Abstract
Background
Colorectal cancer (CRC) diagnosed before age 50 years, or young-onset CRC, is increasing globally with undefined etiology. A sedentary lifestyle is an emerging risk factor for CRC after age 50 years, but its role in young-onset CRC is unknown.
Methods
We prospectively evaluated sedentary behaviors, primarily time watching television (TV), and risk of young-onset CRC among 89 278 women in the Nurses’ Health Study II ages 25–42 years at recruitment (1991–2011). We used Cox proportional hazards modelling to estimate relative risks (RR) and 95% confidence intervals (CIs). Statistical tests were two-sided.
Results
We documented 118 young-onset CRCs over 1 262 540 person-years. Sedentary TV viewing time was statistically significantly associated with increased risk of young-onset CRC, after adjusting for putative risk factors, including obesity and physical activity. Compared to no more than 7 hours per week, women with 7.1–14 hours per week of TV time had a multivariable relative risk (RR) of 1.12 (95% confidence interval [CI] = 0.72 to 1.75), further increased for greater than 14 hours per week (RR = 1.69, 95% CI = 1.07 to 2.67, Ptrend = .03). This association was observed among participants without a CRC family history and was more pronounced for rectal cancer (RR for >14 vs ≤7 hours per week 2.44, 95% CI = 1.03 to 5.78, Ptrend = .04). Overweight or obese participants may be more susceptible.
Conclusion
Independent of exercise and obesity, prolonged sedentary TV viewing time, a surrogate for a more inactive lifestyle, was associated with increased risk of young-onset CRC, particularly of the rectum. These findings provide further evidence on the importance of maintaining an active lifestyle.
Contrasting with decreases in overall colorectal cancer (CRC) incidence, CRC rates have increased dramatically in those ages 20–49 years in the United States, parts of Europe, and Asia (1–5). In the United States, distal colon and rectal cancers are disproportionately driving these alarming trends (2,6). Young-onset CRC, or CRC before age 50 years, is typically diagnosed at a more advanced stage than conventional CRC (mean age 65 years at diagnosis), with more aggressive tumors, different clinicopathological characteristics, and greater years of life lost (7–9). This emerging public health concern has resulted in updated guidelines from the American Cancer Society advising that average-risk screening begins at age 45 years, rather than 50 years (10). While biological differences may account for some distinctive features of young-onset vs conventional CRC, delays in diagnosis may result from challenges in identifying those most at risk. Given such disparities, elucidating the role of traditional CRC risk factors in young-onset CRC is a high unmet need.
Despite public health campaigns that have increased physical activity (11,12), sedentary time is increasing due to the rising prevalence of deskbound work and passive media consumption, comprising half of all waking hours (11,13,14). Independent of other appraisals of energy expenditure, including vigorous physical activity, sedentary behavior has recently been linked to several chronic health outcomes, including obesity (15), diabetes (15,16), cardiovascular disease (17,18), CRC (13,19) and its most common precursor, colonic adenomas (19,20).
While a sedentary lifestyle is one of the leading suspected culprits contributing to this increase (21), the association between sedentary behaviors and young-onset CRC remains to be elucidated. Thus, we investigated the association between several sedentary behaviors and risk of young-onset CRC among women enrolled in a large, prospective US cohort with information on lifestyle and other putative CRC risk factors.
Methods
Study Population
Nurses’ Health Study II (NHS II) is a prospective cohort study of 116 430 female nurses ages 25–42 years at enrollment in 1989. Participants are followed with detailed biennial questionnaires on lifestyle factors, medical diagnoses, medication use, and other exposures of interest including regular and validated self-assessments of anthropometrics, such as body mass index (BMI), physical activity, and quadrennial semiquantitative food frequency questionnaires (FFQ). Cumulative follow-up exceeds 90% of available person-time (22). This study was approved by the human research committees at the Brigham and Women’s Hospital. Informed consent was implied through voluntary return of study questionnaires.
Study baseline was defined as the first year NHS II (1991) collected information on sedentary behaviors, including television (TV) watching. At baseline, we excluded participants with CRC or with missing information on sedentary behaviors. We also excluded women reporting implausible energy intake (< 500 or > 3500 kcal/day) or a prior diagnosis of cancer apart from non-melanoma skin cancer at baseline. Participants who developed inflammatory bowel disease prior to baseline or during follow-up were also excluded. In total, 89 278 women comprised our final study population.
Assessment of Colorectal Cancer
The primary endpoint was incident CRC diagnosed prior to age 50 years. Upon report of CRC on biennial questionnaires, we requested permission to review relevant medical records. Additional cases of lethal CRC were identified through reporting from family, postal authorities, or through the National Death Index. Physicians blinded to self-reported risk factor status reviewed retrieved records to ascertain information on anatomic site, histology, and stage at presentation.
Assessment of Sedentary Behaviors
In NHS II, information on sedentary behaviors was first collected in 1991 and updated in 1997, 2001, 2005, and 2009 in nine categories ranging from 0 to more than 90 hours per week. Our primary exposure was sedentary TV viewing time (ie, watching TV broadcasts or other forms of media associated with TV screen use like video tapes and DVDs). This exposure was selected a priori based on prior investigations demonstrating that among sedentary behaviors, TV viewing time was the strongest predictor of adverse health outcomes (15). We also examined other time spent sitting at home (eg, reading, during meals, time spent at a desk) and collective hours spent sitting at work, while driving, or otherwise out of the home.
