Abstract
Background
Globally, cannabis use is prevalent and widespread. There are currently no pharmacotherapies approved for treatment of cannabis use disorders.
This is an update of a Cochrane Review first published in the Cochrane Library in Issue 12, 2014.
Objectives
To assess the effectiveness and safety of pharmacotherapies as compared with each other, placebo or no pharmacotherapy (supportive care) for reducing symptoms of cannabis withdrawal and promoting cessation or reduction of cannabis use.
Search methods
We updated our searches of the following databases to March 2018: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycINFO and Web of Science.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs involving the use of medications to treat cannabis withdrawal or to promote cessation or reduction of cannabis use, or both, in comparison with other medications, placebo or no medication (supportive care) in people diagnosed as cannabis dependent or who were likely to be dependent.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 21 RCTs involving 1755 participants: 18 studies recruited adults (mean age 22 to 41 years); three studies targeted young people (mean age 20 years). Most (75%) participants were male. The studies were at low risk of performance, detection and selective outcome reporting bias. One study was at risk of selection bias, and three studies were at risk of attrition bias.
All studies involved comparison of active medication and placebo. The medications were diverse, as were the outcomes reported, which limited the extent of analysis.
Abstinence at end of treatment was no more likely with Δ9‐tetrahydrocannabinol (THC) preparations than with placebo (risk ratio (RR) 0.98, 95% confidence interval (CI) 0.64 to 1.52; 305 participants; 3 studies; moderate‐quality evidence). For selective serotonin reuptake inhibitor (SSRI) antidepressants, mixed action antidepressants, anticonvulsants and mood stabilisers, buspirone and N‐acetylcysteine, there was no difference in the likelihood of abstinence at end of treatment compared to placebo (low‐ to very low‐quality evidence).
There was qualitative evidence of reduced intensity of withdrawal symptoms with THC preparations compared to placebo. For other pharmacotherapies, this outcome was either not examined, or no significant differences was reported.
Adverse effects were no more likely with THC preparations (RR 1.02, 95% CI 0.89 to 1.17; 318 participants; 3 studies) or N‐acetylcysteine (RR 0.94, 95% CI 0.71 to 1.23; 418 participants; 2 studies) compared to placebo (moderate‐quality evidence). For SSRI antidepressants, mixed action antidepressants, buspirone and N‐acetylcysteine, there was no difference in adverse effects compared to placebo (low‐ to very low‐quality evidence).
There was no difference in the likelihood of withdrawal from treatment due to adverse effects with THC preparations, SSRIs antidepressants, mixed action antidepressants, anticonvulsants and mood stabilisers, buspirone and N‐acetylcysteine compared to placebo (low‐ to very low‐quality evidence).
There was no difference in the likelihood of treatment completion with THC preparations, SSRI antidepressants, mixed action antidepressants and buspirone compared to placebo (low‐ to very low‐quality evidence) or with N‐acetylcysteine compared to placebo (RR 1.06, 95% CI 0.93 to 1.21; 418 participants; 2 studies; moderate‐quality evidence). Anticonvulsants and mood stabilisers appeared to reduce the likelihood of treatment completion (RR 0.66, 95% CI 0.47 to 0.92; 141 participants; 3 studies; low‐quality evidence).
Available evidence on gabapentin (anticonvulsant), oxytocin (neuropeptide) and atomoxetine was insufficient for estimates of effectiveness.
Authors' conclusions
There is incomplete evidence for all of the pharmacotherapies investigated, and for many outcomes the quality of the evidence was low or very low. Findings indicate that SSRI antidepressants, mixed action antidepressants, bupropion, buspirone and atomoxetine are probably of little value in the treatment of cannabis dependence. Given the limited evidence of efficacy, THC preparations should be considered still experimental, with some positive effects on withdrawal symptoms and craving. The evidence base for the anticonvulsant gabapentin, oxytocin, and N‐acetylcysteine is weak, but these medications are also worth further investigation.
Keywords: Adult, Female, Humans, Male, Young Adult, Acetylcysteine, Acetylcysteine/adverse effects, Acetylcysteine/therapeutic use, Anticonvulsants, Anticonvulsants/adverse effects, Anticonvulsants/therapeutic use, Antidepressive Agents, Antidepressive Agents/adverse effects, Antidepressive Agents/therapeutic use, Buspirone, Buspirone/adverse effects, Buspirone/therapeutic use, Dronabinol, Dronabinol/adverse effects, Dronabinol/therapeutic use, Marijuana Abuse, Marijuana Abuse/drug therapy, Randomized Controlled Trials as Topic, Selective Serotonin Reuptake Inhibitors, Selective Serotonin Reuptake Inhibitors/therapeutic use, Serotonin Receptor Agonists, Serotonin Receptor Agonists/adverse effects, Serotonin Receptor Agonists/therapeutic use
Plain language summary
Medicines for the treatment of cannabis dependence
Background
Cannabis use is relatively common and widespread worldwide. Demand by cannabis users for treatment has been increasing in most regions of the world. Moves in some countries to decriminalise or legalise cannabis use is likely to result in this trend continuing. Currently there are no medicines specifically for the treatment of cannabis use. This review sought to assess the effectiveness and safety of medicines for the treatment of cannabis dependence.
Search date
We searched the scientific literature in March 2018.
Study characteristics
We identified 21 randomised controlled trials (clinical studies where people are allocated at random to one of two or more treatment groups) involving 909 participants treated with active medicines, and 846 who received placebo (a pretend treatment). Key features of dependent drug use are compulsive use, loss of control over use and withdrawal symptoms on cessation of drug use. This review included studies where participants were described as dependent or were likely to be dependent based on cannabis use occurring several days a week, or daily.
The mean age of participants in individual studies ranged from 22 to 41 years, excluding three studies that targeted young people. Most (75%) study participants were male. Most (16) of the studies were undertaken in the USA, with three occurring in Australia, one in Canada and one in Israel. The studies tested a wide range of medicines to reduce the symptoms of cannabis withdrawal and to promote cessation or reduction of cannabis use.
Four studies received study medicines from the manufacturing pharmaceutical company but none were funded by pharmaceutical companies. One study did not report funding or medicine source.
Key results
For the outcome of abstinence at the end of treatment, Δ9‐tetrahydrocannabinol (THC, the major constituent in cannabis) preparations were probably ineffective; antidepressants called selective serotonin reuptake inhibitors, mixed action antidepressants, a medicine called buspirone and a medicine called N‐acetylcysteine may also have been ineffective; and we are uncertain about the effect of anticonvulsants and mood stabilisers.
For the outcome of completion of the scheduled period of treatment, THC preparations, mixed action antidepressants, anticonvulsants and mood stabilisers may not have been effective, we were uncertain about the effect of SSRI antidepressants, and N‐acetylcysteine probably did not support treatment completion. The use of anticonvulsants and mood stabilisers may have increased the likelihood that people left treatment early.
THC preparations and N‐acetylcysteine were probably no more likely to cause side effects than placebo, mixed action antidepressants and buspirone may have been no more likely to cause side effects than placebo, and we were uncertain about SSRI antidepressants.
Based on current research, all medicines should be considered still experimental.
Quality of the evidence
The quality of the evidence for many of the outcomes in this review was low or very low because each medicine was investigated by a small number of studies (ranging from one to four), each study involved small numbers of participants, there was some inconsistency in the findings and there was a risk of bias due to study participants dropping out of treatment.
Summary of findings
Summary of findings for the main comparison. Δ9‐Tetrahydrocannabinol preparation compared to placebo for cannabis dependence.
| THC preparation compared to placebo for cannabis dependence | |||||
| Patient or population: cannabis dependence Setting: inpatient or outpatient Intervention: THC preparation Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with THC preparation | ||||
| Participants abstinent at end of treatment | Study population | RR 0.98 (0.64 to 1.52) | 305 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| 204 per 1000 | 200 per 1000 (131 to 310) | ||||
| Participants experiencing adverse effects | Study population |
RR 1.02 (0.89 to 1.17) |
318 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| 690 per 1000 | 704 per 1000 (614 to 807) | ||||
| Participants withdrawn due to adverse effects | Study population |
RR 2.72 (0.51 to 14.59) |
318 (3 RCTs) | ⊕⊕⊝⊝ Lowb | |
| 13 per 1000 | 34 per 1000 (6 to 185) | ||||
| Completion of scheduled treatment | Study population |
RR 1.10 (0.88 to 1.37) |
369 (4 RCTs) | ⊕⊕⊝⊝ Lowa,c | |
| 648 per 1000 | 0 per 1000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; THC: Δ9‐tetrahydrocannabinol. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level for imprecision: very few events and small group sizes.
bDowngraded two levels for imprecision: very few events and small group sizes.
cDowngraded one level for inconsistency: Studies differed in direction of effect without significant heterogeneity.
Summary of findings 2. Selective serotonin reuptake inhibitor antidepressant compared to placebo for cannabis dependence.
| SSRI antidepressant compared to placebo for cannabis dependence | |||||
| Patient or population: cannabis dependence Setting: outpatient Intervention: SSRI antidepressant Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with SSRI antidepressant | ||||
| Participants abstinent at end of treatment | Study population |
RR 1.73 (0.61 to 4.89) |
128 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| 82 per 1000 | 142 per 1000 (50 to 401) | ||||
| Participants experiencing adverse effects | Study population |
RR 0.76 (0.57 to 1.02) |
76 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c | |
| 800 per 1000 | 608 per 1000 (456 to 816) | ||||
| Participants withdrawn due to adverse effects | Study population |
RR 1.71 (0.16 to 18.04) |
76 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c | |
| 29 per 1000 | 49 per 1000 (5 to 515) | ||||
| Completion of scheduled treatment | Study population |
RR 0.79 (0.49 to 1.27) |
198 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,d | |
| 680 per 1000 | 538 per 1000 (333 to 864) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SSRI: selective serotonin reuptake inhibitor. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level for risk of bias: one study at high risk of bias due to differences in appointment attendance, one study at high risk of attrition bias.
bDowngraded one level for imprecision: very few events and small group sizes.
cDowngraded two levels for imprecision: very few events and small group sizes.
dDowngraded one level for inconsistency: significant heterogeneity between studies.
Summary of findings 3. Mixed action antidepressant compared to placebo for cannabis dependence.
| Mixed action antidepressant compared to placebo for cannabis dependence | |||||
| Patient or population: cannabis dependence Setting: outpatient Intervention: mixed action antidepressant Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with mixed action antidepressant | ||||
| Participants abstinent at end of treatment | Study population |
RR 0.82 (0.12 to 5.41) |
179 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| 250 per 1000 | 205 per 1000 (30 to 1000) | ||||
| Participants experiencing adverse effects | Study population |
RR 0.93 (0.55 to 1.55) |
76 (1 RCT) | ⊕⊕⊝⊝ Lowc | |
| 450 per 1000 | 419 per 1000 (248 to 698) | ||||
| Participants withdrawn due to adverse effects | Study population |
RR 1.44 (0.11 to 18.90) |
179 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| 11 per 1000 | 16 per 1000 (1 to 205) | ||||
| Completion of scheduled treatment | Study population |
RR 0.93 (0.71 to 1.21) |
169 (2 RCTs) | ⊕⊕⊝⊝ Lowc | |
| 573 per 1000 | 533 per 1000 (407 to 694) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level for inconsistency: significant heterogeneity between studies.
bDowngraded one level for imprecision: very few events and small group sizes.
cDowngraded two levels for imprecision: very few events and small group sizes.
cStudies differed in direction of effect without significant heterogeneity.
Summary of findings 4. Anticonvulsants and mood stabilisers compared to placebo for cannabis dependence.
| Anticonvulsants and mood stabilisers compared to placebo for cannabis dependence | |||||
| Patient or population: cannabis dependence Setting: inpatient or outpatient Intervention: anticonvulsants and mood stabilisers Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with anticonvulsants and mood stabilisers | ||||
| Participants abstinent at end of treatment | Study population |
RR 1.14 (0.64 to 2.04) |
48 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | |
| 440 per 1000 | 502 per 1000 (282 to 898) | ||||
| Participants withdrawn due to adverse effects | Study population |
RR 3.67 (0.41 to 32.69) |
116 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c | |
| 39 per 1000 | 144 per 1000 (16 to 1000) | ||||
| Completion of scheduled treatment | Study population |
RR 0.66 (0.47 to 0.92) |
141 (3 RCTs) | ⊕⊕⊝⊝ Lowb | |
| 556 per 1000 | 367 per 1000 (261 to 511) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level for risk of bias: One study at high risk of attrition bias.
bDowngraded two levels for imprecision: Very few events and small group sizes.
cDowngraded one level for inconsistency: Studies differ in direction of effect without significant heterogeneity.
Summary of findings 5. Buspirone compared to placebo for cannabis dependence.
| Buspirone compared to placebo for cannabis dependence | |||||
| Patient or population: cannabis dependence Setting: outpatient Intervention: buspirone Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with buspirone | ||||
| Participants abstinent at end of treatment | Study population |
RR 1.98 (0.62 to 6.33) |
175 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| 46 per 1000 | 91 per 1000 (29 to 291) | ||||
| Participants experiencing adverse effects | Study population |
RR 1.14 (1.00 to 1.29) |
225 (2 RCTs) | ⊕⊕⊝⊝ Lowa | |
| 763 per 1000 | 870 per 1000 (763 to 984) | ||||
| Participants withdrawn due to adverse effects | Study population |
RR 0.63 (0.15 to 2.60) |
225 (2 RCTs) | ⊕⊕⊝⊝ Lowa | |
| 44 per 1000 | 28 per 1000 (7 to 114) | ||||
| Completion of scheduled treatment | Study population |
RR 0.96 (0.74 to 1.23) |
225 (2 RCTs) | ⊕⊕⊝⊝ Lowa | |
| 526 per 1000 | 505 per 1000 (389 to 647) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels for imprecision: very few events and small group sizes.
Summary of findings 6. N‐acetylcysteine compared to placebo for cannabis dependence.
| N‐acetylcysteine compared to placebo for cannabis dependence | |||||
| Patient or population: cannabis dependence Setting: outpatient Intervention: N‐acetylcysteine Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with N‐acetylcysteine | ||||
| Participants abstinent at end of treatment | Study population |
RR 0.89 (0.59 to 1.35) |
302 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| 242 per 1000 | 215 per 1000 (143 to 326) | ||||
| Participants experiencing adverse effects | Study population |
RR 0.94 (0.71 to 1.23) |
418 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| 329 per 1000 | 309 per 1000 (233 to 404) | ||||
| Participants withdrawn due to adverse effects | Study population |
RR 3.00 (0.12 to 72.15) |
116 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Completion of scheduled treatment | Study population |
RR 1.06 (0.93 to 1.21) |
418 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| 652 per 1000 | 691 per 1000 (607 to 789) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels for imprecision: single study, few events.
bDowngraded one level for imprecision: very few events and small group sizes.
Background
Description of the condition
Cannabis production and consumption is highly prevalent and widespread globally (World Drug Report 2017). It is estimated that 3.8% of the global adult population used cannabis in the past year (World Drug Report 2017).
Cannabis use disorders are the reason for treatment in around half the people seeking treatment for the first time at the global level (World Drug Report 2017). Cannabis is identified as the primary drug of concern for more than half of people in treatment for drug use in Africa and Oceania (World Drug Report 2017). Cannabis use within some indigenous communities in North America and Australia may be more prevalent than for their non‐indigenous counterparts (Beauvais 2004; Clough 2004).
The main psychoactive compound in all cannabis products is Δ9‐tetrahydrocannabinol (THC; EMCDDA Cannabis Drug Profile). Cannabis use causes significant adverse effects (Budney 2007a). The acute effects of short‐term cannabis use include impaired memory (Solowij 2008); impaired motor co‐ordination with an associated increased risk of involvement in motor vehicle accidents (Hall 2009); altered judgement; and, in high doses, paranoia and psychosis (Volkow 2014). Long‐term or heavy use of cannabis has been associated with: the development of dependence (Budney 2007a), chronic bronchitis and increased risk of chronic psychotic disorders in people with a predisposition for development of such disorders (Volkow 2014). When use is commenced early in adolescence, long‐term or heavy cannabis use has also been associated with altered brain development, poor educational outcome (Silins 2014; Silins 2015), cognitive impairment (Solowij 2008), and diminished life satisfaction and achievement (Gruber 2003).
One study using a large epidemiological survey in the USA estimated that 47.4% of males and 32.5% of females exposed to cannabis in their lifetime will develop a cannabis use disorder. For most people, the disorder would be cannabis abuse by Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) criteria, but approximately 7.0% of males and 5.3% of females who use cannabis at some point in their life would be likely to develop cannabis dependence (Lev‐Ran 2013a). It has been estimated that, globally in 2017, more than 19 million people were cannabis dependent (Peacock 2018).
As with other drugs of dependence, the risk of developing dependency is influenced by multiple factors. However, intensive use of cannabis, that is daily or near daily use, is likely to increase the risk of cannabis dependence (EMCDDA 2004). It has been suggested that the earlier initiation of cannabis use (Copeland 2014), use of more potent forms of cannabis (e.g. the flowering heads of the female cannabis plant) and the greater use of water‐pipes may have led to an increased amount of THC consumption by some cannabis users and, therefore, possibly greater rates of cannabis dependence (Hall 2001).
The use of cannabis has consistently been associated with psychotic symptoms (Minozzi 2010), and may be associated with the earlier onset of psychotic illness in some people (Large 2011). Cannabis use has been associated with a range of mental health disorders, such as anxiety and mood disorders (Lev‐Ran 2013b). These associations are particularly pronounced with bipolar disorder, substance use disorders and specific (antisocial, dependant and histrionic) personality disorders (Lev‐Ran 2013b).
Estimates of the number of people who use cannabis and experience withdrawal are variable (Agrawal 2008; Budney 2006; Chung 2008; Copersino 2006; Cornelius 2008; Hasin 2008). Evidence regarding factors influencing the severity of cannabis withdrawal remains limited, but there is evidence that the amount of cannabis smoked is predictive of the intensity of withdrawal during abstinence from cannabis (McClure 2012). Smoking behaviour also appears to be a strong predictor for the severity of cannabis dependence (van der Pol 2014).
General acceptance of a specific cannabis withdrawal syndrome is indicated by the inclusion of diagnostic criteria for cannabis withdrawal in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). The DSM‐5 defines cannabis withdrawal by development of three or more of the following signs and symptoms within approximately one week of cessation of heavy and prolonged cannabis use: 1. irritability, anger or aggression; 2. nervousness or anxiety; 3. sleep difficulty; 4. decreased appetite or weight loss; 5. restlessness; 6. depressed mood; and 7. at least one of the following physical symptoms causing significant discomfort: stomach pain, shakiness or tremors, sweating, fever, chills or headache (DSM‐5). Onset of symptoms is usually within 24 to 48 hours of abstinence, reaching peak intensity within the first week (Budney 2007a). Symptoms may persist for up three to four weeks (Milin 2008), although there appears to be significant individual variability. Cannabis withdrawal is not life threatening, neither is it associated with significant medical or psychiatric consequences (Budney 2003).
Demand for treatment by people who use cannabis has generally increased worldwide since the mid to late 2000s, albeit with significant regional variation. The World Drug Report gives data on treatment demand in terms of the proportion of treatment services provided for the major drugs of dependence. People who use cannabis have dominated demand for drug treatment in Africa since the mid to late 2000s with treatment rates consistently over 60%. Demand for cannabis treatment has grown significantly in some regions, more than doubling in Europe and South America and more than trebling in Oceania (World Drug Report 2017). North America as a whole was the only region to see a decrease in the contribution of cannabis to treatment demand (World Drug Report 2017), but within the USA, cannabis admissions increased by 32% between 1996 and 2006 (SAMHSA 2008). With moves to decriminalise or legalise cannabis use in some parts of the world, the trend of increasing demand for treatment is likely to continue.
Increases in the THC content of cannabis may be a factor in the increasing demand for treatment. In the USA, THC content, as detected in confiscated samples, increased from about 3% in the 1980s to 12% in 2012 (Volkow 2014). Cannabis users adjust their smoking behaviour when smoking stronger cannabis but the adjustment does not fully compensate for the increased strength (van der Pol 2014). Hence, cannabis users would be expected to be exposed to higher doses of THC as a result of the increasing potency of cannabis preparations. Cannabis users who seek treatment typically have a long history of cannabis use disorder and multiple previous attempts to quit (Copeland 2014).
Description of the intervention
There are currently no accepted pharmacotherapies for the treatment of cannabis withdrawal or cessation. The identification and development of medications to fill this gap has long been a priority among researchers (Vandrey 2009), and a number of pharmacotherapies have been proposed as possible experimental interventions to attenuate the symptoms and signs of cannabis withdrawal and to promote cessation.
These medications are diverse in nature, encompassing medications that affect cannabinoid receptor systems (e.g. preparations of THC), medications that affect dopamine pathways, medications that affect the specific symptoms of cannabis withdrawal or that have been used in managing withdrawal from other substances, and medications that affect mental health conditions, such as depression, that may be factors contributing to cannabis use.
How the intervention might work
The proposed pharmacological interventions may potentially lessen the symptoms and signs of cannabis withdrawal, including craving. The availability of effective pharmacotherapy for cannabis withdrawal may encourage people who are cannabis dependent to enter treatment, and may increase the rates of completion of withdrawal, cessation of cannabis use and entry into relapse prevention treatment.
It has been reported that the experience of cannabis withdrawal symptoms may be a significant obstacle to the achievement of abstinence by people who are cannabis dependent (Budney 2006; Copeland 2001; Hart 2005). Therefore, the effective treatment of the cannabis withdrawal syndrome may promote cessation of cannabis use and provide a first step towards abstinence and recovery.
Why it is important to do this review
As discussed above, there is increasing recognition that cannabis use and dependence is an important public health issue.
Not all people who use cannabis will need pharmacotherapies to manage withdrawal or support cessation of their use. However, it is important that effective pharmacotherapies are identified for the treatment of cannabis withdrawal, especially in intensive cannabis users who describe withdrawal symptoms on cessation. As such, with the increase in the number of pharmacotherapies tested, this review sought to establish current knowledge on the effectiveness of different medications in the treatment of cannabis dependence.
Objectives
To assess the effectiveness and safety of pharmacotherapies as compared with each other, placebo or no pharmacotherapy (supportive care) for reducing symptoms of cannabis withdrawal and promoting cessation or reduction of cannabis use.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials that provided detailed information on the type and dose of intervention medication used and the characteristics of participants treated.
Types of participants
We included studies that involved participants diagnosed as cannabis dependent or who were likely to be dependent based on reported dose, duration and frequency of use (daily or multiple days per week).
We included studies involving participants dependent on, and withdrawing from, both cannabis and nicotine, but excluded studies involving participants dependent on and withdrawing from substances other than cannabis and nicotine.
We included studies undertaken in either inpatient or outpatient settings. We excluded studies undertaken in purely research settings, such as residential research laboratories. Some of these studies provide insight into the effect of different medications on signs and symptoms of cannabis withdrawal and are considered in the 'Discussion' section. However, such studies generally involved participants who were not seeking treatment for cannabis use and cessation of cannabis use was not the goal of the interventions provided, and the nature of outcomes assessed were generally different to those expected of treatment interventions. For these reasons, we excluded such studies from this review.
We excluded studies involving participants with diagnosed schizophrenia and cannabis use disorder. The primary therapeutic goal in these studies was management of psychotic symptoms, with consideration of the effect of different antipsychotic medications on cannabis use. This limits the application of findings of such studies to the general group of people with cannabis use disorders. Cannabis use in schizophrenia is considered by a separate Cochrane Review (McLoughlin 2014).
Types of interventions
Experimental interventions involved the administration of any medication with the aim of reducing the symptoms and signs of cannabis withdrawal or promoting cessation of cannabis use.
Comparison interventions involved the use of different pharmacotherapies, placebo or no pharmacotherapy (supportive care).
Types of outcome measures
Primary outcomes
Number of participants abstinent from cannabis at the end of treatment as determined by self‐report or urine drug screens, or both.
Intensity of withdrawal as determined by scores on withdrawal scales, the need for symptomatic medications in addition to the experimental intervention or overall assessments by clinicians and participants.
Nature, incidence and frequency of adverse effects and whether the planned medication regimen was modified in response to adverse effects.
Completion of scheduled treatment.
Secondary outcomes
Level of cannabis use at the end of treatment as measured via participant‐reported level of use or urine drug screens, or both.
Number of participants engaged in further treatment following completion of the withdrawal intervention. As discussed in the 'Background' section, treatment of the cannabis withdrawal period may be considered as the first step in treatment, therefore engagement in further relapse prevention treatment may be considered to be a valid outcome of interest.
Search methods for identification of studies
All searches included non‐English language literature. We found no studies in languages other than English.
Electronic searches
We searched:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library 2018, Issue 2;
MEDLINE (1946 to week 3 March 2018) via Ovid Online;
Embase Online (26 March 2018);
PsycINFO (1806 to week 3 March 2018) via Ovid Online;
Web of Science, online (26 March 2018).
We developed a search strategy to retrieve references relating to the pharmacological treatment of cannabis withdrawal. This strategy was adapted to each of the databases listed above.
For details see Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5.
We also searched the following electronic sources of ongoing trials:
ANZCTR registry (January 2018);
ClinicalTrials.gov (www.clinicaltrials.gov; January 2018).
Searching other resources
We checked the reference lists of relevant review articles and retrieved studies to identify any further studies of interest that were not retrieved by the electronic search. We contacted selected researchers who were active in the area seeking information about unpublished study reports. We also checked conference proceedings likely to contain trials relevant to the review.
Data collection and analysis
Selection of studies
Two authors (LG and SN or PS) independently assessed the titles and abstracts of records retrieved from the systematic search according to the identified inclusion and exclusion criteria. All authors agreed on the inclusion and exclusion decisions. We made no attempt to blind the authors to the names of the study authors, institutions, journal of publication and results when applying eligibility criteria.
Data extraction and management
Two authors (LG and SN) extracted key information from the included studies using a data collection form to record information against the outcome measures (abstinence, intensity of withdrawal, adverse effects, completion of treatment, change in cannabis use and engagement in follow‐up treatment) and study characteristics (location, participant characteristics, interventions, study design and source of funding). We confirmed data by consultation with the other review authors. We summarised key findings of studies descriptively in the first instance and considered the capacity for quantitative meta‐analysis.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies according to the approach recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This was based on the evaluation of seven specific methodological domains (namely, sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other bias). For each study, we analysed the seven domains, described them as reported in the study and provided a final judgement on the likelihood of bias in terms of low, high or unclear risk of bias. We based these judgements on the criteria indicated by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and their applicability to the addiction field.
In general, subjective outcomes are more prone to performance and detection bias than objective outcomes. The outcome measures in this review that included a subjective component were self‐reported cannabis use (including abstinence at the end of treatment) and intensity of withdrawal. When considering the risk of bias due to blinding of participants and personnel (performance bias) and blinding of outcome assessment (detection bias), we based the judgement on the outcomes most prone to bias, and also considered the use of measures, such as urinalysis, to support the subjective assessment. We only considered incomplete outcome data for the intensity of withdrawal, change in cannabis use, and nature and incidence of adverse effects. Retention in treatment (duration of treatment) and completion of treatment are frequently primary outcome measures in addiction research. See Appendix 6 for a detailed description of the criteria we considered in the 'Risk of bias' assessment.
Details of the assessments of risk of bias are included in the Characteristics of included studies table.
Measures of treatment effect
Where possible, for dichotomous outcomes (e.g. number completing treatment), we calculated risk ratios (RR) with 95% confidence intervals (CI). There were no continuous data but the intention was to express continuous outcomes as a mean difference where there was a comparable outcome measure (e.g. time in treatment) or as a standardised mean difference where there was variability in the outcome measure (e.g. withdrawal assessment scales), with 95% CIs.
Unit of analysis issues
One included study involved three treatment arms (two different active medications and placebo) (Carpenter 2009). The active medications, compared to placebo, were included in separate comparisons thereby avoiding the unit of analysis error of double‐counting participants. Where urine drug screens were reported in studies, the unit of analysis was the number of study participants and not the number of tests performed.
Dealing with missing data
We contacted original investigators to request missing data, and, where unpublished data were available, included these in the analyses and noted in the study record. We also checked Clinicaltrials.gov where data were missing. It was also intended to use sensitivity analysis to assess the impact of different approaches to handling missing data, but there were insufficient data for this.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by reviewing the variation between studies in terms of the characteristics of participants included, the interventions and the reported outcomes. We grouped studies for analyses by the nature of the medication used (experimental intervention).
We assessed statistical heterogeneity using the Chi2 test and its P value, by visual inspection of the forest plots and the I2 statistic. A P value of the Chi2 test lower than 0.10 or an I2 statistic of at least 50% indicated significant statistical heterogeneity.
Data synthesis
We used Review Manager 5 for statistical analyses (Review Manager 2014). In all analyses, we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
This review aimed to consider the following potential sources of heterogeneity through subgroup analyses:
patterns of cannabis use and the estimated level of THC intake (as indicated by duration and level of use, number of days of use, number of uses per day (frequency), modality of use or route of administration, age at initiation of use);
concurrent tobacco smoking;
concurrent psychiatric illness and current treatment for a psychiatric illness;
the nature of the treatment setting;
the nature of adjunct treatment.
None of these analyses were possible due to limitations of the studies that met the inclusion criteria.
Sensitivity analysis
We did not use risk of bias as a criterion for inclusion in this review. We intended to assess the impact of risk of bias through sensitivity analysis. This would have involved considering the overall estimate of effect with studies with a high risk of bias included or excluded. Limitations of data reported by the studies that met the inclusion criteria meant that sensitivity analysis was not possible. However, we discussed the risk of bias when presenting the results.
Grading of evidence
We assessed the overall quality of the evidence for the primary outcomes using the system developed by the GRADE Working Group for grading the quality of evidence (Schűnemann 2013). GRADE takes into account issues not only related to internal validity but also to external validity, such as directness, consistency, imprecision of results and publication bias. The 'Summary of findings' tables present the main findings of a review in a transparent and simple tabular format. In particular, they provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on the main outcomes.
The GRADE system uses the following criteria for assigning grades of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Grading of the quality of randomised controlled trials is decreased for the following reasons.
Serious (–1) or very serious (–2) study limitation for risk of bias.
Serious (–1) or very serious (–2) inconsistency between study results.
Some (–1) or major (–2) uncertainty about directness (the correspondence between the population, the intervention or the outcomes measured in the studies actually found and those under consideration in our systematic review).
Serious (–1) or very serious (–2) imprecision of the pooled estimate (–1).
Publication bias strongly suspected (–1).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies tables.
Results of the search
Our search strategy identified 1364 records through database searching and five additional records from other sources. After removing duplicates, there were 809 unique records. We excluded 720 records based on title and abstract and obtained 89 full‐text for further assessment. On reading the full text, we excluded 43 reports (relating to 34 studies) with reasons (see Characteristics of excluded studies table). We included 21 studies (45 reports) in the review (see Figure 1).
1.

