“At this point, I am willing to try anything just to keep him safe from hurting himself or anyone else. I hate the feeling of being scared to death of him.”—Lewy Body Dementia Association, “Behavioral Issues” forum participant (9 January 2009).
Recurrent visual hallucinations are a core feature of dementia with Lewy bodies (DLB).1 While the spectrum of behavioral and psychological symptoms of dementia in DLB is broader than psychosis alone—also including anxiety, depression, apathy, and agitation2—hallucinations and delusions (i.e., psychosis) are common in patients with DLB and are a key issue in living with the disease. Hallucinations were described in 76% and delusions in 57% of patients with DLB in 1 cross‐sectional study.3 When comparing mild versus moderate‐severe DLB (as determined by the severity of cognitive impairment), psychosis frequency increased from 50% to over 75%. Specifically, hallucinations increased from 30% to 70%, and delusions increased from 20% to over 50%.2 Other research shows that delusion and hallucination scores on the Neuropsychiatric Inventory are high in DLB regardless of dementia severity.4
Delusions are an important determinant of quality of life (QoL) in DLB as assessed by both patients and caregivers.5 Behavioral disturbances are associated with depression in DLB caregivers6 and are major contributors to caregiver distress and strain.7, 8, 9 Although research is lacking with regard to the determinants of institutionalization in DLB, Parkinson's disease (PD) studies show that psychosis is the main risk factor for nursing home placement.10, 11
The management of hallucinations and delusions in DLB is challenging, however. Antipsychotics have risks in patients with DLB. “Severe neuroleptic sensitivity” is a suggestive feature in DLB clinical diagnostic criteria,1 in part based on research showing that 81% of patients with DLB who received typical antipsychotics had adverse reactions, one‐half of which were severe.12 Atypical antipsychotics received a black‐box warning from the US Food and Drug Administration (FDA) in 2005 due to an increased risk of death in patients with dementia‐related behavioral disturbances. This warning was extended to typical antipsychotics in 2008. Studies demonstrate an increased risk of death in patients with dementia who are treated with antipsychotics in both the short and long term.13, 14 Recent evidence also shows an increased mortality risk in patients with PD who are treated with typical and atypical antipsychotics.15 Besides the obvious risk of worsened parkinsonism with antipsychotics, some studies suggest that antipsychotic use is associated with an increased rate of cognitive decline in dementia in both community settings16 and Alzheimer's disease clinical trials.17
The benefits of antipsychotics in DLB are uncertain, but few alternatives exist. A meta‐analysis of pharmacologic strategies for the closely related Lewy body dementias (LBDs) (i.e., both DLB and PD dementia [PDD]) found few studies of antipsychotic agents in these conditions. In the small studies cited, often with limited methodologic rigor, there was no evidence for efficacy and also a limited ability to exclude potentially beneficial effects.18 When considering PDD with psychosis, a Movement Disorder Society evidence‐based medicine review concluded that clozapine was “efficacious” and had an acceptable risk with specialized monitoring; quetiapine was considered “investigational” given a lack of supporting evidence; and olanzapine was characterized as “unlikely efficacious” and as having “unacceptable risk” given clearly demonstrated worsening of motor function.19
In considering antipsychotic alternatives, a systematic review of 22 pharmacologic strategies for DLB found that only cholinesterase inhibitors (donepezil and rivastigmine) had high‐level evidence for either cognitive or psychiatric symptoms in DLB, but evidence of benefit on neuropsychiatric symptoms was limited and included the possibility of no effect.18 Furthermore, 25% of patients with DLB and PDD who were receiving cholinesterase inhibitors withdrew from treatment trials,18 suggesting that substantial numbers of patients with DLB may not tolerate these drugs. Numerous other agents have been tried in DLB, including dopaminergic agents, antidepressants, and antiepileptics, all without good evidence. Nonpharmacologic approaches to managing the behavioral and psychological symptoms of dementia symptoms are encouraged20 but have little research evidence to support efficacy in DLB. Pimavanserin, a selective serotonin (5‐HT)2A‐inverse agonist, was FDA approved for the treatment of PD psychosis in April 2016.21 Pimavanserin is not yet studied in DLB but will likely be relevant and a phase II trial of another (5‐HT)2A‐inverse agonist is currently underway for patients with LBD.
