Abstract
Purpose:
Osteoblasts and their precursors support hematopoiesis in the bone marrow. We hypothesized that declines in Hgb levels are associated with bone mineral density (BMD).
Methods:
The Cardiovascular Health Study is a prospective longitudinal study that enrolled 5,888 community-dwelling adults aged >65 years and measured hemoglobin twice, in 1989–90 and 1992–93, as well as BMD by dual-energy X-ray absorptiometry (DXA) in 1994–95. In a subset of 1,513 men and women with a Hgb in 1992–93 and BMD, we used linear regression to estimate associations of Hgb (per standard deviation (SD)) with total hip (TH), lumbar spine (LS) and total body (TB) BMD, and used Poisson regression to estimate associations of anemia (in 1992–93; Hgb <13 g/dL in men; <12 g/dL in women) with “low BMD” defined as T-score less than −1 at the TH. In 1,277 participants with Hgb measured on average 2.9 years apart and BMD, we used linear regression to estimate the associations of annualized change in Hgb with TH, LS and TB BMD. All models included age, sex, study-site, race, smoking, alcohol use, weight, height, steroid use, physical activity score, self-reported health, previous cardiovascular disease and prior anti-fracture medication use.
Results:
No significant association was observed between Hgb, measured a mean 2.2 years prior to BMD, and BMD at the TH and LS in men (TH beta = −0.60 [x 10−2g/cm2per 1.1 g/dL Hgb], 95% CI: −1.88 to 0.68; LS beta = −1.69, 95% CI: −3.83 to 0.45) or women (TH beta = −0.49 [x 10−2g/cm2per 1.3 g/dL Hgb], 95% CI: −1.57 to 0.59; LS beta = −0.40, 95% CI: −2.57 to 1.76). Anemia was not observed to be significantly associated with low BMD in men (RR = 0.99, 95% CI: 0.72– 1.40) nor women (RR = 0.98, 95% CI: 0.82– 1.17). The mean change in Hgb was a loss of 0.06 g/dL/year (SD = 0.32). Change in Hgb was not observed to be significantly associated with BMD in men (TH beta = −0.55[x 10−2g/cm2per 1g/dL annualized Hgb change], 95% CI: −4.28 to 3.19; LS beta = 0.63, 95% CI: −5.38 to 6.65) or women (TH beta = 0.92, 95% CI: −1.96 to 3.79; LS beta = −1.77, 95% CI: −7.52 to 3.98). No significant association was observed between anemia and low bone density by T-score in men and women.
Conclusions:
These findings support neither the hypothesis that low Hgb prior to bone density or decreases in Hgb are associated with bone density in older community-dwelling adults nor the use of Hgb level as a case-finding tool to prompt BMD measurement.
Keywords: Hemoglobin, Bone Mineral Density, White Blood Cells, Hematopoiesis, Aging
Introduction
Osteoblasts and their precursors support hematopoiesis in the bone marrow1,2. In animal models, osteoblasts can directly influence red blood cell (RBC) production through erythropoietin3,4. In transgenic mouse models, induced osteoblast deficiency leads to decreased bone marrow cellularity with loss of erythroid and lymphoid progenitors5. We have previously shown that osteoblasts and osteoprogenitor cells regulate lymphopoiesis, while osteocytes within bone regulate myelopoiesis6,7,8.
Given the apparent inter-relationship of bone and hematopoietic cells in mouse models, we and others have explored whether this relationship is observed clinically in humans. Thalassemia and sickle cell anemia are two hematopoietic disorders that have striking skeletal phenotypes. In thalassemia, low bone mineral density (BMD) is common and skeletal fracture prevalence can be as high as 36% by age 239. Patients with sickle cell anemia also have increased prevalence of osteopenia, osteoporosis and fractures10. Data on the impact of altered blood counts on bone phenotypes in relatively healthy adults is less abundant. We recently demonstrated in a prospective observational study that older men with greater hip bone loss have an increased risk of anemia, low lymphocytes and high neutrophils11. These findings parallel changes in RBCs and white blood cells (WBCs) observed in certain animal models of bone disease3,6–8. A few other studies have examined the relationship between hemoglobin (Hgb) concentration levels and BMD. A population-based study of older adults in Italy revealed that increased Hgb levels were associated with increased trabecular and cortical bone density by peripheral quantitative computed tomography (pQCT) in women, and with increased cortical bone density in men12. Lower Hgb has also been associated with decreased ultrasound derived T-scores in an elderly population of men and women13. One retrospective study found that anemia, defined by the World Health Organization (WHO), was 100% specific for low bone density by dual energy X-ray absorptiometry (DXA) in Turkish women14, highlighting the potential clinical utility of a Hgb measurement as a marker of low BMD. Evidence regarding an association between WBCs and BMD in humans is lacking. Other than our prior study in older men11 one small study in postmenopausal women with osteoporosis, determined by DXA, had decreased numbers of B lymphocytes compared to those without15. These findings support the notion that a broader relationship between hematopoiesis and bone health can be observed in humans through blood count parameters and bone density testing. However, the clinical utility of these observations remains to be determined.
