Significance
Transient receptor potential melastatin type 2 (TRPM2) is an oxidative stress-sensing calcium-permeable channel that is thought to contribute to the calcium influx associated with neurodegenerative diseases. Here we show that TRPM2 deficiency lowers chronic unpredictable stress (CUS)-induced reactive oxygen species (ROS) and calpain activation and prevents aberrant hyperactivation of cyclin-dependent kinase 5 (Cdk5). In mice models of CUS, genetic elimination of TRPM2 normalized behavioral deficits. Moreover, removal of Cdk5 reversed the antidepressant-like behaviors observed in Trpm2−/− mice. Our results reveal the important roles of TRPM2 in raising ROS levels and aberrantly hyperactivating Cdk5 in mouse models of CUS and suggest that TRPM2 could be a target for treating ROS-induced neurodegenerative diseases such as depression.
Keywords: TRPM2, depression, ROS, Cdk5, neurogenesis
Abstract
Major depressive disorder (MDD) is a devastating disease that arises in a background of environmental risk factors, such as chronic stress, that produce reactive oxygen species (ROS) in the brain. The chronic stress-induced ROS production involves Ca2+ signals; however, the mechanism is poorly understood. Transient receptor potential melastatin type 2 (TRPM2) is a Ca2+-permeable cation channel that is highly expressed in the brain. Here we show that in animal models of chronic unpredictable stress (CUS), deletion of TRPM2 (Trpm2−/−) produces antidepressant-like behaviors in mice. This phenotype correlates with reduced ROS, ROS-induced calpain activation, and enhanced phosphorylation of two Cdk5 targets including synapsin 1 and histone deacetylase 5 that are linked to synaptic function and gene expression, respectively. Moreover, TRPM2 mRNA expression is increased in hippocampal tissue samples from patients with MDD. Our findings suggest that TRPM2 is a key agent in stress-induced depression and a possible target for treating depression.
One environmental risk factor that has been linked to the pathogenesis of major depressive disorder (MDD) is chronic stress, which can be modeled in rodents (1). Recent studies have shown that stress-induced animal models mimic MDD in humans, suggesting that stress may be a major cause of MDD (2). Some of the physiological hallmarks of stress are reactive oxygen species (ROS) production, inflammation, and increased intracellular calcium (3–5). The pathways responsible for these effects are incompletely understood but are known to include a reduction in hippocampal neurogenesis as well as activation of ion channels (6, 7). Although blockers of ion channels have shown some efficacy in certain models of MDD (8), the molecular identity of the specific ion channels that mediate MDD in vivo remains unknown.
Transient receptor potential melastatin type 2 (TRPM2) is a nonselective cation channel permeable to calcium (Ca2+), sodium, and potassium and activated by oxidant stress, ADP ribose, and intracellular calcium (9, 10). It is abundantly expressed in the central nervous system (CNS), including the hippocampus, substantia nigra, striatum, cortex, and dorsal root ganglion sensory neurons in the spinal cord (11, 12). Several studies have demonstrated a role for TRPM2 in cell death in response to oxidative stress in a variety of cell types, including neurons (13, 14), implying a role for TRPM2 in various neurological disorders (15). In the CNS, TRPM2 affects neurite growth and spine formation (16) and links ROS to calcium-signaling responses (17, 18) that can lead to a variety of neurological disorders.
A reasonable hypothesis linking TRPM2 and depression is that TRPM2 represents a unique channel in that it is activated by a rise in ROS and that this leads to Ca2+ influx, thereby inducing neuronal injury. Cyclin-dependent kinase 5 (Cdk5) is expressed throughout the adult brain, particularly in the hippocampus (19), where it is reportedly activated by various stress conditions, including chronic mild stress in rodents (20). Its activity is dependent upon its direct association with the noncyclin cofactor p35 (21). Cofactor p35 is converted to p25 by the calcium-dependent protease calpain, resulting in the formation of Cdk5/p25 complexes that engender aberrant activity, which may in turn lead to neuronal death, neurodegeneration, and disease (22).
Despite substantial progress, the pathophysiological basis of MDD, which may be triggered or exacerbated by severe or chronic stress, remains unclear. We have identified a previously unknown mechanism involving TRPM2 signaling in the hippocampus that is associated with Cdk5 activation and that affects behavioral responses to acute and chronic stress. As our understanding of its role in stress and depression expand, TRPM2 will likely prove to be a powerful tool in profiling behavioral and disease states.
Results
TRPM2 Mediates the Chronic Stress-Induced ROS Response and Antidepressant-Like Behaviors.
