Abstract
The chemokine CCL22 is predominantly produced by dendritic cells (DCs) and macrophages. CCL22 acts on CCR4-expressing cells including Th2 and Treg. Although a correlation between the CCL22-CCR4 axis and allergic diseases has been established, the mechanism of monocyte lineage-specific Ccl22 gene expression is largely unknown. In the current study, we investigated transcriptional regulation of the Ccl22 gene in DCs and macrophages. Using reporter assays, we identified the critical cis-enhancing elements at 21/−18 and −10/−4 in the Ccl22 promoter. Electrophoretic mobility shift assays proved that transcription factor PU.1 directly binds to the cis-elements. Knockdown of PU.1 markedly decreased Ccl22 expression in bone marrow-derived DCs (BMDCs) and BM macrophages (BMDMs). Chromatin immunoprecipitation assays revealed that PU.1 bound to the Ccl22 promoter in not only BMDCs and BMDMs, but also splenic DCs and peritoneal macrophages. LPS stimulation increased the amount of PU.1 recruited to the promoter, accompanied by upregulation of the Ccl22 mRNA level, which was diminished by Spi1 knockdown. We identified similar cis-elements on the human CCL22 promoter, which were bound with PU.1 in human monocytes. Taken together, these findings indicate that PU.1 transactivates the Ccl22 gene in DCs and macrophages by directly binding to the two elements in the promoter.
Introduction
Chemokines are chemo-attractant proteins which regulate the migration of different subsets of leukocytes during immune responses. CCL22 (also called macrophage-derived chemokine, MDC) is one of the chemokines mainly produced by myeloid cells such as macrophages and DCs in the steady state1. CCL22 and a closely related chemokine, CCL17, tandemly locate on human chromosome 16 and mouse chromosome 8. These chemokines share the same receptor, CCR4, predominantly expressed by Th2 cells. Therefore, these chemokines are considered to be key mediators in the development of Th2-dominant diseases such as atopic dermatitis (AD) and asthma. Indeed, serum levels of CCL22 and CCL17 are significantly elevated in AD patients2. It has been reported that CCL22 and CCR4 are also expressed in several types of tumor cells and Foxp3+ Treg, respectively. This CCL22-CCR4 axis is thought to attract Treg into the tumor microenvironment to evade the immune attack3–5. A CCR4 antagonist is useful for alleviating immune suppression and preventing tumor growth6,7.
PU.1 is a transcription factor specifically expressed in hematopoietic lineages. It is well known that expression level of PU.1 determines the cell fate during development of B cells, macrophages, and granulocytes from hematopoietic stem cells8–10. A study using PU.1 reporter mice demonstrated that PU.1 is highly expressed in myeloid DCs, in contrast to a low level expression of PU.1 in plasmacytoid DCs11. PU.1 binds to Ets motifs as a monomer and binds to EICE motifs as a heterodimer PU.1/IRF4 or PU.1/IRF8. C/EBPα and β, and c-Jun are also known to form a complex with PU.112. We have demonstrated that PU.1 regulates the expression of DC-characteristic genes including Cd80, Cd86, Ciita, Tnf-α, and Tnfsf413–16. Moreover, previous studies have shown that PU.1 is involved in macrophage-specific gene expression17–19.
Despite its critical involvement in several allergic diseases, the expression mechanism of the Ccl22 gene is not fully understood. In the current study, we found that PU.1 transactivates the Ccl22 gene both in DCs and macrophages via the two Ets motifs in the promoter.
Results
Ccl22 promoter activity in DC- and Macrophage-cell lines
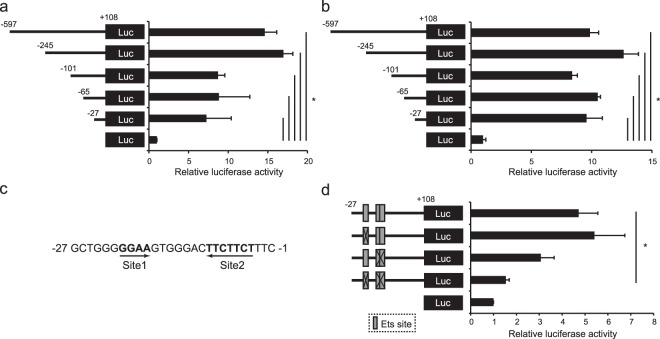
To determine the region critical for promoter activity, we generated a series of reporter vectors, and carried out luciferase assays by transfection of monocyte cell lines with the reporter plasmids. A luciferase assay using JAWSII, a mouse DC cell line, as the host showed that the luciferase activities were apparently detected as long as the segment from −27 to +108 were contained (Fig. 1a). A similar result was obtained by a reporter assay using a mouse macrophage cell line, RAW264.7 (Fig. 1b). These results indicate that −27/−1 region of the promoter plays a pivotal role in the gene expression of Ccl22 in both DCs and Macrophages. Then, we searched possible transcription factor-binding sites in this region using a database, JASPAR (http://jaspar.genereg.net/), and found that three Ets motifs: a typical Ets motif GGAA at −21/−18 (termed Site 1), and TTCTTCT containing two overlapping Ets-like motifs at −10/−4 (termed Site 2), are located within this region (Fig. 1c). To assess the necessity of these putative transcription factor-binding sites for promoter activity, we constructed mutant reporter vectors lacking Site1 and/or Site2. Although the introduction of a mutation at either Site 1 or Site 2 did not affect the luciferase activity, double mutation of both sites significantly reduced the activity (Fig. 1d). These results indicate that two sites containing Ets motifs, −21/−18 and −10/−4, are critical for transcriptional activity of the Ccl22 promoter.
