Abstract
Epidermal growth factor receptor (EGFR) overexpression is common in head and neck squamous cell carcinoma. Targeted therapy specifically directed towards EGFR has been an area of keen interest in head and neck cancer research, as EGFR is potentially an integration point for convergent signaling. Despite the latest advancements in cancer diagnostics and therapeutics against EGFR, the survival rates of patients with advanced head and neck cancer remain disappointing due to anti-EGFR resistance. This review article will discuss recent multilateral efforts to discover and validate actionable strategies that involve signaling pathways in heterogenous head and neck cancer and to overcome anti-EGFR resistance in the era of precision medicine. Particularly, this review will discuss in detail the issue of cancer metabolism, which has recently emerged as a novel mechanism by which head and neck cancer may be successfully controlled according to different perspectives.
Subject terms: Cancer metabolism, Targeted therapies, Head and neck cancer, Cancer therapeutic resistance
Cancer therapy: Combination strategies for head and neck cancer
South Korean researchers propose novel combination strategies for overcoming drug resistance and halting the progression of head and neck cancer (HNC). Although high levels of epidermal growth factor receptor (EGFR) protein in HNC correlate with reduced survival, patients’ response to the EGFR inhibitor cetuximab often declines rapidly after a short period of effectiveness. Hyung Kwon Byeon at Korea University College of Medicine in Seoul and colleagues review current knowledge of the mechanisms underlying cetuximab resistance. They suggest that evaluating a patient’s genetic profile and combining cetuximab with drugs that enhance the effects of inhibiting EGFR signaling pathways (with inhibitors of other EGFR family members or proteins that mediate EGFR entry to the cell nucleus, for example) as well as with agents that inhibit cancer cell metabolism could be a more effective approach for treating HNC.
Introduction
Head and neck cancer (HNC) is the sixth most common cancer worldwide, as 40,000 new patients are diagnosed every year in the United States, and over 600,000 are diagnosed worldwide1. Despite the recent advancements in cancer diagnostics and therapeutics, the survival rates of patients with advanced HNC remain disappointing, and ~300,000 patients worldwide die from this disease every year. The anatomy of the head and neck is especially important since it is responsible for many vital functions such as respiration, phonation, and swallowing. Since locoregional invasion and metastases are relatively common and because esthetic or functional disabilities are inevitable following treatment, many difficulties are associated with the treatment of HNC. Conventional treatment modalities for HNC comprise surgery, chemotherapy, and radiotherapy. Although surgery still plays a definitive role in cases of resectable tumors, limitations in surgical resection clearly exist. Aggressive surgical resection itself would be most troublesome due to the complex and difficult anatomy, especially in cases of locally advanced tumors or recurrent tumors which have been treated with prior chemoradiotherapy. Chemotherapy and radiotherapy are routinely administered to HNC patients in primary definitive, adjuvant, or salvage treatment settings, but advanced cases are typically refractory. Therefore, novel treatment strategies are imperative for the management of HNC, especially in cases where the cancer has progressed beyond an initial stage of resection.
HNC has certain notable characteristics. For example, over 90% of all HNCs are pathologically squamous cell carcinomas, and 80–100% of HNCs feature epidermal growth factor receptor (EGFR) overexpression. Overexpression of EGFR is correlated with decreased survival, resistance to radiation, local treatment failure, and increased distant metastasis. Cetuximab, an EGFR monoclonal antibody, is the only FDA-approved targeted agent for HNC. However, treatment results were quite disappointing, unlike the initial expectations for this agent, as monotherapy responses were shown in only 10–30%, which suggests some form of intrinsic resistance. Moreover, patients who do achieve a clear tumor response eventually manifest disease progression due to acquired resistance to cetuximab. Numerous complex mechanisms underlie this treatment resistance. A low response rate to anti-EGFR targeted therapy, distinct inter- and intratumoral heterogeneity, relatively aggressive clinical features, and the functional and esthetic importance of head and neck anatomy are features that make HNC a challenging cancer to treat. This review article will discuss recent efforts in the discovery and validation of actionable targets in heterogenous HNC and methods to overcome anti-EGFR resistance in the era of precision medicine.
The structure and biology of EGFR
EGFR is a 170 kDa transmembrane glycoprotein cell surface receptor that constitutes the ErbB/HER family, together with ErbB2 (HER2/neu), ErbB3 (HER3), and ErbB4 (HER4). All members of the HER family except for HER2 have known ligands. Six main ligands are known to bind to EGFR: EGF, heparin binding-EGF, TGF-α, amphiregulin, betacellulin, and epiregulin2. When EGFR binds to its ligand, it causes homodimerization or heterodimerization with other HER receptors (HER2, HER3) or other receptor tyrosine kinases (RTKs) such as MET or IGF-1 receptor. The activated EGFR affects four major signaling pathways: MAPK, PI3K/AKT/mTOR, PLCγ/PKC, and the JAK/STAT pathway2. Several studies have reported that some EGFRs exist as tetramers, which results in their inactivation, but the significance of this form has yet to be revealed3,4. EGFR can also act as a membrane-bound chaperone protein for the sodium-glucose cotransporter, SGLT15,6. In HNC, known mutations in EGFR are rare, but the overexpression of EGFR together with one of its ligands, such as TGF-α, is relatively common. Autocrine or paracrine activation by EGFR ligands is important for EGFR activation in HNC. Tobacco smoke, a classic contributor to HNC can increase amphiregulin and TGF-α production, which results in direct EGFR activation. Another route of EGFR stimulation is by the indirect activation of G-protein-coupled receptors (GPCRs). GPCR ligands such as PGE2 or gastrin-releasing peptide (GRP) are increased in HNC, and consequent GPCR activation results in Src-mediated MMP activation; this causes the cleavage and release of EGFR proligands (TGF-α, amphiregulin), which ultimately leads to EGFR transactivation2,7. Furthermore, following EGFR activation, the expression of COX2 and its downstream product PGE2 is increased; PGE2 in turn transactivates EGFR, which establishes a positive feedback loop2,8.
The biological implications of EGFR are most important when EGFR is in its membrane-bound form as described above, where its activity is regulated by the quantity/quality of available receptors (overexpression or gain-of-function mutations in EGFR), interactions with other RTKs, and ligand availability. However, the EGFR signaling may also be spatially regulated by dynamic receptor cellular localization and recycling. EGFR itself can pose distinct signaling effects on different cellular compartments. Some have named these proteins ‘moonlighting proteins,’ where a single protein may have distinct functions according to its subcellular localization9. EGFR has several consequences within the cell once it has been engulfed during endocytosis. EGFR can enter the nucleus where it can serve several roles, be returned to the cell surface to continue its signaling function in its membrane-bound form, be directed to lysosomes for degradation, or remain active in endosomal compartments by mammary-derived growth factor inhibitor (MDG1), which leads to the activation of various downstream signals. Among these routes, the nuclear translocation of EGFR can play several important roles in anti-EGFR resistance10. Nuclear EGFR is known to be associated with poor survival, worse prognosis, and resistance to therapy. Several triggering mechanisms of EGFR nuclear translocation in HNC are known: EGFR ligands, cetuximab, EBV, radiation, and Src family kinase (SFK). Once in the nucleus, EGFR has two distinct functions. First, it acts as a transcription factor that binds to the promoters of multiple genes (iNOS, COX2, Aurora kinase A, B-myb, and cyclin D1) along with DNA-binding transcription cofactors (STAT3, STAT5, and E2F1). Second, nuclear EGFR can directly cause PCNA and DNA-PK phosphorylation due to its intrinsic tyrosine kinase activity. This increased EGFR activity can induce cell proliferation and promote the repair of DNA damage caused by chemoradiotherapy, which results in therapeutic resistance and ultimately cancer progression (Fig. 1).
Fig. 1. The biology of EGFR in head and neck cancer (HNC).