We discretized our exposures of interests at 7 and 14 hours in order to improve interpretability of risk estimates (ie, 1 or 2 hours of TV screen time per day), while utilizing a multiple of the 30-minute TV broadcast. This facilitates more actionable real-world recommendations and is consistent with prior studies by our group and others (19,20,23,24).
To limit reverse causation related to behavior change from undiagnosed CRC, we measured sedentary habits at least one biennial cycle prior to assessment of CRC case and noncase status. We also examined light-intensity activities (standing or walking around at home, standing or walking around at work).
Assessment of Covariates
Height and weight were reported at baseline, and weight was updated biennially. BMI was calculated as weight/height2 (kg/m2). Physical activity was self-reported with validated questionnaires every 2–4 years (25). We assessed total caloric intake, red meat, fiber, calcium, folate, and alcohol consumption using 133-item FFQs every 4 years since 1991 (26). Diet quality was assessed using the Alternative Healthy Eating Index 2010 for which a higher score has been associated with reduced risk of chronic diseases (27,28). Participants reported the number of cigarettes smoked daily and the age they started and stopped (29). Cumulative exposure to smoking in pack-years was derived by multiplying daily packs by years during which that quantity was smoked.
We also assessed and updated family history of CRC among first-degree relatives; regular use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), or multivitamins; diabetes mellitus; menopausal status; and the use of menopausal hormone therapy (30–33). Every two years, we asked participants to report receipt of lower gastrointestinal endoscopies. The indication for endoscopy, including symptoms (eg, bleeding or abdominal pain), family history of CRC, or asymptomatic screening, was also collected.
Statistical Analysis
Follow-up began upon return of the 1991 questionnaire. Person-time accrued until CRC diagnosis, death from any cause, 50th birthdate, or the end of follow-up, whichever occurred first. We employed Cox proportional hazards modelling to estimate age-adjusted and multivariable-adjusted relative risks (RRs) and 95% confidence intervals (95% CIs). We used Schoenfield residual testing to confirm no violations of the proportional hazards assumption.
To represent long-term habits and reduce random within-person variation, exposures of interest were updated at each follow-up cycle using the cumulative average method, calculated using the midpoint of each frequency category as a continuous variable and averaged across all assessments prior to the current questionnaire. Covariates were chosen a priori based on putative CRC risk factors on a time-varying basis, including height (continuous, meters); BMI (continuous); family history of CRC, diabetes, lower endoscopy due to screening or other indications within 10 years (each yes/no); smoking (continuous pack-years); physical activity (metabolic equivalent task hours per week [METs-hours/week], continuous); regular use of aspirin, NSAIDs, multivitamins (each yes/no); menopausal status (pre- or postmenopausal), menopausal hormone use (premenopausal, never, and current use of menopausal hormones); and diet (total calories, red meat, fiber, folate, calcium, and alcohol consumed, Alternate Healthy Eating Index 2010, all continuous). Cumulative average was used when appropriate to represent long-term behaviors.
We also performed subgroup and sensitivity analyses to support our findings. We further limited our analysis to subjects without a family history of CRC or a personal history of recent lower endoscopy to ensure findings were not restricted to those with a suspected genetic predisposition for CRC. We evaluated the association between sedentary behaviors and conventional CRC. Finally, we examined the association between TV viewing time and young-onset CRC according to strata defined by measures of an unhealthy lifestyle, including BMI (<25 vs ≥25 kg/m2), physical activity (<15 vs ≥15 METs-hours/week [median]), and smoking status (ever vs never). Log-likelihood ratio tests were used to estimate P interaction. All P values were two-sided with values less than .05 considered statistically significant. SAS version 9.4 (Cary, NC) was used to perform all statistical analyses.
Results
We documented 118 incident cases of young-onset CRC over 22 years of follow-up encompassing 1 262 540 person-years. Median follow-up time was 13.9 years, and median age of CRC diagnosis was 45 years (interquartile range 41–47 years). Women reporting more time watching TV were more frequently postmenopausal, smoked a greater number of pack-years, had a higher rate of diabetes mellitus, more frequently used aspirin and NSAIDs, used less multivitamins, were less physically active, and had generally poorer indices of healthy dietary intake (Table 1).
Table 1.
