Abstract
The foreign body response (FBR) to implantable materials can negatively impact performance of medical devices such as the cochlear implant. Engineering surfaces that resist the FBR could lead to enhanced functionality including potentially improving outcomes for cochlear implant recipients through reduction in fibrosis. In this work, we coat poly(dimethyl siloxane) (PDMS) surfaceswith two zwitterionic polymers, poly(sulfobetaine methacrylate) (pSBMA) and poly(carboxybetaine methacrylate) (pCBMA), using a simultaneous photografting/photocross-linking process to produce a robust grafted zwitterionic hydrogel. reduce nonspecific protein adsorption, the first step of the FBR. The coating process uses benzophenone, a photografting agent and type II photoinitiator, to covalently link the crosslinked zwitterionic thin film to the PDMS surface. As the concentration of benzophenone on the surface increases, the adhesive strength of the zwitterionic thin films to PDMS surfaces increases as determined by shear adhesion. Additionally, with increased concentration of the adsorbed benzophenone, failure of the system changes from adhesive delamination to cohesive failure within the hydrogel, demonstrating that durable adhesive bonds are formed from the photografting process. Interestingly, anti-fouling properties of the zwitterionic polymers are preserved with significantly lower levels of nonspecific protein adsorption on zwitterion hydrogel-coated samples compared to uncoated controls. Fibroblast adhesion is also dramatically reduced on coated substrates. These results show that crosslinked pSBMA and pCBMA hydrogels can be readily photografted to PDMS substrates and show promise in potentially changing the fibrotic response to implanted biomaterials.
Keywords: neural prosthetic, zwitterionic polymer, foreign body response, photopolymerization
Introduction
Implantable medical devices including pacemakers, myringotomy tubes, joint replacements, and neural prosthetics have dramatically increased quality of life over the past several decades. The function of many of these implants is significantly limited by the formation of fibrotic tissue (fibrosis) around the devices1–3 that occurs weeks to months after implantation.4 Fibrosis is particularly problematic for neural prosthetic devices as it impedes the transmission of electrical current between stimulating electrodes and the target neurons.5 For example, fibrotic tissue formation in the scala tympani is an expected consequence of cochlear implantation but is detrimental to hearing outcomes.6,7 A foreign body response (FBR) occurs with an initial inflammatory response that progresses to fibrosis that encapsulates the implant.8 Fibrotic capsule formation around the electrode array increases electrical impedance, necessitating use of higher current thresholds resulting in increased current spread. More recently intracochlear fibrosis in response to the implanted electrode array has been linked to loss of residual acoustic hearing in the implanted ear.1 Other neural prostheses and implanted medical devices face similar challenges to the FBR.
The initial step in the FBR is nonspecific adsorption of protein to device surfaces.9 Mitigation of protein adsorption to reduce fibrosis has recently become an active area of research. Traditional anti-fouling materials are uncharged hydrophilic polymers, such as poly(ethylene glycol) (PEG) or poly(hydroxyethyl methacrylate) (pHEMA). However both still allow some degree of protein adsorption and ultimately induce a fibrotic response.4 Zwitterionic polymers, such as carboxybetaines and sulfobetaines, have emerged as a new class of anti-fouling materials. These polymers are generated from monomers that have a positively charged group and a negatively charged group on the same repeat unit. A unique aspect of these materials is that they are hydrophilic yet retain a net neutral charge. It has been proposed that charged groups on the zwitterionic repeat units tightly bind water molecules, forming a layer of hydration that makes displacement of the water energetically unfavorable, resulting in significantly reduced protein and other biomolecule adsorption.10,11 Polymers formed from sulfobetaine methacrylate (SBMA) and carboxybetaine methacrylate (CBMA) have shown promise in a variety of applications including glucose sensing in blood serum,12,13 antigen detection in complex media,14 and prolonging the blood circulation time of coated drugs,15 among others.16 Importantly, SBMA- and CBMA-based polymers have shown resistance to cell17,18 and bacterial19,20 adhesion in vitro in addition to mitigating the FBR in vivo.2 Thus, SBMA and CBMA are ideally suited monomers for generating polymers to prevent biofouling on implantable materials.
Many implantable devices utilize poly(dimethyl) siloxane (PDMS), a highly flexible, chemically inert, and easy to fabricate material used in urinary catheters and to house cochlear implant electrode arrays.21,22 While this silicone-based polymer is not toxic to cells, the hydrophobic surface is especially prone to protein adsorption, resulting in a significant FBR and ultimate formation of a fibrotic capsule. Therefore, durable zwitterionic materials for coating PDMS surfaces have the potential to significantly reduce the FBR to implantable materials and improve implant quality and longevity. Silane coupling agents have previously been used to graft zwitterionic polymer brushes onto the surface of PDMS which involves plasma/O3 treatment of the material followed by grafting a reactive group to the surface.23,24 For example, Jiang et. al. functionalized the surface of PDMS with an atom transfer radical polymerization (ATRP) initiator using O3 treatment followed by application of a silane coupling agent to generate carboxybetaine methacrylate (CBMA) polymer brushes showing dramatic reduction in protein adsorption.24 Further, Huang and coworkers immobilized a zwitterionic silane coupling agent with a sulfobetaine group onto a silicone substrate which showed resistance to protein, cellular, and bacterial adhesion.23
Another potential approach would be to use a photochemical process to graft zwitterionic polymers to the surface of PDMS. Research has shown that a type II photoinitiator, such as benzophenone (BP), can be adsorbed to a polymer surface followed by irradiation with UV light. The type II photoinitiator, is excited with the light to a highly reactive diradical state when a photon is absorbed which then abstracts a hydrogen from the grafting surface, producing a radical that allows polymerization to occur from the surface.25 Using this method, polymers can be covalently grafted from a variety of polymeric surfaces including PDMS. Furthermore, this approach also offers the inherent spatial and temporal control of photopolymerization. For example, Ishihara et. al. grafted phosphoryl choline methacrylate polymerized from PDMS surfaces demonstrating decreased contact angles and reduced protein adsorption.26
In this work, we describe a process to simultaneously form and graft a durable zwitterionic cross-linked coating for PDMS surfaces that resists protein and cell adsorption showing potential to mitigate FBR. Our approach uses a one-step photoinitiated grafting process to covalently link poly(sulfobetaine methacrylate) (pSBMA) and poly(carboxybetaine methacrylate) (pCBMA) crosslinked hydrogel polymers to PDMS. The relationship between the amount of the hydrogen abstraction agent (BP) adsorbed on the surface and the concentration of the BP in the feed was examined. The role of BP concentration on adhesion/delamination from the PDMS substrate was characterized by scanning electron microscopy (SEM). Adhesion of these pSBMA and pCBMA hydrogels was examined using shear adhesion for both as prepared and swollen samples. . The anti-fouling properties of the grafted surfaces are also tested using protein adsorption. To demonstrate the efficacy of these materials to potentially resist the FBR, cell density of fibroblasts cultured on these substrates was measured. These findings demonstrate that durable zwitterionic hydrogels can be grafted to implant materials using a one-step and rapid process that significantly decreases biofouling of PDMS.