Schematic diagram of the EGFR signaling network, its various interactions and mode of actions according to cellular localization. Numbers indicate relevant references in the text
Tumors with EGFR overexpression and their characteristics
Overexpression or upregulated activity of EGFR is an important molecular characteristic that has been noted in numerous epithelial solid tumors such as colorectal cancer (CRC), non-small cell lung cancer (NSCLC), HNC, pancreatic cancer, breast cancer, and brain cancer. However, distinguishable mechanisms exist within these EGFR-overexpressing tumors. For instance, EGFR amplification and dysregulated EGFR expression together with KRAS mutations are commonly found in CRC whereas EGFR-activating mutations are important characteristics in NSCLC. Of the diverse somatic mutations in EGFR in NSCLC, exon 19 deletion and L858R mutation of the EGFR kinase domain are the most common forms, as they account for 85% of all EGFR mutations11. In HNC, however, EGFR overexpression is more commonly observed with rare events of EGFR mutations or EGFR amplifications. EGFR overexpression in HNC is also observed in normal tissue adjacent to the cancer, which supports the notion of field cancerization12. In short, EGFR functions more as a driver oncogene in NSCLC, while EGFR plays a role as the component of one of the many pathways that contribute to tumor growth in CRC and HNC.
Approaches to EGFR inhibition in cancer
Two main classes of inhibitors target EGFR: monoclonal antibody (mAb)-based drugs and small molecule tyrosine kinase inhibitors (TKIs). The main action of mAbs is to bind to the extracellular domain (ECD) of EGFR, which blocks ligand-receptor binding and consequently results in the abrogation of EGFR dimerization. The mAb-receptor complex is then internalized after which it is consequently degraded, ultimately resulting in the downregulation of EGFR overexpression. The most well-known anti-EGFR mAb is cetuximab (chimeric mouse-human IgG1 antibody), which is the only FDA-approved targeted agent for HNC, but other agents such as panitumumab (fully humanized IgG2 antibody) are also under intense evaluation in HNC-based clinical trials13,14. In contrast the primary site of action of TKIs is within the intracellular tyrosine kinase domain of EGFR, where they compete with ATP to eliminate EGFR downstream signaling. TKIs are usually short-acting drugs since they tend to have a much shorter half-life than mAbs. TKIs have several advantages over mAbs such as oral administration and fewer hypersensitivity reactions. Reversible acting EGFR TKIs such as gefitinib and erlotinib have not shown a clinical benefit in HNC, but multitarget TKIs such as lapatinib (reversible dual EGFR and HER2 TKI), afatinib and dacomitinib (both irreversible EGFR, HER2, and HER4 pan-HER TKIs) have shown promise in various clinical trials15–18.
EGFR-targeted mAbs
Anti-EGFR mAbs are generally used in cases of CRC and HNC. However, despite the overexpression of EGFR in these cancers, the initial response rates to cetuximab monotherapy are far from encouraging, and furthermore, treatment responses rapidly decline after a short period of effect. Generally, targeted drug resistance can be divided into the following two types: primary (intrinsic) and secondary (acquired) resistance. Naturally, resistance mechanisms vary among different cancers and the type of EGFR-directed agents used.
The major resistance mechanisms to EGFR-targeted mAbs that have been identified thus far are summarized in Table 1. In CRC in particular, the activation of a bypass signaling pathway, also referred to as ‘oncogenic shift,’ is a major mechanism of resistance to cetuximab. KRAS activation is an important mechanism of innate and acquired drug resistance, but resistance may also be mediated through other signaling networks such as MET, HER2/3, BRAF, and PIK3CA, which share the same mechanisms in other cancers. Additionally, in CRC, some have reported an acquired EGFR mutation in the ECD region (S492R), which hinders cetuximab binding. Unlike the oncogenic addiction of EGFR-mutant NSCLC, EGFR, as one of many pathways that contributes to tumor growth in CRC, leads to certain clinical implications. Treatment responses to EGFR inhibitor monotherapy will be relatively less pronounced and overcoming EGFR resistance may be less feasible due to alternate crosstalk mechanisms in CRC. Therefore, a combinatorial treatment strategy may be more applicable in CRC compared with NSCLC in which a single driver oncogene is responsible. These specific considerations in CRC have similar implications in HNC, which will be discussed in more detail below.
Table 1.
Resistance mechanisms to anti-EGFR monoclonal antibodies
| Major mechanisms | Action | References |
|---|---|---|
| Overexpressions of EGFR/ligands | Overexpressions of EGFR and TGF-α | 24,112 |
| Dysregulation of EGFR internalization and degradation by ubiquitination | EGFR is downregulated but its affinity to other activating signals are strengthened | 24,113 |
| MDG1 binding | MDG1-bound intracellular EGFR avoids extracellular targeting | 114 |
| Nuclear translocation of EGFR | Transcription of multiple genes or directly phosphorylates PCNA and DNA-PK | 87 |
| Enhanced SFK-mediated signaling | Promotion of EGFR nuclear translocation | 113,115 |
| EGFRvIII | Constitutively activated EGFR in a ligand-independent manner | 116 |
| KRAS mutation | Constant activation of EGFR downstream signals | 117 |
| PTEN loss | PI3K/AKT signal activation | 117 |
| Increased heterodimerization of EGFR or HER2 with HER3 | PI3K/AKT pathway signal enhanced | 24,115 |
| Crosstalks | Crosstalk with HGF-MET | 24,37 |
| Crosstalk with VEGF-VEGFR1 | 118,119 | |
| EMT | Local invasion and distant metastasis | 120 |
EGFR-targeted TKIs
EGFR TKIs are more commonly applied in NSCLCs, which exhibit oncogene addiction to EGFR signaling. The most common EGFR-activating mutation, L858R, is considered a predictor of sensitivity to EGFR TKIs. As with mAbs, EGFR-targeted TKIs also manifest various resistance mechanisms (Table 2). The most common mechanism of TKI resistance in NSCLC is the EGFR T790M ‘gatekeeper’ mutation, which is found in nearly 60% of patients who present with acquired resistance. This secondary kinase mutation results in a drug-resistant state of the cancer, where the actions of EGFR inhibitors are abrogated while its intrinsic EGFR kinase activity is maintained; this in turn contributes to ‘oncogenic drift’. This acquired resistance to first-generation EGFR TKIs such as erlotinib and gefitinib led to the clinical development of second-generation EGFR TKIs19. Second-generation TKIs such as afatinib and dacomitinib were designed specifically to enhance the treatment efficacy via the formation of irreversible covalent attachments to the EGFR kinase domain and action against a broader range of targets such as other HER family receptors (HER2, HER4) and structurally similar receptors (VEGFR). Their stronger binding activity to this secondary EGFR mutation revealed relatively more robust EGFR targeting ability, but these drugs are still limited. Therefore, third-generation TKIs were developed to specifically act against the T790M EGFR mutation. Osimertinib (AZD9291) has been recently approved by the FDA for NSCLCs harboring the EGFR T790M mutation20. Its primary mode of action is irreversible binding to EGFR with the T790M-mutation, but its effects against EGFR with a L858R mutation or an exon 19 deletion have also been demonstrated. However, a new form of tertiary EGFR C797S mutation has recently emerged, and ways to overcome resistance conferred by this mutation are currently being investigated21–23.
Table 2.
