from which measures of central tendency for MAP might be calculated, but not corresponding measures of distribution ‐ these studies were therefore excluded from the analysis of MAP.
Serum [Na+]
Low dialysate [Na+] probably reduced serum predialysis serum [Na+] compared to neutral or high dialysate [Na+] (Analysis 1.6.1 (7 studies, 258 participants):MD ‐1.69 mM, 95% CI ‐2.36 to ‐1.02; I2 = 0%; moderate certainty evidence), reflecting an effect that can be regarded as being clinically small in size (Hecking 2012; Hecking 2015; Mc Causland 2012). It is uncertain whether low dialysate [Na+] changed Intradialytic serum [Na+] (Analysis 1.6.2 (1 study, 20 participants): MD ‐4.37 mM, 95% CI ‐6.24 to ‐2.40; very low certainty evidence).
1.6. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 6 Serum [Na+].
Low dialysate [Na+] probably reduced postdialysis serum [Na+] compared to neutral or high dialysate [Na+] (Analysis 1.6.3 (3 studies, 99 participants): MD ‐4.74 mM, 95% CI ‐8.30 to 1.77; I2= 86% moderate certainty evidence), reflecting an effect that can be regarded as being moderate in size (Hecking 2012; Hecking 2015; Mc Causland 2012). Heterogeneity was high almost certainly as a result of the different "doses" of dialysate [Na+] between the three studies, and corresponding "dose effect" for interventions on the outcome. For instance, the difference in dialysate [Na+] between the intervention and control arms was ˜15 mM in Ogden 1978, ˜10 mM in Van Kuijk 1996 and only ˜2 mM in Liu 2016, as expected leading to the sub‐physiologic low postdialysis serum [Na+] in Ogden 1978 and relatively normal one in Liu 2016, with Van Kuijk 1996 being in between.
Intradialytic cramps
Low dialysate [Na+] probably increased intradialytic cramp compared to neutral or high dialysate [Na+] (Analysis 1.8 (6 studies, 12,186 HD sessions): RR 1.77, 95% CI 1.15 to 2.73; I2= 89%; moderate certainty evidence). Of note, Akdag 2015 did not quantify this outcome (i.e. over how many sessions), but merely stated it as a reason for withdrawal from the study and exclusion from their analysis; this study was not included in our analysis. There was high heterogeneity between the studies, contributed by the inconsistency of a single study (Liu 2016), again almost certainly due to the relatively smaller "dose effect" for the intervention of only ˜2 mM difference in dialysate [Na+] between arms in that that study.
1.8. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 8 HD sessions complicated by intradialytic hypotension.
Intradialytic hypotension
Low dialysate [Na+] probably increased intradialytic hypotension compared to neutral or high dialysate [Na+] (Analysis 1.8 (9 studies, 12,681 HD sessions): RR 1.56, 95% CI 1.17 to 2.07; I2= 81%; moderate certainty evidence) reflecting an effect that can be regarded as being large in size. Of note, Akdag 2015 did not quantify this outcome (i.e. over how many sessions), but merely stated it as a reason for withdrawal from the study and exclusion from their analysis; this study was not included in our analysis.
Of note, there was considerable variability between studies around how intradialytic hypotension was assessed that might have contributed to the observed high heterogeneity between studies, and a summary is tabulated in additional Table 6 ("Definition of intradialytic hypotension in included studies"). Although these different definitions can give different results, it is likely that this feature did not reduce the internal validity the studies, nor hamper consistent interpretation of the literature ‐ in each study, the patients' responses to therapy in both the intervention and control groups were assessed in the same manner, and are therefore directly comparable. The differences between arms in each of the studies can be synthesised without issue.
3. Definitions of intradialytic hypotension in included studies.
| Study ID | Definition |
| Akdag 2015 | Fall in intradialytic systolic BP by ≥ 20 mmHg associated with symptoms requiring medical attention |
| Beduschi 2013 | Fall in intradialytic BP to < 90/60 |
| Boquin 1977 | “Hypotension requiring intervention” |
| Chambers 2002 | Fall in intradialytic systolic BP by ≥ 30 mmHg or to < 90 mmHg |
| Daugirdas 1985 | Fall in intradialytic MAP by > 15% plus intervention (defined as reduction in UF, administration of medication/intravenous saline) |
| Henrich 1982 | Fall in intradialytic systolic BP to < 90 mmHg |
| Liu 2016 | Fall in intradialytic systolic BP to < 90 mmHg or a decrease in MAP by ≥10 mmHg associated with symptoms or the need for nursing interventions |
| MATCH‐NA 2015 | Fall in intradialytic systolic BP to < 90 mmHg |
| Ogden 1978 | Not reported |
| Quereda 1988 | Fall in intradialytic systolic BP to < 90 mmHg |
| Suckling 2013 | Not reported |
| Van Kuijk 1996 | Fall in intradialytic systolic BP by ≥ 20 mmHg |
BP ‐ blood pressure; UF ‐ ultrafiltration
Postdialysis extracellular fluid status
It is uncertain whether low dialysate [Na+] changed postdialysis extracellular fluid status (Analysis 1.9 (1 study, 38 participants): MD ‐0.30 L, 95% CI ‐2.07 to 1.47); very low certainty evidence). The extracellular fluid status was as assessed by bioimpedance analysis (Beduschi 2013).
1.9. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 9 Postdialysis extracellular fluid status.
Dietary salt intake
Low dialysate [Na+] may have decreased dietary salt intake compared to neutral or high dialysate [Na+] (Analysis 1.10 (2 studies, 95 participants): MD 0.21 g/d, 95% CI ‐0.48 to 0.06; I2= 0%; low certainty evidence. In these two studies intake was assessed from three‐day food diaries, and reported as salt intake in g/d (note: Beduschi 2013 reported intake as sodium/day, but it is in fact salt/day as confirmed with the authors ‐ the corrected data were used in this analysis).
1.10. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 10 Dietary salt intake.
Left ventricular structure
It is uncertain whether low dialysate [Na+] changed left ventricular mass index compared to neutral or high dialysate [Na+] (Analysis 1.11.1 (1 study, 57 participants): MD ‐8.00 g/m2, 95% CI ‐17.11 to 1.11; very low certainty evidence) . It is also uncertain whether low dialysate [Na+] changed either end‐diastolic dimension (Analysis 1.11.2 (1 study, 57 participants): MD 0.40 cm, 95% CI ‐3.18 to 3.98; very low certainty evidence) or end‐systolic dimension, with a and Analysis 1.11.3 (1 study, 57 participants): 0.4 cm, 95% CI ‐2.59 to 3.39; very low certainty evidence). Of note, the single study in this analysis (Liu 2016) was subject to high risk ascertainment bias, since the outcome was determined by single operator, albeit blinded to allocation, at two points separated at 12 months with no control for potential observer drift, using echocardiography as opposed to the research standard of cardiac magnetic resonance imaging.
1.11. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 11 Left ventricular structure.
Antihypertensive medication
Low dialysate [Na+] may have reduced antihypertensive medication burden compared to neutral or high dialysate [Na+] (Analysis 1.12 (2 studies, 103 participants): SMD ‐0.67, 95% CI ‐1.07 to ‐0.28; I2= 0%; low certainty evidence). Of note, SMD was used for this analysis rather than MD ‐ antihypertensive medication burden was quantified as number of antihypertensive drugs in Akdag 2015, and as aggregated equivalent dose units in Liu 2016.
1.12. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 12 Antihypertensive medication.
Fatigue
It is uncertain whether low dialysate [Na+] changed fatigue compared to neutral or high dialysate [Na+] (Analysis 1.13 (1 study, 32 participants): MD 6.52, 95% CI ‐18.57 to 31.60; very low certainty evidence) on a 100‐point fatigue scale (100 most fatigued).
1.13. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 13 Fatigue.
Thirst
It is uncertain whether low dialysate [Na+] changed thirst compared to neutral or high dialysate [Na+] (Analysis 1.14 (1 study, 14 participants): MD ‐0.60, 95% CI ‐2.07 to 0.87; very low certainty evidence), on a 9‐point thirst scale (9 most thirsty).
1.14. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 14 Thirst.
Pulse wave velocity
It is uncertain whether low dialysate [Na+] changed pulse wave velocity compared to neutral or high dialysate [Na+] (Analysis 1.15 (1 study, 57 participants): MD ‐0.20 m/s, 95% CI ‐1.45 to 1.05; very low certainty evidence),
1.15. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 15 Pulse wave velocity.
Vascular resistance
It is uncertain whether low dialysate [Na+] changed vascular resistance compared to neutral or high dialysate [Na+] (Analysis 1.16 (1 study, 18 participants): MD ‐17 mmHg/mL/100mL/s, 95% CI ‐867.03 to 901.03; very low certainty evidence).
1.16. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 16 Arterial vascular resistance [mmHg/mL/I00mL/s].
Venous tone
It is uncertain whether low dialysate [Na+] changed venous tone compared to neutral or high dialysate [Na+] (Analysis 1.17 (1 study, 18 participants): MD 0.40 mmHg/mL/100mL, 95% CI ‐2.28 to 3.08; very low certainty evidence).
1.17. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 17 Venous tone [mmHg/mL/I00mL].
Outcomes not reported
Predialysis extracellular fluid status, hospitalisation, myocardial infarction, stroke, CV death, and death (all causes) were not reported.
Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM)
See Table 2.
Data synthesis for the available outcomes is presented in additional Table 7 ("Outcomes reported for low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM)"). The following text summarises the data synthesis only briefly, listing only the outcomes and where there are no available data, and highlighting differences between this comparison and the main one.
4. Outcomes reported for low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM).
| Outcomes | No. of studies | Low dialysate [Na+] | Neutral dialysate [Na+] | MD or RR (95% CI) | I2 | Certainty of evidence |
| IDWG (kg) | 6 | 131 | 132 | ‐0.33 (‐0.51 to ‐0.14) | 0% | High |
| Predialysis MAP (mmHg) | 2 | 57 | 55 | ‐3.52 (‐5.46 to ‐1.57) | 0% | Low |
| Predialysis systolic BP (mmHg) | 1 | 20 | 18 | ‐12.02 (‐24.78 to 0.74) | ‐ | Very low |
| Predialysis diastolic BP (mmHg) | 1 | 20 | 18 | ‐4.21 (‐15.70 to 7.28) | ‐ | Very low |
| Intradialytic MAP (mmHg) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Intradialytic systolic BP (mmHg) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Intradialytic diastolic BP (mmHg) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Postdialysis MAP (mmHg) | 2 | 57 | 55 | ‐3.01 (‐4.69 to ‐1.34) | 0% | Low |
| Postdialysis systolic BP (mmHg) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Postdialysis diastolic BP (mmHg) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Interdialytic MAP (mmHg) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Interdialytic systolic BP (mmHg) | 2 | 50 | 53 | ‐0.12 (‐6.45 to 6.21) | 0% | Low |
| Interdialytic diastolic BP (mmHg)) | 2 | 51 | 52 | ‐2.00 ‐4.85 to 0.85) | 0% | Low |
| Predialysis serum [Na+] (mM) | 3 | 85 | 84 | ‐1.59 (‐2.40 to ‐0.78) | 0% | Low |
| Intradialytic serum [Na+] (mM) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Postdialysis serum [Na+] (mM) | 1 | 28 | 29 | ‐1.80 (‐2.66 to ‐0.94) | ‐ | Very low |
| Intradialytic cramps | 4 | 5820 sessions | 5880 sessions | 1.66 (0.92 to 2.98) | 91% | Low |
| Intradialytic hypotension | 5 | 6012 sessions | 6072 sessions | 1.49 (1.09 to 2.03) | 88% | Moderate |
| Predialysis ECF status (L) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Postdialysis ECF status (L) | 1 | 20 | 18 | ‐0.30 (‐2.11 to 1.51) | ‐ | Very low |
| Dietary salt intake | 2 | 48 | 47 | ‐0.21 (‐0.48 to 0.06) | 0% | Low |
| LVMI (g/m2) | 1 | 28 | 29 | ‐8.00 (‐17.11 to 1.11) | ‐ | Very low |
| LVEDD (cm) | 1 | 28 | 29 | 0.40 (‐3.18 to 3.98) | ‐ | Very low |
| LVESD (cm) | 1 | 28 | 29 | 0.40 (‐2.59 to 3.39) | ‐ | Very low |
| Antihypertensive medication | 2 | 50 | 53 | ‐0.67 (‐1.07 to ‐0.28) | 0% | Low |
| Fatigue | 1 | 16 | 16 | 6.52 (‐18.57 to 31.60) | ‐ | Very low |
| Thirst | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pulse wave velocity (m/s) | 1 | 28 | 29 | ‐0.20 (‐1.45 to 105) | ‐ | Very low |
| Vascular resistance (mmHg/mL/100 mL) | 1 | 9 | 9 | 0.02 (‐0.91 to 0.94) | ‐ | Very low |
| Venous tone (mmHg/mL/100 mL) | 1 | 9 | 9 | 0.40 (‐2.28 to 3.08) | ‐ | Very low |
| Hospitalisation | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Myocardial infarction | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Stroke | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cardiovascular death | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Death (all causes) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
BP ‐ blood pressure; ECF ‐ extracellular fluid; IDWG ‐ intradialytic weigh gain; LVMI ‐ left ventricular mass index; LVED ‐ left ventricular end diastolic dimension, LVESD ‐ left ventricular end systolic dimension; MAP ‐ mean arterial pressure
Data were available for IDWG, predialysis BP, postdialysis BP, interdialytic BP, serum [Na+], intradialytic cramps, intradialytic hypotension, postdialysis ECF status, dietary salt intake, left ventricular structure, antihypertensive medication, fatigue, and pulse wave velocity. For all of these outcomes, the effect of the intervention was directionally similar to that identified in the main comparison, and the size of the effect also similar.
