Abstract
Apoptosis is a well-conserved cellular destructive event which has been implicated in a variety of diseases such as cancers and neurodegenerative diseases. The comprehensive investigation of apoptosis has been emerged in the field of skeletal muscle biology. Results have been consistent in demonstrating the activation of apoptotic machinery in different pathologic and physiologic muscle atrophic conditions including muscle disuse, hindlimb unloading, muscle dystrophy, sarcopenia, and neuromuscular diseases. Together with the other identified muscle atrophy-related signaling mechanisms such as NFκB, FOXOs/MuRFl/MAFbx and ubiquitin-proteasome, apoptosis has been advocated as an important candidate in regulating denervation-induced muscle loss. The purpose of this article is to review the role and signaling mechanisms of apoptosis during denervation in skeletal muscle including myofibers and satellite cells.
Keywords: Apoptosis, Skeletal Myofiber, Satellite Cell, Nerve Innervation, Denervation, Review
2. INTRODUCTION
Early investigation of apoptosis was exclusively accomplished in mitotic, single nucleated cell population in which apoptosis was demonstrated to function to attain self-dismissal or self-degeneration of cells (1). The designation of “programmed cell death” noticeably indicates that the machinery of apoptosis involves tightly controlled processes of induction, execution, and degradation which are regulated under a highly coordinated and sequential manner (1–6). It is widely accepted that along with proliferation, apoptosis is another indispensable biological event for numerous vital cellular procedures, for example maintenance of tissue homeostasis, immunological defence, and embryonic development (7). In general, three classes of apoptotic signaling pathways: mitochondria-, death receptor- and calcium-mediated pathways have been broadly categorized (8–13). Given that the cellular traits of proliferating capacity and cellular nuclei content of skeletal myofibers are completely different from mitotic single nucleated cell types, it is noteworthy that research has provided evidences showing that these apoptotic machineries and the related components are well conserved in post-mitotic multinucleated skeletal myofibers (14–22). Although the exact physiologic functions of apoptosis in skeletal muscle have not been completely elucidated, significant research progress has been made which has unveiled the potential role of apoptosis in muscle plasticity including both the physiologic and pathologic remodeling. This review will summarize the existing data that have contributed to understanding of the role of apoptosis in skeletal muscle loss associated with denervation.
3. APOPTOSIS: A BIOLOGICAL DISMISSAL EVENT
The phenomenon of necrosis refers to an unorderly cell dismissal process which invokes immunological response and severe cellular damage. In contrast, apoptosis is an energy-dependent internally-encoded biological destructive process that involves tightly regulated cellular signaling to coordinate the sequential apoptotic cascades (2–6). Generally, necrosis leads to cell death caused by accidental lethal insult whereas apoptosis results in cell dismissal by devised plan and with specific intention (1). Apoptosis is occasionally referred to the term “programmed cell death”. Indeed, apoptosis belongs to one of the main types of programmed cell death. Other than apoptosis, there are other types of programmed cell death such as autophagy (23;24).
The word “apoptosis” originates from Greek (apo - from; ptosis - falling) which means “falling off’. It represents a fundamental biological process that is highly conserved among species ranging from worms to humans (3;25). The phenomenon of apoptosis was first systematically defined more than 30 years ago by Kerr and colleagues (1), who described the distinctive morphological characteristics of cell death in the nematode Caenorhabditis elegans (C. elegans). The documented apoptosis-related morphological characteristics include cell shrinkage, cell membrane blebbing, chromatin condensation, intemucleosomal degradation of chromosomal DNA, and formation of membrane-bound fragments called apoptotic bodies (1). Following the demonstration of the phenomenon of apoptosis in C. elegans, homologous apoptotic regulatory death genes have been successfully identified in a variety of organisms including mammals and humans (26). In the past decades, there has been much interest in understanding the biological role and the regulatory mechanisms of apoptosis in life science and disease pathophysiology. Functionally, it has been clearly demonstrated that apoptosis is related to the elimination of damaged, aberrant, or harmful cells. A vital role is carried out by the operation of apoptosis in allowing normal embryonic development, tissue turnover, and immunological function (7). Based on the crucial physiologic role of apoptosis in coordinating the fundamental homeostatic balance among cell proliferation, differentiation, and cell death in multicellular organisms, health would be threatened if this homeostatic balance is not adequately maintained or being disrupted. In fact, aberrant regulation of apoptosis has been demonstrated to contribute to the pathogenesis of severe diseases including viral infections, cancers, neurodegenerative diseases (Alzheimer’s and Parkinson’s diseases), autoimmune diseases (e.g., systemic lupus erythematosus and rheumatoid arthritis), myocardial and cerebral ischemic injuries, loss of pancreatic beta-cell in diabetes mellitus, toxin-induced liver disease, and acquired immune deficiency syndrome (AIDS) (7;27–30). Aberrantly accelerated or the incapability to activate apoptosis can result in the pathogenesis of disorders such as neurodegenerative diseases and cancers development, respectively.
4. SIGNALING MECHANISMS REGULATING APOPTOSIS
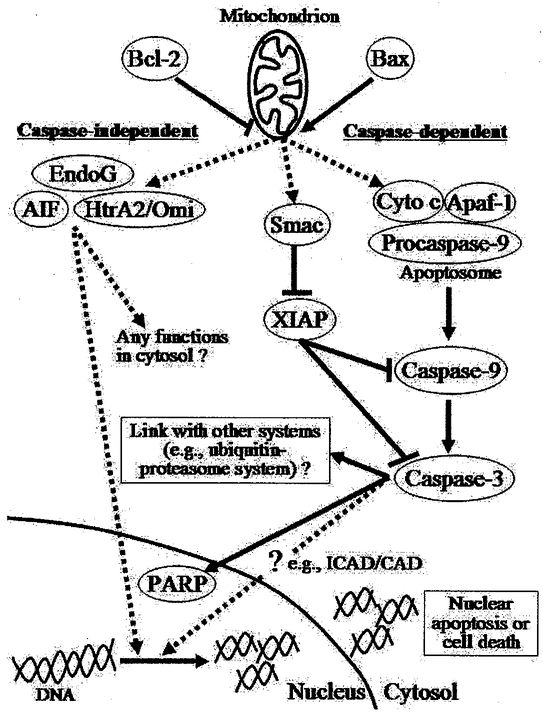
In single nucleated cell populations, apoptosis functions to destroy an intact cell through a cascade of cellular suicide machineiy. One of the distinctive characteristics of apoptosis is that it allows the execution of cell dismissal to occur in the absence of an inflammatory response and therefore causes no disturbance to the neighboring cells. This characteristic of apoptosis permits highly selective cell dismissal of certain designated individual cells among the whole cell population. So far, several apoptotic pathways have been identified in mediating cellular signaling transduction leading to the implementation of apoptosis. These apoptotic pathways include mitochondria-dependent, death receptor-mediated, and endoplasmic reticulum stress-induced pathways (8–13). It is noted that mitochondria-dependent and death receptor-mediated pathways are also known as intrinsic and extrinsic apoptotic pathways, respectively. These apoptotic pathways are named based on the origin of stimulus and the subcellular site that carries out the signaling events. Various gene products have been identified in playing a role in regulating the process of apoptosis and these gene products include B-cell leukemia/lymphoma-2 (BCL-2) family proteins, caspases, inhibitors of apoptosis proteins (IAPs), caspase-independent apoptotic factors including apoptosis inducing factor (AIF), endonuclease G (EndoG) and heat requirement A2 protein (HtrA2/Omi), and other apoptosis-related proteins like cytochrome c, apoptosis protease activating factor-1 (Apaf-1), apoptosis repressor with caspase recruitment domain (ARC), second mitochondria-derived activator of caspase/direct IAP-binding protein with low PI (Smac/DLABLO), p53, heat shock proteins (HSPs) and others. The participation of these apoptotic factors is selective in nature and is largely dependent on the apoptotic pathway being invoked. For example, initiator caspases-8, −9, and −12 are activated when the cell is exposed to corresponding stimuli. When apoptosis is stimulated by TNF-α and FasL which subsequently activate the death receptor apoptotic pathway, caspase-8 is the initiator caspase being triggered and responsible for the mediation of the corresponding subsequent signaling transduction (10;31). Apoptotic signaling initiated by intracellular calcium disturbance and endoplasmic reticulum stress is attributed to initial activation of caspase-12 (11;32), whereas caspase-9 mediates the mitochondria-dependent apoptosis through the interaction of procaspase-9 with Apaf-1, dATP/ATP, and cytochrome c. Although different initiator caspases (caspase-8, −12, and −9) are responsible for the initial signaling transduction in different apoptotic pathways, the signals eventually converge on the activation of common effector caspases-3, −6, or −7, which function to progress to the final dismissal of the target cell. Additionally, based on the involvement of caspase proteases, apoptotic signaling can also be classified into caspase-dependent and caspase-independent pathways (Figure 1).
Figure 1.
Caspase-dependent and -independent apoptotic pathways. Apoptotic signaling can be classified into caspase-dependent and caspase-independent pathways based on the presence of the involvement of caspase proteases. BCL-2 family proteins play an important role in the regulation of apoptotic signaling because they can influence the formation of permeability transition pore on mitochondria and therefore regulate the mitochondrial membrane permeability and the release of mitochondria-housed apoptogenic factors including cytochrome c (cyto c), Smac, A1F, EndoG, and HtrA2/Omi, etc. The caspase-independent apoptotic factors AIF, EndoG, and HtrA2/Omi have been demonstrated to have the ability to induce DNA fragmentation and therefore their nuclear translocation has been related to the occurrence of apoptosis. Caspase-9 functions to assemble a protein complex named the apoptosome through the interaction of procaspase-9 with Apaf-1, mitochondrial released cytochrome c (cyto c), and ATP/dATP in the cytosol. With the caspase-9-mediated proteolytic cleavage ability, the apoptosome protein complex subsequently activates caspase-3 by a proteolytic cleavage of procaspase-3. Caspase-3 is an effector caspase which executes a wide-range of cellular destructive events. It has been proposed to have a proteolytic function in breaking down the actomyosin myofibillaiy complex structure, which is an important initial step in proteolytic degradation of myofibillary proteins by ubiquitin-proteasome system. Further investigations are warrant in examining any linkages between apoptotic signaling and the other muscle degradation pathways.
4.1. BCL-2 protein family
The BCL-2 family serves as an important upstream intracellular checkpoint which plays a crucial role in the coordination of the apoptotic signaling (2). Mammalian cells encode for the entire family of BCL-2 proteins in the genome. Different classes of BCL-2 family members are defined by the homology shared within four conserved sequence motifs namely BH1, BH2, BH3, and BH4. In general, the BCL-2 family is divided into three subclasses of proteins: (i) anti-apoptotic (e.g., Bcl-2, Bcl-XL, Bcl-W, A1, and Mcl-1), (ii) multidomain pro-apoptotic (Bax, Bak, and Bok), and (iii) BH3-only pro-apoptotic (Bid, Bad, Bim, Bik, Dp5/Hrk, Noxa, and Puma) (2;33–35). Among the four identified BH domains, all pro-apoptotic members and most anti-apoptotic members contain the BH3 domain and therefore BH3 domain is believed to be essentially important for the interactions among the family members (33;36;37). The BH3 sequence motif has a hydrophobic α-helix which is favorable for protein interaction, and this is the putative region responsible for the association among the BCL-2 family members through homo- or hetero-oligomerization (33–35;38). The strict control on the balance of cell survival and apoptotic cell death is believed to be principally regulated by the relative ratio of pro- and anti-apoptotic BCL-2 members (2;33;39;40). Among the BCL-2 family members, proapoptotic Bax and anti-apoptotic Bcl-2 have been relatively well-studied. These proteins are thought to constitute the main protagonists in the regulation of apoptotic signaling because it has been exhibited that they can influence the formation of permeability transition pore on mitochondria and therefore regulate the mitochondrial membrane permeability and the release of mitochondria-housed apoptogenic factors including cytochrome c, AIF, and EndoG, etc (41–46). Upon the induction of apoptosis, Bax translocates to mitochondria and subsequently undergoes conformational change to expose its N-terminus (47–51). This conformational change has been suggested to allow the Bax-Bax-oligomerization and insertion of Bax into the outer mitochondrial membrane (52), which mediates the subsequent release of the apoptogenic factors (e.g., cytochrome c and AIF) from the mitochondrial intermembrane space. Regarding the interaction of Bax and Bcl-2, Bax oligomerization is critical for mitochondrial membrane permeabilization whereas Bcl-2 functions to prevent the Bax-Bax-oligomerization and therefore opposes the pro-apoptotic activity of Bax (36;37;39;41;43;53–57). The exact mechanisms on how the BCL-2 family proteins control the release of the mitochondrial apoptogenic factors are still under active investigation.
