Abstract
Background
Computerized multi-model training has been widely studied for its effect on delaying cognitive decline. In this study, we designed the first Chinese-version computer-based multi-model cognitive training for mild cognitive impairment (MCI) patients. Neuropsychological effects and neural activity changes assessed by functional MRI were both evaluated.
Method
MCI patients in the training group were asked to take training 3–4 times per week for 6 months. Neuropsychological and resting-state fMRI assessment were performed at baseline and at 6 months. Patients in both groups were continuously followed up for another 12 months and assessed by neuropsychological tests again.
Results
78 patients in the training group and 63 patients in the control group accomplished 6-month follow-up. Training group improved 0.23 standard deviation (SD) of mini-mental state examination, while control group had 0.5 SD decline. Addenbrooke's cognitive examination-revised scores in attention (p = 0.002) and memory (p = 0.006), as well as stroop color-word test interference index (p = 0.038) and complex figure test-copy score (p = 0.035) were also in favor of the training effect. Difference between the changes of two groups after training was not statistically significant. The fMRI showed increased regional activity at bilateral temporal poles, insular cortices and hippocampus. However, difference between the changes of two groups after another 12 months was not statistically significant.
Conclusions
Multi-model cognitive training help MCI patients to gained cognition benefit, especially in memory, attention and executive function. Functional neuroimaging provided consistent neural activation evidence. Nevertheless, after one-year follow up after last training, training effects were not significant. The study provided new evidence of beneficial effect of multi-model cognitive training.
Keywords: Mild cognitive impairment, Cognitive therapy, Neuroimaging, Neuropsychology
Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; ChEIs, cholinesterase inhibitors; MMSE, mini mental state examination; ACER, Addenbrooke's cognitive examination-revised; STT, shape trail test; CFT, complex figure test; SDS, symbol digit substitution test; SCWT, Stroop Color-Word Test; BOLD, blood oxygen level dependent; MRI, magnetic resonance imaging; fALFF, fractional amplitude of low frequency fluctuation; Reho, regional homogeneity; ROI, region of interest; SD, standard deviation
Highlights
-
•
The cognitive training for mild cognitive impairment had positive effect on memory, attention and executive function.
-
•
The beneficial effect was observed immediately after six-month training and confined to trained cognitive domains.
-
•
In the 12 months after training, training and control group had no difference in cognitive decline.
-
•
Functional neuroimaging showed increased regional activity at bilateral temporal poles, insular cortices and hippocampus.
1. Introduction
Alzheimer's disease (AD) develops from preclinical state, mild cognitive impairment (MCI) to dementia. The prevalence of MCI ranges from 3% to 20% in adults over 65 years old (Gauthier et al., 2006; Jia et al., 2014; Ravaglia et al., 2008). However, approximate 50% of MCI patients (roughly 12% per year) will progress to AD in the following 4–5 years. Dementia due to AD has caused a high medical and economic burden on both community and families. If any interventions could delay disease onset or slow its progression by 1 year, there would be much fewer cases in 2050 with reduction by nearly 9.2 million, and thus less expenditure on care and treatment (Brookmeyer et al., 2007).
Non-pharmacological interventions have shown advantages in delaying cognitive ability declining (Li et al., 2017). There are various approaches, including cognitive training, physical exercise, and diet modification (Olazaran et al., 2010; Buschert et al., 2010). One famous large-scale, randomized trial (the Advanced Cognitive Training for Independent and Vital Elderly study, ACTIVE) in 2001 (Jobe et al., 2001) had three models of cognitive training: speed of processing, memory, and reasoning. Each intervention modified the corresponding ability, and the effect existed for 2 years. Donostia longitudinal study designed a structured training program covering the cognitive functions of attention, orientation, memory, language, visuo-constructive ability, executive functions, visuo-manual coordination, and praxis. The group that received structured intervention got higher scores in nearly all cognitive tests (Buiza et al., 2008).
Fewer cognitive interventions were specially designed for MCI patients. Memory training helped better memory performance in the following six months after training (Rapp et al., 2002). Rozzini et al. treated a group of MCI patients with cognitive training and cholinesterase inhibitors (ChEIs), and these patients showed greater improvement in memory, abstract reasoning, and behavioral disturbances than the only ChEIs group (Rozzini et al., 2007). Computerized cognitive training has been considered as a convenient and flexible method to protect cognition (Barban et al., 2016). A meta-analysis suggested its efficacy on global cognition, select cognitive domains, and psychosocial functioning for MCI patients (Hill et al., 2017).
Regarding the potential neural mechanism, it is suggested that cognitive training would modulate neural plasticity by measuring regional oxygen consumption or neural synchronization (Li et al., 2016; Hotting et al., 2013; Chapman et al., 2015). These results were based on healthy elders, while neural mechanism for MCI needs more evaluation.
In this study, we designed the first Chinese-version computerized cognitive training for MCI patients. The training program comprised eight separate tasks: visual working memory, 30-second memory, episodic memory, speed of calculation, visual search, alertness, mental rotation, and images re-arrangement task. Both neuropsychological and neuroimaging assessments were performed to explore the training effect and related neural activities.
2. Material and methods
2.1. Study design and patients
The study was a randomized controlled pilot trial in Rui Jin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China. All patients were screened by the Mini Mental State Examination (MMSE, Chinese Version) (Katzman et al., 1988), Zung Self-rating Anxiety Scale and Zung Self-rating Depression scale firstly. According to ATN criteria for MCI due to AD, our diagnosis was based on a detailed medical history, neurological examinations, global score of clinical dementia rating scale (global score = 0.5) (Morris, 1997) and medial temporal lobe atrophy (Alberta et al., 2011). Exclusion criteria included evidence of stroke, Parkinson's disease, human immunodeficiency virus infection, and mood problems, as well as treatment with benzodiazepines, antipsychotic or antiepileptic medications. Patients with poor vision or hearing were also excluded.