Data were not available for intradialytic BP, predialysis, thirst, vascular resistance, venous tone, hospitalisation, myocardial infarction, stroke, CV death, and death (all causes).
Low dialysate [Na+] (<138 mM) versus high dialysate [Na+] (>140 mM)
See Table 3.
Data synthesis for the available outcomes is presented in additional Table 8 ("Outcomes reported for low dialysate [Na+] (< 138 mM) versus high dialysate [Na+] (> 140 mM)"). The following text summarises the data synthesis only briefly, listing only the outcomes and where there are no available data, and highlighting differences between this comparison and the main one.
5. Outcomes reported for low dialysate [Na+] (< 138 mM) versus high dialysate [Na+] (> 140 mM).
| Outcome | No. of studies | Low dialysate [Na+] | High dialysate [Na+] | MD or RR (95% CI) | I2 | Certainty of evidence |
| IDWG (kg) | 4 | 44 | 45 | ‐0.42 (‐0.80 to ‐0.05) | 0% | Moderate |
| Predialysis MAP (mmHg) | 2 | 22 | 22 | ‐4.48 (‐12.07 to 3.10) | 0% | Low |
| Predialysis systolic BP (mmHg) | 2 | 23 | 23 | ‐4.22 (‐15.17 to 6.72) | 0% | Low |
| Predialysis diastolic BP (mmHg) | 1 | 7 | 7 | ‐1.70 (‐14.89 to 11.49) | ‐ | Very low |
| Intradialytic MAP (mmHg) | 2 | 17 | 17 | ‐0.04 (‐10.32 to 10.24) | 0% | Low |
| Intradialytic systolic BP (mmHg) | 2 | 17 | 17 | ‐3.99 (‐17.96 to 9.99) | 0% | Low |
| Intradialytic diastolic BP (mmHg) | 2 | 17 | 17 | 1.33 (‐6.29 to 8.95) | 0% | Low |
| Postdialysis MAP (mmHg) | 2 | 19 | 19 | ‐4.85 (‐9.10 to ‐0.60) | 0% | Low |
| Postdialysis systolic BP (mmHg) | 2 | 25 | 25 | ‐10.74 (‐24.04 to 2.57) | 0% | Low |
| Postdialysis diastolic BP (mmHg) | 1 | 9 | 9 | 0 (‐13.98 to 13.98) | ‐ | Very low |
| Interdialytic MAP (mmHg) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Interdialytic systolic BP (mmHg) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Interdialytic diastolic BP (mmHg)) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Predialysis serum [Na+] (mM) | 4 | 44 | 45 | ‐1.92 (‐3.15 to ‐0.70) | 0% | Low |
| Intradialytic serum [Na+] (mM) | 1 | 10 | 10 | ‐4.37 (‐4.79 to ‐3.95) | ‐ | Very low |
| Postdialysis serum [Na+] (mM) | 2 | 21 | 21 | ‐6.75 (‐11.32 to ‐2.18) | 66% | Very low |
| Intradialytic cramps | 2 | 243 sessions | 243 sessions | 1.94 (1.54 to 2.44) | 0% | Low |
| Intradialytic hypotension | 2 | 243 sessions | 243 sessions | 1.71 (0.57 to 5.07) | 78% | Low |
| Predialysis ECF status (L) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Postdialysis ECF status (L) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Dietary salt intake | 0 | ‐ | ‐ | ‐ | ‐ | |
| LVMI (g/m2) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| LVEDD (mm) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| LVESD (mm) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Antihypertensive medication | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Fatigue | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Thirst | 1 | 7 | 7 | ‐0.40 (‐1.46 to 0.66) | ‐ | Very low |
| Pulse wave velocity (m/s) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Vascular resistance (mmHg/mL/100 mL) | 1 | 9 | 9 | 0.02 (‐0.91 to 0.94) | ‐ | Very low |
| Venous tone (mmHg/mL/100 mL) | 1 | 9 | 9 | 0.40 (‐2.28 to 3.08) | ‐ | Very low |
| Hospitalisation | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Myocardial infarction | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Stroke | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cardiovascular death | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Death (all causes) | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
BP ‐ blood pressure; ECF ‐ extracellular fluid; IDWG ‐ interdialytic weigh gain; LVMI ‐ left ventricular mass index; LVEDD ‐ left ventricular end diastolic dimension; LVESD ‐ left ventricular end systolic dimension; MAP ‐ mean arterial pressure
Data were available for IDWG, predialysis BP, intradialytic BP, postdialysis BP, predialysis serum [Na+], intradialytic cramps, intradialytic hypotension, thirst, vascular resistance, and venous tone. For all of these outcomes, the effect of the intervention was directionally similar to that identified in the main comparison, and the size of the effect was also similar.
Data were not available for interdialytic BP, predialysis extracellular fluid status, postdialysis extracellular fluid status, dietary salt intake, left ventricular structure, antihypertensive medication, fatigue, pulse wave velocity, hospitalisation, myocardial infarction, stroke, CV death, death (all causes).
Additional Table 9 ("Dose effect" for intervention of lower versus higher dialysate [Na+]) examines 7 key outcomes and compares:
6. "Dose effect" for intervention of lower versus higher dialysate [Na+].
| Outcome | Overall effect | Neutral dialysate [Na+] (138 to 140 mM) | High dialysate [Na+] | P value* |
| IDWG | ‐0.35 [‐0.51, ‐0.18] | ‐0.33 [‐0.51, ‐0.14] | ‐0.42 [‐0.80, ‐0.05] | 0.65 |
| Predialysis mean MAP | ‐3.58 [‐5.46, ‐1.69] | ‐3.52 [‐5.46, ‐1.57] | ‐4.48 [‐12.07, 3.10] | 0.74 |
| Postdialysis mean MAP | ‐3.26 [‐4.82, ‐1.70] | ‐3.01 [‐4.69, ‐1.34] | ‐4.85 [‐9.10, ‐0.60] | 0.34 |
| Predialysis serum [Na+] | ‐1.69 [‐2.36, ‐1.02] | ‐1.59 [‐2.40, ‐0.78] | ‐1.92 [‐3.15, ‐0.70] | 0.65 |
| Postdialysis serum [Na+] | ‐4.74 [‐8.30, ‐1.17] | ‐1.80 [‐2.66, ‐0.94] | ‐6.75 [‐11.32, ‐2.18] | 0.02 |
| Intradialytic cramps | 1.77 [1.15, 2.73] | 1.66 [0.92, 2.98] | 1.94 [1.54, 2.44] | 0.63 |
| Intradialytic hypotension | 1.52 [1.14, 2.02] | 1.49 [1.09, 2.03] | 1.71 [0.57, 5.07] | 0.81 |
IDWG ‐ interdialytic weigh gain; MAP pressure
*P values refer to hypothesis testing of the means for "vs. neutral dialysate [Na+]" compared to "vs. high dialysate [Na+]"
the effect of the intervention in comparison to neutral dialysate [Na+] (138 to 140 mM), versus,
the corresponding effect of the intervention in comparison to high dialysate [Na+] (> 140 mM).
As can be seen in additional Table 9, the point estimates in each comparison directionally show a greater effect size with the intervention in the latter comparison compared to the former one, although formal hypothesis testing identifies that the difference is only certain for postdialysis serum [Na+].
Discussion
Summary of main results
This review has synthesised data from 12 studies randomising 310 patients, with 266 analysed after attrition due to dropout. The key findings from our meta‐analysis relevant to clinical practice are as follows.
Low dialysate [Na+] compared to higher dialysate [Na+] reduces IDWG
Low dialysate [Na+] compared to higher dialysate [Na+] probably reduces BP
Low dialysate [Na+] compared to higher dialysate [Na+] probably reduces serum [Na+] concentration
Low dialysate [Na+] compared to higher dialysate [Na+] probably increases intradialytic hypotension
Low dialysate [Na+] compared to higher dialysate [Na+] probably increases intradialytic cramp
Point estimates suggest that the effect of low dialysate [Na+] may be smaller relative to neutral dialysate [Na+], and greater in comparison to high dialysate [Na+]
There are insufficient data to inform on other patient‐centred outcomes and dietary sodium intake.
There are insufficient data to inform on cardiac structure and function.
There are no data to inform on hospitalisation and death.
Only one study reported health related quality of life, and they did not report the results by arm but for the cohort as a whole (Chambers 2002).
In the included studies, lower dialysate [Na+] led to lower IDWG and probably BP. These outcomes are both key clinical indicators in the routine care of HD patients, and a reduction in IDWG is associated with improvements in long‐term morbidity and death (Charra 2004; Cabrera 2015; Kalantar‐Zadeh 2009; Kimura 1984; London 2001; Matsuoka 1990; Movilli 2013; Ozkahya 2002; Shepherd 1987; Van Stone 1982), and reduction in BP lowers the risk of death in clinical trials (Heerspink 2009). In the studies we reviewed, it can be concluded that lower dialysate [Na+] has some effects that are very likely to be beneficial ones.
On the other hand, lower dialysate [Na+] also probably led to increased intradialytic hypotension. This is also a key clinical indicator in routine care, and an increase is associated with reduced patient satisfaction with care (Amro 2014; Caplin 2011; Schipper 2011), development of cardiac end‐organ disease (Boon 2004; Bos 2000; Burton 2009; McIntyre 2008; Selby 2007a; Sherman 2011), and increased hospitalisation, major CV events and death (Flythe 2015; Sands 2014; Shoji 2004; Stefansson 2014; Tisler 2003; McIntyre 2014). In addition, lower dialysate [Na+] probably led to a decrease in serum [Na+], albeit a modest one that remained within the normal reference range (135 to 145 mM) in the majority of studies. The only exception was Ogden 1978, where the low dialysate [Na+] was amongst the lowest in the review, and sub‐physiologic at 131 mM. Excluding all the studies with sub‐physiologic dialysate [Na+] from the analysis (Boquin 1977; Ogden 1978; Quereda 1988) did not meaningfully change the direction or size of the effect of low dialysate [Na+] on predialysis serum [Na+] (MD ‐1.49 mM, 95% CI ‐2.35 to ‐0.63]). In the studies we reviewed, it can be concluded that lower dialysate [Na+] also has some effects that are likely to be harmful ones.
The only patient‐centred outcome that we could evaluate was intradialytic cramps. In the included studies, lower dialysate [Na+] probably led to more cramps. In addition to cramps being painful and distressing to patients, cramps can also reduce the effectiveness of HD in a similar way to intradialytic hypotension, through the use of saline boluses to alleviate symptoms, or through early abandonment of HD sessions before therapeutic targets are met. As such, this often‐trivialised adverse event must be considered an important harmful effect.
In the studies we reviewed, there was a suggestion of a "dose‐effect" relating to the intervention, insofar as the point estimates of the effect size of the intervention tended to be larger when the comparator was high dialysate [Na+], and relatively more modest when the comparator was neutral dialysate [Na+] (see additional Table 9 ""Dose effect" for intervention of lower versus higher dialysate [Na+]"). When rudimentary hypothesis testing is applied, however, these effect size estimates do not appear to be significantly different between comparisons other than for postdialysis serum [Na+], where a greater difference in dialysate [Na+] between the intervention and control arms resulted in a greater difference in postdialysis serum [Na+]. Our hypothesis of a "dose‐effect" is consistent with biological models of sodium kinetics, whereby the [Na+] gradient between dialysate and blood defines mass transport, therefore determining body Na+ content, and from there the physiological effect of sodium loading on physiological function and outcomes (Dunlop 2012; Gotch 1990; Keen 2007).
Only single studies were available for the outcomes of thirst and measures of CV structure and function; only two studies were available for dietary sodium intake. No data were available on the "hard" clinical outcomes of hospitalisation and death. Data are therefore insufficient or absent to allow a robust evaluation of these outcomes.
Overall completeness and applicability of evidence
In terms of external validity, many of the studies were old, with half being from last century. This raises the potential for an era effect, since the studies report now‐obsolete HD practices such as the use of cellulosic dialysers (Boquin 1977; Henrich 1982; Ogden 1978; Quereda 1988), acetate‐buffered dialysate (Boquin 1977; Daugirdas 1985; Henrich 1982; Ogden 1978), and parallel plate dialysers (Daugirdas 1985; Ogden 1978; Quereda 1988). The age of these studies means that the studies were undertaken in an era of non‐computerised HD monitors and manual ultrafiltration systems. These characteristics all lead to a greater risk of HD‐induced haemodynamic instability during treatment in the reviewed studies compared to the contemporary setting. The analysis of older studies therefore overestimates of risks related to intradialytic hypotension in modern practice. Patient characteristics in the reviewed study also reflect an era effect, since the study populations (and likely source populations) were younger and healthier than those of today. For instance, the weighted mean age of patients included in this meta‐analysis is 57.9 years, with a weighted mean average vintage on dialysis of 51.5 months, indicating that many started dialysis with an age in their early 50's, between 10 and 20 years earlier than the typical patient these days (see Table 5). Moreover, the percentage of those with end‐stage diabetic kidney disease was 27.6%, which is much lower than that in most parts of the world (United States Renal Data System Report 2015 ‐ International Comparisons). Compared to the participants in the reviewed studies, contemporary HD patients with relatively higher co‐morbid burden are likely to have less robust compensatory mechanisms in the face of ultrafiltration, as well as less tolerance to end‐organ ischaemia.
