Abstract
Background:
Conjoined flap viability is associated with arterial blood supply and venous return. This study aimed to assess the effects of venous drainage position on arterial blood supply and venous return within the conjoined flap.
Methods:
Fifty-four rats were divided randomly into three groups (n = 18 per group). In experimental group 2, only the right intercostal posterior artery and the left iliolumbar vein were maintained; meanwhile, only the right intercostal posterior artery and the left intercostal posterior vein were preserved in experimental group 1. The control group had only the right intercostal posterior artery and vein preserved. The distances between angiosomes were measured. At 7 days after surgery, flap survival was evaluated, lead oxide–gelatin flap angiography was performed, and average microvessel density was assessed by hematoxylin and eosin staining, and lactate levels were assessed.
Results:
The distance between angiosomes I and II was the shortest, whereas angiosomes I and III were most distant (p < 0.05). At 7 days after surgery, survival rates in experimental group 2 and experimental group 1 were both 100 percent, whereas 86.5 ± 1.6 percent of controls survived. Furthermore, angiogenesis was more obvious in experimental group 2 than in experimental group 1 and controls. Moreover, lactate levels were lower in experimental group 2 (7.47 ± 0.17 mM) and experimental group 1 (8.03 ± 0.31 mM) compared with control values (9.98 ± 0.37 mM; p < 0.05).
Conclusion:
Changes in position of venous drainage might cause continuous arterial high-pressure perfusion and venous superdrainage, which improves flap survival.
During flap transplantation, complications associated with insufficient arterial blood supply1,2 and obstructed venous return3–7 are not uncommon, and could result in ischemia, congestion, and necrosis. To solve these problems, arterial supercharging or venous superdrainage is widely used. Some studies have demonstrated that arterial supercharging is more effective than venous superdrainage for flap survival,8,9 whereas others hold the opposite view.10,11 In the clinic, complications resulting from venous problems are more common than those caused by arterial ailments.12–14 Kaplan et al.15 demonstrated that with more veins, venous drainage is better and congestion is less. Some studies have also suggested significantly reduced flap failure and venous thrombosis after anastomosis involving two veins compared with one.16,17 However, Ehrl et al.18 showed that flap survival is independent of the number of venous anastomoses. This study maintained the number of veins, but changed the positions of venous drainage, to assess the effects of venous drainage position on arterial blood supply and venous return within the flap, providing a theoretical basis for the clinical management of conjoined flaps.
MATERIALS AND METHODS
Animals
Fifty-four Sprague-Dawley rats aged 2 months and weighing 250 ± 10 g were obtained from the Laboratory Animal Center of Wenzhou Medical University, Zhejiang Province, People’s Republic of China (license no. SCXK2010-0044). Only male animals were used in this study to avoid gender-related differences. The rats were maintained under a normal 12-hour/12-hour dark/light cycle at 23° to 25°C with a relative humidity of 45 to 55 percent, in individual cages with soft bedding, and given standard laboratory food (Laboratory Animal Center of Zhejiang Province) and water before and after the procedure. The experiments were performed in accordance with ethical guidelines for the use and care of animals; the study was approved by the ethics committee of Wenzhou Medical University.
Surgical Procedure
There are four angiosomes in the dorsum of Sprague-Dawley rats, including the right and left posterior intercostal angiosomes, and the right and left iliolumbar angiosomes19; anastomoses between the four angiosomes are termed the choke area (Fig. 1). Fifty-four rats were divided randomly into three groups (n = 18 per group): (1) in experimental group 2 group, only the right posterior intercostal artery and the left iliolumbar vein were preserved; (2) in experimental group 1 group, only the right posterior intercostal artery and the left posterior intercostal vein were preserved; and (3) the control group had only the right posterior intercostal artery and vein preserved (Fig. 2). All surgical instruments were autoclaved before use. After anesthesia with 5% chloral hydrate (6 ml/kg administered intraperitoneally),20 the entire back was shaved, and the rats were placed in the prone position. The flap measured approximately 6 × 7 cm, with the following borders: (1) cranial, 2.5 cm below the shoulder blade; (2) caudal, at the iliac crest level; (3) intermediate, at the middle axillary level. Then, a sterile towel was placed on the back, and the operative area was sterilized with iodine and 75% alcohol. The flap containing the skin and superficial fascia was dissected completely from the underling soft tissue, and the arteries and veins were ablated or preserved according to grouping. After careful hemostasis, the flap was restored into the orthotopic site using interrupted 4-0 nylon sutures (Pudong Jinhuan Medical Products, Shanghai, People’s Republic of China).
