Abstract
Dimethylarginine dimethylaminohydrolases (DDAHs) are known to degrade asymmetric dimethylarginine, an endogenous inhibitor of NOS, and maintain vascular homeostasis; however, the regulatory pathways of DDAHs remain unclear. In this study, we aimed to define the role of transmembrane glycoprotein neuropilin-1 (NRP1) in the expression of DDAHs and investigate the potential roles of NRP1 in regulation of blood pressure. Short hairpin RNA–mediated knockdown of NRP1 reduced the level and mRNA stability of DDAH1 but not DDAH2 in HUVECs, whereas overexpression of NRP1 increased the mRNA stability of DDAH1. Meanwhile, mesenteric arteries and lung vascular endothelial cells of tamoxifen-inducible endothelial cell–specific NRP1 knockout mice exhibited decreased expression of DDAH1 and slightly increased expression of DDAH2. Mechanistically, the regulation of NRP1 on DDAH1 expression is mediated by a posttranscriptional mechanism involving miR-219-5p in HUVECs. Although the endothelial cell–specific NRP1 knockout mice did not exhibit any significant change in blood pressure at the basal level, they were more sensitive to low-dose angiotensin II infusion–induced increases in blood pressure. Our results show that NRP1 is required for full expression of DDAH1 in endothelial cells and that NRP1 contributes to protection from low-dose angiotensin II–induced increases in blood pressure.—Wang, Y., Wang, E., Zhang, Y., Madamsetty, V. S., Ji, B., Radisky, D. C., Grande, J. P., Misra, S., Mukhopadhyay, D. Neuropilin-1 maintains dimethylarginine dimethylaminohydrolase 1 expression in endothelial cells, and contributes to protection from angiotensin II–induced hypertension.
Keywords: endothelium, NO, high blood pressure, miRNA
Endothelial cell dysfunction is defined by an imbalance in production and consumption of NO (1, 2), which has been shown to be inhibited by asymmetric dimethylarginine (ADMA) (3). ADMA is produced by proteolysis of proteins containing methylarginine residues and competes with l-arginine to inhibit the activity of 3 isoforms of NOS (3). Two isoforms of the dimethylarginine dimethylaminohydrolase (DDAH) enzyme (DDAH1 and DDAH2) degrade ADMA to control its intracellular and plasma levels (4, 5). Mutation of DDAH is correlated with increased susceptibility to thrombosis, stroke, and coronary heart disease (6); the mechanism mediating expressions of DDAH remains unclear, however.
Studies have reported that the ADMA/DDAH1 pathway is involved in the motility of endothelial cells (7) and angiogenic response is impaired in endothelial cell–specific DDAH1 knockout mice (8), suggesting crosstalk between angiogenesis-related pathways and ADMA/DDAH. In the present study, we investigated the effect of neuropilin-1 (NRP1), a coreceptor of several structurally diverse ligands [including VEGF (9) and semaphorins (10, 11)], on the expression of DDAHs (including DDAH1 and DDAH2) in endothelial cells in vitro and endothelial cell–specific NRP1 knockout mice in vivo. Our results identified miR-219-5p as a novel downstream target of NRP1 to control the mRNA stability of DDAH1 but not DDAH2 in endothelial cells. We also examined the role of endothelial NRP1 in a murine angiotensin II (ang II)-induced hypertension model. Our results show that NRP1 controls expression of DDAH1 in endothelial cells and that endothelial NRP1 is essential to controlling blood pressure upon ang II infusion.
MATERIALS AND METHODS
Mice
Tamoxifen-inducible endothelial-specific NRP1 knockout mice were generated and administered tamoxifen (MilliporeSigma, Burlington, MA, USA) as previously described (12). Animal care and experimental procedures were performed under protocols approved by the Institutional Animal Care and Use Committees of Mayo Clinic. The mice were housed in a conventional facility at Mayo Clinic with a reverse 12-h light/12-h dark cycle and ad libitum access to food and water. Animals were allocated to experimental groups according to their predetermined type, and no randomization was used. All animals were included in the study, and the definition of inclusion and exclusion criteria was not applicable. All blood pressure measurements and surgical procedures were performed during daylight hours. The investigators who performed the blood pressure measurements and micro-osmotic pump implantation were blinded to the genotypes of the mice.
