Mycobacterium tuberculosis, the causative agent of human tuberculosis (TB), has surpassed HIV/AIDS as the leading cause of death from a single infectious agent. The increasing occurrence of drug-resistant strains has become a major challenge for health care systems and, in some cases, has rendered TB untreatable.
KEYWORDS: HAMLET, α-lactalbumin, liprotides, macrophages, multidrug resistance, oleic acid, potentiation, sensitization
ABSTRACT
Mycobacterium tuberculosis, the causative agent of human tuberculosis (TB), has surpassed HIV/AIDS as the leading cause of death from a single infectious agent. The increasing occurrence of drug-resistant strains has become a major challenge for health care systems and, in some cases, has rendered TB untreatable. However, the development of new TB drugs has been plagued with high failure rates and costs. Alternative strategies to increase the efficacy of current TB treatment regimens include host-directed therapies or agents that make M. tuberculosis more susceptible to existing TB drugs. In this study, we show that HAMLET, an α-lactalbumin–oleic acid complex derived from human milk, has bactericidal activity against M. tuberculosis. HAMLET consists of a micellar oleic acid core surrounded by a shell of partially denatured α-lactalbumin molecules and unloads oleic acid into cells upon contact with lipid membranes. At sublethal concentrations, HAMLET potentiated a remarkably broad array of TB drugs and antibiotics against M. tuberculosis. For example, the minimal inhibitory concentrations of rifampin, bedaquiline, delamanid, and clarithromycin were decreased by 8- to 16-fold. HAMLET also killed M. tuberculosis and enhanced the efficacy of TB drugs inside macrophages, a natural habitat of M. tuberculosis. Previous studies showed that HAMLET is stable after oral delivery in mice and nontoxic in humans and that it is possible to package hydrophobic compounds in the oleic acid core of HAMLET to increase their solubility and metabolic stability. The potential of HAMLET and other liprotides as drug delivery and sensitization agents in TB chemotherapy is discussed here.
INTRODUCTION
Mycobacterium tuberculosis, the causative agent of human tuberculosis (TB), has surpassed HIV/AIDS as the leading cause of death worldwide from a single infectious agent. In 2016, 10.4 million people were infected with M. tuberculosis, resulting in 1.7 million deaths (1, 2). A concerning development is that multidrug-resistant TB (MDR-TB) cases tripled between 2009 and 2016 (3). Treatment of MDR-TB and extensively drug-resistant TB (XDR-TB) requires the use of less efficient and more expensive drugs, which often have side effects (4–6). This increases treatment times and the risk of developing new drug-resistant M. tuberculosis strains. A striking example is bedaquiline, which was the first new TB drug approved by the FDA since 1974. Bedaquiline resistance was reported only 3 years after its approval in 2012 (7). Furthermore, drug-resistant TB poses a large economic burden on health care systems. In the United States, the average patient treatment costs are approximately $80,000 and $250,000 for MDR- and XDR-TB, respectively (8). In developing countries, the treatment of MDR- and XDR-TB alone can consume up to one-third of the TB program resources (6). Thus, new TB treatment strategies including new drugs are urgently needed (9). However, TB is notoriously difficult to treat due to the slow growth of M. tuberculosis, a formidable permeation barrier established by its outer membrane in combination with other resistance mechanisms (10, 11), and its ability to survive in a persistent drug-resistant state after infection (12, 13). A consequence of the unusually high intrinsic resistance of M. tuberculosis to toxic compounds is that many current antibiotics are not efficient in treating TB (14, 15). Furthermore, high-affinity inhibitors of essential targets developed by in vitro drug screening approaches were not active against M. tuberculosis because of permeability problems (9). In contrast, compounds identified in phenotypic screens using whole bacterial cells are active against M. tuberculosis but may work via nonspecific mechanisms (9). These challenges increase the failure rate and costs of TB drug development.
A promising alternative strategy is to make M. tuberculosis more susceptible to existing TB drugs by increasing their efficacy. The most widely studied approach is the use of drug efflux pump inhibitors (16, 17). For example, the efflux pump inhibitor verapamil reduced the MIC of bedaquiline against M. tuberculosis in vitro (18, 19) and augmented its bactericidal activity in mice (20, 21). Thioridazine is an FDA-approved drug to treat psychotic disorders, enhances the activity of a standard regimen to treat susceptible M. tuberculosis in mice (22), and was used to cure 10 of 12 XDR-TB patients in Buenos Aires, Argentina (23). Thioridazine appears to have multiple beneficial effects in TB chemotherapy, as follows (24): it has moderate activity against M. tuberculosis in vitro (25, 26), enhances the cell permeability of M. tuberculosis (27), and inhibits Ca2+ channels in the phagosomal membrane, leading to an acidification of the phagosome and increased killing of M. tuberculosis (28).
In this study, we examined the activity of HAMLET (human α-lactalbumin made lethal against tumor cells) against M. tuberculosis. HAMLET is a protein-lipid complex with anticancer activity, derived from human milk, and composed of α-lactalbumin and milk fatty acids (29–31). Surprisingly, HAMLET is bactericidal against some bacteria (32) and has also been shown to reverse the antibiotic resistance of both HAMLET-sensitive and -resistant species (33, 34). Here, we show that HAMLET has bactericidal activity against M. tuberculosis in vitro and at sublethal concentrations potentiates the effect of several first- and second-line TB drugs both in in vitro and inside macrophages.
RESULTS
Activity of HAMLET against M. tuberculosis.
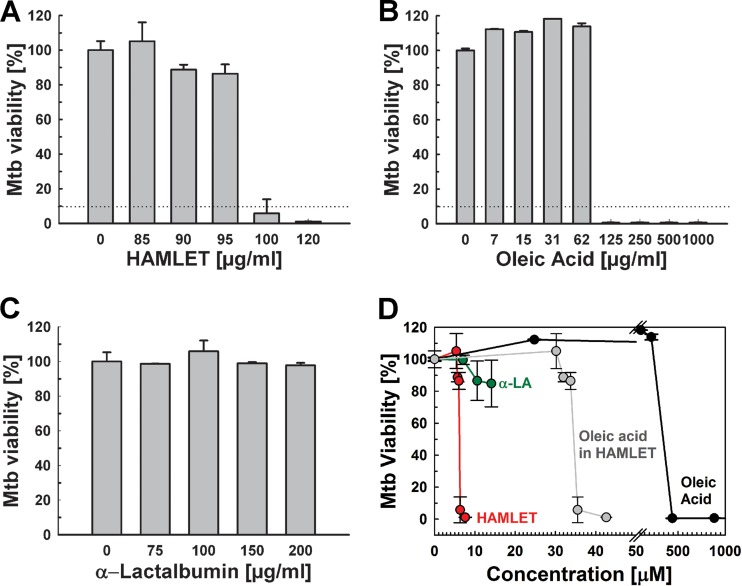
Previous studies showed that HAMLET has bactericidal activity against a select set of Gram-positive and Gram-negative bacteria, such as Streptococcus pneumoniae and Haemophilus influenzae, respectively (32, 35). However, HAMLET was not active against Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterobacter cloacae, or Enterococcus faecalis (32). The first aim of this study was to examine whether HAMLET exhibits direct antibacterial activity against Mycobacterium tuberculosis. To this end, HAMLET was prepared from α-lactalbumin obtained from human milk and complexed with oleic acid, as described previously (36). We used microplate alamarBlue assays, growth curve measurements, and agar plate-based drop assays to examine whether HAMLET has any activity against M. tuberculosis. The virulent strain M. tuberculosis H37Rv was first grown in Hartmans-de Bont (HdB) minimal medium containing increasing concentrations of HAMLET, and the bacterial viability was determined using the alamarBlue assay. Surprisingly, considering the extraordinary low permeability of the mycobacterial outer membrane, HAMLET inhibited the growth and/or viability of M. tuberculosis, with an MIC90 of 100 μg/ml (Fig. 1A). Since HAMLET is prepared through complexing of α-lactalbumin with oleic acid (36) and oleic acid is known to be toxic for M. tuberculosis (37–40), we determined the antibacterial activities of oleic acid and α-lactalbumin separately against M. tuberculosis. While the MIC90 of oleic acid was lower than 125 μg/ml for M. tuberculosis H37Rv (Fig. 1B), no significant activity of human α-lactalbumin was observed up to concentrations of 200 μg/ml (Fig. 1C). Fatty acid quantification revealed that, on average, a single α-lactalbumin protein bound 5.6 oleic acid molecules in the HAMLET preparation used in these experiments. The growth-inhibitory concentration of HAMLET was 6.3 μM, which corresponds to an average concentration of 35 μM oleic acid (Fig. 1D). This is ∼13-fold less than the MIC90 of 443 μM of oleic acid alone against M. tuberculosis (Fig. 1B). This result suggested that α-lactalbumin most likely acts by more efficiently delivering oleic acid to the bacteria and thereby increases its activity against M. tuberculosis. The increased activity of HAMLET against M. tuberculosis compared to that of oleic acid was described previously for other bacteria (32, 41).
FIG 1.
HAMLET is active against M. tuberculosis. The viability of M. tuberculosis H37Rv in Hartmans-de Bont (HdB) minimal medium in the presence of increasing concentrations of HAMLET (A), oleic acid (B), and human α-lactalbumin (C) was determined by the microplate alamarBlue assay. Error bars represent standard errors of the mean values of biological triplicates. The MIC90 is represented by dotted lines in panels A and B. (D) Analysis of the viability of M. tuberculosis H37Rv with increasing molar concentrations of HAMLET (A), oleic acid (B) and human α-lactalbumin (α-LA) (C), with data obtained from the experiments shown in panels A to C.
HAMLET kills M. tuberculosis in vitro.
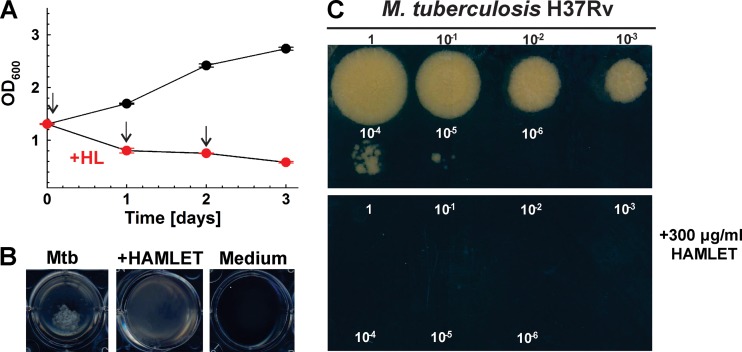
To examine whether HAMLET is bacteriostatic or has bactericidal activity, M. tuberculosis was first grown in HdB minimal medium to an optical density at 600 nm (OD600) of 1.3. This culture was then split into two separate cultures, one of which was treated three times with 300 μg/ml HAMLET at 24-h intervals. On day 3, the OD600 of the treated culture declined to 0.58, while the untreated culture continued to grow to an OD600 of 2.75 (Fig. 2A). A visual inspection of the wells showed the complete absence of the typical cell clumps in the cultures treated with HAMLET, indicating a drastic change or death of M. tuberculosis cells (Fig. 2B). Each day, aliquots were removed from the HAMLET-treated and untreated cultures, and the growth of M. tuberculosis was examined by adding drops of serially diluted samples on agar plates. While growth of untreated M. tuberculosis was detected using a 10,000-fold diluted sample (Fig. 2C), no growth was observed for any HAMLET-treated culture even when an undiluted sample was plated after only one treatment (day 1). These results indicated that a single treatment with 300 μg/ml HAMLET killed M. tuberculosis. Bactericidal activity of HAMLET was previously also observed for S. pneumoniae (32).
