Abstract
Eggs of teleost fish, unlike those of many other animals, allow sperm entry only at a single site, a narrow canal in the egg's chorion called the micropyle. In some fish (e.g., flounder, herring, and Alaska pollock), the micropyle is a narrow channel in the chorion, with or without a shallow depression around the outer opening of micropyle. In some other fish (e.g., salmon, pufferfish, cod, and medaka), the micropyle is like a funnel with a conical opening. Eggs of all the above fish have a glycoprotein tightly bound to the chorion surface around the micropyle. This glycoprotein directs spermatozoa into the micropylar canal in a Ca2+-dependent manner. This substance, called the micropylar sperm attractant or MISA, increases fertilization efficiency and is essential in herring. In flounder, salmon, and perhaps medaka, fertilization is possible without MISA, but its absence makes fertilization inefficient because most spermatozoa swim over the micropyle without entering it. The mechanism underlying sperm–MISA interactions is yet to be determined, but at least in herring the involvement of Ca2+ and K+ channel proteins, as well as CatSper and adenylyl cyclase, is very likely. In some other fish (e.g., zebrafish, loach, and goldfish), the chorion around the micropyle is deeply indented (e.g., zebrafish and loach) or it has radially or spirally arranged grooves around the outer opening of the micropyle (e.g., goldfish). MISA is absent from the eggs of these fish and sperm entry into micropylar canal seems to be purely physical.
Keywords: chemotaxis, egg, fertilization, fish, micropyle, spermatozoa
Summary Sentence
In fish, sperm entry into the egg through the micropyle is guided either chemically or physically depending on the species
Introduction
During spawning of oviparous fish, eggs and spermatozoa are released into hypertonic seawater or hypotonic freshwater. Even in brackish water fish, gametes are seldom laid in isotonic water. In general, the fertile lifespan of fish gametes is rather short once they are in water. For example, eggs of the freshwater fish medaka become completely infertile within 6 min in water [1]. Spermatozoa of most marine and freshwater fish become vigorously motile upon contact with water, but they become immotile in less than a few minutes in many species [2–5]. Black flounder (a marine species) spermatozoa, used often in our studies, move very actively for only 30 s in seawater [6]. While a male fish releases billions of spermatozoa during spawning, fertilizing spermatozoa must quickly enter eggs through micropyles before eggs lose their fertilization competence.
How do fish spermatozoa find the micropyle? In herring, the chorion around the micropylar canal bears a specific glycoprotein that activates spermatozoa, which we previously called “sperm motility initiation factor” or SMIF [7–9]. This factor is required for herring fertilization since its absence renders eggs nonfertilizable [9–11]. It should be stressed that herring is unusual among most fish species in that their spermatozoa are virtually motionless and live in seawater for days. Spermatozoa of most fish become actively motile upon contact with water [5]. Eggs of some other fish (e.g., flounder and salmon) also have a specific glycoprotein tightly bound to the surface of the chorion around the outer opening of the micropyle which “attracts or directs” spermatozoa into the micropylar canal [6]. At least in flounder, fertilization can occur without this micropylar sperm attractant or MISA (previously abbreviated as MSA), but its absence makes fertilization inefficient [6]. Here we report that eggs of some fish have MISA, while others do not. In the latter, the chorion around the micropyle is deeply indented or it has radially or spirally arranged grooves that can function as a physical mechanism to direct spermatozoa into the micropyle.
Materials and methods
Species
Seawater-spawning fish used in this study were Pacific herring (Clupea pallasii), anchovy (Engraulis japonicus), flyingfish (Cypselurus heterurus), black flounder (Pleuronectes obscurus), barfin flounder (Verasper moseri), cresthead flounder (Pleuronectes schrenki), bastard halibut (Paralichthys olivaceus), Pacific cod (Gadus macrocephalus), Alaska pollock (Gadus chalcogrammus), Arctic cod (Boreogadus saida), sailfin sandfish (Actoscopus japonicus), canal rockfish (Sebastolobus macrochir), smooth lumpsucker (Aptocyclus ventricosus), red seabream (Pagrus major), Japanese eel (Anguilla japonica), and tiger pufferfish (Takifugu rubripes).
Freshwater-spawning fish used were coho salmon (Oncorhynchus kisutch), steelhead (ocean-run rainbow) trout (Oncorhynchus mykiss), chum salmon (Onchorhynchus keta), cutthroat trout (Onchorhynchus clarkii), surf smelt (Hypomerus nipponensis), rainbow smelt (Osmerus eperlanus), dace (Leuciscus leuciscus), zebrafish (Danio rerio), goldfish (Carassius auratus), carp (Cyprinus carpio), loach (Misgurnus anguillicaudatus and Lefua nikkonis), stickleback (Gasterosteus aculeatus), mummichog (Fundulus heteroclitus), and medaka (Oryzias latipes).
In addition, spermatozoa from the sea urchin Tripneustes gratilla were used to see how spermatozoa of unrelated organisms behave toward the micropyles of fish eggs.
Gametes from the loach, goldfish, and medaka were collected according to the Guide for the Care and Use of Laboratory Animals of Hokkaido University (approval numbers 19-2 and 27-3). Those of the salmon/trout were collected from live fish in two hatcheries (Shibetsu Salmon Hatchery-Museum, Shibetsu, Hokkaido, Japan and Warm Springs Fish Hatchery, Geyserville, California, USA). Flounder and eel gametes were collected from fish maintained in aquaculture tanks in the Tokyo University of Agriculture at Abashiri, Hokkaido, Japan, and the National Research Institute of Aquaculture, Mimami-ise, Japan, respectively. Gametes of all other fish were collected from fish purchased at fish markets in Kushiro, Abashiri, and Shimonoseki, Japan.
Reagents and media
All inorganic salts and organic compounds were purchased from the Sigma-Aldrich Corp (St. Louis, MO, USA and Japan) unless otherwise stated.
The Ringer's solution originally developed for flounder [6, 12] was used here, except that glucose was omitted from the original medium. Its composition was as follows: 150.0 mM NaCl, 2.5 mM KCl, 3.5 mM CaCl2, 1.0 mM MgCl2.6H2O, 0.7 mM NaH2PO4.H2O and 7.0 mM NaHCO3. The pH value was adjusted to 7.4 with HCl or NaOH. Ca2+-free Ringer's contained 155.5 mM NaCl and 0.1 mM ethylene glycol-bis (beta-aminoethyl ether)-N,N,N΄,N΄-tetraaacetic acid tetrasodium salt (EGTA), other components being the same as normal Ringer's. For freshwater fish, Ringer's diluted 1/100 with distilled water was used instead of tap or river water. For saltwater fish, natural seawater (NSW) (0.45 μm Millipore-filtered) or artificial seawater (ASW) [6] was used, with or without a 1/2–1/3 dilution with distilled water (1/2 dilution denotes mixing one part seawater with one part distilled water; 1/3 dilution denotes mixing one part seawater with two parts distilled water). 1/3 diluted seawater was nearly isotonic with fish plasma and reproductive fluids. Ca2+-free Ringer's and Ca2+-free ASW contained 0.1 and 0.5 mM EGTA, respectively. For herring, we often used 1/2 ASW because eggs in this medium have been shown to have optimal fertilization under this salinity.
Collection and storage of gametes
Mature eggs and spermatozoa were collected from fish by either dissecting the entire isolated gonad or gently squeezing the abdomen of the fish. Eggs and dense sperm (milt) were kept separately in covered plastic petri dishes and maintained at ∼4°C. Gametes were used for experiments within 1–2 days. Herring and pufferfish spermatozoa suspended in Ringer's and kept at ∼4°C retained their fertility for several days. Goldfish spermatozoa in artificial seminal plasma retained their fertility for about 1 week at ∼4°C. Artificial seminal plasma consisted of 96.1 mM NaCl, 7.0 mM KCl, 0.2 mM CaCl2, 0.1 mM MgCl2, and 2.4 mM NaHCO3 (pH 8.2).
Staining of eggs with Coomassie brilliant blue G or fluorescein-conjugated lectin
Fresh live eggs were stained with Coomassie brilliant blue G (CB) [6] to determine if CB-affinity substance existed around or in the micropyle. Phosphate-buffered saline (PBS) was routinely used to dilute stock CB solution for egg staining as well as suspending herring and steelhead trout spermatozoa prior to detection of CatSper-like protein. Some eggs were stained with fluorescein isothiocyanate (FITC)-conjugated plant lectins prior to examination with an epifluorescence microscope [6]. FITC conjugate of wheat germ agglutinin (WGA) was most commonly used. Other FITC-conjugated lectins tested included those of Ricinus communis agglutinin (RCA), jackbean agglutinin (Con A), Ulex europaeus agglutinin (UEA), and Lens culinaris agglutinin (LCA). Washing eggs with Ringer's thoroughly before staining with CB or FITC-lectin was important to remove mucus (ovarian fluid) around the eggs, which may contribute to nonspecific staining. This was particularly evident in the case of the herring. We rinsed herring eggs repeatedly with Ca2+-free Ringer's to remove viscous ovarian fluid before staining them with CB.
Scanning electron microscopy of eggs
Some mature unfertilized eggs, after washing in Ringer's, were fixed in either 2% glutaraldehyde or 10% formalin in 0.1 M PBS, washed in the buffer, dehydrated with an ethanol series, and dried in a Tousimis Samdri-795 critical point dryer. Specimens were mounted on aluminum stubs and sputter coated with gold/palladium in a Hummer 6.2 sputter coater and viewed with a Hitachi S-4800 Field Emission Scanning Electron Microscope at an accelerating voltage of 5 kV.
Insemination and examination of sperm entry in eggs
Table 1 lists media used for treatments of eggs and/or spermatozoa before insemination and for examination of spermatozoa entering the egg's micropyle. Media for pretreatment of eggs and spermatozoa were those that maintained these cells in an “unactivated” state for at least a few hours. Media used for insemination were those that allowed spermatozoa to become actively motile and successfully fertilize.
Table 1.
Media used for gamete pretreatments, insemination, and examination of sperm entry into the micropyle.
| Treatment prior to insemination | Medium that initiates sperm movement | Medium for insemination and examination of sperm entry in micropyle | ||
|---|---|---|---|---|
| Species | Egg | Sperm | ||
| Herring | Ringer's | Ringer's or ASWa | _ | ASWa or Ringer's |
| Flounder | Ringer's | Ringer's | ASW | ASW |
| Trout Salmon | Ringer's | K-rich Ringer's | Ringer's or 1/100 Ringer ’s | Ringer's or 1/100 Ringer's |
| Pufferfish | Ringer's | Ringer's | ASW | ASW |
| Medaka | Ringer's | – | Ringer's or 1/100 Ringer's | Ringer's or 1/100 Ringer's |
| Zebrafish Goldfish Loach | Ringer'sb | – | 1/100 Ringer's | 1/100 Ringer's |
a1/2 ASW is preferable.
bSpontaneous activation of zebrafish eggs in Ringer's can be prevented at least for 1 h by 5–10% chicken egg white in Ringer's.
Sperm entry into the micropyle was examined using three different methods. For eggs with a “sticky” (adhesive) outer layer of the chorion (e.g., those of herring, black flounder, cod, and goldfish), eggs were first allowed to adhere to the bottom of a plastic dish containing Ringer's. After selecting an egg with a clearly visible micropyle (either top or semiprofile view) under a 40 or 50x water-immersion objective lens, the Ringer's was replaced with 1/2 NSW or 1/2 ASW (for herring), or full-strength NSW or ASW (for flounder, and cod) or 1/100 Ringer's (for goldfish) before adding a suspension of actively motile spermatozoa. For eggs of some fish that were not adhesive or floated in water (e.g., zebrafish, medaka), glass or plastic dishes were coated with poly-l-lysine [13] to facilitate attachment of the eggs to dishes. Alternatively, a group of eggs or mechanically isolated chorions in a drop of Ringer's were mounted between a slide and a coverslip that was supported with four dots of vaseline-paraffin-bee's wax (9: 1: 0.5) mixture [14]. Eggs were compressed slightly under the coverslip before searching for an egg with a clearly visible micropyle under a 40x ordinary objective lens. Ringer's was then replaced with either seawater or 1/100 Ringer's, depending on the fish species used (Table 1). Insemination was accomplished by adding freshly diluted sperm suspension from one side of the coverslip and pulling the suspension through the eggs under the coverslip by applying a piece of filter paper on the other side from the sperm addition. Spermatozoa, before and after entering the micropyle, were imaged using the 40 or 50x objective lens.
In some experiments, the number of spermatozoa in the micropyle was determined. In the black flounder, for example, unfertilized eggs were allowed to stick to the bottom of plastic dishes (55 × 12 mm) containing Ringer's. Using a water-immersion objective lens (40 or 50×), an egg with a clearly visible micropyle (semiprofile or top view: see Figures 1–3) was selected. The Ringer's was replaced by ASW containing freshly suspended spermatozoa (3 × 107 cells/ml). The number of spermatozoa within the micropylar canal was determined 20 and 30 s after insemination. It was important to do this quickly because in flounder, all spermatozoa within the canal were extruded from it as early as 40 s (mostly by 1 min) after insemination. Sperm extrusion from the micropyle was due to an outflow of colloidal material of the cortical granule origin, which was released from the egg upon its activation by the fertilizing spermatozoon [15, 16].
