Abstract
Objective:
A strong relation between cognition and mobility has been identified in aging, supporting a role for enhancement mobility through cognitive-based interventions. However, a critical evaluation of the consistency of treatment effects of cognitive-based interventions is currently lacking. The objective of this study was 2-fold: (1) to review the existing literature on cognitive-based interventions aimed at improving mobility in older adults and (2) to assess the clinical effectiveness of cognitive interventions on gait performance.
Design:
A systematic review of randomized controlled trials (RCT) of cognitive training interventions for improving simple (normal walking) and complex (dual task walking) gait was conducted in February 2018.
Setting and Participants:
Older adults without major cognitive, psychiatric, neurologic, and/or sensory impairments were included.
Measures:
Random effect meta-analyses and a subsequent meta-regression were performed to generate overall cognitive intervention effects on single- and dual-task walking conditions.
Results:
Ten RCTs met inclusion criteria, with a total of 351 participants included in this meta-analysis. Cognitive training interventions revealed a small effect of intervention on complex gait [effect size (ES) = 0.47, 95% confidence interval (CI) 0.13 to 0.81, P = .007, I2 = 15.85%], but not simple gait (ES = 0.35, 95% CI e0.01 to 0.71, P = .057, I2 = 57.32%). Moreover, a meta-regression analysis revealed that intervention duration, training frequency, total number of sessions, and total minutes spent in intervention were not significant predictors of improvement in dual-task walking speed, though there was a suggestive trend toward a negative association between dual-task walking speed improvements and individual training session duration (P = .067).
Conclusions/Implications:
This meta-analysis provides support for the fact that cognitive training interventions can improve mobility-related outcomes, especially during challenging walking conditions requiring higher-order executive functions. Additional evidence from well-designed large-scale randomized clinical trials is warranted to confirm the observed effects.
Keywords: Gait control, computerized cognitive training, fall risk, elderly, neurophysiological plasticity
Mobility-related impairments are common in older adults and result in poor quality of life, increased morbidity, and mortality rates.1,2 Although exercise interventions improve mobility, clinicians are faced with a myriad of barriers implementing such programs. For instance, older adults often have limited access and few opportunities to engage in physical exercise programs. A 50% dropout rate in the first 3 to 6 months of commencement of physical exercise programs has been reported.3 Hence, exploration of alternate or complementary approaches to physical exercise to improve mobility is warranted.
A growing body of research reveals involvement of higher-order cognitive processes in demanding postural and gait situations like standing and memorizing or walking while performing additional cognitive tasks.4,5 These mental processes that aid in the allocation of attention among simultaneous tasks (divided attention), adaptation to changing situations, and the inhibition of irrelevant information or distractors (in working memory) are collectively known as executive functions (EFs).6 EFs are critically dependent on the prefrontal cortex, which is vulnerable to both normal and diseased aging.6 Strong interrelation between EFs (such as divided attention) and mobility has been proposed in multiple expert reviews,7,8 suggesting novel opportunities to potentially enhance mobility-related outcomes via cognitive-based approaches.
The ACTIVE study was the first large-scale cognitive intervention designed to ameliorate cognitive and functional disabilities in 2,832 healthy older adults.9 Findings from the ACTIVE study revealed significantly improved long-term cognitive performance, accompanied by enhanced instrumental activities of daily living, health-related quality of life, and driving capabilities.10 In a small pilot study, Verghese and colleagues8 reported far transfer effects of cognitive training to a distal untrained domain such as mobility. They demonstrated enhanced walking performance of sedentary seniors after 8 weeks of computerized training that specifically targeted improvement of EFs. These results were independently replicated by other groups in community-dwelling older adults,11 healthy older adults during prolonged bed-rest,12 and in patients with Parkinson’s disease.13
Although these results are promising, to date no critical evaluation of such cognitive-based approaches on mobility-related outcomes has been performed. A systematic evaluation of this approach could prove useful in designing future intervention studies8 to test a new low-risk and accessible treatment opportunity serving as an alternative or even supplemental strategy for those older adults who do not or cannot engage in physical exercise regimens as a result of physical, motivational, medical, or socioeconomic limitations. Thus, the objective of the current study was to systematically review the existent literature in an effort to ascertain the impact and clinical effectiveness of cognitive training approaches on both simple and complex gait performance in the older adult population.
Methods
Search Strategy
This systematic review was performed by searching PubMed/MEDLINE (NLM), Embase, and Web of Science databases. Manuscripts written in English language and including humans with specific deviations of keyword combinations comprising “cognitive training,” “mobility,” and “older adults” were included (for details, see Table A1 in appendix). Database searches were supplemented by Google Scholar database and review of authors’ personal files with additional screening of the reference list of each included article. Titles and abstracts that did not meet inclusion criteria were excluded. Remaining full texts were screened by 2 independent reviewers (U.M. and J.R.M.), with disagreements resolved through discussion with an expert (J.V.), when necessary. In sum, only peer-reviewed randomized controlled trials that met the above listed inclusion criteria and that were accessible up until February 1, 2018 were included in this review.