Study flow diagram.
Included studies
Twenty‐one randomised controlled trials (45 reports) involving 1755 participants met the inclusion criteria for this review (Allsop 2014; Carpenter 2009; Cornelius 2010; Frewen 2007; Gray 2012; Gray 2017; Johnston 2014; Levin 2004; Levin 2011; Levin 2013; Levin 2016; Mason 2012; McRae‐Clark 2009; McRae‐Clark 2010; McRae‐Clark 2015; McRae‐Clark 2016; Miranda 2017; Penetar 2012; Sherman 2017; Trigo 2018; Weinstein 2014; see Characteristics of included studies table). In total, 909 participants received active medication and 846 participants received placebo. One study reported only the total number of participants (81) and we assumed group sizes of 41 and 40 (Frewen 2007). All studies offered participants some form of psychological therapy in addition to medication or placebo.
All studies involved a comparison between an active medication and placebo but medications were diverse in nature. The medications investigated, grouped according to type and mechanism of action, were:
preparations containing THC: dronabinol (Levin 2011), dronabinol plus lofexidine (Levin 2016), nabiximols (Allsop 2014; Trigo 2018);
selective serotonin reuptake inhibitor (SSRI) antidepressants: fluoxetine (Cornelius 2010), escitalopram (Weinstein 2014), vilazodone (McRae‐Clark 2016);
mixed action antidepressants (noradrenergic and serotonergic effects): nefazodone (Carpenter 2009), mirtazapine (Frewen 2007), venlafaxine (Levin 2013);
anticonvulsant and mood stabilisers: divalproex sodium (Levin 2004), gabapentin (Mason 2012), lithium (Johnston 2014), topiramate (Miranda 2017);
atypical antidepressant (dopamine reuptake inhibitor and weak noradrenaline reuptake inhibitor): bupropion (Carpenter 2009; Penetar 2012);
anxiolytic (serotonin 5‐HT1A partial agonist): buspirone (McRae‐Clark 2009, McRae‐Clark 2015);
selective noradrenaline reuptake inhibitor: atomoxetine (McRae‐Clark 2010);
supplement promoting glutamate release and modulating N‐methyl‐D‐aspartate (NMDA) receptor, N‐acetylcysteine: (Gray 2012; Gray 2017); and
neuropeptide, oxytocin (Sherman 2017).
All except two of the studies were undertaken in outpatient settings. Allsop 2014 and Johnston 2014 were primarily studies of cannabis withdrawal, with medication administered in an inpatient (hospital) setting over six or seven days, with follow‐up interviews after discharge. The scheduled duration for outpatient studies ranged from three weeks (Penetar 2012) to 13 weeks (Carpenter 2009), with 11 to 13 weeks being most common.
Sixteen studies were undertaken in the USA, with three studies in Australia (Allsop 2014; Frewen 2007; Johnston 2014), one study in Israel (Weinstein 2014), and one in Canada (Trigo 2018). Twenty studies reported the source of funding to be (government) research grants; the funding source was unclear for one study (Frewen 2007). Five studies received medications from the manufacturing company (Allsop 2014; Levin 2016; McRae‐Clark 2010; McRae‐Clark 2016; Trigo 2018). Researchers associated with 10 studies declared past or current associations with pharmaceutical companies. Researchers associated with eight studies declared no conflict of interest. Authors of the remaining studies made no declarations.
Four studies included participants with cannabis use disorders as well as cannabis dependence, but the majority of participants met diagnostic criteria for cannabis dependence (Cornelius 2010; Levin 2013; Miranda 2017; Penetar 2012). In the other studies, all participants were cannabis dependent.
The mean age of study participants ranged from 22 to 41 years, and for 12 studies it was between 30 and 35 years; one study did not provide data on age (Penetar 2012). The target population for three studies was adolescents and young adults (Cornelius 2010; Gray 2012; Miranda 2017). The mean age of participants in these studies was 21 (Cornelius 2010), 19 (Gray 2012), and 20 years (Miranda 2017).
One study did not provide information on the gender of participants (Penetar 2012); the majority (61% to 93%) of participants in 19 studies were male. Miranda 2017 was notable in having a smaller proportion (48%) of males.
Participants in two studies had comorbid depression and cannabis use disorders (Cornelius 2010; Levin 2013), and in one study participants met diagnostic criteria for attention deficit hyperactivity disorder (ADHD) as well as cannabis dependence (McRae‐Clark 2010). Gray 2012 reported that 13.8% of participants had some psychiatric comorbidity, but 16 studies excluded people with significant or unstable psychiatric conditions. One study did not report on this aspect (Penetar 2012).
Excluded studies
We excluded 34 studies (43 reports) that were potentially relevant to the review and assessed in detail from the review (see Figure 1; Characteristics of excluded studies table). The reasons for exclusion were: study was exploratory (mostly laboratory‐based) research with participants who were not seeking treatment (13 studies); minority of participants were cannabis dependent, or dependence was unclear (six studies); no treatment comparison (six studies); comparison of antipsychotic drugs for treatment of schizophrenia with concurrent cannabis use (five studies); no treatment intervention, or no medication treatment (five studies); and cannabis use was not the main focus of the treatment intervention (three studies). Five studies were excluded for more than one reason.
Risk of bias in included studies
For summary results of the judged risk of bias across the included studies for each domain, see Figure 2 and Figure 3.
2.
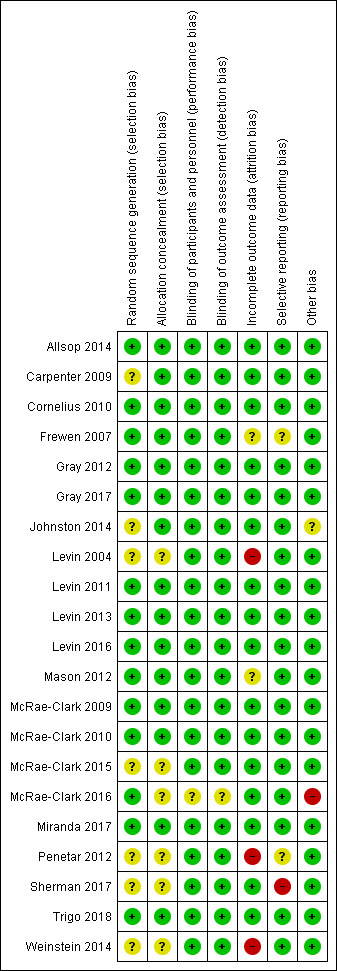
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
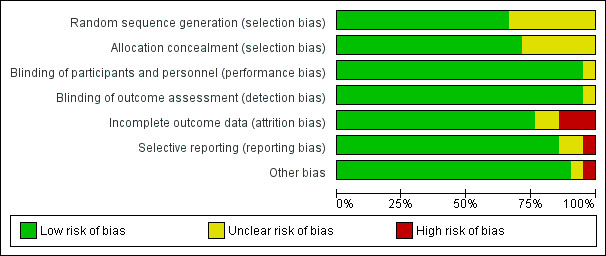
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
We judged seven studies at unclear risk of bias due to the reporting of insufficient information on the method of random sequence generation (Carpenter 2009; Johnston 2014; Levin 2004; McRae‐Clark 2015; Penetar 2012; Sherman 2017; Weinstein 2014). We judged six studies at unclear risk of bias due to the reporting of insufficient information on the method of allocation concealment (Levin 2004; McRae‐Clark 2015; McRae‐Clark 2016; Penetar 2012; Sherman 2017; Weinstein 2014). We judged the remaining studies at low risk of allocation bias.
Blinding
We considered one study to have an unclear risk of both performance and detection bias because insufficient information was reported on the blinding of participants and personnel to make a judgement (McRae‐Clark 2016). All other studies were at low risk of performance and detection bias.
Incomplete outcome data
Completion of treatment is a primary outcome measure for this review. Hence, we only considered the risk of bias due to incomplete data for the outcomes of intensity of withdrawal, adverse effects and abstinence (or use of cannabis). We judged the risk of bias to be unclear for two studies (Frewen 2007; Mason 2012), and high for three studies (Levin 2004; Penetar 2012; Weinstein 2014).
Selective reporting
Frewen 2007 was a secondary analysis of data from a randomised controlled trial and reported some but not all findings from the main study. The full report of the study was not available and hence the risk of reporting bias was unclear. Penetar 2012 did not discuss adverse effects making it unclear whether adverse effects were systematically assessed during the study (unclear risk of reporting bias). On ClinicalTrials.gov, Sherman 2017 indicated "satisfaction with therapy" as a primary outcome but this was not reported in the publication associated with the study (high risk of reporting bias).
Other potential sources of bias
Johnston 2014 administered two‐thirds of participants a benzodiazepine for disturbed sleep. Although the use of such medications were stated to be similar in the two groups, the effect of this additional medication was unclear. We judged McRae‐Clark 2016 to have a high risk of other bias as the placebo group attended a greater proportion of scheduled visits and hence may have received more adjunct interventions.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Results are presented for the outcomes identified as relevant to this review by medication type. Very few studies reported on the secondary outcome regarding the level of cannabis use; where this outcome was reported, it was included in the narrative summary of abstinence at end of treatment. No studies reported on the secondary outcome of participation in further treatment.
1. Preparations containing Δ9‐tetrahydrocannabinol
Four studies compared preparations containing THC with placebo (Allsop 2014; Levin 2011; Levin 2013; Trigo 2018; Table 1).
Participants abstinent at end of treatment
We found no significant difference between THC and placebo in the proportion of participants abstinent at the end of treatment (RR 0.98, 95% CI 0.64 to 1.52; 305 participants; 3 studies; I2 = 0%; Analysis 1.1).
1.1. Analysis.

Comparison 1 Δ9‐Tetrahydrocannabinol (THC) preparation versus placebo, Outcome 1 Participants abstinent at end of treatment.
Withdrawal symptoms and cravings
Preparations containing THC may have been more effective than placebo in reducing cannabis withdrawal symptoms and cravings. Allsop 2014 reported that on average it took 3.1 (standard deviation (SD) 3.0) days for withdrawal scores to fall below baseline with the THC preparation, nabiximols (27 participants), compared with 4.9 (SD 3.16) days for placebo (24 participants). Nabiximols reduced the withdrawal score 66% on average from baseline compared to 52% for placebo. The group receiving nabiximols had significantly lower levels of cravings, irritability, anger and aggression. Levin 2011 similarly reported a reduction in the withdrawal discomfort scores for both the dronabinol (79 participants) and placebo (77 participants) groups, but found that participants receiving dronabinol experienced a significantly greater drop in their withdrawal scores over time. However, Levin 2016 reported that withdrawal scores decreased over time with no significant effect of treatment for dronabinol plus lofexidine compared to placebo and in Trigo 2018, total withdrawal scores progressively decreased with time in treatment, with no significant difference between nabiximols and placebo groups. In Trigo 2018, craving similarly decreased in both groups with time, with no difference between the groups except around week seven when craving was higher in the placebo group.
Participants experiencing adverse effects
We found no significant difference between THC and placebo in the proportion of participants experiencing adverse effects (RR 1.02, 95% CI 0.89 to 1.17; 318 participants; 3 studies; I2 = 7%; Analysis 1.2). THC preparations were associated with a higher likelihood of adverse effects, but these adverse effects were not sufficiently severe to cause withdrawal from treatment.
1.2. Analysis.

Comparison 1 Δ9‐Tetrahydrocannabinol (THC) preparation versus placebo, Outcome 2 Participants experiencing adverse effects.
Participants withdrawn due to adverse effects
We found no significant difference between THC and placebo in the proportion of participants withdrawn from treatment due to adverse effects (RR 2.72, 95% CI 0.51 to 14.59; 318 participants; 3 studies; I2 = 0%; Analysis 1.3), but the number of events was small resulting in the very wide CIs.
1.3. Analysis.

Comparison 1 Δ9‐Tetrahydrocannabinol (THC) preparation versus placebo, Outcome 3 Participants withdrawn due to adverse effects.
Completion of scheduled treatment
We found no significant difference between THC and placebo in the proportion of participants completing the scheduled period of treatment (RR 1.10, 95% CI 0.88 to 1.37; 369 participants; 4 studies; Analysis 1.4). There was some heterogeneity between studies (I2 = 53%).
1.4. Analysis.