4.2. Caspases
Caspases belong to a specific protein family of broadly conserved proteases called cysteine-dependent aspartate proteases (58–60). Since the identification of the destructive role of caspases in apoptosis, caspases have been extensively studied and advocated to have a critical role in apoptotic signaling transduction (58–61). Excluding caspase-9 (62), caspases are normally synthesized in the cell as inactive zymogens named procaspases. According to the data from biochemical and structural studies, it is revealed that following the trigger by an apoptotic signal, inactive procaspases undergo scaffold-mediated transactivation, upstream proteases-mediated proteolytic cleavage, or initiator caspases oligomerization-mediated self-/auto-activation for the acquisition of the active protease activity (58–60;63;64). Caspase-9 is identified as an initiator caspase which has been shown to mediate the signaling transduction of mitochondria-mediated apoptosis. Caspase-9 functions to assemble a protein complex named the apoptosome through the interaction of procaspase-9 with Apaf-1, mitochondrial released cytochrome c, and ATP/dATP in the cytosol. With the caspase-9-mediated proteolytic cleavage ability, the apoptosome protein complex subsequently activates caspase-3 by a proteolytic cleavage of procaspase-3 (58;65–69). Caspase-3 is a common downstream effector caspase which executes a wide-range of cellular destructive events once it becomes activated. So far, there have been a variety of cellular molecules being demonstrated to be proteolytic substrates for caspase-3. These include proteins responsible for cell cycle regulation (e.g., p21Cip1/Waf1), apoptotic cell death (e.g., BcJ-2 and LAP), DNA repair (e.g., poly(ADP-ribose) polymerase (PARP) and inhibitor of caspase-activated DNase (ICAD), cell signal transduction (e.g., Akt/PKB), and cytoskeletal structural scaffold (e.g., gelsolin), etc. (58).
4.3. Caspase-independent apoptotic factors: AIF, EndoG AND HtrA2/OMI
Caspases have long been considered to be the central critical component for apoptosis execution (58–60). However, other than the caspase-dependent apoptotic pathways, several studies have shown that apoptosis can operate without the involvement of caspases (70–72). Besides the intrinsic mitochondrial and extrinsic death receptor apoptotic pathways, apoptotic signaling can be classified into caspase-dependent and caspase-independent pathways. For the caspase-independent apoptosis, a cluster of mitochondria-housed cellular factors including apoptosis-inducing factor (AIF), endonuclease G (EndoG) and high temperature requirement protein A2 (HtrA2/Omi) have been shown to be able to induce apoptosis without the involvement of caspases (70–72). AIF is a mitochondrial flavoprotein that has both oxidoreductase and apoptosis-inducing activities (71; 73–75). Although the exact physiologic function of AIF is not yet completely known, the apoptosis-executing ability of AIF has been well demonstrated. The apoptotic trait of AIF is believed to be related to the presence of a putative DNA binding site resulting in chromatin condensation and DNA fragmentation (76;77). EndoG is an evolutionarily conserved nuclear-encoded endonuclease which has also been demonstrated to have chromosomal DNA cleavage ability in a caspase-independent manner (72). In contrast, the mechanistic properties of another caspase-independent apoptotic factor, serine protease HtrA2/Omi, in causing apoptosis are relatively ambiguous. It has been thought that HtrA2/Omi induces apoptosis via the mechanism adopted by Smac/DIABLO which is to restrain the apoptosis-suppressing activities of LAPs through a caspase-involved process (78). However, it has also been shown that the apoptosis-inducing ability of HtrA2/Omi can operate by means of its proteolytic activity in the absence of caspase activation (70;79). It is worth noting that the precise regulatory mechanisms and the interdependence of these caspase-independent factors in the pro-apoptotic signaling are largely unknown and further investigations are warranted to folly understand their apoptotic role. Nonetheless, these caspase-independent factors are known to be normally resided in the mitochondrial intermembrane space and are released into cytosol once being triggered by the apoptotic signal (70–72;75).
4.4. Apoptotic suppressors: XIAP, ARC and FLIP
Among various identified apoptosis-regulating proteins, there is a group of endogenous proteins which function in suppressing the pro-apoptotic signaling. These apoptotic suppressor proteins include X-linked inhibitor of apoptosis (XIAP), apoptosis repressor with caspases recruitment domain protein (ARC), and Fas-associated death domain protein-like interleukin 1β-converting enzyme-like inhibitory protein (FLIP). Among IAP family proteins, XIAP is one of the potent apoptosis-inhibiting members. XIAP has been reported to be a fundamental conserved gene product among species (80;81). The anti-apoptotic ability of XIAP is attributed to the conserved baculovirus inhibitor of apoptosis repeat (BIR) motif which is the essential part for the inhibition of initiator as well as effector caspases and all protein members in IAP family are found to carry at least one of this BIR motif (82–85). ARC and FLIP are two endogenous apoptosis-suppressing proteins with high expression levels in muscle tissue (86;87). It is intriguing but not evidently proved that the known high resistance of mature muscle tissues to apoptosis is related to the abundant expressions of these two apoptotic suppressors. The apoptotic suppressive effects of ARC and FLIP have been suggested to be associated with their inhibiting interactions with selective caspases, in particular, caspase-8 which is the initiator caspase in death receptor-mediated apoptosis (88–90). However, later findings indicated that ARC is able to interact with pro-apoptotic Bax protein and so exhibits the apoptosis suppressive effect by influencing the mitochondria-mediated apoptotic signaling (91). In regard to FLIP, its apoptosis modulating effect is suggested to be related to the individual splice variants (i.e., protein isoforms), for instance FLIPS versus FLIPL or FLIPα.
5. APOPTOSIS IN SKELETAL MUSCLE
5.1. Apoptosis in postmitotic myofiber
Since its discovery, investigation of apoptosis has been primarily conducted in mitotic cell types. This occurred because the role of apoptosis was initially proposed to be responsible for the degeneration of intact cells, which is essential in maintaining the vital homeostatic balance between cell growth and death. It is well-known that the mechanism of cell removal by apoptosis is indispensable in many tissues under actively proliferative growth. For example, apoptosis is responsible for the continuous loss of intestinal epithelium which counterbalances the rapid proliferation of epithelial cells (92;93). In contrast, the mechanism of apoptosis is relatively unclear in terminally postmitotic cell populations including cardiomyocytes and skeletal myofibers. In particular, mature skeletal muscle is a highly specialized postmitotic tissue which has the characteristics of elongated cylindrical cell shape and multinucleate in each individual myofiber. Recently, apoptosis has been suggested to have a role in mediating the process of muscle loss. It has been shown that the machinery of apoptosis is very well conserved in skeletal myofibers. Although the exact physiologic functions of apoptosis in skeletal myofibers have not been completely revealed, activation of apoptosis and the corresponding pro-apoptotic signaling have been consistently demonstrated under different pathologic and physiologic muscle wasting conditions including neuromuscular disorders, hindlimb unweighting, muscle disuse/unloading, muscle dystrophy, muscle denervation, and sarcopenia (94–112); see for review (14–22). According to these scientific findings, speculation that apoptosis should have some role in mature skeletal myofiber is reasonable. However, the mechanism whereby apoptosis participates in mediating the process of muscle wasting is not completely understood and this has become an emerging topic in the area of the study of muscle biology.
In single cell systems, apoptosis functions to eliminate nuclei and results in cellular dismissal. However, in skeletal muscle, there can be loss of one or more nuclei through an apoptotic mechanism without loss of the entire muscle cell. The process of apoptotic loss of myonuclei in skeletal muscle has been described as “nuclear apoptosis” (113). The idea of “nuclear apoptosis” is intriguing and exciting because apoptosis can selectively identify designated nuclei for destruction in multinucleated skeletal myofiber without targeting other nuclei. The findings that not all myonuclei in a single myofiber become apoptotic during muscle loss has been observed in experimental denervation and denervation-associated disease (e.g., infantile spinal muscular atrophy), which further substantiates the idea of “nuclear apoptosis” in modulating the myofiber volume by controlling the successive myofiber segments. This idea is indeed complementary for the proposed “nuclear domain hypothesis” which explains the phenomenon of cell size remodeling of myofiber by each nuclei controlling a defined cytoplasmic area.
The skeletal myofiber is a differentiated but highly plastic cell type which adapts considerably in response to loading and unloading. In regard to the plasticity of myofiber size, skeletal myofibers increase myofiber size and volume (i.e., hypertrophy) and reduce myofiber size and volume (i.e., atrophy) when exposed to chronic loading and unloading, respectively. According to the nuclear domain hypothesis, a nucleus controls a defined volume of cellular territory in each myofiber exists. Therefore, addition of extra nuclei into the myofiber is required to support the increment of cell size in order to achieve muscle hypertrophy and removal of the myonuclei is needed to allow the muscle to atrophy. Therefore, based on the nuclear domain hypothesis, the processes of hypertrophy and atrophy are presumed to be regulated (directly and/or indirectly) by controlling the number of myonuclei present in myofibers. In the other words, addition of extra nuclei leads to muscle hypertrophy whereas removal of nuclei results in muscle atrophy. While further substantial evidences are needed to prove the nuclear domain hypothesis, it has been demonstrated that the myogenic satellite cell, which is a muscle-specific adult stem cell, plays an important role in promoting muscle hypertrophy by providing the additional nuclei (114–117). The importance of satellite cells in mediating muscle hypertrophy has been in line with the nuclear domain hypothesis. In response to muscle loading, satellite cells are activated, proliferate, and fuse into the existing myofibers which results in the increment of the number of nuclei in myofiber. This proposition has been supported by the observation that inactivation of satellite cell proliferation can prevent or lessen the hypertrophic response to muscle loading. Consistent with this hypothesis, it has been suggested that muscle atrophy due to chronic disuse/unloading or certain cachexia-causing diseases is associated with the reduction of the existing myonuclei in muscle cell (i.e., decrease in the number of myonuclei) (113). Although the mechanism responsible for the loss of myonuclei during muscle atrophy is unknown, elimination of myonuclei by nuclear apoptosis is a rational idea to explain the reduction of myonuclei number in atrophying myofibers. This idea is substantiated by data showing that some myonuclei undergo apoptosis (mostly determined by the technique of TUNEL assay) in many muscle wasting- and disease cachexia-associated conditions including muscle disuse/unloading/unweighting (94;100;102;111;112), muscular dystrophies (118; 119), neuromuscular disorders (21;22;120;121), HIV-acquired immunodeficiency syndrome (AIDS) (122), cancers (123;124), diabetes, chronic heart failure (125–127), chronic obstructive pulmonary disease (COPD) (128), chronic kidney disease (129), sepsis (130), burns (131;132), chronic alcoholic myopathy (133), aging associated sarcopenia (14;15;17–19;100;102;134;135), and muscle denervation (136;137).
5.2. Apoptosis in myogenic satellite cell
Satellite cells are a population of proliferative myoblast cells found in skeletal muscle and their discovery was reported by Mauro in 1961 (138). Satellite cells are normally located between the basal lamina and sarcolemma of myofiber and are mitotically quiescent under the non-stimulated inactive status. Since myonuclei are postmitotic and incapable of undergoing cell division, muscle satellite cells provide the only known important source for adding new nuclei. The addition of nuclei by satellite cells is an important event in contributing to muscle hypertrophy, postnatal muscle growth of mature myofiber as well as regeneration from injury/trauma (117). Due to the proliferative and regenerative nature, satellite cells have been suggested to be a potential valuable cell source for cell-based therapy or regenerative medicine. Experimental data have shown positive results in the outcomes following skeletal myoblast transplantation for diseases including Duchenne muscular dystrophy and heart failure (139;140). Nevertheless, the long-term practical value of satellite cell therapy is uncertain until the results of ongoing clinical trials are known. Nonetheless, the significant innate role of satellite cells in supporting muscle plasticity and adaptation, and particularly in muscle enlargement/hypertrophy has been well established. Given that satellite cells regulate the adaptive and regenerative ability of skeletal muscle (117), it has been suggested that the depletion of the number and the decline in the proliferative potential of muscle satellite cells contribute to the impairment of muscle regenerative capacity and reduction of contractile function in muscular degenerative situations (e.g., denervation, aging, Duchenne muscular dystrophy, and unloading-induced muscle atrophy) (141–146).
The depletion of satellite cells under degenerative states has been attributed to increased apoptosis. Although the mechanisms of the precise regulation of apoptosis in muscle satellite cells have not been elucidated, there has been some data showing that the apoptotic susceptibility of satellite cells is influenced by denervation and aging, two conditions that are associated with considerable muscle mass loss and functional decline (143;144;147–149). Jejurikar and colleagues have demonstrated the denervation-induced changes in satellite cell apoptosis by using rodent model of sciatic nerve transaction and these denervation-induced changes will be further discussed in the subsequent section “Denervation-induced apoptosis in satellite cell” (143). Subsequently, the same group of investigators demonstrated age-dependent differences in apoptotic susceptibility by showing that satellite cells isolated from the old rats have a greater response to proapoptotic agents by increasing apoptosis and caspases when compared to young rats (144). Furthermore, the age-related decrement in muscle recovery during stretch shortening cycles (SSCs) in a rodent muscle injury model has also been shown to be associated with the increase in satellite cell apoptosis (150). Data from an in vitro investigation demonstrated that Erb2-mediated anti-apoptotic signaling is required in the process of satellite cell activation when transited from quiescence state (151). It is noted that the exact molecular and signaling mechanisms in the regulation of apoptosis in satellite cell biology is still largely unknown. Addition investigation is needed to completely understand the physiologic role of apoptosis in the depletion of satellite cell during pathologic muscle wasting conditions.