Selection Criteria
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines as well as Problem/population, Intervention, Comparison, and Outcome (PICO) framework were followed for the literature search strategy:
Population: healthy older adults
Intervention: cognitive-based approaches
Comparison: experimental vs control group
Outcome measures: gait performance
Only randomized clinical trials (RCTs) were included in this systematic review and subsequent meta-analysis. We further restricted selection to trials that only assessed the effect of cognitive-based approaches on gait in older adults (mean participant age ≥60 years) who lacked major cognitive, psychiatric, neurologic, and/or sensory impairments. That is, trials addressing a combination of cognitive-motor and/or physical exercise interventions on mobility were not included. Studies included in this systematic review also needed to employ a control group, either active (eg, educative training, sham/nonprogressive cognitive training) or passive (wait-list or no-contact). Observational or quasi-experimental studies, which provide a lower level of evidence, were not included in this review.
Our primary outcome relied on gait-related assessments, such as gait speed (ie, velocity) in single- and/or dual-task conditions given the above-referenced link between high-order cognition and gait control in older adults.7,14 Similar to recent aging meta-analyses assessing the impact of cognitive interventions,15,16 our objective was to pool multiple outcome measures and obtain the most homogeneous data as possible. In studies where several dual-task outcomes were reported on the same participants, the most complex dual-task (in terms of cognitive demands) was chosen for the purpose of this meta-analysis.
Statistical Analysis
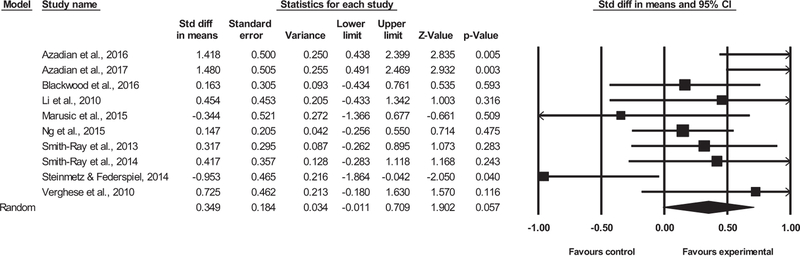
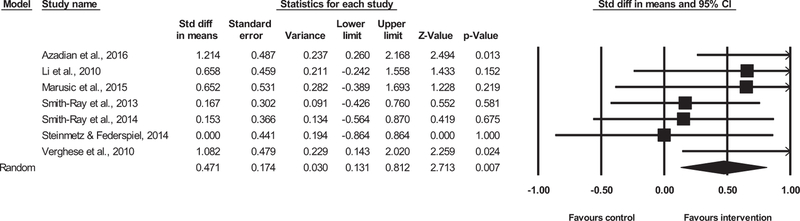
Coding of outcome measures into mobility-related domains was done by 2 reviewers (U.M. and J.R.M.) and approved by an expert (J.V.). In most cases data were extracted from trials reporting baseline and follow-up means, standard deviations, and total number of included participants; however, results from Smith-Ray, Hughes, Prohaska, Little, Jurivich, and Hedeker17 were reported as postintervention mean change for control and intervention groups. Data were entered into Comprehensive Meta-analysis software (Version 3.0, Biostat Inc, Englewood, NJ). Because different RCTs investigated diverse outcome measures for “mobility-related performance,” the unified measure of treatment effect was the standardized mean difference in gait velocity for single and dual-task conditions, which was obtained through the inverse variance random-effects method. However, additional sensitivity analysis was conducted using both fixed and random effects, as well as by excluding each individual study from the model. Change in standard deviations was calculated with an actual correlation between the measures at baseline and posttraining. The following established criteria were used to interpret the magnitude of cognitive-based interventions for mobility-related improvements: trivial (<0.20), small (0.21–0.60), moderate (0.61–1.20), large (1.21–2.00), very large (2.01–4.00), and extremely large (>4.00) changes.18 Heterogeneity across studies was assessed using the I2 statistics, which is a measure of inconsistency used to quantify between-study variability. Values of 25%, 50%, and 75% are recommended to represent low, moderate, and high statistical heterogeneity, respectively.19 Methodological quality within studies was assessed with Physiotherapy Evidence Database Rating Scale. Overall estimates of differences between trial treatments are presented in forest plots (Figures 2 and 3) and summarized in Table 1. Additionally, a meta-regression analysis was performed in order to detect potential predictors of observed effect when the heterogeneity of studies was considered high.26
Fig. 2.
Single-task walking outcomes.
Fig. 3.
Dual-task walking outcomes.
Table 1.
