Abstract
Background
Cellular senescence is a stable cell-cycle arrest induced by telomere shortening and various types of cellular stress including oxidative stress, oncogene activation, DNA damage etc. Heme oxygenase-1 (HO-1) is an inducible stress-response protein that plays antioxidant and anti-apoptotic effects. However, the role and underlying mechanisms of HO-1 in cellular senescence in heart are largely unknown.
Methods
Echocardiography was employed to detect the effect of HO-1 on heart function in adult mice with myocardial infarction (MI) and aged mice. The senescence markers, p53, p16 and LaminB, were analyzed by western blot. The immunofluorescence and immunohistochemical staining were applied to analyze the expression level of p16. SA-β-Gal staining showed the level of cardiomyocyte senescence.
Findings
We found that hemin significantly induced the expression of HO-1, which notably suppressed cardiomyocyte senescence containing the secretion of senescence-associated secretory phenotype. Further studies showed that systemic HO-1 transgenic overexpression improved heart function by inhibiting aging-induced extracellular matrix deposition and fibrogenesis. More importantly, treatment of hemin improved heart function in MI mice. Furthermore, forced expression of HO-1 blunted cardiomyocyte senescence in natural aged mice and in primary cultured neonatal mouse cardiomyocytes.
Interpretation
Our study revealed that HO-1 improved heart function and attenuated cardiomyocyte senescence triggered by ischemic injury and aging. In addition, HO-1 induction alleviated H2O2-induced cardiomyocyte senescence. Finally, our study suggested a novel mechanism of HO-1 to play cardioprotective effect.
Fund
This study was supported by the National Natural Science Foundation of China (81770284 to Hongli Shan); and the National Natural Science Foundation of China (81673425, 81872863 to Yuhong Zhou). The National Natural Science Foundation of China (81473213 to Chaoqian Xu). National Key R&D Program of China (2017YFC1307403 to Baofeng Yang), National Natural Science Foundation of China (81730012 to Baofeng Yang).
Keywords: Myocardial infarction, Heme oxygenase-1, Senescence, Senescence-associated secretory phenotype, Extracellular matrix
Research in context.
Evidence before this study
Cellular senescence is a stable cell-cycle arrest that is induced by damage in many biological and pathological settings. The study about senescence are almost in aged organs. Molecular biological change during the development of myocardial infarction are marked by oxidative stress, DNA damage and so on, which were also part of the mechanisms of senescence. Seldom research focus on the role of cellular senescence in adult mammals. The cardioprotective effect of HO-1 has been widely reported. But the interaction between HO-1 and senescence is poorly described.
Added value of this study
The age of onset of myocardial infarction is earlier than before and it is important to open a new window for the prevention and cure of myocardial infarction. This study can reveal the role of senescence during the development of myocardial infarction and explore the interaction between HO-1 induction and cardiomyocyte senescence.
Implications of all the available evidence
In this study, we found that MI induced ischemic injury triggered cardiomyocyte senescence and which can be attenuated by overexpression of HO-1. Moreover, aged heart was accompanied by the accumulation of senescent cardiomyocytes and which also was relieved by forced expression of HO-1. More importantly, increased expression of HO-1 improved heart function in mice with MI or aging.
Alt-text: Unlabelled Box
1. Introduction
Cellular senescence, a stable cell-cycle arrest that is induced by damage in many biological and pathological settings, consist of replicative senescence, DNA-damage-induced senescence, oxidative stress-induced senescence, and so on [1]. Senescent cells display an enlarged and irregularly shaped cell body and are also characterized by increased enzymatic activities of lysosomal hydrolase senescence-associated β-galactosidase (SA-β-gal), upregulated pro-survival pathways to resist apoptosis and the activation of senescence-associated secretory phenotype (SASP) [2]. SASP are distinctive secretome consisting of various proinflammatory molecules and growth factors [3,4]. The development of cellular senescence contributes to the decline of regenerative potential, decrease of tissues function and inflammation as well as tumorigenesis in aged organisms [5,6]. Interestingly, senescent cells have also been observed in tissue remodeling during development and after injury [7]. However, the functional contribution of cellular senescence to ischemic heart diseases has been poorly examined.
Heme oxygenase-1 (HO-1) is a rapidly inducible cytoprotective factor that catalyzes the oxidative cleavage of heme into equimolar amounts of carbon monoxide (CO), iron, and biliverdin, which is then converted to bilirubin by biliverdin reductase. There are two isoforms of HO: HO-1 is an inducible isoform, while HO-2 is constitutively expressed. HO-1 mitigates cellular injury by exerting antioxidative, anti-apoptotic and anti-inflammatory effects [[8], [9], [10], [11]]. Growing evidences have shown the critical cardioprotective effects of HO-1. Sharma et al. found that ischemia/reperfusion substantially enhances HO-1 expression in the porcine heart, suggesting a potential role of HO-1 in the defense against pathophysiological stress [12]. In a genetic loss-of-function approach, Yet et al. demonstrated that in contrast to wild type mice, hypoxia induces severe right ventricular dilatation and infarction in HO-1−/− mice [13]. In addition, an absence of HO-1 exacerbates ischemia/reperfusion-induced myocardial injury [14]. HO-1 induction reduced apoptosis, increased proliferation and repair of cardiomyocytes in rats suffered from myocardial infarction (MI) [15]. Mice with cardiac-restricted HO-1 overexpression resisted ischemia/reperfusion (I/R) injury, with improved contractibility and reduction of infarct size, oxidative damage, inflammatory cell infiltration and apoptosis. However, the role and mechanism of HO-1 in the regulation of cellular senescence during cardiac injury and aging remains largely unknown.
In this study we identified the crucial role of cellular senescence in MI-induced ischemic injury and elucidated the protective effect of HO-1 against MI- and aging-induced cardiomyocyte senescence. This study provides a novel direction for the prevention and treatment of MI and heart aging.