Figure 1.
Analysis of cis-element in the Ccl22 promoter. JAWSII cells (a) and RAW264.7 cells (b) were transfected with reporter plasmids for renilla luciferase as an internal control and with reporter plasmids for firefly luciferase containing various lengths of the mouse Ccl22 promoter. (c) Sequences of the −27/−1 region of the mouse Ccl22 promoter. Two Ets motifs, designated Site1 and Site 2, are indicated in bold. (d) JAWSII cells were transfected with reporter plasmids of WT or mutant promoters lacking the Ets motif(s) at the indicated sites. All results are shown as means + S.D.s (n = 3). Similar results were obtained in three independent experiments. *p < 0.05.
PU.1 binding directly at the two proximal sites in the mouse Ccl22 promoter
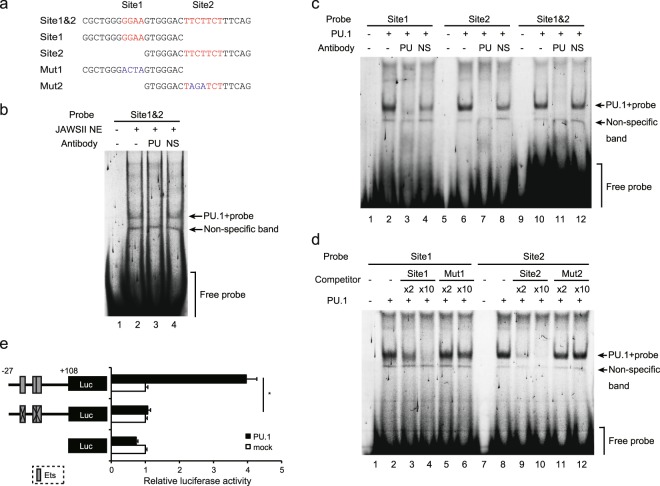
To identify transcription factor binding to the cis-enhancing elements containing Ets motifs (Site1 at −21/−18 and Site2 at −10/−4), EMSAs were performed using probes described in Fig. 2a. When a FLO-labeled probe containing two sites was mixed with the nuclear proteins extracted from JAWSII cells, several bands appeared on an electrophoretic gel (Fig. 2b lane 2). Among members of the Ets transcription factor family, PU.1/Spi1 especially plays critical roles in differentiation and cell type-specific gene expression of DCs and macrophages. Therefore, we hypothesized that PU.1 transactivates the Ccl22 gene that is apparently expressed in monocytic lineages. To confirm that PU.1 binds to the probe, an anti-PU.1 Ab was added into the mixture of the probe and nuclear extracts. As shown in Fig. 2b lane 3, the most major band disappeared in the presence of an anti-PU.1 Ab, whereas the addition of a non-specific Ab did not affect this major band (lane 4). This result indicates that PU.1 dominantly binds to the Ccl22 minimum promoter region in transcription factors expressed in monocytes. A clearer shift band containing PU.1 was detected in an EMSA, which was carried out by using a recombinant PU.1 protein generated using an in vitro transcription/translation system (Fig. 2c, lanes 10–12, and Supplemental Fig. 1). The specific band was also observed when FLO-labeled probes containing either Site 1 or Site 2 were used (Fig. 2c, lanes 2–4 and 6–8). We then performed an EMSA with competitors to identify the PU.1 binding sites in the probe sequence. The shifted band completely disappeared in the presence of an excess amount of the wild-type (WT) competitors, but not mutant competitors (Fig. 2d). These results suggest that PU.1 is capable of binding to −21/−18 GGAA and −10/−4 TTCTTCT sequences in the mouse Ccl22 promoter. To further elucidate the involvement of PU.1 and the cis-elements in mouse Ccl22 promoter activity, a luciferase assay was carried out using 293 T cells, because this non-hematopoietic cell line, in which PU.1 is not detected, is useful to evaluate the effect of exogenously expressed PU.1. Whereas the luciferase activity driven by the WT promoter was enhanced by co-expression of PU.1, introduction of mutations into both Site 1 and Site 2 completely diminished the transactivation effect of PU.1 on promoter activity (Fig. 2e). This result indicates that PU.1 transactivates the mouse Ccl22 gene through binding to Site 1 and Site 2.
Figure 2.
Identification of the PU.1 binding site. (a) Probes used in EMSAs. (b) The FLO-labeled Site 1&2 was incubated with the nuclear extracts prepared from JAWSII cells in the presence of either anti-PU.1 (PU) or non-specific (NS) Abs. (c) The FLO-labeled indicated probes were incubated with recombinant PU.1 protein in the presence of either anti-PU.1 (PU) or non-specific (NS) Abs. (d) The FLO-labeled indicated probes were incubated with recombinant PU.1 protein in the presence of 2-fold (×2) or 10-fold (×10) amounts of non-labeled identical WT or mutated competitor. After electrophoresis in 5% acrylamide gels, fluorescence was detected. (e) 293 T cells were transfected with either empty (mock) or PU.1-expression (PU.1) plasmids together with either WT or mutant reporter plasmids described in Fig. 1. At 48 h after transfection, luciferase and β-galactosidase activities were measured. Luciferase activities were normalized to β-galactosidase activities. Data are expressed as the ratio of the luciferase activity of the respective promoter-less plasmid-transfected cells. Results are shown as means + S.D.s (n = 3). Similar results were obtained in three independent experiments. *p < 0.05. Full-length gels with lower contrasts for (b–d), are included in Supplemental Information.