We recruited MCI patients from January 2016 to December 2016, and the last MCI patient was enrolled on December 20, 2016. A total of 160 MCI patients were finally enrolled in Shanghai. They were randomized to the training or control group. All patients were of unrelated Chinese Han descent with >6 years of education. In order to minimize Hawthorne effect (McCarney et al., 2007), both groups were told the study purpose as observation, follow-up and early diagnosis. Only the training group was given the information about computer-based cognitive training.
Neuropsychological assessment was done at baseline, 6-month, and 18-month. Baseline demographics included gender, age, education level, occupation, concomitant diseases, and medications. A validated neuropsychological battery for multiple cognitive domains was performed by trained researchers (Y. Qiao and YZ. Lu) who were blind to grouping. It included the Chinese version of Addenbrooke's cognitive examination-revised (ACER) (Fang et al., 2014), the auditory verbal learning test (AVLT)-Huashan version (Zhao et al., 2012), the shape trail test (STT, including Part A and B) (Zhao et al., 2013), the Rey-Osterrieth complex figure test (CFT) (Zhao et al., 2015), symbol digit substitution test (SDS), and the Stroop Color-Word Test (SCWT).
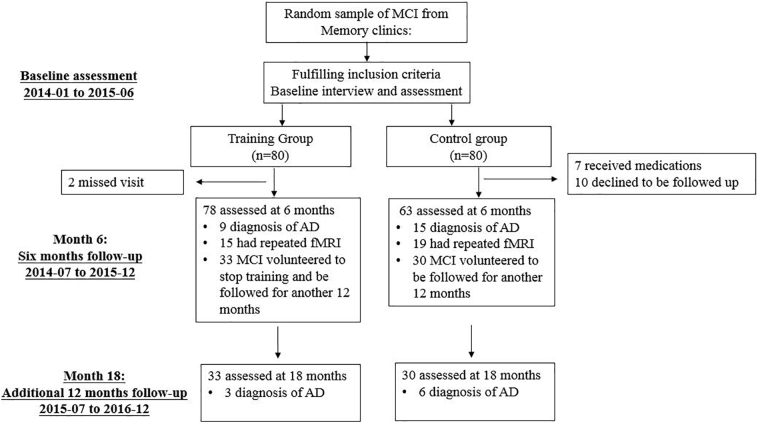
After six-month training, some patients in the training group stopped training and tended to be followed up continuously (Fig. 1). During the follow-up period, medications for dementia (cholinesterase inhibitors or memantine) were not prescribed.
Fig. 1.
Trial profile. AD, Alzheimer's disease; MCI, mild cognitive impairment.
Our study received ethical approval from the Research Ethics Committee, Rui Jin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China. Informed consent was obtained from all patients. The study was registered with Chinese Trial Registry.gov (Register number: ChiCTR-IPR-16008383).
2.2. MRI acquisition and processing
At baseline and 6-month, repeated magnetic resonance imaging (MRI) was performed for neuroimaging evaluation. High-resolution T1-weighted fast spoiled gradient echo (FSPGR) sequence was acquired on a 3.0 Tesla MR system (GE Signa HDxt; GE Healthcare, Milwaukee, WI) equipped with an eight-channel phased array head coil at the department of radiology (sagittal slice orientation; matrix = 256 × 256; repetition time = 5.5 ms; echo time = 1.7 ms; inversion time = 450 ms; flip angle = 12°; voxel size = 1 × 1 × 1 mm3). Resting-state blood oxygen level dependent (BOLD) MRI using echo planar imaging (EPI) weighted sequence (210 functional images, sagittal slice orientation; 35/37/39 slices; slice thickness = 4.0 mm; matrix = 64 × 64; repetition time = 2000 ms; echo time = 30 ms; flip angle = 90°; voxel size = 3.75 × 3.75 × 4 mm3) was also performed at baseline and 6-months if patients consented.
Preprocessing procedures of BOLD included slice timing, realignment, co-registration, normalization by T1 unified segmentation using a modified MATLAB toolbox “Data Processing & Analysis of Brain Imaging (version 3.0)” (Yan et al., 2016). After exclusion cases due to head motion (translation or rotation head motion parameters larger than 2 mm or 2°), 34 patients (15 in the training group and 19 in the control group) were qualified for further analysis.
We calculated mean fractional amplitude of low frequency fluctuation (fALFF) from BOLD signals to represent individuals' regional neural activities (Zou et al., 2008). Voxel based morphometry of gray matter was also calculated as covariate volume.
2.3. Training
Cognitive training was carried out at patients' home online. Training program is comprised of the following eight tasks.
Visual working memory task: In one session, patients were asked to memorize several (2–8 cards, started from 2) playing cards (targets) within some time (time (second) =2 × the number of targets). Then they did a simple calculation, such as “4 + 6=” or “5–3=”. They would be presented with another group of playing cards (probes) until they calculated correctly. Then they needed to judge whether any card (target) seen before was among the probes. The number of probes is twice as many as targets. The difficulty level was self-adaptive. The number of targets doubled (two successive correct responses), or remained (one incorrect response) or decreased (two or more successive incorrect responses) in the next session.
30-second memory task: It is similar to visual working memory task, except for the calculation process. Patients enjoyed 30-second music and scenery pictures instead of calculating. Self-adaptation was identical to visual working memory task.
Episodic memory task: Pictures (1–4 pictures) depicting an event (e.g. Miss Wang is doing physical exercise in the morning in the park) were presented for a few seconds. Then patients enjoyed 30-second music and scenery pictures as an interruption. After music, patients were asked questions about the event (e.g. What is Miss Wang doing in the morning at the park: doing exercise? fishing? sleeping?). Similarly, the number of pictures doubled (two successive correct responses) or remained (one incorrect response) or decreased (two or more successive incorrect responses) in the next session.
