Abstract
Recurrence of disease due to chemotherapy drug resistance remains a major obstacle to a more successful survival outcome of multiple myeloma (MM). Overcoming drug resistance and salvaging patients with relapsed and/or refractory (R/R) MM is an urgent and unmet medical need. Several new personalized treatment strategies have been developed against molecular targets to overcome this drug resistance. There are several targeted therapeutics with anti-MM activity in clinical pipeline, including inhibitors of anti-apoptotic proteins, monoclonal antibodies, antibody-drug conjugates, bispecific antibodies, fusion proteins, and various cell therapy platforms. For example, B-cell maturation antigen (BCMA)-specific CAR-T cell platforms showed promising activity in heavily pretreated R/R MM patients. Therefore, there is renewed hope for high-risk as well as R/R MM patients in the era of personalized medicine.
Keywords: Personalized medicine, Multiple myeloma, Stem cell transplantation, Immuno-oncology, Immunotherapy, Biotherapy
Highlights
-
•
The therapeutic landscape for MM is rapidly evolving in the era of personalized medicine
-
•
New treatment strategies have markedly improved the survival of MM patients and caused a paradigm shift in therapy
1. Introduction
Multiple myeloma (MM) represents the second most common hematologic malignancy after non-Hodgkin lymphoma with ~30,000 new cases per year [1]. MM is a molecularly, biologically and clinically heterogeneous group of diseases with some high risk cytogenetic subsets having a very poor prognosis [[2], [3], [4]]. The survival of MM patients has significantly improved due in part to the application of autologous hematopoietic stem cell transplantation (ASCT) and the introduction of immunomodulatory drugs (IMIDs) lenalidomide and pomalidomide as well as the proteasome inhibitors (PI) bortezomib and carfilzomib as initial induction treatment and as maintenance therapy [[5], [6], [7]]. These improved outcomes have caused a paradigm shift in treatment of MM, from a palliative approach toward more active management, including the use of sequential therapies, with the goal of prolonging progression-free survival (PFS) and OS as well as further therapy at relapse. There are several other new promising drugs/biologics with anti-MM activity in clinical pipeline, including tyrosine kinase inhibitors, inhibitors of anti-apoptotic proteins, monoclonal antibodies (MoAb), antibody-drug conjugates (ADC), bispecific antibodies (bsAbs), fusion proteins, and various cell therapy platforms, including BCMA-directed CAR-T cells [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]].
2. Role of autologous stem cell transplantation (ASCT) in high-risk and R/R MM
Autologous stem cell transplantation (ASCT) offers the best chance of achieving a minimal residual disease (MRD)-negative deep complete response (CR), which is associated with better survival outcomes of newly diagnosed patients with high-risk MM, as defined by staging, chromosomal abnormalities, disease biology, and gene expression [9] and can also be performed upon first relapse [5,[20], [21], [22], [23], [24], [25]]. The role of ASCT may evolve as new strategies are developed including biotherapeutic agents such as bispecific T-cell engagers (BiTEs) and CAR-T cells, which are showing promising results as salvage treatment in patients with R/R MM [26]. Table S1 lists ongoing Phase 2 and Phase 3 clinical trials evaluating multi-modality treatment strategies for high-risk or R/R MM in the context of ASCT.
3. Therapeutic landscape of high risk and relapsed/refractory multiple myeloma in the era of personalized medicine
Intrinsic as well as acquired drug resistance of cancer cells to standard drugs is a major obstacle for a more successful survival outcome of MM patients being treated on contemporary clinical protocols [[5], [6], [7], [8], [9],19,26,27]. Almost all patients with MM who survive initial treatment will eventually relapse and require further therapy. Treatment in R/R MM is often complicated by increased frailty and additional co-morbidities, more aggressive disease refractory to commonly used modalities and unresolved toxicity from previous treatments. The main treatment options for R/R MM patients include ASCT, PIs (bortezomib, carfilzomib, ixazomib), IMiDs (e.g., lenalidomide, pomalidomide, MoAb (e.g. daratumumab [DARA], elotuzumab), alkylating chemotherapy drugs, anthracyclines, panobinostat, and corticosteroids, administered alone, or more commonly as part of two- or three-drug combinations [19,[26], [27], [28], [29], [30], [31], [32], [33], [34], [35]]. The combination of daratumumab DARA, lenalidomide, and dexamethasone (Dex) has been proposed as the most effective regimen in the population of R/R MM patients. According to the clinicaltrials.gov data repository, interventional trials in R/R MM patients are playing an increasingly important role in the clinical development path of oncology drugs that are being developed to overcome chemotherapy drug resistance in MM (Fig. S1).
3.1. Immunomodulatory drugs (IMiDs)
IMiDs are thalidomide analogues with pleiotropic anti-MM activities including immune modulation (enhanced T-cell mediated and NK mediated immunity), pro-apoptotic activity as well as anti-angiogenic, anti-inflammatory (e.g. blocking of the proinflammatory cytokines TNF and IL6) and anti-proliferative effects. Lenalidomide (Revlimid; Celgene) has remarkable activity in patients with newly diagnosed MM and R/R MM and it has contributed to significantly improved survival of MM patients. Pomalidomide (Pomalyst; Celgene) is a 2nd generation IMiD that showed excellent activity alone as well as when combined with low dose Dex in R/R MM patients and appears to have stronger immune modulatory effects compared to lenalidomide [36]. FDA also approved the use of Pom in combination with DARA (Darzalex) and Dex for the treatment of patients with MM who have received at least two prior therapies including lenalidomide (Revlimid) and a PI based on an overall response rate (ORR) of 59% with a median time to response of only one month and a median duration of response of 13.6 months [36].
3.2. Proteasome inhibitors (PIs)
PIs act through multiple mechanisms to suppress tumor survival pathways and to arrest tumor growth, tumor spread and angiogenesis. Two prospective, Phase 2 trials and two randomized Phase 3 trials have evaluated the efficacy of the PI bortezomib in the treatment of patients with R/R MM. ORRs for single agent bortezomib are approximately 30%. Bortezomib has also been evaluated in combination therapy with ORRs of approximately 65%. Carfilzomib is a second generation selective PI that has demonstrated excellent activity in patients with R/R MM [37]. Carfilzomib is FDA-approved for R/R MM patients who have received at least two prior therapies, including bortezomib and an IMiD. Carfilzomib is also approved for use in combination with lenalidomide and Dex (KRd) for the treatment of patients with relapsed MM who have received one to three prior lines of therapy. Ixazomib (Ninlaro) is the first oral PI that has demonstrated activity in patients with MM when given with Dex or in combination with lenalidomide and Dex (IRd) [31]. Ixazomib in combination with lenalidomide and Dex (IRd) is FDA-approved for the treatment of R/R MM patients who have received at least one prior therapy [31]. In a randomized Phase 2 study in newly diagnosed MM patients not eligible for ASCT, the combination of Ixazomib plus thalidomide and Dex for induction followed by maintenance therapy with Ixazomib was highly effective with a high ORR of 81% [31].
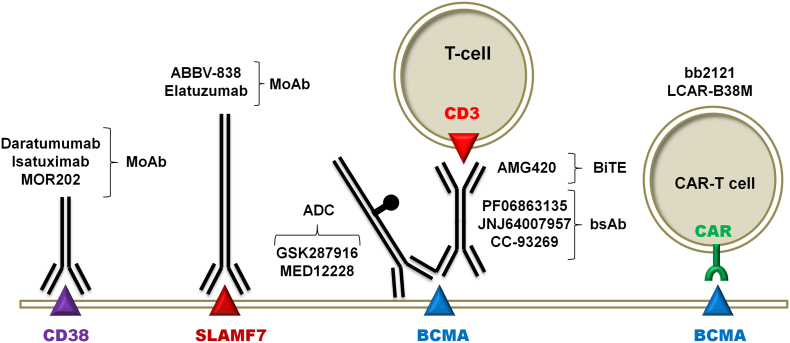
3.3. Biotherapy with monoclonal antibodies (MoAb), antibody-drug conjugates (ADCs), bispecific antibodies (bsAb), bispecific T-cell engagers (BiTE), Chimeric Antigen Receptor (CAR) T-cells (Fig. 1; Fig. S2)
Fig. 1.
