Abstract
Diabetic foot ulcers (DFUs) are a major complication of diabetes, and there is a critical need to develop novel cell- and tissue-based therapies to treat these chronic wounds. Induced pluripotent stem cells (iPSCs) offer a replenishing source of allogeneic and autologous cell types that may be beneficial to improve DFU wound-healing outcomes. However, the biologic potential of iPSC-derived cells to treat DFUs has not, to our knowledge, been investigated. Toward that goal, we have performed detailed characterization of iPSC-derived fibroblasts from both diabetic and nondiabetic patients. Significantly, gene array and functional analyses reveal that iPSC-derived fibroblasts from both patients with and those without diabetes are more similar to each other than were the primary cells from which they were derived. iPSC-derived fibroblasts showed improved migratory properties in 2-dimensional culture. iPSC-derived fibroblasts from DFUs displayed a unique biochemical composition and morphology when grown as 3-dimensional (3D), self-assembled extracellular matrix tissues, which were distinct from tissues fabricated using the parental DFU fibroblasts from which they were reprogrammed. In vivo transplantation of 3D tissues with iPSC-derived fibroblasts showed they persisted in the wound and facilitated diabetic wound closure compared with primary DFU fibroblasts. Taken together, our findings support the potential application of these iPSC-derived fibroblasts and 3D tissues to improve wound healing.—Kashpur, O., Smith, A., Gerami-Naini, B., Maione, A. G., Calabrese, R., Tellechea, A., Theocharidis, G., Liang, L., Pastar, I., Tomic-Canic, M., Mooney, D., Veves, A., Garlick, J. A. Differentiation of diabetic foot ulcer–derived induced pluripotent stem cells reveals distinct cellular and tissue phenotypes.
Keywords: chronic wound healing, 3D in vitro skin tissue, extracellular matrix, migration, reprogramming
Diabetic foot ulcers (DFUs) represent a major complication of diabetes. DFUs are linked to cellular alterations that lead to impaired progenitor cell recruitment to the wound site (1–5), aberrant inflammatory cell infiltration (6, 7), diminished extracellular matrix (ECM) production by fibroblasts (8, 9), and compromised angiogenesis (10). Although our understanding of the pathophysiology of neuropathy and ischemia leading to DFUs has increased in recent years, existing therapies, such as growth factor treatment and nonintegrating bioactive dressings harboring naive fibroblasts, are not always successful (11). In light of that, there is a compelling need to develop new cell-based therapies to treat diabetic complications, such as DFUs.
During the past decade, a tremendous amount of attention has been directed toward the development of human induced pluripotent stem cells (iPSCs) as a potent, replenishing source of autologous and allogeneic cell and tissue types for regenerative therapies. For example, somatic cells have been reprogrammed to iPSCs and then differentiated into therapeutically relevant cells to treat Parkinson’s disease (12), amyotrophic lateral sclerosis (13, 14), liver damage (15), spinal cord injury (16), and hematopoietic disorders (17). In addition, autologous iPSC-derived cells are being evaluated in clinical trials for treatment of macular degeneration (18–20).
However, even though they hold great promise for these therapeutic applications, iPSC-derived cells have yet to be developed to treat recalcitrant DFUs. Although it is now possible to differentiate many cell types from iPSCs such as fibroblasts, keratinocytes, endothelial cells, neurons, and adipocytes (21–25), which are critical for various stages of DFU healing, the differentiated phenotype and biologic potency of iPSC-derived cells has not been exploited for repair of chronic wounds.
We have previously shown that iPSCs derived from foreskin fibroblasts trigger a repair-promoting phenotype, whereas others have shown that these cells can acquire an extended replicative potential (26, 27) and improved mitochondrial function (28) when compared with fibroblasts from which they were initially reprogrammed. Because reprogramming to iPSCs results in large-scale epigenetic remodeling, it may be a critical mechanism in the acquisition of improved biologic function in iPSC-derived fibroblasts (29). This is particularly significant for treating diabetic wounds because stable molecular changes in gene expression, which are induced by prolonged hyperglycemia, persist even after stabilization and normalization of blood sugar levels and thus may be regulated by epigenetic mechanisms (30). It is not known whether this “metabolic memory” in gene expression would persist in iPSCs reprogrammed from fibroblasts derived from patients with diabetes or from DFUs. Thus, it would be helpful to understand whether the impaired wound-repair functions found in DFU-derived fibroblasts (8, 9, 31) would be modified after reprogramming to iPSCs and subsequent differentiation to fibroblasts.
Tissue engineering approaches have not been optimally leveraged to analyze or screen functional outcomes of tissues harboring cells derived from iPSCs to glean biologically meaningful and predictive readouts on their potency. The use of bioengineered 3-dimensional (3D) tissue models would help predict whether the acquisition of differentiated, functional cell types from iPSCs provides improved cellular functions that would be well-suited for the clinical use of iPSC-derived cells for DFU therapy. To meet that growing need, we have developed in vitro 3D tissue models of human skin that mimic chronic wound characteristics to identify tissue and cell phenotypes of primary fibroblasts derived directly from patients with DFUs (8). These cellular and tissue-based readouts are useful in streamlining the preclinical screening of potential DFU treatments because they show organ-level functions, including cell and tissue phenotypes that are not detectable using conventional 2-dimensional (2D) monolayer cultures. These chronic wound-tissue models can be used to complement existing diabetic animal models that often fail to predict many human-specific properties of DFUs that occur in vivo.
We have recently reported the generation of iPSCs from primary, DFU-derived fibroblasts (32). In the current study, we have characterized the cellular and 3D tissue phenotypes associated with iPSC-derived fibroblasts that were initially reprogrammed from DFUs, by comparing them to site- and age-matched fibroblasts from patients with diabetes but no ulcers and from nondiabetic patients. We asked whether it is possible to modify cellular functions associated with wound repair in DFU-derived fibroblasts upon reprogramming and subsequent differentiation into iPSC-derived fibroblasts. Through the detailed, side-by-side comparison of iPSC-derived fibroblasts to the parental DFU fibroblasts (DFUFs) from which they were initially reprogrammed, we have identified important differences in gene expression profiles, ECM production, and other biologic properties of iPSCs that are known to be important for wound repair in 2D culture and 3D tissues. By characterizing these iPSC-derived fibroblasts, we have taken an important first step toward development of novel and more-effective cellular therapies for DFUs, which can deliver repair-competent fibroblasts to stimulate recalcitrant, nonhealing ulcers that do not respond to existing therapies. This may introduce novel treatments toward wound repair and significantly reduce long-term care for patients suffering from these conditions.
MATERIALS AND METHODS
Differentiation of iPSCs into fibroblasts
Differentiation of iPSCs into iPSC-derived fibroblasts was performed as previously described (33). Briefly, iPSC colonies, grown on Matrigel (354277; Corning, Corning, NY, USA), were dislodged with type IV collagenase (17104-019; Thermo Fisher Scientific, Waltham, MA, USA) and passaged onto mouse embryonic fibroblast feeder layer fixed with 4% paraformaldehyde. For the first 3 d, the cells were fed daily with WWE medium containing DMEM (11885; Thermo Fisher Scientific) and F12 (11765; Thermo Fisher Scientific) at a 3:1 ratio, supplemented with 5% Fetal Clone II (HyClone SH30066.03; GE Healthcare Life Sciences, Little Chalfont St. Giles, United Kingdom), 0.18 mM adenine (100190; MP Biomedicals, Santa Ana, CA, USA), 8 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, H-4034; MilliporeSigma, Burlington, MA, USA), 0.5 μg/ml hydrocortisone (H4881; MilliporeSigma), 10−10 M cholera toxin (0219032901; MP Biomedicals), 10 ng/ml epidermal growth factor (Austral Biologicals, San Ramon, CA, USA), 5 μg/ml insulin (407709; MilliporeSigma), and penicillin–streptomycin (15140; Thermo Fisher Scientific). For the next 4 d, cells were fed with WWE medium with freshly prepared 0.5 nM BMP-4 (314-BP; R&D Systems, Minneapolis, MN, USA). On d 7, cells were split onto fixed mouse embryonic fibroblast or Matrigel-coated plates, and for the next week, they were fed with serum-containing embryonic stem cell medium containing 1:1 DMEM:F12, 5% Fetal Clone II, and 1% nonessential amino acids (11140-050; Thermo Fisher Scientific). Next, cells were split 1:3 onto tissue culture plastic (∼1 × 105 cells/well of a 6-well plate) and grown for the next week in WWE medium. At d 21, the cells were split into type I collagen-coated plates (356450; Thermo Fisher Scientific) at a density of 5 × 105 cells/plate and fed with WWE medium. Starting at the next passage, cells were passage 0. The schematic of the differentiation protocol can be found in Fig. 1A and the derivation of the specific iPSC-derived fibroblast lines is shown in Supplemental Fig. S1A.
Figure 1.
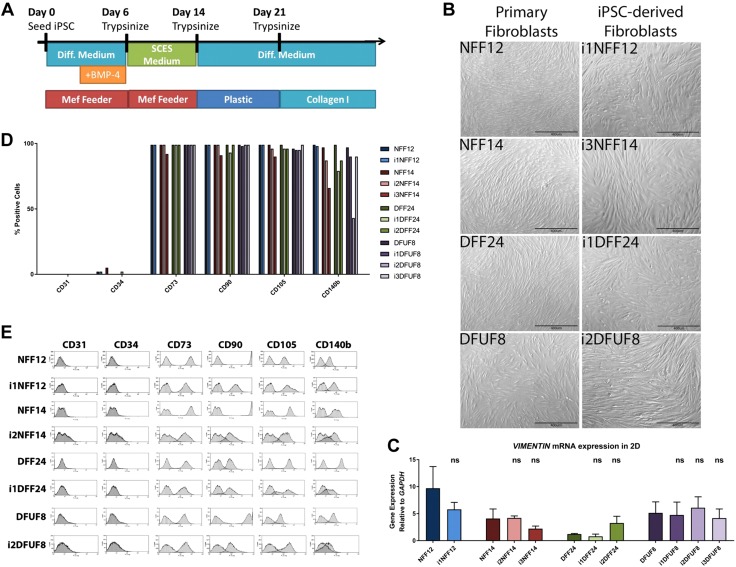
Nondiabetic and diabetic iPSCs were differentiated into iPSC-derived fibroblasts. A) Schematic of the differentiation (Diff.) protocol. MEF, mouse embryonic fibroblast. B) iPSC-derived fibroblasts show typical fibroblast morphology by microscopy. C) iPSC-derived fibroblasts express mesenchymal marker, vimentin. D) iPSC-derived fibroblasts are positive for mesenchymal markers CD73, CD90, CD105, and CD140b and are negative for endothelial markers CD31 and CD34. E) Flow cytometry profiles of the mesenchymal and endothelial markers.