Effect of Various Cognitive Training Approaches on Mobility-Related Outcomes
| Study | Characteristics of Included Group, Study Design, Total N (Intervention/Control), Mean Age, % Female, PEDro Score | Cognitive Training Approach: Type and Length | Targeted Functions With Cognitive Training Approach vs Control Group | Outcome Measures for Both Groups and Measurement Settings | Impact of Cognitive Training Approaches on Mobility-Related Measures |
|---|---|---|---|---|---|
| Azadian et al20 (2016) | Older adults (mean BBS score = 44.5, mean MMSE score = 24.5); RCT; N = 20 (I: 10/C: 10); mean age = 74 y; 0% female; PEDro score = 9 |
Computerized cognitive training (designed by Sina Institute of Psychology, Tehran, Iran); 24 sessions, 8 weeks, 3×/wk, 45 min each, total ~1080 min of training |
Executive functions (working memory, inhibition, speed of processing); control group: pre-post measurements only |
ST and DT (counting backwards) gait speed; once 10-m walk. |
↑ ST gait speed; ↑ DT gait speed |
| Azadian et al21 (2017) | Older adults (mean BBS score = 44.9, mean MMSE score = 24.5); RCT; N = 20 (I: 10/C: 10); mean age = 74 y; 0% female; PEDro score = 9 |
Computerized cognitive training (designed by Sina Institute of Psychology, Tehran, Iran); 18 sessions, 6 weeks, 3×/wk, 45 min each, total ~810 min of training |
Working memory (visual, auditory and stabilized tasks); control group: pre-post measurements only |
ST gait speed; six 12-m walk with 2-min rest between conditions |
↑ ST gait speed |
| Blackwood et al22 (2016) | Community-dwelling older adults; Quasi-RCT; N = 44 (I: 19/C: 25); mean age = 75 y; 73% female; PEDro score = 8 |
Home-based computerized cognitive training (Lumosity); 18 sessions, 6 wk; 3×/wk, 20–25 min each; total ~405 min of training |
Executive function (set shifting, visual spatial ability and attention, recall/memory); control group: pre-post measurements only |
ST gait speed; twice 3.048-m walk with 1.524 m of space on each side for acceleration/deceleration |
= ST gait speed |
| Li et al23 (2010) | Healthy older adults (community- dwelling); RCT, N = 20 (I: 10/C: 10); mean age = 76 y; 65% female; PEDro score = 9 |
Computerized dual-task training; 5 sessions, 2.5 wk, 2×/wk, 60 min each, total ~300 min of training |
Dual-task color and letter discrimination | ST and DT (2-back) gait speed; once 12.2-m walk with 180° turn at 6.1 m |
=ST gait speed; =DT gait speed |
| Marusic et al12 (2015) | Healthy older adults during bed rest; RCT; N = 15 (I: 7/C: 8); 55–65 y (mean age = 60 y); 0% female; PEDro score = 7 |
Computerized cognitive training with spatial navigation task of increasing difficulty (Epic Games, Inc); 12 sessions, 2 wk, 6×/wk, 45 min each, total ~540 min of training |
Executive functions, working memory, navigational skills, attention; control group: watching documentaries at the same time and for the same amount of time |
ST and DT (serial threes) gait speed and swing time variability; 1-minute walk in each condition, each 10 m 180° turn |
=ST gait speed; =DT gait speed; ↓ DTC gait speed; =swing time variability; =DTC swing time variability |
| Ng et al24 (2015) | Community-living prefrail and frail old adults; RCT; N = 95 (I: 47/C: 48); mean age = 70 y; 66% female; PEDro score = 9 |
Cognitive training; 12 sessions, 12 wk, 1×/wk, 120 min each, total ~1440 min of training |
Short-term memory, attention, information-processing skills, reasoning and problem solving abilities; control group: usual care control group (no specific activities, placebo nutritional supplements) |
ST 6-m fast gait speed test; average of 2 times 6-m walk |
=ST gait speed |
| Smith-Ray et al17(2013) | Older adults from independent living facilities; RCT; N = 51 (I: 27/C: 24); mean age = 82 y; 77% female; PEDro score = 7 |
Computerized cognitive training (Insight computer software by Posit Science); 30 sessions, 10 wk, 3×/wk, 60 min each, total ~1800 min of training |
Executive functions, working memory, speed of processing, and inhibition; control group: pre-post measurements only (received materials on falls prevention) |
ST and DT (visuospatial task, Brooks Matrices) gait speed; average of 3 times 10-m walk |
=ST gait speed; =DT gait speed |
| Smith-Ray et al11 (2014) | Community-dwelling black adults with history of falls; RCT; N = 45 (I: 23/C: 22); mean age = 73 y); 91% female; PEDro score = 7 |
Computerized cognitive training (Insight computer software by Posit Science); 20 sessions, 10 wk, 2×/wk, 60 min each, total ~1200 min of training |
Executive functions with visuospatial working memory, speed of processing, and inhibition; control group: pre-post measurements only |
ST and DT (visuospatial task, Brooks Matrices) gait speed; average of 3 times 10-m walk |
↑ ST gait speed; =DT gait speed |
| Steinmetzand Federspiel25 (2014) | Frail old nursing home residents; Quasi-RCT; N = 21(I:12/C:9); mean age=85 y; 81% female; PEDro score = 5 |
Cognitive training composed of basic theory learning, group and individual exercises, cognitive games and home assignments; 12 sessions, 6 wk, 2×/wk, 90 min each, total ~1080 min of training |
Attentional capacities, working memory, the ability to plan, verbal fluency, learning and memory; control group: pre-post measurements only |
ST and DT (serial twos) gait speed and swing time variability; in each condition 5.18-m walk with 2 m of space on each side for acceleration/deceleration |
=ST gait speed; =DT gait speed; ↓ DTC gait speed; =DTC swing time variability |
| Vergheseeta18 (2010) | Sedentary seniors; RCT; N = 20 (I: 10/C: 10); meanage = 79 y; 63% female; PEDro score = 10 |
Computerized brain fitness program (Mindfit; CogniFit Inc, Yokneam, Israel); 24 sessions, 8 wk, 3×/wk, 4560 min each, total ~1260 min of training |
Attention and executive functions; control group: pre-post measurements only (wait-list control) |
ST and DT (reciting alternate letters of the alphabet) gait speed; 7-m walk with 0.91 m of space on each side for acceleration/deceleration | ↑ ST gait speed; ↑ DT gait speed |
BBS, Berg Balance Scale; C, control; DT, dual-task; DTC, dual-task costs; I, intervention; MMSE, Mini-Mental State Examination; PEDro score, Physiotherapy Evidence Database scale; RCT, randomized controlled trial; ST, single-task.
