Abstract
Mesenchymal stem/stromal cells (MSCs) provide an attractive cell source for cartilage repair and cell therapy; however, the underlying molecular pathways that drive chondrogenesis of these populations of adult stem cells remain poorly understood. We generated a rich data set of high-throughput RNA sequencing of human MSCs throughout chondrogenesis at 6 different time points. Our data consisted of 18 libraries with 3 individual donors as biologic replicates, with each library possessing a sequencing depth of 100 million reads. Computational analyses with differential gene expression, gene ontology, and weighted gene correlation network analysis identified dynamic changes in multiple biologic pathways and, most importantly, a chondrogenic gene subset, whose functional characterization promises to further harness the potential of MSCs for cartilage tissue engineering. Furthermore, we created a graphic user interface encyclopedia built with the goal of producing an open resource of transcriptomic regulation for additional data mining and pathway analysis of the process of MSC chondrogenesis.—Huynh, N. P. T., Zhang, B., Guilak, F. High-depth transcriptomic profiling reveals the temporal gene signature of human mesenchymal stem cells during chondrogenesis.
Keywords: RNA-Seq, chondrocyte, pericyte, lncRNA, miRNA
Articular cartilage serves as a low-friction and load-bearing tissue, uniquely providing for joint motion for daily activities in life. However, articular cartilage has little or no capacity to heal or regenerate itself with injury or disease. Therefore, diseases associated with cartilage loss or degeneration often lead to progressive long-term pain and disability (1). Thus, there has been significant effort to develop new tissue-engineering approaches to enhance cartilage repair, often based on combinations of biomaterials and stem cells, to regenerate new cartilage to repair focal defects or resurface entire joints (2).
Mesenchymal stem/stromal cells (MSCs) were originally identified as a multipotent cell type found in the bone marrow (3), and more recently, similar cells have been identified throughout the body as populations of pericytes (4, 5). MSCs are easily accessible, expandable, and capable of differentiating into multiple skeletal lineages (6). For these reasons, they have been intensively investigated as a cell source for tissue engineering of cartilage, as well as a variety of other tissues (7–19). A number of in vitro studies of MSC-derived engineered cartilage have been performed in so-called pellet cultures, which are 3-dimensional aggregates of cells that produce cartilage-like structure after ∼3 wk (20, 21). Several molecular pathways have been proposed to play an important role during this process (22). However, the time course of transcriptomic events involved in the interplay and dynamic regulation of these pathways during MSC chondrogenesis has not been fully elucidated. While a number of individual genes [e.g., transcription factors (TFs), growth factors, and signaling molecules] have been characterized at specific time points, it is likely that the coordinated activity of a number of factors make up the gene regulatory network governing MSC chondrogenesis (23). For example, a recent study has examined this underlying process by differential gene expression analysis on microarray platforms (24).
To examine quantitatively the transcriptomic landscape of MSC chondrogenesis, we performed RNA-Seq at multiple time points during human MSC chondrogenesis in pellet culture. Compared to microarray analysis, RNA-Seq presents several additional advantages. For example, RNA-Seq possesses greater precision due to higher signal-to-noise ratio, independence from manufacturer’s limitation in the number of designed probes, a broader dynamic range to identify low-abundance transcripts and highly differentially expressed genes, and the ability to probe for novel genes (25). Bioinformatic analysis of this RNA-Seq data has uncovered several dominant gene signatures, including intertwining changes of specific molecular pathways, subsets of highly correlated genes of the chondrogenic functional module, and identification of long noncoding RNA (lncRNA) and microRNA (miRNA) candidates as regulators of chondrogenesis. Furthermore, we have assembled this information into an online searchable encyclopedia of MSC chondrogenesis, thus providing a comprehensive knowledge database of both coding and noncoding RNAs that describes the transcriptome of MSCs through progression toward a chondrocytic fate.
MATERIALS AND METHODS
MSC isolation and expansion
Discarded and deidentified waste tissue from the iliac crests of deidentified adult bone marrow transplant donors were collected in accordance with the institutional review board of Duke University Medical Center. Bone marrow–derived MSCs were isolated by their physical adherence to plastic. Cells were expanded and maintained in expansion medium: DMEM-low glucose (Thermo Fisher Scientific, Waltham, MA, USA), 1% penicillin/streptomycin (Thermo Fisher Scientific), 10% lot-selected fetal bovine serum (FBS; Thermo Fisher Scientific), and 1 ng/ml basic fibroblast growth factor (Roche, Basel, Switzerland). Three individual donors were used as biologic replicates in subsequent experiments.
Chondrogenic differentiation
At the end of passage 4, MSCs were dissociated from the culture vessels with 0.05% trypsin-EDTA (Sigma-Aldrich, St. Louis, MO, USA). Trypsin was subsequently inactivated with an equal volume of expansion medium. Detached cells were collected by centrifugation (200 g for 5 min), followed by 3 washes in prewarmed DMEM-high glucose (Thermo Fisher Scientific). The resulting cell mixtures were resuspended at 5.0 × 105 cells/ml in chondrogenic medium: DMEM-high glucose (Thermo Fisher Scientific), 1% penicillin/streptomycin (Thermo Fisher Scientific), 1% Insulin-Transferrin-Selenium Plus Premix (ITS+) (Corning, Corning, NY, USA), 100 nM dexamethasone (Sigma-Aldrich), 50 µg/ml ascorbic acid (Sigma-Aldrich), 40 µg/ml l-proline (Sigma-Aldrich), and 10 ng/ml recombinant human transforming growth factor beta 3 (rhTGF-β3) (R&D Systems, Minneapolis, MN, USA). To form pellets, 2.5 × 105 cells (500 µl) was dispended into each 15 ml conical tube, then centrifuged at 200 g for 5 min. Pellets were cultured for 21 d at 37°C and 5% CO2 with medium exchange every 3 d. Pellets were collected on d 0, 1, 3, 7, 14, and 21 for RNA isolation, and on d 3, 14, and 21 for histologic and biochemical analyses.
Flow cytometry
Passage 4 MSCs were digested with 0.05% trypsin-EDTA (Sigma-Aldrich) and washed 3 times in PBS/1% FBS. Cells were incubated with a cocktail of positive surface markers (CD44, CD73, CD105, CD90) (BD Biosciences, Franklin Lakes, NJ, USA) or with a negative surface marker (CD45) (BioLegend, San Diego, CA, USA) for 45 min. Unstained samples and samples with appropriate isotypes were used as controls. Samples with staining on separate channels were used for compensation. After incubation, cells were washed 3 times with PBS/1% FBS, and flow cytometry was performed. Data were analyzed by FlowJo (Treestar, Ashland, OR, USA) and are reported as means ± sd (n = 3 independent specimens for each donor).
Biochemical analyses for DNA and glycosaminoglycan content
Collected pellets were washed once in PBS, and stored at −20°C until processing. Pellets were digested in 125 µl/ml papain at 60°C overnight for biochemical analyses. DNA content was measured with the PicoGreen assay (Thermo Fisher Scientific), and glycosaminoglycan (GAG) was measured as previously described (26) using the 1,9-dimethylmethylene blue assay at 525 nm wavelength.
Histologic analyses for GAG and collagen content
Collected pellets were washed once in PBS, fixed in 4% paraformaldehyde for 48 h, embedded in paraffin, and sectioned at 10 µm thickness. A standard protocol for Safranin O/Fast Green staining was performed (27). For immunohistochemistry, the following primary mAb were used: type I collagen (#ab90395; Abcam, Cambridge, United Kingdom), type II collagen (#II-II6B3; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA), and type X collagen (#C7974; Sigma-Aldrich). After clearing and rehydration, slides were digested in pepsin (Thermo Fisher Scientific) for epitope retrieval, then treated with methanol/hydrogen peroxide for endogenous peroxidase quenching. The following steps were performed at room temperature: slides were incubated in blocking goat serum (Invitrogen; Thermo Fisher Scientific) for 30 min, followed by the appropriate primary antibodies for 1 h, then with biotinylated goat anti-mouse secondary antibodies (#ab97021; Abcam) for 30 min. Streptavidin–horseradish peroxidase conjugate (Thermo Fisher Scientific) with aminoethyl carbazole chromogen (Thermo Fisher Scientific) was used to develop signals. Staining was performed on human osteochondral sections as positive controls. Slides were also incubated without primary antibodies as negative controls.
RNA isolation, library preparation, and sequencing
On the day of collection, pellets were washed once with PBS, snap frozen in liquid nitrogen, and stored at −80°C until processing. Isolation of RNA was performed according to the manufacturer’s instructions (#48500; Norgen Biotek, Thorold, ON, Canada) with the following modifications: for each time point, 3 pellets were homogenized with a pestle before addition of lysis buffer; RNA was eluted in 20 µl of diethylpyrocarbonate-treated water. Samples were stored at −80°C and submitted to the Duke Center for Genomic and Computational Biology for library preparation and sequencing. Information on sample quality control is provided in Supplemental Table S1. Libraries were prepared using TruSeq Stranded Total RNA with Ribo-Zero Gold kit (Illumina, San Diego, CA, USA). Sequencing was performed on a HiSeq 2500 instrument (Illumina) (paired end and 100 bp reads) with a sequencing depth of 100 million reads/sample.
