Abstract
The relentless rise of antibiotic resistance is considered one of the most serious problems facing mankind. This mini-review will cover three cutting-edge approaches that use light-based techniques to kill antibiotic-resistant microbial species, and treat localized infections. Firstly, we will discuss antimicrobial photodynamic inactivation using rationally designed photosensitizes combined with visible light, with the added possibility of strong potentiation by inorganic salts such as potassium iodide. Secondly the use of blue and violet light alone that activates endogenous photoactive porphyrins within the microbial cells. Thirdly the use of “safe UVC” at wavelengths between 200–230 nm that can kill microbial cells without damaging host mammalian cells. We have gained evidence that all these approaches can kill multi-drug resistant bacteria in vitro, do not themselves induce any resistance, and moreover can treat animal models of localized infections caused by resistant species that can be monitored by non-invasive bioluminescence imaging. Light-based antimicrobial approaches are becoming a growing translational part of anti-infective treatments in the current age of resistance.
1. Introduction to Microbial Drug Resistance
Alexander Fleming discovered the first antibiotic called “penicillin” in 1928 (1), and when mass production became possible, it was widely used by Allied Forces in World War II for treating wounds and post-surgical infections. After the war, penicillin began to be prescribed to the general population at large where it was widely hailed as a “wonder drug”, and commentators predicted a future free from the threat of infectious disease caused by bacteria. However when Fleming won the Nobel Prize in 1945 for his discovery, in his Nobel lecture he already warned of bacteria becoming “easily resistant to penicillin” (2).This resistance had been observed even before the antibiotic entered into broad clinical use. Since then a large number of antibiotics have been discovered, and dread infectious diseases caused by bacteria (pneumonia, tuberculosis, cholera, typhoid, diphtheria) do not wreak the large death toll they once did. Surgery, accidental trauma and childbirth have become much safer as a result of antibiotics.
However, over the last 60 years the development of antibiotic resistance has accelerated rather than slowed down (see Table 1). The reasons for this are many and various. Global consumption of antibiotics in clinical medicine rose by nearly 40% between 2000 and 2010. but this figure masks patterns of declining use in some countries and rapid growth in others. There are large variations between different countries; the annual per-person consumption of antibiotics varies by more than a factor of 10 even in developed countries. Overuse and inappropriate use of antibiotics is facilitated in many places by their availability over-the-counter without prescription, and counterfeit and sub-standard antibiotics dominate the market in some regions. The speed and volume of international travel creates new opportunities for antibiotic-resistant pathogens to spread globally. Bacteria can easily share their genetic material with each other, creating new resistant strains at an unprecedented pace. The biggest problem of all is the fact that almost 80% of all antibiotics in the United States are not taken by people but rather used in livestock feedstuff, which was pointed out by Gloria Guglielmi (3). They are administered to cows, pigs, and chickens to make them grow more quickly, and to keep these animals healthy, in intrinsically unhealthy factory farming conditions. In 2013, more than 131,000 tons of antibiotics were used in food animals worldwide; by 2030, it is expected to be more than 200,000 tons.
Table 1.
Timeline of the introduction of new antibiotics and the first reports of resistance emerging.
| Antibiotic introduced | Antibiotic resistance identified | ||
|---|---|---|---|
| 1943 | penicillin | 1940 | penicillin-resistant Staphylococcus |
| 1950 | tetracyline | 1959 | tetracycline-resistant Shigella |
| 1953 | erythromycin | 1978 | erythromycin-resistant Pneumococcus |
| 1960 | methicillin | 1962 | methicillin-resistant Staphylococcus |
| 1967 | gentamycin | 1979 | gentamycin-resistant Enterococcus |
| 1972 | vancomycin | 1988 | vancomycin-resistant Enterococcus |
| 1985 | ceftazidime | 1987 | ceftazidime-resistant Enterobacteriaceae |
| 1985 | imipenem | 1998 | imipenem-resistant Enterobacteriaceae |
| 1996 | lefloxacin | 1996 | lefloxacin-resistant Pneumococcus |
| 2000 | linezolid | 2001 | linezolid-resistant Staphylococcus |
| 2003 | daptomycin | 2005 | daptomycin-resistant Staphylococcus |
| 2010 | ceftaroline | 2011 | ceftaroline-resistant Staphylococcus |
The real value of antibiotics goes beyond simply preventing death and illness due to infection, in that antibiotics also permit the serious iatrogenic assault on the immune system that occurs in cancer treatment by chemotherapy or radiation therapy, or in organ transplantation, where they have helped to keep complication rates down (4). Therefore, antibiotics are an extremely valuable resource across the whole spectrum of modern medicine (5). However, multidrug-resistant (MDR) and pandrug-resistant (PDR) bacterial strains and the related infections they cause, have become emerging threats to public health throughout the world (6). These infections are associated with an approximately two-fold higher death rate, and with considerably prolonged hospital stays (7). Infections caused by antibiotic resistant microbes are often exceptionally hard to treat due to the limited range of therapeutic options (8). Therefore, there is an urgent need for an all-out search for alternative antimicrobial approaches to kill MDR strains, concentrating on methods that are unlikely to cause resistance to develop (9–11). Recently, Karen Bush et al. pointed out that novel non-antibiotic approaches to prevent and treat infectious disease should be considered high-priority international research and development goals (12).
A promising, innovative approach to achieve this goal is the use of light-based approaches to inactivate pathogenic and resistant microbes infecting living tissue, without causing any unacceptable damage to that tissue.
2. Light-based antimicrobial approaches
The part of the electromagnetic spectrum that is known as “light” ranges from UVC (200–280 nm), UVB (280 – 320 nm), UVA (320 – 400 nm), visible (400 – 750 nm), near infrared (NIR, 750 – 1200 nm) and mid/far IR (1200 – 10,000 nm). All these different wavelengths have been used in one form or another for killing various types of microbes. While bacteria have been by the most often studied microbial grouping, fungi, viruses and parasites have also been killed by light-based techniques.
Light based approaches must draw a balance between: (a) the quantum yield of microbial inactivation, in other words how many photons does it require to kill or inactivate a single microbe? (b) the penetration depth of the light into the tissue which increases with wavelength; (c) the propensity of the light to damage the host cells and tissues. UVC is highly active with a lethal dose of a few mJ of energy to kill or inactivate all known types of pathogens, but has poor penetration into tissue and a high possibility for to damage host cells. However so-called “safe UVC” at 220 nm does not damage host cells. Blue light has moderate activity needing 100s of J of energy, and also has relatively poor penetration, but does not much damage host tissue. Red light has no activity at all unless it is combined with a photosensitizing dye (PS), when the activity increases dramatically needing only a few J of energy. Penetration into tissue is good and damage to normal tissue is controllable depending on the PS structure. NIR light has moderate activity (100s of J) but has good penetration, and any damage to tissue depends on the heat produced which in turn depends on the power density.
One of the main advantages of light-based approaches is that most of them (but not all of them) are broad-spectrum in nature and can effectively destroy all kinds of microbes including bacteria (Gram-positive, Gram-negative, mycobacteria), fungi (yeasts and filamentous fungi), viruses (DNA and RNA) and parasites.
Another important advantage of light-based approaches is that the effectiveness of microbial destruction appears to be largely unaffected by the antibiotic resistance status of the particular microbe. Moreover, with the possible exception of UVC, it has proved impossible to artificially generate resistant microbes by the deliberate repeated administration of cycles of sub-lethal killing followed by regrowth. As many as 20 of these repeated cycles have been carried out with any significant resistance developing (13, 14).
3. Antimicrobial photodynamic inactivation (aPDI)
The discovery of photodynamic therapy (PDT) can be dated back to the year 1900, when workers in Munich, Germany observed that single-celled Paramecia (amoebae) were inactivated by the dye, acridine orange in the presence of light, but not in the dark (15). When it was found a few years later that the presence of oxygen was necessary for this effect to occur, the term “photodynamic” was coined (16). PDT was mainly developed as a cancer therapy in the 1960s and 1970s (17, 18). The photochemical mechanism of PDT was first reported in 1977 by Weishaupt et al (19). They identified singlet molecular oxygen as the most important cytotoxic agent in the destruction of cancer cells incubated with hematoporphyrin and exposed to red light. It was later realized that the key feature of compounds (dyes) such as the porphyrins used for PDT, was the existence of a long-lived triplet state that could be formed by an “intersystem crossing process” from the excited singlet state porphyrin, formed when the ground state porphyrin absorbed a photon of energy (20). Since ground state oxygen has a triplet electronic configuration, the excited porphyrin triplet state is allowed to undergo energy transfer with oxygen to produce the ground state singlet porphyrin and the excited state singlet oxygen (1O2). 1O2 is a highly reactive molecule that can oxidize lipids, proteins and nucleic acids, thus causing the death of any kind of cells (bacteria, fungus, cancer, normal cells, etc). Because 1O2 is highly reactive, it can only damage tissue or cells in the precise location where it is produced (i.e. where both dye and light are present at the same time).
In addition to 1O2, (which became known as the Type II photochemical mechanism), it was realized that other different reactive oxygen species (ROS) were also involved in the PDT effect depending on a number of factors. These ROS (including hydroxyl radicals, hydrogen peroxide, and superoxide anion) were produced by what became known as the Type I photochemical mechanism (21). It is thought that this process initially involves a 1-electron transfer from the PS triplet state to oxygen to form superoxide anion. Figure 1 shows a Jablonski diagram illustrating the photochemical mechanisms of PDT.
Figure 1. Jablonski diagram.
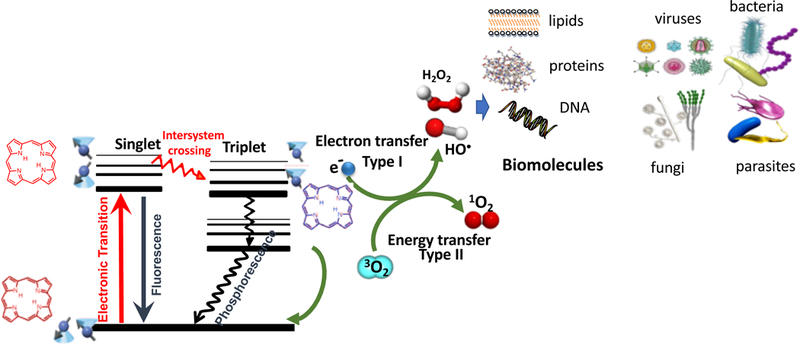
A ground-state PS absorbs a photon, transitions to the short-lived (nsec) excited singlet state that can undergo intersystem crossing to the long-lived (μsec) excited triplet state. The triplet PS can undergo energy transfer with ground state triplet oxygen (3O2) to form reactive singlet oxygen (1O2, Type 2) or else can undergo an electron transfer reaction to form HO•, superoxide and H2O2 (Type 1). These ROS (1O2 and HO•) can damage lipids, proteins and nucleic acids leading to destruction of all types of microbial cells, viruses and parasites.
PDT was not much used as an antimicrobial approach for many years, because the PS that were commonly used for cancer therapy were not particularly effective at killing bacteria, especially Gram-negative strains (22). It was realized around 1990 that, although many Gram-negative bacteria are resistant to aPDI with commonly employed anionic or neutral PS (23), this resistance could be overcome by the use of PS with pronounced cationic charges, or the use of other means of permeabilizing Gram-negative cells (24, 25). These cationic charged PS can be taken up by Gram-negative cells by the process called “self-promoted uptake” (26). In this process, the cationic PS can displace the divalent cations (Ca2+ and Mg2+) that hold together the lipopolysaccharide (LPS) structure forming part of the outer membrane permeability barrier in Gram-negative cells (27). The loss of the LPS allows much more PS to be taken up, and furthermore initial light-mediated damage also increases PS uptake allowing greater PDT damage to occur (28).
The vast majority of PS structures that have been tested for antimicrobial applications are molecules that possess intrinsic cationic charges. These molecules may be cationic porphyrins, chlorins, bacteriochlorins, phthalocyanines, phenothiazinium dyes, fullerenes, BODIPY-dyes, as well as some natural products (29).
There is one major difference between PDT, when it is used as an anticancer treatment, and when it is used as an anti-infection treatment (30). This concerns the mode of administration and the drug-light interval (time between applying the PS and shining the light). In order to treat cancer, it has been well established that the PS functions best when it is injected intravenously into the circulation and allowed to accumulate at the site of the tumor. Indeed, many PS were first clinically tested as fluorescent contrast agents that could “light up” undiscovered tumors in difficult anatomical locations (31, 32). In some cases drug-light intervals as long as 96 hours have been allowed to elapse between injection of the PS (m-tetrahydroxyphenylchlorin) and switching on the red laser light (33). In sharp contrast is the situation when PDT is used to treat localized infections. Here the PS is topically introduced into the infected area. This may be done by painting or dropping the PS solution onto the infected tissue surface, or by for instance a mouth rinse, or instilling into a hollow organ. A short amount of time (minutes) is allowed for the PS to attach and penetrate the microbial cells, which is a relatively rapid process, but not long enough for the PS to be taken up by host cells which is a relatively slow process. Maisch et al even used as short a time as 10 seconds for incubation of the PS with the microbial cells (34). Then when light is delivered the ROS are preferentially generated inside the microbial cells, and no harm is done to host tissue.