Results
Study Selection
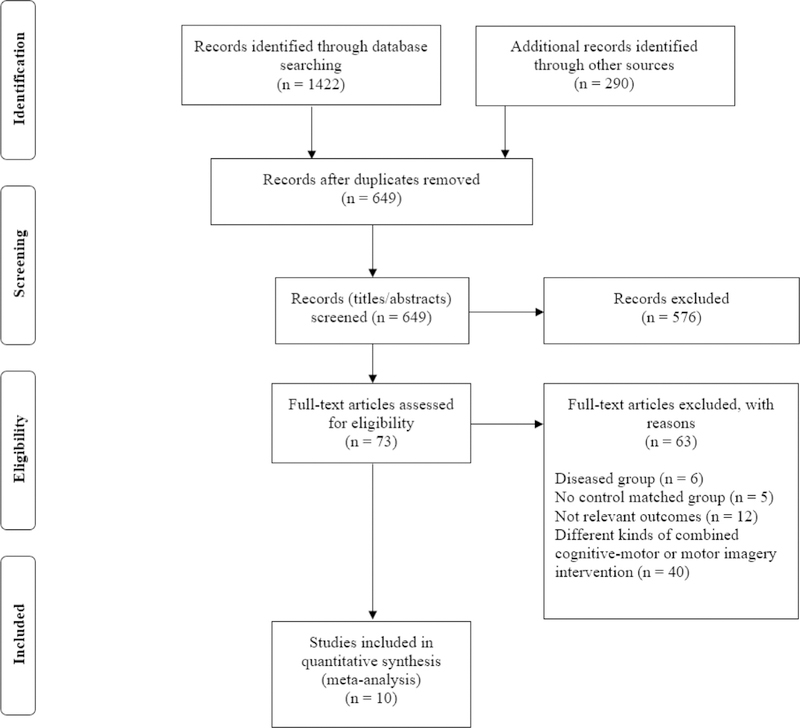
The initial search yielded a total of 1712 results, which was subsequently reduced to 649 after duplicate publications were removed. Further screening resulted in elimination of 576 studies that did not meet our inclusion criteria based on review of study title and/or abstract. Of the remaining 73 studies, comprehensive review of the full text revealed that 63 did not meet inclusion criteria for the following reasons: (1) inclusion of diseased older adults (n = 6), (2) no control group (n = 5), (3) assessment of irrelevant mobility-related outcome measures (n = 12), and/or (4) no pure cognitive training group (n = 40). Thus, a total of 10 trials were included in this systematic review and meta-analysis. Additional explanation of the employed protocols was requested from all 8 authors (there were 2 trials each with the same first authors Smith-Ray et al11,17 and Azadian et al20,21), of which 7 provided feedback. Details of the study selection process are presented in Figure 1.
Fig. 1.
PRISMA flow diagram.
Characteristics of Included Studies
Overall, the 10 trials included in this review encompassed 351 participantsd175 in the intervention group and 176 in the control group. Participants’ mean chronological age ranged from 60 to 85 years, and 62% were women. Five studies were conducted in North America, 2 in Europe, and 3 in Asia. The average methodological quality of included studies, assessed with Physiotherapy Evidence Database Rating Scale score, was high (8/11), with the range of study ranking from fair (5)25 to high (10) quality.8
Cognitive-based intervention design varied across the included studies (see Table 1). The majority used a computerized version of cognitive training (8/10), as well as administered supervised cognitive training with the exception of Blackwood et al,22 who employed a home-based unsupervised cognitive training approach. Training session length varied from 20 to 120 minutes for 1 to 6 training sessions per week, yielding a total training time range of 5 to 30 hours for the entire intervention duration. Included studies had well-matched control groups, which were randomly assigned as wait-list,8 a measurement-only control condition,11,17,20–25 or a non-stimulating condition (watching documentaries) for the same duration of time as the intervention group.12
Characteristics of 10 RCTs that assessed single-task walking outcomes included an average intervention duration of 7.1 weeks (range 2–12 weeks), with 60.0 minutes per training session (range 22.5–120) and 2.8 sessions per week (range 1–6 sessions/wk). Average number of sessions included in the entire study was 17.5 (range 5–30 sessions), with a mean total duration of the entire intervention of 991.5 minutes (range 300–1800 minutes). Similarly, the 7 RCTs that assessed dualtask walking outcomes had on average a study duration of 6.6 weeks (range 2–10 weeks), with 58.9 minutes per training session (range 45–90 minutes) and 3 sessions per week (range 2–6 sessions/wk). The average number of sessions included in the entire study was 18.1 (range 5–30 sessions), with a total duration of 1037.1 minutes (range 300–1800 minutes).