Resistance mechanisms to anti-EGFR tyrosine kinase inhibitors
| Major mechanisms | Action | References |
|---|---|---|
| EGFR mutations | T790M, C797S mutations | 21, 121,122 |
| EGFRvIII | Constitutively activated EGFR in a ligand-independent manner | 123 |
| PTEN mutation/loss | PI3K/AKT signal activation | 124,125 |
| KRAS mutations | Constant activation of EGFR downstream signals | 1 26 |
| Crosstalk | Increased expressions of HER2/HER3 | 2 6 |
| ADAM17 mediated NRG1 release leading to autocrine activation of HER2/HER3 | 127 | |
| Crosstalk with MET | 128 | |
| HGF overexpression | 129 | |
| Crosstalk with AXL | 41 | |
| Crosstalk with VEGF-VEGFR | 119,130 | |
| IGF-1R activation | Crosstalk, upregulation of IGF-1R | 131 |
| Decreased expressions of regulators of IGF-1R ligands (IGFBP3/IGFBP4) leading to increased availability of IGF-1/IGF-2 | 132 | |
| EMT | Local invasion and distant metastasis | 120 |
| Histologic transformation | NSCLC to small cell lung cancer | 133,134 |
EGFR inhibitor resistance in HNC
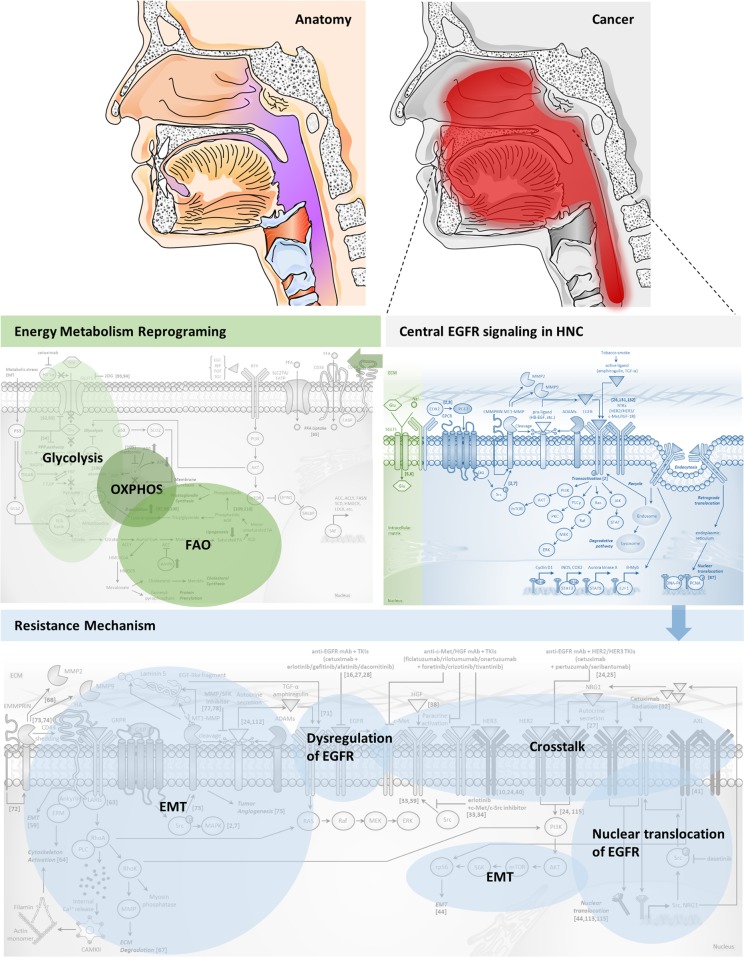
In HNC, most research has focused mainly on the underlying mechanisms of cetuximab resistance since it is the only FDA-approved targeted agent that is currently used in the clinic. From elucidation of the resistance mechanisms, many strategies to overcome such resistance have been be proposed (Fig. 2).
Fig. 2. Major resistance mechanisms against EGFR inhibition in HNC.
This schematic diagram illustrates reported resistance mechanisms to anti-EGFR monoclonal antibodies (mAbs) or tyrosine kinase inhibitors (TKIs) that are relevant in HNC. Inhibitors of specific targets are highlighted in red. Numbers indicate relevant references in the text
HER3
Additional activation of HER3 signaling has been elucidated as one of the major, prominent mechanisms that underlies acquired resistance to cetuximab in HNC10,24,25. Upregulated HER3 signaling has also been recognized as a resistance mechanism to the EGFR TKI, gefitinib26. Phosphorylated HER3 in turn mediates potent activation of PI3K/AKT signaling. The activity of HER3 is dependent on EGFR and HER2, and HER2/HER3 heterodimerization is the main form contributing to cetuximab resistance in HNC. Therefore, simultaneous blocking of EGFR with either HER2 or HER3 has exhibited promising antitumor effects and has been proposed as an important strategy to overcome resistance to cetuximab. Combinatorial treatment of cetuximab with pertuzumab (2C4; HER2 monoclonal antibody)24 or seribantumab (MM-121; HER3 monoclonal antibody)25 results in effective blocking of both EGFR and HER3 signals and potent tumor suppression. Likewise, it has been demonstrated that lapatinib can effectively disrupt HER3 activation by blocking HER2/HER3 heterodimerization, either as monotherapy in intrinsically NRG1/HER3-enriched HNC27 or as a combinatorial treatment with cetuximab in cetuximab-resistant HNC (authors’ unpublished data). Pan-HER TKIs such as dacomitinib have also shown superior treatment efficacies compared with cetuximab or erlotinib alone in HNC cell lines28. Furthermore, the EGFR- and HER3-bispecific monoclonal antibody duligotuzumab (MEHD7945A) has recently been developed and holds promise29. Due to the high expression of NRG1 in HER3-enriched HNC, a significant role of NRG1-mediated autocrine signaling has been suggested in HER3-mediated cetuximab resistance. Therefore, inhibition with NRG1-neutralizing antibodies could be a potential treatment strategy30,31.
SFKs
The SFKs are another important mediator of resistance to EGFR-targeted therapy in HNC. Generally, the SFKs are involved in anti-EGFR resistance and progression of HNC via three primary mechanisms. The first mechanism of EGFR resistance by SFKs is the mediation of cetuximab- or radiation-induced EGFR nuclear translocation, which leads to cetuximab resistance32. The blockade of SFKs by dasatinib treatment abrogates the process of EGFR nuclear translocation promoted by cetuximab or radiation. The second mode of action is that SFKs mediate the cleavage of EGFR proligands and consequent EGFR hyperactivation7. More specifically, Src is activated by the GRP/GRP receptor and contributes to the cleavage and extracellular release of TGF-α and amphiregulin, a process that is mediated by MMPs; this leads to EGFR and downstream MAPK activation2,7. The proteolytic release of TGF-α and amphiregulin by GRP stimulation is blocked not only by a MMP inhibitor but also by a SFK inhibitor. The third role of SFKs concerns ligand-independent activation of MET33,34. Notably, this mode of resistance is specifically relevant to erlotinib and not cetuximab33. In one study, Src inhibition resulted in MET inhibition34. Therefore, the addition of a MET or Src inhibitor to erlotinib treatment may lead to a synergistic effect in erlotinib-resistant HNC.
HGF/MET
MET is involved in another well-established resistance mechanism of EGFR inhibition. HGF is the sole known ligand of MET. Genomic data of HNC reveals that gene amplifications or mutations in the MET gene are relatively rare, with ~20% of HNC presenting either an amplification or copy number gain and fewer than 1% harboring a gene mutation. However, HGF (50%)/MET (80%) overexpression is relatively common in HNC35,36. Compensatory activation of MET is the key mechanism that contributes to acquired resistance to cetuximab24,37. HGF acts mainly as a paracrine factor rather than as an autocrine activator of MET in HNC, and because it is secreted by cancer-associated fibroblasts, it is abundant in the tumor microenvironment38. Although this paracrine effect of HGF is the primary activating mode of MET, it can also be activated in a ligand-independent manner through the mediation of Src, particularly in erlotinib- or gefitinib-resistant tumors33,39. Furthermore, MET can also be activated to some degree by heterodimerization with HER310,24,40, and therefore, blocking MET would be an important strategy to overcome resistance to anti-EGFR therapies. Three approaches have been established to target MET: anti-MET or anti-HGF mAbs such as ficlatuzumab, rilotumumab, and onartuzumab; TKIs such as foretinib, crizotinib, tivantinib, cabozantinib; a NK4 decoy, which is a truncated, soluble MET receptor that acts as an HGF antagonist.