PU.1/Spi1 knockdown by siRNA transfection
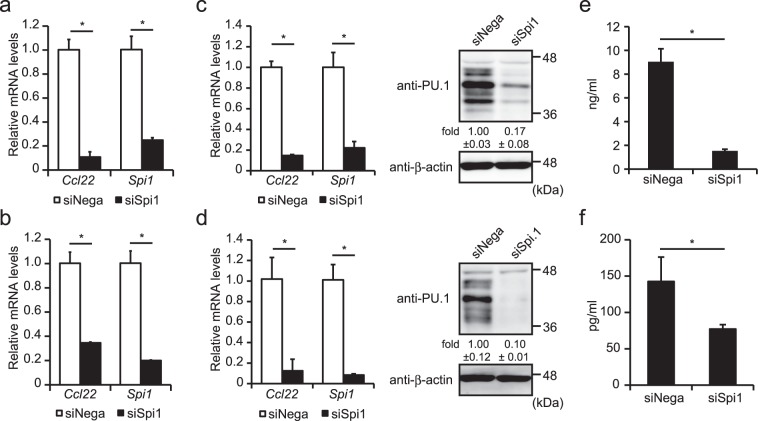
In order to determine the role of PU.1 in the expression of the Ccl22 gene, we introduced Spi1 siRNA into JAWSII and RAW264.7 cells. The introduction of Spi1 siRNA markedly decreased mRNA levels of Spi1 and Ccl22 in JAWSII (Fig. 3a) and RAW264.7 (Fig. 3b). Further, we confirmed the effect of Spi1 knockdown on Ccl22 expression in primary mouse DCs and macrophages generated from bone-marrow cells by cultivation in the presence of GM-CSF and M-CSF, respectively. Under the PU.1-knocked-down condition (both of mRNA and protein levels), the Ccl22 mRNA level was significantly decreased in BMDCs (Fig. 3c) and BMDMs (Fig. 3d). Furthermore, ELISA showed that the amount of CCL22 protein produced from BMDCs (Fig. 3e) and BMDMs (Fig. 3f) was markedly reduced by PU.1 knockdown. Although CCL22 production is enhanced during polarization to alternative activation macrophages (AAM), mRNA levels of other AAM makers, Fizz-1 and Ym-1 were not decreased but rather increased by Spi1 knockdown (Supplemental Fig. 2), suggesting that reduction of Ccl22 expression in Spi1 knockdown cells is not simply due to a reduction of these cells from a less of an AAM phenotype. To evaluate the effect of Spi1 knockdown-mediated suppression of CCL22 production on the capacity to cause migration of Th2 cells, we performed a migration assay. We observed that the number of Th2 cells migrated to Spi1-knocked-down DCs was moderately lower than that to control DCs (Supplemental Fig. 3). We introduced other Spi1 siRNAs into BMDCs to exclude the possibility of an off-target effect of Spi1 siRNA and found that these two siRNAs also significantly suppressed the Ccl22 mRNA level in parallel with the Spi1 knockdown level (Supplemental Fig. 4). Taken together, these results suggest that PU.1 is involved in the gene expression of mouse Ccl22 in DCs and macrophages.
Figure 3.
Effects of PU.1 knockdown on Ccl22 expression in mouse DCs and macrophages. JAWSII (a), RAW264.7 (b), BMDCs (c,e), and BMDMs (d and f) were transfected with either negative control siRNA (siNega) or Spi1 siRNA (siSpi1). At 48 h after transfection, relative mRNA levels were determined by quantitative RT-PCR after normalizing to mouse Gapdh mRNA levels. Data are expressed as the ratio of the expression level of the respective control siRNA-transfected cells. Western blotting analyses using anti-PU.1 Ab and anti-β-actin Ab were performed to evaluate the effect of Spi1 siRNA on PU.1 protein levels in BMDCs (c right) and BMDMs (d right). (e,f) The concentration of the CCL22 protein produced from either siNega or siSpi1 transfected BMDCs (e) or BMDMs (f) was determined as described in the Materials and Methods. Results are shown as means + S.D.s (n = 3). Similar results were obtained in three independent experiments. *p < 0.05.
Binding of PU.1 to the mouse Ccl22 promoter in DCs and macrophages
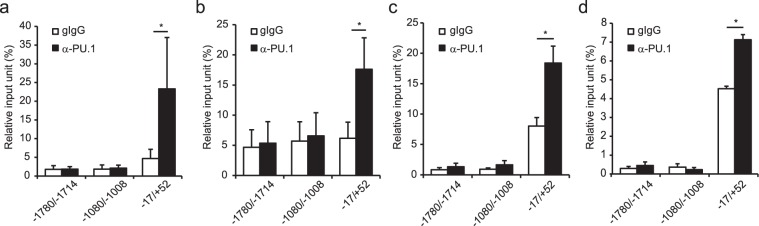
To investigate whether PU.1 binds to the Ccl22 gene in CCL22-expressing cells, we performed ChIP assays using BMDCs (Fig. 4a) and BMDMs (Fig. 4b). A significant amount of PU.1 binding to −17/+52 was detected in DCs and macrophages, whereas PU.1 did not bind to further upstream regions, −1780/−1714 and −1080/−1008 (Fig. 4a,b). These results demonstrate the specific binding of PU.1 to the proximal region of the transcription start site of the mouse Ccl22 gene.
