Summary
Inflammation and oxidative stress are major problems in peripheral nerve injury. Nanoceria can manipulate antioxidant factor expression, stimulate angiogenesis, and assist in axonal regeneration. We fabricate collagen/nanoceria/polycaprolactone (COL/NC/PCL) conduit by asymmetrical three-dimensional manufacture and find that this scaffold successfully improves Schwann cell proliferation, adhesion, and neural expression. In a 15-mm rat sciatic nerve defect model, we further confirm that the COL/NC/PCL conduit markedly alleviates inflammation and oxidative stress, improves microvessel growth, and contributes to functional, electrophysiological, and morphological nerve restoration in the long term. Our findings provide compelling evidence for future research in antioxidant nerve conduit for severe neurological defects.
Subject Areas: Biomaterials, Nanotechnology, Neurosurgery
Graphical Abstract
Highlights
-
•
Collagen/nanoceria/polycaprolactone conduit was prepared by asymmetrical fabrication
-
•
The scaffold induced proliferation, adhesion, and angiogenesis in nerve repair
-
•
The scaffold alleviated oxidative stress and inflammation in the microenvironment
Biomaterials; Nanotechnology; Neurosurgery
Introduction
Wallerian degeneration (WD) occurs at the beginning of acute peripheral nerve injury (PNI), accompanied by recruitment of macrophages. The communication between Schwann cells and macrophages helps to clear myelin debris and to initiate axon growth in the early phase of PNI. Inflammation is a double-edged sword for nerve regeneration. Contradictory effects are associated with the timing of inflammatory process in severe nerve injury. After the early phase of WD, macrophages continue to express pro-inflammatory cues that lead to the transcription of reactive oxygen species (ROS) and activation of secondary cascade of events. These events include hemorrhage, edema, inflammation via inhibition of blood flow, and release of anti-regenerative substances. The consequent effects result in the activation of the cell death machinery and compromise of the neural regeneration process.
Bioactive nerve conduit should effectively repair nerve injury by inhibiting oxidative stress and inflammation. Melatonin-based polycaprolactone nerve conduit reduced oxidative stress and inflammation in lengthy sciatic nerve defects. In addition, it alleviated Schwann cell apoptosis by mediating autophagy-related signaling (Qian et al., 2018a). Poly(D,L-lactic acid)/poly(lactic acid)-co-(glycolic acid)-alt-(L-lysine)/β-tricalcium phosphate (PDLLA/PRGD/β-TCP) conduits can regulate neurotrophin excretion into the microenvironment and reduce the oxidative stress in sciatic nerve regeneration (Qiu et al., 2014). PRGD/PDLLA capping conduit defeated inflammatory infiltration and hazardous collagen deposition as a physical barrier (Yi et al., 2018). Green synthesis of cerium oxide nanoparticle (nanoceria) has aroused interest in nontoxic nanoparticle application for tissue engineering. Nanoceria have two oxide states, Ce3+ (reduced) and Ce4+ (oxidized) on the surface. The state is changed by external stimulation and causes switch-on and switch-off for the catalytic antioxidant potential via oxidation and deoxidation. Nanoceria acts as a superoxide dismutase or catalase (CAT) mimetic when surface Ce3+ is at a high concentration. Exposure of pancreas to diazinon leads to significant oxidative stress. Nanoceria administration can reduce apoptosis of islets, reduce blood sugar level, and control weight loss and thus inhibit oxidative stress (Khaksar et al., 2017). Nanoceria has also significantly restored muscle strength and extended life expectancy in amyotrophic lateral sclerosis study via the increased CAT and oxidase activities (DeCoteau et al., 2016). Apart from the high antioxidant potential, it also has excellent biocompatibility, regenerative capacity, high stability, low cost of synthesis, and ease of storage. In the nervous system, nanoceria is considered to exert positive effects on nerve regeneration after acute or chronic oxidant insult (Najafi et al., 2017). Nanoceria had no adverse effects on the viability of PC12 cells. Instead, it improved neuronal differentiation by extending neurite length (Ciofani et al., 2013). Nanoceria modified with the functional group of polyacrylic acid alleviated H2O2-induced oxidative stress in PC12 cells by regulating cleaved caspase-3 and mitochondrial cytochrome c in the apoptosis-related pathways (Jia et al., 2018). Ceria/polyoxometalate hybrid can degrade amyloid-β aggregates, reduce intracellular ROS, and deactivate microglial cells, which leads to decreased neurotoxicity (Guan et al., 2016). Nanoceria/gelatin scaffolds fabricated by electrospinning display strong ROS capacity, slow the cell aging, and improve neurite sprouting (Marino et al., 2017). However, nanoceria was also reported to pose hazard to the neural stem cells by inhibiting their differentiation and interfering with the cytoskeletal organization (Gliga et al., 2017). In previous findings, nanoceria displays better antioxidant potential in clearing ROS and restoring immune balance. However, the cytotoxicity for neurons is questionable. Therefore, in this study, we will further explore the cytotoxicity, antioxidant, and regenerative functions of nanoceria in nerve tissue engineering, especially for severe nerve defects.
Results and Discussion
Three-Dimensional Asymmetrical Manufacture of COL/NC/PCL Nerve Conduit
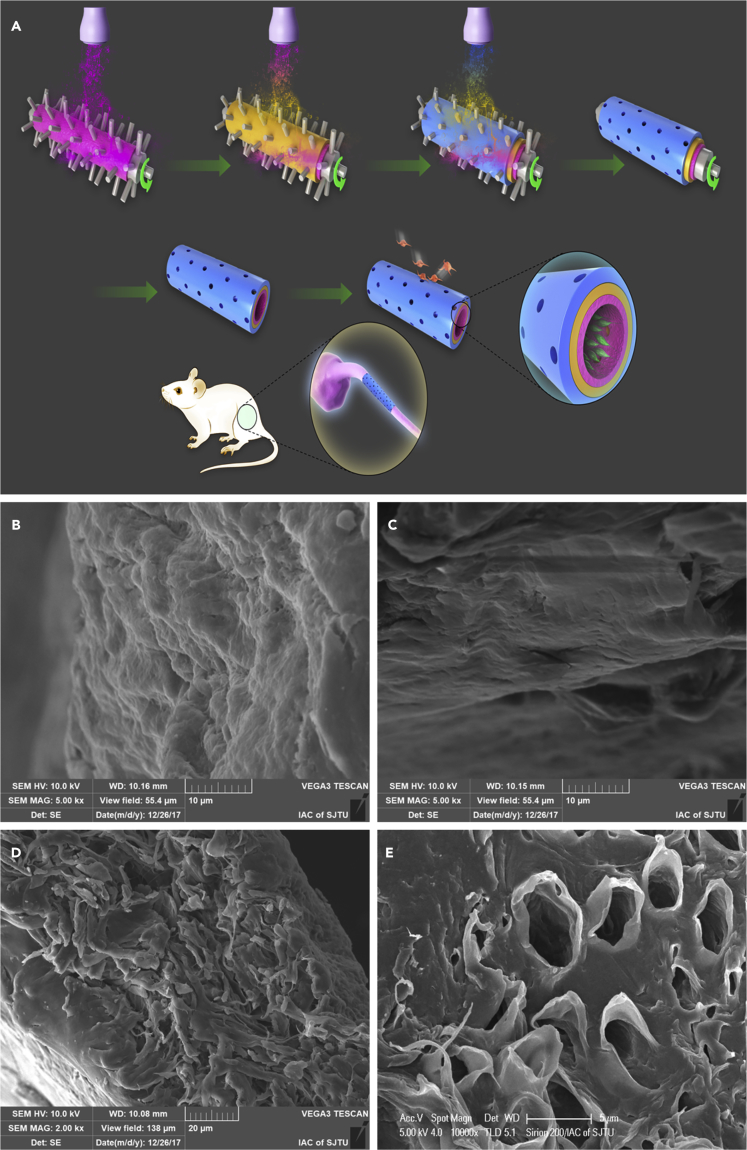