Effective Biotherapy Targets on the Surface of Multiple Myeloma Cells. Abbreviations: MoAb: monoclonal antibody; BiTE: bispecific T-cell engager; ADC: antibody-drug conjugate; bsAb: bispecific antibody; CAR-T: chimeric antigen receptor carrying T-cell. There are 2 ADCs targeting BCMA in clinical development, namely GSK2857916 and MEDI2228. GSK2857916 is a humanized anti-BCMA MoAb conjugated to the cytotoxic agent monomethyl auristatin-F, via the non-cleavable linker maleimidocaproyl. GSK2857916 monotherapy has demonstrated a 60% ORR and a median PFS of 7.9 months in a group of hard to treat and heavily pretreated R/R MM [18]. It has recently received Breakthrough Therapy designation from FDA and also received PRIME designation from the European Medicines Agency (EMA). MEDI2228, a fully human MoAb that is conjugated to pyrrolobenzodiazepine dimer via a protease-cleavable linker is being evaluated in the Phase 1 study NCT03489525 for the treatment of MM. BiTEs are composed of two single-chain variable fragments (scFvs) connected by a flexible linker. One scFv fragment binds to a T cell-specific antigen (typically CD3), whereas the other scFv fragment binds to a tumor-specific antigen. This bispecificity allows BiTEs to juxtapose T-cells and tumor cells physically and promotes the formation of immunological synapses by the simultaneous binding of multiple BiTEs, leading to T-cell activation, cytokine production and cytotoxicity of the tumor cells. BI 836909 (AMG 420) is a BiTE targeting BCMA and CD3ɛ. BsAbs are a class of engineered antibody and antibody-like proteins that, in contrast to ‘regular’ monospecific antibodies, combine two or more different specific antigen binding elements in a single construct. Since bsAbs do not typically occur in nature, they are constructed either chemically or biologically, using techniques such as cell fusion or recombinant DNA technologies. There are 3 clinical stage anti-CD3xBCMA bsAbs, namely Johnson and Johnson's JNJ64007957 (NCT03145181), Pfizer's PF-06863135 (NCT03269136), and Celgene's CC-93269/EM901 (NCT03486067) that have entered Phase 1 testing. See the text for a detailed discussion of the CAR-T cell platforms.
The ideal antigen for effective biotherapy of MM would be protein receptors highly expressed on tumor cell membrane during all stages of MM development. In MM, BCMA, CS1, CD19, CD33, CD38, CD138, and CD56 among others are being evaluated as target antigens for biotherapy.
3.3.1. Targeting CD38 with MoAb
CD38-targeting MoAb such as DARA have single agent activity in heavily pretreated MM patients [[38], [39], [40]] (Fig. 1). DARA is a first-in-class human anti-CD38 MoAb. DARA mediates complement-dependent cytotoxicity (CDC), induces antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and tumor cell apoptosis [[38], [39], [40]]. In the randomized, open-label, multicenter Phase 3 ALCYONE trial (NCT02195479), DARA (Darzalex) in combination with bortezomib (Velcade), melphalan, and prednisone (DVMP) reduced the risk of disease progression or death by 50%, compared to treatment with VMP alone, in patients with newly diagnosed MM who were not eligible for ASCT [39], and FDA approved DARA for this indication.
DARA received initial approval as monotherapy in patients with heavily pretreated R/R MM who were refractory to PIs and IMiDs. In 2 randomized Phase 3 trials of R/R MM patients, addition of DARA to standard of care regimens improved outcomes regardless of cytogenetic risk status (NCT02136134 and NCT02076009). DARA in combination with lenalidomide and Dex (DaraRD regimen) and DARA in combination with bortezomib and Dex (DaraVD regimen) [40] are approved by the US FDA for use in patients with MM who have received at least one prior therapy. In a prospective trial of DARA, Pom, and Dex in multiply relapsed MM (NCT01998971), the ORR was 60% and the median PFS was 8.8 months [36]. FDA has approved this regimen for patients with MM who have received at least two prior therapies including lenalidomide and a PI. In the ongoing Phase 2 and Phase 3 clinical studies, DARA is being evaluated in novel combinations with other treatment modalities, including PI's, IMiDs and Dex (Table 1). Notably, a recent observational study indicated that the immunomodulatory effects of DARA may contribute to a high objective response rate of previously IMiD-refractory or PI-refractory MM patients who are treated with DARA monotherapy to retreatment with IMiD/PI-based salvage regimens [29]. Likewise, other anti-CD38 MoAb, including Isatuximab (ISA) [41] and MOR202 [42], have shown promising activity in clinical trials of R/R MM patients.
Table 1.
Randomized Phase 3 Clinical Trials Evaluating Anti-CD38 Monoclonal Antibodies in High-Risk or R/R Multiple Myeloma.
| Study NCT# | Reference URL | Study title | Phase of trial |
|---|---|---|---|
| NCT02541383 | https://ClinicalTrials.gov/show/NCT02541383 | Study of Daratumumab in Combination With Bortezomib (VELCADE), Thalidomide, and Dexamethasone (VTD) in the First Line Treatment of Transplant Eligible Subjects With Newly Diagnosed Multiple Myeloma | 3 |
| NCT02136134 | https://ClinicalTrials.gov/show/NCT02136134 | Phase 3 Study Comparing Daratumumab, Bortezomib and Dexamethasone (DVd) vs Bortezomib and Dexamethasone (Vd) in Subjects With Relapsed or Refractory Multiple Myeloma | 3 |
| NCT02076009 | https://ClinicalTrials.gov/show/NCT02076009 | Phase 3 Study Comparing Daratumumab, Lenalidomide, and Dexamethasone (DRd) vs Lenalidomide and Dexamethasone (Rd) in Subjects With Relapsed or Refractory Multiple Myeloma | 3 |
| NCT03234972 | https://ClinicalTrials.gov/show/NCT03234972 | A Randomized, Multicenter, Open-label, Phase 3 Study to Compare Daratumumab, Bortezomib, and Dexamethasone (DVd) vs Bortezomib and Dexamethasone (Vd) in Chinese Subjects With Relapsed or Refractory Multiple Myeloma | 3 |
| NCT03180736 | https://ClinicalTrials.gov/show/NCT03180736 | A Phase 3 Study Comparing Pomalidomide and Dexamethasone With or Without Daratumumab in Subjects With Relapsed or Refractory Multiple Myeloma Who Have Received at Least One Prior Line of Therapy With Both Lenalidomide and a Proteasome Inhibitor. | 3 |
| NCT03158688 | https://ClinicalTrials.gov/show/NCT03158688 | A Randomized, Open-label, Phase 3 Study Comparing Carfilzomib, Dexamethasone, and Daratumumab to Carfilzomib and Dexamethasone for the Treatment of Patients With Relapsed or Refractory Multiple Myeloma CANDOR Study of Carfilzomib and Daratumumab for Relapsed Myeloma | 3 |
| NCT03277105 | https://ClinicalTrials.gov/show/NCT03277105 | A Phase 3 Randomized, Multicenter Study of Subcutaneous vs. Intravenous Administration of Daratumumab in Subjects With Relapsed or Refractory Multiple Myeloma | 3 |
| NCT02195479 | https://ClinicalTrials.gov/show/NCT02195479 | A Phase 3, Randomized, Controlled, Open-label Study of VELCADE (Bortezomib) Melphalan-Prednisone (VMP) Compared to Daratumumab in Combination With VMP (D-VMP), in Subjects With Previously Untreated Multiple Myeloma Who Are Ineligible for High-dose Therapy | 3 |
| NCT03275285 | https://ClinicalTrials.gov/show/NCT03275285 | Randomized, Open Label, Multicenter Study Assessing The Clinical Benefit Of Isatuximab Combined With Carfilzomib (Kyprolis®) And Dexamethasone Versus Carfilzomib With Dexamethasone In Patients With Relapsed And/Or Refractory Multiple Myeloma Previously Treated With 1 to 3 Prior Lines | 3 |
| NCT02990338 | https://ClinicalTrials.gov/show/NCT02990338 | A Phase 3 Randomized, Open-label, Multicenter Study Comparing Isatuximab (SAR650984) in Combination With Pomalidomide and Low-Dose Dexamethasone Versus Pomalidomide and Low-Dose Dexamethasone in Patients With Refractory or Relapsed and Refractory Multiple Myeloma | 3 |
3.3.2. Targeting SLAMF7 with MoAb
Elotuzumab (Empliciti) is a first-in-class humanized MoAb targeting SLAMF7 [18,43,44] (Fig. 1). The mechanisms of the antitumor effects of elotuzumab include disrupting MM cell adhesion to bone marrow stromal cells, enhancing NK cell cytotoxicity, and mediating ADCC but not CDC. Elotuzumab is FDA-approved for use in combination with lenalidomide and Dex for the treatment of patients with MM who have received one to three prior therapies. In an open-label, multicenter, Phase 3 trial (ELOQUENT-2, NCT01239797), 646 patients with MM relapsed after one to three prior lines of therapy were randomly assigned to receive standard dose oral lenalidomide plus Dex (Ld) with or without elotuzumab [44]. After a median follow-up of 24 months, Ld plus elotuzumab (ELd) resulted in higher ORR (79 versus 66%) and better PFS when compared with Ld alone. Elotuzumab in combination with Pom-Dex is being examined for relapsed MM in an ongoing Phase 2 randomized trial (NCT02654132). Elotuzumab is being studied in combination with lenalidomide as maintenance after high-dose therapy as well (NCT02420860) (Table 2).
