Abstract
Pollen is the male gametophyte of higher plants. Its major function is to deliver sperm cells to the ovule to ensure successful fertilization. During this process, many interactions occur among pollen tubes and pistil cells and tissues, and calcium ion (Ca2+) dynamics mediate these interactions among cells to ensure that pollen reaches the embryo sac. Although the precise functions of Ca2+ dynamics in the cells are unknown, we can speculate about its roles on the basis of its spatial and temporal characteristics during these interactions. The results of many studies indicate that calcium is a critical element that is strongly related to pollen germination and pollen tube growth.
Keywords: calcium, pollen germination, pollen tube growth, stigma, style
1. Introduction
The fertilization process of high plants begins with pollen grains landing on the stigma and germination of the pollen tubes. If the pollen is compatible, then pollen tubes grow through the pistil tissues of the stigma and style, over the surface of the placenta, and then through the micropyle of the ovule to reach the female gametophyte in the embryo sac. Two male gametes are discharged from the pollen tube into the degenerated synergid where one fuses with the egg cell and the other fuses with the central cell. A series of interactions occur between male and female cells and tissues while pollen tubes are elongating through the diploid sporophytic tissue of the pistil. It is these interactions that ensure the pollen tube enters the embryo sac and that both deposited sperm cells fuse with the egg and central cell successfully. The role of the calcium ion (Ca2+) in these interactions has received particular attention, as it appears to provide a universal signal with pleiotropic effects on attraction, long and short-distance communication, cellular fusion, and cell signaling [1,2,3].
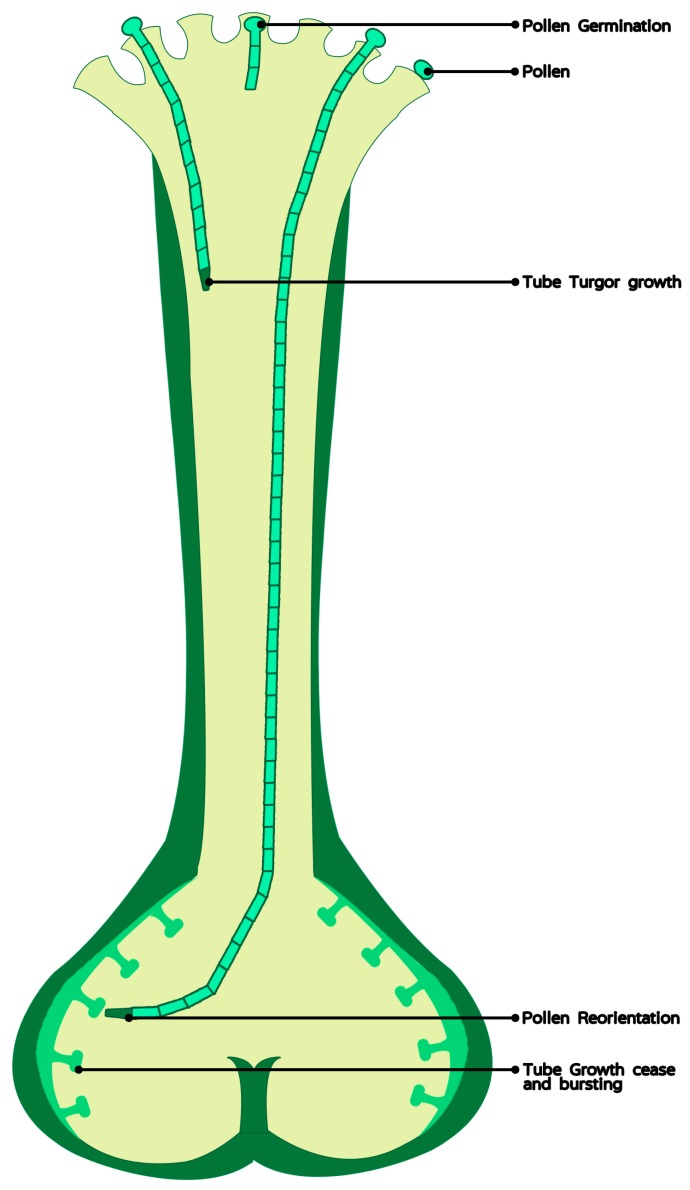
Pollen is the male gametophyte of angiosperms and the only cell that can move and leave the parent plant body. Although pollen is very simple in its composition (2 or 3 cells), its germination and pollen tube growth are very complex mechanisms that ensure the tube reaches its target cell. The results of many studies indicate that Ca2+ dynamics in pollen and the gynoecium are important for these mechanisms to operate (Figure 1) (discussed in detail later). Calcium ions show different temporal and spatial distribution in the cells of male and female tissues during development. We can speculate on the functions of Ca2+ in controlling pollen germination and pollen tube growth on the basis of its temporal and spatial changes in reproductive cells and organs. Although there are continually reports about control mechanisms of pollen tube growth [4,5,6,7], the most results are from in vitro assays. In this review, we focus on the functions of Ca2+ in pollen germination and pollen tube growth in the stigma and style tissues of higher plants. We discuss the relationships between tissue-level changes in Ca2+ uptake and pollen and style maturation during the progamic phase.
Figure 1.
The role of calcium in pollen germination and tube elongation in vivo.
2. Characteristics of Pollen Tube Growth
When pollen grains land on the stigma, compatible grains germinate and an elongating tube forms and grows through the aperture of the pollen grain. Pollen germination is closely related to its hydration to produce turgor, which pushes the pollen cytoplasm to protrude through the aperture to form the pollen tube. Pollen tube growth is both polar and directional. The polar growth of the tube is limited to its tip, and is closely related to the tube’s internal structure [8]. The bipartite pollen tube wall consists of an inner callose sheath and an outer region of pectin-rich fibrillar layers. The pollen tube tip comprises a single pectin layer [9]. Immunocytochemical studies have labelled at least two types of pectin in the pollen tube wall: polygalacturonic acid pectin and methyl-esterified pectin. Acid pectins capable of crosslinking Ca2+ are localized in the subapical region of the pollen tube, whereas richly methyl-esterified pectins are abundant at the tube tip. Calcium-crosslinked pectin in the subapical region of the pollen tube becomes a rigid and insoluble gel. The tube tip region consists of richly methyl-esterified pectins, and is the most elastic region in the tube. It is likely to be the expansion point under internal turgor pressure [10].
