Abstract
Persistence of IDH1 or IDH2 mutations in remission bone marrow specimens of patients with acute myeloid leukemia has been observed, but the clinical impact of these mutations is not well known. In this study, we evaluated 80 acute myeloid leukemia patients with known IDH1 R132 or IDH2 R140/R172 mutations and assessed their bone marrow at the time of remission to determine the potential impact of persistent IDH1/2 mutations. Approximately 40% of acute myeloid leukemia patients given standard treatment in this cohort had persistent mutations in IDH1/2. Patients with an IDH1/2 mutation had an increased risk of relapse after 1 year of follow-up compared to patients without a detectable IDH1/2 mutation (59% versus 24%; P<0.01). However, a persistent mutation was not associated with a shorter time to relapse. High IDH1/2 mutation burden (mutant allelic frequency ≥10%) did not correlate with relapse rate (77% versus 86% for patients with a low burden, i.e., mutant allelic frequency <10%; P=0.66). Persistent mutations were also observed in NPM1, DNMT3A and FLT3 during remission, but IDH1/2 mutations remained significant in predicting relapse by multivariate analysis. Flow cytometry was comparable and complementary to next-generation sequencing-based assay for predicting relapse. Monitoring for persistent IDH1/2 mutations in patients with acute myeloid leukemia in remission can provide information that could be used to justify early interventions, with the hope of facilitating longer remissions and better outcomes in these patients.
Introduction
Acute myeloid leukemia (AML), defined as more than 20% of myeloblasts in blood and/or bone marrow, is heterogeneous and complex at the genomic level. Data from The Cancer Genome Atlas show that many genes are recurrently mutated in patients with AML, including NPM1, FLT3, DNMT3A, IDH1/2, and KRAS/NRAS.1 IDH1 and IDH2 mutations are found in 6-16% and 8-19% of AML patients, respectively.2–7 Collectively, IDH1/2 mutations are observed in 16-20% of AML patients and are enriched (25-30%) in cases of AML with a normal karyotype.6,8,9 IDH1/2 mutations are acquired early in the natural history of AML and can be present in the founding clone.10 There are known mutational hot spots in these genes: codon 132 (Arg) in IDH1 and codons 140 (Arg) and 172 (Arg) in IDH2. IDH2 R140 mutations occur more commonly than R172 mutations in AML.5 IDH1 and IDH2 mutations can also infrequently occur together at presentation.11 The presence of an IDH1/2 mutation alone is not sufficient for the development of AML.12 IDH2 mutations can be associated with clonal hematopoiesis of indeterminate potential (CHIP) in the older population.13 Moreover, IDH1/2 mutations occur together with mutations of other genes, at frequencies that depend on the IDH allele, suggesting that additional genomic insults are needed for AML to develop fully. For example, IDH2 R140 mutations are strongly associated with NPM1 mutations.10
IDH1 and IDH2 encode NADP+-dependent isocitrate dehydrogenases, converting isocitrate to α-ketoglutarate, while reducing NADP+ to NADPH with the production of CO2. IDH1 is present in the cytoplasm and peroxisome, whereas IDH2 resides in mitochondria and is a component of the Krebs’ cycle.14 IDH1 R132 mutation and IDH2 R140/R172 mutations reduce α-ketoglutarate to the oncometabolite D-2-hydroxyglutarate (also known as R-2-hydroxyglutarate).14,15 D-2-hydroxyglutarate has structural similarities to α-ketoglutarate and can competitively inhibit enzymes dependent on α-ketoglutarate, such as the TET enzyme family and histone lysine demethylases, and indeed IDH1/2 mutations in AML are associated with global DNA hypermethylation and impaired hematopoietic differentiation.16,17
Persistent IDH1 or IDH2 mutations have been observed in AML patients at the time of clinical and morphological remission.18,19 Debarri et al. reported that persistent IDH1/2 mutations in AML at the time of remission could predict relapse.19 However, their study cohort was small with only eight patients in complete remission with persistent IDH1/2 mutations, precluding a definitive conclusion. In this study, we explored the utility of mutant IDH1 and IDH2 as minimal residual disease markers in predicting relapse in a large cohort of AML patients.
