Abstract
Background
Patients admitted to intensive care and on mechanical ventilation, are administered sedative and analgesic drugs to improve both their comfort and interaction with the ventilator. Optimizing sedation practice may reduce mortality, improve patient comfort and reduce cost. Current practice is to use scales or scores to assess depth of sedation based on clinical criteria such as consciousness, understanding and response to commands. However these are perceived as subjective assessment tools. Bispectral index (BIS) monitors, which are based on the processing of electroencephalographic signals, may overcome the restraints of the sedation scales and provide a more reliable and consistent guidance for the titration of sedation depth.
The benefits of BIS monitoring of patients under general anaesthesia for surgical procedures have already been confirmed by another Cochrane review. By undertaking a well‐conducted systematic review our aim was to find out if BIS monitoring improves outcomes in mechanically ventilated adult intensive care unit (ICU) patients.
Objectives
To assess the effects of BIS monitoring compared with clinical sedation assessment on ICU length of stay (LOS), duration of mechanical ventilation, any cause mortality, risk of ventilator‐associated pneumonia (VAP), risk of adverse events (e.g. self‐extubation, unplanned disconnection of indwelling catheters), hospital LOS, amount of sedative agents used, cost, longer‐term functional outcomes and quality of life as reported by authors for mechanically ventilated adults in the ICU.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, ProQuest, OpenGrey and SciSearch up to May 2017 and checked references citation searching and contacted study authors to identify additional studies. We searched trial registries, which included clinicaltrials.gov and controlled‐trials.com.
Selection criteria
We included all randomized controlled trials comparing BIS versus clinical assessment (CA) for the management of sedation in mechanically ventilated critically ill adults.
Data collection and analysis
We used Cochrane's standard methodological procedures. We undertook analysis using Revman 5.3 software.
Main results
We identified 4245 possible studies from the initial search. Of those studies, four studies (256 participants) met the inclusion criteria. One more study is awaiting classification. Studies were, conducted in single‐centre surgical and mixed medical‐surgical ICUs. BIS monitor was used to assess the level of sedation in the intervention arm in all the studies. In the control arm, the sedation assessment tools for CA included the Sedation‐Agitation Scale (SAS), Ramsay Sedation Scale (RSS) or subjective CA utilizing traditional clinical signs (heart rate, blood pressure, conscious level and pupillary size). Only one study was classified as low risk of bias, the other three studies were classified as high risk.
There was no evidence of a difference in one study (N = 50) that measured ICU LOS (Median (Interquartile Range IQR) 8 (4 to 14) in the CA group; 12 (6 to 18) in the BIS group; low‐quality evidence).There was little or no effect on the duration of mechanical ventilation (MD ‐0.02 days (95% CI ‐0.13 to 0.09; 2 studies; N = 155; I2 = 0%; low‐quality evidence)). Adverse events were reported in one study (N = 105) and the effects on restlessness after suction, endotracheal tube resistance, pain tolerance during sedation or delirium after extubation were uncertain due to very low‐quality evidence. Clinically relevant adverse events such as self‐extubation were not reported in any study. Three studies reported the amount of sedative agents used. We could not measure combined difference in the amount of sedative agents used because of different sedation protocols and sedative agents used in the studies. GRADE quality of evidence was very low. No study reported other secondary outcomes of interest for the review.
Authors' conclusions
We found insufficient evidence about the effects of BIS monitoring for sedation in critically ill mechanically ventilated adults on clinical outcomes or resource utilization. The findings are uncertain due to the low‐ and very low‐quality evidence derived from a limited number of studies.
Plain language summary
Comparing BIS monitoring with clinical assessment for determining the level of sedation of mechanically ventilated adults in intensive care units
Review question
We reviewed the evidence for benefits of bispectral index (BIS) monitoring compared to clinical assessment (CA) methods in adults connected to a breathing machine (ventilator) in the intensive care unit (ICU).
Background
BIS monitoring follows brain electrical activity to produce scores. These scores may help hospital staff decide whether a person in ICU who is on a ventilator is receiving enough sedative to make them comfortable and accept the ventilator. Sedatives are drugs taken for their calming and sleep‐inducing effects. Giving of too much, or too little, sedative could lead to harm. In the CA method, observing clinical factors such as consciousness, understanding and response to commands helps to assess the depth of sedation or sleep. The score provided by the BIS monitor is not dependent on a person. Monitoring by CA might vary between caregivers. Our aim was to find out if BIS monitoring is beneficial compared to CA for critically ill adults on a ventilator.
Study characteristics
The evidence identified from our literature search is current to May 2017. Four randomized controlled studies met the inclusion criteria for this review (involving 256 adults). One more study is awaiting classification. These studies were conducted in adult surgical and mixed medical‐surgical ICUs, and compared BIS monitoring with various measures for CA.
Study funding sources
For one study, the BIS monitoring devices manufacturer provided equipment. The company had no role in the conduct of the study. Another study was funded as part of a scientific and technological project. No funding information was available for the other two studies.
Key results
With BIS monitoring, we found no significant differences in ICU length of stay (one study, 50 adults), duration of ventilation (two studies, 155 adults) and the risk of adverse events (one study, 105 adults) compared with CA. Clinically relevant adverse events, for example, accidental self‐removal of the breathing tube, were not reported. We could not measure combined difference in amount of sedative use because of the different sedation protocols and sedatives used. None of the other outcomes of interest for the review, for example, death, ventilator‐associated pneumonia, quality of life etc. were reported in any of the studies.
Quality of evidence
The findings of our review are from a limited number of studies which provided 'low to very low' GRADE quality of evidence.
Conclusion
The authors of this review conclude that we found insufficient evidence about the effects of BIS monitoring compared with CA of sedation in critically ill adults who were on a ventilator.
Summary of findings
Summary of findings for the main comparison. BIS monitoring compared to clinical assessment for sedation in mechanically ventilated adults in the intensive care unit and its impact on clinical outcomes and resource utilization.
| BIS monitoring compared to clinical assessment for sedation in mechanically ventilated adults in the intensive care unit and its impact on clinical outcomes and resource utilization | ||||||
| Patient or population: Mechanically ventilated adults in the intensive care unit Setting: Medical and surgical patients in intensive care unit in hospitals in China, Japan and Australia Intervention: BIS monitoring Comparison: Clinical assessment | ||||||
| Outcomes | Anticipated absolute effects* | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with Clinical assessment | Risk with BIS monitoring | |||||
| Intensive care unit length of stay (ICU LOS) (measured in days) | Median ICU LOS was 8 Days | Median ICU LOS was 4 Days higher | Mdn D 4 [Range 4 to 18] | 50 (1 RCT) | ⊕⊕⊕⊝ LOW 1 | |
| Duration of mechanical ventilation (measured in days) | Mean duration of mechanical ventilation was 2.49 days | Mean duration of mechanical ventilation was 0.02 days lower | MD ‐0.02 (‐0.13, 0.09) | 155 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 | |
| Adverse events: Measured as number of patients with adverse events | 105 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 3 | Clinically relevant adverse events such as self‐extubation or unplanned disconnection of indwelling catheters were not reported in any study. | |||
| 809 patients with restlessness after suction per 1000 patients | 16 less patients with restlessness after suction | RR 1.11 (0.90,1.37) | ||||
| 714 patients with endotracheal tube resistance per 1000 patients | 32 more patients with endotracheal tube resistance | RR 0.96 (0.75, 1.22) | ||||
| 928 patients with pain tolerance during sedation per 1000 patients | 8 more patients with pain tolerance during sedation | RR 0.99 (0.89, 1.10) | ||||
| 47 patients with delirium after extubation per 1000 patients | 32 less patients with delirium after extubation | RR 3 (0.28, 32.04) | ||||
| Other important secondary outcomes like Any‐cause mortality, ventilator‐associated pneumonia, hospital LOS, amount of sedative agents used, long term functional outcomes and quality of life were not reported in any studies | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Mdn D: Median difference; CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to very serious concerns about imprecision (very small sample size of the study and large confidence interval).
2 Downgraded two levels due to serious concerns about risk of bias (Zhao 2011 which carries 98.3% weight for this outcome, Random sequence generation, Allocation concealment and selective reporting were graded as unclear risk of bias) and imprecision (Difference in duration of mechanical ventilation was less than one day which is clinically insignificant).
3 Downgraded three levels due to serious concerns about risk of bias (Random sequence generation, Allocation concealment and Selective reporting were assessed as unclear risk of bias), indirectness (Clinically relevant adverse events were not reported) and imprecision (Small number of patients in the study Zhao 2011).
Background
Description of the condition
A significant proportion of the patients admitted to an intensive care unit (ICU) undergo mechanical ventilation (Esteban 2002; Metnitz 2009). It is common practice to administer sedative and analgesic drugs to these patients, to improve their comfort and their interaction with the ventilator. Different sedative and analgesic drugs are used for this purpose (Gommers 2008; Patel 2012). Careful titration of analgesia and sedation is important to prevent pain and discomfort in this population of patients, but oversedation has been associated with increased mortality and morbidity (Kollef 1998; Kress 2000). Optimizing sedation practice may reduce mortality, and may reduce the duration of mechanical ventilation and ICU length of stay, resulting in reduced costs and improved resource utilization (Jackson 2010). The recommended strategy to titrate sedation is to use scales or scores based on clinical criteria (Jacobi 2002). Many sedation tools have been developed, but not all have been validated and tested in clinical practice (Barr 2013). There is variability in the specific domains (e.g. consciousness, cognition, and comprehension) they assess (Sessler 2008), and in their implementation (about 88% of units use a sedation scale, with variability in the sedation scale used) (Martin 2007; Reschreiter 2008; Soliman 2001). Furthermore, these scales are perceived to provide a subjective assessment of patient sedation, also their usefulness in patients receiving neuromuscular blocking medications or requiring deep sedation may be limited.
Description of the intervention
With the aim to overcome the restraints of the subjective sedation scales, many techniques and devices (e.g. Bispectral Index (BIS) monitoring, State Entropy (SE), Auditory evoked potentials (AEPs), Narcotrend Index (NI), Patient State Index (PSI)) have been developed with the purpose of providing an objective measurement of patient's sedation (Carrasco 2000). The BIS monitoring is possibly the most studied and adapted.
BIS monitoring is based on the processing of electroencephalographic signals from the brain. The device uses three or four electrodes applied to the patient's forehead. The electrodes record the raw electroencephalogram (EEG) signal and process it through a proprietary algorithm, producing a dimensionless number, ranging from zero to 100, where 90 to100 indicates a state of wakefulness and zero represents absence of brain electrical activity. BIS monitoring is available in different hardware and software versions (LeBlanc 2006). The set up and maintenance cost of BIS monitoring is quite high. The monitor cost is around USD 6500.00 and a sensor, which includes four electrodes costs around USD 25.00 per set (Sedation Equipment & Supplies 2017), but this cost may be offset by a reduction in the usage of sedative drugs. In one study, titration of sedation with BIS monitoring in ICU patients resulted in an 18% reduction in cost over two months period (about USD 150.00 per patient) mainly as a result of reduction in lorazepam, midazolam and propofol usage (Kaplan 2000).
