Abstract
Currently, there does not exist a strategy that can reduce diabetes and scientists are working towards a cure and innovative approaches by employing stem cell-based therapies. On the other hand, bioprinting technology is a novel therapeutic approach that aims to replace the diseased or lost β-cells, insulin-secreting cells in the pancreas, which can potentially regenerate damaged organs such as the pancreas. Stem cells have the ability to differentiate into various cell lines including insulin‐producing cells. However, there are still barriers that hamper the successful differentiation of stem cells into β-cells. In this review, we focus on the potential applications of stem cell research and bioprinting that may be targeted towards replacing the β-cells in the pancreas and may offer approaches towards treatment of diabetes. This review emphasizes on the applicability of employing both stem cells and other cells in 3D bioprinting to generate substitutes for diseased β-cells and recover lost pancreatic functions. The article then proceeds to discuss the overall research done in the field of stem cell-based bioprinting and provides future directions for improving the same for potential applications in diabetic research.
Keywords: Bioprinting, Tissue engineering, Pluripotent stem cells, Mesenchymal stem cells, Human embryonic stem, Adult human liver cells, β-cells, Islet cells, Biomaterials, Bioink, Stem cell, Diabetes
Core tip: The shortage of strategies that can potentially reduce diabetes has prompted scientists to employ stem-cell based therapies that could help generate pancreatic β- cells that can regenerate damaged pancreas. The present review article discusses the potential applications of stem cell research by incorporating 3D bioprinting technology. The article also elaborates the research that has been previously and provides future directions for enhancing the potential applications in diabetic research.
INTRODUCTION
Diabetes has become a major cause of concern owing to its serious repercussions health and its increasing occurrence at alarming rates. According to the World Health Organization, the number of people with diabetes rose to 422 million and caused 1.6 million deaths in the recent past. Diabetes, a non-communicable disease, is considered as a huge economic burden, for instance in 2010, approximately $376 billion dollars were used to treat and prevent the disease and its complications[1,2]. Over time, diabetes can permanently damage the body organs and is the major cause of kidney failures, heart attacks, strokes, blindness, and lower limb amputations[1,3]. Diabetes is a chronic metabolic disease that can be divided into two main etiopathogenetic categories: Type 1 diabetes mellitus (T1DM), which is the autoimmune destruction of insulin in the pancreas and type 2 diabetes mellitus (T2DM) which occurs when the body uses insulin ineffectively[1].
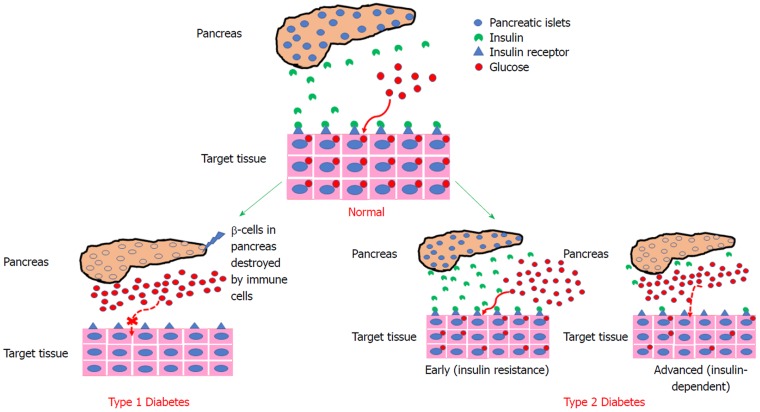
T1DM, also known as juvenile-onset diabetes, is identified by serological evidence and is most commonly found in infants and children[4]. T1DM is a metabolic disease characterized by the autoimmune destruction of islet beta cells (β-cells) and their secretory functions that result in a deficiency of insulin production[5] (Figure 1A). T1DM involves genetic factors such as human leukocytes, antigen class II genes and environmental factors that initiate autoimmunity[6]. The pathogenesis of T1DM is caused by cellular and humoral immune pathways where CD8+T lymphocytes kill β-cells[1]. T1DM patients do not produce insulin and exogenous insulin administration is required to mimic insulin release to control glucose levels during mealtimes. Patients with T1DM have been treated with immunosuppressant agents in the past, but this type of treatment does not maintain the function of β-cells rendering insulin replacement therapy as the only treatment effective for the restoration of metabolic disturbances in T1DM patients. The treatment for T1DM requires administering a long acting insulin dosage (once or twice a day with each meal)[7]. Furthermore, treatment for T1DM is based on a rigorous monitoring of blood glucose levels and intravenous insulin injections. The management of T1DM requires significant patient compliance, which is associated with an increased risk of hypoglycemia.
Figure 1.
Schematic representation differentiating between normal and diabetic (Type 1 and Type 2) pancreas.
T2DM or adult-onset diabetes is a more prevalent category caused by a combination of insulin resistance and inadequate insulin secretory responses and functions[1,4] (Figure 1B). T2DM is asymptomatic as its progression causes hyperglycemia, which triggers pathological and functional changes in target tissues. Patients with T2DM and T1DM are at risk of developing micro- and macro-vascular complications[8-13]. These are associated with atherosclerotic disease affecting arteries that supply blood to the heart and increase the risk of cardiovascular disease in which death from myocardial infarction and strokes is the leading cause of mortality in T1DM and T2DM patients[2,14]. Patients with T2DM are subjected to a non-insulin-based therapy and in some patients with T2DM; the insulin requirements are similar to those with T1DM necessitating daily injections for long-acting insulin during mealtimes[15].
Researchers have intensely studied diabetes and have decades of experience investigating means to replace β-cells of the pancreas that are destroyed by the immune system. Current procedures involve allotransplantation, which requires passing a catheter through the liver, involving high risks of bleeding and blood clots, and is categorized under extremely invasive surgeries[16]. A very common treatment for diabetes is the transplantation of the pancreas, but an extreme shortage of donors still exists[9]. According to United Network for Organ Sharing, a person is added to the national transplant waiting list every ten minutes. Moreover, the other hurdles related to pancreas and islet transplantation are associated with alloimmune responses[9,16].
Current treatment strategies have not been able to successfully maintain or replace the function of β-cells, thereby seeking alternative therapies such as regenerative medicine using stem cells, in combination with bioprinting technologies to cure and diminish the health challenges of diabetes. The efficacy of combining stem cells and additive manufacturing in the field of regenerative medicine has been established in prior studies and has prompted further research for scientists, worldwide. This review discusses stem cell-based therapies and the applications of bioprinting in regenerative medicine, which can be directed towards strategizing potential treatments for regenerating the pancreas affected during diabetes, either in part or as a whole.
STEM CELL-BASED THERAPIES
The main goal of diabetes therapy is to attain normoglycemia through the replacement of the diseased or lost cells of the pancreas with new cells. Scientists have attained success in producing insulin-secreting cells from different types of cells. This section focuses on the current types of stem cell research to treat diabetes and past research relating to novel applications of stem cell therapies for diabetes.
Stem cell-based therapy
Induced pluripotent stem cells: Ever since Takahashi et al[17] demonstrated that induced pluripotent stem cells (iPSCs) could be generated from differentiated somatic cells through the reprogramming of adult and embryonic mouse fibroblasts by transfecting the cells with plasmids, they have opened up a possibility for replacement in cell-based therapy. iPSCs are also favored for their capacity to self-renew infinitely and their potential for differentiation into a wide variety of cell types[18]. The maintenance of undifferentiated iPSCs as cell lines holds great promise for modeling diseases and to generate personalized stem cells for cell therapies[19]. According to studies done by Alipio et al[20], hyperglycemia in diabetic mice was found to be controlled by mouse skin fibroblast-derived iPSCs that differentiated into β-like cells, which were morphologically identical to normal, endogenous cells that secreted insulin. Mature pancreatic cells that had the ability to secrete insulin and C-peptide were generated by the differentiation of human embryonic stem cells (ESCs) and iPSCs[21]. Patients suffering from T1DM and T2DM diabetes were employed as sources to produce iPSCs[22]. In vitro production of insulin-secreting cells was also achieved by the directed differentiation of iPSCs using small molecules and growth factors in the culture[23]. The primary advantages of employing iPSCs are that they do not present ethical concerns and only pose a low risk of teratoma formations[24]. However, the reprogramming of somatic cells into iPSCs achieved with the aid of viral transfection of transcription factors requires the use of genomes[25]. These genomes are harmful as they can trigger mutations and hamper the normal function of iPSCs and their ability to differentiate, in addition to causing the formation of tumors[25].
Mesenchymal stem cells: The method for isolating mesenchymal stem cells (MSCs) from the rat bone marrow was first described by Friedenstein as explained in previous studies[26]. Although the bone marrow is the richest source of MSCs[27-29], they have also been successfully isolated from adipose tissues[30,31], fetal liver[32], umbilical cord and its blood[33,34], fibroblasts[35], endometrium[36], placenta[37], trabecular and compact bone[38]. MSCs have been found to be able to differentiate into mesodermal, endodermal and ectodermal cells under suitable culture conditions[39]. MSCs are suitable for the regeneration of tissues, as they do not result in teratoma formation[39]. Other advantages of using MSCs for stem cell-based therapy include the ease of isolation, expansion to large quantities and their multipotential differentiation capacity[40]. In addition, their ability to circumvent immune recognition and inhibit immune responses also makes them ideal candidates for immunomodulatory cell therapy in immune-mediated diseases[41].
According to studies performed by Xu et al[42], the direct injection of MSCs into the pancreas had helped alleviate diabetes symptoms by improving the metabolic control in animal models, counteracting autoimmunity, enhancing islet engraftment and survival, besides serving as a source of growth factors and cytokines. Direct injection of MSCs has not only been found to be effective in improving the functions of the pancreas but also healed related symptoms like diabetic foot and neuropathy[43]. The main limitation posed by MSCs is their potential to differentiate into unwanted mesenchymal lineages, which can be detrimental to their therapeutic applications[44]. The possibility of malignant transformations and cytogenetic aberrations of MSCs may also considered drawbacks[44]. Results of some MSCs clinical trials in T1DM are shown in Table 1[45-51].
Table 1.