2. Results
2.1. HO-1 represses natural senescence of cardiomyocytes
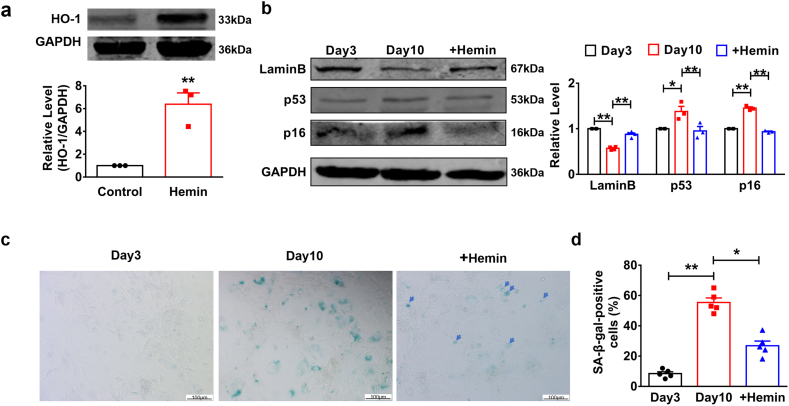
To explore the role of HO-1 in the development of heart aging, we cultured primary neonatal mouse cardiomyocytes (NMCMs) for 10 days to induce the natural senescence and treated with hemin (10 μM) every other day to induce the production of HO-1. Western blot showed the overexpression of HO-1 after the hemin treatment (Fig. 1a). LaminB is a kind of nuclear protein. The decrease of LaminB indicates the DNA damage and it also acts as an important regulator of senescence [16,17]. We observed an increased expression level of LaminB in NMCMs after 10 days of hemin treatment (Fig. 1b). Cyclin-Dependent Kinase Inhibitor 2A (p16) is a senescence related gene, which is an important activator and effector of senescence [18]. In addition, TP53 (p53) plays an important role in the induction of senescence [19]. Western blot showed a decrease levels of both p16 and p53 in NMCMs treated with hemin on the day 10 (Fig. 1b). We also found that SA-β-gal activity, one of the key biomarkers of cellular senescence, was up-regulated in NMCMs cultured for 10 days compared to control group and which was inhibited after treatment with hemin (Fig. 1c–d). Then, we further detected the role of HO-1 by genetic modification. SA-β-gal staining showed that knockdown of HO-1 by siRNA reversed the inhibited effects of hemin on β-galactosidase (Fig. S1a). More importantly, western blot analysis showed the same results that si-HO-1 restored the change of two senescence relevant markers, p53 and LaminB (Fig. S1b). These data suggest that HO-1 can suppress natural senescence of NMCMs.
Fig. 1.
HO-1 repressed replicative cardiomyocytes senescence. Primary cardiomyocytes were cultured for 3 or 10 days and treated with or without hemin (10 μM) every other day. (a) Hemin induced production of HO-1 taken at the 10-day time point was shown by western blot. n = 3. **P < .05. Data are mean ± SEM; Two-tailed t-test was used for the statistical analysis. (b) The effect of HO-1 on expression levels of senescence markers containing LaminB, p53 and p16 were analyzed by western blot. n = 3. *P < .01, **P < .05. Data are mean ± SEM; one-way ANOVA was used for the statistical analysis. (c) and (d) SA-β-gal activity was determined using Senescence β-Galactosidase Staining Kit. Scale bars represent 100 μm. n = 5. *P < .01, **P < .05. Data are mean ± SEM; Two-tailed t-test was used for the statistical analysis.
2.2. Forced expression of HO-1 improves heart function of aging mice
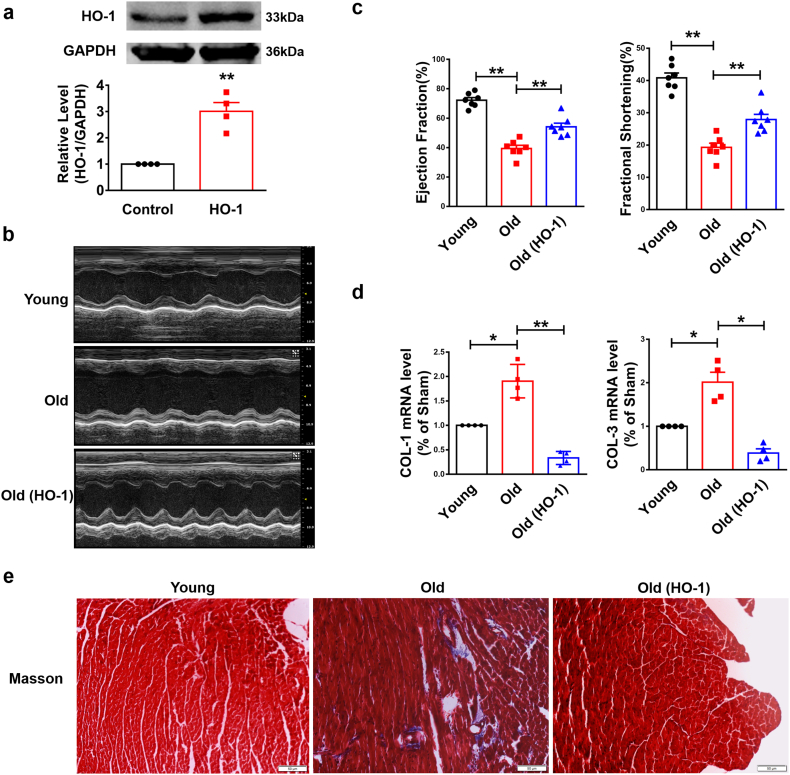
To examine whether HO-1 affects the heart function of aged mice, we used transgenic HO-1 (HO-1+/+) mice. Overexpression of HO-1 was determined by western blot in HO-1+/+ mice (Fig. 2a). Echocardiographic assessment showed the improvement of Ejection Fraction (EF) and Fractional Shortening (FS) in HO-1 transgenic mice compared with WT mice in same age (Fig. 2b–c). qRT-PCR analysis detected the up-regulation of collagen 1α1 and collagen 3α1 (Col 1α1, Col 3α1) of WT mice and forced expression of HO-1 inhibited the production of Col 1α1 and Col 3α1 (Fig. 2d). Furthermore, masson staining showed an accumulation of extracellular matrix (ECM), a crucial phenomenon during the process of aging, in the heart of WT mice and a reduction in HO-1 transgenic mice (Fig. 2e). These data indicate induction of HO-1 improves the heart function of aged mice.
Fig. 2.
Forced expression of HO-1 improves heart function. The systemic HO-1 transgenic overexpression aged mice were 16 months. (a) Overexpression of HO-1 was detected by western blot. n = 4. **P < .05. Data are mean ± SEM; Two-tailed t-test was used for the statistical analysis. (b) Representative short-axis M-mode images from Young, Old and Old (HO-1) group. (c) Ejection Fraction (EF) and Fractional Shortening (FS) of Young, Old and Old (HO-1) group were detected by Echocardiographic assessment. n = 7. **P < .05. Data are mean ± SEM; Two-tailed t-test was used for the statistical analysis. (d) Relative mRNA levels of Col 1α1and Col 3α1 analyzed by qRT-PCR. n = 4. *P < .01, **P < .05. Data are mean ± SEM; Two-tailed t-test was used for the statistical analysis. (e) Masson staining detected the deposition of ECM. Scale bars represent 50 μm. n = 3.
