Abstract
A major component of the neuroendocrine system is the hypothalamus-pituitary adrenal (HPA) axis. HPA axis genes are also known to play a role in placental physiology. Thus, disruptions in the signaling of HPA axis-associated genes may adversely impact the placenta as well as fetal development, with adverse consequences for health and development of the child. In support of this, recent studies have shown that placental epigenetic methylation of HPA axis genes has an impact on infant behavior. In this study, we evaluated CpG methylation of 14 placental HPA axis-associated genes from a subcohort (n=228) of the Extremely Low Gestational Age Newborns (ELGAN) cohort in relation to cognitive function in mid-childhood (e.g. 10 yrs). Multivariable logistic regression revealed that placental CpG methylation of 10 HPA-axis associated genes were significantly associated with cognition at age 10. Specifically, placental CpG methylation levels of the glucocorticoid receptor gene, Nuclear Receptor Subfamily Group 3 C Member 1 (NR3C1) and Brain-derived Neurotropic Factor (BDNF) were significantly associated with increased odds in developing moderate/severe adverse cognitive impairment at age 10. Methyl-CpG Binding Protein 2 (MECP2) was the major transcriptional regulator of the ten identified HPA genes. The data suggest that placental CpG methylation is associated with cognitive outcomes in mid-childhood.
Keywords: HPA axis, placenta, CpG DNA methylation, epigenetics
Introduction
The neuroendocrine system serves as an interface between the brain and many of the peripheral endocrine systems and is integral for maintaining homeostasis throughout the body (Tsigos and Chrousos, 2002). Exposure to exogenous chemicals such as endocrine disrupting compounds (EDCs) in the environment may alter the neuroendocrine system resulting in detrimental health effects, including reproductive disorders and cancer (Toni, 2004; Uzumcu et al., 2012). One of the major components of the neuroendocrine system is the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis comprises interactions among the hypothalamus, the pituitary gland and the adrenal glands and plays a critical role in regulating physiological and biological responses to stressors (Kinlein et al., 2015). Importantly, it has been shown that components of the HPA system are sensitive to disruption by various environmental toxicants and stressors (Kitraki et al., 2015; Lee and Sawa, 2014).
Several genes are key to functioning of the HPA axis system (Lee and Sawa, 2014). Among these are Nuclear Receptor Subfamily Group 3 C Member 1 (NR3C1), FK506 Binding Protein 5 (FKPB5), and Brain-Derived Neurotrophic Factor (BDNF). NR3C1 encodes for the glucocorticoid receptor, which regulates mechanistic negative feedback actions that inhibit HPA axis activity (Keller-Wood and Dallman, 1984). FKPB5 is involved in glucocorticoid signaling (Wochnik et al., 2005). BDNF promotes synaptic plasticity and regulation of Corticotropin-Releasing Hormone (CRH) in the hypothalamus, and is involved in regulating and maintaining homeostasis in the HPA axis during times of stress (Cowansage et al., 2010; Jeanneteau et al., 2012; Naert et al., 2015). Importantly, all of these genes are critical to the brain’s defenses against stress-inducing exposures (Lee and Sawa, 2014).
In addition to their role in neuronal processes, an interesting feature is that many HPA axis-associated genes also control fetal readiness for birth, survival after birth and timing of birth (Wood and Keller-Wood, 2016). The mechanistic basis for this is that HPA axis-associated genes control multiple biological functions in the placenta such as cellular proliferation, nutrient transport, and trophoblast growth (Gao et al., 2012; Kawamura et al., 2009; Padmini et al., 2012) (Figure 1). In the context of environmental exposures, the placenta regulates gene expression and hormone production in response to exposures and thus functions as an environmentally-responsive biosensor during fetal development (Gheorghe et al., 2010). There is evidence that disruption of normal placenta physiology is associated with later life health effects. Specifically, placental physiological measures such as weight and vascularity have been linked to later life health such as cardiovascular disease and hypertension (Barker et al., 1990).
Fig. 1.

The HPA axis involves interactions among the hypothalamus, anterior pituitary, and adrenal cortex. Genes that are critical to the HPA pathway include, Brain-derived neurotrophic factor (BDNF), FK506 binding protein 5 (FKBP5), Corticotropin-releasing hormone/factor (CRF/CRH), and Glucocorticoid receptor (NR3C1). Ultimately, the HPA axis cascade results in the production of cortisol, which has the potential to cross the placental barrier. Cortisol has been shown to regulate fetal readiness for birth and infant survival. HPA-axis associated genes are also involved in trophoblast growth and proliferation as well as nutrient transport.
Furthermore, recent studies have shown that epigenetic marks (i.e. DNA methylation) in the placenta of HPA-axis genes are associated with neurobehavioral outcomes in infants (Appleton et al., 2015; Conradt et al., 2013; Monk et al., 2016; Paquette et al., 2014). The placenta is thus of great interest for study as it: (1) mediates fetal exposures to exogenous compounds, (2) regulates fetal nutrition, (3) controls the production of fetal and maternal cortisol, (4) produces additional hormones key for fetal development, and (5) is a key regulator of the fetal environment.
