Abstract
Paclitaxel (PTX) is an anticancer agent that is used to treat many cancers but it has a very low oral bioavailability due, at least in part, to the drug efflux transporter, P-glycoprotein (P-gp). Therefore, this study was performed to enhance oral bioavailability of PTX. In this study, we investigated the effects of several piperazine derivatives on P-gp function in vitro. Compound 4 was selected as the most potent P-gp inhibitor from the in vitro results for examining the pharmacokinetic (PK) changes of PTX in rats. Compound 4 increased the AUCinf of PTX without alterations in the Cmax value. The elimination half-life was extended and the oral clearance decreased. Additionally, the Tmax was delayed or widened in the treatment groups. Therefore, the bioavailability (BA) of PTX was improved 2.1-fold following the co-administration of 5 mg/kg of the derivative. A piperazine derivative, compound 4, which was confirmed as a substantial P-gp inhibitor in vitro increased the BA of PTX up to 2-fold by a lingering absorption, in part due to inhibition of intestinal P-gp and a low oral clearance of PTX. These results suggest that co-administering compound 4 may change the PK profile of PTX by inhibiting P-gp activity in the body.
Keywords: piperazine derivatives, P-glycoprotein inhibitor, pharmacokinetics, bioavailability, paclitaxel
1. Introduction
Paclitaxel (PTX) is a widely-used anticancer drug that is known to have poor bioavailability (below 10%) due to its low solubility and membrane permeability [1]. Further, the interaction of PTX with the membrane drug efflux transporter functions as an additional barrier to gastrointestinal (GI) PTX absorption [2]. It has also been reported that approximately 50% of orally administered PTX is excluded from the GI track by P-glycoprotein (P-gp) [3]. There have been many studies on how to improve the bioavailability (BA) of PTX by inhibiting P-gp function and expression over the last two decades [2,4,5,6,7,8,9].
P-gp, a well-known crucial member of the ATP-binding cassette (ABC) superfamily and a well-characterized transporter, has broad substrate specificity and affects the pharmacokinetic (PK) behavior of many drugs that are P-gp substrates. It is highly expressed in important organs such as the intestine [10], liver [11], and kidney [12], which are involved in drug absorption, metabolism and excretion. Because the efflux transporter is the primary cause of multi-drug resistance (MDR) and alters the pharmacokinetics of various drugs, P-gp modulation could counter the MDR and increase the BA of orally delivered drugs. Identification and development of novel P-gp modulators have therefore been studied for over three decades [13,14].
Piperazine is an important six-membered, N-heterocyclic compound found in the structure of a number of pharmaceuticals used against many different diseases, such as multiple cancers, primary hypertension, HIV/AIDS, and inflammation [15,16]. However, only a limited number of studies have assessed P-gp modulation with piperazine or its derivatives. A five-hydroxychromone linked piperazine unit was identified as a highly active P-gp-mediated MDR modulator [17]. Some novel compounds with 10-beta-phenyl ethers of dihydroartemisinin (DHA) with piperazine substitutions have demonstrated significant effects on transporter-mediated antiproliferation in human breast cancer cells. Among them, two DHA compounds with piperazine substitution had a 1.5–2-fold greater effect on antiproliferative activity than the DHA compounds without piperazine substitution [18]. These reports have suggested the possibility of piperazine or its analogues as potential P-gp modulators.
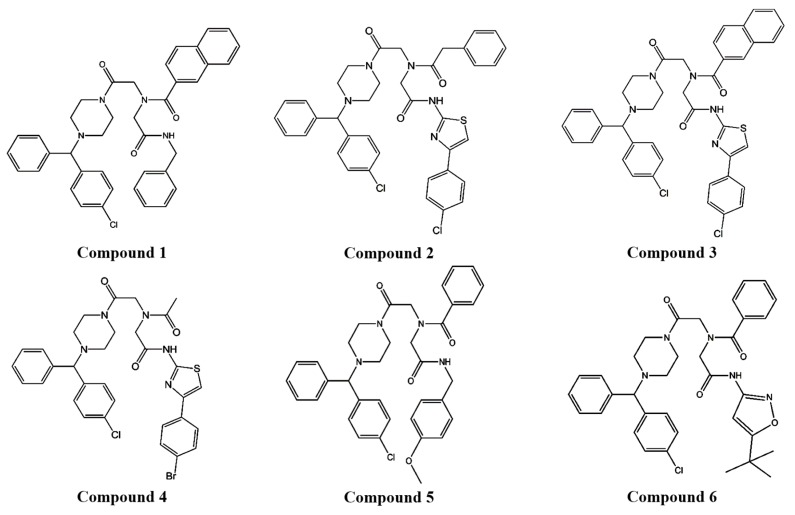
We demonstrated that compound 4, piperazine iminodiacetic acid triamide, which was selected as a potential P-gp inhibitor from several piperazine derivative candidates in vitro (Figure 1), increased the BA of PTX, known to have poor BA (below 10%), as well as a P-gp substrate anticancer drug [1] with a draggy absorption and a slow elimination in rats.