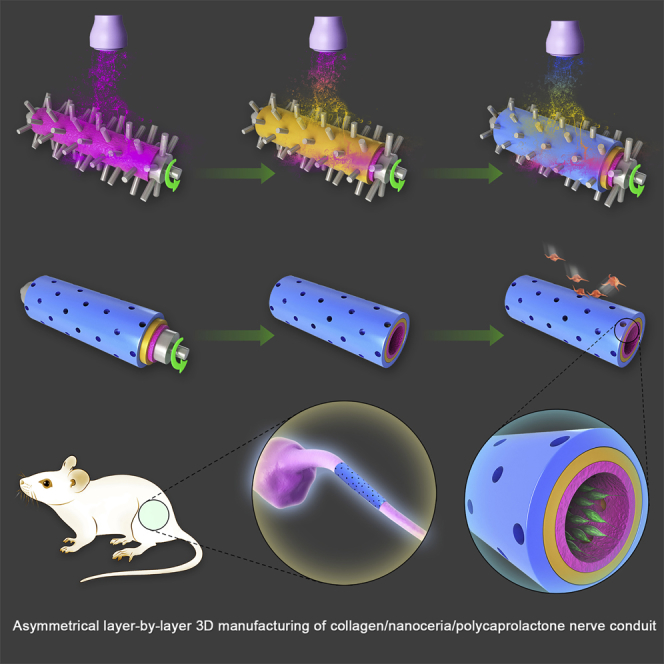
In this study, we fabricated a collagen/nanoceria/polycaprolactone (COL/NC/PCL) nerve conduit via asymmetrical layer-by-layer 3D manufacture. The conduit was composed of three layers: the innermost NC/PCL mixed layer, the outermost COL layer, and the middle PCL layer. A tube mold was rolling counterclockwise, under a sprayer that injected different solutions layer by layer on the rolling tube. A microneedle on the tube assured even pore size, which allowed free exchanges of nutrients into the conduit. Microstructured PCL filaments can increase bands of Büngner and improve nerve regeneration (Carrier-Ruiz et al., 2015). We fabricated PCL layer and NC/PCL layer via 3D manufacture, which added to its granular sensation on the surface. In addition, PCL in 3D structure can significantly improve cell attachment, proliferation, and differentiation (Rasekh et al., 2013, Sharifi et al., 2016). The adhesive effect was further enhanced by the micro/nanosurface structure of NC/PCL topography in the innermost layer. Schwann cells can adhere firmly to the inner conduit and secrete neurotrophic factors to facilitate axonal regrowth and remyelination. An agarose/collagen composite sheet can prevent adhesion of mesenchymal cells and extracellular matrix (ECM) on the lesion tissues (Tang et al., 2007). Hyaluronic acid and collagen can be fabricated as a spongy sheet to stop peritoneal adhesion to peripheral organs (Kuroyanagi et al., 2014). The dense outermost collagen layer prevented tissue adhesion in the surroundings. Therefore, we developed an adhesion gradient from the innermost to the outermost layer, supported by asymmetrical layer-by-layer 3D manufacture technique (Figure 1).
Figure 1.
Schematic Illustration of COL/NC/PCL Nerve Conduit Fabrication and Implantation into a Rat Model
(A) Asymmetrical three-dimensional layer-by-layer manufacture of COL/NC/PCL nerve conduit. It was composed of three layers: the innermost NC/PCL mixed layer, the outermost COL layer, and the middle PCL layer. A tube mold was rolling counterclockwise, under a sprayer that injected different solutions layer by layer on the rolling tube. A microneedle on the tube assured even pore size that allowed free exchanges of nutrients into the conduit. The schematic illustration showed Schwann cell adhesion to the innermost layer and fibroblast detachment from the outermost layer.
(B) Rough innermost layer; scale bar, 10 μm.
(C) Smooth outermost layer; scale bar, 10 μm.
(D) Multilayered structure and an increasing gradient change in roughness inside out; scale bar, 20 μm.
(E) Microporous structure in the COL/NC/PCL nerve conduit; scale bar, 5 μm.
Mechanical and Structural Characteristics of the COL/NC/PCL Conduit
We characterized the COL/NC/PCL nerve conduit via scanning electron microscopy (SEM). The innermost layer was significantly rougher than the outermost layer. The cross-sectional view showed multilayered structure and an increasing gradient change in roughness inside out (Figure 1). We also observed NC morphology and distribution in the scaffold using transmission electron microscopy (TEM) and SEM, respectively. The cerium oxide nanoparticles were relatively evenly distributed in the scaffold and were around 10–50 nm in diameter (Figure S1). Also, they displayed excellent antioxidant properties. The antioxidant activity of 1 mg nanoceria corresponds to that of 100 nmol Trolox. We evaluated the mechanical properties, including conduit thickness and elastic modulus, and found that the COL/NC/PCL conduit was thicker, softer, and less elastic than the NC/PCL conduit, owing to collagen application (Figure S2). Both conduits displayed good elasticity that could support long-term in vivo nerve regeneration, free from conduit collapse. In the fabrication course, microneedles on the mold facilitated microporous architecture, which introduced free exchanges of nutrients, water, and other macromolecules into the conduit lumen (Figure 1; Qian et al., 2018b).
Excellent Biocompatibility of COL/NC/PCL Conduit for RSCs
We assessed the biocompatibility of COL/NC/PCL and NC/PCL conduits to determine their appropriate concentration for neural growth, from 0.5%, 1%, 2% to 4% nanoceria in different conduits using cell counting kit 8 (CCK8) assay. It showed that 1% nanoceria was much less cytotoxic than 2% and 4% and was slightly more toxic than the 0.5% counterpart after 24 h (Figure S3). This was consistent with a previous finding that 1 μg mL−1 nanoceria was the optimal concentration of biological effectiveness for hippocampal neurons (Estevez et al., 2011). We further used 1% nanoceria in the following experiments.
Firm Attachment and Neural Expression of RSCs on the COL/NC/PCL Conduit
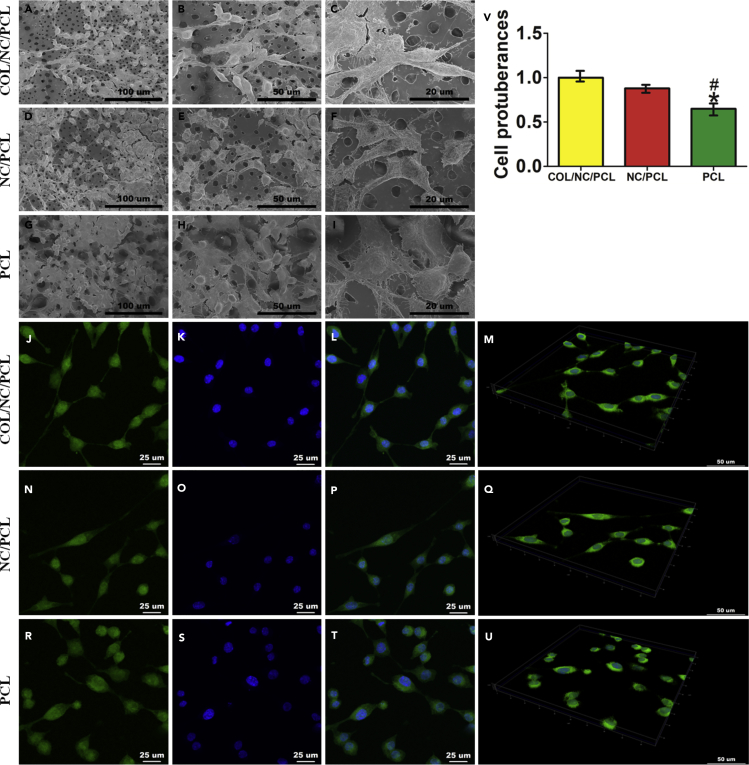
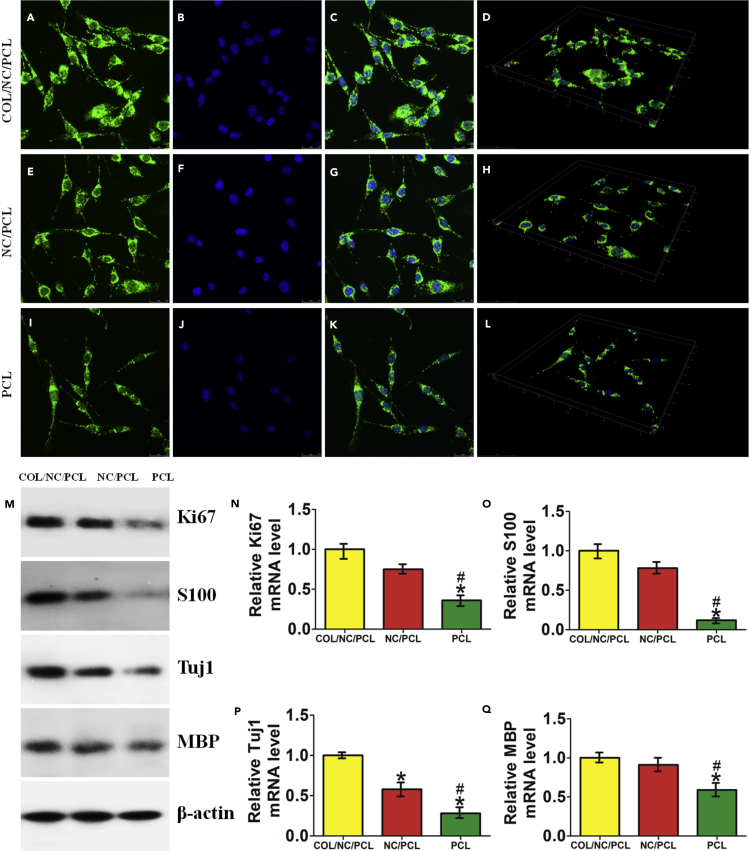
We observed rat Schwann cell (RSC) morphology in different conduits. Cell protuberances were more extended in COL/NC/PCL conduit than in the NC/PCL and PCL counterparts (Figure 2). Excellent cellular adhesion and elongation was supported by tensile stresses originating from the micro- and nanostructured pores in the conduit (Marino et al., 2013). Cell proliferation assay was performed using CCK8 at 24, 72, 120, and 168 h, showing that COL/NC/PCL and NC/PCL conduits improved cell growth compared with the PCL counterpart. We also evaluated Ki67 expression of cells from different conduits. It also showed a higher expression from COL/NC/PCL and NC/PCL conduits than the PCL counterpart (p < 0.05, Figure 3).