Methods
Patients
We searched the database of The University of Texas MD Anderson Cancer Center from November 1, 2012 to December 31, 2017 and identified 80 newly diagnosed AML patients with IDH1 R132 or IDH2 R140/R172 mutations who achieved complete remission (CR) or CR with incomplete hematologic recovery (CRi), according to the 2017 European LeukemiaNet (ELN) recommendations for the diagnosis and management of AML,20 in bone marrow at any time-point of their treatment. To investigate the effect of predominant and well-established mutant IDH1/2 clones in AML, only cases with a mutant allelic frequency (MAF) ≥10% in a pre-treatment sample were included. All cases were collected consecutively and classified according to the 2017 World Health Organization (WHO) classification system.21 Patients with therapy-related AML were excluded from this study. Clinical, laboratory and cytogenetic data were collected from the patients’ electronic medical records. This study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center (Houston, TX, USA) and was conducted in accordance with the Declaration of Helsinki.22
IDH1/2 sequencing
IDH1/2 sequencing was performed on all patients as a part of clinically validated next-generation sequencing-based (NGS) assay (a 53-gene panel, a 28-gene panel or an 81-gene panel) as described previously.23 The limit of detection was 1% for the NGS assay. A sequencing library was prepared using 250 ng of genomic DNA and respective sequencing libraries were subjected to a MiSeq sequencer (Illumina Inc.). NGS data were analyzed using MiSeq Reporter (TruSeq) or SureCall (Haloplex). The Integrative Genomics Viewer (IGV, Broad Institute) was used to visualize read alignment and confirm variant calls.24 A custom-developed, in-house software package (OncoSeek) was used to annotate sequence variants and to interface the data with the IGV. Nomenclature of genetic variants was designated following the Human Genome Variation Society recommendations.25
FLT3 analysis
The presence of internal tandem duplications or point mutations at codon 835 or 836 in FLT3 was determined as described previously.26
Cytogenetic analysis
Conventional chromosome analysis (karyotyping) was performed on G-banded metaphase cells prepared from unstimulated 24-hour and 48-hour bone marrow cultures as described previously.27 Twenty metaphases were analyzed in most cases, but fewer than 20 metaphases were analyzed in some cases when inadequate metaphases were available for complete analysis. The results were reported using the current International System for Human Cytogenetic Nomenclature.28 Cytogenetic risk stratification was assessed in each patient using the United Kingdom Medical Research Council (UKMRC) system.29
Statistical analysis