6. APOPTOSIS IN DENERVATED MUSCLE
6.1. Denervation-induced apoptosis in myofiber
Innervation is a critical constituent in the development and maintenance of postnatal growth of skeletal muscle. Moreover, innervation is needed to attain development of the mature myofiber phenotype and normal physiologic functioning (152;153). Following the removal of innervation, muscle mass is quickly lost and the resultant contractile force markedly declines. In general, denervation is broadly involved in the pathogenesis of many severe neuromuscular diseases including spinal muscular atrophy, peripheral neuropathies and amyotrophic lateral sclerosis (ALS). Examination of the muscle samples collected from patients who have neuromuscular diseases have revealed a considerable degree of denervated muscle fibers. As proposed by Tews and colleagues, defective innervation or a lack of innervation likely prompts myofibers to activate an intrinsic suicide program (i.e., apoptosis) meaning that myofibers are incapable of surviving and are programmed to die if they are not under adequate nervous stimulation (22;120;121;136).
Muscle mass loss is the consequence of increased protein degradation and decreased protein synthesis, which results in considerable myofiber atrophy. When catabolic events overwhelm the anabolism in muscle, the overall rate of proteolysis increases but protein synthesis reduces. This results in a net loss of muscle protein content in response to denervation. Several research studies have identified potentially important signaling pathways responsible for muscle atrophy including IGF/PI3-kinase/AKTl, NFkappaB, ubiquitin-proteasome, FOXOs/MuRFl/MAFbx(atrogin-1), lysosomal calcium-related calpain, and caspase signaling (154–157). The data have consistently shown that denervation activates the program of apoptosis in skeletal muscle. Indeed, there are several review articles which have addressed the role of muscle apoptosis in neuromuscular diseases (21;22;158). While other molecular mechanisms contributing to muscle loss (e.g., ubiquitin-proteasome system) have been comprehensively reviewed (154–157;159–161), the scope of apoptosis in skeletal muscle during experimental denervation has not been previously reviewed and will be discussed here.
From developmental and survival standpoints, myogenesis is necessary in the formation of mature myofíbers and remodeling process for muscle plasticity. Along with the finding that apoptosis contributes to the process of myogenesis in skeletal muscle (162–166), apoptosis has been proposed to have an important role in the development of the pathologic condition leading to severe muscle/neuromuscular diseases. An increase in DNA fragmentation as an indicator of nuclei that are undergoing apoptosis in response to denervation has been shown in both human and animal studies. Compelling evidence suggests that the apoptotic signaling pathway and the related apoptotic components (e.g., caspases) that have been identified in mitotic cell types are fully conserved in mature postmitotic skeletal myofíbers.
Many studies in both patients with neuromuscular diseases (120;121;167–170) and in experimental muscle denervation animal models (95;136;171–176) suggest that apoptosis has an important role in skeletal muscle loss as induced by defective innervation. Although the exact physiologic function of apoptosis in muscle plasticity is not completely understood, it is logical to propose that apoptosis contributes to muscle mass loss during complete or partial denervation. This is based on the consistent demonstration of the activation of apoptosis in denervated atrophying skeletal myofiber. Nonetheless, TUNEL-positive myonuclei or the features of activation of apoptosis are occasionally not found in atrophying muscle during denervation but in myofíbers undergoing the process of regeneration following denervation. These observations suggest that apoptosis may have some unidentified physiologic function in coordinating the remodeling of myofiber in response to tremendous stress and apoptotic machinery may be related to the regenerative process during the denervation-induced muscle adaptation/remodeling (148; 176; 177). The early demonstration of myofiber apoptosis at the ultrastructural level in human denervation disorders has been reported in a study examining infantile spinal muscular atrophy where muscle apoptosis was proposed as a mechanism contributing to the pathogenesis of this disease (168). The specificity of apoptosis was shown ultrastructurally by the presence of apoptosis-specific morphological properties like the formation of membrane-bound muscle cell fragments and apoptotic bodies in the examined muscle samples collected from a child who died eight weeks after birth from acute spinal muscular atrophy (168). These observations indicated that denervated atrophying myofiber degradation involves an apoptotic mechanism in which degrading myofíbers form apoptotic bodies and are phagocytosed by neighboring myofíbers (168). Subsequent studies have provided further evidence of apoptotic-induced DNA fragmentation from chromatin cleavage as detected by the technique of in situ end labeling, in muscles that were sampled from spinal muscular atrophy patients (120;170). Moreover, some data have demonstrated the changes of apoptotic regulatory factors including pro-apoptotic Bax, anti-apoptotic Bcl-2, and caspase proteases in human muscle denervation disorder (120;178;179). The protein product of survival motor neuron (SMN) gene has been shown to be involved in the regulation of apoptosis in motoneurons in muscle of spinal muscular atrophy patients (180–182). Further investigations revealed that SMN is also related to skeletal myofiber apoptosis in addition to the apoptotic cell death of neurons in spinal muscular atrophy (167;183). In addition to spinal muscular atrophy, the activation of apoptosis has also been shown in other human denervation-associated neuromuscular diseases including amyotrophic lateral sclerosis and peripheral neuropathies (21;120;121;177;179), but not in dystrophinopathies, myotonic dystrophy and inflammatory myopathies (170; 184).
Denervation-induced apoptosis has been investigated in experimental animal models in addition to human denervation-related neuromuscular disorders and mixed results are found. DNA breaks as determined by in situ nick translation and tailing assay were reported to be absent in the myonuclei of rodent hindlimb muscle following 30 weeks of denervation (148). But, in another animal study, the characteristics of apoptotic cell death were reported in skeletal muscle after several months of denervation (2,4 or 7 months) and the observed apoptotic events were suggested to be distinct from classical apoptosis that have been shown in other cell type like thymocytes and lymphoid cells (171). By using electron microscopy, differences at the ultrastructural level were found between 2-month denervated and normal muscle tissues. These differences include loss of significant amounts of myofibrils, misalignment of myofibrils and loss of their longitudinal orientation, formation of myofibril-free zones locating mostly at the peripheral areas of sarcoplasm around myonuclei with the presence of degenerated organelles, secondary lysosomes, residual bodies and lipofuscin granules (171). Some changes were found to be more apparent and frequent in muscle after 4 months of denervation. These include degenerative changes as indicated by myofíbers with tortuosity and blebbing of the sarcolemma, presence of myonuclei with increased levels of chromatin condensation, myonuclei that carried convoluted, crescent-like and lobulated nuclear patterns, deep elongated and rounded protrusions budding from myonuclei, and the presence of clusters of spatially isolated rounded bodies consisting of condensed nuclear material (171). Moreover, in 4-month denervated muscle, dead cells with highly condensed nuclei and cytoplasm and evidence showing muscle cell fragmentation into several electron dense bodies were reported (171). However, in this study, apoptosis as detected by the TUNEL assay, showed inconsistency with the ultrastructural morphological apoptotic characteristics. Strong TUNEL-positive labeling was found only in a very small fraction of myonuclei in 2- and 4-months denervated muscle (171). Furthermore, the TUNEL reactivity was observed in a fraction of myocytes presenting abnormal changes (e.g., hypercondensation of chromatin and nuclear fragmentation). Nevertheless, based on their double staining results of DNA fragmentation and nuclear DNA, the number of myonuclei with abnormal morphology and/or condensed chromatin far exceeded the number of TUNEL-positive myonuclei (171). This represents the inconsistent apoptotic results between the morphological and biochemical detection. The authors explained that the observed inconsistency between the morphology- and TUNEL/DNA fragmentation-detected apoptosis exists also in the nonmuscle tissues (e.g., thymocytes and cerebellum) (171;185;186). This study speculated that cell death in skeletal muscle during months of experimental denervation is distinct from classical apoptosis in the form being described in mammalian lymphoid cells (171). However, different levels or types of apoptosis have not been shown in skeletal muscle. One should keep in mind that denervation-activated muscle apoptosis in human denervating disorders has been generally replicated in the animal experimental denervation model. These data are valuable as they provided clear morphological and TUNEL evidences confirming that the mature postmitotic skeletal myofiber is fully capable of activating the cellular program of apoptosis.
Understanding the signaling events responsible for the activation of apoptosis is important because this would result in revealing the physiologic role and the regulatory mechanisms of apoptosis during muscle denervation. However, there is a scarcity of data describing the muscle apoptotic signaling in denervation. Some understanding of the apoptotic regulatory factors in denervation disorders has been obtained from investigating denervating diseases including spinal muscular atrophy, peripheral neuropathy, amyotrophic lateral sclerosis (ALS) and polyneuropathy. The change in the expressions of pro-apoptotic Bax and anti-apoptotic Bcl-2 has been demonstrated in muscle samples collected from 15 months old children who suffered from infantile spinal muscular atrophy (120;167;178). The immunohistochemical analysis revealed that the majority of infantile spinal muscular atrophy muscle specimens exhibited no expression of anti-apoptotic Bcl-2 protein in myofibers (120). Some but very few muscle samples showed immunoreactivity to a Bcl-2 antibody. Some myofiber nuclei demonstrated a peripheral rim of Bcl-2 expression and these myofibers were predominantly small size myofibers (120). This study showed that the number of detected myofibers with DNA fragmentation and Bcl-2 expression was not correlated with the extent of myofiber atrophy (120). This study showed that myofibers from peripheral neuropathy muscle specimens had strong expression of Bcl-2 protein and the Bcl-2 expression was found in small atrophic, angulated myofibers (120). However, no correlation was found between the extent of DNA fragmentation and the Bcl-2 protein expression (120). In a subsequent study conducted by the same group of investigators, pro-apoptotic Bax, in addition to Bcl-2 were examined in skeletal myofibers of spinal muscular atrophy patients (178). Particularly, the authors provided a description of the expression pattern of Bcl-2, Bax, Bcl-x and interleukin-1 beta converting enzyme (ICE) in the onset stage of spinal muscular atrophy. A positive correlation was reported between the Bax expression and defective innervation of myofibers (178). Different expression patterns of Bax protein were observed. Bax expressed in all myofibers including normal, hypertrophic, and atrophic myofibers in early-onset spinal muscular atrophy. In late-onset spinal muscular atrophy, the expression of Bax was only found in atrophic myofibers (178). The restricted expression of Bax in atrophic myofibers was also observed in muscle samples from peripheral neuropathies patients (178). Consistent with the previous Bcl-2 observation (120), Bcl-2 and Bcl-x were expressed only in atrophic myofibers and their expressions were predominantly restricted in late-onset spinal muscular atrophy and peripheral neuropathies (178). According to these results, it seems that the expression of pro-apoptotic Bax is upregulated in parallel with the myofiber atrophy in denervation disorders whereas the expression of anti-apoptotic Bcl-2 is also upregulated but in a less intense fashion and is lacking in some early stage of the disease (i.e., early infantile spinal muscular atrophy). The authors stated that the lack of expression of the anti-apoptotic proteins in infantile spinal muscular atrophy was associated with the immaturity of myofibers which was due to defects in innervation and they assumed that immature myofibers are less efficient in generating strong expression of anti-apoptotic Bcl-2 (120; 178). Since the activation of anti-apoptotic proteins (e.g., expression of Bcl-2) is present together with the apoptosis-promoting proteins including elevated expression of Bax, the balance between the apoptosis-promoting factors and anti-apoptotic strategies seems to determine the fate of myofibers during denervation and data indicated that denervated myofibers eventually undergo apoptosis-related degenerative process and therefore atrophy (22;120;178). The lack of anti-apoptotic Bcl-2 expression in the early stages of muscle development has been proposed as a secondary inducer of apoptotic cell death in denervated muscle which may exacerbate the further pathogenesis of the disease (22;120;178). While the presence of apoptotic nuclei with DNA fragmentation has been confirmed in a study examining the muscle tissues sampled from patients of spinal muscular atrophy I (aged 3 to 5 months) and spinal muscular atrophy III (aged 15 to 19 years) using the technique of in situ end labeling, ICE-like protease immunopositive myofibers were not found in spinal muscular atrophy muscles (170). The expression patterns of apoptotic factors including Bcl-2 and ICE have been particularly inconsistent in data obtained from human denervation disorders. For instance, strong expression of Bcl-2, Bax and ICE as measured by immunohistochemical techniques was found in muscles of patients with ALS and polyneuropathy (121). However, in another study, examination of survival motor neuron (SMN) protein in patients with dermatomyositis, polymyositis, morphologically non-specific myopathy, Duchenne muscular dystrophy, ALS, chronic peripheral neuropathies, rod myopathy, and mitochondrial myopathy revealed that SMN might play a role in denervation diseases but the strong expression of Bcl-2 in muscle with amyotrophic lateral sclerosis was not supported (167). These results showed that SMN, Bcl-2 and Bax were generally associated with the regenerating human myofibers, although the specific physiologic role was not identified (167). The response of caspases to muscle denervation was examined by demonstrating the expression patterns of initiator and effector caspases in denervated human muscle (179). In muscle biopsies obtained from patients with neurogenic muscular atrophy, denervated muscle was shown to have distinct elevation of initiator caspase-9 and effector caspase-7 but there was no change of effector caspase-2 and caspase-3 based on the results obtained by the immunohistochemistiy and western immunoblotting analyses (179). The data also showed different expression patterns of caspase-7 and caspase-9 where the expression of caspase-9 was observed in normal-sized/non-atrophic myofibers with confinement to single myofiber segments but the caspase-7 was only found in atrophic myofibers (179). The lack of the expression of Apaf-1 was reported in both the examined denervated and control human muscles (179). Although caspase-3 was not found to be upregulated in muscle of neurogenic muscular atrophy (179), there are some data to show that caspase-3, which is considered as the main downstream apoptotic executor and the converging downstream target of the initiator caspases, is upregulated in some other neuromuscular disorders including muscular dystrophy and mitochondria myopathies (108;187;188). Caspase-3 has been suggested to be more related to the process of myofiber regeneration rather than apoptosis (179). This suggestion was based on the observations that those neuromuscular disorders with demonstrated activation of caspase-3 were associated with the condition of dynamic regeneration of myofibers such as resembling of embryonic myofiber development. Therefore, it is suspected that the upregulation of caspase-3 might have a regenerative purpose (179).