During pollen tube formation, almost the entire contents of the pollen grain move into the tube. Within the growing tube, most of the cytoplasm is confined to the apex, and a large vacuole fills the grain and the older region of the tube. To restrict the cytoplasm to the apical region of the growing tube, a series of callose plugs are formed at a regular distance behind the tip [11]. The formation of vacuoles in the tube cytoplasm is a persistent process that maintains tube turgor during pollen germination and tube growth [12]. The vacuoles move to the basal part to push the cytoplasm to the apex of the tube. Thus, the pollen tube shows polar growth that is mediated by this internal movement mechanism and the composition of the pollen wall.
Pollen tubes grow down the style and then precisely target a single cell within the female embryo sac. Molecular changes that occur before pollen tube curvature include protein interactions between male and female cells [13,14,15], but Ca2+ also plays a role in determining the orientation of tube growth [16,17], as discussed in detail later. Pollen germination and pollen tube growth are special processes during the early stage of fertilization, and require unique control mechanisms, including a series of interactions among the pollen, pollen tube, pistil cells, and other tissues. Calcium dynamics in pollen and pistil cells represent an interaction between pollen and pistil cells.
3. Calcium Distribution in the Pollen Germination
When pollen grains germinate in small numbers in vitro, the germination and elongation rates are often slower than those observed in larger-scale settings. This population effect can be overcome by adding more pollen, or by adding Ca2+ to the medium. This demand for Ca2+ for pollen germination was confirmed for 86 species in 39 plant families [18], indicating that Ca2+ dependency is widespread among flowering plants. The pollen grains of some plants can germinate on media without Ca2+; however, it is presumed that some Ca2+ stored in the pollen wall is released into the medium during pollen hydration. In rice, for example, pollen grains contain abundant Ca2+ in the cell walls before germination [19].
External Ca2+ not only affects pollen germination but also pollen tube growth. Mascarenhas and Machlis (1962) observed a chemotropic response of Antirrhinum majus pollen tubes to an external Ca2+ gradient in vitro [20]. In another study, lower Ca2+ concentrations appeared to decrease tube elongation via excess accumulation of vesicles at the tip, inducing apical swelling. Increased Ca2+ concentrations appeared to accelerate vesicle fusion at the pollen tube tip, but may have also altered cytoskeletal elements to contribute to a thickened wall at the tube tip [12]. The results of many early studies indicated that Ca2+ is required for the processes of pollen germination and pollen tube growth [1,15].
4. Calcium Distributions in Pollen Tubes
Calcium shows special distribution patterns in the pollen tube. In pollen tubes of Lilium longiflorum, concentrations of free Ca2+ estimated using quin-2 fluorescence were ~10−7 mol/L near the tip and 2 × 10−8 mol/L at the base [21]. The free Ca2+ concentrations measured using the fura-2 fluorescence indicator were 1.7 to 2.6 μM at the tip and 60 to 100 nM at 100 μm behind the tip [22]. The membrane-impermeable injected label fura-2 dextran estimated the Ca2+ concentration to be approximately one-third lower at the tube tip (490 nM at the extreme tip) and 170 nM at 10–20 μm behind the tip. The free Ca2+ gradient was observed to drop off sharply within 10–20 μm of the tube tip [23]. Although the estimated values of free Ca2+ differed markedly among those reports, a free Ca2+ gradient restricted to the apical 20 μm of the pollen tube tip was consistently detected, regardless of the technique. Changes in free Ca2+ beyond the first 20 μm of the pollen tube were simply not observed [24,25,26,27,28]. In all of those studies, the Ca2+ gradient was detected only in growing pollen tubes. Upon death or the addition of Ca2+ channel blockers or antagonists to the medium, the Ca2+ gradient disappeared from the tip of the pollen tube, and tube growth stopped. When such arrested tubes were transferred to medium without Ca2+ blockers or antagonists, they rapidly reestablished a Ca2+ gradient and elongation was reinitiated. The strongly concentrated Ca2+ gradient in the pollen tube tip corresponded closely with vigorous tip elongation [7].
5. Calcium Gradient Oscillation and Pulsatory Growth of the Pollen Tube
Pierson et al. (1994) used more rapid ratiometric calculations based on confocal microscopy data to confirm the steepness and depth of the tip-focused intracellular Ca2+ gradient in lily pollen tubes, which ranged from >3.0 μM at the apex to 0.2 μM within 20 μm from the tip [29]. Although the levels of intracellular Ca2+ were assumed to remain essentially stable over time, further analyses based on measurements using a Ca2+-specific vibrating electrode revealed that extracellular Ca2+ entered the pollen tube at influx rates varying between 1.4 and 14 pmol cm−2 s−1. These rates of extracellular Ca2+ influx into the pollen tube were uneven but reasonably periodic. The tubes displayed pulsed growth, with pulses of pollen tube elongation that corresponded with the periodic deposition of cell wall components [30]. Ratiometric ion imaging of the intracellular Ca2+ gradient indicated that the high point of the gradient fluctuated in magnitude from 0.75 to > 3μM during the 60-s measurement intervals. The elevation of the Ca2+ gradient appeared to be correlated with an increased rate of tube growth [27]. Holdaway-Clarke et al. (1997) also found that the tip-focused Ca2+ gradient oscillated with the same periodicity as pollen tube growth, but the pulses were slightly out of phase. The extracellular Ca2+ influx was delayed by about 11 s compared with the oscillation of the Ca2+ gradient [26].
The relationships among Ca2+ oscillations, pulsed tube growth, and extracellular Ca2+ influx involve dynamic spatial and temporal characteristics of Ca2+ signaling. Pulsed pollen tube elongation, however, is not a requirement for normal pollen tube elongation, even in plants with pollen tubes that show characteristic pulse growth. Geitmann and Cresti (1998) found that the inorganic Ca2+ channel inhibitors La3+ and Gd3+ caused pulsating pollen tubes to abandon their rhythm and elongate steadily. The organic inhibitors of Ca2+ channels, nifedipine and verapamil, slowed the pulse frequency, but did not inhibit pulsed growth [31]. These results strongly suggested that at least two types of Ca2+ channels are present in the plasma membrane of the tube tip. When apical elongation of the pollen tube was inhibited, periodic membrane traffic still occurred with nearly the same periodicity as that during normal tube elongation [32]. Dynamic Ca2+ concentrations play a central role in increasingly complicated models of growth oscillations in pollen tubes, both in a direct physiological role and more importantly, in a signaling role [6,7,33]. The occurrence of Ca2+ oscillations in pollen tubes has become an important model for understanding how such oscillations regulate growth in higher plants.
