Abstract
US Asian/Pacific Islander (API) communities experience high air pollution levels. APIs may be predisposed to pancreatic β-cell dysfunction and have the highest prevalence of gestational diabetes mellitus (GDM) compared with other racial/ethnic groups. Exposure to high levels of volatile organic compounds (VOCs) impairs pancreatic β-cell function, leading to insulin resistance, but racial/ethnic differences in this association are unexamined. We analyzed singleton deliveries (n = 220,065) from the Consortium on Safe Labor (2002–2008). Exposure to 14 VOCs in each hospital referral region was based on modified Community Multiscale Air Quality models. Logistic regression estimated odds ratios for GDM associated with high (≥75th percentile) versus low (<75th percentile) VOC exposure 3 months before conception and during the first trimester of pregnancy. Preconception and first-trimester exposure to high VOC levels was associated with increased odds of GDM among whites and APIs. GDM risk was significantly higher for APIs than whites for most VOCs. Preconception benzene exposure was associated with 29% (95% confidence interval: 12, 47) increased odds of GDM among whites compared with 45% (95% confidence interval: 16, 81) increased odds among APIs. These findings highlight environmental health disparities affecting pregnant women. Increased focus on GDM risk in US API communities is warranted.
Keywords: air pollution, Asian/Pacific Islanders, gestational diabetes mellitus, pregnancy, volatile organic compounds
In the United States, racial/ethnic minorities are overburdened with air pollution exposure because minorities typically reside closer to air pollution sources than do white populations (1–4). US Asian/Pacific Islander (API) communities are exposed to air pollution to a similar degree as black and Hispanic communities, yet API communities are underrepresented in environmental justice research (3, 5, 6). The disproportionate exposure to high levels of air pollution among API communities may be associated with health disparities (7, 8). Because the API population is the fastest growing racial/ethnic group in the US (9), environmental risks for US API populations warrant additional research.
Gestational diabetes mellitus (GDM) prevalence in the United States is estimated to be as high as 9.2%, and rates are increasing (10, 11). According to national, state, and local data, the highest prevalence of GDM is in API populations (10, 12–19). For example, in 2010 Pregnancy Risk Assessment Monitoring System data, APIs had the highest prevalence of GDM (16.3%), followed by Hispanics (12.1%), blacks (10.5%), and whites (6.8%) (10). Despite a focus on racial/ethnic differences in maternal risk factors (12, 13, 16) and calls for increased screening of women in high-risk racial/ethnic groups (12, 17–19), racial/ethnic disparities in prevalence of GDM persist.
GDM has implications beyond pregnancy; GDM is associated with an increased risk of maternal, fetal, and neonatal complications (20). Women in whom GDM develops have an increased risk of developing type 2 diabetes mellitus, and their offspring have an increased risk of obesity and diabetes (20). Racial/ethnic disparities in GDM (10), obesity, and diabetes are persistent (21); thus, potentially modifiable GDM risk factors, such as ambient air pollution, merit attention. Preconception and prenatal exposure to high ambient air pollution levels have been linked with GDM (22–25), yet race/ethnicity-specific associations between air pollution and GDM are unexamined.
Although the mechanisms by which ambient air exposures increase GDM risk are not known, increased inflammation and oxidative stress are potential pathways (25–28). Exposure to high levels of benzene, a volatile organic compound (VOC), may induce oxidative stress (26), which is associated with pancreatic β-cell dysfunction and/or insulin resistance in rats and humans (26, 29, 30). Furthermore, APIs of various ancestries (31–36) have increased risk of insulin resistance, and results of genome-wide association studies suggest this may be a genetic predisposition to pancreatic β-cell failure (31, 37). Given this suggestive evidence, APIs may be uniquely susceptible to developing insulin resistance when exposed to high levels of VOCs.
To better understand racial/ethnic disparities in GDM in the United States, we used data from 220,065 singleton deliveries from the Consortium on Safe Labor (CSL), linked with modified Community Multiscale Air Quality data, to examine race-specific associations between GDM and exposure to high levels of VOCs. We predicted that APIs would have highest GDM risk associated with exposure to high (≥75%), compared with low (<75%), VOC levels.
METHODS
The CSL was an electronic medical record–based, national, retrospective cohort study from 2002 to 2008 that included 19 hospitals in 15 hospital referral regions (HRRs). Data extracted for deliveries at 23 weeks or later included maternal sociodemographic characteristics; medical, reproductive, and prenatal history; and labor and delivery summaries (38). A total of 228,438 deliveries were included in the study. We excluded multifetal pregnancies (n = 5,053; 2.21%), mothers with pre-existing diabetes (n = 3,309; 1.44%), singleton pregnancies with missing data on air pollution exposure (n = 10; 0.004%), and 1 delivery with missing data on maternal race/ethnicity; the final analytic sample comprised 220,065 deliveries. Institutional review boards at all participating sites approved the CSL, and data are anonymous.
GDM was recorded in the medical record data or in discharge summaries using International Classification of Diseases, Ninth Revision, code 648.8. In the United States, during 2002–2008, when births from the CSL occurred, the American Diabetes Association recommended universal screening for GDM between 24 and 28 weeks of gestation, using the Carpenter and Coustan criteria (39).
The Air Quality and Reproductive Health Study linked CSL data to air pollution exposures using a modified version of the Community Multiscale Air Quality Model (40). Individual addresses are not available; therefore, maternal exposure estimates are based on the average exposure for the birth HRR (40, 41). The Community Multiscale Air Quality Model is a 3-dimensional, multipollutant air quality model used to predict ambient pollutant levels on the basis of emissions data from the National Emission Inventory and meteorological data from the Weather Research Forecasting Model. Exposure was based on the predicted hourly ambient pollutant concentrations within HRRs, weighted to reflect population concentration, and accounting for places where women were unlikely to reside, as described elsewhere (40).
