Abstract
Background
Most people admitted to hospitals worldwide require a vascular access device (VAD). Hundreds of millions of VADs are inserted annually in the USA with reports of over a billion peripheral intravenous catheters used annually worldwide. Numerous reports suggest that a team approach for the assessment, insertion, and maintenance of VADs improves clinical outcomes, the patient experience, and healthcare processes.
Objectives
To compare the use of the vascular access specialist team (VAST) for VAD insertion and care to a generalist model approach for hospital or community participants requiring a VAD in terms of insertion success, device failure, and cost‐effectiveness.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1); Ovid MEDLINE (1950 to 7 February 2018); Ovid Embase (1980 to 7 February 2018); EBSCO CINAHL (1982 to 7 February 2018); Web of Science Conference Proceedings Citation Index ‐ Science and Social Science and Humanities (1990 to 7 February 2018); and Google Scholar. We searched the following trial registries: Australian and New Zealand Clinical Trials Register (www.anzctr.org.au); ClinicalTrials.gov (www.clinicaltrials.gov); Current Controlled Trials (www.controlled‐trials.com/mrct); HKU Clinical Trials Registry (www.hkclinicaltrials.com); Clinical Trials Registry ‐ India (ctri.nic.in/Clinicaltrials/login.php); UK Clinical Trials Gateway (www.controlled‐trials.com/ukctr/); and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch). We searched all databases on 7 February 2018.
Selection criteria
We planned to include randomized controlled trials (RCTs) that evaluated the effectiveness of VAST or specialist inserters for their impact on clinical outcomes.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane and used Covidence software to assist with file management.
Main results
We retrieved 2398 citations: 30 studies were eligible for further examination of their full text, and we found one registered clinical trial in progress. No studies could be included in the analysis or review. We assigned one study as awaiting classification, as it has not been accepted for publication.
Authors' conclusions
This systematic review failed to locate relevant published RCTs to support or refute the assertion that vascular access specialist teams are superior to the generalist model. A vascular access specialist team has advanced knowledge with regard to insertion techniques, clinical care, and management of vascular access devices, whereas a generalist model comprises nurses, doctors, or other designated healthcare professionals in the healthcare facility who may have less advanced insertion techniques and who care for vascular access devices amongst other competing clinical tasks. However, this conclusion may change once the one study awaiting classification and one ongoing study are published. There is a need for good‐quality RCTs to evaluate the efficacy of a vascular access specialist team approach for vascular access device insertion and care for the prevention of failure.
Plain language summary
Vascular access specialist teams for insertion of and prevention of vascular access device failure
Review question
We reviewed the evidence concerning the effectiveness of vascular access specialist teams (VAST) compared with generalist models in terms of vascular access device insertion success, device failure, and cost‐effectiveness.
We did not find any eligible studies for our review.
Background
We describe a VAST as the grouping of healthcare personnel who have advanced knowledge and skills in the assessment, insertion, care, and management of vascular access devices, such as infusion/intravenous, intravenous therapy teams, as well as individual vascular access specialists (nurse, doctor, therapist, technician, and physician assistant).
Our goal was to evaluate if the VAST approach is superior to a generalist approach.
We define a generalist model or approach as a larger groups of nurses, doctors, or other designated healthcare professionals in the healthcare facility who have less advanced skills and knowledge in inserting and managing vascular access devices.
We define a vascular access device (VAD) as a catheter (thin tube) inserted into veins or ports that can be implanted under the skin, allowing fluids and medicines to be delivered into veins. Catheters inserted into arteries can be used to monitor therapy. The most common VAD, the peripheral intravenous catheter (PIVC), may remain in place for many days before removal. Implanted VADs or catheters in central veins can usually remain for many weeks, months, and in some cases, particularly with ports, years. Vascular access devices are used for giving fluids (infusion therapy) and intravenous (injected into a vein) medicines, taking blood samples, and invasive monitoring, and are often crucial in providing treatment and care. The use of VADs and infusion therapy extends across almost all medical, surgical, and critical care specialties, and occurs in hospital, long‐term care, and home care settings.
There are several risks related to the insertion of a VAD and its ongoing care that can cause the device to fail (to become no longer suitable for care). One significant postinsertion complication includes catheter‐related venous thrombosis (clot formation). People with cancer or who are critically ill and may require additional medical interventions to treat thrombosis are particularly at risk for this complication. A risk of infusion‐related inflammation of the vein (phlebitis or thrombophlebitis) exists for the PIVC when the cannulated vein becomes painful with other potential signs such as a red appearance at the insertion site. Infection risks such as catheter‐related bloodstream infections are associated with all VADs; preventing such occurrences is a healthcare priority. Catheter‐related bloodstream infections are associated with longer hospital stay, serious illness, death, and increased health service costs.
Study characteristics
We searched a wide range of medical databases on 7 February 2018. We identified 2398 potential studies, 30 of which we looked at in detail. We found one suitable study, however although the study is complete the manuscript has not yet been accepted for publication, and so we were unable to analyse the data. We have assigned the study as awaiting classification; once its results are published we will evaluate it again and decide if it is eligible for inclusion in the review. We found one registered trial that is investigating our review question, but it is still ongoing and not yet completed or published.
Key results
We failed to locate any published randomized controlled trials (RCTs) to support or refute the assertion that vascular access specialist teams are superior to the generalist model for device insertion and prevention of failure. However, this conclusion may change once the one study awaiting classification and the one ongoing study are published. There is a need for good‐quality RCTs to evaluate the efficacy of a VAST approach for VAD insertion and prevention of failure. An RCT is a study (or trial) that aims to reduce bias when testing a new treatment. The people taking part in the trial are randomly allocated to either the group receiving the treatment under investigation or to a group receiving standard treatment (or placebo treatment) as the control.
Quality of evidence
We did not analyse the quality of the evidence as we did not find any suitable studies to include in our review.
Background
Most people admitted to hospital will require a vascular access device (VAD). Hundreds of millions of VADs are inserted annually in the USA alone (O'Grady 2011), with billions inserted in patients worldwide (Rickard 2015). Improving the patient journey with an appropriately placed VAD has been argued as a priority for healthcare delivery services (Moureau 2012). A variety of professionals currently provide VAD insertion and ongoing management of the device, but there is some evidence to suggest that this can contribute to fragmentation of the patient's overall care (Castro‐Sánchez 2014). The question underlying this review was whether a specialist rather than a generalist approach is likely to produce better VAD insertion and care and, therefore, a reduction in complications that contribute to VAD failure.
Description of the condition
The term 'vascular access device' represents a variety of catheters commonly used to access the circulatory system for healthcare treatments. Most VADs used for intravenous therapy are classified by two distinct insertion routes: via peripheral or central veins; however, they can also include arterial, intraosseous, and umbilical routes (Green 1998; Kelly 2009; Reades 2011; Scheer 2002).
Function of a vascular access device
Vascular access devices permit infusion therapy via the circulatory system, blood sampling analysis, and invasive monitoring, and are often crucial in providing treatment and care (Gorski 2016). The use of VADs and infusion therapy extends across almost all medical, surgical, and critical care specialties, and occurs in hospital, long‐term care, and home care settings.
Insertion of vascular access devices
Vascular access device insertion is a patient safety issue (Castro‐Sánchez 2014; Moureau 2013). Several attempts may be made to insert a VAD successfully, with each attempt involving a puncture of the skin by a needle in an attempt to cannulate the desired vessel. Identified rates of first‐time peripheral insertion failure are 12% to 26% in adults and 24% to 54% in children (Sabri 2013). A variety of strategies to improve insertion outcomes for peripheral routes exist, such as assessment tools and clinical prediction rules (Carr 2017), as failure can lead to bruising and pain at the insertion sites (Webster 2008), and multiple punctures of the skin predispose micro‐organism entry into the bloodstream (Mermel 2017). Centrally inserted VADs are associated with more critical procedural complications (e.g. pneumothorax or arterial puncture) and contribute to patient morbidity and mortality, although specialized technology such as ultrasound can be used to reduce the risk of such complications (Wu 2013).
Complications associated with vascular access devices
There are several risks related to VAD insertion and ongoing care. These risks can be either operator or patient related. Postinsertion complications including catheter‐related venous thrombosis (clot formation) can necessitate further medical intervention (Ge 2012). Particularly at risk are people with cancer or who are critically ill (Chopra 2013). A risk of infusion‐related phlebitis (or thrombophlebitis) with peripheral intravenous catheters (PIVCs) exists when the cannulated vein becomes painful with other potential signs such as erythema (red appearance) at the insertion site (Tagalakis 2002). Infection risks such as catheter‐related bloodstream infections are a significant hospital burden and are associated with all VADs, but particularly with central venous catheters (CVCs); preventing such occurrences is another healthcare priority (O'Grady 2011). Catheter‐related bloodstream infection increases hospital stay, morbidity, mortality, and health service costs (Ge 2012).
Vascular access specialist team
For the purposes of this review, the term vascular access specialist team (VAST) represents any grouping of personnel specifically associated with VAD insertion and care, and is synonymous with titles such as infusion teams, intravenous teams, or intravenous therapy teams, as well as individual vascular access specialists (nurse, doctor, respiratory therapist, technician, and physician assistant) who have advanced knowledge and skills and who frequently insert or manage VADs, or both. Positive reports of a VAST approach include the use of nurse‐led teams and advanced nurse practitioners who have inserted CVCs in critical care environments (Alexandrou 2014; Gopal 2006; Yacopetti 2010). The use of a team approach for inserting PIVCs has increased first‐time insertion success (Carr 2010), and historically is associated with decreased device‐related complications (Tomford 1984). The alternative to VAST is the generalist model, where larger groups of nurses, doctors, or other designated healthcare professionals in the healthcare facility who have less advanced skills, insert and care for VADs.