6. Calcium and Turgor Formation during Pollen Tube Growth
The production and maintenance of internal turgor in pollen and the pollen tube provide the driving force for pollen germination and tube growth. However, it is unknown how turgor formation is controlled when pollen absorbs water (hydration). During pollen germination, turgor is produced by the formation of vacuoles. Although some reports have described vacuole formation during microspore development [34,35,36,37], less is known about vacuole metabolism during pollen germination. The rate and type of vacuole metabolism differ among developmental stages (e.g., between the microspore and mature pollen). Vacuole metabolism is a developmental continuation in the microspore, but a new development in mature pollen.
Similar to pollen germination, the pollen tube also requires turgor to push the cytoplasm to the front of the tube. In a growing tube, most of the cytoplasm is confined to the apex region, and large vacuoles fill the older region. Vacuole metabolism produces turgor in the pollen tube. In vigorously growing pollen tubes, vacuole formation is active and continuous [12]. However, it is unknown how vacuoles are generated in pollen and the pollen tube. Recent studies on wheat [37] and Bauhinia blakeana [38] showed that Ca2+ affects the composition of the microspore cytoplasm, primarily by accumulating in mitochondria and destroying their inner membranes (cisterns) to form small vacuoles. These vacuoles then expand and fuse to become large vacuoles during microspore vacuolisation. However, more research is needed to explore the similarities and differences in vacuole formation among plant species.
7. Calcium Dynamics and Reorientation of Pollen Tube during Elongation
The ability of the pollen tube to reorient tip growth is an interesting phenomenon. Pollen tubes precisely target a single cell within the female embryo sac, and the molecular changes that occur before pollen tube curvature are a topic of great interest. Malhó’s group showed that the directionality of Agapanthus umbellatus pollen tubes could be modified by iontophoretic introduction of Ca2+ and by weak electrical fields, which caused pollen tubes to elongate toward the cathode. Introducing a localized gradient of the ionophore A23187, which is believed to open Ca2+ channels, caused the pollen tube tip to reorient towards A23187. When the Ca2+ channel blocker GdCl3 was added to the growth medium, the pollen tube tips elongated away from the GdCl3. An accumulation of cytosolic free Ca2+ preceded the reorientation of the tip and predicted the location of future elongation [39]. A further demonstration of the effect of free Ca2+ was obtained by microinjecting caged Ca2+ into living pollen tubes. The injected pollen tubes were irradiated eccentrically with ultraviolet light near their tip, causing photolysis of the cages and the release of free Ca2+ at that location. The resulting transient rise in free Ca2+ induced a reorientation of tip growth towards the irradiated site. Tip growth resumed near the irradiated region and caused a sustained reorientation of the elongating tip. The site of tip reorientation corresponded closely with the local release of Ca2+. This pattern was reinforced by a decline in Ca2+ levels on the opposite side of the tube, completing the reorientation [27,40]. Thus, Ca2+-rich areas within a gradient can reorient tip elongation, thereby establishing the directionality of future pollen tube elongation. Related studies demonstrated that a kinase present in the pollen tube apex might also be involved in regulating localized Ca2+ channel activity [41,42].
8. Calcium Distribution in the Stigma
The stigma and style of higher plants are the pathway of the growing pollen tube. The characteristics of Ca2+ distribution in the stigma and style reflect the interaction between pistil tissue and the regulation of pollen germination and tube growth.
The stigmas of higher plants intercept pollen grains from many sources, and are the first site where pollen screening occurs. Stigmas are diverse and vary widely among plant species. When pollen grains land on the stigma, compatible grains germinate and pollen tubes elongate from them. The process of pollen germination is related to Ca2+ metabolism of pistil tissues. Tirlapur and Shiggaon (1988) found abundant membrane Ca2+ in the papillae of Ipomoea batatas using chlorotetracycline (CTC) [43]. Bednarska (1989) confirmed this result in Ruscus aculeatus [44] using CTC and X-ray microanalysis, and further observed that germinating pollen of Primula officinalis and R. aculeatus absorbed Ca2+ from the stigma [45]. Studies using antimonate precipitation indicated that, in sunflower, Ca2+ was more abundant on the receptive surfaces of the stigma, especially outside and inside the papillae, than on non-receptive surfaces [46]. Abundant calcium precipitates were also detected in the intercellular matrix of stigmatic tissues of cotton [47] and on the surface of Brassica oleracea stigma after pollination, particularly where pollen grains had landed and tubes had germinated on the stigmatic papillae [48]. Ge et al. (2009) analyzed pollen tube growth in the tobacco stigma. In tobacco, the stigma is a rod-like structure with an enlarged top, and it becomes wet with a thick layer of glycoprotein exudate at anthesis. This layer contains abundant vesicles [49]. Ge et al. (2009) detected abundant calcium precipitates in these vesicles. When pollen grains arrived at the tobacco stigma, they became hydrated and swelled. The accumulation of Ca2+ precipitates at the pollen aperture suggested that the germinating pollen grains absorbed Ca2+. The calcium precipitates that accumulated in pollen cytoplasm were initially concentrated within small vacuoles. The small vacuoles fused as germination proceeded to form prominent large vacuoles, which created the turgor to push the cytoplasm along the forming pollen tube [49]. The lettuce stigma is composed of two, dry slivers, each with a receptive plane consisting of papillae cells, and a non-receptive plane. Before anthesis, few calcium precipitates were visible in the papillae cells. At anthesis, abundant calcium precipitates accumulated in the wall between papillae cells, the site of pollen tube penetration into the stigma. In the epidermal cells of the non-receptive portion of the stigma, few calcium precipitates were observed in the cell and cell walls [50]. These observations indicated that absorption of Ca2+ by pollen grains is related to vacuole genesis. The accumulation of abundant Ca2+ in and on stigmatic surfaces was consistent with the need for extracellular Ca2+ for pollen germination and tube elongation in vitro, providing further evidence that the pistil affects pollen growth in vivo.