Monolayer cell culture
Primary fibroblasts were grown on tissue culture plastic, whereas iPSC-derived fibroblasts were grown on tissue culture plastic, coated with rat-tail collagen type I (08-115; MilliporeSigma) at a density of 7 mg/cm2 in medium containing DMEM (11885; Thermo Fisher Scientific), 10% fetal bovine serum (HyClone SH30071.03; GE Healthcare), HEPES (H-4034; MilliporeSigma) and antibiotic–antimycotic (15240; Thermo Fisher Scientific).
Flow cytometry analysis
Cells from each primary and iPSC-derived fibroblast cell line (5 × 105) were used per analysis. Cells were trypsinized, counted, washed with PBS, and blocked with 2% heat-inactivated fetal bovine serum in PBS. The samples were then incubated with the following phycoerythrin-conjugated primary antibodies: anti-CD31 (ab27334; Abcam, Cambridge, United Kingdom), anti-CD34 (550619; BD Biosciences, San Jose, CA, USA), anti-CD73 (550257; BD Biosciences), anti-CD90 (555596; BD Biosciences), anti-CD105 (ab53321-100; Abcam), and anti-CD140b (558821; BD Biosciences). The data were collected with FACSCalibur (BD Biosciences) and analyzed with Summit software (v.4.3; Agilent Technologies).
RNA isolation
RNA was isolated from fibroblasts grown in 2D monolayer and 3D tissues using RNeasy Mini Kit (74104; Qiagen, Hilden, Germany), following manufacturer’s protocol.
Microarray analysis
For microarray analysis, quality of RNA was assessed with a Nanodrop spectrophotometer (Thermo Fisher Scientific) and a Bioanalyzer (Agilent Technologies). Samples with RNA integrity numbers of ≥9 were used. Microarray hybridization onto HumanHT-12 v4 BeadChip (Illumina, San Diego, CA, USA) containing 47,231 gene probes was performed in the Yale Center for Genome Analysis (Orange, CT, USA). Raw data was analyzed with R (R Foundation for Statistical Computing, Vienna, Austria)/Bioconductor (Seattle, WA, USA) and MicroarrayRUS software (34). Data were log transformed and quantile normalized. Differentially expressed genes were determined with limma (Bioconductor) (35) 1-way ANOVA with 1.5-fold change and false-discovery rate (FDR) of P < 0.5 as a cutoff value. Gene ontology analysis was performed with GOrilla (36, 37) and visualized with Revigo (38). Pathway enrichment was performed with Ingenuity Pathway Analysis (IPA; Qiagen). IPA core analysis was performed on differentially expressed genes after reprogramming, and comparison analysis was used to detect jointly regulated pathways and biologic processes in response to reprogramming. Enrichment P values were calculated using Fisher’s exact test with the Benjamini-Hochberg correction for multiple testing. Pathway regulation was determined via IPA-calculated absolute z score >2, which reflects a statistically significant pattern match of up- or down-regulated genes with their known regulatory effects in other biologic contexts (39).
Quantitative RT-PCR
RNA was reverse transcribed with the iScript cDNA Synthesis Kit (170-8891; Bio-Rad Laboratories, Hercules, CA, USA). For each PCR reaction, 0.5 μl of cDNA was used. PCR was performed with iQ SYBR Green (170-8880; Bio-Rad Laboratories) and the Bio-Rad instrument. Gene expression was quantified by the cycle threshold ΔΔCt method. Gene expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primer sequences can be found in Table 1.
TABLE 1.
Primers
| Item no. | Gene | Primer Sequence, 5′–3′ |
Amplicon size (bp) | |
|---|---|---|---|---|
| Forward | Reverse | |||
| 1 | GAPDH | TGCACCACCACCTGCTTAGC | GGCATGGACTGTGGTCATGAG | 87 |
| 2 | COL1A1 | GGACAGCGTGGTGTGGTCGG | CCTTGGCGCCAGGAGAACCG | 235 |
| 3 | COL1A2 | TACCTCCGCCGGTGACCCAG | GGTGGACCAGGAGGGCCTGT | 256 |
| 4 | COL4A1 | CCAGGATTTCAAGGTCCAAA | TCATTGCCTTGCACGTAGAG | 503 |
| 5 | FN1 | TGACCCCTACACAGTTTCCCA | TGATTCAGACATTCGTTCCCAC | 61 |
2D migration assay
The migration activity of primary and iPSC-derived fibroblasts was determined with the Radius 24-Well Assay Kit (CBA-125-col; Cell Biolabs, San Diego, CA, USA), consisting of a biocompatible gel in each well, according to the manufacturer’s instructions. Briefly, cells were seeded in the assay plates and cultured to reach confluency. The circular biocompatible gels were then removed, and the cell migration was recorded by photography immediately at 0 h and at every 4 h during the next 48 h of incubation. The rate of cell migration was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA) and expressed as a percentage of the area coverage by cells moving into a cell-free region.
WST-8 assay for proliferation quantification
Primary and iPSC-derived fibroblasts were grown in a monolayer, and the WST-8 Kit (CK04; Dojindo Laboratories, Kumamoto, Japan) was used to quantify cell proliferation rates according to manufacturer’s instructions. Briefly, WST-8 reagent was added to the cells and incubated for 2 h at 37°C. Next, reagent was transferred to the 96-well plate and read at 450 nm with a plate reader.
Formation of 3D in vitro self-assembled tissues
The 3D in vitro self-assembled (SA) tissue production was performed as previously described (8, 9, 40, 41). Briefly, 16,000 cells were seeded onto polyethylene terephthalate membranes with 1 μm pores (MCRP24H48; MilliporeSigma) in the WWE medium and were grown in the presence of 10 μg/ml ascorbic acid (A8960-5G; MilliporeSigma) for 5 wk.
Protein isolation and Western blotting
Primary and iPSC-derived fibroblasts grown as 3D tissues were lysed in sodium deoxycholate lysis buffer containing 4% sodium deoxycholate (D-6750; MilliporeSigma) in 20 mM Tris, pH 8.8, and 1% SDS with the addition of protease inhibitor cocktail (CLS8160; MilliporeSigma). Protein concentration was determined using a Bicinchoninic Acid Protein Assay Kit (23225; Thermo Fisher Scientific). Protein samples were resolved on 4–20% TGX Stain-Free Protein Gels (4568096; Bio-Rad Laboratories), under denaturing and reducing conditions, and transferred to a 0.2-μm nitrocellulose membrane (CAS9004-70-0; Bio-Rad Laboratories). The membranes were blocked with 5% milk in Tris-buffered saline and Tween 20 for 2 h at room temperature and incubated with primary antibodies overnight at 4°C. The primary antibodies were as follows: anti-GAPDH (ab9484; Abcam), anti–type I collagen (ab138492; Abcam), anti–type III collagen (ab7778; Abcam), anti-fibronectin (610077; BD Biosciences), anti-decorin (3B3; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA), anti–type VI collagen α-3 chain (sc-515335; Santa Cruz Biotechnology, Dallas, TX, USA), anti–type VI collagen α-2 chain (14853-1-AP; Proteintech Group, Rosemont, IL, USA), anti–type VI collagen α-1 chain (17023-1-AP; Proteintech Group). For type IV collagen, samples were run under native conditions, and the following antibodies were used: anti-GAPDH (ab125247; Abcam), anti–type IV collagen (M3F7; Developmental Studies Hybridoma Bank, University of Iowa). The membranes were washed with Tris-buffered saline and Tween 20 and incubated for 1 h at room temperature in respective secondary antibodies: goat anti-rabbit (111-035-046; Jackson ImmunoResearch Laboratories, West Grove, PA, USA), and goat anti-mouse (115-035-164; Jackson ImmunoResearch Laboratories). The membranes were developed using Clarity Western Enhanced Chemiluminescent Substrate (170-5060; Bio-Rad Laboratories).
Dimethylmethylene blue assay
The 3D tissues were digested with 0.5 mg/ml pronase (165921; F. Hoffmann-La Roche, Basel, Switzerland) at 37°C overnight. Amounts of sulfated glycosaminoglycans (GAGs) were analyzed by incubating samples with dimethylmethylene blue (DMMB) reagent prepared by dissolving 16 mg of 1,9-dimethylmethylene blue zinc chloride double salt (341088; MilliporeSigma) in 1 L of water containing 3.04 g glycine, 1.6 g sodium chloride, and 95 ml of 0.1 M acetic acid. The standard curve was prepared using chondroitin sulfate A sodium salt from bovine trachea (C9819; MilliporeSigma). Amounts of sulfated GAGs were normalized to DNA content determined using Quant-iT PicoGreen double-stranded DNA reagent (P7589; Thermo Fisher Scientific).
Analysis of hyaluronic acid
The 3D tissues were digested with 0.5 mg/ml pronase (165921; Hoffmann-La Roche) at 37°C overnight. Pronase-digested tissues were analyzed for hyaluronic acid (HA) content with an HA ELISA kit (029-001; Corgenix, Broomfield, CO, USA). HA amounts were normalized to DNA content as determined with Quant-iT PicoGreen double-stranded DNA reagent (P7589; Thermo Fisher Scientific).
Mass-spectrometry analysis
Protein lysate (10 µg) was prepared by lysing 3D in vitro SA tissue secreted by DFUF8 and i2DFUF8 in the sodium deoxycholate lysis buffer supplemented with protease inhibitor cocktail (CLS8160; MilliporeSigma). The samples were separated on the 7.5% gel (4561023; Bio-Rad Laboratories) under denaturing and reduced conditions, the bands were excised at the 120 kDa and sent out for mass spectrometry analysis at the Beth Israel Deaconess Medical Center Mass Spectrometry Core (Boston, MA, USA).
In vivo grafting of 3D in vitro SA tissues
Male nonobese-diabetic–severe combined immunodeficiency mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). To induce diabetes, 8-wk-old mice (n = 4/group) received 50 mg/kg streptozotocin (STZ) intraperitoneally daily for 5 d in saline. Fasting blood glucose levels were monitored 10 d post-STZ, and mice with levels >250 mg/dl were considered to have diabetes. After 8 wk of diabetes, the dorsum of the mice was shaved, depilated and decontaminated before wound induction under isoflurane anesthesia. A 6 mm biopsy punch was used to create 2 cutaneous wounds on each mouse. 3D tissues were placed onto the wound beds and then bandaged to be held in place. Mice were monitored daily, and measurements of the diameter of the mouse wounds were performed at d 0 and 10. On d 10 the mice were euthanized, and the wound area was excised for histologic analysis.