Table 2.
Phase 2 and Phase 3 Clinical Trials Evaluating Elotuzumab in High-Risk or R/R Multiple Myeloma.
| Study NCT# | Reference URL | Study title | Phase of Trial |
|---|---|---|---|
| NCT02718833 | https://ClinicalTrials.gov/show/NCT02718833 | A Phase 2 Study of Elotuzumab in Combination With Pomalidomide, Bortezomib, and Dexamethasone in Relapsed and Refractory Multiple Myeloma | 2 |
| NCT03155100 | https://ClinicalTrials.gov/show/NCT03155100 | Phase 2 Study of Carfilzomib + Elotuzumab + Dexamethasone for Relapsed or Progressed Multiple Myeloma After 1–3 Prior Treatment Lines | 2 |
| NCT03030261 | https://ClinicalTrials.gov/show/NCT03030261 | A Phase 2 Study of Elotuzumab, Pomalidomide, & Dexamethasone (Elo-Pom-Dex) With Second Autologous Stem Cell Transplantation for Relapsed Multiple Myeloma | 2 |
| NCT02654132 | https://ClinicalTrials.gov/show/NCT02654132 | An Open Label, Randomized Phase 2 Trial of Pomalidomide/Dexamethasone With or Without Elotuzumab in Relapsed and Refractory Multiple Myeloma (ELOQUENT-3) | 2 |
| NCT02612779 | https://ClinicalTrials.gov/show/NCT02612779 | A Phase 2, Multiple Cohort Study of Elotuzumab in Combination With Pomalidomide and Low-Dose Dexamethasone (EPd), and in Combination With Nivolumab (EN), in Patients With Multiple Myeloma Relapsed or Refractory to Prior Treatment With Lenalidomide. | 2 |
| NCT03361306 | https://ClinicalTrials.gov/show/NCT03361306 | LCI-HEM-MYE-CRD-002: A Phase 2 Study of Carfilzomib- Revlimid-Dexamethasone-Elotuzumab in Relapsed/Refractory Multiple Myeloma | 2 |
| NCT02843074 | https://ClinicalTrials.gov/show/NCT02843074 | Phase 2 Study to Assess the Feasibility and Tolerance of the Combination of Elotuzumab, Lenalidomide and Dexamethasone (ERd) in the Induction, Consolidation and Maintenance Treatment of Transplant-Eligible Patients Newly Diagnosed With Multiple Myeloma | 2 |
| NCT03411031 | https://ClinicalTrials.gov/show/NCT03411031 | A Randomized Parallel Phase 2 Study of Elotuzumab Plus Lenalidomide (Elo/Rev) for the Treatment of Serologic Relapse/Progression While on Lenalidomide Maintenance for Multiple Myeloma | 2 |
| NCT01891643 | https://ClinicalTrials.gov/show/NCT01891643 | A Phase 3, Randomized, Open Label Trial of Lenalidomide/Dexamethasone With or Without Elotuzumab in Subjects With Previously Untreated Multiple Myeloma | 3 |
| NCT03104270 | https://ClinicalTrials.gov/show/NCT03104270 | A Phase 2 Trial of the Efficacy and Safety of Elotuzumab in Combination With Pomalidomide, Carfilzomib and Dexamethasone Among High Risk Relapsed/Refractory Multiple Myeloma Patients | 2 |
| NCT02420860 | https://ClinicalTrials.gov/show/NCT02420860 | Phase 2 Study of the Combination of Elotuzumab With Lenalidomide as Maintenance Therapy Post Autologous Stem Cell Transplant in Patients With Multiple Myeloma | 2 |
| NCT02495922 | https://ClinicalTrials.gov/show/NCT02495922 | A Randomized Phase 3 Trial on the Effect of Elotuzumab in VRD Induction/Consolidation and Lenalidomide Maintenance in Patients With Newly Diagnosed Myeloma | 3 |
| NCT02726581 | https://ClinicalTrials.gov/show/NCT02726581 | An Open-Label, Randomized Phase 3 Trial of Combinations of Nivolumab, Pomalidomide and Dexamethasone in Relapsed and Refractory Multiple Myeloma | 3 |
3.3.3. Targeting B-cell maturation antigen (BCMA) with BsAbs, BiTEs, and Chimeric Antigen Receptor (CAR) T-cells (Fig. 1, Fig. S2)
BCMA is a transmembrane glycoprotein in the TNF receptor superfamily 17 (TNFRSF17) that has a very restricted expression pattern and is undetectable on normal human tissues except for PCs. Current BCMA-targeting biotherapeutic agents in clinical development include ADCs, BiTEs, bsAbs, and CAR-T cell therapy [17,45,46] (Fig. 1). There are several CAR-T platforms targeting BCMA in development [[45], [46], [47], [48], [49], [50]] and several have demonstrated activity in MM. In the first-in-human study of CAR-T cells targeting BCMA, the overall response rate was 81% with 63% very good partial response or complete response for R/R MM patients who had a median of 9.5 prior lines of MM therapy [36]. In a Phase 1 study (CRB-401), b2121 (Bluebird Bio and Celgene), 2nd generation autologous T-cells transduced with a lentiviral vector, showed an ORR of 94% with a CR rate of 56% [50]. These CAR-T cells produced deep and durable responses with 50% of the evaluable patients having ongoing responses >1 year. Notably, the clinical responses have continued to improve even after a year [45,48]. A global pivotal Phase 2 KarMMa trial evaluating bb2121 is open for enrollment (NCT03361748) (Table 3). Likewise, investigators from Jiatong University in China reported at the 2017 ASCO meeting that their BCMA-“bispecific” CAR-T cells (LCAR-B38M, Nanjing Legend Biotech) binding to 2 key BCMA epitopes yielded a 100% ORR with 74% stringent CR rate in R/R MM patients [49].
Table 3.
Interventional Clinical Trials Evaluating CAR-T Cell Platforms in Relapsed or Refractory Multiple Myeloma.
