Abstract
B7‐H5 and its cognate receptor CD28H are T lymphocyte second signaling transduction molecules. Here we aimed to explore the function of this pathway in pancreatic cancer in vitro and in vivo, and evaluated the clinical significance in 136 patients with pancreatic ductal adenocarcinoma enrolled from January 2012 to February 2017 in our hospital. Surgical tumor specimens were collected for immunohistochemical staining to evaluate B7‐H5 expression. Patients’ baseline characteristics, including gender, age, tumor size, tumor location, tumor grading, clinical TNM staging, tumor infiltrating lymphocytes, CA19‐9 and chemotherapy treatment, along with the subsequent follow‐up data, were documented and analyzed. When co‐cultured with T cells, pancreatic cancer PC cells with high B7‐H5 expression induced a more potent immune reaction, indicated by elevated cytokine release and increased proliferation of T lymphocytes compared with cells exhibiting low B7‐H5 expression. Xenograft pancreatic tumors derived from high B7‐H5 expression PC cells exhibited attenuated growth compared to tumors from low B7‐H5 expression cells after transfusion with T lymphocytes in immune‐deficient mice. Of the 136 PDAC tumor tissues, 93 (68.38%) were strong and 43 (31.62%) were weak B7‐H5 expression. Patients with strong B7‐H5 expression had significantly longer overall survival than those with weak expression (median: 16.5 vs 11.5 months, P = .017). TNM staging, tumor location and subsequent chemotherapy were also prognostic factors in these patients. Collectively, B7‐H5/CD28H is a co‐stimulatory signal pathway, and expression of B7‐H5 is associated with improved disease prognosis in patients with pancreatic cancer.
Keywords: B7‐H5, CD28H, immunotherapy, pancreatic cancer, survival
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is among the deadliest cancers due to its delayed diagnosis, low surgical resection rate and limited response to current treatment modalities.1 PDAC is the third leading cause of cancer‐related death in the United States, and most patients survive <12 months after diagnosis.2 Despite decades of efforts, the 5‐year overall survival rate remains lower than 10%,3 and there is an urgent need to develop novel treatment strategies, especially for patients who are refractory to current treatments. Nevertheless, durable responses to immunotherapy have been reported in pancreatic cancer. These responses were often associated with significant infiltration of cytotoxic T lymphocytes (CTL) into the tumor tissue, suggesting that effective immunotherapies may improve patient outcomes.4 Although early clinical trials evaluating the efficacy of immune checkpoint inhibitors, such as those targeting PD1, PD‐L1 or CTLA4, failed in patients with pancreatic cancer,5, 6, 7 new immune targets are promising.
The B7/CD28 families of immune‐regulatory ligands/receptors play crucial roles in the acquisition of effector functions of immune system, there has recently been great interest in the role of these molecules in cancer pathogenesis.8 Notably, B7‐H5 (also known as B7‐H7, HHLA2) and its receptor CD28H (also known as TMIGD2, IGPR‐1) have been identified as the second signaling transduction molecules of T lymphocytes.9 The specific role of B7‐H5/CD28H receptor/ligand pair in immune signaling is controversial, as it has been reported to have both co‐inhibitory and co‐stimulatory functions.10, 11 B7‐H5 expression has been reported in numerous cancers, including non‐small cell lung cancer,12 osteosarcoma,8 melanoma and cancers of the breast, bladder, prostate, esophagus and colon.13 However, there is lack of detailed studies of B7‐H5 expression in pancreatic adenocarcinoma, especially its prognostic value. Aside from clinical TNM stage, few prognostic factors have been identified for the outcomes of pancreatic cancer,14, 15 highlighting the need to discover better prognostic indicators. In this study, we assessed the biological function of B7‐H5/CD28H pathway both in vitro and in vivo, and the expression patterns and clinical significance of this pathway in patients with pancreatic cancer.
2. MATERIALS AND METHODS
2.1. Cell culture and reagents
Panc‐1, Bxpc‐3, Miapaca‐2 and Aspc‐1 pancreatic cancer (PC) cell lines were purchased from ATCC (Manassas, VA, USA). Si187 cell is a primary pancreatic cancer cell line that was derived from a patient with pancreatic ductal adenocarcinoma from our institution. The Si187 cell line has been propagated continuously for more than 50 generations, and a patent application in China is currently ongoing. Human peripheral blood mononuclear cells (PBMC) were obtained from the Blood Center of Zhejiang Provincial (Hangzhou, China). Panc‐1, Bxpc‐3, Aspc‐1 and Si187 cells were cultured in RPMI‐1640 medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). Miapaca‐2 cells were cultured in DMEM (Gibco). PBMC (including all T lymphocytes derived from PBMC) were cultured in improved modified Dulbecco's medium (IMDM; Gibco). All culture media were supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin (Genom, Hangzhou, China). For Miapaca‐2 cells, culture media were supplemented with an additional 2.5% horse serum (Gibco).
