Abstract
Hypoxia is a state of low oxygen tension found in numerous solid tumours. It is typically associated with abnormal vasculature, which results in a reduced supply of oxygen and nutrients, as well as impaired delivery of drugs. The hypoxic nature of tumours often leads to the development of localized heterogeneous environments characterized by variable oxygen concentrations, relatively low pH, and increased levels of reactive oxygen species (ROS). The hypoxic heterogeneity promotes tumour invasiveness, metastasis, angiogenesis, and an increase in multidrug-resistant proteins. These factors decrease the therapeutic efficacy of anticancer drugs and can provide a barrier to advancing drug leads beyond the early stages of preclinical development. This review highlights various hypoxia-targeted and activated design strategies for the formulation of drugs or prodrugs and their mechanism of action for tumour diagnosis and treatment.
1. Introduction
A global increase in cancer diagnoses, combined with moderate clinical response rates of conventional chemotherapies, necessitates the discovery of novel drugs and delivery vehicles that modulate their anticancer effects via tumour specific mechanisms. Characterized by rapid cellular proliferation, cancer malignancies can be triggered through a variety of mechanisms, including exogenous agents, altered gene expression, or protein dysfunction. For instance, a single genetic alteration may lead to the development of a malignant tumour. Consequently, current therapeutic regimens are largely focused on genetic-based cancer taxonomy in addition to basic morphological aspects. Advances in cancer genomics, proteomic biomarker technologies, and cancer therapeutics have enabled the progression from a “one drug for all” approach to an expanding cadre of “personalized medicines”. This evolution of drug discovery reflects progress towards a new paradigm that has been proposed as a therapeutic ideal.1–4 In January 2015, the introduction of the Precision Medicine Initiative (PMI) by the then-President Barack Obama served to broaden the concept of personalized medicine to encompass an extensive range of determinants that could lead to improved health impacts.5
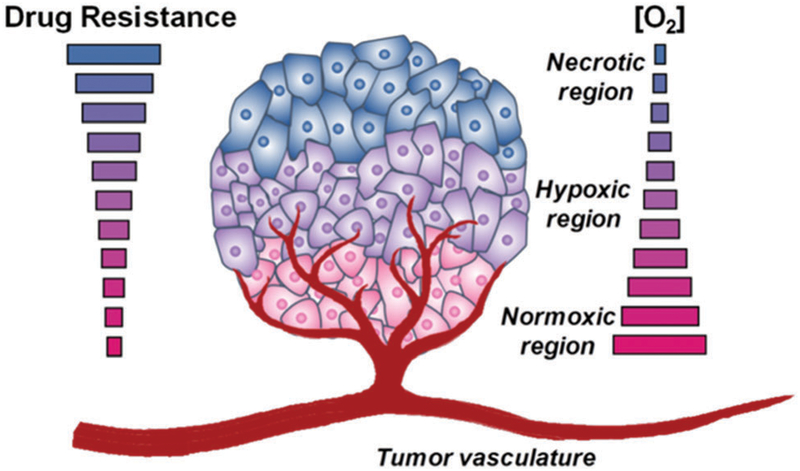
Tumour hypoxia refers to solid tumour regions characterized by low oxygen levels. The origins of hypoxia can be traced in large measure to the abnormal vascularization, which results in an insufficient oxygen and nutrient supply to interior regions of the tumour.6,7 The existence of hypoxia within human tumours was first postulated almost 60 years ago by Thomlinson and Gray,8 who observed that tumour hypoxia imparted resistance to chemo- and radiation therapy (Fig. 1). Owing to the highly dynamic tumour microenvironment, the oxygen concentration can vary from 0.02–2% O2 (below 2.5 mmHg pO2), as compared to 2–9% in normal cells (40 mmHg pO).9 2 The recent development of analytical tools, such as the Eppendorf electrode for the assessment of molecular oxygen levels in tumours and biomarkers for non-invasive hypoxia imaging, have helped to underscore the common existence of hypoxia in human solid tumours.10–14 Relative to oxygenated healthy cells and tissues, hypoxic tumours are generally characterized by a lower pH (acidic environment) as a result of increased anaerobic respiration,15 high levels of reactive oxygen species (ROS),16,17 enhanced local invasiveness,18–20 altered metabolism,21,22 unregulated angiogenesis,23,24 incipient metastases,25 and down-regulated DNA repair pathways.26 Hypoxia also provides conditions favourable for the spread of cancer stem cells.27 Moreover, it can result in the suppression of innate and adaptive immune response mechanisms in the tumour microenvironment.28,29 Hypoxia-related intratumoural heterogeneity plays a pivotal role in metastatic tumour progression and can lead to different dose–response profiles for drugs used in clinical practice.
Fig. 1.
Hypoxia-associated tumour microenvironment. Generally, solid tumours are characterized by unusual tumour vasculature. When the rate of angiogenesis cannot support the increased rate of tumour growth, the oxygen supply to tumour cells is restricted, and regions of hypoxia develop. Hypoxic tumours exhibit enhanced aggressiveness, metastasis, and resistance to radiation and chemotherapy.
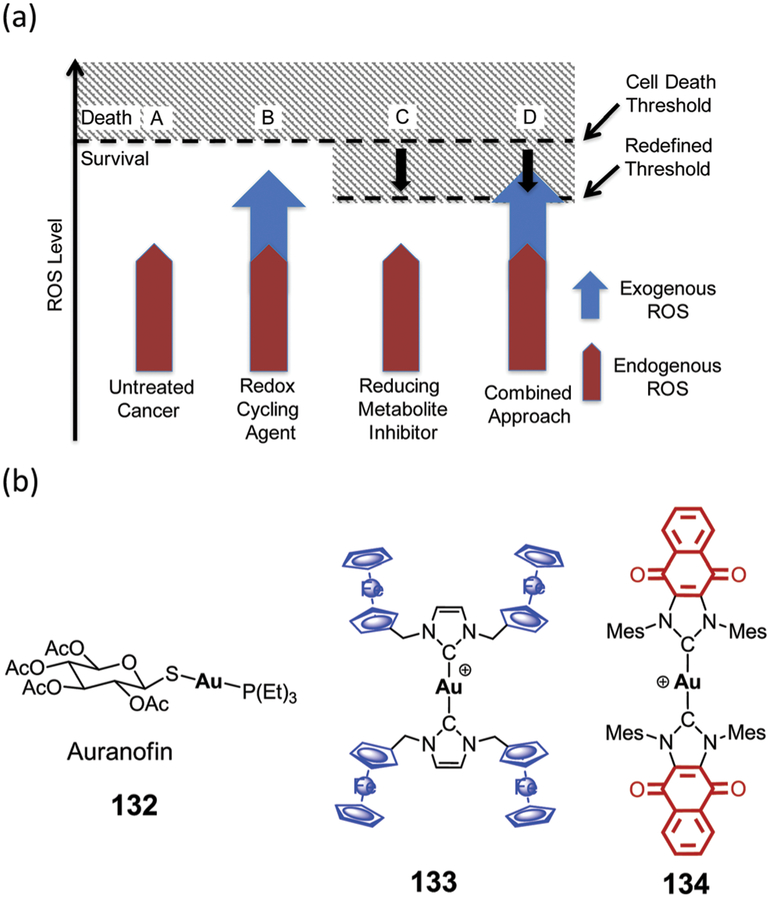
Many of these deleterious effects are ascribed to (a) an insufficient supply of nutrients and anticancer drugs,30 (b) the development of multidrug-resistant proteins,31 (c) cellular proliferation, which is increased after re-oxygenation of hypoxic cells,32 and (d) enhanced expression of various genes responsible for the upregulation of angiogenesis, tumour invasion, and meta-stases.9,25,27,33–35 As normal cells do not typically contain hypoxic regions, these unique and unfavourable features of hypoxic tumours may be exploited as an approach to developing cancer-selective therapies. In addition, hypoxic regions represent an environment in which the normal antioxidant pathways are off balance. This result in a higher level of oxidative stress. A number agents can accentuate exogenous ROS levels via redox cycling or other mechanisms thus increasing oxidative stress. In this review, we summarize the most promising strategies being pursued in the context of hypoxia-targeting drug discovery and development. Not covered are approaches to hypoxia-selective sensors. However, systems that show promise for both therapy and sensing, so-called theranostics, are discussed where hypoxia is critical to effective function.
2. Hypoxia-targeted and activated prodrugs
Continuous changes in the field of pharmacoeconomics and a growing understanding of cancer biology have led the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to encourage drug developers to identify new diagnostic tools based on predictive biomarkers with the goal of achieving specific and selective personalized cancer treatments.36–39 This effort has inter alia generated an increased understanding of the role hypoxia plays in tumour metastatic progression and in the regulation of drug action. Against this background, hypoxia-targeted therapeutics have emerged as a promising strategy in the development of personalized medicine, particularly in the area of cancer. The drug release profile and pharmacokinetics of various prodrugs in hypoxic environments have been monitored by their conjugation to diagnostic units including fluorophores, PET, or MRI agents, which are termed as “all-in-one” theranostic agents.40–42 The theranostic strategy is a “smart” combination of diagnosis and therapy, which provides positive and negative feedback information after cancer therapy, through visualization of disease status; it can lead directly to increases in drug efficacy. For an overview from a clinical perspective, the reader is referred to several excellent reviews and monographs.43–45 In this Review, hypoxia-activated/bioreductive prodrugs, sometimes termed hypoxia-selective cytotoxins, will be discussed in detail. Based on their design and activation mechanism, these strategies have been divided into four categories, which are treated in turn.
3. Quinone-based therapeutics and theranostics
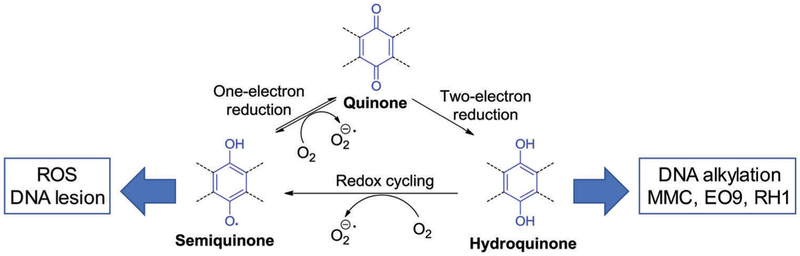
Quinones are common subunits found in numerous natural and synthetic compounds that include anticancer and antimicrobial agents, dyes, vitamin Ks, and cofactors (topaquinone, pyrroloquinone).46–49 Because of their propensity to undergo facile reduction, quinones play fundamental roles in various cellular functions, including redox cycling50–52 and energy transduction.53,54 Quinones can undergo a one-electron reduction mediated by various reductive enzymes, such as cytochrome reductase (P-450R, CPR), NADH-cytochrome b5 reductase (CB5R), and NADPH:ubiquinone oxidoreductase (complex I). This results in the formation of semiquinone radicals. These radical anions can react with molecular oxygen, which results in enhanced ROS production and increased oxidative stress. Generally, the formation of semiquinone radical anions is suppressed in normoxic environment. In hypoxic environments, the semiquinone radicals can be further reduced to hydroquinones (Fig. 2). However, the resulting hydroquinone species are not benign. They are, for instance, associated with toxicity related to DNA cross-linking. Two-electron reduction processes are often correlated with the presence of DT-diaphorase [also referred as NAD(P)H:(quinone-acceptor)oxidoreductase (NQO1) in later discussions], an enzyme that promotes two-electron reduction and which is commonly overexpressed in many human solid tumours, such as breast, ovarian, thyroid, and colon cancer.55–61 The use of special design strategies has enabled the production and selective activation of quinone-based prodrug systems, either in a hypoxic tumour environment, through a one-electron reduction pathway, or by DT-diaphorase (NQO1), a two-electron reduction enzyme.
Fig. 2.
Quinone reduction routes mediated by cytochrome reductase, ubiquinone reductase, and DT-diaphorase.
3.1. Quinone as a drug core scaffold
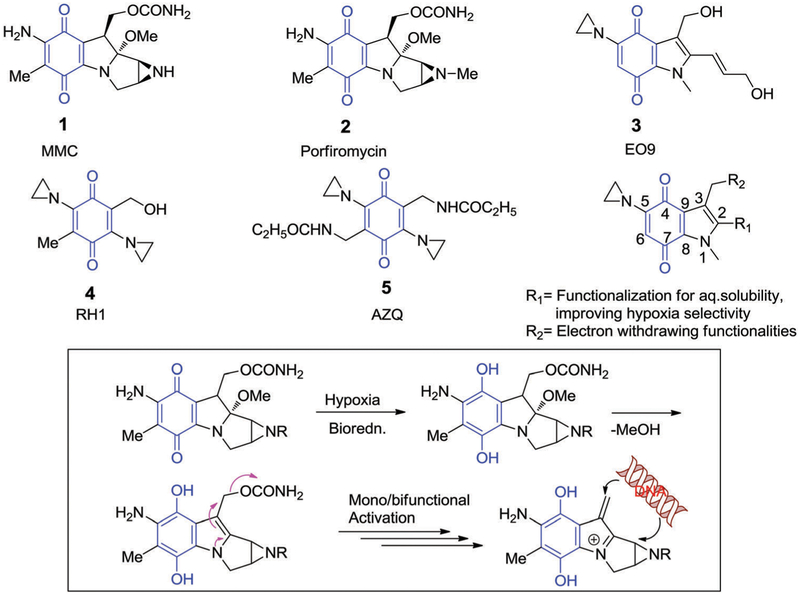
The first quinone-based drug, mitomycin (1, MMC),62,63 was recognized in the 1960s and proposed to induce hypoxia-selective cytotoxicity through a reductive mechanism that was followed by DNA alkylation (Fig. 3).64,65 NQO1 proved capable of activating 1. However, the tumour response towards 1 proved unrelated to NQO1 expression levels as inferred from many studies that failed to demonstrate a correlation between these two variables.66 On the other hand, 1 was found to be responsive to several cancer-associated reductases, including NADPH: cytochrome P450 reductase (P-450R), NADPH:cytochrome b5 reductase (b-5R), xanthine oxidase (XO), and NADPH:cytochrome b5 reductase (b-5R), and NAD(P)H:quinone oxidoreductase 2 (NQO2). Moreover, activation of 1 by NQO1 was found to be pH dependent,67 providing a rationale for why it was less sensitive towards hypoxic cells.68
Fig. 3.
Quinone-based bioreductive compounds and proposed activation mechanism for MMC (1) and porfiromycin (2). As shown schematically, this activation results in the formation of a DNA alkylating agent.
The above findings prompted further development of an MMC analogue, porfiromycin (2, Fig. 3), as a hypoxia-sensitive quinone-based drug; its sensitivity was confirmed through clinical evaluation.69–71 Further efforts were also made to develop aziridinylquinone-based prodrugs, namely EO9 (3),72,73 RH1 (4),74–78 and AZQ (5),79–81 as analogues of 1 with enhanced selectivity toward hypoxic tumour environments. In these agents, the thought was that the aziridine ring attached directly to indolequinone and benzoquinone would become activated upon bioreduction, and could serve as a potent alkylating agent that reacts with nucleophilic sites within DNA strands (Fig. 3).82
The mode of action of 3 has been extensively investigated under both hypoxic and normoxic conditions.72,73,83 Three major pathways are believed to be involved: (i) ROS generation by redox cycling to induce DNA strand breaks followed by quinone moiety reduction promoted by a one-electron reductase, (ii) DNA alkylation by the electrophilic centres at C3 and C2 indole position upon quinone reduction by one- and two-electron reductase, and (iii) DNA alkylation by aziridine ring opening after protonation.84 Under normoxic conditions, tumours expressing NQO1 displayed good response towards 3. However, under hypoxic condition, one-electron reductase (P450R) activity was predominant and resulted in enhanced sensitivity to 3 in tumours expressing low levels of NQO1. These results led to the suggestion that the cytotoxicity of 3 under both aerobic and anaerobic conditions reflects the NQO1 expression levels.55 Animal studies confirmed that 3 exhibited activity against solid tumours with minimal bone marrow toxicity. Lead 3 was evaluated in a phase II clinical trial for the intravesical treatment of advanced breast, bladder, colorectral, gastric and non-small lung cancers. Ultimately, 3 failed to demonstrate a significant therapeutic response in humans, a result ascribed to its poor pharmacokinetic properties.85 Recent phase III clinical trails (NCT00461591 and NCT00598806) with 3 for nonmuscle invasive bladder cancer (NMIBC) resulted in a minimal difference in the two-year recurrence rate between the treatment and placebo group. Consequently, significant efforts have been made to improve the pharmacokinetic features of 3 through examination of structure–activity relationships (SARs) involving the indolequinone scaffold.86–90
One effort to prepare an improved quinone-based cytotoxic agent led to the synthesis of the bis-aziridine, RH1 (2,5-diaziridinyl-3-(hydroxymethyl)-6-methyl-1,4-benzoquinone, 4). This water-soluble compound was expected to display particular selectivity toward NQO1 reductase (Fig. 3).74–78,91,92 Compound 4 was found to exhibit nanomolar activity in vitro, with its cytotoxicity ascribed to the formation of the semiquinone form upon reductive activation and the subsequent formation of DNA inter-strand cross-linked adducts.93 Compared with the corresponding species derived from 1, the semiquinones formed after bioreduction of 4 are quite stable. Both in vitro and in vivo cytotoxicity of 4 was found to correlate well with NQO1 activity.93,94 A formulation of compound 4 with cyclodextrin (20%) was selected for consideration by the National Cancer Institute (NCI) and Cancer Research UK (CR-UK) (NCT00558727) for clinical dose-escalation studies (phase I) in advanced solid tumour patients. However, the paired biopsy data was not encouraging as the DNA cross-linking was observed in even low NQO1 level regions.95 It was proposed that activation of 4 at non-cancerous sites was facilitated by alternative activation mechanisms, including those involving P-450R and NQO2.96,97
Also, subject to initial clinical development was 2,5-bis-(carboethoxyamino)-3,6-diaziridinyl-1,4-benzoquinone (AZQ, 5), a second-generation analogue of 4.74–77 In phase I clinical studies, 5 was found to produce partial and complete responses in patients with malignant astrocytomas and meningeal leukemia. Further, phase I studies with 5 revealed promise of activity in patients with CNS and recurrent or resistant brain tumours. Unfortunately, phase III studies of brain tumour patients confirmed that compound 5 was less effective than other tested chemotherapeutic agents (nitroureas, BCNU (1,3-bis(2-chloroethyl)-1-nitrosourea), or PCNU(1-(2-chloroethyl)-1-nitrosourea)), although activity in resistant brain tumours and nonlymphocytic leukemias was seen. Eventually, clinical studies were terminated due to poor pharmacokinetics, severe toxicity, and lack of sufficient efficacy relative to existing approved anticancer agents.98,99
3.2. Quinones as a trigger for targeted chemotherapeutic drug delivery
Quinone based moieties have been exploited in a number of drug release scenarios due to their reductive capabilities. Reduction to the hydroquinone can facilitate internal electronic rearrangements resulting in covalent bond cleavage and drug release. To date, two major release mechanisms have dominated the quinone-based drug release literature. These limiting approaches are discussed below.
3.2.1. Hypoxia selective drug release via tandem benzoquinone reduction and iminium ion generation.
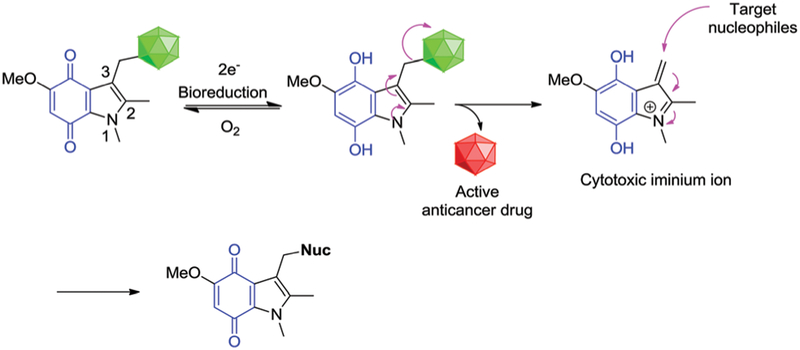
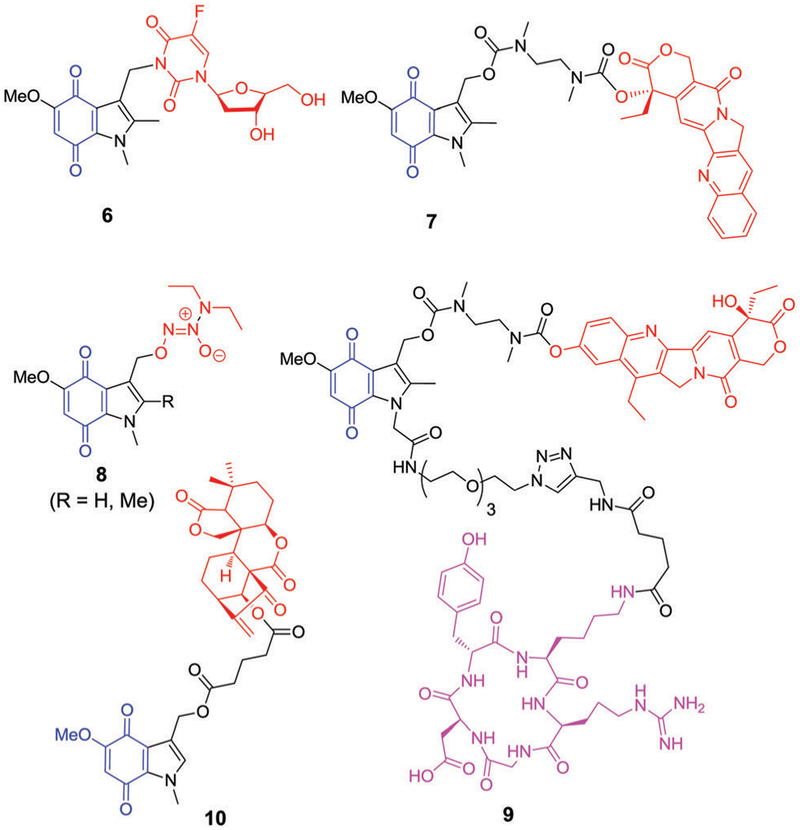
Indolequinones have been extensively explored to effect delivery of cytotoxic agents.85,100–102 Typically, the cytotoxic agents in question are tethered at the C-3 position. Upon bio- or radiolytic reduction, the tethered cytotoxic agent is released as the indolequinone is converted to an electrophilic iminium species (Fig. 4). The iminium daughter product can promote DNA alkylation or other cellular damage resulting in synergistic cytotoxicity. Nishimoto et al. employed indolequinone prodrug 6, to deliver an active drug, 5-fluorodeoxyuridine, upon radiolytic reduction (G value 0.38 × 107 mol J−1) under hypoxic conditions (Fig. 5). Prodrug 6 exhibited minimal cytotoxicity under aerobic conditions toward EMT6/KU cells. However, under hypoxic irradiation, prodrug 6 undergoes a one-electron reduction leading to biomolecular disproportion of the semiquinone radical anion intermediate. The resulting electrophilic iminium cation and active drug displayed synergistic activity and potency in a radiation dose-dependent manner that was superior to that seen for the parent drug alone.101
Fig. 4.
Bioreductive activation of a generalized indolequinone prodrug and proposed mechanism of drug release under hypoxic conditions.
Fig. 5.
Chemical structures of indolequinone-tethered drug conjugates. Hypoxia selective indolequinone = blue, conjugated drug = red, integrin selective cyclic peptide = pink.
Camptothecin (CPT, an irinotecan derivative) is a quinolone alkaloid that acts as a potent DNA enzyme topoisomerase I inhibitor. It displays anticancer activity that is ascribed to lethal DNA strand breakage that occurs during the replication process.103,104 Despite promising preclinical results, the unfavourable physiochemical properties of camptothecin, including poor water solubility and lactone ring instability, have restricted its clinical use. Nevertheless, considerable effort has been devoted to developing new camptothecin derivatives (Topotecan, SN-38). Work has also focused on creating prodrug forms and using it to produce drug delivery vehicles. For instance, Nishimoto et al. synthesized the water-soluble prodrug 7 (Fig. 5) with a CPT and an indolequinone that is conjugated at the C-3 position through an N,N′-dimethyl-aminoethylcarbamate side chain.102 Relative to what was seen with camptothecin, prodrug 7 exhibited slightly improved activity in HT-29 tumour cells under hypoxic conditions (1.8 fold) compared to normoxic conditions. This result was ascribed to fast bioreductive activation followed by cyclization of the chemical linker, a chemical reaction that serves to release the parent drug camptothecin in its active cytotoxic form.
In separate work, Chakrapani et al. employed the indolequinone motif (Fig. 5) to improve the hypoxia-selective delivery of nitric oxide (NO), a potent tumour-static species.105 Conju gate 8 (R = H) exhibited a hypoxia-selective antiproliferative effect in various cell lines, including DLD-1 (IC50 = 0.25 μM), HeLa (IC50 = 0.96 μM), and human urinary bladder cancer cells T-24 (IC50 = 0.56 μM). When methyl substituents were introduced onto the C-2 position of the active agents containing a 4,7-dioxoindole moiety (8, R = Me), the inhibitory potency of the conjugates was diminished by 10–16 times. This result was ascribed to a reduction in the metabolic rate.106,107 Mechanistic studies revealed that conjugate 8 induced nuclear DNA double-strand damage with the simultaneous formation of γ-H2AX foci.
In an effort to improve further the efficacy of CPT, its analogue SN-38 was conjugated to a peptide bearing an indolequinone subunit. This gave structure 9, which incorporates an integrin-selective peptide (shown in pink in Fig. 5). Integrins are highly expressed in tumour vasculature during angio-genesis;108 thus, conjugate 9 containing both an αvβ3 integrin-selective cyclic tetrameric peptide c(RGDfK) and a CPT subunit was expected to target angiogenic tumour cells over normal proliferating endothelial cells. In the absence of DT-diaphorase, conjugate 9 was nontoxic up to concentrations of 300 nM in human cervical carcinoma KB cells. However, in the presence of DT-diaphorase reductase, about 50–70% inhibition in cell growth was observed when cells were exposed to 300 nM of 9. This was taken as evidence that the activity of the conjugate was mediated by recognition of αvβ3 integrin receptors on the cancer cell surface and that CPT drug release is achieved upon reduction.109
Xu et al. reported a series of C-3 functionalized indolequinone-based diterpenoids (orisonin analogues) as hypoxia selective prodrugs. Oridonin is an ent-kaurene diterpenoid natural product that is extracted from Radosia rubescens and which was found to exhibit antitumour activity in various cancer cell lines.110,111 For instance, compared to taxol (used as a positive control), various orisonin analogues were found to exhibit higher toxicity profiles in NQO1-overexpressing human colon carcinoma (HT-29) cell and lung cancer (A549) cell lines. Moreover, relatively low toxicity was seen in the NQO1-deficient lung adenosquamous carcinoma (H596) cell line. A lead compound (10, Fig. 5) was found to have good antiproliferation activity in NQO1-overexpressing HT-29 and A549 cells. In the presence of dicoumarol (an NQO1 inhibitor), the activity of 10 in A549 cells was largely repressed. Further, 10 exhibited synergistic cytotoxi-city in these cells when compared with equimolar combination of oridonin and 10-hydroxy indolequinone. Detailed mechanistic studies, including DCF-ROS staining, mitochondrial membrane potential (MMP) measurement, DNA-based cell cycle analysis and western blotting analysis (caspase 3/9, cytochrome C, Bax, Bcl-xl) revealed that compound 10 induced NQO1-dependent apoptosis through the ROS-triggered mitochondrial apopotic pathway. Efficacy was also verified in liver cancer (H22) xeno-grafted mouse models.112
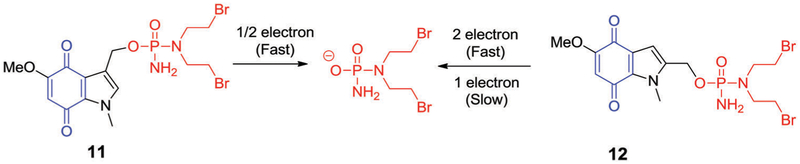
In 2003, Borch et al. demonstrated that the C-2, as well as the C-3, position of indolequinone scaffolds could be modified to deliver phosphoramide mustards after DT-diaphorase reductive activation (Fig. 6).100,113 It was observed that the C-2 and C-3 substituted indolequinone analogues 11 and 12 (Fig. 6) underwent rapid activation following two-electron reduction and exhibited excellent selectivity toward human DT-diaphorase isoenzymes. In contrast, the C-3-substituted indolequinone derivative 11 was preferentially activated via a one electron reduction pathway. It was also found that prodrug 11 would undergo nucleophilic activation to release a phosphoramidate anion when exposed to glutathione (GSH) or dimethyldithionatecarbamate (DDTC) as monitored by31P NMR spectroscopy. In contrast, the corresponding C-2 substituted analogue 12 displayed an extremely low rate of activation when exposed to GSH.113 In model experiments that involved reacting sodium dimethyldithiocarbamate (DDTC) with 11 and 12, direct displacement of the bromide substituents present in the parent molecule was also observed. However, substitution of the chloro analogue was not observed, a finding that was taken as highlighting the choice of chloro analogues for prodrug development. In vitro cytotoxicity assays revealed that both C-2 and C-3 substituted indolequinone-derived prodrugs exhibited nanomolar toxicities against the HT-29 and BE human colon cancer cell lines. As a general rule, the C-2 substituted systems (i.e., 12) displayed a cytotoxicity profile consistent with DT-diaphorase activity, while no such correlation was observed in the case of the C-3 substituted analogues. Furthermore, comparative cytotoxicity studies involving 11 and an acetoxy functionalized derivative led to the suggestion that the indolequinone moiety and phosphoramidate anion act in synergistic fashion. No evidence of such a benefit was seen in the case of the C-2 substituted compounds.
Fig. 6.
C-2 and C-3-substituted indolequinone phosphoramide prodrugs and their proposed modes of activation.
Conjugate 13 (Fig. 7), a benzoquinone scaffold linked to a nitrogen mustard analogue, was investigated in the context of hypoxia-responsive drug activation. This agent was designed to function as a dual mode of action prodrug. After bio-reduction in hypoxic cells, conjugate 13 was expected to release the active mustard, 4-[bis(2-chloroethyl)amino]benzoic acid, along with an intermediate bearing an electrophilic site that was expected to be susceptible to nucleophilic attack by DNA leading to alkylation-based DNA damage.114
Fig. 7.
Tripartite prodrug activation pathway and quinone/trimethyl tripartite quinone-based prodrug structures. Hypoxia selective indolequinone = blue, conjugated drug = red, tumour localizing biotin = pink.