In this study, we examined whether change in Hgb levels over time is associated with bone density in older men and women in the Cardiovascular Health Study (CHS) and whether a low Hgb level is associated with low bone density by WHO criteria. We also examined whether total WBC counts are associated with BMD, based on our prior findings of lower lymphocytes and greater neutrophils in older men with bone loss. Associations between platelet counts and bone density were evaluated in exploratory analyses.
Methods
Study Population
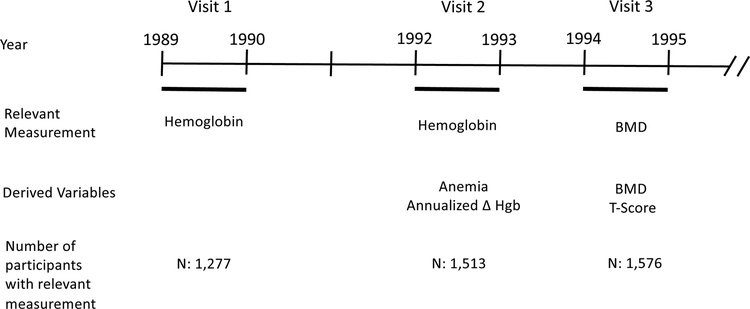
The Cardiovascular Health Study is a prospective observational cohort study designed to determine the risk factors for development and progression of cardiovascular disease (CVD) in older adults. Older men and women were recruited from 4 communities across the United States (Sacramento County, CA; Washington County, MD; Forsyth County, NC; Pittsburgh [Allegheny County], PA). To be eligible, non-institutionalized participants ages 65 or older had to be able to give informed consent, could not be wheelchair bound, could not require a proxy respondent at baseline and could not be receiving active treatment for cancer16. Between 1989 and 1990, 5,201 participants were enrolled, an additional 687 participants were enrolled between 1992 and 1993 to provide increased representation of African-Americans. Participants visited a clinic each year and had telephone contact annually at the midpoint between clinic visits. For the purposes of this analysis, we referred to clinic visits in 1989–1990, 1992–1993 and 1994–1995 as visit 1, visit 2 and visit 3, respectively (Figure 1).
Figure 1. “Study Timeline”.
Relevant measurements, variables and numbers of participants per study visit.
The analytic cohort of the current study consisted of 1,513 participants who had Hgb measured at visit 2 and valid bone density measurements by DXA at visit 3. The analyses of change in Hgb in relation to BMD consisted of 1,277 participants who had Hgb measured in both visits 1 and 2 and BMD in visit 3. Most participants excluded from these analyses of change in Hgb were African American, since they were enrolled in CHS at visit 2.
Participant characteristics
Anthropometric measurements including weight and height were performed during clinic visits by study staff using standardized methods. Body mass index (BMI) was calculated by dividing the baseline weight in kilograms by the height in meters squared. Medical history and personal habits, such as alcohol use and smoking, were ascertained by study staff at home interviews or clinic visits. Cardiovascular disease (CVD) and osteoporosis may be related17. We defined CVD as a “yes” answer to heart failure, stroke, claudication, coronary heart disease, atrial fibrillation, or transient ischemic attack in the medical history. Prescription medication used in the preceding two weeks was documented directly from medication containers. Non-prescription medication was documented from questionnaires. “Steroid use” was defined by any corticosteroid use documented in medication questionnaires. Bisphosphonates, estrogens and selective estrogen receptor modulators were considered “anti-fracture medications” for this analysis. Self-reported walking pace was determined by questionnaire, self-reported physical activity was calculated using a modified Minnesota leisure time activities questionnaire18. Leisure-time activity score (ordinal score for quintiles) and pace of walking (ordinal score for pace <2, 2–3, and >3 mph) were combined into a single physical activity score ranging from 2–8 according to a previous CHS analysis19. To determine self-reported health, participants were asked to rate “health in general”. Answers were dichotomized into “good” (excellent, very good and good) and “not good” (fair and poor).