9. Calcium Distribution in the Style
After pollen germinates, the tube penetrates the stigmatic surface and grows into the transmitting tissue of the style. Mascarenhas and Machlis (1962) found a chemotropic response of pollen tubes to Ca2+ and observed a gradient of Ca2+ concentrations from the stigma to the ovule in the pistil of A. majus. On the basis of in vitro assays, they speculated that the pollen tube responds to a Ca2+ gradient in the pistil, which attracts the tube to elongate towards the ovary [20]. However, Ca2+ gradients were not detected in several other examined flowering plants [51,52]. After those reports, few studies focused on this topic. However, the development of a more sensitive technique to detect Ca2+ in multicellular tissues (i.e., precipitation of Ca2+ using antimonate) led to renewed interest in Ca2+ distribution in the stigma and style. In Brassica napus [53], Helianthus annuus L. [46], and Gossypium hirsutum L. [47], abundant Ca2+ stores were detected in the transmitting tissues. In these plants, calcium-induced antimonate precipitates were mainly detected in the apoplastic system of the transmitting tissue, i.e., the intercellular matrix and cell wall, where pollen tubes elongate. Calcium is an essential requirement for pollen tube growth in vitro. Similarly, the abundant Ca2+ in the transmitting tissue of the style was suggested to meet the requirements for pollen tube growth in vivo. In tobacco, abundant calcium precipitates were detected in the stigma at anthesis, but less were detected in the transmitting tissue from anthesis until 11 h after pollination. At 22 h after pollination, Ca2+ concentrations were found to increase distally from the stigmatic interface with the transmitting tissue through the length of the style to the ovary, resulting in the formation of a Ca2+ gradient in the transmitting tissue [49]. In lettuce, Ca2+ formed a gradient from the top to the base of the style in the transmitting tissue and parenchyma cells before pollination. After pollination, the Ca2+ levels increased in the transmitting tissue, and the gradient distribution became stronger. A Ca2+ gradient was also detected in the tracheae of the vascular bundle of the style. These results indicated that pollination induces an increase in stylar Ca2+ levels [50].
10. Calcium Dynamic in Self-Incompatibility Response
Calcium not only promotes, but also inhibits pollen tube growth. In self-incompatible plants, Ca2+ dynamics are related to the self-incompatibility response. When S-glycoprotein was extracted from self-incompatible stigmas of Papaver rhoeas and added to the culture medium, the Ca2+ concentration increased in pollen tubes and their elongation was inhibited. However, when either compatible or heat-denatured incompatible stigmatic S-glycoprotein was introduced into the culture medium, the Ca2+ levels did not increase in pollen tubes and elongation continued normally [54]. When caged Ca2+ was introduced into pollen tubes, their elongation also proceeded normally. However, cage photolysis resulted in a similar inhibition of tube elongation, which was related to the artificially elevated internal Ca2+ levels. Photoactivation of caged InsP3 elicited a similar response [55]. To eliminate the effect of potential contamination by other stylar components, the SI-gene was cloned and expressed in Escherichia coli. The purified gene product elicited the same result. Thus, the S-protein alone was found to be capable of triggering the Ca2+ signal during the pollen self-incompatible response [56].
The direct imaging of Ca2+ confirmed that the addition of S-protein resulted in an influx of extracellular Ca2+ at the “shank” of the pollen tube. The influx of extracellular Ca2+ was confirmed to play a role in the self-incompatible response [57], because the addition of the Ca2+-antagonists Verapamil or the Ca2+ channel blocker La3+ allowed pollen to overcome the self-incompatible response and elongate into the style [58]. The Ca2+ influx was found to differ between compatible and self-incompatible plants, further confirming the importance of extracellular Ca2+ influx in the self-incompatible response [59]. In Nicotiana alata, self-pollen tube elongation was inhibited by a ribonuclease in the stylar transmitting tissue [60]. Presumably, Ca2+-dependent protein kinases from the pollen tube activated the S-ribonuclease in the transmitting tissue, resulting in the digestion of RNA in the pollen tube and the inhibition of tube growth [61]. Immunological studies have suggested that calmodulin, calmodulin-like, and calreticulin-like proteins are involved in Ca2+ related cell signaling during pollen–pistil interactions [62,63].
Recent studies on the self-incompatibility response of higher plants have revealed interesting characteristics of Ca2+ distribution in stigma tissues. Iwano et al. (2014) found that, in Brassica rapa, the compatible pollen (cross) induced a greater Ca2+ increase in papilla cells of the stigma, while incompatible pollen (self) induced a smaller Ca2+ increase. They speculated that the compatible pollen coat contains signaling molecules that stimulate Ca2+ export from papilla cells [64]. Iwano et al. (2015) used self-incompatible Arabidopsis thaliana expressing S-locus (Self-incompatibility) protein 11 (SP11), S-locus cysteine-rich protein (SCR), and S-receptor kinase, and found that self-pollination specifically induced an increase in cytoplasmic Ca2+ in papilla cells. Direct application of SP11/SCR to the papilla cell protoplasts induced a Ca2+ increase, which was inhibited by the glutamate receptor channel blocker AP-5. An artificial increase in Ca2+ in papilla cells arrested hydration of wild-type pollen. Treatment of papilla cells with AP-5 interfered with self-incompatibility, and the Ca2+ increase during the self-incompatibility response was reduced in gene mutants of the glutamate receptor-like channel. It was speculated that the Ca2+ influx mediated by the glutamate receptor-like channel is essential for the self-incompatibility response leading to self-pollen rejection [65]. The results of those two studies revealed different Ca2+ dynamics; that is, an increase in the Ca2+ concentration in papilla cells during the self-incompatibility response in A. thaliana, but not in B. rapa. These differences indicate that the self-incompatibility response in higher plants is complex and diverse. Also, both B. rapa and A. thaliana have papilla cells in their stigmas. Further studies are required to explore Ca2+ dynamics in stigmas without papilla cells.
11. Calcium Dynamics during Interaction of Pollen Tube with Synergids
Synergid cells in the embryo sac are the final destination for pollen tube growth. The attraction function of the tube entrance of synergids is a hot point in plant reproductive biology. Early studies investigated Ca2+ dynamics in ovaries and ovules of wheat [66], pearl millet [67], tobacco [68], B. napus [69], Plumbago zeylanica [70], and rice [71]. These studies revealed high levels of Ca2+ in the synergids, which may function to attract the pollen tube to the embryo sac. Using Arabidopsis expressing the GFP-based Ca2+-sensor yellow chameleon 3.60 (YC3.60) in pollen tubes and synergid cells, Iwano et al. (2012) investigated Ca2+ dynamics in ovules during pollen tube guidance and reception. In the pollen tube growing towards the micropyle, pollen tubes started turning within 150 µm of the micropylar opening; the Ca2+ concentration was higher in these pollen tube tips than in those not growing towards an ovule. The results suggested that attractants secreted from the ovules affect Ca2+ dynamics in the pollen tube [72].