Age-standardized characteristics according to average sedentary TV viewing time among women younger than 50 years, NHS II 1991–2011*
| Characteristics | Sedentary TV viewing time, hours per week |
||
|---|---|---|---|
| ≤7 | 7.1–14 | >14 | |
| Age, y | 42.3 (5.1) | 41.9 (5.3) | 41.2 (5.4) |
| BMI, kg/m2 | 25.2 (5.5) | 26.3 (6.1) | 27.5 (7.0) |
| Height, cm | 165 (7) | 165 (7) | 165 (7) |
| White race, % | 92.4 | 93.6 | 93.0 |
| Postmenopausal, % | 8.8 | 9.2 | 9.5 |
| Current hormone therapy among postmenopausal, % | 64 | 64 | 67 |
| Family history of colorectal cancer, % | 5.6 | 5.7 | 5.9 |
| Screening lower endoscopy within past 10 years, % | 3.6 | 3.2 | 3.2 |
| Lower endoscopy due to other indications within past 10 years†, % | 7.6 | 7.4 | 7.1 |
| History of diabetes, % | 1.6 | 2.1 | 2.7 |
| Ever smokers, % | 31 | 34 | 36 |
| Pack-years among ever smokers | 11.5 (9.1) | 12.5 (9.7) | 14.3 (10.6) |
| Alcohol intake, g/day | 3.6 (6.8) | 3.8 (7.1) | 3.7 (7.5) |
| Physical activity, MET-hours/week | 22.6 (28.4) | 19.8 (24.9) | 17.2 (23.9) |
| Regular aspirin use, % | 9.9 | 11 | 12 |
| Regular nonaspirin NSAID use, % | 26 | 28 | 29 |
| Current use of multivitamin, % | 46 | 44 | 41 |
| Calories, kcal/day | 1798 (559) | 1828 (558) | 1855 (569) |
| Red meat intake, servings/week | 5.9 (4.7) | 6.4 (4.7) | 7.0 (5.0) |
| Fiber intake, g/day | 19.6 (6.1) | 18.6 (5.6) | 17.7 (5.5) |
| Folate intake, μg/day | 546 (307) | 522 (297) | 495 (292) |
| Calcium intake, mg/day | 1133 (535) | 1092 (513) | 1046 (495) |
| Alternate Healthy Eating Index 2010 | 46.8 (11.1) | 44.8 (10.6) | 43.1 (10.5) |
All values other than age have been directly standardized to age distribution (in 5-year age group) of all the participants. Mean (SD) is presented for continuous variables. BMI = body mass index; CRC = colorectal cancer; MET = metabolic equivalent of tasks; NSAID = nonsteroid anti-inflammatory drug.
Lower endoscopy due to indications other than for screening (eg, having symptoms, family history of colorectal cancer, follow-up endoscopy, or positive fecal occult blood test).
Prolonged sedentary TV viewing time was associated with an increased risk of young-onset CRC, after adjusting for putative CRC risk factors (Table 2). As physical activity may confound the relationship between TV watching and young-onset CRC, we conducted further analyses to adjust for weekly physical energy expenditure, which did not materially alter our risk estimates. Further, our findings were slightly attenuated but still robust after additional adjustment for a possible mediator of this association, BMI. Compared to 7 or fewer, women with 7.1–14 hours of sedentary TV viewing time per week had a multivariable RR of 1.12 (95% CI = 0.72 to 1.75), with risk increasing among those reporting more than 14 hours per week (RR = 1.69, 95% CI = 1.07 to 2.67, Ptrend = .03).
Table 2.
Sedentary TV viewing time and risk of young-onset CRC diagnosed prior to age 50 years
| Sedentary TV viewing time, hours per week |
Ptrend§ | |||
|---|---|---|---|---|
| Young-onset CRC | ≤7 | 7.1–14 | >14 | |
| Cases | 52 | 33 | 33 | |
| Person-years | 629 656 | 367 368 | 265 516 | |
| Age-adjusted RR (95% CI) | 1 (referent) | 1.12 (0.72 to 1.74) | 1.69 (1.09 to 2.63) | .02 |
| Multivariable model 1 RR (95% CI)* | 1 (referent) | 1.15 (0.74 to 1.78) | 1.75 (1.12 to 2.76) | .02 |
| Multivariable model 2 RR (95% CI)† | 1 (referent) | 1.15 (0.74 to 1.79) | 1.77 (1.12 to 2.78) | .02 |
| Multivariable model 3 RR (95% CI)‡ | 1 (referent) | 1.12 (0.72 to 1.75) | 1.69 (1.07 to 2.67) | .03 |
Adjusted for height (continuous), family history of CRC (yes/no), diabetes (yes/no), screening lower endoscopy within past 10 years (yes/no), lower endoscopy due to other indications within past 10 years (yes/no), smoking (continuous pack-years), alcohol intake (continuous), regular use of aspirin (yes/no), non-steroidal anti-inflammatory drugs (yes/no), race, multivitamin (yes/no), menopausal status and menopausal hormone use (premenopausal, never, and current use of menopausal hormone), and dietary intake (total calories, red meat, fiber, folate, calcium, Alternate Healthy Eating Index 2010, continuous). CI= confidence interval; CRC = colorectal cancer; RR, relative risk.
Additionally adjusted for physical activities (continuous).
Additionally adjusted for physical activities (continuous) and body mass index (continuous).
Calculated using the median of each sedentary behavior category as a continuous variable.
We observed similar risk among subjects reporting our highest category of excessive TV viewing time (>14 hours per week) when we restricted our analysis to individuals with no family history of CRC (multivariable RR = 1.83, 95% CI = 1.15 to 2.95; Ptrend = .02; Table 3) and no lower endoscopy in the past 10 years (multivariable RR = 1.76, 95% CI = 1.10 to 2.83; Ptrend = .02).