Another important factor was that the median (interquartile range) for follow‐up in the studies was low at 3 (3, 8.5) weeks. Two studies were of only one HD session per intervention (Suckling 2013; Van Kuijk 1996), and two others were of only one week's HD per intervention (Chambers 2002; MATCH‐NA 2015; Ogden 1978). This is important since a pre‐ post‐study by Thein 2007 demonstrated that BP was still falling four months after a low dialysate [Na+] intervention was made, presumably on the basis of ongoing depletion of participants' non‐osmotic sodium stores (Titze 2008; Titze 2009). Therefore, the short‐term follow‐up of the studies we reviewed may have underestimated the treatment effect of low dialysate [Na+] on outcomes, especially those pertaining to fluid volume status and BP.
Finally, the effect of the intervention might be dependent on the baseline serum [Na+] and co‐morbidities of individual patients. No study reported enough data to perform subgroup analyses according to patient characteristics, which is an important gap in the synthesis.
A comment is warranted about the safety outcomes in this review, and the ascertainment or measurement of both intradialytic hypotension and serum [Na+] in the included studies. With respect to intradialytic hypotension, the various definitions in the literature are tabulated in additional Table 10 ("A priori definitions of intradialytic hypotension in the literature"), although the weight of evidence shows that either the Nadir90/Nadir100 definition or the KDOQI definition are the most appropriate ones with which to assess intradialytic hypotension, with the strongest associations with poorer outcomes (Flythe 2015; Sands 2014; Shoji 2004; Stefansson 2014; Tisler 2003). The various definitions of intradialytic hypotension in the included studies are tabulated in in additional Table 6 ("Definitions of intradialytic hypotension in included studies") . It should be noted that all of them include key aspects Nadir90/Nadir100 and KDOQI definitions, and as such the assessment of intradialytic hypotension in this synthesis is likely to be robust. With respect to serum [Na+], there was considerable variability between studies around how measurements were made, and a summary is tabulated in additional Table 11 ("Methods for measuring [Na+] in included studies"). Although these methods can give slightly different results (Ekbal 2016), it is likely that this feature did not reduce the internal validity the studies, nor hamper consistent interpretation of the literature ‐ in each study, the patients' responses to therapy in both the intervention and control groups were assessed in the same manner, and are therefore directly comparable. The differences between arms in each of the studies can be synthesised without issue, since these values were determined "within‐(calibrated)‐assay", not "between‐(calibrated)‐assay".
7. A priori definitions of intradialytic hypotension in the literature.
| Source | Definition |
| Nadir90 | Minimum intradialytic SBP < 90 mmHg |
| Nadir100 | Minimum intradialytic SBP < 100 mmHg |
| Fall20 | Pre‐HD SBP minus minimum intradialytic SBP ≥ 20 mmHg |
| Fall30 | Pre‐HD SBP minus minimum intradialytic SBP ≥ 30 mmHg |
| Fall20Nadir90 | Minimum intradialytic SBP < 90 mmHg and pre‐HD SBP‐minimum intradialytic SBP ≤ 20 mmHg |
| Fall30Nadir90 | Minimum intradialytic SBP < 90 mmHg and pre‐HD SBP‐minimum intradialytic SBP ≤ 30 mmHg |
| KDOQI Guideline | Pre‐HD SBP minus minimum intradialytic SBP ≥ 20 mmHg plus symptoms of cramping, headache, light‐headedness, vomiting or chest pain during dialysis |
| HEMO Study | Fall in SBP resulting in intervention of UF reduction, blood flow reduction, or saline administration. |
Adapted from Flythe 2015
HD ‐ haemodialysis; SBP ‐ systolic blood pressure; UF ‐ ultrafiltration
8. Methods for measuring [Na+] in included studies.
| Study ID | Serum [Na+] | Dialysate [Na+] |
| Akdag 2015 | Not reported | Not reported |
| Beduschi 2013 | Reflectance spectrophotometrya | Dialysate conductivitya |
| Boquin 1977 | Not reported | Not reported |
| Chambers 2002 | Not reported | Not reported |
| Daugirdas 1985 | Flame photometryb | Flame photometryb |
| Henrich 1982 | Flame photometry | Flame photometry |
| Liu 2016 | Ion selective electrode | Dialysate conductivity |
| MATCH‐NA 2015 | Ion selective electrodec | Dialysate conductivityc |
| Ogden 1978 | Flame photometry | Dialysate conductivity |
| Suckling 2013 | Ion selective electrode | Ion selective electrode |
| Van Kuijk 1996 | Ion selective electrode | Dialysate conductivity |
| Quereda 1988 | Not reported | Not reported |
aPersonal communication Pasqual Barretti 18 July 2018
bPersonal communication John T Daugirdas 17 July 2018
cPersonal communication Jula Inrig 17 July 2018
In terms of completeness, only two of the reviewed studies evaluated antihypertensive usage, and this may have clouded the size of the treatment effect with respect to the outcome of BP. It is plausible that improvement in BP control will manifest as a reduction in antihypertensive requirement, rather than as an overall lowering of BP. For instance, in the Frequent Hemodialysis Network trials of frequent in‐centre and home dialysis, there was a 6.8% and 5.4% lowering of predialysis systolic BP with intensive HD, respectively (Chertow 2010; Rocco 2011). However, a less well reported finding is that there was a 40.8% and 32.3% concurrent reduction in the number of antihypertensive's used by participants (Kotanko 2015) . The lack of reporting of antihypertensive usage may have under‐estimated the treatment effect of low dialysate [Na+] on BP. There were few studies that reported dialysate temperature, which would also influence intradialytic hypotension event rates.
Quality of the evidence
We assessed the quality of study evidence using standard risks of bias domains within the Cochrane tool together with GRADE methodology. Confidence in IDWG results was high, BP and sodium intake results was moderate, but confidence in intradialytic cramps and hypotension as low. We downgraded for the possibility of publication bias due to the very low numbers of data observations for each outcome, precluding formal testing. Data summary was also difficult due to the variable methods of reporting in the individual studies, especially the heterogeneous manner of reporting intradialytic cramps and hypotension. Some studies did not report an estimate of variance (SE or SD) and some provided data in descriptive or figure format only. For the cross‐over studies, we had to combine data from both arms, despite the general recommendation to use just the first part, since these latter data were not available. This increases the chances of a carry‐over effect, especially if there was not washout clear period to separate the two treatment periods (Boquin 1977; Chambers 2002; Henrich 1982; Ogden 1978; Quereda 1988; Van Kuijk 1996). For these studies, the intervention of higher dialysate [Na+] was similar if not identical to their usual HD prescription, and the effect of lower dialysate [Na+] on outcomes may have been under‐estimated.
A comment is warranted about the issue of dialysate [Na+] settings on HD machines. There are only a few studies that compare the accuracy of dialysate [Na+] settings on HD machines with direct measurements using other methods (Ekbal 2016; Hecking 2011; Gul 2016; Descombes 2014 ). However, these studies themselves are contradictory. In one such study, the difference between prescribed and measured dialysate [Na+] concentrations was on average very close to zero, and its distribution was not skewed (Hecking 2011). In the other studies, the measured dialysate [Na+] was either much higher than the prescribed dialysate [Na+] (Ekbal 2016), or slightly higher (Gul 2016; Descombes 2014). To complicate things further, these comparisons differed markedly according to the brand of machinery. There are many reasons for this variation in prescribed versus delivered dialysate [Na+]. The majority appears to be due to variation in operating characteristics for HD treatments: irrespective of the type of machine, facilities that use a wide variation is dialysis prescription and temperature or those that mix their concentrates themselves will test the limits of calibration for their machines, with a sacrifice in accuracy. Another source of variation is the machines themselves. Machines automatically convert the user prescription of sodium and bicarbonate concentration in dialysate to conductivity. These algorithms vary between machines, and are proprietary and not published. In addition, different machines proportion dialysate differently, some with volumetric and some with conductometric systems. The International Standard on concentrate for HD (ISO 13958) limits the concentration variation to 5% for all components, except for sodium, for which it is only 2.5%. Notwithstanding, a variation in 2% in a volumetric systems is an error in dialysate [Na+] of ± 5 mM. When well calibrated, the errors of both of these systems are low (< 1%) but careful dialysis machine maintenance is essential to preserve reliability and precision of systems (Stragier 2018). This may not always happen in "real world" settings. While these differences might seem small, there are potentially different associations between dialysate [Na+] and various outcomes according to different machines (Stragier 2018) and different regions of the world (Hecking 2017). These issues raise concerns about the applicability of the entire body of literature on prescribed dialysate [Na+] as a whole.
Of the included studies, about half measured dialysate [Na+] and delivered the intervention and control treatment according to these measurements as shown in Table 11 ("Methods for measuring [Na+] in included studies"). This will paradoxically decrease the applicability of these studies in "real‐world" settings, where without exception prescribed dialysate [Na+] is performed using the machine settings. The internal validity of included studies is not affected, however ‐ in each study, the patients' responses to therapy in both the intervention and control groups were tested in the same manner, and are therefore directly comparable. The differences between arms in each of the studies can therefore be synthesised without issue. The only caution that is required is around interpretation of the dialysate [Na+] itself ‐ although we have separated the studies in sensitivity analyses into neutral dialysate [Na+] (138 to 140 mM) versus high dialysate [Na+] (> 140 mM), this categorisation is of course according to how the study investigators assessed dialysate [Na+], and may not translate to machine settings in "real world" settings.
Potential biases in the review process
Potential biases in this review relate to the data availability in the individual studies. First, there was heterogeneity in treatment interventions and comparisons; due to the small number of data observations, robust statistical estimates of heterogeneity could not be estimated. Second, we could not assess for potential reporting bias due to the small number of studies in the review. Third, studies were frequently at high risk of bias, but poorer quality studies could not be excluded from sensitivity analyses due to the limited number of data observations. Fourth, the treatment endpoints were principally surrogate markers of health (IDWG, BP, serum [Na+]) and the effects of low dialysate [Na+] on cardiac structure and function and "hard" clinical outcomes remains uncertain. Fifth, most follow‐up was short, and the longer term effects of low dialysate [Na+] on outcomes remains uncertain. Sixth, adverse event reporting in the available studies was infrequent and incomplete. Finally, there is dearth of reporting on patient‐centred outcomes such as quality of life and treatment satisfaction. Notwithstanding, we have attempted to avoid any bias in our review process. Wherever there was any uncertainty regarding methodology of RCT conduct we contacted the study authors directly to seek further clarification before deciding on whether to a study should be included or not.
Agreements and disagreements with other studies or reviews
A narrative review of 23 studies evaluating high versus low dialysate [Na+] has been published (Basile 2015). In contrast to this review, it included observational studies and non‐randomised interventional studies. Overall, their conclusions were similar to ours regarding high heterogeneity between studies and a lack of data about antihypertensive use and "hard" clinical outcomes.
Many narrative reviews or editorials have been written on the subject of low versus high dialysate [Na+] over the years (Hecking 2015; Marshall 2012; Weiner 2014). Most are opinion‐based, and do not include meta‐analysis methodology. Nonetheless most draw the conclusion that the intervention is a promising one, and that definitive clinical trial‐based evidence is required to inform practice. Of note, some opinion leaders advocate utilising a universally individualised dialysate [Na+], calculated around patients' current or recent predialysis serum [Na+] measurements (Lomonte 2011; Penne 2011; Santos 2008). However, the practicality or necessity of such a specific intervention is uncertain, and it is likely that considerations other than just serum [Na+] should determine optimal sodium balance for patients, and therefore the required dialysis [Na+].
Authors' conclusions
Implications for practice.
On the basis of this review, lower dialysate [Na+] reduces IDWG and BP, but probably decreases serum [Na+] and increases intradialytic adverse events. The combined effect of these factors on overall patient experience and clinical outcome is unknown. The implications of this treatment effect need careful consideration. On the one hand it may be beneficial, as these changes may represent a reduction in total body sodium content and thus an improvement in CV structure and function, and reduction in CV morbidity and death. However, the alternative consideration is that a lowering of serum [Na+] may represent a signal of harm, and that an increase in intradialytic hypotension is a cause of decreasing effectiveness of HD, cardiac damage, and ultimately increasing CV morbidity and death. Moreover, there are few data to provide insights into patient‐centred outcomes other than cramps and intradialytic hypotension, and what low‐quality evidence exists is in favour of higher rather than lower dialysate [Na+].