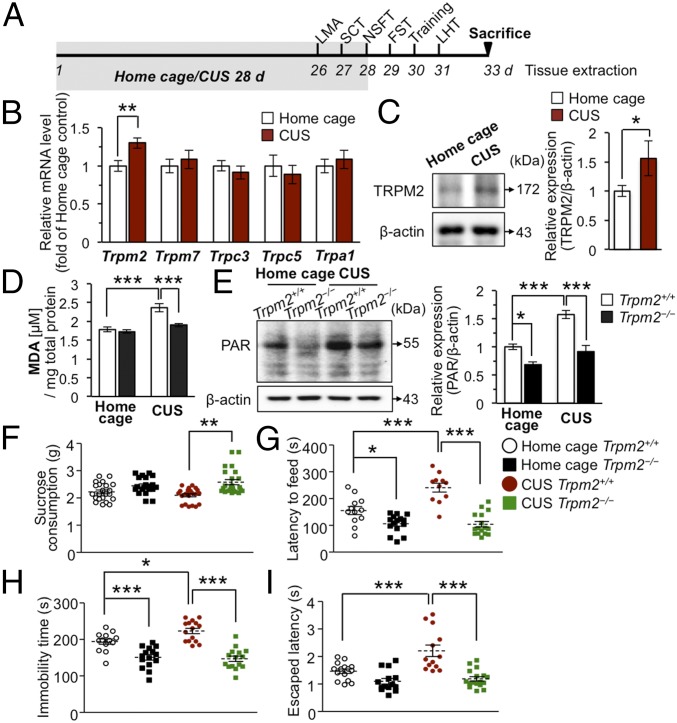
To approach the basis of MDD experimentally, we determined if expression of TRPM2 increased in a chronic unpredictable stress (CUS) model of depression in mice. To induce CUS, we subjected mice to a series of CUS paradigms (hereafter simply referred to as CUS) (23) (Fig. 1A) and measured mRNA and protein levels of TRPM2 in the hippocampus, where TRPM2 is abundant (24). Quantitative real-time PCR analysis did indeed reveal increased TRPM2 levels in the hippocampus of these mice, whereas no changes in other redox-sensitive TRP channels including TRPM7, TRPC3, TRPC5, and TRPA1 (25, 26) were detected (Fig. 1B). Consistent with this, CUS increased TRPM2 protein levels in the hippocampus (Fig. 1C). These findings are in line with the previous finding that TRPM2 was up-regulated in brain samples from mice treated with a ROS generator (27). To see if our findings could be translated to human patients, we evaluated TRPM2 mRNA expression in postmortem hippocampal tissue from subjects with MDD using the gene expression dataset from GEO accession no. GSE53987 of patients with MDD (National Center for Biotechnology Information Gene Expression Omnibus). TRPM2 mRNA expression was significantly higher in the hippocampus of MDD patients than in those of healthy controls (P = 0.004) (SI Appendix, Fig. S1 A–C). No significant difference was observed for other TRP channels that are linked to ROS in the hippocampus (SI Appendix, Fig. S1 B, D–G). Together, these results indicate that TRPM2 may be at least partly specific to depression.
Fig. 1.
TRPM2 modulates chronic stress-induced ROS responses in the hippocampus and depressive-like behaviors in mice. (A) Timeline of experimental procedures. (B) mRNA levels were measured by real-time PCR in the whole hippocampi of Trpm2+/+ mice (n = 4–6 per group). (C) Representative immunoblots (Left) and quantitative data (Right) for TRPM2 protein levels normalized to the level of β-actin (n = 4–6 per group). (D) CUS significantly increased MDA levels in the hippocampus of Trpm2+/+ mice, but not in the Trpm2−/− mice (n = 10 and 7 for Trpm2+/+ and Trpm2−/− mice, respectively; genotype × stress interaction F1,30 = 6.578, P = 0.0156). (E) Representative immunoblots (Left) and quantitative data (Right) of PAR normalized with the level of β-actin (n = 6 per group; genotype × stress interaction F1,20 = 5.805, P = 0.0257). (F) Sucrose consumption test (SCT) (n = 10–12 per group). (G) NSFT (n = 11–16 per group; genotype × stress interaction F1,50 = 12.32, P = 0.001). (H) FST (n = 13–15 per group; genotype × stress interaction F1,54 = 5.006, P = 0.0294). (I) LHT (n = 13–17 per group; genotype × stress interaction F1,55 = 7.286, P = 0.0092). Data are means ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001. Statistical analysis (D, E, G, and I) was performed using two-way ANOVA followed by Bonferroni posttest. Other statistical parameters are listed in SI Appendix, Table S1.
Next, we examined chronic stress-induced ROS accumulation in the hippocampus of Trpm2+/+ and Trpm2−/− mice by measuring lipid peroxidation levels based on malondialdehyde (MDA) production. Before this, we confirmed that TRPM2 was not expressed in the Trpm2 knockout (Trpm2−/−) mice (SI Appendix, Fig. S2 A and B). Similar levels of MDA were found in the hippocampus of control Trpm2+/+ and Trpm2−/− mice (Fig. 1D). However, in CUS (Fig. 1A), MDA increased in the Trpm2+/+ but not in the Trpm2−/− hippocampus (Fig. 1D), suggesting that TRPM2 channels are involved in the ROS accumulation in CUS. Oxidative stress can activate poly ADP ribose polymerase (PARP), which produces a branched polymer of poly ADP ribose (PAR) that activates the TRPM2 channel (9, 10). We therefore investigated whether PAR production is altered in Trpm2−/− mice. PAR increased substantially in stressed Trpm2+/+ hippocampus but less so in stressed Trpm2−/− hippocampus (Fig. 1E), consistent with a lower MDA level in Trpm2−/− mice compared with Trpm2+/+ mice.
It has been reported that stress promotes oxidative metabolic activity (4), which is implicated in depressive-like behaviors (28). Since CUS-induced ROS accumulation was diminished in Trpm2−/− mice (Fig. 1 D and E), we hypothesized that TRPM2 might be an important mediator of the depressive-like behaviors triggered by CUS. We first analyzed depression in TRPM2-deficient mice under nonstressed (home cage) conditions using four behavioral models of antidepressant activity (Fig. 1A). We found a lower immobility time and a shorter latency to feed in the forced swim test (FST) and novelty-suppressed feeding test (NSFT), respectively, in the Trpm2−/− mice than in their Trpm2+/+ littermates (Fig. 1 G and H). However, Trpm2−/− mice did not differ significantly from Trpm2+/+ mice in the learned helplessness test (LHT), another model of behavioral despair (29) (Fig. 1I) or in sucrose consumption (Fig. 1F), spontaneous locomotor activity, or anxiety (SI Appendix, Fig. S2 C–E), although speed and movement distance were slightly higher during the initial block of tests (SI Appendix, Fig. S2 F–H). Evidently, TRPM2 deficiency is moderately associated with antidepressant-like effects under nonstressed conditions. Considering that FST and NSFT involve locomotor activity, we could not rule out the possibility that the reduced immobility time and latency time in the FST and NSFT, respectively, might be contributed by slightly higher locomotor activity in Trpm2−/− mice. This led us to analyze depression-related behaviors under stressed conditions. Trpm2+/+ mice exposed to CUS, but not similarly treated Trpm2−/− mice, had a higher immobility time and a longer latency to feed in the FST and the NSFT, respectively, than home-caged control mice, thus displaying significant depressive responses to CUS (Fig. 1 G and H). Stressed Trpm2−/− mice also tended to have shorter escape latencies in LHT and higher sucrose consumption than Trpm2+/+ mice (Fig. 1 F and I); these findings all indicate that TRPM2 deficiency has a pronounced antidepressant-like effect under stressful conditions. They also suggest that TRPM2 is responsible for the depressive-like behaviors triggered by CUS and that this process might involve ROS produced in CUS.