Table 3.
Sedentary TV viewing time and risk of young-onset CRC diagnosed prior to age 50 years among subjects without family or endoscopy history
| Sedentary TV viewing time (hours per week) |
Ptrend§ | |||
|---|---|---|---|---|
| Young-onset CRC | ≤7 | 7.1–14 | >14 | |
| No family history of CRC | ||||
| Cases | 47 | 26 | 32 | |
| Person-years | 593 474 | 346 385 | 250 593 | |
| Age-adjusted RR (95% CI) | 1 (referent) | 0.99 (0.61 to 1.60) | 1.84 (1.17 to 2.91) | .01 |
| Multivariable model 1 RR (95% CI)* | 1 (referent) | 1.02 (0.63 to 1.66) | 1.92 (1.21 to 3.06) | .01 |
| Multivariable model 2 RR (95% CI)† | 1 (referent) | 0.99 (0.61 to 1.61) | 1.83 (1.15 to 2.95) | .02 |
| No endoscopy in past 10 years | ||||
| Cases | 47 | 30 | 31 | |
| Person-years | 564 521 | 332 045 | 242 157 | |
| Age-adjusted RR (95% CI) | 1 (referent) | 1.13 (0.71 to 1.78) | 1.76 (1.11 to 2.78) | .02 |
| Multivariable model 1 RR (95% CI)*,‡ | 1 (referent) | 1.15 (0.72 to 1.82) | 1.84 (1.15 to 2.94) | .01 |
| Multivariable model 2 RR (95% CI)† | 1 (referent) | 1.11 (0.70 to 1.77) | 1.76 (1.10 to 2.83) | .02 |
Adjusted for height (continuous), diabetes (yes/no), screening lower endoscopy within past 10 years (yes/no), lower endoscopy due to other indications within past 10 years (yes/no), smoking (continuous pack-years), alcohol intake (continuous), regular use of aspirin (yes/no), nonsteroidal anti-inflammatory drugs (yes/no), race, multivitamin (yes/no), menopausal status and menopausal hormone use (premenopausal, never, and current use of menopausal hormone), and dietary intake (total calories, red meat, fiber, folate, calcium, Alternate Healthy Eating Index 2010, continuous). CI = confidence interval; CRC = colorectal cancer; RR = relative risk.
Additionally controlled for body mass index (continuous) and physical activities (continuous).
Additionally adjusted for family history of CRC (yes/no) but not screening lower endoscopy within past 10 years and lower endoscopy due to other indications within past 10 years.
Calculated using the median of each sedentary behavior category as a continuous variable.
Subgroup analyses by anatomic site, although limited by case numbers, demonstrated that sedentary TV viewing time had an outsized influence on risk of young-onset rectal cancer, with elevated multivariable hazards of 1.91 (95% CI = 0.86 to 4.25) for our moderate (7.1–14 hours per week) group and 2.44 (95% CI = 1.03 to 5.78) for our excessive group (Ptrend = .04; Table 4).
Table 4.
Sedentary TV viewing time and risk of young-onset CRC diagnosed prior to age 50 years by anatomic site
| Sedentary TV viewing time (hours per week) |
Ptrend† | |||
|---|---|---|---|---|
| Young-onset CRC | ≤7 | 7.1–14 | >14 | |
| Colon cancer | ||||
| Cases | 40 | 20 | 22 | |
| Person-years | 629 664 | 367 382 | 265 527 | |
| Age-adjusted RR (95% CI) | 1 (referent) | 0.88 (0.51 to 1.51) | 1.42 (0.84 to 2.40) | .25 |
| Multivariable model 1 RR (95% CI)* | 1 (referent) | 0.90 (0.52 to 1.55) | 1.47 (0.85 to 2.54) | .22 |
| Rectal cancer | ||||
| Cases | 12 | 13 | 11 | |
| Person-years | 629 696 | 367 385 | 265 534 | |
| Age-adjusted RR (95% CI) | 1 (referent) | 1.92 (0.87 to 4.22) | 2.62 (1.15 to 6.00) | .02 |
| Multivariable model 1 RR (95% CI)* | 1 (referent) | 1.91 (0.86 to 4.25) | 2.44 (1.03 to 5.78) | .04 |
Adjusted for height (continuous), diabetes (yes/no), family history of CRC, screening lower endoscopy within past 10 years (yes/no), lower endoscopy due to other indications within past 10 years (yes/no), smoking (continuous pack-years), alcohol intake (continuous), regular use of aspirin (yes/no), nonsteroidal anti-inflammatory drugs (yes/no), race, multivitamin (yes/no), menopausal status and menopausal hormone use (premenopausal, never, and current use of menopausal hormone), dietary intake (total calories, red meat, fiber, folate, calcium, Alternate Healthy Eating Index 2010, continuous), physical activities (continuous), and body mass index (continuous). CI = confidence interval; CRC = colorectal cancer; RR, relative risk.
Calculated using the median of each sedentary behavior category as a continuous variable.