Hourly exposure estimates for ambient air pollutants were averaged across exposure windows for each woman. Routine screening for GDM is recommended to occur between 24 and 28 weeks of gestation; thus, we focused on average exposure before conception (3 months before conception) and during the first trimester of pregnancy (through 13 weeks’ gestation). Ambient VOC concentrations in parts per billion were estimated for benzene; 1,3-butadiene; ethylbenzene; cyclohexane; methyl-tertiary-butyl ether; N-hexane; ethyl-methyl ketone; m-xylene; o-xylene; p-xylene; propene; sesquiterpene; styrene; and toluene for each exposure window. Exposure to VOC concentrations at the 75th percentile or greater level was considered high exposure.
Statistical analysis
Demographic characteristics were summarized for women with (n = 11,340) and without (n = 231,405) GDM by race/ethnicity (Web Table 1, available at https://academic.oup.com/aje). Spearman rank correlations between each of the VOCs were calculated (Web Tables 2 and 3). Distribution of women by level of VOC exposure and race/ethnicity was reported (Web Table 4). Ordinal logistic regression was used to estimate the odds ratios and 99% confidence intervals for the association between high VOC levels and GDM. We used 99% confidence intervals to account for multiple testing.
An interaction term between race/ethnicity and exposure to high levels of VOCs was used to estimate race/ethnicity-specific odds ratios and 99% confidence intervals. The odds ratio was interpreted as the risk of GDM with exposure to high VOC exposure among blacks, Hispanics, Asians/Pacific Islanders, and other races/ethnicities compared with white women. A statistically significant interaction term (P < 0.01) suggests the association between high levels of VOCs and GDM is different among blacks, APIs, Hispanics, or other races/ethnicities compared with white women. We assessed the multiplicative interaction because our primary interest was to describe potentially differential associations among racial/ethnic groups and determine whether the data would support the notion of a biologically plausible differential association among APIs (42). Although the race/ethnicity group referred to as “other” here was included in the analysis, specific results are not reported, because of heterogeneity of the population. Robust standard errors accounted for multiple deliveries for the same woman.
Models were adjusted for HRR, maternal age in years, maternal race (white, black, API, Hispanic, other), prepregnancy body mass index (BMI; calculated as weight (kg)/height (m2) and categorized as 11.20–18.49, 18.50–24.99, 25.00–29.99, and 30.00–77.80), insurance status (public, private, other), marital status (married, single, divorced, unknown), parity (nulliparous or multiparous), season of conception (winter, spring, summer, fall), and hospital type (university-affiliated teaching hospital, community teaching hospital, community nonteaching hospital). Socioeconomic status indicators like family income or education level are not available, so insurance (reflecting access to resources (43)) and marital status (married couples typically have higher income compared with other families (44)) were proxies of socioeconomic status for the purposes of this study. Missing data on individual-level covariates were retained as their own category. Separate models were run for preconception (i.e., 3 months before conception) and first trimester of pregnancy (i.e., through 13 weeks’ gestation) windows of exposure. GDM is typically diagnosed during gestational weeks 24–28 (39); thus, examining preconception and first-trimester exposure windows allows for temporality and aligns with our prior work that showed preconception and first-trimester exposure to criteria air pollutants was associated with GDM (25).
Prepregnancy BMI is a key risk factor for GDM (20). In sensitivity analyses, to examine the influence of BMI on the association between VOCs and GDM, we excluded BMI from the final models.
In post hoc analysis, to better understand the API population included in the CSL, we examined the percentage of the API population by ancestry for the CSL hospital metropolitan statistical areas locations. Data were obtained from the 2005 to 2010 American Community Survey, which overlaps the CSL data collection period and covers all relevant geographies. Estimates for API populations were drawn from the “Native Hawaiian and Other Pacific Islander Alone by Selected Groups” and “Asian Alone by Selected Groups” estimates.
RESULTS
Web Table 1 presents data on prevalence of GDM by race/ethnicity and demographic characteristics. APIs had the highest rate of GDM (n = 899, 9.9%), followed by Hispanics (n = 2,462, 6.4%), Other races/ethnicities (n = 854, 5.9%), whites (n = 4,977, 4.5%), and blacks (n = 2,148, 4.3%). For all races/ethnicities, older women and multiparous women had a higher rate of GDM. For all racial/ethnic groups, the rate of GDM increased as prepregnancy BMI increased. Among whites and APIs, divorced women had higher rates of GDM, whereas GDM rates among blacks and Hispanics did not greatly differ by marital status. There were no differences in GDM rates by insurance status for whites or blacks, yet among Hispanic and API women, those in the “other payment” group had lower rates of GDM.
VOC levels for 3 months before conception and during the first trimester of pregnancy are included in Table 1. Generally, VOCs were positively correlated with one another in preconception (0.20 ≤ r ≤ 0.99) and first-trimester (0.21 ≤ r ≤ 0.99) exposure windows (Web Tables 2 and 3).
Table 1.