Fig. 1.
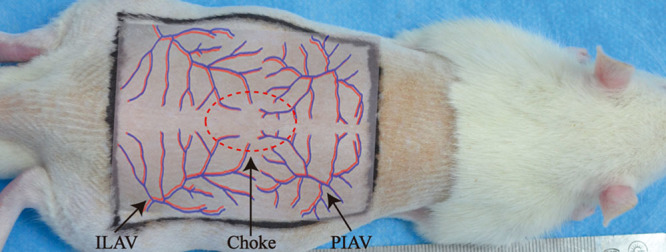
The four angiosomes of the rat dorsum. PIAV, posterior intercostal artery and vein; ILAV, iliolumbar artery and vein; Choke, anastomosis area between the four angiosomes.
Fig. 2.
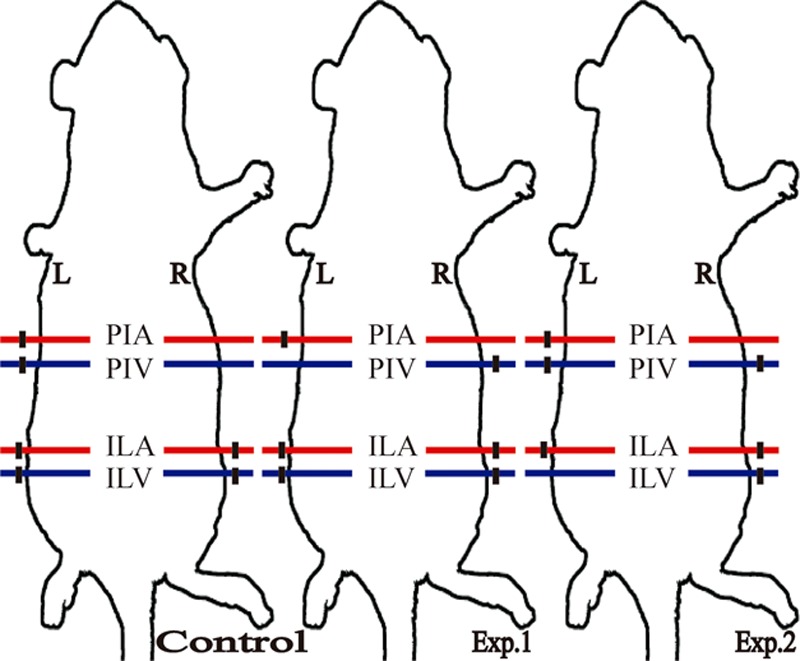
The three flap models. (Left) Control group, preserving only the right posterior intercostal artery and vein; (center) experimental group 1, preserving only the right posterior intercostal artery and the left posterior intercostal vein; (right) experimental group 2 group, preserving only the right posterior intercostal artery and the left iliolumbar vein. PIA, posterior intercostal artery; PIV, posterior intercostal vein; ILA, iliolumbar artery; ILV, iliolumbar vein; R, right; L, left; red, artery; blue, vein; black, ablation.
Distances between Angiosomes
First, angiosome positions were determined in the flap, followed by measurements of the distances between the angiosomes obtained with Vernier calipers. The right posterior intercostal, left posterior intercostal, left iliolumbar, and right iliolumbar angiosomes were angiosomes I to IV, respectively.