Because HPA axis-associated genes are known to regulate placental growth and function and these are known to influence overall fetal development, we hypothesized that placental DNA (CpG) methylation of targeted genes is predictive of later life cognitive function. To address this, we utilized placental samples from the Extremely Low Gestational Age Newborns (ELGAN) cohort to investigate the relationship between placental CpG methylation changes in HPA axis-associated genes and cognitive functioning at age 10. Our study is among the first to investigate the use of placental CpG methylation to predict later life cognitive function in mid-childhood. The data provide novel insights into potential mechanistic relationship of CpG methylation as a driver of fetal development and later life cognition in mid-childhood.
Methods
ELGANs Study Subject Recruitment and Sample Collection
Details regarding the recruitment of ELGAN participants have been discussed elsewhere (O’Shea et al., 2009). Briefly, infants born before 28 weeks of gestation at one of the 14 ELGAN sites between 2002–2004 were eligible for enrollment in the study. Participating mothers provided informed consent following admission to the hospital, before birth, or immediately following birth. Study procedures were approved by the Institutional Review Board at each of the 14 participating ELGAN sites (O’Shea et al., 2009). After recruitment, a total of 1506 infants and 1249 mothers enrolled in the ELGAN study (ELGAN1). Of 1200 ELGAN survivors, 1102 (92%) underwent clinical evaluations at age 2 years. For the second clinical evaluation at age 10, 889 returned for follow up (ELGAN2). Although placental specimens were collected from the large majority of ELGAN participants, specimens of sufficient size for epigenetic analysis include 438 children. For the current study, a total of 228 mother-infant pairs were selected from the 889 ELGAN2 cohort based on the availability of placental samples with data on CpG methylation and cognitive function. Thus, the data presented here represents a subset of placenta from children who display deficits in cognitive function and controls.
Participating women gave permission for collection of a sample of their placenta for the ELGAN study. Upon delivery, placentas were placed into a sterile exam basin and taken to the sampling room, at which point, biopsies of the placentas were collected. To expose the chorion, the amnion was pulled back using sterile technique at the midpoint of the longest distance between the cord insertion and the edge of the placental disk. A tissue sample was collected by applying traction to the chorion and the underlying trophoblast tissue and cutting a sample out at the base of this tissue structure. The tissue sample was subsequently placed into a cryo-vial that was immediately submerged in liquid nitrogen. Placental samples were shipped to the University of North Carolina at Chapel Hill for processing and were stored at −80°C prior to shipment (Onderdonk et al., 2008).
DNA extraction and assessment of DNA methylation
A small subsample of placental tissue (~0.2g) was cut from the frozen biopsy sample and rinsed with sterile 1 X PBS to wash away any residual blood. Samples were then homogenized in Buffer RLT with β -mercaptoethanol (Qiagen, Valencia CA). An AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, Valencia CA) was utilized to extract DNA and RNA sequences that were greater than 18 nucleotides in length, according to the manufacturer’s instructions. To analyze placental CpG methylation, extracted DNA sequences were bisulfate-converted using the EZ DNA methylation kit (Zymo Research, Irvine, CA) and then subsequently hybridized on the Illumina HumanMethylation 450 BeadChip© array (n=132) and the Illumina HumanMethylation850 Bead Chip array (n=96) (Illumina, Inc., San Diego, CA), which assesses the DNA methylation levels of 486,428 and 853,307 individual probes at single nucleotide resolution, respectively. Data were integrated as it has been proposed that high variability methylation sites are well conserved between the two platforms (Logue et al., 2017). Methylation levels were calculated and expressed as β values (β = intensity of the methylated allele (M) / (intensity of the unmethylated allele (U) + intensity of the methylated allele (M) + 100). Data were normalized for both arrays using the minfi package in R (Aryee et al., 2014). Specifically, image files were used to produce background-corrected and quantile normalized β-values. Subsequently, β-values with a p>0.01 were removed from analysis, leaving a total of 237 HPA-axis associated probes available for analysis. To demonstrate that the sites included in the analysis were suitable for integration, a regression analysis was performed on all HPA axis-associated gene probes (n=237). It demonstrated strong concordance of the average beta values at the tested loci (R=0.98, p<0.001) for samples analyzed on the 450k as compared to those analyzed to those on the 850k (Supplemental Figure 1).
Cognitive Function Assessment at Ten Years of Age
General cognitive ability (or IQ) was assessed with the School-Age Differential Ability Scales-II (DAS-II) Verbal and Nonverbal Reasoning scales. Two subtests from the DAS-II and five subtests from the NEPSY-II were used to assess executive function. Working memory was evaluated with the DAS-II Recall of Digits Backwards, Recall Sequential Order test. The NEPSY-II Auditory Attention and Auditory Response Set, the NEPSY-II Animal Sorting test, and the NEPSY-II Inhibition and Inhibition Switching test were utilized to examine auditory attention and set switching, concept generation and mental flexibility, and simple inhibition and inhibition shifting, respectively (Bright et al., 2017). As a unitary measure of cognitive and executive function, we used Latent Profile Analysis (LPA), which empirically identifies subgroups of children who share similar profiles on a set of measures, to classify study participants into groups based on IQ and executive functioning (Heeren et al., 2017). For the current analyses participants were classified into two previously validated distinct profile groups (Heeren et al., 2017): normal or low normal cognitive function (n=158) and moderate/severe cognitive impairment (n=70).