Figure 2.
Schwann Cell Morphology in Different Conduits
(A–U) Cell morphology in different nerve conduits evaluated by SEM (A–I) and immunofluorescence (J–U).
(A–C) Cell morphology in COL/NC/PCL conduit.
(D–F) Cell morphology in NC/PCL conduit.
(G–I) Cell morphology in PCL conduit. Scale bars, 100 μm in (A, D, and G); 50 μm in (B, E, and H); and 20 μm in (C, F, and I).
(J–M) Phalloidin staining showing cell attachment in COL/NC/PCL conduit.
(J, N, and R) Phalloidin staining; scale bars, 25 μm.
(K, O, and S) DAPI staining; scale bars, 25 μm.
(L, P, and T) Merged images; scale bars, 25 μm.
(M, Q, and U) 3D display for cell attachment in different conduits; scale bars, 50 μm.
(V) Cell protuberances in different conduits, *p < 0.05 compared with COL/NC/PCL, #p < 0.05 compared with NC/PCL.
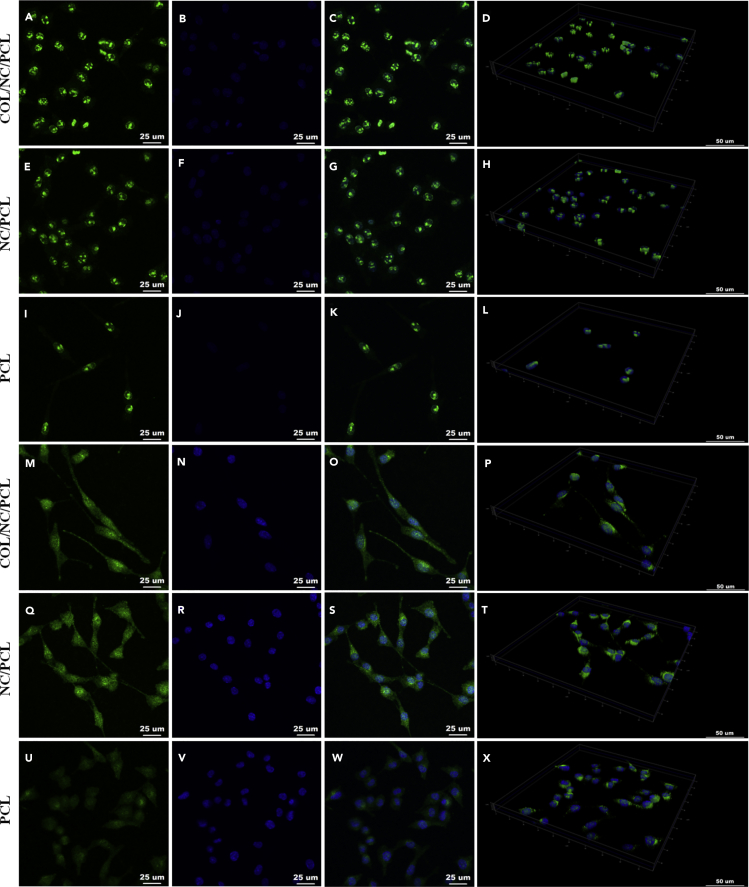
Figure 3.
Immunofluorescence of Ki67 and GFAP
(A–D) Ki67 expression in COL/NC/PCL.
(E–H) Ki67 expression in NC/PCL.
(I–L) Ki67 expression in PCL.
(M–P) GFAP expression in COL/NC/PCL.
(Q–T) GFAP expression in NC/PCL.
(U–X) GFAP expression in PCL.
(A, E, and I) Ki67 staining; scale bars, 25 μm.
(M, Q, and U) GFAP staining; scale bars, 25 μm.
(B, F, J, N, R, and V) DAPI staining; scale bars, 25 μm.
(C, G, K, O, S, and W) Merged images; scale bars, 25 μm.
(D, H, L, P, T, and X) 3D display; scale bars, 50 μm. GFAP, glial fibrillary acidic protein.
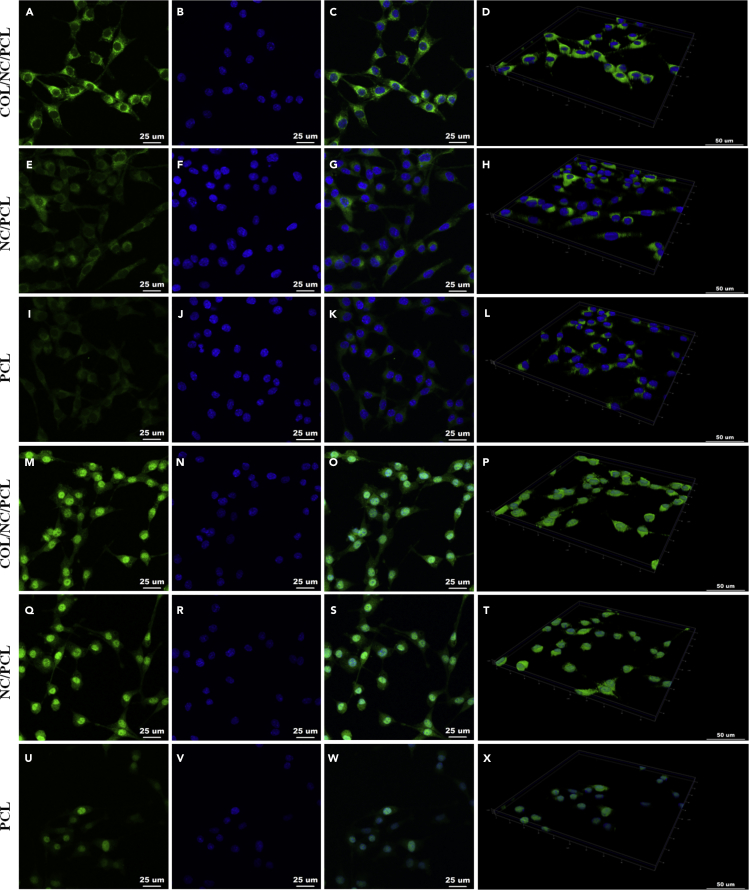
Nanoceria can regulate neuronal cell fate. It promoted neuronal repair by regulating brain-derived neurotrophic factor signaling in Alzheimer disease (D'Angelo et al., 2009). It also improved neurogenesis by ameliorating hypoxia-dependent memory loss (Arya et al., 2016). In this study, we evaluated the neural expression of RSCs in COL/NC/PCL conduit. The axonal orientation and myelination states were evaluated by S100, β-III-tubulin (Tuj1), myelin basic protein (MBP), and glial fibrillary acidic protein (GFAP) expression in vitro (Figures 3, 4, and 5). Their expression from COL/NC/PCL conduit was similar to NC/PCL and significantly higher than the PCL counterpart (p<0.05) confirmed by immunofluorescent assay. Moreover, we also performed western blot (WB) and quantitative PCR (qPCR) to revalidate our findings in vitro (Figure 5).
