Abstract
Purpose of Review
This paper describes recent advances in understanding the mechanisms that drive fracture pain and how these findings are helping develop new therapies to treat fracture pain.
Recent Findings
Immediately following fracture, mechanosensitive nerve fibers that innervate bone are mechanically distorted. This results in these nerve fibers rapidly discharging and signaling the initial sharp fracture pain to the brain. Within minutes to hours, a host of neurotransmitters, cytokines, and nerve growth factor are released by cells at the fracture site. These factors stimulate, sensitize, and induce ectopic nerve sprouting of the sensory and sympathetic nerve fibers which drive the sharp pain upon movement and the dull aching pain at rest. If rapid and effective healing of the fracture occurs, these factors return to baseline and the pain subsides, but if not, these factors can drive chronic bone pain.
Summary
New mechanism-based therapies have the potential to fundamentally change the way acute and chronic fracture pain is managed.
Keywords: Skeletal, Nociceptors, Nerve growth factor, Pediatric, Genetic disorders, Geriatric
Introduction
Fractures and fracture pain are two of the most common and costly problems caused by bone injury or diseases [1, 2]. Fractures that are severe and/or do not heal appropriately can be highly debilitating and have a remarkably negative impact on an individual’s quality of life and functional status [3–5]. Unlike the skin, where nonuse promotes healing, effective healing of a fractured load-bearing bone (i.e., femur, hip, vertebrae) demands the patient to move, use, and mechanically load the injured bone. To optimize bone healing, while minimizing loss of bone and muscle mass, minimal bed rest is recommended following fracture. Thus, in both young and old patients alike, the rehabilitation regimens require the patient to move and place weight on the fractured bone in the first day after fracture stabilization. The most common reason that many patients cannot fully participate in this rehabilitation is that we currently do not have effective side effect-free analgesics that can attenuate bone fracture pain [6••, 7, 8]. If this pain cannot be effectively attenuated, the necessary bone loading and bone healing can be delayed, or does not occur at all, resulting in loss of muscle and bone mass, loss of mobility, and a significant increase in morbidity and mortality [9].
Currently, the treatment of pain following skeletal fracture of a load-bearing bone involves stabilization of the fractured bone, minimal bed rest, and usually the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and opiates to control the pain [6••, 10]. While NSAIDs can be effective in attenuating musculoskeletal pain, reports suggest that they can inhibit fracture healing in mice, rats, and humans [8, 11, 12]. Thus, NSAIDs and cyclooxygenase-2 (COX-2) inhibitors can retard callus formation and bone formation at the fracture site. In turn, this can result in delayed bone healing, increased incidence of fracture non-union, and decreased bone strength [13••, 14, 15]. While this issue as to the extent to which NSAIDs inhibit human bone healing remains unclear, many orthopedic surgeons believe that the use of NSAIDs is contraindicated in patients with bone fracture [16–18].
Although opiates are commonly used to control significant bone fracture pain, compared to placebo, opioids are not very effective in controlling chronic skeletal pain and long-term opiate use in patients with chronic skeletal pain interferes with functional status and the ability of the patient to return to work [19, 20, 21••, 22]. In young individuals with painful bone fractures, long-term opiate use can result in dependence, reduced functional status, and are less likely to return to work [21••]. In light of the above listed problems with both NSAIDS and opiates, development of novel, mechanism-based therapies to attenuate fracture pain is a clear priority for young, adult, and aging patients with acute and chronic bone fracture pain [6••].
Models of Bone Fracture Pain
Given the enormous consequences that fracture pain can have in terms of human suffering and medical costs, it is surprising that until recently, there was not an established animal model for studying bone fracture pain. To develop such a model, the rodent closed femur fracture model [23–25] was used as a starting point, as this model had been successfully employed by the bone research community to explore the effects of various anabolic and anti-resorptive therapies on bone remodeling and bone healing (Fig. 1). To develop a fracture pain model, indices of fracture pain such as nocifensive behaviors and activity monitoring were added. Once these models of bone fracture pain were established [26, 27••, 28] and validated [29], in many ways, these models are similar to the sequence of events observed in humans following bone fracture. Thus, in rodents, pain behaviors were apparent immediately following fracture which included increased guarding and flinching, reduced load bearing on the fractured bone, and an overall decrease in horizontal activity (Fig. 2). If rapid and effective healing occurred, these behaviors were generally extinguished by 14–28 days post-fracture in young adult male and female rodents [26, 27••, 28–30, 31••, 32••]. However, if effective fracture healing did not occur, many of these fracture pain-related behaviors were still present at 6–9 months post-fracture [33•].
Fig. 1.