Statistical Analysis
Logistic regression analysis was performed in SAS (Cary, NC) to test whether methylation levels at 237 CpG sites associated with 14 HPA axis genes that predicted cognitive function at mid-childhood. These genes were selected based on their known involvement in the HPA axis and known relationship to environmental stressors (Lee and Sawa, 2014). Socioeconomic and clinical covariates were selected a priori based on known associations with both methylation and cognitive function including: race, public insurance, maternal education, sex of the infant, and gestational age. While methylation levels (β-values) are calculated as a proportion between 0 and 1, for the purposes of this analysis, β-value were adjusted to β-value* 100 in order to examine the change in the odds ratio (OR) for each percent increase in methylation. This transformation does not change the underlying distribution of the data or the sensitivity of the model. For the purposes of logistic regression, the dependent variable for this model was the binary outcome of either (i) normal or low normal cognitive function (n = 158) or (ii) moderate or severe cognitive impairment (n = 70), which were coded from four groups of no impairment, low impairment, moderate impairment, and severe impairment as derived using LPA. Sites of CpG methylation were considered to be significantly associated with moderate/severe cognitive impairment if the associated p-value was < 0.05. P-values, beta estimates, parameter-likelihood ORs, and 95% confidence intervals (C.I.) for ORs are reported.
To gain insight on the potential functional outcomes that are associated with the identified HPA axis genes, network analysis was conducted using Ingenuity Pathway Analysis (IPA) (Ingenuity Systems®, Redwood City, CA, USA). Enrichment in canonical pathways associated with selected genes and behavioral outcomes were analyzed and reported. Significance was defined as a right-tailed Fisher’s Exact test p-value < 0.0001.
Results
Study Cohort
Demographic information for the ELGAN subcohort (n=228) and the larger ELGAN2 cohort were collected at childbirth and are provided in Table 1. The data on children’s cognitive impairment was collected at age 10 as described in the Methods section above. In the ELGAN subcohort for the current study (n=228), there were 136 (60.2%) males and 92 (39.8%) females. The average maternal age was 29.8 years and the average gestational age was 25.7 weeks. In this subcohort 158 (69.3%) displayed normal or low normal cognitive function while 70 (30.7%) were classified as having moderate/severe cognitive impairment. Approximately 42% of the births in the subcohort were multigestation, and 16% of pregnancies involved assisted reproductive technology (ART). This subcohort displayed similar characteristics as the larger ELGAN2 cohort with respect to race, public insurance, maternal education, and gestational age. There is an enrichment of males in the 228 subcohort likely because of the known sexual dimorphism in neurocognitive outcomes of ELGANs (Kuban et al., 2016).
Table 1.
Study subject characteristics.
| Variable Name | ELGAN subcohort subjects (n=228) N (%) Mean (Range) |
ELGAN2 subjects (n=889) N (%) Mean (Range) |
|---|---|---|
| Infant Sex | ||
| Male | 136 (60.2%) | 471 (53.0%) |
| Female | 92 (39.8%) | 418 (47.0%) |
| Cognitive Impairment | ||
| No/Low | 158 (69.3%) | 660 (74.3%) |
| Moderate/Severe | 70 (30.7%) | 214 (24.1%) |
| Not reported | 15 (1.6%) | |
| Maternal Age | 9.8 (14.6–45.8) | 29.2 (13.2–47.3) |
| Gestational Age | 25.7 (23.0–27.6) | 25.9 (23.0–27.6) |
| Maternal Education | ||
| High School and above | 184 (80.7%) | 686 (77.2%) |
| Below High School | 32 (14.1%) | 123(13.8%) |
| Not reported | 12 (5.2%) | 80 (9%) |
| Smoking | ||
| Yes | 23 (10.1%) | 111 (12.5%) |
| No | 197 (86.4%) | 719 (80.9%) |
| Not reported | 8 (3.5%) | 59 (6.6%) |
| Race | ||
| White | 136 (59.7%) | 515 (59.1%) |
| Non-white | 92 (40.3%) | 357 (40.2%) |
| Not Reported | 17 (1.9%) | |
| Public Insurance | ||
| Yes | 76 (33.2%) | 314 (35.3%) |
| Multiple births | ||
| Yes | 97 (42.5%) | 308 (34.6%) |
| Pregnancies with assisted | ||
| reproductive technology | ||
| Yes | 37 (16%) | 144 (13%) |
Placental CpG methylation predicts children’s cognitive outcomes at age 10
A total of 14 genes comprising 237 probes were selected for analysis as they are known to play a role in the HPA axis, and have a known relationship to environmental stressors (Lee and Sawa, 2014). To test the hypothesis that placental CpG methylation of these genes is predictive of moderate/severe cognitive impairment multivariable logistic regression modeling was used across 228 placentas. These models assessed whether a 1% increase in placental methylation is associated with a change (either an increase or decrease) in the odds of moderate/severe cognitive impairment.
