Abstract
Background
Given the substantial period of time adults spend in their workplaces each day, these provide an opportune setting for interventions addressing modifiable behavioural risk factors for chronic disease. Previous reviews of trials of workplace‐based interventions suggest they can be effective in modifying a range of risk factors including diet, physical activity, obesity, risky alcohol use and tobacco use. However, such interventions are often poorly implemented in workplaces, limiting their impact on employee health. Identifying strategies that are effective in improving the implementation of workplace‐based interventions has the potential to improve their effects on health outcomes.
Objectives
To assess the effects of strategies for improving the implementation of workplace‐based policies or practices targeting diet, physical activity, obesity, tobacco use and alcohol use.
Secondary objectives were to assess the impact of such strategies on employee health behaviours, including dietary intake, physical activity, weight status, and alcohol and tobacco use; evaluate their cost‐effectiveness; and identify any unintended adverse effects of implementation strategies on workplaces or workplace staff.
Search methods
We searched the following electronic databases on 31 August 2017: CENTRAL; MEDLINE; MEDLINE In Process; the Campbell Library; PsycINFO; Education Resource Information Center (ERIC); Cumulative Index to Nursing and Allied Health Literature (CINAHL); and Scopus. We also handsearched all publications between August 2012 and September 2017 in two speciality journals: Implementation Science and Journal of Translational Behavioral Medicine. We conducted searches up to September 2017 in Dissertations and Theses, the WHO International Clinical Trials Registry Platform, and the US National Institutes of Health Registry. We screened the reference lists of included trials and contacted authors to identify other potentially relevant trials. We also consulted experts in the field to identify other relevant research.
Selection criteria
Implementation strategies were defined as strategies specifically employed to improve the implementation of health interventions into routine practice within specific settings. We included any trial with a parallel control group (randomised or non‐randomised) and conducted at any scale that compared strategies to support implementation of workplace policies or practices targeting diet, physical activity, obesity, risky alcohol use or tobacco use versus no intervention (i.e. wait‐list, usual practice or minimal support control) or another implementation strategy. Implementation strategies could include those identified by the Effective Practice and Organisation of Care (EPOC) taxonomy such as quality improvement initiatives and education and training, as well as other strategies. Implementation interventions could target policies or practices directly instituted in the workplace environment, as well as workplace‐instituted efforts encouraging the use of external health promotion services (e.g. gym membership subsidies).
Data collection and analysis
Review authors working in pairs independently performed citation screening, data extraction and 'Risk of bias' assessment, resolving disagreements via consensus or a third reviewer. We narratively synthesised findings for all included trials by first describing trial characteristics, participants, interventions and outcomes. We then described the effect size of the outcome measure for policy or practice implementation. We performed meta‐analysis of implementation outcomes for trials of comparable design and outcome.
Main results
We included six trials, four of which took place in the USA. Four trials employed randomised controlled trial (RCT) designs. Trials were conducted in workplaces from the manufacturing, industrial and services‐based sectors. The sample sizes of workplaces ranged from 12 to 114. Workplace policies and practices targeted included: healthy catering policies; point‐of‐purchase nutrition labelling; environmental supports for healthy eating and physical activity; tobacco control policies; weight management programmes; and adherence to guidelines for staff health promotion. All implementation interventions utilised multiple implementation strategies, the most common of which were educational meetings, tailored interventions and local consensus processes. Four trials compared an implementation strategy intervention with a no intervention control, one trial compared different implementation interventions, and one three‐arm trial compared two implementation strategies with each other and a control. Four trials reported a single implementation outcome, whilst the other two reported multiple outcomes. Investigators assessed outcomes using surveys, audits and environmental observations. We judged most trials to be at high risk of performance and detection bias and at unclear risk of reporting and attrition bias.
Of the five trials comparing implementation strategies with a no intervention control, pooled analysis was possible for three RCTs reporting continuous score‐based measures of implementation outcomes. The meta‐analysis found no difference in standardised effects (standardised mean difference (SMD) −0.01, 95% CI −0.32 to 0.30; 164 participants; 3 studies; low certainty evidence), suggesting no benefit of implementation support in improving policy or practice implementation, relative to control. Findings for other continuous or dichotomous implementation outcomes reported across these five trials were mixed. For the two non‐randomised trials examining comparative effectiveness, both reported improvements in implementation, favouring the more intensive implementation group (very low certainty evidence). Three trials examined the impact of implementation strategies on employee health behaviours, reporting mixed effects for diet and weight status (very low certainty evidence) and no effect for physical activity (very low certainty evidence) or tobacco use (low certainty evidence). One trial reported an increase in absolute workplace costs for health promotion in the implementation group (low certainty evidence). None of the included trials assessed adverse consequences. Limitations of the review included the small number of trials identified and the lack of consistent terminology applied in the implementation science field, which may have resulted in us overlooking potentially relevant trials in the search.
Authors' conclusions
Available evidence regarding the effectiveness of implementation strategies for improving implementation of health‐promoting policies and practices in the workplace setting is sparse and inconsistent. Low certainty evidence suggests that such strategies may make little or no difference on measures of implementation fidelity or different employee health behaviour outcomes. It is also unclear if such strategies are cost‐effective or have potential unintended adverse consequences. The limited number of trials identified suggests implementation research in the workplace setting is in its infancy, warranting further research to guide evidence translation in this setting.