Flap Survival Assessment
At 7 days postoperatively, the rats were anesthetized, and the flaps were photographed with a digital camera (Nikon, Tokyo, Japan); image analysis was performed with ImageJ software (National Institutes of Health, Bethesda, Md.). The area of survival relative to the total area of the flap was considered flap survival, and was computed by dividing the number of pixels constituting the survival area by that of pixels in the whole flap.21
Perforator Flap Angiography
After flap survival assessment, 20 to 30 ml/kg of lead oxide–gelatin (Wenzhou Jinshan Chemical Reagent Co., Ltd, Wenzhou, People’s Republic of China) was injected into the right common carotid artery of the animals through a 22-gauge silicone rubber catheter (Pudong Jinhuan Medical Products, Shanghai, People’s Republic of China). The perfused rat cadavers were frozen at −20°C overnight to allow lead oxide–gelatin to solidify.22 An abdominal midline incision was made, and the trunk skin was stripped for radiography (XG-1; Fujifilm, Tokyo, Japan) to assess vessel changes attributable to the flap.
Average Microvessel Density
At 7 days after surgery, skin tissue samples (1 × 1 cm) were harvested from the choke area, fixed with 4% paraformaldehyde, and embedded in paraffin. Then, 4-μm slices were stained with hematoxylin and eosin (Solarbio, Beijing, People’s Republic of China). Average microvessel density was assessed according to Weidner et al.23 Briefly, hematoxylin and eosin–stained skin tissue samples were screened at low power (100× magnification) to identify three hot spots of typical angiogenesis. Within the hot spots, microvessels were counted at high power (200× magnification), and average vessel count in three hot spots was considered as average microvessel density. All counts were performed by two investigators in a blinded manner; the counts were compared between them, and discrepant results were reassessed. The consensus was used for average microvessel density analysis.
Biochemical Analysis
At 7 days after surgery, lactate levels in tail vein blood samples were assessed on an automatic biochemical analyzer (Vitros5.1FS; Ortho Clinical Diagnostics, Rochester, N.Y.).
Statistical Analysis
Data are mean ± standard error, and SPSS 17.0 software (SPSS, Inc., Chicago, Ill.) was used for statistical analysis. One-way analysis of variance followed by the Tukey test was used to compare the three groups. Values of p < 0.05 were considered statistically significant.
RESULTS
Distances between Angiosomes
The distances between angiosomes I and II, I and III, and I and IV were 2.495 ± 0.063 cm, 4.862 ± 0.066 cm, and 3.684 ± 0.051 cm, respectively. Angiosomes I and II were the closest, whereas the distance from I to III was the longest (p < 0.05) (Fig. 3).
Fig. 3.
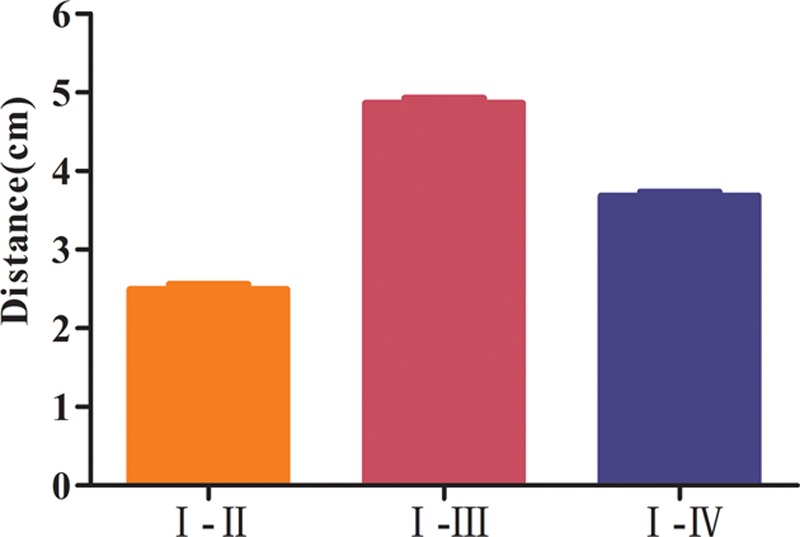
Distances between the angiosomes (n = 16). The distance between angiosomes I and II was the shortest distance, whereas the distance between angiosomes I and III was the longest. All showed statistical significance (p < 0.05).
Changes in Venous Drainage Position Increase Flap Survival
At 7 days after surgery, survival rates were 100 percent for both experimental group 2 and experimental group 1 and 86.5 ± 1.6 percent for the control group (Fig. 4).
