Abstract
Aspirin (acetylsalicylic acid) inhibits prostaglandin (PG) synthesis by transfer of its acetyl group to a serine residue in the cyclooxygenase (COX) active site. Acetylation of Ser530 inhibits catalysis by preventing access of arachidonic acid substrate in the COX-1 isoenzyme. Acetylated COX-2, in contrast, gains a new catalytic activity and forms 15R hydroxy-eicosatetraenoic acid (15R-HETE) as alternate product. Here we show that acetylated COX-2 also retains COX activity, forming predominantly 15R-configuration PGs (70 or 62% 15R, respectively, determined using radiolabeled substrate or LC-MS analysis). Although the Km of arachidonic acid for acetylated COX-2 was ∼3-fold lower than for uninhibited COX-2, the catalytic efficiency for PG formation by the acetylated enzyme was reduced 10-fold due to a concomitant decrease in Vmax. Aspirin increased 15R-PGD2 but not 15R-PGE2 in isolated human leukocytes activated with LPS to induce COX-2. 15R-PGD2 inhibited human platelet aggregation induced by the thromboxane receptor agonist U46,619, and this effect was abrogated by an antagonist of the DP1 prostanoid receptor. We conclude that acetylation of Ser530 in COX-2 not only triggers formation of 15R-HETE but also allows oxygenation and cyclization of arachidonic acid to a 15R-PG endoperoxide. 15R-PGs are novel products of aspirin therapy via acetylation of COX-2 and may contribute to its antiplatelet and other pharmacologic effects.—Giménez-Bastida, J. A., Boeglin, W. E., Boutaud, O., Malkowski, M. G., Schneider, C. Residual cyclooxygenase activity of aspirin-acetylated COX-2 forms 15R-prostaglandins that inhibit platelet aggregation.
Keywords: arachidonic acid, stereocontrol, biosynthesis, leukocytes
Aspirin inhibits the cyclooxygenase (COX) enzymes via a unique mechanism (1). Aspirin covalently modifies the enzyme by transfer of its acetyl group to Ser530 in the COX active site (2, 3). Ser530 lines the fatty acid binding channel across from Tyr385, the residue that initiates oxygenation of arachidonic acid by abstraction of the pro-S hydrogen at C-13 in COX-1 and COX-2 (4, 5). Subsequent oxygenation at C-11, radical cyclization reactions, and final oxygenation at C-15 in the S-configuration yield the prostaglandin (PG) endoperoxide (PGH2) (6, 7). Acetylation of Ser530 in COX-1 blocks all catalytic activity, likely because increased bulk prevents access of the methyl end of arachidonic acid in the hydrophobic top channel beyond Tyr385 (8). Covalent inhibition of COX-1 is the molecular basis of the unique therapeutic effect of low-dose aspirin that selectively affects COX-1 in circulating platelets (9, 10). Acetylation of platelet COX-1 prevents formation of thromboxane A2, a major agonist of platelet aggregation in cardiovascular disease (11, 12). Once acetylated, COX-1 is inhibited for the lifetime of the anucleate platelets that are unable to generate new protein (13).
In contrast, acetylation of Ser530 in COX-2 does not inhibit the enzyme completely but changes the catalytic activity to form 15R hydroxy-eicosatetraenoic acid (15R-HETE) (3, 14, 15). Formation of 15R-HETE is mechanistically intriguing because it suggests that arachidonic acid can adopt a conformation in the active site that is productive yet does not allow the initial oxygenation at C-11, and subsequent cyclization reactions to occur (7). Furthermore, the absolute configuration of C-15 in 15R-HETE is opposite compared with 15S in the prostaglandin products of the unacetylated enzyme (3, 14, 15), raising the question about the mechanism used by the enzyme to control stereochemistry of the oxygenation reaction (7). Mutational analyses have shown a role of active site residues Ser530 and Val349 in controlling the stereochemistry at C-15 during 15-HETE as well as prostaglandin synthesis, but by what mechanisms is not clear (15–17).
The question of how COX-2 controls the stereochemistry at C-15 of its products has triggered a number of mechanistic hypotheses (5, 7, 16, 18–25). Mechanistic analyses and studies on the therapeutic role of aspirin (26–28) were conducted under the premise that acetylation of Ser530 prevents the formation of prostaglandins by COX-2. However, an inspection of chromatograms shown in a few reports on acetylated COX-2 may suggest otherwise (3, 5, 14, 25). In addition to confirming that 15-HETE is the dominant product, these analyses show a radioactive signal that matches the migration or elution time of prostaglandins. We took these findings as a starting point to explore whether COX-2 retains COX activity after acetylation by aspirin.
MATERIALS AND METHODS
Materials
[1-14C]Arachidonic acid (58 mCi/mmol) was purchased from PerkinElmer (Waltham, MA, USA). PG standards, including 15R-PGE2 and 15R-PGD2, and COX inhibitors were from Cayman Chemicals (Ann Arbor, MI, USA). Recombinant human COX-2 and its S530T mutant for use with [1-14C]arachidonic acid were expressed in Sf9 cells and purified as described in Schneider et al. (29). For other experiments, recombinant human COX-2 was expressed in insect cells, solubilized using n-octyl β-d-glucopyranoside, and purified using affinity and size exclusion chromatography in the presence of n-octyl β-d-glucopyranoside as described by Lucido et al. (30).
Incubations with COX-2
Incubations with radiolabeled substrate were conducted in 100 μl of 100 mM Tris-HCl buffer (pH 8.0) containing phenol (500 μM), hematin (2 μM), and recombinant human COX-2 or S530T mutant (1 μM). For preparation of acetylated COX-2, aspirin (2 mM) was added and incubated at room temperature for 1 h, except for the experiment in which incubation time was varied from 15 min to 2 h. The reaction was initiated by adding [1-14C]arachidonic acid (1 × 106 cpm, 100 μM), conducted for 15 min, and terminated by the addition of methanol (50 μl) and 0.1% acetic acid (850 μl). To some samples, unlabeled PGE2 (1 μg) was added. Reactions were extracted using preconditioned 30 mg HLB cartridges (Waters, Milford, MA, USA), washed with water, and eluted with methanol. The eluate was concentrated under a stream of nitrogen and dissolved in 50 μl methanol for RP-HPLC analysis.
Conversion of the PGE diastereomers into PGB2 enantiomers was achieved by treatment in mild alkali (200 mM NaOH) for 20 min (31). Products from a scaled-up 2 ml enzymatic reaction were extracted using 30 mg HLB cartridges (Waters), and the peak corresponding to PGB2 was isolated from RP-HPLC.
For analysis of 12-hydroxy-heptadecatrienoic acid (12-HHT), COX-2 (100 nM) was incubated in 2 ml buffer [100 mM NH4OAc (pH 8.0) containing 500 μM phenol and 2 μM hematin] for 1 h with glutathione (1 mM) in the presence or absence of aspirin (1 mM). The reaction was initiated by adding AA (30 μM final concentration), and after 15 min the samples were extracted and diluted in mobile phase for chiral-phase LC-MS analysis.
For kinetic analyses, COX-2 (100 nM) was incubated in 50 μl buffer [100 mM NH4OAc (pH 8.0) containing 500 μM phenol and 2 μM hematin] at 37°C for 5 min. The reaction was started by adding arachidonic acid (0.1–60 μM) in the same volume of ethanol and stopped after 10 s by the addition of 0.2% acetic acid in acetonitrile (50 μl) containing d4-PGE2 (20 ng). Samples were analyzed by RP-LC-MS extraction. The kinetic parameters were calculated using Prism 5.0 (GraphPad Software, La Jolla, CA, USA).