Experimental
Materials and synthesis
2-(N,N’-dimethylamino)ethyl methacrylate (DMAEM), β-propiolactone, triethyl amine, hydroquinone, [2-(Methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide (SBMA), benzophenone (BP), poly(ethylene glycol) diacrylate (PEGDA, MW 575), paraformaldehyde, collagenase, Dulbecco’s modified eagle medium (DMEM), human fibrinogen, human anti-fibrinogen, and all organic solvents were purchased from Sigma Aldrich. 1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one (HEPK, photoinitiator) was purchased from Ciba. Alexa 488 labeled phalloidin and Alexa 488 conjugated goat anti-rabbit secondary antibody were purchased from ThermoFisher Scientific (Waltham, MA). Hank’s balanced salt solution (HBS), fetal bovine serum (FBS), poly-L-ornithine, trypsin-EDTA dissociation reagent, DAPI-containing mounting medium, were purchased from Gibco (Carlsbad, CA).
2-Carboxy-N,N-dimethyl-N-(2’-(methacryloyloxy)ethyl)ethanaminium inner salt (carboxybetaine methacrylate,CBMA) was synthesized as previously described.17 Briefly, β-propiolactone was added to 10 mL of acetone. The solution was then added dropwise to DMAEM dissolved in acetone at 0o C. The reaction was stirred overnight, after which a small amount of hydroquinone (radical inhibitor) was added. The solvent was removed under reduced pressure with the residual oil dissolved in methanol. Triethylamine was added to the solution to quench any side reactions and the CBMA product was precipitated into cold diethyl ether and vacuum filtered to yield a white solid. The product was then used without further purification. NMR spectra were collected on a Bruker Spectrometer (Avance 300) to confirm chemical structure. 1H NMR (D2O, 300 MHz), δ 6.06 (s, 1H, =CH), δ 5.68 (s, 1H, =CH), δ 4.55 (t, 2H, OCH3), δ 3.70 (t, 2H, NCH2), δ 3.59 (t, 2H, NCH2), δ 3.10 (s, 6H, NCH3), δ 2.64 (t, 2H CH2COO), δ 1.84 (s, 3H =CCH3).
Zwitterion-functionalized PDMS substrate fabrication and characterization
PDMS substrates were fabricated using Sylgard 184 (Dow Corning) by thoroughly mixing 10 parts base to 1 part curing agent. For shear adhesion tests the mixture was cured in an aluminum mold with 4 mm depth and 8 mm width. All other samples were cured in Petri dishes. The PDMS was cured by heating to 80o C for 2 hours. Once the samples cooled, slabs in the aluminum molds were cut to approximately 25 mm long strips. Samples cured in Petri dishes were cut into 15 mm x 15 mm squares. To allow photografting, PDMS was coated with BP by submersing in a solution of BP in acetone (0.01 to 50 mg/mL) for 1 hour. The BP-coated samples were removed and dried in a nitrogen stream to remove solvent then dried in a vacuum for 1 hour. The BP-coated PDMS slabs were kept in a sealed container until directly before film grafting and polymerization.
The concentration of BP adsorbed onto PDMS surfaces was determined using UV/Vis measurements. Coated samples were sonicated in an ethanol solution for 30 minutes to remove the adsorbed BP. The concentration of BP in the ethanol solution was then determined from UV/Vis absorbance at 253 nm as compared to a calibration curve of known concentrations. The calibration curve showed excellent linearity (R2=0.9989) and all BP concentration measurements were evaluated within the calibrated concentrations.
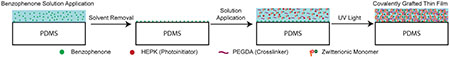
The process for photografting the zwitterionic polymers to PDMS is shown in Fig. 1. Zwitterionic polymers were generated from solutions containing 50 wt% SBMA or CBMA, 2.5 wt% poly(ethylene) glycol diacrylate (PEGDA, crosslinker), 1 wt% 1-[4-(2-hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one (HEPK, photoinitiator), and 46.5 wt% water. To test the adhesion of the zwitterionic thin films to the PDMS substrate, 2.5 μL of monomer solution was pipetted onto a glass substrate, the PDMS was placed on top, and capillary action dispersed the solution evenly between the glass and PDMS to form a 8 mm x 8 mm contact area. The solution was then polymerized using a mercury vapor arc lamp (Omnicure S1500, Lumen Dynamics, Ontario, Canada) at 30 mW/cm2 measured at 365 nm for 10 min. Standard 2.54 × 7.62 cm glass microscope slides used for shear adhesion experiments were functionalized with a silane coupling agent, (3-(trimethoxysilyl)propyl methacrylate) as previously described.27 The coupling agent contains a reactive methacrylate group which forms a covalent bond between the glass substrate and the crosslinked film upon polymerization. Shear adhesion of as prepared samples was measured directly after polymerization with a dynamic mechanical analyzer (DMA; Q800 DMA TA instruments). Swollen samples were immersed in deionized water for 48 hr prior to testing. The adhered glass and PDMS samples were fractured in the DMA using a controlled force ramp rate of 0.5 N/min. at room temperature. The force at break was recorded and normalized to the area of adhesion to the glass substrate.
Fig. 1.
Schematic of the photografting process. UV-light is used to initiate polymerization and grafting of zwitterionic monomers from the PDMS surface forming a polymer thin film hydrogel covalently attached to the material substrate.
Thickness of the zwitterion-coated thin films were measured using scanning electron microscopy (SEM, S-4800, Hitachi). Zwitterion-coated PDMS substrates were cut and mounted vertically on specimen stages. Prior to imaging, samples were sputter coated with gold. Electron accelerating voltage was set at 2.0 kV.
Protein adsorption
Immunofluorescence was used to measure protein adsorption to substrates. The PDMS and zwitterion-coated samples were hydrated overnight in PBS (room temperature) prior to protein exposure. Human fibrinogen (1 mg/mL) was pipetted onto substrates and dispersed by placing a glass coverslip on the solution followed by a 1 hour incubation at room temperature. The samples were then soaked in PBS for three hours, changing the PBS hourly. After rinsing, the samples were incubated in a blocking buffer solution to block the areas unoccupied by fibrinogen for 1 hour at room temperature. An anti-fibrinogen antibody (dilution 1:500) was applied to the samples overnight at 2o C. The substrates were then immersed in PBS for 45 minutes with the solution PBS being exchanged every 15 minutes. The samples were incubated with Alexa 488 conjugated secondary antibody (dilution 1:1000) for one hour at room temperature. The samples were then again rinsed in PBS for 45 minutes with the PBS, changing the PBS every 15 min. and coverslips were applied before epifluorescent imaging. Digital epifluorescent images were captured on a Leica DMIRE2 microscope (Leica Microsystems, Bannockburn, IL) with Leica DFC350FX digital camera and Metamorph software (Molecular Devices, Silicon Valley, CA). Gray-scale images were used to measure relative fluorescence intensity using Image J software (NIH, Bethesda, MD). All conditions were repeated in triplicate, and five representative images were used for analysis at each condition.
Cell culture and density quantification
Dissociated fibroblast cultures were obtained from p4–7 perinatal rat skin as previously described.28 The tissue was scraped to remove subcutaneous fat, morcellized, then digested in 0.125% trypsin with EDTA and 0.2% collagenase for one hour at 37oC. Enzymatic dissociation was stopped with FBS and the cell suspension was spun, rinsed twice, then triturated. The resultant suspension was then seeded into poly-L-ornithine and laminin coated culture flasks. Fibroblasts were maintained in DMEM with 10% FBS, splitting the cells when cultures became confluent but not splitting more than twice prior to use. Prior to seeding onto experimental substrates, the fibroblasts were dissociated from the culture plastic.