How the intervention might work
The argument for VAD insertion by a VAST is that best‐practice care is supported by a consistent, knowledgeable, and skilled approach. Higher levels of inserter knowledge and confidence, built upon experience and procedural competence, suggest the VAST approach has positive insertion outcomes for patients (Alexandrou 2014; Harnage 2012; Jackson 2012). While some VAST models focus on VAD insertion only, others include follow‐up care, which can include clinical tasks such as dressing replacement and daily assessment for potential removal. Even with a limited scope of 'insertion only', VASTs have reported better outcomes for first‐time insertion success (Carr 2010). Reducing the number of failed needle insertions is an important infection prevention strategy (da Silva 2010), and one that can reduce patient stress and length of hospital stay (Barton 1998).
Vascular access specialist team impact on device‐related complications
Peripheral intravenous catheters inserted using a VAST approach have been associated with less phlebitis, erythema, induration, and infiltration (Soifer 1998). This may be related to an increased first‐time insertion success with a VAST, since multiple insertion attempts have been associated with complications and failure (Wallis 2014). Central venous catheters inserted using the VAST approach have low iatrogenic complications with reports of as little as 1% of insertions developing pneumothorax, arterial puncture, and subsequent catheter‐related infection (Alexandrou 2012). Reduced catheter‐related bloodstream infection rates and VAD bacteraemia rates have been attributed to the adoption of a VAST approach (Brunelle 2003; Legemaat 2015)
Why it is important to do this review
Vascular access device insertion success and reduction of subsequent failure are important objectives that can positively impact on patient experience and clinical outcome. It is important to understand if the VAST approach does improve insertion success and reduce VAD failure, iatrogenic procedural complications, and device‐related infection. However, there is no clear evidence to date of the effectiveness of VAST compared with the generalist approach to VAD insertion and no prior systematic review on this topic. Although VASTs themselves do incur costs to pay staff, considering the adverse outcomes that may be avoided, VAST may be the more cost‐effective model. Establishing whether clinical outcomes of VAST are superior to generalist VAD insertion and management is of initial importance for clinicians, consumer groups, policymakers, and healthcare systems.
Objectives
To compare the use of the vascular access specialist team for vascular access device insertion and care to a generalist model approach for hospital or community participants requiring a vascular access device in terms of insertion success, device failure, and cost‐effectiveness.
Methods
Criteria for considering studies for this review
Types of studies
We intended to include all randomized controlled trials (RCTs) that evaluated the effectiveness of VASTs or specialist inserters for their impact on clinical outcomes. We excluded cluster RCTs, where the cluster represented randomization at the ward or hospital level.
We intended to include controlled clinical trials if we did not find any RCTs. Controlled clinical trials refer to quasi‐randomized studies where, although the trial involves testing an intervention with a control, with concurrent enrolment and follow‐up of test and control‐treated groups, the method of allocation is not considered truly random.
Types of participants
We included hospitalized or community participants requiring vascular access. Age was not an excluding factor.
Types of interventions
Intravenous/vascular access teams or specialist inserters (as in VAST) providing insertion or maintenance (or both) of VADs.
Types of outcome measures
Primary outcomes
First‐time insertion success (generally insertion success is reported as a percentage): insertion of a PIVC where venous return occurs, a saline flush passes, and the PIVC is secured and ready for use (Riker 2011), or as defined by the study authors; in CVCs, the number of reported insertion attempts (generally insertion success is reported as a percentage) and where the tip of the CVC is confirmed in the lower third of the superior vena cava (Moureau 2013), or as defined by the study authors.
-
Insertion‐related adverse events/complications:
pneumothorax: inadvertent injury to the pleura of the lung during cannulation of a large vein (defined by each study) (Ayas 2007);
arterial puncture: inadvertent puncture of an artery during cannulation of a large vein (defined by each study) (Guilbert 2008);
nerve damage: inadvertent damage to the nerve that is adjacent to the vein/artery (defined by each study) (Guilbert 2008);
other: as defined by the study author(s).
Cost as defined by the study authors (in the currency of the country where the publication originated).
Secondary outcomes
-
Premature device failure rates as a result of the following:
phlebitis/thrombophlebitis: pain, induration, and erythema with a palpable thrombosis of the cannulated vein (Tagalakis 2002), or as defined by the study authors;
infiltration/extravasation: infiltration is the unintentional leaking of non‐vesicant medication or solution into surrounding tissues, and extravasation is the unintentional leaking of vesicant medication or solution into surrounding tissues (Dougherty 2008);
occlusion: inability to infuse fluid into the VAD or to aspirate blood (Camp‐Sorrell 2007);
thrombosis: central venous thrombosis characterized by neck and arm swelling with associated pain (Ge 2012);
catheter‐related or catheter‐associated bloodstream infection: laboratory‐confirmed bloodstream infection attributed to the catheter (Chopra 2013; Maki 2006);
dislodgement or accidental removal: as defined by the study authors.
Patient satisfaction: as defined by the study authors.
Staff satisfaction: as defined by the study authors.
Dwell time of VAD: in hours from insertion to removal.
Length of hospital stay: in days from hospital admission to discharge.
Search methods for identification of studies
We adapted an Ovid MEDLINE search strategy to search CENTRAL, Ovid Embase, and EBSCO CINAHL and ISI Web of Science. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE and Embase: sensitivity and precision‐maximizing version (Lefebvre 2011). We placed no date, language, or publication restrictions. We performed our search on 7 February 2018.
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We applied no restrictions to language or publication status.
We searched the following databases for relevant trials.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1) (Appendix 1)
MEDLINE (Ovid SP, 1966 to 7 February 2018) (Appendix 2)
Embase (Ovid SP, 1988 to 7 February 2018) (Appendix 3)
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EBSCO, 1982 to 7 February 2018) (Appendix 4)
ISI Web of Science (1990 to 7 February 2018) (Appendix 5)
We developed a subject‐specific search strategy in MEDLINE and used that as the basis for the search strategies in the other databases listed. Where appropriate, we expanded the search strategy with search terms for identifying RCTs.
We scanned the following trials registries for ongoing and unpublished trials (7 February 2018).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch)
ClinicalTrials.gov (www.clinicaltrials.gov)
Australian and New Zealand Clinical Trials Register (www.anzctr.org.au)
Current Controlled Trials (www.controlled‐trials.com/mrct)
HKU Clinical Trials Registry (www.hkclinicaltrials.com)
Clinical Trials Registry ‐ India (ctri.nic.in/Clinicaltrials/login.php)
UK Clinical Trials Gateway (www.controlled‐trials.com/ukctr/)
We developed the search strategy in consultation with the Information Specialist.
Searching other resources
We scanned the reference lists and citations of included trials and any relevant systematic reviews identified for further references to additional trials. We contacted trial authors for additional information when necessary.
Data collection and analysis
Selection of studies
We merged all the electronically retrieved studies from individual databases into Covidence. Following this we removed any duplicate references found employing the filter using icons imports; manage imports then check duplicates. Two review authors (PC, NH) independently assessed titles and abstracts of retrieved studies for relevance. We retrieved full versions of all potentially eligible studies, which the same two review authors independently checked for eligibility. Any discrepancies between review authors were resolved either through mutual discussion, or on two occasions by consulting other review authors (CR, MC) to arbitrate.
Data extraction and management
Two review authors (PC, NH) intended to extract data from each study using our data extraction sheet (Appendix 6). The data extraction sheet was developed in conjunction with the Cochrane Anaesthesia, Critical and Emergency Care Group, and we had planned to pilot test the first two identified studies. Both PC and NH intended to independently extract data and then perform cross‐checking for accuracy and agreement. We intended to include only studies reported in one publication. If we located studies that had been published in duplicate, we intended to maximally extract data from all relevant publications but not to duplicate data in analyses. If we believed any data were missing from the papers, we planned to contact study authors to retrieve this missing information. See Appendix 6 for more information regarding the data that we intended to extract. As we did not locate any published studies, we could not perform data extraction.
Assessment of risk of bias in included studies
Two review authors (PC, NH), intended to independently assess the risk of bias for each of the studies using the 'Risk of bias' assessment tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The tool addresses six specific domains: sequence generation, allocation and concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential sources of bias (Higgins 2011). We planned to express judgements as 'low risk', 'high risk', or 'unclear risk' of bias. We intended to resolve any disagreements by discussion; if we could not reach consensus, a third review author (CR) would arbitrate. We intended to conduct sensitivity analyses to determine whether excluding studies at high risk of bias affected the results of a planned meta‐analysis. We intended to report the 'Risk of bias' table as part of the 'Characteristics of included studies' table and present a 'Risk of bias' summary figure detailing all of the judgements made for all studies included in the review. As we did not locate any published studies, we were unable to assess the risk of bias.
Measures of treatment effect
We intended to calculate dichotomous outcomes using risk ratio (RR) with 95% confidence intervals (CI). We intended to calculate continuous outcomes using the mean difference (MD) with 95% CI. If the results were expressed as rate data (e.g. incidence rates), we planned to calculate a rate ratio. We intended to extract data from time‐to‐event (i.e. dwell time) studies, if the estimates were presented as log‐rank or Cox proportional models. We would not analyse time‐to‐event data that were incorrectly presented as continuous data. As we did not locate any published studies, we could not measure treatment effect.
Unit of analysis issues
Ideally, a study would be designed with participant‐level randomization and analysis, and only one device per participant (adjustment for clustering not necessary in this case). However, we expected to find studies that reported on multiple devices per participant, randomized or analysed at device level, or both, and unadjusted for clustering. We expected to find the following differences.
Number of devices and observed per participant: one or more than one (e.g. after the removal of the initial device the consecutive device(s) was (were) also observed).
Randomization methods: device level, participant level, or ward (or similar) level.
Unit of analysis: device level, participant level, or ward (or similar) level.
Analysis methods: adjusted for clustering or not adjusted for clustering.
In such cases, we intended to attempt to obtain the following from the study authors: participant‐level data or results; data or results for one device per participant; or device‐level data and perform multilevel regression to calculate the adjusted effect. We intended to combine the adjusted results in the meta‐analysis with those of participant‐level trials (using the generic inverse method) and perform sensitivity analyses (Higgins 2011). If we were unsuccessful in obtaining the additional necessary data, then we planned to exclude the study from the meta‐analysis. We excluded cluster RCTs where the cluster represents randomization at the ward or hospital level. We did not locate any published studies to assess unit of analysis.
