Abstract
The purpose of this study was to assess the significance of tenascin‐C (Tnc) expression in steatotic liver ischemia and reperfusion injury (IRI). The critical shortage in donor organs has led to the use of steatotic livers in transplantation regardless of their elevated susceptibility to hepatic IRI. Tnc is an endogenous danger signal extracellular matrix (ECM) molecule involved in various aspects of immunity and tissue injury. In the current study, mice were fed with a steatosis-inducing diet and developed approximately 50% hepatic steatosis, predominantly macrovesicular, before being subjected to hepatic IRI. We report here that lipid accumulation in hepatocytes inflated the production of Tnc in steatotic livers and in isolated hepatic stellate cells (HSCs). Moreover, we show that the inability of Tnc-/- deficient steatotic mice to express Tnc significantly protected these mice from liver IRI. Compared to fatty controls, Tnc-/- steatotic mice showed significantly reduced serum transaminase levels and enhanced liver histological preservation at both 6h and 24h after hepatic IRI. The lack of Tnc expression resulted in impaired Ly-6G neutrophil and Mac-1 leukocyte recruitment as well as in decreased expression of pro-inflammatory mediators (IL‐1β, TNF-α, and CXCL2) after liver reperfusion. Myeloperoxidase (MPO) is the most abundant cytotoxic enzyme secreted by neutrophils and a key mediator of neutrophil-induced oxidative tissue injuries. Using an in vitro model of steatosis, we also show that Tnc markedly potentiated the effect of steatotic hepatocytes on neutrophil-derived MPO activity. In conclusion, our data support the view that inhibition of Tnc is a promising therapeutic approach to lessen inflammation in steatotic livers, and to maximize their successful use in organ transplantation.
Keywords: extracellular matrix, endogenous danger molecules, organ shortage, inflammation, knockout mice
INTRODUCTION
Hepatic ischemia and reperfusion injury (IRI) is a pathological condition characterized by an initial insult caused by the restriction of blood supply to liver, which is further accentuated by the restoration of blood flow to the compromised organ.1 Hepatic IRI remains a major clinical limitation in orthotopic liver transplantation,2 and its negative impact is highly aggravated in steatotic livers.3–6 Steatosis, characterized by the deposition of triglyceride-rich lipid droplets in the cytoplasm of hepatocytes, is a global health problem affecting a very large number of potential donor livers.7–11 Steatosis when present in moderate to severe levels (>30%), particularly macrosteatosis,6 leads to elevated rates of primary non-function,12 and decreased graft and patient survival post-transplantation.13 Nevertheless, the shortage of organ donors has led to the use of these suboptimal steatotic livers in transplantation.8, 9 In 2015 alone, ~2,900 patients died on, or were removed from (because of further health deterioration), the waiting list before having a chance to undergo liver transplantation.14 Hence, there is a need for finding protective strategies effective in fatty liver IRI.
Tenascin-C (Tnc) is a large hexameric extracellular matrix (ECM) molecule composed of multiple fibronectin (FN)‐type III and epidermal growth factor (EGF)‐like repeats and a single fibrinogen‐like domain (FBG).15 Tnc is abundantly expressed during embryogenesis and is normally undetectable in most healthy adult tissues.15 Notably, Tnc is rapidly induced in adult tissues in response to pathological stress.16 While Tnc is necessary for physiological tissue repair, its misregulated expression has been associated with a variety of pathological conditions which include rheumatoid arthritis, cancer, myocardial infarction, hepatitis, and hepatic IRI.17–22 Tnc is a pleiotropic molecule implicated in various cellular functions, being regulation of cell adhesion and migration among the most characterized.18 Growing evidence now points to Tnc as a key regulator of both innate and adaptive immunity. A number of studies have reported that Tnc affects leukocyte recruitment, T cell activation and proliferation, and the production of several inflammatory cytokines.17, 18, 23–26 Tenascin-C is an immunomodulatory protein with complex tissue-specific interactions and functions which are dependent on the type of tissue injury.22, 24 Normal and steatotic livers undergo different mechanisms of ischemic injury.4 The present study assesses the effect of hepatic lipid accumulation on Tnc expression and it tests whether Tnc deletion ameliorates the outcome of steatotic liver IRI.
EXPERIMENTAL PROCEDURES
Animals, Diet, and Model of Hepatic IRI
Tenascin-C deficient (Tnc-/-) knockout (KO) mice (C57BL/6N‐TgH) were obtained from Riken, Japan,27 rederived by sterile embryo transfer to surrogate mothers, and housed in the UCLA animal facility under specific pathogen‐free conditions. To achieve hepatic steatosis, Tnc-/- mice and matched Tnc+/+ wild-type (WT) littermates were fed a HFD diet (HFD; 60% kcal in fat; Research Diets, D12492) from 4 to 12 weeks of age, as previously described.28 Twelve-week-old male mice were subjected to hepatic IRI according to previously published surgical procedures.28, 29 Briefly, arterial and portal venous blood supplies were interrupted to the cephalad lobes of the liver for 60 minutes using an atraumatic clip, and mice were sacrificed after reperfusion. All animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Serum Transaminases
Serum alanine transaminase (ALT) and serum aspartate transaminase (AST) levels were measured using a commercially available kit (Teco Diagnostics, Anaheim, CA), according to the manufacturer’s instructions.
Histology and Immunohistochemistry
Liver histology and immunohistochemistry were performed in paraffin or cryostat sections as previously published.28 Liver specimens were fixed with a 10% buffered formalin solution, embedded in paraffin, and processed for hematoxylin and eosin (H&E) staining. To access the percentage of steatosis, livers were fixed in 4% parafomaldehyde in PBS, embedded in OCT, and processed for Oil Red O staining. Immunohistochemistry was performed using Mac-1 (M1/70) and Ly-6G (1A8) from BD Biosciences, MMP-9 (AF909; R&D Systems), and Tnc (MTn-12: Abcam) antibodies at optimal dilutions. Triple staining of liver tissues and isolated HSCs was detected by immunofluorescence with Alexa Fluor 488 (green) for Tnc and Alexa Fluor 594 (red) for GFAP (D1F4Q; Cell Signaling), Alexa Fluor 594 (red) for CD31 (Santa Cruz Biotechnology) and Alexa Fluor 594 (red) for CD68 (Abcam). Vectashield mounting media with DAPI (Vector Laboratories) was used for nuclear staining. Sections were blindly evaluated by counting 10 high-powered fields (HPFs)/section in triplicate.
RNA Extraction and Reverse Transcriptase PCR
RNA was extracted from livers, hepatocytes, and hepatic stellate cells (HSCs) with Trizol (Life Technologies) as described.30 Reverse transcription was performed using 5 μg of total RNA in a first-strand cDNA synthesis reaction with SuperScript III RNaseH Reverse Transcriptase (LifeTechnologies), as recommended by the manufacturer. The cDNA product was amplified by PCR using primers specific for each target cDNA.