Plain language summary
Improving the implementation of health‐promoting policies and practices in workplaces
The review question
Implementation strategies are meant to improve the adoption and integration of evidence‐based health interventions into routine policies and practices within specific settings. This review examined whether using these strategies improved the implementation of policies and practices in the workplace promoting healthy eating, physical activity, weight control, tobacco cessation and prevention of risky alcohol consumption. We also wanted to know if these strategies changed employees' health behaviours, caused any unintended effects, and were good value for money.
Background
Workplaces are a good setting for programmes that aim to improve health‐related behaviours like diet, physical activity and tobacco use, as adults spend a long time at work each day. However, these kinds of workplace‐based interventions are often poorly implemented, limiting their potential impact on employee health. Identifying strategies that are effective in improving the implementation of workplace‐based interventions has the potential to increase their impact on chronic disease prevention.
Study characteristics
We looked for studies that compared strategies to support the implementation of health‐promoting policies and practices in workplaces versus either no implementation strategy or different implementation strategies. Implementation strategies could include quality improvement initiatives, education, and training, among others. They could target policies or practices directly instituted in the workplace (e.g. workplace healthy catering policy), as well as workplace‐led efforts to encourage the use of external health promotion services (e.g. employee gym membership subsidies).
We found six eligible studies that investigated these strategies. Most took place in the USA, and workplaces were in the manufacturing, industrial and services‐based sectors. The number of workplaces examined in the studies ranged from 12 to 114. Implementation strategies in the six studies targeted different workplace policies and practices: healthy catering; point‐of‐purchase nutrition labelling; environmental prompts and supports for healthy eating and physical activity; tobacco control policies; sponsorship of employee weight management programmes; and adherence to national guidelines for staff health promotion. All studies used multiple strategies to improve the implementation of these policies and practices, including: educational meetings, interventions tailored to the specific needs of the workplace, and workplace consensus processes to implement a policy or practice. Four studies compared implementation strategies versus no intervention, one study compared different implementation strategies, and one study compared two implementation strategies with each other and a control. Researchers used surveys, audits and observations in workplaces to evaluate the effect of the strategies on the implementation of workplace policies and practices.
Search date
The evidence is current to 31 August 2017.
Key results
When we combined findings from three studies, we did not find any difference in the level of implementation of health‐promoting policies or practices between workplaces that received implementation strategy support versus those that did not, indicating that these strategies may make little to no difference. In the two trials comparing different implementation strategies, both reported improvements in implementation, favouring the more intensive implementation support group. Findings for effects on employee health behaviours were inconsistent and based on very low to low certainty evidence, so it is unclear whether the implementation strategies improved these outcomes. One of the included studies reported on cost, and none on the unintended adverse consequences of implementation strategies.
Certainty of evidence
There were few included studies, and they used inconsistent terminology to describe implementation strategies, limiting the strength of the evidence. We rated the certainty of the evidence as low for the effect of implementation strategies on policy and practice implementation, based on four randomised studies (where groups are randomly assigned to different study groups), and very low based on two non‐randomised studies. We also graded evidence on employee health behaviours and cost outcomes as low and very low. The findings of the review do not provide clear evidence regarding the impact of implementation strategies on workplace health‐promoting policy and practice implementation or on employee health behaviours. Further research is needed.
Summary of findings
Summary of findings for the main comparison. Summary of findings: strategies to improve the implementation of workplace‐based health promotion versus no implementation strategy.
| Strategies to improve the implementation of workplace‐based health promotion versus no implementation strategy: findings from randomised controlled trials | ||||||
|
Patient or population: workplace employees Settings: any work setting, of any employment sector and geographical location, staffed by employees Intervention: any strategy (e.g. educational materials; educational meetings; audit and feedback; local opinion leaders; tailored intervention) with the intention of improving the implementation of health‐promoting policies or practices targeting diet, physical activity, obesity, tobacco use and alcohol use in the workplace setting Comparison: no intervention e.g. wait‐list, usual practice or minimal support control (4 trials) Summary of findings for the main comparison were based on included randomised trials only. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with implementation interventions | |||||
| Implementation of workplace‐based policies or practices targeting diet, physical activity, obesity, tobacco use or alcohol use | The mean implementation score was 42.1a | The implementation score in the intervention group was 0.1 lower (3.8 lower to 3.5 higher) | Scores estimated using a standardised mean difference of −0.01 (−0.32 to 0.30) and a standard deviation of 11.8a | 191 workplaces (3 RCTs) |