Speed of calculation task: Flowers with arithmetic questions (e.g. 4 + 6 =? 5–3 =?, 6 × 5 =? 6÷3 =?) were moving downwards from the top of the screen (one flower with one question on it). Patients were asked to answer these questions with keyboard as soon as possible. If the answer was correct, the flower (question) disappeared. If no correct answer was given before the flower moved to the bottom of screen, the flower stayed on the bottom. The task was over when the bottom line of screen was fully occupied by the flowers. The speed of flowers gradually increased during the task.
Visual search task: Patients performed a classic visual search task by searching a letter “T” among “L”s. They were asked to choose “target on” or “no target” as soon as possible (Horowitz and Wolfe, 1998).
Alertness task: In one trial, a red box was presented in the random location as an alert for a while. After it disappeared, a golden coin was presented in the identical location. The time between box disappearance and presentation of the coin randomly varied. Patients were asked to keep alert when box presented and press the “Enter” key as quickly as they saw the coin.
Mental rotation task: Two “R”s (identical or mirrored “R”) of different angle were presented. Patients were asked to judge whether the two letters were the same or mirrored as soon as possible.
Image re-arrangement task: The procedure of several daily activities (e.g. shopping, washing) was separated into six steps depicted by six pictures. All pictures were randomly sequenced. Patients were asked to re-arrange the pictures into a reasonable sequence based on their knowledge.
Patients in the training group were asked to perform all these tasks 3–4 times (about 120–160 min training in total) per week for 6 months. Their login information and training performance on the web server were checked weekly, and telephone interview was used to make sure all participants in the training group got equally amounts of training.
2.4. Statistical analyses
We used per-protocol sets in order to strictly estimate the immediate and late effect of training. If patients were indicated cholinesterase inhibitors or memantine, or they could not insist on training, their data would be ruled out from analysis.
The differences of age, years of education, neuropsychological score, gender, hypertension, and diabetes between two groups at baseline were compared by the independent t-test or chi-square test.
We used mixed-effect regression model with maximum likelihood estimation to compare changes of cognitive scores between two groups. The interaction effect between time and group represented the training effect. In the training group, linear regression was used to estimate the relationship between the training duration and the outcomes.
We defined standardized change scores as changes between endpoint and baseline scores divided by standard deviation (SD) for all patients combined. For comparison with other trials, we calculated effect size by subtracting mean standardized change scores in the control group from those in the training group (Barnes et al., 2009). Significance of <5% was used in all analyses, and SPSS 22 program (IBM Corporation, Armonk, NY, USA) was used for statistical data analysis.
For the fMRI data analysis, a mixed model was also used to estimate interaction between time and group. Standardized gray matter volumes of the whole brain were used as covariates.
Regarding to multiple comparison correction, we used permutation test (Winkler et al., 2016) with threshold-free cluster enhancement (TFCE) to reach the best balance between family-wise error rate (under 5%) and test-retest reliability (Chen et al., 2018). In this way, the distribution of the maximum was used as reference, and the rank of a given voxel in relation to that distribution was used to obtain p values. Significant voxels in the cerebral would be marked by BrainNet Viewer (Xia et al., 2013).
3. Results
3.1. Clinical and cognitive results
A total of 141 MCI patients finished the 6-month follow-up. In the training group, 78 patients completed the assessments at 6-month. Among them, 3 patients (3.84%) converted to AD and 33 MCI patients were continuously observed for another 12 months. In the control group, 63 patients finished. Among them, 4 patients progressed to AD (6.35%) and 30 MCI patients were followed up for another 12 months. Dropout rate was higher in the control group (2.5% in the training group and 21.25% in the control group). The main reason (10 patients in 17) for dropout in the control group was that they contacted with other physicians for medications.
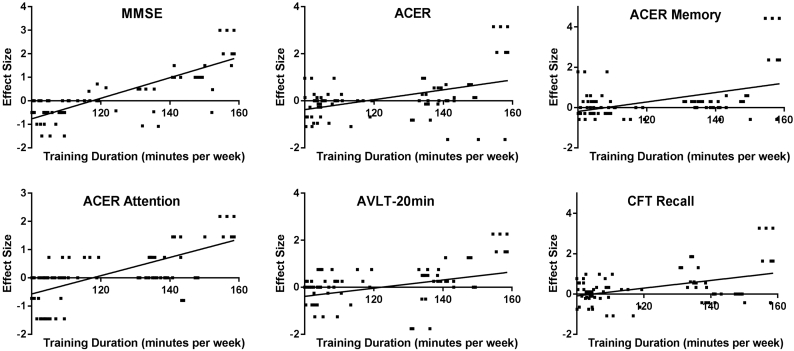
The mean age of the population was 70.4 at baseline (SD 7.1), and two groups were similar to each other (p = .1). Both groups were also of similar level of education (p = .5), as well as hypertension and diabetes. Global cognition assessed by MMSE was equivalent between groups. Training group showed advantages in fluency, language and visuospatial ability assessed by ACER, recall in CFT and symbol digit substitution test (p < .05, Table 1). In the training group, the averaged training duration was 122.8 min every week, ranged from 100.0 to 158.6 min. The relationship between training duration and the effect size was found significant in the following tests: MMSE, ACER, ACER attention, ACER memory, CFT recall and AVLT 20-min recall (Fig. 2).
Table 1.