Meta-Analysis Outcomes (Domain-specific Efficacy)
Single-task walking outcomes
All 10 trials reported single-task gait speed-related outcomes. The combined effect size (ES) was small [ES = 0.35, 95% confidence interval (CI) —0.01 to 0.71, P = .057, I2 = 57.32%; see Figure 2]. The magnitude of effect derived from random and fixed method varied significantly. The magnitude of effect assessed by random method was insignificant (ES = 0.35, 95% CI –0.01 to 0.71, P = .057); however, the fixed method revealed a significantly small effect size (ES = 0.30, 95% CI –0.08 to 0.52, P = .008). Sensitivity analysis revealed that magnitude of effect was modified after each study was excluded, ranking from small (ES = 0.25, 95% CI –0.08 to 0.60, P = .139) to small (ES = 0.44, 95% CI 0.13 to 0.76, P = .006). The results of the funnel plot asymmetry indicated no publication bias for single-task walking outcomes (P = .466; see Figure A1 in appendix).
Because the significance of the observed effect considerably varied between the random and fixed methods, we further performed a meta-regression analysis in order to detect possible predictors of this observed effect. Table A2 (in appendix) shows that none of the selected variables were significant predictors (P ≥ .098).
Dual-Task Walking Outcomes
Seven of 10 trials reported dual-task gait speed-related outcomes. The combined effect size was small (ES= 0.47, 95% CI 0.13–0.81, P = .007, I2 15.85%; Figure 3). There were only small differences in magnitude of effect derived from both random (ES = 0.47) and fixed method (ES = 0.45). Sensitivity analysis revealed that magnitude of effect was not modified after each study was excluded, and it remained small (ES = 0.36, 95% CI 0.04–0.69, P =.029; to ES = 0.56, 95% CI 0.20–0.92, P = .002). The results of funnel plot asymmetry indicated publication bias for dual-task walking outcomes (P = .086; see Figure A2 in appendix).
As reported above, all included studies gave a small effect size (ES = 0.47, 95% CI 0.13–0.81, P = .007, I2 15.85%; Figure 3). However, a meta-analysis of 2 studies8,20 with similar length of single training sessions (~45 minutes/session) produced the largest (interpreted as moderate) effect (ES = 1.15, 95% CI 0.48–1.82, P = .001), on dual-task walking speed parameter.
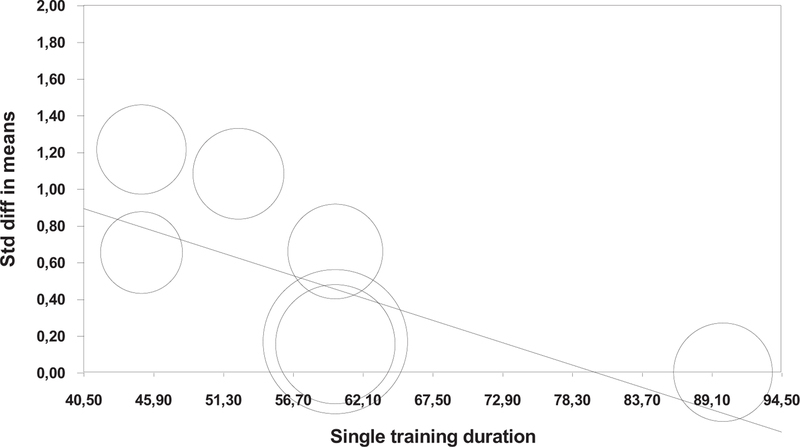
An additional meta-regression analysis revealed a negative trend (P =.067) of relationship between improvements in dual-task walking speed and duration of the individual training session (Figure A3 in appendix). In other words, the shorter the individual training sessions, the greater the improvement in dual-task walking speed at the end of the intervention. The 4 other selected predictors were nonsignificant (P = .304): study duration in weeks (P = .404), training frequency, trainings/wk (P = .411), total number of sessions (P = .865), and total minutes spent in whole study (P =.304). Table A2 (in appendix) shows the meta-regression results for all the subcategories.
Discussion
The present systematic review and meta-analysis provides evidence that cognitive-based interventions can improve mobility-related outcomes in older adults. Because of several limitations in implementation of physical exercise regimens in older adults, alternate or supplementary intervention strategies to improve mobility such as cognitive intervention needed to be identified. Our results show that the cognitive training-related effects were small and statistically significant only for complex walking conditions, such as dual-task walking. The present meta-analysis showed a trend for single-task walking conditions. The main findings of this meta-analysis therefore reveal that cognitive training (nonphysical practice) can improve physical performance in older adults during complex walking conditions (eg, walking while talking or walking while subtracting numbers). Previous studies have linked impairments in dual-task performance to risk of developing several adverse health outcomes such as falls, frailty, disability, and death in older adults,27–29 indicating that improvements in complex walking performance is a clinically relevant outcome.