Of the 237 tested probes, 41 probes representing 10 HPA genes were identified to have CpG methylation that was significantly associated with moderate/severe cognitive impairment (Figure 2). Increases in CpG methylation of a probe in the TSS200 region of NR3C1 displayed the highest OR of 1.876 (CI: 1.067–3.298). The CpG probe (p =0.0012) for which the association of increased CpG methylation and moderate/severe cognitive impairment was most significant was for Heat Shock Protein 90 Alpha Family Class A Member 1 (HSP90AA1) that displayed an OR of 1.061 (CI: 1.024–1.099). Increases in CpG methylation of two probes for Corticotropin Releasing Hormone Binding Protein (CRHBP) were consistently associated with moderate/severe cognitive impairment with OR ranging from 1.029–1.534.
Fig. 2.
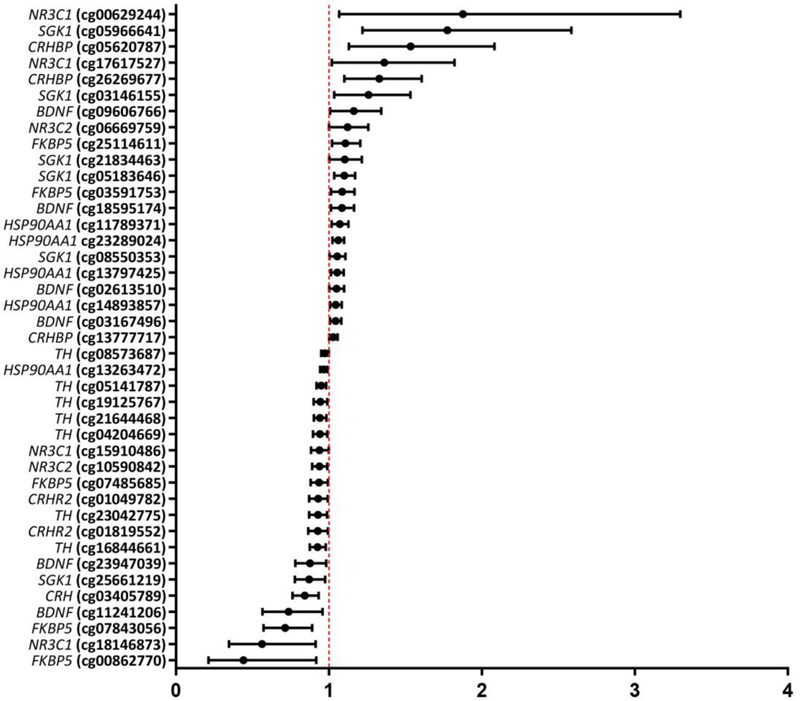
Odds ratios and 95% confidence intervals for 41 CpG probes within 1G HPA axis-associated genes that displayed an association between increased placental CpG methylation and either moderate/severe cognitive impairment or low/low normal cognitive function at age 10 years.
Several probes displayed a negative association between CpG methylation and moderate/severe cognitive impairment, thus representing methylation that was associated with reduced risk of cognitive impairment. Among the probes where increases in CpG methylation were associated with reduced risk was FKBP5 with an OR of 0.441 (CI: 0.212–0.917). The probe that displayed the most significant association (p = 0.0009) with a reduced risk was in the 3’ UTR region of Corticotropin Releasing Hormone (CRH) with an OR of 0.843 (CI: 0.762–0.933). Additionally, multiple probe sites on CRHR2 (OR: 0.927–0.930) and multiple probe sites on TH (OR: 0.926–0.974) displayed consistent negative associations between CpG methylation and moderate/severe cognitive impairment. Genomic locations of the CpG sites for all tested probes (n=237) and their CIs are provided in Supplemental Table 1. To summarize, increases in placental CpG methylation of HPA-axis related genes were associated with both higher and lower risk of cognitive impairment at age 10.
Pathway Analysis Results
To identify canonical pathways, upstream targets, and functional outcomes associated with the 10 significant genes identified network analysis was performed. For this analysis, networks were constructed based on connectivity through the literature, as enabled through IPA where over-represented pathways were determined using a right-tailed Fisher’s Exact test.
The following genes that displayed altered methylation enrich for the glucocorticoid signaling pathway FKBP5, NR3C1, NR3C2, and SGK1 (p = 7.38 • 10−6). Additionally, NR3C1 and NR3C2 enrich for the IL-4 signaling pathway (p = 6.07 • 10−4). In terms of environmental exposures, it has been postulated that environmentally-responsive transcription factors influence CpG methylation patterns (Martin and Fry, 2016). Thus, to explore enrichment for transcription factor binding sites within the genes that displayed altered CpG methylation transcriptional regulators of HPA genes were subsequently identified. Results revealed that the promoter region of the genes that displayed altered CpG methylation including TH, SGK1, CRH, BDNF, and FKBP5 were enriched for binding sites of Methyl-CpG Binding Protein 2 (MECP2) (p = 6.18 • 10−10) (Figure 3).
