Abstract
Background
Adoptive immunotherapy using T cells expressing chimeric antigen receptors (CARs) targeting CD19 has produced remarkable clinical outcomes. However, much of the mechanisms of action, such as the development of memory responses and sources of immune cytokines, remain elusive largely due to the challenge of characterizing human CAR T cell function in vivo. The lack of a suitable in vivo model also hinders the development of new CAR T cell therapies.
Methods
We established a humanized mouse (hu-mouse) model with a functional human immune system and genetically-matched (autologous) primary acute B-lymphoblastic leukemia (B-ALL) that permits modeling of CD19-targeted CAR T cell therapy in immunocompetent hosts without allogeneic or xenogeneic immune responses.
Findings
Anti-CD19 CAR T cells were detected in blood of leukemic hu-mice with kinetics and levels similar to those seen in patients receiving CAR T cell therapy. The levels of CAR T cells were correlated inversely with the burden of leukemia cells and positively with the survival times in anti-CD19 CAR T cell-treated leukemic hu-mice. Infusion of anti-CD19 CAR T cells also resulted in rapid production of T cell- and monocyte/macrophage-derived cytokines and an increase in frequency of regulatory T cells as reported in clinical studies.
Interpretation
These results provide a proof-of-principle that this novel preclinical model has the potential to be used to model human CAR T cell therapy and facilitate the design of new CARs with improved antitumor activity.
Research in context.
Evidence before this study
Anti-CD19 CAR T cell therapy has produced remarkable results in patients with B-cell malignancies. However, much of the mechanisms of action, such as the development of memory responses and sources of immune cytokines, remain elusive largely due to the challenge of characterizing human CAR T cell function in vivo. To date, anti-CD19 CAR-engineered human T cells have been characterized mainly by in vitro assays prior to clinical use. Although some mouse models were used to assess antitumor responses of human CD19-targeted CAR T cell therapy, these models are either immune-compromised or involve allogeneic and/or xenogeneic immune responses, creating a host environment differing from that of patients.
Added value of the study
Here we report a useful hu-mouse model with a functional human immune system and genetically-matched (autologous) primary B-ALL, which permits the modeling of CD19-targeted CAR T cell therapy in immunocompetent hosts without allogeneic or xenogeneic immune responses. We show that anti-CD19 CAR T cells were detected in the peripheral blood with kinetics and levels similar to those seen in patients receiving anti-CD19 CAR T cell therapy, and that the extent of CAR T cell survival and expansion is positively associated with the therapeutic outcome. Furthermore, unlike the currently available patient derived xenograft (PDX) models, our model makes it possible to assess cytokine production by both infused CAR T cells and the recipient immune cells, and alterations in human immune cell profiles following infusion of anti-CD19 CAR T cells.
Implications of all the available evidence
Our data demonstrate a proof-of-principle that this leukemic hu-mouse model is valuable in modeling anti-CD19 CAR T cell therapy and mechanistically understanding the antitumor responses of CAR T cells. Thus, this preclinical model has the potential to facilitate the design of new CARs with improved antitumor activity.
Alt-text: Unlabelled Box
1. Introduction
Adoptive immunotherapy using T cells, which are genetically modified to express chimeric antigen receptors (CARs) targeting CD19, has produced remarkable results in patients with B-cell malignancies [[1], [2], [3]]. Despite the impressive response rates, relapse was detected in patients following CD19-targeted CAR T cell therapy [4,5]. Multiple mechanisms have been thought to be responsible for relapse, including immune escape resulting from the development of CD19-negative tumor cells. In addition, anti-CD19 CAR T cell therapy was also associated with toxicity [6]. Although the establishment of memory CAR T cells in patients was reported [7], the characteristics of these memory T cells remain largely unknown, including their development, function, capacity for self-renewal, and survival factors/signaling. Thus, new mechanistic studies are urgently needed to further elucidate the mechanisms of relapse and toxicity following CAR T cell therapy, and for developing effective strategies of improving the therapeutic outcomes. However, to date, the function and antitumor activity of CAR-engineered human T cells have been characterized mainly by in vitro assays or in immunodeficient mice engrafted with human tumor cell lines [8]. Although a mouse model, produced through the transfer of human CD19-transduced mouse tumor cells in syngeneic human CD19-transgenic mice, made it possible to assess antitumor responses of human CD19-targeted CAR T cell therapy in an immunocompetent syngeneic setting [9], this model tests the responses against mouse tumors of mouse T cells.
Patient-derived xenografts (PDX), which are created by grafting patient-derived cancer cells in immunodeficient mice, have been increasingly used in an attempt to address clinically relevant questions by directly assessing human immune responses to human primary cancer cells, a setting closely recapitulating the features of human diseases [10]. PDX models with human B-ALL were also used in evaluating the therapeutic efficacy of CAR T cells [11]. However, most PDX models to date are either immune-compromised or involve allogeneic and/or xenogeneic immune responses, creating a host environment differing from that of patients. In this study, we establish a humanized mouse (hu-mouse) model with a functional human immune system and genetically-matched (autologous) primary B-ALL, which permits the modeling of CD19-targeted CAR T cell therapy in immunocompetent hosts without allogeneic or xenogeneic immune responses. We show that the extent of CAR T cell survival and expansion is positively associated with the therapeutic outcome. Furthermore, infusion of anti-CD19 CAR T cells results in a significant increase in not only proinflammatory cytokines (IFN-γ and TNFα), but also anti-inflammatory cytokine IL-10 and regulatory T (Treg) cells. Our data demonstrate a proof-of-principle that this leukemic hu-mouse model is valuable in modeling anti-CD19 CAR T cell therapy, mechanistically understanding the antitumor responses of CAR T cells, and testing new strategies of improving the therapeutic outcome.
