Abstract
Insulin-like growth factors (IGFs) are essential for local skeletal muscle growth and organismal physiology, but these actions are entwined with glucose homeostasis through convergence with insulin signaling. The objective of this work was to determine whether the effects of IGF-I on growth and metabolism could be separated. We generated muscle-specific IGF-I–deficient (MID) mice that afford inducible deletion of Igf1 at any age. After Igf1 deletion at birth or in young adult mice, evaluations of muscle physiology and glucose homeostasis were performed up to 16 wk of age. MID mice generated at birth had lower muscle and circulating IGF-I, decreased muscle and body mass, and impaired muscle force production. Eight-wk-old male MID had heightened insulin levels with trends of elevated fasting glucose. This phenotype progressed to impaired glucose handling and increased fat deposition without significant muscle mass loss at 16 wk of age. The same phenotype emerged in 16-wk-old MID mice induced at 12 wk of age, compounded with heightened muscle fatigability and exercise intolerance. We assert that muscle IGF-I independently modulates anabolism and metabolism in an age-dependent manner, thus positioning muscle IGF-I maintenance to be critical for both muscle growth and metabolic homeostasis.—Vassilakos, G., Lei, H., Yang, Y., Puglise, J., Matheny, M., Durzynska, J., Ozery, M., Bennett, K., Spradlin, R., Bonanno, H., Park, S., Ahima, R. S., Barton, E. R. Deletion of muscle IGF-I transiently impairs growth and progressively disrupts glucose homeostasis in male mice.
Keywords: exercise intolerance, diabetes, force generation
Insulin-like growth factor (IGF)-I and insulin are 2 structurally related molecules with convergent and divergent functional roles (1). The liver is considered the major production site of endocrine IGF-I (2, 3), but other tissues, including muscle, produce IGF-I, and this local source acts in a paracrine fashion that is sufficient to maintain growth, even in the absence of liver production. The beneficial effects of IGF-I for muscle growth and repair have been demonstrated in several animal models, including tissue-specific transgenic expression (4–6), viral-mediated gene transfer (7–10), and directed recombinant IGF-I delivery (11). These results have positioned this growth factor as a central therapeutic target for enhancing muscle anabolic function in aging and disease.
In addition to driving growth, IGF-I contributes to glucose homeostasis (12). It has indirect effects through its interplay with insulin and growth hormone (GH), enhancing insulin actions and suppressing GH (13). It also can have direct actions on the glucose handling that may occur through activation of insulin receptors by insulin or hybrid receptors by IGF-I (those comprising insulin/IGF-I hemireceptors) (14). Because skeletal muscle is a primary tissue for glucose disposal (15), the hypertrophic effects of IGF-I on this tissue also contribute to glucose handling by altering the bulk mass available for glucose clearance. We, and others, have proposed that in addition to the paracrine action of IGF-1 in promoting muscle growth, muscle also secretes factors that act in an endocrine manner to regulate organismal growth. Because muscle contributes to the circulating IGF-I pool and is a major sink for glucose uptake, it also has a significant impact on metabolism through both mechanisms.
These actions occur, but distinguishing the direct effects of muscle IGF-I on glucose homeostasis from those indirectly mediated via its paracrine effects on skeletal muscle tissue have been challenging. Further, the problem increases in complexity because of the presence of multiple ligands (IGF-II and insulin) that can interact with IGF-I receptors (IGF-IRs), insulin receptors (IRs), and the pool of hybrid receptors. Removal or inactivation of these receptors in muscle demonstrates the necessity of this signaling pathway for maintaining muscle growth (16, 17). However, mice lacking all muscle Igf-IR/IR complexes exhibit compensation of the glucose transport system, which preserves glucose handling through increased glucose transporter (Glut)-1/4 modulation, despite severe muscle atrophy (16), suggesting that glucose uptake by muscle is an essential property that is maintained regardless of muscle size. This premise is challenged by findings in mice harboring dominant negative expression of Igf1r in muscle, which blocks IGF-IR and hybrid receptor activity (17), where mice exhibit impaired glucose tolerance. In both cases, all ligands are available to bind to any active receptors, thus leaving open the question as to whether all circulating and locally produced ligands can mediate growth and glucose handling in muscle. The goal of the current study was to address the importance of the muscle IGF-I ligand in regulating anabolic and metabolic processes, both locally in muscle and systemically, and whether these actions are dependent on muscle mass.
MATERIALS AND METHODS
Animal studies
Animal studies were performed in accordance with the Institutional Animal Care and Use Committee and approved by the University of Florida and University of Pennsylvania. Male and female mice were used for all studies, unless otherwise stated. Mice were maintained in the animal facility in a 12-h light–dark cycle. Animals had ad libitum access to food and water, and food was withdrawn only if required for an experimental procedure.
Muscle IGF-I–deficient mice (MID) were generated by crossing mice harboring the floxed exon 4 of Igf1 (Igf1fl/fl) (18) (12663; all strains from The Jackson Laboratory, Bar Harbor, ME, USA), with mice carrying the doxycycline (Dox)-inducible Cre recombinase, driven by the human skeletal actin (HSA) promoter (HSA-rtTA/Dox-Cre) (012433). The MID mouse line was crossed with ROSA26 (003474) reporter mice, to monitor deletion, and was backcrossed more than 5 times onto the C57Bl/6 strain (000664) to eliminate strain-dependent variability.
The tetracycline analog Dox was administered to induce Cre transgene expression. Dox was dissolved in drinking water (2 mg/ml) supplemented with 5% sucrose and provided ad libitum to Dox-treated (Dox+) animals in light-proof water bottles for up to 5 d. For birth induction (bMID), Dox was provided to the mothers on postpartum d 0–5. For young adult induction (aMID), Dox was provided to male mice at 12 wk of age. HSA-rtTA/Dox-Cre+ animals lacking floxed Igf1 alleles receiving Dox were used as controls and were randomly assigned to experimental groups.
Body composition analysis by time domain-NMR
The LF90 Minispec Time Domain (TD)-NMR analyzer (Bruker, Spring, TX, USA) was used to determine body composition (fat, lean, and fluid) in conscious live mice (without anesthesia). During this procedure, the mice were placed into a Plexiglas sample holder (90 mm in diameter and 250 mm in length) with ventilation holes provided at both ends and around the tube circumference. The sample holder containing the mouse was then inserted into the 0.5 T magnet bore. The total measurement time was ∼2 min for measurements in triplicate. After the measurements were obtained, the mice were returned to their cages.
Mouse growth measurements
After weaning, male and female mice were weighed twice a week. Complementation of growth deficits in male and female bMID mice was performed by daily subcutaneous injections of mecasermin rinfabate (Iplex; a generous gift from Insmed, Inc., Monmouth Junction, NJ, USA), a complex of recombinant IGF-I and IGF binding protein (IGFBP)-3. The complex was injected at 50 mg/kg and compared with saline injections, within littermate controls, starting after weaning at 4 wk of age to avoid the contribution of circulating IGF-II (19), as well as IGF-I found in mother’s milk (20). Three litters were used to examine the growth phenotype by mecasermin rinfabate.
Plasma measurements
Plasma was obtained from blood samples of male and female control and bMID mice at 4, 8, and 16 wk, and from aMID mice at 16 wk. Blood IGF-I levels were determined with the Rat/Mouse IGF-I ELISA Kit (MG100; R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s recommendations and as described in refs. 4, and 21. This kit detects total rodent Igf-I, and there is no cross-reactivity or interference with Igf-II or Igfbps. The assay can detect Igf-I at 30–2000 pg/ml, with an intra-assay precision of 4.3% and an interassay precision of 5.9%. Data were acquired in duplicate with a SpectraMax M5 Plate Reader (Molecular Devices, Sunnyvale, CA, USA) at 450 nm, and the results were averaged. Data are expressed in nanograms per milliliter. Blood insulin levels (EZRMI-13K; MilliporeSigma, Burlington, MA, USA) were measured according to the manufacturer’s instructions and as described by in another of our studies (22). Data are expressed in picograms per milliliter. Glucose was measured in a tail blood sample by glucometer. Data are expressed in milligrams per deciliter. Blood cholesterol and triglyceride levels were determined in randomly fed mice by Clinical Pathology (University of Florida Veterinary Diagnostics Laboratory, Gainesville, FL, USA). Measurements from randomly fed mice were performed on blood samples obtained between 10 am and 4 pm in fed conditions; unfed measurements were performed at 9 am after 16 h of food deprivation; and fed measurements were performed at 9 am on animals with ad libitum access to food and water.
Tissue IGF-I content
Tissue IGF-I levels were determined with the Rat/Mouse IGF-I ELISA-Kit (MG100; R&D Systems), as previously described and according to the manufacturer’s instructions. Data are expressed as nanograms per gram tissue. Because in previous studies we had observed that the ELISA procedure underestimates IGF-I content when there is a large proportion of glycosylated pro-IGF-I (21), additional muscle lysates from 4-wk-old mice were subjected to immunoblot analysis, as described in a subsequent section, to provide qualitative assessment of IGF-I forms in control and MID mice.
Glucose tolerance test
MID and control mice (8, 12, and 16 wk of age) were transferred to a clean-cage microenviroment and remained unfed for 16 h (5 pm–9 am). Glucose tolerance tests (GTTs) were performed at 9 am. Mice were injected with an intraperitoneal injection of glucose at 2 g/kg body weight (20% glucose w/v in sterile saline), and glucose levels were measured at 0, 15, 30, 60, 90, 120, and 150 min on a tail blood sample.