Blood Count Measurements
Fasting blood was collected at visits 1 and 2. Citrated samples were sent for complete blood count analyses (without differential white blood cell count) using standardized methods at laboratories near the field centers. Pertinent measurements included Hgb level, total white blood cell counts and platelet counts. Anemia was defined by sex-specific 1968 World Health Organization (WHO) Hgb cutoffs, with Hgb <13 g/dL in men and Hgb <12 g/dL in women, based on Hgb levels at visit 2. White blood cell counts and platelet counts were not categorized.
Bone Mineral Density Measurements
Bone density scans by DXA were performed on 1,591 participants in the Pittsburgh, PA and Sacramento, CA sites at visit 3. Total hip and lumbar spine BMD (grams/cm2) were measured on Hologic QDR-2000 or 2000+ densitometers (Hologic, Inc., Bedford, MA). All scans were completed using the array beam mode. Standardized positioning and use of QDR software was based on the manufacturer’s protocol. Scans were read centrally and monitored for quality control at the University of California, San Francisco, reading center with Hologic software version 7.10, as described previously20. The coefficient of variation for the total hip and lumbar spine BMD was <0.75%21. Total body BMD (grams/cm2), derived from the whole body scan data, was evaluated as an exploratory outcome. T-scores at the total hip were calculated using sex- and race/ethnicity-matched NHANES III data as the reference population22. The following equation was used for total hip T-score calculation: [(TH BMD – mean TH BMD for young-adult reference population) / standard deviation of TH BMD for young-adult reference population].
Statistical analyses
Because men and women have different factors affecting bone density, we stratified all analyses a priori by sex. Participant characteristics from visit 2 were computed across sex-specific quartiles of Hgb. We compared characteristics with trend test for continuous variables and χ2 tests for binary and categorical variables.
We analyzed the association of visit 2 Hgb levels with visit 3 total hip, lumbar spine, and total body BMD. We estimated the association of each 1 SD difference in Hgb with BMDs in linear regression. Further, with Poisson regression we estimated the relative risk for low bone density at the total hip defined by T-score lower than −1 associated with anemia. We used two nested models with covariates from visit 2. Model 1 was adjusted for age, race and study site. Model 2 was additionally adjusted for current smoking, alcohol use (0, 1–7, more than 7 drinks per week), weight, height, steroid use, physical activity score, self-reported health, previous CVD and prior anti-fracture medication use. We used a similar approach to evaluate the association of visit 2 WBC and of platelet count with visit 3 BMD.
We defined annualized change in Hgb as visit 2 Hgb minus visit 1 Hgb divided by the time gap between those visits in years. We used linear regression to analyze the relationship of annualized change in Hgb level with BMDs. Models were analogous to those used above except that models were adjusted for visit 1 covariates and model 2 was additionally adjusted for visit 1 Hgb. Again, we used a similar approach to evaluate the association of change in WBC and platelet counts between visits 1 and 2 with visit 3 BMD.
To address the functional form of Hgb and the annualized change in Hgb in the linear regression, we used generalized additive models with smoothers. We did not find any departures from linearity. All analyses were performed using R23.
Results:
Characteristics of women and men by Hgb quartiles are presented in Table 1. Among the 874 women in our study, the median Hgb was 14.5 g/dL (SD: 1.27) and 8.7% met WHO-defined Hgb criteria for anemia. Women in the lowest quartile of Hgb were more often African American and current smokers, and less often reported having “good” health. Women in the highest quartile of Hgb had lower total hip, lumbar spine and total body BMD. There were no significant differences observed in age and in BMI among the quartile groups. Only 4.3% of all women in our study used anti-fracture medications.
Table1.