FIG 2.
Bactericidal activity of HAMLET (HL) against M. tuberculosis. (A) M. tuberculosis H37Rv was grown in HdB minimal medium to an OD600 of 1.3, and then 5 ml of culture was seeded into 12-well microplates. M. tuberculosis was treated with 300 μg/ml HAMLET on days 0, 1, and 2 as indicated by the arrows. The bactericidal activity of HAMLET was determined by measuring the optical density at 600 nm. Error bars represent standard errors of mean values of biological triplicates. (B) Images of the wells were taken on the second day of HAMLET treatment. (C) Tenfold serial dilutions of cultures of untreated and HAMLET-treated M. tuberculosis strains were plated on Middlebrook 7H10 agar after the first treatment with HAMLET (day 0). Agar plates were imaged after incubation at 37°C for 15 days.
HAMLET enhances the activity of antibiotics and TB drugs against M. tuberculosis.
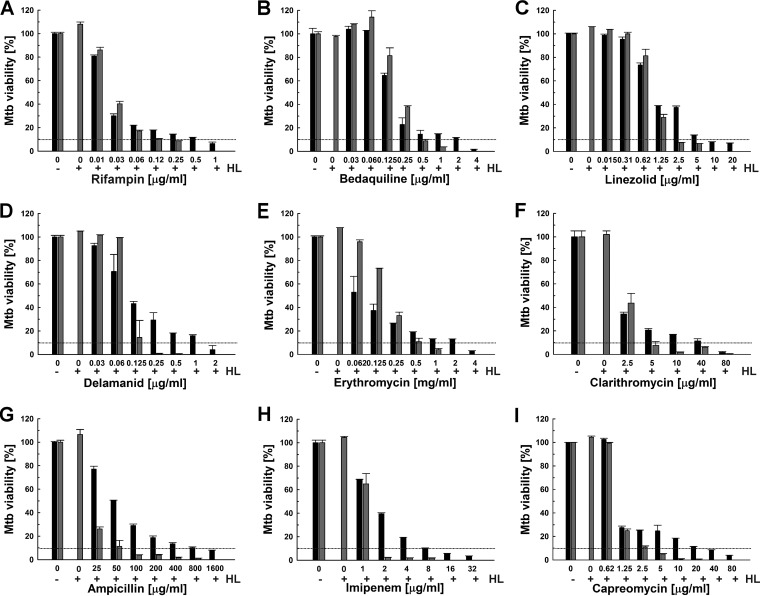
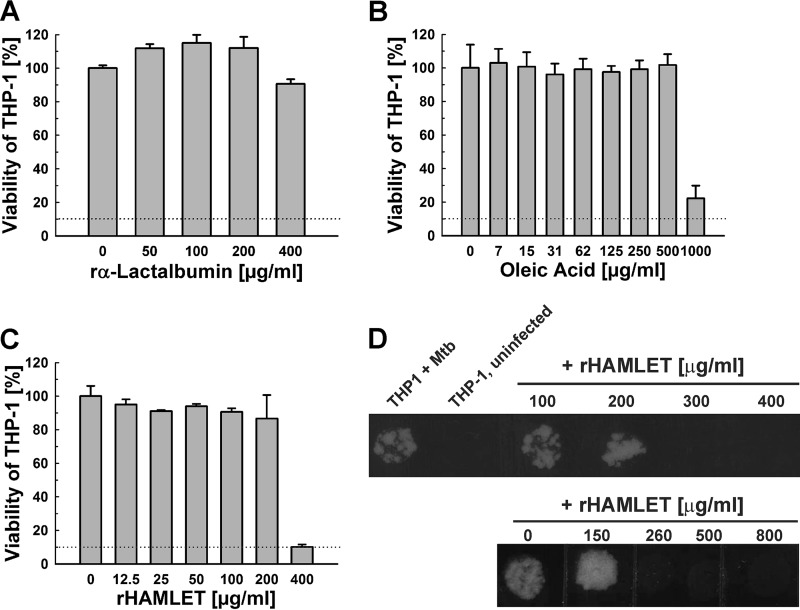
Previous studies showed that HAMLET enhances the activity of some antibiotics against S. pneumoniae and S. aureus (33, 34). After we established that HAMLET is active against M. tuberculosis, we examined whether HAMLET would also potentiate the efficacy of antibiotics and TB drugs against M. tuberculosis. First, we determined that the MIC90 of the new HAMLET preparation against the susceptible M. tuberculosis H37Rv strain was 125 μg/ml, using the microplate alamarBlue assay. Thus, this HAMLET preparation was slightly less active than that used in the previous experiments (Fig. 1). To examine whether HAMLET synergizes with antibiotics and TB drugs, we kept the concentration of HAMLET below its MIC to observe synergistic effects without the confounding factor of the direct bactericidal activity of HAMLET against M. tuberculosis. To this end, we tested several sublethal HAMLET concentrations for synergistic effects with rifampin and a few other TB drugs. The highest HAMLET concentration which did not affect the viability of M. tuberculosis was 90 μg/ml (∼75% of the MIC90), while no synergizing effect was observed for 60 μg/ml HAMLET (∼50% of the MIC90). Then, the viability of M. tuberculosis H37Rv treated with antibiotics and TB drugs alone or in combination with 90 μg/ml HAMLET was measured using the microplate alamarBlue assay. This sublethal concentration of HAMLET sensitized M. tuberculosis to a large number of structurally diverse antibiotics and TB drugs by lowering the MIC90 between 2- and 16-fold (Fig. 3 and Table 1). Importantly, the activity of rifampin, one of the first-line TB drugs, and of its analog rifabutin were enhanced 4- to 8-fold in the presence of HAMLET (Fig. 3A and Table 1; see also Fig. S2 in the supplemental material). Surprisingly, large sensitization effects between 8- and 16-fold-reduced MIC90 values were also observed for the new TB drugs bedaquiline and delamanid, the important second-line TB drug capreomycin (42), and ampicillin (Table 1 and Fig. 3B, D, G, and I). Bedaquiline, delamanid, and capreomycin are used in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis (43–45), while ampicillin has been proposed as an alternative treatment option in combination with β-lactamase inhibitors. HAMLET potentiated the efficacy of the macrolide antibiotics erythromycin and clarithromycin by 8- and 16-fold (Table 1 and Fig. 3E and F), respectively, whereas the efficacies of second-line β-lactam imipenem (Fig. 3H) and the oxazolidone linezolid (Fig. 3C) were both potentiated by 4-fold (Table 1). The efficacies of fluoroquinolones (levofloxacin and ofloxacin) and aminoglycosides (streptomycin and amikacin) were only slightly enhanced or did not change. The first-line TB drugs isoniazid, ethambutol, and pyrazinamide were not compatible with the alamarBlue assay and were therefore not included in this analysis. Taken together, these results show that HAMLET enhances the efficacy of several broad-spectrum antibiotics and TB drugs. It should be noted that the susceptibility of M. tuberculosis to oleic acid (46), the active component in HAMLET, and to drugs (47) depends on the growth medium.
FIG 3.
HAMLET potentiates antibiotics and TB drugs against M. tuberculosis. M. tuberculosis H37Rv was incubated with increasing concentrations of rifampin (A), bedaquiline (B), linezolid (C), delamanid (D), erythromycin (E), clarithromycin (F), ampicillin (G), imipenem (H), and capreomycin (I) in the absence (black bars) or presence (gray bars) of 90 μg/ml HAMLET (75% of the MIC90). The viability of M. tuberculosis H37Rv was determined by the microplate alamarBlue assay. In each panel, the MIC90 is represented by dotted lines. Error bars represent standard errors of the mean values of biological triplicates.
TABLE 1.
Potentiating effect of HAMLET on drugs and antibiotics against M. tuberculosisa
| Antibiotic | MIC90 (μg/ml) for: |
Potentiation factor | |
|---|---|---|---|
| Drug | Drug + HAMLET | ||
| Ampicillin | 1,600 | 100 | 16 |
| Bedaquiline | 4 | 0.5 | 8 |
| Capreomycin | 40 | 2.5 | 16 |
| Clarithromycin | 80 | 5 | 16 |
| d-Cycloserine | 12.5 | 6.25 | 2 |
| Delamanid | 2 | 0.25 | 8 |
| Erythromycin | 4,000 | 500 | 8 |
| Imipenem | 8 | 2 | 4 |
| Linezolid | 10 | 2.5 | 4 |
| Rifampin | 1 | 0.125–0.25 | 4–8 |
| Rifabutin | 1.2 | 0.15 | 8 |
| Streptomycin | 1 | 0.5 | 2 |
| Tetracycline | 8 | 4 | 2 |
The MIC90s of the antibiotics and TB drugs against M. tuberculosis H37Rv were determined using the microplate alamarBlue assay from experiments shown in Fig. 3. HAMLET was added at a concentration of 90 μg/ml (75% of the MIC90). This concentration did not affect the viability of M. tuberculosis (Fig. 3).
Production and activity of recombinant HAMLET.
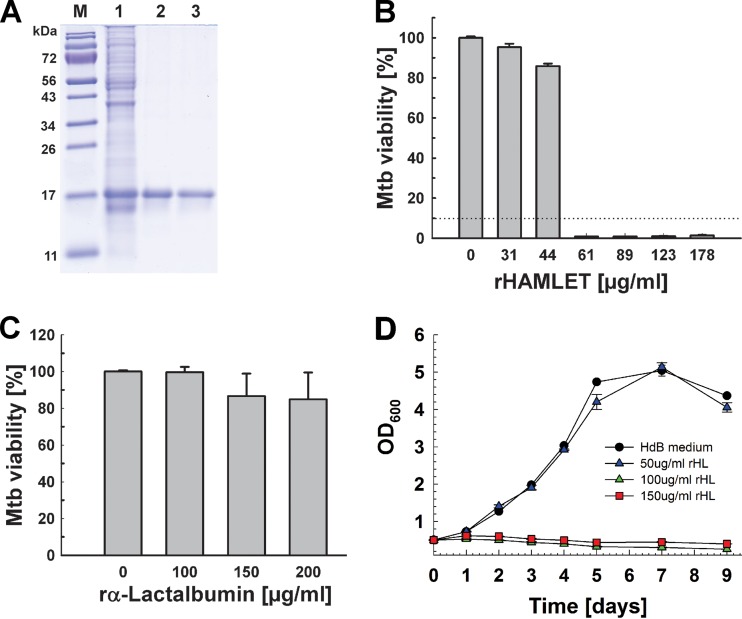
The HAMLET preparation used in the experiments described above was produced from α-lactalbumin obtained from human milk, a heterogenous fluid in limited supply that may not be suitable for future drug development. In order to avoid using human samples and to have easy access to large quantities of HAMLET, we developed a standardized method of producing HAMLET from recombinant α-lactalbumin. First, we synthesized a gene encoding modified human α-lactalbumin. Human α-lactalbumin is a Ca2+-binding metalloprotein whose native state is stabilized by four disulfide bonds (48). It has been shown that all cysteine residues in α-lactalbumin can be mutated to alanines and that this mutated α-lactalbumin forms a complex with oleic acid and retains the tumoricidal activity of HAMLET produced from α-lactalbumin obtained from human milk (49). Since serine is structurally similar to cysteine, we opted to substitute all cysteines of α-lactalbumin with serines (C6S, C28S, C61S, C73S, C77S, C91S, C111S, and C120S) to maintain the chain size and hydrophobic properties, while eliminating the formation of the disulfide bridges. Moreover, a mutation of aspartate 87 eliminates Ca(II) binding and results in partial unfolding, with a loss of tertiary structure (50). A bovine α-lactalbumin with aspartate 87 substituted for alanine was also shown to bind oleic acid and to form a complex with oleic acid and have tumoricidal activity (50). Thus, the D87A mutation was included in our recombinant protein because α-lactalbumin unfolding is required for the conversion to HAMLET (50). The human α-lactalbumin gene encodes a signal peptide which is not part of the mature protein in human milk (51) and was therefore omitted in our construct. Finally, we added an N-terminal histidine tag for affinity purification. This synthetic gene encodes a recombinant α-lactalbumin with a theoretical molecular mass of 15 kDa and was cloned into the T7 polymerase-driven expression vector pET21a+ to yield pML3438 (Fig. S1). Recombinant α-lactalbumin was produced in E. coli BL21(DE3) and was purified from urea-solubilized inclusion bodies by nickel affinity chromatography under denaturing conditions to apparent homogeneity (Fig. 4A). We obtained ∼43 mg recombinant α-lactalbumin from a 250-ml culture of E. coli. To produce recombinant HAMLET (rHAMLET), oleic acid (OA) was added to purified recombinant α-lactalbumin at a 5-fold molar excess in the presence of 2 M urea. This was followed by dialysis against phosphate-buffered saline (PBS) buffer with 2 M urea to remove free oleic acid (Fig. 4A, lane 3). After the second dialysis against only PBS, oleic acid was not detected anymore in the dialysis buffer. Furthermore, increasing the dialysis by 24 h did not change the oleic acid bound to α-lactalbumin. Both results indicated that the oleic acid in the rHAMLET preparation was strongly bound to lactalbumin and not freely available. The oleic acid bound to rHAMLET was determined using a coupled enzyme assay based on reactions catalyzed by acyl coenzyme A synthetase and acyl coenzyme A oxidase and a fluorescent probe which yielded a 6.5-fold molar excess of oleic acid over α-lactalbumin. Recombinant HAMLET (HLML1; final yield, 6.4 mg) was then lyophilized and stored as a powder at −80°C. We also prepared HAMLET from recombinant α-lactalbumin using 3- and 10-fold molar excesses of oleic acid. However, these preparations were less and overly active, respectively, compared to HLML1 and were not used in subsequent experiments.