Figure 1.
The structures of piperazine derivatives.
2. Materials and Methods
2.1. Materials
Synthesized piperazine derivatives were provided by Dr. Hea-Young Park Choo [19]. Daunomycin (DNM) and [3H]-DNM (16 Ci/mmol) were purchased from Merck (Darmstadt, Germany) and PerkinElmer Life and Analytical Sciences (Boston, MA, USA), respectively. PTX was obtained by Samyang Genex (Daejeon, Korea), and (±)-Verapamil hydrochloride was provided by Sigma-Aldrich (St. Louis, MO, USA). Human P-gp membrane and BD GentestTM ATPase assay kits were purchased from BD Bioscience (Woburn, MA, USA). All reagents and solvents were commercially provided at molecular biology and cell culture grades or HPLC analysis grade. A P-gp overexpressing cell line, MCF-7/ADR, was provided by Dr. Marilyn E. Morris (University of Buffalo, NY, USA). Laboratory animals were purchased from Orient Bio (Seongnam, Korea).
2.2. Cell Culture
The MDR human breast cancer cell line MCF-7/ADR was maintained in RPMI 1640, supplemented with 2 mM L-glutamine, 10 mM HEPES, 24 mM NaHCO3, 10% fetal bovine serum, and 1% antibiotic-antimycotic agent at 37 °C in a humidified 5% CO2 atmosphere.
2.3. Cytotoxicity Assay
The cytotoxicity of each piperazine derivative was examined in the cells using the sulforhodamine B (SRB) assay [20]. The changes in IC50 values of DNM, P-gp substrate anticancer drug were estimated in the presence of piperazine derivatives at a concentration of 100 μM. The details of the cytotoxicity assay were described in our previous report [9,21]. Verapamil (VER), a representative P-gp substrate and inhibitor, was used as a positive control at a concentration of 100 μM. The IC50 values were calculated with the Table Curve Windows program. The assay was performed in triplicate.
2.4. [3H]-DNM Uptake and Efflux Studies
Tritium-labeled P-gp substrate uptake and efflux studies were performed using the modified Harker and Sikic method [22]. The pre-selected derivatives, compounds 2 and 4, were applied at a final concentration of 100 μM with 0.05 μM of tritium-labeled DNM for 2 h for the uptake study. For the efflux study, cells were incubated with 0.05 μM tritium-labeled DNM for 1 h and subsequently in the absence or presence of 100 μM of pre-selected derivatives for another 1 h after removing 0.05 μM of the tritium-labeled DNM. The uptake and efflux values based on the radioactivity estimated by Liquid Scintillation Counting were normalized to those of 1 × 106 cells. The detailed procedures have been reported previously [9,21,23]. All the experiments were performed in triplicate and verapamil (VER) was used as a positive control.
2.5. P-Glycoprotein ATPase Activity Assay
Using human P-gp membrane, the P-gp ATPase activity of pre-selected derivatives, compounds 2 and 4, was estimated at a concentration of 100 μM [9,21,23]. The rate of phosphate release per mg membrane protein represented the ATPase activity by converting to the relative ratio versus control value. The assay was performed in duplicate and VER, a P-gp inhibitor and ATPase stimulator was used as the positive control.
2.6. Animals
All the animal experiments were performed using six-week-old male Sprague-Dawley rats (200–235 g) under the procedures granted by the Ewha Womans University Institutional Animal Care and Use Committee (IACUC) (No. 2012-01-019), Korea [23].