TRPM2 Deficiency Reduces ROS Accumulation.
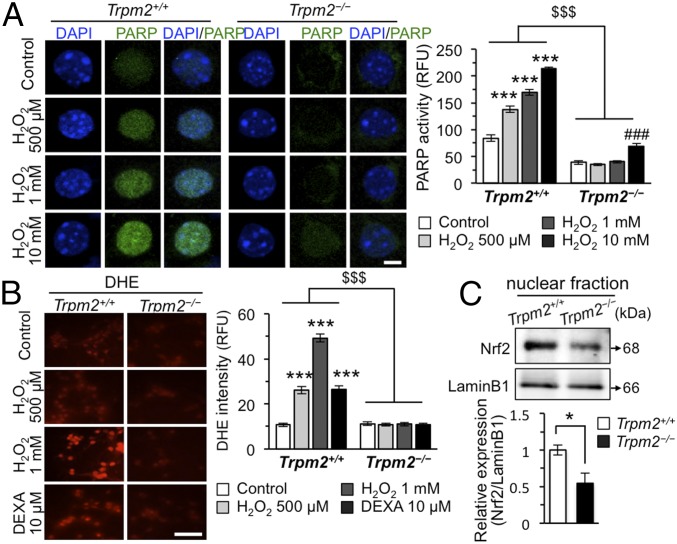
Next, we focused on the role of TRPM2 in the accumulation of ROS in CUS. To this end, we used a cytochemical method to see whether hydrogen peroxide (H2O2), a well-established oxidative stress inducer, induces PARP activity. H2O2 induced PARP activity in Trpm2+/+ hippocampal neurons (Fig. 2A), but was much less effective in Trpm2−/− hippocampal neurons (Fig. 2A). Dihydroethidium (DHE)-reactive superoxide levels, reflecting ROS accumulation, were also lower in the Trpm2−/− neurons (Fig. 2B). Similarly, dexamethasone (10 μM), a synthetic glucocorticoid (30), induced superoxide only in Trpm2+/+, and not Trpm2−/− neurons (Fig. 2B). Nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) is an emerging regulator of cellular resistance to oxidants (31) that would be expected to be translocated to the nucleus and activated when ROS increase, and indeed, we found a higher level of nuclear Nrf2 in Trpm2+/+ than in Trpm2−/− hippocampus (Fig. 2C). Taken together, these results suggest that TRPM2 deficiency prevents stress-induced increases in ROS. Also TRPM2-deficient neurons produced lower PARP activity followed by lower PAR levels than Trpm2+/+ neurons at the same level of oxidative stress, further supporting that ROS levels are basically decreased in TRPM2-deficient neurons.
Fig. 2.
TRPM2 mediates ROS accumulation in the mouse hippocampus. (A) Representative confocal images (Left) and bar graph (Right) showing the average fluorescence intensities of PARP activity (green) in mouse hippocampal primary cultures. RFU, relative fluorescence units (n = 38–53 neurons per group; genotype × drug interaction F3,380 = 45.17, P < 0.0001). (Scale bar, 5 μm.) * vs. Trpm2+/+ control, # vs. Trpm2−/− control. $$$ denotes the difference between genotypes at each concentration of H2O2. (B) Representative images of live cells (Left) and bar graph (Right) showing the average fluorescence intensities of DHE dye (red) upon treatment with H2O2 for 30 min and dexamethasone (DEXA) for 2 h (n = 50 neurons per group; genotype × drug interaction F3,392 = 96.43, P < 0.0001). (Scale bar, 50 μm.) * vs. Trpm2+/+ control. $$$ denotes a significant difference between genotypes at each concentration of drug. (C) Representative immunoblots of the mouse hippocampal lysates (Top). Nuclear protein levels of Nrf2 normalized to LaminB1 (Bottom) (n = 3 per group). Data are means ± SEM; *P < 0.05, ***P < 0.001, ###P < 0.001, $$$P < 0.001. Statistical analysis (A and B) was performed using two-way ANOVA followed by Bonferroni posttest. Other statistical parameters are listed in SI Appendix, Table S1.
Mature hippocampal neurons and proliferating cells are TRPM2-positive (SI Appendix, Fig. S3 A–D). We found that TRPM2 deficiency had beneficial effects on adult hippocampal neurogenesis (SI Appendix, Fig. S4 A–D), a form of neural plasticity that is thought to be relevant to stress (32) and is diminished by oxygen radicals (33), under both home cage and CUS conditions (SI Appendix, Fig. S4 C and D). We observed no effects on glia differentiation and inflammation in Trpm2−/− mice (SI Appendix, Fig. S4 E–G). To ensure that TRPM2 deficiency modified the differentiation potential of neurons, the expression of calbindinD28k, a marker for mature granule cells (34), was examined. The fraction of cells colabeled with calbindinD28k and BrdU+ was higher in Trpm2−/− mice than in Trpm2+/+ mice (SI Appendix, Fig. S4H). In addition, we found that the volume of granule cell layer in the dentate gyrus (DG) was higher in Trpm2−/− mice than in Trpm2+/+ mice (SI Appendix, Fig. S4I). These findings collectively demonstrate that TRPM2 deficiency results in decreased ROS levels and increased ROS-sensitive neuronal plasticity in chronically stressed mice.
TRPM2 Deficiency Blocks Ca2+-Dependent Calpain Activation.