A question might be asked about a third approach to dialysate [Na+]: "In practice, why not avoid high or low dialysate [Na+] altogether, and simply match dialysate [Na+] to serum [Na+] to avoid any disruption on HD?" (Keen 2007; Raimann 2018; Santos 2010). This so‐called "isonatraemic HD" requires individualisation of dialysate [Na+] so that it is close to the patient's predialysis serum [Na+], and is based upon the sodium "set‐point" paradigm: people maintain themselves in optimal sodium balance above and below which health is compromised. Indeed, serum [Na+] is observed to be very stable across time in both healthy and HD patients (Peixoto 2010; Zhang 2014). There are two issue with this approach, one philosophical, and the other logistic. The philosophical issue arises from assuming that an individual's observed sodium balance is optimal. In fact, sodium intake in modern society is largely dictated by conditions other than metabolic need, and determined largely by palatability and custom. Moreover, an individual's sodium "set point" can be easily "re‐set" (Braunwald 1965; Hollenberg 1972; Hollenberg 1980; Strauss 1958): normal people who are established on low sodium diets will promptly excrete any administered sodium. For this reason, attempts to define a "healthy" sodium balance from an observed state of balance is meaningless. In an analogous argument, isocaloric nutrition is not automatically ideal for HD patients, despite a stable weight over time, unless they are in nutritionally optimal to start with. The second objection to isonatraemic HD is logistic. As described above, there is wide variation in the accuracy by which HD machines measure and manage sodium, and there are at present no reliable technical means which would automatically ensure isonatremic HD.
No strong recommendation for practice can be made on the basis of these data. These preliminary findings represent potential mechanisms for both benefit and harm from lower dialysate [Na+], but the net effect of these physiological processes on patient experiences and outcomes at a population level remains unknown. Until the results of a definitive study are available, a decision on what dialysate [Na+] to use will have to be made between every patient and doctor, without definite knowledge of whether lower or higher dialysate [Na+] is better for the average patient. If the patient has a high BP, fluid overload, or high IDWG, then it is probably helpful to consider a lower dialysate [Na+] ‐ the evidence from this review suggests that this strategy will improve those problems. If these problems are not an issue, then perhaps it is better to place priority on avoiding intradialytic hypotension, and advise a higher dialysate [Na+] ‐ the evidence from this review suggests that this strategy will reduce the likelihood of that problem. Even when the results of a definitive study are known, this individualised approach should probably still continue, except that at that time will know for sure whether the starting point for shared decision making should be a lower dialysate [Na+] at a population )i.e. dialysis unit) level, or a higher one.
Implications for research.
Further research is needed to define longer‐term outcomes with lower dialysate [Na+], using modern HD machinery and customary practices, in participants who are reflective of modern patients, examining both mechanistic and clinical outcomes including:
cardiac structure and function as assessed by optimal methods such as magnetic resonance imaging,
fluid status as assessed by optimal methods such as bioimpedence analysis,
hospitalisation,
CV morbidity and death,
patient symptoms scores, satisfaction with care, and quality of life.
Sufficiently powered and well‐designed clinical studies are necessary to provide definitive results. There is an ongoing medium‐sized but short‐term clinical study exploring the effect of low dialysate [Na+] on BP, IDWG and intradialytic hypotension (NCT00724633), a medium‐sized and medium‐term study exploring the effect of low dialysate [Na+] on these outcomes as well as cardiac structure and function and patient‐centred outcomes SoLID 2013, and a large pragmatic study of dialysate [Na+] of 137mM versus 140 mM on clinical outcomes (NCT02823821). A large gap in research is the absence of studies to provide definitive results around the net effect of low dialysate [Na+] on CV morbidity and death, and this gap must be filled before clear recommendations can be made for clinical practice. It would be important for this research to include appropriate predefined subgroup analysis by predialysis serum [Na+] and comorbidity, and economic analyses since this is one of few interventions that may be able to provide benefit at no cost.
Acknowledgements
We wish to thank the referees for their comments and feedback during the preparation of this review. The authors would also like to thank Pasqual Barretti, Irvin Cohen, John Daugirdas, Bill Henrich, Nick Hoenich, Jula Inrig, Nathan Levin, and Rebecca Suckling for their time and effort in providing additional data and information on their respective studies.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention Mean difference; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention Mean difference; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Interdialytic weight gain | 10 | 352 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.51, ‐0.18] |
| 2 Predialysis BP | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Mean arterial pressure | 4 | 156 | Mean Difference (IV, Random, 95% CI) | ‐3.58 [‐5.46, ‐1.69] |
| 2.2 Systolic BP | 3 | 83 | Mean Difference (IV, Random, 95% CI) | ‐7.56 [‐15.92, 0.80] |
| 2.3 Diastolic BP | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐3.13 [‐11.79, 5.54] |
| 3 Intradialytic BP | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Mean arterial pressure | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐18.52, 10.52] |
| 3.2 Systolic BP | 2 | 34 | Mean Difference (IV, Random, 95% CI) | ‐3.99 [‐17.96, 9.99] |
| 3.3 Diastolic BP | 2 | 34 | Mean Difference (IV, Random, 95% CI) | 1.33 [‐6.29, 8.95] |
| 4 Postdialysis BP | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Mean arterial pressure | 4 | 150 | Mean Difference (IV, Random, 95% CI) | ‐3.26 [‐4.82, ‐1.70] |
| 4.2 Systolic BP | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐31.86, 21.86] |
| 4.3 Diastolic BP | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐13.98, 13.98] |
| 5 Interdialytic BP | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Mean arterial pressure | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Systolic BP | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐5.42, 5.08] |
| 5.3 Diastolic BP | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐4.84, 0.84] |
| 6 Serum [Na+] | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Predialysis | 7 | 258 | Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐2.36, ‐1.02] |
| 6.2 Intradialytic | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐4.37 [‐6.24, ‐2.50] |
| 6.3 Postdialysis | 3 | 99 | Mean Difference (IV, Random, 95% CI) | ‐4.74 [‐8.30, ‐1.17] |
| 7 HD sessions complicated by intradialytic cramps | 6 | 12186 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.15, 2.73] |
| 8 HD sessions complicated by intradialytic hypotension | 9 | 12681 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.17, 2.07] |
| 9 Postdialysis extracellular fluid status | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Dietary salt intake | 2 | 95 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.48, 0.06] |
| 11 Left ventricular structure | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Mass index [g/m2] | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐8.0 [‐17.11, 1.11] |
| 11.2 End‐diastolic dimension [mm] | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐3.18, 3.98] |
| 11.3 End‐systolic dimension [mm] | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.59, 3.39] |
| 12 Antihypertensive medication | 2 | 103 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.07, ‐0.28] |
| 13 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14 Thirst | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15 Pulse wave velocity | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16 Arterial vascular resistance [mmHg/mL/I00mL/s] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17 Venous tone [mmHg/mL/I00mL] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
1.7. Analysis.
Comparison 1 Low dialysate [Na+] (< 138 mM) versus neutral dialysate [Na+] (138 to 140 mM) or high dialysate [Na+] (> 140 mM), Outcome 7 HD sessions complicated by intradialytic cramps.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akdag 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | HD regimen
Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation clearly stated in published article, along with mechanism of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clearly stated to be double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 dropouts in the intervention group due to intradialytic hypotension and cramps, which are likely to be related to the intervention. The study is not analysed as intention to treat, and data from those who dropped out are excluded from analysis. There is a high risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of reporting bias |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Beduschi 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | HD regimen
Treatment group
Control group
Duration
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation clearly stated in published article, along with mechanism of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients not blinded, although outcomes are unlikely to be biased by their nature |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14/52 (27%) participants were withdrawn because of acute infections during the study period, 9 from the treatment group and 5 from the control group. The study is not analysed as intention to treat, and data from those who dropped out are omitted from analysis. Although the reasons for dropout do not appear to be related to the treatment, there is still a possibility of attrition bias. In addition, this large number of dropouts may have affected balance of patient characteristics by arm. The groups appeared balanced on measured confounders in Table 1, but an effect of these dropouts on unmeasured confounders is unknown |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of reporting bias |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Boquin 1977.
| Methods |
|
|
| Participants |
|
|
| Interventions | HD regimen
Treatment group
Control group
Duration
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation clearly stated in published article, but not the mechanism of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single (patient) blinding clearly stated in published article |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14/51 patients were removed from the study, 4 because of hospitalisation not otherwise specified, 3 because of transplantation, and 7 because they could not clinically tolerate the treatment. The study is not analysed as intention to treat, and data from those who dropped out are omitted from analysis. There is a high risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of reporting bias |
| Other bias | High risk |
|
Chambers 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | HD regimen
Treatment group 1 (called B in abstract)
Treatment group 2 (called C in abstract)
Treatment group 3 (called D in abstract)
Control group
Duration
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation stated in published article, but not mechanism of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study appears to be free of attrition bias |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of reporting bias |
| Other bias | High risk |
|
Daugirdas 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | HD regimen
Treatment group 1
Treatment group 2
Treatment group 3
Duration
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation clearly stated in published article, but not mechanism of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinding clearly stated in published article and confirmed by the lead author |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 patient dropouts "were judged to be treatment‐unrelated, and accordingly, their data were excluded from analysis". The study is not analysed as intention to treat, and data from those who dropped out is omitted from analysis. Although the reasons for dropout do not appear to be related to the treatment, there is still risk of attrition bias. In addition, this large number of dropouts may have affected balance of patient characteristics by arm. The groups appeared balanced on measured confounders in table 1, but an effect of these dropouts on unmeasured confounders is unknown |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of reporting bias |
| Other bias | High risk | Obsolete dialysis practice patterns (acetate buffered dialysate, parallel plate dialysers), with a patient sample that is not reflective of modern populations. Risk of poor external validity (indirectness) |
Henrich 1982.
| Methods |
|
|
| Participants |
|
|
| Interventions | HD regimen
Treatment group
Control group
Duration
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was not reported in published article, but confirmed by the lead author upon direct contact. The mechanism of randomisation is unknown / unrecalled |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinding clearly stated in published article and confirmed by the lead author |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study appears to be free of attrition bias |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of reporting bias |
| Other bias | High risk |
|
Liu 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | HD regimen
Treatment group
Control group
Duration
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation clearly stated in published article, along with mechanism of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding included outcomes assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7/64 patients were removed from the study, 5 because of death, 2 because of transplantation, and 1 because of loss to follow‐up. These serious adverse events are not otherwise reported. The study is not analysed as intention to treat, and data from those who dropped out is omitted from analysis. The reasons for dropout might be related to the treatment, and there is a risk of attrition bias. In addition, dropouts may have affected balance of patient characteristics by arm, although they are only a small number |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of reporting bias |
| Other bias | High risk | PWV and echocardiography performed by a single, albeit blinded assessor. There is potential for ascertainment bias, and also potential for drift with respect to the echocardiography, which was assessed 12 months apart |
MATCH‐NA 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | HD regimen
Treatment group 1
Treatment group 2
Duration
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation clearly stated in published article, but not mechanism of randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation concealment clearly stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single (patient) blinding clearly stated in published article |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All available data included including those from dropouts, analysed as intention to treat |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of reporting bias |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ogden 1978.