2. Materials and methods
2.1. Animals and human tissues
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NOD/SCID/γc−/− or NSG) mice were purchased from the Jackson Laboratory (Bar Harbor, ME), and were housed in a specific pathogen-free micro-isolator environment and used as recipients of human tissue and cell transplantation at 6 to 10 weeks of age. Discarded human fetal thymus (FTHY) and liver tissues of gestational age of 17 to 20 weeks were obtained from Advanced Bioscience Resource (Alameda, CA). FTHY tissues were cut into small fragments (about 1 mm3). CD34+ fetal liver cells (FLCs) were isolated by the magnetic-activated cell sorter (MACS) separation system using anti-CD34 microbeads (Miltenyi Biotec, Auburn, CA). The prepared FTHY tissue fragments and CD34+ FLCs were cryopreserved immediately in liquid nitrogen until use. Protocols involving the use of animals and discarded human tissues/cells were approved by the institutional animal care and use committee and the human research committee of Columbia University.
2.2. Flow cytometry analysis
Flow cytometry was used in detection and phenotypic analysis of human leukemia and lymphohematopoietic cells in blood and various tissues as indicated where appropriate. All antibodies used in this study were purchased from BD or BioLegend. Analysis was performed on a LSR II or Fortessa (Becton Dickinson, Mountain View, CA), and dead cells were excluded from the analysis by gating out lower forward scatter and high propidium iodide or DAPI retaining cells.
2.3. Generation of human primary B-cell acute lymphoblastic leukemia (B-ALL)
Human B-ALL was generated by transplantation of human fetal liver-derived CD34+ cells that were transduced with retroviruses carrying MLL-AF9 fusion gene and GFP (linked by an IRES sequence) into sublethally (1.2 Gy)-irradiated NSG mice as previously described [12]. Blood cells were collected periodically and examined for leukemia cells (GFP+CD19+) by flow cytometry. GFP+ human B-ALL cells were harvested from bone marrow (BM) and spleen when the mice becoming moribund and cryopreserved at liquid nitrogen until use. Human B-ALL cells were characterized further by flow cytometric analysis using the following mAbs: anti-human CD45, CD19, CD20, CD10, IgM, IgD, CD44, CD33, HLA-DR, HLA-A/B/C; anti-mouse CD45 and Ter119; and isotype control mAbs.
3. Humanized mouse preparation
NSG mice were conditioned with sublethal (1.2 Gy) total body irradiation (TBI), and transplanted intravenously (i.v.) with human CD34+ FLCs (1 × 105/mouse) and FTHY fragment measuring about 1 mm3 (under mouse kidney capsule) from the same fetal donor, as previously described [13,14]. Levels of human hematopoietic cells in hu-mice were determined by flow cytometric analysis using various combinations of the following mAbs: anti-human CD45, CD3, CD4, CD8, CD45RA, CD45RO, and CD19; anti-mouse CD45 and Ter119. The hu-mice were used as donor mice to provide human T cells for preparing CAR T cells (see below) and as recipients for preparing leukemic hu-mice when a significant human immune cell reconstitution was established (approximately 10–12 weeks after humanization). All hu-mice within this experiment were made with human thymic fragments and CD34+ FLCs from a single fetus and randomly grouped.
3.1. Human CAR T cell preparation and in vitro expansion
Human T cells were isolated from hu-mouse spleens by MACS using anti-human CD3 micro-beads (Miltenyi Biotech, Aubum, CA), and then transduced with anti-CD19 CAR- or control CAR-expressing lentiviral or retroviral particles as previously described [7,15]. Two different anti-CD19 CARs were used: one consisting of anti-CD19 single chain antibody with 4-1BB costimulatory and GFP genes (19/BBz/GFP) [15]; and the other consisting of anti-CD19 single chain antibody with CD28 costimulatory genes (19/28z) [1]. The control vectors for 19/BBz/GFP and 19/28z express GFP or 4H11/28z (specific to an ovarian carcinoma antigen [16]), respectively, in the corresponding plasmid backbone. The transduced T cells were identified using flow cytometry by GFP expression, or by staining with anti-idiotype antibodies that detect the CD19-targeted CAR 19/28z (clone 19E3) or the ovarian tumor-antigen targeted CAR 4H11/28z (clone 22G3) as previously reported [7,17]. The virally transduced human T cells were then expanded for about 2 weeks using a previously described protocol with some modifications [18,19]. Briefly, purified T cells were cultured in medium containing pooled allogeneic PBMCs (4 × 106/mL, 40 Gy irradiated) and Epstein-Barr virus-transformed lymphoblastoid cell lines (EBV-LCL, 4 × 105/mL, 60 Gy irradiated), 100 ng/mL anti-huCD3 (OKT3), 50 U/mL IL-2 (R&D, Minneapolis, MN), 5 ng/mL IL-7 (R&D, Minneapolis, MN) and 10 ng/mL IL-15 (R&D, Minneapolis, MN). The expanded T cells were used immediately in adoptive immunotherapy in leukemic hu-mice.
3.2. Leukemic hu-mouse preparation and CAR T cell therapy
Hu-mice with established human immune system (by co-transplantation of human thymus and CD34+ FLCs; as described above) were used to prepare leukemic hu-mice by injection of B-ALL cells (1 × 105/mouse; i.v.), which were generated using MLL-AF9-transduced CD34+ FLCs from the same donor from which tissues/cells were used to make the hu-mice. These leukemic hu-mice were then infused with anti-CD19 or control CAR T cells (5 × 106/ mouse; i.v.) 1–2 weeks after B-ALL cell injection. Levels of CAR T cells and human B-ALL cells were determined as described above; normal human hematopoietic and lymphoid cells were examined by flow cytometric analysis using various combinations of the following mAbs against human (CD45, CD3, CD4, CD8, CD19, CD25, and FoxP3) and mouse (CD45 and Ter119) antigens. Plasmas were harvested for cytokine detection by luminex assay as previously described [7].