FIG 4.
Production of recombinant HAMLET (rHL) from E. coli. (A) Analysis of purified recombinant HAMLET by gel electrophoresis. Lane 1, cell lysate of E. coli BL21(DE3) with the plasmid pML3438 encoding recombinant human α-lactalbumin (rα-lactalbumin); lane 2, pooled α-lactalbumin fractions after nickel affinity purification; lane 3, purified α-lactalbumin after complexation with a 5-fold molar excess of oleic acid to generate HAMLET; lane M, molecular mass in kilodaltons. (B and C) The viability of M. tuberculosis H37Rv grown in Hartmans-de Bont (HdB) minimal medium in the presence of increasing concentrations of recombinant HAMLET (B) and α-lactalbumin (C) was determined using the microplate alamarBlue assay. Error bars represent standard errors of mean values of biological triplicates. The MIC90 is represented by a dotted line. Tukey’s HSD following an F-test (P < 0.02) revealed that the differences between 100, 150, and 200 μg/ml α-lactalbumin are not significant (P = 0.16). (D) M. tuberculosis H37Rv was grown in HdB medium to an optical density at 600 nm of 0.5. Cells were harvested, split, and then treated with fresh HdB medium alone or with 50, 100, and 150 μg/ml rHL at day 0.
The activity of recombinant HAMLET against M. tuberculosis was then determined by an alamarBlue assay, as described above. Recombinant HAMLET strongly inhibited the growth of M. tuberculosis, with an MIC90 of <60 μg/ml (Fig. 4B), whereas we did not observe a significant effect on the viability of M. tuberculosis using recombinant α-lactalbumin up to concentrations of 200 μg/ml (Fig. 4C). Recombinant HAMLET also inhibited the growth of M. tuberculosis in liquid cultures at a concentration of 100 μg/ml (Fig. 4D). These results demonstrated that the mutations of recombinant α-lactalbumin compared to the human protein and that the affinity tag did not interfere with the activity of recombinant HAMLET against M. tuberculosis. The higher activity of recombinant HAMLET compared to HAMLET derived from human α-lactalbumin might be due to its larger oleic acid load, based on the observation that the bactericidal activity of HAMLET is directly correlated with the type and the amount of complexed fatty acid (52, 53). This assumption is supported by our finding that a larger molar excess of oleic acid during the formation of recombinant HAMLET increased its activity. However, we cannot exclude that some mutations might also increase the activity of recombinant HAMLET. Our approach to use recombinant α-lactalbumin modified to associate with oleic acid without additional treatment is fast and yields large quantities of HAMLET, thus providing an efficient alternative to produce HAMLET which does not require human materials.
HAMLET is active against M. tuberculosis in macrophages.
To examine the activity of recombinant HAMLET in macrophages, we first determined its cytotoxicity. Human THP-1 cells were treated with increasing concentrations of α-lactalbumin, oleic acid, and HAMLET, and the viability of the THP-1 cells was determined using an alamarBlue assay, as described above. Recombinant α-lactalbumin was not cytotoxic for THP-1 cells up to concentrations of 400 μg/ml (Fig. 5A), whereas oleic acid exhibited cytotoxic effects at concentrations greater than 500 μg/ml (Fig. 5B). The toxicity of recombinant HAMLET for THP-1 cells was observed at 400 μg/ml (Fig. 5C). To examine whether recombinant HAMLET is active against M. tuberculosis within macrophages, THP-1 cells were infected with M. tuberculosis H37Rv at a multiplicity of infection (MOI) of 10. The infected THP-1 cells were then treated with 2-fold increasing concentrations of HAMLET for 3 days. After lysis of the THP-1 cells, the viability of M. tuberculosis was determined by plating on agar plates. In a first experiment, we observed that HAMLET concentrations up to 245 μg/ml did not affect the growth of M. tuberculosis (not shown). In a subsequent experiment, we found that 260 μg/ml HAMLET completely inhibited the growth of M. tuberculosis (Fig. 5D). It cannot be excluded that the viability of some macrophages was affected by HAMLET at this concentration (Fig. 5C).
FIG 5.
Activity of recombinant HAMLET against M. tuberculosis in macrophages. The viability of differentiated THP-1 macrophages in the presence of increasing concentrations of recombinant α-lactalbumin (A), oleic acid (B), and recombinant HAMLET (C) was determined using the microplate alamarBlue assay. Error bars represent standard errors of mean values of biological triplicates. The MIC90 is represented by dotted lines. (D) Differentiated THP-1 macrophages were infected with M. tuberculosis H37Rv at an MOI of 10 for 4 h. Then, recombinant HAMLET was added at the indicated amounts, and the viability of M. tuberculosis was determined for two days using a drop assay on Middlebrook 7H10 agar plates. The uninfected macrophages are shown as a negative control.
HAMLET enters the cytoplasm of macrophages.
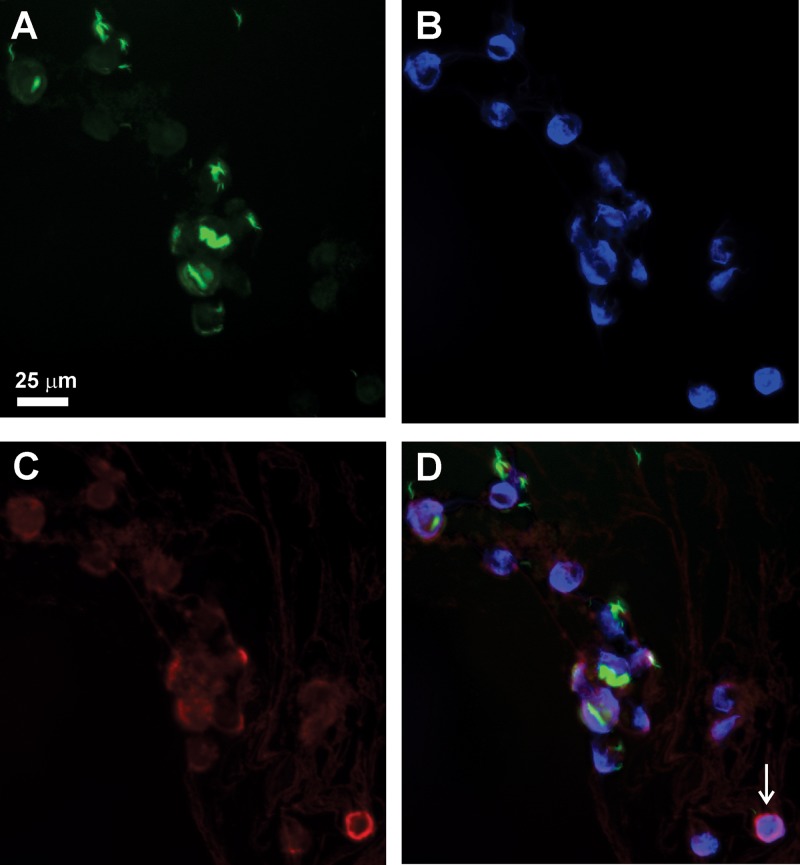
It has been previously observed that HAMLET produced from human milk binds to eukaryotic and prokaryotic cell membranes, is rapidly internalized, and accumulates in the nucleus (54–56). In order to examine whether this is also the case for recombinant HAMLET, we first confirmed that an antiserum against human α-lactalbumin recognized the mutated recombinant α-lactalbumin in an immunoblot (Fig. S1B). Then, human THP-1 cells were infected with gfp expressing M. tuberculosis and treated with 150 μg/ml recombinant HAMLET for 16 h. The THP-1 cells were then stained with the anti-α-lactalbumin antibody (red) and 4′,6-diamidino-2-phenylindole (DAPI; blue) to visualize the nucleus. The immunofluorescence images clearly showed that M. tuberculosis (green) is found in the cytoplasm of the THP-1 cells, presumably in phagosomes, and that recombinant HAMLET accumulates around the nucleus (Fig. 6). The accumulation of α-lactalbumin around the nucleus was not caused by the infection of the macrophages with M. tuberculosis, since the same observation was made with uninfected THP-1 cells (not shown). These experiments demonstrate that recombinant HAMLET is capable of entering macrophages similar to HAMLET produced from human milk.
FIG 6.
HAMLET enters the cytoplasm of macrophages. THP-1 macrophages were infected with green fluorescent protein (GFP)-expressing M. tuberculosis mc26206 at an MOI of 10:1 and treated with HAMLET (150 μg/ml) for 16 h. The fluorescence is shown for the individual channels: (A) M. tuberculosis (green, GFP); (B) nuclei (blue, DAPI staining); (C) monoclonal human α-lactalbumin antibody coupled with Alexa Fluor 594 (red). The overlay is shown in panel D.
HAMLET enhances the activities of bedaquiline, clarithromycin, and delamanid against M. tuberculosis in macrophages.