| Methods |
|
|
| Participants |
|
|
| Interventions | HD regimen
Treatment group
Control group
Duration
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation has been confirmed by the authors, although the mechanism of randomisation is unclear |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinding (participants and investigators) clearly stated in the published article |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study appears to be free of attrition bias |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of reporting bias |
| Other bias | High risk |
|
Quereda 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | HD regimen
Treatment (performed in 8 phases)
Duration
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation clearly stated in published article, but not mechanism of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study appears to be free of attrition bias |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of reporting bias |
| Other bias | High risk |
|
Suckling 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | HD regimen
Treatment group
Control group
Duration
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation stated in published article, but not mechanism of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blinding (participant) clearly stated in the published article |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study appears to be free of attrition bias |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of reporting bias |
| Other bias | Unclear risk | Is a study of treatment over a single dialysis session, and the effect size attributable to the treatment is likely to be attenuated ‐ risk of ascertainment bias |
Van Kuijk 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | HD regimen
Treatment group
Control group
Duration
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation clearly stated in published article, but not mechanism of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study appears to be free of attrition bias |
| Selective reporting (reporting bias) | Low risk | The study appears to be free of reporting bias |
| Other bias | Unclear risk |
|
BP ‐ blood pressure; CrCl ‐ creatinine clearance; CU ‐ cuprammonium cellulose; DBP ‐ diastolic BP; EDD ‐ end‐diastolic diameter; ESD ‐ end‐systolic diameter; Hb ‐ haemoglobin; HCT ‐ haematocrit; HD ‐ haemodialysis; HRQoL ‐ health‐related quality of life; IDWG ‐ interdialytic weight gain; IQR ‐ interquartile range; Kt/V ‐ dialyser clearance adequacy; LVMI ‐ left ventricular mass index; M/F ‐ male female; MAP ‐ mean arterial pressure; MI ‐ myocardial infarction; NYHA ‐ New York Heart Association; PAN ‐ polyacrylonitrile membrane; PWT ‐ posterior wall thickness; PWV ‐ pulse wave velocity; QB ‐ blood (pump) flow rate; QD ‐ dialysate flow rate; RCT ‐ randomised controlled trial; SBP ‐ systolic BP; SD ‐ standard deviation; SEM ‐ standard error of the mean; SQRT ‐ square root; SWT ‐ septal wall thickness; UF ‐ ultrafiltration; URR ‐ urea reduction ratio; UTI ‐ urinary tract infection; VAS ‐ visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acchiardo 1975 | Wrong intervention: study of what solution to infuse for treatment of cramps |
| AIMS 2011 | Wrong population: HDF rather than HD |
| Bachtiar 2005 | Wrong intervention: tested low dialysate [Na+] versus BIS‐guided target weight adjustment on BP. The dialysate [Na+] in the latter group was not specified. The authors were emailed for clarification but never replied |
| Barre 1988 | Wrong intervention: high dialysate [Na+] versus higher dialysate [Na+] |
| CARNIDIAL 2012 | Wrong intervention: carnitine supplementation |
| Coli 2000 | Wrong intervention: dialysate [Na+] with a co‐intervention of UF profiling |
| De Nicola 2000 | Wrong intervention: neutral dialysate [Na+] versus high dialysate [Na+] |
| de Vries 1990 | Wrong intervention: dialysate [Na+] with a co‐intervention of UF profiling |
| Ebrahimi 2017 | Wrong intervention: dialysate [Na+] not < 138 mM |
| Ekart 2015 | Wrong population: HDF rather than HD |
| Enia 1998 | Wrong population: CAPD patients |
| Ficociello 2012 | Wrong intervention: specific alignment of dialysate [Na+] to serum [Na+] |
| HEMATOL 2013 | Wrong population: critically ill rather than maintenance HD patients |
| Henrich 1983 | Wrong intervention: high sodium bicarbonate and acetate HD |
| Lambie 2005 | Wrong intervention: on‐line conductivity monitoring; did not compare low dialysate [Na+] to medium or high dialysate [Na+] |
| Levin 1996 | Wrong intervention: did not test low dialysate [Na+] |
| Macon 1995 | Wrong intervention: slow HD versus dialysate [Na+] modelling |
| Mahiout 1987 | Wrong intervention: high dialysate [Na+] versus higher dialysate [Na+] |
| Meira 2010 | Wrong intervention: high dialysate [Na+] versus profiled dialysate [Na+] with an average dialysate [Na+] that was also high |
| Moret 2006 | Wrong intervention: neutral dialysate [Na+] versus high dialysate [Na+] |
| NCT01015313 | This study has been withdrawn prior to enrolment |
| NCT01168947 | Wrong intervention: saline versus 5% dextrose as priming and rinsing fluids |
| NCT01766882 | Wrong intervention: goal weight challenging versus dietary sodium restriction in the setting of a fixed dialysate [Na+] of 137 mM |
| Oliver 2001 | Wrong population: effect of low dialysate [Na+] on patients with high or low baseline IDWG |
| Oliver 2001a | Wrong intervention: dialysate [Na+] with a co‐intervention of UF profiling |
| Robberechts 2013 | Wrong intervention: the authors did not define low dialysate [Na+], although the study meets the other criteria to be included in the review. The authors were emailed for clarification but never replied |
| Sandy 1996 | Wrong population: critically ill rather than maintenance HD patients |
| Sang 1997 | Wrong intervention: high dialysate [Na+] to higher dialysate [Na+] |
| Selby 2007 | Wrong intervention: low dialysate [Na+] to lower dialysate [Na+] |
BP ‐ blood pressure; CAPD ‐ continuous ambulatory peritoneal dialysis; HDF ‐ haemodiafiltration; HD ‐ haemodialysis; IDWG ‐ interdialytic weigh gain; UF ‐ ultrafiltration
Characteristics of studies awaiting assessment [ordered by study ID]
ISRCTN71215609.
| Methods | RCT The two groups will both undergo a 1‐week period of initial data collection while being maintained on routine dialysis. |
| Participants | HD patients |
| Interventions | Dialysis will be performed using a default dialysate [Na+] of 140 mmol/L. The first group will then continue to dialyse against a standard dialysate [Na+] of 140 mmol/l, while the second group undergoes a period of 1 month of sequential reduction of dialysate [Na+] according to online conductivity monitoring, aiming for isonatric dialysis (i.e. Ionic mass balance of 0‐100 mmol of sodium). This will then be maintained for a period of one month. After that time, there will be a crossover period of adjustment, during which the first group will have their dialysate [Na+] tailored to their requirements, and the second group will revert in a gradual manner to a dialysate [Na+] of 140 mmol/L. Again the two groups will be maintained for a further month, before both groups revert to a standard dialysate [Na+] at the end of the study. |
| Outcomes | Primary outcome measure Neutral sodium balance as assessed by ionic mass balance reduction in IDWG. Secondary outcome measures Pre‐ and postdialysis BP, number of antihypertensive agents stability on dialysis, thirst score. |
| Notes | Primary contact Dr Chris W McIntyre Derby Hospitals NHS Foundation Trust Department of Nephrology Derby City General Hospital Uttoxeter Road Derby DE22 3NE United Kingdom |
HD ‐ haemodialysis; RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT00724633.
| Trial name or title | Effect of lowering the dialysate sodium on blood pressure in haemodialysis patients: a randomized controlled trial |
| Methods | Randomised, parallel‐assignment, double‐blind clinical trial |
| Participants | Canada Sample size 150 patients Eligibility criteria: aged 18 years and older; on 3 times weekly HD of at least 3 months; elevated average ambulatory BP; current dialysate [Na+] prescription 140 mEq/L; average predialysis serum [Na+] < 140 mEq/L Exclusion criteria: frequent intradialytic hypotension; estimated life expectancy < 1 year; non‐adherence to dialysis prescription pregnancy; inability or unwillingness to complete study measures |
| Interventions | HD regimen: not reported Control: dialysate [Na+] 140 mM Intervention 1: dialysate [Na+] = patient predialysis serum [Na+] Intervention 2: dialysate [Na+] < patient predialysis serum [Na+] Duration: 3 months |
| Outcomes | Primary outcome is ambulatory BP Secondary outcomes Thirst HRQoL scores by KDQoL IDWG Intradialytic hypotension EFV Sodium ionic mass |
| Starting date | November 2011 |
| Contact information | rita.suri@lhsc.on.ca |
| Notes | The recruitment status of this study is unknown because the information has not been verified recently. Was due for completion November 2014. |
NCT02145260.
| Trial name or title | Trial of dialysate sodium in chronic hospitalized haemodialysis patients |
| Methods | Single‐centre, prospective, randomised, double‐blind, controlled parallel assignment 4‐year clinical study |
| Participants | USA Sample size 200 Eligibility criteria: chronic HD (> 90 days), age ≥ 18 years, informed consent, first admission during study period. Exclusion criteria: use of pressors, predialysis serum sodium ≤ 128 mmol/L or > 145 mmol/L, predialysis SBP >180 mmHg, intensive care stay earlier in admission, expected length of stay < 24 hours (e.g. admission for HD access procedure), acute coronary syndrome within seven days, acute stroke, institutionalised individuals, pregnancy |
| Interventions | HD regimen: not reported Control: dialysate [Na+] 138 mM Intervention: dialysate [Na+] 142 mM Duration: 6 treatments |
| Outcomes | Primary outcome is magnitude of intradialytic decline in SBP Secondary outcome is change in predialysis high‐sensitivity troponin I |
| Starting date | July 2014 |
| Contact information | fmccausland@partners.org |
| Notes | Due for completion July 2018 |
RESOLVE 2016.
| Trial name or title | Randomised Evaluation of SOdium dialysate Levels on Vascular Events (RESOLVE) |
| Methods | Pragmatic, cluster‐randomized, prospective, open label, controlled parallel arm 7 years clinical study |
| Participants | Australia, New Zealand, UK, India, China, Canada, Germany Sample size 51,520 Inclusion criteria
Exclusion criteria
|
| Interventions | Participating dialysis sites will be randomised to a default dialysate [Na+] of 137 mM versus 140 mM |
| Outcomes | Time to first occurrence of an event in the primary composite outcome, Time frame: through to study completion (estimated to occur after an average of 5 years follow up) Primary outcome is a composite of major CV events (hospitalised acute myocardial infarction, hospitalised stroke) and all‐cause death. Study completion is endpoint driven, but is expected to be when the average follow up is around 5 years |
| Starting date | June 2016 |
| Contact information | mjardine@georgeinstitute.org.au |
| Notes | Due for completion 2023 |
SoLID 2013.
| Trial name or title | A randomised, controlled trial of low sodium dialysate versus conventional sodium dialysate to reduce left ventricular mass index in patients receiving home haemodialysis: The Sodium Lowering In Dialysate (SOLID) Trial |
| Methods | Multicentre, prospective, randomised (permuted randomly sized blocks), single‐blind (outcomes assessor), controlled parallel assignment 3‐year clinical study |
| Participants | NZ Sample size 96 patients Eligibility criteria: incident or prevalent patients treated with maintenance home or self‐care HD; aged 18 years or older; suitable for both low and standard dialysate [Na+] in the view of their treating physician; predialysis plasma [Na+] ≥ 135 mM; willing to participate and able to provide consent Exclusion criteria will include HD treatments at a frequency greater than 3.5 times per week; treatment with maintenance HDF; life expectancy of less than 12 months; scheduled for live donor kidney trans‐plantation within 12 months of entry to the study; considered by the treating nephrologist to have concomitant illnesses or conditions that limit or contraindicate study procedures and follow‐up (e.g. frequent intradialytic hypotension requiring fluid resuscitation); considered by the treating nephrologist to have a high chance of non‐adherence to study treatments and non‐attendance for procedures and follow up; current enrolment in clinical studies involving antihypertensive medications, change sin HD operating parameters, or any other intervention that is likely to confound the outcome of the study; currently using sodium profiling during HD treatments; documented infiltrative cardiomyopathies |
| Interventions | HD regimen: no limits on session length or dialyser flux, bicarbonate buffer, not to increase HD session frequency to greater than 3.5 times a week Intervention: dialysate [Na+] 135 mM Control: dialysate [Na+] 140 mM Duration: 12 months |
| Outcomes | Primary outcome is left ventricular mass index, as measured by cardiac magnetic resonance imaging Secondary outcomes LV volumes, geometry, and regional wall motion score IDWG Intradialytic hypotension NT‐pro‐BNP levels Troponin‐T levels ECF volume by BIA Intra‐ and interdialytic ambulatory BP Number and dose of antihypertensives PWV and PWA HRQoL by EQ5D and KDQOL Xerostomia and thirst inventory scores Predialysis plasma γNa Dietary sodium intake via 3 day food diary Safety and tolerability |
| Starting date | March 2012, completed recruitment |
| Contact information | joanna.dunlop@middlemore.co.nz; mrmmarsh@inspire.net.nz |
| Notes | Full results will be available in late 2017 |
ECF ‐ extracellular fluid; HD ‐ haemodialysis; HRQoL ‐ health‐related quality of life; IDWG ‐ interdialytic weigh gain; LV ‐ left ventricular; PWA ‐ pulse wave amplitude; PWV ‐ pulse wave velocity; SBP ‐ systolic blood pressure
Differences between protocol and review
In the review; due to the small number of eligible studies, the profiled and non‐profiled dialysate sodium interventions were combined and not analysed separately, as was planned in the protocol.
In the review; low (< 138 mM) dialysate [Na+] was compared with the pooled results from both neutral (138‐140mM) and high (>140mM) dialysate [Na+] interventions. This comparison was not pre‐specified in the protocol.
Contributions of authors
Draft the protocol: JD, MM
Study selection: JD, MM
Extract data from studies: MM, JD, AV
Enter data into RevMan: MM, JD
Carry out the analysis: JD, MM, AV
Interpret the analysis: JD, MM, AV
Draft the final review: JD, MM
Disagreement resolution: AV
Update the review: MM
Sources of support
Internal sources
No sources of support supplied
External sources
-
Royal Australasian College of Physicians, New Zealand.
Jacquot Research Establishment Award (MM)
Jacquot Research Entry Award (JD)
-
Health Research Council of New Zealand, New Zealand.
