Abstract
We report two cases of Emergomyces pasteurianus infection in the Netherlands. Both patients were immunocompromised and had pulmonary symptoms. The first patient died due to a pulmonary infection with Es. pasteurianus, concomitant listeriosis, Pseudomonas aeruginosa sepsis and invasive pulmonary aspergillosis. The second patient had pulmonary and subcutaneous lesions, and recovered completely after treatment with posaconazole for 14 months. In both cases, diagnosis of Es. pasteurianus was made with internal transcribed spacer rRNA PCR and culture.
Keywords: Emergomyces, Immunocompromised, Mycosis, Disseminated infection, Dimorphic fungi
1. Introduction
Emergomyces is a recently proposed new genus within the family Ajellomycetaceae (Onygenales), to accommodate the recently described and globally emerging Emmonsia-like fungi which cause disease in immunocompromised hosts [1], [2]. Patients can be infected with Emergomyces species, which are saprophytic dimorphic fungi, presumably by inhaling dust-borne conidia. In the genus Emergomyces five species are described: Es. africanus, Es. pasteurianus, Es. europeaus, Es. orientalis and Es. canadensis. All species lack adiaspores, classically associated with the genus Emmonsia [2]. Emergomyces species have been found in immunocompromised hosts and mostly present as a disseminated infection, with over 80 reported cases of Es. africanus in HIV-patients in South Africa [3], [4], [5].
To date, only a handful of cases of Es. pasteurianus infection have been reported, distributed over Africa, Asia and Europe [6], [7], [8], [9], [10], [11]. We report, to the best of our knowledge, the first two cases with an Es. pasteurianus infection in the Netherlands.
2. Case
2.1. Case A
In November 2017 an Iraqi male, in the eight decade of life, was admitted to a tertiary academic hospital in the Netherlands with a decreased level of consciousness and fever (day 0). The patient, with a medical history of B-cell chronic lymphocytic leukemia and chronic kidney failure, had developed chronic neutropenia due to his hematological condition and therapy (cyclophosphamide and prednisone since eleven months). The patient had returned two months earlier (day −60) from visiting relatives in Iraq.
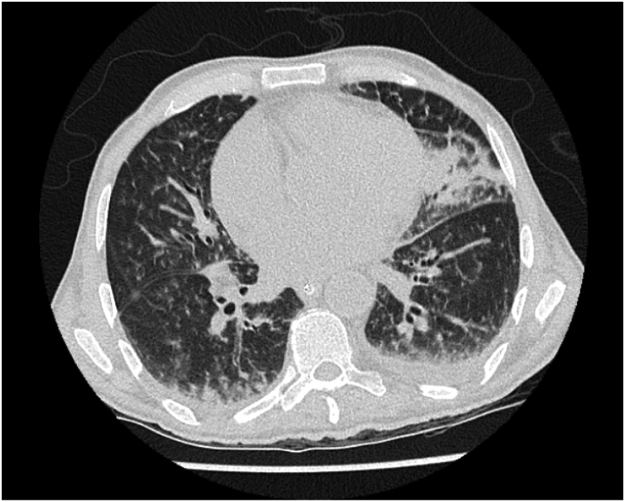
Upon admission he was evaluated for meningitis; cerebrospinal fluid (CSF) analysis (day 0) showed pleocytosis (WBC 613×106/L), normal glucose (0.43 g/L) and an elevated protein concentration (3.6 g/L). Gram staining of CSF (day 0) showed Gram positive rods and treatment with high dose amoxicillin was started; cultures of CSF and blood showed Listeria monocytogenes (day +1). The following days there was some moderate neurological improvement and the fever subsided. On day + 11 fever and tachypnea developed, no skin abnormalities were seen. Broad spectrum antibiotics (cefuroxime/gentamicin) were added to amoxicillin treatment. A thoracic high resolution computed tomography (HRCT) scan (day +11) (Fig. 1) showed extensive bilateral infiltrative abnormalities, consistent with a broad differential diagnosis, including (fungal) infection, but pathognomonic signs for a fungal infection were absent. A bronchoalveolar lavage (BAL) was performed on day + 12 and liposomal amphotericin B 3 mg/kg was started as empirical therapy. The patient was transferred to the Intensive Care Unit (day +12) for respiratory insufficiency, but died within 36 h after onset of fever and tachypnea on day +13, because of respiratory insufficiency, cardiac failure and sepsis caused by Pseudomonas aeruginosa.