Characteristics of Cardiovascular Health Study participants by quartiles of sex-specific hemoglobin level at visit 2 (1992–1993).
| Hemoglobin quartile (g/dL) | Women (n=874) | Men (n=639) | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤12.8 (n=220) |
12.9–13.6 (n=245) |
13.7–14.2 (n=200) |
≥14.3 (n=209) |
≤13.1 (n=168) |
13.2–13.9 (n=165) |
14.0–14.7 (n=152) |
≥14.8 (n=154) |
|
| N (%) or mean ± SD | ||||||||
| Age (Years) | 74.21 ± 4.84 | 74.1 ± 4.79 | 73.38 ± 4.26 | 74.05 ± 4.18 | 75.7 ± 5.91 | 74.53 ± 4.39 | 74.64 ± 4.66 | 74.18 ± 4.81 |
| African American | 65 (29.5%) | 57 (23.3%) | 36 (18.2%) | 22 (10.5%) | 34 (20.2%) | 22 (13.3%) | 22 (14.5%) | 32 (20.8%) |
| Height (meter) | 1.59 ± 0.07 | 1.58 ± 0.06 | 1.58 ± 0.06 | 1.59 ± 0.06 | 1.72 ± 0.07 | 1.72 ± 0.06 | 1.73 ± 0.06 | 1.72 ± 0.07 |
| Weight (kg) | 67.4± 14.5 | 69.0 ± 13.7 | 68.2 ± 13.1 | 68.3 ± 12.6 | 77.7 ± 11.9 | 78.6 ± 10.9 | 79.4 ± 11.8 | 80.9 ± 11.8 |
| BMI (kg/m2) | 26.7 ± 5.5 | 27.5 ± 4.9 | 27.3 ± 5.1 | 26.9 ± 4.5 | 26.2 ± 3.7 | 26.5 ± 3.5 | 26.5 ± 3.6 | 27.3 ± 3.6 |
| Physical Activity Scorea | ||||||||
| 2–3 | 9 (5.5%) | 13 (6.7%) | 13 (6.7%) | 15 (9%) | 13 (9%) | 5 (3.5%) | 8 (6%) | 17 (11.9%) |
| 4–6 | 110 (67.5%) | 122 (63.2%) | 113 (68.9%) | 104 (62.3%) | 77 (53.1%) | 76 (53.5%) | 63 (47.0%) | 86 (60.1%) |
| 7–8 | 44 (27.0%) | 58 (30.1%) | 38 (23.2%) | 48 (28.7%) | 55 (37.9%) | 61 (43.0%) | 63 (47.0%) | 40 (28.0%) |
| Good Self−Reported Healthb | 170 (77.6%) | 201 (82.0%) | 172 (86.0%) | 179 (85.6%) | 135 (80.4%) | 144 (87.3%) | 133 (87.5%) | 130 (84.4%) |
| Cardiovascular Disease | 64 (39.8%) | 62 (25.7%) | 32 (16.1%) | 38 (18.3%) | 44 (20.4%) | 61 (37.7%) | 52 (34.7%) | 43 (29.1%) |
| Prior anti−fx medication | 14 (6.4%) | 7 (2.9%) | 8 (4%) | 9 (4.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Current Smoking | 144 (68.2%) | 125 (52.7%) | 123 (63.4%) | 125 (61.6%) | 65 (40.4%) | 61 (37.4%) | 48 (32%) | 62 (41.3%) |
| Alcohol Use (drinks/week) | ||||||||
| 0 | 127 (58%) | 124 (50.8%) | 87 (43.5%) | 95 (45.5%) | 66 (39.3%) | 65 (39.4%) | 55 (36.4%) | 53 (34.6%) |
| 1–7 | 72 (32.9%) | 92 (37.7%) | 95 (47.5%) | 94 (45%) | 78 (46.4%) | 65 (39.4%)5 | 60 (39.7%) | 66 (43.1%) |
| >7 | 20(9.1%) | 28 (11.5%) | 18(9%) | 20 (9.6%) | 24 (14.3%) | 35 (21.2%) | 36 (23.8%) | 34 (22.2%) |
| Steroid Use (Ever) | 6 (2.7%) | 3 (1.2%) | 2 (1%) | 5 (2.4%) | 4 (2.4%) | 3 (1.8%) | 2 (1.3%) | 5 (3.2%) |
| TH BMD (g/cm2) | 0.75 ± 0.15 | 0.77 ± 0.16 | 0.76 ± 0.14 | 0.73 ± 0.13 | 0.94 ± 0.18 | 0.94 ± 0.16 | 0.93 ± 0.17 | 0.96 ± 0.15 |
| LS BMD (g/cm2) | 0.94 ± 0.28 | 0.95 ± 0.23 | 0.95 ± 0.23 | 0.93 ± 0.11 | 1.16 ± 0.24 | 1.11 ± 0.24 | 1.14 ± 0.27 | 1.12 ± 0.23 |
| TH T−Score | −1.77 ± 1.13 | −1.57 ± 1.18 | −1.61 ± 1.06 | −1.83 ± 1.02 | −0.84 ± 1.14 | −0.80 ± 1.07 | −0.88 ± 1.13 | −0.75 ± 0.97 |
TH: Total Hip, LS: Lumbar Spine, BMD: Bone Mineral Density, fx: fracture, BMI: body mass index
Leisure-time activity score (ordinal score for quintiles) and pace of walking (ordinal score for pace <2, 2–3, and >3 mph) were combined into a single physical activity score ranging from 2–8
Participants were asked to rate “health in general” and answers were dichotomized into “good” (excellent, very good and good) and “not good” (fair and poor) for the purposes of this analysis.