We observed no clear increase in risk for other forms of sitting at home, such as meal time or time spent at a desk (Supplementary Table 1, available online). No obvious association between sitting away from home and risk of young-onset CRC was observed. A trend toward reduced risk of young-onset CRC was observed among participants reporting greater light intensity behaviors, such as standing or walking at work or home (Supplementary Table 2, available online). Consistent with prior work, we detected an elevated risk of CRC diagnosed after age 50 years with prolonged sedentary TV viewing time, though this did not reach statistical significance (Supplementary Table 3, available online).
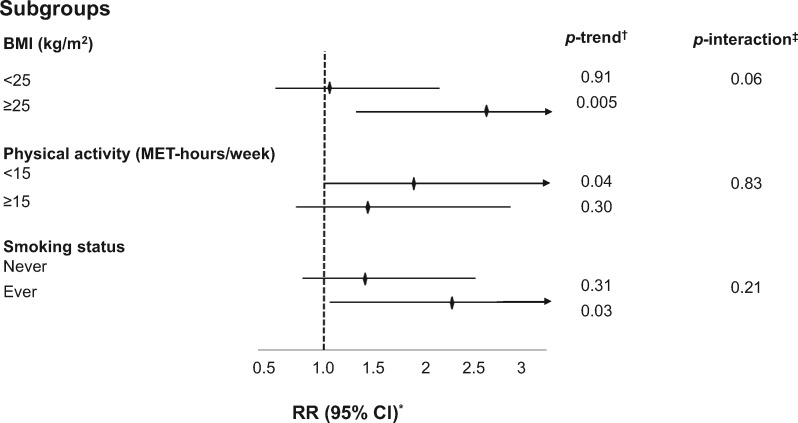
Finally, we evaluated differences in the association between sedentary TV viewing time and CRC prior to age 50 years by several strata. Overweight or obese subjects, those who engaged in less physical activity, and those with a prior history of smoking had elevated risks for young-onset CRC compared to their referent counterparts, with BMI and TV screen time demonstrating the strongest statistical interaction (Pinteraction = .06; Figure 1). A joint classification analysis of both TV screen time and BMI suggests risk of young-onset CRC is further augmented in the presence of both prolonged screen time and elevated BMI (Supplementary Table 4, available online).
Figure 1.
Subgroup analyses of sedentary TV viewing time and risk of young-onset CRC diagnosed prior to age 50 years. BMI = body mass index; MET = metabolic equivalent of tasks; CI = confidence interval; RR = relative risk. *RR was for the comparison of >14 versus 0-7 hours per week of sedentary TV viewing time; was adjusted for the same set of covariates as denoted in model 3 of Table 2 with the exception of each strata-defining covariate. †Calculated using the median of each sedentary behavior category as a continuous variable. ‡Calculated using log likelihood ratio test.
Discussion
Our study provided an unprecedented opportunity to investigate young-onset CRC with long-term prospective follow-up of participants ages 25–42 years at enrollment with a median age at CRC diagnosis of just 45 years. We found that increased TV viewing time was statistically significantly associated with risk of young-onset CRC, particularly rectal cancer. This association was independent of other traditional CRC risk factors, although risk appeared greater in subgroups defined by high BMI, less physical activity, and a positive smoking history. Collectively, these findings are among the first to prospectively link specific sedentary behavioral patterns with risk of young-onset CRC.
Evidence on the association between time spent watching TV and conventional CRC has been accumulating, but data on young-onset CRC remains scarce. A recent meta-analysis demonstrated that sedentary TV viewing time was associated with a 54% increased risk of colon cancer, but included just two studies (one case-control [34] and one cohort study [35]) composed largely of older participants (13). Prior findings from our group leveraging two cohorts of older adults (Nurses’ Health Study and Health Professionals Follow-Up Study; mean age 64–68 years) showed a positive association between sedentary TV viewing time and CRC risk (19). Notably, this association appeared greater among women younger than age 65 years, supporting our findings in this third, younger, independent cohort.
Although our subgroup analysis demonstrated a trend toward elevated risk of conventional CRC with prolonged TV viewing, this was not statistically significant. It is possible that TV watching serves as a better surrogate for a more sedentary lifestyle among younger populations, as they are more likely to be employed full-time with excess TV consuming a comparatively larger proportion of waking, nonworking hours, compared to those older and retired. Moreover, our results also suggest the risk of young-onset CRC attributable to sedentary TV viewing may not be fully mediated through obesity and is independent of physical activity, as a possible confounder.
To improve detection of young-onset CRC, the American Cancer Society recently recommended that routine screening begins at age 45 years, rather than 50 years (10). However, adherence to prior, less strict guidelines is just 62% (36). This low uptake suggests cases in young people will continue to be identified late in real-world settings, particularly among those less than age 45 years who would not benefit from this change. Taken together, targeted identification of those most at risk is critically important to help mitigate this rising burden. Our findings indicate that reducing sedentary behaviors, particularly TV watching, may be an effective, low-risk, and actionable risk reduction measure.