Fig. 1.
Thoracic HRCT scan, showing extensive bilateral infiltrative abnormalities, pathognomonic signs for a fungal infection were absent.
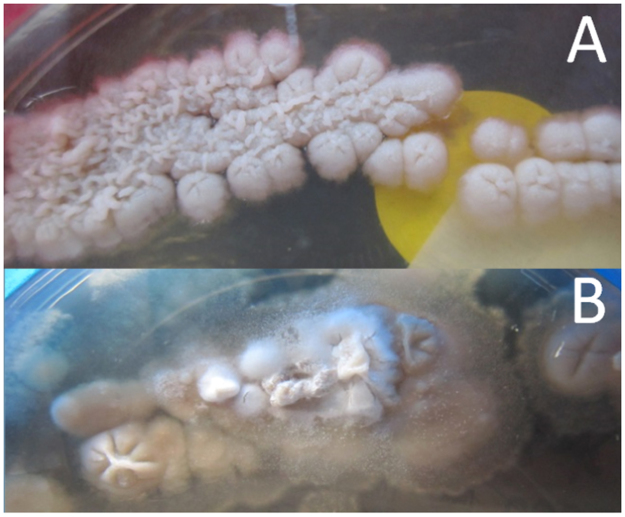
Post-mortem, BAL fluid and serum tested strongly positive in the galactomannan assay (Platelia Aspergillus, Bio-Rad). No Aspergillus could be cultured, but polymerase chain reaction (PCR) on BAL fluid was positive for Aspergillus fumigatus (AsperGenius, PathoNostics, performed by ErasmusMC, Rotterdam, the Netherlands). Culture of BAL fluid showed some small colonies suspected for a fungus after three days of incubation. After three weeks of incubation, a dimorphic fungus was diagnosed without production of adiaspores at 25 °C (Fig. 2). Molecular identification by in-house internal transcribed spacer rRNA (ITS) sequencing revealed Emergomyces pasteurianus (ITS1: GenBank accession number KP260922, length 239 nucleotides, similarity 99.2%; ITS2: GenBank accession number KP260922, length 332 nucleotides, similarity 100%), which was confirmed by additional analysis (ITS and large subunit rRNA (LSU) sequencing) at the CBS-KNAW Fungal Biodiversity Center, Utrecht, The Netherlands. The strain of Es. pasteurianus was negative in the Aspergillus species PCR (AsperGenius, PathoNostics, performed by Leiden University Medical Center).
Fig. 2.
Yeast (A, 35 °C) and mold (B, 28 °C) phase of Es. pasteurianus on Sabouraud agar, after 3 weeks of inoculation.