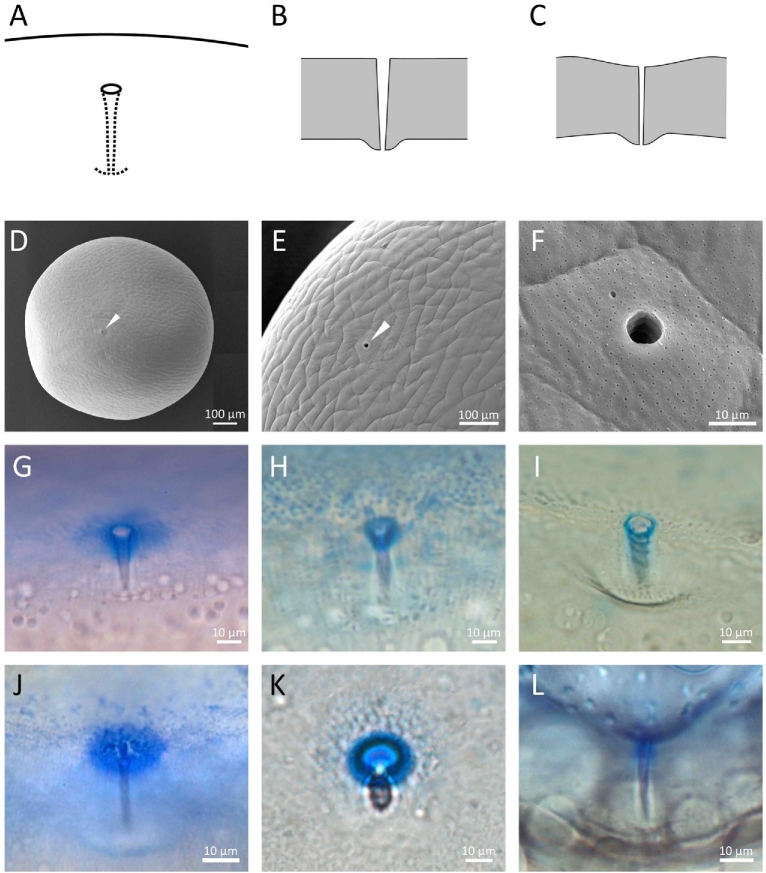
Figure 1.
Type I micropyles. (A–C) Diagrams of semiprofile and crosssection views. In some species, the chorion around the outer opening of the micropylar canal is slightly indented (C). (D–F) Scanning electron micrographs of the micropyle of a black flounder egg; white arrowhead indicates the outer opening of the micropyle. (G–L) Micropyle region of eggs stained with Coomassie Blue. (G) Black flounder, (H) cresthead flounder, (I) barfin flounder, (J) herring, (K) Alaska pollock, (L) mummichog (Fundulus). Figure 1F is a duplicate of Figure 1D of our previous publication in Biol. Reprod. 88, (47) 1–7, 2013, with permission of Biol. Reprod.
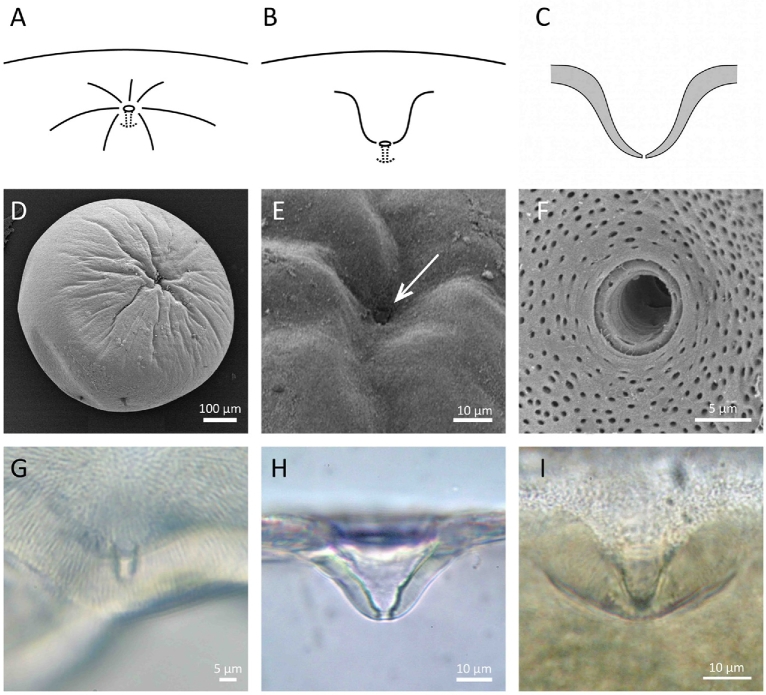
Figure 3.
Type III micropyles. (A–C) Diagrams of semiprofile and crosssection views. (D–F) Scanning electron micrographs of a goldfish egg with radially arranged grooves in the chorion around the micropyle. An arrow in (E) indicates the outer opening of the micropyle. (G) Isolated chorion of a goldfish egg after staining with CB; no obvious staining of the micropyle at the center of this micrograph. (H) Side view of the micropyle of loach, M. anguillicaudatus. (I) Side view of zebrafish micropyle. Note that there is no obvious staining of the micropyle in G–I.
Examination of the effect of pretreatment of eggs with trypsin on sperm entry into the micropyle and fertilization
Herring eggs in Ringer's were treated with or without 0.01–0.1% trypsin for 0.5–2 min at 15–20oC, rinsed thoroughly and inseminated in Ringer's. Sperm concentration in the insemination medium was approximately 107 cells/ml. The behavior of spermatozoa in the egg's micropylar region was examined from the moment of insemination, as previously described [6]. Eggs were considered fertilized when they were cleaving normally 2–4 h later; however, preliminary assessment of fertilization rates of herring eggs could be done 15–30 min after insemination by determining the proportion of eggs with clear egg cortexes and an enlarged perivitelline space, indicative of cortical granule exocytosis.
Black flounder and barfin flounder eggs were treated with 0.003% trypsin for 1 min in Ringer's at ∼15oC, rinsed thoroughly and inseminated in ASW at three different sperm concentrations. Eggs were considered fertilized when they were undergoing normal cleavages 7–15 h later. Similarly, goldfish eggs were treated with or without 0.003–0.01% trypsin in Ringer's for 2 min, rinsed, inseminated in 1/100 Ringer's and examined 2–3 h later. Those undergoing normal cleavages were recorded as fertilized.
Examination of the effects of serine protease inhibitors on herring sperm activation and fertilization
We found that trypsin inhibitors blocked herring sperm activation in the micropylar region. Herring spermatozoa were washed in 1/2 ASW or Ringer's by centrifugation (2000 × g, 1 min), and suspended in 1/2 ASW or Ringer's containing 0.2% soybean trypsin inhibitor, 1 mM benzamidine hydrochloride hydrate, protease inhibitor cocktail (Sigma: 1/200 dilution with Ringer's) or 1 mM tosyl-L-lysine-chloromethyl ketone hydrochloride (TLCK). Ten minutes later, sperm suspension was added to eggs in 1/2 ASW or Ringer's containing the same concentration of inhibitor to see if sperm activation takes place in the micropyle region of eggs. In another experiment, the spermatozoa exposed to inhibitors were washed by centrifugation, and added to eggs in 1/2 ASW or Ringer's without inhibitors. The concentration of spermatozoa in the insemination medium was not strictly controlled, but was approximately 107 cells/ml. Eggs were examined 1–3 min later for sperm activation in the micropylar region. Eggs were recorded as fertilized when they were undergoing normal cleavages 2–3 h after insemination.
Examination of Ca2+ and K+ dependence of sperm entry into the micropyle and fertilization success
The importance of extracellular Ca2+ for efficient sperm entry into the micropyle of herring, flounder, and salmon eggs has already been reported [6, 17]. We confirmed this in pufferfish, bastard halibut, goldfish and loach. For the pufferfish and halibut (marine fish), eggs were washed and kept in Ca2+-free Ringer's with 0.1 mM EGTA. Spermatozoa were washed by centrifugation (2000 × g, 1 min) in Ca2+-free Ringer's with 0.1 mM EGTA. The behavior of spermatozoa in the micropyle region was examined as described previously. For the loach and goldfish, eggs were washed thoroughly in normal or Ca2+-free Ringer's, and inseminated in either normal or Ca2+-free 1/100 Ringer's. The spermatozoa used for insemination in Ca2+-free 1/100 Ringer's had been washed in Ca2+-free Ringer's before they were suspended in 1/100 Ca2+-free Ringer where they were highly motile. Eggs inseminated in Ca2+-free 1/100 Ringer's were examined 1–2 min later for the presence or absence of spermatozoa in the micropylar region before they were transferred to normal 1/100 Ringer's 15 min later. Eggs were considered fertilized when they were undergoing normal cleavages 2–4 h later. For the flounder, spermatozoa were washed in Ca2+-free Ringer's by centrifugation (2000 × g, 1 min) and added to dishes of normal and Ca2+-free ASW containing fresh, unfertilized eggs. Thirty minutes later, all eggs were returned to normal ASW. Those undergoing normal cleavages 24 h later were considered to be fertilized. Since zebrafish eggs activate “spontaneously” very quickly in 1/00 Ringer's (or freshwater), mechanically isolated chorions were used for examination of sperm entry into the micropyle.
We previously reported [6] and reconfirmed in this study that herring spermatozoa require extracellular K+ to be activated in the micropylar region. First, eggs and spermatozoa were washed separately in K+-free 1/2 ASW, and then mixed together. Swimming behavior of spermatozoa in the medium as well as in the micropylar region of eggs was observed before and after the addition of 4.5 mM KCl to the K+-free ASW.
Examination of the effects of Ca2+-mobilizing agents on sperm motility
In herring, extracellular Ca2+ is essential for sperm motility initiation by SMIF and therefore fertilization cannot take place in Ca2+-free seawater [6, 17, 18]. In flounder and salmon, spermatozoa are able to move actively in Ca2+-free water, but they are unable to enter the micropyle efficiently without extracellular Ca2+ [6, 18]. Here, we studied how ionomycin and thimerosal affect the movement of spermatozoa of herring, flounder, and steelhead trout. These reagents are known to increase intracellular Ca2+ levels. To test the effects of these agents, a slide glass with four small dots of vaseline-paraffin mixture was prepared [14]. After a small drop (1 μl) of milt was placed at the center of the four vaseline dots, a coverslip with hanging seawater (for herring and flounder) or 1/100 Ringer's (for trout) with or without the above reagents was laid on the vaseline dots. When the coverslip was gently pressed down at the four dots until the milt touched seawater or Ringer's, spermatozoa were seen dispersing quickly from milt into seawater or Ringer's. The motility patterns of spermatozoa were observed and recorded under dark-field illumination during the first 15 s or so.
Detection of CatSper-like protein in spermatozoa
The presence or absence of CatSper-like proteins in spermatozoa of some selected fish species was examined by western blot analyses and immunocytochemistry. Steelhead trout and herring spermatozoa were separately suspended in PBS and centrifuged at 700 × g for 5 min. Sperm pellets were lysed in a mixture of 40 μl of 4% sodium dodecyl sulfate (SDS) and 40 μl of lysis/binding buffer from mirVana miRNA isolation kit (Cat #AM2561, Life Technologies, Carlsbad, CA). The sperm lysates were examined under a microscope to ensure that all spermatozoa were completely lysed. An amount of 200 μl of protein lysis buffer (50 mM HCl, 60 mM ß-glycerophosphate, 100 mM sodium fluoride (NaF), 2 mM EGTA, 25 mM Na-pyrophosphate, 1 mM DL-dithiothreitol with 0.5% nonyl phenoxypoly ethoxylethanol (NP-40), 0.2% SDS, and 5 mg/ml protease inhibitor tablet) was added to lysates followed by vortexing. Tubes containing the lysates were centrifuged at 13 000 × g for 10 min at room temperature, and the supernatants were carefully collected for protein assay. Protein concentrations were determined with the Bradford assay, and an aliquot of lysate (150 μg) was boiled in SDS sample buffer containing 2% SDS, followed by incubation on ice for 15 min. The denatured protein samples were fractionated using Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred onto a nitrocellulose membrane. Western blot analyses were performed as described [19]. Rabbit polyclonal anti-CatSper 3 prepared by Jin et al. [20, 21] or mouse anti-beta-actin antibodies were used as primary antibodies (Supplemental Table 1). Antirabbit-HRP (for CarSper3) and antimouse-HRP (for actin) from Thermo Scientific were used as the secondary antibodies.
For immunocytochemical demonstration of CatSper, spermatozoa in Ringer's were smeared on slides, air-dried and fixed with 2–4% paraformaldehyde (PF; Ted Pella Inc., Redding, CA) in either 0.1 M cacodylate buffer or PBS for 30 min. Slides were then stored in the buffer with ∼0.001% PF. Some slides were treated for 10 min with 0.5% Triton X-100 in PBS and stored in a refrigerator. Slides were treated with 1 ml blocking buffer (30 μl fetal bovine serum, 30 μl normal goat serum, 940 μl 1% bovine serum albumin in PBS), rinsed with fresh PBS, incubated in anti-CatSper3 and/or anti-CatSper4 antibodies (Supplemental Figure 1) (antisera diluted 1/200–1/400 with PBS), washed in PBS, and stained with either 3 μg/ml of fluorescein isocyanate-conjugated goat antirabbit IgG [20, 21] or immunoperoxidase detection kit (Vecstain ABC Kit; Vector Lab., Burlingame, CA).
Determination of the concentrations of cyclic adenosine monophosphate (cAMP) and adenylyl cyclase in spermatozoa
The concentration of cAMP was measured as described by Harumi et al. [22], with slight modifications. Fish milt (20 μl) was put directly in 10% (w/v) trichloroacetic acid (TCA) in seawater. After centrifugation at 1000 × g for 10 min, the resulting supernatant was extracted four times with an equal volume of water-saturated ether to remove TCA. The aqueous layer was lyophilized and the lyophilisate was kept at –80°C until use. The amount of cAMP was determined by radioimmunoassay with cAMP assay kits (Yamasa Shoyu Co, Chiba, Japan).