Distribution and Mean Levels (parts per billiona) of Volatile Organic Compounds, Consortium on Safe Labor, United States, 2002–2008
| Volatile Organic Compound | 25th Percentile | 50th Percentile | 75th Percentile | Interquartile Range | Mean | Minimum, Maximum |
|---|---|---|---|---|---|---|
| 3 Months Before Conception | ||||||
| Benzene | 0.12 | 0.21 | 0.33 | 0.21 | 0.24 | 0.04, 0.71 |
| 1,3 Butadiene | 5.5 × 10−15 | 3.0 × 10−3 | 6.8 × 10−3 | 6.8 × 10−3 | 4.1 × 10−3 | 1.00 × 10−27, 2.16 × 10−2 |
| Ethylbenzene | 0.03 | 0.07 | 0.13 | 0.09 | 0.10 | 7.1 × 10−3, 0.47 |
| Cyclohexane | 0.01 | 0.02 | 0.03 | 0.02 | 0.03 | 1.79 × 10−3, 0.13 |
| MTB ether | 2.4 × 10−3 | 0.006 | 0.01 | 0.01 | 0.01 | 2.2 × 10−4, 0.04 |
| N-hexane | 0.04 | 0.09 | 0.14 | 0.09 | 0.10 | 9.2 × 10−3, 0.38 |
| Ethyl methyl ketone | 0.03 | 0.07 | 0.12 | 0.09 | 0.09 | 5.3 × 10−3, 0.54 |
| m-Xylene | 0.04 | 0.08 | 0.14 | 0.09 | 0.11 | 8.0 × 10−3, 0.54 |
| o-Xylene | 0.04 | 0.07 | 0.13 | 0.08 | 0.11 | 9.0 × 10−3, 0.49 |
| p-Xylene | 0.03 | 0.07 | 0.13 | 0.09 | 0.10 | 6.0 × 10−3, 0.49 |
| Propene | 2.4 × 10−6 | 0.05 | 0.11 | 0.11 | 0.06 | 0.00, 0.23 |
| Sesquiterpene | 2.1 × 10−7 | 1.2 × 10−6 | 7.5 × 10−6 | 7.3 × 10−6 | 9.4 × 10−6 | 2.09 × 10−10, 1.03 × 10−4 |
| Styrene | 2.0 × 10−17 | 5.1 × 10−4 | 1.0 × 10−3 | 1.5 × 10−3 | 9.7 × 10−4 | 1.00 × 10−27, 6.70 × 10−3 |
| Toluene | 0.29 | 0.50 | 0.83 | 0.54 | 0.71 | 5.00 × 10−2, 3.77 |
| First Trimester of Pregnancy | ||||||
| Benzene | 0.12 | 0.21 | 0.34 | 0.21 | 0.24 | 0.04, 0.70 |
| 1,3 Butadiene | 1.1 × 10−9 | 3.0 × 10−3 | 7.0 × 10−3 | 7.0 × 10−3 | 4.6 × 10−3 | 1.00 × 10−27, 2.15 × 10−2 |
| Ethylbenzene | 0.04 | 0.08 | 0.13 | 0.09 | 0.10 | 7.0 × 10−2, 0.48 |
| Cyclohexane | 0.01 | 0.02 | 0.03 | 0.01 | 0.02 | 1.85 × 10−3, 0.13 |
| MTB ether | 3.0 × 10−3 | 8.0 × 10−3 | 0.01 | 0.01 | 0.01 | 2.0 × 10−4, 0.04 |
| N-hexane | 0.05 | 0.09 | 0.14 | 0.09 | 0.10 | 9.0 × 10−3, 0.37 |
| Ethyl methyl ketone | 0.03 | 0.08 | 0.12 | 0.09 | 0.10 | 5.4 × 10−3, 0.54 |
| m-Xylene | 0.05 | 0.08 | 0.14 | 0.09 | 0.11 | 9.0 × 10−3, 0.49 |
| o-Xylene | 0.04 | 0.07 | 0.12 | 0.08 | 0.11 | 9.0 × 10−3, 0.54 |
| p-Xylene | 0.04 | 0.08 | 0.13 | 0.09 | 0.10 | 6.0 × 10−3, 0.49 |
| Propene | 7.7 × 10−6 | 0.07 | 0.11 | 0.11 | 0.07 | 0.00, 0.22 |
| Sesquiterpene | 2.8 × 10−7 | 1.4 × 10−6 | 9.1 × 10−6 | 8.8 × 10−6 | 10.5 × 10−6 | 2.4 × 10−6, 1.09 × 10−4 |
| Styrene | 2.2 × 10−10 | 6.1 × 10−4 | 1.0 × 10−3 | 1.7 × 10−3 | 1.0 × 10−3 | 1.00 × 10−27, 6.67 × 10−3 |
| Toluene | 0.28 | 0.50 | 0.80 | 0.51 | 0.70 | 5.1 × 10−2, 3.72 |
Abbreviation: MTB, methyl tert-butyl.
a Volume of gaseous pollutant per 109 volumes of ambient air.
API women were exposed to higher levels of VOCs in both the preconception and first-trimester windows. For example, in during the preconception and first-trimester exposure windows, approximately 51% of API women were exposed to high benzene levels, compared with 24% of whites, blacks, and Hispanics (Web Table 4). A similarly high percentage of API women were exposed to high levels of 11 of 14 (78%) VOCs before conception, and to 12 of 14 (85%) VOCs in the first trimester of pregnancy. In contrast, among other racial/ethnic groups, only up to 34% of women were exposed to high levels of VOCs in either the preconception or first-trimester exposure window (Web Table 4).
In preconception models (Table 2), exposure to high levels of VOCs was associated with increased odds of GDM among whites and APIs, except for exposure to 1,3-butadiene and styrene. No statistically significant associations were observed among blacks or Hispanics. Interaction terms suggest the association between GDM and exposure to high levels of VOCs was typically stronger among APIs than among whites. For example, exposure to high levels of benzene was associated with 41% (odds ratio (OR) = 1.41, 99% confidence interval (CI): 1.12, 1.77) increased odds of GDM among APIs and 25% (OR = 1.25, 99% CI: 1.08, 1.43) increased odds among whites.
Table 2.