Isolation and Culture of Mouse Cells
Isolation of neutrophils, hepatocyte, and HSCs was performed according to previously published methods.30–32 Briefly, to isolate primary murine hepatocytes and HSCs, anesthetized mice were subject to a midline laparotomy and cannulation of the inferior vena cava (IVC) followed by liver perfusion with an EGTA-chelating perfusion buffer (EGTA:190mg/L, Glucose:900mg/L, HEPES:10ml of 1M stock solution/L, KCl:400mg/L, Na2HPO4-2H2O:151mg/L, NaCl:8g/L, NaH2PO4-H2O:7mg/L, NaHCO3:350mg/L) After perfusion with 0.4 % collagenase buffer (CaCl2-2H2O:560mg/L, HEPES:10ml of 1M stock solution/L, KCl:400mg/L, Na2HPO4×2H2O:151mg/L, NaCl:8g/L, NaH2PO4-H2O:7mg/L, NaHCO3:350mg/L, Collagenase P: 400mg/L), livers were minced and cells dispersed in culture medium; hepatocyte and nonparenchymal cells were separated by low-speed centrifugation methods. After isolating hepatocytes, HSCs were then purified from nonparenchymal cells using a Nicodenz gradient (8%, 1500g). Purified HSCs were resuspended in the DMEM supplemented with 10% FBS. To isolate adult murine neutrophils, mouse femurs and tibias were stripped of all muscle and sinew, and bone marrow was flushed with Hanks’ balanced saline solution (HBSS). Cells were pelleted and erythrocytes were removed by hypotonic lysis. The bone marrow preparation was resuspended at 5×107 cells/mL in HBSS and the cells were layered on a Percoll gradient (Sigma-Aldrich). Mature neutrophils were recovered at the interface of the 65% and 80% fractions and were more than 90% pure and more than 95% viable in the neutrophil-rich fraction.
Hepatocyte Conditioned Medium Preparation and HSCs Activation in vitro
The preparation of a palmitic acid (PA) (P0500, Sigma) stock solution and a palmitate-induced in vitro fatty liver model were carried out as previously described.33 Briefly, isolated mouse hepatocytes (2 x105 cells/well) were incubated with DMEM plus 10% FBS for 12 hours and then treated with or without 400uM of PA for 24 hours to induce intracellular lipid accumulation. The supernatants were clarified by centrifugation at 6000 g to remove cell debris, filtered and stored in aliquots at −80°C until use. Supernatants harvested from control and steatotic hepatocytes were used as hepatocyte conditioned medium (HCM) and steatotic HCM (S-HCM), respectively. HSCs (10×104 cells/well) were stimulated with HCM or S-HCM for 24 hours. In additional experiments, isolated primary HSCs were stimulated for 24 hours with varying concentrations of recombinant mouse IL-1β (1, 5 and 10ng/ml; EMD Millipore). After stimulation cell samples were harvested either for RNA isolation or for immunofluorescence staining.
MPO Activity Analysis
Isolated neutrophils were resuspended in DMEM, HCM or S-HCM at a final concentration of 10×105 cells/ml. Neutrophils were then seeded in rTnc-coated (Millipore; 20ug/ml) or control tissue culture plates for 4 hours at 37°C in 5% CO2. Supernatant were harvested and mixed in a solution of hydrogen peroxide–sodium acetate and tetra-methyl benzidine (Sigma). The quantity of enzyme degrading in 1 mol/L of peroxide per minute at 25°C was defined as 1U of MPO activity.
Data Analysis
Results are presented as means ± standard deviation. Differences between groups were compared using Student’s t test and a two‐tailed p value < 0.05 was considered significant. Calculations were made using SPSS software (Chicago, IL).
RESULTS
Tenascin-C expression was predominantly upregulated in steatotic livers post-IRI
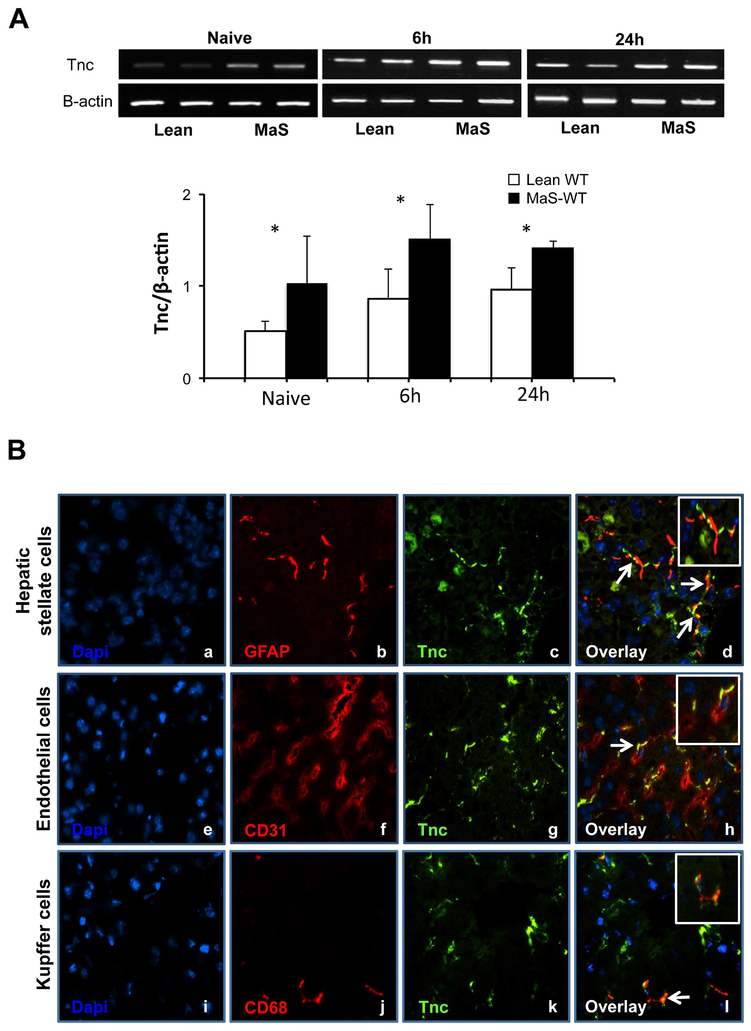
To assess Tnc expression in steatotic livers, we used an established mouse model of steatotic hepatic IRI, in which mice fed with a high-fat diet develop approximately 50% liver steatosis, with macrovesicular triglyceride-rich droplets prior to surgery.28 In contrast to naïve WT normal livers, in which Tnc expression is minimally detected,19 Tnc was quite readily noticeable in naïve WT steatotic livers (Fig. 1A). Moreover, it was significantly increased in steatotic livers after 6h and 24h post-hepatic IRI, compared with their lean counterparts (Fig. 1A). We have used immunofluorescence staining of selected cell markers to identify the sources of Tnc in steatotic liver IRI. As shown in figure 1, triple staining of Tnc, GFAP (a marker of HSCs) and Dapi showed co-localization of Tnc and GFAP in steatotic livers post-IRI. Steatotic liver sections also exhibited simultaneous staining of Tnc and α-SMA after reperfusion (not shown). In addition to HSCs, Tnc staining was detected in some scattered endothelial and Kupffer cells in the injured steatotic livers after IRI (Fig. 1B).