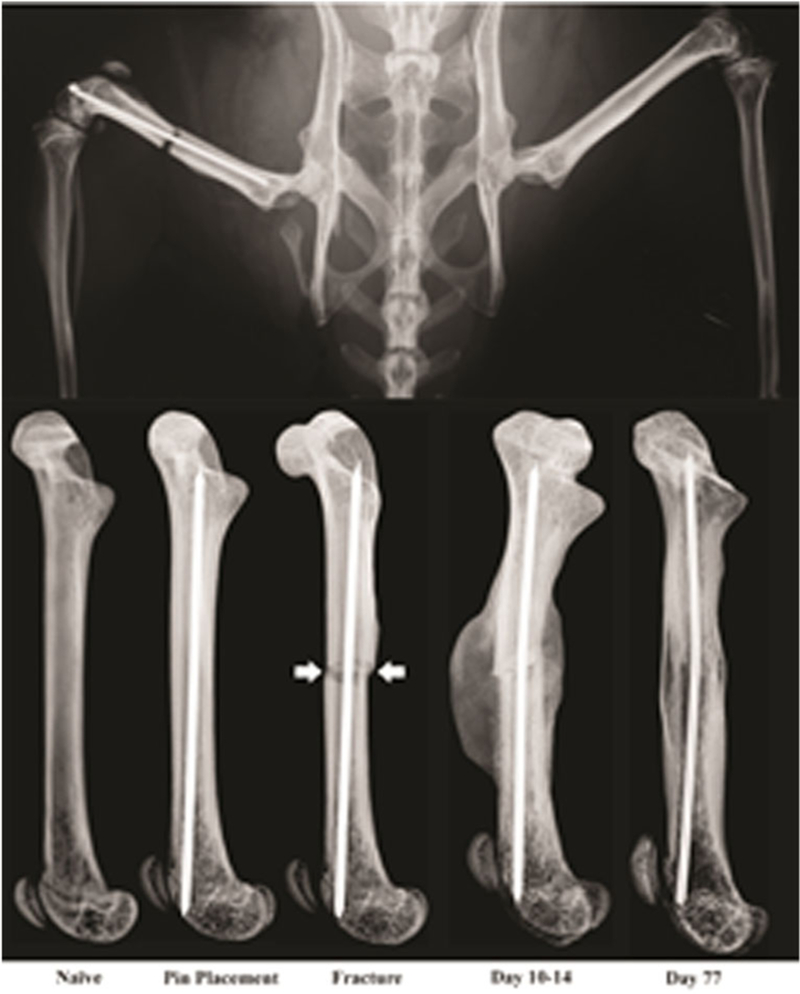
Representative radiographs showing the rodent model of bone fracture pain. Note that in the top image, a titanium pin has been placed in the intramedullary space of the right femur before the fracture (to stabilize the fractured bone) and a fracture has been made in the middle of the femur. Pain is immediately evident following bone fracture and with normal bone healing (callous formation, mineralization, resorption, and cortical union), the fracture pain subsides. These images are from a mouse but a nearly identical model has also been developed in rats
Fig. 2.
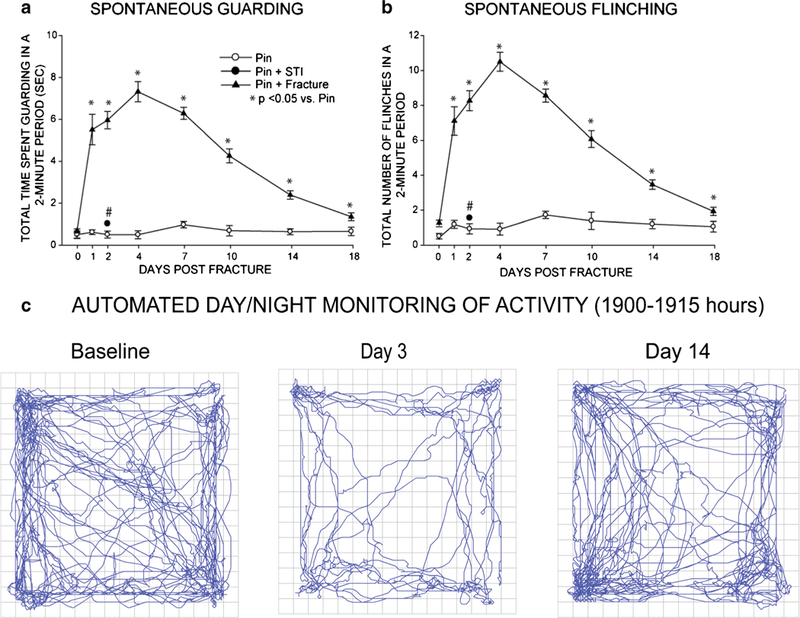
Quantification of pain-related behaviors following bone fracture. Bone fracture pain can be assessed by measuring spontaneous guarding (a) and spontaneous flinching (b) of the right hindlimb over a 2-min observation period during the day. Pain behaviors can also be assessed by using 20 h day/night activity monitoring of horizontal (c), vertical and velocity of movement of the mice following fracture. The pictographs in (c) show the spontaneous horizontal activity at baseline (1 day prefracture), day 3 post-fracture, and day 14 post-fracture. In general, in young mice, if effective bone healing occurs, fracture-induced pain-related behaviors peak at 1–4 days post-fracture and return to baseline by day 21 days post-fracture. Note that in mice, placement of the stainless-steel pin or impactor-induced soft tissue injury (STI) alone with no bone fracture shows little change in either spontaneous guarding (a) or flinching (b)
Mechanisms that Drive Bone Fracture
Fracture Activates Mechanosensitive Channels that Are Expressed by Sensory Neurons which Innervate Bone
Immediately following bone fracture, mechanosensitive nerve fibers that innervate bone are mechanically distorted resul ting in these nerve fibers rapidly discharging and signaling the initial injury to the spinal cord and brain [34–36]. Many of these mechanosensitive nerve fibers that detect and signal the initial fracture pain are located in the periosteum which is tightly opposed to the outer cortical wall of mineralized bone [36]. Previous studies have shown that many of the sensory nerve fibers that innervate the periosteum are mechanosensitive C and A-delta nociceptors [34, 37, 38, 39•] that rapidly respond to mechanical distortion of the adjacent bone or increased intraosseous pressure [37, 40, 41]. Following bone fracture, any movement or loading of the fractured bone would be expected to result in mechanical stimulation of mechanosensitive sensory nerve fibers that innervate the periosteum, mineralized bone, and marrow [27••, 28, 37, 42, 43]. Thus, normally, non-noxious loading of the bone will distort the mechanosensitive nerve fibers so that even normally innocuous movement or loading of the fractured bone will now be perceived as a highly noxious event.