Figure 3:
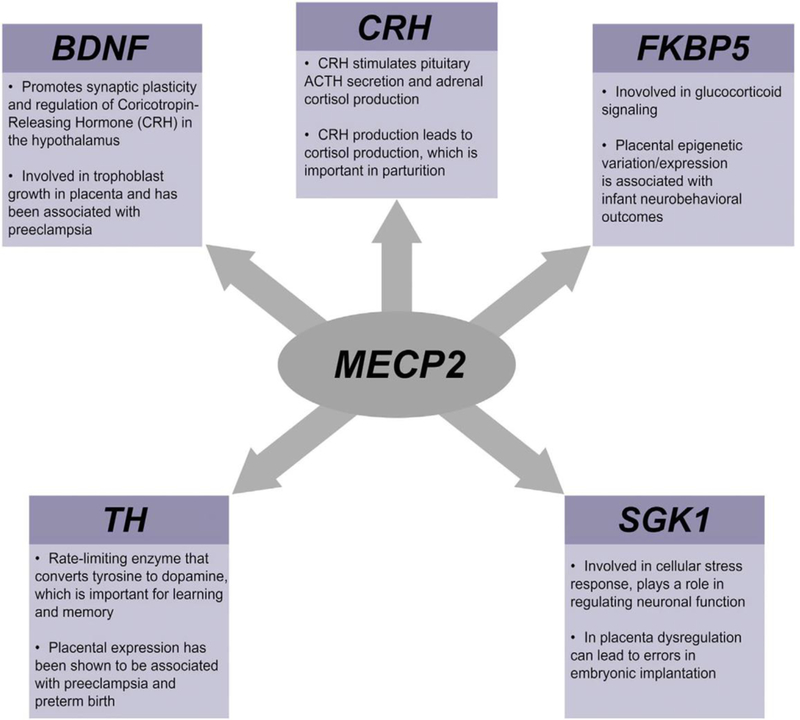
Pathway analysis identifying Methyl-CpG Binding Protein 2 (MECP2) as an enriched transcriptional regulator of HPA axis genes. The function of these genes in the placenta and the HPA axis are detailed.
Discussion
Physiological signals within the placenta such as DNA methylation, gene expression and measures of placental physiology are associated with later life disease such as cognitive impairment, cardiovascular disease, and hypertension (Barker et al., 1990; Bromer et al., 2013; Paquette et al., 2015). Because placental physiology is dependent on transcriptional signals that may be controlled through epigenetic mechanisms, we set out to examine whether placental CpG methylation is predictive of children’s cognitive outcomes at age 10 years. We analyzed 228 placentas from infants enrolled in the ELGAN cohort with a focus on 14 HPA axis-associated genes. These genes were selected for their known roles in stress response in the HPA axis as well as their roles in the placenta. Using multi-variable logistic regression modeling, the methylation levels for a set 237 probes representing 14 HPA axis genes were tested. Of these, 41 probes representing 10 HPA axis-associated genes were identified to be significantly associated with moderate/severe cognitive impairment at 10 years of age. The study is among the first to provide support for the placental epigenome as a potential biological “recording” of the in utero environment that is related to cognition at age 10.
Our findings show that increased CpG methylation in the placenta was associated with increased odds of developing moderate to severe cognitive function at age 10. This was observed for multiple probes for the gene CRHBP. In this regard it is notable that CRHBP is involved in cortisol signaling in the placenta and has been associated with early onset preeclampsia a disorder known to be associated with preterm birth (Hobel et al., 1999; Hogg et al., 2013). These data are important as they demonstrate that epigenetic marks of HPA axis genes in the placenta are associated with cognitive outcomes in midchildhood, and these epigenetic marks may have functional impacts on HPA axis function and activity.
Increased CpG methylation of some CpG sites in the placenta was associated with decreased odds of developing moderate to severe cognitive impairment at age 10. This relationship was observed for CpG sites in TH, CRH, and CRHR2. In the placenta TH has been shown to be associated with preeclampsia and preterm birth (Manyonda et al., 1998). Likewise, CRH receptors, such as CRHR2, have been postulated to be involved in glucose transport across the placenta, which plays a major role in fetal development, nutrition, and cellular signaling (Gao et al., 2012). While in this study environmental toxicant exposure during pregnancy has not yet been assessed, it is interesting to note that dioxin exposures have been associated with induction of TH mRNA levels in rodent neuronal cells and may play a role in neurological dysfunction as TH is the major rate-limiting enzyme that converts tyrosine to dopamine throughout the body (Akahoshi et al., 2009; Daubner et al., 2011). These data, in conjunction with existing literature, demonstrate the potential ability of placental CpG methylation to predict cognitive function and development in mid-childhood.