Our results so far suggest that TRPM2 deficiency abrogates stress-induced increases in ROS and subsequent signaling events. Therefore, it is plausible that ROS are somehow eliminated in TRPM2-deficient neurons. Cdk5 is involved in adult neurogenesis (19), depressive-like behaviors, and stress (35). Moreover, aberrant hyperactivation of Cdk5/p25 due to cleavage of its regulator p35 to p25 by Ca2+-dependent calpain (22) during oxidative stress results in antioxidant enzyme inhibition and ROS accumulation (36, 37). To examine a possible link between TRPM2 and Cdk5, we first compared Cdk5 levels in Trpm2+/+ and Trpm2−/− hippocampal neurons since hippocampal neurons express high levels of TRPM2 channels (SI Appendix, Fig. S3 A and D). Levels of Cdk5 and its activator p35 were elevated both in DG of Trpm2−/− mice (SI Appendix, Fig. S5A) and in cultured Trpm2−/− hippocampal neurons (SI Appendix, Fig. S6A). Moreover, more p35 was coimmunoprecipitated with Cdk5 in Trpm2−/− than in Trpm2+/+ hippocampal tissue (SI Appendix, Fig. S5B), which would imply that Cdk5 kinase activity is higher in the former than the latter due to the increased Cdk5/p35 binding. In vivo kinase assays indeed revealed twofold higher Cdk5 activity in Trpm2−/− mice (SI Appendix, Fig. S5C). This, together with the enhanced Cdk5 expression, suggests that Cdk5/p35 is more active in Trpm2−/− mice than in Trpm2+/+ mice. Levels of other kinases, such as extracellular signal-regulated kinase (ERK), that are involved in stress (38) did not differ between Trpm2+/+ and Trpm2−/− mice (SI Appendix, Fig. S6B).
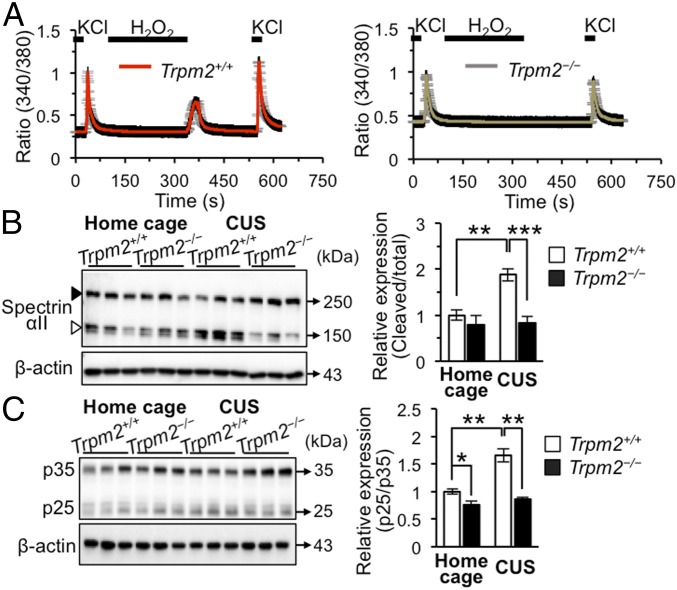
Conversion of p35 to p25 is known to be induced by Ca2+-dependent calpain and to result in aberrantly active Cdk5/p25 (22). To evaluate the role of TRPM2 in Ca2+-dependent calpain activation, we first performed ratiometric Ca2+ imaging. We found that KCl (50 mM) induced substantial Ca2+ influx in both Trpm2+/+ and Trpm2−/− hippocampal neurons (Fig. 3A). However, H2O2 (1 mM) increased intracellular calcium ([Ca2+]i) only in Trpm2+/+ hippocampal neurons (Fig. 3A), consistent with the fact that H2O2 is a well-known TRPM2 activator (11, 14) and confirming that TRPM2 mediates the ROS-induced Ca2+ increase. This led us to examine whether Ca2+-dependent calpain activation is inhibited in Trpm2−/− hippocampal neurons. H2O2 treatment produced a dose-dependent decrease of p35 with a concomitant increase of p25 in Trpm2+/+ neurons but not in Trpm2−/− neurons (SI Appendix, Fig. S7A), consistent with the previously reported ROS-induced p35 degradation via calpain (22) and suggesting that TRPM2 activation promotes conversion of p35 to p25. This finding also accounts for why there is less p35 in Trpm2+/+ than in Trpm2−/− mice (SI Appendix, Fig. S5A). Taken together, our results show that TRPM2 deficiency blocks ROS-induced Ca2+ influx and subsequent p35 degradation to p25.
Fig. 3.
TRPM2 facilitates Ca2+-dependent calpain activation. (A) Cultured hippocampal neurons responded to H2O2 (1 mM) with increases in [Ca2+]i as assessed by Fura-2–based calcium imaging (n = 53, and 62 for Trpm2+/+ and Trpm2−/− neurons, respectively). (B) Immunoblots of Spectrin αII (solid arrowhead, total Spectrin αII; open arrowhead, cleaved form) in the hippocampal DG (n = 4–5 per group; genotype × stress interaction F1,15 = 7.659, P = 0.0144, two-way ANOVA followed by Bonferroni posttest). (C) Immunoblots of p35 and p25 in the hippocampal DG (n = 4 per group). Data are means ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001. Other statistical parameters are listed in SI Appendix, Table S1.
It has been reported that the aberrant activity of Cdk5/p25 reduces the peroxidase activity required for ROS scavenging, thereby resulting in elevation of ROS (36). Cdk5/p25 phosphorylates peroxiredoxin 2 (Prx2), a cellular peroxireductase that serves as an essential oxygen free-radical scavenger, at Thr89, resulting in inhibition of Prx2 activity (36). We found that TRPM2 deficiency reduced the phosphorylation of Prx2 in vivo (SI Appendix, Fig. S8A), consistent with a lower expression of p25 relative to p35 in Trpm2−/− than in Trpm2+/+ mice. We then further investigated whether TRPM2 deficiency is involved in reducing oxidative stress via Cdk5 by measuring PAR production with the help of roscovitine, a small-molecule inhibitor of Cdk5. We confirmed that the basal level of PAR seen in TRPM2-deficient neurons was raised by roscovitine (50 μM) to the level of Trpm2+/+ neurons, indicating that Cdk5 activity is required for reducing oxidative stress in Trpm2−/− neurons (SI Appendix, Fig. S8B).