Comparison 1 Δ9‐Tetrahydrocannabinol (THC) preparation versus placebo, Outcome 4 Completion of scheduled treatment.
2. Selective serotonin reuptake inhibitor antidepressants
Three studies compared SSRI antidepressants with placebo, including fluoxetine (Cornelius 2010), vilazodone (McRae‐Clark 2016), and escitalopram (Weinstein 2014) (Table 2).
Participants abstinent at end of treatment
We found no significant difference between SSRI antidepressants and placebo in the proportion of participants abstinent at the end of treatment (RR 1.73, 95% CI 0.61 to 4.89; 128 participants; 2 studies; Analysis 2.1). On cannabis use outcomes more generally, in McRae‐Clark 2016, cannabis use decreased in the vilazodone and placebo groups by urine tests and self‐report, with no group differences. In Weinstein 2014, there was a tendency towards participants receiving escitalopram being abstinent at the end of treatment compared to those receiving placebo. However, the high rates of dropout from treatment in this study introduced a high risk of bias for this outcome. Cornelius 2010 compared fluoxetine with placebo and reported that the mean count of criteria for cannabis abuse or dependence at the end of treatment was 3.88 (SD 2.51) for 34 participants treated with fluoxetine compared to 3.61 (SD 1.92) for 36 participants receiving placebo. There were no significant group by time interactions for cannabis or depression outcomes in this study.
2.1. Analysis.

Comparison 2 Selective serotonin‐reuptake inhibitor (SSRI) antidepressant versus placebo, Outcome 1 Participants abstinent at end of treatment.
Withdrawal symptoms and craving
Two studies did not consider the intensity of withdrawal (Cornelius 2010; Weinstein 2014). McRae‐Clark 2016 found no difference in the mean withdrawal score at the end of treatment between vilazodone (6.8, SD 5.6; 28 participants) and placebo (6.7, SD 7.5; 31 participants). Craving also did not differ between the groups (mean 49.9, 95% CI 44.8 to 51.9 for vilazodone, 41 participants; 46.7, 95% CI 41.5 to 51.9 for placebo, 35 participants).
Participants experiencing adverse effects
We found no difference between SSRI antidepressants and placebo in the proportion of participants reporting adverse effects (RR 0.76, 95% CI 0.57 to 1.02; 76 participants; 1 study; Analysis 2.2).
2.2. Analysis.

Comparison 2 Selective serotonin‐reuptake inhibitor (SSRI) antidepressant versus placebo, Outcome 2 Participants experiencing adverse effects.
Participants withdrawn due to adverse effects
We found no difference between SSRI antidepressants and placebo in the proportion of participants withdrawn from treatment due to adverse effects, but the CI is very wide due to the small number of events (RR 1.71, 95% CI 0.16 to 18.04; 76 participants; 1 study; Analysis 2.3).
2.3. Analysis.

Comparison 2 Selective serotonin‐reuptake inhibitor (SSRI) antidepressant versus placebo, Outcome 3 Participants withdrawn due to adverse effects.
Completion of scheduled treatment
We found no difference between SSRI antidepressants and placebo in the proportion of participants completing the scheduled period of treatment (RR 0.79, 95% CI 0.49 to 1.27; 198 participants; 3 studies; Analysis 2.4). There was substantial heterogeneity (I2 = 74%). Weinstein 2014 reported a high dropout rate with escitalopram and Cornelius 2010 found no significant difference in rates of completion of treatment for fluoxetine compared to placebo. McRae‐Clark 2016 reported high levels of attrition in both the vilazodone and placebo groups.
2.4. Analysis.

Comparison 2 Selective serotonin‐reuptake inhibitor (SSRI) antidepressant versus placebo, Outcome 4 Completion of scheduled treatment.
3. Mixed action antidepressants
Three studies examined mixed action antidepressants including nefazodone (Carpenter 2009), mirtazapine (Frewen 2007), and venlafaxine (Levin 2013) (Table 3).
Participants abstinent at end of treatment
We found no significant difference between mixed‐action antidepressants and placebo in the proportion of participants abstinent at the end of treatment (RR 0.82, 95% CI 0.12 to 5.41; 179 participants; 2 studies; Analysis 3.1). There was substantial heterogeneity (I2 = 87%).
3.1. Analysis.

Comparison 3 Mixed action antidepressant versus placebo, Outcome 1 Participants abstinent at end of treatment.
In Levin 2013, significantly fewer participants treated with venlafaxine were abstinent at the end of treatment compared to participants receiving placebo. In contrast, in Carpenter 2009, there was a tendency towards abstinence being more likely with nefazodone compared to placebo. However, there was no significant difference in the severity of dependence rating (mean, SD) at the end of treatment for the nefazodone group (2.5, SD 1.4) compared to the placebo group (2.3, SD 1.6). Frewen 2007 did not report data suitable for inclusion in the meta‐analysis but stated that mirtazapine had no effect on cannabis use, with less than 20% of participants reporting abstinence at day 56.
Withdrawal symptoms and cravings
There was no difference between mirtazapine and placebo (Frewen 2007), or nefazodone and placebo (Carpenter 2009), in the effect on cannabis withdrawal symptoms. Mirtazapine improved sleep duration and quality but not sleep disturbances (Frewen 2007).
Participants experiencing adverse effects
We found no significant difference between nefazodone and placebo in the proportion of participants experiencing adverse effects (RR 0.93, 95% CI 0.55 to 1.55; 76 participants; 1 study; Analysis 3.2). However, Carpenter 2009 reported that adverse effects were more likely to be moderate or severe with nefazodone, with diarrhoea most common with nefazodone and gastrointestinal upset most common with placebo.
3.2. Analysis.

Comparison 3 Mixed action antidepressant versus placebo, Outcome 2 Participants experiencing adverse effects.
Participants withdrawn due to adverse effects
We found no significant difference between mixed‐action antidepressants and placebo in the proportion of participants withdrawn from treatment due to adverse effects (RR 1.44, 95% CI 0.11 to 18.90; 179 participants; 2 studies; I2 = 28%; Analysis 3.3), but the CIs were very wide due to the small number of events.
3.3. Analysis.

Comparison 3 Mixed action antidepressant versus placebo, Outcome 3 Participants withdrawn due to adverse effects.
Completion of scheduled treatment
We found no significant difference between mixed‐action antidepressants and placebo in the proportion of participants completing the scheduled period of treatment (RR 0.93, 95% CI 0.71 to 1.21; 169 participants; 2 studies; I2 = 0%; Analysis 3.4).
3.4. Analysis.

Comparison 3 Mixed action antidepressant versus placebo, Outcome 4 Completion of scheduled treatment.
4. Anticonvulsants and mood stabilisers
The four studies in this group used diverse medications: lithium (Johnston 2014), divalproex (Levin 2004), gabapentin (Mason 2012), and topiramate (Miranda 2017) (Table 4).
Participants abstinent at end of treatment
We found no significant difference between anticonvulsants or mood stabilisers and placebo in the proportion of participants abstinent at the end of treatment (RR 1.14, 95% CI 0.64 to 2.04; 48 participants; 2 studies; I2 = 0%; Analysis 4.1).
4.1. Analysis.

Comparison 4 Anticonvulsants and mood stabilisers versus placebo, Outcome 1 Participants abstinent at end of treatment.
On outcomes of cannabis use more generally, at follow‐up participants in Johnston 2014 reported reductions in the mean number of days of cannabis use in the previous week and mean quantity of cannabis use, with no significant differences between lithium and placebo. Levin 2004 reported that at the end of treatment (weeks seven and eight), participants in the divalproex group reported using cannabis on (mean, SD) 2.75 (SD 3.55) days per week, compared to 1.56 (SD 2.34) days per week for the placebo group, and 4.88 (SD 7.58) joints per week compared to 0.99 (SD 1.18) joints per week for the placebo group. The group by time interaction was not statistically significant.
Mason 2012 reported a significant reduction in the grams of cannabis smoked per week, by self‐report and urinalysis, and in the days of use per week for gabapentin compared to placebo.
Miranda 2017 used linear modelling (imputation analysis) to assess cannabis use during treatment with motivational enhancement therapy (MET) plus topiramate or placebo. While the group differences were not statistically significant, the data suggested that participants in the topiramate group smoked fewer grams of cannabis when they used during the final week of the trial (week six). However, this finding was at risk of bias due to higher rates of dropout from the topiramate group.
Withdrawal symptoms and cravings
Gabapentin may have ameliorated cannabis withdrawal symptoms (Mason 2012), but it appeared that divalproex did not (Levin 2004), and lithium affected only some symptoms (Johnston 2014). Lithium (19 participants) did not significantly reduce the total scores on the cannabis withdrawal scale relative to placebo (19 participants), but did significantly reduce the items "loss of appetite", "stomach aches" and "nightmares or strange dreams" (Johnston 2014). The mean change in total withdrawal score from baseline to the last day of inpatient stay was –1.96 (SD 1.66) for lithium (16 participants), and –2.45 (SD 1.97) for placebo (22 participants). There were no significant group differences between divalproex (13 participants) and placebo (12 participants) (Levin 2004). In Mason 2012, gabapentin (25 participants) was associated with significant reductions in acute withdrawal symptoms compared to placebo (25 participants). Miranda 2017 did not report data on intensity of withdrawal in their study of topiramate.
Participants experiencing adverse effects
There were no data suitable for inclusion in meta‐analyses on the adverse effects of anticonvulsants or mood stabilisers. Johnston 2014 stated there were no significant differences in the number or severity of adverse effects for lithium compared to placebo and reported no serious adverse effects. Levin 2004 noted that medication compliance was low for divalproex, based on blood levels, but it was not clear whether the low rate of compliance was related to adverse effects. For gabapentin compared to placebo, Mason 2012 reported no differences between the groups in the type, number and severity of adverse events reported.
Participants withdrawn due to adverse effects
We found no significant difference between anticonvulsants or mood stabilisers and placebo in the proportion of participants withdrawn due to adverse effects (RR 3.67, 95% CI 0.41 to 32.69; 116 participants; 2 studies; I2 = 43%; Analysis 4.2); however, the CIs were very wide due to the small number of events. In Johnston 2014, no participants in either group withdrew due to adverse effects.
4.2. Analysis.

Comparison 4 Anticonvulsants and mood stabilisers versus placebo, Outcome 2 Participants withdrawn due to adverse effects.
Completion of scheduled treatment
We found a significant reduction in treatment completion in participants who received anticonvulsants or mood stabilisers compared with those who received placebo (RR 0.66, 95% CI 0.47 to 0.92; 141 participants; 3 studies; I2 = 0%; Analysis 4.3). Data on topiramate contributed substantially to this result (weight 71.9%) (Miranda 2017). Participants who received gabapentin were also less likely to complete treatment compared to those receiving placebo (Mason 2012).
4.3. Analysis.

Comparison 4 Anticonvulsants and mood stabilisers versus placebo, Outcome 3 Completion of scheduled treatment.
5. Atypical antidepressant (bupropion)
Two studies examined bupropion compared to placebo (Carpenter 2009; Penetar 2012).
Participants abstinent at end of treatment
Neither study reported data on cannabis use outcomes in a form suitable for inclusion in meta‐analysis. Carpenter 2009 reported no difference between the bupropion and placebo groups in terms of the severity of dependence rating at the completion of treatment.
Withdrawal symptoms and cravings
In Penetar 2012, following cessation of cannabis (days eight to 21 of the scheduled treatment protocol), withdrawal discomfort scores increased significantly for the placebo group (12 participants) but not the bupropion group (10 participants) based on change from baseline. Craving scores also increased more for the placebo group. Carpenter 2009 did not report data on withdrawal intensity.
Participants experiencing adverse effects
There were no data suitable for inclusion in meta‐analyses for the adverse effects of bupropion. Carpenter 2009 reported that adverse effects were more likely to be moderate or severe with bupropion compared to placebo.
Participants withdrawn due to adverse effects
There were no data on withdrawal due to adverse effects.
Completion of scheduled treatment
We found no significant difference between bupropion and placebo in the proportion of participants completing the scheduled period of treatment (RR 1.06, 95% CI 0.67 to 1.67; 92 participants; 2 studies; I2 = 0%; Analysis 5.1).
5.1. Analysis.

Comparison 5 Bupropion versus placebo, Outcome 1 Completion of scheduled treatment.
6. Anxiolytic (buspirone)
Two studies examined buspirone compared to placebo (McRae‐Clark 2009; McRae‐Clark 2015; Table 5).
Participants abstinent at end of treatment
Very few participants in McRae‐Clark 2015 achieved abstinence, with no significant difference between the buspirone and placebo groups (RR 1.98, 95% CI 0.62 to 6.33; 175 participants; 1 study; Analysis 6.1). However, the results of this study suggested that gender may be a factor in the response to buspirone with women having worse cannabis use outcomes with buspirone compared to placebo.
6.1. Analysis.

Comparison 6 Buspirone versus placebo, Outcome 1 Participants abstinent at end of treatment.
Withdrawal symptoms and cravings
Both studies found buspirone to have no advantage over placebo on cannabis withdrawal symptoms (McRae‐Clark 2009; McRae‐Clark 2015). McRae‐Clark 2009 reported no significant difference between buspirone and placebo in terms of change in the mean withdrawal checklist score (–10.87, SD 8.99; 23 participants for buspirone and –10.4, SD 7.73; 27 participants for placebo). In McRae‐Clark 2015, the craving score decreased significantly with time in treatment, but with no differences between buspirone and placebo.
Participants experiencing adverse effects
We found that participants were somewhat more likely to experience adverse effects with buspirone compared to placebo (RR 1.14, 95% CI 1.00 to 1.29; 225 participants; 2 studies; I2 = 0%; Analysis 6.2). In McRae‐Clark 2009, gastrointestinal symptoms, headache, dizziness and drowsiness were the most common adverse effects reported. Nearly all were noted as being mild to moderate in severity. In McRae‐Clark 2015, 2/88 participants receiving buspirone, and 1/87 participants receiving placebo, experienced moderate‐to‐severe adverse effects, but these were unrelated to the medication.
6.2. Analysis.

Comparison 6 Buspirone versus placebo, Outcome 2 Participants experiencing adverse effects.
Participants withdrawn due to adverse effects
We found no significant difference between buspirone and placebo in the proportion of participants withdrawn from treatment due to adverse effects (RR 0.63, 95% CI 0.15 to 2.60; 225 participants; 2 studies; I2 = 0%; Analysis 6.3).
6.3. Analysis.

Comparison 6 Buspirone versus placebo, Outcome 3 Participants withdrawn due to adverse effects.
Completion of scheduled treatment
We found no significant difference between buspirone and placebo in the proportion of participants completing the scheduled period of treatment (RR 0.96, 95% CI 0.74 to 1.23; 225 participants; 2 studies; I2 = 0%; Analysis 6.4).
6.4. Analysis.

Comparison 6 Buspirone versus placebo, Outcome 4 Completion of scheduled treatment.
7. Noradrenaline reuptake inhibitor (atomoxetine)
One study compared atomoxetine to placebo in adults with ADHD (McRae‐Clark 2010).
Participants abstinent at end of treatment
The study did not report data on abstinence, but 13/19 participants receiving atomoxetine and 9/19 participants receiving placebo had no days with heavy cannabis use during treatment. The atomoxetine group had mean 60.1% (SD 31.5%) days with cannabis use compared to 68.1% (SD 31.3%) for the placebo group (McRae‐Clark 2010). The authors concluded that atomoxetine may have improved some ADHD symptoms but did not reduce cannabis use.
Withdrawal symptoms and cravings
McRae‐Clark 2010 reported no significant difference between atomoxetine and placebo in terms of change in marijuana craving score.
Participants experiencing adverse effects
We found no significant difference between atomoxetine and placebo in the proportion of participants experiencing adverse effects (RR 1.18, 95% CI 0.95 to 1.46; 38 participants; 1 study; Analysis 7.1). McRae‐Clark 2010 reported that all adverse effects were mild to moderate in severity.
7.1. Analysis.

Comparison 7 Atomoxetine versus placebo, Outcome 1 Participants experiencing adverse effects.
Participants withdrawn due to adverse effects
We found no significant difference between atomoxetine and placebo in the proportion of participants withdrawn from treatment due to adverse effects (RR 3.00, 95% CI 0.13 to 69.31; 38 participants; 1 study; Analysis 7.2), but the CI was wide due to the small number of events.
7.2. Analysis.

Comparison 7 Atomoxetine versus placebo, Outcome 2 Participants withdrawn due to adverse effects.
Completion of scheduled treatment
We found no significant difference between atomoxetine and placebo in the proportion of participants who completed the scheduled period of treatment (RR 1.29, 95% CI 0.60 to 2.74; 38 participants; 1 study; Analysis 7.3).
7.3. Analysis.
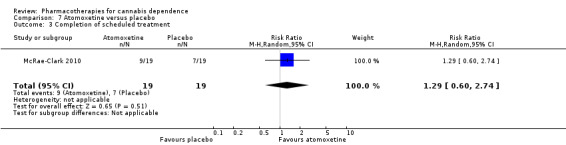
Comparison 7 Atomoxetine versus placebo, Outcome 3 Completion of scheduled treatment.
8. Glutamatergic modulator (N‐acetylcysteine)
Two studies examined N‐acetylcysteine compared to placebo, one in young people (Gray 2012), and one in adults (Gray 2017) (Table 6).
Participants abstinent at end of treatment
We found no significant difference between N‐acetylcysteine and placebo in the proportion of participants abstinent at the end of treatment (RR 0.89, 95% CI 0.59 to 1.35; 302 participants; 1 study; Analysis 8.1). On the broader outcome of cannabis use, Gray 2012 found a reduced likelihood of cannabis‐positive urine drug tests in young people treated with N‐acetylcysteine, but the subsequent, larger study with adults did not replicate this finding (Gray 2017).
8.1. Analysis.

Comparison 8 N‐acetylcysteine versus placebo, Outcome 1 Participants abstinent at end of treatment.
Withdrawal symptoms and cravings
The studies reported no data on the intensity of withdrawal symptoms.
Participants experiencing adverse effects
We found no significant difference between N‐acetylcysteine and placebo in the proportion of participants experiencing adverse effects (RR 0.94, 95% CI 0.71 to 1.23; 418 participants; 2 studies; I2 = 0%; Analysis 8.2). Gray 2017 reported that adverse effects were generally infrequent, with no significant group differences. Of seven serious adverse events reported, six occurred in the placebo group, and none were deemed to be related to medication.
8.2. Analysis.

Comparison 8 N‐acetylcysteine versus placebo, Outcome 2 Participants experiencing adverse effects.
Participants withdrawn due to adverse effects
We found no significant difference between N‐acetylcysteine and placebo in the proportion of participants withdrawn due to adverse effects (RR 3.00, 95% CI 0.12 to 72.15; 116 participants; 1 study; Analysis 8.3) but the small number of events resulted in a very wide CI.
8.3. Analysis.
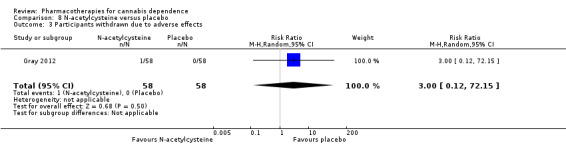
Comparison 8 N‐acetylcysteine versus placebo, Outcome 3 Participants withdrawn due to adverse effects.
Completion of scheduled treatment
We found no significant difference between N‐acetylcysteine and placebo in the proportion of participants completing the scheduled period of treatment (RR 1.06, 95% CI 0.93 to 1.21; 418 participants; 2 studies; I2 = 0%; Analysis 8.4).
8.4. Analysis.

Comparison 8 N‐acetylcysteine versus placebo, Outcome 4 Completion of scheduled treatment.
9. Neuropeptide (oxytocin)
One study of 16 people compared oxytocin and placebo in conjunction with MET (Sherman 2017).
Participants abstinent at end of treatment
All participants had urine drug screens that were positive for THC at each visit, with no participants in either group confirmed to be abstinent at end of treatment. On the outcome of cannabis use in general, although an overall effect of oxytocin on mean daily cannabis use was not detected, there was a decrease in cannabis use from the first to last session of MET in the oxytocin group that was not found in the placebo group.
Withdrawal symptoms and cravings
The study reported no data on withdrawal symptoms and cravings.
Participants experiencing adverse effects
We found no significant difference between oxytocin and placebo in participants experiencing adverse effects (RR 0.50, 95% CI 0.06 to 4.47; 16 participants; 1 study; Analysis 9.2).
9.2. Analysis.

Comparison 9 Oxytocin versus placebo, Outcome 2 Participants experiencing adverse effects.
Participants withdrawn due to adverse effects
The study reported no data on participants withdrawn due to adverse effects.
Completion of scheduled treatment
We found no significant difference between oxytocin and placebo in the proportion of participants completing the scheduled period of treatment (RR 0.86, 95% CI 0.53 to 1.38; 16 participants; 1 study; Analysis 9.3).
9.3. Analysis.

