Abstract
Essential oils are widely used as functional ingredients for potential multi-purpose functional uses. Hydrosols, co-products of the distillation of plant material, are used in food and cosmetic industries and in biological agriculture, but their volatile composition is poorly investigated. The volatile fractions of essential oils and hydrosols from four less-studied 1,8-cineol-rich Eucalyptus species (E. parvula L.A.S. Johnson & K.D. Hill, E. cinerea F. Muell, E. pulverulenta Sims and E. pulverulenta baby blue Sims), cultivated in Tuscany in a system of organic farming, were characterized by solvent dilution (essential oils) or extraction (hydrosols) followed by GC-MS and by HS-SPME-GC×GC-TOFMS analysis. GC-MS analysis showed that essential oils were mainly constituted by oxygenated monoterpenes, particularly 1,8-cineole, with monoterpenes hydrocarbons up to 10.8%. Relative differences in the abundance of minor terpenes as limonene, α-pinene, γ-terpinene, p-cymene, terpinen-4-ol, α-terpineol, and alloaromandrene were pointed out and seem to be suitable for differentiation among EOs of the four different Eucalyptus species. Hydrosols of these species were characterized for the first time: they were mainly constituted by oxygenated monoterpenes (97.6–98.9%), with 1,8-cineole up to 1.6 g/L, while monoterpene and sesquiterpene hydrocarbons were detected only in traces. HS-SPME-GC×GC-TOFMS analysis also allowed providing metabolic profiling of hydrosols for the direct comparison and visualization of volatile components, pointing out the potentially different uses of these products as functional ingredients in food, beverage, and cosmetic industries.
Keywords: aromatic water, hydrolat, volatile compounds, metabolic fingerprint, eucalyptol
1. Introduction
The discovery of the genus Eucalyptus (Myrtaceae) came about when James Cook, an explorer, and Sir Joseph Banks, an expert botanist, travelled in Australia in 1770. This genus comprises more than 800 species of native trees and shrubs from Australia belonging to the Myrtaceae family, which are widely grown in many parts of the world [1].
The aromatic volatile oil (essential oil, EO), which is steam-distilled from the foliage, is among the world’s most traded essential oils in terms of volume. The study of EO has attracted much attention for its anti-microbial, antibacterial, antiseptic, fungicidal, and nematicidal activities [2,3,4,5]. EO has a long history of use against the effect of cold, flu, sinusitis, rhinitis and other respiratory infections [6]. Tests in vitro showed that EO from E. globulus leaves might be exploited as natural antibiotic for the treatment of several infectious diseases caused by Escherichia coli and Staphylococcus aureus [7]. Treatment of refrigerated pork with EO led to a significant decrease in Pseudomonas spp. count and to an increase of customer acceptance [8]. EO from E. globulus and its major compound 1,8-cineole were tested against A. flavus and A. parasiticus: it was found that the antifungal activity was due not only to the 1,8-cineol, but to the whole phytocomplex [9]. A common need is the availability of natural extracts with pleasant taste and/or smell, combined with a preservative action aimed at avoiding lipid deterioration, fungal growth, oxidation and spoilage by microorganisms. The use of essential oils as functional ingredients in food, beverages and cosmetics is gaining increasing interest because of their relatively safe status, their wide acceptance by consumers, and their exploitation for potential multi-purpose functional use [10,11].
To date, the commercial EOs are mainly obtained from the leaves of the most common species of the genus Eucalyptus (i.e., E. globulus), which, according to the Standards ISO, must contain 1,8-cineole in percentages higher than 80–85%.
Hydrosols (EW), also known as hydrolats, floral waters, distillate waters or aromatic waters, are the co-products or the by-products of hydro- and steam distillation of plant material. Hydrosols are used in food and cosmetic industries for their organoleptic and biological properties. They are also used in biological agriculture against mushrooms, mildew and insects and for fertilization of soils [12]. Commercial EWs from Eucalyptus are currently available in the market, even though their volatile compositions have been poorly investigated to date [13,14]. The major components are generally the same present in oxygenated fraction of the corresponding essential oils [15].
In this study, we took into account four less studied 1,8-cineol-rich Eucalyptus species cultivated in Tuscany (central Italy) in a system of organic farming, namely Eucalyptus parvula L.A.S. Johnson & K.D. Hill, Eucalyptus cinerea F. Muell, Eucalyptus pulverulenta Sims and Eucalyptus pulverulenta baby blue Sims. The characterization of EOs from Eucalyptus cinerea [16,17,18] and Eucalyptus pulverulenta [16] has been reported in the literature while, to the authors’ knowledge, no reports on EO from Eucalyptus parvula have been published, to date. Essential oil obtained from the leaves of these Eucalyptus species could potentially be employed for therapeutic ends and as natural additives for use in the food, cosmetics and perfume industries, extending the use of the plant beyond the predominantly ornamental one.
We aimed to evaluate the content and chemical composition of essential oils (EOs) and, for the first time, leaf hydrosols (EWs) obtained by steam distillation of these species. The evaluation of the content and chemical composition of both EOs and EWs was carried out using optimized Gas-Chromatography coupled with Mass Spectrometry (GC-MS). Head-Space Solid Phase Micro Extraction followed by comprehensive two-dimensional Gas-Chromatography (HS-SPME-GC×GC-TOFMS) analyses allowed providing a fast and direct comparison and visualization of the volatile components (fingerprint) of the EWs, also pointing out the presence of some VOCs not detectable only using the GC-MS. To the author’s knowledge, this work is the first report regarding the characterization of the aroma components of EW from these four Eucalyptus species. Due to the fact that these Eucalyptus species are 1,8-cineol-rich, we hypothesized that 1,8-cineol was the main volatile of EOs and EWs, and that the main differences among both EOs and EWs from different species were due to relative amounts of minor volatile compounds.
2. Results and Discussion
Essential oils and leaf hydrosols were analyzed using integrated sampling and chromatographic techniques. In particular, the volatile organic compounds (VOCs) were extracted (from EWs) or diluted (from OEs) with organic solvent and analyzed by GC-MS and the volatile profile of EWs was also analyzed by GC×GC-TOFMS after extraction of VOCs by HS-SPME. GC-MS is the well-recognized technique of choice for analysis of VOCs from plant material and plant extracts [19,20] and, in this work, we applied this technique for the evaluation of the content and chemical composition of both EWs and EOs. HS-SPME is well recognized as a widespread and convenient sampling tool for VOCs and it is increasingly used coupled with GC-MS in analysis of food and more [21]. However, for quantitation purposes, several issues [22,23] (e.g., differences that arise from different absorption capacity of different fibers, changes in sorption temperature, competition between different molecules at different affinities for the absorpting material, fiber wearing) led to the need of using several devices (e.g., the use of a pool of suitable internal standards [23]) to ensure unbiased quantification. For these reasons, we decided to apply HS-SPME-GC×GC-TOFMS analysis on EWs to better elucidate the volatile profile, thus providing a tool for the direct comparison and visualization of plant volatile components and pointing out the presence of molecules not detectable only with GC-MS. Further quantitative evaluation via HS-SPME-GC-MS analysis can be further investigated in future researches. To the authors’ knowledge, the characterization of EWs of these E. species was not been reported in the literature, to date.