For ang II infusion, 10-wk-old animals (male) were anesthetized with an i.p. injection of ketamine/xylazine. Micro-osmotic pumps (Alzet model 1007D; Durect, Cupertino, CA, USA) were implanted subcutaneously in the midscapular region and delivered ang II at a rate of 500 ng/kg/min for 7 d. With the use of a tail cuff noninvasive blood pressure system (Kent Scientific, Torrington, CT, USA), blood pressure was measured at two occasions before the implantation of osmotic pumps and on the seventh day after ang II infusion. Awake mice were placed into the holder for at least 10 min before measurement to minimize the anxiety. Temperature was monitored throughout the experiment. Systolic blood pressure and diastolic blood pressure values were derived from an average of 15–25 measurements per animal. The change in blood pressure was calculated according to the changes in blood pressure from d 0 to d 7.
Quantitative PCR and Western blotting
Quantitative PCR (qPCR) and Western blotting were performed as previously described (12). For microRNA (miRNA) quantification, total mRNA was reverse transcribed with the NCode Vilo miRNA cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). Primer sequences are shown in Supplemental Table 1.
Cells and reagents
HUVECs (Lonza, Basel, Switzerland) were cultured in Endothelial Basal Medium with the Endothelial Cell Growth Medium BulletKit (Lonza). Mouse lung vascular endothelial cells were isolated with a Lung Dissociation Kit (Miltenyi, Bergisch Gladbach, Germany) followed by selection with cluster of differentiation 31 (CD31) MicroBeads (Miltenyi) as the manufacturer described. Short hairpin RNA (shRNA) for human NRP1 and controls were from Open Biosystems (Huntsville, AL, USA). The NRP1 shRNA targeting sequence was 5′-CCCTGTTGGTTTCATTTGAATA-3′. Lentivirus for NRP1 shRNA and control shRNA was prepared in 293T cells, which were transfected with targeted gene (pGIPZ-NRP1 shRNA and pGIPZ-control shRNA), pGag.Pol, and pVSV-G (encoding the cDNAs of the proteins that are required for virus packing) as previously described (13). After infection, 2 μg/ml of puromycin was added to the medium for antibiotic selection, and cell clones were selected for further experiments. Retroviruses expressing NRP1 or LacZ were prepared to overexpress NRP1 in HUVECs as previously described (13). The miR-219-5p inhibitor (MH10664) was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Antibodies against NRP1 (3725) were purchased from Cell Signaling Technology (Danvers, MA, USA). Tamoxifen, actinomycin D (final concentration: 5 μg/ml), cycloheximide (final concentration: 20 μg/ml), and β-actin antibody (A2228) were purchased from MilliporeSigma. DDAH1 antibody (#ab82908) was purchased from Abcam (Cambridge, MA, USA). The specificity of DDAH1 antibody was confirmed with lysates of DDAH1 siRNA (SI04224808; Qiagen, Germantown, MD, USA)-transfected HUVECs. DDAH2 antibody (14966-1-AP) was purchased from ProteinTech (Rosemont, IL, USA).
Statistical analyses
All analyses were performed with GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Experiments were routinely repeated at least 3 times, and the number was increased according to effect size or sample variation. Values are expressed as means ± sem. In Figs. 1A and 2F, the values in the control group were normalized to 1, and other groups were expressed as relative folds/percentages of the control group. Statistical differences were determined to be significant at values of P < 0.05. For comparison between 2 groups, an unpaired 2-tailed Student’s t test was performed. For comparison among ≥3 groups, 1-way ANOVA followed by the Newman-Keuls test was used.
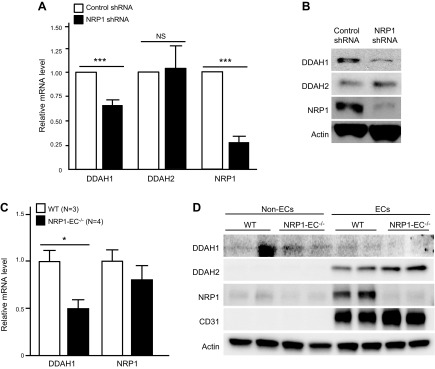
Figure 1.