Comparison 9 Oxytocin versus placebo, Outcome 3 Completion of scheduled treatment.
Discussion
Summary of main results
The medications considered by the studies that met the inclusion criteria for this review were diverse in nature. This, and the variability in the nature of data reported, limited the extent of meta‐analysis that was possible, and the strength of conclusions.
The quality of evidence available for assessment of effectiveness against the defined outcomes was generally very low to moderate (see Table 1; Table 2; Table 3; Table 4; Table 5; Table 6). We did not include 'Summary of findings' tables for bupropion, atomoxetine or oxytocin as data on these medications were sparse, the studies were largely preliminary in nature and, as such, the findings were of limited clinical relevance.
This section summarises the main results by medication type. Additional information is considered where appropriate from studies that were excluded from this review so as to form a more complete view of the potential value of medications for the treatment of cannabis dependence.
1. Preparations containing Δ9‐tetrahydrocannabinol
Preparations containing THC appeared to effectively suppress cannabis withdrawal symptoms and craving, but THC alone or in combination with lofexidine was not associated with reductions in cannabis use or increased rates of completion of treatment in the time frames of the studies included in this review (see Table 1). Data from one ongoing study of longer‐term treatment with nabiximols for cannabis dependence may change these findings (Bhardwaj 2018). The use of medications such as lofexidine (Haney 2008) and zolpidem (Herrmann 2016) as adjuncts have been proposed to enhance the effectiveness of THC preparations in attenuating cannabis withdrawal and improving sleep, though the first randomised controlled trial of a dronabinol plus lofexidine combination appeared not to improve treatment outcomes (Levin 2016). It remains possible that effectiveness may vary with the type of THC preparation.
2. Selective serotonin reuptake inhibitor antidepressants
SSRI antidepressants did not appear to affect cannabis use, withdrawal symptoms or treatment completion (see Table 2).
3. Mixed action antidepressants
The included studies found that the mixed action antidepressants nefazodone, mirtazapine and venlafaxine were of little value in the treatment of cannabis dependence (see Table 3). As with SSRI antidepressants, the mixed action antidepressants may be of value in the treatment of depressive symptoms with comorbid substance use disorder but appeared to have little value specifically for the treatment of cannabis dependence.
4. Anticonvulsants and mood stabilisers
The quality of evidence for this group of medication was low to very low (see Table 4). Gabapentin but not divalproex had some capacity to ameliorate cannabis withdrawal symptoms and promote reduction in cannabis use compared to placebo. Lithium affected only some cannabis withdrawal symptoms and had no effect on retention in treatment. Topiramate may have had some promise in reducing cannabis use, though its poor tolerability may limit its clinical usefulness. Overall, the negative effect on treatment retention with anticonvulsants and mood stabilisers may limit their clinical utility.
5. Atypical antidepressant (bupropion)
The included studies indicated that bupropion may have had some effect on cannabis withdrawal symptoms, but the data were inconclusive on other outcomes. A 'Summary of findings' table was not prepared because of the data limitations.
6. Anxiolytic (buspirone)
Buspirone had little value in the treatment of cannabis dependence (see Table 5). However, it may be useful for the treatment of anxiety in cannabis users.
7. Noradrenaline reuptake inhibitor (atomoxetine)
Atomoxetine is used for the treatment of ADHD and the included study investigated the effectiveness of atomoxetine in a population of cannabis users with ADHD. This study found atomoxetine to have little value in the treatment of cannabis dependence, but it may still be useful for the treatment of ADHD in cannabis users. A 'Summary of findings' table was not prepared because of the data limitations.
8. Glutamatergic modulator (N‐acetylcysteine)
This dietary supplement may have had some effectiveness in the treatment of cannabis dependence but available data were not conclusive (see Table 6).
9. Neuropeptide (oxytocin)
Results from one small pilot study suggested oxytocin may be of interest to study in further trials, though the results of the pilot study were not conclusive. A 'Summary of findings' table was not prepared because of the data limitations.
Overall completeness and applicability of evidence
Most studies conducted were small, the majority of participants were men, and most conducted in the US, Canada and Australia. All of these factors may potentially limit the generalisability of the data. Studies have considered both adult and adolescent populations, although there were differing results in the case of N‐acetylcysteine. As many studies were smaller pilot studies with short time frames, larger studies with longer‐term follow‐up are still required. Greater harmonisation in measures used in different studies to capture changes in cannabis use (in addition to abstinence at end of treatment) may assist in understanding the potential role for different medications, as abstinence is just one aspect of substance use, and increasingly other measures of reduced substance use are being considered for medication trials in substance use disorders (McCann 2015).
Quality of the evidence
The studies included in this review were mostly small, the quality of evidence was assessed as generally very low to moderate (see Table 1; Table 2; Table 3; Table 4; Table 5; Table 6) and the capacity for meta‐analysis was limited. As a result, the conclusions of this review should be considered tentative at best. Nonetheless, the review provides an overview of the current status of evidence and points to future directions for research on the development of pharmacotherapies for cannabis dependence.
Potential biases in the review process
Pharmacological approaches to the management of cannabis withdrawal are still in an experimental phase with a diverse array of medications being explored, many of which have shown limited effectiveness. Studies with negative or neutral findings are less likely to be published and we identified two studies with only limited information available (Frewen 2007; Johnston 2014). It is possible that there are further such studies that we did not locate.
Agreements and disagreements with other studies or reviews
We have identified eight reviews of treatments for cannabis dependence (Benyamina 2008; Copeland 2014; Danovitch 2012; Gorelick 2016; Nordstrom 2007; Sherman 2016; Vandrey 2009; Walther 2016). All are in agreement that several pharmacotherapies, in particular preparations of THC and gabapentin show promise for the treatment of cannabis dependence; but there is currently insufficient evidence to support their broad therapeutic use. These reviews also identify psychotherapies, such as MET and cognitive‐behavioural therapy, as having demonstrated efficacy in decreasing cannabis use and cannabis‐related consequences. Hence, these reviews support the conclusion that psychological approaches should continue to be the mainstay of treatment for cannabis use disorders, with pharmacotherapies continuing to be experimental.
Authors' conclusions
Implications for practice.
Studies undertaken to date on pharmacotherapies for cannabis dependence are insufficient to guide clinical practice. There is incomplete evidence for all of the pharmacotherapies investigated in this review. Quantitative analysis was not possible for most of the outcomes and was limited for most of the pharmacotherapies investigated. The quality of evidence for many of the outcomes was low or very low due to small sample size, inconsistency and risk of attrition bias. The quantitative analyses that were possible, in combination with the general findings reported by the studies reviewed, indicate that selective serotonin reuptake inhibitor (SSRI) antidepressants, mixed action antidepressants, atypical antidepressants (bupropion), anxiolytics (buspirone) and noradrenaline reuptake inhibitors (atomoxetine) are probably of little value in the treatment of cannabis dependence. THC may be of potential value based on qualitative data from individual studies, though meta‐analyses found no significant effect on treatment outcomes, and THC preparations should be considered to still be experimental. The evidence base for THC, the anticonvulsant gabapentin, the glutamatergic modulator N‐acetylcysteine and oxytocin is weak and at this time it is not possible to quantitatively estimate their effectiveness.
At this point in time, psychological approaches such as MET and cognitive‐behavioural therapy remain the mainstay of treatment for cannabis use disorders (Gates 2016).
Implications for research.
There is sufficient evidence to indicate that preparations containing THC and gabapentin may have therapeutic potential, and further research is warranted. N‐acetylcysteine and oxytocin are also worth further consideration to provide alternative medication approaches, but SSRI and mixed action antidepressants, the atypical antidepressant bupropion, the anxiolytic buspirone, anticonvulsants and mood stabilisers, and the selective noradrenaline reuptake inhibitor atomoxetine appear to be of limited value in the treatment of cannabis dependence other than for the management of relevant concomitant conditions. Given that psychological approaches are currently the mainstay of treatment for cannabis use disorders, research into interactions of pharmacotherapies and psychological approaches would also be of value. Oxytocin in particular may enhance psychological therapies through the promotion of prosocial behaviours (Sherman 2017).
What's new
| Date | Event | Description |
|---|---|---|
| 2 March 2020 | Amended | Declarations of interest revised |
History
Protocol first published: Issue 1, 2011 Review first published: Issue 12, 2014
| Date | Event | Description |
|---|---|---|
| 15 May 2018 | New citation required but conclusions have not changed | The updated search found seven additional studies. |
| 9 April 2018 | New search has been performed | New search, new studies, change in authorship |
Acknowledgements
Kushani Marshall and Robert Ali contributed to an earlier version of this review.
Appendices
Appendix 1. Search strategy for CENTRAL via the Cochrane Library online
(cannabis or marijuana or marihuana):ti,ab,kw in Trials
MeSH descriptor: [Marijuana Abuse] explode all trees
(withdrawal or detoxification or cessation or abstinence):ti,ab,kw in Trials
MeSH descriptor: [Substance Withdrawal Syndrome] explode all trees
MeSH descriptor: [Drug Therapy] explode all trees
#1 or #2
#3 or #4 or #5
#6 and #7 in Trials
Appendix 2. Search strategy for MEDLINE via Ovid Online
Marijuana Smoking/
Marijuana Abuse/
(cannabis or mari#uana).mp.
(abuse or depend$).mp.
Substance‐Related Disorders/
1 or 2 or 3
4 or 5
6 and 7
Substance Withdrawal Syndrome/
Drug Therapy/
(detoxif$ or cessation or abstinence).mp.
9 or 10 or 11
8 and 12
randomized controlled trial.pt
controlled clinical trial.pt
random$.ab
(double adj2 blind).ti,ab.
placebo.ti,ab
14 or 15 or 16 or 17 or 18
13 and 19
limit 20 to humans
Appendix 3. Search strategy for Embase (Online)
‘cannabis addiction’/exp or ‘cannabis use’/exp
cannabis:ti,ab or marijuana:ti,ab or marihuana:ti,ab
abuse:ab,ti or dependence:ab,ti
‘drug dependence’:de
#1 or #2
#3 or #4
#5 and #6
‘withdrawal syndrome’/exp
detox*:ab,ti
cessation:ti,ab or abstinence:ti,ab
‘drug therapy’/de
#8 or #9 or #10 or #11
#7 and #12
‘randomized controlled trial’/exp
‘controlled clinical trial’/exp
random*:ti,ab
(double NEXT/2 blind):ti,ab
placebo:ti,ab
#14 or #15 or #16 or #17 or #18
#13 and #19
#13 and #19 and [humans]/lim
Appendix 4. Search strategy for PsycINFO via Ovid Online
marijuana usage/
(cannabis or mari#uana) .mp.
(abuse or depend$).mp.
exp Drug Dependency/
Drug Abuse/
1 or 2
3 or 4 or 5
6 and 7
Drug Withdrawal/
Detoxification/
Drug Therapy/
(detoxifi$ or cessation or abstinence).mp.
9 or 10 or 11 or 12
8 and 13
exp Clinical Trials/
random$.ti,ab
(double adj2 blind).ti,ab
placebo.ti,ab
15 or 16 or 17 or 18
14 and 19
limit 20 to human
Appendix 5. Search strategy for Web of Science
ts=(cannabis or marijuana or marihuana)
ts=cannabis addiction
ts=cannabis abuse
ts=(abuse or addiction or dependence)
#4 and #1
#5 or #3 or #2
ts=drug withdrawal
ts=substance withdrawal syndrome
ts=(detoxification or cessation or abstinence)
ts=drug therapy
#10 or #9 or #8 or #7
#11 and #6
ts=randomized controlled trial
ts=controlled clinical trial
#14 or #13
#15 and #12
Appendix 6. Criteria for risk of bias assessment
| Item | Judgement | Description |
| 1. Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation. |
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention. | |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk. | |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because 1 of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| High risk | Investigators enrolling participants could possibly foresee assignments because 1 of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear risk | Insufficient information to permit judgement of low or high risk. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement. | |
| 3. Blinding of participants and personnel (performance bias) | Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel, and unlikely that the blinding could have been broken. |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding. Blinding of participants and key study personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 4. Blinding of outcome assessment (detection bias) | Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding. Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk | No blinding or incomplete blinding of outcome assessment, and the measurement is likely to be influenced by lack of blinding. Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 5. Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or drop out |
Low risk | No missing outcome data. Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias). Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size. Missing data have been imputed using appropriate methods. All randomised patients are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and cointerventions (intention to treat). |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size. 'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of drop out not reported for each group). | |
| 6 Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| High risk | Not all of the study's prespecified primary outcomes have been reported. 1 or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified. 1 or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect). 1 or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 7. Other bias | Low risk | Potential confounding factors identified but evenly distributed between groups. Study ceased early but with no indications of selection bias. Interventions delivered consistently. |
| High risk | Potential confounding factors unequally distributed between groups. Study ceased early with risk of selection bias. Differences in aspects of delivery of interventions. Mandatory treatment. |
|
| Unclear risk | Confounding possible but not able to be assessed. Study ceased early and unable to determine possible bias. Unclear if delivery of interventions was equivalent. |
Data and analyses
Comparison 1. Δ9‐Tetrahydrocannabinol (THC) preparation versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants abstinent at end of treatment | 3 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.64, 1.52] |
| 2 Participants experiencing adverse effects | 3 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.89, 1.17] |
| 3 Participants withdrawn due to adverse effects | 3 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [0.51, 14.59] |
| 4 Completion of scheduled treatment | 4 | 369 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.88, 1.37] |
Comparison 2. Selective serotonin‐reuptake inhibitor (SSRI) antidepressant versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants abstinent at end of treatment | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.61, 4.89] |
| 2 Participants experiencing adverse effects | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 1.02] |
| 3 Participants withdrawn due to adverse effects | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.16, 18.04] |
| 4 Completion of scheduled treatment | 3 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.49, 1.27] |
Comparison 3. Mixed action antidepressant versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants abstinent at end of treatment | 2 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.12, 5.41] |
| 2 Participants experiencing adverse effects | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.55, 1.55] |
| 3 Participants withdrawn due to adverse effects | 2 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.11, 18.90] |
| 4 Completion of scheduled treatment | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.21] |
Comparison 4. Anticonvulsants and mood stabilisers versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants abstinent at end of treatment | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.64, 2.04] |
| 2 Participants withdrawn due to adverse effects | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 3.67 [0.41, 32.69] |
| 3 Completion of scheduled treatment | 3 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.47, 0.92] |
Comparison 5. Bupropion versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Completion of scheduled treatment | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.67, 1.67] |
Comparison 6. Buspirone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants abstinent at end of treatment | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [0.62, 6.33] |
| 2 Participants experiencing adverse effects | 2 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.00, 1.29] |
| 3 Participants withdrawn due to adverse effects | 2 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.15, 2.60] |
| 4 Completion of scheduled treatment | 2 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.74, 1.23] |
Comparison 7. Atomoxetine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants experiencing adverse effects | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.95, 1.46] |
| 2 Participants withdrawn due to adverse effects | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.31] |
| 3 Completion of scheduled treatment | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.60, 2.74] |
Comparison 8. N‐acetylcysteine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants abstinent at end of treatment | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.35] |
| 2 Participants experiencing adverse effects | 2 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.23] |
| 3 Participants withdrawn due to adverse effects | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.15] |
| 4 Completion of scheduled treatment | 2 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.21] |
Comparison 9. Oxytocin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants abstinent at end of treatment | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Participants experiencing adverse effects | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.06, 4.47] |
| 3 Completion of scheduled treatment | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.53, 1.38] |
9.1. Analysis.

