Abstract
Background
Nicotine replacement therapy (NRT) aims to temporarily replace much of the nicotine from cigarettes to reduce motivation to smoke and nicotine withdrawal symptoms, thus easing the transition from cigarette smoking to complete abstinence.
Objectives
To determine the effectiveness and safety of nicotine replacement therapy (NRT), including gum, transdermal patch, intranasal spray and inhaled and oral preparations, for achieving long‐term smoking cessation, compared to placebo or 'no NRT' interventions.
Search methods
We searched the Cochrane Tobacco Addiction Group trials register for papers mentioning 'NRT' or any type of nicotine replacement therapy in the title, abstract or keywords. Date of most recent search is July 2017.
Selection criteria
Randomized trials in people motivated to quit which compared NRT to placebo or to no treatment. We excluded trials that did not report cessation rates, and those with follow‐up of less than six months. We recorded adverse events from included and excluded studies that compared NRT with placebo. Studies comparing different types, durations, and doses of NRT, and studies comparing NRT to other pharmacotherapies, are covered in separate reviews.
Data collection and analysis
Screening, data extraction and 'Risk of bias' assessment followed standard Cochrane methods. The main outcome measure was abstinence from smoking after at least six months of follow‐up. We used the most rigorous definition of abstinence for each trial, and biochemically validated rates if available. We calculated the risk ratio (RR) for each study. Where appropriate, we performed meta‐analysis using a Mantel‐Haenszel fixed‐effect model.
Main results
We identified 136 studies; 133 with 64,640 participants contributed to the primary comparison between any type of NRT and a placebo or non‐NRT control group. The majority of studies were conducted in adults and had similar numbers of men and women. People enrolled in the studies typically smoked at least 15 cigarettes a day at the start of the studies. We judged the evidence to be of high quality; we judged most studies to be at high or unclear risk of bias but restricting the analysis to only those studies at low risk of bias did not significantly alter the result. The RR of abstinence for any form of NRT relative to control was 1.55 (95% confidence interval (CI) 1.49 to 1.61). The pooled RRs for each type were 1.49 (95% CI 1.40 to 1.60, 56 trials, 22,581 participants) for nicotine gum; 1.64 (95% CI 1.53 to 1.75, 51 trials, 25,754 participants) for nicotine patch; 1.52 (95% CI 1.32 to 1.74, 8 trials, 4439 participants) for oral tablets/lozenges; 1.90 (95% CI 1.36 to 2.67, 4 trials, 976 participants) for nicotine inhalator; and 2.02 (95% CI 1.49 to 2.73, 4 trials, 887 participants) for nicotine nasal spray. The effects were largely independent of the definition of abstinence, the intensity of additional support provided or the setting in which the NRT was offered. Adverse events from using NRT were related to the type of product, and include skin irritation from patches and irritation to the inside of the mouth from gum and tablets. Attempts to quantitatively synthesize the incidence of various adverse effects were hindered by extensive variation in reporting the nature, timing and duration of symptoms. The odds ratio (OR) of chest pains or palpitations for any form of NRT relative to control was 1.88 (95% CI 1.37 to 2.57, 15 included and excluded trials, 11,074 participants). However, chest pains and palpitations were rare in both groups and serious adverse events were extremely rare.
Authors' conclusions
There is high‐quality evidence that all of the licensed forms of NRT (gum, transdermal patch, nasal spray, inhalator and sublingual tablets/lozenges) can help people who make a quit attempt to increase their chances of successfully stopping smoking. NRTs increase the rate of quitting by 50% to 60%, regardless of setting, and further research is very unlikely to change our confidence in the estimate of the effect. The relative effectiveness of NRT appears to be largely independent of the intensity of additional support provided to the individual. Provision of more intense levels of support, although beneficial in facilitating the likelihood of quitting, is not essential to the success of NRT. NRT often causes minor irritation of the site through which it is administered, and in rare cases can cause non‐ischaemic chest pain and palpitations.
Plain language summary
Can nicotine replacement therapy (NRT) help people quit smoking?
Background
We reviewed the evidence about whether NRT helps people who want to quit smoking to stop smoking at six months or longer. NRT aims to reduce withdrawal symptoms associated with stopping smoking by replacing the nicotine from cigarettes. NRT is available as skin patches that deliver nicotine slowly, and chewing gum, nasal and oral sprays, inhalators, and lozenges/tablets, all of which deliver nicotine to the brain more quickly than skin patches, but less rapidly than from smoking cigarettes.
Study characteristics
This review includes 136 trials of NRT, with 64,640 people in the main analysis. All studies were conducted in people who wanted to quit smoking. Most studies were conducted in adults and had similar numbers of men and women. People enrolled in the studies typically smoked at least 15 cigarettes a day at the start of the studies. The evidence is current to July 2017. Trials lasted for at least six months.
Key results
We found evidence that all forms of NRT made it more likely that a person's attempt to quit smoking would succeed. The chances of stopping smoking were increased by 50% to 60%. NRT works with or without additional counselling, and does not need to be prescribed by a doctor. Side effects from using NRT are related to the type of product, and include skin irritation from patches and irritation to the inside of the mouth from gum and tablets. There is no evidence that NRT increases the risk of heart attacks.
Quality of evidence
The overall quality of the evidence is high, meaning that further research is very unlikely to change our conclusions.
Summary of findings
Summary of findings for the main comparison. Nicotine replacement therapy.
| Nicotine replacement therapy versus control for smoking cessation | ||||||
| Patient or population: people who smoke cigarettes Settings: clinical and non‐clinical, including over the counter Intervention: nicotine replacement therapy of any form | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Nicotine replacement therapy of any form | |||||
| Smoking cessation at 6+ months follow‐up Follow‐up: 6 to 24 months | Study population | RR 1.55 (1.49 to 1.61) | 64,640 (133 studies) | ⊕⊕⊕⊕ high1, 2 | ||
| 105 per 1000 | 162 per 1000 (156 to 168) | |||||
| Limited behavioural support | ||||||
| 40 per 1000 | 62 per 1000 (60 to 64) | |||||
| Intensive behavioural support | ||||||
| 150 per 1000 | 232 per 1000 (224 to 242) | |||||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Most studies are judged to be at unclear or high risk of bias, but restricting to only studies at low risk of bias did not significantly alter the effect. 2There are likely to be some unpublished trials with less favourable results that we were unable to identify, and a funnel plot showed some evidence of asymmetry. However, given the large number of trials in the review, this does not suggest the results would be altered significantly were smaller studies with lower RRs included.
Background
Nicotine replacement therapy (NRT) aims to reduce motivation to smoke and the physiological and psychomotor withdrawal symptoms often experienced during an attempt to stop smoking, and therefore increase the likelihood of remaining abstinent (West 2001). Nicotine undergoes first‐pass metabolism in the liver, reducing the overall bioavailability of swallowed nicotine pills. A pill that could reliably produce high enough nicotine levels in the central nervous system would risk causing adverse gastrointestinal effects. To avoid this problem, nicotine replacement products are formulated for absorption through the oral or nasal mucosa (chewing gum, lozenges, sublingual tablets, inhalator, spray) or through the skin (transdermal patches).
Nicotine patches differ from the other products in that they deliver the nicotine dose slowly and passively. They do not replace any of the behavioural activities of smoking. In contrast the other types of NRT are faster‐acting, but require more effort on the part of the user. Transdermal patches are available in several different doses, and deliver between 5 mg and 52.5 mg of nicotine over a 24‐hour period, resulting in plasma levels similar to the trough levels seen in heavy smokers (Fiore 1992). Some brands of patch are designed to be worn for 24 hours whilst others are to be worn for 16 hours each day. Nicotine gum is available in both 2 mg and 4 mg strengths, and nicotine lozenges are available in 1 mg, 1.5 mg, 2 mg and 4 mg strengths. Nicotine nasal sprays are available in either 0.5 mg or 1 mg per spray strengths, and nicotine inhalators are available in both 10 mg and 15 mg strengths. The amount of nicotine absorbed by the user is less than the original dose. None of the available products deliver such high doses of nicotine as quickly as cigarettes. An average cigarette delivers between 1 and 3 mg of nicotine and a person who smokes 20 cigarettes per day absorbs 20 to 40 mg of nicotine each day (Henningfield 2005).
The availability of NRT products on prescription or for over‐the‐counter purchase varies from country to country. Table 2 summarises the products currently licensed in the United Kingdom.
1. Nicotine replacement therapies available in the UK.
| Type | Available doses |
| Nicotine transdermal patches | Worn over 16 hours: 5 mg, 10 mg, 15 mg, 25 mg doses Worn over 24 hours: 7 mg, 14 mg, 20 mg, 21 mg, 30 mg doses* |
| Nicotine chewing gum | 2 mg and 4 mg doses |
| Nicotine sublingual tablet | 2 mg dose |
| Nicotine lozenge | 1 mg, 1.5 mg, 2 mg and 4 mg doses |
| Nicotine inhalation cartridge plus mouthpiece | Cartridge containing 10 mg |
| Nicotine metered nasal spray | 0.5 mg dose/spray |
| Nicotine oral spray | 1 mg dose/spray |
Information extracted from British National Formulary
* 35 mg/24‐hour and 53.5 mg/24‐hour patches available in other regions.
This review was first published over 20 years ago, in 1996, and has been regularly updated since. In previous versions, this review addressed not only the effect of NRT in comparison to placebo for helping people stop smoking, but also looked at comparisons between different forms and doses of NRT, and between NRT and different pharmacotherapies. The evidence that NRT helps some people to stop smoking is now well accepted, and many clinical guidelines recommend NRT as a first‐line treatment for people seeking pharmacological help to stop smoking (Fiore 2008; Italy ISS 2004; Le Foll 2005; NICE 2008; NZ MoH 2014; Woolacott 2002; Zwar 2011). We have therefore split the previous version of the review; this review now only looks at NRT versus placebo or no pharmacotherapy, with the intention that, given the stability of this comparison, this review will no longer require regular updates. Studies which compare doses, delivery, forms, and schedules of NRT will now be covered in a companion review, which will continue to be regularly updated, and is in development at the time of writing. Comparisons between NRT and other frontline pharmacotherapies are covered in separate Cochrane Reviews (Cahill 2016; Hughes 2014). Where they meet our other inclusion criteria, studies of NRT in pregnancy are included in the main analysis of this review but are covered comprehensively in a separate Cochrane Review (Coleman 2015), which will continue to be updated. Readers specifically interested in NRT in pregnancy should refer to Coleman 2015.
Objectives
To determine the effectiveness and safety of nicotine replacement therapy (NRT), including gum, transdermal patch, intranasal spray and inhaled and oral preparations, for achieving long‐term smoking cessation, compared to placebo or 'no NRT' interventions.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials. We also include trials where allocation to treatment was by a quasi‐randomized method, but use appropriate sensitivity analysis to determine whether their inclusion alters the results.
Types of participants
We include men or women who smoked and were motivated to quit, irrespective of the setting from which they were recruited or their initial level of nicotine dependence, or both. We included studies that randomized therapists, rather than smokers, to offer NRT or a control, provided that the specific aim of the study was to examine the effect of NRT on smoking cessation. We have not included trials that randomized physicians or other therapists to receive an educational intervention, which included encouraging their patients to use NRT, but have reviewed them separately (Carson 2012).
Types of interventions
Comparisons of NRT (including chewing gum, transdermal patches, nasal and oral spray, inhalators and tablets or lozenges) versus placebo or no NRT control. The terms 'inhaler' and 'inhalator' (an oral device which delivers nicotine to the buccal mucosa by sucking) are used interchangeably in the literature. We have used the term 'inhalator' throughout the rest of this review.
In some analyses we categorized the trials into groups depending on the level of additional support provided (low or high). The definition of the low‐intensity category was intended to identify a level of support that could be offered as part of the provision of routine medical care. If the duration of time spent with the smoker (including assessment for the trial) exceeded 30 minutes at the initial consultation or the number of further assessment and reinforcement visits exceeded two, we categorized the level of additional support as high. The high‐intensity category included trials where there were a large number of visits to the clinic or trial centre, but these were often brief, spread over an extended period during treatment and follow‐up, and did not include a specific counselling component. To provide a more fine‐grained analysis and to distinguish between high‐intensity group‐based support and other trials within the high‐intensity category, we have therefore specified where the support included multi‐session group‐based counselling with frequent sessions around the quit date.
Previously, this review had also included studies where all arms received NRT (e.g. testing different doses, types) and studies comparing NRT with bupropion. These comparisons are now covered elsewhere; comparisons between different NRT treatments are covered in a companion review, currently under development, and comparisons between NRT and bupropion are found in Hughes 2014.
Types of outcome measures
The review evaluates the effects of NRT versus control on smoking cessation, rather than on withdrawal symptoms. We excluded trials that followed up participants for less than six months, except for trials amongst pregnant women, where the interval between enrolment and delivery may have been shorter (if less than six months, these were excluded from the main analysis). For each study we chose the strictest available criteria to define abstinence. For example, in studies where biochemical validation of cessation was available, we regard only those participants who met the criteria for biochemically‐confirmed abstinence as being abstinent. Wherever possible we chose a measure of sustained cessation rather than point prevalence. We regard people who were lost to follow‐up as being continuing smokers. For the 2012 update and for this current update we collected data on adverse events in both the included and excluded studies, where they were reported. We have not attempted to pool these findings, apart from one meta‐analysis of reports of palpitations, tachycardia or chest pains. We have not included trials that evaluated the effect of NRT for individuals who were attempting to reduce the number of cigarettes smoked rather than to quit in this review. They are covered by a separate review on harm reduction approaches (Lindson‐Hawley 2016).
Search methods for identification of studies
We searched the specialized register of the Cochrane Tobacco Addiction Group on 6 July 2017 for any reports of trials making reference to the use of nicotine replacement therapy of any type, by searching for 'NRT', or 'nicotine' near to terms for nicotine replacement products in the title, abstract or keywords. The most recent issues of the databases included in the register as searched for the current update of this review were:
Cochrane Central Register of Controlled trials (CENTRAL), issue 11, 2016;
MEDLINE (via OVID) to update 20170526;
Embase (via OVID) to week 201724;
PsycINFO (via OVID) to update 20170529.
The search strategy for the Register is given in Appendix 1. For details of the searches used to create the specialized register see the Tobacco Addiction Group Module in the Cochrane Library. The trials register also includes trials identified by handsearching of abstract books from meetings of the Society for Research on Nicotine and Tobacco.
For earlier versions of this review we performed searches of additional databases: Cancerlit, Health Planning and Administration, Social Scisearch, Smoking & Health, and Dissertation Abstracts. Since the searches did not produce any additional trials we did not search these databases after December 1996. During preparation of the first version of this review, we also sent letters to manufacturers of NRT preparations. Since this did not result in additional data we have not repeated the exercise for subsequent updates.
Data collection and analysis
Selection of studies
In previous versions of this review, one review author screened records retrieved by searches, to exclude papers that were not reports of potentially relevant studies. For the last two updates, two review authors independently screened references. Reports that linked to potentially relevant studies but did not report the outcomes of interest are listed along with the main study report in the 'References to Studies' section. The primary reference to the study is indicated, and for most studies the first author and year used as the study identifier corresponds to the primary reference. Where we extracted data for a study from more than one report we have noted this in the Characteristics of included studies table.
Data extraction and management
Two review authors independently extracted data from the published reports and abstracts. We resolved disagreements by discussion or by referral to a third party. We made no attempt to blind these review authors either to the results of the primary studies or to which treatment participants received. We examined reports published only in non‐English language journals with the assistance of translators.
Assessment of risk of bias in included studies
We assessed included studies for risks of selection bias (methods of randomized sequence generation, and allocation concealment), performance and detection bias (the presence or absence of blinding), attrition bias (levels and reporting of loss to follow‐up), and any other threats to study validity, using the Cochrane 'Risk of bias' tool.
Measures of treatment effect
We extracted smoking cessation rates in the intervention and control groups from the reports at six or 12 months. Since not all studies reported cessation rates at exactly these intervals, we allowed a window period of six weeks at each follow‐up point. For trials which also reported follow‐up for more than a year we used 12‐month outcomes in most cases. (We note length of follow‐up for each study in the Characteristics of included studies table). For trials of NRT in pregnant women, we extracted smoking cessation outcomes at the closest follow‐up to end of pregnancy, and also at longest follow‐up post‐partum if reported. We only included studies in pregnant women in the main analysis if they reported results at six months or longer. Following the Cochrane Tobacco Addiction Group's recommended method of data analysis, we use the risk ratio (RR) for summarizing individual trial outcomes and for estimates of pooled effect. Whilst there are circumstances in which odds ratios may be preferable, there is a danger that they will be interpreted as if they are risk ratios, making the treatment effect seem larger (Deeks 2005).
Dealing with missing data
We treated participants who dropped out or who were lost to follow‐up after randomization as being continuing smokers. We noted in the 'Risk of bias' table the proportion of participants for whom the outcome was imputed in this way, and whether there was either high or differential loss to follow‐up. The assumption that 'missing = smoking' will give conservative absolute quit rates, and will make little difference to the risk ratio unless dropout rates differ substantially between groups.
Assessment of heterogeneity
To assess heterogeneity we use the I2 statistic, given by the formula [(Q ‐ df)/Q] x 100%, where Q is the Chi2 statistic and df is its degrees of freedom (Higgins 2003). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). A value greater than 50% may be considered to indicate substantial heterogeneity. When there are many trials, as in this review, the Chi2 test for heterogeneity will be unduly powerful and may identify statistically significant but clinically unimportant heterogeneity.
Data synthesis
We estimated a pooled weighted average of risk ratios using a fixed‐effect Mantel‐Haenszel method, with 95% confidence intervals.
Subgroup analysis and investigation of heterogeneity
In comparing NRT to placebo or control, we performed subgroup analysis for each form of NRT. We did additional subgroup analyses within type of NRT (gum, patch, etc.) to investigate whether the relative treatment effect differed according to the way in which smoking cessation was defined, the intensity of behavioural support, and the recruitment/treatment setting.
Summary of findings table
Following standard Cochrane methodology, we created a 'Summary of findings' table. Also following standard Cochrane methodology, we used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome, and to draw conclusions about the quality of evidence within the text of the review.
The Cochrane Tobacco Addiction Group's Glossary of smoking‐related terms is included in this review (Appendix 2).
Results
Description of studies
Included studies
The review includes 136 studies, 18 of which are new in this update (Anthenelli 2016; Berlin 2014; Cummins 2016; Cunningham 2016; El‐Mohandes 2013; Fraser 2014; Gallagher 2007; Graham 2017; Hasan 2014; Heydari 2012; Heydari 2013; Johns 2017; Lerman 2015; NCT00534404; Scherphof 2014; Stein 2013; Tuisku 2016; Ward 2013). Two studies which gave different doses of NRT based on level of dependency are treated as four separate trials for the purpose of this review (Shiffman 2002 (2 mg); Shiffman 2002 (4 mg); Shiffman 2009 (2 mg); Shiffman 2009 (4 mg)). For this update, we also added longer follow‐up data for one previously included study (Coleman 2012). The most recent search screened 1059 studies. Along with the 18 new included studies, there were three ongoing studies, and 124 studies excluded at full‐text screening. The most common reasons for exclusion were ineligible study design and using an irrelevant comparison (NRT vs NRT rather than control). See Figure 1 for study flow information relating to the most recent search presented in a PRISMA diagram. Trials were conducted in North America (62 studies), Europe (56 studies), Australasia (two studies), Japan (two studies), South America (two studies), Iran (two studies), in multiple regions (two studies), and in India, Syria, Taiwan, and Thailand (one study each). The median sample size was 257 but ranged from fewer than 50 to over 8000 participants. We treated each of the intervention groups in the two studies by Shiffman in 2002 and 2009 separately in the meta‐analysis (Shiffman 2002 (2 mg); Shiffman 2002 (4 mg); Shiffman 2009 (2 mg); Shiffman 2009 (4 mg)), and listed Brantmark 1973b, CEASE 1999, Bolliger 2000b, Wennike 2003b, Bullen 2010, Schnoll 2010 in the Characteristics of included studies tables, despite being excluded studies, because they provided data on adverse events.
1.
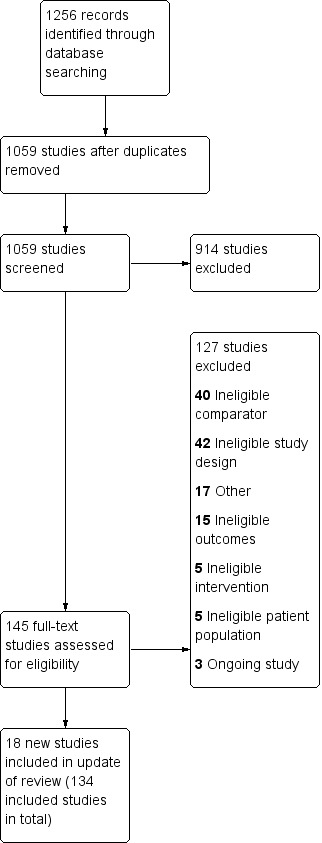
Study flow diagram for most recent update
Participants
Participants were typically adult cigarette smokers with an average age of 40 to 50. Two trials recruited adolescents (Moolchan 2005; Scherphof 2014). Most trials had approximately similar numbers of men and women. Six trials recruited only pregnant women (Berlin 2014; Coleman 2012; El‐Mohandes 2013; Oncken 2008; Pollak 2007;Wisborg 2000); a further four recruited only women (Cooper 2005; Oncken 2007; Pirie 1992; Prapavessis 2007). Two trials recruited African‐American smokers (Ahluwalia 1998; Ahluwalia 2006).
Trials typically recruited people who smoked at least 15 cigarettes a day. Although some trials included lighter smokers as well, the average number smoked was over 20 a day in most studies. Ahluwalia 2006 recruited only people who smoked 10 or fewer cigarettes a day and two trials recruited only people smoking 30 or more a day (Hughes 1990; Hughes 2003). One trial recruited people with a history of alcohol dependence (Hughes 2003), one recruited methadone‐maintained smokers (Stein 2013), and one recruited people with a history of drug abuse including opiates or narcotics (Heydari 2013). Joseph 1996 recruited people with a history of cardiac disease, Hasan 2014 recruited people admitted to hospital with a cardiac or pulmonary illness, Gallagher 2007 recruited people diagnosed with psychotic‐spectrum or affective disorders resulting in long‐term mental illness and experiencing significant symptoms and functional impairment, and Gourlay 1995 recruited relapsed smokers.
Type and dose of nicotine replacement therapy
One hundred and thirty‐three studies contribute to the primary analysis of the efficacy of one or more types of NRT compared to a placebo or other control group not receiving any type of NRT. In this group of studies there were 56 trials of nicotine gum, 51 of transdermal nicotine patch, eight of an oral nicotine tablet or lozenge, seven offering a choice of products, four of intranasal nicotine spray, four of nicotine inhalator, two providing patch and gum (Hasan 2014; Stein 2013), one of oral spray (Tønnesen 2012), one providing patch and inhalator (Hand 2002), one providing patch and lozenge (Piper 2009), and one providing patch, gum and lozenge (Heydari 2013).
Three studies did not contribute to the primary analysis; two were conducted in pregnant women and did not follow up participants at six months or longer (Berlin 2014; El‐Mohandes 2013), and one was conducted in recently relapsed smokers and is hence reported narratively in the text (Gourlay 1995).
Most trials comparing nicotine gum to control provided the 2 mg dose. A few provided 4 mg gum to more highly addicted smokers, and two used only the 4 mg dose (Blondal 1989; Puska 1979). In three trials the physician offered nicotine gum but participants did not necessarily accept or use it (Ockene 1991; Page 1986; Russell 1983). In one trial participants self‐selected 2 mg or 4 mg doses; we treat the two groups as separate trials in the meta‐analysis (Shiffman 2009 (2 mg); Shiffman 2009 (4 mg)). The treatment period was typically two to three months, but ranged from three weeks to 12 months. Some trials did not specify how long the gum was available. Many of the trials included a variable period of dose tapering, but most encouraged participants to be gum‐free by six to 12 months.
In nicotine patch trials the usual maximum daily dose was 15 mg for a 16‐hour patch, or 21 mg for a 24‐hour patch. Thirty‐two studies used a 24‐hour formulation and nine a 16‐hour product; the rest did not specify. One study offered, among other dosage options, a 52.5 mg/24‐hour patch (Wittchen 2011). If studies tested more than one dose we combined all active arms in the comparison to placebo. For one study we included an arm with a lower maximum dose of 14 mg but excluded a 7 mg‐dose arm (TNSG 1991). The minimum duration of therapy ranged from three weeks (Glavas 2003a, half the participants of Glavas 2003b), to three months.
There are eight studies of nicotine sublingual tablets or lozenges. Three used 2 mg sublingual tablets (Glover 2002; Tønnesen 2006; Wallstrom 2000). One used a 1 mg nicotine lozenge (Dautzenberg 2001). One used 2 mg or 4 mg lozenges according to dependence level based on manufacturers' instructions (Piper 2009), and one used 2 mg or 4 mg based on participants' time to first cigarette of the day (TTFC); smokers whose TTFC was more than 30 minutes were randomized to 2 mg lozenges or placebo (Shiffman 2002 (2 mg)), whilst smokers with a TTFC less than 30 minutes had higher‐dose 4 mg lozenges or placebo (Shiffman 2002 (4 mg)). The two groups are treated in the meta‐analysis as separate trials. One trial did not report the lozenge dose (Fraser 2014). There are four trials of intranasal nicotine spray (Blondal 1997; Hjalmarson 1994; Schneider 1995; Sutherland 1992), one trial of oral nicotine spray (Tønnesen 2012), and four trials of nicotine inhalator (Hjalmarson 1997; Leischow 1996a; Schneider 1996; Tønnesen 1993).
As described above, seven studies tested combinations of patch and a short‐acting form of NRT (Hand 2002; Hasan 2014; Heydari 2013; Kornitzer 1995; Moolchan 2005; Stein 2013; Tønnesen 2000). Six studies offered participants a choice of products (Graham 2017; Johns 2017; Kralikova 2009; Molyneux 2003; Ortega 2011; Pollak 2007).
Treatment setting (studies in main comparison)
Twenty‐one trials in the main comparison recruited participants from primary care practices. A further two gum trials were undertaken in workplace clinics (Fagerström 1984; Roto 1987), and one in a university clinic (Harackiewicz 1988). One trial recruited through community physicians (Niaura 1994). Since participants in these trials were recruited in a similar way to primary care, we have aggregated them in the subgroup analysis by setting. We also included one patch trial conducted in Veterans Affairs Medical Centers and recruiting people with cardiac diseases in the primary care category (Joseph 1996). We kept four trials recruiting pregnant women in antenatal clinics in a separate category (Coleman 2012; Oncken 2008; Piper 2009; Wisborg 2000). Six of the gum trials, two of the nasal spray trials, an inhalator trial, an oral spray trial, and a patch trial were carried out in specialized smoking cessation clinics to which participants had usually been referred. Thirteen trials (five patch, three gum, three giving a combination of products and two giving a choice of products) were undertaken with hospital in‐ or outpatients, some of whom were recruited because they had a co‐existing smoking‐related illness. Three patch trials (Davidson 1998; Hays 1999; Sønderskov 1997) and one gum trial (split into Shiffman 2009 (2 mg) and Shiffman 2009 (4 mg)) were undertaken in settings intended to resemble 'over‐the‐counter' (OTC) use of NRT. Two trials were undertaken in drug abuse treatment centres (Heydari 2013; Stein 2013), one in schools (Scherphof 2014), and one in a psychiatric treatment setting. The remaining trials were undertaken in participants from the community, most of whom had volunteered in response to media advertisements, but who were treated in clinical settings.
Excluded studies
Thirty‐four previously included studies were removed from this update, as they did not contain a NRT‐versus‐control comparison. As described in the Methods, studies which contribute to comparisons between multiple forms of NRT are now found in a separate Cochrane Review, in development at the time of publication. Previously‐included studies that compare NRT with bupropion can be found in Hughes 2014. Other studies that were potentially relevant but excluded are listed with reasons in the Characteristics of excluded studies table. Some studies contribute to the adverse events meta‐analysis but not to the main analysis (e.g. due to short follow‐up or short duration of time where comparison was NRT versus control); these are listed in the Characteristics of included studies but we do not count them as included studies. Some studies were excluded due to short follow‐up. Some of these had as their primary outcome withdrawal symptoms rather than cessation. We exclude studies that provided NRT or placebo to people trying to cut down their smoking but not to make an immediate quit attempt, and we consider them in detail in a separate review of interventions for reduction (Lindson‐Hawley 2016). We excluded two trials in which NRT was provided to encourage a quit attempt but participants did not need to be planning to quit: Velicer 2006 proactively recruited people by telephone, with those in one intervention group being mailed a six‐week course of nicotine patches if they were judged to be in the preparation stage or in contemplation and had more pros than cons for quitting; Carpenter 2011 encouraged all participants to make a practice quit attempt, and gave the intervention group trial samples of nicotine lozenges. We excluded one trial in which callers to the NHS Quitline were randomized to be offered free NRT or not to receive the offer; the control group had access to and used free NRT and other stop‐smoking medication at high levels (Ferguson 2012).
Risk of bias in included studies
Six trials are included based only on data available from abstracts, conference presentations, or trial registries (Dautzenberg 2001; Johns 2017; Kralikova 2009; Mori 1992; Nakamura 1990; NCT00534404), so had limited methodological details.
Overall, we judged 12 studies to be at low risk of bias (low risk of bias across all domains), 36 at high risk of bias (high risk of bias in at least one domain), and the rest at unclear risk of bias. The main findings were not sensitive to the exclusion from the meta‐analysis of trials at unclear risk, or of trials at unclear and at high risk of bias. A summary illustration of the risk of bias profile across trials is shown in Figure 2.
2.
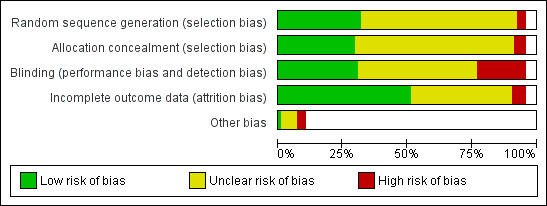
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Thirty‐nine studies (29%) reported allocation procedures in sufficient detail to be rated as being at low risk for their attempts to control selection bias, by using a system of treatment allocation which could not be known or predicted until a participant is enrolled and assigned to a study condition. Twenty‐four of these low‐risk trials (62%) also reported adequate sequence generation procedures. Most studies either did not report how randomization was performed and allocation concealed, or reported them in insufficient detail to determine whether a satisfactory attempt to control selection bias had been made (rated as being at unclear risk). A small number of nicotine gum trials randomized to treatment according to day or week of clinic attendance (Page 1986; Richmond 1993; Russell 1983), or to birth date (Fagerström 1984), and were consequently rated as being at high risk of bias. In one study (Gallagher 2007), study staff oversaw allocation and hence we rated this at high risk of bias. One study randomized by physician and there was no information about avoidance of selection bias in enrolment of smokers (Nebot 1992), so we also rated this as being at high risk.
We judged 44 of the included studies to be at low risk of performance and detection bias (33%). We judged 23 (17%) to be at high risk of bias in this domain, most commonly because they were not blinded (although we judged some studies which were not double‐blind to be at low risk in this domain due to other study factors). Forty‐three trials did not have a matched placebo control (24 gum trials, nine patch trials, six choice of product trials, three combination trials, and one lozenge trial). A further two had both a placebo and a non‐placebo control which we combined for the meta‐analysis control group (Buchkremer 1988; Russell 1983). Approximately one‐third of the trials reported some measure of blinding, but we did not assess whether the integrity of the procedure was tested, in line with the CONSORT guidelines (CONSORT 2001). Where they are done, assessments of blinding integrity should always be carried out before the clinical outcome has been determined, and the findings reported (Altman 2004). Mooney 2004 notes that few published trials report this information. While those that do provide some evidence that participants are likely to assess their treatment assignment correctly, it is insufficient to assess whether this is associated with differences in treatment effects. Further, there may be an apparent breaking of the blinding in trials where the treatment effect is marked, for either an intended outcome or an adverse event, but participants who successfully decipher assignment may disguise their unblinding actions (Altman 2004). It is also possible that those who believe that they are receiving a placebo may be more likely to stop trying to quit.
Definitions of abstinence varied considerably. Eighty‐nine trials (66%) reported some measure of sustained abstinence, which included continuous abstinence with not even a slip since quit day, repeated point prevalence abstinence (with or without biochemical validation) at multiple follow‐ups, or self‐reported abstinence for a prolonged period. Thirty‐nine (29%) reported only point prevalence abstinence at the longest follow‐up. In six studies it was unclear exactly how abstinence was defined. In four trials, participants who smoked two or three cigarettes a week were still classified as abstinent (Abelin 1989; Ehrsam 1991; Glavas 2003a; Glavas 2003b). Sensitivity analyses excluding these four trials made no difference to the overall findings. Most studies reported follow‐up at least 12 months from start of treatment. Fifteen gum trials, 19 patch trials, four combination trials, and one lozenge trial in the primary analysis had only six months follow‐up. We report the findings of a subgroup analysis by type of abstinence and length of follow‐up in the Results section.
One hundred and seventeen (87%) of the trials used biochemical validation of self‐reported smoking cessation at longest follow‐up. The most common form of validation was measurement of carbon monoxide (CO) in expired air. The 'cut‐off' level of CO used to define abstinence varied from less than 4 to 11 parts per million. Some of the 21 trials that did not validate all self‐report at longest follow‐up did use biochemical confirmation at earlier points, or validated some self‐reports. The main findings were not sensitive to the exclusion of 17 studies contributing to that analysis that did not attempt to validate all reported abstinence (Ahluwalia 1998; Buchkremer 1988; Clavel‐Chapelon 1992; Daughton 1991; Fraser 2014; Graham 2017; Huber 1988; NCT00534404; Otero 2006; Page 1986; Puska 1979; Roto 1987; Sønderskov 1997; Tuisku 2016; Villa 1999; Wisborg 2000; Wittchen 2011).
Effects of interventions
See: Table 1
Any type of NRT versus placebo or no NRT control, six months or longer follow‐up
Analysis 1.1 included 131 trials (133 comparisons), with over 64,000 participants (Table 1). A small number of trials contributed to more than one subgroup and two trials were treated as two separate studies in the analyses. Each of the six forms of nicotine replacement therapy (NRT) significantly increased the rate of cessation compared to placebo or no NRT, as did a choice of product. The pooled risk ratio (RR) for abstinence for any form of NRT relative to control was 1.55 (95% confidence interval (CI) 1.49 to 1.61; 64,640 participants). The I2 statistic was 39%, indicating that little of the variability was attributable to between‐trial differences. The risk ratio and 95% CI for each type are tabulated below.
1.1. Analysis.
Comparison 1 Any type of NRT versus placebo/no NRT control, Outcome 1 Smoking cessation at 6+ months follow up.
| Type of NRT | RR | 95% CI | I² | N of studies | N of participants Intervention/Control |
| Gum | 1.49 | 1.40 to 1.60 | 40% | 56* | 10,596 / 11,985 |
| Patch | 1.64 | 1.53 to 1.75 | 24% | 51 | 13,773 / 11,981 |
| Inhalator | 1.90 | 1.36 to 2.67 | 0% | 4 | 490 / 486 |
| Intranasal spray | 2.02 | 1.49 to 2.73 | 0% | 4 | 448 / 439 |
| Tablets/lozenges | 1.52 | 1.32 to 1.74 | 71% | 8* | 2326 / 2113 |
| Oral spray | 2.48 | 1.24 to 4.94 | N/A | 1 | 318 / 161 |
| Choice of product | 1.37 | 1.25 to 1.52 | 42% | 7 | 4179 / 4109 |
| Patch and inhalator | 1.07 | 0.57 to 1.99 | NA | 1 | 136 / 109 |
| Patch and lozenge | 1.83 | 1.01 to 3.31 | N/A | 1 | 267 / 41 |
| Patch and gum | 1.15 | 0.64 to 2.06 | 50% | 2 | 173 / 86 |
| Patch, gum and lozenge | 15.00 | 2.00 to 112.54 | N/A | 1 | 212 / 212 |
| * includes 1 study treated as 2 for analysis; N/A: not applicable | |||||
Although the estimated effect sizes varied across the different products, confidence intervals were wide for the products with higher estimates which had small numbers of trials. One subgroup based on product type had a confidence interval which did not overlap with the pooled estimate; this group consisted of only one study in which only one participant in the control group had successfully quit smoking (Heydari 2013). In the tablets/lozenges subgroup, the I2 statistic was 71%, indicating substantial statistical heterogeneity. In all trials in this subgroup, more participants quit in the intervention arm than in control, but in one study new for this update the point estimate was considerably lower (RR 1.08) (Fraser 2014); this study drove the observed statistical heterogeneity.
Twelve studies had lower quit rates in the treatment than in the control group at the end of follow‐up (all of which had confidence intervals which crossed the line of no effect), and in a further 73% of trials the 95% confidence interval for the RR included 1 (i.e. the trials did not detect a significant treatment effect). Many of these trials had small numbers of smokers, and hence insufficient power to detect a modest treatment effect with reasonable certainty. One large trial of nicotine patches for people with cardiovascular disease had lower quit rates in the intervention than in the control group (Joseph 1996); at six months the quit rates were 14% for active patch and 11% for placebo, but after 48 weeks there had been greater relapse in the active group and rates were 10% and 12% respectively.
3.
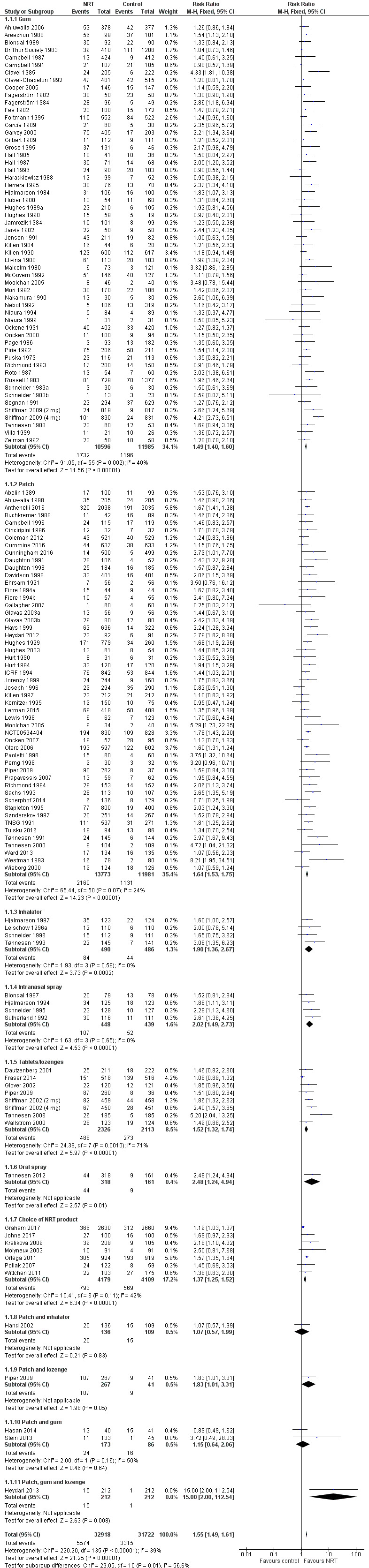
Forest plot of comparison: 1 Any type of NRT versus placebo/no NRT control, outcome: 1.1 Smoking cessation at 6+ months follow up.
Sensitivity to definition of abstinence
For nicotine gum and patch we assessed whether trials that reported sustained abstinence at 12 months had different treatment effects from those that only reported a point prevalence outcome, or had shorter follow‐up (Analysis 2.1; Analysis 2.2). Subgroup categories were sustained abstinence at 12 months or more, sustained abstinence at six months, point prevalence or unclear definition at 12 months, and point prevalence/unclear at six months. For nicotine gum 32/55 studies (56 comparisons) (58%) reported sustained 12‐month abstinence and the estimate was similar to that for all 55 studies: sustained 12‐month RR 1.43, 95% CI 1.31 to 1.56 (13,737 participants), compared with RR 1.49, 95% CI 1.40 to 1.60. The highest estimate was for the subgroup of eight studies reporting sustained abstinence at six months, where confidence intervals did not overlap: RR 2.77, 95% CI 2.14 to 3.59; 4187 participants. This seems to be attributable to one study (Shiffman 2009 (2 mg); Shiffman 2009 (4 mg)), and is unlikely to be of methodological or clinical significance. For nicotine patch, 21/49 studies (43%) reported sustained 12‐month abstinence, and the RR was also similar to that for all 49 studies: sustained 12‐month RR 1.52, 95% CI 1.34 to 1.74 (7622 participants), compared with RR 1.64, 95% CI 1.53 to 1.75 (25,754 participants) overall). For patch studies there was no evidence that the RRs differed significantly between subgroups.
2.1. Analysis.
Comparison 2 Subgroup: Definition of abstinence, Outcome 1 Nicotine gum. Smoking cessation.
2.2. Analysis.
Comparison 2 Subgroup: Definition of abstinence, Outcome 2 Nicotine patch: Smoking cessation.
Sensitivity to intensity of behavioural support
All trials provided the same behavioural support in terms of advice, counselling, and number of follow‐up visits to the active pharmacotherapy and control groups, but different trials provided different amounts of support. We conducted subgroup analyses by intensity of support for gum and patch trials separately (Analysis 3.1; Analysis 3.2). There was no evidence of a significantly different effect between groups. For nicotine gum the RR was similar across all three subgroups. The control group quit rates varied as expected, averaging 3.5% with low‐intensity support, 9% with high‐intensity individual support and 11.7% with group‐based support. Nicotine patch trials showed the same pattern; the RRs were similar for each subgroup and the average control group quit rates were 9.0% with low‐intensity support, 9.5% with high‐intensity individual support and 17.0% with group‐based support.
3.1. Analysis.
Comparison 3 Subgroup: Level of behavioural support, Outcome 1 Nicotine gum. Smoking cessation.
3.2. Analysis.
Comparison 3 Subgroup: Level of behavioural support, Outcome 2 Nicotine patch. Smoking cessation.
Sensitivity to treatment settings
We conducted further subgroup analyses for each type of setting in which smokers were recruited or treated (with type of NRT as a subgroup beneath setting). The pooled RR for trials in community volunteers where care was provided in a medical setting was 1.62 (95% CI 1.53 to 1.72, 65 trials, 24,597 participants; Analysis 4.1) and was similar to that of trials conducted in smoking clinics (RR 1.70, 95% CI 1.48 to 1.96, 12 trials, 3300 participants; Analysis 4.2), trials conducted in primary care settings (RR 1.50, 95% CI 1.33 to 1.69, 24 trials, 11,974 participants; Analysis 4.3), trials conducted in hospitals (RR 1.39, 95% CI 1.24 to 1.55, 13 trials, 7037 participants; Analysis 4.4), and trials conducted in settings similar to 'over the counter' (OTC) (RR 1.40, 95% CI 1.26 to 1.55, 9 trials, 13,163 participants; Analysis 4.5). Pooled results from four trials in antenatal clinics were lower than in other settings (RR 1.22, 95% CI 0.92 to 1.62, 1675 participants; Analysis 4.6); this was the only setting in which results did not show a statistically significant effect of the intervention. In a meta‐regression we checked whether there was any evidence of interaction between the treatment setting and type of NRT used. The effect of nicotine gum was highest in the OTC setting and this seems to be attributable to the same study that contributed heterogeneity in the abstinence subgroup analysis above (Shiffman 2009 (2 mg); Shiffman 2009 (4 mg)).
4.1. Analysis.
Comparison 4 Subgroup: Recruitment/treatment setting, Outcome 1 Community volunteer (treatment provided in medical setting).
4.2. Analysis.
Comparison 4 Subgroup: Recruitment/treatment setting, Outcome 2 Smoking clinic.
4.3. Analysis.
Comparison 4 Subgroup: Recruitment/treatment setting, Outcome 3 Primary care.
4.4. Analysis.
Comparison 4 Subgroup: Recruitment/treatment setting, Outcome 4 Hospitals.
4.5. Analysis.
Comparison 4 Subgroup: Recruitment/treatment setting, Outcome 5 Community volunteer (treatment provided in 'over‐the‐counter' setting).
4.6. Analysis.
Comparison 4 Subgroup: Recruitment/treatment setting, Outcome 6 Antenatal clinic.
Control group quit rates varied by setting; the lowest rates were found in OTC (8.4%) and primary care (6.9%) studies, and the highest rate in smoking clinics (14.3%). Falling within this range, control group rates were 9.3% in antenatal clinics, 12.5% in community volunteers where treatment was provided in a medical setting, and 12.3% in hospitals.
Sensitivity to risk of bias and study methods
Excluding those studies at high risk of bias did not significantly alter the point estimate for the main comparison: RR 1.61, 95% CI 1.52 to 1.69, analysis not shown. Similarly, restricting the main analysis to only those 12 studies at low risk of bias across all domains led to results consistent with the main analysis: RR 1.53, 95% CI 1.37 to 1.71, analysis not shown. Removing those studies without biochemical validation did not substantially influence the effect estimate: RR 1.62, 95% CI 1.55 to 1.70, analysis not shown, nor did restricting the analysis to only placebo‐controlled studies: RR 1.61, 95% CI 1.53 to 1.70, analysis not shown.
Relapsed smokers
Although many of the trials reported here did not specifically exclude people who had previously tried and failed to quit with NRT, one trial recruited people who had relapsed after patch and behavioural support in an earlier phase of the study but were motivated to make a second attempt (Gourlay 1995). This study did not detect an effect on continuous abstinence (RR 1.25, 95% CI 0.34 to 4.60, analysis not shown), although it did detect a significant increase in 28‐day point prevalence abstinence (RR 2.49, 95% CI 1.11 to 5.57). Quit rates were low in both groups with either definition of abstinence.
Adverse events
We have made no systematic attempt in this review to synthesize quantitatively the incidence of the various adverse events reported with the different NRT preparations. This was because of the extensive variation in reporting of the nature, timing and duration of symptoms. In the included studies, attrition rates in NRT groups were generally similar to or lower than in control groups. Appendix 3 summarises the main adverse events reported in the included and excluded studies, where the data were available.
The most common adverse events usually reported with nicotine gum include hiccoughs, gastrointestinal disturbances, jaw pain, and orodental problems (Fiore 1992; Palmer 1992). The only adverse event that appears to interfere with use of the patch is skin sensitivity and local skin irritation; this may affect up to 54% of patch users, but it is usually mild and rarely leads to withdrawal of patch use (Fiore 1992). The major adverse events reported with the nicotine inhalator and nasal and oral sprays are related to local irritation at the site of administration (mouth and nose respectively). For example, symptoms such as throat irritation, coughing, and oral burning were reported significantly more frequently with participants allocated to the nicotine inhalator than to placebo control (Schneider 1996); none of the experiences, however, were reported as severe. With the nasal spray, nasal irritation and runny nose are the most commonly reported adverse events. In the study of oral spray, hiccoughs and throat irritation were the most commonly reported adverse events (Tønnesen 2012). Nicotine sublingual tablets have been reported to cause hiccoughs, burning and smarting sensation in the mouth, sore throat, coughing, dry lips and mouth ulcers (Wallstrom 1999). Adolescents report similar adverse events to adults (Bailey 2012).
A review of adverse events based on 35 trials with over 9000 participants did not find evidence of excess adverse cardiovascular events amongst those assigned to nicotine patch, and the total number of such events was low (Greenland 1998). A meta‐analysis of adverse events associated with NRT included 92 RCTs and 28 observational studies, and addressed a possible excess of chest pains and heart palpitations among users of NRT compared with placebo groups (Mills 2010). The authors report an OR of 2.06 (95% CI 1.51 to 2.82) across 12 studies. We replicated this data collection exercise and analysis where data were available (included and excluded) in this review, and detected a similar but slightly lower estimate, OR 1.88 (95% CI 1.37 to 2.57; 15 studies; 11,074 participants; OR rather than RR calculated for comparison; Analysis 6.1). Chest pains and heart palpitations were an extremely rare event, occurring at a rate of 2.5% in the NRT groups compared with 1.4% in the control groups in the 15 trials in which they were reported at all. A recent network meta‐analysis of cardiovascular events associated with smoking cessation pharmacotherapies (Mills 2014), including 21 RCTs comparing NRT with placebo, found statistically significant evidence that the rate of cardiovascular events with NRT was higher (RR 2.29 95% CI 1.39 to 3.82). However, when only serious adverse cardiac events (myocardial infarction, stroke and cardiovascular death) were considered, the finding was not statistically significant (RR 1.95 95% CI 0.26 to 4.30). A sensitivity analysis demonstrated that lower‐level events, predominantly tachycardia and arrhythmia, accounted for the observed increased risk of cardiovascular events. Chest pains and palpitations are the only clinically significant adverse events to emerge from the trials, and no evidence of significant harm has been identified.
6.1. Analysis.
Comparison 6 Palpitations in NRT vs placebo users, Outcome 1 Palpitations/chest pains.