2.7. Pharmacokinetic Studies
PTX at 2 mg/mL was prepared immediately prior to use with a commercial formulation of Taxol® (Cremophor® EL, anhydrous ethanol and isotonic saline (1:1:4, v/v/v)) [23]. Compound 4 (N-(4-(4-bromophenyl)thiazol-2-yl)-2-(N-(2-(4-((4-chlorophenyl)(phenyl)methyl)piperazin-1-yl)-2-oxoethyl)acetamido)acetamide) was prepared using a process similar to PTX formulation for oral administration.
For intravenous injection of 2 mg/kg of PTX (IV) and oral administration of 25 mg/kg of PTX (PO) with 0, 2, or 5 mg/kg of compound 4, the rats were divided into 4 groups on the day. The common carotid artery was catheterized for blood sampling. Blood sampling was conducted at 0, 0.033, 0.083, 0.25, 0.5, 1, 2, 3, 4, 6, 10, and 24 h for the IV group and 0, 0.25, 0.5, 1, 2, 3, 4, 6, 8, 10, and 24 h for PO groups [21,23].
Prepared plasma samples mixed with internal standard (4-hydroxybenzoic acid n-hexyl ester) were analyzed by the HPLC system [21,23].
WinNonlin® Professional version 5.2 software (Pharsight Corporation, Mountain View, CA, USA) was used to estimate the PK parameters following intravenous or oral PTX administrations to the rats. Non-compartmental analysis was performed using the plasma PTX concentration–time profiles to obtain the following PK parameters: Area under the plasma concentration–time curve from zero time to infinity (AUCinf), elimination half-life (t1/2), apparent volume of distribution after oral administration (Vz/F), and oral clearance (Cl/F). The maximum plasma concentration (Cmax) and the time required to reach Cmax (Tmax) were directly measured from the plasma PTX concentration–time curve. The absolute BA and relative BA (AB and RB, respectively) of PTX were calculated using the respective formulas below:
| AB (%) = AUC po/AUC iv × IV dose/PO dose × 100 |
| RB (%) = AUC po co–administration/AUC po control × 100 |
2.8. Statistical Analysis
Tukey’s HSD test in conjunction with an ANOVA was used for statistical analysis of uptake and efflux and for pharmacokinetics studies. Statistical significance was represented as ‘p value < 0.05.’
3. Results
3.1. Effect of Piperazine Derivatives on Uptake and Efflux of P-gp Substrate
To determine the influence of the derivatives on P-gp function, a P-gp substrate drug, DNM was applied with piperazine derivatives to MCF-7/ADR cells. Initially, four of 6 piperazine derivatives (Figure 1) were selected to examine their effect on DNM, a P-gp substrate drug, cytotoxicity in P-gp-overexpressing cells, because the selected derivatives, compounds 1–4 were not toxic to the cells at five different concentrations (5, 10, 25, 50, and 100 μM), but compounds 5–6 were toxic to the cells to some degree (Table S1).
All the tested derivatives decreased the IC50 value of DNM to less than half that with the negative control (31.7 μM) (Table 1). Compounds 1, 2, and 4 reduced the DNM IC50 values (5.7, 7.6 and 2.7 μM, respectively) similarly to the positive control, VER (3.1 μM). Among all derivatives, compound 4 appeared to be the most effective for reducing the IC50 value of the P-gp substrate drug. Although compound 1 decreased the IC50 value of DNM more than compound 2, compound 2 was relatively less toxic to the cells at all concentrations tested, from 5 μM to 100 μM (Table S1). Based on these results, compounds 2 and 4, which had similar structures, were selected for further in vitro studies.
Table 1.
The change in DNM IC50 value by 100 μM piperazine derivatives in vitro (The numerical data were represented the cell survival ratio).
| Category | Derivatives | DNM IC50 (μM) |
|---|---|---|
| Control | Negative control | 31.7 |
| Positive control * | 3.1 | |
| Piperazine derivatives | 1 | 5.4 |
| 2 | 7.6 | |
| 3 | 11.5 | |
| 4 | 2.7 |
* Positive control: VER, a P-gp inhibitor.
