The wealth of studies on microbial communities has revealed the complexity and dynamics of the composition of communities in many ecological settings. Fewer studies probe the functional interactions of the community members. Function of the community as a whole may not be fully revealed by characterizing the individuals. In our two-species model community, we find an emergent trait resulting from the interaction of the soil bacteria Pseudomonas fluorescens Pf0-1 and Pedobacter sp. V48. Observation of emergent traits suggests there may be many functions of a community that are not predicted based on a priori knowledge of the community members. These types of studies will provide a more holistic understanding of microbial communities, allowing us to connect information about community composition with behaviors determined by interspecific interactions. These studies increase our ability to understand communities, such as the soil microbiome, plant-root microbiome, and human gut microbiome, with the final goal of being able to manipulate and rationally improve these communities.
KEYWORDS: Pseudomonas fluorescens, interspecies interaction, motility, sociomicrobiology
ABSTRACT
Bacteria often live in complex communities in which they interact with other organisms. Consideration of the social environment of bacteria can reveal emergent traits and behaviors that would be overlooked by studying bacteria in isolation. Here we characterize a social trait which emerges upon interaction between the distantly related soil bacteria Pseudomonas fluorescens Pf0-1 and Pedobacter sp. strain V48. On hard agar, which is not permissive for motility of the monoculture of either species, coculture reveals an emergent phenotype that we term “interspecies social spreading,” where the mixed colony spreads across the hard surface. We show that initiation of social spreading requires close association between the two species of bacteria. Both species remain associated throughout the spreading colony, with reproducible and nonhomogenous patterns of distribution. The nutritional environment influences social spreading: no social behavior is observed under high-nutrient conditions, but low-nutrient conditions are insufficient to promote social spreading without high salt concentrations. This simple two-species consortium is a tractable model system that will facilitate mechanistic investigations of interspecies interactions and provide insight into emergent properties of interacting species. These studies will contribute to the broader knowledge of how bacterial interactions influence the functions of communities they inhabit.
IMPORTANCE The wealth of studies on microbial communities has revealed the complexity and dynamics of the composition of communities in many ecological settings. Fewer studies probe the functional interactions of the community members. Function of the community as a whole may not be fully revealed by characterizing the individuals. In our two-species model community, we find an emergent trait resulting from the interaction of the soil bacteria Pseudomonas fluorescens Pf0-1 and Pedobacter sp. V48. Observation of emergent traits suggests there may be many functions of a community that are not predicted based on a priori knowledge of the community members. These types of studies will provide a more holistic understanding of microbial communities, allowing us to connect information about community composition with behaviors determined by interspecific interactions. These studies increase our ability to understand communities, such as the soil microbiome, plant-root microbiome, and human gut microbiome, with the final goal of being able to manipulate and rationally improve these communities.
INTRODUCTION
Within soils live a plethora of microbial species that form complex communities responsible for important ecological functions, such as nutrient cycling and plant health. Omics approaches have given us a wealth of information on the composition, diversity, metabolic potential, and ecology of plant- and soil-associated microbial communities (1, 2). However, to get a complete understanding of microbial functions and interactions within these environments, we must look at every layer, from the full community in vivo to the individual microbe in vitro (3). Historically, research has focused on the study of single species in pure culture, but bacteria are social organisms. Thus, the study of the mechanisms and consequences of multispecies interactions is necessary for us to understand the function of microbial communities as a whole. Investigating entire soil communities in situ presents considerable challenges because of fluctuating soil conditions and the wide range of relevant scales, ranging from particulate to ecological levels (2). Reducing the microbial community to pairwise interactions or small consortia allows for a detailed mechanistic study. This reduction is also an essential link between studying isolated microbes in the laboratory and understanding the collective activities of natural microbial communities (4).
Recent work has considered the social environment of bacteria, investigating altered behaviors and production of secondary metabolites when cocultured with other organisms. Some bacteria exhibit emergent behaviors when presented with other species, likely the result of induction of genes that are not expressed in pure culture. For example, some Pseudomonas fluorescens strains produce an antifungal compound during interactions with other species (5–9). The coculture of different actinomycete species results in the production of secondary metabolites, changes in pigment, and sporulation (10–12). The presence of Escherichia coli or Pseudomonas species affects sporulation and biofilm formation in Bacillus subtilis (13, 14). One subset of social interactions are those which alter the motility behaviors and capabilities of other species. For example, physical association with Saccharomyces cerevisiae results in Streptomyces venezuelae consuming the yeast and triggers “exploratory growth” of the bacteria (15). This exploration is not observed when S. venezuelae is grown in monoculture, under the same environmental conditions. In another example, B. subtilis moves away from a Streptomyces competitor across a solid surface but does not do so in isolation (16, 17). Other behaviors appear less competitive, where a motile species will travel with a nonmotile species that can degrade antibiotics, allowing the consortium to colonize hostile environments (18, 19). Xanthomonas perforans can even change the behavior of Paenibacillus vortex, producing a signal that induces P. vortex to swarm toward it so it can hitchhike (20).
Pseudomonas fluorescens Pf0-1 and Pedobacter sp. strain V48 are known to interact though diffusible and volatile signals, which induce production of an antifungal compound by P. fluorescens (6–8). Previous studies with Pedobacter and a strain closely related to P. fluorescens Pf0-1 (AD21) found that the mixture of the strains showed reciprocal gene expression changes and antagonistic behavior toward the plant pathogen Rhizoctonia solani (5, 9). The initial study noted expansion of the mixed strains beyond the initial area of inoculation (5), but the phenotype was not characterized and has not been the focus of any further studies. We investigated this observation using a new assay. Instead of culturing P. fluorescens Pf0-1 and Pedobacter without contact, as was done in the antagonism assays (6), we mix them together. We hypothesized that, while antibiotic production can be induced at a distance through diffusible or volatile signals, the motility behavior requires close contact and is therefore controlled in a manner distinct from the other two forms of communication.
In this study, we describe an interaction between two distantly related soil bacteria, P. fluorescens Pf0-1 (phylum: Proteobacteria) and Pedobacter sp. V48 (phylum: Bacteroidetes). This interaction produces an emergent behavior, which we term “interspecies social spreading,” in which the bacteria move together across a hard agar surface. When grown in isolation, neither species moves beyond the typical amount of colony expansion. In coculture, both bacteria are present throughout the spreading colony, and fluorescent imaging shows a nonhomogenous distribution. We demonstrate that a close association between the colonies of the two species is required for spreading to initiate and that the levels of nutrients and salts in the medium affect the development of the spreading phenotype.
RESULTS
Interspecies social spreading arises when mixing two distantly related bacteria.