3.2.2. Hydroquinone assisted cyclization-based release mechanisms.
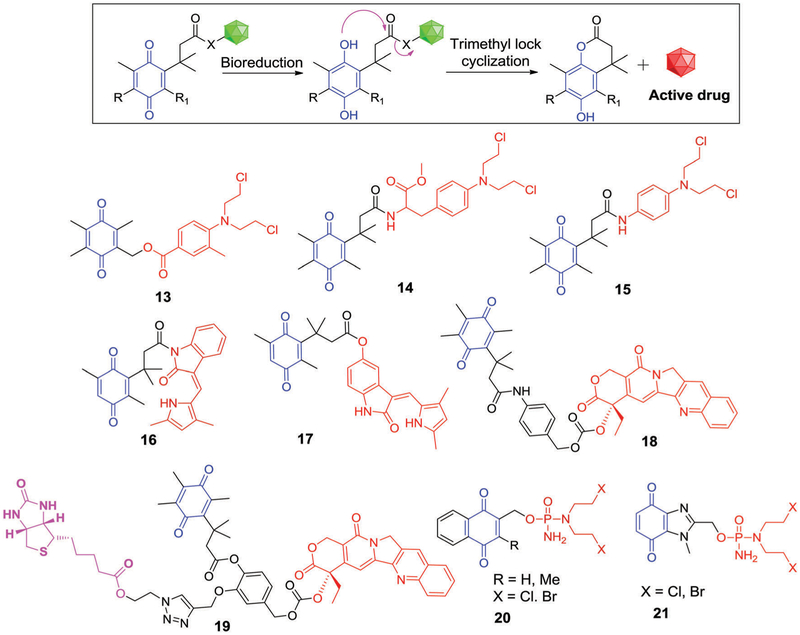
Benzoquinones incorporating three methyl groups (one at the ortho-position of the quinone ring and two at the β-carbonyl positions) can be considered as possessing “trimethyl locks” that result in restricted conformational motion. These “locked” cores have been studied in the context of drug release applications. Under hypoxic conditions, the quinone form is reduced to the corresponding hydroquinone, which leads to severe steric repulsion between the three methyl groups. This is a design feature that can favour release of tethered alcohols or amines.115–118 Taking advantage of this strategy, Carpino et al.115 introduced a tripartite quinone-based prodrug approach, which was further developed by other research groups.116–118 One embodiment is the melphalan-quinone derivative 14. Upon reductive activation, the parent alkylating agent is released via lactonization (Fig. 7).114
Phillips et al. reported a 4-aminophenyl nitrogen mustard-benzoquinone conjugate 15, which proved to be a good substrate for DT-diaphorase with Vmax = 11.86 ± 3.09 mmol min−1 mg−1 and Km = 2.70 ± 1.14 mmol L−1 (Fig. 7).116 The nitrogen mustard released upon bioreduction displayed significant toxicity towards T47D cells overexpressing both DT-diaphorase under aerobic conditions and cytochrome P-450 enzyme under hypoxic condition. A favourable hypoxia cytotoxicity ratio (HCR) relative to normoxia of about 15.8 was noted.116
Moody et al. utilized the same strategy to create the trimethyl quinone derivatives 16 and 17 (Fig. 7) containing semaxanib (SU5416) and its corresponding 6-hydroxy derivative, both of which are potent VEGF receptor tyrosine kinase Flk-KDR inhibitors.117 Preliminary in vitro assays revealed that derivatives 16 and 17 (at 10 μM) exhibited VEGF-stimulated angiogenesis inhibition upon bioreduction, comparable to equimolar treatments of the parent SU5416 (semaxanib) and 6-hydroxy SU5416 drug systems in cultured human umbilical vein endothelial cells (HUVECs). These results provided support for the suggestion that both derivatives 16 and 17 release the active drugs in these biological milieus.
Another investigation involved structure 18 wherein a CPT is conjugated to a quinone propionic acid (Fig. 7). The resulting system displayed significant cytotoxicity in DT-diaphoraseoverexpressing cells and permitted the simultaneous tracking of the drug activation process in vitro.118 To improve the cancer-targeting ability of this system, Kim et al. developed prodrug 19, composed of three moieties, an anticancer drug SN38 (a topoisomerase I inhibitor), Q3PA (trimethyl-locked quinone propionic acid), and a biotin moiety as a cancer-targeting unit. After preferential uptake in cancer cells (presumably biotin-mediated), prodrug 19 is activated by NQO1. This leads to the release of active SN38 and results in cancer cell apoptosis. Drug release and the determinants of apoptosis of cancer cells were further studied in the case of cells expressing high levels of NQO1 and biotin receptors.119
Borch et al. reported a related class of compounds, namely structures 20 and 21 (Fig. 7). Here, a phosphoramide mustard is tethered to either a naphthoquinone or benzimidazole quinone scaffold.120 Upon bioreduction by human DT-diaphorase, the naphthoquinone prodrugs were found to release the parent drugs rapidly (kcat/Km 8 = 3 × 107−3 × 108 M−1 s−1). Clonogenic assays provided support for the notion that the naphthoquinone derivative 21 exhibited potent cytotoxicity in both the HT-29 (DT-diaphorase +ve) and BE (DT-diaphorase −ve) colon cancer cell lines. However, the cytotoxicity profile did not correlate well with the DT-diaphorase activity. Nucleophilic attack by GSH at the C-3 position of naphthoquinone, followed by parent drug release, was proposed as an alternate prodrug activation pathway for these derivatives. The corresponding benzimidazole quinone analogues 20 did not display appreciable cytotoxicity in vitro. This could reflect the stability of the reduced quinone form and a lack of promoted release of the active phosphorodiamidate species.
4. Nitro/azo-based therapeutics and theranostics
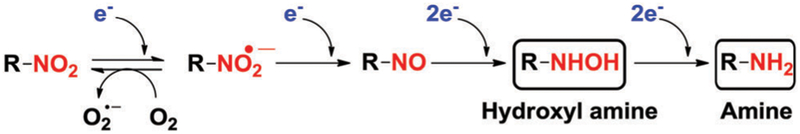
Over the past few decades, nitroaromatics have been extensively utilized in the development of non-invasive probes for imaging tumour hypoxia. Nitroaromatics are readily reduced to corresponding amines by nitroreductases (NTRs), a family of flavin-containing enzymes. NADH and NADPH can provide the requisite reducing equivalents (Fig. 8). Under hypoxic conditions, several intracellular reductases such as NTRs, DT-diaphorase, and azoreductase are overexpressed.121 A direct correlation between NTR levels and solid tumour hypoxia has been established. In this section, the focus is on hypoxia-responsive nitroaromatic (pro)-drugs, with a particular emphasis on their specific biological and medicinal features. The discussion is organized in terms of the proposed mode of action.
Fig. 8.
Stepwise reduction of nitroaromatics under hypoxic conditions.
4.1. Nitroaromatics as “oxygen-mimetic radiosensitizers”
Radiation therapy (RT) has a time-honoured role in cancer therapy. Utilization analyses have revealed that about 30% of the cancer patients received RT either alone or in combination with other treatments.122 In RT, ionizing radiation is used to control or kill the malignant cells. Oxygen, being an extremely electron-affinic molecule, plays a key role in the chemical reactions that lead to DNA damage after a given dose of ionization radiation.123,124 The resulting DNA lesions are thought to result in cell death. Typically, hypoxic tumour regions are 2–3 times more resistant to radiation damage than cells in normal oxygenated environments.125,126 In clinical practice, this results in so-called fraction of the radiation dose, administrating it in a discontinuous fashion over considerable time. This is done in part so as to allow oxygenation of erstwhile hypoxic regions.
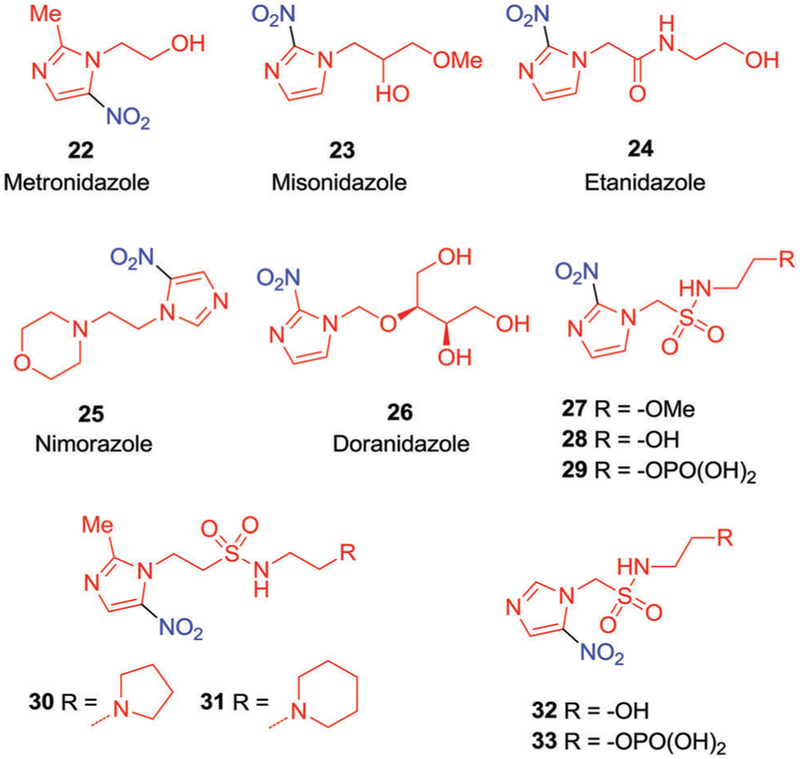
In 1976, Adams et al. suggested that certain compounds, characterized by a high electron affinity, could improve the efficacy of RT127 by acting as mimetics of molecular oxygen. These compounds, termed “oxygen mimetic radiosensitizers,” were found to stabilize the DNA lesions induced by RT.128 Nitroimidazoles have received considerable attention as oxygen mimetic radiosensitizers. Metronidazole (22) was the first nitroimidazole derivative to be explored in this context (Fig. 9).129,130 It was found to provide radiosensitization both in vitro and in vivo, exclusively under hypoxic conditions, and became the subject of various clinical studies.131–133 Initially positive results were seen and this prompted a search for other high electron-affinity agents with enhanced radiosensitizing ability, e.g., misonidazole (23, Fig. 9). Compound 23 exhibited high potency in various animal tumour models and human malignancies after the administration of a single dose of radiation. Extensive clinical trials were conducted worldwide. Unfortunately, phase II trials revealed severe dose-limiting encephalopathy and peripheral neurotoxicity.134–138
Fig. 9.
Chemical structures of oxygen-mimetic radiosensitizers and novel nitroimidazole alkyl sulfonamide radiosensitizers that have been investigated clinically.
In an effort to reduce the neurological toxicity associated with nitroimidazole, Brown et al.139 reported a more polar amide analogue of misonidazole (23), namely etanidazole (24, Fig. 9). Etanidazole proved more effective than misonidazole for two major reasons. First, it displayed reduced toxicity, presumably due to its shorter half-life and relatively rapid excretion rate. Second, the more hydrophilic nature of etanidazole resulted in a slower uptake by neural tissues and a subsequent reduction in neurotoxicity relative to 23.139,140 As reported by Coleman et al.,141,142 etanidazole (24) was well tolerated at a dosage level three times higher than that of misonidazole (23). However, etanidazole (24) was not developed as it failed to meet clinical endpoints in trials involving head and neck cancer.143
Another hypoxia sensitizer, nimorazole (25, Fig. 9),144,145 was found to exhibit considerably lower clinical toxicity and has been registered for use in the treatment of head and neck cancer in Denmark.146–150 Another agent, doranidazole (26, Fig. 9) demonstrated reduced neurotoxicity, presumably owing to its impermeability to the blood–brain barrier (BBB).151,152 This agent demonstrated radiosensitization under hypoxic conditions, both in mouse tumour models and human tumour xenografts.153–155 To date, 26 has been evaluated clinically in Japan, where it showed encouraging results in phase I/II studies for advanced non-small-cell lung cancer (NSCLC).156
Further developments in RT have produced a highly precise and potentially more effective radiation therapy modality termed stereotactic body radiotherapy (SBRT). In SBRT, advanced imaging technologies and sophisticated computer programs are used to guide the delivery of high doses of radiation directly to tumour site. Clinical results utilizing SBRT to treat various solid tumours revealed efficacy profiles equal or superior to fractionated RT.157 Moreover, SBRT offers many advantages over conventional methods, such as a shortened treatment course, faster pain relief, fewer patient visits, and reduced need for surgical operations. This evolution in the standard of care (SOC) has provided an incentive to test novel putative radiation sensitizers in conjunction with SBRT. A summary of these efforts is given below. Unfortunately, to the authors’ knowledge, there are no reports where a putative radiation sensitizer has been tested in a comparative fashion under conditions of both RT and SBRT.
Hay et al.158 reported the alkyl sulfonamide substituted nitroimidazole derivatives 27, 28, 30, and 31 (Fig. 9) and tested them as possible novel radiosensitizers in conjunction with SBRT. Preliminary in vitro clonogenic assays revealed that derivatives bearing 2-nitroimidazole functionality induced significant hypoxia-selective cytotoxicity (6 to 64-fold) against a panel of human cancer cells lines (HT29, H1299, HCT116, PC3, and 22RV1) relative to analogues based on a 5-nitroimidazole unit. Compared with 23, compounds 27 and 28 displayed only modest sensitization, while compounds 30 and 31 displayed sensitization that was equal or superior to that of 23 in HCT-116 human colorectal cell lines. The enhanced radiosensitization observed in 30 and 31 was ascribed to the presence of basic side chains, which was thought to lead to an increase in cellular uptake.
Recently, these researchers utilized a phosphate prodrug strategy to improve the aqueous solubility of conjugates 28 and 32 and compared their equimolar cytotoxicity to 24, as well as the prodrugs 29 and 33 (Fig. 9).159 Although 29 possess similar polarity to 24 (log D values = 1.53 and 1.37, respectively), it displayed enhanced HCR (40) compared to 24 (HC = 19) in HCT116 colon cancer cells. Compound 33, having properties similar to those of 25, displayed only a modest HCR (4). Relative to 24 and 25, prodrugs 29 and 33 showed improved drug delivery, enhanced peak tumor drug concentration, and substantial radiosensitization in combination with RT and delayed the tumor regrowth (3 folds) as compared to radiation (12.5 Gy) alone in HCT116 xenografts.
4.2. Nitroaromatic subunits as integral components of hypoxia-activated prodrugs
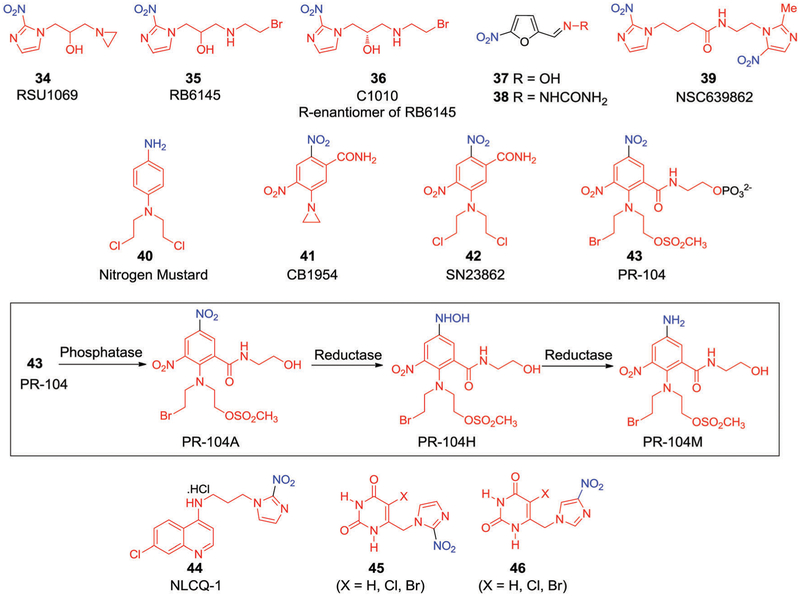
During the development of radiosensitizers, a number of compounds were identified as being substantially more cytotoxic than would be predicted based on their radiosensitization properties alone.160,161 The hypoxia-selective toxicity of these agents, among the first of which was metronidazole (22), was initially noticed by Sutherland et al.162 This observation is considered a watershed moment in the development of second-generation drugs and triggered the initial development of what are now referred to as “hypoxia-activated prodrugs (HAPs)” or “hypoxia-selective cytotoxins (HSCs)”. The rationale for the development of these drugs is based on the concept that hypoxia-selective metabolic reduction of the nitro group by an intracellular nitroreductase via stepwise electron transfer converts the compounds into a cytotoxic species as illustrated in Fig. 8. These cytotoxins can form strong DNA inter-strand cross-links and ultimately lead to cancer cell death. Although the concepts underpinning HAPs were established over 40 years ago, it was only over the course of time that refinements in structural design led to the development of a number of bioreductive drugs. Some of these later systems moved into clinical trials. An example is RSU1069 (34, Fig. 10),163–165 which contains an aziridine moiety on the N1 side chain of the nitroimidazole core and acts as weak monofunctional alkylating agent. In light of the promising in vitro and in vivo results obtained in combination with RT,166 compound 34 was evaluated clinically in 1985. However, it caused severe emesis and was withdrawn from the further clinical trials.167 RB6145 (35, Fig. 10), a prodrug of compound 34, emerged as a more suitable hypoxia-selective candidate168,169 since it displayed a systemic toxicity roughly 2.5-times lower than 35. However, further development of 35 was limited due to the high retinal toxicity of its R-enantiomer (compound 36, also known as C-1010, Fig. 10).170,171
Fig. 10.
Structures of some hypoxia-selective cytotoxins.
In an effort of develop more acceptable agents, compounds 37 and 38 were developed (Fig. 10). These agents are relatively electron-affinic due to the incorporation of 5-nitrofuran moieties in lieu of 2-nitroimidazole cores. In fact, compounds 37 and 38 proved effective as radiosensitizers in tissue culture under hypoxic conditions.172,173 Naylor et al. conducted detailed SAR investigations to understand the effect of varying the electronic character of the side chains on the radiosensitizing and HAP activity within a series of 5-nitrofuran, 2-carboxamide, and 3-carboxamide derivatives.174 It was found that in hypoxic V79 Chinese hamster cells, compared with analogues 34 and 35, the derivatives with greater electron affinity exhibited higher radiosensitization levels in vitro. However, these trends were not translated into in vivo studies; in fact, across the board these systems exhibited low sensitizing activity in murine KHT tumours. This was attributed to several factors including, poor distribution and tumour uptake, rapid metabolism, and undesired interactions with other noncritical targets in animal models.
In 1992 Wilson et al. suggested175 that the hypoxia selectivity and cytotoxicity of this class of bioreductive drug might be further enhanced by incorporating two redox centres within the same molecule to produce so-called “bis-bioreductive drugs”. This approach relies mainly on the principle that both redox centres must be reduced independently by oxygen-inhibitable processes for full activation. As a consequence of this design principle, it was expected that the bis-bioreductive drugs in question would display particularly high hypoxia selectivity compared to their non-activated congeners. This led to the preparation of bis-nitroimidazole N-[2-(2-methyl-5-nitroimidazolyl)ethyl]-4-(2-nitroimidazolyl)butanamide (NSC639862, 39)176 (Fig. 10). Compound 39, with 2-nitro and 5-nitroimidazole units connected through a carboxamide linker, showed 200-fold higher toxicity towards AA8 Chinese hamster cells compared with mononitroimidazoles. In vivo studies revealed that compound 39 was active against KHT177 and MDAH-MCa-4176 tumours when administered in combination with radiation.
An unfortunate corollary of producing bioreductive drugs that are only fully active under conditions of extreme hypoxia,178,179 is that oxygenated tumour regions might remain resistant.180 One conceivable approach to circumventing this problem would be to develop bioreductive drugs that, upon reduction, produce cytotoxic species with sufficiently long half-lives to diffuse into the relatively well-oxygenated cells that surround the chronically hypoxic region. The strategy is known as the “bystander effect” and was investigated in detail by Wilson et al.181 They used nitrogen mustard 40182 (Fig. 10) as the cytotoxin. A number of bioreductive drug candidates based on nitrogen mustards,183 such as CB1954 (41)184 and SN23862 (42),185 are now known (Fig. 10). Compound 42 was found to be a more promising HSC than the corresponding aziridine analogue 41.186 The limited hypoxia-selective cytotoxicity of 41 was attributed to oxygen-insensitive bioreduction by NAD(P)H:quinone oxidoreductase (DT-diaphorase), unlike 42, which undergoes preferential reductive activation by cytochrome P-450 reductase. The active meta-bolite of 42 (i.e., the 2-amine product), was reduced under hypoxic conditions and displayed exceptional bystander activity in multicellular layer (MCL) cultures and was found to be 2000-fold more potent than the parent prodrug.187 Although 41 displayed limited hypoxia selectivity, promising results were obtained when used in combination with gene-dependent enzyme prodrug therapy (GDEPT). It was found to serve as an efficient substrate for Escherichia coli nitroreductase (NTR). This enzyme converts the prodrug into a bifunctional alkylating agent, resulting in DNA cross-links and cell apoptosis.188–191 In combination with RT, the combination of NTR and 41 was found to induce synergistic cytotoxicity in HeLa cells. Phase I/II clinical trials for virus directed enzyme-prodrug therapy (VDEPT) with 41 were carried out in patients with prostate-specific antigen (PSA). Unfortunately, only 7 out of 19 patients showed greater than 10% PSA reduction.192
Denny et al.193,194 conducted extensive SAR studies on 42 in order to find a compound with improved cytotoxicity and improved aqueous solubility that simultaneously retained hypoxic selectivity. Structural optimization resulted in the development of a promising HAP, namely PR-104 (43, Fig. 10),195 designed to serve as a water-soluble phosphate prodrug. Mechanistic studies196 revealed that rapid hydrolysis of the phosphate group by systemic phosphatases serves to generate the alcohol prodrug PR-104A. The nitro group of compound 43 is then reduced by one- or two-electron oxidoreductases. This results in the formation of the hypoxia-selective DNA interstrand cross-linking metabolites, hydroxylamine PR-104H and amine PR-104M (Fig. 10).197,198 Various oxidoreductases were considered responsible for the reduction of 43, including FAD-dependent oxidoreductase, cytochrome P-450 reductase, and aldoketoreductase 1C3 (AKR1C3).199–202 The majority of the activity ascribed to 43 under hypoxic conditions is considered to be the result of a one-electron pathway catalyzed by cytochrome P-450 reductase. In contrast, the activity observed under aerobic conditions was believed to result from a two-electron process catalyzed by AKRIC3.
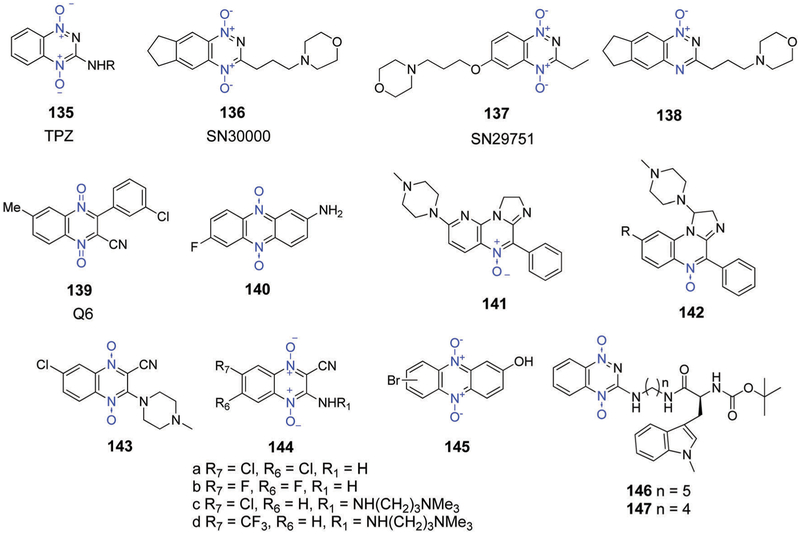
Recently, Wilson et al.203 demonstrated the potential of compound 43 in the inhibition of triple-negative breast cancers via the presumed dual targeting of hypoxia and homologous recombination dysfunction repair. In vitro studies revealed excellent hypoxia selectivity in human tumour derived cancer cell lines (SiHa, HT29, and H460) relative to tirapazamine (TPZ, Fig. 29) or conventional mustards.204 Compound 43 showed promising results in various in vivo preclinical models. For instance, Konopleva et al.205 demonstrated the high efficacy of 43 in murine models for human leukemia. A report by Lock et al.206 also revealed that compound 43 had significant efficacy in T-ALL xenografts and was superior to a combination regimen consisting of dexamethasone, vincristine, and L-asparaginase; furthermore, the activity of 43 in T-ALL cells was well correlated with AKR1C3 expression. In clinical phase I/II studies conducted in acute lymphoblastic leukemia patients with 43, a significant decrease in leukemic hypoxic cells was observed. However, toxicities, such as myelosuppression and gastrointestinal effects, led to investigations of 43 at lower doses than used originally and in combination with other cytotoxins.207 Unfortunately, a first combination trial of 43 with docetaxel in non-small cell lung cancer (NSCLC) was terminated due to the unfavourable nature of the clinical results (NCT00862134). Recent preclinical studies of compound 43 revealed efficacy against breast cancer xenografts in combination with RT. The sensitization induced by 43 was particularly noticeable in BRCA2-knockout mutants. To date, no further clinical trials of 43 in combination with RT have been reported.
Fig. 29.
Structures of hypoxia-activated aromatic N-oxide prodrugs.
To enhance the potency of hypoxia-selective cytotoxins, Papadopoulou and Bloomer tethered DNA intercalating moieties to nitroimidazoles, as exemplified by NLCQ-1 (44, Fig. 10).208 Compound 44 displayed significant in vitro hypoxic selectivity (30-fold) in several rodent (V79, EMT6, and SCCVII) and human (A549 and OVCAR-3) tumour cell lines as compared with normoxic conditions. Perhaps the most striking feature of 44 to emerge from these and other studies was the finding that an increase in exposure time led to dramatically enhanced hypoxic selectivity (up to 386-fold). In vitro studies with isolated rat-liver microsomes and NADPH/NADH led to the suggestion that cytochrome P-450 and b5 reductases were primarily involved in the reductive activation of 44;209 however, other enzymes were also implicated.210 Under conditions of radiation therapy, 44 displayed a synergistic benefit against hypoxic V79 cells both in vitro and in vivo.211 More importantly, 44 was found to enhance the antitumour effect of various chemotherapeutic agents212–217 in murine tumours and human xenografts, and did so without increasing bone marrow suppression or retinal toxicity. These latter adverse events represent two common dose-limiting toxicities associated with this class of therapeutics.
Thymidine phosphorylase (TP), a platelet-derived endothelial cell growth factor (PD-ECGF), plays an important role in angiogenesis, tumour growth, and metastasis. Various immunohistochemical and TP-activity studies have served to confirm its elevated expression in a wide range of solid tumours compared to normal tissues; this is particularly true in hypoxic regions.218 Appreciating this, Jaffar et al.219,220 reported that the 2-nitroimidazolylmethyl uracil derivative 45 and 46 (Fig. 10) acts as novel hypoxia-activated prodrugs. Bioreduction of the nitro group in these prodrugs by cytochrome P-450 reductase produces the corresponding amine forms. The reduced forms of 46 (X = Cl, Br) were found to be potent inhibitors of E. coli and human TP (IC50 = 20 nm) at 40 nM enzyme concentration, while the corresponding amino-46 species (X = Cl, Br) proved 350 fold less reactive (IC50 = 7 μM), In contrast, the 5-unsubstituted analogues (45 and 46, X = H) were less reactive than the corresponding 5-halo analogues. The reduced forms of 45 (X = Cl, Br) were roughly 1000 times more potent than the corresponding parent system. An evaluation of TP inhibition and comparison with other known TP inhibitors, such as 6-amino-5-bromouracil (6A5BU) and 5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]uracil hydrochloride (TPI), led to the conclusion that under conditions of administration, prodrug 45 gives rise to a TP inhibition level that is comparable to that of TPI and 100 times greater than 6A5BU.
Although most of the bioreductive drugs discussed above are promising HAPs, most of the clinical trials conducted with the drug candidates discussed above did not meet their target endpoints.221 It has been proposed that one important limitation is the failure to identify target patient populations likely to benefit from HAP therapy. Thus, to the extent it is possible, developing a correlation between tumour hypoxia and HAP clinical response appears warranted. Other means for improvement would include achieving the selective delivery of HAPs to target cells. It would also be beneficial to create conjugates whose active payloads released after activation, currently species such as, e.g., nitrogen mustards and topoisomerase II inhibitors, are not subject to multidrug resistance.
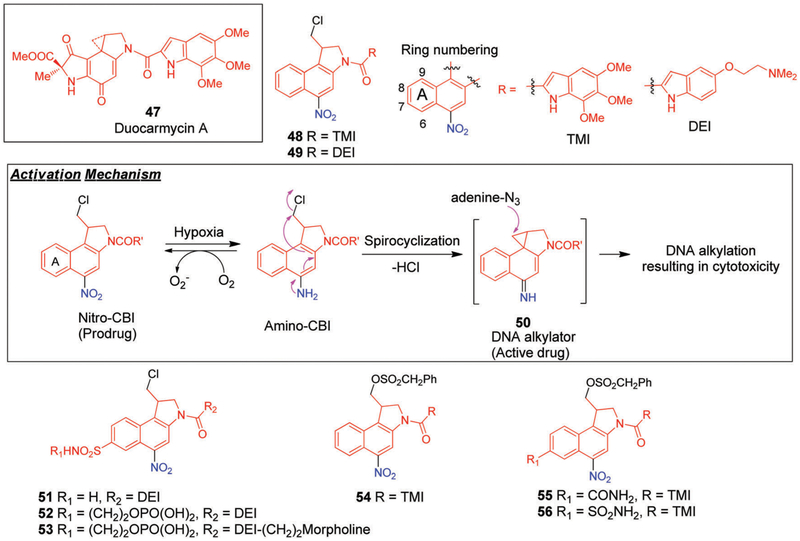
Addressing these limitations has led efforts to the discovery and development of new classes of cytotoxic agents. In this context, Duocarmycin A (47, Fig. 11) has received considerable attention. It has been identified as being a DNA alkylating agent with remarkable cytotoxic potency.222,223 Mechanistic studies revealed that the extreme cytotoxicity of this agent arises from sequence-selective alkylation at the N3 position of adenine within the minor groove of DNA. To date, several analogues of 47 were found to demonstrate promising antitumour activity in preclinical studies.224 Nonetheless, clinical evaluations of 47 and its derivatives have been limited by myelotoxicity, a dose-limiting side effect to which humans appear to be more sensitive than mice.225,226
Fig. 11.
Structures of 7-substituted hypoxia-activated prodrugs of nitro-chloromethylbenzindolines (nitro-CBIs) bearing 5,6,7-trimethoxyindole (TMI) and 5-[(dimethylamino)ethoxy]indole (DEI) side chains.
A desire to circumvent these limitations has led to the development of new prodrug systems that could undergo selective action at the intended tumour site. For instance, Tercel et al.227 reported naphthyl-based alkylating agents obtained by replacing the phenolic hydroxyl group in 47 with a nitro group (giving 48 and 49; cf. Fig. 11). Under conditions of bioreduction, the nitro group is converted to the corresponding amino-derivative followed by a subsequent spiro-cyclization to form the potent DNA alkylating agent 50 (Fig. 11). The nitro-chloromethylindolines derivatives 48 and 49 differ from one another in that they incorporate a neutral 5,6,7-trimethoxyindole (TMI) side chain and a basic 5-[(dimethylamino)ethoxy]indole (DEI) side chain, respectively.228 They were both found to be promising HAPs with the cytotoxic potency (measured as nitro/amino IC50 ratios) of the amino analogues being approximately 480-fold (for 49) and 3000-fold (for 48) higher than the corresponding nitro analogues. In vitro clonogenic survival assays revealed that both 48 and 49 produced HCR values on the order of 300-fold and 200-fold in the case of UV4 and RIF-1 cells, respectively. However, much lower potency and selectivity was observed against HT29 cells.
An investigation of the bystander effect in multicellular layer (MCL) cultures229,230 grown from HCT116 cells revealed that an active metabolite is produced from 49 that is highly diffusible, and which induces toxicity in the surrounding cells. Furthermore, the combination of 49 and RT gave rise to two-fold increase in hypoxia-selective toxicity in vivo against RIF-1 tumours compared with the prodrug itself.
Tercel et al.231 subsequently reported a series of analogs of 49 wherein different electron withdrawing groups (EWGs) were introduced in an effort to raise the one-electron reduction potentials and improve the cytotoxicity. In an independent investigation, the same researchers conducted detailed solvolysis and NMR spectral studies to validate the formation of short-lived DNA alkylating intermediate 50 (Fig. 11), which is presumably an active species formed during bioreduction of prodrugs 48 and 49.232
The most promising analogue contained a sulfonamide (−SO2NH2) group at the 7-position and a DEI side chain (51, Fig. 11). In vitro studies with two human tumour cell lines, ovarian carcinoma SKOV3 and colon carcinoma HT29, yielded HCRs of 275 and 330, respectively. These values are quite high relative to those displayed by the well-established HAPs, TPZ (discussed in later sections)233 and compound 43 (PR-104)234 under similar experimental conditions. Unfortunately, poor aqueous solubility limited the study of sulfonamide 49 in vivo.
To improve the water solubility, a phosphate analogue of 51 (i.e., 52, Fig. 11) was synthesized.235 Prodrug 52 readily undergoes phosphate group hydrolysis to generate the corresponding alcohol, followed by bioreduction to generate a strong DNA alkylating agent. In vitro studies revealed that 52 is about 150-fold more potent in eradicating colony-forming human cervical carcinoma cells (SiHa) under hypoxic conditions as compared to normoxic ones. In vivo studies performed with mice bearing SiHa tumours revealed that prodrug 52, when administrated in combination with high-dose radiation, completely eliminated the tumour burden in three out of five mice tested. Prodrug 52 was also found to exhibit high selectivity in other human xenografts models (e.g., colon, cervix, and lung).
