Abstract
Betulinic acid (BA) and its natural analogues betulin (BN), betulonic (BoA), and 23-hydroxybetulinic (HBA) acids are lupane-type pentacyclic triterpenoids. They are present in many plants and display important biological activities. This review focuses on the chemical transformations used to functionalize BA/BN/BoA/HBA in order to obtain new derivatives with improved biological activity, covering the period since 2013 to 2018. It is divided by the main chemical transformations reported in the literature, including amination, esterification, alkylation, sulfonation, copper(I)-catalyzed alkyne-azide cycloaddition, palladium-catalyzed cross-coupling, hydroxylation, and aldol condensation reactions. In addition, the synthesis of heterocycle-fused BA/HBA derivatives and polymer‒BA conjugates are also addressed. The new derivatives are mainly used as antitumor agents, but there are other biological applications such as antimalarial activity, drug delivery, bioimaging, among others.
Keywords: triterpenes, betulinic acid, betulin, betulonic acid, 23-hydroxybetulinic acid, synthesis, functionalization, derivatization
1. Introduction
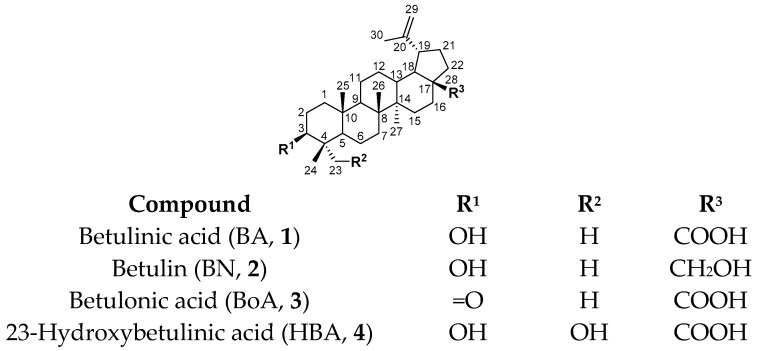
Betulinic acid [3β-hydroxylup-20(29)-en-28-oic acid, BA, 1] and its natural analogues, betulin (BN, 2), betulonic acid (BoA, 3) and 23-hydroxybetulinic acid (HBA, 4), are lupane-type pentacyclic triterpenoids (Figure 1). These triterpenes are widespread in the plant kingdom including the bark of birch trees and the rizoma of Pulsatilla chinensis (Bunge) Regel, and display important biological properties such as anticancer, antiviral, antibacterial and antimalarial activities, among others [1,2,3]. In particular, the antitumor properties of BA and its natural analogues have attracted considerable attention worldwide since many synthetic derivatives of these triterpenes have shown promising results as chemotherapeutic agents for different types of cancer [4,5,6]. An important proof of this are the patents on BA derivatives for cancer chemotherapy, which were reviewed in 2014 by Csuk [4]. Then, in 2015, Zhang et al. reported the recent research on isolation, synthesis, and derivatization of BA 1 and its natural analogues BN 2 and HBA 4, and their antitumor properties, combination treatments, and pharmacological mechanisms [5]. Additionally, Ali-Seyed et al. reviewed the anticancer activities, therapeutic efficacy, and mechanism of action of BA and its derivatives in 2016 [6].
Figure 1.
Structures and numbering system of betulinic acid (BA, 1), betulin (BN, 2), betulonic acid (BoA, 3), and 23-hydroxybetulinic acid (HBA, 4).
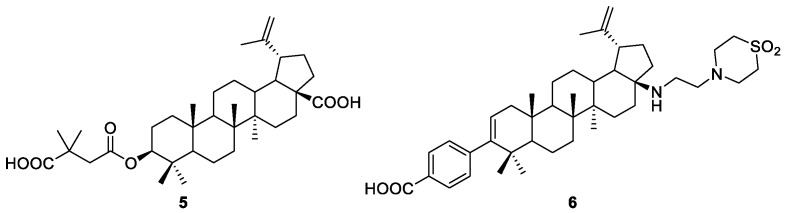
As natural BA also retains antiviral properties against human immunodeficiency virus subtype 1 (HIV-1) [1], the so-called bevirimat 5 and BMS-955176 6 (Figure 2), which are BA-derived synthetic compounds, were originally developed as anti-HIV drugs [7,8]. These compounds are HIV-1 maturation inhibitors, and both have reached phase IIb clinical trials. However, single nucleotide polymorphisms in the CA/SP1 cleavage site of the viral polyprotein have resulted in resistance to bevirimat 5, which led to the discovery of a second-generation maturation inhibitors with broad polymorphic coverage, such as BMS-955176 6. Nevertheless, the development of this compound was also discontinued by the pharmaceutical company GSK, because of gastrointestinal intolerance and treatment-emergent drug resistance by patients. The synthetic pathway to produce BMS-955176 6 became public in 2016 [8], and will be discussed later in this review.
Figure 2.
Structures of bevirimat 5 and BMS-955176 6.
Due to the proven biological properties demonstrated by these natural and synthetic triterpenes, many studies involving them have been reported in the literature. In 2014, Shi et al. published a review covering the synthesis of novel triterpenoids derived from BN 2 and BA 1 calling upon different methodologies from 2006 to 2012, excluding the synthesis of triterpenoid glycosides [9]. Other reviews deal with transformations of triterpenes in general, but also included BA and its analogues, namely recent advances in the synthesis and biological activity of triterpenic acylated oximes [10] and pentacyclic triterpenoids with nitrogen- and sulfur-containing heterocycles [11]. In addition, in 2017, Zhou et al. summarized the advances in triterpenic-based prodrug strategies, including lupane-type triterpenes [12]. More recently, Borkova and co-workers reviewed the advances in the synthesis of A-ring modified BA derivatives and their application as potential therapeutic agents [13]. Furthermore, Pokorny et al. reviewed all reports on click reaction in the chemistry of triterpenes, including a number of derivatives of BA 1 and its analogues [14].
This bibliographic appraisal is organized as follows: in Section 2, the functionalization of the referred triterpenes through simple transformations such as amination, esterification, sulfonation, and alkylation reactions are addressed. Section 3 is devoted to the synthesis of 1,2,3-triazole-linked BA/BN/BoA/HBA-based hybrid compounds by click chemistry. In Section 4, the decoration of BA/BN/BoA/HBA carbon skeletons by palladium-catalyzed cross-coupling reactions is reported. In Section 5, C(2)-hydroxy-BA/BN/BoA/HBA are prepared through hydroxylation reactions. In Section 6, traditional aldol condensation reactions performed on 3-oxotriterpenes are shown. In Section 7 and Section 8, the synthesis of heterocycle-fused BA/HBA derivatives and polymer‒BA conjugates is discussed, respectively. In Section 9, BA/BN/BoA/HBA-based compounds prepared by different methodologies, which do not fit in the previous sections, are presented, while Section 10 presents the main conclusions. Some insights about the biological properties of some of the compounds prepared through the referred methodologies will also be given throughout this review.
2. Simple Transformations
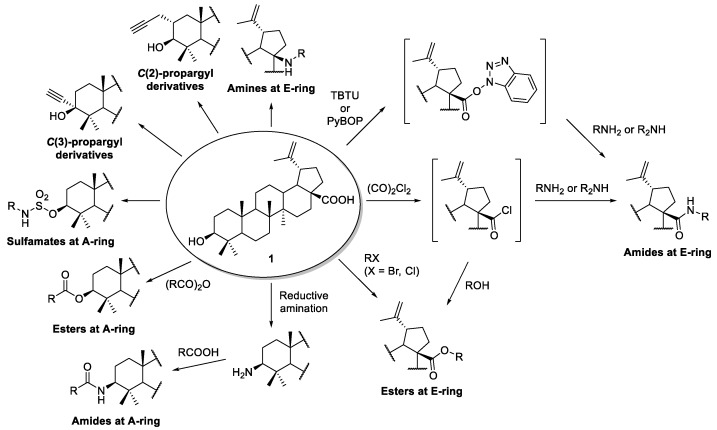
BA possesses two functional groups, namely the 3-OH and 17-COOH groups, which are prone to functionalization through simple transformations such as amination, esterification, sulfonation, and alkylation. A summary of these transformations performed on the BA skeleton is presented in Scheme 1. Many examples of these approaches were found in the literature, and the most interesting ones are discussed in subsections Section 2.1, Section 2.2, Section 2.3 and Section 2.4. In addition, many of these derivatives will be used as useful key intermediates for further functionalization, as described in the following sections.
Scheme 1.
Synthesis of BA-based amide, amine, ester, sulfamate, and alkylated derivatives (appropriate references to each transformation will be put forward along the manuscript).
2.1. Amination
2.1.1. Synthesis of Amide Derivatives
There are two main methodologies to prepare BA/BoA/HBA-based amide derivatives at position 28 [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. The first methodology is based on the formation of an acyl chloride (RCOCl, R = BA/BoA/HBA) as key intermediate using oxalyl chloride [(CO)2Cl2], followed by amination reactions with primary or secondary amines usually in the presence of a base (e.g., Et3N). This procedure allows the synthesis of amide derivatives containing 1,3,4-oxadiazoles, aminobisphosphonic units, cisplatin, and other simpler substituents as shown in the following examples.
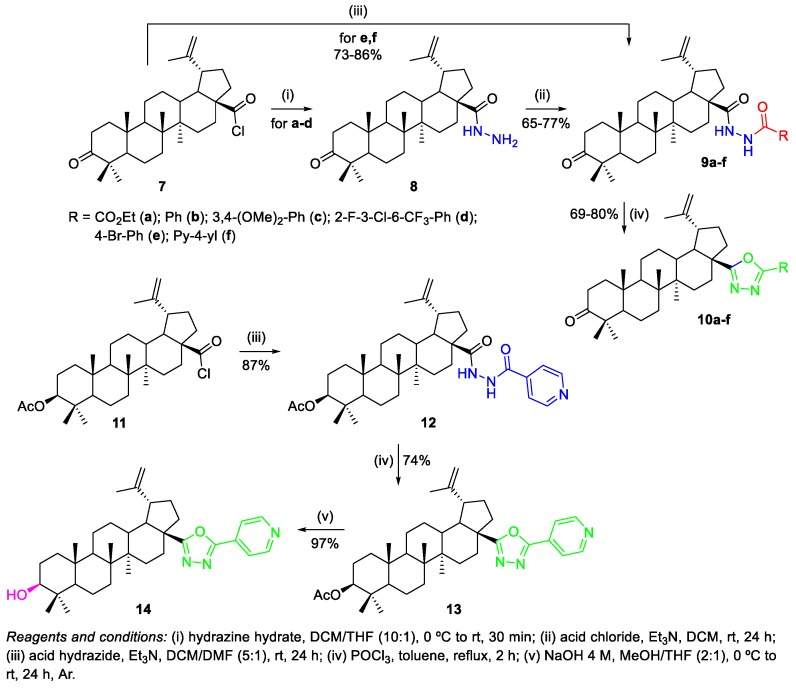
Antimonova et al. reported the synthesis of BoA and BA derivatives containing a 1,3,4-oxadiazole moiety at C-17 and studied their cytotoxic activity in three different human tumor cell models (CEM-13, MT-4, and U-937), revealing that BoA hydrazide intermediate 9d (CCID50 = 12 µM) and 1,3,4-oxadiazole derivative 10f (CCID50 = 15.4 µM) were the most active compounds, being slightly more active than the pristine BoA 3 (CCID50 = 19 µM) against U-937 tumor cells [16]. The employed methodology to prepare the referred compounds consisted in the reaction of acid chlorides (BoA chloride 7 and 3-β-O-acetyl-BA chloride 11 previously synthesized) with acid hydrazides and subsequent cyclization of the resulting acylhydrazides 9a–f and 12, affording the 1,3,4-oxadiazole derivatives 10a–f, 13, and 14 (Scheme 2).
Scheme 2.
Synthesis of BoA and BA derivatives with 1,3,4-oxadiazole substituents at C-17.
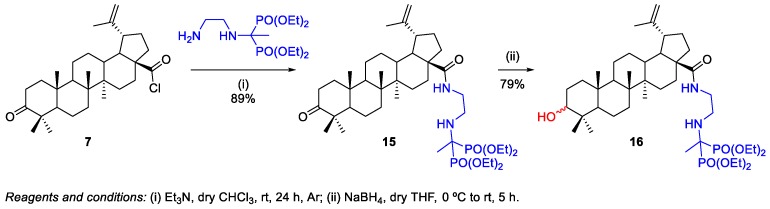
Becker et al. prepared aminobisphosphonate‒BoA 15 and aminobisphosphonate‒BA 16 conjugates, using the methodology described above, in order to obtain potential anticancer agents [23]. Conjugate 15 was synthesized in 89% yield through the reaction of BoA chloride 7 with tetraethyl 1-(2-aminoethylamino)ethane-1,1-diyldiphosphonate in the presence of Et3N (Scheme 3). Then, the reduction of compound 15 with NaBH4 in tetrahydrofuran (THF) gave bisphosphonate derivative of BA 16 in excellent yield (79%).
Scheme 3.
Synthesis of aminobisphosphonate conjugates of BoA and BA 15 and 16.
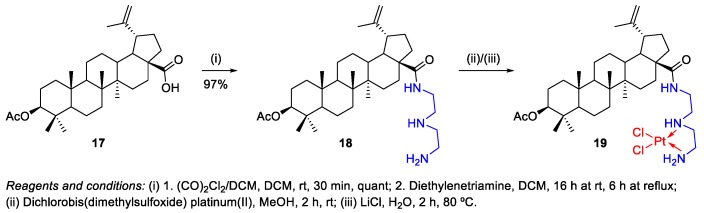
Emmerich et al. published the synthesis of a series of BA‒cisplatin complexes (Scheme 4 and Scheme 11), which were assessed for their cytotoxicity and selectivity against five different tumor cell lines (A549, A2780, 8505C, 518A2 and MCF-7), revealing that the synthesized conjugates 19 (IC50 = 11.57–17.32 µM) and 64 (IC50 = 13.13–29.83 µM) showed similar cytotoxicity to the pristine BA 1 (IC50 = 8.75–14.8 µM) and in all the cases, these complexes were found to be less cytotoxic than cisplatin (IC50 = 0.33–1.34 µM) against all the used tumor cells [17]. The synthetic methodology used to obtain the BA‒cisplatin complexes started with the preparation of the alkyl amide 18 by the reaction of the acid chloride of 3-O-acetyl-BA 17 with diethylenetriamine in dichloromethane (DCM) (Scheme 4). Then, this intermediate 18 was used as ligand in the complexation with dichlorobis(dimethylsulfoxide) platinum(II) in methanol (MeOH), followed by reaction of the appropriate dimethylsulfoxide (DMSO) platinum complexes with an aqueous LiCl solution, affording the BA‒cisplatin conjugate 19.
Scheme 4.
Synthesis of 3-O-acetyl-BA‒cisplatin complex 19.
Simpler amide derivatives were prepared through the referred methodology, starting from 23-O-acetyl-3-oxo-HBA 20 [18] and 3-O-acetyl-BA 17 [15,19] (Scheme 5).
Scheme 5.
Alkyl amide derivatives of 23-O-acetyl-3-oxo-HBA 20 and 3-O-acetyl-BA 17.
The second methodology commonly used to prepare BA-based amide derivatives involves the use of a coupling reagent such as (benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate (PyBOP) and N,N,N’,N’-tetramethyl-O-(benzotriazol-1-yl)uronium tetrafluoroborate (TBTU), which creates intermediates with a benzotriazole-leaving group that easily undergo amination reactions with different amines.
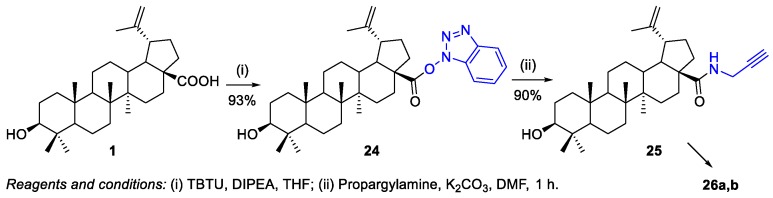
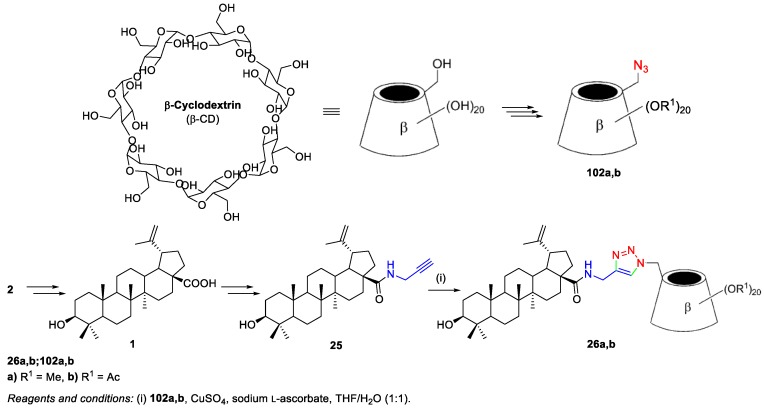
Xiao et al. used this procedure to prepare compound 25 [20] (i.e., through the activation of the carboxyl group of BA 1 with TBTU to obtain the stable intermediate 24), which was then treated with propargylamine under basic conditions to afford derivative 25 (Scheme 6). This compound, bearing a terminal triple bond, underwent a click reaction with cyclodextrin azides in order to prepare triazole-bridged β-cyclodextrin (β-CD)‒BA conjugates 26a,b, as will be shown in Scheme 21.