For incubations with unlabeled substrate, aspirin dose-response effect, and COX-2 inhibition studies, the enzyme (75 nM) was preincubated for 1 h with vehicle (1% ethanol or DMSO), aspirin (1 μM to 1 mM), indomethacin, lumiracoxib, or NS-398 (all at 10 μM). Stock solutions were 100 mM in ethanol or DMSO, respectively. Incubations with arachidonic acid (30 μM) were conducted for 15 min, combined with d4-PGE2 standard (20 ng), extracted, and dissolved in 100 μl straight-phase (SP)-HPLC solvent for SP-LC-MS analysis.
Human leukocytes
Leukocytes were isolated from peripheral blood from normal healthy volunteers (n = 10) as described in Giménez-Bastida et al. (32). Volunteers gave written informed consent before enrolling in the study, which was approved by the Vanderbilt University Institutional Review Board (No. 091243). Leukocytes were used as a mixture after sedimentation (6% dextran, 250 kD average molecular mass) and hypotonic lysis of the remaining red blood cells. Leukocytes were seeded in a 6-well plate at a density of 2 × 107 cells per well. Cells were treated with LPS (10 μg/ml) for 23 h followed by the addition of aspirin (1 mM) or vehicle for 1 h. The culture medium was replaced, and cells were incubated with A23187 (5 μM) for 15 min. Incubations were terminated by pelleting of the cells. Deuterated standard d4-PGE2 (20 ng) was added, and samples were extracted, dissolved in 100 μl SP-HPLC solvent, and analyzed by SP-LC-MS.
Platelet aggregation
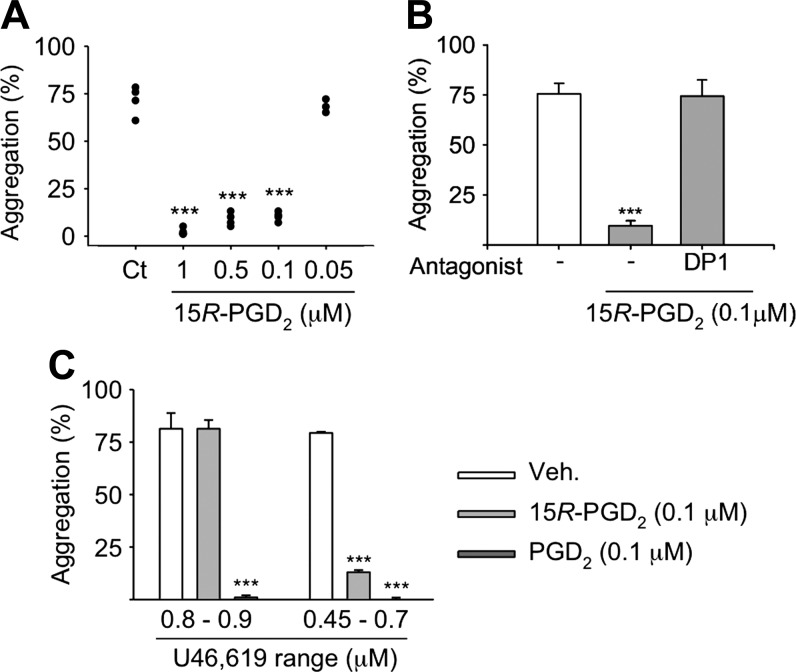
Platelet-rich plasma was obtained from 3–6 human volunteers for the different experiments after obtaining written informed consent (Vanderbilt University Institutional Review Board). Platelet aggregation was measured by light transmission aggregometry using a ChronoLog lumi-aggregometer (460VS) using platelet-rich plasma as described (33). 15R-PGE2 and 15R-PGD2 (50 nM to 5 μM) or PGD2 (0.1 μM) were added and incubated for 2 min. Aggregation was induced by addition of thromboxane receptor agonist U46,619 (0.45–0.9 μM, depending on the platelet sample) and recorded for 6 min. Some samples were incubated with the DP1 receptor antagonist BW A868C for 2 min prior to 15R-PGD2 and U46,619. The receptor antagonist was also included in the control samples. Aggregation was calculated using Aggrolink software (Chrono-Log, Havertown, PA, USA).
HPLC
RP-HPLC analyses of the enzymatic reactions used a diode array system (Agilent 1200; Agilent Technologies, Santa Clara, CA, USA) connected to a Packard Radiomatic A100 Radioactive Flow Detector (Meriden, CT, USA). A Symmetry C18 5-μm column (250 × 4.6 mm; Waters) was eluted at a flow rate of 1 ml/min with a solvent of methanol/water/acetic acid 80:20:0.01 (v/v) for 28 min followed by 100% methanol for 10 min. PGB2 was isolated by RP-HPLC using isocratic elution with acetonitrile/water/acetic acid 55:45:0.01 (v/v). SP-HPLC for the resolution of PGE2 and 15R-PGE2 used an Zorbax RX-SIL 5-μm column (250 × 4.6 mm; Agilent Technologies) eluted with hexane/isopropanol/acetic acid 90:10:0.1 (v/v) at a flow rate of 1 ml/min and monitored using diode array and radioactive flow detectors.
LC-MS
Samples were analyzed using a Thermo TSQ Vantage Triple Quadrupole MS instrument (Thermo Fisher Scientific, Waltham, MA, USA). RP-LC-MS analysis of kinetic parameters was performed with electrospray ionization in negative ionization mode. A Zorbax Eclipse Plus C18 1.8-μm column (2.1 × 50 mm; Agilent Technologies) was eluted with water/acetonitrile (95:5, v/v) as solvent A and acetonitrile/water (95:5, v/v) as solvent B, both containing 0.1% formic acid. A gradient was programmed from 100% A within 5 min to 100% B, which was held for 1 min before returning to starting conditions. The flow rate was 0.5 ml/min. The electrospray voltage was set at 4.0 kV; vaporizer temperature at 350°C; sheath and auxiliary gas pressure at 35 and 10 ψ, respectively; and capillary temperature at 300°C.
SP-LC-MS analysis used atmosphere pressure chemical ionization in negative ionization mode. The column was a Grace Alltima Silica 3-μm column (Grace, Columbia, MD, USA) (100 × 2.1 mm) eluted with hexane/isopropanol/acetic acid 90:10:0.1 (v/v) at a flow rate of 0.5 ml/min. Chiral-phase HPLC analysis of the enantiomers of 12-HHT and PGB2 used a Daicel Chiralpak AD-H column (Chiral Technologies, West Chester, PA, USA) (150 × 2.1 mm) eluted with hexane/ethanol/methanol/acetic acid 90:5:5:0.1 (v/v) at a flow rate of 0.2 ml/min (34). The discharge current was established at 22 mA; vaporizer temperature at 199°C; and sheath and auxiliary gas at 10 and 35 psi, respectively. The ion transfer tube was operated at 300°C.
The following transitions were used in RP-LC-MS in the selected reaction monitoring (SRM)-negative ionization mode: PGE2, m/z 351→271 [collision energy (CE) = 15 eV]; 15-HETE, m/z 319→219 (CE = 15 eV); d4-PGE2, and m/z 355→275 (CE = 15 eV). In SP-LC-MS, 3 diagnostic ion transitions were monitored in parallel for the identification of 15(R,S)-PGE2 and D2 in SRM in negative ion mode: m/z 351→271 (CE = 20 eV), m/z 351→315 (CE = 20 eV), and m/z 351→189 (CE = 20 eV). The ion transition for d4-PGE2 was m/z 355→275 (CE = 20 eV). The ion transition for 12-HHT was m/z 279→179 (CE = 15 eV) and for PGB2 was m/z 333→175 (CE = 25 eV).