Notably, rectal cancer in young people is rising faster than colon cancer, particularly in the United States (6). By 2030, each will increase by 124% and 90%, respectively, among individuals ages 20–34 years, and 46% and 28% among those ages 35–49 years (2,4). Prevention of rectal cancer remains challenging, because the majority of risk factors for conventional CRC have been more strongly associated with colon, rather than rectal cancer (37). Although our finding of disproportionately elevated risk of rectal cancer requires validation, it may help partially explain this phenomenon.
TV viewing, even among comparable behaviors, has been consistently linked to adverse health outcomes (15–18,38,39), including CRC (13,19) and precursor adenomas (19,20). Several biological mechanisms support our observations. Sedentary TV viewing may be particularly deleterious given the absence of social or occupational cues to unbroken sitting, resulting in extended exposure to fecal carcinogens, such as secondary bile acids (40,41). Prolonged sitting has also been shown to impair glucose homeostasis and decrease vitamin D levels (42–45). In contrast, standing and other light activities can improve blood flow and muscle contraction and result in improved glucose regulation and endothelial function (46,47). Sedentary behaviors have been linked to gut dysbiosis (48,49), a recognized determinant in CRC incidence and outcomes (50–52). Increased TV viewing may result in lower energy use, higher caloric intake, and less healthy diets, for which all are CRC risk factors. However, in our study, the association remained after adjusting for each. Finally, the trend toward stronger associations among people who were obese or overweight, less physically active, and had a prior smoking history suggest that individuals with prolonged sitting may have other factors that enhance the detrimental effects of being sedentary.
The strengths of this study include the use of a large prospective cohort with more than 20 years of follow-up, which was necessary because although young-onset CRC is increasing, it is still relatively rare. Information on sedentary TV viewing time was regularly updated prospectively and uncoupled from other comparable activities including TV with nonsedentary behavior (eg, during exercise or while performing chores), as well as occupational time spent sitting. This careful assessment limits recall and ascertainment bias. Quantification of sedentary behavior was conducted at least one biennial cycle prior to assessment of CRC status, making reverse causation less likely. We collected detailed information on a variety of known risk factors for traditional CRC, and their inclusion in our multivariable models did not materially alter our estimated effects, lending credence to our findings.
We acknowledge several limitations. Time using a computer or other devices, including tablets and smartphones, was not assessed. However, these habits, and the use of handheld devices in particular, were not as common during the majority of our study period (1991–2011). Additionally, the use of such devices typically occurs in settings distinct from those associated with watching TV, which often occurs at home. Limited evidence suggests that when compared, TV viewing, but not computer use, is more closely associated with chronic disease risk (53–55). The reasons for this may be because of a decrease in energy expenditure from TV watching compared to other media like video games (54,56,57) coupled with an increase in energy intake associated with staying home and a susceptibility to TV programming cues for unhealthy eating (15,58). It is possible that the lack of association between other sedentary behaviors could be due not to physiologic differences, but rather to measurement error of habits harder to quantify by recall (ie, when compared to TV broadcasts with predetermined start and end times). However, others have demonstrated the reliability and validity of TV time in comparison to other sedentary behaviors (59,60). Additionally, such bias was minimized by use of the cumulative average and would have resulted in attenuation toward the null from random misclassification. Our study was comprised mainly of white female nurses, an accurate reflection of the profession’s demographics at cohort conception, and a racial group disproportionately burdened by young-onset CRC (3,21). While validation in other ethnic populations and among men would be important, these characteristics serve to increase internal validity given the participants high rate of follow-up and ability to provide self-reported health data. As with any observational study, there remains the possibility of unmeasured confounding. Finally, some subgroup analyses were characterized by low case numbers, necessitating cautious interpretation.
In closing, we found that increased sedentary TV viewing time was associated with elevated risk of young-onset CRC. These findings provide further evidence on the importance of maintaining an active lifestyle. Minimizing inactivity may offer protection against young-onset CRC beyond any risk reduction gained from the prevention of other major chronic diseases. Further studies are needed both to elucidate the underlying biological mechanism that may explain this phenomenon and to determine whether a more intensive screening program for sedentary persons at-risk may be of benefit.
Funding
This work was supported by the National Institutes of Health (UM1CA176726, R01CA137178, R01CA205406, R35CA197735, K24DK098311 to ATC, T32CA009001 to LHN); the Crohn's and Colitis Foundation (Senior Investigator Award to ATC, Research Fellowship Award to LHN); National Research Foundation of Korea (NRF-2018R1C1B6008822 & NRF-2018R1A4A1022589 to NK); Massachusetts General Hospital (Stuart and Suzanne Steel Research Scholars Award to ATC); the Raymond P. Lavietes Foundation (Young Investigator Award to YC).