Among the 639 men in our study, the median Hgb was 13.5 g/dL (SD: 1.1), and 10.8% met WHO-defined criteria for anemia. Men in the lowest quartile of Hgb were slightly older, while those in the highest quartile of Hgb had higher BMI and less often had CVD. Men in the highest and lowest quartiles of Hgb did not have observed differences in BMD at any site.
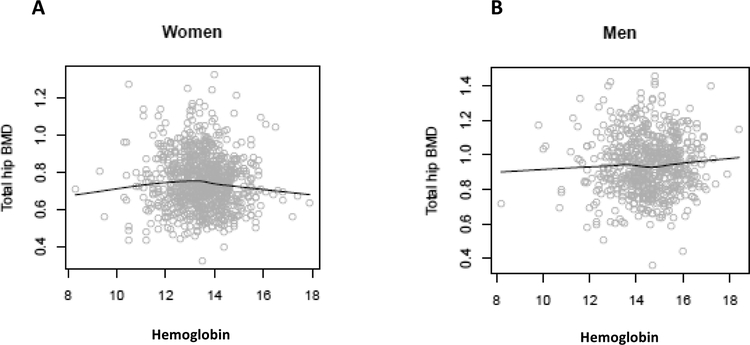
Hgb at visit 2 was not observed to be significantly associated with visit 3 total hip or lumbar spine BMD in either men or women. Visit 2 Hgb had non-significant multivariate-adjusted associations with BMD obtained in visit 3 (mean of 2.2 years after Hgb measurement) at the total hip, lumbar spine and total body measurement sites (Table 2). We found no meaningful departures from linearity when we explored generalized additive models (Figure 2).
Table 2.
Site-specific bone mineral density (BMD) at visit 3** in relation to hemoglobin value and separately white blood cell (WBC) count in visit 2**
| BMD | Women | Men | ||
|---|---|---|---|---|
| Age, race, study site-adjusted β (95% CI) | Full model-adjusted β (95% CI)* | Age, race, study site-adjusted β (95% CI) | Full model-adjusted β (95% CI)* | |
| The association of Hgb and three BMD sites (in 102g/cm2), visit 2 (1992–1993) | ||||
| Per 1. SD (1.14g/dL) increase in Hgb | Per 1 SD (1.27g/dL) increase in Hgb | |||
| Total hip | 0.13 (−0.89, 1.16) | −0.49 (−1.57, 0.59) | −0.29 (−1.53, 0.96) | −0.60 (−1.88, 0.68) |
| Lumbar spine | 0.22 (−1.56, 2.00) | −0.40 (−2.57, 1.76) | −1.12 (−3.07, 0.82) | −1.69 (−3.83, 0.45) |
| Total body | −0.32 (−1.15, 0.50) | −0.64 (−1.57, 0.30) | −0.67 (−1.65, 0.3) | −0.91 (−1.98, 0.16) |
| The association of WBC and three BMD sites (in 102g/cm2), visit 2 (1992–1993) | ||||
| Per 1 SD (1.60×103μL) increase in WBC | Per 1 SD (1.54×103μL) increase in WBC | |||
| Total hip | 0.50 (−0.36, 1.37) | 0.74 (−0.22, 1.69) | −0.28(−1.53, 0.98) | −0.23(−1.51, 1.05) |
| Lumbar spine | 1.79 (0.29, 3.29) | 2.10 (0.19, 4.00) | −0.52 (−2.49, 1.44) | −0.47 (−2.62, 1.68) |
| Total body | 0.52 (−0.18, 1.21) | 0.64 (−0.19, 1.46) | −0.20 (−1.19, 0.78) | −0.47 (−2.62, 1.68) |
Full model adjusted for age, study site, African American race, current smoking, alcohol use (0, 1–7, more than 7), weight, height, steroid use (ever), estrogen use, thiazide use, physical activity score and self-reported health.
visit 2: 1992–1993, visit 3: 1994–1995
Figure 2. “Association between hemoglobin at visit 2a and total hip bone mineral density (BMD) at visit 3a”.