Results of some mesenchymal stem cells clinical trials in diabetes mellitus type 1[45]
| Types | Routes of transplantation | Outcome |
| Human MSCs | Intravenously introduced to Non-obese diabetic/Severe combined immunodeficiency mice with total body irradiation or local abdominal or leg irradiation | Safe and efficient for the long-term treatment of severe complication after radiotherapy[46] |
| Umbilical cord derived MSCs | Injected directly into the pancreas | Improvement of metabolic control. Enhancement of islet engraftment and survival[42] |
| Bone marrow-derived MSC | Differentiated in vivo into functioning β-cells | Normalization of chronic hyperglycemia in a diabetic rat[47] |
| Human placenta ‑derived MSCs | Differentiated into islet-like cell clusters and transplanted into streptozocin-induced diabetic mice | Restoration of normoglycemia in diabetic mice[48] |
| Human umbilical cord blood derived MSCs | Differentiated into IPC through intravenous administration | Improvement in glycemic profiles, histological improvement of insulates[49] |
| Wharton's jelly and amniotic membrane derived MSCs | (1) Differentiated into IPC and transplanted into the liver; (2) Infected with PDX1 gene and differentiated to IPC; and (3) Differentiated into IPC and transplanted into the liver of STZ-induced diabetic rats | Expression of insulin Secretion of C-peptide; expression of pancreas-specific genes[49]; correspondence to high concentrations of glucose[50]; reduction of blood glucose levels after 4 wk of transplantation[51] |
MSCs: Mesenchymal stem cells; IPC: Insulin-producing cells.
Human embryonic stem cells (hESCs): hESCs are characterized by properties such as pluripotency of gene expression, self-renewal ability, and high proliferative capacity[52,53] thereby making them a valuable treatment option in all types of medicine. Numerous in vivo and in vitro differentiation strategies have been adopted for the production of functional pancreatic islets. Generally, hESCs are initially harvested from the inner cell mass of the blastula post fertilization when the cells are still capable of differentiation into all types of germ layers and there is a high level of telomerase activity[52]. This is followed by the differentiation of the hESCs into definitive endoderm, which further undergo differentiation into functional β-cells, through a chain of endodermal intermediates[54,55]. These techniques cause the hESCs to be exposed to specific transcription factors that can facilitate coordinated activation and inhibit intracellular signaling pathways. Although cell signaling and epigenetic factors involved in the differentiation process remain to be studied and understood, the detection of markers such as pancreatic and duodenal homeobox gene 1 (PDx1), insulin gene enhancer protein (Isl-1), and Forkhead box protein A2 validate the endodermic differentiation into endocrine and exocrine pancreatic β-cells[56,57].
Non-stem cell-based therapy
Adult human liver cells: The liver has been extensively studied as a potential source for pancreatic β-cells that can help cure diabetes. It has an added advantage over other organs as it has been derived from the endoderm along with the pancreas[58,59]. A comprehensive developmental shift of adult human liver cells into insulin-producing cells was induced with the help of PDx1 and other soluble factors[59]. Studies conducted by Yang et al[60] provide evidence that purified adult rat hepatic oval ‘‘stem’’ cells transdifferentiate into pancreatic endocrine hormone-producing cells when subjected to culture in a high-glucose environment. These differentiated cells then self-assemble forming three-dimensional islet cell-like clusters that express pancreatic islet cell differentiation-related transcripts which can be validated by reverse transcription–PCR/nested PC and islet-specific hormones detectable by immunohistochemistry[60]. Hepatic oval cell activation through hepatic trans-differentiation and pancreatic islet regeneration was also successfully reversed for streptozotocin-induced diabetes[61]. Although these methods differed in terms of their approaches, they were successful in ameliorating hyperglycemia in the mouse models. This further led to a search for alternate pancreatic sources of insulin as can be seen from the studies conducted by Zalzman et al[62], which demonstrated the reversal of hyperglycemia in mice by employing human expandable insulin-producing cells that were generated by the differentiation of fetal liver progenitor cells .
β-cells: The pancreas is the first choice for harvesting potential stem cells for the treatment of diabetes[63]. Bonner-Weir et al[63] demonstrated through their experiments that the availability of small amounts of pancreatic tissue could help to restore the maximum pancreatic β-cell mass. This has been attributed to the replication and de-differentiation of differentiated β-cells of the pancreatic ducts, which in turn triggers the production of more β-cells. Further studies conducted showed that these ductal cell populations could be cultivated and directed into forming cell-clusters secreting insulin[63,64]. A clonal population of adult pancreatic precursor cells, that had the ability to produce both insulin and C-peptide, were generated from ductal cells by Seaberg et al[65]. Although there were debates in the past about the existence of pancreatic adult stem cells despite their progress and potential, strong evidence indicating that the pancreatic ducts of mice contained multipotent progenitor stem cells, which could generate new β-cells, was given by Xu et al[66]. However, more research needs to be done for the promotion of β-cell formation in diabetic patients by finding and activating pancreatic stem cells. This necessitates the development of better experimental strategies to come up with suitable methods to overcome the issues of isolation and ex-vivo expansion of these stem cells for transplantation.
Islet cells: The pancreatic islets, also termed, as the islets of Langerhans, constitute regions of the pancreas that contain the hormone-producing cells (endocrine cells) and were first described by Paul Langerhans in 1869, a German pathological anatomist[67]. The relation between the pancreas and diabetes was established much later by Minkowski and von Mering[68]. The islets of Langerhans were first isolated from the pancreas of a guinea pig by Moskalewski et al[69] by employing an enzymatic digestion technique. Studies conducted by Bottazzo et al[70] indicated the possibility that islet cell transplantation would be a very suitable option for people who were suffering from T1DM poorly controlled with insulin. The challenges of transplanting islet cells include finding compatible donors, ensuring the survival of the new islets and side effects induced by medications administered to prevent immune rejection[71]. Azarpira et al[72] successfully isolated islet cells from cadaveric donors which were then administered via injections into the recipient’s portal vein. The study showed that there was a reduction in the initial β-cell mass attributed to instant blood-mediated inflammatory reactions, immune responses resulting from the transplantation of the islet cells and diabetogenic effects triggered by the immunosuppressive medications[72]. This necessitated the need for repeated episodes of cell transplantation to ensure significant outcomes[73]. According to studies conducted by Bennet et al[74], it was established that the exposure of isolated islets to ABO-compatible blood resulted in an immediate thrombotic reaction and hence required multiple transplants to reduce the insulin shots. There was also the possibility of the impairment in insulin production of the transplanted islets due to their entrapment by blood clots, which could shut them off from oxygen and attract immunocytes[75]. This motivated scientists to seek alternative cell sources such as pluripotent and multipotent stem cells, to generate pancreatic cells and aid in diabetes therapy by replacing the diseased or lost pancreatic cells[76].
3D BIOPRINTING
Bioprinting techniques had emerged in 1988, as demonstrated by Klebe[10] using cytoscribing technology, a method that requires mispositioning of the cells to construct synthetic tissues using a Hewlett Packard inkjet printer. 3D bioprinting is a revolutionary field that is utilized in biomedical engineering and sciences. The difference between 3D printing and 3D bioprinting is that bioprinting technologies utilize living cells, which are printed layer by layer to form a 3D structures[11,12] with the ultimate goal to regenerate the diseased or damaged tissue and reduce organ shortage[2]. Currently in the United States, there is a great need for an alternative to organ transplants, due to the limited availability of organ donors[77]. A potential solution for this problem is tissue engineering by developing organs that can be built with the patients' genetics to eliminate the chances of rejection, relieve suffering, and save lives[78]. The purpose of tissue engineering and state-of-the-art 3D printing is to develop a degradable scaffold, that will allow cells to proliferate and regenerate through pores to replace the damaged organ or tissue. These characteristics provide the cells with viability and functionality, in addition to the ability to attach and mimic the native organ environment[13].
3D printing tissue engineering and regenerative medicine holds great promise for building and assembling viable and functional tissues and organs. 3D printing involves a combination of scaffold and biomolecules that sustain the cells, to improve or regenerate specific tissue or the whole organ[13]. Researches had encountered challenges while trying to develop the accurate scaffold materials for manual cell seeding[79]. Difficulties in seeding the cells manually limit the cells’ precise placement and ability to proliferate inside the scaffold[79]. Despite the great advantages of biofabrication of scaffolds, another limitation is that cells need to grow in high density to develop the thickness of the organ or tissue, which is difficult to achieve because the cells only attach to the surface and do not penetrate the entire scaffold[79]. Furthermore, the difficulty and need to achieve vascularization and anastomosis is critical. These challenges have led to the development of optimization of bioprinting technologies and cell seeding protocol where scientists encapsulate large numbers of cells to achieve density and promote oxygenation, vascularization and the desired pattern through the scaffold[80,81].
3D bioprinting technologies involve the design of unconventional scaffolds where the design is inspired by the patient's own anatomy for developing a correct shape for the tissue construct. Bioprinting technology can develop a porous construct to allow media and nutrients to reach the cells. Bioprinting technologies are based on three major steps for the design of tissue regeneration. To develop a medical image of the desired area of the body, a blueprint is created using a software system, which is followed by toolpath planning and finally 3D bioprinting, which is divided, into three major categories depending on the technique employed to print (Figure 2)[13,79,82].
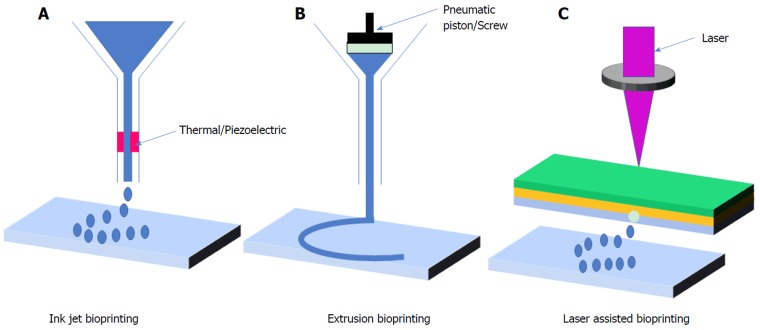
Figure 2.
Classification of bioprinting techniques. Three major classifications of bioprinting modalities are A: Inkjet-based printing, which air-pressure pulses that force droplets from nozzle by heating up the printhead; B: Exzrusion-based printing,using pneumatic or mechanical dispensing systems for extruding continuous beads of materials and/or cells; C: Laser-based bioprinting that uses lasers focused on an absorbing substrate for generating pressure that compels the bioink to be extruded onto a collector substrate.
The first category is extrusion-based bioprinting that uses a combination of automated robotic and fluid allotting system of pneumatic, mechanical force or solenoid micro-extrusion to continuously extrude bioink on the biopaper[13,79]. The second category is inkjet-based
bioprinting, in which small droplets of cells are ejected to fabricated tissues[83]. This method involves electro-hydrodynamic jetting, acoustic droplet ejection, thermal, piezoelectric, or electrostatic energy for printing[79]. The third category is laser-based bioprinting; which incolves cell-transfer and a photo-polymerization process using digital light to crosslink the bioink (Figure 2)[13,79].