The development of resistance to aPDI has not yet been observed despite attempts to deliberately encourage it. If aPDI was used in the treatment of infections, and the PS could only reach the target site at sub-lethal concentrations, any microorganisms surviving would have been exposed to sub-lethal doses of oxidative stress that would not result in total cell death. Since it is known that aPDI can damage DNA, it might also lead to increased mutational events, which could lead to selection for survival of more resistant or less susceptible strains (35). The most frequently employed method of testing for a microbe’s ability to become resistant to a particular agent is by subjecting the said microbe to routine continual exposure to a particular agent.
Giuliani et al. analyzed whether S. aureus, and P. aeruginosa could become resistant to aPDI via repeated exposure to a tetracationic PS Zn(II) phthalocyanine derivative in combination with 30 J/cm2 of 600–700 nm light. After 20 consecutive treatments at PS concentrations corresponding to the previously determined minimum inhibitory concentration (MIC), S. aureus, and P. aeruginosa were all incapable of developing resistance to aPDI. However, when the 20 exposures were repeated without light the MIC for S. aureus of the Zn (II) phthalocyanine derivative in the dark did increase. This shows that S. aureus may be able to develop some ability to protect itself against the dark toxic effect of a PS, perhaps by up-regulating efflux pumps or altering its membrane structure (14).
Cassidy et al. exposed S. aureus and P. aeruginosa to the PS meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate (TMP) and methylene blue (MB) for 72 h in an effort to “habituate” cells. It was shown that sub-lethal aPDI did not decrease the susceptibility to commonly employed antibiotics. Similarly, habituation with sub-lethal aPDI did not reduce susceptibility of P. aeruginosa isolates to aPDI protocols previously determined as lethal (eradication). A reduction in susceptibility to aPDI following habituation was apparent for two S. aureus isolates with MB and for 1 S. aureus isolate with TMP as the photosensitizer (36).
Pourhajibagher et al. studied the effects of aPDI with toluidine blue O (TBO) and light emitting diode irradiation, on virulence features and expression profiling of genes encoding potential virulence factors in a colistin-resistant extensively-drug resistant (XDR) clinical isolate of A. baumannii (CR-XDR-AB) and in A. baumannii ATCC 19606 by studying the cells surviving aPDI. Their results showed that the aPDI could lead to modulation of the virulence of A. baumanii strains in surviving cells in planktonic growth mode by suppressing the expression of the genes (csuE, epsA, and abaI) associated with biofilm formation, but not blsA (the gene corresponding to attenuation in biofilm formation) at the transcriptional level. Following aPDI, there was a significant up-regulation of blsA in CR-XDR-AB, but no up-regulation in the 19606 strain. Insignificant changes in the gene encoding bacterial heat shock protein (dnaK) and DNA repair gene (recA) in surviving cells demonstrated that sAPDI did not induce the typical cell stress fund with other antibacterial treatments, and there was no observable DNA damage when applied to A. baumanii strains in the planktonic growth mode. CR-XDR-AB cells surviving aPDI showed a reduction of cell metabolic activity, increase in outer membrane permeability, and inhibition of efflux pump systems. aPDI reduced the minimum inhibitory concentrations of the most tested antimicrobials by ≥2-fold in CR-XDR-AB strain (37).
4. Potentiation of aPDI by addition of inorganic salts
4.1. In vitro studies
The potentiation of aPDI by addition of inorganic salts was discovered in our laboratory It started when we used sodium azide as a quencher of singlet oxygen (38). Azide has long been known as a physical quencher of singlet oxygen (39). However, when we tested whether azide would quench the bacterial killing mediated by the phenothiazinium dye, methylene blue (MB) and red light (660 nm), we were surprised to find that the killing was paradoxically potentiated (1–2 logs more killing), rather than the expected inhibition (40). Mechanistic investigations showed that there occurred a photoinduced electron transfer to form azide radicals, which could damage the bacterial cells. There was even some oxygen-independent photokilling in the presence of azide, but no killing at all in the absence of oxygen as expected. aPDI mediated by other phenothiazinium dye structures could also be potentiated by addition of azide (41). It is known that phenothiazinium dyes can carry out a mixture of Type 1 and Type 2 photochemistry (38). The fact that oxygen-independent photokilling could also be achieved in the presence of azide when functionalized fullerenes were used as the PS, supported this hypothesis (42). We then hypothesized that electron transfer reactions could occur from the photoexcited PS to other inorganic anions to produce reactive radicals. We tested this hypothesis using photoexcited titanium dioxide which is known as antimicrobial photocatalysis. Photocatalysis largely works by production of Type 1 ROS such as hydrogen peroxide and hydroxyl radicals, This occurs because TiO2 can act as a large band-gap semiconductor, and when photoexcited by short-wavelength light (UVA, 360 nm). it can transfer electrons to oxygen reducing it to superoxide, while at the same time the positive holes can oxidize water to hydroxyl radicals. We showed that potassium iodide could produce dramatic potentiation of the microbial killing (up to six extra logs) of Gram positive and Gram-negative bacteria and also fungi (43). We needed to use relatively high concentrations of iodide (up to 100 mM) to achieve the maximum potentiation. We also showed that TiO2 antimicrobial photocatalysis could be potentiated (although not to the same extent) by addition of potassium bromide (44). The mechanism involved the production of hypobromite (OBr-), a well-known antimicrobial species. The mechanism of iodide potentiation was a combination of the production of relatively stable species (free iodine and hypoiodide) and reactive iodine radicals (43).
We went on to show that aPDI mediated by MB could also be potentiated by KI (45). In this case the mechanism appeared to be a combination of the production of stable antimicrobial species (free iodine and hypoiodite) and short lived reactive species (iodine radicals). The concentration of KI is critical in determining the mechanism. If the concentration is relatively low (up to 10 mM) then iodine radicals are mainly responsible, but if the KI concentration is increased up to 100 mM (or even higher) then free iodine is mainly responsible.
Because of our results with TiO2 photocatalysis, we assumed that the mechanism was via electron transfer (Type 1) but we subsequently revised our theory when we found that the antimicrobial effects of other different PS could also be dramatically potentiated by addition of KI (46). The first indication that the antimicrobial action of Type 2 PS could be dramatically potentiated by addition of KI came with Photofrin (47). Photofrin is a well-known PS used for many years to treat cancer with PDT. It is a derivative of hematoporphyrin which is water soluble, and has been found to localize in tumors when injected intravenously. Photofrin is considered to be a typical Type 2 PS which works mainly by the generation of singlet oxygen (48). Because Photofrin is an anionic compound it does not bind, penetrate or photokill Gram-negative bacteria, although it is quite effective at killing Gram-positive bacteria and fungi (49). When we added 100 mM KI to Photofrin and excited it with blue light (405 nm) we were able to eradicate several different species of Gram-negative bacteria (E. coli, Proteus mirabilis, Klebsiella pneumoniae, Acinetobacter baumannii and Pseudomonas aeruginosa) which were completely unharmed in the absence of KI (47).
To explain this impressive result, we compared the effects of three different formats. The first was termed “In” where all components of the system (bacteria, PS, salt) are present together during the light delivery. The second was “Spin” where the bacteria and PS were incubated together, centrifuged to remove unbound PS, resuspended in KI or other salt solution, and the light delivered. The idea here is to ensure that the PS is in contact with the bacteria, so that if iodine radicals are produced during illumination they will be close to their target. Lastly we have “After”, where the PS and KI (or other salt) were mixed together, illuminated, and either immediately or after different times the bacterial cells were added and mixed. Here the idea is to detect killing caused by stable (free iodine) or semi-stable (hypoiodite) species as compared to short-lived radicals. The results showed that Gram-negative bacteria were killed by free iodine or hypoiodite, while Gram-positive bacteria were more likely to be killed by iodine radicals. The overall chemical equation proposed to explain the oxidation of iodide by singlet oxygen is as follows:
| (1) |
It should be noted that hydrogen peroxide (another stable species) is also produced during this reaction, and there is evidence that hydrogen peroxide and iodine combine to exert synergistic effects against bacterial and yeast species (50).
We next went on to confirm our findings by testing a different (but still well-known) PS that has high activity against Gram-positive species, but no activity against Gram-negative species, namely Rose Bengal (RB). RB was also strongly potentiated by the addition of 100 mM KI producing eradication of several different Gram-negatives, under conditions where no killing was seen without KI (51). We then went on to test a hypothesis that the strength of the binding of the PS to the bacterial cell would govern the amount of potentiation that was obtained by addition of KI. If the PS bound strongly to the bacteria, we argued that potentiation should be found at lower KI concentrations because reactive iodine radicals could carry out the killing. On the other hand, if the PS did not bind to the bacteria and remained in solution, then illumination would generate free iodine which would need a much higher KI concentration. We decided to test this by comparing two porphyrins with different charges, cationic TMPyP4 and anionic TPPS4 (52). Initially the results were as expected, with TMPyP4 (no salt) eradicating Gram-negatives and TPPS4 producing no killing. As expected both porphyrins eradicated bacteria when 100 mM KI was added. An unexpected result was seen when TPPS4 was tested in the “spin” format and eradicated E. coli when KI was added. This seemed to imply that an anionic porphyrin can bind to Gram-negative bacteria. The mystery was solved when we realized that although TPPS4 is a tetrasulfonic acid, it is not actually anionic, but rather behaves as if it is cationic, because of the presence of two ring pyrrole nitrogen atoms that can form hydrochloride salts. Hence although TPPS4 is not sufficiently cationic to kill Gram-negative bacteria in the absence of KI, it is sufficiently cationic to bind to Gram-negatives and kill the bacteria in the “after” format combined with the addition of KI.
There are other inorganic salts besides azide, iodide, and bromide that can potentiate aPDI. We showed that potassium thiocyanate KSCN could potentiate the killing of both Gram-positives and Gram-negatives by MB-aPDI (53). We originally hypothesized that the active species might be thiocyanate radicals (SCN•) formed by a Type 1 electron-transfer mechanism. However mechanistic investigations led us to revise that theory when we found that SCN- quenched singlet oxygen luminescence, dramatically increased oxygen consumption, and produced the spin-trapped ESR signal of an unexpected radical, sulfur trioxide radical anion (SO3•-) (53).
We then tested another different pseudohalide salt which is isoelectronic with KSCN, namely potassium selenocyanate, KSeCN (54). KSeCN at concentrations up to 100 mM could potentiate aPDI giving up to 6 logs of extra killing of E. coli and MRSA. This was observed with aPDI mediated by several different PS structures: MB, RB and TPPS4. When a mixture of selenocyanate with these PS in solution was illuminated and then bacteria were added after the light in the “after format”, there was up to 6 logs of killing (Gram-negative > Gram-positive) but the antibacterial species decayed rapidly (by 20 minutes). Our hypothesis to explain this antibacterial activity was the formation of selenocyanogen (SeCN)2 by oxidation of SeCN- by singlet oxygen (1O2) as shown by quenching of the 1O2 1270 nm luminescence signal by SeCN- and increased photoconsumption of oxygen. The fact that lead tetraacetate mixed with SeCN- which is a literature preparation of (SeCN)2, also produced a short-lived antibacterial species, supported this hypothesis.
4.2. In vivo studies using potentiation of aPDI by inorganic salts.
Up to now we have demonstrated the in vivo applicability of inorganic salt potentiation of aPDT, using potassium iodide. The reason for choosing KI was as follows. KI is non toxic, and saturated KI solution is prescribed orally as a treatment for diverse fungal diseases and inflammatory conditions in dermatology (55–57). KI also has regulatory approval as a prophylactic medicine for release of radioactive iodine in cases of potential nuclear contamination (58).
For some years we have been utilizing a technology that employs stable bioluminescent bacteria or fungi, combined with sensitive low-light imaging (59) to test in vivo treatment of localized infections in small animal models by aPDT (60) (and some other different antimicrobial technologies (61). The advantages of this approach are: (1) it provides real-time information on bacterial viability and distribution within the tissue; (2) it is non-invasive and does not require sacrifice of animals or removal of biopsies; (3) it dramatically reduces animal numbers and each animal can be repeatedly imaged to follow the time course of infections. Figure 2 illustrates a typical experiment in which aPDT is carried out on an infected 3rd degree burn on the mouse back.