Dealing with missing data
Whenever possible, we intended to contact the original investigators to request missing data and methodological details. If we considered that data were missing at random, we intended to analyse the available information. If we considered that data were not missing at random, we intended to analyse the available information and assess the potential impact of the missing data on the findings of the review in the Discussion section. However, we did not locate any published studies.
Assessment of heterogeneity
We intended to consider clinical, methodological, and statistical heterogeneity. We planned to undertake an assessment of comparability of the studies prior to meta‐analysis. We planned to assess heterogeneity of selected studies visually and by using the Chi2 test with significance level set at P value less than 0.10. This assesses whether observed differences in results are compatible with chance alone. In addition, we planned to investigate the degree of heterogeneity by calculating the I2 statistic (an equation combining the Chi2 statistic relative to its degree of freedom). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). If studies were sufficiently similar to consider pooling, we planned to use a fixed‐effect model for low‐to‐moderate levels of heterogeneity (I2 = 0% to 50%). Where appropriate, in the absence of clinical heterogeneity and in the presence of statistical heterogeneity (I2 greater than 50%), we planned to use a random‐effects model. However, studies where heterogeneity exceeded 75% were not going to be pooled (Higgins 2011). As we did not locate any published studies, we did not need to assess heterogeneity.
Assessment of reporting biases
We intended to use visual asymmetry on funnel plots to assess reporting biases if at least 10 studies were available for a meta‐analysis (Sterne 2011). We intended to report each outcome separately. We planned to undertake an observation of small‐study effects if required.
We did not locate any published studies to assess reporting biases.
Data synthesis
We intended to enter into Review Manager 5 all trials included in the systematic review and insert and analyse quantitative data (RevMan 2014). The decision to pool data in a meta‐analysis depended upon the availability of outcome data and assessment of between‐trial heterogeneity. If we identified evidence of substantial heterogeneity (i.e. greater than 50%), we planned to explore potential causes and use a random‐effects model, and a fixed‐effect model to explore any differences between these two estimates. As no studies were included in the review, and synthesis was inappropriate, we have presented a structured narrative review in the Results of the search and Discussion sections.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we planned to perform the following subgroup analyses.
Adult VAST versus paediatric VAST.
Device type: peripheral versus central.
Central device type: peripherally inserted central catheter versus CVC versus tunnelled VAD versus totally implanted.
VAST model: insertion only versus insertion and follow‐up care services.
VAST team versus individual specialists.
As we did not locate any studies, we did not undertake any subgroup analysis and investigation of heterogeneity.
Sensitivity analysis
We planned to initially perform a sensitivity analysis by excluding studies at high risk of bias. We intended to only include studies that were assessed as having a low risk of bias for the estimates of treatment effect in all key domains, namely adequate generation of the randomization sequence, adequate allocation concealment, and blinding of outcome assessor. We also planned to perform sensitivity analysis on:
size of study (fewer than 100 participants);
missing data (worst‐case/best‐case scenario).
'Summary of findings' table and GRADE
We intended to present the main results of the review using the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes in our review (Guyatt 2008), and construct a 'Summary of findings' table using GRADEpro GDT software (Appendix 7) (GRADEpro GDT).
We planned to present the following specific primary outcomes of interest in the 'Summary of findings' tables.
First‐time insertion success.
Insertion‐related adverse events/complications.
Cost.
Device failure with dwell time.
Patient satisfaction.
Staff satisfaction.
Length of hospital stay.
We intended to present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). We intended that the 'Summary of findings' tables would include an overall grading of the body of evidence related to each of the main outcomes using the GRADE approach (Schünemann 2011b). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias (Schünemann 2011b).
Results
Description of studies
See Figure 1.
1.
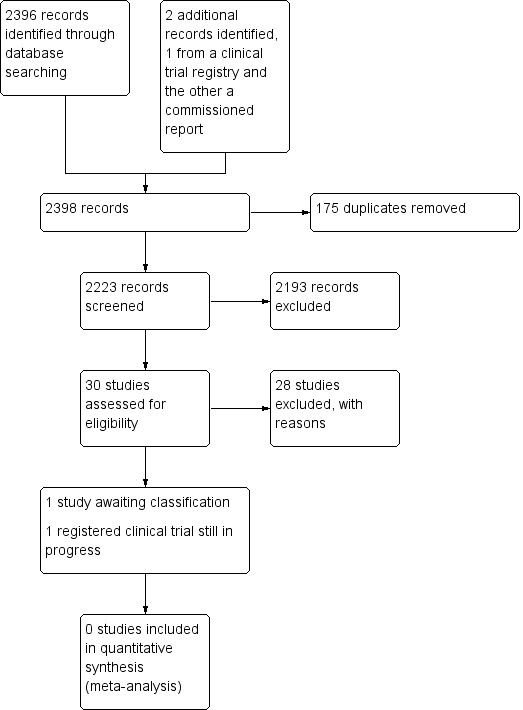
Study search flowchart.
Results of the search
We aimed to identify RCTs pertaining to VAST where the unit of randomization was the participant. We updated our search on the 7 February 2018 as per our protocol search strategy (Carr 2014).
We identified 30 studies that were potentially relevant to the interventional review question posed. We were unable to locate two papers (Tomford 1982; Ward 2000). We retrieved full‐text versions of 26 studies (Boland 2003; Casey 2003; Fong 2001; Gilbert 2016; Hammes 2015; Hockley 2007; Huraib 1994; Keohane 1983; King 2010; Larson 1984; Meier 1998; Mokrzycki 2006; Moretti 2005; Møller 2005; Nehme 1980; Neuman 1998; Puri 1982; Robinson 2005; Secola 2011; Secola 2012; Sherertz 1997; Soifer 1998; Tadokoro 2015; Taylor 2011; Tomford 1984; Treacy 2002).
We obtained one unpublished manuscript (Garate‐Echenique 2014), and we found one ongoing clinical trial (ACTRN12616001675415). Two review authors (PC, NH) independently checked the 26 full‐text papers for eligibility using the Covidence tool (Covidence), and excluded all of them. For transparency, we have provided a summary of studies assessed for full‐text review in our PRISMA flowchart as recommended (see Figure 1) (Liberati 2009).
Included studies
We included no studies in this review.
Excluded studies
We excluded 28 studies (Boland 2003; Casey 2003; Fong 2001; Gilbert 2016; Hammes 2015; Hockley 2007; Huraib 1994; Keohane 1983; King 2010; Larson 1984; Meier 1998; Mokrzycki 2006; Moretti 2005; Møller 2005; Nehme 1980; Neuman 1998; Puri 1982; Robinson 2005; Secola 2011; Secola 2012; Sherertz 1997; Soifer 1998; Tadokoro 2015; Taylor 2011; Tomford 1982; Tomford 1984; Treacy 2002; Ward 2000).
We have documented our reasons for excluding studies in the Characteristics of excluded studies table.
Studies awaiting classification
One unpublished study is awaiting classification, as the manuscript has not yet been accepted for publication, and further data analysis is pending (Garate‐Echenique 2014). See Characteristics of studies awaiting classification.
Ongoing studies
We identified one ongoing clinical trial that has not finished recruitment (ACTRN12616001675415). See Characteristics of ongoing studies.
Risk of bias in included studies
As no studies fulfilled the inclusion criteria for the review, we were unable to assess risk of bias.
Effects of interventions
As we identified no studies to include in the meta‐analysis, we were unable to generate an analysis.
Discussion
Summary of main results
The effectiveness of the VAST approach for VAD insertion and prevention of failure has not yet been evaluated in randomized controlled trials where the unit of randomization represents the participant.
Overall completeness and applicability of evidence
At present, there are no published RCTs evaluating the efficacy of VAST versus the generalist approach. We assumed given the proliferation and ubiquity of both VADs in health care and the various VAST models in use that interventional studies would exist. It may be the case that controlled clinical trials, prospective studies, and quality initiatives are sufficient to convince some healthcare institutions to invest in the VAST concept. Given that VADs are used ubiquitously in health care, that peripheral insertion failure ranges from 12% to 54% (Sabri 2013), and that postinsertion failure rates are reported as up to 50% (Helm 2015), it is unclear why health service researchers have not investigated this topic more stringently. Two early studies (Soifer 1998; Tomford 1984), a cluster RCT and a controlled clinical trial, respectively focused on PIVCs with the primary outcome of device infections, yet many advances since that time limit their current applicability. Both studies took place when routine time‐based removal and insertion of replacement PIVCs was commonly practiced, yet a Cochrane Review now supports clinical indication for removal (Webster 2015). Additionally, vessel‐locating technology to support successful insertions is increasingly reported in the literature, and include ultrasound, transilluminators, and near infrared technology. However, systematic reviews and meta‐analysis have not overwhelmingly proved a clinical benefit with respect to the number of attempts required for success (Egan 2013; Heinrichs 2013; Parker 2016; Stolz 2015). High first‐time insertion has been associated with a VAST approach (Carr 2010; Sabri 2013). Other technological advances such as impregnated catheters and securement technologies are in existence, and may assist with reducing postinsertion VAD complications such as infections (Gilbert 2016). However, a Cochrane Review found no strong evidence to support a particular dressing or securement device technology for the prevention of PIVC dislodgement (Marsh 2015). Other VADs, such as the peripherally inserted central catheter and acute central venous catheter, lack interventional evidence evaluating the VAST approach, despite the increasing prevalence of a specialist team approach with their use (Chopra 2017).
There is an absence of published RCTs evaluating the impact of VAST using current practices and technologies. This systematic review identified one RCT presented at a World Congress in Vascular Access in 2014, which has yet to be published (Garate‐Echenique 2014). Waste in clinical research is of concern (Glasziou 2014), but more importantly if evidence supportive of a VAST approach is left unpublished, it limits application to benefit health services wanting to consider implementing a VAST. An updated review will likely be improved if the work of Garate‐Echenique 2014 (which evaluates a VAST approach with peripherally inserted central catheters) is published, and by expected publications from the pilot RCT registered by Marsh and colleagues (ACTRN12616001675415).