2.1. Chemical Composition of Essential Oils and Hydrolats by GC-MS
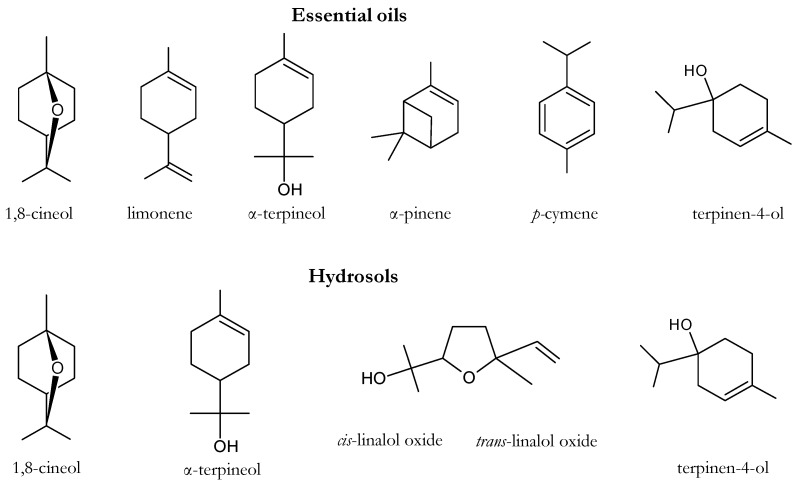
Table 1 shows the chemical composition by GC-MS of the EOs and EWs summarized in Table 3 (see experimental section). Overall, 10 monoterpene hydrocarbons, 19 oxygenated monoterpenes, 2 sesquiterpene hydrocarbons, 2 aromatic monoterpenes (one of which oxygenated), 1 ester, 4 ketones, 1 aldehyde and 5 alcohols were identified. Some of the main molecules detected in the EWs and EOs are reported in Figure 1. Relative abundance of each of the molecules identified by GC-MS was calculated as a percentage of the peak area on the total area of the identified peaks. Peak areas from the total ion current were normalized by the use of the area of internal standard (tridecane).
Table 1.
Volatile organic compounds in hydrosols (EW) and essential oils (EO) of four Eucalyptus species identified by liquid injection GC-MS analysis as described in paragraph 3.4.1. For each compound, concentration is expressed as area % on the total area after normalization with ISTD. Data are the mean of three determinations. Retention Indices (RIcal): Non-isothermal Kovats retention indices from temperature-programming, using the definition of Van den Dool and Kratz, 1963. Retention Indices (RIref): Non-isothermal Kovats retention indices from temperature-programming from Chemistry WebBook. For each compound, different letters indicate significant differences by Fisher’s LSD test (z, y, x, w for aromatic waters; a, b, c, d, e for essential oils).
| n° | Compound | RIcal | RIref | Hydrosols (Area %) | Essential Oils (Area %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-16-EW | 2-16-EW | 3-16-EW | 4-16-EW | 1-17-EW | 1-1617-EW | 1-16-EO | 2-16-EO | 3-16-EO | 4-16-EO | 1-17-EO | 1-1617-EO | ||||
| Monoterpene Hydrocarbons | 0.22 | 0.02 | 0.00 | 0.02 | 0.01 | 0.00 | 8.05 | 10.76 | 7.10 | 8.34 | 6.74 | 7.54 | |||
| 1 | α-pinene | 1030 | 1026 | 0.09 | - | - | - | - | - | 1.20 b | 4.45 e | 2.38 d | 2.15 c | 0.95 a | 1.12 ab |
| 2 | camphene | 1067 | 1065 | - | - | - | - | - | - | 0.03 bc | 0.04 c | 0.02 ab | 0.02 ab | 0.01 a | 0.02 ab |
| 3 | β-pinene | 1122 | 1118 | - | - | - | - | - | - | 0.11 ab | 0.17 cd | 0.14 bc | 0.19 d | 0.09 a | 0.10 a |
| 7 | β-myrcene | 1168 | 1167 | - | - | - | tr | tr | - | 0.12 a | 0.22 c | 0.17 b | 0.25 c | 0.09 a | 0.11 a |
| 8 | α-phellandrene | 1177 | 1177 | - | - | - | - | - | - | 0.15 b | 0.13 b | 0.04 a | 0.01 a | 0.15 b | 0.14 b |
| 12 | Limonene | 1214 | 1210 | 0.08 y | 0.02 z | tr | 0.02 z | 0.01 z | - | 3.60 b | 4.65 d | 3.66 b | 4.29 c | 3.00 a | 3.41 b |
| 14 | Z-ocimene | 1241 | 1242 | - | - | - | - | - | - | 0.36 c | 0.17 b | 0.07 a | 0.07 a | 0.45 d | 0.39 c |
| 15 | γ-terpinene | 1259 | 1254 | tr | - | - | - | - | Tr | 0.54 b | 0.13 a | 0.10 a | 0.07 a | 0.48b | 0.50 b |
| 16 | p-cymene | 1286 | 1281 | 0.05 | - | - | tr | tr | - | 1.78 e | 0.73 b | 0.50 a | 1.23 c | 1.42 cd | 1.63 de |
| 20 | alloocimene | 1383 | 1377 | - | - | - | - | - | - | 0.15 d | 0.07 b | 0.03 a | 0.04 a | 0.12 c | 0.13 c |
| Oxygenated Monoterpenes | 98.62 | 98.50 | 98.29 | 97.64 | 98.91 | 98.80 | 91.30 | 88.74 | 91.99 | 90.60 | 92.71 | 91.89 | |||
| 11 | 2,3-dehydro-1,8-cineole | 1202 | 1197 | - | - | - | - | - | - | - | 0.09 c | 0.08 bc | 0.07 b | 0.05 a | 0.05 a |
| 13 | 1,8-cineol | 1225 | 1221 | 89.53 y | 88.40 z | 90.78 x | 89.17 zy | 90.22 yx | 89.35 zy | 87.06 b | 83.80 a | 87.72 bc | 85.14 a | 88.66 c | 87.75 bc |
| 23 | cis-linalool oxide (furanoid) | 1453 | 1453 | 0.5 zy | 0.77 x | 0.47 zy | 0.44 z | 0.52 zy | 0.55 y | - | - | - | - | - | - |
| 25 | trans-linalool oxide (furanoid) | 1481 | 1482 | 0.49 y | 0.68 x | 0.36 z | 0.43 zy | 0.46 zy | 0.52 y | - | - | - | - | - | - |
| 26 | linalool | 1545 | 1544 | - | - | - | - | - | - | 0.05 b | 0.09 c | 0.09 c | 0.11 d | 0.02 a | 0.03 a |
| 27 | fenchyl alcohol | 1595 | 1571 | tr | tr | - | tr | tr | - | 0.06 b | 0.08 c | 0.05 b | 0.06 b | 0.06 b | 0.03 a |
| 28 | pinocarvone | 1600 | 1575 | - | - | - | - | - | - | 0.06 b | 0.03 a | 0.05 b | 0.06 b | 0.06 b | 0.05 b |