Longer periods of cognitive training have been postulated to have larger training gains as compared to those with short-term cognitive training exposure.25 Yet, the current meta-analysis failed to determine significant predictors of improvement in dual-task performance following cognitive training, including length of single training session, study duration, training frequency, total number of sessions, as well as total study training duration. However, there was an observed trend for greater dual-task walking speed improvements and shorter length of individual cognitive training session, suggesting the potential implementation of shorter (eg, 45 minutes or less per session8,20) sessions when dealing with the older adult population, which may also help improve acceptability in clinical practice.
This quantitative evaluation of cognitive training-related improvements in gait performance supports far transfer of cognitive training to distal untrained mobility processes. Most studies that evaluated different cognitive training programs in the older adult population have reported significant improvements in cognitive functions directly associated with the specifically targeted cognitive areas.9,30–32 Transfer of learning to nonspecifically trained testing situations has been conceptualized as a form of neuroplasticity in overlapping brain areas or specific networks that are important for the untrained task.30,33 The generalization of cognitive training effects on other nonspecifically trained functions or improved everyday life of the elderly should be a priority to optimize daily functioning, especially in complex cognitive-motor (dual- and multitask) situations, namely, in dual-task conditions where there is a constant interplay between attentional resources, which can cause a deterioration on either task (walking/postural or cognitive). Optimized walking performance, especially under more complex/dual-task conditions after cognitive training, has been associated with lower fall risk.34 Cognitive training programs could therefore serve as a promising approach to fall prevention especially for those participants who are reluctant to complete a physical activity intervention.12,17
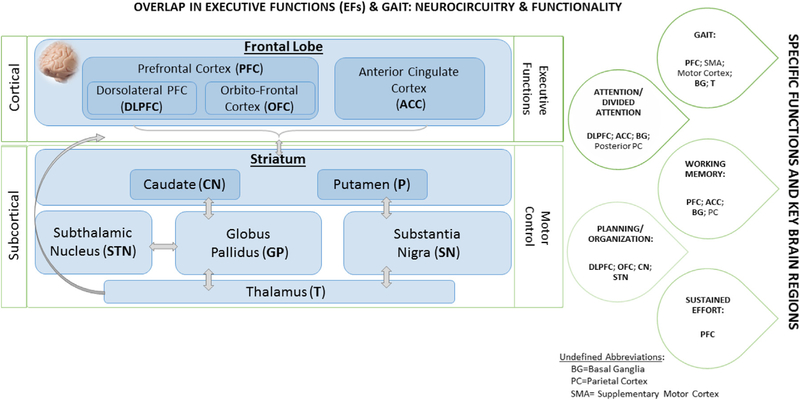
Our meta-analytic findings are in agreement with previous scientific attempts that report a positive transfer of cognitive-based interventions to a distal untrained domain, such as mobility.8,11,12,17,21–24 It is hypothesized that specialized cognitive training, with a strong emphasis on executive control training, will provide beneficial effects on gait and executive functions through its impact on this frontosubcortical circuit.35 Frontosubcortical circuits link specific regions of the frontal cortex (namely, prefrontal cortex) to the basal ganglia (motor control).36 That is, given its plastic properties, the brain is able to continuously change in response to engagement in repeated sensory, motor, and/or cognitive activities.37 Alterations in prefrontal activation patterns have been documented after relatively short-training periods (5 hours over 2–3 weeks) in both healthy young and older adults that received dual-task (DT) training compared to controls.38,39 Figure 4 depicts potential pathways, circuits, and brain substrates involved in both cognitive (executive functions) and mobility (gait) processes and therefore details the potential underlying mechanisms involved in gait-related improvements after cognitive training with an emphasis on executive function training in older adults.
Fig. 4.
Proposed model of cognitive trainingerelated gait improvements.
Some potential limitations of this systematic review and meta-analysis need to be considered. First, relatively nonhomogeneous cognitive approaches were used in terms of duration and frequency, restricting our ability to evaluate a dose-response relationship. Second, although we attempted to include only healthy older adults, it is well known that the vast majority of the elderly population manifest aspects of frailty. Some of the studies included in this meta-analysis reported inclusion of “healthy older adults” who were sedentary or community-dwelling individuals, which may not be considered as “purely” healthy older adults. Further research should focus on a meta-analytic evaluation of the far transfer of cognitive training on mobility domain in frail and symptomatic seniors. In the study by Smith-Ray and colleagues11 a subanalysis of only slow-walkers (<1 m/s) revealed an even stronger training effect as compared with their whole study population. The other 2 included studies showed that the highest effect size also included sedentary seniors8 or older adults with balance impairments.20 Finally, because of insufficient data on other important gait variables such as gait variability, we were not able to evaluate the effects of cognitive training on these specific gait parameters, but future investigations should include broader measures of gait performance.
In conclusion, the present article provides evidence that cognitive training interventions can improve mobility-related outcomes, especially those that require higher-order cognitive abilities (such as walking while talking). To date, the literature suggests that cognitive remediation programs targeting improvements in mobility for older adults are quite promising; however, lower sample sizes and heterogeneity of available trials are still limiting factors to provide unequivocal conclusions.
Acknowledgments
We would like to thank all contacted authors who provided feedback and row data for the calculation of effect sizes. An additional thank-you to Armin H. Paravlic for the various methodological suggestions.