Functional analysis of extensor digitorum longus and soleus
Isolated muscle function testing was performed on the extensor digitorum longus (EDL) and soleus from 16-wk-old mice, according to published protocols (23). After anesthesia with ketamine and xylazine, the EDL and soleus were removed from the mouse hind limbs and placed in a bath of oxygenated Ringer’s solution (in mM) [120 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgSO4, 25 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 5.5 glucose] gas equilibrated with 95% O2 and 5% CO2. The proximal and distal muscle tendons were tied by nonabsorbable braided silk sutures (Fine Science Tools, Foster City, CA, USA) and the muscle was transferred into a 50 ml Radnoti tissue bath in a mouse in vitro apparatus (Model 800A, Dual Mode Muscle Lever 300C; Aurora Scientific, Aurora, ON, Canada) filled with gas equilibrated Ringer’s solution at 22°C. The distal tendon was attached to a rigid post, and the proximal tendon was attached to a force transducer while resting muscle length was maintained. The optimal muscle length (Lo) was established by adjusting the muscle length in isometric twitch conditions, until maximum force was obtained. At Lo, each muscle was stimulated for a period of 500 ms, with a series of 0.5 ms pulses at supramaximum stimulation and at a fusion frequency (120 Hz for the EDL, and 100 Hz for the soleus), to determine maximum isometric tetanic force. Measurements were performed 3 times with resting periods of 5 min between tetanic stimulations. Fatigue tests were then performed, using a modification of the Burke protocol, with 1 Hz trains of pulses, which were 200 µs pulses at 100 Hz and 330 ms duration, for up to 10 min. To determine resistance to fatigue, the forces relative to the initial force were calculated for each muscle.
After the mechanical procedures, muscles were blotted, weighed, and rapidly frozen for subsequent assays. Muscle-specific forces (N/cm²) were calculated by normalizing the maximum muscle tension to the muscle cross-sectional area (CSA). Physiologic CSA was estimated using the following formula: CSA = muscle mass (g)/[Lo (cm) × (L/Lo) × 1.06 g/cm2], where L/Lo is the fiber length to muscle length ratio (0.69 for soleus, and 0.45 for EDL), and 1.06 is the density of the muscle.
Treadmill exercise
Mice were habituated to the Exer3/6 treadmill system with a shock detector (Columbus Instruments, Columbus, OH, USA) by running at 10 m/min for 15 min at a 0° incline 1 d before the test. To test exercise tolerance, mice were left unfed but with access to water for 3 h midday before simultaneously running a treadmill protocol of 0–25 m/min at a 0° incline. Exhaustion was defined as 50 cumulative electrical stimuli (73 V, 0.97 m, 1 Hz). Exhausted mice were removed from the treadmill and allowed access to food and water. Blood glucose measurements were obtained before and immediately after the treadmill protocol.
Immunofluorescence and immunohistochemistry
Fiber size and type were determined an EDL cryosections with BF-F3 [myosin heavy chain (MHC)-IIb], SC-71 (MHC-IIa), BA-F8 (MHC 1) (Developmental Studies Hybridoma Bank, Iowa City, IA, USA), and anti-laminin (FB-082A; Thermo Fisher Scientific, Waltham, MA, USA), as described by us (22). For Glut4, sections were fixed for 5 min in 75% acetone and 25% ethanol and immunostained with polyclonal rabbit anti-Glut4 (Ab654; Abcam, Inc., Cambridge, MA, USA). Secondary antibodies used were AlexaFluor 488 goat anti-mouse IgM (A-21042), AlexaFluor 488 goat anti-mouse IgG (H+L) (A-11029), and AlexaFluor 568 goat anti-rabbit IgG (H+L) (A-11011; all from Thermo Fisher Scientific). Negative control slides, incubated with the secondary antibodies only, were included in each immunostaining. At least 3 animals per group were used.
According to standard protocols, sections were stained with hematoxylin and eosin to determine their structure and periodic acid-Schiff to access the storage of glycogen. For X-gal staining, a double-staining approach was used. Initially, cross sections were fixed for 10 min in 2% paraformaldehyde in PBS and then incubated in X-gal reaction solution [1 mg/ml X-gal, 4 mM potassium ferrocyanide, 4 mM potassium ferricyanide, 2 mM MgCl2, and PBS (pH 7.2–7.4)] overnight at 37°C. Afterward, the cross sections were stained by hematoxylin (Ricca, Arlington, TX, USA) and eosin (250 ml eosin Y 0.25%, 750 ml 80% ethanol and 5 ml glacial acid), to determine the muscle structure.
Samples were visualized in a DMR fluorescence phase contrast microscope, equipped with a DFC7000T camera (Leica Microsystems, Buffalo Grove, IL, USA). Images were acquired and processed with the Leica Application Suite and Microscope Imaging software (Leica Microsystems). Fiber typing and fiber quantification were achieved with semiautomatic muscle analysis using segmentation of histology (known as SMASH) software (24).
Immunoblot analysis
Tissues extracted for immunoblot analysis were snap frozen in liquid nitrogen and stored at −80°C until further processing. Quadriceps were mechanically ground by mortar and pestle in dry ice and homogenized in RIPA buffer (9806; Cell Signaling Technology, Danvers, MA, USA), with the addition of PMSF (36978; Thermo Fisher Scientific), protease (P8340; MilliporeSigma), and phosphatase (P5726; Millipore) inhibitors. Homogenates were incubated in ice for 30 min with periodical pipetting and centrifuged at 15,000 g for 15 min. Proteins were quantified by the Bradford assay (1863028; Thermo Fisher Scientific), and equal amounts were loaded in 12 or 16.5% precast acrylamide gels for SDS-PAGE (5671043; Bio-Rad, Hercules, CA, USA) and transferred to Immobilon-Fl PVDF membrane (IPFL00010; MilliporeSigma). Each membrane was blocked for 1.5 h at room temperature with either Tris-buffered saline (Odyssey Blocking Buffer; 927-50000; Li-Cor Biosciences, Lincoln, NE, USA) or 5% milk in Tris-buffered saline–Tween. The following antibodies were used for overnight incubation at 4°C: phospho-Akt (Ser473) (9271; Cell Signaling Technology), phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (9101; Cell Signaling Technology), protein kinase B (Akt) (pan) (40D4) (2920; Cell Signaling Technology), p44/42 Erk1/2 (3A7) (9107; Cell Signaling Technology), anti-IGF-I (AF791; R&D Systems), anti-Glut4 (Ab654; Abcam, Inc.), and anti-GAPDH (2118; Cell Signaling Technology).
Blots were incubated for 1.5 h at room temperature with the corresponding secondary antibody (Li-Cor Biosciences). After incubation with the secondary antibody, the blots were scanned with the Odyssey CLx Imaging System (Li-Cor Biosciences). The band intensity was automatically determined by the accompanying software Image Studio v.5.2 (Li-Cor Biosciences). Values are expressed as the ratio of the phosphorylated form of protein of interest to its total form, or in the case of Glut4, normalized to total protein by Ponceau S staining. In all figures showing images of gels, all the bands for each picture come from the same gel, to ensure clarity the contrast of an entire gel image was enhanced.
Gene expression analysis
Total RNA and genomic DNA were extracted from tissues with Trizol Reagent (15596018) and DNAse treated with recombinant RNase- free DNase I (04716728001; Roche, Basel, Switzerland). cDNA was prepared with the High-Capacity cDNA Reverse Transcription Kit (4368814). Duplicates of cDNA samples were amplified on the Step One Plus Real-Time PCR System, using Power SYBR Green PCR Master Mix (4367659) (all from Thermo Fisher Scientific). All data were normalized to 18S and plotted as fold changes (means ± sem). The oligonucleotide primers are shown in Supplemental Table 1.
Statistical analysis
All data are presented as means ± sem. All analyses were performed with Prism 7 (GraphPad, La Jolla, CA, USA). A Student’s t test was performed for comparison of 2 groups, and 1- and 2-way ANOVAs were performed for comparison of ≥3 groups to determine significance, followed by Tukey’s or Sidak’s multiple-comparisons post hoc test. The effect size was presented as Cohen’s d. A value of P < 0.05 was considered significant.
RESULTS
Muscle-specific deletion of Igf1 reduces muscle and total body growth
Tissue specific ablation of Igf1 was achieved by crossing mice harboring a floxed Igf1 gene (18) with mice expressing Cre recombinase driven by an inducible promoter (HSA-Cre) (25) (Supplemental Fig. S1). We have named mice that harbor the floxed Igf1 and the HSA-Cre transgene as MID mice, according to the nomenclature of previous tissue-specific deletion (2, 3, 26). PCR analysis using both genomic DNA (Supplemental Fig. S1B) and RNA (Supplemental Fig. S1C) isolated from several MID muscles, liver, heart, brain, and kidney confirmed that Igf1 deletion takes place only in muscles and not in other tissues in a Dox-dependent manner. Further confirmation of tissue specificity of deletion was performed in MID mice crossed with the ROSA26 Cre reporter line. Consistent with the PCR results, we observed X-gal staining in all skeletal muscles after Dox treatment, but not in the liver or heart (Supplemental Fig. S1D). It has been reported that a minimal expression of Cre, without Dox induction, occurs in rare fibers (25). To avoid any unexpected phenotypes, we included male and female mice harboring only the HSA-Cre allele with Dox induction as our experimental controls.