A Fisher exact test was used when comparing categorical variables. Mann-Whitney and Kruskal-Wallis tests were used when comparing numerical variables in two groups or three or more groups, respectively. The cumulative incidence rate of relapse was determined using the competing risk method. The association between an IDH1/2 mutation and the cumulative incidence outcome was determined using a proportional subdistribution hazards regression model (Fine and Gray regression model).30 Differences in the cumulative incidence among patients with different mutations were assessed using the Gray test.31 Time to relapse was calculated from the date of morphological remission to the date of relapse. All variables with a P value <0.05 (two-tailed) were considered to be statistically significant. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA) and SAS 9.4 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Patients
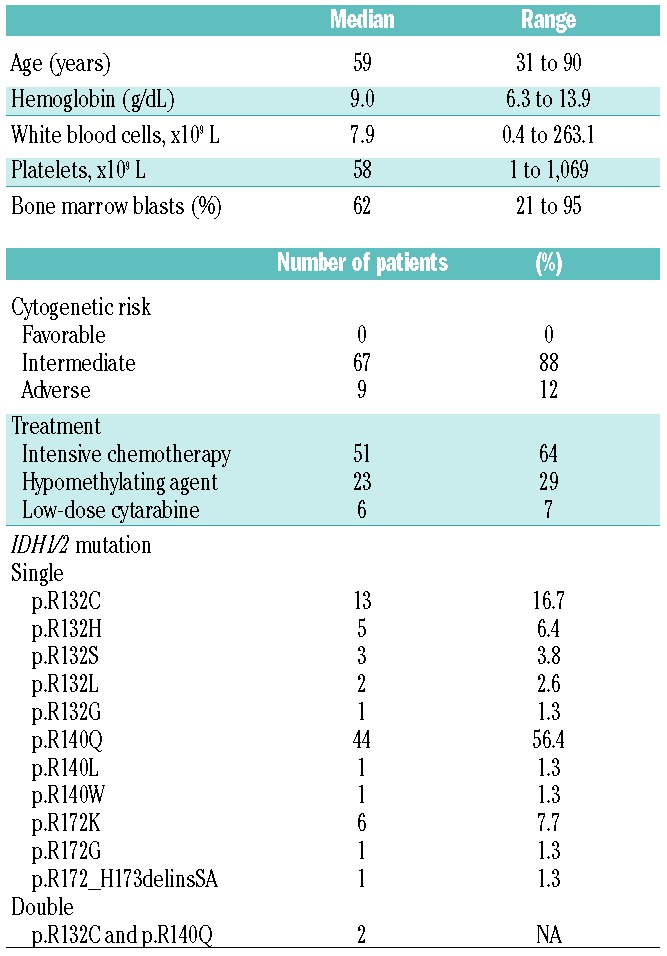
The study group included 80 patients (37 men and 43 women) with a median age of 59 years (range, 31 to 90) at diagnosis. The median hemoglobin concentration, white blood cell count and platelet count were 9.0 g/dL (range, 6.3 to 13.9), 7.9×109/L (range, 0.4 to 263.1×109/L) and 58×109/L (range, 1 to 1,069×109/L), respectively (Table 1). The median bone marrow blast count was 62% (range, 21 to 95%). Among 76 patients with cytogenetic information available, 88% (n=67) and 12% (n=9) had intermediate and adverse cytogenetic risk, respectively. There were no patients with favorable cytogenetic risk. A diploid karyotype was seen in 52 (68%) patients. Various frontline therapies were administered to this cohort of patients, but no patients received an IDH inhibitor as frontline therapy. All patients younger than 60 years of age (n=41) were treated with intensive chemotherapy including 7+3 (idarubicin and cytarabine), CIA (clofarabine, idarubicin and cytarabine), FIA (fludarabine, idarubicin and cytarabine), or CLIA (cladribine, idarubicin and cytarabine with or without sorafenib). The patients over 60 years old (n=39) were treated with intensive chemotherapy (n=10), hypomethylating agents (n=23) or low-dose cytarabine with or without nucleoside analogs (n=6). The median clinical follow-up was 17.5 months (range, 4.9 to 77.5 months).
Table 1.
Laboratory, cytogenetic and IDH1/2 mutation data of patients with acute myeloid leukemia (n=80).
IDH mutations in pretreatment samples
All 80 patients harbored IDH1 and/or IDH2 mutations: 78 patients had a single IDH1 or IDH2 mutation and two patients had both IDH1 and IDH2 mutations. The two patients who had two different IDH1/2 mutations had major (20~34%) and minor (1~14%) clones represented by MAF. As a single mutation, IDH2 R140 mutations were most common (n=46), followed by IDH1 R132 (n=24) and IDH2 R172 (n=7) mutations. IDH2 R172_H173delinsSA was found in one patient. Detailed information regarding the IDH1/2 mutations is presented in Table 1. The median MAF of IDH1/2 mutations in pretreatment samples was 43.8% (range, 12.3% to 62.7%). The median MAF of the IDH1 R132 mutation (39.2%) was similar to that of IDH2 R140 (44.1%) and IDH2 R172 (42.5%) mutation (P=0.31). There were no significant differences in median bone marrow blast count among the three groups (P=0.54).