3.3. Statistical analysis
The level of significant differences in group means was determined by the Student's t-test for parametric data sets. The overall difference between groups was determined by one-way ANOVA with repeated measures. All analysis was performed using Prism 6 (GraphPad Software). A P value of ≤0.05 was considered significant in all analyses.
4. Results
4.1. Modeling anti-CD19 CAR T cell therapy in humanized mice without allogeneic or xenogeneic immune responses
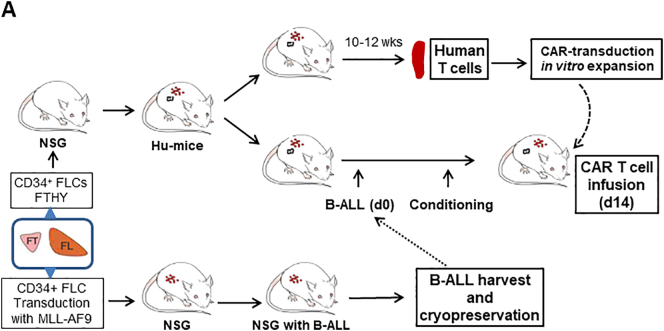
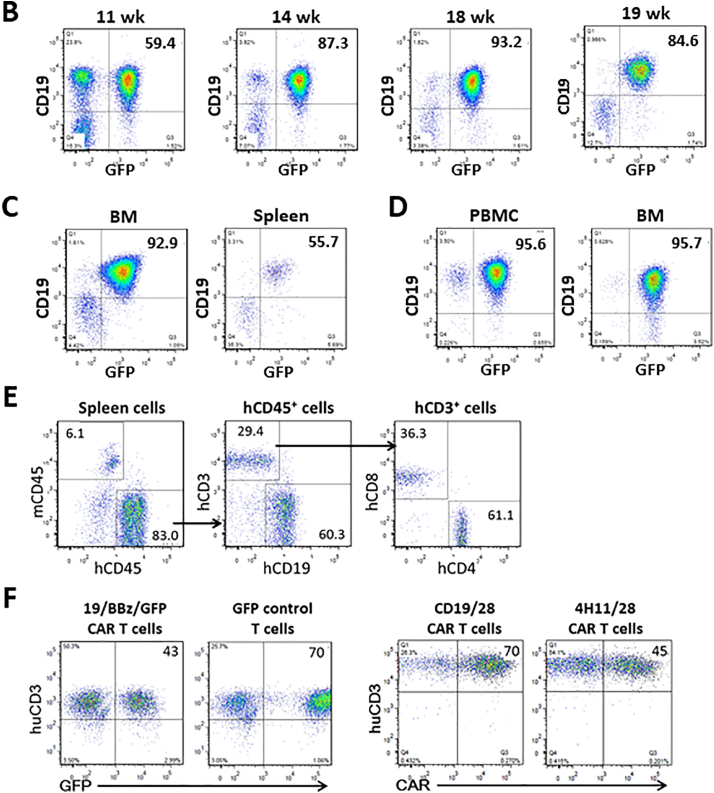
Fig. 1A summarizes the experimental strategy. We first created human B-cell lymphoblastic leukemia (B-ALL) mice by injection of fetal liver-derived CD34+ cells (CD34+ FLCs) that were transduced with retrovirus containing a mixed-lineage leukemia (MLL) fusion gene MLL-AF9 into sublethally-irradiated NSG mice [12,19]. The transplanted NSG mice showed a gradual increase in the levels of CD19+GFP+ human B-ALL cells (Fig. 1B). Bone marrow and spleen cells from the moribund mice (around 19 weeks) were composed largely of CD19+GFP+ leukemia cells (Fig. 1C), which were transplantable in sublethally (1.2 Gy)-irradiated NSG mice (Fig. 1D). Although small numbers of CD19−GFP+ cells were detected (Fig. 1C,D), these cells were CD33− (Fig. S1), consistent with previous report [19]. To perform CAR T cell therapy in immunocompetent recipients and avoid both allogeneic and xenogeneic (anti-mouse) immune responses, we used FTHY and CD34+ FLCs from the same fetus from which CD34+ FLCs were used to make the leukemic hu-mice, in constructing hu-mice with a functional human immune system (Fig. 1A). Two of these hu-mice were sacrificed around 10–12 weeks later when significant human immune cell reconstitution was established (Fig. 1E), and splenic human CD3+ T cells were isolated, transduced with viral vectors encoding human CD19-sepecific CAR, and expanded in vitro for about 2 weeks prior to infusion into leukemic hu-mouse recipients. To firmly the potential of this leukemic hu-mouse model for evaluating the antitumor responses of CAR T cells, we assessed the effects of CAR T cells made by transduction with two different viral constructs in leukemic hu-mice with or without conditioning by cyclophosphamide. One is a lentiviral construct consisting of anti-CD19 single chain antibody with 4-1BB costimulatory and GFP genes (19/BBz/GFP) [15], and the other is a retroviral construct consisting of anti-CD19 single chain antibody with CD28 costimulatory genes (19/28z) [1]. The control vectors for 19/BBz/GFP and 19/28z express GFP or 4H11/28z (specific to an ovarian carcinoma antigen [16]), respectively, in the corresponding plasmid backbone. All these viral constructs demonstrated efficient transduction of hu-mouse-derived human T cells (Fig. 1F). The remaining hu-mice were used to prepare leukemic hu-mice by injection (i.v.) of autologous B-ALL cells 2 weeks prior to infusion of CAR T cells (Fig. 1A). Because human immune cells and leukemia cells were derived from the same human fetal liver-derived CD34+ hematopoietic stem cells, these cells are considered autologous. Furthermore, human T cells from hu-mice, which are tolerant of recipient mouse xenoantigens [20,21], were used to generate CAR-T cells (Fig. 1A). Therefore, this model permits the exploration of CAR T cell therapy in the absence of both allogeneic and xenogeneic immune responses.
