Abstract
Objective: To review the pharmacology, efficacy, and safety of the first nebulized long-acting muscarinic antagonist (LAMA), glycopyrrolate (GLY)/eFlow closed system (CS) nebulizer, approved for maintenance treatment of chronic obstructive pulmonary disease (COPD). Data Sources: A PubMed search was conducted (January 2000 to July 2018) using the following terms/phrases: nebulized glycopyrrolate, inhalation devices in COPD, long-acting muscarinic antagonists COPD, and COPD survey. Retrieved articles were reviewed to identify additional references. Study Selection and Data Extraction: Primary and review articles on GLY/eFlow CS and other treatment options for patients with COPD were selected. Data Synthesis: Guidelines recommend the use of LAMAs, alone or in combination with long-acting β2-agonists, as maintenance therapy for the majority of patients with COPD. With the range of different devices and bronchodilators now available, treatment can be tailored based on individual needs. The eFlow CS nebulizer delivers GLY rapidly over a 2- to 3-minute period and provides bronchodilation within 30 minutes, lasting 12 hours. Phase 2 dose-finding and phase 3 studies demonstrated sustained statistically significant and clinically important improvements in pulmonary function and patient-reported outcomes with GLY/eFlow CS. Relevance to Patient Care and Clinical Practice: GLY/eFlow CS provides a novel, portable, efficient, and rapid drug delivery system. Conclusions: The recently approved GLY/eFlow CS drug-device combination provides a viable treatment option for patients with COPD, particularly those with conditions that may impair proper use of traditional handheld inhalers.
Keywords: chronic obstructive pulmonary disease, inhalers, bronchodilators, pharmaceutical care, drug development and approval, anticholinergics
Background
Chronic obstructive pulmonary disease (COPD) is characterized by persistent dyspnea, cough, sputum production, and airflow obstruction.1 COPD is usually a consequence of smoking or exposure to toxic inhalants causing airway narrowing because of inflammation and alveolar abnormalities, including emphysematous changes of alveolar wall destruction, alveolar space enlargement, and decreased alveolar wall attachment, causing loss of lung elastic recoil and alveolar support.2 Excessive bronchial mucus secretion and wall thickening also contribute to airflow obstruction.
In the United States, approximately 15.7 million adults have a confirmed diagnosis of COPD, with high rates of underdiagnosis.3 COPD represents the third leading cause of mortality in the United States, with the majority of >155 000 deaths in 2015 caused by lower respiratory tract disease attributable to COPD.4,5 COPD typically progresses with age, especially among those with continued exposure to inhaled toxins such as tobacco smoke. Initiatives are under way to decrease disease burden, including the National Heart, Lung, and Blood Institute’s COPD action plan and the Centers for Medicare and Medicaid Services’ 30-day hospital readmissions reduction program. Pharmacists are an integral part of drug therapy management to deliver continual optimal care to COPD patients.6
Data Selection
A PubMed search (English only) was conducted (January 2000 to July 2018) using the following terms/phrases: nebulized glycopyrrolate, inhalation devices in COPD, long-acting muscarinic antagonists COPD, and COPD survey. Studies of glycopyrrolate (GLY)/eFlow closed system (CS) and articles relating to available treatment and device options in COPD were identified, and references from retrieved articles were reviewed.
COPD Classification and Available Treatment Options
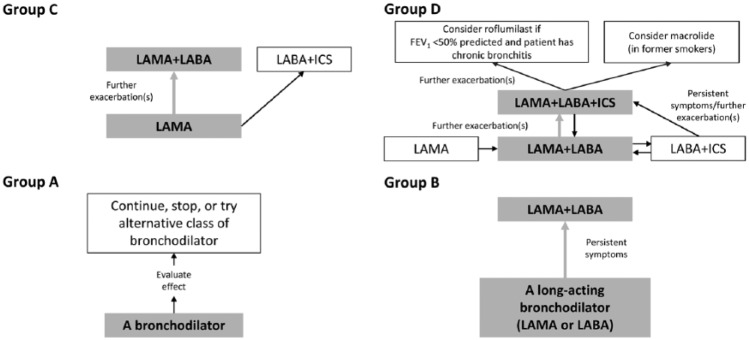
After first demonstrating the presence of airflow obstruction by spirometry and staging of severity according to forced expiratory volume in 1 s (FEV1), the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classifies COPD patients into 4 groups (A, B, C, and D; Figure 1) based on severity of symptoms using the modified British Medical Research Council questionnaire or the COPD Assessment Test, coupled with a patient’s history of exacerbations.1
Figure 1.
Schematic overview of Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018 treatment algorithms by ABCD categories.7 Produced with permission from GOLD.
Abbreviations: FEV1, forced expiratory volume in 1 s; ICS, inhaled corticosteroid; LABA, long-acting β2-agonists; LAMA, long-acting muscarinic antagonists.