2.2. Case B
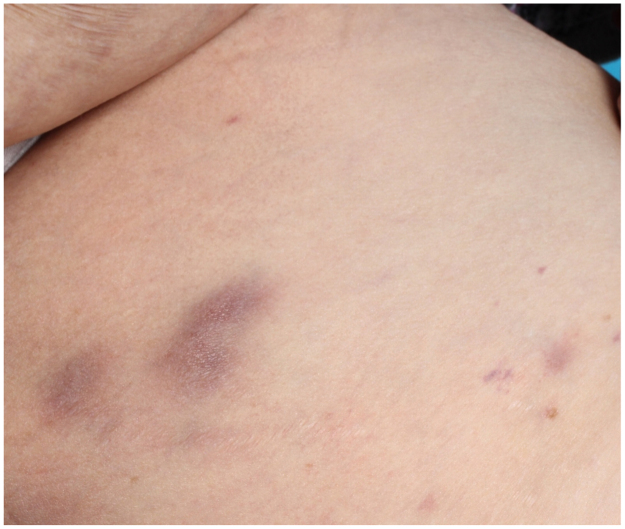
In December 2016 a 62-year old woman of Moroccan descent was admitted to a secondary care hospital in the Netherlands with shortness of breath (day 0). Physical examination showed multiple pink to purple coloured, firm, raised, non-tender, 0.5–2 cm in diameter, subcutaneous lesions on trunk, arms and legs (Fig. 3). She had a medical history of large B-cell non-Hodgkin's lymphoma (2013, in complete remission since 2014), biliary cirrhosis, chronic kidney failure due to diabetes mellitus type 2 and auto-immune haemolytic anaemia treated with 50 mg/day of prednisolone. The patient frequently visited relatives in Morocco and had returned four months earlier from such visit (day −120).
Fig. 3.
Two subcutaneous lesions, approximately 1–2 cm in diameter on the left leg of Case B.
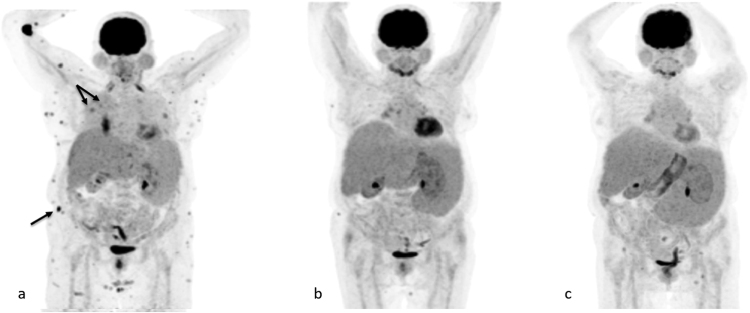
On hospital admission, chest X-ray showed a density in the right upper lobe. Further analysis with a positron emission tomography–computed tomography (PET-CT) scan was performed on day +6, showing two PET positive lesions in the right upper lobe and multiple PET positive subcutaneous lesions, suggestive of malignancy (Fig. 4a).
Fig. 4.
Position emission tomography–computed tomography images at initial presentation (a), after 6 months (b) and after 14 months (c) of posaconazole treatment. The arrows indicate the two lung lesions and the largest subcutaneous lesion.
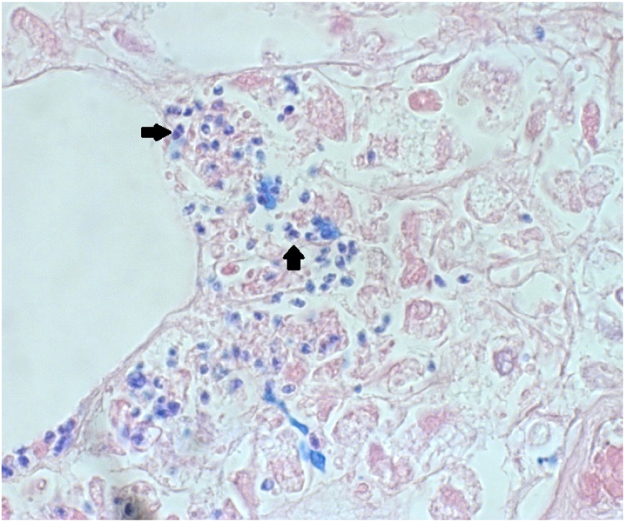
Biopsy of two subcutaneous lesions showed no malignant cancer cells, but infiltration of predominantly histiocytes and within the histiocytes infiltration of structures, 2 µm in diameter, resembling yeast cells (Fig. 5). Both the size of the structures and its localization within histiocytes could be compatible with histoplasmosis. Additional molecular fungal identification directly on paraffin-embedded tissue biopsy material with ITS PCR revealed Emergomyces pasteurianus (GenBank accession number NR_137149, length 261 nucleotides, similarity 100%). On day +26, after 12 days of incubation, cultures of these subcutaneous lesions showed growth of a dimorphic fungus and molecular identification (ITS and LSU sequencing) at the Center of Expertise in Mycology Radboudumc/CWZ, Nijmegen, the Netherlands, confirmed Emergomyces pasteurianus. Additional antifungal susceptibility testing was performed by broth microdilution according to CLSI standards [6]. The following MICs were determined (mg/L): amphotericin B 0.031, anidulafungin 0.016, micafungin <0.008, itraconazole 0.063, voriconazole 0.25, posaconazole 0.063, isavuconazole 1 and fluconazole 64. Patient tested negative for HIV infection and bone marrow biopsy showed no hematological malignancy.