When first licensed there was concern about the safety of NRT in smokers with cardiac disease (TNWG 1994). A trial of nicotine patch that recruited smokers aged over 45 with at least one diagnosis of cardiovascular disease found no evidence that serious adverse events were more common in smokers in the nicotine patch group (Joseph 1996). Events related to cardiovascular disease, such as an increase in angina severity, occurred in approximately 16% of participants, but did not differ according to whether or not they were receiving NRT. A review of safety in people with cardiovascular disease found no evidence of an increased risk of cardiac events (Joseph 2003). This included data from two randomized trials with short‐term follow‐up that we excluded from the present review (Tzivoni 1998; Working Group 1994), and a case‐control study in a population‐based sample. An analysis of 187 smokers admitted to hospital with acute coronary syndromes who received nicotine patches showed no evidence of difference in short‐ or long‐term mortality compared to a propensity‐matched sample of smokers in the same database who did not receive NRT (Meine 2005). A subgroup analysis within a network meta‐analysis of cardiovascular events (Mills 2014), found no increased risk of cardiovascular events with NRT amongst individuals with predisposing conditions that placed them at an increased risk of having an event (RR 1.24, 95% CI 0.77 to 2.02). Another recent network meta‐analysis in people with cardiovascular disease found a slightly higher number of cardiovascular events with NRT but was not able to draw quantitative conclusions due to the low number of trials reporting adverse events and the variation in adverse event definitions used (Suissa 2017).
The six trials assessing NRT use in pregnant women did not detect significant increases in serious adverse events amongst the treatment groups (Berlin 2014; Coleman 2012; El‐Mohandes 2013; Oncken 2008; Pollak 2007; Wisborg 2000). The effects of NRT use on neonatal health are discussed further in a separate Cochrane Review, which found no statistically significant differences in rates of any serious adverse events between treatment and control groups (Coleman 2015). As mentioned previously, this separate review covers this topic comprehensively and will be regularly updated, so interested readers should refer to Coleman 2015 for more information on the adverse events profile of NRT in pregnancy.
Discussion
This review provides high‐quality evidence from trials including over 64,000 participants that offering nicotine replacement therapy (NRT) to dependent smokers who are prepared to try to quit increases their chance of success over that achieved with the same level of support but without NRT. This applies to all forms of NRT and is independent of any variations in methodology or design characteristics of trials included in the meta‐analysis. In particular we did not find evidence that the relative effect of NRT was smaller in trials with longer follow‐up beyond our six‐month minimum for inclusion. We did not compare end‐of‐treatment risk ratios with post‐treatment follow‐up, and relapse rates may be higher in active treatment participants once they stop using NRT products, but later relapse is probably unrelated to NRT use (Etter 2006).
The absolute effects of NRT use will depend on the baseline quit rate, which varies in different clinical settings. Studies of people attempting to quit on their own suggest that success rates after six to 12 months are 3% to 5% (Hughes 2004). Use of NRT might be expected to increase the rate by 2% to 3%, giving a number needed to treat for an additional beneficial outcome (NNTB) of 56. If, however, the quit rate without pharmacotherapy was estimated to be 15%, either because the population had other predictors of successful quitting or received intensive behavioural support, then another 8% might be expected to quit, giving an NNTB of 11.
Intensity of additional support and treatment setting
We did not detect important differences in relative effect within patch or gum studies by our classification of level of support. A letter prior to the previous update of this review identified inconsistencies in the classification of low‐ and high‐intensity support in this review (Walsh 2007). In response, we changed the classification of a small number of trials. This did not alter the conclusion that intensity of support does not appear to be an important moderator of NRT effect.
We also did not detect differences in relative effect according to the setting of recruitment and treatment. This subgroup analysis had considerable overlap with the support subgroup since, for example, people recruited in primary care settings typically had lower‐intensity support.
There has been continuing debate about the amount of evidence for the efficacy of NRT when obtained OTC without advice or support from a healthcare professional (Hughes 2001; Walsh 2000; Walsh 2001). The small number of placebo‐controlled trials in settings intended to replicate OTC settings support the conclusion that the relative effect of NRT is similar to settings where more advice and behavioural support is provided, although quit rates in both control and intervention groups have been low. One other meta‐analysis supports the conclusion of efficacy, although it differs in its inclusion criteria (Hughes 2003). In addition to the same three trials comparing nicotine patch to placebo in an OTC setting (Davidson 1998; Hays 1999; Sønderskov 1997), that review includes one study excluded here due to short follow‐up (Shiffman 2002a). It also pools four trials comparing NRT provided OTC to NRT provided under prescription. We exclude one trial that compared both gum and patch in these settings, but was not randomized (Shiffman 2002b), and another that has not been published and for which we have been unable to obtain reliable data for inclusion (Korberly 1999). The abstract reported that there were no significant differences in quit rates between users of nicotine patch who purchased it through a non‐healthcare facility, and those receiving it on prescription. It has also been suggested that the 'real world' effectiveness of NRT declines or disappears once it becomes available to purchase without requiring contact with a health professional who can offer behavioural support and guidance on appropriate use (Kotz 2014; Pierce 2002). A comparison of two cross‐sectional surveys in California found that quit rates for self‐selected NRT users were higher than rates for non‐users prior to OTC availability, but after the switch to OTC this difference disappeared (Pierce 2002). In addition, a prospective cohort study found that the odds of cessation in people who had used OTC NRT were lower than in people who had not used any cessation pharmacotherapy or accessed a national stop‐smoking service (OR 0.69, 95% CI 0.49 to 0.94) (Kotz 2014). However, these observational studies are at risk of residual confounding from unmeasured confounders, such as psychological factors, as participants self‐selected their treatment. These studies are also at risk of bias, as unaided quit attempts are less likely to be recalled than those involving NRT.
A report of a prospective cohort study questioned the effectiveness of NRT outside of the clinical trial setting after finding no difference in relapse rates between smokers trying to quit who used NRT and those who did not use NRT (Alpert 2012). However, the design of this study has been criticized for not addressing initial quit rates in the two groups (Stapleton 2012). Furthermore, two multi‐country prospective cohort studies observed that NRT users had higher quit rates than non‐users (Kasza 2012; West 2007), although in the former study this effect was limited to NRT patches, with no effect detected for oral nicotine products. Again, these are observational studies and are at risk of confounding and bias.
Trials in special populations
Trials generally restricted recruitment to adults over the age of 18; in a small number of trials the age range was not specified. Two relatively small studies in adolescents did not detect an effect of NRT on quitting at six months or longer (Moolchan 2005; Scherphof 2014). A separate Cochrane Review of tobacco cessation interventions for young people did not detect an effect of NRT, although confidence intervals were wide and did not preclude the possibility of a clinically important effect (Fanshawe 2017). This is likely to remain an active area of research.
This review previously conducted a separate analysis on a subgroup of studies evaluating NRT in pregnant women. This is covered by a separate Cochrane review (Coleman 2015) which provides more comprehensive analysis of these studies and will be regularly updated (whereas this review is now marked as stable and will no longer be updated). Therefore, readers interested in this topic should refer to (Coleman 2015).
Evidence for differential treatment effects in different subgroups
We made no attempt to conduct separate analyses for any subgroups of trial participants, because subgroup results are uncommon in trial reports, and where data cannot be obtained from all studies there is a risk of bias from using incomplete data. Munafó 2004a has reported the results of a meta‐analysis of nicotine patch by sex. The researchers were able to include data from 11 out of 31 eligible trials (35%) and 36% of study participants. They found no evidence that the nicotine patch was more effective for men than for women, as has been hypothesized; although men showed a somewhat bigger benefit from NRT at 12 months, the difference was not significant. There was also no difference in average placebo quit rates between men and women, which has been reported in some studies. In a commentary some additional data were identified (Perkins 2004), but this did not alter the conclusions (Munafó 2004b). A second meta‐analysis of any type of NRT reported that in women the odds ratio for cessation declined with increasing length of follow‐up, with a non‐significant difference at 12 months (Cepeda‐Benito 2004). Amongst men the odds ratio declined less over time and remained significant. Based on a further subgroup analysis, they also reported that the decline in long‐term efficacy in women was greater in trials with low‐intensity support than with high‐intensity support, suggesting that the more intensive support helped prevent late relapse in women who had initially received NRT. Although there was no evidence of bias, the review could only include a subset of published studies, so the finding should be regarded as hypothesis‐generating. All review authors agreed that trials are underpowered to identify any interaction between treatment and any type of individual characteristics, and recommended public archiving of data from studies, as well as new research specifically designed to test group‐by‐treatment interactions. At the moment there does not appear to be sufficient evidence of clinically important differences between men and women to guide treatment matching.
Re‐treating relapsed smokers
Whilst end‐of‐treatment success rates may be quite high, many people relapse after the end of therapy. There is suggestive evidence that repeated use of NRT in people who have relapsed after an initial course may produce further quitters, although the absolute effect is small (Gourlay 1995). A subgroup analysis in another trial indicated that the relative effect of treatment with nicotine patch compared to placebo was at least as high for people who had used NRT before (Jorenby 1999, reported in Durcan 2002). The authors noted that there was no way to distinguish between people who had completely failed to quit using NRT and those who had been initially successful but relapsed.
Limitations of the evidence base
Two possible limitations to this evidence base need to be borne in mind: risk of bias in individual studies and publication bias. For the former, although we judged most of our included studies to be at unclear or high risk of bias in at least one domain, restricting the analysis to only those studies at low risk of bias overall did not significantly alter the pooled effect. For the latter, we tried to partly address any shortcomings from having limited our analysis to reported data by approaching investigators, where necessary, to obtain additional unpublished data or to clarify areas of uncertainty. Although we took steps to minimize publication bias by writing to the manufacturers of NRT products when this review was first prepared, the response was poor and we have not repeated this exercise, although we have searched clinical trials registries. It is therefore possible that there are some unpublished trials, with less favourable results, that we have not identified despite our efforts to do so. A funnel plot (Figure 4) shows some evidence of asymmetry for trials in the main comparison; however, given the large number of trials in the review, the funnel plot does not suggest that results would be altered significantly were smaller studies with lower RRs included. A meta‐analysis has also demonstrated that nicotine gum and patch studies that received pharmaceutical industry funding have on average slightly higher effect sizes than other studies after controlling for some trial characteristics (Etter 2007). The practical effect of these considerations is that the magnitude of the effectiveness of NRT may be smaller than our estimates suggest.
4.
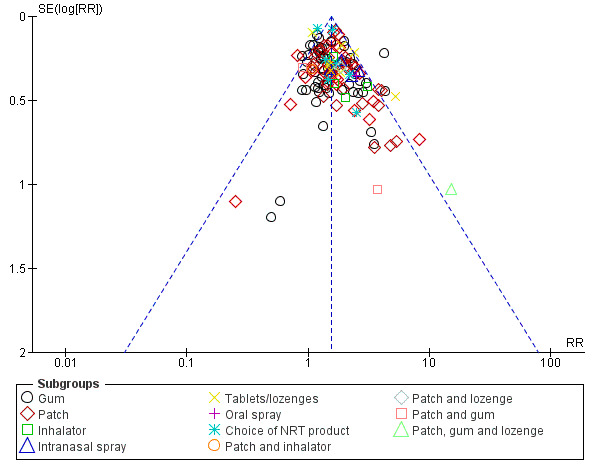
Funnel plot of comparison: 1 Any type of NRT versus placebo/no NRT control, outcome: 1.1 Smoking cessation at 6+ months follow up.
A possible further limitation relates to length of follow‐up. This review excludes studies with less than a six‐month follow‐up from the start of treatment; the outcome used reflects the effect of NRT after the end of active treatment. A comparison of abstinence rates during treatment and abstinence at one year suggests that the relative effect of NRT declines once active therapy stops (Fagerström 2003), i.e. people who quit with the help of NRT are a little more likely to relapse after they discontinue treatment than those on placebo. The relative effect of NRT could continue to decline even after a year of follow‐up. However, a meta‐analysis comparing one‐year and long‐term outcomes in 12 NRT trials with follow‐up beyond one year suggested that the relative efficacy did not change, with similar relapse rates in the active and placebo groups, but further relapse does reduce the absolute difference in quit rates (Etter 2006).
Stability of the evidence base
This review was first published in 1996. Despite the number of included studies more than doubling over this time, the effect estimate has remained remarkably stable, and our intention is that this publication is the final time the Cochrane Tobacco Addiction Group will review the evidence comparing NRT to placebo or to no pharmacotherapy. This is not to say that all questions about NRT have been answered; evidence is still needed comparing different forms, doses, and durations of NRT, comparing NRT to other pharmacotherapies, and testing NRT in special populations where we may reasonably hypothesize that its effectiveness differs from that in the general population (e.g. pregnant women, adolescents). Further studies are also needed of electronic cigarettes containing nicotine, which some consider a form of NRT (but which we have never included in this review). However, we will cover these in separate reviews which we will continue to update regularly (Cahill 2016; Coleman 2015; Fanshawe 2017; Hartmann‐Boyce 2016; Hughes 2014). In summary, based on 20 years of research and 136 randomized controlled trials in over 64,000 participants, we believe the question of whether NRT helps people to quit smoking to be definitively answered. We consider that further research is highly unlikely to change our confidence in the effect of NRT, and funders and researchers should give careful thought before pursuing further studies comparing established forms of NRT with control.
Authors' conclusions
Implications for practice.
All of the commercially available forms of nicotine replacement therapy (NRT), i.e. gum, transdermal patch, nasal spray, inhalator, oral spray, lozenge and sublingual tablet, are effective as part of a strategy to promote smoking cessation. They increase the rate of long‐term quitting by approximately 50% to 60%, regardless of setting. These conclusions apply to smokers who are motivated to quit. There is little evidence about the role of NRT for individuals smoking fewer than 10 to 15 cigarettes a day.
The form of delivery of NRT is unrelated to effectiveness, so other considerations such as preferences, availability, or cost might determine the form of NRT chosen.
The effectiveness of NRT, in terms of the risk ratio, appears to be largely independent of the intensity of additional support provided. Intensive behavioural support is not essential for NRT to be effective. However, it should be noted that the absolute increase in success rates attributable to the use of NRT will be larger when the baseline chance of success is already raised by the provision of intensive behavioural support.
NRT causes non‐ischaemic chest pain and palpitations in a minority of users but there is no evidence of an excess of serious cardiac problems, even in people with established cardiac disease.
NRT commonly leads to minor adverse reactions which reflect irritation of the site of use of the form of NRT. These reactions are usually not severe enough to prompt discontinuation of treatment
Implications for research.
There is high‐quality evidence that nicotine replacement therapy increases quit rates at six months or longer in adults motivated to quit. We consider that further research is highly unlikely to change our confidence in the effect of NRT in this population.
Feedback
How should efficacy be measured?
Summary
The comment (December 2002) states that NRT is not more effective than abrupt cessation. We summarise the supporting arguments and our response to each below:
Reply
1. Pierce & Gilpin (Pierce JP, Gilpin EA. Impact of over‐the‐counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA 2002;288:1260‐4) found no difference in long‐term cessation rates between those who did and who did not use NRT.
This point is addressed in a letter commenting on the study (Stead LF et al. Effectiveness of over‐the‐counter nicotine replacement therapy. JAMA 2002;288:3109‐10). The main limitation of their study is that the comparison between groups of people who chose or did not chose to use NRT, These two groups probably differ in many respects related to their chance of successful quitting, and it is impossible to adjust for these possible confounders. Therefore the conclusions of the study are stronger than the evidence justifies.
The criticism authors also cite the Minnesota insurance review (Boyle RG et al. Does insurance coverage for drug therapy affect smoking cessation? Health Affairs 2002 Nov‐Dec;21:162‐8) but it does not seem to give further support to the point made. The main finding of Boyle et al was that introducing an insurance benefit did not increase use of NRT.
2. In the real‐world those relying exclusively upon NRT are relapsing and dying at pre‐NRT rates.
This is an assertion which is not supported by evidence.
3. NRT study instruction is designed and sequenced in order to foster device transfer. In fact the placebo group must be deprived of critical abrupt cessation instructional tips because if given and followed many could have a negative impact upon the active group.
The review does not make the assertion or implication attributed to it. In the studies involving behavioural support as well as active versus placebo NRT, both active and placebo groups are typically given instructions designed to maximise their chances of success. In these circumstances NRT if anything shows a larger advantage over placebo than it does in minimal support settings. If it is being asserted that placebo groups are being deprived of progressive cigarette weaning or some form of lapse management strategy, there is no evidence to suggest that this approach is effective.
4. The duration of abstinence for NRT groups should begin from the time they stop using NRT.
In response to this it should be noted that it is cigarettes which are causing the harm to health and the aim is to help people stop smoking. Secondly, studies that have followed up smokers long‐term show that the medication genuinely improves long‐term cessation rates and does not simply set the relapse clock back by the time period when nicotine replacement is being used.
5. There are clinic programmes achieving success rates at least as good as those using NRT.
It is necessary to make direct comparisons ensuring that the same criteria are applied to both groups to be able to draw conclusions.
Finally it must be noted that the Cochrane review shows that NRT is estimated to help some 7% smokers to stop long‐term who would not have stopped had they used a similar approach but without NRT. This effect is small but given the health benefits from stopping smoking it is a highly cost‐effective life‐preserving medication. That is not to say that other interventions, including a different kind of behavioural intervention that was incompatible with NRT could not get better results. However, it is not enough just to assert the possibility; with so many lives at stake it would be imperative to demonstrate the effectiveness of such approaches.
Contributors
Comment by John R. Polito. Response by Tim Lancaster & Lindsay Stead on behalf of review authors. Criticism editor Robert West.
How should effectiveness be measured
Summary
The comment (October 2003) suggests that randomised controlled trials (RCTs) alone cannot establish the effectiveness of an intervention in a population.
Reply
RCTs establish the size of effect of an intervention in a particular context in a sample who are eligible and willing to receive the intervention. It always remains possible that the effect size would be different in a different population under different conditions which is why it is important to assess in RCTs how representative the samples are, and how far the context of the trial represents the likely clinical scenarios in which the intervention will be applied. In other words an RCT seeks to achieve internal validity (corresponding to efficacy) and aspires to maximise external validity (corresponding to effectiveness). A 'real‐world' comparison of two groups that are not comparable, and where the differences are not adequately controlled for by design or analysis, does not permit attribution of differences or similarities in outcome to the intervention under investigation.
Contributors
Comment by John Pierce. Reply by Lindsay Stead & Tim Lancaster on behalf of review authors. Criticism Editors: Robert West (internal), Lisa Bero (external).
Impact of failure to assess blinding on validity
Summary
The comment (May 2004) drew attention to a recent paper (Mooney M, White T, Hatsukami D. The blind spot in the nicotine replacement therapy literature: assessment of the double‐blind in clinical trials. Addictive Behaviors 2004; 29(4):673‐684) that notes that most NRT trials do not report whether blinding was maintained, and of those that did, blinding failure was common. The comment also suggests that smokers failing to quit with an NRT‐assisted attempt will not benefit from NRT use in subsequent attempts, and questions whether people who quit smoking but continue to use NRT should be regarded as having quit or not.
Reply
The issue of possible failure of blinding, and hence of possible bias in estimates of treatment effect, is a potential problem in many areas of medicine. Failure to report whether the success of blinding has been tested is widespread (1). There are problems with how best to test the effectiveness of blinding. If participants' guesses are influenced by their success in quitting, then apparent breaking of the blind might be more common where treatment was effective (2).
Where there is evidence that blinding has failed, there still needs to be an assessment of whether this has lead to bias in effect estimates. Mooney's paper makes it clear that there are insufficient data to try to assess whether there was evidence of a bias in treatment estimates in the existing trials. There are many potential sources of bias in trials, and we don't have any evidence to suggest that failure of blinding is more of a problem in trials of NRT. We focus on outcomes at least six months after the quit attempt, so that any differential effect of guessing the treatment assignment on the likelihood of successful quitting would need to be long lasting.
Small amounts of nicotine have been used in placebo products in attempts to improve maintenance of the blind by giving a characteristic taste or smell. In most cases the amounts are small. If there were sufficient nicotine to be pharmacologically active it would seem more likely to decrease the effect of active NRT than inflate the treatment effect.
We do not think there is evidence to state that an initial failure with NRT means that subsequent attempts will also fail. People who have a failed quit attempt in a trial seem to have a low chance of success if they immediately try again, as noted in the studies by Gourlay, and Tonnesen (which was uncontrolled ). A recent study found a similar poor outcome when people who had failed to quit using nicotine patch were randomized to second line therapy with bupropion or placebo (5). In contrast, two recent studies have found that people who reported failed quit attempts using NRT do at least as well when enrolled in trials and treated with NRT as do NRT‐naïve participants. (6,7).
It is important that smokers realise that their chance of a successful long‐term quit from each attempt is low and that NRT, although increasing the likelihood of success, is not a 'magic bullet', and this point is made in the review.
We do not agree that people who give up smoking cannot regard themselves as quitters whilst they are using NRT. In the context of a history of chronic smoking over a period of years we do not think that it is a major concern that 6.7% of new gum users may be still using it after six months. The rate of persistent use appears to fall rapidly, with the same study noting a rate of 2.8% for use after a year or more. Rates of persistent patch use are lower.
References (1) Fergusson D, Glass KC, Waring D, Shapiro S. Turning a blind eye: the success of blinding reported in a random sample of randomised, placebo controlled trials. BMJ. 2004 Feb 21;328(7437):432 2004 (2) Altman DG, Schulz KF, Moher D. Turning a blind eye: testing the success of blinding and the CONSORT statement. BMJ. 2004 May 8;328(7448):1135 (3) Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers, BMJ 1995;311:363‐366 (4) Tonnesen P, Norregaard J, Sawe U, Simonsen K. Recycling with nicotine patches in smoking cessation. Addiction. 1993 Apr;88(4):533‐9 (5) Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. J Clin Oncology 2003; 21(5):914‐920. (6) Durcan MJ, White J, Jorenby DE, Fiore MC, Rennard SI, Leischow SJ et al. Impact of prior nicotine replacement therapy on smoking cessation efficacy. Am J Health Behav 2002; 26(3):213‐220. (7) Shiffman S, Dresler CM, Rohay JM. Successful treatment with a nicotine lozenge of smokers with prior failure in pharmacological therapy. Addiction 2004; 99(1):83‐92.
Contributors
Comment by John R. Polito. Reply by Lindsay Stead, Tim Lancaster Criticism editor Robert West
What's new
| Date | Event | Description |
|---|---|---|
| 11 January 2019 | Amended | Signposts added to more comprehensive and updated data on NRT in pregnancy |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 2, 1996
| Date | Event | Description |
|---|---|---|
| 4 September 2018 | Amended | CENTRAL search date corrected |
| 8 November 2017 | New search has been performed | Searches updated, 18 new studies added. Review split into NRT versus control and NRT versus NRT. |
| 8 November 2017 | New citation required but conclusions have not changed | No changes to conclusions for NRT versus control (other comparisons moved elsewhere). Review marked as stable. Changes to authorship. |
| 19 September 2012 | New search has been performed | Searches updated, 18 new studies added. Table of adverse events added. |
| 19 September 2012 | New citation required but conclusions have not changed | Additional author. No major changes to conclusions. |
| 22 June 2011 | Amended | Additional table converted to appendix to correct pdf format |
| 16 April 2008 | Amended | Converted to new review format. |
| 1 November 2007 | New citation required and conclusions have changed | New studies added, some comparisons reorganised, effect measure changed from odds ratio to risk ratio. Minor changes made to the conclusions about the evidence for combinations of NRT types. Authors changed. |
| 7 April 2004 | New citation required and minor changes | Twelve new studies added, no changes to main conclusions. |
Notes
Prof Chris Silagy died in December 2001. In recognition of his major contribution he remained as first author until 2007. The authorship changed from 2008 issue 1.
Acknowledgements
Chris Silagy was originally the first author, contributed to updates until his death in 2001, and was listed as an author until 2008. Godfrey Fowler was also an author until 2008. Lindsay Stead, Rafael Perrera, and David Mant were authors until 2012. Mark Lodge assisted in the preparation of the initial version of this review. Ruth Ashenden provided technical support. Drs. Tjeder‐Burton, Campbell, Hjalmarson, Fagerström, Mori, Glover, Hughes, Fortmann, Killen, Varady, Ortega, Rose, Cunningham, Wilcox, and Graham co‐operated with our requests for clarification of previously‐reported data. Z. Ilic and L. Silagy assisted with translation of foreign language reports. P. Yudkin provided statistical advice on early updates. Marc Mooney provided copies of two papers we had not been able to obtain. We thank Annette Pluddemann for help in translating study reports. Jonathan Livingstone‐Banks assisted in finding studies for the current update.
JHB is funded in part by the NIHR Oxford Biomedical Research Centre (BRC).
Appendices
Appendix 1. Specialized Register search strategy
#1 NRT: TI,AB,KY,XKY,MH,EMT
#2 (nicotine NEAR2 patch*):TI,AB,KY,XKY,MH,EMT
#3 (nicotine NEAR2 gum):TI,AB,KY,XKY,MH,EMT
#4 (nicotine NEAR2 nasal spray):TI,AB,KY,XKY,MH,EMT
#5 (nicotine NEAR2 lozenge*):TI,AB,KY,XKY,MH,EMT
#6 (nicotine NEAR2 tablet*):TI,AB,KY,XKY,MH,EMT
#7 (nicotine NEAR2 sublingual):TI,AB,KY,XKY,MH,EMT
#8 (nicotine NEAR2 inhal*):TI,AB,KY,XKY,MH,EMT
#9 (nicotine NEAR2 replacement):TI,AB,KY,XKY,MH,EMT
#10 (nicotine NEAR3 therap*):TI,AB,KY,XKY,MH,EMT
#11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
The specialised register was transferred from Reference Manager to the CRS in May 2012. This is the search used for the CRS: KY, XKY, MH & EMT are keyword fields.
Appendix 2. Glossary of terms
| Term | Definition |
| Abstinence | A period of being quit, i.e. stopping the use of cigarettes or other tobacco products, May be defined in various ways; see also: point prevalence abstinence; prolonged abstinence; continuous/sustained abstinence |
| Biochemical verification | Also called 'biochemical validation' or 'biochemical confirmation': A procedure for checking a tobacco user's report that he or she has not smoked or used tobacco. It can be measured by testing levels of nicotine or cotinine or other chemicals in blood, urine, or saliva, or by measuring levels of carbon monoxide in exhaled breath or in blood. |
| Bupropion | A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation; trade names Zyban, Wellbutrin (when prescribed as an antidepressant) |
| Carbon monoxide (CO) | A colourless, odourless highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence. |
| Cessation | Also called 'quitting' The goal of treatment to help people achieve abstinence from smoking or other tobacco use, also used to describe the process of changing the behaviour |
| Continuous abstinence | Also called 'sustained abstinence' A measure of cessation often used in clinical trials involving avoidance of all tobacco use since the quit day until the time the assessment is made. The definition occasionally allows for lapses. This is the most rigorous measure of abstinence |
| 'Cold Turkey' | Quitting abruptly, and/or quitting without behavioural or pharmaceutical support. |
| Craving | A very intense urge or desire [to smoke]. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
| Dopamine | A neurotransmitter in the brain which regulates mood, attention, pleasure, reward, motivation and movement |
| Efficacy | Also called 'treatment effect' or 'effect size': The difference in outcome between the experimental and control groups |
| Harm reduction | Strategies to reduce harm caused by continued tobacco/nicotine use, such as reducing the number of cigarettes smoked, or switching to different brands or products, e.g. potentially reduced exposure products (PREPs), smokeless tobacco. |
| Lapse/slip | Terms sometimes used for a return to tobacco use after a period of abstinence. A lapse or slip might be defined as a puff or two on a cigarette. This may proceed to relapse, or abstinence may be regained. Some definitions of continuous, sustained or prolonged abstinence require complete abstinence, but some allow for a limited number or duration of slips. People who lapse are very likely to relapse, but some treatments may have their effect by helping people recover from a lapse. |
| nAChR | [neural nicotinic acetylcholine receptors]: Areas in the brain which are thought to respond to nicotine, forming the basis of nicotine addiction by stimulating the overflow of dopamine |
| Nicotine | An alkaloid derived from tobacco, responsible for the psychoactive and addictive effects of smoking. |
| Nicotine Replacement Therapy (NRT) | A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco‐free The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges. |
| Outcome | Often used to describe the result being measured in trials that is of relevance to the review. For example smoking cessation is the outcome used in reviews of ways to help smokers quit. The exact outcome in terms of the definition of abstinence and the length of time that has elapsed since the quit attempt was made may vary from trial to trial. |
| Pharmacotherapy | A treatment using pharmaceutical drugs, e.g. NRT, bupropion |
| Point prevalence abstinence (PPA) | A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long‐term quitters. cf. prolonged abstinence, continuous abstinence |
| Prolonged abstinence | A measure of cessation which typically allows a 'grace period' following the quit date (usually of about two weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging. See: Hughes et al 'Measures of abstinence in clinical trials: issues and recommendations'; Nicotine & Tobacco Research, 2003: 5 (1); 13‐25 |
| Relapse | A return to regular smoking after a period of abstinence |
| Secondhand smoke | Also called passive smoking or environmental tobacco smoke [ETS] A mixture of smoke exhaled by smokers and smoke released from smouldering cigarettes, cigars, pipes, bidis, etc. The smoke mixture contains gases and particulates, including nicotine, carcinogens and toxins. |
| Self‐efficacy | The belief that one will be able to change one's behaviour, e.g. to quit smoking |
| SPC [Summary of Product Characteristics] | Advice from the manufacturers of a drug, agreed with the relevant licensing authority, to enable health professionals to prescribe and use the treatment safely and effectively. |
| Tapering | A gradual decrease in dose at the end of treatment, as an alternative to abruptly stopping treatment |
| Tar | The toxic chemicals found in cigarettes. In solid form, it is the brown, tacky residue visible in a cigarette filter and deposited in the lungs of smokers. |
| Titration | A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit adverse events. |
| Withdrawal | A variety of behavioural, affective, cognitive and physiological symptoms, usually transient, which occur after use of an addictive drug is reduced or stopped. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
Appendix 3. Main adverse events by study
| Adverse Event |
RCTs P = patch, G = gum, S = spray, I = inhalator, L = lozenge, T = tablet. EX = excluded study |
Active n events | Active total | Control n events | Control total | Notes |
| Headache | Anthenelli 2016 (P) | 233 | 2022 | 199 | 2014 | Totals are numbers assessed for adverse events |
| Areechon 1988 (G) | 1 | 98 | 0 | 101 | ‐ | |
| Berlin 2014 (P) | 12 | 203 | 9 | 199 | ‐ | |
| Blondal 1989 (G) | 14 | 92 | 14 | 92 | From % | |
| Coleman 2012 (P) | 25 | 521 | 16 | 529 | Pregnant women | |
|
Daughton 1991 (P) 24 h 16 h |
8 3 |
51 55 |
5 | 52 | ‐ | |
| Gourlay 1995 (P) | 8 | 315 | 13 | 314 | ‐ | |
| Harackiewicz 1988 (G) | 6 | 99 | 8 | 85 | First 6 weeks | |
| Hays 1999 (P) | 24 | 321 | 24 | 322 | Excludes pay group | |
| Hjalmarson 1994 (S) | 27 | 116 | 18 | 107 | First 2 weeks | |
| Hurt 1994 (P) | 14 | 120 | 21 | 120 | ‐ | |
| Jarvis 1982 (G) | 14 | 47 | 17 | 44 | ‐ | |
| Jorenby 1999 (P) | 69 63 |
243 244 |
52 | 159 | P vs placebo P + B vs placebo |
|
| Lerman 2015 (P) | 139 | 418 | 169 | 408 | Number of events summed over time, not number of people | |
| Lewis 1998 (P) | 1 | 62 | 1 | 62 | ‐ | |
| Llivina 1988 (G) | 11 | 113 | 8 | 101 | From % | |
| Paoletti 1996 (P) | 19 | 147 (LC15 + HC25) |
15 | 150 (LCP + HC15) |
Active vs placebo (Pl + Pl or lowA+Pl) | |
| Puska 1979 (G) | 20 | 80 | 14 | 74 | From %; missing data removed from denominator | |
| Sachs 1993 (P) | 7 | 113 | 5 | 107 | ‐ | |
| Schneider 1995 (S) | 41 | 128 | 32 | 127 | From % | |
|
Shiffman 2002 (2 mg) (L) Shiffman 2002 (4 mg) (L) |
23 36 |
459 450 |
27 15 |
458 451 |
From % | |
| Stapleton 1995 (P) | 84 | 761 | 30 | 364 | ‐ | |
| Stein 2013 (P) | 10 | 104 | 6 | 33 | ‐ | |
| Sutherland 1992 (S) | 49 | 111 | 41 | 103 | ‐ | |
| Tønnesen 1991 (P) | 6 | 145 | 6 | 144 | From % | |
| Ward 2013 (P) | < 5% | 134 | < 5% | 135 | ‐ | |
| EX Batra 2005 (G) | 43 | 184 | 52 | 180 | ‐ | |
| EX CEASE 1999 (P) 25 mg 15 mg |
80 76 |
1430 1431 |
28 | 714 | ‐ | |
| EX Ebbert 2009 (L) | 10 | 136 | 7 | 134 | Smokeless (from %) | |
| EX Hanson 2003 (P) | 27 | 50 | 34 | 50 | adolescents | |
| EX Mulligan 1990 (P) | 1 | 39 | 0 | 36 | ‐ | |
| EX Rigotti 2009 (P) | 31 | 367 | 22 | 362 | All were on rimonabant | |
| EX Schnoll 2010 (P) | 0 | 182 | 2 | 134 | At 12 weeks | |
| EX Stapleton 2011 (S) | 320 | 506 | 154 | 255 | ‐ | |
| Dizziness/light‐headedness | Ahluwalia 1998 (P) | 0 | 174 | 1 | 168 | ‐ |
| Anthenelli 2016 (P) | 85 | 2022 | 66 | 2014 | Totals are numbers assessed for adverse events | |
| Areechon 1988 (G) | 2 | 98 | 0 | 101 | ‐ | |
| Berlin 2014 (P) | < 5% | 203 | < 5% | 199 | ‐ | |
|
Daughton 1991 (P) 24 h 16 h |
7 4 |
51 55 |
6 | 52 | ‐ | |
| Gourlay 1995 (P) | 5 | 315 | 4 | 314 | ‐ | |
| Harackiewicz 1988 (G) | 9 | 99 | 12 | 85 | First 6 weeks | |
| Hjalmarson 1994 (S) | 24 | 116 | 16 | 107 | First 2 weeks | |
| Hughes 1989a (G) | 71 | 210 | 18 | 105 | From % | |
| Jarvis 1982 (G) | 15 | 47 | 11 | 44 | ||
| Jorenby 1999 (P) | 8 20 |
243 244 |
10 | 159 | P vs placebo P + B vs placebo |
|
| Lerman 2015 (P) | 42 | 418 | 56 | 408 | Number of events summed over time, not number of people | |
| Lewis 1998 (P) | 0 | 62 | 1 | 62 | ‐ | |
| Puska 1979 (G) | 16 | 80 | 16 | 74 | From %; | |
| Sachs 1993 (P) | 1 | 113 | 0 | 107 | ‐ | |
| Schneider 1995 (S) | 61 | 128 | 69 | 127 | From % | |
| Stapleton 1995 (P) | 46 | 761 | 24 | 364 | ‐ | |
| Stein 2013 (P) | 5 | 104 | 1 | 33 | ‐ | |
| Sutherland 1992 (S) | 61 | 111 | 50 | 103 | ‐ | |
| Tønnesen 1991 (P) | 6 | 145 | 0 | 144 | From % | |
| Ward 2013 (P) | <5% | 134 | <5% | 135 | ‐ | |
| EX Hanson 2003 (P) | 20 | 50 | 22 | 50 | adolescents | |
| EX Mulligan 1990 (P) | 1 | 39 | 0 | 36 | ‐ | |
| EX Oncken 2009 (P, S) | P3 S0 |
7 7 |
3 | 7 | Pregnant women | |
| EX Rigotti 2009 (P) | 25 | 367 | 16 | 362 | All were on rimonabant | |
| EX Schnoll 2010 (P) | 2 | 182 | 1 | 134 | At 12 weeks | |
| EX Stapleton 2011 (S) | 308 | 506 | 139 | 255 | ‐ | |
| Nausea/vomiting | Ahluwalia 1998 (P) | 1 | 174 | 3 | 168 | ‐ |
| Anthenelli 2016 (P) Nausea | 199 | 2022 | 137 | 2014 | Totals are numbers assessed for adverse events | |
| Areechon 1988 (G) | 2 | 98 | 2 | 101 | ‐ | |
| EX Batra 2005 (G) | 19 | 184 | 11 | 180 | ‐ | |
|
Berlin 2014 (P) Nausea Vomiting Total |
4 5 9 |
203 | 3 8 11 |
199 | ‐ | |
| Campbell 1996 (P) | 14 | 115 | 4 | 119 | ‐ | |
| Coleman 2012 (P) | 16 | 521 | 19 | 529 | Pregnant women | |
| Dautzenberg 2001 (L) | 7 | 214 | 11 | 222 | ‐ | |
| Garvey 2000 (G) | 11 | 209 | 1 | 69 | (2 mg + 4 mg) % | |
| Glover 2002 (T) | 14 | 120 | 3 | 121 | ‐ | |
| Gourlay 1995 (P) | 10 | 315 | 7 | 314 | ‐ | |
| Harackiewicz 1988 (G) | 17 | 99 | 6 | 85 | First 6 weeks | |
| Hays 1999 (P) | 19 | 321 | 16 | 322 | Excludes pay group | |
| Heydari 2012 (P) Nausea | 0 | 92 | 0 | 91 | ‐ | |
| Hjalmarson 1994 (S) | 16 | 116 | 7 | 107 | First 2 weeks | |
| Hughes 1989a (G) | 69 | 210 | 18 | 105 | From % | |
| Hurt 1994 (P) | 6 | 120 | 3 | 120 | ‐ | |
| Jarvis 1982 (G) | 20 | 47 | 9 | 44 | ‐ | |
|
Jorenby 1999 (P) P P + B |
19 28 |
243 244 |
8 | 159 | ‐ | |
|
Lerman 2015 (P) nausea vomiting |
88 10 |
418 | 111 16 |
408 | Number of events summed over time, not number of people | |
| Lewis 1998 (P) | 4 | 62 | 3 | 62 | P + counselling vs Pl + counselling | |
| Richmond 1994 (P) | 9 | 156 | 2 | 157 | From % | |
| Sachs 1993 (P) | 4 | 113 | 10 | 107 | ‐ | |
| Schneider 1995 (S) | 24 | 128 | 11 | 127 | From % | |
| Schneider 1996 (I) | 14 | 112 | 13 | 111 | ‐ | |
|
Shiffman 2002 (2 mg) (L) Shiffman 2002 (4 mg) (L) |
56 68 |
459 450 |
22 24 |
458 451 |
From % | |
|
Stapleton 1995 (P) (= Russell 1993) |
34 | 761 | 12 | 364 | ‐ | |
| Stein 2013 (P) Nausea | 9 | 104 | 2 | 33 | ‐ | |
| Sutherland 1992 (S) | 26 | 111 | 20 | 103 | ‐ | |
|
Tønnesen 1988 (G) 2 mg 4 mg |
1 0 |
87 27 |
0 | 47 | ‐ | |
| Tønnesen 1991 (P) | 6 | 145 | 1 | 144 | From % | |
| Tønnesen 1993 (I) | 1 | 145 | 1 | 141 | severe | |
| Wallstrom 2000 (T) | 30 | 123 | 9 | 124 | From % | |
| Ward 2013 (P) | < 5% | 134 | < 5% | 135 | ‐ | |
| EX Bolliger 2000a (I) | 9 | 200 | 8 | 200 | ‐ | |
| EX CEASE 1999 (P) 25 mg 15 mg |
104 77 |
1430 1431 |
26 | 714 | ‐ | |
| EX McRobbie 2010 (L,G,S) | L17 G15 S16 |
45 45 45 |
2 |
47 |
‐ | |
| EX Rennard 2006 (I) | 11 | 215 | 5 | 214 | ‐ | |
| EX Rigotti 2009 (P) | 54 | 367 | 36 | 362 | All were on rimonabant | |
| EX Roddy 2006 (P) | 2 | 49 | 3 | 49 | “Dizziness, nausea or headache” | |
| EX Schnoll 2010 (P) | 1 | 182 | 1 | 134 | At 12 weeks (i.e. 4 weeks on placebo or patch) | |
| EX Stapleton 2011 (S) | 336 | 506 | 168 | 255 | ‐ | |
| EX Tsukahara 2010 (P) | 4 | 16 | 0 | 16 | V vs Gum, no placebo | |
| Gastro‐intestinal symptoms |
Berlin 2014 (P) Reflux Pyrosis Total |
8 4 12 |
203 | 5 4 9 |
199 | ‐ |
| Campbell 1991 (G) | 3 | 107 | 7 | 103 | From % | |
|
Daughton 1991 (P) 24 h 16 h |
1 4 |
51 55 |
0 | 52 | ‐ | |
| Dautzenberg 2001 (L) | 2 | 214 | 8 | 222 | ‐ | |
| Glover 2002 (T) | 11 | 120 | 6 | 121 | ‐ | |
| Harackiewicz 1988 (G) | 23 | 99 | 8 | 85 | First 6 weeks | |
| Hjalmarson 1984 (G) | 25 | 92 | 11 | 91 | ‐ | |
| Hurt 1994 (P) | 4 | 120 | 6 | 120 | ‐ | |
| Hughes 1989a (G) | 65 | 210 | 18 | 105 | ‐ | |
| Jarvis 1982 (G) | 24 | 47 | 12 | 44 | ‐ | |
| Joseph 1996 (P) | 5 | 294 | 6 | 290 | ‐ | |
|
Lerman 2015 (P) Gas Abdominal pain Constipation Diarrhoea Flatulence Indigestion |
199 53 100 54 154 81 |
418 | 211 41 111 77 159 92 |
408 | Number of events summed over time, not number of people | |
| Lewis 1998 (P) | 1 | 62 | 2 | 62 | ‐ | |
| Llivina 1988 (G) | 11 | 113 | 6 | 101 | From % | |
| Paoletti 1996 (P) | 16 | 147 (LC15+HC25) |
11 | 150 (LCP+HC15) |
(Pl+Pl or lowA+Pl) | |
| Puska 1979 (G) | 12 | 80 | 13 | 74 | From % | |
| Sachs 1993 (P) | 2 | 113 | 4 | 107 | ‐ | |
|
Shiffman 2002 (2 mg) (L) Shiffman 2002 (4 mg) (L) |
16 24 |
459 450 |
10 17 |
458 451 |
From % | |
|
Shiffman 2009 (2 mg) (G) Shiffman 2009 (4 mg) (G) |
213 216 |
819 830 |
118 120 |
817 831 |
From % | |
| Schneider 1996 (I) | 16 | 112 | 11 | 111 | ‐ | |
| Sønderskov 1997 (P) | 7 | 255 | 9 | 267 | First 4 wks | |
| Stein 2013 (P) Diarrhoea | 0 | 104 | 1 | 33 | ‐ | |
|
Tønnesen 1988 (G) 2 mg 4 mg |
11 4 |
87 27 |
5 | 47 | ‐ | |
| Wallstrom 2000 (T) | 22 | 123 | 11 | 124 | From % | |
| Ward 2013 (P) | < 5% | 134 | < 5% | 135 | ‐ | |
| EX Batra 2005 (G) | 12 | 184 | 5 | 180 | ‐ | |
|
Bullen 2010 (P,G) serious non‐serious |
24 19 |
249 249 |
12 26 |
246 249 |
‐ | |
| EX Ebbert 2010 (L) | 3 | 30 | 0 | 30 | Smokeless (from %) | |
| EX Ebbert 2009 (L) | 15 | 136 | 1 | 134 | Smokeless (from %) | |
| EX Molander 2000 (T) | 1 | 20 | 1 | 20 | ‐ | |
| EX Mulligan 1990 (P) | 3 | 39 | 0 | 36 | ‐ | |
| EX Oncken 2009 (P, S) | P3 S0 |
7 7 |
1 | 7 | Pregnant women | |
| EX Tsukahara 2010 (P) | 14 | 16 | 1 | 16 | V vs Gum, no placebo | |
| Sleep/dream problems | Ahluwalia 1998 (P) | 0 | 174 | 0 | 168 | In first week |
|
Anthenelli 2016 (P) Insomnia Initial insomnia Sleep disorder Nightmare |
195 20 45 56 |
2022 | 139 6 42 17 |
2014 | Totals are numbers assessed for adverse events | |
| Berlin 2014 (P) | 7 | 203 | 5 | 199 | ‐ | |
| Dautzenberg 2001 (L) | 2 | 214 | 3 | 222 | ‐ | |
| Gourlay 1995 (P) | 43 | 315 | 19 | 314 | ‐ | |
| Hays 1999 (P) | 30 | 321 | 20 | 322 | Excludes pay group | |
| Heydari 2012 (P) Abnormal dreams | 0 | 92 | 0 | 91 | ‐ | |
| Hurt 1994 (P) | 9 | 120 | 5 | 120 | ‐ | |
|
ICRF 1994 (P) Mild Moderate Severe |
45 95 32 |
842 |
10 40 13 |
844 |
‐ | |
|
Jorenby 1999 (P) P P+B |
73 116 |
243 244 |
31 | 159 | ‐ | |
| Joseph 1996 (P) | 10 | 294 | 6 | 290 | ‐ | |
|
Lerman 2015 (P) Sleep Abnormal dreams |
203 182 |
418 | 190 132 |
408 | Number of events summed over time, not number of people | |
| Llivina 1988 (G) | 7 | 113 | 10 | 101 | From % | |
| Oncken 2007 (P) | 5 | 57 | 2 | 95 | ‐ | |
| Paoletti 1996 (P) | 19 | 147 (LC15+HC25) |
36 | 150 (LCP+HC15) |
(Pl+Pl or lowA+Pl) | |
| Perng 1998 (P) | 2 | 30 | 0 | 32 | ‐ | |
| Puska 1979 (G) | 26 | 80 | 20 | 74 | From %; | |
| Richmond 1994 (P) | 41 | 156 | 25 | 157 | From % | |
| Sachs 1993 (P) | 4 | 113 | 5 | 107 | ‐ | |
|
Stein 2013 (P) Insomnia/sleep problems/ awakening Dreaming or nightmares |
38 19 |
104 | 12 8 |
33 | ‐ | |
| Ward 2013 (P) | 11 | 134 | 14 | 135 | ‐ | |
| EX CEASE 1999 (P) 25 mg 15 mg |
70 77 |
1430 1431 |
42 | 714 | ‐ | |
| EX Ebbert 2009 (L) | 15 | 136 | 1 | 134 | Smokeless (from %) | |
| EX Ebbert 2010 (L) | 0 | 30 | 3 | 30 | Smokeless (from %) | |
| EX Hanson 2003 (P) | 30 | 50 | 23 | 50 | Adolescents | |
| EX Mulligan 1990 (P) | 2 | 39 | 0 | 36 | ‐ | |
| EX Rigotti 2009 (P) | 35 | 367 | 11 | 362 | All were on rimonabant | |
| EX Schnoll 2010 (P) | 2 | 182 | 6 | 134 | At12 weeks | |
| EX Tsukahara 2010 (P) | 6 | 16 | 2 | 16 | V vs Gum | |
| CV (palpitations, chest pain) |
Berlin 2014 (P) palpitations CV other |
< 5% < 5% |
203 | < 5% < 5% |
199 | ‐ |
| Gourlay 1995 (P) | 5 | 179 | 3 | 143 | ‐ | |
| Hays 1999 (P) | 5 | 321 | 2 | 322 | Excludes pay group | |
| Hjalmarson 1994 (S) | 9 | 116 | 2 | 107 | First 2 weeks | |
|
Lerman 2015 (P) Chest pain Palpitations Irregular heartbeat |
18 35 11 |
418 | 26 50 19 |
408 | Number of events summed over time, not number of people, therefore excluded from meta‐analysis 6.1. | |
| Oncken 2007 (P) | 1 | 57 | 1 | 95 | ‐ | |
| Schneider 1995 (S) | 23 | 128 | 10 | 127 | From % | |
|
Shiffman 2009 (2 mg) (G) Shiffman 2009 (4 mg) (G) |
3 3 |
819 830 |
3 3 |
817 831 |
From % | |
|
Sønderskov 1997 (P) “cardiac” |
1 | 255 | 4 | 267 | First 4 weeks | |
| Sutherland 1992 (S) | 26 | 111 | 15 | 103 | ‐ | |
|
Tønnesen 1988 (G) 2 mg 4 mg |
0 1 |
87 27 |
0 | 47 | ‐ | |
|
Ward 2013 (P) Palpitations CV other |
< 5% < 5% |
134 | < 5% < 5% |
135 | ‐ | |
| EX Bolliger 2000a (I) | 1 | 200 | 2 | 200 | ‐ | |
| EX Brantmark 1973a (G) | 3 | 46 | 4 | 42 | ‐ | |
| EX Bullen 2010 (P,G) | 9 | 249 | 1 | 246 | ‐ | |
| EX CEASE 1999 (P) 25 mg 15 mg |
32 37 |
1430 1431 |
6 | 714 | ‐ | |
| EX Schnoll 2010 (P) | 0 | 182 | 2 | 134 | At12 weeks (i.e. 4 weeks on placebo or patch) | |
| EX Wennike 2003a (G) | 6 | 205 | 4 | 206 | ‐ | |
| Wisborg 2000 states 5 women had palpitations, but no distribution info | ||||||
| Skin reactions | Abelin 1989 (P) | 12 | 156 | 1 | 155 | Combined studies |
| Ahluwalia 1998 (P) | 8 | 174 | 5 | 168 | ‐ | |
| Anthenelli 2016 (P) | 109 | 2022 | 16 | 2014 | Totals are numbers assessed for adverse events | |
| Berlin 2014 (P) | 23 | 203 | 8 | 199 | ‐ | |
| Buchkremer 1988 (P) | 6 | 42 | 6 | 43 | From % | |
| Campbell 1996 (P) | 54 | 115 | 40 | 119 | ‐ | |
| Coleman 2012 (P) | 97 | 521 | 28 | 529 | Pregnant women | |
| Daughton 1991 (P) | 1 3 |
51 (24 h) 55 (16 h) |
1 | 52 | ‐ | |
| Dautzenberg 2001 | 0 | 214 | 2 | 222 | ‐ | |
| Davidson 1998 (P) | 100 | 401 | 52 | 401 | From % | |
| Gourlay 1995 (P) | 44 | 315 | 27 | 314 | ‐ | |
| Hays 1999 (P) | 124 | 321 | 48 | 322 | Excludes pay group | |
| Hurt 1990 (P) | 19 | 31 | 10 | 31 | Over 6 weeks | |
|
Hurt 1994 Mild Moderate Severe |
68 5 1 |
120 |
24 3 0 |
120 |
‐ | |
|
ICRF 1994 (P) Mild Moderate Severe |
18 75 40 |
842 |
8 26 9 |
844 |
‐ | |
|
Jorenby 1999 (P) P P+B |
45 37 |
243 244 |
11 | 159 | ‐ | |
| Joseph 1996 (P) | 6 | 294 | 3 | 290 | ‐ | |
| Kornitzer 1995 (P,G) | 9 7 |
149 150 |
1 | 75 | P+G vs Pl P+PlG vs Pl |
|
|
Lerman 2015 (P) Redness Swelling/rash |
68 48 |
418 | 32 44 |
408 | Number of events summed over time, not number of people | |
| Lewis 1998 (P) | 16 | 62 | 11 | 62 | ‐ | |
| Oncken 2007 (P) | 8 | 57 | 2 | 95 | ‐ | |
| Paoletti 1996 (P) | 59 | 147 (LC15+HC25) |
30 | 150 (LCP+HC15) |
Active vs placebo (Pl+Pl or lowA+Pl) | |
| Perng 1998 (P) | 7 | 30 | 5 | 32 | ‐ | |
| Richmond 1994 (P) | 36 | 156 | 19 | 157 | From % | |
| Sønderskov 1997 (P) | 75 | 255 | 49 | 267 | First 4 weeks | |
| Stapleton 1995 (P) | 108 | 761 | 18 | 364 | ‐ | |
| Stein 2013 (P) Flushing or sweating | 30 | 104 | 11 | 33 | ‐ | |
| Ward 2013 (P) | 12 | 134 | 16 | 135 | ‐ | |
| EX Bullen 2010 (P,G) | 6 5 |
249 249 |
8 3 |
246 246 |
Skin SAEs | |
| EX CEASE 1999 (P) 25 mg 15 mg |
206 185 |
1430 1431 |
36 | 714 | ‐ | |
| EX Hanson 2003 (P) | 31 | 50 | 24 | 50 | adolescents | |
| EX Levin 1994 (P) | 24 | 31 | 21 | 31 | ‐ | |
| EX Mulligan 1990 (P) | 10 | 39 | 10 | 36 | ‐ | |
| EX Oncken 2009 (P, S) | P3 S0 |
7 7 |
0 | 7 | Pregnant women | |
| EX Roddy 2006 (P) | 16 | 49 | 7 | 49 | ‐ | |
| EX Rose 1990 (P) mod. severe |
12 4 |
33 | 0 0 |
32 | From % | |
| Ex Schnoll 2010 (P) | 1 | 182 | 1 | 134 | @12wks | |
| EX Tsukahara 2010 (P) | 0 | 16 | 9 | 16 | V vs Gum, no placebo | |
| Oral/nasal reactions | Areechon 1988 (G) | 1 | 98 | 2 | 101 | ‐ |
| Berlin 2014 (P) | < 5% | 203 | < 5% | 199 | ‐ | |
| Campbell 1991 (G) | 9 | 107 | 6 | 105 | From % | |
| Dautzenberg 2001 (L) | 5 | 214 | 0 | 222 | ‐ | |
| Garvey 2000 (G) | 2 | 209 | 0 | 69 | (2 mg + 4 mg) | |
| Glover 2002 (T) | 24 | 120 | 23 | 121 | Weeks 1 to 2 | |
| Harackiewicz 1988 (G) | 34 | 99 | 1 | 85 | First 6 wks | |
| Hjalmarson 1984 (P) | 24 | 92 | 14 | 91 | ‐ | |
| Hjalmarson 1994 (S) | 85 | 116 | 40 | 107 | First 2 wks | |
| Hughes 1989a (G) | 160 | 210 | 56 | 105 | From % | |
| Jarvis 1982 (G) | 28 | 47 | 23 | 44 | ‐ | |
|
Jorenby 1999 (P) P P+B |
16 25 |
243 244 |
10 | 159 | ‐ | |
| Lerman 2015 (P) | 173 | 418 | 160 | 408 | Number of events summed over time, not number of people | |
| Llivina 1988 (G) | 13 | 113 | 4 | 101 | From % | |
| Perng 1998 (P) | 4 | 30 | 0 | 32 | ‐ | |
| Schneider 1995 (S) | 125 | 128 | 65 | 127 | From % | |
| Schneider 1996 (I) | 47 | 112 | 26 | 111 | ‐ | |
|
Shiffman 2002 (2 mg) (L) Shiffman 2002 (4 mg) (L) |
12 23 |
459 450 |
12 18 |
458 451 |
From % | |
|
Stein 2013 (P) Dry mouth Change in taste |
17 14 |
104 | 4 1 |
33 | ‐ | |
| Sutherland 1992 (S) | 105 | 111 | 67 | 103 | ‐ | |
|
Tønnesen 1988 (G) 2 mg 4 mg |
20 8 |
87 27 |
11 | 47 | ‐ | |
| Tønnesen 1993 (I) | 72 | 145 | 24 | 141 | From % | |
| Wallstrom 2000 (T) | 66 | 123 | 62 | 124 | From % | |
| Ward 2013 (P) | < 5% | 134 | < 5% | 135 | ‐ | |
| EX Adelman 2009 (S) | 7 | 20 | 0 | 20 | Open‐label, no spray for controls | |
| EX Batra 2005 (G) | 8 | 184 | 33 | 180 | ‐ | |
| EX Bolliger 2000a (I) | 14 | 200 | 4 | 200 | ‐ | |
| EX Brantmark 1973a (G) | 6 | 46 | 3 | 42 | ‐ | |
| EX McRobbie 2010 (L,G,S) | L8 G6 S16 |
45 45 45 |
2 |
47 |
‐ | |
| EX Molander 2000 (T) | 5 | 20 | 0 | 20 | ‐ | |
| EX Oncken 2009 (P, S) | P1 S2 |
7 7 |
0 | 7 | Pregnant women | |
| EX Rigotti 2009 (P) | 23 | 367 | 24 | 362 | All were on rimonabant | |
| EX Stapleton 2011 (S) | 194 | 506 | 135 | 255 | ‐ | |
| Hiccups | Berlin 2014 (P) | < 5% | 203 | < 5% | 199 | ‐ |
| Blondal 1989 (G) | 13 | 90 | 0 | 92 | ‐ | |
| Glover 2002 (T) | 18 | 120 | 1 | 121 | ‐ | |
| Harackiewicz 1988 (G) | 8 | 99 | 1 | 85 | First 6 weeks | |
| Hjalmarson 1984 (P) | 7 | 92 | 0 | 91 | ‐ | |
| Hughes 1989a (G) | 103 | 210 | 22 | 105 | From % | |
| Jarvis 1982 (G) | 14 | 47 | 2 | 44 | ‐ | |
| Schneider 1996 (I) | 3 | 112 | 0 | 111 | ‐ | |
|
Shiffman 2002 (2 mg) (L) Shiffman 2002 (4 mg) (L) |
16 38 |
459 450 |
0 0 |
458 451 |
From % | |
|
Tønnesen 1988 (G) 2 mg 4 mg |
2 4 |
87 27 |
0 | 47 | ‐ | |
| Wallstrom 2000 (T) | 14 | 123 | 0 | 124 | From % | |
| Ward 2013 (P) | < 5% | 134 | < 5% | 135 | ‐ | |
| EX Batra 2005 (G) | 28 | 184 | 3 | 180 | ‐ | |
| EX Brantmark 1973a (G) | 11 | 46 | 2 | 42 | ‐ | |
| EX McRobbie 2010 (L,G,S) | L17 G15 S16 |
45 45 45 |
2 |
47 |
‐ | |
| EX Molander 2000 (T) | 1 | 20 | 0 | 20 | ‐ | |
Data and analyses
Comparison 1. Any type of NRT versus placebo/no NRT control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at 6+ months follow up | 133 | 64640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.49, 1.61] |
| 1.1 Gum | 56 | 22581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.40, 1.60] |
| 1.2 Patch | 51 | 25754 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.53, 1.75] |
| 1.3 Inhalator | 4 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.36, 2.67] |
| 1.4 Intranasal spray | 4 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.49, 2.73] |
| 1.5 Tablets/lozenges | 8 | 4439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.32, 1.74] |
| 1.6 Oral spray | 1 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.24, 4.94] |
| 1.7 Choice of NRT product | 7 | 8288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.25, 1.52] |
| 1.8 Patch and inhalator | 1 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.57, 1.99] |
| 1.9 Patch and lozenge | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.01, 3.31] |
| 1.10 Patch and gum | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.64, 2.06] |
| 1.11 Patch, gum and lozenge | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.0 [2.00, 112.54] |
Comparison 2. Subgroup: Definition of abstinence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nicotine gum. Smoking cessation | 56 | 22581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.40, 1.60] |
| 1.1 Sustained 12 months | 32 | 13737 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.31, 1.56] |
| 1.2 Sustained 6 months | 8 | 4187 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [2.14, 3.59] |
| 1.3 PP/uncertain 12 months | 8 | 2501 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.12, 1.55] |
| 1.4 PP/uncertain 6 months | 8 | 2156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.20, 1.68] |
| 2 Nicotine patch: Smoking cessation | 49 | 23976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.52, 1.75] |
| 2.1 Sustained 12 months | 21 | 7622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.34, 1.74] |
| 2.2 Sustained 6 months | 9 | 8613 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.51, 1.92] |
| 2.3 PP/uncertain 12 months | 9 | 3856 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.44, 1.93] |
| 2.4 PP/uncertain 6 months | 10 | 3885 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.32, 2.04] |
Comparison 3. Subgroup: Level of behavioural support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nicotine gum. Smoking cessation | 55 | 21759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.40, 1.61] |
| 1.1 Low intensity support | 17 | 11257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.46, 1.88] |
| 1.2 High intensity individual support | 18 | 6891 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.18, 1.49] |
| 1.3 High intensity group‐based support | 20 | 3611 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.40, 1.76] |
| 2 Nicotine patch. Smoking cessation | 49 | 23657 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.56, 1.79] |
| 2.1 Low intensity support | 15 | 7310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.54, 2.02] |
| 2.2 High intensity individual support | 25 | 12709 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.47, 1.81] |
| 2.3 High intensity group‐based support | 10 | 3638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.43, 1.90] |
Comparison 4. Subgroup: Recruitment/treatment setting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Community volunteer (treatment provided in medical setting) | 65 | 24597 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.53, 1.72] |
| 1.1 Nicotine gum | 28 | 8336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.28, 1.53] |
| 1.2 Nicotine patch | 27 | 11214 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.59, 1.91] |
| 1.3 Nicotine inhalator | 2 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.98, 3.27] |
| 1.4 Nicotine tablet/lozenge | 7 | 3405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.61, 2.36] |
| 1.5 Nicotine intranasal spray | 2 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.16, 2.95] |
| 1.6 Combination of NRT | 1 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.01, 3.31] |
| 1.7 Nicotine oral spray | 1 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.24, 4.94] |
| 2 Smoking clinic | 12 | 3300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.48, 1.96] |
| 2.1 Nicotine gum | 6 | 1283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.30, 1.91] |
| 2.2 Nicotine inhalator | 2 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.30, 2.95] |
| 2.3 Nicotine intranasal spray | 2 | 475 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.44, 3.20] |
| 2.4 Nicotine patch | 2 | 1009 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.18, 2.19] |
| 3 Primary care | 24 | 11974 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.33, 1.69] |
| 3.1 Nicotine gum | 16 | 7277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.35, 1.85] |
| 3.2 Nicotine patch | 7 | 4419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.15, 1.71] |
| 3.3 Choice of NRT products | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.83, 2.30] |
| 4 Hospitals | 13 | 7037 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.24, 1.55] |
| 4.1 Nicotine gum | 3 | 2194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.86, 1.43] |
| 4.2 Nicotine patch | 6 | 2492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.10, 1.78] |
| 4.3 Combination of NRT | 2 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.64, 1.52] |
| 4.4 Choice of NRT products | 2 | 2025 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.36, 1.86] |
| 5 Community volunteer (treatment provided in 'over‐the‐counter' setting) | 9 | 13163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.26, 1.55] |
| 5.1 Nicotine gum | 2 | 3297 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.79 [2.60, 5.52] |
| 5.2 Nicotine patch | 5 | 3542 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.38, 2.55] |
| 5.3 Tablets/lozenges | 1 | 1034 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.32] |
| 5.4 Choice of product | 1 | 5290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.03, 1.37] |
| 6 Antenatal clinic | 4 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.92, 1.62] |
| 6.1 Nicotine gum | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.50, 2.65] |
| 6.2 Nicotine patch | 2 | 1300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.85, 1.66] |
| 6.3 Choice of NRT products | 1 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.69, 3.03] |
Comparison 5. NRT in pregnancy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Abstinence at end of pregnancy | 6 | 2129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.04, 1.69] |
| 1.2 Abstinence at longest post partum follow‐up | 4 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.90, 1.86] |