Baseline characteristics of training and control groups.
| Baseline | Training (Mean ± SD) | Control (Mean ± SD) | P-value |
|---|---|---|---|
| Number (n) | 78 | 63 | – |
| Sex (% female)a | 33/84 | 42/63 | 0.006⁎ |
| Ageb | 69.5 ± 7.3 | 71.5 ± 6.8 | 0.100 |
| Years of educationb | 13.8 ± 2.5 | 13.5 ± 2.5 | 0.500 |
| Diabetesa | 21/72 | 18/60 | >0.99 |
| Hypertensiona | 33/72 | 24/60 | 0.597 |
| MMSEb | 28.0 ± 1.7 | 28.0 ± 1.7 | 0.921 |
| ACE-Rb | 87.3 ± 5.7 | 89.3 ± 6.8 | 0.063 |
| Attention | 17.1 ± 1.1 | 17.2 ± 1.0 | 0.833 |
| Memory | 22.3 ± 4.5 | 23.0 ± 2.6 | 0.283 |
| Fluency | 9.9 ± 2.1 | 8.9 ± 2.0 | 0.008⁎ |
| Language | 24.5 ± 1.7 | 23.2 ± 1.9 | 0.001⁎ |
| Visuospatial ability | 15.5 ± 0.9 | 14.9 ± 1.2 | 0.004⁎ |
| Complex figure testb | |||
| Copy | 34.1 ± 4.6 | 34.3 ± 2.6 | 0.643 |
| Recall | 17.0 ± 9.2 | 12.5 ± 8.3 | 0.003⁎ |
| AVLTb | |||
| Immediate recall | 20.3 ± 5.0 | 16.9 ± 6.6 | 0.001⁎ |
| 5-min recall | 6.1 ± 3.6 | 4.9 ± 3.4 | 0.053 |
| 20-min recall | 6.5 ± 4.2 | 4.1 ± 3.9 | 0.001⁎ |
| 20-min recognition | 20.9 ± 3.2 | 19.5 ± 5.3 | 0.061 |
| SCWTb | |||
| Word | 25.1 ± 4.0 | 25.1 ± 4.0 | 0.359 |
| Color | 38.9 ± 8.5 | 38.1 ± 7.5 | 0.562 |
| Interference | 77.2 ± 21.8 | 84.7 ± 29.3 | 0.081 |
| Index | 3.1 ± 0.7 | 3.1 ± 1.2 | 0.393 |
| Shape trail testb | |||
| STT-A | 67.0 ± 22.2 | 73.5 ± 18.7 | 0.067 |
| STT-B | 151.3 ± 74.5 | 168.6 ± 56.5 | 0.119 |
| SDSb | 40.1 ± 10.9 | 36.3 ± 11.0 | 0.038⁎ |
MMSE, mini mental state examination; ACER, Addenbrooke's cognitive examination-revised; AVLT, auditory verbal learning test; STT, shape trail test; SDS, symbol digit substitution test; SD, standard deviation; SCWT, stroop color-word test.
Chi-square test.
Independent t-test.
p < .05.
Fig. 2.
The significant relationship between training duration and the effect size in the following tests: MMSE, ACER, ACER attention, ACER memory, CFT recall and AVLT 20-min recall (p < .05). MMSE, Mini mental state examination; ACER, Addenbrooke's cognitive examination-revised; AVLT, auditory verbal learning test; CFT, complex figure test.
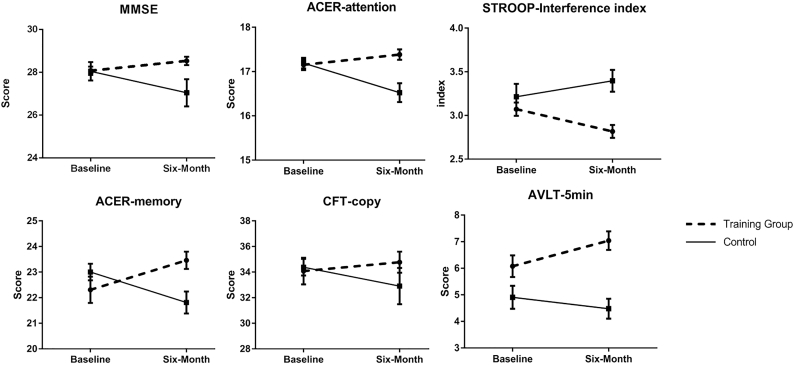
After six months follow-up, significant beneficial effect of the training was found for the primary outcome. The mean difference between groups in change of MMSE in the half year was 0.73 SD (group×time interaction effect, p = .002). MMSE improved 0.23 SD in the training group compared to −0.5 SD in the control group (Fig. 3, Table 2).
Fig. 3.
Significant changes in cognitive performance during the 6 months training. Figure showed statistically significant changes in cognitive performance between baseline and 6 months (higher scores suggest better performance, except Stroop interference index) in the training and control group. Error bars were standard errors. The significance is based on mixed-model repeated-measures analyses of between-group differences (group × time interaction) in changes from baseline to 6 months. MMSE, Mini mental state examination; ACER, Addenbrooke's cognitive examination-revised; AVLT, auditory verbal learning test; CFT, complex figure test.
Table 2.
Mean standardized change in training and control groupsa.