Persistent IDH mutations in complete remission or complete remission with incomplete hematologic remission
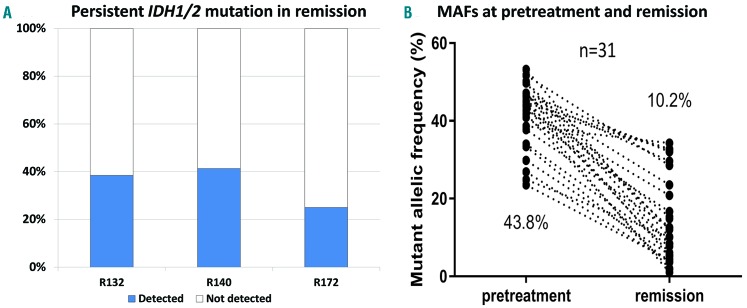
The mutational status of IDH1/2 was available in first CR (n=36) or CRi (n=44) for all patients. In 51 patients treated with intensive chemotherapy, analysis of IDH1/2 was performed after the first, second and third or beyond cycles of therapy in 21, 16 and 14 patients, respectively. In 23 patients treated with hypomethylating agents, analysis of IDH1/2 was performed before and after the fourth cycle of therapy in nine and 15 patients, respectively. The latter also included six patients who had received six or more cycles of therapy. In patients treated with low-dose cytarabine with or without additional drugs (n=6), one, one and four patients were tested for IDH1/2 after, respectively, their first, second and fourth or beyond cycles of therapy. A total of 31 (39%) patients had persistent IDH1/2 mutations in CR or CRi (CRIDH+). Among the patients in CR (n=36), 12 (33%) had persistent IDH1/2 mutations. Similarly, among patients in CRi (n=44), 19 (43%) had persistent IDH1/2 mutations (P=0.49). IDH1 R132, IDH2 R140 and IDH2 R172 mutations were observed in, respectively, ten (38.5%), 19 (41.3%), and two (25%) patients with mutations in a pretreatment bone marrow specimen (P=0.68) (Figure 1A). Compared to the MAF values in pretreatment samples, the MAF of IDH1/2 mutations in CR or CRi were reduced in all patients (median MAF: 10.2%, range, 1% to 34.3%) (Figure 1B). CRIDH+ was not correlated with cytogenetic abnormalities. CRIDH+ was observed in patients with diploid karyotype and those with any cytogenetic abnormalities with similar frequency (40.4% and 37.5%, respectively; P=0.99). Of 24 patients with cytogenetic abnormalities in pretreatment samples, only two had persistent cytogenetic abnormalities.
Figure 1.
Persistent IDH1/2 mutations in remission and changes in mutant allelic frequencies in pretreatment and remission samples. (A) Percentages of persistent IDH1/2 mutations in patients with acute myeloid leukemia in remission. Persistent mutations occur at similar frequencies for the different mutations. (B) Mutant allelic frequency (MAF) of IDH mutations present in pretreatment samples and remission samples. The median mutant allelic frequency is shown for each occasion.
IDH mutations in remission are associated with an increased risk of relapse
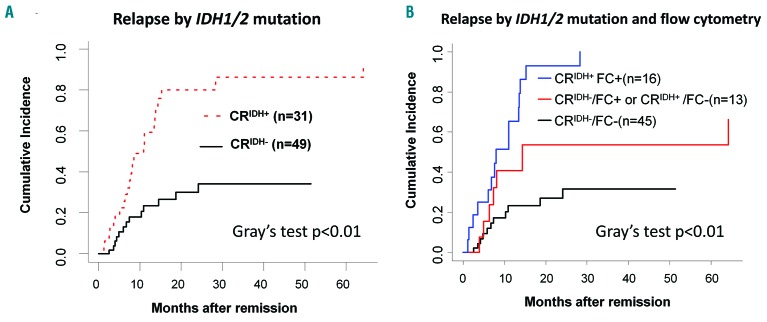
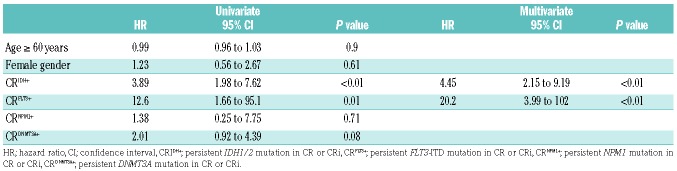
The cumulative incidence rate of relapse in patients with CRIDH+ was 59% at 12 months and 80% at 24 months. The cumulative incidence rate was significantly higher in patients with CRIDH+ than in patients without a detectable IDH1/2 mutation in remission (CRIDH−) (Figure 2A). Using the Fine and Gray regression model, the risk of relapse at 1 year of follow-up was higher for patients with CRIDH+ than patients in the CRIDH− group (59% versus 24%; hazard ratio, 3.89; 95% confidence interval: 1.98 to 7.62; P<0.01) (Table 2). Regarding mutation type, 90%, 74% and 100% of patients with persistent IDH1 R132, IDH2 R140 and IDH2 R172 clones relapsed, respectively (P=0.44). There were no differences regarding relapse between patients treated with intensive chemotherapy and hypomethylating agents (47.1% and 43.5%, respectively) (P=0.77). The median time to relapse in patients with CRIDH+ was not significantly different from that of CRIDH− patients (median: 8.1 and 6.9 months, respectively) (P=0.71).