5.1. Analysis.
Comparison 5 NRT in pregnancy, Outcome 1 Smoking cessation.
Comparison 6. Palpitations in NRT vs placebo users.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Palpitations/chest pains | 15 | 11074 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.37, 2.57] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abelin 1989.
| Methods | Country: Switzerland Recruitment: 21 primary care clinics | |
| Participants | 199 primary care patients 40% female, average age 41, average cpd 27 Participants were motivated to quit. | |
| Interventions | 1. Nicotine patch, 24 h, 12 weeks with weaning; 21 mg smokers of > 20 cpd, 14 mg for < 20 cpd 2. Placebo patch Level of support: low (number of visits unclear) | |
| Outcomes | Sustained abstinence at 12 months (0 to 3 cigarettes/week) Validation: expired CO | |
| Notes | Methods in Lancet paper, final follow up in Muller 1990. Sources of support not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated; described as "randomised, between‐subjects, double‐blind, and placebo‐controlled" |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind", no further details. 75% of NRT group and 76% of placebo group correctly guessed their assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts similar between groups (NRT 20, placebo 21); 36/41 dropouts continued to smoke, but all 41 counted as treatment failures in ITT analysis |
| Other bias | High risk | If smoking from 0 to 3 cigarettes/week, and CO 0 to 11 ppm, counted as abstinent |
Ahluwalia 1998.
| Methods | Country: USA Recruitment: hospital in‐ and outpatients | |
| Participants | 410 African‐American smokers Average age 47, FTND 6 Participants were motivated to quit | |
| Interventions | 1. Nicotine patch (21 mg with weaning, 10 weeks) 2. Placebo patch Level of support: high (1 h initial visit and brief follow‐up visits) | |
| Outcomes | Prolonged abstinence at 6 months (self‐report of no smoking since end of treatment) Validation: none | |
| Notes | Study funded by American Cancer Society Career Development Award, Marion Merrell Dow Inc, and Emory Medical Care Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A "computer‐generated random numbers table with a block size set at 20" |
| Allocation concealment (selection bias) | Low risk | Study staff blinded ‐ see below |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Both study staff and patients were blinded to patch treatment" 63% of NRT participants and 44% of placebo participants correctly guessed their assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses similar between groups at 6 months: NRT 53, placebo 58. Counted as treatment failures for ITT analyses |
Ahluwalia 2006.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 755 African‐American light smokers (≤ 10 cpd) 67% female, average age 45, average cpd 8 Participants were motivated to quit | |
| Interventions | Factorial trial, behavioural interventions collapsed for this review 1. Nicotine gum (2 mg), recommended use tailored to cpd. Highest 10/day for 4 weeks, tapering for 4 weeks 2. Placebo gum, 8 weeks Level of support: high: 3 in‐person visits at randomization, week 1, week 8, and phone contact at week 3, week 6, week 16, content based on either motivational interviewing or health education principles | |
| Outcomes | PP abstinence at 6 months (7‐day PP) Validation: cotinine ≤ 20 ng/ml | |
| Notes | Study funded by National Cancer Institute; products supplied by Glaxo‐SmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization codes were generated in blocks of 36". For counselling support "a sealed envelope with pre‐assigned randomization numbers was drawn" |
| Allocation concealment (selection bias) | Low risk | Quote: "Study staff ... were blinded" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Study staff and participants were blinded". "Assignment to MI counselling versus HE was not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants receiving active gum and HE counselling were more likely to remain in the study, but interaction not statistically significant. Losses to follow up at week 26: NRT + MI: 32; NRT + HE 21; Placebo + MI 39; Placebo + HE 26. |
Anthenelli 2016.
| Methods | Countries: Argentina, Australia, Brazil, Bulgaria, Canada, Chile, Denmark, Finland, Germany, Mexico, New Zealand, Russian Federation, Slovakiam South Africa, Spain, USA Recruitment: community (media advertisements, posters, fliers) | |
| Participants | 8144 smokers (≥ 10 cpd), treatment‐seeking, exhaled CO > 10 ppm at screening. Participants in the psychiatric disorder cohort had to have a current or lifetime stable psychiatric diagnosis 44% men, mean age 46, mean CPD 20.7, mean FTND 5.8 |
|
| Interventions | 1. Varenicline, 1 mg x 2/day (1 week titrated, then 11 weeks full dose)
2. Bupropion SR, 150 mg x 2/day (titrated for 3 days, then full dose for 11 weeks) 3. Nicotine patch, 21 mg x 7 weeks, 14 mg x 2 weeks, 7 mg x 2 weeks (11 weeks, 24 v 16 h not specified) 4. Triple‐dummy placebo for each arm of the trial (12 weeks) Level of support: high (counselling (up to 10 mins) at all contacts: up to 15 face‐to‐face visits and 11 telephone visits) |
|
| Outcomes | 6 months continuous abstinence weeks 9 to 24 Validation: CO < 10 ppm |
|
| Notes | New for 2017 update. For this review, arm 3 v 4 only Trial funded by Pfizer and GlaxoSmithKline Some data extraction and risk of bias taken from Cahill 2016. N quit extrapolated from percentages given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomisation schedule ... using a block size of 8 (1:1:1:1 ratio) for each of the 20 diagnosis by region combinations" |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators obtained participant identification numbers via a web‐based or telephone call‐in drug management system" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Study product kit codes did not allow deciphering of randomised treatment or block size. As such, participants, investigators, and research personnel were masked to treatment assignment" "The triple dummy design feature required participants to take study medication as masked tablets dispensed in separate varenicline and bupropion pill bottles each with matching placebo along with with either applying active or placebo patches on a daily basis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses fully accounted for; ITT analysis conducted throughout. 790/1025 NRT and 765/1036 placebo completed study |
Areechon 1988.
| Methods | Country: Thailand Recruitment: community volunteers | |
| Participants | 200 smokers (≥ 15 cpd) 6% female, average age 39, average cpd 24 Participants were motivated to quit | |
| Interventions | 1. Gum (2 mg) x 8 boxes 2. Placebo gum x 8 boxes Level of support: high (weekly visits with physician, unspecified frequency and duration) | |
| Outcomes | PP abstinence at 6 months Validation: CO | |
| Notes | Support level reclassified as high, 2008 Study funded by Merrel Dow (Bangkok, Thailand), with products supplied by A.B. Leo, Helsinborg, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind. "Neither the investigators nor the subjects knew which subjects received the active gum and which received the placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Significant differences between NRT (20 dropouts) and placebo (37 dropouts; P < 0.01) at 6 months |
| Other bias | High risk | 10/93 quitters did not provide CO validation, but distribution not reported. All are included in MA |
Berlin 2014.
| Methods | Country: France Recruitment: multicentre, advertisements and letters from participating healthcare settings |
|
| Participants | 403 pregnant smokers (≥ 5 cpd) at 9 to 20 weeks amenorrhoea, motivated to quit 100% female, average age 29, average cpd 11, median FTND 4.5, median gestational age 17 weeks |
|
| Interventions | 1. Nicotine patch, 16 h, from TQD to delivery. Daily dose 10 to 30 mg/day based on salivary cotinine, adjusted at 6 and 12 weeks post‐randomization 2. Placebo on same schedule Level of support: high (1 h behavioural counselling at baseline, at least 10 mins counselling at following 6 visits) |
|
| Outcomes | Continuous abstinence at 20 weeks post‐TQD Validation: CO ≤ 8 ppm |
|
| Notes | New for 2017 update 2‐month cessation data post‐delivery also collected but not reported. Data at 20 weeks post‐TQD included in Analysis 5.1.1. Not included in main analysis because follow‐up was less than 6 months Funding: Ministry of Health, France and Assistance publique‐Hôpitaux de Paris |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A computer generated randomisation list (allocation ratio 1:1) in blocks of four was prepared and kept double blinded.” |
| Allocation concealment (selection bias) | Low risk | Quote: “…the randomisation number was attributed automatically at the completion of the randomisation visit. A statistician…who was fully independent of the trial, prepared the random, computer generated allocation sequence. The randomisation code was kept in a sealed envelope in a safe. A copy of the randomisation code was kept separately in case of a serious adverse event necessitating exposure of a participant’s group assignment. Investigators, members of the coordination centre, hospital pharmacists, and the study statistician were kept blinded until the code was opened before witnesses on 19 February 2013.” |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “All study staff (investigators, pharmacists, members of the coordination centre and of the drug safety monitoring board, laboratory staff, statistician) were double blinded to treatment allocation.” “The placebo patches were manufactured by the same company, with specific quality control guidelines to ensure double blinding.” “Determinations of saliva cotinine levels were carried out blinded. The investigators were not aware of the results” “Data were analysed blinded to treatment” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 92/203 and 113/199 withdrew, 107/203 and 123/199 not followed up at every visit (needed for strictest measure) (> 50% attrition overall) |
Blondal 1989.
| Methods | Country: Iceland Recruitment: community volunteers invited to attend a smoking cessation clinic | |
| Participants | 182 smokers (included pipe and cigar users, smoked at least once a day) 57% female, average age 42, average tobacco use 21 g/day Participants were volunteers, but motivation not required or assessed | |
| Interventions | 1. Gum (4 mg) for at least 1 month 2. Placebo gum (containing pepper) for 1 month or more Level of support: high (group therapy, 5 x 1‐h sessions, TQD at session 1) | |
| Outcomes | Lapse‐free abstinence at 12 months (24 months also reported, no validation) Validation: CO < 10 ppm | |
| Notes | Lapse‐free abstinence used since 2008 Study funded by Icelandic Ministry of Health and Social Security | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assignment was by group (6 to active gum, 6 to placebo); whether randomized or not is unclear |
| Allocation concealment (selection bias) | Unclear risk | Probably. "Each subgroup knew they would either get nicotine gum or a placebo" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/59 claiming abstinence at 12 months were not CO‐confirmed (4 missing and 3 > 10 ppm), and counted as continuing smokers |
| Other bias | Low risk | 44/92 in NRT group were highly nicotine‐dependent, compared with 28/90 in placebo group (P = 0.03) |
Blondal 1997.
| Methods | Country: Iceland Recruitment: community volunteers | |
| Participants | 159 smokers (≥ 1 cpd) 44% female, average age 42, average tobacco use 25 g/day Participants had to be motivated to quit | |
| Interventions | 1. Nicotine nasal spray (NNS) ad lib use. Each dose (2 squirts) delivered 1 mg nicotine. Maximum dose 5 mg/h and 40 mg/day. Recommended duration of use 3 months 2. Placebo nasal spray containing piperine to mimic sensory effect of nicotine Level of support: high (Group therapy 6 x 1‐h sessions) | |
| Outcomes | Sustained abstinence at 1 year (continuous abstinence from quit day, follow‐up also at 2 years) Validation: CO < 10 ppm at each of 5 follow‐ups | |
| Notes | Abstinence at 24 months 15/79 vs 11/78. OR 1.4 Study funded by Icelandic Ministry of Health and Social Security, with consumables supplied by Pharmacia & Upjohn | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization code", with spray dispensed by University pharmacy |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Subject and therapist were blind to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant lost to follow‐up, assumed to be a smoker. Dropout rates not reported |
Bolliger 2000b.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Excluded study, but contributing data on adverse events | |
Br Thor Society 1983.
| Methods | Country: UK (95 centres) Recruitment: hospital chest clinics (80%) and inpatient wards | |
| Participants | 1618 clinic patients age 18 to 65 with a smoking‐related illness (pulmonary or vascular) 39% female, average age 49, average cpd 24 | |
| Interventions | 1. Brief advice from physician 2. Brief advice + booklet 3. Brief advice + booklet + placebo chewing gum 4. Brief advice + booklet + nicotine chewing gum (2 mg for up to 3 months, up to 6 months on request) Level of support: low (1 month and 3 month follow‐up visits) | |
| Outcomes | Sustained validated abstinence at 6 months and 12 months Validation: Venous carboxyhaemoglobin | |
| Notes | Includes both placebo and no‐placebo groups. 4 vs 1 + 2 + 3 used in main comparison. 4 vs 3 has lower OR (0.8) but does not alter MA notably Study was funded by Health Education Council and Lundbeck Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each physician had a balanced block of 12 treatments. Assignment was by numbered envelope |
| Allocation concealment (selection bias) | Low risk | Physician opened envelope at first treatment session |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Placebo and nicotine gums were indistinguishable in appearance and taste, and neither the physician nor the patient knew which gum had been issued" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lower losses from gum groups (10 and 10) than from Advice groups (24 and 24), but 18 VA and VAB participants were prescribed Nicorette in error; removing these made differences non‐significant |
Brantmark 1973b.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Excluded study, but contributing data on adverse events | |
Buchkremer 1988.
| Methods | Country: Germany Recruitment: community volunteers | |
| Participants | 131 smokers 50% female, average age 35, average cpd 29 Participants were motivated to give up | |
| Interventions | 1. Nicotine patch (24 h/day, 8 weeks, 15 cm with weaning) + behavioural therapy 2. Placebo patch + behavioural therapy 3. Behavioural therapy alone Level of support: high (9 weekly group sessions) | |
| Outcomes | Abstinence (not stated how assessed) at 12 months Validation: none | |
| Notes | Placebo and no‐placebo groups. 1 vs 2 + 3 used in main comparison Study was funded by Deutsche Forschungsgemeinschaft | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "smokers were randomly assigned ... Randomization included matching by age, sex and initial cigarette consumption" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind; "checked by questioning both the training personnel and the probands of nicotine‐ and placebo‐groups". No significant differences in right and wrong guesses |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates not reported |
Bullen 2010.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Excluded study, but contributing data on adverse events | |
Campbell 1987.
| Methods | Country: UK Recruitment: primary care (45 GPs in 11 centres) | |
| Participants | 836 primary care patients agreeing to try to stop smoking after brief advice from their doctor 61% female, average age 39 | |
| Interventions | 1. Nicotine gum (2 mg) x 6 boxes 2. Placebo gum x 6 boxes Level of support: low (no further face‐to‐face contact, ⅔ received a letter after 1 month) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO | |
| Notes | Study funded by Chest, Heart and Stroke Association; discounted Nicorette gum supplied by Lunbeck, free chewing gum by Wrigleys | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "in a double‐blind random fashion". Control participants were recruited sequentially after the gum cohort had been assembled |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 37% losses at 12 months |
| Other bias | Unclear risk | Placebo gum was actually Wrigleys gum, repackaged and labelled |
Campbell 1991.
| Methods | Country: UK Recruitment: hospital inpatients | |
| Participants | 212 patients with smoking‐related diseases 44% female, 53% aged 50+, 61% smoked > 15 cpd | |
| Interventions | 1. Nicotine gum 2 to 4 mg (3 months) 2. Placebo gum Level of support: high (support at 2, 3, 5 weeks, 3 months, 6 months) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO | |
| Notes | Study was supported by Pharmacia LEO | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "those who had agreed were given packages of identical appearance randomly containing either nicotine (2 mg) or placebo gum" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Non‐attenders were classified as failures"; rate of dropouts not reported |
Campbell 1996.
| Methods | Country: UK Recruitment: hospital inpatients and outpatients | |
| Participants | 234 adult smokers (> 1 cpd in previous week) (172 outpatients, 62 inpatients) Stratified on FTND. Participants were motivated to quit 54% female, average age 49 | |
| Interventions | 1. Nicotine patch (21 mg, 24 h, 12 weeks with dose tapering) 2. Placebo patch Level of support: high (counselling at 2, 4, 8,12 weeks) | |
| Outcomes | Continuous abstinence at 12 months Validation: CO | |
| Notes | Originally included as Burton 1992 which was an abstract of the same trial Study was funded and supplied by Ciba‐Geigy Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants stratified by inpatient/outpatient status, and outpatients also by FTND score. Participants "were randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Abstract describes the trial as "double‐blind", but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 57 NRT and 56 placebo participants did not complete the 12‐week course. By 52 weeks, 28 participants had dropped out of the NRT group, and 40 from the placebo group |
CEASE 1999.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Excluded study, but contributing data on adverse events | |
Cinciripini 1996.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 64 smokers (> 15 cpd) 70% female, average cpd 29/22 | |
| Interventions | 1. Nicotine patch (21 mg, 12 weeks incl weaning) 2. Behaviour therapy only (no placebo) Level of support: High (group therapy weekly for 9 weeks) | |
| Outcomes | Sustained abstinence, 12 months post‐treatment and all previous points (EOT, 1, 3, 6 months) Validation: CO < 6 ppm at each point | |
| Notes | 121 smokers recruited but only the first 64 followed up for 1 year. 6‐month quit rates for whole cohort were approximately 53% vs 30% (personal communication 2004) Study was supported by a DHHS grant, and by Ciba Geigy Corporation and Marion Merrell Dow | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Sixty‐four participants ... were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not reported, but failures and missing were counted as non‐abstinent |
Clavel 1985.
| Methods | Country: France Recruitment: community volunteers | |
| Participants | 427 smokers (≥ 5 cpd) 51% female, average age 34, average cpd 22 for intermediate group (Clavel 1984). Participants were motivated to quit | |
| Interventions | 1. Nicotine gum (2 mg) x 1 box 2. Control group (time lock‐controlled cigarette case) (Acupuncture arm not included in this review) Level of support: High (3 x 1 h group therapy sessions in first month) | |
| Outcomes | Sustained abstinence at 13 months Validation: "Smoking cessation adjusted using exhaled CO figures from published trials" | |
| Notes | Classification of support corrected to high in 2008 update Study was supported by the Haut Comité d'Aide à la Lutte Contre le Cancer, and Laboratoire Léo, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Treatment ... was allocated by balanced randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Those still smoking at 1 month were not followed and were counted as failures, as were the 6% non‐responders. Half the abstainers were visited at home at 13 months and tested for expired CO |
Clavel‐Chapelon 1992.
| Methods | Country: France Recruitment: community volunteers | |
| Participants | 996 smokers (≥ 10 cpd) 45% female, average age 34 | |
| Interventions | Factorial trial with active/placebo acupuncture arms, collapsed for this review 1. Nicotine gum (2 mg) for up to 6 months, max 30/day 2. Placebo gum (contained 1 mg unbuffered nicotine) Level of support: high (3 acupuncture session at 0, 7, 28 days) | |
| Outcomes | Abstinence at 13 months (1‐month quitters followed up). 4‐year follow‐up reported in 1997 with different 1‐year results Validation: none at 1 year | |
| Notes | Question over inclusion because placebo contained small amount of nicotine Abstinence at 4 years 30/481 vs 32/515 Study was supported by CIBA‐GEIGY | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Treatments were administrated blindly" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants abstinent at 1 month were followed up. 2 participants were lost between months 9 and 12, and 32 between year 1 and year 4. Losses "were considered successes until the date of the last follow‐up and afterwards were not considered anymore" |
Coleman 2012.
| Methods | Country: UK Recruitment: pregnant women attending hospital clinics |
|
| Participants | 1050 pregnant women at 12 to 24 weeks gestation smoking ≥ 5 cpd Average age 26, average cpd at time of recruitment 14, average cpd before pregnancy 20 |
|
| Interventions | 1. Nicotine patch 15 mg/16 h for 8 weeks (participants given 4 week supply at outset, if not smoking at 4 weeks given another 4‐week supply) 2. 'Visually identical' placebo on same schedule Level of support: high. Behavioural cessation support ≤ 1 h at enrolment + 3 phone calls (on quit date, 3 days after quit date, 4 weeks after quit date). If collecting another 4‐week supply of NRT/placebo, participants given another face‐to‐face session |
|
| Outcomes | Continuous abstinence from quit date to delivery and prolonged abstinence at 2 years from delivery. Lapses of up to 5 cigarettes (on 5 occasions) permitted Validation: at delivery: salivary cotinine < 10 ng/ml, CO ≥ 8 ppm, primary outcomes required saliva cotinine validation, with or without CO. At 2 years, no validation |
|
| Notes | Funded by NIHR Health Assessment Technology Programme Similar rates of adverse pregnancy and birth outcomes in both groups; at 2 years, infants born to women who used NRT during pregnancy were more likely to have unimpaired development Low compliance in both arms (7.2% active treatment and 2.8% placebo group reported using patch for more than 1 month) Longer‐term follow‐up data (2 year post‐delivery) added for 2017 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated sequence, in random permuted blocks of randomly varying size and with stratification by recruiting site" |
| Allocation concealment (selection bias) | Low risk | Quote: "eligibility criteria were entered into a secure online database before randomization" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "identically packaged study patches were dispensed, and all participants and study personnel were unaware of the study assignments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 981/1050 participants provided data at delivery; participants missing data counted as smokers |
Cooper 2005.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 439 female smokers (≥ 10 cpd) Average age 38, average cpd 23 | |
| Interventions | 1. Nicotine gum (2 mg), 10 to 12 pieces/day recommended, for 9 weeks, weaning last 3 weeks 2. Placebo gum Level of support: high. 13 x 1‐h weekly cognitive behavioural group sessions. Reduction prior to TQD week 5 (3rd arm tested phenylpropanolamine gum, not included in review) | |
| Outcomes | PP abstinence at 12 months Validation: CO < 10 ppm Weight change in quitters was also a primary outcome in the trial | |
| Notes | First included as Cooper 2003. Published report from 2007 Sources of support not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible participants ... were randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not reported, all analyses conducted as ITT. Dropouts (if any) counted as treatment failures in our analysis |
Cummins 2016.
| Methods | Country: USA Recruitment: inpatients at participating hospitals (multicentre) |
|
| Participants | 1270 hospitalied smokers (excl. obstetrics, surgery and behavioural health patients), smoked in last 30 days and at least 6 cpd on days smoked 57% male, average age 50, average cpd 15 |
|
| Interventions | 1. NRT patches for 8 weeks, doses based on cpd. If 6 cpd to 10 cpd: 14 mg for 6 weeks, 7 mg for 2 weeks. If > 10 cpd: 21 mg for 4 weeks, 14 mg for 2 weeks, 7 mg for 2 weeks. (NS if 16‐h or 24‐h patches) 2. No NRT Level of support: varied. All were provided quitline number. Hospital systems, individual hospitals, and even individual units had their own approach to usual care for smokers, with differences in providing counselling or prescribing quitting aids during hospitalisation. There was no attempt to constrain these activities. Some participants in the NRT and the no‐NRT groups also received counselling due to factorial design (2 x 2 factorial design: NRT/counselling/NRT and counselling/usual care). Counselling was by the Quitline service. Authors tested for an interaction between NRT and counselling and this was not significant, therefore results collapsed for this review |
|
| Outcomes | 7‐day PP at 6 months validation: saliva cotinine < 10 ng/ml |
|
| Notes | New for 2017 update Funding: National Cancer Institute (CA159533) N quit extrapolated from percentages given. Not included in support subgroups as support varied by study centre |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomly assigned by computer to one of four groups” |
| Allocation concealment (selection bias) | Low risk | Recruiters “entered study‐related information into a secure website that randomised the subject.” |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo. Participants therefore aware if on NRT or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | NRT 205/637, no‐NRT 208/633 dropout < 50%. (return of saliva kits at 6 months for validation 57%, still > 50%) |
Cunningham 2016.
| Methods | Country: Canada Recruitment: by random digit dialling |
|
| Participants | 1000 smokers (≥ 10 cpd) 51% female, average age 49, average cpd 18, mean FTND 5 |
|
| Interventions | 1. Nicotine patches. 5 weeks total, tapered: 3 weeks 21 mg, 1 week 14 mg, 1 week 7 mg (unclear if 16 or 24 h) 2. No intervention Level of support: low; no support provided (patches mailed to intervention participants) |
|
| Outcomes | 30‐day PP at 6 months Validation: Saliva cotinine < 15 µg/L |
|
| Notes | New for 2017 update Total n followed up from author correspondence Funding: Canadian Institutes of Health Research, Centre for Addiction and Mental Health, Canada Foundation for Innovation, Ontario Ministry of Research and Innovation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomized "using a random number generator contained in the computer assisted telephone interview program" This was "conducted in blocks of 10 with a 1:1 allocation to the experimental group within each block and no stratification" |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) All outcomes | High risk | The participants knew which group they were in, although the interviewers were masked to the experimental group at each follow‐up point "(ensured through use of the computer‐assisted telephone interview program)” No placebo control |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 389/500 and 415/499 followed up at 6 months |
Daughton 1991.
| Methods | Country: USA Recruitment: community volunteers at 2 sites | |
| Participants | 158 smokers (at least 1 pack cpd) 53% female, average age 42, average cpd 33 | |
| Interventions | 1. Nicotine patch (15 cm², 4 weeks) worn for 16 h/day 2. Nicotine patch (15 cm², 4 weeks) worn for 24 h/day 3. Placebo patch, 4 weeks Level of support: unclear and differed between sites | |
| Outcomes | Sustained abstinence at 6 months Validation: None after 4 weeks (CO at 2 to 4 weeks) | |
| Notes | 1 + 2 vs 3 in Analysis 1.1. Not used in support intensity subgroup analysis Study was funded by ALZA Corp, Palo Alto, CA, through a contract with the Merrel Dow Research Institute, Cincinnati, OH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All 158 study‐eligible volunteers were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as "double‐blind"; "All of the patches were physically identical in appearance". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts (if any) not reported; included as treatment failures in our analysis; results presented on an ITT basis |
Daughton 1998.
| Methods | Country: USA (21 sites) Recruitment: patients at family practices ‐ self‐referred to study or recruited by physician | |
| Participants | 369 smokers (> 20 cpd) Average age 37, average cpd 27 to 30; participants were variously motivated to quit | |
| Interventions | 1. Nicotine patch (21 mg, 16 h, 10 weeks with weaning) 2. Placebo patch Level of support: low (Nicoderm Committed Quitters Programme support booklet + follow‐up visit 1 week after quit day) | |
| Outcomes | Sustained abstinence (continuous self‐reported from quit day) at 12 months Validation: CO ≤ 8 ppm and saliva cotinine < 20 mg/mL | |
| Notes | There were differences in quit rates between self‐referred and physician‐selected recruits and between smokers recruited during an illness and at another visit Study was funded by Marion Merrell Dow Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a random code was generated" for equal numbers of active and placebo within blocks of 10 |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Participants were assigned randomly, in a double‐blind fashion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses low at 3 months (1.1%), 6 months (1.6%) and 12 months (2.2%). Those lost to follow‐up were included as failures |
Dautzenberg 2001.
| Methods | Country: France Recruitment: community volunteers | |
| Participants | 433 smokers (excludes 25 from ITT population) 52% female, average age 39, average cpd 21 | |
| Interventions | 1. Nicotine lozenge (1 mg, 8 to 24/day, 6 weeks + 6 weeks weaning for quitters) 2. Placebo lozenge Level of support: not stated | |
| Outcomes | PP abstinence at 26 weeks Validation: CO < 10 ppm | |
| Notes | Based on published abstract Study was funded by Novartis Consumer Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double‐blind", but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Higher losses in placebo than active group (44% vs 37%); analyses conducted as ITT counting dropouts as treatment failures |
Davidson 1998.
| Methods | Country: USA (4 centres) Recruitment: community volunteers in shopping malls (OTC setting) | |
| Participants | 802 smokers (> 20 cpd) who scored 5+ on a questionnaire assessing motivation 54% female, average age 39, average cpd 29 | |
| Interventions | 1. Nicotine patch (22 mg, 24 h, for up to 6 weeks) 2. Placebo patch Level of support: low (self‐help book provided. Participants visited mall weekly to obtain patches. CO levels were monitored) | |
| Outcomes | Sustained abstinence at 24 weeks (from week 2) Validation: Expired CO ≤ 8 ppm at each weekly visit, but 24 week quit based on self‐report | |
| Notes | 541/802 did not complete the 6 weekly visits Study was funded by Elan Pharmaceutical Corporation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses were included as failures. 67.5% withdrew before study completion; placebo losses higher than active, but differences not statistically significant |
Ehrsam 1991.
| Methods | Country: Switzerland Recruitment: University (primary care) | |
| Participants | 112 smokers at 2 universities Average age 26, average cpd 23 | |
| Interventions | 1. Nicotine patch (21 or 14 mg/24 h, 9 weeks, tapered) 2. Placebo patch Level of support: high (no counselling) | |
| Outcomes | Sustained abstinence at 12 months (0 to 3 cigarettes per week) Validation: urinary cotinine | |
| Notes | Study funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "doppelblinden" but no further information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts included as failures. 36% dropped out of active group, and 55% out of placebo group |
| Other bias | Unclear risk | Abstinence defined as 0 to 3 cigarettes a week, with CO < 12 ppm. Relapse defined as ≥ 1 cpd, or ≥ 14 cigarettes over 2 weeks |
El‐Mohandes 2013.
| Methods | Country: USA Recruitment: healthcare (3 prenatal care sites) |
|
| Participants | 52 pregnant (< 30 weeks gestation) smokers motivated to quit, self‐identified as ethnic minority 100% female, average age 28, average cpd 6, mean gestational age at baseline 9 weeks |
|
| Interventions | 1. Nicotine patch, 10 weeks. Dose based on baseline salivary cotinine: if baseline salivary cotinine level ≥ 100 ng/ml then 21 mg patches for 2 weeks, 14 mg patches for 4 weeks and 7 mg patches for 4 weeks. If baseline salivary cotinine level 20 to 99 ng/ml then 14 mg patches for 6 weeks and 7 mg patches for 4 weeks 2. No pharmacotherapy Level of support: high (6 individual in person counselling visits) |
|
| Outcomes | Abstinence since last visit (approximately 3 weeks) at 20 weeks Validation: CO ≤ 8 ppm |
|
| Notes | New for 2017 update Does not contribute to primary analyses as follow‐up < 6 months. 20‐week abstinence (pre‐delivery) included in Analysis 5.1.1 Funding: Eunice Kennedy Shriver National Institute of Child Health and Human Development |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Using a 1:1 ratio, women were randomized to either the NRT patch and continued CBT (Group 1) or CBT only (Group 2)… The web‐based database management system was programmed to randomize after entering the necessary data to verify eligibility and administration of the baseline survey.” |
| Allocation concealment (selection bias) | Low risk | Quote: “The web‐based database management system was programmed to randomize after entering the necessary data to verify eligibility and administration of the baseline survey.” |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “Telephone interviewers were blinded to group assignment.” “The intervention specialists were blinded to group assignment.” No placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At the strictest quit timepoint (salivary continine levels at final visit), 34/52 participants lacked data (> 50%). If already delivered before 20 week follow‐up, did not have a visit 6 and smoking status not known |
Fagerström 1982.
| Methods | Country: Sweden Recruitment: smoking cessation clinic | |
| Participants | 100 consecutive smokers; 43 referred by physician, 57 applied by phone to SC clinic 59% female | |
| Interventions | 1. Nicotine gum (2 mg) for at least 4 weeks 2. Placebo gum for at least 4 weeks Level of support: high (individual counselling, average 7.7 sessions) | |
| Outcomes | PP abstinence at 6 months Validation: CO | |
| Notes | Study funding source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned... in blocks of ten" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All patients were told that the chewing gum they received contained nicotine"; participants did not know that they were involved in a study "the experimenter's guess of nicotine or placebo gum was in the direction of better than chance, but not significantly so". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 early dropouts (3 active, 1 placebo) excluded from analysis; all other dropouts counted as smokers in final analysis |
Fagerström 1984.
| Methods | Country: Sweden Recruitment: general practices and industrial clinics (primary care) | |
| Participants | 145 smokers motivated to quit 56% female, average age 40 years, average cpd 19 Therapists: 10 Swedish GPs, 3 Swedish industrial physicians | |
| Interventions | 1. Short follow‐up (advice plus 1 appointment) 2. Long follow‐up (advice plus 2 appointments, phone call + letter) 3. Short follow‐up plus nicotine gum (2 mg or 4 mg) 4. Long follow‐up plus nicotine gum Level of support: low | |
| Outcomes | Sustained abstinence at 12 months (and at 1, 6 months) Validation: 15% deception rate detected by expired CO > 4 ppm in a random subset of claimed non‐smokers at 6 months. Self‐reported 12 month rates used in MA | |
| Notes | 3 and 4 vs 1 and 2 in Comparison 1 Study funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were randomly assigned" by birthdate; participants born 1st to 20th received active gum, 21st to 31st no gum. Those born on even dates got long follow‐up, odd dates short follow‐up. |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants abstinent at 1 follow‐up were seen again for the next one. All losses counted as failures |
| Other bias | High risk | Physicians selected for the study were personal acquaintances of the author, and all except 1 were non‐smokers |
Fee 1982.
| Methods | Country: UK Recruitment: smoking cessation clinic | |
| Participants | 352 smokers, no other demographic data | |
| Interventions | 1. Gum (2 mg) given for 5 weeks 2. Placebo gum given for 5 weeks Level of support: high (10 group therapy sessions) | |
| Outcomes | PP abstinence at 12 months Validation: Blood carboxyhaemoglobin | |
| Notes | Study was supported by LEO Laboratories, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation was carried out by external staff, using a random selection procedure unknown to the authors" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Significantly higher losses from placebo (47.7%) than from active group (36.7%). Losses taken as failures |
Fiore 1994a.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 88 smokers (> 15 cpd), motivated to quit. | |
| Interventions | 1. Nicotine patch (22 mg/24 h, 8 weeks, no weaning) 2. Placebo patch Level of support: high (intensive group counselling) | |
| Outcomes | PP abstinence at 6 months (7‐day PP) Validation: CO | |
| Notes | Reported in same paper as Fiore 1994b Studies supported by Elan Pharmaceutical Research Corporation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a pregenerated computer sequence" and stratified by FTQ score |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Ten placebo and 1 active participant failed to complete the NRT course. 25 participants lost to follow‐up at 6 months were included as failures. |
Fiore 1994b.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 112 smokers (> 15 cpd) | |
| Interventions | 1. Nicotine patch (22 mg/24 h, 6 weeks including weaning) 2. Placebo patch Level of support: high (8 weekly 10 min to 20 min individual counselling) | |
| Outcomes | PP abstinence at 6 months (7 days PP) Validation: CO | |
| Notes | Reported in same paper as Fiore 1994a. Studies supported by Elan Pharmaceutical Research Corporation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a pregenerated computer sequence" and stratified by FTQ score |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29% did not complete treatment phase and were included as failures (15 on active patch, 18 on placebo). 36% lost to follow‐up, and were included as failures |
Fortmann 1995.
| Methods | Country: USA Setting: community volunteers (telephone recruitment) | |
| Participants | 1044 smokers aged 18 to 65, able to quit for 24 h, and without serious illness. Motivated to maintain abstinence 42% female, average age 40, average cpd 20 | |
| Interventions | 1. Nicotine gum (2 mg, 1/h, at least 10/day and not more than 30/day) 2. Self‐help materials 3. Nicotine gum plus materials 4. Incentive alone All groups offered incentive of USD 100 for quitting at 6 months Level of support: low | |
| Outcomes | PP abstinence at 12 months Validation: CO < 9 ppm/salivary cotinine < 20 ng/ml | |
| Notes | Until 2008 only groups 1 and 4 compared. Since the trial was factorial and shows no evidence of interaction, both gum groups now used; 1 and 3 vs 2 and 4. The RR is unaltered but CIs narrow Study was funded by the National Heart, Lung, and Blood Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was stratified by gender and cigarette consumption". No further detail |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3.9% dropped out at 6 months, and 6.2% at 12 months. Unclear whether dropouts were included, although disconfirmations were reclassified as smokers |
Fraser 2014.