To determine whether TRPM2 regulates Cdk5 activity via calpain in vivo, we again turned to hippocampal tissues obtained from animals that had experienced CUS and monitored p25 as well as spectrin αII, a marker of calpain activity (22). Stress led to an increase in spectrin αII breakdown products in protein extracts from stressed Trpm2+/+ mice hippocampus, but not from Trpm2−/− mice hippocampus (Fig. 3B). Consistent with this, higher p25 levels relative to p35 were apparent in the hippocampus of stressed Trpm2+/+ mice than in those of stressed Trpm2−/− mice (Fig. 3C), indicating that CUS strongly activates calpain in Trpm2+/+ mice but not in Trpm2−/− mice and hence that TRPM2 is involved in the stress-induced activation of calpain. Collectively, these results demonstrate that TRPM2 induces Ca2+-dependent calpain activation; the increased level of Cdk5/p35 relative to Cdk5/p25 in TRPM2-deficient neurons may contribute to efficient scavenging of ROS in these neurons.
TRPM2 Deficiency Increases Cdk5-Driven Gene Expression and Synaptic Transmission.
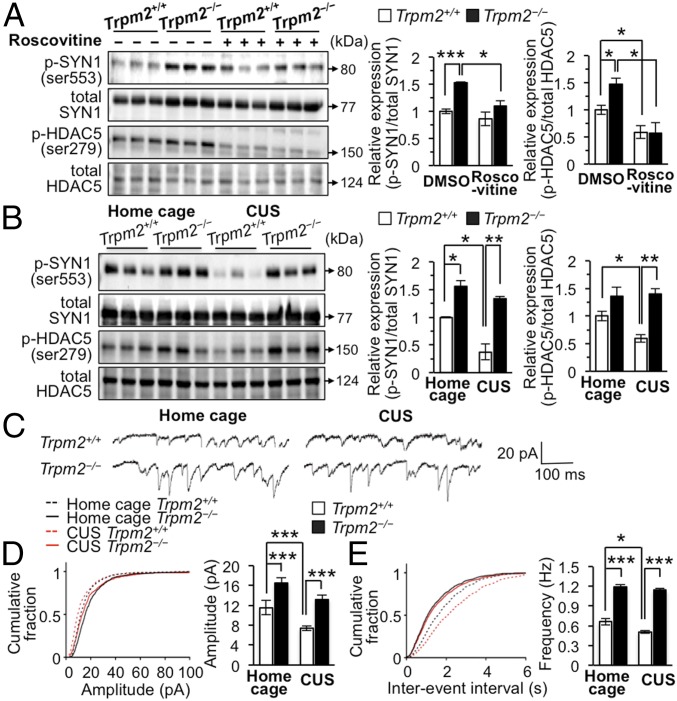
Since TRPM2 influences Cdk5 activity, which can affect neuronal activity, we assessed whether Cdk5 regulates synapsin (SYN) 1 and histone deacetylase (HDAC) 5, known endogenous substrates for Cdk5 phosphorylation (39, 40). H2O2 decreased the expression of p-SYN1 and p-HDAC5 in Trpm2+/+ but not in Trpm2−/− neurons (SI Appendix, Fig. S7B), indicating that TRPM2 activation regulates the phosphorylation of SYN1 and HDAC5. Treatment of neurons with roscovitine (50 μM) decreased levels of p-SYN1 and p-HDAC5 in Trpm2−/− neurons, indicating that the increases in p-SYN1 and p-HDAC5 in these neurons are Cdk5-dependent (Fig. 4A).
Fig. 4.
Mice lacking TRPM2 exhibit enhanced Cdk5-specific phosphorylation and synaptic properties. (A) Representative immunoblots of the lysates from mice hippocampal primary neurons treated with roscovitine (50 μM) for 12 h (n = 3 per group). (B) Representative immunoblots of the hippocampal DG lysates (n = 3 per group). (C) Representative traces of mEPSCs recorded from slices of DG neurons of Trpm2+/+ and Trpm2−/− mice under home cage (Left) and CUS conditions (Right), respectively. (D) Cumulative probability plots of the peak amplitudes of mEPSC recordings from Trpm2+/+ and Trpm2−/− DG neurons (n = 3–4 mice per group). (E) Cumulative probability plots of the interevent intervals of mEPSC recordings from Trpm2+/+ and Trpm2−/− DG neurons (n = 3–4 mice per group). Data are means ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001. Other statistical parameters are listed in SI Appendix, Table S1.
To further evaluate the role of hippocampal TRPM2 in Cdk5 activation, we examined whether TRPM2 knockdown up-regulates Cdk5 activity in cultured primary neurons using a lentivirus encoding an shRNA specific for Trpm2 mRNA (lenti-shTRPM2) (SI Appendix, Fig. S9A). Infection of neurons with lenti-shTRPM2 indeed increased the phosphorylation of SYN1 and HDAC5 (SI Appendix, Fig. S9B).
We next investigated whether ROS-induced TRPM2 activation produces similar alterations in mice experiencing CUS and found that CUS markedly decreased p-SYN1 and p-HDAC5 levels in the hippocampal DG of Trpm2+/+ mice (Fig. 4B). However, in Trpm2−/− mice, p-SYN1 and p-HDAC5 remained elevated under CUS (Fig. 4B), consistent with their high Cdk5 activity. Taken together, these results indicate that TRPM2 is responsible for the CUS-induced Cdk5-dependent signaling.
Since phosphorylation of SYN1 at Ser553 is implicated in transmitter release via physiological regulation of cytoskeletal elements (39), we examined spontaneous miniature excitatory postsynaptic currents (mEPSC) and found significantly increased amplitudes and frequencies of these currents in Trpm2−/− neurons (Fig. 4 C–E), indicative of increased synapse number and enhanced synaptic transmission, respectively. These results were also observed in mice that had experienced CUS (Fig. 4 C–E), supporting the view that TRPM2 deficiency promotes synaptic transmission under stressful conditions. In line with these results, the expression of synaptic molecules such as NR1, NR2B, GluR2, and Shank2 was increased in Trpm2−/− neurons (SI Appendix, Fig. S10A). Taken together, these results reveal that TRPM2 deficiency enhances Cdk5, p35, and Cdk5/p35 activity, which in turn leads to increase in synaptic strength.