Fig. 4.
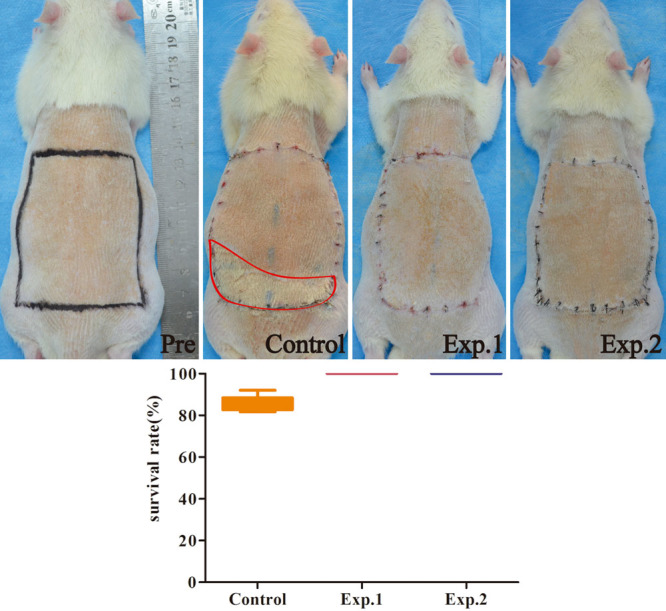
Flap survival at 7 days after surgery (n = 6). Red box, necrosis area. The survival rate of the control group was 86.5 ± 4.3 percent, whereas 100 percent of animals in experimental groups 1 and 2 survived.
Changes in Venous Drainage Position Enhance Angiogenesis
At 7 days after surgery, angiography demonstrated that angiogenesis between angiosomes I and II was obvious in all groups; the number of blood vessels in experimental group 2 was the highest, followed by experimental group 1, and then the control group, in the region of angiosomes III and IV (Fig. 5). Hematoxylin and eosin staining revealed a higher frequency of microvessels in experimental group 2 (29.5 ± 1.6) compared with experimental group 1 (24.0 ± 1.6) and controls (18.4 ± 0.9) (p < 0.05). A significant difference was also found between experimental group 1 and controls (p < 0.05) (Fig. 6).
Fig. 5.
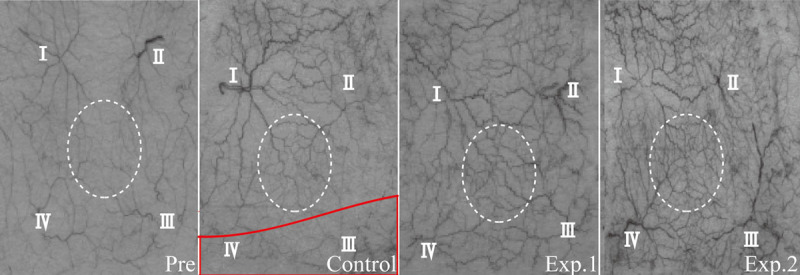
Angiography at 7 days after surgery. White dotted circle, choke area; red box, necrosis area. Angiogenesis between angiosomes I and II was obvious in all groups. The number of blood vessels was relatively low in the control group, larger in experimental group 1, and largest in experimental group 2 in the region of angiosomes III and IV.
Fig. 6.
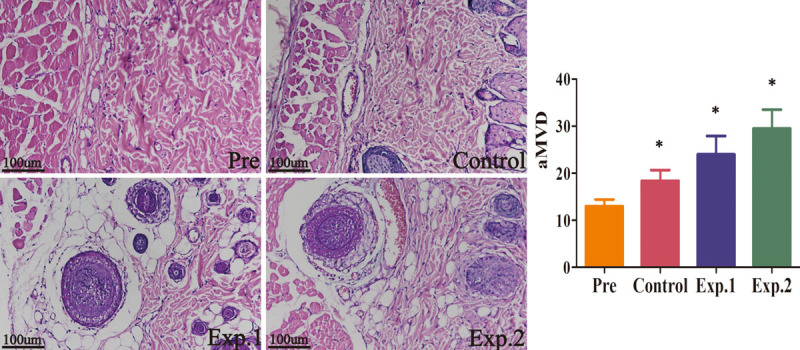
Hematoxylin and eosin staining for angiogenesis detection at 7 days after surgery (n = 6). More microvessels were found in experimental group 2 compared with experimental group 1 and the control group (p < 0.05). Differences were also noted between experimental group 1 and the control group (p < 0.05). Scale bar = 100 μm.