Fig. 1.
Modeling anti-CD19 CAR T cell therapy in humanized mice without allogeneic or xenogeneic immune responses. (A) Schematic outline of the experimental design. (B—C) Representative FACS profiles showing the presence of human CD19+GFP+ leukemia cells in blood (PBMC) at the indicated times (B), and in the bone marrow (BM) and spleen at week 19 (C) from NSG mice receiving MLL-AF9-transduced CD34+ FLCs. (D) FACS profiles showing the presence of human CD19+GFP+ leukemia cells in PBMC and BM from a representative NSG mouse receiving GFP+ leukemia cells from the primary leukemic mice 15 weeks after adoptive transfer. (E-F) For each experiment, we sacrificed 2 hu-mice for generating CAR T cells. From two hu-mice we could obtain 1.5 × 107 or more human CD3+ T cells, and from which we could generate >15 × 107 CAR T cells after transduction and expansion (sufficient for >30 mice at the dose of 5 × 106 per mouse). Human CD3+ T cells were isolated from hu-mouse spleens by FACS and transduced with anti-CD19 CAR or control viruses. (E) FACS profiles showing human immune cell chimerism in the spleens of hu-mice. (F) Transduced hu-mouse splenic CD3+ T cells were expanded in vitro for 2 weeks and analyzed for the expression of 19/BBz/GFP CAR and GFP (Left), or CD19/28 CAR and control 4H11/28 CAR (Right). Numbers on the figure indicate the percentages of positively-stained cells.
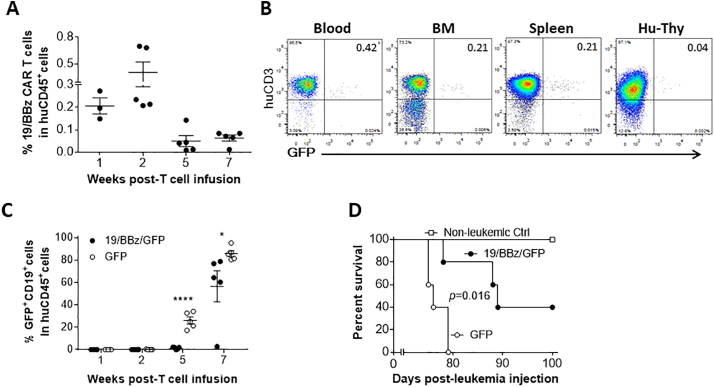
We first evaluated anti-leukemia responses in leukemic hu-mice that were conditioned with 2 injections of cyclophosphamide (50 mg/kg per day; i.v.) 4 and 3 days prior to infusion of 19/BBz/GFP CAR T cells or control T cells transduced with GFP only (Fig. 2). The levels of 19/BBz/GFP CAR T (i.e., CD3+GFP+) cells in blood peaked around 2 weeks and gradually declined thereafter (Fig. 2A), with both the levels and kinetics similar to those seen in patients receiving anti-CD19 CAR T cell therapy [7]. 19/BBz/GFP CAR T cells were also detected in bone marrow and spleen, and at a very low level in the human thymic graft (Fig. 2B). Infusion of 19/BBz/GFP CAR T cells achieved significant anti-leukemia responses, as shown by significantly lowered leukemia burdens (Fig. 2C) and prolonged survival time (Fig. 2D) compared to those receiving control T cells.
Fig. 2.
Survival and antitumor responses of 19/BBz/GFP CAR T cells in cyclophosphamide-conditioned hu-mice with autologous human immune system and B-ALL. Leukemic hu-mice were treated with 19/BBz/GFP CAR- or GFP-transduced human T cells 2 weeks after injection of human B-ALL cells (n = 5 per group). All mice were conditioned with 2 injections of cyclophosphamide (50 mg/kg per day; i.v.) 4 and 3 days prior to CAR T cell therapy. (A) Levels (%) of 19/BBz/GFP (CD3+GFP+) T cells in PBMCs (each symbol represents an individual mouse). (B) Representative FACS profiles for 19/BBz/GFP CAR T cells in the indicated tissues 3 weeks after infusion of 19/BBz/GFP CAR T cells. (C) Levels (%) of human CD19+GFP+ leukemia cells in PBMCs (each symbol represents an individual mouse). (D) Survivals of leukemic hu-mice treated with 19/BBz/GFP CAR T cells or GFP-transduced control human T cells. The data shown are from one of two independent experiments with similar results.
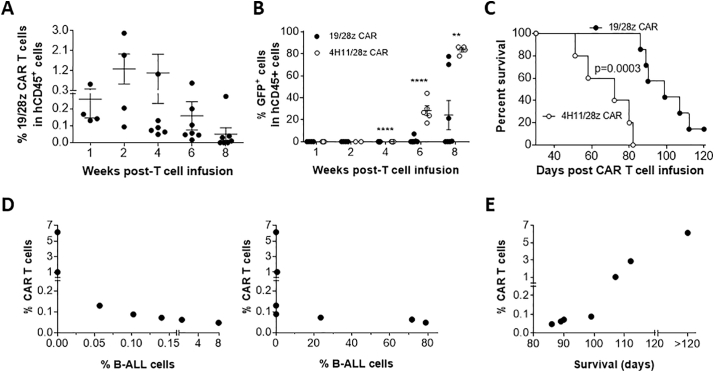
Similar results were observed in leukemic hu-mice that were infused with 19/28z CAR T cells without prior conditioning (Fig. 3). Again, anti-CD19 CAR T cells were detected and peaked around 2 weeks in blood (Fig. 3A), and infusion of 19/28z CAR T cells achieved significant anti-leukemia responses, as shown by significantly lowered leukemia burdens (Fig. 3B) and prolonged survival time (Fig. 3C) compared to the controls treated with 4H11/28z CAR T cells. Furthermore, higher levels of anti-CD19 CAR T cells were associated with lower burdens of leukemia cells (Fig. 3D) and longer survival times (Fig. 3E). Although CD19−GFP+ cells were detectable, the majority of GFP+ leukemia cells were CD19+ in both 19/28z and 4H11/28z CAR T cell-treated mice, and there was no difference in the ratios of CD19−GFP+ or CD19+GFP+ leukemia cells between two groups (Fig. S2), suggesting that the CD19−GFP+ leukemia cells were likely derived from CD19+GFP+ leukemia cells and/or may gain CD19 expression.