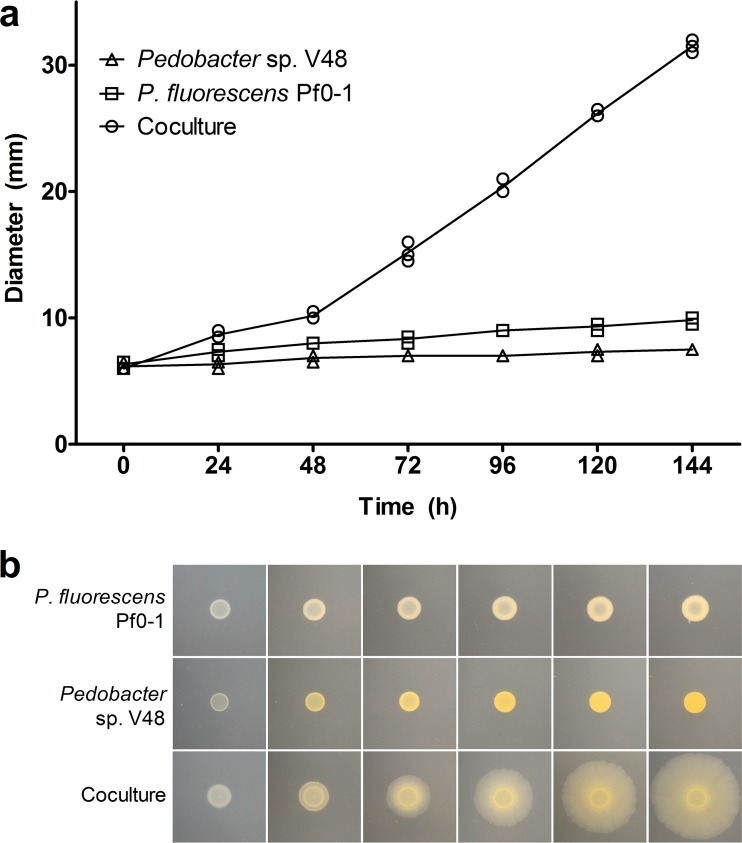
In previous studies, antifungal activity was observed when P. fluorescens Pf0-1 and Pedobacter sp. V48 were cultured 15 mm apart (6). In addition to this interaction-induced trait, the possibility of motility was noted, but not further investigated, in a mixture of Pseudomonas sp. strain AD21 and Pedobacter (5, 9). To explore this phenomenon, we developed an assay in which the induced motility is greater and more easily observed. Our approach differed from the conditions of the original observation in inoculation method, strain combination, and medium composition. When we plated P. fluorescens Pf0-1 and Pedobacter on TSB-NK medium solidified with 2% agar, a mixed colony of the two bacteria expanded across the surface of the agar, an environment in which neither monoculture exhibited motility. The emergent social spreading is shown in Fig. 1.
FIG 1.
Mixed colony of P. fluorescens Pf0-1 and Pedobacter sp. V48 spreads across a hard surface (2% agar), a behavior not observed in the monoculture of either species. (a) Diameter of colonies at 24-h intervals for three independent experiments. (b) Phenotypes of mono- and cocultures at 24-h intervals, starting at 24 h. Contrast and brightness levels were adjusted for optimal viewing.
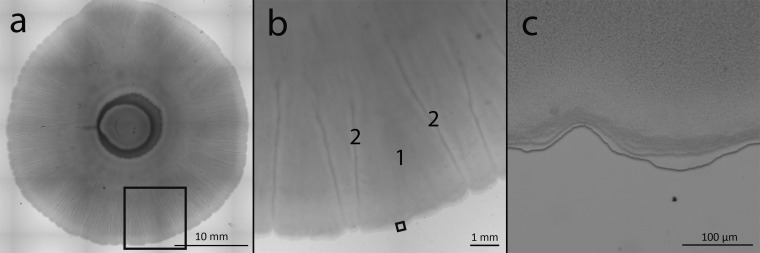
Social spreading becomes apparent between 24 and 48 h after inoculation, when the colony begins to spread from the edge of the inoculum (Fig. 1b, 48 h). The diameter of the spreading coculture is significantly different from the colony expansion of the monocultures starting at the 24-h time point (P < 0.001) (Fig. 1a). Once the spreading phenotype is fully visible (around 72 h), the average speed of expansion is 1.69 ± 0.09 (SEM) µm/min. At the onset of movement, the leading edge has a visibly thicker front (Fig. 1b, 48 h). As the colony spreads, the thick front disappears and small “veins” radiating from the center develop. Over time, the “veins” become more pronounced toward the leading edge, making a “petal” pattern (Fig. 2a and b). The leading edge is characterized by a distinctive, terraced appearance comprised of three to six layers (Fig. 2c). Varying the initial Pseudomonas/Pedobacter ratios between 5:1 and 1:5 did not have a visible effect on spreading across the plate (data not shown).
FIG 2.
Mixed colony of P. fluorescens Pf0-1 and Pedobacter sp. V48 at different magnifications. (a) Image of the whole coculture colony created by stitching an ×8 magnification mosaic. (b) ×16 magnification of the leading edge showing the patterns of “petals” (1) in between “veins” (2) visible near the edge of the colony. (c) ×112 magnification shows a terraced appearance of the leading edge. Colony imaged 144 h after inoculation. Black boxes indicate area enlarged in the adjacent panel. Scale bars are noted at the bottom of each image. Contrast and brightness levels were adjusted for optimal viewing.
P. fluorescens Pf0-1 and Pedobacter sp. V48 comigrate.
The previously observed “bacterial expansion” of Pedobacter when interacting with Pseudomonas sp. AD21 was suggested to be gliding motility, triggered as a mechanism to escape competition from AD21 (5, 9). We examined the possibility that the spreading observed when coinoculating Pedobacter and P. fluorescens Pf0-1 was a result of Pedobacter moving away from P. fluorescens. Bacteria were collected from the center, middle, and edge of a 7-day-old motile colony. The presence or absence of each species was tested by culturing these samples on selective media. We recovered both species from each point in the spreading colony (data not shown), showing comigration rather than an escape strategy by Pedobacter.
Interspecies social spreading shows reproducible spatial organization.
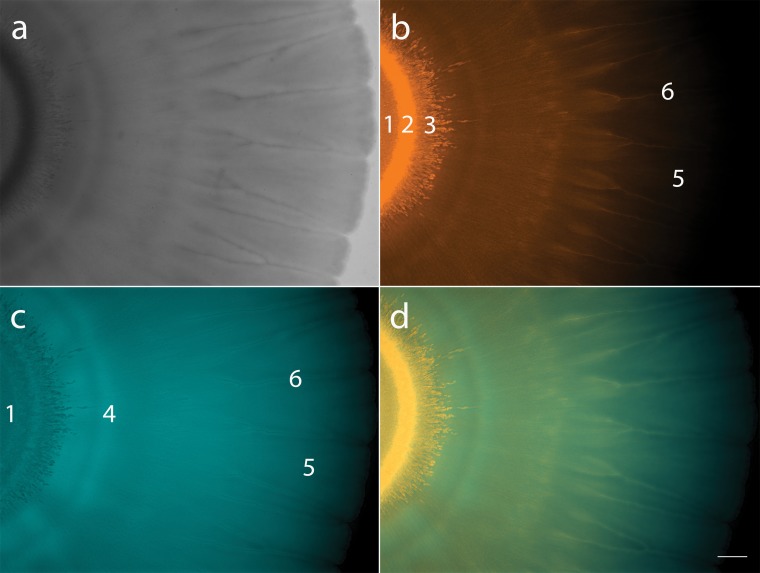
To obtain a more detailed look at the spatial relationships within the spreading colony, we tagged P. fluorescens with a cyan fluorescent protein (eCFP [21]) and Pedobacter with a red fluorescent protein (dsRedEXPRESS [21]), integrated into the chromosome. In P. fluorescens, eCFP carried by a mini-Tn7 transposon was integrated upstream of glmS (22), creating Pf0-ecfp. In Pedobacter, dsRedEXPRESS carried by the HimarEm transposon (23) was integrated at random locations in the chromosome, resulting in 16 independently derived mutants with an insert. Each tagged Pedobacter strain (V48-dsRed) was indistinguishable from the wild type in social assays with P. fluorescens, indicating no deleterious impact of the insertions. We picked one strain with an insert in locus N824_RS25465 (GenBank accession no. NZ_AWRU01000034) and no apparent defect in social spreading. The initiation of social spreading appeared slightly delayed in a mixture of the tagged strains, but the visible patterns and stages of development looked identical, and speed was not significantly different once spreading initiated.
