Abstract
Background and Objectives:
The mesenchymal stem cells derived from peripheral blood (PB) have been recognized as a promising source for allogeneic cell therapy. The aim of this study was to investigate the isolation, growth and differentiation ability of peripheral blood-isolated mesenchymal stem cells.
Methods:
The mononuclear cells were purified from fresh peripheral blood using density gradient centrifugation then cultured in a suitable medium, expanded and characterized. In the following, these cells were cultured in specific adipogenic and osteogenic differentiation media.
Results and Conclusion:
In spite of the absence of any stimulating factor, the cells adhered to the flasks and developed a rather homogeneous, spindle-shaped morphology after consecutive passages. The cells were confirmed to have mesenchymal phenotype by expression of specific markers (CD90, CD105, and CD73) and absence of CD45 marker, which is specific for hematopoietic stem cells. They could differentiate into lineage-specific committed cells (osteoblasts and adipocytes).
According to the findings, the conventional, labour-intensive and time-consuming approaches are not necessary to obtain an optimal number of cells from peripheral blood. This relatively accessible and minimally invasive source of stem cells may open a new era for practical exploitation in regenerative medicine.
Key Words: Peripheral blood, Mesenchymal stem cells, Differentiation, Regenerative medicine
Introduction
Nowadays, isolation of stem cells having the capacity to differentiate into numerous cell types is interestingly noteworthy in regenerative medicine and tissue engineering (1-3). In this context, identification of new stem cell sources, minimally invasive isolation procedures and optimized cell culture conditions are needed for clinical applications. Due to the effective clinical features of mesenchymal stem cells (MSCs), numerous studies have focused on isolation and differentiation of these multipotent cells (1, 2, 4). Extraction from bone marrow (as a main source) is an invasive and a high-risk approach that gives low frequency and heterogeneous population (5). To circumvent these problems, researchers have attempted to isolate mesenchymal stem cells from alternative accessible tissue sources (4, 6-8). Accessibility and a high differentiation potential, introduce blood-derived stem cells as a promising source for medicinal applications (6, 9). However, there is controversy about whether MSC can be detected in the blood circulation in human. Some studies failed to detect MSC in peripheral blood. To dominate this problem, some alterations in isolation methods were done. Using large amount of blood for isolation of these cells is one of these alterations. Zvaifler et al. tried to isolate stem cells from sterile blood packages obtained from blood transfusion services (10). The next strategy was using mobilization protocols such as granulocytecolony stimulating factor (G-CSF) to stimulate stem cells and release them from bone marrow to peripheral blood. For example, Tondreau et al. could successfully isolate stem cells using G-CSF mobilization (11). However, G-CSF mobilization protocol is timeconsuming and not affordable. It is also associated with side effects such as nausea and vomiting (10, 12). Yet, it has been reported that cells with fibroblast morphology which are derived from peripheral blood mononuclear cells in culture, express a hematopoietic immunophenotype. Therefore, they do not fulfill the criteria for MSC set by International Society for Cellular Therapy (ISCT). In this study, the researchers tried to introduce a modified procedure for isolation of peripheral blood-derived mesenchymal stem cells. The novelty of this study was non-mobilization approach and also minimal quantity of blood usage. The isolated cells were analyzed based on ISCT criteria and the findings were compared with other studies.
Materials and Methods
Isolation and expansion of peripheral blood mononuclear cells
Blood sample (10 ml) was taken from a healthy young female after obtaining informed consent form. The density gradient centrifugation was used for the pre-enrichment of mononuclear cells to improve the recovery of rare stem cells. For this purpose, acidcitratedextrose (ACD)-treated blood was centrifuged at 3500 rpm for 20 min. The obtained buffy coat was diluted (1:1) with phosphate-buffered saline (PBS, pH 7.4, Gibco, BRL) and layered on the Ficoll Paque solution (Biosera, France). After centrifugation at 1500 rpm for 15 min, the isolated mononuclear cells were plated out in DMEM-F12 (Gibco, BRL), 15% FBS (Gibco, BRL) and 1% penicillin-streptomycin antibiotic (Gibco, BRL) at a seeding density of 20 × 106 cells per cell-culture dish. Culture medium was changed every 2 days and suspended cells were discarded by each medium exchange. Then the supernatant was aspirated and cells were harvested by centrifugation. The final pellet was transferred into one dish containing DMEM-F12, FBS (15%) and antibiotics. Serial passages were performed using trypsin enzyme (Gibco, BRL).