Fig. 3.
Survival and antitumor responses of 19/28z CAR T cells in hu-mice with autologous human immune system and B-ALL. Hu-mice were injected with B-ALL cells followed two weeks later by infusion of 19/28z (n = 7) or control 4H11/28z (n = 5) CAR-transduced T cells. (A) Levels (%) of 19/28z CAR T cells in PBMCs (each symbol represents an individual mouse). (B) Levels (%) of GFP+ leukemia cells in PBMCs (each symbol represents an individual mouse). (C) Survivals of leukemic hu-mice treated with 19/28z or 4H11/28z CAR T cells. (D) Correlation between the level (%) of CAR T cells at week 4 and the levels of CD19+GFP+ B-ALL cells at weeks 6 (Left) and 8 (Right) in peripheral blood of leukemic hu-mice treated with 19/28z CAR T cells (n = 7). (E) Correlation between the level (%) of CAR T cells at week 4 and the survival times of leukemic hu-mice treated with 19/28z CAR T cells (n = 7). The data shown are from one of two independent experiments with similar results.
4.2. Infusion of anti-CD19 CAR T cells results in a rapid decrease in normal B cells
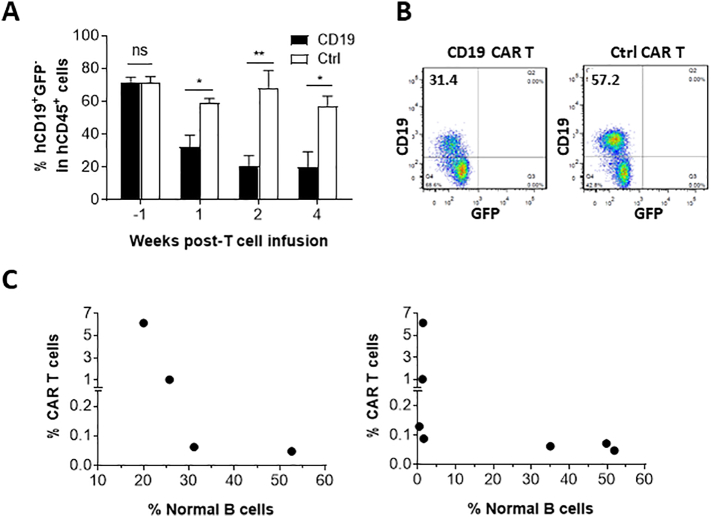
We also compared the levels of non-leukemic human B cells (i.e., CD19+GFP− cells) between leukemic hu-mice receiving anti-CD19 CAR (19/28z) T cells and those injected with control CAR (4H11/28z) T cells. Flow cytometry analysis revealed that anti-CD19 CAR T cell infusion was associated with a significant decrease in the levels of normal human B cells. Anti-CD19 CAR T cell-treated mice showed a rapid and significant reduction in blood CD19+GFP− cells by 1 week post-CAR T cell infusion (Fig. 4A,B). The levels of normal B cells were inversely associated with the levels of anti-CD19 CAR T cells in leukemia mice infused with anti-CD19 CAR T cells (Fig. 4C). Thus, the levels of normal human B cells provide a potential early indicator of CAR T cell-mediated immune responses.
Fig. 4.
Infusion of anti-CD19 CAR T cells results in a rapid decrease in normal human B cells. (A) Levels (%) of human CD19+GFP− B cells in PBMCs at the indicated times after infusion of 19/28z (n = 7) or control (4H11/28z; n = 5) CAR T cells. (B) Representative FACS profiles for human CD19+ (GFP−) B cells in PBMCs (at week 1) from anti-CD19 CAR or control T cell-treated leukemic hu-mice. (C) Correlation between the level (%) of anti-CD19 CAR T cells at week 4 and the levels (%) of normal human CD19+GFP− B cells at weeks 1 (Left) and 4 (Right) after infusion of anti-CD19 (19/28z) CAR T cells (each symbol represents an individual mouse). The data shown are from one of three independent experiments with similar results.
5. Anti-CD19 CAR T cell therapy associated changes in regulatory T cells and cytokine production
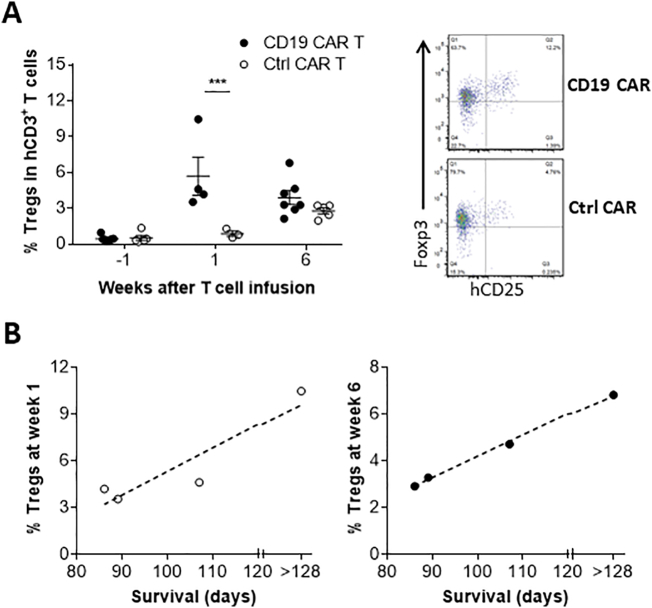
To determine the overall immune responses associated with anti-CD19 CAR T cell treatment, we compared the levels of regulatory T cells (Tregs) and cytokine production between anti-CD19 and control CAR T cell-injected leukemic hu-mice. A significant early increase in the levels of CD4+CD25+FoxP3+ Tregs was detected in leukemic hu-mice treated with 19/28z CAR T cells compared to those receiving control 4H11/28z CAR T cells (Fig. 5A). Interestingly, the levels of Tregs were positively correlated with the survival times in anti-CD19 CAR T cell-treated mice (Fig. 5B), suggesting that the observed increase in Tregs is likely to be a consequence of immune responses induced by anti-CD19 CAR T cells. In agreement with this possibility, an increase in Tregs was found during acute allograft rejection [[22], [23], [24]].