Interestingly, probes sites within three genes, namely NR3C1, BDNF, and FKBP5, displayed a unique pattern where depending on the CpG site location, increased placental methylation was associated with either reduced or increased odds for moderate/severe cognitive impairment at age 10. Differences in OR for these CpG sites could be due to the impact of the location of the methylation mark as a differential driver of gene expression (Rojas et al., 2015). For example, CpG methylation of probes within the promoter regions of genes tend to be associated with gene silencing, while methylation of probes within the gene body are associated with gene activation (Rojas et al., 2015). Related to placental function, NR3C1 is highly expressed and is thought to play a role in regulating fetal exposure to cortisol (Conradt et al., 2013). BDNF has been shown to promote trophoblast growth, and cell survival during placental development and low expression levels of BDNF in the placenta have been associated with pregnancy complications such as preeclampsia and preterm birth (D’Souza et al., 2014; Kawamura et al., 2009). Of relevance to cognitive outcomes, hypo/hyper methylation and subsequent altered expression of NR3C1 and FKBP5 in the placenta have been associated with adverse neurobehavioral outcomes (Appleton et al., 2015; Bromer et al., 2013; Conradt et al., 2013; Paquette et al., 2014; Paquette et al., 2015). In further support of our findings, hypermethylation of BDNF in hippocampal tissues and peripheral blood have been associated with depression, bipolar disorder, autism, and schizophrenia (Kundakovic et al., 2015).
In the placenta, with relevance to environmental EDCs, prenatal exposures to Bisphenol A (BPA) has been associated with increases in global methylation, which may ultimately alter expression of HPA genes, thus, altering both cognitive outcomes and placental development (Nahar et al., 2015). In relation to other tissues, it is interesting that expression levels of NR3C1, FKPB5, and BDNF are altered by exposure to BPA. For example, perinatal BPA exposures in rats induce anxiety-related disorders as a result of decreased expression in NR3C1 as well as alter methylation and expression levels of FKBP5 in hippocampal tissues (Chen et al., 2015; Kitraki et al., 2015). Additionally, studies in humans and rat blood and hippocampal tissues have confirmed that prenatal exposures to BPA alter expression as well as methylation levels, and may lead to adverse cognitive functioning (Kundakovic et al., 2015). Understanding the function that these identified genes serve in the HPA axis is important as their dysregulation has been implicated in the development of numerous cognitive outcomes and may provide evidence for potential therapeutic techniques to prevent these outcomes (de Kloet et al., 2006; Schatzberg et al., 2014).
When interpreting the results of this study, several factors should be considered. The present study focuses on CpG methylation in the placenta. Unfortunately, we do not have access to brain tissue in the ELGAN study. It is established that there are tissue-specific patterns of CpG methylation (Ghosh et al., 2010) as well as CpG sites that display conserved methylation patterns across tissues (Woodfine et al., 2011). Thus, it is not the intent of this study to convey that CpG methylation in the placenta would be similar to CpG methylation in the fetal brain. Rather, the CpG methylation in the placenta is viewed as a “biological recording” of placental signaling pathways that are critical for fetal growth and development. This work contributes to a growing body of literature showing altered placental CpG methylation HPA axis genes that are associated with altered infant behavior (Bromer et al., 2013; Conradt et al., 2013; Monk et al., 2016; Paquette et al., 2014; Paquette et al., 2015). The present study is among the first to demonstrate that methylation of HPA axis-related genes in the placenta is critically related to neurocognitive outcomes in children born preterm. It must also be noted that the ELGAN cohort consists only of preterm births. Further research must be carried out to determine the generalizability of these findings in a non-preterm cohorts. Finally, placental RNA or protein are not currently available to functionally validate the expression levels in response to increases in CpG methylation. Similarly, access to environmental exposure data was not currently available for study. Future studies should integrate mRNA and protein expression with CpG methylation and environmental exposure data to gain further insight on how CpG methylation in the placenta impacts gene expression levels.
In summary, the results of this study highlight a set of 10 HPA axis-associated genes that displayed an association between increased placental CpG methylation and either moderate/severe cognitive impairment or low/low normal cognitive function at age 10 years. Many of these genes regulate both placental function and HPA axis function. The identified genes are also known to play integral roles in memory, learning, and the development of psychological disorders and there is evidence that exposure to EDCs may influence their expression as well. Furthermore, given the plasticity of the epigenome during the prenatal period, these alterations could be influenced by exposure to environmental contaminants, including EDCs. Growing research supports that exposure to common EDCs, including estrogenic compound like BPA, may dysregulate several genes involved in regulation of the HPA axis (Kundakovic et al., 2015; Weiser and Handa, 2009). This work provides a basis for which to subsequently investigate the role of EDCs on the HPA axis. Future work should incorporate exposure data as it relates to epigenetic modifications of the HPA axis-associated genes, as these data could provide more information as to how EDCs mechanistically disrupt the HPA axis and potentially provide biomarkers for exposure and later-life cognitive impairments in mid-childhood.