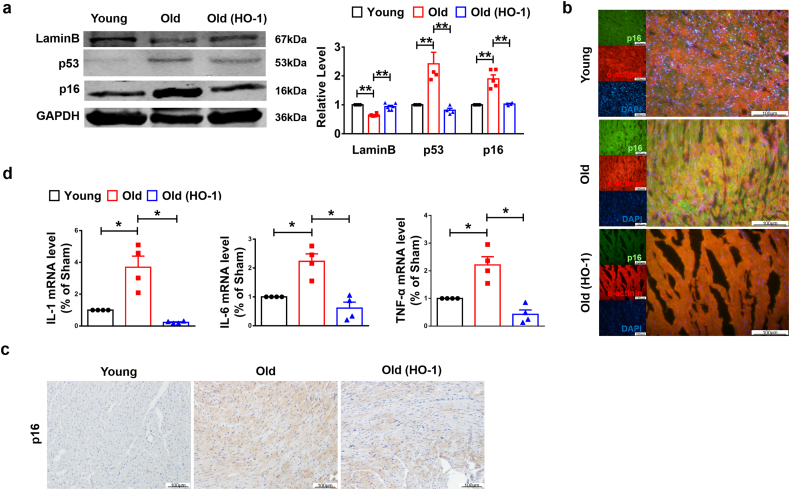
To further detect whether HO-1 improves the heart function by acting on cellular senescence, we analyzed the senescence markers and found increased LaminB expression in old HO-1+/+ mice (Fig. 3a). In addition, protein levels of p53 and p16 were down-regulated due to HO-1 overexpression in aged mice compared to WT group (Fig. 3a). Immunofluorescence and immunohistochemistry staining of histological sections also showed the down-regulation of p16 in HO-1 transgenic mice compared with WT mice in same age (Fig. 3b–c). Numerous activities of senescent cells depend on the aptitude of these cells to secrete many kinds of bioactive molecules, a behavior termed the senescence-associated secretory phenotype (SASP) [20]. IL-1 and IL-6 are important factors of SASP [21,22]. qRT-PCR analysis was used to determine the increased mRNA levels of IL-1, IL-6 and TNF-α of aged mice and which were alleviated after forced expression of HO-1(Fig. 3d). Collectively, these data indicate that forced expression of HO-1 protects the heart function by repressing cardiomyocytes natural senescence.
Fig. 3.
Up-regulation of HO-1 attenuated heart aging in vivo. The systemic HO-1 transgenic overexpression aged mice were 16 months. (a) The protein levels of LaminB, p53 and p16 were analyzed by western blot. n = 3. **P < .05. Data are mean ± SEM; one-way ANOVA was used for the statistical analysis. (b) Immunofluorescence staining using anti-p16 and anti-α-actinin antibodies showed the level change of p16. The tissue section thickness is 6 μm. Scale bars represent 100 μm. (c) Immunohistochemistry staining using anti-p16 antibody showed the level change of p16. The tissue section thickness is 6 μm. Scale bars represent 100 μm. (d) Relative levels of SASP containing IL-1, IL-3 and TNF-α were analyzed by qRT-PCR. n = 3 to 4. *P < .01. Data are mean ± SEM; Two-tailed t-test was used for the statistical analysis.
2.3. HO-1 attenuates cardiomyocytes senescence under stimulation of H2O2
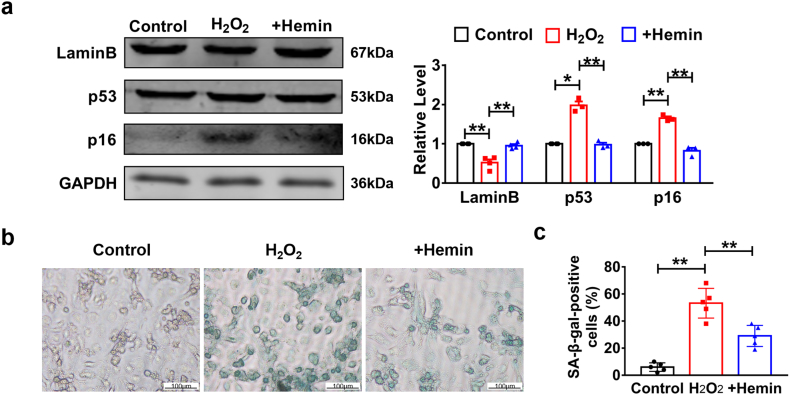
Aging is a physiological and pathological process. It occurs during the entire life of beings and is made up of cellular senescence. As the growth of organism, accumulation of massive damaged and dead cardiomyocytes leads to heart aging and dysfunction. Although the mechanism of cellular senescence has been clearly understood in aged mice, the role it plays in MI mice is still unknown. To explore the role of senescence in vitro, NMCMs were treated with H2O2. Characterization of p53 and p16 in the presence of H2O2 in NMCMs revealed the existence of senescence in vitro (Fig. 4a). Furthermore, we found an increase in expression level of LaminB and decrease in p53 and p16 after treatment of hemin (10 μM) for 24 h compared to H2O2 group (Fig. 4a). Specific SA-β-gal staining of NMCMs with H2O2 treatment also indicated that cardiomyocytes senescence played an important role in MI injury in vitro and could be attenuated by HO-1 induced with hemin treatment (Fig. 4b–c). To prove that heme dependent effects on cardiomyocytes senescence are primarily due to HO-1 induction, we designed RNA interference experiments. SA-β-Gal staining showed that transfection of HO-1 siRNA significantly reversed the effects of hemin and significantly inhibited the activation of β-galactosidase induced by H2O2 while NC group had no such effect (Fig. S1a). In addition, western blot analysis showed that knockdown of HO-1 abolished the effects induced by hemin pretreatment (Fig. S1c). These data show that hemin plays the protective role, at least partially, by induction of HO-1 in cardiomyocytes exposed to H2O2.
Fig. 4.
HO-1 repressed cardiomyocytes senescence under the treatment of H2O2 in vitro. Primary cardiomyocytes were treated with H2O2 (50 μM) for 3 h and hemin (10 μM) for 24 h. (a) The expression levels of LaminB, p53 and p16 analyzed by western blot. n = 3. *P < .01, **P < .05. Data are mean ± SEM; one-way ANOVA was used for the statistical analysis. (b) and (c) SA-β-gal activity analyzed by Senescence β-Galactosidase Staining Kit. Scale bars represent 100 μm n = 4. **P < .05. Data are mean ± SEM; Two-tailed t-test was used for the statistical analysis.
The products of heme degradation, CO and bilirubin, catalyzed by HO-1 also have significant effects in heart diseases. Increasing evidence suggests that CO plays an important homeostatic and cytoprotective role. CO-releaser CORM-2 provides cardioprotective effect against acute doxorubicin-induced cardiotoxicity in mice by implementing antioxidant and antiapoptotic effects [23]. Bilirubin is a potent antioxidant generated intracellularly during the degradation of heme by HO-1. Clark et al. found that exogenously administered bilirubin significantly restored myocardial function, alleviated infarct size and improved mitochondrial dysfunction in I/R rats [24]. According to the previous studies, we further detected the effects of CO and bilirubin in cardiomyocytes senescence. NMCMs were pretreated with CORM-2 or bilirubin respectively. SA-β-gal staining indicated that the treatment of CORM-2 or bilirubin significantly attenuated the activation of β-galactosidase induced by H2O2 (Fig. S2a). Western blot showed that pretreatment of CORM-2 or bilirubin reversed the dysregulation of LaminB and p53 triggered by H2O2 stimulation (Fig. S2b-c). These results suggest that the products of HO-1, CO and bilirubin, have the potential to attenuate cardiomyocytes senescence.