Figure 1.
Tenascin-C expression in steatotic livers. (A) Tnc mRNA was significantly upregulated in steatotic livers (closed bars) before and after hepatic IRI, compared to their lean counterparts (open bars). (B) Hepatic stellate cells were a source of Tnc expression in steatotic livers after IRI; Tnc protein expression (c, g, and k, green; Alexa Fluor 488) co-localized with GFAP staining (b, red; Alexa Fluor 594) in the hepatic peri-portal areas; nuclear dapi stain is shown in blue (a, e, and i). Moreover, Tnc expression was also detected in scattered CD31 (f, red; Alexa Fluor 594) and CD68 (j, red; Alexa Fluor 594) cells in steatotic livers post-IRI. The respective overlay images (d, h, and l) are shown at the right (white arrows point to co-localization of Tnc and GFAP, Tnc and CD31, and Tnc and CD68; n=4–6/group; *p<0.05).
Lipid accumulation in hepatocytes regulated Tnc expression in HSCs
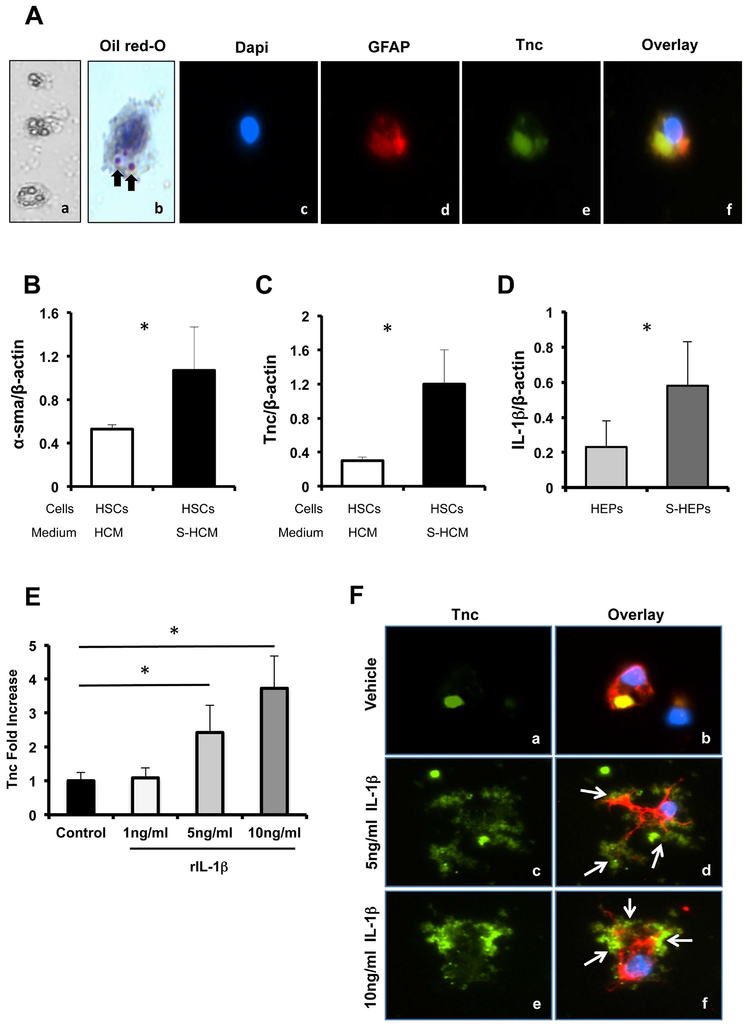
Hepatic stellate cells (HSCs) are considered to be major producers of extracellular matrix proteins,34 and we have confirmed that they were significant sources of Tnc in our liver settings. We then used an established palmitate-induced in vitro fatty liver model to test whether hepatic steatosis can affect Tnc expression by HSCs. In this model, steatotic hepatocytes are generated by incubation with saturated fatty acid palmitate (to induce intracellular lipid accumulation) and their conditioned media (S-HCM) is used for cell stimulation.33 Triple immunofluorescence staining of DAPI, Tnc, and GFAP showed co-localization of Tnc and GFAP in isolated HSCs, which were also characterized by Vitamin A deposition in their cytoplasm (Fig. 2A). Compared with HSCs incubated with control HCM (conditioned media from non-steatotic hepatocytes), incubation of HSCs with S-HCM resulted in increased α-SMA (~2-fold, p<0.05) expression and in a striking upregulation of Tnc (~4-fold, p<0.05) levels, (Fig. 2B and C). IL-1β is an inflammatory cytokine known to activate HSCs.35 We observed that lipid accumulation in isolated hepatocytes resulted in a significant increase of IL-1β expression (~2.5-fold increase, p<0.05) in these cells (Fig. 2D). IL-1β was also found to be upregulated in naïve WT steatotic livers (~3-fold increase, p<0.05) and in WT steatotic livers after IRI (~1.7-fold increase, p<0.05) when compared to their respective WT lean counterparts. We next examined whether IL-1β is capable of regulating Tnc expression in HSCs using varying concentrations of exogenous IL-1β; we observed that IL-1β induced Tnc expression by HSCs in a dose-dependent fashion (Fig. 2E). Moreover, we detected a particularly robust Tnc staining in the IL-1β-stimulated HSCs (Fig. 2F). Taken together, the above results suggest that lipid accumulation in liver leads to an increase of pro-inflammatory cytokines such as IL-1β which, in turn, favors Tnc overproduction.
Figure 2.