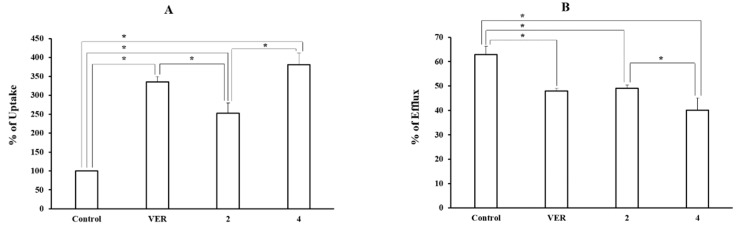
The pre-selected two piperazine derivatives (compounds 2 and 4) were used to measure the cellular uptake and efflux of tritium-labeled DNM at a concentration of 100 μM in order to confirm the P-gp inhibitory function (Figure 2). Both derivatives significantly increased the tritium-labeled DNM uptake to 2.5–3.8-fold (252.9 ± 26.8% and 380.8 ± 31.5% for compounds 2 and 4, respectively) compared with the control and decreased the efflux up to 0.6–0.8 fold (40.2 ± 4.9% and 49.1 ± 1.4% for compounds 4 and 2, respectively) compared with the control (63.0 ± 3.3%). Compound 4 showed a more significant effect on DNM uptake and efflux than compound 2. Compound 4 showed a stronger effect on both uptake and efflux functions of the P-gp substrate compared to VER, the positive control (335.1 ± 14.7% in uptake and 48.0 ± 1.1% in efflux).
Figure 2.
The Effect of 2 pre-selected derivatives on the uptake (A) and efflux (B) of [3H]-DNM in MCF-7/ADR cells. [3H]-DNM uptake and efflux were assayed after the incubation with each derivative (100 μM) for 2 h and 1 h, respectively. VER was used as a positive control. Control represents uptake or efflux of tritium-labeled P-gp substrate alone. Each data represents the mean ± S.D. (*, p < 0.05) from triplicate experiments.
3.2. Effect of Piperazine Derivatives on P-gp ATPase Activity
To examine the modulation mechanism on P-gp activity, the effect of compounds 2 and 4 on ATPase activity was estimated using an ATPase assay kit and a human P-gp membrane (Table 2). Compounds 2 and 4 did not exhibit a significant modulating effect on ATPase activity (1.3- and 1.2-fold, respectively) compared to the control, whereas VER (100 µM) increased the activity by more than 2-fold compared to the control.
Table 2.
Effect of piperazine derivatives and verapamil on P-gp ATPase activity.
| Comp Conc. (μM) |
Blank | VER a | 2 | 4 |
|---|---|---|---|---|
| 100 | 1.00 | 2.24 | 1.29 | 1.18 |
a VER: ATPase stimulator; the values are expressed as a ratio of the control (blank).
3.3. Effect of Compound 4 on P-gp Function In Vivo: PTX Pharmacokinetics
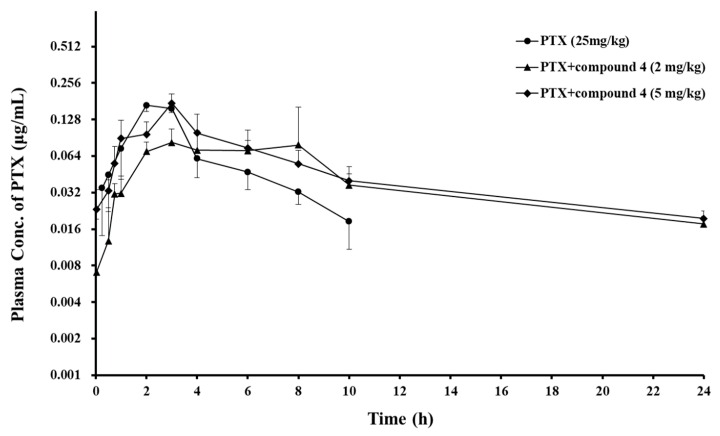
Based on the results from the in vitro studies, compound 4 was selected to examine the P-gp modulating effect in an animal model. PTX was orally administered to rats to determine the effect of compound 4 on the PK profile of the P-gp substrate drug. Figure 3 and Table 3 show the mean plasma PTX concentration–time profiles and PK parameters of PTX, respectively. The AUCinf and t1/2 of the anticancer drug increased with an increasing dose of compound 4, whereas the total clearance was reduced with an increasing dose of the compound. The Cmax and Vz/F following oral administration of PTX were not significantly different among the control group and treatment groups, but Tmax was delayed or widened. As a result, the BA of PTX was enhanced 1.6- and 2.1-fold at 2 and 5 mg/kg doses of compound 4, respectively (Table 3).