Denny et al.236 also investigated the effect of the leaving group by preparing a series of nitro-chloromethylbenzindoline (nitro-CBI) prodrugs containing sulfonate leaving groups and comparing their cytotoxicity profiles and HCRs to the corresponding chloro-analogues. In the SKOV3 and HT29 cell lines under both aerobic and hypoxic conditions, the sulfonate analogues with a neutral side chain 5,6,7-trimethoxyindole (TMI) displayed higher cytotoxicities than the chloro derivatives. In vitro studies with the most effective prodrug (i.e., 54, Fig. 11) revealed a large variation in the HCR values that were found to depend upon the choice of the cell line. For instance, the HCR values varied from 246 for the SKOV3 cell line to 3.6 in the HT29 cell line. This difference was first assumed to arise from the different expression levels of P-450R across these cell lines. However, the absence of an observable correlation between P-450R expression levels and IC50 values led the authors to exclude this possibility.
In another investigation, Stevenson et al.237 reported a series of nitro-CBI-based HAPs (55 and 56, Fig. 11) that contain a sulfonate leaving group with an electron-withdrawing substituent at the 7 position of the naphthyl core. Prodrugs 55 and 56, which contain a 7-substituted primary carboxamide and sulfon-amide, respectively, as well as a neutral side chain (TMI), displayed high in vitro toxicity in the HT29 human cancer cell lines with HCR values of 370 and 1010, respectively.
Tercel et al. reported a series of nitro-duocarmycins analogues bearing amine-containing side chains of varying pKa (9.64 to 5.24) and evaluated their hypoxia-selective cytotoxicity in various tumour models.238 It was initially proposed that the presence of additional amine functionality would contribute to overall toxicity by binding to the minor grove of the DNA. In vitro cytotoxicity assays conducted in hypoxic HT29 cells revealed that most of the derivatives delivered the cytotoxic aminoCBIs to the cell. Moreover, it was found that the toxicity of these analogues was dependent more on the structural features than on the choice of particular basic substituents. The highest hypoxia potencies and largest HCR values were found in the case of the derivatives bearing the most basic chains. However, in vivo studies conducted either as a single agent or in combination with chemotherapy (gemcitabine or docedexal) revealed that the highest cytotoxicities were observed in the case of the analogues carrying weak basic side chains. Compared with compound 52, compound 53 (Fig. 11) displayed efficacy in a resistant H460 tumour model and also proved efficacious towards a hypoxic prostate cancer 22Rv1 xenograft (14 mmol kg−1) when administered as a single dose in combination with docetaxel (32 mmol kg−1) (n = 6–7). Moreover, 53 proved capable of eliminating detectable clonogens of radioresistant SiHa cervical tumours when used in conjunction with a single dose of gamma radiation. Lead 53 was also tested for enhanced therapeutic effects in A2780 ovarian xenograft models with gemcitabine (anticancer drug) in a multi-dose treatment schedule. In this study, 3 out of 7 tested animals were found completely tumour free at 100 days following treatment.
4.3. Nitroaromatics as hypoxia-activated prodrugs with drug elimination
In recent years, a modular concept for HAP design that relies on prodrug activation and active species release under hypoxic conditions has attracted considerable interest in the area of chemotherapeutic drug design.239 The approach is appealing since in principle it may be used to enhance the favourable biodistribution of drugs and reduce off-target toxic effects by promoting the selective release of an active drug form within tumour tissues.
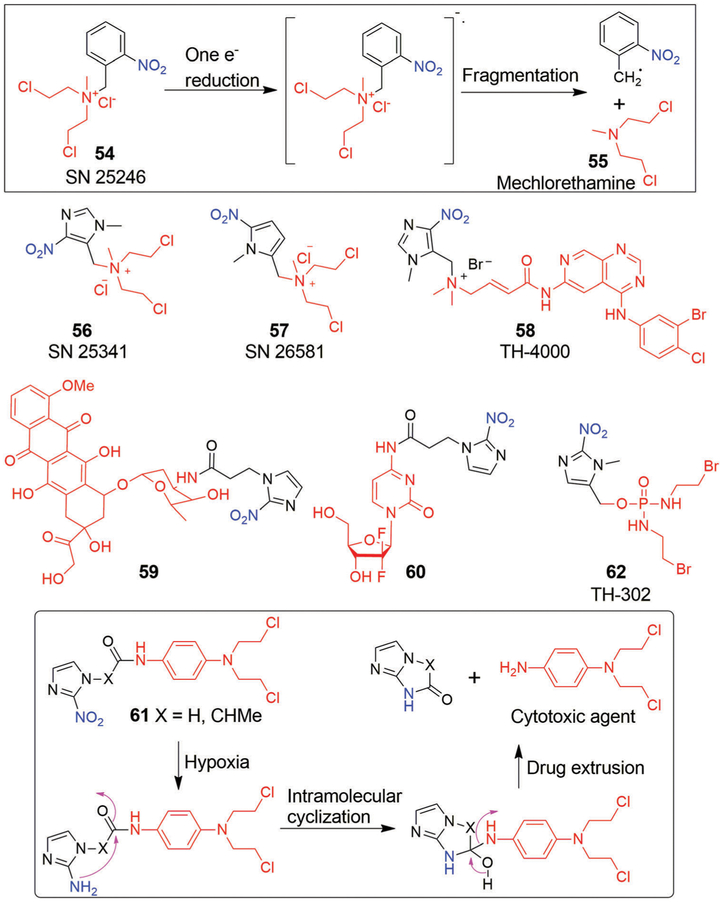
Denny et al.240 reported the benzylic quaternary ammonium mustard, SN 25246 (57), as a new potential class of HAPs (Fig. 12). This prodrug releases the highly cytotoxic agent methylbischloroethylamine (58, mechlorethamine; an aliphatic nitrogen mustard) upon one-electron reduction of the nitro group under hypoxic conditions followed by fragmentation. In vitro studies served to confirm that prodrug 57 displayed significant selectivity (200-fold) against hypoxic UV4 cells. The relatively long half-life of 58 (ca. 15 min) allows prodrug 57 to exert a strong bystander effect. Support for this conclusion came from the finding that prodrug 57 displayed greater cytotoxicity against intact EMT6 spheroids than against dissociated ones.241 Unfortunately, the prodrug showed only marginal cytotoxicity in vivo against hypoxic cells in KHT tumours.
Fig. 12.
Hypoxia-activated prodrugs based upon nitrogen mustard and their proposed activation mechanism.
Wilson et al.242 reported a detailed mechanistic study of the release of mechlorethamine (58) from nitroarylmethyl quaternary prodrugs that involved the use of steady-state radiolysis combined with high-performance liquid chromatography (HPLC). On the basis of their findings, the authors suggested that 57 undergo multiple electron reduction processes before releasing mechlorethamine (58) through fragmentation. As part of this investigation, two heterocyclic analogues 59 (SN 25341, 1-methyl-4-nitro-5-imidazolyl derivative) and 60 (SN 26581, (1-methyl-5-nitro-2-pyrrolyl derivative) were identified (Fig. 12). Unlike 60, these latter agents undergo clean radiolytic one-electron reductive fragmentation to release a high dose of mechlorethamine. A dinitrobiimidazole (DNBI) dimer, which arises from the arylmethyl radical, is also produced. Within this set of derivatives, the imidazole 59 was found to display strong hypoxic selectivity in EMT6 cells and RIF-1 cells.243 Prodrug 59 also produced promising in vivo results in RIF-1 tumour models when tested in combination with either RT or cisplatin. However, these findings were tempered by unpredictable systemic toxicities and nonspecific release of 58.
Another HAP agent to advance into clinical studies is TH-4000 (61, Fig. 12). TH-4000, termed tarloxotinib, is a bioreductive pan-HER inhibitor. In most cancers, hyperactivation of HER family receptors is common and leads to downstream upregulation of several signaling pathways, such as MAPK, PI3K/AKT and JAK/STAT, that are closely linked with tumour progression and metastasis. TH-4000 (61) undergoes a one-electron reduction under hypoxia conditions resulting in a nitro radical that subsequently releases an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TK1).244 The tyrosine kinase inhibitor in question, erlotinib, is used clinically for treating NSCLC patients.245 Promising preclinical results were obtained with TH-4000,246,247 and mechanistic studies conducted on panel of NSCLC cell lines revealed that 61 is metabolized under hypoxic conditions. It was also found to be more effective than the parent drug erlotinib in both wild-type as well as EGFR-mutant xenografts. Subsequently, clinical trials with 61 were conducted in NSCLC, HNSCC, and skin sarcoma cell carcinoma (NCT02454842 and NCT02449641). Encouraging treatment responses were seen in the case of the skin carcinoma patients. However, the trial was terminated due to insignificant responses in patients suffering from HNSCC and NSCLC.
Nagasaki et al.248 proposed two novel HAPs, namely 62 and 63 (Fig. 12). These conjugates contain doxorubicin and 5-FU linked to a 2-nitroimidazole core through an amide linkage designed to prevent undesirable drug release before the hypoxic region is reached. Compared to the parent drugs, these HAPs displayed drastically improved penetration in the hypoxic region, a finding ascribed to their increased hydrophobicity and metabolic stability. In the hypoxic region, cytotoxic drug release from 62 and 63 was thought to involve the formation of a 6-membered cyclic structure. In vitro studies led to the suggestion that both pro-drugs are highly cytotoxic in the MIA PaCa-2 cell line, particularly under hypoxic conditions. An investigation of the in vivo anti-tumour activity of 62 in colon 26 tumour-bearing mice revealed an improved survival rate relative to doxorubicin alone. A reduction in adverse events was also noted.
Hay et al.249 reported an alternative approach to delivering cytotoxins to hypoxic cells. In this case, a nitroimidazole moiety was conjugated to a 4-carboxamidophenyl nitrogen mustard (as the cytotoxic agent) via the N1 side chain; this gave HAP 64 (Fig. 12). Reduction of the nitro group favours intramolecular cyclization, resulting in the formation of a tetrahedral intermediate that collapses and expels the active nitrogen mustard. An in vitro cytotoxicity assay using the AA8 cell line revealed that conjugate 64 was 3.3-fold more toxic under hypoxic than normoxic conditions.
TH-302 (65, evofosfamide) is a HAP that consists of a brominated analogue of isophosphoramide mustard (Br-IPM) linked to a 2-nitroimidazole moiety (Fig. 12). The proposed mechanism of action for 65 includes reduction of the nitro group by one-electron oxidoreductases under hypoxic conditions. This is followed by fragmentation to release the bromoisophosphoramide mustard, a species that is recognized as being a potent DNA alkylating agent. Prodrug 65 was first synthesized and evaluated preclinically by Duan et al.,250 and was reported to be 400-fold more cytotoxic in the H460 human non-small-cell lung cancer cell line under hypoxic conditions than normoxic conditions. In vivo studies in mouse xenograft models bearing MIA PaCa-2 human pancreatic cancers251,252 revealed that 65 possessed high antitumour efficacy when administered either alone or in combination with gemcitabine. It was found to extend survival relative to controls with one out of eight of the test animals remaining tumour free at day 44. Other enhancements in chemotherapeutic benefit were reported.253 For instance, it was found that treating nude mice bearing human breast MCF-7 or prostate PC-3 tumour xenografts with 65 4 h before the subsequent administration of doxorubicin or docetaxel significantly enhanced the anticancer effects of these classic chemotherapeutics. This proved true both in the perivascular and hypoxic regions. On the basis of this and other promising preclinical studies, 65 advanced into clinical trials and was tested as both a monotherapy and in combination with other chemotherapy regimens in more than 1500 patients. Early on, 65 provided promising results in phase II clinical trials in combination with doxorubicin (soft tissue sarcoma)254,255 and gemcitabine (pancreatic cancer).256 However, hematologic toxicity was observed in the case of the doxorubicin trial. Recently, two large phase III clinical studies of 65 were carried out in combination with other anticancer drugs; these were focused on soft tissue sarcoma (NCT0144088) and advanced pancreatic cancer (NCT01746979) patients. Unfortunately, little improvement in the overall survival was seen.
Lu et al. developed compound 66 (Fig. 12) as a hypoxia responsive prodrug of SN-38.257 As compared with SN-38 [HCR = 0.74 (in HT29 cancer cells), 0.83 (in H460 cancer cells)], 66 displayed slightly improved HCR in the HT29 (3.82) and H460 (3.71) cancer cell lines.
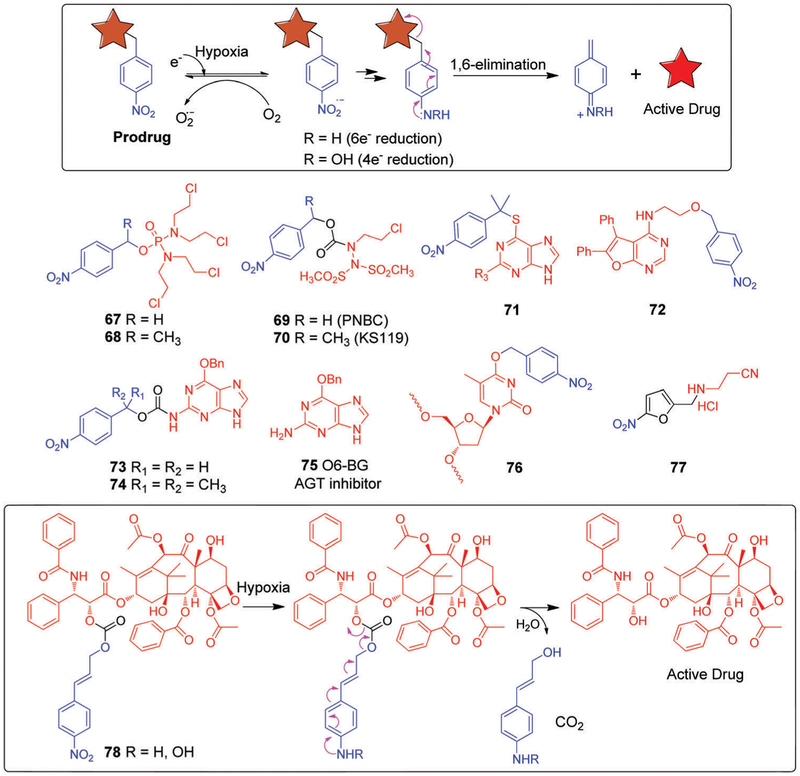
Nitroaromatics have been widely used to prepare hypoxia selective prodrugs. In hypoxic environments, the NO2 unit is readily reduced to the corresponding aniline species. Often this is used to unlock the active cytotoxin. The 4-nitrobenzyl moiety has received considerable attention in this regard. It has been widely investigated as a so-called self-immolative linker in various prodrug designs, as well as in the context of other therapeutic modalities, including antibody-directed enzyme prodrug therapy (ADEPT) and gene-directed enzyme prodrug therapy (GDEPT) development. Enzymes that metabolize the aromatic nitro groups are particularly important in the context of these efforts owing to the large electronic changes that are produced as a result. Reduction of the nitro group (Hammett σp electronic parameter = 0.78) produces either hydroxylamine (4e−reduction, σp = −0.34) or aniline (6e−reduction, σp = −0.66), which then undergo a spontaneous 1,6-elimination to release the active drug (Fig. 13). Most ADEPT and GDEPT studies relying on this mechanism have exploited the nitroreductase codified by the nfsB gene of E. coli, an oxygen-insensitive flavin mononucleotide (FMN) nitroreductase (NTR) having a close sequence homology to the NTR of S. typhimurium.258 Single crystal structural studies revealed that this NTR is a homodimer have one FMN unit per monomer, with two channels leading to the active site. There are few contacts with the ligands that can contribute to its observed substrate selectivity.259 NTRs efficiently reduces the nitroaromatics to hydroxylamines in a two-step ping-pong bi–bi mechanism.260 The following examples illustrate the use of the 4-nitrobenzyl moiety as a basis for creating hypoxia selective prodrugs.
Fig. 13.
Nitrobenzyl-based hypoxia-activated prodrugs. Also shown is their proposed mechanism of drug release, which involves 1,6-elimination under hypoxic conditions.
Borch et al.261 employed a 4-nitrobenzyl linker to generate the nitrophenyl phosphorodiamidate prodrugs 67 and 68 (Fig. 13). Prodrug 68, with an a-methyl substituent, was found to be selective to HT-29 cells with an aerobic/hypoxic toxicity ratio of approximately 90. An ex vivo assay revealed toxicity to murine bone marrow granulocyte/macrophage progenitors (GM-CFC) that was comparable with the hypoxic toxicity against HT-29 cells. This led to the conclusion that another mechanism for drug activation exists in addition to the expected nitro group bioreduction-based pathway the linker was designed to exploit.
Other nitrobenzyl-based HAPs, 69 and 70 (Fig. 13), were described by Sartorelli et al.262 Upon enzymatic reduction, both of these agents led to the formation of 90CE (1,2-bis(methylsulfonyl)-1-(2-chloroethyl)hydrazine), an antineoplastic DNA crosslinking cytotoxin.263,264 Mechanistic studies revealed that 69 (PNBC) could reductively release the cytotoxin 90CE by either enzymatic nitro reduction or nucleophilic attack by thiols such as GSH or glutathione S-transferase (GST), which are often overexpressed in tumours. In contrast, prodrug 70 (KS119) appeared to be activated exclusively via nitro group reduction. Presumably, nucleophilic attack by a thiol is suppressed for steric reasons as the result of the methyl group on the methylene carbon spacer. In vitro cytotoxicity studies revealed that prodrug 70 was more potent than 69 with high hypoxia selectivity being seen in the EMT6 tumour cell line. Similar promising results were observed in CHO K1dhfr parental cells. However, 69 was less efficacious against hypoxic cells and also displayed some toxicity in aerobic cells, presumably as a result of GST thiol activation. An analogue, HAP 71 (Fig. 13),265 was designed to deliver 6-thioguanine (6-TG), an antileukemic drug, deep into solid tumours. In vitro studies with A549 cells, which are known to express a high level of P450 reductase,266 led to the suggestion that 6-TG is released efficiently under anoxic conditions in the case of 71.
The therapeutic effect of a majority of the bioreductive prodrugs described above occurs through the release of DNA-damaging agents. As a consequence, there is a risk that using these prodrugs in combination with standard chemotherapies may increase the possibility of off-target toxicities. An alternative approach, which represents a significant departure from the above-mentioned mechanism, involves a strategy wherein an active drug that acts as a strong inhibitor of a protein or enzyme required for tumour progression, is masked by a hypoxia-responsive linker.
Hammond et al.267 utilized this approach to create the bioreductive prodrug 72 (Fig. 13), which contains a checkpoint kinase 1 (Chk1)/Aurora A inhibitor. Chk1/Aurora A is serine/threonine-specific protein kinase that may promote tumour growth, as inferred from the positive correlation of Chk1 expression and tumour grade.268,269 The inhibitor in 72 was rendered inactive by tethering it to a nitrobenzyl group. Reduction of the nitro group prompts release of the active Chk1/Aurora inhibitor. Presumably as a result, prodrug 72 was found to display high potency against severely hypoxic cells in vitro.
A number of alkylating agents mediate their antitumour effect by damaging DNA through guanine O-6 alkylation. However, this DNA damage is repaired by the protein O6-alkylguanine-DNA alkyltransferase (AGT). This enzyme transfers alkyl groups from an alkylated guanine O-6 position to the cysteine residue in its active site.270 Therefore, it follows that a high level of AGT increases resistance to guanine O-6 alkylating agents.271 O-6-Benzylguanine (O6-BG, 75, Fig. 13) is a known inhibitor of AGT. It deactivates the enzyme through the formation of an S-benzyl cysteine derivative in its active site. It has also been found to sensitize cancer cells to alkylating agents.272,273 Unfortunately, O6-BG not only sensitizes cancer cells, it also potentiates normal cells. To address this latter limitation, Penketh et al.274 utilized a prodrug strategy to develop conjugates 73 and 74 (Fig. 13). In 73 and 74, the 2-amino group, which is essential for the AGT inhibitory activity of 75, is protected in the form of a carbamate. Bioreduction of the nitro group by NADPH:cytochrome P-450 under hypoxic conditions facilitated the unmasking of the amino group. This allowed the site-specific release of O6-BG within hypoxic regions and served to reduce aerobic toxicity. Furthermore, in vitro studies performed against DU145 human prostate carcinoma cells led to the suggestion that 74 (40 μM) sensitizes DU145 cells to the levels of cytotoxicity displayed by laromustine (an alkylating agent) under hypoxic, but not normoxic conditions.
In another report, Saneyoshi et al.275 described the synthesis of an oligonucleotide-based HAP, 76 (Fig. 13), in which the thymine base is protected using a hypoxia-labile nitrobenzyl linker. Under hypoxic conditions, deprotection of the thymine group serves to convert 76 to an active oligonucleotide that is able to form stable duplexes with target oligonucleotides, thereby inhibiting mRNA translation.
In separate work, Minutolo et al.276 developed a bioreductively activatable inhibitor 77 (Fig. 13) of lysyl oxidase (LOX). This enzyme is overexpressed in hypoxic tumour cells and promotes the metastatic spread of several solid tumours.277 As inferred from an in vitro invasion analysis, prodrug 77 exerts a 6-fold higher anti-invasion effect in breast cancer MDA-MB-231 cells under hypoxic conditions than it does under normoxic conditions.
In an effort to develop a hypoxia-responsive paclitaxel pro-drug, Scheeren et al. designed a series of paclitaxel analogues conjugated with various nitro/azo-based functional groups. All the compounds generated in the context of this study exhibited good stability in Tris buffer. Moreover, a selected lead, compound 78 (Fig. 13), showed diminished aerobic toxicity in various human cancer cell lines (H226, MCF7, EVSA-T, WIDR, IGROV, MI9, and A498).278
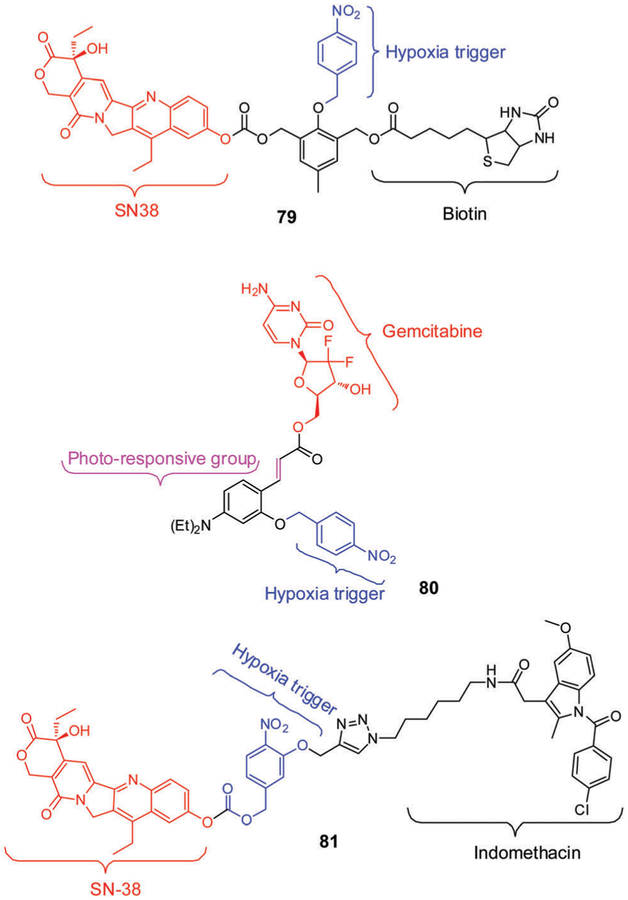
Recently, Kim et al.279 developed the hypoxia-activated conjugate 79 (Fig. 14) for the diagnosis and treatment of solid tumours. This system contains a cancer-localizing biotin subunit, the anticancer drug SN38, and 4-nitrobenzyl (a hypoxia-responsive self-immolative linker). In accord with the design expectations, conjugate 79 showed a preference for cancer cells. This preference was ascribed to the cancer-targeting ability of biotin. Activation of 79 by nitroreductase (NTR, E. coli), particularly under hypoxic conditions, was expected to promote release of SN38 through 4- or 6-electron reductive elimination of the 4-nitrobenzyl linker. Prodrug 79 displayed high selectivity for, and greater cytotoxicity against, A549 and HeLa cells (biotin-positive) under hypoxic conditions compared to BJ and WI-38 cells (normal cells). In vivo studies involving HeLainoculated xenograft mouse models served to confirm that 79 accumulates specifically in these particular solid tumours. Its activation under conditions of hypoxia produced a strong anticancer effect, a finding ascribed to the inhibition of the growth factor HIF-1α.
Fig. 14.
Structures of hypoxia-activated theranostics 79, 80, and 81.
Theranostic 80 (Fig. 14), based on a nitrobenzyl trigger, was developed by Zhang et al.280 This dual action agent was designed to release selectively both the anticancer drug gemcitabine (GMC) and a coumarin (CM) fluorescent probe following hypoxic bioreduction, photo-activation, and demasking in sequence. Evidence in support of this proposition came from the observation of enhanced CM-derived fluorescence under conditions of expected release. In fact, the levels of discharged drug were found to reflect the extent of hypoxia and the exposure time. Further support for this conclusion came from cell viability data involving the MCF-7 cell line studied under hypoxic conditions.
Angiogenesis, a tightly regulated process for new blood vessel formation in solid tumours, can influence the activation and therapeutic efficacy of hypoxia responsive prodrugs. Hence blocking angiogenic pathways in combination with hypoxia responsive prodrugs could be beneficial. Recently, Kim et al. developed a theranostic 81 (Fig. 14), where a nonsteroidal anti-inflammatory drug (NSAID) indomethacin (COX-2 inhibitor) was utlilized for two separate and complementary purposes i.e., cancer targeting as well as angiogenesis inhibition.281 Compared with a control analogue lacking indomethacin unit (SN-38, 81), theranostic 81 exhibited preferential targeting ability and cytotoxicity in COX-2 positive cancer cells (HeLa, A549) as compared to normal COX-2 negative cells (WI-38 and BJ) under hypoxic conditions. Further, 81 showed prolonged tumour retention and improved therapeutic response in HeLa inoculated xeno-graft animal models. The improved therapeutic efficacy of 81 compared to SN-38 and other controls was ascribed to its both cancer-targeting ability and COX-2 knockdown mediated anti-angiogenesis ability, as confirmed by decreased expression of angiogenic genes (VEGFA, CD31, ANGPT v2, and ANGPT v3) in tumour tissues.
4.4. Azoaromatics as hypoxia-activated prodrugs with drug elimination
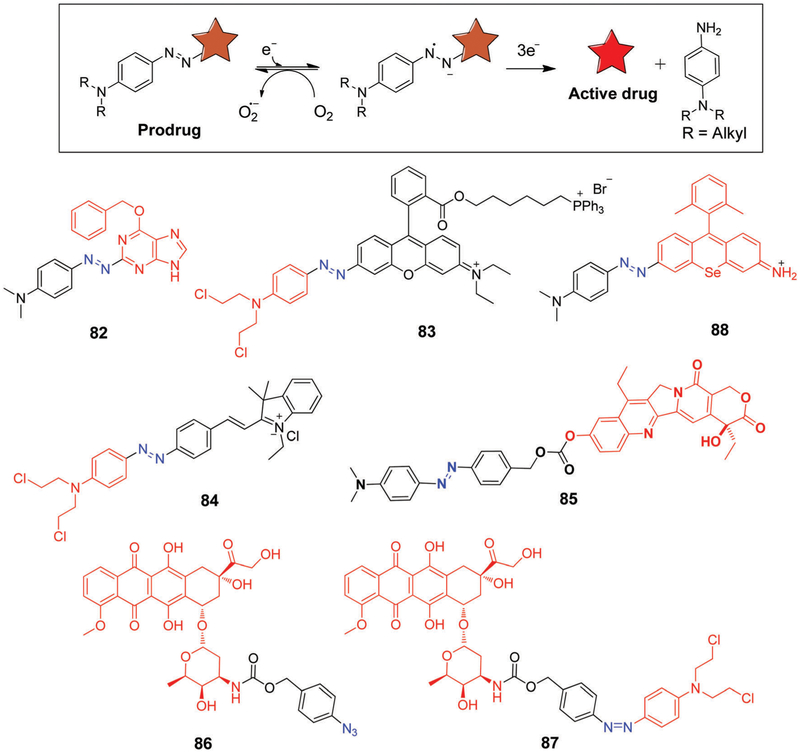
The overwhelming majority of bioreductive prodrugs reported to date have been based on the use of quinones, nitroaromatics, and N-oxides as the initial release triggers. Considerably less work has been devoted to incorporating redox active azo groups into hypoxia-sensitive prodrugs.282,283 In contrast, the use of azo moieties to create hypoxia-responsive imaging probes is relatively well developed.284 Baumann et al.285 reported 6-(benzyloxy)-2-(aryldiazenyl)-9H-purine 77 (Fig. 15) as an azo-based pro-drug of the AGT inhibitor, O6-BG (75, Fig. 13), discussed above.273 Hypoxia-driven azo-reduction of 82 by AGT-expressing DU145 cells resulted in the release of the active inhibitor O6-BG in excellent yield. This release, in turn, served to enhance the cytotoxic effect of laromustine in DU145 cells.
Fig. 15.
Mechanism of azo-based prodrug activation and structures of azo-based drug delivery systems.
Kim et al.286 explored the use of an azobenzene scaffold to create the hypoxia-selective theranostic 83 (Fig. 15). This prodrug specifically targeted the mitochondria in cancer cells where it was expected that hypoxia-mediated reduction of the azobenzene moiety would result in release of N,N-bis(2-chloroethyl)-1,4-benzenediamine, a DNA alkylating agent, as well as a fluorescent rhodamine analogue. Theranostic 83 showed good in vitro cytotoxicity against the DU145 and MDA-MB-231 cell lines under hypoxic conditions (3% O2). Furthermore, in vivo and ex vivo studies involving xenografted mouse models revealed a significant reduction in tumour burden, along with a decrease in angiogenic markers and suppressed cell proliferation. As prepared 83 is non-fluorescent. However, hypoxia activation via azo-reduction leads to release of rhodamine, a fluorescent moiety that allowed for spectroscopic-based monitoring of the release process. Recently, azo-based prodrugs, 84 and 85 were also developed and tested for both hypoxia selective in vitro and in vivo anticancer activity (Fig. 15).287,288
Most early efforts centered around prodrug activation strategies involving enzymatic activation. However, in 2008, Robillard et al.289 proposed an alternative chemical-based route wherein the selective Staudinger reaction was used to achieve prodrug activation. This strategy is embodied in prodrug 86 (Fig. 15), which undergoes hydrolysis spontaneously under aqueous conditions followed by self-immolation to release free doxorubicin. Prodrug 86 displayed a 176-fold higher IC50 compared to free doxorubicin in tests involving the A431 human vulvar skin squamous carcinoma cell line.
Recently, Cheng et al. developed a heteromeric prodrug 87 (Fig. 15) containing two chemotherapeutic drugs, doxorubicin and nitrogen mustard, linked through an azo moiety.290 Compound 87 displayed good hypoxia responsive toxicity in 4T1-tumour bearing mice models as compared with controls (PBS, doxorubicin) with minimal side effects.
In another investigation, Urano et al.291 has reported the development of activatable photosensitizer 88 (Fig. 15), which relies on an azo subunit linked to a conjugated selenorosamine dye. Compound 88 was not found to generate singlet oxygen when irradiated under normoxia condition, a result ascribed to the fact that in the excited state, the system undergoes a very fast rotation around –N=N– bond thus reducing the intersystem crossing needed to generate the triplet state and promote formation of singlet oxygen. However, even under mildly hypoxic condition reduction of the azo group occurs. This activates the photosensitizer leading to a significant singlet oxygen quantum yield. In vitro cytotoxicity studies further demonstrated that compound 88 is highly selective in that it induces the photo-dynamic ablation of human lung cancer-derived A549 cells much more effectively under hypoxic as opposed to normoxic conditions.