Immunoprofiling
Flow cytometry was used for immunoprofiling of stem cells. The cells were harvested, pelleted and resuspended in PBS. They were stained for 30 min at 4°C with anti-human CD45 (BD-Biosciences, USA; 555482), CD73 (BD-Biosciences, USA; 561254),
CD105 (BD-Biosciences, USA; 561443) and CD90 (BD-Biosciences, USA; 555596) antibodies. An appropriate isotype-matched control antibody was used in all analyses. The incubated cells (n=10000) were analyzed using FACSCalibur flow cytometer (BDBiosciences) and CellQuest Pro software (version 5.1).
Assessing differentiation potential
The obtained cells were assessed for osteogenic and adipogenic differentiation potentials. The cells of passage 3 were seeded in T-25 flasks (5×103/cm2) and grown in DMEM-F12 and FBS until reaching desired confluency (90%). At this point, the cells were incubated with an appropriate differentiation (osteogenic or adipogenic) medium for 21 days (n=1). Besides high glucose DMEM (Gibco, BRL), FBS (2%), ascorbic acid 2-Phosphate (50 µg) (Sigma, Germany) and dexamethasone (10 nM and 100 nM for osteogenic and adipogenic differentiation, respectively) (Sigma, Germany) that are common compounds of various differentiation media, β-Glycerol Phosphate (10 mM) (Sigma, Germany) and indomethacin (50 µg) (Sigma, Germany) are another components of osteogenic and adipogenic differentiation media, respectively. Controls were grown in DMEM-F12 and FBS (2%). The media was changed every 2 days. To evaluate the differentiation process, cells were fixed (10 min, at room temperature) with PBS-buffered formalin (10%) and stained for 1 hr with oil-Red O (Sigma, Germany) and Alizarin red (Merck, USA) to confirm adipogenesis and osteogenesis, respectively.
Results & Discussion
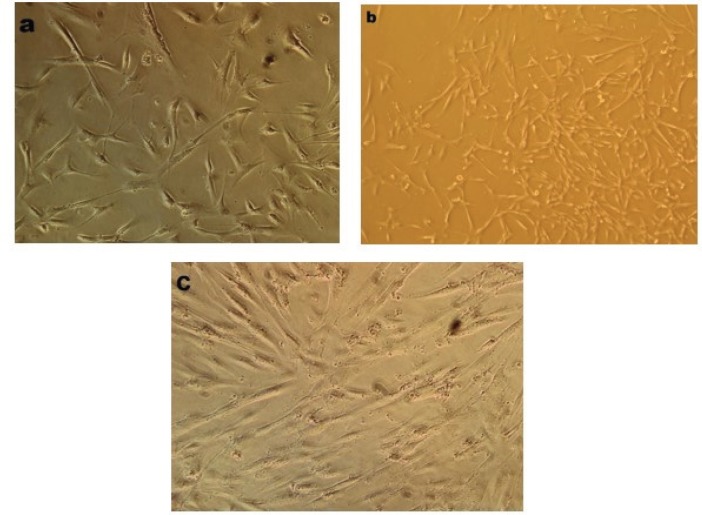
MSCs were successfully isolated from peripheral blood of donor. The number of 20 million PB-derived mononuclear cells was isolated and grown in the appropriate medium. In order to reach the desired confluency following the appearance of mesenchymal-like adherent cells (7th day), some inducible and growth factors were added to the medium. Approximately 0.5-1 million adherent cells were obtained at the end of passage 0 on the 15th day, which were passaged. Phase contrast microscope was used for regularly monitoring cultured cells morphology. Figure 1 shows the phase contrast images of these elongated and spindle-shaped cells in different passages. As shown in Figure 1, PB-MSCs were initially mixed up with small population of round cells which were gradually omitted from medium by each passage (Figure 1).
Figure 1.