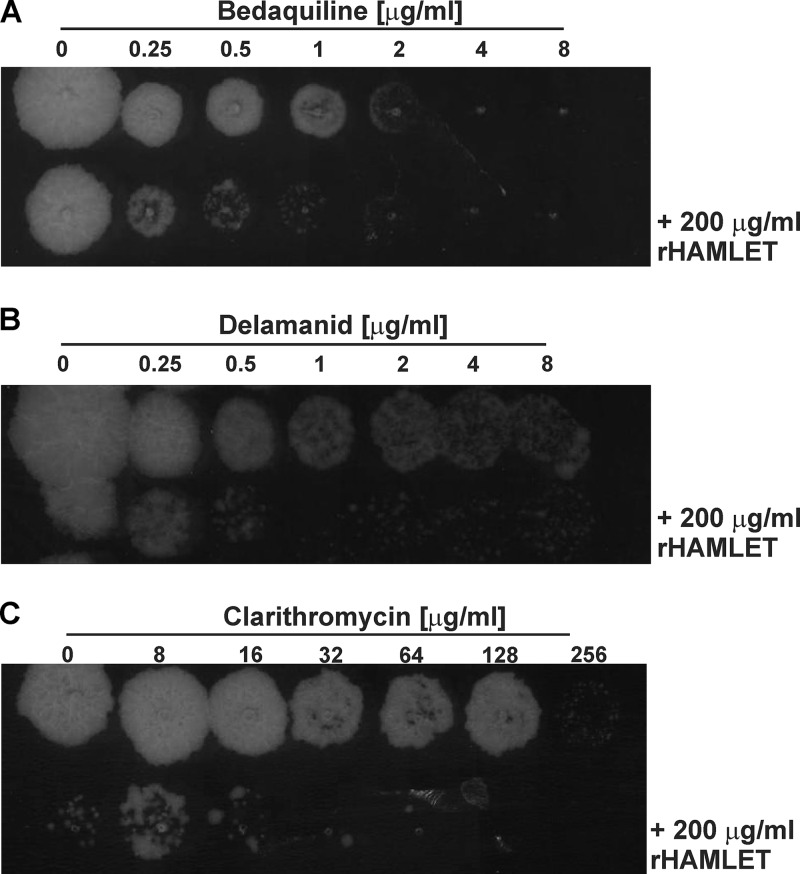
Since we established that recombinant HAMLET is active against M. tuberculosis in macrophages (Fig. 5), we examined whether it also potentiates the efficacies of antimycobacterial compounds within macrophages. To this end, we determined the effect of noninhibitory concentrations of recombinant HAMLET (200 μg/ml) in the presence of TB drugs against M. tuberculosis within infected macrophages. M. tuberculosis-infected THP-1 cells were treated with 200 μg/ml recombinant HAMLET in combination with various concentrations of each antibiotic in microtiter plates. After 3 days of treatment, the viability of M. tuberculosis was determined by drop assays on Middlebrook 7H10 agar plates. Recombinant HAMLET increased the activities of the novel TB drugs bedaquiline and delamanid by 4-fold and 8-fold, respectively (Fig. 7), similar to the potentiation effect of HAMLET in vitro (Fig. 3B and D). The MIC90 values of clarithromycin were previously found to be between 64 and 128 μg/ml against different strains of M. tuberculosis (57), consistent with the MIC90 of 80 μg/ml determined in our study (Table 1). Since the efficacy of clarithromycin against M. tuberculosis in vitro was strongly enhanced by HAMLET, we included clarithromycin in our experiments. The MIC90 of clarithromycin for M. tuberculosis in THP-1 cells was reduced from 256 μg/ml to 16 to 32 μg/ml in the presence of 200 μg/ml recombinant HAMLET (Fig. 7C). Thus, HAMLET enhances the efficacy of clarithromycin against M. tuberculosis by 8- to 16-fold both in vitro and in macrophages. In contrast, and similar to the in vitro experiments performed with HAMLET derived from human milk, we did not observe any effect of recombinant HAMLET on the efficacies of levofloxacin, d-cycloserine, and isoniazid against M. tuberculosis in THP-1 macrophages (data not shown).
FIG 7.
Recombinant HAMLET increases the efficacy of bedaquiline, delamanid, and clarithromycin against M. tuberculosis in infected macrophages. Differentiated THP-1 cells were infected with M. tuberculosis H37Rv at an MOI of 10, washed, and treated with serial dilutions of bedaquiline (A), delamanid (B), and clarithromycin (C) in the presence and absence of 200 μg/ml recombinant HAMLET for two days. Ten-microliter drops of lysed macrophages were then plated on Middlebrook 7H10 agar plates which were incubated for 13 days at 37°C.
DISCUSSION
HAMLET has bactericidal activity against M. tuberculosis.
In this study, we showed that HAMLET, a complex of α-lactalbumin and oleic acid derived from human milk, has bactericidal activity against M. tuberculosis (MIC90, 100 to 125 μg/ml, or 6 to 7.5 μM), as previously found for S. pneumoniae and other bacteria (32, 33, 56). This effect is specific for HAMLET, as the individual components of the complex had no activity under the same conditions. At first glance, this is a surprising result considering that mycobacteria have the lowest cell permeability known among bacteria (10, 58), while Gram-positive bacteria, such as S. pneumoniae, have a rather high cell permeability (59). However, it is known that M. tuberculosis is susceptible to low concentrations of oleic acid (60). HAMLET and similar protein-oleic acid complexes, called liprotides, form structures consisting of a micellar oleic acid core surrounded by a shell of partially denatured protein (61, 62). When liprotides contact lipid membranes, they transfer their entire fatty acid content within 20 s (63) and release the protein monomers (62). Numerous studies indicate that the fatty acids are the toxic components in HAMLET (52, 53, 64) and in other liprotides (41, 65). This is consistent with our finding that HAMLET was more active against M. tuberculosis with increasing oleic acid content. The function of the protein component, e.g., α-lactalbumin in HAMLET, is believed to increase the solubility of the fatty acid by shielding it from water (61, 62, 66). Our observation that HAMLET is approximately 10-fold more efficient in killing M. tuberculosis than the same molar amount of oleic acid, while α-lactalbumin had no significant effect on the viability of M. tuberculosis in vitro (Fig. 1D) is consistent with the liprotide model, in which α-lactalbumin increases the solubility and stability of the oleic acid micelle core and transports it to lipid membranes. The bactericidal activity of oleic acid against M. tuberculosis (67) might also explain the toxicity of HAMLET for M. tuberculosis.
It is conceivable that the lack of activity of HAMLET against many other bacteria (32) is related to their resistance to oleic acid. For example, Staphylococcus aureus and Streptococcus pyogenes (68) are resistant to oleic acid concentrations up to 6 mM. Escherichia coli (69), Pseudomonas aeruginosa (70), and Enterococcus faecalis (71) are used in large-scale conversions of oleic acid to hydroxy fatty acids, also indicating a significant resistance to oleic acid. A prominent exception appears to be S. pneumoniae, which is susceptible to HAMLET (32) but resistant to 6 mM oleic acid (68).
HAMLET synergizes with TB drugs.
In our study, we showed that HAMLET at concentrations which did not affect the growth of M. tuberculosis (Fig. 3) potentiates the efficacies of a remarkably broad array of TB drugs and antibiotics by 2- to 16-fold against M. tuberculosis H37Rv (Table 1). The capability of HAMLET to sensitize bacteria to antibiotics was observed previously (33, 34). For example, sublethal concentrations of HAMLET increased the efficacy of penicillin, erythromycin, and gentamicin against sensitive S. pneumoniae strains between 3- and 5-fold (33). HAMLET increased the efficacies of methicillin, erythromycin, and vancomycin against S. aureus strains sensitive to these antibiotics by 2-fold, although HAMLET showed no detectable activity by itself (34). Remarkably, HAMLET showed larger synergy effects between 8- and 300-fold against drug-resistant S. pneumoniae strains and 4- and 32-fold against drug-resistant strains of S. aureus. The rather large synergy of HAMLET with TB drugs and antibiotics against the drug-susceptible M. tuberculosis H37Rv strain suggests that HAMLET has the potential to make drug-resistant strains of M. tuberculosis susceptible to standard TB chemotherapy regimens. The molecular mechanism by which HAMLET augments the efficacy of antibiotics and drugs against M. tuberculosis is unknown. It is conceivable that HAMLET might increase the membrane permeability of M. tuberculosis, as has been described for other cells (55, 72, 73). HAMLET was also shown to decrease the bacterial proton motive force that may reduce the activity of efflux pumps and cause an accumulation of antibiotics within bacterial cells (32, 33). However, intracellular effects of HAMLET might also play a role, e.g., HAMLET-induced sensitization of S. pneumoniae was found to require calcium influx and kinase activation that may synergize with antibiotics and other drugs to increase their bactericidal activity (33).
Sensitization of bacterial pathogens to existing antibiotics has been mainly explored by using drug efflux pump inhibitors (74, 75). This approach also appears to be promising for M. tuberculosis, which encodes the largest number of drug efflux pumps compared to its genome size among known bacteria (76). Indeed, numerous studies have shown the role of efflux pumps in drug resistance of M. tuberculosis (16, 77). In particular, thioridazine was shown to synergize with TB drugs by enhancing the efficacy of isoniazid and rifampin by 64- and 128-fold (78), has sterilizing activity in combination with a first-line regimen against acute murine tuberculosis (79), and cured 17 out of 18 XDR-TB patients in Argentina (80). However, thioridazine and other phenothiazines inhibit Ca2+ ion channels and are used as antipsychotic drugs (24); hence, they have side effects and are cytotoxic at low concentrations. For example, the 50% inhibitory concentration (IC50) of thioridazine for human macrophages is 5 to 8 μg/ml (24, 78, 81). An additional cytotoxic mechanism of many drug efflux pump inhibitors is that they impair host transporter proteins (82).
HAMLET is active against M. tuberculosis in macrophages.
The activity of HAMLET against M. tuberculosis in THP-1 cells (Fig. 5D) is remarkable because it indicates that oleic acid, the toxic component of HAMLET (52, 64), is released in sufficient quantities into the macrophage cell to impair growth or kill M. tuberculosis. Interestingly, we also observed that α-lactalbumin accumulated around the nucleus of both uninfected and M. tuberculosis-infected THP-1 cells after treatment with HAMLET (Fig. 6). Since THP-1 cells were derived from the peripheral blood of a patient with acute monocytic leukemia (83), cell entry of α-lactalbumin and accumulation in the nucleus of THP-1 cells after treatment with HAMLET is consistent with previous observations for tumor cells (30, 84). Treatment of tumor cells with HAMLET leads to a loss of the mitochondrial membrane potential, induces mitochondrial swelling and the release of cytochrome c (31), disrupts chromatin by strongly interacting with histones (54), perturbs the structure and integrity of lipid membranes (55, 72), and triggers signaling cascades resulting in apoptotic cell death (85, 86). However, it is unknown whether and how these mechanisms contribute to the growth restriction of M. tuberculosis in HAMLET-treated THP-1 cells. Further experiments are needed to establish whether HAMLET is also active against M. tuberculosis in primary human macrophages.
Can HAMLET be used in TB treatment regimens?