Activation and Sensitization of Bone Nociceptors
Within minutes of bone fracture, a wide variety of stromal and inflammatory cells release mediators that can directly activate or sensitize nociceptors that normally innervate the bone. These mediators include prostaglandins [6••, 44], bradykinin [45], endothelins [46], and nerve growth factor [27••, 31••] which have all been shown to excite and/or sensitize nociceptors that innervate the bone. Interestingly, therapies targeting these mediators have been shown to relieve a variety of human skeletal pains in animals and humans including osteoarthritis, low back pain, and bone cancer pain [47, 48••]. One molecule that appears to be particularly effective in sensitizing bone nociceptors is nerve growth factor (NGF) [32••, 37]. When NGF binds to its cognate receptor tropomyosin receptor kinase A (TrkA), a variety of mechanotransducers, ion channels, receptors, and neurotransmitters expressed by nociceptors appear to be sensitized and/or upregulated [32••, 49] so that normally innocuous stimulation of a bone nociceptor is now per-ceived as a noxious event (Fig. 3).
Fig. 3.
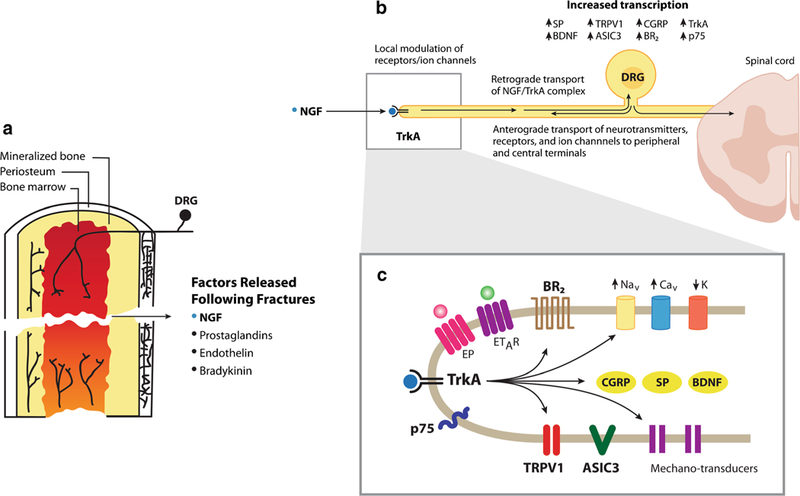
Schematic illustrating the sensory nerve fibers that innervate the femur and some of the factors that may contribute to fracture-induced skeletal pain. Sensory nerve fibers that innervate the bone (a) are generally mechanosensitive and are present in the periosteum (the thin cellular and fibrous sheath that surrounds the outer surface of the mineralized bone), cortical bone, and bone marrow. Following bone fracture, a variety of factors are released at the fracture site including nerve growth factor (NGF), prostaglandins, endothelins, and bradykinin which activate and/or sensitize neurons that convey information from the bone to the spinal cord (b) in that NGF binds to its cognate receptor tropomyosin receptor kinase A (TrkA) and the NGF/TrkA complex is then retrogradely transported to the cell body of the sensory neuron where it induces upregulation of a variety of neurotransmitters, receptors, and ion channels involved in detecting and transmitting noxious stimuli from the bone to the spinal cord and brain. In sensory nerve fibers at the fracture site (c), NGF also directly sensitizes a variety of receptors, ion channels, and mechanotransducers expressed by sensory nerve fibers that innervate the bone so that normally innocuous stimulation of the bone is now perceived as noxious stimuli
Nerve Injury and Ectopic Nerve Sprouting Following Bone Fracture
Following bone fracture, mechanical injury to sensory or sympathetic nerve fibers that innervate the bone may occur generating a neuropathic pain state [27••, 28]. One other mechanism that may be involved in driving bone fracture pain is ectopic nerve sprouting (Fig. 4). Following bone fracture, several neurotrophic factors, including NGF, are released by stromal and inflammatory cells, which can induce an exuberant and highly ectopic sprouting resulting in hyper-innervation of the marrow, mineralized bone, and periosteum [33•]. Importantly, NGF can not only induce ectopic nerve sprouting into areas of bone that are normally poorly innervated, but can also sensitize these newly sprouted nerve fibers so normal loading or movement of the fractured bone is perceived as a highly noxious event [6••, 37]. While ectopic sprouting of sensory/sympathetic nerve fibers probably occurs in the callus at most fracture sites [50], with rapid and effective bone healing, NGF levels decline and these newly sprouted nerve fibers are “pruned” back resulting in a normal innervation of the bone and non-sensitized nociceptors [51]. However, if normal and effective fracture healing does not occur, this ectopic nerve sprouting in the non-resorbed callus can persist [33•, 51] so that mechanical strain and/or distortion of still weak and non-healed bone may result in normally innocuous movement and loading of the fracture site now being perceived as a noxious event.