AXL
Together with Tyro-3 and MerTK, AXL constitutes the TAM family of RTKs. Previously, the oncogenic RTK AXL was implicated in resistance to EGFR TKIs such as erlotinib in HNC41, but AXL was also found to be both overexpressed and hyperactivated in cetuximab-resistant HNC42. More importantly, the elimination of HER2 or HER3 receptors in cetuximab-resistant HNC cells has no effect on EGFR phosphorylation, whereas AXL knockdown causes a prominent decrease in EGFR activity and significant inhibition of tumor proliferation. From these findings, additionally targeting the AXL appears to be a rational approach to overcome EGFR resistance, since HER2/HER3 signaling inhibition is not sufficient for complete tumor suppression42,43. Furthermore, AXL promotes EGFR nuclear translocation and transcriptional induction of NRG1 and SFKs, which leads to autocrine activation of HER3, EGFR-HER3 interaction, and EGFR activation44. This provides compelling evidence that the previously described major resistance mechanisms to EGFR inhibitors are all intimately connected to one another. AXL has further roles in the activation of rapamycin/ribosomal protein S6 signaling and the induction of epithelial to mesenchymal transition (EMT), which mediates resistance to PI3K and EGFR inhibition44.
p53
The p53 protein is a tumor suppressor that plays a vital role in the suppression of cancer progression by promoting cell-cycle arrest, apoptosis, and senescence. The TP53 gene is the most commonly mutated gene in HNC45,46, and loss of p53 function is found in more than 90% of HNC cases35,47. TP53 mutations in HNC are correlated with poor clinical outcomes48, and p53 protein also plays a significant role in acquired resistance to EGFR inhibitors based on the identification of a robust loss of p53 in HNC cells that are resistant to cetuximab or erlotinib49. Furthermore, the loss of p53 also demonstrates cross-resistance to radiation, and therefore, restoration of p53 function resensitizes HNC to cetuximab and radiation, just as dasatinib enhances both cetuximab therapy and radiotherapy32. p53 regulates sensitivity to EGFR inhibitors by controlling EGFR downstream pathways such as ERK signaling and the PI3K/AKT pathway50,51. Targeting the cell cycle, including p53, therefore seems to be a promising therapeutic strategy in HNC.
The function of p53 is also important in cancer metabolism because it modulates glycolysis in several ways. p53 inhibits glycolysis by reducing the gene expression of GLUT1 and increasing gene expressions of TP53-induced glycolysis and apoptosis regulator and phosphatase and tensin homolog deleted from chromosome 1052,53. In addition, p53 inhibits the pentose phosphate pathway, which is involved in nucleotide biosynthesis54. Furthermore, p53 induces the expression of synthesis of cytochrome c oxidase deficient homolog 2 and glutaminase 2 in the mitochondria for oxidative phosphorylation (OXPHOS)55,56. In cancer cells with functional loss of p53, the Warburg effect will therefore be accentuated, and thus targeting the p53 protein will have further important implications in HNC.
EMT
For the progression and dissemination of cancer, tumor cells need to migrate, invade, and metastasize, as well as proliferate. Clinically, the invasiveness of a tumor is directly related to patient prognosis57. It is widely accepted that these processes are executed by EMT induction. During EMT, tumor cells at the primary site lose cell–cell contacts, engage in cytoskeletal remodeling, acquire mesenchymal and stem cell signatures, and display migratory phenotypes. As stated above, EMT is another mechanism that contributes to resistance to anti-EGFR therapies in HNC58.
In particular, expression of a stemness marker CD44 is increased during EMT59, and plays significant roles in HNC progression. The exons of the CD44 gene are alternatively spliced to produce multiple variant isoforms of CD44 (CD44v), and of these, the CD44 isoforms v3, v6, and v10 are particularly significant in HNC60. The expression of these variant isoforms in HNC were found to be related to lymph node/distant metastasis, advanced disease, poor survival, and chemoresistance, and generally, CD44 expression is primarily concentrated at the invasive fronts of tumors60,61. CD44 has a principle role in the mediation of resistance to drug therapy including EGFR-targeted agents61,62. Hyaluronan (HA), which is a major constituent of the extracellular matrix (ECM), is the primary ligand of CD44. As HA binds to the CD44 receptor, which is localized at the cell surface, a CD44-EGFR complex is formed. This complex in turn initiates various downstream signals mediated by leukemia-associated Rho-guanine (LARG) nucleotide exchange factor. The HA/CD44-EGFR-LARG complex can activate Ras-mediated MAPK signaling or RhoA-mediated RhoK signaling, which leads to either myosin light chain phosphatase and MMP activation and consequent ECM degradation, or PI3K signaling activation63. Moreover, HA/CD44 signaling can induce cytoskeleton activation, either by RhoA/PLC-mediated intracellular Ca2+ release and subsequent calcium/calmodulin-dependent protein kinase type II activation, or by direct interaction of ankyrin and ezrin-radixin-moesin proteins with CD4464. The role of CD44 in metastasis has also been rigorously investigated in a recent report65. In that study, a subpopulation of CD44high oral carcinoma cells was slow-cycling, exhibited overexpression of genes related to fatty acid metabolism, and was involved in lymphatic/distant metastasis rather than tumor proliferation. Furthermore, these specific cancer cells exhibited CD36 overexpression, a cell surface receptor which uptakes extracellular lipid to obtain ATP energy through lipid β-oxidation66. The contribution of CD44 to CD36-mediated fatty acid oxidation (FAO) in ‘initiating metastasis’ suggests a novel, alternative strategy of FAO suppression in HNC.
The interaction of tumor cells with the ECM is an integral factor in local invasion and metastasis of cancer. ECM degradation is caused by proteolytic enzymes, typically MMPs, that are secreted by tumor cells. MMPs are classified into collagenases (MMP-1, -8, and -13), gelatinases (MMP-2 and -9), stromelysins (MMP-3, -10, -11, and -27), matrilysins (MMP-7 and -26), enamelysin (MMP-20), metalloelastase (MMP-12), membrane-type MMPs (MMP-14 to 17, -24, and -25), and others (MMP-19, -21, -23, and -28) by dependent substrates67. In particular, MT1-MMP (MMP-14) is a zinc-dependent proteinase expressed in the cell membrane that is involved in the promotion of tumor growth and metastasis68. EGFR is activated by the cleavage of activating growth factor molecules or by the dispersion of receptor ligands within the ECM onto the cell surface69. Several proteinases such as MMPs and ADAMs regulate growth factors70. Specifically, MT1-MMP is involved in the dispersion of EGF ligands such as HB-EGF and EGF-like fragment released from the γ2 chain of laminin 5, which activate EGFR71. The activated EGFR signals in turn induce MT1-MMP expression and promote invasion of HNC by EMMPRIN-mediated MMP-2 and MMP-9 expression72. MT1-MMP also promotes intracellular signaling through Src and MAPK, and the shedding of CD4473,74. It was also reported that MT1-MMP modulates tumor-induced angiogenesis75. More noteworthy is that MT1-MMP supports the maintenance of energy metabolism via the mediation of the direct or indirect uptake of glucose and lipoproteins76. In short, MT1-MMP plays many key roles in growth, invasion, metastasis, and energy metabolism of cancer cells. Therefore, EGFR signaling and downstream gene expression could be regulated by a broad spectrum of MMP inhibitors such as clinically tested batimastat (BB-94), ilomastat (GM6001) and marimastat (BB-2516) or by the MT1-MMP-specific inhibitor NSC40502077,78.