While the bactericidal activity of HAMLET against M. tuberculosis is surprising, the potentiating effect for a broad range of antibiotics and TB drugs is certainly more useful from a therapeutic point of view. It should be noted that it is unlikely that HAMLET alone will play a role in TB chemotherapy due to the rather high concentration needed to kill M. tuberculosis in macrophages (∼260 μg/ml). In addition, these concentrations might be cytotoxic for human cells, as we found that HAMLET kills THP-1 cells at concentrations between 200 and 400 μg/ml. For medical applications, the finding that HAMLET augmented the activities of bedaquiline, delamanid, and clarithromycin inside macrophages by factors similar to those in vitro is more relevant because lung macrophages constitute the initial habitat of M. tuberculosis after infection in the host lung (87). Importantly, clinical studies showed that topical applications of HAMLET were safe and that HAMLET had tumor-specific activity, resulting in an effective treatment of bladder and skin cancer in humans (88, 89). In vitro studies showed that HAMLET is assembled at low pH and is protease resistant (90), suggesting that it can survive the harsh conditions in the stomach. Indeed, oral applications of HAMLET not only reduced tumor progression and mortality in mice with colon cancer, but it also reduced tumor development when given prophylactically in drinking water (91, 92). Taken together, these results suggest that HAMLET might be a useful reagent in current TB regimens or in new combinations including drugs which are more efficient in the presence of HAMLET (Table 1). Probably the most exciting potential application is based on the recent findings that protein complexes, such as HAMLET, can package hydrophobic molecules, such as cholesterol, tryptophan, and the hydrophobic vitamins D and E in their fatty acid micelle core (93–95). Packaging into liprotides not only drastically increased the solubility and stability of these molecules in water but also delivered them to lipid membranes (63, 93, 95). Thus, incorporation of the very hydrophobic TB drugs, such as rifampin, rifabutin, bedaquiline, and/or delamanid, into HAMLET would not only make these drugs more water-soluble and likely increase their chemical and metabolic stability, but it would also provide a transport vehicle to cells and a mechanism to exploit the considerable synergy of HAMLET with these drugs (Table 1). Another beneficial property of HAMLET is its potential to reduce the emergence of drug-resistant strains by interfering with the slow evolution of drug resistance in M. tuberculosis (96) in a manner similar to that shown for the drug efflux pump inhibitors verapamil (97) and thioridazine (98). Indeed, HAMLET inhibited resistance development when S. aureus was repeatedly exposed to methicillin in the presence of HAMLET (34). However, access of drugs to M. tuberculosis in granulomas, the primary sites of M. tuberculosis in the lungs of TB patients, and the phenotypic resistance of M. tuberculosis in the cavity of granulomas are key determinants of the efficacy of TB drugs (13, 99) and represent major hurdles in TB drug development. The unique property of HAMLET and other liprotides to interact with lipid membranes and rapidly unload their content into target cells (63) might improve granuloma penetration of drug cargo and might also reduce potential side effects.
Conclusions.
HAMLET is a promising new agent that directly kills M. tuberculosis and augments the activity of several TB drugs and antibiotics against M. tuberculosis. The synergistic effect of HAMLET with hydrophobic drugs could be exploited in coencapsulation formulations (93) and might enable the use of drugs which have become ineffective against drug-resistant strains. The observations that HAMLET is stable after oral delivery in mice (91) and is nontoxic in human studies (88) highlight the potential of HAMLET as a TB drug delivery and sensitization agent. These findings justify experiments to examine the activity of HAMLET coadministered with current TB drugs and of HAMLET-drug complexes in animal models of TB.
MATERIALS AND METHODS
Chemicals and enzymes.
All chemicals were purchased from AdooQ Bioscience, ABM, GE Healthcare, or Sigma at the highest purity available. Enzymes for DNA restriction and modification were purchased from New England BioLabs. Isolation and modification of DNA were performed as described previously (100). The α-lactalbumin genes were obtained from GenScript USA, Inc. Oligonucleotides were purchased from Integrated DNA Technologies, Inc. (Table S1).
Bacterial strains and growth conditions.
All bacterial strains used in this study are listed in Table S2. Escherichia coli DH5α was used for all cloning experiments and was routinely grown in LB medium at 37°C. Initial cultures of M. tuberculosis were grown at 37°C in Middlebrook 7H9 liquid medium (Difco Laboratories) supplemented with 10% Middlebrook OADC enrichment (0.5 g/liter oleic acid, 50 g/liter albumin, 20 g/liter dextrose, 0.04 g/liter catalase, 8.5 g/liter sodium chloride; Difco Laboratories), 0.2% glycerol, and 0.025% tyloxapol. Then, M. tuberculosis was grown in Hartmans-de Bont (HdB) minimal medium (101) with 0.02% tyloxapol for in vitro susceptibility experiments or suspended in cell culture medium (RPMI 1640) for experiments with macrophages. For testing the viability of M. tuberculosis, samples were plated on Middlebrook 7H10 agar (Difco Laboratories) supplemented with 0.2% glycerol, unless stated otherwise. Antibiotics were used as described in the text.
Production and characterization of HAMLET from human milk.
Human α-lactalbumin purified from human milk was converted into HAMLET by complexing EDTA-treated apo-protein with oleic acid (C18:1; Sigma) on an ion-exchange matrix, and the HAMLET complex was eluted with salt, as described previously (32). The complexed material was dialyzed with water to remove salt, and the protein-lipid complex was lyophilized and saved at −80°C until use. The oleic acid content of the HAMLET batches was determined using a coupled enzymatic reaction (catalog no. MAK044; Sigma-Aldrich). The fluorescence at 585 nm was measured using an excitation at 535 nm. Before dilution of HAMLET for each experiment, the concentration of the stock solution was determined by measuring the absorption at 280 nm using the theoretical extinction coefficient for α-lactalbumin in a NanoDrop spectrophotometer (Thermo Scientific).
Susceptibility of M. tuberculosis to HAMLET, antibiotics, and TB drugs by the alamarBlue assay.
To determine the MICs of HAMLET, oleic acid, α-lactalbumin, antibiotics, and TB drugs, we used the microplate alamarBlue assay (MABA) as described previously (102, 103). Briefly, M. tuberculosis was grown at 37°C in supplemented Middlebrook 7H9 liquid medium. Then, a 500-μl aliquot of the culture was added to 10 ml of Hartmans-de Bont (HdB) minimal medium (101) with 0.02% tyloxapol until the culture reached an OD600 of 1. The culture was then diluted by 20-fold in HdB medium with 0.02% tyloxapol to an OD600 of 0.05. Aliquots of 100 μl were added to the wells of a 96-well microplate containing 100 μl of HAMLET, oleic acid, α-lactalbumin, antibiotics, antibiotics plus HAMLET, or HdB medium as a control. Dilutions of HAMLET, oleic acid, α-lactalbumin, and antibiotics were prepared in Hdb medium. Antibiotics and drugs were serially 2-fold diluted from the following starting concentrations: rifampin, 1 μg/ml; bedaquiline, 4 μg/ml; linezolid, 20 μg/ml; delamanid, 2 μg/ml; erythromycin, 4 mg/ml; clarithromycin, 80 μg/ml; ampicillin, 1,600 μg/ml; imipenem, 32 μg/ml; capreomycin, 80 μg/ml; 4-salicylic acid, 80 μg/ml; streptomycin, 32 μg/ml; tetracycline, 64 μg/ml; rifabutin, 1.2 μg/ml; d-cycloserine, 100 μg/ml; ofloxacin, 4 μg/ml; amikacin, 1.25 μg/ml; and levofloxacin, 128 μg/ml. After incubation of the microplates for 3 days at 37°C, resazurin was added to each well at a final concentration of ∼90 μM. After an additional incubation of 6 h, the fluorescence of the metabolically converted resazurin dye was measured at 590 nm after excitation at 530 nm using a Cytation3 imaging reader (BioTek). The MICs (Table 1) were defined as the lowest concentration of antibiotic which reduced the viability of M. tuberculosis by at least 90%.
Activity of HAMLET against M. tuberculosis in liquid medium.
M. tuberculosis H37Rv was grown in a 60-ml bottle with Hartmans-de Bont (HdB) minimal medium to an OD600 of 1.3. Then, 10 ml of the culture was centrifuged, and the pelleted cells were resuspended in the same volume of fresh HdB medium. The culture was split, and 2.5 ml was transferred to each of two wells of a 12-well microplate. One culture was treated with a final concentration of 300 μg/ml HAMLET each day for 3 days. The microplates were incubated at 37 °C for 14 days. HdB medium in a third well served as a background control for the optical density of medium alone. Each day, the OD600 was measured, and 10 μl of each sample of serial 10-fold dilutions was dropped on Middlebrook 7H10 agar plates. The agar plates were incubated for 15 days at 37°C.
The activity of recombinant HAMLET against M. tuberculosis was determined in 30-ml bottles, each containing 5 ml of HdB medium. After M. tuberculosis was grown to an OD600 of 0.5, recombinant HAMLET was added to final concentrations of 50, 100, and 150 μg/ml. The OD600 was measured every day for 10 days.
Cytotoxicity of oleic acid, α-lactalbumin, and HAMLET for macrophages.
To determine the cytotoxicity of oleic acid, α-lactalbumin, and HAMLET for macrophages, we used the microplate alamarBlue assay. THP-1 cells were grown in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 10 mM HEPES, and 0.5% nonessential amino acids in a 5% CO2 environment at 37°C. Cells were seeded at 100,000 per well in 96-well plates, washed in supplemented RPMI 1640 medium without fetal bovine serum, and differentiated by the addition of 0.4% phorbol 12-myristate 13-acetate (PMA) for 17 h. To determine the toxicity of oleic acid in macrophages, a 100 mg/ml solution of oleic acid in 100% ethanol was diluted 100-fold in the supplemented RPMI 1640 medium without fetal bovine serum, as described above. The serial dilutions were prepared in tubes before addition to the macrophages. As a negative control, ethanol was diluted in the same medium and added to macrophages. Oleic acid micelles were visualized using a light microscope. Serial dilutions of recombinant α-lactalbumin in RPMI 1640 medium supplemented as described above were added to the macrophages. To determine the toxicity of HAMLET or TB drugs, alone or in combination, compounds were diluted in same supplemented RPMI 1640 medium and then added to the macrophages. The microplates were incubated at 37°C in a 5% CO2 incubator for up to 48 h. Resazurin dye was added to each well at a final concentration of ∼90 μM. After an additional incubation of 6 h, the fluorescence of the metabolically converted resazurin dye was quantified using a Cytation3 imaging reader (BioTek).
Intracellular activity of HAMLET against M. tuberculosis.
To determine the intracellular activity of HAMLET, M. tuberculosis H37Rv was grown in Middlebrook 7H9 medium to an OD600 of about 1.0, pelleted, and resuspended in the supplemented RPMI 1640 medium. THP-1 cells were grown in a flask and then differentiated with 0.4% PMA in 96-well microplates, as described above. Then, the differentiated THP-1 cells were infected with M. tuberculosis H37Rv at a multiplicity of infection (MOI) of 10:1 for 4 h. Uninfected THP-1 cells were used as a control. The microplate was washed two times with RPMI 1640 medium, and 200 μl RPMI 1640 medium alone or dilutions of different HAMLET concentrations in RPMI 1640 medium were added to the wells and incubated at 37°C in a 5% CO2 for 2 days. Then, the macrophages were lyzed by adding 100 μl of 1× PBS and 0.025% SDS per well. Serial 10-fold dilutions in 1× PBS were made in another 96-well microplate. Five microliters of each dilution was dropped on Middlebrook 7H10 agar plates, which were incubated for 15 days at 37°C. The agar plates were imaged using a scanner.
Construction of an expression vector for production of recombinant human α-lactalbumin from E. coli.