Figure 2. PDT experiment.
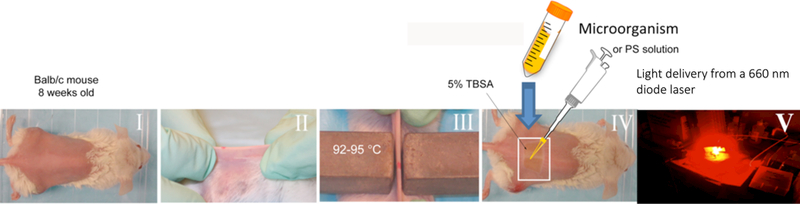
Illustration of an aPDT experiment in a mouse model of an infected burn produced by two heated brass blocks. Generally the bacteria are applied to the surface of the burn by a pipette, followed after a short time by application of the PS in a similar fashion, and after another relatively short time by light delivery.
The combination of aPDI mediated by MB together with KI was studied in a mouse model of a murine burn infection using bioluminescent MRSA (45). The infected burn was treated with 50 μM MB, with and without the addition of 10 mM KI, and excited with 660 nm light at up to 150 J/cm2, while the control groups were dark controls with the same amount of MB plus KI and the light-alone control received 660 nm light to 150 J/cm2. Figure 3 shows a set of five representative bioluminescence image time courses from burns (each time course from a single mouse in each of the 5 groups) infected with MRSA. The amount of light required to eradicate bacteria from the wound was much less when the MB plus KI solution was irradiated than when MB alone was irradiated.
Figure 3. PDT mediated by MB plus KI in vivo.
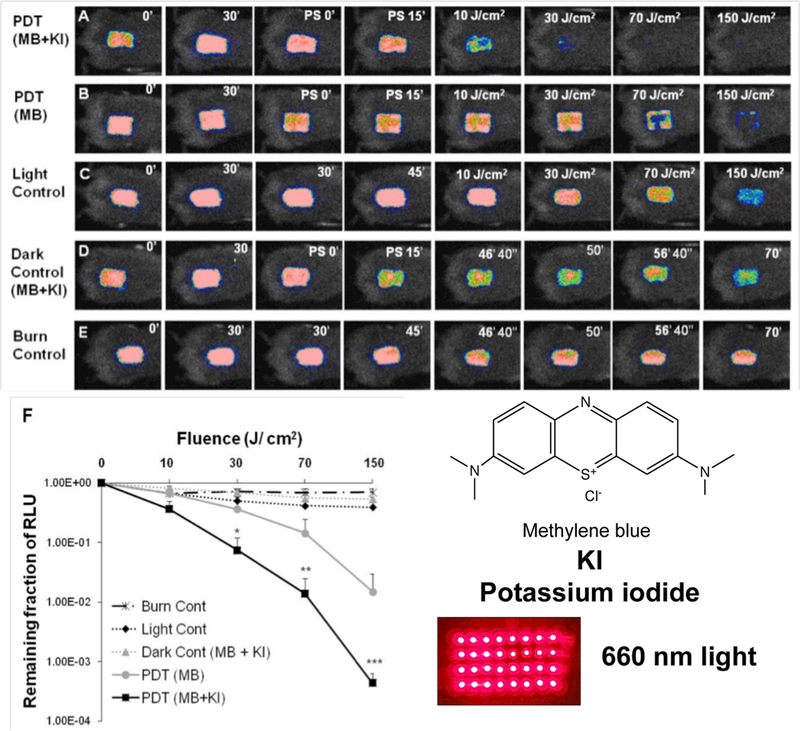
(A-E) Successive bacterial bioluminescence images of representative mouse burns infected with 10(8) CFU of luminescent MRSA (USA 300) treated with: (A) PDT using mixture of MB (50 μM) + KI (10 mM) or (B) PDT using MB (50 μM) at 30 min after bacterial inoculation + 15 min from PS application. PDT was carried out with a combination of 50 μL of a mixture containing MB + KI or MB alone and 150 J/cm2 red light (660 ± 15 nm, 100 mW/cm2). (C) Light alone; (D) applied with mixture of MB + KI, but without red light illumination (dark control); (E) burn control without any treatment. (F). Dose-response plot of mean bacterial bioluminescence of mouse burns infected with MRSA (USA 300) after treatment with: light alone, mixture of MB (50 μM) + KI (10 mM) (dark control), PDT using MB (50 μM) alone or mixture of MB (50 μM) + KI (10 mM). From (45) no permission necessary.
Other similar studies used an abrasion wound infected with bioluminescent A. baumannii and treated with aPDT mediated by a functionalized fullerene excited by UVA or white light combined with KI (62), and an abrasion wound infected with bioluminescent P. aeruginosa and treated with aPDT mediated by RB excited by green light combined with KI (51). A mouse model of oral candidiasis caused by bioluminescent C. albicans was treated by MB-mediated aPDT combined with KI (63). A model of urinary tract infection in the female rat bladder caused by a bioluminescent strain of uropathogenic E. coli was treated by instillation of MB solution followed by a KI solution and then intravesicular delivery of red light using a fiber optic (64).
5. Blue light antimicrobial photoinactivation
5.1. In vitro studies
The advantages of using blue light alone to kill resistant microbes, is that the light is not as harmful to the host tissue or to the surroundings compared to UV light and moreover no added exogenous PS or dye is required (65, 66). Firstly, we must define what wavelengths can be called “blue light”. The most effective range is from ~390 nm to 420 nm (more accurately termed “violet” light), the next most effective range is from 450–480 nm and possible the least effective range is from 420–450 nm. Over the last five years a wide range of microbial cells, including Gram-positive bacteria, Gram-negative bacteria, mycobacteria, molds, yeasts and dermatophytes have been shown to be susceptible to blue light (66). Studies have been carried out in vitro using planktonic cells or biofilms, ex vivo, and in vivo using animal models (pre-clinical) and even in patients (clinical trials). The biological response to blue-light was firstly reported in 1881 by Charles Darwin when he described a blue light induced phototropic response in plants (67), and has since been identified in all three domains (eukaryotes, bacteria and archaea). Recent discoveries have been made showing that within the genomes of many bacteria and fungi, are sequences encoding photoreceptor proteins. Other photoreceptors have been studied in E.coli (68) and A. baumannii (69) that possess a functional blue-light-sensing A (bslA) gene, coding for a protein called BLUF (blue light sensing using flavins. Blue light can regulate bacterial motility, suppress biofilm formation, and subsequently potentiate light inactivation of bacteria. On the other hand, the presence of blue light may also activate or increase bacterial virulence (69).
The lethality of blue light for bacteria has been reported both in vitro and in vivo. Blue light can mediate a broad-spectrum antimicrobial effect on both Gram-negative and Gram-positive bacteria. While the wavelength range of 390–420 nm has been reported to be the most effective antimicrobial spectral range, both 455 nm and 470 nm have also been found to have some antimicrobial effects on some bacterial species (e.g., S. aureus). The mechanism of the antimicrobial effect of blue light is that blue light excites endogenous intracellular metal-free porphyrins to behave as PS as described above for the case of aPDI. This photon absorption then leads to energy transfer from the porphyrin triplet state to oxygen producing 1O2 in a similar manner to PDT (70). However, different bacteria demonstrate variable susceptibilities to blue light. Studies have reported that Gram-positive species, in general, were more susceptible to 405 nm light inactivation than Gram-negative species, which is generally consistent with the results obtained in a recent study (71, 72). It has also been theorized that the differences in inactivation kinetics may be due to organism-specific differences in porphyrin levels, different individual porphyrin sub-types (protoporphyrin, coproporphyrin or uroporphyrin), or different porphyrin subcellular localizations (9, 71). Moreover, it has been speculated that less oxygen-tolerant bacterial species may be particularly susceptible to the effects of ROS as some microaerophilic species have been found to possess fewer key antioxidant defenses than most aerobes (73, 74). For example, studies have shown blue light to be capable of inactivating the anaerobic oral pathogens Prevotella, Porphyromonas, and Fusobacterium as well as microaerophilic pathogens such as P. acnes and H. pylori (9, 75). However, inactivation results achieved with anaerobic/microaerophilic bacteria have not provided conclusive evidence as to whether oxygen-sensitive anaerobic bacteria are any more susceptible than aerobes as many of these anaerobic bacteria are also known to accumulate high levels of porphyrins.
Blue light is also lethal to many species of fungi, including filamentous fungi, although the effectiveness is dependent on the light wavelength, energy levels and microbial genus and species (76). Blue non-coherent light sources, such as halogen lamps, light-emitting diodes (LED), and plasma-arc curing (PAC) lights, are often used in vivo and in vitro studies. In vitro bactericidal efficacy of blue light for the bacteria Escherichia coli, MRSA and P. aeruginosa, even Streptococcus mutans in biofilms has been reported (77). Studies on the in vitro antimicrobial effects of blue light are summarized in Table 2.
Table 2.
Studies of the in vitro antimicrobial effect of blue light
| Light Source | Radiant exposure | Bacterial species/strains | Inactivation efficacy | Ref |
|---|---|---|---|---|
| 405-nm diode laser | 20 J/cm2 | H. pylori | >99.9% | Hamblin et al (70) |
| 405 nm light-emitting device | 15 J/cm2 at lamp aperture | P. gingivalis | >75% | Fukui et al. (122) |
| 380–520 nm broadband light | 4.2–42 J/cm2 | P. gingivalis, P. intermedia, P. nigrescens, P. elaninogenica, S. constellatus |
P. intermedia and P. nigrescens: >5 log10 at 4.2 J/cm2; P. melaninogenica: >5 log10 at 21 J/cm2; P. gingivalis:1.83 log10 at 42 J/cm2 | Soukos et al. (123) |
| 400–500 nm lamps | 260 and 1300 mW/cm2 for up to 3 min | P. gingivalis,
F. nucleatum, S. mutans, E. faecalis |
The minimal inhibitory dose for P. gingivalis and F. nucleatum was 16–62 J/cm2, for S. mutans and S. faecalis was 159–212 J/cm2 | Feuerstein et al. (124) |
| 405-nm superluminous diode light |
50.4–55.2 J/cm2 | MRSA USA 300; MRSA IS-853 | 92.1% for USA 300; 93.5% for IS-853 | Enwemeka et al. (125) |
| 470-nm superluminous diode light | 55 J/cm2 | MRSA USA 300; MRSA IS-853 | 90.4% for both strains | Enwemeka et al. (126) |
| 405 and 470 nm light | 15 J/cm2 | S. aureus,
P. aeruginosa |
S. aureus: 90% at 405 nm, 62% at 470 nm; P. aeruginosa: 95.1% at 405 nm, 96.5% at 470 nm | Guffey and Wilborn (127) |
| 407–420 nm | five P. acnes strains | decreased by 15.7% immediately and 24.4% at 60 min after the irradiation. | Kawada et al. (128) | |
| 407–420 nm | 75J/cm2 | P. acnes | less than 2-log10 units (99%) illuminated once; decreased by 4-log10 units (99.99%) after two illuminations and by 5-log10 units (99.999%) after three illuminations | Ashkenazi et al.(129) |
| 390–410 nm LED | 54–108 J/cm2 | A. baumannii, Enterobacter cloacae, Stenotrophomonas maltophilia, P. aeruginosa, E. coli, S. aureus, E. faecium, Klebsiella pneumoniae, Elizabethkingia meningoseptica. | Most (71%) showed a decrease of 5 log10 units | Halstead et al (130) |
| 405–425nm LED | 110 J/cm2 | A. baumannii | 7.64-log10 CFU | Dai et al (89) |
| 405–425nm LED | 70 J/cm2 |
P. aeruginosa,
A. baumannii, methicillin-resistant S. aureus (MRSA), Candida albicans |
> 4-log10 CFU | Zhang et al (90, 91) |
| 393–413 nm LED | 133 J/cm2 |
S. aureus,
S. pneumonia, E. coli, P. aeruginosa |
4.75-log10 | Barneck et al (131) |
| 118 – 2214 J/cm2 |
S. aureus,
S. epidermidis, E. faecalis, S. pneumoniae, Corynebacterium striatum, E. coli, K. pneumoniae, P. aeruginosa, Serratia marcescens C. albicans. |
Complete inactivation (> 4-log10 CFU) in suspension was achieved in all of the isolates tested. | Gupta et al (132) | |
| 395–414 nm LED | 68 J/cm2 | β-lactam-resistant E. coli | > 6-log10 CFU reduction | Rhodes et al (133) |
5.2. Mechansisms of aBL
Broadly speaking the different wavelengths of aBL can be divided into two broad classes. The chromophores that have been proposed to be responsible for the aBL killing of bacteria are shown in Figure 4. Firstly, wavelengths in the range of 390–420 nm are absorbed by the Soret band of metal-free porphyrins. The molar extinction coefficient of the porphyrin Soret band can be large, around 150,000–250,000 M-1cm-1. However, the specific types of porphyrins can lead to different photo-inactivation rates in different bacteria (i.e. Staphylococcus, Streptococcus, Bacillus, Escherichia, Acinetobacter, and Aeromonas) (78). Both S. aureus and S. epidermidis produced coproporphyrin as the predominant porphyrin species, whereas there was no predominant porphyrin produced in the Gram-negative E. coli, Acinetobacter, and Aeromonas strains. The amount of coproporphyrin produced by the Staphylococcal strains was two to three times higher than in the Gram-negative strains. Therefore, different bacteria produce different porphyrins and the peak absorption wavelengths of these porphyrins may differ. Therefore, different wavelengths may be required for their optimum photo-stimulation. The above-mentioned factors may be responsible for the variation in the bactericidal efficacy of blue light. There has been no comprehensive study of the amounts and identities of the porphyrins produced by bacteria in laboratory cultivation conditions as well as their natural physiological status in the environment, especially bacteria that are infecting a mammalian host (79). Do bacteria that can feed on blood and blood products such as hemin, accumulate more or less porphyrins compared to bacteria that grow on laboratory media? Secondly, wavelengths around 450 nm are absorbed by flavins such as (FMN) and flavoproteins. Although flavins have a double absorption band in the UVA and the blue, they have a minimum about 400 nm. While there are numerous studies looking at aPDI with exogenously added riboflavin and other flavins (80, 81), unequivocal proof that endogenous flavins are responsible for aBL killing is scarce (82). Thirdly, there was an interesting report that in the case of S. aureus, the yellow-orange pigment known as staphyloxanthin could act as a blue-light absorbing lethal chromophore (83).