Appendix
Table A1.
Search Strategy of the Systematic Review
| Item | Details |
|---|---|
| Keywords used | ((“cognitive training” OR “cognitive stimulation” OR “cognitive simulation” OR “cognitive rehabilitation” OR “cognitive remediation” OR “cognitive enhancement” OR “cognitive restructuring” OR “cognitive activity” OR “mental training” OR “mental stimulation” OR “mental simulation” OR “mental rehabilitation” OR “mental remediation” OR “psychological training” OR “psychological stimulation” OR “psychological simulation” OR “psychological rehabilitation” OR “psychological remediation” OR “brain training” OR “attention training” OR “reasoning training” OR “neurocognitive training” OR “neurocognitive intervention” OR “mental skills training” OR “memory training” OR “videogame” OR “video game” OR “computer game” OR “virtual reality”) AND (mobility OR gait OR walking OR locomotion OR locomotor OR locomotory OR balance OR postural OR “postural control” OR posture) AND (“older adults” OR “older adult” OR “elderly” OR “aging” OR “ageing” OR “elder adults” OR “elders” OR “old” OR “old-olds”)) |
| Databases | PubMed/MEDLINE (NLM), Embase, Web of Science |
| Time filter | Accessible/published articles until February 1, 2018 |
| Language | English |
Table A2.
Meta-regression for Cognitive Training Variables to Predict Mobility-Related Outcomes
| Coefficient | Standard Error | 95% Lower CI | 95% Upper CI | Z Value | P Value | |
|---|---|---|---|---|---|---|
| Single-task walking outcomes | ||||||
| Study duration, wk | 0.0042 | 0.0357 | −0.0657 | 0.0740 | 0.1164 | .907 |
| Single training duration, min | −0.0047 | 0.0032 | −0.0110 | 0.0017 | −1.4465 | .148 |
| Training frequency, sessions/wk | 0.0372 | 0.0958 | −0.1505 | 0.2250 | 0.3888 | .697 |
| Number of sessions in the whole study, n | 0.0263 | 0.0159 | −0.0049 | 0.0575 | 1.6524 | .098 |
| Total training duration in the whole study, min | −0.0001 | 0.0002 | −0.0005 | 0.0005 | −0.0570 | .955 |
| Dual-task walking outcomes | ||||||
| Study duration, wk | −0.0443 | 0.0531 | −0.1484 | 0.0598 | −0.8340 | .404 |
| Single training duration, min | −0.0225 | 0.0123 | −0.0466 | 0.0015 | −1.8340 | .067 |
| Training frequency, sessions/wk | 0.1185 | 0.1441 | −0.1639 | 0.4010 | 0.8226 | .411 |
| Number of sessions in the whole study, n | −0.0032 | 0.0187 | −0.0399 | 0.0335 | −0.1697 | .865 |
| Total training duration in the whole study, min | −0.0003 | 0.0003 | −0.0010 | 0.0003 | −1.0282 | .304 |
Bold values indicate a nonsignificant trend.
Fig. A1.
Funnel plot of standard difference in means vs standard error for single-task walking outcomes.
Fig. A2.
Funnel plot of standard difference in means vs standard error for dual-task walking outcomes.
Fig. A3.
Regression of single training duration on standard difference in means.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc 2000;48:493–498. [DOI] [PubMed] [Google Scholar]
- 2.Topinkova E, Aging disability and frailty. Ann Nutr Metab 2008;52(suppl 1): 6–11. [DOI] [PubMed] [Google Scholar]
- 3.Allen K, Morey MC. Physical activity and adherence. In: Bosworth H, editor. Improving Patient Treatment Adherence: A Clinician’s Guide New York, NY: Springer New York; 2010. [Google Scholar]
- 4.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord 2008;23:329–342; quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boisgontier MP, Beets IA, Duysens J, et al. Age-related differences in attentional cost associated with postural dual tasks: Increased recruitment of generic cognitive resources in older adults. Neurosci Biobehav Rev 2013;37: 1824–1837. [DOI] [PubMed] [Google Scholar]
- 6.Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms Hoboken, NJ: Taylor and Francis; 2007. p. 3–20. [Google Scholar]
- 7.Montero-Odasso M, Verghese J, Beauchet O, et al. Gait and cognition: A complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc 2012;60:2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verghese J, Mahoney J, Ambrose AF, et al. Effect of cognitive remediation on gait in sedentary seniors. J Gerontol A Biol Sci Med Sci 2010;65:1338–1343. [DOI] [PubMed] [Google Scholar]
- 9.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA 2006;296: 2805–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tennstedt SL, Unverzagt FW. The ACTIVE Study: Study Overview and Major Findings Los Angeles, CA: Sage Publications; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith-Ray RL, Makowski-Woidan B, Hughes SL. A randomized trial to measure the impact of a community-based cognitive training intervention on balance and gait in cognitively intact black older adults. Health Educ Behav 2014;41(1 suppl):62S–69S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marusic U, Kavcic V, Giordani B, et al. Computerized spatial navigation training during 14 days of bed rest in healthy older adult men: Effect on gait performance. Psychol Aging 2015;30:334–340. [DOI] [PubMed] [Google Scholar]
- 13.Milman U, Atias H, Weiss A, et al. Can cognitive remediation improve mobility in patients with Parkinson’s disease? Findings from a 12 week pilot study. J Parkinsons Dis 2014;4:37–44. [DOI] [PubMed] [Google Scholar]