Disk substrates (10 mm diameter) for cell adsorption determination were punched from the 15×15 mm samples prepared as described above. These substrates were soaked overnight either in PBS or FBS prior to cell plating. In all cases 0.5 mL of suspension with a density of 1×107 cells/mL was placed onto each sample in a 24 well plate. These cultures were maintained for 48 hours prior to fixing with 4% paraformaldehyde in PBS. All samples were then labeled with Alexa 488 conjugated phalloidin (0.165 μM for 30 minutes) followed by three washes with PBS. Coverslips with DAPI-containing mounting medium were placed onto the stained cultures to label nuclei.
Cell density was determined by counting the total number of nuclei from digital images of randomly selected 20x microscopic fields using the cell counting feature of MetaMorph software package. At least 10 images were taken for each culture, with at least 3 substrates per experiment. All experimental conditions were repeated in triplicate.
Statistics
Statistical analysis was conducted using Graphpad Prism 7.01 software. To compare cell density between uncoated, pCBMA-coated, and pSBMA-coated, a one-way ANOVA with post hoc Tukey test was used.
Results and Discussion
Surface-initiated zwitterionic graft polymerization
Engineering coatings for existing implantable material may represent a practical and quickly implemented approach to mitigating FBR without compromising the inherent mechanical properties. Because the surface is the only portion of a device that will come into direct contact with host fluids and tissues, functionalizing the substrate with a zwitterionic polymer thin film (<25 μm) may significantly reduce the FBR. Introducing hydrophilic polymers (poly(ethylene glycol) (PEG) or zwitterionic polymers) onto the surface of hydrophobic materials such as PDMS or other polymers may prevent the accumulation of proteins, other biomolecules, and cells on the surface.26,29
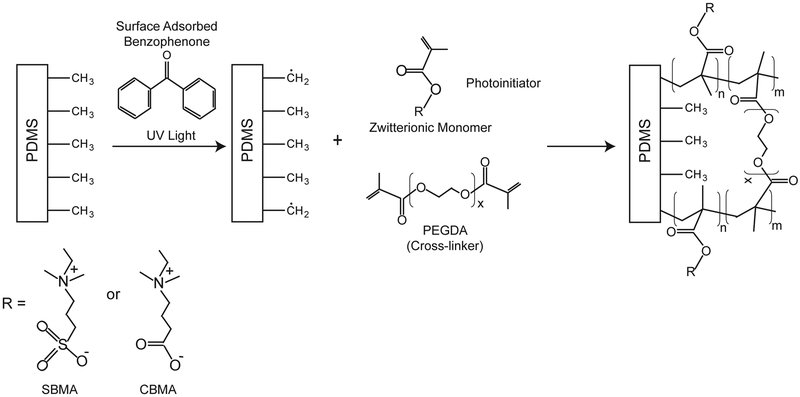
In this work, sulfobetaine methacrylate (SBMA) and carboxybetaine methacrylate (CBMA) were chosen as potential photograftable monomers due to their excellent anti-fouling properties including ultra-low protein adsorption and cell adhesion as reported elsewhere.30,31 SBMA and CBMA contain a methacrylate group which can be polymerized through a radical chain polymerization mechanism. To engineer a durable zwitterionic cross-linked zwitterionic hydrogel coating, poly(ethylene glycol) diacrylate (PEGDA, MW 575), a crosslinking molecule, was incorporated into the formulation. The crosslinker allows the zwitterionic polymer to form a covalent network, which enables thicker film fabrication and is more resistant to shear forces. These formulations were photografted to poly(dimethyl siloxane) (PDMS), which is used extensively in implantable devices such as orthopedics, shunts, and catheters.32 To covalently graft the pSBMA and pCBMA thin films to PDMS substrates, a photografting process was used. Benzophenone (BP), a type II photoinitiator, can be applied to substrates to covalently photograft (meth)acrylates to a variety of polymers.25 Adsorbing BP onto the surface of PDMS enables photografting/polymerization reaction resulting in covalent grafting to the substrate. Type II photoinitiators are excited to a short-lived singlet state by absorption of UV light which is quickly transformed to a triplet state.33 This highly-reactive triplet state initiates the grafting process by abstracting aliphatic hydrogens from the substrate and transferring the radicals to the surface. After this step, polymerization is initiated from the surface and covalently bonds the polymer chain to the substrate (See Fig. 2). A second water-soluble photoinitiator is added to the solution that also initiates polymerization in the bulk. Thus, initiation of polymerization simultaneously occurs in the bulk and at the surface to graft the film to the surface and form a strongly adhered thin film in a single step. One major advantage of this grafting mechanism is that it is effective for nearly all polymer surfaces because most polymers contain aliphatic hydrogens which can be abstracted to initiate polymerization.34
Fig. 2.
Schematic representing the basic chemistry of the photografting process. Benzophenone (BP) adsorbed on the substrates initiates covalent grafting and polymerization of the polymer to the PDMS.
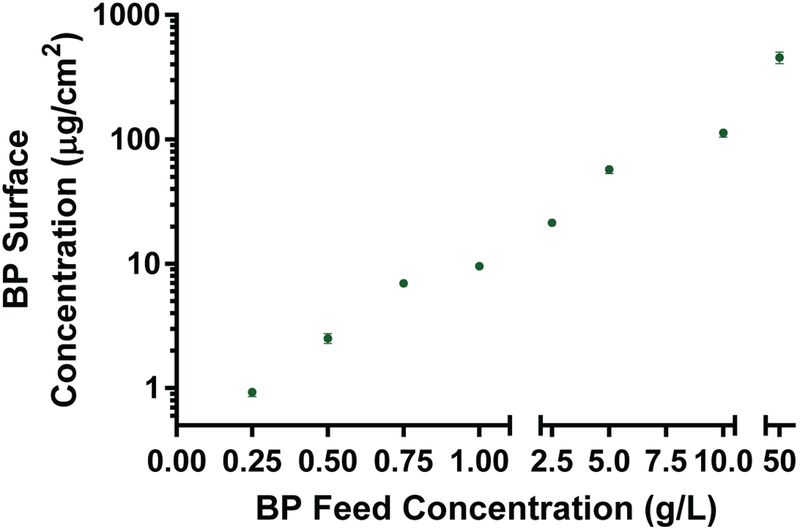
The ability of BP to abstract hydrogens from the surface plays a critical role in the process of grafting zwitterionic networks to PDMS surfaces. PDMS substrates were submersed in a BP/acetone solution with varying feed concentrations. Concentrations of 0.25 to 50 mg/mL were used to adsorb different amounts of BP on the surface required to effectively graft the zwitterionic polymer to the PDMS. After submersion in the BP solution and subsequent drying under vacuum, the coated samples were sonicated in an ethanol solution for 30 minutes to remove all adsorbed BP. The solution concentration was quantified by comparing absorbance of the BP at 253 nm to a calibration curve of known concentration via UV/Vis spectroscopy. The relationship between feed BP concentration and amount of BP physically adsorbed to the PDMS surface is shown in Fig. 3. As the feed concentration increased, the amount of adsorbed BP increased by several orders of magnitude, ranging from just under 1 μg/cm2 to over 500 μg/cm2. At concentrations above 50 mg/mL BP visible crystals started to form on the surface and as such were not used in thin film preparation. These findings demonstrate that the surface concentration of BP can be controlled by altering the feed concentration of BP. By changing the surface concentration of BP, the amount of initiation occurring at the surface is likely to change which will alter the degree of photografting to the surface.
Fig. 3.
Quantification of BP surface concentration with respect to BP feed concentration. The surface concentration increases with increasing feed concentration.