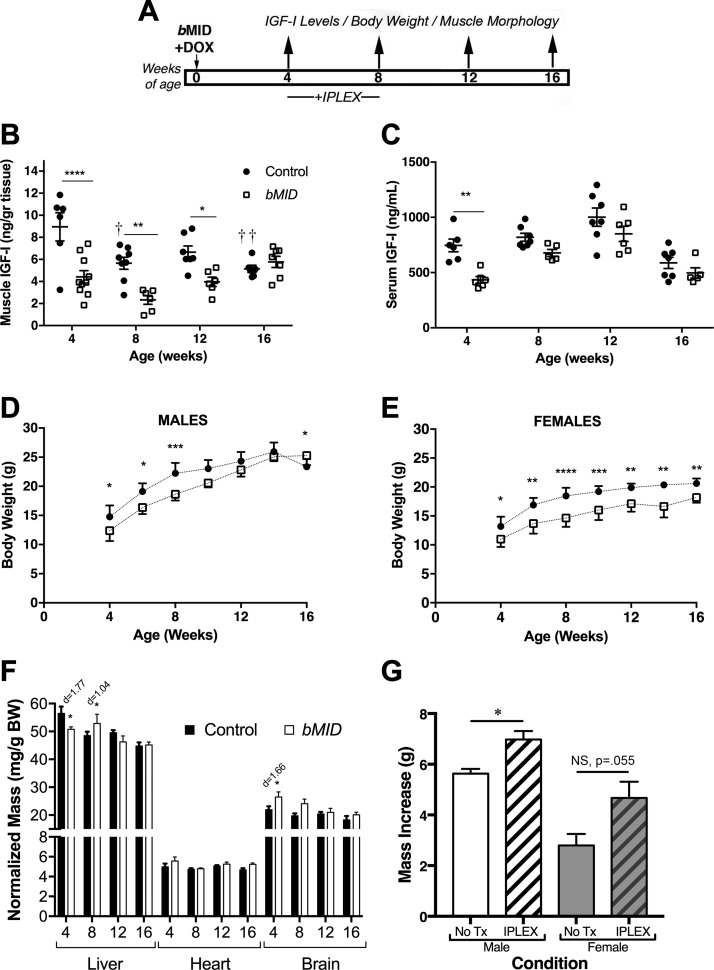
To examine the effects of muscle IGF-I deletion from birth, MID mice were treated with Dox as neonates (bMID) and evaluated from 4 to 16 wk of age (Fig. 1A). We measured IGF-I content in quadriceps protein extracts by ELISA. Muscles of bMID mice of both genders exhibited a significant reduction in muscle IGF-I at 4, 8, and 12 wk of age compared to sex- and age-matched controls (Fig. 1B). However, by 16 wk of age, no significant difference was observed in muscle IGF-I content. The predominant form of IGF-I in skeletal muscle is glycosylated pro-IGF-I (21), and in the bMID mice, this form was notably reduced, as shown by immunoblot analysis (Supplemental Fig. S1E). Because the ELISA measurements are more sensitive to the mature IGF-I form and less sensitive to the glycosylated forms (21), these findings suggest that the decrease in IGF-I levels in muscle was more extensive than that measured by ELISA. Taking into consideration the dual actions (endocrine vs. autocrine/paracrine) of IGF-I, ELISA was also used to determine circulating levels of IGF-I. As anticipated from previous studies (22), there was a ∼40% reduction of serum IGF-I in bMID mice observed at 4 wk of age (Fig. 1C). However, no difference was observed in circulating IGF-I levels at the other time points examined.
Figure 1.
Muscle Igf1 deletion leads to impaired body growth. A) Experimental design involving deletion of Igf1 at birth (bMID). B) IGF-I content of quadriceps was measured by ELISA in male and female bMID and control mice (4 wk, n = 6–10; 8 wk, n = 6–8, 12 wk, n = 6–7; and 16 wk, n = 6–9). bMID muscles had significantly lower IGF-I content at 4, 8, and 12 wk than age-matched control muscles. Control muscles exhibited an age-dependent decrease in Igf-I content. C) IGF-I levels quantified by ELISA in serum from male and female bMID and control mice (4 wk, n = 5–6; 8 wk, n = 5–6; 12 wk, n = 5–7; and 16 wk, n = 7–8). Circulating IGF-I was 40% lower in 4-wk-old bMID mice. D, E) Growth curves of male (n = 7–9) (D) and female (n = 5–10) (E) bMID and control mice from 4 to 16 wk of age show impaired growth of bMID mice until 8 wk in males and throughout the window of assessment for females. Male bMID mice became larger than control mice at 16 wk of age. F) Representative liver, heart, and brain dissections from 4-, 8-, 12-, and 16-wk-old bMID and control mice (n = 4–15), showed that loss of muscle IGF-I has only minimal effect on normalized mass of other tissues. G) Restoration of body growth after exogenous administration of mecasermin rinfabate. Histogram depicts the body mass increase of bMID males and females when injected with mecasermin rinfabate or vehicle between 4 and 8 wk of age. Data are means ± sem. Statistical analysis was performed with 2-way ANOVA, followed by Sidak’s multiple-comparisons test, or 2-tailed Student’s t text with effect size presented as Cohen’s d. *P < 0.05 (for bMID vs. control under same condition/treatment), **P < 0.01, ***P < 0.001, ****P < 0.0001, ††P < 0.01 (within-strain comparison).
The reduction of muscle IGF-I resulted in reduced body weight in both males and females (Fig. 1D, E). Male bMID mice exhibited a transient decrease in body weight up to 9 wk of age, after which they gained more mass, leading to higher body weights at 16 wk of age. In contrast, female bMID mice remained smaller than the control cohorts through 16 wk of age, which was the oldest age of observation in the study. To examine what contributed to the reduced body mass, mice were euthanized at 4, 8, 12, and 16 wk of age, and several tissues, including liver, heart, fat, and brain, were harvested and weighed (Supplemental Table S2). Similar to our previous findings (22), all tissues exhibited diminished sizes in bMID mice, except for fat. Tissue masses were normalized to body weight to determine whether the tissues contributed to overall impairments of growth. There was a significant increase in normalized mass of brain at 4 wk, but not at 8 or 12 wk (Fig. 1F). There was a significant decrease in liver mass at 4 wk and an increase at 8 wk in normalized mass (Fig. 1F). To determine whether the changes in mass were dependent on local stores of IGF-I, liver and brain IGF-I contents were assessed by ELISA. IGF-I content was lower in liver from bMID mice, but did not differ in brain (Supplemental Fig. S2). It appears that the changes in liver mass did not correlate with IGF-I content, whereas the brains may have been protected from growth deficits in 4-wk-old bMID mice because of retention of local IGF-I stores. To examine the potential disruption in the GH/IGF-I/insulin axis, we measured expression of acid labile subunit (Als) and Igfbp1 in liver. At 8 wk of age, liver from bMID mice displayed a significant increase in Als expression, which disappeared by 16 wk of age (Supplemental Fig. S2), supporting a transient compensatory increase in GH (27). Igfbp1 expression was highly variable in bMID livers, ranging from 1.2- to 19-fold higher than that in age-matched controls, which was significant by 2-way ANOVA for strain, but not significant in post hoc analysis (Supplemental Fig. S2), supporting high fluctuations in insulin levels (28).
To determine whether the growth defect could be rescued through a boost in circulating IGF-I, 4-wk-old bMID mice from 3 litters were treated with mecasermin rinfabate or vehicle (PBS) daily until 8 wk of age (Fig. 1G). This time window was chosen to capture the period of most active growth in the absence of any contribution from circulating IGF-II or -I delivered by mother’s milk (19, 20). Compared to littermate bMID controls, the body mass of the males increased by 24% and that of the females by 66%. A small cohort of these mice was analyzed for normalized muscle mass and signaling (Supplemental Fig. S3). The normalized mass of quadriceps and tibialis anterior muscles in mecasermin rinfabate–treated bMID mice were 10–12% lower than their litter mate controls, and there was no significant change in p-Akt levels associated with mecasermin rinfabate treatment. Although increased circulating IGF-I can compensate for the diminished muscle IGF-I production in terms of organismal growth, the skeletal muscles appeared to be refractory to improvements in mass within the period studied.
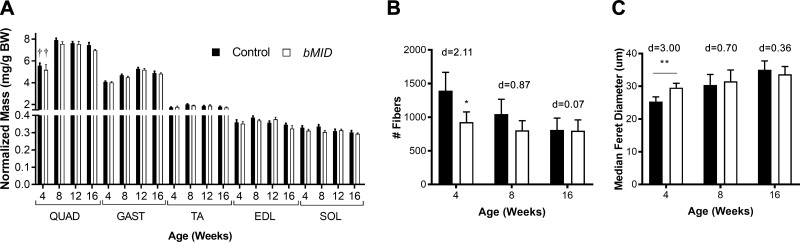
Because skeletal muscle was the site of diminished IGF-I production, we determined the mass of hind limb muscles as representatives of the entire musculature. The loss of muscle IGF-I production in the bMID mice resulted in 25% lower mass of all muscles examined, regardless of fiber type (Supplemental Table S3). The most profound reduction was observed at 4 and 8 wk of age. Further, when muscle masses were normalized to body weight, there were no differences between bMID and control mice (Fig. 2A), supporting that the reduced body weight was caused primarily by diminished muscle mass. To investigate whether the observed loss of muscle mass was related to reduction of muscle fiber number or fiber size, immunohistologic analysis of EDL muscles using laminin and MHC-specific antibodies was performed (Supplemental Fig. S4A). Muscle fiber quantification displayed a decrease in muscle fiber number of bMID EDL muscles of 30% at 4 wk and 20% at 8 wk and, finally, no reduction at 16 wk (Fig. 2B), concurrent with the time course of muscle mass loss (Supplemental Table S3). Median Feret diameter of fibers showed an opposite pattern compared with muscle fiber number. In 4 wk bMID mice, the diameter increased compared to control, whereas it remained unchanged at 8 and 16 wk (Fig. 2C). Fiber size distribution analysis of bMID mice (Supplemental Fig. S4C) revealed a bimodal distribution of fibers at younger ages, with a higher proportion of small fibers and of large fibers in bMID EDL muscles. The broader fiber distribution disappeared with age progression (8 wk, Supplemental Fig. S3E; 16 wk, Supplemental Fig. S4G). In examining myonuclei in the fibers, we found no apparent differences in the number of nuclei per fiber or in the proportion of central nuclei in muscle cross sections (data not shown). Further, we did not observe a shift in fiber type at 4, 8, and 16 wk (Supplemental Fig. S4B, D, F, respectively), yet there was an increased rare appearance of type I/slow fibers in 4-wk-old bMID EDL muscles (data not shown). Thus, muscle mass reduction in bMID EDL muscles is primarily related to the lower number of muscle fibers and is coupled with compensatory fiber hypertrophy in young mice.