Statistical analysis
Data were analyzed using Prism 5.0 (GraphPad Software). Kolmogorov-Smirnov analysis was performed to determine normal distribution of data. Normally distributed data were analyzed by a Student’s t test or ANOVA, followed by Newman-Keuls post hoc analysis. When normal distribution was not assumed, the data were analyzed by Wilcoxon signed rank and Mann-Whitney U tests. Values are given as means ± sd, and P < 0.05 was considered statistically significant.
RESULTS
Acetylated COX-2 forms 15R-prostaglandins
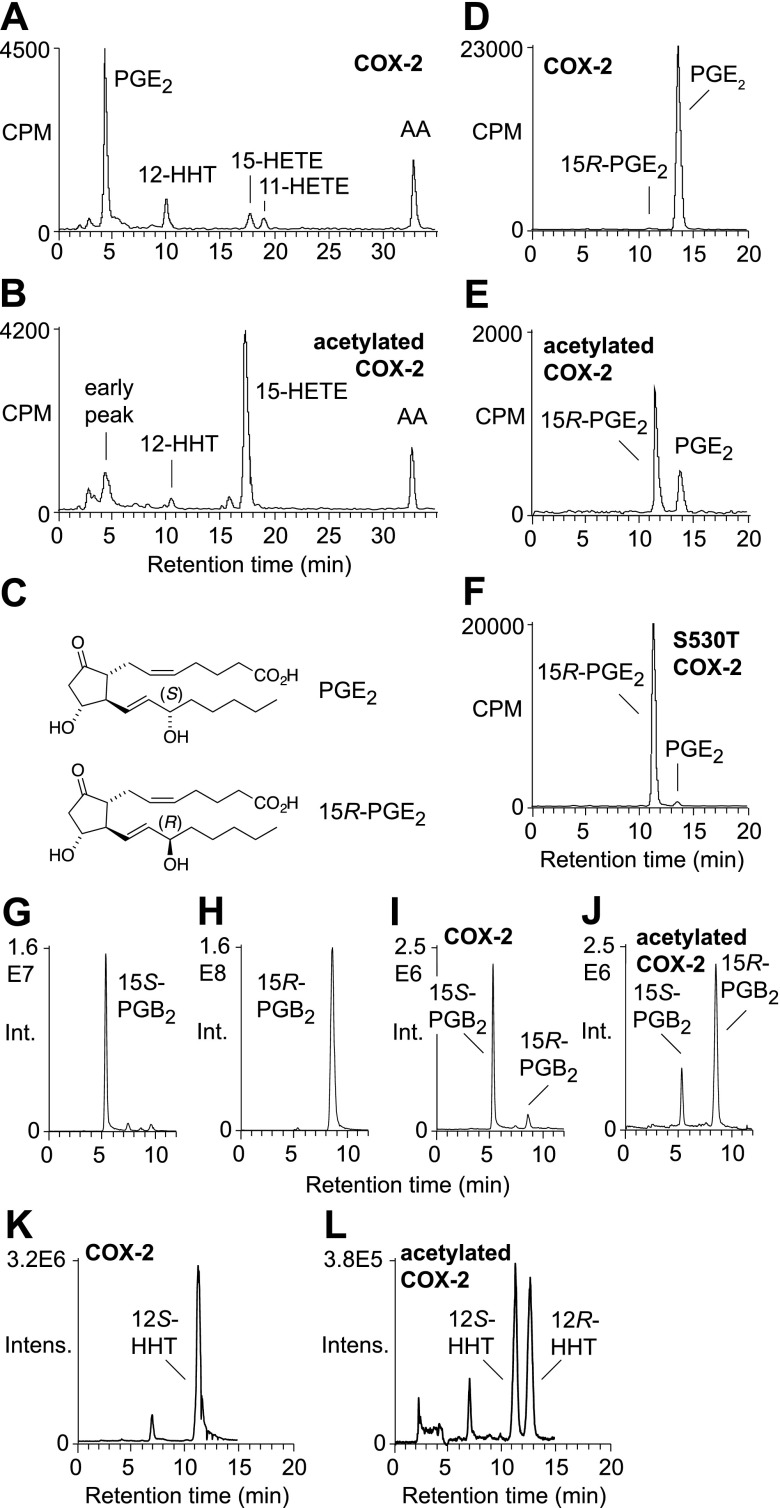
Recombinant human COX-2 was pretreated with aspirin or vehicle for 1 h and reacted with [1-14C]arachidonic acid. Product formation was analyzed by RP-HPLC with a radioactive flow detector. PGE2 was the major product of COX-2 together with the expected minor by-products 12-HHT and 15- and 11-HETE (Fig. 1A). Aspirin-acetylated COX-2 formed 15-HETE as the major product (Fig. 1B) (5). In addition, the chromatogram showed early eluting radioactive material at the retention time of prostaglandins (4–5 min), which accounted for ∼15% of all enzymatic products detected.
Figure 1.
Aspirin-acetylated recombinant human COX-2 forms 15R-configuration prostaglandins. A, B) RP-HPLC analysis of the products formed from [1-14C]arachidonic acid by COX-2 (A) and acetylated COX-2 (B). C) Structures of PGE2 and 15R-PGE2. D–F) SP-HPLC analysis of PGE formed by uninhibited (D), acetylated (E), and S530T mutant (F) COX-2. G–J) Chiral phase LC-SRM-MS analysis of PGB2 formed by based-catalyzed transformation of PGE2 and 15R-PGE2 standards, as well as PGE formed in the reaction of uninhibited and acetylated COX-2, monitoring the transition m/z 333 to 175 in negative ion mode. K, L) Chiral phase LC-SRM-MS analysis of 12-HHT formed by uninhibited COX-2 (K) and acetylated COX-2 (L) monitoring the transition of m/z 279 to 179 in negative ion mode.
We tested whether the early eluting radioactive products of acetylated COX-2 contained PGE2. Unlabeled PGE2 was added as a marker to the samples, and the corresponding peaks were isolated using RP-HPLC and further analyzed using SP-HPLC with radiodetection. SP-HPLC, in contrast to RP-HPLC, achieves resolution of the 15R- and 15S-epimers of PGE2 (Fig. 1C), enabling assignment of the absolute configuration by chromatographic analysis (17). PGE2 formed in the COX-2 reaction was >98% of the 15S-configuration (Fig. 1D). Analysis of PGE2 from acetylated COX-2 gave 2 peaks, and the later eluting peak coeluted with PGE2 (Fig. 1E). The earlier eluting peak was identified as 15R-PGE2, based on identical retention time as an authentic standard generated using S530T mutant COX-2 (Fig. 1F) (17). Integration of the peak areas showed that aspirin-acetylated recombinant human COX-2 formed PGE2 in a 70:30 ratio of the 15R/15S epimers (Fig. 1E).
We next performed a direct analysis of the C-15 configuration of the PGE products in the enzymatic reactions. 15R-PGE2 and PGE2 from the acetylated and uninhibited COX-2 reactions as well as the standards PGE2 and 15R-PGE2 were converted to the corresponding PGB2 enantiomers by treatment with a mild base (31). Base-catalyzed dehydration removes all stereocenters from the molecule besides C-15, enabling configurational analysis by chiral-phase chromatography. LC-MS analysis using a chiral-phase HPLC column resolved the 15S- and 15R-PGB2 enantiomers (Fig. 1G, H). PGB2 obtained from the reaction of uninhibited COX-2 was >98% 15S, whereas PGB2 from acetylated COX-2 was 80% of the 15R configuration (Fig. 1I, J).