Treatment with inhaled bronchodilators provides symptom relief through improved lung function, patient quality of life, exercise tolerance, and reducing COPD exacerbations.1 Although either class of bronchodilator may provide benefit for patients, initial therapy for GOLD group A includes short- and/or long-acting bronchodilators, whereas long-acting muscarinic antagonists (LAMAs) or long-acting β2-agonists (LABAs) are initially recommended for those in group B, LAMAs for group C, and combinations of LAMA/LABA, LAMA and/or inhaled corticosteroids (ICS)/LABA for group D (Figure 1).7 The GOLD guidelines do not specify one LAMA over any other LAMA or LABA. The GOLD guidelines also recommend personalization of treatment escalation and de-escalation based on patient symptoms and exacerbation risk. For patients in groups B and C, combination LAMA/LABA treatment is recommended when monotherapies do not provide sufficient symptom relief, whereas addition of ICS to LABA is an additional approach in group C for patients with persistent or severe exacerbations.7 Addition of ICS—via one single inhaler triple therapy which has been recently approved in the United States8 or through the use of an ICS/LABA plus a LAMA — is recommended for patients in group D who exhibit disease progression or insufficient symptom relief. For patients in group D who experience further exacerbations following triple therapy, roflumilast or chronic macrolides should be considered, depending on patient characteristics.7 Trials of treatment de-escalation are limited and mainly involve the withdrawal of ICS.1,9
Selection of Inhalation Devices in COPD
Inhalation drug therapies are the principal pharmacological means to treat obstructive lung diseases. Aerosol and device characteristics of inhalation therapies are important determinants of drug deposition in the respiratory tract and oropharyngeal cavity, thereby affecting treatment efficacy and safety. Deposition is a function of aerosol particle size, shape and density, anatomy of the lungs, and inhalation pattern.10 Aerosol particle size is usually described on the basis of mass median aerodynamic diameter (MMAD), and the optimal range is 1 to 5 µm (also referred to as the respirable particle range). It has been reported that medium-sized particles (~3 µm) may be more efficacious for bronchodilation than smaller particles.11,12 The geometric standard deviation (GSD) is a measure of the dispersion of particle diameter.10 Aerosols with a GSD of ⩾1.22 are considered polydisperse and are more inclined to be delivered throughout the lungs compared with a monodisperse solution.10 Inhalation devices with a higher proportion of aerosol particles >5 µm in size emit doses less efficiently, with greater oropharyngeal deposition and lower delivery to the lungs, compared with those with a smaller aerosol particle size and more efficient emission.10
Currently available inhalation devices include pressurized metered dose inhalers (pMDIs), dry powder inhalers (DPIs), soft mist inhalers, and nebulizers. There is no single type of device that is preferred over any other in COPD, rather selection is “personalized” for the patient based on the patient’s and prescriber’s preferences, formulary considerations, and the patient’s ability to correctly administer any specific device. Patient characteristics such as age, peak inspiratory flow, baseline lung function, and physical and cognitive disabilities are essential considerations in the selection of the most appropriate device.13-15 Handheld inhalers are by far more widely used compared with nebulizers; however, they are sometimes associated with suboptimal outcomes, especially in patients with hand-breath coordination difficulties or physical and/or cognitive impairments.13 Incorrect pMDI technique has been associated with increased risk of hospitalization, emergency department visits, and oral corticosteroid use.16
Nebulizers, which produce a fine mist and use tidal breathing (normal inspiration and expiration effort), are an alternative to handheld inhalers and may be particularly useful for those patients who have difficulties using handheld inhalers.13 They vary in their efficiency and consistency of drug delivery at the correct dose,10,17 and drugs should be administered using the nebulizer(s) recommended by the manufacturer, unless other sound scientific evidence is available, to ensure best treatment outcomes. The typical time for drug administration through most nebulizers is 10 to 15 minutes. Proper patient education, including repetitive feedback, regarding device use is essential for optimal treatment response.7,10 A survey of pulmonologists showing that whereas 70% discussed proper use of nebulizers with their patients, only 9% provided cleaning and maintenance instructions, highlight some of the gaps in care regarding inhalational therapies.18 Thus, proper patient/device pairing, training, and education are important to ensure proper device use and treatment benefit.1,19-21
LAMAs for the Treatment of COPD
LAMAs are one of the most widely prescribed bronchodilators for maintenance treatment of COPD. The earliest inhaled LAMAs used clinically were parenteral formulations of atropine and GLY. Nebulized atropine, which crosses the blood-brain barrier, was poorly tolerated, whereas GLY, with relatively poor penetration, was better tolerated.22,23 In the early 2000s, tiotropium (TIO), a scopolamine derivative, became the first LAMA available; there are currently 3 additional LAMAs available in the United States (aclidinium, umeclidinium, and GLY), all of which are delivered via handheld inhalers. LAMAs are now also available as fixed-dose combinations with LABAs (formoterol, vilanterol, and olodaterol) and most recently as a triple inhaler of fluticasone furoate, vilanterol, and umeclidinium. Inhaled LAMAs appear to provide similar efficacy and adverse event profiles; selection tends to be based more on the inhalation device, insurance coverage, and consideration of a once-daily or twice-daily dosing regimen, which may affect patient adherence and daytime/nighttime symptom control. Tiotropium has received Food and Drug Administration (FDA) approval for prevention of COPD exacerbations,24 and a meta-analysis of umeclidinium showed that it results in a decreased risk of exacerbations.25 GLY currently does not have an FDA indication for COPD exacerbations because of the lack of a clinical study conducted to determine its effectiveness for this purpose.