⊕⊕⊝⊝ Lowb,c | One RCT that compared a workplace cafeteria nutrition intervention to a wait‐list control could not be synthesised in the meta‐analysis (Bandoni 2010). The trial reported a significant improvement on the single primary measure of implementation included in the review. One RCT reported additional dichotomous implementation outcomes that could not be synthesised in the meta‐analysis (Biener 1999). The trial reported a significant improvement on 1 out of 3 implementation outcomes included in the review. |
| Employee dietary intake | — | — | — | 19,419 participants (2 RCTs) |
⊕⊝⊝⊝ Very lowb,d,e | Mixed results were reported for this outcome. One RCT found a workplace cafeteria nutrition intervention effective in increasing fruit and vegetable consumption (Bandoni 2010). The other RCT found a worksite cancer control intervention effective in decreasing dietary intake of fat and increasing fruit and vegetable intake; however, it was not effective in increasing fibre consumption (Biener 1999). |
| Employee tobacco use | — | — | — | 18,205 participants (1 RCT) |
⊕⊕⊝⊝ lowb,c | One RCT which compared a worksite cancer control intervention to a minimal support control group reported no effect on smoking prevalence or the proportion of smokers who quit (Biener 1999). |
| Employee physical activity, weight status, and alcohol use | No RCTs reported these outcomes. | |||||
| Cost or cost‐effectiveness | — | — | — | 46 workplaces (1 RCT) |
⊕⊕⊝⊝ Lowc,f | One RCT reported an increase in employer costs in the implementation intervention group compared to the control group (Hannon 2012). |
| Unintended adverse effects | No RCTs reported this outcome. | |||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aWe used the postintervention mean and standard deviation of the control group from Hannon 2012 for the risk with no intervention to re‐express the SMD in terms of a mean implementation score. bDowngraded one level for risk of bias – most information comes from studies at unclear or high risk of bias for most criteria. cDowngraded one level for imprecision – sample size < 400. dDowngraded one level for inconsistency – results in both directions. eDowngraded one level for imprecision – the confidence intervals contained the null value and upper CI crosses SMD of 0.5. fDowngraded one level for high probability of publication bias – no other studies reported assessing cost‐effectiveness, selective reporting suspected.
Background
Description of the condition
Globally, approximately 40 million people die from chronic diseases each year (Haidong 2016). Some of the most prevalent modifiable risk factors for chronic disease are poor diet, physical inactivity, obesity, tobacco use and alcohol use (Lim 2012). Recent estimates across countries of the Organisation for Economic Co‐operation and Development (OECD) indicate that 40% and 43% of adults, respectively, do not consume vegetables or fruit on a daily basis (OECD 2017). International research suggests that 31% of adults globally are physically inactive (Hallal 2012), 13% are obese (body mass index (BMI) of 30 kg/m2 or more) (WHO 2016), and nearly one quarter (22%) smoke tobacco (WHO 2016). Moreover, the prevalence of heavy episodic alcohol use amongst adults is estimated to be 7.5% globally (WHO 2014). Cumulatively, these health risks represent a considerable burden to the community (Gakidou 2017).
The World Health Organization (WHO) has identified workplaces as valuable access points for providing interventions targeting chronic disease prevention (WHO 1981). As is the case in the community, modifiable, behavioural risk factors for chronic disease are prevalent in the workplace population, particularly among those with low‐income occupations (Scollo 2015). Workplaces provide an opportunity to reach a large number of adults for prolonged periods each working day. In 2014 alone, adults from OECD countries spent an average 36.8 hours per week in paid employment (OECD 2015). Furthermore, workplaces have existing infrastructure to provide multi‐level chronic disease prevention interventions to workers (Pelletier 2011). As such, interventions in this setting could make a significant contribution to population level reductions in chronic disease risk.
A number of systematic reviews and meta‐analyses have been published in the last 10 years regarding the effectiveness of workplace interventions for influencing health behaviours (Anderson 2009; Barr‐Anderson 2011; Benedict 2008; Cahill 2014; Fichtenberg 2002; Fishwick 2013; Freak‐Poli 2013; Geaney 2013; Kahn‐Marshall 2012; Maes 2012; Malik 2014; Mhurchu 2010; Rongen 2013; To 2013; Vuillemin 2011; Wong 2012). Reviews of workplace interventions targeting dietary behaviour have typically reported that such interventions yield modest improvements (Anderson 2009; Geaney 2013; Maes 2012; Mhurchu 2010), with similar results for interventions targeting tobacco use (Cahill 2014; Fichtenberg 2002; Fishwick 2013; Freak‐Poli 2013). Reviews of interventions targeting physical inactivity (Barr‐Anderson 2011; Malik 2014; To 2013; Vuillemin 2011; Wong 2012), obesity (Benedict 2008; Vuillemin 2011), and risky alcohol use (Ames 2011; Kolar 2015; Lee 2014) have reported mixed results, although such reviews have identified some effective programmes.
Description of the intervention
Implementation of effective workplace interventions is required if they are to benefit public health (Bero 1998). 'Implementation' is defined as the use of strategies to adopt and integrate evidence‐based health interventions to change practice patterns within specific settings (Glasgow 2012). Specifically, implementation research is the study of strategies designed to integrate health policies, practices or programmes within specific settings (e.g. workplaces) (Schillinger 2010). The US National Institutes of Health recognises implementation research as a component of the third stage ('T3') of the research translation process and as being essential if health innovations are to generate health improvements in the community (Glasgow 2012).