| Study NCT# | Reference URL | Study title | Phase of trial | CAR-T platform |
|---|---|---|---|---|
| NCT03287804 | https://ClinicalTrials.gov/show/NCT03287804 | A Single-Arm, Open-Label, Multi-Center, Phase 1/2 Study Evaluating the Safety and Clinical Activity of AUTO2, a CAR-T Cell Treatment Targeting BCMA and TACI in Patients With Relapsed or Refractory Multiple Myeloma | 1/2 | CAR-T BCMA + TACI |
| NCT03322735 | https://ClinicalTrials.gov/show/NCT03322735 | A Study of BCMA CAR-T Cells for Patients With Relapsed and Refractory Multiple Myeloma | 1/2 | CAR-T BCMA |
| NCT03661554 | https://ClinicalTrials.gov/show/NCT03661554 | BCMA Nano Antibody CAR-T Cells for Patients With Refractory and Relapsed Multiple Myeloma | 1 | CAR-T Nanobody/CAR-T BCMA |
| NCT03664661 | https://ClinicalTrials.gov/show/NCT03664661 | A Single-center, One Arm, Open-Label Clinical Study of BCMA Nanobody CAR-T Cells in Refractory/Relapsed Myeloma | 1 | CAR-T BCMA |
| NCT02546167 | https://ClinicalTrials.gov/show/NCT02546167 | Pilot Study of Redirected Autologous T Cells Engineered To Contain an Anti-BCMA scFv Coupled To TCRζ And 4-1BB Signaling Domains in Patients With Relapsed and/or Refractory Multiple Myeloma | 1 | CAR-T BCMA |
| NCT03548207 | https://ClinicalTrials.gov/show/NCT03548207 | A Phase 1b-2, Open-Label Study of JNJ-68284528, A Chimeric Antigen Receptor T-Cell (CAR-T) Therapy Directed Against BCMA in Subjects With Relapsed or Refractory Multiple Myeloma | 1/2 | JNJ-68284528/CAR-T BCMA |
| NCT03448978 | https://ClinicalTrials.gov/show/NCT03448978 | Phase 1 Safety and Feasibility Study of Autologous CD8+ T-cells Transiently Expressing a Chimeric Antigen Receptor Directed to B-Cell Maturation Antigen in Patients With Multiple Myeloma | 1 | CAR-T BCMA |
| NCT03318861 | https://ClinicalTrials.gov/show/NCT03318861 | A Phase 1 Multicenter Study of KITE-585, an Autologous Anti-BCMA CAR-T Cell Therapy, in Subjects With Relapsed/Refractory Multiple Myeloma | 1 | KITE-585/CAR-T BCMA |
| NCT03274219 | https://ClinicalTrials.gov/show/NCT03274219 | A Phase 1 Study of bb21217, an Anti-BCMA CAR- T Cell Drug Product, in Relapsed and/or Refractory Multiple Myeloma | 1 | bb21217/CAR-T BCMA |
| NCT03361748 | https://ClinicalTrials.gov/show/NCT03361748 | A Phase 2, Multicenter Study to Determine the Efficacy and Safety of bb21217 in Subjects With Relapsed and Refractory Multiple Myeloma | 2 | bb21217/CAR-T BCMA |
| NCT03430011 | https://ClinicalTrials.gov/show/NCT03430011 | Protocol H125001: An Open-Label Phase 1/2 Study of JCARH125, BCMA-targeted Chimeric Antigen Receptor (CAR)-T Cells, in Subjects With Relapsed or Refractory Multiple Myeloma | 1/2 | JCARH125/CAR-T BCMA |
| NCT03288493 | https://ClinicalTrials.gov/show/NCT03288493 | Open-Label, Multicenter, Single Ascending Dose Study to Assess the Safety of P-BCMA-101 in Subjects With Relapsed and/or Refractory Multiple Myeloma (MM) | 1 | P-BCMA-101 CAR-T/CAR-T BCMA |
| NCT03196414 | https://ClinicalTrials.gov/show/NCT03196414 | Study of T Cells Targeting CD138/BCMA (CAR-T CD138/BCMA) for Chemotherapy Refractory and Relapsed Multiple Myeloma | 1/2 | CAR-T CD138/BCMA |
| NCT03464916 | https://ClinicalTrials.gov/show/NCT03464916 | A Phase 1, Open-Label, Dose-Escalation, Pharmacokinetic and Pharmacodynamic Study of the Safety and Efficacy of CAR2 Anti-CD38 A2 CAR-T Cells in Patients With Relapsed or Refractory Multiple Myeloma | 1 | CAR-T CD38 |
3.4. Immune-checkpoint inhibitors
Immune-checkpoint inhibitors (ICI) targeting the PD-1/PD-L1 axis have recently emerged as promising agents against hematologic malignancies, including MM [51]. Early clinical trials of IMiDs combined with PD-1 pathway blocking MoAb have shown promising preliminary results. For example, the Phase 1 KEYNOTE-023 (NCT02036502) study of the anti-PD1 MoAb pembrolizumab plus lenalidomide and Dex in relapsed MM showed an ORR of 76%. Unfortunately, excessive and unpredictable toxicity has raised serious safety concerns about anti-PD1 MoAb, as reflected by several advanced clinical trials in R/R MM as well as newly diagnosed high-risk MM having been suspended by the FDA (e.g. NCT02579863, NCT02576977, NCT02906332) [52]. Further investigation and randomized trials are needed to further evaluate the safety and effectiveness of the ICI targeting the PD-1/PD-L1 axis [52,53].
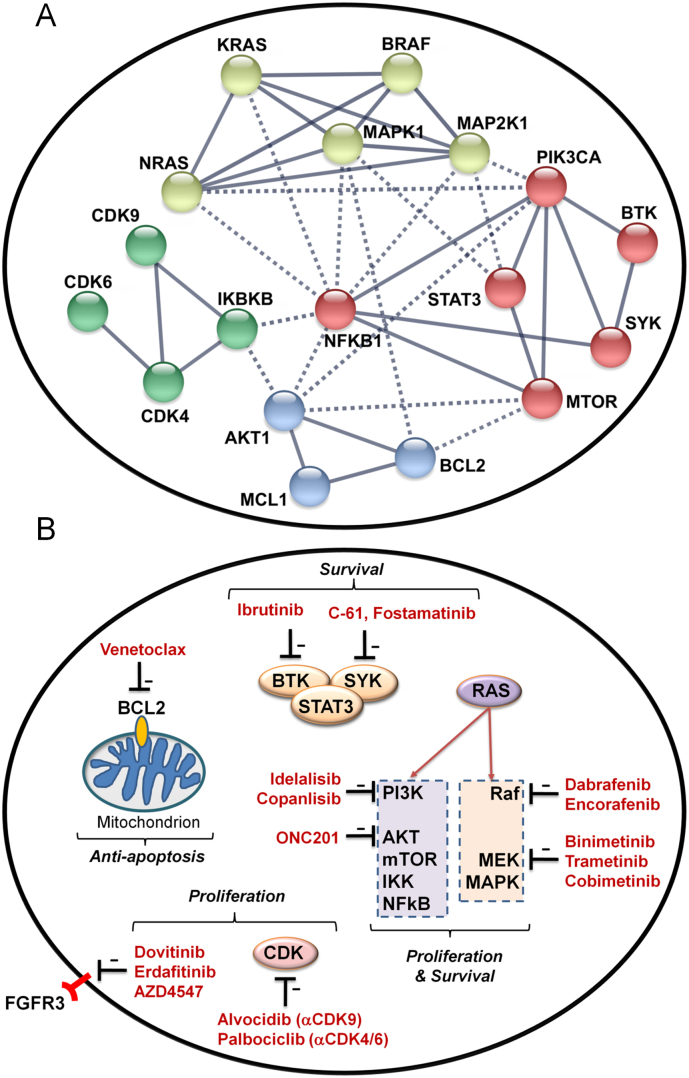
3.5. Targeted therapeutics and personalized medicine for MM (Fig. 2)
Fig. 2.
Molecular Targets and Targeted Therapeutics in R/R MM and High-Risk MM. [A] Network of Molecular Targets. The publicly available STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) database (http: //string-db.org) provides a critical assessment and integration of protein–protein interactions, including direct (physical) as well as indirect (functional) associations. The depicted STRING network view of the protein-protein interaction network for molecular targets in MM cells was constructed using STRING10 algorithm and the STRING database. Nodes show the protein identifiers and lines depict known interactions between the proteins. The lines represent experimental associations between the proteins, as determined by the STRING data mining algorithm. Associations were filtered with confidence parameter >90% (highest confidence from the datamining algorithm) and depicted by the connecting lines. Clustering algorithm was performed on the association scores to group protein-protein interaction networks for the most closely networked interactions (“MCL clustering algorithm” provided by the software). The MCL inflation parameter was set to a value of 2.5 to distinguish 4 inter-connected cluster groupings. Solid lines represent connections within clusters and dotted lines depict connections between clusters. [B] Targeted Therapeutics and Their Targets. Depicted are the molecular targets according to their functional significance and available targeted medicines that inhibit their function.