Fig. 4.
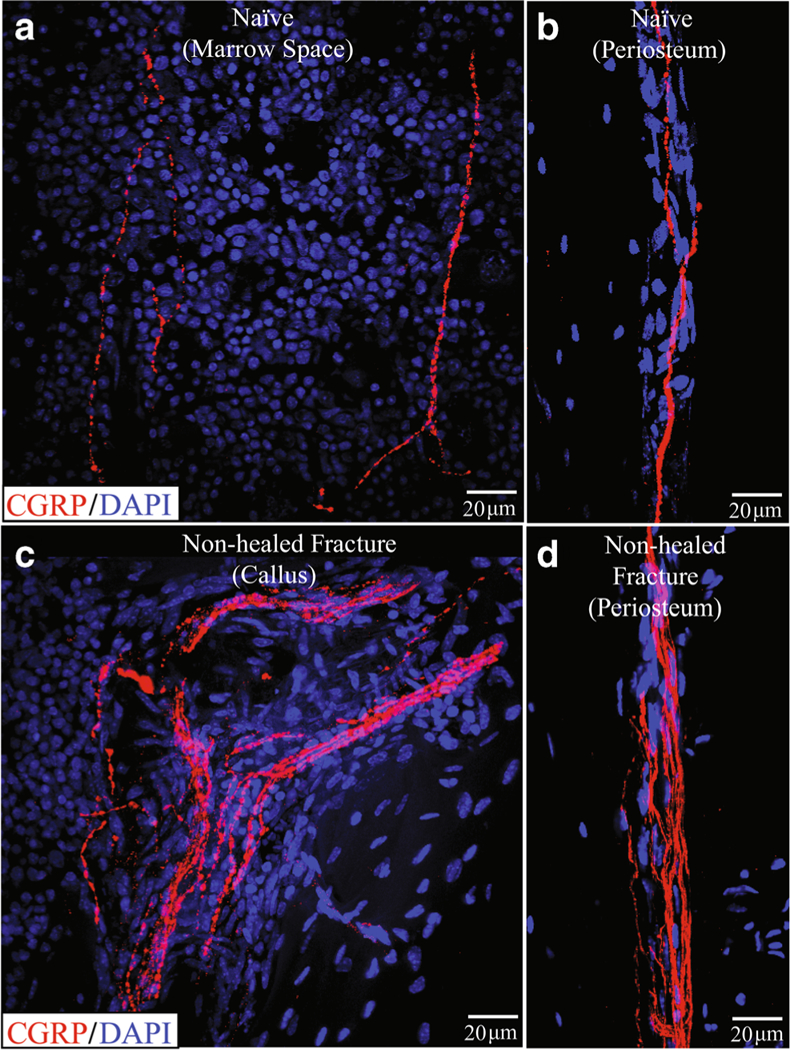
Sprouting of sensory nerve fibers at an unhealed fracture site 6 months following bone fracture where desired healing of the fracture has not occurred. In these confocal images, sensory nerve fibers are labeled with an antibody raised against calcitonin gene-related peptide (CGRP, red). Note that in the normal uninjured femur (a, b), there are a few new fibers in the bone marrow (a) and in the periosteum (b). However, in the non-healed fracture, there is a marked increase in the density of sensory nerve fibers in both the callous (c) and in the periosteum (D). This “hyper-innervation” of the bone at the unhealed fracture site is never observed in the normal femur and may contribute to the chronic limping and pain-related behaviors observed in animals with non-healed fractures
Central Sensitization
While the present review has focused on advances made in understanding the peripheral mechanisms that drive bone fracture pain, what is also clear is that following bone fracture, the brain will also undergo sensitization (i.e., “central sensitization”) that amplifies the perception and severity of pain [52••, 53]. Central sensitization is thought to occur when the chemical, electrophysiological, and pharmacological systems that transmit and modulate pain are altered in the spinal cord and brain so that normal use and movement of the bone is now perceived as a noxious event [54].