Project grant 11/583, 13/442
Declarations of interest
JD: None known
AV: None known
MM: is currently employed by Baxter Healthcare as the Director of Medical Affairs Asia‐Pacific, Renal Therapeutic Area
New
References
References to studies included in this review
Akdag 2015 {published data only}
- Akdag S, Akyol A, Cakmak HA, Tosu AR, Asker M, Yaman M, et al. The effect of low‐sodium dialysate on ambulatory blood pressure measurement parameters in patients undergoing hemodialysis. Therapeutics & Clinical Risk Management 2015;11:1829‐35. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beduschi 2013 {published and unpublished data}
- Beduschi GC, Telini LS, Caramori JC, Martin LC, Barretti P. Effect of dialysate sodium reduction on body water volume, blood pressure, and inflammatory markers in hemodialysis patients‐‐a prospective randomized controlled study. Renal Failure 2013;35(5):742‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boquin 1977 {published and unpublished data}
- Boquin E, Parnell S, Grondin G, Wollard C, Leonard D, Michaels R, et al. Crossover study of the effects of different dialysate sodium concentrations in large surface area, short‐term dialysis. Proceedings of the Clinical Dialysis & Transplant Forum 1977;7:48‐52. [MEDLINE: ] [PubMed] [Google Scholar]
Chambers 2002 {published and unpublished data}
- Chambers G, Hoenich NA. Is stepwise profiling of ultrafiltration and sodium beneficial to the elderly patient receiving haemodialysis therapy? [abstract]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):580a. [CENTRAL: CN‐00444738] [Google Scholar]
Daugirdas 1985 {published data only (unpublished sought but not used)}
- Daugirdas JT, Al‐Kudsi RR, Ing TS, Norusis MJ. A double‐blind evaluation of sodium gradient hemodialysis. American Journal of Nephrology 1985;5(3):163‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Henrich 1982 {published data only (unpublished sought but not used)}
- Henrich WL, Woodard TD, McPhaul JJ Jr. The chronic efficacy and safety of high sodium dialysate: double‐blind, crossover study. American Journal of Kidney Diseases 1982;2(3):349‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liu 2016 {published data only}
- Liu J, Sun F, Ma LJ, Shen Y, Mei X, Zhou YL. Increasing dialysis sodium removal on arterial stiffness and left ventricular hypertrophy in hemodialysis patients. Journal of Renal Nutrition 2016;26(1):38‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MATCH‐NA 2015 {published and unpublished data}
- Inrig JK, Molina C, D'Silva K, Kim C, Buren P, Allen JD, et al. Effect of low versus high dialysate sodium concentration on blood pressure and endothelial‐derived vasoregulators during hemodialysis: a randomized crossover study. American Journal of Kidney Diseases 2015;65(3):464‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ogden 1978 {published and unpublished data}
- Ogden DA. A double blind crossover comparison of high and low sodium dialysis. Proceedings of the Clinical Dialysis & Transplant Forum 1978;8:157‐65. [MEDLINE: ] [PubMed] [Google Scholar]
- Ogden DA, Cohen IM. High and low sodium hemodialysis ‐ a twelve week double blind crossover comparison [abstract]. Kidney International 1979;16(6):895. [Google Scholar]
Quereda 1988 {published data only (unpublished sought but not used)}
- Quereda C, Orofino L, Marcen R, Lamas S, Sabater J, Ortuno J. Contribution of membrane and dialysate to the incidence of hemodialysis (HD)‐induced symptomatic hypotension (SH) [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:167.
- Quereda C, Orofino L, Marcen R, Sabater J, Matesanz R, Ortuno J. Influence of dialysate and membrane biocompatibility on hemodynamic stability in hemodialysis. International Journal of Artificial Organs 1988;11(4):259‐64. [MEDLINE: ] [PubMed] [Google Scholar]
- Quereda C, Orofino L, Marcen R, Sabater J, Ortuno J. Combined use of low temperature and high sodium dialysate improves symptomatic hypotension (SH) in hemodialysis (HD) [abstract]. 24th Annual Congress. EDTA‐ERA; 1987 Oct 25‐29; West Berlin, Germany. 1987:132.
Suckling 2013 {published and unpublished data}
- Suckling RJ, Swift PA, He FJ, Markandu ND, Macgregor GA. Altering plasma sodium concentration rapidly changes blood pressure during haemodialysis. Nephrology Dialysis Transplantation 2013;28(8):2181‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Kuijk 1996 {published data only}
- Kuijk WH, Wirtz JJ, Grave W, Heer F, Menheere PP, Hooff JP, et al. Vascular reactivity during combined ultrafiltration‐haemodialysis: influence of dialysate sodium. Nephrology Dialysis Transplantation 1996;11(2):323‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acchiardo 1975 {published data only}
- Acchiardo SR, Skoutaski VA, Hatch FE. Management of muscle cramps in hemodialysis patients. Controlled prospective study. Proceedings of the Clinical Dialysis & Transplant Forum 1975;5:6‐8. [MEDLINE: ] [PubMed] [Google Scholar]
AIMS 2011 {published data only}
- Locatelli F, Stefoni S, Petitclerc T, Coli L. HFR‐Acquilibrium International Multicentric Study (AIMS): focus on intradialytic cardiovascular stability [abstract no: LB‐PO3148]. Journal of the American Society of Nephrology 2011;22(Abstracts):3B. [Google Scholar]
- Locatelli F, Stefoni S, Petitclerc T, Coli L, Filippo S, Andrulli S, et al. Effect of a plasma sodium biofeedback system applied to HFR on the intradialytic cardiovascular stability. Results from a randomized controlled study. Nephrology Dialysis Transplantation 2012;27(10):3935‐42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bachtiar 2005 {published data only}
- Bachtiar NS, Khairuddin K, Hoenich N, Hussain R, Tan SY. Management of hypertension in patients on chronic haemodialysis: modulation of dialysate sodium compared to adjustment of dry weight as determined by bioimpedance analysis [abstract no: W‐PO40028]. Nephrology 2005;10(Suppl):A286‐7. [Google Scholar]
Barre 1988 {published data only}
- Barre PE, Brunelle G, Gascon‐Barr M. A randomized double blind trial of dialysate sodiums of 145 mEq/L, 150 mEq/L, and 155 mEq/L. ASAIO Transactions 1988;34(3):338‐41. [MEDLINE: ] [PubMed] [Google Scholar]
CARNIDIAL 2012 {published data only}
- Mercadal L, Coudert M, Vassault A, Pieroni L, Debure A, Ouziala M, et al. L‐carnitine treatment in incident hemodialysis patients: the multicenter, randomized, double‐blinded, placebo‐controlled CARNIDIAL trial. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(11):1836‐42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coli 2000 {published data only}
- Coli L, Dalmastri V, Pascalis A, Isola E, Magelli C, Magnani G, et al. Modulating dialysate sodium and ultrafiltration: various profiles and their clinical effectiveness [abstract]. Nephrology Dialysis Transplantation 2000;15(9):219. [CENTRAL: CN‐00460570] [Google Scholar]
De Nicola 2000 {published data only (unpublished sought but not used)}
- Nicola L, Bellizzi V, Minutolo R, Cioffi M, Giannattasio P, Terracciano V, et al. Effect of dialysate sodium concentration on interdialytic increase of potassium. Journal of the American Society of Nephrology 2000;11(12):2337‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Vries 1990 {published data only}
- Vries PM, Kouw PM, Olthof CG, Solf A, Schuenemann B, Oe LP, et al. The influence of dialysate sodium and variable ultrafiltration on fluid balance during hemodialysis. ASAIO Transactions 1990;36(4):821‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ebrahimi 2017 {published data only}
- Ebrahimi H, Safavi M, Saeidi MH, Emamian MH. Effects of sodium concentration and dialysate temperature changes on blood pressure in hemodialysis patients: a randomized, triple‐blind crossover clinical trial. Therapeutic Apheresis & Dialysis 2017;21(2):117‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ekart 2015 {published data only}
- Ekart R, Volgemut Z, Bevc S, Stropnik‐Galuf T, Hojs R. Effect of different dialysate sodium concentration on blood pressure in hemodialysis patients: a randomized study [abstract no: PP.11.03]. Journal of Hypertension 2015;33(Suppl 1):e224‐5. [EMBASE: 71935304] [Google Scholar]
Enia 1998 {published data only}
- Enia G, Panuccio V, Parlongo S, Crucitti S, Biondo A, Pustorino D, et al. Hypotensive effect of ultra‐low sodium dialysate in CAPD patients: a double‐blind, randomized, crossover study [abstract no: T271]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):282A. [CENTRAL: CN‐00445236] [Google Scholar]
Ficociello 2012 {published data only}
- Ficociello LH, Usvyat L, Ginsberg N, Moore S, Pacelli L, Taylor P, et al. Alignment of dialysate sodium to serum sodium in dialysis patients with serum sodium <137 mmol/L [abstract]. American Journal of Kidney Diseases 2012;59(4):A35. [EMBASE: 71074694] [Google Scholar]
HEMATOL 2013 {published data only}
- Du Cheyron D, Terzi N, Seguin A, Valette X, Prevost F, Ramakers M, et al. Use of online blood volume and blood temperature monitoring during haemodialysis in critically ill patients with acute kidney injury: a single‐centre randomized controlled trial. Nephrology Dialysis Transplantation 2013;28(2):430‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Henrich 1983 {published data only}
- Henrich WL, Woodard TD, Meyer BD, Chappell TR, Rubin LJ. High sodium bicarbonate and acetate hemodialysis: double‐blind crossover comparison of hemodynamic and ventilatory effects. Kidney International 1983;24(2):240‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lambie 2005 {published data only}
- Lambie SH, McIntyre CW. Use of an on‐line conductivity monitoring to study individually set dialysate sodium concentrations: a randomised controlled study [abstract no: SU‐FC253]. Journal of the American Society of Nephrology 2003;14(Nov):55A‐6A. [Google Scholar]
- Lambie SH, Taal MW, Fluck RJ, McIntyre CW. Online conductivity monitoring: validation and usefulness in a clinical trial of reduced dialysate conductivity. ASAIO Journal 2005;51(1):70‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levin 1996 {published data only}
- Levin A, Carlisle EF, Ansell J, Goldstein MB. Double blind crossover trial (DBCT) of ramped hypertonic sodium dialysis (RHTD): beneficial effects for specific clinical problems [abstract]. Kidney International 1990;37(1):306. [CENTRAL: CN‐00626025] [Google Scholar]
- Levin A, Goldstein MB. The benefits and side effects of ramped hypertonic sodium dialysis. Journal of the American Society of Nephrology 1996;7(2):242‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Macon 1995 {published data only}
- Macon EJ, Campbell OC. A randomized comparison between sodium modeling and slow dialysis in the prevention of the dialysis disequilibrium syndrome (DES) [abstract]. Journal of the American Society of Nephrology 1995;6(3):547. [CENTRAL: CN‐00484936] [Google Scholar]
Mahiout 1987 {published data only}
- Mahiout A, Ludat K, Gahl GM, Schultze G. Effect of sodium dialysate concentration on beta2 microglobulin during haemodialysis [abstract]. Nephrology Dialysis Transplantation 1987;2(5):448. [CENTRAL: CN‐00260334] [Google Scholar]
Meira 2010 {published data only}
- Meira FS, Figueiredo AE, Zemiarcki J, Pacheco J, Poli‐de‐Figueiredo CE, d'Avila DO. Two variable sodium profiles and adverse effects during hemodialysis: a randomized crossover study. Therapeutic Apheresis & Dialysis 2010;14(3):328‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moret 2006 {published data only}
- Moret K, Aalten J, Wall Blake W, Gerlag P, Beerenhout C, Sande F, et al. The effect of sodium profiling and feedback technologies on plasma conductivity and ionic mass balance: a study in hypotension‐prone dialysis patients. Nephrology Dialysis Transplantation 2006;21(1):138‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT01015313 {unpublished data only}
- Kotanko P, Levin N. Effects of intensified sodium management in hemodialysis patients. www.clinicaltrials.gov/ct2/show/NCT01015313 (first received 18 November 2009).
NCT01168947 {unpublished data only}
- Kotanko P. Effect of sodium concentration of priming and rinsing fluids on weight gain. www.clinicaltrials.gov/ct2/show/NCT01168947 (first received 23 July 2010).
NCT01766882 {unpublished data only}
- Saran R. Volume, sodium and blood pressure management in HD (VSBP). www.clinicaltrials.gov/ct2/show/NCT01766882 (first received 11 January 2013).
Oliver 2001 {published data only}
- Oliver A, Wright M, Matson A, King N, Woodrow G, Dye L, et al. The effect of low sodium dialysis on fluid consumption and thirst scores in haemodialysis patients with high and low baseline interdialytic weight gain [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):273A. [CENTRAL: CN‐00447029] [Google Scholar]
Oliver 2001a {published data only}
- Oliver MJ, Edwards LJ, Churchill DN. Impact of sodium and ultrafiltration profiling on hemodialysis‐related symptoms. Journal of the American Society of Nephrology 2001;12(1):151‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Oliver MJ, Edwards LJ, Churchill DN. Randomized study of the effect of sodium profiled dialysis with blood volume monitoring on dialysis related symptoms [abstract no:A0989]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):194A. [Google Scholar]
Robberechts 2013 {published data only}
- Robberechts T, Allamani M, Marmitte N, Ingelgem D, Niepen P. The effect of hyponatremic versus isonatremic dialysate in patients with intradialytic hypertension [abstract]. Acta Clinica Belgica 2013;68(6):460. [EMBASE: 71520514] [Google Scholar]
- Robberechts T, Allamani M, Niepen P. The effect of hyponatremic versus isonatremic dialysate in patients with intradialytic hypertension [abstract]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii241‐2. [EMBASE: 72206929] [Google Scholar]
Sandy 1996 {published data only}
- Sandy D, Kozlowski L, Bednarz D, Heyka R, Paganini E. A stratified prospective randomized cross‐over comparison of variable (VNA) vs fixed (FNA) sodium dialysate during ICU ARF IHD therapy [abstract no: A1379]. Journal of the American Society of Nephrology 1996;7(9):1525. [Google Scholar]
Sang 1997 {published data only}
- Sang GL, Kovithavongs C, Ulan R, Kjellstrand CM. Sodium ramping in hemodialysis: a study of beneficial and adverse effects. American Journal of Kidney Diseases 1997;29(5):669‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Selby 2007 {published data only}
- Selby N, Taal M, McIntyre C. Reduction of dialysate sodium concentration using conventional HD or prescribed end dialysis plasma conductivity (Diacontrol©) [abstract no: SP678]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv244. [Google Scholar]
- Selby NM, Taal MW, McIntyre CW. Comparison of progressive conductivity reduction with Diacontrol and standard dialysis. ASAIO Journal 2007;53(2):194‐200. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Selby NM, Taal MW, McIntyre CW. Reduction of dialysate sodium concentration using conventional HD or prescribed end plasma conductivity (Diacontrol©) [abstract no: SA‐PO1140]. Journal of the American Society of Nephrology 2006;17(Abstracts):813A. [Google Scholar]
References to studies awaiting assessment
ISRCTN71215609 {published data only}
- ISRCTN71215609. Study into achievement of isonatric haemodialysis by individual tailoring of dialysate sodium according to ionic mass balance derived from online conductance monitoring. www.controlled‐trials.com/ISRCTN71215609 (first received 30 September 2005).