| Methods | Country: USA Recruitment: community (individuals who spontaneously accessed smokefree.gov portal) |
|
| Participants | 1034 smokers of ≥ 5 cpd, motivated to quit, no prior use of smokefree.gov website 68% female, average age 39, average cpd 19.3, mean FTND 5.3 |
|
| Interventions | 1. Nicotine mini‐lozenge for 2 weeks (mailed). 162 lozenges received (dosage not given but based on time to first cigarette), instructed to use 6 to 10 lozenges per day 2. No pharmacotherapy or placebo Level of support: variable (factorial study resulting in 32 distinct experimental conditions, behavioural elements varied on quitline counselling, messaging, brochures) |
|
| Outcomes | 7‐day PP at 7 months (by e‐mail) Validation: none |
|
| Notes | New for 2017 update Factorial trial, NRT versus no NRT compared in main analyses, other factors related to behavioural support (authors tested for interaction. No interaction found between NRT and behavioural components, results therefore collapsed for our analysis) N quit extrapolated from percentages given Funding: Matthews Media Group, National Cancer Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Through automated system: “Randomization occurred immediately after the confirmation call, and participants completing this step were sent an automated email welcoming them to the study and outlining services they would receive (based on their randomization).” |
| Allocation concealment (selection bias) | Low risk | Automated, see above |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants. “Follow‐up interviewers were blind as to treatment assignment”. No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 7 months, 828/1034 participants were followed up (> 50%). Dropout for each group not given but states follow‐up across 5 different treatment factors 76% to 81%. Difference between groups not statistically significant |
Gallagher 2007.
| Methods | Country: USA Recruitment: 3 psychiatric case management sites in La Frontera, Arizona | |
| Participants | 180 smokers, aged 18+, English‐speaking, smoked at least 10 cpd for at least 3 years, CO > 10 ppm. Diagnosed with DSM‐IV Axis 1 psychotic‐spectrum or affective disorders resulting in long‐term mental illness and experiencing significant symptoms and functional impairment 52% male, average age 43, av FTQ 6.1, average cpd 24.8 | |
| Interventions | 1. Contingency reinforcement (CR): Weekly visits weeks 1 to 4 (Phase 1), fortnightly weeks 6 to 12 (Phase II), monthly weeks 16 to 24 (Phase III). Payments USD 25 for baseline assessment and USD 5 per visit, plus USD 20 per abstinent visit in Phase I, USD 40 in Phase II, USD 60 in Phase III, and USD 80 if abstinent at 36‐week follow‐up. Max payable USD 580 for attendance + abstinence. At each visit weight, pulse rate, smoking status, intention to quit, withdrawal symptoms, CO, BP measured
2. CR + NRT: As CR Group, plus 16‐week course of 21 mg NRT patches (16 h or 24 h not stated), plus supporting instructions
3. Control: Visits at baseline and weeks 20 and 36, plus encouraged to use the community smoker helpline, ALA and ACS self‐help information Level of support: high (contingency reinforcement) |
|
| Outcomes | PP abstinence at week 36 Verified by expired CO < 10 ppm and by salivary cotinine < 15 ng/mL |
|
| Notes | New for 2017 update. Analysis compares 2 vs 1; 3 not included as comparison with NRT confounded Not required to commit to cessation, but 98% expressed interest either in quitting or in reducing Additional information supplied by the author. N quit extrapolated from percentages given Study funded by Arizona Biomedical Research Commission. Risk of bias and some data extraction from Cahill 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unconcealed computer‐generated random number lists (personal communication) |
| Allocation concealment (selection bias) | High risk | Study staff oversaw allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant differences between groups: Attrition for CR at weeks 20 and 36 was 37% and 43%; CR+NRT at weeks 20 and 36 was 35% and 36% |
García 1989.
| Methods | Country: Spain Recruitment: primary care | |
| Participants | 106 adult smokers (excludes 81 not beginning treatment) 65% female, average age 36, average cpd 25 | |
| Interventions | 1. Gum (2 mg) for 3 to 4 months 2. Placebo gum for 3 to 4 months Level of support: high (group therapy, 7 sessions over 3 months) | |
| Outcomes | Sustained abstinence at 6 months Validation: CO ≤ 7 ppm | |
| Notes | Sources of support not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "La asignación a los grupos de estudio se realizaba aleatoriamente al acudir a la primera entrevista" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind ("doble ciego") |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts reported at 1, 3 and 6 months. Analyses appear to be ITT‐based, counting dropouts as failures |
Garvey 2000.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 608 smokers, aged > 20, smoking > 5 cpd 51% female, average cpd 23 | |
| Interventions | 1. 4 mg nicotine gum (recommended 9 to 15 pieces), weaning from 2 months 2. 2 mg nicotine gum, use as 1 3. Placebo gum All received brief counselling (5 to 10 mins) at each study visit (1, 7, 14, 30 days, 2, 3, 6, 9, 12 months) Level of support: high | |
| Outcomes | Sustained abstinence at 12 months (relapse defined as 7+ consecutive days or episodes of smoking) Validation: CO ≤ 8 ppm | |
| Notes | 4 + 2 mg doses combined in main comparison Study was funded by National Institute of Drug Abuse and Department of Veterans Affairs. Gum supplied by Marion Merrell Dow | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified by dependence level (high/low) and then allocated "using a randomized, double‐blind procedure" |
| Allocation concealment (selection bias) | Unclear risk | No further detail |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind, but no further information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Relapsers were included as failures. Dropout rates not reported |
Gilbert 1989.
| Methods | Country: Canada Recruitment: primary care | |
| Participants | 223 patients presenting to primary care doctors and smoking at least 1 cpd (not selected by motivation) | |
| Interventions | 1. Support from physician plus offer of nicotine gum prescription (2 mg) 2. Support from physician (no placebo) Level of support: low (enrolment, quit day, offer of 4 support visits, 2 in week 1, 1 month, 2 months) | |
| Outcomes | Sustained abstinence at 12 months (for 3 months) Validation: salivary cotinine | |
| Notes | ˜30% of gum group did not use any, 14% of support only group did use gum. ˜70% attended quit day visit, ˜43% attendance for follow‐up visits Study was funded by US National Insititutes of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "physicians were presented with a sealed envelope indicating treatment allocation by the receptionist"; "allocation was balanced within each block of four patients for each physician" |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo gum used. Control group participants could request gum, and physician would decide whether or not to prescribe |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up at 1 year of 91.5%; those lost to follow‐up were included as failures |
| Other bias | Unclear risk | Participants using gum were required to pay for their prescription Participants claiming abstinence were visited for validation test without being aware this would happen |
Glavas 2003a.
| Methods | Country: Croatia Recruitment: hospital health professionals | |
| Participants | 112 healthcare professionals smoking at least 1 cpd. 26% had FTND score 6+ 66% female, average age 34, average cpd: 24 | |
| Interventions | 1. Nicotine patch, 24 h, 25 mg/15 mg/8 mg starting dose depending on baseline cpd. 3 weeks 2. Placebo patch Level of support: low (visits to pick up patch at 7, 14, 21 days, no details about advice given) | |
| Outcomes | Sustained abstinence (3 or fewer cigarettes/week) at 1 year (5‐year abstinence also reported, not used in MA) Validation: CO < 11 ppm | |
| Notes | Study was supported by Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Low risk | Quote: "each examinee received a presealed envelope, labeled after random numbering, which contained either 8 transdermal nicotine system patches or matching placebo stickers" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind, but no further detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 dropouts by year 1 and year 5, classified as failures |
Glavas 2003b.
| Methods | Country: Croatia Recruitment: community volunteers | |
| Participants | 160 smokers | |
| Interventions | 1. Nicotine patch, 24 h, 25 mg/15 mg/8 mg starting dose depending on baseline cpd. 6 weeks 2. Nicotine patch, 24 h, 25 mg/15 mg starting dose depending on baseline cpd. 3 weeks 3. Placebo patch. 6 weeks 4. Placebo patch 3 weeks Level of support: low | |
| Outcomes | Abstinence at 6 months after EOT Validation: CO < 11 ppm | |
| Notes | Both durations pooled for main comparison Study funding information not reported Author supplied additional details in personal communication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Low risk | Quote: "presealed numbered envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "The envelopes were prepared well in advance and the distribution was commissioned to a nurse not taking part in the evaluation process" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Other bias | Unclear risk | Abstinence defined as ≤ 2 cigarettes per week |
Glover 2002.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 241 smokers (≥ 10 cpd), motivated to quit 54% female, average age 42, average cpd 29 | |
| Interventions | 1. Nicotine sublingual tablet (2 mg). Recommended dosage 1 tablet/h for smokers with FTND < 7, 2 tablets/h for scores ≥ 7. After 3 months treatment, tapering period of 3 months if necessary 2. Placebo tablet Level of support: high (brief counselling at all visits 1, 2, 3, 6 weeks, 3, 6,12 months) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO < 10 ppm | |
| Notes | Study was funded by Pharmacia & Upjohn | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated randomization code" |
| Allocation concealment (selection bias) | Low risk | Quote: "subjects were sequentially randomized" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All tablets were identical in appearance... each placebo tablet contained 3 μg of capsaicin to mimic the oral effects of nicotine and to maintain blinding" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up included as failures. Dropout rates not reported |
Gourlay 1995.
| Methods | Country: Australia Recruitment: community volunteers | |
| Participants | 629 smokers (> 15 cpd) who had relapsed after transdermal nicotine and behavioural counselling in an earlier phase of the study Minimal additional support | |
| Interventions | 1. Nicotine patch 30 cm² (21 mg/24 h) for 4 weeks, 20cm² (14 mg/24 h) for 4 weeks, 10 cm² (7 mg/24 h) for 4 weeks. 2. Placebo patch | |
| Outcomes | Sustained abstinence at 6 months Validation: expired CO < 10 ppm | |
| Notes | Does not contribute to main comparison. Test of patches vs placebo in recently relapsed smokers. Results given in text. Study was funded by Ciba‐Geigy Australia, the Anti‐Cancer Council of Australia and the Victorian Health Promotion Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were randomised" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants invited at week 11 to guess their assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts at each stage reported in full. Losses to follow‐up included as failures |
Graham 2017.
| Methods | Country: USA Recruitment: smoking cessation website |
|
| Participants | 5290 current smokers 61% female, average age 42, average cpd 17, mean FTND 5.3 |
|
| Interventions | 1. 4 weeks of NRT patch, gum or lozenge depending on participant preference, mailed to participants. Standard dosing protocol as per labelling instructions 2. No NRT Level of support: low (use of interactive website. Some participants also received web‐based social network intervention. 2 x 2 factorial design. No evidence of interaction between NRT and web‐based social network intervention, therefore results collapsed for our analysis) |
|
| Outcomes | 30‐day PP at 9 months Validation: none |
|
| Notes | New for 2017 update 9‐month data obtained from authors Funding: National Cancer Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomisation is stratified by gender and baseline motivation to quit. Within‐strata randomisation assignments are automated using a computer algorithm” |
| Allocation concealment (selection bias) | Low risk | Central computer‐based allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded but if research staff contacted participants by phone, they were blinded to treatment condition. No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 50% followed up at 9 months (1600/2630 Intervention, 1418/2660 control) |
Gross 1995.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 177 smokers 51% female, average age 42, average cpd 33, average FTND 7.8 | |
| Interventions | 1. Nicotine gum (2 mg), tapered from week 12. Active gum groups further randomized to chew 7, 15 or 30 pieces of gum 2. No gum Level of support: high (1 pre‐quit group counselling session, 14 clinic visits in 10 weeks) | |
| Outcomes | Continuous abstinence at 6 months (up to 3 cigarettes allowed) Validation: CO ≤ 10 ppm. Saliva thiocyanate in week 2 | |
| Notes | No placebo. Long‐term abstinence rates not affected by amount of gum, so these groups collapsed for comparison with no‐gum condition Study was funded by National Institute of Drug Abuse, and supported by Marion Merrell Dow | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | "Random assignment", stratified by dependence measures |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Relapsers or non‐quitters included as failures |
Hall 1985.
| Methods | Country: USA Recruitment: community volunteers and physician referrals | |
| Participants | 120 smokers (77 in arms contributing to MA) 47% female, average age 38, average cpd 31 | |
| Interventions | 1. Intensive behavioural treatment (14 group sessions over an 8‐week period) 2. Combined ‐ 2 mg nicotine gum (period of use not specified) and intensive behavioural treatment 3. Low‐contact behavioural treatment (4 meetings over 3 weeks) and 2 mg gum Level of support: high | |
| Outcomes | Abstinence at 12 months Validation: CO < 10 ppm and blood thiocyanate < 85 mg/mL | |
| Notes | No placebo. 2 vs 1 in main comparison. 3 not used in MA. Quit rate higher than arm 1 Study was funded by National Institute of Drug Abuse and Department of Veterans Affairs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned within time constraints to one of the three treatment conditions" |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo; no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts reported |
Hall 1987.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 139 adult smokers 47% female, average age 39, average cpd 30 | |
| Interventions | 2 x 2 factorial trial of gum and behavioural support 1. Nicotine gum (2 mg) up to 12 months 2. Placebo gum up to 12 months Both levels of behavioural support classified as high intensity and collapsed in analysis (both group‐based, 14 x 75‐min sessions, or 5 x 60‐min sessions) | |
| Outcomes | PP abstinence at 12 months Validation: CO < 8 ppm and serum thiocyanate < 95 mm/l | |
| Notes | Study funded by National Institute of Drug Abuse and Department of Veterans Affairs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Within their time constraints, subjects were randomly assigned to 5 to 6 member groups across conditions" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Group leaders blinded to gum use. Leaders and participants tried to guess assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates reported, but no detail |
Hall 1996.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 207 smokers of which 6 excluded from analyses because of protocol breaches 52% female, average age 40, average cpd 24 | |
| Interventions | 2 x 2 factorial trial of gum and psychological treatment 1. Nicotine gum (2 mg) for 8 weeks, 1 piece/h for 12 h/day recommended 2. Placebo gum, same schedule Both levels of behavioural support classified as high intensity and collapsed in analysis (both group‐based, 10 sessions over 8 weeks, TQD session 3) | |
| Outcomes | Sustained abstinence at 12 months (abstinent at all assessments) Validation: CO ≤ 10 ppm at 8, 12, 26 weeks and urinary cotinine ≤ 60 ng/ml at 52 weeks | |
| Notes | Psychological treatment arms collapsed, no evidence of a significant interaction Study was funded by National Institute of Drug Abuse and Department of Veterans Affairs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were stratified according to depression history and number of cigarettes smoked per day; they were then randomly assigned from within stratified blocks" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 participants excluded from analyses for protocol violations. No further information on dropouts |
Hand 2002.
| Methods | Country: UK Recruitment: hospital in‐ or outpatients referred by hospital doctor | |
| Participants | 245 patients with smoking‐related disease 46% male, typically aged 50+, smoking 15+ cpd; participants were motivated to try and quit | |
| Interventions | 1. Nicotine patch (initially 30 or 20 mg based on smoking rate) and inhaler for 3 weeks including patch tapering. Same counselling as control 2. Individual counselling, 4 sessions in 4 weeks. No placebo Level of support: high | |
| Outcomes | Sustained abstinence at 12 months (abstinent at all assessments) Validation: CO < 10 ppm | |
| Notes | No placebo. Compliance with NRT was low, 28% did not use, 30% used full supply Used in main comparisons Study was funded from one author's endowment fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | High risk | Quote: "randomised, according to month of entry"; unequal months, with imbalance in favour of NRT group |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo, so not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates not reported, but all included in analyses |
Harackiewicz 1988.
| Methods | Country: USA Recruitment: primary care (University Health Centre) | |
| Participants | 197 smokers (151 used in MA), motivated to quit 63% female, average age 36, average cpd 26 | |
| Interventions | 1. Nicotine gum (2 mg, 6 weeks initial supply, suggested tapering after 3 months, available for 6 months) plus self‐help manual 2. Self‐help manual 3. Control (booklet) Level of support: low (single appointment with doctor or nurse, length not specified) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO in all participants, cotinine and carboxyhemaglobin in a subsample of participants | |
| Notes | No placebo. Arm 3 not included in MA control group ‐ it had a lower quit rate so inclusion would increase the gum treatment effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly assigned to one of three conditions" |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo, so not applicable; but researchers were blinded to treatment condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% of participants did not return for any follow‐up, and were not included in the analyses. Remaining 175 included in all analyses, whether or not they attended all follow‐ups |
Hasan 2014.
| Methods | Country: USA Recruitment: hospital |
|
| Participants | 122 (81 to relevant arms) smokers admitted with a cardiac or pulmonary illness 48% female, average age 55, average cpd 20 |
|
| Interventions | 1. Patch and gum/lozenges as per participant preference. Patch dose dependent on cpd prior to hospitalization; exact dose not specified but participants smoking 10 to 20 cpd on 21 mg/day initially 2. No NRT Level of support: high. 90‐min individualized hypnotherapy session with a certified hypnotist and a tobacco treatment specialist, plus self‐help materials and counselling (intensive counselling for 30 mins in hospital, with 5 follow‐up 15‐min phone calls with additional counselling at 1, 2, 4, 8, and 12 weeks after hospital discharge) |
|
| Outcomes | 7‐day PP at 6 months Validation: Urinary cotinine < 15 ng/ml |
|
| Notes | New for 2017 update Funding: Norman H. Read Charitable Trust Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “We randomized participants to one of three treatment groups: NRT only (NRT), hypnotherapy only (H), and a group receiving both hypnotherapy and NRT (HNRT)… Randomization assignments were performed in permuted blocks of three (ratio 1:1:1) with assignments sequentially numbered” Not clear how sequence generated |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization assignments were performed in permuted blocks of three (ratio 1:1:1) with assignments sequentially numbered, and schedule was maintained independent of the study by the project coordinator. Randomized assignments were concealed from both patients and research staff until patients had signed the informed consent document and were enrolled in the study" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “Due to the nature of the intervention conditions, counselors could not be blinded to the modality of intervention.” “Our analysis is somewhat limited by the fact that comparing two vastly different modalities such as hypnosis and pharmacotherapy represents a randomization challenge, as participants and interventionists cannot be blinded to treatment conditions.” No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33.9% lost to follow‐up overall. In relevant treatment arms: 14/41 in hypno, 13/38 in hypno‐plus‐NRT |
Hays 1999.
| Methods | Country: USA (3 sites) Recruitment: community volunteers | |
| Participants | 958 smokers, > 15 cpd, motivated to quit 50% female, average age 44, typically smoked 21 to 40 cpd | |
| Interventions | 1. Nicotine patches (22 mg, 24 h for 6 weeks) purchased by participants, open‐label 2. Nicotine patches (22 mg, 24 h for 6 weeks) provided, double‐blind 3. Placebo patches provided The intervention replicated an OTC environment, with no counselling intervention and minimal study recording. Weekly visits required for CO measurement and adverse experience recording, but study sites were not in medical centres and there was no advice, counselling or interaction with medical personnel Level of support: low | |
| Outcomes | Abstinence at 6 months (7‐day PP) Validation: CO ≤ 8 ppm | |
| Notes | 1 and 2 vs 3 in patch vs placebo comparisons Study was supported by Elan Pharmaceutical Research Corp | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random schedule" |
| Allocation concealment (selection bias) | Low risk | 2‐stage process. 1. random allocation to 1 of 2 trials, i.e. open‐label pay trial or placebo‐controlled. 2. Those in placebo trial were then assigned "by means of a computer‐generated code, in blocks of 20". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The randomization code was not revealed to any of the investigators until completion of the study." Packaging identical |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants who missed follow‐up visits classified as failures. Dropout rates not reported |
Herrera 1995.
| Methods | Country: Venezuela Recruitment: community volunteers | |
| Participants | 322 smokers > 10 cpd, scoring ≥ 4 on FTND, no serious illness. Only those who were ready to quit after 4 weeks of behavioural treatment were randomized 43% female, average age ˜38, average cpd 33 for high dependence, 16 for low dependence | |
| Interventions | Low‐dependence smokers (FTND 4 to 6): 1. 2 mg nicotine gum 2. Placebo gum High‐dependence smokers (FTND 7 to 11): 1. 4 mg nicotine gum plus 2. 2 mg nicotine gum Level of support: high for all (12 group sessions over 6 weeks + 6 weekly maintenance sessions) Participants also randomized to starting medication with increasing dose for 1 week before TQD, or to start at full dose on TQD ‐ there was no blinding for this | |
| Outcomes | Sustained abstinence at 2 years (1 year also reported) Validation: expired CO < 6 ppm | |
| Notes | Low‐dependence smokers included in comparison 1 Relapse between 1 and 2 years similar between low‐dependence groups. Higher relapse in 4 mg high dependence than 2 mg No information on support or funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Stratified on dependency scores, to determine dosage. Then "randomly assigned" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 68 participants dropped out in Phase 1 (weeks 1 to 2) and 10 participants in Phase 2 (weeks 4 to 6), i.e. before randomization. Dropout rates not reported, but classified as relapsed "and not further analyzed" |
Heydari 2012.
| Methods | Country: Iran Setting: Smoking cessation clinics | |
| Participants | 272 treatment‐seeking participants: Brief advice (91), NRT (92), varenicline (89). 41.2% women, mean age 42.5 years, mean FTND 5.5 |
|
| Interventions | 1. NRT: 8 weeks of 15 mg/24 h NRT patches 2. 8 weeks of 1 mg x 2/day varenicline (titrated 1st week) 3. Control group: no pharmacotherapy Level of support: high (all received brief (5 mins) education and counselling at 4 x weekly sessions.) |
|
| Outcomes | 12 months PPA, in person or by phone, verified by expired CO (cut‐off value not given) | |
| Notes | New for 2017 update. Our analyses only include 1 vs 3 Funding: Masih Daneshvari Hospital Research Institute, Tehran Risk of bias and some data extraction taken from Cahill 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Smokers who attended the clinic for help in quitting were divided randomly" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label; blinding of outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition: "Participants entered the study of their own accord and none left the study" |
| Other bias | Unclear risk | Abstinence‐by‐gender data (Table 2) appears to contain an error for women on NRT at 12 months; we have ignored this finding in favour of the combined‐genders data |
Heydari 2013.
| Methods | Country: Iran Recruitment: drug abuse treatment centres |
|
| Participants | 424 smokers ("habitual smokers" ≥ 1 year), history of drug abuse including opiates or narcotics for ≥ 1 year prior to referral to drug treatment centre 100% male, average age 44, average cpd NS (majority smoked 11 to 30 cpd), mean FTND 5.3 |
|
| Interventions | 1. NRT patch, gum and lozenges over 6 weeks. Step down 30 mg, 20 mg and 10 mg patches, and supply of 4 mg chewing gum and 1 mg pills 2. No pharmacotherapy or placebo Level of support: high (individual behavioural therapy, further detail not provided) |
|
| Outcomes | Abstinence at 6 months (type of measure not specified) Validation: exhaled CO (cut off not specified) |
|
| Notes | New for 2017 update. Not included in Analysis 4 as only study to be conducted in drug abuse treatment setting Funding: not specified, NRT provided free of charge by Meliora Health Corporation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “424 persons were assigned in a simple randomisation process into intervention and control groups using a computer generated list of random numbers” |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “researchers informed clinicians as to the type of treatment to administer to assigned subjects. Clinicians were not blinded…at the point at which treatment was administered” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants followed up at 6 months |
Hjalmarson 1984.
| Methods | Country: Sweden Recruitment: smoking cessation clinic | |
| Participants | 206 smokers 56% female, average age 42, average cpd 24 | |
| Interventions | 1. Nicotine gum (2 mg) (no restrictions on amount or duration of use) 2. Placebo gum Level of support: high (6 group sessions in 6 weeks) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO | |
| Notes | No information on support or funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | 26 groups "were randomly assigned" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both therapists and nurse distributing gum were blinded to assignment of groups. Placebo gum was flavoured with capsaicin to mimic nicotine |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 dropouts from each cohort during follow‐up; they were counted as smokers. 3 more from each cohort relapsed and were retreated, but counted as smokers within the study |
Hjalmarson 1994.
| Methods | Country: Sweden Recruitment: smoking cessation clinic | |
| Participants | 248 smokers 57% female, average age 45, average cpd 22 | |
| Interventions | 1. Nicotine nasal spray (0.5 mg/spray) used as required up to 40 mg/day for up to 1 year 2. Placebo spray Level of support: high (8 x 45‐ to 60‐min group sessions over 6 weeks with clinical psychologist) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO < 10 ppm | |
| Notes | Study was supported by Kabi Pharmacia AB, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects ... were randomized" to 26 groups |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Procedure was blind to both subject and therapist", but where more than 1 household member was enrolled all members got the same treatment (6 couples thus affected, 3 in active and 3 in placebo). At 12 months, 60% of responders correctly guessed their assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | By 12 months, 20% had relapsed |
Hjalmarson 1997.
| Methods | Country: Sweden Recruitment: smoking cessation clinic | |
| Participants | 247 smokers (> 10 cpd) who had previously made a serious attempt to stop using nicotine gum, and were motivated to quit 64% female, average age 48, average cpd 21 | |
| Interventions | 1. Nicotine inhaler (recommended minimum 4/day, tapering after 3 months, use permitted to 6 months) 2. Placebo inhaler Level of support: high (8 group meetings over 6 weeks) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO < 10 ppm at 2 and 6 weeks and 3, 6, 12 months | |
| Notes | Study was funded by Pharmacia & Upjohn, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All numbers were on a list for random allocation to medication" |
| Allocation concealment (selection bias) | Low risk | Participants received "a subject number consecutively" at the first group session |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The randomization was blinded to both the participant and the therapist", but members of the same household received the same treatment. At 12 month follow‐up, 86% of the active group and 90% of placebo group correctly guessed their assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and relapsers all counted as failures. Details fully reported |
Huber 1988.
| Methods | Country: Germany Recruitment: community volunteers | |
| Participants | 225 smokers (109 contribute to MA) No demographic information | |
| Interventions | 1. Nicotine gum alone 2. Behaviour therapy, 5 weekly group meetings 3. Nicotine gum (no details of dose) and behaviour therapy Level of support: high 4. 6‐month waiting‐list control | |
| Outcomes | Abstinence at 12 months Validation: none | |
| Notes | 3 vs 2 in comparison 1. No placebo. Quit rates derived from graphs. The nicotine‐alone group was not used in the MA; quit rates were higher than intervention 2 Study funding and support not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "225 interested subjects ... were randomly assigned" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10% had dropped out after 1 year |
Hughes 1989a.
| Methods | Country: USA Recruitment: primary care | |
| Participants | 315 daily smokers, motivated to quit 56% female, average age 37, average cpd 29 | |
| Interventions | 1. Nicotine gum (2 mg for 3 to 4 months) 2. Placebo gum Level of support: low (29 to 35 mins at 1st visit including nurse and physician advice and materials, follow‐up appointment 1 to 2 weeks later) | |
| Outcomes | Sustained abstinence at 12 months Validation: salivary cotinine < 15 ng/mL or thiocyanate < 1.6 mmol/L | |
| Notes | Time spent at 1st visit is marginal for inclusion in low‐intensity support category Study was funded by National Institute on Drug Abuse; gum supplied by Merrel‐Dow Research Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A 4th random digit (1 to 9) was added to their 3‐digit subject ID number. Only exception was members of same household got the same treatment |
| Allocation concealment (selection bias) | Low risk | 2:1 randomization scheme. Quote: "Subjects were assigned randomly in a double‐blind manner" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Pharmacists dispensed gum from numbered bins, and were unaware of assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and lost to follow‐up were included as smokers. Full details of losses reported |
Hughes 1990.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 78 smokers, motivated to quit 54% female, average age 34 to 44, average cpd 24 to 30 | |
| Interventions | 1. Placebo gum 2. 1 mg nicotine gum (unbuffered formula, available dose approximately 0.5 mg) 3. 2 mg nicotine gum 4. 4 mg nicotine gum Gum use not recommended for longer than 3 months Level of support: low (similar to Hughes 1989a) | |
| Outcomes | Sustained abstinence at 6 months Validation: Independent observer report | |
| Notes | 2 + 3 + 4 vs 1 in Comparison 1. Excluding the lowest dose would increase the treatment effect Study was funded by National Institute on Drug Abuse | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "in a double‐blind manner"; participants guessed which group they had been assigned to |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Subjects unable to be contacted were counted as smokers". Losses not reported |
Hughes 1999.
| Methods | Country: USA (12 sites), Australia (1 site) Recruitment: community volunteers and referrals | |
| Participants | 1039 smokers (≥ 30 cpd) who had made a prior quit attempt, motivated to try again 50% male, average age 43, average cpd 38 | |
| Interventions | 1. 42 mg nicotine patch (24 h, 6 weeks + 10 weeks tapering) 2. 35 mg nicotine patch 3. 21 mg nicotine patch 4. Placebo patch Level of support: high (group behaviour therapy for 7 weeks, brief individual counselling at 5 dose‐tapering meetings. Self‐help booklet) | |
| Outcomes | Prolonged abstinence at 6 months (from 2 weeks post‐quit) verified at each follow‐up visit (12‐month follow‐up only completed for 11/13 sites) Validation: CO ≤ 10 ppm | |
| Notes | All doses pooled in Analysis 1.1 against placebo 6‐month abstinence rates used in analyses since not all centres completed 12‐month follow‐up due to sponsor termination of study. Denominators confirmed by author Study was funded by National Institute on Drug Abuse, ALZA and Hoechst Marion Roussel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned in a double‐blind manner" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double‐blind" but no further detail |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Early termination by sponsor, resulting in incomplete long‐term follow‐up data collection. Losses were included as failures |
Hughes 2003.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 115 smokers with a history of alcohol dependence, motivated to quit, ≥ 30 cpd 68% male, average cpd 30 | |
| Interventions | 1.Nicotine patch (21 mg, 24 h, 6 weeks + 4 weeks tapering + 2 weeks placebo) 2. Placebo patch 12 weeks Level of support: high (Group behaviour therapy x 6, brief individual counselling x 3) | |
| Outcomes | Sustained abstinence at 6 months (from 2 weeks post‐quit) Validation: CO ≤ 10 ppm at each follow‐up visit | |
| Notes | Unadjusted ORs used in MA not significant, significant when adjusted for smoking variables Study was supported by GlaxoSmithKline, and funded by National Institute on Drug Abuse | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "we assumed that missing data indicated smoking". Losses reported, but not distribution across groups |
Hurt 1990.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 62 adult smokers (> 20 cpd); only accepted if willing to make a quit attempt 53% female, average age 39, average cpd 30 | |
| Interventions | 1. Nicotine patch (30 mg 24 h, 6 weeks + option of further 12 weeks ± tapering) 2. Placebo patch (continuing smokers at 6 weeks were offered active patch) Level of support: high (brief advice from nurse co‐ordinator at 6 weekly visits) | |
| Outcomes | Sustained abstinence at 12 months (quit by week 6, and all subsequent visits) Validation: CO ≤ 8 ppm | |
| Notes | Study was in part supported by Elan Pharmaceutical Research Corporation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "subjects were assigned randomly" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated; initial double‐blind was broken after 6 weeks of treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 dropouts from each group in first 6 weeks; smoking status of all dropouts ascertained "at last contact". Early dropouts were excluded from later analyses |
Hurt 1994.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 240 adult smokers (> 20 cpd), motivated to quit 53% female, average age 43, average cpd 30 | |
| Interventions | 1. Nicotine patch (22 mg/24 h, 8 weeks, no tapering) 2. Placebo patch Level of support: high (nurse counselling at 8 weekly visits, weekly phone calls to week 12) | |
| Outcomes | Abstinence at 12 months (no puff since 9‐month visit) Validation: CO ≤ 8 ppm | |
| Notes | Study was supported by Lederle Laboratories, NY | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "subjects were randomly assigned to active or placebo patches" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind; no further information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "subjects with missing information or who dropped out... were considered to be smoking". Dropout rates and reasons fully reported |
ICRF 1994.
| Methods | Country: UK Setting: primary care (19 general practices) | |
| Participants | 1686 smokers (> 15 cpd), not necessarily motivated to quit. 55% female, average age 43, average cpd 24 | |
| Interventions | 1. Nicotine patch (21 mg/24 h, 12 weeks incl tapering) 2. Placebo patch Level of support: high (brief advice from nurse at 4 study visits) | |
| Outcomes | Sustained abstinence at 12 months (from week 1) Validation: Salivary cotinine or CO | |
| Notes | 8‐year follow‐up in Yudkin 2003, OR remained similar Study supported by Ciba‐Geigy Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Quote: "prior random allocation of study numbers to each intervention group and by sequential allocation of a study number to patients on entry" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and nurses blinded to patches but not to support materials. Participants invited to guess assignment at end of treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Disconfirmations and dropouts counted as smokers |
Jamrozik 1984.
| Methods | Country: UK Recruitment: primary care (6 general practices) | |
| Participants | 200 adult smokers who had failed to stop smoking during a previous study of the effect of physician advice No demographic information | |
| Interventions | 1. Nicotine gum (2 mg) for 3 months+ 2. Placebo gum Level of support: low (follow‐up visits at 2, 4, 12 weeks for data collection, no counselling reported) | |
| Outcomes | PP abstinence at 6 months Validation: expired CO ≤ 12 ppm | |
| Notes | Study was funded by Oxford District Research Committee and Nuffield Dominions Trust, and supported by Lundbeck Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The codes were balanced to give equal numbers of patients receiving either the active gum ... or a placebo". |
| Allocation concealment (selection bias) | Low risk | Quote: "allocated to next available of ten alphabetical codes" from lists held in each practice |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Treatments were "identical in appearance and packaging". "No one doctor or member of staff was likely to see sufficient numbers of patients to be able to break the 10 code system" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up included as failures |
Jarvis 1982.
| Methods | Country: UK Recruitment: smoking cessation clinic | |
| Participants | 116 clinic attenders, motivated to quit 55% female, average age 41/38, average cpd 31/27 (P < 0.05) | |
| Interventions | 1. Nicotine gum (2 mg) unrestricted amount for at least 3 months 2. Placebo gum (1 mg unbuffered nicotine) Level of support: high (group therapy 6 x 1 h weekly) | |
| Outcomes | Sustained abstinence at 12 months (6‐month and 12‐month PP) Validation: CO (small number by confirmation from friend/relative only) | |
| Notes | The placebo gum was intended to match the active gum in taste but deliver minimal amounts of nicotine Study was funded by Medical Research Council and Dept of Health and Social Security, and supported by AB Leo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "treated in groups of about ten, taken in order from the waiting list" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Therapists and subjects were blind to the allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One sparticipant lost to follow‐up counted as a failure |
| Other bias | High risk | "Placebo" patch contained nicotine |
Jensen 1991.
| Methods | Country: Denmark Recruitment: smoking cessation clinic | |
| Participants | 293 adult smokers (> 10 cpd) in relevant arms 54% female, average age 42, average cpd 21 to 22 | |
| Interventions | 1. Nicotine gum (2 mg for 3 months) 2. Silver acetate chewing gum (not used in MA) 3. Standard chewing gum Level of support: high (9 group meetings over a year, weekly to week 4) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO | |
| Notes | 12 month data reported in Jensen 1990, used from 2008 Sources of support not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "smokers were randomised to 24 smaller groups and each group was randomly allocated to treatment". No further information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The study was not blind", because of restrictions on use of silver acetate gum |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 21 trial‐wide losses reported, but not included in the analyses. Distribution not stated, so not possible to include those lost to follow‐up in the final denominator |
Johns 2017.
| Methods | Country: India Recruitment: unclear |
|
| Participants | 300 (200 to relevant arms) smokers prone to lung cancer (previous lung disease/family history of lung cancer/past cancer treatment/lowered immunity/previous smoking‐related cancers/exposure to certain chemicals/radon gas) Other characteristics unknown |
|
| Interventions | 1. NRT: patch, gum, inhalator, sublingual tablet or nasal spray for 6 weeks (no further detail provided) 2. No NRT Level of behavioural support: low (20 mins intervention, no further detail given) |
|
| Outcomes | PP (length NS) at 12 months Validation: CO < 10 ppm |
|
| Notes | New for 2017 update Conference abstract only so limited information available, hence only in primary analysis N quit extrapolated from percentages given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number followed up not reported |
Jorenby 1999.
| Methods | Country: USA (4 sites) Recruitment: community volunteers | |
| Participants | 893 smokers, motivated to quit, (> 15 cpd) 52% female, average age 42 to 44, average cpd 25 to 28 |
|
| Interventions | 1. Nicotine patch (21 mg/24 h for 6 weeks, tapered for 2 weeks) and sustained release bupropion 300 mg for 9 weeks from 1 week before quit day 2. Bupropion 300 mg and placebo patch 3. Nicotine patch and placebo tablets 4. Placebo patch and placebo tablets Level of support: high, < 15 min individual counselling session at each weekly assessment. 1 phone call 3 days after quit day | |
| Outcomes | Abstinence at 12 months (primary outcome for study was PP abstinence; this analysis uses continuous abstinence since quit day) Validation: Expired CO < 10 ppm at each clinic visit | |
| Notes | 3 vs 4 in main analyses Study was funded by Glaxo Wellcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "subjects were randomly assigned to one of four treatments with use of an unequal‐cell design... Randomization was not balanced within sites" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Medications were identical, but other blinding procedures not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 311 discontinued treatment, with 177 withdrawing completely from the trial. Full details reported. All were included in ITT analyses with losses to follow‐up counted as smokers |
Joseph 1996.
| Methods | Country: USA, multicentre trial Recruitment: 10 Veterans Affairs Medical Centers | |
| Participants | 584 smokers (> 15 cpd) with a history of cardiac disease. Patients with cardiac events within the last 2 weeks were excluded | |
| Interventions | 1. Nicotine patch, (21 mg/24 h for 6 weeks, 14 mg for 2 weeks, 7 mg for 2 weeks) 2. Placebo patch Level of support: High (self‐help pamphlets and brief behavioural counselling on 3 occasions) | |
| Outcomes | PP abstinence at 6 months (Joseph 1996), 12 months (Joseph 1999) Validation: CO ≤ 10 ppm | |
| Notes | Study was funded by Hoechst Marion Roussel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated schedule" at the Minneapolis VAMC Co‐ordinating Center |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned in blocks of 10 |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind, but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses and withdrawals fully reported, as primary and secondary endpoints |
Killen 1984.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 64 adult smokers 72% female, average age 44, average cpd 32 | |
| Interventions | 1. Nicotine gum (2 mg) for 7 weeks 2. Skills training 3. Skills training plus nicotine gum Level of support: high (group therapy) | |
| Outcomes | Sustained abstinence at 10½ months Validation: CO | |
| Notes | 1 + 3 vs 2 used in comparison. 3 vs 2 would increase effect Study was funded by the National Institute of Health, and supported by Merrell‐Dow Pharmaceuticals Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Participants "were blocked on sex and Fagerström score and assigned randomly to treatment group". "Therapists were assigned randomly to treatment conditions" |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding reported. "Interpretation of this data is hampered by the lack of a placebo control condition." Unclear if therapists aware of gum allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11/75 recruited dropped out before full treatment, and are excluded from analyses |
Killen 1990.
| Methods | Country: USA Recruitment: community volunteers who had abstained from smoking for 48 h | |
| Participants | 1218 adult smokers 52% female, average age 43, average cpd 25 | |
| Interventions | 1. Nicotine gum (2 mg, 8 weeks) ad lib dosing 2. Nicotine gum on a fixed dose 3. Placebo gum 4. No gum Each group was also factorially randomized to 1 of 3 psychological interventions (all high support) | |
| Outcomes | PP abstinence at 12 months (7‐day PP) Validation: cotinine, except participants who moved away | |
| Notes | Quit rates were higher on fixed dose than ad lib gum Quit rates identical (18%) in placebo and no‐gum groups at 12 months Study was funded by National Cancer Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Assignment to gum condition was double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 deaths removed from final analyses. Participants moving out of the area were removed from the analyses. Unconfirmed claims of abstinence counted as smokers |
Killen 1997.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 424 smokers ˜50% female, average age ˜45, average cpd ˜23 | |
| Interventions | 2 x 2 factorial design, comparison between video & self‐help manuals and manuals alone collapsed 1. Nicotine patch (21 mg/24 h) for 8 weeks, 14 mg for 4 weeks, 7 mg for 4 weeks 2. Placebo patch 3. Nicotine patch and video (The video was shown at initial visit and a copy supplied for home use) 4. Placebo patch and video Level of support: low (All treatment groups received a self‐help treatment manual designed to develop self‐regulatory skills | |
| Outcomes | Sustained abstinence at 12 months (7‐day PP at 6 and 12 months) Validation: saliva cotinine < 20 ng/ml with the exception of participants living outside the area | |
| Notes | There was evidence of an interaction between NRT and video/self‐help conditions but this does not alter the MA so the conditions are combined from 2007. Both self‐help conditions treated as low intensity ‐ classifying video as high intensity would marginally reduce effect in high‐intensity subgroup Study was funded by National Heart, Lung, and Blood Institute, and supported by Hoechst Marion Roussel Inc and Blue Shield Management | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Participants "were randomized to treatment conditions" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Assignment to the patch condition was double‐blind"; participants invited to guess assignment at 6 month follow‐up |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants leaving the area (10) were excluded from analyses; all other unconfirmed claims of abstinence were counted as failures |
Kornitzer 1995.
| Methods | Country: Belgium Recruitment: worksite volunteers | |
| Participants | 374 healthy smokers (> 10 cpd for > 3 years), motivated to quit 61% male, average age 40, average cpd 25 | |
| Interventions | 1. Nicotine patch (12 weeks 15 mg/16 h, 6 weeks 10 mg, 6 weeks 5 mg) and nicotine gum (2 mg, as required) 2. Nicotine patch and placebo gum 3. Placebo patch and placebo gum. Level of support: high (nurse counselling) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO < 10 ppm | |
| Notes | Contributes data to main comparison (2 vs 3) Study was supported by Pharmacia Consumer Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See below |
| Allocation concealment (selection bias) | Low risk | Quote: "randomized list generated by a computer program". Randomization balanced between companies 2:2:1 |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The investigator and the subjects were completely blind concerning treatment". "unblinding was never requested during the whole study" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals counted as treatment failures. All analyses conducted on ITT basis. Dropout and withdrawal rates not reported |
Kralikova 2009.
| Methods | Country: Czech Republic Recruitment: community volunteers "wanting to reduce" | |
| Participants | 314 smokers (≥ 15 cpd) 58% female, average age 46, average cpd 25 | |
| Interventions | 1. Choice of 4 mg nicotine gum (up to 24/day) or 10 mg inhaler (6 to 12 daily) for up to 6 months with further 3 months tapering 2. Placebo gum or inhaler Common components: brief behavioural cessation/reduction support at clinic visits (9 scheduled) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO < 10 ppm | |
| Notes | Trial also included assessment of reduction. Reduction outcomes contribute to Cochrane Review on harm reduction Study details are taken from a conference abstract. Published 2009 Study supported by Pharmacia CHC, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double‐blind" ‐ no further details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
Leischow 1996a.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 222 smokers (> 20 cpd). (2 excluded from analysis having received incorrect prescription) 55% female, average age 44, average cpd 26 | |
| Interventions | 1. Nicotine Inhaler (10 mg). Advised to use 4 to 20 cartridges/day for 3 months. After this tapering was encouraged until 6 months 2. Placebo inhaler Participants received advice and watched a video showing proper use of the inhaler Level of support: high (brief individual smoking cessation support at each study visit, 10 in all) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO < 10 ppm at each follow‐up | |
| Notes | Study was funded by Pharmacia Upjohn, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomization code was generated by computer" |
| Allocation concealment (selection bias) | Low risk | Quote: "subjects were sequentially and randomly assigned" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts reported at 12‐month visit. Losses to follow‐up counted as failures |
Lerman 2015.
| Methods | Country: USA and Canada Recruitment: community (multicentre) |
|
| Participants | 1246 (826 to relevant arms) smokers of at least 10 cpd for at least 6 months 44% female, average age 46, average cpd 18, mean FTND 5.3 |
|
| Interventions | 1. NRT patch, 11 weeks. 21 mg for 6 weeks, 14 mg for 2 weeks, 7 mg for 3 weeks 2. Placebo Level of support: high (1 h in‐person pre‐quit group behavioural counselling, brief (˜15 minute) telephone counselling at weeks 0, 1, 4, 8) |
|
| Outcomes | 7‐day PP at 12 months Validation: CO < 8 ppm |
|
| Notes | New for 2017 update Funding: National Institute on Drug Abuse, National Cancer Institute, National Human Genome Research Institute, National Institute on General Medical Sciences, Abramson Cancer Center at the University of Pennsylvania, Commonwealth of Pennsylvania Department of Health, Canadian Institutes of Health Research, Canada Foundation for Innovation, Ontario Ministry of Research and Innovation. Pfizer Inc. provided varenicline and placebo pills at no cost N quit extrapolated from percentages given As combination of group and indiv. support, not included in support subgroup analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified Quote: "biostatistician, independent of study investigators, developed the randomisation procedure which was integrated into a centralised data management system. Subjects were randomised to the treatment arms in a 1:1:1 ratio. Randomisation was stratified by baseline NMR status and study site, and blocked in blocks of 12 patients (4/treatment block) to ensure approximate balance" |
| Allocation concealment (selection bias) | Low risk | Allocation was done by centralised data management system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding of researchers and participants "participants, study investigators, and personnel…were masked to treatment arm allocation and NMR status". Data were only unmasked following collection of all 6‐month follow‐up data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 50% followed up by 12 months (280/418 I, 264/408 C) |
Lewis 1998.
| Methods | Country: USA Recruitment: hospitalised patients willing to make a quit attempt | |
| Participants | 185 smokers (≥ 10 cpd), motivated to quit 46% female, average age 43 to 44, cpd 23 to 24 | |
| Interventions | 1. Minimal intervention, 2 to 3 mins motivational message and self‐help pamphlet 2. As 1. plus placebo patch. Nurse provided brief telephone counselling at 1, 3, 6 and 24 weeks 3. As 2. plus nicotine patch (22 mg/ 24 h for 3 weeks, tapered to 11 mg for 3 weeks) Level of support: low (since initial support was brief and further contacts in 2 were by phone | |
| Outcomes | PP abstinence at 6 months Validation: CO ≤ 10 ppm | |
| Notes | 3 vs 1 + 2 used in MAs Study was funded by Elan Pharmaceutical Research Corporation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See below |
| Allocation concealment (selection bias) | Low risk | Quote: "using a predetermined computer‐generated randomization code" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Both patients and study staff were blinded with respect to patch dose" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates not reported, but analyses count those lost to follow‐up as treatment failures |
Llivina 1988.
| Methods | Country: Spain Recruitment: smoking cessation clinic | |
| Participants | 216 smokers Average cpd 28 to 30 | |
| Interventions | 1. Nicotine gum (dose not stated) for 1 month 2. Placebo gum Level of support: high (group support) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO | |
| Notes | Reclassified as high support 2008 Study was funded by el Fondo de Investigaciones Sanitarias de la Seguridad Social, la Sociedad Española de Patologia Respiratoria, and los Laboratorios PENSA‐ESTEVE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "asignados al azar" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "doble ciego" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and withdrawals reported (Tabla 2) |
Malcolm 1980.
| Methods | Country: UK Recruitment: community volunteers | |
| Participants | 194 smokers 40% to 43% female, average age 44 to 46, average cpd 25 to 26 | |
| Interventions | 1. Nicotine gum (2 mg) at least 10/day for at least 3 months 2. Placebo gum 3. Control Level of support: high (weekly individual counselling for 1 month) | |
| Outcomes | Sustained abstinence at 6 months Validation: venous carboxyhaemoglobin ≤ 1.6% | |
| Notes | Study was supported by AB Leo & Company, Helsinborg, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly allocated" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "The trial was double blind between the gum groups" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only the 1‐month quitters were followed up at 6 months (77/82 participants) |
| Other bias | Unclear risk | 16 participants with dentures who could not chew gum were allocated to Controls but analysed separately |
McGovern 1992.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 293 adult smokers. Average cpd not stated. 58% smoked > 25 cpd | |
| Interventions | 1. ALA Freedom from Smoking clinic program plus nicotine gum (2 mg for 3 months) 2. ALA Freedom from Smoking clinic program alone (no placebo gum) Level of support: high (group) | |
| Outcomes | PP abstinence at 12 months Validation: salivary thiocyanate | |
| Notes | Study was supported by Merrell‐Dow Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Participants were randomly assigned .... Assignment to condition was by clinic group rather than individual subject". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not relevant, as no placebo gum used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Percentage response rates at follow‐up reported, with no differences between groups |
Molyneux 2003.