Figure 2.
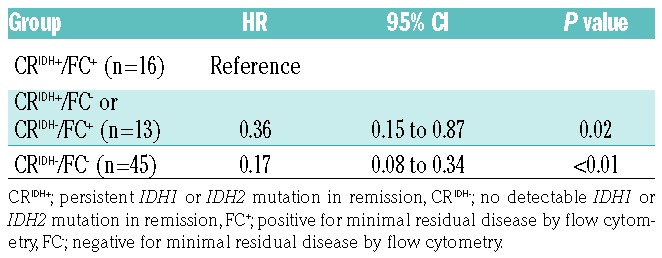
Cumulative incidence rates of relapse in patients with persistent IDH1/2 mutations in remission. (A) Cumulative incidences of relapse in patients with persistent IDH1/2 mutation and patients without detectable IDH1/2 mutation. The cumulative incidence rate is significantly higher in patients with persistent IDH1/2 mutation in remission (CRIDH+) than in patients without detectable IDH1/2 mutation in remission (CRIDH−). (B) Cumulative incidence rate of relapse in patients with respect to mutational status in IDH1/2 and flow cytometrically determined presence of minimal residual disease in remission. The cumulative incidence of relapse was significantly higher in patients who were positive in both molecular and flow cytometry tests compared to patients with a positive result in either of the tests or negative in both. CRIDH+; persistent IDH1/2 mutation in remission, CRIDH−; non-detectable IDH1/2 mutation in remission, FC+; positive for minimal residual disease by flow cytometry, FC−; negative for minimal residual disease by flow cytometry.
Table 2.
Risk of relapse according to the presence of persistent mutations in different genes (Fine and Gray regression model).
IDH1/2 mutation burden does not correlate with relapse
Among patients with CRIDH+, the MAF values for CRIDH+ were not significantly different between relapsed (median MAF: 10.0%) and non-relapsed patients (median MAF: 20.5%) (P=0.19). To further evaluate the correlation between IDH1/2 mutation burden and relapse, we arbitrarily divided patients according to whether they had a high MAF (≥10%) or low MAF (<10%). Among 17 patients with a high MAF, 13 (77%) patients relapsed, whereas 12 of 14 (86%) patients with a low MAF relapsed (P=0.66). The difference in the median time to relapse between patients in the high and low MAF groups (6.2 and 9.9 months, respectively) was not statistically significant (P=0.18). We also assessed different MAF as cutoffs for high versus low mutation burden (5%, 20% and 30%), but correlation with relapse was not observed using any of these cutoffs (data not shown).
IDH mutations in remission predict relapse in the context of co-mutations
Other gene mutations were detected in 89% of patients who had IDH1/2 mutations in a pre-treatment sample. NPM1 (n=38) was the most frequently co-mutated gene followed by DNMT3A R882 (n=25), FLT3-ITD (internal tandem duplication) (n=22), and KRAS/NRAS (n=12). Few (<5) patients had mutations in ASXL1, BRAF, CEBPA, JAK2, RUNX1, TET2 or TP53. CRIDH+ was significantly more common (39%) than CRFLT3+ (n=3, 14%), CRNPM1+ (n=4, 11%), and CRKRAS/NRAS+ (n=0, 0%) in CR or CRi (P>0.05). CRDNMT3A+ was present with a similar frequency, being seen in 36% of patients (n=9). We assessed the effect of CRIDH+ in the context of co-mutations using the Fine and Gray regression model. By univariate analysis, CRFLT3+ also demonstrated an increased risk of relapse. By multivariate analysis, CRIDH+ and CRFLT3+ remained significant for an increased risk of relapse.
We also assessed the dynamic changes of clonal architecture in 25 CRIDH+ patients who relapsed. Comparing mutational profiles at CR/CRi and relapse, four patients acquired novel mutations at relapse. These mutations were ERBB2 p.R784C (MAF: 12.3%), FLT3 p.D835Y (1.5%), TP53 p.G245D (3.8%) and WT1 p.K467fs (12.2%). These mutations showed subclonal fraction patterns compared with the MAF of IDH1/2 mutation at relapse.