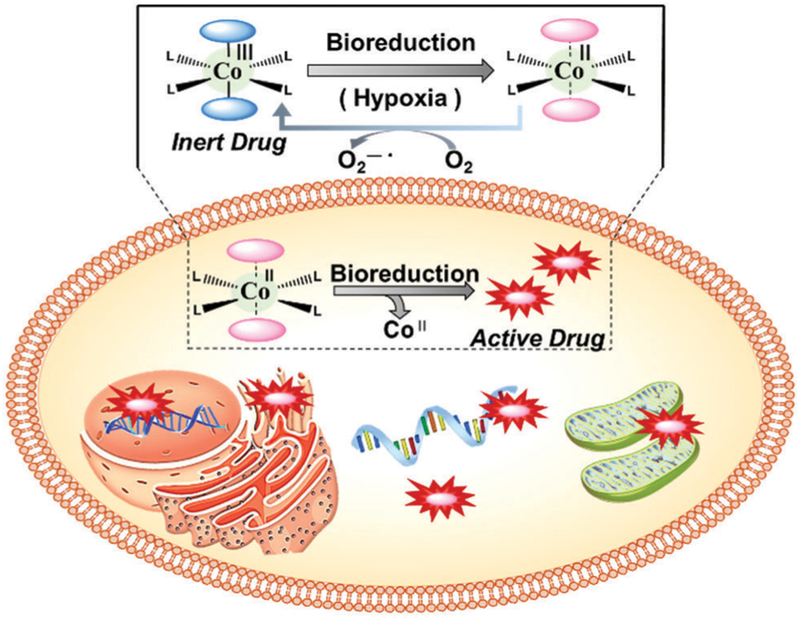
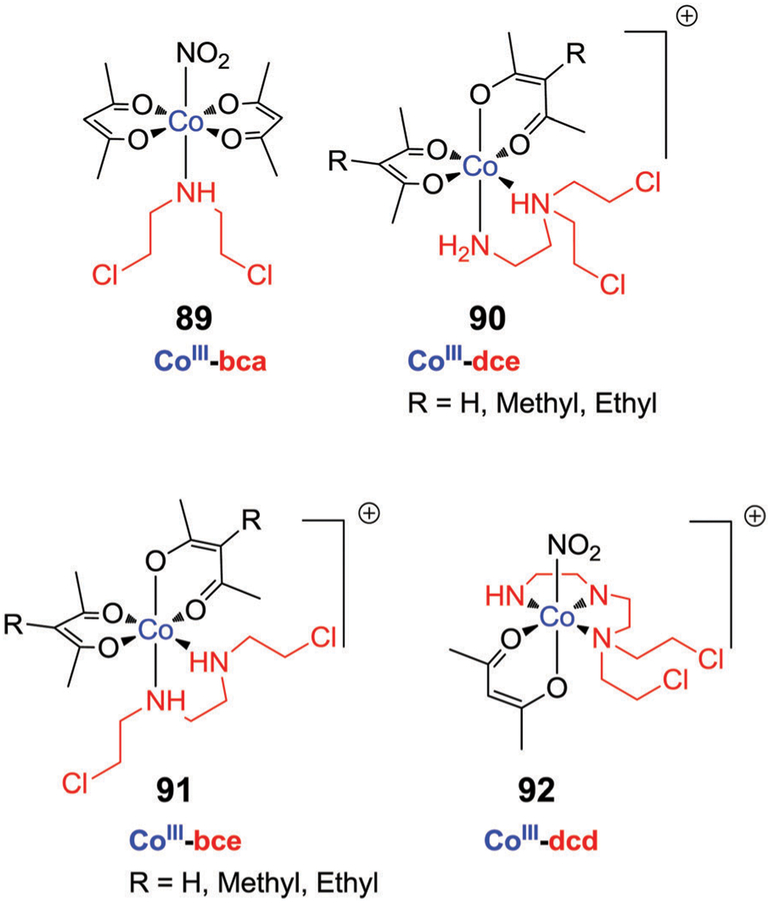
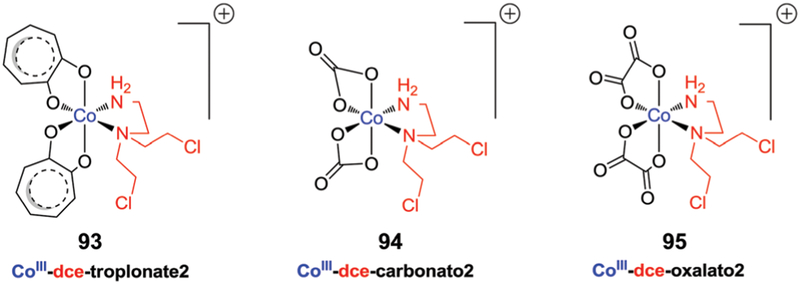
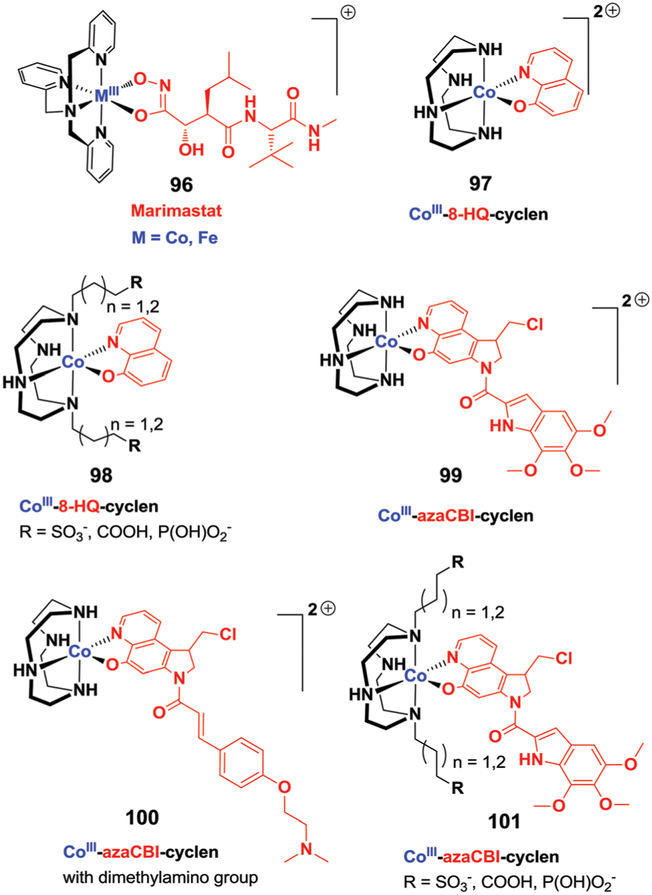
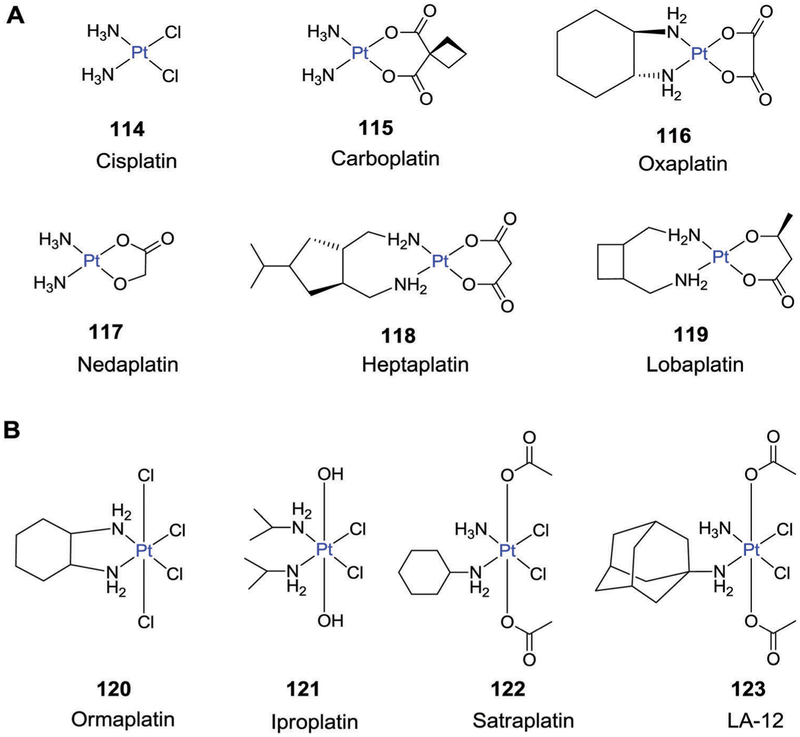
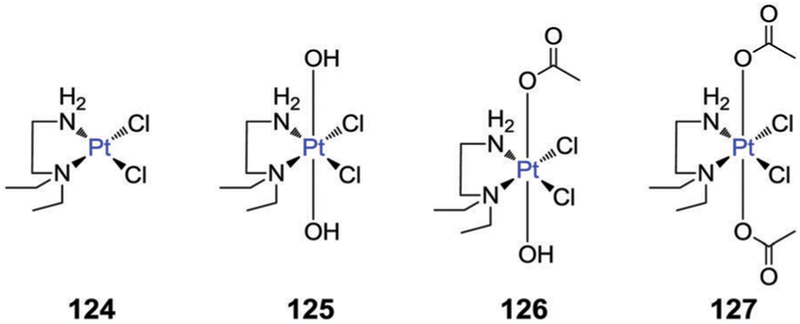
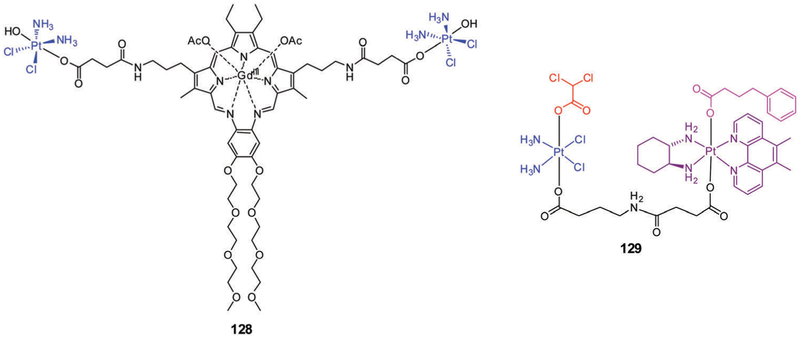
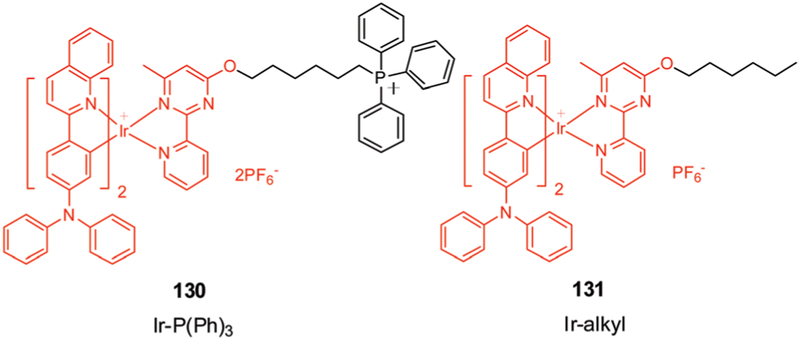
5. Metal-based therapeutics
The majority of dynamic pharmaceutical drug ingredients are organic and biologically driven compounds. Consequently, an enormous effort has been devoted to making and testing potential organic chemistry-based drug leads. However, the serendipitous discovery of cisplatin and its eventual approval as a therapeutic in 1978 has changed the cancer treatment landscape over the last 40 years. It cannot be overstated that cisplatin and carboplatin are two of the most important anti-tumour agents in the clinic.292 They are used as front line therapies by oncologists, with roughly half of all patients undergoing chemotherapy receiving one of these complexes, alone or in combination with other agents.293 Unfortunately, despite their success and history, small molecule platinum agents suffer from multiple limitations: (1) lack of tumour selectivity, (2) high systemic toxicity, (3) multiple mechanisms of resistance (inborn and acquired) and (4) a narrow spectrum of activity.294 Overcoming such limitations has been an interdisciplinary challenge.294,295 In cancers such as NSCLC, mesothelioma, and ovarian, these limitations are manifest in low 5-year survival rates of 5–45% in patients treated with cisplatin and carboplatin as frontline therapies.
The success and limitations of cisplatin has prompted an ongoing search for yet-improved new metal-based drug candidates.296–298 In contrast with organic-based drugs, metal-based drug candidates possess potential advantages, such as an ability to adjust their respective geometries, vary their coordination numbers, and undergo changes in redox states. One of the most promising avenues is the use of metal-based prodrugs to target tumour hypoxia. The canonical rationale behind this approach is as follows: cytotoxic agents may be tethered to metal complexes that are stable in relatively high oxidation states under aerobic conditions. This imparts stability to the active cytotoxic agents and shields them from rapid metabolism. Upon bioreduction, the metal complexes are reduced to a lower oxidation state, became relatively unstable, and release the active form of the cytotoxic agent.
5.1. Cobalt-based therapeutics
A number of cobalt complexes have been explored as hypoxia-sensitive prodrugs. In general, Co(III) complexes are moderately inert, adopt octahedral geometries (low spin, d6). Upon bio-reduction, the complexes are reduced to their more labile Co(II) forms. As a general rule, Co(II) complexes are re-oxidized back to Co(III) complexes in the presence of molecular oxygen. However, in hypoxic environments, the labile Co(II) forms (high spin, d7) can dominate, allowing release of the active drug.299,300 The requisite bioreduction processes are typically mediated by endogenous reductases, such as P-450R and xanthine oxidase (XO). This assumes the reduction potential of the Co(III) complex is compatible with that of the cytosolic environment (−200 mV to −400 mV).301 In addition, ligand alterations can influence the reduction potential of the Co(III) complexes. This fine-tuning has inspired the synthesis of a variety of promising hypoxia selective prodrug frameworks (Fig. 16).
Fig. 16.
Proposed mechanism of reductive activation of Co(III) prodrugs.
In 1987, Teicher et al. reported that Co(III) complexes containing both nitro and amine ligands served as promising radio-sensitizers toward hypoxic mammary carcinoma cells (EMT6).302 Further investigations of Co(III) complexes containing acetylacetonate (acac) and a mustard, bis(2-chloroethyl)amine (bca), revealed significant cytotoxicity towards murine cancer cells.303 A specific complex of this class, [Co(acac)2(NO2)(bca)] (89, Fig. 17, CoIII-bca), proved inactive in its oxidized state. This inactivity was ascribed to the fact that the lone pair of the nitrogen is coordinated to the cobalt center. This potential nucleophilic center can thus not participate in an intra-molecular cyclization, which is deemed necessary to produce the active aziridinium species considered responsible for DNA alkylation-induced cytotoxicity. Upon reduction, the nitrogen mustard is released from the more labile Co(II) form of the complex, as evidenced by cytotoxicity being observed in hypoxic mammary carcinoma cells (EMT6). Unfortunately, screening assays on SCC-25/HN2 cells revealed that 89 underwent overly facile reduction, obviating its presumed bioreduction-mediated selectivity.
Fig. 17.
Chemical structures of hypoxia-activated cobalt(III) complexes incorporating various ligands.
Co(III) complexes containing bidentate mustards N,N′-bis-(chloroethyl)ethylenediamine (bce) and N,N′-bis(chloroethyl)-ethylenediamine (dce) were also investigated.304–306 In vitro analyses revealed that the cytotoxicity of CoIII-dce and CoIII-bce (90 and 91, Fig. 17) resulted from the released mustard agents only. Further SAR studies provided support for the conclusion that in addition to mustard agents, the acetoacetonate ancillary ligand also played a crucial role in mediating the observed hypoxia selectivity. Compared with the unsubstituted acetylacetonateincorporated Co(III) complexes, the corresponding methyl and ethyl analogues displayed substantially improved hypoxia selectivity. Furthermore, a lead complex that emerged from these efforts, [Co(Meacac)2dce]+, SN 24771, was found to exhibit cytotoxicity against Chinese hamster ovary fibroblast cells (AA8, UV4) that was enhanced by 6-fold under hypoxic conditions. Significant selectivity was also observed against multicellular spheroids in solid tumours. The mechanism for hypoxia selectivity was investigated in detail using pulse and steady state radiolysis techniques and led to the suggestion that more complex processes were involved than the expected redox cycling.307 A Co(III) complex with tridentate mustard, N,N′-bis(2-chloroethyl)-diethylenetriamine, CoIII-dcd (92, Fig. 17) was also developed with the hope of enhancing the metabolic stability.308 Despite exhibiting modest hypoxia selectivity, the potency was significantly reduced (up to 20-fold) compared to the corresponding bidentate Co(III) analogs.
A series of Co(III) complexes with various ancillary ligands (bis-tropolonato, anionic carbonato, and oxalato) were used to investigate in systematic fashion the impact of these ligands on the metabolic stability of the complexes (Fig. 18).309–311 The cobalt bis-troplonate complex, CoIII-dce-troplonate2 (93), exhibited a relatively high reduction potential but a toxicity profile comparable to that of free mustard against UV4 cells and the Chinese hamster ovary fibroblast subline AA8, without any marked hypoxic selectivity. The corresponding anionic Co(III)-dce complexes [CoIII-dcecarbonato2 (94) and CoIII-dce-oxalato2 (95)] exhibited lower toxicity compared with the free N,N-bis(2-chloroethyl)-1,2-ethanediamine (dce) species and possessed lower hypoxia selectivity compared with the corresponding acac complex. The hypoxia-selective cytotoxicity of these family of analogues (Fig. 17 and 18) was investigated by pulse radiolysis techniques. These studies led to suggestion that upon bioreduction, the bidentate alkylating mustards and the acac ligands are released giving rise to a Co(II)–hexaaqua complex. The observed hypoxia-selective cytotoxicity of the most promising Co(III) complexes was thus considered to be the result of competition between molecular oxygen and the reactivity of the corresponding Co(III) species towards intracellular reductants.
Fig. 18.
Chemical structures of hypoxia activated cobalt(III) complexes.
Using the same general reduction-based release strategy, Hambley et al.312 created a Co(III)–tris-(2-methylpyridyl)amine complex designed to deliver matrix metalloproteinase (MMP) inhibitors specifically at hypoxic tumour sites. MMPs are a diverse class of zinc containing endopeptidases, which play a vital role in many biological processes, including arthritis, cancer progression, and tissue ulcerations. So far, several MMP-selective inhibitors have been reported for the treatment of cancer; however, the mixed preclinical and clinical responses have limited their potential utility.313–315 One of the perceived impediments is the presence of hydroxamic functionality in the structural designs of the most potent MMPs inhibitors reported to date. The hydroxamic group, which is exceptionally and non-specifically reactive toward various biomolecules (especially metal centres), is thought to diminish the drug efficacy. To overcome this constraint, Hambley and coworkers coupled a Co(III)-tris(2-methylpyridyl)amine (tpa) complex to MMP inhibitors via a hydroxamic acid moiety (96, Fig. 19). The goal was to minimize side reactions in well oxygenated regions prior to drug activation at hypoxic tumour sites. Initial structural and electrochemical investigations of Co(III)-tpa complexes with alkyl and aryl hydroxamic acid ligands as MMP inhibitors provided support for the design hypothesis, namely that Co(III)-complexes could be used to transport hydroxamic acid-containing compounds specifically to hypoxic tumour sites.316 In the context of these studies, a Co(III)-tpa complex with marimastat (a potent MMP inhibitor) was prepared and tested (Fig. 19). In vivo toxicity studies provided results inconsistent with the conclusion that the Co(III)-tpa-marimastat complex, 96 was relatively more effective at reducing the growth of implanted 4T1.2 tumours in mice compared with free marimastat. Later, Co(III)-tpa complexes containing both hydroximate and hydroxamate forms have been developed. Interestingly, both protonated forms exhibited different reduction potentials and thus varying abilities to release bound hydroxamic acid groups. As most tumours are both acidic and hypoxic, this feature was noted as one that could be manipulated to develop new tumour-specific Co(III) complexes for use in delivering drug candidates to cancerous lesions.317
Fig. 19.
Chemical structures of Co(III) complexes bearing tridentate and tetradentate ligands.
Other Co(III) complexes composed of tetradentate ligands, including 8-hydroxyquinoline (8-HQ, 97, 98) and azachloromethylbenzindoline (azaCBI, 99, 100 & 101) as biologically active agents, have been investigated (Fig. 19).318,319 Both Co(III) complexes undergo efficient reduction through radiolytic and nonradiolytic pathways in hypoxic cells. Interestingly, the 99 complex displayed a 20-fold increase in cytotoxicity against HT29 cells in hypoxia environments relative to the same cells cultured under normoxic conditions. Further efforts were devoted to the improvement of hypoxic selectivity, complex stability, and solubility. It was found that the incorporation of the dimethylaminoethanol moiety on the pyrroloquinoline ring (100) gives rise to an approximately 30- and 40-fold hypoxia selectivity relative to normoxic conditions in lung cancer cells (A549) and ovarian cancer cells (SKOV3), respectively.320 Conversely, the incorporation of a 1,7-substituted cyclen as an ancillary ligand with anionic carboxylate, phosphonato, or sulfonate ligands (101) resulted in a reduction in the hypoxic selectivity, along with a slight improvement in solubility. Possibly this may be due to the higher lipophilic character of complexes that allows them to enter the cell via passive diffusion through the cell membrane. Alternatively, the authors suggested that these complexes may be subject to reduction by outer membrane nitroreductases, thus accounting for the low observed HCRs (hypoxic cytotoxicity ratios).321
A series of Co(III)-azaCBI complexes with cyclam (1,4,8,11-tetraazacyclotetradecane) ligands were designed with the aim of increasing the electron density at the metal center (102, Fig. 20). Coordination with the trans-like bridged nitrogen atoms within the cyclam serves to enforce a cis coordination about the metal center while enhancing the relatively stability of the Co(III) oxidation state relative to the Co(II) form (i.e., decreasing the reduction potential). In vitro cytotoxicity studies against a panel of human cancer cell lines confirmed that CoIII-azaCBI-cyclam (102, R = H) exhibited high HCRs in the range of 81–212 in anoxic environment (in a Pd/H2 catalyzed anaerobic chamber) as compared to those containing 20% oxygen. Interestingly, the cytotoxicity to 102 was not found to be correlated with one-electron reductase (POR) expression levels, a well-known reductase for hypoxia selective toxins. Moreover, 102 (R = H) displayed poor toxicity against hypoxic and radiation resistant HT-29 tumour models when compared with tirapazamine (TPZ, Fig. 29).322 The presumed nonenzymatic reductants responsible for the activation of 102 are still unknown, and this presents a major hurdle in the pharmacological application of 102 as a hypoxia-selective cytotoxin.
Fig. 20.
Hypoxia activated Co(III) cytotoxin complexes bearing various tethered ligands.
Curcumin, a natural product derived from Curcuma longa (turmeric), a nontoxic yellow pigment, displays a number of biological effects, including anti-inflammatory, antioxidant, ant-proliferative, and antimetastatic activity, both in vitro and in vivo. However, poor solubility and rapid metabolism has limited the clinical potential of curcumin.323–325 Renfrew et al.326 created a Co(III)-based curcumin complex (CoIII–Curcumin, 103) with the aim of improving the aqueous solubility, stability, and preferential uptake in hypoxic tumour regions (Fig. 20). Both fluorescence lifetime imaging and X-ray absorption spectroscopy revealed its reductive activation and ability to release curcumin in various tumour models. Using the same strategy, the hypoxia-activated, tumour targeted delivery of the EGFR inhibitor (ELK-785) was attempted (Fig. 20).327 In vitro and in vivo studies of the agent produced for the purpose, CoIII-EGFR (104), revealed improved antitumour efficacy against A431 xenografts under conditions of hypoxia as compared with normoxic conditions. Encouragingly, 104 produced distinct and reproducible tumour regression in Calu3 xenograft mouse models.
5.2. Ruthenium-based therapeutics
The chemistry of ruthenium (Ru) complexes has been known since the 1950s;328 however, their antitumour properties were first observed only in the late 1960s.329 Several Ru metal-based complexes developed to date have displayed promising toxicity profiles against various cancer cell lines, both in vitro and in vivo.330–335
Ruthenium can exist in several stable oxidation states (i.e., II, III, and IV) under physiological conditions, and a minimal energy barrier exists for the interconversion between these different oxidation states. This has made ruthenium complexes attractive as potential drug candidates. To date, Ru(II) arenebased complexes and bioreducible Ru(III) complexes have engendered considerable interest in this context (Fig. 21). Currently, two Ru(III) complexes [ImH][trans-RuCl4(DMSO)Im] (Im = imidazole) (NAMI-A, 105) and [Na][trans-RuCl4In2] (Na = sodium) (NKP-1339, 107) are in phase II clinical trials (Fig. 22). These complexes display significant cytotoxicity in both in vitro and in in vivo models.336–342 However, their exact mechanism of action remains unknown. In 105 and KP1019 (106), i.e., [InH][trans-RuCl4In2] (In = indazole), the octahedral Ru(III) complex produced through synthesis is reduced to a more labile Ru(II) form by ascorbate or GSH under hypoxic conditions, a transformation that is thought to result in increased DNA adduct-induced toxicity.343 However, in spite of being similar in structure, these two complexes exhibit different biological activity.344–348 Generally, the antitumour activity of these and other higher valent ruthenium complexes appears correlated with the ease of bioreduction to the corresponding Ru(II) forms.349
Fig. 21.
Proposed reduction mechanism for Ru(III) and Pt(IV) prodrugs inside and outside the cell.
Fig. 22.
Chemical structures of ruthenium complexes of interest as potential anticancer agents. Currently, NAMI-A and NKP-1339 are under clinical evaluation.
Keppler et al. reported a homologous series of [RuIIICl(6–n)-(ind)n](3–n) (n = 0–4; ind = indazole) complexes designed to improve the hypoxia-sensitive response. Collectively, electro-chemical studies and in vitro assays revealed that tuning the reduction potential of the Ru(III) derivative through structural modification resulted in a two-fold enhancement of cytotoxicity in the colon cancer (SW480) and ovarian cancer (CH1) cells.350 Likewise, Clarke et al. reported that two ruthenium-based anticancer complexes, cis-[Ru(NH3)4Cl2]Cl (CCR) and (ImH)trans-[RuCl4(Im)2] (ICR), displayed enhanced DNA–Ru adduct formation produced and toxicity against HeLa cells that was 150-fold higher under hypoxic, as compared to normoxic conditions.349
In another comparative study, the chemically reduced form of 105 (produced by ascorbic acid, cysteine, or GSH reduction) was found to display greater tumour regrowth inhibition than the oxidized form of 105 in mammary carcinoma (MCa)-bearing mice.351 Further support for the “activation by reduction” hypothesis being critical to the action of Ru(III) antitumour complexes came from the finding that 106 displayed an enhanced affinity for GMP upon treatment with two equivalents of GSH. However, increasing the GSH levels further (up to 10-fold), led to a reduction in the GMP binding affinity owing to the formation of a GSH–Ru adduct.352,353
The promising activity exhibited by 106 led to the development of an analogue, NKP-1339 [sodium trans-[tetrachloridobis(1H-indazole)ruthenate(III)], 107], which was prepared in an effort to enhance the water solubility of the parent system (Fig. 22).354 Comparatively strong tumour targeting was seen for NKP-1339, an effect ascribed to an ability to bind to serum proteins (albumin and transferrin) coupled with reductive activation within the reducing tumour environment. Detailed mechanistic and cytotoxicity studies revealed that the reduction of the Ru(III) center was favored under hypoxic conditions and the resulting complex perturbed the cellular redox balance and induced apoptosis.355,356 A phase I clinical study involving 16 patients revealed that 107 is well-tolerated (dose = 20–420 mg m−2) in patients with various solid tumour types (lung, neuroendocrine, colon, ovarian, cervical, pancreatic, and head and neck cancer) (NCT01415297). Two patients with stage IV neuroendocrine tumors were noted as having experienced disease stabilization. Complex 107 was found to downregulate GRP78 (protein mis-folding processing regulator) activity. Enhanced GRP78 activity is seen in many tumours and has been associated with both intrinsic and conventional chemotherapy-induced drug resistance. In a study reported in 2012, complex 107 was also found to be potent against many tumour types, including those resistant to standard anticancer drugs.357 Further clinical studies of this agent thus appear warranted.
The preclinical anticancer activity of 107 was further studied in combination with sorafenib (a multi-kinase inhibitor, Nexavar™). Nexavar targets diverse oncogenic and pro-angiogenic receptor tyrosine kinases, including vascular endothelial growth factor receptor (VEGFR-1/23), platelet-derived growth factor receptor (PDGFR-1), Raf serine/threonine kinases, and RET proto-oncogene. With NKP-1339, synergistic cytotoxic effects were observed against both sorafenib-resistant (HCC1.1, Hep3B, VL-8 and VM-1) and sorafenib-responsive (PLC/PRL/5, HepG2 and HCC2) cancer cells. Enhanced rates of apoptosis with complete loss of mitotic cell population was seen both in vitro and in vivo in Hep3B hepatoma and VM-1 xenograft mouse models.358
Mukherjee et al. studied a sterically hindered imidazole-based Ru(II)–arene complex [(L)RuII(η6-p-cym)(Cl)]PF6 (L = N-((1H-imidazol-2-yl)methylene)-2,6-diisopropylaniline), 108 and investigated the mechanism of complex activation, as well as its activity profile in the MCF-7 and A549 cancer cell lines (Fig. 23). This sterically hindered metal complex exhibited high stability in 110 mM NaCl solution, underscoring its expected stability in extracellular matrix and resistance to deactivation through GSH. Interestingly, this complex displayed enhanced cytotoxi-city towards the MCF-7 and A549 cell lines under hypoxic conditions, with or without GSH (up to 1 mM) relative to normoxic conditions. This enhanced activity was attributed to the slow rate of complex hydrolysis. Cell cycle arrest in the sub G1 and G2/M phases, as well as apoptosis, was observed after activation.359
Fig. 23.
Chemical structures of ruthenium complexes (102–107).
Further examples of hypoxia-responsive metal complexes with DNA-based cytotoxicity were reported by MacDonnell et al. (Fig. 23).360,361 A series of cationic metal complexes in which two ruthenium cations are chelated to tetrapyrido[3,2-a:2′,3′-c:3″,2″’-h:2‴,3‴-j]phenazine) (tpphz) (109), 9,11,20,22-tetraazatetrapyrido[3,2-a:2′,3′-c:3″,2″−1:‴,3‴-n]pentacene (P4+, tatpp) (110), and 1,10-phenanthroline (phen) (111) moieties were synthesized. The monomeric [(Phen)2Ru(tatpp)]2+ (110) and dimeric [(phen)2Ru(tatpp)Ru(phen)2]4+ (111) complexes, in the form of their chloride salts, exhibited good aqueous solubility. They displayed cytotoxicity with corresponding G2-M cell cycle arrest362,363 under both normoxic and hypoxic conditions across a panel of cancer cell lines. In vivo studies further demonstrated significant tumour growth inhibition (83%) in human lung cancer H358 xenograft mouse models. Supporting electrochemical studies revealed enhanced hypoxic sensitivity that was attributed to concomitant formation of carbon-based radical species.
Another series of ruthenium and iridium b-ketoiminato complexes exhibiting significant toxicity against a panel of cancer cell lines were reported by McGowan et al. (Fig. 23).364
Compared to normoxic conditions, the [Ru(b-ketoiminato)] complexes (112, X = F, Br) displayed two-fold higher sensitivity under hypoxic conditions than in normoxic environments. They also were found to inhibit thioredoxin reductase effectively (i.e., at nanomolar concentrations). Detailed mechanistic studies revealed that these complexes exhibited a different mode of action from cisplatin drugs. Specifically, they were believed to induce single-strand DNA breakage and, as a result, trigger apoptosis.
In another report, Chao et al. presented a series of ruthenium(II) complexes containing anthraquinone subunits. These systems exhibited significant potency that was ascribed in part to their lipophilicity and good cellular uptake.365 In vitro toxicity studies revealed that in contrast with cisplatin, the most active analog, [Ru(pbqy)(adtpy)(ClO4)] (pdpy = 6-phenyl-2,2′-bipyridine) (adtpy = 2-([2,2′:6′,2″-terpyridin]-4′-yl)anthracene-9,10-dione) (113, Fig. 23), was up to 46-fold more potent in hypoxic HeLa cells and 65-fold more potent in 3D multicellular tumour spheroids than in normoxic controls. Further studies confirmed a correlation between the hydrophobicity and cellular uptake behaviour of the complexes with hypoxic toxicity. Under hypoxic condition exposure (8 h), this agent was found to localize preferentially to both the mitochondria (50%) and the nucleus (32%), which is a slight shift compared to what is seen under normoxic condition (60% mitochondrial and 28% nucleus localization). In contrast, cisplatin exhibited preferential accumulation in the cytoplasm with only 8% of the drug being found in the nucleus under both normoxic and hypoxic conditions. Mechanistic studies revealed that conjugate 113 triggered apoptosis through multiple synergistic pathways, including DNA damage, DNA replication inhibition, HIF-α inhibition, and mitochondrial dysfunction.