Clinicians, patients, and families are often left wondering what to do, particularly when hallucinations and delusions are distressing or when they contribute to behaviors that put patients and family members at risk. In this scenario, lack of treatment evidence is unsatisfying and untenable; something has to be tried. As suggested in the opening quote, when patients or families are at risk, families are willing to consider medications with risks, as the risks of doing nothing are also high. Another Lewy Body Dementia Association forum participant expressed this view:
“Risperdal is the only thing that worked… It is on the no‐no list for LBD, but her psychosis got so bad without it that it affected her physical health and well‐being. If she wasn't on this medication, she would not sleep, eat, or trust any of her family… The side effects from the Risperdal were minimal and worth it so she could have peace and we could keep her home with us” (“Behavioral Issues” forum, 9 January 2009).
In making decisions regarding the treatment of psychosis in DLB, physicians, caregivers/family, and patients (when able) should partner in shared decision making (SDM) to consider values and preferences alongside evidence to make the best decision in each unique situation,22 with physicians helping patients and families to understand the potential benefits and risks of antipsychotic use.
The approach to antipsychotic use in patients with DLB starts with avoidance (Fig. 1). Addressing underlying medical conditions and weaning medications that may exacerbate hallucinations and delusions are important first steps. Evidence from PDD suggests that treating systemic illness, adjusting medications, and monitoring over weeks may preclude the need for antipsychotic therapy in the short term in as many as two‐thirds of patients, though one‐half of these patients required antipsychotic medication on long‐term follow‐up.23 When discontinuing medications, the approach favored in PDD with psychosis is also relevant in DLB: start by discontinuing medications with anticholinergic properties, including tricyclic antidepressants and bladder antispasmodics. Sequential consideration is then given to discontinuing amantadine, monoamine oxidase B inhibitors, and dopamine agonists (all of which are less commonly used in DLB than in PD). Finally, if needed and tolerated, wean levodopa/carbidopa to the dose that best balances cognitive/behavioral and motor effects.24
Figure 1.
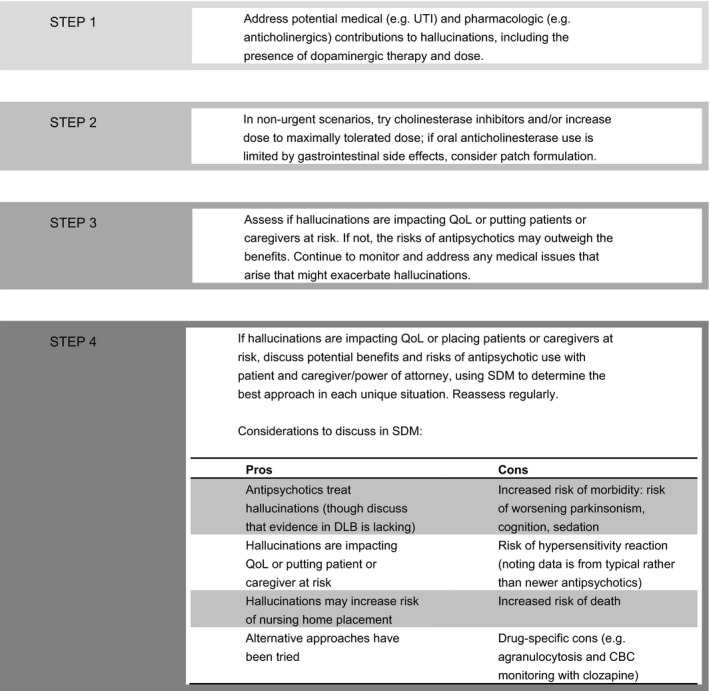
Approach to antipsychotic use in patients who have dementia with Lewy bodies. UTI, urinary tract infection; QoL, quality of life; DLB, dementia with Lewy bodies; CBC, complete blood count; SDM, shared decision making.
While robust evidence for a beneficial effect of cholinesterase inhibitors on hallucinations and delusions is lacking, there is some evidence that these medications may help people with DLB and improve psychiatric symptoms in those with PDD.18 Thus, cholinesterase inhibitors can be tried for overall benefit with a hoped‐for impact on hallucinations and delusions.
When antipsychotics are indicated, personal values and needs play a role in antipsychotic selection. Expert consensus favors clozapine use given its evidence in PD psychosis and a lower risk of worsening parkinsonism than other antipsychotics. However, some patients and families are unwilling to accept even the low risk of agranulocytosis (0.38%19) or feel unable to commit to the demands of routine blood work and pharmacy visits, although home nursing visits can alleviate some of the laboratory burden, and the frequency of blood draws now is less than in the past.