Quality of the evidence
We could not analyse the quality of the evidence as one study is awaiting classification (Garate‐Echenique 2014), and an ongoing trial is not yet completed (ACTRN12616001675415).
Potential biases in the review process
Publication bias may have occurred where only negative studies on this topic have not been published. This seems unlikely given that we found only one registered clinical trial, and it is not yet published. Additionally, and with respect to our correspondence with Garate‐Echenique 2014, non‐publication of their revised manuscript may be owing to resource limitations to complete reviewers' suggestions. Finally, we may have missed a VAST trial represented by a different synonym. However, this is unlikely given that two research librarians assisted with the search strategy. One potential bias is that our inclusion criteria limited the review to RCTs where the unit of randomization was the participant. However, given the lack of eligible studies found with this approach, an opportunity exists for a greater number of similar RCTs on this topic. We attempted to conduct a comprehensive search for studies, but the fact that no studies have as yet been incorporated may be a source of potential bias.
Agreements and disagreements with other studies or reviews
We found reports of the efficacy of a VAST approach using a cluster randomized trial design (Tomford 1984), or quasi‐experimental studies (Soifer 1998). It is unclear if this and other evidence are enough to support change, but it is worthwhile noting that not all VAST approaches have the desired impact in reducing catheter‐related bloodstream infections, even if clinical guidelines are rigorously followed (Secola 2012). Despite a health technology assessment programme in the UK commissioning an RCT investigation examining the clinical and cost‐effectiveness of tunnelled central venous access device insertion with or without vessel‐locating technology in adult cancer patients (Boland 2003), little progress has been made in assessing the effectiveness of VAST. Furthermore, the most recent survey of 'vascular access specialist' reported that clinical practice is not always consistent with contemporary evidence‐based recommendations (Chopra 2017). Additionally, the recent recommendation for defining VASTs by the largest vascular access society (Davis 2016), is perhaps more proof that there exists disconnectedness with this clinical aspect of health care (Castro‐Sánchez 2014). It is conceivable that RCTs would go some way to substantiate or refute the evidence for VAST.
Authors' conclusions
Implications for practice.
This review failed to locate any published randomized controlled trials to support or refute the assertion that vascular access specialist teams for device insertion and prevention of failure are superior to the generalist model. However, this conclusion may change once the one study awaiting classification (Garate‐Echenique 2014), and the one ongoing study (ACTRN12616001675415), are published.
Implications for research.
There is a need for good‐quality randomized controlled trials to evaluate the efficacy of a vascular access specialist team approach for vascular access device insertion and care for the prevention of failure.
What's new
| Date | Event | Description |
|---|---|---|
| 3 January 2019 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Jane Cracknell, Managing Editor (Cochrane Anaesthesia, Critical and Emergency Care Group) for editorial guidance in preparing this review, and Bronagh Blackwood (content editor), Evan Alexandrou, Nancy Moureau (peer reviewers), and Marina Sartini (consumer referee) for their time and effort in evaluating and critiquing this systematic review.
We would like to acknowledge Karen Hovhannisyan (former Cochrane Anaesthesia, Critical and Emergency Care Group Trials Search Co‐ordinator) for assistance with the MEDLINE search strategy. Additionally, we thank Mr Simon Lewis, Librarian Faculty of Health and Medical Sciences, The Unviersity of Western Australia for updating the search strategy. We would also like to thank Bronagh Blackwood (content editor), Nathan Pace (statistical editor), and Nancy Moureau, Linda Kelly, and Evan Alexandrou (peer reviewers) for their help and editorial advice during the preparation of the protocol for this systematic review (Carr 2014).
Finally, we acknowledge colleagues in sharing their unpublished study manuscript and data (Garate‐Echenique 2014).
Appendices
Appendix 1. CENTRAL (the Cochrane Library) search strategy
#1 MeSH descriptor: [Catheterization, Central Venous] explode all trees #2 MeSH descriptor: [Catheterization, Peripheral] explode all trees #3 MeSH descriptor: [Catheters, Indwelling] explode all trees #4 MeSH descriptor: [Catheterization, Swan‐Ganz] explode all trees #5 MeSH descriptor: [Catheterization] explode all trees #6 (#1 or #2 or #3 or #4 or #5) and (team* or clinician* or specialist*):ti,ab #7 ((clinician* or specialist*) near/4 (inserter* or mainte* or vascular access*)) or ((vascular access or intravenous therap* or IV) near/4 team*) #8 #6 or #7
Appendix 2. MEDLINE (Ovid SP) search strategy
1 ((Catheterization, Central Venous/ or Catheterization, Peripheral/ or Catheters, Indwelling/ or Catheterization/ or Catheterization, Swan‐Ganz/) and (team* or clinician* or specialist*).ti,ab.) or ((clinician* or specialist*) adj5 (inserter* or mainte* or vascular access*)).mp. or ((vascular access or intravenous therap* or IV) adj5 team*).mp. 2 ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 3 1 and 2
Appendix 3. Embase (Ovid SP) search strategy
1. ((central venous catheterization/ or indwelling catheter/ or catheterization/ or pulmonary artery catheterization/) and (team* or clinician* or specialist*).ti,ab.) or ((clinician* or specialist*) adj4 (inserter* or mainte* or vascular access*)).mp. or ((vascular access or intravenous therap* or IV) adj5 team*).mp. 2. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 3. 1 and 2
Appendix 4. CINAHL (EBSCO) search strategy
S1 ( (MM "Catheterization") OR (MM "Catheterization, Central Venous") OR (MM "Catheterization, Peripheral") OR (MM "Catheterization, Peripheral Central Venous") ) AND ( team* or clinician* or specialist* ) or (AB ((clinician* or specialist*) N5 (inserter* or mainte* or vascular access*)) or ((vascular access or intravenous therap* or IV) N5 team*) ) OR ( TI ((clinician* or specialist*) N5 (inserter* or mainte* or vascular access*)) or ((vascular access or intravenous therap* or IV) N5 team*) ) S2 random* or multicenter* or prospective* or ((clinical or controlled) and trial*) or ((blind* or mask*) and (single or double or triple or treble)) S3 S1 and S2
Appendix 5. ISI Web of Science search strategy
#1 TI=(catheter* SAME (team* or clinician* or specialist*)) or TI=((clinician* or specialist*) SAME (inserter* or mainte* or vascular access*)) or TS=((vascular access or intravenous therap* or IV) SAME team*) #2 TS=(random* or multicenter* or prospective* or ((clinical or controlled) SAME trial*) or ((blind* or mask*) SAME (single or double or triple or treble))) #3 #1 and #2
Appendix 6. Data extraction tool
Data extraction sheet
| Review title or ID |
| Study ID(surname of first author and year first full report of study was published (e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
| Notes: |
1. General information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/ abstract/ report that data are extracted from) |
|
|
Report ID (ID for this paper/ abstract/ report) |
|
| Reference details | |
| Report author contact details | |
|
Publication type (e.g. full report/abstract/letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
| Notes: | |
2. Study eligibility
| Study characteristics |
Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the protocol) |
Yes | No | Unclear |
Location in text (pg & ¶/fig/table) |
|
| Type of study | Randomized controlled trial | |||||
| Participants | Adult/paediatric recipients of VAD Hospital/community setting |
|||||
| Types of intervention | VAD insertion by a VAST Designation of VAST (infusion therapist, IV team, VA team, other specialist inserting VADs) |
|||||
| Types of comparison | VAD insertion by medical doctor VAD insertion by nurse VAD insertion by physician assistant VAD insertion by technician |
|||||
| Types of outcome measures |
Primary outcomes First‐time insertion success Cost Complication or adverse events and time of
Secondary outcomes Device failure and time of as a result of
Patient satisfaction Staff satisfaction Length of hospital stay |
|||||
| INCLUDE | EXCLUDE | |||||
| Reason for exclusion | ||||||
| Notes: | ||||||
¶: paragraph; fig: figure; IV: intravenous; pg: page; VA: vascular access; VAD: vascular access device; VAST: vascular access specialist team.
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
|
Description Include comparative information for each group (i.e. intervention and controls) if available |
Location in text (pg & ¶/fig/table) |
||
|
Population description (from which study participants are drawn) |
|||
|
Setting (including location and social context) |
|||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method/s of recruitment of participants | |||
| Informed consent obtained | Yes No Unclear | ||
| Notes: | |||
¶: paragraph; fig: figure; pg: page.
4. Methods
| Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
| Aim of study | |||
| Design(e.g. parallel, cross‐over, cluster) | |||
|
Unit of allocation (by individuals, cluster/ groups or body parts) |
|||
| Start date | |||
| End date | |||
| Total study duration | |||
| Ethical approval obtained for study | Yes No Unclear | ||
| Notes: | |||
¶: paragraph; fig: figure; pg: page.
5. Risk of bias assessment
| Domain | Risk of bias | Support for judgement |
Location in text (pg & ¶/fig/table) |
||
| Low risk | High risk | Unclear | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of participants and personnel (performance bias) |
Outcome group: First time insertion success |
||||
| Cost | |||||
| Complications/adverse events from VAD | |||||
| Device failure | |||||
|
Blinding of outcome assessment (detection bias) |
Outcome group: First‐time insertion success |
||||
| Cost | |||||
| Complications/adverse events from VAD | |||||
| Device failure | |||||
|
Incomplete outcome data (attrition bias) |
First‐time insertion success | ||||
| Cost | |||||
| Complications/adverse events from VAD | |||||
| Device failure | |||||
|
Selective outcome reporting? (reporting bias) |
|||||
| Other bias | |||||
| Notes: | |||||
¶: paragraph; fig: figure; pg: page; VAD: vascular access device.