As the end point of pollen tube growth, the synergids are the site where pollen tube growth must stop. Still in A. thaliana, the Ca2+ concentration in synergid cells did not change when the pollen tube grew towards the micropyle or entered the ovule. When the pollen tube arrived at the synergid cell, a Ca2+ oscillation was produced at the micropylar pole of the synergid, and spread towards the chalazal pole. Finally, the Ca2+ concentration in the synergid cell peaked at pollen tube rupture. The authors speculated that signals from the pollen tube induced the Ca2+ oscillations in synergids, and the peak Ca2+ content in synergids induced pollen tube rupture, indicating an interaction between the pollen tube and the synergids [72]. This was the first experimental evidence that synergids can arrest pollen tube growth by calcium dynamic. Then, Hamamura et al. (2014) found that after pollen tube discharge and plasmogamy, the egg and central cells of A. thaliana displayed transient spikes in Ca2+ concentrations, but not oscillations. In contrast, the synergid cells displayed Ca2+ oscillations on pollen tube arrival. The two synergid cells showed distinct Ca2+ dynamics depending on their respective roles in tube reception [73]. Generally, there are two synergids beside the egg in the embryo sac: the degenerated synergid that accepts the pollen tube, and the persistent synergid. An experiment using two genetically encoded Ca2+ sensors with non-overlapping emission spectra showed that the Ca2+ oscillations increased in only one synergid following pollen tube entrance [74]. The synergids controlled this process by coordinating their distinct Ca2+ signatures in response to the Ca2+ dynamics and the growth behavior of the pollen tube. In addition, the Ca2+ signatures were interchangeable between the two synergids, implying that their fates of death or survival were determined by reversible interactions with the pollen tube [74]. Denninger et al. (2014) reported that Ca2+ oscillations were initiated in synergid cells after physical contact with the pollen tube apex. In egg and central cells, a short Ca2+ transient was associated with pollen tube burst and sperm cell arrival. A second extended Ca2+ transient solely in the egg cell was correlated with successful fertilization [75]. In all of those studies, interactions between the pollen tube and synergids were found to be mediated by Ca2+ dynamics in both cells, confirming that the synergids function in attracting pollen tube entrance and stopping pollen tube growth [76,77].
12. Conclusions and Prospects
Calcium distribution in the pollen tube is closely related to pollen tube function, polarity, and tip elongation. The asymmetrical Ca2+ distribution in the tube tip triggers reorientation of the axis of elongation. The subcellular distribution of Ca2+ in the pollen tube occurs along a steep dynamic gradient that is related to tube elongation. The most pistils of flowering plants contain abundant Ca2+ that meets the requirements for tube elongation in vivo and attracts pollen tubes to the ovule and embryo sac. Besides the spatial and temporal characteristics of Ca2+ distribution in the stigma and style, the Ca2+ dynamics in papilla cells of the stigma and pollen also indicate a function in controlling the self-incompatibility response. High levels of Ca2+ in degenerated synergids not only guide the entry of the pollen tube, but also stop tube growth. All of these results indicate that Ca2+ plays critical roles in pollen tube growth in vivo.
Significant progress has been made in understanding the role of Ca2+ in pollen tube growth, but numerous questions remain unanswered. First, the exact function of Ca2+ in the growing pollen tube has not been proven. Some reports have indicated that Ca2+ associates with the pollen tube wall to confer rigidity and mechanical strength, but does not drive pollen tube growth. The origin of tube growth is its internal turgor, which is related to vacuole genesis. However, the genesis of vacuoles in the pollen tube has not been confirmed by any assay method. Second, pollen tubes grow in plant pistils in vivo, but many reported results were obtained in vitro. In vitro conditions are assumed to mimic the natural state of pollen tubes and represent gamete behavior, but significant and perhaps critical functional differences may be overlooked. Pollen tubes elongate faster, tubes live longer, and sperm cells (in bicellular species) form sooner and more reliably in vivo than in vitro. Pollen tube growth in vivo involves interactions with pistil tissues, especially at critical stages such as tube reorientation and the cessation of growth. Breaking of the pollen tube also involves an interaction between the pollen tube and the synergids mediated by Ca2+. Confirmation of the roles of Ca2+ in pollen tube growth in vivo would reinforce and extend the current state of knowledge.
Funding
This work was supported by the Fujian Province Science and Technology Research Funding on the fifth Tree Breeding Program of Chinese fir (Min Lin Ke 2016-35).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ge L.L., Tian H.Q., Russell S.D. Calcium function and distribution during fertilization process of angiosperms. Am. J. Bot. 2007;94:1046–1060. doi: 10.3732/ajb.94.6.1046. [DOI] [PubMed] [Google Scholar]
- 2.Damineli D.S.C., Portes M.T., Feijó J.A. One thousand and one oscillators at the pollen tube tip: The quest for a central pacemaker revisited. In: Obermeyer G., Feijó J.A., editors. Pollen Tip Growth—From Biophysical Aspects to Systems Biology. Springer; Berlin/Heidelberg, Germany: 2017. pp. 391–414. [Google Scholar]