The process of bioprinting involves two components, namely the bioink and the biopaper. The bioink is a biomaterial in which live cells are embedded to print on the biopaper to mimic the extracellular matrix of the desired tissue. The biopaper is another important component of 3D bioprinting because it serves as the substrate on which cells (bioink) are deposited in an organized pattern[13]. Currently, hydrogels are popularly employed as bioinks as they facilitate effective oxygen, nutrient and metabolite transportation, besides providing great permeability to water[13,79,84].
However, synthetic bioinks struggle to achieve high printability and biocompatibility, thereby strengthening the need for developing naturally derived bioinks. A novel furfuryl-gelatin based bioink was developed and found to exhibit a highly porous networked structure, and co-culture feasibility when C2C12 myoblasts and STO fibroblasts were printed in a double-layered structure[85]. These structures, cross-linked by exposure to visible light, have been successful in preserving the viability of both cells types, showing that this bioink can be used for tissue engineering applications for developing complex tissues to help study cellular communication in a disease or normal models[85]. Comparison of cell viabilities for ink jet based-, extrusion based- and laser assisted bioprinting is shown in Table 2[86-90].
Table 2.
Comparison of cell viabilities for ink jet based-, extrusion based- and laser assisted bioprinting[90]
Other properties of the bioink, such as transfer of thermal energy into kinetic energy and high viscosity, rapid gelation mechanism by enzymatic, physical, or chemical crosslinking processes are important for consideration to develop the ideal scaffolds[91].
APPLICATIONS OF BIOPRINTING
3D bioprinting has the ability to write living cells in a stackable layer-by-layer organizational pattern using biomaterials to engineer a specific construct for the use of tissue regeneration, surgery procedures, drug and medical studies to treat disease and health-related complications[84,92,93]. This computer-assisted technology is a powerful tool that has obtained attention worldwide[79] and 3D bioprinting modalities are driven by endless possibilities of innovative use in regenerative medicine and tissue engineering. This technology offers the advantage of placing cells in a precise location and specific fashion to create a cellular models[84,92,93].
Tissue engineering and regenerative medicine
Current translational benefits in 3D bioprinting are in tissue engineering and regenerative medicine i.e., bone tissue engineering for the development of the specific tissue construct by recreating the unique patients’ anatomy[79,94]. Another such benefit of 3D bioprinting is the ability to develop a cardiac patch with the ability to synchronously beat, which has great promise in regenerating a specific area of the heart[95]. Anil Kumar et al[85] developed a novel furfuryl-gelatin based hydrogel that was bioprinted into cell-laden rectangular constructs and may potentially be implanted on post infarcted hearts. Cartilage tissue has been successfully bioprinted, to solve cartilage defect repair[65]. Furthermore, the progress made in creating an organ-in-a-chip helps to simulate the mechanisms and functions of a specific body area[92]. This approach also provides the opportunity to perform drug screening studies for diseases[96]. However, the difficulty in incorporating vascularization simultaneously with the 3D bioprinting of tissues, gives rise to challenges in the fabrication of bone tissues, to treat major defects or bone loss[97]. The use of hydrogels for bone tissue in bioprinting approaches makes it difficult to implant into a load-bearing site in the patient’s body[98]. Thus, the hydrogels need to be mechanically robust and possess the characteristics to support large-scale regeneration of bone tissue in vivo[98].
3D bioprinting for bone tissue reconstruction presents major challenges related to vascularization. Considerable progress has been made in skin bioprinting, but improvement in scar-less tissue formation need to be implemented[79]. Another challenge that needs to be overcome involves in vivo studies for bioprinted blood vessel and the organ fabrication. Moreover, the availability of technologies capable of bioprinting vascular networks in high density and generate organ constructs integrating different tissues together, are also needed[79]. For this, co-culture of different cell types for the development and reconstitution of the functionality of a whole organ is necessary. Despite the progress in 3D bioprinting technologies translated from bench to bedside, the aforementioned applications still have limitations and challenges that need to be overcome, especially in the fabrication of functional tissues with long-term viability[99]. For instance, the heart, pancreas, and liver are the organs that are the most difficult to fabricate due to the need for metabolic functions and vascularization[79]. Metabolically highly active organs are a great challenge to reconstruct because their complexity requires molecular networks from arteries, veins and cell communication of different cell types in order to mimic the identical long-term functionality[99].
Currently, bioprinted living constructs have been acutely investigated and transplanted in vivo (animal models)[100]. Animal studies provided the opportunity and insights into evaluation of engraftment of the implant with the host anastomosis, vascularization, and regeneration of functionality[101]. 3D-printed metallic, plastics, and ceramics have been developed as successful constructs for bone tissue replacement and these constructs have been transplanted into humans[97]. Bioprinting is a powerful tool for medical procedures, especially for a near future with possible in situ bioprinting[79]. In situ bioprinting is an attractive application for 3D bioprinting that has provided a major advantage in regenerative medicine over traditional procedures. Recently, the use of in situ bioprinting was applied in skin regeneration for large wounds on pig models[102] and skull defects in rodents[101,103]. The advancement of in situ bioprinting can be applied in the regeneration of a variety of tissues and organs such as plastic surgery, maxillo- and craniofacial reconstruction[103].
Screening and drug toxicity testing
Another benefit in using bioprinting technologies is the application of bioprinted tissue and organ models for potential pharmaceutics use and for screening and drug toxicity testing[96]. This application relieves the time consumption and cost related to drug discovery, which entails financial investment and human resources. In addition, 3D bioprinted tissues have the ability to bioprint in microarrays and develop in vitro 3D-pinted models that mimic the native human tissue[79,96]. This approach provides the opportunity to use a 3D-assay system that may contribute to a possible solution to lower the cost and financial investment in pharmaceutics. Bioprinting has also offered other great advantages for testing toxicity; for instance, the development of liver-on-a-chip for testing hepatic toxicity of acetaminophen[104] and the test of antitumor drugs for breast cancer[105].
Future concerns
Regenerative medicine is a rapidly expanding area of research that deals with repairing or replacing damaged tissues and organs[106]. Tissue engineering may one day put an end to allogenic organ transplantation and the need for immunosuppression. Stem cells are a cornerstone to this process, as they possess the ability to differentiate into nearly any cell type[107]. Combining the abovementioned research fields with 3D bioprinting will allow for in vitro tissue creation. Bioprinting uses the 3D additive manufacturing process while utilizing biomaterials, growth factors, or different cell types as the printing medium[108].
Computed tomography or magnetic resonance imaging scans can be used to create a digital blueprint of the desired organ[109,110]. This computer created file is then converted into thin slices that can be layered on top of one another. When the 3D printing process is done the tissue still needs to undergo a maturation process before it can be implanted. Over time the tissue will start to develop its own extracellular matrix and any temporary scaffolding is degraded[110].
One of the largest challenges in 3D printing human tissue and organs is to implement and promote vascularization[111]. Researches have tried to overcome this obstacle by printing sacrificial mediums embedded in endothelial cells, which can mature into blood vessels as the original medium slowly degrades over time[112]. However, these constructs are extremely fragile and require mechanical and chemical stimulation to undergo maturation and capable of implantation into the body[113]. Once in the body, the new tissue must generate its own extracellular matrix to be fully incorporated. Trauma and tumor growth can lead to substantial amounts of bone loss[114]. Traditional bone grafts are limited < 5 cm in size and often fail due to residual stress[115]. Gao et al[87] used an inkjet printer to print peptides and PEG with simultaneous photo-polymerization using bone marrow mesenchymal stem cells, which showed significantly enhanced osteogenic differentiation.
Liver transplantation is the only cure for liver failure. However, there are more people waiting for livers than there are donors, leading to many deaths while waiting for a transplant[116]. Faulkner-Jones et al[117] differentiated iPSCs into hepatocytes after bioprinting showed that stem cells maintain their pluripotency during the printing process. Ahn et al[118] printed a multilayer porous mesh structure made with alginate and ADSCs, which they successfully differentiated into a hepatogenic lineage expressing liver-specific genes.
THERAPEUTIC APPLICATIONS OF STEM CELLS AND BIOPRINTING TOWARDS DIABETES
Around 15 different types of tissues have been studied in bioprinting technology but there are other tissues types that are part of the human body, which are unexplored and need more investigation[79]. In addition, the innovation of bionic organs or new types of organs is a possible direction for the future in bioprinting to solve organ shortage and alleviate patients’ suffering[119]. Bioprinting research involves multiple cell types patterned to mimic the complex anatomy of the human body and the understanding for an optimal protocol for culture conditions with multiple cell types; these optimizations should include the correct medium and nutrients to promote growth and viability of multiple cell types[79,81].
For T2DM
An example of an application of bioprinting with cells is a pancreatic model bioprinted with pancreatic islets that was implanted into a diabetic murine model leading to regulated insulin secretion. However, the size of the mouse model of study was significantly different, about 100000 times smaller than a human model[79,100,120]. Hence, the 3D bioprinted models of study need to have relevant dimensions for clinical use i.e., the simulation of human size, a larger animal model needs to be used that can possibly represent human physiology[121].
A recent study had reported translational benefits of adult and embryonic stem cell in which stem cells can be used to produce insulin-like secreting cells known as β-cells[76]. The translational benefits provided evidence towards the existence of new β-cells generated by the replication of pre-existing β-cells from the adult pancreas or partial removal of the pancreas[76]. Cells used to reconstruct and regenerate the pancreas after implantation must be pathogen free. Ideally, the cells that will differentiate into β-cells should not only be able to reconstitute the function of the pancreas but also maintain long-term and normal activity[122]. It has been shown that mature exocrine cells of the pancreas can be reprogrammed to become β-like-cells in vivo with a combination of 3 transcription factors[123]. Another challenge that needs to be addressed is that the differentiated β-cells persist as individual cells or small clusters and do not reorganize into islets before clinical therapy is induced[122]. The viruses that are used to reprogram factors needed for induction of differentiation should be replaced with safer reagents to produce β-cells[124].