Therapeutic strategies beyond simple EGFR inhibition in HNC
Reinforcement of oncogenic signaling inhibition
Although many important mechanisms contribute to resistance to EGFR-targeted therapies in HNC, EGFR is still a significant therapeutic target. The importance of EGFR overexpression aside, EGFR is an integral point for convergent signaling pathways, and EGFR targeting should form the basis of oncogenic signaling inhibition. Therefore, multilateral strategies that strengthen the inhibition of oncogenic signals should be employed.
Simultaneous inhibition of both the ECD and the intracellular tyrosine kinase domain of EGFR by combination of a mAb and a TKI can be a rational approach to enhance EGFR inhibition. The complementary actions of a mAb and TKI can be combined to present a deadly blow to the tumor by throwing a ‘HER1-2 punch’79. The synergistic effect of combining cetuximab with erlotinib or gefitinib have been extensively studied in many EGFR-dependent cancers80–82. One study showed a marked increase in EGFR mRNA in erlotinib-resistant tumors, which could be abrogated by cetuximab treatment81. Therefore, both EGFR downregulation and suppression of EGFR activity can be achieved. This dual inhibition of EGFR was also shown to be effective in HNC, where gefitinib or erlotinib still retained its antitumor activity in cetuximab-resistant cells83.
In consideration of crosstalk mechanisms with other HER family receptors following EGFR inhibition, the inhibition of a multitude of HER receptors would be an effective treatment strategy to overcome resistance. This so-called ‘horizontal targeting’ strategy in combination with EGFR inhibitors has been intensively investigated in NSCLC and has shown promising outcomes in both preclinical and clinical studies84–86. The combination of afatinib and cetuximab exerted a synergistic effect in EGFR TKI-resistant NSCLC from not only the inhibitory actions of multi-HER receptors but also from the dual inhibition of the extracellular and intracellular domains of EGFR85. Furthermore, this combinatorial strategy even showed promising results when administered as a first-line therapy in TKI-naïve NSCLC86. Multi-HER inhibitors such as lapatinib, afatinib, and dacomitinib have also shown encouraging results as single agents in HNC16,27,28, and combinatorial treatment of afatinib and cetuximab has shown promise in HNC16. For all the reasons discussed above, the combined treatment of cetuximab with either afatinib or dacomitinib deserves attention as an active area of investigation. Additionally, horizontal targeting of another major resistance molecule, MET could also be considered.
In distinction to the horizontal targeting strategy, vertical targeting of EGFR can be achieved by combinatorial inhibition of EGFR and RTKs such as SFKs or AXL, which mediate the nuclear translocation of EGFR87. This inhibition would be particularly advantageous since they are also involved in signaling crosstalk with molecules such as MET and HER3, which play mechanistically important roles in conferring anti-EGFR resistance in HNC as discussed above.
Targeting cancer metabolism
Deregulating cellular energetics or reprogramming of cellular metabolism is an important emerging hallmark of cancer88. Normally, eukaryotic cells under aerobic conditions utilize glucose to produce pyruvate by glycolysis, after which the pyruvate then enters the tricarboxylic acid cycle in the mitochondria and consequently yields 36 molecules of ATP by OXPHOS. However, in anaerobic conditions, glycolysis is favored instead of OXPHOS and glucose is catabolized to lactate, which results in the generation of 2 molecules of ATP. Common features of the altered energy metabolism in cancer cells are increased glucose uptake and preference for glycolysis even in the presence of oxygen (hence the term ‘aerobic glycolysis’), which is known as the ‘Warburg effect’89. Biological advantages which cancer cells expect from this metabolic rewiring despite its rather inefficient, counterintuitive process are faster rate of ATP production, reduced generation of reactive oxygen species, stability of glycolytic fueling under hypoxic conditions which many tumors lie, and increased shunting of glycolytic intermediates into various biosynthetic pathways to accomplish nucleotide, amino acid, and lipid synthesis needed for active cell proliferation. Cancer cells therefore enhance glycolysis by various compensatory mechanisms such as increasing glucose uptake by upregulating glucose transporter GLUT1 and activation of oncogenes (RAS, MYC) or mutation of tumor suppressor genes (TP53)88,90,91. Clinical implications of the Warburg effect are the incorporation of the glucose analog 18F-fluorodeoxyglucose, which is used in cancer diagnostics in positron emission tomography92, and the administration of yet another glucose analog, 2-deoxyglucose (2DG), as a therapy against many cancers including HNC93,94.
More recently, there has been an accumulation of refuting evidences against the Warburg effect. Unlike the initial understanding that cancer cells possess defective OXPHOS (hence the predominance of glycolysis), it is now widely accepted that cancer cells have normally functioning mitochondria capable of OXPHOS95,96. Especially under metabolic stress conditions such as restricted glucose supply, cancer cells can generate ATP through mitochondrial oxidation of cellular fuels other than glucose, most typically glutamine and fatty acids97,98. Whatsmore, unlike the anabolic metabolism with glycolysis, OXPHOS is mainly promoted for catabolic metabolism in cancer cells undergoing EMT. There are reports that in solid tumors, when cancer cells lose ECM attachment or migrate for metastasis, the cells markedly decrease glucose uptake and undergo energetic stress. The cells depend on FAO instead of glycolysis to meet the high demand for ATP required for cell survival97,99,100. Tumors with a lipogenic phenotype are associated with disease aggressiveness, worse prognosis, chemoresistance, and protection of cells against oxidative stress101,102. Therefore, therapies that act against mitochondrial DNA or OXPHOS should be viable anti-cancer strategies. Previous studies reported a reduction in the tumorigenic potential of cancer cells by targeting the mitochondrial DNA103,104, and agents such as metformin (an OXPHOS inhibitor) and etomoxir (an FAO inhibitor) are already being investigated as promising therapeutic options. Numerous studies have been conducted and have demonstrated that metformin is a potentially effective agent in HNC105. Specific suppression of FAO by CPT-1 inhibition with etomoxir demonstrates antitumor effects in pancreatic ductal adenocarcinoma and restores sensitivity to a conventional chemotherapeutic agent, gemcitabine106. Its effect on HNC has yet to be studied, but positive results are anticipated. Since cancer cells adapt to glycolysis inhibition by utilizing alternative nutrients to increase OXPHOS, the combinatorial treatment strategy of using both a glycolytic inhibitor (2DG) and an OXPHOS inhibitor (metformin) rather than either agent alone, has been studied as an effective treatment because it exerts a synergistic therapeutic effect in the induction of cell death103,107.
Alteration in cellular energy metabolism is considered to be a universal hallmark of cancer. Increased glucose uptake, enhanced glycolysis and FAO are also distinctive hallmarks of HNC (Fig. 3)108. As previously discussed, mutant p53 plays many central roles in not only the initiation and progression of HNC but also in the context of cancer metabolism, where it modulates the glycolysis pathway in multiple ways. In addition, based on the association of CD44 and the initiation of metastasis of HNC cells by CD36-mediated fatty acid uptake, this HNC-specific FAO could be a potentially effective target by which metastasis and progression of HNC can be controlled65. This widespread but distinctive trait of cancer can be exploited as a therapeutic target and it is expected that it will complement the former targeted therapy by overcoming its current limitations. Recently, some have suggested a close relationship between oncogenic signaling such as the AKT-mTOR axis, which is the downstream signaling pathway of EGFR and other RTKs, and lipid metabolism, which is centered on oncogenic activation of sterol regulatory element-binding proteins109,110. A recent report has linked cetuximab resistance with the rewiring mechanism of cancer metabolism in HNC111. Cellular stress exerted by cetuximab reverses the Warburg effect, which in turn induces FAO stimulation and fatty acid synthesis inhibition through AMP-activated protein kinase activation. Therefore, future research should be directed toward this innovative approach to achieve novel insights and to advance HNC treatment. By simultaneously targeting two distinct areas of cancer, signaling networks and metabolism, the therapeutic effects would be maximized.