Adenylyl cyclase activity was determined as described by Harumi et al. [23] with some modifications. After frozen spermatozoa (50 μl) were homogenized with 1 ml of 1 mM 3-isobutyl-1-methylxanthine, 1 mM ATP was added. Ten minutes later, the reaction was terminated by adding 0.1 ml of 100% (w/v) TCA. The concentration of cAMP was measured as described above.
Examination of possible involvement of cAMP-dependent protein phosphorylation in sperm motility initiation, sperm entry into the micropyle and fertilization
A potent inhibitor of cAMP-dependent protein kinase, N-[2-(p-bromocinnamylamino) ethyl] 5 isoquinolinesulfonamide dihydrochloride hydrate) (H-89) was tested to see if it blocks sperm motility initiation and/or sperm entry into the micropyle. Herring eggs and spermatozoa were separately kept in 1/2 ASW with or without 10 μM H-89 for 10 min. Insemination was accomplished by adding a sperm suspension to a dish containing eggs. Motility and the behavior of spermatozoa in ASW and around the micropyle were examined as described previously. For flounder, spermatozoa and eggs were separately kept in Ringer's with or without 10–50 μM H-89 for 10 min. In Ringer's, flounder spermatozoa remained motionless. Insemination was accomplished by transferring eggs from Ringer's to ASW with or without H-89 and inseminated with spermatozoa with or without H-89 treatment. Thus, in one set of experiments, eggs and spermatozoa were kept in the medium with H-89 all the time, whereas in another set of experiments, they were never exposed to this reagent. Motility of spermatozoa in Ringer's or ASW as well as around the micropyle was examined as described previously. The effect of H-89 on the motility of pufferfish spermatozoa was examined in the same way. Steelhead trout spermatozoa were suspended in K+-rich Ringer's [24] with or without 10 μM H-89. Spermatozoa were immotile in these media. Two to five minutes later, 2 μl of the sperm suspension was mixed with 200 μl of 1/100 Ringer's with or without H-89 to see if spermatozoa begin to move actively. For goldfish and zebrafish, spermatozoa were first kept in Ringer's with or without 50 μM H-89 for 5–20 min (spermatozoa remained immotile), then transferred to 1/100 Ringer's with or without the same concentration of H-89 to see if they become actively motile.
Examination of sperm entry into the micropyle of eggs of unrelated species
In general, spermatozoa enter eggs of the same species much more readily than those of other species. We studied whether spermatozoa can enter the micropyle of eggs of phylogenetically distant species. First, the chorions of cutthroat trout eggs were each cut in half using iridectomy scissors, rinsed in Ringer's and kept frozen. When needed, chorions were defrosted and transferred to ASW and inseminated with spermatozoa of black flounder or Alaska pollock to see if and how spermatozoa enter the micropyle. Second, the eggs of various fish (black flounder, herring, pufferfish, smelt, steelhead trout, medaka, goldfish, and zebrafish) were fixed and stored in 2% PF in PBS or 10% formalin in water. When needed, eggs were rinsed with PBS and cut in half. After the egg proper was removed by vigorous pipetting, chorions were washed with PBS before they were kept overnight in 1 M glycine to block free aldehyde groups. Chorions were thoroughly rinsed in seawater and mounted between a glass slide and a coverslip as previously described. After finding a chorion with a clearly visible micropyle, a freshly prepared suspension of sea urchin spermatozoa was introduced under the coverslip. The sea urchin we used was the collector sea urchin, Tripneustes gratilla, which spawn in Hawaii all year around. Incidentally, the width of the sperm head of the fish and the sea urchin was about the same (1.5–2.0 μm), and the width of the micropylar canal at its bottom was the same or slightly larger than the width of the sperm head.
Results
Variation in motile lifespan of spermatozoa of fish used in this study
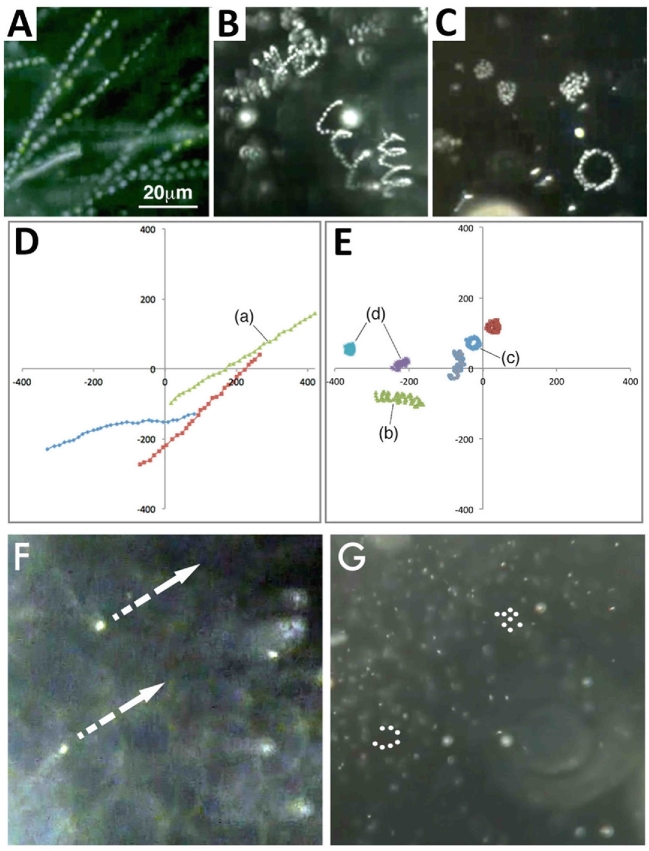
The motile lifespan of spermatozoa in media varied among different species. Herring spermatozoa were unique in that they were intrinsically motionless and lived in seawater for hours and even days. It is interesting that male herring spawn first and the release of milt in water attracts and induces spawning of females as well as other males [25, 26]. In other fish, spermatozoa did not live long in water. Black flounder spermatozoa, for example, moved vigorously upon contact with seawater; nearly 100% were actively motile in the beginning, but 30 s later only about 50% were motile. Only 5–10% spermatozoa were motile at 1 min, and none at 2 min. Barfin flounder spermatozoa remained motile a little longer than black flounder spermatozoa. About 50% were actively motile at 3 min in seawater; all became motionless by 40 min. Pufferfish spermatozoa were actively motile in seawater during the first 30 s, but became motionless by 1 min or so. The spermatozoa of freshwater fish used in this study (e.g., steelhead trout, goldfish, loach, and zebrafish) exhibited vigorous motility during the first 10–20 s after contact with water, thereafter in most cases they lost their progressive motility by 1–3 min.
Structural classification of the micropyle and localization of Coomassie blue- and lectin-affinity material(s) around/in the micropyle
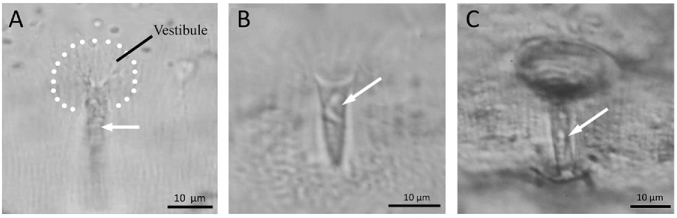
The structure of the micropyle (and chorion) of fish eggs varies to a great extent among different species of fish [27, 28]. Here, we arbitrarily classified it into three groups (Table 2). In the first (Type I), the micropyle is a narrow, tapered canal; the chorion around the outer opening of the micropyle is either flat or slightly indented (Figure 1A–C). Flounders, herring, pollock, seabream, and eel belong to this group. In the second (Type II), the micropyle is like a funnel with a wide, conical mouth and a narrow long stem, with or without indentation of the chorion around the outer opening of the micropyle (Figure 2A–C). The conical mouth is commonly referred to as the vestibule. Micropyles of salmon, trout, medaka, and anchovy are in this group. In the third (Type III), the chorion around the micropyle has radially arranged grooves or is deeply indented (“sinkhole-like”) (Figure 3A–C). Micropyles of goldfish, zebrafish, loach, and smelts belong to this group.
Table 2.
Characteristics of chorion and micropyle of fish eggs studied.
| Type | Chorion around the micropyle | Micropyle itself | For diagrams and/or photos, see | Example of species |
|---|---|---|---|---|
| I | Flat or slightly depressed | Long or short (manhole-like) | Figure 1 | Flounders, bastard halibut, Alaska pollock, mummichog, eel, herring, pufferfish, cods, red seabream, and flying fish |
| II | Flat or slightly depressed | Long or short (funnel-like) | Figure 2 | Salmon, trout, medaka, anchovy, canal rockfish, sailfin sandfish, and lumpsucker |
| III | Deeply depressed like “sinkhole” or has distinct grooves | Short | Figure 3 | Goldfish, carp, loach, zebrafish, smelts, dace, and stickleback |
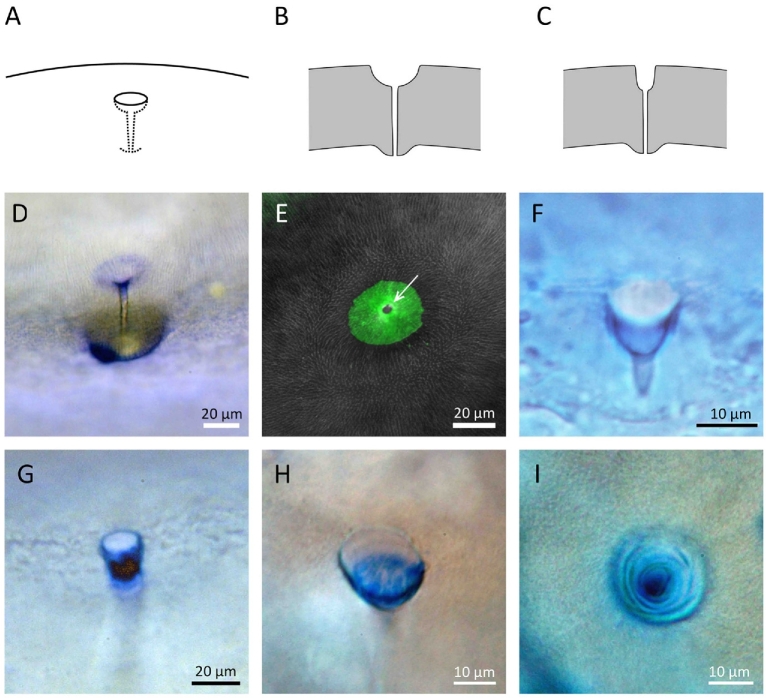
Figure 2.
Type II micropyles. (A–C) Diagrams of semiprofile and crosssection views. (D) Semiprofile view of the micropyle of steelhead trout egg stained with CB, showing that the bottom of the micropylar mouth (vestibule) is darkly stained. (E) Top view of micropyle of steelhead egg, showing that the vestibule of the micropyle is weakly stained by FITC-WGA, whereas the outer opening of the micropylar canal is strongly stained (white arrow). (F) Micropyle of cod egg stained with CB. (G) Micropyle of pufferfish egg stained with CB. (H and I) Profile and top views of medaka micropyle stained with CB.
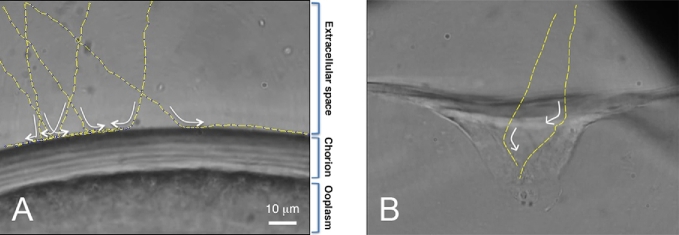
In Type I micropyles, the surface of the chorion around the outer opening of the micropyle as well as in the upper region of the canal are typically stained with CB (Figure 1G–L; also see Figure 4D of reference [6]) as well as lectins (e.g., WGA; Figure 5 of this study and Con A: data not shown in this study). In Type II micropyles, CB- (Figure 2D, F–I) and lectin (WGA)-affinity material (Figures 2E and 4) was observed on the wall of the upper and/or lower portion of the conical mouth as well as the upper region of the micropylar canal. In Type III micropyles, CB-affinity material was absent (Figure 3G–I). The micropylar region of the zebrafish egg (with Type III micropyle) was not stained by any of the FITC-conjugated lectins we tested (WGA, Con-A, RCA, UEA, and LCA) (data not shown).
Figure 4.
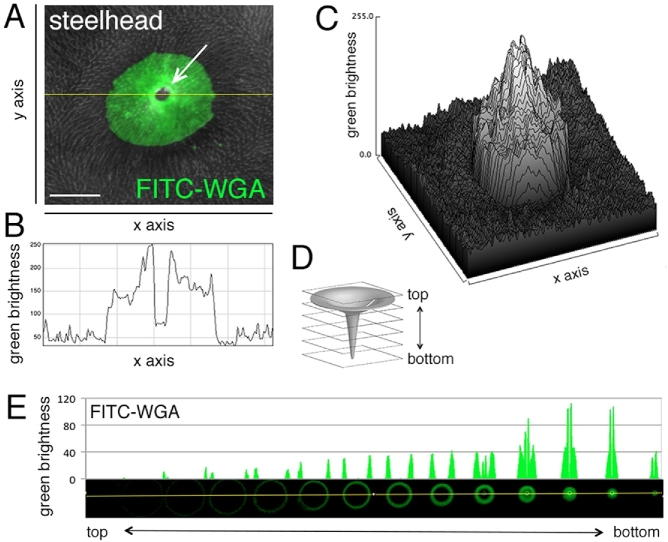
A gradient staining of the micropyle of a live steelhead trout egg by FITC-WGA. (A) The mouth (“vestibule”) of a funnel-like micropyle, showing that WGA staining is most intense around the outer opening (a white arrow) of the micropylar canal. (B) Crosssectional WGA labeling intensity (across yellow line in A) revealed by scanning laser confocal images of the micropylar region, showing a 2.5 times increase in label from the outer edge (∼100 pixels) to the most intense site immediately at the edge of the micropylar canal (∼250 pixels). (C) Three-dimensional representation of WGA fluorescence from (A) showing a 360 degree increase in intensity in WGA labeling. (D, E) Profiling of green fluorescence of confocal sections. Sliced images were aligned from top (left) to bottom (right).