General antipsychotic considerations are class‐wide, but agent‐specific data exist. Concerns regarding antipsychotic hypersensitivity were established with case series involving typical antipsychotics; the risks of hypersensitivity with newer antipsychotics are unknown. Risks of parkinsonism are higher with typical antipsychotics and higher potency atypical antipsychotics (e.g., olanzapine and risperidone) and are less with clozapine and quetiapine, with limited data for other agents. In a retrospective case‐control study of antipsychotic use in patients with general dementia, haloperidol was associated with the highest 6‐month absolute mortality risk (3.9%; 95% confidence interval [CI], 1.0%–6.6%), quetiapine had the lowest risk (2.0%; 95% CI, 0.7%–3.3%), and risperidone (3.7%; 95% CI, 2.2%–5.3%) and olanzapine (2.5%; 95% CI, 0.3%–4.7%) fell in between.13 Similarly, in patients with PD, the absolute mortality risk (converted from person‐years to 6‐month risks assuming linear death rates) was highest in those exposed to haloperidol (26%; 95% CI, 18%–36%) and lowest in those who received quetiapine (8%; 95% CI, 7%–9%), with risperidone (14%; 95% CI, 12%–17%) and olanzapine (11%; 95% CI, 8%–15%) in between.15 Epidemiologic mortality data rarely include clozapine given its infrequent use.
When performing SDM regarding the management of problematic psychosis, discussions of antipsychotic use should include both pros and cons (Fig. 1). When available, using absolute risks improves patient and family understanding of the risks to inform decision making.25 Absolute risks are available for agranulocytosis with clozapine, and there are mortality data for some antipsychotics (above), but the absolute risks of worsened cognition, parkinsonism, and serious hypersensitivity reactions are not well‐defined, and this needs to be part of the discussion. If trying an antipsychotic, it should be titrated to either improvement or side effects that prompt discontinuation.
Effective and safe treatment options for psychosis in patients with DLB remain an unmet need. In the interim, the use of antipsychotics in this population is driven not by evidence but by clinical need. While strategies to avoid antipsychotic use are important, should these fail, antipsychotics can be a reasonable strategy after SDM if the risks of ongoing symptoms outweigh those associated with treatment.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
M.J.A.: 1A, 1B, 1C, 3A, 3B
D.W.: 1A, 1B, 1C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding sources and conflicts of interest: Melissa J. Armstrong is supported by an Agency for Healthcare Research and Quality career‐development grant (K08HS24159‐01). She reports no conflicts of interest related to this article. Daniel Weintraub reports a Veterans Health Administration (VHA) Merit Review Award (IIR 12‐144‐2). Support for Veterans Affairs/Centers for Medicare and Medicaid Services data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02‐237 and 98‐004).
Funding sources and conflicts of interest for the previous 12 months: Melissa J. Armstrong was a local investigator for studies sponsored by the Parkinson Study Group, the Huntington Study Group, the CHDI Foundation, AbbVie, Biotie, and Insightec, and she received funding as a sub‐investigator or local investigator on National Institutes of Health grants U01 AR057967‐01, U01NS080818‐01A1, and U01NS080840‐01A1; she receives royalties for publication of Parkinson's Disease: Improving Patient Care (Oxford University Press); she serves as an evidence‐based medicine methodology consultant for the American Academy of Neurology (AAN); and she has received stipends for serving as faculty at the annual meeting of the AAN and as faculty on an AAN online course on evidence‐based medicine. Daniel Weintraub has received research funding or support from the Michael J. Fox Foundation for Parkinson's Research, the National Institutes of Health (National Institute of Neurological Disorders and Stroke), Novartis Pharmaceuticals, the Department of Veterans Affairs, Avid Radiopharmaceuticals, Alzheimer's Disease Cooperative Study, and the International Parkinson and Movement Disorder Society; honoraria from AbbVie, Acadia, Biotie, Clintrex LLC, Novartis, Teva Pharmaceuticals, UCB, and the CHDI Foundation; license fee payments from the University of Pennsylvania for the QUIP and QUIP‐RS questionnaires; royalties from Wolters Kluweland; and fees for legal consultation for a lawsuit related to antipsychotic prescribing in a patient with Parkinson's disease.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 2. Borroni B, Agosti C, Padovani A. Behavioral and psychological symptoms in dementia with Lewy‐bodies (DLB): frequency and relationship with disease severity and motor impairment. Arch Gerontol Geriatr 2008;46:101–106. [DOI] [PubMed] [Google Scholar]