NRP1 maintains DDAH1 expression in vascular endothelial cells in vitro and in vivo. A) HUVECs were infected with lentivirus expressing control shRNA or NRP1 shRNA. Expression of DDAH1, DDAH2, and NRP1 were examined with qPCR. n = 5 for each group. B) HUVECs were infected with lentivirus expressing control shRNA or NRP1 shRNA, Western blotting was performed to examine expression of DDAH1, NRP1, and β-actin. Images are representative of 3 independent experiments. C) mRNA levels of DDAH1 and NRP1 in mesenteric arteries of control mice and NRP1EC−/− mice were quantified with qPCR [n = 3 for wild-type (WT) group, n = 4 for knockout group]. D) Lung endothelial cells of control mice and NRP1EC−/− mice were isolated with CD31 Dynabeads and subjected to Western blotting with the indicated antibodies. Images are representative of 2 independent experiments (n = 4 each group). NS, not significant. *P < 0.05, ***P < 0.001.
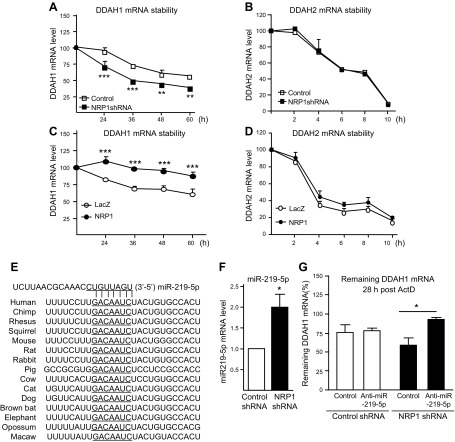
Figure 2.
NRP1 controls mRNA stability of DDAH1 via miR-219-5p in endothelial cells. A–D) mRNA stability of DDAH1 and DDAH2 was quantified with qPCR in NRP1 knockdown HUVECs (A, B) and NRP1 overexpressing HUVECs (C, D) after administration with actinomycin D (ActD; 5 μg/ml) (n = 4 for each group). E) Sequence alignment of the miR-219-5p base-pairing sites in the 3′-untranslated region of DDAH1 mRNAs, which are highly conserved among human, chimpanzee, rhesus monkey, squirrel, mouse, rat, rabbit, pig, and cow, among others. The “seed” sequences of miR-219-5p complementary to DDAH1 are underlined. F) The miR-219-5p mRNA level was validated in NRP1 knockdown HUVECs with qPCR (n = 7 for each group). G) Control HUVECs and NRP1 knockdown HUVECs were transfected with an miR219-5p inhibitor or corresponding control, and then administered ActD (5 μg/ml). mRNA was collected at time 0 and 28 h post-ActD administration, and mRNA stability of DDAH1 was examined (n = 4 for each group). *P < 0.05, **P < 0.01, ***P < 0.001.
RESULTS
NRP1 maintains expression of DDAH1 but not DDAH2 in vitro and in vivo
To define the effect of NRP1 on the expression of DDAHs in endothelial cells, expression of DDAH1 and DDAH2 was examined upon knockdown and overexpression of NRP1 in HUVECs. As shown in Fig. 1A, B, knockdown of NRP1 significantly decreased the mRNA and protein levels of DDAH1. Meanwhile, knockdown of NRP1 did not affect the DDAH2 mRNA level but slightly increased its protein level. Conversely, overexpression of NRP1 did not significantly affect levels of DDAH1 or DDAH2 (Supplemental Fig. 1A, B).
To further define the regulation of NRP1 on DDAHs in vivo, expression of DDAH1 was examined in the tamoxifen-inducible endothelial cell–specific NRP1 knockout (NRP1EC−/−) mice, which were generated by crossing mice expressing tamoxifen-inducible Cre-recombinase (Cre-ERT2) under the control of the vascular endothelial-cadherin promoter with NRP1f/f floxed mice. Our results (Fig. 1C) showed that expression of DDAH1 was significantly reduced in the mesenteric arteries of NRP1EC−/− mice. Furthermore, lung vascular endothelial cells, characterized by expression of cluster of differentiation 31 (CD31), were isolated and exhibited decreased expression of NRP1 and DDAH1 in the NRP1EC−/− mice (Fig. 1D). DDAH2 was observed to be mainly expressed in the vascular endothelial cells of lung tissues and was slightly increased in the NRP1EC−/− group (Fig. 1D).