Figure 3.
Mean plasma concentration–time profiles of the oral co-administration of PTX with piperazine derivative, compound 4 to rats. Bars represent S.D. (n = 3–4). (●) PTX (25 mg/kg, PO) alone, PTX (25 mg/kg) co-administrated with compound 4 (▲) 2, or (◆) 5 mg/kg.
Table 3.
PK parameters of PTX orally co-administrated with compound 4 (2 and 5 mg/kg, PO) in rats.
| PK Parameters | PTX Oral Control | Co-Administration of PTX with Compound 4 | PTX IV Control | |
|---|---|---|---|---|
| 2 mg/kg | 5 mg/kg | |||
| Cmax (ng/mL) | 189 ± 76 | 112 ± 55 | 175 ± 0.032 | 2968 ± 428 |
| Tmax (h) | 2.0 | [2.0–8.0] | 3.0 | |
| AUCinf (ng·h/mL) | 745 ± 208 | 1162 ± 322 | 1539 ± 209 * | 835 ± 155 |
| t1/2 (h) | 3.1 ± 0.8 | 7.9 ± 2.0 * | 11.0 ± 2.7 * | 2.06 ± 0.8 |
| ke (1/h) | 0.233 ± 0.062 | 0.092 ± 0.027 * | 0.066 ± 0.016 * | 0.37 ± 0.12 |
| Vz/F (mL) | 40,589 ± 16,353 | 56,582 ± 25,172 | 53,466 ± 15,335 | 1754 ± 419 |
| Cl/F (mL/h) | 8721 ± 2040 | 4811 ± 1406 * | 3356 ± 455 * | 614 ± 123 |
| AB (%) | 7.1 | 11.1 | 14.7 | 100.0 |
| RB (%) | 156.0 | 206.6 * | ||
(* p < 0.05 compared with PTX oral control). Data are presented as mean ± S.D. (3–4 rats per group).
4. Discussion
We selected the synthesized piperazine derivative, N-(4-(4-bromophenyl)thiazol-2-yl)-2-(N-(2-(4-((4-chlorophenyl)(phenyl)methyl)piperazin-1-yl)-2-oxoethyl)acetamido)acetamide (compound 4) as a potential P-gp inhibitor based on in vitro results. The effect of compound 4 on the BA of PTX was then monitored. Compound 4 increased the BA of the anticancer drug PTX by as much as 56–106.6%, where the greatest effect was seen when the compound was co-administered orally at a dose of 5 mg/kg (Table 3). The absolute BA of PTX was only 7.1% when it was orally administered alone.
P-gp modulation mediated by piperazine derivatives has not been studied substantially, and most studies have been performed in vitro. Therefore, P-gp inhibition by piperazine or its derivatives has not yet been elucidated. One study reported that a radio-iodinated piperazine derivative, 5-[3-{4-(2-phenyl-2-(4-[125I]iodo-phenyl)acetyl)piperazin-1-yl}-2-hydroxypropoxy] quinoline, induced approximately a 10-fold shorter absorption half-life and a 50% shorter mean residence time in xenograft mice inoculated with P-gp overexpressed in human uterine sarcoma cells (MES-SA/Dx5) than those in the parental cells [24]. This observation was differed from our results, which could be attributed to different animal species, chemical structure, and/or formulation.
However, although piperazine or its derivatives did not demonstrate a powerful effect on P-gp modulation, it is still considered a potential approach because piperazine is the basic core structure of a number of pharmaceuticals for many diseases [15,16]. Moreover, in vitro investigations have shown the possibility of piperazine or derivative use for modulating MDR mediated by P-gp [17,18] and other ABC transporters, such as breast cancer resistance protein (BCRP) [25] and multidrug resistance-associated protein 1 (MRP1) [26].
In the present study, compounds 1–4 (Figure 1) were selected to examine their effect on DNM cytotoxicity in MCF-7/ADR cells. These selected compounds were not toxic to the P-gp overexpressing cells at five different concentrations (5, 10, 25, 50, and 100 μM) (Table S1). Previously, we examined P-gp inhibitory effect of a positive control, VER at two different concentrations (50 and 100 uM) in in vitro studies. Because VER at the concentration of 50 uM did not fully inhibit P-gp function, as compared to 100 uM concentration, based on the results of cytotoxicity and uptake studies [9,27], the cells were treated with 100 uM of the piperazine derivatives and VER to investigate their P-gp inhibitory activity in this study. Among the four piperazine derivatives, the chemical structures of compounds 2–4 were similar (Figure 1). Based on chemical structure and reversing effect on resistance to DNM by decreasing IC50 value in the cells, compounds 2 and 4 were chosen to examine their effects on uptake and efflux functions of P-gp substrate and P-gp ATPase activity.