Association Between Preconception Exposure to High Volatile Organic Compounds (≥75th Percentile) and Gestational Diabetes in the Consortium on Safe Labor, United States, 2002–2008a
| VOC | White (n = 109,396) | Black (n = 49,093) | Hispanic (n = 38,241) | Asian/Pacific Islander (n = 9,068) | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 99% CI | OR | 99% CI | OR | 99% CI | OR | 99% CI | |
| Benzene | 1.25 | 1.08, 1.43b | 1.07 | 0.89, 1.30 | 1.01 | 0.84, 1.23 | 1.41 | 1.12, 1.77b,c |
| 1,3 Butadiene | 1.01 | 0.92, 1.10 | 0.98 | 0.86, 1.12 | 0.92 | 0.81, 1.04 | 1.01 | 0.81, 1.27 |
| Ethylbenzene | 1.38 | 1.17, 1.63b | 1.16 | 0.95, 1.43 | 1.18 | 0.95, 1.47 | 1.57 | 1.22, 2.01b,c |
| Cyclohexane | 1.09 | 0.99, 1.21 | 0.96 | 0.82, 1.12 | 0.97 | 0.84, 1.13 | 1.25 | 1.02, 1.53b,c |
| MTB ether | 1.15 | 1.06, 1.24b | 1.06 | 0.93, 1.21 | 0.95 | 0.84, 1.07 | 1.27 | 1.05, 1.53b |
| N-hexane | 1.30 | 1.16, 1.46b | 1.11 | 0.94, 1.31 | 1.08 | 0.92, 1.28 | 1.46 | 1.18, 1.80b,c |
| Ethyl methyl ketone | 1.18 | 1.08, 1.28b | 1.05 | 0.91, 1.21 | 1.00 | 0.88, 1.15 | 1.24 | 1.03, 1.51b |
| m-Xylene | 1.32 | 1.12, 1.56b | 1.15 | 0.93, 1.41 | 1.12 | 0.90, 1.38 | 1.47 | 1.15, 1.89b,c |
| o-Xylene | 1.16 | 0.99, 1.35 | 0.97 | 0.79, 1.19 | 0.99 | 0.81, 1.22 | 1.32 | 1.04, 1.67b,c |
| p-Xylene | 1.31 | 1.15, 1.50b | 1.12 | 0.94, 1.35 | 1.12 | 0.94, 1.34 | 1.45 | 1.16, 1.82b,c |
| Propene | 1.12 | 1.04, 1.21b | 1.02 | 0.90, 1.16 | 0.87 | 0.76, 1.00c | 1.29 | 1.07, 1.56b,c |
| Sesquiterpene | 1.16 | 1.06, 1.26b | 1.09 | 0.95, 1.25 | 0.96 | 0.84, 1.09c | 1.38 | 1.13, 1.68b,c |
| Styrene | 1.02 | 0.93, 1.12 | 0.99 | 0.87, 1.12c | 0.94 | 0.83, 1.07c | 0.98 | 0.77, 1.24 |
| Toluene | 1.13 | 0.97, 1.31 | 0.95 | 0.78, 1.16 | 0.96 | 0.79, 1.18 | 1.28 | 1.02, 1.62b,c |
Abbreviations: CI, confidence interval; MTB, methyl tert-butyl; OR, odds ratio.
a Model was adjusted for maternal race, maternal age, insurance status, marital status, season of conception, parity, site, hospital type, and prepregnancy body mass index. Other race/ethnicity groups were included in the analysis, but stratum-specific results are not reported here because of heterogeneity of the population.
b Statistically significant estimate (P < 0.01).
c Significant interaction term (P < 0.01) for VOCs and race/ethnicity (reference: non-Hispanic white).
In first-trimester models (Table 3), exposure to high levels of 6 of the 14 VOCs was associated with increased odds of GDM among whites and APIs: ethylbenzene, methyl tert-butyl ether, N-hexane, ethyl methyl ketone, propene, and sesquiterpene. The association between benzene and GDM was statistically significant among APIs (OR = 1.29, 99% CI: 1.04, 1.59) but not among whites (OR = 1.13, 99% CI: 0.99, 1.29; P for interaction < 0.01). No statistically significant associations were observed among blacks or Hispanics. The association between GDM and exposure to high levels of VOCs was typically stronger among APIs than among whites. For example, N-hexane exposure was associated with 34% (OR = 1.34, 99% CI: 1.10, 1.65) increased odds of GDM among APIs and only a 15% (OR = 1.15, 99% CI: 1.03, 1.29) increased odds among whites (P for interaction < 0.01).
Table 3.
Association Between First-Trimester Exposure to High Volatile Organic Compounds (≥75th Percentile) and Gestational Diabetes in the Consortium on Safe Labor, United States, 2002–2008a
| VOC | White (n = 109,396) | Black (n = 49,093) | Hispanic (n = 38,241) | Asian/Pacific Islander (n = 9,068) | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 99% CI | OR | 99% CI | OR | 99% CI | OR | 99% CI | |
| Benzene | 1.13 | 0.99, 1.29 | 0.98 | 0.81, 1.17 | 0.95 | 0.79, 1.15 | 1.29 | 1.04, 1.59b,c |
| 1,3 Butadiene | 0.97 | 0.88, 1.07 | 0.87 | 0.76, 1.00c | 0.88 | 0.77, 1.01c | 0.91 | 0.71, 1.16c |
| Ethylbenzene | 1.13 | 0.95, 1.33b | 0.96 | 0.77, 1.18 | 0.95 | 0.76, 1.17 | 1.28 | 1.00, 1.64b,c |
| Cyclohexane | 1.03 | 0.93, 1.14 | 0.97 | 0.83, 1.13 | 0.95 | 0.82, 1.10 | 1.13 | 0.92, 1.39c |
| MTB ether | 1.10 | 1.01, 1.19b | 1.00 | 0.88, 1.15 | 0.90 | 0.79, 1.01 | 1.28 | 1.06, 1.54b,c |
| N-hexane | 1.15 | 1.03, 1.29b | 0.99 | 0.84, 1.18 | 0.97 | 0.82, 1.14 | 1.34 | 1.10, 1.65b,c |
| Ethyl methyl ketone | 1.13 | 1.03, 1.24b | 1.01 | 0.87, 1.17 | 0.93 | 0.81, 1.08 | 1.21 | 1.01, 1.46b |
| m-Xylene | 1.07 | 0.89, 1.27 | 0.90 | 0.72, 1.13 | 0.89 | 0.71, 1.11 | 1.21 | 0.94, 1.56c |
| o-Xylene | 1.02 | 0.86, 1.21 | 0.86 | 0.69, 1.07 | 0.86 | 0.69, 1.07 | 1.17 | 0.91, 1.51c |
| p-Xylene | 1.08 | 0.93, 1.25 | 0.93 | 0.76, 1.14 | 0.90 | 0.74, 1.10 | 1.23 | 0.97, 1.55c |
| Propene | 1.10 | 1.02, 1.19b | 0.97 | 0.85, 1.11 | 0.95 | 0.82, 1.09 | 1.28 | 1.06, 1.54b,c |
| Sesquiterpene | 1.13 | 1.03, 1.23b | 1.06 | 0.92, 1.22 | 0.91 | 0.79, 1.02 | 1.36 | 1.12, 1.65b,c |
| Styrene | 1.00 | 0.91, 1.10 | 0.93 | 0.81, 1.06 | 0.90 | 0.75, 1.20c | 0.95 | 0.75, 1.20 |
| Toluene | 1.08 | 0.92, 1.28 | 0.91 | 0.73, 1.13 | 0.92 | 0.74, 1.14 | 1.23 | 0.96, 1.50c |
Abbreviations: CI, confidence interval; MTB, methyl tert-butyl; OR, odds ratio.