Figure 2.
Muscle Igf1 deletion induces loss of muscle mass. A) Normalized masses of hind limb muscles were not different between bMID and control mice at all time points examined (4, 8, 12, and 16 wk) across all muscle groups (n = 8–15). B) Total number of fibers decreased in bMID EDL muscles (n = 3–7) at 4 wk of age. C) There was an increase in median Feret diameter at 4 wk between bMID and control EDL fibers. Values are means ± sem. Statistical analysis was performed with a 2‐tailed Student’s t test with effect size presented as Cohen’s d. *P < 0.05, **P < 0.01 (bMID vs. control), ††P < 0.01 (comparisons within strain).
Loss of muscle IGF-I leads to transient changes in signaling and atrogene expression
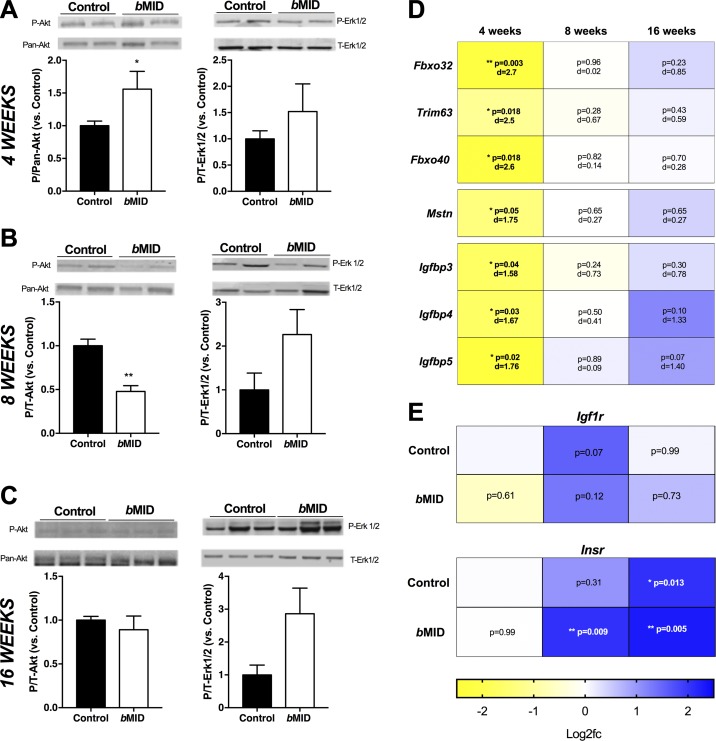
Although IGF-IR phosphorylation was undetectable in resting muscles without exogenous stimulation by its ligand, we examined the resting phosphorylation levels of Akt and Erk (p-Akt and p-Erk1/2) as representative downstream elements of the pathway (29). p-Akt levels were higher in 4 wk bMID muscles compared to control (Fig. 3A), but basal p-Akt diminished in bMID muscles at 8 wk (Fig. 3B), followed by no change at 16 wk of age (Fig. 3C). In contrast to Akt, Erk1/2 showed a trend of hyperphosphorylation in bMID muscles at all age groups (Fig. 3A–C).
Figure 3.
Muscle Igf1 deletion modulates IGF-I signaling pathways and gene expression. A–C) Akt and Erk1/2 phosphorylation in quadriceps lysates of bMID compared to control mice show transient increases in p-Akt at 4 wk of age (A), with lower p-Akt at 8 wk of age (B), followed by a return to control levels by 16 wk of age (C). D) Heat map of semiquantitative real time-PCR analysis using values of log2 of fold change vs. control at the same age. Negative regulators of muscle mass (Fbxo32, Trim63, Fbxo40, and myostatin/Gdf8) demonstrate significant decreases in bMID quadriceps samples at 4 wk of age, but no marked changes at 8 or 16 wk of age. IGF-I binding proteins (Igfbp3, -4, and -5) showed significantly decreased expression at 4 wk of age, with no significant changes at 8 or 16 wk of age. E) Heat map of semiquantitative real-time PCR analysis using values of log2 of fold change vs. control at 4 wk of age. Igf1r and Insr expression did not differ between age-matched bMID and control samples, but Insr expression increased significantly with age in both strains. Data are means ± sem (n = 3–7 samples/strain and time point pooled for males and females). *P < 0.05, **P < 0.01 (bMID vs. control) by Student’s t test (A–C); 2-way ANOVA followed by Sidak’s multiple-comparisons test (D, E), with effect size presented as Cohen’s d.
IGF-I activity is a positive regulator of muscle growth that counters loss of muscle mass mediated by regulating expression of the E3 ubiquitin ligases, including F-box (Fbxo)32, Trim63, and Fbxo40 (30, 31). We found significantly lower expression of these genes at 4 wk of age in bMID muscles, with 4-fold lower Fbxo32 and 3-fold lower Fbxo40 than in control tissues (Fig. 3D); all returned to control levels at 8 and 16 wk. In addition, we examined the expression of the myostatin (Mstn) gene, a negative regulator of muscle growth (32). Consistent with the atrogene profile, Mstn gene expression was 2-fold lower in bMID muscles at 4 wk of age, with no differences between bMID and control muscles at 8 and 16 wk (Fig. 3D). As Mstn (33) and Fbxo40 (31) actions lead to diminished p-Akt levels, the heightened p-Akt we observed in 4 wk bMID muscle lysates can be attributed to disinhibition of IGF-IR activity via reduced Fbxo40 and Mstn, ultimately leading to a transient down-regulation of Fbxo32 and Trim63.
The bioavailability of IGF-I is regulated, in part, by the family of Igfbps (34). We evaluated the expression of Igfbp3, -4, and -5, the major binding proteins in muscle, across the different age groups of bMID and control mice. There was a common pattern of expression for all Igfbps, with down-regulation at 4 wk, no change at 8 wk, and modest up-regulation at 16 wk (Fig. 3D). As the combined ablation of IGFBP3, -4, and -5 results in growth retardation, we cannot exclude a direct effect of the transient reduction in expression on the bMID growth phenotype (35).
To determine whether the diminished IGF-IR in muscle led to alterations in receptor expression, we measured Igf1r and insulin receptor (Insr) gene expression from 4 to 16 wk of age. We did not observe differences in expression between muscles of bMID mice and age-matched controls. However, there was a progressive increase in Insr expression, where at 16 wk of age, the levels were more than 4-fold higher than at 4 wk of age, regardless of genotype (Fig. 3E), but age-dependent changes were not apparent for Igf1r.
Muscle Igf1 deletion in young adult mice increases body weight with minimal change in muscle mass
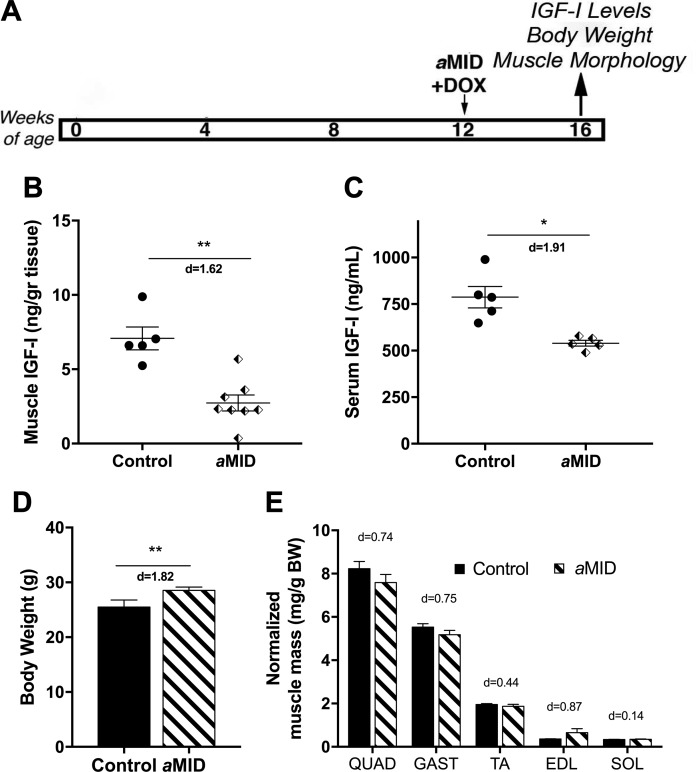
The male bMID mice displayed a transient impairment in growth, leading to increased body weight at 16 wk of age. To minimize the effects of the most active growth period in the mice, we induced Igf1 deletion in 12-wk-old male mice (aMID) and evaluated them at the same end point of 16 wk (Fig. 4A). Quadriceps of aMID mice exhibited a significant reduction in IGF-I content compared to their controls (Fig. 4B). Circulating IGF-I levels were also significantly reduced in aMID mice (Fig. 4C). Even with diminished IGF-I levels, there was a paradoxical increase in aMID body weight at 16 wk of age (Fig. 4D). Assessment of muscles at euthanasia revealed minimal change in mass (Fig. 4E). Thus, muscle mass could not account for the increased body weight in male MID mice, which occurred regardless of the age at Igf1 deletion.