Data processing for computational analysis
Raw fastq files were processed with Trim Galore! (Babraham Bioinformatics, Cambridge, United Kingdom) to eliminate low-quality reads and remnant adapter sequences. After trimming, processed reads were aligned to the human reference genome (v.GRCh38) by STAR (28), and the number of aligned reads to each annotated genes or transcripts (GENCODE v21) was counted using HTseq (29). Normalized reads for each sample were performed with DESeq (29). Data were deposited in the Gene Expression Omnibus (GEO accession number GSE109503).
Computational analysis
At each pair of time points, we collected 2 lists of differentially expressed genes (up-regulated and down-regulated) using DESeq (30). Gene lists were uploaded to DAVID Bioinformatics Resources (https://david.ncifcrf.gov/) to perform gene ontology enrichment analysis (31, 32). The following parameters were used for enrichment annotation: Homo sapiens was chosen as background, and results were obtained for biologic pathways (GOTERM_BP_DIRECT) and functional categories (UP_KEYWORD). After functional annotation clustering, an enrichment score cutoff of 2.5 was imposed to extract meaningful clusters out of resulting lists. For each cluster, pathways or terms with the highest Benjamini-Hochberg adjusted P values were chosen. We also recorded pathways or terms that appear multiple times across gene lists (enriched at various time point transitions), provided their adjusted P values were the second highest in each cluster. Bar graphs were generated on the basis of log transforms of adjusted P values. A similar pipeline was also used to compare the transcriptomic landscapes between MSC-derived pellets and human cartilage samples (downloaded from GSE106292) using DESeq and DAVID Bioinformatics Resources with the same parameters. Weighted gene correlation network analysis (WGCNA) was performed on normalized counts (as calculated by DESeq) of RNA-Seq data. The d 0 samples were excluded from this analysis for 2 reasons: their long distance from the other samples, and the lack of biochemical data at earlier time points for accurate correlation of gene expression and trait. An adjacency matrix was built with a soft thresholding value of 7, based on recommendation from the WGCNA tutorial. Gene cluster dendrogram was performed with a height cutoff of 0.25. Resulting gene lists of interesting modules were extracted and uploaded to DAVID for gene ontology analysis with the aforementioned parameters. TFs from each module were extracted with information curated by JASPAR (33). Gene coexpression networks were prepared with Cytoscape (34) representing the top 20 hub genes of each module or TFs in each module with their top 3 connected genes.
Determination of K-mean value
A list of unique genes identified after DESeq analysis was clustered using the K-means clustering algorithm. K-mean value was determined by the elbow method.
Web tool development
The web page was developed using the Shiny R package (35). The graph depicts normalized gene counts, with error bars representing the sd of each gene at each time point. Data were fitted for a polynomial function of power 3. The heat map depicts relative expression of transcript variants across time points. The color panel was scaled for each row, with dark purple being the highest expression and white being the lowest.
Statistical analysis
Statistical analysis was performed by R statistical software (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/). Biochemical data were analyzed by 2-way ANOVA with Tukey post hoc test (α = 0.05). Results were reported as mean values ± sd.
RESULTS
Surface marker distribution of mesenchymal stem cells
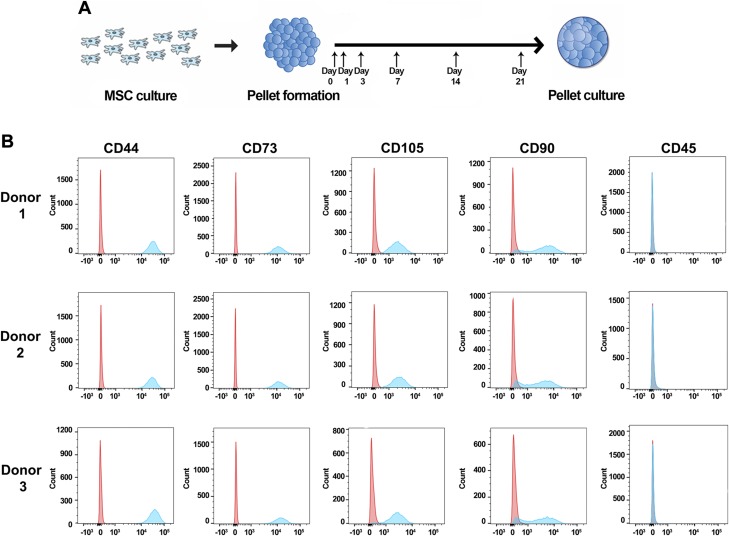
An overview of our experimental strategy is outlined in Fig. 1A. MSCs from 3 separate donors were cultured and expanded in monolayer for pellet formation, with the pellets subsequently collected at 6 different time points for RNA isolation and sequencing. At 3 of the collection time points (d 3, 14, and 21), biochemical and histologic analyses were also completed. In addition, we collected MSCs cultured in monolayer for flow cytometry to characterize their surface marker distribution. MSCs from all 3 donors exhibited similar patterns of surface markers (Fig. 1B): high in CD44, CD73, CD105, and CD90, and low or nonexpressive in CD45. Our MSC populations were mainly positive for CD44 (>97%), CD73 (>97%), and CD105 (>93%), with a majority (>75%) expressing CD90 (Table 1). More importantly, the pattern was conserved across all 3 donors, suggesting a certain level of similarity in surface marker distribution of our biological replicates (Table 1).
Figure 1.
Distribution of MSC surface markers. A) Experimental design. MSCs were formed into pellets after passage 4 and cultured under chondrogenic conditions for up to 21 d. Pellets were collected at d 0, 1, 3, 7, 14, and 21. B) Representative histograms from 3 donors. Control samples stained with isotypes are depicted in red; samples stained with surface markers are depicted in blue.
TABLE 1.
Percentage of cell populations positive for specific markers
| Donor | CD44 | CD73 | CD105 | CD90 | CD45 |
|---|---|---|---|---|---|
| 1 | 99.0 ± 0.5 | 99.1 ± 0.5 | 94.3 ± 0.1 | 78.0 ± 3.3 | 0.1 ± 0.1 |
| 2 | 97.2 ± 4.5 | 97.3 ± 4.5 | 93.0 ± 5.8 | 75.9 ± 1.6 | 0.4 ± 0.1 |
| 3 | 98.7 ± 1.2 | 98.7 ± 1.0 | 96.2 ± 0.6 | 77.2 ± 2.2 | 0.1 ± 0.0 |
Data are expressed as means ± sd; n = 3 for each donor.
MSCs synthesized a cartilaginous matrix under chondrogenic conditions
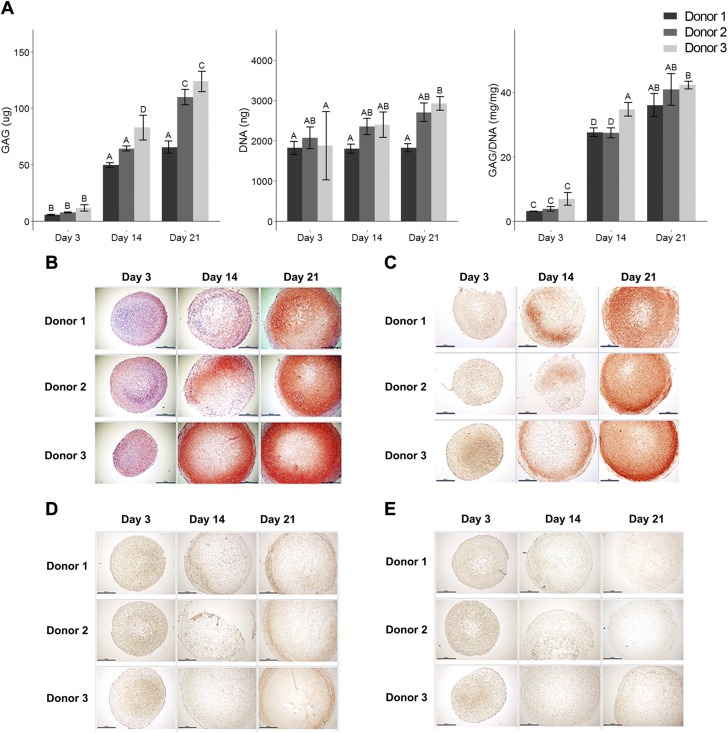
Pellets exhibited an accumulation of GAG over our time course of analysis (Fig. 2A), with a significant increase from d 3 (8.4 ± 3.1 µg; average from 3 donors) to d 14 (65.6 ± 15.5 µg; average from 3 donors). At later time points (d 14 and 21), there was a slight increase in the amount of GAG produced within individual donors. In addition, on d 21, donor 3 exhibited the highest level of GAG (123.6 ± 9.0 µg), followed by donor 2 (109.7 ± 6.8 µg), then donor 1 (65.5 ± 5.3 µg). In contrast, the level of DNA was similar between days. When GAG was normalized to DNA, we observed the same phenomenon: a significant boost from d 3 to d 14 (4.6 ± 2.1 mg/mg compared to 29.9 ± 3.9 mg/mg; average from 3 donors), and a slight increase from d 14 to d 21 (29.9 ± 3.9 mg/mg compared to 39.7 ± 4.2 mg/mg; average from 3 donors). Interestingly, normalized data showed little donor to donor variability, with donor 3 still exhibiting the highest GAG per DNA amount on d 21 (42.2 ± 1.2 mg/mg).