E. coli DH5α (Table S2) was used for plasmid construction. The DNA encoding the mutated human α-lactalbumin (C6S, C28S, C61S, C73S, C77S, D87A, C91S, C111S, and C120S) with an N-terminal histidine tag comprising eight histidines was synthetized by GenScript. This DNA fragment was cloned into the pET-21(b)+ plasmid using the restriction sites NdeI and HindIII, resulting in the expression plasmid pML3438 (Table S3). The DNA sequence of the mutated α-lactalbumin gene was confirmed by sequencing (Heflin Center for Genomic Science, UAB). The sequence of the final recombinant α-lactalbumin protein is HHHHHHHHKQFTKSELSQLLKDIDGYGGIALPELISTMFHTSGYDTQAIVENNESTEYGLFQISNKLWSKSSQVPQSRNISDISSDKFLDDDITADIMSAKKILDIKGIDYWLAHKALSTEKLEQWLSEKL. The positions of the mutated residues are in bold and underlined. The calculated isotopically averaged molecular mass of the recombinant α-lactalbumin protein is 15,003 kDa.
Purification of recombinant lactalbumin from E. coli and production of HAMLET.
E. coli BL21(DE3) carrying the α-lactalbumin expression plasmid pML3438 was cultured for 24 h in ZYP-5052 autoinduction medium (104) supplemented with 100 μg/ml ampicillin at 37°C. The cells were harvested by centrifugation, washed, and lysed by sonication in PBS (pH 7.5) containing 10 mg/ml lysozyme, 200 mM phenylmethylsulfonyl fluoride (PMSF), and a protease inhibitor cocktail (cOmplete Mini; Sigma). The insoluble fraction was collected by centrifugation and dissolved in 6 M urea in PBS (pH 7.5). The protein solution was loaded onto a Ni(II) affinity column (HisTrap HP; GE Healthcare) in the same buffer, and the protein was eluted using 250 mM imidazole. The fractions containing α-lactalbumin (15 kDa) were pooled, and oleic acid dissolved in 100% ethanol was added at a 5-fold molar excess over α-lactalbumin. This mixture was dialyzed against PBS (pH 7.5) containing 2 M urea for 24 h. This step was followed by a second dialysis against only PBS (pH 7.5) for 24 h to remove free oleic acids and urea. Recombinant HAMLET was then lyophilized and kept as a powder at −80°C until used. Before performing the experiments, the biological activity of HAMLET was tested as described above. HAMLET was dissolved in the appropriate medium or buffer and then filtered using a polyvinylidene difluoride (PVDF) filter with a pore size of 0.22 μM (Millipore). Additionally, the concentration of HAMLET was determined photometrically before each experiment by measuring the absorption at 280 nm using the theoretical extinction coefficient of 22,460 M−1 cm−1 for recombinant α-lactalbumin and a NanoDrop spectrophotometer (Thermo Scientific).
Fluorescence microscopy of macrophages.
Uninfected THP-1 cells and THP-1 cells infected with gfp-expressing M. tuberculosis (105) were treated with 150 μg/ml human α-lactalbumin or HAMLET. The cells were incubated in an 8-well glass slide (Lab-Tek II Chamber Slide) for 16 h at 37°C. The coverslips were washed with warm fresh RPMI 1640 medium and fixed for 10 min at room temperature with 4% paraformaldehyde. The THP-1 cells were washed three times with PBS and then incubated with 0.2% Triton X-100 for 5 min to permeabilize the cells. The cells were washed again with PBS, blocked with 3% normal goat serum (NGS) in 3% bovine serum albumin (BSA) for 45 min at room temperature, and then incubated with a 1:300 dilution of an anti-α-lactalbumin monoclonal mouse antibody overnight at 4°C. The cells were washed three times with PBS and incubated for 1 h with a 1:500 dilution of a goat anti-mouse antibody coupled with Alexa Fluor Plus 594 (gift from Elizabeth Sztul) at room temperature. Then, the cells were washed three times with PBS, incubated with 300 nM 4′,6-diamidino-2-phenylindole (DAPI) for 5 min, washed with PBS, and kept at 4°C in PBS. For fluorescence microscopy, the THP-1 cells were covered with FluorSave medium (Calbiochem) and a coverslip. Images were taken using Zeiss AxioXam MRm Rev3 and/or MRc cameras attached to a Zeiss AxioImager Z1 epifluorescence microscope (Carl Zeiss, Thornwood, NY).
Statistical analysis.
SigmaPlot (Systat Software) was used for graph development and statistical analysis. Where applicable, statistical significance was determined by Tukey’s honestly significant difference (HSD) test following an F-test. P values less than 0.05 are considered significant. All data presented are mean values, with error bars representing the standard error of the mean values of biological triplicates.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jim Sun for initial macrophage experiments and Norberto Gonzales Juarbe for help with fluorescence microscopy.
These studies were supported by the research grant K2015-99X-22878-01-6 from the Swedish Medical Research Council to M.N. and A.P.H. and by a grant from The Royal Physiographic Society, Lund, Sweden, to A.P.H.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01846-18.
REFERENCES
- 1.WHO. 2017. Global tuberculosis report. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf. [Google Scholar]
- 2.Gawad J, Bonde C. 2018. Current affairs, future perspectives of tuberculosis and antitubercular agents. Indian J Tuberc 65:15–22. doi: 10.1016/j.ijtb.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Lange C, Chesov D, Heyckendorf J, Leung CC, Udwadia Z, Dheda K. 2018. Drug-resistant tuberculosis: an update on disease burden, diagnosis and treatment. Respirology 23:656–673. doi: 10.1111/resp.13304. [DOI] [PubMed] [Google Scholar]
- 4.Dheda K, Gumbo T, Gandhi NR, Murray M, Theron G, Udwadia Z, Migliori GB, Warren R. 2014. Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med 2:321–338. doi: 10.1016/S2213-2600(14)70031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dheda K, Cox H, Esmail A, Wasserman S, Chang KC, Lange C. 2018. Recent controversies about MDR and XDR-TB: global implementation of the WHO shorter MDR-TB regimen and bedaquiline for all with MDR-TB? Respirology 23:36–45. doi: 10.1111/resp.13143. [DOI] [PubMed] [Google Scholar]
- 6.Dheda K, Gumbo T, Maartens G, Dooley KE, McNerney R, Murray M, Furin J, Nardell EA, London L, Lessem E, Theron G, van Helden P, Niemann S, Merker M, Dowdy D, Van Rie A, Siu GK, Pasipanodya JG, Rodrigues C, Clark TG, Sirgel FA, Esmail A, Lin HH, Atre SR, Schaaf HS, Chang KC, Lange C, Nahid P, Udwadia ZF, Horsburgh CR Jr, Churchyard GJ, Menzies D, Hesseling AC, Nuermberger E, McIlleron H, Fennelly KP, Goemaere E, Jaramillo E, Low M, Jara CM, Padayatchi N, Warren RM. 2017. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med 5:291–360. doi: 10.1016/S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 7.Bloemberg GV, Keller PM, Stucki D, Stuckia D, Trauner A, Borrell S, Latshang T, Coscolla M, Rothe T, Hömke R, Ritter C, Feldmann J, Schulthess B, Gagneux S, Böttger EC. 2015. Acquired resistance to bedaquiline and delamanid in therapy for tuberculosis. N Engl J Med 373:1986–1988. doi: 10.1056/NEJMc1505196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks SM, Hirsch-Moverman Y, Salcedo K, Graviss EA, Oh P, Seaworth B, Flood J, Armstrong L, Armitige L, Mase S, Consortium TBES. 2016. Characteristics and costs of multidrug-resistant tuberculosis in-patient care in the United States, 2005–2007. Int j Tuber Lung Dis 20:435–441. doi: 10.5588/ijtld.15.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koul A, Arnoult E, Lounis N, Guillemont J, Andries K. 2011. The challenge of new drug discovery for tuberculosis. Nature 469:483–490. doi: 10.1038/nature09657. [DOI] [PubMed] [Google Scholar]
- 10.Brennan PJ, Nikaido H. 1995. The envelope of mycobacteria. Annu Rev Biochem 64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 11.Koch A, Cox H, Mizrahi V. 2018. Drug-resistant tuberculosis: challenges and opportunities for diagnosis and treatment. Curr Opin Pharmacol 42:7–15. doi: 10.1016/j.coph.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart GR, Robertson BD, Young DB. 2003. Tuberculosis: a problem with persistence. Nat Rev Microbiol 1:97–105. doi: 10.1038/nrmicro749. [DOI] [PubMed] [Google Scholar]
- 13.Sarathy JP, Via LE, Weiner D, Blanc L, Boshoff H, Eugenin EA, Barry CE III, Dartois VA. 2018. Extreme drug tolerance of Mycobacterium tuberculosis in caseum. Antimicrob Agents Chemother 62:e02266-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarlier V, Nikaido H. 1994. Mycobacterial cell wall: structure and role in natural resistance to antibiotics. FEMS Microbiol Lett 123:11–18. doi: 10.1111/j.1574-6968.1994.tb07194.x. [DOI] [PubMed] [Google Scholar]
- 15.Xu W, DeJesus MA, Rucker N, Engelhart CA, Wright MG, Healy C, Lin K, Wang R, Park SW, Ioerger TR, Schnappinger D, Ehrt S. 2017. Chemical genetic interaction profiling reveals determinants of intrinsic antibiotic resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 61:e01334-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viveiros M, Martins M, Rodrigues L, Machado D, Couto I, Ainsa J, Amaral L. 2012. Inhibitors of mycobacterial efflux pumps as potential boosters for anti-tubercular drugs. Expert Rev Anti Infect Ther 10:983–998. doi: 10.1586/eri.12.89. [DOI] [PubMed] [Google Scholar]
- 17.Spengler G, Kincses A, Gajdacs M, Amaral L. 2017. New roads leading to old destinations: efflux pumps as targets to reverse multidrug resistance in bacteria. Molecules 22:468. doi: 10.3390/molecules22030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9:e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Cohen KA, Winglee K, Maiga M, Diarra B, Bishai WR. 2014. Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:574–576. doi: 10.1128/AAC.01462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Tyagi S, Almeida DV, Maiga MC, Ammerman NC, Bishai WR. 2013. Acceleration of tuberculosis treatment by adjunctive therapy with verapamil as an efflux inhibitor. Am J Respir Crit Care Med 188:600–607. doi: 10.1164/rccm.201304-0650OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Tyagi S, Bishai WR. 2015. Verapamil increases the bactericidal activity of bedaquiline against Mycobacterium tuberculosis in a mouse model. Antimicrob Agents Chemother 59:673–676. doi: 10.1128/AAC.04019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Soolingen D, Hernandez-Pando R, Orozco H, Aguilar D, Magis-Escurra C, Amaral L, van Ingen J, Boeree MJ. 2010. The antipsychotic thioridazine shows promising therapeutic activity in a mouse model of multidrug-resistant tuberculosis. PLoS One 5:e12640. doi: 10.1371/journal.pone.0012640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amaral L, Boeree MJ, Gillespie SH, Udwadia ZF, van Soolingen D. 2010. Thioridazine cures extensively drug-resistant tuberculosis (XDR-TB) and the need for global trials is now! Int J Antimicrob Agents 35:524–526. doi: 10.1016/j.ijantimicag.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Amaral L, Viveiros M. 2017. Thioridazine: a non-antibiotic drug highly effective, in combination with first line anti-tuberculosis drugs, against any form of antibiotic resistance of Mycobacterium tuberculosis due to its multi-mechanisms of action. Antibiotics (Basel) 6:3. doi: 10.3390/antibiotics6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amaral L, Kristiansen JE, Viveiros M, Atouguia J. 2001. Activity of phenothiazines against antibiotic-resistant Mycobacterium tuberculosis: a review supporting further studies that may elucidate the potential use of thioridazine as anti-tuberculosis therapy. J Antimicrob Chemother 47:505–511. doi: 10.1093/jac/47.5.505. [DOI] [PubMed] [Google Scholar]