2.4. Hemin promotes HO-1 production and inhibits cardiomyocytes senescence in mice with MI
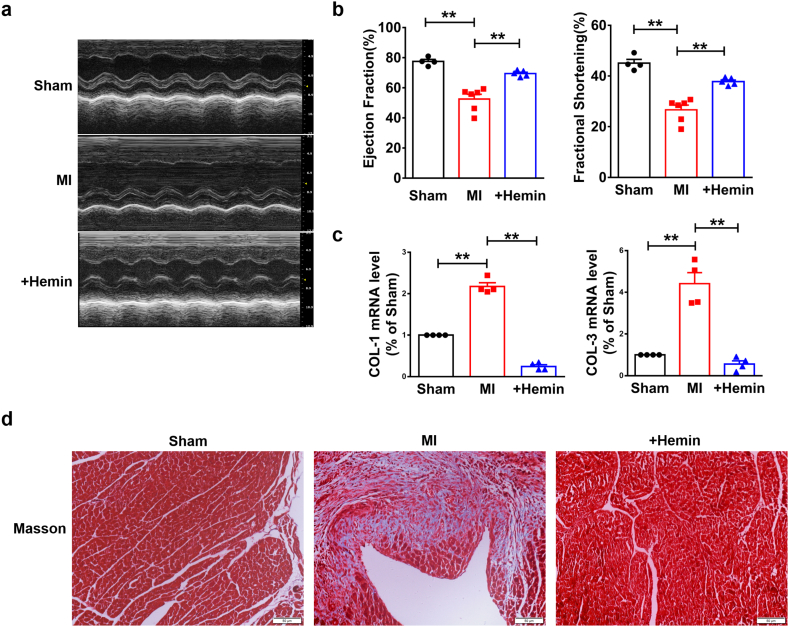
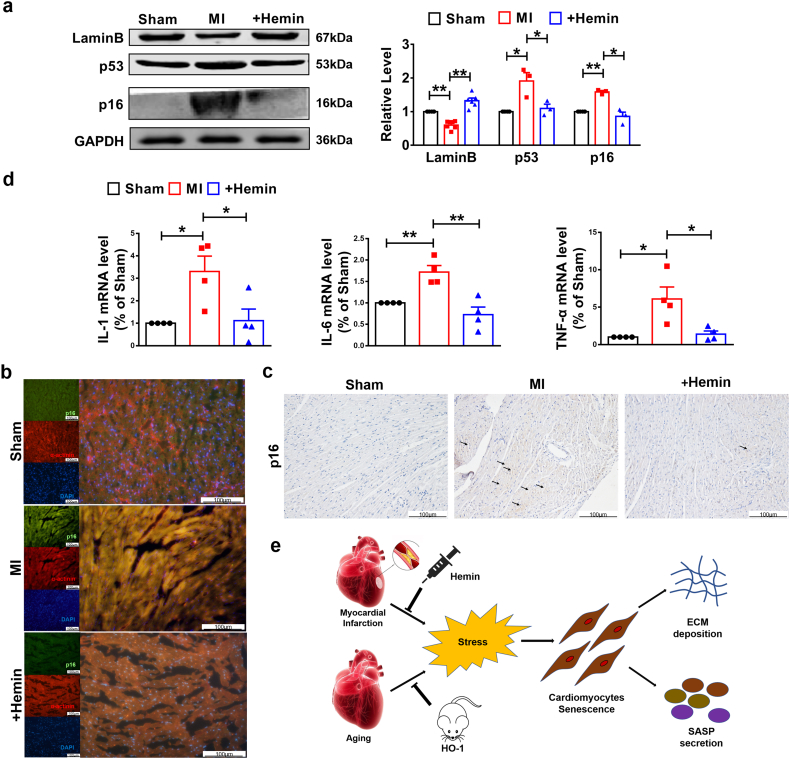
Next, we examined whether HO-1 played the same role in MI mice. Firstly, echocardiographic assessment showed the impaired EF and FS in MI mice, which were improved after treatment with hemin (Fig. 5a–b). Meanwhile, qRT-PCR analysis showed that treatment of hemin significantly ablated the up-regulated mRNA levels of Col 1α1 and Col 3α1 in MI mice (Fig. 5c). Masson staining indicated that hemin-driven HO-1 overexpression repressed the deposition of ECM in WT mice compared to MI mice (Fig. 5d). These data demonstrate that HO-1 protects heart function against MI. More importantly, the protein level of LaminB was down-regulated in MI mice, along with the up-regulation of p16 and p53, which were reversed by treatment with hemin (Fig. 6a). In addition, increased protein level of p16 was detected in MI mice by immunofluorescence and immunohistochemistry staining (Fig. 6b–c). Furthermore, qRT-PCR analysis determined increase of SASP levels containing IL-1, IL-6 and TNF-α, which were inhibited by treatment with hemin (Fig. 6d). All these results demonstrate that enhanced expression of HO-1 induced by hemin attenuates MI injury triggered cardiomyocytes senescence.
Fig. 5.
Overproduction of HO-1 induced by hemin improves heart function of mice with MI injury. Mice were 6–8 weeks. The model of MI was established by ligating left anterior descending coronary artery for a week. Mice in MI + Hemin group were i.p. injected with 20 mg/kg (once every other day) of hemin. (a) Representative short-axis M-mode images from Sham, MI and MI + Hemin group. (b) Ejection Fraction (EF) and Fractional Shortening (FS) of Sham, MI and MI + Hemin group were detected by Echocardiographic assessment. n = 4. **P < .05. Data are mean ± SEM; Two-tailed t-test was used for the statistical analysis. (c) Relative mRNA levels of Col 1α1and Col 3α1 analyzed by qRT-PCR. n = 4. **P < .05. Data are mean ± SEM; Two-tailed t-test was used for the statistical analysis. (d) Masson staining showed the deposition of ECM. Scale bars represent 50 μm. n = 3.
Fig. 6.
Enhanced expression of HO-1 inhibited senescence induced MI injury. Mice were 6–8 weeks. The model of MI was established by ligating left anterior descending coronary artery for a week. Mice in MI + Hemin group were i.p. injected with 20 mg/kg (once every other day) of hemin (a) Western blot detected the expression level of LaminB, p53 and p16. n = 3. *P < .01, **P < .05. Data are mean ± SEM; one-way ANOVA was used for the statistical analysis. (b) and (c) p16 level was analyzed by immunofluorescence and immunohistochemistry staining. The tissue section thickness is 6 μm. Scale bars represent 100 μm. (d) Relative mRNA levels of IL-1, IL-3 and TNF-α were analyzed by qRT-PCR. n = 4. *P < .01, **P < .05. Data are mean ± SEM; Two-tailed t-test was used for the statistical analysis. (e) Model of the role of HO-1 in cardiomyocytes senescence induced by MI injury and heart aging.