Figure 2.
MSC chondrogenesis in pellet culture. A) Biochemical assays measuring GAG and DNA over time course of analysis. There was increase in GAG accumulation from d 3 to later time points (left and right), while DNA levels remained similar across days (middle). Mean ± sd. n = 3 independent specimens per donor (3 donors as biologic replicates). Two-way ANOVA followed by Tukey post hoc test (α = 0.05). Groups of different letters are statistically different from one another. B) Safranin O/Fast Green staining on pellets from 3 donors at different time points. C–E) Immunohistochemistry staining on pellets from 3 donors at different time points: collagen type II (C); collagen type I (D); collagen type X (E). Scale bars, 500 µm.
Increased GAG accumulation over time was also reflected in Safranin O/Fast Green staining (Fig. 2B). While pellets from d 3 exhibited little or no GAG staining, d 21 pellets from all 3 donors exhibited high level of GAG. Immunohistochemistry indicated that in addition to GAG accumulation, these pellets were also synthesizing type II collagen—another marker of chondrogenesis—with defined positive staining on d 21 compared to d 3 (Fig. 2C). In addition, we also performed immunohistochemistry on type I collagen (a marker for fibrosis) (Fig. 2D) and type X collagen (a marker for cartilage hypertrophy) (Fig. 2E). The protein levels of type I and type X collagen observed in our pellets were low during our time course of analysis.
Interactions of biologic pathways during chondrogenesis
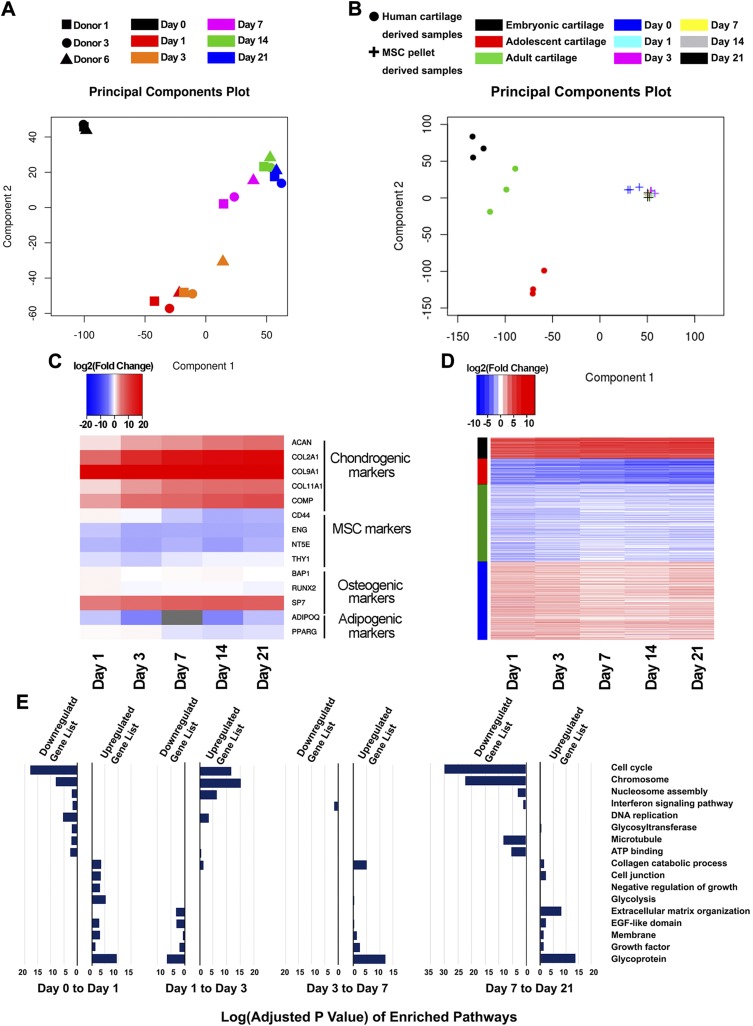
We performed principal component analysis (PCA) of RNA-Seq data on normalized samples to explore their interrelationships (Fig. 3A). All of the biologic replicates were clustered together by time points, showing that significant changes in the transcriptome were observed over time in culture as the samples underwent chondrogenesis. In this analysis, d 0 samples were separate from the other days. The d 1 and d 3 samples appeared similar to one another compared to those of later time points. The d 7 samples signified the transition moving from the multipotent to a more defined, differentiated chondrocyte-like state. There was no apparent difference among d 14 and d 21 samples.
Figure 3.
Differential expression (DE) analysis. A) PCA of 18 MSC-derived pellet samples. Samples were closely related by days, and there was minor donor-to-donor variation. B) PCA of MSC-derived pellet samples vs. human cartilage at different developmental stages. C) Expression patterns of MSC markers and markers of potential lineages. Gene expression was represented as log2 fold change compared to d 0 samples. D) K-means clustering grouped DE genes into 4 patterns. Gene expression was represented as log2 fold change compared to d 0 samples. E) Bar graphs of enriched pathways from gene ontology analysis. Datasets for human cartilage were retrieved using GEO accession number GSE106292 (79).
PCA between our acquired data set and publicly available RNA-Seq data the NIH Gene Expression Omnibus (GEO) accession number GSE106292 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE106292) indicated that engineered cartilage is transcriptomically distinct from human articular cartilage of all stages reported (embryonic, adolescent, and adult) (Fig. 3B), although MSC pellets at all time points appeared more similar to human adult cartilage (compared to human embryonic cartilage or human adolescent cartilage). In-depth analysis of differentially expressed genes revealed similar patterns in gene ontologies. Compared to human cartilage samples, d 21 MSC-derived pellets contained highly expressed genes in collagen organization, extracellular matrix organization, and skeletal system development (Table 2 and Supplemental Table S2). In addition, low-expressed genes in pellets pertained to transcriptional regulation (Supplemental Table S2). MSC-derived pellets were different from human embryonic samples at the limb bud stage (samples collected at 6 wk), indicating that the in vitro system utilized to engineer cartilage followed a distinct path and thus may resemble other pathways in skeletal system development.
TABLE 2.
Differentially expressed genes between MSC-derived pellets and human cartilage samples
| Day 21 MSC pellet comparison | Up-regulated genes (n) | Down-regulated genes (n) |
|---|---|---|
| Embryonic cartilage | 1332 | 764 |
| Adolescent cartilage | 2075 | 798 |
| Adult cartilage | 1017 | 566 |
To further analyze the transcriptomic profiles of MSC chondrogenesis, we used RNA-Seq to investigate the expression patterns of markers of MSC and their potential lineages (Fig. 3C). As expected, in our defined induction medium, the expression of chondrogenic markers was significantly up-regulated (e.g., COL2A1, ACAN, COL9A1, COL11A1, COMP). This process occurred concurrently with a decrease in the expression of MSC markers (e.g., CD44, ENG, NT5E, THY1). In addition, we examined how markers of other potential mesenchymal lineages responded, especially osteogenesis and adipogenesis. Almost all nonchondrogenic cell-fate markers either remained constant or were down-regulated in our time course differentiation, with the exception of SP7. While SP7 was up-regulated, its expression, as measured by RPKM values (reads per kilobase per million mapped reads), was relatively lower compared to those of chondrogenic markers (Supplemental Table S1). Overall, transcriptomes recapitulated the trend detected on a protein level (Fig. 2A), suggesting robust MSC chondrogenesis, with RNA-Seq data similarly reflecting the regulation of gene expression during chondrogenesis.