The issue of apoptotic signaling in denervated muscle from animal studies has been relatively well studied. Tews and colleagues denervated rodent facial muscles followed by reinnervation of these muscles in order to investigate the causal effect of denervation on the activation of myofiber apoptosis (136). They demonstrated that nuclei DNA-fragmentation was first elevated in experimentally denervated rodent facial muscle and successful subsequent reinnervation reduced the chromatin breakdown in the previously denervated facial muscle. Subsequently, after 10 weeks of reinnervation, the elevated rate of DNA fragmentation returned and resembled the normal control muscle (136). The results of immunohistochemical analyses demonstrated strong protein expressions of Bcl-2, Bcl-xL, and Bax in experimentally denervated facial muscle whereas Bcl-xL and Bax were diminished after 7 weeks of reinnervation (136). However, Bcl-2 was continuously upregulated throughout the whole 20 weeks of the study period, which included both denervation and reinnervation (136). The authors suggested that the balance of apoptosis-inhibiting and -promoting factors (i.e., Bcl-2/Bcl-xL and Bax, respectively) played a role in determining the ability of myofibers to withstand apoptotic cell death until reinnervation (136). In addition to the demonstration of the change of expression of apoptotic factors including Bcl-2, Bcl-xL and Bax, this study provided evidence confirming that denervation causes the occurrence of DNA-fragmentation in myofibers and that can be reversed by reinnervating the muscle (136). By using sciatic nerve transection as the model of denervation-reinnervation neurological disorders, significant upregulation of Bax but unchanged Bcl-2 and lack of TUNEL-positive labeling were reported in rat tibialis anterior muscle after weeks of surgical procedure (176). Regarding the observations of elevation of Bax in the absence of apoptotic morphological features and DNA-fragmentation as determined by TUNEL-positive myonuclei, the authors suggested that upregulation of Bax might not be related to the process of cell death but rather the regeneration process of myofibers following denervation (176). It is not known whether the lack of DNA-fragmentation and features of apoptosis, which was conflicting with the previous animal denervationreinnervation findings and human denervating disorders, was due to the specific muscle degeneration conditions examined in this study. Nevertheless, responsive changes of Bax, Bcl-2 and Bcl-xL in myofibers have been clearly demonstrated during denervation.
By using the technique of arbitrarily primed PCR-mediated RNA fingerprinting, the differentially expressed genes were examined in rodent gastrocnemius muscle after tetrodotoxin (TTX)-induced sciatic nerve paralysis (173). This model simulates the denervation-like loss of neural activity to the muscle, but axonal transport stays undisrupted. This study demonstrated the upregulation of the transcript abundance of two apoptosis-related genes, TRAP-2 and calpain-3 in muscle after the loss of neural input. These data are consistent with the idea that apoptotic processes in the denervation-induced muscle atrophy is the result of loss of neural activity to the muscle rather than any disruption in axonal transport from the motoneuron (173). In a study in which denervation of rodent hindlimb gastrocnemius and soleus muscles was performed, caspase-8 enzymatic activity and Bax protein were reported to increase after 14 days of denervation of the medial and lateral branches of tibial nerve (which innervate the gastrocnemius and soleus muscles) in both young adult and old rodent muscles (95). The enzymatic activity of caspase-3,−7,−10 was shown to increase in the 14 day-deήervated young adult gastrocnemius and old soleus muscles (95). MyoD is a muscle-specific transcriptional regulator and a myogenic regulatory factor which plays an essential role in regulating the development and plasticity of skeletal muscle. While it has been demonstrated that MyoD expression is rapidly induced in skeletal muscle in response to the removal of innervation (189–191), MyoD may act as a protective factor against the denervation-induced apoptosis via the activation of p21 and Rb proteins (192). The immunhistochemical results of this study demonstrated the presence of co-localization of MyoD, p21 and Rb in myonuclei of plantaris muscle which has been denervated for days (192). In a subsequent study, rat gastrocnemius muscle was denervated by transecting the medial and lateral branches of tibial nerve and apoptotic DNA-fragmentation and the apoptotic factors involved in the mitochondrial apoptotic pathway were measured in muscle following 14 days of denervation (110). As indicated by the results of cytosolic nucleosome ELISA measurement, apoptotic DNA fragmentation was reported to be elevated by one-fold after 14-days of denervation (110). Reverse transcription-polymerase chain reaction (RT-PCR) and western immunoblot analyses revealed an increase of the Bax/Bcl-2 ratio both at the mRNA and protein levels. This was due to the magnitude of the elevation of the transcript and protein expressions of pro-apoptotic Bax which were relatively greater than that of anti-apoptotic Bcl-2. While both proapoptotic Bax and anti-apoptotic Bcl-2 were found to be upregulated in response to 14-days of denervation (110), these findings support previous observations that both proapoptotic and anti-apoptotic strategies in BCL-2 family proteins are simultaneously initiated in human denervated muscle (121;178). Denervated rodent muscle has also shown the mitochondrial release of cytochrome c, Smac/DIABLO and AIF, upregulation of caspase-3 and caspase-9 mRNA, active protein fragment and protease enzymatic activity, reduction of XIAP, and the presence of cleaved PARP protein fragment (110). No denervation-associated change was reported on Apaf-1, Id2 and c-Myc. However, the protein content of tumor suppressor protein p53 was observed to be dramatically elevated in both the extracted subcellular nuclear and cytosolic protein fractions in denervated muscle (110). Moreover, changes were found in the protein levels of stress/oxidative stress-related proteins including increased heat shock protein-70 (HSP70) and decreased manganese superoxide dismutase (MnSOD) together with no change in copper-zinc superoxide dismutase (CuZnSOD) in the denervated muscle (110).
Together these data provide direct descriptive evidence demonstrating the activation of the mitochondria-associated apoptotic signaling in skeletal muscle in response to denervation (110). The findings substantiated the idea that the “classical” mitochondria-mediated apoptosis is generally very well conserved in mature postmitotic muscle as indicated by the observed apoptotic signaling (110). The existence of mitochondria-associated apoptotic machinery has been further supported by data showing that the opening of the mitochondrial permeability transition pore (PTP) was promoted by denervation in skeletal muscle (193). By examining the mitochondria isolated from rodent hindlimb plantar muscles following 21 days of denervation by transecting sciatic nerve, Csukly and colleagues demonstrated that muscle denervation increased the sensitivity to calcium-induced opening of mitochondria PTP (193). This was partly attributed to the in vivo mitochondrial and whole muscle calcium overload, which increased the protein abundance of the PTP opening facilitating protein CypD relative to other mitochondrial marker proteins (193). The authors suggested that these changes may predispose the mitochondria to permeability transition in skeletal muscle in response to denervation (193). Because the opening of the mitochondria PTP contributes to part of the mechanism responsible for the release of several mitochondria-resided apoptotic factors such as cytochrome c and AIF, these data have accounted, at least partly, for the activation of apoptosis mediated by mitochondria. Furthermore, the mitochondrial PTP opening supports the notion that mitochondria-associated apoptosis is involved in the muscle remodeling process as induced by denervation (193). The activation of mitochondria-mediated apoptotic signaling by muscle denervation was consistent with data from rodent hindlimb muscles that were denervated by transecting sciatic or peroneal nerve for up to 42 days (137). Adhihetty and coworkers showed that the ratio of Bax/Bcl-2 was increased dramatically along with evidence of myonuclear apoptosis as indicated by TUNEL-positive nuclei labeling deep to the sarcolemmal membrane (identified via laminin staining). In the denervated muscle, they also found a reduction of mitochondrial content with decreases in mitochondrial transcription factor Tfam and transcriptional activator PGC-1α, elevated maximal rate of intermyofibillar mitochondrial pore opening (Vmax) and reduced intermyofibillar mitochondrial time to Vmax, decreased MnSOD protein abundance, and increased subsarcolemmal mitochondrial reactive oxygen species production (137). However there was a decreased production of reactive oxygen species in intermyofibillar mitochondria from denervated muscles (137). The large increase in the Bax/Bcl-2 ratio was attributed to elevation of pro-apoptotic Bax. However, a reduction in anti-apoptotic Bcl-2 was inconsistent with the previously reported both elevations of Bax and Bcl-2 in denervated muscles from animals and human denervation disorder muscles (110;120;121;136;137;194). It is not known whether different experimental conditions such as the way by which muscle denervation was induced or the duration of denervation have contributed to the conflicting Bcl-2 results. Intriguing findings also indicated that the two different morphologically distinct subfractions of mitochondria respond differently in terms of apoptotic signaling. These include subsarcolemmal mitochondria which are the mitochondria found immediately underneath the sarcolemmal membrane and intermyofibillar mitochondria which are mitochondria intermingled within the myofibrils in skeletal muscle. These subsets of mitochondria have differential susceptibility to apoptotic stimuli (195). The differential susceptibility of subsarcolemmal and intermyofibillar mitochondria to apoptotic stimuli has been demonstrated by different mitochondrial release of cytochrome c and AIF, rate of opening of permeability transition pore Vmax, expression of the mitochondrial permeability transition pore component voltage-dependent anion channel (VDAC) and cyclophilin D (195).
The causal role of the activation of apoptosis in mediating denervation muscle loss was investigated in a study using a genetic knockout (194). Because Bax has been suggested to play an essential role in coordinating the proapoptotic signaling and has been shown to be markedly upregulated in skeletal muscle following denervation, Bax may be a crucial candidate in the activation of denervation-induced apoptosis. This possibility was examined by testing the hypothesis that eliminating the Bax gene would suppress the pro-apoptotic signaling in the atrophying muscle during denervation and therefore attenuate the extent of the resultant muscle loss (194). Apoptotic DNA fragmentation, apoptotic regulatory factors and oxidative/cellular stress markers were examined in gastrocnemius muscle following 14 days of denervation in mice that were lacking of pro-apoptotic Bax gene (194). After 14 days of denervation, the extent of the decrease in muscle mass was found to be attenuated in Bax knockout mice when compared to the wild-type mice. While similar changes in mitochondrial AIF and Smac/DIABLO releases, Bcl-2, XIAP, HSP27, p53, and MnSOD were observed in Bax knockout and wild-type muscles, some different observations on the apoptotic markers were found exclusively in Bax knockout denervated muscle. These events included non-significant change of DNA fragmentation, unchanged caspase-3 and −9 activities, suppressed mitochondrial release of cytochrome c, unchanged oxidative stress markers comprising hydrogen peroxide, MDA/4-HAE and nitrotyrosine, and upregulation of ARC (194). These data have provided direct evidence indicating that pro-apoptotic signaling is involved in the atrophying process that leads to muscle loss during denervation. More importantly, strategies such as genetically removal of pro-apoptotic Bax gene as exhibited in this study is useful in ameliorating denervation-induced pathologic muscle wasting by interfering on the pro-apoptotic signaling (194). While the study of apoptosis in muscle wasting has just begun, it is warranted to have more intervention-based investigations on the causal effect of apoptosis on denervation muscle loss by using genetic, pharmaceutical, or other approaches. The precise physiologic role of apoptosis in muscle plasticity during denervation and the feasibility of the application of regimens or interventions aimed to inhibit apoptosis-related muscle wasting should therefore be explored.
Death receptor-mediated apoptosis contributes to an alternative pathway in executing the apoptotic program. Indeed, the activation of apoptosis appears to be related, at least in part, to the death receptor-mediated apoptotic signaling pathway in skeletal muscle during denervation. There have been some limited data reported the evidence of the activation of death receptor apoptotic events in both human and rodent denervated muscles (95;173;177;196). Jin and colleagues have demonstrated that the increased percentage of apoptotic muscle cells, decreased expression of Bcl-2 protein and mRNA as well as upregulated expression of the death receptor apoptotic factors including Fas, Fas-associated protein with death domain (FADD) and caspase-8 in atrophying rat muscle which has been denervated by severing the brachial plexus of forelimb (196). It is worth noting that this study also provided data showing the increase in apoptosis in skeletal myoblast isolated from atrophying muscle following weeks of denervation as determined by flow cytometry. By using the technique of RNA fingerprinting by arbitrarily primed PCR (RAP-PCR) and Northern blotting, Tang and coworkers have identified the upregulation of death receptor apoptotic gene, TNF type 1 receptor associate protein (TRAP-2) in response to nerve injury induced by sciatic nerve paralysis by tetrodotoxin (TTX) in rat muscle (173). In an experimental denervation rat model, Alway and colleagues demonstrated the activation of caspase-8 which is an essential apoptotic enzyme protease in mediating the death receptor apoptotic pathway (95). In addition, the death receptor apoptotic factors have been investigated in motor neuron disorder such as amyotrophic lateral sclerosis and polyneuropathy in human skeletal muscle (177). By using the technique of immunocytochemistry, Schoser and coworkers have shown immunopositive staining of Fas, Bax and Bcl-2 and they concluded that the FasL/Fas system and Bcl-2/Bax system were apparently active independently of DNA fragmentation and apoptosis in human denervating and atrophying muscle taken from sporadic amyotrophic lateral sclerosis and polyneuropathy patients (177). Overall, the documented data indicated that death receptor-mediated apoptotic pathway is possibly involved in muscle denervation apoptosis. Nonetheless, more investigations on this apoptotic signaling pathway are required with the aim to folly reveal the regulatory mechanism of apoptosis in mediating muscle loss with denervation.