Changes in Venous Drainage Position Improve Ischemia and Hypoxia
Lactate levels in experimental group 2, experimental group 1, and controls were 7.47 ± 0.17 mM, 8.03 ± 0.31 mM, and 9.98 ± 0.37 mM, respectively, indicating lower amounts in experimental groups 2 and 1 compared with control values (p < 0.05) (Fig. 7).
Fig. 7.
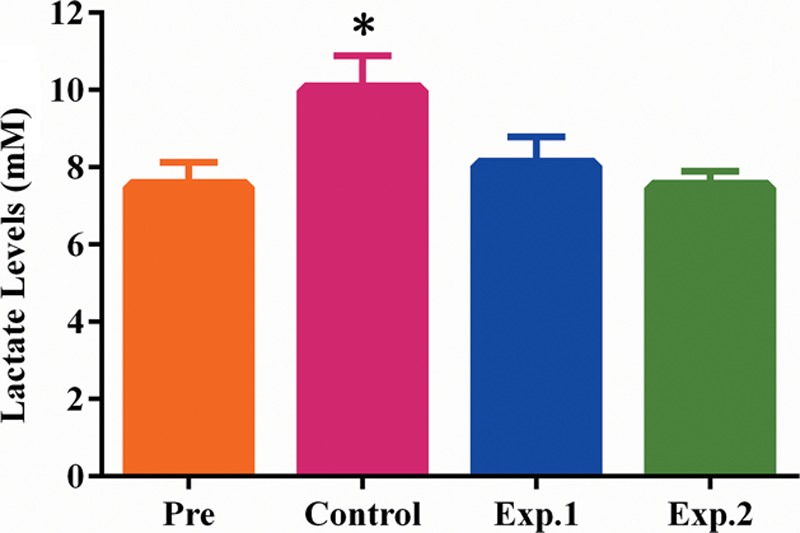
Lactate levels at 7 days after surgery (n = 6). Lactate levels in experimental group 1 and experimental group 2 were reduced to preoperative levels, but remained high in the control group, with statistically significant differences (p < 0.05).
DISCUSSION
Traditional supercharging improves flap complications by anastomosing an additional artery or vein in the distal part of the flap24–26; however, which of the two ways of supercharging is superior remains unclear. One view is that flap complications are mainly caused by venous congestion. An additional vein in the distal part of the flap could promote venous drainage and improve flap complications.27,28 Another view is that an additional artery in the distal part of the flap could increase arterial blood supply, thus improving flap complications.29–32 However, arterial supercharging also affects the vein, increasing the burden of venous drainage, which could lead to flap necrosis if used improperly.33 The best flap survival occurs with both arterial supercharging and venous superdrainage. In the present study, arterial high-pressure perfusion and venous superdrainage were achieved by venous drainage position changes, and acted together on the same flap; this not only improved arterial blood supply to the distal part of the flap, but also promoted venous drainage, thereby improving flap survival.