Morphology of isolated cells. (a): The adherent cells were observed 7 days after primary culture and displayed spindle-shaped morphology; (b), (c): Morphology of the cells at the first and second passages. The pictures were taken using phase contrast microscope at ×200 magnification
Immunoprofiling
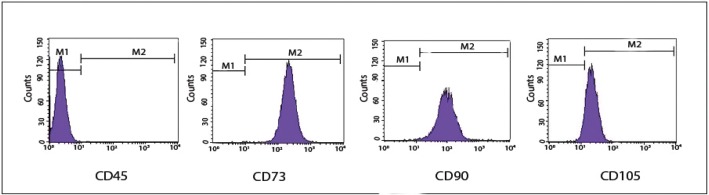
The obtained cells were further characterized by their immunophenotypic antigen profile via flow cytometry (Figure 2). The antigen profiling data revealed that these cells were negative for differentiated cell marker, i.e., CD45 but positive for mesenchymal stem cell markers; CD90 (99.72%), CD73 (99.97%), and CD105 (92.74%).
Figure 2.
Immunophenotypic profile of stem cells. The cells were labeled with conjugated antibodies against the indicated antigens (CD45 as a hematopoietic cell marker and CD105, CD90, and CD73 as mesenchymal stem cell markers), then analyzed by flow cytometry
In Vitro differentiation into adipocyte and osteocyte
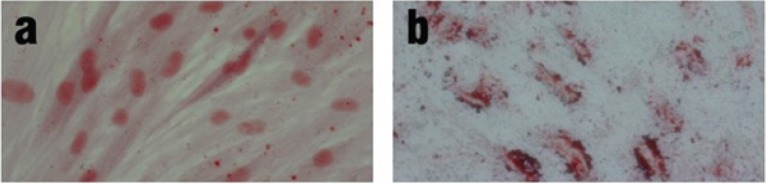
Isolated cells were further evaluated by their differentiation potential, a hallmark of MSCs. To investigate the osteogenic and adipogenic differentiation potential, cells were cultured in the specific induction medium and characterized by Alizarin Red and Oil Red O stainings, respectively (Figure 3). These findings indicated that obtained cells were able to differentiate into osteogenic (display calcium deposits) and adipogenic (display small fat vacuoles) cells.
Figure 3.
Osteogenic and adipogenic differentiation potential of peripheral blood-derived MSCs. The cells were induced to form osteoblasts and adipocytes by culturing in the specific induction media. Differentiation was confirmed by staining with Alizarin red (a) and Oil-red O (b). Images were taken using phase contrast microscope at ×200 magnification
Peripheral blood has been used as one of the sources for MSCs due to its ease of access and having suitable stem cells. It seems that bone marrow is a main origin of these cells (6, 10, 13); however, additional evaluations are required to substantiate this possibility. The isolation and culture of MSCs from peripheral blood has been reported by some research groups. However, some of these studies have used bone marrow stimulators such as granulocyte-colony stimulating factor (G-CSF) and large amounts of peripheral blood (14-16). Although encouraging initial results have been observed in the application of growth factors for blood-derived MSCs isolation, advances in therapeutic efficacy have been hampered by the various side effects of these agents (6, 17). In some studies, like those conducted by Trivanovic et al. (14) and PP-Chong et al. (7), neither large amount of blood nor any stimulator was used. In the first study, MSCs were successfully isolated from 2 out of 6 specimens. To characterize the isolated cells, and to detect special stem cells related genes and multilineage differentiation, they used flow cytometry analysis and RT-PCR, respectively. However, the researchers did not mention the amount of blood they obtained from donors. According to the results, the isolation method they used was not suitable to isolate MSCs from all donors. They suggested further research about extraction and ex-vivo expansion for their safe and effective use. In the second study, the MSCs were extracted from bone marrow and peripheral blood of 20 donors. The researchers mentioned that donors were all patients as some studies have proved that patients with organ injury have increased levels of circulating mesenchymal stem cells. However, consistent with the first study, the researchers did not mention the amount of blood they obtained from donors. According to their results, they could isolate MSCs from all 20 donors. They used only flow cytometry and evaluated the differentiation ability for characterizing the isolated cells. The main aim of their study was to show that peripheral blood MSCs can differentiate into chondrocyte and these cells can be used for some defects.