There are a range of potential strategies that can improve the likelihood of implementation of interventions to address diet, physical activity, obesity, tobacco use and alcohol use. In health services research, for example, the Cochrane Effective Practice and Organisation of Care (EPOC) Group has developed a taxonomy to characterise educational, behavioural, financial, regulatory and organisational strategies that can improve professional practice and health care (EPOC 2015). Specific implementation strategies included in the taxonomy include continuous quality improvement, educational materials, performance monitoring, local consensus processes and educational outreach visits (EPOC 2015). Schools (Nathan 2012), childcare services (Finch 2012; Jones 2015b), and sporting clubs (Kingsland 2015), among other settings, have utilised strategies to improve implementation of evidence‐based health interventions, and these could similarly be applied to workplaces to improve implementation of chronic disease prevention policies and practices.
How the intervention might work
Strategies that improve the implementation of workplace‐based health related policies and practices may be effective if they address the determinants impeding implementation. However, the determinants of policy and practice implementation are complex. A number of factors can impede implementation of health promotion initiatives in the workplace settings (Cherniack 2010). For example, when the US National Institutes of Health and the Centers for Disease Control and Prevention convened a workshop to advance utilisation of effective strategies to reduce chronic disease risks in the workplace, participants identified many barriers to worksite programme implementation (Sorensen 2011), including lack of employee interest, limited staff resources, cost, misalignment of incentives and insufficient support from management, while others have identified workplace financial, structural and cultural issues (Cherniack 2010). Moreover, theoretical implementation frameworks, including Damschroder's Consolidated Framework for Implementation Research (CFIR) (Damschroder 2009), the Theoretical Domains Framework (TDF) (Cane 2012) and the 'behaviour change wheel', also suggest that barriers to implementation are complex, operate at multiple levels and include individual, organisational, cultural, social, political and other macro‐levels factors (Damschroder 2009; Michie 2011). Similarly, such frameworks suggest that a sound understanding of implementation context and barriers is required in order to correctly apply implementation frameworks and select strategies that best address the determinants of implementation (Michie 2008; Michie 2011).
Why it is important to do this review
The lack of evidence regarding effective strategies to improve the implementation of health‐promoting policies and practices in workplaces represents a significant gap in the health promotion and implementation science literature. Future workplace interventions will benefit significantly from a comprehensive review of strategies to improve the implementation of evidence‐based interventions targeting diet, physical activity, obesity, tobacco use and alcohol use. This review will provide a summary of the current evidence base for health promotion practitioners, as well as other end‐users including employers or insurers, regarding the design and implementation of interventions to promote healthy behaviours within workplaces.
Objectives
To assess the effectiveness of strategies for improving the implementation of workplace‐based policies or practices targeting diet, physical activity, obesity, tobacco use and alcohol use.
Secondary objectives were to:
examine the impact of implementation strategies on employee health behaviours including diet, physical activity, weight status, tobacco use and alcohol use;
describe the cost or cost‐effectiveness of such strategies; and
describe any unintended adverse effects on workplaces or workplace staff.
Review conceptual model
We developed this review based on the conceptual model of implementation research that Proctor 2009 proposed. In the logic model (Figure 1), it is first necessary to identify workplace‐based interventions (policies or practices) to promote health, before then applying an implementation strategy to improve the likelihood of uptake and integration of the intervention into usual workplace practice ('implementation'). Implementation outcomes are used to assess the effects of the implementation strategy in achieving intervention implementation. The logic model assumes that intervention implementation is required for any benefits on individual employee health outcomes to be attributed to the intervention. The primary focus of the review, however, is the effects of implementation strategies on implementation outcomes. The model provides a broad logic to support evidence synthesis and interpretation and is not intended to represent a determinant or explanatory model of implementation interventions.
1.
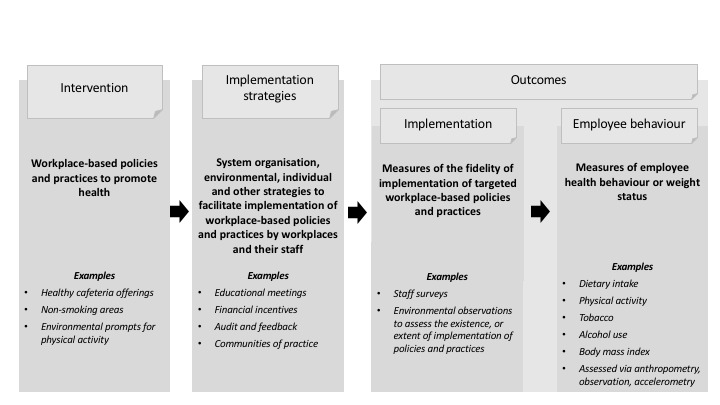
Review logic model
Methods
Criteria for considering studies for this review
Types of studies
A protocol prospectively describing the review methods has been previously published (Wolfenden 2016b).
Strategies to improve the implementation of policies or practices targeting settings‐based health promotion are often complex in nature, and researchers have evaluated them using a wide variety of methods and designs in settings such as schools and childcare services (Wolfenden 2016; Wolfenden 2017). While randomised controlled trials (RCTs) are considered to be the most reliable and robust studies for establishing intervention effectiveness, applying this design in complex public health interventions is often impractical or inappropriate (Glasgow 1999). Consequently, we anticipated there would be a paucity of randomised trials relevant to the review question. To overcome this, we included any trial (randomised or non‐randomised) with a parallel control group including the following trial designs.