Notes
Affiliations of authors: Division of Gastroenterology (LHN, ATC) and Clinical and Translational Epidemiology Unit (LHN, PHL, ATC), Massachusetts General Hospital and Harvard Medical School, Boston, MA; Division of Public Health Sciences, Department of Surgery, Washington University School of Medicine, St Louis, MO (XZ, XZ, XL, YC); Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases XBZ and Department of Colorectal Surgery XBZ, the Six Affiliated Hospital, Sun Yat-sen University, Guangzhou, P.R. China; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA (NK, KW, WCW, ELG); Department of Food Science and Biotechnology, Dongguk University, Goyang, Republic of Korea (NK); Yale Cancer Center, New Haven, CT (CSF); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (SO, WCW, ELG); Program in MPE Molecular Pathological Epidemiology, Department of Pathology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (SO); Department of Oncologic Pathology, Dana-Farber Cancer Institute and Harvard Medical School, Boston, MA (SO, ELG); Broad Institute of MIT and Harvard, Cambridge, MA (SO, ATC); Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA (KN); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (WCW, ATC); Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, MA (ATC); Siteman Cancer Center, Washington University School of Medicine, St Louis, MO (YC).
Author Contributions: Study concept and design: LHN, ATC, ELG, and YC. Acquisition of data: KW, CSF, WCW, ATC, ELG. Analysis and interpretation of data: all coauthors. Drafting of the manuscript: LHN and YC. Critical revision of the manuscript for important intellectual content: all coauthors. Statistical analysis: XYZ and YC. Obtained funding: CSF, WCW, ATC, ELG, and YC. Administrative, technical, or material support: YC. Study supervision: YC.
We would like to thank the participants and staff of the Nurses’ Health Study II for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Supplementary Material
References
- 1. Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89(2):216–224. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A.. Colorectal cancer mortality rates in adults ages 20 to 54 years in the United States, 1970-2014. JAMA. 2017;318(6):572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larsen IK, Bray F.. Trends in colorectal cancer incidence in Norway 1962-2006: an interpretation of the temporal patterns by anatomic subsite. Int J Cancer. 2010;126(3):721–732. [DOI] [PubMed] [Google Scholar]
- 6. Lui RN, Tsoi K, Sung JJ.. Global increasing incidence of colorectal cancer in younger individuals and 55 years: temporal trends of 65,909 cases spanning 3 continents. Gastroenterology. 2018;154(6):S–70. [Google Scholar]
- 7. Pearlman R, Frankel WL, Swanson B, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017;3(4):464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25(8):1128–1139. [DOI] [PubMed] [Google Scholar]
- 9. Lortet-Tieulent J, Soerjomataram I, Lin CC, et al. U.S. burden of cancer by race and ethnicity according to disability-adjusted life years. Am J Prev Med. 2016;51(5):673–681. [DOI] [PubMed] [Google Scholar]
- 10. Wolf Andrew MD, Fontham Elizabeth TH, Church Timothy R, et al. Colorectal cancer screening for average‐risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–281. [DOI] [PubMed] [Google Scholar]
- 11. Kerr J, Anderson C, Lippman SM.. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. 2017;18(8):e457–e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iannotti RJ, Wang J.. Trends in physical activity, sedentary behavior, diet, and BMI among US adolescents, 2001–2009. Pediatrics. 2013. http://pediatrics.aappublications.org/content/early/2013/09/11/peds.2013-1488.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmid D, Leitzmann MF.. Television viewing and time spent sedentary in relation to cancer risk: a meta-analysis. J Natl Cancer Inst. 2014;106(7). [DOI] [PubMed] [Google Scholar]
- 14. Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123–132. [DOI] [PubMed] [Google Scholar]
- 15. Hu FB, Li TY, Colditz GA, et al. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–1791. [DOI] [PubMed] [Google Scholar]
- 16. Hu FB, Leitzmann MF, Stampfer MJ, et al. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161(12):1542–1548. [DOI] [PubMed] [Google Scholar]
- 17. Warren T, Barry V, Hooker S, et al. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med Sci Sports Exerc. 2010;42(5):879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stamatakis E, Hamer M, Dunstan DW.. Screen-based entertainment time, all-cause mortality, and cardiovascular events: population-based study with ongoing mortality and hospital events follow-up. J Am Coll Cardiol. 2011;57(3):292–299. [DOI] [PubMed] [Google Scholar]
- 19. Keum N, Cao Y, Oh H, et al. Sedentary behaviors and light-intensity activities in relation to colorectal cancer risk. Int J Cancer. 2016;138(9):2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao Y, Meyerhardt JA, Chan AT, et al. Television watching and colorectal cancer survival in men. Cancer Causes Control. 2015;26(10):1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. [DOI] [PubMed] [Google Scholar]
- 22. Davenport ER, Cusanovich DA, Michelini K, et al. Genome-wide association studies of the human gut microbiota. PLoS One. 2015;10(11):e0140301.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma P, Yao Y, Sun W, et al. Daily sedentary time and its association with risk for colorectal cancer in adults: a dose–response meta-analysis of prospective cohort studies. Medicine (Baltimore). 2017;96(22):e7049.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morris JS, Bradbury KE, Cross AJ, et al. Physical activity, sedentary behaviour and colorectal cancer risk in the UK Biobank. Br J Cancer. 2018;118(6):920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. [DOI] [PubMed] [Google Scholar]
- 26. Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–1271. [DOI] [PubMed] [Google Scholar]