NRP1 controls mRNA stability of DDAH1 via miR-219-5p in endothelial cells
To investigate the mechanism through which NRP1 regulates DDAH expression, mRNA stability of DDAHs was examined in NRP1-silenced and NRP1-overexpressed HUVECs. Our results showed that DDAH1 is stable in the control group when NRP1 levels are unaltered. However, upon NRP1 knockdown, DDAH1 mRNA stability was significantly decreased (68.5 ± 4.4 h vs. 43.5 ± 0.5 h; P < 0.01) (Fig. 2A); conversely, overexpression of NRP1 significantly increased DDAH1 mRNA stability (79.12 ± 11.27 h vs. 213.6 ± 56.7 h; P < 0.05) (Fig. 2C). No differences in DDAH2 mRNA stability were observed upon knockdown or overexpression of NRP1 (Fig. 2B, D). Protein stability assay was also performed and showed that the basal level of DDAH1 was decreased in NRP1 knockdown HUVECs, but its protein stability was not significantly affected after knocking down NRP1 (Supplemental Fig. 1C, D). These results suggest that a posttranscriptional mechanism is involved in the NRP1-mediated regulation of DDAH1.
Because miRNAs play an important role in translation inhibition and mRNA degradation, the 3′-untranslated region of DDAH1 mRNA was analyzed, and a conserved miRNA binding site of miR-219-5p among species was identified (Fig. 2E). Indeed, increased expression of miR-219-5p was present in NRP1 knockdown endothelial cells (Fig. 2F). Furthermore, transfection of an miR-219-5p inhibitor significantly increased the mRNA stability in NRP1 knockdown endothelial cells (Fig. 2G). These results suggest that NRP1 regulates DDAH1 expression through a posttranscriptional mechanism involving miR-219-5p.
Endogenous endothelial NRP1 is involved in the regulation of blood pressure in an experimental hypertension model induced by low-dose ang II infusion
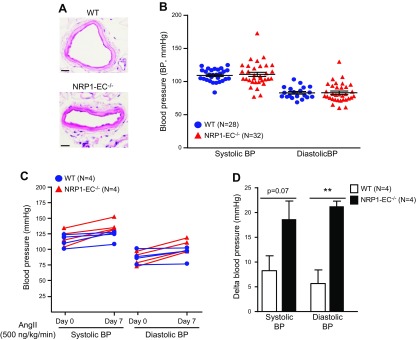
Because DDAHs have been reported previously to play important roles in the regulation of blood pressure (8, 14, 15), we then sought to investigate the effect of endothelial NRP1 on the blood pressure levels in vivo. The vascular endothelial–cadherin Cre-ERT2/NRP1f/f mice were administrated tamoxifen and used as the control group. The morphology and structures of mesenteric arteries did not exhibit significant differences between control and NRP1EC−/− mice (Fig. 3A). Meanwhile, the average level of each readout of blood pressure was not significantly changed in the NRP1EC−/− mice (Fig. 3B).
Figure 3.
Knockout of endothelial NRP1 aggravates a low-dose ang II infusion–induced hypertension. Male vascular endothelial–cadherin Cre-ERT2/NRP1f/f [wild-type (WT)] mice and vascular endothelial–cadherin Cre-ERT2+/NRP1f/f (NRP1-EC−/−) mice were administered tamoxifen through gavage feeding for 5 consecutive days and then subjected to all the experiments. A) Hematoxylin and eosin staining of mesenteric arteries. Images are representative of 4 mice in each group. B) Basal level blood pressure of NRP1-EC−/− (n = 32) and WT mice (n = 28) was measured with tail-cuff system. C, D) WT mice (n = 4) and NRP1-EC−/− mice (n = 4) were implanted with osmotic pumps releasing ang II (500 ng/kg/min) for 7 d. Blood pressure was measured at d 0 and 7 after ang II infusion (C). The change in blood pressure was calculated according to the changes in blood pressure levels from d 0 to 7 after ang II infusion (D). The delta blood pressure of each mouse is expressed as the increased blood pressure level from d 0 to 7 (D). **P < 0.01.