6.2. Denervation-induced apoptosis in satellite cell
The myogenic satellite cell has an important role in mediating muscle development, postnatal growth, regeneration, and muscle plasticity (116;117;138). Satellite cells are normally quiescent. But when they are stimulated by loading, muscle trauma or damage, they are capable of proliferation. In response to muscle denervation, satellite cells are activated initially but the amount and the proliferative potential of satellite cells are found to be considerably declined during long-term chronic denervation (147; 148;197; 198). It has been reported that the percentage of satellite cell nuclei decreases to less than 20% of control values after 20–30 weeks of denervation, whereas 10 weeks of neonatal denervation has been shown to deplete almost all satellite cells (148). Similar findings show that satellite cells are reduced to 1% of all cells present within a skeletal muscle cross-section after 7 to 20 weeks of denervation (148;197). Lu et al. have used ultrastructural observations to show that 18 months of denervation in rodent skeletal muscle reduced the restorative capacity of skeletal muscle as demonstrated by significant myofiber atrophy, increase in the amount of interstitial collagen deposition, increased myofiber cell death, reduced capillarity, and lowered activation of satellite cells (198).
Apoptosis has been suggested to be related to the depletion of satellite cells during chronic muscle denervation (147). There have been some in vitro data demonstrating the activation of apoptosis in satellite cells in response to muscle denervation. A report examining muscle following 2 months of denervation has shown increases in nuclei with hypercondensed chromatin and fragmented DNA which were found diffusely throughout the examined muscle cross-section (171). Jejurikar et al. investigated the apoptotic responses of cultured satellite cells which were isolated from the rodent hindlimb muscle after 2 to 20 weeks of denervation as induced by sciatic nerve transaction. The cultured satellite cells were then subjected to apoptogenic stimulants including tumor necrosis factor (TNF)-α and actinomycin D (143). Satellite cells from denervated muscle were found to be more susceptible to apoptogenic stimulation when compared to the satellite cells isolated from non-denervated muscle. Furthermore, satellite cells that were isolated from muscles following 6 and 10 weeks of denervation were reported to have a greater extent of DNA fragmentation as determined by TUNEL and caspase activity (143). It was shown that both Bax and Bcl-2 were expressed in only the adherent satellite cells isolated from muscles following 6 and 10 weeks of denervation and non-denervated muscle (143). The authors concluded that these data supported the causative role of apoptosis in the depletion of satellite cells in chronic prolonged denervated skeletal muscle based on the findings that the susceptibility to apoptosis increased in muscle after denervation (143). Since the phenomenon of continuous denervation-reinnervation occurs with aging in skeletal muscle, it is worth mentioning that the same group of investigators have also demonstrated that the satellite cells isolated from old rat muscles have a greater susceptibility to apoptogenic stimulation when compared to satellite cells isolated from young muscle (144). Moreover, a study conducted by Siu and co-workers has provided data indicating that apoptotic signaling contributed to the elimination of the previously stretch-induced activated myogenic satellite cell nuclei during unloading-induced muscle atrophy (111;199). These data are consistent with the idea that apoptosis plays a role in regulating the process of muscle atrophy including when atrophy occurs in previously hypertrophied skeletal muscle.
6.3. Potential role of oxidative stress in denervation-associated muscle apoptosis
Oxidative stress is probably a potential activator and important mediator of muscle apoptosis during denervation-induced muscle loss. Oxidative stress has been investigated in the muscle following the removal of nerve supply and the results indicate that the redox homeostasis as indicated by the oxidative stress markers and antioxidant enzymes responds to muscle denervation (110;194;200–202). According to the investigations on oxidative stress and apoptosis during muscle denervation, oxidative stress is proposed to be a triggering factor in the activation of apoptosis in muscle denervation (22). Skeletal muscle has showed down regulation of the expression of sarcolemmal neuronal nitric oxide synthase (nNOS) and calpain-3 which are the factors associated with oxidative stress and apoptotic signaling pathway following denervation (203–205). The influence of denervation in the free radical scavenging system has been studied in slow-twitch soleus and fast-twitch extensor digitorum longus muscles in rodents (202). After 2 or 5 weeks of denervation as induced by crushing the sciatic nerve, reduction of copper zinc superoxide dismutase (CuZnSOD) was reported in denervated slow muscle. Manganese superoxide dismutase (MnSOD), fumarase and glutathione peroxidase were markedly decreased after 2 weeks of denervation but their levels returned back to control levels by 5 weeks of denervation (202). These findings of the observed selective alteration of antioxidant enzymes CuZnSOD and MnSOD and differential regulation of glutathione peroxidase in denervated muscle suggest that redox balance is disturbed during muscle denervation and oxidative stress is provoked in the process (202). Additionally, the response of mitochondria-associated apoptotic signaling components and antioxidant enzyme MnSOD and CuZnSOD to muscle denervation has been studied (110). Along with the data demonstrating the activation of the mitochondria-associated apoptosis and the related pro-apoptotic signaling, the expression of MnSOD was found to be concomitantly reduced in the muscle after 14 days of denervation (110). It is worth noting that these simultaneous changes of apoptotic signaling and oxidative stress-related markers observed in denervated muscle have been documented in other muscle atrophic conditions (137). The pattern of the activation of apoptosis concomitant with the alteration of oxidative stress markers has also been reported in atrophic conditions including hindlimb unweighting (94;100;109;206) and unloading of stretch-induced hypertrophied muscle (111;199). While the exact relationship of oxidative stress and apoptosis in muscle loss is not fully understood, the simultaneous activation of apoptosis and oxidative stress initiates the hypothesis that the activation of apoptosis is somehow related to oxidative stress in denervated skeletal muscle.
The deficiency in a pro-apoptotic gene product Bax has been shown to attenuate the pro-apoptotic signaling and reduce the extent of muscle loss in mouse skeletal muscles after 14 days of denervation. Furthermore, the changes in oxidative stress markers and antioxidant enzymes were shown to be different between wild-type and Bax-deficient muscle in response to denervation (194). While the antioxidant enzyme MnSOD was diminished in a similar extent in wild-type and Bax knockout muscles with denervation, oxidative stress markers including hydrogen peroxide, malondialdehyde/4-hydroxyalkenals (MDA/4-HAE) and nitrotyrosine contents were elevated in wild-type muscle in response to denervation but the denervation-induced elevations of all these oxidative stress-related markers were not seen in Bax knockout muscle (194). Evidence showing that there is no elevation of the oxidative stress markers including hydrogen peroxide, MDA/4-HAE and nitrotyrosine in the denervated Bax knockout muscle indicated that Bax is upstream and sensitive to redox homeostatic control during denervation-inducèd muscle atrophy (194). These observations are consistent with the proposition that oxidative stress has a role in mediating the process of muscle atrophy as well as the corresponding apoptotic mechanism. These results revealed that Bax participates and is able to affect the oxidative stress-related events during muscle denervation implying that apoptotic signaling probably acts upstream of the redox events. However, it is unlikely that the effect of oxidative stress is simply downstream of apoptosis and it would be more likely and practical if apoptosis and redox event communicate in a complex connection in which these two cellular events might exist in an interactive cooperative manner in the skeletal muscle. Nonetheless, it is not known if the feedback and/or feed forward mechanisms might exist in coordinating the regulations of redox and apoptosis and this would need further investigation to explore. Another possibility is that Bax knockout muscle may have offered a more favorable cellular environment against pro-oxidants by the better balance with Bcl-2 which is the intimate apoptotic working partner of Bax (194;207). Since anti-apoptotic Bcl-2 protein has been demonstrated to have antioxidant promoting ability which provides resistance to apoptosis as induced by oxidative stress (208), Bax null muscles might have allowed Bcl-2 to decrease the elevation of oxidative stress at a higher efficiency during denervation. Although further experimental work is required to reveal the exact relationship between Bax (and other apoptotic components) and oxidative stress in muscle denervation, these data strongly suggested that oxidative stress and apoptotic signaling are linked and coordinated with each other, at least in the muscle remodeling process during muscle denervation.
7. SUMMARY AND PERSPECTIVE
Existing evidence together indicates that apoptosis is consistently activated in response to denervation and has a role in denervation-associated muscle wasting. Although the precise physiologic function of apoptotic mechanism in denervation-induced muscle adaptation has yet to be explored, apoptosis probably participates in the muscle remodeling process associated with muscle wasting and denervation. Overall, although there are some preliminary data on apoptosis and oxidative stress in muscle denervation wasting, there are still some unresolved issues and several important questions remain and should be the focus of future study before substantial conclusions concerning the role of apoptosis in skeletal muscle denervation can be determined. There is a need to have more data directly addressing the activation of apoptosis during denervation. For example, is apoptosis a cause or simply a consequence of the process of muscle remodeling? The exact functional effect of apoptosis is unknown in the process of muscle atrophy during denervation. Other potential signaling pathways (e.g., IGF/PI3-kinase/AKTl, NFkappaB, ubiquitin-proteasome, FOXOs/MuRFl/MAFbx (atrogin-1), and lysosomal calcium-dependent calpain system) have been identified as important mechanisms for muscle atrophy. However, the linkage and relationship of these identified atrophic/proteolytic mechanisms with the demonstrated activated apoptotic signaling during muscle denervation-induced muscle loss are largely unclear and would need to be addressed in the future. The exploration of the precise triggering cellular events responsible for the activation of apoptotic machinery in muscle denervation should also be another research topic needs to be investigated.
8. ACKNOWLEDGEMENTS
The authors apologize to the researchers whose scientific contributions were not included in this article due to the space constraint. During the written process of the present article, the research work of SE Alway was supported by National Institute on Aging Grant R01 AG-021530 and PM Siu was supported by The Hong Kong Polytechnic University ICRG funds.