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Total no. randomized (or total population at start of study for NRCTs) |
||
|
Clusters (if applicable, number, type, number of people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| Race/ethnicity | ||
|
Severity of illness Community/ inpatient Critical care area |
||
| Co‐morbidities | ||
| Other treatment received(additional to study intervention) | ||
| Other relevant socio‐demographics | ||
| Subgroups measured | ||
| Subgroups reported | ||
| Notes: | ||
¶: paragraph; fig: figure; NRCT: non‐randomized controlled trial; pg: page.
7. Intervention groups
Copy and paste table for each intervention and comparison group
Intervention group 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Intervention (e.g. VAD inserted by member of VAST) |
||
| VAD(i.e. type of VAD) | ||
| Description(e.g. clinical practice such as advanced technology ultrasound used) | ||
| Number randomized to group(specify whether number of people, VADs or clusters) | ||
| Training and education of VAST clinician | ||
| Timing(e.g. 5 day service, on call or 24/7) | ||
| Delivery(e.g. role and responsibility of VAST, autonomous/independent clinicians) | ||
|
Providers and model (e.g. profession, nurse led/ medical model) |
||
| Co‐interventions (e.g. VAST patients all have chlorhexidine impregnated discs, and generic do not) | ||
| Economic variables (i.e. intervention cost, changes in other costs as result of intervention) | ||
|
Resource requirements to replicate intervention (e.g. staff numbers, equipment) |
||
| Notes: | ||
¶: paragraph; fig: figure; pg: page; VAD: vascular access device; VAST: vascular access specialist team.
8. Outcomes
We will utilize this table for each primary and secondary outcome.
Outcome 1
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name Primary 1 (P1) Secondary 1 (S1) |
|||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power (sample size calculations) | |||
| Notes: | |||
¶: paragraph; fig: figure; ITT: intention to treat; pg: page.
9. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
9.1 Dichotomous outcome
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| Number missing participants and reasons | ||||||
| Number participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, cluster/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Re‐analysis required?(specify) | Yes No Unclear | |||||
| Re‐analysis possible? | Yes No Unclear | |||||
| Re‐analysed results | ||||||
| Notes: | ||||||
¶: paragraph; fig: figure; pg: page.
9.2 Continuous outcome (repeat as necessary)
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
|
Results (We will specify is our results are catheters or days) |
Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| Number missing participants and reasons | ||||||||||
| Number participants moved from other group and reasons | ||||||||||
| Any other results reported | ||||||||||
|
Unit of analysis (individuals, cluster/ groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Re‐analysis required?(specify) | Yes No Unclear | |||||||||
| Re‐analysis possible? | Yes No Unclear | |||||||||
| Re‐analysed results | ||||||||||
| Notes: | ||||||||||
¶: paragraph; fig: figure; pg: page; SD: standard deviation.
Other information
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention result | SD (or other variance) | Control result | SD (or other variance) | ||
| Overall results | SE (or other variance) | |||||
| Number participants | Intervention | Control | ||||
| Number missing participants and reasons | ||||||
| Number participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, cluster/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Re‐analysis required?(specify) | Yes No Unclear | |||||
| Re‐analysis possible? | Yes No Unclear | |||||
| Re‐analysed results | ||||||
| Notes: | ||||||
¶: paragraph; fig: figure; pg: page; SD: standard deviation; SE: standard error.
10. Applicability
| Have important populations been excluded from the study?(consider high‐risk populations, and possible differences in the intervention effect) | Yes No Unclear | |
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | Yes No Unclear | |
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear | |
| Notes: | ||
¶: paragraph; fig: figure; pg: page.
11. Other information
| Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | ||
| Notes: | ||
¶: paragraph; fig: figure; pg: page.
Appendix 7. 'Summary of findings' and quality of evidence
Insertion of a vascular access device by a VAST
Patients or population: people receiving vascular access device.
Setting: inpatient setting and outpatient/community setting.
Intervention: insertion or care (or both) of a vascular access device by a member of a VAST.
Comparison: clinicians inserting these devices who exist outside of a defined VAST.
Definitions: CVC: central venous catheter; IO: intraosseous; PICC: peripherally inserted central catheter; VAD: vascular access device; VAST: vascular access specialist team.
| Primary outcomes | Illustrative comparison risk | Relative effect | Number of participants | Quality of evidence | Comments |
| First‐time insertion success | |||||
| Economic cost‐benefit analysis | |||||
| Adverse events/complications (occlusion, phlebitis, infiltration/extravasation, thrombosis, catheter‐related bloodstream infection, pneumothorax/haemothorax, arterial puncture) | |||||
| Device failure with dwell time | |||||
| Secondary outcomes | Illustrative comparison risk | Relative effect | Number of participants | Quality of evidence | Comments |
| Patient satisfaction | |||||
| Staff satisfaction | |||||
| Length of hospital stay |
Sensitivity analysis
| Effectiveness: Paediatric Adult |
|||||
| Specific VAD inserted by VAST (arterial, peripheral, PICC, CVC, tunnelled, IO, other ‐ will specify) |
|||||
| VAST make‐up (model and members such as nursing/medical/technicians/physician assistants) |
CVC: central venous catheter; IO: intraosseous; PICC: peripherally inserted central catheter; VAD: vascular access device; VAST: vascular access specialist team.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boland 2003 | Wrong comparator: compared blind percutaneous insertion versus image guided |
| Casey 2003 | Non‐randomized controlled study |
| Fong 2001 | Non‐randomized controlled study |
| Gilbert 2016 | Wrong intervention: compared impregnated catheter versus non‐impregnated |
| Hammes 2015 | Wrong study design: incidence case control study |
| Hockley 2007 | Wrong intervention: catheter tip positioning system versus blind positioning |
| Huraib 1994 | Controlled clinical trial design |
| Keohane 1983 | Wrong intervention: compared tunnelled versus untunnelled catheters |
| King 2010 | Non‐randomized controlled study |
| Larson 1984 | Non‐randomized controlled study |
| Meier 1998 | Wrong study design: time series design |
| Mokrzycki 2006 | Non‐randomized controlled study |
| Moretti 2005 | Wrong intervention: antimicrobial central venous catheter coating versus non‐coated central venous catheter |
| Møller 2005 | Wrong intervention: patient education |
| Nehme 1980 | Non‐randomized controlled study |
| Neuman 1998 | Prospective study design with no randomization |
| Puri 1982 | Non‐randomized controlled study |
| Robinson 2005 | Wrong design: prospective quality assurance study |
| Secola 2011 | Initial preliminary pilot data randomized at the unit level |
| Secola 2012 | Randomized at the unit level |
| Sherertz 1997 | Wrong intervention: compared coated catheter versus non‐coated catheter |
| Soifer 1998 | Controlled clinical trial design |
| Tadokoro 2015 | Not a randomized controlled trial design |
| Taylor 2011 | Wrong study design: before‐and‐after intervention study |
| Tomford 1982 | Unable to locate any text or data |
| Tomford 1984 | Non‐randomized controlled study |
| Treacy 2002 | Not a randomized controlled trial design |
| Ward 2000 | Unable to locate any text or data |
Characteristics of studies awaiting assessment [ordered by study ID]
Garate‐Echenique 2014.
| Methods | Randomized controlled trial |
| Participants | Patients receiving intravenous therapy treatment for > 6 days |
| Interventions | Nurse‐led intravenous therapy team providing: vascular access device selection; insertion of the device; patient education for care and maintenance |
| Outcomes | Length of hospital stay; vascular access device survival; complications; patient satisfaction |
| Notes | The study presented as an oral abstract has been reviewed by International Journal of Nursing Studies; it will be considered for publication once a revised manuscript is accepted. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616001675415.
| Trial name or title | Reliable Intravenous Access by Line Experts (RELIABLE): a pilot randomised controlled trial to compare peripheral venous catheter insertion by vascular access specialists with any clinician (generalist model, standard practice) on clinical and economic outcomes among medical and surgical patients |
| Methods | Randomized controlled trial |
| Participants | Patients from the medical and surgical departments who require a PVC for longer than 24 hours will be eligible for inclusion in this study. Consenting patients will have their PVC inserted by a vascular access specialist (intervention) or a generalist clinician (control). |
| Interventions | Arm 1 (control): PVC inserted as per hospital policy by a credentialed PVC inserter (generalist approach). Arm 2 (intervention): vascular access specialist, defined as a registered nurse with advanced knowledge of vascular access including catheter technology, dressings, modalities of catheter access, and intravenous therapy management. A substudy will consider use of ultrasound for assessment of veins, but not for the insertion procedure. |
| Outcomes | The primary outcome is to establish the feasibility of conducting an adequately powered randomized controlled trial in the future. Feasibility measures will include: patient eligibility (more than 90% of those screened); consent (more than 90% agree to enrol); protocol adherence (more than 90% receive the allocated intervention); and retention (less than 5% of enrolled participants lost to follow‐up). Secondary outcomes include: phlebitis: defined as 2 or more of pain, redness, swelling, palpable cord, or purulent discharge. Infiltration and extravasation: defined as the movement of IV fluids into the surrounding tissue with/without resulting tissue breakdown. Occlusion: defined as the PVC will not infuse, or leakage occurs when fluid is infused. Dislodgement (either partial or total): partial: change in PVC length at insertion site (inner catheter visible); total: PVC completely leaves the vein. Infection (laboratory‐confirmed local or bloodstream infection): PVC skin swabs, PVC tip and blood cultures may be collected as per usual clinical practice if clinical suspicion of local infection or systemic infection. PVC dwell time: from the time of PVC insertion until removal from either device failure, routine replacement, or the completion of IV therapy. Cost‐effectiveness: estimates of costs of staff resources, equipment, and PVC failure resource usage with previously developed cost estimations. Detailed resources used for a PVC insertion and removal will be recorded for a subset of 15 participants per study group. Staff and patient acceptability of the intervention assessed on a 0‐to‐10 Likert scale |
| Starting date | Not yet recruiting |
| Contact information | Ms Nicole Marsh Nursing and Midwifery Research Centre Level 2, Building 34 Royal Brisbane and Women's Hospital Cnr Bowen Bridge Road & Butterfield Street Herston, QLD, 4029 |
| Notes |
IV: intravenous PVC: peripheral venous catheter
Differences between protocol and review
We made the following changes to the published protocol (Carr 2014).