2.2. Panc‐1 H149 and Panc‐1 H5351
Panc‐1 cells in the logarithmic growth phase and in good condition were seeded into a 6‐well plate at a density of 1 × 105/mL. When the cells had completely adhered to the wall, the medium was replaced with 2 mL of a fresh DMEM. The cells in two wells were then added with 20‐30 μL B7‐H5‐GFP‐carrying or negative control lentivirus (Oobio, Shanghai, China), respectively. Following complete mixing by agitation, the cells were incubated. Approximately 12‐16 hours after the transduction, the DMEM was replaced and transduction was continued for 48 hours. Following this, transduction results for the cells were examined using an inverted fluorescence microscope (Leica, Wetzlar, Germany). Overgrown cells were inoculated onto a 6‐well plate for drug screening using DMEM containing 2 μg/mL puromycin solutions (Sigma‐Aldrich, St. Louis, MO, USA). The medium was replaced every 2 days. The expression of green fluorescent protein (GFP) was observed using inverted fluorescence microscopy. After 10‐15 days of screening, cell lines with stable expression of B7‐H5‐GFP and negative control vector were obtained, and named as Panc‐1 H5351 and Panc‐1 H149, respectively.
2.3. T lymphocyte functional assays
To evaluate T lymphocyte proliferation, 24‐well plates were pre‐coated overnight with anti‐human CD3 (clone OKT3, Biolegend, San Diego, CA, USA) at 5 μg/mL. Panc‐1 H149 and Panc‐1 H5351 cells were pre‐coated at 1 × 104 cells/well in 24 well plates. Human peripheral blood T cells were negatively selected and purified using a human Pan‐T‐cell selection kit (Miltenyi Biotec, Auburn, CA, USA). After sorted from PBMCs, T cells were stained with CFSE (Biolegend) reagent in a final concentration of 2.5 μg/mL. T cells were added into each well at 1 × 105 cells per well. Co‐stimulatory anti‐human CD28 (clone 28.2, Biolegend) was added at the beginning of the co‐culture at 5 μg/mL. Suspended T lymphocytes were collected for CFSE analysis by flow cytometry after co‐culture for 5 days.
To evaluate the cytokine release from T lymphocytes, 96‐well plates were pre‐coated overnight with anti‐human CD3 (clone OKT3, Biolegend) at 5 μg/mL. Panc‐1 H149 and Panc‐1 H5351 cells were pre‐coated at 2 × 103 cells/well in 96 well plate. Human peripheral blood T cells were positively selected and purified using a human CD8+ naive‐T‐cell selection kit (Miltenyi Biotec). T cells were added into each well at 1 × 104 or 2 × 104 cells per well. Co‐stimulatory anti‐human CD28 (clone 28.2, Biolegend) was added at the beginning of the co‐culture at 5 μg/mL. Suspended T lymphocytes were collected for CD28H analysis by flow cytometry after co‐culture for 3 days. Culture supernatants were collected for ELISA to evaluate cytokine expression.
For injection with human T lymphocytes in mice, 96‐well plates were pre‐coated overnight with anti‐human CD3 (clone OKT3, Biolegend) at 5 μg/mL. Human peripheral blood T cells were positively selected and purified using a human CD8+ naive‐T‐cell selection kit (MiltenyiBiotec). T cells were added into each well at 2 × 104 cells per well. Co‐stimulatory anti‐human CD28 (clone 28.2, Biolegend) was added at the beginning of the co‐culture at 5 μg/mL. After 5 days in culture, suspended T lymphocytes were collected to inject into mice.
2.4. Assessment of tumor cell viability using the CCK8 assay
The viability of Panc‐1 H149 and Panc‐1 H5351 cells in the co‐culture system was evaluated using the CCK8 cell counting kit. After suspended T lymphocytes and supernatants were removed, serum free media were added, and the viability of tumor cells was assessed using the CCK8 cell counting kit (Keygen Biotech, Nanjing, China), according to the manufacturer's instructions.
2.5. Immunohistochemistry and ELISA
In brief, formalin‐fixed paraffin‐embedded PDAC tissue samples were cut into 5‐μm sections and subjected to heat‐induced antigen retrieval. The following antibodies were used: anti‐HHLA2 (1:100, AP10650a, Abgent, San Diego, CA, USA), anti‐human‐CD3 epsilon (1:200, Abcam, Cambridge, MA, USA) and anti‐human CD8a (1:200, Cell Signaling Technology, Carlsbad, CA, USA). Slides were then incubated with HRP‐conjugated antibodies against rabbit or mouse IgG, as appropriate, using a Histostain‐Plus Kit (ZSGB‐BIO, Beijing, China). Slides were then counterstained with hematoxylin and evaluated under a microscope (Leica). Negative controls were performed without the application of primary antibodies. Cell cytoplasm and membrane staining were indicated by brown particles that defined cells as B7‐H5 positive. Strong expression was defined as more than 25% cancer cells express this B7‐H5 molecular, while weak expression was defined as less than 25% cancer cells express this B7‐H5 molecular. CD3 and CD8 were employed for tumor infiltrated lymphocytes (TIL) markers, and Image J (version Java 1.8.0_112, NIH, Bethesda, MD, USA) analysis was performed for measurement of positive staining cells. More than 1500 cells/mm3 CD3+ or CD8+ T cells was defined as high TIL status, otherwise would be defined as low TIL, as previously reported.16, 17
For the ELISAs, tumor cells and CD8+ naïve T cells co‐culture supernatants were collected by centrifugation and stored at −80°C. Concentrations of IL‐2, TNF‐a, and IFN‐γ were detected using specific ELISA kits (Biolegend), according to the manufacturer's instructions.