BIS monitoring is quite well established for monitoring anaesthesia depth (Punjasawadwong 2014), but there are differences in patient characteristics in critical care compared to anaesthesia. A critical care patient's brain may be abnormal. Delirium and neurological impairment are extremely common in the intensive care setting (Singhal 2014). Sepsis is often characterized by an acute brain dysfunction (Sonneville 2013). There are several other conditions that can also cause encephalopathy in critical care patients (Fugate 2013; Hu 2014; Ma 2013; Stevens 2008; Ziaja 2013). The effect of hypoglycaemia (low blood sugar level), temperature, nerve‐muscle electrical activity and drugs such as catecholamines on BIS monitoring scores might vary (Barr 2013; LeBlanc 2006). Also, there are already well‐established validated clinical sedation scores, such as the Richmond Agitation Sedation scale (RASS) and Sedation Agitation Scale (SAS) available in critical care, hence it is not clear if BIS monitoring in critically ill patients is equally as effective as in anaesthesia.
How the intervention might work
Significant under‐sedation occurs using subjective analysis of sedation in the ICU (Kaplan 2000). BIS monitoring has been reported to be better than clinical assessment (CA) methods for ICU patients undergoing short‐term mechanical ventilation in terms of reduction in the amount of sedative use and time to wakefulness (Zhao 2011). It has also been reported that BIS monitoring can reliably differentiate between inadequate and adequate sedation (Karamchandani 2010); helps in faster emergence and improved recovery from sedation; and reduces recall phenomenon thereby, reducing the posttraumatic stress disorder (PTSD) (Kaplan 2000). When compared with four commonly used subjective clinical scales (Ramsay Sedation Scale (RSS), RASS, SAS and Adaptation to Intensive Care Environment scale), BIS monitoring showed significant correlation with all the scales (Yaman 2012). In another study comparing BIS monitoring with RASS in mechanically ventilated critically ill patients, BIS monitoring correlated well with RASS (Karamchandani 2010). With the production of an objective measurement in the form of a dimensionless number, BIS monitoring might be able to overcome some of the limitations of the subjective clinical sedation scales and provide a more reliable and consistent guidance for the titration of sedation in ICU.
Why it is important to do this review
The benefits of BIS monitoring in patients undergoing general anaesthesia for surgical procedures have been confirmed by a Cochrane review (Punjasawadwong 2014). The use of BIS monitoring in intensive care has many advantages. Using BIS monitoring to guide sedative administration would allow optimizations of drug delivery to the needs of the individual patients in order to avoid unnecessary deep or light sedation. Compared to CA, BIS monitoring can distinguish between lightly and deeply sedated patients (Dewhurst 2000). It has a special role in critically ill brain injured patients with or without sedation (Deogaonkar 2004). It has also been reported to reduce consumption of sedative drugs (Kaplan 2000). All this may lead to reduced duration of mechanical ventilation, ICU length of stay, hospital length of stay and ultimately result in cost saving. Although several studies have evaluated the use of BIS monitoring in the ICU, there are only two systematic reviews that have been undertaken to establish its benefit for ICU patients (Finger 2016; Bilgili 2017). However both of these reviews included studies where sedation monitoring based on CA was used in both the intervention and control arm (i.e. BIS monitoring and CA versus CA alone). By undertaking a well‐conducted systematic review we aim to answer the question, does the use of BIS monitoring alone compared to clinical sedation assessment lead to improvement in clinical outcomes and resource utilisation.
Objectives
To assess the effects of BIS monitoring compared with clinical sedation assessment on intensive care unit (ICU) length of stay (LOS), duration of mechanical ventilation, any cause mortality, risk of ventilator‐associated pneumonia (VAP), risk of adverse events (e.g. self‐extubation, unplanned disconnection of indwelling catheters), hospital LOS, amount of sedative agents used, cost, longer‐term functional outcomes as reported by authors and quality of life as reported by authors for mechanically ventilated adult study participants in the ICU.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) comparing BIS monitoring versus clinical assessment (CA) for the management of sedation in mechanically ventilated critically ill adults, regardless of language and publication status.
We planned to include cluster‐randomized trials in our review but none were identified .
Non‐randomized and quasi‐randomized trials were not eligible for inclusion because of the significant risk of bias.
Cross‐over trials were also not eligible for inclusion because this methodology is not suitable for investigating the intervention topic of our study.
Types of participants
We included trials involving adults undergoing mechanical ventilation in ICUs, irrespective of the admission diagnosis.
Types of interventions
The intervention group comprised all participants whose sedation was managed by a strategy based on BIS monitoring with, or without, the use of a protocol to titrate the sedation level. The control group included all participants whose sedation was managed by monitoring with any clinical method (using clinical judgement or a specific clinical sedation scoring tool), with or without the use of a titration protocol.
Types of outcome measures
Primary outcomes
Intensive care unit (ICU) length of stay (LOS), measured in days.
Secondary outcomes
Duration of mechanical ventilation, measured in days.
Any‐cause mortality.
Risk of ventilator‐associated pneumonia (VAP).
Risk of adverse events (e.g. self‐extubation, unplanned disconnection of indwelling catheters).
Hospital LOS in days.
Amount of sedative agents used. (See Differences between protocol and review).
Cost.
Longer‐term functional outcomes as reported by study authors.
Quality of life as reported by study authors using SF36 or similar tools.
Search methods for identification of studies
Electronic searches
We searched the latest issue of the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 6 of 12, June 2017; Appendix 1), MEDLINE (Ovid SP, from 1994 to May 2017 Appendix 2), Embase (Ovid SP, from 1994 to May 2017; Appendix 3) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost, from 1994 to May 2017; Appendix 4).
We searched the databases from 1994 onwards, because BIS monitor was introduced by Aspect Medical Systems, Inc. (Norwood, Massachusetts, USA) for the first time in 1994.
In the relevant databases (MEDLINE and Embase) the sensitivity‐maximizing strategy was applied as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We adopted our ProQuest search strategy in searching all other databases (Appendix 5).
We also searched clinicaltrials.gov, controlled‐trials.com and other national and regional registries for ongoing trials.
We did not impose any language restrictions.
Searching other resources
In addition to searches of electronic databases;
we searched OpenGrey for Information on grey literature (up to June 2017);
screened the reference lists of all eligible trials and relevant reviews;
undertook cited reference searching using SciSearch (up to June 2017);
identified relevant studies published in dissertations or theses by searching ProQuest Dissertations and Theses database (up to June 2017);
we tried to contact experts in the field and the manufacturer of the device, however we did not receive any response from them.
Data collection and analysis
Selection of studies
We merged the results of the searches (described above) using reference management software, and removed all duplicates.
Two review authors (RS, AB) independently examined the titles and abstracts of identified studies and removed obviously irrelevant reports. We (RS, AB) were not blinded to any details of the published study. After this first screening process, we (RS, AB) compared our results and were able to resolve disagreements by discussion. In cases of inability to reach a consensus, we consulted a third review author (RJ).
We produced a list of potentially relevant studies. The same two review authors independently assessed studies for potential inclusion in the review by using the Cochrane Anaesthesia, Critical and Emergency Review Group's (ACE's) study selection and data extraction form (Appendix 6). We independently noted the reasons for exclusion.
We resolved disagreements in study selection by discussion. In cases of inability to reach a consensus, we consulted a third review author (AK). We contacted the journal/ corresponding author of the relevant studies for additional data or clarifications.
We compiled a list of all eligible studies, along with a list of excluded studies.
Data extraction and management
Two review authors (RS, AB) extracted data independently according to the predetermined criteria provided on the ACE study selection and data extraction form (Appendix 6). If any relevant data were missing, we contacted the first author or corresponding author of the study to obtain this information. Data extraction or translation from studies of languages other than English were undertaken by Cochrane experts arranged by the Cochrane Anaesthesia, Critical and Emergency Review Group. One Japanese article (Inaba 2007), was translated and data extracted by two Japanese speaking healthcare professionals in addition to the Cochrane organized expert.
We (RS, AB) resolved disagreements by discussion. If we were unable to reach an agreement, we consulted the third review author (AK).
We collected the following information about study context where available.
Country where the study was conducted.
Number of beds in the hospital.
Number of beds in the Intensive care unit (ICU).
Number of admissions to the ICU per year.
Nurse‐to‐patient ratio.
Type of ICU (medical, surgical, cardiac, neurological, trauma, burn).
Type of sedation used in both groups, as well as dose and total amount given.
Whether paralytics were used in both groups.
Confounders: drugs (e.g. catecholamines, aminophylline), electromyography (EMG), sleep, temperature, hypoglycaemia, excessive muscle movement, etc.
Diagnosis.
Severity of illness scoring.
Assessment of risk of bias in included studies
Two review authors (RS, AK) independently assessed risk of bias using the Cochrane 'Risk of bias' tool (Higgins 2011). We were not blinded to the names of the study authors, institutions, journal and results. We judged the quality of studies on the basis of risk of bias in the following domains.
-
Selection bias.
Random sequence generation.
Allocation concealment.
-
Detection bias.
Blinding of outcome assessors.
Blinding of personnel.
-
Attrition bias.
Incomplete outcome data.
-
Reporting bias.
Selective reporting.
We classified studies as low risk, high risk or unclear risk of bias for the above domains using information available from the studies. We considered a study as having low risk of bias if all domains (except blinding of personnel, as blinding is not possible because of the nature of the study), were assessed as adequate (low risk). We considered a study as having high risk of bias if one or more domains (except blinding of personnel) were assessed as inadequate (high or unclear risk), and as having an unclear risk if insufficient detail of what happened in the study was reported. Primary analysis was planned to be restricted to studies at low risk of bias. We planned to perform a sensitivity analysis excluding studies assessed as having high risk of bias. We (RS, AK) resolved any cases of disagreement about classification of risks by discussion. If we were unable to reach an agreement, we planned to consult a third review author (MH), however this was not required.
We constructed a 'Risk of bias' table as part of the 'Characteristics of included studies,' a 'Risk of bias' summary figure (Figure 1) and a 'Risk of bias' graph (Figure 2), with details of all judgements made for all studies included in the review. For the 'Risk of bias' table, we have provided a text box that includes a description of the design, conduct or observations that underline the judgement.
1.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
2.
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We undertook analysis using RevMan 5.3 software.
For continuous outcomes (duration of mechanical ventilation), we presented the treatment effect as a mean difference (MD). ICU LOS is presented as median with range as only one study reported this outcome (Weatherburn 2007) and it was reported as median. For dichotomous outcomes (risk of adverse events), we presented treatment effect as a risk ratio (RR). We presented effect estimates along with 95% confidence intervals (CI).
Unit of analysis issues
We included in our review only randomized controlled trials with a parallel‐group design. The issue of repeated measures is not relevant for the outcomes under investigation.
We planned, if the review included cluster‐randomized studies, to perform a sensitivity analysis that excludes cluster‐randomized studies to determine the impact of including them in the analysis. Our search did not find any cluster‐randomized trials.