Although 3D bioprinting has been successfully applied to fabricate tissues such as blood vessels[125,126], skin[127,128], bone and cartilage[93,122,125-130] and liver[13], the bioprinting of pancreatic islet tissues to treat diabetes remains to be explored. However, other techniques such as stereolithography have shown promise, in this regard. According to work done by Gallego-Perez et al[131], microwell arrays were created with stereolithography and electrospinning, and structurally interfaced with a porous sheet of micro/nano-scale polyblend fibers. These arrays served as a platform for the anchoring and subsequent assemblage of human pancreatic ductal epithelial cells into insulin-expressing 3D clusters occurred[131]. Given that cluster size and uniformity are known to influence islet cell behavior, the ability to effectively control these parameters could find applications in the development of anti-diabetic therapies[131]. Immunoreactivity for insulin, C-peptide and glucagon was detected on both the platform and control surfaces; however, intracellular levels of C-peptide/cell were approximately 60% higher on the platform[131]. Alginate-based porous scaffolds as extra-hepatic islet delivery systems were successfully developed through 3D plotting by Marchioli et al[99]. INS1E β-cells, human and mouse islets were successfully embedded in these 3D-plotted constructs without affecting their morphology and viability while preventing their aggregation[99]. Studies such as these show that there is a definite possibility of treating diabetes by incorporating 3D printing technology, but rigorous research is in order before that can be achieved.
Investigations led by Dor et al[132] provided conclusive evidence that terminally differentiated β-cells could retain a significant proliferative capacity in vivo and could be used as a major source for new β-cells during adult life and following pancreatectomy in mice.
A scalable differentiation protocol to generate millions of glucose-responsive β-cells from hPSC in vitro was reported by Pagliuca et al[54] as the, insulin-producing cells that were previously generated from human pluripotent stem cells (hPSC) were found to lack many functional characteristics exhibited by bona fide β-cells.
Ozbolat et al[14] proposed the concept of miniature organs, that could potentially be fabricated on a smaller scale in comparison to their natural counterparts and closely mimic the most vital function of the associated organ, such as a pancreatic organ. This organ could be placed in a less immune-responsive site in the body to effectively produce and secrete insulin in the desired quantities into the bloodstream to regulate glucose levels to normoglycemia in the human body[14].
Chen et al[133] investigated the possibility of differentiating rat marrow MSCs in vitro into functional islet-like cells and to confirm their diabetes therapeutic potential. Insulin mRNA and protein expressions were observed in the resulting typical islet-like clustered cells[133]. The insulin excreted from the differentiated cells was found to be much higher than the undifferentiated MSCs[133]. The injected differentiated MSCs were also found to downregulate glucose levels in diabetic rats when diabetic rat models were made to test the in vivo function of the differentiated MSCs[133].
Jiang et al[56] established a novel serum-free protocol to generate insulin-producing islet-like clusters (ILCs) from hESCs grown under feeder-free conditions. The hESCs were treated with sodium butyrate and activin A to generate definitive endoderm[56]. The endoderm population was then converted into cellular aggregates which were further differentiated into Pdx1-expressing pancreatic endoderm in the presence of epidermal and basic fibroblast growth factors[56]. The aggregates were finally allowed to mature and the temporal pattern of pancreas-specific expression in the hESC-derived ILCs showed considerable resemblance to in vivo pancreas development, and the final population contained representatives of the ductal, exocrine, and endocrine pancreas[56].
Ferrell et al[134] successfully developed a technique that could enable the active patterning of individual cells and groups of cells in a polymer-based microdevice using vacuum-assisted cell seeding. Polymer microwells with various geometries on top of commercially available porous membranes were moulded by employing soft lithography[134]. This method was used to determine the number of cells in a microwell for given cell seeding density and microwell geometry and tested successfully with pancreatic ductal epithelial-like cells indicating potential applications in tissue engineering[134].
Patients with diabetes mellitus are at a greater risk of developing heart failure such as hypertension and coronary artery disease[135]. Diabetic patients may develop a diabetic heart disease (DHD) in which progresses with cardiac hypertrophy where the thickness of the left ventricular wall is increased and caused diastolic dysfunctions and other abnormalities[2]. Myocardial dysfunctions and impaired coronary perfusions in DHD are dependent pathologies associated with endothelial dysfunction initiated by diabetes[136]. Previous studies had showed that T2DM disrupts mitochondrial proteomic associated with protein import efficiency, which triggers mitochondrial dysfunction in diabetic patients leading to heart problems[4]. Further studies need to be explored in order to understand the causes of DHD; for instance, the development of an organ-on-a-chip can be established to construct experiments for deficiency of signaling pathways, drugs screening through systemic interactions by interconnecting different organs such as the pancreas and the heart or other organs affected by diabetes[137,138]. In addition, organ-on-a-chip can help to develop devices with sensors that can read glucose levels or increased proteins levels in the heart that may trigger heart failure; moreover, these state-of-the-art devices can also help to manage skin wound in risk of bacterial infections on those diabetic patients[9,139,140]. Status of stem cell therapies and bioprinting in tissue repair and regeneration are shown in Table 3[141-181].
Table 3.
Status of stem cell therapies and bioprinting in tissue repair and regeneration
| Organs | Stem cell | Bioprinting |
| Heart | (1) Combination of Mesenchymal and c-kit (+) Cardiac stem cell[141]; and (2) Human embryonic stem cell–derived cardiomyocytes[142] | (1) 3D bioprinting approach for vascularized heart tissue engineering based on human umbilical vein endothelial cells and induced pluripotent stem cells-derived cardiomyocytes[143]; (2) 3D-printed patch composed of human cardiac-derived progenitor cells in a hyaluronic acid/gelatin (HA/gel) based matrix[144]; and (3) 3D endothelial bed was seeded with cardiomyocytes to generate aligned myocardium capable of spontaneous and synchronous contraction[145] |
| Blood vessels | (1) Endothelial cells derived from human embryonic stem cells[146]; and (2) Human Pluripotent Stem cells[147] | (1) Pluronic F127 was used as a sacrificial material for the formation of the vasculature through a multi-nozzle 3D bioprinting system[148]; and (2) Drop-on-demand bioprinting technique to generate in vitro blood vessel models[149] |
| Nerves | Mesenchymal stem cell[150,151] | (1) Novel technique for bioprinting of fibrin scaffolds by extruding fibrinogen solution into thrombin solution, utilizing hyaluronic acid (HA) and polyvinyl alcohol[152]; and Production of high-resolution 3D structures of polylactide-based materials via multi-photon polymerization and explores their use as neural tissue engineering scaffolds[153] |
| Eyes | (1) Embryonic stem cell[154]; and (2) Limbal stem-cell[155] | (1) Produced 3D cornea-mimicking tissues using human stem cells and laser-assisted bioprinting[156]; and (2) Physical and chemical signals through 3D-bioprinting of HA hydrogels and co-differentiation of retinal progenitor cells into photo receptors [157] |
| Kidneys | (1) Embryonic stem cell[158]; and (2) Human pluripotent stem cells[159,160] | Bioprinting method for creating 3D human renal proximal tubules in vitro that are fully embedded within an extracellular matrix[161] |
| Skin | Mesenchymal stem cells[102,162] | (1) Amniotic fluid-derived stem cells printed in a set of pressure-driven nozzles through hydrogel solutions[102]; (2) Novel bioink made of gelatin methacrylamide and collagen doped with tyrosinase is presented for the 3D bioprinting of living skin tissues[163]; and (3) 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink[164] |
| Pancreas | (1) Embryonic stem cells[165]; and (2) Human embryonic stem cells[166,167] | (Not fully developed) reviews[168,169] |
| Brain | (1) Multipotent adult stem cells[170]; and (2) Endogenous neural stem cells[171] | (1) Method for fabricating human neural tissue by 3D printing human neural stem cells with a bioink, and subsequent gelation of the bioink for cell encapsulation[172]; and (2) 3D bioprinted glioma stem cell model, using modified porous gelatin/alginate/fibrinogen hydrogel that mimics the extracellular matrix[173] |
| Lungs | (1) Distal airway stem cell[174]; (2) Pluripotent stem cells[175]; and (3) Exogenous stem/progenitor cells[176] | Reviews[177,178] |
| Liver | (1) Mesenchymal stem cells[179]; and (2) Induced pluripotent stem cells-derived organ bud transplant[180] | (1) Human embryonic stem cells-derived hepatocyte-like cells were 3D printed using alginate hydrogel matrix[117]; (2) Development of a liver-on-a-chip platform for long-term culture of 3D human HepG2/C3A spheroids for drug toxicity assessment[104]; and (3) Liver tissue model conducive to hepatotoxicity testing was developed by bioprinting hepatic spheroids encapsulated in a hydrogel scaffold into a microfluidic device[181] |
For T1DM
Besides providing for a constant source of β-cells, for serving therapeutic benefits in T1DM there is a need for a protective shell, which can house the newly regenerated β-cells while preventing antibodies from destroying them, thereby retaining their functionality.
Although T1MD has been treated by the transplantation of islets of Langerhans into the pancreas, it has necessitated the need to administer immunosuppressive drugs to the patients[182]. Since the side effects of these drugs have not been understood completely, cell transplantation therapy without the use of immunosuppressive drugs is preferred. Bioartificial pancreas has been fabricated by the encapsulation of islet cells within a semi-permeable membrane for the resolution of this issue[182]. Prior research has reported that these models function well with small animal models, but their clinical outcome on human patients remains to be studied further[182].
Scaffold-free tissue strands, expressing high levels of insulin, were microfabricated for extrusion based bioprinting by Akkouch et al[183]. These tissue strands were composed of rat fibroblasts and mouse insulinoma TC-3 β cells in the core and shell, respectively and were developed for scale up tissue engineering purposes[183].
Microscale organoids in which heterocellular aggregates possessed organ-like functions, have been successfully generated in vitro for pancreatic tissues by Greggio et al[184]. Efficient expansion of dissociated mouse embryonic pancreatic progenitors was enabled by establishing three-dimensional culture conditions in Matrigel[184]. Hollow spheres, composed of pancreatic progenitors, or complex organoids spontaneously undergoing pancreatic morphogenesis and differentiation, were generated by the manipulation of the medium composition[184]
Hiscox et al[185] successfully developed a tissue engineered pre-vascularized pancreatic encapsulating device (PPED) using collagen gels. It was observed that isolated islets that were placed in collagen gels exhibited fourfold more insulin release than islets not in collagen. Subsequently, a sandwich comprised of two layers of pre-vascularized collagen gels around a central collagen gel containing islets was also developed and implanted. In vitro characterization of the islets showed that islets were functional and responded to glucose stimulation[185]. Insulin and the presence of intra-islet endothelial cells were detected by performing immunohistochemical analysis. The results of the study indicated that PPED was able to enhance the islet survival by supporting islet viability and maintaining intra-islet endothelial cell structures[185]. Bloch et al[186] developed a technology to overcome the immunoisolation of pancreatic islets that leads to severe cell hypoxia and dysfunction. A thermophylic strain of the unicellular alga Chlorella was used as a natural photosynthetic oxygen generator to supply oxygen to the islets encapsulated in alginate[186]. The results of the study indicated that photosynthetic-dependent oxygen generation induced higher glucose-stimulated insulin response when compared to normoxic perfusion[186].