Figure 4.
Immunofluorescence of S100 and Tuj1
(A–D) S100 expression in COL/NC/PCL.
(E–H) S100 expression in NC/PCL.
(I–L) S100 expression in PCL.
(M–P) Tuj1 expression in COL/NC/PCL.
(Q–T) Tuj1 expression in NC/PCL.
(U–X) Tuj1 expression in PCL.
(A, E, and I) S100 staining; scale bars, 25 μm.
(M, Q, and U) Tuj1 staining; scale bars, 25 μm.
(B, F, J, N, R, and V) DAPI staining; scale bars, 25 μm.
(C, G, K, O, S, and W) Merged images; scale bars, 25 μm.
(D, H, L, P, T, and X) 3D display, scale bars, 50 μm.
Figure 5.
Immunofluorescence of MBP
(A–D) MBP expression in COL/NC/PCL.
(E–H) MBP expression in NC/PCL.
(I–L) MBP expression in PCL.
(A, E, and I) MBP staining; scale bars, 25 μm.
(B, F, and J) DAPI staining; scale bars, 25 μm.
(C, G, and K) Merged images; scale bars, 25 μm.
(D, H, and L) 3D display; scale bars, 50 μm.
(M) Western blots of Ki67, S100, Tuj1, and MBP compared among COL/NC/PCL, NC/PCL, and PCL conduits.
(N) Relative Ki67 mRNA level.
(O) Relative S100 mRNA level.
(P) Relative Tuj1 mRNA level.
(Q) Relative MBP mRNA level.
*p < 0.05 compared with COL/NC/PCL, #p < 0.05 compared with NC/PCL.
Antioxidant and Anti-inflammatory Roles of COL/NC/PCL Conduit
In addition to neural protection, the ability of nanoceria to scavenge oxidative stress is very significant for the oxygen-enriched organs and systems. Different manufacturing techniques contributed to nanoceria fabrication with the unique capability of oxygen storage, release, and clearance (Ishikawa et al., 2016, Yao et al., 2018). Nanoceria reduced ROS production and DNA and protein damages to repair and restore main cell activities in cardiac engineering (Pagliari et al., 2012). Water-soluble nanoceria could scavenge ROS and accumulative oxidative insult in age-associated macular diseases (Mitra et al., 2017). In the presence of a H2O2 oxidant insult, we performed 2′,7′-dichlorofluorescein diacetate (DCFDA) flow cytometry assay and evaluated some antioxidant and inflammatory markers' expression, such as hemeoxygenase 1 (HO-1), manganese-dependent superoxide dismutase (MnSOD), glutamate-cysteine ligase catalytic (GCLC), and interleukin 6 (IL-6), using WB and qPCR assays. The oxidative stress state in cells cultured in COL/NC/PCL and NC/PCL conduits was significantly lower than that in PCL counterpart, indicating that nanoceria could reduce the oxidative level by scavenging ROS (Figure S4). The effect was further enhanced by collagen addition.
Nanoceria has diverse sizes varying from a few to hundreds of nanometers. Larger nanoceria was found to scavenge intracellular ROS to a greater extent than the smaller nanoceria, and ROS scavenging was found to increase with treatment time (Vassie et al., 2017). In this study, we purchased 20-nm-diameter nanoceria, which improved its antioxidative stress ability.
In Vivo Regenerative Capacity of COL/NC/PCL Conduit
The antioxidant and neural protective role of nanoceria-based conduit was further evaluated in Sprague Dawley (SD) rat sciatic nerve injury model. We included COL/NC/PCL conduit, NC/PCL conduit, PCL conduit, and autograft and assessed their performances in peripheral nerve regeneration at 6, 12, and 18 weeks after surgery. We did not notice ulcer, delayed wound healing, or severe infection at each time point. Walking track analysis and withdrawal latency measurement were adopted for locomotor and sensory performance evaluation. The sciatic function index was the highest in autograft, and the results were better in COL/NC/PCL and NC/PCL conduits than the PCL counterpart at 6 and 12 weeks after surgery. At 18 weeks, the value of COL/NC/PCL conduit was similar to that of autograft, and higher than those of the others (p<0.05). As for withdrawal latency, the reaction time was prolonged evidently in COL/NC/PCL, NC/PCL, and PCL conduits when compared with the autograft at 6 and 12 weeks after surgery. At 18 weeks, the reaction time of COL/NC/PCL conduit was still longer than that of the autograft but much shorter than that of the rest (p < 0.05) (Figure S5). It showed that the COL/NC/PCL conduit promoted locomotor and sensory recovery to a certain extent after severe traumatic injury.
Operated nerves were dissected from rats and subjected to electromyography testing, such as nerve conducting velocity (NCV) and distal compound motor action potential (DCMAP) (Kerasnoudis et al., 2014, Yarar et al., 2015). At 6 and 12 weeks after surgery, the NCV of COL/NC/PCL conduit (13.6 m s−1, 20.4 m s−1) was significantly higher than that of NC/PCL (10.1 m s−1, 15.2 m s−1) and PCL (7.3 m s−1, 11.8 m s−1) counterparts, but was still lower than that of the autograft (16.6 m s−1, 25.9 m s−1). Nevertheless, there was a slight difference between the COL/NC/PCL conduit (32.1 m s−1) and autograft (34.7 m s−1) at 18 weeks. However, the results were much better than those of the NC/PCL (25.8 m s−1) and PCL (19.5 m s−1) conduits. DCMAP is the total action potential of every motor endplate and an important indicator for muscle contraction gain. Like NCV, autograft outnumbered other groups in DCMAP at 6 and 12 weeks after surgery (p < 0.05). However, the DCMAP value of the COL/NC/PCL conduit was close to that of the autograft at 18 weeks and evidently higher that of the rest (p < 0.05, Figure S5). It indicated that the COL/NC/PCL conduit significantly elevated electrophysiological performance and restored electrical transduction.
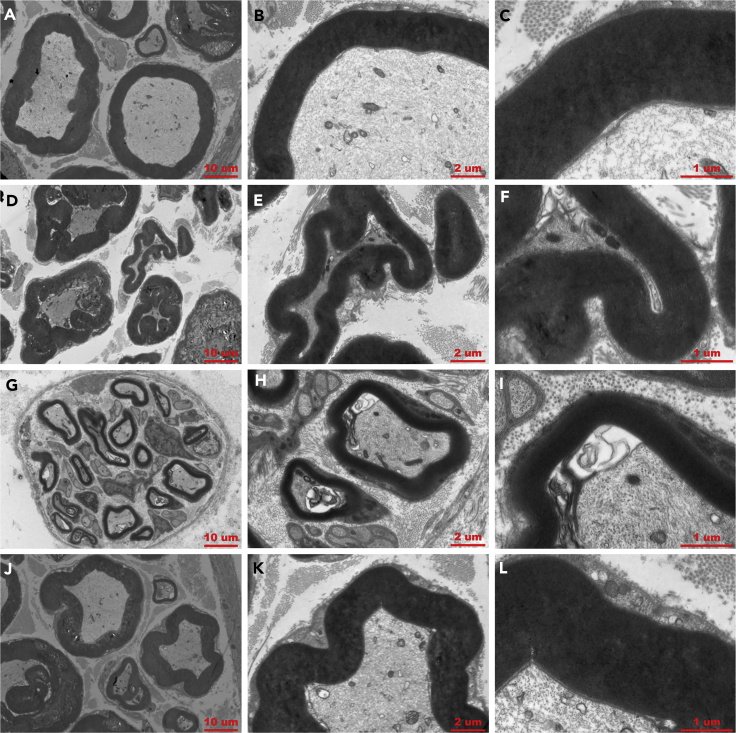
After functional and electrophysiological evaluation, we prepared semithin sections from the middle portion of 15-mm regenerated nerve samples in each group and performed hematoxylin and eosin (H&E) staining, 1% toluidine blue (TB) staining, and TEM. We evaluated several indicators for peripheral nerve regeneration, such as the number of myelinated axons, thickness of myelin sheath, regenerated axon area, and average myelinated axon diameter (Qian et al., 2018c). H&E and TB staining showed that the total axon number and area results were similar between COL/NC/PCL and NC/PCL at 6 weeks. However, the diameter and thickness of regenerated nerves and myelinated axons of COL/NC/PCL conduit were higher than those of the remaining conduits at 6 and 12 weeks after surgery (p < 0.05, Figures S6–S8). None of them could match the results of autograft. However, at 18 weeks, COL/NC/PCL conduit and autograft showed similar values and were significantly better than NC/PCL and PCL conduits (p < 0.05, Figure S9). By TEM observation, the thickness and average diameter of myelinated fibers were evaluated. In COL/NC/PCL conduit, the average myelinated axon diameter and myelin thickness were evidently increased when compared with the NC/PCL and PCL counterparts (p<0.05) at three time points, but were lower than those in autograft at 6 and 12 weeks (Figures S10 and S11). However, no significant difference was observed between the COL/NC/PCL conduit and autograft at 18 weeks (p > 0.05, Figure 6).