Comparison 9 Oxytocin versus placebo, Outcome 1 Participants abstinent at end of treatment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allsop 2014.
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | Setting: inpatient (2 hospitals), New South Wales, Australia. Total duration of inpatient admission: 9 days 51 adults seeking treatment for cannabis use, dependent by DSM‐IV‐TR Group sizes: group 1, 27; group 2, 24 Groups well matched apart from differences in baseline withdrawal score and disability scale scores. Mean age 35 years 76% male 53% unemployed; 25% married or in de facto relationship On average using 23 g cannabis per day, mean duration of use 20 years; 71% also nicotine dependent Dependence on alcohol or other drugs except nicotine or caffeine and unstable medical or psychiatric conditions were exclusion criteria. |
|
| Interventions | Group 1: nabiximols (cannabis extract, Sativex®), maximum dose THC 86.4 mg, cannabidiol 80 mg; medication: 6 days, washout: 3 days Group 2: placebo Cognitive‐behavioural therapy tailored to inpatient cannabis withdrawal as adjunct intervention Follow‐up interview after 28 days. Participants compensated AUD 40 for follow‐up interviews |
|
| Outcomes | Overall withdrawal score, irritability, craving and depression reported as graphs and results of statistical analyses with imputation for missing data Number retained in treatment at all time points, median days inpatient stay Withdrawal and craving assessed with Cannabis Withdrawal Scale (19 items on 11‐point Likert scale for the previous 24 hours) Drug use by modified TLFB Change in amount of cannabis use from baseline to 28‐day follow‐up |
|
| Notes | Funding: research grant (Australian National Health and Medical Research Council), with study drugs provided by manufacturer (GW Pharmaceuticals, UK). Declaration of conflict of interest not published. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician generated a randomization list for each site using random block sizes in Stata, version 11.1 …" |
| Allocation concealment (selection bias) | Low risk | Comment: method of allocation concealment not reported, but generation of lists by independent statistician and use of matching placebos would be expected to provide good control of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, investigators, and outcome assessors were blind to treatment allocation until all research procedures were complete. Blinding was maintained by the use of a matched placebo … The success of patient blinding was formally assessed before hospital discharge." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients, investigators, and outcome assessors were blind to treatment allocation until all research procedures were complete. Blinding was maintained by the use of a matched placebo … The success of patient blinding was formally assessed before hospital discharge." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Statistical methods used to impute missing data and assess data as missing at random. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Carpenter 2009.
| Methods | Double‐blind, randomised, placebo‐controlled trial. All participants received placebo for 1 week before random allocation. | |
| Participants | Setting: outpatient clinic, New York, USA. Scheduled duration 13 weeks. Participants: 106, seeking treatment for problems related to cannabis use, cannabis dependent by DSM‐IV and smoking at least 5 cannabis joints per week. Group sizes: group 1, 36; group 2, 40; group 3, 30 Groups similar on demographics and cannabis use at baseline, except more males in group 3 (placebo) Mean age 32 years 76% male (group 1 ‐ nefazodone 78%, group 2 ‐ bupropion 63%, group 3 ‐ placebo 93%) 34% Caucasian, 28% Hispanic, 27% African‐American; 91% employed Mean age 18 years at first regular cannabis use; at baseline used on average 28 days in last 30 days Exclusion criteria: "significant and unstable psychiatric condition," "chronic organic mental disorder" and "DSM‐IV dependence criteria for another substance" |
|
| Interventions | Group 1: oral nefazodone, 150 mg/day to maximum 600 mg/day Group 2: oral bupropion SR 150 mg to maximum of 300 mg/day Group 3: oral placebo for 10 weeks Riboflavin added to medication to monitor adherence All participants received placebo for 2 weeks after medication phase Participants attended treatment clinic twice weekly (paid USD 5 for transport costs at each visit); medications dispensed weekly Weekly individual psychosocial intervention based on coping skills as adjunct therapy |
|
| Outcomes | Number completing 13 weeks of study, number abstinent at week 10, dependence severity at baseline and week 10 (and improvement), withdrawal symptoms, sleep, Hamilton Anxiety Rating Scale at baseline and week 10 Total adverse effects during study. Cannabis use assessed by self‐report and urine toxicology of observed samples provided at each clinic visit, with a cut‐off of 100 ng/mL (rather than usual 50 ng/mL) to minimise false positives. Severity of dependence symptoms assessed by Clinical Global Impression (scores from 1 = no pathology to 7 = extreme pathology) Sleep quality self‐reported once a week using the St Mary's Hospital Sleep Questionnaire Irritability self‐reported every other week with the Snaith Irritability Scale (4 items each rated 0 to 3) Hamilton Anxiety Rating Scale (14 items each rated 0 to 4) administered by clinician every other week If either urine or self‐report data were missing for a given week, it was considered a non‐abstinent week. |
|
| Notes | Funding from research grant (NIDA) 1 author declared past associations with pharmaceutical companies. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "A research pharmacist who was independent of the research team, conducted the randomisation." "All capsules were prepared at the research pharmacy and looked identical for all three treatment conditions." Comment: methods for allocation concealment not reported but the involvement of an independent pharmacist would be expected to protect against bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All capsules were prepared at the research pharmacy and looked identical for all three treatment conditions." Comment: study stated to have been conducted double‐blind. The provision of active and placebo medications in identical capsules and the use of riboflavin to confirm medication adherence would help to reduce the risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study conducted double‐blind, as indicated above, and the use of urine screening to support self‐report data would be expected to be associated with a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was substantial dropout from all 3 groups, with only 52/106 (49%) participants randomised completing the 10‐week medication phase and 43% completing the full 13‐week trial. Quote: "Survival analysis revealed no statistically significant group differences on treatment retention … there were no differences between those participants who completed the trial and those who did not on demographic indices or baseline substance use measures." Comment: missing data on cannabis use regarded as indicative of "non‐abstinence;" statistical methods used to allow for missing data. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Cornelius 2010.
| Methods | Double‐blind, randomised, placebo‐controlled trial. 1 physician remained non‐blinded to handle any potential problems. | |
| Participants | Setting: outpatient clinic, Pittsburgh, USA. Scheduled duration 12 weeks Participants: 70 adolescents and young adults (aged 14–25 years at baseline) with comorbid major depression and cannabis use disorder by DSM‐IV criteria Group sizes: group 1, 34; group 2, 36 Groups similar on demographics and clinical characteristics Mean age 21.1 years 61% male 56% Caucasian, 37% African‐American 94% cannabis dependent, using on average of 76% of days in prior month; 28.6% also alcohol dependent Exclusion criteria: bipolar disorder; schizoaffective disorder; schizophrenia; substance abuse or dependence other than alcohol, nicotine or cannabis; history of IV drug use |
|
| Interventions | Group 1: fluoxetine, 10 mg/day increasing to 20 mg/day after 2 weeks Group 2: placebo 9 sessions (delivered at each clinic visit) of manual‐based cognitive‐behavioural therapy for depression and cannabis use and motivation enhancement therapy for cannabis use as adjunct intervention |
|
| Outcomes | Severity of abuse or dependence (criteria count), days cannabis used in past week, number completing treatment Depressive symptoms rated by observer with Hamilton Rating Scale for Depression and by participants with Beck Depression Inventory Cannabis use behaviours assessed by TLFB method |
|
| Notes | Funding from research grants (NIDA, NIAAA, Veterans Affairs). All authors declared no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patient randomization was conducted by urn randomization stratified by gender …" |
| Allocation concealment (selection bias) | Low risk | Quote: "Active medication and matching placebo were prepared by the research pharmacy …" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was conducted in a double‐blind fashion, though [one] physician … remained non‐blinded in order to handle any problems which may have arisen." Comment: considered likely that participants and treating personnel were blind to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study conducted double‐blind, as indicated above. It is likely that blinding was maintained for outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors noted: "low percentage of missing data." Missing data handled by last observation carried forward. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Frewen 2007.
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | Setting: outpatient, Sydney, Australia. Scheduled duration of medication 4 weeks, with follow‐up after 28 days Participants: 81 adults seeking treatment for cannabis use, used cannabis in 72 hours prior to assessment interview, dependent by DSM‐IV‐TR in previous 3 months Group sizes: not reported Similarity of groups not assessed. Characteristics of participants stated as similar to characteristics of general population seeking treatment for cannabis use Mean age 31.4 years 81% male. 78% Australian‐born; 64% employed; 92% living in stable accommodation; 63% not in a relationship Mean 12 years of cannabis use; 97% daily smokers; 63% daily tobacco smokers Exclusion criteria: psychiatric or medical instability |
|
| Interventions | Group 1: oral mirtazapine 30 mg/day Group 2: placebo Weekly individual cognitive‐behavioural therapy as adjunct intervention Reimbursement of AUD 30 for expenses at the day 56 interview |
|
| Outcomes | Withdrawal symptoms in first 7 days related to subsequent cannabis use for groups combined (effect of medication not considered in this analysis) Measures of sleep quality and disruption Withdrawal symptoms measured daily for 14 days with the Marijuana Withdrawal Scale (32 items, rated from 0 = "none" to 3 = "severe") Cannabis use assessed with the drug scale of the Opiate Treatment Index Sleep problems recorded with the Karolinska Sleep Questionnaire for 7 days, and the Pittsburgh Sleep Quality Index (24 items, global score 0 to 21, with higher scores indicative of poorer sleep) at baseline and days 28 and 56 |
|
| Notes | Funding: not reported. No declaration of conflict of interest made | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomized … using permuted block randomisation." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was independently assigned by pharmacy staff offsite." Comment: as independent pharmacy staff controlled the randomisation process, it is likely to have prevented investigators and participants from foreseeing allocation assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "… the placebo was identically matched in colour, shape, size and taste to the medication." "All treating physicians, psychologists and research staff were blind to the randomisation until all participants had completed the final research interview." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study conducted double‐blind, as indicated above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information available to form a view |
| Selective reporting (reporting bias) | Unclear risk | Limited study results available |
| Other bias | Low risk | None apparent |
Gray 2012.
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | Setting: outpatient, university clinic, South Carolina, USA. Scheduled duration 8 weeks Participants: 116 adolescents (aged 13–21 years), cannabis‐dependent and using cannabis regularly Group sizes: group 1, 58; group 2, 58 Groups similar on demographics and baseline clinical characteristics Mean age 18.9 years 73% male 83.5% white; 73.9% enrolled in school Mean 22.6 days with cannabis use in 30 days prior to baseline; 57% smoked tobacco; 13.8% had a psychiatric comorbidity Exclusion criteria: dependence on other substances (except nicotine) and unstable psychiatric or medical illness |
|
| Interventions | Group 1: N‐acetylcysteine 1200 mg twice daily Group 2: placebo Twice‐weekly contingency management and weekly brief (10 minute) individual cessation counselling as adjunct therapies. Initial contingent reward USD 5 (cash) for both adherence and abstinence with amount increased by USD 2 for each successive visit; reward reset to baseline if conditions not met Seen in clinic weekly. Follow‐up 4 weeks after treatment conclusion |
|
| Outcomes | Likelihood of negative urine test reported as odds ratio and 95% confidence interval Occurrence of adverse events (number of events and number of participants) Proportion of medication doses consumed, discontinuation of medication due to adverse effects Number completing treatment, median days in treatment, contingency rewards earned Urine cannabinoid testing at all visits. Self‐reported cannabis use by TLFB Medication diaries and weekly tablet counts used to determine adherence Participants lost to follow‐up or absent for visits were coded as having a positive urine test |
|
| Notes | Funding: research grants (NIDA, National Center for Research Resources) Authors declared "no competing interests." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in 1:1 parallel group allocation stratified by age and baseline cannabis use. No significant group differences at baseline suggesting appropriate sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "university investigational drug service oversaw randomization, encased medication in identical‐appearing capsules, and dispensed them in weekly blister packs …" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "university investigational drug service … encased medication in identical‐appearing capsules." "Participants, investigators and clinical staff remained blind to treatment assignment throughout the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study conducted double‐blind as indicated above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data and non‐attendance regarded as indicating non‐abstinence. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Gray 2017.
| Methods | Multisite, double‐blind randomised, placebo‐controlled trial | |
| Participants | Setting: outpatient, 6 sites within the National Drug Abuse Treatment Clinical Trials Network, USA. Scheduled duration 12 weeks Participants: 302 adults aged 18–50 years, seeking treatment, cannabis dependent by DSM‐IV‐TR and positive urine test during the initial screening visit Group sizes: group 1, 153; group 2, 149 Groups similar on demographics and baseline clinical characteristics, except employment (group 1, 42.5%; group 2, 60.4% working) and education (group 1, 28.5%; group 2, 14.8% graduated from high school) Mean age 30.3 years 71.5% male 58.3% white Mean cannabis use 26.0/30 days at baseline. Exclusion criteria: unstable psychiatric conditions, dependence on substances other than cannabis or tobacco, recent synthetic cannabinoid use |
|
| Interventions | Group 1: N‐acetylcysteine, 1200 mg twice daily Group 2: placebo All participants received contingency management and medical management |
|
| Outcomes | Odds of negative urine cannabinoid tests during treatment Cannabis urine screens by central laboratory during weekly clinic visits and at post‐treatment follow‐up Adverse effects at each study visit Adherence defined as taking ≥ 80% of prescribed medication each study week, confirmed by riboflavin level |
|
| Notes | Funding: research grants (US National Institutes of Health) 5/23 authors declared research support, or unrestricted grants from pharmaceutical companies, or the provision of advisory and consultancy services to pharmaceutical companies, none of which were involved in this trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization, conducted centrally through the CTN Data and Statistics Center, was on a 1:1 ratio, with stratification by study site and self‐reported binary tobacco smoking status." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization, conducted centrally through the CTN Data and Statistics Center;" "United States Pharmacopeia grade NAC [N‐acetylcysteine] powder was encapsulated in 600 mg quantities (two 600 mg capsules per twice‐daily dose). Matched placebo capsules were also prepared." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Among participants assigned to NAC, 46.5% guessed they were receiving NAC and 53.5% guessed they were receiving placebo, and the medical clinician guessed that 52% were receiving NAC and 48% were receiving placebo. Among those assigned to placebo, 53.7% guessed they were receiving NAC and 46.3% guessed they were receiving placebo, and the medical clinician guessed that 57.3% were receiving NAC and 42.7% were receiving placebo. These differences were not statistically significant, and the participant and medical clinician agreed on guesses more often than by chance (p < 0.0001)." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As indicated above, participant may have been able to accurately guess their group allocation, but the outcomes reported were objective in nature and these are less likely to be affected if the blind is broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Retention rates and data availability similar in the 2 groups Missing urine tests imputed as positive |
| Selective reporting (reporting bias) | Low risk | Published protocol, reported outcomes consistent with protocol |
| Other bias | Low risk | Riboflavin added to all capsules as a biomarker for medication adherence. Criteria defining adherence specified in advance. Analyses comparing outcomes for those meeting or not meeting these criteria were undertaken. |
Johnston 2014.
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | Setting: inpatient withdrawal unit; Sydney, Australia. Scheduled duration of inpatient treatment: 8 days. Follow‐up at 14, 30 and 90‐days after discharge Participants: 38 adults, cannabis dependent by DSM‐IV‐TR, seeking treatment Group sizes: group 1, 16; group 2, 22 Higher proportion of group 2 (placebo) had completed education to year 10 or higher, were married or in de facto relationship and had used amphetamines in past month, but differences not significant (possibly due to small sample size) Mean age 40.5 years 65.8% male 26.3% married or in de facto relationship Mean age at first cannabis use 14.8 years Exclusion criteria: current alcohol dependence; frequent use of drugs other than cannabis, caffeine or tobacco; significant psychiatric conditions |
|
| Interventions | Group 1: lithium carbonate, 500 mg twice daily Group 2: placebo Standard withdrawal care and symptomatic medications available to both groups |
|
| Outcomes | Mean daily withdrawal score as graph, and analysis of change in score Completion of withdrawal treatment, adverse effects Frequency of cannabis use, severity of dependence, continuous and point‐prevalence abstinence, score on Cannabis Problems Questionnaire Withdrawal severity assessed by Cannabis Withdrawal Scale (19‐items, scored 0–10, completed by participants; score averaged across items to give daily score in range 0–10) |
|
| Notes | Included in previous review as Johnson 2012, based on conference abstract only. Full report now available Funding: research grant (Australian National Health & Medical Research Council) Authors declared no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Only the independent trial pharmacist had access to the randomization schedule," but method of sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the independent trial pharmacist had access to the randomization schedule" suggests central process such that investigators could not foresee assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All participants, researchers and clinicians involved in the direct care of patients were blind to treatment condition." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All participants, researchers and clinicians involved in the direct care of patients were blind to treatment condition." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "To satisfy intention‐to‐treat, missing data were imputed using multiple imputation …, generating five different plausible datasets allowing for the uncertainty inherent in the predictions …" "… retention according to protocol was relatively low (41–50 %) … the majority of patients left the study at medication cessation (day 7), which was only 1 day short of discharge." Comment: retention is a primary outcome for this review, imputation methods for missing data in other outcomes acceptable. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Unclear risk | Quote: "two thirds of study participants were administered a benzodiazepine (nitrazepam) at some point during their inpatient stay for disturbed sleep. Given that sleep problems (insomnia) are one of the most prominent cannabis withdrawal symptoms, the administration of nitrazepam may well have significantly subdued the withdrawal experience, especially sleep problems." |
Levin 2004.
| Methods | Double‐blind, randomised, placebo‐controlled trial. 2‐week single‐blind placebo lead‐in phase prior to random allocation. Study included a cross‐over phase which was not included in this review due to substantial dropout (> 30%) in the first 2 weeks. | |
| Participants | Setting: outpatient with 2 clinic visits per week; New York, USA. Scheduled duration 8 weeks (plus subsequent cross‐over phase that was excluded from this review) Participants: 27 enrolled, 25 randomised; cannabis dependent by DSM‐IV, using daily Group sizes: group 1, 13; group 2, 12 Groups similar on demographics and clinical characteristics at baseline, except for somewhat higher (not statistically significant) use of cannabis by group 1 Mean age 32 years 92% male 56% Caucasian, 20% Hispanic, 24% African American Mean (± SD) joints smoked per week at baseline: group 1, 28.3 (SD 23.2); group 2, 19.4 (SD 16.4) Exclusion criteria: dependence on other substances, except caffeine and nicotine, and psychiatric disorder requiring medical intervention |
|
| Interventions | Group 1: oral divalproex sodium commenced at 500 mg/day, increasing to maximum of 2 g/day, depending on response Group 2: placebo Medication in 2 doses per day Weekly individual cognitive‐behavioural relapse prevention therapy as adjunct |
|
| Outcomes | Outcomes reported for 19 participants who completed 8 weeks of study: frequency and amount of cannabis use and craving score at baseline and weeks 7 and 8; number completing scheduled treatment; number with ≥ 2 weeks of assumed abstinence Urine samples collected and analysed at each visit Participants reported cannabis use and completed a visual analogue scale of intensity and desire for cannabis each week Clinician‐rated global impression assessment for cannabis use completed weekly "Strict abstinence" defined as ≥ 1 negative urine sample and no self‐reported cannabis use for that week. "Assumed abstinence" if patient reported no cannabis use and urine samples had THC‐COOH levels ≥ 50% below the previous week. |
|
| Notes | Funding: Research grants (NIDA) Declaration of conflict of interest not published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twenty‐seven participants were enrolled and 25 were randomized." Comment: method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: [participants] "… were randomly assigned to receive either divalproex or a matching placebo." Comment: method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Following randomization, patients received … either divalproex sodium or a placebo using a double‐blind design." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study conducted double‐blind. Use of urine screening to support determination of "abstinence" would be expected to help reduce bias in subjective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Rates of dropout were similar in the 2 groups, but there was no discussion of possible differences between those retained and those who dropped out of the study. Cannabis use outcomes were reported only for those who completed treatment. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | The cross‐over phase of the trial was excluded from analyses and this review due to high rates of dropout in the first 2 weeks |
Levin 2011.
| Methods | Randomised, double‐blind, placebo‐controlled, trial. 1‐week placebo lead‐in phase; those who used cannabis less than twice a week during this phase were not randomised. | |
| Participants | Setting: outpatient with clinic attendance twice weekly, New York, USA. Scheduled duration 12 weeks Participants: 156 adults seeking outpatient treatment for problems related to cannabis use, dependent by DSM‐IV‐TR, using cannabis ≥ 5 days/week in prior 28 days Group sizes: group 1, 79; group 2, 77 No significant group differences in demographic or clinical characteristics at baseline Mean age 38 years 82% male 60% employed full‐time, 13% part‐time; 31% married Exclusion criteria: significant psychiatric condition and dependence on other substances except nicotine |
|
| Interventions | Group 1: oral dronabinol, commenced at 10 mg/day, titrated to 20 mg twice a day or the maximum tolerated Group 2: placebo Medication maintained to end of week 8 then tapered over 2 weeks Weekly individual therapy based on coping skills plus MET as adjunct intervention. Participants earned vouchers with value increased by USD 1.50 for each consecutive visit, with value reset for non‐attendance, and USD 10 for returning their tablet bottle and remaining medication. Maximum possible earnings were USD 570. Cash payments of USD 5–20 were made at each visit for transport costs. |