Tnc expression by hepatic stellate cells. (A) Isolated primary HSCs, which were positive for vitamin A (a, phase contrast; b, Oil-red-O) and GFAP staining (d, red; Alexa Fluor 594), stained also positive for Tnc (e, green; Alexa Fluor 488); nuclear dapi stain is shown in blue (c). (B) α-sma and (C) Tnc mRNA expressions were markedly increased in isolated HSCs treated with conditioned media from steatotic hepatocytes (S-HCM; filled bars), compared to control HSCs treated with conditioned media from non-steatotic hepatocytes (HCM; open bars). (D) IL-1β mRNA expression was significantly upregulated in steatotic hepatocytes (S-HEP; dark grey bar), compared to non-steatotic hepatocytes (HEP; light grey bar). (E) Exogenous recombinant IL-1β (1–10ng/ml) induced the expression of Tnc by HSCs in a dose-dependent fashion. (F) Tnc protein expression (green; Alexa Fluor 488) in vehicle (a, and b) and in IL-1β (5ng/ml, c, and d; 10ng/ml, e, and f) treated HSCs. Overlay of Tnc (green), GAFP (red; Alexa Fluor 594), and dapi (blue) staining (b, d, and f) confirms a widespread Tnc expression in IL-1β-stimulated HSCs (white arrows point to co-localization of Tnc and GAFP; black arrows point to vitamin A stored in HSCs; in vitro data is expressed as mean ± SD of four independent experiments; *p<0.05).
Tnc deficiency didn’t interfere with the development of hepatic steatosis in HFD-fed mice
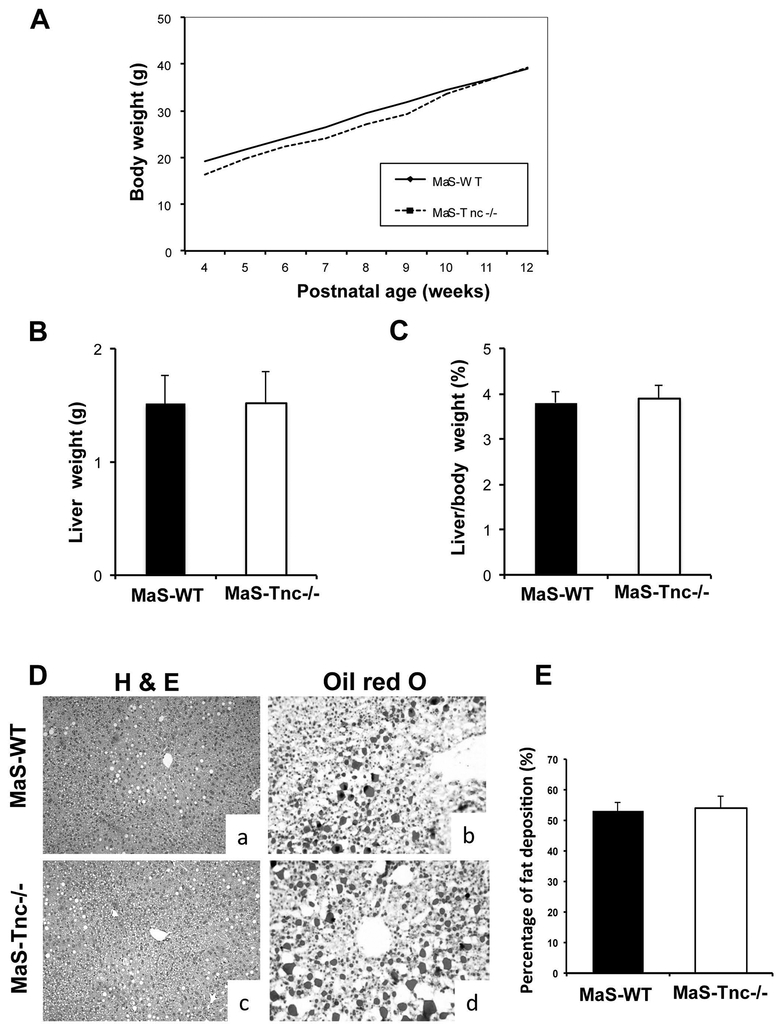
HFD-fed C57BL6 mice develop fatty livers resembling human obesity,36 and provide a good model to study steatotic liver IRI.28 To test whether Tnc deficiency affects the development of hepatic steatosis, Tnc-/- deficient mice and respective Tnc+/+ wild-type control littermates were fed a high fat diet for eight weeks;28 Tnc-/- deficient and WT mice developed comparable body weight (Fig. 3A), liver weight (Fig. 3B), and liver/body weight ratios (Fig. 3C). Moreover, Tnc-/- and WT livers showed similar levels of fat deposition (~50%), with predominantly macrovesicular fatty infiltration (MaS) after the feeding period (Fig. 2D and E) and, therefore, revealing that HFD-fed Tnc-/- and WT (Tnc+/+) mice developed comparable levels of liver steatosis.
Figure 3.
HFD-fed Tenascin-C deficient and wild-type mice developed comparable hepatic steatosis. (A) Tnc-/- mice (dotted line) and Tnc+/+ littermates (full line) showed comparable body weight gains during the HFD-feeding period. Tnc-/- (open bars) and Tnc +/+ (filled bars) mice achieved comparable (B) liver weights and (B) liver/body weight ratios after the HFD-feeding period. (D) H&E (a, c) and Oil-red-O (b, d) stainings demonstrated similar (E) fat deposition (%) in livers of Tnc-/- (c, d; open bars) and Tnc+/+ (a, b; filled bars) steatotic mice after the HFD-feeding period (n=6/group).