| Methods | Country: UK Recruitment: hospital | |
| Participants | 274 smokers (182 in relevant arms) admitted to medical and surgical wards, smoked in last 28 days 60% male, average age 60, median cpd 17, 81% had previous quit attempt | |
| Interventions | 1. Choice of NRT products (15 mg 16‐h patch/2 mg or 4 mg gum, 10 mg inhalator/2 mg sublingual tablet, 0.5 mg spray), Brief (20 min) bedside counselling from a research doctor or nurse 2. Brief counselling only 3. Usual care, no smoking advice (not used in MA) Level of support: low | |
| Outcomes | Continuous abstinence at 12 months Validation: CO < 10 ppm | |
| Notes | No placebo. 63% chose patch, 13% inhalator, 11% gum, 8% tablets and 1% nasal spray, 4% declined use Study was supported by Pharmacia Consumer Healthcare, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised ... using a list generated for each centre, allocating equally in random permuted blocks of nine". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not relevant to participants, as NRT group chose their own type. Assessment and delivery blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up counted as failures. All losses fully detailed in flow chart |
| Other bias | Unclear risk | 4% of counselling + NRT group refused NRT, and counselling‐only group were advised about NRT but not given it; usage across groups not reported |
Moolchan 2005.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 120 adolescent (age 13 to 17) smokers (≥ 10 cpd), motivated to quit 70% female, average age 15, average cpd 19 | |
| Interventions | 1. Nicotine patch (21 mg, or 14 mg for < 20 cpd) for 6 weeks + placebo gum 2. Nicotine gum (4 mg, or 2 mg for < 24 cpd) for 6 weeks + placebo patch 3. Double placebo Level of support: high (11 x 45‐min individual counselling over 12 weeks) | |
| Outcomes | PP abstinence at 6 months Validation: CO and cotinine | |
| Notes | Placebo group contributes twice to MA ‐ too small to affect total Sustained abstinence at 3 and 6 months could be derived from text, relative effect greater since no quitters on placebo Study was funded by National Institute on Drug Abuse, and supported by GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized ... according to an algorithm held by the National Institute on Drug Abuse Pharmacy, with true replacement of the non‐completers". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double‐blind, double‐dummy", but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were included as failures for cessation. Losses fully reported |
Mori 1992.
| Methods | Country: Japan Recruitment: hospital | |
| Participants | 364 smokers with smoking‐related illness. Number of cpd not stated. Motivation to quit probably not required. |
|
| Interventions | 1. Nicotine gum 2 mg for 3 months 2. Placebo gum Level of support: low | |
| Outcomes | Abstinence (not defined) at 6 months Validation: serum thiocyanate | |
| Notes | "Supported partially by FISss 90/0431 and SEPAR". Trial report was abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double blind", but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
Nakamura 1990.
| Methods | Country: Japan Recruitment: community volunteers | |
| Participants | 60 adult smokers. Average cpd 31 | |
| Interventions | 1. Nicotine gum (2 mg, 2 months or longer) 2. Non‐placebo control group received counselling Level of support: high | |
| Outcomes | Sustained abstinence at 6 months Validation: CO | |
| Notes | Study was supported by Merrell‐Dow Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Assignment was done ... by individual randomisation based on their screen's numbers [or] by group randomisation by worksite unit". 15 members for Group 3 were chosen from 19 applicants, based on distribution of employment |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Described as an "open controlled trial" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analyses conducted, with all dropouts and non‐compliers included as failures. But "smoking on one or two occasions in a single day was not considered a failure ... although occasional smoking was considered a failure" |
NCT00534404.
| Methods | Country: USA Recruitment: not specified |
|
| Participants | 2485 (1658 in relevant arms) smokers of at least 10 cpd | |
| Interventions | 1. NRT patch, 8 weeks. 21 mg for 4 weeks, 14 mg for 2 weeks, 7 mg for 2 weeks 2. No NRT Level of support: low (internet assisted tobacco treatment) |
|
| Outcomes | 6 months prolonged abstinence at 9 months Validation: none |
|
| Notes | New for 2017 update Funding: not specified Data from clinical trials registry so limited information available, for this reason not included in setting subgroup |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not specified but as not placebo‐controlled presumably unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 50% participants followed up (687/830 I, 586/828 C) |
Nebot 1992.
| Methods | Country: Spain Recruitment: primary care | |
| Participants | 425 unselected smokers. 60% to 70% smoking > 15 cpd | |
| Interventions | A. Brief counselling from physician B. Physician counselling plus nicotine gum C. Health education from nurse Level of support: low | |
| Outcomes | PP abstinence at 12 months Validation: CO | |
| Notes | Study was supported by the Fondo de Investigaciones Sanitarias de la Seguridad Social | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not applicable; "each PCT was randomly allocated to perform the three different interventions successively". No information about avoidance of selection bias |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only those quit at 2 months were followed up at 12 months. All non‐responders were included as failures |
| Other bias | Unclear risk | Unequal assignments to the 3 groups, with nurse and NRT groups outnumbered 1:2 by the medical advice group |
Niaura 1994.
| Methods | Country: USA Recruitment: outpatient settings and physician referrals (primary care subgroup) | |
| Participants | 77 low‐dependence (FTND ≤ 6) and 96 high‐dependence smokers 50% female, average age 42, average cpd 29, FTND 4.7 for low dependence, 8.0 for high dependence | |
| Interventions | 1. Nicotine gum 2 mg, ad lib for up to 4 months (participants given prescription for gum, not free) 2. No gum Level of support: high (4 individual counselling sessions and ALA self‐help treatment manuals) | |
| Outcomes | Continuous abstinence at 12 months Validation: saliva cotinine, or CO for gum users | |
| Notes | No placebo used. Data collapsed across dependence levels. As predicted by the study, smokers with lower dependence had lower quit rates with than without gum. The point estimate would be higher if inclusion restricted to the high‐dependence group Study was supported by National Cancer Institute and National Heart, Lung, and Blood Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants stratified on level of nicotine dependence. "Within each of the high‐ and low‐dependence groups, subjects were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No placebo ‐ not relevant. But therapist and participant were blinded to FTQ score (level of dependency), and to match or mismatch status for gum use |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates fully reported |
Niaura 1999.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 62 smokers in relevant arms 50% female, average cpd 28, average age 43.5 | |
| Interventions | 1. Brief cognitive behavioral relapse prevention (CBRP) , 15‐min sessions 2. Intensive CBRP with nicotine gum (2 mg) 3. Intensive CBRP with cue exposure 4. Intensive CBRP with cue exposure + nicotine gum Level of support: high (5 group sessions within 3 weeks of TQD) | |
| Outcomes | Sustained abstinence, 12 months and all previous follow‐ups (1, 3, 6 months) Validation: CO < 8 ppm | |
| Notes | 4 vs 3, behavioural support not identical in others. No placebo Study was supported by Department of Veterans Affairs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Counselors were kept blind to the relapse prevention condition to which subjects were assigned". Participants not blinded, and no placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up fully reported |
Ockene 1991.
| Methods | Country: USA Recruitment: primary care | |
| Participants | 1223 unselected smokers 57% female, average age 35, average cpd 22 to 23 |
|
| Interventions | 1. Advice only 2. Participant‐centred counselling 3. Participant‐centred counselling and offer of nicotine gum (2 mg) plus minimal or intensive follow‐up by telephone Level of support: mixed (not used in subgroup analysis) | |
| Outcomes | Sustained abstinence at 12 months (quit at 6 months and 12 months, reported in Ockene 1994) Validation: none | |
| Notes | 69% of group 3 accepted prescription and received at least 1 box of gum 12‐month sustained rates, 3 vs 2, used in MA since 2008 Study was funded by the National Cancer Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to the physician and follow‐up conditions" |
| Allocation concealment (selection bias) | Low risk | Physicians opened "a packet containing the intervention materials, which they received at the beginning of the clinic encounter" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses and dropouts were included as failures. 62 participants removed from denominator (4 deaths, 58 not contacted by study staff) |
Oncken 2007.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 152 post‐menopausal women (≥ 10 cpd) Average cpd 22, average age 54/56.6 | |
| Interventions | 1. Nicotine patch (21 mg for 13 weeks including 4 weeks tapering) 2. Placebo patch Level of support: high (7 visits including 4 x 2‐h group counselling, 1 pre‐TQD) | |
| Outcomes | PP abstinence at 16 months (12 months post‐EOT) Validation: CO < 8 ppm | |
| Notes | Study was supported by The Patrick and Catherine Weldon Donaghue Foundation, The University of Connecticut Center on Aging, University of Connecticut General Clinical Research Center and the National Institute for Health. Pharmaceuticals supplied by GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assignment ratio was 3:5; "152 women were randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind, but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts or missed visits included as failures. Losses at each follow‐up fully reported |
Oncken 2008.
| Methods | Country: USA Recruitment: volunteers from antenatal clinics |
|
| Participants | 194 pregnant women smoking at least 1 cpd Average age 25, average cpd 10 in week before study enrolment, average cpd 18 prepregnancy, mean FTND < 4 |
|
| Interventions | 1. 2 mg nicotine gum (first 6 weeks: instructed to chew 1 piece for every cigarette usually smoked per day, not exceeding 20, followed by 6‐week tapering period) 2. Placebo gum, dosing and duration as above Level of support: high. In‐person and telephone individual smoking cessation counselling |
|
| Outcomes | Abstinence at 32 to 34 weeks of gestation and 7‐day PP at 6 to12 weeks post‐partum (abstinence at 6 weeks post‐quit date also reported) Validation: CO and urinary cotinine |
|
| Notes | Varying lengths of follow‐up. Longest follow‐up used in primary analysis NRT group had significantly higher birth weight and gestational age than placebo group. NRT group significantly more likely to attend follow‐up visits. Funded by the National Institutes of Health. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerized urn randomization program to balance participant assignment in the two treatment groups" |
| Allocation concealment (selection bias) | Low risk | Urn randomization procedure implies that allocation not known until after enrolment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double blind", methods not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significantly higher loss to follow‐up in placebo group (50% as opposed to 35%). Those lost to follow‐up considered to be smoking |
Ortega 2011.
| Methods | Country: Spain Recruitment: hospital inpatients |
|
| Participants | 1843 hospital inpatients who identified as smokers 88% male, average age 62, average 56 packs/year |
|
| Interventions | 1. Nicotine patch or gum (max 12 weeks; participant's choice) + CBT 2. CBT only 3. Declined to participate Level of support: high (standardized 30‐ to 45‐min sessions every 3 days until participant discharged from hospital; post‐discharge participant could have telephone or in‐person sessions at 1 week, 15 days, 2, 3, 6, and 12 months) |
|
| Outcomes | Continuous abstinence from quit day at 12 months Validation: 34% of participants verified with CO measurement |
|
| Notes | No placebo. Groups 1 and 2 included in primary analysis under 'choice of NRT'. "No significant outcome differences between NRT types" (personal communication from author) 717 declined to participate but followed up at 12 months Funding not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized "using a "computerized algorithm." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants not blinded; no placebo group. Not specified as to whether study personnel were blinded. Quote: "...the one‐year abstinence in the telephone follow‐up group was self declared and not validated, which may entail bias when evaluating whether these patients truly had stopped smoking." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost not specified. Participants lost to follow‐up included as smokers in outcome data |
Otero 2006.
| Methods | Country: Brazil Recruitment: community volunteers | |
| Participants | 1199 smokers (includes 254 non‐attenders), motivated to quit 63% female, average age 42, 46% smoked > 20 cpd | |
| Interventions | Factorial design with multiple levels of behavioural support 1. Nicotine patch (21 mg, 14 mg for FTND < 5) 8 weeks including tapering + behavioural support 2. Cognitive behavioural support only Level of support: Mixed ‐ low = single 20‐min session. High = 1, 2, 3 or 4 weekly 1‐h sessions. Maintenance or recycling sessions provided at 3, 6, 12 months | |
| Outcomes | PP abstinence at 12 months Validation: none | |
| Notes | Contributes to both high‐ and low‐support subgroups No placebo Study was supported by the Institute for Global Tobacco Control and the Fogarty International Center of the National Institutes of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. Randomization was stratified by age and sex by an independent specialist |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | 29% of control group participants asked for nicotine patch after the 3‐month follow‐up which might have increased control group quit rates at 12 months |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
Page 1986.
| Methods | Country: Canada Recruitment: primary care (5 family practices in Ontario) | |
| Participants | 275 unselected smokers. Primary care attenders aged 18 to 65 years Number of cpd not stated | |
| Interventions | 1. No advice 2. Advice to quit 3. Advice to quit plus offer of nicotine chewing gum prescription (2 mg) Level of support: low | |
| Outcomes | Sustained abstinence at 6 months Validation: none | |
| Notes | 3 vs 1 + 2 No placebo. Study was funded by the Canadian College of Family Physicians of Canada and by the University of Waterloo Social Sciences and Humanities Research Grant Fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomized by day of attendance. Post hoc tests of results by day of attendance showed no interaction |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single‐blinding: Quote: "subjects were not aware of their treatment group nor the fact that they were being evaluated against other experimental groups". Follow‐up interviewers "remained blind to the patient's experimental group until the final section in the interview" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses reported, but not included in analyses |
Paoletti 1996.
| Methods | Country: Italy Recruitment: community volunteers | |
| Participants | 297 smokers (≥ 10 cpd), motivated to quit Stratified according to baseline cotinine levels 40% female, average age 43, average cpd 24 in low‐cotinine group (n = 120), 30 in high group (n = 177) | |
| Interventions | Stratum A (Baseline cotinine < 250 ng/ml) 1. Nicotine patch (15 mg/16 h, 18 weeks incl taper) 2. Placebo patch Stratum B (Baseline cotinine > 250 ng/ml) 3. Nicotine patch 15 mg 4. Nicotine patch 25 mg Level of support: low | |
| Outcomes | PP abstinence at 12 months Validation: CO and plasma cotinine | |
| Notes | Stratum A in Comparison 1 Study was funded by Pharmacia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization stratified on plasma cotinine levels. No detail on methods used |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind. All participants got 2 patches, to ensure maintenance of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up fully reported |
Perng 1998.
| Methods | Country: Taiwan Recruitment: outpatient chest clinics, volunteers | |
| Participants | 62 smokers (> 20 cpd) 94% male, average age 62, average cpd 26 | |
| Interventions | 1. Nicotine patch (24 mg/24 h for 6 weeks, no weaning) 2. Placebo patch Level of support: high (weekly visit to outpatient department for assessment, unclear if counselling was provided) | |
| Outcomes | Abstinence at 12 months Validation: CO < 10 ppm during patch use, but no validation at 12 months | |
| Notes | Level of support reclassified as high, 2008 update Study was funded by Orient Europharma Company Ltd, Taipei, Taiwan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed by an independent outside facility" |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind. No further detail |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
Piper 2009.
| Methods | Country: USA Participants: community volunteers |
|
| Participants | 1504 smokers motivated to quit 58% female, average age 45, average cpd 21.4 |
|
| Interventions | 1. Nicotine lozenge 2 or 4 mg for 12 weeks (based on dose‐for‐dependence level as in instructions) 2. Nicotine patch (24 h, 21, 14, and 7 mg titrated down over 8 week period post‐quit) 3. Bupropion SR (150 mg bid, 1 week pre‐quit, 8 weeks post‐quit) 4. Lozenge + patch (duration and dosage as above) 5. Bupropion + lozenge (duration and dosage as above) 6. Placebo (5 groups matched to above 5 interventions) Level of support: high. All participants received 7 one‐to‐one 10‐ to 20‐min counselling sessions |
|
| Outcomes | 7‐day PP abstinence at 6 months; initial cessation Validation: CO < 10 ppm |
|
| Notes | Placebo outcomes not reported by subgroup; outcomes generated by applying overall percentage of events in placebo group to individual subgroups. 1, 2, 4 and 6 included in primary analysis Analyses conducted using ITT Most of the funding from National Institute on Drug Abuse and National Center for Research Resources. Medication provided to participants at no extra cost by GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was double‐blind and used a block randomization scheme with sex and self‐reported race as the blocking variables." |
| Allocation concealment (selection bias) | Low risk | Quote: "Staff did not know to which type(s) of medication a participant would be assigned until the moment of randomization, and study staff were blinded to whether the medication was active or placebo." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Double blind." "Study staff were blinded to whether the medication was active or placebo". Type of medication (i.e. patch, gum, pill) would have been apparent to both groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90 dropouts (out of 1504). Analyses conducted using ITT. Individuals with missing data considered to be smoking |
Pirie 1992.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 417 women smokers, average cpd 25 to 27 | |
| Interventions | 1. Group therapy 2. Group therapy plus weight control programme 3. Group therapy plus nicotine gum 4. Group therapy plus weight control programme and nicotine gum Gum type: 2 mg ad lib Level of support: high | |
| Outcomes | Sustained abstinence at 12 months Validation: CO | |
| Notes | 3 and 4 compared to 1 and 2 Study was funded by the National Cancer Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomized to one of four groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No placebo. No detail reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. Moved away completed assessments by phone or mail |
Pollak 2007.
| Methods | Country: USA Recruitment: volunteers from antenatal clinic |
|
| Participants | 181 pregnant women smoking at least 5 cpd Average age 27, average cpd pre‐pregnancy 19 |
|
| Interventions | 1. CBT 2. CBT + free NRT (choice of patch, gum, lozenge or no NRT. Patch: 16 h, encouraged to use for 6 weeks, dose based on woman's smoking level, < 10 cpd = 7 mg/day, 10 to 14 cpd = 14 mg/day, ≥ 15 cpd = 21 mg/day; gum or lozenge: 2 mg for every cpd) Level of support: high (6 one‐to‐one counselling sessions) |
|
| Outcomes | 7‐day PP at 38 weeks of gestation and 3 months post‐partum Validation: salivary cotinine |
|
| Notes | Varying lengths of follow‐up Recruitment suspended early when interim analysis found higher rate of negative birth outcomes in CBT+NRT arm; not statistically different when adjusted for previous history of birth outcomes in final analysis 6 in NRT group opted to use no NRT; 4 in CBT‐only arm reported use of NRT Funded by the National Cancer Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerised random number generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "each support specialist had a handheld device that contained a randomization list" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label, unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women lost to follow‐up considered smokers; similar numbers in both groups |
| Other bias | Unclear risk | Women in CBT+NRT group significantly more likely to attend CBT sessions |
Prapavessis 2007.
| Methods | Country: New Zealand Recruitment: community volunteers | |
| Participants | 121 women smokers (> 10 cpd) (excludes dropouts not starting programme) | |
| Interventions | NRT as adjunct to either CBT or exercise programmes, collapsed for this review 1. Nicotine patch (21 mg/24 h for 10 weeks, no weaning) 2. No patch Level of support: high (36 x 45‐min session over 12 weeks of group CBT or supervised vigorous exercise, starting 6 weeks before TQD) | |
| Outcomes | Continuous abstinence since TQD at 12 months from end of programme Validation: CO < 10 ppm, cotinine < 10 ng/mL | |
| Notes | No placebo Study was funded by the National Heart Foundation of New Zealand, and supported by GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Using a computer‐generated program, participants were then randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Analyses were conducted by intent to treat". Missing data on smoking abstinence were counted as failures. % losses reported |
Puska 1979.
| Methods | Country: Finland Recruitment: community volunteers | |
| Participants | 229 adult smokers, 80% smoking > 5 cpd | |
| Interventions | 1. Nicotine gum (4 mg) for 3 weeks 2. Placebo gum for 3 weeks Level of support: high (group therapy) | |
| Outcomes | PP abstinence at 6 months Validation: none | |
| Notes | Study was supported by AB Leo and Co, Helsinborg, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Neither the subjects nor the course leaders were aware who received active and who placebo gum" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up were reported, but were not included in the analyses |
Richmond 1993.
| Methods | Country: Australia Recruitment: primary care | |
| Participants | 450 adult smokers (350 in included arms) Average cpd 15 to 21 |
|
| Interventions | 1. Smokescreen programme plus nicotine gum, dose and duration not stated 2. Smokescreen programme alone 3. Brief advice and gum (not included in MA) Level of support: high (5 visits during first 3 months) | |
| Outcomes | Continuous abstinence (from week 1) at 12 months Validation: expired CO < 14 ppm | |
| Notes | No placebo Continuous abstinence rates from Richmond 1993 paper used from 2007. Group 3 not included Study was funded by the Department of Health, Housing and Community Services, Community Health Anti‐Tuberculosis Association, Glaxo Australia, and the Drug and Alcohol Directorate, NSW Department of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "random weekly assignment" |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up were included as failures |
Richmond 1994.
| Methods | Country: Australia Recruitment: community volunteers | |
| Participants | 315 smokers average cpd 29 |
|
| Interventions | 1. Nicotine patch (24 h, 22 mg/24 h, 10 weeks incl tapering) 2. Placebo patch Level of support: high (group therapy) | |
| Outcomes | Sustained abstinence at 12 months (reported in Richmond 1997, which also reports 3‐year follow‐up, not used in MA) Validation: CO | |
| Notes | 3‐year abstinence 21/153 vs 8/152, OR 2.9 ‐ higher than at 12 months Study was funded by Marion Merrell Dow, and supported by the Drug and Alcohol Directorate, NSW Department of Health, and the Lifestyle Unit, Prince of Wales Hospital, Sydney | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment and control patches were arranged in random order by Marion Merrell Dow, Sydney, then issued sequentially to patients as they attended"; married couples were assigned to same condition |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up included as failures. Dropout rates fully reported |
Roto 1987.
| Methods | Country: Finland Recruitment: primary care (occupational health centres) | |
| Participants | 121 smokers > 10 cpd, > 1 year, 43% female |
|
| Interventions | 1. Nicotine gum (2 mg and 4 mg) + advice 2. Advice only (no placebo) Level of support: low | |
| Outcomes | Abstinence at 6 months (not defined) Validation: not described | |
| Notes | Study funding and support not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts classified as smokers |
Russell 1983.
| Methods | Country: UK Recruitment: primary care ‐ consecutive attenders admitting to being cigarette smokers and consenting to participate at 6 general practices | |
| Participants | 2106 unselected adult smokers average cpd 17.5 |
|
| Interventions | 1. No intervention 2. Advised to stop smoking plus provided with a "give up smoking" booklet 3. As group 2, plus offer of nicotine gum prescription, individual therapy, single visit, 1 minimal content, 1 more intensive content, untrained therapist Level of support: low | |
| Outcomes | Sustained abstinence at 4 months and 12 months Validation: 66% of those claiming to have quit validated with CO | |
| Notes | 3 vs 2 + 1 used in comparison. Using only 2 as control has negligible effect on point estimate Only 53% of group 3 tried the gum Use of quit rates adjusted for estimated validation failure and protocol violation would increase relative effect of gum Study was funded by the Medical Research Council, and the AB Leo Research Foundation, Sweden | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants assigned "according to their week of attendance" |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. Correct procedure was not followed by 10.4% in Grp 1, 15.4% in Grp 2 and 16.2% in Grp 3. Only 53% of Grp 3 ever tried the gum |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 deaths and 152 who moved away were excluded from analyses. 327 with no or inadequate data at follow‐up were included as failures |
Sachs 1993.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 220 adult smokers average cpd 28 to 29 |
|
| Interventions | 1. Nicotine patch (15 mg/16 h, 12 weeks + 6 weeks tapering) 2. Placebo patch Level of support: high (physician advice, 8 visits during treatment period) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO | |
| Notes | Study was funded by National Institute on Drug Abuse, Kabi Pharmacia AB and Parke‐Davis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were sequentially and randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates not fully reported, but all participants included in ITT analyses with dropouts counted as smokers |
Scherphof 2014.
| Methods | Country: Netherlands Recruitment: schools |
|
| Participants | 265 adolescents (12 to 18 years old), smoking ≥ 7 cpd, motivated to quit 52.9% female, mean age 16.5, mean cpd 16.7 |
|
| Interventions | 1. 24‐h patch, dose and length depending on baseline cpd. If > 20 cpd, 3 weeks 21 mg/day, 3 weeks 14 mg/day; 3 weeks 7 mg/day; if < 20 cpd, 3 weeks 14 mg/day, 3 weeks 7 mg/day 2. Control: placebo patch control, otherwise identical to intervention Level of support: low (one‐off "short behavioral intervention aimed at quitting smoking (e.g. preparations and expectations)" at study start) |
|
| Outcomes | 30‐day PP abstinence at 12 months Verification: salivary cotinine measured using a NicAlert saliva strip (Nymox) |
|
| Notes | New for 2017 update Funding: ZonMw – The Netherlands Organization for Health Research and Development; Novartis provided study medication and placebo Risk of bias and some data extraction from Fanshawe 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized according to a computer‐generated randomization list by the pharmacy of the University Medical Centre to either (1) active study medication (nicotine patch) or (2) an identically appearing placebo (placebo patch)." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "participants and research assistants were blind to treatment allocation"; however, does not specify how this occurred |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Placebo‐controlled, but no further detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up not reported by trial arm, but 10.1% overall at 12 months |
Schneider 1983a.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 60 heavy smokers (> 1 pack/day) 60% female, average age 40/37, average cpd 35/31 | |
| Interventions | Study A (clinic support): 1. Nicotine gum, (2 mg duration not stated) 2. Placebo gum Level of support: high (individual support at multiple clinic assessment visits, daily during week 1, weekly to week 5) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO | |
| Notes | Reported in same papers as Schneider 1983b. Shared study ID until 2008. Schneider 1983 provides demographic data so now used as primary reference Jarvik 1984 reports outcomes by dependency score for 48/60 participants Study was funded by National Institute on Drug Abuse and by the Medical Research Service of the Veterans Administration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "gum was dispensed in a double‐blind procedure" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
Schneider 1983b.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 36 heavy smokers (> 1 pack/day) no demographic details | |
| Interventions | Study B (pilot dispensary study): 1. Nicotine gum, (2 mg, duration not stated) 2. Placebo gum Level of support: low (weekly laboratory visits for 5 weeks but no support provided) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO | |
| Notes | Reported in same papers as Schneider 1983a. Shared study ID until 2008 Study was funded by National Institute on Drug Abuse and by the Medical Research Service of the Veterans Administration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "gum was dispensed in a double‐blind procedure" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
Schneider 1995.
| Methods | Country: USA Recruitment: community volunteers (radio and newspaper ads) | |
| Participants | 255 adults with no serious illness, motivated to quit, smoking > 15 cpd for > 2 years with baseline CO level > 20 ppm average cpd 28 to 29 | |
| Interventions | 1. Nicotine nasal spray 2. Placebo spray Nicotine dosage: 0.5 mg of nicotine per spray. No fewer than 8 and no more than 32 doses/day for 6 weeks, with free use for further 6 months Level of support: high (repeated clinic visits for assessment) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO < 8 ppm. | |
| Notes | Study was funded by Veteran Affairs and Pharmacia (Sweden) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned to conditions" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "the trial was double‐blind". Participant guesses reported as confirmation of blinding success |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Limited information |
Schneider 1996.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 223 adult smokers (≥10 cpd) 37% female, average age 44, average cpd 29/26 (significantly higher in active group) | |
| Interventions | 1. Nicotine inhaler (4 to 20 inhalers per day) for up to 6 months, with weaning from 3 months 2. Placebo inhaler Level of support: high (repeated clinic visits for assessment) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO and salivary cotinine | |
| Notes | Study was funded by Veteran Affairs and by Pharmacia & Upjohn (Sweden) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer generated randomization list was prepared by the manufacturers" |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent "randomizer" packaged drug from the list." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Subjects and all personnel connected with the trial (including the PI) were kept blind". Participants guessed their allocation as a test of the blinding at final assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
Schnoll 2010.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Excluded study, but contributing data on adverse events | |
Segnan 1991.
| Methods | Country: Italy Recruitment: primary care ‐ consecutive patients attending 44 general practices | |
| Participants | 923 practice attenders aged 20 to 60 average cpd not stated Therapists: GPs who had undergone a 3‐h training session |
|
| Interventions | 1. Advice and leaflet 2. Repeated counselling (follow‐up at 1, 3, 6, 9 months) 3. Repeated counselling plus prescription for nicotine gum unless contraindicated, dose not stated, up to 3 months 4. Repeated counselling plus spirometry Level of support: high | |
| Outcomes | Sustained abstinence at 12 months Validation: urinary cotinine | |
| Notes | 3 vs 1 + 2 + 4. Study was supported by SIMG (Italian Association of General Practice), and by Serono SPA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a predetermined randomized sequence of the four interventions" |
| Allocation concealment (selection bias) | Low risk | Quote: "a package of closed numbered envelopes ... was provided to each GP". Research staff checked the integrity of the process |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Interviews were conducted by "trained interviewers, independent of the study staff" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates reported |
Shiffman 2002 (2 mg).
| Methods | Country: USA and UK (15 sites) Recruitment: community volunteers | |
| Participants | 917 smokers, motivated to quit, time to first cigarette > 30 mins 58% female, average age 41, cpd 17 | |
| Interventions | 1. Nicotine lozenge, 2 mg. Recommended dose 1 every 1 to 2 h, min 9, max 20/day for 6 weeks, decreasing 7 to 12 weeks, available as needed 13 to 24 weeks 2. Placebo lozenge, same schedule Level of support: high (brief advice at 4 visits in 4 weeks from enrolment) | |
| Outcomes | Continuous abstinence at 12 months (sustained from 2 weeks, no slips allowed) Validation: CO ≤ 10 ppm at all follow‐ups. (only abstainers continued in study) | |
| Notes | Dose based on dependence level. Low‐dependence group here. High‐dependence group in Shiffman 2002 (4 mg). Study was supported by GlaxoSmithKline Consumer Healthcare | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "smokers were randomized" after stratification for dependency by time to first cigarette of the day |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind, but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only abstainers were followed up. "Participants who did not appear for a visit were counted as treatment failures". Losses fully reported |
Shiffman 2002 (4 mg).
| Methods | Country: USA and UK (15 sites) Recruitment: community volunteers | |
| Participants | 901 smokers, time to first cigarette < 30 mins 55% female, average age 44, cpd 26 | |
| Interventions | 1. Nicotine lozenge, 4 mg. Recommended dose 1 every 1 to 2 h, min 9, max 20/day for 6 weeks, decreasing 7 to 12 weeks, available as needed 13 to 24 weeks 2. Placebo lozenge, same schedule | |
| Outcomes | Continuous abstinence at 12 months (sustained from 2 weeks, no slips allowed) Validation: CO ≤ 10 ppm at all follow‐ups (only abstainers continued in study) | |
| Notes | Dose based on dependence level. High‐dependence group here. Low‐dependence group in Shiffman 2002 (2 mg) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See above (Shiffman 2002 (2 mg)) |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See above |
Shiffman 2009 (2 mg).
| Methods | Country: USA Recruitment: community volunteers |
|
| Participants | 1636 smokers wishing to quit by gradual reduction (RTQ technique) 64% female, average age 42, average cpd 9.4, average FTND 4.4 |
|
| Interventions | 1. Nicotine gum 2 mg. Instructed to gradually reduce smoking while increasing gum use for up to 8 weeks. Post‐quit instructed to use 1 piece every 1 to 2 h for first 6 weeks; 1 every 2 to 4 h for next 3 weeks; 1 every 4 to 8 hours for final 3 weeks 2. Placebo gum, same schedule as above Level of support: low (designed to simulate OTC setting) |
|
| Outcomes | Abstinence at 6 months from start of treatment (initial abstinence had to be achieved within 8 weeks of start of treatment, so duration of abstinence was at least 4 months) Validation: CO ≤ 10 ppm |
|
| Notes | Included in main analyses Dose based on dependence level. Participants read labelling which recommended 4 mg dose for smokers of > 25 cpd and selected appropriate dose. Low‐dependence group here. High‐dependence group reported in Shiffman 2009 (4 mg). Funding provided by GlaxoSmithKline Consumer Healthcare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a 1:1 computer‐generated randomization scheme, balanced across study sites and generated separately for the 2‐ and 4‐mg groups" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind", method not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Those who had not succeeded at 28 days follow‐up not followed up at 6 months. All missing data considered to be smoking |
Shiffman 2009 (4 mg).
| Methods | Country: USA Recruitment: community volunteers |
|
| Participants | 1661 smokers wishing to quit by gradual reduction (RTQ technique) 50% female, average age 46, average cpd 32, average FTND 6.9 |
|
| Interventions | 1. Nicotine gum 4 mg. Instructed to gradually reduce smoking while increasing gum use for up to 8 weeks. Post‐quit instructed to use 1 piece every 1 to 2 hours for first 6 weeks; 1 every 2 to 4 hours for next 3 weeks; 1 every 4 to 8 hours for final 3 weeks 2. Placebo gum, same schedule as above Level of support: low (designed to simulate OTC setting) |
|
| Outcomes | Abstinence at 6 months from start of treatment (initial abstinence had to be achieved within 8 weeks of start of treatment, so duration of abstinence was at least 4 months) Validation: CO ≤ 10 ppm |
|
| Notes | Dose based on dependence level. High‐dependence group here. Low‐dependence group reported in Shiffman 2009 (2 mg). Funding provided by GlaxoSmithKline Consumer Healthcare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See above (Shiffman 2009 (2 mg)) |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See above |
Stapleton 1995.
| Methods | Country: UK Setting: primary care | |
| Participants | 1200 smokers considered by GP to be highly dependent and motivated to give up average cpd 23 to 24 |
|
| Interventions | 1. Nicotine patch standard dose (15 mg/16 h for 18 weeks) 2. Nicotine patch with dose increase to 25 mg at 1 week if required 3. Placebo patch group The nicotine patch groups were further randomized to gradual tapering or abrupt withdrawal at week 12 Level of support: high (physician advice and brief support at 1, 3, 6, 12 weeks) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO | |
| Notes | The dose increase after 1 week did not affect cessation, 1 + 2 vs 3 in comparison 1 Study was funded by Kabi Pharmacia (Sweden) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer generated list, complied in blocks of six (four active, two placebo)" |
| Allocation concealment (selection bias) | Low risk | Numbered packages |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Both subjects and their doctors or nurses were blind to whether the dose increase was real or placebo". Study conduct throughout was monitored by clinical research associates of the pharmaceutical company. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analyses, with losses/failures included as smokers. Number of dropouts not specified |
Stein 2013.
| Methods | Country: USA Recruitment: methadone‐maintained treatment centres in New England | |
| Participants | 315 adult methadone‐maintained smokers, smoking 10+ cpd, willing to set a quit date within the 1st week Mean age 39.9, 47.6% female, 78.5% white, mean cpd 20, mean FTND 5.7 |
|
| Interventions | 1. Combination NRT: 24‐week course of NRT patch (42 mg for > 30 cpd, 21 mg if < 30 cpd), + ad lib nicotine gum (4 mg) as needed 2. Varenicline: 24‐week course of varenicline tablets, 1st week titrated 3. Placebo: 24‐week course of identical tablets and regimen Level of support: high (all received standardized 15‐min session of advice to quit (5As model) and made monthly visits for support and top‐up medication) |
|
| Outcomes | 7‐day PP at 6 months (continuous abstinence also reported from 2 weeks to 6 months but unclear if this was biochemically verified) Validation: CO < 8 ppm; urinary cotinine in varenicline and placebo participants claiming abstinence |
|
| Notes | New for 2017 update. Analysis uses only 1 v 3 Funding: NCI grant RO1 CA129226; MDS supported by a NIDA mid‐career investigator award K24 DA000512 Risk of bias and some data extraction from Cahill 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Quote: Participants were randomized to treatment after completing the baseline assessment". No further information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind"; research assistants were "blind to participant group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26/133 NRT and 10/45 placebo lost to follow‐up |
Sutherland 1992.
| Methods | Country: UK Recruitment: smoking cessation clinic | |
| Participants | 227 smokers, motivated to quit. Average cpd 25 to 27 | |
| Interventions | 1. Nicotine nasal spray, maximum 40 mg/day 2. Placebo spray Level of support: High (4 weeks group support) | |
| Outcomes | Sustained abstinence at 12 months Validation: CO | |
| Notes | Follow‐up beyond 1 year reported in Stapleton 1998 Study was funded by the Medical Research Council and by the Imperial Cancer Research Fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They drew a card marked A or P for allocation to active or placebo group" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Subjects and therapists were blind to spray assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up briefly reported |
Sønderskov 1997.
| Methods | Country: Denmark Recruitment: customers seeking to buy nicotine patches OTC at 42 pharmacies | |
| Participants | 522 smokers of > 10 cpd. Smokers of > 20 cpd used a higher‐dose patch than lower‐rate smokers 50% female, average age 39 | |
| Interventions | 1. Nicotine patch (24 h). > 20/day smokers used 21 mg for 4 weeks, 14 mg for 4 weeks, 7 mg for 4 weeks. Smokers of < 20/day used 14 mg for first 8 weeks, 7 mg for 4 weeks 2. Placebo patches Level of support: Low (brief instructions on patch use at baseline, visit to collect further patches at 4 and 8 weeks, no behavioural support) | |
| Outcomes | Abstinence at 6 months ‐ no reported smoking in the last 4 weeks, by telephone interview with neutral independent assessor Validation: none | |
| Notes | Study was partly funded by Ciba‐Geigy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized sequential treatment packages", stratified by smoking level |
| Allocation concealment (selection bias) | Low risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo patches contained "a pharmacologically negligible amount of nicotine". "The blinding procedure was not broken until all the main results were tabulated". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Participants lost to follow‐up (n = 19) were classified as smokers". Losses and reasons fully reported |
TNSG 1991.
| Methods | Country: USA (9 sites) Recruitment: community volunteers (treated at smoking cessation clinics) | |
| Participants | 808 unselected smokers 60% female, average age 43, average cpd 31 | |
| Interventions | 1. Nicotine patch (21 mg/24 h, 6 weeks+) 2. Nicotine patch 14 mg 3. Placebo patch Abstainers at end of week 6 entered a randomized blinded trial of weaning Level of support: high (group therapy, 6+ sessions) | |
| Outcomes | Sustained abstinence at 6 months Validation: CO | |
| Notes | 2 trials pooled and data relating to a 7 mg patch group used in only 1 trial omitted Long‐term (4 to 5 year) follow‐up data reported for 7/9 sites (Daughton 1999). Data not used in MA ‐ point estimate would be higher Study was supported by Alza Corp | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated: "patients were ... randomized", but members of same household received same assignment, with one randomly selected for inclusion in the analyses |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All participants were included in outcome evaluations except for the excluded members of couples (49 participants) and nine participants with major protocol infractions". Losses and withdrawals were included as treatment failures |
Tuisku 2016.
| Methods | Country: Finland Recruitment: community |
|
| Participants | 180 (in relevant arms) 18 to 26 years old, smoked daily for at least past month, smoked > 100 cigarettes in life, light smokers (as per Heaviness of Smoking Index based on cpd and time to first cigarette) only included in this review 52% female, median age 21, median cpd 10 |
|
| Interventions | 1. NRT patch (10 mg/16 h) for 8 weeks 2. Placebo Level of support: high (individual smoking cessation counselling of 30 mins (and planned for week 52)) |
|
| Outcomes | 7‐day PP at 6 months (Methods section also states 12 months follow‐up but results not reported) Validation: none |
|
| Notes | New for 2017 update Funding: Ministry of Social Affairs and Health, Finland; Finnish Research Foundation of the Pulmonary Disease; Finnish Medical Society Duodecim Participants were assessed as light or heavy smokers. Light smokers were randomized to placebo or 10 mg NRT patches. Heavy smokers were randomized to varenicline or 15 mg NRT patches. First comparison is eligible for inclusion in this review (NRT vs no NRT). Second comparison is not (NRT vs varenicline). Cannot combine NRT 15 mg group with 10 mg group – different populations randomized |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “After assessment… at the baseline visit, simple randomisation with a computer‐generated random list… was used to allocate study subjects into the different treatment groups” |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “The placebo patch was not identical to the nicotine patch" “the study was not conducted in a blinded manner” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts: 22/86 for placebo, 18/94 for NRT |
| Other bias | High risk | 12‐month cessation measured but not reported |
Tønnesen 1988.
| Methods | Country: Denmark Recruitment: primary care | |
| Participants | 113 low‐ to medium‐dependence smokers, motivated to quit (19 or less on Horn‐Russell scale) 56% female, average age 45, average cpd 20 60 highly‐dependent smokers 58% female, average age 45, average cpd 26 to 28 | |
| Interventions | Group A: Low/medium dependence 1. Nicotine gum (2 mg) for 16 weeks 2. Placebo Group B: High dependence 1. Nicotine gum 4 mg for 6 weeks then 2 mg 2. Nicotine gum 2 mg Level of support: high (informal group support, 6 sessions) | |
| Outcomes | Sustained abstinence at 12 months (24 months also reported) Validation: CO | |
| Notes | Group A in comparison 1 Abstinence at 24 months 17/60 vs 5/53, OR 3.8, relative effect greater than at 12 months Study was supported by AB Leo (Sweden) and H. Lundbeck A.S. (Denmark) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants stratified by dependence, then "subjects on each list were then randomly assigned to treatment in blocks of two" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Gum was packaged and produced to be indistinguishable between 2 mg, 4 mg and placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who attended 1st counselling session were included in analyses, regardless of attendance or level of gum use. Only 2/173 were lost to follow‐up |
Tønnesen 1991.
| Methods | Country: Denmark Recruitment: community volunteers | |
| Participants | 289 smokers (≥ 10 cpd) 70% female, average age 45, average cpd 22 | |
| Interventions | 1. Nicotine patch (15 mg/16 h for 12 weeks with tapering) 2. Placebo patch Level of support: high (7 clinic visits including a few minutes of advice) | |
| Outcomes | Sustained abstinence at 12 months (also reported 24 months in Tønnesen 1992, 3 years in Mikkelsen 1994) Validation: CO | |
| Notes | Classification of support corrected to high in 2008 update Study was supported in part by Kabi Pharmacia Therapeutics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were sequentially and randomly assigned to either active treatment or placebo according to a computer‐generated randomization code" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patches were packaged and labeled with consecutive numbers" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The placebo patches were identical to the active patches in appearance, packaging and labeling, but contained no nicotine" Blinding code was broken after week 26 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All who attended the 1st session were included in the analyses. Losses to follow‐up were included as smokers |
Tønnesen 1993.
| Methods | Country: Denmark Recruitment: community volunteers | |
| Participants | 286 smokers (≥ 10 cpd) 60% female, average age 39, average cpd 20 | |
| Interventions | 1. Nicotine inhaler (2 to 10/day) up to 6 months 2. Placebo inhaler Level of support: high (brief advice at 8 clinic visits, 0, 1, 2, 3, 6,12, 24, 52 weeks) | |
| Outcomes | Sustained abstinence at 12 months (from week 2, paper also reports with‐slips outcome) Validation: CO | |
| Notes | Study was supported by Kabi Pharmacia Therapeutics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization code for assignment to either active or placebo inhaler was generated by a computer program" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The placebo inhaler contained only the additive and was identical in appearance to the active inhaler". Participants were asked at 12 months to guess their assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Subjects unavailable for follow‐up were assumed to be smokers". Relapsers were dropped from the study, but were all contacted at 1 year. 6 were lost to follow‐up and 7 excluded for protocol violations |
Tønnesen 2000.
| Methods | Country: Denmark Recruitment: referrals to lung clinic | |
| Participants | 446 smokers ≥ 10 cpd 52% female, average age 49, average cpd 18 | |
| Interventions | 1. 5 mg nicotine patch (placebo) 2. 15 mg (16 h) nicotine patch for 12 weeks (up to 9 months on request) 3. Nicotine inhaler (4 to 12/day ad lib) 4. Combination, 15 mg patch and inhaler Level of support: high (Physician advice at baseline, brief (15 minute) nurse counselling at 2, 6 weeks, 3, 6, 9, 12 months) | |
| Outcomes | Sustained abstinence at 12 months, (from week 2, paper also reports PP and with‐slips rates) Validation: CO < 10 ppm at all visits | |
| Notes | In main comparison for patch vs placebo but not inhaler Study funding and support not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated list with random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not used ‐ open trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Non‐attenders or lost to follow‐up were included as smokers |
Tønnesen 2006.
| Methods | Country: Denmark Recruitment: lung clinic patients and newspaper adverts | |
| Participants | 370 smokers (at least 1 cpd) with COPD (Mean FEV1 was 56% of predicted) 52% female, average age 61, average cpd 20 (8% < 7/day), 71% had previously tried NRT | |
| Interventions | 2 x 2 factorial trial of lozenge and behavioural support 1. Nicotine sublingual tablet (2 mg), recommended dose depended on baseline cpd, from min 3 to max 40 per day 2. Placebo Level of support: high: Either 4 clinic visits (0, 2 weeks, 6, 12 months) and 6 phone calls, total time 2½ h, or 7 visits (0, 2, 4, 8, 12 weeks) and 5 calls, total 4½ h | |
| Outcomes | Sustained abstinence at 12 months (from 2 weeks) Validation: CO < 10 ppm at all visits | |
| Notes | New for 2008 update
Behavioural support arms collapsed. Both involved multiple clinic visits Study was funded by the Danish Medical Research Council, and supported by Pfizer Consumer Healthcare (Sweden) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated to one of the four treatment groups using a block randomization list at each center" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind, but no further detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up fully reported |
Tønnesen 2012.
| Methods | Country: Germany (2 sites) and Denmark (1 site) Recruitment: community volunteers |
|
| Participants | 479 adult smokers of ≥ 1 cpd, motivated to quit 56% male, average age 47, average cpd 22.7, average FTND 5.3 |
|
| Interventions | 1. Active: weeks 1 to 6: 1 to 2 sprays when participants would normally have smoked a cigarette or experienced a craving, up to 4 sprays/hour and 64 sprays/day. Tapered down weeks 7 to 12 (end of week 9 instructed to be using half as much as in weeks 1 to 6, reducing to max 4 sprays/day by week 12). Occasional use (max 4 sprays/day) permitted weeks 13 to 24. 1 mg/spray oral nicotine spray (in development, name not provided) 2. Control: placebo on same schedule Level of support: high. General written and oral advice (< 10 mins) at study start and < 3 mins at subsequent visits up to and including week 24 (9 visits total) |
|
| Outcomes | Prolonged abstinence from week 2 to 52 (also recorded AEs and prolonged abstinence to weeks 6 and 24) Validation: CO < 10 ppm |
|
| Notes | Funded by McNeil AB, Sweden Setting: smoking cessation clinics |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subject randomization list stratified by study site” |
| Allocation concealment (selection bias) | Low risk | Quote: "The supply or resupply of study medication to a subject was determined via an Interactive Voice Response System involving a dispenser pack number randomization list. Both randomization lists were computer‐generated." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double blind....The supply or resupply of study medication to a subject was determined via an Interactive Voice Response System...the placebo was identical in appearance, but contained capsaicin instead of nicotine to mimic the taste of nicotine" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar percentage lost in both groups (151/318 active, 81/161 placebo). 9% of active group and 7.5% of placebo group withdrew due to adverse events. Those not present at 52‐week follow‐up counted as smokers |
Villa 1999.
| Methods | Country: Spain Recruitment: volunteers working in a university health and safety department | |
| Participants | 47 smokers (excludes 5 who did not attend at least 2 sessions) 72% female, average age 36, cpd 24 to 26 | |
| Interventions | 1. Nicotine gum (2 mg) 2. No gum Level of support: high (8 weekly group sessions, 5 before TQD. Reduction prior to quitting) | |
| Outcomes | Abstinence at 12 months (not defined) Validation: none | |
| Notes | No placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Los participantes fueron distribuidos aleatoriamente" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
Wallstrom 2000.
| Methods | Country: Sweden Recruitment: community volunteers | |
| Participants | 247 smokers (≥ 10 cpd), motivated to quit 59% female, average age 45, average cpd 1 to 20 | |
| Interventions | 1. Nicotine sublingual tablet, 2 mg. Recommended dosage 1/h for smokers with FTND < 7, 2/h for scores ≥ 7. After 3 months treatment, tapering period of 3 months if necessary 2. Placebo tablet Level of support: high (brief 5‐mins counselling at study visits (0, 1, 2, 3, 6 weeks, 3, 6 months) | |
| Outcomes | Sustained abstinence at 12 months (from week 2, paper also reports with‐slips rates) Validation: CO < 10 ppm | |
| Notes | Study was supported by Pharmacia & Upjohn Consumer Health Care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomized... using a computer program". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All medication was dispensed by staff who were not involved in treating the subjects"; placebo tablets identical, but without nicotine and with capsaicin |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analyses were based on ITT. Losses not reported in detail |
Ward 2013.
| Methods | Country: Syria Recruitment: primary care centres (3 clinics were operated by NGOs for low‐middle income patients, 4th clinic is private) |
|
| Participants | 269 smokers (≥ 5 cpd, > 1 year) 22% female, average age 40, average cpd 28, mean FTND 5.8 |
|
| Interventions | 1. Nicotine patch, 24 h for 6 weeks. Participants who smoked ≥ 10 cpd given 2 weeks at 21 mg, 2 weeks 14 mg, 2 weeks 7 mg. Participants who smoked 5 to 9 cpd given 4 weeks 14 mg, 2 weeks 7 mg 2. Placebo on same schedule Level of support: high (3 x 30 mins individual face‐to‐face counselling plus 5 x 10‐min phone calls, from 4 days prior to TQD to 45 days post‐TQD) |
|
| Outcomes | Prolonged abstinence at 12 months Validation: CO < 10 ppm |
|
| Notes | New for 2017 update N quit extrapolated from percentages given Funding: "This work was supported by PHS grant 1R01DA024876" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified: “random permuted blocks stratified according to clinic and patient gender” |
| Allocation concealment (selection bias) | Low risk | Quote: “Allocation assignments were contained in opaque, sequentially‐numbered envelopes and were maintained in the biostatistics unit of the SCTS, a facility geographically separated from the clinics. A statistician, not otherwise involved in the trial, made each allocation after receiving a request from a cessation coordinator, prepared the treatment package, including patches, and had it delivered to the clinic. Patients, interventionists and data collectors were blind to allocation” |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “Patients, interventionists and data collectors were blind to allocation” placebo‐controlled: “62% of those on NRT correctly guessed treatment group, compared to 40% on placebo”. However, no effect detected so judged as low risk of bias for this domain |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 85% I and 79% C follow‐up at 12 months |
Wennike 2003b.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Excluded study, but contributing data on adverse events | |
Westman 1993.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 158 smokers motivated to quit (excludes 1 participant who used nicotine gum throughout) 57% female, average age 41, average cpd 30 | |
| Interventions | 1. Nicotine patch (25 mg/24 h, 6 weeks incl weaning) 2. Placebo patches Level of support: high (brief counsellor support at 3 clinic visits, 4 telephone counselling sessions, self‐help materials) | |
| Outcomes | Sustained abstinence at 6 months (from 2 weeks post‐TQD) Validation: CO < 8 ppm | |
| Notes | Study was supported by TBS Laboratories, Piscataway, NJ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Using simple randomization, the subjects were assigned to active or placebo treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "At all times, the subjects and study staff were masked to the treatment assignments". Participant blinding was assessed at week 6 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts fully reported |
Wisborg 2000.