The flap was perfused by arteries and drained by the accompanying veins of the four angiosomes preoperatively, in a state of dynamic equilibrium. In the control group, the right posterior intercostal artery and vein were preserved, with the remaining arteries and veins ablated; arterial blood from the right posterior intercostal artery was forced to flow across, to the adjacent angiosomes II, III, and IV because of the pressure difference, and drained through the right posterior intercostal vein. Vascular dilation and angiogenesis followed the direction of blood flow, and the flap was partially necrotic. Compared with the control group, the accompanying right posterior intercostal vein was ablated in experimental group 1; the blood could not drain, and the arterial pressure of angiosome I was increased, forcing the blood to perfuse under high-pressure to the adjacent angiosomes, and superdrained by means of the left posterior intercostal vein because of the ablation of the accompanying left posterior intercostal artery. Such a flap had effects of arterial high-pressure perfusion and venous superdrainage at the early stage because of venous drainage position changes, aiming to establish the normal artery-vein path. Once established, the above effects disappeared. In this group, the flap survived completely. Compared with controls and experimental group 1, experimental group 2 had the left iliolumbar vein, with a relatively larger vascular caliber, preserved. The distance between the inflow artery and outflow vein was longer (Fig. 3), and the artery-vein path could not be built quickly. This flap had not only effects of arterial high-pressure perfusion and venous superdrainage at the early stage, but also venous superdrainage later. Arterial high-pressure perfusion and venous superdrainage lasted longer, and changes to the vascular rete within the flap were more pronounced in experimental group 2 compared with experimental group 1; therefore, the experimental group 2 flaps totally survived (Fig. 4). Compared with the traditional supercharging flap, arterial high-pressure perfusion and venous superdrainage of the current flap in both experimental groups were caused by changes of venous drainage position; not only it did not increase the burden of venous drainage, but also promoted venous drainage, with no additional vessels needed.
Improved flap survival is accompanied by an increased number of microvessels.34–36 Angiography showed overt angiogenesis between angiosomes I and II in all groups, because the distance between angiosomes I and II was the shortest, and arterial blood easily flowed to angiosome II. Once the artery-vein approach was established, the effects of arterial high-pressure perfusion and venous superdrainage in experimental group 1 disappeared, but this lasted longer in experimental group 2; therefore, the most pronounced angiogenesis was observed in experimental group 2, in the region of angiosomes III and IV (Fig. 5). Hematoxylin and eosin staining also revealed more microvessels in experimental group 2 compared with experimental group 1 and controls, with more in experimental group 1 in comparison with the control group (Fig. 6). Thus, changes of venous drainage position are beneficial for flap survival, and involve a mechanism dependent on angiogenesis.
The lactate level is an indicator of tissue ischemia and hypoxia.37,38 Lactate levels in experimental groups 2 and 1 returned to normal values, but remained high in the control group at 7 days after surgery (Fig. 7). These findings indicated that ischemia and hypoxia had largely disappeared in experimental groups 2 and 1. There was no congestion at the distal end of the flap in experimental groups 2 and 1, whereas this was a serious problem in the control group.
This was just a preliminary study and had several limitations. First, it focused on phenomenon description, and expression levels of related proteins were not assessed; therefore, the underlying molecular mechanisms remain unclear. Second, the associations of the duration limit of arterial high-pressure perfusion and venous superdrainage with the distance from the artery to the vein deserve further attention.
CONCLUSIONS
Changes in venous drainage position might result in arterial high-pressure perfusion and venous superdrainage, which alter the vascular rete, thereby improving survival. The longer it lasts, the more obvious the effects are that are obtained.
ACKNOWLEDGMENTS
This study was supported by the National Nature Science Foundation of China (grant nos. 31371214 and 81472104) and the Student Science and Technology Innovation Project of Zhejiang Province (grant no. 2017R413044). The authors would like to thank B. W. Xue (Radiology Department, First Affiliated Hospital of Wenzhou Medical University) for assistance with radiography.