These results prompted us to evaluate the blood pressure of NRP1EC−/− mice in the murine experimental hypertension models. To induce hypertension, we implanted osmotic minipumps in both control and NRP1EC−/− mice to deliver recombinant ang II, the most commonly used vasopressor in experimental models of hypertension (16); a low dose of 500 ng/kg per minute was used. Infusion of ang II led to increased blood pressure in both groups; however, NRP1EC−/− mice showed a greater change than the control group (Fig. 3C, D). These results suggest that endogenous endothelial NRP1 has a suppressive effect on hypertension induced by low-dose ang II infusion.
DISCUSSION
In this study, we identified that NRP1 mediates the expression of DDAH1 through a mechanism involving miR-219-5p in vascular endothelial cells. The important role of the NRP1-DDAH1 pathway in endothelial cell homeostasis was further supported by the aggravated increase of blood pressure in NRP1EC−/− mice. Taken together, our results suggest that NRP1 is essential to maintain vascular function upon infusion of low-dose ang II.
NRP1 has been reported to mediate endothelial cell chemotaxis, cell adhesion, axon guidance, and transduction of multiple signaling pathways (17, 18). Embryonic deletion of endothelial NRP1 led to severe vascular defects and embryonic lethality; however, mice deficient in endothelial NRP1 in adulthood were viable but exhibited an attenuated response to semaphorin 3A, decreased VEGF-induced vascular permeability, and reduced inflammatory responses in a mouse neuroinflammatory disease model (12, 19, 20). Although NRP1EC−/− mice did not show any significant change in the basal blood pressure level, they exhibited significantly elevated blood pressure after the ang II infusion compared with control mice (Fig. 3). Increased ang II has been shown to induce hypertension through several mechanisms, including endothelial cell dysfunction (21) and ADMA (22, 23). In light of our results showing decreased expression of DDAH1 in the endothelial cells of NRP1EC−/− mice (Fig. 1), decreased expression of DDAH1 may contribute to the ADMA levels and blood pressure control in NRP1EC−/− mice upon ang II infusion. Although DDAH2 was observed to be slightly increased in both NRP1 knockdown HUVECs and lung endothelial cells of NRP1EC−/− mice, the level of DDAH2 may not sufficiently compensate the deceased level of DDAH1. Indeed, our results (data not shown) suggest that circulating ADMA is potentially increased in the NRP1EC−/− mice. ADMA also exhibited increased trends in the adipose tissues of mice lacking NRP1 in cardiomyocytes and vascular smooth muscle cells (SM22-α-Nrp-1 knockout) (24). Taken together, these results suggest that it will be of importance to maintain endothelial NRP1 signaling to control blood pressure under certain proconstrictive stimuli, such as ang II infusion.
DDAH1 functions as one of the major enzymes responsible for the degradation of ADMA, which has been shown to be an independent risk factor for coronary heart disease and many other diseases (25–27). The role of endothelial DDAH1 in blood pressure regulation remains controversial. Knockout of endothelial DDAH1 in mice was first reported to elevate circulating ADMA levels and increase systolic blood pressure (14); however, a study showed that deletion of endothelial DDAH1 in mice did not correlate to an increase in ADMA or change in blood pressure but rather led to an impaired angiogenic response (8). Our study raises the possibility that DDAH1 may be essential for blood pressure maintenance in response to certain types of vasopressors. Meanwhile, NRP1 reportedly potentiates VEGF-stimulated production of prostacyclin I2 in endothelial cells (28), indicating that multiple downstream mediators of NRP1 may be involved in the regulation of blood pressure.
Our results show that NRP1 regulates expression of DDAH1 through a posttranscriptional mechanism in vascular endothelial cells under basal conditions absent ligand-mediated stimulation of NRP1 (Fig. 2). Interestingly, we noticed that the DDAH1 mRNA level was stable in HUVECs, which was confirmed with 2 different sets of DDAH1 primers (Fig. 2 and data not shown). One study reported that the half-life of DDAH1 mRNA was ~1 h in normal pulmonary artery endothelial cells and smooth muscle cells under normoxic conditions (29), suggesting that cell type–specific variability of DDAH1 mRNA stability may exist. Notably, the opposite changes in DDAH1 and DDAH2 protein levels in the NRP1-ablated endothelial cells indicate that DDAH2 is differently regulated by NRP1 in endothelial cells (Fig. 1). Knockdown of NRP1 did not significantly alter the mRNA level and stability of DDAH2 but slightly increased the DDAH2 protein levels (Figs. 1 and 2), suggesting that a posttranscriptional mechanism may be involved. Furthermore, although the DDAH2 level did not show a significant change in the tissues of DDAH1 knockout mice in the previous studies (30), DDAH2 was observed to be increased in the rat diabetic kidney tissues, which had decreased DDAH1 and elevated ADMA levels (31). These studies raise possibilities that the compensatory up-regulation of DDAH2 may be induced in NRP1 knockdown endothelial cells, which have decreased DDAH1.