Fig. 3. Metabolic patterns against bioenergetic stress in progressive HNC.
Increased aerobic glycolysis or Warburg effect is a characteristic pattern of metabolism in cancers including HNC. However, due to metabolic stress, EMT changes, or certain drug treatments, cancer cells undergo metabolic rewiring where oxidative phosphorylation is favored instead of glycolysis. Specific actions and their consequences are highlighted in red. Additionally, possible connections between signaling pathways in cancer and cancer metabolism are suggested as shown. Numbers indicate relevant references in the text
Conclusion
In this article, studies concerning targeted therapies that involve central EGFR signaling in HNC have been comprehensively reviewed. Although numerous resistance mechanisms to EGFR-targeted therapies have been reported in HNC, EGFR is still important as an integral point for convergent signaling pathways, and therefore, EGFR targeting should form the basis of oncogenic signaling inhibition. As previously suggested, strategies that reinforce oncogenic signaling inhibition by dual inhibition of the ECD and the intracellular tyrosine kinase domain of EGFR or horizontal/vertical targeting should be considered.
Herein, one notable characteristic feature of HNC is reprogrammed energy metabolism, which involves enhanced glycolysis and alternative activation of OXPHOS. Targeting cancer metabolism would be a distinctive strategy from targeting signaling pathways, and the combination of these two different approaches holds novel promise in exerting a multilateral combat strategy against HNC (Fig. 4). From several clues that suggest connections between cancer metabolism and signaling pathways in HNC, a more detailed understanding of how cancer metabolism is mechanistically involved in EGFR-mediated signaling in HNC is required. In the era of precision medicine and multiomics, evaluating the genetic profile of each individual patient and incorporating the patient profile into therapeutics form the basis of modern cancer treatment and preparing a multilateral strategy would be feasible for HNC patients in the next future.
Fig. 4. Multilateral treatment strategy based on EGFR signaling and cancer metabolism to stop the progression of HNC.
From various resistance mechanisms against EGFR-targeted therapy and energy metabolism reprogramming in EGFR-centered HNC undergoing progression, the targeting of both EGFR central signaling and metabolism may be a rational treatment strategy
Conflict of interest
H.K.B. is supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2016R1C1B2011211). M.K. is supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No.2017R1C1B2010867). J.Y. is supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C0179).
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hyung Kwon Byeon, Jaemoon Yang
Contributor Information
Hyung Kwon Byeon, Phone: +82-2-920-5486, Email: ewellcastle@gmail.com.
Jaemoon Yang, Phone: +82-2-2228-0832, Email: 177hum@yuhs.ac.
References
- 1.Ferlay J, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J. Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J. Clin. Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 3.Clayton AH, et al. Ligand-induced dimer-tetramer transition during the activation of the cell surface epidermal growth factor receptor: a multidimensional microscopy analysis. J. Biol. Chem. 2005;280:30392–30399. doi: 10.1074/jbc.M504770200. [DOI] [PubMed] [Google Scholar]
- 4.Furuuchi K, Berezov A, Kumagai T, Greene MI. Targeted antireceptor therapy with monoclonal antibodies leads to the formation of inactivated tetrameric forms of ErbB receptors. J. Immunol. 2007;178:1021–1029. doi: 10.4049/jimmunol.178.2.1021. [DOI] [PubMed] [Google Scholar]
- 5.Weihua Z, et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008;13:385–393. doi: 10.1016/j.ccr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dittmann K, Mayer C, Rodemann HP, Huber SM. EGFR cooperates with glucose transporter SGLT1 to enable chromatin remodeling in response to ionizing radiation. Radiother. Oncol. 2013;107:247–251. doi: 10.1016/j.radonc.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, et al. SRC family kinases mediate epidermal growth factor receptor ligand cleavage, proliferation, and invasion of head and neck cancer cells. Cancer Res. 2004;64:6166–6173. doi: 10.1158/0008-5472.CAN-04-0504. [DOI] [PubMed] [Google Scholar]
- 8.Harari PM, Wheeler DL, Grandis JR. Molecular target approaches in head and neck cancer: epidermal growth factor receptor and beyond. Semin. Radiat. Oncol. 2009;19:63–68. doi: 10.1016/j.semradonc.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min KW, Lee SH, Baek SJ. Moonlighting proteins in cancer. Cancer Lett. 2016;370:108–116. doi: 10.1016/j.canlet.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Saba NF, Chen GZ, Shin DM. Targeting HER (ERBB) signaling in head and neck cancer: an essential update. Mol. Asp. Med. 2015;45:74–86. doi: 10.1016/j.mam.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 2013;19:1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–3584. [PubMed] [Google Scholar]
- 13.Wirth LJ, et al. Phase I dose-finding study of paclitaxel with panitumumab, carboplatin and intensity-modulated radiotherapy in patients with locally advanced squamous cell cancer of the head and neck. Ann. Oncol. 2010;21:342–347. doi: 10.1093/annonc/mdp477. [DOI] [PubMed] [Google Scholar]
- 14.Vermorken JB, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 15.Harrington K, et al. Randomised phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: rationale for future randomised trials in human papilloma virus-negative disease. Eur. J. Cancer. 2013;49:1609–1618. doi: 10.1016/j.ejca.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Seiwert TY, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann. Oncol. 2014;25:1813–1820. doi: 10.1093/annonc/mdu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HS, et al. Phase II clinical and exploratory biomarker study of dacomitinib in patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Clin. Cancer Res. 2015;21:544–552. doi: 10.1158/1078-0432.CCR-14-1756. [DOI] [PubMed] [Google Scholar]
- 18.Elicin O, Ozsahin M. Current role of dacomitinib in head and neck cancer. Expert Opin. Investig. Drugs. 2016;25:735–742. doi: 10.1080/13543784.2016.1177022. [DOI] [PubMed] [Google Scholar]
- 19.Yu HA, Riely GJ. Second-generation epidermal growth factor receptor tyrosine kinase inhibitors in lung cancers. J. Natl. Compr. Canc. Netw. 2013;11:161–169. doi: 10.6004/jnccn.2013.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J. Hematol. Oncol. 2016;9:34. doi: 10.1186/s13045-016-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thress KS, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat. Med. 2015;21:560–562. doi: 10.1038/nm.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia Y, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature. 2016;534:129–132. doi: 10.1038/nature17960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Song Y, Liu D. EAI045: the fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett. 2017;385:51–54. doi: 10.1016/j.canlet.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler DL, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, et al. HER3 targeting sensitizes HNSCC to cetuximab by reducing HER3 activity and HER2/HER3 dimerization: evidence from cell line and patient-derived xenograft models. Clin. Cancer Res. 2017;23:677–686. doi: 10.1158/1078-0432.CCR-16-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erjala K, et al. Signaling via ErbB2 and ErbB3 associates with resistance and epidermal growth factor receptor (EGFR) amplification with sensitivity to EGFR inhibitor gefitinib in head and neck squamous cell carcinoma cells. Clin. Cancer Res. 2006;12:4103–4111. doi: 10.1158/1078-0432.CCR-05-2404. [DOI] [PubMed] [Google Scholar]