To examine the biologic pathways involved in this process, we performed differential gene expression analysis between pairs of consecutive time points. Table 3 summarizes the number of genes that are significantly up- or down-regulated for at least 2 folds (|log2(foldchange)| >1 and P ≤ 0.05). MSCs exhibited the greatest change from d 0 to d 1, with 2087 genes being up-regulated and 1860 genes being down-regulated. On the contrary, the least noticeable difference was between d 14 and d 21, with only 11 genes being up-regulated and 5 genes being down-regulated. A complete list of differentially expressed genes is provided in Supplemental Table S3. Differentially expressed genes emerged into 4 main expression patterns as identified by the K-means clustering algorithm (Fig. 3D). The first cluster was composed of genes that were continuously up-regulated, while the second cluster contained genes that were continuously down-regulated during chondrogenesis. The third and the fourth clusters were constituted by genes that were more dynamically regulated during earlier time points (d 1 and d 3), but subsequently returned to d 0 expression levels past the d 7 time point. Subsequently, we performed gene ontology analysis with DAVID (31, 32) to pinpoint the orchestration of biologic pathways involved during chondrogenesis. Figure 3E depicts the most enriched pathways as MSCs were differentiating toward a chondrocyte-like state. Because the number of differentially expressed genes from d 14 to d 21 was small, we did not find any significantly enriched pathways for this pair of time points (adjusted values P > 0.05). Therefore, we examined whether there would be any significant pathways from d 7 to d 21. Importantly, pathways related to chondrogenic development were enriched from lists of up-regulated genes (glycoprotein, growth factor, extracellular matrix organization, collagen catabolic process). In addition, cellular proliferation was highly up-regulated during d 1 to d 3, and subsequently down-regulated at later time points (DNA replication, nucleosome assembly, chromosome, cell cycle), suggesting a switch from a proliferative to a biosynthetic state during chondrogenic differentiation.
TABLE 3.
Differentially expressed genes through MSC chondrogenesis
| Days | Up-regulated genes (n) | Down-regulated genes (n) |
|---|---|---|
| 0 to 1 | 2087 | 1860 |
| 1 to 3 | 322 | 170 |
| 3 to 7 | 354 | 518 |
| 7 to 14 | 32 | 135 |
| 14 to 21 | 11 | 5 |
| 7 to 21 | 326 | 452 |
Modules of gene coexpression networks highly correlated with GAG accumulation
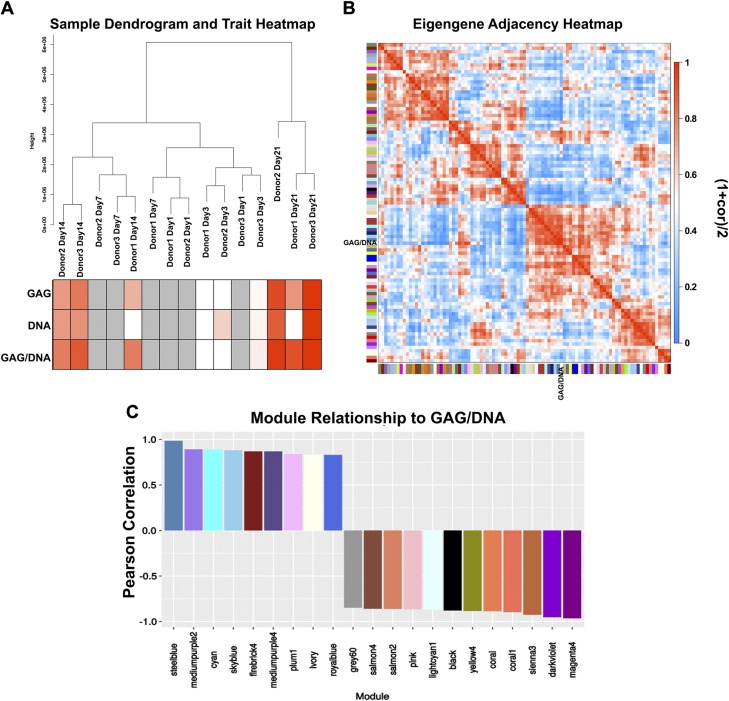
Hierarchical clustering of RNA-Seq data of all samples recapitulated that samples showed greater similarity by days of chondrogenic induction than by donor of origin, particularly at earlier time points. The d 21 samples separate themselves from the rest, signifying a distinct state in transcriptomic composition. The content of GAG, DNA, and GAG per DNA increased over time and correlated with sample groups going from d 3 to d 14 to d 21 (Fig. 4A heat map). Because donor 1 had the least chondrogenic potential (lowest GAG level) (Fig. 2A), samples derived from donor 1 at mid time points (d 7 and 14) were associated with samples of earlier time points from the other 2 donors: donor 1–d 14 transcriptome was more similar to those of donor 2–d 7 and donor 3–d 7.
Figure 4.
Modules and their relationships to biochemical changes. A) Dendrogram of RNA-Seq samples and corresponding changes in traits. Lowest values are depicted in white; highest values are depicted in red; missing values are depicted in gray. B) Eigengene adjacency heat map with blocks of metamodules. C) Modules whose eigengenes are highly correlated with changes in GAG per DNA and their corresponding Pearson correlation values.
Unsigned WGCNA (36, 37) was utilized to identify subsets of genes that were highly correlated with changes in GAG, DNA, and GAG per DNA, hereafter referred to as traits. Genes were next clustered into 94 modules of correlated expression patterns (Supplemental Fig. S1A and Supplemental Table S4). Subsequently, the eigengene of each module was investigated for correlation with changes in GAG per DNA. WGCNA defines eigengene as the first principal component of a gene cluster, and it thus effectively represents the gene expression profile of that module. For ease of tracking and visualization, modules are designated with arbitrary colors. The adjacency heat map (Fig. 4B) grouped modules whose eigengenes are highly correlated with the trait of interest into blocks of metamodules. WGCNA defines the adjacency value as follows: Aj = [(1 + correlation)/2]. Because a module is highly correlated with the investigated trait (GAG per DNA), its adjacency value will either be close to 0 or close to 1. With an absolute correlation value cutoff of 0.8 (Ai < 0.1 or Aj > 0.9), 21 interesting modules emerged and required further investigation (Fig. 4C). Module steel blue was the most positively correlated with the traits, and module magenta4 (hereafter referred to as magenta) was the most negatively correlated. We also examined the relationships between these modules to changes in GAG (Supplemental Fig. S1B) and changes in DNA (Supplemental Fig. S1C). As expected, correlations between modules and GAG as trait were similar to those between modules and GAG per DNA as trait. Interestingly, with DNA as trait, we observed a different order in correlated modules, possibly due to a relatively moderate increase in DNA compared to the magnitude of increase in GAG over time.
A limitation in our above analysis of gene–trait correlation was the missing values from biochemical data, which could potentially lead to identification of insignificant gene clusters. To validate our finding, we extracted the expression values of chondrogenic markers (COL2A1 and ACAN) and performed WGCNA with these vectors as traits (Supplemental Fig. S2A, B). Our rationale was that modules highly correlated to chondrogenic expression patterns would also be composed of genes heavily involved in the process. Consistent with our initial findings, module steel blue and module magenta were again established as the most interesting gene clusters that were highly correlated to the expression patterns of both COL2A1 and ACAN (Supplemental Fig. S2C, D).