5.3. Other metal-based therapeutics
Platinum(II) metal complexes are a mainstay of cancer chemo-therapy (114–119, Fig. 24). Three platinum(II) complexes have received FDA approval, cisplatin, carboplatin, and oxaplatin. A number of analogues designed to minimize the toxicities (nephrotoxicity and gastrointestinal toxicity) induced by cisplatin are in the development stage. The currently accepted mechanism of action is that the FDA-approved Pt(II) complexes target fast proliferating cells by binding to DNA after cellular uptake.366–371 Although in routine use worldwide, the FDA-approved platinum(II) cancer drugs are far from ideal. They suffer from high intrinsic toxicity and their efficacy in many tumour types is limited by various factors, such as low intra-cellular accumulation, upregulated DNA repair mechanisms, and drug inactivation by various thiol reductants, including GSH and metallothioneins. In part because of these considerations, considerable attention has focused on platinum(IV) complexes in recent years (Fig. 24). Relative to their Pt(II) congeners, Pt(IV) complexes are relatively non-toxic. Several Pt(IV) variations are stable in biological fluids, allowing many undesired deactivation pathways to be overcome and clinically attractive strategies, such as oral administration, to be explored.
Fig. 24.
Chemical structures of (A) clinically approved/marketed platinum(II) complexes and (B) platinum(IV) complexes under clinical investigations.
In platinum(IV) complexes, the metal center typically adopts an octahedral geometry with a low-spin d6 electron configuration. The presence of two extra ligands relative to typical Pt(II) complexes imparts enhanced kinetic inertness towards biological nucleophiles. In addition, the axial ligands offer the possibility of tuning various pharmacokinetic parameters, such as lipophilicity, reduction potential, and cancer microenvironment-targeting. Although the Pt(IV) complexes can interact with DNA in their oxidized forms, adduct formation is relatively slow.372 Thus, Pt(IV) complexes are generally considered to be prodrugs that need to undergo reduction to produce a more active Pt(II) form. Since Pt(II) complexes are typically square planar, reduction generally leads to expulsion of the two axial ligands.373 The reduction rates are normally correlated with the reduction potentials. Thus, they can often be tuned through modification of the Pt(IV) complex composition. Once reduced, the active Pt(II) forms are thought to generate a cytotoxic response through DNA binding in analogy to what is proposed for the classic, FDA-approved platinum drugs.
Ormaplatin (tetrachloro(trans-1,2-diaminocyclohexane)-platinum(IV), 120) was the first Pt(IV) complex to undergo clinical trials (Fig. 24). Upon bioreduction, an active dichloro(trans-1,2-diaminocyclohexane)platinum(II) agent is produced from ormaplatin. This particular Pt(IV) complex was found to be active against cisplatin-resistant cell lines both in vitro and in vivo. In phase I clinical studies, various dose patterns and administration modes were investigated. Unfortunately, ormaplatin engendered severe neurotoxicity at the maximum tolerated dose (MTD), presumably reflecting its fast reduction from Pt(IV) to Pt(II) form in vivo.374,375 Another Pt(IV) complex, iproplatin (cis,trans,cis- dichlorodihydroxobis(isopropylamine)platinum(IV), 121) was also investigated clinically.376 Compared to ormaplatin, iproplatin was found to be less prone to reduction owing to the presence of axial hydroxyl groups. Additionally, the higher water solubility (44.1 mM) allowed explorations of various formulations and modes of administration. To date, a total 38 clinical trials, ranging from phase I to phase III, have been conducted with iproplatin targeting a range of cancer types.377–379 However, little benefit was seen for ormaplatin in these trials relative to the FDA-approved Pt(II) agents (e.g., cisplatin and carboplatin).
Another Pt(IV) analogue, satraplatin [trans,cis,cis-bis(acetate)-aminecyclohexylaminedichloroplatinum(IV)], 122 was developed as an anticancer platinum agent suitable for oral administration (Fig. 24).380 In the bloodstream 122 undergoes bioreduction to furnish six distinct Pt(II) species with ammine(cylcohexylamine)dicholorplatinum(II) being the major daughter product.381 In pre-clinical studies, 122 displayed a better toxicity profile compared to cisplatin in several ovarian cancer cell lines in vitro. It also exhibited good activity against the cisplatin-resistant cancer cell lines 41McisR and HX/155cisK.382 In vivo studies involving ADJ/PC6 plasmacytoma-bearing mice, revealed an improved antitumour efficacy for satraplatin relative to cisplatin, carboplatin and ormaplatin.382 A phase II clinical study, involving patients with squamous head and neck cancer and small cell lung cancer, revealed a 38% response rate in the case of satraplatin. The response rate was similar to that of cisplatin, but without the severe toxicity (neuro/nephrotoxicity) seen for this and other Pt(II) drugs.383 In other phase II trials, involving patients with advanced and recurrent squamous cervix cancer, more mixed therapeutic responses were observed.384 Satraplatin was also extensively investigated in patients with metastatic castration-resistant prostate cancer where a 62% response rate (stable disease or partial response) was seen.385,386 Bristol-Myers commissioned a phase III trials for satraplatin as a combination anticancer therapy with prednisone (synthetic corticosteroid drug, an immunosuppressant agent). However, the studies were terminated prior to completion since the combination of satraplatin and prednisone was found to be less efficacious than prednisone alone.387 Another study commissioned by GPC Bioteck for the same combination was conducted in 950 patients with refractory cancers. It was found that the combined treatment protocol resulted in a 36% reduction in pain progression and an improvement in patient survival as compared with prednisone alone.388 However, FDA approval was not forthcoming based on a lack of sufficient efficacy.389 Currently, another Pt(IV) agent, LA-12 (123, Fig. 24) is being developed.390 It is in phase I clinical studies.391
Keppler et al. reported392,393 the cytotoxic profiles of a series of platinum(IV) complexes 124–127 designed to be active against cisplatin sensitive (CH1) and resistant (A549 and SW480) cancer cell lines, under both hypoxic and normoxic conditions (Fig. 25). In vitro studies demonstrated that these complexes exhibited a significant toxicity profile (IC50) in low molecular to nanomolar concentration, which was found to be either better or comparable with cisplatin [SW480/CH1 IC50 values for 126 (13), 127 (4)]. These results were found to correlate with lipophilicity and with drug accumulation in a panel of tested cancer cell lines in a non-hypoxia monolayer (2D), as well as in hypoxic spheroid (3D) models. This set of complexes also displayed significant inhibition of tumour growth in hypoxic xenografted SCID mouse models as compared to satraplatin (122).
Fig. 25.
Chemical structures of hypoxia-responsive Pt(IV) complexes.
Texaphyrins are macrocyclic scaffolds capable of coordinating various metal cations, including trivalent lanthanides.394 A particular gadolinium(III) complex, i.e., Gd3+-texaphyrin (motexafin gadolinium, MGd), proved water soluble and capable of targeting preferentially cancerous lesions.395,396 To improve the cancer targeting of Pt(IV) complexes, Sessler et al. developed the MGd– Pt(IV) conjugates by tethering gadolinium–texaphyrin complex with inert Pt(IV) centers prodrug through an axial succinate linker (128, Fig. 26).397 The resulting conjugate, complex 128, was found to be light sensitive, resulting in photoreduction upon exposure to glass-filtered daylight. In vitro studies confirmed that prodrug 128 exhibited good cytotoxicity toxicity in both platinum-sensitive ovarian A2780 cells and cisplatin-resistant 2780CP cell lines. In follow up in vitro studies, it was discovered that texaphyrin participated as redox mediators in the reduction and activation of Pt(IV) prodrugs in the presence of cancer cells.398
Fig. 26.
Chemical structures of platinum(IV) complexes.
Over the past several years, a number of other efforts beyond those associated with platinum complex and its analogues have been devoted with a view to meeting the challenge of producing modified platinum agents with targeting capabilities. Generally, one or two biologically active non-DNA targeting moieties are included within the Pt(IV) coordination sphere. Compared with the corresponding Pt(II) complexes, these so-called dual action prodrugs have been found to display enhanced potency in drug resistance cell lines. Much of this work has been reviewed elsewhere.369 Here, we discuss only a few representative recent examples.
Gibson et al. reported a quadruple action Pt(IV) prodrug (129, Fig. 26) obtained by combining a Pt(IV) analogue of cisplatin-containing an axial dichloroacetic acid ligand (DCA, a pyruvate dehydrogenase kinase (PDK) inhibitor) with another platinum(IV) agent, Pt56MeSS [platinum(1S,2S-diaminocyclohexane)(5,6-dimethyl-1,10-phenanthroline)]2+ containing phenylbutyrate (a histone deacetylase and pyruvate dehydrogenase kinase inhibitor) linked at an axial position through a succinate ligand.399 Upon bioreduction, four different bioactive molecules are produced. Compared with cisplatin and other tested controls (analogues lacking either of PDK inhibitor, phenylbutyrate and Pt56MeSS), conjugate 129 exhibited an improved cytotoxi-city profile in both monolayer (2D) and spheroid (3D) cultured cancer cells. Prodrug 129 was found to be 200–450 fold more potent in KRAS (Kirsten rat sarcoma) mutated colon and pancreatic cancer cells as compared to cisplatin itself. It was also found to be 40 times more selective towards KRAS mutated cancer cell than towards normal cells.
Photodynamic therapy (PDT) is a clinically approved noninvasive method for the treatment of various types of cancer. It relies on the activation of molecular oxygen present in the tumour environment to generate singlet oxygen. As the concentration of O2 in the hypoxic tumour is low compared to normal tissues, the efficiency of singlet oxygen generation is low; this significantly limits the utility of the PDT method for cancer therapy. In an attempt to increase PDT efficiency, a mitochondriallytargeted PDT agent, Ir–P(Ph)3 (130, Fig. 27), was developed.400 Its phototoxicity was tested in HeLa cells under both normoxic and hypoxic conditions. Compared with the lysosomal targeted complex Ir–alkyl, 131 (a reference PDT agent), incubation with the mitochondrial-targeted agent 130 resulted in an increased intracellular oxygen concentration in HeLa cells under hypoxic conditions, a finding ascribed to respiration inhibition. Both 130 and 131 exhibited low dark toxicity as determined by methyl thiazolyltetrazolium (MTT) assays. The phototoxicity of these two agents was further tested by flow cytometry. Compared with 131, HeLa cells pretreated with 130 under hypoxia condition (5% O2) exhibited a greater level of cell death upon irradiation at 475 nm (xenon lamp, laser power 22 mW cm−2). Agent 130 also gave rise to higher cell viability ratios (CVR = cell death without/with light irradiation) of 39 and 30 in normoxic and hypoxic conditions, respectively, compared with the lysosomal targeted control 131 (CVR < 1.5).
Fig. 27.
Chemical structures of (a) Ir–P(Ph)3 124 and (b) Ir–alkyl 125.
In an effort to expand the periodic table of inorganic anti-cancer therapeutics, significant work has been devoted to realizing the therapeutic potential of gold-based agents. Gold complexes were originally developed clinically as anti-rheumatic agents. However, as their mechanism of action was elucidated, their anticancer potential began to be appreciated.401 Early complexes, such as auranofin (132) and aurothioglucose, paved the way for the discovery of the next generation of gold(I) and gold(III) complexes with potential utility in oncology (Fig. 28). The field of anticancer gold-based therapeutics is broad, and the majority of recently published complexes do not fall within the scope of this review. For a recent review on gold based anticancer complexes, please refer to Porchia et al.402 and an earlier seminal review by Susan Berners-Price.403 However, discussed below are examples of gold complexes designed to target the cancer antioxidant pathway.
Fig. 28.
(a) Mechanism-based rationale for a dual targeting approach to drug design. (A) Elevated endogenous ROS can be tolerated by cancer cells. (B) Accentuating exogenous ROS by a single mechanism may not reach the cell death threshold. (C) Antioxidant inhibitors reduce the concentrations of reducing metabolites, thus lowering the cell death threshold. (D) The dual targeting approach involves the use of (1) a socalled redox cycling agent to accentuate exogenous ROS that is combined with (2) a reducing metabolite inhibitor to lower the cell death threshold. This combination is expected to overwhelm the system and drive it towards death. (b) Chemical structures of auranofin 132 and dual targeting redox active Au(I) N-heterocyclic carbene-based complexes 133 and 134. The portion of this figure appearing in (a) was first published in ref. 407 and reproduced with permission. Copyright Royal Society of Chemistry.
The majority of gold-based complexes with therapeutic potential are believed to elicit their antiproliferative response via the selective inhibition of the selenoenzyme, thioredoxin reductase (TrxR), which is overexpressed in several cancer indications.404 Despite the selectivity of Au towards TrxR, the therapeutic utility of this approach is limited due to the network robustness of the cancer antioxidant pathway. This unfortunate result recapitulates what has become a recurring finding in drug development; specifically, it has been found that singular random deletions or inhibition of specific proteins often gives rise to poor phenotypic outputs and poor preclinical or clinical outcomes due to the scale-free nature of biological networks.405 As a consequence, even the most effective protein targeting small molecular approaches (i.e., targeted therapies) do not always lead to viable drug candidates. For example, it was shown that knockdown of TrxR by 90% (via siRNA), provided little to no phenotypic change in cell proliferation.406 Further treatment with 132 resulted in no differences in cell proliferation between TrxR knockdown A549 and A549 cells treated with mock siRNA. It was proposed by Arumugam and Arambula that this finding and the inferred robustness of TrxR is consistent with a highly networked endogenous antioxidant system that would require multiple modes of drug targeting to be suppressed in a therapeutically useful manner (Fig. 28a).407
To test this hypothesis, N-heterocyclic carbene (NHC) Au(I) systems were developed that contain the redox active moieties ferrocene and naphthoquinone (Fig. 28b).407,408 Both systems were characterized by submicromolar potency levels across several human cancer cell lines, including A549 lung, A2780 ovarian, 2780CP ovarian, and PC-3 prostate. These fused systems were found to be significantly more potent than the constituent Au(I)–NHC complex or redox active cores. Furthermore, both 133 and 134 were found to be synergistic as regard their anticancer potency as compared to control combinations that were equimolar in the respective components (i.e., 1 equiv. Au(I)–NHC and 2 or 4 equiv. of the appropriate redox active core). These systems were also found to accentuate mitochondrial ROS in a synergistic fashion relative to their constituents and inhibit TrxR effectively. Differential gene expression of A549 cells treated with the ferrocenylated gold complex 133 showed activation of several genes associated with endoplasmic reticulum (ER) stress and unfolded protein responses. Such activation profiles are consistent with prior findings with other systems that led to the suggestion that metal-based therapeutics could act as inducers of cancer-specific immunogenic cell death.409
To examine further the selectivity towards the cancer cellular environment over healthy tissue, complex 134 was evaluated in zebrafish embryos bearing A549 lung cancer xenografts. This fluorescence based in vivo assay allows for the detection of drug-induced cellular death. It was found that complex 134 induced xenograft specific cell death at drug levels that were well tolerated by healthy tissue (i.e., no healthy tissue specific death was detected). Taken in concert, the results obtained with 133 and 134 provide support for the notion that a good anti-cancer response may be obtained by using a dual targeting approach to attack the cancer antioxidant network.
6. N-Oxide-based therapeutics
Compounds bearing N-oxide units have been developed as potential HAPs. N-Oxide prodrugs are susceptible to enzyme-mediated reduction. This reduction can be used as activation strategy to release cytotoxic drugs.410 Based on their specific structures and mode of activation, N-oxide drugs may be divided into three main categories. These are discussed below.
6.1. Aromatic N-oxide containing aromatics
Tirapazamine, TPZ (135, R = H, Fig. 29)411 a benzotriazine-1,4-dioxide, is an extensively studied prodrug that generates reactive radical species by intracellular reductases under hypoxic conditions.412 The formation of TPZ radicals was found to be mediated by one-electron oxidoreductases. The radicals generated in this way are thought to cause irreversible damage to DNA through single and double strand breaks, thereby triggering apoptosis. Gates et al. conducted an extensive study in an effort to determine whether ●OH radicals are the major DNA-damaging species.413 Separately, Anderson et al.414 employed spin trapping agents, such as 5,5′-dimethylpyrroline 1-N-oxide (DMPO) and 5-diethoxyphosphoryl-5-methyl-pyrroline N-oxide (DEPMPO), to show that TPZ produces C- and N-centered radicals, but not the ●OH radical, upon reduction with P-450R. Further support for this latter conclusion came from electron paramagnetic resonance (EPR), pulse radiolysis (PR), steady-state radiolysis, and theoretical calculations (DFT).415
TPZ (135) exhibited promising activity against different tumors, including cervical cancer, head and neck cancer, non-small cell lung cancer, metastatic melanoma, and ovarian and primary peritoneal cancer in a number of phase I and phase II clinical trials alone or in combination with other antitumor agents or photodynamic therapy (PDT).416,417 Unfortunately, TPZ yielded poor outcomes in phase III clinical trials when tested as a combination regimen (chemo/radiotherapy) against HNSCC (head and neck squamous cell carcinoma).418–420 In fact, little if any improvement in patient survival, whether or not TPZ was used alone or in combination with radio- or chemotherapy.421–423 This failure was attributed to the low aqueous solubility, poor extravascular transport, and modest radiotherapeutic quality control.
To improve the hypoxic selectivity and efficacy of TPZ, various derivatives of 3-amino-1,2,4-benzotriazine-1,4-dioxide with enhanced solubility and lipophilicity have been synthesized and evaluated under hypoxic and normoxic conditions.424,425 In vitro analyses demonstrated that substitution at the 3- and 7-positions was particularly effective under hypoxic conditions in terms of modulating the overall potency. In the most favorable cases, HCR values as high 35 and 23 were seen in HT-29 and SCCVII cells, respectively.
Among the TPZ analogues reported to date are the morpho-line based benzotriazine di-oxide HAPs, SN30000 and SN29751 (136 and 137, Fig. 29). These derivatives were found to display promising pharmacokinetic behavior.424,426 HAP 136 showed better activity than TPZ427,428 and was considered promising. During subsequent preclinical assessments, 136 was found to be light-sensitive under normal laboratory conditions.429 Since this could have adverse implications in terms of clinical development, the photo-degradation behaviour of 136 was investigated in aqueous solution.430 First-order photo-degradation kinetics were seen in dilute aqueous solution with a quantum yield of 0.016. Compared with 136, the degradation product formed (morpholine N-oxide analogue of 136) displayed reduced cytotoxicity towards human cancer cell lines (HT29 and H1299) under both hypoxic (>2000-fold) and aerobic conditions (6 fold).
Wouters et al. utilized genome-scale shRNA screens and oxidoreductase-focused libraries to identify the flavoprotein POR (cytochrome P-450 oxidoreductase) as a potential determinant biomarker for benzotriazine di-oxide and 136 in hypoxic environments.431 These researchers found that genetic knockout or knockdown of POR expression diminished the activity of 136 and TH-302 (65, Fig. 12) (see discussion in Section 4.3 above) in vitro under hypoxic conditions in a range of cancer cell lines. In a follow-up investigation, Wilson et al.432 investigated the toxicokinetics of 136 in NIH-III mice. Compound 136 was found to undergo reduction to give 138. Oxidation of the morpholine side chain and subsequent glucuronidation were also seen. The plasma pharmacokinetics (PK) of 136 and its intermediates were essentially the same when HT-29 tumor xenografts were used as the model. This was taken as an indication that the metabolic transformations were largely occurring in normal tissues. The bioreduction modeled by hepatic S9-preparations to give 138 was strongly inhibited by oxygen. Plasma PK of 136 and 138 corresponded closely to timing of reversible acute clinical signs and marked hypothermia (rectal temperature drop of ~ 8 °C at nadir at maximum dose). Similar acute toxicity was elicited by dosing with TPZ or 138. Notably, the daughter product 138 did not induce the kidney and lung histopathology seen in the case of 136. It was also devoid of appreciable toxicity as inferred from studies involving hypoxic cell cultures. Further, 138 exhibited minimal redox cycling ability in normoxic cultures. Hence 138 was not considered to be responsible for the acute toxicity seen with 136. On the other hand, a two-compartment model, wherein the clearance of 136 was proposed to involve bioreductive metabolism to 138, was found to account for the non-linear PK features of 136 seen in mice.
In another study, Q6 (7-methyl-3-(3-chlorophenyl)-quinoxaline-2-carbonitrile 1,4-dioxide) (139, Fig. 29)433 was shown to exhibit preferential hypoxic cell targeting and enhanced anti-tumor efficacy via autophagic degradation of HIF-1α relative to the parent molecule, TPZ (135). Zhu et al. investigated the mechanism of Q6-induced cell death under hypoxic conditions and observed that Q6 interacts directly with topoisomerase II (topo II) and inhibits its catalytic activity.434 It was proposed that Q6 stabilizes various topo II-DNA ternary complexes, resulting in DNA double-strand breaks (DSB). This, in turn, leads to cell cycle arrest and apoptosis, as demonstrated in the HCC cell line, both in vitro and in vivo. The improved efficacy of Q6 relative to TPZ was ascribed to the induction of autophagic degradation of HIF-1 α, in addition to the topo II targeting effects. It was concluded that Q6 thus represents a promising HAP candidate that warrants further evaluation.
Phenazine N,N′-dioxides (PDOs) are another class of bioreductive agents with redox properties that display toxicity towards hypoxic cells.435,436 To increase further the potency of PDOs, a number of derivatives have been synthesized by (a) varying the 7- and 8-substituents of PDOs and (b) developing the corresponding aza-analogs; and (c) changing the OH-substituents.437 Many of the resulting derivatives were tested against V79 cells under normoxic and hypoxic conditions. One of these derivatives, conjugate 140 (Fig. 29) bearing fluoro-amino units, was found to exhibit strong hypoxic potency (P = 2.5 μM) with an appreciable HCR value (6.8). The beneficial features of compound 140 were attributed to its ability to interact with DNA.
A number of other heterocyclic N-oxides, including those based on imidazopyridopyrazine 141,438–441 imidazoquino xaline 142,442 quinoxaline 1,4-dioxide 143,443–447 and phenazine 5,10-dioxides 144448 cores, have also been explored as hypoxia-activated therapeutics (Fig. 29). Monge et al. conducted an extensive SAR study to develop the potent hypoxia selective quinoxaline 1,4-dioxide based derivatives. The presence of electron withdrawing groups at the 6- and/or 7-position of the 3-amino scaffold significantly improved the reduction potential of derivatives as compared with 135. Among the analogues tested, derivatives 144a and 144b exhibited the greatest hypoxia selectivity. They also displayed a 30-fold higher toxicity than 135.449 In a separate study, the water-soluble 3-[[(N,N′-dialkyl-amino)alkyl]amino]-2-quinoxalinecarbonitrile 1,4-di-N-oxides derivatives 144c and 144d bearing 7-chloro and 7-trifluoromethyl substituents, respectively, provided higher potency and great selectively (HCR values of ca. 250 and 340, respectively).450 Similarly, compound 145 showed selective toxicity against hypoxic V79 cells in vitro and MCF breast cancer xenograft models. An alkaline comet assay confirmed the exclusive production of ●OH radicals under hypoxic conditions and corresponding DNA damage.451
Hori et al. developed a series of hypoxic-neoplastic cells targeted hybrid analogues of 1-methyltryptophan (1MT, 2,3-dioxygenase inhibitor) and tirapazamine linked through flexible alkyl chains.452 The lead compounds in question, 146 and 147, displayed improved HCR values of about 4.9 and 5.0, respectively against EMT6/KU cells as compared with 135 alone (HCR = 3.2). The improved HCR values seen for these prodrugs was ascribed to their dual biological function i.e., hypoxic cytotoxin (TPZ) and IDO inhibition by IMT respectively. Other hybrids of 135 with DNA interchelators have also been developed and found to exhibit enhanced hypoxic cytotoxicity (1–56 fold), but with HCR values (8–51) that are more modest than those of 135 (HCR = 62) in HT-29 cancer cell lines. Several analogues with a different range of DNA-binding affinities are currently the subject of further investigation.453,454
Gates et al.455 reported the TPZ analogue di-N-oxide 148 that contains a nitrogen mustard subunit (Fig. 30). This derivative was found to be 30-fold more potent than the mono-N-oxide 149 under hypoxic condition. Based on control experiments, the difference in potency was found to be due to overlapping mechanisms of action. Bioreduction of 148 produces the daughter system 149 (Fig. 30). The TPZ subunit in 149 was thought to be more susceptible to hydrolytic release of the reactive mustard as the result of the change in the electronic structure. As a result, under hypoxic conditions, compound 148 (as a prodrug for 149) was expected to induce oxidative DNA damage via the triazine ring, whereas the nitrogen mustard it carries would act as a strong DNA-alkylating agent.
Fig. 30.
Structure and activation of hypoxia-activated aromatic N-oxide prodrug 140.
6.2. Aliphatic N-oxides
Tertiary alkyl amine N-oxides have also shown cytotoxicity toward hypoxic cells. The oxygen sensitive two-electron reduction of an alkyl tertiary amine N-oxide in the presence of cytochrome C P-450 reductase or the CYP3A isozyme of NADPH produces a tertiary amine. Alkylamines are protonated under physiological pH and within the nucleus bind to DNA as the result of electrostatic interactions; this can trigger apoptosis.456,457 As N-oxidation masks the cationic nature of amines, N-oxide prodrugs usually exhibited low toxicity and DNA binding ability. Under hypoxic conditions, these prodrugs get activated to produce corresponding amine drugs, hence imparting a degree of selectivity.458
A well-investigated alkyl N-oxide prodrug is AQ4N (150, Fig. 31). In reducing environments, it is activated to produce AQ4, (151, Fig. 31). This bis-amine is a strong topoisomerase II inhibitor with DNA-intercalating properties similar to those of mitoxantrone (152).459 AQN4 (150) exhibits low cytotoxicity in hypoxic cells; however, its conversion to 151 under hypoxic conditions leads to ca. 100× higher toxicity. Preclinical and phase I investigations provided support for the notion that 150 produces antitumor effects when used as a single agent or in combination with radio/chemotherapy.460–463 Unfortunately, development of 150 has apparently not progressed past a phase II trial initiated in 2006 that compared 150 against temozolomide in glioblastoma in conjunction with RT (NCT00394628).
Fig. 31.
Structures of alkyl tertiary amine-based N-oxide prodrugs.
Multi-ring scaffolds, such as 9-aminoacridine (153),464–466 naphthalamide (155 and 156),467 anthrapyrazole (159),468 azaanthrapyrazole (160),469 and mitoxantrone (161),470 have been explored as hypoxia-selective prodrugs. These systems were generally prepared by appending tertiary N-oxides to the corresponding amines (viz., 154, 157, 158, and 162; Fig. 31). The cancer cell selectivity of the N-oxides, relative to their amino counterparts was found to be 10–1000-fold greater under hypoxic conditions across a number of cell lines. This was taken as an early indication that topoisomerase II inhibitors may be directed towards hypoxic regions via N-oxide prodrug formation.
6.3. Discrete N-oxide prodrugs
Recognizing the canonical redox chemistry of N-oxides, several HAPs containing this moiety were developed in an effort to obtain prodrugs that would be activated under hypoxic conditions. As above, agents of this class have generally been obtained by tethering an N-oxide moiety to a cytotoxic agent. Nitromin (163, Fig. 32) represents the first example of this type of putative prodrug. This agent showed a 4× increased inhibition of Walker cells growth under conditions of hypoxia than normoxia. Activity was ascribed to the bioreductive production of nitrogen mustard, a bifunctional alkylating agent.471
Fig. 32.
Structures of hypoxia-activated prodrugs (HAPs) based on masked cytotoxic agents.
Another example of this type of HAP, namely chlorambucil N-oxide (164, Fig. 32), was reported by Denny et al.472 Compared to chlorambucil, prodrug 164 displayed a lower level of hypoxic toxicity in AA8 cells; however, it showed some toxicity in the presence of rat liver microsomes and NADPH.473
In separate study, Wardman et al.474 reported a potential HAP prodrug 165 (Fig. 32), which upon activation under hypoxic conditions forms a complex with the Fe(II) cation. Even under conditions of low oxygen tension, this promotes production of hydroxyl radicals, thereby accounting for the cytotoxicity seen in vitro (Chinese hamster V79 cell line). This mode of action mirrors that proposed for bleomycin (an antibiotic that has been used to treat skin, head, and neck cancers).475 Prodrug 165 itself contains a hypoxia-responsive pyridine N-oxide moiety and per se is inactive towards DNA damage. In contrast, its reduced form proved very active, as noted above.
7. Summary and outlook
Cancer is caused by various factors that disrupt the balance of cell survival, proliferation, and differentiation. Over the past 60 years, the influential role of tumour hypoxia in oncology, and its potential as a therapeutic target have become increasingly well recognized. Exploiting tumour hypoxia as a means of targeting tumours and releasing active drugs selectively may allow new drug discovery strategies to be pursued that could in due course lead to improved cancer therapy regimens. In this Review, we have highlighted chemical and preclinical research developments in the area of hypoxia-responsive therapeutics. Particular emphasis has been placed on bioreductive approaches since these currently appear to have the greatest promise for success.
As the reductive pathways involved for prodrug activation are hypoxia dependent and are in turn inversely correlated with available oxygen concentrations, normal cells are largely spared from the side effects that might be expected in the absence of hypoxia targeting and reductive drug release. The hope is that the present summary will prove a useful guide to established researchers, allowing them to extend their understanding while inspiring investigators new to the area to take up the challenges and opportunities associated with hypoxia as a drug discovery strategy. Ultimately, these efforts are expected to lead to new and more effective therapeutic strategies while advancing the rapidly emerging area of personalized cancer medicine.
Acknowledgements
This work was supported by the CRI project (grant no. 2018R1A3B1052702 to J. S. K.) from the National Research Foundation (NRF) of Korea, Shangha University (center funds to J. L. S.), the U.S. National Institutes of Health (grant no. CA68682 to J. L. S., grant no. CA232765 to J. F. A.), and the Robert A. Welch Foundation (chair F-0018 to J. L. S.). Support from Shanghai University is also gratefully acknowledged.
Abbreviations
- BBB
Blood brain barrier
- BCNU
1,3-Bis(2-chloroethyl)-1-nitrosourea
- CYP450
Cytochrome P450
- CNS
Central nervous system
- DNA
Deoxyribonucleic acid
- DDTC
Sodium dimethyldithiocarbamate
- DMSO
Dimethyl sulfoxide
- DEI
5-[(Dimethylamino)ethoxy]indole
- EMA
European Medicines Agency
- ECGF
Endothelial cell growth factor
- FDA
Food Drug and Administration
- FMN
Flavin mononucleotide
- GSH
Glutathione
- GST
Glutathione S-transferase
- GDEPT
Gene-directed enzyme prodrug therapy
- HSCs
Hypoxia selective cytotoxins
- HAPs
Hypoxia activated prodrugs
- HCR
Hypoxia cytotoxic ratio
- HIF
Hypoxia-inducible factor
- HDR
Homology-directed repair
- MRI
Magnetic resonance imaging
- 1MT
I-Methyltryptophan
- NTR
Nitroreductase
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NCI
National Cancer Institute
- NHC
N-Heterocyclic carbine
- NSCLC
Non-small-cell lung cancer
- PET
Positron emission tomography
- PCNU
1-(2-Chloroethyl)-1-nitrosourea
- P450
Cytochrome P450
- PDT
Photodynamic therapy
- Q3PA
Trimethyl-locked quinone propionic acid
- RT
Radiation therapy
- ROS
Reactive oxygen species
- SRBT
Stereotactic body radiotherapy
- NaCl
Sodium chloride
- SCID
Severe combined immunodeficiency
- SOC
Standard of care
- SAR
Structure–activity relationship
- Trx
Thioredoxin
- TrxR
Thioredoxin reductase
- TMI
5,6,7-Trimethoxyindole
- US
United States
- VEFG
Vascular endothelial growth factor
- XO
Xanthine oxidase
Biographies

From left to right: Seyoung Koo, Prof. Jong Seung Kim & Dr Amit Sharma
Seyoung Koo has been working at the Department of Chemistry, Korea University as a PhD student under the supervision of Prof. Jong Seung Kim since 2014. Her research interests involve the design and synthesis of imaging agents and drug delivery systems for cancer therapy.