RCTs and cluster‐RCTs.
Quasi‐RCTS and cluster quasi‐RCTs.
Controlled before‐and‐after trials (CBAs) and cluster‐CBAs.
Trials assessing any strategy to improve the implementation of policies or practices in workplace settings targeting diet, physical activity, obesity, tobacco use or alcohol use (or a combination of these) were eligible. To be included, trials were required to report the impact of a defined implementation strategy on an implementation outcome between experimental groups.
Types of participants
We included trials undertaken in any workplace setting, in any location and country, staffed by paid employees (who may or may not have also included unpaid volunteers). Workplaces could be from any employment sector, for example: manufacturing, health, education, business, information technology, retail, agriculture, construction or mining. Participants in trials could be those representing organisations, paid employees at any level of the workplace organisation, or other officials or organisations who could influence the implementation of workplace health‐promoting practices or policies. We excluded trials or arms of trials assessing implementation performed by research staff.
Types of interventions
We included trials that compared a strategy designed to improve the implementation of workplace‐based health‐promoting policies and practices targeting diet, physical activity, obesity, tobacco use and alcohol use versus either no intervention (i.e. wait‐list, usual practice or minimal support control) or a different implementation strategy. To be eligible for inclusion, trials had to include strategies to improve implementation by those involved in the delivery, uptake or use of policies or practices in workplaces. Implementation strategies could include quality improvement initiatives, education and training, performance feedback, prompts and reminders, implementation resources, financial incentives, penalties, communication and social marketing strategies, professional networking, the use of opinion leaders or implementation consensus processes, as well as other strategies included in the Effective Practice and Organisation of Care (EPOC) taxonomy (EPOC 2015). Implementation strategies could employ a single strategy (e.g. the use of educational materials only) or be multi‐component, employing several strategies (e.g. audit and feedback, educational materials and educational meetings). Additionally, implementation strategies could target policies and practices directly instituted in the workplace environment, as well as workplace‐instituted efforts to encourage the use of external services to promote employee health behaviour change (e.g. workplace subsidies for employee gym memberships fees). We still included strategies to support the implementation of workplace policies and practices that did not clearly fit within the predefined EPOC implementation strategy subcategories, classifying them as 'other' strategies.
Types of outcome measures
The review examined a range of primary and secondary outcomes relating to the implementation of workplace‐based policies and practices for health promotion. We defined 'implementation' as the use of strategies to integrate evidence‐based health interventions and to change practice patterns within specific settings (Glasgow 2012). We included implementation outcomes if they represented a measure of implementation fidelity, that is, a measure of delivery or execution of a workplace policy or practice. Such implementation outcomes typically represent assessments of the organisational environment, workplace policies, or professional behaviour of staff. To be included, outcomes had to report an action undertaken by a workplace or by workplace personnel. Outcomes could be categorical (e.g. the presence or absence of smoke‐free signage) or continuous (e.g. the number of healthy menu items in the workplace cafeteria). Implementation outcomes, expressed as a score, have been frequently reported in trials of implementation strategies in other settings (Alaimo 2015; Benjamin 2007; Saunders 2006; Sutherland 2017; Ward 2008). Often scores are derived by simply summing the number of targeted policies or practices that have been implemented (Jones 2015b); however, other tools combine items assessing implementation quality (e.g. a rating of how well a programme or policy was implemented), frequency (how often an organisational practice occurs) and other constructs such as duration (Naylor 2006; Perry 2004; Sallis 1997; Story 2000). We included any score‐based measure of implementation. Implementation outcome measures were not required to report any psychometric properties to be included.
We did not consider measures of individual employee health behaviours (e.g. proportion of employees with dietary intakes consistent with nutrition guidelines) to be implementation outcomes. Implementation could have occurred at any scale (local, national or international) and include any length of follow‐up of the implementation outcome. We included trials that reported only follow‐up data of an implementation outcome (i.e. no baseline data) in instances where the trial utilised a randomised design, as baseline values were assumed to have been equivalent (or differ only due to chance).
Primary outcomes
Any objective or subjective (self‐reported) measure of the implementation of a workplace policy or practice targeting diet, physical activity, obesity, tobacco use or alcohol use.
Such measures could include, for example, the percentage of workplaces implementing a healthy catering policy, or the mean number of health‐promoting practices implemented by workplaces to promote physical activity. Data on these outcomes could come from self‐reports (e.g. completed by workplace staff), direct observations by researchers, audits of workplace records or the workplace environment, or audits of data collected by external organisations (e.g. parent company or government). We excluded indirect measures of implementation, such as an intention to implement a workplace policy or practice, or change in attitude towards the implementation of a workplace policy or practice.
Secondary outcomes
We extracted data on secondary outcomes only for measures corresponding to reported implementation outcomes. For example, in a trial targeting workplace policies and practices to promote physical activity and healthy eating where trialists reported an implementation strategy and implementation outcome data only for the healthy eating aspect, we extracted secondary trial outcomes relating only to diet (e.g. foods or beverages consumed by workplace employees). Secondary outcomes could be measured objectively or subjectively (self‐reported), and they included the following.