Figure 4.
Analysis of the PU.1 binding region in the mouse Ccl22 promoter. ChIP assay was performed using either goat IgG (gIgG) or anti-PU.1 Ab (PU.1) in BMDCs (a), BMDMs (b), splenic DCs (c), and peritoneal macrophages (d). The amounts of immunoprecipitated chromatin were determined by quantitative PCR amplifying the indicated region of the Ccl22 promoter. Data are expressed as the percentage of the input for each ChIP assay. Results are shown as means + S.D.s (n > 3). Similar results were obtained in more than three independent experiments. *p < 0.05.
Furthermore, we carried out ChIP assays using DCs and macrophages freshly isolated from mice. As expected, significant and specific binding of PU.1 to −17/+52 of the Ccl22 promoter was detected in splenic DCs (Fig. 4c) and peritoneal macrophages (Fig. 4d). These results indicate that PU.1 trasactivates the Ccl22 gene by binding to the proximal region of the promoter in DCs and macrophages in vivo.
Involvement of IRFs in the gene expression of Ccl22
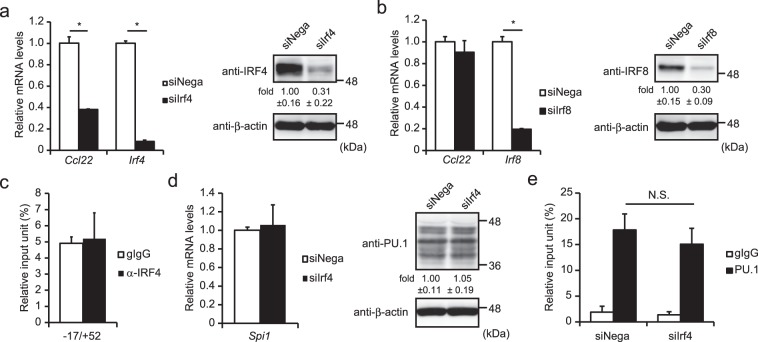
In addition to the role as a monomeric transcription factor, PU.1 also regulates the target genes forming a heterodimer with IRF4 or IRF8. In order to investigate the involvement of IRFs in the transcriptional regulation of Ccl22, we introduced siRNA against either Irf4 or Irf8 into BMDCs. Quantitative PCR showed that Irf4 knockdown significantly decreased the mRNA level of Ccl22 under the condition, in which levels of Irf4 mRNA and IRF4 protein were reduced (Fig. 5a). In contrast, Irf8 siRNA did not affect the Ccl22 mRNA level even though levels of Irf8 mRNA and IRF8 protein were decreased (Fig. 5b). To examine whether IRF4 co-localizes to the minimum promoter region of the Ccl22 gene with PU.1, we performed a ChIP assay using an anti-IRF4 Ab and quantified the amount of the immunoprecipitated chromosomal DNA by amplifying the region −17/+52. However, we did not detect significant binding of IRF4 around the identified PU.1 binding sites (Fig. 5c). We evaluated the effect of Irf4 siRNA on the expression and/or binding of PU.1 to −17/+52. As shown in Fig. 5d, levels of mRNA and protein of PU.1 were not affected by Irf4 siRNA. The amount of PU.1 binding to the region −17/+52 was not significantly reduced by Irf4 siRNA (Fig. 5e). These observations suggest that the involvement of IRF4 in the gene expression of Ccl22 is not due to the formation of a transcription complex with PU.1 around the transcriptional start site. IRF4 may transactivate the Ccl22 gene via another region on this gene or may indirectly regulate Ccl22 gene expression through transactivation of another transcription factor(s).
Figure 5.
Involvement of IRFs in the expression of Ccl22 in BMDCs. (a,b) BMDCs were transfected with negative control siRNA (siNega), Irf4 siRNA (siIrf4) (a), or Irf8 siRNA (siIrf8) (b). At 48 h after transfection, relative mRNA levels were determined by quantitative RT-PCR after normalizing to mouse Gapdh mRNA levels. Data are expressed as the ratio of the expression level of the respective control siRNA-transfected cells. Western blotting analyses were performed using transfectants, which were harvested at 48 h after transfection (right in a,b). (c) ChIP assay was performed by using either goat IgG (gIgG) or anti-IRF4 Ab (IRF4) in BMDCs. The amounts of immunoprecipitated chromatin were determined by quantitative PCR targeting the −17/+52 region of the Ccl22 promoter. Data are expressed as the percentage of the input for each ChIP assay. (d) mRNA level (left) and protein level (right) of PU.1 in control (siNega) or Irf4 knockdown cells (siIrf4). (e) PU.1 binding degree to −17/+52 in control (siNega) or Irf4 knockdown cells (siIrf4) determined by ChIP assay. Results are shown as means + S.D.s (n = 3). Similar results were obtained in three independent experiments. *p < 0.05.