3. Discussion
Collectively, these data suggested that MI induced ischemic injury triggered cardiomyocyte senescence which can also be attenuated by overexpression of HO-1. Moreover, the aging of heart was accompanied by the accumulation of senescent cardiomyocytes and forced expression of HO-1 in transgenic mice therefore relieving the development of aging. More importantly, increased expression of HO-1 improved heart function in mice with MI and in HO-1 transgenic mice (Fig. 6e).
Cellular senescence plays an important role in tissue remodeling during development and in organ damage. Accelerated senescence of epithelial cells plays a role in IPF pathogenesis by perpetuating abnormal epithelial-mesenchymal interactions [25]. A recent study by Wiley CD et al. indicated that mitochondrial dysfunction induced a senescence response which includes the abnormal secretion of SASP and the activation of AMPK and p53 [26]. However, the role of senescence in MI was poorly reported. Here we firstly found that ischemic injury induced cardiomyocyte senescence in MI mice. Ischemic injury caused by MI initiated cell autophagy, apoptosis and immune-inflammatory reaction for clearance of damaged organelles and cells due to DNA damage, oxidative stress and mitochondrial dysfunction, which were also the reasons, at least partially, for cellular senescence [27]. Therefore, we detected the expression levels of p16 and p53, two canonical senescence related pathway that trigger the activation of cellular senescence and found an increased level of p16 and p53 in MI mice. Furthermore, up-regulated enzyme activity of SA-β-gal and secretion of SASP, a critical marker of senescence, including IL-1, IL-6 and TNF-α were also detected. These data show that cardiomyocyte senescence participates in the development of MI. Consistent with previous reports, MI was characterized by cardiomyocyte death and ventricular remodeling, as well as abnormal proliferation and transdifferentiation of fibroblasts. We firstly demonstrated that cardiomyocytes senescence was involved in the progress of myocardial ischemic injury and contributed to the decrease of heart function shown by the reduced EF and FS. A recent study showed that injury induced cellular senescence enhanced in vivo reprogramming in skeletal muscle [28]. On the other hand, Baker et al. found the negative effects of senescence on organ functionality during aging [29]. These finds suggest that cellular senescence is not only a reason for organ aging, but also plays more function than we considered during the development of different organs. Collectively, senescence is a double-edged sword that participates in opposite effects in different pathophysiologic processes. More works needs to be done to further explore the mechanism senescence played in MI.
The mechanisms underlying the cardioprotective actions of HO-1 have not been fully elucidated. Chronic HO-1 activation by prolonged administration of hemin improved survival and exerted protective effects in a rat model of myocardial ischemia by exerting a potent antioxidant activity and disrupting multiple levels of the apoptotic and inflammatory cascade [30]. HO-1 induction improved cardiac function following MI by upregulating p-AKT levels with a concomitant inhibition of pGSK3β leading to preserved mitochondrial membrane potential [31]. Here we demonstrated that the cardioprotective effects of HO-1, at least in part, by inhibiting cellular senescence. These findings are somewhat surprising in light of previous studies. It was reported that the enhanced expression of HO-1 protected chondrocytes against stress induced cellular senescence [32]. Moreover, Clérigues V et al. found that HO-1 mediated the protective effects on senescence in osteoarthritic osteoblasts [33]. These findings suggest the possibility of HO-1 to take effect by the regulation of senescence in heart disease. We identified that induction of HO-1 by treatment with hemin inhibited the secretion of SASP in MI mice. Furthermore, the increased expression of HO-1 blunted the deposition of ECM and improved heart function in mice suffering from ischemic injury. In vitro studies demonstrated that hemin pretreatment significantly inhibited the activation of β-galactosidase and prevented cardiomyocytes from H2O2 -induced senescence. Transfection of HO-1 siRNA abolished the protective effect of hemin administration, which indicated that hemin played the cardioprotective role, at least in part, by overexpression of HO-1. Issan Y et al. found that HO-1 induction played a role in cardioprotection against hypoxic damage in cardiomyocytes [31]. Another study showed that HO-1 promoted neovascularization after myocardial infarction by modulating the expression of HIF-1α, SDF-1α and VEGF-B [15]. However, our findings firstly indicated that overexpression of HO-1 improved heart function following MI via repressing cardiomyocyte senescence. Antioxidative effect of HO-1 is widely known and oxidative stress is, in part, a reason for senescence. Therefore, we hypothesized that HO-1 attenuated cellular senescence by modulating the degradation of ROS. However, the underlying mechanism is still poorly understood and need more exploration. In a previous report, Ben Mordechai and colleagues have demonstrated that a targeted hemin-dependent intervention, in which hemin was specifically targeted with a liposome-based approach, can have protective effects in a similar in vivo model of MI. Lipid-based drug carriers offered great potential for improving the therapeutic efficacy in MI. More work is required to do, including the use of this liposome-based approach, in our further study that helps us to target a single type of cell and detect the function of the HO-1 induction on cardiomyocytes in vivo.
On the other hand, products of hemin degradation, CO and bilirubin, were reported to mediate the cardioprotective effect. A previous study by Clark et al. shown that CO-releaser CORM-3 protects isolated myocytes against hypoxia/reoxygenation injury and isolated rat hearts against ischemia-reperfusion injury [34]. Yiru Guo et al. found that CORM-3 plays the cardioprotective role by decreasing the myocardial infarct size in vivo [35]. Bilirubin is regarded as a waste product but it is increasingly appreciated that endogenous bilirubin has strong anti-oxidative properties, which are attributed to its ability to scavenge peroxyl radicals and to inhibit low density lipoprotein (LDL) oxidation [36]. In our study, we found that CO and bilirubin attenuated the activation of β-gal induced by H2O2 stimulation. Pretreatment of CORM-2 and bilirubin reversed the dysregulation of senescence related proteins, p53 and LaminB. It described a new mechanism of HO-1 products and suggested that hemin has a cardioprotective effect which depends on the action of HO-1 and its degraded products, CO and bilirubin.
A main feature of aged organisms is the accumulation of cellular senescence [37], a state of permanent cell cycle arrest in response to different damaging stimuli. Wang et al. evaluated that HO-1 induction in the failing heart was an important cardioprotective adaptation that attenuated pathological left ventricular remodeling by inhibition of apoptosis and mitochondria function [38]. We found that forced expression of HO-1 inhibited cardiomyocyte senescence of aged heart. Overexpression of HO-1 significantly reversed the decline of heart function in aging mice. These findings revealed a novel way that HO-1 played in heart aging.
In conclusion, the present study describes that ischemic injury triggered cardiomyocyte senescence in mice with MI and highlights the beneficial role of HO-1 on attenuation of cellular senescence during the development of MI and heart aging. These findings may provide a new sight to the connection between cellular senescence and heart dysfunction and have implication for the treatment of MI and improvement of heart aging.