2.6. Flow cytometry
Fresh tumor tissue was washed 3 times with PBS (Genom). Tumor samples were then cut into small pieces with scissors and then minced thoroughly using sterile scalpel blades. The minced tumor pieces were incubated with ultrapure collagenase IV (Worthington Biochemicals, Freehold, NJ, USA) at 37°C for 1 hour, with manual dissociation every 15‐20 minutes by pipetting. Single cells were filtered through a 70‐μm nylon mesh (Corning, NY, USA) and washed with PBS supplemented with 2% FBS. For patient blood samples, PBMCs were extracted with Ficoll solution (Haoyang Bio, Tianjing, China), according to the manufacturer's instructions. Gradient centrifugation was carried out at 400× g with low acceleration and zero brake for 20 minutes at 4°C. Cells were stained with specific antibodies, according to the manufacturer's instructions. The mouse anti‐human B7‐H5 (clone 5B6‐2) and hamster anti‐human CD28H (clone 4‐5) primary antibodies were a kind gift from Dr. Yuwen Zhu. And other antibodies, including FITC mouse anti‐human CD45 (clone H130), PE mouse anti human CD3 (clone HIT3a), APC goat anti‐mouse IgG (clone poly4053), APC mouse isotype (clone MOPC‐21), Alexa Fluor goat anti‐hamster IgG (clone poly4055), hamster isotype controls (clone HTK888), mouse Fc block (2.4G2) and 7‐AAD, were purchased from either Biolegend or BD (BD Biosciences, San Diego, CA, USA). Flow cytometry was performed on a BD FACS Canto‐II Flow Cytometer (BD Biosciences), and data were analyzed using FlowJo software (version 10.6, Ashland, OR, USA).
2.7. Real time quantitative polymerase chain reaction (real time qPCR)
Fresh tumor tissue was minced in liquid nitrogen and total RNA was harvested using Trizol LS Reagent (Invitrogen, Carlsbad, CA, USA), and 1 μg total RNA was reverse transcribed into single‐stranded cDNA using QuantScript RT Kit (Takara Biotechnology, Dalian, China). The cDNA was used to measure the relative expression of genes using SYBR Premix Ex TaqTM II (Takara) according to the manufacturer's protocol;qPCR assays were performed in 20 μL reactions with Applied Biosystems 7500 Fast Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA) using the standard method. The mRNA expression was normalized to ß‐actin. The gene expression values were expressed as the relative mRNA expression (fold change) compared to the corresponding controls. All primers were synthesized by Xiangyin Biotech Inc. (Shanghai, China; Table S1)
2.8. Mouse models and experiments
Six‐week old B‐NSG (NOD‐Prkdcscid IL2rgtm1/Bcgen) mice were purchased from Biocytogen Animal Center (Beijing, China). Experiments were performed according to the ethical guidelines established by the Ethics Committee of our hospital. Half a million Panc‐1 H149 or Panc‐1 H5351 cells were re‐suspended in 50 μL PBS and subcutaneously injected in B‐NSG, then those mice were divided into treatment and control group. After 2 weeks, the treatment group received injections of 1 ×106 active healthy human naïve CD8+ T lymphocytes through the tail vein weekly for 6 weeks, while the control group received PBS vehicle injection. One week after the 6th injection, mice were sacrificed and tumors were harvested for evaluation.
2.9. Acquisition of human tissue and clinical information
Fresh pancreatic cancer tumor tissue and blood samples were obtained from patients with PDAC who underwent curative resection between January 2012 and February 2017 at our hospital. Patients were treatment‐naïve prior to surgery. The diagnosis of pancreatic adenocarcinoma was confirmed by pathological examination of tumor tissue after surgery. Baseline clinical data, including gender, age, tumor size, tumor location, tumor grade, clinical TNM staging, CA19‐9, and subsequent chemotherapy, were documented. Subsequent follow‐up data were collected and analyzed. Patient's survival was defined as the duration between the operation of curative resection and death. All patients provided written informed consent. The protocol of this study was approved and supervised by the Ethics Committee of our hospital.
2.10. Statistical analysis
Data were analyzed using SPSS (version 24, SPSS Inc., Chicago, IL, USA) and GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). Data are presented as mean±standard deviation (SD) or 95% CI. Continuous variables were analyzed using an unpaired Student's t‐test to compare between 2 groups, or one‐way ANOVA for multiple groups. Categorical variables were compared using a chi‐square test or Fisher exact test, as appropriate. A P value of <.05 was considered to be statistically significant.
3. RESULTS
3.1. B7‐H5/CD28H acts as a co‐stimulatory pathway in vitro
CD28H was previously found on naïve T cells as a receptor for B7‐H511; thus, the function of this pathway was evaluated by modulating the expression of the ligand B7‐H5 on cancer cells which interact with T cells. Flow cytometry was used to assess the expression of B7‐H5 on PC cells (Figure S1). No significant B7‐H5 expression was observed on Panc‐1, Bxpc‐3, Aspc‐1, Miapaca‐2, or Si187 cells. A stable B7‐H5 transfection system was utilized to further study the function of B7‐H5 on PC cells. Panc‐1 H5351 cells were transfected with the B7‐H5‐GFP plasmid, and Panc‐1 H149 cells were transfected with a negative control plasmid; B7‐H5 expression was confirmed by flow cytometry (Figure S2A,B). These stable transfectants were used to determine the biologic function of B7‐H5. Naïve CD8+ T cells were sorted using magnetic beads, which were then co‐cultured with either Panc‐1 H149 or Panc‐1 H5351. The supernatant from the Panc‐1 H5351 co‐culture system had elevated IL‐2, TNF‐a, and IFN‐γ levels compared with that from Panc‐1 H149 cells (Figure 1A), as determined by ELISA.