We have enhanced and strengthened the wording of the Objectives and Methods sections.
Changes to Objectives include the following.
The previous "To evaluate studies that describe and/or analyse the efficacy of VAST compared with generalist models with regard to insertion success, device failure and cost‐effectiveness", was changed to
"To compare the use of vascular access specialist teams for VAD insertion and care to a generalist model approach for hospital or community participants requiring a VAD in terms of insertion success, device failure, and cost‐effectiveness."
Changes to Methods include the following.
Since we registered our protocol (Carr 2014), we used a different bibliography software to sort and screen studies. We therefore did not import our searches into Endnote 2012 as per initial protocol, and instead used Covidence.
On the advice of the editorial team and the Information Specialist we changed the electronic search to the following.
We searched the following databases for relevant trials:
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1) (Appendix 1)
MEDLINE (Ovid SP, 1966 to 7 February 2018) (Appendix 2)
EMBASE (Ovid SP, 1988 to 7 February 2018) (Appendix 3)
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EBSCO, 7 February 2018) (Appendix 4)
ISI Web of Science (7 February 2018) (Appendix 5)
We developed a subject‐specific search strategy in MEDLINE and used that as the basis for the search strategies in the other databases listed. Where appropriate, we expanded the search strategy with search terms for identifying RCTs.
We scanned the following trials registries for ongoing and unpublished trials (7 February 2018).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/trialsearch)
ClinicalTrials.gov (www.clinicaltrials.gov)
Australian and New Zealand Clinical Trials Register (www.anzctr.org.au)
Current Controlled Trials (www.controlled‐trials.com/mrct)
HKU Clinical Trials Registry (www.hkclinicaltrials.com)
Clinical Trials Registry ‐ India (ctri.nic.in/Clinicaltrials/login.php)
UK Clinical Trials Gateway (www.controlled‐trials.com/ukctr/)
We developed the search strategy in consultation with the Information Specialist.
We changed the following
"We handsearched bibliographies of all retrieved and relevant publications identified by the above strategies for further studies. We searched online thesis repositories for submissions related to this review. We contacted experts in the field to ask for information relevant to this review. Where the full details of a trial were absent and required we attempted to contact the study authors to retrieve information..."
to
"We scanned the reference lists and citations of included trials and any relevant systematic reviews identified for further references to additional trials. We contacted trial authors for additional information when necessary."
Additional changes include updated author affiliations and declarations of interest.
Contributions of authors
Conceiving the review: Peter J Carr (PC), Claire M Rickard (CR).
Co‐ordinating the review: PC.
Undertaking manual searches: PC, Niall S Higgins (NH).
Screening search results: PC, NH.
Organizing retrieval of papers: PC, NH.
Screening retrieved papers against inclusion criteria: PC, NH.
Appraising quality of papers: PC, NH.
Abstracting data from papers: PC, NH.
Writing to authors of papers for additional information: PC.
Providing additional data about papers: PC, NH.
Obtaining and screening data on unpublished studies: PC, NH.
Data management for the review: PC, NH, Gabor Mihala (GM).
Entering data into Review Manager 5 (RevMan 2014): PC, NH, GM, Marie L Cooke (MC).
Review Manager 5 statistical data: PC, NH, GM.
Other statistical analysis not using Review Manager 5: GM.
Interpretation of data: PC, NH, MC, GM, CR.
Statistical inferences: PC, GM.
Writing the review: PC, NH, MC, CR.
Securing funding for the review: PC, CR.
Performing previous work that was the foundation of the present study: PC, CR.
Guarantor for the review: PC.
Reading and checking review before submission: PC, NH, MC, GM, CR.
Sources of support
Internal sources
Division of Emergency Medicine, School of Medicine, The University of Western Australia, Australia.
External sources
No sources of support supplied
Declarations of interest
Peter J Carr received a grant from CareFusion (facilitated by his institution at the time) to attend a scientific meeting on vascular access in the USA in 2012. He received speakers bureau payment from CareFusion in 2013 and BD in 2014 for lectures on the subject of vascular access. His PhD research was supported by a BD contribution to the AVATAR group based at Griffith University. No funding was allocated for the review, with no influence over the design of this review. All of the aforementioned have not biased or influenced this review.
Niall S Higgins has no conflicts of interest to declare.
Marie L Cooke is an academic researcher. Griffith University (not Prof Cooke) has received an unrestricted educational grant from Baxter to support the development of educational materials on peripheral intravenous catheter insertion, maintenance, and removal. Prof Cooke has not undertaken any research specifically into IV teams (the topic of this review).
Gabor Mihala has no conflicts of interest to declare.
Claire M Rickard is an academic researcher and speaker in the field of vascular access. Griffith University (not Prof Rickard) has received payments from manufacturers of intravenous (IV) catheters and related equipment for educational lectures or expert opinion on products (3M, Bard, B.Braun, BD, CareFusion, Mayo, ResQDevices, Smiths Medical) and for one consultancy research project on the topic of a simulated time‐in‐motion study on flushing of IV catheters (BD) (Keogh 2014). Griffith University (not Prof Rickard) has also received unrestricted, grant‐in‐aid donations from manufacturers of IV catheters and related equipment (3M, Adhezion, Angiodynamics, Bard, Baxter, BD, Centurion, CareFusion, Cook, Entrotech, FloMedical, Medtronic, Smiths Medical, and Teleflex) to 1) support Prof Rickard's independent research (manufacturers have no involvement in study design, execution, data handling, publication preparation, or approval), and 2) to support travel costs for research staff and students to present their independent research at conferences. Prof Rickard is a PhD supervisor and co‐investigator on the registered trial (ACTRN12616001675415), investigating vascular access specialist team (the topic of this review), for which there is no commercial funding and with an ultrasound machine loaned by Bard (manufacturer has no involvement in study design, execution, data handling, publication preparation, or approval). Prof Rickard has published government‐funded research that identified IV team/expert vascular access specialist team insertion as one of many factors statistically linked to fewer IV catheter complications (Wallis 2014).
Edited (no change to conclusions)
References
References to studies excluded from this review
Boland 2003 {published data only}
- Boland A, Haycox A, Bagust A, Fitzsimmons L. A randomised controlled trial to evaluate the clinical and cost‐effectiveness of Hickman line insertions in adult cancer patients by nurses. Health Technology Assessment (Winchester, England) 2003;7(36):iii, ix‐x, 1‐99. [PUBMED: 14611735] [DOI] [PubMed] [Google Scholar]
Casey 2003 {published data only}
- Casey J, Davies J. A nurse led central line insertion service. European Dialysis and Transplant Nurses Association. European Renal Care Association Journal 2003;29(4):203‐5. [PUBMED: 14748429] [DOI] [PubMed] [Google Scholar]
Fong 2001 {published data only}
- Fong NI, Holtzman SR, Bettmann MA, Bettis SJ. Peripherally inserted central catheters: outcome as a function of the operator. Journal of Vascular and Interventional Radiology 2001;12(6):723‐9. [PUBMED: 11389224] [DOI] [PubMed] [Google Scholar]
Gilbert 2016 {published data only}
- Gilbert RE, Mok Q, Dwan K, Harron K, Moitt T, Millar M, et al. Impregnated central venous catheters for prevention of bloodstream infection in children (the CATCH trial): a randomised controlled trial. Lancet 2016;387(10029):1732‐42. [PUBMED: 26946925] [DOI] [PubMed] [Google Scholar]
Hammes 2015 {published data only}
- Hammes M, Desai A, Pasupneti S, Kress J, Funaki B, Watson S, et al. Central venous catheters: incidence and predictive factors of venous thrombosis. Clinical Nephrology 2015;84(1):21‐8. [PUBMED: 25997503] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hockley 2007 {published data only}
- Hockley SJ, Hamilton V, Young RJ, Chapman MJ, Taylor J, Creed S, et al. Efficacy of the CathRite system to guide bedside placement of peripherally inserted central venous catheters in critically ill patients: a pilot study. Critical Care and Resuscitation: Journal of the Australasian Academy of Critical Care Medicine 2007;9(3):251‐5. [PUBMED: 17767451] [PubMed] [Google Scholar]
Huraib 1994 {published data only}
- Huraib S, Askar A, Abu‐Aisha H, al‐Wakeel J. Prevalence of infection from subclavian dialysis catheters with two different postinsertion catheter cares: a randomized comparative study. Angiology 1994;45(12):1047‐51. [DOI: 10.1177/000331979404501208; PUBMED: 7985832] [DOI] [PubMed] [Google Scholar]
Keohane 1983 {published data only}
- Keohane PP, Jones BJ, Attrill H, Cribb A, Northover J, Frost P, et al. Effect of catheter tunnelling and a nutrition nurse on catheter sepsis during parenteral nutrition. A controlled trial. Lancet 1983;2(8364):1388‐90. [PUBMED: 6140494] [DOI] [PubMed] [Google Scholar]
King 2010 {published data only}
- King DS, Cruz E, Kaufman J. A model for a nurse‐led programme of bedside placement of peripherally inserted central catheters in neonates and infants with congenital cardiac disease. Cardiology in the Young 2010;20(3):302‐7. [PUBMED: 20346200] [DOI] [PubMed] [Google Scholar]
Larson 1984 {published data only}
- Larson E, Hargiss C. A decentralized approach to maintenance of intravenous therapy. American Journal of Infection Control 1984;12(3):177‐86. [PUBMED: 6565469] [DOI] [PubMed] [Google Scholar]
Meier 1998 {published data only}
- Meier PA, Fredrickson M, Catney M, Nettleman MD. Impact of a dedicated intravenous therapy team on nosocomial bloodstream infection rates. American Journal of Infection Control 1998;26(4):388‐92. [PUBMED: 9721390] [DOI] [PubMed] [Google Scholar]