- 26.Vesenbeckh S, Krieger D, Bettermann G, Schonfeld N, Bauer TT, Russmann H, Mauch H. 2016. Neuroleptic drugs in the treatment of tuberculosis: minimal inhibitory concentrations of different phenothiazines against Mycobacterium tuberculosis. Tuberculosis (Edinb) 98:27–29. doi: 10.1016/j.tube.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 27.de Keijzer J, Mulder A, de Haas PE, de Ru AH, Heerkens EM, Amaral L, van Soolingen D, van Veelen PA. 2016. Thioridazine alters the cell-envelope permeability of Mycobacterium tuberculosis. J Proteome Res 15:1776–1786. doi: 10.1021/acs.jproteome.5b01037. [DOI] [PubMed] [Google Scholar]
- 28.Martins M, Viveiros M, Amaral L. 2008. Inhibitors of Ca2+ and K+ transport enhance intracellular killing of M. tuberculosis by non-killing macrophages. In Vivo 22:69–75. [PubMed] [Google Scholar]
- 29.Håkansson A, Zhivotovsky B, Orrenius S, Sabharwal H, Svanborg C. 1995. Apoptosis induced by a human milk protein. Proc Natl Acad Sci U S A 92:8064–8068. doi: 10.1073/pnas.92.17.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Håkansson A, Andreasson J, Zhivotovsky B, Karpman D, Orrenius S, Svanborg C. 1999. Multimeric alpha-lactalbumin from human milk induces apoptosis through a direct effect on cell nuclei. Exp Cell Res 246:451–460. doi: 10.1006/excr.1998.4265. [DOI] [PubMed] [Google Scholar]
- 31.Köhler C, Gogvadze V, Hakansson A, Svanborg C, Orrenius S, Zhivotovsky B. 2001. A folding variant of human alpha-lactalbumin induces mitochondrial permeability transition in isolated mitochondria. Eur J Biochem 268:186–191. doi: 10.1046/j.1432-1327.2001.01870.x. [DOI] [PubMed] [Google Scholar]
- 32.Håkansson A, Svensson M, Mossberg AK, Sabharwal H, Linse S, Lazou I, Lonnerdal B, Svanborg C. 2000. A folding variant of alpha-lactalbumin with bactericidal activity against Streptococcus pneumoniae. Mol Microbiol 35:589–600. doi: 10.1046/j.1365-2958.2000.01728.x. [DOI] [PubMed] [Google Scholar]
- 33.Marks LR, Clementi EA, Håkansson AP. 2012. The human milk protein-lipid complex HAMLET sensitizes bacterial pathogens to traditional antimicrobial agents. PLoS One 7:e43514. doi: 10.1371/journal.pone.0043514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marks LR, Clementi EA, Håkansson AP. 2013. Sensitization of Staphylococcus aureus to methicillin and other antibiotics in vitro and in vivo in the presence of HAMLET. PLoS One 8:e63158. doi: 10.1371/journal.pone.0063158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clementi EA, Marks LR, Duffey ME, Håkansson AP. 2012. A novel initiation mechanism of death in Streptococcus pneumoniae induced by the human milk protein-lipid complex HAMLET and activated during physiological death. J Biol Chem 287:27168–27182. doi: 10.1074/jbc.M112.371070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svensson M, Håkansson A, Mossberg AK, Linse S, Svanborg C. 2000. Conversion of alpha-lactalbumin to a protein inducing apoptosis. Proc Natl Acad Sci U S A 97:4221–4226. doi: 10.1073/pnas.97.8.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondo E, Kanai K. 1972. The lethal effect of long-chain fatty acids on mycobacteria. Jpn J Med Sci Biol 25:1–13. doi: 10.7883/yoken1952.25.1. [DOI] [PubMed] [Google Scholar]
- 38.Kondo E, Kanai K. 1976. Further studies on the lethal effect of long-chain fatty acids on mycobacteria. Jpn J Med Sci Biol 29:25–37. doi: 10.7883/yoken1952.29.25. [DOI] [PubMed] [Google Scholar]
- 39.Kondo E, Kanai K. 1977. The relationship between the chemical structure of fatty acids and their mycobactericidal activity. Jpn J Med Sci Biol 30:171–178. doi: 10.7883/yoken1952.30.171. [DOI] [PubMed] [Google Scholar]
- 40.Carballeira NM. 2008. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog Lipid Res 47:50–61. doi: 10.1016/j.plipres.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clementi EA, Wilhelm KR, Schleucher J, Morozova-Roche LA, Hakansson AP. 2013. A complex of equine lysozyme and oleic acid with bactericidal activity against Streptococcus pneumoniae. PLoS One 8:e80649. doi: 10.1371/journal.pone.0080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dijkstra JA, van der Laan T, Akkerman OW, Bolhuis MS, de Lange WCM, Kosterink JGW, van der Werf TS, Alffenaar JWC, van Soolingen D. 2018. In vitro susceptibility of Mycobacterium tuberculosis to amikacin, kanamycin, and capreomycin. Antimicrob Agents Chemother 62:e01724-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta R, Geiter LJ, Wells CD, Gao M, Cirule A, Xiao H. 2015. Delamanid for extensively drug-resistant tuberculosis. N Engl J Med 373:291–292. doi: 10.1056/NEJMc1415332. [DOI] [PubMed] [Google Scholar]
- 44.Seaworth BJ, Griffith DE. 2017. Therapy of multidrug-resistant and extensively drug-resistant tuberculosis. Microbiol Spectr 5:TNMI7-0042-2017. doi: 10.1128/microbiolspec.TNMI7-0042-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pontali E, Visca D, Centis R, D'Ambrosio L, Spanevello A, Migliori GB. 2018. Multi and extensively drug-resistant pulmonary tuberculosis: advances in diagnosis and management. Curr Opin Pulm Med 24:244–252. [DOI] [PubMed] [Google Scholar]
- 46.Lyon RH, Lichstein HC, Hall WH. 1963. Effect of Tween 80 on the growth of tubercle bacilli in aerated cultures. J Bacteriol 86:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pethe K, Sequeira PC, Agarwalla S, Rhee K, Kuhen K, Phong WY, Patel V, Beer D, Walker JR, Duraiswamy J, Jiricek J, Keller TH, Chatterjee A, Tan MP, Ujjini M, Rao SP, Camacho L, Bifani P, Mak PA, Ma I, Barnes SW, Chen Z, Plouffe D, Thayalan P, Ng SH, Au M, Lee BH, Tan BH, Ravindran S, Nanjundappa M, Lin X, Goh A, Lakshminarayana SB, Shoen C, Cynamon M, Kreiswirth B, Dartois V, Peters EC, Glynne R, Brenner S, Dick T. 2010. A chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-dependent growth inhibitors devoid of in vivo efficacy. Nat Commum 1:1. doi: 10.1038/ncomms1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hiraoka Y, Segawa T, Kuwajima K, Sugai S, Murai N. 1980. alpha-Lactalbumin: a calcium metalloprotein. Biochem Biophys Res Commun 95:1098–1104. doi: 10.1016/0006-291X(80)91585-5. [DOI] [PubMed] [Google Scholar]
- 49.Pettersson-Kastberg J, Mossberg AK, Trulsson M, Yong YJ, Min S, Lim Y, O'Brien JE, Svanborg C, Mok KH. 2009. alpha-Lactalbumin, engineered to be nonnative and inactive, kills tumor cells when in complex with oleic acid: a new biological function resulting from partial unfolding. J Mol Biol 394:994–1010. doi: 10.1016/j.jmb.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 50.Svensson M, Fast J, Mossberg AK, Duringer C, Gustafsson L, Hallgren O, Brooks CL, Berliner L, Linse S, Svanborg C. 2003. Alpha-lactalbumin unfolding is not sufficient to cause apoptosis, but is required for the conversion to HAMLET (human alpha-lactalbumin made lethal to tumor cells). Protein Sci 12:2794–2804. doi: 10.1110/ps.0231003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Findlay JB, Brew K. 1972. The complete amino-acid sequence of human -lactalbumin. Eur J Biochem 27:65–86. doi: 10.1111/j.1432-1033.1972.tb01812.x. [DOI] [PubMed] [Google Scholar]
- 52.Permyakov SE, Knyazeva EL, Khasanova LM, Fadeev RS, Zhadan AP, Roche-Hakansson H, Hakansson AP, Akatov VS, Permyakov EA. 2012. Oleic acid is a key cytotoxic component of HAMLET-like complexes. Biol Chem 393:85–92. doi: 10.1515/BC-2011-230. [DOI] [PubMed] [Google Scholar]
- 53.Brinkmann CR, Brodkorb A, Thiel S, Kehoe JJ. 2013. The cytotoxicity of fatty acid/alpha-lactalbumin complexes depends on the amount and type of fatty acid. Eur J Lipid Sci Technol 115:591–600. doi: 10.1002/ejlt.201200165. [DOI] [Google Scholar]
- 54.Düringer C, Hamiche A, Gustafsson L, Kimura H, Svanborg C. 2003. HAMLET interacts with histones and chromatin in tumor cell nuclei. J Biol Chem 278:42131–42135. doi: 10.1074/jbc.M306462200. [DOI] [PubMed] [Google Scholar]
- 55.Mossberg AK, Puchades M, Halskau O, Baumann A, Lanekoff I, Chao Y, Martinez A, Svanborg C, Karlsson R. 2010. HAMLET interacts with lipid membranes and perturbs their structure and integrity. PLoS One 5:e9384. doi: 10.1371/journal.pone.0009384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hakansson AP, Roche-Hakansson H, Mossberg AK, Svanborg C. 2011. Apoptosis-like death in bacteria induced by HAMLET, a human milk lipid-protein complex. PLoS One 6:e17717. doi: 10.1371/journal.pone.0017717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Truffot-Pernot C, Lounis N, Grosset JH, Ji B. 1995. Clarithromycin is inactive against Mycobacterium tuberculosis. Antimicrob Agents Chemother 39:2827–2828. doi: 10.1128/AAC.39.12.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nikaido H, Jarlier V. 1991. Permeability of the mycobacterial cell wall. Res Microbiol 142:437–443. doi: 10.1016/0923-2508(91)90117-S. [DOI] [PubMed] [Google Scholar]
- 59.Lambert PA. 2002. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. J Appl Microbiol 92:46S–54S. doi: 10.1046/j.1365-2672.92.5s1.7.x. [DOI] [PubMed] [Google Scholar]
- 60.Dubos RJ. 1946. Effect of long chain fatty acids on bacterial growth. Proc Soc Exp Biol Med 63:56–58. doi: 10.3181/00379727-63-15491P. [DOI] [PubMed] [Google Scholar]
- 61.Kaspersen JD, Pedersen JN, Hansted JG, Nielsen SB, Sakthivel S, Wilhelm K, Nemashkalova EL, Permyakov SE, Permyakov EA, Pinto Oliveira CL, Morozova-Roche LA, Otzen DE, Pedersen JS. 2014. Generic structures of cytotoxic liprotides: nano-sized complexes with oleic acid cores and shells of disordered proteins. Chembiochem 15:2693–2702. doi: 10.1002/cbic.201402407. [DOI] [PubMed] [Google Scholar]