Figure 1.
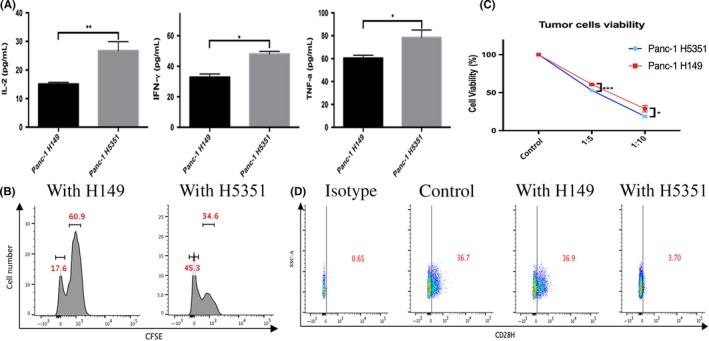
B7‐H5 co‐stimulates the functions of T lymphocytes in vitro. A, ELISA analysis reveals that the supernatant of the co‐culture system from pancreatic cancer cells with high B7‐H5 expression (Panc‐1 H5351) had elevated IL‐2, TNF‐a and IFN‐γ expressions. The data are expressed as mean ± SD. B, Flow cytometry analysis reveals an increased proliferation of Pan T lymphocytes co‐cultured with pancreatic cancer cells with high B7‐H5 expression (Panc‐1 H5351). C, The viability of pancreatic cancer cells was measured by CCK8 after co‐culture with naïve CD8+ T cells. Tumor cells with high B7‐H5 expression had lower viability. The data are expressed as mean ± SD. D, T lymphocytes co‐cultured with pancreatic cancer cells with high B7‐H5 expression (Panc‐1 H5351) had lower CD28H expression. Representative data from at least 3 independent experiments are shown. (***P < .001; **P < .01; *P < .05; NS no significance)
The effects of B7‐H5 on the proliferation of T cells were evaluated next. Pan T cells were sorted by magnetic beads and stained with CFSE, followed by co‐culture with Panc‐1 H149 or Panc‐1 H5351 cells. Flow cytometry analysis revealed that T cells co‐cultured with Panc‐1 H5351 cells exhibited increased proliferation compared to those co‐cultured with Panc‐1 H149 cells (Figure 1B). Furthermore, Panc‐1 H5351 had a lower viability when compared with Panc‐1 H149 at the indicated tumor cells/T cells ratios (Figure 1C). Interestingly, after co‐culture with Panc‐1 H5351 cells, T lymphocytes had lower CD28H expression compared with those co‐cultured with Panc‐1 H149 cells (Figure 1D). Similar results were also obtained when the extracellular function domain of the human B7‐H5 protein was added to cultures of naïve CD8+ T cells, comparing to cells treated with control IgG (data not shown). These in vitro data indicate that B7‐H5 acts as a co‐stimulatory signal and enhances proliferation and cytokine secretion of T cells.
3.2. B7‐H5/CD28H acts as a co‐stimulatory pathway in vivo
As this B7‐H5/CD28H signaling is absent in mice or rat,10, 11 B‐NSG mice were employed to research the function of B7‐H5/CD28H pathway in vivo. Xenografts were established in B‐NSG mice using Panc‐1 H149 cells or Panc‐1 H5351 cells. In the treatment group, hematoxylin and eosin (HE) staining revealed no obvious differences in tumor phenotypes of Panc‐1 H149 and Panc‐1 H5351 tumors in B‐NSG mice which received T cells transfusion (Figure 2A). Additionally, no significant difference was observed in tumor metastases between both cell lines. The distribution patterns of CD8+ human T cells in the B‐NSG primary xenograft tumors and circulation were analyzed by immunohistochemistry (IHC) staining; no obvious change in distribution of CD8+ human T cells was found between the Panc‐1 H149 and Panc‐1 H5351 xenografts (Figure 2B). However, after the 4th injection with CD8+ human T cells, xenograft Panc‐1 H5351 tumors were smaller in size than Panc‐1 H149 tumors (Figure 2C). Upon necropsy, Panc‐1 H5351 xenograft tumors exhibited reduced tumor volume and weight compared with Panc‐1 H149 xenograft tumors (Figure 2D,E). No difference between Panc‐1 H5351 control group and Panc‐1 H149 control group was found (Figure 2C,D). Similarly, no significant difference was detected between the Panc‐1 H149 treat group which had received the T cells transfusion and the control group (Figure 2C,D). It is possible that the total number of additional CD8+ human T lymphocytes injected into each mouse was too small, which would make it very challenging to obtain a sufficient amount of T cells for the analysis of phenotypic changes or functions. Along with the in vitro data, the in vivo data might indicate that B7‐H5 acts as a co‐stimulatory signal and can subsequently activate T cell proliferation and cytokine secretion.
Figure 2.