Figure 5.
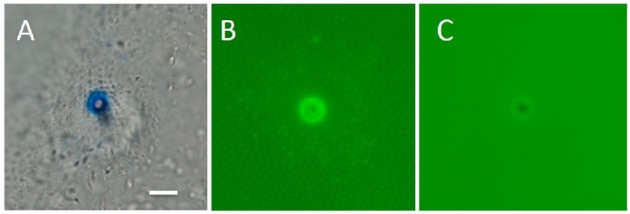
Barfin flounder micropyle stained with CB (A) and FITC-WGA (B). (C) WGA affinity of the micropylar opening was lost after 1 min treatment of eggs with 0.001% trypsin.
Trypsin treatment of eggs reduces the fertilization rate in herring and flounders, but not in goldfish
We previously reported that the fertility of black flounder eggs was drastically reduced by 1 min treatment with 0.001–0.003% trypsin [6]. This was confirmed in the present study (Table 3). Trypsin treatment of black flounder eggs removed CB-affinity material around the outer opening of the micropyle and most spermatozoa swam over the micropyle without entering. In controls where eggs were not treated with trypsin, the micropylar canal was filled with as many as ∼20 spermatozoa within 20 s after insemination. In trypsin-treated eggs, usually none or only a few entered the micropyle even though the micropylar structure remained unchanged. In no instance was the micropyle filled with spermatozoa.
Table 3.
Proportions (mean ± SD)a of black flounder eggs fertilized after 1 min treatment with 0.003% trypsin in Ringer's.
| Sperm concentration at insemination | |||
|---|---|---|---|
| Egg treatment with trypsin | 2.7 × 107/ml | 2.7 × 106/ml | 0.4 × 106/ml |
| No (control) | 87.7 ± 3.8 | 75.7 ± 6.3 | 58.2 ± 6.7 |
| Yes | 46.3 ± 5.1* | 34.7 ± 5.9* | 9.3 ± 4.5* |
aMean ± SD of three experiments using a single male and a single female. About 100 eggs were used for each determination. Fertilization rates for all trypsin treated eggs were significantly different (P < 0.05; asterisk) from the untreated eggs, regardless of the sperm concentration used.
Drastic reduction of fertilization after trypsin treatment of eggs also occurred in barfin flounder (Table 4). When eggs were treated with 0.001% trypsin for 1 min, spermatozoa seldom entered the micropyles, which remained morphologically intact. When inseminated with 107 spermatozoa/ml in seawater, the majority of control (untreated) eggs were fertilized, whereas only 15% were fertilized following trypsin treatment. The loss or sharp reduction of CB- and lectin-affinity material from the micropylar region was obvious (Figure 5).
Table 4.
Fertilization of flounder, herring, and goldfish eggs with or without trypsina treatment.
| Treatment of unfertilized eggs with trypsin | ||||
|---|---|---|---|---|
| Fish | Conc. | Duration (min) | No. of exp. | % eggs fertilizedb |
| Black flounder | 0.003% | 1 | 2 | 22 (53/241) |
| 0 (control) | – | 2 | 88 (163/186) | |
| Barfin flounder | 0.001% | 1 | 3 | 15 (48/321) |
| 0 (control) | – | 3 | 82 (256/314) | |
| Herring | 0.1% | 0.5 | 2 | 0 (0/455) |
| 0.01% | 2 | 1 | 37 (58/155) | |
| 0 (control) | – | 2 | 89 (169/190) | |
| Goldfishc | 0.01% | 2 | 1 | 80 (192/239) |
| 0.003% | 2 | 1 | 93 (175/189) | |
| 0 (control) | – | 1 | 93 (228/245) | |
aSigma trypsin (T4665) for herring and flounder; Difco trypsin-250(215240) for goldfish.
bSperm concentrations at insemination were approximately 107/ml (herring and flounder) and 106/ml (goldfish).
cRinger's solution used for this fish consisted of 128 mM NaCl, 2.8 mM KCl, and 1.8 mM CaCl2. The bottom of the dish was covered with Saran (plastic) Wrap to prevent adhesion of eggs to the dish. Trypsin treatment was done in this solution. After rinsing with Ringer's, eggs were transferred to 1/100 Ringer's and inseminated.
Herring spermatozoa need to be activated by a micropylar glycoprotein (SMIF) before entering the micropyle [18], and therefore the removal of SMIF from eggs by 0.1% trypsin treatment resulted in fertilization failure even before the structure of the chorion was visibly altered (Table 4). In goldfish, the fertilization rate was not affected by trypsin treatment (Table 4).
Sperm entry into the micropyle and fertilization is Ca2+-dependent in some fish
We previously reported that spermatozoa of salmon/trout and flounder swam actively in Ca2+-free water, but were unable to enter the egg's micropyle efficiently, with most spermatozoa swimming over the outer opening of the micropyle [6, 18]. We found that the same was true for the pufferfish, bastard halibut, and anchovy. In normal ASW, spermatozoa that came into contact or very close to the outer opening of the micropyle almost always entered the micropyle, and therefore the micropylar canal was quickly filled with many spermatozoa. In contrast, when eggs were in Ca2+-free ASW, only one of ∼30–50 spermatozoa that came very close to the micropyle entered it. In black flounder, the average numbers of spermatozoa that entered the micropyle after insemination in normal and Ca2+-free ASW were 16 ± 2.8 (n = 6) and 0.8 ±1.0 (n = 8), respectively, when the sperm concentration in the insemination medium was approximately 5 × 107 spermatozoa/ml.
In goldfish, loach, and zebrafish, the presence or absence of Ca2+ in the medium made no difference in sperm entry into the micropyle. The chorion of the goldfish egg, unlike that of the other two, has radially arranged grooves around the micropyle (Figure 3A and D). We observed many goldfish spermatozoa swimming along the grooves toward the micropyle. When inseminated in 1/100 Ringer's, the micropyle was quickly filled with spermatozoa regardless of the presence or absence of Ca2+ in the medium. The chorion of zebrafish and loach has no grooves around the micropyle, but instead has a deep indentation like a sinkhole (Figure 3B, C, H and I). In both normal and Ca2+-free media, zebrafish and loach spermatozoa were seen sliding along the surface of the deeply indented chorion to enter the micropyle.
Table 5 shows the proportion of black flounder, goldfish, and loach eggs fertilized in normal and Ca2+-free media, indicating that fertilization (normal cleavage) did not take place at all in Ca2+-free medium. It should be stressed that extracellular Ca2+ was needed for spermatozoa to enter the egg proper (sperm–egg fusion) in all fish we studied (i.e., herring, trout, flounders, goldfish, and loach). Although we saw spermatozoa reaching the bottom of the micropylar canal after insemination in Ca2+-free media, all the spermatozoa within the micropylar canal were pushed out of it within a few minutes.
Table 5.
Fertilization of black flounder, goldfish, and loach eggs in normal and Ca2+-free media.
| Total no. of eggs | ||||
|---|---|---|---|---|
| Species | Insemination medium | No. of exp. | Inseminated | Fertilized (%) |
| Black flounder | ASW | 3 | 396 | 360 (91) |
| Ca2+-free ASW | 3 | 404 | 0 (0) | |
| Goldfish | 1/100 Ringer's | 2 | 135 | 128 (95) |
| Ca2+-free 1/100 Ringer's | 2 | 127 | 0 (0) | |
| Loach | 1/100 Ringer's | 2 | 87 | 77 (89) |
| Ca2+-free 1/100 Ringer's | 2 | 66 | 0 (0) | |
CatSper-like protein is present in spermatozoa of some fish, but not in others
A Ca2+-specific channel of sperm, CatSper, is essential for the initiation of the vigorous, hyperactive motility of mammalian spermatozoa prior to fertilization [29–31]. By western blot analysis of extracts of trout and herring spermatozoa using antimouse CatSper3 antibody, we detected an immunoreactive band that was similar to that in mouse CatSper3 (∼44 kD) (Figure 6). Immunocytochemical studies using CatSper antibodies (3 and 4) revealed that these antibodies bind to the midpiece region of spermatozoa of fish with Type I and II micropyles (flounders, medaka, herring, trout) (Figure 7A–E). The intensity and pattern of the reaction to antibody were the same in both Triton-treated and nontreated sperm preparations. The spermatozoa of fish with Type III micropyles (loach, zebrafish, and goldfish) did not react to the antibody (Figure 7F–H).
Figure 6.
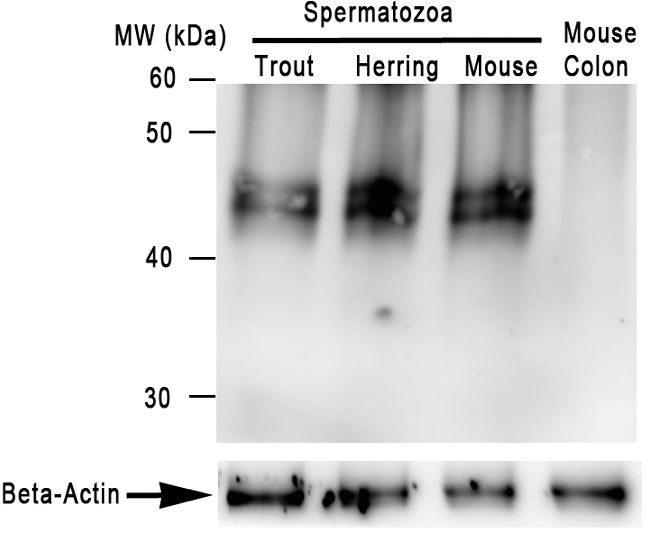
Detection of CatSper-like protein in trout, herring, and mouse spermatozoa using western blot analysis. Mouse spermatozoa were used as a positive control, whereas mouse colon was used as a negative control. Beta-actin was used as a loading control.
Figure 7.
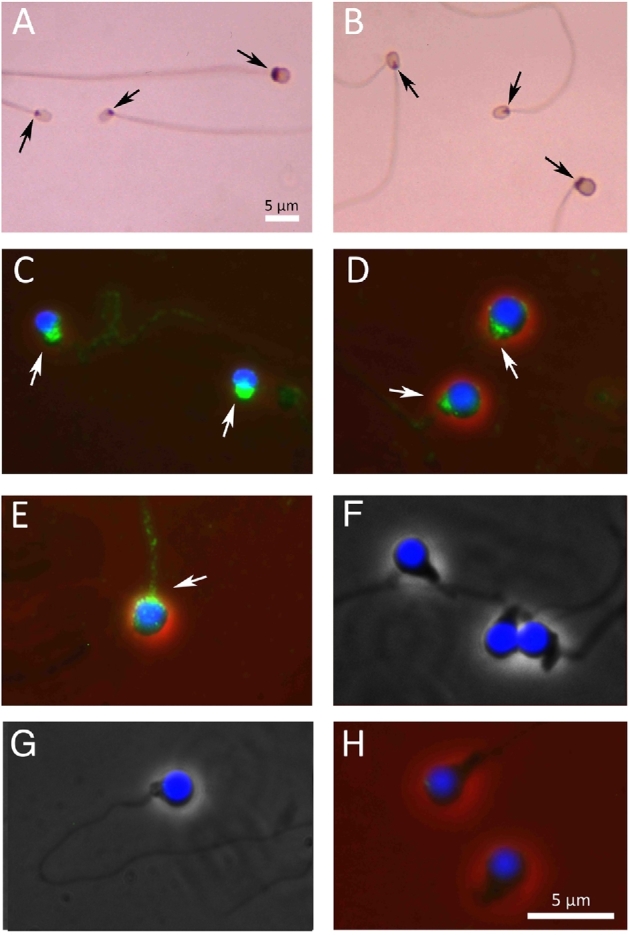
Localization of CatSper. (A and B) Spermatozoa stained by immunoperoxidase method; (C–H) spermatozoa stained by immunofluorescence method. An arrow indicates the midpiece region of each spermatozoon. (A) Two black flounder (smaller) and one barfin flounder (larger) spermatozoa. (B) Two black flounder (smaller) and one medaka (larger) spermatozoa. (C) Herring spermatozoa. (D) Medaka spermatozoa. (E) Steelhead trout spermatozoon. Note that the midpiece regions of spermatozoa of the flounder, medaka, steelhead trout, and herring all reacted intensely to anti-CatSper antibody. (F) Loach spermatozoa. (G) Zebrafish spermatozoon. (H) Goldfish spermatozoa; none of the last three reacted to the antibody.
The swimming pattern of fish spermatozoa is changed by Ca2+-mobilizing reagents
Flounder spermatozoa exposed to NSW or ASW began to swim rapidly and almost straightforward (Figure 8A, D and F; Supplemental Movie 1) or spirally with a large wavelength. They became motionless by 1 min. Steelhead trout spermatozoa became vigorously motile upon exposure to both normal (isotonic) and 1/100 (hypotonic) Ringer's. Those in 1/100 Ringer's became motionless by 1 min, while those in normal Ringer's remained motile for up to 3 min. A Ca2+-ionophore, ionomycin, which mobilizes Ca2+ across the plasma membrane and/or mobilizes Ca2+ from intracellular stores [32], altered swimming patterns of fish spermatozoa. In media containing 1–10 μM ionomycin, cresthead flounder and steelhead trout spermatozoa displayed spiral, circulatory, spinning, or whiplash motions (Figure 8B, C, E, and G; Supplemental Movie 2). Such altered movements were not seen in Ca2+-free media containing the same concentrations of ionomycin.