Effect of TLR-mediated stimulation on the recruitment of PU.1 to the gene
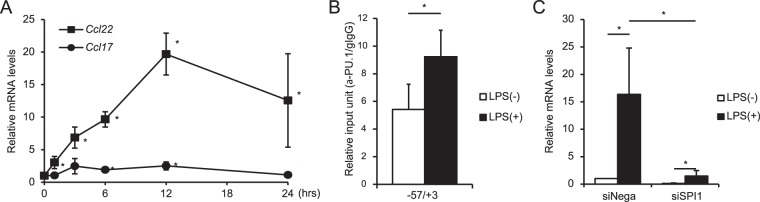
DCs and macrophages dramatically alter their gene expression in response to stimulation signaling such as TLR ligands. As shown in Fig. 6a, the mRNA level of Ccl22 was markedly increased in response to LPS stimulation, in contrast to a slight increase in the Ccl17 mRNA level. Thus, we performed a ChIP assay to investigate whether the LPS-induced upregulation of Ccl22 transcription reflects the PU.1 binding ability to the Ccl22 promoter in DCs. As shown in Fig. 6b, the PU.1 binding level at the region −17/+52 in mature BMDCs was approximately 1.8-fold of that of immature BMDCs. We also confirmed that the LPS-induced increase of the Ccl22 mRNA level was significantly attenuated by siRNA-mediated Spi1 knockdown (Fig. 6c). These results demonstrate that PU.1 recruitment to the Ccl22 promoter is augmented by LPS-dependent maturation, thereby increasing the transcription of Ccl22.
Figure 6.
Involvement of PU.1 in the expression of Ccl22 in mature BMDCs. (a) BMDCs were stimulated with 1 μg/ml LPS for the indicated period. Relative Ccl22 and Ccl17 mRNA levels were determined by quantitative RT-PCR after normalizing to mouse Gapdh mRNA levels. Data are expressed as the ratio of the expression level of the non-stimulated cells. (b) ChIP assay was performed using either goat IgG (gIgG) or anti-PU.1 Ab (α-PU.1) in BMDCs stimulated with 1 μg/ml LPS for 24 hours. The amounts of immunoprecipitated chromatin were determined by quantitative PCR targeting the −17/+52 region of the Ccl22 promoter. Data are expressed as the ratio of α-PU.1 to gIgG (α-PU.1/gIgG). (c) BMDCs were transfected with either negative control siRNA (siNega) or Spi1 siRNA (siSpi1). At 24 hours after transfection, cells were stimulated with 1 μg/ml LPS and further incubated for 24 hours. Relative mRNA levels were determined by quantitative RT-PCR after normalizing to mouse Gapdh mRNA levels. Data are expressed as the ratio of the expression level of the control siRNA-transfected immature BMDCs. Results are shown as means + S.D.s (n > 3). Similar results were obtained in more than three independent experiments.
Involvement of PU.1 in expression of the human CCL22 gene
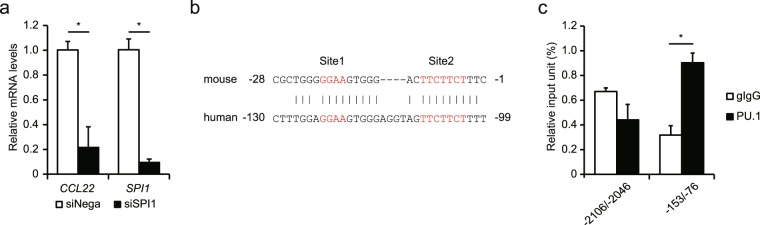
To investigate whether the role of PU.1 in Ccl22 expression in mouse monocytes is translatable to human biology, we evaluated the effect of SPI1 siRNA on CCL22 expression level in human monocyte cells, THP-1. As shown in Fig. 7a, SPI1 siRNA effectively suppressed SPI1 mRNA level and subsequently reduced CCL22 mRNA level. When nucleotide sequence of the human CCL22 gene was compared with that of mouse, we found that the elements similar to Site1 and Site2 exist on the human CCL22 promoter (Fig. 7b). By ChIP assay, we confirmed that PU.1 bound to this region in human monocyte cells (Fig. 7c). These results suggest that PU.1 is a common transactivator for the CCL22 (Ccl22) gene in human and mouse.
Figure 7.
Involvement of PU.1 in the expression of the human CCL22 gene. (a) mRNA levels of CCL22 and SPI1 in SPI1 siRNA (siSPI1) or control siRNA (siNega) introduced THP-1 cells. (b) Alignment of nucleotide sequences of human and mouse CCL22 genes. (c) ChIP assay was performed using either control IgG (gIgG) or anti-PU.1 Ab (α-PU.1) in THP-1 cells. The amounts of immunoprecipitated chromatin were determined by quantitative PCR targeting the −153/−76 (including cis-elements) or −2106/−2046 (cis-control) of the CCL22 promoter. Results are shown as means + S.D.s of three independent experiments.
Discussion
The Ets family transcription factor PU.1 is essential for myeloid cell development. Several studies have demonstrated that PU.1 plays critical roles in DC and macrophage functions13,14,16,20. In the current study, we provided evidence that PU.1 is involved in Ccl22 expression in DCs and macrophages.