Scheme 6.
BA-based amide derivative 25 as intermediate in the synthesis of triazole-bridged β-CD‒BA conjugates.
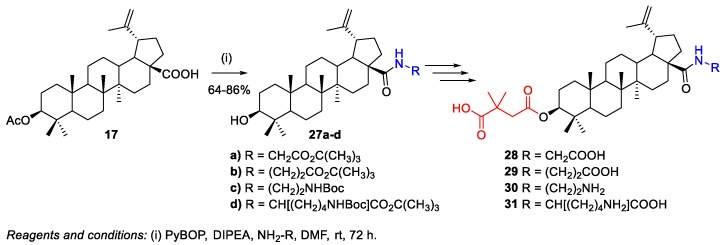
Coric et al. reported the synthesis of bevirimat derivatives 28–31 containing various substituents at C-28 in order to obtain conjugates with improved water solubility [21]. Compound 30 showed a higher hydrosolubility associated with a 2.5-fold increase in anti-HIV-1 activity (IC50 = 0.016 µM) compared with bevirimat 5 (IC50 = 0.040 µM). Different hydrophilic substituents were introduced in 3-O-acetyl-BA 17 by one-pot peptide coupling with PyBOP and N,N-diisopropylethylamine (DIPEA) in the presence of an appropriate amine, affording the intermediates 27a–d in 64%–86% yield (Scheme 7). After three additional steps, bevirimat derivatives 28–31 were obtained.
Scheme 7.
Synthesis of bevirimat derivatives 28–31.
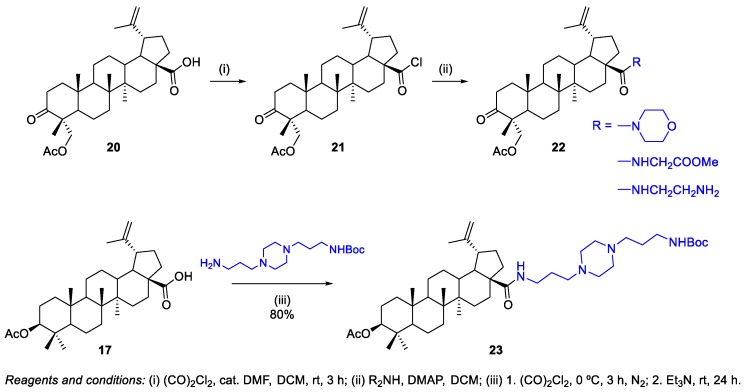
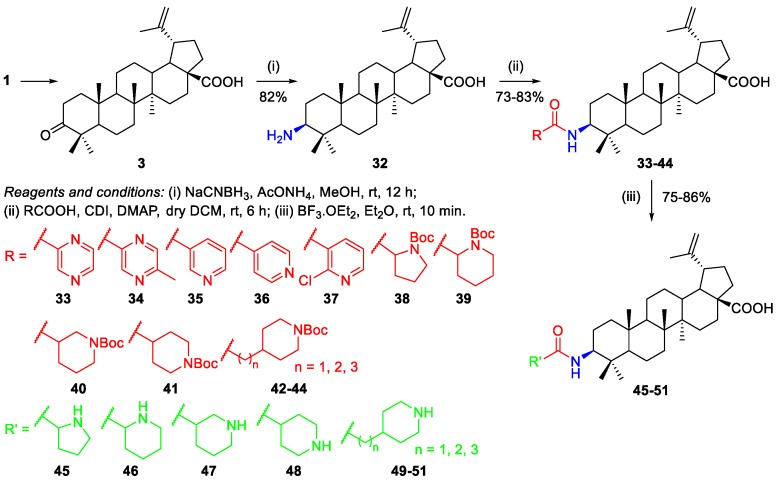
A different methodology was employed to prepare BA-based amide derivatives at C-3 [31]. The key intermediate 32, a BA-based primary amine, was obtained in very good yield (82%) by Borch reduction of BoA 3 with sodium cyanoborohydride and ammonium acetate in methanol (Scheme 8). Then, the authors found that N,N’-carbonyldiimidazole (CDI) was the optimal condensation agent and provided compounds 33–37 and intermediates 38–44 in good yields (73%–83%). Intermediates 38–44 were converted into compounds 45–51 by deprotection of the tert-butyloxycarbonyl (Boc) group in the presence of mild boron trifluoride etherate. Furthermore, the synthesized compounds were investigated for their activity against the growth of eight non-drug resistant and one multidrug-resistant tumor cell lines. Compound 48 was the most active one (IC50 = 0.33–2.45 µM), being on average 20-fold more potent than the parent BA 1 (IC50 = 14.04–38.50 µM).
Scheme 8.
Synthesis of a series of BA derivatives containing nitrogen heterocycles at C-3, linked through amide linkages.
2.1.2. Synthesis of Amine Derivatives
Amination reactions have also been used to prepare BA-based amine derivatives [8,32,33], as shown in the following examples.
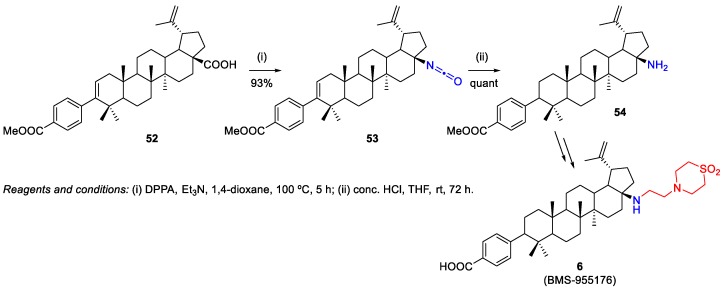
Regueiro-Ren et al. reported the synthesis and preclinical characterization of the potent and orally active BMS-955176 6, a second generation HIV-1 maturation inhibitor [8]. Its synthetic procedure involved a Suzuki coupling at C-3 starting from BA 1, which will be described in Scheme 24, and a Curtius rearrangement using diphenyl phosphoryl azide (DPPA) to afford the C-17 primary amine 54 (Scheme 9). This method could be carried out either in a single step or stepwise via isolation of the corresponding C-28 isocyanate 53.
Scheme 9.
Synthesis of BMS-955176 6, a second generation HIV-1 maturation inhibitor [8].
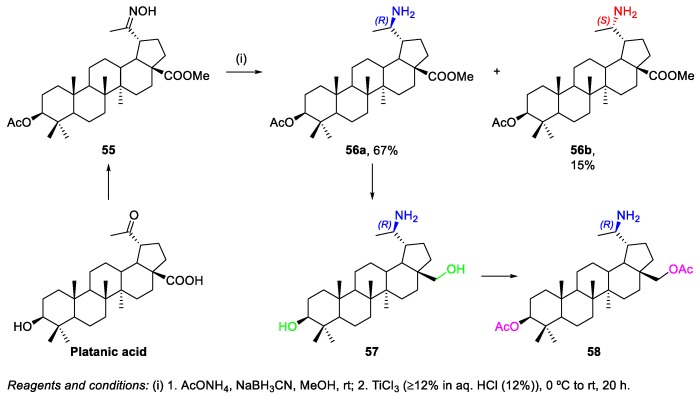
Kahnt et al. prepared the BN-derived amine 58, starting from platanic acid, which is the C(20)=O BA analogue [32]. The reduction of oxime 55 with NaBH3CN/TiCl3/NH4OAc in methanol gave the amines 56a,b in 67% and 15% yields, respectively (Scheme 10); these compounds were separated by chromatography, and their absolute configuration at C-20 was determined using 1H NMR spectroscopy. Then, BA-derived amine 56a was converted into BN-derived amine 58 in a two-step sequence. Furthermore, the synthesized 20-amino derivatives were screened for their cytotoxicity against five human cancer cell lines [32]. Derivative 58 was the most active compound (EC50 = 2.1–4.0 µM), being more potent than deprotected 20-amino-BN derivative 57 (EC50 = 16.0 to >30 µM) and even parents platanic acid (EC50 > 30 µM) and BA 1 (EC50 = 11.0–18.9 µM).
Scheme 10.
Synthesis of amines 56a,b, 57, and 58.
2.2. Esterification
Positions 3 and 28 of BA skeleton are easily functionalized through esterification reactions and, consequently, many studies involving this transformation have been reported [17,22,27,31,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. The used methodologies involve: (i) The reaction of 17-COOH group of BA 1 with alkyl halides in the presence of a base (mainly, K2CO3), or (ii) the reaction of BA-based acyl chloride intermediate with alcohols, or even (iii) the reaction of 3-OH group of BA 1 with acid anhydrides in the presence of a catalyst [usually, 4-(dimethylamino)pyridine (DMAP)] (Scheme 11). Some of these reports will be highlighted in the following examples.
Scheme 11.
Synthesis of BA- and BoA-based ester derivatives [17,34,35,36,37,38,39].
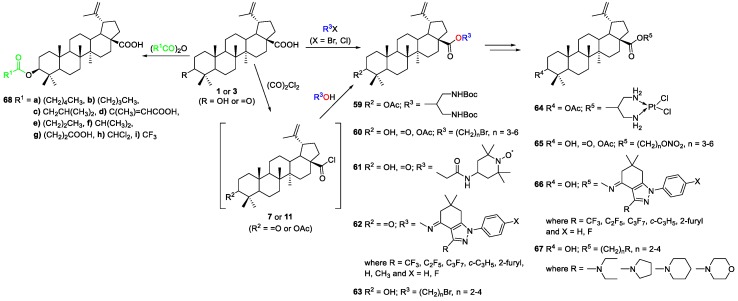
Emmerich et al. prepared a new series of BA‒cisplatin conjugates, as shown previously in Scheme 4 [17]. The synthesis of these conjugates also involved an esterification reaction. It started with the reaction of 3-O-acetyl-BA 17 with oxalyl chloride, obtaining the BA-based acyl chloride 11 intermediate, which then underwent reaction with N,N’-(Boc)2-1,3-diaminopropan-2-ol in the presence of Et3N, in DCM, affording the [1,3-(t-butylcarboxyamino)-2-propyl] 3-O-acetylbetulinate 59 in quantitative yield. After two subsequent steps, the authors obtained the BA‒cisplatin conjugates 64 (Scheme 11).
Liu et al. reported the connection of BA and nitric oxide donors via different linkers in order to prepare NO‒BA hybrids 65 and to improve the antitumor activity of BA [34]. However, the results of their biological activity showed that none of them revealed cytotoxicity against human hepatocellular carcinoma (HepG2) cells in vitro. Regarding their synthesis, BA 1 was first modified with an appropriate dibromoalkane in the presence of K2CO3, in DMF to give the corresponding C-28 esters 60 in good yields (63%–70%), which then were transformed into compounds 65 (Scheme 11).
Da Silva and co-workers performed esterification reactions at C-3 of BA in an attempt to improve its antimalarial activity, reduce cytotoxicity, and search for new targets [35]. The authors synthesized a series of BA analogues bearing ester substituents at C-3 68a–i through the reaction of BA 1 with appropriate substituted acid anhydrides in the presence of DMAP (Scheme 11). The BA derivatives with ester substituents at C-3 68a–i were obtained in poor to quantitative yields (24%–100%). Moreover, derivatives 68e (IC50 = 5 µM) and 68f (IC50 = 8 µM) were the most effective against CQ-sensitive Plasmodium falciparum 3D7, and were non-cytotoxic against a HEK293T cell line, being two to four times more active than BA 1 (IC50 = 18 µM).
Popov et al. reported the preparation of radical-containing substituted esters of triterpenic acids [36]. Thus, the reaction of BoA 3 and BA 1 with 4-(2-chloroacetamido)-2,2,6,6-tetramethylpiperidin-1-oxyl in the presence of K2CO3, in DMF at room temperature, formed substituted esters of the referred triterpenic acids with the nitroxyl radical 4-amino-2,2,6,6-tetramethylpiperidin-1-oxyl 61 in very good yields (81%) (Scheme 11).
Khlebnicova et al. synthesized novel BA‒indazolone hybrids 66 with an oxime ester linkage [37]. A series of BoA‒indazolone hybrids 62 were obtained in 33%–83% yield via alcoholysis of the BoA-based acyl chloride intermediate 7 with 6,7-dihydro-1H-indazol-4(5H)-one oximes (Scheme 11). After diastereoselective reduction of the obtained BoA conjugates 62, the BA‒indazolone hybrids 66 were obtained in excellent yields (94%–97%).
Saha and co-workers reported the synthesis, characterization, and tumoricidal potential of a co-drug 68h [39]. This ester derivative of BA 68h was synthesized in good yield (75%) by treating BA 1 with dichloroacetyl chloride in the presence of pyridine (Py) and DMAP, in dry DCM and under N2-atmosphere (Scheme 11). In vitro studies revealed high cytotoxicity (IC50 = 9.46–14.4 µM) and selectivity of 68h against several cancer cells (MCF-7, MDA-MB-231, MDA-MB-468, DU-145, PC-3, and B16-F10) in comparison with BA 1 (IC50 = 51.29–70 µM). In addition, in vivo studies exhibited tumor inhibitory potential of 68h, and clinically achievable doses did not produce any apparent toxicity.
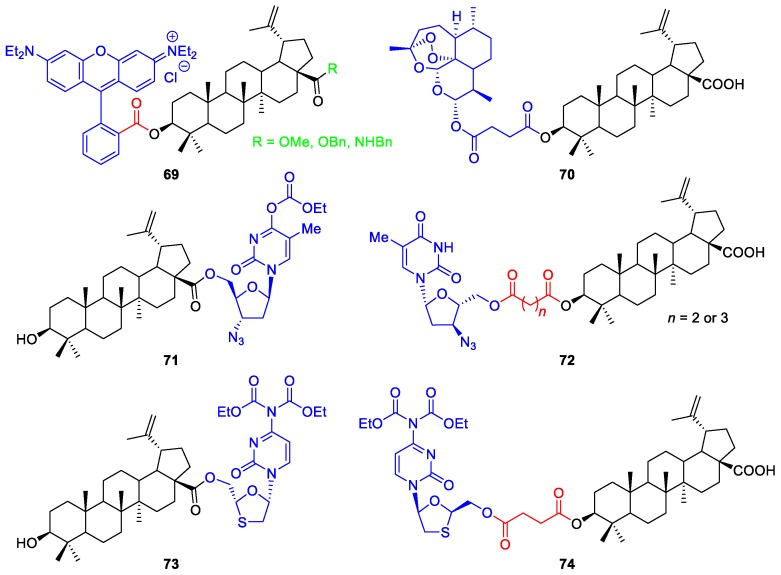
Other interesting examples involving esterification reactions are the synthesis of esters of rhodamine B with triterpenoids 69 [44], an artesunic acid–BA hybrid 70 [40], and ester-linked conjugates of BA with anti-HIV drugs [azidothymidine (AZT) and lamivudina (3TC)] 71–74 [43] (Figure 3).
Figure 3.
Chemical structures of BA esters derivatives bearing a rhodamine B moiety 69, an artesunic acid unit 70, and anti-HIV drugs 71–74.
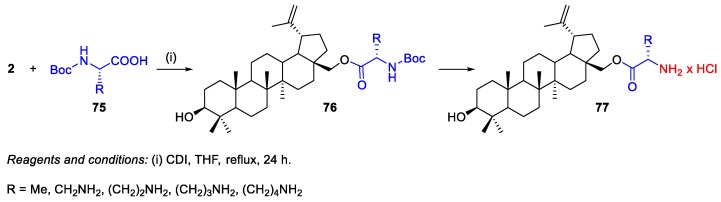
BN 2 was also used as starting material to prepare ester derivatives [46,48,49]. For instance, BN derivatives bearing amino acids 77 such as alanine (Boc-L-Ala-OH), lysine [Boc-L-Lys(Boc)-OH], and three of its unnatural derivatives [2,3-diaminopropionic acid, Boc-L-Dap(Boc)-OH; 2,4-diaminobutyric acid, Boc-L-Dab(Boc)-OH; and ornithine, Boc-L-Orn(Boc)-OH] were synthesized by Drąg-Zalesińska and co-workers [48]. The general procedure for the synthesis of these five amino acid esters of BN 77 consisted in the reaction between BN 2 and Boc-protected amino acids 75 in the presence of CDI, in THF at reflux (Scheme 12). The obtained BN esters present one (BN‒L-Ala-NH2) or two free amino groups, which gives them improved properties such as increased solubility in water and easy transportation through the cell membrane. In addition, their in vitro cytotoxicity was tested in human epidermoid carcinoma cells (A431), revealing enhanced antitumor activity compared to BN 2 (IC50 = 80.2 µM) [48]. In particular, the highest cytotoxicity was achieved by compounds bearing Lys (IC50 = 7.3 µM) and Orn (IC50 = 10.1 µM) side chains. Furthermore, incubation with compounds containing Orn, Dab, and Dap side chains led to the highest number of apoptotic cells.