Figure 4. Chromophores for blue light inactivation of bacteria.
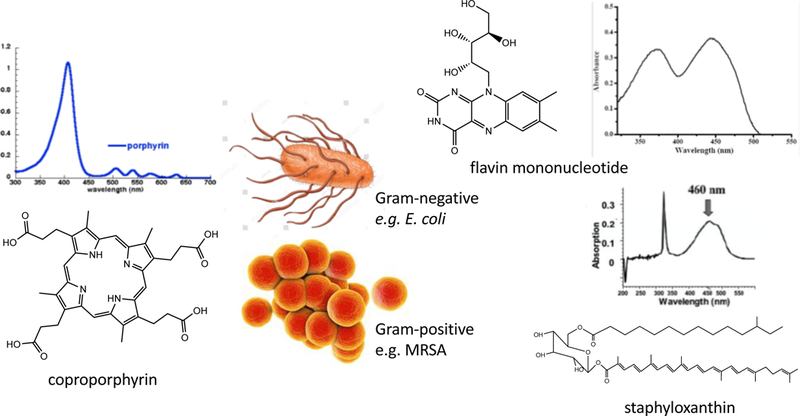
Cemical structures and absorption spectra of coproporphyrin, flavin mononucleotide, and staphyloxanthin.
5.3. Resistance to aBL
In order to assess the possible development of tolerance or resistance to antimicrobial blue light alone in P. aeruginosa, 10 repeated cycles of sub-lethal inactivation of bacteria in vitro, followed by bacterial re-growth, were carried out by Amin et al (13). This tolerance study showed no evidence of the development of resistance by P. aeruginosa to blue light after 10 consecutive cycles of sub-lethal inactivation. Their finding was in agreement with similar findings obtained previously when an A. baumannii strain was treated with blue light for 10 consecutive cycles of sub-lethal inactivation (84).
5.4. Blue light toxicity towards host cells and tissue
If the use of blue light for the treatment of infectious disease is to become accepted, it is important to understand the effects of blue light on host cells and tissues so that non-specific damage to the patient can be avoided (or at least not be a concern). However, only limited studies have been reported so far in this area.
In one in vitro study, Hockberguer et al. (85) showed that mouse (3T3 fibroblasts), monkey (kidney epithelial cells), and human (foreskin keratinocytes) irradiated with blue light (450nm-nm at 6.3 W/cm2) for 20 min produced hydrogen peroxide (H2O2), an important ROS which can cause cellular damage and could contribute to pathologies associated with exposure blue light. Another study was carried out by Wataha et al. (86) The investigators treated 3T3 mouse fibroblasts with three dental blue (400nm-500 nm) light sources, and detected succinate dehydrogenase (SDH) activity of mitochondria after a range of fluences from 1.3 to 60 J/cm2. Results showed that fluences ranging from 5 J/cm2 to 15 J/cm2 irreversibly suppressed SDH activity nearly 100% when compared to no-light controls up to 72 h post-exposure. A recent clinical study by Kleinpenning et al. (87) found no significant side effects of blue light on human skin. Eight healthy volunteers were irradiated with a blue light source (390–460 nm) with a peak emission of 420 nm on 5 consecutive days at 20 J/cm2 daily. To analyze the irradiation effects, skin biopsies were analyzed for p53 expression, vacuolization, sunburn cells, elastosis, matrix metalloproteinase-1 (MMP-1), and melan-A expression. No inflammatory nor sunburn cells were found and no significant changes in p53 and MMP-1 expressions or sign of elastosis were observed after blue light exposure.
5.5. Animal studies of antimicrobial blue light for localized infections
As discussed above in the section about aPDT, we have developed several different small animal models of localized infections produced by stable bioluminescent bacteria and fungi that are suitable for testing light-mediated antimicrobial approaches. These models were ideal to test the in vivo ability of aBL to treat real life infections. Firstly, we tested community acquired MRSA infection of a skin abrasion on the mouse back. Exposure to 40 J/cm2 of 415 nm LED on the same day of the infection, or to 108 J/cm2 on the day after the infection both led to a reduction of 99% in the bioluminescence signal (88). Next, we studied 3rd degree burns on the mouse back infected with P. aeruginosa (89). Application of 55 J/cm2 of 415 nm LED on the same day of infection led to a reduction of 99% in the bioluminescence signal, and importantly 100% of the mice survived, as opposed to only 18% of mice in the untreated control group (see Figure 5).
Figure 5. Wound infection treated with aBL.
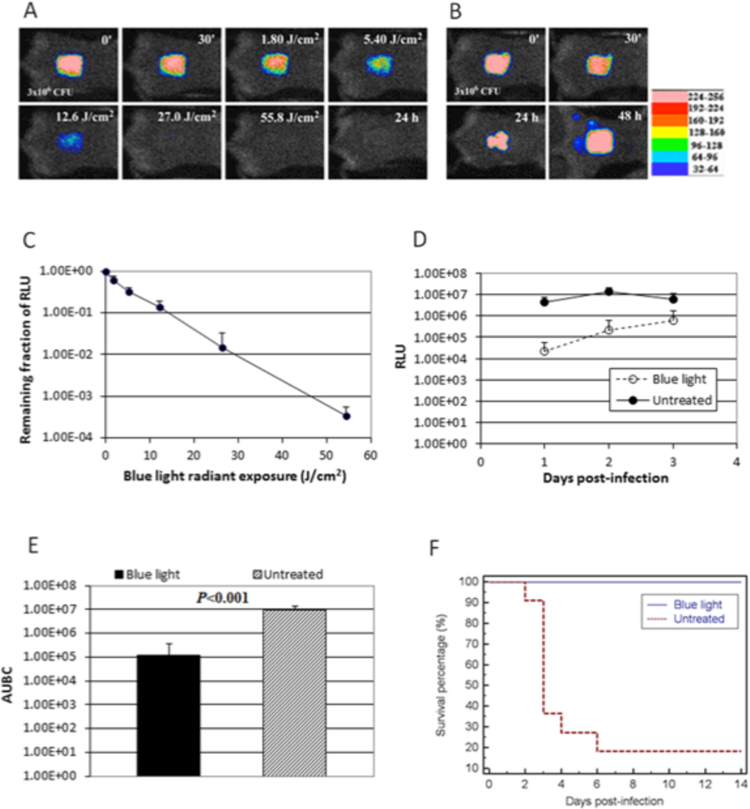
A, B. Successive bacterial luminescence images of representative mouse burns infected with 3 X 10(6) CFU of luminescent P. aeruginosa with and without blue light (415 nm) exposure, respectively. Blue light was delivered at 30 min after bacterial inoculation. (C) Dose response of mean bacterial luminescence of mouse burns infected with 3 X 10(6) CFU of P. aeruginosa and exposed to blue light (415 nm) at 30 min after bacterial inoculation. (D) Time courses of mean bacterial luminescence of the infected skin abrasions with and without blue light exposure, respectively (E) Mean areas under the bioluminescence versus time curves (from day 1 to day 2 in the two-dimensional coordinate system in panel D), representing the overall bacterial burden of mouse wounds. Bars, standard deviations. (F) Kaplan-Meier survival curves of blue light-treated (n = 11) and untreated (n =11) mouse burns (P = 0.0001). From (89) no permission necessary.
A similar result was achieved with A. baumannii infecting a 3rd degree mouse burn (90). C. albicans infection in a mouse burn could be reduced by 1.75 log10 units by 432 J/cm2 of 415 nm LED (91). The last study utilized an interesting animal model of infectious keratitis caused by inoculation of bioluminescent P. aeruginosa onto the surface of the mouse cornea (92) and is shown in Figure 6. The effectiveness of aBL (415 nm LED) was evaluated as a function of radiant exposure when aBL was delivered at 6 or 24 hours after bacterial inoculation. The aBL exposure calculated to reach the retina was within the safety limits laid down by the American National Standards Institute. Bacterial burden in the infected corneas was rapidly and significantly reduced (>2-log10) after a single exposure of 180 J/cm2 at 6 hours and 372 J/cm2 at 24 hours. These animal studies are summarized in Table 3.
Figure 6. aBL for infectious keratitis.
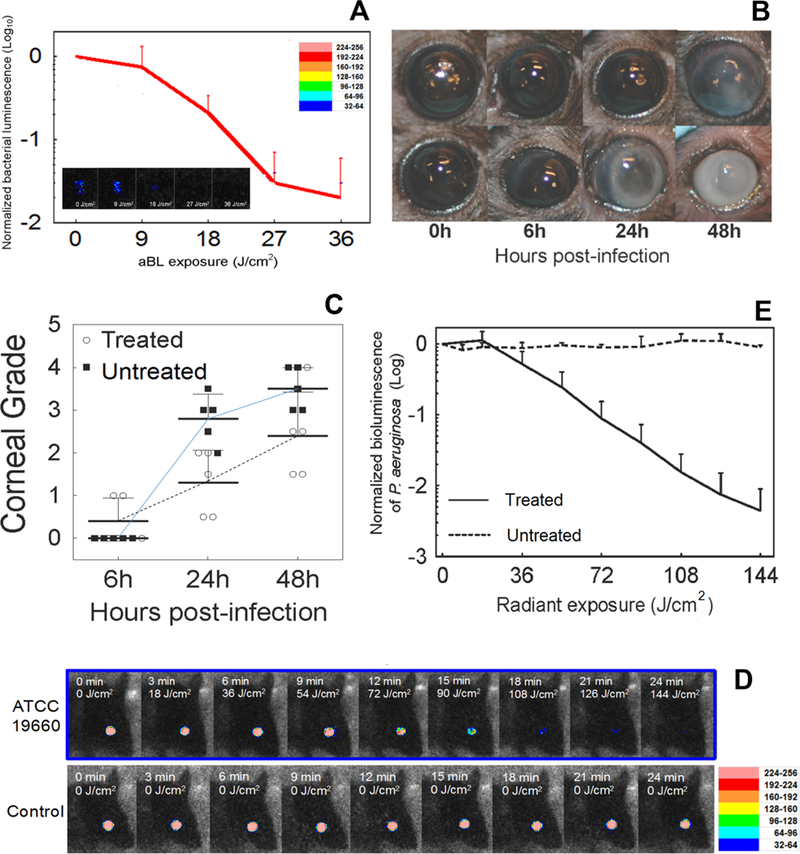
(A) Influence of radiant exposure of aBL on the bacterial luminescence intensity of mouse eyes infected with 5X10(5) CFU P. aeruginosa and treated with 36 J/cm2 aBL at 6 hours post inoculation. The inset shows the bacterial luminescence images from a representative mouse eye infected with 5X10(5) CFU P. aeruginosa and treated with 36 J/cm2 aBL at 6 hours post inoculation. Bacterial luminescence images were taken when 0, 9, 18, 27, and 36 J/cm2 aBL had been delivered. The pseudo-color scale bar indicates the bioluminescence intensity value of a single pixel in the bioluminescence images. (B) Photographs of two representative mouse eyes infected with 5X10(5) CFU P. aeruginosa treated with aBL (36 J/cm2) at 6 hours post infection (top row) or not treated (bottom row), indicating less opacity and damage in the aBL-treated mouse eye than in the untreated one. (C) Corneal pathology scores of the infected mouse eyes treated with 36 J/cm2 aBL at 6 hours post infection or not treated showing the higher mean corneal pathology scores of the untreated mouse eyes than the aBL-treated mouse eyes Analysis of the interaction between aBL treatment and time post infection shows that the effect of aBL treatment increased with the time post infection. Data were analyzed using the general linear model. (D) The bacterial luminescence images of representative mouse eyes infected with 5X10(5) CFU P. aeruginosa, treated with 144 J/cm2 aBL 24 hours later or left untreated. (E) Influence of radiant exposure of aBL on the bacterial luminescence intensity of the mouse eyes infected with 5X10(5) CFU P. aeruginosa, treated with 144 J/cm2 aBL at 24 hours post inoculation or left untreated. Bacterial luminescence images were taken when 0, 18, 36, 54, 72, 90, 108, 126, and 144 J/cm2 aBL had been delivered. From (92) no permission necessary.