Figure 8.
Tracks of cresthead flounder spermatozoa in ASW with or without ionomycin. (A and D) Tracks of spermatozoa in normal ASW, moving straightforward (a). (B, C, and E) Tracks of spermatozoa in ASW containing ionomycin, moving spirally (b), circularly (c), or in a spinning or whiplash fashion (d). (F) A frame captured from Supplemental Movie 1, showing spermatozoa in normal ASW that move straightforward, as shown by the dotted arrow. (G) A frame captured from Supplemental Movie 2, showing that spermatozoa in ASW containing ionomycin display spiral, circular, spinning, or whiplash movement.
Thimerosal, which releases Ca2+ from intracellular calcium stores, also altered the motility pattern of spermatozoa. Cresthead flounder and barfin flounder spermatozoa exhibited a spinning motion in the presence of 2.5 μM thimerosal in both normal and Ca2+-free ASW (data not shown). Steelhead trout spermatozoa also displayed spinning and zigzag motions in normal and Ca2+-free Ringer's containing 2.5 μM thimerosal. Herring spermatozoa, which were intrinsically motionless in seawater or Ringer's, exhibited an active zigzag forward motion in both normal and Ca2+-free ASW containing 1 μM thimerosal. The movement, however, continued for only a few minutes.
H-89, a potent inhibitor of cAMP-dependent protein kinase, inhibits sperm motility initiation and fertilization in some fish, but not in others
When herring eggs and spermatozoa were separately treated for 5 min in Ringer's containing 10 μM H-89, then mixed, none of the eggs were fertilized (0/539; two experiments). Sperm activation in the micropyle region of the eggs was not observed. H-89 also inhibited motility initiation of steelhead trout spermatozoa. When they were kept in K+-rich Ringer's containing 10 μM H-89 for 10 min, then transferred to normal Ringer's with the same concentration of H-89, none of spermatozoa became motile. Motility inhibition of herring and steelhead trout spermatozoa by H-89 was reversible. When H-89 was removed by several washings, spermatozoa moved actively in the eggs' micropylar region (herring) or in normal Ringer's (steelhead).
Table 6.
Nucleotidyl cyclase and cyclic nucleotide in fish spermatozoa: mean values of samples from 4 to 6 fishmean values of samples from 4 to 6 fish.
ap mol cAMP produced/mg sperm protein/min at 20oC.
bf mol cAMP/109 sperm.
In other fish, including black flounder, pufferfish, goldfish, and zebrafish, H-89 had no effect on sperm motility. When black flounder spermatozoa were suspended in Ringer's with H-89 (10–50 μM) for 10 min, then transferred to ASW with the same concentration of H-89, they began to move vigorously. Sperm entry into the micropyle and fertilization occurred normally in ASW containing H-89. Likewise, spermatozoa of pufferfish, goldfish, and zebrafish moved vigorously in ASW (pufferfish) or 1/00 Ringer's (goldfish and zebrafish) containing 10–50 μM H-89.
The presence of adenylyl cyclase and cAMP in herring and flounder spermatozoa were confirmed (Table 6). Note that herring spermatozoa have higher concentrations of adenylyl cyclase and cAMP than flounder spermatozoa. As of today, we have no data on adenylyl cyclase and cAMP levels in other fish.
Spermatozoa of unrelated species can enter and pass through micropyles of fish eggs
When chorions of cutthroat trout eggs (with Type II micropyle) were isolated, rinsed, and inseminated in ASW with spermatozoa of black flounder or Alaska pollock, spermatozoa of these foreign species entered the trout's micropyle one by one after their random, thigmotactic movement along the chorion surface (Figure 9A; Supplemental Movie 3). When chorions of various fish eggs were isolated, put in seawater and inseminated with sea urchin spermatozoa, the spermatozoa swam along the chorion surface, some entering the micropylar canal after random, thigmotactic movement on the chorion surface and even passing through the canal. Fish whose egg micropyles allowed the entry and passage of sea urchin spermatozoa included black flounder (Figure 9B), medaka (Figure 9C), steelhead salmon, pond smelt, goldfish, and zebrafish.
Figure 9.
Sperm entry into the micropyle of eggs of unrelated species. (A) A frame captured from Supplemental Movie 3, showing black flounder spermatozoa (white arrow) that entered the micropyle of a cutthroat egg. (B) Sea urchin spermatozoa (white arrow) that entered the micropyle of a black flounder egg. (C) Sea urchin spermatozoa (white arrow) that entered the micropyle of a medaka egg.
Some unique features of herring spermatozoa
As stated earlier, herring spermatozoa are unique in that they are virtually immotile in natural and 2-fold diluted seawater (the optimum fertilization media) yet remain fertile for days. No greater than 5% of spermatozoa collected from freshly captured males displayed an active “sporadic” movement that continued for less than 2–3 s (occasionally 2–3 min) before becoming quiescent (Figure 10A; Supplemental Movie 4). The incidence of spermatozoa with sporadic motion increased noticeably when the medium was agitated. Sporadic movement of spermatozoa was also seen in Ca2+-free 1/2 ASW. When spermatozoa were loaded with the intracellular Ca2+ indicator fluo-4 AM and examined [6], we observed green fluorescence “flashes” in the spermatozoa with sporadic motion (data not shown). When spermatozoa were in seawater for one or more days, virtually all of them became motionless, yet when they were brought (by water current) in contact with the micropylar region of the chorion where SMIF is located, they became vigorously motile (Figure 10B; Supplemental Movie 5). It is known that in spawning grounds of herring, males first release milt in the water, which attracts and induces spawning of females as well as other males [25, 26]. To date, we have not observed herring spermatozoa activated in the micropylar regions of other marine fish we have studied (including flounders, cod, and Alaska pollock), indicating that sperm activation in the micropylar region of herring eggs is species-specific. It is of interest that herring spermatozoa were not activated at all in the micropylar region of herring eggs in K+-free 1/2 ASW [18] and no fertilization occurred (present study). Apparently, both Ca2+ and K+ in the medium are essential for herring sperm activation in the micropylar region.
Figure 10.
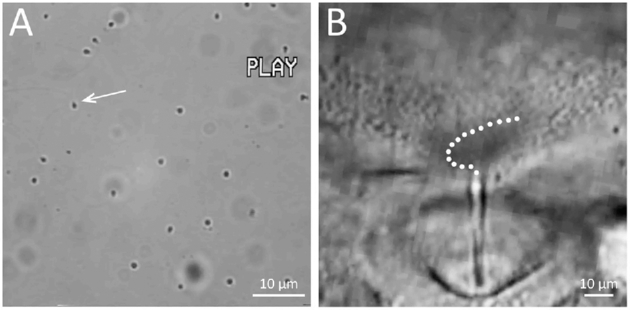
Herring spermatozoa. (A) A frame captured from Supplemental Movie 4, showing that most spermatozoa in seawater are intrinsically motionless, but a few display sporadic, temporal movement. An arrow indicates the spermatozoon that exhibited a sporadic motion in the Movie. (B) A frame captured from Supplemental Movie 5, showing that spermatozoa exhibit active movement on the chorion around the micropyle and enter the micropyle one by one as shown by a dotted line.
The reagents other than H-89 (inhibitor of cAMP-dependent protein kinase) that inhibited herring sperm motility activation in the micropylar region of the chorion were thimerosal and LY-83583 (6-anilinoquinoline-5,-8 quinone). The former is known to activate the inositol trisphosphate receptor, thereby triggering the release of Ca2+ from intracellular stores, whereas the latter inhibits soluble guanylate cyclase and blocks Ca2+ release from internal stores. Although thimerosal (1–10 μm) rendered herring spermatozoa motile (zigzag motion) for a few minutes in both normal and Ca2+-free 1/2 ASW, none of 174 eggs were fertilized in seawater containing this reagent because no spermatozoa were able to enter the micropyle. In 1/2 ASW containing 0.1 mM LY-83583, spermatozoa were motionless even if they drifted to the micropylar region. None of the herring eggs (0/972; two experiments) were fertilized in 1/2 ASW containing LY-83583.
The presence of trypsin-like serine protease inhibitors in seawater blocked motility activation of herring spermatozoa in the micropylar region. Sperm activation was inhibited reversibly by 0.2% soybean trypsin inhibitor (SBTI) and 1 mM benzamidine hydrochloride hydrate. In contrast, a 1 min treatment of spermatozoa with 1 mM TLCK irreversibly rendered them incapable of activation in the micropylar region. None of eggs were fertilized in 1/2 ASW containing these inhibitors (0/758; six experiments).
Discussion
This study showed that the micropylar region of the eggs of some fish possesses a glycoprotein that “attracts” or “directs” spermatozoa into the micropyle. We assessed the presence or absence of this micopylar sperm attractant or MISA by (a) the staining reaction of egg to CB and FITC-lectin, (b) loss of staining ability of the micropylar region to CB/lectin after trypsin treatment, and (c) extracellular Ca2+ dependence of efficient sperm entry into the micropyle (Table 7). Apparently, MISA is absent in some other fish. Since the vast majority of investigators still assume, without direct evidence, that all fish spermatozoa enter the micropyle by a “random” movement, we first discuss how fish spermatozoa swim in water before and after contacting the egg surface.
Table 7.
Parameters used for estimation of the presence or absence of micropylar sperm attractant (MISA) in fish egg.
| Species (Micropyle type) | Staining (+) or no staining (–) of micropylar region by treatment | Loss of staining ability of micropylar region by trypsin | Efficiency of sperm entry in micropyle is affected (+) or not affected (–) by extracellular Ca2+ | Presence (+) or absence (–) of MISA | |
|---|---|---|---|---|---|
| CB | FITC-WGA | ||||
| Herring (I) | + | + | Yes | + | + |
| Flounder (I) | + | + | Yes | + | + |
| Trout (II) | + | + | Yes | + | + |
| Anchovy (II)a | – | + | Yes | + | + |
| Goldfish (III) | – | –b | NA | – | – |
| Loach (III) | – | –b | NA | – | – |
| Zebrafish (III) | – | –b | NA | – | – |
aNote that anchovy micropyle is not stained by CB, but is stained by FITC-WGA.
bOther FITC-conjugated lectins tested (ConA, UEA, RCA, and LCA) did not stain micropylar region of eggs of these fish.
NA: not applicable
Movement of fish spermatozoa in water and on the egg surface
Spermatozoa of most oviparous fish begin to swim rapidly upon contact with water [3, 33]. They typically swim almost in a straight line, or helically with a large wavelength (pitch). Upon contact with the chorion (or any solid substrate), they swim along its surface (Figure 11; Supplemental Movie 6). Such a thigmotactic movement of animal spermatozoa on the surface of solid substrates has been known for many years [34]. Fish spermatozoa moving along the surface of the chorion would have a greater chance of entering the micropylar region than those swimming freely and randomly in the water column.
Figure 11.
(A) A frame captured from Supplemental Movie 6A. Black flounder spermatozoa in water move freely. Upon contact with the chorion, they move along the chorion surface for some time (as shown by dotted arrows) before swimming away. This type of sperm movement (thigmotaxis) is seen on the surface of any solid material or in the water–air interface as well. (B) A frame captured from Supplemental Movie 6B. Loach spermatozoa move toward the micropyle (at 6:00) after colliding with the chorion surface, which is deeply concave around the micropyle.
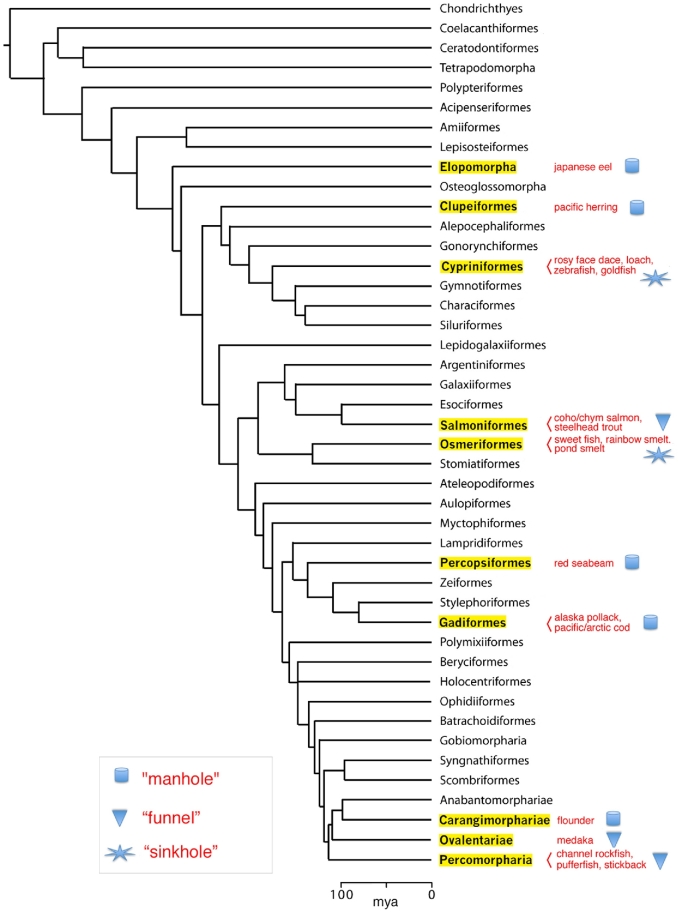
We reported here that the micropyle facilitates sperm entry into fish egg in three different ways. In the first (Type I), the chorion surrounding the outer opening of the micropyle has MISA, which alters the sperm motility pattern and facilitates sperm entry in the micropylar canal. Micropyles of herring, flounder, and pufferfish eggs are representative. In the second (Type II), the micropyle itself possesses a distinct funnel-like opening (a vestibule) that seems to increase the chance of sperm entry into the micropylar canal. MISA is located on the walls of the funnel-like opening of the micropyle. Micropyles of salmon, trout, and medaka eggs belong to this group. In the third way (Type III), the chorion around the micropyle is deeply indented like a “sinkhole” or it has radially or spirally arranged grooves [27, 35–37], both of which seem to guide spermatozoa physically into the micropylar canal. MISA is absent in the micropylar region. Micropyles of goldfish, zebrafish, and loach are examples. When fish with these three types of micropyle are mapped on a fish phylogenetic tree [38] (Figure 12), Type I (“manhole-like”) micropyles are found in species of a relatively ancient clade, and Type II (“funnel-like”) micropyles appeared in recently diversified species (>130 Mya). Types II and III micropyles seem to have emerged independently from the phylogenetic lineage and perhaps as the result of adaptation of each species to a particular environment. Thus far, Type III micropyle has been found only in freshwater fish, although some marine fish may have this type of micropyle.