Fluorescent microscopy verified culturing data that showed both bacteria are present throughout the spreading colony, but we also found that population density varies across distinct areas within the colony. These distribution patterns were highly reproducible and show six distinct zones (Fig. 3). At zone 1, the point of inoculation, fluorescent imaging shows a homogenous mix of the two bacteria (Fig. 3b and c). Zone 2, the coffee ring effect formed at the edge of the point of inoculation (24–26), is bright orange, indicating that Pedobacter dominates this region (Fig. 3b). Pedobacter spreads out from this dense area into zone 3, in a starburst pattern (Fig. 3b). Just outward from the starburst, we see a blue ring (zone 4), where P. fluorescens appears more abundant (Fig. 3c and d). In the main body of the coculture, a thin motile section spreads out, making “petals” (zone 5), with “veins” (zone 6) between them (Fig. 2b and Fig. 3a). The “veins” between the “petals” appear to have high Pedobacter populations (Fig. 3b), while the areas directly surrounding them are dominated by P. fluorescens (Fig. 3c). The flat areas of the “petals” appear more well-mixed, though the red signal becomes difficult to detect toward the edge of the colony (Fig. 3d). Overall, imaging data show that we can find both species throughout the colony, but the distribution is not homogenous. Rather, we observed reproducible patterns with some well-mixed areas and others of high spatial assortment.
FIG 3.
Mixed colony of fluorescently tagged P. fluorescens Pf0-1 (Pf0-ecfp) and Pedobacter sp. V48 (V48-dsRed). (a) Coculture colony viewed with white light. (b) Coculture imaged using DsRed filter (filter set 43 HE), pseudocolored in orange, showing V48-dsRed distribution throughout the colony. (c) Coculture imaged using CFP filter (filter set 47 HE), pseudocolored in turquoise, showing Pf0-ecfp distribution throughout the colony. (d) Merged images of DsRed and CFP filters. Numbers in panels b and c indicate six zones of distinct patterns: 1, point of inoculation; 2, coffee ring; 3, starburst; 4, P. fluorescens ring; 5, petals; 6, veins. Colonies imaged at ×7 magnification; scale bar represents 1 mm. Colony imaged 144 h after inoculation.
Diffused compounds and heat-killed cells do not trigger interspecies social spreading.
Previous studies demonstrated that interactions between P. fluorescens and Pedobacter were mediated via both diffusible and volatile signals (6–8). We first asked whether spreading could be triggered by diffusible compounds produced by one of the partner species or by the coculture. Mono- and cocultures grown on cellophane membranes were used to precondition our spreading assay agar. After 2 days, cellophane membranes (and the bacteria growing on them) were peeled off the agar. Plates were then inoculated with one of the partner strains to evaluate development of the social spreading phenotype. After 7 days of growth, no sign of spreading beyond normal colony expansion was observed (change in diameter was not significantly different from negative control), indicating that no motility-inducing compounds had been secreted into the agar.
As the signal did not appear to diffuse through cellulose, we next asked if inactive cells or cell fragments of each species could trigger spreading in the other species. To address this, we used dead cells from one species, or from the spreading coculture. Mono- and cocultures were grown on TSB-NK medium (as previously described) for 4 days, suspended in phosphate buffer, and heat killed at 65°C for 15 min. This heat-killed suspension was added directly on top of growing colonies of each species, or to wells adjacent to the colony being tested. Heat-killed suspension was added every 24 h for 5 days. The plates were monitored for 10 days, but no social spreading was observed under any condition, beyond that due to physical disruption which is also present in the buffer control.
Physical association of P. fluorescens Pf0-1 and Pedobacter sp. V48 is required for interspecies social spreading.
Because a diffusible signal was unlikely to be triggering social spreading, we asked whether a close association between the two bacteria was a necessary condition for the interaction. To answer this question, we used assays in which the bacterial participants were plated side by side with no physical barrier and in which they were separated only by semipermeable membranes.
When colonies were adjacent, rather than mixed, no social spreading was observed while the P. fluorescens and Pedobacter colonies were visibly separate (data not shown). However, once the colonies grew sufficiently to make contact (Fig. 4, 24 h), the colony started to spread out from the point of contact (72 h). The spreading front radiates outward (96 h), first developing around the P. fluorescens colony (144 h) and then proceeding to surround the Pedobacter colony (192 h). At this level of resolution, contact between the colonies appears to occur before any social spreading can be seen.
FIG 4.
Social spreading emerges after contact between colonies of P. fluorescens Pf0-1 and Pedobacter sp. V48, left and right in each panel, respectively. Colonies come into contact 24 h after inoculation; the motile front becomes visible 48 h after contact and spreads outward and around the P. fluorescens colony before surrounding the Pedobacter colony. Black spots indicate sampling locations. Pictures taken every 24 h. Scale bar represents 10 mm.
Samples were collected from the edge of the moving front every 24 h after contact, both on a y axis from the point of contact and following the moving front as it wrapped around the P. fluorescens colony (Fig. 4). The presence of each species was tested by culturing these samples on selective media. Both species were culturable at every point sampled (data not shown), showing that Pedobacter is present in the moving front behind the P. fluorescens colony (Fig. 4, 144 h), on the opposite side of where they initially came into contact. This indicates that Pedobacter moves around the P. fluorescens colony on the motile front.
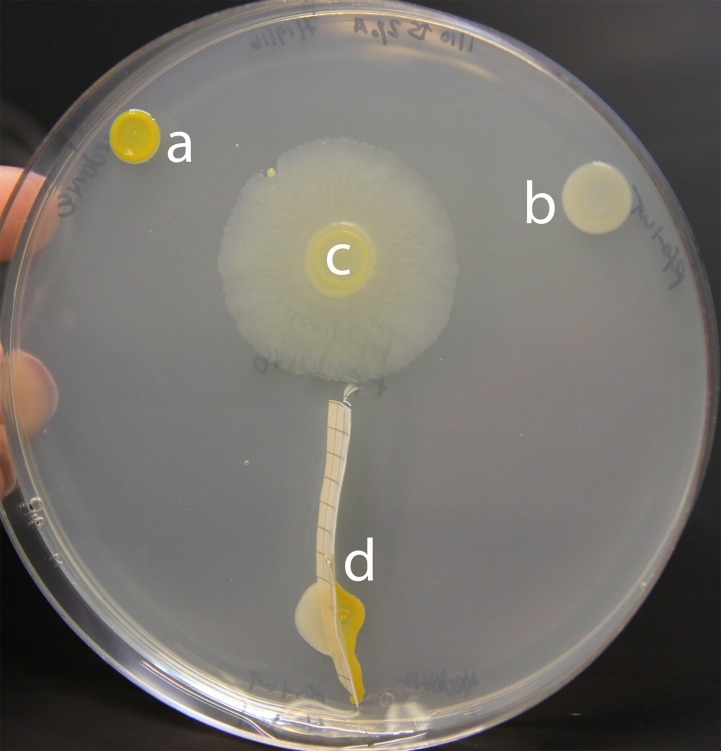
To further evaluate the requirement that P. fluorescens and Pedobacter be physically associated, we inoculated both strains immediately adjacent to each other but separated by either semipermeable mixed-ester cellulose or PES (polyether sulfone) membranes. When inoculated this way, individual colony growth continued as normal, but these bacteria were unable to trigger social spreading despite their close proximity. After 6 days of growth, no sign of interspecies social spreading was observed (Fig. 5).
FIG 5.
A semipermeable barrier prevents development of the interspecies social spreading phenotype. (a) Pedobacter sp. V48 monoculture; (b) P. fluorescens Pf0-1 monoculture; (c) a mixed colony; (d) P. fluorescens and Pedobacter separated by a mixed-ester cellulose membrane. Pictures taken 144 h after inoculation. Colonies were grown on a 100-mm petri dish.
Nutritional environment influences interspecies social spreading.
Conditions in soil and rhizosphere environments fluctuate, with bacteria subjected to a wide range of environmental stressors, including limited nutrient and water availability (2). Because such fluctuations may influence expression of traits, we examined the effect of nutrient level on interspecies social spreading. Our standard assay condition, TSB-NK, consists of 10% strength tryptic soy (3 g/liter) supplemented with NaCl (5 g/liter) and KH2PO4 (1 g/liter).