MM is characterized by a heterogeneous mutational landscape [[2], [3], [4]]. Some of the molecular mutations which are believed to be among the driver mutations of MM are druggable with available targeted therapeutics that can be matched to a specific targeted therapy for that aberration, thereby providing the foundation for a patient-tailored precision medicine program for MM [6,13]. Mutations involving CCND1 and CDKN2C are found in 18% of MM patients. Palbociclib (PD0332991) selectively inhibits CDK4/6, causing a G1 arrest in primary myeloma cells [54] (Fig. 2). Activated RAS initiates the RAS-RAF-MAPK signaling cascade by activating the protein kinase activity of the RAF kinase. RAF phosphorylates and activates the serine/tyrosine/threonine kinase MAP2K (also known as MAPKK or MEK - MEK1 and MEK2). MEK phosphorylates and activates the serine/threonine-selective MAPK. RAF kinase inhibitors as well as MEK kinase inhibitors disrupt the activation of MAPK and therefore serve as functional MAPK inhibitors (MAPKi) [13]. In the NCT02834364 (GMMG-BIRMA) Phase 2 study in R/R MM patients with the BRAF V600E/K mutation are being treated with the kinase inhibitors Encorafenib (LGX818; RAF kinase inhibitor) in combination with the MEK inhibitor Binimetinib (MEK162) (Fig. 2). Other RAF kinase inhibitors include dabrafenib, encorafenib, vemurafenib, XL281. Other MEK inhibitors include binimetinib (MEK162), trametinib (GSK1120212), and cobimetinib [13] (Fig. 2). In 2013/2014 FDA approved trametinib and dabrafenib combination for treatment of metastatic melanoma patients with BRAF mutations. It has been reported that MM cases with BRAF V600E mutations can respond to vemurafenib [55]. However, NRAS mutations have been shown to confer resistance to vemurafenib in BRAF-mutated MM [56] and spatial genomic heterogeneity in MM has also been associated with delayed onset treatment failure in vemurafenib-treated BRAF-mutated MM patients [57]. Inhibition of BRAF using BRAF V600E inhibitors can result in paradoxical activation of the MAPK pathway, a phenomenon that is exaggerated in KRAS-mutated cancers. Inhibition of MAPKK/MEK has emerged as a viable strategy to treat patients with BRAF-mutated cancers and to overcome paradoxical activation in the setting of therapy with BRAF V600E-directed agents. Trametinib is an oral, allosteric inhibitor of MEK1/2 that has shown early clinical activity in tumors with activating BRAF mutations (Fig. 2). In the NCI-MATCH study (NCT02465060), R/R MM patients with BRAF V600E/R/K/D mutation receive in a Phase 2 setting dabrafenib and trametinib, whereas patients with BRAF fusion or BRAF non-V600 mutation receive trametinib and patients with NRAS mutation in codon 12, 13, or 61 receive binimetinib. Likewise, dabrafenib plus trametinib, dabrafenib alone, or trametinib alone are being evaluated in patients with R/R MM and BRAF/NRAS/KRAS mutations (NCT03091257). The t(4;14) translocation is associated with upregulation of the FGFR3. Patients with t(4;14) demonstrate an overall poor prognosis. There may be additional mutations by which FGFR3 is dysregulated in MM that are independent of t(4;14) translocation. There are several FGFR inhibitors (FGFRi), including non-selective inhibitors (e.g. Dovitinib, Lenvatinib, Nintedanib, Ponatinib, Lucitanib, Pazopanib) and selective inhibitors (e.g. AZD4547, BGJ398, ARQ-087, JNJ 42756493, BAY-1163877) (Fig. 2). Dovitinib (NCT01058434) has been evaluated in MM patients with FGFR3 gene alterations. NCT02952573 is an ongoing proof of concept study of the oral FGFRi JNJ 42756493/Erdafitinib comparing R/R MM patients with and without FGFR3 mutations. In the NCI-MATCH study (NCT02465060), R/R MM patients with FGFR pathway alterations are treated with the selective FGFR3i AZD4547. BTK also emerged as a promising new molecular target in R/R MM (Fig. 2). In primary myeloma-bearing SCID-rab mice, the rationally designed non-covalent Bruton's tyrosine kinase (BTK) inhibitor LFM-A13 inhibited osteoclast activity, prevented myeloma-induced bone resorption and moderately suppressed myeloma growth [58]. Ibrutinib, a first-in-class, once-daily, oral covalent inhibitor of BTK, exhibited promising clinical activity in R/R MM patients who had received ≥2 prior lines of therapy, including an immunomodulatory agent [10]. Spleen tyrosine kinase (SYK) inhibitors also show potential as new therapeutic agents for R/R MM [11,15] (Fig. 2). While these TKI, especially the FGFRi and BTKi hold clinical promise based on preliminary clinical trial data, currently, there is insufficient information regarding the clinical potential of TKI as possible components of future personalized treatment strategies for R/R MM.
The BCL-2-specific BH3 mimetic Venetoclax (ABT-199) is a selective, orally bioavailable BCL-2 inhibitor that induces cell death in MM cells, particularly in those harboring t(11;14), which express high levels of BCL-2 relative to BCL-XL and MCL-1 [14,59] (Fig. 2). Intrinsic resistance to Venetoclax treatment observed in MM patient samples has been attributed to a low BCL-2-to-MCL-1 gene expression ratio, suggesting a central role for MCL-1 [60]. Livingston et al. reported that the CDK9 inhibitor Alvocidib suppresses MCL-1 expression via CDK9-mediated regulation of RNA polymerase II and potentiates the activity of venetoclax against MM cells, reminiscent of the findings reported for AML cells [61]. Furthermore, Dexamethasone has been reported to enhance the sensitivity of MM cells to venetoclax [62]. FDA previously granted accelerated approval to Venetoclax (Venclexta, AbbVie/Roche) in 2016 for the treatment of patients with recurrent CLL who have 17p deletion. Kumar et al. reported that Venetoclax monotherapy exhibited an acceptable safety profile and evidence of single-agent activity in patients with heavily pre-treated R/R MM, predominantly in patients with t(11;14) abnormality (NCT01794520) [14]. Most responses (86%) were reported in patients with t(11;14). In this group, ORR was 40%, with 27% of patients achieving very good partial response or better. A Phase 3, multicenter, randomized, double blind, placebo-controlled study is active and will evaluate the efficacy and safety of Venetoclax plus bortezomib and Dex in subjects with R/R MM who are considered sensitive or naïve to PI and received 1 to 3 prior lines of therapy for MM (NCT02755597). Other combinations are being explored in separate clinical trials (e.g. NCT03399539, NCT0331418, NCT03539744). The pending clinical data from the ongoing clinical trials of venetoclax in R/R MM patients are anticipated to provide critical insights regarding the clinical potential of this promising agent and how to best integrate it into the standard of care for R/R MM patients.
4. Future directions and outstanding questions
4.1. Detection of MRD
Recently, a EuroFlow-based next generation flow (NGF) approach was reported for highly sensitive and standardized detection of MRD in MM, and the results of its validation vs. a conventional 8-color flow-MRD method and NGS [63]. Such standardized and validated NGF-MRD methods can be applied to virtually every MM patient for MRD monitoring in BM after therapy and provide the opportunity for adding MRD negativity to criteria for response to treatment assessments and including it (together with PFS and OS) as an efficacy endpoint [[63], [64], [65]]. Even in patients who achieve an early CR, the use of MRD detection methodologies would allow clinical researchers to assess the contribution of additional interventions and time to the quality (i.e. “depth”) of CR. The use of fused functional and anatomic imaging using positron emission tomography-computed tomography (18F-FDG PET/CT) [[66], [67], [68]] combined with MRD monitoring using highly sensitive new detection methods [[63], [64], [65]] provides a unique opportunity to better evaluate clinical activity of innovative treatment platforms and new combination regimens in R/R MM as well as newly diagnosed high-risk MM patients.
4.2. Optimizing risk mitigation to maximize patient safety and quality of life
Protocols of targeted therapeutics should consider incorporating specific monitoring and management guidelines for specific toxicities for risk mitigation based on the most recent and up to date integrated safety analysis of the particular drug that will be used [[69], [70], [71]]. The higher mortality rate for early toxicity in patients ≥80 years emphasizes the need for a careful frailty assessment and more effective risk-mitigation measures for elderly.
4.3. Proof-of-concept studies with specific cellular PD/PK endpoints
Secondary plasma cell leukemia (PCL) is observed in advanced MM with a frequency of 1–4% [72]. The circulating PCL cells in blood can be easily collected before, during and after treatment with the different single agent as well as combination modalities for (i) cellular PK analyses, (ii) evaluation of on-target and off-target effects at the cellular level using phosphoproteomics; (iii) evaluation of the kinetics of cytotoxic activity against MCL cells using multi-color flow cytometry in a quantitative apoptosis assay. Well-designed mechanism of action (MOA) studies that leverage PCL cells can provide clinically meaningful new insights that inform the design of future MM studies. In view of the oligoclonality of MM, it is likely to be helpful to characterize the dynamics of the mutational profile of persistent/outgrowing MM clones, elucidate the clonal diversity, persistence or disappearance of multi-mutated clones, acquisition of additional mutations. Rashid et al. National University of Singapore recently reported an artificial intelligence (AI) technology based experimental platform labeled as the Quadratic Phenotypic Optimization Platform (QPOP) to rapidly identify patient-tailored novel drug combinations for R/R MM [73] Notably, Lagana et al. reported an integrated multiomics approach for personalized therapy of MM and its application in a pilot precision medicine clinical trial in R/R MM patients. Their data appeared to demonstrate the feasibility of NGS-guided personalized therapy [27].