Characterization of the photografted zwitterionic polymer
To understand the dependence of the photografting process on the concentration of adsorbed BP, pSBMA and pCBMA thin films were photopolymerized onto PDMS substrates using feed concentrations as above. pSBMA- and pCBMA-grafted thin films were fabricated by sandwiching 2.5 μL of zwitterionic monomer solution between a piece of methacrylate-functionalized glass and an 8 mm wide PDMS sheet. Capillary action allowed the monomer to disperse evenly between the two substrates. After polymerization, the glass slide was removed leaving a crosslinked thin film that may be covalently grafted to the PDMS substrate (Fig. 1). pSBMA- and pCBMA-coated PDMS substrates were sliced and then imaged using scanning electron microscopy (SEM) to reveal cross-sectional images of the adhesion.
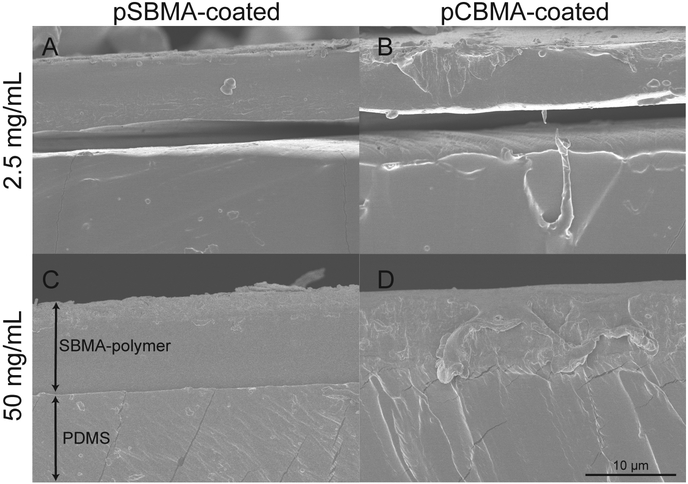
At concentrations at or less than 2.5 mg/mL, the pSBMA and pCBMA polymers began to peel from the PDMS substrate as shown by SEM images in Fig. 4A–B. While the polymer remained attached to the substrate after preparation, it only weakly adhered and could be easily removed. At these concentrations, insufficient covalent bonds were formed from the photografting reaction to create strong adhesion between the film and the surface, causing delamination after short-term use. As the amount of BP adsorbed onto the surface was increased, both the pSBMA- and pCBMA-coated surfaces demonstrated stronger adhesion to the PDMS, with no delamination observed as shown in Fig. 4C–D. At higher BP solution concentrations, and thus greater available BP at the surface, the number of surface radicals generated upon illumination increases, allowing more covalent bonds to form between the substrate and the polymer chains in the thin film. A greater number of covalent bonds between the zwitterionic thin film and the PDMS prevents film delamination. At higher concentrations of BP (10 or 50 mg/mL), delamination from the substrates was not observed during subsequent substrate testing, suggesting that these concentrations are more appropriate for further testing.
Fig. 4.
SEM images of photografted zwitterionic polymers. Shown are pSBMA-coated PDMS using A) 2.5 and C) 50 mg/mL BP feed concentration and pCBMA-coated PDMS using B) 2.5 and D) 50 mg/mL BP feed concentration.
Additionally, the thicknesses of both the pSBMA and pCBMA films prepared as outlined were measured using SEM imaging. pSBMA-coated samples were 7.3 (±2.8) μm thick while pCBMA-coated samples were similarly 7.5 (±1.7) μm thick. Interestingly, the thickness of the thin films was independent of the surface concentration of BP. Because of the fabrication process, the thin film thickness is dictated by the amount of monomer in contact with the surface. A crosslinked network is formed upon polymerization, which will gel the monomer on the PDMS substrate. Thus, it is reasonable that no changes in thickness were observed with different concentrations of benzophenone as the concentration and amount of monomer solution did not change. If desired, film thickness could easily be changed by increasing or decreasing the amount of monomer solution contacting the surface during polymerization.
To quantitatively assess the adhesion of the pSBMA- and pCBMA-grafted polymers to PDMS surfaces, shear adhesion to the PDMS was measured. This test evaluated how the materials responded to shear by measuring the force at which failure occurs along a plane parallel to the direction the force was applied. The strength of the bond between the thin film and the substrate is estimated by measuring the maximum force per unit area required to fracture the sample or delaminate the coating. Samples that require more force per unit area indicate stronger adhesion to the substrate. Fig. 5 illustrates how this method was used to evaluate the adhesion of the zwitterionic polymers to the PDMS substrates. A solution of zwitterionic monomer (SBMA or CBMA), crosslinker (PEGDA, MW 575), and photoinitiator (HEPK) in aqueous solution was applied to a functionalized glass surface. A PDMS substrate was then placed on top of the liquid and capillary action was used to disperse the solution evenly between the substrates. The glass used was functionalized using a methacrylated silane coupling agent to ensure adhesion between the polymer and the glass.
Fig. 5.
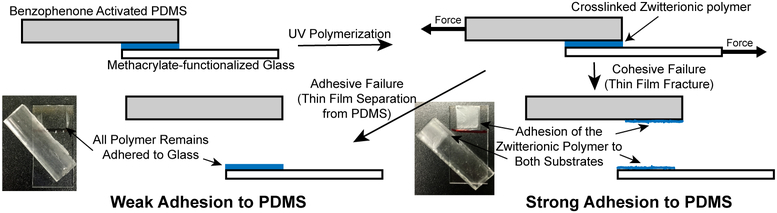
Schematic representing shear adhesion experiments. A benzophenone activated sample was covalently bound to methacrylate-functionalized glass by photografting/photopolymerizing the zwitterionic polymer between substrates. The samples were strained until fracture by applying a force to both ends of the substrates. The samples showed either adhesive failure (left image), where the polymer remains adhered to the glass, or cohesive failure (right image), where the film itself fractures yet remains adhered to both substrates.
As the bond between the glass and polymer is much stronger than what would be expected from the hydrogel coating or the adhesion between the coating and PDMS substrate, three potential modes of failure could occur using this method: 1) substrate/backing failure, 2) adhesive failure, and 3) cohesive failure. Substrate failure occurs when the substrate fractures, and indicates that the substrate is weaker than the forces from either adhesive or cohesive bonds from the coating. This type of failure would indicate a high degree of adhesion and high cohesive strength of the adhesive relative to the mechanical strength of the substrate. Adhesive failure occurrs when the bond between the substrate and the adhesive is broken. The forces exerted on the connection are greater than the forces created between the substrate and the adhesive, which cause the adhesive to peel from the substrate (see left image in Fig. 5). When failure of this type is observed, it often indicated relatively low levels of adhesion between the substrate and the adhesive. Finally, cohesive failure occurs when the bonds within the adhesive brake because the external force exceeded the cohesive bond (see right image in Fig. 5). When this failure is observed, the adhesive is strongly adhered to the substrate, but the mechanical strength of the adhesive may have been relatively weak.
For the system in study, cohesive and adhesive failure were the only types observed due to the relatively high strength of the glass and PDMS compared to the zwitterionic crosslinked hydrogel. Further, adhesive failure would indicate that the grafted polymer was only weakly bonded to the PDMS. Adhesive failure between the glass and the zwitterionic polymer was never observed. The surface methacrylate groups provided a high degree of covalent grafting between the zwitterionic polymer and the glass which was stronger than the cohesive strength of the films. The strong bond between the zwitterionic thin film and the glass ensured that measurements would only measure failures between the PDMS and thin film or failures within the thin film.