|
| Outcomes | Number achieving 2 weeks' abstinence in weeks 7 and 8 and median maximum consecutive days abstinence Number retained in study to week 8 Mean number of therapy sessions attended Number experiencing any adverse effects, requiring dose reduction, serious adverse events and number withdrawn due to adverse events Withdrawal scores reported as graph and results of statistical modelling Medication compliance Cannabis use assessed by TLFB. Urine samples tested at each clinic visit for confirmation of self‐report Withdrawal symptoms assessed twice weekly using the Withdrawal Discomfort Score (10 items, scores 0–30) Craving by MCQ with the 47‐item version completed once a month, and the 12‐item version weekly Adverse effects assessed twice a week using the Modified Systematic Assessment for Treatment and Emergent Events (SAFTEE) |
|
| Notes | Funding: research grant (NIDA) 1 author declared prior associations with pharmaceutical companies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomized … using a fixed block size of 4, stratified by joints used per week … and whether or not they were receiving a psychotropic medication." |
| Allocation concealment (selection bias) | Low risk | Quote: "A research pharmacist, who was independent of the research team, conducted the randomization." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Donabinol … or matching placebo … was prepared by the pharmacy … packaged in matching gelatin capsules with lactose filler and an equal amount of riboflavin. All capsules looked identical …" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study conducted double‐blind as indicated above. Participants may have been able to distinguish the effects of dronabinol, but use of urine screening to support self‐report would be expected to reduce risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All analyses were conducted on the intent‐to‐treat population." "… missing data in weeks 7 and 8 were scored as indicating cannabis use …" |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Levin 2013.
| Methods | Randomised, double‐blind, placebo‐controlled trial. 1‐week placebo lead‐in phase; those who improved as assessed by Clinical Global Impression rating were not randomised. | |
| Participants | Setting: Outpatient with twice weekly clinic attendance, New York, USA. Scheduled duration 12 weeks. Participants: 103 seeking treatment for problems related to cannabis use, cannabis dependence and major depressive disorder or dysthymia by DSM‐IV Group sizes: group 1, 51; group 2: 52 No significant group differences on demographic or clinical characteristics at baseline Mean age 35 years 74% male 40% working full‐time; 18% currently married Mean 27.4 days of use in month prior to baseline Exclusion criterion: physical dependence on substances other than cannabis or nicotine |
|
| Interventions | Group 1: venlafaxine‐extended release, up to 375 mg on a fixed‐flexible schedule Group 2: placebo Medication dose titrated over 3 weeks, then maintained for 8 weeks Weekly individual cognitive behavioural therapy that primarily targeted cannabis use as adjunct intervention. Participants received USD 5–20 per visit for transport costs, and USD 10 per week if they returned their tablet bottles and any remaining medication. |
|
| Outcomes | Abstinence defined by ≥ 2 consecutive weeks without cannabis use, confirmed by urine tests Improvement in depressive symptoms by Hamilton Depression Rating Scale Cannabis use assessed by TLFB. Urine THC levels tested at each visit, with cut‐off of 100 ng/mL to decrease the probability of false positives Adverse effects assessed weekly using the Modified Systematic Assessment for Treatment and Emergent Events |
|
| Notes | Funding: research grants (NIDA) 2 authors declared prior associations with pharmaceutical companies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized at the end of the [placebo] lead‐in phase using a computer‐generated fixed‐block size of 4, with a 1:1 allocation ratio, and stratified by joints used per week … and severity of depression." Comment: similarities of groups at baseline suggest adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "A research pharmacist, who was independent of the research team, conducted the randomization and maintained the allocation sequence." Venlafaxine or placebo "was prepared by the pharmacy … packaged in matching gelatin capsules with lactose filler." Comment: allocation by pharmacy would support adequate concealment of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, care providers and outcome assessors were kept blinded to the allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Participants, care providers and outcome assessors were kept blinded to the allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants who dropped out were significantly younger and less likely to be married, but rates of dropout were similar in the 2 groups. Those who dropped out without achieving 2 continuous weeks of abstinence were classified as not abstinent. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Levin 2016.
| Methods | Randomised double‐blind, placebo‐controlled trial. 1‐week placebo lead‐in phase prior to randomisation | |
| Participants | Setting: outpatient, New York, USA. Scheduled duration 11 weeks Participants: 122 adults seeking treatment, cannabis dependent by DSM‐IV, daily smoking in 28 days prior to study entry and THC‐positive urine sample on day of study entry Group sizes: 61 in each group Groups similar on demographic and clinical characteristics at baseline Mean age 35.2 years 68.9% male 19.7% currently married, 41.0% unemployed Exclusion criteria: severe mental illness, dependence on other drugs except nicotine |
|
| Interventions | Group 1: dronabinol 20 mg + lofexidine 0.6 mg 3 times a day; medications combined in capsule Group 2: placebo Medications were maintained until the end of week 8, were then tapered over 2 weeks and participants were monitored off medications during the last study week. All participants received weekly motivational enhancement and relapse prevention therapy. |
|
| Outcomes | Number achieving "consecutive abstinence" (defined as 21 consecutive days of abstinence based on TLFB self‐report during titration and maintenance phase) Marijuana craving assessed weekly using the modified 12‐item MCQ Marijuana withdrawal assessed using a 10‐item self‐report checklist with each item rated 0–3 for a possible total score of 30 |
|
| Notes | Funding: research grant (NIDA) 2 authors declared associations with pharmaceutical companies Additional data on clinicaltrials.gov/ct2/show/results/NCT01020019 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomized at the end of the placebo lead‐in phase using computer generated random blocks of sizes 4, 6, and 8, with a 1:1 allocation ratio stratified by joints used per week." |
| Allocation concealment (selection bias) | Low risk | Quote: "A Ph.D. statistician at Columbia University independent of the research team conducted the randomization and maintained the allocation sequence." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, investigators and study staff were blind to allocation" … "Lofex–Dro or matching placebo (PBO) was prepared by the un‐blinded pharmacy …, packaged in matching gelatin capsules with lactose filler and an equal amount of riboflavin …" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study undertaken double‐blind as indicated above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The observed proportion of subjects completing the titration and maintenance medication phases of the trial were 37/61 (60.66%) in Lofex–Dro and 42/61 (68.85%) in PBO. " "There was not a significant difference in retention between treatment groups." Unclear how missing data were handled but would equally effect both groups. |
| Selective reporting (reporting bias) | Low risk | None apparent, reported outcomes consistent with clinical trials registration |
| Other bias | Low risk | None apparent |
Mason 2012.
| Methods | Randomised, double‐blind, placebo controlled trial | |
| Participants | Setting: outpatient with weekly clinic visits, California, USA. Scheduled duration 12 weeks Participants: 50 adults, seeking treatment, current cannabis dependence by DSM‐IV, smoked cannabis at least once in week prior to randomisation Group sizes: 25 in each group No significant group differences on demographic or clinical variables at baseline Mean age 33.9 years 88% male 62% employed full‐time; 40% married Mean 11.6 years of daily cannabis use, smoking a mean of 11.0 g/week Exclusion criteria: abuse or dependence on substances other than cannabis or nicotine, and significant psychiatric disorders |
|
| Interventions | Group 1: oral gabapentin 300 mg/day, increasing to 1200 mg/day Group 2: placebo Abstinence‐oriented individual counselling weekly |
|
| Outcomes | Change in amount of cannabis use, frequency of use and withdrawal symptoms, as graphs and results of statistical tests Number completing treatment Cannabis use by weekly urine toxicology and self‐report by TLFB interview Withdrawal symptoms by Marijuana Withdrawal Checklist Marijuana Problems Scale completed at baseline and end of treatment |
|
| Notes | Funding: research grants (NIDA) 1 author declared past associations with pharmaceutical companies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomly assigned … in a 1:1 ratio, on the basis of a computer‐generated randomization code." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization code was kept by the study pharmacist, who provided subjects with a 1‐week supply of medication in a blister card package at each weekly study visit …" Comment: allocation by pharmacy would support adequate concealment of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects, care providers, and those assessing outcomes were blinded to the identity of drug assignment. Gabapentin was purchased and over‐encapsulated to match placebo capsules." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Subjects, care providers, and those assessing outcomes were blinded to the identity of drug assignment. Gabapentin was purchased and over‐encapsulated to match placebo capsules." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High rate of dropout. Extent of missing data, and adjustments for missing data unclear |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
McRae‐Clark 2009.
| Methods | Randomised, double‐blind, placebo‐controlled trial. 93 participants randomised; 34 did not receive study drug (21 failed to return for second baseline visit); analysis based on those randomised who received study drug and completed at least 1 visit after baseline. | |
| Participants | Setting: outpatient with clinic visits 1–2 times per week, South Carolina, USA. Scheduled duration 12 weeks Participants: 50 adults, current cannabis dependence by DSM‐IV Group sizes: group 1, 23; group 2, 27 Treatment groups similar on baseline characteristics Mean age 31.6 years 90% male 86% Caucasian On average used cannabis on 89% of days prior to study entry, using mean 3.8 g/day Exclusion criteria: dependence on other substances except caffeine or nicotine, history of psychotic disorder, current major depression |
|
| Interventions | Group 1: oral buspirone, initiated at 5 mg twice a day, increased 5–10 mg every 3– 4 days as tolerated to maximum 60 mg per day Group 2: placebo Motivational interviewing (3 sessions) as adjunct intervention for first 4 weeks. Participants received USD 10 for time and travel associated with study visits |
|
| Outcomes | Urinalysis data reported as percent of screens that were negative, not participants with negative screens Mean change in withdrawal score Number experiencing any adverse effect Number completing treatment Change in reported cannabis use per using day, % days abstinent during study Cannabis use by TLFB for 90 days prior to study entry, and weekly throughout the study Craving by MCQ, withdrawal, by Marijuana Withdrawal Checklist Urine drug screens at baseline and weekly during study Adverse effects evaluated weekly with open‐ended questions Adjustment for missing data by last observation carried forward |
|
| Notes | Funding: research grant (NIDA) 2 authors declared past associations with pharmaceutical companies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Urn randomization … was used to determine treatment assignment. Urn variables used were age … gender, and [anxiety] score …" |
| Allocation concealment (selection bias) | Low risk | Quote: [participants] "Randomized at central pharmacy …" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Buspirone and placebo tablets were packaged in identical opaque gelatin capsules … Comment: double‐blind stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind stated and urinalysis to support self‐report data would be expected to reduce bias, although authors noted some inconsistencies between urine screen and self‐report data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High rate of dropout but statistical methods used to adjust for missing data (Generalized Estimating Equation modelling and last observation carried forward) |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
McRae‐Clark 2010.
| Methods | Randomised, double‐blind, placebo‐controlled trial; 78 participants were randomised but only 46 received study medication and only 38 returned for ≥ 1 post‐baseline assessment. Analyses based on this group | |
| Participants | Setting: outpatient, South Carolina, USA. Scheduled duration 12 weeks Participants: 38 adults, cannabis dependence and attention deficit hyperactivity disorder (with age of onset before 12 years) by DSM‐IV Group sizes: 19 in each group No significant group differences on baseline characteristics Mean age 29.9 years 76% male 92% Caucasian Used cannabis on average 87% of days prior to baseline, using mean of 4.1 times per day Exclusion criteria: dependence on other substances except caffeine or nicotine, and other psychiatric disorders |
|
| Interventions | Group 1: oral atomoxetine started at 25 mg/day, increased to 40 mg/day in week 2, and to 80 mg/day in week 3 as tolerated, with further increase to 100 mg/day in week 4 if required Group 2: placebo Motivational interviewing (3 sessions) as adjunct intervention. Nominal monetary reimbursement for completion of study assessments |
|
| Outcomes | Self‐reported cannabis use during week 12 (last observation carried forward for participants who did not complete the trial) Number completing treatment Change in craving scores Number experiencing adverse effects and type of adverse effects Cannabis use self‐reported by TLFB weekly and assessed by Clinical Global Impression of Severity and Improvement Scales Urine drug screens at baseline and then weekly Medication side effects weekly by standard checklist Craving by MCQ Compliance assessed by patient report and tablet count |
|
| Notes | Funding: research grants (NIDA), with medication and placebo provided by manufacturer (Eli Lilly and Company) 2 authors declared past associations with pharmaceutical companies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomization was used to assign treatments to participants using a 1:1 allocation ratio." |
| Allocation concealment (selection bias) | Low risk | Quote: "… participants were randomized at the central pharmacy …" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind stated, and use of matching capsules would support maintenance of the blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind stated. Use of urine screening to validate self‐report data would be expected to reduce the risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High rates of dropout in both groups. Last observation carried forward and statistical techniques used to allow for missing data. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
McRae‐Clark 2015.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Setting: outpatient (no further details on site, appears to be a single site). Scheduled duration 12 weeks Participants: 175 adults, current cannabis dependence by DSM‐IV Group sizes: group 1, 88; group 2, 87 Groups similar on demographics and clinical characteristics at baseline Mean age 24 years 77% male 64% Caucasian Exclusion criteria: current dependence on other substances except caffeine and nicotine, and significant psychiatric condition |
|
| Interventions | Group 1: buspirone, up to 60 mg/day Group 2: placebo Brief MET intervention and contingency management to encourage study retention as adjunct interventions |
|
| Outcomes | Participants with ≥ 1 negative urine test during treatment Point prevalence of abstinence by urine test at week 12 Number reporting adverse events |
|
| Notes | Funding: research grant (NIDA) 2 authors report previous pharmaceutical company funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Buspirone and placebo tablets were packaged in identical opaque gelatin capsules with lactose." Comment: it was likely that participants and treating clinicians were blind to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind likely as indicated above, and only objective outcomes reported which are less likely to be affected by knowledge of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Rates of attrition high, but similar in both groups. Missing data on cannabinoid urine tests counted as positive. |
| Selective reporting (reporting bias) | Low risk | None apparent, published outcomes accorded with prospectively registered outcomes. |
| Other bias | Low risk | None apparent |
McRae‐Clark 2016.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Setting: outpatient, South Carolina, USA. Scheduled duration 8 weeks Participants: 76 adults, current cannabis dependence by DSM‐IV Group sizes: group 1, 41; group 2, 35 Groups similar on demographics and clinical characteristics at baseline, except somewhat higher baseline cannabinoid levels in placebo group (adjusted for in analysis) Mean age 22 years 79% male 55% Caucasian Exclusion criteria: current dependence on other substances except caffeine and nicotine, and significant psychiatric conditions |
|
| Interventions | Group 1: vilazodone, flexible dose up to 40 mg/day Group 2: placebo Medications commenced at 10 mg/day for 7 days, increased to 20 mg/day for 7 days, then up to 40 mg/day as tolerated Brief MET (3 sessions) and contingency management to encourage study retention as adjuncts |
|
| Outcomes | Weekly urine tests Self‐report cannabis use by TLFB (weekly) Craving by MCQ (weekly) Adverse effects assessed weekly by clinician with open‐ended questions Medication compliance by weekly patient report and tablet count Those lost to follow‐up or missing study visits coded as positive urine screen results Numbers attending ≥ 1 visit, and proportion of scheduled visits attended |
|
| Notes | Funding: research grant (NIH). Vilazodone and matching placebo were provided by Forest Pharmaceuticals. The authors reported no conflict of interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Urn randomization was used to determine treatment assignment. Randomization variables included gender and presence or absence of anxiety or depressive disorders." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information reported to permit judgement of low or high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information reported to permit judgement of low or high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow‐up or missing study visits coded as urine drug screen failures. Groups differed in retention rates, but this is primary outcome measure for this review. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | High risk | Placebo group attended greater proportion of scheduled visits and hence may have received more adjunct interventions. |
Miranda 2017.
| Methods | Randomised placebo‐controlled pilot study. Participants stratified on sex, cannabis dependence and baseline working memory function prior to randomisation | |
| Participants | Setting: outpatient, Providence, USA. Scheduled duration 6 weeks Participants: 66 adolescents (aged 15–24 years), used cannabis at least twice weekly in prior 28 days and experiencing some clinically significant problems Group sizes: group 1, 40; group 2, 26 Groups similar on demographics (except mean age) and baseline clinical characteristics Mean age: group 1, 20.3 years, group 2, 18.8 years 48.5% male 80% met DSM‐IV‐TR criteria for cannabis abuse or dependence Exclusion criteria: significant psychiatric conditions. |
|
| Interventions | Group 1: topiramate, titrated over 4‐weeks then stabilised at 200 mg/day for 2 weeks Group 2: placebo MET biweekly for 3 sessions |
|
| Outcomes | Number completing study, days in treatment, sessions of MET attended Cannabis use (grams per use day and % days with use) Medication compliance Adverse effects |
|
| Notes | Funding: research grant (NIDA) 1 author declared associations with pharmaceutical companies. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An investigator with no direct participant contact used a computer‐generated random allocation sequence to assign participants to treatment conditions on a 2:1 (topiramate to placebo) ratio." |
| Allocation concealment (selection bias) | Low risk | Quote: "An investigator with no direct participant contact used a computer‐generated random allocation sequence to assign participants to treatment conditions." "An independent compounding pharmacy provided topiramate and placebo capsules, which were identical in appearance. Capsules were prepackaged in 7‐day blister packaging cards consecutively numbered according to a computer‐generated randomization schedule to ensure that the researchers who enrolled and assessed participants were blind to treatment assignments." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "An independent compounding pharmacy provided topiramate and placebo capsules, which were identical in appearance;" "Participants and study personnel in direct contact with participants were blind to treatment assignments." "Counselors were blind to participants' medication condition and did not conduct any research assessments with participants." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent compounding pharmacy provided topiramate and placebo capsules, which were identical in appearance;" "Participants and study personnel in direct contact with participants were blind to treatment assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 66 participants, 39 youth (59.1 percent) completed the trial. As expected, attrition occurred disproportionally in the topiramate condition (52.5 percent) compared with the placebo condition (23.1 percent)." Comment: attrition is an outcome for this review. Missing data were imputed in analyses of cannabis use outcomes. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
Penetar 2012.
| Methods | Randomised, double‐blind, placebo‐controlled trial. Participants used cannabis as usual for 7 days prior to randomisation. | |
| Participants | Setting: outpatient with daily clinic attendance Monday to Friday, Harvard Medical School, USA. Scheduled duration 21 days Participants: 22 adults, seeking treatment, cannabis abuse or dependence by DSM‐IV, ≥ 3 years of heavy use (smoking on ≥ 5 days a week or > 25 times per month) and with ≥ 2 negative symptoms in previous quit attempts Group sizes: group 1, 10; group 2: 12 Demographic data provided only for 9 who completed the study (5 male, mean age 31.2 years, 7 met criteria for dependence) Exclusion criterion: abuse or dependence on any other drug (including nicotine) |
|
| Interventions | Group 1: oral bupropion SR 150 mg/day for days 1–3, then 150 mg twice a day Group 2: placebo Riboflavin added to medication capsules to measure compliance Weekly individual MET (3 sessions) as adjunct intervention |
|
| Outcomes | Number completing study, change in withdrawal discomfort and change in craving Data reported as graphs and results of statistical tests Withdrawal by Marijuana Withdrawal Checklist (29 items each rated 0–3). Withdrawal discomfort score calculated from 10 items (maximum score 30) Drug use, sleep and withdrawal recorded by participants in daily diary. Urine testing to confirm cannabis use |
|
| Notes | Funding: research grant (NIDA). No conflicts of interest reported Disclosures of interests according to ICMJE criteria were a requirement of publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation to treatment group stated, but method of sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Bupropion tablets were repackaged into gelatin capsules … Placebo consisted of identical appearing gelatin capsules." Comment: double‐blind stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind stated as indicated above. Use of urine screening to verify self‐report expected to reduce risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of dropout and demographics reported only for those who completed treatment. Unclear whether there were differences between the groups, or between those who did and did not complete the study. Unclear how missing data were handled. |
| Selective reporting (reporting bias) | Unclear risk | Data on adverse effects not reported. |
| Other bias | Low risk | None apparent |
Sherman 2017.
| Methods | Randomised, double‐blind, placebo‐controlled trial. Oxytocin and placebo compared as adjuncts to MET. | |
| Participants | Setting: outpatient with 3 MET sessions over 4 weeks at the Medical University of South Carolina, South Carolina, USA Participants: 16 adults, seeking treatment, cannabis dependence by DSM‐IV, cannabis as the primary substance of abuse (no criteria for dependence on any other substance except nicotine in the 60 days prior to enrolment). Group sizes: 8 in each group Mean age 25.5 years 62.5% male Exclusion criteria: history of or current psychotic disorder or bipolar affective disorder |
|
| Interventions | Group 1: oxytocin 40 IU as nasal spray prior to first 1 sessions of MET Group 2: placebo (matching saline spray) Both groups received 45‐ to 60‐minute sessions of MET at study weeks 1, 2 and 4. |
|
| Outcomes | Therapy Session Satisfaction and Cannabis use measured via self‐report of number of smoking sessions per day (TLFB) | |