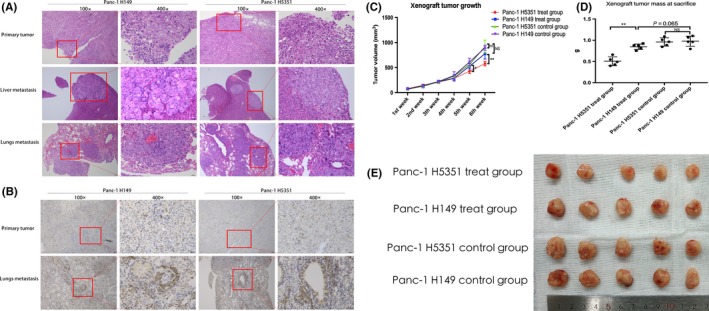
B7‐H5 co‐stimulates the functions of T lymphocytes in vivo. Panc‐1 H149 and Panc‐1 H5351 xenograft tumors established in B‐NSG mice. A, HE staining was performed on primary subcutaneous tumors and their liver or lung metastases; no obvious phenotype differences were found between the Panc‐1 H149 and Panc‐1 H5351 groups. Scale bar 25 μm. B, IHC staining was used to compare the distribution of the injected naïve human CD8+ T cells in B‐NSG mice. No distribution differences were found between the Panc‐1 H149 and Panc‐1 H5351 groups in primary tumors or in circulation. Scale bar 25 μm. C, The primary tumor diameter in the two groups was measured weekly after tail vein injection. The data are expressed as mean ± SD. D, The primary tumor weight was measured in the two groups. The data are represented by mean ± SD. E, Panc‐1 H149 and Panc‐1 H5351 groups of B‐NSG mice were treated and harvested. Representative data from two independent experiments are shown. (***P < .001; **P < .01; *P < .05; NS no significance)
3.3. B7‐H5 is expressed in human pancreatic cancer and is associated with prognosis
We evaluated the expression pattern of B7‐H5 in human PC tumors using immunohistochemistry. The 136 patients with PDAC (Table S2) enrolled in this study were divided into 2 groups based on the expression status of B7‐H5. (Figure 3A): A total of 93 patients were defined to have strong expression, while other 43 subjects had weak expression (strong expression rate 68.38%; Table 1). Further mRNA expression from 43 cases of fresh samples were confirmed by real time quantitative PCR, which reveled that B7‐H5 strong expression group had a higher mRNA level than it in the weak expression group (Figure 3B). No obvious relation between B7‐H5 expression and the clinical TNM staging, status of TIL, or the tumor grading was found (Table S3). But in univariate survival analysis, overall survival was associated with the expression of B7‐H5, clinical TNM staging, and receipt of adjuvant chemotherapy, status of TIL (Table 1). Multivariable COX analysis further revealed no significant correlations between the survival of pancreatic cancer patients and factors such as gender, age, tumor size, tumor differentiation grade, or serum CA19‐9 levels (Table S4). However, the expression of B7‐H5, clinical TNM staging, receipt of adjuvant chemotherapy and tumor location were found to be independent prognostic factors for survival of patients with PDAC. Those who had strong B7‐H5 expression, lower TNM staging, completed chemotherapy, and tumors located in the pancreatic body or tail might have a better prognosis (Table 2). Furthermore, Kaplan‐Meier survival analysis revealed that patients with PDAC who are strong expression for B7‐H5 had significantly better median OS than those lacking B7‐H5 expression (median OS of 16.5 months in the strong group and 11.5 months in the weak group; log rank P = .017; Figure 3C). Nevertheless, despite having high B7‐H5 expression in PC tumor tissue, patients in the B7‐H5 strong expression group had dismal prognosis still. To elucidate the underlying mechanism, the expression pattern of the B7‐H5 receptor, CD28H, was further assessed in fresh tumor tissue and in the blood sample of those 43 cases. Because CD28H functions as a co‐stimulatory receptor on T cells, a CD28H expression gating strategy was carried out by focusing on CD3+ T lymphocytes (Figure 4A). Patients had distinct levels of CD28H (Figure 4B), but CD28H expression was much lower in tumor tissue than in blood samples (Figure 4C). Considering that immune cells, including the CD3+ T lymphocytes, migrated to the tumor microenvironment from the peripheral circulation, the CD28H down‐regulation status could partially account for the impaired function of B7‐H5/CD28H co‐stimulatory pathway in pancreatic cancer.
Figure 3.