Dealing with missing data
We performed quantitative analysis on an intention‐to‐treat (ITT) basis and planned to contact the study authors for missing data. Data for Zhao 2011, was converted from hours to days and the standard deviations (SD) calculated from the reported 95% CI.
Assessment of heterogeneity
We had planned not to perform meta‐analysis if we suspected important clinical heterogeneity on examination of the included studies. We used the Chi2statistic to test statistical heterogeneity between studies and considered a P value ≤ 0.10 as indicating significant heterogeneity; we used the I2 statistic to assess the magnitude of heterogeneity (Higgins 2002). We considered an I2 > 50% would indicate problematic heterogeneity between studies and in such case we would carefully consider the value of any pooled analysis. We planned to use a random‐effects model analysis if an I2 was greater than 30%. We planned to use a fixed‐effect model of analysis to determine the best estimate of the intervention effect. If the two did not coincide, we would not consider the random‐effects estimate as the actual intervention effect in the population under study. We constructed forest plots to summarize findings from the included studies.
Assessment of reporting biases
We undertook a comprehensive electronic search and a search of other sources such as trial registries, as described above, to minimize the effects of publication bias. We planned to construct a contour‐enhanced funnel plot to differentiate asymmetry due to publication bias. As we had less than 10 studies, funnel plots of effect estimates against their standard errors (on a reversed scale) were not created as per the guideline.
Data synthesis
We quantitatively reviewed the included data and combined the data by intervention, outcome and population using the Cochrane's statistical software (Revman 5.3). We synthesized the data only in the absence of important clinical or statistical heterogeneity, and we expressed pooled estimates of the mean difference for continuous variables and risk ratios for proportions.
We planned to use the inverse‐variance fixed‐effect method of meta‐analysis for continuous variables. For studies reporting median and range, we took estimation of the mean and standard deviation using the method described by Hozo and colleagues (Hozo 2005).
Had we identified cluster‐randomized studies, we planned to determine whether the results had been correctly analysed by using an appropriate method such as a multi‐level mode, variance component analysis or generalized estimating equations (GEEs). Had this been done, we would have included in the meta‐analysis the effect estimates from these studies and their standard errors.
If substantial heterogeneity was present, and if sufficient studies were available, we planned to perform a random‐effects meta‐analysis.
We have presented the results in the form of a forest plot.
Subgroup analysis and investigation of heterogeneity
When appropriate, with obvious clinical or statistical (I2 > 50%) heterogeneity, we planned to consider subgroup analysis based on participants with neurological injury, including:
head injury;
cardiopulmonary bypass; and
use of neuromuscular blocking agents.
if the data had indicated heterogeneity on that basis, patients with neurological injury were excluded from our selected studies. Not enough data were available to undertake subgroup analysis based on patients on cardiopulmonary bypass or the use of neuromuscular blocking agents.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the consistency of effect size measures in studies with low risk of bias versus those with high risk of bias. We did not perform a sensitivity analysis, as there were not enough studies included in the review.
'Summary of findings' table and GRADE
We present study findings in a standard 'Summary of findings' table (Table 1), which includes a list of all important outcomes; a measure of the typical burden of these outcomes; the absolute and relative magnitude of effect; the numbers of participants and studies addressing each outcome and a grade for the overall quality of the body of evidence for each outcome.
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes (intensive care unit length of stay, duration of mechanical ventilation and risk of adverse events (e.g. self‐extubation, unplanned disconnection of indwelling catheters)) and constructed Table 1 using GRADE software. The GRADE approach appraises the quality of a body of evidence according to the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of the body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
Results of the search
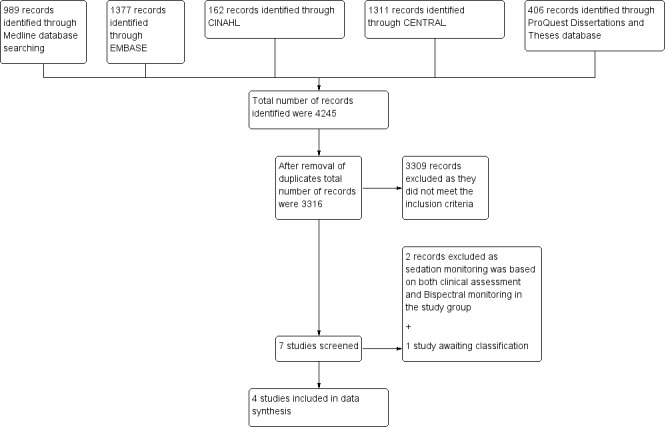
We identified 4245 possible studies from the initial search. From these studies we identified seven potentially relevant studies and retrieved them for further assessment (Figure 3).
3.
Study flow diagram.
Included studies
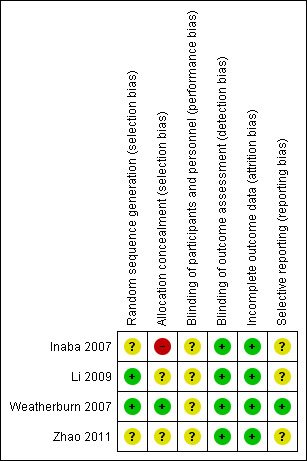
Of the seven identified studies, we included four trials with 256 participants (Inaba 2007; Li 2009; Weatherburn 2007; Zhao 2011) that fulfilled the inclusion criteria and compared Bispectral Index (BIS) versus clinical assessment (CA) method in monitoring sedation in adult mechanically ventilated Intensive care unit (ICU) participants. We excluded two studies because sedation monitoring was based on CA in addition to BIS monitoring in the intervention group and hence did not fit with the aim of our review (Binnekade 2009; Olson 2009). One study is awaiting classification (Ou 2016). In all the included studies, sedation was assessed with BIS monitoring in the intervention group. BIS monitoring was assessed hourly in all studies but one (Li 2009), where it was assessed four times in a 48‐hour period. In the control group, sedation was assessed using a variety of methods. In Inaba 2007, the Ramsay score was used, in Zhao 2011 , the Sedation Agitation Scale (SAS) was used, and in Li 2009, both the SAS and the Ramsay score were used. In Weatherburn 2007, sedation assessment was conducted clinically, based on heart rate, blood pressure, conscious level and pupillary size. In the control group, frequency of sedation assessment was conducted hourly in Inaba 2007 and Zhao 2011, four times in an 48‐hour period in Li 2009, and not reported in Weatherburn 2007.
Participants and settings
We reported full participant details in the Characteristics of included studies. All were single‐centre studies. Inclusion and exclusion criteria were fairly similar across studies. Main differences included study sample size (ranging from 18 (Inaba 2007), to 105 (Zhao 2011)), age (39.3 years in Zhao 2011, and 53 years in Weatherburn 2007), and duration of mechanical ventilation (immediate postoperative period in Inaba 2007 and longer than 12 hours in Weatherburn 2007 and Zhao 2011). Trials were conducted in different parts of the world; China (Li 2009; Zhao 2011), Japan (Inaba 2007), and Australia (Weatherburn 2007). Three of the four studies were published in languages other than English: two in Chinese (Zhao 2011; Li 2009), and one in Japanese (Inaba 2007).
Interventions
Intervention was sedation titration based on BIS monitoring. Target BIS score varied between studies; it was 40 to 70 in Inaba 2007, greater than 70 in Weatherburn 2007, 50 to 70 in Zhao 2011. Target BIS score was not mentioned in the Li 2009 study. There were large differences in the sedation protocol used in different studies. Both sedative drugs and administration methods varied. In Inaba 2007, fentanyl and propofol were administered as an infusion, in Li 2009, midazolam was given both as boluses and infusion, propofol and midazolam infusion were given in Zhao 2011. In Weatherburn 2007, morphine and midazolam were given, however the exact protocol was not described.
Control group
The same sedatives were given in the control group compared to intervention group in all the studies with similar bolus and infusion protocols. In Inaba 2007, the target Ramsay score was four to five, in Li 2009, the target SAS was three to four, but the target for Ramsay score was not described. In Zhao 2011, the target SAS was three to four. In Weatherburn 2007, the target for sedation with CA was not described. Muscle relaxants were used in both groups in Li 2009; no information was available about use of paralytics in other studies.
Funding sources
Funding sources for Weatherburn 2007 included Abbott Australasia and manufacturers of the device. Authors reported that funders of the study had no role in the study concept, design, data collection, data analysis, data interpretation or writing of the reports. Funding for Li 2009 was from Scientific and technological project Chengdu Sichuan. No information was given about the role of the funders. No information about funding was given for Inaba 2007 and Zhao 2011. Author conflict of interest was not reported in the studies.
Excluded studies
We excluded two studies as sedation monitoring was based on CA in addition to BIS monitoring in the study group and hence did not fit with the aim of our review (Binnekade 2009; Olson 2009) (Characteristics of excluded studies).
Studies awaiting classification
Ou 2016 is only published as an abstract, not enough data are provided for analysis. No contact details were provided for authors. Publishers when contacted did not provide authors' contact details.
Ongoing studies
We found no ongoing studies
Risk of bias in included studies
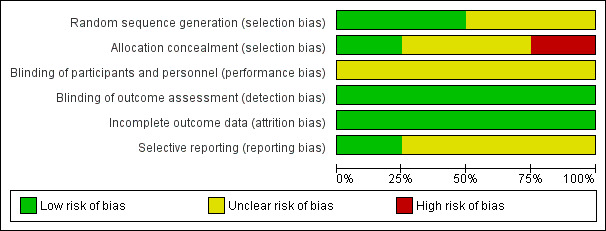
All studies were randomized controlled trials. Risk of bias has been described in the 'Risk of bias' table for each study (Characteristics of included studies). Figure 1 and Figure 2 summarize the risk of bias within and across studies, respectively.
Allocation
Allocation concealment was classified as 'low risk' in one study (Weatherburn 2007). Allocation concealment was classified as high risk in Inaba 2007 and unclear risk in Li 2009 and Zhao 2011.
Blinding
Because of the nature of the intervention, it was not possible to blind participants and personnel (performance bias). No information was reported about blinding of outcome assessment in any of the studies, but review authors judge that the outcome measurements of interest are unlikely to be influenced by lack of blinding of outcome assessment.
Incomplete outcome data
All four studies were classified as 'low risk' as all the participants completed the study and there was no loss to follow‐up.
Selective reporting
One study was classified as 'low risk' because they had published the protocol (Weatherburn 2007), and the study's pre‐specified (primary and secondary) outcomes were reported. The remaining three studies were classified as 'unclear risk' as we could not find a record in the trials registry.
Effects of interventions
See: Table 1
See Summary of findings table 1 (Table 1)
Primary outcomes
1. Intensive care unit (ICU) length of stay (LOS), measured in days
One study reported this outcome (N = 50) (Weatherburn 2007). There was no significant difference in ICU length of stay in days between the two arms of the study (Median (Interquartile Range IQR) 8 (4, 14) in the clinical assessment (CA) group; 12 (6, 18) in the BIS group; P = 0.20). ). The GRADE quality of evidence was downgraded by two levels to low due to concerns about imprecision (because of small size of the study and large confidence interval (CI)).