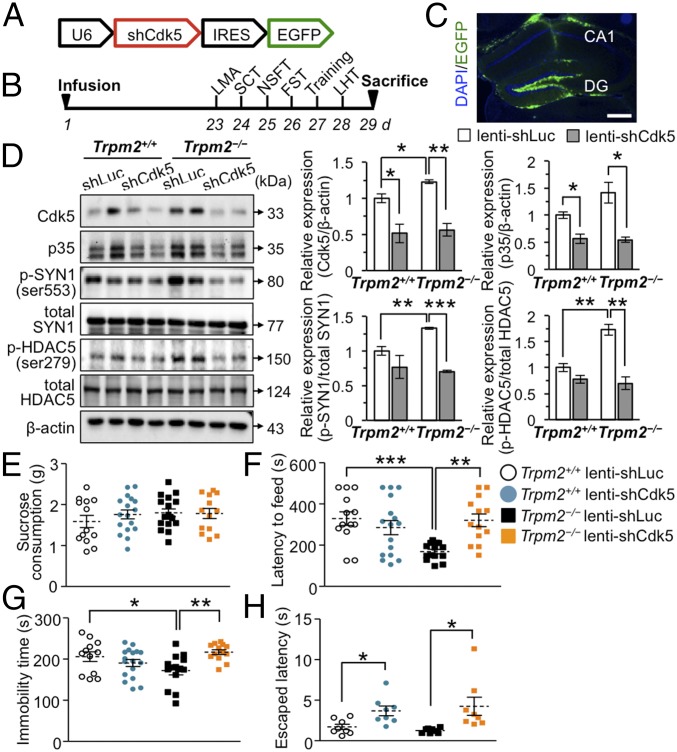
Antidepressant-Like Behaviors in Trpm2−/− Mice Are Blocked by Knockdown of Cdk5 in the DG of Hippocampus.
Given the previous results, we investigated whether Cdk5 knockdown attenuates the antidepressant-like effects seen in Trpm2−/− mice (Fig. 5 A and B). Infusion of lenti-shCdk5 into the hippocampal DG (Fig. 5C) attenuated Cdk5 in both Trpm2+/+ and Trpm2−/− mice (Fig. 5D) and was accompanied by reduced phosphorylation of SYN1 and HDAC5 (Fig. 5D). If Cdk5 activity is involved in reducing ROS (36), lenti-shCdk5 should increase ROS-dependent PARP activity and PAR production, and this was the case in hippocampal neurons (SI Appendix, Fig. S11A). The reason why the effects of Cdk5 knockdown were higher in the Trpm2−/− than in the Trpm2+/+ mice might possibly be because basal levels were also high in the Trpm2−/− neurons.
Fig. 5.
Antidepressant-like behaviors in Trpm2−/− mice are blocked by deletion of Cdk5 in the DG. (A) Lentiviral vector expressing shRNAs targeted against mouse Cdk5 (lenti-shCdk5). (B) Timeline of experimental procedures. (C) GFP immunostaining confirmed localization of lentivirus infection in the adult mouse hippocampal DG. (Scale bar, 400 μm.) (D) Representative immunoblots of the hippocampal DG lysates (n = 3 per group). (E) Sucrose consumption test (SCT) (n = 13–17 per group). (F) NSFT (n = 13–17 per group; genotype × knockdown interaction F1,52 = 11.19, P = 0.0015). (G) FST (n = 12–17 per group; genotype × knockdown interaction F1,52 = 10.03, P = 0.0026). (H) LHT (n = 8 per group). Data are means ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001. Statistical analysis (F and G) was performed using two-way ANOVA followed by Bonferroni posttest. Other statistical parameters are listed in SI Appendix, Table S1.
These results led us to predict that Cdk5 knockdown would block the antidepressant-like effects seen in Trpm2−/− mice, and this proved to be the case: immobility, latency to feed, and escape latency in the FST, NSFT, and LHT, respectively, were all increased by infusing the Trpm2−/− mice with lenti-shCdk5 (Fig. 5 F–H) whereas shCdk5 only slightly increased latency time in the LHT in Trpm2+/+ mice (Fig. 5H). The behavioral effects of lenti-shCdk5 occurred without changes in sucrose consumption (Fig. 5E), total locomotor activities, or anxiety (SI Appendix, Fig. S12 A–C). Using the CUS approach, we also assessed the effect of Cdk5 knockdown in Trpm2−/− mice on chronic stress-induced behavior (SI Appendix, Fig. S13A). Trpm2−/− mice infused with lenti-shLuc and then exposed to CUS displayed the predicted antidepressant-like behaviors in the FST and LHT compared with Trpm2+/+ mice infused with lenti-shLuc (SI Appendix, Fig. S13 D and E). However, these antidepressant-like effects of TRPM2 deficiency were completely absent in Trpm2−/− mice infused with lenti-shCdk5; they displayed similar immobility and latency to escape in the FST and LHT, respectively, as Trpm2+/+ mice infused with lenti-shLuc (SI Appendix, Fig. S13 C–E), further indicating that Cdk5 plays a role in the antidepressant-like effects of TRPM2 deficiency. The behavioral effects of lenti-shCdk5 under stressed conditions occurred without changes in sucrose consumption (SI Appendix, Fig. S13B), total locomotor activities, or anxiety (SI Appendix, Fig. S13 F–H).
Collectively, our findings support the idea that ROS produced during CUS induce PARP activity and PAR production; PAR activates TRPM2, and the resulting Ca2+ influx causes calpain activation and aberrant Cdk5/p25 formation. As a result, neurons are unable to scavenge ROS, and the ROS induce the various deleterious effects of CUS and cause depression.