4.4. Real-world data (RWD) and real-world evidence (RWE) to evaluate the relative effectiveness, safety and utility of new anti-MM treatment strategies
The 21st Century Cures Act (“Cures Act”) encourages the Food and Drug Administration to consider RWE of the safety and comparative effectiveness of drugs in its approval process [74]. Additional RWD will be essential to an improved understanding of the current clinical practice patterns, so that new agents can be effectively incorporated into existing treatment strategies for MM or used instead of less effective treatments to ensure that patients receive the best possible care. Randomized adaptive clinical trials aimed at comparing the efficacy of different treatment strategies with respect to their achieved rate of sustained deep responses with MRD negativity as well as their tolerability as well as randomized hybrid interventional x observational studies that collect real-world data regarding their real-world effectiveness/treatment burden ratio and relative tolerability are needed to reach a consensus regarding the “best” treatment strategies for high-risk and R/R MM.
5. Conclusion
The therapeutic landscape for MM is rapidly evolving in the era of personalized medicine. New treatment strategies have markedly improved the survival of MM patients and caused a paradigm shift in therapy in treatment of MM, from a palliative approach for an incurable hematologic malignancy to a more patient-tailored active management strategy of a potentially curable disease, including the use of sequential therapies, with the goal of prolonging PFS and OS as well as further salvage therapy at relapse. The observation that some of the innovative treatment platforms have resulted in durable MRD-negative deep CRs even in heavily pretreated R/R MM patients has renewed the hope for development of potentially curative therapies for MM as predicted by Barlogie et al. [75]. There is a growing list of promising targeted therapeutics with anti-MM activity in clinical pipeline, including TKI, inhibitors of anti-apoptotic proteins, MoAbs, ADC, bsAbs, fusion proteins, and various cell therapy platforms. Which of these treatment modalities will ultimately evolve into components of a new standard of care regimen for R/R or high-risk MM patients remains to be determined. Several challenges remain and require a multi-stake holder collaboration for successful integration of new technology platforms as well as clinical management guidelines into the contemporary MM treatment programs.
Search strategy and selection criteria
Data for this Review were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles using the search terms “multiple myeloma”, “clinical trial”, and “therapy”. Abstracts and reports from meetings were included only when they related directly to previously published work. Only articles published in English between 2010 and 2018 were included. We also interrogated the clinicaltrials.gov data repository (https://clinicaltrials.gov/) to determine the number of interventional trials in R/R MM patients that were initiated from 1993 to 2018. Search terms to identify the trials were “Relapse OR Refractory”, “Interventional studies”, and “Multiple Myeloma”. The following search terms were used for the Table 1, Table 2, Table 3: Recruiting, Active not recruiting, completed; Interventional Studies; Multiple Myeloma; CD38; Start date from 09/01/2013 to 09/01/2018. Additional terms were: CD38 (Table 1), Elotuzumab (Table 2), CAR-T (Table 3).
Funding
The project described was supported in part by DHHS grants P30 CA014089, U01-CA-151837, R01CA-154471, R21-CA-164098 (F.M.U) and P30 CA16672 (R.E.C) from the National Cancer Institute, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. TD was supported in part by research fund of the Turkish Academy of Sciences, Turkey. F.M.U is the laureate of the 2018 TUBA Academy Prize in Health and Life Sciences of the Turkish Academy of Sciences, Turkey. The funders had no role in paper design, data collection, data analysis, interpretation, writing of the paper.
Conflict-of-interest disclosure
The authors declare no competing financial interests or other conflict of interests.
Authorship contribution
All authors have made significant and substantive contributions to the study. All authors have participated in the review of relevant literature, drafting of the manuscript, review and revisions of the final draft. F.M.U. and SQ have equally contributed to this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.12.004.
Appendix A. Supplementary data
Supplementary material
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Robiou du Pont S., Cleynen A., Fontan C., Attal M., Munshi N., Corre J. Genomics of multiple myeloma. J Clin Oncol. 2017;35:963–967. doi: 10.1200/JCO.2016.70.6705. [DOI] [PubMed] [Google Scholar]
- 3.Hoang P.H., Dobbins S.E., Cornish A.J., Chubb D., Law P.J., Kaiser M. Whole-genome sequencing of multiple myeloma reveals oncogenic pathways are targeted somatically through multiple mechanisms. Leukemia. 2018 doi: 10.1038/s41375-018-0103-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manier S., Salem K.Z., Park J., Landau D.A., Getz G., Ghobrial I.M. Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2017;14:100–113. doi: 10.1038/nrclinonc.2016.122. [DOI] [PubMed] [Google Scholar]
- 5.Gay F., Engelhardt M., Terpos E., Wäsch R., Giaccone L., Auner H.W. From transplant to novel cellular therapies in multiple myeloma: European Myeloma Network guidelines and future perspectives. Haematologica. 2018;103(2):197–211. doi: 10.3324/haematol.2017.174573. [Epub 2017 Dec 7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramson H.N. The multiple myeloma drug pipeline 2018: A review of small molecules and their therapeutic targets. Clin Lymphoma Myeloma Leuk. 2018;18(9):611–627. doi: 10.1016/j.clml.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Mohty M., Terpos E., Mateos M.V., Cavo M., Lejniece S., Beksac M. Multiple myeloma treatment in real-world clinical practice: results of a prospective, multinational, noninterventional study. Clin Lymphoma Myeloma Leuk. 2018 doi: 10.1016/j.clml.2018.06.018. pii: S2152-2650(18)30230-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Cavo M., Terpos E., Bargay J., Einsele H., Cavet J., Greil R. The multiple myeloma treatment landscape: international guideline recommendations and clinical practice in Europe. Expert Rev Hematol. 2018;11(3):219–237. doi: 10.1080/17474086.2018.1437345. [DOI] [PubMed] [Google Scholar]
- 9.Lonial S., Boise L.H., Kaufman J. How I treat high-risk myeloma. Blood. 2015;126:1536–1543. doi: 10.1182/blood-2015-06-653261. [DOI] [PubMed] [Google Scholar]
- 10.Richardson P.G., Bensinger W.I., Huff C.A., Costello C.L., Lendvai N., Berdeja J.G. Ibrutinib alone or with dexamethasone for relapsed or relapsed and refractory multiple myeloma: phase 2 trial results. Br J Haematol. 2018;180(6):821–830. doi: 10.1111/bjh.15058. [Epub 2018 Feb 13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uckun F.M., Qazi S., Ma H., Tuel-Ahlgren L., Ozer Z. STAT3 is a substrate of SYK tyrosine kinase in B-lineage leukemia/lymphoma cells exposed to oxidative stress. Proc Natl Acad Sci U S A. 2010;107(7):2902–2907. doi: 10.1073/pnas.0909086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahbari K.J., Nosrati J.D., Spektor T.M., Berenson J.R. Venetoclax in Combination with Bortezomib, Dexamethasone, and Daratumumab for Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2018;18(9):e339–e343. doi: 10.1016/j.clml.2018.06.003. [Epub 2018 Jun 18] [DOI] [PubMed] [Google Scholar]
- 13.Heuck C.J., Jethava Y., Khan R., van Rhee F., Zangari M., Chavan S. Inhibiting MEK in MAPK pathway-activated myeloma. Leukemia. 2016;30:976–980. doi: 10.1038/leu.2015.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S., Kaufman J.L., Gasparetto C., Mikhael J., Vij R., Pegourie B. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130(22):2401–2409. doi: 10.1182/blood-2017-06-788786. [DOI] [PubMed] [Google Scholar]