| Mean changes (95% CI) |
|||
|---|---|---|---|
| Training group (N = 78) | Control group (N = 63) | Difference (Effect size) | |
| MMSE | 0.23 (0.01 to 0.44) | −0.50 (−0.72 to −0.27) | 0.73 (0.42 to 1.04)⁎ |
| ACER | 0.11 (−0.11 to 0.32) | −0.20 (−0.46 to 0.06) | 0.31 (−0.02 to 0.64) |
| Attention | 0.17 (−0.03 to 0.37) | −0.48 (−0.74 to −0.23) | 0.67 (0.33 to 0.97)⁎ |
| Memory | 0.34 (0.11 to −0.58) | −0.35 (−0.55 to −0.15) | 0.69 (0.38 to 1.00)⁎ |
| Fluency | −0.20 (−0.41 to 0.02) | 0.15 (−0.11 to 0.41) | −0.35 (−0.68 to −0.02)⁎⁎ |
| Language | 0.01 (−0.16 to 0.18) | −0.05 (−0.37 to 0.26) | 0.06 (−0.27 to 0.40) |
| Visuospatial ability | −0.05 (−0.27 to 0.17) | 0.13 (−0.13 to 0.39) | −0.18 (−0.49 to 0.18) |
| Complex figure test | |||
| Copy | 0.12 (−0.11 to 0.35) | −0.25 (−0.49 to −0.01) | 0.37 (0.04 to 0.70)⁎ |
| Recall | 0.34 (0.14 to 0.55) | 0.19 (−0.08 to 0.47) | 0.15 (−0.19 to 0.48) |
| AVLT | |||
| Immediate recall | 0.07 (−0.15 to 0.29) | 0.23 (−0.03 to 0.48) | −0.16 (−0.49 to 0.18) |
| 5-min recall | 0.28 (0.07 to 0.48) | −0.12 (−0.39 to 0.15) | 0.40 (0.07 to 0.73)⁎⁎ |
| 20-min recall | 0.01 (−0.22 to 0.24) | 0.11 (−0.14 to 0.36) | −0.10 (−0.43 to 0.24) |
| Recognition | 0.02 (−0.12 to 0.17) | −0.23 (−0.56 to 0.10) | 0.25 (−0.08 to 0.59) |
| Stroop test | |||
| Word | 0.25 (0.01 to 0.49) | 0.11 (−0.11 to 0.34) | 0.14 (−0.20 to 0.47) |
| Color | −0.15 (−0.36 to 0.05) | 0.09 (−0.18 to 0.36) | −0.25 (0.58 to 0.09) |
| Interference | −0.09 (−0.33 to 0.15) | 0.17 (−0.05 to 0.40) | −0.26 (−0.60 to 0.07) |
| Index | −0.26 (−0.44 to −0.08) | 0.19 (−0.11 to 0.48) | −0.45 (−0.78 to −0.12)⁎ |
| STT | |||
| STT-A | −0.15 (−0.36 to 0.06) | −0.22 (−0.49 to 0.05) | 0.07 (−0.27 to 0.41) |
| STT-B | −0.16 (−0.39 to 0.07) | −0.01 (−0.25 to 0.23) | −0.15 (−0.48 to 0.18) |
| SDS | 0.07 (−0.16 to 0.30) | 0.13 (−0.11 to 0.38) | −0.06 (−0.40 to 0.27) |
MMSE, Mini mental state examination; ACER, Addenbrooke's cognitive examination-revised; AVLT, auditory verbal learning test; STT, shape trail test; SDS, symbol digit substitution test; CI, Confidence interval; SCWT, stroop color-word test.
Mean changes are changes between endpoint and baseline scores divided by standard deviation for all subjects combined. Difference is training - control; positive values favor training group, negative values favor control group.
p < .05.
p < .1.
We also found significant training effects of ACER attention (p = .002) and memory (p = .006), as well as SCWT interference index (p = .038) and CFT copy (p = .035). Effect size of verbal memory (5-minute recall in AVLT) was marginally significant in favor of the training group (p = .080, range from 0.07 to 0.73 SD) (Table 2). However, effect size of ACER fluency tended to favor the control group (p = .073, ranged from −0.68 to −0.02 SD). Other differences of changes in secondary outcomes between the groups were not statistically significant.
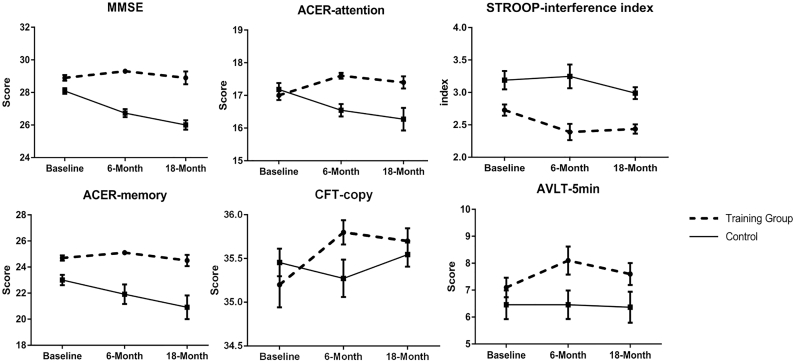
Thirty-three patients in the training group and 30 patients in the control group were observed for another 12 months. In this sub-population, we firstly found similar improvement in the training group (33 patients) at 6-month: MMSE (p < .001), attention in ACER (p < .001), CFT copy (p = .05), SDS (p = .027), SCWT-color (p = .013) and STT-B (p = .020). However, in the following 12 months, all differences between the changes in two groups were not statistically significant (Fig. 4).
Fig. 4.
Changes in cognitive performance during the 18 months follow-up. Figure illustrates changes in cognitive performance at baseline, 6 months and 18 months (higher scores suggest better performance, except stroop interference index) in the training and control group. Training group stopped training during 6 and 18 months. Error bars were standard errors. MMSE, Mini mental state examination; ACER, Addenbrooke's cognitive examination-revised; AVLT, auditory verbal learning test; CFT, complex figure test.
3.2. fMRI results
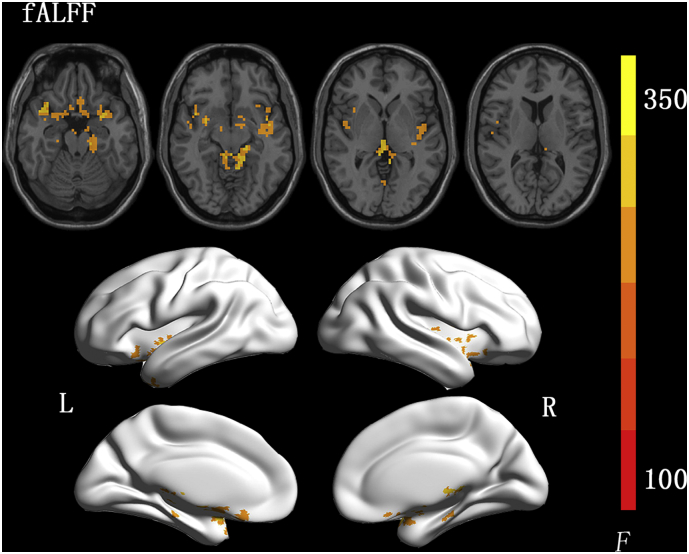
The interaction effect (group×time) in mixed model analysis was depicted in the Fig. 5. It represented the difference between changes of two groups at two time points (Difference = (training group at 6 months-training group at baseline)−(control group at 6 months-control group at baseline)). In fALFF results, clusters of positive difference were detected at bilateral temporal poles, insular lobes and left parahippocampal gyrus (Fig. 5, corrected voxel level: p < .05). Clusters found in bilateral temporal and insular lobes merged, with total size of 643 voxels. No significantly negative difference was detected.