Figure 6.
TEM for Axonal Regeneration and Remyelination State at 18 Weeks Postoperatively
(A–C) COL/NC/PCL conduit.
(D–F) NC/PCL conduit.
(G–I) PCL conduit.
(J–L) Autograft.
Scale bars, 10 μm in (A, D, G, and J); 2 μm in (B, E, H, and K); and 1 μm in (C, F, I, and L).
COL/NC/PCL Alleviates Muscle Atrophy and Restores Muscle Viability
Muscle viability is lost when nerve injury compromises neurological and electrical stimulation. Prolonged nerve denervation can lead to chronic muscle atrophy (Sun et al., 2017, Kollitz et al., 2018). Sciatic nerves manipulate gastrocnemius muscle, which in turn can reflect the neurological status. In addition, delayed muscle regeneration results in reduction in muscle contraction speed and force, along with a transformation from fast-twitch-type to slow-twitch-type muscle fiber (Zimowska et al., 2017). Therefore we measured gastrocnemius muscle weight and evaluated muscle morphology and muscle fiber type. At 6 and 12 weeks after surgery, the gastrocnemius muscle weight from COL/NC/PCL conduit was significantly higher than those from NC/PCL and PCL conduits (p<0.05), but it was significantly lower than that from the autograft (p < 0.05). There was no difference between the values of the COL/NC/PCL conduit and autograft at 18 weeks (p > 0.05, Figure S12). From the muscle fiber H&E and TB staining, a well-structured muscle fiber morphology was presented from the COL/NC/PCL conduit. The majority of area was filled with fast twitch phenotype fiber. In contrast, muscle samples from NC/PCL and PCL conduits displayed relatively irregular and incomplete morphology. Most of the areas were occupied by slow-twitch-type muscle fiber. Laminin attaches the cytoskeleton structure to the ECM. Therefore it is an important protein in the process wherein skeletal muscle spreads forces and resists pressure in the surroundings (Holmberg and Durbeej, 2013). Better results were achieved from autograft and COL/NC/PCL conduit than the others (Figures S13–S18). It indicated that the COL/NC/PCL conduit evidently improved skeletal muscle restoration by ameliorating muscle atrophy.
COL/NC/PCL Inhibits Oxidative Stress and Inflammation in Long-Term Repair
Lasting oxidative stress and inflammatory reaction are harmful for effective axonal and myelin sheath regeneration. We evaluated the antioxidant and anti-inflammatory roles of nanoceria in long-range nerve defect for the first time, although nanoceria was confirmed to reduce ROS production and inflammatory adhesion previously (Oró et al., 2016). HO-1 can regulate redox homeostasis against ROS production and oxidant insult (Min et al., 2011). Its increased expression contributes to ROS clearance and cell protection (Xu et al., 2015). Nuclear factor-like 2 (Nrf2) is an upstream regulator of HO-1 family. Therefore, under oxidative environment, Nrf2 can activate HO-1 to strengthen antioxidative and anti-inflammatory reactions (Bao et al., 2016). We evaluated Nrf2 and HO-1 expression from different groups at three time points. Both COL/NC/PCL conduit and autograft showed the highest expression of both markers. NC/PCL conduit displayed more Nrf2 and HO-1 than PCL counterpart. In addition, we also included tumor necrosis factor α, Toll-like receptor 4, and IL-6. These markers are representatives for inflammatory status. Inflammation is the consequence of trauma-induced hypoxia in the nerve injury site. Macrophages gathered and released some pro-inflammatory molecules, leading to persistent tissue adhesion and compromised nerve regeneration (Lanza et al., 2012). We noticed that the inflammatory reaction was the most severe in PCL conduit, followed by NC/PCL counterpart. Nevertheless, COL/NC/PCL conduit and autograft only exhibited very mild inflammation, especially at 18 weeks after surgery (Figures S19–S22). It indicated that COL/NC/PCL conduit significantly reduced oxidative stress and inflammation after traumatic peripheral nerve damage.
Nanoceria Stimulates Ideal Angiogenesis in Nerve Tissue Surroundings
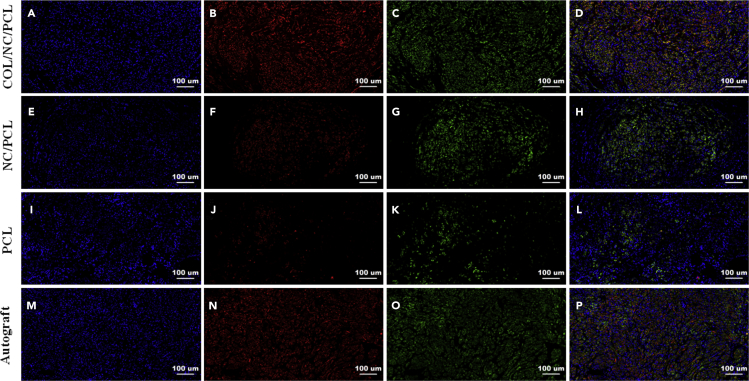
Angiogenesis is the process wherein new vessels form and provide nutrition to the surrounding tissues, like bones and nerves (Baker et al., 2011). Therefore, it is very vital to functional peripheral nerve regeneration. Nanoceria has a unique characteristic of inducing angiogenesis in vivo. This capability is also related to oxygen transfer and modulation in the intracellular environment (Das et al., 2012). Low ROS level is beneficial to angiogenesis. In contrast, high ROS level reduces angiogenic state. Nanoceria creates relatively low ROS microenvironment to stimulate ideal new vessel formation. In this study, we analyzed the angiogenic potential of nanoceria in long-range nerve defect in vivo for the first time. We included CD31 and CD34, two markers for angiogenesis (Luedi et al., 2018, Wang et al., 2017). The neovascularization was assessed upon several indicators, including microvessel density (MVD), vessel-like structure (VLS) area, and density ((VLS area+ CD31/CD34+ area)/total scaffold area) at 6, 12, and 18 weeks after surgery. The angiogenic status was very close between COL/NC/PCL and NC/PCL conduits, which was better than that of the PCL counterpart. However, autograft displayed the most prominent angiogenesis in all groups (Figures S23–S26). It was probably the higher vascular endothelial growth factor secretion from autologous nerves that contributed to better neovascularization in the surroundings (Hoben et al., 2015).