We first asked if interspecies social spreading could initiate under richer nutrient conditions. No social spreading was apparent when P. fluorescens and Pedobacter were mixed on full-strength TSB (30 g/liter) (Fig. 6a), with the coculture exhibiting the same characteristics and colony expansion as the P. fluorescens monoculture. We next asked whether the salt amendments to TSB-NK influence interspecies social spreading, using assays without the addition of salts, and with the addition of NaCl and KH2PO4 individually. When grown on 10% TSB, the coculture is motile, but the distance spread is modest compared to when the medium is supplemented with both salts (Fig. 6c). The individual P. fluorescens colony expands similarly to the coculture, suggesting minimal social behavior under these conditions. Growth on TSB-K changes neither pattern nor rate of mono- and coculture expansion compared to 10% TSB (data not shown). On TSB-N, the mixed culture spreads and develops the patterns characteristic of interspecies social spreading, while the P. fluorescens monoculture does not expand (Fig. 6d). The phenotype and diameter of the spreading colony are most similar to those observed under TSB-NK conditions (Fig. 6b).
FIG 6.
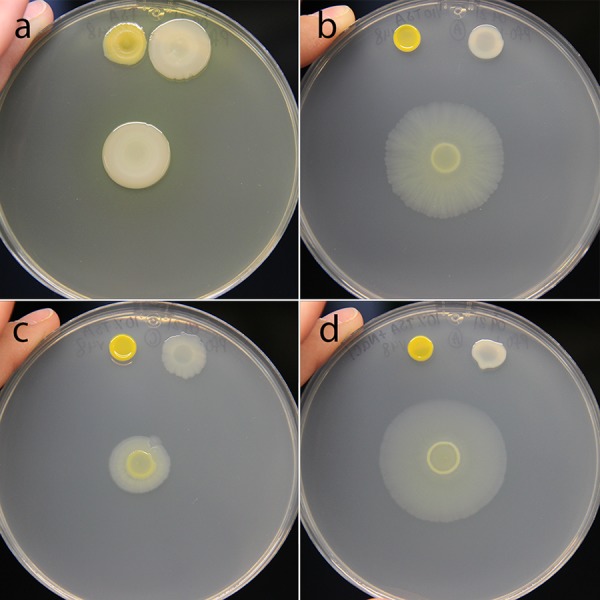
Low-nutrient and high-salt conditions are required for interspecies social spreading. (a) Mixed colony on full-strength TSB does not show social spreading. (b) Mixed colony on TSB-NK (10% tryptic soy supplemented with both NaCl and KH2PO4) shows social spreading. (c) Mixed colony on 10% strength TSB shows impaired social spreading. (d) Mixed colony on TSB-N (supplemented with NaCl) exhibits the interspecies social spreading phenotype. For all panels, Pedobacter sp. V48 monoculture is on the top left of the plate, P. fluorescens Pf0-1 is on the top right of the plate, and the mixed colony is in the center. Pictures were taken 144 h after inoculation. Colonies were grown on a 100-mm petri dish.
In the previous experiment, we observed that variations of tryptic soy medium led to altered social phenotypes. To assess the influence of each component of TSB on interspecies social spreading, we utilized a medium in which these were individually manipulated. We made eight combinations of media to vary d-glucose, tryptone, and NaCl in concentrations equivalent to those in full-strength and 10% TSB. On media with d-glucose or tryptone at full-strength concentrations, we did not observe social spreading regardless of the concentration of the other components (Fig. 7a to f). Under these conditions, the appearance and expansion of the coculture resembled those of the P. fluorescens monoculture, with notably greater biomass in media with full-strength tryptone (Fig. 7a to d). When the concentration of all three components was reduced to 10%, we observed social spreading, but the migration distance of the coculture was modest, and P. fluorescens monoculture expanded to a similar extent (Fig. 7h). On media containing 10% strength d-glucose, 10% strength tryptone, and full-strength NaCl, interspecies social spreading emerged when P. fluorescens and Pedobacter were cocultured (Fig. 7g). Unique to this condition, the monocultures of both strains are immotile, indicating a dramatic change in behavior when strains are mixed. The observations under this condition are most similar to those observed on TSB-N and TSB-NK (Fig. 6b and d).
FIG 7.

Effect of nutrient levels on the interaction between P. fluorescens Pf0-1 and Pedobacter sp. V48, looking at 3 core components of TSB: tryptone (20 g/liter), d-glucose (2.5 g/liter), and NaCl (5 g/liter) for “high” concentrations. Components were reduced to 1/10 for “low” concentrations. For all panels, Pedobacter monoculture is on the top left of the plate, P. fluorescens is on the top right of the plate, and the mixed colony is in the center. Pictures were taken 144 h after inoculation. Colonies were grown on 100-mm petri dishes.
Based on these results, we conclude that full interspecies social spreading was observed only in low-nutrient media supplemented with NaCl (Fig. 6b and d and Fig. 7g). We observed reduced social spreading on low-nutrient media without salt supplementation (Fig. 6c and Fig. 7h), and an absence of social behavior on rich media (Fig. 6a and Fig. 7a to f). While we can implicate salt as an important factor in social spreading, high salt concentrations alone are not sufficient to induce social behavior, as we do not see social behavior under rich medium conditions. This indicates that there may be more than one important nutritional component factored in the decision of these bacteria to socialize.
DISCUSSION
In this study, we investigate interspecies social spreading, a phenomenon that emerges from the interaction of two distantly related soil bacteria. Neither species moves on its own under the conditions of our study, but a mixture of the two species can spread across a hard surface (2% agar). Contact between the two bacterial colonies is required for spreading to initiate, and this association is maintained as the coculture expands. The social phenotype could be observed only under specific nutritional conditions, indicating an interplay between environmental and biological factors. The interaction between Pedobacter and P. fluorescens serves as a simple and tractable model for investigating interspecies interactions. Our research contributes to the growing body of work studying bacteria in social contexts to investigate emergent traits and behaviors.
Surface motility is a trait that could be beneficial to bacteria under a range of environmental conditions. Species related to Pedobacter sp. V48 use gliding motility on 1% agar or glass surfaces (27–29). V48 has not been observed to engage in gliding motility, but we have observed phenotypes similar to sliding motility in other species (30, 31), when inoculated on semisolid agar (unpublished observations). P. fluorescens Pf0-1 is capable of flagella-driven swimming in and swarming motility on semisolid agar (0.3% and 0.6%, respectively) without the need for a partner bacterium (32, 33). Interspecies social spreading is distinct from Pseudomonas flagellar motility in its requirement of the presence of a second species. Additionally, media with higher agar percentages form environments that are nonpermissive for flagella-driven motility in P. fluorescens, as well as most species, but together, Pf0-1 and V48 appear to employ an alternative strategy for movement across hard surfaces.
De Boer et al. (5) suggested that in water agar, the sporadic occurrence of movement they observed indicated a strategy by Pedobacter to escape competition. However, the comigration under our conditions does not support this hypothesis, as the two species remain associated throughout the colony. Our contact experiments provide further evidence, as the presence of Pedobacter in the motile areas surrounding the P. fluorescens colony shows it has moved toward its partner, rather than away from it. The pattern of Pedobacter migration clearly indicates that it is not escaping.
Evidence, both from culturing and from fluorescent imaging, shows that P. fluorescens and Pedobacter comigrate across the hard agar surface. Initiation of the process requires physical contact, as motility is precluded when a semipermeable membrane is placed between the two colonies. We suggest that the nature of this interaction is distinct from contact-dependent toxin delivery systems, such as type VI secretion and contact-dependent growth inhibition, as they commonly mediate signal exchange between closely related species and are involved in competition between more distantly related strains (34–36). While our results do not rule out quorum sensing for communication between the two species (37), a diffusible signal (if it exists) does not appear be sufficient to trigger the motility response. Additionally, our experiment in which bacteria are pregrown on cellophane indicates that social spreading is not triggered by a change in the medium caused by metabolic activity of one of the two species. Our data indicate that physical association is required for social spreading between P. fluorescens and Pedobacter. The question remains: are the bacteria producing a signal which induces an already-present motility mechanism in one species, or are they directly manipulating the environment in a way which facilitates comigration, such as by production of a surfactant? Regardless of which mechanism is used, close association is still a prerequisite for either induction or facilitation of social spreading.