| Notes | Data from published report, clinicaltrials.gov and provided by investigators. Study aimed to examine if oxytocin administration prior to MET would enhance the outcomes of psychosocial treatment for cannabis dependence. Funding: research grant (NIDA) Authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported, described as 'randomized.' |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching saline spray was compounded by the pharmacy who held the blinding key until the study was over. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Matching saline spray was compounded by the pharmacy who held the blinding key until the study was over. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13/16 participants completed study |
| Selective reporting (reporting bias) | High risk | Primary outcome in clinicaltrials.gov described as satisfaction with therapy; details on primary outcome not reported in main publication. |
| Other bias | Low risk | None apparent |
Trigo 2018.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Setting: outpatient, Toronto, Canada. Scheduled duration 12 weeks + 12 weeks follow‐up Participants: 40 adults, cannabis dependent by DSM‐IV, seeking treatment Group sizes: 20 in each group Groups similar on demographic and clinical characteristics Mean age 32 years 72% male Exclusion criteria: substance use disorders other than cannabis, nicotine, caffeine, psychotropic medication for other indications |
|
| Interventions | Group 1: nabiximols, oral spray administered as needed up to THC 113.4 mg + cannabidiol 105 mg Group 2: placebo MET and cognitive behavioural therapy weekly as adjunct |
|
| Outcomes | Medication use determined by weighing vials Abstinence by self‐report (TLFB) and smoking diaries, with urine and blood tests for THC and metabolites as confirmation Number completing treatment, cannabis use, craving and adverse effects |
|
| Notes | Data extracted from unpublished manuscript and clinicaltrials.gov Funding source: research grant (NIH) Nabiximols and placebo sprays provided by manufacturer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible participants were enrolled by the principal investigator … and randomized in blocks of 10 … in a 1:1 ratio and in a double blind manner by the participating pharmacy." |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible participants were enrolled by the principal investigator … and randomized in blocks of 10 … in a 1:1 ratio and in a double blind manner by the participating pharmacy." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All study staff except for the participating pharmacy were blinded after assignment to interventions." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All study staff except for the participating pharmacy were blinded after assignment to interventions." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Rates of attrition similar in two groups, and attrition is an outcome measure for this review. Missing data managed appropriately through statistical analysis. |
| Selective reporting (reporting bias) | Low risk | Main outcomes registered prospectively on clinicaltrials.gov |
| Other bias | Low risk | None apparent |
Weinstein 2014.
| Methods | Randomised, double‐blind, placebo‐controlled trial. 1‐week "induction" with placebo prior to randomisation | |
| Participants | Setting: outpatient, Tel Aviv, Israel. Scheduled duration 9 weeks Participants: 52 adults, regular cannabis users, dependent by DSM‐IV Group sizes: 26 in each group Similarity of groups not reported Mean age 32.7 years 75% male Exclusion criteria: dependence on other drugs or alcohol and significant psychiatric disorder |
|
| Interventions | Group 1: escitalopram 10 mg/day Group 2: placebo Medication for 9 weeks, follow‐up sessions for further 14 weeks. Blinding broken after 9 weeks; participants able to continue open‐label escitalopram use. Participants instructed to stop cannabis use after 4 weeks of medication. Weekly (9 sessions) cognitive‐behaviour (relapse prevention) and MET in combination with medication |
|
| Outcomes | Number completing treatment, number abstinent, number reporting not taking medication, results of statistical analyses of withdrawal scores Urine samples collected every second week Questionnaires administered to assess anxiety and depression Revised Clinical Institute Withdrawal Assessment Scale adapted for assessment of cannabis withdrawal (score ≥ 10 indicated significant withdrawal) |
|
| Notes | Funding: research grant (Israeli anti‐drug authority) Authors declared no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "participants were blindly randomized …" Comment: method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "participants were blindly randomized …" Comment: method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind stated and only objective outcomes reported which are less likely to be affected by knowledge of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High (50%) rate of dropout. Those who did not complete study were younger, and more likely to be daily alcohol drinkers. Non‐completers marginally more depressed, but difference not statistically significant. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders 4th Edition (Text Revision); ICMJE: International Committee of Medical Journal Editors; IV: intravenous; MCQ: Marijuana Craving Questionnaire; MET: motivational enhancement therapy; NIAAA: National Institute on Alcohol Abuse and Alcoholism; NIDA: National Institute on Drug Abuse; NIH: National Institutes of Health; SD: standard deviation; SR: sustained release; THC: Δ9‐tetrahydrocannabinol; THC‐COOH: 11‐nor‐9‐carboxy‐Δ9‐tetrahydrocannabinol; TLFB: timeline follow‐back.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2018 | Participants recruited from opioid treatment programme; all receiving medication‐assisted treatment. Cross‐over design, not randomised controlled trial, comparing medication‐assisted treatment with no medication, or with varenicline. Total 7 participants, 4 of whom were cannabis dependent, 3 met criteria for cannabis abuse. |
| Akerele 2007 | Participants diagnosed with abuse or dependence on marijuana or cocaine. Data reported separately for cocaine and marijuana use, but it was not possible to extract data just for those dependent on marijuana. All participants were diagnosed with schizophrenia; the management of substance use in the context of schizophrenia was the main focus of the study. |
| Brown 2013 | Secondary analysis of data from a randomised controlled trial comparing 2 behavioural interventions. No use of medications. |
| Budney 2007b | Laboratory study involving non‐treatment seeking cannabis users. Not all users were cannabis dependent, and participants were not trying to reduce their cannabis use. |
| Cooper 2013 | Laboratory study involving marijuana smokers who were not seeking treatment. Investigation of research model of withdrawal and relapse rather than treatment intervention. |
| Cornelius 1999 | Randomised controlled trial comparing fluoxetine and placebo for treatment of alcohol dependence with comorbid major depression. Effect on subgroup with diagnosed marijuana abuse considered as secondary analysis. |
| Cornelius 2008 | Reported cannabis withdrawal symptoms in participants entering 2 separate trials of fluoxetine. No treatment intervention for cannabis dependence. |
| Cornelius 2015 | Open‐label study of mirtazapine and motivation therapy for people with major depressive disorder and substance use disorder. Most study participants had used cannabis, but unclear how many were dependent. No treatment comparison. |
| Findling 2009 | Randomised controlled trial comparing fluoxetine and placebo for treatment of depressive symptoms in adolescents with comorbid substance use disorder. Cannabis use reported by 88.2% of participants (41.2% dependent). The emphasis of this study was on the amelioration of depression. Outcome data not reported separately for the subset of cannabis‐dependent participants. |
| Geller 1998 | Randomised controlled trial comparing lithium and placebo for treatment of adolescents with bipolar disorder and comorbid substance use disorder. Majority of participants were polydrug users; 2/25 were dependent on cannabis only. |
| Gillman 2006 | Reported the use of nitrous oxide for treatment of withdrawal associated with the smoking of methaqualone combined with cannabis. Unclear how many participants were cannabis dependent. All participants received placebo then analgesic nitrous oxide. Effectiveness assessed only in terms of improvement in withdrawal symptoms. |
| Gray 2010 | Open‐label single group study investigating the effectiveness of N‐acetylcysteine in promoting cessation of cannabis use. No treatment comparison. |
| Haney 2001 | Comparison of bupropion and placebo in terms of effect on mood when administered in conjunction with active or placebo cannabis cigarettes. Laboratory study that aimed to assess the therapeutic potential of bupropion, but not a treatment intervention. |
| Haney 2003a | Laboratory study comparing the effect of nefazodone 450 mg/day and placebo on the acute effects of cannabis, and on cannabis withdrawal symptoms. The study aimed to assess the therapeutic potential of nefazodone in cannabis withdrawal but was not a treatment intervention. |
| Haney 2003b | Investigation of mechanism of effects of cannabis through comparison of naltrexone and methadone, administered prior to oral THC, and different doses of oral THC administered in combination with naltrexone or placebo. No treatment intervention. |
| Haney 2004 | 2 separate laboratory‐based studies, 1 assessing THC and 1 assessing divalproex, compared to placebo, in terms of effects on cannabis withdrawal. Studies aimed to assess the therapeutic potential of THC and divalproex but were not treatment interventions. |
| Haney 2008 | Laboratory study investigating the effect of lofexidine and THC (separately and in combination) compared with placebo on cannabis withdrawal symptoms and a model of cannabis relapse. The study aimed to test the therapeutic potential of lofexidine in cannabis withdrawal but was not a treatment intervention. |
| Haney 2010 | Controlled laboratory study investigating the effects of baclofen or mirtazapine on cannabis smoking, craving and withdrawal. Exploratory study of the potential therapeutic value of baclofen and mirtazapine, but not a treatment intervention. |
| Haney 2013 | Laboratory study assessing effect of nabilone on marijuana withdrawal symptoms, and laboratory measure of relapse. Study aimed to test the therapeutic potential of nabilone but was not a treatment intervention. |
| Haney 2015 | Laboratory study comparing impact of naltrexone and placebo on effects of active or inactive cannabis. Participants not seeking treatment. |
| Haney 2016 | Laboratory dose‐ranging study of effects of cannabidiol. Participants not seeking treatment. |
| Herrmann 2016 | Laboratory study investigating effect of zolpidem and nabilone (separately and in combination) compared with placebo on marijuana withdrawal symptoms and a model of marijuana relapse. The study aimed to test the therapeutic potential of zolpidem in marijuana smokers but was not a treatment intervention. |
| Nanjayya 2010 | Open‐label study investigating the use of baclofen for the treatment of cannabis dependence. No treatment comparison. |
| Notzon 2018 | Single group study of injectable naltrexone for treatment of cannabis dependence. No treatment comparison. |
| Rubio 2006 | Comparison of antipsychotic drugs for treatment of schizophrenia, with consideration of effects on cannabis use. |
| Schnell 2014 | Comparison of antipsychotic drugs for treatment of schizophrenia, with consideration of effects on cannabis use. |
| Sevy 2011 | Comparison of antipsychotic drugs for treatment of schizophrenia, with consideration of effects on cannabis use. |
| Sugarman 2011 | Controlled study assessing the safety of modafinil in combination with THC. While the study contributed to assessment of the therapeutic potential of modafinil, this study did not involve a treatment intervention. Participants were occasional cannabis users (people who were heavy users or dependent were excluded). |
| Trigo 2016 | Laboratory study assessing effects of Sativex on cannabis withdrawal and craving. Not a treatment intervention. Preliminary study to Trigo 2018. |
| Van Nimwegen 2008 | Randomised controlled trial comparing olanzapine and risperidone for treatment of schizophrenia. Majority of participants were not using cannabis and cannabis dependence was not assessed. |
| Vandrey 2011 | Cross‐over study comparing zolpidem and placebo during short (3‐day) periods of abstinence from cannabis in terms of sleep parameters. Not a full treatment intervention for cannabis dependence. |
| Vandrey 2013 | Comparison of dronabinol and placebo in terms of effect on cannabis withdrawal and subjective effects of smoked cannabis, but without providing a treatment intervention for cannabis dependence. |
| Vandrey 2016 | Laboratory study of effect of zolpidem on sleep during cannabis withdrawal. Not a full treatment intervention. |
| Winstock 2009 | An open‐label study investigating the use of lithium carbonate for the management of cannabis withdrawal. No treatment comparison. |
THC: Δ9‐tetrahydrocannabinol.
Characteristics of ongoing studies [ordered by study ID]
Bhardwaj 2018.
| Trial name or title | Randomised controlled trial (RCT) of cannabinoid replacement therapy (nabiximols) for the management of treatment‐resistant cannabis dependent patients |
| Methods | Randomised controlled trial |
| Participants | 142 cannabis‐dependent adults |
| Interventions | Experimental: nabiximols Comparison: placebo |
| Outcomes | Non‐prescribed cannabis (self‐reported cannabis use days, urine toxicology) |
| Starting date | 2016 |
| Contact information | Nicholas Lintzeris, University of Sydney |
| Notes | Recruitment complete, protocol published and results pending (requested) |
D'Souza 2015.
| Trial name or title | Safety and efficacy of a FAAH‐inhibitor to treat cannabis withdrawal |
| Methods | Randomised controlled trial |
| Participants | 120 cannabis‐dependent participants |
| Interventions | Experimental: FAAH inhibitor PF‐04457845 Comparison: placebo |
| Outcomes | Withdrawal score, cannabis use, craving, relapse rates |
| Starting date | June 2012 |
| Contact information | Deepak C D'Souza, Yale University |
| Notes |
NCT00974376.
| Trial name or title | Gabapentin treatment of cannabis dependence |
| Methods | Randomised controlled trial |
| Participants | 150 cannabis‐dependent participants |
| Interventions | Experimental: gabapentin Comparison: placebo |
| Outcomes | Negative urine drug screens at 12 weeks' follow‐up |
| Starting date | 2009 |
| Contact information | Barbara J Mason |
| Notes |
NCT01598896.
| Trial name or title | Combination of dronabinol and clonidine for cannabis dependence in patients with schizophrenia |
| Methods | Randomised controlled trial |
| Participants | 18 cannabis‐dependent participants with schizophrenia |
| Interventions | Experimental: dronabinol and clonidine Comparison: placebo |
| Outcomes | Change in cannabis use |
| Starting date | May 2012 |
| Contact information | William M Hurley‐Welljams‐Dorof, McLean Hospital, USA |
| Notes |
NCT02044809.
| Trial name or title | Cannabidiol, a novel intervention for cannabis use problems? |
| Methods | Randomised controlled trial |
| Participants | 96–168 young people who want to quit cannabis |
| Interventions | Experimental: cannabidiol Comparison: placebo |
| Outcomes | Stage 1: identification of most effective dose |
| Starting date | March 2014 |
| Contact information | Clinical Psychopharmacology Unit, UK |
| Notes |
NCT02579421.
| Trial name or title | Hormones and reduction in co‐users of marijuana and nicotine |
| Methods | Randomised controlled trial |
| Participants | 100 marijuana‐dependent participants |
| Interventions | Experimental: progesterone Comparison: placebo |
| Outcomes | Change in marijuana use by timeline follow‐back method |
| Starting date | 2015 |
| Contact information | Sharon Allen, University of Minnesota |
| Notes |
FAAH: fatty acid amide hydrolase.
Differences between protocol and review
The protocol focused on the management of cannabis withdrawal. When it became clear that very few studies considered withdrawal as a distinct phase, the review was broadened to include interventions to support cessation or reduction of cannabis use as well as management of withdrawal symptoms. The broadening of the review made the specification of "the portion of the scheduled treatment episode that is completed on average" less relevant; hence this was dropped from the review.
The protocol stipulated the inclusion of studies that involve participants who are diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) or 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD‐10) criteria as cannabis dependent, or where dependence is likely based on reported dose, duration and frequency of use (daily or multiple days per week). Given the qualifier of "where dependence is likely" the specification of DSM‐IV or ICD‐10 criteria would not have resulted in the exclusion of any included studies and was dropped from the methods of the review in the interests of simplicity.
The approach to heterogeneity specified in the protocol (use of a random‐effects model in the presence of statistical heterogeneity) was changed based on statistical advice received in the interim. The routine use of a random‐effects model is preferred and was the approach used for the review.
This version of the review specifically excluded studies involving participants with diagnosed schizophrenia and cannabis use disorder. The primary therapeutic goal in these studies was management of psychotic symptoms, with consideration of the effect of different antipsychotic medications on cannabis use.
Contributions of authors
All authors contributed to the review concept and design.
SN, LG and PS undertook literature searches, assessed studies for inclusion, and wrote a first draft of the text.
BLF provided comments at all stages of the review.
Sources of support
Internal sources
DASSA‐WHO Collaborating Centre in the Treatment of Drug and Alcohol Problems, Australia.
External sources
No sources of support supplied
Declarations of interest
SN: was supported by an National Health and Medical Research Council Fellowship while completing this review. SN is an investigator on untied educational grants Indivior on studies unrelated to this work.
LG: none known.
PS: none known.
BLF: received funding from Pfizer in the form of Global Research Awards On Nicotine Dependence or GRAND Awards (awards provided independently from Pfizer by a panel of international experts). The GRAND awards notifications were obtained in 2008, 2009, 2010, 2011, 2016. Dr Le Foll received some salary support on some of those grants to compensate for time spent on research (less than 5% of his income). The salary support was received by CAMH and transferred to Dr Le Foll and CAMH controlled the use of the funds. As some of the salary support was received around the time of the previous version of the review (Cochrane Database Syst Rev. 2014;(12)), the Funding Arbiters reviewed the case and determined that, because the award followed open competition judged by an independent panel, the funding did not constitute a relevant conflict.
Edited (no change to conclusions)
References
References to studies included in this review
Allsop 2014 {published data only}
- Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry 2014;71(3):281‐91. [DOI: 10.1001/jamapsychiatry.2013.3947] [DOI] [PubMed] [Google Scholar]
- Bruno R, Allsop DJ, Copeland J, McGregor L, Norberg MM, Dunlop AJ, et al. The impact of Sativex (R) on cognitive function during treatment for cannabis withdrawal. Drug and Alcohol Review 2013;32:27. [Google Scholar]
- Kevin RC, Allsop DJ, Lintzeris N, Dunlop AJ, Booth J, McGregor IS. Urinary cannabinoid levels during nabiximols (Sativex®)‐medicated inpatient cannabis withdrawal. Forensic Toxicology 2017;35(1):33‐44. [Google Scholar]
- Montebello M, Allsop D, Copeland J, Lintzeris N, Dunlop A, Sadler C, et al. Cannabinoid replacement therapy for management of cannabis withdrawal: a randomized controlled trial of nabiximols (Sativex®). Australian and New Zealand Journal of Psychiatry 2014;48(Suppl 1):79. [Google Scholar]
Carpenter 2009 {published data only}
- Carpenter KM, McDowell D, Brooks DJ, Cheng WY, Levin FR. A preliminary trial: double‐blind comparison of nefazodone, bupropion‐SR, and placebo in the treatment of cannabis dependence. American Journal on Addictions 2009;18(1):53‐64. [DOI: 10.1080/10550490802408936] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornelius 2010 {published data only}
- Cornelius JR, Bukstein OG, Douaihy AB, Clark DB, Chung TA, Daley DC, et al. Double‐blind fluoxetine trial in comorbid MDD‐CUD youth and young adults. Drug and Alcohol Dependence 2010;112(1‐2):39‐45. [DOI: 10.1016/j.drugalcdep.2010.05.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Ferrell R, Douaihy AB, Hayes J, Kirisci L, et al. Treatment trial and long‐term follow‐up evaluation among co‐morbid youth with major depression and a cannabis use disorder. Advances in Psychology Research 2012;93:109‐21. [PMC free article] [PubMed] [Google Scholar]
Frewen 2007 {published and unpublished data}
- Frewen A, Montebello ME, Baillie A, Rea F. Effects of mirtazapine on withdrawal from dependent cannabis use. 69th Annual Scientific Meeting of the College on Problems of Drug Dependence; 2007 June 16‐21; Quebec City, Canada. 2007:21. [MEDLINE: ]
- Frewen AR. An Examination of Withdrawal Symptoms and their Relationship with Outcomes in a Combined Behavioural and Pharmacological Intervention for Dependent Cannabis Users [thesis]. North Ryde (Australia): Macquarie University, 2009. [Google Scholar]
Gray 2012 {published data only}
- Bentzley JP, Tomko RL, Gray KM. Low pretreatment impulsivity and high medication adherence increase the odds of abstinence in a trial of N‐acetylcysteine in adolescents with cannabis use disorder. Journal of Substance Abuse Treatment 2016;63:72‐7. [DOI: 10.1016/j.jsat2015.12.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, et al. A double‐blind randomized controlled trial of N‐acetylcysteine in cannabis‐dependent adolescents. American Journal of Psychiatry 2012;169(8):805‐12. [DOI: 10.1176/appi.ajp.2012.12010055; EMBASE: AN 2012469181] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Baker NL, Gray KM. Cigarette smoking during an N‐acetylcysteine‐assisted cannabis cessation trial in adolescents. American Journal of Drug and Alcohol Abuse 2014;40(4):285‐91. [DOI: 10.3109/00952990.2013.878718] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roten A, Baker NL, Gray KM. Cognitive performance in a placebo‐controlled pharmacotherapy trial for youth with marijuana dependence. Addictive Behaviors 2015;45:119‐23. [DOI: 10.1016/j.addbeh.2015.01.013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addictive Behaviors 2013;38(3):1788‐91. [DOI: 10.1016/j.addbeh.2012.11.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L, Baker N, McClure E, Gray K. Alcohol use during an N‐acetylcysteine cannabis cessation trial in adolescents. Drug and Alcohol Dependence 2017;171:e195. [DOI: 10.1016/j.drugalcdep.2016.08.533] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Baker NL, McClure EA, Tomko RL, Adisetiyo V, Gray KM. Alcohol use during a trial of N‐acetylcysteine for adolescent marijuana cessation. Addictive Behaviors 2016;63:172‐7. [DOI: 10.1016/j.addbeh.2016.08.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray 2017 {published data only}
- Gray KM, Sonne SC, McClure EA, Ghitza UE, Matthews AG, McRae‐Clark AL, et al. A randomized placebo‐controlled trial of N‐acetylcysteine for cannabis use disorder in adults. Drug and Alcohol Dependence 2017;177:249‐57. [DOI: 10.1016/j.drugalcdep.2017.04.020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Sonne SC, Winhusen T, Carroll KM, Ghitza UE, McRae‐Clark AL, et al. Achieving Cannabis Cessation – Evaluating N‐acetylcysteine Treatment (ACCENT): design and implementation of a multi‐site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemporary Clinical Trials 2014;39(2):211‐23. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BJ, McRae‐Clark AL, Baker NL, Sonne SC, Killeen TK, Cloud K, et al. Gender differences among treatment‐seeking adults with cannabis use disorder: Clinical profiles of women and men enrolled in the Achieving Cannabis Cessation – Evaluating N‐acetylcysteine Treatment (ACCENT) study. American Journal on Addictions 2017;26(2):136‐44. [DOI: 10.1111/ajad.12503] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L, Tomko R, Baker N, McClure E, Book G, Gray K. The effect of N‐acetylcysteine on alcohol use during a cannabis cessation trial. Drug and Alcohol Dependence 2018;185:17‐22. [DOI: 10.1016/j.drugalcdep.2017.12.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2014 {published data only}
- Allsop DJ, Bartlett DJ, Johnston J, Helliwell D, Winstock A, McGregor IS, et al. The effects of lithium carbonate supplemented with nitrazepam on sleep disturbance during cannabis abstinence. Journal of Clinical Sleep Medicine 2015;11(10):1153‐62. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J, Lintzeris N, Allsop DJ, Suraev A, Booth J, Carson DS, et al. Lithium carbonate in the management of cannabis withdrawal: a randomized placebo‐controlled trial in an inpatient setting. Psychopharmacology 2014;231(24):4623‐36. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Johnston J, Lintzeris N, McGregor I, Allsop DJ, Helliwell D, Winstock A. A double blind, randomised, placebo controlled trial of lithium carbonate for the management of cannabis withdrawal [conference abstract]. Drug and Alcohol Review 2013;32:43. [Google Scholar]
- Johnston J, Lintzeris N, McGregor I, Guastella A, Allsop D, Helliwell D, et al. Preliminary findings from a double blind, randomised, placebo controlled trial of lithium carbonate for the management of cannabis withdrawal [conference abstract]. Drug and Alcohol Review 2012;31:45. [Google Scholar]
Levin 2004 {published data only}
- Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, et al. Pharmacotherapy for marijuana dependence: a double‐blind, placebo‐controlled pilot study of divalproex sodium. American Journal on Addictions 2004;13:21‐32. [DOI: 10.1080/10550490490265280] [DOI] [PubMed] [Google Scholar]
Levin 2011 {published data only}
- Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double‐blind, placebo‐controlled trial. Drug and Alcohol Dependence 2011;116:142‐50. [DOI: 10.1016/j.drugalcdep.2010.12.010; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levin 2013 {published data only}