The ratio of 70:30 for 15R-PGE2 to PGE2 by the acetylated enzyme was obtained in 3 independent incubations using different preparations of recombinant human COX-2 solubilized with n-octyl β-d-glucopyranoside. Acetylation by aspirin was less efficient when the enzyme was solubilized with CHAPS (25), and a ratio of 50:50 for 15R-PGE2 to PGE2 was observed after 1 h of aspirin treatment. Prolonged incubation with a higher concentration of aspirin resulted in more complete acetylation of CHAPS-solubilized COX-2. Under these conditions, a ratio of 70:30 for 15R-PGE2 to PGE2 was also observed for the CHAPS-solubilized enzyme (data not shown).
Nonenzymatic rearrangement of highly unstable PGH2 yields not only PGE2 and PGD2 but also 12-HHT (35). The 12-hydroxy group of 12-HHT is identical to the 15-hydroxy group of PGH2; the change in numbering is due to the loss of carbons 9–11 as malondialdehyde. 12-HHT formed by uninhibited COX-2 was >99% 12S (Fig. 1K). 12-HHT from acetylated COX-2 was 47% 12R (Fig. 1L), which was less R-enantiomer than in the PG products. The decrease of the R-enantiomer can be explained by the reduced propensity of 15R-PGH2 to break down to 12-HHT compared with PGH2 (15S-configuration). This has originally been shown for products of the 15R-specific COX in the coral Plexaura homomalla (31). Although the coral COX formed PGH2 that was 97% 15R, the corresponding 12-HHT was only 90% 12R (31).
Kinetic analysis
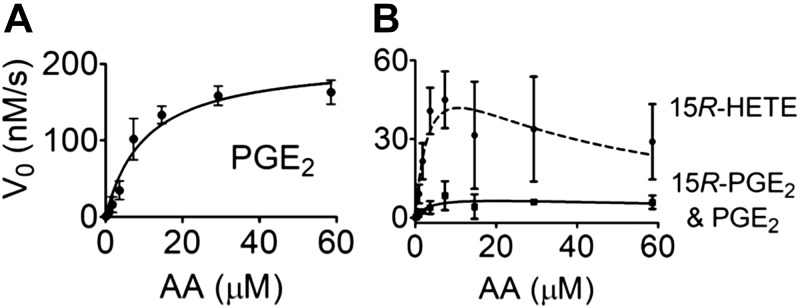
We determined the catalytic efficiency of uninhibited and acetylated COX-2. The concentration of arachidonic acid varied from 0.1 to 60 μM, and the reaction time with the enzyme was 10 s to prevent depletion of the substrate. Formation of PG and HETE products was quantified using reversed-phase LC-MS. Vmax for formation of the mixture of PGE2 C-15 epimers (8.7 ± 3.7 nM/s) by acetylated COX-2 was almost 25-fold less than for PGE2 by COX-2 (206.4 ± 15.8 nM/s) and ∼8-fold less than for 15R-HETE (71.6 ± 28.5 nM/s) (Fig. 2). The Km values of arachidonic acid were significantly reduced for the acetylated enzyme forming PG and 15-HETE (3.7 ± 3.4 and 3.8 ± 2.8 μM, respectively) compared with uninhibited COX-2 (10.4 ± 2.3 μM). For acetylated COX-2, arachidonic acid concentrations above 10 μM showed substrate inhibition in the formation of 15R-HETE but not for PG products, resulting in increased uncertainty of the calculated kinetic parameters. The catalytic efficiency kcat/Km for PG formation by the acetylated enzyme (0.02/s) vs. uninhibited COX-2 (0.20/s) was reduced 10-fold.
Figure 2.
Kinetic analysis of the reaction of COX-2 and acetylated COX-2. A) Formation of PGE2 from arachidonic acid (AA) by COX-2. B) Formation of 15R-HETE and PGE2/15R-PGE2 by acetylated COX-2. Products were quantified by direct analysis of reaction mixtures using RP-LC-MS. Values are means ± sd (n = 3 independent experiments).
Time-course of acetylation
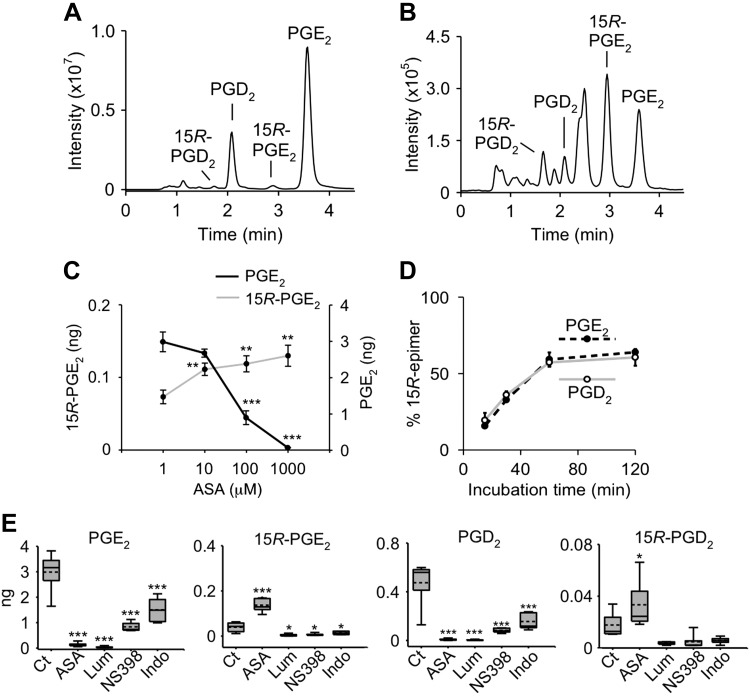
We developed a method for resolution of PGE2, PGD2, and their 15R-epimers by SP-HPLC and detection using LC-MS (Fig. 3A). Aspirin treatment of recombinant COX-2 resulted in an increase of the 15R-epimers, although, as expected, the overall amount of PG products was decreased (Fig. 3B).
Figure 3.
Effect of aspirin and other inhibitors on PG formation by recombinant COX-2. A, B) SP-LC-MS analysis of reactions of COX-2 (A) and acetylated COX-2 with arachidonic acid (B) monitoring the transition of m/z 351 to 271 (loss of 2 × H2O and CO2) in negative ion SRM mode. C) Dose-response curve of aspirin in the formation of 15R-PGE2 and inhibition of PGE2 by COX-2. D) Relative proportion (%) of 15R-PGE2 and 15R-PGD2 of total PGE or PGD, respectively, as a function of the incubation time with aspirin. E) Formation of PGE2, 15R-PGE2, PGD2, and 15R-PGD2 by COX-2 in a control reaction (Ct) or in the presence of aspirin (ASA), lumiracoxib (Lum), NS-398, or indomethacin (Indo). Reactions were conducted in 100 μl of buffer, and products were quantified by LC-SRM-MS analysis using d4-PGE2 as internal standard. Values are means ± sd (n = 3 independent experiments). **P < 0.01, ***P < 0.001 (statistically significant reduction of PGE2 or increase of 15R PGE2 compared with control).
We established the dose-response relationship for aspirin in the product formation by COX-2. Increased formation of 15R-PGE2 was paralleled by the decrease of formation of PGE2, and both occurred with an estimated half maximal inhibitory concentration (IC50) of ∼50 μM (Fig. 3C).