References to ongoing studies
NCT00724633 {unpublished data only}
- Suri R, Lindsay R, Rehman F. Effect of lowering the dialysate sodium on blood pressure in hemodialysis patients. www.clinicaltrials.gov/ct2/show/NCT00724633 (first received 29 July 2008).
NCT02145260 {unpublished data only}
- McCausland F, Waikar S. Trial of dialysate sodium in chronic hospitalized hemodialysis patients. www.clinicaltrials.gov/ct2/show/NCT02145260 (first received 22 May 2014).
RESOLVE 2016 {unpublished data only}
- Jardine M. Randomised evaluation of sodium dialysate levels on vascular events (RESOLVE). www.clinicaltrials.gov/ct2/show/NCT02823821 (first received 6 July 2016).
SoLID 2013 {published data only}
- Dunlop JL, Vandal AC, Zoysa JR, Gabriel RS, Gerber LM, Haloob IA, et al. Rationale and design of the Myocardial Microinjury and Cardiac Remodeling Extension Study in the Sodium Lowering in Dialysate trial (Mac‐SoLID study). BMC Nephrology 2014;15:120. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop JL, Vandal AC, Zoysa JR, Gabriel RS, Haloob IA, Hood CJ, et al. Rationale and design of the Sodium Lowering In Dialysate (SoLID) trial: a randomised controlled trial of low versus standard dialysate sodium concentration during hemodialysis for regression of left ventricular mass. BMC Nephrology 2013;14:149. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop JL, Vandal AC, Zoysa JR, Gabriel RS, Haloob IA, Hood CJ, et al. Update: Rationale and design of the Sodium Lowering In Dialysate (SoLID) trial: a randomised controlled trial of low versus standard dialysate sodium concentration during hemodialysis for regression of left ventricular mass. BMC Nephrology 2015;16:120. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall MR, Vandal AC, Dunlop JL, Zoysa JR, Haloob IA, Hood CJ, et al. The sodium lowering in dialysate (SoLID) trial: a randomized controlled trial of low versus standard dialysate sodium concentration (DNa) during hemodialysis (HD) for regression of left ventricular (LV) mass [abstract no: HI‐OR04]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):1B. [Google Scholar]
Additional references
Agarwal 2003
- Argarwal R. Systolic hypertension in hemodialysis patients.[Erratum appears in Semin Dial. 2003 Jul‐Aug;16(4):334]. Seminars in Dialysis 2003;16(3):208‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amro 2014
- Amro A, Waldum B, Dammen T, Miaskowski C, Os I. Symptom clusters in patients on dialysis and their association with quality‐of‐life outcomes. Journal of Renal Care 2014;40(1):23‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Basile 2015
- Basile C, Pisano A, Lisi P, Rossi L, Lomonte C, Bolignano D. High versus low dialysate sodium concentration in chronic haemodialysis patients: a systematic review of 23 studies. Nephrology Dialysis Transplantation 2015;31(4):1‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Basri 2002
- Basri NA, Shaheen FA. Visual loss in uremic patients on dialysis: a case report and review of literature. Saudi Journal of Kidney Disease & Transplantation 2002;13(1):45‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Boon 2004
- Boon D, Montfrans GA, Koopman MG, Krediet RT, Bos WJ. Blood pressure response to uncomplicated hemodialysis: the importance of changes in stroke volume. Nephron 2004;96(3):c82‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bos 2000
- Bos WJ, Bruin S, Olden RW, Keur I, Wesseling KH, Westerhof N, et al. Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. American Journal of Kidney Diseases 2000;35(5):819‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Braunwald 1965
- Braunwald E, Plauth WH Jr, Morrow AG. A method for the detection and quantification of impaired sodium excretion. Results of an oral sodium tolerance test in normal subjects and in patients with heart disease. Circulation 1965;32:223‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burton 2009
- Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis‐induced cardiac injury: determinants and associated outcomes. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(5):914‐20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cabrera 2015
- Cabrera C, Brunelli SM, Rosenbaum D, Anum E, Ramakrishnan K, Jensen DE, et al. A retrospective, longitudinal study estimating the association between interdialytic weight gain and cardiovascular events and death in hemodialysis patients. BMC Nephrology 2015;16:113. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caplin 2011
- Caplin B, Kumar S, Davenport A. Patients' perspective of haemodialysis‐associated symptoms. Nephrology Dialysis Transplantation 2011;26(8):2656‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chang 2011
- Chang TI, Paik J, Greene T, Desai M, Bech F, Cheung AK, et al. Intradialytic hypotension and vascular access thrombosis. Journal of the American Society of Nephrology 2011;22(8):1526‐33. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Charra 2004
- Charra B, Chazot C, Jean G, Hurot JM, Terrat JC, Vanel T, et al. Role of sodium in dialysis. Minerva Urologica e Nefrologica 2004;56(3):205‐13. [MEDLINE: ] [PubMed] [Google Scholar]
Chertow 2010
- FHN Trial Group, Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, et al. In‐center hemodialysis six times per week versus three times per week.[Erratum appears in N Engl J Med. 2011 Jan 6;364(1):93]. New England Journal of Medicine 2010;363(24):2287‐300. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cybulsky 1985
- Cybulsky AV, Matni A, Hollomby DJ. Effects of high sodium dialysate during maintenance hemodialysis. Nephron 1985;41(1):57‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davenport 2008
- Davenport A. Potential adverse effects of replacing high volume hemofiltration exchanges on electrolyte balance and acid‐base status using the current commercially available replacement solutions in patients with acute renal failure. International Journal of Artificial Organs 2008;31(1):3‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davies 2016 [pers comm]
- Davies E. (Kidney Research UK). re: Future priorities for renal research: survey report: a report on the findings from a patient survey exercise assessing kidney patients' views on research priorities. Letter to: E Davies (Kidney Research UK) 2016.
Descombes 2014
- Descombes E, Fellay B, Riedo E, Hemett OM, Magnin, J‐L, Fellay G. Comparison of sodium conductivity prescription and dialysate sodium concentration with three different hemodialysis (HD) monitors: not all the monitors are equal [abstract no: P71]. Swiss Med Weekly 2014;144(Suppl 208):31S. [EMBASE: 71713933] [Google Scholar]
Dheenan 2001
- Dheenan S, Henrich WL. Preventing dialysis hypotension: a comparison of usual protective maneuvers. Kidney International 2001;59(3):1175‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dubin 2011
- Dubin R, Owens C, Gasper W, Ganz P, Johansen K. Associations of endothelial dysfunction and arterial stiffness with intradialytic hypotension and hypertension. Hemodialysis International 2011;15(3):350‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunlop 2012
- Marshall MR, Dunlop JL. Are dialysate sodium levels too high?. Seminars in Dialysis 2012;25(3):277‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ekbal 2016
- Ekbal NJ, Consalus A, Persaud J, Davenport A. Reliability of delivered dialysate sodium concentration. Hemodialysis International 2016;20(Suppl 1):S2‐6. [EMBASE: 612346884] [DOI] [PubMed] [Google Scholar]
Eknoyan 2002
- Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. New England Journal of Medicine 2002;347(25):2010‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eldehni 2012
- Eldehni MT, Mcintyre CW. Are there neurological consequences of recurrent intradialytic hypotension?. Seminars in Dialysis 2012;25(3):253‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eldehni 2015
- Eldehni MT, Odudu A, McIntyre CW. Randomized clinical trial of dialysate cooling and effects on brain white matter. Journal of American Society of Nephrology 2015;26(4):957‐65. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischbach 1988
- Fischbach M, Tarral E, Geisert J. Sequential hypertonic haemodialysis in children. Pediatric Nephrology 1988;2(4):442‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Flanigan 1998
- Flanigan MJ. Sodium flux and dialysate sodium in hemodialysis. Seminars in Dialysis 1998;11(5):298‐304. [DOI: 10.1111/j.1525-139X.1998.tb00372.x] [DOI] [Google Scholar]
Flanigan 2008
- Flanigan MJ. How should dialysis fluid be individualized for the chronic hemodialysis patient? Sodium. Seminars in Dialysis 2008;21(3):226‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Flythe 2015
- Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. Journal of the American Society of Nephrology 2015;26(3):724‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gotch 1990
- Gotch F, Evans M, Metzner K, Westphal D, Polaschegg H. An on‐line monitor of dialyzer Na and K flux in hemodialysis. ASAIO Transactions 1990;36(3):M359‐61. [MEDLINE: ] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grassi 2002
- Grassi G, Dell'Oro R, Seravalle G, Foglia G, Trevano FQ, Mancia G. Short‐ and long‐term neuroadrenergic effects of moderate dietary sodium restriction in essential hypertension. Circulation 2002;106(15):1957‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gul 2016
- Gul A, Miskulin DC, Paine SS, Narsipur SS, Arbeit LA, Harford AM, et al. Comparison of prescribed and measured dialysate sodium: a quality improvement project. American Journal of Kidney Diseases 2016;67(3):439‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hecking 2011
- Hecking M, Kainz A, Horl WH, Herkner H, Sunder‐Plassmann G. Sodium setpoint and sodium gradient: influence on plasma sodium change and weight gain. American Journal of Nephrology 2011;33(1):39‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hecking 2012
- Hecking M, Karaboyas A, Saran R, Sen A, Inaba M, Rayner H, et al. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clinical Journal of The American Society of Nephrology: CJASN 2012;7(1):92‐100. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hecking 2015
- Hecking M, Rayner H, Port FK. More evidence needed before lower dialysate sodium concentrations can be recommended. American Journal of Kidney Diseases 2015;65(3):519‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hecking 2017
- Hecking M, Wong MM, Port FK, Robinson BM, McCullough KP. Regional differences in the associations between prescribed dialysate sodium concentration and interdialytic weight gain in the dialysis outcomes and practice patterns study. American Journal of Kidney Diseases 2017;70(3):450‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heerspink 2009
- Heerspink HJ, Ninomiya T, Zoungas S, Zeeuw D, Grobbee DE, Jardine MJ, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta‐analysis of randomised controlled trials. Lancet 2009;373(9668):1009‐15. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollenberg 1972
- Hollenberg NK, Adams DF, Solomon HS, Abrams HL, Merrill JP. What mediates the renal vascular response to a salt load in normal man?. Journal of Applied Physiology 1972;33(4):491‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hollenberg 1980
- Hollenberg NK. Set point for sodium homeostasis: surfeit, deficit, and their implications. Kidney International 1980;17(4):423‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hoorn 2013
- Hoorn EJ, Zietse R. Hyponatremia and mortality: moving beyond associations. American Journal of Kidney Diseases 2013;62(1):139‐49. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
K/DOQI Workgroup 2005
- K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. American Journal of Kidney Diseases 2005;45(4 Suppl 3):S1‐153. [MEDLINE: ] [PubMed] [Google Scholar]
Kalantar‐Zadeh 2009
- Kalantar‐Zadeh K, Regidor DL, Kovesdy CP, Wyck D, Bunnapradist S, Horwich TB, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long‐term hemodialysis. Circulation 2009;119(5):671‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keen 2007
- Keen ML, Gotch FA. The association of the sodium "setpoint" to interdialytic weight gain and blood pressure in hemodialysis patients. International Journal of Artificial Organs 2007;30(11):971‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kimura 1984
- Kimura G, Gotch FA. Serum sodium concentration and body fluid distribution during interdialysis: importance of sodium to fluid intake ratio in hemodialysis patients. International Journal of Artificial Organs 1984;7(6):331‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Kotanko 2015
- Kotanko P, Garg AX, Depner T, Pierratos A, Chan CT, Levin NW, et al. Effects of frequent hemodialysis on blood pressure: results from the randomized frequent hemodialysis network trials. Hemodialysis International 2015;19(3):386‐401. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kyriazis 2002
- Kyriazis J, Glotsos J, Bilirakis L, Smirnioudis N, Tripolitou M, Georgiakodis F, et al. Dialysate calcium profiling during hemodialysis: use and clinical implication. Kidney International 2002;61(1):276‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Locatelli 1984
- Locatelli F, Ponti R, Pedrini L, Costanzo R, Filippo S, Marai P, et al. Sodium kinetics across dialysis membranes. Nephron 1984;38(3):174‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lomonte 2011
- Lomonte C, Basile C. Do not forget to individualize dialysate sodium prescription. Nephrology Dialysis Transplantation 2011;26(4):1126‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
London 2001
- London GM, Pannier B, Guerin AP, Blacher J, Marchais SJ, Darne B, et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow‐up of an interventional study. Journal of American Society of Nephrology 2001;12(12):2759‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mandai 2013
- Mandai S, Kuwahara M, Kasagi Y, Kusaka K, Tanaka T, Shikuma S, et al. Lower serum sodium level predicts higher risk of infection‐related hospitalization in maintenance hemodialysis patients: an observational cohort study. BMC Nephrology 2013;14:276. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manlucu 2010
- Manlucu J, Gallo K, Heidenheim PA, Lindsay RM. Lowering postdialysis plasma sodium (conductivity) to increase sodium removal in volume‐expanded hemodialysis patients: a pilot study using a biofeedback software system. American Journal of Kidney Diseases 2010;56(1):69‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marshall 2012
- Marshall MR, Dunlop JL. Are dialysate sodium levels too high?. Seminars in Dialysis 2012;25(3):277‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marshall 2017
- Marshall MR, Chan CT. The evolution of home HD ‐ meeting modern patient needs. Contributions to Nephrology 2017;189:36‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matsuoka 1990
- Matsuoka H, Kimura G, Sanai T, Kojima S, Kawano Y, Imanishi M, et al. Normalization of increased sodium sensitivity by maintenance hemodialysis. American Journal of Hypertension 1990;3(8 Pt 1):628‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mc Causland 2012
- Causland FR, Brunelli SM, Waikar SS. Dialysate sodium, serum sodium and mortality in maintenance hemodialysis. Nephrology Dialysis Transplantation 2012;27(4):1613‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntyre 2008
- McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, et al. Hemodialysis‐induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(1):19‐26. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntyre 2011
- McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clinical Journal of American Society of Nephrology: CJASN 2011;6(1):133‐41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntyre 2014
- McIntyre CW, Odudu A. Hemodialysis‐associated cardiomyopathy: a newly defined disease entity. Seminars in Dialysis 2014;27(2):87‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Movilli 2013
- Movilli E, Camerini C, Gaggia P, Zubani R, Feller P, Poiatti P, et al. Role of dialysis sodium gradient on intradialytic hypertension: an observational study. American Journal of Nephrology 2013;38(5):413‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Munoz Mendoza 2011
- Munoz Mendoza J, Bayes LY, Sun S, Doss S, Schiller B. Effect of lowering dialysate sodium concentration on interdialytic weight gain and blood pressure in patients undergoing thrice‐weekly in‐center nocturnal hemodialysis: a quality improvement study. American Journal of Kidney Diseases 2011;58(6):956‐63. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oberleithner 2007
- Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proceedings of the National Academy of Sciences of the United States of America 2007;104(41):16281‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliver 2001b
- Oliver MJ, Edwards LJ, Churchill DN. Impact of sodium and ultrafiltration profiling on hemodialysis‐related symptoms. Journal of the American Society of Nephrology 2001;12(1):151‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ozkahya 2002
- Ozkahya M, Toz H, Ozerkan F, Duman S, Ok E, Basci A, Mees EJ. Impact of volume control on left ventricular hypertrophy in dialysis patients. Journal of Nephrology 2002;15(6):655‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Peixoto 2010
- Peixoto AJ, Gowda N, Parikh CR, Santos SF. Long‐term stability of serum sodium in hemodialysis patients. Blood Purification 2010;29(3):264‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peixoto 2011
- Peixoto AJ, Santos SF. How should the predialysis plasma sodium level be interpreted in hemodialysis patients?. Seminars in Dialysis 2011;24(4):409‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Penne 2011
- Penne EL, Sergeyeva O. Sodium gradient: a tool to individualize dialysate sodium prescription in chronic hemodialysis patients?. Blood Purification 2011;31(1‐3):86‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Polaschegg 1985
- Polaschegg HD, Husar D (inventors), Fresenius AG (assignee). Apparatus with regulated mixing of the dialysis solution. US patent 4508622. Patent date 02.04.85. Priority DE21.06.82 3223051 International classification B01D13/00. www.patents.justia.com/patent/4508622 (date accessed 7 November 2018).