Flow cytometry is comparable to next-generation sequencing in predicting relapse
We compared molecular test results to those of multi-parametric flow cytometry (FC) immunophenotyping in CR/CRi bone marrow specimens. Flow cytometric results were available for a total of 79 patients. Minimal residual disease (MRD) determination by FC has been described previously.32,33 The sensitivity of the flow cytometry was validated to 0.1% − 0.01% depending on the leukemic cell phenotype. According to FC, 19 (26%) patients had MRD, 55 (76%) were MRD-negative and results were indeterminate in five patients. Among 74 patients in whom both FC and molecular MRD tests were performed, the results were concordant in 61 (82%) patients and discordant in 13 (18%) patients with a statistically significant association (P<0.01). Of the 13 patients with discordant FC and molecular testing MRD results, ten patients were positive by FC only and three patients were positive by molecular testing only. Four of the five patients for whom FC was indeterminate with regards to MRD had a persistent IDH2 p.R140Q mutation with various MAF values (median 12.5%; range, 1 to 28.5%) and all of them relapsed. Similarly to CRIDH+ patients, those who were positive for MRD assessed by flow cytometry (FC+) showed an increased risk of relapse compared to patients who were negative for MRD by flow cytometry (FC−) after 1 year of follow-up (63% versus 27%; hazard ratio, 4.24; 95% confidence interval: 2.22 to 8.13; P<0.01). Patients with positive results in both methods (CRIDH+/FC+) had a significantly higher risk of relapse compared to those with discordant results (CRIDH+/FC− or CRIDH−/FC+) or negative results in both methods (CRIDH−/FC−) (Figure 2 and Table 3).
Table 3.
Risk of relapse with respect to presence or absence of a persistent IDH1/2 mutation and flow cytometry determined minimal residual disease status (Fine and Gray regression model).
Discussion
IDH1 and IDH2 mutations are not uncommon in AML. In addition, IDH1/2 mutations can be found in the pre- leukemic clone in individuals without pathology-proven AML or even in healthy individuals as a sign of age-related clonal hematopoiesis.13,34–36 For these reasons, in this study, we only selected AML patients with an IDH1/2 mutation as a predominant clone (MAF >10%) in pretreatment samples to minimize the effect on our analysis of subclonal IDH1/2 mutations.
In patients with AML associated with IDH1/2 mutations, karyotyping is not a preferred method for monitoring persistent aberrancy during remission for at least two reasons: cytogenetic analysis is less sensitive and IDH1/2 mutations are enriched in AML with a normal karyotype.5,8,11,37 In support of this statement, 68% of patients had a diploid karyotype at diagnosis. Out of 24 patients who had cytogenetic abnormalities in pretreatment samples, only two had persistent cytogenetic abnormalities in the remission sample. Therefore, follow-up with more sensitive methods is necessary to monitor for MRD.
In this cohort of patients, IDH2 mutations (69%) were more common than IDH1 mutations (31%). In the IDH2 group, mutations at codon 140 were far more frequent than R172 mutations in an approximately 5.8 to 1 ratio. IDH1 mutations were present in almost one-third of patients. These frequency data are consistent with those from other studies of AML in the literature.5,11,37 The median MAF of an IDH1/2 mutation was 43.8%, indicating that the IDH1/2 mutation was the predominant clone in most patients. The median MAF of the IDH1 R132 mutation (39.2%) was slightly lower than that of IDH2 mutations (44.1% and 42.5% for IDH2 R140 and R172 mutations, respectively).