Fig. 5.
Anti-CD19 CAR T cell therapy-associated changes in Tregs. (A) Levels (%; Left) and representative staining profiles (CD25 and Foxp3 expression in gated human CD3+CD4+ cells at week 6; Right) of Tregs in PBMCs of anti-CD19 (19/28z) or control (4H11/28z) CAR T cell-treated leukemic hu-mice. (B) Correlation between the survival times of leukemic hu-mice and their levels (%) of human Tregs in PBMCs at weeks 1 (Left) and 6 (Right) after infusion of anti-CD19 (19/28z) CAR T cells. Each symbol represents an individual mouse and 4–7 mice per group were examined at each time point.
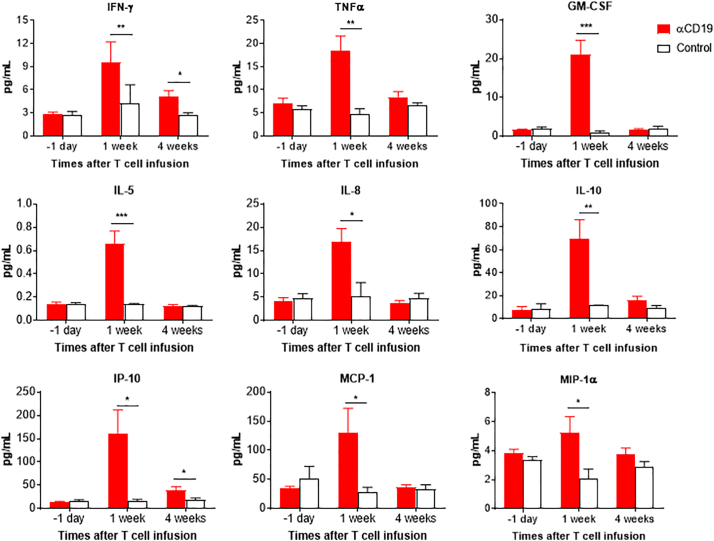
Plasma were collected from the leukemic hu-mice 1 day prior to, and weeks 1 and 4 after infusion of CAR T cells, and the levels of human cytokines were measured by luminex assay as previously described [7]. Leukemic hu-mice treated with anti-CD19 CAR T cells showed a significant increase in serum levels of multiple T-cell and monocyte/macrophage-derived human cytokines/chemokines, including IFN-γ, TNFα, GM-CSF, IL-5, IL-8, IL-10, IP-10, MCP-1, and MIP-1α, compared to control CAR T cell-injected mice (Fig. 6). For all these cytokines, the highest levels were seen at week 1, revealing that the production of these cytokines occurred rapidly following infusion of anti-CD19 CAR T cells. These results indicate that not only the infused CAR T cells, but also the recipient monocytes/macrophages are important in cytokine production following CAR T cell therapy, similar to previous reports for patients [7,25].
Fig. 6.
Anti-CD19 CAR T cell therapy-induced cytokine production. Shown are serum levels of the indicated human cytokines in leukemic hu-mice at the indicated times after infusion of anti-CD19 (19/28z; n = 7) or control (4H11/28z; n = 5) CAR T cells. The data shown are from one of two independent experiments with similar results.
6. Discussion
In this study, we report a useful hu-mouse model that permits modeling of anti-CD19 CAR T cell immunotherapy in a more clinically-relevant setting. An important advantage of this model, over previously published models, is that the recipient hu-mice have a functional human lymphohematopoietic system consisting of multilineage human immune cells and autologous primary B-ALL driven by a patient-derived fusion MLL-AF9 oncogene, thus permitting the evaluation of antitumor responses in immunocompetent hosts. Another unique feature of this model is the use of anti-CD19 CAR-expressing human T cells without allogeneic responses against the human components (either normal or malignant human cells) in the hu-mouse recipients, and without anti-mouse xenoreactivity. These features make this model highly valuable for mechanistic and preclinical studies of anti-CD19 CAR T cell adoptive therapy. The level of leukemia cells and the survival time of the leukemic recipients are two important indicators of the antitumor effects in patients receiving CAR T cell therapy. In our model, anti-CD19 CAR T cells were detected in the peripheral blood with kinetics and levels similar to those seen in patients receiving anti-CD19 CAR T cell therapy [7]. Furthermore, the level of CAR T cells correlated positively with survival times and inversely with the burden of leukemia cells in anti-CD19 CAR T cell-treated leukemic hu-mice, consistent with the results of clinical studies [1,2].