| Methods | Country: Denmark Recruitment: volunteers, antenatal clinic | |
| Participants | 250 pregnant women who continued to smoke after 1st trimester Average age 28, average cpd 14; 43% primiparous | |
| Interventions | 1. Nicotine patch (15 mg/16 h, tapering to 10 mg, 11 weeks total) 2. Placebo patch Level of support: high. 4 x 15‐ to 20‐min sessions of midwife counselling at 0, 4,11 weeks from enrolment, and 4 weeks before expected delivery | |
| Outcomes | Abstinence at 4 weeks prior to delivery and at 1 year post‐partum (telephone interview). (Rates at 3 months post‐partum also reported) Validation: Cotinine < 26 ng/ml at 4 weeks pre‐delivery visit only | |
| Notes | First long‐term study of nicotine patch in pregnancy. Compliance with patch use was low. Only 17% of active and 8% of placebo used all patches. Data used in Analysis 5.1 from 2012 is abstinence at 4th prenatal visit rather than continuous abstinence from 2nd to 4th prenatal visit, for consistency with Coleman 2015. The effect estimate is not altered Study was funded by the Danish Cancer Society and the Department of Health, and supported by Pharmacia & Upjohn | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization. Quote: "Pharmacia & Upjohn ... generated the randomization list, supplied the patches with randomization numbers, and kept the code between patch number and the specific treatment until data collection was finished". |
| Allocation concealment (selection bias) | Low risk | Quote: "Women ... were assigned consecutive numbers on the randomization list" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Treatment status was not known by the women or the midwife throughout the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data reported, and included as smokers. Analyses were ITT |
Wittchen 2011.
| Methods | Country: Germany Recruitment: 167 primary care clinics |
|
| Participants | 467 'current regular smokers' attending primary care clinic for any reason and willing to consider treatment in next 7 days 48% male, average age 43, average cpd 20 |
|
| Interventions | 1. Minimal intervention (not used in review) 2. CBT 3. CBT + bupropion SR (9 to 12 weeks, 150 mg;1/day for first 6 days; 2/day thereafter) 4. CBT + NRT for 9 to 12 weeks, participant's choice of patch (7 mg to 52.5 mg), gum (2 or 4 mg) or spray (10 mg/ml) Level of support: high for 2, 3 and 4 (1 excluded from analysis). 4 to 5 one‐on‐one counselling sessions for 20 to 30 mins |
|
| Outcomes | Abstinence at 12 months (from EOT) Validation: none |
|
| Notes | 4 vs 2 included in primary analyses. 1 not used as results vs NRT would be confounded with CBT Participants covered all costs for pharmaceutical treatments Sponsored by the Federal Ministry of Education and Research; additional support provided by GlaxoSmithKline GmbH & Co and Pharmacia GmbH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Generated by the study center"; used to put 4 different coloured questionnaires in random order |
| Allocation concealment (selection bias) | High risk | No concealment Quote: "questionnaires were distributed consecutively to all attending patients on the target days by nurses. Thus, the assignment of patients was entirely dependent on the consecutive attendance of patients and the random assignment of a color. Doctors were not allowed to interfere with this study procedure." But numbers allocated to groups very uneven and discussion states: "Random checks of this procedure [randomization] and quality assurance tests by study monitors revealed that in some cases in the latter part of the study treatment was based on patient and physician preferences." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither participants nor providers were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar number of dropouts between groups; participants lost to follow‐up considered smokers for MA |
Zelman 1992.
| Methods | Country: USA Recruitment: community volunteers | |
| Participants | 116 smokers (excludes 10 early treatment dropouts evenly distributed across conditions) 54% female, average age 29 to 35, average cpd 25 to 27 | |
| Interventions | 1. Rapid smoking + support counselling 2. Rapid smoking + skills training 3. Nicotine gum 2 mg, average 10 pieces/day, duration not stated + skills training 4. Nicotine gum + support counselling Level of support: high (6 x 60‐ to 75‐min group sessions over 2 weeks, starting on quit day) | |
| Outcomes | Sustained abstinence at 12 months (not more than 2 consecutive days of smoking) Validation: Independent observer report | |
| Notes | No placebo. Group support variants collapsed; 3 and 4 compared to 1 and 2 Study was funded by National Institutes of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Placebos not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Early dropout rates reported, but not included in the analyses. 4 12‐month dropouts included as smokers |
AE = adverse event; ALA = American Lung Association; C = control; CBT = cognitive behavioural therapy; CO = carbon monoxide in exhaled air; cpd = cigarettes per day; COPD = chronic obstructive pulmonary disease; EOT = end of treatment; FTND = Fagerström Test for Nicotine Dependence; FTQ = Fagerström Tolerance Questionnaire; I = intervention; ITT = intention to treat; MA = meta‐analysis; RTQ = reduce‐to‐quit; OTC = over‐the‐counter; PP = point prevalence; SC = smoking cessation; TQD = target quit date
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adelman 2009 | Study of nicotine nasal spray in adolescents. 12 weeks follow‐up |
| Allen 2005 | Short‐term study of effect of nicotine patch on weight change during early abstinence |
| Allen 2011 | Trial of NRT for reduction of agitation and aggression in smokers with schizophrenia |
| Aubin 2006 | Short‐term study of the effect of different types of nicotine patch on sleep and smoking urges |
| Batra 2005 | Trial of nicotine gum for smoking reduction in people not making a quit attempt. See Cochrane Review of harm reduction interventions, Lindson‐Hawley 2016 |
| Berlin 2011 | Trial of standard NRT dosing vs dose adaptation according to salivary cotinine |
| Bock 2010 | Trial of computer software quit programme, treatment group offered free NRT. Control group could also use NRT (unsubsidized) |
| Bolliger 2000a | Trial of nicotine inhaler for smoking reduction in people not making a quit attempt. See Cochrane Review of harm reduction interventions, Lindson‐Hawley 2016 |
| Bolliger 2007 | Pilot study, not powered to detect efficacy differences between gum, inhaler and mouth spray |
| Brantmark 1973a | Double‐blind gum/placebo only for 1st week of clinic, then both groups offered active gum during 6‐month follow‐up period |
| Caldwell 2016 | All arms received pharmacotherapy |
| Carpenter 2003 | Compared 2 methods of reducing smoking. Control group also offered NRT if a quit attempt planned |
| Carpenter 2011 | Measured effect of providing NRT samples on participants not initially motivated to quit. Participants were encouraged but not required to make a practice quit attempt. Intervention participants were provided with up to 2 boxes of nicotine lozenges |
| Chan 2010 | Measured effect of counselling + 2 weeks free NRT. No data on whether control group also using NRT; unclear if outcome due to counselling or free NRT |
| Chan 2011 | Measured effect of adherence counselling as opposed to effect of NRT itself |
| Chou 2004 | Only 3 months follow‐up |
| Christen 1984 | Only 15 weeks follow‐up |
| Cohen 1989a | Primarily a trial of training dentists. Included in Cochrane Review of training of health professionals (Carson 2012) |
| Cohen 1989b | Primarily a trial of training doctors. Included in Cochrane Review of training of health professionals (Carson 2012) |
| Croghan 2007 | Provides a short‐term comparison between nicotine patch, bupropion, and combination therapy. Initial failures randomized to retreatment so no long‐term control group |
| Cummings 2011 | Compared provision of free NRT, but participants able to use additional NRT as desired |
| Dey 1999 | Compared free and paid prescription for nicotine patch. Only 14 weeks follow‐up |
| Donny 2009 | Endpoint not cessation |
| Ebbert 2009 | Study of NRT for smokeless tobacco users |
| Ebbert 2010 | Study of mailed NRT for smokeless tobacco users |
| Elan Pharm 88‐02 | No long‐term follow‐up. Long‐term follow‐up for 1 site included as Hurt 1990 |
| Elan Pharm 90‐03 | No long‐term follow‐up. Long‐term follow‐up for 1 site included as Fiore 1994a |
| Etter 2004 | Trial of a choice of NRT products for smoking reduction in people not making a quit attempt. See Cochrane Review of harm reduction interventions, Lindson‐Hawley 2016 |
| Fagerström 1993 | Endpoint withdrawal symptoms, not cessation |
| Fagerström 1997 | Short‐term cross‐over trial of different types of NRT. For 2 weeks smokers could choose a method, for other 2 they were randomly assigned to one of gum, patch, spray, inhaler or tablet. Smoking reduction assessed |
| Fagerström 2000 | Short‐term cross‐over trial comparing 2 nicotine delivery devices |
| Ferguson 2012 | Study of offer of free NRT via NHS Quitline services. Control group had access to and used free NRT and other stop‐smoking medications at high levels; study conditions were very similar for both groups |
| Finland unpublished | Only 3‐month follow‐up. Comparison of patch and nasal spray (n = 51) versus nasal spray alone (n = 50). Sustained abstinence rates 18% in each group. Used in a sensitivity analysis of combination therapies |
| Foulds 1993 | Follow‐up less than 6 months |
| Garvey 2006 | Not enough information currently available (abstract only) |
| Glover 1992 | Follow‐up less than 6 months |
| Gross 1989 | Study of weight gain. Abstinence outcomes not reported |
| Guo 2006 | Only 3 months follow‐up |
| Hajek 1999 | Follow‐up less than 6 months |
| Hanson 2003 | Follow‐up only 10 weeks; primary outcomes were withdrawal, craving, safety and compliance among adolescents |
| Haustein 2003 | Trial of nicotine gum for smoking reduction in people not making a quit attempt. See Cochrane Review of harm reduction interventions, Lindson‐Hawley 2016 |
| Hoch 2006 | Not enough information currently available (abstract only) |
| Hotham 2006 | RCT of nicotine patch as adjunct to counselling for pregnant smokers. Only 20 people in each condition, with high withdrawal and low compliance. |
| Hughes 1989b | No long‐term follow‐up, primarily a trial of the effect of instructions |
| Hurt 1995 | Analysis of prior nicotine patch studies (to determine if recovering alcoholic smokers were more nicotine‐dependent than non‐alcoholics and whether the efficacy of nicotine patch therapy was comparable) |
| Hurt 2003 | All participants received nicotine patch |
| Jarvik 1984 | Reports subgroup analysis by level of nicotine dependence. See Schneider 1983a for main outcomes |
| Jibrail 2010 | Only 12 weeks follow‐up. Study of NRT for smoking abstinence and relationship between c‐reactive protein and depressed mood during nicotine abstinence |
| Kapur 2001 | Only 12 weeks follow‐up. Trial of nicotine patch in pregnant smokers. 30 participants |
| Korberly 1999 | Insufficient data in unpublished abstracts to include |
| Kozak 1995 | Open‐label study in which smokers with higher nicotine dependence scores were given higher patch doses |
| Kras 2010 | Study of NRT and hypericum perforatum extract. Only 10 weeks follow‐up |
| Krumpe 1989 | Only 10 weeks follow‐up |
| Krupski 2016 | All arms received pharmacotherapy |
| Kupecz 1996 | Participants were randomized by month of treatment to group therapy with nicotine patch (n = 21) or gum (n = 17) |
| Landfeldt 1998 | Only 12 weeks follow‐up reported in abstract |
| Leischow 1996b | Only 10 weeks follow‐up |
| Levin 1994 | Only 9 weeks follow‐up |
| Lin 1996 | Only 8 weeks follow‐up |
| Marsh 2005 | Only 3 months follow‐up, safety study comparing 4 mg lozenge to 4 mg gum |
| McCarthy 2006 | Only 3 months follow‐up, study of withdrawal symptoms |
| McRobbie 2010 | Short‐term cross‐over study assessing withdrawal symptoms and user satisfaction |
| Meier 1990 | Short‐term follow‐up. Compared dependence individualized to standard dose patch. |
| Merz 1993 | Only 3 months follow‐up |
| Miller 2009 | 1377 low‐income smokers with quitline and subsidized NRT. Participants informed what group they would be in when first invited to participate |
| Millie 1989 | Only 2 months follow‐up |
| Minneker 1989 | Only 9 weeks follow‐up |
| Molander 2000 | Cross‐over study with 2‐day smoke‐free periods |
| Mooney 2005 | All participants used nicotine gum |
| Mulligan 1990 | Only 6 weeks follow‐up |
| Nackaerts 2009 | Insufficient data in published abstract to include (longest follow‐up reported in abstract 1m); NRT delivered for maximum 7 days |
| NCT00000437 | 3‐month follow‐up only. Thank you to Barbara Mason for confirming |
| Okuyemi 2007 | Intervention combined nicotine gum and multiple sessions of motivational interviewing |
| Oncken 2009 | Study of short‐term effects of NRT in pregnant smokers |
| Piper 2016 | All arms received pharmacotherapy |
| Pomerleau 2003 | Compared extended treatment (18 weeks) to 10‐week treatment with nicotine patch. No follow‐up beyond 18 weeks |
| Rennard 2006 | Trial of nicotine inhaler for smoking reduction in people not making a quit attempt. See Cochrane Review of harm reduction interventions, Lindson‐Hawley 2016 |
| Rey 2009 | All study participants received nicotine nasal spray. Comparison between different types of instructional guidance for dosing |
| Rigotti 2009 | Assessed effectiveness of adding NRT to rimonabant which has not been licensed for smoking cessation and results may not be generalizable |
| Roddy 2006 | Only 13 weeks follow‐up. At this point there were no quitters in either the treatment or control group. There were particularly high losses to follow‐up (64% overall) and low compliance (median duration of patch use 1 week) |
| Rose 1990 | Only 3 weeks follow‐up |
| Rubinstein 2008 | Only 12 weeks follow‐up |
| Sachs 1995 | Only 6 weeks follow‐up |
| Schlam 2016 | All arms received pharmacotherapy |
| Schneider 2004 | Short‐term cross‐over study |
| Schneider 2008 | Outcome was craving and withdrawal, not abstinence |
| Schnoll 2015 | All arms received pharmacotherapy |
| Shahab 2011 | Short‐term cross‐over trial of withdrawal symptom relief |
| Shiffman 2000a | Compared 10 and 6 weeks of patch treatment without longer follow‐up. Main outcome was craving and withdrawal |
| Shiffman 2000b | Comparison between 24‐h and 16‐h patches. Assessment of craving and abstinence over 2 weeks |
| Shiffman 2002a | Only 10 weeks follow‐up |
| Shiffman 2002b | Not a randomized trial. Compared prescription and OTC patch in different populations using different methods |
| Shiffman 2006 | Only 6 weeks follow‐up. High‐dose (35 mg) patch |
| Stapleton 2011 | Only 12 weeks follow‐up |
| Sun 2009 | Only 3 months follow‐up |
| Sussman 2004 | Presents Project EX program for adolescent tobacco use cessation. Mentions trial of nicotine gum vs herbal gum but insufficient detail provided |
| Sutherland 1999 | Only 3 months follow‐up. Comparison of patch and nasal spray (n = 104) versus patch alone (n = 138) or nasal spray alone (n = 138). Used in a sensitivity analysis of combination therapies |
| Sutherland 2005 | Only 12 weeks follow‐up |
| Sutton 1987 | Control group received no treatment so effect of nicotine gum is confounded with the brief counselling |
| Sutton 1988 | Control group received no treatment so effect of nicotine gum is confounded with the behavioural support |
| Thorsteinsson 2001 | No long‐term follow‐up reported |
| Tsukahara 2010 | Follow‐up less than 6 months. Direct comparison of varenicline and nicotine patch for smoking cessation |
| Tundulawessa 2010 | Only 4 weeks follow‐up |
| Tzivoni 1998 | Follow‐up less than 6 months |
| Tønnesen 1996 | All study participants received nicotine nasal spray. Comparison between ad lib and fixed schedule dosing |
| Uyar 2005 | Unpublished, insufficient detail in abstract on nicotine patch dose, length of treatment, level of support |
| Velicer 2006 | Participants were sent nicotine patches if they were assessed as potentially ready to quit. They did not have to set a quit date |
| Vial 2002 | Treatment groups differed from control in amount of counselling as well as use of NRT |
| Vikhireva 2003 | Trial of free choice of NRT product vs assigned NRT product from the outcome; no control group |
| Warner 2005 | Goal of intervention was relief of stress and withdrawal postoperatively |
| Wennike 2003a | Trial of nicotine gum for smoking reduction in people not making a quit attempt. See Cochrane Review of harm reduction interventions, Lindson‐Hawley 2016 |
| Williams 2007 | Only short‐term outcomes reported in conference abstract. Trial terminated early when no benefit of higher dose detected in interim analysis |
| Wiseman 2005 | 2‐week cross‐over study |
| Working Group 1994 | Follow‐up less than 6 months |
h = hour; OTC = over the counter;
Characteristics of ongoing studies [ordered by study ID]
NCT01010477.
| Trial name or title | Double‐blind, placebo‐controlled trial of nicotine nasal spray as an aid for smoking cessation in schizophrenia |
| Methods | RCT |
| Participants | 60 individuals with schizophrenia |
| Interventions | Nicotine nasal spray or placebo spray with behavioural intervention |
| Outcomes | Abstinence at 12 months |
| Starting date | August 2009 |
| Contact information | Mia H Zimmerman, hanosma@umdnj.edu |
| Notes |
NCT01484340.
| Trial name or title | A smoking cessation trial in HIV‐infected patients in South Africa (JHU) |
| Methods | RCT |
| Participants | HIV‐infected patients in South Africa |
| Interventions | 1. intensive anti‐smoking counseling + NRT (patches) 2. intensive anti‐smoking counseling only |
| Outcomes | 6‐month and 12‐month cessation, CO‐validated |
| Starting date | March 2014 |
| Contact information | Johns Hopkins University |
| Notes |
NCT02918500.
| Trial name or title | Effect of pre‐op NRT on peri‐operative complications and long‐term abstinence: a pilot trial in patients undergoing CABG surgery |
| Methods | Single site, double‐blind RCT |
| Participants | Smokers of > 5 cpd scheduled for CABG surgery |
| Interventions | 1. NRT patch 2. Placebo |
| Outcomes | Smoking cessation (CO‐validated) at time of surgery and 6 month post‐op; peri‐operative complications |
| Starting date | Oct 2017 |
| Contact information | Evyanne Wooding, ewooding@ottawaheart.ca |
| Notes |
CABG = coronary artery bypass graft; RCT = randomized controlled trial
Contributions of authors
For the most recent version of this review: JHB and SC screened studies. Data extraction and risk of bias assessment was conducted by SC, WY and JHB. The review text was updated by JHB, SC and WY with review and suggestions from all other authors.
Sources of support
Internal sources
-
Nuffield Department of Primary Care Health Sciences, University of Oxford, UK.
Editorial base for the Cochrane Tobacco Addiction Group
-
National Institute for Health Research School for Primary Care Research, UK.
Support for the Nuffiled Department of Primary Health Care Health Sciences, University of Oxford
External sources
-
National Institute for Health Research (NIHR), UK.
Infrastructure funding for the Cochrane Tobacco Addiction Group
Declarations of interest
CB was involved in a trial on pre‐cessation use of NRT (Bullen 2010)
SCC none known
JHB none known
TL none known
WY none known
Edited (no change to conclusions)
References
References to studies included in this review
Abelin 1989 {published data only}
- Abelin T, Buehler A, Muller P, Vesanen K, Imhof PR. Controlled trial of transdermal nicotine patch in tobacco withdrawal. Lancet 1989;1(8628):7‐10. [DOI] [PubMed] [Google Scholar]
- Abelin T, Ehrsam R, Buhler‐Reichert A, Imhof PR, Muller P, Thommen A. Effectiveness of a transdermal nicotine system in smoking cessation studies. Methods and Findings in Experimental and Clinical Pharmacology 1989;11(3):205‐14. [PubMed] [Google Scholar]
- Muller P, Abelin T, Ehrsam R, Imhof P, Howald H, Mauli D. The use of transdermal nicotine in smoking cessation. Lung 1990;168 Suppl:445‐53. [DOI] [PubMed] [Google Scholar]
Ahluwalia 1998 {published data only}
- Ahluwalia JS, McNagny SE, Clark WS. Smoking cessation among inner‐city African Americans using the nicotine transdermal patch. Journal of General Internal Medicine 1998;13(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahluwalia 2006 {published data only}
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 x 2 factorial design. Addiction 2006;101(6):883‐91. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Thomas JL, Guo H, An LC, Okuyemi KS, Collins TC, et al. Predictors of smoking reduction among Blacks. Nicotine & Tobacco Research 2010;12(4):423‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen NL, Mayo MS, Sanderson Cox L, Okuyemi KS, Choi WS, Kaur H, et al. Predictors of quitting among African American light smokers enrolled in a randomized, placebo‐controlled trial. Journal of General Internal Medicine 2006;21(6):590‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Cox LS, Nollen NL, Snow TM, Kaur H, Choi W, et al. Baseline characteristics and recruitment strategies in a randomized clinical trial of African‐American light smokers. American Journal of Health Promotion 2007;21(3):183‐91. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction 2007;102(12):1979‐86. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Pulvers KM, Cox LS, Thomas JL, Kaur H, Mayo MS, et al. Nicotine dependence among African American light smokers: a comparison of three scales. Addictive Behaviors 2007;32(10):1989‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Thomas JL, Warren J, Guo H, Ahluwalia JS. Relationship between smoking reduction and cessation among light smokers. Nicotine & Tobacco Research 2010;12(10):1005‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Zheng H, Guo H, Ahluwalia JS. Predictors of adherence to nicotine gum and counseling among African‐American light smokers. Journal of General Internal Medicine 2010;25(9):969‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JL, Bronars CA, Stewart DW, Okuyemi KS, Befort CA, Nazir N, et al. Psychometric properties of a brief Smoking Consequences Questionnaire for Adults (SCQ‐A) among African American light smokers. Substance Abuse 2009;30(1):14‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JR, Okuyemi KS, Guo H, Thomas JL, Ahluwalia JS. Predicting home smoking restrictions among African American light smokers. American Journal of Health Behavior 2010;34(1):110‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JR, Thomas JL, Okuyemi KS, Lindgren B, Ahluwalia JS. Development and validation of a multidimensional measure of stress among African American light smokers. Journal of the National Medical Association 2010;102(10):890‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whembolua GL, Davis JT, Reitzel LR, Guo H, Thomas JL, Goldade KR, et al. Subjective social status predicts smoking abstinence among light smokers. American Journal of Health Behavior 2012;36(5):639‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anthenelli 2016 {published and unpublished data}
- Anthenelli R, Benowitz N, West R, Aubin L, McRae T, Lawrence D, et al. Reports of suicidal ideation and behavior in the EAGLES trial. Society for Research on Nicotine and Tobacco; 2016 March 03‐05; USA. 2016; Vol. SYM5D:8.
- Anthenelli RM, Benowitz NL, West R, Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double‐blind, randomised, placebo‐controlled clinical trial. Lancet 2016; Vol. 387, issue 10037:2507‐20. [DOI: ] [DOI] [PubMed]
- Benowitz N, Evins AE, West R, Aubin L, McRae T, Lawrence D, et al. EAGLES trial: study design and neuropsychiatric safety results. Society for Research on Nicotine and Tobacco; 2016 March 03‐05; USA. 2016; Vol. SYM5B:7.
- NCT01456936. A phase 4, randomized, double blind, active and placebo controlled multicenter study evaluating the neuropsychiatric safety and efficacy of 12 weeks varenicline tartrate 1mg BID and bupropion hydrochloride 150 mg BID for smoking cessation in subjects with and without a history of psychiatric disorders [EAGLES]. clinicaltrials.gov/ct2/show/NCT01456936 (first received 21 October 2011).
- Prochaska J. Neuropsychiatric risk concerns in the context of smoking, quitting, and cessation pharmacotherapy use. Society for Research on Nicotine and Tobacco; 2016 March 03‐05; USA. 2016; Vol. SYM5A:7.
- Prochaska J, Benowitz N, West R, Anthenelli R. Evaluating adverse events in a global smoking cessation study (EAGLES): a randomized controlled trial comparing the safety and efficacy of the first‐line smoking cessation aids in smokers with and without pychiatric disorders. Society for Research on Nicotine and Tobacco; 2016 March 03‐05; USA. 2016; Vol. SYM5:7.
- West R, Benowitz N, Evins AE, Aubin L, McRae T, Lawrence D, et al. Relative efficacy of varenicline, bupropion SR, and nicotine transdermal patch in aiding smoking cessation in the EAGLES trial. Society for Research on Nicotine and Tobacco; 2016 March 03‐05. 2016; Vol. SYM5C:8.
Areechon 1988 {published data only}
- Areechon W, Punnotok J. Smoking cessation through the use of nicotine chewing gum: a double‐blind trial in Thailand. Clinical Therapeutics 1988;10:183‐6. [PubMed] [Google Scholar]
Berlin 2014 {published data only}
- Berlin I, Grange G, Jacob N, Tanguy ML. Nicotine patches in pregnant smokers: Randomised, placebo controlled, multicentre trial of efficacy. BMJ (online) 2014;348:1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster T. In pregnant smokers, the nicotine patch did not increase abstinence or birthweight more than placebo. Annals of Internal Medicine 2014;160(12):JC11. [DOI] [PubMed] [Google Scholar]
Blondal 1989 {published data only}
- Blondal T. Controlled trial of nicotine polacrilex gum with supportive measures. Archives of Internal Medicine 1989;149:1818‐21. [DOI] [PubMed] [Google Scholar]
Blondal 1997 {published data only}
- Blondal T, Franzon M, Westin A. A double‐blind randomized trial of nicotine nasal spray as an aid in smoking cessation. European Respiratory Journal 1997;10:1585‐90. [DOI] [PubMed] [Google Scholar]
- Blondal T, Olafsdottir I, Gunnarsdottir R, Franzon M, Westin A. Controlled trial of nicotine nasal spray as an aid to stopping smoking [abstract]. European Respiratory Journal 1993;6(Suppl 17):631S. [Google Scholar]
Bolliger 2000b {published data only}
- Bolliger CT. Practical experiences in smoking reduction and cessation. Addiction 2000;95(1 S1):S19‐S24. [DOI] [PubMed] [Google Scholar]
- Bolliger CT, Zellweger JP, Danielsson T, Biljon X, Robidou A, Westin A, et al. Influence of long‐term smoking reduction on health risk markers and quality of life. Nicotine & Tobacco Research 2002;4:433‐9. [DOI] [PubMed] [Google Scholar]
- Bolliger CT, Zellweger JP, Danielsson T, Biljon X, Robidou A, Westin A, et al. Smoking reduction with oral nicotine inhalers: double blind, randomised clinical trial of efficacy and safety. BMJ 2000;321(7257):329‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brantmark 1973b {published data only}
- Brantmark B, Ohlin P, Westling H. Nicotine‐containing chewing gum as an anti‐smoking aid. Psychopharmacologia 1973;31:191‐200. [DOI] [PubMed] [Google Scholar]
Br Thor Society 1983 {published data only}
- Research Committee of the British Thoracic Society. Comparison of four methods of smoking withdrawal in patients with smoking related diseases. Report by a subcommittee of the Research Committee of the British Thoracic Society. British Medical Journal (Clinical research ed.) 1983;286(6365):595‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchkremer 1988 {published data only}
- Buchkremer G, Bents H, Horstmann M, Opitz K, Tolle R. Combination of behavioral smoking cessation with transdermal nicotine substitution. Addictive Behaviors 1989;14:229‐38. [DOI] [PubMed] [Google Scholar]
- Buchkremer G, Bents H, Minneker E, Opitz K. Long‐term effects of a combination of transdermal nicotine administration with behavior therapy for smoking cessation [Langfristige Effekte einer Kombination von transdermaler Nikotinzufuhr mit Verhaltenstherapie zur Raucherentwohnung]. Nervenarzt 1988;59:488‐90. [PubMed] [Google Scholar]
Bullen 2010 {published data only}
- Bullen C, Howe C, Lin RB, Grigg M, Laugesen M, McRobbie H, et al. Pre‐cessation nicotine replacement therapy: pragmatic randomized trial. Addiction 2010;105(8):1474‐83. [DOI] [PubMed] [Google Scholar]
Campbell 1987 {published data only}
- Campbell IA, Lyons E, Prescott R. Do nicotine chewing‐gum and postal encouragement add to doctors' advice. Practitioner 1987;231:114‐7. [PubMed] [Google Scholar]
Campbell 1991 {published data only}
- Campbell IA, Prescott RJ, Tjeder‐Burton SM. Smoking cessation in hospital patients given repeated advice plus nicotine or placebo chewing gum. Respiratory Medicine 1991;85:155‐7. [DOI] [PubMed] [Google Scholar]
Campbell 1996 {published data only}
- Burton S, Campbell IA, Prescott RJ. Nicotine patches versus placebo in 235 hospital patients [abstract 191]. 8th World Conference on Tobacco or Health; 1992 Mar 30‐Apr 03; Buenos Aires, Argentina. 1992.
- Campbell IA, Prescott RJ, Tjeder‐Burton SM. Transdermal nicotine plus support in patients attending hospital with smoking‐related diseases: a placebo‐controlled study. Respiratory Medicine 1996;90:47‐51. [DOI] [PubMed] [Google Scholar]
CEASE 1999 {published data only}
- Tønnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A, et al. Higher dosage nicotine patches increase one‐year smoking cessation rates: Results from the European CEASE trial. European Respiratory Journal 1999;13:238‐46. [DOI] [PubMed] [Google Scholar]
Cinciripini 1996 {published data only}
- Cinciripini PM, Cinciripini LG, Wallfisch A, Haque W. Behavior therapy and the transdermal nicotine patch: Effects on cessation outcome, affect, and coping. Journal of Consulting and Clinical Psychology 1996;64:314‐23. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Wetter DW, Fouladi RT, Blalock JA, Carter BL, Cinciripini LG, et al. The effects of depressed mood on smoking cessation: mediation by postcessation self‐efficacy. Journal of Consulting and Clinical Psychology 2003;71:292‐301. [DOI] [PubMed] [Google Scholar]
Clavel 1985 {published data only}
- Clavel F, Benhamou S. Tobacco withdrawal. Comparison of the efficacy of various methods. Intermediate results of a comparative study [Désintoxcation tabagique. Comparison de l'efficacité de differentes méthodes. Résultats intermédiaires d'une étude comparative]. Presse Médicale 1984;13:975‐7. [PubMed] [Google Scholar]
- Clavel F, Benhamou S, Company Huertas A, Flamant R. Helping people to stop smoking: randomised comparison of groups being treated with acupuncture and nicotine gum with control group. British Medical Journal 1985;291:1538‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clavel‐Chapelon 1992 {published data only}
- Clavel‐Chapelon F, Paoletti C, Benhamou S. A randomised 2 x 2 factorial design to evaluate different smoking cessation methods. Revue d'Epidemiologie et de Sante Publique 1992;40:187‐90. [PubMed] [Google Scholar]
Coleman 2012 {published data only}
- Bowker KA, Lewis S, Coleman T, Vaz LR, Cooper S. Comparison of cotinine levels in pregnant women while smoking and when using nicotine replacement therapy. Nicotine & Tobacco Research 2014;16(6):895‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K Britton J, et al. A randomized trial of nicotine‐replacement therapy patches in pregnancy. The New England Journal of Medicine 2012;366(9):808‐19. [DOI] [PubMed] [Google Scholar]
- Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K, Britton J, et al. A randomized trial of nicotine‐replacement therapy patches in pregnancy. Obstetrical & Gynecological Survey 2012;67(7):387‐8. [Google Scholar]
- Coleman T, Thornton J, Britton J, Lewis S, Watts K, Coughtrie MWH, et al. Protocol for the Smoking, Nicotine and Pregnancy (SNAP) trial: Double‐blind, placebo‐randomised, controlled trial of nicotine replacement therapy in pregnancy. BMC Health Services Research 2007;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Lewis S, Thornton JG, Marlow N, Watts K, Britton J, et al. The SNAP trial: a randomised placebo‐controlled trial of nicotine replacement therapy in pregnancy‐‐clinical effectiveness and safety until 2 years after delivery, with economic evaluation. Health Technology Assessment (Winchester, England) 2014;18(54):1‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Taggar J, Lewis S, Marlow N, Dickinson A, Whitemore R, et al. Effect of nicotine patches in pregnancy on infant and maternal outcomes at 2 years: follow‐up from the randomised, double‐blind, placebo‐controlled SNAP trial [Erratum appears in Lancet Respiratory Medicine. 2014 Nov;2(11):e22]. Lancet Respiratory Medicine 2014;2(9):728‐37. [DOI] [PubMed] [Google Scholar]
- Essex HN, Parrott S, Wu Q, Li J, Cooper S, Coleman T. Cost‐effectiveness of nicotine patches for smoking cessation in pregnancy: a placebo randomized controlled trial (SNAP). Nicotine & Tobacco Research 2015;17(6):636‐42. [DOI] [PubMed] [Google Scholar]
- Grainge M, Coleman T, Cooper S, Thornton J, Watts K, Britton J, et al. Double‐blind, placebo‐controlled, randomised trial of nicotine replacement therapy patches in pregnancy. Journal of Perinatal Medicine 2011;39:Suppl:172. [Google Scholar]
- Thornton JG, Coleman T, Britton J, Cooper S, Watts K, Lewis S, et al. The smoking, nicotine and pregnancy (SNAP) trial: Main results. Archives of Disease in Childhood: Fetal and Neonatal Edition 2011;96:Fa109. [Google Scholar]
- Vaz LR, Aveyard P, Cooper S, Leonardi‐Bee J, Coleman T, Watts K, et al. The association between treatment adherence to nicotine patches and smoking cessation in pregnancy: a secondary analysis of a randomized controlled trial. Nicotine & Tobacco Research 2016;18(10):1952‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz LR, Coleman T, Cooper S, Aveyard P, Leonardi‐Bee J, Snap trial team. The nicotine metabolite ratio in pregnancy measured by trans‐3'‐hydroxycotinine to cotinine ratio: characteristics and relationship with smoking cessation. Nicotine & Tobacco Research 2015;17(11):1318‐23. [DOI] [PubMed] [Google Scholar]
- Vaz LR, Leonardi‐Bee J, Aveyard P, Cooper S, Grainge M, Coleman T. Factors associated with smoking cessation in early and late pregnancy in the smoking, nicotine, and pregnancy trial: a trial of nicotine replacement therapy. Nicotine & Tobacco Research 2014;16(4):381‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2005 {published data only}
- Cooper TV, Klesges RC, Debon MW, Zbikowski SM, Johnson KC, Clemens LH. A placebo controlled randomized trial of the effects of phenylpropanolamine and nicotine gum on cessation rates and postcessation weight gain in women. Addictive Behaviors 2005;30:61‐75. [DOI] [PubMed] [Google Scholar]
- Cooper TV, Montgomery GV, Debon MW, Zbikowski SM, Klesges RC, Johnson KC. The effects of PPA and nicotine gum on cessation rates and post cessation weight gain in women [POS3‐46]. Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003; New Orleans, LA. 2003.
Cummins 2016 {published data only}
- Cummins S, Zhu SH, Gamst A, Kirby C, Brandstein K, Klonoff‐Cohen H, et al. Nicotine patches and quitline counseling to help hospitalized smokers stay quit: study protocol for a randomized controlled trial. Trials 2012;13(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins SE, Gamst AC, Brandstein K, Seymann GB, Klonoff‐Cohen H, Kirby CA, et al. Helping hospitalized smokers: a factorial RCT of nicotine patches and counseling. American Journal of Preventive Medicine 2016;51(4):578‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunningham 2016 {published data only}
- Cunningham JA, Kushnir V, Selby P, Tyndale RF, Zawertailo L, Leatherdale ST. Effect of mailing nicotine patches on tobacco cessation among adult smokers: a randomized clinical trial. JAMA Internal Medicine 2016;176(2):184‐90. [DOI] [PubMed] [Google Scholar]
- Kushnir V, Sproule BA, Cunningham JA. Mailed distribution of free nicotine patches without behavioral support: Predictors of use and cessation. Addictive Behaviors 2017;67:73‐8. [DOI] [PubMed] [Google Scholar]
- Kushnir V, Sproule BA, Zawertailo L, Selby P, Tyndale RF, Leatherdale ST, et al. Impact of self‐reported lifetime depression or anxiety on effectiveness of mass distribution of nicotine patches. Tobacco Control 2016;26(5):526‐33. [DOI] [PubMed] [Google Scholar]
- Wise J. Mailing free nicotine patches to smokers reduces smoking rates, study shows. BMJ (Clinical research ed.) 2016;352:i468. [DOI] [PubMed] [Google Scholar]
Daughton 1991 {published data only}
- Daughton DM, Heatley SA, Prendergast JJ, Causey D, Knowles M, Rolf CN, et al. Effect of transdermal nicotine delivery as an adjunct to low‐intervention smoking cessation therapy. A randomized, placebo‐controlled, double‐blind study. Archives of Internal Medicine 1991;151:749‐52. [PubMed] [Google Scholar]
- Daughton DM, Heatley SA, Prendergast JJ, Causey D, Knowles M, Rolf CN, et al. Effects of transdermal nicotine as an adjunct in smoking cessation therapy. A double‐blind randomized study controlled with placebo [Effetti del rilascio transdermico di nicotina come terapia di supporto per lo svezzamento dal fumo di sigaretta. Uno studio randomizzato in doppio cieco con controlli trattati con placebo.]. Archivio Monaldi 1992;47:17‐29. [PubMed] [Google Scholar]
Daughton 1998 {published data only}
- Daughton D, Susman J, Sitorius M, Belenky S, Millatmal T, Nowak R, et al. Transdermal nicotine therapy and primary care. Importance of counseling, demographic, and participant selection factors on 1‐year quit rates. The Nebraska Primary Practice Smoking Cessation Trial Group. Archives of Family Medicine 1998;7:425‐30. [DOI] [PubMed] [Google Scholar]
Dautzenberg 2001 {published and unpublished data}
- Dautzenberg B, Peiffer G, Toulouse F, Yvinec MJ, Jacob N, Kienzler JL. Randomized trial assessment of nicotinell lozenge 1mg, a new oral nicotine replacement therapy. Society for Research on Nicotine and Tobacco 3rd European Conference; 2001; Paris, France. 2001:55.
- Dautzenberg B, Ruff F, Vaucher M, Maillon P, Jacob N, Kienzler JL, et al. First demonstration of the good efficacy/safety ratio of Nicotinell® 1mg Lozenge (NL 1mg), a new form of nicotine substitution, by randomised clinical trial. European Respiratory Journal 2001;18 Suppl 33:12s. [Google Scholar]
Davidson 1998 {published data only}
- Davidson M, Epstein M, Burt R, Schaefer C, Whitworth G, McDonald A. Efficacy and safety of an over‐the‐counter transdermal nicotine patch as an aid for smoking cessation. Archives of Family Medicine 1998;7:569‐74. [DOI] [PubMed] [Google Scholar]
Ehrsam 1991 {published data only}
- Abelin T, Ehrsam R, Buhler‐Reichert A, Imhof PR, Muller P, Thommen A, et al. Effectiveness of a transdermal nicotine system in smoking cessation studies. Methods and Findings in Experimental and Clinical Pharmacology 1989;11:205‐14. [PubMed] [Google Scholar]
- Abelin T, Ehrsam R, Imhof P, Muller P, Howald H. Clinical experience with a transdermal nicotine system in healthy nicotine‐dependent smokers. In: Wilhemsen L editor(s). Smoking as a Cardiovascular Risk Factor ‐ New Strategies for Smoking Cessation. Hogrefe & Huber, 1991:35‐46. [Google Scholar]
- Ehrsam RE, Buhler A, Muller P, Mauli D, Schumacher PM, Howald H, et al. Weaning of young smokers using a transdermal nicotine patch [Entwohnung junger Raucher mit Hilfe eines transdermalen Nikotinpflasters]. Schweizerische Rundschau fur Medizin Praxis 1991;80:145‐50. [PubMed] [Google Scholar]
- Muller P, Abelin T, Ehrsam R, Imhof P, Howald H, Mauli D. The use of transdermal nicotine in smoking cessation. Lung 1990;168:445‐53. [DOI] [PubMed] [Google Scholar]
El‐Mohandes 2013 {published data only}
- El‐Mohandes AA, Windsor R, Tan S, Perry DC, Gantz MG, Kiely M. A randomized clinical trial of trans‐dermal nicotine replacement in pregnant African‐American smokers. Maternal and Child Health Journal 2013;17(5):897‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fagerström 1982 {published data only}
- Fagerström KO. A comparison of psychological and pharmacological treatment in smoking cessation. Journal of Behavioral Medicine 1982;5:343‐51. [DOI] [PubMed] [Google Scholar]
- Fagerström KO. Tolerance, withdrawal and dependence on tobacco and smoking termination. International Review of Applied Psychology 1983;32:29‐52. [Google Scholar]
Fagerström 1984 {published data only}
- Fagerström KO. Effects of nicotine chewing gum and follow‐up appointments in physician‐based smoking cessation. Preventive Medicine 1984;13:517‐27. [DOI] [PubMed] [Google Scholar]
Fee 1982 {published data only}
- Fee WM, Stewart MJ. A controlled trial of nicotine chewing gum in a smoking withdrawal clinic. Practitioner 1982;226:148‐51. [PubMed] [Google Scholar]
Fiore 1994a {published data only}
- Elan Pharmaceutical Research Corp. NDA 19‐983 for Approval of PROSTEP. Study 90‐03 1992.
- Fiore MC, Kenford SL, Jorenby DE, Wetter DW, Smith SS, Baker TB. Two studies of the clinical effectiveness of the nicotine patch with different counseling treatments. Chest 1994;105:524‐33. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA 1994;271:589‐94. [DOI] [PubMed] [Google Scholar]
Fiore 1994b {published data only}
- Fiore MC, Kenford SL, Jorenby DE, Wetter DW, Smith SS, Baker TB. Two studies of the clinical effectiveness of the nicotine patch with different counseling treatments. Chest 1994;105:524‐33. [DOI] [PubMed] [Google Scholar]
Fortmann 1995 {published data only}
- Fortmann SP, Killen JD. Nicotine gum and self‐help behavioral treatment for smoking relapse prevention ‐ resuIts from a trial using population‐based recruitment. Journal of Consulting and Clinical Psychology 1995;63:460‐8. [DOI] [PubMed] [Google Scholar]
Fraser 2014 {published data only}
- Fraser D, Kobinsky K, Smith SS, Kramer J, Theobald WE, Baker TB. Five population‐based interventions for smoking cessation: a MOST trial. Translational Behavioral Medicine 2014;4(4):382‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 2007 {published data only}
- Gallagher SM, Penn PE, Schindler E. Smoking cessation in persons with schizophrenia and other serious mental illness (PA1‐5). Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006; Orlando, Florida, USA. 2006.
- Gallagher SM, Penn PE, Schindler E, Layne W. A comparison of smoking cessation treatments for persons with schizophrenia and other serious mental illnesses. Journal of Psychoactive Drugs 2007;39(4):487‐97. [DOI] [PubMed] [Google Scholar]
García 1989 {published data only}
- Quílez García C, Hernando Arizaleta L, Rubio Díaz A, Estruch Riba J, Fornés Ramis MV. Treatment for smoking with nicotine gum in primary care. A double blind trial [Tratamiento del tabaquismo, con chicle de nicotina, en atencion primaria. Estudio a doble ciego]. Revista Clinica Espanola 1993;192(4):157‐61. [PubMed] [Google Scholar]
- Quílez García C, Hernando Arizaleta L, Rubio Díaz A, Granero Fernández EJ, Vila Coll MA, Estruch Riba JSO. Double‐blind study of the efficacy of nicotine chewing gum for smoking cessation in the primary care setting [Estudio doble ciego de la eficacia del chicle de nicotina en la deshabituacion tabaquica dentro del ambito de la atencion primaria]. Atencion Primaria 1989;6(10):719‐26. [PubMed] [Google Scholar]
Garvey 2000 {published data only}
- Doherty K, Militello FS, Kinnunen T, Garvey AJ. Nicotine gum dose and weight gain after smoking cessation. Journal of Consulting and Clinical Psychology 1996;64:799‐807. [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Rohay JM, Gitchell JG, Garvey AJ. Effect of compliance with nicotine gum dosing on weight gained during a quit attempt. Addiction 2011;106:651‐6. [DOI] [PubMed] [Google Scholar]
- Garvey AJ, Kinnunen T, Nordstrom BL, Utman CH, Doherty K, Rosner B, et al. Effects of nicotine gum dose by level of nicotine dependence. Nicotine & Tobacco Research 2000;2:53‐63. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation ‐ characteristics of depressed smokers and effects of nicotine replacement. Journal of Consulting and Clinical Psychology 1996;64:791‐98. [DOI] [PubMed] [Google Scholar]
- Nordstrom BL, Kinnunen T, Utman CH, Garvey AJ. Long‐term effects of nicotine gum on weight gain after smoking cessation. Nicotine & Tobacco Research 1999;1:259‐68. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Sembower MA, Rohay JM, Gitchell JG, Garvey AJ. Assigning dose of nicotine gum by time to first cigarette. Nicotine & Tobacco Research 2013;15(2):407‐12. [DOI] [PubMed] [Google Scholar]
Gilbert 1989 {published data only}
- Gilbert JR, Wilson DM, Best JA, Taylor DW, Lindsay EA, Singer J, et al. Smoking cessation in primary care. A randomized controlled trial of nicotine‐bearing chewing gum. Journal of Family Practice 1989;28:49‐55. [PubMed] [Google Scholar]
Glavas 2003a {published data only}
- Glavas D, Rumboldt M, Rumboldt Z. Smoking cessation with nicotine replacement therapy among health care workers: randomized double‐blind study. Croatian Medical Journal 2003;44:219‐24. [PubMed] [Google Scholar]
Glavas 2003b {published data only}
- Glavas D, Rumboldt Z. Smoking cessation using the transdermal nicotine system [Odvikavanje od pusenja transdermalnim nikotinskim sustavom]. Lijecnicki Vjesnik 2003;125(1‐2):8‐12. [PubMed] [Google Scholar]
Glover 2002 {published data only}
- Glover E, Glover P, Franzon M, Sullivan R, Cerullo C. Safety and efficacy of a nicotine sublingual tablet for smoking cessation [abstract]. Smoke Free 21st Century: 2nd European Conference on Tobacco or Health; 1999; Las Palmas de Gran Canaria. 1999.
- Glover ED, Franzon M, Sullivan CR, Cerullo CL. A nicotine sublingual tablet for treating tobacco dependence [Abstract]. Society for Research on Nicotine and Tobacco 3rd Europe Conference; 2001 September; Paris, France. 2001:48.
- Glover ED, Glover PN, Franzon M, Sullivan CR, Cerullo CC, Howell RM, et al. A comparison of a nicotine sublingual tablet and placebo for smoking cessation. Nicotine & Tobacco Research 2002;4:441‐50. [DOI] [PubMed] [Google Scholar]
- Glover ED, Glover PN, Franzon M, Sullivan R, Sullivan P, Howell R, et al. A nicotine sublingual tablet for smoking cessation: 6‐month data [Abstract]. 10th World Conference on Tobacco or Health; 1997 August 24‐28; Beijing, China. 1997.