Characteristics of highly correlated modules
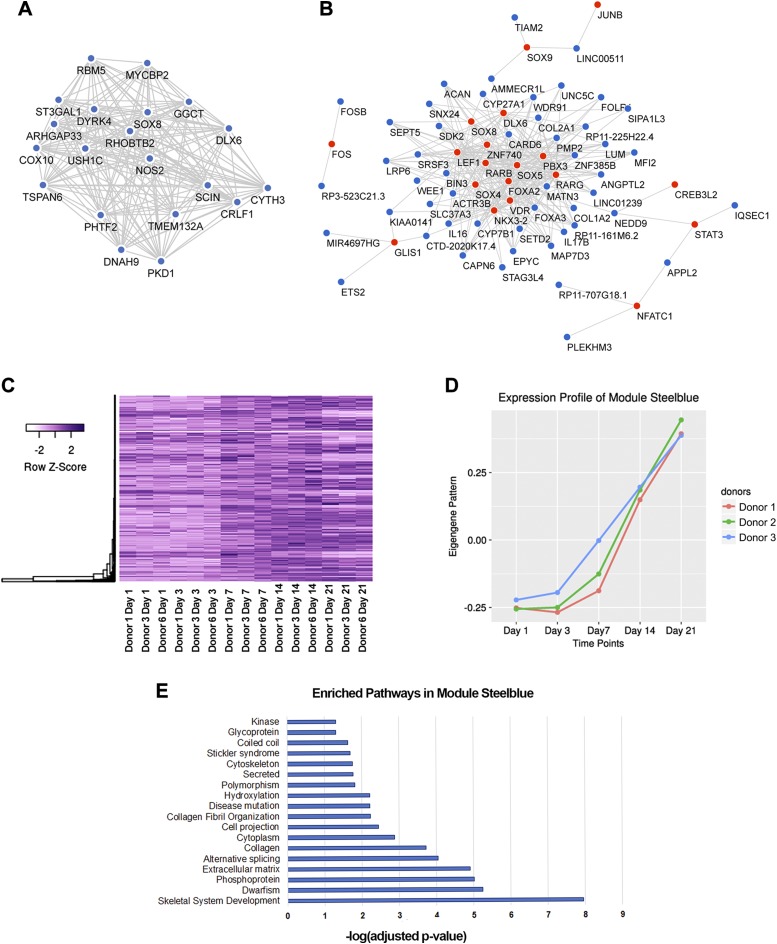
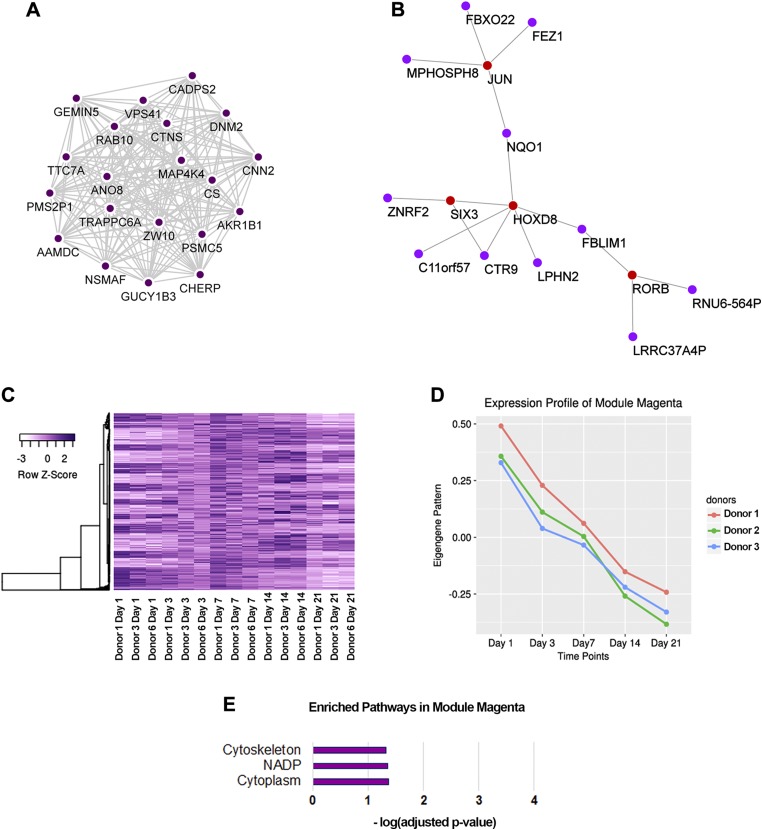
From WGCNA, we identified the modules steel blue and magenta as most relevant for further investigation. Coexpression networks were built for the 20 top hubs (Figs. 5A and 6A), where genes with the most connections were centered inside the networks. It has been proposed that centrally located hubs are essential as shared links among interacting pathways (38), and that these hubs are key control points in module formation (39). We were also interested in the relationship between TFs and their potential downstream effectors. Thus, we constructed a gene coexpression network of all TFs with defined DNA binding motifs identified in each module with the top 3 genes that were most correlated to each TF (Figs. 5B and 6B). For ease of visualization, connections among non-TF genes are hidden in represented figures. Highlights within the network from module steel blue involved TFs established for their roles in skeletal system development (e.g., FOS, JUNB, FOXA2, SOXs, RARs) (40–45). In contrast, TFs involved in module magenta mostly have unknown functions in the skeletal tissue, except for JUN and HOXD8. While JUN has been suggested to form complexes with FOS to regulate bone development (44), HOXD8 has been shown to affect mesodermal axial patterning and positioning of the hind limbs (46).
Figure 5.
Characteristics of module steel blue. A) Coexpression network of 20 top hubs. B) Coexpression network of involved TFs (red) and their top 3 connected genes (steel blue). C) Heat map of steel blue modular gene expression. D) Eigengene vector of module steel blue. E) Enriched Gene Ontology terms and pathways from modular genes with gene ontology analysis.
Figure 6.
Characteristics of module magenta. A) Coexpression network of 20 top hubs. B) Coexpression network of involved TFs (red) and their top 3 connected genes (magenta). C) Heat map of magenta modular gene expression. D) Eigengene vector of module magenta. E) Enriched Gene Ontology terms and pathways from modular genes with gene ontology analysis.
Defined expression patterns for each module (Figs. 5C, D and 6C, D) were visualized by a heat map of modular genes and the pattern of eigengene vector. It is important to note that by constructing unsigned networks, negatively and positively correlated genes would be clustered into a single module, provided they passed the thresholding cutoff. Therefore, although heat maps of modular genes showed patterns that closely resemble the eigengene, there were subsets of genes whose expressions were reversed compared to the eigengene pattern. The distinction between module steel blue and module magenta lay within modular gene dynamics between d 1 and d 7. Magenta modular genes exhibited a more rapid, steeper change in their expression pattern (rapid changes) compared to those of module steel blue (delayed changes) during these earlier time points.
We also examined the next 5 most correlated modules (mediumpurple2, cyan, sky blue, dark violet, sienna3). Their top 20 hub genes and TF networks are depicted in corresponding gene coexpression networks (Supplemental Fig. S3A, D, E, H, I, L, O) with accompanying characteristic expression patterns for each module (Supplemental Fig. S3B, C, F, G, J, K, M, N, P, Q).
To elucidate the biologic pathways underlying module steel blue and module magenta, we performed enrichment analysis on modular genes with DAVID (Figs. 5E and 6E). There were 1172 genes for module steel blue and 395 genes for module magenta that were used as independent inputs to DAVID. Encouragingly, skeletal system development was detected as the most enriched biologic pathway involved in module steel blue. Pathways previously suggested to be connected to musculoskeletal diseases such as dwarfism, polymorphism, and Stickler syndrome were also enriched in our analysis. For these reasons, we propose that this cluster of genes represent crucial players in chondrogenesis, and we thus hereby refer to it as the chondrogenic functional module. The genes comprising module magenta were mainly involved in cytoskeletal organization, although possessing a relatively smaller significant value.
Identification of miRNAs and lncRNAs from chondrogenic functional module
We proceeded to further investigate module steel blue. Within this module, we identified 230 annotated lncRNA genes (Supplemental Table S5). In addition, we examined whether known targets of miRNA would be enriched in module steel blue because RNA-Seq data do not capture small noncoding information. We selected miRNAs with fewer than 50 predicted targets and high prediction scores utilizing data provided by the miR database (47, 48). By this method, we could detect 498 associated upstream miRNAs whose target mRNAs were enriched in our chondrogenic functional module (Supplemental Table S5).
Web-based encyclopedia of MSC chondrogenesis
Our processed RNA-Seq data were developed into a web-based encyclopedia for easy access and user-friendly interface (http://msc-browser.guilaklab.com). Here, we have arranged data into 2 categories: expression of genes and expression of transcript variants over the time course of chondrogenesis. Their patterns can be readily retrieved from a drop-down menu of gene names of interest. As an example, we looked up ACAN in our web browser (Supplemental Fig. S4). The resulting graph portrays the expression pattern of ACAN during chondrogenesis, and the accompanying heat map reveals that there were 4 splice variants of ACAN, whose patterns conform to that of the gene itself.
GEO accession number
The data in this manuscript can be retrieved using Geo accession number GSE109503.
DISCUSSION
Advances in high-throughput sequencing have provided critical new methods for rapid and cost-effective genomic and transcriptomic research. Our study provides an extensive RNA-Seq database that followed MSC chondrogenesis in vitro at a deep sequencing level (100 million reads per library). This depth of sequencing is particularly valuable because it provides the possibility to identify novel, unknown transcripts, as well as the capability to distinguish differential levels of lowly expressed transcripts (49). With 3 biologic replicates and little donor-to-donor variation, our data presents an in-depth encyclopedia of the gene expression changes induced during MSC chondrogenesis. Our study demonstrated that this was a complicated process with many concerted pathways, beginning with a transient up-regulation in cellular proliferation and transitioning toward the building of collagen, glycoprotein, and extracellular matrix. Differentially expressed genes were divided into 4 main expression patterns, exhibiting continuous or transient up-regulation and down-regulation. Furthermore, gene coexpression network analysis on this data set led us to identify a chondrogenic functional module composed of 1172 genes. The eigengene (first principal component) of this module is highly correlated with changes in primary chondrocytic markers, including GAG protein level, COL2A1 expression, and ACAN expression over time, signifying that corresponding modular genes may be heavily involved in chondrogenesis. We speculate that in-depth investigation of this gene cluster will identify many more crucial players and potentiate our ability to understand the signaling axes involved in chondrogenesis. In this respect, a more comprehensive understanding of this process will, we hope, provide important insights that improve MSC-based cartilage tissue engineering. More importantly, we identified a list of noncoding genes (small noncoding and long noncoding) that could potentially be both prognostic and therapeutic. For example, understanding of the gene regulatory networks or specific noncoding RNAs that regulate chondrogenesis may allow for development of new protocols for more rapid or complete differentiation of MSCs in this context.