Secondary outcomes
1. Duration of mechanical ventilation, measured in days
This outcome was reported in two studies (N = 155) (Weatherburn 2007; Zhao 2011) (Analysis 1.1). The pooled analysis showed no effect in the duration of mechanical ventilation between the BIS monitoring group and the CA group (mean difference (MD) ‐0.02 days (95% CI ‐0.13 to 0.09; Chi2 = 0.01; I2= 0%). The GRADE quality of evidence was judged as low due to serious concerns about risk of bias (Zhao 2011, which carries 98.3% weight for this outcome, random sequence generation, allocation concealment and selective reporting were graded as unclear risk of bias) and imprecision (the difference in duration of mechanical ventilation is less than one day which is not clinically significant).
1.1. Analysis.
Comparison 1 Bispectral Index versus Clinical assessment, Outcome 1 Duration of mechanical ventilation.
2. Any cause mortality
This outcome was not reported in included studies.
3. Risk of ventilator‐associated pneumonia
This outcome was not reported in included studies.
4. Risk of adverse events
This outcome was reported by only one study (N = 105) (Zhao 2011). The number of patients with adverse events analysed included restlessness after suction, endotracheal tube resistance, pain tolerance during sedation and delirium after extubation. There was no significant difference between the two groups. Restlessness after extubation: risk ratio (RR) 1.11 (95% CI 0.90 to 1.37), endotracheal tube resistance: RR 0.96 (95% CI 0.75 to 1.22), pain tolerance during sedation: RR 0.99 (95% CI 0.89 to 1.10), delirium after extubation: RR 3 (95% CI 0.28 to 32.04), all P > 0.05. The GRADE quality of evidence was downgraded to very low due to serious concerns about risk of bias (random sequence generation, allocation concealment and selective reporting were assessed as unclear risk of bias), indirectness (clinically relevant adverse events were not reported) and imprecision (small number of patients in the study).
Other clinically important adverse events such as self‐extubation and unplanned disconnection of indwelling catheters were not reported.
5. Hospital LOS in days
This outcome was not reported in included studies.
6. Amount of sedative agents used
This outcome was reported in three studies (Inaba 2007; Weatherburn 2007; Zhao 2011, ). We could not pool results because the studies used different sedation protocols and sedative agents. Results are presented in Additional Table 2. The GRADE quality of evidence was judged as very low due to serious concerns about risk of bias (allocation concealment and selective reporting in Zhao 2011, and Inaba 2007 was assessed as either high risk or unclear risk), inconsistency (because of heterogeneity of data) and imprecision (effect estimate of amount of sedative agents used was imprecise).
1. Other Data.
| Study | BIS group | Clinical assessment group | |||||
| N | Mean (SD) | N | Mean (SD) | Mean difference | 95% CI | P value | |
| Inaba 2007 | |||||||
| Average propofol dose (mg/kg/hour) | 9 | 5.3 (1) | 9 | 5.1 (0.9) | 0.2 | ‐0.68, 1.08 | 0.670 |
| Time to eye opening (minutes) | 9 | 5.7 (5.7) | 9 | 4.1 (2.8) | 1.6 | ‐2.55, 5.75 | 0.771 |
| Time to consciousness (minutes) | 9 | 7.6 (5.3) | 9 | 7.6 (3.6) | 0 | ‐4.19, 4.19 | NA |
| Number of flow rate changes | 9 | 4.4 (2.5) | 9 | 3.6 (1.7) | 0.8 | ‐1.18, 2.78 | 0.779 |
| Number of boluses | 9 | 1.4 (2.3) | 9 | 0.89 (1.4) | 0.51 | ‐1.25,2.27 | 0.719 |
| Weatherburn 2007 | |||||||
| Mean morphine total daily dosage (mg) | 25 | 22.6* | 25 | 26.6* | 0.67 | ||
| Mean midazolam total daily dosage (mg) | 25 | 18.4* | 25 | 14.6* | 0.85 | ||
| Zhao 2011 | |||||||
| Mean midazolam dose (mg/kg/hour) | 42 | 0.10 (0.02) | 63 | 0.09 (0.02) | 0.01 | 0.00, 0.02 | 0.993 |
| Mean propofol dose (mg/kg/hour) | 42 | 0.95 (0.23) | 63 | 0.86 (0.20) | 0.09 | 0.00, 0.18 | 0.979 |
| Mean time to wake up (minutes) | 42 | 0* | 63 | 15* | <0.05 | ||
* Standard deviation not reported
7. Cost
This outcome was not reported in the included studies.
8. Longer‐term functional outcomes as reported by study authors
This outcome was not reported in included studies.
9. Quality of life as reported by study authors
This outcome was not reported in the included studies.
Discussion
This review includes randomized controlled trials (RCTs) comparing bispectral index (BIS) monitoring versus clinical assessment (CA) for sedation in mechanically ventilated adult intensive care unit (ICU) patients. We collected data on clinically relevant outcomes such as ICU length of stay (LOS), which was the primary outcome and the secondary outcomes such as duration of mechanical ventilation, any‐cause mortality, risk of ventilator‐associated pneumonia (VAP), risk of adverse events, hospital LOS, amount of sedative agents used, cost, longer‐term functional outcomes and quality of life. Data on the primary and secondary end points were available for only ICU LOS, duration of mechanical ventilation, risk of adverse events and amount of sedative agents used.
Summary of main results
Our primary objective was to assess the effect of mode of sedation assessment on ICU LOS. Evidence from one study (Weatherburn 2007), with 50 participants showed no statistically and clinically significant difference between the BIS monitoring and CA group. The GRADE quality of evidence was low for this outcome.
Of our secondary objectives, only duration of mechanical ventilation, risk of adverse events and amount of sedative agents used were reported. Two studies (155 participants) reported the duration of mechanical ventilation (Weatherburn 2007; Zhao 2011), with no significant difference between the groups (GRADE Low quality of evidence). The number of patients with adverse events (restlessness after suction, endotracheal tube resistance, pain tolerance during sedation and delirium after extubation) was reported in only one study (105 participants) (Zhao 2011). There was no statistically significant difference between the two groups (GRADE very low quality of evidence). Adverse events of interest for the review, such as self‐extubation and unplanned disconnection of indwelling catheters, were not reported. Three studies (173 participants) reported the amount of sedative agents used (Inaba 2007; Weatherburn 2007;Zhao 2011). The studies used different sedation protocol and sedative agents; therefore it was not possible to pool results (GRADE very low‐quality of evidence)(Table 2).
Overall completeness and applicability of evidence
Our protocol proposed the following outcomes: ICU LOS, duration of mechanical ventilation, any cause mortality, risk of VAP, risk of adverse events, hospital LOS, amount of sedative agents used, cost, long‐term functional outcomes and quality of life. The outcomes we sought are consistent with the recommended four core areas of outcomes: death, life impact, pathological manifestations, and resource used by other specialties such as rheumatology (The OMERACT Handbook 2014). Most of the studies included in our review did not report many of these outcomes. However some of the outcomes even though reported were not defined (duration of mechanical ventilation), or they used different methods of measurements (sedation) leading to the possibility of inconsistency in outcomes between trials. Development and utilization of core outcome sets (COS) may help to prevent these issues in the future. Several COS for critical care research are still in various stages of development (Blackwood 2015).
There are some outcomes, which were not mentioned in the protocol, but may be of importance for patients on sedation in ICU. Posttraumatic stress disorder (PTSD) is one such example. Systematic review of studies has shown that one‐fifth of general ICU survivors have either substantial PTSD symptoms or clinician‐diagnosed PTSD (Davydow 2008). Another systematic review showed that early post‐ICU memories of in‐ICU frightening or psychotic experiences were associated with increased risk of post‐ICU PTSD in over 80% of the studies that examined this factor (Parker 2015). Therefore PTSD may be a useful outcome to look for in studies assessing depth of sedation monitoring. Delirium and mild cognitive impairment in ICU survivors may be other useful outcome measures.
Quality of the evidence
Our review included four studies with 256 patients. Only one study (Weatherburn 2007) was judged to be at low risk of bias. Other studies were judged to be at high risk of bias. The GRADE quality of evidence ranked from low to very low across the different outcomes. Methodological limitations of the studies included small numbers (256 patients), risk of bias (random sequence generation, allocation concealment and selective reporting), inconsistency (duration of mechanical ventilation not defined) and imprecision (large confidence interval).
External validity of this review may be limited because there was a large heterogeneity in the patient population. Zhao 2011 and Inaba 2007 enrolled patients who were admitted postoperatively and required ventilation for less than 24 hours, whereas Weatherburn 2007 included patients from a mixed medical‐surgical ICU who required ventilation for longer duration of time.
Potential biases in the review process
We followed the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane 2008). The eligibility for inclusion and exclusion and assessment for risk of bias was carried out independently by two review authors (RS, AB). In our protocol (Shetty 2014), we stated that we would include all adults (18 years of age or older) undergoing mechanical ventilation in ICU for longer than 24 hours, irrespective of the admission diagnosis. We made two changes to this section. We removed the criterion: "longer than 24 hours" because three of the four included studies otherwise could not fulfil the criteria. We changed "18 years of age or older" to only 'adults' because all of the included studies mentioned adults, but did not provide the exact range and we were unable to obtain additional data from the study authors. Hence the criteria for types of participants now reads "We included all adults undergoing mechanical ventilation in an ICU, irrespective of the admission diagnosis" (Differences between protocol and review).There were no other major departures from the protocol (Shetty 2014), that could have affected our findings or introduced any risk of bias. However difference in duration of mechanical ventilation less than one day is clinically insignificant. Hence inclusion of three more studies with less than 24 hours of mechanical ventilation may not result in clinically significant difference in duration of mechanical ventilation.
Agreements and disagreements with other studies or reviews
Our Cochrane review compared BIS monitoring versus clinical assessment for sedation in mechanically ventilated adult ICU patients. BIS monitoring and clinical assessment versus clinical assessment alone was investigated in two recently published meta‐analysis/systematic reviews (Bilgili 2017; Finger 2016). In these reviews there was no benefit of adding BIS monitoring to clinical assessment. Also ICU LOS was actually better in the control group (mean difference (MD) 1.4; 95% confidence interval (CI) 0.29, 0.5; P = 0.01) indicating addition of BIS monitoring to usual clinical monitoring could be harmful (Finger 2016). In our review median ICU LOS was four days higher in the BIS monitoring group even though this was not statistically significant. We are not aware of any other systematic review or meta‐analysis comparing BIS monitoring versus clinical assessment in this patient group. The American College of Chest Physicians, American College of Critical Care Medicine, Society of Critical Care Medicine, and the American Society of Health System Pharmacists clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill patient (Barr 2013), recommend that the routine use of BIS is not recommended (moderate quality of evidence rated as strongly against the intervention).
The benefits of BIS monitoring in patients undergoing general anaesthesia for surgical procedures have been confirmed by a Cochrane review (Punjasawadwong 2014). This benefit is not shown in our review. The reason for this may be the difference in level of target sedation (anaesthesia needs deeper level of sedation). Also endpoints are different; the aim in anaesthesia is avoiding awareness, whereas target of ICU sedation is keeping patient alert and calm to lightly sedated and hence the patient is always aware.