Jong Seung Kim was born in Daejon, Korea in 1963. He received PhD from the Department of Chemistry and Biochemistry, Texas Tech University and completed a one-year postdoctoral fellowship at University of Houston. In 2007, he moved to the Department of Chemistry at Korea University in Seoul as a full professor.
Amit Sharma was born in Nagrota Bagwan, Kangra, H.P., India in 1983. He received his PhD from Guru Nanak Dev University, Amritsar, India under the supervision of Prof. Kamaljit Singh. Thereafter, he joined Sphaera Pharma Pvt. Ltd, India as a research scientist (2011–2014). In 2014, he joined Prof. J. S. Kim’s research group as research professor. His research interests concern the development of smart biomarkers and drug delivery systems for chemotherapy.

From left to right: Prof. Jonathan L. Sessler and Dr Jonathan F. Arambula
Jonathan L. Sessler received a BSc degree in Chemistry in 1977 from the University of California, Berkeley. He obtained his PhD from Stanford University in 1982. After postdoctoral stays in Strasbourg and Kyoto, he accepted a position as an Assistant Professor of Chemistry at the University of Texas at Austin, where he is currently the Doherty-Welch Chair. Prof. Sessler was a co-founder of Pharmacyclics, Inc. and Cible, Inc. He was also a WCU Professor at Yonsei University and recently accepted a laboratory directorate at Shanghai University. Prof. Sessler has published > 720 papers (cited > 37 000 times) and his h-index is 100.
Jonathan F. Arambula received his PhD in Chemistry from the University of Illinois and was an American Cancer Society Postdoctoral Fellow at the University of Texas at Austin and M.D. Anderson Cancer Center. Following this, he held an assistant professor position in the Department of Chemistry at Georgia Southern University. In 2016, he co-founded Cible Inc., whose focus is the development of tumor localizing therapies that overcome drug resistance. With research efforts focused on early stage translational science, he is the co-inventor and author of multiple metal-based discovery platforms focused on overcoming cancer drug resistance.

From left to right: Dr Hardev Singh & Dr Rajesh Kumar
Hardev Singh was born in Amritsar, India, in 1982. He received his PhD degree in Organic Chemistry under the supervision of Prof. Vandana Bhalla, Department of Chemistry, Guru Nanak Dev University, Amritsar, India. After a two-year post-doctoral fellowship at Ajou University with Prof. Hwan Myung Kim, he joined Prof. Jong Seung Kim’s lab as a research professor in 2015. His current research interests include the development of novel molecular imaging probes and targeted drug delivery systems (DDS’s) for cancer therapy.
Rajesh Kumar was born in Pathankot, India in 1978. He received his PhD degree in organic chemistry under the supervision of Prof. Manoj Kumar from the Department of Chemistry, Guru Nanak Dev University, Amritsar, India. From Sept 2011 to June 2015, he worked in Prof. Jong Seung Kim’s lab, Korea University, Seoul as a research professor. In June of 2015, he moved to the University of Calgary as a postdoctoral fellow and then Canam Bioresearch as a research scientist. His research interests concern the development of small molecular biomarkers, drug delivery systems, and nanomedicines for cancer therapy.
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.Schilsky RL, Nat. Rev. Drug Discovery, 2010, 9, 363–366. [DOI] [PubMed] [Google Scholar]
- 2.Chin L, Andersen JN and Futreal PA, Nat. Med, 2011, 17, 297–303. [DOI] [PubMed] [Google Scholar]
- 3.Chari RV, Acc. Chem. Res, 2008, 41, 98–107. [DOI] [PubMed] [Google Scholar]
- 4.Fine BM and Amler L, Clin. Pharmacol. Ther, 2009, 85, 535–538. [DOI] [PubMed] [Google Scholar]
- 5.Obama BH, 2015, 2015 State of the Union Address, https://www.whitehouse.gov/thepress-office/2015/01/20/remarks-president-state-unionaddress-january-20-2015.
- 6.Vaupel P, Kallinowski F and Okunieff P, Cancer Res, 1989, 49, 6449–6465. [PubMed] [Google Scholar]
- 7.Vaupel P and Harrison L, Oncologist, 2004, 9(suppl 5), 4–9. [DOI] [PubMed] [Google Scholar]
- 8.Thomlinson RH and Gray LH, Br. J. Cancer, 1955, 9, 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertout JA, Patel SA and Simon MC, Nat. Rev. Cancer, 2008, 8, 967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaupel P, Schlenger K, Knoop C and Hockel M, Cancer Res, 1991, 51, 3316–3322. [PubMed] [Google Scholar]
- 11.Vaupel P, Hockel M and Mayer A, Antioxid. Redox Signaling, 2007, 9, 1221–1235. [DOI] [PubMed] [Google Scholar]
- 12.Lv PC, Roy J, Putt KS and Low PS, Mol. Pharmaceutics, 2016, 13, 1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piao W, Tsuda S, Tanaka Y, Maeda S, Liu FY, Takahashi S, Kushida Y, Komatsu T, Ueno T, Terai T, Nakazawa T, Uchiyama M, Morokuma K, Nagano T and Hanaoka K, Angew. Chem., Int. Ed, 2013, 52, 13028–13032. [DOI] [PubMed] [Google Scholar]
- 14.Zheng X, Wang X, Mao H, Wu W, Liu B and Jiang X, Nat. Commun, 2015, 6, 5834. [DOI] [PubMed] [Google Scholar]
- 15.Chiche J, Brahimi-Horn MC and Pouyssegur J, J. Cell. Mol. Med, 2010, 14, 771–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D and Schumacker PT, Circ. Res, 2010, 106, 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM and Schumacker PT, J. Biol. Chem, 2000, 275, 25130–25138. [DOI] [PubMed] [Google Scholar]
- 18.Cairns RA, Khokha R and Hill RP, Curr. Mol. Med, 2003, 3, 659–671. [DOI] [PubMed] [Google Scholar]
- 19.Luo Y, He DL, Ning L, Shen SL, Li L, Li X, Zhau HE and Chung LW, BJU Int, 2006, 98, 1315–1319. [DOI] [PubMed] [Google Scholar]
- 20.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S and Comoglio PM, Cancer Cell, 2003, 3, 347–361. [DOI] [PubMed] [Google Scholar]
- 21.Cairns RA, Harris IS and Mak TW, Nat. Rev. Cancer, 2011, 11, 85–95. [DOI] [PubMed] [Google Scholar]
- 22.Kim JW, Gao P and Dang CV, Cancer Metastasis Rev, 2007, 26, 291–298. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Sun M, Wang L and Jiao B, J. Cell. Biochem, 2013, 114, 967–974. [DOI] [PubMed] [Google Scholar]
- 24.Semenza GL, Oncogene, 2013, 32, 4057–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rankin EB and Giaccia AJ, Science, 2016, 352, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bristow RG and Hill RP, Nat. Rev. Cancer, 2008, 8, 180–192. [DOI] [PubMed] [Google Scholar]
- 27.Gammon L and Mackenzie IC, J. Oral Pathol. Med, 2016, 45, 77–82. [DOI] [PubMed] [Google Scholar]
- 28.Palazon A, Aragones J, Morales-Kastresana A, de Landazuri MO and Melero I, Clin. Cancer Res, 2012, 18, 1207–1213. [DOI] [PubMed] [Google Scholar]
- 29.Kumar V and Gabrilovich DI, Immunology, 2014, 143, 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minchinton AI and Tannock IF, Nat. Rev. Cancer, 2006, 6, 583–592. [DOI] [PubMed] [Google Scholar]
- 31.Szakacs G, Hall MD, Gottesman MM, Boumendjel A, Kachadourian R, Day BJ, Baubichon-Cortay H and Di Pietro A, Chem. Rev, 2014, 114, 5753–5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatenby RA and Gillies RJ, Nat. Rev. Cancer, 2004, 4, 891–899. [DOI] [PubMed] [Google Scholar]
- 33.Mizusawa K, Takaoka Y and Hamachi I, J. Am. Chem. Soc, 2012, 134, 13386–13395. [DOI] [PubMed] [Google Scholar]
- 34.Strese S, Fryknas M, Larsson R and Gullbo J, BMC Cancer, 2013, 13, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song X, Liu X, Chi W, Liu Y, Wei L, Wang X and Yu J, Cancer Chemother. Pharmacol, 2006, 58, 776–784. [DOI] [PubMed] [Google Scholar]
- 36.Goodsaid FM and Mendrick DL, Sci. Transl. Med, 2010, 2, 47ps44. [DOI] [PubMed] [Google Scholar]
- 37.Kneller R, Nat. Rev. Drug Discovery, 2010, 9, 867–882. [DOI] [PubMed] [Google Scholar]
- 38.Goodsaid F and Papaluca M, Nat. Biotechnol, 2010, 28, 441–443. [DOI] [PubMed] [Google Scholar]
- 39.Woosley RL, Myers RT and Goodsaid F, Clin. Pharmacol. Ther, 2010, 87, 530–533. [DOI] [PubMed] [Google Scholar]
- 40.Paulmurugan R, Oronsky B, Brouse CF, Reid T, Knox S and Scicinski J, Theranostics, 2013, 3, 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penet MF, Krishnamachary B, Chen ZH, Jin JF and Bhujwalla ZM, Adv. Cancer Res, 2014, 124, 235–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee MH, Sharma A, Chang MJ, Lee J, Son S, Sessler JL, Kang C and Kim JS, Chem. Soc. Rev, 2018, 47, 28–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips RM, Cancer Chemother. Pharmacol, 2016, 77, 441–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunter FW, Wouters BG and Wilson WR, Br. J. Cancer, 2016, 114, 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mistry IN, Thomas M, Calder EDD, Conway SJ and Hammond EM, Int. J. Radiat. Oncol., Biol., Phys, 2017, 98, 1183–1196. [DOI] [PubMed] [Google Scholar]
- 46.Patai S, The Chemistry of the Quinoid Compounds, John Wiley & Sons, London, 1st edn, 1974, vol. 1 and 2. [Google Scholar]
- 47.Abdella BRJ and Fisher J, Environ. Health Perspect, 1985, 64, 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujiwara A, Mori T, Iida A, Ueda S, Hano Y, Nomura T, Tokuda H and Nishino H, J. Nat. Prod, 1998, 61, 629–632. [DOI] [PubMed] [Google Scholar]
- 49.Duine JA, Eur. J. Biochem, 1991, 200, 271–284. [DOI] [PubMed] [Google Scholar]
- 50.Brisach-Wittmeyer A, Souna Sido AS, Guilini P and Desaubry L, Bioorg. Med. Chem. Lett, 2005, 15, 3609–3610. [DOI] [PubMed] [Google Scholar]
- 51.Guillen F, Martinez MJ, Munoz C and Martinez AT, Arch. Biochem. Biophys, 1997, 339, 190–199. [DOI] [PubMed] [Google Scholar]
- 52.Hay S, Westerlund K and Tommos C, J. Phys. Chem. B, 2007, 111, 3488–3495. [DOI] [PubMed] [Google Scholar]
- 53.Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S and Jap BK, Science, 1998, 281, 64–71. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, Huang L, Shulmeister VM, Chi YI, Kim KK, Hung LW, Crofts AR, Berry EA and Kim SH, Nature, 1998, 392, 677–684. [DOI] [PubMed] [Google Scholar]
- 55.Kepa JK and Ross D, Br. J. Cancer, 1999, 79, 1679–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Danson S, Ward TH, Butler J and Ranson M, Cancer Treat. Rev, 2004, 30, 437–449. [DOI] [PubMed] [Google Scholar]
- 57.Schlager JJ and Powis G, Int. J. Cancer, 1990, 45, 403–409. [DOI] [PubMed] [Google Scholar]
- 58.Workman P, Oncol. Res, 1994, 6, 461–475. [PubMed] [Google Scholar]
- 59.Malkinson AM, Siegel D, Forrest GL, Gazdar AF, Oie HK, Chan DC, Bunn PA, Mabry M, Dykes DJ, Harrison SD Jr. and Ross D, Cancer Res, 1992, 52, 4752–4757. [PubMed] [Google Scholar]
- 60.Belinsky M and Jaiswal AK, Cancer Metastasis Rev, 1993, 12, 103–117. [DOI] [PubMed] [Google Scholar]
- 61.Fitzsimmons SA, Workman P, Grever M, Paull K, Camalier R and Lewis AD, J. Natl. Cancer Inst, 1996, 88, 259–269. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz HS, Sodergren JE and Philips FS, Science, 1963, 142, 1181–1183. [DOI] [PubMed] [Google Scholar]
- 63.Iyer VN and Szybalski W, Science, 1964, 145, 55–58. [DOI] [PubMed] [Google Scholar]
- 64.Cummings J, Spanswick VJ, Tomasz M and Smyth JF, Biochem. Pharmacol, 1998, 56, 405–414. [DOI] [PubMed] [Google Scholar]
- 65.Tomasz M and Palom Y, Pharmacol. Ther, 1997, 76, 73–87. [DOI] [PubMed] [Google Scholar]
- 66.Sharp SY, Kelland LR, Valenti MR, Brunton LA, Hobbs S and Workman P, Mol. Pharmacol, 2000, 58, 1146–1155. [DOI] [PubMed] [Google Scholar]
- 67.Siegel D, Beall H, Kasai M, Arai H, Gibson NW and Ross D, Mol. Pharmacol, 1993, 44, 1128–1134. [PubMed] [Google Scholar]
- 68.Rauth AM, Mohindra JK and Tannock IF, Cancer Res, 1983, 43, 4154–4158. [PubMed] [Google Scholar]
- 69.Keyes SR, Rockwell S and Sartorelli AC, Cancer Res, 1985, 45, 3642–3645. [PubMed] [Google Scholar]
- 70.Baker LH, Izbicki RM and Vaitkevicius VK, Med. Pediatr. Oncol, 1976, 2, 207–213. [DOI] [PubMed] [Google Scholar]
- 71.Belcourt MF, Hodnick WF, Rockwell S and Sartorelli AC, Adv. Enzyme Regul, 1998, 38, 111–133. [DOI] [PubMed] [Google Scholar]
- 72.Walton MI, Smith PJ and Workman P, Cancer Commun, 1991, 3, 199–206. [DOI] [PubMed] [Google Scholar]
- 73.Robertson N, Haigh A, Adams GE and Stratford IJ, Eur. J. Cancer, Part A, 1994, 30, 1013–1019. [DOI] [PubMed] [Google Scholar]
- 74.Winski SL, Hargreaves RH, Butler J and Ross D, Clin. Cancer Res, 1998, 4, 3083–3088. [PubMed] [Google Scholar]
- 75.Ward TH, Coe N, Hargreaves R, Butler J and McGown AT, Clin. Cancer Res, 2000, 6(suppl), 4527s. [Google Scholar]
- 76.Loadman PM, Phillips RM, Lim LE and Bibby MC, Biochem. Pharmacol, 2000, 59, 831–837. [DOI] [PubMed] [Google Scholar]
- 77.Hasinoff BB and Begleiter A, Free Radical Res, 2006, 40, 974–978. [DOI] [PubMed] [Google Scholar]
- 78.Begleiter A, Leith MK, Patel D and Hasinoff BB, Cancer Chemother. Pharmacol, 2007, 60, 713–723. [DOI] [PubMed] [Google Scholar]
- 79.Khan AH and Driscoll JS, J. Med. Chem, 1976, 19, 313–317. [DOI] [PubMed] [Google Scholar]
- 80.Chou F, Khan AH and Driscoll JS, J. Med. Chem, 1976, 19, 1302–1308. [DOI] [PubMed] [Google Scholar]
- 81.King CL, Wong SK and Loo TL, Eur. J. Cancer Clin. Oncol, 1984, 20, 261–264. [DOI] [PubMed] [Google Scholar]
- 82.Xing CG and Skibo EB, Biochemistry, 2000, 39, 10770–10780. [DOI] [PubMed] [Google Scholar]
- 83.Plumb JA, Gerritsen M, Milroy R, Thomson P and Workman P, Int. J. Radiat. Oncol., Biol., Phys, 1994, 29, 295–299. [DOI] [PubMed] [Google Scholar]
- 84.Phillips RM, Hendriks HR, Sweeney JB, Reddy G and Peters GJ, Expert Opin. Drug Metab. Toxicol, 2017, 13, 783–791. [DOI] [PubMed] [Google Scholar]
- 85.Phillips RM, Loadman PM and Cronin BP, Br. J. Cancer, 1998, 77, 2112–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naylor MA, Swann E, Everett SA, Jaffar M, Nolan J, Robertson N, Lockyer SD, Patel KB, Dennis MF, Stratford MRL, Wardman P, Adams GE, Moody CJ and Stratford IJ, J. Med. Chem, 1998, 41, 2720–2731. [DOI] [PubMed] [Google Scholar]
- 87.Newsome JJ, Swann E, Hassani M, Bray KC, Slawin AMZ, Beall HD and Moody CJ, Org. Biomol. Chem, 2007, 5, 1629–1640. [DOI] [PubMed] [Google Scholar]
- 88.Swann E, Barraja P, Oberlander AM, Gardipee WT, Hudnott AR, Beall HD and Moody CJ, J. Med. Chem, 2001, 44, 3311–3319. [DOI] [PubMed] [Google Scholar]
- 89.Phillips RM, Naylor MA, Jaffar M, Doughty SW, Everett SA, Breen AG, Choudry GA and Stratford IJ, J. Med. Chem, 1999, 42, 4071–4080. [DOI] [PubMed] [Google Scholar]
- 90.Jaffar M, Phillips RM, Williams KJ, Mrema I, Cole C, Wind NS, Ward TH, Stratford IJ and Patterson AV, Biochem. Pharmacol, 2003, 66, 1199–1206. [DOI] [PubMed] [Google Scholar]
- 91.Dehn DL, Winski SL and Ross D, Clin. Cancer Res, 2004, 10, 3147–3155. [DOI] [PubMed] [Google Scholar]
- 92.Ward TH, Danson S, McGown AT, Ranson M, Coe NA, Jayson GC, Cummings J, Hargreaves RH and Butler J, Clin. Cancer Res, 2005, 11, 2695–2701. [DOI] [PubMed] [Google Scholar]
- 93.Berardini MD, Souhami RL, Lee CS, Gibson NW, Butler J and Hartley JA, Biochemistry, 1993, 32, 3306–3312. [DOI] [PubMed] [Google Scholar]
- 94.Kim JY, Patterson AV, Stratford IJ and Hendry JH, Anticancer Drugs, 2004, 15, 71–77. [DOI] [PubMed] [Google Scholar]
- 95.Danson SJ, Johnson P, Ward TH, Dawson M, Denneny O, Dickinson G, Aarons L, Watson A, Jowle D, Cummings J, Robson L, Halbert G, Dive C and Ranson M, Ann. Oncol, 2011, 22, 1653–1660. [DOI] [PubMed] [Google Scholar]
- 96.Hasinoff BB and Begleiter A, Free Radical Res, 2006, 40, 974–978. [DOI] [PubMed] [Google Scholar]
- 97.Yan C, Kepa JK, Siegel D, Stratford IJ and Ross D, Mol. Pharmacol, 2008, 74, 1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Begleiter A, Front. Biosci, 2000, 5, E153–E171. [DOI] [PubMed] [Google Scholar]
- 99.Lee EJ, George SL, Amrein PC, Paciucci PA, Allen SL and Schiffer CA, Leukemia, 1998, 12, 139–143. [DOI] [PubMed] [Google Scholar]
- 100.Hernick M, Flader C and Borch RF, J. Med. Chem, 2002, 45, 3540–3548. [DOI] [PubMed] [Google Scholar]
- 101.Tanabe K, Makimura Y, Tachi Y, Imagawa-Sato A and Nishimoto S, Bioorg. Med. Chem. Lett, 2005, 15, 2321–2324. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Z, Tanabe K, Hatta H and Nishimoto S, Org. Biomol. Chem, 2005, 3, 1905–1910. [DOI] [PubMed] [Google Scholar]
- 103.Wall ME, Wani MC, Cook CE, Palmar KH, McPhail AT and Sim GA, J. Am. Chem. Soc, 1966, 88, 3888–3890. [Google Scholar]
- 104.Hsiang YH, Hertzberg R, Hecht S and Liu LF, J. Biol. Chem, 1985, 260, 14873–14878. [PubMed] [Google Scholar]
- 105.Sharma K, Iyer A, Sengupta K and Chakrapani H, Org. Lett, 2013, 15, 2636–2639. [DOI] [PubMed] [Google Scholar]
- 106.Colucci MA, Moody CJ and Couch GD, Org. Biomol. Chem, 2008, 6, 637–656. [DOI] [PubMed] [Google Scholar]
- 107.Naylor MA, Jaffar M, Nolan J, Stephens MA, Butler S, Patel KB, Everett SA, Adams GE and Stratford IJ, J. Med. Chem, 1997, 40, 2335–2346. [DOI] [PubMed] [Google Scholar]
- 108.Desgrosellier JS and Cheresh DA, Nat. Rev. Cancer, 2010, 10, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang B, Desai A, Tang S, Thomas TP and Baker JR Jr., Org. Lett, 2010, 12, 1384–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ding Y, Ding C, Ye N, Liu Z, Wold EA, Chen H, Wild C, Shen Q and Zhou J, Eur. J. Med. Chem, 2016, 122, 102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ikezoe T, Chen SS, Tong XJ, Heber D, Taguchi H and Koeffler HP, Int. J. Oncol, 2003, 23, 1187–1193. [PubMed] [Google Scholar]
- 112.Xu S, Yao H, Pei L, Hu M, Li D, Qiu Y, Wang G, Wu L, Yao H, Zhu Z and Xu J, Eur. J. Med. Chem, 2017, 132, 310–321. [DOI] [PubMed] [Google Scholar]
- 113.Hernick M and Borch RF, J. Med. Chem, 2003, 46, 148–154. [DOI] [PubMed] [Google Scholar]
- 114.de Groot FM, Damen EW and Scheeren HW, Curr. Med. Chem, 2001, 8, 1093–1122. [DOI] [PubMed] [Google Scholar]
- 115.Carpino LA, Triolo SA and Berglund RA, J. Org. Chem, 1989, 54, 3303–3310. [Google Scholar]
- 116.Volpato M, Abou-Zeid N, Tanner RW, Glassbrook LT, Taylor J, Stratford I, Loadman PM, Jaffar M and Phillips RM, Mol. Cancer Ther, 2007, 6, 3122–3130. [DOI] [PubMed] [Google Scholar]
- 117.Maskell L, Blanche EA, Colucci MA, Whatmore JL and Moody CJ, Bioorg. Med. Chem. Lett, 2007, 17, 1575–1578. [DOI] [PubMed] [Google Scholar]
- 118.Liu PL, Xu JS, Yan DH, Zhang PS, Zeng F, Li BW and Wu SZ, Chem. Commun, 2015, 51, 9567–9570. [DOI] [PubMed] [Google Scholar]
- 119.Shin WS, Han J, Verwilst P, Kumar R, Kim JH and Kim JS, Bioconjugate Chem, 2016, 27, 1419–1426. [DOI] [PubMed] [Google Scholar]
- 120.Flader C, Liu JW and Borch RF, J. Med. Chem, 2000, 43, 3157–3167. [DOI] [PubMed] [Google Scholar]
- 121.Zhang J, Liu HW, Hu XX, Li J, Liang LH, Zhang XB and Tan W, Anal. Chem, 2015, 87, 11832–11839. [DOI] [PubMed] [Google Scholar]
- 122.Smith BD, Haffty BG, Wilson LD, Smith GL, Patel AN and Buchholz TA, J. Clin. Oncol, 2010, 28, 5160–5165. [DOI] [PubMed] [Google Scholar]
- 123.Quintiliani M, Int. J. Radiat. Biol. Relat. Stud. Phys., Chem. Med, 1986, 50, 573–594. [DOI] [PubMed] [Google Scholar]
- 124.Ward JF, Prog. Nucleic Acid Res. Mol. Biol, 1988, 35, 95–125. [DOI] [PubMed] [Google Scholar]
- 125.Teicher BA, Hematol. Oncol. Clin. North Am, 1995, 9, 475–506. [PubMed] [Google Scholar]
- 126.Evans SM, Jenkins WT, Shapiro M and Koch CJ, Adv. Exp. Med. Biol, 1997, 411, 215–225. [DOI] [PubMed] [Google Scholar]
- 127.Adams GE, Dische S, Fowler JF and Thomlinson RH, Lancet, 1976, 307, 186–188. [DOI] [PubMed] [Google Scholar]
- 128.Adams GE and Cooke MS, Int. J. Radiat. Biol. Relat. Stud. Phys., Chem. Med, 1969, 15, 457–471. [DOI] [PubMed] [Google Scholar]
- 129.Asquith JC, Foster JL, Willson RL, Ings R and McFadzean JA, Br. J. Radiol, 1974, 47, 474–481. [DOI] [PubMed] [Google Scholar]
- 130.Willson RL, Cramp WA and Ings RMJ, Int. J. Radiat. Biol, 1974, 26, 557–569. [DOI] [PubMed] [Google Scholar]
- 131.Urtasun R, Band P, Chapman JD, Feldstein ML, Mielke B and Fryer C, N. Engl. J. Med, 1976, 294, 1364–1367. [DOI] [PubMed] [Google Scholar]
- 132.Deutsch G, Foster JL, McFadzean JA and Parnell M, Br. J. Cancer, 1975, 31, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roland M, JAMA, J. Am. Med. Assoc, 1962, 180, 242–244. [DOI] [PubMed] [Google Scholar]
- 134.Grigsby PW, Winter K, Wasserman TH, Marcial V, Rotman M, Cooper J, Keys H, Asbell SO and Phillips TL, Int. J. Radiat. Oncol., Biol., Phys, 1999, 44, 513–517. [DOI] [PubMed] [Google Scholar]
- 135.Dische S, Saunders MI, Lee ME, Adams GE and Flockhart IR, Br. J. Cancer, 1977, 35, 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saunders M and Dische S, Br. J. Cancer, Suppl, 1996, 27, S271–S278. [PMC free article] [PubMed] [Google Scholar]
- 137.Overgaard J, Hansen HS, Andersen AP, Hjelm-Hansen M, Jorgensen K, Sandberg E, Berthelsen A, Hammer R and Pedersen M, Int. J. Radiat. Oncol., Biol., Phys, 1989, 16, 1065–1068. [DOI] [PubMed] [Google Scholar]
- 138.Kun LE, Ho KC and Moulder JE, Cancer, 1982, 49, 423–426. [DOI] [PubMed] [Google Scholar]
- 139.Brown JM, Yu NY, Brown DM and Lee WW, Int. J. Radiat. Oncol., Biol., Phys, 1981, 7, 695–703. [DOI] [PubMed] [Google Scholar]
- 140.Coleman CN, Wasserman TH, Urtasun RC, Halsey J, Noll L, Hancock S and Phillips TL, Int. J. Radiat. Oncol., Biol., Phys, 1990, 18, 389–393. [DOI] [PubMed] [Google Scholar]
- 141.Coleman CN, Wasserman TH, Urtasun RC, Halsey J, Hirst VK, Hancock S and Phillips TL, Int. J. Radiat. Oncol., Biol., Phys, 1986, 12, 1105–1108. [DOI] [PubMed] [Google Scholar]
- 142.Coleman CN, Halsey J, Cox RS, Hirst VK, Blaschke T, Howes AE, Wasserman TH, Urtasun RC, Pajak T, Hancock S, Phillips TL and Noll L, Cancer Res, 1987, 47, 319–322. [PubMed] [Google Scholar]
- 143.Eschwege F, SanchoGarnier H, Chassagne D, Brisgand D, Guerra M, Malaise EP, Bey P, Busutti L, Cionini L, NGuyen T, Romanini A, Chavaudra J and Hill C, Int. J. Radiat. Oncol., Biol., Phys, 1997, 39, 275–281. [DOI] [PubMed] [Google Scholar]
- 144.Timothy AR, Overgaard J and Overgaard M, Int. J. Radiat. Oncol., Biol., Phys, 1984, 10, 1765–1768. [DOI] [PubMed] [Google Scholar]
- 145.Overgaard J, Overgaard M and Timothy AR, Br. J. Cancer, 1983, 48, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Henk JM, Bishop K and Shepherd SF, Radiother. Oncol, 2003, 66, 65–70. [DOI] [PubMed] [Google Scholar]
- 147.Overgaard J, Hansen HS, Lindelov B, Overgaard M, Jorgensen K, Rasmusson B and Berthelsen A, Radiother. Oncol, 1991, 20, 143–149. [DOI] [PubMed] [Google Scholar]
- 148.Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, Lindelov B and Jorgensen K, Radiother. Oncol, 1998, 46, 135–146. [DOI] [PubMed] [Google Scholar]
- 149.Bentzen J, Toustrup K, Eriksen JG, Primdahl H, Andersen LJ and Overgaard J, Acta Oncol, 2015, 54, 1001–1007. [DOI] [PubMed] [Google Scholar]
- 150.Thomson D, Yang H, Baines H, Miles E, Bolton S, West C and Slevin N, Clin. Oncol, 2014, 26, 344–347. [DOI] [PubMed] [Google Scholar]
- 151.Oya N, Shibamoto Y, Sasai K, Shibata T, Murata R, Takagi T, Iwai H, Suzuki T and Abe M, Int. J. Radiat. Oncol., Biol., Phys, 1995, 33, 119–127. [DOI] [PubMed] [Google Scholar]
- 152.Kuwabara M, Iida Y, Inanami O, Sawamura S, Yokoyama K and Tsujitani M, J. Radiat. Res, 2002, 43, 77–88. [DOI] [PubMed] [Google Scholar]
- 153.Aoki M, Furusawa Y, Shibamoto Y, Kobayashi A and Tsujitani M, J. Radiat. Res, 2002, 43, 161–166. [DOI] [PubMed] [Google Scholar]
- 154.Yahiro T, Masui S, Kubota N, Yamada K, Kobayashi A and Kishii K, J. Radiat. Res, 2005, 46, 363–372. [DOI] [PubMed] [Google Scholar]
- 155.Shibamoto Y, Kubota T, Kishii K and Tsujitani M, Radiother. Oncol, 2000, 56, 265–270. [DOI] [PubMed] [Google Scholar]
- 156.Nishimura Y, Nakagawa K, Takeda K, Tanaka M, Segawa Y, Tsujino K, Negoro S, Fuwa N, Hida T, Kawahara M, Katakami N, Hirokawa K, Yamamoto N, Fukuoka M and Ariyoshi Y, Int. J. Radiat. Oncol., Biol., Phys, 2007, 69, 786–792. [DOI] [PubMed] [Google Scholar]
- 157.Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, Teh BS, McGarry RC, Cardenes HR and Timmerman RD, Nat. Rev. Clin. Oncol, 2010, 7, 44–54. [DOI] [PubMed] [Google Scholar]