It should be emphasized that we do not yet know the specific mechanisms that generate central sensitization. However, what we do know is that injury to the skeletal system seems to be much more effective at inducing central sensitization as compared to injury to skin. For example, in 1986, Woolf and Wall noted “…a twisted ankle invokes relatively little destruction of tissue and elicits an abrupt localized stabbing pain that dies down quickly but is followed by a prolonged period of spreading, poorly localized deep pain, and tenderness that affects reflexes and gait. In contrast, localized skin damage produces an acute burst of pain that gradually dies down over minutes but is associated with a spatially restricted response of flair, wheal and surrounding tenderness” [55]. These authors also noted that small skin lesions produce comparatively less widespread and prolonged disturbances to sensation and reflex patterns than injuries to the skeleton [55].
Mechanism-Based Therapies to Control Bone Fracture Pain
Although bone fractures are a common cause of chronic pain and long-term physical disability (especially in the elderly where bone healing is slower and frequently incomplete), there are currently relatively few pharmacological therapies that can fully manage the pain and stimulate fracture repair without significant, unwanted side effects. However, in the last decade, significant progress has been made. Thus, in terms of bone fracture pain, we now know that the bone is innervated by a limited repertoire of sensory nerve fibers [37, 49, 56] (Fig. 5) and that many forms of skeletal pain have both a nociceptive and a neuropathic component [6••]. We also know that the majority of sensory nerve fibers that innervate the bone express TrkA and thus respond to NGF that is released at the site of bone fracture. Furthermore, we know that sensory and sympathetic nerve fibers in an injured or diseased skeleton also display a remarkable neurochemical and morphological plasticity by upregulating neurotransmitters, cytokines, growth factors, and receptors and undergoing an exuberant nerve sprouting that is never observed in the normal skeleton [33•, 57]. Additionally, sequestration of NGF or inhibition of TrkA can result in significant attenuation of skeletal pain [27••, 29, 32••, 47, 48••, 57–62].
Fig. 5.
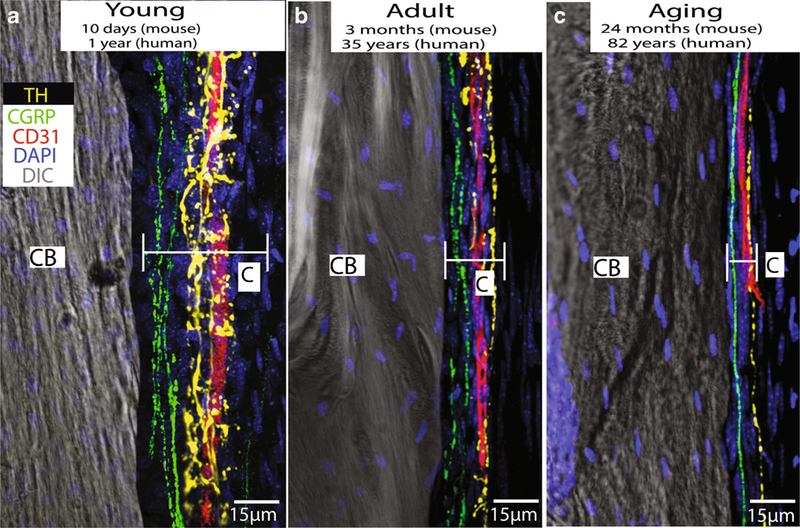
Images showing that sympathetic nerve fibers (yellow), sensory nerve fibers (green), and blood vessels (red) are present and have a similar organization in the periosteum of the young, adult, and aging mouse femur. The periosteum is a thin cellular and fibrous sheath that is tightly opposed to the outer surface of all bones of the body and probably plays a major role in detecting bone fracture and the generation of acute and chronic fracture pain. These data would suggest that even in the very young and very old, the periosteum is richly innervated by sensory and sympathetic nerve fibers and these nerve fibers probably play a significant role in the detection and signaling of fracture pain throughout the lifespan. In these images, blood vessels are labeled with an antibody raised against CD-31, primary afferent sensory nerve fibers are labeled with an antibody raised against calcitonin gene-related peptide (CGRP), and sympathetic nerve fibers are labeled with an antibody raised against tyrosine hydroxylase (TH)
Progress has also been made in beginning to identify molecules that may speed up fracture healing. Thus, sclerostin has now been identified as a protein that is expressed and released by osteocytes [3, 63] and when it is present in high levels, it inhibits bone formation and slows down fracture healing [64••, 65]. With aging, the incidence of low-trauma fractures increases and the rate of rapid and effective bone fracture healing decreases. Inhibition of sclerostin (with antibodies that sequester sclerostin) may reduce the likelihood of fragility fractures, stimulate more rapid and effective fracture healing, and thereby reduce the incidence and duration of skeletal pain that frequently accompanies failed healing of bone fractures. Thus, preclinical and human studies suggest that inhibition of endogenous sclerostin builds bone and promotes fracture healing in the young and aging bone [3••, 64••].