Discussion
Causative evidence linking TRPM2 levels with the pathogenesis of MDD stems from our findings that the TRPM2 deletion reduces ROS levels and ameliorates CUS-induced behavioral phenotypes. First, we have used in vivo and in vitro approaches to investigate the downstream effects of TRPM2 on ROS levels during chronic stress. We established that the presence of TRPM2 induced PAR and p25 formation in the hippocampus, effects known to be triggered by oxidative stress. PAR formation in turn provided an autoregulatory feed-forward loop provoking further-increased ROS levels by activating TRPM2 and thereby triggering calpain-induced p35 degradation (SI Appendix, Fig. S14). Thus, it is tempting to speculate that ROS-induced Cdk5/p25 activity provides a feed-forward signaling response that strengthens oxidative stress effects leading to processes causing neuronal injury in Trpm2+/+ neurons. Second, TRPM2 deficiency has an antidepressant-like effect. Hippocampal knockdown of Cdk5 by shCdk5 efficiently counteracted the antidepressant-like effects of Trpm2−/−, and since shCdk5 did locally affect Cdk5 expression/activity, we can rule out the idea that the Cdk5 effects on behavior are secondary consequences of oxidative stress. These observations demonstrate that TRPM2 channels and Cdk5/p25 synergize to produce deleterious effects during stress and provide evidence for a previously unappreciated mechanism by which CUS-induced oxidative stress alters neuronal regulation in models of depression.
The mechanism by which CUS affects neurons is not well understood. Papadopoulou et al. (20) reported that CUS strongly increased Cdk5 activity in the hippocampus. In contrast, we consistently found that higher Cdk5 catalytic activity was associated with antidepressant-like behaviors. The source of the discrepancy between our results and those of Papadopoulou et al. (20) remains unknown. However, since Cdk5/p35 and Cdk5/p25 have the same kinase activity, their results might reflect the combined activities of Cdk5/p35 and Cdk5/p25. Our own data, which show that CUS-induced p25 formation is blocked in Trpm2−/− neurons, explain how CUS normally leads to degradation of p35 to p25. Cdk5 activity may be altered by binding to activators such as p25 and p35 during stress, in line with previous observations that Cdk5 is a component of the adaptive response to chronic stress (20). Cdk5/p35 is a cytoplasmic protein kinase anchored to neuronal membranes or the cytoskeleton whereas Cdk5/p25 is found in the nuclear fraction (41). Therefore, it is possible that Cdk5/p35 contributes to the phosphorylation of SYN1 and HDAC5 in the cytosol, thereby enhancing synaptic function and retaining HDAC5 in the cytoplasm (40).
Inhibitors of Cdk5/p25 are presently being sought as potential treatments for neurodegeneration, and Cdk5/p35 may be essential in adult neurons to prevent cell death. A useful inhibitor must discriminate between Cdk5 bound to p25 as opposed to p35. However, all known conventional inhibitors of Cdk5 are expected to target both Cdk5/p25 and Cdk5/p35. Furthermore, the antidepressant-like behaviors in Trpm2−/− mice may not be completely explained by the actions of p35 or p25 especially in the presence of Cdk5 knockdown. Therefore, further study of the roles of p35 and p25 in the downstream TRPM2 pathway is needed. From this point of view, Cdk5−/− mice could provide valuable models of stress-induced depression and may further help delineate whether p35 and p25 play a role in the TRPM2 pathway in the absence of Cdk5. Our present work suggests that inhibiting TRPM2 may provide a therapeutic strategy for reducing the toxic effects of Cdk5/p25 without affecting the normal function of Cdk5/p35.
As elevated markers of oxidative stress have been noted in brains of MDD patients (42) and those with neurodegenerative diseases (43), it is possible that oxidative stress leads to aberrant Cdk5 activity and TRPM2 expression in these disorders. We report that stress-induced ROS produce depressive-like behaviors via the TRPM2-Cdk5 pathway. Given the substantial evidence for a role of oxidative stress in depression (44), further research on oxidative stress-induced alterations of TRPM2 activity and intracellular calcium signaling in this serious neuropsychiatric disorder is warranted.
Materials and Methods
A detailed description of the materials and methods is provided in SI Appendix, SI Materials and Methods.
Mice.
TRPM2 knockout mice were generated and characterized previously (45). All experiments were performed with 8- to 12-wk-old male TRPM2 knockout mice, and age-matched male wild-type littermates were set for each experiment. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Hanyang University.
CUS Procedure.
We used various stressors and deliberately designed the sequence of them to maximize unpredictability (23). All CUS mice were exposed to two or three stressors a day for 28 d (for details, see SI Appendix, Table S2).
Viral-Mediated Gene Transfer.
Three microliters of lentivirus was injected bilaterally into each DG of the dorsal hippocampus at a rate of 0.15 μL/min (stereotaxic coordinates in millimeters with reference to the bregma: anteroposterior, −2.0; mediolateral, ±1.5; dorsoventral, −2.4) using a 26s gauge syringe needles (Hamilton).
Statistical Analysis.
Statistical analysis was performed using GraphPad Prism 5.0 software. Two-way ANOVA followed by Bonferroni’s posttest was used for multiple comparisons between groups when assessing the effect of genotype on stress and the effect of lenti-shRNA infusion on behaviors. The results were presented as mean ± SEM. The level of statistical significance was set at P < 0.05 using two-tailed tests. All experiments were carried out at least three times. Related statistical parameters are specified in SI Appendix, Table S1.
Supplementary Material
Acknowledgments
We thank Christopher W. Cowan and David S. Park for providing anti-HDAC5(Ser279) and anti-Prx2(Thr89), respectively. This work was supported by National Research Foundation of Korea Grants 2016R1A2B2006474 (to H.S.) and 2016R1A2B4013332 (to S.J.J.); Basic Science Research Program Grants 2017R1A6A3A01005765 (to S.E.W.) and 2017R1D1A1B03032858 (to M.C.); and Medical Research Center Grant 2017R1A5A2015395 (to H.S.) funded by the Ministry of Science and Technology, Republic of Korea.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814335116/-/DCSupplemental.