Spline for hemoglobin addressing the functional form of hemoglobin in linear regression of total hip in women (Panel A) and men (Panel B), using generalized additive model adjusted for age, race and study site.aVisit 2: 1992–1993, visit 3: 1994–1995.
WBC count at visit 2 was significantly associated with visit 3 BMD only at the lumbar spine in women (LS beta = 2.10; 95% CI 0.19 to 4.00 per 10−2 g/cm2). There were no significant associations observed between WBC counts and BMD in men, or between platelet counts and BMD in men or women. No changes in Hgb, WBC count, or platelet count between visits 1 and 2 (a mean of 2.9 years apart) were observed to be significantly associated with visit 3 total hip, lumbar spine BMD in men or women. Additional adjustment for Visit 1 Hgb did not change these estimates (Table 3). Anemia at visit 2 was not significantly associated with low bone density by T-score at visit 3 in women (adjusted RR 0.98, 95% CI: 0.82– 1.17) or men (adjusted RR 1.00, 95% CI: 0.72– 1.40).
Table 3.
Association of annualized change in Hemoglobin between visits 1 and 2** with bone mineral density (BMD) at visit 3**.
| BMD | Women | Men | ||
|---|---|---|---|---|
| Age, race and study site-adjusted β (95% CI) | Full model-adjusted β (95% CI)* | Age, race and study site adjusted β (95% CI) | Full model-adjusted β (95% CI)* | |
| Annual change (g/dL/year) in Hgb from visit 1 to visit 2 and three BMD sites (in 102g/cm2) | ||||
| Total hip | 1.67 (−1.70, 5.04) | 0.22 (−2.95, 3.38) | −2.06 (−6.10, 1.99) | −1.18 (−5.12, 2.76) |
| Lumbar spine | 2.25 (−3.64, 8.13) | −2.12 (−8.44, 4.19) | −3.45 (−9.70, 2.81) | −1.49 (−7.80, 4.81) |
| Total body | 0.45 (−2.29, 3.18) | −1.35 (−4.20, 1.50) | −0.92 (−4.05, 2.20) | 0.04 (−3.10, 3.18) |
Full model adjusted for age, study site, race, current smoking, alcohol (0, 1–7, more than 7), weight, height, steroid use (ever), estrogen use, thiazide use, Physical activity score, self-reported health, and Hemoglobin at visit 1
visit 1: 1989–1990, visit 2: 1992–1993, visit 3: 1994–1995
Discussion:
Our findings do not support the hypothesis that changes in hemoglobin are related with bone density or that frank anemia could identify those with low bone density. To our knowledge this is the first evaluation of Hgb change in association with BMD. In 1,576 older women and men who participated in the prospective Cardiovascular Health Study, one-time Hgb level or annualized change in Hgb across an average duration of 2.9 Years were not observed to be associated with BMD of the lumbar spine or total hip measured a mean of 2.2 years later. Anemia by WHO classification was not found to be associated with low bone density by T-score. One-time or annualized changes in WBC or platelet counts were also not observed to be associated with BMD.
The current study is consistent with our previous report that bone loss over a mean 6.9 years was associated with anemia in older men, but cross-sectional bone density by DXA was not11. In an Italian cohort, anemia was found to be correlated with low BMD by pQCT in cross-section12. The difference in resolution between pQCT, which measures bone in 2.1 mm-thick transverse scans, and conventional DXA could account for the discrepancy in our findings. We and others have shown that a low Hgb level is associated with increased fracture risk24–27, which was largely independent of BMD or osteoporosis diagnosis. While change in hemoglobin was not associated with BMD this does not preclude that Hgb change may predict fractures. Future studies should evaluate the relationship of change in Hgb and fractures directly.
The current study contrasts with a previous study that reported that anemia was 100% sensitive and 33.5% specific for “low bone mass” in Turkish women14. Differences in findings may be due at least in part to the Turkish study’s retrospective design, smaller sample size with younger participants, Turkish origin, and the use of patient charts as the data source. Our data are derived from a large prospective observational cohort using standardized methods for blood count analysis and DXA procedures, and DXA scans were read centrally.