Persistent IDH1/2 mutations in patients with AML who are in complete remission (CRIDH+) have been reported by others.18,19 We observed that approximately 40% of AML patients in remission had persistent IDH1/2 mutations with decreased MAF regardless of IDH mutation subtype (IDH1 R132, IDH2 R140 and IDH2 R172) or treatment type (intensive chemotherapy versus hypomethylating agents). Approximately 50% of patients with CRIDH+ had MAF below the assay sensitivity of the Sanger sequencing (<10%). This indicates that a NGS-based approach is necessary to monitor persistent IDH1/2 mutations. CRIDH+ was associated with an increased risk of relapse (hazard ratio, 3.89; 95% confidence interval: 1.98-7.62; P<0.01) compared to patients with CRIDH−. However, CRIDH+ was not associated with a shorter time to relapse (median 8.1 months versus 6.9 months in patients with CRIDH−; P=0.71). Interestingly, high mutation burden did not correlate with relapse in this study because patients with lower IDH1/2 mutation burden (MAF <10%) relapsed with a similar frequency as patients with a higher mutation burden (MAF ≥10%) (77% and 86%, respectively, P=0.66). Accordingly, these data suggest that presence of persistent IDH1/2 mutation in remission is per se associated with relapse in AML patients and that mutation burden does not have an additive predictive effect.
Focusing on a single event (IDH1/2 mutation) as a predictive marker of relapse in AML is potentially problematic because of frequent co-mutations in other genes including FLT3, NPM1 and DNMT3A. Indeed, co-mutations in other genes were detected in the majority of patients (89%) in this study cohort. However, persistent mutation in other genes in remission was rare, except for DNMT3A. By univariate analysis, CRFLT3+ also showed an increased risk of relapse. This result might not be reliable because only a few patients (n=3) had persistent FLT3 mutation in remission.
We noticed that a few AML patients acquired novel mutations at relapse, but at a relatively low burden (MAF range: 1.5% – 12.2%). These mutations occurred in genes in the activated signaling (FLT3 or KRAS) and tumor suppressor (TP53 and WT1) classes, apparently providing either proliferative or survival signals to the IDH-mutated clone.
FC is a powerful tool for detecting residual leukemic cells and can be used to predict relapse in patients with AML. The concordance rate for detecting MRD between FC and molecular methods was 82%, similar to earlier studies.38,39 Positivity for MRD determined by FC was also associated with an increased risk of relapse in this cohort. Interestingly, patients with positive results according to both methods (CRIDH+/FC+) had a significantly higher risk of relapse compared to those with discordant results (CRIDH+/FC− or CRIDH−/FC+) or negative results by both methods (CRIDH−/FC−). These findings suggest that mutational analysis and FC are complementary methods useful for predicting relapse in AML patients in CR or CRi.
The data we present are in accordance with those of a recent study by Jongen-Lavrencic et al., who investigated 430 patients with AML or refractory anemia with excess blasts treated according to the clinical protocol of either the HOVON or SAKK with achievement of either CR or CRi after two cycles of induction chemotherapy.39 Mutational screening was performed at the time of diagnosis and at CR/CRi using a targeted, 54-gene NGS panel (limit of detection: ≤1% of mutant allele). Their study showed that persistent mutation in genes other than DNMT3A, TET2 and ASXL1 in CR/CRi was an independent risk factor for relapse. IDH1 and IDH2 mutations were included in their study and showed a similar frequency of persistent mutation in CR/CRi (28%). However, the authors did not focus on particular genes with respect to the increased risk of relapse.
To the best of our knowledge, our study is the largest cohort (n=80) investigating the impact of persistent IDH1/2 mutations in CR/CRi in AML patients. However, our study does have some limitations: (i) the time point of IDH1/2 analysis in remission was not uniform, and (ii) our cohort was not sufficiently large to reliably investigate the effect of co-mutations in remission. Larger-scale studies are necessary to reproduce the results of our study.