An accompanying phenotype of chondrogenically induced MSCs is their potential for hypertrophic differentiation with an up-regulation of COL10A1, MMP13, and sometimes fibroblastic markers such as COL1A1, particularly when implanted in vivo. In our study, pellets collected on d 21 showed increased staining for collagen type I and collagen type X compared to earlier time points, signifying that at the protein level, the cells had started to express a hypertrophic or fibroblastic phenotype in this defined culture system. Similarly, at the gene expression level, we observed a gradual increase in COL1A1, COL10A1, and MMP13. This suggests that these MSC pellets may be on the way to becoming endochondral cartilage, as has been reported in the literature (50, 51). Further investigation of the pathways involved in these processes may provide important insights into regulating MSC hypertrophic differentiation, either positively or negatively, for tissue engineering applications.
Pellet culture has been extensively used as an in vitro model for chondrogenesis in cartilage tissue engineering (20, 21). Under appropriate culture conditions, these 3-dimensional aggregates usually exhibit extracellular matrix rich in GAG and collagen type II, concomitant with an increase in gene expression of well-defined chondrogenic markers such as COL2A1, ACAN, and SOX9 (52). In contrast, it has been suggested that there was little proliferation and, to some extent, gradual cell loss within these pellets (52). Conforming to existing literature, our biochemical and histologic assays confirmed robust chondrogenesis with progressive accumulation of GAG and collagen type II in the extracellular matrix, with relatively stable DNA content over time. Nonetheless, we observed via PCA that at these early time points (up to 21 d), cartilage engineered from MSCs is transcriptomically distinct from human articular cartilages (Fig. 3B). This observation reiterates current opinions that early MSC-derived pellet aggregates form a tissue in vitro that is distinct from native cartilage, indicating that further studies of MSC differentiation or longer maturation times may be necessary for improving the hyaline-like quality of cartilage engineered from MSCs.
While pellet culture was originally thought to mimic embryonic limb development where mesenchymal condensation initiates chondrogenesis (53), the interplay between pathways and dynamics of chondrogenic differentiation has not been fully elucidated. Here, transcriptomic profiling of MSC chondrogenesis has identified several key logical pathways, such as glycoprotein, extracellular matrix organization, and collagen catabolic process. At first glance, it was counterintuitive to establish collagen catabolic process—the breakdown of collagen—as one of the up-regulated pathways in our analysis. However, further investigation indicated that genes identified within this ontology term were mostly collagen (COL2A1, COL11A1, COL9A1) instead of proteases or collagenases, so we propose that the ontology term would be more correctly categorized as “collagen regulation.” It is important to note that there were also a few matrix metalloproteinases within this gene ontology term that were up-regulated from d 0 to d 1, and MMP13 was up-regulated from d 7 to d 21—a phenomenon that has been reported previously (50, 51).
Although several cell surface markers are often used to identify MSCs, it is widely accepted that the distribution of surface markers can be influenced by many factors, including the culture conditions (54), isolation techniques (55, 56), and cell source (57). Therefore, it is valuable to characterize the surface marker distribution of specific MSCs populations. Here, we showed that MSCs from 3 donors are relatively homogeneous for 3 surface markers (CD44+/CD73+/CD105+) (>95% of cell populations), although CD90—another characteristic cluster of differentiation—was only positive in about 80% of the populations. Early publications on isolated human MSCs indicated that cells were at least homogeneous at passage 1 and passage 2 (6) for a composition of surface markers, including CD44, CD73, CD105, and CD90 (58, 59). While CD73 and CD105 appeared to be stable after monolayer expansion, some cases indicated that MSCs in fact gained CD44 expression (60). Complementary to the MSC markers, we found that MSCs were negative for expression of hematopoietic stem-cell–specific CD45. Furthermore, the cell surface marker profiles in the present study are consistent with those reported under similar expansion culture condition, and which have subsequently been identified to have enhanced chondrogenic potential (54).
WGCNA is a powerful tool for extracting meaningful biologic cues from large, high-throughput data sets. The use of a gene coexpression network is most advantageous in its ability for global interpretation without reliance on prior knowledge (61). It has been extensively documented that coexpressed genes are functionally related and coregulated (62, 63). Identification of genes within the same module can offer profound insight into uncharacterized genes with likely similar biologic functions or involvement of new genes in previously curated pathways. With WGCNA, we obtained a chondrogenic functional module of 1172 genes. This module contains characteristic markers for cartilage matrix: COL2A1, ACAN, COMP, COL9A1, and COL11A1. Mechanosensitive ion channels TRPV4 and PIEZO1, crucial for cartilage maintenance, homeostasis, and injury response, were also present in this module (64–67).
A subset of TFs and their highly coexpressed genes in module steel blue was highlighted. Among the participants were members of the SOX family, retinoic acid receptors, FOS/JUN complex, and FOXA2. While it is widely accepted that the SOX trio (SOX5/6/9) controls chondrogenesis (40–42), SOX6 was surprisingly not a steel blue modular gene. We speculate that SOX6 may be regulated by a different mechanism, and thus it exhibited a different expression behavior. The other interesting TFs had been established to function in skeletal system development. For example, mice deficient in retinoic acid receptors (RARB/RARG double knockout) displayed stunted skeletal growth (45). FOS and JUN proteins form the TF complex AP1 and play an important role in osteoblast maturation and osteoclastogenesis (44). FOXA2 factor regulates chondrocyte hypertrophy by up-regulating collagen type X and MMP13 (43). TFs crucial for chondrogenesis and hypertrophy were highly connected to one another, reiterating the entangled pathways that essentially result in endochondral ossification (51, 68). Hence, the ability to dissect and separate these routes will provide insight into preventing unwanted differentiation pathways such as MSC hypertrophy.
By further examining this module, we identified 230 lncRNAs and 498 associated miRNAs. Evident physiologic connection between correlated genes indicates the significance of these noncoding transcripts and their roles in chondrogenic regulation. Initially referred to as genomic remnants, lncRNAs are gaining remarkable attention as crucial molecules in cellular maintenance and lineage commitment (69, 70). Recently, a growing number of lncRNAs have been identified as important players in musculoskeletal development and diseases (71). In this study, we identified 230 modular lncRNA candidates. Among these were a few whose functions in skeletal system development had been elucidated, such as H19 (72, 73) and HIF1A-AS1 (74, 75). This list therefore represents a composition of long noncoding genes that requires further investigation to fully understand their underlying mechanisms.
Historically, miRNAs represent a major class of noncoding RNAs that are more established and extensively investigated. In the realm of musculoskeletal system development, let-7, miR-20b, miR-363 had been shown to up-regulate during limb formation in chick embryos (76). miR-31 and miR-338 were differentially expressed during blastema formation in appendage regeneration and were conserved across evolutionarily distant species (77). miR-145 was up-regulated during MSC chondrogenic differentiation and regulated Sox9 expression in mice (78). It was promising that we identified the aforementioned miRNAs in our list of module-associated miRNAs. Thus, further investigation is needed to harness the full potential of manipulating these miRNAs for functional cartilage tissue engineering.
CONCLUSIONS
In summary, we have provided an in-depth database of MSC chondrogenesis. Rigorous analysis of this data set identified several subsets of interesting genes: protein coding, long noncoding, and small noncoding. Routinely, genes are studied as individual entities, and while a singular pathway can be examined in detail, their integration into larger genomic/transcriptomic scheme is often lost. Thus, a more robust gene signature like the chondrogenic functional module could broaden the knowledge of chondrogenesis and cartilage pathology. The data have been developed into an easily accessible web-based encyclopedia that can act as a reference point for additional pathway analysis and hypothesis-driven experiments designed to improve MSC differentiation.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank the Duke University Genomics Core Facility, the Washington University in St. Louis Pathology Core Labs, and the Washington University Center of Regenerative Medicine for their resources and support. The authors also wish to thank S. Oswald (Washington University in St. Louis) for assistance with technical writing. This work was supported by the Arthritis Foundation, U.S. National Institutes of Health (NIH) Grants AR50245, AR48852, AG15768, AR48182, and AR067467, the Nancy Taylor Foundation for Chronic Diseases, the Collaborative Research Center of the AO Foundation, Davos, Switzerland, and the Washington University Musculoskeletal Research Center (NIH P30 AR057235). B.Z. was supported by NIH grant DA027995 and the Goldman Sachs Philanthropy Fund. The authors declare no conflicts of interest.
Glossary
- FBS
fetal bovine serum
- GAG
glycosaminoglycan
- lncRNA
long noncoding RNA
- miRNA
microRNA
- MSC
mesenchymal stem/stromal cell
- PCA
principal component analysis
- TF
transcription factor
- WGCNA
weighted gene correlation network analysis
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
N. P. T. Huynh and F. Guilak designed research; N. P. T. Huynh analyzed data, and wrote the report; B. Zhang provided expertise and advice on computational analysis; and all authors edited the report.