Using a reporter assay, we demonstrated that transcriptional regulatory elements of the Ccl22 gene located within −27/+108 and two putative PU.1 binding elements were critical for transcriptional activity. Ccl22 expression was markedly decreased by Spi1 knockdown in all investigated cell types. ChIP assays showed that PU.1 bound to the proximal region of the Ccl22 promoter. From these results, we demonstrate that PU.1 is one of the most important transcription factors in the gene expression of Ccl22. Although transcriptional activity was significantly suppressed by mutation of the two PU.1 binding motifs in the luciferase assay, the activity was still higher than that of those without the promoter. This result indicates that another transcription factor(s) is involved in the gene expression of Ccl22 by binding within the −27/+108 region. Indeed, CCL22 is also expressed in non-hematopoietic cells, such as keratinocytes, which do not express PU.1. Previous studies have demonstrated that NF-κB and STAT1 are involved in the IFN-γ/TNF-α-dependent upregulation of CCL22 in human keratinocytes21,22. Considering that our reporter assay was carried out in a steady state, these molecules were not likely to enhance the transcriptional activity via binding to the −27/+108 region. It will be intriguing to further investigating which transcription factors regulate Ccl22 gene expression.
Even though ChIP assays were carried out using JAWSII and BMDCs without inducing maturation, we detected significant amount of PU.1 binding to the Ccl22 promoter. These results suggest that PU.1 plays a role as an activator of basal transcription of the Ccl22 gene in DCs, which produce a large amount of CCL22 even in the absence of inflammatory signaling. We also exhibited that PU.1 binding levels at the identified region were increased in response to LPS stimulation and subsequent upregulation of Ccl22 was significantly attenuated by Spi1 knockdown. These results suggest that PU.1 also plays an important role in the transactivation of Ccl22 upon DC maturation.
Here we used BMDMs and peritoneal macrophages as macrophage models. Macrophages are classified into two groups, M1 macrophages and M2 macrophages, depending on their expression of surface makers, cytokines, and chemokines. Ccl22 is one of the M2 macrophage-markers, and BMDMs generated with M-CSF are thought to exhibit an M2-like phenotype23. Indeed, we demonstrated that PU.1 is involved in the transcriptional regulation of Ccl22 by binding to the promoter in BMDMs. Recent studies have demonstrated that characteristic transcription factors like Spi-C and GATA6 are essential for differentiation of splenic and peritoneal macrophages, respectively24,25, suggesting that tissue-resident macrophages are functionally distinct. Although we showed that PU.1 marginally bound to the Ccl22 promoter in peritoneal macrophages, it is possible that PU.1 binding level is altered depending on where macrophages reside. Further analyses are required to elucidate this.
Previously, Chang et al. demonstrated that PU.1 promotes the development of Th926 and subpopulation of Th227 that express CCL22. PU.1 has also been described in promoting alternative activation of macrophages that produce CCL2228. In the current study, it was revealed that the CCL22 (Ccl22) gene, which has been reported to be under the control of PU.1, is directly transactivated by PU.1 via the cis-elements. Although Th cells were not examined in our study, the CCL22 gene may be a direct target of PU.1 in Th cells.
Previous studies have demonstrated that serum levels of CCL22 and CCL17 are significantly elevated and correlated with disease severity in patients with Atopic dermatitis2,29–31. Moreover, administration of CCR4 blocking antibody to the airway inflammation model abolished asthmatic symptoms32. According to these studies, targeting the interactions between CCL17/CCL22 and CCR4 may be a useful approach for controlling allergic diseases. We previously reported that injection of Spi1 siRNA significantly suppressed contact hypersensitivity of mice13. Considering that Th2-related genes such as Tnsf4 and Ccl22 are also transactivated by PU.1 in DCs, Spi1 knockdown may be a favorable strategy for allergic diseases.
Materials and Methods
Mice and cells
Bone marrow-derived DCs (BMDCs) and macrophages (BMDMs) were generated from BALB/c mice (Japan SLC, Hamamatsu, Japan) as previously described16,33,34.
Mouse DC line JAWSII, mouse macrophage line RAW264.7, and human monocyte cell line THP-1 were maintained as previously described13,16,35. All animal experiments were performed in accordance with the approved guidelines of the Institutional Review Board of Tokyo University of Science, Tokyo, Japan. The Animal Care and Use Committees of Tokyo University of Science specifically approved this study.
Isolation of splenic DCs and peritoneal macrophages
CD11c microbeads and an auto MACS Pro separator (Miltenyi Biotech, Tubingen, Germany) were used to isolate DCs from mouse spleen. Peritoneal macrophages were obtained as adhesive cells by incubating mouse peritoneal cells with 10% FCS-DMEM for 1 hour.
Introduction of siRNA into cells
Spi1 siRNA (Stealth Select RNAi, Sfpi1-MSS247676), Irf4 siRNA (MSS205499), Irf8 siRNA (MSS236848), SPI1 siRNA (SFPI1-HSS186060) and control siRNA purchased from Invitrogen (Carlsbad, CA) were introduced into BMDCs or BMDMs as previously described16.