Fig. 5.
Histopathologic section (Giemsa stain, x400) of a subcutaneous lesion, showing yeast-like structures (arrows, approximately 2 µm in diameter).
She was treated with posaconazole and the daily prednisolone dose was gradually lowered to 5 mg. A PET-CT scan conducted after 6 months of posaconazole treatment (day +209) showed a decline in number and PET intensity of all lesions (Fig. 4b) and after 14 months of antifungal treatment (day +424) all subcutaneous and lung lesions disappeared (Fig. 4c). Posaconazole treatment was stopped on day +434.
3. Discussion
To our knowledge, we report the first two cases of Emergomyces pasteurianus infection in the Netherlands. Both our patients were immunocompromised: due to a hematological condition (Case A) or therapy (Cases A and B). Pulmonary symptoms were present in both cases, cutaneous lesions as described in previously reported Es. pasteurianus cases [7], [8], [9], [10], [11], [12] and in most cases of disseminated Es. africanus [3], [13], were only present in Case B. Es. pasteurianus infection was diagnosed by culture and molecular diagnostic techniques of tissue biopsy material. Case A died on day +13 due to respiratory failure, whereas Case B was successfully treated with long-term posaconazole treatment.
Like Blastomyces dermatidis and Histoplasma capsulatum, Es. pasteurianus is a dimorphic fungus. Emergomycosis seems to be a disease of the immunocompromised host, with few case reports of immunocompetent patients [13], [14]. The transmission route of Emergomyces species is still unknown. Probably the natural reservoir is soil and infection is presumed to occur via inhalation of conidia [15], [16]. In histoplasmosis it is known that reactivation is possible when patients become immunocompromised. The first reported case of Es. pasteurianus (previously known as Emmonsia pasteuriana) was an Italian women with AIDS with no history of travel abroad [7]. Our patients may have acquired colonization or infection outside Europe, during a visit to their country of origin. It is not known whether Emergomyces remains present in the patient after initial infection or colonization and if it can reactivate if the patient becomes immunocompromised. So a more prolonged course of an imported infection in our patients cannot be excluded.
Currently, there are no treatment guidelines for patients with emergomycosis. Guidelines for blastomycosis and histoplasmosis recommend liposomal amphotericin B as initial therapy, followed by itraconazole and therapy duration should be at least 12 months or lifelong when immunosuppression cannot be reversed [17], [18]. In Case A the patient was treated with liposomal amphotericin B and in Case B, the patient was successfully treated with 14 months of posaconazole and tapering daily prednisolone dose. A recent in vitro study of 11 strains of Emergomyces species showed that posaconazole had the lowest geometric mean MIC [19], supporting that posaconazole may be an alternative for liposomal amphotericin B or itraconazole.
The reported cases emphasize that clinicians should be aware of the presence of mixed, invasive fungal infections with fungi other than Aspergillus and Zygomycetes in immunocompromised patients. They should also be aware that invasive fungal infections may mimic malignancy. Molecular diagnostic techniques like ITS PCR could accurately and rapidly diagnose these fungi from clinical specimens.