Hematoxylin and eosin staining and anti–type I collagen staining
Mouse tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 7 μm thickness, and stained with hematoxylin and eosin (H&E) to assess tissue morphology. For immunohistochemistry, antigen retrieval was performed by steaming tissue sections in citrate buffer for 15 min and immunostaining was performed with antibodies against human type I collagen (ab138492; Abcam). Staining localization was detected with 3,3′-diaminobenzidine (sk-4100; Vector Laboratories, Burlingame, CA, USA), and tissues were counterstained with hematoxylin (MilliporeSigma).
RESULTS
iPSC-derived cells from nondiabetic and diabetic patients display a fibroblast phenotype
We characterized the cellular features of iPSC-derived fibroblasts by comparing them with age- and location-matched fibroblasts of nondiabetic and diabetic origin from which they were initially reprogrammed. Primary fibroblasts were isolated from the foot skin of patients without diabetes [nondiabetic foot fibroblasts (NFFs)], foot skin of patients with type 2 diabetes [diabetic foot fibroblasts (DFFs)], and DFUFs from patients with type 2 diabetes (8, 9, 31) and were reprogrammed into iPSCs that met all criteria of pluripotency (32). Using a differentiation protocol that we previously developed (Fig. 1A) for human embryonic stem cells and iPSCs derived from foreskin fibroblasts (33), 4 iPSC lines (iPSC-NFF12, iPSC-NFF14, iPSC-DFF24, and iPSC-DFUF8) were differentiated into multiple iPSC-derived fibroblast lines as follows: iPSC-NFF12 was differentiated into 1 fibroblast line (i1NFF12), iPSC-NFF14 was differentiated into 2 fibroblast lines (i2NFF14 and i3NFF14), iPSC-DFF24 was also differentiated into 2 fibroblast lines (i1DFF24 and i2DFF24), and iPSC-DFUF8 was differentiated into 3 fibroblast lines (i1DFUF8, i2DFUF8, and i3DFUF8) (Supplemental Fig. S1A).
All iPSC-derived fibroblasts showed typical fibroblast morphology (Fig. 1B) and expressed the mesenchymal marker vimentin (Fig. 1C), indicating that all iPSC-derived cells were directed toward a mesenchymal cell fate. We then performed flow cytometry to further evaluate the lineage of those iPSC-derived cells. We found that those iPSC-derived cell lines did not express the endothelial or hematopoietic markers CD31 or CD34 (Fig. 1D). Mesenchymal markers, such as CD73, CD90, CD105, and CD140b, were detected and were stable over the course of multiple passages (Fig. 1D, E). Taken together, these findings reveal that stable fibroblasts could be differentiated from iPSCs of both nondiabetic and diabetic origin, including those from DFUs.
Comparison of global gene expression between iPSC-derived fibroblasts and those derived from patients with DFUs reveals differences in genes involved in wound healing
We used microarray analysis to analyze the full complement of genes differentially regulated in iPSC-derived fibroblasts. Gene expression profiles were compared between primary patient-derived fibroblast lines from 3 patient types (NFF12 and NFF14, DFF24, and DFUF8) and corresponding iPSC-derived fibroblast lines (i1NFF12, i2NFF14, and i3NFF14; i1DFF24 and i2DFF24; and i1DFUF8, i2DFUF8, and i3DFUF8). These cells were grown in 2D monolayer culture to determine whether reprogramming to iPSCs and subsequent differentiation into this fibroblast lineage fate altered patterns of gene expression.
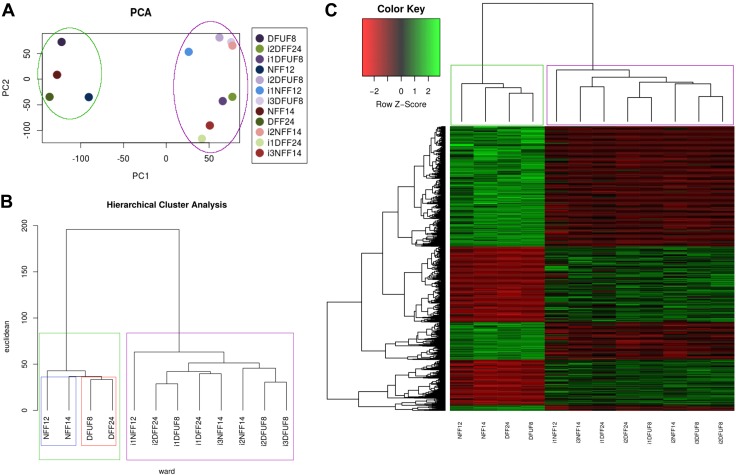
Principal component analysis was performed to assess overall separation of the cell lines based on the global gene expression. Principle components 1 and 2 together accounted for 25% of the variance, with 10 principle components being needed to explain 93% of the variance, which suggests that there are multiple different sources of the variance that cannot be explained by the first 2 principle components (Supplemental Fig. S1B). At the same time, plotting against principle components 1 and 2 visualized all the cell lines based on the gene expression data and showed 2 major groups: one containing primary fibroblasts (circled in green) and another containing iPSC-derived fibroblasts (circled in purple) (Fig. 2A). This suggests that iPSC-derived cells acquired unique properties when compared with primary fibroblast cell lines.
Figure 2.
Global gene expression analysis reveals that both nondiabetic and diabetic iPSC-derived fibroblasts differ in gene expression profile from primary fibroblasts. A) Principal component analysis clusters primary and iPSC-derived fibroblasts separately. B) Hierarchical clustering shows 2 distinct clusters of gene expression: primary and iPSC-derived fibroblasts. C) Heat map showing 1304 DEGs between primary and iPSC-derived fibroblasts.
In parallel, another unsupervised method of data analysis, hierarchical clustering, was used to further analyze the global gene expression data. Hierarchical clustering was performed with Ward’s method, and Euclidean distances were used as a measure of distance between clusters. This analysis revealed 2 main clusters: one containing primary fibroblasts (boxed in green) and the second containing iPSC-derived fibroblasts (boxed in purple) (Fig. 2B). Although hierarchical cluster analysis showed separation of nondiabetic primary fibroblasts (blue) from diabetic primary fibroblasts (red), there were no distinct clusters denoting nondiabetic when compared with diabetic iPSC-derived cell lines. This lack of segregation of iPSC-derived fibroblasts based on their cell of origin (NFF vs. DFUF and DFF), suggests that iPSC reprogramming and differentiation resulted in a unique gene expression signature in those fibroblasts.
Because both principle cluster analysis and hierarchical cluster analysis showed that the differences in gene expression between primary and iPSC-derived fibroblasts were larger than the differences between diabetic (DFUF and DFF) and nondiabetic (NFF) cells, we further investigated the genes that were differently expressed between primary and corresponding iPSC-derived fibroblasts. Linear models for microarray data analysis identified 1304 differentially expressed genes (DEGs) between primary and iPSC-derived fibroblasts based on the threshold values of 1.5-fold change and false discover rate (FDR)- adjusted P value <0.05 (Fig. 2C and Supplemental Table S1 and Table 2). These DEGs represent the genes whose expression was changed when primary fibroblasts were reprogrammed and differentiated to iPSC-derived fibroblasts. To evaluate phenotypic significance of these DEGs and how changes in gene expression possibly contribute to wound healing, we performed gene ontology (GO) analysis to identify statistically overrepresented groups of genes in 3 categories: biologic process, molecular function, and cellular component. In the “molecular function” category, GO analysis showed that 1304 DEGs that differed between primary and iPSC-derived fibroblasts were involved in regulation of cell migration, cell proliferation, ECM organization, response to endogenous stimulus, developmental process, and cell adhesion (Supplemental Fig. S1C and Supplemental Table S2). This indicates that reprogramming to iPSCs and subsequent differentiation to fibroblasts changed expression of the relevant for wound healing genes in iPSC-derived fibroblasts, regardless of the primary cell diabetic background.
TABLE 2.
Top 20 differentially expressed genes between primary and iPSC-derived fibroblasts
| Item no. | Probe ID | Symbol | Sequence reference | iPSC-derived mean | Primary mean | FC | FDRP |
|---|---|---|---|---|---|---|---|
| 1 | ILMN_1795325 | ACTG2 | NM_001615.3 | 12.22 | 6.36 | 58.03 | 0.000366205 |
| 2 | ILMN_1694778 | LOC646723 | XR_017241.1 | 12.44 | 7.26 | 36.22 | 1.25E−06 |
| 3 | ILMN_1750324 | IGFBP5 | NM_000599.2 | 12.61 | 7.84 | 27.38 | 0.000357686 |
| 4 | ILMN_2132982 | IGFBP5 | NM_000599.2 | 12.36 | 7.74 | 24.65 | 0.000574478 |
| 5 | ILMN_1671971 | LOC644743 | XR_016703.1 | 10.91 | 6.41 | 22.59 | 1.45E−05 |
| 6 | ILMN_2041222 | FLJ40504 | NM_173624.1 | 10.98 | 6.51 | 22.21 | 4.78E−06 |
| 7 | ILMN_1753584 | KRT8 | NM_002273.2 | 10.58 | 6.46 | 17.48 | 2.27E−05 |
| 8 | ILMN_3263974 | KRT18P13 | XM_001726959.1 | 10.24 | 6.41 | 14.19 | 3.13E−05 |
| 9 | ILMN_1759097 | MLLT11 | NM_006818.3 | 10.83 | 7.02 | 14.07 | 4.29E−09 |
| 10 | ILMN_2067656 | CCND2 | NM_001759.2 | 10.36 | 6.60 | 13.53 | 0.000202056 |
| 11 | ILMN_1677636 | COMP | NM_000095.2 | 6.69 | 10.28 | −12.09 | 0.001284741 |
| 12 | ILMN_1813704 | KIAA1199 | NM_018689.1 | 7.97 | 11.70 | −13.26 | 0.000350598 |
| 13 | ILMN_2388800 | PPAP2B | NM_003713.3 | 7.21 | 11.03 | −14.08 | 3.41E−06 |
| 14 | ILMN_1681780 | MKX | NM_173576.1 | 6.78 | 10.67 | −14.87 | 6.75E−07 |
| 15 | ILMN_1680110 | C10orf116 | NM_006829.2 | 6.95 | 10.87 | −15.10 | 7.79E−06 |
| 16 | ILMN_1790338 | PRRX2 | NM_016307.3 | 6.45 | 11.42 | −31.38 | 1.58E−08 |
| 17 | ILMN_1768227 | DCN | NM_133503.2 | 6.82 | 11.81 | −31.93 | 5.95E−07 |
| 18 | ILMN_1684306 | S100A4 | NM_019554.2 | 7.02 | 12.37 | −40.93 | 9.74E−06 |
| 19 | ILMN_1688780 | S100A4 | NM_019554.2 | 7.21 | 12.67 | −43.99 | 4.67E−05 |
| 20 | ILMN_1784459 | MMP3 | NM_002422.3 | 6.60 | 12.64 | −65.41 | 7.65E−07 |
iPSC-derived fibroblasts acquired migration and proliferation phenotypes distinct from parent cells
Migratory and proliferative properties of dermal fibroblasts are important for cutaneous wound healing and are impaired in chronic wounds (8, 31, 42–44). Expression of genes comparing DFU-derived, primary, and iPSC-derived fibroblasts were found to differentially express genes that regulate cell migration and cell proliferation (Supplemental Fig. S1C). This was also confirmed by IPA, which showed many migration-related genes being induced after reprogramming in i1DFF24, i2DFF24, and i2DFUF8 (Supplemental Fig. S1D) and uniquely in i2DFUF8 (Supplemental Fig. S1E). Thus, we next performed functional studies in a monolayer, 2D culture to characterize migratory and proliferative capacity of iPSC-derived fibroblasts compared with that primary cell lines from which they were derived.