We treated recombinant COX-2 with aspirin (1 mM) for 15, 30, 60, and 120 min prior to addition of substrate to determine the time course of acetylation and formation of 15R-PGs relative to PGs (Fig. 3D). The ratio of the 15R-epimers of both PGE2 and PGD2 relative to PGE2 and PGD2, respectively, plateaued at 62:38 at 1 h incubation time with aspirin. These values were similar but not identical to the results obtained with radiolabeled substrate, suggesting that there may be minor unidentified coeluting products in either or both analytical methods.
Other NSAIDs and coxibs do not induce 15R-PG formation
We tested whether other COX inhibitors induce formation of 15R-PGs. The nonselective inhibitor indomethacin as well as COX-2 selective NS-398 and lumiracoxib inhibit the enzyme by binding in the COX active site and preventing access of arachidonic acid but do not target Ser530 or covalently bind to the enzyme (1). All inhibitors significantly inhibited formation of PGE2 and PGD2 by recombinant human COX-2 (Fig. 3E). Aspirin was unique in inducing synthesis of 15R-PGE2 and 15R-PGD2, resulting in an ∼2-fold increase (P < 0.05); none of the other inhibitors changed the amount of 15R-PG formed (Fig. 3E). The small amount of baseline formation of 15R-PGE2 was likely due to imperfect catalysis by COX-2 and/or nonenzymatic reactions (36, 37).
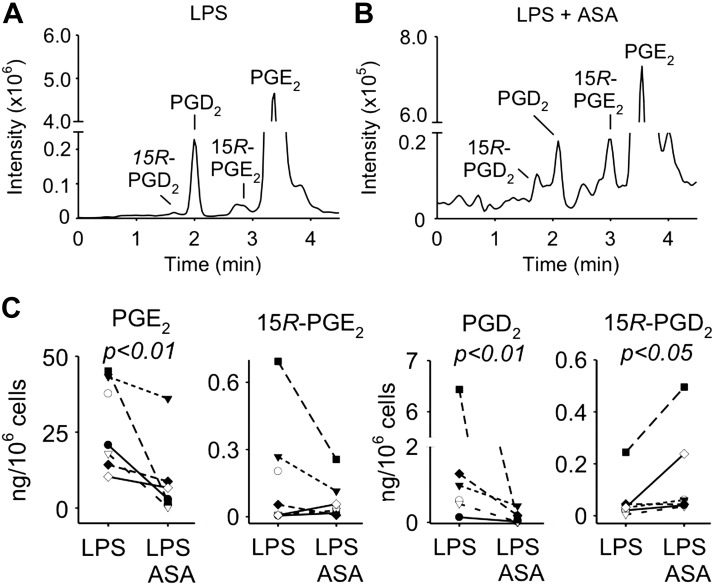
15R-PGs are formed in isolated human leukocytes
We isolated leukocytes from 10 healthy human volunteers to analyze formation of PG and their 15R-epimers. Peripheral blood leukocytes were activated with LPS for 23 h to induce expression of COX-2, followed by treatment with aspirin (1 mM) or vehicle for 1 h. PGE2 was the most abundant prostanoid in LPS-activated cells and remained the major prostanoid in cells treated additionally with aspirin, although it was reduced significantly (Fig. 4A, B). 15R-PGE2 was present in LPS-activated leukocytes, but treatment with aspirin did not change the levels. PGD2 was likewise reduced, and 15R-PGD2 was increased by aspirin in leukocytes obtained from 7 out of 10 volunteers. 15R-PGD2 was undetectable in 3 volunteers (Fig. 4C).
Figure 4.
Formation of 15R-PGs in human leukocytes. A, B) Freshly isolated peripheral blood leukocytes were treated for 23 h with LPS followed by treatment with vehicle (A) or aspirin (B) for 1 h. Samples were analyzed by SP-LC-MS monitoring the transition of m/z 351 to 271 in the negative ion SRM mode. C) Quantification of PGE2, 15R-PGE2, PGD2, and 15R-PGD2 in leukocytes treated with LPS and aspirin or vehicle. Samples from 7–10 human volunteers were analyzed. P < 0.01 (statistically significant reduction of PGE2 and PGD2), P < 0.05 (statistically significant increase of 15RPCD2).
15R-PGD2 inhibits platelet aggregation
Human platelets express receptors for PGD2 (DP1), PGE2 (EP2, EP3, EP4), thromboxane A2, and PGI2, and all are involved in the regulation of aggregation (38). Because there was indication that 15R-PG can bind to EP and DP receptors (30, 39), we tested whether 15R-PGD2 and 15R-PGE2 regulate platelet aggregation. Platelet aggregation was induced by the thromboxane receptor agonist U46,619. 15R-PGD2 inhibited aggregation between 58 and 69% at 0.1, 0.5, and 1 μM but was not active at 0.05 μM (Fig. 5A). The inhibitory activity of 15R-PGD2 (at 0.1 μM) was abrogated by BWA 868C, an antagonist of the DP1 receptor (Fig. 5B). 15R-PGE2 was less potent, and consistent inhibition of aggregation required ≥5-μM concentration.
Figure 5.
Inhibition of platelet aggregation by 15R-PGD2. A) Human platelets were pretreated with 15R-PGD2, and aggregation was induced by the thromboxane receptor agonist U46,619 (n = 5 volunteers). B) Platelets were treated with 15R-PGD2 (0.1 μM) and U46,619 in the absence and presence of the DP1 receptor antagonist BWA 868C (n = 6 volunteers). C) Platelets were pretreated with 15R-PGD2 or PGD2 (0.1 μM), and aggregation was induced by U46,619 at 2 different concentrations (n = 3 volunteers). Values are means ± sd.
To compare the inhibitory potency of 15R-PGD2 with PGD2, aggregation was induced using 2 different concentrations of U46,619. At the lower concentration (0.45–0.7 μM) that was just sufficient to induce aggregation, both 15R-PGD2 and PGD2 were inhibitory, whereas at the higher concentration of U46,619 (0.8–0.9 μM), only PGD2 was able to inhibit aggregation (Fig. 5C). The IC50 of PGD2 for inhibition of platelet aggregation stimulated by PGG2 or ADP is ∼30 nM (40, 41).
DISCUSSION
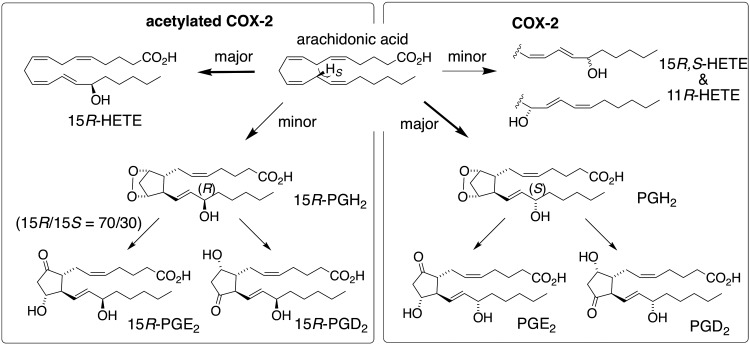
Formation of 15R-HETE as a result of aspirin acetylation of COX-2 was first reported 25 yr ago (14) and has since been used as a model to explore how COX-2 controls the reaction with arachidonic acid. A number of hypotheses were developed to explain how altered binding conformation of arachidonic acid induced by acetylated Ser530 results in inverted stereochemistry at C-15 (5, 7, 16, 18–25). A common facet to these hypotheses was the consideration of 15R-HETE as the sole catalytic product—formation of a prostaglandin product was not anticipated, let alone included in any model. Here we show that acetylated human COX-2 retains the ability to form prostaglandins and that the prostaglandins formed are predominantly of the 15R-configuration (Fig. 6), challenging the current understanding of reaction control in COX catalysis.