Abbreviations:
- AIDS
acquired immune deficiency syndrome
- AIF
apoptosis inducing factor
- ALS
amyotrophic lateral sclerosis
- Apaf-1
apoptosis protease activating factor-1
- ARC
apoptosis repressor with caspase recruitment domain
- BCL-2
B-cell leukaemia/lymphoma-2
- BIR
baculovirus inhibitor of apoptosis repeat
- COPD
chronic obstructive pulmonary disease
- CuZnSOD
copper zinc superoxide dismutase
- EndoG
endonuclease G
- FLIP
Fas-associated death domain protein-like interleukin lβ-converting enzyme-like inhibitory protein
- FOXO
forkhead box O
- HSP
heat shock protein
- HtrA2/Qmi
heat requirement A2 protein
- IAP
inhibitor of apoptosis protein
- ICAD
inhibitor of caspase-activated DNase
- ICE
interleukin-1 beta converting enzyme
- IGF
insulin-like growth factor
- MAFbx
muscle atrophy F-box
- MDA/4-HAE
malondialdehyde/4-hydroxyalkenals
- MnSOD
manganese-superoxide dismutase
- MuRFl
muscle RING fingerl
- NFkappaB
nuclear factor kappaB
- nNOS
neuronal nitric oxide synthase
- PARP
poly(ADP-ribose) polymerase
- PI3-kinase
phosphatidyliiιositol-3 kinase
- PTP
permeability transition pore
- RT-PCR
reverse transcription-polymerase chain reaction
- SMN
survival motor neuron
- SSC
stretch shortening cycle
- TNF-α
tumor necrosis factor-alpha
- TTX
tetrodotoxin
- TUNEL
TdT-mediated dUTP nick end labeling
- VDAC
voltage-dependent anion channel
- XLAP
X-linked inhibitor of apoptosis
9. REFERENCES
- 1.Kerr JF, Wyllie AH & Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26, 239–257 (1972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danial NN & Korsmeyer SJ. Cell death: critical control points. Cell 116,205–219 (2004) [DOI] [PubMed] [Google Scholar]
- 3.Ellis RE, Yuan JY & Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol 7, 663–698 (1991) [DOI] [PubMed] [Google Scholar]
- 4.Steller H Mechanisms and genes of cellular suicide. Science 267, 1445–1449(1995) [DOI] [PubMed] [Google Scholar]
- 5.Yuan J Molecular control of life and death. Curr Opin Cell Bio 7, 211–214 (1995) [DOI] [PubMed] [Google Scholar]
- 6.Yuan J Transducing signals of life and death. Curr Opin Cell Biol 9, 247–251 (1997) [DOI] [PubMed] [Google Scholar]
- 7.Thompson CB Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456–1462 (1995) [DOI] [PubMed] [Google Scholar]
- 8.Gorman AM, Ceccatelli S & Orrenius S. Role of mitochondria in neuronal apoptosis. Dev Neurosci 22, 348–358 (2000) [DOI] [PubMed] [Google Scholar]
- 9.Green DR & Kroemer G. The pathophysiology of mitochondrial cell death. Science 305, 626–629 (2004) [DOI] [PubMed] [Google Scholar]
- 10.Li H, Zhu H, Xu CJ & Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94,491–501 (1998) [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA & Yuan J. Caspase-12 mediates endoplasmicreticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403, 98–103 (2000) [DOI] [PubMed] [Google Scholar]
- 12.Phaneuf S & Leeuwenburgh C. Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol 282, R423–R430 (2002) [DOI] [PubMed] [Google Scholar]
- 13.Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U & Green DR. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science 310, 66–67(2005) [DOI] [PubMed] [Google Scholar]
- 14.Dirks AJ & Leeuwenburgh C. The role of apoptosis in age-related skeletal muscle atrophy. Sports Med 35, 473–483 (2005) [DOI] [PubMed] [Google Scholar]
- 15.Dupont-Versteegden EE Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol 40,473–481 (2005) [DOI] [PubMed] [Google Scholar]
- 16.Dupont-Versteegden EE Apoptosis in skeletal muscle and its relevance to atrophy. World J Gastroenterol 12, 7463–7466 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leeuwenburgh C Role of apoptosis in sarcopenia. J Gerontol A Biol Sci Med Sci 58, 999–1001 (2003) [DOI] [PubMed] [Google Scholar]
- 18.Marzetti E & Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol 41, 1234–1238 (2006) [DOI] [PubMed] [Google Scholar]
- 19.Pollack M, Phaneuf S, Dirks A & Leeuwenburgh C. The role of apoptosis in the normal aging brain, skeletal muscle, and heart. Ann N Y Acad Sci 959, 93–107 (2002) [DOI] [PubMed] [Google Scholar]
- 20.Sandri M & Carraro U. Apoptosis of skeletal muscles during development and disease. Int J Biochem Cell Biol 31, 1373–1390 (1999) [DOI] [PubMed] [Google Scholar]
- 21.Tews DS Apoptosis and muscle fibre loss in neuromuscular disorders. Neuromuscul Disord 12, 613–622 (2002) [DOI] [PubMed] [Google Scholar]
- 22.Tews DS Muscle-fiber apoptosis in neuromuscular diseases. Muscle Nerve 32, 443–458 (2005) [DOI] [PubMed] [Google Scholar]
- 23.Tsujimoto Y & Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ 12 Suppl 2, 1528–1534 (2005) [DOI] [PubMed] [Google Scholar]
- 24.Schwartz LM, Smith SW, Jones ME & Osborne BA. Do all programmed cell deaths occur via apoptosis? Proc Natl Acad Sci U S A 90, 980–984 (1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan J Evolutionary conservation of a genetic pathway of programmed cell death. J Cell Biochem 60,4–11 (1996) [DOI] [PubMed] [Google Scholar]
- 26.Sulston JE & Horvίtz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56, 110–156(1977) [DOI] [PubMed] [Google Scholar]
- 27.Duke RC, Ojcius DM & Young JD. Cell suicide in health and disease. Sci Am 275, 80–87 (1996) [DOI] [PubMed] [Google Scholar]
- 28.Williams GT Programmed cell death: apoptosis and oncogenesis. Cell 65, 1097–1098 (1991) [DOI] [PubMed] [Google Scholar]
- 29.Yuan J & Yankner BA. Apoptosis in the nervous system. Nature 407, 802–809 (2000) [DOI] [PubMed] [Google Scholar]
- 30.Lee SC & Pervaiz S. Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol 39,497–504 (2007) [DOI] [PubMed] [Google Scholar]
- 31.Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR & Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem 274, 5053–5060 (1999) [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa T & Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol 150, 887–894 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao DT & Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol 16, 395–419 (1998) [DOI] [PubMed] [Google Scholar]
- 34.Mikhailov V, Mikhailova M, Degenhardt K, Venkatachalam MA, White E & Saikumar P. Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bäk reorganization and cytochrome c release. J Biol Chem 278, 5367–5376 (2003) [DOI] [PubMed] [Google Scholar]
- 35.Mikhailov V, Mikhailova M, Pulkrabek DJ, Dong Z, Venkatachalam MA & Saikumar P. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem 276, 18361–18374 (2001) [DOI] [PubMed] [Google Scholar]
- 36.Korsmeyer SJ BCL-2 gene family and the regulation of programmed cell death. Cancer Res 59, 1693s–1700s (1999) [PubMed] [Google Scholar]
- 37.Korsmeyer SJ Regulators of cell death. Trends Genet 11, 101–105 (1995) [DOI] [PubMed] [Google Scholar]
- 38.Er E, Lalier L, Cartron PF, Oliver L & Vallette FM. Control of Bax homo-dimerization by its carboxy-terminal. J Biol Chem (2007) [DOI] [PubMed] [Google Scholar]
- 39.Korsmeyer SJ, Yin XM, Oltvai ZN, Veis-Novack DJ & Linette GP. Reactive oxygen species and the regulation of cell death by the Bcl-2 gene family. Biochim Biophys Acta 1271, 63–66 (1995) [DOI] [PubMed] [Google Scholar]
- 40.Chao DT, Linette GP, Boise LH, White LS, Thompson CB & Korsmeyer SJ. Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med 182, 821–828 (1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroemer G, Galluzzi L & Brenner C. Mitochondrial membrane permeabilizatίon in cell death. Physiol Rev 87, 99–163 (2007) [DOI] [PubMed] [Google Scholar]
- 42.Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ,Matsuda H & Tsujimoto Y. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci U S A 95, 14681–14686 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed JC, Jurgensmeier JM & Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim Biophys Acta 1366,127–137 (1998) [DOI] [PubMed] [Google Scholar]
- 44.Shimizu S, Narita M & Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399, 483–487 (1999) [DOI] [PubMed] [Google Scholar]
- 45.Tsujimoto Y & Shimizu S. VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ 7, 1174–1181 (2000) [DOI] [PubMed] [Google Scholar]
- 46.Tsujimoto Y, Nakagawa T & Shimizu S. Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta 1757, 1297–1300 (2006) [DOI] [PubMed] [Google Scholar]
- 47.Basanez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood KA, Hsu Y, Zimmerberg J & Youle RJ. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc Natl Acad Sci U S A 96, 5492–5497 (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cartron PF, Moreau C, Oliver L, Mayat E, Meflah K & Vallette FM. Involvement of the N-terminus of Bax in its intracellular localization and function. FEBS Lett 512, 95–100 (2002) [DOI] [PubMed] [Google Scholar]
- 49.Desagher S & Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol 10, 369–377 (2000) [DOI] [PubMed] [Google Scholar]
- 50.Hsu YT, Wolter KG & Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A 94, 3668–3672 (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG & Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 139, 1281–1292 (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zha H, ime-Sempe C, Sato T & Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem 271, 7440–7444 (1996) [DOI] [PubMed] [Google Scholar]
- 53.Lalier L, Cartron PF, Juin P, Nedelkina S, Manon S, Bechinger B & Vallette FM. Bax activation and mitochondrial insertion during apoptosis. Apoptosis 12, 887–896 (2007) [DOI] [PubMed] [Google Scholar]
- 54.Yin XM, Oltvai ZN & Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369, 321–323 (1994) [DOI] [PubMed] [Google Scholar]
- 55.Reed JC Double identity for proteins of the Bcl-2 family. Nature 387,773–776 (1997) [DOI] [PubMed] [Google Scholar]
- 56.Reed JC Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ 13, 1378–1386 (2006) [DOI] [PubMed] [Google Scholar]
- 57.Antonsson B, Montessuit S, Lauper S, Eskes R & Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem J 345 Pt 2, 271–278 (2000) [PMC free article] [PubMed] [Google Scholar]
- 58.Chang HY & Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev 64, 821–846 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Degterev A, Boyce M & Yuan J. A decade of caspases. Oncogene 22, 8543–8567 (2003) [DOI] [PubMed] [Google Scholar]
- 60.Eamshaw WC, Martins LM & Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68, 383–424 (1999) [DOI] [PubMed] [Google Scholar]
- 61.Grutter MG Caspases: key players in programmed cell death. Curr Opin Struct Biol 10, 649–655 (2000) [DOI] [PubMed] [Google Scholar]
- 62.Stennicke HR, Deveraux QL, Humke EW, Reed JC, Dixit VM & Salvesen GS. Caspase-9 can be activated without proteolytic processing. J Biol Chem 274, 8359–8362 (1999) [DOI] [PubMed] [Google Scholar]
- 63.Grutter MG Caspases: key players in programmed cell death. Curr Opin Struct Biol 10, 649–655 (2000) [DOI] [PubMed] [Google Scholar]
- 64.Stennicke HR, Deveraux QL, Humke EW, Reed JC, Dixit VM & Salvesen GS. Caspase-9 can be activated without proteolytic processing. J Biol Chem 274, 8359–8362(1999) [DOI] [PubMed] [Google Scholar]
- 65.Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X & Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell 9, 423–432 (2002) [DOI] [PubMed] [Google Scholar]
- 66.Shi Y Apoptosome: the cellular engine for the activation of caspase-9. Structure 10, 285–288 (2002) [DOI] [PubMed] [Google Scholar]
- 67.Shi Y Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell 9,459–470 (2002) [DOI] [PubMed] [Google Scholar]
- 68.Shi Y Caspase activation, inhibition, and reactivation: a mechanistic view. Protein Sci 13, 1979–1987 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shiozaki EN, Chai J & Shi Y. Oligomerization and activation of caspase-9, induced by Apaf-1 CARD. Proc Natl Acad Sci U S A 99, 4197–4202 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blink E, Maianski NA, Alnemri ES, Zervos AS,Roos D & Kuijpers TW. Intramitochondrial serine protease activity of Omi/HtrA2 is required for caspase-independent cell death of human neutrophils. Cell Death Differ 11, 937–939 (2004) [DOI] [PubMed] [Google Scholar]
- 71.Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G & Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410, 549–554 (2001) [DOI] [PubMed] [Google Scholar]
- 72.Li LY, Luo X & Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412, 95–99(2001) [DOI] [PubMed] [Google Scholar]
- 73.Joza N, Oudit GY, Brown D, Benit P, Kassiri Z, Vahsen N, Benoit L, Patel MM, Nowikovsky K, Vassault A, Backx PH, Wada T, Kroemer G, Rustin P & Penninger JM. Muscle-specific loss of apoptosis-inducing factor leads to mitochondrial dysfunction, skeletal muscle atrophy, and dilated cardiomyopathy. Mol Cell Biol 25, 10261–10272(2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cande C, Cecconi F, Dessen P & Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci 115, 4727–4734(2002) [DOI] [PubMed] [Google Scholar]