In summary, our results show that NRP1 maintains DDAH1 expression in endothelial cells and deletion of endothelial NRP1 leads to increased blood pressure upon infusion of low-dose ang II.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Alex Kolodkin (Johns Hopkins University, Baltimore, MD, USA) and Dr. Ralf H. Adams (Max Planck Institute, Münster, Germany) for the NRP1flox/flox mice and vascular endothelial–cadherin/Cre-ERT2 mice, respectively. They also thank Dr. Jordan D. Miller and Mr. Bruce E. Knudsen (Mayo Clinic) for assistance with the mouse blood pressure measurements. The authors thank the Microarray Core Facility at Mayo Clinic for their support and Brandy H. Edenfield (Mayo Clinic Jacksonville Histopathology Facility) for the kind help. The authors thank Dr. Luke H. Hoeppner (The Hormel Institute, University of Minnesota, Austin, MN, USA) for critically reviewing the manuscript, and Drs. DeLisa Fairweather and Katelyn A. Bruno (Mayo Clinic) for assistance with mouse lung endothelial cell isolation. This work was supported by U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grant HL140411, and NIH National Cancer Institute Grant CA78383-20 (both to D.M.), the Florida Department of Health Cancer Research Chair’s Fund Florida Grant 3J-02 (to D.M.), and the American Heart Association Grant AHA-14POST20390029 (to Y.W.). The authors declare no conflicts of interest.
Glossary
- ADMA
asymmetric dimethylarginine
- ang II
angiotensin II
- CD31
cluster of differentiation 31
- DDAH
dimethylarginine dimethylaminohydrolase
- miRNA
microRNA
- NRP1
neuropilin-1
- qPCR
quantitative PCR
- shRNA
short hairpin RNA
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Wang and D. Mukhopadhyay designed the research, analyzed the data, and wrote the paper; Y. Wang, E. Wang, Y. Zhang, and V. S. Madamsetty performed research; D. C. Radisky, B. Ji, and S. Misra analyzed the data; and J. P. Grande contributed analytic tools for experiments.
REFERENCES
- 1.Hadi H. A., Carr C. S., Al Suwaidi J. (2005) Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 1, 183–198 [PMC free article] [PubMed] [Google Scholar]
- 2.Liao J. K. (2013) Linking endothelial dysfunction with endothelial cell activation. J. Clin. Invest. 123, 540–541 10.1172/JCI66843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallance P., Leone A., Calver A., Collier J., Moncada S. (1992) Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339, 572–575 10.1016/0140-6736(92)90865-Z [DOI] [PubMed] [Google Scholar]
- 4.Achan V., Broadhead M., Malaki M., Whitley G., Leiper J., MacAllister R., Vallance P. (2003) Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler. Thromb. Vasc. Biol. 23, 1455–1459 10.1161/01.ATV.0000081742.92006.59 [DOI] [PubMed] [Google Scholar]
- 5.Ogawa T., Kimoto M., Sasaoka K. (1987) Occurrence of a new enzyme catalyzing the direct conversion of NG,NG-dimethyl-L-arginine to L-citrulline in rats. Biochem. Biophys. Res. Commun. 148, 671–677 10.1016/0006-291X(87)90929-6 [DOI] [PubMed] [Google Scholar]
- 6.Ding H., Wu B., Wang H., Lu Z., Yan J., Wang X., Shaffer J. R., Hui R., Wang D. W. (2010) A novel loss-of-function DDAH1 promoter polymorphism is associated with increased susceptibility to thrombosis stroke and coronary heart disease. Circ. Res. 106, 1145–1152 10.1161/CIRCRESAHA.109.215616 [DOI] [PubMed] [Google Scholar]
- 7.Wojciak-Stothard B., Torondel B., Tsang L. Y., Fleming I., Fisslthaler B., Leiper J. M., Vallance P. (2007) The ADMA/DDAH pathway is a critical regulator of endothelial cell motility. J. Cell Sci. 120, 929–942 10.1242/jcs.002212 [DOI] [PubMed] [Google Scholar]