COL/NC/PCL Conduit Improves Axonal Regeneration, Remyelination, and Neural Viability
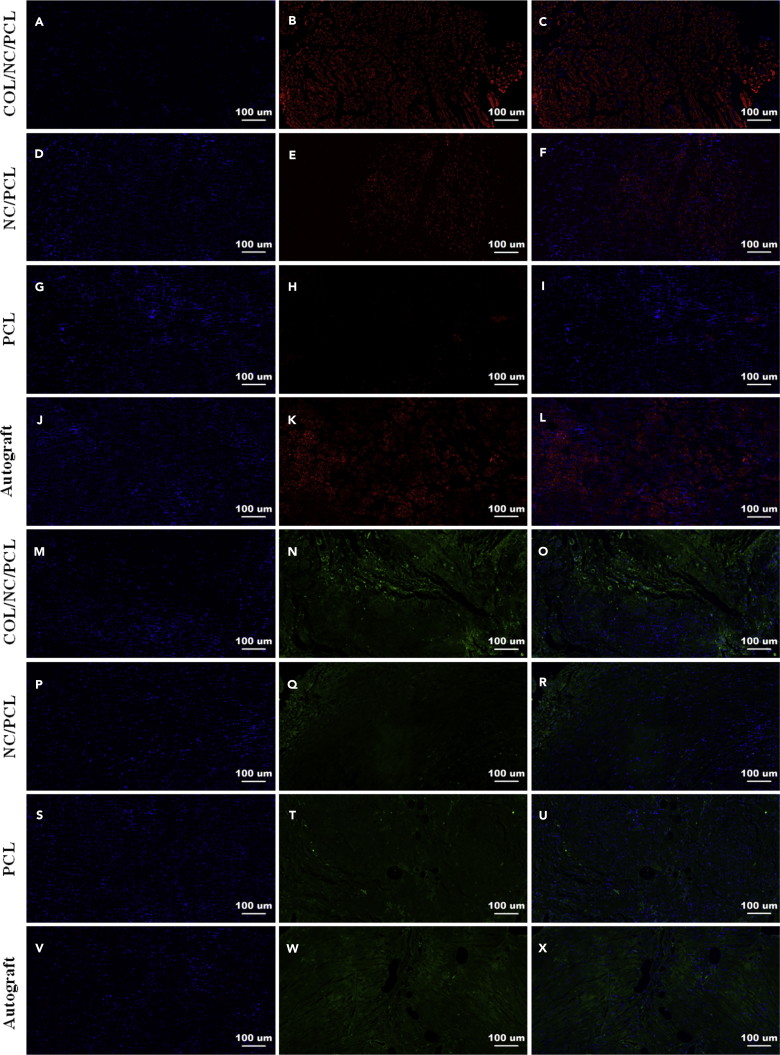
In addition to morphological evaluation of axonal and myelin regeneration, we also evaluated some markers, sox10, growth-associated protein-43 (GAP-43), S100, Tuj1, MBP, and neurofilament 200 (NF200). Sox10 promotes neurogenesis in peripheral nerve restoration after trauma (Dyachuk et al., 2014). GAP-43 is an important biomarker in the entire course of peripheral nerve regrowth (Kaneda et al., 2010). S100, Tuj1, MBP, and NF200 are common markers involved in assessment of sciatic nerve recovery (Qian et al., 2018d). NF200 and Tuj1 can reflect axonal regeneration (Tian et al., 2017, Ansari et al., 2017). S100 indicates Schwann cell migration, and MBP represents neural fiber myelination (Uz et al., 2017, Feliú et al., 2017). At 6 and 12 weeks after surgery, sox10 and GAP-43 expression was higher in autograft than COL/NC/PCL conduit (p<0.05), and did not show significant differences between NC/PCL and PCL conduits (p>0.05). At 18 weeks, autograft and COL/NC/PCL conduit showed significantly higher sox10 and GAP-43 expression than the remaining groups (p < 0.05, Figures S27–S30). At 6 and 12 weeks after surgery, the autograft group showed better results in Tuj1, NF200, S100, and MBP expression than the remaining conduits (p<0.05). At 18 weeks, the expression of Tuj1, NF200, S100, and MBP was evidently upregulated in COL/NC/PCL conduit. It was notoriously higher than those of the NC/PCL and PCL conduits (p < 0.05) and was slightly lower than those of the autograft at 6, 12 (p>0.05, Figures S31–S34), and 18 weeks (p > 0.05, Figures 7 and 8). It displayed that the COL/NC/PCL conduit significantly contributed to axonal regrowth and remyelination after severe long-range nerve defect.
Figure 7.
Triple Immunofluorescence of Regenerated Nerve Tissues Showing Axonal Restoration at 18 Weeks Postoperatively
(A–D) COL/NC/PCL.
(E–H) NC/PCL.
(I–L) PCL.
(M–P) Autograft.
(A, E, I, and M) DAPI staining.
(B, F, J, and N) NF200 staining.
(C, G, K, and O) Tuj1 staining.
(D, H, L, and P) Merged images.
Scale bars, 100 μm.
Figure 8.
Triple Immunofluorescence Showing Schwann Cell Viability and Remyelination at 18 Weeks Postoperatively
(A–X) (A–C and M–O) COL/NC/PCL. (D–F and P–R) NC/PCL. (G–I and S–U) PCL. (J–L and V–X) Autograft. (A, D, G, J, M, P, S, and V) DAPI staining. (B, E, H, and K) MBP staining. (N, Q, T, and W) S100 staining. (C, F, I, L, O, R, U, and X) Merged images.
Scale bars, 100 μm.
Conclusions
In summary, we fabricated COL/NC/PCL nerve conduit via asymmetrical layer-by-layer 3D manufacture. The conduit was composed of three layers: the innermost NC/PCL mixed layer, the outermost COL layer, and the middle PCL layer. A microneedle on the tube assured even pore size, which allowed free exchanges of nutrients into the conduit. We evaluated the antioxidative stress and anti-inflammatory potential of COL/NC/PCL conduit both in vitro and in vivo. It evidently promoted better ROS clearance and inflammation inhibition. In addition, nanoceria-based conduit also contributed to satisfactory axonal and myelin regeneration in severe sciatic nerve defect model at 18 weeks after surgery. This effect was further enhanced by collagen in the outermost layer via asymmetrical 3D manufacture because it assured fewer fibroblasts adhered either to the conduit or to the regenerated nerves. Moreover, we found that nanoceria stimulated angiogenesis after nerve injury, probably due to the proangiogenic microenvironment resulting from the low ROS production (Tojo et al., 2005). We believe our asymmetrical manufacture will provide more insights into the design of nerve conduit that combines suitable structural cues and controlled drug release. In addition, it paves a promising avenue to the integrated and fast fabrication of nerve scaffold. Nanoceria is characteristic of several important factors involved in nerve regeneration, such as oxygen modulation, angiogenic stimulation, and neural morphology reconstruction. Nanoceria-based biomaterials are considered to provide compelling evidence for future studies in antioxidant conduit and severe neurological defects in the nerve tissue engineering.
Limitations of the Study
In this study, we evaluated the asymmetrical fabrication of COL/NC/PCL composite channel for repairing large sciatic nerve defects. Although many advantages and functions were displayed, including its potential in improving tissue proliferation, adhesion, and antioxidant capacity, the possible mechanism behind these facts was not comprehensively investigated. In addition, we did not observe complete scaffold degradation at 18 weeks. It required further evaluation at longer time points in vivo.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The study was supported by the Projects of National Science Foundation of China (Grant Nos. 81830076, 81672146, 81570992 and 81571261), the Projects of National Science Foundation of Shanghai, China (Grant No. 17401901000), SUMHS Seed Foundation Project (Grant Nos. HMSF-16-21-010), Science and Technology Development Foundation of Pudong New District, Shanghai, China (Grant Nos. PKJ2016-Y55 and PWZxq2017-03), the Program of Shanghai Sixth People’s Hospital East Campus Foundation (No.2019YY001), and The Program of Shanghai Sixth People’s Hospital Foundation (No.LY2Y0272). The study was also partly sponsored by the Interdisciplinary Program of Shanghai Jiao Tong University (Nos. ZH2018QNA56 and YG2017MS22). We appreciate the help from the faculty of the Instrumental Analysis Center (IAC) of Shanghai Jiao Tong University.
Author Contributions
W.E.Y. conceived the initial idea and the conceptualization. C.F. and W.E.Y. designed the study, participated in data extraction and analysis, and revised the manuscript. Y.Q., X.Z., Q.H., H.L., W.E.Y., and C.F. participated in the study design, searched databases, extracted and assessed studies, and helped draft the manuscript. Y.Q. wrote the manuscript. All authors have read and approved the final manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: February 22, 2019
Footnotes
Supplemental Information includes Transparent Methods and 34 figures and can be found with this article online at https://doi.org/10.1016/j.isci.2019.01.013.
Contributor Information
Wei-En Yuan, Email: yuanweien@sjtu.edu.cn.
Cunyi Fan, Email: cyfan@sjtu.edu.cn.