Table 3.
Reports of the in vivo use of aBL in animal models of localized infection
| Light Source | Animal model | Bacterial species/strains | Inactivation efficacy | Ref |
|---|---|---|---|---|
| 405–425 nm LED | Infected skin abrasion on mouse back | Bioluminescent MRSA USA300LAC::lux |
> 2 log10 reduction of bioluminescence after 40 J/cm2 (day 0) or 108 J/cm2 (day 1) | Dai et al (88) |
| 405–425 nm LED | Infected 3rd degree burn on mouse back | Bioluminescent P. aeruginosa | Bioluminescence was reduced 100-fold by 55 J/cm2. Mouse survival was 100% vs 18.2% in untreated mice | Dai et al (89) |
| 405–425 nm LED | Infected 3rd degree burn on mouse back | Bioluminescent A. baumannii | 55.8 J/cm2 reduced bacterial levels in burn | Zhang et al (90) |
| 405–425 nm LED | Infected 3rd degree burn on mouse back | Bioluminescent C. albicansi | A single exposure of 432 J/cm2 aBL reduced the fungal burden in infected mouse burns by 1.75-log10 | Zhang et al (91) |
| 405–425 nm LED | Infectious keratitis in mice | Bioluminescent P. aeruginosa | Bacterial luminescence in the infected corneas was reduced (>2-log10) after a single exposure of aBL. | Zhu et al (92) |
5.6. Clinical application of blue light for infectious diseases
Several studies have reported the benefits of blue light on acne vulgaris, an important dermatologic disorder in which the major pathogen is P. acnes. It has long been known that when acne lesions are examined under blue light or UVA light, distinct red fluorescence is observed (93). This fluorescence arises from porphyrins that have been synthesized by the P. acnes bacteria and have accumulated in the sebum secretions of the sebaceous glands. Fluorescence correlates better with the amount of sebum, rather than with the amount of P. acnes bacteria (94). Therefore, it is at present unclear if the beneficial effects of blue light on acne rely mainly on PDT killing of the actual bacteria, or on some PDT effects on the sebaceous glands. Nevertheless, studies have shown that blue light does produce a clinically significant effect on acne (95).
Blue light also can inactive H. pylori, which is the major pathogen causing atrophic gastritis and peptic ulcers and is increasingly resistant to antibiotics (96). Ganz et al. (97) treated 10 patients with blue light (405 nm, 40.5J/cm2) via an optical fiber inserted in the endoscope channel that illuminated a 1-cm diameter area of the gastric3mucosa. Significant decreases (91%) in H. pylori colonies per gram of tissue in treated sites was observed by comparing biopsies from treated and adjacent untreated sites. Some patients showed reductions of bacterial burden approaching 99%. Another pilot trial was carried out by Lembo et al. (98) using a blue (408 nm) laser source with multi-segmented balloon that allowed whole stomach illumination to treat 18 patients with H. pylori infection. There was a generalized reduction in bacterial load in the stomach. The largest reduction in bacterial load was found in the antrum (>97%), followed by the body (>95%) and the fundus (>86%) with no apparent dose-response correlation. However, repeated breath tests showed H. pylori repopulated in days following illumination. These clinical studies showing that blue light can treat inflammatory and non-inflammatory acne lesions caused by P. acnes, and possibly digestive disorders caused by H. pylori are summarized in Table 4.
Table 4.
Clinical studies with antimicrobial blue light
| Light source | Clinical trial type | Disease | Treatment regimen | No. of patients/acne location | Total follow-up time | outcome | Side-effects | Ref |
|---|---|---|---|---|---|---|---|---|
| 407–420 nm metal halide lamp | open clinical trial | Mild to moderate acne | twice a week up to 5 weeks | 30 | 8 weeks | Acne lesions were reduced by 64%. | Two patients experienced dryness. | (128) |
| Handheld 412 nm LED | prospective, single-center, open-label study | Mild-to-moderate facial acne, | 2J/cm2/day full-face treatment | 32 | 8 weeks | 100 percent of subjects improved, |
Three minimal adverse events | (134) |
| home use blue-LED | IRB approved randomized self-control study | Inflammatory facial acne | Four Treatments twice daily | 30 | until resolution | significant reduction in lesion size and erythema and improvement | N/A | (135) |
| hand-held, blue- LED | open clinical trial | mild-to-moderate facial acne | twice daily dose of ~29 J/cm2, | 33 | 8 weeks | 90% improvements | N/A | (136) |
| 407 to 420 nm LED | prospective, randomized, open comparative study | inflammatory facial acne grades II or III | 8 sessions of light therapy for 15 minutes each, twice a week, | 60 | 4 weeks | Equivalent improvement to benzoyl peroxide | 23.3% patients with mild desquamation and/or dryness | (137) |
| 415 nm LED | open-label study | 48 J/cm2, twice a week for 4–8 weeks | 45 | 8 weeks | mean improvement score was 3.14 at 4 weeks and 2.90 at 8 weeks | N/A | (138) | |
| 409–419 nm LED | open-label study | mild to moderate acne | eight 10–20 treatments over 4 weeks | 30 | 12 weeks | Significant decrease in lesion count continued to week 12. | N/A | (139) |
| 405 nm laser | controlled, prospective, blinded, trial |
H. pylori stomach infection | 40 J/cm2 was delivered to a 1-cm diameter spot in the gastric antrum via optical fiber passed through an endoscope |
10 | Comparison of biopsies removed at treatment | mean reduction in CFU/g tissue was 91% |
none | (97) |
| 408 nm laser | controlled, prospective pilot trial |
H. pylori stomach infection | Whole stomach illumination via endoscopic balloon and diffusing fibers 10–30 kJ |
18 | Comparison of biopsies removed at treatment and urea breath test | Reduction in CFU/g tissue ranged from 93% to 86% depending on location. Urea breath test dropped after Tx but recurred after 5 weeks |
Transient, minor | (98) |
5.7. Other applications of aBL
Many of the proposed applications of aBL have been concerned with areas of antimicrobial killing or sterilization that are not directly connected to the goal of this review (prevention and treatment of antibiotic resistant infections) and might be considered off-topic. Therefore, we will not cover these in detail, but rather give one or two examples.
Medical applications include inactivation of bacterial contamination in ex vivo stored plasma (99), and sterilization of contact lenses (100). Agricultural produce and foodstuffs have been one of the most investigated areas so far. aBL has been studied for killing E. coli in milk and Salmonella in orange juice (101). There have also been studies of aBL for bacterial contamination on the surfaces of foodstuffs such as fresh-cut papaya (102), fresh-cut mango (103), and processed meat (hot dogs) (104). Another possible application has been to extend the life-time of fruits and vegetables post-harvest, which is usually restricted by the growth of molds or the development of rot (105).
6. Safe UVC
6.1. 254 nm UVC
UVC light is electromagnetic irradiation between the wavelengths of 200 to 280 nm. It has been known for more than 100 years that UV light, particularly UVC is highly germicidal (106). UVC light with a wavelength 254-nm is readily produced from a low-pressure mercury vapor lamp, and is commonly used to inactivate and kill many microbial species, where it is popularly known as germicidal UV (107). The antimicrobial mechanism of UVC light relies on its ability to damage DNA caused by production of a variety of mutagenic and cytotoxic DNA lesions such as cyclobutane pyrimidine dimers (CPD) (108) and 6,4 photoproducts (109). CPD are known to interrupt the transcription, translation, and replication of DNA, leading to cell death (110). It has been reported that irradiation of operating rooms with germicidal UVC during orthopedic surgical procedures reduced the rate of surgical site infections (111).
Using mouse models, Dai et al. (112) investigated the potential of UVC light for the traetment of infections in highly contaminated superficial cutaneous wounds. Mouse models of partial-thickness skin abrasions infected with bioluminescent P. aeruginosa and S. aureus were developed. Approximately 107 bacterial cells were inoculated onto wounds measuring 1.2 × 1.2 cm on the dorsal surfaces of mice. UVC light was delivered at 30 min after bacterial inoculation. It was found that for both bacterial infections, UVC light at 2.59 J/cm2 significantly reduced the bacterial burden in the infected mouse wounds by 10-fold in comparison to untreated wounds (112) Furthermore, UVC light increased the survival rate of mice infected with P. aeruginosa (58%) and increased the wound healing rate in mice infected with S. aureus (31%). In another study, Dai et al. (113) investigated the use of UVC irradiation (254 nm) for treatment of bioluminescent C. albicans infection in mouse third degree burns. A single exposure carried out on day 0 (30 min post-infection) gave an average 2.16-log10 (99%) loss of fungal luminescence when 2.92 J/cm2 UVC had been delivered, while UVC at 24 h post-infection gave 1.94-log10 (96%) reduction of fungal luminescence after 6.48 J/cm2 UVC was found to be superior to a topical anti-fungal drug, nystatin cream.
Nevertheless, it has also been shown that exposure of human cells to 254-nm UVC causes the formation of mutagenic and cytotoxic DNA lesions, which (if repeated for a sufficiently long time) can lead to the initiation and progression of skin cancer [12]. Although these DNA lesions can be rapidly and efficiently repaired by DNA repair enzymes usually by 24 hours and entirely by 48 hours, it would indeed be preferable if these DNA lesions could be avoided.
6.2. Safe short wavelength UVC
A new approach uses excimer lamps, which are quasimonochromatic light sources that can operate over a wide range of wavelengths in the UV and vacuum ultraviolet (VUV) spectral regions. The operation of excimer lamps is based on the formation of excited dimmers (excimers) between two of the same atoms, or exciplexes formed between two or more dissimilar atoms (114). The best-known examples include rare gas excimers and rare-gas-halogen excimers. When the excited state semi-stable dimer splits apart returning to the ground state, a UV-photon is emitted. For example, Xe2*, Kr2*, Ar2* are excimer molecules, but XeCl*, KrCl*, XeBr*, ArCl*, Xe2Cl* are exciplex molecules. The spectrum of excimer lamp radiation is characterized by an intense narrow emission band (115). The full-width at half maximum of these emission bands depends on the kind of working molecule and excitation conditions and ranges from 2 to 15 nm.
The underlying rationale for “safe UVC” is that as the wavelength drops below that of the most commonly used UVC wavelength (namely the low-pressure mercury lamp at 254 nm) then the absorption coefficient of protein rises sharply, while the absorption coefficient of nucleic acids remains about the same. The consequence of this change in absorption, is that a 222-nm photon has the same chance of damaging a DNA molecule as a 254 nm photon, but cannot penetrate as well through the protein that composes the cytosol of an eukaryotic cell. Since eukaryotic cells are considerable larger than prokaryotic bacterial cells, there is much more protein inside them. In eukaryotic cells the DNA is concentrated inside the nucleus which is separated from the plasma membrane. In prokaryotic cells the DNA is contained in the nucleoid, a region of cytoplasm where the chromosomal DNA is located. Bacteria do not have a membrane bound nucleus, but simply an area of the cytoplasm where the strands of DNA are found. Most bacteria have a single, circular chromosome that undergoes replication, although a few species do have two or more chromosomes. Smaller circular DNA strands, called plasmids, are also found in the cytoplasm.
It has been reported that the penetration ability of short-UVC light is reduced by half in only about 0.3 um of tissue (116). Buananno and co-workers undertook a series of studies with 207-nm UVC light produced by a krypton-bromine excimer lamp (117, 118). They showed that 207 nm can inactivate bacteria efficiently (>99.9% killing), moreover this wavelength also showed less cytotoxic and mutagenic damage to human keratinocytes compared to 254 nm on the basis of equal energy density delivered.