- 14.Segev-Jacubovski O, Herman T, Yogev-Seligmann G, et al. The interplay between gait, falls and cognition: Can cognitive therapy reduce fall risk? Expert Rev Neurother 2011;11:1057–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly ME, Loughrey D, Lawlor BA, et al. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: A systematic review and meta-analysis. Ageing Res Rev 2014;15: 28–43. [DOI] [PubMed] [Google Scholar]
- 16.Martin M, Clare L, Altgassen AM, et al. Cognition-based interventions for older people and people with mild cognitive impairment. Cochrane Database Syst Rev; 2011:CD006220. [DOI] [PubMed]
- 17.Smith-Ray RL, Hughes SL, Prohaska TR, et al. Impact of cognitive training on balance and gait in older adults. J Gerontol B Psychol Sci Soc Sci 2013;70: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins WG, Marshall SW, Batterham AM, et al. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 2009;41: 3–13. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azadian E, Torbati HRT, Kakhki ARS, et al. The effect of dual task and executive training on pattern of gait in older adults with balance impairment: A randomized controlled trial. Arch Gerontol Geriatr 2016;62:83–89. [DOI] [PubMed] [Google Scholar]
- 21.Azadian E, Majlesi M, Jafarnezhadgero AA. The effect of working memory intervention on the gait patterns of the elderly. J Bodywork Mov Ther; 2017. [in press]. [DOI] [PubMed]
- 22.Blackwood J, Shubert T, Fogarty K, et al. The impact of a home-based computerized cognitive training intervention on fall risk measure performance in community dwelling older adults, a pilot study. J Nutr Health Aging 2016;20:138–145. [DOI] [PubMed] [Google Scholar]
- 23.Li KZ, Roudaia E, Lussier M, et al. Benefits of cognitive dual-task training on balance performance in healthy older adults. J Gerontol A Biol Sci Med Sci 2010;65:1344–1352. [DOI] [PubMed] [Google Scholar]
- 24.Ng TP, Feng L, Nyunt MS, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: A randomized controlled trial. Am J Med 2015;128 1225.e1–1236.e1. [DOI] [PubMed] [Google Scholar]
- 25.Steinmetz JP, Federspiel C. The effects of cognitive training on gait speed and stride variability in old adults: Findings from a pilot study. Aging Clin Exp Res 2014;26:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borde R, Hortobágyi T, Granacher U. Doseeresponse relationships of resistance training in healthy old adults: A systematic review and meta-analysis. Sports Med 2015;45:1693–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verghese J, Holtzer R, Lipton RB, et al. Mobility stress test approach to predicting frailty, disability, and mortality in high functioning older adults. J Am Geriatr Soc 2012;60:1901–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montero-Odasso M, Muir SW, Speechley M. Dual-task complexity affects gait in people with mild cognitive impairment: The interplay between gait variability, dual tasking, and risk of falls. Arch Phys Med Rehabil 2012;93: 293–299. [DOI] [PubMed] [Google Scholar]
- 29.Beauchet O, Annweiler C, Allali G, et al. Recurrent falls and dual task-related decrease in walking speed: Is there a relationship? J Am Geriatr Soc 2008; 56:1265–1269. [DOI] [PubMed] [Google Scholar]
- 30.Marusic U, Giordani B, Moffat SD, et al. Computerized cognitive training during physical inactivity improves executive functioning in older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2018;25:49–69. [DOI] [PubMed] [Google Scholar]
- 31.Edwards J, Wadley V, Myers R, et al. Transfer of a speed of processing intervention to near and far cognitive functions. Gerontology 2002;48:329–340. [DOI] [PubMed] [Google Scholar]
- 32.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA 2002;288:22712281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry AS, Zanto TP, Clapp WC, et al. The influence of perceptual training on working memory in older adults. PLoS One 2010;5:e11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verghese J, Buschke H, Viola L, et al. Validity of divided attention tasks in predicting falls in older individuals: A preliminary study. J Am Geriatr Soc 2002;50:1572–1576. [DOI] [PubMed] [Google Scholar]
- 35.Verghese J, Ayers E, Mahoney JR, et al. Cognitive remediation to enhance mobility in older adults: The CREM study. Neurodegener Dis Manag 2016;6:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9:357–381. [DOI] [PubMed] [Google Scholar]
- 37.Chalupa LM, Berardi N, Caleo M, et al. Cerebral Plasticity: New Perspectives Cambridge, MA: MIT Press; 2011. [Google Scholar]
- 38.Erickson KI, Colcombe SJ, Wadhwa R, et al. Training-induced functional activation changes in dual-task processing: An FMRI study. Cerebral Cortex 2006; 17:192–204. [DOI] [PubMed] [Google Scholar]
- 39.Erickson KI, Colcombe SJ, Wadhwa R, et al. Training-induced plasticity in older adults: Effects of training on hemispheric asymmetry. Neurobiol Aging 2007; 28:272–283. [DOI] [PubMed] [Google Scholar]