Fig. 5.
Voxel-wise results of mixed-model analyses of interaction differences (group × time interaction) in fALFF changes from baseline to 6 months. Difference = (training group at 6 months - training group at baseline) - (control group at 6 months - control group at baseline). Results are shown in neurologic convention. Corrected by permutation test with threshold-free cluster enhancement (TFCE), with voxel level: p < .05. fALFF, amplitude of low frequency fluctuation.
4. Discussion
In the trial, we estimated the effectiveness of the Chinese-version computer-based cognitive training in MCI patients. Regarding global cognitive function, we observed an effect size of 0.73 SD in MMSE and 0.31 SD in ACER in favor of the training group. MMSE decline in the control group was about 1 point per 6-month. The Chinese-version MMSE has been validated in China for >20 years and its norm and longitudinal changes in MCI patients are relatively more clear than other cognitive tests. The only difference between the Chinses-version MMSE and the original one is the phrase which should be repeated (“No ifs, ands or buts”). It was replaced by a Chinese sentence. According to MMSE studies in China, MMSE decreased by 0.6 to 2 per year in MCI patients (Gao et al., 2011; Xiao et al., 2006), which was consistent with our data in the control group. Though effect size of ACER was not statistically significant, it is similar in magnitude to other cognitive training for normal aging (Lampit et al., 2014), MCI (Barnes et al., 2009), and cholinesterase inhibitors (ChEIs) for treatment of Alzheimer's disease (Birks, 2006).
As for secondary outcomes, we observed effect sizes for attention, memory, visuospatial configuration and executive function tests in ACER consistently favored the training group. Attention test included orientation (time and place), immediate memory, and successive calculation. Memory test in ACER had a relatively short interval before recall, which was similar to 5-minute recall in AVLT. It is suggested that our training tended to benefit short-term memory. In contrast, effect size of verbal fluency test tended to favor the control condition. Since the training program did not include verbal training, the result suggested the hypothesis that training program may have domain-specific effects. The transfer effect observed in other studies was confined in related abilities, such as visual working memory and spatial working memory (Li et al., 2008), speed of processing and driving mobility (Ross et al., 2016). No transfer effect was observed between language and other cognitive domains. Regarding to improvement of verbal fluency test of the control group, more studies are needed to determine whether the effect was due to random variation. In the subsample of patients who tended to be followed up for another 12 months, similar effects on global cognition, attention, executive function, and visuo-spatial tests were found immediately after the end of training. Nevertheless, after one-year follow up without training, effects were not significant. The results were partially consistent with other training studies. In a memory training study, training effect (working memory ability) and transfer effect were found improved immediately after training. Nevertheless, after one-year follow up, both effects were not significant (Buschkuehl et al., 2008). Spatial working memory task also has effects transferred to more demanding spatial tasks, with benefits lasting only for 3 months (Li et al., 2008).
In the resting-state fMRI, fractional ALFF (fALFF) approach, the ratio of the power spectrum of low-frequency (0.01–0.08 Hz) to that of the entire frequency range, is suggested better sensitivity and specificity in detecting spontaneous brain activities (Zou et al., 2008). MCI patients showed decreased fALFF mainly in the medial prefrontal cortex, posterior cingulate cortex/precuneus and hippocampus/parahippocampal gyrus, while there was increased activity in some temporal regions (Wang et al., 2016; Liang et al., 2014; Han et al., 2011).
In a cognitive interventional study for healthy older adults, ALFF in the middle frontal gyrus, superior frontal gyrus, and anterior cerebellum lobe was enhanced, corresponding to improved memory and social support (Yin et al., 2014). Working memory training for young adults increased fALFF in the right dorsal lateral prefrontal cortex, frontopolar area and medial prefrontal cortex (Takeuchi et al., 2017). These results suggested training-induced spontaneous brain activities during the resting state. In our study, clusters in the bilateral temporal lobes and insular cortices were enhanced measured by fALFF, as well as clusters in the bilateral hippocampus.
We only observed positive interaction effect, indicating positive correlation between training and regional neural activity. The fMRI results were partly consistent with neuropsychological outcomes immediately after training. We observed that attention, short-term memory, visuospatial configuration, and executive function gained from the training. Temporal poles were found as “semantic hub” of the brain, and its neural codes were related with idiosyncratic patterns of memory errors, as well as variation in true-memory performance (Chadwick et al., 2016). Ventral regions of the temporal pole and para-hippocampal gyri were also related to visual semantic representations (Peelen and Caramazza, 2012). Besides, the integrity including bilateral hippocampus and para-hippocampal gyri could enhance emotion in memory processes (Kumfor et al., 2014).
The insular cortex has connections with the anterior cingulate cortex, temporal pole, prefrontal cortex, frontal and parietal opercula, visual association cortex, hippocampus, and entorhinal cortex (Nagai et al., 2007). Bilateral insular cortices and nearby angular gyri were involved in various cognitive and mood processes, and its network with hippocampus was related with attention (Loitfelder et al., 2012) and visual cortex responses (Petro et al., 2017). Functional neuroimaging studies have found insula hyper-activations for tasks including those involving perception, intentional action, and consciousness (Gasquoine, 2014). It also demonstrated causal influences on all other frontal-cingulate-parietal regions, serving as a major causal control hub during multisensory attention (Chen et al., 2015). The neural activation in insular cortex may help to explain the benefit for visual and semantic memory related tasks. Since insular cortex did not suffer injury in MCI or early AD, it may also work as compensatory neural activities after training.