Figure 4.
Muscle Igf1 deletion in young adult male mice leads to increased body weight and minimal changes in muscle mass. A) Schematic of experimental design using deletion of Igf1 in young adult males (aMID). B, C) IGF-I content of quadriceps (n = 5–7) (B) and serum (n = 5) (C) of male aMID and control was measured by ELISA. aMID mice had significantly lower quadriceps and serum IGF-I content than their age-matched controls. D) Body weight was significantly higher in 16-wk-old aMID mice than in control mice (n = 7/group). E) Normalized muscle mass was not affected by Igf1 deletion, and all comparisons had minimal effect size. Data are represented as means ± sem. *P < 0.05, **P < 0.01 (aMID vs. control), by Student’s t test, with effect size presented as Cohen’s d.
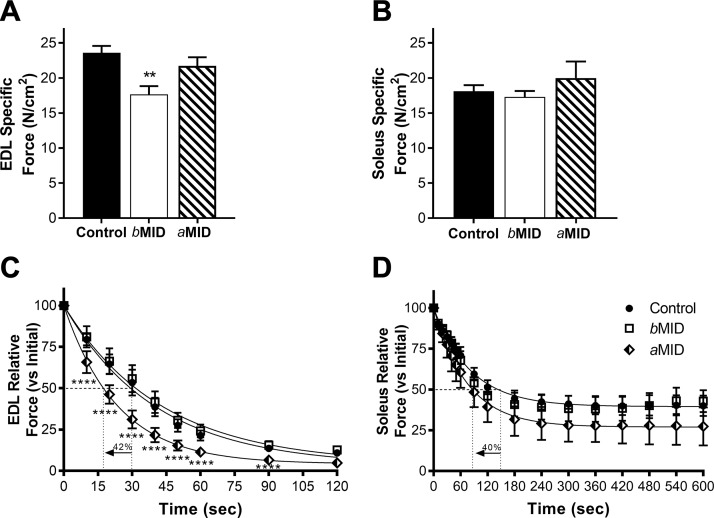
Male MID mouse muscles exhibit impaired muscle function
To evaluate the functional impact of reduced IGF-I, we assessed isometric force production and fatigue in 16 wk male MID mice. A ∼30% loss of maximal force generating capacity was observed in EDL muscles from bMID mice (Fig. 5A), whereas soleus did not exhibit the same weakness (Fig. 5B). To determine whether this was an age- or duration-dependent effect of Igf1 deletion, aMID muscles were also examined. Neither EDL nor soleus were weaker than muscles from control mice (Fig. 5A, B). However, both EDL and soleus from aMID mice were significantly more susceptible to fatiguing contractions, as measured by the time taken for the force produced by each muscle to decrease to 50% of its maximum force. aMID EDL and soleus reached this point 40–42% earlier than controls (Fig. 5C, D). No changes were observed in the fatigability of either EDL or soleus of bMID mice. It appears that timing- and duration-dependent effects on strength and fatigue susceptibility emerge after deletion of muscle Igf1.
Figure 5.
Muscle Igf1 deletion leads to diminished muscle function and reduced fatigue resistance. Isometric tetanic force measurements of isolated EDL (A) and soleus (B) from 16-wk-old control and MID male mice reveal significantly lower specific force in bMID EDL, but no differences in force production between MID and control soleus (n = 5–10). Fatigue tests of EDL (C) and soleus (D) caused a more rapid loss of relative force in both muscles from aMID mice (n = 5–11), as shown by the 42 and 40% reduction, respectively, in the time to reach 50% of initial force. Data are represented as means ± sem. Statistical analysis was performed by 1-way ANOVA, followed by a Tukey multiple-comparison test. Nonlinear fits of fatigue tests assumed 1-phase decay. Multiple Student’s t tests were performed between aMID, bMID, and control at the different time points during the fatigue run. **P < 0.01 (bMID vs. control in the same condition), ****P < 0.001 (aMID vs. control).
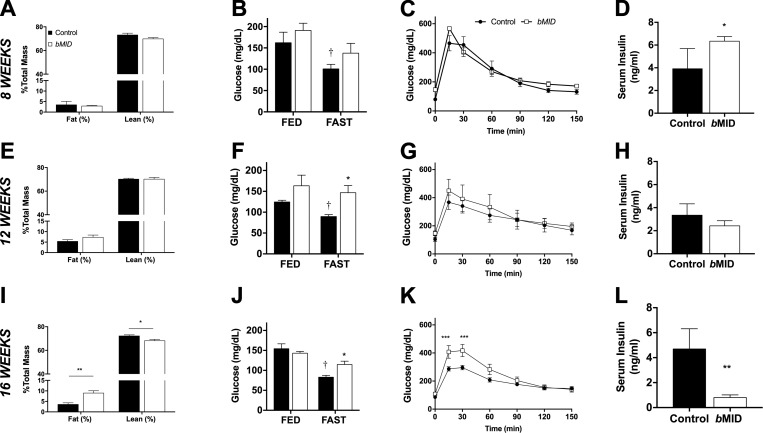
Altered glucose homeostasis after muscle Igf1 deletion is age and duration dependent
IGF-I is a well-established growth factor, and has been shown to stimulate the growth of all cell types (1). It also has a major metabolic effect, which, to some extent, complements the metabolic action of insulin (13). To clarify the putative dual role of IGF-I in anabolic and metabolic processes, we evaluated body composition and indices of glucose handling of male bMID mice at 8, 12, and 16 wk of age. In 8-wk-old male bMID and control mice, no significant differences in relative body composition were observed based, on time-domain–NMR analysis (Fig. 6A). Blood glucose levels dropped after food deprivation in control but not in bMID mice (Fig. 6B). Glucose sensitivity by GTT displayed no differences between strains (Fig. 6C), but increased insulin levels in random fed bMID mice occurred (Fig. 6D). At 12 wk of age, no change in body composition was evident (Fig. 6E), but bMID mice continued to show impaired reduction in fasting glucose levels (Fig. 6F). Total body glucose clearance (Fig. 6G) and insulin levels (Fig. 6H) were similar to their respective control values.
Figure 6.
Muscle Igf1 deletion alters body composition and glucose handling in an age-dependent manner. Body composition analysis by time-domain–NMR in 8 wk (n = 4) (A), 12 wk (n = 6) (E), and 16 wk (n = 6) (I) male bMID mice revealed a progressive increase in their fat levels with age without a significant alteration in their lean mass percentage until 16 wk of age. No changes in either the fat or lean mass percentage was observed in control mice, at the time points examined. Blood glucose levels were measured in mice aged 8 wk (n = 7–8) (B), 12 wk (n = 4) (F), and 16 wk (n = 6–7) (J) bMID mice and their age-matched controls when unfed overnight or randomly fed. Control but not bMID mice responded to food deprivation, with reduced glucose levels. Intraperitoneal GTTs showed no significant differences in 8 wk (n = 7–8) (C) and 12 wk (G) (n = 4–5) bMID mice, and delayed glucose clearance in 16-wk-old bMID mice (K) vs. age-matched control mice (n = 5–8). Serum insulin was elevated in 8-wk-old bMID (D), did not change in 12-wk-old bMID (H), and was reduced in 16-wk-old bMID (L) mice compared with their age-matched controls) (n = 4–5). Data are represented as means ± sem. Analysis by 1-way ANOVA, followed by Sidak’s multiple comparisons test. Insulin level comparisons were analyzed by a Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001 (bMID vs. control), †P < 0.05 (fed vs. unfed) by Student’s t test.
At 16 wk of age, male bMID mice exhibited a 3-fold increase in fat and significantly lower lean mass than did control mice (Fig. 6I). Glucose levels in bMID mice did not respond to food deprivation, leading to elevated fasting glucose levels compared with those in control mice (Fig. 6K). Clearance of glucose according to GTT results revealed a delayed response in bMID mice vs. control mice (Fig. 6L). Insulin levels in randomly fed mice showed a substantial decrease in bMID mice at 16 wk of age (Fig. 6M). There was a progressive response to loss of muscle IGF-I affecting both body composition and glucose homeostasis in male mice.