Mokrzycki 2006 {published data only}
- Mokrzycki MH, Zhang M, Golestaneh L, Laut J, Rosenberg SO. An interventional controlled trial comparing 2 management models for the treatment of tunneled cuffed catheter bacteremia: a collaborative team model versus usual physician‐managed care. American Journal of Kidney Diseases 2006;48(4):587‐95. [PUBMED: 16997055] [DOI] [PubMed] [Google Scholar]
Moretti 2005 {published data only}
- Moretti EW, Ofstead CL, Kristy RM, Wetzler HP. Impact of central venous catheter type and methods on catheter‐related colonization and bacteraemia. Journal of Hospital Infection 2005;61(2):139‐45. [DOI: 10.1016/j.jhin.2005.02.012; PUBMED: 16026898] [DOI] [PubMed] [Google Scholar]
Møller 2005 {published data only}
- Møller T, Borregaard N, Tvede M, Adamsen L. Patient education ‐ a strategy for prevention of infections caused by permanent central venous catheters in patients with haematological malignancies: a randomized clinical trial. Journal of Hospital Infection 2005;61(4):330‐41. [PUBMED: 16005107] [DOI] [PubMed] [Google Scholar]
Nehme 1980 {published data only}
- Nehme AE. Nutritional support of the hospitalized patient. The team concept. JAMA 1980;243(19):1906‐8. [PUBMED: 6767864] [PubMed] [Google Scholar]
Neuman 1998 {published data only}
- Neuman ML, Murphy BD, Rosen MP. Bedside placement of peripherally inserted central catheters: a cost‐ effectiveness analysis. Radiology 1998;206(2):423‐8. [PUBMED: 9457195] [DOI] [PubMed] [Google Scholar]
Puri 1982 {published data only}
- Puri P. Total parenteral nutrition in the newborn using peripheral veins: role of I.V. nursing team. European Journal of Pediatric Surgery 1982;37(10):50‐2. [DOI: 10.1055/s-2008-1059815; PUBMED: 6818788 ] [DOI] [PubMed] [Google Scholar]
Robinson 2005 {published data only}
- Robinson MK, Mogensen KM, Grudinskas GF, Kohler S, Jacobs DO. Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. Journal of Parenteral and Enteral Nutrition 2005;29(5):374‐9. [DOI: 10.1177/0148607105029005374; PUBMED: 16107601] [DOI] [PubMed] [Google Scholar]
Secola 2011 {published data only}
- Secola R. Preliminary results from a randomized prospective pilot study evaluating a Central venous catheter (Cvc) team in reducing Cvc‐related bloodstream infections in pediatric oncology patients. Pediatric Blood & Cancer 2011;57(5):856. [Google Scholar]
Secola 2012 {published data only}
- Secola R, Azen C, Lewis MA, Pike N, Needleman J, Sposto R, et al. A crossover randomized prospective pilot study evaluating a central venous catheter team in reducing catheter‐related bloodstream infections in pediatric oncology patients. Journal of Pediatric Oncology Nursing 2012;29(6):307‐15. [PUBMED: 23087249] [DOI] [PubMed] [Google Scholar]
Sherertz 1997 {published data only}
- Sherertz RJ, Stephens JL, Marosok RD, Carruth WA, Rich HA, Hampton KD, et al. The risk of peripheral vein phlebitis associated with chlorhexidine‐coated catheters: a randomized, double‐blind trial. Infection Control Hospital Epidemiology 1997;18(4):230‐6. [PUBMED: 9131364] [DOI] [PubMed] [Google Scholar]
Soifer 1998 {published data only}
- Soifer NE, Borzak S, Edlin BR, Weinstein RA. Prevention of peripheral venous catheter complications with an intravenous therapy team: a randomized controlled trial. Archives of Internal Medicine 1998;158(5):473‐7. [PUBMED: 9508225] [DOI] [PubMed] [Google Scholar]
Tadokoro 2015 {published data only}
- Tadokoro T, Tokumine J, Lefor AT, Kawabata T, Yoza K, Kinjo T. The three‐step method for ultrasound‐guided pediatric internal jugular venous catheterization: a clinical trial. Journal of Anesthesia 2015;29(1):131‐3. [PUBMED: 24981562] [DOI] [PubMed] [Google Scholar]
Taylor 2011 {published data only}
- Taylor T, Massaro A, Williams L, Doering J, McCarter R, He J, et al. Effect of a dedicated percutaneously inserted central catheter team on neonatal catheter‐related bloodstream infection. Advances in Neonatal Care 2011;11(2):122‐8. [PUBMED: 21730901] [DOI] [PubMed] [Google Scholar]
Tomford 1982 {published data only}
- Tomford JW, Hershey CO. The effect of an intravenous therapy team on peripheral venous catheter associated phlebitis ‐ a controlled trial. Clinical Research 1982;30(4):770A. [Google Scholar]
Tomford 1984 {published data only}
- Tomford JW, Hershey CO, McLaren CE, Porter DK, Cohen DI. Intravenous therapy team and peripheral venous catheter‐associated complications. A prospective controlled study. Archives of Internal Medicine 1984;144(6):1191‐4. [PUBMED: 6375611] [PubMed] [Google Scholar]
Treacy 2002 {published data only}
- Treacy PJ, Snelling P, Ragg J, Carson P, O'rourke I. Impact of a multidisciplinary team approach upon patency rates of arteriovenous fistulae. Nephrology 2002;7(2):66‐71. [DOI: 10.1046/j.1440-1797.2002.00089.x] [DOI] [Google Scholar]
Ward 2000 {published data only}
- Ward K, Strain NC, Wright C, James M, Makin AJ. A prospective economic evaluation of the cost of a catheter related sepsis: best case scenario V worst case scenario and the impact of a nutrition support team on cost savings. Proceedings of the Nutrition Society 2000;59(Suppl OCB):170A. [Google Scholar]
References to studies awaiting assessment
Garate‐Echenique 2014 {published data only}
- Garate‐Echenique L, Armenteros‐Yeguas V, Tomas‐Lopez M‐A, Moraza‐Dulanto I, Miranda‐Serrano E, Pena‐Tejera C‐M. The effectiveness of a nurse‐led intravenous therapy team in terms of length of stay, venous access complications and satisfaction of patients requiring long‐term intravenous therapy: a randomized controlled trial. Journal of Vascular Access 2014;15(3):209. [DOI: 10.5301/jva.5000275] [DOI] [Google Scholar]
References to ongoing studies
ACTRN12616001675415 {published data only}
- ACTRN12616001675415. Reliable Intravenous Access by Line Experts (RELIABLE): a pilot randomised controlled trial to compare peripheral venous catheter insertion by vascular access specialists with any clinician (generalist model, standard practice) on clinical and economic outcomes among medical and surgical patients. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371162 (first received 7 November 2016).
Additional references
Alexandrou 2012
- Alexandrou E, Murgo M, Calabria E, Spencer TR, Carpen H, Brennan K, et al. Nurse‐led central venous catheter insertion ‐ procedural characteristics and outcomes of three intensive care based catheter placement services. International Journal of Nursing Studies 2012;49(2):162‐8. [PUBMED: 21944565] [DOI] [PubMed] [Google Scholar]
Alexandrou 2014
- Alexandrou E, Spencer TR, Frost SA, Mifflin N, Davidson PM, Hillman KM. Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates ‐ a report from 13 years of service. Critical Care Medicine 2014; Vol. 42, issue 3:536‐43. [PUBMED: 24145843] [DOI] [PubMed]
Ayas 2007
- Ayas NT, Norena M, Wong H, Chittock D, Dodek PM. Pneumothorax after insertion of central venous catheters in the intensive care unit: association with month of year and week of month. Quality and Safety in Health Care 2007;16:252‐5. [PUBMED: 17693670 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barton 1998
- Barton AJ, Danek G, Johns P, Coons M. Improving patient outcomes through CQI: vascular access planning. Journal of Nursing Care Quality 1998;13(2):77‐85. [PUBMED: 9842178] [DOI] [PubMed] [Google Scholar]
Brunelle 2003
- Brunelle D. Impact of a dedicated infusion therapy team on the reduction of catheter‐related nosocomial infections. Journal of Infusion Nursing 2003;26(6):362‐6. [PUBMED: 14624176] [DOI] [PubMed] [Google Scholar]
Camp‐Sorrell 2007
- Camp‐Sorrell D. Clinical dilemmas: vascular access devices. Seminars in Oncology Nursing 2007;23(3):232‐9. [PUBMED: 17693350] [DOI] [PubMed] [Google Scholar]
Carr 2010
- Carr PJ, Glynn RW, Dineen B, Kropmans TJB. A pilot intravenous cannulation team: an Irish perspective. British Journal of Nursing 2010;19(10):s19‐27. [PUBMED: 20622770] [DOI] [PubMed] [Google Scholar]
Carr 2017
- Carr PJ, Higgins NS, Cooke ML, Rippey J, Rickard CM. Tools, clinical prediction rules, and algorithms for the insertion of peripheral intravenous catheters in adult hospitalized patients: a systematic scoping review of literature. Journal of Hospital Medicine 2017;12(10):851‐8. [DOI: 10.12788/jhm.2836; PUBMED: 28991954] [DOI] [PubMed] [Google Scholar]
Castro‐Sánchez 2014
- Castro‐Sánchez E, Charani E, Drumright LN, Sevdalis N, Shah N, Holmes AH. Fragmentation of care threatens patient safety in peripheral vascular catheter management in acute care ‐ a qualitative study. PLoS ONE 2014;9(1):1‐6. [PUBMED: 24454958] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chopra 2013
- Chopra V, Anand S, Hickner A, Buist M, Rogers MA, Saint S, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet 2013;382(9889):311‐25. [PUBMED: 23697825] [DOI] [PubMed] [Google Scholar]
Chopra 2017
- Chopra V, Kuhn L, Ratz D, Shader S, Vaughn VM, Saint S, et al. Vascular access specialist training, experience, and practice in the United States: results from the national PICC1 survey. Journal of Infusion Nursing 2017;40(1):15‐25. [PUBMED: 28030479] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence 2017. Version accessed 8 February 2017. Melbourne, Australia: Veritas Health Innovation.
da Silva 2010
- Silva GA, Priebe S, Dias FN. Benefits of establishing an intravenous team and the standardization of peripheral intravenous catheters. Journal of Infusion Nursing 2010;33(3):156‐60. [PUBMED: 20442599] [DOI] [PubMed] [Google Scholar]
Davis 2016
- Davis L, Owens AK, Thompson J. Defining the specialty of vascular access through consensus: shaping the future of vascular access. Journal of the Association for Vascular Access 2016;21(3):125‐30. [Embase: 20160904859] [Google Scholar]
Dougherty 2008
- Dougherty L. IV therapy: recognizing the differences between infiltration and extravasation. British Journal of Nursing 2008;17(14):896‐901. [PUBMED: 18935841] [DOI] [PubMed] [Google Scholar]
Egan 2013
- Egan G, Healy D, O'Neill H, Clarke‐Moloney M, Grace PA, Walsh SR. Ultrasound guidance for difficult peripheral venous access: systematic review and meta‐analysis. Emergency Medicine Journal 2013;30(7):521‐6. [PUBMED: 22886890] [DOI] [PubMed] [Google Scholar]
Endnote 2012 [Computer program]
- Endnote. Thomson Reuters [Endnote X6]. Version X6. Thomson Reuters, 2012.