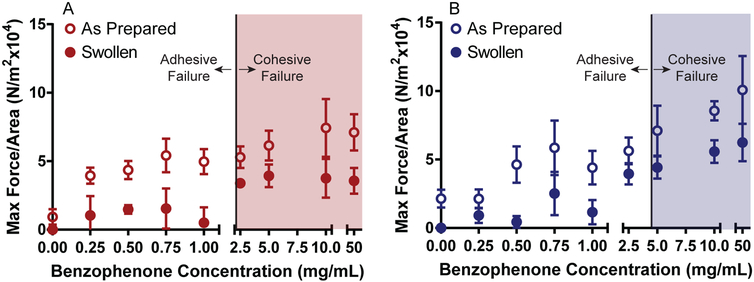
Shear adhesion strength was examined for pSBMA and pCBMA grafted polymers that were either tested without further treatment after photografting (as prepared) or swollen to equilibrium in water. The zwitterionic hydrogels were fabricated with 50% water from the polymerization solution, but will absorb additional water. Upon swelling, the hydrogel thin films increased in volume inducing changes in mechanical properties. As fully swollen thin films more closely approximates in vivo conditions, it was important to identify changes in adhesion properties in the fully hydrated state. The same BP feed concentrations were evaluated to assess the role of BP in the photografting process. Examination of shear adhesion strength data as a function of BP concentration is shown in Fig. 6. For as prepared pSBMA-coated samples, an upward trend in maximum force per area is observed as BP feed concentrations were increased as would be expected with more covalent bonds formed during the photografting/polymerization process (Fig. 6A). For BP feed concentrations below 2.5 mg/mL, adhesive failure between the PDMS and pSBMA thin film was observed. At or above 2.5 mg/mL, cohesive failure occurred indicating a critical amount of BP is on the surface and therefore sufficient covalent bonds have formed to prevent delamination. Swollen pSBMA samples follow a similar trend, but require less force per area to fracture. It is important to note that many of the samples below 2.5 mg/mL fractured adhesively before sample loading and were recorded as 0 N/m2. Similar to the as prepared samples, at and above 2.5 mg/mL cohesive failure was observed. As the thin films absorbed water, the modulus and force required for fracture decreased even when cohesive failure was observed. When cohesive failure occurred, the force required to fracture the thin film was measured rather than force required to overcome adhesion between the PDMS and zwitterionic thin film. Therefore, no significant changes in maximum force per area were observed above concentrations where cohesive failure was recorded.
Fig. 6.
Maximum force per area results as measured using shear adhesion experiments as a function of BP feed concentration for zwitterion-grafted thin films to PDMS substrates. A) Shear adhesion test data for pSBMA-grafted thin films. B) Shear adhesion results for pCBMA-coated PDMS. Max force per area increases with increasing BP feed concentration. Shaded area represents samples that failed cohesively.
Adhesion of the pCBMA thin films to PDMS followed a similar trend to the pSBMA. For as prepared samples, the maximum force per area required to fracture the samples increased with increasing BP concentrations (Fig. 6B). While the general trend was the same, cohesive failure was not observed until BP feed concentrations were greater than or equal to 5 mg/mL. pCBMA swollen samples were very similar to the pSBMA below 2.5 mg/mL, with many of the samples failing before testing. A decrease in maximum force per area was also evident compared to the as prepared samples across all concentrations, even when cohesive failure was observed. It is notable that the pCBMA hydrogels in general exhibited higher maximum force per area values than pSBMA at higher feed concentrations. This increase is likely due to the fact that pCBMA forms a higher modulus hydrogel than pSBMA.35,36 At concentrations where cohesive failure was observed, the cohesive strength of the pCBMA thin film was measured; as expected the maximum force required to cause failure was higher for pCBMA than for pSBMA. These findings demonstrate that the adhesion of the crosslinked zwitterionic films increases with higher BP surface concentrations and that durable bonds can be formed between the PDMS and the hydrogel films.
Cell and protein adsorption to zwitterion-coated PDMS
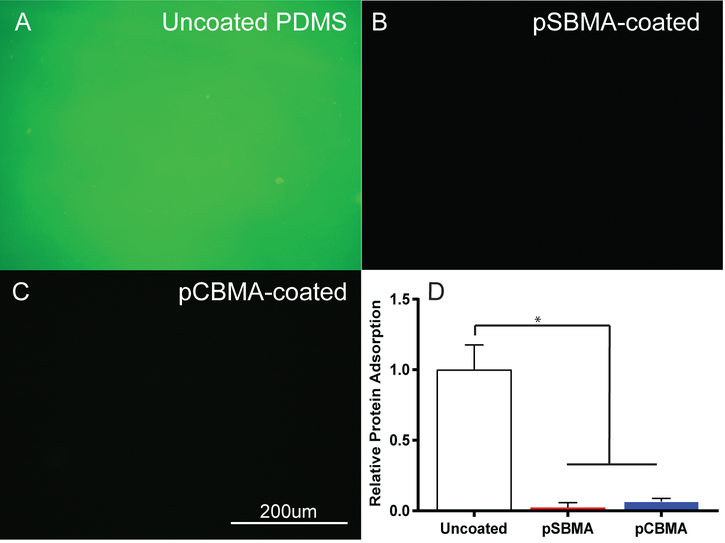
Resistance to protein adsorption is a key indicator of anti-fouling properties and such resistance could significantly change FBR.4,9,37 Fibrinogen is abundant in serum, and was therefore used as a representative protein. For all experiments evaluating anti-fouling properties of the zwitterion-coated PDMS, 50 mg/mL BP feed was used to prevent delamination from the substrate. Epifluorescent images of immunostained adsorbed protein after incubation for 1 hour in a 1 mg/mL fibrinogen solution are shown in Fig. 7A–C. The uncoated PDMS exhibited a bright green color where the pSBMA- and pCBMA-coated surfaces showed almost no fluorescence. Quantification of the adsorbed protein by measuring fluorescence intensity indicated a significant reduction in protein adsorption for both pSBMA- and pCBMA-coated surfaces compared to bare PDMS (Fig. 7D). No statistically significant difference was observed between pCBMA-coated compared to pSBMA-coated samples (One way ANOVA, p=0.9585). These results demonstrate the efficacy of both pSBMA and pCBMA in preventing the adsorption of protein and potentially mitigating the FBR.
Fig. 7.
Characterization of protein adsorption. Representative epifluorescent images of immunostained fibrinogen (green) on A) uncoated, B) pSBMA-coated, and C) pCBMA-coated PDMS. D) Fibrinogen adsorption on uncoated, pSBMA-coated and pCBMA-coated PDMS as measured by epifluorescent microscopy. pSBMA- and pCBMA-coated substrates significantly reduce fibrinogen adsorption (*p < 0.001 one way ANOVA). Error bars represent standard deviation of the mean.