Scheme 12.
Synthesis of monosubstituted BN esters containing L-amino acids 77.
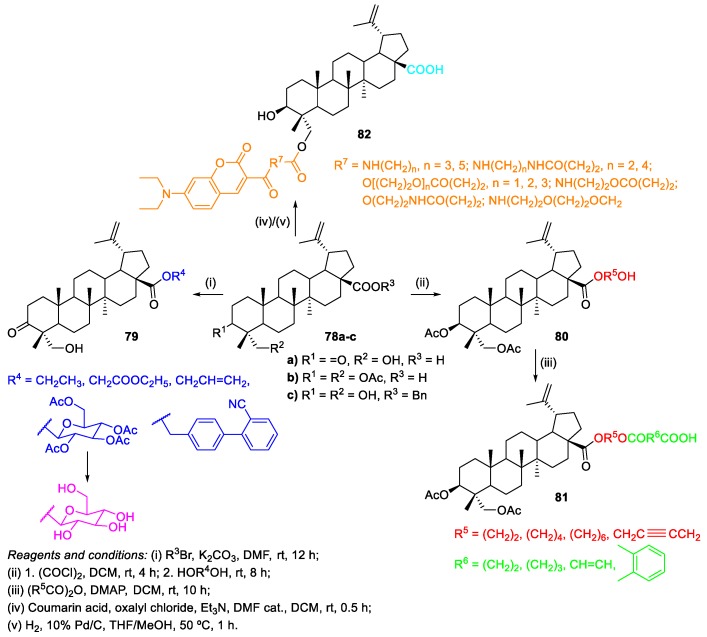
HBA 4 was also used to perform esterification reactions [18,50,51]. Zhang et al. prepared C-28 ester derivatives of HBA bearing different substituents 79 by the treatment of 3-oxo-HBA 78a with appropriate halides under basic conditions (Scheme 13) [18].
Scheme 13.
Synthesis of HBA-based ester derivatives.
Furthermore, Bi et al. synthesized HBA C-28 ester derivatives 81 as antitumor agent candidates [50]. The target compounds were evaluated for their antitumor activities in vitro against five cancer cell lines (A549, BEL-7402, SF-763, B16 and HL-60). Among the synthesized compounds, the derivative bearing the substituent –O(CH2)6OCO(CH2)3COOH had the most potent antitumor activity (IC50 = 8.35–14.05 µM), in comparison with HBA 4 (IC50 = 75.64-90.09 µM), and showed similar antitumor activity in vivo to cyclophosphamide in H22 liver tumor and to 5-fluorouracil in B16 melanoma. Regarding the synthesis of the desired HBA C-28 ester derivatives 81, they were readily prepared in two steps, starting from 3,23-O-diacetyl-HBA derivative 78b (Scheme 13). The first step involved the formation of HBA-based acyl chloride with oxalyl chloride, followed by the reaction of this intermediate with an appropriate alcohol. Then, these compounds 80 were used as starting materials for the preparation of 81.
Another interesting report involving esterification reactions at 23-OH group of HBA 4 was published by Yao et al. [51]. The authors synthesized a series of fluorescent HBA derivatives conjugated with coumarin dyes 82 and evaluated them for their antiproliferative activity. The HBA probe bearing –NH(CH2)2NHCO(CH2)2– as substituent was the most antiproliferative compound (IC50 = 4.07 and 6.26 µM) against two tumor cell lines (B16F10 and MCF-7), having been further studied for live cell imaging in order to elucidate the mechanisms of anticancer action of HBA 4. The synthesis of the fluorescent HBA probes 82 consisted in the reaction of the HBA benzyl ester derivative 78c with the acyl chloride derivatives of coumarin dyes (which were prepared in situ using oxalyl chloride and DMF as catalyst) in the presence of Et3N, in dry DCM, followed by debenzylation with 10% Pd/C as catalyst under atmospheric pressure of hydrogen (Scheme 13). The desired compounds 82 were obtained in poor to fair yields (20%–60%).
2.3. Sulfonation
Sulfonation reactions are another example of a simple transformation, which demonstrate that simple modifications of the parent structures of BA 1 and its analogues result in highly potent derivatives as anticancer agents [52,53].
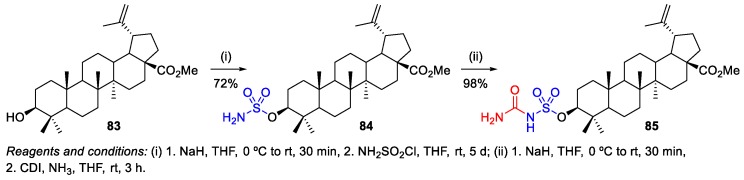
Sommerwerk et al. synthesized and evaluated the cytotoxic activity of several methyl esters of natural triterpenic acids, including BA 1 [52]. Sulfamate 84 was evaluated for its cytotoxic activity against six human tumor cell lines (518A2, FaDu, HT-29, MCF-7, A549, and SW1736). Although this sulfamate has demonstrated high cytotoxic activity (EC50 = 6.7–10.1 µM), it was less active than the corresponding C(3)-sulfamate of methyl maslinoate (EC50 = 2.9–4.0 µM). The employed methodology consisted in the conversion of methyl betulinate 83 into its corresponding sulfamate 84 by deprotonation of the 3-hydroxyl group followed by the addition of freshly prepared sulfamoyl chloride (Scheme 14). Then, the sulfamate 84 was treated with sodium hydride, CDI, and ammonia, in THF, leading to the formation of the corresponding carbamoylsulfamate 85 in excellent yield.
Scheme 14.
Synthesis of methyl triterpenoates derived from natural triterpenic acids.
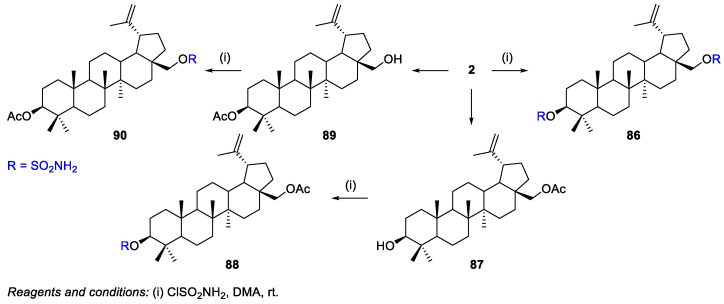
A series of betulinyl sulfamates 86, 88, and 90 have been synthesized and evaluated for their in vitro anticancer activity and carbonic anhydrase IX (CAIX) inhibition (an attractive target for tumor-selective therapy strategies in cancer cells) [53]. The positions 3 and 28 of BN were modified by the reaction of BN 2 with sulfamoyl chloride, affording a di-substituted BN-sulfamate derivative 86 and mono-substituted derivatives 88 and 90 (Scheme 15). The presence of different substitution patterns allow the establishment of structure-activity relationships [53]. Among the synthesized sulfamates, C(28)-mono-substituted derivative 90 (IC50 = 4.87–9.94 µM) was the most cytotoxic compound against five tumor cell lines (518A2, 8505C, A2780, MCF-7, and A549) and possessed high inhibitory activity towards CAIX (Ki = 1.25 nM).
Scheme 15.
Synthesis of sulfamate–BN conjugates.
2.4. Alkylation
Another example of a simple transformation that has been very useful to functionalize BA 1 is alkylation reactions [54,55,56,57]. The obtained C(2)- or C(3)-alkylated compounds were used as important intermediates to prepare more complex ones as shown in the following sections.
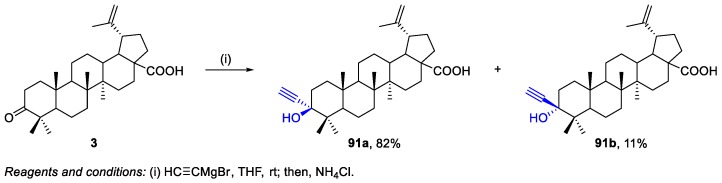
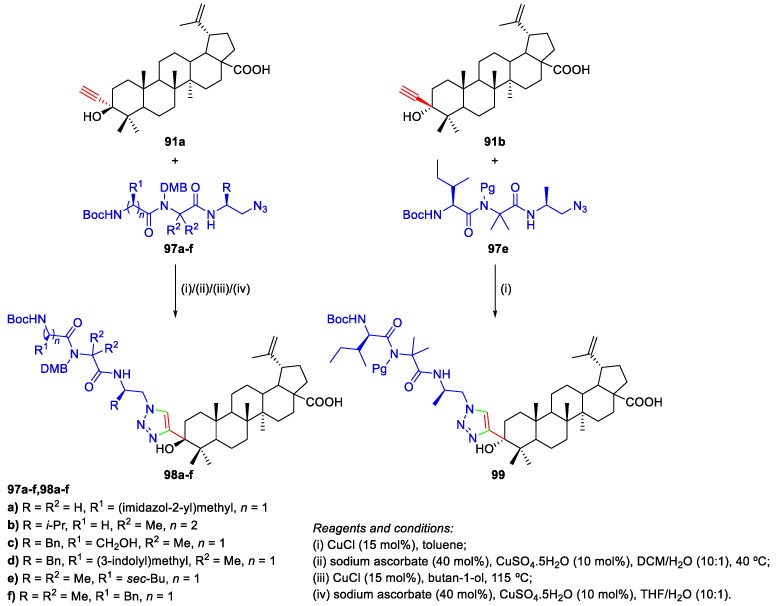
Govdi et al. prepared BA derivatives 91a,b bearing an alkyne group at C-3 via the reaction of ethynylmagnesium bromide with BoA 3 (Scheme 16) [54]. This reaction afforded the major diastereomer 91a with the axially oriented alkynyl group, in 82% yield, together with the minor isomer 91b, having the equatorially oriented ethynyl group (11% yield). These compounds were used as starting materials to synthesize new BA derivatives containing 1,2,3-triazole peptide fragments at C-3, as shown in Scheme 19.
Scheme 16.
Synthesis of 3-ethynyl-BA derivatives 91a,b.
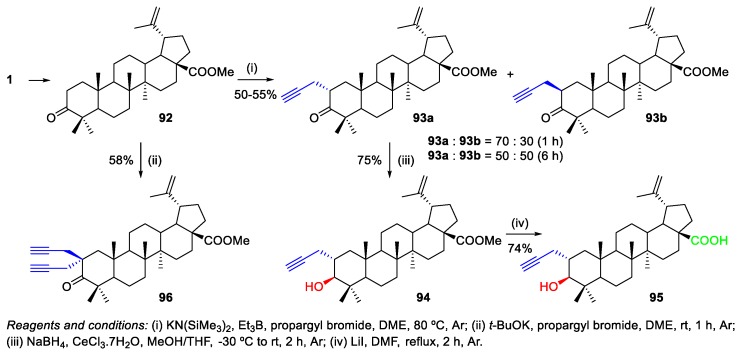
Spivak et al. developed a chemoselective method for the synthesis of C(2)-propargyl-BA derivatives (Scheme 17) and the subsequent transformation of these compounds into conjugates with 1,2,3-triazole glucopyranosides via click chemistry (Scheme 22) [55]. These authors performed the reaction of propargyl bromide with the enolate formed by treating methyl betulonate 92 with KN(SiMe3)2‒Et3B in 1,2-dimethoxyethane (DME) at room temperature (Scheme 17). Over a short period of time (1 h), diastereomer 93a with the equatorial-oriented propynyl group was the major product obtained from this reaction. Then, C(2)-alkynyl BA derivative 95 was synthesized from 93a in two-steps. In addition, elimination of the methine H-2 proton in 92 induced by potassium t-butoxide (t-BuOK) in DME gave rise to potassium enolate, which reacted with an excess of propargyl bromide to give 2,2-bis-alkylated product 96 in 58% yield (Scheme 17).
Scheme 17.
Synthesis of C(2)-propargyl-BA derivatives.
3. Click Chemistry—Copper-Catalyzed Azide-Alkyne Cycloaddition
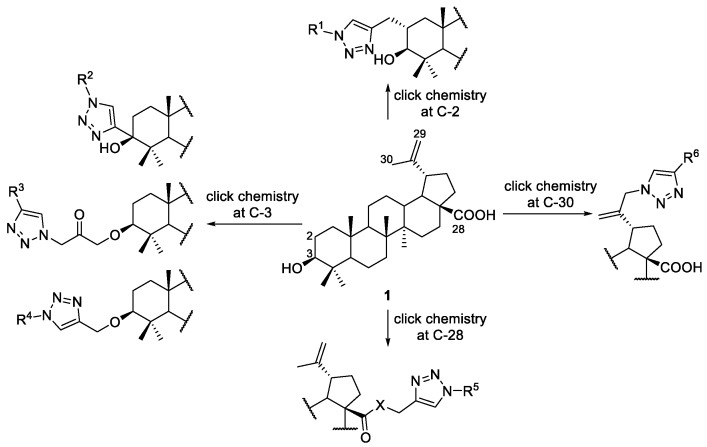
The copper-catalyzed azide-alkyne cycloaddition (CuAAC) is one of the most used click reactions to date [58]. This cycloaddition reaction gives 1,2,3-triazoles and has been widely used to synthesize 1,2,3-triazole-linked BA derivatives [14] (Scheme 18). This methodology allowed the functionalization of BA at positions 2 [55,59], 3 [54,60,61,62], 28 [20,26,63,64,65,66,67,68,69,70], and 30 [71,72,73], with different substrates, including peptides, approved drugs as AZT, β-cyclodextrins, sugars, and other triterpenes, as shown in the following examples.
Scheme 18.
Functionalization of BA at C-2, C-3, C-28, and C-30 by CuAAC.
Govdi et al. reported the synthesis of new BA–peptide conjugates linked through a 1,2,3-triazole moiety using the CuAAC methodology (Scheme 19) [54]. These authors prepared the required BA derivatives bearing an alkyne group at C-3 91a,b (Scheme 16) and, then, these compounds reacted with azidopeptides 97a–f to give the desired conjugates 98a–f and 99 in 60%–88% and 73% yield, respectively. All obtained compounds were tested for their anti-inflammatory activity. In particular, BA–peptide conjugates 98a,b,d,e were found to exhibit high anti-inflammatory activity, comparable to that of indomethacin (a nonsteroidal anti-inflammatory drug), and the introduction of these amino acid residues in BA core enhanced its anti-inflammatory properties by 14.3%–32.4% [54].
Scheme 19.
Synthesis of BA–peptide conjugates 98a–f and 99.
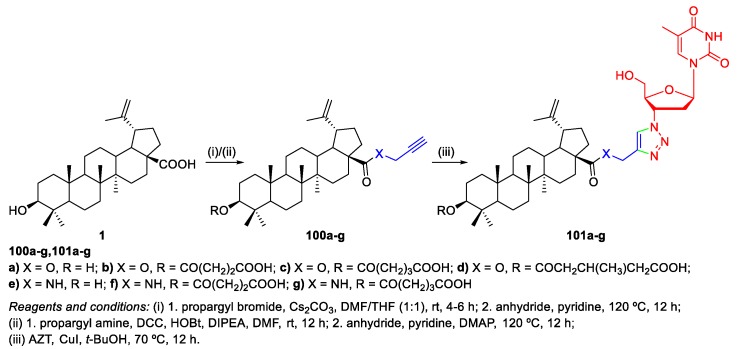
Thi et al. described a synthetic approach toward novel triazole-linked BA–AZT hybrids 101a–g (Scheme 20) [63,66]. Once again, the used methodology consisted on a Cu(I)-catalyzed 1,3-cycloaddition between propargyl-substituted BA derivatives 100a–g and AZT (an antiretroviral drug used in the treatment of HIV/AIDS). The synthesized BA–AZT hybrids 101a–g were studied for their anticancer activity and revealed moderate to good cytotoxicity against two human tumor cell lines (KB and Hep-G2). In addition, amide–triazole-linked hybrids seemed to be less potent than the corresponding ester–triazole-linked analogues. For instance, derivative 101a (IC50 = 5.9 and 7.0 µM) showed higher cytotoxicity than 101e (IC50 = 126.1 and 92.5 µM). The parent pharmacophores BA (IC50 = 27.5 and 23.9 µM) and AZT (IC50 > 479 µM) also showed considerably less potent cytotoxic activities as compared to the most promising BA–AZT conjugate 101a.
Scheme 20.
Synthesis of BA–AZT hybrids 101a–g.
Xiao et al. synthesized a series of water-soluble 1,2,3-triazole-bridged β-cyclodextrin–BA conjugates via click chemistry (Scheme 21) [20]. Firstly, they prepared BA 1, starting from BN 2, and then, the propargyl-substituted BA derivative 25 (whose synthesis was describe in Scheme 6) underwent a click reaction with the previously prepared azido-substituted β-CDs 102a,b. The BA–β-CDs conjugates 26a,b were obtained in 72% and 79% yield, respectively. These conjugates were synthesized as a new class of anti-hepatitis C virus (HCV) entry inhibitors, exhibiting greater activity (%inhibition = 81.7% and 98.2%) than parent BA 1 (%inhibition = 65.2%).