- 75.Cande C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N & Kroemer G. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie 84, 215–222 (2002) [DOI] [PubMed] [Google Scholar]
- 76.Lipton SA & Bossy-Wetzel E. Dueling activities of AIF in cell death versus survival: DNA binding and redox activity. Cell 111, 147–150 (2002) [DOI] [PubMed] [Google Scholar]
- 77.Ye H, Cande C, Stephanou NC, Jiang S, Gurbuxani S, Larochette N, Daugas E, Garrido C, Kroemer G & Wu H. DNA binding is required for the apoptogenic action of apoptosis inducing factor. Nat Struct Biol 9, 680–684 (2002) [DOI] [PubMed] [Google Scholar]
- 78.Hegde R, Srinivasula SM, Zhang Z, Wassell R, Mukattash R, Cilenti L, DuBois G, Lazebnik Y, Zervos AS, Femandes-Alnemri T & Alnemri ES. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J Biol Chem 277,432–438 (2002) [DOI] [PubMed] [Google Scholar]
- 79.Suzuki Y, Takahashi-Niki K, Akagi T, Hashikawa T & Takahashi R. Mitochondrial protease Omi/HtrA2 enhances caspase activation through multiple pathways. Cell Death Differ 11,208–216 (2004) [DOI] [PubMed] [Google Scholar]
- 80.Shi Y Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell 9,459–470 (2002) [DOI] [PubMed] [Google Scholar]
- 81.Deveraux QL, Roy N, Stennicke HR, Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS & Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J 17, 2215–2223 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi Y Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell 9, 459–470 (2002) [DOI] [PubMed] [Google Scholar]
- 83.Deveraux QL, Stennicke HR, Salvesen GS & Reed JC. Endogenous inhibitors of caspases. J Clin Immunol 19, 388–398 (1999) [DOI] [PubMed] [Google Scholar]
- 84.Deveraux QL & Reed JC. LAP family proteins-suppressors of apoptosis. Genes Dev 13, 239–252 (1999) [DOI] [PubMed] [Google Scholar]
- 85.Salvesen GS & Duckett CS. LAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol 3, 401–410 (2002) [DOI] [PubMed] [Google Scholar]
- 86.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Bums K, Mattmann C, Rimoldi D, French LE & Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature 388, 190–195 (1997) [DOI] [PubMed] [Google Scholar]
- 87.Koseki T, Inohara N, Chen S & Nunez G. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci U S A 95, 5156–5160 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Bums K, Mattmann C, Rimoldi D, French LE & Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature 388, 190–195 (1997) [DOI] [PubMed] [Google Scholar]
- 89.Koseki T, Inohara N, Chen S & Nunez G. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci U S A 95, 5156–5160 (1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abmayr S, Crawford RW & Chamberlain JS. Characterization of ARC, apoptosis repressor interacting with CARD, in normal and dystrophin-deficient skeletal muscle. Hum Mol Genet 13,213–221 (2004) [DOI] [PubMed] [Google Scholar]
- 91.Gustafsson AB, Tsai JG, Logue SE, Crow MT & Gottlieb RA. Apoptosis repressor with caspase recruitment domain protects against cell death by interfering with Bax activation. J Biol Chem 279, 21233–21238(2004) [DOI] [PubMed] [Google Scholar]
- 92.Edelblum KL, Yan F, Yamaoka T & Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium, lnflamm Bowel Dis 12, 413–424 (2006) [DOI] [PubMed] [Google Scholar]
- 93.Hall PA, Coates PJ, Ansari B & Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci 107 (Pt 12), 3569–3577 (1994) [DOI] [PubMed] [Google Scholar]
- 94.Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V & Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol 273, C579–C587 (1997) [DOI] [PubMed] [Google Scholar]
- 95.Alway SE, Degens H, Krishnamurthy G & Chaudhrai A. Denervation stimulates apoptosis but not Id2 expression in hindlimb muscles of aged rats. J Gerontol A Biol Sci Med Sci 58, 687–697 (2003) [DOI] [PubMed] [Google Scholar]
- 96.Dirks A & Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol 282, R519–R527 (2002) [DOI] [PubMed] [Google Scholar]
- 97.Dirks AJ & Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radio Biol Med 36, 27–39 (2004) [DOI] [PubMed] [Google Scholar]
- 98.Dirks AJ & Leeuwenburgh C. Tumor necrosis factor alpha signaling in skeletal muscle: effects of age and caloric restriction. J Nutr Biochem 17, 501–508 (2006) [DOI] [PubMed] [Google Scholar]
- 99.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Remmen H. Van, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C & Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484 (2005) [DOI] [PubMed] [Google Scholar]
- 100.Leeuwenburgh C, Gurley CM, Strotman BA & Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol 288, R1288–R1296 (2005) [DOI] [PubMed] [Google Scholar]
- 101.Phillips T & Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J 19, 668–670 (2005) [DOI] [PubMed] [Google Scholar]
- 102.Pistilli EE, Siu PM & Alway SE. Molecular regulation of apoptosis in fast plantaris muscles of aged rats. J Gerontol A Biol Sci Med Sci 61,245–255 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pistilli EE, Jackson JR & Alway SE. Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis 11, 2115–2126 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pistilli EE, Siu PM & Alway SE. Interleukin-15 responses to aging and unloading-induced skeletal muscle atrophy. Am J Physiol Cell Physiol 292, C1298–C1304 (2007) [DOI] [PubMed] [Google Scholar]
- 105.Sandri M, Carraro U, Podhorska-Okolov M, Rizzί C, Arslan P, Monti D & Franceschi C. Apoptosis, DNA damage and ubiquitin expression in normal and mdx muscle fibers after exercise. FEES Lett 373, 291–295 (1995) [DOI] [PubMed] [Google Scholar]
- 106.Sandri M, Podhorska-Okolow M, Geromel V, Rizzi C, Arslan P, Franceschi C & Carraro U. Exercise induces myonuclear ubiquitination and apoptosis in dystrophin-deficient muscle of mice. J Neuropathol Exp Neurol 56, 45–57 (1997) [DOI] [PubMed] [Google Scholar]
- 107.Sandri M, Minetti C, Pedemonte M & Carraro U. Apoptotic myonuclei in human Duchenne muscular dystrophy. Lab Invest 78, 1005–1016 (1998) [PubMed] [Google Scholar]
- 108.Sandri M, El Meslemani AH, Sandri C, Schjerling P, Vissing K, Andersen JL, Rossini K, Carraro U & Angelini C. Caspase 3 expression correlates with skeletal muscle apoptosis in Duchenne and facioscapulo human muscular dystrophy. A potential target for pharmacological treatment? J Neuropathol Exp Neurol 60, 302–312 (2001) [DOI] [PubMed] [Google Scholar]
- 109.Siu PM, Pistilli EE & Alway SE. Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am J Physiol Regul Integr Comp Physiol 289, R1015–R1026 (2005) [DOI] [PubMed] [Google Scholar]
- 110.Siu PM & Alway SE. Mitochondrίa-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol 565, 309–323 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Siu PM, Pistilli EE, Butler DC & Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol 288, C338–C349 (2005) [DOI] [PubMed] [Google Scholar]
- 112.Dupont-Versteegden EE, Strotman BA, Gurley CM, Gaddy D, Knox M, Fluckey JD & Peterson CA. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am J Physiol Regul Integr Comp Physiol 291, R1730–R1740 (2006) [DOI] [PubMed] [Google Scholar]
- 113.Allen DL, Roy RR & Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22, 1350–1360(1999) [DOI] [PubMed] [Google Scholar]
- 114.Adams GR Satellite cell proliferation and skeletal muscle hypertrophy. Appl Physiol Nutr Metab 31, 782–790 (2006) [DOI] [PubMed] [Google Scholar]
- 115.Charge SB & Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84, 209–238 (2004) [DOI] [PubMed] [Google Scholar]
- 116.Hawke TJ & Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91, 534–551 (2001) [DOI] [PubMed] [Google Scholar]
- 117.Schultz E & McCormick KM. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol 123, 213–257 (1994) [DOI] [PubMed] [Google Scholar]
- 118.Tews DS & Goebel HH. DNA-fragmentation and expression of apoptosis-related proteins in muscular dystrophies. Neuropathol Appl Neurobiol 23, 331–338 (1997) [PubMed] [Google Scholar]
- 119.Yamada H, Nakagawa M, Higuchi I, Horikiri T & Osame M. Detection of DNA fragmentation of myonuclei in myotonic dystrophy by double staining with anti-emerin antibody and by nick end-labeling. J Neurol Sci 173, 97–102(2000) [DOI] [PubMed] [Google Scholar]
- 120.Tews DS & Goebel HH. DNA fragmentation and BCL-2 expression in infantile spinal muscular atrophy. Neuromuscul Disord 6,265–273 (1996) [DOI] [PubMed] [Google Scholar]
- 121.Tews DS, Goebel HH & Meinck HM. DNA-fragmentation and apoptosis-related proteins of muscle cells in motor neuron disorders. Acta Neurol Scand 96, 380–386 (1997) [DOI] [PubMed] [Google Scholar]
- 122.Miro O, Lopez S, Femandez-Sola J, Garrabou G, Pedrol E, Badia E, Martinez E, Cardellach F, Gatell JM & Casademont J. Short communication: HIV infection, antiretrovirals, and apoptosis: studies on skeletal muscle. AIDS Res Hum Retroviruses 21, 702–705 (2005) [DOI] [PubMed] [Google Scholar]
- 123.Ishiko O, Yoshida H, Sumi T, Hirai K, Honda K, Hyun Y, Nakagawa E, Hattori K & Ogita S. Expression of skeletal muscle cells apoptosis regulatory proteins in plasma-perfused VX2 carcinoma-bearing rabbits. Anticancer Res 21,2363–2368 (2001) [PubMed] [Google Scholar]
- 124.Ishiko O, Sumi T, Yoshida H, Hyun Y & Ogita S. Expression of apoptosis regulatory proteins in the skeletal muscle of tumor-bearing rabbits compared with diet-restricted rabbits. Int J Mol Med 8, 279–283 (2001) [DOI] [PubMed] [Google Scholar]
- 125.Filippatos GS, Kanatselos C, Manolatos DD, Vougas B, Sideris A, Kardara D, Anker SD, Kardaras F & Uhal B. Studies on apoptosis and fibrosis in skeletal musculature: a comparison of heart failure patients with and without cardiac cachexia. Int J Cardiol 90, 107–113 (2003) [DOI] [PubMed] [Google Scholar]
- 126.Strassburg S, Springer J & Anker SD. Muscle wasting in cardiac cachexia. Int J Biochem Cell Biol 37, 1938–1947(2005) [DOI] [PubMed] [Google Scholar]
- 127.Vescovo G, Volterrani M, Zennaro R, Sandri M, Ceconi C, Lorusso R, Ferrari R, Ambrosio GB & Libera L Dalla. Apoptosis in the skeletal muscle of patients with heart failure: investigation of clinical and biochemical changes. Heart 84, 431–437 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Agusti AG, Sauleda J, Miralles C, Gomez C, Togores B, Sala E, Batle S & Busquets X. Skeletal muscle apoptosis and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166,485–489 (2002) [DOI] [PubMed] [Google Scholar]
- 129.Mitch WE Proteolytic mechanisms, not malnutrition, cause loss of muscle mass in kidney failure. J Ren Nutr 16, 208–211 (2006) [DOI] [PubMed] [Google Scholar]
- 130.Almendro V, Carbo N, Busquets S, Figueras M, Tessitore L, Lopez-Soriano FJ & Argiles JM. Sepsis induces DNA fragmentation in rat skeletal muscle. Eur Cytokine Netw 14,256–259 (2003) [PubMed] [Google Scholar]
- 131.Yasuhara S, Kanakubo E, Perez ME, Kaneki M, Fujita T, Okamoto T & Martyn JA. The 1999 Moyer award. Bum injury induces skeletal muscle apoptosis and the activation of caspase pathways in rats. J Burn Care Rehabil 20, 462–470 (1999) [DOI] [PubMed] [Google Scholar]
- 132.Astrakas LG, Goljer I, Yasuhara S, Padfíeld KE, Zhang Q, Gopalan S, Mindrinos MN, Dai G, Yu YM, Martyn JA, Tompkins RG, Rahme LG & Tzika AA. Proton NMR spectroscopy shows lipids accumulate in skeletal muscle in response to bum trauma-induced apoptosis. FASEB J 19, 1431–1440 (2005) [DOI] [PubMed] [Google Scholar]
- 133.Femandez-Sola J, Nicolas JM, Fatjo F, Garcia G, Sacanella E, Estruch R, Tobias E, Badia E & Urbano-Marquez A. Evidence of apoptosis in chronic alcoholic skeletal myopathy. Hum Pathol 34, 1247–1252 (2003) [DOI] [PubMed] [Google Scholar]
- 134.Dirks AJ, Hofer T, Marzetti E, Pahor M & Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev 5, 179–195 (2006) [DOI] [PubMed] [Google Scholar]
- 135.Pollack M & Leeuwenburgh C. Apoptosis and aging: role of the mitochondria. J Gerontol A Biol Sci Med Sci 56, B475–B482 (2001) [DOI] [PubMed] [Google Scholar]
- 136.Tews DS, Goebel HH, Schneider I, Gunkel A,Stennert E & Neiss WF. DNA-fragmentation and expression of apoptosis-related proteins in experimentally denervated and reinnervated rat facial muscle. Neuropathol Appl Neurobiol 23, 141–149 (1997) [PubMed] [Google Scholar]
- 137.Adhihetty PJ, O’Leary MF, Chabi B, Wicks KL & Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol 102, 1143–1151 (2007) [DOI] [PubMed] [Google Scholar]
- 138.Mauro A Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9, 493–495 (1961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, Schwartz K, Vilquin JT & Marolleau JP. Myoblast transplantation for heart failure. Lancet 357,279–280 (2001) [DOI] [PubMed] [Google Scholar]