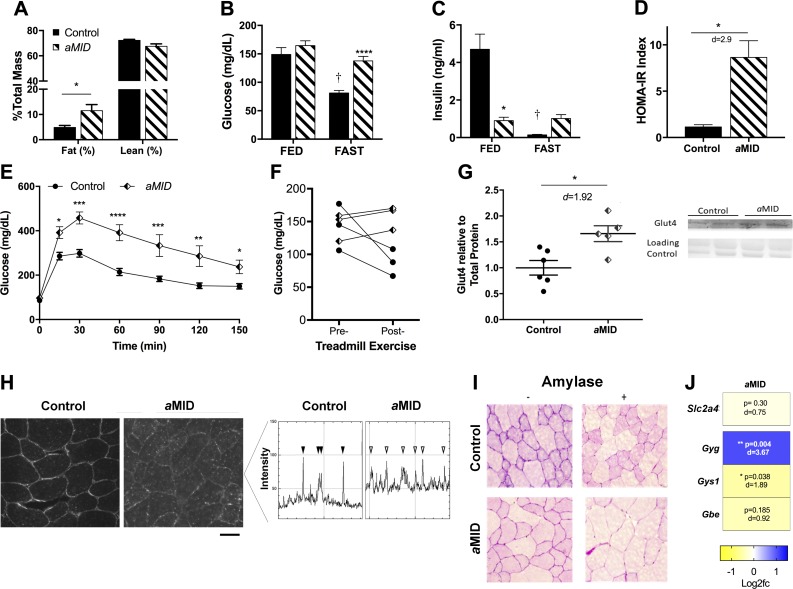
Adult deletion of muscle Igf1 impairs glucose handling and exercise tolerance
After deletion of Igf1 at birth, we observed a prominent effect on glucose homeostasis that emerged over time. To address whether this effect is dependent on age or duration of Igf1 deletion, aMID mice were evaluated according to the same outcome measures. At 16 wk of age, male aMID mice displayed increased adiposity (40% greater fat) compared with control mice (Fig. 7A) and elevated glucose levels after overnight food deprivation (Fig. 7B). These changes were accompanied by substantially decreased insulin levels in fed aMID mice and no insulin response upon withholding of food (Fig. 7C). Calculation of the homeostatic model assessment for insulin resistance revealed a more than 4-fold elevation in male aMID mice (Fig. 7D). Clearance of glucose by GTT revealed a marked glucose intolerance in aMID compared to controls (Fig. 7E). In addition to disruptions in glucose homeostasis, total circulating cholesterol levels were elevated ∼50% in male aMID mice (control 104.5 ± 16.9; aMID 155.8 ± 15.1 mg/dl, n = 4 per strain; P = 0.004), although there was no change in triglyceride levels (control 172.7 ± 42.6, aMID 158.3 ± 33.6 mg/dl, P = 0.64), suggesting that HDL and LDL are the contributors to elevated cholesterol. Thus, even though the duration of Igf1 deletion was only 1 mo, the aMID mice were similar to the age- and sex-matched bMID mice in the disruption of glucose homeostasis.
Figure 7.
Muscle Igf1 deletion in male aMID mice alters body composition and glucose handling and increases Glut4 protein levels. A) The 16-wk-old aMID mice had greater fat levels than control mice by time-domain–NMR (n = 9). B) Blood glucose levels in 16-wk-old aMID mice is elevated in unfed states compared to control mice. C) Insulin levels were lower in aMID mice under random feeding conditions; there was no response to food deprivation. D) Homeostatic model assessment-insulin resistance of aMID male mice was significantly elevated (n = 3/strain). E) Glucose clearance by GTT was delayed in 16-wk-old aMID mice vs. control mice (n = 8–11). F) Glucose levels after 60 min treadmill running decreased in control mice, but remained unchanged in aMID mice (n = 3). G) Immunoblots of quadriceps lysates showed ∼60% increase in Glut4 content in aMID muscles (n = 5–6). H) Immunostaining for Glut4 (left) and analysis of signal intensities in horizontal scans across the image (right) showed sarcolemmal localization in both control (solid arrowheads) and aMID (open arrowheads) muscles but greater cytoplasmic levels in aMID muscles. Scale bar, 25 μm. I) Histologic analysis with periodic acid-Schiff after digestion with amylase from quadriceps of control and aMID mice. J) Heat map of semiquantitative real-time PCR analysis using values of log2 of fold change vs. control. Expression of the glycogenin gene was significantly higher and Gys1 was significantly lower in aMID muscles, with no change in Slc2a4 and glycogen branching enzyme (n = 4–5). Note that control values for glucose and insulin are from the same cohorts as are presented in Fig. 6. Data are means ± sem. Analysis by 2-way ANOVA, followed by Sidak’s multiple-comparisons test. Insulin levels, exercise changes in glucose, and Glut4 comparisons were analyzed by a Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001 (bMID vs. control).
The glucose-handling effects were more profound in male aMID mice in the absence of a transient growth phenotype, so we focused our attention on this group to resolve underlying mechanisms leading to glucose-handling defects. Because increased activity can also trigger glucose clearance, we subjected a small cohort of mice to exhaustive treadmill running. In a 60 min treadmill running test, blood glucose diminished by ∼40% in control mice, but levels in aMID mice did not change from pre-exercise values after treadmill running (Fig. 7F). Further, 2 of the aMID mice could not complete the task, suggesting that exercise intolerance occurs in the absence of muscle IGF-I. As glucose uptake in skeletal muscle is primarily dependent upon Glut4, we determined whether there were changes in its abundance in quadriceps of aMID and control mice. We found a ∼60% increase in Glut4 protein by immunoblot analysis, but no change at the mRNA level, and so the levels of Glut4 in muscle do not explain the reduced glucose uptake (Fig. 7G, J). Immunostaining for Glut4 showed that both control and aMID muscles displayed membrane localization of the protein, but aMID muscles also contained stronger cytoplasmic staining (Fig. 7H). The increased Glut4 content in aMID muscles likely resides in the cytoplasm and the impaired glucose clearance observed in male MID mice cannot be attributed to a loss of Glut4.
Upon entry into muscle, glucose can be used immediately for energy production or stored as glycogen. We found that male aMID quadriceps exhibited less intense glycogen staining, suggesting that storage of glucose may be less efficient in aMID muscles (Fig. 7I). To clarify whether glycogen metabolism was altered, we measured expression of key enzymes regulating glycogen storage and found increased glycogenin gene, diminished glycogen synthase (Gys1), and no differences in glycogen branching enzyme-1 expression in aMID muscles (Fig. 7J), suggesting that initiation of glycogen synthase is enhanced, but that the reduction in Gys1 led to inefficient glycogen formation with the loss of muscle IGF-I production.
DISCUSSION
The term IGF encompasses more than the structural similarity between insulin and IGF-I. This similarity is particularly relevant for skeletal muscle, in which anabolic and metabolic processes are coupled (36). Although there is clear evidence that receptors for both IGF-I and insulin are key players in these actions, the importance of the ligand and its source remain unresolved. To address growth and glucose handling actions, we generated a mouse model with inducible skeletal muscle specific ablation of Igf1: the MID mouse. Specifically, we observed diminished growth of mice when muscle Igf1 was deleted at birth. This growth defect was associated with heightened insulin levels in 8-wk-old male mice, an indicator of insulin resistance (37), which was similar to our previous findings with striated muscle deletion of Grp94 (22). This observation is confounded by the fact that there is a reduction in muscle mass, as muscle itself is a major glucose sink. The situation differed dramatically 2 mo later, when the male MID mice grew fat, displayed impaired glucose clearance, all in the absence of significant muscle mass loss. Related mouse models manipulating the IGF-I pathway include targeted ablation of IGF-I production from liver, brain, or pancreas (2, 3, 26, 38, 39). In addition, extensive study has focused on the receptor pool in striated muscle, using either transgenic expression of dominant negative IGF-IR (40), or ablation of IGF-IR or IR, or both at birth (17, 41). The ability to selectively induce deletion of the ligand enables the distinction between metabolic and anabolic actions, and demonstrates that, in the absence of skeletal muscle IGF-I production, a disintegration of glucose homeostasis is evident in the entire organism that appears separate from the growth defects.
In terms of its effects on growth, IGF-I is widely accepted to promote cell proliferation and increase protein synthesis in virtually all cell types (42). Thus, with total ablation of Igf1, there is a reduced drive for growth, resulting in a smaller tissue and body size (43). The seminal papers that demonstrated that deletion of liver Igf1 in mice did not alter growth (2, 3, 38) substantiated that local pools of IGF-I are sufficient to support the needs of those tissues. In bMID mice, impairment of growth was primarily in skeletal muscle, the site of deletion. As a large proportion of body mass comprises muscle, a local effect on muscle contributed to a global effect on body weight. Even though muscles were smaller at 4 wk of age and IGF-I content was 50% lower than in controls, p-Akt levels were elevated, and fiber sizes were larger. These findings can be attributed to the diminished expression of several negative regulators of muscle growth—namely Mstn and Fbxo40—that normally suppress p-Akt levels (31, 33). Likewise, the general reduction of Igfbp expression could contribute to enhanced IGF-I signaling (35). This effect disappeared by 8 wk of age, where p-Akt levels in muscle were significantly diminished, and there were no changes in the expression of these factors. High circulating levels of insulin were evident at that age, but p-Akt levels remained depressed, suggesting that insulin could not rescue signaling, or that there was postreceptor desensitization in the muscle (37). Boosting circulating levels of IGF-I via mecasermin rinfabate also failed to rescue the muscle growth deficits. This outcome suggests that circulating IGF-I is insufficient to drive muscle growth within the period examined and that the local source predominates in driving anabolic actions. Longer duration treatments will help to verify these findings. Our next question was whether a local ablation of IGF-I could have a systemic growth impairment. Evidence of a disruption of the GH/IGF-I axis included increased liver mass. Although liver IGF-I content was lower at 8 wk of age, the increased mass combined with heightened Als expression indicated GH-dependent effects in the liver. Increased pancreatic β-cell production of insulin could also arise from high GH levels (44), but it is thought that secretion is regulated by paracrine actions of local pancreatic IGF-I rather than circulating IGF-I levels (26, 45). Nevertheless, the GH response was transient, and it therefore does not explain the eventual metabolic disturbances that manifested later.
These progressive metabolic changes occur, not only at the local level, but also systemically. We focused our evaluation of these effects on male aMID mice, to remove the confounding factor of transient growth impairment, yet retain the diminished IGF-I levels in muscle and blood. Local muscle changes included impaired fatigue resistance in isolated muscles, which was independent of shifts in fiber type distributions and occurred in both fast (EDL) and slow (soleus) muscles. These results suggest the presence of a metabolic defect endogenous to the isolated aMID muscles, which were bathed in physiologic oxygenated Ringer’s solution containing glucose, but were more susceptible to fatigue tests. In contrast to the differential oxidative and glycolytic needs of specific fiber types, a common feature of all muscle types is the need for fuel uptake. Thus, one possibility is that aMID muscles could not use glucose for fuel, or failed to store sufficient glycogen to withstand the fatigue test, despite the elevated Glut4 levels. Consistent with this interpretation is the reduced expression of Gys1 in aMID, a key enzyme needed for glycogen formation.