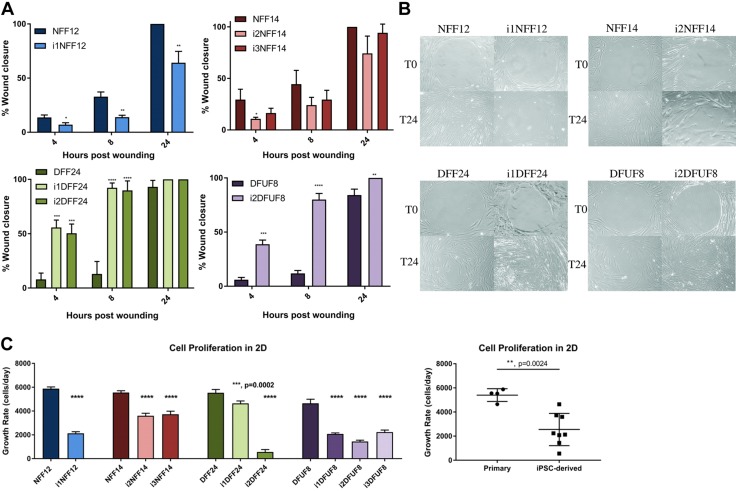
In a 2D gap-closure cell-migration assay, i1NFF12 and i2NFF14 and i3NFF14 migrated slower than their primary counterparts NFF12 and NFF14, respectively (Fig. 3A, B). In contrast, i1DFF24 and i2DFF24 and i2DFUF8 migrated faster than their primary cell counterparts, DFF24 and DFUF8, respectively (Fig. 3A, B). Overall, DFF24 and DFUF8 migrated slower than NFF12 and NFF14, which supported our earlier data that primary DFUFs and DFFs have impaired motility in the scratch-wound assay (8, 31). All tested iPS cell lines, except i2NFF14 and i1NFF12, migrated faster than DFUF8 and DFF24, which suggests that DFU-derived, iPSC-derived fibroblasts have improved migration properties.
Figure 3.
iPSC-derived fibroblasts have improved migratory functions. A) The percentage of wound closure in the 2D migration assay. i1NFF12 was compared with NFF12 using t test, all other iPSC-derived cell lines were compared with its parent primary fibroblast cell line using 1-way ANOVA with Tukey’s correction for multiple comparisons. B) Representative images of the 2D migration assay. C) Quantification of fibroblast proliferation using the WST-8 assay. Each iPSC-derived cell line was compared with its parent primary fibroblast cell line using 1-way ANOVA with Tukey’s correction for multiple comparisons; n = 3 for each cell line. Error bars represent sd. Primary and iPSC-derived fibroblasts were compared using Student’s t tests. P > 0.05 (not significant), *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
Migration was not affected by proliferation because iPSC-derived fibroblasts proliferated less than the primary fibroblasts from which they were derived (Fig. 3C). In addition, DFUF8 showed decreased proliferation rate compared with NFF12, NFF14, and DFF24, whereas i1DFF24 proliferated at the same rate as DFUF8. All other iPSC-derived lines showed decreased proliferation compared with DFUF8.
Together, these findings indicate that reprogramming and differentiation improved migratory, but not proliferative, properties of both DFF- and DFU-derived primary fibroblasts.
iPSC-derived fibroblasts express and deposit unique ECM proteins in the 3D in vitro SA tissue model, regardless of the source of primary fibroblasts
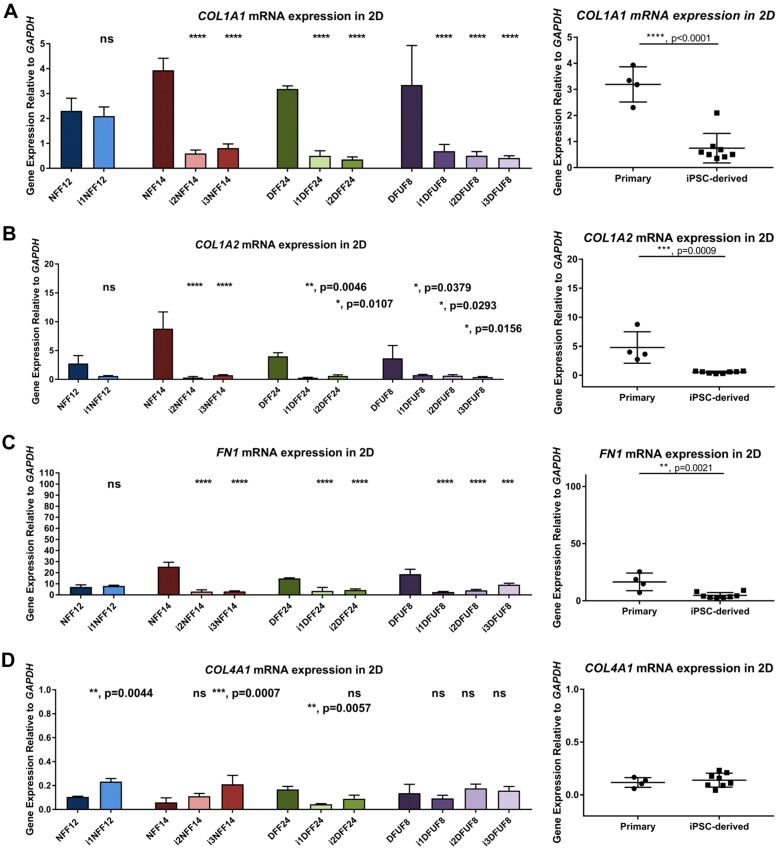
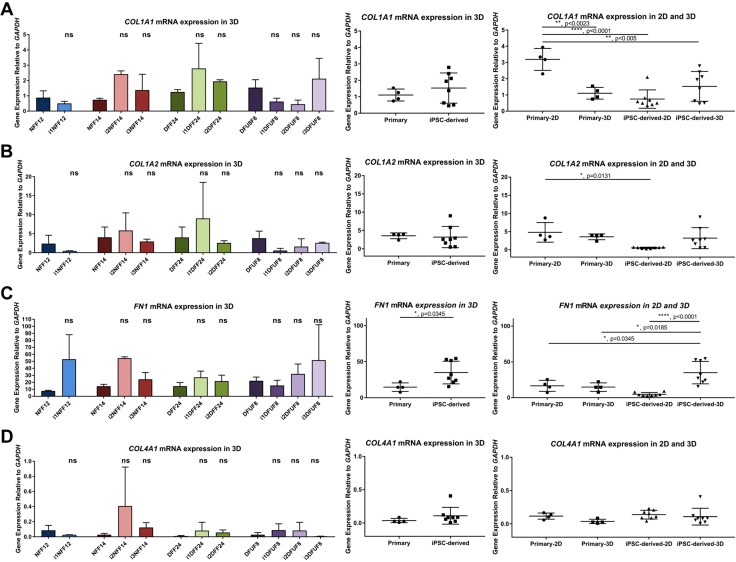
We confirmed microarray data with quantitative PCR analysis of the expression of multiple DEGs that belong to GO term “extracellular matrix organization” by comparing primary and iPSC-derived fibroblasts grown in monolayer, 2D cultures. All iPSC-derived fibroblasts, except i1NFF12, showed decreased expression of the genes coding for the ECM proteins that are typically seen in the healthy dermis, type I collagen pro-α 1(I) chain (COL1A1), type I collagen pro-α 2(I) chain (COL1A2), and fibronectin 1 (FN1) (Fig. 4A–C). In contrast, 3 iPSC-derived fibroblast cell lines (i1NFF12, i3NFF14, and i1DFF24) were characterized by elevated COL4A1 gene expression, which is usually limited to basement membranes in human skin (45–47) (Fig. 4D). Together, these findings suggested a shift in the pattern of ECM gene expression from that typically seen in the dermis before reprogramming to an atypical pattern of ECM expression in fibroblasts after reprogramming and differentiation. We further focused on the ECM properties of iPSC-derived fibroblasts because of the observed shift in the ECM gene expression and because of the importance of ECM for fibroblast function in wound healing.
Figure 4.
Gene expression of iPSC-derived fibroblasts grown in 2D monolayer culture shows profiles distinct from primary fibroblasts. COL1A1 (A), COL1A2 (B), FN1 (C), and COL4A1 (D) in iPSC-derived fibroblasts grown in 2D monolayer culture are expressed at different levels compared with primary fibroblasts. Each iPSC-derived cell line was compared with its parent primary fibroblast cell line using 1-way ANOVA with Tukey’s correction for multiple comparisons; n = 3 for each cell line. Error bars represent sd. Primary and iPSC-derived fibroblasts were compared using Student’s t tests. P > 0.05 (not significant), *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
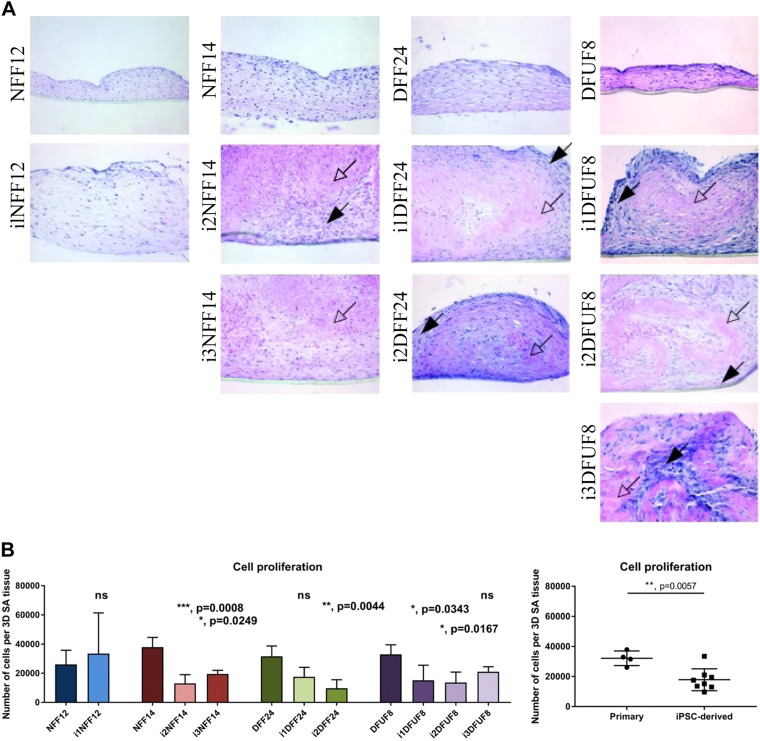
We used a 3D approach to study whether the shift in ECM gene expression observed in iPSC-derived fibroblasts, compared with their primary counterparts, had an effect on the functional properties of those cells. We, therefore, constructed 3D SA tissues to compare the organization and composition of the ECMs generated from primary NFF fibroblasts to iPSC-derived fibroblasts and to compare primary and diabetic fibroblasts (DFUFs and DFFs) to iPSC-derived fibroblasts. Primary fibroblasts generated a fibrillar ECM that showed well-aligned and organized fibers and contained many fibroblasts (Fig. 5A). In contrast, iPSC-derived fibroblasts deposited a thicker ECM tissue (Fig. 5A) with a distinct morphologic appearance that organized into “spheroids” (Supplemental Fig. S2A, black arrows). All iPSC-derived cell lines, including those reprogrammed from NFFs (i1NFF12, i2NFF14, and i3NFF14), and from DFUFs and DFFs (i1DFF24, i2DFF24, i1DFUF8, i2DFUF8, and i3DFUF8) demonstrated a thin peripheral rim consisting of fibrillar, cellular ECM (Fig. 5A, black arrows) with a large central region consisting of nonfibrillar, acellular ECM that appeared homogeneous (Fig. 5A, white arrows).