Quantitative RT-PCR
Quantitative RT-PCR was performed as previously described36. The TaqMan primers are listed as follows: Spi1, Mm00488142_m1; Irf4, Mm00516431_m1; Irf8, Mm00492567_m1; Gapdh, 4352339E; SPI1, Hs02786711_m1; GAPDH, 4326317E. The nucleotide sequences of primers used for detection of the mouse Ccl22 and human CCL22 mRNAs are as follows: mCcl22-forward; 5′-AAGCCTGGCGTTGTTTTGAT-3′, mCcl22-reverse; 5′-CCTGGGATCGGCACAGATA-3′, hCCL22-forward; 5′-CCCTACGGCGCCAACAT-3′, and hCCL22-reverse; 5′-CAGACGGTAACGGACGTAATCA-3′.
Western blotting
Western blotting analysis was performed as previously described20. Following antibodies were used: anti-PU.1 antibody (D-19), anti-IRF4 antibody (M-17), and anti-IRF8 antibody (C-19), (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-β-actin antibody (AC-15, Sigma-Aldrich).
ELISA
The concentration of mouse CCL22 protein was determined using an ELISA kit (MCC220, R&D systems, Minneapolis, MN).
Luciferase assay
A series of reporter plasmids carrying the wild type or mutant mouse Ccl22 promoters were generated as previously described16 using primer sets listed in Supplemental Table 1.
Transfections of JAWSII, RAW264.7, and 293 T cells were performed as previously described16. After 48 hours, luciferase activity was determined using ARVO Light (Perkin Elmer, Waltham, MA) and a Dual-luciferase assay kit (Promega), as previously described37.
EMSA
Nuclear proteins were prepared from JAWSII cells. PU.1 protein was synthesized using a TNT T7 quick-coupled transcription/translation system (Promega) and pCR-PU.1 as a template. After incubation of the proteins and fluorescein-labeled oligonucleotides, electrophoretic mobility shift assay was performed as previously described16,38.
ChIP assay
ChIP assays were performed as previously described37. Antibodies against PU.1 and IRF4 were the same as those used in Western blotting analysis. Goat IgG (#02-6202, Invitrogen) was used as control antibody. The amount of immunoprecipitated or input DNA was quantified by a real-time PCR system using the primer sets (see Supplementary Table 2).
Statistical analysis
Statistical analysis was performed as previously described16.
Supplementary information
Acknowledgements
We are grateful to the members of Laboratory of the Molecular and Cellular Immunology (Tokyo University of Science) for constructive discussions and technical support. This work was supported by the Funding Program for Next-Generation World-Leading Researchers from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to CN #LS111), a Grant-in-Aid for Challenging Exploratory Research (CN), Grant-in-Aid for Young Scientists (B) (KK and TY), the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (Translational Research Center, Tokyo University of Science), the Tokyo Biochemical Research Foundation (CN), and the Tojuro Iijima Foundation for Food Science and Technology (CN and TY), and the Takeda Science Foundation (CN). K.K. is supported by Postdoctoral Fellowships from Tokyo University of Science (from 2014 to 2015) and by JSPS Research Fellowships for Young Scientists (#3241; from 2016).
Author Contributions
T.Y. designed research, performed experiments, and prepared the manuscript; S.N., K.N., Y.U. and K.K. performed experiments and analyzed data; C.N. designed research and prepared the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37894-9.
References
- 1.Vissers JL, et al. Quantitative analysis of chemokine expression by dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2001;69:785–793. [PubMed] [Google Scholar]
- 2.Shimada Y, Takehara K, Sato S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J Dermatol Sci. 2004;34:201–208. doi: 10.1016/j.jdermsci.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Li YQ, et al. Tumor secretion of CCL22 activates intratumoral Treg infiltration and is independent prognostic predictor of breast cancer. PLoS One. 2013;8:e76379. doi: 10.1371/journal.pone.0076379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizukami Y, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3 + regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama T, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to infiltration of regulatory T cells in esophageal squamous cell carcinoma. Dis Esophagus. 2010;23:422–429. doi: 10.1111/j.1442-2050.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 6.Bayry J, Tartour E, Tough DF. Targeting CCR4 as an emerging strategy for cancer therapy and vaccines. Trends Pharmacol Sci. 2014;35:163–165. doi: 10.1016/j.tips.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Pere H, et al. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood. 2011;118:4853–4862. doi: 10.1182/blood-2011-01-329656. [DOI] [PubMed] [Google Scholar]
- 8.DeKoter, R. P. & Singh, H. Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science288, 1439–1441, doi:8531 (2000). [DOI] [PubMed]
- 9.Singh H, DeKoter RP, Walsh JC. PU.1, a shared transcriptional regulator of lymphoid and myeloid cell fates. Cold Spring Harb Symp Quant Biol. 1999;64:13–20. doi: 10.1101/sqb.1999.64.13. [DOI] [PubMed] [Google Scholar]
- 10.Dahl R, et al. Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPalpha ratio and granulocyte colony-stimulating factor. Nat Immunol. 2003;4:1029–1036. doi: 10.1038/ni973. [DOI] [PubMed] [Google Scholar]