Bacteria dwelling in soil experience variations in a wide range of abiotic conditions, including the key parameters we have tested: salinity and available carbon and nitrogen (2). Environmental conditions have previously been shown to affect motility of individual species; gliding motility in some Flavobacterium species increases with reduced nutrient concentration (38, 39). Changes in behavior resulting from environmental fluctuations can affect how species interact with one another. The ability of P. fluorescens and Pedobacter to spread socially is dependent upon the conditions under which they are growing. In general, high concentrations of glucose and amino acids lead to a buildup of biomass and no apparent social movement. Lower glucose and amino acid concentrations are associated with interspecies social spreading across the plate, but decreasing the salt concentration of the medium slows expansion of the colony. Social spreading resulting from the interaction is conditional, with alteration of just a subset of environmental factors resulting in dramatic changes in behavior. It is tempting to speculate that the consortium of P. fluorescens and Pedobacter can integrate signals from each other’s presence and from the nutrient conditions of their environment to determine whether to behave socially. We see similar examples of intraspecies social behaviors being influenced both by biotic factors (quorum sensing) and by abiotic factors (nutrient conditions) in P. aeruginosa (40), Bacillus subtilis (41), and yeast (42).
There are a wide variety of examples of motility resulting from interspecies interactions, where the presence of a motile partner fosters the motility of an immotile participant. Nonmotile Staphylococcus aureus hitchhikes on swimming P. aeruginosa (43), and Burkholderia cepacia coswarms with P. aeruginosa in environments where it cannot do so independently (19). X. perforans induces motile P. vortex to swarm toward it, which allows it to hitchhike on top of P. vortex rafts (20). P. vortex is also capable of carrying fungal spores or antibiotic-degrading cargo bacteria to cross unfavorable environments (18, 44). In an even more complex system, Dyella japonica can migrate on fungal hyphae, but some strains can do so only in the presence of a Burkholderia terrae helper (45, 46). All of these examples of “hitchhiking” phenomena require one species to already be motile, and stand in contrast to the behavior we have investigated, where social spreading emerges from two conditionally nonmotile participants. The fact that both species are present at the edge of the spreading colony suggests that both have an active role in the behavior, though it does not rule out the possibility of one species inducing motility in the other and hitchhiking, as seen in other systems (20).
In addition to describing a new mode of motility, this discovery highlights the possibility that many functions and behaviors of bacteria in complex communities may be triggered by interactions between different species or even domains. Studying interactions between two or more microorganisms may lead to the discovery of emergent traits that would be impossible to predict based on the study of each organism in isolation. Alongside approaches that characterize the members and connectedness of microbial communities, tools to decipher the phenotypic outcomes of interactions are needed in order to develop a full appreciation of microbiomes. Studies of this type are important for understanding the role of microbial communities within an ecological context.
We have investigated an interaction-dependent trait which emerges under particular nutritional conditions when distantly related bacteria come into close physical contact. This interaction gives the participating bacteria the ability to spread on a hard agar surface, which neither can do alone. This strategy of comigration may serve as an additional mechanism by which plant- and soil-associated bacteria can move in their natural environments, when the conditions do not favor the modes of single-species motility previously described. Given the distant and different locations from which these two strains were isolated, we hypothesize this is not a unique interaction between this pair but rather has evolved between various Pedobacter and Pseudomonas species. To understand the phenomenon, several lines of investigation should be pursued: mechanistic studies which explore the factors each species is contributing to social spreading, the process by which contact triggers motility, whether there are important metabolic interactions, and the way in which environmental conditions are integrated into the decision to move together. The system we study is a tractable model for studying interspecies interactions, giving us the opportunity to answer questions about the nature of interspecies social spreading and ask questions about the broader field of bacterial communities. Models such as these will ultimately lead to a greater understanding of the functions of communities as a whole rather than as collections of individuals.
MATERIALS AND METHODS
Bacterial strains, primers, plasmids, and culture conditions.
Bacterial strains and plasmids are described in Table 1. E. coli was grown at 37°C in LB broth, Miller (Fisher Scientific). Pseudomonas fluorescens Pf0-1 and Pedobacter sp. V48 were routinely grown at 30°C or 20°C, respectively, in 10% strength tryptic soy broth (BD Difco) amended with NaCl and KH2PO4, as described by de Boer et al. (5). This medium is referred to throughout the text as TSB-NK. To differentiate the two species from mixed cultures, we used Pseudomonas minimal medium (PMM) with 25 mM succinate (47) for P. fluorescens and 14.6 mM lactose for Pedobacter. Media were solidified with BD Difco Bacto agar (1.5%, wt/vol) when required, except for social spreading assays, for which 2% agar was used. For experiments with variations in nutrients, we used full-strength TSB (30 g/liter), 10% TSB (3 g/liter), and 10% TSB amended with NaCl or KH2PO4 (called TSB-N or TSB-K, respectively), and a medium composed of d-glucose (2.5 g/liter), tryptone (20 g/liter), and NaCl (5 g/liter). These individual components were used at those concentrations or reduced to 10% concentrations in all eight combinations. For selection of transposon insertions carrying fluorescent protein genes, kanamycin (50 µg/ml), gentamicin (50 µg/ml), or erythromycin (100 µg/ml) was added to the growth medium.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid |
Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli S17-1 | recA thi pro hsdR RP4-2-Tc::Mu-Km::Tn7λpir Smr Tpr | 53 |
| P. fluorescens | ||
| Pf0-1 | Wild type, Apr | 54 |
| Pf0-ecfp | Pf0-1::mini-Tn7 ecfp Gmr | This study |
| Pedobacter | ||
| V48 | Wild type | 55 |
| V48-dsRed | V48 N824_RS25465T899::HimarEm1 PompA-dsRedEXPRESS Emr; NCBI accession no. NZ_AWRU00000000.1, as of 25 September 2017 |
This study |
| Plasmids | ||
| pUC18T-mini-Tn7T-Gm-dsRedEXPRESS | Gmr | 21 |
| pUC18T-mini-Tn7T-Gm-ecfp | Gmr | 21 |
| pUX-BF13 | R6K replicon-based helper plasmid carrying Tn7 transposase genes | 56 |
| pHimarEm1 | Plasmid carrying HimarEm1; Kmr (Emr) | 23 |
| pHimarEm1-dsRed | pHimarEm1Ω(3.529 kb::PompA-dsRedEXPRESS) | This study |
Interspecies social spreading assays.
P. fluorescens and Pedobacter for use in social spreading assays were incubated in 2 ml TSB-NK at 20°C for 24 h, with shaking (160 rpm). Social spreading assays were carried out on TSB-NK solidified with 2% agar. Plates were poured at a temperature of ∼62°C in a single layer and allowed to set for ∼15 min before inoculation. Inoculation was done on freshly poured plates.
(i) Mixed inoculum assays. Assays were started by combining 5 μl of each participant in one spot on the agar surface. As controls, 10-μl spots of each bacterial isolate were plated distant from each other and the coculture, all on the same plate. Once the inoculation liquid had dried, plates were incubated at 20°C. Measurements of the colony diameter were taken every 24 h. Experiments were performed in triplicate.
(ii) Direct contact assay—adjacent plating. P. fluorescens and Pedobacter were grown as described above. The aliquots of bacteria were plated adjacent but without the drops touching. Once the inoculation liquid had dried, plates were incubated at 20°C and monitored daily to determine the time at which colony growth led to contact between the isolates, and when spreading phenotypes developed.