4. Material and Methods
4.1. Animal experiments
All animal experiments were performed according to the protocols approved by the Institutional Animal Ethics Committee of Harbin Medical University. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (2011, 8th Ed.). Male C57BL/6 mice (20–30 g; 6–8 weeks old) were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and randomly divided into three groups: CTL (control; sham-operated), MI (MI model with LAD for 7 days), and MI + Hemin. MI model was established as described previously [39,40]. Briefly, all mice were anesthetized under intraperitoneal (i.p.) avertin and placed in a supine position on a heating pad (37 °C). Animals were intubated with a 19 G stump needle and ventilated with room air using a MiniVent Type 845 mouse ventilator (Hugo Sachs Elektronik-Harvard Apparatus, Germany; stroke volume 250 μl, respiratory rate 120 breaths/min). MI was induced by permanent ligation of the left anterior descending artery (LAD) with a 7–0 prolene suture for 7 days. Sham-operated animals served as surgical controls and were subjected to the same procedures as the experimental animals with the exception that the LAD was not ligated. Mice in MI + Hemin group were intraperitoneal injected with hemin (20 mg/kg) from Sigma-Aldrich (Cambridge, MA, USA) at the day before MI surgery and administrated hemin every other day. Hemin was prepared as follows: solutions in 0.2 M NaOH were freshly prepared before administration, adjusted to pH 7.4 with phosphate buffer and diluted with saline. Transgenic mice were generated by the standard pronuclear injection technique using C57BL/6 mice as we previously reported [41]. For construction of the transgene, mouse HO-1 cDNA was cloned into a pCAGG plasmid. Expression of mouse HO-1 cDNA was driven by the chicken β-actin promoter linked to a human cytomegalovirus immediate-early enhancer, followed by first exon and intron of chicken β-actin. Mouse HO-1 transcript, with its own stop codon and poly(A) signal, was followed by a rabbit β-globin poly(A) sequence. The mRNA transcript of the transgene therefore consists of part of the first exon of chicken β-actin, which is transcribed but not translated, followed by mouse HO-1 cDNA. All of the animals were genotyped by PCR amplification of DNA extracted from the tails of mice to assess the presence of the mouse HO-1 transgene. The region from the exon of the pCAGG vector to exon 1 of HO-1 was amplified with the following primers: 5′ GCC TTC TTC TTT TTC CTA CAG CTC 3′ and 5′ GGC ATG TCG GGC TGT GGAC 3′.
All mice were housed under identical conditions, and were given water and food ad libitum. LV tissues were harvested for biochemical assays from 7-days MI or sham surgeries mice and 16-months old mice. We collected border area from LV tissues post LAD ligation and tissues were immediately snap frozen.
4.2. Cell culture and treatment
Primary mice cardiomyocytes were cultured as previously described [42]. 1-to 3-day-old C57BL/6 mouse were anesthetized using 4–5% isoflurane-inhalation anesthesia. Cardiac tissues were digested by trypsin. After centrifugal selection, digested cells were resuspended with DMEM (Biological Industries, Shanghai, China) containing 10% fetal bovine serum (FBS, Biological Industries) and cultured in culture flask (NEST Biotechnology Co. Ltd., Jiangsu, China) for 1.5 h. And then, cardiomyocytes were purified by differential adhesion and treated with 0.1 mmol/l 5-bromo-2-deoxyuridine (BrdU, Sigma-Aldrich,) to inhibit the growth of cardiac fibroblast. Cells were cultured for 48 h to grow adhered to the wall. After 48 h, cardiomyocytes were treated with hemin (10 μM) to induce the expression of HO-1. Then, cells were treated with H2O2 (Sigma-Aldrich) 24 h after treatment of hemin at a final concentration of 50 μM for 3 h. For the H2O2 + CORM-2 group, cardiomyocytes were pretreated with CORM-2 (100 μM) for 1 h before the administration of H2O2. For the H2O2 + Bilirubin group, bilirubin (20 μM) was treated before the administration of H2O2 for 12 h [43]. Cardiomyocytes were cultured 10 days to induce aging and hemin was administrated every other day. Hemin and bilirubin were prepared as follows: solutions in 0.2 M NaOH were freshly prepared before administration, adjusted to pH 7.4 with phosphate buffer and diluted with saline. CORM-2 was dissolved in DMEM.
4.3. siRNA Transfection
For transfection, cardiomyocytes were incubated with serum-free medium. The siRNA and Lipofectamine 2000 (Invitrogen, Carlsbad, CA) were separately mixed with 500 μl of Opti-MEM ® I Reduced Serum Medium (Gibco, Grand Island, NY) for 5 min. Then, the two mixtures were combined and incubated for 15 min. The Lipofectamine; siRNA mixture was added to the cells and incubated at 37 °C for 6 h. Then, we changed the serum-free medium to 10% serum medium and prepared for further study. The siRNA of HO-1 synthesized by GenePharma (shanghai, China) and the sequence of siRNA refers to the previous study by Chao XJ et al. [44]. HO-1 siRNA: sense: 5′- CCACACAGCACUAUGUAAATT-3′, antisense 5′- UUUACAUAGUGCUGUGUGGTT-3′. NC: sense: 5′- UUCUCCGAACGUGUCACGUTT-3′. antisense: 5′- ACGUGACACGUUCGGAGAATT-3′.
4.4. Immunofluorescence
Frozen sections of C57BL/6 mice heart were prepared using Olympus IX73 microscope (Olympus, Valley, PA). The sections were fixated in 4% paraformaldehyde (Solarbio, Beijing, China) at room temperature for 20 min and washed 3 times for 5 min each wash. Penetration were performed with 0.5% Triton X-100 at room temperature for 1 h. Sections were washed 3 times and blocked with normal goat serum at 37 °C for 1.5 h. After 3 times' washes, sections were incubated with anti-p16 (Santa Cruz Biotechnology, Texas, USA) antibody at 4 °C overnight. The next day, sections were incubated with the FITC-conjugated goat anti-mouse antibodies at room temperature for 1 h, followed by staining of DAPI (F. Hoffmann-La Roche, Basel, Switzerland) for 5 min. Immunostaining was observed under microscope (Olympus America Inc., Center Valley, USA).
4.5. Immunohistochemistry
Deparaffinization and rehydration of cardiac tissue sections were reached in dimethylbenzene and graded ethanol, followed by treatment of sections with 3% H2O2 for 10 min. The sections were heated with antigen retrieval buffers to repair antigen for 10 min at 95 °C and cooled to room temperature. Sections were blocked with 5% bovine serum albumin at 37 °C for 1.5 h. After 3 times' washes, sections were incubated with anti-p16 antibody (Affinity Biosciences, Cat# AF-0288, 1:250) at 4 °C overnight. The next day, sections were incubated with the HRP conjugated secondary antibodies (ZsBio, Beijing, China) at room temperature for 1 h and colored by DAB (ZsBio). Nucleus were stained with Mayers hematoxylin from Solarbio (Beijing, China).