Figure 6.
Transformation of arachidonic acid by COX-2 and aspirin acetylated COX-2.
Our analyses indicate that both 15R- and 15S-prostaglandins were formed by acetylated COX-2. An alternative explanation (i.e., that only 15R-prostaglandins were formed by the acetylated enzyme and 15S-prostaglandins were formed by residual, unacetylated enzyme) appears less likely. X-ray structural analysis indicates that aspirin acetylates both subunits of the recombinant COX-2 homodimer (25). Our analyses further showed that the ratio of 15R- to 15S-epimers plateaued with increasing time and dose of treatment with aspirin, which would not be expected if 15S oxygenation were an artifact of incomplete acetylation. Thus, arachidonic acid binds in the acetylated COX-2 active site in a conformation that allows COX catalysis to proceed normally up to the final oxygenation at C-15, which is largely inverted from S- to R-configuration. More likely than not this conformation involves binding of the tail end of arachidonic acid in the top channel as occurs in the nonacetylated enzyme (42–44). Formation of 15R-prostaglandins is not unprecedented in nature, and a similar binding mode has been inferred for the biosynthesis of 15R-prostaglandins in coral (31). In the absence of an experimental structure of arachidonic acid binding in the active site of acetylated COX-2, the factors that guide C-15 stereocontrol and allow 11R-oxygenation and cyclization remain elusive.
15R-PG formation was not a mere artifact of the purified enzyme but also occurred in primary human leukocytes that were activated to express COX-2. In fact, both 15R-PGE2 and 15R-PGD2 were detected even without aspirin treatment, possibly due to incorrect COX catalysis or nonenzymatic formation (36). Only 15R-PGD2, however, was increased by aspirin. This was surprising because LPS results in strong induction of COX-2 and PGE2 is the major prostanoid formed by activated leukocytes (32). Thus, enzymatic transformation of 15R-PGH2 may favor 15R-PGD2 and discriminate against 15R-PGE2, possibly due to a putative ability of PGD synthase to transform 15R-PGH2, whereas it may not be a substrate for PGE synthase. Preferential metabolism of 15R-PGE2 over 15R-PGD2 is a less likely explanation because the major inactivating enzyme, 15-hydroxy prostaglandin dehydrogenase, is stereospecific for the 15S-hydroxyl and unlikely to react with the 15R-epimers (45). Nevertheless, 15R-PGs were detected for the first time in primary human cells, justifying studies on their functional role in human physiology.
Information on the roles of 15R-PGs in humans and other mammals is scant. The 15R epimer of PGE2 binds to the human EP1 receptor with almost 1000-fold less affinity than PGE2 (46), and a similar loss in potency has been invoked for the other EP receptors (47, 48). In contrast, there is some indication that 15R-PGE2 may bind to mouse EP3 and EP4 receptors with low nanomolar affinity (39). 15R-PGE2 isolated from the coral P. homomalla showed anti-inflammatory activity by inhibiting phorbol ester–induced mouse ear edema and by inhibiting degranulation of human neutrophils and release of myeloperoxidase (49). Bioactivity data for 15R-PGD2 were not available in the PubChem database (50). In one study, 15R-PGD2 was described as equal in potency to PGD2 as an agonist of the DP2 receptor (EC50, 0.5–1 nM), and it stimulated various responses in basophils and eosinophils, including CD11b expression, actin polymerization, and migration, processes that are regulated by DP2 (30).
Because human platelets express a large number of prostanoid receptors, we investigated the effects of 15R-PGE2 and 15R-PGD2 on platelets as a means to explore whether the 15R-epimers may be involved in mediating one of aspirin’s recognized activities, inhibition of platelet aggregation. Proaggregatory thromboxane A2, a COX-1 metabolite, and antiaggregatory PGI2, a COX-2 metabolite, are key regulators of platelet aggregation. PGE2 and PGD2 play more subtle and complex roles (38). We found that 15R-PGD2 inhibited platelet aggregation induced by the thromboxane receptor agonist U46,619, and this involved the DP1 receptor. With an estimated IC50 of ∼70 nM, 15R-PGD2 was about half as potent as PGD2 (IC50, ∼30 nM) (40, 41), but in the absence of additional studies it is unclear whether 15R-PGD2 may be formed in sufficient amounts to activate the receptor. Activation of DP1 inhibits platelet aggregation (51), and our finding that a selective DP1 antagonist inhibited 15R-PGD2–induced aggregation suggested DP1 as a major target of 15R-PGD2 in platelets. This finding was in contrast to studies by Cossette et al. (30), who reported that 15R-PGD2 only weakly induced release of cAMP from human platelets, an effect mediated by DP1, although the study did not report further target validation by using a receptor agonist or antagonist.
Several mechanisms contribute to the therapeutic benefits of aspirin. The anti-inflammatory and analgesic effects of regular strength aspirin are due to inhibition of PGE2 downstream of COX-2 (26, 52). The antithrombotic effect of low-dose aspirin is due to inhibition of COX-1 in platelets (9, 53). Inhibition of COX-1 and COX-2 is implicated in the cancer-chemopreventive effects of aspirin, although it is less well established through what mechanisms (54). A further mechanism for therapeutic effects of aspirin is suggested to involve formation of 15R-HETE by acetylated COX-2. Oxidation of 15R-HETE by 5-LOX gives rise to aspirin-triggered lipoxins that have anti-inflammatory activities in vitro and in animal models (55). Our studies suggest the possibility that aspirin exerts yet another therapeutic mechanism by triggering formation of anti-aggregatory 15R-PGD2.
In summary, we have shown that acetylation of recombinant human COX-2 results in the synthesis of 15R-PGH2, which was detected as its stable 15R-PGE2 and 15R-PGD2 transformation products. Although 15R-PGH2 was a minor product compared with 15R-HETE, its formation is of major significance for understanding reaction control in COX catalysis. We established baseline levels of 15R-PGs in leukocytes activated ex vivo and provide evidence that aspirin triggers formation of 15R-PGD2 in these cells, a novel potential mechanism by which aspirin controls platelet aggregation.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grants R01GM076592 and R01GM118412 (to C.S.) and R01GM115386 (to M.G.M.). O.B. was supported, in part, by American Heart Association Grant 14GRNT20460090. J.A.G.-B. was supported by a postdoctoral award from the American Heart Association (16POST30690001). Mass spectrometric analyses were in part performed through Vanderbilt University Medical Center’s Digestive Disease Research Center, supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant P30DK058404 Core Scholarship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the NIH. The authors declare no conflicts of interest.
Glossary
- 12-HHT
12-hydroxy-heptadecatrienoic acid
- CE
collision energy
- COX
cyclooxygenase
- HETE
hydroxy-eicosatetraenoic acid
- IC50
half maximal inhibitory concentration
- PG
prostaglandin
- PGH2
prostaglandin endoperoxide
- SP
straight-phase
- SRM
selected reaction monitoring
AUTHOR CONTRIBUTIONS
J. A. Giménez-Bastida performed cell and enzyme incubations and analyzed products by LC-MS; J. A. Giménez-Bastida and O. Boutaud performed platelet aggregation; W. E. Boeglin and C. Schneider performed incubations of enzymes with arachidonic acid and HPLC analyses; M. G. Malkowski provided enzyme; O. Boutaud, M. G. Malkowski, and C. Schneider designed experiments; C. Schneider wrote the manuscript with help from O. Boutaud and M. G. Malkowski; and all authors analyzed results and approved the final version of the manuscript.