The local responses in aMID mice that lowered IGF-I content in muscles were coupled with systemic consequences, including impaired glucose clearance both at rest and during exercise. Furthermore, aMID mice could not complete a treadmill running test, suggesting that the fuel source is not sufficient to support exercise activity. Because the mice are weaker and fatigue more rapidly, they may be unable to move to the same extent as their control counterparts. This may have led to increases in fat deposition detected by time-domain–NMR. At this point, we have not evaluated alternate substrates for supporting isolated muscle function or whole animal activity. However, it appears that a change in body composition and fuel utilization is instigated by a reduction in IGF-I production solely by muscle.
Receptor populations within muscle regulate IGF-I actions mechanistically. These include not only IGF-IRs, but also insulin receptors and hybrid receptors formed by association of insulin and IGF-I hemireceptors (17, 46). The proportion of these receptors varies across tissues, but prior estimates suggest that more than half of the IGF-IR pool are hybrid receptors in adult skeletal muscle (47), in part, because of the pronounced expression of INSR in adult muscle (48). In mice, we observed an age-dependent increase in Insr expression, which was independent of genotype. Assuming random assembly of receptor dimers, the proportion of IGF-IR in young mice may be greater than in older mice, whereas the proportion of hybrid/insulin receptors likely increases with age. Hybrid receptors have been shown to bind IGF-I with greater affinity than insulin (49), but their downstream actions could mimic both IGF- and insulin-mediated effects (50). The greater prevalence of hybrid/insulin receptors at 16 wk compared with younger ages may underlie the emergence of a metabolic disturbance with loss of muscle IGF-I in aMID mice. Previous studies targeting the IGF, insulin, and hybrid receptor pools in murine muscle have also observed changes in both growth and metabolism, which is consistent with this interpretation. First, ablation of IGF-IR in skeletal muscle impaired only growth (41). In contrast, ablation of both IGF-IR and insulin receptors resulted in deficits in both mass and glucose homeostasis (16). Further, manipulation of both receptor pools in muscle, according to the dominant negative IGF-IR (MKR mouse) model, generated a metabolic phenotype where a global disruption of glucose handling occurred (17, 41). The findings in these studies implicate the IGF-IR as the anabolic receptor and muscle hybrid and IR as the metabolic receptors. We have extended these findings to demonstrate that muscle IGF-I is the ligand driving both processes. Specifically, we observed glucose-handling impairments in both the presence and absence of muscle mass reductions, but the metabolic disruption progresses with age, and this factor must be considered when interpreting these results. Pointing to the same studies described above, similar findings occurred in evaluating more mature animals (17, 41), in contrast to those in which mice were as young as 8 wk (16). These results point to a greater complexity in an already intricate balance of multiple ligand and receptor interactions.
This study leaves open several interesting questions. First, increased adiposity, which has also been observed transiently in mice (51), as well in mice with transgenic expression of Igfbp2 (52), illustrate that fat may act as a buffer to the impaired glucose uptake by muscle, even though it is insufficient to fully correct glucose handling. What is remarkable is that in 16-wk-old bMID mice, IGF-I levels returned to normal, yet a strong metabolic effect persisted. This suggests that a loss of muscle IGF-I triggered a cascade of events that resulted in a metabolic disturbance. The consequences of muscle Igf1 deletion should be examined across a broader range of ages and durations to fully understand how loss of IGF-I from muscle sets in motion a more fundamental impact on glucose homeostasis in the organism. Second, metabolic consequences of loss of muscle IGF-I were not apparent in female mice, supporting a clear sex-related difference in the response of deleted muscle IGF-I production (53). There are many potential causes, including the presence of estrogen, given that it is well known to have beneficial effects on glucose homeostasis and plays a protective role in female mice (54, 55). None of these was directly addressed in our model; further study is needed to understand the basis for this difference.
The physiologic changes we found in response to a loss of muscle IGF-I production mirror many features of type II diabetes, including progressive changes in insulin levels, impaired glucose handling, higher cholesterol, and increased adiposity (56). The effects may be compounded by a catabolic drive to reduce muscle mass, and as a consequence, the loss of one of the major sites of glucose uptake (57). Therefore, not only the levels of IGF-I and insulin, but also the proportions of fat and muscle can modulate glucose handling in the body. The endocrine network surrounding the regulation of blood glucose can become dysregulated with age, poor nutrition, and obesity. Our findings implicate muscle IGF-I production as an important component of this network, clearly acting in a paracrine manner on muscle, but potentially driving systemic changes in response to the altered metabolism of muscle. Thus, it is apparent that maintenance of IGF-I levels in muscle is important, not only for preservation of muscle mass, but also for general metabolic health.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank the Penn Mouse Phenotyping, Physiology and Metabolism (MPPM) Core (Philadelphia, PA, USA) for treadmill exercise testing and Insmed, Inc. (Monmouth Junction, NJ, USA) for providing mecasermin rinfabate (Iplex). The research was supported, in part, by U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grant P30-DK19525; NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR057363 (to E.R.B.) and AR052646 to the Physiological Assessment Core of the Paul D. Wellstone Muscular Dystrophy Cooperative Research Center. The authors declare no conflicts of interest.
Glossary
- Als
acid labile subunit
- CSA
cross-sectional area
- Dox
doxycycline
- EDL
extensor digitorum longus
- Fbxo
F-box
- GH
growth hormone
- Glut
glucose transporter
- GRP
glucose-regulated protein
- GTT
glucose tolerance test
- Gys
glycogen synthase
- HAS
human α-skeletal actin
- IGF
insulin-like growth factor
- IGFBP
IGF binding protein
- IGF-IR
insulin-like growth factor I receptor
- Insr
insulin receptor
- IR
insulin receptor
- Lo
optimal muscle length
- MHC
myosin heavy chain
- MID
muscle Igf-I deficient
- Slc2a4
glucose transporter
- TD-NMR
time domain-NMR
- Trim
tripartite motif–containing
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
E. R. Barton designed the research; G. Vassilakos, H. Lei, Y. Yang, J. Durzynska, M. Ozery, K. Bennett, S. Park, R. S. Ahima, and E. R. Barton analyzed data; G. Vassilakos and E. R. Barton wrote the paper; and all authors performed the research.
REFERENCES
- 1.Laron Z. (2001) Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol. Pathol. 54, 311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yakar S., Liu J. L., Stannard B., Butler A., Accili D., Sauer B., LeRoith D. (1999) Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 96, 7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sjögren K., Liu J. L., Blad K., Skrtic S., Vidal O., Wallenius V., LeRoith D., Törnell J., Isaksson O. G., Jansson J. O., Ohlsson C. (1999) Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc. Natl. Acad. Sci. USA 96, 7088–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman M. E., DeMayo F., Yin K. C., Lee H. M., Geske R., Montgomery C., Schwartz R. J. (1995) Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J. Biol. Chem. 270, 12109–12116 [DOI] [PubMed] [Google Scholar]
- 5.Musarò A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., Barton E. R., Sweeney H. L., Rosenthal N. (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 27, 195–200 [DOI] [PubMed] [Google Scholar]
- 6.Barton E. R., Morris L., Musaro A., Rosenthal N., Sweeney H. L. (2002) Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J. Cell Biol. 157, 137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton-Davis E. R., Shoturma D. I., Sweeney H. L. (1999) Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol. Scand. 167, 301–305 [DOI] [PubMed] [Google Scholar]
- 8.Lee S., Barton E. R., Sweeney H. L., Farrar R. P. (2004) Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J. Appl. Physiol. (1985) 96, 1097–1104 [DOI] [PubMed] [Google Scholar]
- 9.Barton E. R. (2006) Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J. Appl. Physiol. (1985) 100, 1778–1784 [DOI] [PubMed] [Google Scholar]
- 10.Stevens-Lapsley J. E., Ye F., Liu M., Borst S. E., Conover C., Yarasheski K. E., Walter G. A., Sweeney H. L., Vandenborne K. (2010) Impact of viral-mediated IGF-I gene transfer on skeletal muscle following cast immobilization. Am. J. Physiol. Endocrinol. Metab. 299, E730–E740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams G. R., McCue S. A. (1998) Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J. Appl. Physiol. (1985) 84, 1716–1722 [DOI] [PubMed] [Google Scholar]
- 12.Clemmons D. R. (2004) Role of insulin-like growth factor iin maintaining normal glucose homeostasis. Horm. Res. 62(suppl 1), 77–82 [DOI] [PubMed] [Google Scholar]
- 13.Clemmons D. R. (2012) Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol. Metab. Clin. North Am. 41, 425–443, vii–viii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Entingh-Pearsall A., Kahn C. R. (2016) Differential roles of the insulin and insulin-like growth factor-I (IGF-I) receptors in response to insulin and IGF-I [published corrections appear in J. Biol. Chem., 279, 38016]. J. Biol. Chem. 291, 22339–22340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klip A., Pâquet M. R. (1990) Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care 13, 228–243 [DOI] [PubMed] [Google Scholar]