Conclusions
We are only beginning to understand the mechanisms that drive fracture pain. Whether mechanism-based therapies such as anti-NGF, TrkA inhibitors, or anti-sclerostin receive approval for broad use in humans will depend on their safety profile. However, NGF and its cognate receptor TrkA clearly play major roles in driving bone fracture pain and sclerostin plays a major role in driving the age-related decline in fracture healing. A better understanding of the mechanisms that drive bone fracture pain as well as the factors that control bone healing in the young, adult, and aging bone has the potential to transform our ability to better manage fracture pain whether the bone fracture is due to injury, disease, or aging.
Acknowledgments
Funding Information
Research supporting this manuscript was funded by NIH grants CA154550, CA157449, and NS023970 to Patrick Mantyh. Dr. Mantyh has served as a consultant and/or received research grants from Abbott (Abbott Park, IL), Adolor (Exton, PA), Array Biopharma (Boulder, CO), Johnson and Johnson (New Brunswick, NJ), Merck (White Plains, New York), Pfizer (New York, NY), Plexxikon (Berkeley, CA), Rinat (South San Francisco, CA), and Roche (Palo Alto, CA).
Footnotes
This article is part of the Topical Collection on Bone and Joint Pain
Compliance with Ethical Standards
Conflict of Interest Stefanie Mitchell and Lisa Majuta declare no conflicts of interest.
Human and Animal Rights and Informed Consent All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Papers of particular interest, published recently, have been highlighted as:
Of importance
Of major importance
References
- 1.Yates D, Smith M. Orthopaedic pain after trauma. In: PD Wall RM, editor. Textbook of pain New York: Churchhill Livingstone; 1994. p. 409–21. [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17(12):1726–33. 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Thompson ML, Chartier SR, Mitchell SA, Mantyh PW. Preventing painful age-related bone fractures: anti-sclerostin therapy builds cortical bone and increases the proliferation of osteogenic cells in the periosteum of the geriatric mouse femur. Mol Pain 2016;12:1–11. 10.1177/1744806916677147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruber R, Koch H, Doll BA, Tegtmeier F, Einhorn TA, Hollinger JO. Fracture healing in the elderly patient. Exp Gerontol 2006;41(11):1080–93. 10.1016/j.exger.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Svensson H, Olofsson E, Karlsson J, Hansson T, Olsson L-E. A painful, never ending story: older women’s experiences of living with an osteoporotic vertebral compression fracture. Osteoporos Int 2016;27(5):1729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.••.Mantyh PW. The neurobiology of skeletal pain. Eur J Neurosci 2014;39(3):508–19. 10.1111/ejn.12462.A recent review of the neurobiology of skeletal pain including new approaches to treatment.
- 7.Feinberg SD. Prescribing analgesics. How to improve function and avoid toxicity when treating chronic pain. Geriatrics 2000;55(11): 44 9–50, 3 passim [PubMed] [Google Scholar]
- 8.Giannoudis PV, MacDonald DA, Matthews SJ, Smith RM, Furlong AJ, De Boer P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br 2000;82(5):655–8. [DOI] [PubMed] [Google Scholar]
- 9.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 1999;353(9156):878–82. 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 10.Alves CJ, Neto E, Sousa DM, Leitao L, Vasconcelos DM, Ribeiro-Silva M, et al. Fracture pain—traveling unknown pathways. Bone 2016;85:107–14. 10.1016/j.bone.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor JP, Capo JT, Tan V, Cottrell JA, Manigrasso MB, Bontempo N, et al. A comparison of the effects of ibuprofen and rofecoxib on rabbit fibula osteotomy healing. Acta Orthop 2009;80(5):597–605. 10.3109/17453670903316769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon AM, O’Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg Am 2007;89(3):500–11. 10.2106/JBJS.F.00127. [DOI] [PubMed] [Google Scholar]
- 13.••.Simon AM, Manigrasso MB, O’Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res 2002;17(6):963–76. 10.1359/jbmr.2002.17.6.963.Reported that Cox2 reduces fracture healing.
- 14.Gerstenfeld LC, Thiede M, Seibert K, Mielke C, Phippard D, Svagr B, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res 2003;21(4):670–5. 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 15.Murnaghan M, Li G, Marsh DR. Nonsteroidal anti-inflammatory drug-induced fracture nonunion: an inhibition of angiogenesis? J Bone Joint Surg Am 2006;88(Suppl 3):140–7. 10.2106/JBJS.F.00454. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharyya T, Levin R, Vrahas MS, Solomon DH. Nonsteroidal antiinflammatory drugs and nonunion of humeral shaft fractures. Arthritis Rheum 2005;53(3):364–7. 10.1002/art.21170. [DOI] [PubMed] [Google Scholar]
- 17.Koester MC, Spindler KP. Pharmacologic agents in fracture healing. Clin Sports Med 2006;25(1):63–73, viii. 10.1016/j.csm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler P, Batt ME. Do non-steroidal anti-inflammatory drugs adversely affect stress fracture healing? A short review. Br J Sports Med 2005;39(2):65–9. 10.1136/bjsm.2004.012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am 2009;91(4):919–27. 10.2106/JBJS.H.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savage SR. Long-term opioid therapy: assessment of consequences and risks. J Pain Symptom Manag 1996;11(5):274–86. [DOI] [PubMed] [Google Scholar]
- 21.••.Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013;154(Suppl 1):S94–100. 10.1016/j.pain.2013.09.009.An excellent review of the effects of opiates and functional status.