There is evidence to show that muscular activity may affect BIS values (Dahaba 2005). The magnitude of BIS overestimation significantly correlates to both BIS and electromyographic activity before neuromuscular blockade (Vivien 2003). BIS monitoring may be a reasonable approach in assessing depth of sedation in ICU patients receiving neuromuscular paralysis. However, no studies so far have looked at outcome benefits in this group of patients.
Authors' conclusions
Implications for practice.
We found insufficient evidence about the effects of bispectral index (BIS) monitoring compared with clinical assessment (CA) of sedation in mechanically ventilated adults in the intensive care unit (ICU). The findings are uncertain due to the low and very low quality evidence derived from a limited number of studies.
Implications for research.
We could not show any benefits of BIS monitoring compared with CA of sedation in mechanically ventilated adults in the ICU. However in certain patient populations it is not possible to perform CA to monitor depth of sedation optimally. Examples include patients who are paralysed. Muscular activity affects BIS values and BIS scores are not overestimated in paralysed patients because of absent muscular activity. A well‐conducted large multi‐centre randomized controlled trial in this specific patient population looking into clinically relevant outcomes, including posttraumatic stress disorder (PTSD) and delirium would clarify further areas of doubt about benefits with the use of this monitoring.
What's new
| Date | Event | Description |
|---|---|---|
| 3 January 2019 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 8, 2014 Review first published: Issue 2, 2018
| Date | Event | Description |
|---|---|---|
| 26 February 2018 | Amended | Typo corrected in acknowledgement section |
Acknowledgements
We would like to thank Jane Cracknell for her editorial support throughout the study.
We thank Liz Bickerdike (editor Cochrane Editorial Unit), Bronagh Blackwood (content editor), Vibeke E Horstmann (statistical editor), Aaron M Joffe, Douglas Coursin, Frank Sasse, Yodying Punjasawadwong, Michael O'Connor (peer reviewers), Janet Wale (consumer editor) and Heather Maxwell (copy editor) for their help and editorial advice during the preparation of this systematic review.
We thank Karen Hovhannisyan for the initial formulation of the search strategy and initial database search and Monika Afzali for her help with translation.
We thank Gonzalo De La Cerda, Sarah Stowell and Nathan Pace (statistical editor) for their help in preparing the protocol (Shetty 2014).
We thank Professor Ling Zhang, Hong Zheng and Lei Rocky for the help with the translation and data extraction from Chinese articles.
We thank Mina Nishimori, Chiho Otani and Yuki Takao for the help with the translation and data extraction from Japanese article.
We thank Celia Burnett for her help with database search.
Appendices
Appendix 1. Search strategy for CENTRAL
#1 MeSH descriptor: [Electroencephalography] explode all trees #2 (EEG or BIS or electroence*):ti,ab or (brain near monitor*) or bispectral index:ti,ab #3 #1 or #2 #4 MeSH descriptor: [Intensive Care] explode all trees #5 MeSH descriptor: [Intensive Care Units] explode all trees #6 MeSH descriptor: [Critical Care] explode all trees #7 MeSH descriptor: [Respiration, Artificial] explode all trees #8 MeSH descriptor: [Ventilators, Mechanical] explode all trees #9 MeSH descriptor: [Propofol] explode all trees #10 MeSH descriptor: [Conscious Sedation] explode all trees #11 ((intensive or critical) near (care or unit*)):ti,ab or sedat*:ti,ab or (ventilat* near (mechanical* or intub*)):ti,ab #12 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 #13 #3 and #12 #14 (child* not (adult* and child*)) #15 #13 not #14
Appendix 2. MEDLINE (Ovid SP) search strategy
1. exp Electroencephalography/ or (EEG or BIS or electroence*).ti,ab. or (brain adj3 monitor*).mp. or bispectral index.mp. 2. Intensive Care/ or Intensive Care Units/ or Critical Care/ or (ICU or ITU or ((intensive or critical) adj3 (care or unit*))).ti,ab. or Respiration, Artificial/ or Ventilators, Mechanical/ or Propofol/ or Conscious Sedation/ or sedat*.ti,ab. or (ventilat* adj3 (mechanical* or intub*)).mp. 3. ((randomised controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 4. (child* not (adult* and child*)).af. 5. (1 and 2 and 3) not 4
Appendix 3. Embase (Ovid SP) search strategy
1. exp electroencephalography/ or (EEG or BIS or electroence*).ti,ab. or (brain adj3 monitor*).ti,ab. or bispectral index.ti,ab. 2. intensive care/ or intensive care unit/ or (ICU or ITU or ((intensive or critical) adj3 (care or unit*))).ti,ab. or artificial ventilation/ or mechanical ventilator/ or propofol/ or conscious sedation/ or sedat*.ti,ab. or (ventilat* adj3 (mechanical* or intub*)).ti,ab. 3. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. 4. (child* not (adult* and child*)).af. 5. (1 and 2 and 3) not 4
Appendix 4. CINAHL (EBSCOhost) search strategy
S1 (MH "Electroencephalography") OR ( (EEG or BIS or electroence*) or (brain N3 monitor*) or bispectral index ) S2 AB ( ((intensive or critical) N3 (care or unit*)) or sedat* or (ventilat* N3 (mechanical* or intub*)) ) OR ( (MH "Critical Care") OR (MH "Intensive Care Units") OR (MH "Respiration, Artificial") OR (MH "Ventilators, Mechanical") OR (MH "Propofol") OR (MH "Conscious Sedation") ) S3 (random* or ((clinical or controlled) N3 trial*) or placebo* or prospective* or crossover or multicenter) or ((blind* or mask*) N3 (single or double or triple or treble)) S4 (child* not (adult* and child*)) S5 (S1 or S2 or S3) not S4
Appendix 5. Details of literature search process
Dates searches were undertaken
Medline 30th May 2017
EMBASE and CINAHL 30th May 2017
CENTRAL 10th June 2017
ProQuest Dissertation and Theses Database 10th June 2017
OpenGrey 11th June 2017
SciSearch 11th June 2017
Clinicaltrials.gov and controlled‐trials.com 10th June 2017
WHO International Clinical Trials Registry platform 10th June 2017
1. ProQuest search strategy
Electroence* OR bis* AND (Intensive care) OR (critical care) OR ventilat* OR respirat* AND propofol OR sedat*
2. OpenGrey search strategy
Bispectr* OR Intensi* OR Critica* OR Sedat*
3. SciSearch search strategy
Bispectr* OR Intensi* OR Critica* OR Sedat*
4. Other sources search strategy
We adopted our ProQuest search strategy in searching all other databases.
Other databases searched include,
Clinicaltrials.gov,
Controlledtrials.com (ISRTCN registry) and
WHO International Clinical Trials Registry Platform Search portal
Appendix 6. ACE study selection and data extraction form
| Review title or ID |
| Study ID(surname of first author and year first full report of study was published e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
|
Notes: |
1. General information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/abstract/report that data are extracted from) |
|
|
Report ID (ID for this paper/abstract/report) |
|
| Reference details | |
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
|
Notes: | |
| First author | Journal/Conference proceedings, etc. | Year |
| |
2. Study eligibility
| Study characteristics |
Eligibility criteria (insert eligibility criteria for each characteristic as defined in the Protocol) |
Yes | No | Unclear |
Location in text (pg & ¶/fig/table) |
|||||||
| Type of study | Randomized controlled trial | |||||||||||
| Controlled clinical trial |
||||||||||||
| Cluster‐randomized trials | ||||||||||||
|
Participants |
Adult patients (18 years of age or older) undergoing mechanical ventilation in an intensive care unit for longer than 24 hours |
|||||||||||
| Types of intervention | BIS monitoring used |
|
||||||||||
| Sedation protocol used |
||||||||||||
| Clinical method used to assess levels of sedation (clinical judgement or specific clinical sedation scoring tool) in the control arm with or without use of a titration protocol | ||||||||||||
| Types of outcome measures | Intensive care unit (ICU) length of stay | |
||||||||||
| Duration of mechanical ventilation | |
|||||||||||
| Longer‐term functional outcomes as reported by study authors | ||||||||||||
| INCLUDE | EXCLUDE | |||||||||||
|
Reason for exclusion |
||||||||||||
| Notes: | ||||||||||||
| Do not proceed if any of the above answers are ‘No.’ If study is to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies.’ |
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
|
Description (include comparative information for each group (i.e. intervention and controls) if available) |
Location in text (pg & ¶/fig/table) |
||||
|
Population description (from which study participants are drawn) |
|||||
| Country where the study was conducted | |||||
|
Setting (including location and social context) |
|||||
| Number of beds in the hospital | |||||
| Number of beds in the ICU | |||||
| Percentage of ventilated beds | |||||
| Nurse‐to‐patient ratio | |||||
| Number of patients admitted to ICU each year | |||||
|
Type of ICU |
Surgical Medical Cardiac Trauma Neurological Burn Other, specify: |
||||
| Inclusion criteria | |||||
| Exclusion criteria | |||||
| Method/s of recruitment of participants | |||||
|
Informed consent obtained |
Yes No Unclear |
||||
| Notes: | |||||
4. Methods
|
Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Aim of study | ||||||
| Design(e.g. parallel, cross‐over, cluster) | ||||||
| Single‐centre/Multi‐centre | ||||||
|
Unit of allocation (by individuals, clusters/groups or body parts) |
||||||
| Start date | |
|||||
| End date | |
|||||
|
Total study duration |
||||||
|
Severity of illness scoring system used |
APACHE SAPS SOFA AIS ISS TISS MPM MODS Other, specify: |
|||||
| Diagnosis | ||||||
|
Sedatives used (name, dosage, range, number and % of patients receiving this drug) |
||||||
| Administration of sedatives | Continuous Bolus |
|||||
| Total number of sedative agents used with unit of measurement | ||||||
| Paralytics used in both groups | Yes No Unclear |
|||||
| Method of sedation assessment used for control group | Sedation and agitation scale (SAS) Visual analogue scale (VAS) Train of Four (TOF) in patient on paralysis Richmond Agitation and Sedation Scale (RASS) Observer's assessment of agitation and sedation Ramsey sedation scale Modified Ramsey sedation scale Cook Motor activity assessment scale (MAAS) Vancouver interactive and calmness scale Adaptation to intensive care environment Minnesota Sedation and Assessment Tool Score of the UK Intensive Care Society Sheffield Bloomsbury Local scoring system Other, specify: |
|||||
| Ethical approval needed/obtained for study | Yes No Unclear |
|||||
| Notes: | ||||||
5. 'Risk of bias' assessment
SeeChapter 8of the Cochrane Handbook for Systematic Reviews of Interventions.
| Domain |
Risk of bias |
Support for judgement |
Location in text (pg & ¶/fig/table) |
||
| Low risk | High risk | Unclear risk | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of participants and personnel (performance bias) |
Outcome group: all/ |
||||
| (if required) |
Outcome group: |
||||
|
Blinding of outcome assessors (detection bias) |
Outcome group: all/ |
||||
| (if required) |
Outcome group: |
||||
|
Incomplete outcome data (attrition bias) |
|||||
|
Selective outcome reporting? (reporting bias) |
|||||
|
Other bias |
|||||
|
Notes: | |||||
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all participants in a trial are analysed according to the intervention to which they were allocated, whether or not they received it . | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
Were withdrawals described? Yes No Not clear
Discuss if appropriate…………………………………………………………………………………………
6. Participants
Provide overall data and, if available, comparative data for each intervention and comparison group.