a Model was adjusted for maternal race, maternal age, insurance status, marital status, season of conception, parity, site, hospital type, and prepregnancy body mass index. Other race/ethnicity groups were included in the overall analysis, but stratum-specific results are not reported here, due to heterogeneity of the population.
b Statistically significant estimate (P < 0.01).
c Significant interaction term (P < 0.01), suggesting the association is different from data on white women.
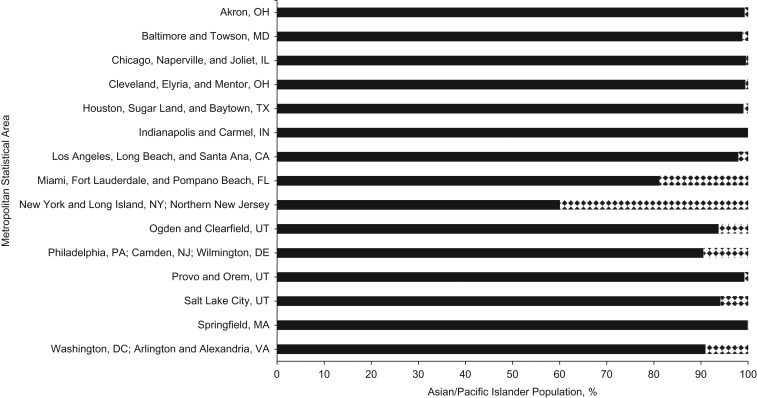
In sensitivity analysis, excluding BMI from the models did not result in any meaningful change in the observed associations for APIs and whites but did result in a greater number of statistically significant associations for blacks and Hispanics (Web Table 5). In post hoc analysis, the API populations residing within the metropolitan areas represented in the CSL were, on average, 93.44% Asian and 6.56% Hawaiian/Pacific Islander. The distribution of APIs by ancestry for each metropolitan area is shown in Figure 1.
Figure 1.
Asian/Pacific Islander population percentage by ancestry in metropolitan statistical areas of hospitals in the Consortium on Safe Labor, 2002–2008. Asian ancestry is represented by solid black; Pacific Islander ancestry is represented by the black-and-white pattern.
DISCUSSION
In this study of ambient VOC exposure and GDM, we consistently observed that high levels of VOCs increased the odds of GDM among both whites and APIs, but not among blacks, and only for preconception ethylbenzene exposure among Hispanics. Furthermore, the associations were typically stronger among APIs than among whites and were not apparently influenced by differences in BMI. These results are in line with our hypothesis that APIs would have the highest GDM risk associated with VOC exposure. Exposure did not appear to influence risk for blacks and Hispanics, perhaps due to limited variation in ambient VOC exposure compared with whites (Web Table 4).
These findings on exposure to ambient VOCs and GDM add to the growing literature regarding associations between adverse pregnancy outcomes and preconception and prenatal exposure to VOCs. For example, preconception exposure to high levels of VOCs is associated with gestational hypertension (45), whereas exposure to high levels of VOCs during pregnancy is associated with higher blood pressure (46), cardiovascular events (47), and decreased birth weight (48).
Our findings are also in line with evidence regarding criteria air pollutant exposure and GDM. For example, preconception and first-trimester exposure to nitrogen oxides and sulfur dioxide was associated with increased GDM risk, according to CSL data (25). Similar observations were made in large cohorts of pregnant women in Denmark, Sweden, and Taiwan (22–24). Our results also suggest that chronic exposure to air pollutants increases risk of GDM, because associations appear consistent across adjacent exposure windows (22–25). For example, among API women, preconception propene exposure was associated with 30% (OR = 1.30, 99% CI: 1.07, 1.57) increased odds of GDM, and first-trimester propene exposure was associated with 28% (OR = 1.28, 99% CI: 1.06, 1.54) increased odds of GDM (Tables 2 and 3). Among white women, associations were similar but weaker across preconception and first-trimester exposure windows.
The results of the present study are consistent with 2 lines of evidence, which, when taken together, suggest APIs may be uniquely susceptible to GDM development associated with VOC exposure. First, relevant to all racial/ethnic groups, exposure to air pollution increases inflammation and oxidative stress, which are linked with pancreatic β-cell dysfunction (25, 27–30, 49). For example, benzene exposure, a VOC examined in the present study, had a dose-response relationship with increased oxidative stress, poor pancreatic β-cell viability, and increased insulin resistance in laboratory animals, but the dosing was not comparable to an ambient air exposure (26). Among humans, excessive oxidative stress also impairs insulin expression, leading to pancreatic β-cell dysfunction and insulin resistance (29, 30). Second, results of genome-wide association studies suggest genetic variants associated with insulin resistance and pancreatic β-cell dysfunction (31, 37) are more common in API populations, supporting the notion of racial/ethnic-specific variation in genetic risks for GDM (37). Given GDM is a multifactorial disease, these observations provide evidence of GDM risk among APIs, and the interaction between environmental and genetic risks merits further attention.
Because APIs are grouped in CSL data, our analyses cannot distinguish various subgroups. Populations of various Asian backgrounds share common genetic risks (31–36), but evidence is less clear among Pacific Islanders. One study found Micronesians had a greater insulin response to oral glucose than did Polynesians, suggesting variability in pancreatic β-cell function by ethnicity among Pacific Islanders (36). This potential susceptibility is compounded by the greater proportion of API women in the CSL exposed to high levels of VOCs compared with other racial/ethnic groups (Web Table 4).