- 27.Wilson TR, Lee DY, Berry L, Shames DS, Settleman J. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell. 2011;20:158–172. doi: 10.1016/j.ccr.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Ather F, et al. Dacomitinib, an irreversible Pan-ErbB inhibitor significantly abrogates growth in head and neck cancer models that exhibit low response to cetuximab. PLoS ONE. 2013;8:e56112. doi: 10.1371/journal.pone.0056112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang S, et al. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res. 2013;73:824–833. doi: 10.1158/0008-5472.CAN-12-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shames DS, et al. High heregulin expression is associated with activated HER3 and may define an actionable biomarker in patients with squamous cell carcinomas of the head and neck. PLoS ONE. 2013;8:e56765. doi: 10.1371/journal.pone.0056765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iida M, et al. Overcoming acquired resistance to cetuximab by dual targeting HER family receptors with antibody-based therapy. Mol. Cancer. 2014;13:242. doi: 10.1186/1476-4598-13-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Iida M, Dunn EF, Wheeler DL. Dasatinib blocks cetuximab- and radiation-induced nuclear translocation of the epidermal growth factor receptor in head and neck squamous cell carcinoma. Radiother. Oncol. 2010;97:330–337. doi: 10.1016/j.radonc.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stabile LP, et al. c-Src activation mediates erlotinib resistance in head and neck cancer by stimulating c-Met. Clin. Cancer Res. 2013;19:380–392. doi: 10.1158/1078-0432.CCR-12-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sen B, Peng S, Saigal B, Williams MD, Johnson FM. Distinct interactions between c-Src and c-Met in mediating resistance to c-Src inhibition in head and neck cancer. Clin. Cancer Res. 2011;17:514–524. doi: 10.1158/1078-0432.CCR-10-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang H, Kiess A, Chung CH. Emerging biomarkers in head and neck cancer in the era of genomics. Nat. Rev. Clin. Oncol. 2015;12:11–26. doi: 10.1038/nrclinonc.2014.192. [DOI] [PubMed] [Google Scholar]
- 36.Hartmann S, Bhola NE, Grandis JR. HGF/Met signaling in head and neck cancer: impact on the tumor microenvironment. Clin. Cancer Res. 2016;22:4005–4013. doi: 10.1158/1078-0432.CCR-16-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madoz-Gurpide J, et al. Activation of MET pathway predicts poor outcome to cetuximab in patients with recurrent or metastatic head and neck cancer. J. Transl. Med. 2015;13:282. doi: 10.1186/s12967-015-0633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knowles LM, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin. Cancer Res. 2009;15:3740–3750. doi: 10.1158/1078-0432.CCR-08-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu H, et al. Dual blockade of EGFR and c-Met abrogates redundant signaling and proliferation in head and neck carcinoma cells. Clin. Cancer Res. 2011;17:4425–4438. doi: 10.1158/1078-0432.CCR-10-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seiwert TY, et al. The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res. 2009;69:3021–3031. doi: 10.1158/0008-5472.CAN-08-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giles KM, et al. AXL mediates acquired resistance of head and neck cancer cells to the epidermal growth factor receptor inhibitor erlotinib. Mol. Cancer Ther. 2013;12:2541–2558. doi: 10.1158/1535-7163.MCT-13-0170. [DOI] [PubMed] [Google Scholar]
- 42.Brand TM, et al. AXL mediates resistance to cetuximab therapy. Cancer Res. 2014;74:5152–5164. doi: 10.1158/0008-5472.CAN-14-0294. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 43.Brand TM, et al. AXL is a logical molecular target in head and neck squamous cell carcinoma. Clin. Cancer Res. 2015;21:2601–2612. doi: 10.1158/1078-0432.CCR-14-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brand TM, et al. The receptor tyrosine kinase AXL mediates nuclear translocation of the epidermal growth factor receptor. Sci. Signal. 2017;10:eaag1064. doi: 10.1126/scisignal.aag1064. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Agrawal N, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stransky N, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat. Rev. Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 48.Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat. Rev. Cancer. 2009;9:701–713. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 49.Huang S, et al. p53 modulates acquired resistance to EGFR inhibitors and radiation. Cancer Res. 2011;71:7071–7079. doi: 10.1158/0008-5472.CAN-11-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sauer L, Gitenay D, Vo C, Baron VT. Mutant p53 initiates a feedback loop that involves Egr-1/EGF receptor/ERK in prostate cancer cells. Oncogene. 2010;29:2628–2637. doi: 10.1038/onc.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh B, et al. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 2002;16:984–993. doi: 10.1101/gad.973602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bensaad K, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 53.Feng Z, et al. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 54.Jiang P, et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 2011;13:310–316. doi: 10.1038/ncb2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matoba S, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 56.Hu W, et al. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc. Natl Acad. Sci. USA. 2010;107:7455–7460. doi: 10.1073/pnas.1001006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holz C, et al. Epithelial-mesenchymal-transition induced by EGFR activation interferes with cell migration and response to irradiation and cetuximab in head and neck cancer cells. Radiother. Oncol. 2011;101:158–164. doi: 10.1016/j.radonc.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 59.Barriere G, et al. Circulating tumor cells and epithelial, mesenchymal and stemness markers: characterization of cell subpopulations. Ann. Transl. Med. 2014;2:109. doi: 10.3978/j.issn.2305-5839.2014.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang SJ, Wong G, de Heer AM, Xia W, Bourguignon LY. CD44 variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope. 2009;119:1518–1530. doi: 10.1002/lary.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang SJ, Bourguignon LY. Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am. J. Pathol. 2011;178:956–963. doi: 10.1016/j.ajpath.2010.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang SJ, Bourguignon LY. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2006;132:771–778. doi: 10.1001/archotol.132.7.771. [DOI] [PubMed] [Google Scholar]
- 63.Torre C, Wang SJ, Xia W, Bourguignon LY. Reduction of hyaluronan-CD44-mediated growth, migration, and cisplatin resistance in head and neck cancer due to inhibition of Rho kinase and PI-3 kinase signaling. Arch. Otolaryngol. Head Neck Surg. 2010;136:493–501. doi: 10.1001/archoto.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang SJ, Bourguignon LY. Hyaluronan-CD44 promotes phospholipase C-mediated Ca2+ signaling and cisplatin resistance in head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2006;132:19–24. doi: 10.1001/archotol.132.1.19. [DOI] [PubMed] [Google Scholar]
- 65.Pascual G, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 66.Pepino MY, Kuda O, Samovski D, Abumrad NA. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev. Nutr. 2014;34:281–303. doi: 10.1146/annurev-nutr-071812-161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muvva C, Patra S, Venkatesan S. MMpI: a wide range of available compounds of matrix metalloproteinase inhibitors. PLoS ONE. 2016;11:e0159321. doi: 10.1371/journal.pone.0159321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poincloux R, Lizárraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J. Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 69.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp. Cell Res. 2003;284:2–13. doi: 10.1016/S0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 70.Shiomi T, Lemaître V, D’Armiento J, Okada Y. Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases. Pathol. Int. 2010;60:477–496. doi: 10.1111/j.1440-1827.2010.02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodríguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: What do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim. Biophys. Acta. 2010;1803:39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki S, Ishikawa K. Combined inhibition of EMMPRIN and epidermal growth factor receptor prevents the growth and migration of head and neck squamous cell carcinoma cells. Int. J. Oncol. 2014;44:912–917. doi: 10.3892/ijo.2013.2238. [DOI] [PubMed] [Google Scholar]
- 73.Pahwa S, Stawikowski MJ, Fields GB. Monitoring and inhibiting MT1-MMP during cancer initiation and progression. Cancers. 2014;6:416–435. doi: 10.3390/cancers6010416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Itoh Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015;44:207–223. doi: 10.1016/j.matbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J. Biol. Chem. 2007;282:6899–6905. doi: 10.1074/jbc.M609570200. [DOI] [PubMed] [Google Scholar]