CONCLUSION
The ever-rising global burden of diabetes and its related complications is predicted to affect about 650 million by 2040 and is a major burden on our economy (American Heart Association). Diabetes mellitus is believed to be the underlying cause of functional and structural changes in the myocardium, that manifests in the condition referred to as diabetic cardiomyopathy (DCM), and may lead to heart failure independent of underlying coronary heart disease[187]. Patients with T2DM are recognized to have an increased risk of cardiovascular morbidity and mortality as hyperglycemia deteriorates endogenous cardiac protection[188]. Although DCM results from various mechanisms including microvascular impairment, metabolic disturbance, subcellular component abnormalities, cardiac autonomic dysfunction, and a maladaptive immune response, the underlying pathogenesis is partially understood. But there are major discrepancies among animal and human studies that leaves an important gap in knowledge[189]. Insights into the pathophysiology of human DCM are critical to discovering standardized targeted therapies. Therefore, there is an urgent need to biofabricate human tissue-on-a-chip models that can serve as a basis for development of novel therapeutic approaches to cure or prevent DCM in vivo. Bioprinting is a promising recent technology, which is likely to play an influential role in regenerative medicine. Many technical challenges still need to be overcome including limitations in resolution, cell distribution, vascularization, and innervation. However, this technology is poised to alleviate the treatment limitations of end-stage organ dysfunction and failure. These challenges can be addressed by using more sophisticated printing technologies. Another possibility for addressing these challenges is through the fabrication and characterization of more sophisticated bioinks that deliver the necessary cues for promoting cell survival and the desired differentiation.
Hinton et al[190] developed a novel 3D printing method using the freeform reversible embedding of suspended hydrogels. This novel printing process generates intricate structures that mimic the properties of native tissues found in vivo, including the structures found in bone and brain.
Human cardiac cells prepared from iPSCs are incredibly useful as tools for generating human models of heart disease to acquire an improved understanding of the underlying mechanisms, and for testing different drugs or other treatments[191,192]. They can also be used to help predict which patients might have toxic cardiac side effects from drugs for other diseases. Such an advancement in stem cell-based tissue engineering will enable building of physiologically relevant cardiac tissue for applications in drug discovery and will further provide the opportunity to create personalized in vitro models from cells derived from patients[193]. The use of stem cell therapies and bioprinting in clinical practice will continue to emerge in the upcoming years. The availability of disease specific iPSCs such as those derived from patients having T1DM and T2DM have a huge potential towards fabrication of disease specific human tissue-on-a-chip models that may be used to model disease progression in vitro[194].
The employment of stem cells for the treatment of diabetes is still at its infancy stage in spite of the magnificent strides that have been taken in the field of stem cell biology and research. The research that has been done over the past decade has established that insulin-producing cells can definitely be derived from stem cells. However, the entire potential of stem cells can be harnessed only upon the resolution of associated issues and hurdles that fall in the way. Some of the key issues that limit the further exploration of stem cells in clinical trials include exploration of stem cells in clinical trials includes safety concerns, formations of teratomas, transplantation issues and autoimmune response, and also ethical dilemmas posed by ESCs. Similarly, the problems associated with the scale up production, further hamper the application of adult stem cells and iPSCs, as a choice of therapeutic resources. The need to formulate newer methods for the differentiation and selection of completely functional β-cell is a priority. The regeneration of these cells can be made possible only by controlling the regulation of various factors. The scientific efforts of the past research have made it possible to generate insulin-secreting cells and have laid the foundation for future research to come up with solutions utilizing stem cells as therapeutic agents to alleviate diabetes.
Footnotes
Conflict-of-interest statement: The authors have no conflicts to declare.
Manuscript source: Invited manuscript
Peer-review started: October 19, 2018
First decision: November 15, 2018
Article in press: January 6, 2019
Specialty type: Cell and tissue engineering
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Saeki K, Tanabe S, Valenti MT S- Editor: Wang JL L- Editor: A E- Editor: Tan WW
Contributor Information
Shweta Anil Kumar, Inspired Materials and Stem-Cell Based Tissue Engineering Laboratory, Department of Metallurgical, Materials and Biomedical Engineering, University of Texas at El Paso, 500 W University Avenue, El Paso, TX 79968, United States.
Monica Delgado, Inspired Materials and Stem-Cell Based Tissue Engineering Laboratory, Department of Metallurgical, Materials and Biomedical Engineering, University of Texas at El Paso, 500 W University Avenue, El Paso, TX 79968, United States.
Victor E Mendez, Inspired Materials and Stem-Cell Based Tissue Engineering Laboratory, Department of Metallurgical, Materials and Biomedical Engineering, University of Texas at El Paso, 500 W University Avenue, El Paso, TX 79968, United States.
Binata Joddar, Inspired Materials and Stem-Cell Based Tissue Engineering Laboratory, Department of Metallurgical, Materials and Biomedical Engineering, University of Texas at El Paso, 500 W University Avenue, El Paso, TX 79968, United States; Border Biomedical Research Center, University of Texas at El Paso, 500 W University Avenue, El Paso, TX 79968, United States. bjoddar@utep.edu.
References
- 1.Cibulskis RE, Alonso P, Aponte J, Aregawi M, Barrette A, Bergeron L, Fergus CA, Knox T, Lynch M, Patouillard E, Schwarte S, Stewart S, Williams R. Malaria: Global progress 2000 - 2015 and future challenges. Infect Dis Poverty. 2016;5:61. doi: 10.1186/s40249-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams R, Van Gaal L, Lucioni C. Assessing the impact of complications on the costs of Type II diabetes. Diabetologia. 2002;45:S13–S17. doi: 10.1007/s00125-002-0859-9. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 5.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005;12:580–591. doi: 10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- 7.Owens DR, Zinman B, Bolli GB. Insulins today and beyond. Lancet. 2001;358:739–746. doi: 10.1016/S0140-6736(01)05842-1. [DOI] [PubMed] [Google Scholar]
- 8.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 9.Bottino R, Trucco M, Balamurugan AN, Starzl TE. Pancreas and islet cell transplantation. Best Pract Res Clin Gastroenterol. 2002;16:457–474. doi: 10.1053/bega.2002.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klebe RJ. Cytoscribing: a method for micropositioning cells and the construction of two- and three-dimensional synthetic tissues. Exp Cell Res. 1988;179:362–373. doi: 10.1016/0014-4827(88)90275-3. [DOI] [PubMed] [Google Scholar]
- 11.Heller M, Bauer HK, Goetze E, Gielisch M, Ozbolat IT, Moncal KK, Rizk E, Seitz H, Gelinsky M, Schröder HC, Wang XH, Müller WE, Al-Nawas B. Materials and scaffolds in medical 3D printing and bioprinting in the context of bone regeneration. Int J Comput Dent. 2016;19:301–321. [PubMed] [Google Scholar]
- 12.Ozbolat IT, Peng W, Ozbolat V. Application areas of 3D bioprinting. Drug Discov Today. 2016;21:1257–1271. doi: 10.1016/j.drudis.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Nishat T, De la Vega L, Anil Kumar S, Abelseth L, Alonzo M, Amereh M, Joddar B, Willerth SM. 3D Bioprinting Stem Cell Derived Tissues. Cell Mol Bioeng. 2018;11:219–240. doi: 10.1007/s12195-018-0530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013;60:691–699. doi: 10.1109/TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- 15.Seshiah V, Kalra S, Balaji V, Balaji M. Insulin aspart for the treatment of Type 2 diabetes. Diabetes Management. 2015;5:127. [Google Scholar]
- 16.Bucher P, Mathe Z, Bosco D, Becker C, Kessler L, Greget M, Benhamou PY, Andres A, Oberholzer J, Buhler L, Morel P, Berney T. Morbidity associated with intraportal islet transplantation. Transplant Proc. 2004;36:1119–1120. doi: 10.1016/j.transproceed.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 19.Jang J, Yoo JE, Lee JA, Lee DR, Kim JY, Huh YJ, Kim DS, Park CY, Hwang DY, Kim HS, Kang HC, Kim DW. Disease-specific induced pluripotent stem cells: a platform for human disease modeling and drug discovery. Exp Mol Med. 2012;44:202–213. doi: 10.3858/emm.2012.44.3.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alipio Z, Liao W, Roemer EJ, Waner M, Fink LM, Ward DC, Ma Y. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proc Natl Acad Sci U S A. 2010;107:13426–13431. doi: 10.1073/pnas.1007884107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 22.Teo AK, Windmueller R, Johansson BB, Dirice E, Njolstad PR, Tjora E, Raeder H, Kulkarni RN. Derivation of human induced pluripotent stem cells from patients with maturity onset diabetes of the young. J Biol Chem. 2013;288:5353–5356. doi: 10.1074/jbc.C112.428979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raikwar SP, Kim EM, Sivitz WI, Allamargot C, Thedens DR, Zavazava N. Human iPS cell-derived insulin producing cells form vascularized organoids under the kidney capsules of diabetic mice. PLoS One. 2015;10:e0116582. doi: 10.1371/journal.pone.0116582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanemura H, Go MJ, Shikamura M, Nishishita N, Sakai N, Kamao H, Mandai M, Morinaga C, Takahashi M, Kawamata S. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS One. 2014;9:e85336. doi: 10.1371/journal.pone.0085336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calafiore R, Basta G. Stem cells for the cell and molecular therapy of type 1 diabetes mellitus (T1D): the gap between dream and reality. Am J Stem Cells. 2015;4:22–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Afanasyev BV, Elstner E, Zander AR. A.J. Friedenstein, founder of the mesenchymal stem cell concept. Cell Ther Transplant. 2009;1:35–38. [Google Scholar]