Ge 2012
- Ge X, Cavallazzi R, Li C, Pan SM, Wang YW, Wang F. Central venous access sites for the prevention of venous thrombosis, stenosis and infection. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD004084.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasziou 2014
- Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014;383(9913):267‐76. [PUBMED: 24411647] [DOI] [PubMed] [Google Scholar]
Gopal 2006
- Gopal K, Fitzsimmons L, Lawrance JA. Nurse‐led central venous catheter service: Christie experience. British Journal of Radiology 2006;79(945):762‐5. [PUBMED: 16641413] [DOI] [PubMed] [Google Scholar]
Gorski 2016
- Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M. Infusion therapy standards of practice. Journal of Infusion Nursing. 2016;39(Suppl 1):S1‐159. [ISSN 1533‐1458] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 8 February 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Green 1998
- Green C, Yohannan GC. Umbilical arterial and venous catheters: placement, use, and complications. Neonatal Network 1998;17(6):23‐8. [PUBMED: 9832755] [PubMed] [Google Scholar]
Guilbert 2008
- Guilbert MC, Elkouri S, Bracco D, Corriveau MM, Beaudoin N, Dubois MJ, et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. Journal of Vascular Surgery 2008;48(4):918‐25. [PUBMED: 18703308] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harnage 2012
- Harnage S. Seven years of zero central‐line‐associated bloodstream infections. British Journal of Nursing 2012;21(21):S6‐12. [PUBMED: 23469515] [DOI] [PubMed] [Google Scholar]
Heinrichs 2013
- Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta‐analysis of new interventions for peripheral intravenous cannulation of children. Pediatric Emergency Care 2013;29(7):858‐66. [PUBMED: 23823270] [DOI] [PubMed] [Google Scholar]
Helm 2015
- Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable. Journal of Infusion Nursing 2015;38(3):189‐203. [Embase: 608443533] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jackson 2012
- Jackson A, Coop S. Zero central‐line infections in a 550‐bedded district general hospital. British Journal of Nursing 2012;21(14):S24, S26‐8. [PUBMED: 23252179] [DOI] [PubMed] [Google Scholar]
Kelly 2009
- Kelly LJ. The family of vascular access devices. Journal of Infection Prevention 2009;10:s7‐12. [DOI: 10.1177/1757177409342156] [DOI] [Google Scholar]
Keogh 2014
- Keogh S, Marsh N, Higgins N, Davies K, Rickard C. A time and motion study of peripheral venous catheter flushing practice using manually and prefilled flush syringes. Journal of Infusion Nursing 2014;37(2):96‐101. [PUBMED: 24583939] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville F. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Legemaat 2015
- Legemaat MM, Jongerden IP, Rens RM, Zielman M, Hoogen A. Effect of a vascular access team on central line‐associated bloodstream infections in infants admitted to a neonatal intensive care unit: a systematic review. International Journal of Nursing Studies 2015;52(5):1003‐10. [PUBMED: 25526669] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [PUBMED: 19621070] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maki 2006
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clinic Proceedings 2006;81(9):1159‐71. [PUBMED: 16970212] [DOI] [PubMed] [Google Scholar]
Marsh 2015
- Marsh N, Webster J, Mihala G, Rickard CM. Devices and dressings to secure peripheral venous catheters to prevent complications. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011070.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mermel 2017
- Mermel LA. Short‐term peripheral venous catheter–related bloodstream infections: a systematic review. Clinical Infectious Diseases 2017;65(10):1757‐62. [DOI: 10.1093/cid/cix562; PUBMED: 29020252 ] [DOI] [PubMed] [Google Scholar]
Moureau 2012
- Moureau N, Trick N, Nifong T, Perry C, Kelley C, Carrico R, et al. Vessel health and preservation (part 1): a new evidence‐based approach to vascular access selection and management. Journal of Vascular Access 2012;13(3):351‐6. [PUBMED: 22307471] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moureau 2013
- Moureau N, Lamperti M, Kelly LJ, Dawson R, Elbarbary M, Boxtel AJH, et al. Evidence‐based consensus on the insertion of central venous access devices: definition of minimal requirements for training. British Journal of Anaesthesia 2013;110(3):347‐56. [PUBMED: 23361124] [DOI] [PubMed] [Google Scholar]
O'Grady 2011
- O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Summary of recommendations: guidelines for the prevention of intravascular catheter‐related infections. Clinical Infectious Diseases 2011;52(9):162‐93. [PUBMED: 21467014] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2016
- Parker SIA, Benzies KM, Hayden KA, Lang ES. Effectiveness of interventions for adult peripheral intravenous catheterization: a systematic review and meta‐analysis of randomized controlled trials. International Emergency Nursing 2016;31:15‐21. [PUBMED: 27411965] [DOI] [PubMed] [Google Scholar]
Reades 2011
- Reades R, Studnek JR, Vandeventer S, Garrett J. Intraosseous versus intravenous vascular access during out‐of‐hospital cardiac arrest: a randomized controlled trial. Annals of Emergency Medicine 2011;58(6):509‐16. [DOI: 10.1016/j.annemergmed.2011.07.020; PUBMED: 21856044 ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rickard 2015
- Rickard CM, Marsh NM, Webster J, Gavin NC, McGrail MR, Larsen E, et al. Intravascular device administration sets: replacement after standard versus prolonged use in hospitalised patients ‐ a study protocol for a randomised controlled trial (The RSVP Trial). BMJ Open 2015;5(2):e007257. [PUBMED: 25649214] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riker 2011
- Riker MW, Kennedy C, Winfrey BS, Yen K, Dowd MD. Validation and refinement of the difficult intravenous access score: a clinical prediction rule for identifying children with difficult intravenous access. Academic Emergency Medicine 2011;18(11):1129‐34. [PUBMED: 22092893] [DOI] [PubMed] [Google Scholar]
Sabri 2013
- Sabri A, Szalas J, Holmes KS, Labib L, Mussivand T. Failed attempts and improvement strategies in peripheral intravenous catheterization. Bio‐Medical Materials and Engineering 2013;23(1‐2):93‐108. [PUBMED: 23442240] [DOI] [PubMed] [Google Scholar]
Scheer 2002
- Scheer B, Perel A, Pfeiffer U. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Critical Care 2002;6(3):199‐204. [PUBMED: 12133178] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings’ tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [PUBMED: 21784880] [DOI] [PubMed] [Google Scholar]
Stolz 2015
- Stolz LA, Stolz U, Howe C, Farrell IJ, Adhikari S. Ultrasound‐guided peripheral venous access: a meta‐analysis and systematic review. Journal of Vascular Access 2015;16(4):321‐6. [DOI: 10.5301/jva.5000346; PUBMED: 25656255] [DOI] [PubMed] [Google Scholar]
Tagalakis 2002
- Tagalakis V, Kahn SR, Libman M, Blostein M. The epidemiology of peripheral vein infusion thrombophlebitis: a critical review. American Journal of Medicine 2002;113(2):146‐51. [PUBMED: 12133753 ] [DOI] [PubMed] [Google Scholar]
Wallis 2014
- Wallis MC, McGrail M, Webster J, Marsh N, Gowardman J, Playford EG, et al. Risk factors for peripheral intravenous catheter failure: a multivariate analysis of data from a randomized controlled trial. Infection Control Hospital Epidemiology 2014;35(1):63‐8. [PUBMED: 24334800] [DOI] [PubMed] [Google Scholar]
Webster 2008
- Webster J, Clarke S, Paterson D, Hutton A, Dyk S, Gale C, et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ 2008;337:a339. [PUBMED: 18614482] [DOI] [PMC free article] [PubMed] [Google Scholar]
Webster 2015
- Webster J, Osborne S, Rickard CM, New K. Clinically‐indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD007798.pub4] [DOI] [PubMed] [Google Scholar]
Wu 2013
- Wu SY, Ling Q, Cao LH, Wang J, Xu MX, Zeng WA. Real‐time two‐dimensional ultrasound guidance for central venous cannulation: a meta‐analysis. Anesthesiology 2013;118(2):361‐75. [PUBMED: 23249991] [DOI] [PubMed] [Google Scholar]
Yacopetti 2010
- Yacopetti N, Alexandrou E, Spencer TR, Frost SA, Davidson PM, O'Sullivan G, et al. Central venous catheter insertion by a clinical nurse consultant or anaesthetic medical staff: a single‐centre observational study. Critical Care and Resuscitation 2010;12(2):90‐5. [PUBMED: 20513216] [PubMed] [Google Scholar]
References to other published versions of this review
Carr 2014
- Carr PJ, Higgins NS, Cooke ML, Mihala G, Rickard CM. Vascular access specialist teams for device insertion and prevention of failure. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD011429] [DOI] [PMC free article] [PubMed] [Google Scholar]