4.6. Senescence associated β-galactosidase (SA-β-Gal) staining
Senescence β-Galactosidase Staining Kit were purchased from Beyotime (Jiangsu, China). Mouse primary cardiomyocytes in six-well culture plates (Nest Biotechnology, Jiangsu, China) were washed in PBS for one time and fixed in staining fixture (1 ml each well) for 15 min in room temperature. After washing 3 times in PBS, cells were incubated with fresh staining solution at 37° overnight. Observation with light microscope (Olympus America Inc.).
4.7. Western blot
Samples were separated on 12% SDS-PAGE gels. Proteins on gel were transferred onto nitrocellulose membranes (Pall Crop., Port Washington, NY, USA). The membranes were blocked in PBS with 5% skimmed milk for 1.5 h at room temperature. Before incubation with the first antibody, the membranes were washed with PBS. Membranes were incubated with diluted antibody at 4 °C overnight. The next day, the membranes were washed with PBST (PBS containing 5% Tween-20) 3 times, which is followed by incubation with the secondary antibody diluted in PBS with 5% skimmed milk for 50 min at room temperature. Then, the membranes were washed 3 times again. Odyssey Infrared Scanning System (Gene Co. Ltd., Hongkong, China) was used to detect membranes. The western blot results were analyzed by Image Studio software and normalized with respect to loading control.
The antibody resources and dilutions are as follows:
Antibody against HO-1 (Cat# 10701-1-AP, RRID: AB_2118685, 1:500) and GAPDH (Cat# 60004-1-Ig, RRID: AB_2107436, 1:2000), p53 (Cat# 10442-1-AP, RRID: AB_2206609, 1:500) and LaminB (Cat# 12987-1-AP, RRID: AB_2136290) were obtained from Proteintech Group (Wuhan, China). Antibody against p16 (Cat# sc-1661, RRID: AB_628067, 1:500) were obtained from Santa Cruz Biotechnology (Texas, USA). The secondary antibodies Goat Anti-Mouse IgG, IRDye® 800CW Conjugated antibody (Cat# 926-32210, RRID: AB_621842, 1:8000) and Goat Anti-Rabbit IgG, IRDye® 800CW Conjugated antibody (Cat# 926-32211, RRID: AB_621843, 1:8000) were obtained from LI-COR Biosciences (Lincoln, Nebraska, USA).
4.8. qRT-PCR analysis
Total RNA was isolated from tissues and cultured cells using a standard protocol (Invitrogen, Carlsbad, USA). Concentration and purity of RNA were detected by Nano-drop 8000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, USA), 500 ng of total RNA were converted into cDNA for each sample. qPCR was performed using SYBR Green (Thermo Fisher Scientific). Relative expression levels of mRNAs were calculated based on Ct values and normalized to GAPDH.
4.9. Statistical analysis
All the data are presented as means ± SEM. Statistical analyses were assessed with t-test of two group comparisons. We used GraphPad Prism 5.0 for statistical analyses. A value P < .05 was considered as statistically significant.
Acknowledgments
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81770284 to Hongli Shan); and the National Natural Science Foundation of China (81673425, 81872863 to Yuhong Zhou). The National Natural Science Foundation of China (81473213 to Chaoqian Xu). National Key R&D Program of China (2017YFC1307403 to Baofeng Yang), National Natural Science Foundation of China (81730012 to Baofeng Yang).
Declaration of interests
All authors have no competing interests to declare, financial or otherwise.
Author contributions
X. Gao and H.-L. Shan contributed to the design of research; H. Liang also contributed to the design of research and performed the data analysis; T. Li, L. Zhang, R. Yang and L. Yue guided the cellular experiments; M. Zhang, Y. Dong and Y. Zhou conducted the animal experiments H. Liang performed the data analysis. H.-T. Shan wrote the manuscript; B. Yang and C. Xu edited the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.11.056.
Contributor Information
Haihai Liang, Email: lianghaihai@ems.hrbmu.edu.cn.
Xu Gao, Email: gaoxu@hrbmu.edu.cn.
Hongli Shan, Email: shanhongli@ems.hrbmu.edu.cn.
Appendix A. Supplementary data
Supplementary material
References
- 1.Hernandez-Segura A., Nehme J., Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28(6):436–453. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Bent E., Gilbert L., Hemann M. A senescence secretory switch mediated by PI3K/AKT/mTOR activation controls chemoprotective endothelial secretory responses. Genes Dev. 2016;30(16):1811–1821. doi: 10.1101/gad.284851.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohanna M., Giuliano S., Bonet C., Imbert V., Hofman V., Zangari J. Senescent cells develop a PARP-1 and nuclear factor-{kappa}B-associated secretome (PNAS) Genes Dev. 2011;25(12):1245–1261. doi: 10.1101/gad.625811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capell B., Drake A., Zhu J., Shah P., Dou Z., Dorsey J. MLL1 is essential for the senescence-associated secretory phenotype. Genes Dev. 2016;30(3):321–336. doi: 10.1101/gad.271882.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demaria M., Ohtani N., Youssef S., Rodier F., Toussaint W., Mitchell J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho J., Yusuf R., Kook S., Attar E., Lee D., Park B. Purinergic P2Y₁₄ receptor modulates stress-induced hematopoietic stem/progenitor cell senescence. J Clin Invest. 2014;124(7):3159–3171. doi: 10.1172/JCI61636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh J., Lee Y., Wagers A. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20(8):870–880. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otterbein L.E., Foresti R., Motterlini R. Heme oxygenase-1 and carbon monoxide in the heart: The balancing act between danger signaling and pro-survival. Circ Res. 2016;118(12):1940–1959. doi: 10.1161/CIRCRESAHA.116.306588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouard S., Otterbein L.E., Anrather J., Tobiasch E., Bach F.H., Choi A.M. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192(7):1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orozco L.D., Kapturczak M.H., Barajas B., Wang X., Weinstein M.M., Wong J. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res. 2007;100(12):1703–1711. doi: 10.1161/CIRCRESAHA.107.151720. [DOI] [PubMed] [Google Scholar]
- 11.Lee T.S., Chau L.Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8(3):240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 12.Sharma H., Maulik N., Gho B., Das D., Verdouw P. Coordinated expression of heme oxygenase-1 and ubiquitin in the porcine heart subjected to ischemia and reperfusion. Mol Cell Biochem. 1996;157(1–2):111–116. doi: 10.1007/BF00227888. [DOI] [PubMed] [Google Scholar]
- 13.Yet S., Perrella M., Layne M., Hsieh C., Maemura K., Kobzik L. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103(8):R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X., Wei J., Peng D.H., Layne M.D., Yet S.F. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes. 2005;54(3):778–784. doi: 10.2337/diabetes.54.3.778. [DOI] [PubMed] [Google Scholar]