- 22.Chau DL, Walker V, Pai L, Cho LM. Opiates and elderly: use and side effects. Clin Interv Aging 2008;3(2):273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manigrasso MB, O’Connor JP. Characterization of a closed femur fracture model in mice. J Orthop Trauma 2004;18(10):687–95. [DOI] [PubMed] [Google Scholar]
- 24.Gerstenfeld LC, Alkhiary YM, Krall EA, Nicholls FH, Stapleton SN, Fitch JL, et al. Three-dimensional reconstruction of fracture callus morphogenesis. J Histochem Cytochem 2006;54(11): 1215–28. 10.1369/jhc.6A6959.2006. [DOI] [PubMed] [Google Scholar]
- 25.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res 1984;2(1):97–101. 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- 26.Jimenez-Andrade JM, Bloom AP, Mantyh WG, Koewler NJ, Freeman KT, Delong D, et al. Capsaicin-sensitive sensory nerve fibers contribute to the generation and maintenance of skeletal fracture pain. Neuroscience 2009;162(4):1244–54. 10.1016/j.neuroscience.2009.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.••.Koewler NJ, Freeman KT, Buus RJ, Herrera MB, Jimenez-Andrade JM, Ghilardi JR, et al. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res 2007;22(11):1732–42. 10.1359/jbmr.070711.Development of the first rodent model of fracture pain.
- 28.Jimenez-Andrade JM, Martin CD, Koewler NJ, Freeman KT, Sullivan LJ, Halvorson KG, et al. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain 2007;133(1–3):183–96. 10.1016/j.pain.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Majuta LA, Longo G, Fealk MN, McCaffrey G, Mantyh PW. Orthopedic surgery and bone fracture pain are both significantly attenuated by sustained blockade of nerve growth factor. Pain 2015; 156(1) : 157–65. 10.1016/j.pain.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman KT, Koewler NJ, Jimenez-Andrade JM, Buus RJ, Herrera MB, Martin CD, et al. A fracture pain model in the rat: adaptation of a closed femur fracture model to study skeletal pain. Anesthesiology 2008;108(3):473–83. 10.1097/ALN.0b013e3181649351. [DOI] [PubMed] [Google Scholar]
- 31.••.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Mantyh WG, Bloom AP, Bouhana KS, et al. Sustained blockade of neurotrophin receptors TrkA, TrkB and TrkC reduces non-malignant skeletal pain but not the maintenance of sensory and sympathetic nerve fibers. Bone 2011;48(2):389–98. 10.1016/j.bone.2010.09.019.Antagonism of Trks attenuates skeletal pain.
- 32.••.Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011;115(1):189–204. 10.1097/ALN.0b013e31821b1ac5.Review summarizing NGF and the relief of skeletal pain.
- 33.•.Chartier SR, Thompson ML, Longo G, Fealk MN, Majuta LA, Mantyh PW. Exuberant sprouting of sensory and sympathetic nerve fibers in nonhealed bone fractures and the generation and mainte-nance of chronic skeletal pain. Pain 2014;155(11):2323–36. 10.1016/j.pain.2014.08.026.Nerve sprouting in a non-healed bone fracture.
- 34.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 2002;113(1):155–66. [DOI] [PubMed] [Google Scholar]
- 35.Hukkanen M, Konttinen YT, Rees RG, Gibson SJ, Santavirta S, Polak JM. Innervation of bone from healthy and arthritic rats by substance P and calcitonin gene related peptide containing sensory fibers. J Rheumatol 1992;19(8):1252–9. [PubMed] [Google Scholar]
- 36.Martin CD, Jimenez-Andrade JM, Ghilardi JR, Mantyh PW. Organization of a unique net-like meshwork of CGRP+ sensory fibers in the mouse periosteum: implications for the generation and maintenance of bone fracture pain. Neurosci Lett 2007;427(3): 148–52. 10.1016/j.neulet.2007.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nencini S, Ringuet M, Kim DH, Chen YJ, Greenhill C, Ivanusic JJ. Mechanisms of nerve growth factor signaling in bone nociceptors and in an animal model of inflammatory bone pain. Mol Pain 2017;13:1744 806917697011. 10.1177/1744806917697011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aso K, Izumi M, Sugimura N, Okanoue Y, Ushida T, Ikeuchi M. Nociceptive phenotype alterations of dorsal root ganglia neurons innervating the subchondral bone in osteoarthritic rat knee joints . Osteoarthr Cartil 2016;24(9):1596–603. 10.1016/j.joca.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 39.•.Ivanusic JJ, Mahns DA, Sahai V, Rowe MJ. Absence of large-diameter sensory fibres in a nerve to the cat humerus. J Anat 2006;208(2):251–5. 10.1111/j.1469-7580.2006.00519.x.Large diameter nerve fibers do not appear to innervate bone.