- 158.Bonnet M, Hong CR, Gu YC, Anderson RF, Wilson WR, Pruijn FB, Wang JL, Hicks KO and Hay MP, Bioorg. Med. Chem, 2014, 22, 2123–2132. [DOI] [PubMed] [Google Scholar]
- 159.Bonnet M, Hong CR, Wong WW, Liew LP, Shome A, Wang J, Gu Y, Stevenson RJ, Qi W, Anderson RF, Pruijn FB, Wilson WR, Jamieson SMF, Hicks KO and Hay MP, J. Med. Chem, 2018, 61, 1241–1254. [DOI] [PubMed] [Google Scholar]
- 160.Mohindra JK and Rauth AM, Cancer Res, 1976, 36, 930–936. [PubMed] [Google Scholar]
- 161.Hall EJ and Roizin-Towle L, Radiology, 1975, 117, 453–457. [DOI] [PubMed] [Google Scholar]
- 162.Sutherland RM, Cancer Res, 1974, 34, 3501–3503. [PubMed] [Google Scholar]
- 163.Stratford IJ, O’Neill P, Sheldon PW, Silver AR, Walling JM and Adams GE, Biochem. Pharmacol, 1986, 35, 105–109. [DOI] [PubMed] [Google Scholar]
- 164.Stratford IJ, Adams GE, Godden J and Howells N, Int. J. Radiat. Biol, 1989, 55, 411–422. [DOI] [PubMed] [Google Scholar]
- 165.Bremner JC, Cancer Metastasis Rev, 1993, 12, 177–193. [DOI] [PubMed] [Google Scholar]
- 166.Chaplin DJ, Durand RE, Stratford IJ and Jenkins TC, Int. J. Radiat. Oncol., Biol., Phys, 1986, 12, 1091–1095. [DOI] [PubMed] [Google Scholar]
- 167.Horwich A, Holliday SB, Deacon JM and Peckham MJ, Br. J. Radiol, 1986, 59, 1238–1240. [DOI] [PubMed] [Google Scholar]
- 168.Jenkins TC, Naylor MA, Oneill P, Threadgill MD, Cole S, Stratford IJ, Adams GE, Fielden EM, Suto MJ and Stier MA, J. Med. Chem, 1990, 33, 2603–2610. [DOI] [PubMed] [Google Scholar]
- 169.Naylor MA, Threadgill MD, Showalter HD, Stratford IJ, Stephens MA, Fielden EM and Adams GE, Drug Des. Discovery, 1993, 10, 249–255. [PubMed] [Google Scholar]
- 170.Breider MA, Pilcher GD, Graziano MJ and Gough AW, Toxicol. Pathol, 1998, 26, 234–239. [DOI] [PubMed] [Google Scholar]
- 171.Lee AE and Wilson WR, Toxicol. Appl. Pharmacol, 2000, 163, 50–59. [DOI] [PubMed] [Google Scholar]
- 172.Chapman JD, Borsa J, Petkau A, Reuvers AP and Mccalla DR, Cancer Res, 1972, 32, 2616–2624. [PubMed] [Google Scholar]
- 173.Sutherland RM and Durand RE, Int. J. Radiat. Biol. Relat. Stud. Phys., Chem. Med, 1972, 22, 613–618. [DOI] [PubMed] [Google Scholar]
- 174.Naylor MA, Stephens MA, Cole S, Threadgill MD, Stratford IJ, Oneill P, Fielden EM and Adams GE, J. Med. Chem, 1990, 33, 2508–2513. [DOI] [PubMed] [Google Scholar]
- 175.Wilson WR, Vanzijl P and Denny WA, Int. J. Radiat. Oncol., Biol., Phys, 1992, 22, 693–696. [DOI] [PubMed] [Google Scholar]
- 176.Moselen JW, Hay MP, Denny WA and Wilson WR, Cancer Res, 1995, 55, 574–580. [PubMed] [Google Scholar]
- 177.Hay MP, Wilson WR, Moselen JW, Palmer BD and Denny WA, J. Med. Chem, 1994, 37, 381–391. [DOI] [PubMed] [Google Scholar]
- 178.Wilson WR, Moselen JW, Cliffe S, Denny WA and Ware DC, Int. J. Radiat. Oncol., Biol., Phys, 1994, 29, 323–327. [DOI] [PubMed] [Google Scholar]
- 179.Koch CJ, Cancer Res, 1993, 53, 3992–3997. [PubMed] [Google Scholar]
- 180.Marshall RS and Rauth AM, Cancer Res, 1988, 48, 5655–5659. [PubMed] [Google Scholar]
- 181.Denny WA and Wilson WR, Cancer Metastasis Rev, 1993, 12, 135–151. [DOI] [PubMed] [Google Scholar]
- 182.Palmer BD, Wilson WR, Pullen SM and Denny WA,J. Med. Chem, 1990, 33, 112–121. [DOI] [PubMed] [Google Scholar]
- 183.Denny WA and Wilson WR, J. Med. Chem, 1986, 29, 879–887. [DOI] [PubMed] [Google Scholar]
- 184.Cobb LM, Connors TA, Elson LA, Khan AH, Mitchley BC, Ross WC and Whisson ME, Biochem. Pharmacol, 1969, 18, 1519–1527. [DOI] [PubMed] [Google Scholar]
- 185.Palmer BD, Wilson WR, Cliffe S and Denny WA,. Med. Chem, 1992, 35, 3214–3222. [DOI] [PubMed] [Google Scholar]
- 186.Wilson WR, Palmer BD, Pullen SM, Cliffe S and Denny WA, 40th Ann. Meeting Radiation Research SOC1.9 92, p. 59, Abstract. [Google Scholar]
- 187.Helsby NA, Wheeler SJ, Pruijn FB, Palmer BD, Yang SJ, Denny WA and Wilson WR, Chem. Res. Toxicol, 2003, 16, 469–478. [DOI] [PubMed] [Google Scholar]
- 188.Searle PF, Chen MJ, Hu LQ, Race PR, Lovering AL, Grove JI, Guise C, Jaberipour M, James ND, Mautner V, Young LS, Kerr DJ, Mountain A, White SA and Hyde EI, Clin. Exp. Pharmacol. Physiol, 2004, 31, 811–816. [DOI] [PubMed] [Google Scholar]
- 189.Chung-Faye G, Palmer D, Anderson D, Clark J, Downes M, Baddeley J, Hussain S, Murray PI, Searle P, Seymour L, Harris PA, Ferry D and Kerr DJ, Clin. Cancer Res, 2001, 7, 2662–2668. [PubMed] [Google Scholar]
- 190.Djeha AH, Hulme A, Dexter MT, Mountain A, Young LS, Searle PF, Kerr DJ and Wrighton CJ, Cancer Gene Ther, 2000, 7, 721–731. [DOI] [PubMed] [Google Scholar]
- 191.Teng G, Ju Y, Yang Y, Hua H, Chi J and Mu X, Mol. Med. Rep, 2016, 14, 5164–5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Patel P, Young JG, Mautner V, Ashdown D, Bonney S, Pineda RG, Collins SI, Searle PF, Hull D, Peers E, Chester J, Wallace DM, Doherty A, Leung H, Young LS and James ND, Mol. Ther, 2009, 17, 1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Palmer BD, Wilson WR, Atwell GJ, Schultz D, Xu XZ and Denny WA, J. Med. Chem, 1994, 37, 2175–2184. [DOI] [PubMed] [Google Scholar]
- 194.Palmer BD, Wilson WR, Anderson RF, Boyd M and Denny WA, J. Med. Chem, 1996, 39, 2518–2528. [DOI] [PubMed] [Google Scholar]
- 195.Patterson AV, Ferry DM, Edmunds SJ, Gu Y, Singleton RS, Patel K, Pullen SM, Hicks KO, Syddall SP, Atwell GJ, Yang S, Denny WA and Wilson WR, Clin. Cancer Res, 2007, 13, 3922–3932. [DOI] [PubMed] [Google Scholar]
- 196.Guise CP, Mowday AM, Ashoorzadeh A, Yuan R, Lin WH, Wu DH, Smaill JB, Patterson AV and Ding K, Chin. J. Cancer, 2014, 33, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gu YC, Patterson AV, Atwell GJ, Chernikova SB, Brown JM, Thompson LH and Wilson WR, Mol. Cancer Ther, 2009, 8, 1714–1723. [DOI] [PubMed] [Google Scholar]
- 198.Singleton RS, Guise CP, Ferry DM, Pullen SM, Dorie MJ, Brown JM, Patterson AV and Wilson WR, Cancer Res, 2009, 69, 3884–3891. [DOI] [PubMed] [Google Scholar]
- 199.Hunter FW, Jaiswal JK, Hurley DG, Liyanage HDS, McManaway SP, Gu YC, Richter S, Wang JL, Tercel M, Print CG, Wilson WR and Pruijn FB, Biochem. Pharmacol, 2014, 89, 224–235. [DOI] [PubMed] [Google Scholar]
- 200.Guise CP, Wang AT, Theil A, Bridewell DJ, Wilson WR and Patterson AV, Biochem. Pharmacol, 2007, 74, 810–820. [DOI] [PubMed] [Google Scholar]
- 201.Guise CP, Abbattista MR, Tipparaju SR, Lambie NK, Su JC, Li D, Wilson WR, Dachs GU and Patterson AV, Mol. Pharmacol, 2012, 81, 31–40. [DOI] [PubMed] [Google Scholar]
- 202.Guise CP, Abbattista MR, Singleton RS, Holford SD, Connolly J, Dachs GU, Fox SB, Pollock R, Harvey J, Guilford P, Donate F, Wilson WR and Patterson AV, Cancer Res, 2010, 70, 1573–1584. [DOI] [PubMed] [Google Scholar]
- 203.Hunter FW, Hsu HL, Su JC, Pullen SM, Wilson WR and Wang JL, Mol. Cancer Ther, 2014, 13, 2501–2514. [DOI] [PubMed] [Google Scholar]
- 204.Hicks KO, Myint H, Patterson AV, Pruijn FB, Siim BG, Patel K and Wilson WR, Int. J. Radiat. Oncol., Biol., Phys, 2007, 69, 560–571. [DOI] [PubMed] [Google Scholar]
- 205.Benito J, Shi YX, Szymanska B, Carol H, Boehm I, Lu HB, Konoplev S, Fang WD, Zweidler-McKay PA, Campana D, Borthakur G, Bueso-Ramos C, Shpall E, Thomas DA, Jordan CT, Kantarjian H, Wilson WR, Lock R, Andreeff M and Konopleva M, PLoS One, 2011, 6, e23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Manesh DM, El-Hoss J, Evans K, Richmond J, Toscan CE, Bracken LS, Hedrick A, Sutton R, Marshall GM, Wilson WR, Kurmasheva RT, Billups C, Houghton PJ, Smith MA, Carol H and Lock RB, Blood, 2015, 126, 1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Konopleva M, Thall PF, Yi CA, Borthakur G, Coveler A, Bueso-Ramos C, Benito J, Konoplev S, Gu YC, Ravandi F, Jabbour E, Faderl S, Thomas D, Cortes J, Kadia T, Kornblau S, Daver N, Pemmaraju N, Nguyen HQ, Feliu J, Lu HB, Wei C, Wilson WR, Melink TJ, Gutheil JC, Andreeff M, Estey EH and Kantarjian H, Haematologica, 2015, 100, 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Papadopoulou MV and Bloomer WD, Clin. Cancer Res, 2003, 9, 5714–5720. [PubMed] [Google Scholar]
- 209.Papadopoulou MV, Ji M, Rao MK and Bloomer WD, Oncol. Res, 2003, 14, 21–29. [DOI] [PubMed] [Google Scholar]
- 210.Tomigahara Y, Onogi M, Saito K, Isobe N, Kaneko H and Nakatsuka I, Xenobiotica, 2000, 30, 395–406. [DOI] [PubMed] [Google Scholar]
- 211.Papadopoulou MV, Ji M, Rao MK and Bloomer WD, Oncol. Res, 2000, 12, 325–333. [DOI] [PubMed] [Google Scholar]
- 212.Workman P, Int. J. Radiat. Oncol., Biol., Phys, 1992, 22, 631–637. [DOI] [PubMed] [Google Scholar]
- 213.Papadopoulou MV, Ji M and Bloomer WD, Int. J. Radiat. Oncol., Biol., Phys, 1998, 42, 775–779. [DOI] [PubMed] [Google Scholar]
- 214.Papadopoulou MV, Ji M, Ji X, Bloomer WD and Hollingshead MG, Cancer Chemother. Pharmacol, 2002, 50, 501–508. [DOI] [PubMed] [Google Scholar]
- 215.Papadopoulou MV, Ji M and Bloomer WD, Oncol. Res, 2002, 13, 47–54. [DOI] [PubMed] [Google Scholar]
- 216.Papadopoulou MV, Ji M and Bloomer WD, Cancer Chemother. Pharmacol, 2001, 48, 160–168. [DOI] [PubMed] [Google Scholar]
- 217.Papadopoulou MV, Ji M, Ji X and Bloomer WD, Cancer Chemother. Pharmacol, 2002, 50, 291–298. [DOI] [PubMed] [Google Scholar]
- 218.Griffiths L and Stratford IJ, Int. J. Radiat. Oncol., Biol., Phys, 1998, 42, 877–883. [DOI] [PubMed] [Google Scholar]
- 219.Cole C, Reigan P, Gbaj A, Edwards PN, Douglas KT, Stratford IJ, Freeman S and Jaffar M, J. Med. Chem, 2003, 46, 207–209. [DOI] [PubMed] [Google Scholar]
- 220.Reigan P, Edwards PN, Gbaj A, Cole C, Barry ST, Page KM, Ashton SE, Luke RWA, Douglas KT, Stratford IJ, Jaffar M, Bryce RA and Freeman S, J. Med. Chem, 2005, 48, 392–402. [DOI] [PubMed] [Google Scholar]
- 221.McKeown SR, Cowen RL and Williams KJ, Clin. Oncol, 2007, 19, 427–442. [DOI] [PubMed] [Google Scholar]
- 222.Boger DL and Johnson DS, Angew. Chem., Int. Ed. Engl, 1996, 35, 1438–1474. [Google Scholar]
- 223.Tichenor MS and Boger DL, Nat. Prod. Rep, 2008, 25, 220–226. [DOI] [PubMed] [Google Scholar]
- 224.Kobayashi E, Okamoto A, Asada M, Okabe M, Nagamura S, Asai A, Saito H, Gomi K and Hirata T, Cancer Res, 1994, 54, 2404–2410. [PubMed] [Google Scholar]
- 225.Pavlidis N, Aamdal S, Awada A, Calvert H, Fumoleau P, Sorio R, Punt C, Verweij J, van Oosterom A, Morant R, Wanders J and Hanauske AR, Cancer Chemother. Pharmacol, 2000, 46, 167–171. [DOI] [PubMed] [Google Scholar]
- 226.Markovic SN, Suman VJ, Vukov AM, Fitch TR, Hillman DW, Adjei AA, Alberts SR, Kaur JS, Braich TA, Leitch JM and Creagan ET, Am. J. Clin. Oncol, 2002, 25, 308–312. [DOI] [PubMed] [Google Scholar]
- 227.Tercel M, Denny WA and Wilson WR, Bioorg. Med. Chem. Lett, 1996, 6, 2741–2744. [Google Scholar]
- 228.Wilson WR, Stribbling SM, Pruijn FB, Syddall SP, Patterson AV, Liyanage HDS, Smith E, Botting KJ and Tercel M, Mol. Cancer Ther, 2009, 8, 2903–2913. [DOI] [PubMed] [Google Scholar]
- 229.Wilson WR, Hicks KO, Pullen SM, Ferry DM, Helsby NA and Patterson AV, Radiat. Res, 2007, 167, 625–636. [DOI] [PubMed] [Google Scholar]
- 230.Singleton DC, Li D, Bai SY, Syddall SP, Smaill JB, Shen Y, Denny WA, Wilson WR and Patterson AV, Cancer Gene Ther, 2007, 14, 953–967. [DOI] [PubMed] [Google Scholar]
- 231.Tercel M, Atwell GJ, Yang SJ, Stevenson RJ, Botting KJ, Boyd M, Smith E, Anderson RF, Denny WA, Wilson WR and Pruijn FB, J. Med. Chem, 2009, 52, 7258–7272. [DOI] [PubMed] [Google Scholar]
- 232.Tercel M, Pruijn FB, O’Connor PD, Liyanage HDS, Atwell GJ and Alix SM, ChemBioChem, 2014, 15, 1998–2006. [DOI] [PubMed] [Google Scholar]
- 233.Le QTX, Moon J, Redman M, Williamson SK, Lara PN, Goldberg Z, Gaspar LE, Crowley JJ, Moore DF and Gandara DR, J. Clin. Oncol, 2009, 27, 3014–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jameson MB, Rischin D, Pegram M, Gutheil J, Patterson AV, Denny WA and Wilson WR, Cancer Chemother. Pharmacol, 2010, 65, 791–801. [DOI] [PubMed] [Google Scholar]
- 235.Tercel M, Atwell GJ, Yang SJ, Ashoorzadeh A, Stevenson RJ, Botting KJ, Gu YC, Mehta SY, Denny WA, Wilson WR and Pruijn FB, Angew. Chem., Int. Ed, 2011, 50, 2606–2609. [DOI] [PubMed] [Google Scholar]
- 236.Ashoorzadeh A, Atwell GJ, Pruijn FB, Wilson WR, Tercel M, Denny WA and Stevenson RJ, Bioorg. Med. Chem, 2011, 19, 4851–4860. [DOI] [PubMed] [Google Scholar]
- 237.Stevenson RJ, Denny WA, Tercel M, Pruijn FB and Ashoorzadeh A, J. Med. Chem, 2012, 55, 2780–2802. [DOI] [PubMed] [Google Scholar]
- 238.Tercel M, Lee HH, Mehta SY, Youte Tendoung JJ, Bai SY, Liyanage HDS and Pruijn FB, J. Med. Chem, 2017, 60, 5834–5856. [DOI] [PubMed] [Google Scholar]
- 239.Denny WA, Wilson WR and Hay MP, Br. J. Cancer, Suppl, 1996, 74, S32–S38. [PMC free article] [PubMed] [Google Scholar]
- 240.Tercel M, Wilson WR and Denny WA, J. Med. Chem, 1993, 36, 2578–2579. [DOI] [PubMed] [Google Scholar]
- 241.Denny WA, Wilson WR, Tercel M, Van Zijl P and Pullen SM, Int. J. Radiat. Oncol., Biol., Phys, 1994, 29, 317–321. [DOI] [PubMed] [Google Scholar]
- 242.Wilson WR, Ferry DM, Tercel M, Anderson RF and Denny WA, Radiat. Res, 1998, 149, 237–245. [PubMed] [Google Scholar]
- 243.Tercel M, Lee AE, Hogg A, Anderson RF, Lee HH, Siim BG, Denny WA and Wilson WR, J. Med. Chem, 2001, 44, 3511–3522. [DOI] [PubMed] [Google Scholar]
- 244.Jackson V, Silva S, Abbattista M, Guise C, Bull M, Ashoorzadeh A, Hart C, Pearce T, Smaill J and Patterson AV, Mol. Cancer Ther, 2016, 14, A66. [Google Scholar]
- 245.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sanchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M and Spanish Lung Cancer Group, N. Engl. J. Med, 2009, 361, 958–967. [DOI] [PubMed] [Google Scholar]
- 246.Patterson AV, Silva S, Guise C, Abbattista M, Bull M, Hsu A, Sun J, Jung D, Grey A, Ashoorzadeh A, Anderson R and Smaill JB, Cancer Res, 2015, 75, 5358. [Google Scholar]
- 247.Patterson AV, Silva S, Guise C, Bull M, Abbattista M, Hsu A, Sun JD, Hart CP, Pearce TE and Smaill JB, J. Clin. Oncol, 2015, 33, e13548. [Google Scholar]
- 248.Ikeda Y, Hisano H, Nishikawa Y and Nagasaki Y, Mol. Pharmaceutics, 2016, 13, 2283–2289. [DOI] [PubMed] [Google Scholar]
- 249.Hay MP, Denny WA and Wilson WR, Anti-Cancer Drug Des, 1996, 11, 383–402. [PubMed] [Google Scholar]
- 250.Duan JX, Jiao H, Kaizerman J, Stanton T, Evans JW, Lan L, Lorente G, Banica M, Jung D, Wang JW, Ma HY, Li XM, Yang ZJ, Hoffman RM, Ammons WS, Hart CP and Matteucci M, J. Med. Chem, 2008, 51, 2412–2420. [DOI] [PubMed] [Google Scholar]
- 251.Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM and Bouvet M, Clin. Exp. Metastasis, 2004, 21, 7–12. [DOI] [PubMed] [Google Scholar]
- 252.Bouvet M, Spernyak J, Katz MH, Mazurchuk RV, Takimoto S, Bernacki R, Rustum YM, Moossa AR and Hoffman RM, Cancer Res, 2005, 65, 9829–9833. [DOI] [PubMed] [Google Scholar]
- 253.Saggar JK and Tannock IF, Int. J. Cancer, 2014, 134, 2726–2734. [DOI] [PubMed] [Google Scholar]
- 254.Ganjoo KN, Cranmer LD, Butrynski JE, Rushing D, Adkins D, Okuno SH, Lorente G, Kroll S, Langmuir VK and Chawla SP, Oncology, 2011, 80, 50–56. [DOI] [PubMed] [Google Scholar]
- 255.Chawla SP, Cranmer LD, Van Tine BA, Reed DR, Okuno SH, Butrynski JE, Adkins DR, Hendifar AE, Kroll S and Ganjoo KN, J. Clin. Oncol, 2014, 32, 3299–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Borad MJ, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL, Schelman WR, Stephenson J, Chiorean EG, Rosen PJ, Ulrich B, Dragovich T, Del Prete SA, Rarick M, Eng C, Kroll S and Ryan DP, J. Clin. Oncol, 2015, 33, 1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jin C, Zhang QM and Lu W, Eur. J. Med. Chem, 2017, 132, 135–141. [DOI] [PubMed] [Google Scholar]
- 258.Anlezark GM, Melton RG, Sherwood RF, Coles B, Friedlos F and Knox RJ, Biochem. Pharmacol, 1992, 44, 2289–2295. [DOI] [PubMed] [Google Scholar]
- 259.Lovering AL, Hyde EI, Searle PF and White SA, J. Mol. Biol, 2001, 309, 203–213. [DOI] [PubMed] [Google Scholar]
- 260.Parkinson GN, Skelly JV and Neidle S, J. Med. Chem, 2000, 43, 3624–3631. [DOI] [PubMed] [Google Scholar]
- 261.Mulcahy RT, Gipp JJ, Schmidt JP, Joswig C and Borch RF, J. Med. Chem, 1994, 37, 1610–1615. [DOI] [PubMed] [Google Scholar]
- 262.Seow HA, Penketh PG, Shyam K, Rockwell S and Sartorelli AC, Proc. Natl. Acad. Sci. U. S. A, 2005, 102, 9282–9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Penketh PG, Shyam K, Baumann RP, Remack JS, Brent TP and Sartorelli AC, Cancer Chemother. Pharmacol, 2004, 53, 279–287. [DOI] [PubMed] [Google Scholar]
- 264.Baumann RP, Shyam K, Penketh PG, Remack JS, Brent TP and Sartorelli AC, Cancer Chemother. Pharmacol, 2004, 53, 288–295. [DOI] [PubMed] [Google Scholar]
- 265.Thomson P, Naylor MA, Stratford MRL, Lewis G, Hill S, Patel KB, Wardman P and Davis PD, Bioorg. Med. Chem. Lett, 2007, 17, 4320–4322. [DOI] [PubMed] [Google Scholar]
- 266.Yu LJ, Matias J, Scudiero DA, Hite KM, Monks A, Sausville EA and Waxman DJ, Drug Metab. Dispos, 2001, 29, 304–312. [PubMed] [Google Scholar]
- 267.Cazares-Korner C, Pires IM, Swallow ID, Grayer SC, O’Connor LJ, Olcina MM, Christlieb M, Conway SJ and Hammond EM, ACS Chem. Biol, 2013, 8, 1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Patil M, Pabla N and Dong Z, Cell. Mol. Life Sci, 2013, 70, 4009–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Goto H, Izawa I, Li P and Inagaki M, Cancer Sci, 2012, 103, 1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Pegg AE, Cancer Res, 1990, 50, 6119–6129. [PubMed] [Google Scholar]
- 271.Gerson SL and Willson JK, Hematol. Oncol. Clin. North Am, 1995, 9, 431–450. [PubMed] [Google Scholar]
- 272.Dolan ME, Moschel RC and Pegg AE, Proc. Natl. Acad. Sci. U. S. A, 1990, 87, 5368–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Dolan ME, Mitchell RB, Mummert C, Moschel RC and Pegg AE, Cancer Res, 1991, 51, 3367–3372. [PubMed] [Google Scholar]
- 274.Zhu R, Liu MC, Luo MZ, Penketh PG, Baumann RP, Shyam K and Sartorelli AC, J. Med. Chem, 2011, 54, 7720–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Saneyoshi H, Hiyoshi Y, Iketani K, Kondo K and Ono A, Bioorg. Med. Chem. Lett, 2015, 25, 5632–5635. [DOI] [PubMed] [Google Scholar]
- 276.Granchi C, Funaioli T, Erler JT, Giaccia AJ, Macchia M and Minutolo F, ChemMedChem, 2009, 4, 1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Erler JT and Giaccia AJ, Cancer Res, 2006, 66, 10238–10241. [DOI] [PubMed] [Google Scholar]
- 278.Damen EWP, Nevalainen TJ, van den Bergh TJM, de Groot FMH and Scheeren HW, Bioorg. Med. Chem, 2002, 10, 71–77. [DOI] [PubMed] [Google Scholar]
- 279.Kumar R, Kim EJ, Han J, Lee H, Shin WS, Kim HM, Bhuniya S, Kim JS and Hong KS, Biomaterials, 2016, 104, 119–128. [DOI] [PubMed] [Google Scholar]
- 280.Feng W, Gao C, Liu W, Ren H, Wang C, Ge K, Li S, Zhou G, Li H, Wang S, Jia G, Li Z and Zhang J, Chem. Commun, 2016, 52, 9434–9437. [DOI] [PubMed] [Google Scholar]
- 281.Kim HS, Sharma A, Ren WX, Han J and Kim JS, Biomaterials, 2018, 185, 63–72. [DOI] [PubMed] [Google Scholar]
- 282.Ross WC and Warwick GP, Nature, 1955, 176, 298–299. [DOI] [PubMed] [Google Scholar]
- 283.Ross WCJ and Warwick GP, J. Chem. Soc, 1956, 1724–1732. [Google Scholar]
- 284.Kiyose K, Hanaoka K, Oushiki D, Nakamura T, Kajimura M, Suematsu M, Nishimatsu H, Yamane T, Terai T, Hirata Y and Nagano T, J. Am. Chem. Soc, 2010, 132, 15846–15848. [DOI] [PubMed] [Google Scholar]
- 285.Zhu R, Baumann RP, Penketh PG, Shyam K and Sartorelli AC, J. Med. Chem, 2013, 56, 1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Verwilst P, Han J, Lee J, Mun S, Kang HG and Kim JS, Biomaterials, 2017, 115, 104–114. [DOI] [PubMed] [Google Scholar]
- 287.Hu MX, Yang C, Luo Y, Chen F, Yang FF, Yang SP, Chen H, Cheng ZQ, Li K and Xie YM, J. Mater. Chem. B, 2018, 6, 4385. [DOI] [PubMed] [Google Scholar]
- 288.Zhou Y, Maiti M, Sharma A, Won M, Yu L, Miao LX, Shin J, Podder A, Bobba KN, Han J, Bhuniya S and Kim JS, J. Controlled Release, 2018, 288, 14–22. [DOI] [PubMed] [Google Scholar]
- 289.van Brakel R, Vulders RCM, Bokdam RJ, Grull H and Robillard MS, Bioconjugate Chem, 2008, 19, 714–718. [DOI] [PubMed] [Google Scholar]
- 290.Li SY, Jiang XY, Zheng RR, Zuo SJ, Zhao LP, Fan GL, Fan JH, Liao YH, Yu XY and Cheng H, Chem. Commun, 2018, 54, 7983–7986. [DOI] [PubMed] [Google Scholar]
- 291.Piao W, Hanaoka K, Fujisawa T, Takeuchi S, Komatsu T, Ueno T, Terai T, Tahara T, Nagano T and Urano Y, J. Am. Chem. Soc, 2017, 139, 13713–13719. [DOI] [PubMed] [Google Scholar]
- 292.Siegel R, Ma J, Zou Z and Jemal A, Ca-Cancer J. Clin, 2014, 64, 9–29. [DOI] [PubMed] [Google Scholar]
- 293.Galanski M, Jakupec MA and Keppler BK, Curr. Med. Chem, 2005, 12, 2075–2094. [DOI] [PubMed] [Google Scholar]
- 294.Rabik CA and Dolan ME, Cancer Treat. Rev, 2007, 33, 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Rosenberg B, VanCamp L, Trosko JE and Mansour VH, Nature, 1969, 222, 385–386. [DOI] [PubMed] [Google Scholar]
- 296.Muhammad N and Guo Z, Curr. Opin. Chem. Biol, 2014, 19, 144–153. [DOI] [PubMed] [Google Scholar]
- 297.Romero-Canelon I and Sadler PJ, Inorg. Chem, 2013, 52, 12276–12291. [DOI] [PubMed] [Google Scholar]
- 298.Hambley TW, Science, 2007, 318, 1392–1393. [DOI] [PubMed] [Google Scholar]
- 299.Chen Y and Hu L, Med. Res. Rev, 2009, 29, 29–64. [DOI] [PubMed] [Google Scholar]
- 300.Hall MD, Failes TW, Yamamoto N and Hambley TW, Dalton Trans, 2007, 3983–3990. [DOI] [PubMed] [Google Scholar]
- 301.Schafer FQ and Buettner GR, Free Radical Biol. Med, 2001, 30, 1191–1212. [DOI] [PubMed] [Google Scholar]
- 302.Teicher BA, Jacobs JL, Cathcart KNS, Abrams MJ, Vollano JF and Picker DH, Radiat. Res, 1987, 109, 36–46. [PubMed] [Google Scholar]
- 303.Teicher BA, Abrams MJ, Rosbe KW and Herman TS, Cancer Res, 1990, 50, 6971–6975. [PubMed] [Google Scholar]
- 304.Ware DC, Palmer BD, Wilson WR and Denny WA, J. Med. Chem, 1993, 36, 1839–1846. [DOI] [PubMed] [Google Scholar]
- 305.Ware DC, Wilson WR, Denny WA and Rickard CEF, J. Chem. Soc., Chem. Commun, 1991, 1171–1173. [Google Scholar]
- 306.Ware DC, Denny WA and Clark GR, Acta Crystallogr., Sect. C: Cryst. Struct. Commun, 1997, 53, 1058–1059. [Google Scholar]
- 307.Anderson RF, Denny WA, Ware DC and Wilson WR, Br. J. Cancer, 1996, 74, S48–S51. [PMC free article] [PubMed] [Google Scholar]
- 308.Ware DC, Brothers PJ, Clark GR, Denny WA, Palmer BD and Wilson WR, J. Chem. Soc., Dalton Trans, 2000, 925–932. [Google Scholar]
- 309.Ware DC, Palmer HR, Pruijn FB, Anderson RE, Brothers PJ, Denny WA and Wilson WR, Anti-Cancer Drug Des, 1998, 13, 81–103. [PubMed] [Google Scholar]
- 310.Ware DC, Palmer HR, Brothers PJ, Rickard CEF, Wilson WR and Denny WA, J. Inorg. Biochem, 1997, 68, 215–224. [DOI] [PubMed] [Google Scholar]
- 311.Craig PR, Brothers PJ, Clark GR, Wilson WR, Denny WA and Ware DC, Dalton Trans, 2004, 611–618. [DOI] [PubMed] [Google Scholar]
- 312.Failes TW, Cullinane C, Diakos CI, Yamamoto N, Lyons JG and Hambley TW, Chem. – Eur. J, 2007, 13, 2974–2982. [DOI] [PubMed] [Google Scholar]
- 313.Nagase H, Visse R and Murphy G, Cardiovasc. Res, 2006, 69, 562–573. [DOI] [PubMed] [Google Scholar]
- 314.Vandenbroucke RE and Libert C, Nat. Rev. Drug Discovery, 2014, 13, 904–927. [DOI] [PubMed] [Google Scholar]
- 315.Hidalgo M and Eckhardt SG, J. Natl. Cancer Inst, 2001, 93, 178–193. [DOI] [PubMed] [Google Scholar]
- 316.Failes TW and Hambley TW, Dalton Trans, 2006, 1895–1901. [DOI] [PubMed] [Google Scholar]
- 317.Bonnitcha PD, Kim BJ, Hocking RK, Clegg JK, Turner P, Neville SM and Hambley TW, Dalton Trans, 2012, 41, 11293–11304. [DOI] [PubMed] [Google Scholar]
- 318.Ahn GO, Botting KJ, Patterson AV, Ware DC, Tercel M and Wilson WR, Biochem. Pharmacol, 2006, 71, 1683–1694. [DOI] [PubMed] [Google Scholar]
- 319.Chang JYC, Stevenson RJ, Lu GL, Brothers PJ, Clark GR, Denny WA and Ware DC, Dalton Trans, 2010, 39, 11535–11550. [DOI] [PubMed] [Google Scholar]
- 320.Milbank JBJ, Stevenson RJ, Ware DC, Chang JYC, Tercel M, Ahn GO, Wilson WR and Denny WA, J. Med. Chem, 2009, 52, 6822–6834. [DOI] [PubMed] [Google Scholar]