- 3. Aarsland D, Ballard C, Larsen JP, McKeith I. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson's disease with and without dementia. Int J Geriatr Psychiatry 2001;16:528–536. [DOI] [PubMed] [Google Scholar]
- 4. Hashimoto M, Yatabe Y, Ishikawa T, et al. Relationship between dementia severity and behavioral and psychological symptoms of dementia in dementia with Lewy bodies and Alzheimer's disease patients. Dement Geriatr Cogn Dis Extra 2015;5:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bostrom F, Jonsson L, Minthon L, Londos E. Patients with dementia with Lewy bodies have more impaired quality of life than patients with Alzheimer disease. Alzheimer Dis Assoc Disord 2007;21:150–154. [DOI] [PubMed] [Google Scholar]
- 6. Lowery K, Mynt P, Aisbett J, Dixon T, O'Brien J, Ballard C. Depression in the carers of dementia sufferers: a comparison of the carers of patients suffering from dementia with Lewy bodies and the carers of patients with Alzheimer's disease. J Affect Disord 2000;59:61–65. [DOI] [PubMed] [Google Scholar]
- 7. Ricci M, Guidoni SV, Sepe‐Monti M, et al. Clinical findings, functional abilities and caregiver distress in the early stage of dementia with Lewy bodies (DLB) and Alzheimer's disease (AD). Arch Gerontol Geriatr 2009;49:e101–e104. [DOI] [PubMed] [Google Scholar]
- 8. Leggett AN, Zarit S, Taylor A, Galvin JE. Stress and burden among caregivers of patients with Lewy body dementia. Gerontologist 2011;51:76–85. [DOI] [PubMed] [Google Scholar]
- 9. Svendsboe E, Terum T, Testad I, et al. Caregiver burden in family carers of people with dementia with Lewy bodies and Alzheimer's disease [published online ahead of print 14 Jan 2016]. Int J Geriatr Psychiatry. doi: 10.1002/gps.4433 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10. Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson's disease. Neurology 1993;43:2227–2229. [DOI] [PubMed] [Google Scholar]
- 11. Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population‐based, prospective study. J Am Geriatr Soc 2000;48:938–942. [DOI] [PubMed] [Google Scholar]
- 12. McKeith I, Fairbairn A, Perry R, Thompson P, Perry E. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ 1992;305:673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry 2015;72:438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Langballe EM, Engdahl B, Nordeng H, Ballard C, Aarsland D, Selbaek G. Short‐ and long‐term mortality risk associated with the use of antipsychotics among 26,940 dementia outpatients: a population‐based study. Am J Geriatr Psychiatry 2014;22:321–331. [DOI] [PubMed] [Google Scholar]
- 15. Weintraub D, Chiang C, Kim HM, et al. Antipsychotic use and mortality risk in Parkinson's disease. JAMA Neurol 2016;73:535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McShane R, Keene J, Gedling K, Fairburn C, Jacoby R, Hope T. Do neuroleptic drugs hasten cognitive decline in dementia? Prospective study with necropsy follow up. BMJ 1997;314:266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vigen CL, Mack WJ, Keefe RS, et al. Cognitive effects of atypical antipsychotic medications in patients with Alzheimer's disease: outcomes from CATIE‐AD. Am J Psychiatry 2011;168:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta‐analysis. Am J Psychiatry 2015;172:731–742. [DOI] [PubMed] [Google Scholar]
- 19. Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society evidence‐based medicine review update: treatments for the non‐motor symptoms of Parkinson's disease. Mov Disord 2011;26(suppl 3):S42–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia [serial online]. BMJ 2015;350:h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson's disease psychosis: a randomised, placebo‐controlled phase 3 trial. Lancet 2014;383:533–540. [DOI] [PubMed] [Google Scholar]
- 22. Armstrong MJ, Shulman LM, Vandigo J, Mullins CD. Patient engagement and shared decision making: what do they look like in neurology practice? Neurol Clin Pract 2016;6:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomsen TR, Panisset M, Suchowersky O, Goodridge A, Mendis T, Lang AE. Impact of standard of care for psychosis in Parkinson disease. J Neurol Neurosurg Psychiatry 2008;79:1413–1415. [DOI] [PubMed] [Google Scholar]
- 24. Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology 2009;72:S1–S136. [DOI] [PubMed] [Google Scholar]
- 25. Fagerlin A, Zikmund‐Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst 2011;103:1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]