- 76.Mori H, et al. New insight into the role of MMP14 in metabolic balance. Peer J. 2016;4:e2142. doi: 10.7717/peerj.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Remacle AG, et al. Novel MT1-MMP small molecule inhibitors based on insights into hemopexin domain function in tumor growth. Cancer Res. 2012;72:2339–2349. doi: 10.1158/0008-5472.CAN-11-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cathcart J, Pulkoski-Grossa A, Cao J. Targeting matrix metalloproteinases in cancer: bringing new life to old ideas. Genes Dis. 2015;2:26–34. doi: 10.1016/j.gendis.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gibbons DL, Byers LAA. HER 1-2 punch: dual EGFR targeting deals resistance a deadly blow. Cancer Discov. 2014;4:991–994. doi: 10.1158/2159-8290.CD-14-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matar P, et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin. Cancer Res. 2004;10:6487–6501. doi: 10.1158/1078-0432.CCR-04-0870. [DOI] [PubMed] [Google Scholar]
- 81.Jimeno A, et al. Epidermal growth factor receptor dynamics influences response to epidermal growth factor receptor targeted agents. Cancer Res. 2005;65:3003–3010. doi: 10.1158/0008-5472.CAN-04-3586. [DOI] [PubMed] [Google Scholar]
- 82.Wheeler JJ, et al. Combining erlotinib and cetuximab is associated with activity in patients with non-small cell lung cancer (including squamous cell carcinomas) and wild-type EGFR or resistant mutations. Mol. Cancer Ther. 2013;12:2167–2175. doi: 10.1158/1535-7163.MCT-12-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64:5355–5362. doi: 10.1158/0008-5472.CAN-04-0562. [DOI] [PubMed] [Google Scholar]
- 84.Janjigian YY, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov. 2014;4:1036–1045. doi: 10.1158/2159-8290.CD-14-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ribeiro Gomes J, Cruz MR. Combination of afatinib with cetuximab in patients with EGFR-mutant non-small-cell lung cancer resistant to EGFR inhibitors. Onco Targets Ther. 2015;8:1137–1142. doi: 10.2147/OTT.S75388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pirazzoli V, et al. Afatinib plus cetuximab delays resistance compared to single-agent erlotinib or afatinib in mouse models of TKI-Naïve EGFR L858R-induced lung adenocarcinoma. Clin. Cancer Res. 2016;22:426–435. doi: 10.1158/1078-0432.CCR-15-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li C, Iida M, Dunn EF, Ghia AJ, Wheeler DL. Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene. 2009;28:3801–3813. doi: 10.1038/onc.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 89.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 92.Cammaroto G, et al. The role of PET/CT in the management of patients affected by head and neck tumors: a review of the literature. Eur. Arch. Otorhinolaryngol. 2016;273:1961–1973. doi: 10.1007/s00405-015-3651-4. [DOI] [PubMed] [Google Scholar]
- 93.Zhang D, et al. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014;355:176–183. doi: 10.1016/j.canlet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 94.Raez LE, et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2016;71:523–530. doi: 10.1007/s00280-012-2045-1. [DOI] [PubMed] [Google Scholar]
- 95.Weinhouse S. Studies on the fate of isotopically labeled metabolites in the oxidative metabolism of tumors. Cancer Res. 1951;11:585–591. [PubMed] [Google Scholar]
- 96.Weinhouse S. Oxidative metabolism of neoplastic tissues. Adv. Cancer Res. 1955;3:269–325. doi: 10.1016/S0065-230X(08)60922-7. [DOI] [PubMed] [Google Scholar]
- 97.Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat. Rev. Cancer. 2016;16:635–649. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017;7:1016–1036. [PMC free article] [PubMed] [Google Scholar]
- 99.Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat. Rev. Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 102.Rysman E, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- 103.Cheong JH, et al. Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol. Cancer Ther. 2011;10:2350–2362. doi: 10.1158/1535-7163.MCT-11-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vyas S, Zaganjor E, Haigis MC. Mitochondria and cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rêgo DF, Elias ST, Amato AA, Canto GL, Guerra EN. Anti-tumor effects of metformin on head and neck carcinoma cell lines: a systematic review. Oncol. Lett. 2017;13:554–566. doi: 10.3892/ol.2016.5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luo J., et al. An indispensable role of CPT-1a to survive cancer cells during energy stress through rewiring cancer metabolism. Tumour Biol. 2016; e-pub ahead of print 13 October 2016; 10.1007/s13277-016-5382-6. [DOI] [PubMed]
- 107.Ben Sahra I, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70:2465–2475. doi: 10.1158/0008-5472.CAN-09-2782. [DOI] [PubMed] [Google Scholar]
- 108.Yamamoto M, Inohara H, Nakagawa T. Targeting metabolic pathways for head and neck cancers therapeutics. Cancer Metastas-. Rev. 2017;36:503–514. doi: 10.1007/s10555-017-9691-z. [DOI] [PubMed] [Google Scholar]
- 109.von Roemeling CA, Copland JA. Targeting lipid metabolism for the treatment of anaplastic thyroid carcinoma. Expert Opin. Ther. Targets. 2016;20:159–166. doi: 10.1517/14728222.2016.1086341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Röhrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer. 2016;16:732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 111.Li X, et al. AMPK-mediated energy homeostasis and associated metabolic effects on cancer cell response and resistance to cetuximab. Oncotarget. 2015;6:11507–11518. doi: 10.18632/oncotarget.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat. Rev. Clin. Oncol. 2010;7:493–507. doi: 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu Y, et al. Epidermal growth factor receptor (EGFR) ubiquitination as a mechanism of acquired resistance escaping treatment by the anti-EGFR monoclonal antibody cetuximab. Cancer Res. 2007;67:8240–8247. doi: 10.1158/0008-5472.CAN-07-0589. [DOI] [PubMed] [Google Scholar]
- 114.Nevo J, et al. Mammary-derived growth inhibitor alters traffic of EGFR and induces a novel form of cetuximab resistance. Clin. Cancer Res. 2009;15:6570–6581. doi: 10.1158/1078-0432.CCR-09-0773. [DOI] [PubMed] [Google Scholar]
- 115.Wheeler DL, et al. Epidermal growth factor receptor cooperates with Src family kinases in acquired resistance to cetuximab. Cancer Biol. Ther. 2009;8:696–703. doi: 10.4161/cbt.8.8.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sok JC, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin. Cancer Res. 2006;12:5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 117.Lièvre A, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;6:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 118.Viloria-Petit A, et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. 2001;61:5090–5101. [PubMed] [Google Scholar]
- 119.Bianco R, et al. Vascular endothelial growth factor receptor-1 contributes to resistance to anti-epidermal growth factor receptor drugs in human cancer cells. Clin. Cancer Res. 2008;14:5069–5080. doi: 10.1158/1078-0432.CCR-07-4905. [DOI] [PubMed] [Google Scholar]
- 120.Fuchs BC, et al. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008;68:2391–2399. doi: 10.1158/0008-5472.CAN-07-2460. [DOI] [PubMed] [Google Scholar]
- 121.Kobayashi S, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 122.Pao W, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ji H, et al. Epidermal growth factor receptor variant III mutations in lung tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc. Natl Acad. Sci. USA. 2006;103:7817–7822. doi: 10.1073/pnas.0510284103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.She QB, Solit D, Basso A, Moasser MM. Resistance to gefitinib in PTEN-null HER overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3'-kinase/Akt pathway signaling. Clin. Cancer Res. 2003;9:4340–4346. [PubMed] [Google Scholar]
- 125.Bianco R, et al. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;22:2812–2822. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
- 126.Pao W, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou BB, et al. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer Cell. 2006;10:39–50. doi: 10.1016/j.ccr.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Engelman JA, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 129.Yano S, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–9487. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 130.Ciardiello F, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin. Cancer Res. 2004;10:784–793. doi: 10.1158/1078-0432.CCR-1100-03. [DOI] [PubMed] [Google Scholar]
- 131.Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002;62:200–207. [PubMed] [Google Scholar]
- 132.Guix M, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J. Clin. Invest. 2008;118:2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu HA, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alam N, et al. Small-cell carcinoma with an epidermal growth factor receptor mutation in a never-smoker with gefitinib-responsive adenocarcinoma of the lung. Clin. Lung Cancer. 2010;11:E1–E4. doi: 10.3816/CLC.2010.n.046. [DOI] [PubMed] [Google Scholar]