| 29 | terpinen-4-ol | 1617 | 1612 | 0.92 y | 0.94 yx | 1.01 x | 1.37 w | 0.75 z | 0.87 y | 0.51 cd | 0.47 bc | 0.56 d | 0.72 e | 0.39 a | 0.44 b |
| 31 | cis-p-mentha-2,8-dienol | 1637 | 1642 | 0.07 zy | 0.06 z | 0.12 x | 0.08 y | 0.12 x | 0.08 y | 0.02 a | 0.03 ab | 0.06 c | 0.08 d | 0.04 b | 0.04 b |
| 33 | trans-pinocarveol | 1683 | 1659 | 0.17 z | 0.24 yx | 0.23 yx | 0.24 yx | 0.25 x | 0.21 y | 0.09 ab | 0.13 c | 0.11 bc | 0.08 a | 0.10 ab | 0.09 ab |
| 34 | trans-p-mentha-2,8-dienol | 1679 | 1670 | 0.11 z | 0.10 z | 0.11 z | 0.12 z | 0.13 z | 0.10 z | - | - | - | - | - | - |
| 35 | terpineol isomer | 1682 | - | 0.25 z | 0.40 y | 0.37 y | 0.42 y | 0.25 z | 0.24 z | 0.14 ab | 0.19 c | 0.17 bc | 0.23 d | 0.12 a | 0.14 ab |
| 36 | citral | 1698 | 1695 | 0.17 x | 0.27 w | 0.19 x | 0.10 z | 0.14 y | 0.19 x | 0.05 ab | 0.07 bc | 0.08 c | 0.05 ab | 0.04 a | 0.05 ab |
| 37 | α-terpineol | 1707 | 1704 | 6.02 x | 6.24 x | 4.19 z | 4.50 z | 5.53 y | 6.21 x | 3.11 c | 2.77 b | 2.00 a | 2.20 a | 3.02 bc | 3.08 bc |
| 38 | borneol | 1718 | 1715 | tr | 0.05 x | - | 0.04 y | 0.02 z | - | - | - | - | - | - | - |
| 39 | α-terpinyl acetate | 1722 | 1721 | - | - | - | - | - | - | 0.02 a | 0.90 b | 0.92 b | 1.71 c | 0.02 a | - |
| 40 | cis-carveol | 1850 | 1847 | 0.14 yx | 0.11 z | 0.12 zy | 0.10 z | 0.18 w | 0.16 xw | - | - | - | - | - | - |
| 42 | exo-2-hydroxycineole | 1870 | 1870 | 0.08 z | 0.13 y | 0.21 x | 0.52 w | 0.10 zy | 0.11 zy | - | - | - | - | - | - |
| 43 | cis-p-mentha-1(7),8-dien-2-ol | 1905 | 1888 | 0.17 y | 0.11 z | 0.13 z | 0.11 z | 0.24 w | 0.21 x | 0.12 bc | 0.09 ab | 0.09 ab | 0.07 a | 0.13 c | 0.14 c |
| Sesquiterpene Hydrocarbons | - | - | - | - | - | - | 0.09 | 0.10 | 0.47 | 0.63 | 0.02 | 0.03 | |||
| 30 | β-caryophyllene | 1631 | 1625 | - | - | - | - | - | - | 0.07 b | 0.07 b | 0.03 a | 0.03 a | 0.02 a | 0.03 a |
| 32 | alloaromandrene | 1640 | 1645 | - | - | - | - | - | - | 0.02 a | 0.02 a | 0.44 b | 0.60 c | - | - |
| Aromatic Monoterpenes | - | - | - | - | - | - | 0.03 | 0.04 | 0.02 | 0.03 | 0.04 | 0.03 | |||
| 24 | p-cymenene | 1455 | 1455 | - | - | - | - | - | - | 0.03 b | 0.04 c | 0.02 a | 0.03 b | 0.04 c | 0.03 b |
| Oxygenated Aromatic Monoterpenes | 0.06 | 0.11 | 0.08 | 0.10 | 0.10 | 0.08 | - | - | - | - | - | - | |||
| 41 | p-cymen-8-ol | 1857 | 1869 | 0.06 z | 0.11 x | 0.08 zy | 0.10 yx | 0.10 yx | 0.08 zy | - | - | - | - | - | - |
| Ester | - | - | - | - | - | - | 0.04 | - | - | - | 0.03 | 0.05 | |||
| 4 | isoamyl acetate | 1125 | 1126 | - | - | - | - | - | - | 0.04 a | - | - | - | 0.03 a | 0.05 a |
| Ketones | 0.21 | 0.22 | 0.20 | 0.23 | 0.20 | 0.27 | 0.24 | 0.20 | 0.25 | 0.24 | 0.22 | 0.22 | |||
| 5 | heptan-4-one | 1132 | - | - | - | - | - | - | - | 0.06 c | 0.03 a | 0.04 ab | 0.04 ab | 0.04 ab | 0.05 bc |
| 6 | heptan-3-one | 1161 | 1163 | 0.05 z | 0.06 zy | 0.06 zy | 0.07 yx | 0.05 z | 0.08 x | 0.07 a | 0.06 a | 0.07 a | 0.06 a | 0.06 a | 0.07 a |
| 9 | heptan-2-one | 1190 | 1185 | 0.12 z | 0.16 z | 0.14 z | 0.16 z | 0.13 z | 0.16 z | 0.12 a | 0.11 a | 0.14 a | 0.14 a | 0.12 a | 0.11 a |
| 19 | 6-methylhept-5-en-2-one | 1346 | 1338 | 0.04 x | - | - | - | 0.02 z | 0.03 y | tr | - | - | - | - | - |
| aldehyde | - | - | tr | - | - | - | - | - | - | - | - | - | |||
| 22 | nonanal | 1405 | 1401 | - | - | tr | - | - | - | - | - | - | - | - | - |
| Alcohols | 0.89 | 1.15 | 1.43 | 2.01 | 0.78 | 0.85 | 0.14 | 0.14 | 0.15 | 0.14 | 0.14 | 0.16 | |||
| 10 | 3-methylbutanol | 1198 | 1210 | 0.46 z | 0.76 y | 0.68 y | 1.07 x | 0.45 z | 0.49 z | - | - | - | - | - | - |
| 17 | heptan-3-ol | 1290 | - | 0.07 zy | 0.08 yx | 0.06 z | 0.08 yx | 0.06 z | 0.09 x | 0.07 a | 0.06 a | 0.08 a | 0.07 a | 0.06 a | 0.08 a |
| 18 | heptan-2-ol | 1314 | 1318 | 0.06 z | 0.09 x | 0.08 yx | 0.09 x | 0.07 zy | 0.08 yx | 0.07 a | 0.08 a | 0.08 a | 0.07 a | 0.07 a | 0.08 a |
| 21 | Z-hex-3-en-1-ol | 1384 | 1384 | 0.14 x | 0.07 z | 0.09 zy | 0.24 w | 0.12 yx | 0.13 yx | - | - | - | - | - | - |
| 44 | 2-phenylethanol | 1928 | 1924 | 0.16 y | 0.15 y | 0.52 x | 0.53 x | 0.08 z | 0.06 z | - | - | - | - | - | - |
Figure 1.
Chemical structure of some of the most abundant molecules in the EWs and EOs.