Figure 5.
The 3D in vitro SA tissues formed by iPSC-derived fibroblasts show unique tissue appearance. A) Representative images of H&E staining of 3D in vitro SA tissues produced by primary and iPSC-derived fibroblasts. B) Quantification of fibroblast proliferation in 3D SA tissues using WST-8 assay. Each iPSC-derived cell line was compared with its parent primary fibroblast cell line using 1-way ANOVA with Tukey’s correction for multiple comparisons. n = 6 for each cell line. Error bars represent sd. Primary and iPSC-derived fibroblasts were compared using Student’s t tests. P > 0.05 (not significant), *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
iPSC-derived 3D SA tissues had fewer cells compared with primary fibroblasts, which was also observed in 2D monolayer cultures (Fig. 5B). This indicates that iPSC-derived cells have slower proliferation rate in both 2D and 3D and that acellular matrix possibly has additional inhibitory effect on the iPSC-derived fibroblasts’ survival and proliferation. Importantly, differences in SA tissue appearance, previously seen between SA tissues fabricated from primary NFFs and primary DFUFs and DFFs (8), were not observed in iPSC-derived fibroblasts reprogrammed from those 2 cell types. Instead, those 2 cell types were similar.
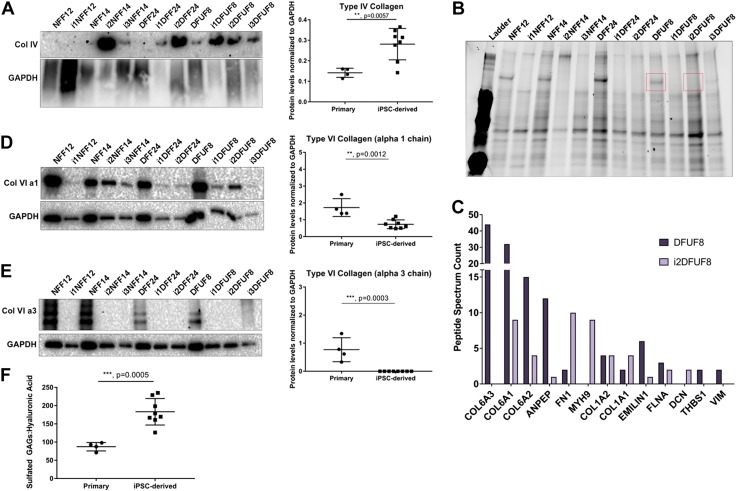
In the 3D in vitro SA tissue model, iPSC-derived fibroblasts showed expression of COL1A1, COL1A2, and COL4A1 similar to levels expressed in primary fibroblasts of all patient origins (Fig. 6A, B, D). This suggests that the environment in 3D tissues regulated expression of these genes shifting iPSC-derived fibroblasts to behave more like primary fibroblasts. Expression of FN1 was greater in iPSC-derived fibroblasts in 3D models (Fig. 6C). Western blot analysis did not demonstrate significant differences in levels of type I or type III collagen (Supplemental Fig. S2B, C) or fibronectin (Supplemental Fig. S2E) between primary and iPSC-derived fibroblasts in 3D SA tissues. In addition, there was no significant difference in the ratio of type I to type III collagen in primary and iPSC-derived fibroblasts (Supplemental Fig. S2D). Type IV collagen was highly enriched in the 3D SA tissues deposited by iPSC-derived fibroblasts when compared with primary cells (Fig. 7A). Taken together, these findings demonstrate that 3D SA tissues harboring iPSC-derived fibroblasts generated tissues with ECM proteins that were similar to each other regardless of the origin of the primary cell type reprogrammed (i.e., nondiabetic and diabetic). This supports our microarray data that iPS cells are more similar to each other than they are to the parental cells from which they were derived. This suggests that the process of reprogramming itself, and not the diabetic nature of the reprogrammed fibroblast, was dominant in determining the tissue phenotype when iPSC-derived fibroblasts were grown as 3D SA tissues.
Figure 6.
Gene expression of iPSC-derived fibroblasts in 3D in vitro SA tissues. COL1A1 (A), COL1A2 (B), FN1 (C), and COL4A1 (D) expression in iPSC-derived fibroblasts in 3D in vitro SA tissues. Each iPSC-derived cell line was compared with its parent primary fibroblast cell line using 1-way ANOVA with Tukey’s correction for multiple comparisons; n = 2 for each cell line. Error bars represent sd. Primary and iPSC-derived fibroblasts were compared using Student’s t tests. Primary and iPSC-derived fibroblasts in both 2D and 3D tissues were compared using 1-way ANOVA with Tukey’s correction for multiple comparisons. P > 0.05 (not significant), *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
Figure 7.
In a 3D in vitro SA tissue model, iPSC-derived fibroblasts deposit ECMs that are distinct from primary fibroblasts. A) iPSC-derived fibroblasts are enriched in type IV collagen. B) Protein gel showing distinct band (red) of around 120 kDa present in primary and absent in iPSC-derived fibroblasts that was excised and analyzed using mass spectrometry analysis. C) Peptide spectrum counts of the identified proteins from the bands shown in B. D) Type VI collagen α-1 chain is down-regulated in iPSC-derived fibroblasts. E) Type VI collagen α-3 chain was not detected in iPSC-derived fibroblasts. F) Sulfated GAGs to HA ratio is higher in iPSC-derived fibroblasts compared with primary fibroblasts. Primary and iPSC-derived fibroblasts were compared using Student’s t tests; n = 2–4 of biologic replicates per each cell line. P > 0.05 (not significant), *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
To further characterize the composition of these iPSC-derived SA tissues and the phenotypic convergence between all types of iPSC-derived cells that was seen, we performed mass spectrometry analysis to acquire a more-detailed understanding of their protein composition. Analysis of the 3D SA tissue extracts by polyacrylamide protein gel revealed the loss of a protein band near 120 kDa in i2DFUF8 iPSC-derived cells when compared with SA tissue extracts from primary, DFUF8 patient-derived fibroblasts (Fig. 7B). These 2 regions from polyacrylamide protein gels were excised from the gel for DFUF8 and i2DFUF8 samples and analyzed using mass spectrometry. Mass spectrometry spectral counts showed that iPSC-derived, i2DFUF8 had no detectable type VI collagen α-3 chains and had lower levels of type VI collagen α-1 and -2 chains. In contrast, primary cells (DFUF8) showed higher levels of 3 type VI collagen chains (Fig. 7C). This suggests loss of assembly and function of the dermal collagen type VI in iPSC-derived SA tissues. To confirm these mass spectrometry results in all cell lines, we then performed Western blot analysis on all iPSC-derived fibroblasts reprogrammed from diabetic and nondiabetic primary cell types (i1NFF12, i2NFF14, i3NFF14, i1DFF24, i2DFF24, i1DFUF8, i2DFUF8, and i3DFUF8). We found that iPSC-derived fibroblasts from both nondiabetic and diabetic backgrounds had reduced levels of type VI collagen α-1 and -3 chains (Fig. 7D, E), whereas type VI collagen α-2 chain remained unchanged (Supplemental Fig. S2F). This supports the “phenotypic convergence” seen in other ECM components of the SA tissue morphology, which also showed ECM components that were similar to each other, regardless of the origin of those primary cell types (i.e., nondiabetic and diabetic). Taken together, these results demonstrate that the repertoire of dermal ECM proteins produced by SA tissues harboring iPSC-derived fibroblasts was distinct from that produced by primary, DFU-derived fibroblasts. The presence of molecular constituents not commonly seen in mature dermal ECM demonstrates that the process of reprogramming and subsequent differentiation of iPSCs to fibroblasts can generate unique tissues with immature ECM phenotypes.
3D in vitro SA tissues from iPSC-derived fibroblasts are enriched in sulfated GAGs
To further characterize the composition of the immature ECM phenotypes observed in 3D in vitro SA tissues constructed with iPSC-derived fibroblasts, we next analyzed tissues for the presence of GAGs. These glycoproteins are involved in ECM assembly and growth factor signaling relevant to tissue repair and regeneration (48–50). We performed a DMMB assay to measure total sulfated GAGs, and an ELISA assay was used to measure the nonsulfated GAGs and HA. iPSC-derived fibroblasts had a higher ratio of sulfated GAGs to HA (Fig. 7F), suggesting that there is a shift in ECM composition in iPSC-derived cells that might affect growth factor signaling. Decorin is a proteoglycan that serves as a core protein for such sulfated GAGs as chondroitin sulfate and dermatan sulfate, and which is needed for collagen fibrillogenesis (49) and, through inhibition of TGF-β1, is associated with decreased fibrosis (51). i2DFUF8 3D tissues showed increased levels of decorin compared with DFUF8 by mass spectroscopy (Fig. 7C), but Western blot showed that decorin was expressed at similar levels in primary and iPSC-derived fibroblasts from both nondiabetic and diabetic backgrounds, suggesting that increased decorin is not responsible for increased sulfated GAGs levels (Supplemental Fig. S2G).