Gourlay 1995 {published data only}
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. BMJ 1995;311(7001):363‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham 2017 {published data only}
- Graham AL, Cha S, Papandonatos GD, Cobb NK, Mushro A, Fang Y, et al. Improving adherence to web‐based cessation programs: a randomized controlled trial study protocol. Trials 2013;14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Papandonatos GD, Cha S, Erar B, Amato MS. Improving adherence to smoking cessation treatment: smoking outcomes in a web‐based randomized trial. Annals of Behavioral Medicine 2018;52(4):331‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Papandonatos GD, Cha S, Erar B, Amato MS, Cobb NK, et al. Improving adherence to smoking cessation treatment: Intervention effects in a web‐based randomized trial. Nicotine & Tobacco Research 2017;19(3):324‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gross 1995 {published data only}
- Gross J, Johnson J, Sigler L, Stitzer ML. Dose effects of nicotine gum. Addictive Behaviors 1995;20:371‐81. [DOI] [PubMed] [Google Scholar]
Hall 1985 {published data only}
- Hall SM, Tunstall C, Rugg D, Jones R, Benowitz N. Nicotine gum and behavioral treatment in smoking cessation. Journal of Consulting and Clinical Psychology 1985;53:256‐8. [DOI] [PubMed] [Google Scholar]
Hall 1987 {published data only}
- Hall SM, Tunstall CD, Ginsberg D, Benowitz NL, Jones RT. Nicotine gum and behavioral treatment: a placebo controlled trial. Journal of Consulting and Clinical Psychology 1987;55:603‐5. [DOI] [PubMed] [Google Scholar]
Hall 1996 {published data only}
- Hall SM, Muñoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, et al. Mood management and nicotine gum in smoking treatment ‐ a therapeutic contact and placebo‐controlled study. Journal of Consulting and Clinical Psychology 1996;64(5):1003‐9. [DOI] [PubMed] [Google Scholar]
Hand 2002 {published data only}
- Hand S, Edwards S, Campbell IA, Cannings R. Controlled trial of three weeks nicotine replacement treatment in hospital patients also given advice and support. Thorax 2002;57:715‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harackiewicz 1988 {published data only}
- Harackiewicz JM, Blair LW, Sansone C, Epstein JA, Stuchell RN. Nicotine gum and self‐help manuals in smoking cessation: an evaluation in a medical context. Addictive Behaviors 1988;13:319‐30. [DOI] [PubMed] [Google Scholar]
Hasan 2014 {published data only}
- Hasan FM, Zagarins SE, Pischke KM, Saiyed S, Bettencourt AM, Beal L, et al. Hypnotherapy is more effective than nicotine replacement therapy for smoking cessation: results of a randomized controlled trial. Complementary Therapies in Medicine 2014;22(1):1‐8. [DOI] [PubMed] [Google Scholar]
Hays 1999 {published data only}
- Hays JT, Croghan GA, Offord KP, Hurt RD, Schroeder DR, Wolter TD, et al. Over‐the‐counter nicotine patch therapy for smoking cessation: Results from randomized, double‐blind, placebo‐controlled and open label trials. American Journal of Public Health 1999;89:1701‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays JT, Croghan GA, Offord KP, Wolter TD, Nides MA, Davidson M. Over‐the‐Counter (OTC) transdermal nicotine patch therapy [abstract]. Journal of Addictive Diseases 1997;16:136. [Google Scholar]
- Hays JT, Croghan IT, Offord KP, Hurt RD, Schroeder DR, Wolter TD, et al. Over the counter 22mg nicotine patch therapy for smoking cessation: results from randomized double‐blind placebo‐controlled and open label trials. Society for Research on Nicotine and Tobacco 5th Annual Meeting; 1999; San Diego, CA, USA 1999. [DOI] [PMC free article] [PubMed]
Herrera 1995 {published data only}
- Herrera N, Franco R, Herrera L, Partidas A, Rolando R, Fagerström KO. Nicotine gum, 2 and 4 mg, for nicotine dependence. A double‐blind placebo‐controlled trial within a behavior modification support program. Chest 1995;108:447‐51. [DOI] [PubMed] [Google Scholar]
Heydari 2012 {published data only}
- Heydari G, Talischi F, Tafti SF, Masjedi MR. Quitting smoking with varenicline: parallel, randomised efficacy trial in Iran. International Journal of Tuberculosis and Lung Disease 2012;16(2):268‐72. [CENTRAL: 814663; CRS: 9400123000012712; EMBASE: 2012026893; PUBMED: 22236931] [DOI] [PubMed] [Google Scholar]
Heydari 2013 {published data only}
- Heydari G, Talischi F, Batmanghelidj E, Pajooh MR, Boroomand A, Zamani M, et al. Dual addictions, parallel treatments: Nicotine replacement therapy for patients receiving methadone treatment in the Islamic Republic of Iran. La Revue de Santé de la Mediterranée Orientale / al‐Majallah al‐sihhiyah li‐sharq al‐mutawassit (Eastern Mediterranean Health Journal) 2013;19(SUPPL 3):S25‐31. [PubMed] [Google Scholar]
Hjalmarson 1984 {published data only}
- Hjalmarson AI. Effect of nicotine chewing gum in smoking cessation. A randomized, placebo‐controlled, double‐blind study. JAMA 1984;252:2835‐8. [PubMed] [Google Scholar]
Hjalmarson 1994 {published data only}
- Hjalmarson AI, Franzon M, Westin A, Wiklund O. Effect of nicotine nasal spray on smoking cessation. A randomized, placebo‐controlled, double‐blind study. Archives of Internal Medicine 1994;154:2567‐72. [PubMed] [Google Scholar]
Hjalmarson 1997 {published data only}
- Hjalmarson A, Nilsson F, Sjöström L, Wiklund O. The nicotine inhaler in smoking cessation. Archives of Internal Medicine 1997;157(15):1721‐8. [PubMed] [Google Scholar]
Huber 1988 {published data only}
- Huber D. Combined and separate treatment effects of nicotine chewing gum and self‐control method. Pharmacopsychiatry 1988;21:461‐2. [DOI] [PubMed] [Google Scholar]
Hughes 1989a {published data only}
- Hughes JR, Gust SW, Keenan R, Fenwick JW, Skoog K, Higgins ST. Long‐term use of nicotine vs placebo gum. Archives of Internal Medicine 1991;151:1993‐8. [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Keenan RM, Fenwick JW, Healey ML. Nicotine vs placebo gum in general medical practice. JAMA 1989;261:1300‐5. [PubMed] [Google Scholar]
Hughes 1990 {published data only}
- Hughes JR, Gust SW, Keenan RM, Fenwick JW. Effect of dose on nicotine's reinforcing, withdrawal‐suppression and self‐reported effects. Journal of Pharmacology and Experimental Therapeutics 1990;252:1175‐83. [PubMed] [Google Scholar]
Hughes 1999 {published data only}
- Hughes JR, Lesmes GR, Hatsukami DK, Richmond RL, Lichtenstein E, Jorenby DE, et al. Are higher doses of nicotine replacement more effective for smoking cessation?. Nicotine & Tobacco Research 1999;1:169‐74. [DOI] [PubMed] [Google Scholar]
Hughes 2003 {published data only}
- Hughes JR, Novy P, Hatsukami DK, Jensen J, Callas PW. Efficacy of nicotine patch in smokers with a history of alcoholism. Alcoholism‐Clinical and Experimental Research 2003;27:946‐54. [DOI] [PubMed] [Google Scholar]
Hurt 1990 {published data only}
- Elan Pharmaceutical Research Corp. NDA 19‐983 for Approval of PROSTEP. Study 88‐02 1992.
- Hurt RD, Lauger GG, Offord KP, Kottke TE, Dale LC. Nicotine‐replacement therapy with use of a transdermal nicotine patch‐a randomized double‐blind placebo‐controlled trial. Mayo Clinic Proceedings 1990;65:1529‐37. [DOI] [PubMed] [Google Scholar]
Hurt 1994 {published data only}
- Hurt RD, Dale LC, Fredrickson PA, Caldwell CC, Lee GA, Offord KP, et al. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow‐up: One‐year outcome and percentage of nicotine replacement. JAMA 1994;271:595‐600. [PubMed] [Google Scholar]
ICRF 1994 {published data only}
- David SP, Munafó MR, Murphy MF, Walton RT, Johnstone EC. The serotonin transporter 5‐HTTLPR polymorphism and treatment response to nicotine patch: follow‐up of a randomized controlled trial. Nicotine & Tobacco Research 2007;9(2):225‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial Cancer Research Fund General Practice Research Group. Effectiveness of a nicotine patch in helping people stop smoking: results of a randomised trial in general practice.. BMJ 1993;306:1304‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial Cancer Research Fund General Practice research Group. Randomised trial of nicotine patches in general practice: results at one year.. BMJ 1994;308:1476‐7. [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Yudkin Pl, Hey K, Roberts SJ, Welch SJ, Murphy MF, et al. Genetic variation in dopaminergic pathways and short‐term effectiveness of the nicotine patch. Pharmacogenetics 2004;14(2):83‐90. [DOI] [PubMed] [Google Scholar]
- Yudkin P, Hey K, Roberts S, Welch S, Murphy M, Walton R. Abstinence from smoking eight years after participation in randomised controlled trial of nicotine patch. BMJ 2003;327(7405):28‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin P, Munafó M, Hey K, Roberts S, Welch S, Johnstone E, et al. Effectiveness of nicotine patches in relation to genotype in women versus men: randomised controlled trial. BMJ 2004;328(7446):989‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamrozik 1984 {published data only}
- Jamrozik K, Fowler G, Vessey M, Wald N. Placebo controlled trial of nicotine chewing gum in general practice. British Medical Journal 1984;289:794‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarvis 1982 {published data only}
- Jarvis MJ, Raw M, Russell MAH, Feyerabend C. Randomised controlled trial of nicotine chewing‐gum. British Medical Journal 1982;285:537‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 1991 {published data only}
- Jensen EJ, Schmidt E, Pedersen B, Dahl R. Effect of nicotine, silver acetate, and ordinary chewing gum in combination with group counselling on smoking cessation. Thorax 1990;45:831‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EJ, Schmidt E, Pedersen B, Dahl R. Effect on smoking cessation of silver acetate, nicotine and ordinary chewing gum. Influence of smoking history. Psychopharmacology (Berl) 1991;104:470‐4. [DOI] [PubMed] [Google Scholar]
- Jensen EJ, Schmidt E, Pedersen B, Dahl R. The effect of nicotine, silver acetate, and placebo chewing gum on the cessation of smoking. The influence of smoking type and nicotine dependence. International Journal of the Addictions 1991;26:1223‐31. [DOI] [PubMed] [Google Scholar]
Johns 2017 {published data only}
- Johns D. Randomised controlled trial comparing nicotine replacement therapy (nrt) and counselling on smoking cessation in patients prone to lung cancer using bed font micro‐smokerlyzer. International MASCC/ISOO Symposium: Supportive Care in Cancer; 2017; USA. 2017:S102.
Jorenby 1999 {published data only}
- Durcan MJ, White J, Jorenby DE, Fiore MC, Rennard SI, Leischow SJ, et al. Impact of prior nicotine replacement therapy on smoking cessation efficacy. American Journal of Health Behaviors 2002;26:213‐20. [DOI] [PubMed] [Google Scholar]
- Jamerson BD, Nides M, Jorenby DE, Donahue R, Garrett P, Johnston JA, et al. Late‐term smoking cessation despite initial failure: an evaluation of bupropion sustained release, nicotine patch, combination therapy, and placebo. Clinical Therapeutics 2001;23:744‐52. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained‐release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine 1999;340:685‐91. [DOI] [PubMed] [Google Scholar]
Joseph 1996 {published data only}
- Joseph AM, Antonnucio DO. Lack of efficacy of transdermal nicotine in smoking cessation. New England Journal of Medicine 1999;341:1157‐8. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Norman SM, Ferry LH, Prochazka AV, Westman EC, Steele BG, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. New England Journal of Medicine 1996;335:1792‐8. [DOI] [PubMed] [Google Scholar]
Killen 1984 {published data only}
- Killen JD, Maccoby N, Taylor CB. Nicotine gum and self‐regulation training in smoking relapse prevention. Behavior Therapy 1984;15:234‐48. [Google Scholar]
Killen 1990 {published data only}
- Fortmann SP, Killen JD, Telch MJ, Newman B. Minimal contact treatment for smoking cessation. A placebo controlled trial of nicotine polacrilex and self‐directed relapse prevention: initial results of the Stanford Stop Smoking Project. JAMA 1988;260:1575‐80. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Newman B, Varady A. Evaluation of a treatment approach combining nicotine gum with self‐guided behavioral treatments for smoking relapse prevention. Journal of Consulting and Clinical Psychology 1990;58:85‐92. [DOI] [PubMed] [Google Scholar]
Killen 1997 {published data only}
- Bailey SR, Fong DM, Bryson SW, Fortmann SP, Killen JD. Perceived drug assignment and treatment outcome in smokers given nicotine patch therapy. Journal of Substance Abuse Treatment 2010;32:150‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Davis L, Varady A. Nicotine patch and self‐help video for cigarette smoking cessation. Journal of Consulting and Clinical Psychology 1997;65:663‐72. [DOI] [PubMed] [Google Scholar]
Kornitzer 1995 {published data only}
- Kornitzer M, Boutsen M, Dramaix M, Thijs J, Gustavsson G. Combined use of nicotine patch and gum in smoking cessation: a placebo‐controlled clinical trial. Preventive Medicine 1995;24:41‐7. [DOI] [PubMed] [Google Scholar]
- Kornitzer M, Boutsen M, Thijs J, Gustavsson G. Efficiency and safety of combined use of nicotine patches and nicotine gum in smoking cessation: a placebo controlled double‐blind trial [abstract]. European Respiratory Journal 1993;6(Suppl 17):630S. [Google Scholar]
Kralikova 2009 {published and unpublished data}
- Kralikova E, Kozak J, Rasmussen T, Cort N. The clinical benefits of NRT‐supported smoking reduction. Nicotine & Tobacco Research 2002;4:243. [Google Scholar]
- Kralikova E, Kozak JT, Rasmussen T, Gustavsson G, Houezec J. Smoking cessation or reduction with nicotine replacement therapy: a placebo‐controlled double blind trial with nicotine gum and inhaler. BMC Public Health 2009;9:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leischow 1996a {published data only}
- Leischow SJ, Nilsson F, Franzon M, Hill A, Otte P, Merikle EP. Efficacy of the nicotine inhaler as an adjunct to smoking cessation. American Journal of Health Behavior 1996;20:364‐71. [Google Scholar]
Lerman 2015 {published data only}
- Lerman C, Schnoll RA, Hawk LW Jr, Cinciripini P, George TP, Wileyto EP, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double‐blind placebo‐controlled trial. Lancet Respiratory Medicine 2015;3(2):131‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin RA, Ashare RL, Schnoll RA, Cinciripini PM, Hawk LW Jr, Lerman C, et al. Does cannabis use moderate smoking cessation outcomes in treatment‐seeking tobacco smokers? Analysis from a large multi‐center trial. American Journal on Addictions/American Academy of Psychiatrists in Alcoholism and Addictions 2016;25(4):291‐6. [DOI] [PubMed] [Google Scholar]
Lewis 1998 {published data only}
- Lewis SF, Piasecki TM, Fiore MC, Anderson JE, Baker TB. Transdermal nicotine replacement for hospitalized patients: A randomized clinical trial. Preventive Medicine 1998;27(2):296‐303. [DOI] [PubMed] [Google Scholar]
Llivina 1988 {published data only}
- Salvador Llivina T, Marin Tuya D, Gonzalez Quintana J, Iniesta Torres C, Castellvi Barrera E, Muriana Saez C, et al. Treatment of smoking: efficacy of the use of nicotine chewing gum. Double‐blind study [Tratamiento del tabaquismo: eficacia de la utilizacion del chicle de nicotina. Estudio a doble ciego]. Medicina Clinica Barcelona 1988;90:646‐50. [PubMed] [Google Scholar]
Malcolm 1980 {published data only}
- Malcolm RE, Sillett RW, Turner JA, Ball KP. The use of nicotine chewing gum as an aid to stopping smoking. Psychopharmacology Series 1980;70:295‐6. [DOI] [PubMed] [Google Scholar]
McGovern 1992 {published data only}
- McGovern PG, Lando HA. An assessment of nicotine gum as an adjunct to Freedom from Smoking cessation clinics. Addictive Behaviors 1992;17:137‐47. [DOI] [PubMed] [Google Scholar]
Molyneux 2003 {published data only}
- Molyneux A, Lewis S, Leivers U, Anderton A, Antoniak M, Brackenridge A, et al. Clinical trial comparing nicotine replacement therapy (NRT) plus brief counselling, brief counselling alone, and minimal intervention on smoking cessation in hospital inpatients. Thorax 2003;58(6):484‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moolchan 2005 {published data only}
- Collins CC, Epstein DH, Parzynski CS, Zimmerman D, Moolchan ET, Heishman SJ. Puffing behavior during the smoking of a single cigarette in tobacco‐dependent adolescents. Nicotine & Tobacco Research 2010;12:164‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken FH, Pickworth WB, Epstein DH, Moolchan ET. Smoking rates and topography predict adolescent smoking cessation following treatment with nicotine replacement therapy. Cancer Epidemiology, Biomarkers & Prevention 2006;15:154‐7. [DOI] [PubMed] [Google Scholar]
- Jaszyna‐Gasior M, Schroeder JR, Thorner ED, Heishman SJ, Collins CC, Lo S, et al. Age at menarche and weight concerns in relation to smoking trajectory and dependence among adolescent girls enrolled in a smoking cessation trial. Addictive Behaviors 2009;34(1):92‐5. [DOI] [PubMed] [Google Scholar]
- Moolchan ET, Robinson ML, Ernst M, Cadet JL, Pickworth WB, Heishman SJ, et al. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics 2005;115(4):e407‐e414. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Schroeder JR, Moolchan ET. Adolescent smokers screened for a nicotine replacement treatment trial: Correlates of eligibility and enrollment. Nicotine & Tobacco Research 2006;8:447‐54. [DOI] [PubMed] [Google Scholar]
- Thorner‐Bantug E, Jaszyna‐Gasior M, Schroeder JR, Collins CC, Moolchan ET. Weight gain, related concerns, and treatment outcomes among adolescent smokers enrolled in cessation treatment. Journal of the National Medical Association 2009;101(10):1009‐14. [DOI] [PubMed] [Google Scholar]
Mori 1992 {unpublished data only}
- Mori T, Shimao T, Yulchiro G, Namiki M, Hyachi T. A clinical trial of nicotine chewing gum for smoking cessation [abstract 428]. 8th World Conference on Tobacco or Health; 1992; Buenos Aires, Argentina. 1992.
Nakamura 1990 {unpublished data only}
- Nakamura M, Saito J, Oshima A, Miyamoto M, Matushita A, Endo S. Effect of nicotine chewing gun in smoking cessation classes. The Global War. Proceedings of the 7th World Conference on Tobacco and Health; 1990; Perth, Western Australia. Perth: Health Department of Western Australia, 1990:665‐7.
NCT00534404 {published data only}
- NCT00534404. iQuit Smoking: a randomized trial of internet access to nicotine patches. clinicaltrials.gov/show/NCT00534404 (first received 24 September 2007).
Nebot 1992 {published data only}
- Nebot M, Cabezas C. Does nurse counseling or offer of nicotine gum improve the effectiveness of physician smoking‐cessation advice?. Family Practice Research Journal 1992;12:263‐70. [PubMed] [Google Scholar]
Niaura 1994 {published data only}
- Niaura R, Goldstein MG, Abrams DB. Matching high and low‐dependence smokers to self‐help treatment with or without nicotine replacement. Preventive Medicine 1994;23:70‐7. [DOI] [PubMed] [Google Scholar]
Niaura 1999 {published data only}
- Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, Sirota AD. Cue exposure treatment for smoking relapse prevention: A controlled clinical trial. Addiction 1999;94(5):685‐96. [DOI] [PubMed] [Google Scholar]
Ockene 1991 {published data only}
- Ockene JK, Kristeller J, Goldberg R, Amick TL, Pekow PS, Hosmer D, et al. Increasing the efficacy of physician‐delivered smoking interventions: a randomized clinical trial. Journal of General Internal Medicine 1991;6:1‐8. [DOI] [PubMed] [Google Scholar]
- Ockene JK, Kristeller J, Pbert L, Hebert JR, Luippold R, Goldberg RJ, et al. The physician‐delivered smoking intervention project: can short‐term interventions produce long‐term effects for a general outpatient population?. Health Psychology 1994;13:278‐81. [DOI] [PubMed] [Google Scholar]
Oncken 2007 {published data only}
- Allen AM, Kleppinger A, Lando H, Oncken C. Effect of nicotine patch on energy intake and weight gain in postmenopausal women during smoking cessation. Eating Behaviors 2013;14(4):420‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppinger A, Litt MD, Kenny AM, Oncken CA. Effects of smoking cessation on body composition in postmenopausal women. Journal of Women's Health 2010;19:1651‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oncken C, Cooney J, Feinn R, Lando H, Kranzler HR. Transdermal nicotine for smoking cessation in postmenopausal women. Addictive Behaviors 2007;32:296‐309. [DOI] [PubMed] [Google Scholar]
- Oncken C, Cooney J, Lando H, Feinn R, Kranzler H. Transdermal nicotine for smoking cessation in postmenopausal women (POS1‐045). Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005; Prague, Czech Republic. 2005.
Oncken 2008 {published data only}
- Dornelas E, Oncken C, Greene J, Sankey HZ, Kranzler HR. Major depression and PTSD in pregnant smokers enrolled in nicotine gum treatment trial. American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2013;22(1):54‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish LJ, Peterson BL, Namenek Brouwer RJ, Lyna P, Oncken CA, Swamy G, et al. Adherence to nicotine replacement therapy among pregnant smokers. Nicotine & Tobacco Research 2009;11(5):514‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oncken C, Dornelas E, Greene J, Sankey H, Glasmann A, Feinn R, et al. Nicotine gum for pregnant smokers: a randomized controlled trial. Obstetrics & Gynecology 2008;112(4):859‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy GK, Roelands JJ, Peterson BL, Fish LJ, Oncken CA, Pletsch P, et al. Predictors of adverse events among pregnant smokers exposed in a nicotine replacement therapy trial. American Journal of Obstetrics & Gynecology 2009;201(4):354‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ortega 2011 {published and unpublished data}
- Ortega F, Vellisco A, Márquez E, López‐Campos JL, Rodríguez A, Ángeles Sánchez M, et al. Effectiveness of a cognitive orientation program with and without nicotine replacement therapy in stopping smoking in hospitalised patients. Archivos de Bronconeumologia 2011;47(1):3‐9. [DOI] [PubMed] [Google Scholar]
- Valencia B, Ortega F, Vellisco A, Márquez‐Martín E, Campos JLL, Rodríguez AM, et al. Effectiveness of a cognitive orientation program with and without nicotine replacement therapy in stopping smoking in hospitalised patients [Abstract]. European Respiratory Journal 2011;38(55):775s [P4224]. [DOI] [PubMed] [Google Scholar]
Otero 2006 {published data only}
- Otero UB, Perez CA, Szklo M, Esteves GA, dePinho MM, Szklo AS, et al. Randomized clinical trial: effectiveness of the cognitive‐behavioral approach and the use of nicotine replacement transdermal patches for smoking cessation among adults in Rio de Janeiro, Brazil [Ensaio clinico randomizado: efetividade da abordagem cognitivo‐comportamental e uso de adesivos transdermicos de reposicao de nicotina, na cessacao de fumar, em adultos residentes no Municipio do Rio de Janeiro, Brasil]. Cadernos de Saude Publica 2006;22:439‐49. [DOI] [PubMed] [Google Scholar]
Page 1986 {published data only}
- Page AR, Walters DJ, Schlegel RP, Best JA. Smoking cessation in family practice: the effects of advice and nicotine chewing gum prescription. Addictive Behaviors 1986;11:443‐6. [DOI] [PubMed] [Google Scholar]
Paoletti 1996 {published data only}
- Cosci F, Corlando A, Fornai E, Pistelli F, Paoletti P, Carrozzi L. Nicotine dependence, psychological distress and personality traits as possible predictors of smoking cessation. Results of a double‐blind study with nicotine patch. Addictive Behaviors 2009;34(1):28‐35. [DOI] [PubMed] [Google Scholar]
- Fornai E, Desideri M, Pistelli F, Carrozzi L, Puntoni R, Avino S, et al. Smoking reduction in smokers compliant to a smoking cessation trial with nicotine patch. Monaldi Archives for Chest Disease 2001;56:5‐10. [PubMed] [Google Scholar]
- Paoletti P, Fornai E, Maggiorelli F, Puntoni R, Viegi G, Carrozzi L, et al. Importance of baseline cotinine plasma values in smoking cessation: results from a double blind study with nicotine patch. European Respiratory Journal 1996;9:643‐51. [DOI] [PubMed] [Google Scholar]
Perng 1998 {published data only}
- Perng RP, Hsieh WC, Chen YM, Lu CC, Chiang SJ. Randomized, double‐blind, placebo‐controlled study of transdermal nicotine patch for smoking cessation. American Journal of Respiratory and Critical Care Medicine 1999;159(3SS):A735. [PubMed] [Google Scholar]
- Perng RP, Hsieh WC, Chen YM, Lu CC, Chiang SJ. Randomized, double‐blind, placebo‐controlled study of transdermal nicotine patch for smoking cessation. Journal of the Formosan Medical Association 1998;97:547‐51. [PubMed] [Google Scholar]
Piper 2009 {published data only}
- Asthana A, Johnson HM, Piper ME, Fiore MC, Baker TB, Stein JH. Effects of smoking intensity and cessation on inflammatory markers in a large cohort of active smokers. American Heart Journal 2010;160(3):458‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Piper ME, Smith SS, Fiore MC, Jorenby DE. Defining and predicting short‐term alcohol use changes during a smoking cessation attempt. Addictive Behaviors 2015;48:52‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. American Heart Journal 2011;161(1):145‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, Baker TB. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. Journal of Consulting & Clinical Psychology 2011;79(1):34‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, et al. Effects of smoking and smoking cessation on endothelial function: 1‐year outcomes from a randomized clinical trial. Journal of the American College of Cardiology 2010;55(18):1988‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction 2011;106(2):418‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, et al. A randomized placebo‐controlled clinical trial of 5 smoking cessation pharmacotherapies. Archives of General Psychiatry 2009;66(11):1253‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pirie 1992 {published data only}
- Pirie PL, McBride CM, Hellerstedt WL, Jeffery RW, Hatsukami DK, Allen S, et al. Smoking cessation in women concerned about weight. American Journal of Public Health 1992;82:1238‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pollak 2007 {published data only}
- Pollak KI, Oncken C, Lipkus IM, Peterson BL, Swamy GK, Pletsch PK, et al. Effectiveness of adding nicotine replacement therapy to cognitive behavioral therapy for smoking cessation in pregnant smokers: the Baby Steps trial (PA6‐3). Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 February 21‐24; Austin, Texas, USA. 2007:25.
- Pollak KI, Oncken CA, Lipkus IM, Lyna P, Swamy GK, Pletsch PK, et al. Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. American Journal of Preventive Medicine 2007;33:297‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prapavessis 2007 {published data only}
- Prapavessis H, Cameron L, Baldi JC, Robinson S, Borrie K, Harper T, et al. The effects of exercise and nicotine replacement therapy on smoking rates in women. Addictive Behaviors 2007;32:1416‐32. [DOI] [PubMed] [Google Scholar]
Puska 1979 {published data only}
- Puska P, Bjorkqvist S, Koskela K. Nicotine‐containing chewing gum in smoking cessation: a double blind trial with half year follow‐up. Addictive Behaviors 1979;4:141‐6. [DOI] [PubMed] [Google Scholar]
Richmond 1993 {published data only}
- Richmond R, Heather N. General Practitioner interventions for smoking cessation: past results and future prospects. Behaviour Change 1990;7:110‐9. [Google Scholar]
- Richmond RL, Makinson RJ, Giugni AA, Webster IW. General Practitioner smoking interventions in Australia: results of studies over the past ten years. The Global War: Proceedings of the 7th World Conference on Tobacco and Health; 1990; Perth, Western Australia. Perth: Health Department of Western Australia, 1990:657‐60.
- Richmond RL, Makinson RJ, Kehoe LA, Giugni AA, Webster IW. One‐year evaluation of three smoking cessation interventions administered by general practitioners. Addictive Behaviors 1993;18:187‐99. [DOI] [PubMed] [Google Scholar]
Richmond 1994 {published data only}
- Richmond RL, Harris K, Almeida Neto A. The transdermal nicotine patch: results of a randomised placebo‐controlled trial. Medical Journal of Australia 1994;161:130‐5. [DOI] [PubMed] [Google Scholar]
- Richmond RL, Kehoe L, Neto ACdA. Effectiveness of a 24‐hour transdermal nicotine patch in conjunction with a cognitive behavioural programme: One year outcome. Addiction 1997;92:27‐31. [PubMed] [Google Scholar]
Roto 1987 {published data only}
- Roto P, Ojala A, Sundman K, Jokinen K, Peltomakl R. Nicotine gum and withdrawal from smoking. Suomen Laakarllehtl 1987;36:3445‐8. [Google Scholar]
Russell 1983 {published data only}
- Russell MA, Merriman R, Stapleton J, Taylor W. Effect of nicotine chewing gum as an adjunct to general practitioner's advice against smoking. British Medical Journal (Clinical Research Ed.) 1983;287:1782‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sachs 1993 {published data only}
- Sachs DPL, Sawe U, Leischow SJ. Effectiveness of a 16‐hour transdermal nicotine patch in a medical practice setting, without intensive group counseling. Archives of Internal Medicine 1993;153:1881‐90. [PubMed] [Google Scholar]
Scherphof 2014 {published data only}
- Scherphof CS, Eijnden RJ, Engels RCME, Vollebergh WA. Short‐term efficacy of nicotine replacement therapy for smoking cessation in adolescents: a randomized controlled trial. Journal of Substance Abuse Treatment 2014;46(2):120‐7. [DOI] [PubMed] [Google Scholar]
- Scherphof CS, Eijnden RJ, Lugtig P, Engels RCME, Vollebergh WA. Adolescents' use of nicotine replacement therapy for smoking cessation: predictors of compliance trajectories. Psychopharmacology 2014;231(8):1743‐52. [DOI] [PubMed] [Google Scholar]
- Scherphof CS, Eijnden RJJM, Engels RCME, Vollebergh WAM. Long‐term efficacy of nicotine replacement therapy for smoking cessation in adolescents: A randomized controlled trial. Drug and Alcohol Dependence 2014;140:217‐20. [DOI] [PubMed] [Google Scholar]
Schneider 1983a {published data only}
- Jarvik ME, Schneider NG. Degree of addiction and effectiveness of nicotine gum therapy for smoking. American Journal of Psychiatry 1984;141:790‐1. [DOI] [PubMed] [Google Scholar]
- Schneider NG, Jarvik ME. Nicotine gum vs. placebo gum: comparisons of withdrawal symptoms and success rates. NIDA Research Monograph 1985;53:83‐101. [PubMed] [Google Scholar]
- Schneider NG, Jarvik ME, Forsythe AB, Read LL, Elliott ML, Schweiger A. Nicotine gum in smoking cessation: a placebo‐controlled, double‐blind trial. Addictive Behaviors 1983;8:253‐61. [DOI] [PubMed] [Google Scholar]
Schneider 1983b {published data only}
- Schneider NG, Jarvik ME. Nicotine gum vs. placebo gum: comparisons of withdrawal symptoms and success rates. NIDA Research Monograph 1985;53:83‐101. [PubMed] [Google Scholar]
- Schneider NG, Jarvik ME, Forsythe AB, Read LL, Elliott ML, Schweiger A. Nicotine gum in smoking cessation: a placebo‐controlled, double‐blind trial. Addictive Behaviors 1983;8:253‐61. [DOI] [PubMed] [Google Scholar]
Schneider 1995 {published data only}
- Schneider NG, Olmstead R, Mody FV, Doan K, Franzon M, Jarvik ME, et al. Efficacy of a nicotine nasal spray in smoking cessation: a placebo‐controlled, double‐blind trial. Addiction 1995;90:1671‐82. [DOI] [PubMed] [Google Scholar]
Schneider 1996 {published data only}
- Schneider NG, Olmstead R, Nilsson F, Mody FV, Franzon M, Doan K. Efficacy of a nicotine inhaler in smoking cessation: a double‐blind, placebo controlled trial. Addiction 1996;91:1293‐306. [PubMed] [Google Scholar]
- Schneider NG, Olmstead R, Nilsson F, Mody FV, Franzon M, Doan K. Efficacy of a nicotine inhaler in smoking cessation: a double‐blind, placebo‐controlled trial [abstract]. Addiction 1997;92:630. [PubMed] [Google Scholar]
Schnoll 2010 {published data only}
- Lerman C, Jepson C, Wileyto EP, Patterson F, Schnoll R, Mroziewicz M, et al. Genetic variation in nicotine metabolism predicts the efficacy of extended‐duration transdermal nicotine therapy. Clinical Pharmacology & Therapeutics 2010;87(5):553‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, et al. Effectiveness of extended‐duration transdermal nicotine therapy: a randomized trial. Annals of Internal Medicine 2010;152(3):144‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Wileyto EP, Lerman C. Extended duration therapy with transdermal nicotine may attenuate weight gain following smoking cessation. Addictive Behaviors 2012;37:565‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Segnan 1991 {published data only}
- Segnan N, Ponti A, Battista RN, Senore C, Rosso S, Shapiro SH, et al. A randomized trial of smoking cessation interventions in general practice in Italy. Cancer Causes and Control 1991;2:239‐46. [DOI] [PubMed] [Google Scholar]
- Senore C, Battista RN, Ponti A, Segnan N, Shapiro SH, Rosso S, et al. Comparing participants and nonparticipants in a smoking cessation trial: Selection factors associated with general practitioner recruitment activity. Journal of Clinical Epidemiology 1999;52:83‐9. [DOI] [PubMed] [Google Scholar]
- Senore C, Battista RN, Shapiro SH, Segnan N, Ponti A, Rosso S, et al. Predictors of smoking cessation following physicians' counseling. Preventive Medicine 1998;27:412‐21. [DOI] [PubMed] [Google Scholar]
Shiffman 2002 (2 mg) {published data only}
- Dresler CM, Shiffman S, Strahs KR. Safety profile of the new nicotine polacrilex lozenge (PO1 36). Society for Research on Nicotine and Tobacco 8th Annual Meeting: 2002; Savannah, Georgia, USA. 2002.
- Ferguson SG, Gitchell JG, Shiffman S. Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction (Abingdon, England) 2012;107(7):1349‐53. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Nicotine lozenge efficacy in light smokers. Drug & Alcohol Dependence 2005;77:311‐4. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction 2007;102:809‐14. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Archives of Internal Medicine 2002;162:1267‐76. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dresler CM, Rohay JM. Successful treatment with a nicotine lozenge of smokers with prior failure in pharmacological therapy. Addiction 2004;99:83‐92. [DOI] [PubMed] [Google Scholar]
Shiffman 2002 (4 mg) {published data only}
- Ferguson SG, Gitchell JG, Shiffman S. Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction (Abingdon, England) 2012;107(7):1349‐53. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dresler CM, Hajek P, Gilburt SJ, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Archives of Internal Medicine 2002;162:1267‐76. [DOI] [PubMed] [Google Scholar]
Shiffman 2009 (2 mg) {published data only}
- Shiffman S, Ferguson SG, Strahs KR. Quitting by gradual smoking reduction using nicotine gum: a randomized controlled trial. American Journal of Preventive Medicine 2009;36(2):96‐104. [DOI] [PubMed] [Google Scholar]
Shiffman 2009 (4 mg) {published data only}
- Shiffman S, Ferguson SG, Strahs KR. Quitting by gradual smoking reduction using nicotine gum: a randomized controlled trial. American Journal of Preventive Medicine 2009;36(2):96‐104. [DOI] [PubMed] [Google Scholar]
Stapleton 1995 {published data only}
- Russell MAH, Stapleton JA, Feyerabend C, Wiseman SM, Gustavsson G, Sawe U, et al. Targeting heavy smokers in general practice: randomised controlled trial of transdermal nicotine patches. BMJ 1993;306(6888):1308‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JA, Russell MAH, Feyerabend C, Wiseman SM, Gustavsson G, Sawe U, et al. Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction 1995;90:31‐42. [DOI] [PubMed] [Google Scholar]
Stein 2013 {published and unpublished data}
- Dios MA, Anderson BJ, Caviness CM, Stein MD. Early quit days among methadone‐maintained smokers in a smoking cessation trial. Nicotine & Tobacco Research 2014;16(11):1463‐9. [CENTRAL: 1042497; CRS: 9400129000003420] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00790569. Varenicline versus nicotine replacement for methadone‐maintained smokers. ClinicalTrials.gov/ct2/ (first received 13 November 2008). [CRS: 9400123000011408]
- Stein MD, Caviness CM, Kurth ME, Audet D, Olson J, Anderson BJ. Varenicline for smoking cessation among methadone‐maintained smokers: A randomized clinical trial. Drug and Alcohol Dependence 2013;133(2):486‐93. [CENTRAL: 870955; CRS: 9400107000001457; EMBASE: 2013694276; PUBMED: 23953658] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sutherland 1992 {published data only}
- Stapleton JA, Sutherland G, Russell MAH. How much does relapse after one year erode effectiveness of smoking cessation treatments? Long term follow up of randomised trial of nicotine nasal spray. BMJ 1998;316(7134):830‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland G, Stapleton JA, Russell MAH, Jarvis MJ, Hajek P, Belcher M, et al. Randomised controlled trial of nasal nicotine spray in smoking cessation. Lancet 1992;340:324‐9. [DOI] [PubMed] [Google Scholar]
Sønderskov 1997 {published data only}
- Sønderskov J, Olsen J, Meillier L, Overvad OK, Sabroe S. The effect of transdermal nicotine patches in smoking cessation. A randomized trial in pharmacy customers in Denmark. Ugeskrift for Laeger 1999;161:593‐7. [PubMed] [Google Scholar]
- Sønderskov J, Olsen J, Sabroe S, Meillier L, Overvad K. Nicotine patches in smoking cessation: A randomized trial among over‐ the‐counter customers in Denmark. American Journal of Epidemiology 1997;145:309‐18. [DOI] [PubMed] [Google Scholar]
TNSG 1991 {published data only}
- Daughton DM, Fortmann SP, Glover ED, Hatsukami DK, Heatley SA, Lichtenstein E, et al. The smoking cessation efficacy of varying doses of nicotine patch delivery systems 4 to 5 years post‐quit day. Preventive Medicine 1999;28:113‐8. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Gitchell JG, Shiffman S, Sembower MA. Prediction of abstinence at 10 weeks based on smoking status at 2 weeks during a quit attempt: secondary analysis of two parallel, 10‐week, randomized, double‐blind, placebo‐controlled clinical trials of 21‐mg nicotine patch in adult smokers. Clinical Therapeutics 2009;31(9):1957‐65. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Ward MM. Subgroups of smokers with different success rates after use of transdermal nicotine. Addiction 1997;92:207‐17. [PubMed] [Google Scholar]
- Transdermal Nicotine Study Group. Transdermal nicotine for smoking cessation. Six‐month results from two multicenter controlled clinical trials. Transdermal Nicotine Study Group. JAMA 1991;266:3133‐8. [PubMed] [Google Scholar]
Tuisku 2016 {published data only}
- Tuisku A, Salmela M, Nieminen P, Toljamo T. Varenicline and nicotine patch therapies in young adults motivated to quit smoking: a randomized, placebo‐controlled, prospective Studys. Basic & Clinical Pharmacology & Toxicology 2016;119(1):78‐84. [DOI] [PubMed] [Google Scholar]
Tønnesen 1988 {published data only}
- Tønnesen P, Fryd V, Hansen M, Helsted J, Gunnersen AB, Forchammer H, et al. Effect of nicotine chewing gum in combination with group counseling on the cessation of smoking. New England Journal of Medicine 1988;318:15‐8. [DOI] [PubMed] [Google Scholar]
Tønnesen 1991 {published data only}
- Mikkelsen KL, Tønnesen P, Norregaard J. Three‐year outcome of two‐ and three‐year sustained abstainers from a smoking cessation study with nicotine patches. Journal of Smoking‐Related Disorders 1994;5:95‐100. [Google Scholar]
- Norregaard J, Tønnesen P, Petersen L. Predictors and reasons for relapse in smoking cessation with nicotine and placebo patches. Preventive Medicine 1993;22:261‐71. [DOI] [PubMed] [Google Scholar]
- Tønnesen P, Norregaard J, Sawe U. Two‐year outcome in a smoking cessation trial with a nicotine patch. Journal of Smoking‐Related Disorders 1992;3:241‐5. [Google Scholar]
- Tønnesen P, Norregaard J, Simonsen K, Sawe U. A double‐blind trial of a 16‐hour transdermal nicotine patch in smoking cessation. New England Journal of Medicine 1991;325:311‐5. [DOI] [PubMed] [Google Scholar]
- Tønnesen P, Norregaard J, Simonsen K, Sawe U. A double‐blind trial of nicotine patches in smoking cessation [En dobbeltblind undersogelse af nikotinplaster ved rygeafvaenning]. Ugeskrift for Laeger 1992;154:251‐4. [PubMed] [Google Scholar]
Tønnesen 1993 {published data only}
- Tønnesen P, Norregaard J, Mikkelsen K, Jorgensen S, Nilsson F. A double‐blind trial of a nicotine inhaler for smoking cessation. JAMA 1993;269:1268‐71. [PubMed] [Google Scholar]
Tønnesen 2000 {published data only}
- Tønnesen P, Mikkelsen KL. Smoking cessation with four nicotine replacement regimes in a lung clinic. European Respiratory Journal 2000;16:717‐22. [DOI] [PubMed] [Google Scholar]
Tønnesen 2006 {published data only}
- Pbert L. Nurse‐conducted smoking cessation in patients with COPD, using nicotine sublingual tablets and behavioral support. Chest 2006;130:314‐6. [DOI] [PubMed] [Google Scholar]
- Tonnesen P, Mikkelsen K, Bremann L. Nurse‐conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006;130:334‐42. [DOI] [PubMed] [Google Scholar]
Tønnesen 2012 {published and unpublished data}
- Tønnesen P, Lauri H, Perfekt R, Mann K, Batra A. Efficacy and safety of a novel nicotine mouth spray in smoking cessation: A randomized, placebo‐controlled, double blind, multicenter study with 52‐week follow up. Poster Presented at SRNT, Feb 16‐19th, 2011, Toronto, Canada.
- Tønnesen P, Lauri H, Perfekt R, Mann K, Batra A. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double blind trial. European Respiratory Journal 2012;40(3):548‐54. [DOI: 10.1183/09031936.00155811] [DOI] [PMC free article] [PubMed] [Google Scholar]
Villa 1999 {published data only}
- Villa RS, Alvarez ABD, Hermida JRF. Effectiveness of a multicomponent program to quit smoking with and without nicotine chewing gum [Eficacia de un programa multicomponente para dejar de fumar con y sin chicle de nicotina]. Psicologia Conductual 1999;7:107‐18. [Google Scholar]
Wallstrom 2000 {published data only}
- Pharmacia, Upjohn. Nicorette Nicotine Microtab Monograph. Chester: Adis International, 1998:Adis International, 1998. [Google Scholar]
- Wallstrom M, Nilsson F, Hirsch JM. A double‐blind placebo controlled clinical evaluation of a nicotine sublingual tablet in smoking cessation [abstract]. European Respiratory Journal 1997; Vol. 10, issue Suppl 25:440S. [PubMed]
- Wallstrom M, Nilsson F, Hirsch JM. A randomized double‐blind placebo‐controlled clinical evaluation of a nicotine sublingual tablet in smoking cessation. Addiction 2000;95(8):1161‐71. [PubMed] [Google Scholar]
Ward 2013 {published data only}
- Ben Taleb Z, Ward KD, Asfar T, Bahelah R, Maziak W. Predictors of adherence to pharmacological and behavioral treatment in a cessation trial among smokers in Aleppo, Syria. Drug and Alcohol Dependence 2015;153:167‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Taleb Z, Ward KD, Asfar T, Jaber R, Auf R, Maziak W. Predictors of nicotine withdrawal symptoms: findings from the first randomized smoking cessation trial in a low‐income country setting. International Journal of Public Health 2016;61(6):701‐8. [DOI] [PubMed] [Google Scholar]
- Ben Taleb Z, Ward KD, Asfar T, Jaber R, Bahelah R, Maziak W. Smoking cessation and changes in body mass index: Findings from the first randomized cessation trial in a low‐income country setting. Nicotine & Tobacco Research 2017;19(3):351‐6. [DOI] [PubMed] [Google Scholar]
- Ben Taleb Z, Ward KD, Asfar T, Jaber R, Bahelah R, Maziak W. Smoking cessation and changes in body mass index: findings from the first randomized cessation trial in a low‐Income country setting. Nicotine & Tobacco Research 2016;19(3):351‐56. [DOI] [PubMed] [Google Scholar]
- Lando H. Commentary on Ward et al. (2013): failure to find a treatment effect for NRT in a low‐income country ‐ implications. Addiction (Abingdon, England) 2013;108(2):404‐5. [DOI] [PubMed] [Google Scholar]
- Ward KD, Asfar T, Al Ali R, Rastam S, Weg MW, Eissenberg T, et al. Randomized trial of the effectiveness of combined behavioral/pharmacological smoking cessation treatment in Syrian primary care clinics. Addiction (Abingdon, England) 2013;108(2):394‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wennike 2003b {published data only}
- Wennike P, Danielsson T, Landfeldt B, Westin A, Tønnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo‐controlled trial of nicotine gum with 2‐year follow‐up. Addiction 2003;98(10):1395‐402. [DOI] [PubMed] [Google Scholar]
Westman 1993 {published data only}
- Westman EC, Levin ED, Rose JE. The nicotine patch in smoking cessation. A randomized trial with telephone counseling. Archives of Internal Medicine 1993;153:1917‐23. [PubMed] [Google Scholar]
Wisborg 2000 {published data only}
- Wisborg K, Henriksen TB, Jespersen LB, Secher NJ. Nicotine patches for pregnant smokers: A randomized controlled study. Obstetrics & Gynecology 2000;96(6):967‐71. [DOI] [PubMed] [Google Scholar]
Wittchen 2011 {published data only}
- Wittchen HU, Hoch E, Klotsche J, Muehlig S. Smoking cessation in primary care ‐ a randomized controlled trial of bupropion, nicotine replacements, CBT and a minimal intervention. International Journal of Methods in Psychiatric Research 2011;20(1):28‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zelman 1992 {published data only}
- Zelman DC, Brandon TH, Jorenby DE, Baker TB. Measures of affect and nicotine dependence predict differential response to smoking cessation treatments. Journal of Consulting and Clinical Psychology 1992;60:943‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adelman 2009 {published data only}
- Adelman WP. Nicotine nasal spray neither effective nor well‐tolerated by adolescent smokers. Journal of Pediatrics 2009;154(3):462‐3. [DOI] [PubMed] [Google Scholar]
Allen 2005 {published data only}
- Allen SS, Hatsukami D, Brintnell DM, Bade T. Effect of nicotine replacement therapy on post‐cessation weight gain and nutrient intake: A randomized controlled trial of postmenopausal female smokers. Addictive Behaviors 2005;30:1273‐80. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatsukami DK, Bade T, Center B. Transdermal nicotine use in postmenopausal women: does the treatment efficacy differ in women using and not using hormone replacement therapy?. Nicotine & Tobacco Research 2004;6(5):777‐88. [DOI] [PubMed] [Google Scholar]
Allen 2011 {published data only}
- Allen MH, Debanne M, Lazignac C, Adam E, Dickinson LM, Damsa C. Effect of nicotine replacement therapy on agitation in smokers with schizophrenia: a double‐blind, randomized, placebo‐controlled study. American Journal of Psychiatry 2011;168(4):395‐9. [DOI] [PubMed] [Google Scholar]
Aubin 2006 {published data only}
- Aubin HJ, Luthringer R, Demazieres A, Dupont C, Lagrue G. Comparison of the effects of a 24‐hour nicotine patch and a 16‐hour nicotine patch on smoking urges and sleep. Nicotine & Tobacco Research 2006;8:193‐201. [DOI] [PubMed] [Google Scholar]
Batra 2005 {published data only}
- Batra A, Klingler K, Landfeldt B, Friederich HM, Westin A, Danielsson T. Smoking reduction treatment with 4‐mg nicotine gum: A double‐blind, randomized, placebo‐controlled study. Clinical Pharmacology & Therapeutics 2005;78:689‐96. [DOI] [PubMed] [Google Scholar]
- Landfeldt B, Batra A, Friederich HM, Klingler K, Westin A. Smoking reduction with a 4 mg nicotine gum ‐ final results from a placebo‐controlled trial over 13 months. Society for Research on Nicotine and Tobacco 5th European Meeting; 2003 November 20‐22; Padua, Italy. 2003.