Potential mechanisms for the relationship of hematopoietic cells and bone metabolism in humans have recently been reviewed28. In brief, osteoblasts may directly stimulate erythropoiesis3,4 or increased bone marrow cellularity could decrease bone volume29. Changes in bone marrow composition associated with low bone density30,31 could also affect the mechanical properties of bone32,33. Osteoblasts and their precursors play a role in the control of myelopoiesis and support of lymphopoiesis in mice1,3,4,7,8,34, but the net effect of changes in the bone microenvironment on circulating total WBC count is uncertain. We found that a higher WBC count was associated with increased lumbar spine BMD in women but otherwise WBC count or change in WBC count over time was not observed to be associated with BMD in men and women. Our previous evaluation of older men revealed that poor bone health was related to different WBC subtypes in opposing directions, as hip bone loss was associated with both high neutrophils and low lymphocytes11. In a cross sectional study of 50 post-menopausal women, those with osteoporosis (DXA BMD T-score under −2.5) had decreased B lymphocytes compared to those with normal bone density15. To our knowledge this is the only other study evaluating the WBC and bone density relationship. WBC differentials were not available in CHS so we could not directly confirm these prior results. Overall, our cohort had WBC counts within the clinically normal range (99% of participants had a WBC count <11×103μL). It may be that more extreme total WBC count values are necessary to identify those with low bone density without knowledge of WBC subtypes.
Our study had several strengths. The Cardiovascular Health Study has a robust prospective design and included measurement of blood counts at different time points which allowed us to evaluate change in blood counts over years. DXA scans were performed on similar Hologic machines using standardized methods and were centrally read, minimizing variability in these assessments. The large cohort included men and women and had a high proportion of both community-based Caucasian and African American participants. Our study also has some limitations. Blood counts were assessed at sites near the respective field centers rather than centrally, which is a potential source of increased variability in these results. The African American cohort only had one blood count measurement so we could not include them in the Hgb change analyses. Due to the low prevalence of anti-fracture medication use in our study (4.3% women, 0% men), we would not expect this to dramatically affect our estimates. We did not have androgen or estrogen levels in our participants, which could have been helpful to evaluate the sex differences noted in our results. We acknowledge that using peripheral blood counts as a representation of hematopoietic health and BMD as a representation of bone cell health has its limitations and could have influenced our results.
Conclusion:
We found that annualized Hgb change and one-time Hgb levels were not associated with BMD a mean of 2.2 years later. Anemia also was not associated with low bone density by T-score. Our findings suggest that neither a single Hgb measurement nor longitudinal change in Hgb would be useful as a marker of low bone density in the short-term in older community dwelling individuals. Future studies should focus on potential sex differences in this relationship and whether change in blood counts, specifically Hgb are associated with fractures.
Acknowledgements:
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. RV was supported by NIDDK grant T32 DK007217. The content is solely our responsibility and does not necessarily represent the official views of the National Institute of Health.
Footnotes
Disclosures:
Declarations of interest: JYW has sponsored research funding from Radius Health, Inc in Waltham, MA.
References
- 1.Panaroni C, Tzeng YS, Saeed H, Wu JY. Mesenchymal progenitors and the osteoblast lineage in bone marrow hematopoietic niches. Curr Osteoporos Rep 2014; 12(1): 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol 2012; 12(1): 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rankin EB, Wu C, Khatri R, et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell 2012; 149(1): 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C, Giaccia AJ, Rankin EB. Osteoblasts: a novel source of erythropoietin. Curr Osteoporos Rep 2014; 12(4): 428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 2004; 103(9): 3258–64. [DOI] [PubMed] [Google Scholar]