In the present study, characterization of MSCs isolated from the low amounts of peripheral blood without any growth factor administration was described. In addition, the osteogenic and adipogenic differentiation potential of these cells was evaluated. Consistent with few previous studies (8, 12, 16), the isolated cells in this study met commonly used criteria for defining MSCs (14, 15, 17) that were described by International Society for Cellular Therapy (ISCT). These spindle-shaped cells being adhered to the flask were negative for the expression of differentiated hematopoietic cell marker (CD45), but positive for MSC markers (CD90, CD105, and CD73). Isolated cells exhibited osteogenic and adipogenic differentiation potential as shown by Alizarin Red and Oil-red O stainings, respectively (Figure 3). This study, using standardized procedures for isolation, culture and differentiation of blood-derived MSCs, aimed to determine the non-invasive and most efficient approach to extract and expand blood-derived MSCs that can be used as an allogeneic source for regenerative medicine.
Conclusion
Mesenchymal stem cells are widely used in cell therapy, thus, choosing the best and safest source is important. Peripheral blood is a safe, available and non-invasive source for isolation of MSCs. In this study, it was tried to isolate MSCs from peripheral blood without using G-CSF and other mobilizing factors. Therefore, this method can be used as a safe procedure for isolation of MSCs from peripheral blood.
Acknowledgements
This study was supported by Kerman University of Medical Sciences, Pathology and Stem Cell Research Center, Kerman, Iran.
Conflict of interest
All authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistrypart I: stem cell sources. J Prosthodont Res. 2012;56(3):151–65. doi: 10.1016/j.jpor.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Lin TC, Lee OK. Stem cells: a primer. Chin J Physiol. 2008;51(4):197–207. [PubMed] [Google Scholar]
- 3.Kadir RA, Ariffin SHZ, Wahab RMA. Senafis Molecular characterisation of human peripheral blood stem cells. S Afr J Sci. 2012;108(5-6):67–73. [Google Scholar]
- 4.Maleki M, Ghanbarvand F, Mohammad Reza Behvarz ME, Ghadirkhomi E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells. 2014;7(2):118–26. doi: 10.15283/ijsc.2014.7.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33(11):1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong PP, Selvaratnam L, Abbas AA, Kamarul Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res. 2012;30(4):634–42. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 8.Bieback K, Kern S, Kocaömer A, Ferlik K, Bugert P. Comparing mesenchymal stromal cells from different human tissues: bone marrow, adipose tissue and umbilical cord blood. Biomed Mater Eng. 2008;18(1Suppl):S71–6. [PubMed] [Google Scholar]
- 9.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–7. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 10.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2(6):477–88. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, et al. Mesenchymal stem cells derived from CD133‐positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23(8):1105–12. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 12.Hoogduijn MJ, Verstegen MM, Engela AU, Korevaar SS, Roemeling-van Rhijn M, Merino A, et al. No evidence for circulating mesenchymal stem cells in patients with organ injury. Stem Cells Dev. 2014;23(19):2328–35. doi: 10.1089/scd.2014.0269. [DOI] [PubMed] [Google Scholar]
- 13.Stoltz JF, de Isla N, Li YP, Bensoussan D, Zhang L, Huselstein C, et al. Stem cells and regenerative medicine: myth or reality of the 21th century. Stem Cells Int. 2015;2015:734731. doi: 10.1155/2015/734731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trivanović D, Kocić J, Mojsilović S, Krstić A, Ilić V, Okić-Đorđević I, et al. Mesenchymal stem cell isolated from peripheral blood and umbilical cord Wharton’s jelly. Srp Arh Celok Lek. 2013;141(3-4):178–86. doi: 10.2298/sarh1304178t. [DOI] [PubMed] [Google Scholar]
- 15.Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, Marx G, et al. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006;37(10):967–76. doi: 10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- 16.Potdar P, Subedi R. Defining Molecular Phenotypes of Mesenchymal and hematopoietic Stem Cells derived from Peripheral blood of Acute Lymphocytic Leukemia patients for regenerative stem cell therapy. J Stem Cells Regen Med. 2011;7(1):29–40. doi: 10.46582/jsrm.0701004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012;5:19. doi: 10.1186/1756-8722-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]