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Total no. randomly assigned (or total population at start of study for NRCTs) |
||
|
Clusters (if applicable, no., type, no. people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age (mean, median, range, etc.) | ||
| Sex (number/%, etc.) | ||
| Race/Ethnicity | ||
| Severity of illness | ||
| Diagnosis | ||
| Co‐morbidities | ||
| Past history of delirium or dementia | ||
| Other treatment received(additional to study intervention) | ||
|
Discharge destination |
Home Rehabilitation facility Skilled nursing facility (nursing home) Long‐term acute care hospital Other, specify: |
|
| Other relevant sociodemographics | ||
| Subgroups measured | ||
| Subgroups reported | ||
| Notes: | ||
7. Intervention groups
Copy and paste table for each intervention and comparison group.
Intervention group 1
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Group name |
||
|
No. randomly assigned to group (specify whether no. people or clusters) |
||
| Theoretical basis(include key references) | ||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| BIS version | ||
| BIS mean, range, etc. | ||
| BIS measurement at each sedation score and correlation | ||
| Hours on BIS | ||
|
Confounders that may effect BIS reading (aminophylline, catecholamines, ketamine, electrical/non‐electrical EMG interference, hypoglycaemia, sleep, sound, temperature, excessive muscle movement) |
||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity etc., if relevant) |
||
| Co‐interventions | ||
| Economic variables (i.e. intervention cost, changes in other costs as result of intervention) | ||
|
Resource requirements to replicate intervention (e.g. staff numbers, cold chain, equipment) |
||
| Notes: | ||
8. Outcomes
|
Outcomes relevant to your review (copy and paste from ‘Types of outcome measures’) | |
| Reported in paper (circle) | |
| Intensive care unit (ICU) length of stay | Yes / No |
| Duration of mechanical ventilation | Yes / No |
| Any‐cause mortality | Yes / No |
| Risk of ventilator‐associated pneumonia | Yes / No |
| Risk of adverse events (self‐extubation, unplanned disconnection of indwelling catheters, etc.) | Yes / No |
| Hospital length of stay | Yes / No |
| Quality of life | Yes / No |
| Longer‐term functional outcomes as reported by study authors | Yes / No |
| Cost | Yes / No |
| Total amount of sedative agents used | Yes / No |
Intensive care unit (ICU) length of stay
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name |
|||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Duration of mechanical ventilation
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Any‐cause mortality
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Risk of ventilator‐associated pneumonia
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Risk of adverse events (e.g. self‐extubation, unplanned disconnection of indwelling catheters)
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Hospital length of stay
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Amount of sedative agents used
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
| Notes: | |||
Cost
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Longer‐term functional outcomes, as reported by study authors
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
Quality of life
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Outcome name | |||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
9. Results
Intensive care unit (ICU) length of stay
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post intervention or change from baseline? | ||||||||||
| Results | Intervention | Control | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| Overall result (comparison) | ||||||||||
| Mean difference | Standard error | 95% confidence interval | ||||||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Any other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||
| Reanalysed results | ||||||||||
|
Notes: |
||||||||||
Duration of mechanical ventilation
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post intervention or change from baseline? | ||||||||||
| Results | Intervention | Control | ||||||||
| Median | IQR (or other variance) | No. participants | Median | IQR (or other variance) | No. participants | |||||
| Overall result (comparison) | ||||||||||
| Mean or median difference | Standard error (or other variance) | 95% confidence interval | ||||||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
| Any other results reported | ||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||
| Reanalysed results | ||||||||||
|
Notes: |
||||||||||
Any‐cause mortality
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Control | ||||
| Risk | Number of participants | Risk | Number of participants | |||
| Overall result (comparison) | ||||||
| Risk ratio (relative risk) | Standard error (or other variance) | 95% confidence interval | ||||
| No. participants | Intervention | Control | ||||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, clusters/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
|
Notes: | ||||||
Risk of ventilator‐associated pneumonia
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Control | ||||
| Risk | Number of participants | Risk | Number of participants | |||
| Overall result (comparison) | ||||||
| Risk ratio (relative risk) | SE (or other variance) | 95% confidence interval | ||||
| No. participants | Intervention | Control | ||||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, clusters/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
|
Notes: | ||||||
Risk of adverse events (e.g. self‐extubation, unplanned disconnection of indwelling catheters)
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Control | ||||
| Risk | Number of participants | Risk | Number of participants | |||
| Overall result (comparison) | ||||||
| Risk ratio (relative risk) | Standard error (or other variance) | 95% confidence interval | ||||
| No. participants | Intervention | Control | ||||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, clusters/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
|
Notes: | ||||||
Hospital length of stay
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post intervention or change from baseline? | ||||||||||
| Results | Intervention | Control | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| Overall result (comparison) | ||||||||||
| Mean difference | Standard error | 95% confidence interval | ||||||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
|
Any other results reported |
||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||
| Reanalysed results | ||||||||||
|
Notes: |
||||||||||
Amount of sedatives used
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post intervention or change from baseline? | ||||||||||
| Results | Intervention | Control | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| Overall result (comparison) | ||||||||||
| Mean difference | Standard error | 95% confidence interval | ||||||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
|
Any other results reported |
||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||
| Reanalysed results | ||||||||||
|
Notes: |
||||||||||
Cost
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| Overall result (comparison) | ||||||||||
| Mean difference | Standard error | 95% confidence interval | ||||||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
|
Any other results reported |
||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||
| Reanalysed results | ||||||||||
|
Notes: |
||||||||||
Longer‐term functional outcomes, as reported by study authors
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post intervention or change from baseline? | ||||||||||
| Results | Intervention | Control | ||||||||
| Mean or median | SD (or other variance) | No. participants | Mean or median | SD (or other variance) | No. participants | |||||
| Overall result (comparison) | ||||||||||
| Mean difference | Standard error | 95% confidence interval | ||||||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
|
Any other results reported |
||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||
| Reanalysed results | ||||||||||
| Notes: | ||||||||||
Quality of life
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post intervention or change from baseline? | ||||||||||
| Results | Intervention | Control | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| Overall result (comparison) | ||||||||||
| Mean difference | Standard error | 95% confidence interval | ||||||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
|
Any other results reported |
||||||||||
|
Unit of analysis (individuals, clusters/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||
| Reanalysed results | ||||||||||
|
Notes: |
||||||||||
Other outcomes
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Correlation with propofol, morphine and midazolam dose |
10. Applicability
| Have important populations been excluded from the study?(consider disadvantaged populations and possible differences in the intervention effect) | Yes No Unclear |
|
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | Yes No Unclear |
|
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear |
|
|
Notes: | ||
11. Other information
References to trial
Check other references identified in searches. If further references to this trial are identified, link the papers now and list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference proceedings, etc. | Year |
| A | Paper listed above | ||
| B | Further papers | ||
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | ||
|
Notes: | ||
|
Other information that you feel is relevant to the results Indicate whether any data were obtained from the primary author; and whether results were estimated from graphs, etc., or were calculated by you using a formula (this should be stated and the formula given). In general, if results not reported in paper(s) are obtained, this should be made clear here to be cited in the review. |
| |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal/Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
Data and analyses
Comparison 1. Bispectral Index versus Clinical assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of mechanical ventilation | 2 | 155 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Inaba 2007.
| Methods | Single‐centre randomized controlled trial. Study period: March 2003 to June 2003 | |
| Participants | Total number of patients 18. All males, age less than 75 years. Undergoing mechanical ventilation until 6:30 AM next day after head and neck surgery in an ICU in Japan. Patients admitted after brain surgery excluded. No critical illness severity score reported. ASA 1‐2 Sedation protocol used: Fentanyl 10 mcg/kg/hour, propofol 6 mg /kg/hour to 10 mg/kg/hour. Target Bispectral Index (BIS) score 40‐70, target Ramsay score 4‐5. Propofol titrated as per BIS monitoring or Ramsay score |
|
| Interventions | BIS monitoring (N = 9) versus Ramsay score (N = 9). Frequency of monitoring every hour | |
| Outcomes | Apart from average propofol dose, no other primary or secondary outcome of interest for the review was reported. Other outcome reported include time to eye‐opening, time to consciousness, number of flow rate changes and number of boluses | |
| Notes | Study funding sources not specified No possible conflict of interest reported We were unable to contact the study authors for more details as no email ID was found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to both BIS and Ramsay" Comment: Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | High risk | Comment: No information given about allocation concealment. Probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned, however not possible to blind in this type of study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | They were probably aware of the allocation, but review authors judge that the outcome reported is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there was no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No published protocol available, however all outcomes mentioned in the methods are reported |
Li 2009.
| Methods | Single‐centre randomized controlled trial. Study period: March 2004 to May 2008 | |
| Participants | Adult patients in general intensive care under mechanical ventilation at the Third People's Hospital of Chengdu in China. Total number of patients 83. Sex not reported. Mean age in years Bispectral Index (BIS) monitoring group 66.23 +/‐ 19.60 and Clinical assessment group 64.07+/‐18.26, APACHE II BIS monitoring group 23.70+/‐2.71 and Clinical assessment group 23.60 +/‐ 2.92. Sedation protocol: Midazolam 2‐5 mg every 5‐15 minutes until sedation target reached and then 0.1 mg/kg/hour. Paralytics were used in both groups when necessary. Target Sedation Agitation Scale (SAS) 3‐4, targets for BIS monitoring and Ramsay scores not found | |
| Interventions | BIS monitoring (N = 42) versus SAS and Ramsay (N = 41). Assessment was recorded before sedation, immediately after sedation, 16, 32 and 48 hours after sedation | |
| Outcomes | No primary or secondary outcome of interest for the review was reported. Other outcomes reported include respiratory rate, circulation, sedation depth, fraction of inspired oxygen, pulse saturation of oxygen before and after sedation | |
| Notes | It was a feasibility study and conclusion was BIS monitoring is feasible for assessing the depth of sedation in mechanically ventilated adult ICU patients. Study funding source from Scientific and technological project, Chengdu Sichuan. No possible conflict of interest reported Contacted authors for more details but no data provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:The random numbers generated by computer were randomly divided into BIS monitoring group and routine group" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk'. No information given about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned, however not possible to blind in this type of study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information given about blinding of outcome assessment, but review authors judge that the outcome reported is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there was no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No published protocol available, however all outcomes mentioned in the methods are reported |
Weatherburn 2007.