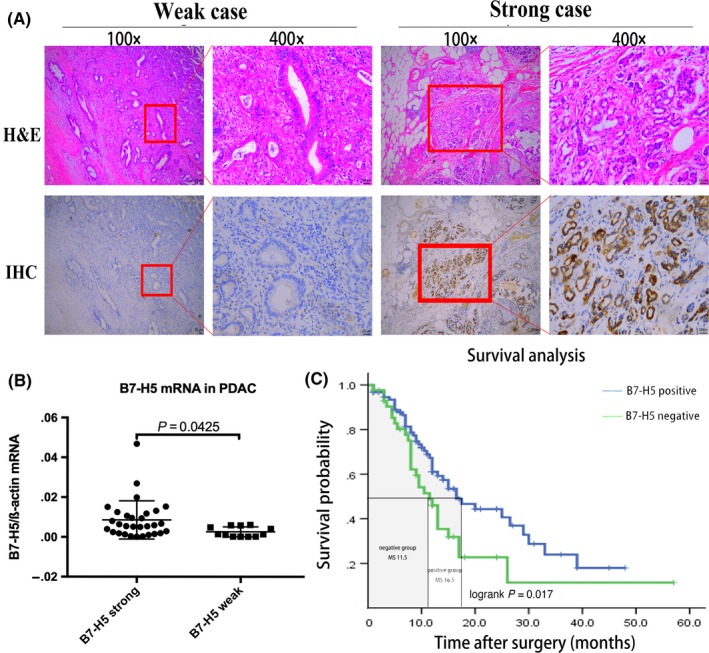
B7‐H5 positivity correlates with prolonged overall survivals in patients with pancreatic cancer. A, Consecutive paraffin slides from two representative pancreatic cancer tissues were evaluated by HE and B7‐H5 IHC staining. B7‐H5 expression was focal in the cytomembrane and cytoplasm of the cancer cells. Scale bar 25 μm. B, B7‐H5 mRNA in fresh patient tumor of both groups were measured by real time quantitative PCR, ß‐actin was employed as corresponding control. C, Kaplan‐Meier survival analysis was carried out, and the median survival in the B7‐H5 strong expression group and the B7‐H5 weak expression group was 16.5 and 11.5 months, respectively. (***P < .001; **P < .01; *P < .05; NS no significance)
Table 1.
Univariate survival analysis
| Characteristics of patient population | No. cases | Median survival (months) | 95%CI | P value |
|---|---|---|---|---|
| Gender | ||||
| Male | 80 | 16.5 | 11.654‐21.346 | .513 |
| Female | 56 | 13 | 10.966‐15.034 | |
| Age | ||||
| ≤60 | 34 | 25 | 6.637‐43.363 | .645 |
| >60, ≤70 | 64 | 13 | 9.348‐16.652 | |
| >70 | 38 | 15 | 11.833‐18.167 | |
| Tumor location | ||||
| Head/neck/uncinate | 103 | 13 | 10.741‐15.259 | .165 |
| Body/tail | 33 | 17 | 5.862‐28.138 | |
| Tumor size | ||||
| ≤10 mm3 | 45 | 13 | 10.216‐15.784 | .340 |
| >10 mm3, ≤40 mm3 | 83 | 15 | 11.019‐18.981 | |
| >40 mm3 | 8 | 7 | .649‐13.351 | |
| Tumor grading | ||||
| No or low | 46 | 13 | 8.799‐17.201 | .320 |
| Moderate | 82 | 15 | 8.316‐21.684 | |
| High | 8 | 15 | / | |
| Clinical TNM staging | ||||
| Ia | 1 | / | / | .002 |
| Ib | 3 | / | / | |
| IIa | 52 | / | / | |
| IIb | 65 | / | / | |
| III | 8 | / | / | |
| IV | 7 | / | / | |
| B7‐H5 expression | ||||
| Strong | 93 | 16.5 | 10.761‐22.239 | .017 |
| Weak | 43 | 11.5 | 8.881‐14.119 | |
| Adjuvant chemotherapy | ||||
| No | 66 | 11.5 | 9.705‐13.295 | .000 |
| Unfinished | 7 | 9 | 6.434‐11.566 | |
| Completed | 63 | 26 | 15.360‐36.640 | |
| CA19‐9 | ||||
| ≤37 | 27 | 16.5 | 10.375‐22.625 | .953 |
| >37 | 109 | 14 | 11.155‐16.845 | |
| Status of TIL | ||||
| High | 24 | 31.29 | 22.197‐40.385 | .029 |
| Low | 34 | 18.29 | 12.595‐23.989 | |
Table 2.
Multivariable COX survival analysis
| Characteristics of patient population | No. cases | Hazard ratio | 95%CI | P value |
|---|---|---|---|---|
| Tumor location | ||||
| Head/neck/uncinate | 103 | .492 | .274‐.885 | .018 |
| Body/tail | 33 | |||
| Clinical TNM staging | ||||
| Ia | 1 | 1.493 | 1.188‐1.876 | .001 |
| Ib | 3 | |||
| IIa | 52 | |||
| IIb | 65 | |||
| III | 8 | |||
| IV | 7 | |||
| B7‐H5 expression | ||||
| Strong | 93 | 2.241 | 1.351‐3.718 | .002 |
| Weak | 43 | |||
| Adjuvant chemotherapy | ||||
| No | 66 | .654 | .511‐.836 | .001 |
| Unfinished | 7 | |||
| Completed | 63 | |||
Figure 4.