- 40.Furusawa S A neurophysiological study on the sensibility of the bone marrow. Nihon Seikeigeka Gakkai Zasshi 1970;44(5):365–70. [PubMed] [Google Scholar]
- 41.Nencini S, Ivanusic J. Mechanically sensitive Adelta nociceptors that innervate bone marrow respond to changes in intra-osseous pressure. J Physiol 2017;595(13):4399–415. 10.1113/JP273877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seike W Electrophysiological and histological studies on the sensibility of the bone marrow nerve terminal. Yonago Acta Med 1976;20(3):192–211. [PubMed] [Google Scholar]
- 43.Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain 2005;115(1–2):128–41. 10.1016/j.pain.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 44.Sabino MA, Ghilardi JR, Jongen JL, Keyser CP, Luger NM, Mach DB, et al. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res 2002;62(24):7343–9. [PubMed] [Google Scholar]
- 45.Sevcik MA, Ghilardi JR, Halvorson KG, Lindsay TH, Kubota K, Mantyh PW. Analgesic efficacy of bradykinin B1 antagonists in a murine bone cancer pain model. J Pain 2005;6(11):771–5. doi.org/10.1016/j.jpain.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Peters CM, Lindsay TH, Pomonis JD, Luger NM, Ghilardi JR, Sevcik MA, et al. Endothelin and the tumorigenic component of bone cancer pain. Neuroscience 2004;126(4):1043–52. 10.1016/j.neuroscience.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 47.Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 2011;152(10):2248–58. 10.1016/j.pain.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 48.••.Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010;363(16):1521–31. 10.1056/NEJMoa0901510.Anti-NGF relieves skeletal pain in humans.
- 49.Castaneda-Corral G, Jimenez-Andrade JM, Bloom AP, Taylor RN, Mantyh WG, Kaczmarska MJ, et al. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience 2011;178:196–207. 10.1016/j.neuroscience.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Ahmad T, Spetea M, Ahmed M, Kreicbergs A. Bone reinnervation after fracture: a study in the rat. J Bone Miner Res 2001;16(8):1505–10. 10.1359/jbmr.2001.16.8.1505. [DOI] [PubMed] [Google Scholar]
- 51.Yasui M, Shiraishi Y, Ozaki N, Hayashi K, Hori K, Ichiyanagi M, et al. Nerve growth factor and associated nerve sprouting contribute to local mechanical hyperalgesia in a rat model of bone injury. Eur J Pain 2012;16(7):953–65. 10.1002/j.1532-2149.2011.00094.x. [DOI] [PubMed] [Google Scholar]
- 52.••.Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 2010;6(10):599–606. 10.1038/nrrheum.2010.107.An excellent review of central sensitization in musculoskeletal pain.
- 53.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149(3):573–81. 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152(3 Suppl):S2–15. 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woolf CJ, Wall PD. Relative effectiveness of C primary afferent fibers of different origins in evoking a prolonged facilitation of the flexor reflex in the rat. J Neurosci 1986;6(5):1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone 2004;35(5):1003–12. 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 57.Jimenez-Andrade JM, Ghilardi JR, Castaneda-Corral G, Kuskowski MA, Mantyh PW. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 2011;152(11):2564–74. 10.1016/j.pain.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, et al. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res 2005;65(20):9426–35. 10.1158/0008-5472.CAN-05-0826. [DOI] [PubMed] [Google Scholar]
- 59.Tanezumab Cattaneo A., a recombinant humanized mAb against nerve growth factor for the treatment of acute and chronic pain. Curr Opin Mol Ther 2010;12(1):94–106. [PubMed] [Google Scholar]
- 60.Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain 2012;13(8):790–8. 10.1016/j.jpain.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Schnitzer TJ, Lane NE, Birbara C, Smith MD, Simpson SL, Brown MT. Long-term open-label study of tanezumab for moderate to severe osteoarthritic knee pain. Osteoarthr Cartil 2011;19(6):639–46. 10.1016/j.joca.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Ekman EF, Gimbel JS, Bello AE, Smith MD, Keller DS, Annis KM, et al. Efficacy and safety of intravenous tanezumab for the symptomatic treatment of osteoarthritis: 2 randomized controlled trials versus naproxen. J Rheumatol 2014;41(11):2249–59. 10.3899/jrheum.131294. [DOI] [PubMed] [Google Scholar]
- 63.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 2008;283(9): 5866–75. 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 64.••.Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 2009;24(4):578–88. 10.1359/jbmr.081206.Anti-sclerostin therapy increases bone formation and strength.
- 65.Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 2010;25(5):948–59. 10.1002/jbmr.14. [DOI] [PubMed] [Google Scholar]