- 3.Feijó J.A., Wudick M.M. Calcium is life. J. Exp. Bot. 2018;69:4147–4150. doi: 10.1093/jxb/ery279. [DOI] [Google Scholar]
- 4.Michard E., Alves F., Feijó J.A. The role of ion fluxes in polarized cell growth and morphogenesis: The pollen tube as an experimental paradigm. Int. J. Dev. Biol. 2009;53:1609–1622. doi: 10.1387/ijdb.072296em. [DOI] [PubMed] [Google Scholar]
- 5.Hepler P.K., Kunkel J.G., Rounds C.M., Winship L.J. Calcium entry into pollen tubes. Trends Plant Sci. 2012;17:32–38. doi: 10.1016/j.tplants.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Michard E., Simon A.A., Tavares B., Wudick M.M., Feijó J.A. Signaling with ions: The keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiol. 2017;173:91–111. doi: 10.1104/pp.16.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konrad K.R., Maierhofer T., Hedrich R. Spatio-temporal aspects of Ca2+-signalling: Lessons from guard cells and pollen tubes. J. Exp. Bot. 2018;69:4195–4214. doi: 10.1093/jxb/ery154. [DOI] [PubMed] [Google Scholar]
- 8.Derksen J., Janssen G., Wolters-Arts M., Lichtscheidl-Schultz I., Adlassnig W., Ovecka M., Doris F., Steer M. Wall architecture with high porosity is established at the tip and maintained in growing pollen tubes of Nicotiana tabacum. Plant J. 2011;68:495–506. doi: 10.1111/j.1365-313X.2011.04703.x. [DOI] [PubMed] [Google Scholar]
- 9.Heslop-Harrison J. Pollen germination and pollen tube growth. Int. Rev. Cytol. 1987;107:1–78. [Google Scholar]
- 10.Li Y.Q., Chen F., Linskens H.F., Cresti M. Distribution of unesterified and esterified pectins in cell walls of pollen tubes of flowering plants. Sex. Plant Reprod. 1994;7:145–152. doi: 10.1007/BF00228487. [DOI] [Google Scholar]
- 11.Bhojwani S.S., Bhatnagar S.P. The Embryology of Angiosperms. Vikas Publishing House Pvt Ltd.; New Delhi, India: Bombay, India: Bangalore, India: Calcutta, India: Kanpur, India: 1974. pp. 102–120. [Google Scholar]
- 12.Steer M.W., Steer J.M. Pollen tube tip growth. New Phytol. 1989;111:323–358. doi: 10.1111/j.1469-8137.1989.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 13.Kasahara R.D., Portereiko M.F., Sandaklie-Nikolova L., Rabiger D.S., Drews G.N. MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell. 2005;17:2981–2992. doi: 10.1105/tpc.105.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okuda S., Tsutsui H., Shiina K., Sprunck S., Takeuchi H. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–36112. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- 15.Steinhorst L., Kudla J. Calcium-a central regulator of pollen germination and tube growth. Biochim. Biophys. Acta. 2013;1833:1573–1581. doi: 10.1016/j.bbamcr.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Barberini M.L., Muschietti J. Imaging of Calcium Dynamics in Pollen Tube Cytoplasm. In: Estevez J.M., editor. Plant Cell Expansion. Humana Press; New York, NY, USA: Heidelberg, Germany: Dordrecht, The Netherlands: London, UK: 2014. pp. 49–57. [Google Scholar]
- 17.Sarah H., Timo G., Konrad K.R. An interconnection between tip-focused Ca2+ and anion homeostasis controls pollen tube growth. Plant Signal. Behav. 2018;13:1–6. doi: 10.1080/15592324.2018.1529521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brewbaker J.L., Kwack B.H. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 1963;50:859–865. doi: 10.1002/j.1537-2197.1963.tb06564.x. [DOI] [Google Scholar]
- 19.Tian H.Q., Kuang A., Musgrave M.E., Russell S.D. Calcium distribution in fertile and sterile anthers of a photoperiod-sensitive genic male-sterile rice. Planta. 1998;204:183–192. doi: 10.1007/s004250050245. [DOI] [Google Scholar]
- 20.Mascarenhas J.P., Machlis L. Chemotropic response of Antirrhinum majus pollen to calcium. Nature. 1962;196:292–293. doi: 10.1038/196292a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nobiling R., Reiss H.D. Quantitative analysis of calcium gradients and activity in growing pollen tubes of Lilium longiflorum. Protoplasma. 1987;139:20–2421. doi: 10.1007/BF01417531. [DOI] [Google Scholar]
- 22.Obermeyer G., Weisenseel M.H. Calcium channel blocker and calmodulin antagonists affect the gradient of free calcium ions in lily pollen tubes. Eur. J. Cell Biol. 1991;56:319–327. [PubMed] [Google Scholar]
- 23.Miller D.D., Callaham D.A., Gross D.J., Hepler P.K. Free Ca2+ in growing pollen tubes of Lilium. J. Cell Sci. 1992;101:7–12. [Google Scholar]
- 24.Franklin-Tong V.E., Hackett G., Hepler P.K. Ratio-imaging of Ca2+ in the self-incompatibility response of Papaver rhoeas. Plant J. 1997;12:1375–1386. doi: 10.1046/j.1365-313x.1997.12061375.x. [DOI] [Google Scholar]
- 25.Hepler P.K. Tip growth in pollen tubes: Calcium leads the way. Trends Plant Sci. 1997;2:79–80. doi: 10.1016/S1360-1385(97)88385-9. [DOI] [Google Scholar]
- 26.Holdaway-Clarke T.L., Feijó J.A., Hackett G.R., Kunkel J.G., Hepler P.K. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell. 1997;9:1999–2010. doi: 10.1105/tpc.9.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malhó R., Trewavas A.J. Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell. 1996;8:1935–1949. doi: 10.1105/tpc.8.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierson E.S., Miller D.D., Callaham D.A., Van Aken J., Hackett G., Hepler P.K. Tip-localized calcium entry fluctuates during pollen tube growth. Dev. Biol. 1996;174:160–173. doi: 10.1006/dbio.1996.0060. [DOI] [PubMed] [Google Scholar]
- 29.Pierson E., Miller D.D., Callaham D.A., Shipley A.M., Rivers B.A., Cresti M., Hepler P.K. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell. 1994;6:1815–1828. doi: 10.1105/tpc.6.12.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierson E.S., Li Y., Zhang H.Q., Willemse M.T.M., Linskens H.F., Cresti M. Pulsatory growth of pollen tubes: Investigation of a possible relationship with the periodic distribution of cell wall components. Plant Biol. 1995;44:121–128. doi: 10.1111/j.1438-8677.1995.tb00774.x. [DOI] [Google Scholar]