- 28. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higuchi LM, Khalili H, Chan AT, et al. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol. 2012;107(9):1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Colditz GA, Stampfer MJ, Willett WC, et al. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987;126(2):319–325. [DOI] [PubMed] [Google Scholar]
- 31. Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giovannucci E, Stampfer MJ, Colditz GA, et al. Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med. 1998;129(7):517–524. [DOI] [PubMed] [Google Scholar]
- 33. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60(4):207–221. [DOI] [PubMed] [Google Scholar]
- 34. Steindorf K, Tobiasz-Adamczyk B, Popiela T, et al. Combined risk assessment of physical activity and dietary habits on the development of colorectal cancer. A hospital-based case-control study in Poland. Eur J Cancer Prev. 2000;9(5):309–316. [DOI] [PubMed] [Google Scholar]
- 35. Howard RA, Freedman DM, Park Y, et al. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2008;19(9):939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White A, Thompson TD, White MC, et al. Cancer screening test use—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murphy CC, Singal AG.. Establishing a research agenda for early-onset colorectal cancer. PLoS Med. 2018;15(6):e1002577.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krishnan S, Rosenberg L, Palmer JR.. Physical activity and television watching in relation to risk of type 2 diabetes: the black women’s health study. Am J Epidemiol. 2009;169(4):428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ford ES, Schulze MB, Kröger J, Pischon T, Bergmann MM, Boeing H.. Television watching and incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition–Potsdam Study. J Diabetes. 2010;2(1):23–27. [DOI] [PubMed] [Google Scholar]
- 40. Aune D, Chan DSM, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagengast FM, Grubben MJ, van Munster IP.. Role of bile acids in colorectal carcinogenesis. Eur J Cancer. 1995;31A(7–8):1067–1070. [DOI] [PubMed] [Google Scholar]
- 42. Edwardson CL, Gorely T, Davies MJ, et al. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS One. 2012;7(4):e34916.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmidt MD, Cleland VJ, Thomson RJ, et al. A comparison of subjective and objective measures of physical activity and fitness in identifying associations with cardiometabolic risk factors. Ann Epidemiol. 2008;18(5):378–386. [DOI] [PubMed] [Google Scholar]
- 44. Balkau B, Mhamdi L, Oppert JM, et al. Physical activity and insulin sensitivity: the RISC study. Diabetes. 2008;57(10):2613–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691–2709. [DOI] [PubMed] [Google Scholar]
- 46. Olufsen MS, Ottesen JT, Tran HT, et al. Blood pressure and blood flow variation during postural change from sitting to standing: model development and validation. J Appl Physiol (1985). 2005;99(4):1523–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thosar SS, Johnson BD, Johnston JD, et al. Sitting and endothelial dysfunction: the role of shear stress. Med Sci Monit. 2012;18(12):RA173–RA180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bressa C, Bailen-Andrino M, Perez-Santiago J, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One. 2017;12(2):e0171352.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mach N, Fuster-Botella D.. Endurance exercise and gut microbiota: a review. J Sport Health Sci. 2017;6(2):179–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brennan CA, Garrett WS.. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol. 2016;70:395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shah MS, DeSantis TZ, Weinmaier T, et al. Leveraging sequence-based faecal microbial community survey data to identify a composite biomarker for colorectal cancer. Gut. 2018;67(5):882–891. [DOI] [PubMed] [Google Scholar]
- 52. Mehta RS, Nishihara R, Cao Y, et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by fusobacterium nucleatum in tumor tissue. JAMA Oncol. 2017;3(7):921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Jong E, Visscher TL, HiraSing RA, et al. Association between TV viewing, computer use and overweight, determinants and competing activities of screen time in 4- to 13-year-old children. Int J Obes. 2013;37(1):47–53. [DOI] [PubMed] [Google Scholar]
- 54. Nang EE, Salim A, Wu Y, et al. Television screen time, but not computer use and reading time, is associated with cardio-metabolic biomarkers in a multiethnic Asian population: a cross-sectional study. Int J Behav Nutr Phys Act. 2013;10(1):70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Altenburg TM, de Kroon ML, Renders CM, et al. TV time but not computer time is associated with cardiometabolic risk in Dutch young adults. PLoS One. 2013;8(2):e57749.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lanningham-Foster L, Foster RC, McCrady SK, et al. Activity-promoting video games and increased energy expenditure. J Pediatr. 2009;154(6):819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Otten JJ, Jones KE, Littenberg B, et al. Effects of television viewing reduction on energy intake and expenditure in overweight and obese adults: a randomized controlled trial. Arch Intern Med. 2009;169(22):2109–2115. [DOI] [PubMed] [Google Scholar]
- 58. Pearson N, Biddle SJ.. Sedentary behavior and dietary intake in children, adolescents, and adults. A systematic review. Am J Prev Med. 2011;41(2):178–188. [DOI] [PubMed] [Google Scholar]
- 59. Lynch BM, Friedenreich CM, Khandwala F, et al. Development and testing of a past year measure of sedentary behavior: the SIT-Q. BMC Public Health. 2014;14(1):899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wijndaele K, De Bourdeaudhuij I, Godino JG, et al. Reliability and validity of a domain-specific last 7-d Sedentary Time Questionnaire. Med Sci Sports Exerc. 2014;46(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.