Monoterpene hydrocarbons (MH): these terpenes were present in the EO samples with relative abundances between 6.74% and 10.76%. Limonene was the most abundant MH, with similar percentages in all the EOs (3.00–4.65%). Different abundances of the other MHs were pointed out for different Eucalyptus species: in Eucalyptus parvula, similar amounts of p-cymene and α-pinene were detected, followed by lower amounts of γ-terpinene and Z-ocimene. In Eucalyptus cinerea, similar amounts of α-pinene and Limonene were detected, followed by lower amounts of p-cymene, β-myrcene, β-pinene and Z-ocimene. Regarding the other two species (Eucalyptus pulverulenta Sims and Eucalyptus pulverulenta baby blue Sims), the α-pinene amount was approximately half the Limonene, with lower amounts of p-cymene, β-myrcene, and β-pinene. Other MH (camphene, α-phellandrene, alloocimene) were in low amounts. Noteworthy, the α-phellandrene content in EOs of all these species was lower than 1%, according to the European Pharmacopoeia specification for 1,8-cineol-rich E. oils [16].
1,8-cineole (eucalyptol) was by far the main component of the analyzed samples (see the next paragraphs); however, the differences in relative abundance of metabolites present in low amount, or even in trace, play a critical role in mediating different activities for EOs from different Eucalyptus species with 1,8-cineole as the main component; indeed, these different activities (i.e., alleophatic [24], protection against Parthenium hysterophorus L. [25]) were reported as only due to differences in the relative abundance of minor components [24,25], likely due to the synergistic effect of these latter compounds with other components [26,27].
Regarding the hydrosols, no significant amounts of MH were detected, due to the hydrophobic nature of these molecules.
Oxygenated monoterpenes (OM): 1,8-cineole is the main component of EOs obtained from the leaves of Eucalyptus globulus, the most common Eucalyptus species [20]. In EOs from these four species, relative abundance of 1,8-cineole ranged between 83.80% and 88.66%, higher than the 80–85% indicated by the standard ISO as the minimum amount of 1,8-cineole for EO from E. globulus. Other papers in the literature reported the characterization of EOs from Eucalyptus cinereal [16,17,18] and Eucalyptus pulverulenta [16], while, to the authors’ knowledge, no reports on EO from Eucalyptus parvula have been published, to date. In such papers, the relative abundance of 1,8-cineole showed great variability ranging usually from 58.0 to 69.0% and sometimes reaching 87.8% in E. cinerea EOs and being approx. 75% in E. pulverulenta EOs. Consequently, also the relative abundances of the other minor terpenes showed a great variability. This variability might be due to the effect of climatic and geographical factors and harvesting season.
In our study, Eucalyptus parvula and Eucalyptus pulverulenta Sims were the species with the highest amount of 1,8-cineole. Since EOs are totally composed by the volatile fraction, the relative abundance of each compound can be assumed as the amount of this molecule in the oil expressed as g/100g.
Regarding the analyzed hydrosols (EWs), 1,8-cineole was in the range 88.40–90.78%. In order to better characterize these hydrosols, 1,8-cineole was quantified using an external calibration curve, as reported in the experimental section. Table 2 shows that the absolute concentration of 1,8-cineole in the EWs extracts varied in the range 0.74–1.58 g/L, highlighting that this molecule was also recovered in water samples.
Table 2.
Content of 1,8-cineole in the hydrosols by GC-MS analysis. Data are expressed in g/L as mean of three independent determinations (SD < 3%). Different letters indicate significant differences at p < 0.05.
| Sample | Kind of Sample | 1,8-cineole (g/L) |
|---|---|---|
| 1-16-EW | hydrosol | 1.58 a |
| 2-16-EW | hydrosol | 1.45 b |
| 3-16-EW | hydrosol | 1.52 a |
| 4-16-EW | hydrosol | 0.74 e |
| 1-17-EW | hydrosol | 0.86 d |
| 1-1617-EW | hydrosol | 1.20 c |
In EWs, OMs constituted 98–99% of the total VOCs, according to their water solubility, higher than MHs. Regarding OMs other than 1,8-cineole, in our samples α-terpineol was the most abundant one (4.19–6.24%), followed by lower amounts of terpinen-4-ol, linalool oxides (furanoid, cis and trans), terpineol isomer, and other minor OMs (<1.5%).
OMs constituted about 90% of the EOs. In these samples, α-terpineol was in the range 2.00–3.11%. Noteworthy, in the EO from Eucalyptus parvula, the highest amount of α-terpineol and no presence of its ester, namely α-terpinyl acetate, were detected. In the other three species, α-terpinyl acetate was detected and the sum of the percentages of α-terpineol and α-terpinyl acetate was similar to that of α-terpineol of EO of the Eucalyptus parvula. The other OMs didn’t exceed 0.72%.
Other terpenes: no sesquiterpene hydrocarbons were identified in EWs, in agreement with their insolubility in water. In EOs, very low percentages of β-caryophyllene (≤0.07%) in all samples, and slightly higher amounts of alloaromandrene in Eucalyptus pulverulenta Sims (0.44%) and Eucalyptus pulverulenta baby blue Sims (0.60%) were detected.
One aromatic monoterpene, namely p-cymenene, was identified in very low amounts (≤0.04%) only in EOs samples, while one oxygenated aromatic monoterpene (p-cymen-8-ol) was identified in low amounts (≤0.11%) only in EWs, according to their different water solubility.
Other compounds: no significant amounts of esters and aldehydes were identified in our samples, the only exceptions being traces of nonanal in one EW sample and very low amounts of isoamyl acetate in EO from Eucalyptus parvula. Ketones (the three linear isomers of heptanone and lower percentages of 6-methylhept-5-en-2-one) were identified in low amounts (0.20–0.27% in both EWs and EOs). Finally, alcohols were identified in very low amounts in EOs (heptan-2-ol and heptan-3-ol for a total amount up to 0.16%), while in EWs they were present in percentages up to 2.01%, with 3-methylbutanol as the main molecule, followed by 2-phenylethanol and Z-hex-3-en-1-ol.
2.2. Fingerprint Analysis by HS-SPME-GC×GC-TOFMS
Solid-phase microextraction (SPME) is a rapid and simple procedure for extraction of volatile fraction from aromatic and medicinal plants [28]. As reported, the divinilbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber is the most effective SPME fiber able to isolate the volatile fraction from commercial hydrosols of several plants [12]. HS-SPME and GC×GC-TOFMS fingerprint analysis are ideal tools to analyze complex volatile fraction from several matrices, and to provide a sensitive method for the direct comparison and visualization of plant volatile components. As previously reported, 1,8-cineole was the major component in the EOs and EWs, but differences in the other metabolites present in low amounts are very important. Utilization of comprehensive two-dimensional GC (GC×GC) increases separation power with respect to that of the one-dimensional GC in complex matrices where the presence of low abundant components is critical, such as Eucalyptus [29]. To our knowledge, there has been no study reporting the volatile profile of EWs from these Eucalyptus species; therefore, hydrosols from the four Eucalyptus species were analyzed by HS-SPME-GC×GC-TOFMS to better elucidate the volatile profile of these by-products, also pointing out the presence of molecules not detectable with only GC-MS. HS-SPME-GC×GC-TOFMS analyses of the complex volatile fraction of EWs were submitted to advanced fingerprinting analysis of 2D chromatographic data.