- 31.Geitmann A., Cresti M. Ca2+ channel control the rapid expansions in pulsating growth of Petunia hybrida pollen tubes. J. Plant Physiol. 1998;152:439–447. doi: 10.1016/S0176-1617(98)80261-7. [DOI] [Google Scholar]
- 32.Parton R.M., Fischwe-Parton S., Trewavas A.J., Watahiki M.K. Pollen tubes exhibit regular periodic membrane trafficking events in the absence of apical extension. J. Cell Sci. 2003;116:2707–2719. doi: 10.1242/jcs.00468. [DOI] [PubMed] [Google Scholar]
- 33.Holdaway-Clarke T.L., Hepler P.K. Control of pollen tube growth: Role of ion gradients and fluxes. New Phytol. 2003;159:539–563. doi: 10.1046/j.1469-8137.2003.00847.x. [DOI] [PubMed] [Google Scholar]
- 34.Marty F. Plant vacuoles. Plant Cell. 1999;11:587–599. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto Y., Nishimura M., Hara-Nishimura I., Noguchi T. Behavior of Vacuoles during Microspore and Pollen Development in Arabidopsis thaliana. Plant Cell Physiol. 2003;44:1192–1201. doi: 10.1093/pcp/pcg147. [DOI] [PubMed] [Google Scholar]
- 36.Pacini E., Jacquard C., Clément C. Pollen vacuoles and their significance. Planta. 2011;234:217–227. doi: 10.1007/s00425-011-1462-4. [DOI] [PubMed] [Google Scholar]
- 37.Li D.X., Hu H.Y., Ru Z.G., Tian H.Q. Calcium controls the formation of vacuoles from mitochondria to regulate microspore development in wheat. Plant Reprod. 2017;30:131–139. doi: 10.1007/s00497-017-0309-y. [DOI] [PubMed] [Google Scholar]
- 38.Zheng R.H., Su S.D., Xiao H., Tian H.Q. Calcium distribution during pollen development in Bauhinia blakeana. Trees. 2018;32:465–472. doi: 10.1007/s00468-017-1643-2. [DOI] [Google Scholar]
- 39.Malhó R., Read N.D., Pais M.S., Trewavas A.J. Role of cytosolic free calcium in the reorientation of pollen tube growth. Plant J. 1994;5:331–341. doi: 10.1111/j.1365-313X.1994.00331.x. [DOI] [Google Scholar]
- 40.Malhó R., Read N.D., Trewavas N.D., Pais M.S. Calcium channel activity during pollen tube growth and reorientation. Plant Cell. 1995;7:1173–1184. doi: 10.1105/tpc.7.8.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moutinho A., Trewavas A.J., Malhó R. Relocation of a Ca2+-dependent protein kinase activity during pollen tube reorientation. Plant Cell. 1998;10:1499–1509. doi: 10.1105/tpc.10.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutermuth T., Herbell S., Lassig R., Brosché M., Romeis T., Feijó J.A., Hedrich Konrad K.R. Tip-localized Ca2+ channels control pollen tube growth via kinase-dependent R- and S-type anion channel regulation. New Phytol. 2018;218:1089–1105. doi: 10.1111/nph.15067. [DOI] [PubMed] [Google Scholar]
- 43.Tirlapur U.K., Shiggaon S.V. Distribution of Ca2+ and calmodulin in the papillae cells of the stigma surface, visualized by chlorotetracycline and fluorescing calmodulin-binding phenothiazines. Ann. Biol. 1988;4:49–53. [Google Scholar]
- 44.Bednarska E. The effects of exogenous Ca2+ ions on pollen grain germination and pollen tube growth: Investigations with 45Ca2+ together with verapamil, La3+ and ruthenium red. Sex. Plant Reprod. 1989;2:53–58. doi: 10.1007/BF00190119. [DOI] [Google Scholar]
- 45.Bednarska E. Calcium uptake from the stigma by germinating pollen in Primula officinalis L. and Ruscus aculeatus L. Sex. Plant Reprod. 1991;4:36–38. doi: 10.1007/BF00194569. [DOI] [Google Scholar]
- 46.Zhang J.S., Yang H.Y., Zhu L., Tong H. Ultracytochemical localization of calcium in the stigma, style and micropyle of sunflower. Acta Bot. Sin. 1995;37:691–696. (In Chinese) [Google Scholar]
- 47.Zhang J.S., Yang H.Y., Zhu L., Tong H. Ultracytochemical localization of calcium in the pollen tube track of cotton gynoecium. Acta Bot. Sin. 1997;39:121–125. (In Chinese) [Google Scholar]
- 48.Elleman C.J., Dickinson H.G. Commonalities between pollen/stigma and host/pathogen interaction: Calcium accumulation during stigmatic penetration by Brassica oleracea pollen tubes. Sex. Plant Reprod. 1999;12:194–202. doi: 10.1007/s004970050192. [DOI] [Google Scholar]
- 49.Ge L.L., Xie C.T., Tian H.Q., Russell S.D. Distribution of calcium in the stigma and style of tobacco during pollen germination and tube elongation. Sex. Plant Reprod. 2009;22:87–96. doi: 10.1007/s00497-009-0094-3. [DOI] [PubMed] [Google Scholar]
- 50.Wei D.M., Qiu Y.L., Liu R.S., Tian H.Q. Calcium distribution in the stigmas and styles of lettuce (Lactuca sativa) Ann. Bot. Fenn. 2010;47:361–366. doi: 10.5735/085.047.0506. [DOI] [Google Scholar]
- 51.Glenk H.O., Eager W., Schimmer O. Can Ca2+ ions act as a chemotropic factor in Oenothera fertilization? In: Heslop-Harrison J., editor. Pollen Development and Physiology. Appleton-Century-Crofts; New York, NY, USA: 1971. pp. 255–261. [Google Scholar]
- 52.Mascarenhas J.P. The biochemistry of angiosperm pollen development. Bot. Rev. 1975;41:259–341. doi: 10.1007/BF02860839. [DOI] [Google Scholar]
- 53.Mao J.Q., Chen Y.Y., Miao Y. Ca2+ ion localization in the path of pollen tube growth within the gynoecium of Brassica napus. Acta Bot. Sin. 1992;34:233–236. (In Chinese) [Google Scholar]
- 54.Franklin-Tong V.E., Ride J.P., Read N.D., Trewavas A.J., Franklin F.C.H. The self-incompatibility response in Papaver rhoeas is mediated by cytosolic free calcium. Plant J. 1993;4:163–177. doi: 10.1046/j.1365-313X.1993.04010163.x. [DOI] [Google Scholar]