Tnc Deficiency Improved Steatotic Liver Function and Histology after Hepatic IRI
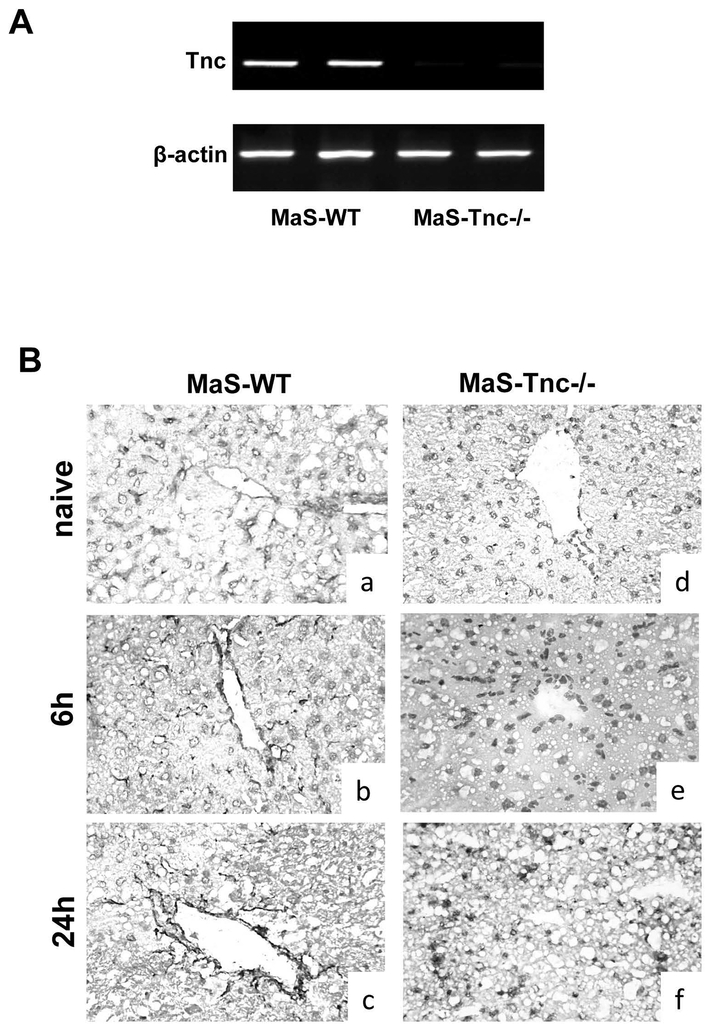
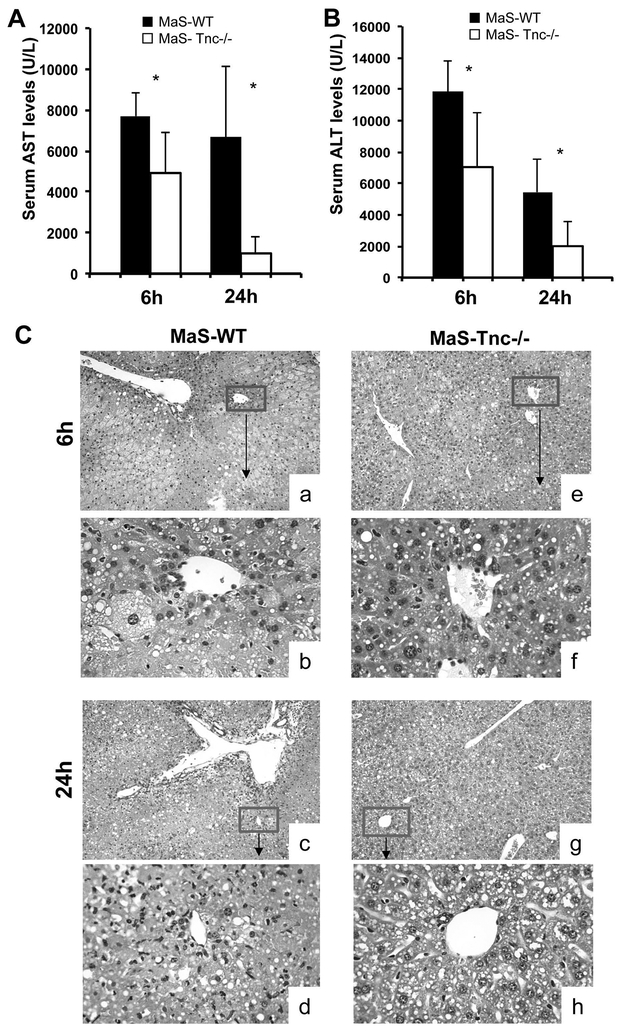
To assess the significance of Tnc overproduction in steatotic liver IRI, we used 8-week HFD fed Tnc-/- mice and respective WT littermates with approximately 50% hepatic steatosis, predominantly macrovesicular. RT-PCR (Fig. 4A) and immunohistochemistry (Fig. 4B) confirmed that Tnc was fully deleted in our Tnc-/- mice. In contrast, Tnc deposition was strongly detected in nonparenchymal liver cells of wild-type MaS control mice before and after hepatic (Fig. 4B). In general, MaS-Tnc-/- mice showed less steatotic liver damage, as evidenced by the significantly reduced serum aminotransaminase levels (U/L) at 6h (p<0.05) and 24h (p<0.05) after hepatic IRI, compared with MaS-Tnc+/+ controls (Fig.5A and B). The lower aminotransferase levels were associated with improved histological preservation in Tnc-/- steatotic mice. MaS-Tnc-/- livers were characterized by decreased sinusoidal congestion and reduced liver necrosis after reperfusion as compared with the highly damaged fatty controls (Fig. 5C).
Figure 4.
Genetic disruption of the Tnc gene eliminated Tnc expression in steatotic mice. (A) Tnc mRNA expression was virtually absent in the livers of Tnc-/- steatotic mice. (B) Immunohistochemistry showed strong non-parenchymal cell Tnc protein deposition in the peri-vascular and peri-sinusoidal areas of Tnc+/+ steatotic livers (a, b, c) before IRI (a, d) and after 6h (b, e) and 24h (c, f) of hepatic IRI, which was clearly absent in all Tnc-/- steatotic livers (d, e, f) (n=5–6/group; magnification x40).
Figure 5.
Serum transaminases and histological preservation in Tnc +/+ and Tnc -/- steatotic mice after liver IRI. (A) sAST and (B) sALT levels measured in blood samples retrieved after 6h and 24h of IRI were significantly lower in Tnc -/- mice (open bars) than in Tnc +/+ mice (filled bars). (C) Representative H&E staining of Tnc +/+ (a, b, c, d) and Tnc -/- (e, f, g, h) steatotic livers after 6h (a, b, e, f) and 24h (c, d g, h) of hepatic IRI. Tnc deficiency was associated with markedly smaller areas of lobular architecture disruption and improved vascular congestion when compared to Tnc expressing steatotic livers (n= 5/group; a, c, e, g: magnification x10; b, d, f, h: magnification x40).
Tnc Deficiency Depressed Leukocyte Infiltration and Activation in Steatotic Liver IRI.
Infiltration of Ly-6G neutrophils and Mac-1 leukocytes was clearly depressed in the portal areas of MaS-Tnc-/- livers at 6h (p<0.05) and 24h (p<0.05) post-hepatic IRI, compared to controls (Fig. 6A and B). In addition, matrix metalloproteinase-9 (MMP-9) positive leukocytes were also depressed in the MaS-Tnc-/- livers at 6h (p< 0.05) and 24h (p<0.05) after reperfusion (Fig. 6E and F). The decrease in leukocyte recruitment correlated with reduced levels of pro-inflammatory TNF-α (24h), IL1-β (6h and 24h), and CXCL-2 (24h) in MaS-Tnc-c-/- livers post- reperfusion (Fig. 6G, H, and I).
Figure 6.
Tnc deficiency reduced inflammatory leukocyte infiltration, cytokine and chemokine expression in steatotic livers post-IRI. Livers harvested at 6h (a, c) and 24h (b, d) after liver IRI showed reduced (A, B) Ly-6G+, (C, D) Mac-1+, and (E, F) MMP-9+ leukocyte infiltration in steatotic Tnc -/- (c, d; open bars) livers, when compared to their respective wild-type Tnc +/+ controls (a, b; filled bars). The expression of pro-inflammatory cytokines (G) TNF-α, (H) IL-1β, and chemokine (I) CXCL-2 was also significantly depressed in Tnc -/- steatotic livers (open bars) post-IRI (n= 5/group; *p<0.05).