Recently, a krypton-chlorine (Kr-Cl) excimer lamp was developed to emit 222-nm UVC light (114). We evaluated the bactericidal and DNA-damaging effects of 222-nm UVC light compared with 254 nm UVC light in MRSA-infected wounds in a mouse model (119). We demonstrated that 222-nm UVC light at 75 and 150 mJ/cm2 showed efficient bactericidal activity on both normal mouse skin and in MRSA-infected wounds. Although the bactericidal activity of 222-nm UVC measured immediately after irradiation in infected wounds was slightly less than that of 254-nm UVC, the bacterial numbers in the wounds on days 5, 8 and 12 in 222-nm UVC wounds were comparable to or lower than those in 254-nm UVC wounds. Importantly, DNA damage to keratinocytes and other cells in the infected wound caused by 222-nm UV light was less severe than that caused by 254-nm UVC. Figure 7 illustrates the use of 222 nm safe UVC to kill bacteria in vitro and in vivo.
Figure 7. Comparison of 222 nm and 254 nm UVC.
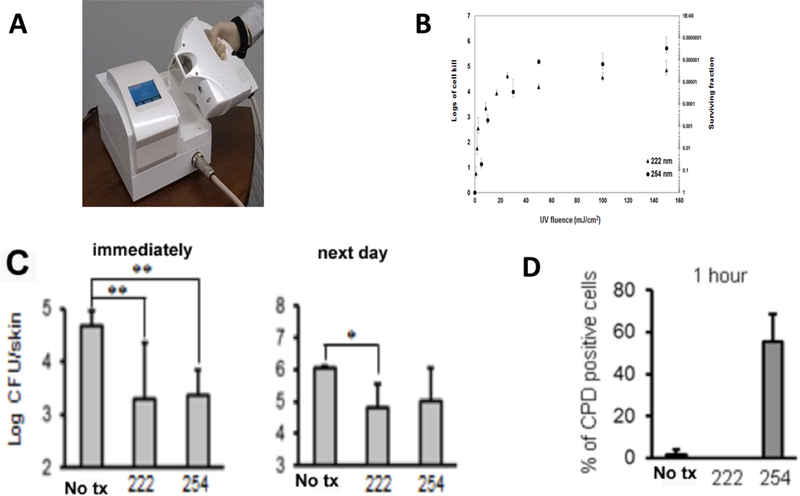
(A) Photograph of 222 nm Kr/Cl excimer lamp. (B) Comparison of 222 nm and 254 nm UVC for in vitro inactivation of S. aureus on an agar plate. (C) S. aureus CFU extracted from a mouse wound either immediately or 24 hours after treatment with 750 mJ/cm2 of either 222 nm or 254 nm UVC. (D) % of CPD-positive cells quantified by counting 10 random high-power (×400) fields of tissue sections removed 1 hour post exposure to 150 mJ/cm2 of either 222 nm or 254 nm UVC. From (119) no permission necessary.
Because the skin surface is not completely smooth and is covered by intersecting grooves called “sulci cutis”, it might be possible that S. aureus cells were distributed in such a way within these sulci cutis grooves, or in hair follicles and were not irradiated with a sufficient dose of UVC to elicit the full bactericidal effect. In other words some bacteria could have been shielded by the skin surface irregularities.
It is known that wound exudate, a protein-rich fluid produced in response to tissue damage, is present in wounds. It contains various types of cells and many kinds of proteins including inflammatory mediators as well as proteolytic enzymes [29]. Thus it might be possible that proteins in the wound exudate absorb UVC light thus protecting the bacteria, and the remaining bacterial cells which were not eradicated, could proliferate and grow in the wound after UV irradiation.
In the wound healing process, epithelial cells from the wound edge begin to migrate over the surface of wound “burrowing a path” beneath the scab and the underlying granulation tissue (120). It has been previously reported that UV exposure might be beneficial for wound healing by inducing hyperplasia, granulation tissue formation, sloughing of necrotic tissue and enhancing re-epithelialization (121).Our results demonstrated that irradiation with 254-nm UVC but not with 222-nm UVC impaired the burrowing or migration of keratinocytes across the wound suggesting that irradiation with 254-nm but not 222-nm UVC can induce DNA damage and inflammation in wounded dermis as well as epidermis. Although further investigation will be required for any application of 222-nm UVC light in clinical settings, the Xe-Cl lamp is promising to reduce SSI and wound infections especially by multi-drug resistant bacteria. It remains to be tested whether “safe UVC” is sufficiently safe to make the adoption of skin and eye protection less critical than it is with 254 nm UVC.
7. Conclusions
This review has highlighted three different applications of light that can all be used in the fight against drug-resistant pathogens. Each of them has their own particular strengths and weaknesses. aPDT is the most complicated as it requires the use of a separate exogenous PS, and moreover if KI potentiation is used, the addition of a sufficient concentration of KI solution. It can however deliver complete eradication at relatively low fluences, and red light can penetrate well into tissue. It is at present uncertain if the PS and the KI can be combined into some type of nano-drug delivery system such as liposomes or micelles. This may be difficult as a high concentration of KI (100s of mM) is required to obtain the best potentiation effect. The use for treating a bladder infection was attractive, as the bladder is ideally suited to contain an aqueous solution at the same time as light is delivered (64). However, the urothelial lining of the bladder may be more sensitive to the damaging effects of PDT compared to skin and soft tissue.
Blue light alone has the advantages of being simple to deliver, and modern blue LEDs can provide substantial power densities from portable devices in a reliable manner. However, the penetration depth of blue light into tissue is very limited (< 1mm) and other approaches will be necessary to get sufficient blue light to infections that are anything else than purely superficial. Interstitial optical fibers, light delivery microneedles, and tissue optical clearing are some possibilities (66). UVC penetrates even less than blue light but its antimicrobial killing efficiency is several orders of magnitude higher. Even though UVC devices are clinically approved for treatment of wounds, they have not been much used in wound care because of fears about the possible toxic and carcinogenic effects of UVC. Perhaps the introduction of “safe UVC” at 207 nm or 222 nm will overcome this reluctance.
Acknowledgements
Research in the Hamblin laboratory is supported by US NIH grants R01AI050875 and R21AI121700. Research in the Abrahamse laboratory is supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant Number 98337).
References
- 1.Fleming A (1929). On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to their Use in the Isolation of B. influenzæ. Br J Exp Pathol 10, 226–236. [Google Scholar]
- 2.Fleming A (December 11, 1945). Penicillin . Nobel Lecture,. [Google Scholar]
- 3.Guglielmi G (2017). Are antibiotics turning livestock into superbug factories? Science. [Google Scholar]
- 4.Youngblood WJ, Gryko DT, Lammi RK, Bocian DF, Holten D and Lindsey JS. (2002). Glaser-mediated synthesis and photophysical characterization of diphenylbutadiyne-linked porphyrin dyads. J Org Chem. 67, 2111–2117. [DOI] [PubMed] [Google Scholar]
- 5.Smith R and Coast J. (2013). The true cost of antimicrobial resistance. Br Med J. 346, f1493. [DOI] [PubMed] [Google Scholar]
- 6.Kraus CN (2008). Low hanging fruit in infectious disease drug development. Curr Opin Microbiol. 11, 434–438. [DOI] [PubMed] [Google Scholar]
- 7.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N and Quinn JP. (2013). Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 13, 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoneyama H and Katsumata R. (2006). Antibiotic resistance in bacteria and its future for novel antibiotic development. Biosci Biotechnol Biochem. 70, 1060–1075. [DOI] [PubMed] [Google Scholar]
- 9.Hamblin MR and Hasan T. (2004). Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 3, 436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maisch T (2007). Anti-microbial photodynamic therapy: useful in the future? Lasers Med Sci. 22, 83–91. [DOI] [PubMed] [Google Scholar]
- 11.Maisch T, Hackbarth S, Regensburger J, Felgentrager A, Baumler W, Landthaler M and Roder B. (2011). Photodynamic inactivation of multi-resistant bacteria (PIB) - a new approach to treat superficial infections in the 21st century. J Dtsch Dermatol Ges. 9, 360–366. [DOI] [PubMed] [Google Scholar]
- 12.Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, Jacoby GA, Kishony R, Kreiswirth BN, Kutter E, Lerner SA, Levy S, Lewis K, Lomovskaya O, Miller JH, Mobashery S, Piddock LJ, Projan S, Thomas CM, Tomasz A, Tulkens PM, Walsh TR, Watson JD, Witkowski J, Witte W, Wright G, Yeh P and Zgurskaya HI. (2011). Tackling antibiotic resistance. Nat Rev Microbiol. 9, 894–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin RM, Bhayana B, Hamblin MR and Dai T. (2016). Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg Med. 48, 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuliani F, Martinelli M, Cocchi A, Arbia D, Fantetti L and Roncucci G. (2010). In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob Agents Chemother. 54, 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raab O (1900). Uber die Wirkung fluoreszierender Stoffe auf Infusorien . Z Biol. 39 524–546. [Google Scholar]
- 16.Von Tappeiner H and Jodlbauer A. (1904). Uber Wirkung der photodynamischen (fluorieszierenden) Stoffe auf Protozoan und Enzyme . Dtsch Arch Klin Med. 80, 427–487. [Google Scholar]
- 17.Lipson RL and Baldes EJ. (1960). The photodynamic properties of a particular hematoporphyin derivative. Arch Dermatol. 82, 508. [DOI] [PubMed] [Google Scholar]
- 18.Dougherty TJ, Grindey GB, Fiel R, Weishaupt KR and Boyle DG. (1975). Photoradiation therapy. II. Cure of animal tumors with hematoporphyrin and light. J Natl Cancer Inst. 55, 115–121. [DOI] [PubMed] [Google Scholar]
- 19.Weishaupt KR, Gomer CJ and Dougherty TJ. (1976). Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 36, 2326–2329. [PubMed] [Google Scholar]
- 20.Ochsner M (1997). Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B. 39, 1–18. [DOI] [PubMed] [Google Scholar]
- 21.da Silva Baptista M, Cadet J, Di Mascio P, Ghogare AA, Greer A, Hamblin MR, Lorente C, Nunez SC, Ribeiro MS, Thomas AH, Vignoni M and Yoshimura TM. (2017). Type I and II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem Photobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik Z, Ladan H and Nitzan Y. (1992). Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J Photochem Photobiol B. 14, 262–266. [DOI] [PubMed] [Google Scholar]
- 23.Malik Z, Hanania J and Nitzan Y. (1990). Bactericidal effects of photoactivated porphyrins--an alternative approach to antimicrobial drugs. J Photochem Photobiol B. 5, 281–293. [DOI] [PubMed] [Google Scholar]
- 24.Nitzan Y, Gutterman M, Malik Z and Ehrenberg B. (1992). Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem Photobiol. 55, 89–96. [DOI] [PubMed] [Google Scholar]
- 25.Bertoloni G, Rossi F, Valduga G, Jori G and van Lier J. (1990). Photosensitizing activity of water- and lipid-soluble phthalocyanines on Escherichia coli. FEMS Microbiol Lett. 59, 149–155. [DOI] [PubMed] [Google Scholar]
- 26.Hancock RE and Bell A. (1988). Antibiotic uptake into gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 7, 713–720. [DOI] [PubMed] [Google Scholar]
- 27.Orekhov PS, Kholina EG, Bozdaganyan ME, Nesterenko AM, Kovalenko IB and Strakhovskaya MG. (2018). Molecular Mechanism of Uptake of Cationic Photoantimicrobial Phthalocyanine across Bacterial Membranes Revealed by Molecular Dynamics Simulations. J Phys Chem B. 122, 3711–3722. [DOI] [PubMed] [Google Scholar]
- 28.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH and Brown ST. (1996). Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-negative and gram-positive bacteria. J Photochem Photobiol B. 32, 159–164. [DOI] [PubMed] [Google Scholar]
- 29.Abrahamse H and Hamblin MR. (2016). New photosensitizers for photodynamic therapy. Biochem J. 473, 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma SK, Mroz P, Dai T, Huang YY, St Denis TG and Hamblin MR. (2012). Photodynamic Therapy for Cancer and for Infections: What Is the Difference? Isr J Chem. 52, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipson RL, Baldes EJ and Olsen AM. (1961). Hematoporphyrin derivative: a new aid for endoscopic detection of malignant disease. J Thorac Cardiovasc Surg. 42, 623–629. [PubMed] [Google Scholar]
- 32.Rassmussan-Taxdal DS, Ward GE and Figge FH. (1955). Fluorescence of human lymphatic and cancer tissues following high doses of intravenous hematoporphyrin. Cancer. 8, 78–81. [DOI] [PubMed] [Google Scholar]
- 33.D’Cruz AK, Robinson MH and Biel MA. (2004). mTHPC-mediated photodynamic therapy in patients with advanced, incurable head and neck cancer: a multicenter study of 128 patients. Head Neck. 26, 232–240. [DOI] [PubMed] [Google Scholar]
- 34.Eichner A, Gonzales FP, Felgentrager A, Regensburger J, Holzmann T, Schneider-Brachert W, Baumler W and Maisch T. (2013). Dirty hands: photodynamic killing of human pathogens like EHEC, MRSA and Candida within seconds. Photochem Photobiol Sci. 12, 135–147. [DOI] [PubMed] [Google Scholar]