- 8.Dowsett L., Piper S., Slaviero A., Dufton N., Wang Z., Boruc O., Delahaye M., Colman L., Kalk E., Tomlinson J., Birdsey G., Randi A. M., Leiper J. (2015) Endothelial dimethylarginine dimethylaminohydrolase 1 is an important regulator of angiogenesis but does not regulate vascular reactivity or hemodynamic homeostasis. Circulation 131, 2217–2225 10.1161/CIRCULATIONAHA.114.015064 [DOI] [PubMed] [Google Scholar]
- 9.Soker S., Takashima S., Miao H. Q., Neufeld G., Klagsbrun M. (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92, 735–745 10.1016/S0092-8674(00)81402-6 [DOI] [PubMed] [Google Scholar]
- 10.Kolodkin A. L., Levengood D. V., Rowe E. G., Tai Y. T., Giger R. J., Ginty D. D. (1997) Neuropilin is a semaphorin III receptor. Cell 90, 753–762 10.1016/S0092-8674(00)80535-8 [DOI] [PubMed] [Google Scholar]
- 11.He Z., Tessier-Lavigne M. (1997) Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90, 739–751 10.1016/S0092-8674(00)80534-6 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Cao Y., Mangalam A. K., Guo Y., LaFrance-Corey R. G., Gamez J. D., Atanga P. A., Clarkson B. D., Zhang Y., Wang E., Angom R. S., Dutta K., Ji B., Pirko I., Lucchinetti C. F., Howe C. L., Mukhopadhyay D. (2016) Neuropilin-1 modulates interferon-γ-stimulated signaling in brain microvascular endothelial cells. J. Cell Sci. 129, 3911–3921 10.1242/jcs.190702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Zeng H., Wang P., Soker S., Mukhopadhyay D. (2003) Neuropilin-1-mediated vascular permeability factor/vascular endothelial growth factor-dependent endothelial cell migration. J. Biol. Chem. 278, 48848–48860 10.1074/jbc.M310047200 [DOI] [PubMed] [Google Scholar]
- 14.Hu X., Xu X., Zhu G., Atzler D., Kimoto M., Chen J., Schwedhelm E., Lüneburg N., Böger R. H., Zhang P., Chen Y. (2009) Vascular endothelial-specific dimethylarginine dimethylaminohydrolase-1-deficient mice reveal that vascular endothelium plays an important role in removing asymmetric dimethylarginine. Circulation 120, 2222–2229 10.1161/CIRCULATIONAHA.108.819912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambden S., Kelly P., Ahmetaj-Shala B., Wang Z., Lee B., Nandi M., Torondel B., Delahaye M., Dowsett L., Piper S., Tomlinson J., Caplin B., Colman L., Boruc O., Slaviero A., Zhao L., Oliver E., Khadayate S., Singer M., Arrigoni F., Leiper J. (2015) Dimethylarginine dimethylaminohydrolase 2 regulates nitric oxide synthesis and hemodynamics and determines outcome in polymicrobial sepsis. Arterioscler. Thromb. Vasc. Biol. 35, 1382–1392 10.1161/ATVBAHA.115.305278 [DOI] [PubMed] [Google Scholar]
- 16.Leong X. F., Ng C. Y., Jaarin K. (2015) Animal models in cardiovascular research: hypertension and atherosclerosis. BioMed Res. Int. 2015, 528757 10.1155/2015/528757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee S., Sengupta K., Dhar K., Mehta S., D’Amore P. A., Dhar G., Banerjee S. K. (2006) Breast cancer cells secreted platelet-derived growth factor-induced motility of vascular smooth muscle cells is mediated through neuropilin-1. Mol. Carcinog. 45, 871–880 10.1002/mc.20248 [DOI] [PubMed] [Google Scholar]