Any measure of diet, physical activity (including sedentary behaviours), weight status, tobacco use or alcohol use. Such measures could be derived from any data source including direct observation, questionnaire, or anthropometric or biochemical assessments. We excluded studies examining malnutrition or malnourishment.
Estimates of absolute costs or any assessment of the cost‐effectiveness of strategies to improve the implementation of policies or practices in workplaces.
Any reported unintended adverse consequences of a strategy to improve the implementation of policies or practices in workplaces. This could include impacts on employee health (e.g. injury following the implementation of physical activity promoting practices), workplace operation or staff attitudes (e.g. impacts on staff motivation or cohesion).
Search methods for identification of studies
We performed a comprehensive search for both published and unpublished peer‐reviewed and grey literature by searching electronic databases, handsearching relevant journals and screening the reference lists of included trials. Articles published in any language were eligible, and there were no restrictions regarding article publication date.
Electronic searches
We searched the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue [8]) in the Cochrane Library (searched 31 August 2017);
MEDLINE Ovid (1946 to 31 August 2017);
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid (1946 to 31 August 2017);
The Campbell Library via Campbell website (2004 to 31 August 2017);
PsycINFO Ovid (1806 to 31 August 2017);
Education Resource Information Center (ERIC) Proquest (1966 to 31 August 2017);
Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO (1937 to 31 August 2017);
SCOPUS via Scopus website (1823 to 31 August 2017); and
Dissertations and Theses (1743 to 21 September 2017).
We adapted the MEDLINE search strategy for each database using database‐specific subject headings (Appendix 1). We included filters used in other systematic reviews for research design (Waters 2011), setting (Cahill 2014; Freak‐Poli 2013), physical activity and healthy eating (Dobbins 2013; Guerra 2014; Jaime 2009), obesity (Waters 2011), tobacco use prevention (Thomas 2013), and alcohol misuse (Foxcroft 2011). We also used a search filter for intervention (implementation strategies) that had been employed in previous Cochrane Reviews (Wolfenden 2016; Wolfenden 2017), and which was originally developed based on common terms in implementation and dissemination research (Rabin 2008; Rabin 2010).
Searching other resources
We screened the reference lists of included trials to identify potentially relevant studies and contacted the authors of included trials for other potentially relevant studies. We handsearched all publications between August 2012 and September 2017 in Implementation Science and the Journal of Translational Behavioral Medicine. We also conducted searches of the WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch) up to 26 September 2017, as well as the US National Institutes of Health registry (clinicaltrials.gov) up to 21 September 2017. We consulted with experts in the field to identify other relevant research and ongoing or unpublished trials and grey literature publications.
Data collection and analysis
Selection of studies
Two review authors (FS, AG, BP and TR) independently screened all titles and abstracts retrieved from the literature search using a standardised screening tool applied by the review team in previous systematic reviews (Wolfenden 2016; Wolfenden 2017), which authors had developed based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We obtained full texts of all potentially relevant or unclear articles, and pairs of authors (FS, AG or SG) independently reviewed each article against the inclusion criteria. At each stage, we resolved disagreements by discussion and, where required, by consulting a third review author (LW). We recorded reasons for exclusion of trials in the Characteristics of excluded studies table.
Data extraction and management
Pairs of review authors (SG, MF, SY, ABC, HTVZ, MK, CW, RH and JJ) independently extracted data using a data extraction form applied by the review team in previous systematic reviews (Wolfenden 2016; Wolfenden 2017), which was adapted from the Cochrane Public Health Group Methods Manual (CPHG 2011). We resolved any disagreements in data extraction by discussion or by consulting a third author (LW) where required. Where key data were missing from the trial reports, we attempted to contact the authors to obtain such information. Where multiple reports of the same trial were published, we extracted data from those deemed the most applicable. We extracted data comprehensively to cover all relevant outcomes and methods reported across studies. Two review authors (SG, SY) independently undertook classification of implementation strategies against the EPOC criteria (EPOC 2015). A third reviewer (LW) helped to resolve disagreements in classification.
We extracted and reported the following study characteristics.
Information regarding study design; date of publication; type of workplace; country; participant and workplace demographic and socioeconomic characteristics; number of experimental conditions; trial numbers and recruitment rate; and information to allow 'Risk of bias' assessment.
Information describing the characteristics of the intervention (i.e. the policy or practice subject to implementation) and the implementation strategy; the theoretical underpinning of the intervention (if noted in the trial); and information to allow implementation strategy classification against the EPOC Group Taxonomy of Interventions (EPOC 2015).
Information on trial primary and secondary outcomes including the data collection method; validity of measures used; unit of allocation and analysis; effect size (with 95% confidence interval and P value); and measures of outcome variability.
Information on the source(s) of research funding and potential conflicts of interest.