Supplementary Material
ACKNOWLEDGEMENTS:
This research was supported by grants from the National Institute of Health including the Environmental Influences on Child Health Outcomes (ECHO) award (1U2COD023375, UG33OD023348 and 1UG3OD023275), the National Institute of Environmental Health Sciences (P42-ES007126, T32-ES007018, T32-ES007126, P42-ES007373, P01-ES022832), the National Institute of Neurological Disorders and Stroke (5U01NS040069 and 2R01NS040069), and from the National Institute for Occupational Safety and Health: T42/OH-008673. Further support was provided by the Wake Forest School of Medicine Innovation Pilot Grant, the Harold M and Mary Earnhardt Eagle Endowed Fund for Pediatric and Neonatal Research, and the EPA (RD83544201). The authors acknowledge Caroline Reed for her assistance with the figure.
Footnotes
Disclosure of potential conflict of interests: The authors claim no competing financial interests.
References
- Tsigos C, Chrousos GP, 2002. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53, 865–871. [DOI] [PubMed] [Google Scholar]
- Toni R, 2004. The neuroendocrine system: organization and homeostatic role. Journal of endocrinological investigation 27, 35–47. [PubMed] [Google Scholar]
- Uzumcu M, Zama AM, Oruc E, 2012. Epigenetic mechanisms in the actions of endocrine-disrupting chemicals: gonadal effects and role in female reproduction. Reproduction in domestic animals = Zuchthygiene 47 Suppl 4, 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlein SA, Wilson CD, Karatsoreos IN, 2015. Dysregulated hypothalamic-pituitary-adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Frontiers in psychiatry 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitraki E, Nalvarte I, Alavian-Ghavanini A, Ruegg J, 2015. Developmental exposure to bisphenol A alters expression and DNA methylation of Fkbp5, an important regulator of the stress response. Molecular and cellular endocrinology 417, 191–199. [DOI] [PubMed] [Google Scholar]
- Lee RS, Sawa A, 2014. Environmental stressors and epigenetic control of the hypothalamic-pituitary-adrenal axis. Neuroendocrinology 100, 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF, 1984. Corticosteroid inhibition of ACTH secretion. Endocrine reviews 5, 1–24. [DOI] [PubMed] [Google Scholar]
- Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T, 2005. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem 280, 4609–4616. [DOI] [PubMed] [Google Scholar]
- Cowansage KK, LeDoux JE, Monfils MH, 2010. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Current molecular pharmacology 3, 12–29. [DOI] [PubMed] [Google Scholar]
- Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, Chao MV, 2012. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proceedings of the National Academy of Sciences of the United States of America 109, 1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert G, Zussy C, Tran Van Ba C, Chevallier N, Tang YP, Maurice T, Givalois L, 2015. Involvement of Endogenous Brain-Derived Neurotrophic Factor in Hypothalamic-Pituitary-Adrenal Axis Activity. Journal of neuroendocrinology 27, 850–860. [DOI] [PubMed] [Google Scholar]
- Wood CE, Keller-Wood M, 2016. The critical importance of the fetal hypothalamus-pituitary-adrenal axis. F1000Res 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Lv C, Xu C, Li Y, Cui X, Gu H, Ni X, 2012. Differential regulation of glucose transporters mediated by CRH receptor type 1 and type 2 in human placental trophoblasts. Endocrinology 153, 1464–1471. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Kawamura N, Sato W, Fukuda J, Kumagai J, Tanaka T, 2009. Brain-derived neurotrophic factor promotes implantation and subsequent placental development by stimulating trophoblast cell growth and survival. Endocrinology 150, 3774–3782. [DOI] [PubMed] [Google Scholar]
- Padmini E, Venkatraman U, Srinivasan L, 2012. Mechanism of JNK signal regulation by placental HSP70 and HSP90 in endothelial cell during preeclampsia. Toxicol Mech Methods 22, 367–374. [DOI] [PubMed] [Google Scholar]
- Gheorghe CP, Goyal R, Mittal A, Longo LD, 2010. Gene expression in the placenta: maternal stress and epigenetic responses. Int J Dev Biol 54, 507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Bull AR, Osmond C, Simmonds SJ, 1990. Fetal and placental size and risk of hypertension in adult life. BMJ 301, 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton AA, Lester BM, Armstrong DA, Lesseur C, Marsit CJ, 2015. Examining the joint contribution of placental NR3C1 and HSD11B2 methylation for infant neurobehavior. Psychoneuroendocrinology 52, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E, Lester BM, Appleton AA, Armstrong DA, Marsit CJ, 2013. The roles of DNA methylation of NR3C1 and 11beta-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics 8, 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Feng T, Lee S, Krupska I, Champagne FA, Tycko B, 2016. Distress During Pregnancy: Epigenetic Regulation of Placenta Glucocorticoid-Related Genes and Fetal Neurobehavior. Am J Psychiatry 173, 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette AG, Lester BM, Koestler DC, Lesseur C, Armstrong DA, Marsit CJ, 2014. Placental FKBP5 genetic and epigenetic variation is associated with infant neurobehavioral outcomes in the RICHS cohort. PloS one 9, e104913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, Leviton A, 2009. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early human development 85, 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A, Extremely Low Gestational Age Newborns Study, I., 2008. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. American journal of obstetrics and gynecology 198, 110 e111–117. [DOI] [PubMed] [Google Scholar]