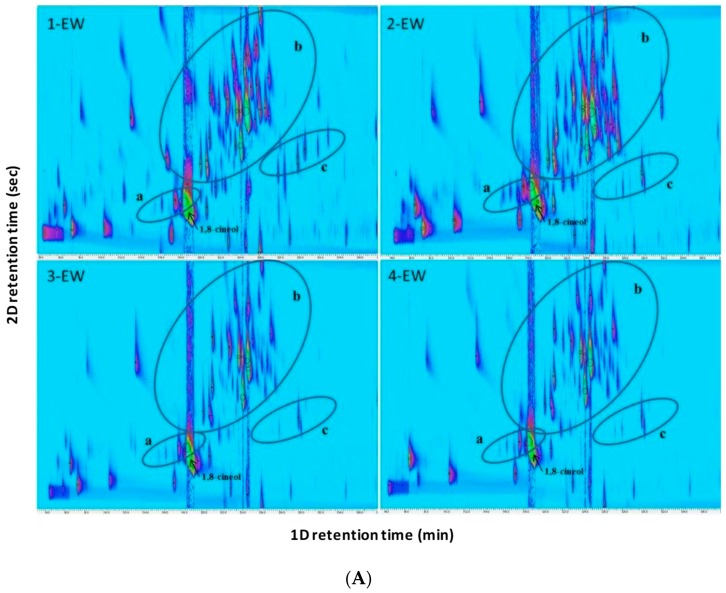
In Figure 2, “contour plots” from HS-SPME-GC×GC-TOFMS analyses of the four Eucalyptus species are reported: each 2D-peak corresponds to a single volatile compound. In this case, SPME and comprehensive comparative analysis of 2D chromatographic data showed visual differences among EW samples. 1-16-EW and 2-16-EW showed a larger number and a higher intensity of peaks, with respect to 3-16-EW and 4-16-EW. The most intense peak corresponded to 1,8-cineole.
Figure 2.
(A) 2D contour plots of the analyzed EWs. Braced region a: monoterpenic hydrocarbons; b: oxygenated monoterpenes; c: oxygenated monoterpenes acetate; (B) comprehensive template matching fingerprinting with the main identified volatile compounds of 1-16-EW: E. parvula L.A.S. Johnson & K.D. Hill; 2-16-EW; E. cinerea F. Muell; 3-16-EW: E. pulverulenta Sims; 4-16-EW: E. pulverulenta baby blue Sims.
For example, a total of about 400 compounds was detected by GC×GC analysis in 1-16-EW (estimated from the number of peak contours in 2D plots) and, after subtracting baseline peaks, corresponding to fiber blending or background interferences, 137 peaks/compounds were identified. These results were in agreement with Wong et al. [20], where the 2D rational separation pattern aids the identification of ca. 400 metabolites in Eucalyptus spp. leaf oils, 183 of which were identified or tentatively identified and represented percentages between 50.8–90.0% of the total ion count, comprising various chemical families.
HS-SPME-GC×GC-TOFMS provided a high metabolic coverage of VOCs: monoterpenes, oxygenated monoterpenes (the main class), oxygenated monoterpenes acetate, and others (ketones, aldehyde, alcohols).
GC×GC is currently adopted as separation technique not only because of its high separation power and sensitivity, but also for its ability to produce more widely distributed and rationalized peak patterns [30] for chemically correlated group of analytes. Terpenic compounds of Eucalyptus hydrosols were organized mainly in three clusters in 2D separation space: monoterpenic hydrocarbons, oxygenated monoterpenes and monoterpenes acetate, except for the 1,8-cineol that wrapped around, resulting in monoterpenes zone (Figure 2A). 1,8-cineol showed high secondary retention and fall outside the range of secondary retention time (wrapped around). As previously reported for volatile oil from leaves of Eucalyptus dunnii [31], one molecule that wraps around, does not affect the separation and identification of the compounds, since the more strongly retained components (those that wrap-around) did not overlap peaks that were weakly retained in the subsequent modulation.
Up to 31 peaks/compounds belonging to the class of oxygenated monoterpenes were distributed in a defined part of the contour plot for 1-16-EW (Figure 2A, braced region “b”). The number of oxygenated monoterpenes were 33 for 2-16-EW, 23 for 3-16EW and 24 for 4-16-EW.
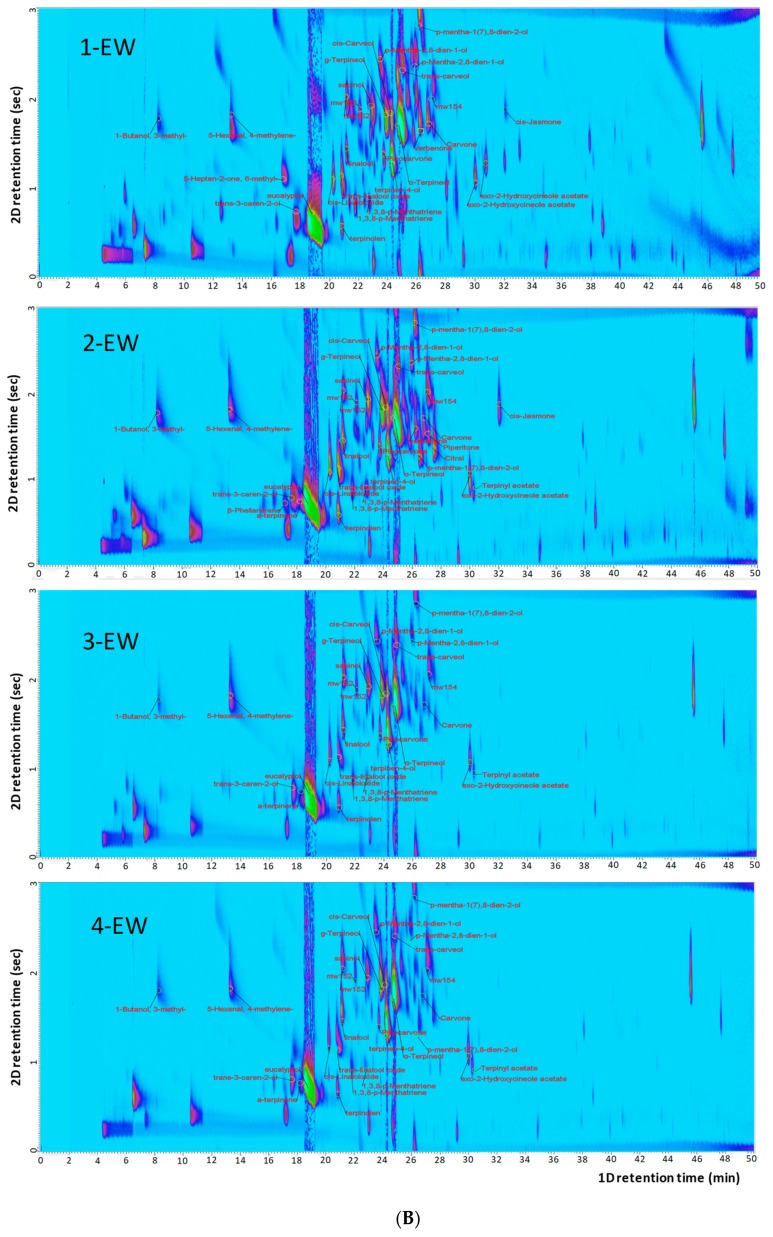
An advanced approach known as comprehensive template matching fingerprinting [32] was adopted (Figure 2B). This method considers, as a comparative feature, each individual 2D peak together with its time coordinates, detector response and MS fragmentation pattern, and includes them in a sample template that is created by the analyst and can be used to compare plots from different samples directly and comprehensively. A template could be used to correctly interpret visual differences in further analyses. To create the template, the peak identification was performed by matching the experimental mass spectra against spectra databases combined with GC-MS data.