Berlin 2011 {published data only}
- Berlin I, Jacob N, Coudert M, Perriot J, Schultz L, Rodon N. Adjustment of nicotine replacement therapies according to saliva cotinine concentration: the ADONIS* trial‐a randomized study in smokers with medical comorbidities. Addiction 2011;106(4):833‐43. [DOI] [PubMed] [Google Scholar]
- Berlin I, Singleton EG, Heishman SJ. Validity of the 12‐item French version of the Tobacco Craving Questionnaire in treatment‐seeking smokers. Nicotine & Tobacco Research 2010;12(5):500‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bock 2010 {published data only}
- Bock BC, Hudmon KS, Christian J, Graham AL, Bock FR. A tailored intervention to support pharmacy‐based counseling for smoking cessation. Nicotine & Tobacco Research 2010;12:217‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolliger 2000a {published data only}
- Bolliger CT. Practical experiences in smoking reduction and cessation. Addiction 2000;95(1 S1):S19‐S24. [DOI] [PubMed] [Google Scholar]
- Bolliger CT, Zellweger JP, Danielsson T, Biljon X, Robidou A, Westin A, et al. Influence of long‐term smoking reduction on health risk markers and quality of life. Nicotine & Tobacco Research 2002;4:433‐9. [DOI] [PubMed] [Google Scholar]
- Bolliger CT, Zellweger JP, Danielsson T, Biljon X, Robidou A, Westin A, et al. Smoking reduction with oral nicotine inhalers: double blind, randomised clinical trial of efficacy and safety. BMJ 2000;321(7257):329‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolliger 2007 {published data only}
- Bolliger CT, Biljon X, Axelsson A. A nicotine mouth spray for smoking cessation: a pilot study of preference, safety and efficacy. Respiration 2007;74(2):196‐201. [DOI] [PubMed] [Google Scholar]
Brantmark 1973a {published data only}
- Brantmark B, Ohlin P, Westling H. Nicotine‐containing chewing gum as an anti‐smoking aid. Psychopharmacologia 1973;31:191‐200. [DOI] [PubMed] [Google Scholar]
Caldwell 2016 {published data only}
- Caldwell BO, Crane J. Combination nicotine metered dose inhaler and nicotine patch for smoking cessation: a randomized controlled trial. Nicotine & Tobacco Research 2016;18(10):1944‐51. [DOI] [PubMed] [Google Scholar]
Carpenter 2003 {published data only}
- Carpenter MJ, Hughes JR, Keely JP. Effect of smoking reduction on later cessation: a pilot experimental study. Nicotine & Tobacco Research 2003;5(2):155‐62. [DOI] [PubMed] [Google Scholar]
Carpenter 2011 {published data only}
- Carpenter MJ, Alberg AJ, Gray KM, Saladin ME. Motivating the unmotivated for health behavior change: a randomized trial of cessation induction for smokers. Clinical Trials 2010;7(2):157‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: a randomized clinical trial. Archives of Internal Medicine 2011;171(21):1901‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2010 {published data only}
- Chan SS, Leung DY, Abdullah AS, Lo SS, Yip AW, Kok WM, et al. Smoking‐cessation and adherence intervention among Chinese patients with erectile dysfunction. American Journal of Preventive Medicine 2010;39(3):251‐8. [DOI] [PubMed] [Google Scholar]
Chan 2011 {published data only}
- Chan SS, Leung DY, Abdullah AS, Wong VT, Hedley AJ, Lam TH. A randomized controlled trial of a smoking reduction plus nicotine replacement therapy intervention for smokers not willing to quit smoking. Addiction 2011;106(6):1155‐63. [DOI] [PubMed] [Google Scholar]
Chou 2004 {published data only}
- Chou KR, Chen R, Lee JF, Ku CH, Lu RB. The effectiveness of nicotine‐patch therapy for smoking cessation in patients with schizophrenia. International Journal of Nursing Studies 2004;41:321‐30. [DOI] [PubMed] [Google Scholar]
Christen 1984 {published data only}
- Christen AG, Drook C, McDonald JL, Stookey G, Olson B. Efficacy of nicotine chewing gum in facilitating smoking cessation. Journal of the American Dental Association 1984;108:594‐7. [DOI] [PubMed] [Google Scholar]
Cohen 1989a {published data only}
- Cohen SJ, Stookey GK, Katz BP, Drook CA, Christen AG. Helping smokers quit: a randomized controlled trial with private practice dentists. Journal of the American Dental Association 1989;118:41‐5. [DOI] [PubMed] [Google Scholar]
Cohen 1989b {published data only}
- Cohen SJ, Stookey GK, Katz BP, Drook CA, Smith DM. Encouraging primary care physicians to help smokers quit. A randomised, controlled trial. Annals of Internal Medicine 1989;110:648‐52. [DOI] [PubMed] [Google Scholar]
Croghan 2007 {published data only}
- Clark MM, Hurt RD, Croghan I, Patten CA, Novotny P, Sloan JA, et al. The prevalence of weight concerns in a smoking abstinence clinical trial (POS2‐84). Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 February 15‐18, Orlando, Florida, USA. 2006.
- Croghan IT, Hurt RD, Croghan GA, Sloan JA. Comparing nicotine inhaler, bupropion and nicotine inhaler plus bupropion in treating tobacco dependence [abstract]. Nicotine & Tobacco Research 2005;7(4):680‐1. [Google Scholar]
- Croghan IT, Hurt RD, Dakhil SR, Croghan GA, Sloan JA, Novotny PJ, et al. Randomized comparison of a nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clinic Proceedings 2007;82:186‐95. [DOI] [PubMed] [Google Scholar]
- Croghan IT, Hurt RD, Ebbert JO, Croghan GA, Polk J, Stella PJ, et al. Racial differences in smoking abstinence rates in a multicenter, randomized, open‐label trial in the United States. Journal of Public Health 2010;18:59‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cummings 2011 {published data only}
- Cummings KM, Hyland A, Carlin‐Menter S, Mahoney MC, Willett J, Juster HR. Costs of giving out free nicotine patches through a telephone quit line. Journal of Public Health Management & Practice 2011;17(3):E16‐23. [DOI] [PubMed] [Google Scholar]
Dey 1999 {published data only}
- Dey P, Foy R, Woodman M, Fullard B, Gibbs A. Should smoking cessation cost a packet? A pilot randomized controlled trial of the cost‐effectiveness of distributing nicotine therapy free of charge. British Journal of General Practice 1999;49:127‐8. [PMC free article] [PubMed] [Google Scholar]
Donny 2009 {published data only}
- Donny EC, Jones M. Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug & Alcohol Dependence 2009;104(1‐2):23‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebbert 2009 {published data only}
- Ebbert JO, Severson HH, Croghan IT, Danaher BG, Schroeder DR. A randomized clinical trial of nicotine lozenge for smokeless tobacco use. Nicotine & Tobacco Research 2009;11(12):1415‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebbert 2010 {published data only}
- Ebbert JO, Severson HH, Croghan IT, Danaher BG, Schroeder DR. A pilot study of mailed nicotine lozenges with assisted self‐help for the treatment of smokeless tobacco users. Addictive Behaviors 2010;35(5):522‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elan Pharm 88‐02 {published data only}
- Elan Pharmaceutical Research Corp. NDA 19‐983 for Approval of PROSTEP. Study 88‐02 1992.
Elan Pharm 90‐03 {published data only}
- Elan Pharmaceutical Research Corp. NDA 19‐983 for Approval of PROSTEP. Study 90‐03 1992.
Etter 2004 {published data only}
- Dar R, Stronguin F, Etter JF. Assigned versus perceived placebo effects in nicotine replacement therapy for smoking reduction in Swiss smokers. Journal of Consulting & Clinical Psychology 2005;73:350‐3. [DOI] [PubMed] [Google Scholar]
- Etter JF, Laszlo E, Perneger TV. Postintervention effect of nicotine replacement therapy on smoking reduction in smokers who are unwilling to quit: Randomized trial. Journal of Clinical Psychopharmacology 2004;24(2):174‐9. [DOI] [PubMed] [Google Scholar]
- Etter JF, Laszlo E, Zellweger JP, Perrot C, Perneger TV. Nicotine replacement to reduce cigarette consumption in smokers who are unwilling to quit: a randomized trial. Journal of Clinical Psychopharmacology 2002;22:487‐95. [DOI] [PubMed] [Google Scholar]
Fagerström 1993 {published data only}
- Fagerström KO, Schneider NG, Lunell E. Effectiveness of nicotine patch and nicotine gum as individual versus combined treatments for tobacco withdrawal symptoms. Psychopharmacology (Berl) 1993;111:271‐7. [DOI] [PubMed] [Google Scholar]
Fagerström 1997 {published data only}
- Fagerström KO, Tejding R, Westin A, Lunell E. Aiding reduction of smoking with nicotine replacement medications: hope for the recalcitrant smoker?. Tobacco Control 1997;6:311‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fagerström 2000 {published data only}
- Fagerström KO, Hughes JR, Rasmussen T, Callas PW. Randomised trial investigating effect of a novel nicotine delivery device (Eclipse) and a nicotine oral inhaler on smoking behaviour, nicotine and carbon monoxide exposure, and motivation to quit. Tobacco Control 2000;9:327‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferguson 2012 {published data only}
- Coleman T, McEwen A, Bauld L, Ferguson J, Lorgelly P, Lewis S. Protocol for the Proactive Or Reactive Telephone Smoking CeSsation Support (PORTSSS) trial. Trials 2009;10(26). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J, Docherty G, Bauld L, Lewis S, Lorgelly P, Boyd KA, et al. Effect of offering different levels of support and free nicotine replacement therapy via an English national telephone quitline: randomised controlled trial. BMJ March 2012;344:e1696. [DOI: 10.1136/bmj.e1696] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finland unpublished {unpublished data only}
- Anon. Combination NRT; Improving efficacy in smoking cessation. McNeil Consumer Healthcare booklet, 2007. Short term outcomes reported on p17.
Foulds 1993 {published data only}
- Foulds J, Stapleton J, Hayward M, Russell MA, Feyerabend C, Fleming T, et al. Transdermal nicotine patches with low‐intensity support to aid smoking cessation in outpatients in a general hospital. A placebo‐controlled trial. Archives of Family Medicine 1993;2:417‐23. [DOI] [PubMed] [Google Scholar]
Garvey 2006 {unpublished data only}
- Garvey AJ, Hoskinson RA, Wadler BM, Kinnunen T, Sachs DPL. Individualising nicotine patch dose to match smokers' usual nicotine intake levels (PA9‐4). Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 February 15‐18; Orlando, Florida, USA. 2006:32.
- Mustonen TK, Spencer SM, Hoskinson RA, Sachs DPL, Garvey AJ. The influence of gender, race, and menthol content on tobacco exposure measures. Nicotine & Tobacco Research 2005;7:581‐90. [DOI] [PubMed] [Google Scholar]
Glover 1992 {published data only}
- Glover ED, Glover PN, Sullivan CR, Sullivan P, Nilsson F, Sawe U. Nicotine inhaler versus placebo in smoking cessation [abstract 237]. 8th World Conference on Tobacco or Health; 1992; Buenos Aires, Argentina. 1992.
Gross 1989 {published data only}
- Gross J, Stitzer ML, Maldonado J. Nicotine replacement: effects on postcessation weight gain. Journal of Consulting and Clinical Psychology 1989;57:87‐92. [DOI] [PubMed] [Google Scholar]
Guo 2006 {published data only}
- Guo S, Sun H, Niu G, Gao J, Yang X, Zhou D. The smoking cessation efficacy of nicotine sublingual tablet: A double‐blind, placebo controlled trial. Chinese Journal of Psychiatry 2006;39(2):102‐5. [Google Scholar]
Hajek 1999 {published data only}
- Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Archives of Internal Medicine 1999;159:2033‐8. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl) 2000;149:198‐202. [DOI] [PubMed] [Google Scholar]
Hanson 2003 {published data only}
- Dickmann PJ, Mooney ME, Allen SS, Hanson K, Hatsukami DK. Nicotine withdrawal and craving in adolescents: effects of sex and hormonal contraceptive use. Addictive Behaviors 2009;34(6‐7):620‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine & Tobacco Research 2003;5(4):515‐26. [DOI] [PubMed] [Google Scholar]
Haustein 2003 {published data only}
- Haustein KO, Batra A, Landfeldt B, Westin A. The effect of short‐term or long‐term reduction on smoking cessation; results from a placebo controlled smoking reduction study with the nicotine gum. Nicotine & Tobacco Research 2003;5:278. [Google Scholar]
- Pfizer. Summary of clinical efficacy. Application for licensing of Nicorette Inhalator/Gum for smoking reduction leading to cessation. Company data NICORE‐1013‐273‐SU.
Hoch 2006 {published data only}
- Hoch E, Wittchen HU. Population health perspective on smoking cessation: A randomized controlled trial of different methods in primary health care (RPOS 3‐71). Society for Research on Nicotine and Tobacco 12th Annual Meeting; 2006 February 15‐18; Orlando, Florida, USA. 2006.
- Sonntag H, Hoch E, Jahn B, Spiegel B, Pfister H, Wittchen HU. Smoking cessation in primary care: implementation effectiveness and optimized allocation. Suchtmedizin in Forschung und Praxis 2003;5(2):137‐41. [Google Scholar]
Hotham 2006 {published data only}
- Hotham ED, Gilbert AL, Atkinson ER. A randomised‐controlled pilot study using nicotine patches with pregnant women. Addictive Behaviors 2006;31:641‐8. [DOI] [PubMed] [Google Scholar]
Hughes 1989b {published data only}
- Hughes JR, Gulliver SB, Amori G, Mireault GC, Fenwick JF. Effect of instructions and nicotine on smoking cessation, withdrawal symptoms and self‐administration of nicotine gum. Psychopharmacology (Berl) 1989;99:486‐91. [DOI] [PubMed] [Google Scholar]
Hurt 1995 {published data only}
- Hurt RD, Dale LC, Offord KP, Croghan IT, Hays JT, Gomez Dahl L. Nicotine patch therapy for smoking cessation in recovering alcoholics. Addiction 1995;90(11):1541‐6. [DOI] [PubMed] [Google Scholar]
Hurt 2003 {published data only}
- Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. Journal of Clinical Oncology 2003;21(5):914‐20. [DOI] [PubMed] [Google Scholar]
Jarvik 1984 {published data only}
- Jarvik ME, Schneider NG. Degree of addiction and effectiveness of nicotine gum therapy for smoking. American Journal of Psychiatry 1984;141:790‐1. [DOI] [PubMed] [Google Scholar]
Jibrail 2010 {published data only}
- Jibrail JJ, Cortas NK, Sarieddine DS, Kanj NA, Zaatari GS, Daher RT. Impact of nicotine metabolite monitoring on the efficacy of smoke cessation and usefulness of sequential CRP measurements. American Journal of Respiratory Critical Care Medicine 2010;181:A2652. [Google Scholar]
Kapur 2001 {published data only}
- Kapur B, Hackman R, Selby P, Klein J, Koren G. Randomized, double‐blind, placebo‐controlled trial of nicotine replacement therapy in pregnancy. Current Therapeutic Research Clinical and Experimental 2001;62:274‐8. [Google Scholar]
Korberly 1999 {unpublished data only}
- Korberly B, Gustavsson G, Kruse E. Over‐the‐counter efficacy of a 15 mg daytime nicotine patch for smoking cessation: a randomized multicenter trial. Society for Research on Nicotine and Tobacco 4th European Conference; 2002 October 03‐05; Santander, Spain. 2002:23.
- Korberly BH, Maguire MK. An open label multicenter trial to evaluate and compare the efficacy of nicotrol 15mg as part of an OTC intervention package or as a prescription as an aid in smoking cessation. Society for Research on Nicotine and Tobacco Fifth Annual Meeting; 1999;San Diego, CA, USA. 1999.
Kozak 1995 {published data only}
- Kozak J, Fagerström KO, Sawe U. High‐dose treatment with the nicotine patch. International Journal of Smoking Cessation 1995;4(2):26‐8. [Google Scholar]
Kras 2010 {published data only}
- Kras M, Stough C, Scholey A, Kure C, Camfield D. Hypericum perforatum, nicotine patches and combination hypericum perforatum/nicotine patches for smoking cessation. European Neuropsychopharmacology 2010;20:S608‐9. [Google Scholar]
Krumpe 1989 {published data only}
- Krumpe P, Malani N, Adler J. Efficacy of transdermal nicotine administration as an adjunct for smoking cessation in heavily nicotine addicted smokers. Annual Review of Respiratory Disease 1989;139:A337. [Google Scholar]
Krupski 2016 {published data only}
- Krupski L, Cummings KM, Hyland A, Mahoney MC, Toll BA, Carpenter MJ, et al. Cost and effectiveness of combination nicotine replacement therapy among heavy smokers contacting a quitline. Journal of Smoking Cessation 2016;11(1):50‐9. [Google Scholar]
Kupecz 1996 {published data only}
- Kupecz D, Prochazka A. A comparison of nicotine delivery systems in a multimodality smoking cessation program. Nurse Practitioner 1996;21(2):73, 77‐8, 81. [DOI] [PubMed] [Google Scholar]
Landfeldt 1998 {unpublished data only}
- Landfeldt B, Kruse E, Westin A, Mattson K, Lojander J. Nicotine replacement treatment in heavy smokers: nicotine nasal spray combined with nicotine patch in a double‐blind controlled study (abstract). European Respiratory Journal 1998;12(Suppl 28):154S. [Google Scholar]
Leischow 1996b {published data only}
- Leischow SJ, Hill A, Cook G. The effects of transdermal nicotine for the treatment of hispanic smokers. American Journal of Health Behavior 1996;20:304‐11. [Google Scholar]
Levin 1994 {published data only}
- Levin ED, Westman EC, Stein RM, Carnahan E, Sanchez M, Herman S, et al. Nicotine skin patch treatment increases abstinence, decreases withdrawal symptoms, and attenuates rewarding effects of smoking. Journal of Clinical Psychopharmacology 1994;14:41‐9. [PubMed] [Google Scholar]
Lin 1996 {published data only}
- Lin HN. The effectiveness of nicotine patch for smoking cessation. Chinese Psychiatry 1996;10:29‐38. [Google Scholar]
Marsh 2005 {published data only}
- Marsh HS, Dresler CM, Choi JH, Targett DA, Gamble ML, Strahs KR. Safety profile of a nicotine lozenge compared with that of nicotine gum in adult smokers with underlying medical conditions: A 12‐week, randomized, open‐label study. Clinical Therapeutics 2005;27(10):1571‐87. [DOI] [PubMed] [Google Scholar]
McCarthy 2006 {published data only}
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. Journal of Abnormal Psychology 2006;115(3):454‐66. [DOI] [PubMed] [Google Scholar]
McRobbie 2010 {published data only}
- McRobbie H, Thornley S, Bullen C, Lin RB, Senior H, Laugesen M, et al. A randomized trial of the effects of two novel nicotine replacement therapies on tobacco withdrawal symptoms and user satisfaction. Addiction 2010;105(7):1290‐8. [DOI] [PubMed] [Google Scholar]
Meier 1990 {published data only}
- Meier Lammermann E, Mayer M, Boleski PL. Combination of transdermal nicotine substitution and behavioural group therapy in smoking cessation. European Respiratory Journal 1990;3(Suppl 10):168S. [Google Scholar]
Merz 1993 {published data only}
- Merz PG, Keller Stanislawski B, Huber T, Woodcock BG, Rietbrock N. Transdermal nicotine in smoking cessation and involvement of non‐ specific influences. International Journal of Clinical Pharmacology Therapy and Toxicology 1993;31:476‐82. [PubMed] [Google Scholar]
Miller 2009 {published data only}
- Miller CL, Sedivy V. Using a quitline plus low‐cost nicotine replacement therapy to help disadvantaged smokers to quit. Tobacco Control 2009;18(2):144‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Millie 1989 {published data only}
- Millie A, Berriau T, Paget D, Philardeau V, Postal MJ, Dautzenberg B, et al. Can weight gain during weaning from smoking be limited using nicotine gum? [Peut‐on limiter la prise de poids chez les fumeurs lors du sevrage tabagique en utilisant la gomme a la nicotine?]. Revue de Pneumonologie Clinique 1989;45:243‐9. [PubMed] [Google Scholar]
Minneker 1989 {published data only}
- Minneker E, Buchkremer G, Block M. The effect of different dosages of a transdermal nicotine substitution system on the success rate of smoking cessation therapy. Methods and Findings in Experimental and Clinical Pharmacology 1989;11:219‐22. [PubMed] [Google Scholar]
Molander 2000 {published data only}
- Molander L, Lunell E, Fagerström KO. Reduction of tobacco withdrawal symptoms with a sublingual nicotine tablet: a placebo controlled study. Nicotine & Tobacco Research 2000;2:187‐91. [DOI] [PubMed] [Google Scholar]
Mooney 2005 {published data only}
- Mooney M, Babb D, Jensen J, Hatsukami D. Interventions to increase use of nicotine gum: A randomized, controlled, single‐blind trial. Nicotine & Tobacco Research 2005;7(4):565‐79. [DOI] [PubMed] [Google Scholar]
Mulligan 1990 {published data only}
- Mulligan SC, Masterson JG, Devane JG, Kelly JG. Clinical and pharmacokinetic properties of a transdermal nicotine patch. Clinical Pharmacology and Therapeutics 1990;47:331‐7. [DOI] [PubMed] [Google Scholar]
Nackaerts 2009 {published data only}
- Nackaerts K, Salhi B, Devolder A, Meysman M, Boudrez H, Van‐Dyck L. A randomised, double‐blind, placebo‐controlled phase 3 study investigating the efficacy of nicotine substitution (NS) on nicotine withdrawal symptoms (NWS) in hospitalised smokers (HS) [Abstract]. European Respiratory Society Annual Congress; 2009 September 12‐16; Vienna, Austria,. 2009:4632.
NCT00000437 {published data only}
- NCT00000437. Tobacco dependence in alcoholism treatment (nicotine patch/naltrexone). ClinicalTrials.gov/show/NCT00000437 (first received 3 November 1999).
Okuyemi 2007 {published data only}
- Okuyemi KS, James AS, Mayo MS, Nollen N, Catley D, Choi WS, et al. Pathways to health: a cluster randomized trial of nicotine gum and motivational interviewing for smoking cessation in low‐income housing. Health Education & Behavior 2007;34:43‐54. [DOI] [PubMed] [Google Scholar]
Oncken 2009 {published data only}
- Oncken C, Campbell W, Chan G, Hatsukami D, Kranzler HR. Effects of nicotine patch or nasal spray on nicotine and cotinine concentrations in pregnant smokers. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(9):751‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piper 2016 {published data only}
- Piper ME, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, et al. Identifying effective intervention components for smoking cessation: a factorial screening experiment. Addiction 2016;111(1):129‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pomerleau 2003 {published data only}
- Lerman C, Audrain J, Patterson F, Kaufmann V, Rukstalis M, Wileyto EP, et al. Differential response to nicotine replacement therapies in obese and non‐obese women (PA2‐6). Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003; New Orleans, LA, USA. 2003.
- Pomerleau OF, Pomerleau CS, Marks JL, Snedecor SM, Mehringer AM, Namenek‐Brouwer RJ, et al. Prolonged nicotine patch use in quitters with past abstinence‐induced depressed mood. Journal of Substance Abuse Treatment 2003;24(1):13‐18. [DOI] [PubMed] [Google Scholar]
Rennard 2006 {published data only}
- Rennard SI, Glover E, Leischow S, Daughton DM, Glover P, Muramoto M. Efficacy of nicotine inhaler in smoking reduction. Nicotine & Tobacco Research 2002;4:380. [DOI] [PubMed] [Google Scholar]
- Rennard SI, Glover ED, Leischow S, Daughton DM, Glover PN, Muramoto M, et al. Efficacy of the nicotine inhaler in smoking reduction: A double‐blind, randomized trial. Nicotine & Tobacco Research 2006;8:555‐64. [DOI] [PubMed] [Google Scholar]
Rey 2009 {published data only}
- Rey L, Vaucher P, Secretan F, Zellweger JP, Bodenmann P. Use of nicotine substitute prescribed at hourly plus ad libitum intake or ad libitum for heavy smokers willing to quit: a randomized controlled trial. Substance Abuse Treatment, Prevention, & Policy 2009;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rigotti 2009 {published data only}
- Rigotti NA, Gonzales D, Dale LC, Lawrence D, Chang Y, CIRRUS Study Group. A randomized controlled trial of adding the nicotine patch to rimonabant for smoking cessation: efficacy, safety and weight gain. Addiction 2009;104(2):266‐76. [DOI] [PubMed] [Google Scholar]
Roddy 2006 {published data only}
- Roddy E, Romilly N, Challenger A, Lewis S, Britton J. Use of nicotine replacement therapy in socioeconomically deprived young smokers: a community‐based pilot randomised controlled trial. Tobacco Control 2006;15:373‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 1990 {published data only}
- Rose JE, Levin ED, Behm FM, Adivi C, Schur C. Transdermal nicotine facilitates smoking cessation. Clinical Pharmacology and Therapeutics 1990;47:323‐30. [DOI] [PubMed] [Google Scholar]
Rubinstein 2008 {published data only}
- Rubinstein ML, Benowitz NL, Auerback GM, Moscicki AB. A randomized trial of nicotine nasal spray in adolescent smokers. Pediatrics 2008;122(3):e595‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sachs 1995 {published data only}
- Sachs DPL. Effectiveness of the 4‐mg dose of nicotine polacrilex for the initial treatment of high‐dependent smokers. Archives of Internal Medicine 1995;155:1973‐80. [PubMed] [Google Scholar]
Schlam 2016 {published data only}
- Schlam TR, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, et al. Comparative effectiveness of intervention components for producing long‐term abstinence from smoking: a factorial screening experiment. Addiction 2016;111(1):142‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2004 {published data only}
- Schneider NG, Olmstead RE, Nides M, Mody FV, Otte Colquette P, Doan K, et al. Comparative testing of 5 nicotine systems: initial use and preferences. American Journal of Health Behavior 2004;28(1):72‐86. [DOI] [PubMed] [Google Scholar]
Schneider 2008 {published data only}
- Schneider NG, Cortner C, Gould JL, Koury MA, Olmstead RE. Comparison of craving and withdrawal among four combination nicotine treatments. Human Psychopharmacology 2008;23(6):513‐7. [DOI] [PubMed] [Google Scholar]
Schnoll 2015 {published data only}
- Schnoll RA, Goelz PM, Veluz‐Wilkins A, Blazekovic S, Powers L, Leone FT, et al. Long‐term nicotine replacement therapy: a randomized clinical trial. JAMA Internal Medicine 2015;175(4):504‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shahab 2011 {published data only}
- Shahab L, McEwen A, West R. Acceptability and effectiveness for withdrawal symptom relief of a novel oral nicotine delivery device: a randomised crossover trial. Psychopharmacology 2011;216(2):187‐96. [DOI] [PubMed] [Google Scholar]
Shiffman 2000a {published data only}
- Shiffman S, Khayrallah M, Nowak R. Efficacy of the nicotine patch for relief of craving and withdrawal 7‐10 weeks after cessation. Nicotine & Tobacco Research 2000;2:371‐8. [DOI] [PubMed] [Google Scholar]
Shiffman 2000b {published data only}
- Shiffman S, Elash CA, Paton SM, Gwaltney CJ, Paty JA, Clark DB, et al. Comparative efficacy of 24‐hour and 16‐hour transdermal nicotine patches for relief of morning craving. Addiction 2000;95(8):1185‐95. [DOI] [PubMed] [Google Scholar]
Shiffman 2002a {published data only}
- Ferguson SG, Gitchell JG, Shiffman S. Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction 2012;107(7):1349‐53. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dresler CM, Gorsline J, Dimarino ME. The efficacy of nicotine patch in an over‐the‐counter environment: results from a randomized, double‐blind, placebo‐controlled trial (PO130). 11th World Conference on Tobacco or Health; 2000 August 06‐11; Chicago, Illinois, USA. 2000; Vol. 1.
- Shiffman S, Gorsline J, Gorodetzky CW. Efficacy of over‐the‐counter nicotine patch. Nicotine & Tobacco Research 2002;4:477‐83. [DOI] [PubMed] [Google Scholar]
Shiffman 2002b {published data only}
- Shiffman S, Rolf CN, Hellebusch SJ, Gorsline J, Gorodetzky CW, Chiang YK, et al. Real‐world efficacy of prescription and over‐the‐counter nicotine replacement therapy. Addiction 2002;97:505‐16. [DOI] [PubMed] [Google Scholar]
Shiffman 2006 {published data only}
- Ferguson SG, Shiffman S. Effect of high‐dose nicotine patch on the characteristics of lapse episodes. Health Psychology 2010;29:358‐66. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. Journal of Consulting and Clinical Psychology 2006;74:1153‐61. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ. Immediate hedonic response to smoking lapses: Relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology 2006;184(3‐4):608‐18. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence‐induced withdrawal and craving using high‐dose nicotine replacement therapy. Psychopharmacology 2006;184(3‐4):637‐44. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kirchner TR. Cigarette‐by‐cigarette satisfaction during ad libitum smoking. Journal of Abnormal Psychology 2009;118(2):348‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, et al. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. Journal of Consulting & Clinical Psychology 2006;74:276‐85. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychology 2003;22:378‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue‐provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting and Clinical Psychology 2004;72(6):1136‐43. [DOI] [PubMed] [Google Scholar]
Stapleton 2011 {published data only}
- Stapleton JA, Sutherland G. Treating heavy smokers in primary care with the nicotine nasal spray: randomized placebo‐controlled trial. Addiction 2011;106(4):824‐32. [DOI] [PubMed] [Google Scholar]
Sun 2009 {published data only}
- Sun HQ, Guo S, Chen DF, Jiang ZN, Liu Y, Di XL, et al. Family support and employment as predictors of smoking cessation success: a randomized, double‐blind, placebo‐controlled trial of nicotine sublingual tablets in Chinese smokers. American Journal of Drug & Alcohol Abuse 2009;35(3):183‐8. [DOI] [PubMed] [Google Scholar]
Sussman 2004 {published data only}
- Sussman S, McCuller WJ, Zheng H, Pfingston YM, Miyano J, Dent CW. Project EX: a program of empirical research on adolescent tobacco use cessation. Tobacco Induced Diseases 2004;2(3):119‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sutherland 1999 {published data only}
- Anon. Combination NRT; Improving efficacy in smoking cessation. McNeil Consumer Healthcare booklet. Short term outcomes reported on p18.
- Sutherland G. A placebo‐controlled double‐blind combination trial of nicotine spray and patch [abstract]. Nicotine & Tobacco Research 1999;1:186. [Google Scholar]
Sutherland 2005 {published data only}
- Sutherland G, Stapleton JA, Russell MA. Randomized placebo‐controlled trial of nicotine nasal spray in general practice. Nicotine & Tobacco Research 2005;7(4):686. [Google Scholar]
Sutton 1987 {published data only}
- Sutton S, Hallett R. Randomized trial of brief individual treatment for smoking using nicotine chewing gum in a workplace setting. American Journal of Public Health 1987;77:1210‐1. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sutton 1988 {published data only}
- Sutton S, Hallett R. Smoking intervention in the workplace using videotapes and nicotine chewing gum. Preventive Medicine 1988;17:48‐59. [DOI] [PubMed] [Google Scholar]
Thorsteinsson 2001 {published data only}
- Thorsteinsson HS, Gillin JC, Patten CA, Golshan S, Sutton LD, Drummond S, et al. The effects of transdermal nicotine therapy for smoking cessation on depressive symptoms in patients with major depression. Neuropsychopharmacology 2001;24(4):350‐8. [DOI] [PubMed] [Google Scholar]
Tsukahara 2010 {published data only}
- Tsukahara H, Noda K, Saku K. A randomized controlled open comparative trial of varenicline vs nicotine patch in adult smokers: efficacy, safety and withdrawal symptoms (the VN‐SEESAW study). Circulation Journal 2010;74(4):771‐8. [DOI] [PubMed] [Google Scholar]
Tundulawessa 2010 {published data only}
- Tundulawessa Y, Yongchaiyud P, Chutrthong W, Tundulawessa K. The bioequivalent and effect of nicotine formulation gum on smoking cessation. Journal of the Medical Association of Thailand 2010;93(5):574‐9. [PubMed] [Google Scholar]
Tzivoni 1998 {published data only}
- Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovascular Drugs and Therapy 1998;12:239‐44. [DOI] [PubMed] [Google Scholar]
Tønnesen 1996 {published data only}
- Tønnesen P, Mikkelsen K, Norregaard J, Jorgensen S. Recycling of hard‐core smokers with nicotine nasal spray. European Respiratory Journal 1996;9:1619‐23. [DOI] [PubMed] [Google Scholar]
Uyar 2005 {unpublished data only}
- Uyar M, Bayram N, Filiz A, Elbek O, Topçu A, Dikensoy O, et al. Comparison of nicotine patch and bupropion in treating tobacco dependence. European Respiratory Journal 2005;26(Suppl 49):388s. [Google Scholar]
Velicer 2006 {published data only}
- Velicer WF, Friedman RH, Fava JL, Gulliver SB, Keller S, Sun X, et al. Evaluating nicotine replacement therapy and stage‐based therapies in a population‐based effectiveness trial. Journal of Consulting & Clinical Psychology 2006;74:1162‐72. [DOI] [PubMed] [Google Scholar]
- Velicer WF, Keller S, Friedman RH, Fava JL, Gulliver SB, Ward RM, et al. Comparing participants and nonparticipants recruited for an effectiveness study of nicotine replacement therapy. Annals of Behavioral Medicine 2005;29:181‐91. [DOI] [PubMed] [Google Scholar]
Vial 2002 {published data only}
- Vial RJ, Jones TE, Ruffin RE, Gilbert AL. Smoking cessation program using nicotine patches linking hospital to the community. Journal of Pharmacy Practice and Research 2002;32(1):57‐62. [Google Scholar]
Vikhireva 2003 {published data only}
- Vikhireva O, Shalnova S, Deev A. Nicotine replacement therapy in Russia: old wine in new skins? Randomized parallel study of nicotine gum/inhaler in smoking cessation/reduction. Society for Research on Nicotine and Tobacco. 5th European Conference; 2003 November 20‐22; Padua, Italy. 2003.
- Vikhireva O, Shalnova S, Deev A, Levshin V, Radkevich N, Kalinina A. NRT‐assisted cessation in Russia: individual and population level benefits. Society for Research on Nicotine and Tobacco 11th Annual Meeting; 2005 March 20‐23; Prague, Czech Republic. 2005.
Warner 2005 {published data only}
- Warner DO, Patten CA, Ames SC, Offord KP, Schroeder DR. Effect of nicotine replacement therapy on stress and smoking behavior in surgical patients. Anesthesiology 2005;102(6):1138‐46. [DOI] [PubMed] [Google Scholar]
Wennike 2003a {published data only}
- Wennike P, Danielsson T, Landfeldt B, Westin A, Tønnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo‐controlled trial of nicotine gum with 2‐year follow‐up. Addiction 2003;98(10):1395‐402. [DOI] [PubMed] [Google Scholar]
Williams 2007 {published and unpublished data}
- Williams JM, Gandhi KK, Foulds J, Steinberg M, Lou S, Masumova F, et al. No advantage for high dose compared to regular dose nicotine patch on short‐term abstinence rates in schizophrenia (PA2‐3). Society for Research on Nicotine and Tobacco 13th Annual Meeting; 2007 February 21‐24, Austin, Texas. 2007.
Wiseman 2005 {published data only}
- Wiseman EJ, Williams DK, McMillan DE. Effectiveness of payment for reduced carbon monoxide levels and noncontingent payments on smoking behaviors in cocaine‐abusing outpatients wearing nicotine or placebo patches. Experimental and Clinical Psychopharmacology 2005;13(2):102‐10. [DOI] [PubMed] [Google Scholar]
Working Group 1994 {published data only}
- Working Group for the Study of Transdermal Nicotine. Nicotine replacement therapy for patients with coronary artery disease. Working Group for the Study of Transdermal Nicotine in Patients with Coronary artery disease. Archives of Internal Medicine 1994;154:989‐95. [PubMed] [Google Scholar]
References to ongoing studies
NCT01010477 {unpublished data only}
- NCT01010477. Trial of nicotine nasal spray as an aid for smoking cessation in schizophrenia. ClinicalTrials.gov/show/NCT01010477 2009.
NCT01484340 {published data only}
- NCT01484340. A smoking cessation trial in HIV‐infected patients in South Africa. ClinicalTrials.gov/show/NCT01484340 (first received 2 December 2011).
NCT02918500 {published data only}
- NCT02918500. Effect of pre‐op NRT on peri‐operative complications and long‐term abstinence: a pilot trial in patients undergoing CABG surgery. ClinicalTrials.gov/show/NCT02918500 (first received 29 September 2016).
Additional references
Alpert 2012
Altman 2004
- Altman DG, Schulz KF, Moher D. Turning a blind eye: testing the success of blinding and the CONSORT statement. BMJ 2004;328(7448):1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 2012
- Bailey SR, Crew EE, Riske EC, Ammerman S, Robinson TN, Killen JD. Efficacy and tolerability of pharmacotherapies to aid smoking cessation in adolescents. Paediatric Drugs 2012;14(2):91‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cahill 2015
- Cahill K, Hartmann‐Boyce J, Perera R. Incentives for smoking cessation. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD004307.pub5] [DOI] [PubMed] [Google Scholar]
Cahill 2016
- Cahill K, Lindson‐Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD006103.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2012
- Carson KV, Verbiest MEA, Crone MR, Brinn MP, Esterman AJ, Assendelft WJJ, et al. Training health professionals in smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD000214.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cepeda‐Benito 2004
- Cepeda‐Benito A, Reynoso JT, Erath S. Meta‐analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. Journal of Consulting and Clinical Psychology 2004;72:712‐22. [DOI] [PubMed] [Google Scholar]
Clavel 1984
- Clavel F, Benhamou S. Tobacco withdrawal. Comparison of the efficacy of various methods. Intermediate results of a comparative study [Désintoxcation tabagique. Comparison de l'efficacité de differentes méthodes. Résultats intermédiaires d'une étude comparative]. Presse Médicale 1984;13:975‐7. [PubMed] [Google Scholar]
Coleman 2015
- Coleman T, Chamberlain C, Davey M, Cooper SE, Leonardi‐Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD010078.pub2] [DOI] [PubMed] [Google Scholar]
CONSORT 2001
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Annals of Internal Medicine 2001;134:663‐94. [DOI] [PubMed] [Google Scholar]
Cooper 2003
- Cooper TV, Montgomery GV, Debon MW, Zbikowski SM, Klesges RC, Johnson KC. The effects of PPA and nicotine gum on cessation rates and post cessation weight gain in women [POS3‐46]. Society for Research on Nicotine and Tobacco 9th Annual Meeting; 2003; New Orleans, LA, USA. 2003.
Daughton 1999
- Daughton DM, Fortmann SP, Glover ED, Hatsukami DK, Heatley SA, Lichtenstein E, et al. The smoking cessation efficacy of varying doses of nicotine patch delivery systems 4 to 5 years post‐quit day. Preventive Medicine 1999;28:113‐8. [DOI] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Higgins JPT, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. in: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org.
Durcan 2002
- Durcan MJ, White J, Jorenby DE, Fiore MC, Rennard SI, Leischow SJ, et al. Impact of prior nicotine replacement therapy on smoking cessation efficacy. American Journal of Health Behaviors 2002;26:213‐20. [DOI] [PubMed] [Google Scholar]
Etter 2006
- Etter JF, Stapleton JA. Nicotine replacement therapy for long‐term smoking cessation: a meta‐analysis. Tobacco Control 2006;15:280‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Etter 2007
- Etter JF, Burri M, Stapleton J. The impact of pharmaceutical company funding on results of randomized trials of nicotine replacement therapy for smoking cessation: a meta‐analysis (PA9‐6). Addiction 2007;102:815‐22. [DOI] [PubMed] [Google Scholar]
Fagerström 2003
- Fagerström KO. Clinical treatment of tobacco dependence: The endurance of pharmacologic efficacy. Journal of Clinical Psychiatry Monograph 2003;18:35‐40. [Google Scholar]
Fanshawe 2017
- Fanshawe TR, Halliwell W, Lindson N, Aveyard P, Livingstone‐Banks J, Hartmann‐Boyce. Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD003289.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiore 1992
- Fiore MC, Jorenby DE, Baker TB, Kenford SL. Tobacco dependence and the nicotine patch. Clinical guidelines for effective use. JAMA 1992;268:2687‐94. [PubMed] [Google Scholar]
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service, May 2008. [Google Scholar]
Greenland 1998
- Greenland S, Satterfield MH, Lanes SF. A meta‐analysis to assess the incidence of adverse effects associated with the transdermal nicotine patch. Drug Safety 1998;18:297‐308. [DOI] [PubMed] [Google Scholar]
Hartmann‐Boyce 2016
- Hartmann‐Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD010216.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henningfield 2005
- Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer Journal for Clinicians 2005;55:281‐99. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2001
- Hughes JR. The effectiveness of over‐the‐counter nicotine replacement: a rebuttal. Drug and Alcohol Review 2001;20:319‐22. [Google Scholar]
Hughes 2004
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long‐term abstinence among untreated smokers. Addiction 2004;99:29‐38. [DOI] [PubMed] [Google Scholar]
Hughes 2014
- Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000031.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Italy ISS 2004
- Osservatorio Fumo, Alcol e Droga. Linee Guida Cliniche per Promuovere la Cessazione dell’Abitudine al Fumo. Rome: Istituto Superiore di Sanita, 2004. [Google Scholar]
Jensen 1990
- Jensen EJ, Schmidt E, Pedersen B, Dahl R. Effect of nicotine, silver acetate, and ordinary chewing gum in combination with group counselling on smoking cessation. Thorax 1990;45:831‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph 1999
- Joseph AM, Antonnucio DO. Lack of efficacy of transdermal nicotine in smoking cessation. New England Journal of Medicine 1999;341:1157‐8. [DOI] [PubMed] [Google Scholar]
Joseph 2003
- Joseph AM, Fu SS. Safety issues in pharmacotherapy for smoking in patients with cardiovascular disease. Progress in Cardiovascular Diseases 2003;45:429‐41. [DOI] [PubMed] [Google Scholar]
Kasza 2012
- Kasza KA, Hyland AJ, Borland R, McNeill AD, Bansal‐Travers M, Fix BV, et al. Effectiveness of stop‐smoking medications: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction 2012. [DOI: 10.1111/j.1360-0443.2012.04009.x] [DOI] [PMC free article] [PubMed]
Kotz 2014
- Kotz D, Brown J, West R. Prospective cohort study of the effectiveness of smoking cessation treatments used in the "real world". Mayo Clinic Proceedings 2014;89(10):1360‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le Foll 2005
- Foll B, Melihan‐Cheinin P, Rostoker G, Lagrue G. Smoking cessation guidelines: evidence‐based recommendations of the French Health Products Safety Agency. European Psychiatry 2005;20:431‐41. [DOI] [PubMed] [Google Scholar]
Lindson‐Hawley 2016
- Lindson‐Hawley N, Hartmann‐Boyce J, Fanshawe TR, Begh R, Farley A, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD005231.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meine 2005
- Meine TJ, Patel MR, Washam JB, Pappas PA, Jollis JG. Safety and effectiveness of transdermal nicotine patch in smokers admitted with acute coronary syndromes. American Journal of Cardiology 2005;95:976‐8. [DOI] [PubMed] [Google Scholar]
Mikkelsen 1994
- Mikkelsen KL, Tønnesen P, Norregaard J. Three‐year outcome of two‐ and three‐year sustained abstainers from a smoking cessation study with nicotine patches. Journal of Smoking‐Related Disorders 1994;5:95‐100. [Google Scholar]
Mills 2010
- Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta‐analysis of one hundred and twenty studies involving 177,390 individuals. Tobacco Induced Diseases 2010;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2014
- Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta‐analysis. Circulation 2014;129(1):28‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mooney 2004
- Mooney M, White T, Hatsukami D. The blind spot in the nicotine replacement therapy literature: assessment of the double‐blind in clinical trials. Addictive Behaviors 2004;29(4):673‐84. [DOI] [PubMed] [Google Scholar]
Muller 1990
- Muller P, Abelin T, Ehrsam R, Imhof P, Howald H, Mauli D. The use of transdermal nicotine in smoking cessation. Lung 1990;168:445‐53. [DOI] [PubMed] [Google Scholar]
Munafó 2004a
- Munafó M, Bradburn M, Bowes L, David S. Are there sex differences in transdermal nicotine replacement therapy patch efficacy? A meta‐analysis. Nicotine & Tobacco Research 2004;6:769‐76. [DOI] [PubMed] [Google Scholar]
Munafó 2004b
- Munafó M, Bradburn M, Bowes L, David S. Investigating subgroups in smoking cessation treatment response: Response to Perkins. Nicotine & Tobacco Research 2004;6:865‐7. [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. Public Health Guideline [PH10] Stop Smoking Services. National Institute for Health and Clinical Excellence, 2008. [Google Scholar]
NZ MoH 2014
- Ministry of Health. The New Zealand Guidelines for Helping People to Stop Smoking.. Wellington, New Zealand: Ministry of Health, 2014. [Google Scholar]
Ockene 1994
- Ockene JK, Kristeller J, Pbert L, Hebert JR, Luippold R, Goldberg RJ, et al. The physician‐delivered smoking intervention project: can short‐term interventions produce long‐term effects for a general outpatient population?. Health Psychology 1994;13:278‐81. [DOI] [PubMed] [Google Scholar]
Palmer 1992
- Palmer KJ, Buckley MM, Faulds D. Transdermal nicotine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy as an aid to smoking cessation. Drugs 1992;44:498‐529. [DOI] [PubMed] [Google Scholar]
Perkins 2004
- Perkins KA. Obstacles to determining individual differences in the efficacy of smoking cessation medications. Nicotine & Tobacco Research 2004;6:765‐7. [DOI] [PubMed] [Google Scholar]
Pierce 2002
- Pierce JP, Gilpin EA. Impact of over‐the‐counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA 2002;288:1260‐4. [DOI] [PubMed] [Google Scholar]
Richmond 1997
- Richmond RL, Kehoe L, Neto ACdA. Effectiveness of a 24‐hour transdermal nicotine patch in conjunction with a cognitive behavioural programme: One year outcome. Addiction 1997;92:27‐31. [PubMed] [Google Scholar]
Schneider 1983
- Schneider NG, Jarvik ME, Forsythe AB, Read LL, Elliott ML, Schweiger A. Nicotine gum in smoking cessation: a placebo‐controlled, double‐blind trial. Addictive Behaviors 1983;8:253‐61. [DOI] [PubMed] [Google Scholar]
Stapleton 1998
- Stapleton JA, Sutherland G, Russell MAH. How much does relapse after one year erode effectiveness of smoking cessation treatments? Long term follow up of randomised trial of nicotine nasal spray. BMJ 1998;316(7134):830‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stapleton 2012
- Stapleton, JA. Perverse conclusion from results. Tobacco Control 2012:np.
Suissa 2017
- Suissa K, Larivière J, Eisenberg MJ, Eberg M, Gore GC, Grad R, et al. Efficacy and safety of smoking cessation interventions in patients with cardiovascular disease: a network meta‐analysis of randomized controlled trials. Circulation: Cardiovascular Quality and Outcomes 2017;10(1):e002458. [DOI] [PubMed] [Google Scholar]
TNWG 1994
- Transdermal Nicotine Working Group. Nicotine replacement therapy for patients with coronary artery disease. Working Group for the Study of Transdermal Nicotine in Patients with Coronary Artery Disease. Archives of Internal Medicine 1994;154:989‐95. [PubMed] [Google Scholar]
Tønnesen 1992
- Tønnesen P, Norregaard J, Sawe U. Two‐year outcome in a smoking cessation trial with a nicotine patch. Journal of Smoking‐Related Disorders 1992;3:241‐5. [Google Scholar]
Wallstrom 1999
- Wallstrom M, Sand L, Nilsson F, Hirsch JM. The long‐term effect of nicotine on the oral mucosa. Addiction 1999;94:417‐23. [DOI] [PubMed] [Google Scholar]
Walsh 2000
- Walsh RA, Penman AG. The effectiveness of nicotine replacement therapy over‐the‐counter. Drug and Alcohol Review 2000;19:243‐7. [Google Scholar]
Walsh 2001
- Walsh RA, Penman AG. The effectiveness of over‐the‐counter nicotine replacement: reply to Hughes. Drug and Alcohol Review 2001;20:322‐4. [Google Scholar]
Walsh 2007
- Walsh RA, Stead L, Lancaster T. The Cochrane review on nicotine replacement therapy: incorrect or uncertain classifications of additional support levels & Authors' response. Tobacco Control 2007;16:215‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2001
- West R, .Shiffman S. Effect of oral nicotine dosing forms on cigarette withdrawal symptoms and craving: a systematic review. Psychopharmacology Berl 2001;155:115‐22. [DOI] [PubMed] [Google Scholar]
West 2007
- West R, Zhou X. Is nicotine replacement therapy for smoking cessation effective in the "real world"? Findings from a prospective multinational cohort study. Thorax 2007;62:998‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woolacott 2002
- Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al. The clinical effectiveness and cost‐effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation. Health Technology Assessment 2002;6:1‐245. [DOI] [PubMed] [Google Scholar]
Zwar 2011
- Zwar N, Borland R, Peters M, Litt J, Bell J, Caldwell B, et al. Supporting Smoking Cessation: A Guide for Health Professionals. Melbourne: The Royal Australian College of General Practitioners, 2011. [Google Scholar]
References to other published versions of this review
Silagy 1994a
- Silagy C, Mant D, Fowler G, Lodge M. Meta‐analysis on efficacy of nicotine replacement therapies in smoking cessation. Lancet 1994;343:139‐42. [DOI] [PubMed] [Google Scholar]
Silagy 1994b
- Silagy C, Mant D, Fowler G, Lancaster T. The effectiveness of nicotine replacement therapies in smoking cessation. Online Journal of Current Clinical Trials 1994;Doc No:113. [DOI] [PubMed] [Google Scholar]
Silagy 1996
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 1996, Issue 2. [DOI: 10.1002/14651858.CD000146] [DOI] [Google Scholar]
Silagy 2001
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD000146] [DOI] [PubMed] [Google Scholar]
Silagy 2002
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD000146] [DOI] [PubMed] [Google Scholar]
Silagy 2004
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000146] [DOI] [PubMed] [Google Scholar]
Stead 2008
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000146.pub3] [DOI] [PubMed] [Google Scholar]
Stead 2012
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]