| Methods | Single‐centre randomized controlled trial. Study period: September 2004 to July 2005 | |
| Participants | Adult mechanically ventilated patients in a surgical and general ICU at the Alfred Hospital, a tertiary level teaching hospital in Melbourne, Australia . Total 50 patients, 66% male, mean age 53 years, median APCHE II score was 14. Sedation protocol: Not described. Sedative agents used were morphine and midazolam. Target Bispectral Index (BIS) score greater than 70 | |
| Interventions | BIS monitoring (N = 25) versus Clinical assessment (N = 25). BIS monitoring readings were recorded hourly. Clinical assessment was done by nurses based on heart rate, blood pressure, conscious level and pupillary size, however frequency of monitoring is not mentioned | |
| Outcomes | Intensive care unit length of stay, duration of mechanical ventilation, amount of sedative agents administered (total daily dosage of morphine and midazolam with mean and range), were reported, no other secondary outcome of interest for the review was reported | |
| Notes | Funding sources included Abbott Australasia and BIS monitors and sensors from the manufacturers. The supporters of the study had no role in the study concept, design, data collection, data analysis, data interpretation or writing of the reports. No conflict of interest reported Contacted authors for more details, author not working in the institution any more and study archived hence no details available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Patients were randomized using sealed opaque pre‐coded envelopes Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: Patients were randomized using sealed opaque pre‐coded envelopes Comment: Done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned, however not possible to blind in this type of study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information given about blinding of outcome assessment, but review authors judge that the outcome reported is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all patients reported |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes were reported . |
Zhao 2011.
| Methods | Single‐centre randomized controlled trial. Study period: March 2008 to February 2009 | |
| Participants | Adult patients aged 18‐60 years after operation receiving mechanical ventilation for longer than 12 hours in an ICU at Beijing Tongren Hospital in China. Total number of patients 105, Male 96.2%, mean age 39.3+/‐9.5 years, APACHE I Bispectral Index (BIS) monitoring group 3.57+/‐2.60 and Clinical assessment group 4.19+/‐2.30 | |
| Interventions | BIS monitoring (N=42) versus Sedation Agitation Scale (SAS) (N = 63) recorded every hour. Sedation protocol: Midazolam 0.10 mg/kg/hour and propofol 1 mg/kg/hour. Target BIS score 50‐70, target SAS grade 3‐4 | |
| Outcomes | Duration of mechanical ventilation, adverse events and amount of sedation (mean midazolam and propofol dose with standard deviation) reported. No other primary or secondary outcome of interest for review reported. Adverse events reported include restlessness after suction, endotracheal tube resistance, pain tolerance during sedation and delirium after extubation. Other outcomes reported include sedation time and time to wake up | |
| Notes | No information given about study funding sources No possible conflict of interest reported Contacted authors for more details but no reply was received from the authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly divided into two groups" Comment: Insufficient information to permit judgement of 'low risk' or 'high risk'. No information given about method of randomizations |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients enrolled in this study were divided into groups using the envelop method" Comment: No information given about whether envelope was opaque or sealed etc |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned, however not possible to blind in this type of study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information given about blinding of outcome assessment, but review authors judge that the outcome reported is not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there was no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No published protocol available, however all outcomes mentioned in the methods are reported |
APACHE= acute physiology and chronic health evaluation (an illness severity scoring system used for intensive care patients); BIS = Bispectral index ; ICU= intensive care unit ; N= number; SAS = Sedation Agitation Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Binnekade 2009 | Excluded as sedation monitoring was based on clinical assessment in addition to Bispectral Index monitoring in the study group |
| Olson 2009 | Excluded as sedation monitoring was based on clinical assessment in addition to Bispectral Index monitoring in the study group |
Characteristics of studies awaiting assessment [ordered by study ID]
Ou 2016.
| Methods | Prospective randomized trial |
| Participants | 60 adults (18‐65 years) mechanically ventilated for more than 48 hours in the induction, maintenance and recovery phase of sedation. |
| Interventions | BIS monitoring versus Sedation Agitation Scale (SAS). Details of sedation protocol not reported. Target BIS score 60‐70. Target SAS grade 3‐4. |
| Outcomes | Primary outcome in the induction phase was haemodynamic changes and in the maintenance and recovery phase was total dose of sedative used. In the induction phase SAS monitoring was associated with more stable haemodynamics (less hypotension and bradycardia). In the maintenance and recovery phase, BIS resulted in a marked reduction in the total dose of propofol and fentanyl but higher use of midazolam. Secondary outcomes (ICU mortality, ICU LOS, length of mechanical ventilation and serious adverse events) were similar between two groups. |
| Notes | Study only published as an abstract, not enough data provided for analysis. No contact details were provided for authors. Publishers when contacted did not provide authors' contact details. |
BIS = Bispectral index; ICU= intensive care unit; LOS = length of stay
Differences between protocol and review
We made the following changes to the published protocol (Shetty 2014)
Our protocol stated that we would include adult patients (18 years of age or older) undergoing mechanical ventilation in ICU for longer than 24 hours, irrespective of the admission diagnosis. We made two changes to this. For duration of mechanical ventilation, the "longer than 24 hours" criterion was removed because three of the four studies otherwise could not be included. The "18 years of age or older" criterion was changed to only 'adult patients' because all of the included studies mentioned adults but did not provide the exact range and we were unable to obtain additional data from the study authors.
The Objective section was changed from "To assess the effects of Bispectral Index (BIS) monitoring compared with clinical sedation assessment on mortality, duration of mechanical ventilation, intensive care unit (ICU) and hospital length of stay (LOS), ventilator‐associated pneumonia, adverse events, amount of sedative agents used, cost and longer‐term functional outcomes and quality of life as reported by study authors for mechanically ventilated adult study participants in the ICU" to "To assess the effects of BIS monitoring compared with clinical sedation assessment on Intensive care unit (ICU) length of stay (LOS), duration of mechanical ventilation, any cause mortality, risk of ventilator‐associated pneumonia (VAP), risk of adverse events (e.g. self‐extubation, unplanned disconnection of indwelling catheters), hospital length of stay, amount of sedative agents used, cost, longer‐term functional outcomes as reported by authors and quality of life as reported by authors for mechanically ventilated adult study participants in the ICU" as it was a typographical error.
In the secondary outcomes "Number of sedative agents used" is changed to "Amount of sedative agents used' as it was a typographical error.
Gonzalo De Cerda and Sarah Stowell's name have been removed from the author list and Arunkumar Namachivayam's name has been added.
Contributions of authors
Rajesh M Shetty (RS), Antonio Bellini (AB), Dhuleep Wijayatilake (DW), Mark A Hamilton (MH), Rajesh Jain (RJ), Arunkumar Namachivayam (AK), Sunil Karanth (SK)
Conceiving of the review: RS.
Co‐ordinating the review: RS, AB.
Undertaking manual searches: RS, AB.
Screening search results: RS, AB.
Organizing retrieval of papers: RS, AB, RJ.
Screening retrieved papers against inclusion criteria: RS, AB.
Appraising quality of papers: RS, AB, AK
Abstracting data from papers: RS, AB, AK
Writing to authors of papers for additional information: RS.
Providing additional data about papers: RS, AB.
Obtaining and screening data on unpublished studies: RS.
Managing data for the review: RS, AB, AK.
Entering data into Review Manager (Revman 5.3): RS, AK.
Calculating RevMan statistical data: RS, AK.
Performing other statistical analysis not using RevMan: RS, AK.
Interpreting data: RS, AB, AK.
Making statistical inferences: RS, AK.
Writing the review: RS.
Securing funding for the review: RS, DW, AB, RJ.
Performing previous work that served as the foundation of the present study: RS.
Serving as guarantor for the review (one author): RS.
Taking responsibility for reading and checking the review before submission: RS, AB, DW, RJ, SK, AK.
Sources of support
Internal sources
No sources of support supplied
External sources
Own funding from authors, UK.
Declarations of interest
Rajesh M Shetty: none known
Antonio Bellini: none known
Dhuleep Wijayatilake: none known
Mark A Hamilton has received lecture fees from Edwards Life Sciences to deliver two lectures which are unrelated to the topics of this review
Rajesh Jain: none known
Sunil Karanth: none known
Arunkumar Namachivayam: none known
Edited (no change to conclusions)
References
References to studies included in this review
Inaba 2007 {published data only}
- Inaba S, Hashimoto M, Takahashi M, Motomura Y, Kochi A. Comparison of BIS and Ramsay score for evaluation of sedation with propofol in ICU. Masui. The Japanese Journal of Anesthesiology 2007;56(1):57‐60. [PUBMED: 17243646] [PubMed] [Google Scholar]
Li 2009 {published data only}
- Li X, Kang Y, Zhang C. A study of bispectral index monitoring in assessing the depth of sedation of patients under mechanical ventilation. Zhongguo Wei Zhong Bing Ji Jiy Yi Xue = Chinese Critical Care Medicine 2009;21(6):361‐3. [ISSN: 1003‐0603; PUBMED: 19570344] [PubMed] [Google Scholar]
Weatherburn 2007 {published data only}
- Weatherburn C, Endacott R, Tynan P, Bailey M. The impact of bispectral index monitoring on sedation administration in mechanically ventilated patients. Anesthesia and Intensive Care 2007;35(2):204‐8. [ISSN: 0310‐057X; PUBMED: 17444309] [DOI] [PubMed] [Google Scholar]
Zhao 2011 {published data only}
- Zhao D, Xu Y, He W, Li T, He Y. A comparison of bispectral index and sedation‐agitation scale in guiding sedation therapy: a randomized controlled study in patients undergoing short‐term mechanical ventilation. Zhongguo Wei Zhong Bing Ji Jiy Yi Xue = Chinese Critical Care Medicine 2011;23(4):220‐3. [ISSN: 1003‐0603; PUBMED: 21473824] [PubMed] [Google Scholar]