Our findings highlight environmental health disparities among the API community, which is often overlooked in environmental justice literature because of small sample sizes and the “model minority” label (3, 5). Small sample sizes of API within population-based datasets may not allow for examination of API health disparities or comparisons within API subpopulations (3, 50, 51). APIs are often labeled as model minorities because of their high average socioeconomic status, which suggests the API population has low risk for poor health outcomes compared with other racial/ethnic groups (3, 52). Reliance on the model minority label would appear to mask systemic discrimination contributing to environmental health disparities faced by US API communities.(3, 52, 53). Characteristics of API women in this analysis are in line with the model minority label: They are of similarly high socioeconomic status as whites with respect to private insurance coverage (74% vs. 78%) and marital status (84% vs. 78% married). However, the stronger associations between GDM and exposure to higher levels of VOCs among API women are in line with accumulating evidence that the model minority label does not accurately describe the health experiences of API populations (54–56). Studies investigating potential interactions between environmental factors and genetic susceptibility are warranted to better understand environmental risk factors among the API community.
Because we were unable to disaggregate Asian and Pacific Islander ancestries from the medical record, we examined the percentage of the API population by ancestry for the metropolitan statistical areas within which the CSL hospitals are located. We found CSL hospitals are in metropolitan areas with substantially larger Asian populations than Pacific Islander populations, suggesting our data largely reflect women of Asian ancestry (Figure 1). And because Asian ancestry may confer higher genetic susceptibility to pancreatic β-cell dysfunction than does Pacific Islander ancestry (31–36), the stronger associations we observed among API women are likely driven by Asian women. Our results may be less applicable to Pacific Islanders, and more research is needed regarding the diversity within the API population.
Our findings are notable for several reasons. To our knowledge, this is the first study to identify the association between GDM and exposure to VOCs, as well as the first study to examine race-specific associations between GDM and VOC exposure. Our findings are consistent with a potential biologic link between GDM risk and exposure to high levels of VOCs among API women. In addition, we used modelled air pollution data to obtain estimates of VOC exposure for the full cohort. The modelled data took population density, spatial and temporal distribution, and weather into account when estimating levels of VOCs (40). This study also benefits from the large amount of clinical data available in the CSL, allowing for a robust examination of the association between VOCs and GDM in a diverse sample of pregnant women in the United States.
This study should also be considered in the context of its limitations. We were unable to examine API women by ancestry, and ancestry may be related to both VOC exposure and pancreatic β-cell function. However, results of post hoc analysis suggest the majority of our sample was of Asian ancestry. The estimates of VOC exposure were averaged over HRRs in which the birth occurred and were not based on participants’ address or neighborhood. Exposure misclassification may have occurred if mothers resided outside the HRR for all or part of their pregnancy. Approximately 10%–30% of pregnant women move during pregnancy, but most moves occur within the same region to areas with similar levels air pollution (57–59). The HRRs are generally similar in size to, and often overlap with, metropolitan statistical areas. Residents within an HHR contribute more than 80% of hospitalizations to that hospital, so mothers are likely to live within the HRR (60). Our models were adjusted for HRR to account for potential spatial variability, which also removes some variation associated with VOC exposure, and we adjusted the models for hospital type, because tertiary care centers might serve a more dispersed population.
Other possible sources of misclassification concern whether pregnant women potentially spend more time indoors than other individuals or vary in their activity patterns. Although we cannot rule out an impact based on these differences, women in their first trimester of pregnancy have been reported to spend a relatively similar amount of time indoors as a general population group (14.4 hours per day vs. 15.5 hours per day, respectively) (61). First-trimester exposure estimates based solely on ambient air pollution measures at the home address also have been strongly correlated with exposure estimates taking into account space-time activity (i.e., time spent commuting, time spent indoors; r = 0.98, P < 0.01) (62).
Overall, we have applied a conservative strategy for estimating ambient VOC exposure, averaging over broader time and space dimensions to provide more stable estimates. As such, these results may be biased toward the null for several reasons. We averaged VOC exposure over the HRR, which reduces the potential impact of small point-source exposure. We examined a dichotomous high/not high exposure variable because we did not assume relationships would be linear and there are no routine monitoring data to fuse to the modeled data. Finally, we believe misclassification would be nondifferential because it is unlikely that later onset of GDM is related to the mother’s residential or local mobility before conception or in early pregnancy.
The results for any individual VOC should be interpreted with caution. Some of the VOCs are highly correlated and VOCs may be correlated with other pollutants associated with GDM risk, such as nitrogen oxides (25), and may share common sources, limiting attribution of risk associated with a specific compound. We report them individually to encourage other researchers who may have specific compound data to attempt to confirm or refute our findings. In this first examination of the association between GDM and exposure to higher levels of VOCs, we found the association between VOCs and GDM was stronger among API women than among women from other racial/ethnic groups. Whether these findings are due to differences in pancreatic β-cell function associated with increased susceptibility to VOCs should be further explored. The US API population often has been overlooked in the environmental health literature; therefore, studies focused on this population are warranted to better understand the environmental health disparities of these communities.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Epidemiology Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland (Andrew D. Williams, Katherine L. Grantz, Cuilin Zhang, Carrie Nobles, Pauline Mendola); and The Emmes Corporation, Rockville, Maryland (Seth Sherman).
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), including funding for the Consortium on Safe Labor (contract no. HHSN267200603425C) and the Air Quality and Reproductive Health Study (contract no. HHSN275200800002I, task order no. HHSN27500008). Data used in this manuscript were obtained from The Dartmouth Atlas, which is funded by the Robert Wood Johnson Foundation and the Dartmouth Clinical and Translational Science Institute under award number UL1TR001086 from the National Center for Advancing Translational Sciences of the NIH.
This paper has been cleared for publication by the NICHD, but the funding source had no role in the design, analysis, interpretation or writing of the manuscript.