Figure 12.
Phylogenetic tree of fish and types of micropyles. Note that the “manhole-like” micropyle (Type I) appeared first during evolution.
Role of sperm motility initiation factor and micropylar sperm attractant in herring and other fish
As stated above, eggs of many fish have MISA, which apparently “guides” motile spermatozoa into the micropyle. It should be re-emphasized that the herring is unique in that their spermatozoa are intrinsically immotile in water and the egg has both SMIF and sperm guidance factor (MISA). Since we have never seen herring spermatozoa being activated in the micropylar region of eggs of any other species of fish (e.g., flounders, cod, Alaska pollock, and pufferfish), herring SMIF must be very species-specific. SMIF is a glycoprotein with molecular weight of approximately 105 kDa [7, 39], the chemical nature of which is yet to be investigated. According to Oda et al. [40], when herring eggs were “soaked” in seawater, the resulting solution (egg water) contained a protein that “activated” herring spermatozoa. This sperm-activating protein, called herring sperm-activation protein (HSAP) [40] was identified as a kazal-type trypsin inhibitor [41]. Although herring “egg water” indeed has the ability to enhance the incidence of spontaneous, temporal, sporadic movement of spermatozoa (see Supplemental data), it must be the chorion-bound SMIF-MISA that “directs” spermatozoa into the micropyle. It is feasible to consider that during natural spawning of herring in seawater, HSAP, SMIF, and MISA all work synergistically to facilitate fertilization. Synergistic action of HSAP and SMIF has been discussed previously [9].
An issue to be resolved first is whether herring MISA and SMIF are identical or two different substances. According to Griffin et al. [7], who used an immunocytochemical method, SMIF is present on the chorion surrounding the depression of the micropyle, but at lower levels near the opening of the canal (figure 4 of [7]), whereas MISA stained by CB is localized immediately around the outer opening of the micropylar canal as well as on its walls (figure 5B of [6] and Figure 1 of this study]. SMIF appears to be unique to the herring, whereas MISA is common in many fish. Nevertheless, MISA in other fish and SMIF in herring are all likely produced by a giant micropylar cell [42, 43] and nearby follicular cells during oocyte maturation. At any rate, methods for collecting and identifying SMIF and MISA must be established to understand the precise relationship between the two.
As we reported previously [6] and confirmed in this study, the fertilization rate of black and barfin flounder eggs was reduced drastically after protease (trypsin) treatment (Table 4). It is known that barfin flounders kept in aquaculture tanks during the spawning reason ovulate every 3–4 days, but often fail to spawn (release eggs from the body cavity to seawater). Eggs retained in the body cavity for 2 days have a very low fertility [44], even though gross morphology of eggs (including micropyle structure) remains unchanged. We saw most spermatozoa swim over the outer opening of the micropyles of such (over-ripe) eggs. This seems to be due to the loss of MISA by cathepsin released from dying or dead eggs ovulated in the previous cycle [45]. Cathepsin within the barfin flounder egg is known to cleave yolk protein during the final stage of egg maturation to increase the buoyancy of mature eggs.
According to Ishijima et al. [46], medaka spermatozoa entering the funnel-shaped opening (vestibule) of the micropyle stall momentarily before advancing into the canal, indicating that some reaction takes place between spermatozoa and the vestibule before spermatozoa advance into micropylar canal. Takano and Onitake [47] noted that medaka spermatozoa seldom entered the micropylar canal when eggs were pretreated with trypsin. It should be noted that the medaka egg has MISA on the inner wall of the vestibule (Figure 2H and I) and medaka MISA is most likely protease sensitive. According to Iwamatsu et al. [48], a “mucus” coating the entire surface of the medaka egg is removed and the inner third of the micropylar canal is closed by a 5–30 min treatment with 0.25% trypsin. It is most likely that the medaka's MISA is removed long before the chorion swells and the micropylar canal is closed by extended egg treatments using such a high concentration of trypsin. It should be noted that the chorion around the outer opening of the micropyles of medaka and flounder eggs no longer “guides” spermatozoa into its canal after fertilization [6, 49]. This must be due to loss (modification) of MISA by a trypsin-like material (of cortical granule origin) released from the egg immediately after fertilization [50].
The prime role of MISA in eggs with Type I and II chorion/micropyles should be considered as increasing the chance of fertilization, particularly when sperm concentrations around the eggs are not very high during spawning. The molecular mechanism by which MISA “guides” spermatozoa into the micropyle is the subject of future investigations. What has been observed in the flounder and salmon is that the spermatozoa coming very close to the outer opening of the micropyle suddenly change their motion from linear to curvilinear fashion before entering the micropylar canal [6, 18]. When MISA is removed, spermatozoa swim linearly, ignoring the micropyle. Although we have not traced the tail beating pattern of individual spermatozoa as they make a sharp turn to enter the micropylar canal, it is well established that spermatozoa (of various species) change their swimming pattern from a linear to a curvilinear fashion when the tail-bending pattern changes from symmetrical to asymmetrical in association with an increase in intracellular Ca2+ [9, 33, 51–53]. We observed a distinct difference in movement patterns of flounder spermatozoa in media with or without Ca2+-mobilizing agents (Figure 8A–C; Supplemental Movie 2).
In eggs with Type III micropyles (i.e., those of zebrafish, goldfish, loach, and smelt), MISA is absent, and therefore trypsin treatment of eggs before insemination made no difference on the outcome of insemination (Table 4). A deep depression of the chorion around the micropyle or grooves in the chorion appear to “guide” spermatozoa physically into the micropylar canal.
Large variations in characteristics of fish eggs and spermatozoa
Although the number of fish species used in this study was rather limited, it is interesting to see that fish use a variety of tactics to facilitate fertilization. In fish with Types I and II micropyles, MISA around the outer opening of micropyle (as well as in the micropylar canal) definitely increases the chance of sperm entry into the canal, whereas those with Type III micropyles exclusively use the physical architecture of the chorion to direct spermatozoa into the micropyle. Sperm physiological and biochemical characteristics seem to be designed to facilitate entry into eggs of their own species quickly and efficiently.
In our previous paper [6], we reported that the chorion around the micropyle of the bitterling egg was stained with CB. Since the micropyle of this fish falls in the category of Type III (sinkhole-like), the presence or absence of MISA must be reinvestigated. It is possible that viewing the micropyle of CB-stained eggs from the side, rather than from the top, gives us a false impression that the micropyle is stained by CB. According to Suzuki [54], bittering spermatozoa remain actively motile for a longer time in the micropylar region than in other regions of the chorion, and a distinct aggregated mass of spermatozoa appears around the micropyle several minutes after insemination. A small molecule associated with a protein is likely the factor responsible for sperm activation and aggregation around the micropyle of this fish [55]. Whether fish with Type III micropyles all have such a molecule must be investigated.
Changes in the motility pattern of fish spermatozoa around the micropyle may have something in common with the “chemotactic” movement of sea urchin spermatozoa toward the egg as well as hyperactivation of mammalian spermatozoa before fertilization. Spermatozoa of hydroids [56], ascidians [57], and corals [58] change their swimming pattern from helical to straight when they approach the egg. This is known as the “chemotactic response” of spermatozoa to sperm attractants released from eggs. Mammalian spermatozoa which are about to fertilize eggs begin a vigorous motion, called hyperactivation (asymmetrical flagellar bending based on increased intracellular Ca2+) which is believed to be essential for sperm ascent from the lower to the upper segment of the oviduct, as well as for sperm passage through the egg's vestments, in particular the zona pellucida [29, 59]. Involvement of cAMP- and cyclic guanosine monophosphate (cGMP)-dependent adenylyl and guanylyl cyclases, intra- and extracellular Ca2+, CatSper and KSper channel proteins for chemotactic movement of invertebrate spermatozoa and in hyperactivation of mammalian spermatozoa have been well established (for reviews, see [52, 60–63]). Here, we demonstrated the presence of cAMP-dependent protein kinase and CatSper-like protein in spermatozoa of many (but not all) fish. The thigmotactic “circular” motion of herring spermatozoa toward the egg's micropylar canal and a sudden change in the direction of sperm movement toward the micropyle of salmon and flounder eggs [6, 18] may be homologous to the chemotactic movement of sea urchin spermatozoa and hyperactivated movement of mammalian spermatozoa that occurs immediately before fertilization.
We predict that fish with Type I and II micropyles have spermatozoa with CatSper channel protein, while those with Type III micropyles do not. It was once thought that fish do not have CatSper [64], but we detected an immunoreactive band (∼44 kD) that was similar to that in mouse CatSper 3 (Figure 6). Recent genome sequencing also revealed the presence of CatSper homologs or orthologs in Atlantic herring [65], salmon (GenBank acc. no. XP_014053736), and spotted gar (GenBank acc. no. XP_015194281). Herring and salmon have Type I and II micropyles, respectively. It would be very interesting to know what type of micropyle the spotted gar has, because this is a “primitive” fish belonging to the order of Lepisosteiformes. Further biochemical and genomic studies are needed to better understand CatSper proteins in fish spermatozoa.
Working hypotheses for the process and mechanism of sperm entry into the fish egg
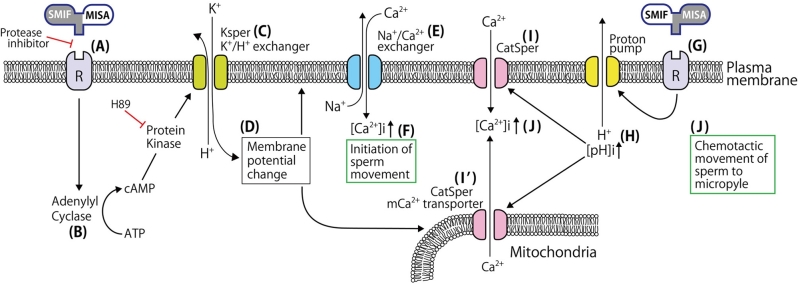
Since we have more information about sperm activation in the micropyle region in the herring as compared to other fish, we first present our working model in this species (Figure 13). Before discussing herring fertilization, we must clarify some confusion about sperm motility-initiating factors in the herring. According to Morisawa [66] and Oda et al. [40], seawater in which herring eggs were washed for 45–60 min contained 8.1 kDa proteins capable of “activating” herring spermatozoa. The proteins were identified as trypsin inhibitor-like proteins [41]. Since herring SMIF is tightly bound to the chorion surface around the micropyle and cannot be removed even by very vigorous washings in seawater [10, 11, 39], the proteins that Oda et al. [41] identified as the sperm-activating factor are likely the ovarian fluid components attached to mature, ovulated eggs. Although we found that herring ovarian fluid was indeed able to increase sporadic, temporal movement of spermatozoa, it is unlikely that this fluid itself is primarily involved in the sperm entry into herring eggs (see Supplemental data 1).
Figure 13.
Working model of the sequence of events that lead to herring spermatozoa motility initiation and entry into egg. For explanation, see the text.
SMIF and MISA could be two separate molecules, but here we hypothesize that SMIF and MISA of the herring egg could be a single molecule with two different domains. We also hypothesize that herring spermatozoa have SMIF-MISA receptors. One of them may have a trypsin-like property because all serine protease inhibitors we tested completely blocked motility activation of herring spermatozoa in the micropylar region (this study). The presence of trypsin-like molecule on eel sperm surface has been reported [67]. Figure 13 illustrates that binding of SMIF-MISA to sperm receptor (A) activates adenylyl cyclase (B) that causes an increase in intracellular cAMP concentrations of spermatozoa. This in turn activates a presumptive KSper [68] or K+/H+ exchanger (C), allowing an influx of K+ from seawater and an elevation in intracellular pH. This causes a marked change in the membrane potential of the plasma and mitochondrial membranes (D), resulting in activation of a Na+/Ca2+ exchanger (E) in the plasma membrane [8, 69]. An increase in intracellular Ca2+ causes activation of immotile spermatozoa into an active state (F). This is the step when immotile herring spermatozoa begin to move. When motile spermatozoa come in contact with egg's SMIF- MISA (G), proton pumps in the sperm plasma membrane are activated. Further increase of intracellular pH (H) activates CatSper (I) in the plasma membrane or CatSper/Ca2+ channel in the outer mitochondrial membrane (I'), resulting in further increase of intracellular Ca2+ concentration (J) by Ca2+ influx from the medium and/or Ca2+ efflux from mitochondria [70]. This secondary increase of intracellular Ca2+ concentration alters the sperm's tail beating pattern (from symmetrical to asymmetrical), which facilitates sperm entry into the micropylar canal.