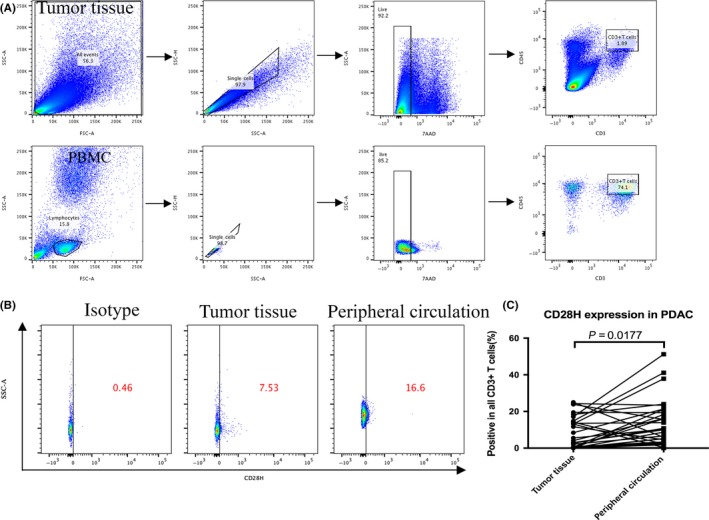
CD28H expression of tumor infiltrated lymphocytes attenuated in patients with pancreatic cancer. A, The flow cytometry gating strategy for CD3+ T lymphocytes from tumor tissue (superior panel) and peripheral circulation (inferior panel). B, The CD28H expression in CD3+ T lymphocytes from fresh tumor tissue and peripheral circulation was measured by flow cytometry. C, The CD28H expression in CD3+ T lymphocytes from fresh tumor tissue and peripheral circulation was analyzed. (***P < .001; **P < .01; *P < .05; NS no significance)
4. DISCUSSION
Despite recent advances in combination chemotherapies, prognosis for patients with pancreatic cancer remains appallingly poor, even among patients who undergo macroscopically curative resection (5‐year survival of 20.7%‐23.9%, median survival of 22.3‐23.6 months) followed by adjuvant chemotherapy.18 In addition to traditional surgical or chemotherapeutical approaches, new treatment strategies, including immunotherapy using checkpoint inhibitors or monoclonal antibodies that have been established as effective cancer therapies for other cancer types, have largely failed to improve survival in pancreatic cancer when used as single agents.19 Considering the miserable prognosis of this disease, the identification of novel immunotherapeutic targets while taking into account the specific microenvironment of PDAC is of great importance and clinical interest.20 There is mounting evidence suggesting that immunotherapy, in conjunction with standard of care, can improve the outcomes of patients with pancreatic cancer.21 Many studies have assessed expressions of inhibitory receptors on exhausted CD8+ T cells, but without specific focus on co‐stimulatory molecules.22 In this study, we demonstrate that B7‐H5 can act as a co‐stimulatory factor via its receptor, CD28H, which is expressed on T lymphocytes. Evaluation of clinical data revealed that B7‐H5 was positively expressed in 68.38% of patients with PDAC, which is in consistence with its expression patterns in other cancers such as breast cancer and osteosarcoma.8 Additionally, study had suggested that in triple negative breast cancer B7‐H5 expression was associated with lymph‐node metastasis and advanced stage at the time of diagnosis.13 In the present study, B7‐H5 expression level did not correlate with the clinical TNM staging, status of TIL and tumor grading, which implied that B7‐H5 might participate the cancer immune evacuation but not the cancer genesis process, acted as an independent prognostic factor in overall survival in pancreatic cancer. Our data suggested that patients with high expression of B7‐H5 in pancreatic cancer tissue may have more favorable survival prognosis compared to those with weak or negative B7‐H5 expression. We further demonstrated that PC may induce immune evasion by attenuating the expression of CD28H on T lymphocytes that migrated to the tumor from the peripheral circulation. Therefore, overcoming the attenuation of CD28H expression in T lymphocytes that have infiltrated tumor tissue, or up‐regulating B7‐H5 expression on pancreatic cancer cells, may constitute a novel mechanism to improve the effects of immunotherapy in pancreatic cancer. Nevertheless, patients who underwent surgery have a much better prognosis when they received subsequent chemotherapy; this is in accordance with the results of the JASPAC‐1 clinical trial, suggesting that a combined approach using multiple strategies including surgery, standard chemotherapy, and B7‐H5 pathway‐targeting therapy might emerge as an effective method for the treatment of pancreatic cancer.
However, the function of B7‐H5/CD28H (HHLA2/TMIGD2) second signal transduction pathway is still shrouded in controversy,8, 10, 11 with little consensus in the extant literature. As far as we know, all these published research results concern about this B7‐H5/CD28H pathway were most descriptive data, little about the detail of the signaling pathway or molecular mechanism was mentioned in these paper. The absence of murine B7‐H5 and CD28H gene expression and the lack of reliable commercial reagents, including specific neutralizing antibodies against human B7‐H5 or CD28H, have significantly dampened the efforts to establish a comprehensive understanding of this B7 family pathway.23 This controversy may be attributed to the possible existence of 2 different receptors or unsubstantiated receptors of the HHLA2 ligand. Besides, the methods for T cells stimulation in this two paper10, 11 were different, one used HHLA2‐Ig,10 the other used CD28H‐mAb.11 And we have noticed that different T cell subpopulations were employed in these two researches, one with total T cells,10 the other with CD4+ or naïve CD4+T cells,11 these subpopulations have very different spectra of receptors on their surface and might have different cytokines and chemokines production, which might conduct the different results. Yet there remains much to be discovered about this pathway. However, the underlying mechanism of the regulation of B7‐H5 or CD28H remains unknown. In our study, we found that CD28H expression in T lymphocytes was down‐regulated after the engagement of T lymphocytes with B7‐H5 expressing PC tumor cells. It has also been reported that alterations in gene methylation patterns are responsible to other co‐stimulatory signals, such as CD137, which could induce changes in B7‐H5 mRNA and protein expression; this was confirmed by pyrosequencing in a series of healthy donors.24
In conclusion, our findings reveal that B7‐H5/CD28H is a co‐stimulatory signal pathway, and expression of B7‐H5 is associated with improved disease prognosis in PDAC, which indicate that the B7‐H5/CD28H pathway might be a potential immunotherapeutic target in pancreatic cancer. Although there are no available agents to target B7‐H5 or CD28H currently, the success of limited number of immunotherapeutic studies on pancreatic cancer has provided considerable evidence with space for improvement.25 Combined with standard chemotherapy or other immunotherapy strategies, targeting the B7‐H5/CD28H pathway may be an effective treatment approach for pancreatic cancer. Due to high frequency of B7‐H5 strong expression in patients with pancreatic cancer, this pathway merits further clinical evaluation.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Chen W, Liang TB and Bai XL conceived the idea. Chen Q, Wang JX, Zhang Q, Wei T, Zhou Y and Xu XY performed the experiments. All authors analyzed and interpreted the data including the clinical parts. Chen Q and Wang JX drafted the manuscript. All authors made critical revisions and approved the final version of the manuscript.