Introduction to Nebulized GLY
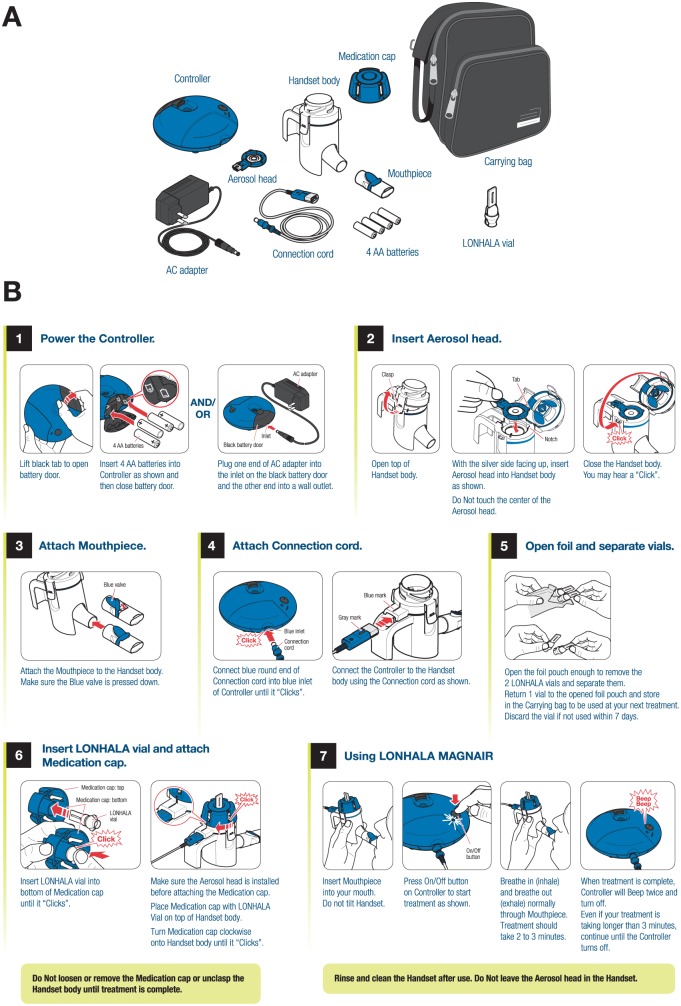
In December 2017, nebulized GLY (LONHALA 25 µg twice daily; Sunovion Pharmaceuticals Inc., Marlborough, MA, USA), administered via the eFlow CS nebulizer (MAGNAIR; PARI Pharma GmbH, Starnberg, Germany), became the first nebulized LAMA approved by the FDA for the maintenance treatment of COPD, including chronic bronchitis and/or emphysema.9 The eFlow CS is a novel, handheld electronic nebulizer that provides short nebulization times (2-3 minutes), silent operation, and portability (direct current [DC] power and battery operated; Figure 2).9,26 The aerosol head consists of a vibrating membrane (piezo electric actuator) with thousands of laser-drilled holes that control particle size and aerosol flow. The vibration aerosolizes the drug solution into a soft mist with a relatively uniform droplet size (GSD of aerosol particles = 1.7). The MMAD of GLY/eFlow CS is 3.7 µm, which is an optimal particle size for bronchodilation.26 It has a relatively high fine-particle fraction (72%) suitable for lung deposition.26 The soft mist allows highly efficient drug deposition, with up to 88% of the nominal drug dose delivered to the central and peripheral lung using tidal breathing.26 The GLY solution is not viscous or a suspension, but cleaning of the aerosol head is needed to minimize clogging, which may cause longer nebulization time. Although this is a very efficient drug delivery system, the cost of the device and the need for cleaning it after each use may affect patient adherence9; however, it is recommended that all nebulizers are cleaned after each use. The aerosol head component may be subject to clogging; however, replacements are provided monthly with each refill. No specific data are currently available from the manufacturer about the effect of not cleaning the aerosol head.
Figure 2.
Components (A) and instructions for use (B) of the GLY/eFlow CS nebulizer (courtesy of Sunovion Pharmaceuticals Inc, Marlborough, MA, and PARI Pharma GmbH, Starnberg, Germany).9
Abbreviations: CS, closed system; GLY, glycopyrrolate.
Currently, the eFlow CS device can administer nebulized GLY only and cannot be used with other nebulized bronchodilators or corticosteroids. In addition, the LONHALA vial should not be used with other nebulizers because administration via other devices has not been studied and it contains too small a volume (1 mL) for delivery through conventional nebulizers.