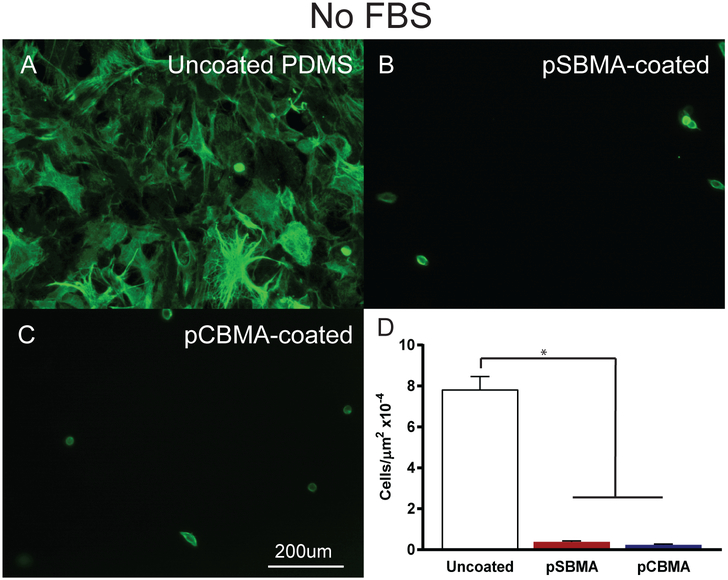
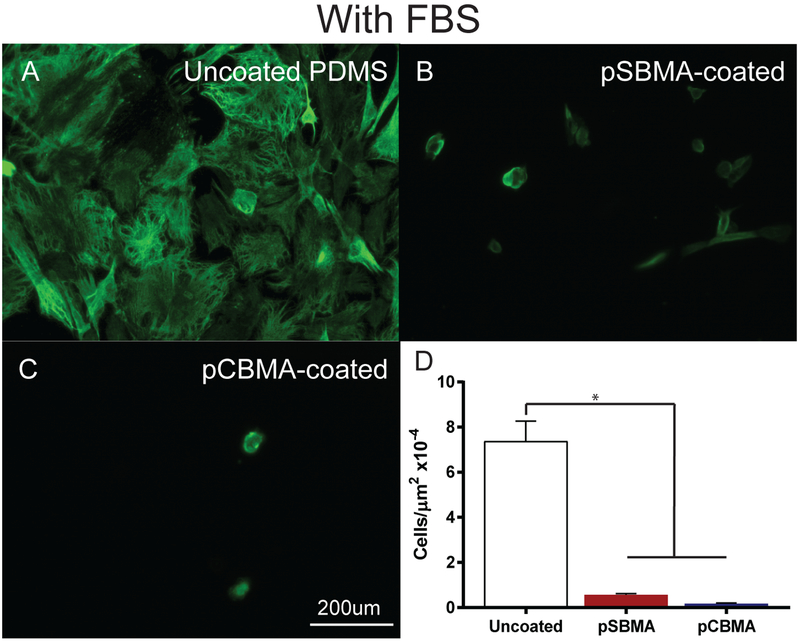
Fibroblasts are the predominant cell type found in the fibrotic capsules that form around implanted materials.37 Thus, to further evaluate the ability of these crosslinked zwitterionic coatings to prevent fouling and the foreign body response, fibroblast adhesion was measured. Accordingly, pSBMA-coated, pCBMA-coated, and uncoated PDMS were evaluated for fibroblast adhesion with and without first incubating the samples in fetal bovine serum (FBS).Cells were allowed to remain in culture for 48 hours and were then fixed, labeled with fluorescently tagged phalloidin which binds cytoskeletal actin, and epifluorescent microscopy was then performed. Images of fibroblast adhesion without FBS are shown in Fig. 8A–C. Phalloidin staining revealed a markedly different cellular morphology on uncoated substrates compared to that seen on coated PDMS (Fig. 8A). When cultured on uncoated PDMS, fibroblasts showed a characteristic elongated multipolar shape, indicating that the cells were healthy and adherent to the underlying substrate. In contrast, pSBMA- and pCBMA-coated substrates exhibited a rounded morphology with few if any cytoplasmic extensions (Fig. 8B–C). Interestingly, fewer cells appeared to adhere to the pCBMA-coated surfaces than the pSBMA-coated substrates. Fibroblast cell density, a direct measure of the cell adhesion, without FBS was dramatically reduced over coated compared to uncoated substrates (Fig. 8D). pSBMA-coated surfaces showed less than 5% of the cells adhered when compared to the PDMS control. The pCBMA-coated substrates had slightly lower cell adhesion with just above 3% of the cell density of the uncoated samples.
Fig. 8.
Representative epifluorescent images of fibroblasts grown on A) uncoated, B) pSBMA-coated, and C) pCBMA-coated PDMS without FBS in the culture medium. Fibroblast cytoskeletal actin is labeled with Alexa 488-phalloidin to demonstrate morphology (green). D) Fibroblast cell density on uncoated pSBMA-coated, and pCBMA-coated PDMS substrates. pSBMA- and pCBMA-coated PDMS significantly reduce fibroblast cell adhesion (*p < 0.001 one way ANOVA). Error bars represent standard error of the mean.
When devices are implanted into the body they are first exposed to serum proteins that can adhered to the implant surfaces. To mimic exposure to serum proteins and to provide a more challenging environment for resistance of fouling, the samples were incubated with FBS prior to cell culture. This did not dramatically alter the cell density and adhesion. Fibroblasts adherent to the uncoated PDMS were dense and evenly distributed across the substrates (Fig. 9A). In contrast, cell density was noticeably lower on pSBMA- and pCBMA-coated surfaces, with the remaining cells exhibiting a rounded morphology (Fig. 9B–C). Quantification of cell density showed significant reductions in cell counts (Fig. 9D). Once again the pSBMA-coated samples contained slightly more adherent cells than the pCBMA-coated PDMS although not significantly (One-way ANOVA, p=0.8489). This result is consistent with prior work that has demonstrated improved efficacy of pCBMA polymers when compared to pSBMA.38,39 This increase in anti-fouling properties is likely due to enhanced structuring and ordering of water molecules around the pCBMA polymer compared to pSBMA, as supported by molecular simulations conducted by Jiang et. al.40 Our lab also found that linear pCBMA polymers photografted to glass were more effective than pSBMA in preventing adhesion of multiple cell types including fibroblasts.28 These results demonstrate the efficacy of crosslinked and photografted zwitterionic coatings in preventing fibroblast adhesion.
Fig. 9.
Representative epifluorescent images of fibroblasts grown on A) uncoated, B) pSBMA-coated, and C) pCBMA-coated PDMS with FBS in culture medium. Fibroblast cytoskeletal actin is labeled with Alexa 488-phalloidin to demonstrate morphology (green). D) Fibroblast cell density on uncoated pSBMA-coated, and pCBMA-coated PDMS substrates. pSBMA- and pCBMA-coated PDMS significantly reduce fibroblast cell adhesion (*p < 0.001 one way ANOVA). Error bars represent standard error of the mean.