In summary, approximately 40% of AML patients with an IDH1/2 mutation at initial diagnosis will have persistent mutations after therapy in remission bone marrow samples. A persistent IDH1/2 mutation is associated with an increased risk of relapse. Monitoring IDH1/2 mutations in AML patients during remission using a highly sensitive NGS-based assay may provide useful information to guide early interventions with the aim of achieving longer remissions and better outcomes.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/2/305
References
- 1.Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou WC, Hou HA, Chen CY, et al. Distinct clinical and biologic characteristics in adult acute myeloid leukemia bearing the isocitrate dehydrogenase 1 mutation. Blood. 2010;115(14):2749–2754. [DOI] [PubMed] [Google Scholar]
- 3.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361(11):1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnittger S, Haferlach C, Ulke M, Alpermann T, Kern W, Haferlach T. IDH1 mutations are detected in 6.6% of 1414 AML patients and are associated with intermediate risk karyotype and unfavorable prognosis in adults younger than 60 years and unmutated NPM1 status. Blood. 2010;116(25):5486–5496. [DOI] [PubMed] [Google Scholar]
- 5.Im AP, Sehgal AR, Carroll MP, et al. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid malignancies: associations with prognosis and potential treatment strategies. Leukemia. 2014;28(9):1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28(22):3636–3643. [DOI] [PubMed] [Google Scholar]
- 7.Patel KP, Ravandi F, Ma D, et al. Acute myeloid leukemia with IDH1 or IDH2 mutation: frequency and clinicopathologic features. Am J Clin Pathol. 2011;135(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medeiros BC, Fathi AT, DiNardo CD, Pollyea DA, Chan SM, Swords R. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia. 2017;31(2):272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43(10):1541–1551. [DOI] [PubMed] [Google Scholar]
- 10.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki M, Knobbe CB, Munger JC, et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488(7413):656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reitman ZJ, Parsons DW, Yan H. IDH1 and IDH2: not your typical oncogenes. Cancer Cell. 2010;17(3):215–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov. 2013;3(7):730–741. [DOI] [PubMed] [Google Scholar]
- 16.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou WC, Peng KY, Lei WC, et al. Persistence of mutant isocitrate dehydrogenase in patients with acute myeloid leukemia in remission. Leukemia. 2012;26(3):527–529. [DOI] [PubMed] [Google Scholar]
- 19.Debarri H, Lebon D, Roumier C, et al. IDH1/2 but not DNMT3A mutations are sui targets for minimal residual disease monitoring in acute myeloid leukemia patients: a study by the Acute Leukemia French Association. Oncotarget. 2015;6(39):42345–42353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 22.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- 23.Ok CY, Patel KP, Garcia-Manero G, et al. TP53 mutation characteristics in therapy-related myelodysplastic syndromes and acute myeloid leukemia is similar to de novo diseases. J Hematol Oncol. 2015;8:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson JT, Thorvaldsdottir H, Winckler W, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.den Dunnen JT, Dalgleish R, Maglott DR, et al. HGVS Recommendations for the description of sequence variants: 2016 update. Hum Mutat. 2016;37(6):564–569. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Jones D, Medeiros LJ, Luthra R, Lin P. Acute myeloid leukaemia with FLT3 gene mutations of both internal tandem duplication and point mutation type. Br J Haematol. 2005;130(5):726–728. [DOI] [PubMed] [Google Scholar]
- 27.Tang Z, Medeiros LJ, Yin CC, et al. Sex chromosome loss after allogeneic hematopoietic stem cell transplant in patients with hematologic neoplasms: a diagnostic dilemma for clinical cytogeneticists. Mol Cytogenet. 2016;9:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGowan-Jordan J, Simons A, Schmid M: ISCN: An International System for Human Cytogenomic Nomenclature (2016). Basel, Switzerland: Karger, 2016. [Google Scholar]
- 29.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. [DOI] [PubMed] [Google Scholar]
- 30.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Am Stat Assoc. 1999;94(446): 496–509. [Google Scholar]
- 31.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 32.Ouyang J, Goswami M, Peng J, et al. Comparison of multiparameter flow cytometry immunophenotypic analysis and quantitative RT-PCR for the detection of minimal residual disease of core binding factor acute myeloid leukemia. Am J Clin Pathol. 2016;145(6):769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Jorgensen JL, Wang SA. How do we use multicolor flow cytometry to detect minimal residual disease in acute myeloid leukemia¿ Clin Lab Med. 2017;37(4):787–802. [DOI] [PubMed] [Google Scholar]
- 34.Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corces-Zimmerman MR, Hong WJ, Weissman IL, Medeiros BC, Majeti R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci U S A. 2014;111(7):2548–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcucci G, Maharry K, Wu YZ, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28(14):2348–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Getta BM, Devlin SM, Levine RL, et al. Multicolor flow cytometry and multigene next-generation sequencing are complementary and highly predictive for relapse in acute myeloid leukemia after allogeneic transplantation. Biol Blood Marrow Transplant. 2017;23(7):1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jongen-Lavrencic M, Grob T, Hanekamp D, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. 2018;378(13):1189–1199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.