- 11.Nutt SL, Metcalf D, D’Amico A, Polli M, Wu L. Dynamic regulation of PU.1 expression in multipotent hematopoietic progenitors. J Exp Med. 2005;201:221–231. doi: 10.1084/jem.20041535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burda P, Laslo P, Stopka T. The role of PU.1 and GATA-1 transcription factors during normal and leukemogenic hematopoiesis. Leukemia. 2010;24:1249–1257. doi: 10.1038/leu.2010.104. [DOI] [PubMed] [Google Scholar]
- 13.Kanada S, et al. Critical role of transcription factor PU.1 in the expression of CD80 and CD86 on dendritic cells. Blood. 2011;117:2211–2222. doi: 10.1182/blood-2010-06-291898. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura N, et al. Role of PU.1 in MHC class II expression through transcriptional regulation of class II transactivator pI in dendritic cells. J Allergy Clin Immunol. 2012;129:814–824.e816. doi: 10.1016/j.jaci.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Fukai T, et al. Involvement of PU.1 in the transcriptional regulation of TNF-alpha. Biochem Biophys Res Commun. 2009;388:102–106. doi: 10.1016/j.bbrc.2009.07.126. [DOI] [PubMed] [Google Scholar]
- 16.Yashiro T, Hara M, Ogawa H, Okumura K, Nishiyama C. Critical Role of Transcription Factor PU.1 in the Function of the OX40L/TNFSF4 Promoter in Dendritic Cells. Sci Rep. 2016;6:34825. doi: 10.1038/srep34825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hume, D. A. et al. Regulation of CSF-1 receptor expression. Mol Reprod Dev46, 46–52; discussion 52–43, https://doi.org/10.1002/(SICI)1098-2795(199701)46:1<46::AID-MRD8>3.0.CO;2-R (1997). [DOI] [PubMed]
- 18.Ross IL, Yue X, Ostrowski MC, Hume DA. Interaction between PU.1 and another Ets family transcription factor promotes macrophage-specific Basal transcription initiation. J Biol Chem. 1998;273:6662–6669. doi: 10.1074/jbc.273.12.6662. [DOI] [PubMed] [Google Scholar]
- 19.Weigelt K, Lichtinger M, Rehli M, Langmann T. Transcriptomic profiling identifies a PU.1 regulatory network in macrophages. Biochem Biophys Res Commun. 2009;380:308–312. doi: 10.1016/j.bbrc.2009.01.067. [DOI] [PubMed] [Google Scholar]
- 20.Yashiro T, Kubo M, Ogawa H, Okumura K, Nishiyama C. PU.1 Suppresses Th2 Cytokine Expression via Silencing of GATA3 Transcription in Dendritic Cells. PLoS One. 2015;10:e0137699. doi: 10.1371/journal.pone.0137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi XF, et al. The adenylyl cyclase-cAMP system suppresses TARC/CCL17 and MDC/CCL22 production through p38 MAPK and NF-kappaB in HaCaT keratinocytes. Mol Immunol. 2009;46:1925–1934. doi: 10.1016/j.molimm.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Kwon DJ, et al. Casuarinin suppresses TARC/CCL17 and MDC/CCL22 production via blockade of NF-kappaB and STAT1 activation in HaCaT cells. Biochem Biophys Res Commun. 2012;417:1254–1259. doi: 10.1016/j.bbrc.2011.12.119. [DOI] [PubMed] [Google Scholar]
- 23.Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178:5245–5252. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 24.Kohyama M, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457:318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang HC, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang HC, et al. PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity. 2005;22:693–703. doi: 10.1016/j.immuni.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Qian F, et al. The transcription factor PU.1 promotes alternative macrophage polarization and asthmatic airway inflammation. J Mol Cell Biol. 2015;7:557–567. doi: 10.1093/jmcb/mjv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakinuma T, et al. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001;107:535–541. doi: 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- 30.Kakinuma T, et al. Serum macrophage-derived chemokine (MDC) levels are closely related with the disease activity of atopic dermatitis. Clin Exp Immunol. 2002;127:270–273. doi: 10.1046/j.1365-2249.2002.01727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jahnz-Rozyk K, Targowski T, Paluchowska E, Owczarek W, Kucharczyk A. Serum thymus and activation-regulated chemokine, macrophage-derived chemokine and eotaxin as markers of severity of atopic dermatitis. Allergy. 2005;60:685–688. doi: 10.1111/j.1398-9995.2005.00774.x. [DOI] [PubMed] [Google Scholar]
- 32.Perros F, Hoogsteden HC, Coyle AJ, Lambrecht BN, Hammad H. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy. 2009;64:995–1002. doi: 10.1111/j.1398-9995.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- 33.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 34.Weischenfeldt J, Porse B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. CSH Protoc 2008. 2008;pdb:prot5080. doi: 10.1101/pdb.prot5080. [DOI] [PubMed] [Google Scholar]
- 35.Takagi A, et al. Prolonged MHC class II expression and CIITA transcription in human keratinocytes. Biochem Biophys Res Commun. 2006;347:388–393. doi: 10.1016/j.bbrc.2006.05.215. [DOI] [PubMed] [Google Scholar]
- 36.Nagaoka, M. et al. The Orphan Nuclear Receptor NR4A3 Is Involved in the Function of DendriticCells. J Immunol, 10.4049/jimmunol.1601911 (2017). [DOI] [PubMed]
- 37.Maeda K, et al. FOG-1 represses GATA-1-dependent FcepsilonRI beta-chain transcription: transcriptional mechanism of mast-cell-specific gene expression in mice. Blood. 2006;108:262–269. doi: 10.1182/blood-2005-07-2878. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama C, et al. Regulation of human Fc epsilon RI alpha-chain gene expression by multiple transcription factors. J Immunol. 2002;168:4546–4552. doi: 10.4049/jimmunol.168.9.4546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.