Conflict of interest: none declared.
Abbreviations
- API
Asian/Pacific Islander
- BMI
body mass index
- CI
confidence interval
- CSL
Consortium on Safe Labor
- GDM
gestational diabetes mellitus
- HRR
hospital referral region
- OR
odds ratio
- VOC
volatile organic compound
REFERENCES
- 1. Brown P. Race, class, and environmental health: a review and systematization of the literature. Environ Res. 1995;69(1):15–30. [DOI] [PubMed] [Google Scholar]
- 2. Clark LP, Millet DB, Marshall JD. National patterns in environmental injustice and inequality: outdoor NO2 air pollution in the United States. PLoS One. 2014;9(4):e94431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grineski SE, Collins TW, Morales DX. Asian Americans and disproportionate exposure to carcinogenic hazardous air pollutants: a national study. Soc Sci Med. 2017;185:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clark LP, Millet DB, Marshall JD. Changes in transportation-related air pollution exposures by race-ethnicity and socioeconomic status: outdoor nitrogen dioxide in the United States in 2000 and 2010. Environ Health Perspect. 2017;125(9):097012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sze J. Asian American activism for environmental justice. Peace Rev. 2004;16(2):149–156. [Google Scholar]
- 6. Zou B, Peng F, Wan N, et al. . Spatial cluster detection of air pollution exposure inequities across the United States. PLoS One. 2014;9(3):e91917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention Leading Causes of Death (LCOD) by Race/Ethnicity, All Males-United States, 2014 2017. https://www.cdc.gov/healthequity/lcod/men/2014/race-ethnicity/index.htm. Accessed August 11, 2017.
- 8. Centers for Disease Control and Prevention Leading Causes of Death (LCOD) by Race/Ethnicity, All Females-United States, 2014 2017. https://www.cdc.gov/women/lcod/2014/race-ethnicity/index.htm. Accessed August 11, 2017.
- 9. Lopez G, Ruiz NG, Patten E. Key Facts About Asian Americans, a Diverse and Growing Population Washington, DC: Pew Research Center; 2017. http://www.pewresearch.org/fact-tank/2017/09/08/key-facts-about-asian-americans/. Accessed August 11, 2017.
- 10. DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis. 2014;11:E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lavery JA, Friedman AM, Keyes KM, et al. . Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124(5):804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hedderson M, Ehrlich S, Sridhar S, et al. . Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35(7):1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol. 2010;24(5):441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(suppl 2):S141–S146. [DOI] [PubMed] [Google Scholar]
- 15. Dabelea D, Snell-Bergeon JK, Hartsfield CL, et al. . Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28(3):579–584. [DOI] [PubMed] [Google Scholar]
- 16. Kim SY, England L, Sappenfield W, et al. . Racial/ethnic differences in the percentage of gestational diabetes mellitus cases attributable to overweight and obesity, Florida, 2004–2007. Prev Chronic Dis. 2012;9: E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hunsberger M, Rosenberg KD, Donatelle RJ. Racial/ethnic disparities in gestational diabetes mellitus: findings from a population-based survey. Womens Health Issues. 2010;20(5):323–328. [DOI] [PubMed] [Google Scholar]
- 18. Savitz DA, Janevic TM, Engel SM, et al. . Ethnicity and gestational diabetes in New York City, 1995–2003. BJOG. 2008;115(8):969–978. [DOI] [PubMed] [Google Scholar]
- 19. Thorpe LE, Berger D, Ellis JA, et al. . Trends and racial/ethnic disparities in gestational diabetes among pregnant women in New York City, 1990–2001. Am J Public Health. 2005;95(9):1536–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Committee on Practice Bulletins–Obstetrics ACOG practice bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–e64. [DOI] [PubMed] [Google Scholar]
- 21. Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev. 2015;11(3):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan SC, Huang CC, Lin SJ, et al. . Gestational diabetes mellitus was related to ambient air pollutant nitric oxide during early gestation. Environ Res. 2017;158:318–323. [DOI] [PubMed] [Google Scholar]
- 23. Pedersen M, Olsen SF, Halldorsson TI, et al. . Gestational diabetes mellitus and exposure to ambient air pollution and road traffic noise: a cohort study. Environ Int. 2017;108:253–260. [DOI] [PubMed] [Google Scholar]
- 24. Malmqvist E, Jakobsson K, Tinnerberg H, et al. . Gestational diabetes and preeclampsia in association with air pollution at levels below current air quality guidelines. Environ Health Perspect. 2013;121(4):488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robledo CA, Mendola P, Yeung E, et al. . Preconception and early pregnancy air pollution exposures and risk of gestational diabetes mellitus. Environ Res. 2015;137:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bahadar H, Maqbool F, Mostafalou S, et al. . Assessment of benzene induced oxidative impairment in rat isolated pancreatic islets and effect on insulin secretion. Environ Toxicol Pharmacol. 2015;39(3):1161–1169. [DOI] [PubMed] [Google Scholar]
- 27. van den Hooven EH, de Kluizenaar Y, Pierik FH, et al. . Chronic air pollution exposure during pregnancy and maternal and fetal C-reactive protein levels: the Generation R Study. Environ Health Perspect. 2012;120(5):746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee PC, Talbott EO, Roberts JM, et al. . Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology. 2011;22(4):524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gerber PA, Rutter GA. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal. 2017;26(10):501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaneto H, Kawamori D, Matsuoka TA, et al. . Oxidative stress and pancreatic β-cell dysfunction. Am J Ther. 2005;12(6):529–533. [DOI] [PubMed] [Google Scholar]
- 31. Bhopal RS. A four-stage model explaining the higher risk of type 2 diabetes mellitus in South Asians compared with European populations. Diabet Med. 2013;30(1):35–42. [DOI] [PubMed] [Google Scholar]