(iii) Direct contact assay—separation by membranes. P. fluorescens and Pedobacter were plated close together, separated only by a membrane. Either Millipore polyether sulfone (PES) Express Plus membranes (0.22-µm pores) or Gelman Sciences mixed-ester cellulose Metricel membranes (0.45-µm pores) were cut into rectangular strips and sterilized by autoclaving. These strips were then embedded into the agar by suspending them perpendicular to the bottom of petri dishes with forceps, as agar was poured into plates. Once set, the filters protruded approximately 5 mm above the agar surface. Bacteria were inoculated on either side of the filter, with 5-µl spots of each species, close enough to touch the filter.
(iv) Cellophane overlay assay. Squares of porous cellophane (GE Healthcare Biosciences Corp.) were placed on top of TSB-NK plates. Cultures of P. fluorescens or Pedobacter or a coculture of the two were placed on top of the cellophane, with cellophane alone used as a negative control. Plates were incubated at 20°C for 2 days, at which point cellophane was removed, and 5-µl spots of either species were placed in the center of the plate, so that cultures were on a plate where cellophane had been (negative control), one where the partner species had been cultured, or one where a mix of the species had been cultured.
(v) Heat-kill assay. Cells were scraped from TSB-NK plates, suspended in PBS buffer, heat killed, and added on top of or adjacent to a colony of Pseudomonas or Pedobacter to test the ability of heat-killed cells to induce movement in the partner species. To place the heat-killed suspension adjacent to living colonies, a well was made in freshly poured agar, by cutting a core using the top end of a 10-µl pipette tip (USA Scientific, Inc.), and partially filling it in using 60 to 70 µl agar. Cultures of Pseudomonas or Pedobacter (5-µl spots) were inoculated adjacent to the well, and the well was filled with the heat-killed suspension. For experiments in which the heat-killed suspension was added directly on top of living colonies, these colonies were initiated with 10-μl spots of liquid culture. The suspensions added directly on top of the colony or to the wells were heat-killed Pseudomonas or coculture on/next to a Pedobacter colony, or heat-killed Pedobacter or coculture on/next to a Pseudomonas colony. These heat-killed cells, or PBS buffer as a negative control, were added to the colonies or wells every 24 h until the end of the experiment. The cells added on top of the colonies or into the wells were extracted from 4-day-old mono- and coculture colonies on TSB-NK, inoculated and cultured as previously described. Whole colonies from these plates were resuspended in 1 ml PBS buffer, vortexed until fully suspended, and then heat killed at 65°C for 15 min. Effectiveness of heat killing was evaluated by plating 100 µl of resuspension on TSB-NK and PMM with succinate or lactose.
Fluorescent protein tagging.
(i) eCFP labeling of P. fluorescens. pUC18T-mini-Tn7T-Gm-ecfp was a gift from Herbert Schweizer (Addgene plasmid no. 65030). A constitutively expressed fluorescent protein gene carried by pUC18T-mini-Tn7T-Gm-ecfp was transferred to P. fluorescens by conjugation from E. coli S17-1, with transposase being provided by pUX-BF13 introduced from a second E. coli S17-1 donor, as previously described (48). Transposon-carrying strains were selected by growth on gentamicin (50 µg/ml), and transposition of the mini-Tn7 element into the target site in the P. fluorescens genome was confirmed by PCR using primers Tn7-F and glmS-R (Table 2). Pf0-1 with fluorescent inserts was tested for alteration in interspecies social spreading by coculturing with Pedobacter, as described above.
TABLE 2.
Primers
| Primer | Sequence (5′–3′) | Purpose | Reference or source |
|---|---|---|---|
| Tn7 F | 5′-CAGCATAACTGGACTGATTTCAG-3′ | Verify integration of transposon into chromosomal glmS locus |
48 |
| glmS R | 5′-TGCTCAAGGGCACTGACG-3′ | Verify integration of transposon into chromosomal glmS locus |
48 |
| PompA-dsRed F | 5′-ACGTTCTCGGAGGAGGCCATCAACGCAACAAAAGAAACTGC-3′ | Amplification of N824_RS25200 promoter to join with dsRed gene |
This study |
| PompA R | 5′-TATGGTACCAGTCATCTAGGCGGCTGTAG-3′ | Amplification of N824_RS25200 promoter to join with dsRed gene; includes KpnI-site for inserting into pHimarEm1 |
This study |
| dsRed F | 5′-TACTCAGGAGAGCGTTCACC-3′ | Amplification of sRed gene with no promoter, to join with V48 N824_RS25200 promoter by SOE PCR |
This study |
| dsRed R | 5′-GCAGTTTCTTTTGTTGCGTTGATGGCCTCCTCCGAGAACGT-3′ | Amplification of dsRed gene with no promoter, to join with V48 N824_RS25200 promoter by SOE PCR; includes KpnI-site for inserting into pHimarEm1 |
This study |
| pHimar KpnI-flank F | 5′-CTGCCCTGCAATCGACCTCG-3′ | Verify ligation of dsRed into pHimarEm1 |
This study |
| pHimar KpnI-flank R | 5′-CAGATAGCCCAGTAGCTGAC-3′ | Verify ligation of dsRed into pHimarEm1 |
This study |
| erm F | 5′-CCGCACCCAAAAAGTTGCAT-3′ | Verify integration of transposon into V48 chromosome |
This study |
| erm R | 5′-GACAATGGAACCTCCCAGAA-3′ | Verify integration of transposon into V48 chromosome |
This study |
| ARB1 | 5′-GGCCACGCGTCGACTAGTACNNNNNNNNNNGATAT-3′ | Find location of transposon integration in V48 chromosome |
52 |
| ARB6 | 5′-GGCCACGCGTCGACTAGTACNNNNNNNNNNACGCC-3′ | Find location of transposon integration in V48 chromosome |
52 |
| ARB2 | 5′-GGCCACGCGTCGACTAGTAC-3′ | Find location of transposon integration in V48 chromosome |
52 |
| Himar Arb1 (TnExt) | 5′-GTGTTGTTCCAGTTTGAGATC-3′ | Find location of transposon integration in V48 chromosome |
This study |
| Himar609 Arb2 (TnInt) | 5′-TGGGAATCATTTGAAGGTTGG-3′ | Find location of transposon integration in V48 chromosome |
23 |
(ii) dsRedEXPRESS labeling of Pedobacter. pUC18T-mini-Tn7T-Gm-dsRedExpress was a gift from Herbert Schweizer (Addgene plasmid no. 65032). To express dsRedEXPRESS in Pedobacter, a Pedobacter promoter was cloned upstream of the dsRedEXPRESS coding sequence. A highly expressed gene from an unpublished RNA-seq experiment was identified (N824_RS25200), and the upstream 300 bp were amplified from Pedobacter genomic DNA using primers PompA and dsRed, designed for splicing-by-overlap extension–PCR (SOE-PCR) (Table 2). The promoter was then spliced with the amplified dsRedEXPRESS coding sequence using SOE-PCR (49). Flanking primers were designed with KpnI restriction sites, enabling cloning of the spliced product into a KpnI site in pHimarEm1 (23). To join compatible ends between the plasmid and the amplicons, we used T4 DNA ligase (New England Biolabs, Inc.). The ligated plasmid was introduced into E. coli S17-1 competent cells by electroporation (Bio-Rad Micropulser). S17-1 colonies carrying the plasmid were selected by plating on LB medium containing kanamycin (50 µg/ml), and the presence of the dsRedEXPRESS gene was confirmed by PCR, using pHimar KpnI-flank primers (Table 2). The resulting plasmid is called pHimarEm1-dsRed.
pHimarEm1-dsRed was transferred to Pedobacter by conjugation using a method adapted from Hunnicutt and McBride (50). Briefly, 20-h-old cultures of E. coli S17-1 (pHimarEm1-dsRed) and Pedobacter were subcultured 1:100 into fresh LB and grown to mid-exponential phase (E. coli) or for 7 h (Pedobacter). Cells were collected by centrifugation, suspended in 100 µl of LB, and then mixed in equal amounts on TSB-NK with 100 µl of 1 M CaCl2 spread on the surface. Following overnight incubation at 30°C, cells were scraped off the surface of the plate, and dilutions were plated on TSB-NK with erythromycin (100 µg/ml) to select for strains that received the plasmid (ermF is not expressed in E. coli). Transconjugants were incubated at 25°C for 3 to 4 days. The presence of the transposon insert in Pedobacter was confirmed using ermF primers (Table 2). Pedobacter with fluorescent inserts was tested for alteration in interspecies social spreading by coculturing with Pseudomonas, as described above.