Our study has limitation in the follow-up. MCI patients in the training group may stop training if they suffer from more severe cognitive decline, and patients in the control group will also drop out and turn to other medical help when their symptoms get severe. The attrition bias would obscure the interventional effect if per-protocol sets were strictly adopted. In further studies, an intention-to-treat analysis would help minimize the bias. In addition, further studies should determine the target population precisely and try to incorporate the reason of dropout into final effect.
Beyond traditional cognitive stimulation, combined assessment and training application by visual reality could be tried due to its more convenience and high classification rate for MCI patients (Zygouris et al., 2017; D et al., 2015). In addition, multidomain intervention, as well as physiological stimulation such as transcranial magnetic stimulation, remains to be well investigated in clinical settings.
5. Conclusion
In summary, we designed the Chinese computer-based cognitive training and found that it was effective for delaying cognitive decline in elders with MCI. The effect was only observed immediately after training and confined to trained domains. Functional MRI suggested corresponding increased local neural activities at bilateral temporal poles, insular cortices and hippocampus.
Ethics approval and consent to participate
The study received ethical approval from the Research Ethics Committee, Rui Jin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China. It was also registered with Chinese Trial Registry.gov (Register number: ChiCTR-IPR-16008383).
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
BY Li and NY He analyzed and interpreted the patient data regarding the cognitive training and wrote the manuscript. Y Quan, PJ Cui and YZ Lu performed the neuropsychological examination of all the patients. NY He, HW Ling and FH Yan performed MRI and its analysis. HD tang and SD Chen designed the trial, interpret the final results and modified the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from Clinical Research Center, Shanghai Jiao Tong University School of Medicine [DLY201603]; the National Natural Science Foundation of China [81501086, 81400888, 91332107]; National Key R&D Program of China [2016YFC1305804]; and Innovation Program of Shanghai Municipal Education Commission [2017-01-07-00-01-E00046].
Acknowledgements
We acknowledged all patients and their caregivers participating in the trial.
Contributor Information
Fu-Hua Yan, Email: yfh11655@rjh.com.cn.
Hui-Dong Tang, Email: funground@163.com.
Sheng-Di Chen, Email: chensd@rjh.com.cn.
References
- Alberta Marilyn S., DeKosky Steven T., Dickson Dennis. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barban F. Protecting cognition from aging and Alzheimer's disease: a computerized cognitive training combined with reminiscence therapy. Int. J. Geriatr. Psychiatry. 2016;31(4):340–348. doi: 10.1002/gps.4328. [DOI] [PubMed] [Google Scholar]
- Barnes D.E. Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Dis. Assoc. Disord. 2009;23(3):205–210. doi: 10.1097/WAD.0b013e31819c6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst. Rev. 2006;(1) doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Buiza C. A randomized, two-year study of the efficacy of cognitive intervention on elderly people: the Donostia Longitudinal Study. Int. J. Geriatr. Psychiatry. 2008;23(1):85–94. doi: 10.1002/gps.1846. [DOI] [PubMed] [Google Scholar]
- Buschert V., Bokde A.L., Hampel H. Cognitive intervention in Alzheimer disease. Nat. Rev. Neurol. 2010;6(9):508–517. doi: 10.1038/nrneurol.2010.113. [DOI] [PubMed] [Google Scholar]
- Buschkuehl M. Impact of working memory training on memory performance in old-old adults. Psychol. Aging. 2008;23(4):743–753. doi: 10.1037/a0014342. [DOI] [PubMed] [Google Scholar]
- Chadwick M.J. Semantic representations in the temporal pole predict false memories. Proc. Natl. Acad. Sci. U. S. A. 2016;113(36):10180–10185. doi: 10.1073/pnas.1610686113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S.B. Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cereb. Cortex. 2015;25(2):396–405. doi: 10.1093/cercor/bht234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. Role of the anterior insular cortex in integrative causal signaling during multisensory auditory-visual attention. Eur. J. Neurosci. 2015;41(2):264–274. doi: 10.1111/ejn.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Lu B., Yan C.G. Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Hum. Brain Mapp. 2018;39(1):300–318. doi: 10.1002/hbm.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D G. Can a virtual reality cognitive training application fulfill a dual role? Using the virtual supermarket cognitive training application as a screening tool for mild cognitive impairment.%A Zygouris S. J. Alzheimers Dis. 2015;44(4):1333–1347. doi: 10.3233/JAD-141260. [DOI] [PubMed] [Google Scholar]
- Fang R. Validation of the Chinese version of Addenbrooke's cognitive examination-revised for screening mild Alzheimer's disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2014;37(3–4):223–231. doi: 10.1159/000353541. [DOI] [PubMed] [Google Scholar]
- Gao Z.B. A follow-up study on cognitive changes of mild cognitive impairment in the elderly. Zhonghua Yi Xue Za Zhi. 2011;91(1):37–39. [PubMed] [Google Scholar]
- Gasquoine P.G. Contributions of the insula to cognition and emotion. Neuropsychol. Rev. 2014;24(2):77–87. doi: 10.1007/s11065-014-9246-9. [DOI] [PubMed] [Google Scholar]
- Gauthier S. Mild cognitive impairment. Lancet. 2006;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Han Y. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI study. NeuroImage. 2011;55(1):287–295. doi: 10.1016/j.neuroimage.2010.11.059. [DOI] [PubMed] [Google Scholar]
- Hill N.T. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am. J. Psychiatry. 2017;174(4):329–340. doi: 10.1176/appi.ajp.2016.16030360. [DOI] [PubMed] [Google Scholar]
- Horowitz T.S., Wolfe J.M. Visual search has no memory. Nature. 1998;394(6693):575–577. doi: 10.1038/29068. [DOI] [PubMed] [Google Scholar]