- 18.Glinka Y., Prud’homme G. J. (2008) Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J. Leukoc. Biol. 84, 302–310 10.1189/jlb.0208090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu C., Rodriguez E. R., Reimert D. V., Shu T., Fritzsch B., Richards L. J., Kolodkin A. L., Ginty D. D. (2003) Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5, 45–57 10.1016/S1534-5807(03)00169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acevedo L. M., Barillas S., Weis S. M., Göthert J. R., Cheresh D. A. (2008) Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood 111, 2674–2680 10.1182/blood-2007-08-110205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta P. K., Griendling K. K. (2007) Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 292, C82–C97 10.1152/ajpcell.00287.2006 [DOI] [PubMed] [Google Scholar]
- 22.Luo Z., Teerlink T., Griendling K., Aslam S., Welch W. J., Wilcox C. S. (2010) Angiotensin II and NADPH oxidase increase ADMA in vascular smooth muscle cells. Hypertension 56, 498–504 10.1161/HYPERTENSIONAHA.110.152959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa K., Wakino S., Tatematsu S., Yoshioka K., Homma K., Sugano N., Kimoto M., Hayashi K., Itoh H. (2007) Role of asymmetric dimethylarginine in vascular injury in transgenic mice overexpressing dimethylarginie dimethylaminohydrolase 2. Circ. Res. 101, e2–e10 10.1161/CIRCRESAHA.107.156901 [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Cao Y., Yamada S., Thirunavukkarasu M., Nin V., Joshi M., Rishi M. T., Bhattacharya S., Camacho-Pereira J., Sharma A. K., Shameer K., Kocher J. P., Sanchez J. A., Wang E., Hoeppner L. H., Dutta S. K., Leof E. B., Shah V., Claffey K. P., Chini E. N., Simons M., Terzic A., Maulik N., Mukhopadhyay D. (2015) Cardiomyopathy and worsened ischemic heart failure in sm22-α cre-mediated neuropilin-1 null mice: dysregulation of pgc1α and mitochondrial homeostasis. Arterioscler. Thromb. Vasc. Biol. 35, 1401–1412 10.1161/ATVBAHA.115.305566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willeit P., Freitag D. F., Laukkanen J. A., Chowdhury S., Gobin R., Mayr M., Di Angelantonio E., Chowdhury R. (2015) Asymmetric dimethylarginine and cardiovascular risk: systematic review and meta-analysis of 22 prospective studies. J. Am. Heart Assoc. 4, e001833 10.1161/JAHA.115.001833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Böger R. H., Bode-Böger S. M., Szuba A., Tsao P. S., Chan J. R., Tangphao O., Blaschke T. F., Cooke J. P. (1998) Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 98, 1842–1847 10.1161/01.CIR.98.18.1842 [DOI] [PubMed] [Google Scholar]
- 27.Leiper J. M. (2005) The DDAH-ADMA-NOS pathway. Ther. Drug Monit. 27, 744–746 10.1097/01.ftd.0000179849.42395.11 [DOI] [PubMed] [Google Scholar]
- 28.Neagoe P. E., Lemieux C., Sirois M. G. (2005) Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin synthesis requires the activation of VEGF receptor-1 and -2 heterodimer. J. Biol. Chem. 280, 9904–9912 10.1074/jbc.M412017200 [DOI] [PubMed] [Google Scholar]
- 29.Iannone L., Zhao L., Dubois O., Duluc L., Rhodes C. J., Wharton J., Wilkins M. R., Leiper J., Wojciak-Stothard B. (2014) miR-21/DDAH1 pathway regulates pulmonary vascular responses to hypoxia. Biochem. J. 462, 103–112 10.1042/BJ20140486 [DOI] [PubMed] [Google Scholar]
- 30.Hu X., Atzler D., Xu X., Zhang P., Guo H., Lu Z., Fassett J., Schwedhelm E., Böger R. H., Bache R. J., Chen Y. (2011) Dimethylarginine dimethylaminohydrolase-1 is the critical enzyme for degrading the cardiovascular risk factor asymmetrical dimethylarginine. Arterioscler. Thromb. Vasc. Biol. 31, 1540–1546 10.1161/ATVBAHA.110.222638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onozato M. L., Tojo A., Leiper J., Fujita T., Palm F., Wilcox C. S. (2008) Expression of NG,NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: effects of angiotensin II receptor blockers. Diabetes 57, 172–180 10.2337/db06-1772 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.