- 27.Gabr MM, Zakaria MM, Refaie AF, Ismail AM, Abou-El-Mahasen MA, Ashamallah SA, Khater SM, El-Halawani SM, Ibrahim RY, Uin GS, Kloc M, Calne RY, Ghoneim MA. Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice. Cell Transplant. 2013;22:133–145. doi: 10.3727/096368912X647162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y, Chen L, Hou XG, Hou WK, Dong JJ, Sun L, Tang KX, Wang B, Song J, Li H, Wang KX. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin Med J (Engl) 2007;120:771–776. [PubMed] [Google Scholar]
- 29.Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837–2844. doi: 10.1634/stemcells.2007-0164. [DOI] [PubMed] [Google Scholar]
- 30.Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, Müller B, Zulewski H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341:1135–1140. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 31.Cao M, Pan Q, Dong H, Yuan X, Li Y, Sun Z, Dong X, Wang H. Adipose-derived mesenchymal stem cells improve glucose homeostasis in high-fat diet-induced obese mice. Stem Cell Res Ther. 2015;6:208. doi: 10.1186/s13287-015-0201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semeraro R, Cardinale V, Carpino G, Gentile R, Napoli C, Venere R, Gatto M, Brunelli R, Gaudio E, Alvaro D. The fetal liver as cell source for the regenerative medicine of liver and pancreas. Ann Transl Med. 2013;1:13. doi: 10.3978/j.issn.2305-5839.2012.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang HS, Shyu JF, Shen WS, Hsu HC, Chi TC, Chen CP, Huang SW, Shyr YM, Tang KT, Chen TH. Transplantation of insulin-producing cells derived from umbilical cord stromal mesenchymal stem cells to treat NOD mice. Cell Transplant. 2011;20:455–466. doi: 10.3727/096368910X522270. [DOI] [PubMed] [Google Scholar]
- 34.Prabakar KR, Domínguez-Bendala J, Molano RD, Pileggi A, Villate S, Ricordi C, Inverardi L. Generation of glucose-responsive, insulin-producing cells from human umbilical cord blood-derived mesenchymal stem cells. Cell Transplant. 2012;21:1321–1339. doi: 10.3727/096368911X612530. [DOI] [PubMed] [Google Scholar]
- 35.Tateishi K, He J, Taranova O, Liang G, D'Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601–31607. doi: 10.1074/jbc.M806597200. [DOI] [PubMed] [Google Scholar]
- 36.Santamaria X, Massasa EE, Feng Y, Wolff E, Taylor HS. Derivation of insulin producing cells from human endometrial stromal stem cells and use in the treatment of murine diabetes. Mol Ther. 2011;19:2065–2071. doi: 10.1038/mt.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadam S, Muthyala S, Nair P, Bhonde R. Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud. 2010;7:168–182. doi: 10.1900/RDS.2010.7.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 39.Wakao S, Kuroda Y, Ogura F, Shigemoto T, Dezawa M. Regenerative Effects of Mesenchymal Stem Cells: Contribution of Muse Cells, a Novel Pluripotent Stem Cell Type that Resides in Mesenchymal Cells. Cells. 2012;1:1045–1060. doi: 10.3390/cells1041045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin Y, Guan J, Zhang C. Mesenchymal stem cells: mechanisms and role in bone regeneration. Postgrad Med J. 2014;90:643–647. doi: 10.1136/postgradmedj-2013-132387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J, Liao W, Gu D, Liang L, Liu M, Du W, Liu P, Zhang L, Lu S, Dong C, Zhou B, Han Z. Neural ganglioside GD2 identifies a subpopulation of mesenchymal stem cells in umbilical cord. Cell Physiol Biochem. 2009;23:415–424. doi: 10.1159/000218188. [DOI] [PubMed] [Google Scholar]
- 43.Bhartiya D. Stem cells to replace or regenerate the diabetic pancreas: Huge potential & existing hurdles. Indian J Med Res. 2016;143:267–274. doi: 10.4103/0971-5916.182615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29:5–10. doi: 10.1002/stem.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Babiker N, Gassoum A, Abdelraheem N, Arbab MA, ALDeaf S, El-Sheikh M, Musa H. The progress of Stem cells in the treatment of diabetes mellitus type 1. Progress in Stem Cell. 2017;4:175–188. [Google Scholar]
- 46.François S, Usunier B, Douay L, Benderitter M, Chapel A. Long-Term Quantitative Biodistribution and Side Effects of Human Mesenchymal Stem Cells (hMSCs) Engraftment in NOD/SCID Mice following Irradiation. Stem Cells Int. 2014;2014:939275. doi: 10.1155/2014/939275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang C, Han P, Oprescu AI, Lee SC, Gyulkhandanyan AV, Chan GN, Wheeler MB, Giacca A. Evidence for a role of superoxide generation in glucose-induced beta-cell dysfunction in vivo. Diabetes. 2007;56:2722–2731. doi: 10.2337/db07-0279. [DOI] [PubMed] [Google Scholar]
- 48.Ende N, Chen R, Reddi AS. Transplantation of human umbilical cord blood cells improves glycemia and glomerular hypertrophy in type 2 diabetic mice. Biochem Biophys Res Commun. 2004;321:168–171. doi: 10.1016/j.bbrc.2004.06.121. [DOI] [PubMed] [Google Scholar]
- 49.Hashemian SJ, Kouhnavard M, Nasli-Esfahani E. Mesenchymal Stem Cells: Rising Concerns over Their Application in Treatment of Type One Diabetes Mellitus. J Diabetes Res. 2015;2015:675103. doi: 10.1155/2015/675103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu LF, Wang NN, Liu YS, Wei X. Differentiation of Wharton's jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue Eng Part A. 2009;15:2865–2873. doi: 10.1089/ten.TEA.2008.0579. [DOI] [PubMed] [Google Scholar]
- 51.He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godfrey KJ, Mathew B, Bulman JC, Shah O, Clement S, Gallicano GI. Stem cell-based treatments for Type 1 diabetes mellitus: bone marrow, embryonic, hepatic, pancreatic and induced pluripotent stem cells. Diabet Med. 2012;29:14–23. doi: 10.1111/j.1464-5491.2011.03433.x. [DOI] [PubMed] [Google Scholar]
- 53.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 56.Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 57.Shim JH, Kim SE, Woo DH, Kim SK, Oh CH, McKay R, Kim JH. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50:1228–1238. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- 58.Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 59.Sapir T, Shternhall K, Meivar-Levy I, Blumenfeld T, Cohen H, Skutelsky E, Eventov-Friedman S, Barshack I, Goldberg I, Pri-Chen S, Ben-Dor L, Polak-Charcon S, Karasik A, Shimon I, Mor E, Ferber S. Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci U S A. 2005;102:7964–7969. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, Peck AB. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci U S A. 2002;99:8078–8083. doi: 10.1073/pnas.122210699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim S, Shin JS, Kim HJ, Fisher RC, Lee MJ, Kim CW. Streptozotocin-induced diabetes can be reversed by hepatic oval cell activation through hepatic transdifferentiation and pancreatic islet regeneration. Lab Invest. 2007;87:702–712. doi: 10.1038/labinvest.3700561. [DOI] [PubMed] [Google Scholar]
- 62.Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, Zern MA, Fleischer N, Efrat S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci U S A. 2003;100:7253–7258. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52:2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- 65.Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 66.Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 67.Sakula A. Paul Langerhans (1847-1888): a centenary tribute. J R Soc Med. 1988;81:414–415. doi: 10.1177/014107688808100718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karamanou M, Protogerou A, Tsoucalas G, Androutsos G, Poulakou-Rebelakou E. Milestones in the history of diabetes mellitus: The main contributors. World J Diabetes. 2016;7:1–7. doi: 10.4239/wjd.v7.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moskalewski S. Isolation and culture of the islets of langerhans of the guinea pig. Gen Comp Endocrinol. 1965;5:342–353. doi: 10.1016/0016-6480(65)90059-6. [DOI] [PubMed] [Google Scholar]
- 70.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2:1279–1283. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 71.Naujok O, Francini F, Picton S, Jörns A, Bailey CJ, Lenzen S. A new experimental protocol for preferential differentiation of mouse embryonic stem cells into insulin-producing cells. Cell Transplant. 2008;17:1231–1242. doi: 10.3727/096368908787236549. [DOI] [PubMed] [Google Scholar]
- 72.Azarpira N, Aghdai MH, Nikeghbalian S, Geramizadeh B, Darai M, Esfandiari E, Bahador A, Kazemi K, Al-Abdullah IH, Malek-Hosseini SA. Human islet cell isolation: the initial step in an islet transplanting program in Shiraz, Southern Iran. Exp Clin Transplant. 2014;12:139–142. doi: 10.6002/ect.2012.0306. [DOI] [PubMed] [Google Scholar]
- 73.Agarwal A, Brayman KL. Update on islet cell transplantation for type 1 diabetes. Semin Intervent Radiol. 2012;29:90–98. doi: 10.1055/s-0032-1312569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, Bretzel RG, Elgue G, Larsson R, Nilsson B, Korsgren O. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907–1914. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 75.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105:125–133. doi: 10.1517/03009734000000059. [DOI] [PubMed] [Google Scholar]
- 76.Sheik Abdulazeez S. Diabetes treatment: A rapid review of the current and future scope of stem cell research. Saudi Pharm J. 2015;23:333–340. doi: 10.1016/j.jsps.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Proneth A, Schnitzbauer AA, Schenker P, Wunsch A, Rauchfuss F, Arbogast H, Manekeller S, Nadalin S, Heise M, Ströhlein MA, Banas B, Schemmer P, Becker T, Bechstein WO, Pascher A, Viebahn R, Geissler EK, Schlitt HJ, Farkas SA. Extended Pancreas Donor Program-The EXPAND Study: A Prospective Multicenter Trial Testing the Use of Pancreas Donors Older Than 50 Years. Transplantation. 2018;102:1330–1337. doi: 10.1097/TP.0000000000002122. [DOI] [PubMed] [Google Scholar]
- 78.Huston C. The impact of emerging technology on nursing care: warp speed ahead. Online J Issues Nurs. 2013;18:1. [PubMed] [Google Scholar]
- 79.Ozbola IT, editor . 3D Bioprinting: fundamentals, principles and applications. London: Academic Press; 2016. [Google Scholar]
- 80.Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip. 2009;9:269–275. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
- 81.Sheng W, Ogunwobi OO, Chen T, Zhang J, George TJ, Liu C, Fan ZH. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip. 2014;14:89–98. doi: 10.1039/c3lc51017d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ozbolat IT, Koc B. 3D hybrid wound devices for spatiotemporally controlled release kinetics. Comput Methods Programs Biomed. 2012;108:922–931. doi: 10.1016/j.cmpb.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 83.Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 84.Ozbolat IT. Scaffold-based or scaffold-free bioprinting: competing or complementing approaches? J Nanotechnol Eng Med. 2015;6:024701. [Google Scholar]