Scheme 21.
Synthesis of BA–β-CD conjugates 26a,b.
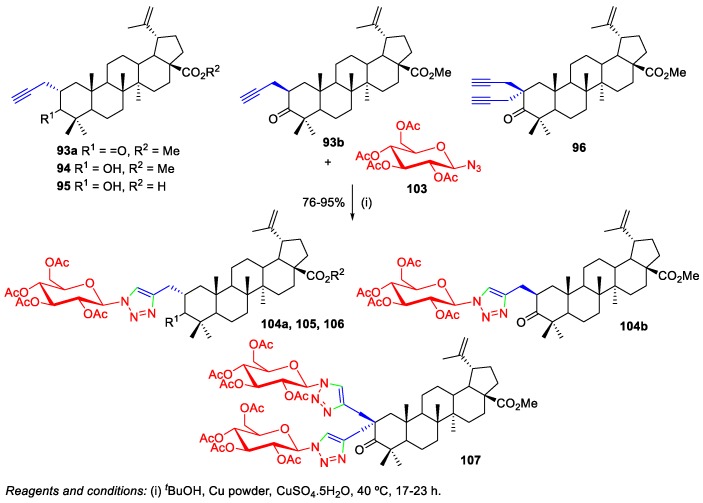
The use of sugars as substrates to perform click chemistry bioconjugation with BA can also be found in the literature [55,60]. Spivak et al. reported the Cu(I)-catalyzed 1,3-dipolar cycloaddition reaction of C(2)-propargyl-substituted BA derivatives (93a,b and 94–96) and the azide-β-D-glucopyranoside 103 to prepare 1,2,3-triazole-containing BA–glucopyranosides conjugates 104a,b and 105–107 (Scheme 22) [55]. The synthesis of the required alkynyl BA derivatives 93a,b and 94–96 was performed via α-alkylation of methyl betulonate with propargyl bromide and was discussed in Section 2.4 of this work.
Scheme 22.
Synthesis of 1,2,3-triazole-containing BA–glucopyranosides conjugates 104a,b and 105–107.
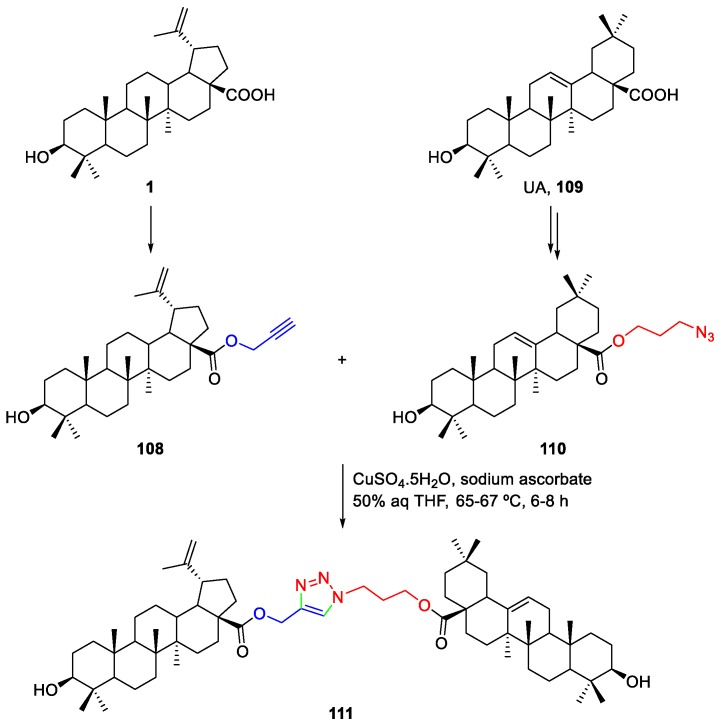
Another interesting example of the use of click chemistry to functionalize BA was reported by Pattnaik et al. [65]. The authors synthesized several triterpenoid dimers linked through a 1,2,3-triazole moiety (Scheme 23). Thus, this synthesis consisted of two major components, such as the propargyl ester of BA 108 and the ursolic acid (UA, 109) C-28 acyl azide 110. Then, the click reaction of both in the presence of copper sulfate and sodium ascorbate, in a mixture of THF and water, afforded the BA–UA dimer 111 in very good yield (84%). Its cytotoxicity against two human breast cancer cell lines (MCF-7 and MDA-MB-231) was evaluated, revealing that dimer 111 is a potent anticancer agent (GI50 = 1.4 µM), showing 2.9-fold more activity that the standard doxorubicin (GI50 = 4.1 µM) against MDA-MB-231.
Scheme 23.
Synthesis of BA–UA triazole dimer 111.
4. Palladium-Catalyzed Cross-Coupling Reactions
The presence of functional groups such as esters or amides in a drug structure can be a problematic issue since C‒O or C‒N bonds are easily hydrolyzed. Thus, Pd-catalyzed cross-coupling reactions are a useful methodology which allow the functionalization of BA and its analogues through C‒C bond formation, as shown in the following examples.
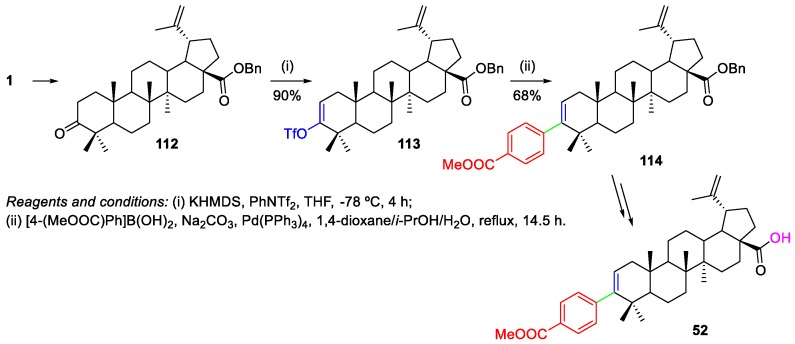
There are only two reports on the functionalization of BA through Pd-catalyzed cross-coupling reactions after 2012. Regueiro-Ren et al. modified the BA skeleton at position 3 by a Suzuki coupling (Scheme 24) [8]. These authors oxidized the 3-OH group to ketone 112 with pyridinium chlorochromate (PCC) and, then, it was converted into the triflate derivative 113, using phenyl triflimide and a strong base. Suzuki coupling of 113 with [4-(methoxycarbonyl)phenyl]boronic acid in the presence of tetrakis(triphenylphosphane)palladium(0) as catalyst afforded the coupling product 114, which was transformed into BA derivative 52 after two additional steps. 52 was used as starting material to prepare BMS-955176 6 as shown in Scheme 9.
Scheme 24.
Suzuki cross-coupling reaction of a BA triflate 113 with a boronic acid.
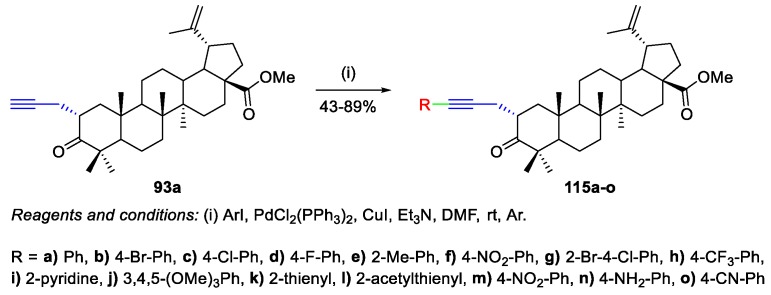
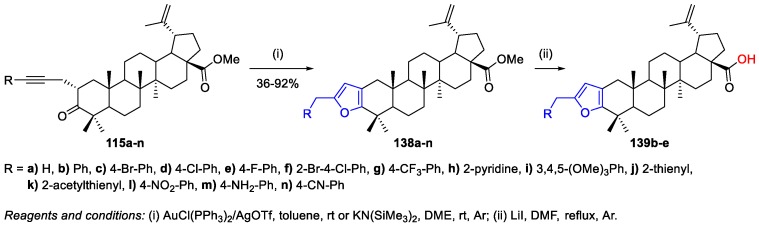
Gubaidullin et al. prepared C(2)-alkynyl derivatives of BoA, presenting several aryl and heterocyclic substituents [74,75] and starting from C(2)-propargyl derivative 93a with an equatorial-oriented α-propynyl group (Scheme 17). The products 115a–o were obtained in fair to very good yields (43%–89%) by Sonogashira reactions in the presence of bis(triphenylphosphane)palladium(II) dichloride, copper(I) iodide, and triethylamine (Scheme 25). Then, the obtained products 115a–o suffered heterocyclization in order to produce [3,2-b]furan-substituted BA derivatives, as will be shown in Scheme 33.
Scheme 25.
Synthesis of C(2)-alkynyl-BoA derivatives 115a–o.
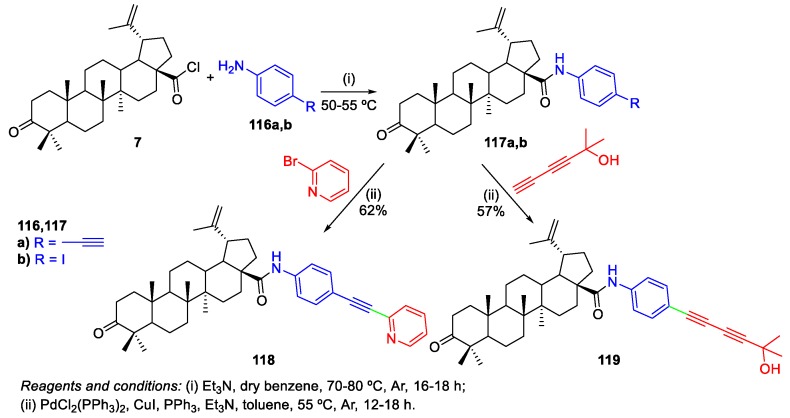
However, a work involving Sonogashira reactions for the preparation of acetylenic derivatives of BoA was published in 2009 and was not referred in the last review about this topic. Vasilevsky et al. prepared acetylene-containing BoA derivatives 118 and 119 by selective Pd-catalyzed cross-coupling reactions (Scheme 26) [25].
Scheme 26.
Synthesis of acetylenic derivatives of BoA 118 and 119 through Sonogashira reactions.
5. Hydroxylation
Hydroxylation reactions are typically defined as chemical transformations which consist in the introduction of one or more hydroxyl groups (-OH) into an organic compound. Compounds bearing hydroxyl groups (hydrogen bond donors/acceptors) present many improved physicochemical properties, especially higher hydrophilicity, than compounds lacking this functional group (e.g., sugars and amino acids). In addition, hydroxy-containing compounds can be useful to bind with enzymatic receptors and transport proteins and, also, can be esterified in order to produce prodrugs [76]. Some authors modified BA and its analogues by introducing a hydroxyl group at C-2 in order to obtain useful intermediates for the synthesis of new derivatives (shown in the following examples), which were assessed for their antitumor activity [18,32,77].
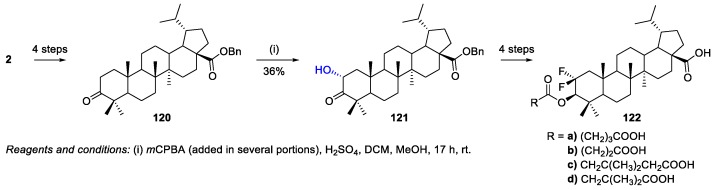
Benzyl dihydrobetulonate 120 was prepared through a four-step process with an overall yield of 64%, starting from BN 2 [77]. Then, this compound was oxidized by m-chloroperoxybenzoic acid (mCPBA) to give benzyl 2α-hydroxydihydrobetulonate 121 in 36% yield (Scheme 27), which was used as intermediate for the synthesis of 2,2-difluorodihydrobetulinic acid ester derivatives 122a–d.
Scheme 27.
Synthesis of benzyl 2α-hydroxydihydrobetulonate 121.
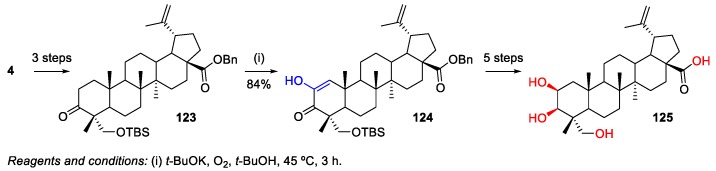
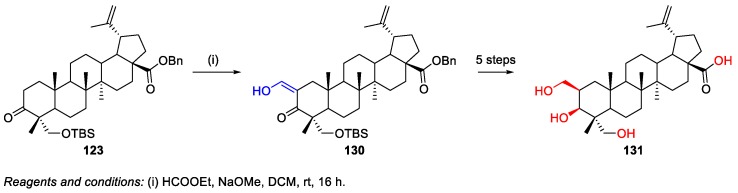
The oxidation of 3-oxo-HBA derivative 123, which was synthesized from natural HBA 4, with potassium t-butoxide in t-butyl alcohol furnished C-2 enol derivative 124 in 84% yield (Scheme 28). After deprotection, acetylation, and hydrogenation (five-step process), this compound was converted into 2-hydroxy-HBA 125 [18].
Scheme 28.
Synthesis of 2-hydroxy-HBA 125.
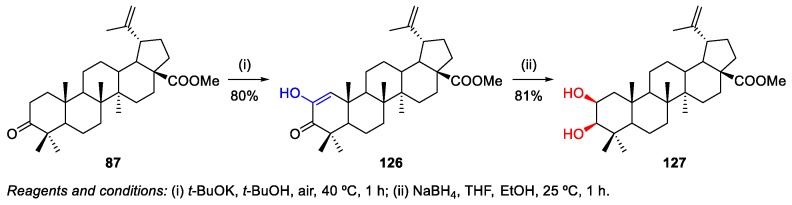
Through a similar procedure to the one described above, methyl betulonate 87 was used as starting material to prepare methyl 2-hydroxybetulinate 127 by the reaction with potassium t-butanolate in dry t-butanol in the presence of air, yielding intermediate 126 in 80% (Scheme 29) [32]. The reduction of 126 with NaBH4 in THF at 0 °C afforded 127 in 81% yield.
Scheme 29.
Synthesis of methyl 2-hydroxybetulinate 127.
6. Aldol Condensation
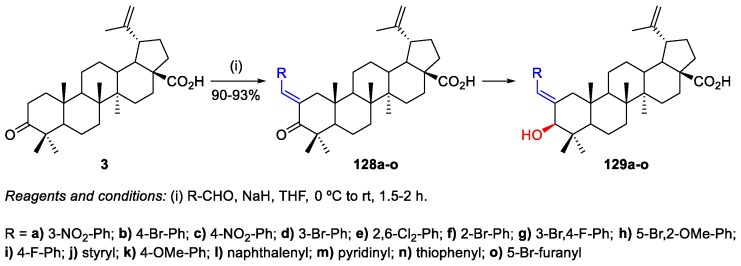
The aldol reactions are a straightforward methodology for C‒C bond formation. BoA 3 can easily undergo aldol condensation with aldehydes/cinnamaldehydes, affording BoA-based compounds bearing a α,β-unsaturated carbonyl system. The obtained α,β-unsaturated carbonyl compounds can be interesting substrates for conjugate addition reactions with several nucleophiles. In the following examples are described the aldol condensations performed in BoA 3 found in the literature [18,78].
Gupta et al. synthesized several benzylidene-BA derivatives 129a–o through a three-step methodology (Scheme 30) [78]. The key step involved aldol condensation reactions between BoA 3 and different aldehydes. The authors optimized the reaction conditions by using different bases, such as K2CO3, KOEt, NaOH, Et3N, DMAP, and NaH, and the best results were obtained in the presence of NaH as a base and THF as a solvent. These compounds were synthesized in an effort to develop potent anticancer agents, having been evaluated for their cytotoxicity against five different human cancer cell lines (A549, PC-3, HCT 116, MCF-7, and MIA PaCa-2). Compound 129c was found to be the most potent derivative among the synthesized series (IC50 = 1.36–3.5 µM).
Scheme 30.
Synthesis of benzylidene-BA derivatives 129a–o.
Another example of an aldol reaction performed on HBA skeleton was reported by Zhang et al. [18]. As described before in Scheme 28, 3-oxo-HBA derivative 123 was used as starting material to prepare C-2 modified derivatives. It was converted into 2-hydroxymethylene-3-oxo-HBA derivative 130 by the reaction with ethyl formate in the presence of sodium methoxide, in DCM (Scheme 31). The C-2 hydroxymethylated product 131 was synthesized by a similar procedure as the one used to prepare compound 127 from 126 (Scheme 28).
Scheme 31.
Synthesis of C-2 modified HBA derivatives 130 and 131.
7. Synthesis of Heterocycle-Fused BA/HBA Derivatives
Oxygen and nitrogen heterocyclic compounds, such as furan, pyrazole, pyrrole, isoxazole, etc., show important biological properties. The fusion between these compounds and other bioactive compounds, such as triterpenes in the current review, can provide new hybrid molecules in accordance with the molecular hybridization concept; i.e., a hybrid drug comprises two pharmacophores in one single molecule and is designed to interact with multiple targets, or to amplify its effect through action on another biotarget as one single molecule, or to counterbalance the known side effects associated with the other hybrid part [79]. In this context, in 2015, Kvasnica et al. published a review which covered the synthesis and medicinal significance of pentacyclic triterpenoids bearing nitrogen- and sulfur-containing heterocycles from 1962 to 2014 [11].