Supporting information
ACKNOWLEDGMENTS
We thank Mrs Lei Ni, Minghua Sun, Liqiang Hu and Yi Wang for their technical support. We would like to give our special thanks to Dr Yuwen Zhu of Anschutz Medical Campus, University of Colorado for providing the B7‐H5‐GFP plasmid, anti‐human B7‐H5 antibody (clone 5B6‐2), and anti‐human CD28H antibody (clone 4‐5).
Chen Q, Wang J, Chen W, et al. B7‐H5/CD28H is a co‐stimulatory pathway and correlates with improved prognosis in pancreatic ductal adenocarcinoma. Cancer Sci. 2019;110:530–539. 10.1111/cas.13914
Chen and Wang equally contributed to this study.
REFERENCES
- 1. Costello E, Greenhalf W, Neoptolemos JP. New biomarkers and targets in pancreatic cancer and their application to treatment. Nat Rev Gastroenterol Hepatol. 2012;9(8):435‐444. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 3. Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63(5):318‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor‐infiltrating macrophages and improves response to T‐cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74(18):5057‐5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti‐CTLA‐4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455‐2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soares KC, Rucki AA, Wu AA, et al. PD‐1/PD‐L1 blockade together with vaccine therapy facilitates effector T‐cell infiltration into pancreatic tumors. J Immunother. 2015;38(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koirala P, Roth ME, Gill J, et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci Rep. 2016;6:31154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janakiram M, Shah UA, Liu W, et al. The third group of the B7‐CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7‐H3. Immunol Rev. 2017;276(1):26‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao R, Chinai JM, Buhl S, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T‐cell function. Proc Natl Acad Sci USA. 2013;110(24):9879‐9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu Y, Yao S, Iliopoulou BP, et al. B7‐H5 costimulates human T cells via CD28H. Nat Commun. 2013;4:2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng H, Janakiram M, Borczuk A, et al. HHLA2, a new immune checkpoint member of the B7 family, is widely expressed in human lung cancer and associated with EGFR mutational status. Clin Cancer Res. 2017;23(3):825‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janakiram M, Chinai JM, Fineberg S, et al. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res. 2015;21(10):2359‐2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605‐1617. [DOI] [PubMed] [Google Scholar]
- 15. Yuan C, Morales‐Oyarvide V, Babic A, et al. Cigarette smoking and pancreatic cancer survival. J Clin Oncol. 2017;35(16):1822‐1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galon J, Pagès F, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schueneman AJ, Sugar EA, Uram J, et al. Low total lymphocyte count is associated with poor survival in patients with resected pancreatic adenocarcinoma receiving a GM‐CSF secreting pancreatic tumor vaccine. Ann Surg Oncol. 2013;20(Suppl 3):S725‐S730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S‐1 versus gemcitabine for resected pancreatic cancer: a phase 3, open‐label, randomised, non‐inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248‐257. [DOI] [PubMed] [Google Scholar]
- 19. Michl P, Krug S. Overcoming immune evasion in pancreatic cancer: the combination matters. Gut. 2018;67(6):997‐999. [DOI] [PubMed] [Google Scholar]
- 20. Benyamine A, Loncle C, Foucher E, et al. BTN3A is a prognosis marker and a promising target for Vγ9Vδ2 T cells based‐immunotherapy in pancreatic ductal adenocarcinoma (PDAC). Oncoimmunology. 2017;7(1):e1372080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Banerjee K, Kumar S, Ross KA, et al. Emerging trends in the immunotherapy of pancreatic cancer. Cancer Lett. 2018;417:35‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamphorst AO, Wieland A, Nasti T, et al. Rescue of exhausted CD8 T cells by PD‐1‐targeted therapies is CD28‐dependent. Science. 2017;355(6332):1423‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crespo J, Vatan L, Maj T, et al. Phenotype and tissue distribution of CD28H+ immune cell subsets. Oncoimmunology. 2017;6(12):e1362529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aznar MA, Labiano S, Diaz‐Lagares A, et al. CD137 (4‐1BB) costimulation modifies dna methylation in CD8+ T cell‐relevant genes. Cancer Immunol Res. 2018;6(1):69‐78. [DOI] [PubMed] [Google Scholar]
- 25. Battaglia S, Muhitch JB. Unmasking targets of antitumor immunity via high‐throughput antigen profiling. Curr Opin Biotechnol. 2016;42:92‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