At present, we are not certain about the location of CatSper-like protein in fish spermatozoa, because we have not examined it in live spermatozoa or at the electron microscopy level. The presence of CatSper in the mitochondria cannot be negated because the midpiece region of fish spermatozoa reacted strongly to CatSper antibody even after treatment of sperm preparations with Triton X-100. The presence of CatSper mRNA and protein in the mitochondria of bovine spermatozoa has been reported by Gur and Breitbart [71], although they did not claim that this protein serves as a Ca2+ channel of sperm's mitochondrial membrane.
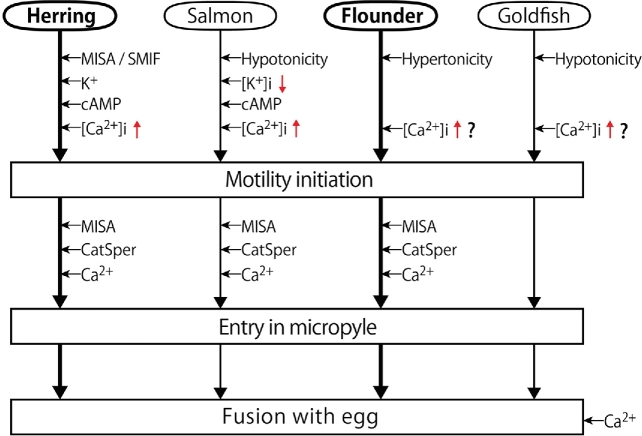
Figure 14 illustrates the presumptive sequence of events that lead fish spermatozoa into the micropyle, culminating in their entry in the egg. Four species are arbitrarily represented here. In herring, SMIF-MISA, K+, Ca2+, cAMP, and CatSper are all involved in sperm motility initiation and entry into the micropyle. In salmon and trout, hypotonic water of the spawning media causes an efflux of the sperm's intracellular K+ [66] and an influx of water [72]. This causes an elevation in cAMP that triggers sperm motility initiation. An increase in intracellular Ca2+ concentration upon sperm motility initiation is well documented [73, 74]. MISA, CatSper, and extracellular Ca2+ are all involved in sperm entry into micropyles. In flounder, hypertonicity of seawater triggers sperm motility initiation without involvement of cAMP. Although spermatozoa are able to enter the micropyle by chance after random, thigmotactic movement on the chorion, their entry into the micropyle is certainly facilitated by MISA and CatSper. In goldfish, hypotonicity of freshwater triggers sperm motility initiation without involvement of cAMP. In carp, closely related to goldfish, intracellular Ca2+ is known to increase during sperm motility initiation in water [75], and sperm entry into the micropyle involves neither MISA nor CatSper.
Figure 14.
Presumptive sequence of events involved in sperm motility initiation and entry into eggs in four representative fish. For explanation, see the text.
According to Creech et al. [76], the chorion around the micropyle of the fathead minnow (a cyprinid fish like the goldfish) has nitric oxide (NO) synthase that might generate NO to enhance sperm motility. As reported in this paper, spermatozoa of the goldfish, zebrafish, and loach were all able to enter micropyles of aldehyde-fixed eggs, and therefore neither nitric oxide synthase nor NO seems to be essential for sperm entry into the micropyle of these fish. Fechner maintain that the zebrafish sperm head possesses a K+ channel that mediates membrane hyperpolarization, Ca2+ influx and “spinning” (circular) motion of spermatozoa. It is unknown at the moment how widespread this phenomenon is among all fish species.
In all fish we have studied, Ca2+ in the medium is essential for successful sperm–egg fusion (sperm entry into egg cytoplasm). The requirement for extracellular Ca2+ for sperm–egg membrane fusion has been established in sea urchin [77], fish [78], and mammals [79]. In fact, Ca2+ seems to be required for all types of membrane fusion [80]. What types of proteins mediate sperm–egg fusion in fish is completely unknown. According to Grayson [81], fish have an ortholog (ancestor gene) of Juno, a glycophosphatidylinositol (GPI)-anchored membrane glycoprotein which functions as the egg's receptor for the sperm receptor protein Izumo that mediates sperm–egg fusion in mammals [82, 83]. Proteins similar to egg's “Juno” and sperm's counterpart “Izumo” may be involved in gamete membrane fusion in fish.
One thing that puzzled us after insemination of eggs in Ca2+-free medium was an extrusion of spermatozoa from the micropylar canal. As stated in the Results section, a few spermatozoa may enter the micropyle of the egg after insemination in Ca2+-free medium. The first spermatozoon may come into contact with the egg plasma membrane at the bottom of the micropylar canal. What was interesting was the extrusion of this and all other followers within the canal. The reason is not clear, but could be due to a flow of invisible colloidal materials constantly released from the egg proper even before egg activation/fertilization.
Supplementary data
Supplementary data are available at BIOLRE online.
Supplemental Table S1. Details of antibodies used throughout this study.
Supplemental Movie 1. Movement of cresthead flounder spermatozoa in normal seawater, exhibiting straightforward motion.
Supplemental Movie 2. Movement of cresthead flounder spermatozoa in seawater containing 1–10 μM ionomycin. Note that many spermatozoa display spiral, circular, spinning, or whiplash movement.
Supplemental Movie 3. Micropyle of cutthroat trout egg filled with black flounder spermatozoa.
Supplemental Movie 4. Herring spermatozoa are intrinsically motionless in normal seawater, but a few display sporadic jumping motion from time to time. This movement can occur in Ca2+-free seawater, perhaps due to temporal Ca2+ release from internal stores.
Supplemental Movie 5. Herring spermatozoa become actively motile on contact with the chorion surrounding the micropyle. The spermatozoon that enters the micropyle first fuses with the egg; the remaining spermatozoa are expelled from the micropylar canal.
Supplemental Movie 6. Spermatozoa of most fish begin to move actively upon contact with the water in which spawning takes place. (A) This movie shows that black flounder spermatozoa swim freely in seawater, but when they collide with the chorion, they begin to swim along the chorion surface (thigmotactically) for some time before leaving it. (B) This movie shows loach (Lefua nikkonis) spermatozoa moving toward the micropyle after colliding with the chorion near the micropyle.
Supplementary data are available at BIOLRE online.
Supplemental Table S1. Details of antibodies used throughout this study.
Supplemental Movie 1. Movement of cresthead flounder spermatozoa in normal seawater, exhibiting straightforward motion.
Supplemental Movie 2. Movement of cresthead flounder spermatozoa in seawater containing 1–10 μM ionomycin. Note that many spermatozoa display spiral, circular, spinning, or whiplash movement.
Supplemental Movie 3. Micropyle of cutthroat trout egg filled with black flounder spermatozoa.
Supplemental Movie 4. Herring spermatozoa are intrinsically motionless in normal seawater, but a few display sporadic jumping motion from time to time. This movement can occur in Ca2+-free seawater, perhaps due to temporal Ca2+ release from internal stores.
Supplemental Movie 5. Herring spermatozoa become actively motile on contact with the chorion surrounding the micropyle. The spermatozoon that enters the micropyle first fuses with the egg; the remaining spermatozoa are expelled from the micropylar canal.
Supplemental Movie 6. Spermatozoa of most fish begin to move actively upon contact with the water in which spawning takes place. (A) This movie shows that black flounder spermatozoa swim freely in seawater, but when they collide with the chorion, they begin to swim along the chorion surface (thigmotactically) for some time before leaving it. (B) This movie shows loach (Lefua nikkonis) spermatozoa moving toward the micropyle after colliding with the chorion near the micropyle.
Acknowledgments
We thank Dr. Masaki Ichimura of the Shibetsu Salmon Hatchery-Museum, Shibetsu, Hokkaido, Japan, who generously provided us with cutthroat trout eggs, and Ms. Ellen McKenna, California Department of Fish and Wildlife, Warm Springs Fish Hatchery, California, who generously provided us with sperm and eggs of steelhead trout. We wish to thank Dr. Hideko Tanaka of the National Research Institute of Aquaculture (Japan) for generously supplying eel gametes. Thanks are also due to the Marine Resources Center of Woods Hole Marine Laboratory, Woods Hole, Massachusetts, for providing us with common mummichog, Fundulus. We thank Drs Jean-Ju Chung and Jae-Yeon Hwang of Yale University for sharing genomic information of the spotted gar.
References
- 1. Yamamoto T. Physiological studies of fertilization and activation of fish egg. I. Response of the cortical layer of the egg of Oryzias latipes to insemination and to artificial stimulation. Annot Zool Japon 1944; 22:109–125. [Google Scholar]
- 2. Scott AP, Baynes SM. A review of the biology, handling and storage of salmonid spermatozoa. J Fish Biol 1980; 17:707–739. [Google Scholar]
- 3. Alavi SMH, Cosson J. Sperm motility in fishes. (II) Effects of ions and osmolality: a review. Cell Biol Int 2006; 30:1–14. [DOI] [PubMed] [Google Scholar]
- 4. Cosson J, Groison A-L, Suquet M, Fauvel C, Dreanno C, Billard R. Studying sperm motility in marine fish: an overview on the state of the art. J Appl Ichthyol 2008; 24:460–486. [Google Scholar]
- 5. Browne RK, Kaurova SA, Uteshev VK, Shishova NV, McGinnity D, Figiel, CR, Mansour N, Agnew D, Wu M, Gakhova EN, Dzyuba B, Cosson J. Sperm motility of externally fertilizing fish and amphibians. Theriogenology 2015; 83:1–13. [DOI] [PubMed] [Google Scholar]
- 6. Yanagimachi R, Cherr G, Matsubara T, Andoh T, Harumi T, Vines C, Pillai M, Griffin F, Matsubara H, Weatherby T, Kaneshiro K. Sperm attractant in the micropylar region of fish and insect eggs. Biol Reprod 2013; 88:1–11. [DOI] [PubMed] [Google Scholar]
- 7. Griffin FJ, Vines CA, Pillai MC, Yanagimachi R, Cherr GN. Sperm motility initiation factor is a minor component of the Pacific herring egg chorion. Dev Growth Diff 1996; 38:193–202. [DOI] [PubMed] [Google Scholar]
- 8. Vines CA, Yoshida K, Griffin FJ, Pillai MC, Morisawa M, Yanagimachi R, Cherr GN. Motility initiation in herring sperm is regulated by reverse sodium-calcium exchange. Proc Natl Acad Sci USA 2002; 99:2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cherr GN, Morisawa M, Vines CA, Yoshida K, Smith EH, Matsubara T, Pillai MC, Griffin FJ, Yanagimachi R. Two egg-derived molecules in sperm motility initiation and fertilization in the Pacific herring (Clupea pallasii). Int J Dev Biol 2008; 52:743–752. [DOI] [PubMed] [Google Scholar]
- 10. Yanagimachi R. Some properties of the sperm-activating factor in the micropyle area of the herring egg. Annot Zool Japon 1957a; 30:114–119. [Google Scholar]
- 11. Yanagimachi R. Studies of fertilization in Clupea pallasi. VI. Fertilization of the egg deprived of the membrane. Jpn J Ichthyol 1957b; 6:41–47. [Google Scholar]
- 12. Hirano T, Johnson DW, Bern HA. Control of water movement in flounder urinary bladder by prolactin. Nature 1971; 230:469–471. [DOI] [PubMed] [Google Scholar]
- 13. Andoh T, Matsubara T, Harumi T, Yanagimachi R. The use of poly-l-lysine to facilitate examination of sperm entry into pelagic, non-adhesive fish eggs. Int J Dev Biol 2004; 52:753–757. [DOI] [PubMed] [Google Scholar]
- 14. Yanagimachi R. Germ cell and fertilization: why I studied these topics and what I learned along the path of my study. Andrology 2014; 2:787–793. [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi W, Yamamoto TS. Light and electron microscopic observations of sperm entry in the chum salmon egg. J Exp Zool 1987; 243:311–322. [Google Scholar]
- 16. Iwamatsu T. Fertilization in fishes. In: Tarin J, Cano A (eds), Fertilization in Protozoa and Metazoan Animals. Berlin Heidelberg: Springer-Verlag; 2000:89–145. [Google Scholar]
- 17. Yanagimachi R, Kanoh Y. Manner of sperm entry in herring egg, with special reference to the role of calcium ions in fertilization. J Fac Sci Hokkaido Univ (Ser 6) 1953; 11:487–494. [Google Scholar]
- 18. Yanagimachi R, Cherr GN, Pillai MC, Baldwin JD. Factors controlling sperm entry into the micropyles of salmonid and herring eggs. Dev Growth Diff 1992; 34:447–461. [DOI] [PubMed] [Google Scholar]
- 19. Yuan S, Zheng H, Zheng Z, Yan W. Proteonomic analyses reveal a role of cytoplasmic droplets as an energy source during epididymal sperm maturation. PLoS One 2013; 8(10):e77466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin JL, O’Doherty AM, Wang S, Zheng H, Sanders KM, Yan W. Catsper3 and Catsper4 encode two cation channel-like proteins exclusively expressed in the testis. Biol Reprod 2005; 73:1235–1242. [DOI] [PubMed] [Google Scholar]
- 21. Jin J, Jin N, Zheng H, Ro S, Tafolla D, Sanders KM, Yan W. Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol Reprod 2007; 77:37–44. [DOI] [PubMed] [Google Scholar]
- 22. Harumi T, Hoshino K, Suzuki N. Effects of sperm-activating peptide I on Hemicentrotus pulcherrimus spermatozoa in high potassium seawater. Dev Growth Diff 1992b; 34:163–172. [DOI] [PubMed] [Google Scholar]