- Logue MW, Smith AK, Wolf EJ, Maniates H, Stone A, Schichman SA, McGlinchey RE, Milberg W, Miller MW, 2017. The correlation of methylation levels measured using Illumina 450K and EPIC BeadChips in blood samples. Epigenomics 9, 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, Irizarry RA, 2014. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30, 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright HR, Babata K, Allred EN, Erdei C, Kuban KCK, Joseph RM, O’Shea TM, Leviton A, Dammann O, Investigators ES, 2017. Neurocognitive Outcomes at 10 Years of Age in Extremely Preterm Newborns with Late-Onset Bacteremia. The Journal of pediatrics. [Google Scholar]
- Heeren T, Joseph RM, Allred EN, O’Shea TM, Leviton A, Kuban KCK, 2017. Cognitive functioning at age 10 years among children born extremely preterm: A latent profile approach. Pediatric research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuban KC, Joseph RM, O’Shea TM, Allred EN, Heeren T, Douglass L, Stafstrom CE, Jara H, Frazier JA, Hirtz D, Leviton A, Extremely Low Gestational Age Newborn Study, I., 2016. Girls and Boys Born before 28 Weeks Gestation: Risks of Cognitive, Behavioral, and Neurologic Outcomes at Age 10 Years. The Journal of pediatrics 173, 69–75 e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Fry RC, 2016. A cross-study analysis of prenatal exposures to environmental contaminants and the epigenome: support for stress-responsive transcription factor occupancy as a mediator of gene-specific CpG methylation patterning. Environmental epigenetics 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromer C, Marsit CJ, Armstrong DA, Padbury JF, Lester B, 2013. Genetic and epigenetic variation of the glucocorticoid receptor (NR3C1) in placenta and infant neurobehavior. Developmental psychobiology 55, 673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette AG, Lester BM, Lesseur C, Armstrong DA, Guerin DJ, Appleton AA, Marsit CJ, 2015. Placental epigenetic patterning of glucocorticoid response genes is associated with infant neurodevelopment. Epigenomics 7, 767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobel CJ, Arora CP, Korst LM, 1999. Corticotrophin-releasing hormone and CRH-binding protein. Differences between patients at risk for preterm birth and hypertension. Ann N Y Acad Sci 897, 54–65. [DOI] [PubMed] [Google Scholar]
- Hogg K, Blair JD, McFadden DE, von Dadelszen P, Robinson WP, 2013. Early onset pre-eclampsia is associated with altered DNA methylation of cortisol-signalling and steroidogenic genes in the placenta. PloS one 8, e62969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manyonda IT, Slater DM, Fenske C, Hole D, Choy MY, Wilson C, 1998. A role for noradrenaline in pre-eclampsia: towards a unifying hypothesis for the pathophysiology. British journal of obstetrics and gynaecology 105, 641–648. [DOI] [PubMed] [Google Scholar]
- Akahoshi E, Yoshimura S, Uruno S, Ishihara-Sugano M, 2009. Effect of dioxins on regulation of tyrosine hydroxylase gene expression by aryl hydrocarbon receptor: a neurotoxicology study. Environ Health 8, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubner SC, Le T, Wang S, 2011. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 508, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas D, Rager JE, Smeester L, Bailey KA, Drobna Z, Rubio-Andrade M, Styblo M, Garcia-Vargas G, Fry RC, 2015. Prenatal arsenic exposure and the epigenome: identifying sites of 5-methylcytosine alterations that predict functional changes in gene expression in newborn cord blood and subsequent birth outcomes. Toxicological sciences : an official journal of the Society of Toxicology 143, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza V, Patil V, Pisal H, Randhir K, Joshi A, Mehendale S, Wagh G, Gupte S, Joshi S, 2014. Levels of brain derived neurotrophic factors across gestation in women with preeclampsia. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 37, 36–40. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA, 2015. DNA methylation of BDNF as a biomarker of early-life adversity. Proceedings of the National Academy of Sciences of the United States of America 112, 6807–6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar MS, Liao C, Kannan K, Harris C, Dolinoy DC, 2015. In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere 124, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Zhou L, Bai Y, Zhou R, Chen L, 2015. Hypothalamic-pituitary-adrenal axis hyperactivity accounts for anxiety- and depression-like behaviors in rats perinatally exposed to bisphenol A. J Biomed Res 29, 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG, 2006. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. Journal of psychiatric research 40, 550–567. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Keller J, Tennakoon L, Lembke A, Williams G, Kraemer FB, Sarginson JE, Lazzeroni LC, Murphy GM, 2014. HPA axis genetic variation, cortisol and psychosis in major depression. Molecular psychiatry 19, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Yates AJ, Fruhwald MC, Miecznikowski JC, Plass C, Smiraglia D, 2010. Tissue specific DNA methylation of CpG islands in normal human adult somatic tissues distinguishes neural from non-neural tissues. Epigenetics 5, 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfine K, Huddleston JE, Murrell A, 2011. Quantitative analysis of DNA methylation at all human imprinted regions reveals preservation of epigenetic stability in adult somatic tissue. Epigenetics Chromatin 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ, 2009. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience 159, 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