- 15.Lakkisto P., Kyto V., Forsten H., Siren J.M., Segersvard H., Voipio-Pulkki L.M. Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1alpha, SDF-1alpha and VEGF-B. Eur J Pharmacol. 2010;635(1–3):156–164. doi: 10.1016/j.ejphar.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 16.Chen H., Zheng X., Zheng Y. Age-associated loss of Lamin-B leads to systemic inflammation and gut hyperplasia. Cell. 2014;159(4):829–843. doi: 10.1016/j.cell.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H., Zheng X., Xiao D., Zheng Y. Age-associated de-repression of retrotransposons in the Drosophila fat body, its potential cause and consequence. Aging Cell. 2016;15(3):542–552. doi: 10.1111/acel.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helman A., Klochendler A., Azazmeh N., Gabai Y., Horwitz E., Anzi S. p16(Ink4a)-induced senescence of pancreatic beta cells enhances insulin secretion. Nat Med. 2016;22(4):412–420. doi: 10.1038/nm.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johmura Y., Shimada M., Misaki T., Naiki-Ito A., Miyoshi H., Motoyama N. Necessary and sufficient role for a mitosis skip in senescence induction. Mol Cell. 2014;55(1):73–84. doi: 10.1016/j.molcel.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Rodier F. Detection of the senescence-associated secretory phenotype (SASP) Methods Mol Biol. 2013;965:165–173. doi: 10.1007/978-1-62703-239-1_10. [DOI] [PubMed] [Google Scholar]
- 21.Kang C., Xu Q., Martin T.D., Li M.Z., Demaria M., Aron L. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349(6255):aaa5612. doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acosta J.C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J.P. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15(8):978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soni H., Pandya G., Patel P., Acharya A., Jain M., Mehta A.A. Beneficial effects of carbon monoxide-releasing molecule-2 (CORM-2) on acute doxorubicin cardiotoxicity in mice: role of oxidative stress and apoptosis. Toxicol Appl Pharmacol. 2011;253(1):70–80. doi: 10.1016/j.taap.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Clark J.E., Foresti R., Sarathchandra P., Kaur H., Green C.J., Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278(2):H643–H651. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 25.Kuwano K., Araya J., Hara H., Minagawa S., Takasaka N., Ito S. Cellular senescence and autophagy in the pathogenesis of chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF) Respir Investig. 2016;54(6):397–406. doi: 10.1016/j.resinv.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Wiley C.D., Velarde M.C., Lecot P., Liu S., Sarnoski E.A., Freund A. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016;23(2):303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorchner H., Poling J., Gajawada P., Hou Y., Polyakova V., Kostin S. Myocardial healing requires Reg3beta-dependent accumulation of macrophages in the ischemic heart. Nat Med. 2015;21(4):353–362. doi: 10.1038/nm.3816. [DOI] [PubMed] [Google Scholar]
- 28.Chiche A., Le Roux I., von Joest M., Sakai H., Aguin S.B., Cazin C. Injury-Induced Senescence Enables in Vivo Reprogramming in Skeletal Muscle. Cell Stem Cell. 2017;20(3):407–414. doi: 10.1016/j.stem.2016.11.020. [e4] [DOI] [PubMed] [Google Scholar]
- 29.Baker D.J., Childs B.G., Durik M., Wijers M.E., Sieben C.J., Zhong J. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collino M., Pini A., Mugelli N., Mastroianni R., Bani D., Fantozzi R. Beneficial effect of prolonged heme oxygenase 1 activation in a rat model of chronic heart failure. Dis Model Mech. 2013;6(4):1012–1020. doi: 10.1242/dmm.011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Issan Y., Kornowski R., Aravot D., Shainberg A., Laniado-Schwartzman M., Sodhi K. Heme oxygenase-1 induction improves cardiac function following myocardial ischemia by reducing oxidative stress. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim K.M., Park S.E., Lee M.S., Kim K., Park Y.C. Induction of heme oxygenase1 expression protects articular chondrocytes against cilostazolinduced cellular senescence. Int J Mol Med. 2014;34(5):1335–1340. doi: 10.3892/ijmm.2014.1918. [DOI] [PubMed] [Google Scholar]
- 33.Clerigues V., Guillen M.I., Castejon M.A., Gomar F., Mirabet V., Alcaraz M.J. Heme oxygenase-1 mediates protective effects on inflammatory, catabolic and senescence responses induced by interleukin-1beta in osteoarthritic osteoblasts. Biochem Pharmacol. 2012;83(3):395–405. doi: 10.1016/j.bcp.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Clark J.E., Naughton P., Shurey S., Green C.J., Johnson T.R., Mann B.E. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93(2):e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 35.Guo Y., Stein A.B., Wu W.J., Tan W., Zhu X., Li Q.H. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004;286(5):H1649–H1653. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitek L., Schwertner H.A. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Adv Clin Chem. 2007;43:1–57. doi: 10.1016/s0065-2423(06)43001-8. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang G., Hamid T., Keith R.J., Zhou G., Partridge C.R., Xiang X. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121(17):1912–1925. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang B., Lin H., Xu C., Liu Y., Wang H., Han H. Choline produces cytoprotective effects against ischemic myocardial injuries: evidence for the role of cardiac m3 subtype muscarinic acetylcholine receptors. Cell. Physiol. Biochem. 2005;16(4–6):163–174. doi: 10.1159/000089842. [DOI] [PubMed] [Google Scholar]
- 40.Qian L., Huang Y., Spencer C.I., Foley A., Vedantham V., Liu L. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hui Y., Wang D., Li W., Zhang L., Jin J., Ma N. Long-term overexpression of heme oxygenase 1 promotes tau aggregation in mouse brain by inducing tau phosphorylation. J. Alzheimer's Dis. 2011;26(2):299–313. doi: 10.3233/JAD-2011-102061. [DOI] [PubMed] [Google Scholar]
- 42.He H., Liu X., Lv L., Liang H., Leng B., Zhao D. Calcineurin suppresses AMPK-dependent cytoprotective autophagy in cardiomyocytes under oxidative stress. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2013.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim D.S., Chae S.W., Kim H.R., Chae H.J. CO and bilirubin inhibit doxorubicin-induced cardiac cell death. Immunopharmacol Immunotoxicol. 2009;31(1):64–70. doi: 10.1080/08923970802354762. [DOI] [PubMed] [Google Scholar]
- 44.Chao X.J., Chen Z.W., Liu A.M., He X.X., Wang S.G., Wang Y.T. Effect of tacrine-3-caffeic acid, a novel multifunctional anti-Alzheimer's dimer, against oxidative-stress-induced cell death in HT22 hippocampal neurons: involvement of Nrf2/HO-1 pathway. CNS Neurosci Ther. 2014;20(9):840–850. doi: 10.1111/cns.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material