REFERENCES
- 1.Nguyen U. S., Zhang Y., Zhu Y., Niu J., Zhang B., Felson D. T. (2011) Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann. Intern. Med. 155, 725–732 10.7326/0003-4819-155-11-201112060-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed T. A., Hincke M. T. (2014) Mesenchymal stem cell–based tissue engineering strategies for repair of articular cartilage. Histol. Histopathol. 29, 669–689 [DOI] [PubMed] [Google Scholar]
- 3.Caplan A. I. (1991) Mesenchymal stem cells. J. Orthop. Res. 9, 641–650 10.1002/jor.1100090504 [DOI] [PubMed] [Google Scholar]
- 4.Caplan A. I. (2008) All MSCs are pericytes? Cell Stem Cell 3, 229–230 10.1016/j.stem.2008.08.008 [DOI] [PubMed] [Google Scholar]
- 5.Crisan M., Yap S., Casteilla L., Chen C. W., Corselli M., Park T. S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P. N., Traas J., Schugar R., Deasy B. M., Badylak S., Buhring H. J., Giacobino J. P., Lazzari L., Huard J., Péault B. (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 6.Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- 7.Abrahamsson C. K., Yang F., Park H., Brunger J. M., Valonen P. K., Langer R., Welter J. F., Caplan A. I., Guilak F., Freed L. E. (2010) Chondrogenesis and mineralization during in vitro culture of human mesenchymal stem cells on three-dimensional woven scaffolds. Tissue Eng. Part A 16, 3709–3718 10.1089/ten.tea.2010.0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunger J. M., Huynh N. P., Guenther C. M., Perez-Pinera P., Moutos F. T., Sanchez-Adams J., Gersbach C. A., Guilak F. (2014) Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc. Natl. Acad. Sci. USA 111, E798–E806 10.1073/pnas.1321744111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass K. A., Link J. M., Brunger J. M., Moutos F. T., Gersbach C. A., Guilak F. (2014) Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 35, 5921–5931 10.1016/j.biomaterials.2014.03.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwong F. N., Richardson S. M., Evans C. H. (2008) Chordin knockdown enhances the osteogenic differentiation of human mesenchymal stem cells. Arthritis Res. Ther. 10, R65 10.1186/ar2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meinel L., Hofmann S., Betz O., Fajardo R., Merkle H. P., Langer R., Evans C. H., Vunjak-Novakovic G., Kaplan D. L. (2006) Osteogenesis by human mesenchymal stem cells cultured on silk biomaterials: comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials 27, 4993–5002 10.1016/j.biomaterials.2006.05.021 [DOI] [PubMed] [Google Scholar]
- 12.Moutos F. T., Glass K. A., Compton S. A., Ross A. K., Gersbach C. A., Guilak F., Estes B. T. (2016) Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc. Natl. Acad. Sci. USA 113, E4513–E4522 10.1073/pnas.1601639113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker B. M., Nathan A. S., Gee A. O., Mauck R. L. (2010) The influence of an aligned nanofibrous topography on human mesenchymal stem cell fibrochondrogenesis. Biomaterials 31, 6190–6200 10.1016/j.biomaterials.2010.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bian L., Zhai D. Y., Mauck R. L., Burdick J. A. (2011) Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng. Part A 17, 1137–1145 10.1089/ten.tea.2010.0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bian L., Guvendiren M., Mauck R. L., Burdick J. A. (2013) Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. Natl. Acad. Sci. USA 110, 10117–10122 10.1073/pnas.1214100110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W. J., Tuli R., Huang X., Laquerriere P., Tuan R. S. (2005) Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials 26, 5158–5166 10.1016/j.biomaterials.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 17.Kuo C. K., Tuan R. S. (2008) Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng. Part A 14, 1615–1627 10.1089/ten.tea.2006.0415 [DOI] [PubMed] [Google Scholar]
- 18.Awad H. A., Butler D. L., Harris M. T., Ibrahim R. E., Wu Y., Young R. G., Kadiyala S., Boivin G. P. (2000) In vitro characterization of mesenchymal stem cell–seeded collagen scaffolds for tendon repair: effects of initial seeding density on contraction kinetics. J. Biomed. Mater. Res. 51, 233–240 [DOI] [PubMed] [Google Scholar]
- 19.Young R. G., Butler D. L., Weber W., Caplan A. I., Gordon S. L., Fink D. J. (1998) Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J. Orthop. Res. 16, 406–413 10.1002/jor.1100160403 [DOI] [PubMed] [Google Scholar]
- 20.Johnstone B., Hering T. M., Caplan A. I., Goldberg V. M., Yoo J. U. (1998) In vitro chondrogenesis of bone marrow–derived mesenchymal progenitor cells. Exp. Cell Res. 238, 265–272 10.1006/excr.1997.3858 [DOI] [PubMed] [Google Scholar]
- 21.Mackay A. M., Beck S. C., Murphy J. M., Barry F. P., Chichester C. O., Pittenger M. F. (1998) Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 4, 415–428 10.1089/ten.1998.4.415 [DOI] [PubMed] [Google Scholar]
- 22.Bhaskar B., Mekala N. K., Baadhe R. R., Rao P. S. (2014) Role of signaling pathways in mesenchymal stem cell differentiation. Curr. Stem Cell Res. Ther. 9, 508–512 10.2174/1574888X09666140812112002 [DOI] [PubMed] [Google Scholar]
- 23.Yang Y., Han L., Yuan Y., Li J., Hei N., Liang H. (2014) Gene co-expression network analysis reveals common system-level properties of prognostic genes across cancer types. Nat. Commun. 5, 3231 10.1038/ncomms4231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somoza R. A., Correa D., Labat I., Sternberg H., Forrest M. E., Khalil A. M., West M. D., Tesar P., Caplan A. I. (2018) Transcriptome-wide analyses of human neonatal articular cartilage and human mesenchymal stem cell–derived cartilage provide a new molecular target for evaluating engineered cartilage. Tissue Eng. Part A 24, 335–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z., Gerstein M., Snyder M. (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 10.1038/nrg2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farndale R. W., Buttle D. J., Barrett A. J. (1986) Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 883, 173–177 10.1016/0304-4165(86)90306-5 [DOI] [PubMed] [Google Scholar]
- 27.Estes B. T., Diekman B. O., Gimble J. M., Guilak F. (2010) Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat. Protoc. 5, 1294–1311 10.1038/nprot.2010.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T. R. (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders S., Pyl P. T., Huber W. (2015) HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anders S., Huber W. (2010) Differential expression analysis for sequence count data. Genome Biol. 11, R106 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang da W., Sherman B. T., Lempicki R. A. (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang da W., Sherman B. T., Lempicki R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 33.Khan A., Fornes O., Stigliani A., Gheorghe M., Castro-Mondragon J. A., van der Lee R., Bessy A., Chèneby J., Kulkarni S. R., Tan G., Baranasic D., Arenillas D. J., Sandelin A., Vandepoele K., Lenhard B., Ballester B., Wasserman W. W., Parcy F., Mathelier A. (2018) JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 46(D1), D260–D266 10.1093/nar/gkx1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., Amin N., Schwikowski B., Ideker T. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang W., Cheng J., Allaire J. J., Xie Y., McPherson J. (2017) Shiny: web application framework for R. R package version 1.0.5. Available at: https://CRAN.R-project.org/package=shiny. Accessed November 1, 2017
- 36.Langfelder P., Horvath S. (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langfelder P., Horvath S. (2012) Fast R functions for robust correlations and hierarchical clustering. J. Stat. Softw. 46, pii: i11 10.18637/jss.v046.i11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong H., Mason S. P., Barabási A. L., Oltvai Z. N. (2001) Lethality and centrality in protein networks. Nature 411, 41–42 10.1038/35075138 [DOI] [PubMed] [Google Scholar]
- 39.Doering T. A., Crawford A., Angelosanto J. M., Paley M. A., Ziegler C. G., Wherry E. J. (2012) Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 37, 1130–1144 10.1016/j.immuni.2012.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeda T., Kamekura S., Mabuchi A., Kou I., Seki S., Takato T., Nakamura K., Kawaguchi H., Ikegawa S., Chung U. I. (2004) The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 50, 3561–3573 10.1002/art.20611 [DOI] [PubMed] [Google Scholar]