Supplementary Material
Footnotes
The first two authors contributed equally to this work.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Roberts AP, Cohen JI, Cook TA. The rat ventral island flap: A comparison of the effects of reduction in arterial inflow and venous outflow. Plast Reconstr Surg. 1996;97:610–615. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y, Tajima S. The influence of arterial inflow and venous outflow on the survival of reversed-flow island flaps: An experimental study. Plast Reconstr Surg. 1997;99:2021–2029. [DOI] [PubMed] [Google Scholar]
- 3.Talbot SG, Pribaz JJ. First aid for failing flaps. J Reconstr Microsurg. 2010;26:513–515. [DOI] [PubMed] [Google Scholar]
- 4.Pannucci CJ, Nelson JA, Chung CU, et al. Medicinal leeches for surgically uncorrectable venous congestion after free flap breast reconstruction. Microsurgery 2014;34:522–526. [DOI] [PubMed] [Google Scholar]
- 5.Novo-Torres A, Fakih I, Aparicio-Alcazar JJ, Garcia-Juarranz J, Navarro-Sempere L, Lorda-Barraguer E. Breast sharing: New perspectives on an old method. J Plast Reconstr Aesthet Surg. 2015;68:1727–1732. [DOI] [PubMed] [Google Scholar]
- 6.Galanis C, Nguyen P, Koh J, Roostaeian J, Festekjian J, Crisera C. Microvascular lifeboats: A stepwise approach to intraoperative venous congestion in DIEP flap breast reconstruction. Plast Reconstr Surg. 2014;134:20–27. [DOI] [PubMed] [Google Scholar]
- 7.Du W, Wu PF, Qing LM, et al. Systemic and flap inflammatory response associates with thrombosis in flap venous crisis. Inflammation 2015;38:298–304. [DOI] [PubMed] [Google Scholar]
- 8.Ueda K, Harashina T, Oba S, Nagasaka S. Which vessel is more important in the supercharged flap: Artery, vein, or both? An experimental study. J Reconstr Microsurg. 1994;10:153–155. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto Y, Sakurai H, Nakazawa H, Nozaki M. Effect of vascular augmentation on the haemodynamics and survival area in a rat abdominal perforator flap model. J Plast Reconstr Aesthet Surg. 2009;62:244–249. [DOI] [PubMed] [Google Scholar]
- 10.Hallock GG, Rice DC. Efficacy of venous supercharging of the deep inferior epigastric perforator flap in a rat model. Plast Reconstr Surg. 2005;116:551–555; discussion 556. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara M, Nagata T, Matsushita Y, Ishikawa K, Yusuke O, Fukamizu H. Delayed distally based sural flap with temporary venous supercharging. Microsurgery 2013;33:534–538. [DOI] [PubMed] [Google Scholar]
- 12.Tran NV, Buchel EW, Convery PA. Microvascular complications of DIEP flaps. Plast Reconstr Surg. 2007;119:1397–1405; discussion 14061408. [DOI] [PubMed] [Google Scholar]
- 13.Yu P, Chang DW, Miller MJ, Reece G, Robb GL. Analysis of 49 cases of flap compromise in 1310 free flaps for head and neck reconstruction. Head Neck 2009;31:45–51. [DOI] [PubMed] [Google Scholar]
- 14.Lie KH, Barker AS, Ashton MW. A classification system for partial and complete DIEP flap necrosis based on a review of 17,096 DIEP flaps in 693 articles including analysis of 152 total flap failures. Plast Reconstr Surg. 2013;132:1401–1408. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan HY, Yaffe B, Borenstein A. Single artery replantation of totally avulsed scalp. Injury 1993;24:488–490. [DOI] [PubMed] [Google Scholar]
- 16.Riot S, Herlin C, Mojallal A, et al. A systematic review and meta-analysis of double venous anastomosis in free flaps. Plast Reconstr Surg. 2015;136:1299–1311. [DOI] [PubMed] [Google Scholar]
- 17.Chaput B, Vergez S, Somda S, et al. Comparison of single and double venous anastomoses in head and neck oncologic reconstruction using free flaps: A meta-analysis. Plast Reconstr Surg. 2016;137:1583–1594. [DOI] [PubMed] [Google Scholar]
- 18.Ehrl D, Heidekrueger PI, Heine-Geldern A, Ninkovic M, Broer PN. One versus two venous anastomoses in microvascular upper extremity reconstruction. J Reconstr Microsurg. 2017;33:502–508. [DOI] [PubMed] [Google Scholar]
- 19.Zhuang Y, Hu S, Wu D, Tang M, Xu DC. A novel in vivo technique for observations of choke vessels in a rat skin flap model. Plast Reconstr Surg. 2012;130:308–317. [DOI] [PubMed] [Google Scholar]