The PK parameters of PTX provided more information about the P-gp modulating effect of the piperazine derivative. Although the Cmax and Vz/F of PTX were not considerably altered after oral co-administration with compound 4, the increased AUCinf, prolonged Tmax and t1/2, and decreased oral clearance of PTX (Table 3) in the presence of compound 4 may result in an enhanced BA of PTX by inhibiting P-gp located in the absorption and elimination organs.
The mean plasma concentration–time profiles of PTX provided additional information and the following points were observed (Figure 3). In the absorption phase of PTX, the piperazine derivative induced to extend or drag the PTX absorption peak in the treatment groups, and in the PTX elimination phase, the derivative led to slower elimination of PTX. Because P-gp is located in whole intestine [28], inhibition of P-gp function by the piperazine derivative can occur through whole intestine, which can induce a lingering absorption peak. This kind of peak was also previously observed when PTX was orally administered with VER at a dose of 5 mg/kg [29].
Compound 4 could affect not only the PTX absorption pathway but also the elimination pathway. The P-gp efflux transporter is related to drug elimination as well as drug absorption following oral administration [2,30]. PTX is eliminated by the hepatic metabolism, renal excretion [30,31], and biliary excretion [32]. Because P-gp is expressed in the hepatic canalicular membrane [11] and the proximal tubule of the kidney [12], the interaction between PTX and compound 4 in the P-gp efflux function can occur, resulting in slow elimination of PTX by P-gp inhibition in the eliminating organs.
5. Conclusions
Four piperazine derivatives showed a P-gp inhibitory activity in cell studies. Among them, compound 4, N-(4-(4-bromophenyl)thiazol-2-yl)-2-(N-(2-(4-((4-chlorophenyl)(phenyl)methyl)piperazin-1-yl)-2-oxoethyl)acetamido)acetamide, was selected as the most potent P-gp inhibitor candidate from in vitro studies. The results indicated that co-administration of 5 mg/kg of compound 4 significantly enhanced the oral BA of PTX by 2.1-fold in rats. Although the interaction between PTX and compound 4 was not fully understood in vivo, this result may be, at least in part, possibly due to the inhibition of P-gp present in absorption and elimination organs. Therefore, the piperazine derivative can be exploited in order to enhance the oral BA of other P-gp substrate drugs as well as PTX.
Acknowledgements
We thank M.E. Morris for providing the MCF-7/ADR cell line.
Supplementary Materials
The following are available online at http://www.mdpi.com/1999-4923/11/1/23/s1, Table S1. The toxic effect of each derivative in MCF-7/ADR cells after 2 h incubation.
Author Contributions
Conceptualization, J.L., S.W.C. and H.J.L.; Methodology, S.W.C., J.L. and H.J.L.; Formal Analysis, J.L., S.W.C. and H.J.L.; Investigation, S.W.C., J.L., H.-Y.P.C. and H.J.L.; Resources, J.H.Y. and H.-Y.P.C.; Data Curation, J.L. and H.J.L.; Writing—Original Draft Preparation, J.L., S.W.C., A.R.O., S.J.R. and H.J.L.; Writing—Review & Editing, J.L., S.J.R. and H.J.L; Visualization, A.R.O.; Supervision, H.J.L.; Project Administration, H.J.L.; Funding Acquisition, J.L., A.R.O. and H.J.L.
Funding
This research work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2017R1A2B4007869).