The transposon insertion sites in the Pedobacter chromosome were amplified by arbitrarily primed PCR (51), using a method adapted from O’Toole et al. (52) (Table 2 lists primers), and identified by sequencing the arb-PCR products. Nucleic acid sequencing was performed by Massachusetts General Hospital CCIB DNA Core. Sequences were analyzed using CLC Genomics Workbench Version 10.1.1 (Qiagen) to find the location of transposon integration.
Imaging.
Still pictures were taken using an EOS Rebel T3i camera (Canon) and processed using Photoshop CC 2017 version: 14.2.1 and Illustrator CC 2017 version: 17.1.0 (Adobe). Using Photoshop, the levels of some images were adjusted to improve contrast.
For microscopy, motile colonies were examined using an Axio Zoom.V16 microscope (Zeiss). To visualize fluorescent strains, filter set 43 HE DsRed was used with a 1.5-s exposure, shown with pseudocolor orange, as well as filter set 47 HE cyan fluorescent protein, with a 600-ms exposure, shown with pseudocolor turquoise. Images were captured using an AxioCam 503 monocamera, with a native resolution of 1,936 by 1,460 pixels. For image acquisition and processing, we used Zen 2 Pro software (Zeiss).
Statistics.
We measured the amount of colony expansion of the monocultures of both P. fluorescens and Pedobacter and the expansion of social spreading in coculture. Colony diameter of three independent experiments was measured every 24 h. To compare the diameters of monocultures and cocultures at each time point, we performed a two-way ANOVA followed by a Bonferroni post hoc test, using GraphPad Prism version 5.04 for Windows (GraphPad Software).
We compared the movement speed between a combination of wild-type P. fluorescens and Pedobacter and a combination of fluorescently tagged Pf0-ecfp and V48-dsRed. Colony diameters of six independent experiments were measured every day, and speed was calculated by dividing the distance traveled by the amount of time elapsed since the last time point. To calculate average speed, we used only time points after the interspecies social spreading phenotype developed. To compare the means of the speed of the wild-type and tagged strains, we conducted an unpaired, two-tailed, Student t test, using GraphPad Prism version 5.04.
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. L.M.M. was supported by a University of Massachusetts Dartmouth Distinguished Doctoral Fellowship.
We thank Brianna Arruda, Michael Baym, Jacob Palmer, Emma Piatelli, and Marian Wahl for constructive criticism and expert advice.
L.M.M. designed and carried out experiments, analyzed data, and wrote the manuscript. A.S.B., S.C.S., and L.M.S. each contributed a key experiment and edited the manuscript. M.W.S. contributed to experimental design, data analysis, and writing and editing of the manuscript.
The authors declare that they have no competing interests.
REFERENCES
- 1.Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. 2013. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- 2.Fierer N. 2017. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol 15:579–590. doi: 10.1038/nrmicro.2017.87. [DOI] [PubMed] [Google Scholar]
- 3.Abreu NA, Taga ME. 2016. Decoding molecular interactions in microbial communities. FEMS Microbiol Rev 40:648–663. doi: 10.1093/femsre/fuw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasche S, Kim Y, Oliveira AP, Patil KR. 2017. Model microbial communities for ecosystems biology. Curr Opin Syst Biol 6:51–57. doi: 10.1016/j.coisb.2017.09.002. [DOI] [Google Scholar]
- 5.de Boer W, Wagenaar A-M, Klein Gunnewiek PJA, van Veen JA. 2007. In vitro suppression of fungi caused by combinations of apparently non-antagonistic soil bacteria. FEMS Microbiol Ecol 59:177–185. doi: 10.1111/j.1574-6941.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 6.Garbeva P, Silby MW, Raaijmakers JM, Levy SB, de Boer W. 2011. Transcriptional and antagonistic responses of Pseudomonas fluorescens Pf0-1 to phylogenetically different bacterial competitors. ISME J 5:973–985. doi: 10.1038/ismej.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garbeva P, Tyc O, Remus-Emsermann MNP, van der Wal A, Vos M, Silby M, de Boer W. 2011. No apparent costs for facultative antibiotic production by the soil bacterium Pseudomonas fluorescens Pf0-1. PLoS One 6:e27266. doi: 10.1371/journal.pone.0027266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garbeva P, Hordijk C, Gerards S, de Boer W. 2014. Volatile-mediated interactions between phylogenetically different soil bacteria. Front Microbiol 5:289. doi: 10.3389/fmicb.2014.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garbeva P, de Boer W. 2009. Inter-specific interactions between carbon-limited soil bacteria affect behavior and gene expression. Microb Ecol 58:36–46. doi: 10.1007/s00248-009-9502-3. [DOI] [PubMed] [Google Scholar]
- 10.Seyedsayamdost MR, Traxler MF, Zheng S-L, Kolter R, Clardy J. 2011. Structure and biosynthesis of amychelin, an unusual mixed-ligand siderophore from Amycolatopsis sp. AA4. J Am Chem Soc 133:11434–11437. doi: 10.1021/ja203577e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traxler MF, Seyedsayamdost MR, Clardy J, Kolter R. 2012. Interspecies modulation of bacterial development through iron competition and siderophore piracy. Mol Microbiol 86:628–644. doi: 10.1111/mmi.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traxler MF, Watrous JD, Alexandrov T, Dorrestein PC, Kolter R. 2013. Interspecies interactions stimulate diversification of the Streptomyces coelicolor secreted metabolome. mBio 4:e00459-13. doi: 10.1128/mBio.00459-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powers MJ, Sanabria-Valentin E, Bowers AA, Shank EA. 2015. Inhibition of cell differentiation in Bacillus subtilis by Pseudomonas protegens. J Bacteriol 197:2129–2138. doi: 10.1128/JB.02535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandchamp GM, Caro L, Shank EA. 2017. Pirated siderophores promote sporulation in Bacillus subtilis. Appl Environ Microbiol 83:e03293-16. doi: 10.1128/AEM.03293-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones SE, Ho L, Rees CA, Hill JE, Nodwell JR, Elliot MA. 2017. Streptomyces exploration is triggered by fungal interactions and volatile signals. Elife 6:e21738. doi: 10.7554/eLife.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stubbendieck RM, Straight PD. 2015. Escape from lethal bacterial competition through coupled activation of antibiotic resistance and a mobilized subpopulation. PLoS Genet 11:e1005722. doi: 10.1371/journal.pgen.1005722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Kyle S, Straight PD. 2018. Antibiotic stimulation of a Bacillus subtilis migratory response. mSphere 3:e00586-17. doi: 10.1128/mSphere.00586-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finkelshtein A, Roth D, Ben Jacob E, Ingham CJ. 2015. Bacterial swarms recruit cargo bacteria to pave the way in toxic environments. mBio 6:e00074-15. doi: 10.1128/mBio.00074-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venturi V, Bertani I, Kerényi Á, Netotea S, Pongor S. 2010. Co-swarming and local collapse: quorum sensing conveys resilience to bacterial communities by localizing cheater mutants in Pseudomonas aeruginosa. PLoS One 5:e9998. doi: 10.1371/journal.pone.0009998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagai E, Dvora R, Havkin-Blank T, Zelinger E, Porat Z, Schulz S, Helman Y. 2014. Surface-motility induction, attraction and hitchhiking between bacterial species promote dispersal on solid surfaces. ISME J 8:1147–1151. doi: 10.1038/ismej.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi KH, Schweizer HP. 2006. Mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc 1:153–161. doi: 10.1038/nprot.2006.24. [DOI] [PubMed] [Google Scholar]
- 22.Lambertsen L, Sternberg C, Molin S. 2004. Mini-Tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environ Microbiol 6:726–732. doi: 10.1111/j.1462-2920.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- 23.Braun TF, Khubbar MK, Saffarini DA, Mcbride MJ, Acteriol JB. 2005. Flavobacterium johnsoniae gliding motility genes identified by mariner mutagenesis. J Bacteriol 187:6943–6952. doi: 10.1128/JB.187.20.6943-6952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanni D, Kalziqi A, Thomas J, Ng SL, Vivek S, Ratcliff WC, Hammer BK, Yunker PJ. 2017. Life in the coffee-ring: how evaporation-driven density gradients dictate the outcome of inter-bacterial competition. arXiv http://arxiv.org/abs/1707.03472.