Tnc potentiates the effect of steatotic hepatocytes on neutrophil-derived MPO activity
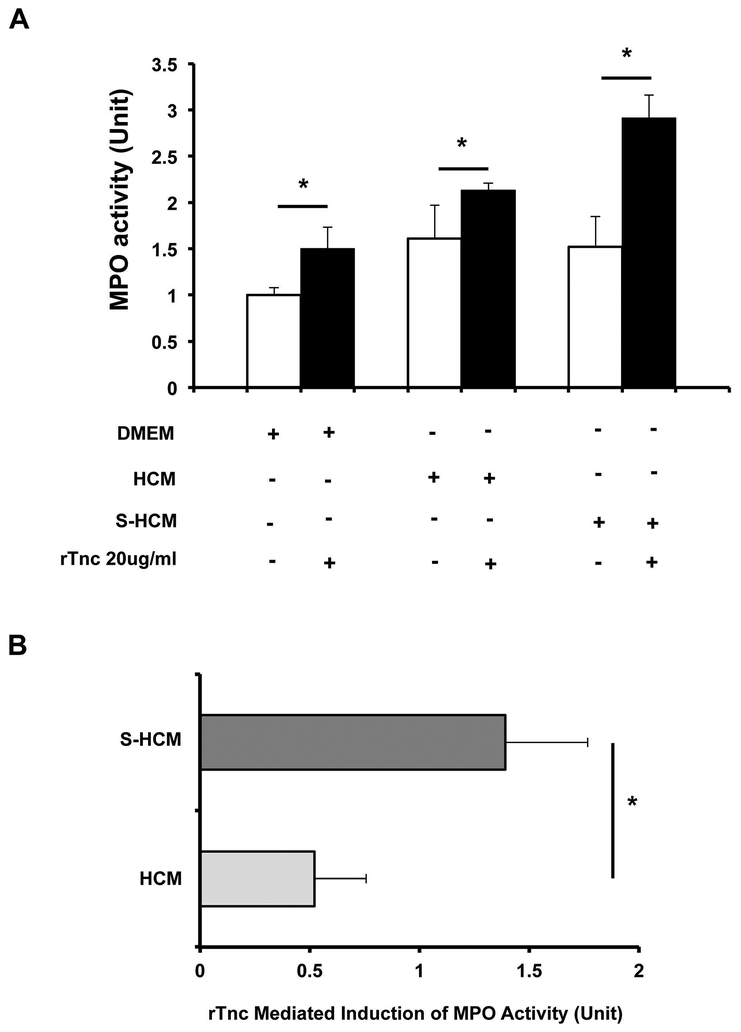
Myeloperoxidase (MPO) is the most cytotoxic and abundant enzyme secreted by activated neutrophils,37 and an important factor in the neutrophil-induced oxidative tissue injuries.38 To test whether Tnc affects MPO activity, neutrophils were incubated with DMEM, HCM, or S-HCM in rTnc-coated or control tissue culture plates. In general, MPO activity was enhanced in neutrophils stimulated by conditioned media from both non-steatotic (HCM) and steatotic (S-HCM) hepatocytes, compared to neutrophils cultured in DMEM. On the other hand, Tnc upregulated MPO activity in all three groups (DMEM, HCM, and S-HCM) of treated neutrophils; however, its effect was particularly noticeable when neutrophils were incubated simultaneously with Tnc and S-HCM (Fig.7 A). Indeed, the difference in MPO activity between rTnc (+) and rTnc (-) in the S-HCM group was about 2.5 fold larger (p<0.05) than the equivalent difference in the HCM group (Fig.7 A), providing the evidence that Tnc enhances neutrophil-mediated injury in steatotic livers.
Figure 7.
Tenascin-C accentuated the effect of steatotic hepatocytes on neutrophil-derived MPO activity. (A) MPO activity was enhanced in neutrophils stimulated by conditioned media from both non-steatotic (HCM) and steatotic (S-HCM) hepatocytes, compared to neutrophils cultured in DMEM. MPO activity was also elevated in all three neutrophils groups (DMEM, HCM, and S-HCM) when cultured in rTnc-coated plates (filed bars), compared with neutrophils cultured in control plates (open bars). However, MPO activity was predominantly increased in neutrophils stimulated by conditioned medium from steatotic hepatocytes in the presence of Tnc. (B) The difference in MPO activity between neutrophils cultured in rTnc-coated plates and neutrophils cultured in control plates in the S-HCM group was about 2.5 fold larger than the equivalent difference in the HCM group (in vitro data is expressed as mean ± SD of three independent experiments; *p<0.05).
DISCUSSION
Here we tested the role of tenascin-C expression in a model of steatotic hepatic IRI; in this model, mice fed with a steatosis-inducing diet develop approximately 50% liver steatosis, with macrovesicular triglyceride-rich droplets, prior to warm hepatic ischemia and reperfusion injury.28 The prevalence of obesity has increased substantially in the last three decades,39 and hepatic steatosis, a major complication of obesity, is associated to higher rates of primary nonfunction and to inferior patient survival after surgery.12, 13 Indeed, the decision about whether or not to transplant a liver is often based on the degree of hepatic steatosis at the time of procurement.40 Along with transplantation, steatosis has a negative impact in other clinical situations, such as shock, and cardiac arrest, which are also subject to warm hepatic IRI.6, 41
Organ steatosis leads to changes in ECM microenvironments,42, 43 which are still poorly understood due to their complexity.21 Tnc is an ECM molecule with a highly restricted expression during embryonic development, and it is virtually undetectable in mostly healthy adult tissues.15 However, de novo expression of Tnc has been detected in several adult tissues under various pathophysiological conditions.15, 26 In contrast to naïve normal (lean) livers, in which Tnc protein deposition is nearly absent,19 Tnc was already readily detected in the vascular areas of naïve steatotic livers. Moreover, Tnc expression was significantly upregulated in steatotic livers after hepatic IRI, when compared to their lean counterparts. Hepatic stellate cells are regarded as key sources of extracellular matrix proteins,34 and eagerly expressed Tnc in our liver settings. In addition to HSCs, scattered endothelial and Kupffer cells also stained positively for Tnc during hepatic IRI. To support the hypothesis that steatosis affects the expression of Tnc in liver, we used a well-established palmitate-induced in vitro fatty liver model,33 in which hepatocellular lipid accumulation resulted in increased HSC activation and Tnc overproduction by isolated HSCs. IL-1β is a proinflammatory cytokine known to activate HSCs,44 and it was significantly upregulated in the settings of hepatic steatosis. Indeed, exogenous administration of IL-1β induced Tnc production by HSCs in a dose-dependent fashion. Taken together, these findings support the concept that lipid accumulation in hepatocytes, likely through upregulation of proinflammatory cytokines, leads to an inflated production of Tnc in liver.