- Kelly MA, Pavlicova M, Glass A, Mariani JJ, Bisaga A, Sullivan MA, et al. Do withdrawal‐like symptoms mediate increased marijuana smoking in individuals treated with venlafaxine‐XR?. Drug and Alcohol Dependence 2014;144:42‐6. [DOI: 10.1016/j.drugalcdep.2014.06.040] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani J, Brooks DJ, Pavlicova M, Nunes EV, Agosti V, et al. A randomized double‐blind, placebo‐controlled trial of venlafaxine‐extended release for co‐occurring cannabis dependence and depressive disorders. Addiction 2013;108(6):1084‐94. [DOI: 10.1111/add.12108] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levin 2016 {published data only}
- Levin FR, Mariani JJ, Pavlicova M, Brooks D, Glass A, Mahony A, et al. Dronabinol and lofexidine for cannabis use disorder: a randomized, double‐blind, placebo‐controlled trial. Drug and Alcohol Dependence 2016;159:53‐60. [DOI: 10.1016/j.drugalcdep.2015.11.025] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2012 {published data only}
- Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, et al. A proof‐of‐concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis‐dependent adults. Neuropsychopharmacology 2012;37:1689‐98. [DOI: 10.1038/npp.2012.14] [DOI] [PMC free article] [PubMed] [Google Scholar]
McRae‐Clark 2009 {published data only}
- McRae‐Clark AL, Baker NL, Sonne SC, DeVane C, Wagner A, Norton J. Concordance of direct and indirect measures of medication adherence in a treatment trial for cannabis dependence. Journal of Substance Abuse Treatment 2015;57:70‐4. [DOI: 10.1016/j.jsat.2015.05.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae‐Clark AL, Carter RE, Killeen TK, Carpenter MJ, Wahlquist AE, Simpson SA, et al. A placebo‐controlled trial of buspirone for the treatment of marijuana dependence. Drug and Alcohol Dependence 2009;105:132‐8. [DOI: 10.1016/j.drugalcdep.2009.06.022] [DOI] [PMC free article] [PubMed] [Google Scholar]
McRae‐Clark 2010 {published data only}
- McRae‐Clark AL, Carter RE, Killeen TK, Carpenter MJ, White KG, Brady KT. A placebo‐controlled trial of atomoxetine in marijuana‐dependent individuals with attention deficit hyperactivity disorder. American Journal on Addictions 2010;19(6):481‐9. [DOI: 10.1111/j.1521-0391.2010.00076.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
McRae‐Clark 2015 {published data only}
- McRae‐Clark AL, Baker NL, Gray KM, Killeen TK, Wagner AM, Brady KT, et al. Buspirone treatment of cannabis dependence: a randomized, placebo‐controlled trial. Drug and Alcohol Dependence 2015;156:29‐37. [DOI: 10.1016/j.drugalcdep.2015.08.013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BJ, Baker NL, McRae‐Clark AL. Gender differences in cannabis use disorder treatment: change readiness and taking steps predict worse cannabis outcomes for women. Addictive Behaviors 2016;60:197‐202. [DOI: 10.1016/j.addbeh.2016.04.014] [DOI] [PMC free article] [PubMed] [Google Scholar]
McRae‐Clark 2016 {published data only}
- McRae‐Clark AL, Baker NL, Gray KM, Killeen T, Hartwell KJ, Simonian SJ. Vilazodone for cannabis dependence: a randomized, controlled pilot trial. American Journal on Addictions 2016;25(1):69‐75. [DOI: 10.1111/ajad.12324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miranda 2017 {published data only}
- Gray JC, Treloar Padovano H, Wemm SE, Miranda R. Predictors of topiramate tolerability in heavy cannabis‐using adolescents and young adults: a secondary analysis of a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Psychopharmacology 2018;38(2):134‐7. [DOI: 10.1097/JCP.0000000000000843] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Treloar H, Blanchard A, Justus A, Monti PM, Chun T, et al. Topiramate and motivational enhancement therapy for cannabis use among youth: a randomized placebo‐controlled pilot study. Addiction Biology 2017;22(3):779‐90. [DOI: 10.1111/adb.12350] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar H, Blanchard A, Justus A, Monti P, Chun T, Swift R, et al. Effects of topiramate on cannabis use among youth: an initial proof‐of concept trial in the natural environment. Alcoholism: Clinical and Experimental Research 2015;39:265A. [Google Scholar]
Penetar 2012 {published data only}
- Penetar DM, Looby AR, Ryan ET, Maywalt MA, Lukas SE. Bupropion reduces some of the symptoms of marihuana withdrawal in chronic marihuana users: a pilot study. Substance Abuse: Research and Treatment 2012;6:63‐71. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 2017 {published data only}
- Sherman BJ, Baker NL, McRae‐Clark AL. Effect of oxytocin pretreatment on cannabis outcomes in a brief motivational intervention. Psychiatry Research 2017;249:318‐20. [DOI: 10.1016/j.psychres.2017.01.027] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trigo 2018 {published data only}
- Trigo J, Soliman A, Staios G, Quilty L, Fischer B, George T, et al. Sativex associated with behavioral‐relapse prevention strategy as treatment for cannabis dependence, a pilot study. Neuropsychopharmacology 2016;41:S422‐S3. [DOI: 10.1038/npp.2016.241] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo JM, Quilty L, Soliman A, Fischer B, Rehm J, Selby P, et al. Sativex associated with behavioral‐relapse prevention strategy as treatment for cannabis dependence: a pilot randomized clinical trial. Neurotherapeutics 2017;14(3):819. [DOI: 10.1007/s13311-017-0543-x] [DOI] [Google Scholar]
- Trigo JM, Soliman A, Quilty LC, Fischer B, Rehm J, Selby P, et al. Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: a pilot randomized clinical trial. PloS One 2018;13(1):e0190768. [DOI: 10.1371/journal.pone.0190768] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinstein 2014 {published data only}
- Weinstein AM, Miller H, Bluvstein I, Rapoport E, Schreiber S, Bar‐Hamburger R, et al. Treatment of cannabis dependence using escitalopram in combination with cognitive‐behavior therapy: a double‐blind placebo‐controlled study. American Journal of Drug and Alcohol Abuse 2014;40(1):16‐22. [DOI: 10.3109/00952990.2013.819362] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adams 2018 {published data only}
- Adams TA, Arnsten JH, Ning Y, Nahvi S. Feasibility and preliminary effectiveness of varenicline for treating co‐occurring cannabis and tobacco use. Journal of Psychoactive Drugs 2018;50(1):12‐8. [DOI: 10.1080/02791072.2017.1370746] [DOI] [PMC free article] [PubMed] [Google Scholar]
Akerele 2007 {published data only}
- Akerele E, Levin FR. Comparison of olanzapine to risperidone in substance‐abusing individuals with schizophrenia. American Journal on Addictions 2007;16:260‐8. [DOI: 10.1080/10550490701389658] [DOI] [PubMed] [Google Scholar]
Brown 2013 {published data only}
- Brown PC, Budney AJ, Thostenson JD, Stanger C. Initiation of abstinence in adolescents treated for marijuana use disorders. Journal of Substance Abuse Treatment 2013;44(4):384‐90. [EMBASE: AN 2013109720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Budney 2007b {published data only}
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Oral delta‐9‐tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug and Alcohol Dependence 2007;86(1):22‐9. [CENTRAL: CN‐00590656] [DOI] [PubMed] [Google Scholar]
Cooper 2013 {published data only}
- Cooper ZD, Foltin RW, Hart CL, Vosburg SK, Comer SD, Haney M. A human laboratory study investigating the effects of quetiapine on marijuana withdrawal and relapse in daily marijuana smokers. Addiction Biology 2013;18(6):993‐1002. [EMBASE: AN 2013769387] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornelius 1999 {published data only}
- Cornelius JR, Salloum IM, Haskett RF, Ehler JG, Jarrett PJ, Thase ME, et al. Fluoxetine versus placebo for the marijuana use of depressed alcoholics. Addictive Behaviours 1999;24(1):111‐4. [DOI] [PubMed] [Google Scholar]
Cornelius 2008 {published data only}
- Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment‐seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addictive Behaviors 2008;33(11):1500‐5. [DOI: 10.1016/j.addbeh.2008.02.001; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornelius 2015 {published data only}
- Cornelius JR, Douaihy A, Daley D, Chung T, Wesesky MA, Wood DS. Mirtazapine pilot trial in comorbid MDD/SUD: long‐term follow‐up results. Drug and Alcohol Dependence 2015;156:e48‐e9. [Google Scholar]
Findling 2009 {published data only}
- Findling RL, Pagano ME, McNamara NK, Stansbrey RJ, Faber JE, Lingler J, et al. The short‐term safety and efficacy of fluoxetine in depressed adolescents with alcohol and cannabis use disorders: a pilot randomized placebo‐controlled trial. Child and Adolescent Psychiatry and Mental Health 2009;3:11. [CENTRAL: CN‐00754249; DOI: 10.1186/1753-2000-3-11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschtritt ME, Pagano ME, Christian KM, McNamara NK, Stansbrey RJ, Lingler J, et al. Moderators of fluoxetine treatment response for children and adolescents with comorbid depression and substance use disorders. Journal of Substance Abuse Treatment 2012;42(4):366‐72. [DOI: 10.1016/j.jsat.2011.09.010; PsycINFO AN 2011‐27672‐001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geller 1998 {published data only}
- Geller B, Cooper TB, Sun K, Zimermann B, Frazier J, Williams M, et al. Double‐blind and placebo‐controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. Journal of the American Academy of Child and Adolescent Psychiatry 1998;37(2):171‐8. [PsycINFO AN 1997‐39092‐009] [DOI] [PubMed] [Google Scholar]
- Geller B, Cooper TB, Watts HE, Cosby CM, Fox LW. Early findings from a pharmacokinetically designed double‐blind and placebo‐controlled study of lithium for adolescents comorbid with bipolar and substance dependency disorders. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 1992;16(3):281‐99. [EMBASE: AN 1992154297] [DOI] [PubMed] [Google Scholar]
Gillman 2006 {published data only}
- Gillman MA, Harker N, Lichtigfeld FJ. Combined cannabis/methaqualone withdrawal treated with psychotropic analgesic nitrous oxide. International Journal of Neuroscience 2006;116:859‐69. [CENTRAL: CN‐00566721; DOI: 10.1080/00207450600753998] [DOI] [PubMed] [Google Scholar]
Gray 2010 {published data only}
- Gray KM, Watson NL, Carpenter MJ, Larowe SD. N‐Acetylcysteine (NAC) in young marijuana users: an open‐label pilot study. American Journal on Addictions 2010;19(2):187‐9. [DOI: 10.1111/j.1521-0391.2009.00027.x; EMBASE: 2010123976] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haney 2001 {published data only}
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology 2001;155(2):171‐9. [DOI] [PubMed] [Google Scholar]
Haney 2003a {published data only}
- Haney M, Hart CL, Ward AS, Foltin RW. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacology 2003;165(2):157‐65. [DOI] [PubMed] [Google Scholar]
Haney 2003b {published data only}
- Haney M, Bisaga A, Foltin RW. Interaction between naltrexone and oral THC in heavy marijuana smokers. Psychopharmacology 2003;166:77‐85. [DOI: 10.1007/s00213-002-1279-8] [DOI] [PubMed] [Google Scholar]
Haney 2004 {published data only}
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, et al. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology 2004;29:158‐70. [DOI] [PubMed] [Google Scholar]
Haney 2008 {published data only}
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology 2008;197(1):157‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haney 2010 {published data only}
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Cooper ZD, et al. Effects of baclofen and mirtazapine on a laboratory model of marijuana withdrawal and relapse. Psychopharmacology 2010;211(2):233‐44. [CENTRAL: CN‐00760900; DOI: 10.1007/s00213-010-1888-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haney 2013 {published data only}
- Haney M, Bedi G, Cooper Z. Nabilone dose‐response in marijuana smokers: comparison to dronabinol. Neuropsychopharmacology 2010;35:S277. [Google Scholar]
- Haney M, Bedi G, Cooper Z, Vosburg S, Comer S, Foltin R. Nabilone decreases marijuana withdrawal and relapse in the human laboratory. Neuropsychopharmacology 2011;36:S177‐8. [DOI: 10.1038/npp.2011.291; EMBASE: AN 70607410] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology 2013;38(8):1557‐65. [DOI: 10.1038/npp.2013.54; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haney 2015 {published data only}
- Haney M, Ramesh D, Glass A, Pavlicova M, Bedi G, Cooper ZD. Naltrexone maintenance decreases cannabis self‐administration and subjective effects in daily cannabis smokers. Neuropsychopharmacology 2015;40(11):2489‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haney 2016 {published data only}
- Babalonis S, Haney M, Malcolm RJ, Lofwall MR, Votaw VR, Sparenborg S, et al. Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug and Alcohol Dependence 2017;172:9‐13. [DOI: 10.1016/j.drugalcdep.2016.11.030] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babalonis S, Lofwall MR, Nuzzo PA, Elayi C, Malcolm RJ, Haney M, et al. Examination of the behavioral effects of oral cannabidiol alone and in combination with smoked marijuana. Drug and Alcohol Dependence 2015;156:e13. [Google Scholar]
- Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology 2016;41(8):1974‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrmann 2016 {published data only}
- Haney M, Cooper Z, Bedi G, Reed SC, Ramesh D, Foltin RW. Marijuana withdrawal and relapse in the human laboratory: effect of zolpidem alone and in combination with nabilone. Neuropsychopharmacology 2013;38:S508‐9. [DOI: 10.1038/npp.2013.281; EMBASE: AN 71278673] [DOI] [Google Scholar]
- Herrmann ES, Cooper Z, Bedi G, Ramesh D, Reed SC, Comer SD, et al. Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and relapse among non‐treatment‐seeking cannabis users. Drug and Alcohol Dependence 2017;171:e88. [DOI: 10.1016/j.drugalcdep.2016.08.247] [DOI] [Google Scholar]
- Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, et al. Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacology 2016;233(13):2469‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nanjayya 2010 {published data only}
- Nanjayya SB, Shivappa M, Chand PK, Murthy P, Benegal V. Baclofen in cannabis dependence syndrome. Biological Psychiatry 2010;68:e9‐e10. [DOI: 10.1016/j.biopsych.2010.03.033; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Notzon 2018 {published data only}
- Notzon DP, Kelly MA, Choi CJ, Pavlicova M, Mahony AL, Brooks DJ, et al. Open‐label pilot study of injectable naltrexone for cannabis dependence. American Journal of Drug and Alcohol Abuse 2018;44(6):612‐29. [DOI: 10.1080/00952990.2017.1423321] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubio 2006 {published data only}
- Rubio G, Martinez I, Recio A, Ponce G, Lopez‐Munoz F, Alamo C, et al. Risperidone versus zuclopenthixol in the treatment of schizophrenia with substance abuse comorbidity: a long‐term randomized, controlled, crossover study. European Journal of Psychiatry 2006;20(3):133‐46. [Google Scholar]
Schnell 2014 {published data only}
- Schnell T, Koethe D, Krasnianski A, Gairing S, Schnell K, Daumann J, et al. Ziprasidone versus clozapine in the treatment of dually diagnosed (DD) patients with schizophrenia and cannabis use disorders: a randomized study. American Journal on Addictions 2014;23(3):308‐12. [DOI] [PubMed] [Google Scholar]
Sevy 2011 {published data only}
- Sevy S, Robinson DG, Sunday S, Napolitano B, Miller R, McCormack J, et al. Olanzapine vs risperidone in patients with first‐episode schizophrenia and a lifetime history of cannabis use disorders: 16‐week clinical and substance use outcomes. Psychiatry Research 2011;188(3):310‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sugarman 2011 {published data only}
- Sugarman DE, Poling J, Sofuoglu M. The safety of modafinil in combination with oral 9‐tetrahydrocannabinol in humans. Pharmacology, Biochemistry and Behavior 2011;98(1):94‐100. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trigo 2016 {published data only}
- Trigo JM, Lagzdins D, Rehm J, Selby P, Gamaleddin I, Fischer B, et al. Effects of fixed or self‐titrated dosages of Sativex on cannabis withdrawal and cravings. Drug and Alcohol Dependence 2016;161:298‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vandrey 2011 {published data only}
- Vandrey R, Smith MT, McCann UD, Budney AJ, Curran EM. Sleep disturbance and the effects of extended‐release zolpidem during cannabis withdrawal. Drug and Alcohol Dependence 2011;117:38‐44. [DOI: 10.1016/j.drugalcdep.2011.01.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vandrey 2013 {published data only}
- Vandrey R, Stitzer ML, Mintzer MZ, Huestis MA, Murray JA, Lee D. The dose effects of short‐term dronabinol (oral THC) maintenance in daily cannabis users. Drug and Alcohol Dependence 2013;128(1‐2):64‐70. [DOI: 10.1016/j.drugalcdep.2012.08.001; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vandrey 2016 {published data only}
- Vandrey R. Cannabis withdrawal and sleep disturbance: implications for the treatment of cannabis use disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(10):S66. [Google Scholar]
- Vandrey R, Budney AJ, Smith M, Herrmann E, Hampson A, Stitzer ML. A pilot study of zolpidem pharmacotherapy in the treatment of cannabis use disorders. Drug and Alcohol Dependence 2015;146:e10. [Google Scholar]
Van Nimwegen 2008 {published data only}
- Nimwegen LJ, Haan L, Beveren NJ, Helm M, Brink W, Linszen D. Effect of olanzapine and risperidone on subjective well‐being and craving for cannabis in patients with schizophrenia or related disorders: a double‐blind randomized controlled trial. Canadian Journal of Psychiatry 2008;53(6):400‐5. [EMBASE: AN 2008305264] [DOI] [PubMed] [Google Scholar]
Winstock 2009 {published data only}
- Winstock AR, Lea T, Copeland J. Lithium carbonate in the management of cannabis withdrawal in humans: an open‐label study. Journal of Psychopharmacology 2009;23(1):84‐93. [CENTRAL: CN‐00714374; DOI: 10.1177/0269881108089584] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Bhardwaj 2018 {published data only}
- Bhardwaj AK, Allsop DJ, Copeland J, McGregor IS, Dunlop A, Shanahan M, et al. Randomised controlled trial (RCT) of cannabinoid replacement therapy (nabiximols) for the management of treatment‐resistant cannabis dependent patients: a study protocol. BMC Psychiatry 2018;18(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Souza 2015 {published data only}
- D'Souza D. FAAH inhibitor PF‐04457845 in the treatment of cannabis dependence. Neuropsychopharmacology 2017;43:S72. [DOI: 10.1038/npp.2017.263] [DOI] [Google Scholar]
- D'Souza D, Creatura G, Cortes‐Briones J, Thurnauer H, Bluez G, Deaso E, et al. FAAH inhibitor treatment for cannabis dependence. Neuropsychopharmacology 2015;40:S437‐8. [Google Scholar]
NCT00974376 {published data only}
- NCT00974376. Gabapentin treatment of cannabis dependence. clinicaltrials.gov/ct2/show/NCT00974376 (first received 1 September 2009).
NCT01598896 {published data only}
- NCT01598896. Combination of dronabinol and clonidine for cannabis dependence in patients with schizophrenia. clinicaltrials.gov/show/NCT01598896 (first received 11 May 2012).
NCT02044809 {published data only}
- NCT02044809. Cannabidiol: a novel intervention for cannabis use problems?. clinicaltrials.gov/ct2/show/NCT02044809 (first received 22 January 2014).
NCT02579421 {published data only}
- NCT02579421. Hormones and reduction in co‐users of marijuana and nicotine. clinicaltrials.gov/show/NCT02579421 (first received 13 October 2015).
Additional references
Agrawal 2008
Beauvais 2004
Benyamina 2008
- Benyamina A, Lecacheux M, Blecha L, Reynaud M, Lukasiewcz M. Pharmacotherapy and psychotherapy in cannabis withdrawal and dependence. Expert Review of Neurotherapeutics 2008;8(3):479‐91. [DOI] [PubMed] [Google Scholar]
Budney 2003
Budney 2006
Budney 2007a
- Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addiction Science & Clinical Practice 2007; Vol. 4, issue 1:4‐16. [DOI] [PMC free article] [PubMed]
Chung 2008
- Chung T, Martin CS, Cornelius JR, Clark DB. Cannabis withdrawal predicts severity of cannabis involvement at 1‐year follow‐up among treated adolescents. Addiction 2008; Vol. 103, issue 5:787‐99. [DOI] [PMC free article] [PubMed]
Clough 2004
Copeland 2001
Copeland 2014
- Copeland J, Clement N, Swift W. Cannabis use, harms and the management of cannabis use disorder. Neuropsychiatry 2014;4(1):55‐63. [DOI: 10.2217/npy.13.90] [DOI] [Google Scholar]
Copersino 2006
Danovitch 2012
- Danovitch I, Gorelick DA. State of the art treatments for cannabis dependence. Psychiatric Clinics of North America 2012;35(2):309‐26. [DOI: 10.1016/j.psc.2012.03.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
DSM‐5
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. dsm.psychiatryonline.org 2013.
EMCDDA 2004
- European Monitoring Centre for Drugs and Drug Addiction. Cannabis problems in context: understanding the increase in European treatment demands. www.emcdda.europa.eu/html.cfm/index34898EN.html 2004, issue Selected Issue 2:81‐92.
EMCDDA Cannabis Drug Profile
- European Monitoring Centre for Drugs and Drug Addiction. Cannabis drug profile. www.emcdda.europa.eu/publications/drug‐profiles/cannabis Accessed prior to: 13 January 2019.
Gates 2016
- Gates PJ, Sabioni P, Copeland J, Foll B, Gowing L. Psychosocial interventions for cannabis use disorder. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD005336.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorelick 2016
- Gorelick DA. Pharmacological treatment of cannabis‐related disorders: a narrative review. Current Pharmaceutical Design 2016;22(42):6409‐19. [DOI: 10.2174/1381612822666160822150822] [DOI] [PubMed] [Google Scholar]
Gruber 2003
Hall 2001
- Hall W, Degenhardt L, Lynskey M. The Health and Psychological Effects of Cannabis Use. 2nd Edition. Canberra (Australia): Commonwealth of Australia, 2001. [National Drug Strategy Monograph No. 44] [Google Scholar]
Hall 2009
Hart 2005
Hasin 2008
- Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF. Cannabis withdrawal in the United States: results from NESARC. Journal of Clinical Psychiatry 2008; Vol. 69, issue 9:1354‐63. [DOI] [PMC free article] [PubMed]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from handbook.cochrane.org.
Large 2011
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis. Archives of General Psychiatry 2011;68(6):555‐61. [DOI] [PubMed] [Google Scholar]
Lev‐Ran 2013a
- Lev‐Ran S, Strat Y, Imtiaz S, Rehm J, Foll B. Gender differences in prevalence of substance use disorders among individuals with lifetime exposure to substances: results from a large representative sample. American Journal on Addictions 2013;22:7‐13. [DOI] [PubMed] [Google Scholar]
Lev‐Ran 2013b
- Lev‐Ran S, Foll B, McKenzie K, George TP, Rehm J. Cannabis use and cannabis use disorders among individuals with mental illness. Comprehensive Psychiatry 2013;54:589‐98. [DOI] [PubMed] [Google Scholar]
McCann 2015
- McCann DJ, Ramey T, Skolnick P. Outcome measures in medication trials for substance use disorders. Current Treatment Options in Psychiatry 2015;2(2):113‐21. [Google Scholar]
McClure 2012
- McClure EA, Stitzer ML, Vandrey R. Characterizing smoking topography of cannabis in heavy users. Psychopharmacology 2012;220:309‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLoughlin 2014
- McLoughlin BC, Pushpa‐Rajah JA, Gillies D, Rathbone J, Variend H, Kalakouti E, et al. Cannabis and schizophrenia. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD004837.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milin 2008
Minozzi 2010
- Minozzi S, Davoli M, Bargagli M, Amato L, Vecchi S, Perucci C. An overview of systematic reviews on cannabis and psychosis: discussing apparently conflicting results. Drug and Alcohol Review 2010;29(3):304‐17. [DOI] [PubMed] [Google Scholar]
Nordstrom 2007
Peacock 2018
- Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018;113(10):1905‐26. [DOI: 10.1111/add.14234] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
SAMHSA 2008
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Treatment Episode Data Set (TEDS): 1996‐2006. National Admissions to Substance Abuse Treatment Services. www.oas.samhsa.gov/copies.cfm. Rockville (MD): Substance Abuse and Mental Health Services Administration, 2008. [DASIS Series: S‐43, DHHS Publication No. (SMA) 08‐4347] [Google Scholar]
Schűnemann 2013
- Schűnemann H, Brozek J, Guyatt G, Oxman A, editor(s). GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group, 2013. gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed prior to 13 January 2019).
Sherman 2016
- Sherman BJ, McRae‐Clarke AL. Treatment of cannabis use disorder: current science and future outlook. Reviews of Therapeutics 2016;36(5):511‐35. [DOI: 10.1002/phar.1747] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silins 2014
- Silins E, Horwood LJ, Patton GC, Fergusson DM, Olsson CA, Hutchinson DM, et al. Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry 2014;1:286‐96. [DOI: 10.1016/S2215-0366(14)70307-4] [DOI] [PubMed] [Google Scholar]
Silins 2015
- Silins E, Fergusson DM, Patton GC, Horwood LJ, Olsson CA, Hutchinson DM, et al. Adolescent substance use and educational attainment: an integrative data analysis comparing cannabis and alcohol from three Australasian cohorts. Drug and Alcohol Dependence 2015;156:90‐6. [DOI: 10.1016/j.drugalcdep.2015.08.034] [DOI] [PubMed] [Google Scholar]
Solowij 2008
- Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Current Drug Abuse Reviews 2008;1(1):81‐98. [DOI] [PubMed] [Google Scholar]
van der Pol 2014
- Pol P, Liebregts N, Brunt T, Amsterdam J, Graaf R, Korf DJ, et al. Cross‐sectional and prospective relation of cannabis potency, dosing and smoking behaviour with cannabis dependence: an ecological study. Addiction 2014;109:1101‐9. [DOI] [PubMed] [Google Scholar]
Vandrey 2009
- Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we?. CNS Drugs 2009; Vol. 23, issue 7:543‐53. [DOI] [PMC free article] [PubMed]
Volkow 2014
- Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. New England Journal of Medicine 2014;370:2219‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walther 2016
- Walther L, Gantner A, Heinz A, Majić T. Evidence‐based treatment options in cannabis dependency. Deutsches Ärzteblatt International 2016;113(39):653‐9. [DOI: 10.3238/arztebl.2016.0653] [DOI] [PMC free article] [PubMed] [Google Scholar]
World Drug Report 2017
- United Nations Office on Drugs and Crime. World Drug Report 2017. www.unodc.org/wdr2017 (accessed prior to 13 January 2019). [eISBN 978‐92‐1‐060623‐3]
References to other published versions of this review
Marshall 2014
- Marshall K, Gowing L, Ali R, Foll B. Pharmacotherapies for cannabis dependence. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD008940.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