The host immune cells and microenvironment play a critical role in cancer immunotherapy. Although PDX models make it possible to study primary human leukemia, the lack of human immune cells in the recipient mice limits their application. Unlike conventional PDX models, our leukemic hu-mice have a functional human immune system consisting of multi-lineages of human immune cells, including T cells, B cells and antigen-presenting dendritic cells. In these hu-mice, we were able to monitor the changes in normal human B cells following CAR T cell therapy. Like patients treated with anti-CD19 CAR T cells [1,2,[26], [27], [28]], the antitumor response was closely associated with destruction of normal human B cells in the leukemic hu-mice following infusion of anti-CD19 CAR T cells. It appeared that the level of human B cells closely paralleled the level of B-ALL cells, and correlated inversely with the level of anti-CD19 CAR T cells. Thus, in our model, the levels of normal human B cells can be used as another informative and clinically relevant parameter for evaluating the efficacy of anti-CD19 CAR T cell therapy.
Another advantage of using leukemic hu-mice with a functional autologous immune system is the possibility of assessing CAR T cell infusion-associated cytokine production by both the infused and host immune cells, and assessing changes in host immune regulatory cells. We observed a significant increase in not only T-cell-derived cytokines, but also multiple cytokines produced by recipient human monocyte/macrophages-derived human cytokines/chemokines, consistent with what has been reported for patients [7,25]. We also detected a rapid increase in Tregs in anti-CD19 CAR T cell-treated leukemic hu-mice. A similar increase in Tregs was also reported in patients treated with anti-CD19 CAR T cells [7]. Although the present study did not delve into the underlying mechanisms, our data provide a proof-of-principle for using this model to characterize the host immunological changes associated with adoptive CAR T cell immunotherapy and the impact of these changes on the therapeutic outcome.
Cytokine-release syndrome (CRS) is one of the reported toxicities associated with anti-CD19 CAR T cell therapy. Considering the limited cross-reactivity of some proinflammatory human cytokines with their corresponding mouse cytokine receptors, hu-mice may not be ideal for exploring CRS-related toxicities. However, hu-mice are still highly useful in understanding cytokine production following CAR T cell infusion. Recent studies showed that human monocyte-derived IL-1 and IL-6 (both are reactive with mouse cells [29,30]) are responsible for CRS and neurotoxicity in anti-CD19 CAR T cell-treated hu-mice, which were made by injection of human CD34+ cells into triple transgenic NSG mice expressing human stem cell factor, GM-CSF and IL-3 (SGM3) [31]. The lack of these toxicities in similarly treated NSG hu-mice suggests that the transgenic human cytokines are likely required for the significantly elevated IL-1 and IL-6 production in SGM3 hu-mice following infusion of anti-CD19 CAR T cells. In support of this possibility, significant human monocyte/macrophage activation and cytokine production (including IL-1 and IL-6) were detected in similarly humanized SGM3, but not NSG mice (Yoshihara S and Yang YG; Unpublished data) [32]. Considering that GM-CSF, a cytokine produced by a variety of immune and nonimmune cells, plays an important role in macrophage and T cell activation, and that its overexpression leads to severe inflammation [33], this cytokine is likely involved in the development of CRS in the humanized SGM3 mice. Here we show that infusion of anti-CD19 CAR T cells induces GM-CSF production, while IL-6 was below the detectable level in most mice. Elevated GM-CSF production was also reported for patients following anti-CD19 CAR T cell therapy [25]. Our data support a recent work in which GM-CSF inhibition was found to abrogate CRS and neuroinflammation [34]. Together, these studies indicate that GM-CSF may play a critical role in CRS following CAR T cell therapy, and provide an explanation why severe toxicities and death still occur from CRS despite the routine use of IL-6 receptor antagonists [35].
We acknowledge that the hu-mouse host environment is not identical to that of a human host. However, previous studies by our group and others have produced sufficient evidence demonstrating the value and feasibility of using hu-mice made of human fetal thymus and CD34+ FLCs to study human immunity [13,14,20,[36], [37], [38]]. These hu-mice show robust reconstitution of human lymphoid and hematopoietic cells, development of secondary lymphoid organs consisting of mainly human immune cells, and the capability to mount antigen-specific T cell and antibody responses to protein antigens [14], viral antigens [37], autoantigens [20], and allogeneic and xenogeneic cells [13,36,38]. In the present study, we extend this model to a new hu-mouse model with both a human immune system and autologous primary human leukemia, and demonstrate its feasibility in modeling anti-CD19 CAR T cell immunotherapy in an autologous setting. These leukemic hu-mice offer a useful model that permits in vivo characterization of antitumor responses against autologous human leukemia in immunocompetent hosts without allogeneic or xenogeneic immune responses.
Authorship
Contribution: CHJ, JX, SR, XH, and XZ performed experiments; CHJ, JX, SR, XZ, RJB, and YGY designed experiments and analyzed data; YGY conceived the research project and directed the research; CHJ, JX and YGY wrote the manuscript; all authors edited and approved the manuscript.
Conflict-of-interest disclosure
The authors declare no competing financial interests.
Acknowledgments
The authors thank Siu-hong Ho for help with flow cytometry and Dr. John E. Dick for providing MLL-AF9 vector. This work was supported by grants from the Ministry of Science and Technology of China (2015CB964400), National Institute of Health (CA205426), and the Natural Science Foundation of China (91642208). Flow Cytometric analysis was performed in the CCTI Flow Cytometry Core funded in part through an NIH Shared Instrumentation Grant (1S10RR027050).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.12.013.