REFERENCES
- 1.Blobaum A. L., Marnett L. J. (2007) Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 50, 1425–1441 [DOI] [PubMed] [Google Scholar]
- 2.Roth G. J., Stanford N., Majerus P. W. (1975) Acetylation of prostaglandin synthase by aspirin. Proc. Natl. Acad. Sci. USA 72, 3073–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lecomte M., Laneuville O., Ji C., DeWitt D. L., Smith W. L. (1994) Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J. Biol. Chem. 269, 13207–13215 [PubMed] [Google Scholar]
- 4.Hamberg M., Samuelsson B. (1967) On the mechanism of the biosynthesis of prostaglandins E-1 and F-1-alpha. J. Biol. Chem. 242, 5336–5343 [PubMed] [Google Scholar]
- 5.Schneider C., Brash A. R. (2000) Stereospecificity of hydrogen abstraction in the conversion of arachidonic acid to 15R-HETE by aspirin-treated cyclooxygenase-2. Implications for the alignment of substrate in the active site. J. Biol. Chem. 275, 4743–4746 [DOI] [PubMed] [Google Scholar]
- 6.Van der Donk W. A., Tsai A. L., Kulmacz R. J. (2002) The cyclooxygenase reaction mechanism. Biochemistry 41, 15451–15458 [DOI] [PubMed] [Google Scholar]
- 7.Schneider C., Pratt D. A., Porter N. A., Brash A. R. (2007) Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem. Biol. 14, 473–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loll P. J., Picot D., Garavito R. M. (1995) The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat. Struct. Biol. 2, 637–643 [DOI] [PubMed] [Google Scholar]
- 9.Harter H. R., Burch J. W., Majerus P. W., Stanford N., Delmez J. A., Anderson C. B., Weerts C. A. (1979) Prevention of thrombosis in patients on hemodialysis by low-dose aspirin. N. Engl. J. Med. 301, 577–579 [DOI] [PubMed] [Google Scholar]
- 10.Preston F. E., Whipps S., Jackson C. A., French A. J., Wyld P. J., Stoddard C. J. (1981) Inhibition of prostacyclin and platelet thromboxane A2 after low-dose aspirin. N. Engl. J. Med. 304, 76–79 [DOI] [PubMed] [Google Scholar]
- 11.Hirsh P. D., Hillis L. D., Campbell W. B., Firth B. G., Willerson J. T. (1981) Release of prostaglandins and thromboxane into the coronary circulation in patients with ischemic heart disease. N. Engl. J. Med. 304, 685–691 [DOI] [PubMed] [Google Scholar]
- 12.Eikelboom J. W., Hirsh J., Weitz J. I., Johnston M., Yi Q., Yusuf S. (2002) Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation 105, 1650–1655 [DOI] [PubMed] [Google Scholar]
- 13.Patrono C., García Rodríguez L. A., Landolfi R., Baigent C. (2005) Low-dose aspirin for the prevention of atherothrombosis. N. Engl. J. Med. 353, 2373–2383 [DOI] [PubMed] [Google Scholar]
- 14.Holtzman M. J., Turk J., Shornick L. P. (1992) Identification of a pharmacologically distinct prostaglandin H synthase in cultured epithelial cells. J. Biol. Chem. 267, 21438–21445 [PubMed] [Google Scholar]
- 15.Mancini J. A., O’Neill G. P., Bayly C., Vickers P. J. (1994) Mutation of serine-516 in human prostaglandin G/H synthase-2 to methionine or aspirin acetylation of this residue stimulates 15-R-HETE synthesis. FEBS Lett. 342, 33–37 [DOI] [PubMed] [Google Scholar]
- 16.Thuresson E. D., Lakkides K. M., Rieke C. J., Sun Y., Wingerd B. A., Micielli R., Mulichak A. M., Malkowski M. G., Garavito R. M., Smith W. L. (2001) Prostaglandin endoperoxide H synthase-1: the functions of cyclooxygenase active site residues in the binding, positioning, and oxygenation of arachidonic acid. J. Biol. Chem. 276, 10347–10357 [DOI] [PubMed] [Google Scholar]
- 17.Schneider C., Boeglin W. E., Prusakiewicz J. J., Rowlinson S. W., Marnett L. J., Samel N., Brash A. R. (2002) Control of prostaglandin stereochemistry at the 15-carbon by cyclooxygenases-1 and -2. A critical role for serine 530 and valine 349. J. Biol. Chem. 277, 478–485 [DOI] [PubMed] [Google Scholar]
- 18.Xiao G., Tsai A. L., Palmer G., Boyar W. C., Marshall P. J., Kulmacz R. J. (1997) Analysis of hydroperoxide-induced tyrosyl radicals and lipoxygenase activity in aspirin-treated human prostaglandin H synthase-2. Biochemistry 36, 1836–1845 [DOI] [PubMed] [Google Scholar]
- 19.Thuresson E. D., Lakkides K. M., Smith W. L. (2000) Different catalytically competent arrangements of arachidonic acid within the cyclooxygenase active site of prostaglandin endoperoxide H synthase-1 lead to the formation of different oxygenated products. J. Biol. Chem. 275, 8501–8507 [DOI] [PubMed] [Google Scholar]
- 20.Rowlinson S. W., Crews B. C., Goodwin D. C., Schneider C., Gierse J. K., Marnett L. J. (2000) Spatial requirements for 15-(R)-hydroxy-5Z,8Z,11Z, 13E-eicosatetraenoic acid synthesis within the cyclooxygenase active site of murine COX-2. Why acetylated COX-1 does not synthesize 15-(R)-hete. J. Biol. Chem. 275, 6586–6591 [DOI] [PubMed] [Google Scholar]
- 21.Tsai A. L., Palmer G., Wu G., Peng S., Okeley N. M., van der Donk W. A., Kulmacz R. J. (2002) Structural characterization of arachidonyl radicals formed by aspirin-treated prostaglandin H synthase-2. J. Biol. Chem. 277, 38311–38321 [DOI] [PubMed] [Google Scholar]
- 22.Peng S., Okeley N. M., Tsai A. L., Wu G., Kulmacz R. J., van der Donk W. A. (2002) Synthesis of isotopically labeled arachidonic acids to probe the reaction mechanism of prostaglandin H synthase. J. Am. Chem. Soc. 124, 10785–10796 [DOI] [PubMed] [Google Scholar]
- 23.Furse K. E., Pratt D. A., Schneider C., Brash A. R., Porter N. A., Lybrand T. P. (2006) Molecular dynamics simulations of arachidonic acid-derived pentadienyl radical intermediate complexes with COX-1 and COX-2: insights into oxygenation regio- and stereoselectivity. Biochemistry 45, 3206–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tosco P. (2013) A mechanistic hypothesis for the aspirin-induced switch in lipid mediator production by cyclooxygenase-2. J. Am. Chem. Soc. 135, 10404–10410 [DOI] [PubMed] [Google Scholar]
- 25.Lucido M. J., Orlando B. J., Vecchio A. J., Malkowski M. G. (2016) Crystal structure of aspirin-acetylated human cyclooxygenase-2: insight into the formation of products with reversed stereochemistry. Biochemistry 55, 1226–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patrignani P., Patrono C. (2015) Cyclooxygenase inhibitors: from pharmacology to clinical read-outs. Biochim. Biophys. Acta 1851, 422–432 [DOI] [PubMed] [Google Scholar]
- 27.Patrignani P., Patrono C. (2016) Aspirin and cancer. J. Am. Coll. Cardiol. 68, 967–976 [DOI] [PubMed] [Google Scholar]
- 28.Rothwell P. M., Fowkes F. G., Belch J. F., Ogawa H., Warlow C. P., Meade T. W. (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377, 31–41 [DOI] [PubMed] [Google Scholar]