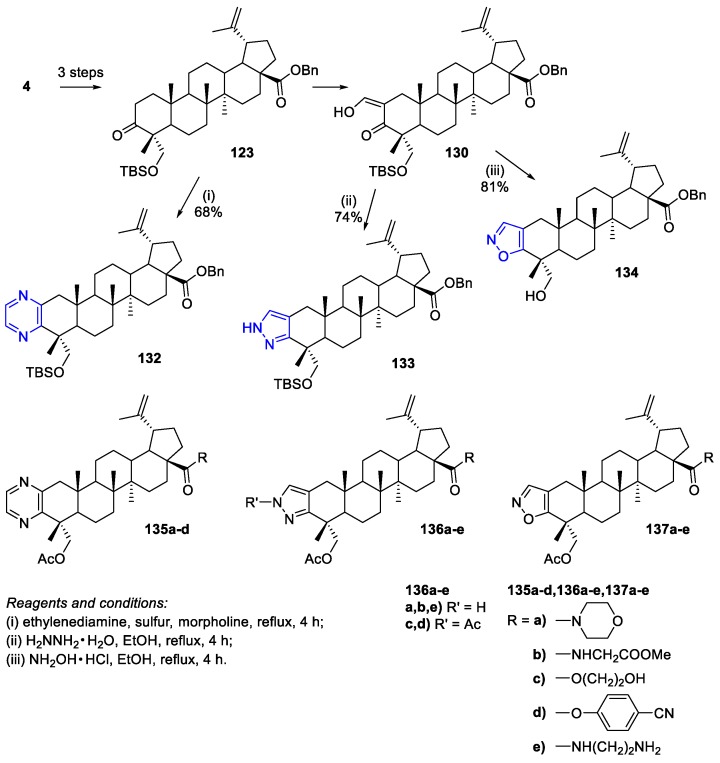
Since then, Zhang and co-workers have been using similar approaches, to the previously described, to synthesize heterocycle-fused HBA derivatives, starting from the protected form of 3-oxo-HBA 123 (Scheme 32) [80,81,82]. They prepared pyrazine-, pyrazole-, and isoxazole-fused HBA derivatives 135–137, which were assessed for their antitumor activity against cancer cell lines. In general, the biological screening results showed that all derivatives exhibited more significant antiproliferative activity than the parent HBA. In particular, compound 135a (IC50 = 3.53 µM, 4.42 µM, and 5.13 µM against cell lines SF-763, B16, and Hela, respectively) exhibited the most potent activity among the pyrazine-fused HBA series [80]. Moreover, compound 136e (IC50 = 5.58 and 6.13 µM against B16 and SF763 cancer cell lines, respectively) displayed the most potent activity among the pyrazole-fused HBA series [81]. In addition, compounds 137a and 137e (IC50 = 6.08–10.04 µM and 6.94–9.74 µM, respectively, against cell lines HL-60, BEL-7402, SF-763, Hela, and B16) were the most potent derivatives among the isoxazole-fused HBA series [82]. Regarding the synthesis of these heterocycle-fused HBA derivatives, the pyrazine-fused HBA derivative 132 was obtained in 68% yield through the reaction of 123 with ethylenediamine and sulfur in refluxing morpholine. In addition, pyrazole 133 and isoxazole 134 were prepared by the treatment of the enol intermediate 130 with hydrazine hydrate or hydroxylamine hydrochloride and were obtained in 74% and 81% yield, respectively. Finally, their ester or amide derivatives were obtained through similar procedures to ones described above in the Section 2.1 and Section 2.2 of this review.
Scheme 32.
Synthesis of heterocyclic ring-fused HBA derivatives 132–134.
Also, Gubaidullin et al. reported the gold-catalyzed intramolecular heterocyclization of compounds 115a–n [74,75], whose synthesis was described previously in Scheme 25. It occurred in the presence of 2 mol% PPh3AuCl and 2 mol% AgOTf in toluene at room temperature and afforded methyl [3,2-b]furan-based betulonate derivatives 138a–n in high yields (36%–92%) over a short period of time (Scheme 33).
Scheme 33.
Synthesis of [3,2-b]furan-substituted BA derivatives 139b–e.
8. Synthesis of Polymer‒BA Conjugates
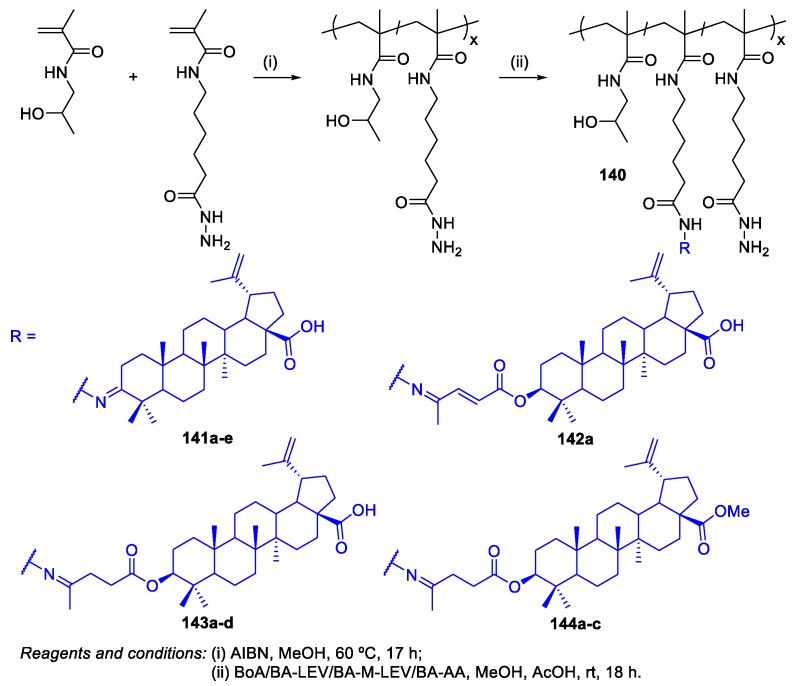
Drug delivery systems based on polymer carriers, for example liposomes, have become the most well-investigated pharmacological approach in recent decades. These carrier systems are usually developed to stabilize therapeutic compounds, overcoming obstacles to cellular and tissue uptake, improving biodistribution, bioavailability, and therapeutic efficacy, and decreasing the side effects of compounds that target specific sites in vivo. Only a few articles have been published describing the preparation of BA‒polymer conjugates since 2013 [83,84,85,86,87]. Although the synthesis of polymer‒BA conjugates involves mainly amination and esterification reactions, it makes more sense to create a specific section to approach this theme.
Lomkova et al. reported the synthesis, physicochemical, and preliminary biological characterization of micellar polymer‒BA conjugates based on N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer carriers, enabling the controlled release of cytotoxic BA derivatives in solid tumors or tumor cells [83]. The synthesis of these polymer‒BA conjugates started with the preparation of BA levulinate (BA-LEV) and BA 3-acetyl acrylate (BA-AA) through esterification reactions of 3-OH group of BA with levulinic acid and 3-acetyl acrylic acid, respectively, using the N,N’-dicyclohexylcarbodiimide (DCC) coupling method. Furthermore, methylated BA levulinate (BA-M-LEV) was prepared by methylation of BA-LEV. Then, the polymer−drug conjugates with BA derivatives differing in their BA content were prepared by the reaction of free hydrazide groups of polymer 140 with keto group-containing BA derivatives, affording the following conjugates: polymer‒BoA 141a–e, polymer‒BA-AA 142a, polymer‒BA-LEV 143a–d, and polymer‒BA-M-LEV 144a–c (Scheme 34). Conjugate 144a bearing 1.0 mol% of BA-M-LEV was the only one which enabled pH-dependent controlled release of the active drug in vitro. In addition, this conjugate exhibited high in vitro cytotoxicity (IC50 = 27.72–64.20 µM) against DLD-1, HT-29, and HeLa cancer cells, higher than that of BA 1 (IC50 = 62.65–87.05 µM) and its parent derivative BA-M-LEV (IC50 = 63.61–105.19 µM). Furthermore, conjugate 144a also enhanced tumor accumulation in HT-29 xenograft in mice.
Scheme 34.
Synthesis of polymer−drug conjugates with BA derivatives.
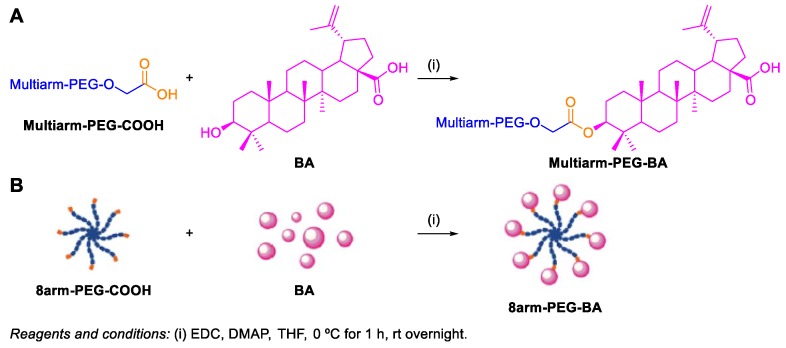
Dai el al. designed, synthesized and evaluated the in vivo effectiveness of water soluble multiarm-polyethylene glycol‒BA (multiarm-PEG–BA) prodrugs [84]. These conjugates were synthesized via an esterification reaction between the carboxy groups of multiarm-PEG–COOH and the 3-OH group of BA 1 (Scheme 35). The obtained 4arm-PEG40K–BA, 8arm-PEG40K–BA, and 8arm-PEG20K–BA prodrugs exhibited high drug loading capacity (3.26–11.81 wt%) and high-water solubility (290–750-fold of free BA 1). Their in vitro cytotoxicity was evaluated against two tumor cell lines (LLC and A549) and the trend for the IC50 values was 4arm-PEG40K–BA (37.03 and 27.19 µg mL−1) > 8arm-PEG40K–BA (47.65 and 39.57 µg mL−1) > 8arm-PEG20K–BA (58.15 and 44.18 µg mL−1). Furthermore, tumor xenograft assays demonstrated the superior therapeutic effect of these multiarm-PEG–BA prodrugs on inhibition of tumor growth when compared with free BA 1.
Scheme 35.
Synthesis of multiarm-PEG‒BA prodrugs.
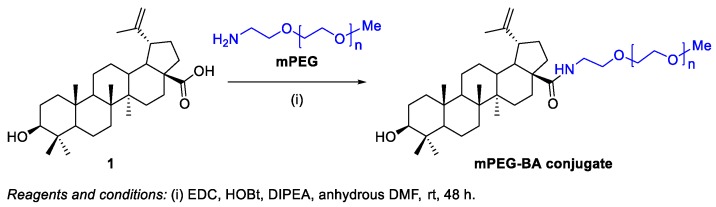
BA‒monomethoxypolyethylene glycol (mPEG) conjugate was synthesized in order to improve BA 1 solubility and anticancer efficacy [87]. PEGylated BA was synthesized by direct coupling of 17-COOH group of BA 1 with NH2 group of mPEG using EDC/HOBt coupling chemistry, as illustrated in Scheme 36. The mPEG‒BA conjugate (IC50 = 17.25–19.93 µM) was compared with the native BA 1 (IC50 = 5.67–7.45 µM) for its antiproliferative activity in the hepatocarcinoma cell lines Hep3b and Huh-7. Although mPEG‒BA conjugate was less cytotoxic than BA 1, it demonstrated significant cytotoxicity and internalization, and induced cell apoptosis in the referred cancer cells. Also, in vivo studies demonstrated significant reduction in tumor volume in case of PEGylated BA as compared to native BA 1.
Scheme 36.
Synthesis of mPEG–BA conjugates.
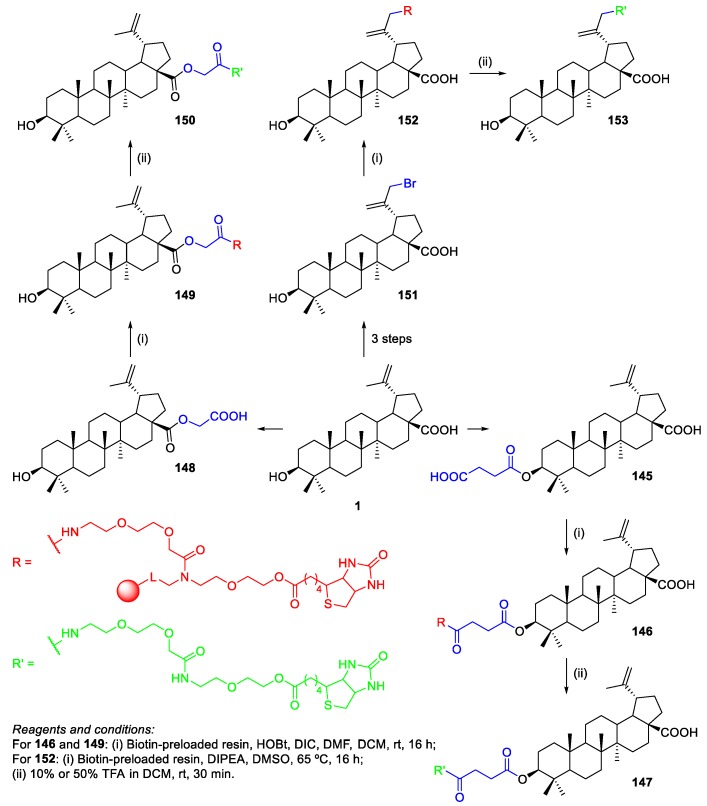
Soural et al. developed a simple and fast synthetic technique to connect BA 1 to biotin through a PEG linker [85]. Three different conjugation sites were suggested: attachment via 3-OH group, position 30, and 17-COOH group. However, there was the need of pre-modifying BA since its COOH group was not reactive under the proposed conditions. For this purpose, BA 1 was converted into the corresponding hemisuccinate 145, glyoxalate 148, and was brominated at C-30 to give an active halogen in compound 151. Then, the obtained BA-based compounds 145 and 148 were subjected to the reaction with a biotin-preloaded resin using HOBt/DIC, followed by cleavage of the polymer support with 10% or 50% TFA in DCM, affording biotinylated BA derivatives at C-3 147 and C-28 150 in overall yields of 31% and 43%, respectively (Scheme 37). Furthermore, brominated BA derivative 151 was converted into biotinylated BA derivative at C-30 153 in an overall yield of 25% (Scheme 37). Finally, cytotoxicity of the biotinylated BA derivatives 147, 150, and 153 was evaluated on two cancer cell lines (CCRF-CEM and HCT116). The most active compound was 150 biotinylated at C-28 (IC50 = 14.85 µM), which showed higher cytotoxicity than BA 1 (IC50 = 30.0 µM) and the intermediate 148 (IC50 = 43.54 µM) against CCRF-CEM cancer cells.
Scheme 37.
Synthesis of biotinated BA derivatives 147, 150, and 153.
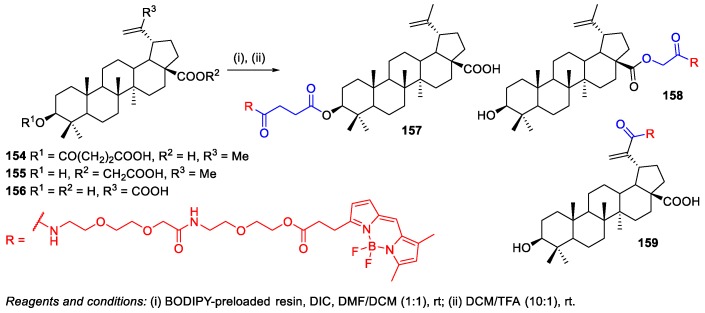
Krajcovicova et al. prepared boron-dipyrromethene (BODIPY)-labeled BA conjugates 157–159 for use in fluorescent microscopy [86]. Live cell studies focused on fluorescence conjugate uptake demonstrated a specific labeling pattern of conjugates 157–159. The authors attached the fluorescent dye at C-3, C-28, and C-30 by a solid-phase synthetic method. Firstly, BA 1 had to be pre-modified by esterification reactions at C-3 and C-28 with succinic anhydride or benzyl bromoacetate, and by a Pinnick oxidation reaction at C-30. Secondly, the BODIPY-preloaded resin was prepared through a similar approach to the biotin-preloaded resins described above, and the subsequent acylation with the pre-modified BA derivatives afforded the final conjugates bearing a BODIPY moiety 157–159 (Scheme 38).
Scheme 38.
Synthesis of BODIPY‒BA conjugates 157–159.
9. Miscellaneous Methodologies
In this section, several reports on BA/BN/BoA/HBA functionalization through different methodologies that do not fit in the previous sections will be presented.