Taken together, the unique 3D ECM tissues composition derived from iPSC-derived fibroblasts suggests that delivery of the patient specific 3D in vitro SA iPSC-derived tissues into the chronic wound could serve as a source of therapy.
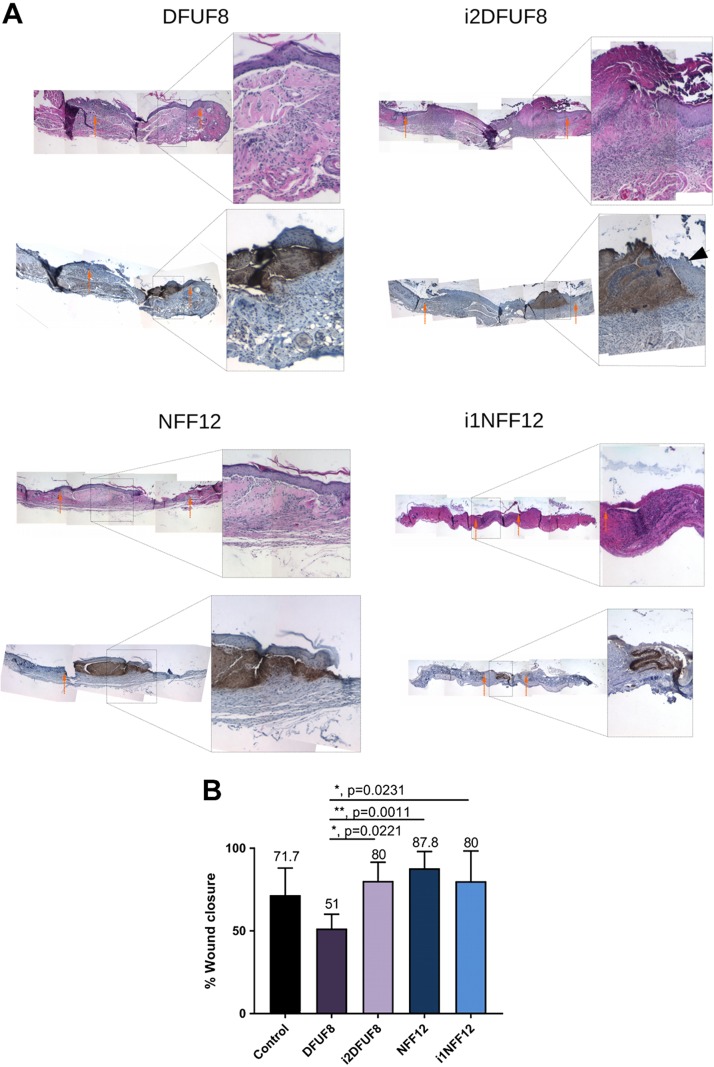
In vivo grafting of 3D SA tissues from iPSC-derived fibroblasts improves reepithelization in a diabetic mouse model
The composition and structure of the iPSC-derived SA tissues, which did not demonstrate a fibrillar structure, suggested they may have atypical tissue function. To explore that tissue function, we next implanted 3D in vitro SA tissues constructed with either primary or iPSC-derived fibroblasts from nondiabetic and diabetic origin (NFF12, i1NFF12, DFUF8, and i2DFUF8) into a mouse model of tissue transplantation. SA tissues were grown for 5 wk in vitro and were transplanted into 6-mm full-thickness wounds created on the back of STZ-induced diabetic mice. Grafted mice were monitored daily, and grafting caused no untoward sequelae. Staining of excised tissues with anti–type I collagen, which binds specifically to human type I collagen, showed that all transplanted 3D SA tissues, regardless of being primary or iPSC-derived cells, persisted intact for 10 d after transplantation (Fig. 8A). H&E staining of those tissues showed that tissues had integrated into the mouse connective tissue and remained intact (Fig. 8A). Both staining for type I collagen distribution and H&E stain showed that 3D SA tissues preserved their morphologic organization and structure after in vivo transplantation. Grafted SA tissues appeared beneath a surface epithelium, indicating that their tissue integrity was maintained during healing. In addition, this suggests that iPSC-derived SA tissues were an effective surface substrate that supported reepithelialization after full-thickness wounding. In i2DFUF8, epithelium, which was migrating over 3D SA tissues, appeared to be more hyperproliferative in comparison with i1NFF12 (Fig. 8A, black arrow). Grafting of the tissues derived from primary, healthy foot fibroblasts (NFF12) significantly improved wound healing in diabetic mice compared with primary DFUFs (DFUF8) (Fig. 8B). Improved healing was also seen in iPSC-derived fibroblasts from both nondiabetic and diabetic backgrounds (i1NFF12 and i2DFUF8) when compared with DFUF8 (Fig. 8B). This demonstrates that DFUF8 results in impaired wound healing, as previously shown in vitro, using 3D wound-healing models (8). Our findings that both iPSC-derived fibroblast types from DFUF and NFF origins (i2DFUF8 and i1NFF12) supported wound healing, whereas primary fibroblasts showed differences between DFUFs and NFFs, further illustrate the phenotypic convergence of fibroblasts after reprogramming into iPSCs and differentiation. The persistence of SA tissues in those wound models suggests their capacity to deliver iPSC-derived cells or iPSC-derived ECM proteins to nonhealing wounds in the future. Taken together, these findings extend the observations in 3D tissues to an in vivo diabetic wound environment.
Figure 8.
In vivo grafting of the 3D SA tissues. A) H&E and anti-human type I collagen staining showing localization of the grafted 3D in vitro SA tissues. Orange arrows indicate wound margins. B) Wound closure in mice with grafting of 3D in vitro SA tissues. All groups were compared using 1-way ANOVA with Tukey’s multiple comparisons test; n = 5–11 wounds. Error bars represent sd. P > 0.05 (not significant), *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
DISCUSSION
iPSCs offer a replenishing source of allogeneic or patient-specific cell types that hold promise to treat many diseases (52). The potential of iPSC-derived cells from DFUs has not, to our knowledge, been exploited to treat chronic wounds.
In the present study, we show that iPSC-derived fibroblasts from DFUs exhibited gene signatures and functional properties that were distinct from the primary DFUFs from which they were initially derived. Genes that we have previously shown to be differentially expressed between primary nondiabetic and diabetic fibroblasts (9, 31) were found to be similarly expressed in iPSC-derived fibroblasts reprogrammed from primary nondiabetic and diabetic fibroblasts. Global gene expression analysis showed that iPSC-derived fibroblasts from both nondiabetic and diabetic backgrounds were more similar to each other than they were to the primary cells from which they were derived. Although we recognize the limitation in analysis of single clones, we interpret this as a process of “phenotypic convergence,” through which, cell types become more similar after reprogramming and differentiation.
This “convergence” may possibly be linked to epigenetic alterations that occur upon iPSC reprogramming. Although it has been previously shown that iPSCs and iPSC-derived cells from patients with genetic insulin-resistance model in vivo defects observed in patients with type 2 diabetes (53, 54), it is known that epigenetic mechanisms occurring during reprogramming can remove epigenetic marks associated with disease phenotypes (55). Our findings are supported by recent studies showing a similar “phenotypic convergence” in iPSC-derived dopaminergic neurons reprogrammed from sporadic Parkinson’s disease (56) and iPSC-derived fibroblasts reprogrammed from scleroderma (57).
It is possible that epigenetic “metabolic memory” can be modified following reprogramming and differentiation to improve cellular repair functions. We have previously shown that demethylation of specific promoter regions improves cellular-repair phenotypes upon differentiation of foreskin fibroblast-derived iPSCs to fibroblasts (58). Our current findings suggest that changes of gene expression in diabetic compared with nondiabetic fibroblasts may be, in future studies, linked to epigenetic alterations. Future in-depth epigenetic analysis of iPSC-derived fibroblasts from DFUs is likely to enhance our understanding of DFU pathogenesis, by identifying repair functions that are linked to DNA methylation, histone modifications, and miRNAs after reprogramming. This will shift viewing epigenetic, “metabolic memory” as a hurdle, thus allowing us to exploit it for clinical benefit that would shift the way we treat DFUs.
“Phenotypic convergence” was also observed in the 3D in vitro SA tissue model, in which iPSC-derived fibroblasts from both nondiabetic and diabetic patients showed similar tissue structure and organization. Beyond that, tissues constructed with both nondiabetic and diabetic iPSC-derived fibroblasts displayed a shift in expression of genes coding for ECM proteins that were distinct in tissues derived from primary cells, which further supports persistence of phenotypic convergence in 3D tissues. The 3D tissues constructed with both nondiabetic and diabetic, iPSC-derived fibroblasts showed novel ECM composition that included elevated type IV collagen and the absence of type VI collagen. The unusual appearance of the iPSC-derived ECM in SA tissues may be attributed, in part, to the elevation of type IV collagen, which is typically restricted in its deposition to a basement membrane (45–47). There was a significant down-regulation of type VI collagen α-1 chain in combination with the absence of type VI collagen α-3 in the ECM of iPSC-derived fibroblasts. A reduction of type VI collagen was previously shown to lead to greater interfibrillar spacing in ECM secreted by fibroblasts and to stimulate fibroblast motility (59). Thus, absence of type VI collagen in iPSC-derived 3D tissues further indicates the unusual composition and organization of their ECM that may be related to a range of biologic functions that remain to be determined. The 3D tissues from both nondiabetic and diabetic, iPSC-derived fibroblasts also showed an increased ratio of sulfated GAGs to HA, which may be related to structural properties and growth factor activity seen in these tissues (48–50). The unusual morphologic and biochemical features seen in 3D tissues suggest that they can serve as a useful platform technology to determine the properties of iPSC-derived cells in tissues with complex, biologically meaningful structure and organization.
Here, we showed that 3D tissues fabricated from iPSC-derived cells reprogrammed from DFUs organize into an ECM that can be delivered to and persist in a cutaneous diabetic wound. This suggests that it may serve as cellularized, scaffold biomaterial that can support ECM organization, remodeling, and maturation. These iPSC-derived 3D tissues could be potentially used in the future for the delivery of cells and growth factors to support healing of chronic wounds. It may be especially helpful to generate these tissue scaffolds from iPSCs initially derived DFUFs, which may be better adapted to integration into a DFU host upon therapeutic transplantation. Additionally, bioengineered skin substitutes containing human foreskin fibroblasts have been shown to not persist in the chronic wounds for longer than a week (60, 61). As a result, iPSC-derived cells and tissues may offer a better source for fibroblasts in bioengineered products. Further testing of the delivery of ECM generated from diabetic iPSC-derived fibroblasts will reveal whether the cellular or ECM components offer benefits in the treatment of recalcitrant DFUs.