Appendix A. Supplementary data
Supplementary material
References
- 1.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177) doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park J.H., Riviere I., Gonen M., Wang X., Senechal B., Curran K.J. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sotillo E., Barrett D.M., Black K.L., Bagashev A., Oldridge D., Wu G. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson H.J., Brentjens R.J. Overcoming antigen escape with CAR T-cell therapy. Cancer Discov. 2015;5(12):1238–1240. doi: 10.1158/2159-8290.CD-15-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neelapu S.S., Tummala S., Kebriaei P., Wierda W., Gutierrez C., Locke F.L. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95) doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brentjens R.J., Latouche J.-B., Santos E., Marti F., Gong M.C., Lyddane C. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 9.Pegram H.J., Lee J.C., Hayman E.G., Imperato G.H., Tedder T.F., Sadelain M. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne A.T., Alférez D.G., Amant F., Annibali D., Arribas J., Biankin A.V. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17:254. doi: 10.1038/nrc.2016.140. [DOI] [PubMed] [Google Scholar]
- 11.Qin H., Cho M., Haso W., Zhang L., Tasian S.K., Oo H.Z. Eradication of B-ALL using chimeric antigen receptor–expressing T cells targeting the TSLPR oncoprotein. Blood. 2015;126(5):629–639. doi: 10.1182/blood-2014-11-612903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barabé F., Kennedy J.A., Hope K.J., Dick J.E. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316(5824):600–604. doi: 10.1126/science.1139851. [DOI] [PubMed] [Google Scholar]
- 13.Lan P., Tonomura N., Shimizu A., Wang S., Yang Y.-G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34<sup>+</sup> cell transplantation. Blood. 2006;108(2):487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 14.Tonomura N., Habiro K., Shimizu A., Sykes M., Yang Y.-G. Antigen-specific human T-cell responses and T cell–dependent production of human antibodies in a humanized mouse model. Blood. 2008;111(8):4293–4296. doi: 10.1182/blood-2007-11-121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tammana S., Huang X., Wong M., Milone M.C., Ma L., Levine B.L. 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies. Hum Gene Ther. 2010;21(1):75–86. doi: 10.1089/hum.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chekmasova A.A., Rao T.D., Nikhamin Y., Park K.J., Levine D.A., Spriggs D.R. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res. 2010;16(14):3594–3606. doi: 10.1158/1078-0432.CCR-10-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rafiq S., Yeku O.O., Jackson H.J., Purdon T.J., van Leeuwen D.G., Drakes D.J. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36(9):847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudley M.E., Wunderlich J.R., Robbins P.F., Yang J.C., Hwu P., Schwartzentruber D.J. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia J., Hu Z., Yoshihara S., Li Y., Jin C.-H., Tan S. Modeling human leukemia immunotherapy in humanized mice. EBioMedicine. 2016;10:101–108. doi: 10.1016/j.ebiom.2016.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan S., Li Y., Xia J., Jin C.-H., Hu Z., Duinkerken G. Type 1 diabetes induction in humanized mice. Proc Natl Acad Sci. 2017;114(41):10954–10959. doi: 10.1073/pnas.1710415114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalscheuer H., Onoe T., Dahmani A., Li H.W., Holzl M., Yamada K. Xenograft tolerance and immune function of human T cells developing in pig thymus xenografts. J Immunol. 2014;192(7):3442–3450. doi: 10.4049/jimmunol.1302886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veronese F., Rotman S., Smith R.N., Pelle T.D., Farrell M.L., Kawai T. Pathological and clinical correlates of FOXP3+ cells in renal allografts during acute rejection. Am J Transplant. 2007;7(4):914–922. doi: 10.1111/j.1600-6143.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 23.Bestard O., Cruzado J.M., Rama I., Torras J., Goma M., Seron D. Presence of FoxP3+ regulatory T Cells predicts outcome of subclinical rejection of renal allografts. J Am Soc Nephrol. 2008;19(10):2020–2026. doi: 10.1681/ASN.2007111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boix-Giner F., Millan O., San Segundo D., Munoz-Cacho P., Mancebo E., Llorente S. High frequency of central memory regulatory T cells allows detection of liver recipients at risk of early acute rejection within the first month after transplantation. Int Immunol. 2016;28(2):55–64. doi: 10.1093/intimm/dxv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teachey D.T., Lacey S.F., Shaw P.A., Melenhorst J.J., Maude S.L., Frey N. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6(6):664–679. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brentjens R.J., Riviere I., Park J.H., Davila M.L., Wang X., Stefanski J. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enomoto T., Sugawa H., Kosugi S., Inoue D., Mori T., Imura H. Prolonged effects of recombinant human interleukin-1 alpha on mouse thyroid function. Endocrinology. 1990;127(5):2322–2327. doi: 10.1210/endo-127-5-2322. [DOI] [PubMed] [Google Scholar]
- 30.Manz M.G. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity. 2007;26(5):537–541. doi: 10.1016/j.immuni.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 32.Wunderlich M., Stockman C., Devarajan M., Ravishankar N., Sexton C., Kumar A.R. A xenograft model of macrophage activation syndrome amenable to anti-CD33 and anti-IL-6R treatment. JCI Insight. 2016;1(15) doi: 10.1172/jci.insight.88181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y., Liu C.H., Roberts A.I., Das J., Xu G., Ren G. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell Res. 2006;16(2):126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 34.Sterner R.M., Sakemura R., Cox M.J., Yang N., Khadka R.H., Forsman C.L. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2018 doi: 10.1182/blood-2018-10-881722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frey N.V., Levine B.L., Lacey S.F., Grupp S.A., Maude S.L., Schuster S.J. Refractory cytokine release syndrome in recipients of chimeric antigen receptor (CAR) T cells. Blood. 2014;124(21):2296. [Google Scholar]
- 36.Lan P., Wang L., Diouf B., Eguchi H., Su H., Bronson R. Induction of human T-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood. 2004;103(10):3964–3969. doi: 10.1182/blood-2003-10-3697. [DOI] [PubMed] [Google Scholar]
- 37.Melkus M.W., Estes J.D., Padgett-Thomas A., Gatlin J., Denton P.W., Othieno F.A. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12(11):1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 38.Rong Z., Wang M., Hu Z., Stradner M., Zhu S., Kong H. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell. 2014;14(1):121–130. doi: 10.1016/j.stem.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material