- 35.Moody CS and Hassan HM. (1982). Mutagenicity of oxygen free radicals. Proc Natl Acad Sci U S A. 79, 2855–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassidy CM, Donnelly RF and Tunney MM. (2010). Effect of sub-lethal challenge with Photodynamic Antimicrobial Chemotherapy (PACT) on the antibiotic susceptibility of clinical bacterial isolates. J Photochem Photobiol B. 99, 62–66. [DOI] [PubMed] [Google Scholar]
- 37.Pourhajibagher M, Boluki E, Chiniforush N, Pourakbari B, Farshadzadeh Z, Ghorbanzadeh R, Aziemzadeh M and Bahador A. (2016). Modulation of virulence in Acinetobacter baumannii cells surviving photodynamic treatment with toluidine blue. Photodiagnosis Photodyn Ther. 15, 202–212. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Xuan Y, Koide Y, Zhiyentayev T, Tanaka M and Hamblin MR. (2012). Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg Med. 44, 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musbat L, Weitman H and Ehrenberg B. (2013). Azide quenching of singlet oxygen in suspensions of microenvironments of neutral and surface charged liposomes and micelles. Photochem Photobiol. 89, 253–258. [DOI] [PubMed] [Google Scholar]
- 40.Huang L, St Denis TG, Xuan Y, Huang YY, Tanaka M, Zadlo A, Sarna T and Hamblin MR. (2012). Paradoxical potentiation of methylene blue-mediated antimicrobial photodynamic inactivation by sodium azide: role of ambient oxygen and azide radicals. Free Radic Biol Med. 53, 2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasimova KR, Sadasivam M, Landi G, Sarna T and Hamblin MR. (2014). Potentiation of photoinactivation of Gram-positive and Gram-negative bacteria mediated by six phenothiazinium dyes by addition of azide ion. Photochem Photobiol Sci. 13, 1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin R, Wang M, Huang YY, Landi G, Vecchio D, Chiang LY and Hamblin MR. (2015). Antimicrobial photodynamic inactivation with decacationic functionalized fullerenes: oxygen independent photokilling in presence of azide and new mechanistic insights. Free Radic Biol Med. 79, 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang YY, Choi H, Kushida Y, Bhayana B, Wang Y and Hamblin MR. (2016). Broad-Spectrum Antimicrobial Effects of Photocatalysis Using Titanium Dioxide Nanoparticles Are Strongly Potentiated by Addition of Potassium Iodide. Antimicrob Agents Chemother. 60, 5445–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Huang YY, Kushida Y, Bhayana B and Hamblin MR. (2016). Broad-spectrum antimicrobial photocatalysis mediated by titanium dioxide and UVA is potentiated by addition of bromide ion via formation of hypobromite. Free Radic Biol Med. 95, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vecchio D, Gupta A, Huang L, Landi G, Avci P, Rodas A and Hamblin MR. (2015). Bacterial photodynamic inactivation mediated by methylene blue and red light is enhanced by synergistic effect of potassium iodide. Antimicrob Agents Chemother. 59, 5203–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamblin MR (2017). Potentiation of antimicrobial photodynamic inactivation by inorganic salts. Expert Rev Anti Infect Ther. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang L, Szewczyk G, Sarna T and Hamblin MR. (2017). Potassium Iodide Potentiates Broad-Spectrum Antimicrobial Photodynamic Inactivation Using Photofrin. ACS Infect Dis. 3, 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim MM, Penjweini R, Gemmell NR, Veilleux I, McCarthy A, Buller GS, Hadfield RH, Wilson BC and Zhu TC. (2016). A Comparison of Singlet Oxygen Explicit Dosimetry (SOED) and Singlet Oxygen Luminescence Dosimetry (SOLD) for Photofrin-Mediated Photodynamic Therapy. Cancers (Basel). 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maisch T, Baier J, Franz B, Maier M, Landthaler M, Szeimies RM and Baumler W. (2007). The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc Natl Acad Sci U S A. 104, 7223–7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zubko EI and Zubko MK. (2013). Co-operative inhibitory effects of hydrogen peroxide and iodine against bacterial and yeast species. BMC Res Notes. 6, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wen X, Zhang X, Szewczyk G, El-Hussein A, Huang YY, Sarna T and Hamblin MR. (2017). Potassium Iodide Potentiates Antimicrobial Photodynamic Inactivation Mediated by Rose Bengal in In Vitro and In Vivo Studies. Antimicrob Agents Chemother. 61, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang L, El-Hussein A, Xuan W and Hamblin MR. (2017). Potentiation by potassium iodide reveals that the anionic porphyrin TPPS4 is a surprisingly effective photosensitizer for antimicrobial photodynamic inactivation. J Photochem Photobiol B. 178, 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.St Denis TG, Vecchio D, Zadlo A, Rineh A, Sadasivam M, Avci P, Huang L, Kozinska A, Chandran R, Sarna T and Hamblin MR. (2013). Thiocyanate potentiates antimicrobial photodynamic therapy: In situ generation of the sulfur trioxide radical anion by singlet oxygen. Free Radic Biol Med. 65C, 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang L, Xuan W, Zadlo A, Kozinska A, Sarna T and Hamblin MR. (2018). Antimicrobial photodynamic inactivation is potentiated by addition of selenocyanate: possible involvement of selenocyanogen? J Biophotonics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costa RO, Macedo PM, Carvalhal A and Bernardes-Engemann AR. (2013). Use of potassium iodide in dermatology: updates on an old drug. An Bras Dermatol. 88, 396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hassan I and Keen A. (2012). Potassium iodide in dermatology. Indian J Dermatol Venereol Leprol. 78, 390–393. [DOI] [PubMed] [Google Scholar]
- 57.Sterling JB and Heymann WR. (2000). Potassium iodide in dermatology: a 19th century drug for the 21st century-uses, pharmacology, adverse effects, and contraindications. J Am Acad Dermatol. 43, 691–697. [DOI] [PubMed] [Google Scholar]
- 58.FDA. (2001). Guidance on potassium iodide as a thyroid blocking agent in radiation emergencies. US Food and Drug Administration. [Google Scholar]
- 59.Avci P, Karimi M, Sadasivam M, Antunes-Melo WC, Carrasco E and Hamblin MR. (2018). In-vivo monitoring of infectious diseases in living animals using bioluminescence imaging. Virulence. 9, 28–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Demidova TN, Gad F, Zahra T, Francis KP and Hamblin MR. (2005). Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. J Photochem Photobiol B. 81, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, Castano AP and Hamblin MR. (2006). Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 27, 4157–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Dai T, Wang M, Vecchio D, Chiang LY and Hamblin MR. (2015). Potentiation of antimicrobial photodynamic inactivation mediated by a cationic fullerene by added iodide: in vitro and in vivo studies. Nanomedicine (Lond). 10, 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freire F, Ferraresi C, Jorge AO and Hamblin MR. (2016). Photodynamic therapy of oral Candida infection in a mouse model. J Photochem Photobiol B. 159, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang YY, Wintner A, Seed PC, Brauns T, Gelfand JA and Hamblin MR. (2018). Antimicrobial photodynamic therapy mediated by methylene blue and potassium iodide to treat urinary tract infection in a female rat model. Sci Rep. 8, 7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP and Hamblin MR. (2012). Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist Updat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Wang Y, Wang Y, Murray CK, Hamblin MR, Hooper DC and Dai T. (2017). Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resist Updat. 33-35, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Darwin C, in ‘The power of movement in plants’, New York, 1881. [Google Scholar]
- 68.Tschowri N, Lindenberg S and Hengge R. (2012). Molecular function and potential evolution of the biofilm-modulating blue light-signalling pathway of Escherichia coli. Molecular microbiology. 85, 893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP and Hamblin MR. (2012). Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 15, 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA and Tolkoff MJ. (2005). Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrobial agents and chemotherapy. 49, 2822–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maclean M, MacGregor SJ, Anderson JG and Woolsey G. (2009). Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl Environ Microbiol. 75, 1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murdoch LE, Maclean M, Endarko E, MacGregor SJ and Anderson JG. (2012). Bactericidal effects of 405 nm light exposure demonstrated by inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium species in liquid suspensions and on exposed surfaces. ScientificWorldJournal. 2012, 137805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jean D, Briolat V and Reysset G. (2004). Oxidative stress response in Clostridium perfringens. Microbiology. 150, 1649–1659. [DOI] [PubMed] [Google Scholar]
- 74.Murphy C, Carroll C and Jordan KN. (2006). Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J Appl Microbiol. 100, 623–632. [DOI] [PubMed] [Google Scholar]
- 75.Feuerstein O, Ginsburg I, Dayan E, Veler D and Weiss EI. (2005). Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem Photobiol. 81, 1186–1189. [DOI] [PubMed] [Google Scholar]
- 76.De Lucca AJ, Carter-Wientjes C, Williams KA and Bhatnagar D. (2012). Blue light (470 nm) effectively inhibits bacterial and fungal growth. Letters in applied microbiology. [DOI] [PubMed] [Google Scholar]
- 77.Guffey JS and Wilborn J. (2006). In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomedicine and laser surgery. 24, 684–688. [DOI] [PubMed] [Google Scholar]
- 78.Nitzan Y, Salmon-Divon M, Shporen E and Malik Z. (2004). ALA induced photodynamic effects on gram positive and negative bacteria. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology. 3, 430–435. [DOI] [PubMed] [Google Scholar]
- 79.Maclean M, MacGregor SJ, Anderson JG and Woolsey G. (2009). Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Applied and environmental microbiology. 75, 1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liang JY, Cheng CW, Yu CH and Chen LY. (2015). Investigations of blue light-induced reactive oxygen species from flavin mononucleotide on inactivation of E. coli. J Photochem Photobiol B. 143, 82–88. [DOI] [PubMed] [Google Scholar]
- 81.Wong TW, Cheng CW, Hsieh ZJ and Liang JY. (2017). Effects of blue or violet light on the inactivation of Staphylococcus aureus by riboflavin-5’-phosphate photolysis. J Photochem Photobiol B. 173, 672–680. [DOI] [PubMed] [Google Scholar]
- 82.Lubart R, Lipovski A, Nitzan Y and Friedmann H. (2011). A possible mechanism for the bactericidal effect of visible light. Laser Ther. 20, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong PT, Mohammad H, Hui J, Li J, Liang L, N M., Seleem MN and Cheng JX. (2017). Annihilation of Methicillin-resistant Staphylococcus aureus via Photobleaching of Staphyloxanthin. bioRxiv. doi: 10.1101/227603, [DOI] [Google Scholar]
- 84.Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS, Sherwood ME, Baer DG, Hamblin MR and Dai T. (2014). Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. J Infect Dis. 209, 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsai T, Chien HF, Wang TH, Huang CT, Ker YB and Chen CT. (2011). Chitosan augments photodynamic inactivation of gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 55, 1883–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsai T, Yang YT, Wang TH, Chien HF and Chen CT. (2009). Improved photodynamic inactivation of gram-positive bacteria using hematoporphyrin encapsulated in liposomes and micelles. Lasers Surg Med. 41, 316–322. [DOI] [PubMed] [Google Scholar]
- 87.Kleinpenning MM, Smits T, Frunt MH, van Erp PE, van de Kerkhof PC and Gerritsen RM. (2010). Clinical and histological effects of blue light on normal skin. Photodermatology, photoimmunology & photomedicine. 26, 16–21. [DOI] [PubMed] [Google Scholar]