Supplemental Information
References
- Ansari S., Diniz I.M., Chen C., Sarrion P., Tamayol A., Wu B.M., Moshaverinia A. Human periodontal ligament- and gingiva-derived mesenchymal stem cells promote nerve regeneration when encapsulated in alginate/hyaluronic acid 3D scaffold. Adv. Healthc. Mater. 2017;6 doi: 10.1002/adhm.201700670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya A., Gangwar A., Singh S.K., Roy M., Das M., Sethy N.K., Bhargava K. Cerium oxide nanoparticles promote neurogenesis and abrogate hypoxia-induced memory impairment through AMPK-PKC-CBP signaling cascade. Int. J. Nanomed. 2016;11:1159–1173. doi: 10.2147/IJN.S102096. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Baker M., Robinson S.D., Lechertier T., Barber P.R., Tavora B., D'Amico G., Jones D.T., Vojnovic B., Hodivala-Dilke K. Use of the mouse aortic ring assay to study angiogenesis. Nat. Protoc. 2011;7:89–104. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- Bao J., Ding R., Zou L., Zhang C., Wang K., Liu F., Li P., Chen M., Wan J.B., Su H. Forsythiae fructus inhibits B16 melanoma growth involving MAPKs/Nrf2/HO-1 mediated anti-oxidation and anti-inflammation. Am. J. Chin. Med. 2016;44:1043–1061. doi: 10.1142/S0192415X16500580. [DOI] [PubMed] [Google Scholar]
- Carrier-Ruiz A., Evaristo-Mendonça F., Mendez-Otero R., Ribeiro-Resende V.T. Biological behavior of mesenchymal stem cells on poly-ɛ-caprolactone filaments and a strategy for tissue engineering of segments of the peripheral nerves. Stem Cell Res. Ther. 2015;6:128. doi: 10.1186/s13287-015-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani G., Genchi G.G., Liakos I., Cappello V., Gemmi M., Athanassiou A., Mazzolai B., Mattoli V. Effects of cerium oxide nanoparticles on PC12 neuronal-like cells: proliferation, differentiation, and dopamine secretion. Pharm. Res. 2013;30:2133–2145. doi: 10.1007/s11095-013-1071-y. [DOI] [PubMed] [Google Scholar]
- D'Angelo B., Santucci S., Benedetti E., Di Loreto S., Phani R., Falone S., Amicarelli F., Ceru M., Cimini A. Cerium oxide nanoparticles trigger neuronal survival in a human Alzheimer disease model by modulating BDNF pathway. Curr. Nanosci. 2009;5:167–176. [Google Scholar]
- Das S., Singh S., Dowding J.M., Oommen S., Kumar A., Sayle T.X., Saraf S., Patra C.R., Vlahakis N.E., Sayle D.C. The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments. Biomaterials. 2012;33:7746–7755. doi: 10.1016/j.biomaterials.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoteau W., Heckman K.L., Estevez A.Y., Reed K.J., Costanzo W., Sandford D., Studlack P., Clauss J., Nichols E., Lipps J. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomedicine. 2016;12:2311–2320. doi: 10.1016/j.nano.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Dyachuk V., Furlan A., Shahidi M.K., Giovenco M., Kaukua N., Konstantinidou C., Pachnis V., Memic F., Marklund U., Müller T. Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science. 2014;345:82–87. doi: 10.1126/science.1253281. [DOI] [PubMed] [Google Scholar]
- Estevez A.Y., Pritchard S., Harper K., Aston J.W., Lynch A., Lucky J.J., Ludington J.S., Chatani P., Mosenthal W.P., Leiter J.C. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic. Biol. Med. 2011;51:1155–1163. doi: 10.1016/j.freeradbiomed.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Feliú A., Bonilla Del Río I., Carrillo-Salinas F.J., Hernández-Torres G., Mestre L., Puente N., Ortega-Gutiérrez S., López-Rodríguez M.L., Grandes P., Mecha M. 2-arachidonoylglycerol reduces proteoglycans and enhances remyelination in a progressive model of demyelination. J. Neurosci. 2017;37:8385–8398. doi: 10.1523/JNEUROSCI.2900-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliga A.R., Edoff K., Caputo F., Källman T., Blom H., Karlsson H.L., Ghibelli L., Traversa E., Ceccatelli S., Fadeel B. Cerium oxide nanoparticles inhibit differentiation of neural stem cells. Sci. Rep. 2017;7:9284. doi: 10.1038/s41598-017-09430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Li M., Dong K., Gao N., Ren J., Zheng Y., Qu X. Ceria/POMs hybrid nanoparticles as a mimicking metallopeptidase for treatment of neurotoxicity of amyloid-β peptide. Biomaterials. 2016;98:92–102. doi: 10.1016/j.biomaterials.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Hoben G., Yan Y., Iyer N., Newton P., Hunter D.A., Moore A.M., Sakiyama-Elbert S.E., Wood M.D., Mackinnon S.E. Comparison of acellular nerve allograft modification with Schwann cells or VEGF. Hand. 2015;10:396–402. doi: 10.1007/s11552-014-9720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg J., Durbeej M. Laminin-211 in skeletal muscle function. Cell Adh. Migr. 2013;7:111–121. doi: 10.4161/cam.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y., Takeda M., Tsukimoto S., Nakayama K.S., Asao N. Cerium oxide nanorods with unprecedented low-temperature oxygen storage capacity. Adv. Mater. 2016;28:1467–1471. doi: 10.1002/adma.201504101. [DOI] [PubMed] [Google Scholar]
- Jia J., Zhang T., Chi J., Liu X., Sun J., Xie Q., Peng S., Li C., Yi L. Neuroprotective effect of CeO2@PAA-LXW7 against H2O2-induced cytotoxicity in NGF-differentiated PC12 cells. Neurochem. Res. 2018 doi: 10.1007/s11064-018-2559-y. [DOI] [PubMed] [Google Scholar]
- Kaneda M., Nagashima M., Mawatari K., Nunome T., Muramoto K., Sugitani K., Kato S. Growth-associated protein43 (GAP43) is a biochemical marker for the whole period of fish optic nerve regeneration. Adv. Exp. Med. Biol. 2010;664:97–104. doi: 10.1007/978-1-4419-1399-9_12. [DOI] [PubMed] [Google Scholar]
- Khaksar M.R., Rahimifard M., Baeeri M., Maqbool F., Navaei-Nigjeh M., Hassani S., Moeini-Nodeh S., Kebriaeezadeh A., Abdollahi M. Protective effects of cerium oxide and yttrium oxide nanoparticles on reduction of oxidative stress induced by sub-acute exposure to diazinon in the rat pancreas. J. Trace Elem. Med. Biol. 2017;41:79–90. doi: 10.1016/j.jtemb.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Kerasnoudis A., Pitarokoili K., Behrendt V., Gold R., Yoon M.S. Multifocal motor neuropathy: correlation of nerve ultrasound, electrophysiological, and clinical findings. Peripher. Nerv. Syst. 2014;19:165–174. doi: 10.1111/jns5.12067. [DOI] [PubMed] [Google Scholar]
- Kollitz K.M., Giusti G., Friedrich P.F., Bishop A.T., Shin A.Y. Validation of isometric tetanic force as a measure of muscle recovery after nerve injury in the rabbit biceps. J. Hand Surg. Am. 2018;43:488.e1–488.e8. doi: 10.1016/j.jhsa.2017.10.037. [DOI] [PubMed] [Google Scholar]
- Kuroyanagi M., Yamamoto A., Shimizu N., Toi A., Inomata T., Takeda A., Kuroyanagi Y. Development of anti-adhesive spongy sheet composed of hyaluronic acid and collagen containing epidermal growth factor. J. Biomater. Sci. Polym. Ed. 2014;25:1253–1265. doi: 10.1080/09205063.2014.926579. [DOI] [PubMed] [Google Scholar]
- Lanza C., Raimondo S., Vergani L., Catena N., Sénès F., Tos P., Geuna S. Expression of antioxidant molecules after peripheral nerve injury and regeneration. J. Neurosci. Res. 2012;90:842–848. doi: 10.1002/jnr.22778. [DOI] [PubMed] [Google Scholar]
- Luedi M.M., Singh S.K., Mosley J.C., Hassan I.S.A., Hatami M., Gumin J., Andereggen L., Sulman E.P., Lang F.F., Stueber F. Dexamethasone-mediated oncogenicity in vitro and in an animal model of glioblastoma. J. Neurosurg. 2018;12:1–10. doi: 10.3171/2017.7.JNS17668. [DOI] [PubMed] [Google Scholar]