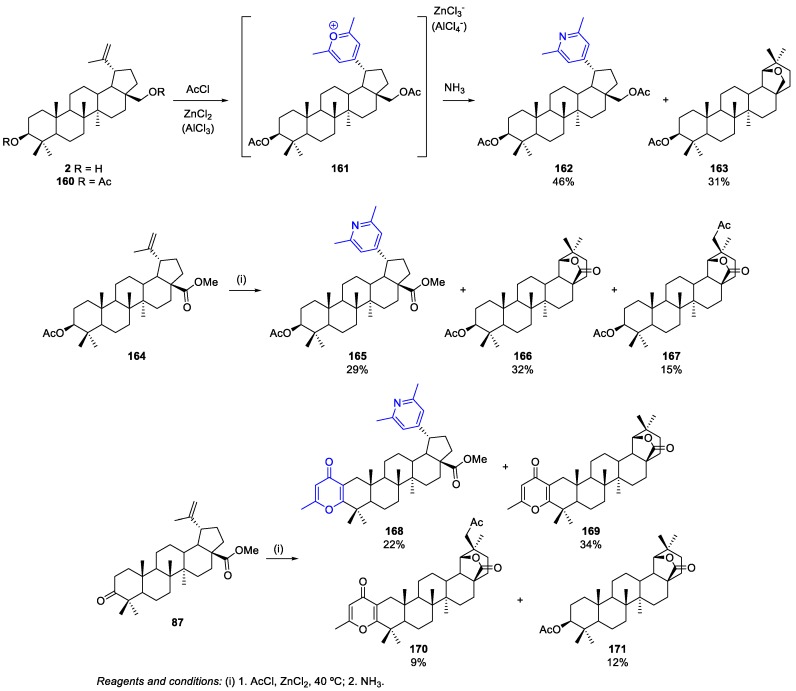
Antimonova et al. reported a methodology to synthesize BN/BA/BoA derivatives containing a pyridine group at position 20 [88]. In this context, the reaction of betulin diacetate 160 with acetyl chloride in the presence of a Lewis acid (ZnCl2, AlCl3), followed by treatment of the pyrilium salt 161 with ammonia formed 3,28-diacetoxy-19-(2,6-dimethylpyridin-4-yl)-20,29,30-trinorlupane 162 (46% yield) and 3-O-acetylallobetulin 163 (31% yield) as byproduct (Scheme 39). In addition, using a similar procedure to perform the reaction of methyl 3-O-acetylbetulinate 164 with acetyl chloride, 19-(pyridin-4-yl)-20,29,30-trinorlupane 165 and two 28-oxoallobetulin derivatives, 166 and 167, were obtained in 29%, 32%, and 15% yields, respectively (Scheme 39). Moreover, the presence of the C-3 ketone in the methyl betulonate 87 was responsible for another side reaction that formed a 4-oxopyran ring fused to the A-ring of the triterpene skeleton (Scheme 39). Consequently, four compounds 168–171 (22%, 34%, 9%, and 12% yields, respectively) were isolated from the reaction of 87 with acetyl chloride in the presence of ZnCl2 (Scheme 39).
Scheme 39.
Synthesis of BN/BA/BoA derivatives containing a pyridine group at C-20.
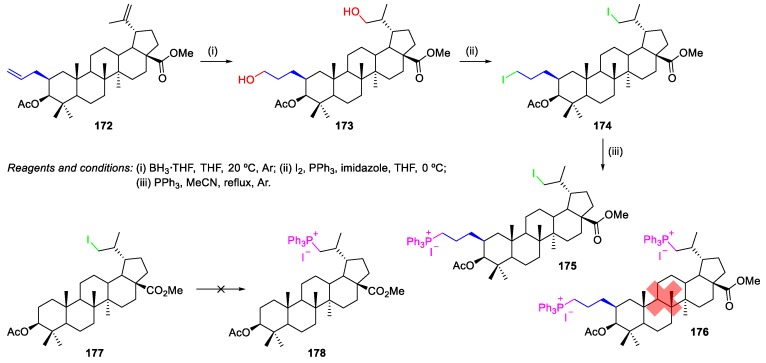
Spivak et al. reported the synthesis of BN- and BA-based triphenylphosphonium derivatives [89,90,91,92]. For instance, BA-based triphenylphosphonium salt 175 was synthesized from 2β-allyl-BA methyl ester 172 (this compound was synthesized through a similar procedure to the one described in Scheme 17 [57]) as follows (Scheme 40): compound 172 underwent hydroboration with BH3·THF, in THF to obtain diol 173 in 55% yield. Treatment of the diol 173 with crystalline iodine in the presence of imidazole and triphenylphosphane gave compound 174 in 68% yield. The resulting diiodide 174 was refluxed in acetonitrile with an excess of triphenylphosphane, affording monophosphonium salt 175 with high selectivity (89% yield). The authors did not observe the formation of bisphosphonium derivative 176. Apparently, steric factors prevented the SN2 substitution reaction of the C(30)-iodide by the bulky triphenylphosphane group. Thus, the reaction between iodide 177 and an excess (10 equiv) of triphenylphosphane did not afford compound 178 even under prolonged refluxing in toluene or acetonitrile (Scheme 40).
Scheme 40.
Synthesis of a BA-based triphenylphosphonium salt 175.
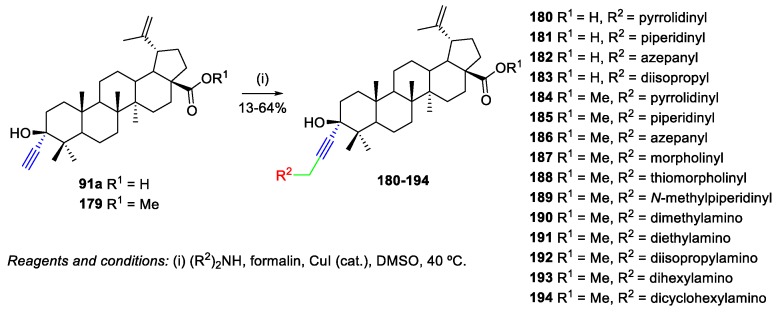
Csuk et al. prepared several BA derivatives through Mannich reactions, which were tested for their cytotoxic activity using a panel of nine human cancer cell lines (SW1736, MCF-7, LIPO, DLD-1, A549, A2780, A253, 8505C, and 518A2) [56]. Many of the synthesized compounds showed increased cytotoxicity over the naturally occurring BA 1 (IC50 = 6.7–17.5 µM) and the highest activity was found for the N-methylpiperazine derivative 189 (IC50 = 2.5–5.8 µM). Regarding the synthesis of the desired compounds and starting from 3-ethynyl-3-hydroxylup-20(29)-ene derivatives 91a and 179, whose synthesis is similar to the one described in Scheme 16, copper-catalyzed Mannich reactions were performed with several acyclic and cyclic secondary amines in the presence of aqueous formalin, in DMSO, for one up to several days (Scheme 41). The BA-derived hydroxypropargylamines 180–194 were obtained in fair to good yields (13%–64%).
Scheme 41.
Copper-catalysed Mannich reactions of BA-based alkynols 91a and 179 with several amines.
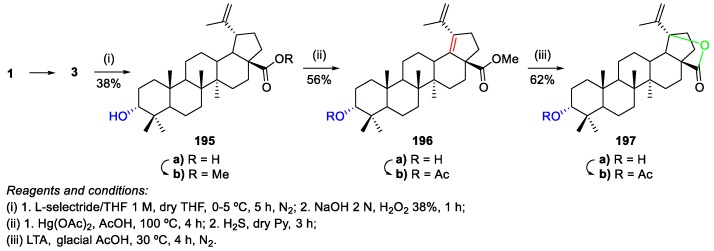
Mandal et al. developed a method for the hemi-synthesis of the rare triterpenoid, 3-epihydroxylup-20(29)-en-19(28)-olide 197, from BA 1 [93]. This triterpene was isolated from the bark of Microtropis fokienensis and Perrottetia arisanensis and has shown significant cytotoxicity against seven different cancer cell lines. Its synthetic methodology involved the BA 1 epimerization, followed by mercuric acetate dehydrogenation and lead tetraacetate (LTA) oxidation (Scheme 42).
Scheme 42.
Partial synthesis of 3α-hydroxylup-20(29)-en-19(28)-olide 197.
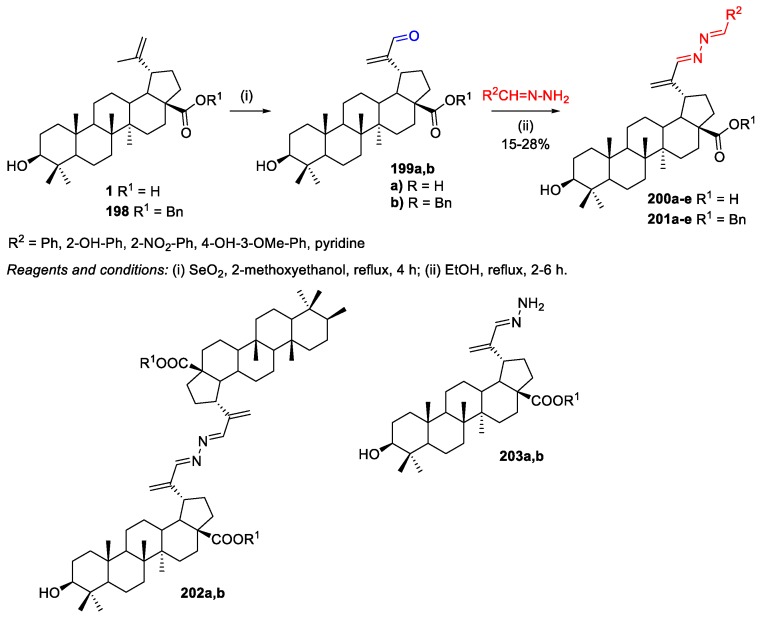
Pokorny et al. prepared BA-based azines as selective cytotoxic agents to leukaemia cells CCRF-CEM [94]. The new azines with a free 28-COOH group 200a–e were highly and selectively cytotoxic (IC50 = 3.4–8.8 µM) against the referred cancer cells and had influence on cell cycle and DNA/RNA synthesis. To synthesize the desired triterpenic azines, BA 1 was oxidized by SeO2 to 30-oxo-BA 199a. Then, this aldehyde was reacted with hydrazones in refluxing ethanol (Scheme 43), affording azines 200a–e in lower yields (15%–28%), whereas most of the starting triterpene gave a mixture of polar compounds that the authors found difficult to separate (Scheme 43). Protected azines 201a–e were isolated in higher yields (24%–45%) than azines of free acids 200a–e, but most of the triterpene was still lost. Three byproducts were isolated: symmetrical bistriterpenic azine 202b, hydrazone 203b, and benzyl betulinate 198 as a product of the Wolff–Kishner reduction.
Scheme 43.
Synthesis of BA-based azines derivatives 200a–e and 201a–e.
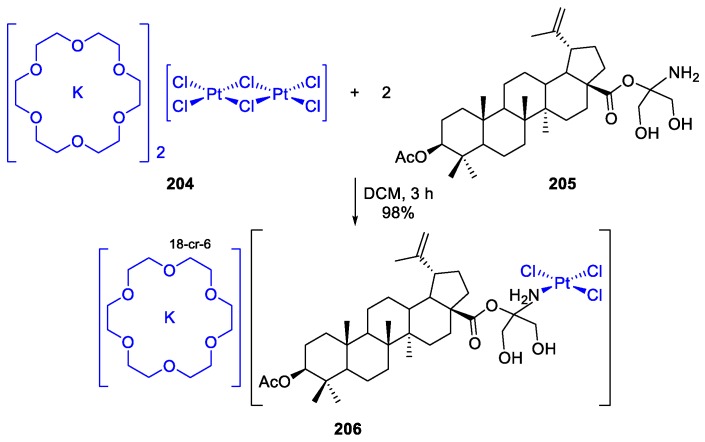
The synthesis, characterization and in vitro antitumor activity of a platinum(II)‒acetylated BA tris(hydroxymethyl)aminomethane ester conjugate 206 were reported [95]. This complex showed lower activity than BA 1, starting BA ester derivative 205, and cisplatin. It was prepared in almost quantitative yield through the reaction of dichloromethane solution of acetylated BA tris(hydroxymethyl)aminomethane ester 205 and [K(18-cr-6)]2[Pt2Cl6] 204 (Scheme 44).
Scheme 44.
Synthesis of the platinum(II) complex 206.
10. Conclusions
In this review, the chemical functionalization of BA and its natural analogues BN, BoA, and HBA was addressed. The most employed chemical transformations used to functionalize these triterpenes are amination and esterification reactions performed on their functional groups (3-OH and 17-COOH), affording new bioactive derivatives or activated intermediates for further transformation. Nevertheless, other interesting transformations were also found in the literature such as CuAAC to obtain 1,2,3-triazole-linked BA conjugates, Pd-catalyzed cross-coupling and aldol condensation reactions, which afford new functionalized BA derivatives through C‒C bond formation instead of C‒O or C‒N bonds. In addition, the preparation of new oxygen (furan) and nitrogen (pyrazine, pyrazole, isoxazole) heterocyclic-fused BA derivatives was also described. Most of the obtained triterpenic derivatives were studied for their biological properties, especially for their antitumor, anti-HIV, and antimalarial activities. Finally, there are also few reports on the incorporation of BA and its analogues in polymer formulations by solid-phase methods for different applications such as drug delivery and bioimaging.
Acknowledgments
Carmen Freire acknowledges FCT/MCTES for a contract under the program Investigador FCT 2012 (IF/01407/2012). Joana Sousa also acknowledges the MultiBiorefinery project (POCI-01-0145-FEDER-016403) for her post-doctoral grant.