- 6.Fulzele K, Krause DS, Panaroni C, et al. Myelopoiesis is regulated by osteocytes through Gsalpha-dependent signaling. Blood 2013; 121(6): 930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu JY, Purton LE, Rodda SJ, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A 2008; 105(44): 16976–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panaroni C, Fulzele K, Saini V, Chubb R, Pajevic PD, Wu JY. PTH Signaling in Osteoprogenitors Is Essential for B-Lymphocyte Differentiation and Mobilization. J Bone Miner Res 2015; 30(12): 2273–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogiatzi MG, Macklin EA, Fung EB, et al. Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res 2009; 24(3): 543–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osunkwo I An update on the recent literature on sickle cell bone disease. Curr Opin Endocrinol Diabetes Obes 2013; 20(6): 539–46. [DOI] [PubMed] [Google Scholar]
- 11.Valderrábano RJ, Lui LY, Lee J, et al. Bone Density Loss Is Associated With Blood Cell Counts. Journal of Bone and Mineral Research 2017; 32(2): 212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesari M, Pahor M, Lauretani F, et al. Bone density and hemoglobin levels in older persons: results from the InCHIANTI study. Osteoporos Int 2005; 16(6): 691–9. [DOI] [PubMed] [Google Scholar]
- 13.Laudisio A, Marzetti E, Pagano F, Bernabei R, Zuccalà G. Haemoglobin levels are associated with bone mineral density in the elderly: a population-based study. Clinical rheumatology 2009; 28(2): 145–51. [DOI] [PubMed] [Google Scholar]
- 14.Korkmaz U, Korkmaz N, Yazici S, et al. Anemia as a risk factor for low bone mineral density in postmenopausal Turkish women. European journal of internal medicine 2012; 23(2): 154–8. [DOI] [PubMed] [Google Scholar]
- 15.Breuil V, Ticchioni M, Testa J, et al. Immune changes in post-menopausal osteoporosis: the Immunos study. Osteoporosis international 2010; 21(5): 805–14. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, et al. The cardiovascular health study: design and rationale. Annals of epidemiology 1991; 1(3): 263–76. [DOI] [PubMed] [Google Scholar]
- 17.Farhat GN, Cauley JA. The link between osteoporosis and cardiovascular disease. Clinical Cases in Mineral and Bone Metabolism 2008; 5(1): 19. [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson-Cohen C, Katz R, Mozaffarian D, et al. Physical activity and rapid decline in kidney function among older adults. Archives of internal medicine 2009; 169(22): 2116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Archives of internal medicine 2009; 169(8): 798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbins J, Hirsch C, Whitmer R, Cauley J, Harris T. The association of bone mineral density and depression in an older population. Journal of the American Geriatrics Society 2001; 49(6): 732–6. [DOI] [PubMed] [Google Scholar]
- 21.Jovanovich A, Bùžková P, Chonchol M, et al. Fibroblast growth factor 23, bone mineral density, and risk of hip fracture among older adults: the cardiovascular health study. The Journal of Clinical Endocrinology & Metabolism 2013; 98(8): 3323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Looker A, Wahner H, Dunn W, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporosis International 1998; 8(5): 468–90. [DOI] [PubMed] [Google Scholar]
- 23.Team RC. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 24.Valderrábano RJ, Lee J, Lui L-Y, et al. Older Men with Anemia Have Increased Fracture Risk Independent of Bone Mineral Density. The Journal of Clinical Endocrinology & Metabolism 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemoglobin Looker A. and hip fracture risk in older non-Hispanic white adults. Osteoporosis International 2014; 25(10): 2389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Thomson CA, Aickin M, et al. The relationship between incidence of fractures and anemia in older multiethnic women. J Am Geriatr Soc 2010; 58(12): 2337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorgensen L, Skjelbakken T, Lochen ML, et al. Anemia and the risk of non-vertebral fractures: the Tromso Study. Osteoporos Int 2010; 21(10): 1761–8. [DOI] [PubMed] [Google Scholar]
- 28.Valderrabano RJ, Wu JY. Bone and blood interactions in human health and disease. Bone 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurevitch O, Khitrin S, Valitov A, Slavin S. Osteoporosis of hematologic etiology. Experimental hematology 2007; 35(1): 128–36. [DOI] [PubMed] [Google Scholar]
- 30.Paccou J, Hardouin P, Cotten A, Penel G, Cortet B. The role of bone marrow fat in skeletal health: usefulness and perspectives for clinicians. The Journal of Clinical Endocrinology & Metabolism 2015; 100(10): 3613–21. [DOI] [PubMed] [Google Scholar]
- 31.Devlin MJ, Rosen CJ. The bone–fat interface: basic and clinical implications of marrow adiposity. The Lancet Diabetes & Endocrinology 2015; 3(2): 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurkan UA, Akkus O. The mechanical environment of bone marrow: a review. Ann Biomed Eng 2008; 36(12): 1978–91. [DOI] [PubMed] [Google Scholar]
- 33.Dickerson D, Sander E, Nauman E. Modeling the mechanical consequences of vibratory loading in the vertebral body: microscale effects. Biomechanics and Modeling in Mechanobiology 2008; 7(3): 191–202. [DOI] [PubMed] [Google Scholar]
- 34.Panaroni C, Wu JY. Interactions between B lymphocytes and the osteoblast lineage in bone marrow. Calcif Tissue Int 2013; 93(3): 261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]