- 23. Harumi T, Kurita M, Suzuki N. Purification and characterization of sperm creatine kinase and guanylate cyclase of sea urchin Hemicentrotus pulcherrimus. Dev Growth Diff 1992a; 34:151–162. [DOI] [PubMed] [Google Scholar]
- 24. Morisawa M, Suzuki K, Morisawa S. Effects of potassium and osmolality on spermatozoan motility of salmonid fishes. J Exp Biol 1983; 107:105–113. [DOI] [PubMed] [Google Scholar]
- 25. Hay DE. Reproductive biology of Pacific herring (Clupea pallasi). Can J Fish Aquat Sci 1985; 42:s111–s126. [Google Scholar]
- 26. Carolsfeld J, Tester M, Kreiberg H, Sherwood NM. Pheromone-induced spawning of Pacific herring. Horm Behav 1997; 31:256–268. [DOI] [PubMed] [Google Scholar]
- 27. Riehl R, Kokoscha M. A unique surface pattern and micropylar apparatus in the eggs of Luciocephalus sp. (Perciformes, Luciocephalidae). J Fish Biol 1993; 43:617–620. [Google Scholar]
- 28. Kunz YW. Developmental Biology of Teleost Fishes. New York, NY USA: Springer; 2004. [Google Scholar]
- 29. Ho HC, Suarez SS. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction 2001; 122:519–526. [DOI] [PubMed] [Google Scholar]
- 30. Ren D, Xia J. Calcium signaling through CatSper channels in mammalian fertilization. Physiology 2010; 25:165–175. [DOI] [PubMed] [Google Scholar]
- 31. Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature 2001; 413:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dedkova EN, Sigova AA, Zinchenko VP. Mechanisms of action of calcium ionophores on intact cells: ionophore-resistant cells. Membr Cell Biol 2000; 13:357–368. [PubMed] [Google Scholar]
- 33. Cosson J. Frenetic activation of fish spermatozoa flagella entails short-term motility, portending their precocious decadence. J Fish Biol 2010; 76:240–279. [DOI] [PubMed] [Google Scholar]
- 34. Lillie FR. Problems of Fertilization. Chicago: University of Chicago Press; 1919. [Google Scholar]
- 35. Amanze D, Iyengar A. The micropyle: a sperm guidance system in teleost fertilization. Development 1990; 109:495–500. [DOI] [PubMed] [Google Scholar]
- 36. Chen CH, Wu CC, Shao KT, Yang JS. Chorion microstructure for identifying five fish eggs of Apogonidae. J Fish Biol 2007; 71:913–919. [Google Scholar]
- 37. Britz R, Toledo-Piza M. Egg surface structure of freshwater toadfish Thalassophryne amazonica (Teleostei: Batrachoididae) with information on its distribution and natural habit. Neotrop Ichthyol 2012; 10:593–599. [Google Scholar]
- 38. Betancur RR, Broughton RE, Wiley EO, Carpenter K, Lopez JA, Li C, Holcroft NI, Arcila D, Sanciangco M, Cureton II JC, Zhang F, Buser T et al. The tree of life and a new classification of bony fishes. PLoS Curr 2013. doi:10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pillai MC, Shields TS, Yanagimachi R, Cherr GN. Isolation and partial characterization of the sperm motility initiation factor from eggs of the Pacific herring, Clupea pallasi. J Exp Zool 1993; 265:336–342. [Google Scholar]
- 40. Oda S, Igarashi Y, Ohtake H, Sakai K, Shimizu N, Morisawa M. Sperm-activating proteins from unfertilized eggs of the Pacific herring, Clupea pallasii. Dev Growth Diff 1995; 37:257–261. [DOI] [PubMed] [Google Scholar]
- 41. Oda S, Igarashi Y, Manaka K, Koibuchi N, Sakai-Sawada M, Morisawa M, Ohtake H, Shimizu N. Sperm-activating proteins obtained from the herring eggs are homologous to trypsin inhibitors and synthesized in follicle cells. Dev Biol 1998; 204:55–63. [DOI] [PubMed] [Google Scholar]
- 42. Ohta H, Takano K. Ultrastructure of micropylar cells in the pre-ovulatory follicles of Pacific herring, Clupea pallasi Valenciennes. Bull Fac Fish Hokkaido Univ 1982; 33:57–64. [Google Scholar]
- 43. Zhang T, Feng S, Pan Z. Scanning electron microscopic observations on the sperm entry into the eggs of goldfish, Carassius auratus. Zool Res 1993; 14:166–170. [Google Scholar]
- 44. Koya Y, Matsubara T, Nakagawa T. Efficient artificial fertilization method based on the ovulation cycle in barfin flounder Verasper moseri. Fish Sci 1994; 60:537–540. [Google Scholar]
- 45. Matsubara T, Koya Y. Course of proteolytic cleavage in three classes of yolk proteins during oocyte maturation in barfin flounder Verasper moseri, a marine teleost spawning pelagic eggs. J Exp Zool 1997; 278:189–200. [Google Scholar]
- 46. Ishijima S, Hamaguchi Y, Iwamatsu T. Sperm behavior in the micropyle of the medaka egg. Zool Sci 1993; 10:179–182. [Google Scholar]
- 47. Takano M, Onitake K. On the mode of sperm entry into the micropylar canal in the medaka, Oryzias latipes. Zool Sci 1989; 6:1169. [Google Scholar]
- 48. Iwamatsu T, Yoshizaki N, Shibata Y. Changes in the chorion and sperm entry into the micropyle during fertilization in the teleost fish, Oryzias latipes. Dev Growth Diff 1997; 39:33–41. [DOI] [PubMed] [Google Scholar]
- 49. Iwamatsu T, Ishijima S, Nakashima S. Movement of spermatozoa and changes in micropyles during fertilization in medaka eggs. J Exp Zool 1993; 266:57–64. [Google Scholar]
- 50. Iwamatsu T, Shibata Y, Kanie T. Changes in chorion proteins induced by the exudate released from the egg cortex at the time of fertilization in the teleost, Oryzias latipes. Dev Growth Diff 1995; 37:747–759. [DOI] [PubMed] [Google Scholar]
- 51. Yoshida M, Ishikawa M, Izumi H, De Santis R, Morisawa M. Store-operated calcium channel regulates the chemotactic behavior of ascidian sperm. Proc Natl Acad Sci USA 2003; 100:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Darszon A, Guerrero A, Galindo BE, Nishigaki T, Wood CD. Sperm-activating peptides in the regulation of ion fluxes, signal transduction and motility. Int J Dev Biol 2004; 52:595–606. [DOI] [PubMed] [Google Scholar]
- 53. Darszon A, Nishigaki T, Beltran C, Trevino CL. Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev 2011; 91:1305–1355. [DOI] [PubMed] [Google Scholar]
- 54. Suzuki R. Sperm activation and aggregation during fertilization in some fishes. I. Behavior of spermatozoa around the micropyle. Embryologia 1958; 4:93–102. [Google Scholar]
- 55. Suzuki R. Sperm activation and aggregation during fertilization in some fishes. VII. Separation of the sperm-stimulating factor and its chemical nature. Jap J Zool 1961;13:79–100. [Google Scholar]
- 56. Miller RL. Sperm chemo-orientation in the metazoa. In: Metz CB, Monroy A (eds), Biology of Fertilization, vol. 2 New York: Academic Press, 1985:275–337. [Google Scholar]
- 57. Yoshida M, Yoshida K. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol Hum Reprod 2011; 17:457–465. [DOI] [PubMed] [Google Scholar]
- 58. Morita M, Nishikawa A, Nakajima Y, Iguchi A, Sakai K, Takemura A, Okuno M. Eggs regulate sperm flagellar motility initiation, chemotaxis and inhibition in the coral Acropora digitifera, A. gemmifera and A. tenuis. J Exp Biol 2006; 209:4574–4579. [DOI] [PubMed] [Google Scholar]
- 59. Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill DJ (eds), The Physiology of Reproduction, 2nd ed New York: Raven Press, 1994:189–317. [Google Scholar]
- 60. Kaupp UB. 100 years of sperm chemotaxis. J Gen Physiol 2012; 140:583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Clapham DE. Sperm BerserKers. eLife 2013, 2:e01469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vacquier VD, Loza-Huerta A, Garcia-Rincon J, Darszon A, Beltran C. Soluble adenylyl cyclase of sea urchin spermatozoa. Biochim Biophys Acta 2014; 1842:2621–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Seifert R, Flick M, Bonigk W, Alvarez L, Trotschel C, Poetsch A, Muller A, Goodwin N, Pelzer P, Kashikar ND, Kremmer E, Jikeli J et al. The CatSper channel controls chemosensation in sea urchin sperm. EMBO J 2015; 34:379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cai X, Clapham DE. Evolutionary genomics reveals lineage-specific gene loss and rapid evolution of a sperm-specific ion channel complex: CatSpers and CatSper β. PLoS One 2008; 3:e3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Barrio AM, Lamichhaney S, Fan G, Rafati N, Pettersson M, Zhang H, Dainat J, Ekman D, Hoppner M, Jern P, Martin M, Nystedt B et al. The genetic basis for ecological adaptation of the Atlantic herring revealed by genome sequencing. eLife 2016; 5:e12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Morisawa M. Cell signaling mechanisms for sperm motility. Zool Sci 1994; 11:647–662. [PubMed] [Google Scholar]
- 67. Miura C, Ohta T, Ozaki Y, Tanaka H, Miura T. Trypsin is a multifunctional factor in spermatogenesis. Proc Natl Acad Sci USA 2009; 106:20972–20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci USA 2007; 104:7688–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cherr G, Vines CA, Smith EH, Pillai M, Griffin F, Yanagimachi R. Sperm motility initiation in Pacific herring. In: Cosson JJ. (ed), Flagellar Mechanics and Sperm Guidance. United Arab Emirates: Bentham Sci Pub; 2015:207–224. [Google Scholar]
- 70. Costello S, Michelangeli F, Nash K, Lefievre L, Morris J, Machado-Oliveira G, Barratt C, Kirkman-Brown J, Publicover S. Ca2+-stores in sperm: their identities and functions. Reproduction 2009;138:425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gur Y, Breitbart H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev 2006; 20:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Takei GL, Mukai C, Okuno M. Regulation of salmonid fish sperm motility by osmotic shock-induced water influx across the plasma membrane. Comp Biochem Physiol (A) 2015; 182:84–92. [DOI] [PubMed] [Google Scholar]
- 73. Cosson MP, Billard R, Letellier L. Rise of intracellular Ca2+ accompanies the initiation of trout sperm motility. Cytoskeleton 1989; 14:424–434. [Google Scholar]
- 74. Boitano S, Omoto CK. Trout sperm swimming patterns and role of intracellular Ca++. Cell Motil Cytoskeleton 1992; 21:74–82. [Google Scholar]
- 75. Krasznai Z, Marian T, Izumi H, Damjanovich S, Balkay L, Tron L, Morisawa M. Membrane hyperpolarization removes inactivation of Ca2+ channels, leading to Ca2+ influx and subsequent initiation of sperm motility in the common carp. Proc Natl Acad Sci USA 2000; 97:2052–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Creech MM, Arnold EV, Boyle B, Muzenich MC, Montvelle C, Bohle DS, Atherton RW. Sperm motility enhancement by nitric oxide produced by the oocytes of fathead minnow, Pimephelas promelas. J Andrology 1998; 19:667–674. [PubMed] [Google Scholar]
- 77. Sano K, Kanatani H. External calcium ions are requisite for fertilization of sea urchin eggs by spermatozoa with reacted acrosomes. Dev Biol 1980; 78:242–246. [DOI] [PubMed] [Google Scholar]
- 78. Ginsburg AS. Sperm-egg association and its relationship to activation of the egg in salmonid fishes. Development 1963; 11:13–33. [PubMed] [Google Scholar]
- 79. Yanagimachi R. Calcium requirement for sperm-egg fusion in mammals. Biol Reprod 1978; 19:949–958. [DOI] [PubMed] [Google Scholar]
- 80. Hay JC. Calcium: a fundamental regulator of intracellular membrane fusion? EMBO Rep 2007; 8:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Grayson P. Izumo 1 and Juno: The evolutionary origins and coevolution of essential sperm-egg binding partners. Roy Soc Open Sci 2015; 2:150296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014; 508:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005; 434:234–238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at BIOLRE online.
Supplemental Table S1. Details of antibodies used throughout this study.
Supplemental Movie 1. Movement of cresthead flounder spermatozoa in normal seawater, exhibiting straightforward motion.
Supplemental Movie 2. Movement of cresthead flounder spermatozoa in seawater containing 1–10 μM ionomycin. Note that many spermatozoa display spiral, circular, spinning, or whiplash movement.
Supplemental Movie 3. Micropyle of cutthroat trout egg filled with black flounder spermatozoa.
Supplemental Movie 4. Herring spermatozoa are intrinsically motionless in normal seawater, but a few display sporadic jumping motion from time to time. This movement can occur in Ca2+-free seawater, perhaps due to temporal Ca2+ release from internal stores.
Supplemental Movie 5. Herring spermatozoa become actively motile on contact with the chorion surrounding the micropyle. The spermatozoon that enters the micropyle first fuses with the egg; the remaining spermatozoa are expelled from the micropylar canal.
Supplemental Movie 6. Spermatozoa of most fish begin to move actively upon contact with the water in which spawning takes place. (A) This movie shows that black flounder spermatozoa swim freely in seawater, but when they collide with the chorion, they begin to swim along the chorion surface (thigmotactically) for some time before leaving it. (B) This movie shows loach (Lefua nikkonis) spermatozoa moving toward the micropyle after colliding with the chorion near the micropyle.