- 41.Lefebvre V., Li P., de Crombrugghe B. (1998) A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 17, 5718–5733 10.1093/emboj/17.19.5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefebvre V., Huang W., Harley V. R., Goodfellow P. N., de Crombrugghe B. (1997) SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol. Cell. Biol. 17, 2336–2346 10.1128/MCB.17.4.2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ionescu A., Kozhemyakina E., Nicolae C., Kaestner K. H., Olsen B. R., Lassar A. B. (2012) FoxA family members are crucial regulators of the hypertrophic chondrocyte differentiation program. Dev. Cell 22, 927–939 10.1016/j.devcel.2012.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner E. F. (2002) Functions of AP1 (Fos/Jun) in bone development. Ann. Rheum. Dis. 61(Suppl 2), ii40–ii42 10.1136/ard.61.suppl_2.ii40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams J. A., Kondo N., Okabe T., Takeshita N., Pilchak D. M., Koyama E., Ochiai T., Jensen D., Chu M. L., Kane M. A., Napoli J. L., Enomoto-Iwamoto M., Ghyselinck N., Chambon P., Pacifici M., Iwamoto M. (2009) Retinoic acid receptors are required for skeletal growth, matrix homeostasis and growth plate function in postnatal mouse. Dev. Biol. 328, 315–327 10.1016/j.ydbio.2009.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van den Akker E., Fromental-Ramain C., de Graaff W., Le Mouellic H., Brûlet P., Chambon P., Deschamps J. (2001) Axial skeletal patterning in mice lacking all paralogous group 8 Hox genes. Development 128, 1911–1921 [DOI] [PubMed] [Google Scholar]
- 47.Wang X. (2016) Improving microRNA target prediction by modeling with unambiguously identified microRNA-target pairs from CLIP-ligation studies. Bioinformatics 32, 1316–1322 10.1093/bioinformatics/btw002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong N., Wang X. (2015) miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 43(D1), D146–D152 10.1093/nar/gku1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sims D., Sudbery I., Ilott N. E., Heger A., Ponting C. P. (2014) Sequencing depth and coverage: key considerations in genomic analyses. Nat. Rev. Genet. 15, 121–132 10.1038/nrg3642 [DOI] [PubMed] [Google Scholar]
- 50.Mueller M. B., Tuan R. S. (2008) Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 58, 1377–1388 10.1002/art.23370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mueller M. B., Fischer M., Zellner J., Berner A., Dienstknecht T., Prantl L., Kujat R., Nerlich M., Tuan R. S., Angele P. (2010) Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs (Print) 192, 158–166 10.1159/000313399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekiya I., Vuoristo J. T., Larson B. L., Prockop D. J. (2002) In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc. Natl. Acad. Sci. USA 99, 4397–4402 10.1073/pnas.052716199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall B. K. (2015) Bones and Cartilage, Academic Press, Cambridge, MA [Google Scholar]
- 54.Hagmann S., Moradi B., Frank S., Dreher T., Kämmerer P. W., Richter W., Gotterbarm T. (2013) FGF-2 addition during expansion of human bone marrow–derived stromal cells alters MSC surface marker distribution and chondrogenic differentiation potential. Cell Prolif. 46, 396–407 10.1111/cpr.12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pösel C., Möller K., Fröhlich W., Schulz I., Boltze J., Wagner D. C. (2012) Density gradient centrifugation compromises bone marrow mononuclear cell yield. PLoS One 7, e50293 10.1371/journal.pone.0050293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mareschi K., Rustichelli D., Calabrese R., Gunetti M., Sanavio F., Castiglia S., Risso A., Ferrero I., Tarella C., Fagioli F. (2012) Multipotent mesenchymal stromal stem cell expansion by plating whole bone marrow at a low cellular density: a more advantageous method for clinical use. Stem Cells Int. 2012, 920581 10.1155/2012/920581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sacchetti B., Funari A., Remoli C., Giannicola G., Kogler G., Liedtke S., Cossu G., Serafini M., Sampaolesi M., Tagliafico E., Tenedini E., Saggio I., Robey P. G., Riminucci M., Bianco P. (2016) No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Reports 6, 897–913 10.1016/j.stemcr.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barry F., Boynton R., Murphy M., Haynesworth S., Zaia J. (2001) The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 289, 519–524 10.1006/bbrc.2001.6013 [DOI] [PubMed] [Google Scholar]
- 59.Barry F. P., Boynton R. E., Haynesworth S., Murphy J. M., Zaia J. (1999) The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem. Biophys. Res. Commun. 265, 134–139 10.1006/bbrc.1999.1620 [DOI] [PubMed] [Google Scholar]
- 60.Bara J. J., Richards R. G., Alini M., Stoddart M. J. (2014) Concise review: bone marrow–derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells 32, 1713–1723 10.1002/stem.1649 [DOI] [PubMed] [Google Scholar]
- 61.Zhao W., Langfelder P., Fuller T., Dong J., Li A., Hovarth S. (2010) Weighted gene coexpression network analysis: state of the art. J. Biopharm. Stat. 20, 281–300 10.1080/10543400903572753 [DOI] [PubMed] [Google Scholar]
- 62.Lee H. K., Hsu A. K., Sajdak J., Qin J., Pavlidis P. (2004) Coexpression analysis of human genes across many microarray data sets. Genome Res. 14, 1085–1094 10.1101/gr.1910904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Månsson R., Tsapogas P., Akerlund M., Lagergren A., Gisler R., Sigvardsson M. (2004) Pearson correlation analysis of microarray data allows for the identification of genetic targets for early B-cell factor. J. Biol. Chem. 279, 17905–17913 10.1074/jbc.M400589200 [DOI] [PubMed] [Google Scholar]
- 64.Lee W., Guilak F., Liedtke W. (2017) Role of piezo channels in joint health and injury. Curr. Top. Membr. 79, 263–273 10.1016/bs.ctm.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Conor C. J., Leddy H. A., Benefield H. C., Liedtke W. B., Guilak F. (2014) TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. USA 111, 1316–1321 10.1073/pnas.1319569111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phan M. N., Leddy H. A., Votta B. J., Kumar S., Levy D. S., Lipshutz D. B., Lee S. H., Liedtke W., Guilak F. (2009) Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 60, 3028–3037 10.1002/art.24799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee W., Leddy H. A., Chen Y., Lee S. H., Zelenski N. A., McNulty A. L., Wu J., Beicker K. N., Coles J., Zauscher S., Grandl J., Sachs F., Guilak F., Liedtke W. B. (2014) Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. USA 111, E5114–E5122 10.1073/pnas.1414298111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ng J. J., Wei Y., Zhou B., Bernhard J., Robinson S., Burapachaisri A., Guo X. E., Vunjak-Novakovic G. (2017) Recapitulation of physiological spatiotemporal signals promotes in vitro formation of phenotypically stable human articular cartilage. Proc. Natl. Acad. Sci. USA 114, 2556–2561 10.1073/pnas.1611771114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guttman M., Donaghey J., Carey B. W., Garber M., Grenier J. K., Munson G., Young G., Lucas A. B., Ach R., Bruhn L., Yang X., Amit I., Meissner A., Regev A., Rinn J. L., Root D. E., Lander E. S. (2011) lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 477, 295–300 10.1038/nature10398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geisler S., Coller J. (2013) RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat. Rev. Mol. Cell Biol. 14, 699–712 10.1038/nrm3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huynh N. P., Anderson B. A., Guilak F., McAlinden A. (2017) Emerging roles for long noncoding RNAs in skeletal biology and disease. Connect. Tissue Res. 58, 116–141 10.1080/03008207.2016.1194406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steck E., Boeuf S., Gabler J., Werth N., Schnatzer P., Diederichs S., Richter W. (2012) Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. J. Mol. Med. (Berl.) 90, 1185–1195 10.1007/s00109-012-0895-y [DOI] [PubMed] [Google Scholar]
- 73.Dudek K. A., Lafont J. E., Martinez-Sanchez A., Murphy C. L. (2010) Type II collagen expression is regulated by tissue-specific miR-675 in human articular chondrocytes. J. Biol. Chem. 285, 24381–24387 10.1074/jbc.M110.111328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen-Kfir E., Artsi H., Levin A., Abramowitz E., Bajayo A., Gurt I., Zhong L., D’Urso A., Toiber D., Mostoslavsky R., Dresner-Pollak R. (2011) Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology 152, 4514–4524 10.1210/en.2011-1128 [DOI] [PubMed] [Google Scholar]
- 75.Bäckesjö C. M., Li Y., Lindgren U., Haldosén L. A. (2009) Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. Cells Tissues Organs 189, 93–97 10.1159/000151744 [DOI] [PubMed] [Google Scholar]
- 76.Darnell D. K., Kaur S., Stanislaw S., Konieczka J. H., Yatskievych T. A., Antin P. B. (2006) MicroRNA expression during chick embryo development. Dev. Dyn. 235, 3156–3165 10.1002/dvdy.20956 [DOI] [PubMed] [Google Scholar]
- 77.King B. L., Yin V. P. (2016) A conserved microRNA regulatory circuit is differentially controlled during limb/appendage regeneration. PLoS One 11, e0157106 10.1371/journal.pone.0157106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang B., Guo H., Zhang Y., Chen L., Ying D., Dong S. (2011) MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS One 6, e21679 10.1371/journal.pone.0021679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hicks M. R., Hiserodt J., Paras K., Fujiwara W., Eskin A., Jan M., Xi H., Young C. S., Evseenko D., Nelson S. F., Spencer M. J., Handel B. V., Pyle A.D. (2018) ERBB3 and NGFR mark a distinct skeletal muscle progenitor cell in human development and hPSCs. Nat. Cell Biol. 20, 46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.