Assessment of risk of bias in included studies
The 'Risk of bias' assessment considered study design and reporting characteristics relevant to implementation outcomes of included trials. We used the Cochrane 'Risk of bias' tool (Appendix 2), which includes assessments based on the following domains: selection bias, performance bias, detection bias, attrition bias, and reporting bias (Higgins 2011). We included an additional criterion, 'potential confounding', for assessing the risk of bias in non‐randomised trial designs, as recommended in Chapter 13 of Higgins 2011, 'Including non‐randomised studies'. We assessed trials as having low, high, or unclear risk of bias for each risk of bias assessment domain, in accordance with Chapter 8 of Higgins 2011, 'Assessing risk of bias in included studies'. Two authors (MK and CW) assessed risk of bias independently for each trial, resolving any disagreement by discussion, or if required, by consulting a third author (JJ). We assessed secondary (non‐implementation) outcomes of the review in the same manner as that for implementation outcomes, and as reported in Appendix 3. We included additional criteria for cluster‐RCT designs in the assessment of these outcomes, including recruitment to cluster, baseline imbalance, loss of clusters, incorrect analysis, contamination and compatibility with individually randomised RCTs, in accordance with Chapter 16 of Higgins 2011, 'Special topics in statistics'.
Measures of treatment effect
We performed meta‐analysis of trials reporting score‐based measures of implementation, expressing treatment effects as a standardised mean difference (SMD) with 95% confidence intervals (CI) given variability in instruments used to assess implementation. We interpreted the magnitude of effect size using the benchmarks suggested by Cohen, considering an SMD of 0.2 a small effect; 0.5 a medium effect; and 0.8 a large effect (Cohen 1988). We performed meta‐analysis with Review Manager 5 (RevMan 5) software using data extracted (e.g. estimate of effect size and effect variability) from the trial reports and the generic inverse variance method using a random‐effects model (Higgins 2011; RevMan 2014). We did not need to transform any data for inclusion in the analyses. We did not undertake pooled analyses for other continuous (non‐score based) or dichotomous implementation outcomes given trial and outcome heterogeneity. For such trials, we reported measures of treatment effect as they were presented in the original manuscripts and synthesised them narratively.
Unit of analysis issues
Clustered studies
Within included trials, the appropriate unit of analysis could vary depending on the outcome reported. For implementation outcomes, workplaces were often the unit of allocation and analysis. However, for secondary outcomes such as measures of employee health behaviours, allocation at the workplace level and collection of data at the individual level (i.e. employees) was common. We examined all trials using cluster designs for any outcome for unit of analysis errors. We identified one trial, Bandoni 2010, that had not appropriately adjusted for clustering in the analysis of secondary trial outcomes, and we noted this in the 'Characteristics of included studies' table.
Studies with more than one treatment group
Two included trials had more than one treatment arm for assessment of implementation outcomes (Jones 2015; Parker 2010). Neither of the trials contributed to meta‐analysis, and we described the effects of the intervention across treatment arms narratively.
Dealing with missing data
In instances where data pertaining to trial participants, interventions, outcomes, results or methods were missing or unclear, we contacted the corresponding authors of the published trial to supply such information, including any additional information provided in the review as appropriate. We documented any evidence of potential selective reporting or incomplete reporting of trial data in the 'Risk of bias' tables.
Assessment of heterogeneity
For implementation outcomes pooled in the quantitative synthesis, we assessed heterogeneity by first visually inspecting forest plots for the extent to which CIs overlapped. Second, we conducted Chi2 tests, considering a P value of less than 0.05 to indicate statistical heterogeneity. Finally, we calculated the I2 statistic, considering an I2 value of more than 50% indicative of substantial heterogeneity. In these cases, review authors discussed the appropriateness of meta‐analysis until reaching a consensus. We did not perform meta‐analysis when the I2 statistic was more than 90%. Given the limited number of trials included in the meta‐analysis, we were unable to explore heterogeneity through subgroup analyses.
Assessment of reporting biases
Given the small number of included trials, we were not able to generate a funnel plot to visually inspect for asymmetry. We therefore assessed reporting bias by comparing published reports with information in trial registers and protocols, where such information was available. Where we suspected reporting bias (via assessment of risk of bias in included studies), we attempted to contact study authors and ask them to provide missing outcome data. We recorded instances of potential reporting bias in the 'Risk of bias' summary.
Data synthesis
Consistent with the approach of previous Cochrane Reviews on implementation strategies in the childcare and school setting (Wolfenden 2016; Wolfenden 2017), we synthesised trial findings based on the outcomes and comparisons reported. We narratively synthesised findings for all included trials by firstly describing trial characteristics, participants, interventions and outcomes. We then described the effects of implementation strategies for individual trials by reporting the effect size of the primary implementation outcomes. We focused on specified primary outcomes where available, as the intervention (implementation strategy) was designed to directly influence this outcome, and thus trials (should have been) powered to detect meaningful effects on these measures. Furthermore, pre‐specified primary (as opposed to secondary) outcomes are considered most appropriate for hypothesis testing. For trials with multiple follow‐up periods, we used data from the final follow‐up period reported.
We performed meta‐analysis where trials were reasonably homogeneous and contained equivalent research designs (e.g. randomised trials) and comparable outcomes measures and comparisons. We conducted meta‐analysis using RevMan 2014 software. We selected reported study estimates that adjusted for potential confounding variables for inclusion in meta‐analysis over reported estimates that did not adjust for potential confounding variables. We pooled data from primary implementation outcomes reported in trials. Where the trial authors in the published manuscripts did not identify a primary outcome measure, we assumed it was the implementation outcome they had used in the trial sample size calculation. In its absence, for trials reporting subscales of an overall implementation score (in addition to a total scale score), we used the total score as the primary outcome to provide a more comprehensive measure of implementation. When a trial reported a large number of implementation outcomes but without an identified primary outcome, we calculated standardised ('d') measures of effect size for each outcome, we ranked measures based on their size of effect, and we used the measure at the median.