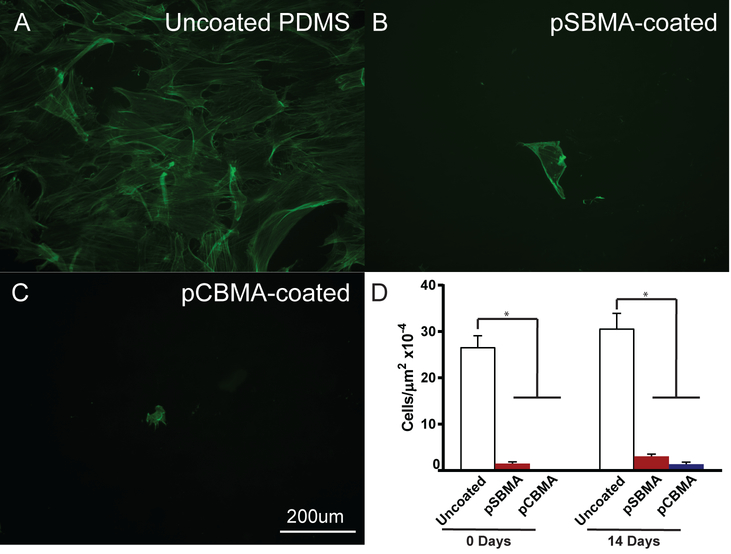
While pSBMA- and pCBMA-coatings on PDMS were effective in preventing protein and fibroblast adhesion over short time frames, it is also important to evaluate the long-term anti-fouling properties of the films. Thus, zwitterion-coated samples were incubated in culture media for 14 days before fibroblasts were seeded onto the substrates to test the efficacy after extended exposure to media. A parallel experiment was conducted where fibroblasts were seeded directly after substrate fabrication as a control (Day 0 samples). For the day 0 experiment, cell density on the uncoated PDMS was similar to the previous experiments with relatively high cell density (Fig. 10D). The pSBMA- and pCBMA-coated surfaces were resistant as before to cell adhesion in the 0 day seeding with pCBMA preventing all fibroblast adhesion. Epifluorescent images of fibroblasts seeded after 14 days are shown in Fig. 10A–C. Cell density is slightly higher on the uncoated PDMS, and cells have spread and elongated. As seen before extensive exposure to culture media, cell density is noticeably reduced on the pSBMA- and pCBMA-coated PDMS. Quantification of the cell density revealed that the number of cells increased on all substrates after the 14 day exposure to media, but not to a statistically significant degree. The number of cells adhered in the uncoated PDMS increased from approximately 11 ×10−4 cells/μm2 to 13 ×10−4 cells/μm2. For the pSBMA-coated samples, cell density doubled from about 0.6 ×10−4 cells/μm2x to 1.3 ×10−4 cells/μm2 while pCBMA-coated PDMS increased from no observable cells to 0.6 ×10−4 cells/μm2, almost 20 times less than the cell density on the PDMS control. For both experiments, cell density was dramatically lower for the zwitterion-coated surface and the incubation time did not result in a significant increase in cell density. Further, for the entirety of the experiment, the pSBMA and pCBMA thin films remained securely adhered to the PDMS. These results show that photografted/polymerized zwitterionic coatings show promise in maintaining anti-fouling properties while remaining durable for potential biological applications.
Fig. 10.
Representative epifluorescent images of fibrobasts grown on A) uncoated, B) pSBMA-coated, and C) pCBMA-coated PDMS after substrate immersion in culture medium for 14 days. Fibroblasts are labeled with Alexa 488-phalloidin (green). D) Fibroblast cell density at 0 and 14 days in culture medium for uncoated, pSBMA-coated, and pCBMA-coated PDMS. A significant difference was observed between uncoated and the zwitterion-coated PDMS at 0 and 14 days. No significant increase in cell density was observed after the 14 day incubation for all substrates. Error bars represent standard error of the mean.
Conclusions
Engineering surfaces that resist biofouling represents a critical first step in developing neural prosthetics that integrate effectively with biological systems. Coating existing implant materials with a zwitterionic coating is one potential approach to mitigate the foreign body response. In this work, we describe a simple method to simultaneously photograft pSBMA and pCBMA zwitterionic crosslinked hydrogels to PDMS, a common implant material. Photografting was achieved by adsorbing the type II photoinitiator benzophenone onto the PDMS surface, which initiated a polymerization reaction from the surface upon UV-light absorption. This process generated a durable covalently-grafted bond between the crosslinked zwitterionic hydrogels and the PDMS substrate. The amount of BP adsorbed on the surface was increased by higher BP feed concentrations. At higher surface BP concentrations, the crosslinked zwitterionic thin films resisted delamination as examined by SEM imaging. Shear adhesion experiments quantitatively revealed that the strength of adhesion increased with higher BP surface concentrations until a critical concentration was reached between 1 and 2.5 g/L above which cohesive instead of adhesive failure was observed. Examination of anti-fouling properties of the crosslinked zwitterionic hydrogels revealed a greater than 90% reduction in fibrinogen adsorption for both pSBMA- and pCBMA-coated substrates. Fibroblast adhesion was also reduced 20 fold or greater for zwitterion-coated PDMS. Finally, the thin films maintained resistance to fibroblast adhesion after exposure to culture media for up to 14 days in vitro. This work demonstrates that a simple and controllable process has been developed to successfully photograft and simultaneously polymerize zwitterionic hydrogelson PDMS substrates. These durable hydrogels resist protein and fibroblast adhesion, demonstrating significant potential to resist fouling in implantable materials.
Acknowledgements
Supported by NIH R01-DC012578, T32DC000040, T35HL007485–35, and P30-DC010362. The authors would like to thank Dr. Eric Nuxoll for use of the UV/Vis Spectrometer.
References
- (1).Quesnel AM; Nakajima HH; Rosowski JJ; Hansen MR; Gantz BJ; Nadol JB Delayed Loss of Hearing after Hearing Preservation Cochlear Implantation: Human Temporal Bone Pathology and Implications for Etiology. Hear. Res 2015, 333, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Zhang L; Cao Z; Bai T; Carr L; Ella-Menye J-R; Irvin C; Ratner BD; Jiang S Zwitterionic Hydrogels Implanted in Mice Resist the Foreign-Body Reaction. Nat. Biotechnol 2013, 31 (6), 553–556. [DOI] [PubMed] [Google Scholar]
- (3).Ishai R; Herrmann BS; Nadol JB; Quesnel AM The Pattern and Degree of Capsular Fibrous Sheaths Surrounding Cochlear Electrode Arrays. Hear. Res 2017, 348, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Ratner BD Reducing Capsular Thickness and Enhancing Angiogenesis around Implant Drug Release Systems. J. Control. Release 2002, 78, 211–218. [DOI] [PubMed] [Google Scholar]
- (5).Nadol JB, J.; Burgess BJ; Gantz BJ; Coker NJ; Ketten DR; Kos I; Roland JT, J.; Shiao Jiun Yih, D. K.; Eddington DK; Montandon P; et al. Histopathology of Cochlear Implants in Humans. Ann. Otol. Rhinol. Laryngol 2001, 110 (9), 883–891. [DOI] [PubMed] [Google Scholar]
- (6).Seyyedi M; Nadol JB Intracochlear Inflammatory Response to Cochlear Implant Electrodes in Humans. Otol. Neurotol 2014, 35 (9), 1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ishiyama A; Doherty J; Ishiyama G; Quesnel AM; Lopez I; Linthicum FH Post Hybrid Cochlear Implant Hearing Loss and Endolymphatic Hydrops. Otol. Neurotol 2016, 1516–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Anderson JM; Rodriguez A; Chang DT Foreign Body Reaction to Biomaterials. Semin. Immunol 2008, 20 (2), 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Rolfe B; Mooney J; Zhang B; Jahnke S; Le S-J; Huang Q; Wang H; Campbell G; Campbell J The Fibrotic Response to Implanted Biomaterials: Implications for Tissue Engineering. Regen. Med. Tissue Eng. - Cells Biomater 2011, 552–568. [Google Scholar]