Because this is the first dedicated drug nebulization device that nonspecialty pharmacies can dispense, it is important for pharmacists to be familiar with the eFlow CS assembly, vial loading, and cleaning. To assemble the eFlow CS nebulizer (Figure 2A), after washing hands, the top of the handset should be unclasped and the aerosol head inserted into the top of the handset; the aerosol head can be inserted only in one position because of a tab on the side.9 The clasp should then be snapped back into position. Then, the patient removes 1 GLY vial from the foil package and places it into the bottom of the medication cap (bottom of vial toward aerosol head) with an audible click to ensure proper placement (Figure 2B). The medication cap with drug vial is then placed onto the handset and twisted clockwise until it clicks. The patient then places the mouthpiece to his or her lips (not covering the blue 1-way valve), pushes the On/Off button, and then begins inhaling and exhaling normally through the mouthpiece for the entire ~2-minute period of nebulization.9 This is in contrast to typical jet nebulizers (that require 10-15 minutes for drug delivery), where the patient moves the nebulizer mouthpiece back and forth to his or her lips throughout the nebulization interval.27 Device cleaning requires careful washing of the handset parts and aerosol head separately in warm soapy water and careful rinsing with warm water to remove all soap.9 Proper nebulizer cleaning and maintenance are essential and decrease the risk of infection.28
The typical monthly retail cost of GLY/eFlow CS is ~$1150, which includes the cost of the nebulizer.29 The costs of other nebulized long-acting bronchodilators Perforomist (formoterol fumarate; Mylan Specialty LP) and Brovana (arformoterol tartrate; Sunovion Pharmaceuticals Inc) are ~$975 (not including the cost of the nebulizer and associated supplies).29 The retail costs of 1 month’s supply of handheld LAMA inhalers are ~$420 for Spiriva Respimat (tiotropium bromide; Boehringer Ingelheim) and ~$330 for Incruse Ellipta (umeclidinium; GlaxoSmithKline).29 These prices vary according to source and payers.
Currently, GLY/eFlow CS is the only nebulized LAMA, and because it is a new product, determining the most appropriate patients for its use will ultimately be determined through application in the patient care setting. As noted previously, personalization of inhalation device is warranted in the management of COPD. Patient types that might benefit from this inhalational product include those with significant cognitive and/or neurological impairment, where the drug is administered by a caregiver or health care provider. Another patient type is one where the administration of the medication must be observed; thus, a shorter administration time may offer an advantage when nebulization is preferred.
Pharmacokinetic/Pharmacodynamic Profile of Nebulized GLY
Similar to other LAMAs, GLY targets M1, M2, and M3 muscarinic receptors, but with a 3- to 5-fold higher affinity for M3 receptors in human airways.30 M3 is the principal receptor responsible for basal tone of airway smooth muscle.7 GLY has a similar onset of effect on human airway smooth muscle as ipratropium, but it dissociates more slowly from receptors and, therefore, has a longer duration of action.30
Prior studies with oral GLY (eg, 4 mg orally as for gastrointestinal disorders) have shown low and variable gastric absorption.31 Therefore, the swallowed fraction will contribute little to the systemic bioavailability of nebulized GLY. Blood levels achieved with the 25-µg inhaled dose are quite low.32 Although nebulized GLY is metabolized by various enzymes, including the cytochrome P (CYP) and cholinesterase families, via first-pass metabolism, it has no in vitro effects on the activity of a wide range of CYP family members; efflux transporters, including MDR1; and uptake transporters such as OATP1.9
In a phase 2, randomized, double-blind, placebo-controlled, dose-ranging study, patients received a single dose of nebulized GLY (range: 12.5-400 µg), with a mean nebulization time of <2 minutes for all doses.33 Absorption occurred rapidly from the lung, with a dose-proportional maximal serum concentration (Cmax) occurring within 15 to 30 minutes of dose administration. The median serum elimination half-life (t1/2) was 1.1 to 1.2 hours for the 0- to 1-hour interval and 2.3 to 7.5 hours for the 0- to 12-hour interval, following administration of 50, 100, 200, or 400 µg doses. All doses of GLY were well tolerated.33
When administered twice daily, nebulized GLY reached steady-state levels and approximately 2- to 3-fold accumulation of systemic GLY within 1 week of continuous treatment.9 At 1 to 10 ng/mL total plasma concentration, approximately 38% to 41% of GLY was bound to plasma proteins. Population pharmacokinetic analyses of COPD patients did not reveal any clinically relevant effects of age (41 to 80 years) or body weight (40.1 to 154.8 kg) on GLY pharmacokinetics.9
Drug Interactions and Dosing in Renal and Hepatic Disease
There are no data available on the effects of renal or hepatic impairment on GLY pharmacokinetics. Similar to other LAMAs,7 GLY is primarily eliminated by renal excretion (85%), with little contribution from metabolism or biliary excretion (5%).9 Although not studied in patients with severe renal impairment,9 careful monitoring is warranted in patients with significant kidney disease. Because hepatic elimination is not a significant contributor to clearance of GLY,9 liver impairment is not expected to alter drug clearance or metabolism. However, when coadministered with cimetidine, there was a 22% increase in systemic exposure to GLY associated with a 23% decrease in clearance.34
Clinical Development: Phase 2 Dose-Finding and Phase 3 Studies
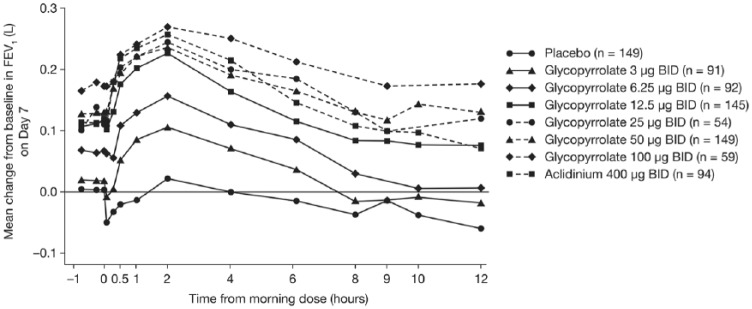
The Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) clinical development program extensively evaluated nebulized GLY.35-37 The primary goal was to achieve an FEV1 increase >100 mL compared with placebo in patients with COPD. Pooled analysis of 2 phase 2 dose-finding studies, GOLDEN 2 and GOLDEN 6,35 in adults with moderate to severe COPD showed that treatment with nebulized GLY resulted in clinically and statistically significant improvements in lung function, at days 7 and 28. The change from baseline in trough FEV1 on day 7 was significantly greater than placebo for all doses of nebulized GLY, except the 3-µg twice-daily dose (Figure 3). The improvements in lung function observed with GLY 25 and 50 µg twice daily were comparable to those seen with aclidinium bromide, and the drug was well tolerated at all doses in both studies.35
Figure 3.