- 16.O’Neill B. T., Lauritzen H. P., Hirshman M. F., Smyth G., Goodyear L. J., Kahn C. R. (2015) Differential role of insulin/IGF-1 receptor signaling in muscle growth and glucose homeostasis. Cell Reports 11, 1220–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández A. M., Kim J. K., Yakar S., Dupont J., Hernandez-Sanchez C., Castle A. L., Filmore J., Shulman G. I., Le Roith D. (2001) Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 15, 1926–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J. L., Grinberg A., Westphal H., Sauer B., Accili D., Karas M., LeRoith D. (1998) Insulin-like growth factor-I affects perinatal lethality and postnatal development in a gene dosage-dependent manner: manipulation using the Cre/loxP system in transgenic mice. Mol. Endocrinol. 12, 1452–1462 [DOI] [PubMed] [Google Scholar]
- 19.Lui J. C., Finkielstain G. P., Barnes K. M., Baron J. (2008) An imprinted gene network that controls mammalian somatic growth is down-regulated during postnatal growth deceleration in multiple organs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R189–R196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura A., Miyado K., Yamatoya K., Kawano N., Umezawa A. (2015) Breast milk stimulates growth hormone secretion in infant mice, and phosphorus insufficiency disables this ability and causes dwarfism-like symptoms. Regen. Ther. 2, 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durzyńska J., Philippou A., Brisson B. K., Nguyen-McCarty M., Barton E. R. (2013) The pro-forms of insulin-like growth factor I (IGF-I) are predominant in skeletal muscle and alter IGF-I receptor activation. Endocrinology 154, 1215–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barton E. R., Park S., James J. K., Makarewich C. A., Philippou A., Eletto D., Lei H., Brisson B., Ostrovsky O., Li Z., Argon Y. (2012) Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production. FASEB J. 26, 3691–3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moorwood C., Liu M., Tian Z., Barton E. R. (2013) Isometric and eccentric force generation assessment of skeletal muscles isolated from murine models of muscular dystrophies. J. Vis. Exp. e50036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith L. R., Barton E. R. (2014) SMASH—semi-automatic muscle analysis using segmentation of histology: a MATLAB application. Skelet. Muscle 4, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao P., Monks D. A. (2009) A tetracycline-inducible and skeletal muscle-specific Cre recombinase transgenic mouse. Dev. Neurobiol. 69, 401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y., Herrera P. L., Guo Y., Sun D., Tang Z., LeRoith D., Liu J. L. (2004) Pancreatic-specific inactivation of IGF-I gene causes enlarged pancreatic islets and significant resistance to diabetes. Diabetes 53, 3131–3141 [DOI] [PubMed] [Google Scholar]
- 27.Daughaday W. H., Rotwein P. (1989) Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr. Rev. 10, 68–91 [DOI] [PubMed] [Google Scholar]
- 28.Brismar K., Fernqvist-Forbes E., Wahren J., Hall K. (1994) Effect of insulin on the hepatic production of insulin-like growth factor-binding protein-1 (IGFBP-1), IGFBP-3, and IGF-I in insulin-dependent diabetes. J. Clin. Endocrinol. Metab. 79, 872–878 [DOI] [PubMed] [Google Scholar]
- 29.Schiaffino S., Mammucari C. (2011) Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet. Muscle 1, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stitt T. N., Drujan D., Clarke B. A., Panaro F., Timofeyva Y., Kline W. O., Gonzalez M., Yancopoulos G. D., Glass D. J. (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 14, 395–403 [DOI] [PubMed] [Google Scholar]
- 31.Shi J., Luo L., Eash J., Ibebunjo C., Glass D. J. (2011) The SCF-Fbxo40 complex induces IRS1 ubiquitination in skeletal muscle, limiting IGF1 signaling. Dev. Cell 21, 835–847 [DOI] [PubMed] [Google Scholar]
- 32.McPherron A. C., Lawler A. M., Lee S. J. (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387, 83–90 [DOI] [PubMed] [Google Scholar]
- 33.Morissette M. R., Cook S. A., Buranasombati C., Rosenberg M. A., Rosenzweig A. (2009) Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am. J. Physiol. Cell Physiol. 297, C1124–C1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baxter R. C. (2014) IGF binding proteins in cancer: mechanistic and clinical insights. Nat. Rev. Cancer 14, 329–341 [DOI] [PubMed] [Google Scholar]
- 35.Ning Y., Schuller A. G., Bradshaw S., Rotwein P., Ludwig T., Frystyk J., Pintar J. E. (2006) Diminished growth and enhanced glucose metabolism in triple knockout mice containing mutations of insulin-like growth factor binding protein-3, -4, and -5. Mol. Endocrinol. 20, 2173–2186 [DOI] [PubMed] [Google Scholar]
- 36.Sheffield-Moore M., Urban R. J. (2004) An overview of the endocrinology of skeletal muscle. Trends Endocrinol. Metab. 15, 110–115 [DOI] [PubMed] [Google Scholar]
- 37.Shanik M. H., Xu Y., Skrha J., Dankner R., Zick Y., Roth J. (2008) Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 31(suppl 2), S262–S268 [DOI] [PubMed] [Google Scholar]
- 38.Yakar S., Liu J. L., Fernandez A. M., Wu Y., Schally A. V., Frystyk J., Chernausek S. D., Mejia W., Le Roith D. (2001) Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes 50, 1110–1118 [DOI] [PubMed] [Google Scholar]
- 39.Cheng C. M., Reinhardt R. R., Lee W. H., Joncas G., Patel S. C., Bondy C. A. (2000) Insulin-like growth factor 1 regulates developing brain glucose metabolism. Proc. Natl. Acad. Sci. USA 97, 10236–10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernández A. M., Dupont J., Farrar R. P., Lee S., Stannard B., Le Roith D. (2002) Muscle-specific inactivation of the IGF-I receptor induces compensatory hyperplasia in skeletal muscle. J. Clin. Invest. 109, 347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mavalli M. D., DiGirolamo D. J., Fan Y., Riddle R. C., Campbell K. S., van Groen T., Frank S. J., Sperling M. A., Esser K. A., Bamman M. M., Clemens T. L. (2010) Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J. Clin. Invest. 120, 4007–4020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barton E. R. (2006) The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl. Physiol. Nutr. Metab. 31, 791–797 [DOI] [PubMed] [Google Scholar]
- 43.Baker J., Liu J. P., Robertson E. J., Efstratiadis A. (1993) Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75, 73–82 [PubMed] [Google Scholar]
- 44.Nielsen J. H., Linde S., Welinder B. S., Billestrup N., Madsen O. D. (1989) Growth hormone is a growth factor for the differentiated pancreatic beta-cell. Mol. Endocrinol. 3, 165–173 [DOI] [PubMed] [Google Scholar]
- 45.Zhao A. Z., Zhao H., Teague J., Fujimoto W., Beavo J. A. (1997) Attenuation of insulin secretion by insulin-like growth factor 1 is mediated through activation of phosphodiesterase 3B. Proc. Natl. Acad. Sci. USA 94, 3223–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Federici M., Porzio O., Lauro D., Borboni P., Giovannone B., Zucaro L., Hribal M. L., Sesti G. (1998) Increased abundance of insulin/insulin-like growth factor-I hybrid receptors in skeletal muscle of obese subjects is correlated with in vivo insulin sensitivity. J. Clin. Endocrinol. Metab. 83, 2911–2915 [DOI] [PubMed] [Google Scholar]
- 47.Bailyes E. M., Navé B. T., Soos M. A., Orr S. R., Hayward A. C., Siddle K. (1997) Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem. J. 327, 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Neill B. T., Lee K. Y., Klaus K., Softic S., Krumpoch M. T., Fentz J., Stanford K. I., Robinson M. M., Cai W., Kleinridders A., Pereira R. O., Hirshman M. F., Abel E. D., Accili D., Goodyear L. J., Nair K. S., Kahn C. R. (2016) Insulin and IGF-1 receptors regulate FoxO-mediated signaling in muscle proteostasis. J. Clin. Invest. 126, 3433–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slaaby R. (2015) Specific insulin/IGF1 hybrid receptor activation assay reveals IGF1 as a more potent ligand than insulin. Sci. Rep. 5, 7911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siddle K. (2012) Molecular basis of signaling specificity of insulin and IGF receptors: neglected corners and recent advances. Front. Endocrinol. (Lausanne) 3, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sjögren K., Wallenius K., Liu J. L., Bohlooly-Y M., Pacini G., Svensson L., Törnell J., Isaksson O. G., Ahrén B., Jansson J. O., Ohlsson C. (2001) Liver-derived IGF-I is of importance for normal carbohydrate and lipid metabolism. Diabetes 50, 1539–1545 [DOI] [PubMed] [Google Scholar]
- 52.Rehfeldt C., Renne U., Sawitzky M., Binder G., Hoeflich A. (2010) Increased fat mass, decreased myofiber size, and a shift to glycolytic muscle metabolism in adolescent male transgenic mice overexpressing IGFBP-2. Am. J. Physiol. Endocrinol. Metab. 299, E287–E298 [DOI] [PubMed] [Google Scholar]
- 53.Ashpole N. M., Logan S., Yabluchanskiy A., Mitschelen M. C., Yan H., Farley J. A., Hodges E. L., Ungvari Z., Csiszar A., Chen S., Georgescu C., Hubbard G. B., Ikeno Y., Sonntag W. E. (2017) IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience 39, 129–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spangenburg E. E., Geiger P. C., Leinwand L. A., Lowe D. A. (2012) Regulation of physiological and metabolic function of muscle by female sex steroids. Med. Sci. Sports Exerc. 44, 1653–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saengsirisuwan V., Pongseeda S., Prasannarong M., Vichaiwong K., Toskulkao C. (2009) Modulation of insulin resistance in ovariectomized rats by endurance exercise training and estrogen replacement. Metabolism 58, 38–47 [DOI] [PubMed] [Google Scholar]
- 56.Wilcox G. (2005) Insulin and insulin resistance. Clin. Biochem. Rev. 26, 19–39 [PMC free article] [PubMed] [Google Scholar]
- 57.Kalyani R. R., Corriere M., Ferrucci L. (2014) Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2, 819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.