- 32. Chen KW, Boyko EJ, Bergstrom RW, et al. . Earlier appearance of impaired insulin secretion than of visceral adiposity in the pathogenesis of NIDDM. 5-Year follow-up of initially nondiabetic Japanese-American men. Diabetes Care. 1995;18(6):747–753. [DOI] [PubMed] [Google Scholar]
- 33. Hulman A, Simmons RK, Brunner EJ, et al. . Trajectories of glycaemia, insulin sensitivity and insulin secretion in South Asian and white individuals before diagnosis of type 2 diabetes: a longitudinal analysis from the Whitehall II cohort study. Diabetologia. 2017;60(7):1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsumoto K, Miyake S, Yano M, et al. . Glucose tolerance, insulin secretion, and insulin sensitivity in nonobese and obese Japanese subjects. Diabetes Care. 1997;20(10):1562–1568. [DOI] [PubMed] [Google Scholar]
- 35. Rattarasarn C, Soonthornpan S, Leelawattana R, et al. . Decreased insulin secretion but not insulin sensitivity in normal glucose tolerant Thai subjects. Diabetes Care. 2006;29(3):742–743. [DOI] [PubMed] [Google Scholar]
- 36. Zimmet P, Whitehouse S, Kiss J. Ethnic variability in the plasma insulin response to oral glucose in Polynesian and Micronesian subjects. Diabetes. 1979;28(7):624–628. [DOI] [PubMed] [Google Scholar]
- 37. Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375(9712):408–418. [DOI] [PubMed] [Google Scholar]
- 38. Zhang J, Troendle J, Reddy UM, et al. . Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203(4):326.e1–326.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. American Diabetes Association Gestational diabetes mellitus. Diabetes Care. 2003;26(suppl 1):S103–S105. [DOI] [PubMed] [Google Scholar]
- 40. Chen G, Li J, Ying Q, et al. . Evaluation of observation-fused regional air quality model results for population air pollution exposure estimation. Sci Total Environ. 2014;485–486:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dartmouth Atlas of Healthcare Working Group Dartmouth Atlas of Healthcare: Geographic Crosswalks and Research Files Lebanon, NH: Dartmouth College; 2013. http://www.dartmouthatlas.org/tools/downloads.aspx?tab=39. Accessed June 30, 2017.
- 42. Ahlbom A, Alfredsson L. Interaction: a word with two meanings creates confusion. Eur J Epidemiol. 2005;20(7):563–564. [DOI] [PubMed] [Google Scholar]
- 43. Cohen RA. Impact of type of insurance plan on access and utilization of health care services for adults aged 18–64 years with private health insurance: United States, 2007–2008. NCHS Data Brief. 2010;(28):1–8. [PubMed] [Google Scholar]
- 44. US Census Bureau Income and Poverty in the United States: 2016. Current Population Reports, P60-259 Washington, DC: US Census Bureau; 2017. https://www.census.gov/content/dam/Census/library/publications/2017/demo/P60-259.pdf. Accessed November 3, 2017.
- 45. Zhu Y, Zhang C, Liu D, et al. . Ambient air pollution and risk of gestational hypertension. Am J Epidemiol. 2017;186(3):334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Männistö T, Mendola P, Liu D, et al. . Acute air pollution exposure and blood pressure at delivery among women with and without hypertension. Am J Hypertens. 2015;28(1):58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Männistö T, Mendola P, Laughon Grantz K, et al. . Acute and recent air pollution exposure and cardiovascular events at labour and delivery. Heart. 2015;101(18):1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chang M, Park H, Ha M, et al. . The effect of prenatal TVOC exposure on birth and infantile weight: the Mothers and Children’s Environmental Health study. Pediatr Res. 2017;82:423–428. [DOI] [PubMed] [Google Scholar]
- 49. Lowe LP, Metzger BE, Lowe WL Jr, et al. . Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab. 2010;95(12):5427–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Holland AT, Palaniappan LP. Problems with the collection and interpretation of Asian-American health data: omission, aggregation, and extrapolation. Ann Epidemiol. 2012;22(6):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palaniappan LP, Wong EC, Shin JJ, et al. . Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond). 2011;35(3):393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yi SS, Kwon SC, Sacks R, et al. . Commentary: persistence and health-related consequences of the model minority stereotype for Asian Americans. Ethn Dis. 2016;26(1):133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chou RS, Feagin JR. Myth of the Model Minority: Asian Americans Facing Racism. Boulder, CO: Paradigm Publishers; 2015. [Google Scholar]
- 54. Saenphansiri X, Wyant DK, Wofford LG. Barriers to health care among Laotian Americans in Middle Tennessee. J Health Care Poor Underserved. 2017;28(4):1537–1558. [DOI] [PubMed] [Google Scholar]
- 55. Pu J, Hastings KG, Boothroyd D, et al. . Geographic variations in cardiovascular disease mortality among Asian American subgroups, 2003–2011. J Am Heart Assoc. 2017;6(7):e005597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mui P, Bowie JV, Juon HS, et al. . Ethnic group differences in health outcomes among Asian American men in California. Am J Men’s Health. 2017;11(5):1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bell ML, Belanger K. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol. 2012;22(5):429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen L, Bell EM, Caton AR, et al. . Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res. 2010;110(2):162–168. [DOI] [PubMed] [Google Scholar]
- 59. Pereira G, Bracken MB, Bell ML. Particulate air pollution, fetal growth and gestational length: the influence of residential mobility in pregnancy. Environ Res. 2016;147:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dartmouth Atlas of Healthcare Working Group The Dartmouth Atlas of Health Care in the United States Hanover, NH; 1996. http://www.dartmouthatlas.org/downloads/atlases/96Atlas.pdf. Accessed June 30, 2017.
- 61. Nethery E, Brauer M, Janssen P. Time–activity patterns of pregnant women and changes during the course of pregnancy. J Expo Sci Environ Epidemiol. 2009;19:317–324. [DOI] [PubMed] [Google Scholar]
- 62. Ouidir M, Giorgis-Allemand L, Lyon-Caen S, et al. . Estimation of exposure to atmospheric pollutants during pregnancy integrating space–time activity and indoor air levels: does it make a difference? Environ Int. 2015;84:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.