- 321.Lu GL, Stevenson RJ, Chang JYC, Brothers PJ, Ware DC, Wilson WR, Denny WA and Tercel M, Bioorg. Med. Chem, 2011, 19, 4861–4867. [DOI] [PubMed] [Google Scholar]
- 322.Chang JYC, Lu GL, Stevenson RJ, Brothers PJ, Clark GR, Botting KJ, Ferry DM, Tercel M, Wilson WR, Denny WA and Ware DC, Inorg. Chem, 2013, 52, 7688–7698. [DOI] [PubMed] [Google Scholar]
- 323.Prasad S, Tyagi AK and Aggarwal BB, Cancer Res. Treat, 2014, 46, 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Aggarwal BB, Kumar A and Bharti AC, Anticancer Res, 2003, 23, 363–398. [PubMed] [Google Scholar]
- 325.Bansal SS, Goel M, Aqil F, Vadhanam MV and Gupta RC, Cancer Prev. Res, 2011, 4, 1158–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Renfrew AK, Bryce NS and Hambley TW, Chem. Sci, 2013, 4, 3731–3739. [Google Scholar]
- 327.Karnthaler-Benbakka C, Groza D, Kryeziu K, Pichler V, Roller A, Berger W, Heffeter P and Kowol CR, Angew. Chem., Int. Ed, 2014, 53, 12930–12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dwyer FP, Gyarfas EC, Rogers WP and Koch JH, Nature, 1952, 170, 190–191. [DOI] [PubMed] [Google Scholar]
- 329.Clarke MJ, Met. Ions Biol. Syst, 1980, 11, 231–283. [Google Scholar]
- 330.Clarke MJ, Coord. Chem. Rev, 2002, 232, 69–93. [Google Scholar]
- 331.Clarke MJ, Sadler PJ and Alessio E, Metallopharmaceuticals, Springer, Berlin, New York, 1999. [Google Scholar]
- 332.Pluim D, van Waardenburg RCAM, Beijnen JH and Schellens JHM, Cancer Chemother. Pharmacol, 2004, 54, 71–78. [DOI] [PubMed] [Google Scholar]
- 333.Harris TV, Szilagyi RK and Holman KLM, J. Biol. Inorg. Chem, 2009, 14, 891–898. [DOI] [PubMed] [Google Scholar]
- 334.Kostova I, Curr. Med. Chem, 2016, 13, 1085–1107. [DOI] [PubMed] [Google Scholar]
- 335.Ang WH and Dyson PJ, Eur. J. Inorg. Chem, 2006, 4003–4018. [Google Scholar]
- 336.Alessio E, Mestroni G, Bergamo A and Sava G, Curr. Top. Med. Chem, 2004, 4, 1525–1535. [DOI] [PubMed] [Google Scholar]
- 337.Hartinger CG, Jakupec MA, Zorbas-Seifried S, Groessl M, Egger A, Berger W, Zorbas H, Dyson PJ and Keppler BK, Chem. Biodiversity, 2008, 5, 2140–2155. [DOI] [PubMed] [Google Scholar]
- 338.Trondl R, Heffeter P, Kowol CR, Jakupec MA, Berger W and Keppler BK, Chem. Sci, 2014, 5, 2925–2932. [Google Scholar]
- 339.Hartinger CG, Zorbas-Seifried S, Jakupec MA, Kynast B, Zorbas H and Keppler BK, J. Inorg. Biochem, 2006, 100, 891–904. [DOI] [PubMed] [Google Scholar]
- 340.Keppler BK, Henn M, Juhl UM, Berger MR, Niebl R and Wagner FE, Prog. Clin. Biochem. Med, 1989, 10, 41–69. [Google Scholar]
- 341.Bergamo A, Masi A, Jakupec MA, Keppler BK and Sava G, Met.-Based Drugs, 2009, 2009, 681270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Clarke MJ, Coord. Chem. Rev, 2003, 236, 209–233. [Google Scholar]
- 343.Trynda-Lemiesz L, Acta Biochim. Pol, 2004, 51, 199–205. [PubMed] [Google Scholar]
- 344.Depenbrock H, Schmelcher S, Peter R, Keppler BK, Weirich G, Block T, Rastetter J and Hanauske AR, Eur. J. Cancer, 1997, 33, 2404–2410. [DOI] [PubMed] [Google Scholar]
- 345.Sava G, Zorzet S, Turrin C, Vita F, Soranzo M, Zabucchi G, Cocchietto M, Bergamo A, DiGiovine S, Pezzoni G, Sartor L and Garbisa S, Clin. Cancer Res, 2003, 9, 1898–1905. [PubMed] [Google Scholar]
- 346.Frausin F, Scarcia V, Cocchietto M, Furlani A, Serli B, Alessio E and Sava G, J. Pharmacol. Exp. Ther, 2005, 313, 227–233. [DOI] [PubMed] [Google Scholar]
- 347.Vacca A, Bruno M, Boccarelli A, Coluccia M, Ribatti D, Bergamo A, Garbisa S, Sartor L and Sava G, Br. J. Cancer, 2002, 86, 993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Pacor S, Zorzet S, Cocchietto M, Bacac M, Vadori M, Turrin C, Gava B, Castellarin A and Sava G, J. Pharmacol. Exp. Ther, 2004, 310, 737–744. [DOI] [PubMed] [Google Scholar]
- 349.Frasca D, Ciampa J, Emerson J, Umans RS and Clarke MJ, Met.-Based Drugs, 1996, 3, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Jakupec MA, Reisner E, Eichinger A, Pongratz M, Arion VB, Galanski M, Hartinger CG and Keppler BK, J. Med. Chem, 2005, 48, 2831–2837. [DOI] [PubMed] [Google Scholar]
- 351.Sava G, Bergamo A, Zorzet S, Gava B, Casarsa C, Cocchietto M, Furlani A, Scarcia V, Serli B, Iengo E, Alessio E and Mestroni G, Eur. J. Cancer, 2002, 38, 427–435. [DOI] [PubMed] [Google Scholar]
- 352.Schluga P, Hartinger CG, Egger A, Reisner E, Galanski M, Jakupec MA and Keppler BK, Dalton Trans, 2006, 1796–1802. [DOI] [PubMed] [Google Scholar]
- 353.Frasca DR and Clarke MJ, J. Am. Chem. Soc, 1999, 121, 8523–8532. [Google Scholar]
- 354.Peti W, Pieper T, Sommer M, Keppler BK and Giester G, Eur. J. Inorg. Chem, 1999, 1551–1555. [Google Scholar]
- 355.Smith CA, SutherlandSmith AJ, Keppler BK, Kratz F and Baker EN, J. Biol. Inorg. Chem, 1996, 1, 424–431. [Google Scholar]
- 356.Clarke MJ, Zhu FC and Frasca DR, Chem. Rev, 1999, 99, 2511–2534. [DOI] [PubMed] [Google Scholar]
- 357.Trondl R, Heffeter P, Jakupec MA, Berger W and Keppler BK, BMC Pharmacol. Toxicol, 2012, 13, A82. [Google Scholar]
- 358.Heffeter P, Atil B, Kryeziu K, Groza D, Koellensperger G, Korner W, Jungwirth U, Mohr T, Keppler BK and Berger W, Eur. J. Cancer, 2013, 49, 3366–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Purkait K, Karmakar S, Bhattacharyya S, Chatterjee S, Dey SK and Mukherjee A, Dalton Trans, 2015, 44, 5969–5973. [DOI] [PubMed] [Google Scholar]
- 360.Janaratne TK, Yadav A, Ongeri F and MacDonnell FM, Inorg. Chem, 2007, 46, 3420–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Yadav A, Janaratne T, Krishnan A, Singhal SS, Yadav S, Dayoub AS, Hawkins DL, Awasthi S and MacDonnell FM, Mol. Cancer Ther, 2013, 12, 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Yamaguchi T, Nomura H, Matsunaga K, Ito S, Takata J and Karube Y, Biol. Pharm. Bull, 2003, 26, 1523–1527. [DOI] [PubMed] [Google Scholar]
- 363.Yamaguchi T, Eto M, Harano K, Kashige N, Watanabe E and Ito S, Tetrahedron, 1999, 55, 675–686. [Google Scholar]
- 364.Lord RM, Hebden AJ, Pask CM, Henderson IR, Allison SJ, Shepherd SL, Phillips RM and McGowan PC, J. Med. Chem, 2015, 58, 4940–4953. [DOI] [PubMed] [Google Scholar]
- 365.Zeng L, Chen Y, Huang H, Wang J, Zhao D, Ji L and Chao H, Chem. – Eur. J, 2015, 21, 15308–15319. [DOI] [PubMed] [Google Scholar]
- 366.Lippert B, Cisplatin: chemistry and biochemistry of a leading anticancer drug, Verlag Helvetica Chimica Acta, Wiley-VCH, Zürich Weinheim, New York, 1999. [Google Scholar]
- 367.Jung Y and Lippard SJ, Chem. Rev, 2007, 107, 1387–1407. [DOI] [PubMed] [Google Scholar]
- 368.Kelland LR and Farrell N, Platinum-based drugs in cancer therapy, Humana Press, Totowa, N.J., 2000. [Google Scholar]
- 369.Johnstone TC, Suntharalingam K and Lippard SJ, Chem. Rev, 2016, 116, 3436–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Huang HF, Zhu LM, Reid BR, Drobny GP and Hopkins PB, Science, 1995, 270, 1842–1845. [DOI] [PubMed] [Google Scholar]
- 371.Johnstone TC, Suntharalingam K and Lippard SJ, Philos. Trans. R. Soc., A, 2015, 373, 20140185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Dolman RC, Deacon GB and Hambley TW, J. Inorg. Biochem, 2002, 88, 260–267. [DOI] [PubMed] [Google Scholar]
- 373.Hall MD and Hambley TW, Coord. Chem. Rev, 2002, 232, 49–67. [Google Scholar]
- 374.Schilder RJ, Lacreta FP, Perez RP, Johnson SW, Brennan JM, Rogatko A, Nash S, Mcaleer C, Hamilton TC, Roby D, Young RC, Ozols RF and Odwyer PJ, Cancer Res, 1994, 54, 709–717. [PubMed] [Google Scholar]
- 375.Tutsch KD, Arzoomanian RZ, Alberti D, Tombes MB, Feierabend C, Robins HI, Spriggs DR and Wilding G, Invest. New Drugs, 1999, 17, 63–72. [DOI] [PubMed] [Google Scholar]
- 376.Pendyala L, Cowens JW, Chheda GB, Dutta SP and Creaven PJ, Cancer Res, 1988, 48, 3533–3536. [PubMed] [Google Scholar]
- 377.de wit R, Tesselaar M, Kok TC, Seynaeve C, Rodenburg CJ, Verweij J, Helle PA and Stoter G, Eur. J. Cancer Clin. Oncol, 1991, 27, 1383–1385. [DOI] [PubMed] [Google Scholar]
- 378.Sessa C, Vermorken J, Renard J, Kaye S, Smith D, Huinink WT, Cavalli F and Pinedo H, J. Clin. Oncol, 1988, 6, 98–105. [DOI] [PubMed] [Google Scholar]
- 379.Anderson H, Wagstaff J, Crowther D, Swindell R, Lind MJ, Mcgregor J, Timms MS, Brown D and Palmer P, Eur. J. Cancer Clin. Oncol, 1988, 24, 1471–1479. [DOI] [PubMed] [Google Scholar]
- 380.Kelland LR, Abel G, Mckeage MJ, Jones M, Goddard PM, Valenti M, Murrer BA and Harrap KR, Cancer Res, 1993, 53, 2581–2586. [PubMed] [Google Scholar]
- 381.Raynaud FI, Mistry P, Donaghue A, Poon GK, Kelland LR, Barnard CFJ, Murrer BA and Harrap KR, Cancer Chemother. Pharmacol, 1996, 38, 155–162. [DOI] [PubMed] [Google Scholar]
- 382.Harrap KR, Cancer Treat. Rev, 1985, 12, 21–33. [DOI] [PubMed] [Google Scholar]
- 383.Choy H, Park C and Yao M, Clin. Cancer Res, 2008, 14, 1633–1638. [DOI] [PubMed] [Google Scholar]
- 384.Trudeau M, Stuart G, Hirte H, Drouin P, Plante M, Bessette P, Dulude H, Lebwohl D, Fisher B and Seymour L, Gynecol. Oncol, 2002, 84, 327–331. [DOI] [PubMed] [Google Scholar]
- 385.Vaishampayan UN, Fontana J, Heilbrun LK, Smith D, Heath E, Dickow B and Figg WD, Urol. Oncol, 2014, 32(31), e25–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Figg WD, Chau CH, Madan RA, Gulley JL, Gao R, Sissung TM, Spencer S, Beatson M, Aragon-Ching J, Steinberg SM and Dahut WL, Clin. Genitourin. Cancer, 2013, 11, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Sternberg CN, Whelan P, Hetherington J, Paluchowska B, Slee PH, Vekemans K, Van Erps P, Theodore C, Koriakine O, Oliver T, Lebwohl D, Debois M, Zurlo A, Collette L and Genitourinary Tract Group of the EORTC, Oncology, 2005, 68, 2–9. [DOI] [PubMed] [Google Scholar]
- 388.Sternberg CN, Petrylak D, Witjes F, Ferrero J, Eymard J, Falcon S, Chatta K, Vaughn D, Berry W and Sartor O, Proc. Am. Soc. Clin. Oncol, 2007, 25, 5019. [Google Scholar]
- 389.European Medicines Agency, “Withdrawal Assessment Report for Orplanta: Procedure No. EMEA/H/C/888”, 2008.
- 390.Bouchal P, Jarkovsky J, Hrazdilova K, Dvorakova M, Struharova I, Hernychova L, Damborsky J, Sova P and Vojtesek B, Proteome Sci, 2011, 9, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Shaili E, Sci. Prog, 2014, 97, 20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Pichler V, Goschl S, Meier SM, Roller A, Jakupec MA, Galanski M and Keppler BK, Inorg. Chem, 2013, 52, 8151–8162. [DOI] [PubMed] [Google Scholar]
- 393.Schreiber-Brynzak E, Pichler V, Heffeter P, Hanson B, Theiner S, Lichtscheidl-Schultz I, Kornauth C, Bamonti L, Dhery V, Groza D, Berry D, Berger W, Galanski M, Jakupec MA and Keppler BK, Metallomics, 2016, 8, 422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Preihs C, Arambula JF, Magda D, Jeong H, Yoo D, Cheon J, Siddik ZH and Sessler JL, Inorg. Chem, 2013, 52, 12184–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Lee MH, Kim EJ, Lee H, Kim HM, Chang MJ, Park SY, Hong KS, Kim JS and Sessler JL, J. Am. Chem. Soc, 2016, 138, 16380–16387. [DOI] [PubMed] [Google Scholar]
- 396.Wei WH, Fountain M, Magda D, Wang Z, Lecane P, Mesfin M, Miles D and Sessler JL, Org. Biomol. Chem, 2005, 3, 3290–3296. [DOI] [PubMed] [Google Scholar]
- 397.Thiabaud G, Arambula JF, Siddik ZH and Sessler JL, Chem. – Eur. J, 2014, 20, 8942–8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Thiabaud G, McCall R, He GG, Arambula JF, Siddik ZH and Sessler JL, Angew. Chem., Int. Ed, 2016, 55, 12626–12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Petruzzella E, Braude JP, Aldrich-Wright JR, Gandin V and Gibson D, Angew. Chem., Int. Ed, 2017, 56, 11539–11544. [DOI] [PubMed] [Google Scholar]
- 400.Lv W, Zhang Z, Zhang KY, Yang HR, Liu SJ, Xu AQ, Guo S, Zhao Q and Huang W, Angew. Chem., Int. Ed, 2016, 55, 9947–9951. [DOI] [PubMed] [Google Scholar]
- 401.Mirabelli CK, Johnson RK, Sung CM, Faucette L, Muirhead K and Crooke ST, Cancer Res, 1985, 45, 32–39. [PubMed] [Google Scholar]
- 402.Porchia M, Pellei M, Marinelli M, Tisato F, Del Bello F and Santini C, Eur. J. Med. Chem, 2018, 146, 709–746. [DOI] [PubMed] [Google Scholar]
- 403.Berners-Price SJ and Filipovska A, Metallomics, 2011, 3, 863–873. [DOI] [PubMed] [Google Scholar]
- 404.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian DL, Lam JS, Ailles LE, Wong MZ, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL and Clarke MF, Nature, 2009, 458, 780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Barabasi AL and Oltvai ZN, Nat. Rev. Genet, 2004, 5, 101–113. [DOI] [PubMed] [Google Scholar]
- 406.Eriksson SE, Prast-Nielsen S, Flaberg E, Szekely L and Arner ESJ, Free Radical Biol. Med, 2009, 47, 1661–1671. [DOI] [PubMed] [Google Scholar]
- 407.McCall R, Miles M, Lascuna P, Burney B, Patel Z, Sidoran KJ, Sittaramane V, Kocerha J, Grossie DA, Sessler JL, Arumugam K and Arambula JF, Chem. Sci, 2017, 8, 5918–5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Arambula JF, McCall R, Sidoran KJ, Magda D, Mitchell NA, Bielawski CW, Lynch VM, Sessler JL and Arumugam K, Chem. Sci, 2016, 7, 1245–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Terenzi A, Pirker C, Keppler BK and Berger W, J. Inorg. Biochem, 2016, 165, 71–79. [DOI] [PubMed] [Google Scholar]
- 410.Cerecetto H and Gonzalez M, Mini-Rev. Med. Chem, 2001, 1, 219–231. [DOI] [PubMed] [Google Scholar]
- 411.Zeman EM, Brown JM, Lemmon MJ, Hirst VK and Lee WW, Int. J. Radiat. Oncol., Biol., Phys, 1986, 12, 1239–1242. [DOI] [PubMed] [Google Scholar]
- 412.Patterson AV, Saunders MP, Chinje EC, Patterson LH and Stratford IJ, Anti-Cancer Drug Des, 1998, 13, 541–573. [PubMed] [Google Scholar]
- 413.Daniels JS and Gates KS, J. Am. Chem. Soc, 1996, 118, 3380–3385. [Google Scholar]
- 414.Shinde SS, Hay MP, Patterson AV, Denny WA and Anderson RF, J. Am. Chem. Soc, 2009, 131, 14220–14221. [DOI] [PubMed] [Google Scholar]
- 415.Shinde SS, Maroz A, Hay MP, Patterson AV, Denny WA and Anderson RF, J. Am. Chem. Soc, 2010, 132, 2591–2599. [DOI] [PubMed] [Google Scholar]
- 416.Miller VA, Ng KK, Grant SC, Kindler H, Pizzo B, Heelan RT, von Roemeling R and Kris MG, Ann. Oncol, 1997, 8, 1269–1271. [DOI] [PubMed] [Google Scholar]
- 417.Treat J, Johnson E, Langer C, Belani C, Haynes B, Greenberg R, Rodriquez R, Drobins P, Miller W, Meehan L, McKeon A, Devin J, von Roemeling R and Viallet J, J. Clin. Oncol, 1998, 16, 3524–3527. [DOI] [PubMed] [Google Scholar]
- 418.Hicks KO, Pruijn FB, Sturman JR, Denny WA and Wilson WR, Cancer Res, 2003, 63, 5970–5977. [PubMed] [Google Scholar]
- 419.Durand RE and Olive PL, Int. J. Radiat. Oncol., Biol., Phys, 1992, 22, 689–692. [DOI] [PubMed] [Google Scholar]
- 420.Durand RE and Olive PL, Radiat. Oncol. Invest, 1997, 5, 213–219. [DOI] [PubMed] [Google Scholar]
- 421.Williamson SK, Crowley JJ, Lara PN, McCoy J, Lau DHM, Tucker RW, Mills GM and Gandara DR, J. Clin. Oncol, 2005, 23, 9097–9104. [DOI] [PubMed] [Google Scholar]
- 422.Rischin D, Peters LJ, O’Sullivan B, Giralt J, Fisher R, Yuen K, Trotti A, Bernier J, Bourhis J, Ringash J, Henke M and Kenny L, J. Clin. Oncol, 2010, 28, 2989–2995. [DOI] [PubMed] [Google Scholar]
- 423.DiSilvestro PA, Ali S, Craighead PS, Lucci JA, Lee YC, Cohn DE, Spirtos NM, Tewari KS, Muller C, Gajewski WH, Steinhoff MM and Monk BJ, J. Clin. Oncol, 2014, 32, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Hay MP, Pchalek K, Pruijn FB, Hicks KO, Siim BG, Anderson RF, Shinde SS, Phillips V, Denny WA and Wilson WR, J. Med. Chem, 2007, 50, 6654–6664. [DOI] [PubMed] [Google Scholar]
- 425.Hay MP, Gamage SA, Kovacs MS, Pruijn FB, Anderson RF, Patterson AV, Wilson WR, Brown JM and Denny WA, J. Med. Chem, 2003, 46, 169–182. [DOI] [PubMed] [Google Scholar]
- 426.Hicks KO, Siim BG, Jaiswal JK, Pruijn FB, Fraser AM, Patel R, Hogg A, Liyanage HDS, Dorie MJ, Brown JM, Denny WA, Hay MP and Wilson WR, Clin. Cancer Res, 2010, 16, 4946–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Chitneni SK, Bida GT, Yuan H, Palmer GM, Hay MP, Melcher T, Wilson WR, Zalutsky MR and Dewhirst MW, J. Nucl. Med, 2013, 54, 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Anderson RF, Yadav P, Patel D, Reynisson J, Tipparaju SR, Guise CP, Patterson AV, Denny WA, Maroz A, Shinde SS and Hay MP, Org. Biomol. Chem, 2014, 12, 3386–3392. [DOI] [PubMed] [Google Scholar]
- 429.Hunter FW, Wang JL, Patel R, Hsu HL, Hickey AJR, Hay MP and Wilson WR, Biochem. Pharmacol, 2012, 83, 574–585. [DOI] [PubMed] [Google Scholar]
- 430.Gu YC, Jaiswal JK, Wang JL, Hicks KO, Hay MP and Wilson WR, J. Pharm. Sci, 2014, 103, 3464–3472. [DOI] [PubMed] [Google Scholar]
- 431.Hunter FW, Young RJ, Shalev Z, Vellanki RN, Wang JL, Gu YC, Joshi N, Sreebhavan S, Weinreb I, Goldstein DP, Moffat J, Ketela T, Brown KR, Koritzinsky M, Solomon B, Rischin D, Wilson WR and Wouters BG, Cancer Res, 2015, 75, 4211–4223. [DOI] [PubMed] [Google Scholar]
- 432.Gu Y, Chang TT, Wang J, Jaiswal JK, Edwards D, Downes NJ, Liyanage HDS, Lynch CRH, Pruijn FB, Hickey AJR, Hay MP, Wilson WR and Hicks KO, Front. Pharmacol, 2017, 8, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Liu XW, Cai TY, Zhu H, Cao J, Su Y, Hu YZ, He QJ and Yang B, Autophagy, 2014, 10, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Chang LL, Liu XW, Wang DD, Ma J, Zhou TY, Chen Y, Sheng R, Hu YZ, Du Y, He QJ, Yang B and Zhu H, PLoS One, 2015, 10, e0144506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Cerecetto H, Gonzalez M, Lavaggi ML, Aravena MA, Rigol C, Olea-Azar C, Azqueta A, Lopez de Cerain A, Monge A and Bruno AM, Med. Chem, 2006, 2, 511–521. [DOI] [PubMed] [Google Scholar]
- 436.Cerecetto H, Gonzalez M, Lavaggi ML and Porcal W, J. Braz. Chem. Soc, 2005, 16, 1290–1296. [Google Scholar]
- 437.Lavaggi ML, Nieves M, Cabrera M, Olea-Azar C, Lopez de Cerain A, Monge A, Cerecetto H and Gonzalez M, Eur. J. Med. Chem, 2010, 45, 5362–5369. [DOI] [PubMed] [Google Scholar]
- 438.Langmuir VK, Laderoute KR, Mendonca HL, Sutherland RM, Hei TK, Liu SX, Hall EJ, Naylor MA and Adams GE, Int. J. Radiat. Oncol., Biol., Phys, 1996, 34, 79–84. [DOI] [PubMed] [Google Scholar]
- 439.Naylor MA, Adams GE, Haigh A, Cole S, Jenner T, Robertson N, Siemann D, Stephens MA and Stratford IJ, Anti-Cancer Drugs, 1995, 6, 259–269. [DOI] [PubMed] [Google Scholar]
- 440.Barham HM and Stratford IJ, Biochem. Pharmacol, 1996, 51, 829–837. [DOI] [PubMed] [Google Scholar]
- 441.Naylor MA, Sutton BM, Nolan J, Oneill P, Fielden EM, Adams GE and Stratford IJ, Int. J. Radiat. Oncol., Biol., Phys, 1994, 29, 333–337. [DOI] [PubMed] [Google Scholar]
- 442.Naylor MA, Stephens MA, Nolan J, Sutton B, Tocher JH, Fielden EM, Adams GE and Stratford IJ, Anti-Cancer Drug Des, 1993, 8, 439–461. [PubMed] [Google Scholar]
- 443.Ortega MA, Morancho MJ, Martinez-Crespo FJ, Sainz Y, Montoya ME, de Cerain AL and Monge A, Eur. J. Med. Chem, 2000, 35, 21–30. [DOI] [PubMed] [Google Scholar]
- 444.Solano B, Junnotula V, Marin A, Villar R, Burguete A, Vicente E, Perez-Silanes S, Aldana I, Monge A, Dutta S, Sarkar U and Gates KS, J. Med. Chem, 2007, 50, 5485–5492. [DOI] [PubMed] [Google Scholar]
- 445.Amin KM, Ismail MMF, Noaman E, Soliman DH and Ammar YA, Bioorg. Med. Chem, 2006, 14, 6917–6923. [DOI] [PubMed] [Google Scholar]
- 446.Zarranz B, Jaso A, Aldana I and Monge A, Bioorg. Med. Chem, 2004, 12, 3711–3721. [DOI] [PubMed] [Google Scholar]
- 447.Monge A, Martinezcrespo FJ, Decerain AL, Palop JA, Narro S, Senador V, Marin A, Sainz Y, Gonzalez M, Hamilton E and Barker AJ, J. Med. Chem, 1995, 38, 4488–4494. [DOI] [PubMed] [Google Scholar]
- 448.Cerecetto H, Gonzalez M, Lavaggi ML, Azqueta A, de Cerain AL and Monge A, J. Med. Chem, 2005, 48, 21–23. [DOI] [PubMed] [Google Scholar]
- 449.Monge A, Palop JA, Lopez de Cerain A, Senador V, Martinez-Crespo FJ, Sainz Y, Narro S, Garcia E, de Miguel C and Gonzalez M, et al. , J. Med. Chem, 1995, 38, 1786–1792. [DOI] [PubMed] [Google Scholar]
- 450.Monge A, Martinez-Crespo FJ, Lopez de Cerain A, Palop JA, Narro S, Senador V, Marin A, Sainz Y, Gonzalez M and Hamilton E, et al. , J. Med. Chem, 1995, 38, 4488–4494. [DOI] [PubMed] [Google Scholar]
- 451.Lavaggi ML, Cabrera M, Pintos C, Arredondo C, Pachon G, Rodriguez J, Raymondo S, Pacheco JP, Cascante M, Olea-Azar C, Lopez de Cerain A, Monge A, Cerecetto H and Gonzalez M, ISRN Pharmacol, 2011, 314209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Nakashima H, Uto Y, Nakata E, Nagasawa H, Ikkyu K, Hiraoka N, Nakashima K, Sasaki Y, Sugimoto H, Shiro Y, Hashimoto T, Okamoto Y, Asakawa Y and Hori H, Bioorg. Med. Chem, 2008, 16, 8661–8669. [DOI] [PubMed] [Google Scholar]
- 453.Delahoussaye YM, Hay MP, Pruijn FB, Denny WA and Brown JM, Biochem. Pharmacol, 2003, 65, 1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Hay MP, Pruijn FB, Gamage SA, Liyanage HD, Kovacs MS, Patterson AV, Wilson WR, Brown JM and Denny WA, J. Med. Chem, 2004, 47, 475–488. [DOI] [PubMed] [Google Scholar]
- 455.Johnson KM, Parsons ZD, Barnes CL and Gates KS, J. Org. Chem, 2014, 79, 7520–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Patterson LH, Craven MR, Fisher GR and Teesdalespittle P, Oncol. Res, 1994, 6, 533–538. [PubMed] [Google Scholar]
- 457.Mehibel M, Singh S, Cowen RL, Williams KJ and Stratford IJ, Oncol. Rep, 2016, 35, 1925–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Wilson WR, Denny WA, Pullen SM, Thompson KM, Li AE, Patterson LH and Lee HH, Br. J. Cancer, 1996, 74, S43–S47. [PMC free article] [PubMed] [Google Scholar]
- 459.Smith PJ, Blunt NJ, Desnoyers R, Giles Y and Patterson LH, Cancer Chemother. Pharmacol, 1997, 39, 455–461. [DOI] [PubMed] [Google Scholar]
- 460.Patterson LH and McKeown SR, Br. J. Cancer, 2000, 83, 1589–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Friery OP, Gallagher R, Murray MM, Hughes CM, Galligan ES, McIntyre IA, Patterson LH, Hirst DG and McKeown SR, Br. J. Cancer, 2000, 82, 1469–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Albertella MR, Loadman PM, Jones PH, Phillips RM, Rarnpling R, Burnet N, Alcock C, Anthoney A, Vjaters E, Dunk CR, Harris PA, Wong A, Lalani AS and Twelves CJ, Clin. Cancer Res, 2008, 14, 1096–1104. [DOI] [PubMed] [Google Scholar]
- 463.Papadopoulos KP, Goel S, Beeram M, Wong A, Desai K, Haigentz M, Milan ML, Mani S, Tolcher A, Lalani AS and Sarantopoulos J, Clin. Cancer Res, 2008, 14, 7110–7115. [DOI] [PubMed] [Google Scholar]
- 464.Wilson WR, Denny WA, Twigden SJ, Baguley BC and Probert JC, Br. J. Cancer, 1984, 49, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Wilson WR, Vanzijl P and Denny WA, Int. J. Radiat. Oncol., Biol., Phys, 1992, 22, 693–696. [DOI] [PubMed] [Google Scholar]
- 466.Lee HH, Wilson WR, Ferry DM, vanZijl P, Pullen SM and Denny WA, J. Med. Chem, 1996, 39, 2508–2517. [DOI] [PubMed] [Google Scholar]
- 467.Yin H, Xu YF, Qian XH, Li YL and Liu HW, Bioorg. Med. Chem. Lett, 2007, 17, 2166–2170. [DOI] [PubMed] [Google Scholar]
- 468.Supino R, Polizzi D, Pavesi R, Pratesi G, Guano F, Capranico G, Palumbo M, Sissi C, Richter S, Beggiolin G, Menta E, Pezzoni G, Spinelli S, Torriani D, Carenini N, Dal Bo L, Facchinetti F, Tortoreto M and Zunino F, Oncology, 2001, 61, 234–242. [DOI] [PubMed] [Google Scholar]
- 469.El-Dakdouki MH, Adamski N, Foster L, Hacker MP and Erhardt PW, J. Med. Chem, 2011, 54, 8224–8227. [DOI] [PubMed] [Google Scholar]
- 470.Pors K, Shnyder SD, Teesdale-Spittle PH, Hartley JA, Zloh M, Searcey M and Patterson LH, J. Med. Chem, 2006, 49, 7013–7023. [DOI] [PubMed] [Google Scholar]
- 471.White INH, Suzanger M, Mattocks AR, Bailey E, Farmer PB and Connors TA, Carcinogenesis, 1989, 10, 2113–2118. [DOI] [PubMed] [Google Scholar]
- 472.Tercel M, Wilson WR and Denny WA, J. Med. Chem, 1995, 38, 1247–1252. [DOI] [PubMed] [Google Scholar]
- 473.Kirkpatrick DL, Schroeder HL and Chandler KJ, Anti-Cancer Drugs, 1994, 4, 467–472. [DOI] [PubMed] [Google Scholar]
- 474.Highfield JA, Mehta LK, Parrick J, Candeias LP and Wardman P, J. Chem. Soc., Perkin Trans. 1, 1999, 2343–2351. [Google Scholar]
- 475.Mir LM, Tounekti O and Orlowski S, Gen. Pharmacol.- Vasc. S, 1996, 27, 745–748. [DOI] [PubMed] [Google Scholar]