- (10).Estephan ZG; Schlenoff PS; Schlenoff JB Zwitteration as an Alternative to PEGylation. Langmuir 2011, 27, 6794–6800. [DOI] [PubMed] [Google Scholar]
- (11).Shao Q; Jiang S Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater 2015, 27 (1), 15–26. [DOI] [PubMed] [Google Scholar]
- (12).Yang W; Xue H; Carr LR; Wang J; Jiang S Zwitterionic Poly(Carboxybetaine) Hydrogels for Glucose Biosensors in Complex Media. Biosens. Bioelectron 2011, 26 (5), 2454–2459. [DOI] [PubMed] [Google Scholar]
- (13).Yang W; Bai T; Carr LR; Keefe AJ; Xu J; Xue H; Irvin C.a. ; Chen S; Wang J; Jiang S The Effect of Lightly Crosslinked Poly(Carboxybetaine) Hydrogel Coating on the Performance of Sensors in Whole Blood. Biomaterials 2012, 33 (32), 7945–7951. [DOI] [PubMed] [Google Scholar]
- (14).Zhu Y; Xu X; Brault ND; Keefe AJ; Han X; Deng Y; Xu J; Yu Q; Jiang S Cellulose Paper Sensors Modified with Zwitterionic Poly(Carboxybetaine) for Sensing and Detection in Complex Media. Anal. Chem 2014, 86 (6), 2871–2875. [DOI] [PubMed] [Google Scholar]
- (15).Cao Z; Zhang L; Jiang S Superhydrophilic Zwitterionic Polymers Stabilize Liposomes. Langmuir 2012, 28 (31), 11625–11632. [DOI] [PubMed] [Google Scholar]
- (16).Cao B; Tang Q; Cheng G Recent Advances of Zwitterionic Carboxybetaine Materials and Their Derivatives. J. Biomater. Sci. Polym. Ed 2014, 25 (14–15), 1502–1513. [DOI] [PubMed] [Google Scholar]
- (17).Zhang Z; Chao T; Chen S; Jiang S Superlow Fouling Sulfobetaine and Carboxybetaine Polymers on Glass Slides. Langmuir 2006, 22 (12), 10072–10077. [DOI] [PubMed] [Google Scholar]
- (18).Li G; Cheng G; Xue H; Chen S; Zhang F; Jiang S Ultra Low Fouling Zwitterionic Polymers with a Biomimetic Adhesive Group. Biomaterials 2008, 29 (35), 4592–4597. [DOI] [PubMed] [Google Scholar]
- (19).Cheng G; Zhang Z; Chen S; Bryers JD; Jiang S Inhibition of Bacterial Adhesion and Biofilm Formation on Zwitterionic Surfaces. Biomaterials 2007, 28 (29), 4192–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Cheng G; Li G; Xue H; Chen S; Bryers JD; Jiang S Zwitterionic Carboxybetaine Polymer Surfaces and Their Resistance to Long-Term Biofilm Formation. Biomaterials 2009, 30 (28), 5234–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Yoda R Elastomers for Biomedical Applications. J. Biomater. Sci. Polym. Ed 1998, 9 (6), 561–626. [DOI] [PubMed] [Google Scholar]
- (22).Diaz Blanco C; Ortner A; Dimitrov R; Navarro A; Mendoza E; Tzanov T Building an Antifouling Zwitterionic Coating on Urinary Catheters Using an Enzymatically Triggered Bottom-up Approach. ACS Appl. Mater. Interfaces 2014, 6 (14), 11385–11393. [DOI] [PubMed] [Google Scholar]
- (23).Yeh S-B; Chen C-S; Chen W-Y; Huang C-J Modification of Silicone Elastomer with Zwitterionic Silane for Durable Antifouling Properties. Langmuir 2014, 30 (38), 11386–11393. [DOI] [PubMed] [Google Scholar]
- (24).Keefe AJ; Brault ND; Jiang S Suppressing Surface Reconstruction of Superhydrophobic PDMS Using a Superhydrophilic Zwitterionic Polymer. Biomacromolecules 2012, 13 (5), 1683–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Rånby B Surface Modification and Lamination of Polymers by Photografting. Int. J. Adhes. Adhes 1999, 19 (5), 337–343. [Google Scholar]
- (26).Goda T; Konno T; Takai M; Moro T; Ishihara K Biomimetic Phosphorylcholine Polymer Grafting from Polydimethylsiloxane Surface Using Photo-Induced Polymerization. Biomaterials 2006, 27 (30), 5151–5160. [DOI] [PubMed] [Google Scholar]
- (27).Tuft BW; Li S; Xu L; Clarke JC; White SP; Guymon BA; Perez KX; Hansen MR; Guymon CA Photopolymerized Microfeatures for Directed Spiral Ganglion Neurite and Schwann Cell Growth. Biomaterials 2013, 34 (1), 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Leigh BL; Cheng EL; Andresen C; Hansen MR; Guymon CA Photopolymerizable Zwitterionic Polymer Patterns Control Cell Adhesion and Guide Neural Growth. Biomacromolecules 2017, 18 (8), 2389–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Goda T; Matsuno R; Konno T; Takai M; Ishihara K Photografting of 2-Methacryloyloxyethyl Phosphorylcholine from Polydimethylsiloxane: Tunable Protein Repellency and Lubrication Property. Colloids Surfaces B Biointerfaces 2008, 63 (1), 64–72. [DOI] [PubMed] [Google Scholar]
- (30).Yang W; Xue H; Li W; Zhang J; Jiang S Pursuing “Zero” Protein Adsorption of Poly(Carboxybetaine) from Undiluted Blood Serum and Plasma. Langmuir 2009, 25 (19), 11911–11916. [DOI] [PubMed] [Google Scholar]
- (31).Jiang S; Cao Z Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater 2010, 22 (9), 920–932. [DOI] [PubMed] [Google Scholar]
- (32).Colas A; Curtis J Silicone Biomaterials: History and Chemistry and Medical Applications of Silicones; 2004.
- (33).Yang WT; Rhnby B The Role of Far UV Radiation in the Photografting Process. Polym. Bull 1996, 37 (1), 89–96. [Google Scholar]
- (34).Bengt R Surface Modification of Polymers by Photointiated Graft Polymerization. Makromol. Chemie, Macromol. Symp 1992, 63, 55–67. [Google Scholar]
- (35).Carr L; Cheng G; Xue H; Jiang S Engineering the Polymer Backbone To Strengthen Nonfouling Sulfobetaine Hydrogels. Langmuir 2010, 26 (18), 14793–14798. [DOI] [PubMed] [Google Scholar]
- (36).Carr LR; Xue H; Jiang S Functionalizable and Nonfouling Zwitterionic Carboxybetaine Hydrogels with a Carboxybetaine Dimethacrylate Crosslinker. Biomaterials 2011, 32 (4), 961–968. [DOI] [PubMed] [Google Scholar]
- (37).Anderson JM Biological Responses to Materials. Annu. Rev. Mater. Res 2001, 31, 81–110. [Google Scholar]
- (38).Zhang Z; Zhang M; Chen S; Horbett TA; Ratner BD; Jiang S Blood Compatibility of Surfaces with Superlow Protein Adsorption. Biomaterials 2008, 29 (32), 4285–4291. [DOI] [PubMed] [Google Scholar]
- (39).Lin W; Ma G; Wu J; Chen S Different in Vitro and in Vivo Behaviors between Poly(Carboxybetaine Methacrylate) and Poly(Sulfobetaine Methacrylate). Colloids Surfaces B Biointerfaces 2016, 146, 888–894. [DOI] [PubMed] [Google Scholar]
- (40).Shao Q; He Y; White AD; Jiang S Difference in Hydration between Carboxybetaine and Sulfobetaine. J. Phys. Chem. B 2010, 114 (49), 16625–16631. [DOI] [PubMed] [Google Scholar]