Donor age, sites of origin, and different types of primary cells used for reprogramming can strongly affect the properties of iPSC-derived cells. For example, in this study, NFFs and DFFs were derived from the dorsum of the foot, whereas DFUFs were derived from the plantar surface of the foot, which are known to have different properties (62–64). Some reports showed that these diverse properties could be erased in late-passage iPSCs compared with early passage iPSCs (65–67). In addition, because reprogramming to iPSCs and subsequent differentiation are highly variable, stochastic processes (68, 69), this can combine with cell modifications induced by cell culture passage, preexisting genetic variability, and differences in reprogramming and differentiation protocols to result in interindividual variability between iPSCs from different patients and intraindividual variability between iPSCs from a common patient (70). This variability and the resultant clonal heterogeneity of iPSC-derived cells have been previously well established for multiple cell types (70–72). Similarly, we have observed clonal heterogeneity of iPSC-derived fibroblasts, which suggests that it will be important to identify the iPSC-derived clones of the greatest therapeutic benefit. To accomplish that will require screening clones with assays, such as 3D in vitro SA tissues, which can determine their functionality using predictive markers for clones that may be of therapeutic value.
Taken together, we present strong evidence that nonhealing, predominantly senescent, slow migratory fibroblasts derived from patients with DFUs can be successfully reprogrammed to generate promigratory fibroblasts that facilitate wound closure. iPSC-derived fibroblasts initially reprogrammed from DFUs also display dramatic shifts in patterns of gene expression and the associated changes in the appearance of the 3D in vitro SA tissues, which suggests the future utility of these cells and the 3D tissues constructed with them to improve wound healing.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This project was supported by U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK98055-06 (to J.A.G.). The authors declare no conflicts of interest.
Glossary
- 2/3D
2/3-dimensional
- ECM
extracellular matrix
- DEG
differentially expressed gene
- DFF
diabetic foot fibroblast
- DFU
diabetic foot ulcer
- DFUF
diabetic foot ulcer fibroblast
- DMMB
dimethylmethylene blue
- FDR
false-discovery rate
- GAG
glycosaminoglycan
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GO
gene ontology
- HA
hyaluronic acid
- H&E
hematoxylin and eosin
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IPA
Ingenuity Pathway Analysis
- iPSC
induced pluripotent stem cell
- NFF
nondiabetic foot fibroblast
- SA
self-assembled
- STZ
streptozotocin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
O. Kashpur conceived and designed the experiments, collected and assembled the data, analyzed and interpreted the data, and wrote the manuscript; A. Smith collected, assembled, and reviewed the manuscript and interpreted data; B. Gerami-Naini collected, assembled, and reviewed the manuscript and interpreted data; A. G. Maione and R. Calabrese collected data; A. Tellechea, G. Theocharidis, and L. Liang collected and assembled data; I. Pastar reviewed the manuscript and collected and assembled data; M. Tomic-Canic, D. Mooney, and A. Veves conceived and designed the study, interpreted the data, obtained financial support, and provided manuscript feedback; and J. A. Garlick conceived and designed the study, interpreted the data, obtained financial support, wrote the manuscript, reviewed the manuscript, and gave final manuscript approval and feedback.
REFERENCES
- 1.Gallagher K. A., Liu Z. J., Xiao M., Chen H., Goldstein L. J., Buerk D. G., Nedeau A., Thom S. R., Velazquez O. C. (2007) Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1α. J. Clin. Invest. 117, 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galkowska H., Wojewodzka U., Olszewski W. L. (2005) Low recruitment of immune cells with increased expression of endothelial adhesion molecules in margins of the chronic diabetic foot ulcers. Wound Repair Regen. 13, 248–254 [DOI] [PubMed] [Google Scholar]
- 3.Wicks K., Torbica T., Mace K. A. (2014) Myeloid cell dysfunction and the pathogenesis of the diabetic chronic wound. Semin. Immunol. 26, 341–353 [DOI] [PubMed] [Google Scholar]
- 4.Margolis D. J., Hampton M., Hoffstad O., Mala D. S., Mirza Z., Woltereck D., Shannon S., Troiano M. A., Mitra N., Yang M., Bhopale V. M., Thom S. R. (2017) NOS1AP genetic variation is associated with impaired healing of diabetic foot ulcers and diminished response to healing of circulating stem/progenitor cells. Wound Repair Regen. 25, 733–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thom S. R., Hampton M., Troiano M. A., Mirza Z., Malay D. S., Shannon S., Jennato N. B., Donohue C. M., Hoffstad O., Woltereck D., Yang M., Yu K., Bhopale V. M., Kovtun S., Margolis D. J. (2016) Measurements of CD34+/CD45dim stem cells predict healing of diabetic neuropathic wounds. Diabetes 65, 486–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetzler C., Kämpfer H., Stallmeyer B., Pfeilschifter J., Frank S. (2000) Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J. Invest. Dermatol. 115, 245–253 [DOI] [PubMed] [Google Scholar]
- 7.Loots M. A., Lamme E. N., Zeegelaar J., Mekkes J. R., Bos J. D., Middelkoop E. (1998) Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J. Invest. Dermatol. 111, 850–857 [DOI] [PubMed] [Google Scholar]
- 8.Maione A. G., Brudno Y., Stojadinovic O., Park L. K., Smith A., Tellechea A., Leal E. C., Kearney C. J., Veves A., Tomic-Canic M., Mooney D. J., Garlick J. A. (2015) Three-dimensional human tissue models that incorporate diabetic foot ulcer-derived fibroblasts mimic in vivo features of chronic wounds. Tissue Eng. Part C Methods 21, 499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maione A. G., Smith A., Kashpur O., Yanez V., Knight E., Mooney D. J., Veves A., Tomic-Canic M., Garlick J. A. (2016) Altered ECM deposition by diabetic foot ulcer-derived fibroblasts implicates fibronectin in chronic wound repair. Wound Repair Regen. 24, 630–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galiano R. D., Tepper O. M., Pelo C. R., Bhatt K. A., Callaghan M., Bastidas N., Bunting S., Steinmetz H. G., Gurtner G. C. (2004) Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 164, 1935–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eming S. A., Martin P., Tomic-Canic M. (2014) Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 6, 265sr6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmqvist S., Lehtonen Š., Chumarina M., Puttonen K. A., Azevedo C., Lebedeva O., Ruponen M., Oksanen M., Djelloul M., Collin A., Goldwurm S., Meyer M., Lagarkova M., Kiselev S., Koistinaho J., Roybon L. (2016) Creation of a library of induced pluripotent stem cells from Parkinsonian patients. NPJ Parkinsons Dis. 2, 16009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulting G. L., Kiskinis E., Croft G. F., Amoroso M. W., Oakley D. H., Wainger B. J., Williams D. J., Kahler D. J., Yamaki M., Davidow L., Rodolfa C. T., Dimos J. T., Mikkilineni S., MacDermott A. B., Woolf C. J., Henderson C. E., Wichterle H., Eggan K. (2011) A functionally characterized test set of human induced pluripotent stem cells. Nat. Biotechnol. 29, 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Balasubramanian U., Cohen D., Zhang P. W., Mosmiller E., Sattler R., Maragakis N. J., Rothstein J. D. (2015) A comprehensive library of familial human amyotrophic lateral sclerosis induced pluripotent stem cells. PLoS One 10, e0118266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H., Kim Y., Sharkis S., Marchionni L., Jang Y. Y. (2011) In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci. Transl. Med. 3, 82ra39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nori S., Okada Y., Yasuda A., Tsuji O., Takahashi Y., Kobayashi Y., Fujiyoshi K., Koike M., Uchiyama Y., Ikeda E., Toyama Y., Yamanaka S., Nakamura M., Okano H. (2011) Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. USA 108, 16825–16830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki N., Yamazaki S., Yamaguchi T., Okabe M., Masaki H., Takaki S., Otsu M., Nakauchi H. (2013) Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol. Ther. 21, 1424–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higuchi A., Kumar S. S., Benelli G., Alarfaj A. A., Munusamy M. A., Umezawa A., Murugan K. (2017) Stem cell therapies for reversing vision loss. Trends Biotechnol. 35, 1102–1117 [DOI] [PubMed] [Google Scholar]
- 19.Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., Fujihara M., Akimaru H., Sakai N., Shibata Y., Terada M., Nomiya Y., Tanishima S., Nakamura M., Kamao H., Sugita S., Onishi A., Ito T., Fujita K., Kawamata S., Go M. J., Shinohara C., Hata K. I., Sawada M., Yamamoto M., Ohta S., Ohara Y., Yoshida K., Kuwahara J., Kitano Y., Amano N., Umekage M., Kitaoka F., Tanaka A., Okada C., Takasu N., Ogawa S., Yamanaka S., Takahashi M. (2017) Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 376, 1038–1046 [DOI] [PubMed] [Google Scholar]
- 20.Sayed N., Liu C., Wu J. C. (2016) Translation of human-induced Pluripotent Stem cells: from clinical trial in a dish to precision medicine. J. Am. Coll. Cardiol. 67, 2161–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Wilgenburg B., Browne C., Vowles J., Cowley S. A. (2013) Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PLoS One 8, e71098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senju S., Haruta M., Matsumura K., Matsunaga Y., Fukushima S., Ikeda T., Takamatsu K., Irie A., Nishimura Y. (2011) Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. 18, 874–883 [DOI] [PubMed] [Google Scholar]
- 23.Lian X., Bao X., Al-Ahmad A., Liu J., Wu Y., Dong W., Dunn K. K., Shusta E. V., Palecek S. P. (2014) Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling; erratum: 13, 170. Stem Cell Reports 3, 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrova A., Celli A., Jacquet L., Dafou D., Crumrine D., Hupe M., Arno M., Hobbs C., Cvoro A., Karagiannis P., Devito L., Sun R., Adame L. C., Vaughan R., McGrath J. A., Mauro T. M., Ilic D. (2014) 3D in vitro model of a functional epidermal permeability barrier from human embryonic stem cells and induced pluripotent stem cells. Stem Cell Reports 2, 675–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh M., Umegaki-Arao N., Guo Z., Liu L., Higgins C. A., Christiano A. M. (2013) Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One 8, e77673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lapasset L., Milhavet O., Prieur A., Besnard E., Babled A., Aït-Hamou N., Leschik J., Pellestor F., Ramirez J. M., De Vos J., Lehmann S., Lemaitre J. M. (2011) Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 25, 2248–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lian Q., Zhang Y., Zhang J., Zhang H. K., Wu X., Zhang Y., Lam F. F., Kang S., Xia J. C., Lai W. H., Au K. W., Chow Y. Y., Siu C. W., Lee C. N., Tse H. F. (2010) Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 121, 1113–1123 [DOI] [PubMed] [Google Scholar]