The main differences that emerged between the four varieties could be summarized as follows: 1-16-EW showed the presence of 6-methylhept-5-en-2-one that was not present in the other species (see also Table 1) and the presence of exo-2-hydroxycineole acetate isomers. 1-16-EW did not show terpinyl acetate and α-terpinene, which instead were found in the other three species. 2-16-EW was the only species that showed the presence of β-phellandrene, piperitone and citral. 1-16-EW and 2-16-EW showed the presence of cis-jasmone and carvone, while 3-16-EW and 4-16-EW didn’t show the presence of these molecules. Volatile profiles presented in 2D contour plots allow visual discrimination of the metabolic composition among interspecies of Eucalyptus aromatic waters, as reported for leaf oils of other different Eucalyptus spp. [20].
3. Materials and Methods
3.1. Chemicals
All chemicals and standards of analytical reagent grade were from Sigma Aldrich (Steinheim, Germany). Tridecane, 1,8-cineole, heptane and a mixture of linear alkanes (C10–C26) in hexane were used. Inert gasses (He and N2 99.999% purity) were supplied by SOL gas company (Monza, Italy).
3.2. Plant Material
In the littoral area of Versilia and Pisa (North-Tuscany-Italy-Latitude: 43.873651; Longitude: 10.328756), Versil Green Società Agricola s.s., a commercial farm, cultivates several species of Eucalyptus for the production of ornamental green fronds and essential oils. The cultivated species are: Eucalyptus parvula L.A.S. Johnson & K.D. Hill, Eucalyptus cinerea F. Muell, Eucalyptus pulverulenta Sims and Eucalyptus pulverulenta baby blue Sims (Table 3). Figure 3a–d show a picture of each plant. All samples are grown by organic practices, accredited according to the UNI EN 45011 standard. During the production of ornamental fronds, leaves and little stems were separated from the young branches of healthy plants of two years and distilled as reported in Section 3.3.
Table 3.
List of the analyzed samples. EW: hydrosol or aromatic water; EO: essential oil. 1, E. parvula L.A.S. Johnson & K.D. Hill; 2, E. cinerea F. Muell; 3, E. pulverulenta Sims; 4, E. pulverulenta baby blue Sims. 16 and 17 indicate the year in which the sample was obtained. 1617 indicates samples obtained as a mixture in equal parts of samples from 2016 and 2017.
| Sample Name | Kind of Sample | Eucalyptus Species | Year | Yields % |
|---|---|---|---|---|
| 1-16-EW | aromatic water | E. parvula L.A.S. Johnson & K.D. Hill | 2016 | |
| 2-16-EW | aromatic water | E. cinerea F. Muell | 2016 | |
| 3-16-EW | aromatic water | E. pulverulenta Sims | 2016 | |
| 4-16-EW | aromatic water | E. pulverulenta baby blue Sims | 2016 | |
| 1-17-EW | aromatic water | E. parvula L.A.S. Johnson & K.D. Hill | 2017 | |
| 1-1617-EW | aromatic water | E. parvula L.A.S. Johnson & K.D. Hill | 2016–2017 | |
| 1-16-EO | essential oil | E. parvula L.A.S. Johnson & K.D. Hill | 2016 | 1.2 |
| 2-16-EO | essential oil | E. cinerea F. Muell | 2016 | 1.1 |
| 3-16-EO | essential oil | E. pulverulenta Sims | 2016 | 1.1 |
| 4-16-EO | essential oil | E. pulverulenta baby blue Sims | 2016 | 1.1 |
| 1-17-EO | essential oil | E. parvula L.A.S. Johnson & K.D. Hill | 2017 | 1.3 |
| 1-1617-EO | essential oil | E. parvula L.A.S. Johnson & K.D. Hill | 2016–2017 |
Figure 3.
(a): Eucalyptus parvula L.A.S. Johnson & K.D. Hill; (b): Eucalyptus cinerea F. Muell; (c): Eucalyptus pulverulenta Sims; (d): Eucalyptus pulverulenta baby blue Sims.
3.3. Obtaining of the Essential Oils and Hydrosols
Essential oils and hydrosols were obtained by steam distillation of the eucalyptus fresh leaves and little stems, within 24 h after harvesting, using the Essenziale 20 extractor (Tred Technology srl, Italy). The system used low working temperatures (always below 80 °C), thus decreasing the consumption energy and minimizing the degradation of the volatile fraction. The process parameters were continuously checked and adjusted during the distillation, e.g., internal pressure inside the boiler, internal boiler temperature, water temperature, oil and hydrosol flow. The starting material vs hydrosol ratio was 3:1 and the recovery of the corresponding essential oil was in variable percentage depending on the collection period and atmospheric conditions. The mean of yields of EO are from 1.1 to 1.3%, as reported in Table 3.
3.4. Analysis of Essential Oils and Aromatic Waters
3.4.1. GC-MS Analysis
EO samples were diluted 10,000 times with heptane, in presence of tridecane (20 ppm), for avoiding saturated signals during the following chromatographic analysis.
Regarding EWs, 0.5 mL of sample were extracted with 0.5 mL of heptane (with 20 ppm, tridecane) for 1 h with an automatic stirrer; water residues were removed using anhydrous sodium sulfate and the obtained organic extracts were diluted 20 times with heptane.
GC-MS analysis of EO and EW solutions, obtained as described above, were carried out by liquid injection on an Agilent 7890a Gas Chromatograph equipped with a Gerstel MPS automatic sampler system and a quadrupole Mass Spectrometer 5975c MSD (Agilent Technologies, Palo Alto, CA, USA) working in split-less mode. The analytes separation was carried out by an Agilent DB InnoWAX column (length, 50 m; i.d., 200 µm, film thickness, 0.4 µm). Initial oven temperature was 40 °C, held for 1 min. Then, it raised to 200 °C at 5 °C min−1, then raised to 260 °C at 10 °C min−1 and finally held at 260 °C for 6 min. Injector temperature was 260 °C, while the carrier gas helium, was at a flow rate of 1.2 mL/min. 1 µL of each sample was injected.
Mass spectrometer worked in the mass range 40–350 m/z and with an electron ionization of 70 eV and the Total Ion Current chromatograms were recorded. Compounds were tentatively identified by comparing the mass spectra of each peak with those reported in mass spectral database as the standard NIST08/Wiley98 libraries; when available, standards were used for confirming the nature of the identified molecules: α-pinene, β-pinene, camphene, β-myrcene, α-phellandrene, limonene, Z-ocimene, γ-terpinene, p-cymene, alloocimene, 1,8-cineol, linalool, terpinen-4-ol, citral, α-terpineol, borneol, β-caryophillene, heptan-2-one, 6-methylhept-5-en-2-one, nonanale, 3-methylbytanol, heptan-2-ol and 2-phenylethanol. Peaks identification was then confirmed by comparing their retention index; to this aim, a mixture of linear alkanes (C10–C26) in hexane (Sigma Aldrich, Saint Louis, MI, USA) was injected in the same condition already described for sample analysis and the retention indexes were calculated by the generalized equation [33] and compared with the literature [34] The relative concentration of each identified compound was calculated as peak area on total area of all the identified peaks (peaks areas were normalized using tridecane as internal standard). 1,8 cineole in EWs was quantified by a six point calibration curve, which were built using 1,8 cineole as external standard (range 10–160 ppm, 0.9936 R2).