- 15.Koerber R.M., Held S.A.E., Heine A., Kotthoff P., Daecke S.N., Bringmann A. Analysis of the anti-proliferative and the pro-apoptotic efficacy of Syk inhibition in multiple myeloma. Exp Hematol Oncol. 2015;4:21. doi: 10.1186/s40164-015-0016-z. [eCollection 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y., Shi J., Gu Z., Salama M.E., Das S., Wendlandt E. Bruton tyrosine kinase is a therapeutic target in stem-like cells from multiple myeloma. Cancer Res. 2015;75(3):594–604. doi: 10.1158/0008-5472.CAN-14-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trudel S., Lendvai N., Popat R., Voorhees P.M., Reeves B., Libby E.N. Deep and durable responses in patients (Pts) with relapsed/refractory multiple myeloma (MM) treated with monotherapy GSK2857916, an antibody drug conjugate against B-cell maturation antigen (BCMA): preliminary results from Part 2 of Study BMA117159. Presented at: ASH Annual Meeting and Exposition; Dec. 9–12, 2017; Atlanta, Georgia. Abstract 741. Blood. 2017;130(Suppl. 1):741. [Google Scholar]
- 18.Varga C., Laubach J.P., Anderson K.C., Richardson P.G. Investigational agents in immunotherapy: a new horizon for the treatment of multiple myeloma. Br J Haematol. 2018;181(4):433–446. doi: 10.1111/bjh.15116. [DOI] [PubMed] [Google Scholar]
- 19.Chim C.S., Kumar S.K., Orlowski R.Z., Cook G., Richardson P.G., Gertz M.A. Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond. Leukemia. 2018;32(2):252–262. doi: 10.1038/leu.2017.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain T., Sonbol M.B., Kolla K.R., Almader-Douglas D., Palmer J., Fonseca R. High dose chemotherapy with early autologous stem cell transplantation compared to standard dose chemotherapy or delayed transplantation in patients with newly diagnosed Multiple Myeloma: A systematic review and meta-analysis. Biology of blood and marrow transplantation 2018. Biol Blood Marrow Transplant. 2018 doi: 10.1016/j.bbmt.2018.09.021. Sep 20. pii: S1083-8791(18)30578-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Jagannath S., Abonour R., Durie B.G.M., Narang M., Terebelo H.R., Gasparetto C.J. Impact of post-ASCT maintenance therapy on outcomes in patients with newly diagnosed multiple myeloma in Connect MM. Blood Adv. 2018;2(13):1608–1615. doi: 10.1182/bloodadvances.2018017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonsalves W.I., Buadi F.K., Ailawadhi S., Bergsagel P.L., Chanan Khan A.A., Dingli D. Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a Mayo Stratification of Myeloma and Risk-adapted Therapy (mSMART) consensus statement. Bone Marrow Transplant. 2018 doi: 10.1038/s41409-018-0264-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gay F., Jackson G., Rosiñol L., Holstein S.A., Moreau P., Spada S. Maintenance Treatment and Survival in patients with Myeloma: a Systematic Review and Network Meta-analysis. JAMA Oncol. 2018 doi: 10.1001/jamaoncol.2018.2961. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandolfi S., Vekstein C., Laubach J.P., O'Brien A., Masone K., Munshi N.C., Anderson K.C. The evolving role of transplantation in multiple myeloma: the need for a heterogeneous approach to a heterogeneous disease. Clin Adv Hematol Oncol. 2018;16(8):564–574. [PubMed] [Google Scholar]
- 25.Kumar S.K., Callander N.S., Alsina M. NCCN guidelines insights: multiple myeloma, version 3.2018. J Natl Compr Canc Netw. 2018;16:11–20. doi: 10.6004/jnccn.2018.0002. [DOI] [PubMed] [Google Scholar]
- 26.Veltri L.W., Milton D.R., Delgado R., Shah N., Patel K., Nieto Y. Outcome of autologous hematopoietic stem cell transplantation in refractory multiple myeloma. Cancer. 2017;123(18):3568–3575. doi: 10.1002/cncr.30770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagana A., Beno I., Melnekoff D., Leshchenko V., Madduri D., Ramdas D. JCO precision oncology. 2018. Precision medicine for relapsed multiple myeloma on the basis of an integrative multiomics approach. [JCO Precision Oncology - published online August 8, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popat R., Brown S.R., Flanagan L., Hall A., Gregory W., Kishore B. Extended follow-up and the feasibility of Panobinostat maintenance for patients with Relapsed Multiple Myeloma treated with Bortezomib, Thalidomide, Dexamethasone plus Panobinostat (MUK six open label, multi-Centre phase I/II Clinical Trial) Br J Haematol. 2018 doi: 10.1111/bjh.15551. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Oostvogels R., Jak M., Raymakers R., Mous R., Minnema M.C. Efficacy of retreatment with immunomodulatory drugs and proteasome inhibitors following daratumumab monotherapy in relapsed and refractory multiple myeloma patients. Br J Haematol. 2018 doi: 10.1111/bjh.15504. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terpos E., Katodritou E., de la Rubia J., Hungria V., Hulin C., Roussou M. Bortezomib-based therapy for relapsed/refractory multiple myeloma in real-world medical practice. Eur J Haematol. 2018 doi: 10.1111/ejh.13147. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Zanwar S., Abeykoon J.P., Kapoor P. Ixazomib: a novel drug for multiple myeloma. Expert Rev Hematol. 2018 doi: 10.1080/17474086.2018.1518129. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Dimopoulos M.A., Goldschmidt H., Niesvizky R., Joshua D., Chng W.J., Oriol A. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(10):1327–1337. doi: 10.1016/S1470-2045(17)30578-8. [DOI] [PubMed] [Google Scholar]
- 33.Ailawadhi S., Mikhael J.R., Laplant B.R., Laumann K.M., Kumar S., Roy V. Pomalidomide-dexamethasone in refractory multiple myeloma: long-term follow-up of a multi-cohort phase II clinical trial. Leukemia. 2018;32(3):719–728. doi: 10.1038/leu.2017.258. [Epub 2017 Sep 1] [DOI] [PubMed] [Google Scholar]
- 34.Siegel D.S., Dimopoulos M.A., Ludwig H., Facon T., Goldschmidt H., Jakubowiak A. Improvement in overall survival with Carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36:1–7. doi: 10.1200/JCO.2017.76.5032. [DOI] [PubMed] [Google Scholar]
- 35.Harousseau J.L., Attal M. How I treat first relapse of myeloma. Blood. 2017;130(8):963–973. doi: 10.1182/blood-2017-03-726703. [DOI] [PubMed] [Google Scholar]
- 36.Chari A., Suvannasankha A., Fay J.W., Arnulf B., Kaufman J.L., Ifthikharuddin J.J. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974–981. doi: 10.1182/blood-2017-05-785246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreau P., Mateos M.V., Berenson J.R., Weisel K., Lazzaro A., Song K. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (a.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018;19(7):953–964. doi: 10.1016/S1470-2045(18)30354-1. [DOI] [PubMed] [Google Scholar]
- 38.Frerichs K.A., Nagy N.A., Lindenbergh P.L., Bosman P., Marin Soto J., Broekmans M. CD38-targeting antibodies in multiple myeloma: mechanisms of action and clinical experience. Expert Rev Clin Immunol. 2018;14(3):197–206. doi: 10.1080/1744666X.2018.1443809. [DOI] [PubMed] [Google Scholar]
- 39.Mateos M.V., Dimopoulos M.A., Cavo M., Suzuki K., Jakubowiak A., Knop S. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med. 2018;378(6):518–528. doi: 10.1056/NEJMoa1714678. [DOI] [PubMed] [Google Scholar]
- 40.Spencer A., Lentzsch S., Weisel K., Avet-Loiseau H., Mark T.M., Spicka I. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018 doi: 10.3324/haematol.2018.194118. Sep 20. pii: haematol.2018.194118. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson P.G., Attal M., Campana F., Le-Guennec S., Hui A.M., Risse M.L. Isatuximab plus pomalidomide/dexamethasone versus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma: ICARIA phase III study design. Future Oncol. 2018 May;14(11):1035–1047. doi: 10.2217/fon-2017-0616. [DOI] [PubMed] [Google Scholar]
- 42.Raab M.S., Chatterjee M., Goldschmidt H., Agis H., Blau I., Einsele H. A phase I/IIa Study of the CD38 antibody MOR202 alone and in combination with pomalidomide or lenalidomide in patients with relapsed or refractory multiple myeloma. Blood. 2016;128(22):1152. [Google Scholar]
- 43.Cella D., McKendrick J., Kudlac A., Palumbo A., Oukessou A., Vij R. Impact of elotuzumab treatment on pain and health-related quality of life in patients with relapsed or refractory multiple myeloma: results from the ELOQUENT-2 study. Ann Hematol. 2018 doi: 10.1007/s00277-018-3469-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimopoulos M.A., Lonial S., Betts K.A., Chen C., Zichlin M.L., Brun A. Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended 4-year follow-up and Analysis of relative progression-free survival from the randomized ELOQUENT-2 trial. Cancer. 2018 doi: 10.1002/cncr.31680. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Brudno J.N., Maric I., Hartman S.D., Rose J.J., Wang M., Lam N. T Cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36(22):2267–2280. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho S.F., Anderson K.C., Tai Y.T. Targeting B Cell maturation antigen (BCMA) in multiple myeloma: potential uses of BCMA-based immunotherapy. Front Immunol. 2018;9:1821. doi: 10.3389/fimmu.2018.01821. [eCollection 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bluhm J., Kieback E., Marino S.F., Oden F., Westermann J., Chmielewski M. CAR T Cells with Enhanced Sensitivity to B Cell Maturation Antigen for the Targeting of B Cell Non-Hodgkin's Lymphoma and Multiple Myeloma. Mol Ther. 2018;26(8):1906–1920. doi: 10.1016/j.ymthe.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jesus G., Berdeja Yi Lin, Raje Noopur S., Siegel David Samuel DiCapua, Munshi Nikhil C., Liedtke Michaela. First-in-human multicenter study of bb2121 anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: Updated results. J Clin Oncol. 2017;35(15) suppl. 3010. [Google Scholar]