References
- 1.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 2.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 3.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aschbacher K, et al. Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013;38:1698–1708. doi: 10.1016/j.psyneuen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satoh E, Tada Y, Matsuhisa F. Chronic stress enhances calcium mobilization and glutamate exocytosis in cerebrocortical synaptosomes from mice. Neurol Res. 2011;33:899–907. doi: 10.1179/1743132811Y.0000000033. [DOI] [PubMed] [Google Scholar]
- 6.Huang TT, Zou Y, Corniola R. Oxidative stress and adult neurogenesis: Effects of radiation and superoxide dismutase deficiency. Semin Cell Dev Biol. 2012;23:738–744. doi: 10.1016/j.semcdb.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nazıroğlu M. Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J Recept Signal Transduct Res. 2012;32:134–141. doi: 10.3109/10799893.2012.672994. [DOI] [PubMed] [Google Scholar]
- 8.Zarate CA, Jr, et al. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perraud AL, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 10.Sano Y, et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- 11.Hara Y, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 12.Fonfria E, et al. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res. 2006;26:159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- 13.Buttyan R, et al. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989;9:3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneko S, et al. A critical role of TRPM2 in neuronal cell death by hydrogen peroxide. J Pharmacol Sci. 2006;101:66–76. doi: 10.1254/jphs.fp0060128. [DOI] [PubMed] [Google Scholar]
- 15.Nazıroğlu M. TRPM2 cation channels, oxidative stress and neurological diseases: Where are we now? Neurochem Res. 2011;36:355–366. doi: 10.1007/s11064-010-0347-4. [DOI] [PubMed] [Google Scholar]
- 16.Jang Y, et al. TRPM2 mediates the lysophosphatidic acid-induced neurite retraction in the developing brain. Pflugers Arch. 2014;466:1987–1998. doi: 10.1007/s00424-013-1436-4. [DOI] [PubMed] [Google Scholar]
- 17.Yermolaieva O, Brot N, Weissbach H, Heinemann SH, Hoshi T. Reactive oxygen species and nitric oxide mediate plasticity of neuronal calcium signaling. Proc Natl Acad Sci USA. 2000;97:448–453. doi: 10.1073/pnas.97.1.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heiner I, et al. Endogenous ADP-ribose enables calcium-regulated cation currents through TRPM2 channels in neutrophil granulocytes. Biochem J. 2006;398:225–232. doi: 10.1042/BJ20060183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagace DC, et al. Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2008;105:18567–18571. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadopoulou A, et al. Acute and chronic stress differentially regulate cyclin-dependent kinase 5 in mouse brain: Implications to glucocorticoid actions and major depression. Transl Psychiatry. 2015;5:e578. doi: 10.1038/tp.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 22.Lee MS, et al. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 23.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagamine K, et al. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics. 1998;54:124–131. doi: 10.1006/geno.1998.5551. [DOI] [PubMed] [Google Scholar]
- 25.Kozai D, Ogawa N, Mori Y. Redox regulation of transient receptor potential channels. Antioxid Redox Signal. 2014;21:971–986. doi: 10.1089/ars.2013.5616. [DOI] [PubMed] [Google Scholar]
- 26.Kitajima N, et al. TRPC3 positively regulates reactive oxygen species driving maladaptive cardiac remodeling. Sci Rep. 2016;6:37001. doi: 10.1038/srep37001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roedding AS, Tong SY, Au-Yeung W, Li PP, Warsh JJ. Chronic oxidative stress modulates TRPC3 and TRPM2 channel expression and function in rat primary cortical neurons: Relevance to the pathophysiology of bipolar disorder. Brain Res. 2013;1517:16–27. doi: 10.1016/j.brainres.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Seo JS, et al. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J Neurosci. 2012;32:9690–9699. doi: 10.1523/JNEUROSCI.0794-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caldarone BJ, George TP, Zachariou V, Picciotto MR. Gender differences in learned helplessness behavior are influenced by genetic background. Pharmacol Biochem Behav. 2000;66:811–817. doi: 10.1016/s0091-3057(00)00271-9. [DOI] [PubMed] [Google Scholar]
- 30.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 31.Itoh K, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 33.Herrera DG, et al. Selective impairment of hippocampal neurogenesis by chronic alcoholism: Protective effects of an antioxidant. Proc Natl Acad Sci USA. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandt MD, et al. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 2003;24:603–613. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 35.Bavley CC, Fischer DK, Rizzo BK, Rajadhyaksha AM. Cav1.2 channels mediate persistent chronic stress-induced behavioral deficits that are associated with prefrontal cortex activation of the p25/Cdk5-glucocorticoid receptor pathway. Neurobiol Stress. 2017;7:27–37. doi: 10.1016/j.ynstr.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Liu W, Szumlinski KK, Lew J. p10, the N-terminal domain of p35, protects against CDK5/p25-induced neurotoxicity. Proc Natl Acad Sci USA. 2012;109:20041–20046. doi: 10.1073/pnas.1212914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu D, et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson’s disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 38.Treccani G, et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol Psychiatry. 2014;19:433–443. doi: 10.1038/mp.2014.5. [DOI] [PubMed] [Google Scholar]
- 39.Matsubara M, et al. Site-specific phosphorylation of synapsin I by mitogen-activated protein kinase and Cdk5 and its effects on physiological functions. J Biol Chem. 1996;271:21108–21113. doi: 10.1074/jbc.271.35.21108. [DOI] [PubMed] [Google Scholar]
- 40.Taniguchi M, et al. Histone deacetylase 5 limits cocaine reward through cAMP-induced nuclear import. Neuron. 2012;73:108–120. doi: 10.1016/j.neuron.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito T, et al. p25/cyclin-dependent kinase 5 promotes the progression of cell death in nucleus of endoplasmic reticulum-stressed neurons. J Neurochem. 2007;102:133–140. doi: 10.1111/j.1471-4159.2007.04540.x. [DOI] [PubMed] [Google Scholar]
- 42.Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67:360–368. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- 43.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 44.Bilici M, et al. Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J Affect Disord. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto S, et al. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.