Mean change from baseline in trough FEV1 in the GOLDEN 2 and GOLDEN 6 studies.35 Redrawn with author’s permission.
Abbreviations: FEV1, forced expiratory volume in 1 s; GOLDEN, Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer.
Based on these data,35 phase 3 studies in patients with moderate to severe COPD evaluated GLY 25- and 50-µg twice-daily doses. GOLDEN 3 and GOLDEN 4 were replicate, placebo-controlled, 12-week studies evaluating both doses in patients with moderate to severe COPD (Table 1).37 Compared with placebo, treatment with GLY resulted in significant improvements in trough FEV1 at every time point evaluated. There was also significant improvement in patient health status, measured using St George’s Respiratory Questionnaire (SGRQ), with 25 µg twice daily in GOLDEN 3 and both doses in GOLDEN 4, compared with placebo. However, these improvements in SGRQ total score were less than the minimum clinically important difference (MCID) for SGRQ of 4 units38 and, as such, may not be considered clinically significant. In a GOLDEN 3 substudy, patients receiving GLY had a rapid and sustained increase in FEV1 AUC0-12h, with similar improvements at week 0 and week 12 (Figure S1; see supplemental material available online at http://journals.sagepub.com/home/aop/supplemental-data).37 This finding was consistent with the absence of tolerance effect over time to the bronchodilator effect with LAMAs, whereas tolerance may occur with LABAs.7
Table 1.
Overview of the Study Design and Outcomes of the GOLDEN Phase 3 Clinical Trials.
| Phase 3 Study | Study Design | Treatments | Patient Population | Outcomes |
|---|---|---|---|---|
| GOLDEN 337 | 12-Week, double-blind, placebo-controlled | • Nebulized GLY 25 or 50 µg bid • Placebo |
Total n = 653 • GLY 25 µg bid (n = 217) • GLY 50 µg bid (n = 218) • Placebo (n = 218) |
Efficacy: • Clinically important improvements in placebo-adjusted change from baseline in trough FEV1 at week 12 (0.105L*** and 0.126 L*** with GLY 25 and 50 µg bid, respectively) • Clinically important improvements in placebo-adjusted change from baseline in trough FVC at week 12 (0.149 L*** and 0.167 L*** with GLY 25 and 50 µg bid, respectively) • Statistically significant improvement in placebo-adjusted SGRQ total score with GLY 25 µg bid (−3.072 units*); change in SGRQ with GLY 50 µg bid (−1.848 units) was not statistically significant Safety: • Frequency of TEAEs was highest in patients receiving placebo (52.3%) compared with GLY 25 and 50 µg bid (39.6% and 48.2%, respectively) • The most common TEAEs were cough (placebo: 10.1%; GLY 25 µg: 7.4%; GLY 50 µg: 9.6%) and worsening of COPD (placebo: 8.3%; GLY 25 µg: 5.1%; GLY 50 µg: 10.6%). Dry mouth occurred in 1.4% and 2.3% of patients receiving GLY 25 and 50 µg, respectively, but not in patients receiving placebo • Discontinuations resulting from AEs were more common with placebo (9.6%) compared with GLY 25 and 50 µg bid (3.2% and 3.7%, respectively) |
| GOLDEN 437 | 12-Week, double-blind, placebo-controlled | • Nebulized GLY 25 or 50 µg bid • Placebo |
Total n = 640 • GLY 25 µg bid (n = 214) • GLY 50 µg bid (n = 214) • Placebo (n = 212) |
Efficacy: • Clinically important improvements in placebo-adjusted change from baseline in trough FEV1 at week 12 (0.084 L*** and 0.082 L*** with GLY 25 and 50 µg bid, respectively) • Clinically important improvements in placebo-adjusted change from baseline in trough FVC at week 12 (0.130 L*** and 0.113 L** with GLY 25 and 50 µg bid, respectively) • Statistically significant improvement in placebo-adjusted SGRQ total score with GLY 25 and 50 µg bid (−3.59** and −3.56** units, respectively) Safety: • Frequency of TEAEs was similar in patients receiving placebo (52.4%) and GLY 50 µg bid (53.3%) and lowest with GLY 25 µg bid (47.2%) • The most common TEAEs were cough (placebo: 6.6%; GLY 25 µg: 6.5%; GLY 50 µg: 8.4%), worsening of COPD (placebo: 9.0%; GLY 25 µg: 7.9%; GLY 50 µg: 6.5%), and dyspnea (placebo: 3.8%; GLY 25 µg: 7.5%; GLY 50 µg: 5.1%). Dry mouth occurred in 0.5%, 0.5%, and 0.9% of patients receiving placebo, GLY 25 µg, and GLY 50 µg, respectively • Discontinuations resulting from TEAEs were more common with placebo (9.0%) compared with GLY 25 and 50 µg bid (7.0% and 4.2%, respectively) |
| GOLDEN 536 | 48-Week, open-label, active-controlled, long-term safety study | • Nebulized GLY 50 µg bid • TIO 18 µg qd DPI |
Total n = 1086 • GLY (n = 620) • TIO (n = 466) |