- 55.Frankin-Tong V.E., Drøbak B.K., Allan A.C., Watkins P.A.C., Trewavas A.L. Growth of pollen tubes of Papaver rhoeas is regulated by a slow-moving calcium wave propagated by inositol 1,4,5-trisphosphate. Plant Cell. 1996;8:1305–1321. doi: 10.1105/tpc.8.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franklin-Tong V.E., Ride J.P., Franklin F.C.H. Recombinant stigmatic self-incompatibility (S-) protein elicits a Ca2+ transient in pollen of Papaver rhoeas. Plant J. 1995;8:299–307. doi: 10.1046/j.1365-313X.1995.08020299.x. [DOI] [Google Scholar]
- 57.Straatman K.R., Dove S.K., Holdaway-Clarke T., Hepler P.K., Kunkel J.G., Franklin-Tong V.E. Calcium signaling in pollen of Papaver rhoeas undergoing the self-incompatibility (SI) response. Sex. Plant Reprod. 2001;14:105–110. doi: 10.1007/s004970100092. [DOI] [Google Scholar]
- 58.Wehling P., Hackauf B., Wriche G. Phosphorylation of pollen proteins in relation to self-incompatibility in rye (Secale cereale L.) Sex. Plant Reprod. 1994;7:67–75. doi: 10.1007/BF00230574. [DOI] [Google Scholar]
- 59.Franklin-Tong V.E., Holdaway-Clarke T.L., Straatman K.R., Kunkel J.G., Hepler P.K. Involvement of extracellular calcium influx in the self-incompatibility response of Papaver rhoeas. Plant J. 2002;29:333–345. doi: 10.1046/j.1365-313X.2002.01219.x. [DOI] [PubMed] [Google Scholar]
- 60.McClure B.A., Gray J.E., Anderson M.A., Clarke A.E. Self-incompatibility in Nicotiana alata involves degradation of pollen rRNA. Nature. 1990;347:757–760. doi: 10.1038/347757a0. [DOI] [Google Scholar]
- 61.Kunz C., Chang A., Faure J.D., Clarke A.E., Poly G.M., Anderson M.A. Phosphorylation of style S-RNases by Ca2+-dependent protein kinases from pollen tubes. Sex. Plant Reprod. 1996;9:25–34. doi: 10.1007/BF00230363. [DOI] [Google Scholar]
- 62.Lenartowska M., Lenartowski R., Smoliński D.J., Wróbel B., Niedojadło J., Jaworski K., Bednarska E. Calreticulin expression and localization in plant cells during pollen-pistil interactions. Planta. 2009;231:67–77. doi: 10.1007/s00425-009-1024-1. [DOI] [PubMed] [Google Scholar]
- 63.Lenartowska R., Suwińska A., Lenartowska M. Calreticulin expression in relation to exchangeable Ca2+ level that changes dynamically during anthesis, progamic phase, and double fertilization in Petunia. Planta. 2015;241:209–227. doi: 10.1007/s00425-014-2178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iwano M., Igarashi M., Tarutani Y., Kaothien-Nakayama P., Nakayama H., Moriyama H., Yakabe R., Entani T., Shimosato-Asano H., Ueki M., et al. A pollen coat–inducible autoinhibited Ca2+-ATPase expressed in stigmatic papilla cells is required for compatible pollination in the Brassicaceae. Plant Cell. 2014;26:636–649. doi: 10.1105/tpc.113.121350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwano M., Ito K., Fujii S., Kakita M., Asano-Shimosato H., Igarashi M., Kaothien-Nakayama P., Entani T., Kanatani A., Takehisa M., et al. Calcium signalling mediates self-incompatibility response in the Brassicaceae. Nat. Plants. 2015;1:1–8. doi: 10.1038/nplants.2015.128. [DOI] [PubMed] [Google Scholar]
- 66.Chaubal R., Reger B.J. Relatively high calcium is localized in synergid cells of wheat ovaries. Sex. Plant Reprod. 1990;3:98–102. doi: 10.1007/BF00198852. [DOI] [Google Scholar]
- 67.Chaubal R., Reger B.J. Calcium in the synergid cells and other regions of pearl millet ovaries. Sex. Plant Reprod. 1992;5:34–46. doi: 10.1007/BF00714556. [DOI] [Google Scholar]
- 68.Tian H.Q., Russell S.D. Calcium distribution in fertilized and unfertilized ovules and embryo sacs of Nicotiana tabacum L. Planta. 1997;202:93–105. doi: 10.1007/s004250050107. [DOI] [Google Scholar]
- 69.Yu F.L., Liang S.P., Yang H.Y., Wang Y. Ultracytochemical localization of calcium in micropyle and embryo sac of Brassica napus before and after pollination. Acta Bot. Sin. 1998;40:591–597. (In Chinese) [Google Scholar]
- 70.Tian H.Q., Zhu H., Russell S.D. Calcium changes in ovules and embryo sacs of Plumbago zeylanica L. Sex. Plant Reprod. 2000;13:11–20. doi: 10.1007/PL00009837. [DOI] [Google Scholar]
- 71.Zhao J., Yu F.L., Liang S.P., Zhou C., Yang H.Y. Changes of calcium distribution in egg cells, zygotes and two-celled proembryos of rice (Oryza sativa L.) Sex. Plant Reprod. 2002;14:331–337. [Google Scholar]
- 72.Iwano M., Ngo Q.A., Entani T., Shiba H., Nagai T., Miyawaki A., Isogai A., Grossniklaus U., Takayama S. Cytoplasmic Ca2+ changes dynamically during the interaction of the pollen tube with synergid cells. Development. 2012;139:4202–4209. doi: 10.1242/dev.081208. [DOI] [PubMed] [Google Scholar]
- 73.Hamamura Y., Nishimaki M., Takeuchi H., Geitmann A., Kurihara D., Higashiyama T. Live imaging of calcium spikes during double fertilization in Arabidopsis. Nat. Commun. 2014;5:4722–4731. doi: 10.1038/ncomms5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ngo Q.A., Vogler H., Lituiev D.S., Nestorova A., Grossniklaus U. A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev. Cell. 2014;29:491–500. doi: 10.1016/j.devcel.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Denninger P., Bleckmann A., Lausser A., Vogler F., Ott T., Ehrhardt D.W., Frommer W.B., Sprunck S., Dresselhaus T., Grossmann G. Male-female communication triggers calcium signatures during fertilization in Arabidopsis. Nat. Commun. 2014;5:4645–4657. doi: 10.1038/ncomms5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li D.X., Lin M.Z., Wang Y.Y., Tian H.Q. Synergid: A key link in fertilization of angiosperms. Biol. Plant. 2009;53:401–407. doi: 10.1007/s10535-009-0078-z. [DOI] [Google Scholar]
- 77.Higashiyama T., Yang W. Gametophytic Pollen Tube Guidance: Attractant Peptides, Gametic Controls, and Receptors. Plant Physiol. 2017;173:112–121. doi: 10.1104/pp.16.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]