- 85.Kumar SA, Tasnim N, Dominguez E, Allen SC, Suggs L, Ito Y, Joddar B. A Comparative Study of a 3D Bioprinted Gelatin-Based Lattice and Rectangular-Sheet Structures. Gels. 2018;4:73. doi: 10.3390/gels4030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact Mater. 2018;3:144–156. doi: 10.1016/j.bioactmat.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saunders RE, Derby B. Inkjet printing biomaterials for tissue engineering: bioprinting. IMRV. 2014;59:430–448. [Google Scholar]
- 88.Gao G, Yonezawa T, Hubbell K, Dai G, Cui X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol J. 2015;10:1568–1577. doi: 10.1002/biot.201400635. [DOI] [PubMed] [Google Scholar]
- 89.Dai X, Liu L, Ouyang J, Li X, Zhang X, Lan Q, Xu T. Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers. Sci Rep. 2017;7:1457. doi: 10.1038/s41598-017-01581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hopp B, Smausz T, Szabó G, Kolozsvári L, Nogradi A, Kafetzopoulos D, Fotakis C. Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer. Optical Engineering. 2012;51:014302. [Google Scholar]
- 91.Jungst T, Smolan W, Schacht K, Scheibel T, Groll J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem Rev. 2016;116:1496–1539. doi: 10.1021/acs.chemrev.5b00303. [DOI] [PubMed] [Google Scholar]
- 92.Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2:022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 94.Coakley MF, Hurt DE, Weber N, Mtingwa M, Fincher EC, Alekseyev V, Chen DT, Yun A, Gizaw M, Swan J, Yoo TS, Huyen Y. The NIH 3D Print Exchange: A Public Resource for Bioscientific and Biomedical 3D Prints. 3D Print Addit Manuf. 2014;1:137–140. doi: 10.1089/3dp.2014.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dababneh AB, Ozbolat IT. Bioprinting technology: a current state-of-the-art review. J Nanotechnol Eng Med. 2014;136:061016. [Google Scholar]
- 96.Peng W, Unutmaz D, Ozbolat IT. Bioprinting towards Physiologically Relevant Tissue Models for Pharmaceutics. Trends Biotechnol. 2016;34:722–732. doi: 10.1016/j.tibtech.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 97.Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013;16:496–504. [Google Scholar]
- 98.Temple JP, Hutton DL, Hung BP, Huri PY, Cook CA, Kondragunta R, Jia X, Grayson WL. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res A. 2014;102:4317–4325. doi: 10.1002/jbm.a.35107. [DOI] [PubMed] [Google Scholar]
- 99.Marchioli G, van Gurp L, van Krieken PP, Stamatialis D, Engelse M, van Blitterswijk CA, Karperien MB, de Koning E, Alblas J, Moroni L, van Apeldoorn AA. Fabrication of three-dimensional bioplotted hydrogel scaffolds for islets of Langerhans transplantation. Biofabrication. 2015;7:025009. doi: 10.1088/1758-5090/7/2/025009. [DOI] [PubMed] [Google Scholar]
- 100.Itoh M, Nakayama K, Noguchi R, Kamohara K, Furukawa K, Uchihashi K, Toda S, Oyama J, Node K, Morita S. Scaffold-Free Tubular Tissues Created by a Bio-3D Printer Undergo Remodeling and Endothelialization when Implanted in Rat Aortae. PLoS One. 2015;10:e0136681. doi: 10.1371/journal.pone.0136681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ozbolat IT. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015;33:395–400. doi: 10.1016/j.tibtech.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 102.Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med. 2012;1:792–802. doi: 10.5966/sctm.2012-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keriquel V, Guillemot F, Arnault I, Guillotin B, Miraux S, Amédée J, Fricain JC, Catros S. In vivo bioprinting for computer- and robotic-assisted medical intervention: preliminary study in mice. Biofabrication. 2010;2:014101. doi: 10.1088/1758-5082/2/1/014101. [DOI] [PubMed] [Google Scholar]
- 104.Bhise NS, Manoharan V, Massa S, Tamayol A, Ghaderi M, Miscuglio M, Lang Q, Shrike Zhang Y, Shin SR, Calzone G, Annabi N, Shupe TD, Bishop CE, Atala A, Dokmeci MR, Khademhosseini A. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication. 2016;8:014101. doi: 10.1088/1758-5090/8/1/014101. [DOI] [PubMed] [Google Scholar]
- 105.Knowlton S, Onal S, Yu CH, Zhao JJ, Tasoglu S. Bioprinting for cancer research. Trends Biotechnol. 2015;33:504–513. doi: 10.1016/j.tibtech.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 106.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 108.Jammalamadaka U, Tappa K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J Funct Biomater. 2018;9:E22. doi: 10.3390/jfb9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun W, Darling A, Starly B, Nam J. Computer-aided tissue engineering: overview, scope and challenges. Biotechnol Appl Biochem. 2004;39:29–47. doi: 10.1042/BA20030108. [DOI] [PubMed] [Google Scholar]
- 110.Thibault JB, Sauer KD, Bouman CA, Hsieh J. A three-dimensional statistical approach to improved image quality for multislice helical CT. Med Phys. 2007;34:4526–4544. doi: 10.1118/1.2789499. [DOI] [PubMed] [Google Scholar]
- 111.Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63:300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 112.Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chung S, King MW. Design concepts and strategies for tissue engineering scaffolds. Biotechnol Appl Biochem. 2011;58:423–438. doi: 10.1002/bab.60. [DOI] [PubMed] [Google Scholar]
- 114.U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville: U.S. Department of Health and Human Services, Office of the Surgeon General, 2004 [Google Scholar]
- 115.Lee JH, Frias V, Lee KW, Wright RF. Effect of implant size and shape on implant success rates: a literature review. J Prosthet Dent. 2005;94:377–381. doi: 10.1016/j.prosdent.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 116.Brown RS., Jr Live donors in liver transplantation. Gastroenterology. 2008;134:1802–1813. doi: 10.1053/j.gastro.2008.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Faulkner-Jones A, Fyfe C, Cornelissen DJ, Gardner J, King J, Courtney A, Shu W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication. 2015;7:044102. doi: 10.1088/1758-5090/7/4/044102. [DOI] [PubMed] [Google Scholar]
- 118.Ahn SH, Lee HJ, Lee JS, Yoon H, Chun W, Kim GH. A novel cell-printing method and its application to hepatogenic differentiation of human adipose stem cell-embedded mesh structures. Sci Rep. 2015;5:13427. doi: 10.1038/srep13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mannoor MS, Jiang Z, James T, Kong YL, Malatesta KA, Soboyejo WO, Verma N, Gracias DH, McAlpine MC. 3D printed bionic ears. Nano Lett. 2013;13:2634–2639. doi: 10.1021/nl4007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bock T, Pakkenberg B, Buschard K. Increased islet volume but unchanged islet number in ob/ob mice. Diabetes. 2003;52:1716–1722. doi: 10.2337/diabetes.52.7.1716. [DOI] [PubMed] [Google Scholar]
- 121.Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. 2012;20:50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Park JY, Choi JC, Shim JH, Lee JS, Park H, Kim SW, Doh J, Cho DW. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication. 2014;6:035004. doi: 10.1088/1758-5082/6/3/035004. [DOI] [PubMed] [Google Scholar]
- 123.Koblas T, Leontovyc I, Loukotova S, Kosinova L, Saudek F. Reprogramming of Pancreatic Exocrine Cells AR42J Into Insulin-producing Cells Using mRNAs for Pdx1, Ngn3, and MafA Transcription Factors. Mol Ther Nucleic Acids. 2016;5:e320. doi: 10.1038/mtna.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Borowiak M, Melton DA. How to make beta cells? Curr Opin Cell Biol. 2009;21:727–732. doi: 10.1016/j.ceb.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, Barabaschi G, Demarchi D, Dokmeci MR, Yang Y, Khademhosseini A. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14:2202–2211. doi: 10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 127.Michael S, Sorg H, Peck CT, Koch L, Deiwick A, Chichkov B, Vogt PM, Reimers K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS One. 2013;8:e57741. doi: 10.1371/journal.pone.0057741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo SS, Dai G, Karande P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods. 2014;20:473–484. doi: 10.1089/ten.tec.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Castilho M, Moseke C, Ewald A, Gbureck U, Groll J, Pires I, Teßmar J, Vorndran E. Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication. 2014;6:015006. doi: 10.1088/1758-5082/6/1/015006. [DOI] [PubMed] [Google Scholar]
- 130.Gao G, Schilling AF, Yonezawa T, Wang J, Dai G, Cui X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol J. 2014;9:1304–1311. doi: 10.1002/biot.201400305. [DOI] [PubMed] [Google Scholar]
- 131.Gallego-Perez D, Higuita-Castro N, Reen RK, Palacio-Ochoa M, Sharma S, Lee LJ, Lannutti JJ, Hansford DJ, Gooch KJ. Micro/nanoscale technologies for the development of hormone-expressing islet-like cell clusters. Biomed Microdevices. 2012;14:779–789. doi: 10.1007/s10544-012-9657-4. [DOI] [PubMed] [Google Scholar]
- 132.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 133.Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10:3016–3020. doi: 10.3748/wjg.v10.i20.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ferrell N, Gallego-Perez D, Higuita-Castro N, Butler RT, Reen RK, Gooch KJ, Hansford DJ. Vacuum-assisted cell seeding in a microwell cell culture system. Anal Chem. 2010;82:2380–2386. doi: 10.1021/ac902596b. [DOI] [PubMed] [Google Scholar]