- 140.Negroni E, Butler-Browne GS & Mouly V. Myogenic stem cells: regeneration and cell therapy in human skeletal muscle. Pathol Biol (Paris) 54,100–108 (2006) [DOI] [PubMed] [Google Scholar]
- 141.Darr KC & Schultz E. Hindlimb suspension suppresses muscle growth and satellite cell proliferation. J Appl Physiol 67, 1827–1834 (1989) [DOI] [PubMed] [Google Scholar]
- 142.Heslop L, Morgan JE & Partridge TA. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci 113 (Pt 12), 2299–2308 (2000) [DOI] [PubMed] [Google Scholar]
- 143.Jejurikar SS, Marcelo CL & Kuzon WM Jr. Skeletal muscle denervation increases satellite cell susceptibility to apoptosis. Plast Reconstr Sw g 110, 160–168(2002) [DOI] [PubMed] [Google Scholar]
- 144.jejurikar SS, Henkelman EA, Cedema PS, Marcelo CL, Urbanchek MG & Kuzon WM Jr. Aging increases the susceptibility of skeletal muscle derived satellite cells to apoptosis. Exp Gerontol (2006) [DOI] [PubMed] [Google Scholar]
- 145.Kadi F, Charifi N, Denis C & Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 29, 120–127(2004) [DOI] [PubMed] [Google Scholar]
- 146.Renault V, Thomell LE, Eriksson PO, Butler-Browne G & Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell 1, 132–139 (2002) [DOI] [PubMed] [Google Scholar]
- 147.Jejurikar SS & Kuzon WM Jr. Satellite cell depletion in degenerative skeletal muscle. Apoptosis 8, 573–578 (2003) [DOI] [PubMed] [Google Scholar]
- 148.Rodrigues Ade C & Schmalbruch H. Satellite cells and myonuclei in long-term denervated rat muscles. Anat Rec 243, 430–437 (1995) [DOI] [PubMed] [Google Scholar]
- 149.de Castro Rodrigues A, Andreo JC & de Mattos Rodrigues SP. Myonuclei and satellite cells in denervated rat muscles: an electron microscopy study. Microsurgery 26,396–398 (2006) [DOI] [PubMed] [Google Scholar]
- 150.Krajnak K, Waugh S, Miller R, Baker B, Geronilla K,Alway SE & Cutlip RG. Proapoptotic factor Bax is increased in satellite cells in the tibialis anterior muscles of old rats. Muscle Nerve 34, 720–730 (2006) [DOI] [PubMed] [Google Scholar]
- 151.Golding JP, Calderbank E, Partridge TA & Beauchamp JR. Skeletal muscle stem cells express anti-apoptotic ErbB receptors during activation from quiescence. Exp Cell Res 313,341–356 (2007) [DOI] [PubMed] [Google Scholar]
- 152.Hughes SM Muscle development: electrical control of gene expression. Curr Biol 8, R892–R894 (1998) [DOI] [PubMed] [Google Scholar]
- 153.Pette D Historical Perspectives: plasticity of mammalian skeletal muscle. J Appl Physiol 90,1119–1124 (2001) [DOI] [PubMed] [Google Scholar]
- 154.Franch HA & Price SR. Molecular signaling pathways regulating muscle proteolysis during atrophy. Curr Opin Clin Nutr Metab Care 8, 271–275 (2005) [DOI] [PubMed] [Google Scholar]
- 155.Kandarian SC & Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33, 155–165(2006) [DOI] [PubMed] [Google Scholar]
- 156.Nader GA Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int J Biochem Cell Biol 37, 1985–1996 (2005) [DOI] [PubMed] [Google Scholar]
- 157.Ventadour S & Attaix D. Mechanisms of skeletal muscle atrophy. Curr Opin Rheumatol 18, 631–635 (2006) [DOI] [PubMed] [Google Scholar]
- 158.Miller JB & Girgenrath M. The role of apoptosis in neuromuscular diseases and prospects for anti-apoptosis therapy. Trends Mol Med 12, 279–286 (2006) [DOI] [PubMed] [Google Scholar]
- 159.Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D & Combaret L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem 41, 173–186(2005) [DOI] [PubMed] [Google Scholar]
- 160.Glass DJ Molecular mechanisms modulating muscle mass. Trends Mol Med 9, 344–350 (2003) [DOI] [PubMed] [Google Scholar]
- 161.Glass DJ Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37, 1974–1984 (2005) [DOI] [PubMed] [Google Scholar]
- 162.Dawson BA & Lough J. Immunocytochemical localization of transient DNA strand breaks in differentiating myotubes using in situ nick-translation. Dev Biol 127, 362–367 (1988) [DOI] [PubMed] [Google Scholar]
- 163.Fidzianska A & Goebel HH. Human ontogenesis. 3. Cell death in fetal muscle. Acta Neuropathol (Berl) 81, 572–577(1991) [DOI] [PubMed] [Google Scholar]
- 164.Webb JN The development of human skeletal muscle with particular reference to muscle cell death. J Pathol 106, 221–228 (1972) [DOI] [PubMed] [Google Scholar]
- 165.Webb JN Muscle cell death in developing human skeletal muscle. J Pathol 104, v (1971) [PubMed] [Google Scholar]
- 166.Webb JN Cell death in developing skeletal muscle: histochemistry and ultrastructure. J Pathol 123, 175–180 (1977) [DOI] [PubMed] [Google Scholar]
- 167.Broccolini A, Engel WK & Askanas V. Localization of survival motor neuron protein in human apoptotic-like and regenerating muscle fibers, and neuromuscular junctions. Neuroreport 10, 1637–1641 (1999) [DOI] [PubMed] [Google Scholar]
- 168.Fidzianska A, Goebel HH & Warlo I. Acute infantile spinal muscular atrophy. Muscle apoptosis as a proposed pathogenetic mechanism. Brain 113 (Pt 2), 433–445 (1990) [DOI] [PubMed] [Google Scholar]
- 169.Fidzianska A Suicide muscle cell programmeapoptosis. Ultrastructural study. Folia Neuropathol 40, 27–32 (2002) [PubMed] [Google Scholar]
- 170.Migheli A, Mongini T, Doriguzzi C, Chiado-Piat L, Piva R, Ugo I & Palmucci L. Muscle apoptosis in humans occurs in normal and denervated muscle, but not in myotonic dystrophy, dystrophinopathies or inflammatoiy disease. Neurogenetics 1, 81–87 (1997) [DOI] [PubMed] [Google Scholar]
- 171.Borisov AB & Carlson BM. Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec 258, 305–318 (2000) [DOI] [PubMed] [Google Scholar]
- 172.Schmalbruch H Natural killer cells and macrophages in immature denervated rat muscles. J Neuropathol Exp Neurol 55, 310–319 (1996) [DOI] [PubMed] [Google Scholar]
- 173.Tang H, Cheung WM, Ip FC & Ip NY. Identification and characterization of differentially expressed genes in denervated muscle. Mol Cell Neurosci 16, 127–140(2000) [DOI] [PubMed] [Google Scholar]
- 174.Trachtenberg JT Fiber apoptosis in developing rat muscles is regulated by activity, neuregulin. Dev Biol 196, 193–203 (1998) [DOI] [PubMed] [Google Scholar]
- 175.Yoshimura K & Harii K. A regenerative change during muscle adaptation to denervation in rats. J Surg Res 81, 139–146(1999) [DOI] [PubMed] [Google Scholar]
- 176.Olive M & Ferrer I. Bcl-2 and bax immunohistochemistry in denervation-reinnervation and necrosis-regeneration of rat skeletal muscles. Muscle Nerve 23, 1862–1867(2000) [DOI] [PubMed] [Google Scholar]
- 177.Schoser BG, Wehling S & Blottner D. Cell death and apoptosis-related proteins in muscle biopsies of sporadic amyotrophic lateral sclerosis and polyneuropathy. Muscle Nerve 24, 1083–1089 (2001) [DOI] [PubMed] [Google Scholar]
- 178.Tews DS & Goebel HH. Apoptosis-related proteins in skeletal muscle fibers of spinal muscular atrophy. J Neuropathol Exp Neurol 56, 150–156 (1997) [DOI] [PubMed] [Google Scholar]
- 179.Tews DS, Behrhof W & Schindler S. Expression patterns of initiator and effector caspases in denervated human skeletal muscle. Muscle Nerve 31, 175–181 (2005) [DOI] [PubMed] [Google Scholar]
- 180.Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M &. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80, 155–165 (1995) [DOI] [PubMed] [Google Scholar]
- 181.Lefebvre S, Burglen L, Frezal J, Munnich A & Melki J. The role of the SMN gene in proximal spinal muscular atrophy. Hum Mol Genet 7, 1531–1536 (1998) [DOI] [PubMed] [Google Scholar]
- 182.Roy N, Mahadevan MS, McLean M, Shutler G, Yaraghi Z, Farahani R, Baird S, Besner-Johnston A, Lefebvre C, Kang X &. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell 80, 167–178 (1995) [DOI] [PubMed] [Google Scholar]
- 183.Gennarelli M, Lucarelli M, Capon F, Pizzuti A, Merliπi L, Angelini C, Novelli G & Dallapiccola B. Survival motor neuron gene transcript analysis in muscles from spinal muscular atrophy patients. Biochem Biophys Res Commun 213, 342–348 (1995) [DOI] [PubMed] [Google Scholar]
- 184.Tews DS & Goebel HH. Cell death and oxidative damage in inflammatory myopathies. Clin Immunol Immunopathol 87,240–247 (1998) [DOI] [PubMed] [Google Scholar]
- 185.Ansari B, Coates PJ, Greenstein BD & Hall PA. In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol 170, 1–8 (1993) [DOI] [PubMed] [Google Scholar]
- 186.Wood KA, Dipasquale B & Youle RJ. In situ labeling of granule cells for apoptosis-associated DNA fragmentation reveals different mechanisms of cell loss in developing cerebellum. Neuron 11, 621–632 (1993) [DOI] [PubMed] [Google Scholar]
- 187.Ikezoe K, Nakagawa M, Yan C, Kira J, Goto Y &I. Nonaka. Apoptosis is suspended in muscle of mitochondrial encephalomyopathies. Acta Neuropathol (Berl) 103, 531–540(2002) [DOI] [PubMed] [Google Scholar]
- 188.Mukasa T, Momoi T & Momoi MY. Activation of caspase-3 apoptotic pathways in skeletal muscle fibers in laminin alpha2-deficient mice. Biochem Biophys Res Commun 260, 139–142 (1999) [DOI] [PubMed] [Google Scholar]
- 189.Hyatt JP, Roy RR, Baldwin KM & Edgerton VR. Nerve activity-independent regulation of skeletal muscle atrophy: role of MyoD and myogenin in satellite cells and myonuclei. Am J Physiol Cell Physiol 285, C l 161–C1173 (2003) [DOI] [PubMed] [Google Scholar]
- 190.Voytik SL, Przyborski M, Badylak SF & Konieczny SF. Differential expression of muscle regulatory factor genes in normal and denervated adult rat hindlimb muscles. Dev Dyn 198,214–224 (1993) [DOI] [PubMed] [Google Scholar]
- 191.Walters EH, Stickland NC & Loughna PT. The expression of the myogenic regulatory factors in denervated and normal muscles of different phenotypes. J Muscle Res Cell Motil 21,647–653 (2000) [DOI] [PubMed] [Google Scholar]
- 192.Ishido M, Kami K & Masuhara M. In vivo expression patterns of MyoD, p21, and Rb proteins in myonuclei and satellite cells of denervated rat skeletal muscle. Am J Physiol Cell Physiol 287, C484–C493 (2004) [DOI] [PubMed] [Google Scholar]
- 193.Csukly K, Ascah A, Matas J, Gardiner PF, Fontaine E & Burelle Y. Muscle denervation promotes opening of the permeability transition pore and increases the expression of cyclophilin D. J Physiol 574,319–327 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Siu PM & Alway SE. Deficiency of the Bax gene attenuates denervation-induced apoptosis. Apoptosis 11, 967–981 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Adhihetty PJ, Ljubicic V, Menzies KJ & Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol 289, C994–C1001 (2005) [DOI] [PubMed] [Google Scholar]
- 196.Jin H, Wu Z, Tian T & Gu Y. Apoptosis in atrophic skeletal muscle induced by brachial plexus injury in rats. J Trauma 50, 31–35 (2001) [DOI] [PubMed] [Google Scholar]
- 197.Viguie CA, Lu DX, Huang SK, Rengen H & Carlson BM. Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat Rec 248, 346–354 (1997) [DOI] [PubMed] [Google Scholar]
- 198.Lu DX, Huang SK & Carlson BM. Electron microscopic study of long-term denervated rat skeletal muscle. Anat Rec 248, 355–365 (1997) [DOI] [PubMed] [Google Scholar]
- 199.Siu PM & Alway SE. Id2 and p53 participate in apoptosis during unloading-induced muscle atrophy. Am J Physiol Cell Physiol 288, C1058–C1073 (2005) [DOI] [PubMed] [Google Scholar]
- 200.Olive M, Unzeta M, Moreno D & Ferrer I. Overexpression of semicarbazide-sensitive amine oxidase in human myopathies. Muscle Nerve 29,261–266 (2004) [DOI] [PubMed] [Google Scholar]
- 201.Tews DS Role of nitric oxide and nitric oxide synthases in experimental models of denervation and reinnervation. Microsc Res Tech 55, 181–186 (2001) [DOI] [PubMed] [Google Scholar]
- 202.Asayama K, Dettbam WD & Burr IM. Differential effect of denervation on free-radical scavenging enzymes in slow and fast muscle of rat. J Neurochem 46, 604–609 (1986) [DOI] [PubMed] [Google Scholar]
- 203.Tews DS, Goebel KH, Schneider I, Gunkel A, Stennert E & Neiss WF. Expression of different isoforms of nitric oxide synthase in experimentally denervated and reinnervated skeletal muscle. J Neuropathol Exp Neurol 56, 1283–1289(1997) [DOI] [PubMed] [Google Scholar]
- 204.Kusner LL & Kaminski HJ. Nitric oxide synthase is concentrated at the skeletal muscle endplate. Brain Res 730,238–242(1996) [DOI] [PubMed] [Google Scholar]
- 205.Stockholm D, Herasse M, Marchand S, Praud C, Roudaut C, Richard I, Sebille A & Beckmann JS. Calpain 3 mRNA expression in mice after denervation and during muscle regeneration. Am J Physiol Cell Physiol 280, C1561–C1569 (2001) [DOI] [PubMed] [Google Scholar]
- 206.Lawler JM, Song W & Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med 35, 9–16 (2003) [DOI] [PubMed] [Google Scholar]
- 207.Sharpe JC, Amoult D & Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta 1644, 107–113 (2004) [DOI] [PubMed] [Google Scholar]
- 208.Kowaltowski AJ, Fenton RG & Fiskum G. Bcl-2 family proteins regulate mitochondrial reactive oxygen production and protect against oxidative stress. Free Radic Biol Med 37,1845–1853 (2004) [DOI] [PubMed] [Google Scholar]