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- 1.Panchagnula R. Pharmaceutical aspects of paclitaxel. Int. J. Pharm. 1998;172:1–15. doi: 10.1016/S0378-5173(98)00188-4. [DOI] [Google Scholar]
- 2.Sparreboom A., van Asperen J., Mayer U., Schinkel A.H., Smit J.W., Meijer D.K., Borst P., Nooijen W.J., Beijnen J.H., van Tellingen O. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc. Natl. Acad. Sci. USA. 1997;94:2031–2035. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo J.S., Lee C.H., Shim C.K., Hwang S.J. Enhanced oral bioavailability of paclitaxel by coadministration of the P-glycoprotein inhibitor KR30031. Pharm. Res. 2003;20:24–30. doi: 10.1023/A:1022286422439. [DOI] [PubMed] [Google Scholar]
- 4.Kwak J.O., Lee S.H., Lee G.S., Kim M.S., Ahn Y.G., Lee J.H., Kim S.W., Kim K.H., Lee M.G. Selective inhibition of MDR1 (ABCB1) by HM30181 increases oral bioavailability and therapeutic efficacy of paclitaxel. Eur. J. Pharmacol. 2010;627:92–98. doi: 10.1016/j.ejphar.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Mo R., Jin X., Li N., Ju C., Sun M., Zhang C., Ping Q. The mechanism of enhancement on oral absorption of paclitaxel by N-octyl-O-sulfate chitosan micelles. Biomaterials. 2011;32:4609–4620. doi: 10.1016/j.biomaterials.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Amini-Fazl M.S., Mobedi H., Barzin J. Investigation of aqueous stability of taxol in different release media. Drug. Dev. Ind. Pharm. 2014;40:519–526. doi: 10.3109/03639045.2013.771646. [DOI] [PubMed] [Google Scholar]
- 7.Wang T., Sun Y., Ma W., Yang Z., Yang J., Liu J., Fan H., Yang Y., Gu J., Fawcett J.P., et al. Trantinterol, a novel beta2-adrenoceptor agonist, noncompetitively inhibits P-glycoprotein function in vitro and in vivo. Mol. Pharm. 2015;12:1–9. doi: 10.1021/mp500239v. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y., Huo M., Fu Y., Xu W., Cai H., Yao L., Chen Q., Mu Y., Zhou J., Yin T. N-Deoxycholic acid-N,O-hydroxyethyl Chitosan with a Sulfhydryl Modification To Enhance the Oral Absorptive Efficiency of Paclitaxel. Mol. Pharm. 2017;14:4539–4550. doi: 10.1021/acs.molpharmaceut.7b00662. [DOI] [PubMed] [Google Scholar]
- 9.Chae S.W., Lee J., Park J.H., Kwon Y., Na Y., Lee H.J. Intestinal P-glycoprotein inhibitors, benzoxanthone analogues. J. Pharm. Pharmacol. 2018;70:234–241. doi: 10.1111/jphp.12832. [DOI] [PubMed] [Google Scholar]
- 10.Li M., Hurren R., Zastawny R.L., Ling V., Buick R.N. Regulation and expression of multidrug resistance (MDR) transcripts in the intestinal epithelium. Br. J. Cancer. 1999;80:1123–1131. doi: 10.1038/sj.bjc.6690475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiebaut F., Tsuruo T., Hamada H., Gottesman M.M., Pastan I., Willingham M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hori R., Okamura N., Aiba T., Tanigawara Y. Role of P-glycoprotein in renal tubular secretion of digoxin in the isolated perfused rat kidney. J. Pharmacol. Exp. Ther. 1993;266:1620–1625. [PubMed] [Google Scholar]
- 13.Palmeira A., Sousa E., Vasconcelos M.H., Pinto M.M. Three decades of P-gp inhibitors: Skimming through several generations and scaffolds. Curr. Med. Chem. 2012;19:1946–2025. doi: 10.2174/092986712800167392. [DOI] [PubMed] [Google Scholar]
- 14.Chung F.S., Santiago J.S., Jesus M.F., Trinidad C.V., See M.F. Disrupting P-glycoprotein function in clinical settings: What can we learn from the fundamental aspects of this transporter? Am. J. Cancer Res. 2016;6:1583–1598. [PMC free article] [PubMed] [Google Scholar]
- 15.Shaquiquzzaman M., Verma G., Marella A., Akhter M., Akhtar W., Khan M.F., Tasneem S., Alam M.M. Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents. Eur. J. Med. Chem. 2015;102:487–529. doi: 10.1016/j.ejmech.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Ye Z., Gettys K.E., Dai M. Opportunities and challenges for direct C-H functionalization of piperazines. Beilstein J. Org. Chem. 2016;12:702–715. doi: 10.3762/bjoc.12.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadjeri M., Barbier M., Ronot X., Mariotte A.M., Boumendjel A., Boutonnat J. Modulation of P-glycoprotein-mediated multidrug resistance by flavonoid derivatives and analogues. J. Med. Chem. 2003;46:2125–2131. doi: 10.1021/jm021099i. [DOI] [PubMed] [Google Scholar]