3.4.2. HS-SPME-GC×GC-TOFMS Analysis
The EWs from the four Eucalyptus species (Table 3) were extracted by solid-phase microextraction (SPME) and analyzed by GC×GC-TOFMS. GC×GC was performed by a flow modulation apparatus consisting on an Agilent 7890B GC (Agilent Technologies, Palo Alto, CA, USA), with flow modulator device for 2D separation, coupled with a time-of-flight mass spectrometer (TOF-DS Markes International Ltd., Llantrisant, UK). After some trials aimed at optimizing amounts of sample, NaCl and water and exposure time and temperature, SPME conditions were set as follow: 1 mL of the EW sample, together with 2 g of NaCl and 4 mL of deionized water were placed into a 20-mL screw cap vial fitted with PTFE/silicone septa. VOCs were absorbed exposing a divinilbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) 2 cm fiber (Supelco) for 10 min into the vial at 60 °C and then immediately desorbed at 280 °C in a gas chromatograph injection port.
Chromatographic separation was performed using a first dimension (1D) HP-5 column (20 m × 0.18 mm I.D. × 0.18 μm film thickness (df); Agilent Technologies, Palo Alto, CA, USA), and a WAX second dimension (2D) column (5 m × 0.32 mm I.D. × 0.15 μm df; Agilent Technologies, Palo Alto, CA, USA).
Flow modulation was performed with a modulation period of 3 s. Helium was used as carrier gas (99.999% purity) at flow rates of 0.4 and 10 mL/min in first and second dimensions, respectively.
The chromatographic conditions were: oven temperature program, 40 °C, increased at 4 °C/min to 220 °C, increased at 10 °C/min to 260 °C (hold 1 min); injector temperature, 260 °C; split ratio 1:5. The inlet of the 2D column was maintained under vacuum by a deactivated fused silica (0.30 m × 0.10 mm I.D.) placed immediately before the column, after the flow modulator. TOFMS parameters: the ion source temperature was 230 °C; the transfer line temperature was 280 °C; ionization, −70 eV. A mass range of 43–500 Da was used, with data rate of 50 Hz. TOF-DS TM software, version 2.0 (Markes International Ltd.; Llantrisant, UK, 2016) was used for data acquisition. GC IMAGE version R2.5 GC×GC (64 bit) software (GC IMAGE; LCC-Lincon, NE, USA, 2014) was used for data processing.
3.5. Statistical Analysis
The semi-quantitative (Table 1) and quantitative data (Table 2) are expressed as the mean of three determinations. Statistical significance was evaluate applying one-way ANOVA and F-test (p < 0.05) using Microsoft Excel statistical software; means were then compared by Fisher’s LSD test using the DSAASTAT excel® VBA macro, version 1.1 (Onofri, A.; Pisa, Italy, 2007).
4. Conclusions
This study reports a first preliminary characterization of the volatile profile of EO and EW of four less studied 1,8-cineol-rich Eucalyptus species (E. parvula L.A.S. Johnson & K.D. Hill, E. cinerea F. Muell, E. pulverulenta Sims and E. pulverulenta baby blue Sims) cultivated in Tuscany (Italy) and intended to be employed as natural additives in the food, cosmetics and perfume industries, as well as for therapeutic ends, beyond the predominantly ornamental one. Chemical differences in VOCs from EWs and EOs were evidenced, providing products for potentially different uses. Further studies will be necessary for the standardization of commercial EOs and EWs optimizing the technological harvesting period.
Regarding the EOs from these Eucalyptus species, GC-MS analysis of diluted samples showed that oxygenated monoterpenes accounted for up to 92.7% and monoterpenes hydrocarbons contributed to volatile fraction for up to 10.8%, with limonene as the most representative MH. The relative abundance of minor terpenes as limonene, α-pinene, γ-terpinene, p-cymene, terpinen-4-ol, α-terpineol and alloaromandrene seems to be suitable for differentiation among EOs of the four different Eucalyptus species.
GC-MS analysis allowed pointing out that the volatile fraction of EWs extracts was mainly constituted by oxygenated monoterpenes (97.6–98.9%), with monoterpene and sesquiterpene hydrocarbons detected only in traces. HS-SPME-GC×GC-TOFMS analysis of EWs extracts also allowed for metabolic profiling of EWs for the direct comparison and visualization of volatile components of these not yet investigated co-products. The GC-MS quantitative evaluation of 1,8-cineole in EWs showed amounts of up to 1.6 g/L; consequently, the studied EWs, co-products of steam distillation of fresh leaves and little stems of the four Eucalyptus species, can be proposed as functional ingredients for the food, beverage, and cosmetic industries.
Acknowledgments
The authors are grateful to ITALCOL Spa, Castelfiorentino (FI), to QuMAP Laboratory of PIN (Prato) and to Fabio Villanelli for technical support.
Author Contributions
Conceptualization, A.R.; Data curation, F.I., L.C. and C.C.; Formal analysis, F.I. and L.C.; Project administration, E.G. and A.R.; Resources, E.G.; Supervision, A.R.; Writing-original draft, F.I. and L.C.
Conflicts of Interest
The authors declare no conflict of interest
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Coppen J.J.W. Eucalyptus: The Genus Eucalyptus. Taylor & Francis; London, UK: 2002. [Google Scholar]
- 2.Ramezani H., Singh H.P., Batish D.R., Kohli R.K. Antifungal activity of the volatile oil of Eucalyptus citriodora. Fitoterapia. 2002;73:261–262. doi: 10.1016/S0367-326X(02)00065-5. [DOI] [PubMed] [Google Scholar]
- 3.Cermelli C., Fabio A., Fabio G., Quaglio P. Effect of eucalyptus essential oil on respiratory bacteria and viruses. Curr. Microbiol. 2008;56:89–92. doi: 10.1007/s00284-007-9045-0. [DOI] [PubMed] [Google Scholar]
- 4.Mulyaningsih S., Sporer F., Zimmermann S., Reichling J., Wink M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine. 2010;17:1061–1066. doi: 10.1016/j.phymed.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Tyagi A.K., Malik A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 2011;26:228–235. doi: 10.1016/j.foodchem.2010.11.002. [DOI] [Google Scholar]
- 6.Sadlon A.E., Lamson D.W. Immune-Modifying and Antimicrobical Effects of Eucalyptus Oil and simple Inhalation Devices. Altern. Med. Rev. 2010;15:33–47. [PubMed] [Google Scholar]
- 7.Bachir Raho G., Benali M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012;2:739–742. doi: 10.1016/S2221-1691(12)60220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu H., Shao X., Cao J., Ou C., Pan D. Antimicrobial activity of eucalyptus essential oil against Pseudomonas in vitro and potential application in refrigerated storage of pork meat. Int. J. Food Sci. Technol. 2016;51:994–1001. doi: 10.1111/ijfs.13052. [DOI] [Google Scholar]