- 29.Schneider C., Boeglin W. E., Brash A. R. (2004) Identification of two cyclooxygenase active site residues, Leucine 384 and Glycine 526, that control carbon ring cyclization in prostaglandin biosynthesis. J. Biol. Chem. 279, 4404–4414 [DOI] [PubMed] [Google Scholar]
- 30.Cossette C., Walsh S. E., Kim S., Lee G. J., Lawson J. A., Bellone S., Rokach J., Powell W. S. (2007) Agonist and antagonist effects of 15R-prostaglandin (PG) D2 and 11-methylene-PGD2 on human eosinophils and basophils. J. Pharmacol. Exp. Ther. 320, 173–179 [DOI] [PubMed] [Google Scholar]
- 31.Valmsen K., Järving I., Boeglin W. E., Varvas K., Koljak R., Pehk T., Brash A. R., Samel N. (2001) The origin of 15R-prostaglandins in the Caribbean coral Plexaura homomalla: molecular cloning and expression of a novel cyclooxygenase. Proc. Natl. Acad. Sci. USA 98, 7700–7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giménez-Bastida J. A., Shibata T., Uchida K., Schneider C. (2017) Roles of 5-lipoxygenase and cyclooxygenase-2 in the biosynthesis of hemiketals E2 and D2 by activated human leukocytes. FASEB J. 31, 1867–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith J. P., Haddad E. V., Downey J. D., Breyer R. M., Boutaud O. (2010) PGE2 decreases reactivity of human platelets by activating EP2 and EP4. Thromb. Res. 126, e23–e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider C., Yu Z., Boeglin W. E., Zheng Y., Brash A. R. (2007) Enantiomeric separation of hydroxy and hydroperoxy eicosanoids by chiral column chromatography. Methods Enzymol. 433, 145–157 [DOI] [PubMed] [Google Scholar]
- 35.Hamberg M., Samuelsson B. (1967) Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J. Biol. Chem. 242, 5344–5354 [PubMed] [Google Scholar]
- 36.Gao L., Zackert W. E., Hasford J. J., Danekis M. E., Milne G. L., Remmert C., Reese J., Yin H., Tai H. H., Dey S. K., Porter N. A., Morrow J. D. (2003) Formation of prostaglandins E2 and D2 via the isoprostane pathway: a mechanism for the generation of bioactive prostaglandins independent of cyclooxygenase. J. Biol. Chem. 278, 28479–28489 [DOI] [PubMed] [Google Scholar]
- 37.Hecker M., Ullrich V., Fischer C., Meese C. O. (1987) Identification of novel arachidonic acid metabolites formed by prostaglandin H synthase. Eur. J. Biochem. 169, 113–123 [DOI] [PubMed] [Google Scholar]
- 38.Friedman E. A., Ogletree M. L., Haddad E. V., Boutaud O. (2015) Understanding the role of prostaglandin E2 in regulating human platelet activity in health and disease. Thromb. Res. 136, 493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tani K., Naganawa A., Ishida A., Egashira H., Sagawa K., Harada H., Ogawa M., Maruyama T., Ohuchida S., Nakai H., Kondo K., Toda M. (2001) Design and synthesis of a highly selective EP2-receptor agonist. Bioorg. Med. Chem. Lett. 11, 2025–2028 [DOI] [PubMed] [Google Scholar]
- 40.Nishizawa E. E., Miller W. L., Gorman R. R., Bundy G. L., Svensson J., Hamberg M. (1975) Prostaglandin d2 as a potential antithrombotic agent. Prostaglandins 9, 109–121 [DOI] [PubMed] [Google Scholar]
- 41.Whittle B. J., Moncada S., Vane J. R. (1978) Comparison of the effects of prostacyclin (PGI2), prostaglandin E1 and D2 on platelet aggregation in different species. Prostaglandins 16, 373–388 [DOI] [PubMed] [Google Scholar]
- 42.Rowlinson S. W., Crews B. C., Lanzo C. A., Marnett L. J. (1999) The binding of arachidonic acid in the cyclooxygenase active site of mouse prostaglandin endoperoxide synthase-2 (COX-2). A putative L-shaped binding conformation utilizing the top channel region. J. Biol. Chem. 274, 23305–23310 [DOI] [PubMed] [Google Scholar]
- 43.Malkowski M. G., Ginell S. L., Smith W. L., Garavito R. M. (2000) The productive conformation of arachidonic acid bound to prostaglandin synthase. Science 289, 1933–1937 [DOI] [PubMed] [Google Scholar]
- 44.Vecchio A. J., Simmons D. M., Malkowski M. G. (2010) Structural basis of fatty acid substrate binding to cyclooxygenase-2. J. Biol. Chem. 285, 22152–22163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shio H., Ramwell P. W., Andersen N. H., Corey E. J. (1970) Stereospecificity of the prostaglandin 15-dehydrogenase from swine lung. Experientia 26, 355–357 [DOI] [PubMed] [Google Scholar]
- 46.Ungrin M. D., Carrière M. C., Denis D., Lamontagne S., Sawyer N., Stocco R., Tremblay N., Metters K. M., Abramovitz M. (2001) Key structural features of prostaglandin E(2) and prostanoid analogs involved in binding and activation of the human EP(1) prostanoid receptor. Mol. Pharmacol. 59, 1446–1456 [DOI] [PubMed] [Google Scholar]
- 47.Main I. H., Whittle B. J. (1975) Potency and selectivity of methyl analogues of prostaglandin E2 on rat gastrointestinal function. Br. J. Pharmacol. 54, 309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller W. L., Sutton M. J. (1976) Relative biological activity of certain prostaglandins and their enantiomers. Prostaglandins 11, 77–84 [DOI] [PubMed] [Google Scholar]
- 49.Reina E., Ramos F. A., Castellanos L., Aragón M., Ospina L. F. (2013) Anti-inflammatory R-prostaglandins from Caribbean Colombian soft coral Plexaura homomalla. J. Pharm. Pharmacol. 65, 1643–1652 [DOI] [PubMed] [Google Scholar]
- 50. National Center for Biotechnology Information. (2018) PubChem Compound Database, U.S. National Library of Medicine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/5283094. Accessed February 13, 2018.
- 51.Giles H., Leff P. (1988) The biology and pharmacology of PGD2. Prostaglandins 35, 277–300 [DOI] [PubMed] [Google Scholar]
- 52.Vane J. R. (1971) Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 231, 232–235 [DOI] [PubMed] [Google Scholar]
- 53.Patrignani P., Filabozzi P., Patrono C. (1982) Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J. Clin. Invest. 69, 1366–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boutaud O., Sosa I. R., Amin T., Oram D., Adler D., Hwang H. S., Crews B. C., Milne G., Harris B. K., Hoeksema M., Knollmann B. C., Lammers P. E., Marnett L. J., Massion P. P., Oates J. A. (2016) Inhibition of the biosynthesis of prostaglandin E2 by low-dose aspirin: implications for adenocarcinoma metastasis. Cancer Prev. Res. (Phila.) 9, 855–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiang N., Fierro I. M., Gronert K., Serhan C. N. (2000) Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J. Exp. Med. 191, 1197–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]