Efficacy: • Sustained improvement in LSM change from baseline in trough FEV1 over 48 weeks of 0.102 L with GLY 50 µg bid compared with 0.093 L with TIO 18 µg qd • Similar improvements in placebo-adjusted change from baseline in trough FEV1 at week 48 (0.069 and 0.089 L with GLY 50 µg bid and TIO 18 µg qd, respectively) • Similar improvement in placebo-adjusted SGRQ total score with GLY 50 µg bid (−3.07 units) and TIO 18 µg qd (−4.08 units) at week 48 Safety: • Frequency of TEAEs was similar between treatments over 48 weeks (69.4% and 67.0% with GLY 50 µg bid and TIO 18 µg qd, respectively) • Discontinuations resulting from TEAEs were more common with GLY 50 µg bid (10.0%) compared with TIO 18 µg qd (2.8%) • Incidence of CV disease among patients receiving either treatment was similar regardless of their CV risk (high risk: 4.7% and 4.4%; low risk: 2.3% and 3.6%; with GLY 50 µg bid and TIO 18 µg qd, respectively) |
Abbreviations: AE, adverse event; bid, twice daily; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; DPI, dry powder inhaler; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GLY, glycopyrrolate; GOLDEN, Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer; LSM, least squares mean; qd, once daily; SGRQ, St George’s Respiratory Questionnaire; TEAE, treatment-emergent adverse event; TIO, tiotropium.
P < 0.05, **P < 0.01, ***P < 0.001 versus placebo.
Incidence of treatment-emergent adverse events (TEAEs) was lowest among patients treated with 25 µg twice daily in both studies (GOLDEN 3: 52.3%, 39.6%, and 48.2%; GOLDEN 4: 52.4%, 47.2%, and 53.3%; with placebo, GLY 25 µg, and GLY 50 µg, respectively).37 The most common TEAEs reported were cough and COPD worsening and occurred to a similar extent in patients receiving placebo or GLY.37 Discontinuations resulting from TEAEs were more common in patients receiving placebo.37 Although very infrequent, urinary tract infections were slightly more common in patients treated with GLY,37 perhaps related to urinary retention observed with LAMAs.7 The incidences of cardiovascular adverse events and major adverse cardiovascular events (MACEs) were low in both studies.37
GOLDEN 5 was an open-label, active-controlled, long-term safety study comparing GLY 50 µg twice daily with TIO 18 µg, administered via HandiHaler (DPI), once daily in adults with moderate to severe COPD (Table 1).36 Incidence of overall and serious TEAEs were similar among patients treated with GLY or TIO, whereas fewer MACEs occurred with GLY.36 More discontinuations occurred with GLY 50 µg twice daily than TIO in the study; this may be partly a result of the fact that ~30% of participants recruited in both treatment arms of GOLDEN 5 had received TIO prior to the study, resulting in a somewhat “selected” patient population with tolerance to TIO but naïve to GLY. The most frequent TEAEs were COPD worsening and cough. Cough appeared to be numerically more common in the GLY group, possibly owing to the aerosol/airway interactions that sometimes occur with nebulized agents, leading to cough. Exacerbations occurred less often in the GLY group. Improvements in trough FEV1 with GLY were similar to that for TIO.36 The SGRQ MCID was met for TIO (−4.07 units) but not with GLY (−3.07 units). When comparing the proportion of SGRQ responders versus nonresponders in GOLDEN 5, the differences were not statistically significant between the 2 LAMAs at 48 weeks (43.8% for GLY and 44.7% for TIO). The number of individuals who were responders can be considered a more meaningful assessment than the SGRQ total score because it represents the number of patients who achieved a clinically important change in SGRQ (greater than MCID), whereas the total score is an average of the changes in all patients. The outcomes of the phase 3 studies led to the approval of GLY/eFlow CS as the first nebulized LAMA for the maintenance treatment of moderate to severe COPD.9
Relevance to Patient Care and Clinical Practice
Long-acting bronchodilators are an essential part of the chronic pharmacological management of COPD. Until GLY/eFlow CS was approved and marketed, a nebulized LAMA was not available in the United States, thus providing an alternative to handheld inhalers. As pointed out by the GOLD COPD guidelines, no LAMA is preferred over any other, and thus, the main differentiation is the inhalational device and cost. In general, the majority of patients can be managed with handheld LAMA inhalers; however, there may be some patients for whom nebulized delivery may be desirable, such as those with cognitive and/or physical limitations that make using traditional inhalers suboptimal.