To assess the significance of Tnc expression in steatotic liver IRI, we fed Tnc-/- deficient mice and Tnc+/+ wild-type littermates with a HFD diet prior to 60 min of hepatic ischemia followed by reperfusion. Mice genetically lacking Tnc have no grossly phenotypic abnormalities.45 Tnc deficiency did not interfere with the ability of mice to develop steatosis; both Tnc-/- and Tnc+/+ HFD-fed mice developed extensive hepatic steatosis, with macrovesicular fat inclusions, after the feeding period. Lack of Tnc expression significantly protected against steatotic hepatic IRI; Tnc-/- steatotic livers were characterized by decreased sinusoidal congestion, reduced necrosis, and improved function after hepatic IRI. We have previously shown that Tnc deficiency is effective in lowering transaminase levels and liver damage at 24 hours after hepatic IRI in normal mice, but it doesn’t significantly affect liver function or damage in the early hours (6 hours) of reperfusion in these mice.19 Rather remarkably, Tnc deficiency significantly depressed the transaminase levels and enhanced liver histological preservation at both 6 and 24 hours post-reperfusion in steatotic mice. Tenascin-C activity is regulated by its production and availability in the extracellular microenvironments.26 These observations suggest that the increased production of Tnc observed in steatotic livers contributes towards a more prominent role for Tnc in these livers and, therefore, to a more noticeable impact for Tnc deficiency in steatotic hepatic IRI.
Leukocyte transmigration across endothelial and ECM barriers is dependent on adhesive events and matrix degradation mechanisms.46 Tnc is a ligand for several integrin receptors present on leukocytes.47 In steatotic livers, leukocyte recruitment and ensuing release of pro-inflammatory cytokines were significantly depressed post-hepatic IRI when Tnc was deleted. These results are in line with growing evidence that local expression of Tnc acts as an important trigger for leukocyte recruitment.25 It is well accepted that neutrophils are major effectors in innate immune responses and are among the first leukocytes to accumulate in tissues during acute inflammation.48 Neutrophils release several enzymes upon activation, being myeloperoxidase the most cytotoxic and abundant enzyme secreted by these cells.37 In addition to triggering neutrophil recruitment, Tnc regulated neutrophil-derived MPO activity. Besides, the upregulation of MPO activity was particularly effective when neutrophils were simultaneously exposed to Tnc and conditioned medium from steatotic hepatocytes. MPO activity is an important factor in the neutrophil-induced oxidative tissue injuries,38 and steatotic livers are particularly susceptible to oxidative stress.49 MPO generates hypochlorous acid (HOCl) from H2O2 and chloride ions, which is a potent oxidant.50 Taken all together, these observations support the view that the increased amounts of Tnc in steatotic livers favor a prominent inflammatory milieu in these livers during hepatic IRI. Hence, our results suggest that inhibition of Tnc is a promising therapeutic strategy to curb excessive inflammation in suboptimal steatotic livers and to maximize their successful use in organ transplantation. In addition to hepatic IRI, Tnc has been considered a viable therapeutic candidate for targeting in several other inflammatory pathologies.22 However, this field is still in its infancy, and more studies aimed at better understanding the biological complexity of Tnc, combined with technological advancements, are needed to generate improved therapeutic modalities to inhibit Tnc in both experimental and clinical settings.22
Acknowledgments
Financial statement: Supported in part by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) R01AI057832 grant.
List of abbreviations:
- ALT
Alanine transaminase
- AST
aspartate transaminase
- ECM
extracellular matrix
- HSC
hepatic stellate cell
- HCM
hepatocyte conditioned medium
- HFD
high fat diet
- IRI
ischemia and reperfusion injury
- KO
knockout
- MPO
myeloperoxidase
- S-HCM
steatotic hepatocyte conditioned medium
- Tnc
tenascin‐C
- WT
wild-type
Footnotes
Conflicts of interest: Nothing to disclose
REFERENCES:
- 1.Oliveira THC, Marques PE, Proost P, Teixeira MMM. Neutrophils: a cornerstone of liver ischemia and reperfusion injury. Lab Invest 2018;98:51–62. [DOI] [PubMed] [Google Scholar]
- 2.de Rougemont O, Dutkowski P, Clavien PA. Biological modulation of liver ischemia-reperfusion injury. Curr Opin Organ Transplant 2010;15:183–9. [DOI] [PubMed] [Google Scholar]
- 3.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl 2003;9:651–63. [DOI] [PubMed] [Google Scholar]
- 4.Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology 2000;32:1280–8. [DOI] [PubMed] [Google Scholar]
- 5.Anderson CD, Upadhya G, Conzen KD, Jia J, Brunt EM, Tiriveedhi V, Xie Y, Ramachandran S, Mohanakumar T, Davidson NO, Chapman WC. Endoplasmic reticulum stress is a mediator of posttransplant injury in severely steatotic liver allografts. Liver Transpl 2011;17:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selzner N, Selzner M, Jochum W, Amann-Vesti B, Graf R, Clavien PA. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J Hepatol 2006;44:694–701. [DOI] [PubMed] [Google Scholar]
- 7.Kopelman PG. Obesity as a medical problem. Nature 2000;404:635–43. [DOI] [PubMed] [Google Scholar]
- 8.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis 2001;21:105–13. [DOI] [PubMed] [Google Scholar]
- 9.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263–73. [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Implications for liver transplantation. Liver Transpl 2018;24:166–170. [DOI] [PubMed] [Google Scholar]
- 11.Dutkowski P, Linecker M, DeOliveira ML, Mullhaupt B, Clavien PA. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology 2015;148:307–23. [DOI] [PubMed] [Google Scholar]
- 12.Verran D, Kusyk T, Painter D, Fisher J, Koorey D, Strasser S, Stewart G, McCaughan G. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl 2003;9:500–5. [DOI] [PubMed] [Google Scholar]
- 13.Marsman WA, Wiesner RH, Rodriguez L, Batts KP, Porayko MK, Hay JE, Gores GJ, Krom RA. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation 1996;62:1246–51. [DOI] [PubMed] [Google Scholar]
- 14.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant 2017;17 Suppl 1:174–251. [DOI] [PubMed] [Google Scholar]
- 15.Tucker RP, Chiquet-Ehrismann R. Tenascin-C: Its functions as an integrin ligand. Int J Biochem Cell Biol 2015;65:165–8. [DOI] [PubMed] [Google Scholar]