- 20.Yu YL, Shao YK, Ding YQ, et al. Decellularized kidney scaffold-mediated renal regeneration. Biomaterials 2014;35:6822–6828. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Chen SY, Gao WY, et al. Experimental study of survival of pedicled perforator flap with flow-through and flow-end blood supply. Br J Surg. 2015;102:375–381. [DOI] [PubMed] [Google Scholar]
- 22.Bergeron L, Tang M, Morris SF. A review of vascular injection techniques for the study of perforator flaps. Plast Reconstr Surg. 2006;117:2050–2057. [DOI] [PubMed] [Google Scholar]
- 23.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis: Correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama Y, Soeda S, Kasai Y. The importance of arterial inflow in the distal side of a flap: An experimental investigation. Plast Reconstr Surg. 1982;69:61–67. [PubMed] [Google Scholar]
- 25.Kim CY, Kim YH. Supermicrosurgical reconstruction of large defects on ischemic extremities using supercharging techniques on latissimus dorsi perforator flaps. Plast Reconstr Surg. 2012;130:135–144. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Feng S, Xia Y, et al. Prefabricated flaps: Identification of microcirculation structure and supercharging technique improving survival area. J Reconstr Microsurg. 2017;33:112–117. [DOI] [PubMed] [Google Scholar]
- 27.Landin L, Bolado P, Casado-Sanchez C, et al. Safety of salvaging impending flap congestion in breast reconstruction by venous supercharging of the cephalic vein. Ann Plast Surg. 2015;74:52–56. [DOI] [PubMed] [Google Scholar]
- 28.Herlin C, Bekara F, Bertheuil N, et al. Venous supercharging reduces complications and improves outcomes of distally based sural flaps. J Reconstr Microsurg. 2017;33:343–351. [DOI] [PubMed] [Google Scholar]
- 29.Chang H, Nobuaki I, Minabe T, Nakajima H. Comparison of three different supercharging procedures in a rat skin flap model. Plast Reconstr Surg. 2004;113:277–283. [DOI] [PubMed] [Google Scholar]
- 30.Vinh VQ, Van Anh T, Ogawa R, Hyakusoku H. Anatomical and clinical studies of the supraclavicular flap: Analysis of 103 flaps used to reconstruct neck scar contractures. Plast Reconstr Surg. 2009;123:1471–1480. [DOI] [PubMed] [Google Scholar]
- 31.Ono S, Chung KC, Takami Y, et al. Perforator-supercharged occipitocervicopectoral flaps for lower face and neck reconstruction. Plast Reconstr Surg. 2012;129:879–887. [DOI] [PubMed] [Google Scholar]
- 32.Vosburg RW, White MJ, Heckler FR. Supercharging of delayed pedicled transverse rectus abdominis myocutaneous flaps: Is it a viable option? Microsurgery 2015;35:204–206. [DOI] [PubMed] [Google Scholar]
- 33.Chiu DT, Hu G, Wu J, Rhee S, Rogers L, Gorlick N. Extended rat-ear flap model: A new rodent model for studying the effects of vessel supercharging on flap viability. J Reconstr Microsurg. 2002;18:503–508. [DOI] [PubMed] [Google Scholar]
- 34.Abbas OL, Borman H, Terzi YK, et al. Inhibition of the notch pathway promotes flap survival by inducing functional neoangiogenesis. Ann Plast Surg. 2015;75:455–462. [DOI] [PubMed] [Google Scholar]
- 35.Yoon AP, Jones NF. Critical time for neovascularization/angiogenesis to allow free flap survival after anastomotic thrombosis without surgical intervention: A review of the literature. Microsurgery 2016;36:604–612. [DOI] [PubMed] [Google Scholar]
- 36.Kira T, Omokawa S, Akahane M, et al. Effectiveness of bone marrow stromal cell sheets in maintaining random-pattern skin flaps in an experimental animal model. Plast Reconstr Surg. 2015;136:624e–632e. [DOI] [PubMed] [Google Scholar]
- 37.Tyner TR, Tong W, Donovan K, McDonald T, Sian K, Yamaguchi KT. Dichloroacetate reduces tissue necrosis in a rat transverse rectus abdominis musculocutaneous flap model. Ann Plast Surg. 2006;56:320–326. [DOI] [PubMed] [Google Scholar]
- 38.Handayani S. A simple method to measure serum lactate concentration as a reliable parameter to detect flaps blood-flow patency. J Plastik Rekonstruksi. 2012;1:435–439. [Google Scholar]