Acknowledgements
The authors are grateful to Stefan Akerboom and Ilja Norbart, microbiology technicians at Leiden University Medical Center, who processed the collected samples of Case A and Judith van Paassen MD, intensivist, for critical reading of the manuscript. The authors thank Dorothee E. Arnold MD PhD, pathologist at Amphia Ziekenhuis for histopathological tissue analysis and Jacques F.G.M. Meis MD PhD, Canisius Wilhelmina Hospital for performing antifungal susceptibility testing and his advice on the antifungal therapy for Case B.
Acknowledgments
Conflict of interest
The authors declare no conflicts of interest.
Funding source
None
References
- 1.Dukik K., Munoz J.F., Jiang Y., Feng P., Sigler L., Stielow J.B., Freeke J., Jamalian A., Gerrits van den Ende B., McEwen J.G., Clay O.K., Schwartz I.S., Govender N.P., Maphanga T.G., Cuomo C.A., Moreno L.F., Kenyon C., Borman A.M., de Hoog S. Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales) Mycoses. 2017;60:296–309. doi: 10.1111/myc.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Y., Dukik K., Muñoz J.F., Sigler L., Schwartz I.S., Govender N.P., Kenyon C., Feng P., Gerrits van den Ende B., Stielow J.B., Stchigel A.M., Lu H., de Hoog S. Phylogeny, ecology and taxonomy of systemic pathogens and their relatives in Ajellomycetaceae (Onygenales): Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis. Fungal Divers. 2018;90:245–291. [Google Scholar]
- 3.Kenyon C., Bonorchis K., Corcoran C., Meintjes G., Locketz M., Lehloenya R., Vismer H.F., Naicker P., Prozesky H., van Wyk M., Bamford C., du Plooy M., Imrie G., Dlamini S., Borman A.M., Colebunders R., Yansouni C.P., Mendelson M., Govender N.P. A dimorphic fungus causing disseminated infection in South Africa. N. Engl. J. Med. 2013;369:1416–1424. doi: 10.1056/NEJMoa1215460. [DOI] [PubMed] [Google Scholar]
- 4.Maphanga T.G., Britz E., Zulu T.G., Mpembe R.S., Naicker S.D., Schwartz I.S., Govender N.P. In vitro antifungal susceptibility of yeast and mold phases of isolates of dimorphic fungal pathogen Emergomyces africanus (formerly Emmonsia sp.) from HIV-infected South African patients. J. Clin. Microbiol. 2017;55:1812–1820. doi: 10.1128/JCM.02524-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz I.S., Kenyon C., Lehloenya R., Claasens S., Spengane Z., Prozesky H., Burton R., Parker A., Wasserman S., Meintjes G., Mendelson M., Taljaard J., Schneider J.W., Beylis N., Maloba B., Govender N.P., Colebunders R., Dlamini S. AIDS-related endemic mycoses in Western Cape, South Africa, and clinical mimics: a cross-sectional study of adults with advanced HIV and recent-onset, widespread skin lesions. Open Forum Infect. Dis. 2017;4:ofx186. doi: 10.1093/ofid/ofx186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute . Clinical and Laboratory Standards Institute; Wayne, USA: 2008. M38-A2 Reference Method for Broth Dilution Antimicrobial Susceptibility Testing of Filamentous Fungi; Approved Standard-Second Edition. [Google Scholar]
- 7.Gori S., Drouhet E. Cutaneous disseminated mycosis in a patient with AIDS due to a new dimorphic fungus. J. Mycol. Med. 1998;8:57–63. [Google Scholar]
- 8.Pelegrin I., Alastruey-Izquierdo A., Ayats J., Cuenca-Estrella M., Cabellos C. A second look at Emmonsia infection can make the difference. Transpl. Infect. Dis. 2014;16:519–520. doi: 10.1111/tid.12214. [DOI] [PubMed] [Google Scholar]