GLY is the newest commercially available LAMA in the United States since umeclidinium; GLY has now been marketed as a DPI, pMDI, and via nebulizer. All formulations of GLY approved in the United States are administered twice daily as compared with once-daily LAMAs such as tiotropium and umeclidinium. The eFlow CS device is portable and easy to use, and allows efficient and rapid drug delivery.
Conclusions
The GLY/eFlow CS drug-device combination represents an important advance in the treatment of COPD. The GOLDEN phase 3 trials demonstrated clinically important efficacy outcomes and that nebulized GLY is well tolerated among patients with COPD.36,37 Whereas the efficacy and safety of LAMA monotherapies are well established in patients with moderate to severe COPD,39 the drug-device combination of GLY/eFlow CS provides a portable, patient-friendly, efficient, and rapid drug delivery system.26,33,35-37
Supplemental Material
Supplemental material, Supplementary_Figure for Glycopyrrolate/eFlow CS: The First Nebulized Long-Acting Muscarinic Antagonist Approved to Treat Chronic Obstructive Pulmonary Disease by Roy A. Pleasants in Annals of Pharmacotherapy
Footnotes
Declaration of Conflicting Interests: The author declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Pleasants has received grants from Boehringer Ingelheim, GlaxoSmithKline, and Teva, speaker fees from Astra-Zeneca and Sunovion, and consultant fees from Teva and Glaxo-SmithKline.
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical writing support was provided by Hashem Dbouk, PhD, of FireKite, an Ashfield company, part of UDG Healthcare plc, and funded by Sunovion Pharmaceuticals Inc.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2018 report. https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf. Accessed August 17, 2018.
- 2. MacNee W. Pathology, pathogenesis, and pathophysiology. BMJ. 2006;332:1202-1204. doi: 10.1136/bmj.332.7551.1202 [DOI] [Google Scholar]
- 3. Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:289-295. [PMC free article] [PubMed] [Google Scholar]
- 4. Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61:1-51. [PubMed] [Google Scholar]
- 5. Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: final data for 2015. Natl Vital Stat Rep. 2017;66:1-75. [PubMed] [Google Scholar]
- 6. American Pharmacists Association Foundation. White paper on expanding the role of pharmacists in chronic obstructive pulmonary disease: American Pharmacists Association Foundation. J Am Pharm Assoc. 2011;51:203-211. doi: 10.1331/JAPhA.2011.11513 [DOI] [PubMed] [Google Scholar]
- 7. Cazzola M, Page CP, Calzetta L, Matera MG. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64:450-504. doi: 10.1124/pr.111.004580 [DOI] [PubMed] [Google Scholar]
- 8. Trelegy Ellipta [pacakge insert]. Research Triangle Park, NC: GlaxoSmithKline; 2018. [Google Scholar]
- 9. Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in COPD patients (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198:329-339. doi: 10.1164/rccm.201803-0405OC [DOI] [PubMed] [Google Scholar]
- 10. Laube BL, Jansses HM, de Jongh FHC, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37:1308-1331. doi: 10.1183/09031936.00166410 [DOI] [PubMed] [Google Scholar]
- 11. Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med. 2005;172:1497-1504. doi: 10.1164/rccm.200410-1414OC [DOI] [PubMed] [Google Scholar]
- 12. Zanen P, Go LT, Lammers JW. Optimal particle size for beta 2 agonist and anticholinergic aerosols in patients with severe airflow obstruction. Thorax. 1996;51:977-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DePietro M, Gilbert I, Millette LA, Riebe M. Inhalation device options for the management of chronic obstructive pulmonary disease. Postgrad Med. 2018;130:83-97. doi: 10.1080/00325481.2018.1399042 [DOI] [PubMed] [Google Scholar]
- 14. Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335-371. doi: 10.1378/chest.127.1.335 [DOI] [PubMed] [Google Scholar]
- 15. Hanania NA, Braman S, Adams SG, et al. The role of inhalation delivery devices in COPD: perspectives of patients and health care providers. Chronic Obstr Pulm Dis. 2018;5:111-123. doi: 10.15326/jcopdf.5.2.2017.0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melani AS, Bonavia M, Cilenti V, et al. ; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi: 10.1016/j.rmed.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 17. Tiffin N, Zeman K, Bennett W. Efficacy and variability of aerosol delivery from portable DC-powered compressor/nebulizer systems. Can J Respir Ther. 2011;47:28-33. [Google Scholar]
- 18. Braman SS, Carlin BW, Hanania NA, et al. Results of a pulmonologist survey regarding knowledge and practices with inhalation devices for COPD. Respir Care. 2018;63:840-848. doi: 10.4187/respcare.05717 [DOI] [PubMed] [Google Scholar]
- 19. Rootmensen GN, van Keimpema AR, Jansen HM, de Haan RJ. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J Aerosol Med Pulm Drug Deliv. 2010;23:323-328. doi: 10.1089/jamp.2009.0785 [DOI] [PubMed] [Google Scholar]