Abbreviations
| Ac | Acetyl |
| AIDS | Acquired immunodeficiency syndrome |
| Ala | Alanine |
| AZT | Azidothymidine |
| BA | Betulinic acid |
| BA-AA | Betulinic acid 3-acetyl acrylate |
| BA-LEV | Betulinic acid levulinate |
| BA-M-LEV | Methylated betulinic acid levulinate |
| BN | Betulin |
| Bn | Benzyl |
| BoA | Betulonic acid |
| Boc | tert-Butyloxycarbonyl |
| BODIPY | Boron-dipyrromethene |
| Bu | Butyl |
| cat | Catalyst |
| CCID50 | Cell culture infectious dose 50% |
| CD | Cyclodextrin |
| CDI | 1,1’-Carbonyl-diimidazole |
| CuAAC | Copper-catalyzed azide-alkyne cycloaddition |
| Dab | 2,4-Diaminobutyric acid |
| Dap | 2,3-Diaminopropionic acid |
| DCC | N,N’-Dicyclohexylcarbodiimide |
| DCM | Dichloromethane |
| DIC | N,N’-Diisopropylcarbodiimide |
| DIPEA | N,N-Diisopropylethylamine |
| DMA | N,N-Dimethylacetamide |
| DMAP | 4-(Dimethylamino)pyridine |
| DME | 1,2-Dimethoxyethane |
| DMF | Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| DPPA | Diphenyl phosphoryl azide |
| EC50 | Half maximal effective concentration |
| EDC | N-(3-Dimethylaminopropyl)-N’-ethylcarbodiimide |
| equiv | Molar equivalents |
| Et | Ethyl |
| GI50 | Growth inhibition of 50% of cells |
| GSK | GlaxoSmithKline |
| HBA | 23-Hydroxybetulinic acid |
| HIV | Human Immunodeficiency Virus |
| HOBt | Hydroxybenzotriazole |
| HPMA | N-(2-Hydroxypropyl)methacrylamide |
| IC50 | Half maximal inhibitory concentration |
| i-Pr | Isopropyl |
| KHMDS | Potassium bis(trimethylsilyl)amide |
| LTA | Lead tetraacetate or lead(IV) acetate |
| Lys | Lysine |
| mCPBA | m-Chloroperoxybenzoic acid |
| Me | Methyl |
| Orn | Ornithine |
| PCC | Pyridinium chlorochromate |
| PEG | Polyethylene glycol |
| Ph | Phenyl |
| Py | Pyridine |
| PyBOP | Benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate |
| quant | Quantitative (yield) |
| rt | Room temperature |
| SN2 | Bimolecular nucleophilic substitution |
| TBS | tert-Butyl(dimethyl)silyl |
| TBTU | N,N,N’,N’-Tetramethyl-O-(benzotriazol-1-yl)uronium tetrafluoroborate |
| t-Bu | tert-Butyl |
| Tf | Triflate |
| TFA | Trifluoroacetic acid |
| THF | Tetrahydrofuran |
| UA | Ursolic acid |
| β-CD | β-Cyclodextrin |
Funding
This work is financed by Portugal 2020 through FEDER in the frame of POCI and in the scope of the projects: MultiBiorefinery (POCI-01-0145-FEDER-016403), CICECO—Aveiro Institute of Materials CTM/50011 (POCI-01-0145-FEDER-007679), and QOPNA Research Unit (UID/QUI/00062/2019), co-financed by FCT/MCTES.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Moghaddam M.G., Ahmad F.B.H., Samzadeh-Kermani A. Biological Activity of Betulinic Acid: A Review. Pharmacol. Pharm. 2012;3:119–123. doi: 10.4236/pp.2012.32018. [DOI] [Google Scholar]
- 2.Ríos J.L., Máñez S. New Pharmacological Opportunities for Betulinic Acid. Planta Med. 2018;84:8–19. doi: 10.1055/s-0043-123472. [DOI] [PubMed] [Google Scholar]
- 3.Sami A., Taru M., Salme K., Jari Y.-K. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006;29:1–13. doi: 10.1016/j.ejps.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Csuk R. Betulinic acid and its derivatives: A patent review (2008–2013) Expert Opin. Ther. Pat. 2014;24:913–923. doi: 10.1517/13543776.2014.927441. [DOI] [PubMed] [Google Scholar]
- 5.Zhang D.-M., Xu H.-G., Wang L., Li Y.-J., Sun P.-H., Wu X.-M., Wang G.-J., Chen W.-M., Ye W.-C. Betulinic Acid and its Derivatives as Potential Antitumor Agents. Med. Res. Rev. 2015;35:1127–1155. doi: 10.1002/med.21353. [DOI] [PubMed] [Google Scholar]
- 6.Ali-Seyed M., Jantan I., Vijayaraghavan K., Bukhari S.N.A. Betulinic Acid: Recent Advances in Chemical Modifications, Effective Delivery, and Molecular Mechanisms of a Promising Anticancer Therapy. Chem. Biol. Drug Des. 2016;87:517–536. doi: 10.1111/cbdd.12682. [DOI] [PubMed] [Google Scholar]
- 7.Martin D.E., Salzwedel K., Allaway G.P. Bevirimat: A Novel Maturation Inhibitor for the Treatment of HIV-1 Infection. Antivir. Chem. Chemother. 2008;19:107–113. doi: 10.1177/095632020801900301. [DOI] [PubMed] [Google Scholar]
- 8.Regueiro-Ren A., Liu Z., Chen Y., Sin N., Sit S.-Y., Swidorski J.J., Chen J., Venables B.L., Zhu J., Nowicka-Sans B., et al. Discovery of BMS-955176, a Second Generation HIV-1 Maturation Inhibitor with Broad Spectrum Antiviral Activity. ACS Med. Chem. Lett. 2016;7:568–572. doi: 10.1021/acsmedchemlett.6b00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi W., Tang N., Yan W. Research and Development in Betulin and Betulinic Acid Derived Triterpenoids. Mini-Rev. Org. Chem. 2014;11:343–354. doi: 10.2174/1570193X1103140915112124. [DOI] [Google Scholar]
- 10.Bednarczyk-Cwynar B., Zaprutko L. Recent advances in synthesis and biological activity of triterpenic acylated oximes. Phytochem. Rev. 2015;14:203–231. doi: 10.1007/s11101-014-9353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kvasnica M., Urban M., Dickinson N.J., Sarek J. Pentacyclic triterpenoids with nitrogen- and sulfur-containing heterocycles: Synthesis and medicinal significance. Nat. Prod. Rep. 2015;32:1303–1330. doi: 10.1039/C5NP00015G. [DOI] [PubMed] [Google Scholar]
- 12.Zhou M., Zhang R.-H., Wang M., Xu G.-B., Liao S.-G. Prodrugs of triterpenoids and their derivatives. Eur. J. Med. Chem. 2017;131:222–236. doi: 10.1016/j.ejmech.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Borkova L., Hodon J., Urban M. Synthesis of Betulinic Acid Derivatives with Modified A-Rings and their Application as Potential Drug Candidates. Asian J. Org. Chem. 2018;7:1542–1560. doi: 10.1002/ajoc.201800163. [DOI] [Google Scholar]
- 14.Pokorny J., Borkova L., Urban M. Click Reactions in Chemistry of Triterpenes - Advances Towards Development of Potential Therapeutics. Curr. Med. Chem. 2018;25:636–658. doi: 10.2174/0929867324666171009122612. [DOI] [PubMed] [Google Scholar]
- 15.Innocente A., Casanova B.B., Klein F., Lana A.D., Pereira D., Muniz M.N., Sonnet P., Gosmann G., Fuentefria A.M., Gnoatto S.C.B. Synthesis of Isosteric Triterpenoid Derivatives and Antifungal Activity. Chem. Biol. Drug Des. 2014;83:344–349. doi: 10.1111/cbdd.12251. [DOI] [PubMed] [Google Scholar]
- 16.Antimonova A.N., Petrenko N.I., Shakirov M.M., Pokrovskii M.A., Pokrovskii A.G., Shul’ts E.E. Synthesis and Cytotoxic Activity of Lupane Triterpenoids Containing 1,3,4-Oxadiazoles. Chem. Nat. Compd. 2014;50:1016–1023. doi: 10.1007/s10600-014-1150-2. [DOI] [Google Scholar]
- 17.Emmerich D., Vanchanagiri K., Baratto L.C., Schmidt H., Paschke R. Synthesis and studies of anticancer properties of lupane-type triterpenoid derivatives containing a cisplatin fragment. Eur. J. Med. Chem. 2014;75:460–466. doi: 10.1016/j.ejmech.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H., Zhu P., Liu J., Yang X., Xu S., Yao H., Jiang J., Ye W., Wu X., Xu J. Synthesis and antitumor activity of novel 3-oxo-23-hydroxybetulinic acid derivatives. Eur. J. Med. Chem. 2014;87:159–167. doi: 10.1016/j.ejmech.2014.09.058. [DOI] [PubMed] [Google Scholar]
- 19.Innocente A., Silva G., Cruz L., Moraes M., Nakabashi M., Sonnet P., Gosmann G., Garcia C., Gnoatto S. Synthesis and Antiplasmodial Activity of Betulinic Acid and Ursolic Acid Analogues. Molecules. 2012;17:12003. doi: 10.3390/molecules171012003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao S., Wang Q., Si L., Shi Y., Wang H., Yu F., Zhang Y., Li Y., Zheng Y., Zhang C., et al. Synthesis and Anti-HCV Entry Activity Studies of β-Cyclodextrin–Pentacyclic Triterpene Conjugates. ChemMedChem. 2014;9:1060–1070. doi: 10.1002/cmdc.201300545. [DOI] [PubMed] [Google Scholar]
- 21.Coric P., Turcaud S., Souquet F., Briant L., Gay B., Royer J., Chazal N., Bouaziz S. Synthesis and biological evaluation of a new derivative of bevirimat that targets the Gag CA-SP1 cleavage site. Eur. J. Med. Chem. 2013;62:453–465. doi: 10.1016/j.ejmech.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Xu K., Xu X., Chu F., Wang M., Wang P., Li G., Song J., Zhang Y., Lei H. Synthesis and biological evaluation of T-OA analogues as the cytotoxic agents. Res. Chem. Intermed. 2015;41:6257–6269. doi: 10.1007/s11164-014-1737-z. [DOI] [Google Scholar]
- 23.Becker C.S., Chukanov N.V., Grigor’ev I.A. New Amino-Bisphosphonate Building Blocks in the Synthesis of Bisphosphonic Derivatives Based on Lead Compounds. Phosphorus Sulfur Silicon Relat. Elem. 2015;190:1201–1212. doi: 10.1080/10426507.2014.979989. [DOI] [Google Scholar]
- 24.Wiemann J., Heller L., Perl V., Kluge R., Ströhl D., Csuk R. Betulinic acid derived hydroxamates and betulin derived carbamates are interesting scaffolds for the synthesis of novel cytotoxic compounds. Eur. J. Med. Chem. 2015;106:194–210. doi: 10.1016/j.ejmech.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 25.Vasilevsky S.F., Govdi A.I., Shults E.v.E., Shakirov M.M., Sorokina I.V., Tolstikova T.G., Baev D.S., Tolstikov G.A., Alabugin I.V. Efficient synthesis of the first betulonic acid–acetylene hybrids and their hepatoprotective and anti-inflammatory activity. Bioorg. Med. Chem. 2009;17:5164–5169. doi: 10.1016/j.bmc.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 26.Suman P., Patel A., Solano L., Jampana G., Gardner Z.S., Holt C.M., Jonnalagadda S.C. Synthesis and cytotoxicity of Baylis-Hillman template derived betulinic acid-triazole conjugates. Tetrahedron. 2017;73:4214–4226. doi: 10.1016/j.tet.2016.11.056. [DOI] [Google Scholar]
- 27.Bębenek E., Chrobak E., Wietrzyk J., Kadela M., Chrobak A., Kusz J., Książek M., Jastrzębska M., Boryczka S. Synthesis, structure and cytotoxic activity of acetylenic derivatives of betulonic and betulinic acids. J. Mol. Struct. 2016;1106:210–219. doi: 10.1016/j.molstruc.2015.10.102. [DOI] [Google Scholar]
- 28.Khlebnikova T.S., Piven Y.A., Lakhvich F.A., Frolova T.S., Sorokina I.V., Tolstikova T.G. Synthesis and Cytotoxicity of Conjugates of Betulinic Acid and F-Containing 2-Acylcycloalkane-1,3-Diones. Chem. Nat. Compd. 2018;54:1100–1105. doi: 10.1007/s10600-018-2565-y. [DOI] [Google Scholar]
- 29.Krishna C., Bhargavi M.V., Krupadanam G.L.D. Design, Synthesis, and Cytotoxicity of Semisynthetic Betulinic Acid-1,2,4-Oxadiazole Amide Derivatives. Russ. J. Gen. Chem. 2018;88:312–318. doi: 10.1134/S1070363218020196. [DOI] [Google Scholar]
- 30.Ali M.T.M., Zahari H., Aliasak A., Lim S.M., Ramasamy K., Macabeo A.P.G. Synthesis, Characterization and Cytotoxic Activity of Betulinic Acid and Seco-Betulinic Acid Derivatives against Human Colorectal Carcinoma. Orient. J. Chem. 2017;33:242–248. [Google Scholar]
- 31.Cui H.-W., He Y., Wang J., Gao W., Liu T., Qin M., Wang X., Gao C., Wang Y., Liu M.-Y., et al. Synthesis of heterocycle-modified betulinic acid derivatives as antitumor agents. Eur. J. Med. Chem. 2015;95:240–248. doi: 10.1016/j.ejmech.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 32.Kahnt M., Heller L., Al-Harrasi A., Schäfer R., Kluge R., Wagner C., Otgonbayar C., Csuk R. Platanic acid-derived methyl 20-amino-30-norlupan-28-oates are potent cytotoxic agents acting by apoptosis. Med. Chem. Res. 2018;27:1757–1769. doi: 10.1007/s00044-018-2189-6. [DOI] [Google Scholar]
- 33.Chen Y., Sit S.-Y., Chen J., Swidorski J.J., Liu Z., Sin N., Venables B.L., Parker D.D., Nowicka-Sans B., Lin Z., et al. The design, synthesis and structure-activity relationships associated with C28 amine-based betulinic acid derivatives as inhibitors of HIV-1 maturation. Bioorg. Med. Chem. Lett. 2018;28:1550–1557. doi: 10.1016/j.bmcl.2018.03.067. [DOI] [PubMed] [Google Scholar]
- 34.Liu J.-H., Tang J., Zhu Z.-F., Chen L. Design, synthesis, and anti-tumor activity of novel betulinic acid derivatives. J. Asian Nat. Prod. Res. 2014;16:34–42. doi: 10.1080/10286020.2013.870998. [DOI] [PubMed] [Google Scholar]
- 35.da Silva G.N., Maria N.R., Schuck D.C., Cruz L.N., de Moraes M.S., Nakabashi M., Graebin C., Gosmann G., Garcia C.R., Gnoatto S.C. Two series of new semisynthetic triterpene derivatives: Differences in anti-malarial activity, cytotoxicity and mechanism of action. Malar. J. 2013;12:89. doi: 10.1186/1475-2875-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popov S.A., Shpatov A.V., Grigor’ev I.A. Synthesis of Substituted Esters of Ursolic, Betulonic, and Betulinic Acids Containing the Nitroxyl Radical 4-Amino-2,2,6,6-Tetramethylpiperidine-1-Oxyl. Chem. Nat. Compd. 2015;51:87–90. doi: 10.1007/s10600-015-1209-8. [DOI] [Google Scholar]
- 37.Khlebnicova T.S., Piven Y.A., Baranovsky A.V., Lakhvich F.A., Shishkina S.V., Zicāne D., Tetere Z., Rāviņa I., Kumpiņš V., Rijkure I., et al. Synthesis of novel lupane triterpenoid-indazolone hybrids with oxime ester linkage. Steroids. 2017;117:77–89. doi: 10.1016/j.steroids.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Yang S., Liang N., Li H., Xue W., Hu D., Jin L., Zhao Q., Yang S. Design, synthesis and biological evaluation of novel betulinic acid derivatives. Chem. Cent. J. 2012;6:141. doi: 10.1186/1752-153X-6-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saha S., Ghosh M., Dutta S.K. A potent tumoricidal co-drug ‘Bet-CA’—An ester derivative of betulinic acid and dichloroacetate selectively and synergistically kills cancer cells. Sci. Rep. 2015;5:7762. doi: 10.1038/srep07762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ackermann A., Karagöz A.Ç., Ghoochani A., Buchfelder M., Eyüpoglu I., Tsogoeva S.B., Savaskan N. Cytotoxic profiling of artesunic and betulinic acids and their synthetic hybrid compound on neurons and gliomas. Oncotarget. 2017;8:61457–61474. doi: 10.18632/oncotarget.18390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu B., Yan W.-Q., Xu X., Wu G.-R., Zhang C.-Z., Han Y.-T., Chu F.-H., Zhao R., Wang P.-L., Lei H.-M. Combination of amino acid/dipeptide with ligustrazine-betulinic acid as antitumor agents. Eur. J. Med. Chem. 2017;130:26–38. doi: 10.1016/j.ejmech.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 42.Xu B., Chu F., Zhang Y., Wang X., Li Q., Liu W., Xu X., Xing Y., Chen J., Wang P., et al. A Series of New Ligustrazine-Triterpenes Derivatives as Anti-Tumor Agents: Design, Synthesis, and Biological Evaluation. Int. J. Mol. Sci. 2015;16:21035. doi: 10.3390/ijms160921035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dang A.T.T., Pham C.T., Le T.A., Truong H.H., Vu H.T.T., Soldatenkov A.T., Van Nguyen T. New hybrids between triterpenoid acids and nucleoside HIV-RT inhibitors. Mendeleev Commun. 2015;25:96–98. doi: 10.1016/j.mencom.2015.03.004. [DOI] [Google Scholar]
- 44.Wolfram R.K., Heller L., Csuk R. Targeting mitochondria: Esters of rhodamine B with triterpenoids are mitocanic triggers of apoptosis. Eur. J. Med. Chem. 2018;152:21–30. doi: 10.1016/j.ejmech.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 45.Eignerova B., Tichy M., Krasulova J., Kvasnica M., Rarova L., Christova R., Urban M., Bednarczyk-Cwynar B., Hajduch M., Sarek J. Synthesis and antiproliferative properties of new hydrophilic esters of triterpenic acids. Eur. J. Med. Chem. 2017;140:403–420. doi: 10.1016/j.ejmech.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 46.da Silva G.N.S., Atik D.M., Fernandes J.L.A., Nascimento D.d.F.d., Fazolo T., Souza A.P.D.d., Gnoatto S.C.B. Synthesis of three triterpene series and their activity against respiratory syncytial virus. Arch. Pharm. 2018;351:1800108. doi: 10.1002/ardp.201800108. [DOI] [PubMed] [Google Scholar]