- 135.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 136.Lew JK, Pearson JT, Schwenke DO, Katare R. Exercise mediated protection of diabetic heart through modulation of microRNA mediated molecular pathways. Cardiovasc Diabetol. 2017;16:10. doi: 10.1186/s12933-016-0484-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abaci HE, Shuler ML. Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr Biol (Camb) 2015;7:383–391. doi: 10.1039/c4ib00292j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015;14:248–260. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ding S, Schumacher M. Sensor Monitoring of Physical Activity to Improve Glucose Management in Diabetic Patients: A Review. Sensors (Basel) 2016;16:E589. doi: 10.3390/s16040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bruen D, Delaney C, Florea L, Diamond D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors (Basel) 2017;17:E1866. doi: 10.3390/s17081866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bolli R, Hare JM, March KL, Pepine CJ, Willerson JT, Perin EC, Yang PC, Henry TD, Traverse JH, Mitrani RD, Khan A, Hernandez-Schulman I, Taylor DA, DiFede DL, Lima JAC, Chugh A, Loughran J, Vojvodic RW, Sayre SL, Bettencourt J, Cohen M, Moyé L, Ebert RF, Simari RD Cardiovascular Cell Therapy Research Network (CCTRN) Rationale and Design of the CONCERT-HF Trial (Combination of Mesenchymal and c-kit+ Cardiac Stem Cells As Regenerative Therapy for Heart Failure) Circ Res. 2018;122:1703–1715. doi: 10.1161/CIRCRESAHA.118.312978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, Couture L, Vogel KW, Astley CA, Baldessari A, Ogle J, Don CW, Steinberg ZL, Seslar SP, Tuck SA, Tsuchida H, Naumova AV, Dupras SK, Lyu MS, Lee J, Hailey DW, Reinecke H, Pabon L, Fryer BH, MacLellan WR, Thies RS, Murry CE. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. 2018;36:597–605. doi: 10.1038/nbt.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maiullari F, Costantini M, Milan M, Pace V, Chirivì M, Maiullari S, Rainer A, Baci D, Marei HE, Seliktar D, Gargioli C, Bearzi C, Rizzi R. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci Rep. 2018;8:13532. doi: 10.1038/s41598-018-31848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gaetani R, Feyen DA, Verhage V, Slaats R, Messina E, Christman KL, Giacomello A, Doevendans PA, Sluijter JP. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015;61:339–348. doi: 10.1016/j.biomaterials.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 145.Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell'Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A, Khademhosseini A. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang ZZ, Au P, Chen T, Shao Y, Daheron LM, Bai H, Arzigian M, Fukumura D, Jain RK, Scadden DT. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 147.Chan XY, Black R, Dickerman K, Federico J, Lévesque M, Mumm J, Gerecht S. Three-Dimensional Vascular Network Assembly From Diabetic Patient-Derived Induced Pluripotent Stem Cells. Arterioscler Thromb Vasc Biol. 2015;35:2677–2685. doi: 10.1161/ATVBAHA.115.306362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu Y, Hu Y, Liu C, Yao H, Liu B, Mi S. A Novel Strategy for Creating Tissue-Engineered Biomimetic Blood Vessels Using 3D Bioprinting Technology. Materials (Basel) 2018;11:E1581. doi: 10.3390/ma11091581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schöneberg J, De Lorenzi F, Theek B, Blaeser A, Rommel D, Kuehne AJC, Kießling F, Fischer H. Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci Rep. 2018;8:10430. doi: 10.1038/s41598-018-28715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Koç ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–222. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 151.Pak HN, Qayyum M, Kim DT, Hamabe A, Miyauchi Y, Lill MC, Frantzen M, Takizawa K, Chen LS, Fishbein MC, Sharifi BG, Chen PS, Makkar R. Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a Swine model of myocardial infarction. J Cardiovasc Electrophysiol. 2003;14:841–848. doi: 10.1046/j.1540-8167.2003.03124.x. [DOI] [PubMed] [Google Scholar]
- 152.England S, Rajaram A, Schreyer DJ, Chen X. Bioprinted fibrin-factor XIII-hyaluronate hydrogel scaffolds with encapsulated Schwann cells and their in vitro characterization for use in nerve regeneration. Bioprinting. 2017;5:1–9. [Google Scholar]
- 153.Melissinaki V, Gill AA, Ortega I, Vamvakaki M, Ranella A, Haycock JW, Fotakis C, Farsari M, Claeyssens F. Direct laser writing of 3D scaffolds for neural tissue engineering applications. Biofabrication. 2011;3:045005. doi: 10.1088/1758-5082/3/4/045005. [DOI] [PubMed] [Google Scholar]
- 154.Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 155.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 156.Sorkio A, Koch L, Koivusalo L, Deiwick A, Miettinen S, Chichkov B, Skottman H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials. 2018;171:57–71. doi: 10.1016/j.biomaterials.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 157.Wang P, Park JH, Sayed M, Chang TS, Moran A, Chen S, Pyo SH. Sustainable Synthesis and Characterization of Bisphenol A-Free Polycarbonate from Six-Membered Dicyclic Carbonate. Polym Chem. 2018;9:3798–3807. doi: 10.1039/C8PY00676H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2014;16:118–126. doi: 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- 159.Mae SI, Shono A, Shiota F, Yasuno T, Kajiwara M, Gotoda-Nishimura N, Arai S, Sato-Otubo A, Toyoda T, Takahashi K, Nakayama N, Cowan CA, Aoi T, Ogawa S, McMahon AP, Yamanaka S, Osafune K. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun. 2013;4:1367. doi: 10.1038/ncomms2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim YK, Nam SA, Yang CW. Applications of kidney organoids derived from human pluripotent stem cells. Korean J Intern Med. 2018;33:649–659. doi: 10.3904/kjim.2018.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci Rep. 2016;6:34845. doi: 10.1038/srep34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 163.Shi Y, Xing TL, Zhang HB, Yin RX, Yang SM, Wei J, Zhang WJ. Tyrosinase-doped bioink for 3D bioprinting of living skin constructs. Biomed Mater. 2018;13:035008. doi: 10.1088/1748-605X/aaa5b6. [DOI] [PubMed] [Google Scholar]
- 164.Kim BS, Kwon YW, Kong JS, Park GT, Gao G, Han W, Kim MB, Lee H, Kim JH, Cho DW. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53. doi: 10.1016/j.biomaterials.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 165.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 166.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 167.Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016;22:306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Orive G, Emerich D, Khademhosseini A, Matsumoto S, Hernández RM, Pedraz JL, Desai T, Calafiore R, de Vos P. Engineering a Clinically Translatable Bioartificial Pancreas to Treat Type I Diabetes. Trends Biotechnol. 2018;36:445–456. doi: 10.1016/j.tibtech.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 169.Karzyński K, Kosowska K, Ambrożkiewicz F, Berman A, Cichoń J, Klak M, Serwańska-Świętek M, Wszoła M. Use of 3D bioprinting in biomedical engineering for clinical application. Medical Studies. 2018;34:93–97. [Google Scholar]
- 170.Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 171.Niimi Y, Levison SW. Pediatric brain repair from endogenous neural stem cells of the subventricular zone. Pediatr Res. 2018;83:385–396. doi: 10.1038/pr.2017.261. [DOI] [PubMed] [Google Scholar]
- 172.Gu Q, Tomaskovic-Crook E, Wallace GG, Crook JM. Engineering Human Neural Tissue by 3D Bioprinting. Methods Mol Biol. 2018;1758:129–138. doi: 10.1007/978-1-4939-7741-3_10. [DOI] [PubMed] [Google Scholar]
- 173.Zhang S, Wang H. Current Progress in 3D Bioprinting of Tissue Analogs. SLAS Technol. 2018:2472630318799971. doi: 10.1177/2472630318799971. [DOI] [PubMed] [Google Scholar]
- 174.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, Crum CP, Xian W, McKeon F. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Warburton D, Perin L, Defilippo R, Bellusci S, Shi W, Driscoll B. Stem/progenitor cells in lung development, injury repair, and regeneration. Proc Am Thorac Soc. 2008;5:703–706. doi: 10.1513/pats.200801-012AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nerger BA, Nelson CM. 3D culture models for studying branching morphogenesis in the mammary gland and mammalian lung. Biomaterials. 2018:In press. doi: 10.1016/j.biomaterials.2018.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.De Santis MM, Bölükbas DA, Lindstedt S, Wagner DE. How to build a lung: latest advances and emerging themes in lung bioengineering. Eur Respir J. 2018;52:1601355. doi: 10.1183/13993003.01355-2016. [DOI] [PubMed] [Google Scholar]
- 179.Despeyroux A, Duret C, Gondeau C, Perez-Gracia E, Chuttoo L, de Boussac H, Briolotti P, Bony C, Noël D, Jorgensen C, Larrey D, Daujat-Chavanieu M, Herrero A. Mesenchymal stem cells seeded on a human amniotic membrane improve liver regeneration and mouse survival after extended hepatectomy. J Tissue Eng Regen Med. 2018;12:1062–1073. doi: 10.1002/term.2607. [DOI] [PubMed] [Google Scholar]
- 180.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 181.Knowlton S, Tasoglu S. A Bioprinted Liver-on-a-Chip for Drug Screening Applications. Trends Biotechnol. 2016;34:681–682. doi: 10.1016/j.tibtech.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 182.Teramura Y, Iwata H. Bioartificial pancreas microencapsulation and conformal coating of islet of Langerhans. Adv Drug Deliv Rev. 2010;62:827–840. doi: 10.1016/j.addr.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 183.Akkouch A, Yu Y, Ozbolat IT. Microfabrication of scaffold-free tissue strands for three-dimensional tissue engineering. Biofabrication. 2015;7:031002. doi: 10.1088/1758-5090/7/3/031002. [DOI] [PubMed] [Google Scholar]
- 184.Greggio C, De Franceschi F, Figueiredo-Larsen M, Gobaa S, Ranga A, Semb H, Lutolf M, Grapin-Botton A. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development. 2013;140:4452–4462. doi: 10.1242/dev.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hiscox AM, Stone AL, Limesand S, Hoying JB, Williams SK. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng Part A. 2008;14:433–440. doi: 10.1089/tea.2007.0099. [DOI] [PubMed] [Google Scholar]
- 186.Bloch K, Papismedov E, Yavriyants K, Vorobeychik M, Beer S, Vardi P. Photosynthetic oxygen generator for bioartificial pancreas. Tissue Eng. 2006;12:337–344. doi: 10.1089/ten.2006.12.337. [DOI] [PubMed] [Google Scholar]
- 187.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation. 2016;133:2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 189.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 190.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue HJ, Ramadan MH, Hudson AR, Feinberg AW. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1:e1500758. doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Davis RP, van den Berg CW, Casini S, Braam SR, Mummery CL. Pluripotent stem cell models of cardiac disease and their implication for drug discovery and development. Trends Mol Med. 2011;17:475–484. doi: 10.1016/j.molmed.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 192.Izpisúa Belmonte JC, Ellis J, Hochedlinger K, Yamanaka S. Induced pluripotent stem cells and reprogramming: seeing the science through the hype. Nat Rev Genet. 2009;10:878–883. doi: 10.1038/nrg2700. [DOI] [PubMed] [Google Scholar]
- 193.Zhang YS, Aleman J, Arneri A, Bersini S, Piraino F, Shin SR, Dokmeci MR, Khademhosseini A. From cardiac tissue engineering to heart-on-a-chip: beating challenges. Biomed Mater. 2015;10:034006. doi: 10.1088/1748-6041/10/3/034006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stepniewski J, Kachamakova-Trojanowska N, Ogrocki D, Szopa M, Matlok M, Beilharz M, Dyduch G, Malecki MT, Jozkowicz A, Dulak J. Induced pluripotent stem cells as a model for diabetes investigation. Sci Rep. 2015;5:8597. doi: 10.1038/srep08597. [DOI] [PMC free article] [PubMed] [Google Scholar]