- 9.Vilela G.R., de Almeida G.S., D’Arce M.A.B.R., Moraes M.H.D., Brito J.O., da Silva M.F.G.F., Silva S.C., de Stefano Piedade S.M., Calori-Domingues M.A., da Gloria E.M. Activity of essential oil and its major compound, 1,8-cineole, from Eucalyptus globulus Labill., against the storage fungi Aspergillus flavus Link and Aspergillus parasiticus Speare. J. Stored Prod. Res. 2009;45:108–111. doi: 10.1016/j.jspr.2008.10.006. [DOI] [Google Scholar]
- 10.Ormancey X., Sisalli S., Coutiere P. Formulation of essential oils in functional perfumery. Parfum. Cosmet. Actual. 2001;157:30–40. [Google Scholar]
- 11.Sacchetti G., Maietti S., Muzzoli M., Scaglianti M., Manfredini S., Radice M., Bruni R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005;91:621–632. doi: 10.1016/j.foodchem.2004.06.031. [DOI] [Google Scholar]
- 12.Paolini J., Leandri C., Desjobert J.M., Barboni T., Costa J. Comparison of liquid–liquid extraction with headspace methods for the characterization of volatile fractions of commercial hydrolats from typically Mediterranean species. J. Chromatogr. A. 2008;1193:37–49. doi: 10.1016/j.chroma.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Edris A.E. Identification and absolute quantification of the major water-soluble aroma components isolated from the hydrosols of some aromatic plants. J. Essent. Oil-Bear. Plants. 2009;12:155–161. doi: 10.1080/0972060X.2009.10643705. [DOI] [Google Scholar]
- 14.Ndiaye H.B., Diop M.B., Gueye M.T., Ndiaye I., Diop S.M., Fauconnier M.L., Lognay G. Characterization of essential oils and hydrosols from senegalese Eucalyptus camaldulensis Dehnh. J. Essent. Oil-Bear. Plants. 2018;30:1–11. doi: 10.1080/10412905.2017.1420554. [DOI] [Google Scholar]
- 15.Price L., Price S. Understanding Hydrolats: The Specific Hydrosols for Aromatherapy. Churchill Livingstone/Elsevier; Amsterdam, The Nederlands: 2004. p. 96. [Google Scholar]
- 16.Zrira S., Bessiere J.M., Menut C., Elamrani A., Benjilali B. Chemical composition of the essential oil of nine Eucalyptus species growing in Morocco. Flavour Fragr. J. 2004;19:172–175. doi: 10.1002/ffj.1289. [DOI] [Google Scholar]
- 17.Elaissi A., Hadj Salah K., Mabrouk S., Larb K.K., Chemli R., Harzallah-Skhiri F. Antibacterial activity and chemical composition of 20 Eucalyptus species essential oils. Food Chem. 2011;129:1427–1434. doi: 10.1016/j.foodchem.2011.05.100. [DOI] [Google Scholar]
- 18.Kahla Y., Zouari-Bouassida K., Rezgui F., Trigui M., Tounsi S. Efficacy of Eucalyptus cinerea as a Source of Bioactive Compounds for Curative Biocontrol of Crown Gall Caused by Agrobacterium tumefaciens Strain B6. BioMed Res. Int. 2017 doi: 10.1155/2017/9308063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ieri F., Cecchi L., Vignolini P., Belcaro M.F., Romani A. HPLC/DAD, GC/MS and GC/GC/TOF analysis of Lemon balm (Melissa officinalis L.) sample as standardized raw material for food and nutraceuticals uses. Adv. Hortic. Sci. 2017;3:141–147. doi: 10.13128/ahs-21091. [DOI] [Google Scholar]
- 20.Wong Y.F., Perlmutter P., Marriott P.J. Untargeted metabolic profiling of Eucalyptus spp. leaf oils using comprehensive two-dimensional gas chromatography with high resolution mass spectrometry: Expanding the metabolic profile. Metabolomics. 2017;13:46. doi: 10.1007/s11306-017-1173-3. [DOI] [Google Scholar]
- 21.Calamai L., Villanelli F., Bartolucci G., Pieraccini G., Moneti G. Comprehensive sampling and sample preparation. Elsevier; Amsterdam, The Nederlands: 2012. Sample preparation for direct ms analysis of food. [Google Scholar]
- 22.Oliver-Pozo C., Aparicio-Ruiz R., Romero I., Garcia-Gonzalez D.L. Analysis of volatile markers for virgin olive oil aroma defects by SPME-GC/FID: Possible sources of incorrect data. J. Agric. Food Chem. 2015;63:10477–10483. doi: 10.1021/acs.jafc.5b03986. [DOI] [PubMed] [Google Scholar]
- 23.Fortini M., Migliorini M., Cherubini C., Cecchi L., Calamai L. Multiple internal standard normalization for improving HS-SPME-GC-MS quantitation in virgin olive oil volatile compounds (VOO-VOCs) profile. Talanta. 2017;165:641–652. doi: 10.1016/j.talanta.2016.12.082. [DOI] [PubMed] [Google Scholar]
- 24.May F., Ash J. An assessment of the allelopathic potential of Eucalyptus. Aust. J. Botany. 1990;38:245–254. doi: 10.1071/BT9900245. [DOI] [Google Scholar]
- 25.Kohli R.K., Batish D.R., Singh H.P. Eucalypt oils for the control of Parthenium (Perthenium hysterophorus L.) Crop Prot. 1998;17:119–122. doi: 10.1016/S0261-2194(97)00095-1. [DOI] [Google Scholar]
- 26.Hummelbrunner L.A., Isman M.B. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae) J. Agric. Food Chem. 2001;49:715–720. doi: 10.1021/jf000749t. [DOI] [PubMed] [Google Scholar]
- 27.Nerio L.S., Olivero-Verbel J., Stashenko E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010;101:372–378. doi: 10.1016/j.biortech.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 28.Spietelun A., Marcinkowski L., De la Guardia M., Namieśnik J. Review recent developments and future trends in solid phase microextraction techniques towards green analytical chemistry. J. Chromatogr. A. 2013;1321:1–13. doi: 10.1016/j.chroma.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 29.Hantao W.L., Toledo B.R., de Lima Ribeiro F.A., Pizetta M., Geraldi Pierozzi C., Luiz Furtado E., Augusto F. Comprehensive two-dimensional gas chromatography combined to multivariate data analysis for detection of disease-resistant clones of Eucalyptus. Talanta. 2013;116:1079–1084. doi: 10.1016/j.talanta.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 30.Cordero C., Bicchi C., Rubiol P. Group-type and fingerprint analysis of roasted food matrices (coffee and hazelnut samples) by comprehensive two-dimensional gas chromatography. J. Agric. Food Chem. 2008;56:7655–7666. doi: 10.1021/jf801001z. [DOI] [PubMed] [Google Scholar]
- 31.Von Mühlen C., Alcaraz Zini C., Bastos Caramaob E., Marriott P.J. Comparative study of Eucalyptus dunnii volatile oil composition using retention indices and comprehensive two-dimensional gas chromatography coupled to time-of-flight and quadrupole mass spectrometry. J. Chromatogr. A. 2008;1200:34–42. doi: 10.1016/j.chroma.2008.05.070. [DOI] [PubMed] [Google Scholar]
- 32.Cordero C., Atsbaha Zebelo S., Gnavi G., Griglione A., Bicchi C., Maffei M.E., Biolo P. HS-SPME-GC×GC-qMS volatile metabolite profiling of Chrysolina herbacea frass and Mentha spp. Leaves. Anal. Bioanal. Chem. 2012;402:1941–1952. doi: 10.1007/s00216-011-5600-4. [DOI] [PubMed] [Google Scholar]
- 33.Van den Dool H., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 34.Chemistry WebBook. [(accessed on 1 December 2018)]; Available online: http://www.nist.gov/index.html.