- 25.Sempels W, De Dier R, Mizuno H, Hofkens J, Vermant J. 2013. Auto-production of biosurfactants reverses the coffee ring effect in a bacterial system. Nat Commun 4:1757. doi: 10.1038/ncomms2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deegan RD, Bakajin O, Dupont TF, Huber G, Nagel SR, Witten TA. 1997. Capillary flow as the cause of ring stains from dried liquid drops. Nature 389:827–829. [Google Scholar]
- 27.McBride MJ. 2001. Bacterial gliding motility: multiple mechanisms for cell movement over surfaces. Annu Rev Microbiol 55:49–75. doi: 10.1146/annurev.micro.55.1.49. [DOI] [PubMed] [Google Scholar]
- 28.McBride MJ, Zhu Y. 2013. Gliding motility and por secretion system genes are widespread among members of the phylum Bacteroidetes. J Bacteriol 195:270–278. doi: 10.1128/JB.01962-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBride MJ. 2004. Cytophaga-flavobacterium gliding motility. J Mol Microbiol Biotechnol 7:63–71. doi: 10.1159/000077870. [DOI] [PubMed] [Google Scholar]
- 30.Martínez A, Torello S, Kolter R. 1999. Sliding motility in Mycobacteria. J Bacteriol 181:7331–7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Rear J, Alberti L, Harshey RM. 1992. Mutations that impair swarming motility in Serratia marcescens 274 include but are not limited to those affecting chemotaxis or flagellar function. J Bacteriol 174:6125–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seaton SC, Silby MW, Levy SB. 2013. Pleiotropic effects of GacA on Pseudomonas fluorescens Pf0-1 in vitro and in soil. Appl Environ Microbiol 79:5405–5410. doi: 10.1128/AEM.00819-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deflaun MF, Tanzer AS, Mcateer AL, Levy SB, Marshall B, Levy SB. 1990. Development of an adhesion assay and characterization of an adhesion-deficient mutant of Pseudomonas fluorescens. Appl Environ Microbiol 56:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallique M, Bouteiller M, Merieau A. 2017. The type VI secretion system: a dynamic system for bacterial communication? Front Microbiol 8:1454. doi: 10.3389/fmicb.2017.01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saak CC, Gibbs KA. 2016. The self-identity protein IdsD is communicated between cells in swarming Proteus mirabilis colonies. J Bacteriol 198:3278–3286. doi: 10.1128/JB.00402-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia EC. 2018. Contact-dependent interbacterial toxins deliver a message. Curr Opin Microbiol 42:40–46. doi: 10.1016/j.mib.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juhas M, Eberl L, Tümmler B. 2005. Quorum sensing: the power of cooperation in the world of Pseudomonas. Environ Microbiol 7:459–471. doi: 10.1111/j.1462-2920.2005.00769.x. [DOI] [PubMed] [Google Scholar]
- 38.Laanto E, Bamford JKH, Laakso J, Sundberg LR. 2012. Phage-driven loss of virulence in a fish pathogenic bacterium. PLoS One 7:e53157. doi: 10.1371/journal.pone.0053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pérez-Pascual D, Menéndez A, Fernández L, Méndez J, Reimundo P, Navais R, Guijarro JA. 2009. Spreading versus biomass production by colonies of the fish pathogen Flavobacterium psychrophilum: role of the nutrient concentration. Int Microbiol 12:207–214. [PubMed] [Google Scholar]
- 40.Boyle KE, Monaco H, van Ditmarsch D, Deforet M, Xavier JB. 2015. Integration of metabolic and quorum sensing signals governing the decision to cooperate in a bacterial social trait. PLoS Comput Biol 11:e1004279. doi: 10.1371/journal.pcbi.1004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazazzera BA. 2000. Quorum sensing and starvation: signals for entry into stationary phase. Curr Opin Microbiol 3:177–182. [DOI] [PubMed] [Google Scholar]
- 42.Chen H, Fink GR. 2006. Feedback control of morphogenesis in fungi by aromatic alcohols. Genes Dev 20:1150–1161. doi: 10.1101/gad.1411806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samad T, Billings N, Birjiniuk A, Crouzier T, Doyle PS, Ribbeck K. 2017. Swimming bacteria promote dispersal of non-motile staphylococcal species. ISME J 11:1933–1937. doi: 10.1038/ismej.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ingham CJ, Kalisman O, Finkelshtein A, Ben-Jacob E. 2011. Mutually facilitated dispersal between the nonmotile fungus Aspergillus fumigatus and the swarming bacterium Paenibacillus vortex. Proc Natl Acad Sci U S A 108:19731–19736. doi: 10.1073/pnas.1102097108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warmink JA, Nazir R, Corten B, van Elsas JD. 2011. Hitchhikers on the fungal highway: the helper effect for bacterial migration via fungal hyphae. Soil Biol Biochem 43:760–765. doi: 10.1016/j.soilbio.2010.12.009. [DOI] [Google Scholar]
- 46.Warmink JA, van Elsas JD. 2009. Migratory response of soil bacteria to Lyophyllum sp. strain Karsten in soil microcosms. Appl Environ Microbiol 75:2820–2830. doi: 10.1128/AEM.02110-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirner S, Krauss S, Sury G, Lam ST, Ligon JM, van Pee K-H. 1996. The non-haem chloroperoxidase from Pseudomonas fluorescens and its relationship to pyrrolnitrin biosynthesis. Microbiology 142:2129–2135. doi: 10.1099/13500872-142-8-2129. [DOI] [PubMed] [Google Scholar]
- 48.Monds RD, Newell PD, Schwartzman JA, O’Toole GA. 2006. Conservation of the Pho regulon in Pseudomonas fluorescens Pf0-1. Appl Environ Microbiol 72:1910–1924. doi: 10.1128/AEM.72.3.1910-1924.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61–68. [DOI] [PubMed] [Google Scholar]
- 50.Hunnicutt DW, McBride MJ. 2000. Cloning and characterization of the Flavobacterium johnsoniae gliding-motility genes gldB and gldC. J Bacteriol 182:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caetano-Anollés G. 1993. Amplifying DNA with arbitrary oligonucleotide primers. PCR Methods Appl 3:85–94. [DOI] [PubMed] [Google Scholar]
- 52.O’Toole GA, Pratt LA, Watnick PI, Newman DK, Weaver VB, Kolter R. 1999. Genetic approaches to study of biofilms. Methods Enzymol 310:91–109. [DOI] [PubMed] [Google Scholar]
- 53.Simon R, Priefer U, Puhler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 54.Compeau G, Al-Achi BJ, Platsouka E, Levy SB. 1988. Survival of rifampin-resistant mutants of Pseudomonas fluorescens and Pseudomonas putida in soil systems. Appl Environ Microbiol 54:2432–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Boer W, Verheggen P, Klein Gunnewiek PJA, Kowalchuk GA, van Veen JA. 2003. Microbial community composition affects soil fungistasis. Appl Environ Microbiol 69:835–844. doi: 10.1128/AEM.69.2.835-844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao Y, Lies DP, Fu H, Roberts GP. 1991. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene 109:167–168. [DOI] [PubMed] [Google Scholar]