- Marino A., Ciofani G., Filippeschi C., Pellegrino M., Pellegrini M., Orsini P., Pasqualetti M., Mattoli V., Mazzolai B. Two-photon polymerization of sub-micrometric patterned surfaces: investigation of cell-substrate interactions and improved differentiation of neuron-like cells. ACS Appl. Mater. Interfaces. 2013;5:13012–13021. doi: 10.1021/am403895k. [DOI] [PubMed] [Google Scholar]
- Marino A., Tonda-Turo C., De Pasquale D., Ruini F., Genchi G., Nitti S., Cappello V., Gemmi M., Mattoli V., Ciardelli G. Gelatin/nanoceria nanocomposite fibers as antioxidant scaffolds for neuronal regeneration. Biochim. Biophys. Acta. 2017;1861:386–395. doi: 10.1016/j.bbagen.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Min K.J., Lee J.T., Joe E.H., Kwon T.K. An IκBα phosphorylation inhibitor induces heme oxygenase-1(HO-1) expression through the activation of reactive oxygen species (ROS)-Nrf2-ARE signaling and ROS-PI3K/Akt signaling in an NF-κB-independent mechanism. Cell Signal. 2011;23:1505–1513. doi: 10.1016/j.cellsig.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Mitra R.N., Gao R., Zheng M., Wu M.J., Voinov M.A., Smirnov A.I., Smirnova T.I., Wang K., Chavala S., Han Z. Glycol chitosan engineered autoregenerative antioxidant significantly attenuates pathological damages in models of age-related macular degeneration. ACS Nano. 2017;11:4669–4685. doi: 10.1021/acsnano.7b00429. [DOI] [PubMed] [Google Scholar]
- Najafi R., Hosseini A., Ghaznavi H., Mehrzadi S., Sharifi A.M. Neuroprotective effect of cerium oxide nanoparticles in a rat model of experimental diabetic neuropathy. Brain Res. Bull. 2017;131:117–122. doi: 10.1016/j.brainresbull.2017.03.013. [DOI] [PubMed] [Google Scholar]
- Oró D., Yudina T., Fernández-Varo G., Casals E., Reichenbach V., Casals G., González de la Presa B., Sandalinas S., Carvajal S., Puntes V. Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J. Hepatol. 2016;64:691–698. doi: 10.1016/j.jhep.2015.10.020. [DOI] [PubMed] [Google Scholar]
- Pagliari F., Mandoli C., Forte G., Magnani E., Pagliari S., Nardone G., Licoccia S., Minieri M., Di Nardo P., Traversa E. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano. 2012;6:3767–3775. doi: 10.1021/nn2048069. [DOI] [PubMed] [Google Scholar]
- Qian Y., Han Q., Zhao X., Song J., Cheng Y., Fang Z., Ouyang Y., Yuan W.E., Fan C. 3D melatonin nerve scaffold reduces oxidative stress and inflammation and increases autophagy in peripheral nerve regeneration. J. Pineal Res. 2018;65:e12516. doi: 10.1111/jpi.12516. [DOI] [PubMed] [Google Scholar]
- Qian Y., Song J., Zhao X., Chen W., Ouyang Y., Yuan W., Fan C. 3D fabrication with integration molding of a graphene oxide/polycaprolactone nanoscaffold for neurite regeneration and angiogenesis. Adv. Sci. 2018;5:1700499. doi: 10.1002/advs.201700499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Song J., Zheng W., Zhao X., Ouyang Y., Yuan W., Fan C. 3D manufacture of gold nanocomposite channels facilitates neural differentiation and regeneration. Adv. Funct. Mater. 2018;28:1707077. [Google Scholar]
- Qian Y., Zhao X., Han Q., Chen W., Li H., Yuan W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018;9:323. doi: 10.1038/s41467-017-02598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu T., Yin Y., Li B., Xie L., Yan Q., Dai H., Wang X., Li S. PDLLA/PRGD/β-TCP conduits build the neurotrophin-rich microenvironment suppressing the oxidative stress and promoting the sciatic nerve regeneration. J. Biomed. Mater.Res. A. 2014;102:3734–3743. doi: 10.1002/jbm.a.35078. [DOI] [PubMed] [Google Scholar]
- Rasekh M., Ahmad Z., Frangos C.C., Bozec L., Edirisinghe M., Day R.M. Spatial and temporal evaluation of cell attachment to printed polycaprolactone microfibres. Acta Biomater. 2013;9:5052–5062. doi: 10.1016/j.actbio.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Sharifi F., Patel B.B., Dzuilko A.K., Montazami R., Sakaguchi D.S., Hashemi N. Polycaprolactone microfibrous scaffolds to navigate neural stem cells. Biomacromolecules. 2016;17:3287–3297. doi: 10.1021/acs.biomac.6b01028. [DOI] [PubMed] [Google Scholar]
- Sun Y., Jin C., Li K., Zhang Q., Geng L., Liu X., Zhang Y. Restoration of orbicularis oculi muscle function in rabbits with peripheral facial paralysis via an implantable artificial facial nerve system. Exp. Ther. Med. 2017;14:5289–5296. doi: 10.3892/etm.2017.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Yang W., Mao X. Agarose/collagen composite scaffold as an anti-adhesive sheet. Biomed. Mater. 2007;2:S129–S134. doi: 10.1088/1748-6041/2/3/S09. [DOI] [PubMed] [Google Scholar]
- Tian T., Yu Z., Zhang N., Chang Y., Zhang Y., Zhang L., Zhou S., Zhang C., Feng G., Huang F. Modified acellular nerve-delivering PMSCs improve functional recovery in rats after complete spinal cord transection. Biomater.Sci. 2017;5:2480–2492. doi: 10.1039/c7bm00485k. [DOI] [PubMed] [Google Scholar]
- Tojo T., Ushio-Fukai M., Yamaoka-Tojo M., Ikeda S., Patrushev N., Alexander R.W. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- Uz M., Büyüköz M., Sharma A.D., Sakaguchi D.S., Altinkaya S.A., Mallapragada S.K. Gelatin-based 3D conduits for transdifferentiation of mesenchymal stem cells into Schwann cell-like phenotypes. Acta Biomater. 2017;53:293–306. doi: 10.1016/j.actbio.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Vassie J.A., Whitelock J.M., Lord M.S. Endocytosis of cerium oxide nanoparticles and modulation of reactive oxygen species in human ovarian and colon cancer cells. Acta Biomater. 2017;50:127–141. doi: 10.1016/j.actbio.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhu H., Guo Q., Qian T., Zhang P., Li S., Xue C., Gu X. Overlapping mechanisms of peripheral nerve regeneration and angiogenesis following sciatic nerve transection. Front. Cell. Neurosci. 2017;11:323. doi: 10.3389/fncel.2017.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Li H., Hou X., Li D., He S., Wan C., Yin P., Liu M., Liu F., Xu J. Punicalagin induces Nrf2/HO-1 expression via upregulation of PI3K/AKT pathway and inhibits LPS-induced oxidative stress in RAW264.7 macrophages. Mediators Inflamm. 2015;2015:380218. doi: 10.1155/2015/380218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C., Wang W., Wang P., Zhao M., Li X., Zhang F. Near-infrared upconversion mesoporous cerium oxide hollow biophotocatalyst for concurrent pH-/H2O2-responsive O2-evolving synergetic cancer therapy. Adv. Mater. 2018;30 doi: 10.1002/adma.201704833. [DOI] [PubMed] [Google Scholar]
- Yarar E., Kuruoglu E., Kocabıcak E., Altun A., Genc E., Ozyurek H., Kefeli M., Marangoz A.H., Aydın K., Cokluk C. Electrophysiological and histopathological effects of mesenchymal stem cells in treatment of experimental rat model of sciatic nerve injury. Int. J. Clin. Exp. Med. 2015;8:8776–8784. [PMC free article] [PubMed] [Google Scholar]
- Yi J., Jiang N., Li B., Yan Q., Qiu T., Swaminatha Iyer K., Yin Y., Dai H., Yetisen A.K., Li S. Painful terminal neuroma prevention by capping PRGD/PDLLA conduit in rat sciatic nerves. Adv. Sci. 2018;5:1700876. doi: 10.1002/advs.201700876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimowska M., Kasprzycka P., Bocian K., Delaney K., Jung P., Kuchcinska K., Kaczmarska K., Gladysz D., Streminska W., Ciemerych M.A. Inflammatory response during slow- and fast-twitch muscle regeneration. Muscle Nerve. 2017;55:400–409. doi: 10.1002/mus.25246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.