- 47.Komissarova N.G., Dubovitskii S.N., Orlov A.V., Shitikova O.V., Abdullin M.F., Spirikhin L.V., Yunusov M.S. Synthesis of Sulfobetaines Based on Betulinic Acid and its Esters. Chem. Nat. Compd. 2015;51:746–751. doi: 10.1007/s10600-015-1399-0. [DOI] [Google Scholar]
- 48.Drąg-Zalesińska M., Wysocka T., Borska S., Drąg M., Poręba M., Choromańska A., Kulbacka J., Saczko J. The new esters derivatives of betulin and betulinic acid in epidermoid squamous carcinoma treatment–In vitro studies. Biomed. Pharmacother. 2015;72:91–97. doi: 10.1016/j.biopha.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Komissarova N.G., Dubovitskii S.N., Shitikova O.V., Vyrypaev E.M., Spirikhin L.V., Eropkina E.M., Lobova T.G., Eropkin M.Y., Yunusov M.S. Synthesis of Conjugates of Lupane-Type Pentacyclic Triterpenoids with 2-Aminoethane- and N-Methyl-2-Aminoethanesulfonic Acids. Assessment of in vitro Toxicity. Chem. Nat. Compd. 2017;53:907–914. doi: 10.1007/s10600-017-2153-6. [DOI] [Google Scholar]
- 50.Bi Y., Xu J., Sun F., Wu X., Ye W., Sun Y., Huang W. Synthesis and Biological Activity of 23-Hydroxybetulinic Acid C-28 Ester Derivatives as Antitumor Agent Candidates. Molecules. 2012;17:8832. doi: 10.3390/molecules17088832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao H., Wei G., Liu Y., Yao H., Zhu Z., Ye W., Wu X., Xu J., Xu S. Synthesis, Biological Evaluation of Fluorescent 23-Hydroxybetulinic Acid Probes, and Their Cellular Localization Studies. ACS Med. Chem. Lett. 2018;9:1030–1034. doi: 10.1021/acsmedchemlett.8b00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sommerwerk S., Heller L., Csuk R. Synthesis and Cytotoxic Activity of Pentacyclic Triterpenoid Sulfamates. Arch. Pharm. 2015;348:46–54. doi: 10.1002/ardp.201400297. [DOI] [PubMed] [Google Scholar]
- 53.Vanchanagiri K., Emmerich D., Bruschke M., Bache M., Seifert F., Csuk R., Vordermark D., Paschke R. Synthesis and biological investigation of new carbonic anhydrase IX (CAIX) inhibitors. Chem.-Biol. Interact. 2018;284:12–23. doi: 10.1016/j.cbi.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 54.Govdi A.I., Sokolova N.V., Sorokina I.V., Baev D.S., Tolstikova T.G., Mamatyuk V.I., Fadeev D.S., Vasilevsky S.F., Nenajdenko V.G. Synthesis of new betulinic acid-peptide conjugates and in vivo and in silico studies of the influence of peptide moieties on the triterpenoid core activity. MedChemComm. 2015;6:230–238. doi: 10.1039/C4MD00236A. [DOI] [Google Scholar]
- 55.Spivak A.Y., Gubaidullin R.R., Galimshina Z.R., Nedopekina D.A., Odinokov V.N. Effective synthesis of novel C(2)-propargyl derivatives of betulinic and ursolic acids and their conjugation with β-d-glucopyranoside azides via click chemistry. Tetrahedron. 2016;72:1249–1256. doi: 10.1016/j.tet.2016.01.024. [DOI] [Google Scholar]
- 56.Csuk R., Nitsche C., Sczepek R., Schwarz S., Siewert B. Synthesis of Antitumor-Active Betulinic Acid-Derived Hydroxypropargylamines by Copper-Catalyzend Mannich Reactions. Arch. Pharm. 2013;346:232–246. doi: 10.1002/ardp.201200428. [DOI] [PubMed] [Google Scholar]
- 57.Spivak A.Y., Shakurova E.R., Nedopekina D.A., Khalitova R.R., Khalilov L.M., Odinokov V.N., Bel’skii Y.P., Ivanova A.N., Bel’skaya N.V., Danilets M.G., Ligacheva A.A. Novel lupane triterpenoids containing allyl substituents in ring A: Synthesis and in vitro study of antiinflammatory and cytotoxic properties. Russ. Chem. Bull. 2011;60:694. doi: 10.1007/s11172-011-0108-9. [DOI] [Google Scholar]
- 58.Liang L., Astruc D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011;255:2933–2945. doi: 10.1016/j.ccr.2011.06.028. [DOI] [Google Scholar]
- 59.Spivak A.Y., Galimshina Z.R., Nedopekina D.A., Odinokov V.N. Synthesis of New C-2 Triazole-Linked Analogs of Triterpenoid Pentacyclic Saponins. Chem. Nat. Compd. 2018;54:315–323. doi: 10.1007/s10600-018-2331-1. [DOI] [Google Scholar]
- 60.Majeed R., Sangwan P.L., Chinthakindi P.K., Khan I., Dangroo N.A., Thota N., Hamid A., Sharma P.R., Saxena A.K., Koul S. Synthesis of 3-O-propargylated betulinic acid and its 1,2,3-triazoles as potential apoptotic agents. Eur. J. Med. Chem. 2013;63:782–792. doi: 10.1016/j.ejmech.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 61.Chakraborty B., Dutta D., Mukherjee S., Das S., Maiti N.C., Das P., Chowdhury C. Synthesis and biological evaluation of a novel betulinic acid derivative as an inducer of apoptosis in human colon carcinoma cells (HT-29) Eur. J. Med. Chem. 2015;102:93–105. doi: 10.1016/j.ejmech.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 62.Wang C., Lu L., Na H., Li X., Wang Q., Jiang X., Xu X., Yu F., Zhang T., Li J., et al. Conjugation of a Nonspecific Antiviral Sapogenin with a Specific HIV Fusion Inhibitor: A Promising Strategy for Discovering New Antiviral Therapeutics. J. Med. Chem. 2014;57:7342–7354. doi: 10.1021/jm500763m. [DOI] [PubMed] [Google Scholar]
- 63.Thi T.A.D., Tuyet N.T.K., The C.P., Nguyen H.T., Thi C.B., Duy T.D., D’hooghe M., Nguyen T.V. Synthesis and cytotoxic evaluation of novel ester-triazole-linked triterpenoid–AZT conjugates. Bioorg. Med. Chem. Lett. 2014;24:5190–5194. doi: 10.1016/j.bmcl.2014.09.079. [DOI] [PubMed] [Google Scholar]
- 64.Khan I., Guru S.K., Rath S.K., Chinthakindi P.K., Singh B., Koul S., Bhushan S., Sangwan P.L. A novel triazole derivative of betulinic acid induces extrinsic and intrinsic apoptosis in human leukemia HL-60 cells. Eur. J. Med. Chem. 2016;108:104–116. doi: 10.1016/j.ejmech.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 65.Pattnaik B., Lakshmi J.K., Kavitha R., Jagadeesh B., Bhattacharjee D., Jain N., Mallavadhani U.V. Synthesis, structural studies, and cytotoxic evaluation of novel ursolic acid hybrids with capabilities to arrest breast cancer cells in mitosis. J. Asian Nat. Prod. Res. 2017;19:260–271. doi: 10.1080/10286020.2016.1240169. [DOI] [PubMed] [Google Scholar]
- 66.Thi T.A.D., Tuyet N.T.K., The C.P., Nguyen H.T., Thi C.B., Phuong H.T., Boi L.V., Nguyen T.V., D’hooghe M. Synthesis and cytotoxic evaluation of novel amide–triazole-linked triterpenoid–AZT conjugates. Tetrahedron Lett. 2015;56:218–224. [Google Scholar]
- 67.Bębenek E., Kadela-Tomanek M., Chrobak E., Latocha M., Boryczka S. Novel triazoles of 3-acetylbetulin and betulone as anticancer agents. Med. Chem. Res. 2018;27:2051–2061. doi: 10.1007/s00044-018-2213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang H., Xu R., Shi Y., Si L., Jiao P., Fan Z., Han X., Wu X., Zhou X., Yu F., et al. Design, synthesis and biological evaluation of novel l-ascorbic acid-conjugated pentacyclic triterpene derivatives as potential influenza virus entry inhibitors. Eur. J. Med. Chem. 2016;110:376–388. doi: 10.1016/j.ejmech.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 69.Dangroo N.A., Singh J., Rath S.K., Gupta N., Qayum A., Singh S., Sangwan P.L. A convergent synthesis of novel alkyne–azide cycloaddition congeners of betulinic acid as potent cytotoxic agent. Steroids. 2017;123:1–12. doi: 10.1016/j.steroids.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Han X., Shi Y., Si L., Fan Z., Wang H., Xu R., Jiao P., Meng K., Tian Z., Zhou X., et al. Design, synthesis and biological activity evaluation of novel conjugated sialic acid and pentacyclic triterpene derivatives as anti-influenza entry inhibitors. MedChemComm. 2016;7:1932–1945. doi: 10.1039/C6MD00292G. [DOI] [Google Scholar]
- 71.Antimonova A.N., Petrenko N.I., Shakirov M.M., Rybalova T.V., Frolova T.S., Shul’ts E.E., Kukina T.P., Sinitsyna O.I., Tolstikov G.A. Synthesis and study of mutagenic properties of lupane triterpenoids containing 1,2,3-triazole fragments in the C-30 position. Chem. Nat. Compd. 2013;49:657–664. doi: 10.1007/s10600-013-0702-1. [DOI] [Google Scholar]
- 72.Shi W., Tang N., Yan W.-D. Synthesis and cytotoxicity of triterpenoids derived from betulin and betulinic acid via click chemistry. J. Asian Nat. Prod. Res. 2015;17:159–169. doi: 10.1080/10286020.2014.979164. [DOI] [PubMed] [Google Scholar]
- 73.Sidova V., Zoufaly P., Pokorny J., Dzubak P., Hajduch M., Popa I., Urban M. Cytotoxic conjugates of betulinic acid and substituted triazoles prepared by Huisgen Cycloaddition from 30-azidoderivatives. PLoS ONE. 2017;12:e0171621. doi: 10.1371/journal.pone.0171621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gubaidullin R.R., Yarmukhametova D.S., Nedopekina D.A., Khalitova R.R., Spivak A.Y. Effective synthesis of novel furan-fused pentacyclic triterpenoids via anionic 5-exo dig cyclization of 2-alkynyl-3-oxotriterpene acids. Arkivoc. 2017;v:100–116. doi: 10.24820/ark.5550190.p010.142. [DOI] [Google Scholar]
- 75.Gubaidullin R.R., Khalitova R.R., Galimshina Z.R., Spivak A.Y. Synthesis of novel [3,2-b]furan-fused pentacyclic triterpenoids via gold-catalyzed intramolecular heterocyclization of 2-alkynyl-3-oxotriterpene acids. Tetrahedron. 2018;74:1888–1899. doi: 10.1016/j.tet.2018.02.056. [DOI] [Google Scholar]
- 76.Patrick G.L. An Introduction to Medicinal Chemistry. Oxford University Press; Oxford, UK: 2017. [Google Scholar]
- 77.Borkova L., Jasikova L., Rehulka J., Frisonsova K., Urban M., Frydrych I., Popa I., Hajduch M., Dickinson N.J., Vlk M., et al. Synthesis of cytotoxic 2,2-difluoroderivatives of dihydrobetulinic acid and allobetulin and study of their impact on cancer cells. Eur. J. Med. Chem. 2015;96:482–490. doi: 10.1016/j.ejmech.2015.03.068. [DOI] [PubMed] [Google Scholar]
- 78.Gupta N., Rath S.K., Singh J., Qayum A., Singh S., Sangwan P.L. Synthesis of novel benzylidene analogues of betulinic acid as potent cytotoxic agents. Eur. J. Med. Chem. 2017;135:517–530. doi: 10.1016/j.ejmech.2017.04.062. [DOI] [PubMed] [Google Scholar]
- 79.Meunier B. Hybrid Molecules with a Dual Mode of Action: Dream or Reality? Acc. Chem. Res. 2008;41:69–77. doi: 10.1021/ar7000843. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H., Wang Y., Zhu P., Liu J., Xu S., Yao H., Jiang J., Ye W., Wu X., Xu J. Design, synthesis and antitumor activity of triterpenoid pyrazine derivatives from 23-hydroxybetulinic acid. Eur. J. Med. Chem. 2015;97:235–244. doi: 10.1016/j.ejmech.2015.04.057. [DOI] [PubMed] [Google Scholar]
- 81.Zhang H., Zhu P., Liu J., Lin Y., Yao H., Jiang J., Ye W., Wu X., Xu J. Synthesis, in vitro and in vivo antitumor activity of pyrazole-fused 23-hydroxybetulinic acid derivatives. Bioorg. Med. Chem. Lett. 2015;25:728–732. doi: 10.1016/j.bmcl.2014.11.058. [DOI] [PubMed] [Google Scholar]
- 82.Zhang H., Li F., Zhu P., Liu J., Yao H., Jiang J., Ye W., Wu X., Xu J. Synthesis and Biological Evaluation of Oxygen-containing Heterocyclic Ring-fused 23-Hydroxybetulinic Acid Derivatives as Antitumor Agents. Chem. Biol. Drug Des. 2015;86:424–431. doi: 10.1111/cbdd.12543. [DOI] [PubMed] [Google Scholar]
- 83.Lomkova E.A., Chytil P., Janoušková O., Mueller T., Lucas H., Filippov S.K., Trhlíková O., Aleshunin P.A., Skorik Y.A., Ulbrich K., Etrych T. Biodegradable Micellar HPMA-Based Polymer–Drug Conjugates with Betulinic Acid for Passive Tumor Targeting. Biomacromolecules. 2016;17:3493–3507. doi: 10.1021/acs.biomac.6b00947. [DOI] [PubMed] [Google Scholar]
- 84.Dai L., Li D., Cheng J., Liu J., Deng L.-H., Wang L.-Y., Lei J.-D., He J. Water soluble multiarm-polyethylene glycol–betulinic acid prodrugs: Design, synthesis, and in vivo effectiveness. Polym. Chem. 2014;5:5775–5783. doi: 10.1039/C4PY00648H. [DOI] [Google Scholar]
- 85.Soural M., Hodon J., Dickinson N.J., Sidova V., Gurska S., Dzubak P., Hajduch M., Sarek J., Urban M. Preparation of Conjugates of Cytotoxic Lupane Triterpenes with Biotin. Bioconjugate Chem. 2015;26:2563–2570. doi: 10.1021/acs.bioconjchem.5b00567. [DOI] [PubMed] [Google Scholar]
- 86.Krajcovicova S., Stankova J., Dzubak P., Hajduch M., Soural M., Urban M. A Synthetic Approach for the Rapid Preparation of BODIPY Conjugates and their use in Imaging of Cellular Drug Uptake and Distribution. Chem. Eur. J. 2018;24:4957–4966. doi: 10.1002/chem.201706093. [DOI] [PubMed] [Google Scholar]
- 87.Saneja A., Sharma L., Dubey R.D., Mintoo M.J., Singh A., Kumar A., Sangwan P.L., Tasaduq S.A., Singh G., Mondhe D.M., Gupta P.N. Synthesis, characterization and augmented anticancer potential of PEG-betulinic acid conjugate. Mater. Sci. Eng. C. 2017;73:616–626. doi: 10.1016/j.msec.2016.12.109. [DOI] [PubMed] [Google Scholar]
- 88.Antimonova A.N., Petrenko N.I., Shakirov M.M., Shul’ts E.E. Synthesis of 19-(2,6-Dimethylpyrid-4-yl)-20,29,30-trinorlupanes. Chem. Nat. Compd. 2014;50:305–310. doi: 10.1007/s10600-014-0938-4. [DOI] [Google Scholar]
- 89.Spivak A.Y., Keiser J., Vargas M., Gubaidullin R.R., Nedopekina D.A., Shakurova E.R., Khalitova R.R., Odinokov V.N. Synthesis and activity of new triphenylphosphonium derivatives of betulin and betulinic acid against Schistosoma mansoni in vitro and in vivo. Bioorg. Med. Chem. 2014;22:6297–6304. doi: 10.1016/j.bmc.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 90.Spivak A.Y., Nedopekina D.A., Shakurova E.R., Khalitova R.R., Gubaidullin R.R., Odinokov V.N., Dzhemilev U.M., Bel’skii Y.P., Bel’skaya N.V., Stankevich S.A., et al. Synthesis of lupane triterpenoids with triphenylphosphonium substituents and studies of their antitumor activity. Russ. Chem. Bull. 2013;62:188–198. doi: 10.1007/s11172-013-0028-y. [DOI] [Google Scholar]
- 91.Spivak A.Y., Nedopekina D.A., Khalitova R.R., Gubaidullin R.R., Odinokov V.N., Bel’skii Y.P., Bel’skaya N.V., Khazanov V.A. Triphenylphosphonium cations of betulinic acid derivatives: Synthesis and antitumor activity. Med. Chem. Res. 2017;26:518–531. doi: 10.1007/s00044-016-1771-z. [DOI] [Google Scholar]
- 92.Nedopekina D.A., Gubaidullin R.R., Odinokov V.N., Maximchik P.V., Zhivotovsky B., Bel’skii Y.P., Khazanov V.A., Manuylova A.V., Gogvadze V., Spivak A.Y. Mitochondria-targeted betulinic and ursolic acid derivatives: Synthesis and anticancer activity. MedChemComm. 2017;8:1934–1945. doi: 10.1039/C7MD00248C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mandal A., Ghosh A., Ghosh S., Shil S., Bothra A.K., Ghosh P. 3-Epihydroxy lup-20(29)-en-19(28)-olide: Partial synthesis, antitopoisomerase activity, and 3D molecular docking. Med. Chem. Res. 2016;25:1087–1095. doi: 10.1007/s00044-016-1551-9. [DOI] [Google Scholar]
- 94.Pokorny J., Krajcovicova S., Hajduch M., Holoubek M., Gurska S., Dzubak P., Volna T., Popa I., Urban M. Triterpenic azines, a new class of compounds with selective cytotoxicity to leukemia cells CCRF-CEM. Future Med. Chem. 2018;10:483–491. doi: 10.4155/fmc-2017-0171. [DOI] [PubMed] [Google Scholar]
- 95.Kaluđerović G., Bulatović M., Krajnović T., Paschke R., B. Zmejkovski B., Maksimović-Ivanić D., Mijatović S. (18-Crown-6)potassium(I) Trichlorido[28-acetyl-3-(tris-(hydroxylmethyl)amino-ethane)betulinic ester-κN]platinum(II): Synthesis and In Vitro Antitumor Activity. Inorganics. 2017;5:56. doi: 10.3390/inorganics5030056. [DOI] [Google Scholar]