- 49.Fan F., Zhao W., Liu J., He A., Chen Y., Cao X. ASCO Annual Meeting. Abstract LBA3001. Presented June 5, 2017. 2017. Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma. [Google Scholar]
- 50.Cornell R.F., Costa L.J. The future of chimeric antigen receptor T cell therapy for the treatment of Multiple Myeloma. Biol Blood Marrow Transplant. 2018 doi: 10.1016/j.bbmt.2018.11.009. pii: S1083-8791(18)30719-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.D'Agostino M., Gazzera G., Cetani G., Bringhen S., Boccadoro M., Gay F. Clinical and pharmacologic features of monoclonal antibodies and checkpoint blockade therapy in Multiple Myeloma. Curr Med Chem. 2018 May 13 doi: 10.2174/0929867325666180514114806. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 52.Jelinek T., Paiva B., Hajek R. Update on PD-1/PD-L1 inhibitors in Multiple Myeloma. Front Immunol. 2018 doi: 10.3389/fimmu.2018.02431. 16 Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paul B., Kang S., Zheng Z., Kang Y. The challenges of checkpoint inhibition in the treatment of multiple myeloma. Cell Immunol. 2018 Dec;334:87–98. doi: 10.1016/j.cellimm.2018.10.003. Epub 2018 Oct 13. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark A.S., Karasic T.B., Demichele A., Vaughn D.J., O'Hara M., Perini R. Palbociclib (PD0332991)-a selective and potent cyclin-dependent kinase inhibitor: a review of pharmacodynamics and clinical development. JAMA Oncol. 2016 Feb;2(2):253–260. doi: 10.1001/jamaoncol.2015.4701. [Review] [DOI] [PubMed] [Google Scholar]
- 55.Raje N., Chau I., Hyman D.M., Ribrag V., Blay J.-Y., Tabernero J. Vemurafenib in patients with relapsed refractory Multiple Myeloma harboring BRAFV600 Mutations: a Cohort of the histology-independent VE-BASKET study. JCO Precision Oncol. 2018 doi: 10.1200/PO.18.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raab M.S., Lehners N., Xu J., Ho A.D., Schirmacher P., Goldschmidt H. Spatially divergent clonal evolution in multiple myeloma: overcoming resistance to BRAF inhibition. Blood. 2016;127:2155–2157. doi: 10.1182/blood-2015-12-686782. [DOI] [PubMed] [Google Scholar]
- 57.Rasche L., Chavan S.S., Stephens O.W., Patel P.H., Tytarenko R., Ashby C. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat Commun. 2017 Aug 16;8(1):268. doi: 10.1038/s41467-017-00296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bam R., Ling W., Khan S., Pennisi A., Venkateshaiah S.U., Li X. Role of Bruton's tyrosine kinase in myeloma cell migration and induction of bone disease. Am J Hematol. 2013 Jun;88(6):463–471. doi: 10.1002/ajh.23433. [Epub 2013 Mar 28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moreau P., Chanan-Khan A., Roberts A.W. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130:2392–2400. doi: 10.1182/blood-2017-06-788323. [DOI] [PubMed] [Google Scholar]
- 60.Gupta V.A., Matulis S.M., Conage-Pough J.E., Nooka A.K., Kaufman J.L., Lonial S. Bone marrow microenvironment–derived signals induce Mcl-1 dependence in multiple myeloma. Blood. 2017;129(14):1969–1979. doi: 10.1182/blood-2016-10-745059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Livingston M., Kim W., Haws H., Peterson P., Whatcott C.J., Siddiqui-Jain A. Proceedings of the American Association for Cancer Research Annual Meeting 2017. 77(13 Suppl) AACR; Cancer Res 2017; Washington, DC. Philadelphia (PA): 2017. Alvocidib potentiates the activity of venetoclax in preclinical models of multiple myeloma [abstract] Abstract nr 1106. Apr 1–5. [Google Scholar]
- 62.Matulis S.M., Gupta V.A., Nooka A.K., Hollen H.V., Kaufman J.L., Lonial S. Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax. Leukemia. 2016;30:1086–1093. doi: 10.1038/leu.2015.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flores-Montero J., Sanoja-Flores L., Paiva B., Puig N., García-Sánchez O., Böttcher S. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31(10):2094–2103. doi: 10.1038/leu.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caers J., Garderet L., Kortüm K.M., O'Dwyer M.E., van de Donk N.W.C.J., Binder M. An European Myeloma network recommendation on tools for diagnosis and monitoring of multiple myeloma: what to use and when. Haematologica. 2018 doi: 10.3324/haematol.2018.189159. pii: haematol.2018.189159. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu B., Thall P., Milton D.R., Sasaki K., Bashir Q., Shah N. High-risk myeloma and minimal residual disease postautologous-HSCT predict worse outcomes. Leuk Lymphoma. 2018:1–11. doi: 10.1080/10428194.2018.1485908. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Aljama M.A., Sidiqi M.H., Buadi F.K., Lacy M.Q., Gertz M.A., Dispenzieri A. Utility and Prognostic Value of 18 F-FDG Positron Emission Tomography-Computed Tomography Scans in patients with newly Diagnosed Multiple Myeloma. Am J Hematol. 2018 Sep 8 doi: 10.1002/ajh.25279. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 67.Jung S.H., Kwon S.Y., Min J.J., Bom H.S., Ahn S.Y., Jung S.Y. 18F-FDG PET/CT is useful for determining survival outcomes of patients with multiple myeloma classified as stage II and III with the revised international staging system. Eur J Nucl Med Mol Imaging. 2018 doi: 10.1007/s00259-018-4114-0. Sep 5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 68.Bailly C., Carlier T., Jamet B., Eugene T., Touzeau C., Attal M. Interim PET analysis in first line therapy of multiple myeloma: Prognostic value of ΔSUVmax in the FDG-avid patients of the IMAJEM study. Clin Cancer Res. 2018 doi: 10.1158/1078-0432.CCR-18-0741. Aug 1. pii: clincanres.0741.2018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69.Bringhen S., Milan A., Ferri C., Wäsch R., Gay F., Larocca A. Cardiovascular adverse events in modern myeloma therapy - Incidence and risks. A review from the European Myeloma Network (EMN) and Italian Society of Arterial Hypertension (SIIA) Haematologica. 2018;103(9):1422–1432. doi: 10.3324/haematol.2018.191288. [Epub 2018 Jul 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bringhen S., Offidani M., Palmieri S., Pisani F., Rizzi R., Spada S. Early mortality in myeloma patients treated with first-generation novel agents thalidomide, lenalidomide, bortezomib at diagnosis: a pooled analysis. Crit Rev Oncol Hematol. 2018;130:27–35. doi: 10.1016/j.critrevonc.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Ludwig H., Delforge M., Facon T., Einsele H., Gay F., Moreau P. Prevention and management of adverse events of novel agents in multiple myeloma: a consensus of the European Myeloma Network. Leukemia. 2018 doi: 10.1038/s41375-018-0040-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mina R., D'Agostino M., Cerrato C., Gay F., Palumbo A. Plasma cell leukemia: update on biology and therapy. Leuk Lymphoma. 2017;58(7):1538–1547. doi: 10.1080/10428194.2016.1250263. [DOI] [PubMed] [Google Scholar]
- 73.Rashid M.B.M.A., Toh T.B., Hooi L., Silva A., Zhang Y., Tan P.F. Optimizing drug combinations against multiple myeloma using a quadratic phenotypic optimization platform (QPOP) Sci Transl Med. 2018;8:10(453). doi: 10.1126/scitranslmed.aan0941. [DOI] [PubMed] [Google Scholar]
- 74.Jarow J.P., Lavange L., Woodcock J. Multidimensional evidence Generation and FDA Regulatory Decision making: defining and using "Real-World" Data. JAMA. 2017;318(8):703–704. doi: 10.1001/jama.2017.9991. [DOI] [PubMed] [Google Scholar]
- 75.Barlogie B., Mitchell A., van Rhee F., Epstein J., Morgan G.J., Crowley J. Curing myeloma at last: defining criteria and providing the evidence. Blood. 2014;124(20):3043–3051. doi: 10.1182/blood-2014-07-552059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material