References to studies excluded from this review
Binnekade 2009 {published data only}
- Binnekade J, Wilde R, Slooter A, Sluijs J, Beenen O, Schultz M, et al. Multicenter randomized trial of sedation using daily wake‐up calls, bispectral index or clinical sedation scores in a mixed medical‐surgical ICU population. Critical Care (London, England) 2009;13 Suppl 1:P396 (S161). [DOI: 10.1186/cc7560; PMC4084282] [DOI] [Google Scholar]
Olson 2009 {published data only}
- Olson M, Thoyre M, Peterson D, Graffagnino C. A randomized evaluation of bispectral index‐augmented sedation assessment in neurological patients. Neurocritical Care 2009;11(1):20‐7. [DOI: 10.1007/s12028-008-9184-6; ISSN: 1541:6933; PMC2706915; PUBMED: 19184556] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ou 2016 {published data only (unpublished sought but not used)}
- Ou X, Hua Y, Zhang W, Zheng R, Lin H. Sedation‐Agitation scale and Bispectral index to monitor sedation depth in mechanically ventilated. Critical Care Medicine 2016;44(12):391. [DOI: 10.1097/01.ccm.0000509938.26705.81] [DOI] [Google Scholar]
Additional references
Barr 2013
- Barr J, Fraser G, Puntillo K, Ely E, Gèlinas C, Dasta J, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Critical Care Medicine 2013;41(1):263‐306. [DOI: 10.1097/CCM.0b013e3182783b72; PUBMED: 23269131] [DOI] [PubMed] [Google Scholar]
Bilgili 2017
- Bilgili B, Montoya JC, Layon AJ, Berger AL, Kirchner HL, Gupta LK, et al. Utilizing bi‐spectral index (BIS) for the monitoring of sedated adult ICU patients‐ A systematic review. Minerva Anestesiologica 2017;83(3):288‐301. [DOI: 10.23736/S0375-9393.16.10886-7; ISSN 0375‐9393; Online ISSN 1827‐1596; PUBMED: 27314595] [DOI] [PubMed] [Google Scholar]
Blackwood 2015
- Blackwood B, Marshall J, Rose L. Progress on core outcome sets for critical care research. Current Opinion in Critical Care 2015;21(5):439‐44. [DOI: 10.1097/MCC.0000000000000232; PMID 26263299] [DOI] [PubMed] [Google Scholar]
Carrasco 2000
- Carrasco G. Instruments for monitoring intensive care unit sedation. Critical Care 2000;4(4):217‐25. [PUBMED: 11094504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane 2008
- Julian P, Higgins T, Sally G. Cochrane Handbook for Systematic Reviews of Interventions. Wiley, September 2008. [ISBN: 978‐0‐470‐69951‐5] [Google Scholar]
Dahaba 2005
- Dahaba AA. Different conditions that could result in Bispectral index indicating an incorrect hypnotic state. Anaesthesia and Analgesia 2005;101(3):765‐73. [DOI: 10.1213/01.ane.0000167269.62966.af; PUBMED: 16115989] [DOI] [PubMed] [Google Scholar]
Davydow 2008
- Davydow DS, Gifford JM, Desai SV. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. General Hospital Psychiatry 2008;30(5):421‐34. [DOI: 10.1016/j.genhosppsych.2008.05.006; PMC2572638; PMID 18774425] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deogaonkar 2004
- Deogaonkar A, Gupta R, DeGeorgia M, Sabharwal V, Gopakumaran B, Shubert A, et al. Bispectral Index monitoring correlates with sedation scales in brain‐injured patients. Critical Care Medicine 2004;32(12):2403‐6. [PUBMED: 15599143] [DOI] [PubMed] [Google Scholar]
Dewhurst 2000
- Dewhurst AT, Chiverley‐Williams S, Goldstone J. The change in bispectral index with stimulation indicates depth of sedation in intensive care. Critical Care 2000;4(Suppl 1):191. [PMC3333115] [Google Scholar]
Esteban 2002
- Esteban A, Anzueto A, Frutos F, Alìa I, Brochard L, Stewart T, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation. A 28 day international study. JAMA 2002;287(3):345‐55. [PUBMED: 11790214] [DOI] [PubMed] [Google Scholar]
Finger 2016
- Finger RG, Mallmann C, Nedel WL. BIS monitoring in sedated, mechanically ventilated patients:right tool in the wrong patients? A meta‐analysis. Intensive Care Medicine 2016;42(6):1086‐7. [DOI: 10.%E2%80%8B1007/%E2%80%8Bs00134-016-4282-y; PUBMED: 26928039] [DOI] [PubMed] [Google Scholar]
Fugate 2013
- Fugate JE, Kalimullah EA, Hocker SE, Clark SL, Wijdicks EF, Rabinstein AA. Cefepime neurotoxicity in the intensive care unit: a cause of severe, under appreciated encephalopathy. Critical Care 2013;17(6):R264. [PUBMED: 24200036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gommers 2008
- Gommers D, Bakker J. Medications for analgesia and sedation in the intensive care unit: an overview. Critical Care 2008;12(Suppl 3):S4. [PUBMED: 18495055] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Spiegelhalter DJ. Being sceptical about meta‐analyses: a Bayesian perspective on magnesium trials in myocardial infarction. International Journal of Epidemiology 2002;31(1):96‐104. [PUBMED: 11914302] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of the sample. BMC Medical Research Methodology 2005;5:13. [DOI: 10.1186/1471-2288-5-13; PUBMED: 15840177 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2014
- Hu CF, Wang CC, Chen SJ, Perng CL, Yang HY, Fan HC. Prognostic values of a combination of intervals between respiratory illness and onset of neurological symptoms and elevated serum IgM titers in Mycoplasma pneumoniae encephalopathy. Journal of Microbilogy, Immunology and Infection = Wei mian yu gan ran za zhi 2014;47(6):497‐502. [ISSN: 1684‐1182; PUBMED: 23968755] [DOI] [PubMed] [Google Scholar]
Jackson 2010
- Jackson D, Proudfoot C, Kann K, Walsh T. A systematic review of the impact of sedation in the ICU on resource use, costs and patient safety. Critical Care 2010;14(2):R59. [PUBMED: 20380720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobi 2002
- Jacobi J, Fraser G, Coursin D, Riker R, Fontaine D, Wittbrodt E, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Critical Care Medicine 2002;30(1):119‐41. [PUBMED: 11902253] [DOI] [PubMed] [Google Scholar]
Kaplan 2000
- Kaplan LJ, Bailey H. Bispectral index (BIS) monitoring of ICU patients on continuous infusion of sedatives and paralytics reduces sedative drug utilization and cost. Critical Care 2000;4(Suppl 1):190. [PMC3333114] [Google Scholar]
Karamchandani 2010
- Karamchandani K, Rewari V, Trikha A, Batra RK. Bispectral index correlates well with Richmond agitation sedation scale in mechanically ventilated critically ill patients. Journal of Anesthesia 2010;24(3):394‐8. [PUBMED: 20225074] [DOI] [PubMed] [Google Scholar]
Kollef 1998
- Kollef M, Levy N, Ahrens T, Schaiff R, Prentice D, Sherman G. The use of continuous iv sedation is associated with prolongation of mechanical ventilation. Chest 1998;114(2):541‐8. [PUBMED: 9726743] [DOI] [PubMed] [Google Scholar]
Kress 2000
- Kress J, Pohlman A, O'Connor M, Hall J. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. New England Journal of Medicine 2000;342(20):1471‐7. [PUBMED: 10816184] [DOI] [PubMed] [Google Scholar]
LeBlanc 2006
- LeBlanc J, Dasta J, Kane‐Gill S. Role of the Bispectral Index in sedation monitoring in the ICU. Annals of Pharmacotherapy 2006;40(3):490‐500. [PUBMED: 16492796] [DOI] [PubMed] [Google Scholar]
Ma 2013
- Ma L, Lin Z, Chen D. Wernicke encephalopathy following nutritional deficiency in a patient with multiple trauma. Anaesthesia and Intensive Care 2013;41(6):816‐7. [PUBMED: 24180735] [PubMed] [Google Scholar]
Martin 2007
- Martin J, Franck M, Sigel S, Weiss M, Spies C. Changes in sedation management in German intensive care units between 2002 and 2006: a national follow up survey. Critical Care 2007;11(6):R124‐30. [PUBMED: 18062820] [DOI] [PMC free article] [PubMed] [Google Scholar]
Metnitz 2009
- Metnitz P, Metnitz B, Moreno R, Bauer P, Sorbo L, Hoermann C, et al. Epidemiology of mechanical ventilation: analysis of the SAPS 3 database. Intensive Care Medicine 2009;35(5):816‐25. [PUBMED: 19288079] [DOI] [PubMed] [Google Scholar]
Parker 2015
- Parker AM, Sricharoenchai T, Raparla S. Posttraumatic stress disorder in critical illness survivors: a meta‐analysis. Critical Care Medicine 2015;43(5):1121‐9. [DOI: 10.1097/CCM.0000000000000882; PMID 25654178] [DOI] [PubMed] [Google Scholar]
Patel 2012
- Patel S, Kress J. Sedation and analgesia in the mechanically ventilated patient. American Journal of Respiratory and Critical Care Medicine 2012;185(5):486‐97. [PUBMED: 22016443] [DOI] [PubMed] [Google Scholar]
Punjasawadwong 2014
- Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD003843.pub3; PMID 24937564] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reschreiter 2008
- Reschreiter H, Maiden M, Kapila A. Sedation practice in the intensive care unit: a UK national survey. Critical Care 2008;12(6):R152‐9. [PUBMED: 19046459] [DOI] [PMC free article] [PubMed] [Google Scholar]
Revman 5.3 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (Revman). Version 5.3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sedation Equipment & Supplies 2017
- 186‐0106E‐Quad Sensor (4 Electrode Sensor). https://www.sedationkit.com/product/quad‐sensor‐4‐electrode‐sensor‐25bx/ (accessed 22 June 2017).
Sessler 2008
- Sessler C, Grap M, Ramsay M. Evaluating and monitoring analgesia and sedation in the intensive care unit. Critical Care 2008;12(Suppl 3):S2‐14. [PUBMED: 18495053] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singhal 2014
- Singhal NS, Josephson SA. A practical approach to neurologic evaluation in the intensive care unit. Journal of Critical Care 2014;29(4):627‐33. [PUBMED: 24636925] [DOI] [PubMed] [Google Scholar]
Soliman 2001
- Soliman H, Mélot C, Vincent JL. Sedative and analgesic practice in the intensive care unit: the results of a European survey. British Journal of Anaesthesia 2001;87(2):186‐92. [PUBMED: 11493487] [DOI] [PubMed] [Google Scholar]
Sonneville 2013
- Sonneville R, Verdonk F, Rauturier C, Klein IF, Wolff M, Annane D, et al. Understanding brain dysfunction in sepsis. Annals of Intensive Care 2013;3(1):15. [PMC3673822] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2008
- Stevens RD, Nyquist PA. Types of brain dysfunction in critical illness. Neurologic Clinics 2008;26(2):469‐86. [PUBMED: 18514822] [DOI] [PubMed] [Google Scholar]
The OMERACT Handbook 2014
- Boers M, Kirwan JR, Tugwell P, Beaton D, Conaghan PG, D'Agostino MA, et al. The OMERACT Handbook. https://www.omeract.org/pdf/OMERACT_Handbook.pdf accessed on 21.06.2017.
Vivien 2003
- Vivien B, Maria S, Ouattara A, Langeron O, Coriat P, Riou B. Overestimation of Bispectral Index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology 2003;99(1):9‐17. [PMID 12826836] [DOI] [PubMed] [Google Scholar]
Yaman 2012
- Yaman F, Ozcan N, Ozcan A, Kaymak C, Basar H. Assesment of correlation between Bispectral Index and four common sedation scales used in mechanically ventilated patients in ICU. European Review for Medical and Pharmacological Sciences 2012;16(5):660‐6. [PUBMED: 22774408] [PubMed] [Google Scholar]
Ziaja 2013
- Ziaja M. Septic encephalopathy. Current Neurology and Neuroscience Reports 2013;13(10):383. [PMC3779311] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Shetty 2014
- Shetty RM, Bellini A, Wijayatilake DS, Hamilton MA, Jain R, Cerda G, et al. BIS monitoring versus clinical assessment for sedation in mechanically ventilated adult patients in the intensive care unit and its impact on clinical outcomes and resource utilization. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD011240] [DOI] [PMC free article] [PubMed] [Google Scholar]