- 20. Pothirat C, Chaiwong W, Phetsuk N, Pisalthanapuna S, Chetsadaphan N, Choomuang W. Evaluating inhaler use technique in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:1291-1298. doi: 10.2147/COPD.S85681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blackstock F, Webster KE. Disease-specific health education for COPD: a systematic review of changes in health outcomes. Health Educ Res. 2007;22:703-717. doi: 10.1093/her/cyl150 [DOI] [PubMed] [Google Scholar]
- 22. Simpson KH, Smith RJ, Davies LF. Comparison of the effects of atropine and glycopyrrolate on cognitive function following general anaesthesia. Br J Anaesth. 1987;59:966-969. [DOI] [PubMed] [Google Scholar]
- 23. Restrepo RD. Use of inhaled anticholinergic agents in obstructive airway disease. Respir Care. 2007;52:833-851. [PubMed] [Google Scholar]
- 24. Spiriva HandiHaler [package insert]. Ridgefield, CT: Boehringer Ingelheim; 2018. [Google Scholar]
- 25. Pleasants RA, Wang T, Gao J, Tang H, Donohue JF. Inhaled umeclidinium in COPD patients: a review and meta-analysis. Drugs. 2016;76:343-361. doi: 10.1007/s40265-015-0532-5 [DOI] [PubMed] [Google Scholar]
- 26. Pham S, Ferguson GT, Kerwin E, Goodin T, Wheeler A, Bauer A. In vitro characterization of the eFlow closed system nebulizer with glycopyrrolate inhalation solution. J Aerosol Med Pulm Drug Deliv. 2018;31:162-169. doi: 10.1089/jamp.2017.1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sims MW. Aerosol therapy for obstructive lung diseases: device selection and practice management issues. Chest. 2011;140:781-788. doi: 10.1378/chest.10-2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alhaddad B, Smith FJ, Robertson T, Watman G, Taylor KMG. Patients’ practices and experiences of using nebuliser therapy in the management of COPD at home. BMJ Open Respir Res. 2015;2:e000076. doi: 10.1136/bmjresp-2014-000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. GoodRx. Home page. https://www.goodrx.com/. Accessed August 17, 2018.
- 30. Haddad EB, Patel H, Keeling JE, Yacoub MH, Barnes PJ, Belvisi MG. Pharmacological characterization of the muscarinic receptor antagonist, glycopyrrolate, in human and guinea-pig airways. Br J Pharmacol. 1999;127:413-420. doi: 10.1038/sj.bjp.0702573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ali-Melkkila T, Kaila T, Kanto J. Glycopyrrolate: pharmacokinetics and some pharmacodynamic findings. Acta Anaesthesiol Scand. 1989;33:513-517. [DOI] [PubMed] [Google Scholar]
- 32. Lonhala Magnair [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc.; 2018. [Google Scholar]
- 33. Leaker BR, Barnes PJ, Jones CR, Tutuncu A, Singh D. Efficacy and safety of nebulized glycopyrrolate for administration using a high efficiency nebulizer in patients with chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2015;79:492-500. doi: 10.1111/bcp.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dumitras S, Sechaud R, Drollmann A, et al. Effect of cimetidine, a model drug for inhibition of the organic cation transport (OCT2/MATE1) in the kidney, on the pharmacokinetics of glycopyrronium. Int J Clin Pharmacol Ther. 2013;51:771-779. doi: 10.5414/CP201946 [DOI] [PubMed] [Google Scholar]
- 35. Donohue JF, Goodin T, Tosiello R, Wheeler A. Dose selection for glycopyrrolate/eFlow® phase III clinical studies: results from GOLDEN (Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer) phase II dose-finding studies. Respir Res. 2017;18:202. doi: 10.1186/s12931-017-0681-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferguson GT, Goodin T, Tosiello R, Wheeler A, Kerwin E. Long-term safety of glycopyrrolate/eFlow® CS in moderate-to-very-severe COPD: results from the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 5 randomized Study. Respir Med. 2017;132:251-260. doi: 10.1016/j.rmed.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 37. Kerwin E, Donohue JF, Goodin T, Tosiello R, Wheeler A, Ferguson GT. Efficacy and safety of glycopyrrolate/eFlow® CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: results from the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 3 and 4 randomized controlled trials. Respir Med. 2017;132:238-250. doi: 10.1016/j.rmed.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 38. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79. [DOI] [PubMed] [Google Scholar]
- 39. Mastrodicasa MA, Droege CA, Mulhall AM, Ernst NE, Panos RJ, Zafar MA. Long acting muscarinic antagonists for the treatment of chronic obstructive pulmonary disease: a review of current and developing drugs. Expert Opin Investig Drugs. 2017;26:161-174. doi: 10.1080/13543784.2017.1276167 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Figure for Glycopyrrolate/eFlow CS: The First Nebulized Long-Acting Muscarinic Antagonist Approved to Treat Chronic Obstructive Pulmonary Disease by Roy A. Pleasants in Annals of Pharmacotherapy