- 9.Malik R., Capoor M.R., Vanidassane I., Gogna A., Singh A., Sen B., Rudramurthy S.M., Honnavar P., Gupta S., Chakrabarti A. Disseminated Emmonsia pasteuriana infection in India: a case report and a review. Mycoses. 2016;59:127–132. doi: 10.1111/myc.12437. [DOI] [PubMed] [Google Scholar]
- 10.Tang X.H., Zhou H., Zhang X.Q., Han J.D., Gao Q. Cutaneous disseminated emmonsiosis due to Emmonsia pasteuriana in a patient with cytomegalovirus enteritis. JAMA Dermatol. 2015;151:1263–1264. doi: 10.1001/jamadermatol.2015.1792. [DOI] [PubMed] [Google Scholar]
- 11.Feng P., Yin S., Zhu G., Li M., Wu B., Xie Y., Ma H., Zhang J., Cheng C., de Hoog G.S., Lu C., Lai W. Disseminated infection caused by Emmonsia pasteuriana in a renal transplant recipient. J. Dermatol. 2015;42:1179–1182. doi: 10.1111/1346-8138.12975. [DOI] [PubMed] [Google Scholar]
- 12.Lavergne R.-A., Kandel-Aznar C., Khatchatourian L., Garcia-Hermoso D., Jeddi F., Boutoille D., Morio F., Le Pape P. Emmonsia pasteuriana: une cause rare d′infection fongique chez l′immunodéprimé. J. Mycol. Méd. 2017;27:e7–e8. [Google Scholar]
- 13.Schwartz I.S., Govender N.P., Corcoran C., Dlamini S., Prozesky H., Burton R., Mendelson M., Taljaard J., Lehloenya R., Calligaro G., Colebunders R., Kenyon C. Clinical characteristics, diagnosis, management, and outcomes of disseminated emmonsiosis: a retrospective case series. Clin. Infect. Dis. 2015;61:1004–1012. doi: 10.1093/cid/civ439. [DOI] [PubMed] [Google Scholar]
- 14.Wang P., Kenyon C., de Hoog S., Guo L., Fan H., Liu H., Li Z., Sheng R., Yang Y., Jiang Y., Zhang L., Xu Y. A novel dimorphic pathogen, Emergomyces orientalis (Onygenales), agent of disseminated infection. Mycoses. 2017;60:310–319. doi: 10.1111/myc.12583. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz I.S., Lerm B., Hoving J.C., Kenyon C., Horsnell W.G., Basson W.J., Otieno-Odhiambo P., Govender N.P., Colebunders R., Botha A. Emergomyces africanus in soil, South Africa. Emerg. Infect. Dis. 2018;24:377–380. doi: 10.3201/eid2402.171351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz I.S., McLoud J.D., Berman D., Botha A., Lerm B., Colebunders R., Levetin E., Kenyon C. Molecular detection of airborne Emergomyces africanus, a thermally dimorphic fungal pathogen, in Cape Town, South Africa. PLoS Negl. Trop. Dis. 2018;12:e0006174. doi: 10.1371/journal.pntd.0006174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman S.W., Dismukes W.E., Proia L.A., Bradsher R.W., Pappas P.G., Threlkeld M.G., Kauffman C.A., Infectious Diseases Society of America Clinical practice guidelines for the management of blastomycosis: 2008 update by the infectious diseases Society of America. Clin. Infect. Dis. 2008;46:1801–1812. doi: 10.1086/588300. [DOI] [PubMed] [Google Scholar]
- 18.Wheat L.J., Freifeld A.G., Kleiman M.B., Baddley J.W., McKinsey D.S., Loyd J.E., Kauffman C.A., Infectious Diseases Society of America Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007;45:807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 19.Dukik K., Al-Hatmi A.M.S., Curfs-Breuker I., Faro D., de Hoog S., Meis J.F. Antifungal susceptibility of emerging dimorphic pathogens in the family Ajellomycetaceae. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.01886-17. [DOI] [PMC free article] [PubMed] [Google Scholar]