- 18.Zhong H., Zhao X., Zuo Z., Sun J., Yao Y., Wang T., Liu D., Zhao L. Combating P-glycoprotein-mediated multidrug resistance with 10-O-phenyl dihydroartemisinin ethers in MCF-7 cells. Eur. J. Med. Chem. 2016;108:720–729. doi: 10.1016/j.ejmech.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Kam Y.L., Rhee S.J., Choo H.Y. Solution-phase combinatorial synthesis of nonpeptide bradykinin antagonists. Bioorg Med. Chem. 2004;12:3543–3552. doi: 10.1016/j.bmc.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 20.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 21.Chae S.W., Woo S., Park J.H., Kwon Y., Na Y., Lee H.J. Xanthone analogues as potent modulators of intestinal P-glycoprotein. Eur. J. Med. Chem. 2015;93:237–245. doi: 10.1016/j.ejmech.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Harker W.G., Sikic B.I. Multidrug (pleiotropic) resistance in doxorubicin-selected variants of the human sarcoma cell line MES-SA. Cancer Res. 1985;45:4091–4096. [PubMed] [Google Scholar]
- 23.Chae S.W., Han A.R., Park J.H., Rhie J.Y., Lim H.J., Seo E.K., Lee H.J. In vitro and in vivo evaluation of phenylbutenoid dimers as inhibitors of P-glycoprotein. J. Nat. Prod. 2013;76:2277–2281. doi: 10.1021/np4004917. [DOI] [PubMed] [Google Scholar]
- 24.Kortylewicz Z.P., Augustine A.M., Nearman J., McGarry J., Baranowska-Kortylewicz J. Radioiodinated agents for imaging multidrug resistant tumors. Med. Chem. 2009;5:171–181. doi: 10.2174/157340609787582954. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt S.M., Stefan K., Wiese M. Pyrrolopyrimidine Derivatives as Novel Inhibitors of Multidrug Resistance-Associated Protein 1 (MRP1, ABCC1) J. Med. Chem. 2016;59:3018–3033. doi: 10.1021/acs.jmedchem.5b01644. [DOI] [PubMed] [Google Scholar]
- 26.Strouse J.J., Ivnitski-Steele I., Khawaja H.M., Perez D., Ricci J., Yao T., Weiner W.S., Schroeder C.E., Simpson D.S., Maki B.E., et al. A selective ATP-binding cassette subfamily G member 2 efflux inhibitor revealed via high-throughput flow cytometry. J. Biomol. Screen. 2013;18:26–38. doi: 10.1177/1087057112456875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee K., Chae S.W., Xia Y., Kim N.H., Kim H.J., Rhie S., Lee H.J. Effect of coumarin derivative-mediated inhibition of P-glycoprotein on oral bioavailability and therapeutic efficacy of paclitaxel. Eur. J. Pharmacol. 2014;723:381–388. doi: 10.1016/j.ejphar.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Mouly S., Paine M.F. P-glycoprotein increases from proximal to distal regions of human small intestine. Pharm. Res. 2003;20:1595–1599. doi: 10.1023/A:1026183200740. [DOI] [PubMed] [Google Scholar]
- 29.Kim K.M., Lee K., Jang K., Moon Y.S., Lee H.J., Rhie S.J. Effects of Adamantyl Derivatives on Pharmacokinetic Behavior of Paclitaxel in Rats. Biomol. Ther. (Seoul) 2017;25:553–558. doi: 10.4062/biomolther.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Weger V.A., Beijnen J.H., Schellens J.H. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—A review. Anticancer Drugs. 2014;25:488–494. doi: 10.1097/CAD.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 31.Rowinsky E.K. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu. Rev. Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 32.Klecker R.W., Jamis-Dow C.A., Egorin M.J., Erkmen K., Parker R.J., Stevens R., Collins J.M. Effect of cimetidine, probenecid, and ketoconazole on the distribution, biliary secretion, and metabolism of [3H]taxol in the Sprague-Dawley rat. Drug Metab. Dispos. 1994;22:254–258. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.