- 16.Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol 2003;200:488–99. [DOI] [PubMed] [Google Scholar]
- 17.Giblin SP, Midwood KS. Tenascin-C: Form versus function. Cell Adh Migr 2015;9:48–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowy CM, Oskarsson T. Tenascin C in metastasis: A view from the invasive front. Cell Adh Migr 2015;9:112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuriyama N, Duarte S, Hamada T, Busuttil RW, Coito AJ. Tenascin-C: a novel mediator of hepatic ischemia and reperfusion injury. Hepatology 2011;54:2125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hesse J, Leberling S, Boden E, Friebe D, Schmidt T, Ding Z, Dieterich P, Deussen A, Roderigo C, Rose CR, Floss DM, Scheller J, Schrader J. CD73-derived adenosine and tenascin-C control cytokine production by epicardium-derived cells formed after myocardial infarction. FASEB J 2017;31:3040–3053. [DOI] [PubMed] [Google Scholar]
- 21.Gocheva V, Naba A, Bhutkar A, Guardia T, Miller KM, Li CM, Dayton TL, Sanchez-Rivera FJ, Kim-Kiselak C, Jailkhani N, Winslow MM, Del Rosario A, Hynes RO, Jacks T. Quantitative proteomics identify Tenascin-C as a promoter of lung cancer progression and contributor to a signature prognostic of patient survival. Proc Natl Acad Sci U S A 2017;114:E5625–E5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzeda AM, Midwood KS. Internal Affairs: Tenascin-C as a Clinically Relevant, Endogenous Driver of Innate Immunity. J Histochem Cytochem 2018;66:289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puente Navazo MD, Valmori D, Ruegg C. The alternatively spliced domain TnFnIII A1A2 of the extracellular matrix protein tenascin-C suppresses activation-induced T lymphocyte proliferation and cytokine production. J Immunol 2001;167:6431–40. [DOI] [PubMed] [Google Scholar]
- 24.Midwood KS, Orend G. The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal 2009;3:287–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbadi D, Laroumanie F, Bizou M, Pozzo J, Daviaud D, Delage C, Calise D, Gaits-Iacovoni F, Dutaur M, Tortosa F, Renaud-Gabardos E, Douin-Echinard V, Prats AC, Roncalli J, Parini A, Pizzinat N. Local production of tenascin-C acts as a trigger for monocyte/macrophage recruitment that provokes cardiac dysfunction. Cardiovasc Res 2018;114:123–137. [DOI] [PubMed] [Google Scholar]
- 26.Momcilovic M, Stamenkovic V, Jovanovic M, Andjus PR, Jakovcevski I, Schachner M, Miljkovic D. Tenascin-C deficiency protects mice from experimental autoimmune encephalomyelitis. J Neuroimmunol 2017;302:1–6. [DOI] [PubMed] [Google Scholar]
- 27.Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Mice develop normally without tenascin. Genes Dev 1992;6:1821–31. [DOI] [PubMed] [Google Scholar]
- 28.Kato H, Kuriyama N, Duarte S, Clavien PA, Busuttil RW, Coito AJ. MMP-9 deficiency shelters endothelial PECAM-1 expression and enhances regeneration of steatotic livers after ischemia and reperfusion injury. J Hepatol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology 2008;47:186–98. [DOI] [PubMed] [Google Scholar]
- 30.Kato H, Duarte S, Liu D, Busuttil RW, Coito AJ. Matrix Metalloproteinase-2 (MMP-2) Gene Deletion Enhances MMP-9 Activity, Impairs PARP-1 Degradation, and Exacerbates Hepatic Ischemia and Reperfusion Injury in Mice. PLoS One 2015;10:e0137642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duarte S, Kato H, Kuriyama N, Suko K, Ishikawa TO, Busuttil RW, Herschman HR, Coito AJ. Hepatic Ischemia and Reperfusion Injury in the Absence of Myeloid Cell-Derived COX-2 in Mice. PLoS One 2014;9:e96913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maschmeyer P, Flach M, Winau F. Seven steps to stellate cells. J Vis Exp 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wobser H, Dorn C, Weiss TS, Amann T, Bollheimer C, Buttner R, Scholmerich J, Hellerbrand C. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res 2009;19:996–1005. [DOI] [PubMed] [Google Scholar]
- 34.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai LJ, Li HY, Guan LX, Ritchie G, Zhou JX. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res 2009;2:16–25. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology 2005;42:880–5. [DOI] [PubMed] [Google Scholar]
- 37.Strzepa A, Pritchard KA, Dittel BN. Myeloperoxidase: A new player in autoimmunity. Cell Immunol 2017;317:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dallegri F, Ottonello L. Tissue injury in neutrophilic inflammation. Inflamm Res 1997;46:382–91. [DOI] [PubMed] [Google Scholar]
- 39.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cameron AM, Ghobrial RM, Yersiz H, Farmer DG, Lipshutz GS, Gordon SA, Zimmerman M, Hong J, Collins TE, Gornbein J, Amersi F, Weaver M, Cao C, Chen T, Hiatt JR, Busuttil RW. Optimal utilization of donor grafts with extended criteria: a single-center experience in over 1000 liver transplants. Ann Surg 2006;243:748–53; discussion 753–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology 2003;125:917–36. [DOI] [PubMed] [Google Scholar]
- 42.Lee MJ, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care 2010;13:371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE Jr., Peterson CA, Kern PA. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab 2011;96:E1990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hellerbrand C, Jobin C, Iimuro Y, Licato L, Sartor RB, Brenner DA. Inhibition of NFkappaB in activated rat hepatic stellate cells by proteasome inhibitors and an IkappaB super-repressor. Hepatology 1998;27:1285–95. [DOI] [PubMed] [Google Scholar]
- 45.Settles DL, Kusakabe M, Steindler DA, Fillmore H, Erickson HP. Tenascin-C knockout mouse has no detectable tenascin-C protein. J Neurosci Res 1997;47:109–17. [PubMed] [Google Scholar]
- 46.Coito AJ. Leukocyte transmigration across endothelial and extracellular matrix protein barriers in liver ischemia/reperfusion injury. Curr Opin Organ Transplant 2011;16:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida T, Akatsuka T, Imanaka-Yoshida K. Tenascin-C and integrins in cancer. Cell Adh Migr 2015;9:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nat Immunol 2018;19:327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soltys K, Dikdan G, Koneru B. Oxidative stress in fatty livers of obese Zucker rats: rapid amelioration and improved tolerance to warm ischemia with tocopherol. Hepatology 2001;34:13–8. [DOI] [PubMed] [Google Scholar]
- 50.Alfakry H, Malle E, Koyani CN, Pussinen PJ, Sorsa T. Neutrophil proteolytic activation cascades: a possible mechanistic link between chronic periodontitis and coronary heart disease. Innate Immun 2016;22:85–99. [DOI] [PubMed] [Google Scholar]