Port 1973
- Port FK, Johnson WJ, Klass DW. Prevention of dialysis disequilibrium syndrome by use of high sodium concentration in the dialysate. Kidney International 1973;3(5):327‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Poulikakos 2014
- Poulikakos D, Marks V, Lelos N, Banerjee D. Low serum sodium is associated with protein energy wasting and increased interdialytic weight gain in haemodialysis patients. Clinical Kidney Journal 2014;7(2):156‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ragon 1985
- Ragon A, Reynier JP, Murisasco A, Elsen R, Leblond G. Dialysate sodium control during modeling in hemodialysis. Artificial Organs 1985;9(1):63‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rahman 1999
- Rahman M, Dixit A, Donley V, Gupta S, Hanslik T, Lacson E, et al. Factors associated with inadequate blood pressure control in hypertensive hemodialysis patients. American Journal of Kidney Diseases 1999;33(3):498‐506. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raimann 2009
- Raimann J, Liu L, Ulloa D, Kotanko P, Levin NW. Consequences of overhydration and the need for dry weight assessment. Contributions to Nephrology 2009;161:99‐107. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raimann 2018
- Raimann JG, Ficociello LH, Usvyat LA, Zhang H, Pacelli L, Moore S, et al. Effects of dialysate to serum sodium (Na(+)) alignment in chronic hemodialysis (HD) patients: retrospective cohort study from a quality improvement project. BMC Nephrology 2018;19(1):75. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rocco 2011
- Rocco MV, Lockridge RS Jr, Beck GJ, Eggers PW, Gassman JJ, Greene T, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney International 2011;80(10):1080‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodrigues Telini 2013
- Rodrigues Telini LS, Carvalho Beduschi G, Caramori JC, Castro JH, Martin LC, Barretti P. Effect of dietary sodium restriction on body water, blood pressure, and inflammation in hemodialysis patients: a prospective randomized controlled study. International Urology & Nephrology 2014;46(1):91‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sands 2014
- Sands JJ, Usvyat LA, Sullivan T, Segal JH, Zabetakis P, Kotanko P, et al. Intradialytic hypotension: frequency, sources of variation and correlation with clinical outcome. Hemodialysis International 2014;18(2):415‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Santos 2008
- Santos SF, Peixoto AJ. Revisiting the dialysate sodium prescription as a tool for better blood pressure and interdialytic weight gain management in hemodialysis patients. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(2):522‐30. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santos 2010
- Santos SF, Peixoto AJ. Sodium balance in maintenance hemodialysis. Seminars in Dialysis 2010;23(6):549‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schipper 2011
- Schipper K, Abma TA. Coping, family and mastery: top priorities for social science research by patients with chronic kidney disease. Nephrology Dialysis Transplantation 2011;26(10):3189‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schreiber 2001
- Schrieber MJ Jr. Clinical case‐based approach to understanding intradialytic hypotension. American Journal of Kidney Diseases 2001;38(4 Suppl 4):S37‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Selby 2007a
- Selby NM, McIntyre CW. The acute cardiac effects of dialysis. Seminars in Dialysis 2007;20(3):220‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shepherd 1987
- Shepherd R, Farleigh CA, Atkinson C, Pryor JS. Effects of haemodialysis on taste and thirst. Appetite 1987;9(2):79‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sherman 2011
- Sherman RA, Kapoian T. Intradialytic hypotension strikes again. Journal of the American Society of Nephrology 2011;22(8):1396‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shoji 2004
- Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis‐associated hypotension as an independent risk factor for two‐year mortality in hemodialysis patients. Kidney International 2004;66(3):1212‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Song 2002
- Song JH, Lee SW, Suh CK, Kim MJ. Time‐averaged concentration of dialysate sodium relates with sodium load and interdialytic weight gain during sodium‐profiling hemodialysis.[Erratum appears in Am J Kidney Dis 2002 Dec;40(6):1357]. American Journal of Kidney Diseases 2002;40(2):291‐301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Song 2005
- Song JH, Park GH, Lee SY, Lee SW, Lee SW, Kim MJ. Effect of sodium balance and the combination of ultrafiltration profile during sodium profiling hemodialysis on the maintenance of the quality of dialysis and sodium and fluid balances. Journal of the American Society of Nephrology 2005;16(1):237‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stefansson 2014
- Stefansson BV, Brunelli SM, Cabrera C, Rosenbaum D, Anum E, Ramakrishnan K, et al. Intradialytic hypotension and risk of cardiovascular disease. Clinical Journal of American Society of Nephrology: CJASN 2014;9(12):2124‐32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 1972
- Stewart WK, Fleming LW, Manuel MA. Muscle cramps during maintenance haemodialysis. Lancet 1972;1(7759):1049‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stiller 2001
- Stiller S, Bonnie‐Schorn E, Grassmann A, Uhlenbusch‐Korwer I, Mann H. A critical review of sodium profiling for hemodialysis. Seminars in Dialysis 2001;14(5):337‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stragier 2018
- Stragier A, Lopot F, Svara F, Polakovic V. Fallacies and pitfalls of dialysis sodium prescription and control. Blood Purification 2018;46(1):27‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strauss 1958
- Strauss MB, Lamdin E, Smith WP, Bleifer DJ. Surfeit and deficit of sodium; a kinetic concept of sodium excretion. A.M.A. Archives of Internal Medicine 1958;102(4):527‐536. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sukhanov 2011
- Sukhanov S, Semprun‐Prieto L, Yoshida T, Michael Tabony A, Higashi Y, Galvez S, et al. Angiotensin II, oxidative stress and skeletal muscle wasting. American Journal of the Medical Sciences 2011;342(2):143‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swart 2011
- Swart RM, Hoorn EJ, Betjes MG, Zietse R. Hyponatremia and inflammation: the emerging role of interleukin‐6 in osmoregulation. Nephron. Physiology 2011;118(2):45‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taratufolo 2001
- Taratufolo A, Egidi S, Grassotti A, Pedulla F, Satriani M, Galeotti P, et al. Clinical impact of residual renal function on patients starting dialysis treatment. Edtna‐Erca Journal 2001;27(2):75‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thein 2007
- Thein H, Haloob I, Marshall MR. Associations of a facility level decrease in dialysate sodium concentration with blood pressure and interdialytic weight gain. Nephrology Dialysis Transplantation 2007;22(9):2630‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tisler 2003
- Tisler A, Akocsi K, Borbas B, Fazakas L, Ferenczi S, Gorogh S, et al. The effect of frequent or occasional dialysis‐associated hypotension on survival of patients on maintenance haemodialysis. Nephrology Dialysis Transplantation 2003;18(12):2601‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Titze 2008
- Titze J. Water‐free Na+ retention: interaction with hypertension and tissue hydration. Blood Purification 2008;26(1):95‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Titze 2009
- Titze J. Water‐free sodium accumulation. Seminars in Dialysis 2009;23(3):253‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Stone 1982
- Stone JC, Bauer J, Carey J. The effect of dialysate sodium concentration on body fluid compartment volume, plasma renin activity and plasma aldosterone concentration in chronic hemodialysis patients. American Journal of Kidney Diseases 1982;2(1):58‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Waikar 2009
- Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. American Journal of Medicine 2009;122(9):857‐65. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waikar 2011
- Waikar SS, Curhan GC, Brunelli SM. Mortality associated with low serum sodium concentration in maintenance hemodialysis. American Journal of Medicine 2011;124(1):77‐84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiner 2014
- Weiner DE, Brunelli SM, Hunt A, Schiller B, Glassock R, Maddux FW, et al. Improving clinical outcomes among hemodialysis patients: a proposal for a "volume first" approach from the chief medical officers of US dialysis providers. American Journal of Kidney Diseases 2014;64(5):685‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wells 2004
- Wells M, Foroozan R. Transient visual loss may anticipate occipital infarction from hemodialysis. American Journal of Kidney Diseases 2004;43(5):e29‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wilkinson 1977
- Wilkinson R, Barber SG, Robson V. Cramps, thirst and hypertension in hemodialysis patients ‐‐ the influence of dialyzate sodium concentration. Clinical Nephrology 1977;7(3):101‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Wong 2017
- Wong MM, McCullough KP, Bieber BA, Bommer J, Hecking M, Levin NW, et al. Interdialytic weight gain: trends, predictors, and associated outcomes in the International Dialysis Outcomes and Practice Patterns Study (DOPPS). American Journal of Kidney Diseases 2017;69(3):367‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zazgornik 1997
- Zazgornik J, Biesenbach G, Forstenlehner M, Stummvoll K. Profile of antihypertensive drugs in hypertensive patients on renal replacement therapy (RRT). Clinical Nephrology 1997;48(6):337‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Zhang 2014
- Zhang Z, Duckart J, Slatore CG, Fu Y, Petrik AF, Thorp ML, et al. Individuality of the plasma sodium concentration. American Journal of Physiology ‐ Renal Physiology 2014;306(12):F1534‐43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2014
- Zhou YL, Liu J, Ma L, Sun F, Shen Y, Huang J, et al. Impact of dry weight determined by calf bioimpedance ratio on carotid stiffness and left ventricular hypertrophy in hemodialysis patients. Artificial Organs 2014;38(4):327‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Dunlop 2014
- Dunlop JL, Vandal AC, Marshall MR. Dialysate sodium levels for chronic haemodialysis. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD011204] [DOI] [PMC free article] [PubMed] [Google Scholar]