- 28.Suhr S. T., Chang E. A., Tjong J., Alcasid N., Perkins G. A., Goissis M. D., Ellisman M. H., Perez G. I., Cibelli J. B. (2010) Mitochondrial rejuvenation after induced pluripotency. PLoS One 5, e14095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hewitt K. J., Garlick J. A. (2013) Cellular reprogramming to reset epigenetic signatures. Mol. Aspects Med. 34, 841–848 [DOI] [PubMed] [Google Scholar]
- 30.Intine R. V., Sarras M. P., Jr (2012) Metabolic memory and chronic diabetes complications: potential role for epigenetic mechanisms. Curr. Diab. Rep. 12, 551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang L., Stone R. C., Stojadinovic O., Ramirez H., Pastar I., Maione A. G., Smith A., Yanez V., Veves A., Kirsner R. S., Garlick J. A., Tomic-Canic M. (2016) Integrative analysis of miRNA and mRNA paired expression profiling of primary fibroblast derived from diabetic foot ulcers reveals multiple impaired cellular functions. Wound Repair Regen. 24, 943–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerami-Naini B., Smith A., Maione A. G., Kashpur O., Carpinito G., Veves A., Mooney D. J., Garlick J. A. (2016) Generation of induced pluripotent stem cells from diabetic foot ulcer fibroblasts using a nonintegrative Sendai virus. Cell. Reprogram. 18, 214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hewitt K. J., Shamis Y., Carlson M. W., Aberdam E., Aberdam D., Garlick J. A. (2009) Three-dimensional epithelial tissues generated from human embryonic stem cells. Tissue Eng. Part A 15, 3417–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wicks K., Torbica T., Umehara T., Amin S., Bobola N., Mace K. A. (2015) Diabetes inhibits Gr-1+ myeloid cell maturation via cebpa deregulation. Diabetes 64, 4184–4197 [DOI] [PubMed] [Google Scholar]
- 35.Ritchie M. E., Phipson B., Wu D., Hu Y., Law C. W., Shi W., Smyth G. K. (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eden E., Navon R., Steinfeld I., Lipson D., Yakhini Z. (2009) GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10, 48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eden E., Lipson D., Yogev S., Yakhini Z. (2007) Discovering motifs in ranked lists of DNA sequences. PLOS Comput. Biol. 3, e39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Supek F., Bošnjak M., Škunca N., Šmuc T. (2011) REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One 6, e21800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krämer A., Green J., Pollard J., Jr., Tugendreich S. (2014) Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30, 523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pouyani T., Papp S., Schaffer L. (2012) Tissue-engineered fetal dermal matrices. In Vitro Cell. Dev. Biol. Anim. 48, 493–506 [DOI] [PubMed] [Google Scholar]
- 41.Shamis Y., Hewitt K. J., Bear S. E., Alt-Holland A., Qari H., Margvelashvilli M., Knight E. B., Smith A., Garlick J. A. (2012) iPSC-derived fibroblasts demonstrate augmented production and assembly of extracellular matrix proteins. In Vitro Cell. Dev. Biol. Anim. 48, 112–122 [DOI] [PubMed] [Google Scholar]
- 42.Hehenberger K., Heilborn J. D., Brismar K., Hansson A. (1998) Inhibited proliferation of fibroblasts derived from chronic diabetic wounds and normal dermal fibroblasts treated with high glucose is associated with increased formation of l-lactate. Wound Repair Regen. 6, 135–141 [DOI] [PubMed] [Google Scholar]
- 43.Hehenberger K., Kratz G., Hansson A., Brismar K. (1998) Fibroblasts derived from human chronic diabetic wounds have a decreased proliferation rate, which is recovered by the addition of heparin. J. Dermatol. Sci. 16, 144–151 [DOI] [PubMed] [Google Scholar]
- 44.Brem H., Golinko M. S., Stojadinovic O., Kodra A., Diegelmann R. F., Vukelic S., Entero H., Coppock D. L., Tomic-Canic M. (2008) Primary cultured fibroblasts derived from patients with chronic wounds: a methodology to produce human cell lines and test putative growth factor therapy such as GMCSF. J. Transl. Med. 6, 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Segal N., Andriani F., Pfeiffer L., Kamath P., Lin N., Satyamurthy K., Egles C., Garlick J. A. (2008) The basement membrane microenvironment directs the normalization and survival of bioengineered human skin equivalents. Matrix Biol. 27, 163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleischmajer R., Utani A., MacDonald E. D., Perlish J. S., Pan T. C., Chu M. L., Nomizu M., Ninomiya Y., Yamada Y. (1998) Initiation of skin basement membrane formation at the epidermo-dermal interface involves assembly of laminins through binding to cell membrane receptors. J. Cell Sci. 111, 1929–1940 [DOI] [PubMed] [Google Scholar]
- 47.Halfter W., Oertle P., Monnier C. A., Camenzind L., Reyes-Lua M., Hu H., Candiello J., Labilloy A., Balasubramani M., Henrich P. B., Plodinec M. (2015) New concepts in basement membrane biology. FEBS J. 282, 4466–4479 [DOI] [PubMed] [Google Scholar]
- 48.Briquez P. S., Hubbell J. A., Martino M. M. (2015) Extracellular matrix-inspired growth factor delivery Systems for skin wound healing. Adv. Wound Care (New Rochelle) 4, 479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tracy L. E., Minasian R. A., Caterson E. J. (2016) Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care (New Rochelle) 5, 119–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz G. S., Wysocki A. (2009) Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 17, 153–162 [DOI] [PubMed] [Google Scholar]
- 51.Mohan R. R., Gupta R., Mehan M. K., Cowden J. W., Sinha S. (2010) Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp. Eye Res. 91, 238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y., Inoue H., Wu J. C., Yamanaka S. (2017) Induced pluripotent stem cell technology: a decade of progress. Nat. Rev. Drug Discov. 16, 115–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iovino S., Burkart A. M., Kriauciunas K., Warren L., Hughes K. J., Molla M., Lee Y. K., Patti M. E., Kahn C. R. (2014) Genetic insulin resistance is a potent regulator of gene expression and proliferation in human iPS cells. Diabetes 63, 4130–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iovino S., Burkart A. M., Warren L., Patti M. E., Kahn C. R. (2016) Myotubes derived from human-induced pluripotent stem cells mirror in vivo insulin resistance. Proc. Natl. Acad. Sci. USA 113, 1889–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saha K., Jaenisch R. (2009) Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell 5, 584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kikuchi T., Morizane A., Doi D., Magotani H., Onoe H., Hayashi T., Mizuma H., Takara S., Takahashi R., Inoue H., Morita S., Yamamoto M., Okita K., Nakagawa M., Parmar M., Takahashi J. (2017) Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 548, 592–596 [DOI] [PubMed] [Google Scholar]
- 57.Wang Z., Nakamura K., Jinnin M., Kudo H., Goto M., Era T., Kira T., Nakashima T., Fukushima S., Ihn H. (2016) Establishment and gene expression analysis of disease-derived induced pluripotent stem cells of scleroderma. J. Dermatol. Sci. 84, 186–196 [DOI] [PubMed] [Google Scholar]
- 58.Hewitt K. J., Shamis Y., Knight E., Smith A., Maione A., Alt-Holland A., Sheridan S. D., Haggarty S. J., Garlick J. A. (2012) PDGFRβ expression and function in fibroblasts derived from pluripotent cells is linked to DNA demethylation. J. Cell Sci. 125, 2276–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theocharidis G., Drymoussi Z., Kao A. P., Barber A. H., Lee D. A., Braun K. M., Connelly J. T. (2016) type VI collagen regulates dermal matrix assembly and fibroblast motility. J. Invest. Dermatol. 136, 74–83 [DOI] [PubMed] [Google Scholar]
- 60.Stone R. C., Stojadinovic O., Rosa A. M., Ramirez H. A., Badiavas E., Blumenberg M., Tomic-Canic M. (2017) A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Sci. Transl. Med. 9, eaaf8611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu S., Kirsner R. S., Falanga V., Phillips T., Eaglstein W. H. (2006) Evaluation of Apligraf persistence and basement membrane restoration in donor site wounds: a pilot study. Wound Repair Regen. 14, 427–433 [DOI] [PubMed] [Google Scholar]
- 62.Sorrell J. M., Caplan A. I. (2004) Fibroblast heterogeneity: more than skin deep. J. Cell Sci. 117, 667–675 [DOI] [PubMed] [Google Scholar]
- 63.Chang H. Y., Chi J. T. Dudoit S., Bondre C., van de Rijn M., Bolstein D., Brown P. O. (2002) Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. USA. 99, 12877–12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillippeos C., Telerman S. B. Oulès B., Pisco A. O., Shaw T. J., Elgueta R., Lombardi G., Driskell R. R., Soldin M., Lynch M. D., Watt F. M. (2018) Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J. Invest. Dermatol. 138, 811–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polo J. M., Anderssen E. Walsh R. M., Schwarz B. A., Nefzger C. M., Lim S. M., Borkent M., Apostolou E., Alaei S., Cloutier J., Bar-Nur O., Cheloufi S. Stadtfeld M., Figueroa M. E., Robinton D., Natesan S., Melnick A., Zhu J., Ramaswamy S., Hochedlinger K. (2012) A molecular roadmap of reprogramming somatic cells into iPS cells. Cell 151, 1617–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim D. S., Lee J. S. Leem J. W., Huh Y. J., Kim J. Y., Park I. H., Daley G. Q., Hwang D. Y., Kim D. W. (2010) Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev. 6, 270–281 [DOI] [PubMed] [Google Scholar]
- 67.Krijger P. H. L., Di Stefano B. de Wit E., Limone F., van Oevelen C., de Laat W., Graf T. (2016) Cell-of-origin-specific 3D genome structure acquired during somatic cell reprogramming. Cell Stem Cell 18 597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahashi K., Yamanaka S. (2016) A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 17, 183–193 [DOI] [PubMed] [Google Scholar]
- 69.Hochedlinger K., Jaenisch R. (2016) Induced pluripotency and epigenetic reprogramming. Cold Spring Harb. Perspect. Biol. 7, 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nityanandam A., Baldwin K. K. (2015) Advances in reprogramming-based study of neurologic disorders. Stem Cells Dev. 24, 12365–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu B. Y., Wick J. P. Yu J., Ma L. X., Zhang X. Q., Thomson J. A., Zhang S. C. (2010) Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA. 107, 4335–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mills J. A., Wang K. Paluru P., Ying L., Galvão A. M., Xu D., Yao Y., Sullivan S. K., Mac H. Omari A., Jean J. C., Shen S., Gower A., Spira A., Motoslavsky G., Kotton D. N., French D. L., Weiss M. J., Gadue P. (2013) Clonal genetic and hematopoietic heterogeneity among human-induced pluripotent stem cell lines. Blood 122, 2047–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.