- 62.Frislev HS, Jessen CM, Oliveira CL, Pedersen JS, Otzen DE. 2016. Liprotides made of alpha-lactalbumin and cis fatty acids form core-shell and multi-layer structures with a common membrane-targeting mechanism. Biochim Biophys Acta 1864:847–859. doi: 10.1016/j.bbapap.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Frislev HS, Jakobsen SCL, Frank SA, Otzen DE. 2018. Dynamic content exchange between liprotides. Biophys Chem 233:13–18. doi: 10.1016/j.bpc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Fontana A, Spolaore B, Polverino de Laureto P. 2013. The biological activities of protein/oleic acid complexes reside in the fatty acid. Biochim Biophys Acta 1834:1125–1143. doi: 10.1016/j.bbapap.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 65.Brinkmann CR, Heegaard CW, Petersen TE, Jensenius JC, Thiel S. 2011. The toxicity of bovine alpha-lactalbumin made lethal to tumor cells is highly dependent on oleic acid and induces killing in cancer cell lines and noncancer-derived primary cells. FEBS J 278:1955–1967. doi: 10.1111/j.1742-4658.2011.08112.x. [DOI] [PubMed] [Google Scholar]
- 66.Brinkmann CR, Thiel S, Otzen DE. 2013. Protein-fatty acid complexes: biochemistry, biophysics and function. FEBS J 280:1733–1749. doi: 10.1111/febs.12204. [DOI] [PubMed] [Google Scholar]
- 67.Minami K. 1957. Bactericidal action of oleic acid for tubercle bacilli. I. Quantitative and analytical survey of the action. J Bacteriol 73:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kabara JJ, Swieczkowski DM, Conley AJ, Truant JP. 1972. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother 2:23–28. doi: 10.1128/AAC.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joo YC, Seo ES, Kim YS, Kim KR, Park JB, Oh DK. 2012. Production of 10-hydroxystearic acid from oleic acid by whole cells of recombinant Escherichia coli containing oleate hydratase from Stenotrophomonas maltophilia. J Biotechnol 158:17–23. doi: 10.1016/j.jbiotec.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Martin-Arjol I, Llacuna JL, Manresa A. 2014. Yield and kinetic constants estimation in the production of hydroxy fatty acids from oleic acid in a bioreactor by Pseudomonas aeruginosa 42A2. Appl Microbiol Biotechnol 98:9609–9621. doi: 10.1007/s00253-014-5996-9. [DOI] [PubMed] [Google Scholar]
- 71.Hudson JA, Mackenzie CA, Joblin KN. 1996. Factors affecting the formation of 10-hydroxystearic acid from oleic acid by a ruminal strain of Enterococcus faecalis. Appl Microbiol Biotechnol 45:404–407. doi: 10.1007/s002530050703. [DOI] [PubMed] [Google Scholar]
- 72.Frislev HS, Boye TL, Nylandsted J, Otzen D. 2017. Liprotides kill cancer cells by disrupting the plasma membrane. Sci Rep 7:15129. doi: 10.1038/s41598-017-15003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wen H, Glomm WR, Halskau O. 2013. Cytotoxicity of bovine alpha-lactalbumin: oleic acid complexes correlates with the disruption of lipid membranes. Biochim Biophys Acta 1828:2691–2699. doi: 10.1016/j.bbamem.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 74.Kourtesi C, Ball AR, Huang YY, Jachak SM, Vera DM, Khondkar P, Gibbons S, Hamblin MR, Tegos GP. 2013. Microbial efflux systems and inhibitors: approaches to drug discovery and the challenge of clinical implementation. Open Microbiol J 7:34–52. doi: 10.2174/1874285801307010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Venter H, Ma S. 2016. Efflux pump inhibitors: a novel approach to combat efflux-mediated drug resistance in bacteria. Curr Drug Targets 17:702–719. doi: 10.2174/1389450116666151001103948. [DOI] [PubMed] [Google Scholar]
- 76.da Silva PE, Von Groll A, Martin A, Palomino JC. 2011. Efflux as a mechanism for drug resistance in Mycobacterium tuberculosis. FEMS Immunol Med Microbiol 63:1–9. doi: 10.1111/j.1574-695X.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- 77.Pule CM, Sampson SL, Warren RM, Black PA, van Helden PD, Victor TC, Louw GE. 2016. Efflux pump inhibitors: targeting mycobacterial efflux systems to enhance TB therapy. J Antimicrob Chemother 71:17–26. doi: 10.1093/jac/dkv316. [DOI] [PubMed] [Google Scholar]
- 78.Pieroni M, Machado D, Azzali E, Santos Costa S, Couto I, Costantino G, Viveiros M. 2015. Rational design and synthesis of thioridazine analogues as enhancers of the antituberculosis therapy. J Med Chem 58:5842–5853. doi: 10.1021/acs.jmedchem.5b00428. [DOI] [PubMed] [Google Scholar]
- 79.Dutta NK, Pinn ML, Karakousis PC. 2014. Sterilizing activity of thioridazine in combination with the first-line regimen against acute murine tuberculosis. Antimicrob Agents Chemother 58:5567–5569. doi: 10.1128/AAC.03408-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abbate E, Vescovo M, Natiello M, Cufre M, Garcia A, Gonzalez Montaner P, Ambroggi M, Ritacco V, van Soolingen D. 2012. Successful alternative treatment of extensively drug-resistant tuberculosis in Argentina with a combination of linezolid, moxifloxacin and thioridazine. J Antimicrob Chemother 67:473–477. doi: 10.1093/jac/dkr500. [DOI] [PubMed] [Google Scholar]
- 81.Scalacci N, Brown AK, Pavan FR, Ribeiro CM, Manetti F, Bhakta S, Maitra A, Smith DL, Petricci E, Castagnolo D. 2017. Synthesis and SAR evaluation of novel thioridazine derivatives active against drug-resistant tuberculosis. Eur J Med Chem 127:147–158. doi: 10.1016/j.ejmech.2016.12.042. [DOI] [PubMed] [Google Scholar]
- 82.Tegos GP, Haynes M, Strouse JJ, Khan MM, Bologa CG, Oprea TI, Sklar LA. 2011. Microbial efflux pump inhibition: tactics and strategies. Curr Pharm Des 17:1291–1302. doi: 10.2174/138161211795703726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer 26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 84.Svensson M, Duringer C, Hallgren O, Mossberg AK, Hakansson A, Linse S, Svanborg C. 2002. Hamlet–a complex from human milk that induces apoptosis in tumor cells but spares healthy cells. Adv Exp Med Biol 503:125–132. doi: 10.1007/978-1-4615-0559-4_14. [DOI] [PubMed] [Google Scholar]
- 85.Gustafsson L, Hallgren O, Mossberg AK, Pettersson J, Fischer W, Aronsson A, Svanborg C. 2005. HAMLET kills tumor cells by apoptosis: structure, cellular mechanisms, and therapy. J Nutr 135:1299–1303. doi: 10.1093/jn/135.5.1299. [DOI] [PubMed] [Google Scholar]
- 86.Hallgren O, Gustafsson L, Irjala H, Selivanova G, Orrenius S, Svanborg C. 2006. HAMLET triggers apoptosis but tumor cell death is independent of caspases, Bcl-2 and p53. Apoptosis 11:221–233. doi: 10.1007/s10495-006-3607-7. [DOI] [PubMed] [Google Scholar]
- 87.Queval CJ, Brosch R, Simeone R. 2017. The macrophage: a disputed fortress in the battle against Mycobacterium tuberculosis. Front Microbiol 8:2284. doi: 10.3389/fmicb.2017.02284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mossberg AK, Wullt B, Gustafsson L, Mansson W, Ljunggren E, Svanborg C. 2007. Bladder cancers respond to intravesical instillation of HAMLET (human alpha-lactalbumin made lethal to tumor cells). Int J Cancer 121:1352–1359. doi: 10.1002/ijc.22810. [DOI] [PubMed] [Google Scholar]
- 89.Gustafsson L, Leijonhufvud I, Aronsson A, Mossberg AK, Svanborg C. 2004. Treatment of skin papillomas with topical alpha-lactalbumin-oleic acid. N Engl J Med 350:2663–2672. doi: 10.1056/NEJMoa032454. [DOI] [PubMed] [Google Scholar]
- 90.Gustafsson L, Aits S, Onnerfjord P, Trulsson M, Storm P, Svanborg C. 2009. Changes in proteasome structure and function caused by HAMLET in tumor cells. PLoS One 4:e5229. doi: 10.1371/journal.pone.0005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puthia M, Storm P, Nadeem A, Hsiung S, Svanborg C. 2014. Prevention and treatment of colon cancer by peroral administration of HAMLET (human alpha-lactalbumin made lethal to tumour cells). Gut 63:131–142. doi: 10.1136/gutjnl-2012-303715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith K. 2013. Therapy: HAMLET takes a leading role on the colorectal cancer stage. Nat Rev Gastroenterol Hepatol 10:126. doi: 10.1038/nrgastro.2013.27. [DOI] [PubMed] [Google Scholar]
- 93.Pedersen JN, Pedersen JS, Otzen DE. 2015. The use of liprotides to stabilize and transport hydrophobic molecules. Biochemistry 54:4815–4823. doi: 10.1021/acs.biochem.5b00547. [DOI] [PubMed] [Google Scholar]
- 94.Pedersen JN, Frislev HS, Pedersen JS, Otzen DE. 2016. Using protein-fatty acid complexes to improve vitamin D stability. J Dairy Sci 99:7755–7767. doi: 10.3168/jds.2016-11343. [DOI] [PubMed] [Google Scholar]
- 95.Frislev HS, Nielsen J, Nylandsted J, Otzen D. 2018. Using liprotides to deliver cholesterol to the plasma membrane. J Membr Biol 251:581–592. doi: 10.1007/s00232-018-0034-y. [DOI] [PubMed] [Google Scholar]
- 96.Szumowski JD, Adams KN, Edelstein PH, Ramakrishnan L. 2013. Antimicrobial efflux pumps and Mycobacterium tuberculosis drug tolerance: evolutionary considerations. Curr Top Microbiol Immunol 374:81–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adams KN, Szumowski JD, Ramakrishnan L. 2014. Verapamil, and its metabolite norverapamil, inhibit macrophage-induced, bacterial efflux pump-mediated tolerance to multiple anti-tubercular drugs. J Infect Dis 210:456–466. doi: 10.1093/infdis/jiu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dutta NK, Pinn ML, Karakousis PC. 2014. Reduced emergence of isoniazid resistance with concurrent use of thioridazine against acute murine tuberculosis. Antimicrob Agents Chemother 58:4048–4053. doi: 10.1128/AAC.02981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dartois V. 2014. The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells. Nat Rev Microbiol 12:159–167. doi: 10.1038/nrmicro3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ausubel FA, Brent R, Kingston RE, Moore DD, Seidmann JG, Smith JA, Struhl K. 1990. Current protocols in molecular biology. Greene Publishing and Wiley-Interscience, New York, NY. [Google Scholar]
- 101.Smeulders MJ, Keer J, Speight RA, Williams HD. 1999. Adaptation of Mycobacterium smegmatis to stationary phase. J Bacteriol 181:270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Collins L, Franzblau SG. 1997. Microplate alamarBlue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 41:1004–1009. doi: 10.1128/AAC.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Danilchanka O, Mailaender C, Niederweis M. 2008. Identification of a novel multidrug efflux pump of Mycobacterium tuberculosis. Antimicrob Agents Chemother 52:2503–2511. doi: 10.1128/AAC.00298-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Studier FW. 2005. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 105.Pajuelo D, Gonzalez-Juarbe N, Tak U, Sun J, Orihuela CJ, Niederweis M. 2018. NAD(+) depletion triggers macrophage necroptosis, a cell death pathway exploited by Mycobacterium tuberculosis. Cell. Rep 24:429–440. doi: 10.1016/j.celrep.2018.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.