- Hotting K. Effects of a cognitive training on spatial learning and associated functional brain activations. BMC Neurosci. 2013;14:73. doi: 10.1186/1471-2202-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J. The prevalence of mild cognitive impairment and its etiological subtypes in elderly Chinese. Alzheimers Dement. 2014;10(4):439–447. doi: 10.1016/j.jalz.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Jobe J.B. ACTIVE: a cognitive intervention trial to promote independence in older adults. Control. Clin. Trials. 2001;22(4):453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R. A Chinese version of the mini-mental state examination; impact of illiteracy in a Shanghai dementia survey. J. Clin. Epidemiol. 1988;41(10):971–978. doi: 10.1016/0895-4356(88)90034-0. [DOI] [PubMed] [Google Scholar]
- Kumfor F. Frontal and temporal lobe contributions to emotional enhancement of memory in behavioral-variant frontotemporal dementia and Alzheimer's disease. Front. Behav. Neurosci. 2014;8:225. doi: 10.3389/fnbeh.2014.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampit A., Hallock H., Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med. 2014;11(11) doi: 10.1371/journal.pmed.1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.C. Working memory plasticity in old age: practice gain, transfer, and maintenance. Psychol. Aging. 2008;23(4):731–742. doi: 10.1037/a0014343. [DOI] [PubMed] [Google Scholar]
- Li T. Cognitive training can reduce the rate of cognitive aging: a neuroimaging cohort study. BMC Geriatr. 2016;16:12. doi: 10.1186/s12877-016-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.Y. The role of cognitive activity in cognition protection: from Bedside to Bench. Transl. Neurodegener. 2017;6:7. doi: 10.1186/s40035-017-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P. Altered amplitude of low-frequency fluctuations in early and late mild cognitive impairment and Alzheimer's disease. Curr. Alzheimer Res. 2014;11(4):389–398. doi: 10.2174/1567205011666140331225335. [DOI] [PubMed] [Google Scholar]
- Loitfelder M. Abnormalities of resting state functional connectivity are related to sustained attention deficits in MS. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0042862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarney R. The Hawthorne effect: a randomised, controlled trial. BMC Med. Res. Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.C. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int. Psychogeriatr. 1997;9(Suppl. 1):173–176. doi: 10.1017/s1041610297004870. (discussion 177-8) [DOI] [PubMed] [Google Scholar]
- Nagai M., Kishi K., Kato S. Insular cortex and neuropsychiatric disorders: a review of recent literature. Eur. Psychiatry. 2007;22(6):387–394. doi: 10.1016/j.eurpsy.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Olazaran J. Nonpharmacological therapies in Alzheimer's disease: a systematic review of efficacy. Dement. Geriatr. Cogn. Disord. 2010;30(2):161–178. doi: 10.1159/000316119. [DOI] [PubMed] [Google Scholar]
- Peelen M.V., Caramazza A. Conceptual object representations in human anterior temporal cortex. J. Neurosci. 2012;32(45):15728–15736. doi: 10.1523/JNEUROSCI.1953-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro N.M. Multimodal imaging evidence for a frontoparietal modulation of visual cortex during the selective processing of conditioned threat. J. Cogn. Neurosci. 2017;29(6):953–967. doi: 10.1162/jocn_a_01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp S., Brenes G., Marsh A.P. Memory enhancement training for older adults with mild cognitive impairment: a preliminary study. Aging Ment. Health. 2002;6(1):5–11. doi: 10.1080/13607860120101077. [DOI] [PubMed] [Google Scholar]
- Ravaglia G. Mild cognitive impairment: epidemiology and dementia risk in an elderly Italian population. J. Am. Geriatr. Soc. 2008;56(1):51–58. doi: 10.1111/j.1532-5415.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- Ross L.A. The transfer of cognitive speed of processing training to older adults' driving mobility across 5 years. J. Gerontol. B Psychol. Sci. Soc. Sci. 2016;71(1):87–97. doi: 10.1093/geronb/gbv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzini L. Efficacy of cognitive rehabilitation in patients with mild cognitive impairment treated with cholinesterase inhibitors. Int. J. Geriatr. Psychiatry. 2007;22(4):356–360. doi: 10.1002/gps.1681. [DOI] [PubMed] [Google Scholar]
- Takeuchi H. Neural plasticity in amplitude of low frequency fluctuation, cortical hub construction, regional homogeneity resulting from working memory training. Sci. Rep. 2017;7(1):1470. doi: 10.1038/s41598-017-01460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.J. Amplitude of low-frequency fluctuation (ALFF) and fractional ALFF in migraine patients: a resting-state functional MRI study. Clin. Radiol. 2016;71(6):558–564. doi: 10.1016/j.crad.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Winkler A.M. Faster permutation inference in brain imaging. NeuroImage. 2016;141:502–516. doi: 10.1016/j.neuroimage.2016.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S.F. Outcome and cognitive changes of mild cognitive impairment in the elderly: a follow-up study of 47 cases. Zhonghua Yi Xue Za Zhi. 2006;86(21):1441–1446. [PubMed] [Google Scholar]
- Yan C.G. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14(3):339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Yin S. Intervention-induced enhancement in intrinsic brain activity in healthy older adults. Sci. Rep. 2014;4:7309. doi: 10.1038/srep07309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q. Short-term delayed recall of auditory verbal learning test is equivalent to long-term delayed recall for identifying amnestic mild cognitive impairment. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q. The Shape Trail Test: application of a new variant of the Trail making test. PLoS One. 2013;8(2):e57333. doi: 10.1371/journal.pone.0057333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q. Auditory verbal learning test is superior to REY-OSTERRIETH complex figure memory for predicting mild cognitive impairment to Alzheimer's disease. Curr. Alzheimer Res. 2015;12(6):520–526. doi: 10.2174/1567205012666150530202729. [DOI] [PubMed] [Google Scholar]
- Zou Q.H. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods. 2008;172(1):137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygouris S. A preliminary study on the feasibility of using a virtual reality cognitive training application for remote detection of mild cognitive impairment. J. Alzheimers Dis. 2017;56(2):619–627. doi: 10.3233/JAD-160518. [DOI] [PubMed] [Google Scholar]