- 88.Dai T, Gupta A, Huang YY, Sherwood ME, Murray CK, Vrahas MS, Kielian T and Hamblin MR. (2013). Blue Light Eliminates Community-Acquired Methicillin-resistant Staphylococcus aureus in Infected Mouse Skin Abrasions. Photomed Laser Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dai T, Gupta A, Huang YY, Yin R, Murray CK, Vrahas MS, Sherwood ME, Tegos GP and Hamblin MR. (2013). Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother. 57, 1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS, Sherwood ME, Baer DG, Hamblin MR and Dai T. (2013). Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii burn infection in mice: Implications for prophylaxis and treatment of combat-related wound infections. J Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y, Zhu Y, Chen J, Wang Y, Sherwood ME, Murray CK, Vrahas MS, Hooper DC, Hamblin MR and Dai T. (2016). Antimicrobial blue light inactivation of Candida albicans: in vitro and in vivo studies. Virulence. 7, 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu H, Kochevar IE, Behlau I, Zhao J, Wang F, Wang Y, Sun X, Hamblin MR and Dai T. (2017). Antimicrobial Blue Light Therapy for Infectious Keratitis: Ex Vivo and In Vivo Studies. Invest Ophthalmol Vis Sci. 58, 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dobrev H (2010). Fluorescence diagnostic imaging in patients with acne. Photodermatol Photoimmunol Photomed. 26, 285–289. [DOI] [PubMed] [Google Scholar]
- 94.Youn SW, Kim JH, Lee JE, Kim SO and Park KC. (2009). The facial red fluorescence of ultraviolet photography: is this color due to Propionibacterium acnes or the unknown content of secreted sebum? Skin Res Technol. 15, 230–236. [DOI] [PubMed] [Google Scholar]
- 95.Pei S, Inamadar AC, Adya KA and Tsoukas MM. (2015). Light-based therapies in acne treatment. Indian Dermatol Online J. 6, 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Megraud F and Lamouliatte H. (2003). Review article: the treatment of refractory Helicobacter pylori infection. Alimentary pharmacology & therapeutics. 17, 1333–1343. [DOI] [PubMed] [Google Scholar]
- 97.Ganz RA, Viveiros J, Ahmad A, Ahmadi A, Khalil A, Tolkoff MJ, Nishioka NS and Hamblin MR. (2005). Helicobacter pylori in patients can be killed by visible light. Lasers in surgery and medicine. 36, 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lembo AJ, Ganz RA, Sheth S, Cave D, Kelly C, Levin P, Kazlas PT, Baldwin PC 3rd, Lindmark WR, McGrath JR and Hamblin MR. (2009). Treatment of Helicobacter pylori infection with intra-gastric violet light phototherapy: a pilot clinical trial. Lasers in surgery and medicine. 41, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maclean M, Anderson JG, MacGregor SJ, White T and Atreya CD. (2016). A New Proof of Concept in Bacterial Reduction: Antimicrobial Action of Violet-Blue Light (405 nm) in Ex Vivo Stored Plasma . J Blood Transfus. 2016, 2920514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoenes K, Stangl F, Gross A and Hessling M. (2016). Improved contact lens disinfection by exposure to violet radiation. Technol Health Care. 24, 145–151. [DOI] [PubMed] [Google Scholar]
- 101.Ghate V, Kumar A, Zhou W and Yuk HG. (2016). Irradiance and Temperature Influence the Bactericidal Effect of 460-Nanometer Light-Emitting Diodes on Salmonella in Orange Juice. J Food Prot. 79, 553–560. [DOI] [PubMed] [Google Scholar]
- 102.Kim MJ, Bang WS and Yuk HG. (2017). 405 +/− 5 nm light emitting diode illumination causes photodynamic inactivation of Salmonella spp. on fresh-cut papaya without deterioration. Food Microbiol. 62, 124–132. [DOI] [PubMed] [Google Scholar]
- 103.Kim MJ, Tang CH, Bang WS and Yuk HG. (2017). Antibacterial effect of 405+/−5nm light emitting diode illumination against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella on the surface of fresh-cut mango and its influence on fruit quality. Int J Food Microbiol. 244, 82–89. [DOI] [PubMed] [Google Scholar]
- 104.Guffey JS, Payne WC, Motts SD, Towery P, Hobson T, Harrell G, Meurer L and Lancaster K. (2016). Inactivation of Salmonella on tainted foods: using blue light to disinfect cucumbers and processed meat products. Food Sci Nutr. 4, 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liao H-L, Alferez F and Burns JK. (2013). Assessment of blue light treatments on citrus postharvest diseases. Postharvest Biol. Technol. 81, 81–88. [Google Scholar]
- 106.Gupta A, Dai T, Avci P, Huang YY and Hamblin MR. (2013). Ultraviolet Radiation in Wound Care: Sterilization and Stimulation Adv Wound Care. doi: 10.1089/wound.2012.0366., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang JC, Ossoff SF, Lobe DC, Dorfman MH, Dumais CM, Qualls RG and Johnson JD. (1985). UV inactivation of pathogenic and indicator microorganisms. Appl Environ Microbiol. 49, 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Poepping C, Beck SE, Wright H and Linden KG. (2014). Evaluation of DNA damage reversal during medium-pressure UV disinfection. Water Res. 56, 181–189. [DOI] [PubMed] [Google Scholar]
- 109.McCready S (2014). An immunoassay for measuring repair of UV photoproducts. Methods Mol Biol. 1105, 551–564. [DOI] [PubMed] [Google Scholar]
- 110.Gurzadyan GG, Gorner H and Schulte-Frohlinde D. (1995). Ultraviolet (193, 216 and 254 nm) photoinactivation of Escherichia coli strains with different repair deficiencies. Radiat Res. 141, 244–251. [PubMed] [Google Scholar]
- 111.Ritter MA, Olberding EM and Malinzak RA. (2007). Ultraviolet lighting during orthopaedic surgery and the rate of infection. J Bone Joint Surg Am. 89, 1935–1940. [DOI] [PubMed] [Google Scholar]
- 112.Dai T, Garcia B, Murray CK, Vrahas MS and Hamblin MR. (2012). UVC Light Prophylaxis for Cutaneous Wound Infections in Mice. Antimicrob Agents Chemother. 56, 3841–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dai T, Kharkwal GB, Zhao J, St Denis TG, Wu Q, Xia Y, Huang L, Sharma SK, d’Enfert C and Hamblin MR. (2011). Ultraviolet-C light for treatment of Candida albicans burn infection in mice. Photochem Photobiol. 87, 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eliasson B and Kogelschatz U. (1988). UV excimer radiation from dielectric-barrier discharges, Appl. Phys. B Lasers Opt. 46, 299–303. [Google Scholar]
- 115.Gellert B and Kogelschatz U. (1991). Generation of Excimer Emission in Dielectric Barrier Discharges. Applied Physics B. 52, 14–21. [Google Scholar]
- 116.Goldfarb AR and Saidel LJ. (1951). Ultraviolet absorption spectra of proteins. Science. 114, 156–157. [DOI] [PubMed] [Google Scholar]
- 117.Buonanno M, Randers-Pehrson G, Bigelow AW, Trivedi S, Lowy FD, Spotnitz HM, Hammer SM and Brenner DJ. (2013). 207-nm UV light - a promising tool for safe low-cost reduction of surgical site infections. I: in vitro studies. PLoS One. 8, e76968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buonanno M, Stanislauskas M, Ponnaiya B, Bigelow AW, Randers-Pehrson G, Xu Y, Shuryak I, Smilenov L, Owens DM and Brenner DJ. (2016). 207-nm UV Light-A Promising Tool for Safe Low-Cost Reduction of Surgical Site Infections. II: In-Vivo Safety Studies . PLoS One. 11, e0138418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Narita K, Asano K, Morimoto Y, Igarashi T, Hamblin MR, Dai T and Nakane A. (2018). Disinfection and healing effects of 222-nm UVC light on methicillin-resistant Staphylococcus aureus infection in mouse wounds. J Photochem Photobiol B. 178, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Evans ND, Oreffo RO, Healy E, Thurner PJ and Man YH. (2013). Epithelial mechanobiology, skin wound healing, and the stem cell niche. J Mech Behav Biomed Mater. 28, 397–409. [DOI] [PubMed] [Google Scholar]
- 121.Dai T, Vrahas MS, Murray CK and Hamblin MR. (2012). Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev Anti Infect Ther. 10, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fukui M, Yoshioka M, Satomura K, Nakanishi H and Nagayama M. (2008). Specific-wavelength visible light irradiation inhibits bacterial growth of Porphyromonas gingivalis. Journal of periodontal research. 43, 174–178. [DOI] [PubMed] [Google Scholar]
- 123.Soukos NS, Som S, Abernethy AD, Ruggiero K, Dunham J, Lee C, Doukas AG and Goodson JM. (2005). Phototargeting oral black-pigmented bacteria. Antimicrobial agents and chemotherapy. 49, 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feuerstein O, Persman N and Weiss EI. (2004). Phototoxic effect of visible light on Porphyromonas gingivalis and Fusobacterium nucleatum: an in vitro study. Photochemistry and photobiology. 80, 412–415. [DOI] [PubMed] [Google Scholar]
- 125.Enwemeka CS, Williams D, Hollosi S, Yens D and Enwemeka SK. (2008). Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med. 40, 734–737. [DOI] [PubMed] [Google Scholar]
- 126.Enwemeka CS, Williams D, Enwemeka SK, Hollosi S and Yens D. (2009). Blue 470-nm light kills methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomed Laser Surg. 27, 221–226. [DOI] [PubMed] [Google Scholar]
- 127.Guffey JS and Wilborn J. (2006). In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed Laser Surg. 24, 684–688. [DOI] [PubMed] [Google Scholar]
- 128.Kawada A, Aragane Y, Kameyama H, Sangen Y and Tezuka T. (2002). Acne phototherapy with a high-intensity, enhanced, narrow-band, blue light source: an open study and in vitro investigation. Journal of dermatological science. 30, 129–135. [DOI] [PubMed] [Google Scholar]
- 129.Ashkenazi H, Malik Z, Harth Y and Nitzan Y. (2003). Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS immunology and medical microbiology. 35, 17–24. [DOI] [PubMed] [Google Scholar]
- 130.Halstead FD, Thwaite JE, Burt R, Laws TR, Raguse M, Moeller R, Webber MA and Oppenheim BA. (2016). Antibacterial Activity of Blue Light against Nosocomial Wound Pathogens Growing Planktonically and as Mature Biofilms. Appl Environ Microbiol. 82, 4006–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barneck MD, Rhodes NLR, de la Presa M, Allen JP, Poursaid AE, Nourian MM, Firpo MA and Langell JT. (2016). Violet 405-nm light: a novel therapeutic agent against common pathogenic bacteria. J Surg Res. 206, 316–324. [DOI] [PubMed] [Google Scholar]
- 132.Gupta S, Maclean M, Anderson JG, MacGregor SJ, Meek RM and Grant MH. (2015). Inactivation of micro-organisms isolated from infected lower limb arthroplasties using high-intensity narrow-spectrum (HINS) light. Bone Joint J. 97-B, 283–288. [DOI] [PubMed] [Google Scholar]
- 133.Rhodes NL, de la Presa M, Barneck MD, Poursaid A, Firpo MA and Langell JT. (2016). Violet 405 nm light: A novel therapeutic agent against beta-lactam-resistant Escherichia coli. Lasers Surg Med. 48, 311–317. [DOI] [PubMed] [Google Scholar]
- 134.Wheeland RG and Koreck A. (2012). Safety and Effectiveness of a New Blue Light Device for the Self-treatment of Mild-to-moderate Acne. The Journal of clinical and aesthetic dermatology. 5, 25–31. [PMC free article] [PubMed] [Google Scholar]
- 135.Gold MH, Sensing W and Biron JA. (2011). Clinical efficacy of home-use blue-light therapy for mild-to moderate acne. Journal of cosmetic and laser therapy : official publication of the European Society for Laser Dermatology. 13, 308–314. [DOI] [PubMed] [Google Scholar]
- 136.Wheeland RG and Dhawan S. (2011). Evaluation of self-treatment of mild-to-moderate facial acne with a blue light treatment system. Journal of drugs in dermatology : JDD. 10, 596–602. [PubMed] [Google Scholar]
- 137.de Arruda LH, Kodani V, Bastos Filho A and Mazzaro CB. (2009). [A prospective, randomized, open and comparative study to evaluate the safety and efficacy of blue light treatment versus a topical benzoyl peroxide 5% formulation in patients with acne grade II and III]. Anais brasileiros de dermatologia. 84, 463–468. [DOI] [PubMed] [Google Scholar]
- 138.Tremblay JF, Sire DJ, Lowe NJ and Moy RL. (2006). Light-emitting diode 415 nm in the treatment of inflammatory acne: an open-label, multicentric, pilot investigation. Journal of cosmetic and laser therapy : official publication of the European Society for Laser Dermatology. 8, 31–33. [DOI] [PubMed] [Google Scholar]
- 139.Morton CA, Scholefield RD, Whitehurst C and Birch J. (2005). An open study to determine the efficacy of blue light in the treatment of mild to moderate acne. The Journal of dermatological treatment. 16, 219–223. [DOI] [PubMed] [Google Scholar]