Subgroup analysis and investigation of heterogeneity
We could not conduct quantitative examination of heterogeneity because there were only three trials in pooled analysis. We described the characteristics of included trials according to population, intervention, comparison, outcome and study design to establish clinical and methodological heterogeneity across included trials narratively. We used a threshold of implementation across 50 or more workplaces to represent implementation 'at scale' consistent with other reviews (Wolfenden 2016; Wolfenden 2017); however, as no trials included interventions delivered to 50 or more workplaces, we did not perform subgroup analyses based on the scale of implementation.
Sensitivity analysis
Given the small number of trials included in the meta‐analysis and the low I2 and lack of statistical heterogeneity, we did not perform sensitivity analysis by removing studies with a high risk of bias.
'Summary of findings' table
We generated a 'Summary of findings' table to present the key findings of included studies (see Table 1), based on recommendations of the Cochrane EPOC group and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included a list of primary and secondary outcomes in the review, a description of the intervention effect, the number of participants and trials addressing the outcome, and a grade for the overall certainty of evidence. We produced the 'Summary of findings' table for studies of RCT design only, which produced a comparison between an implementation intervention and a no‐intervention control (i.e. wait‐list, usual practice or minimal support control).
We graded the certainty of the body of evidence for each individual outcome from high to very low in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We conducted separate GRADE assessments for randomised trials that compared an implementation intervention versus no intervention and for non‐randomised trials that compared an implementation intervention versus an alternate intervention. Two review authors (RH and LW) used the GRADE system to independently assess the certainty of the body of evidence through consideration of study limitations, consistency of effect, imprecision, indirectness and publication bias. When these authors could not reach a consensus, a third review author (JJ) was consulted to resolve discrepancies.
Results
Description of studies
See Characteristics of included studies; Table 2 and Table 3: 'Summary of workplace settings, interventions, outcomes and effects for included trials'; Characteristics of excluded studies; and Characteristics of ongoing studies.
1. Summary of workplace settings, interventions, outcomes and effects for included trials: implementation strategies versus no intervention.
| Trial (study design) | Workplace setting | Intervention and comparison (sample sizes) | Implementation outcomes and effects | Secondary outcomes and effects |
|
Bandoni 2010 (RCT) |
Workplaces predominantly from industrial sector Region: Brazil |
Educational meetings and educational materials (15 workplaces; 630 employees) vs Wait‐list control (14 workplaces; 584 employees) |
Quantity fruits and vegetables in lunch meals (g/meal), measured via food service manager self‐reported survey (validity NR). Greater increase in intervention (adjusted MD 49.05 g, 95% CI 8.38 to 89.71) | Employee fruit and vegetable consumption (g/day), measured via self‐reported survey (validity NR). Slightly greater increase in intervention (adjusted effect estimate 11.75 g, 95% CI 2.73 to 20.77) |
|
Beresford 2010 (RCT) |
Small‐ to medium‐sized workplaces in manufacturing, transportation and utilities, and personal and household services industries Region: USA |
Tailored intervention; local opinion leaders; local consensus process and educational materials (17 workplaces; n employees NR) vs Wait‐list control (17 workplaces; n employees NR) |
Implementation of 11 practices supportive of healthy eating, physical activity and weight control, measured via scores derived from environmental assessment checklist (validity NR). NS difference 9/11 practices. Higher scores in intervention for notices encouraging physical activity (adjusted effect estimate 0.33, 95% CI 0.00 to 0.85) and healthy eating (0.40, 95% CI 0.00 to 1.46) | NR |
|
Biener 1999 (RCT) |
Workplaces from manufacturing, communications, public service and utilities sectors Region: USA |
Local opinion leaders; local consensus process; educational meetings; and educational outreach visits (55 workplaces; 8914 employees) vs Minimal support control comprising printed health promotion materials (56 workplaces; 9291 employees) |
Workplace tobacco control policy restrictiveness and compliance, measured via scores derived from employee self‐reported survey (validity NR). NS difference restrictiveness: adjusted difference 0.01 (SE 0.09) or compliance: 0.03 (SE 0.07) % workplaces reporting improvement in cafeteria and vending machine nutrition labelling and healthy catering policy, measured via organisational informant interview (validity NR). NS difference cafeteria labelling (MD 13.4%, P = 0.72) or catering policy (MD 10.9%, P = 0.30). Greater improvement in intervention vending machine labelling (MD 39.6%, P < 0.01) |
Employee smoking prevalence and % of quitters, measured via self‐reported survey (validity NR). NS difference in prevalence (difference −0.66%, 95% CI −3.0 to 1.2) or quit rate (1.53%, 95% CI −1.0 to 3.7) % dietary energy from fat, % increase in fibre (g/1000 kcal, and % increase in fruit and vegetables (servings/day), measured via Block FFQ (validated). Greater increase in intervention fruit and vegetables (adjusted increase 5.6%, SE 1.3, P < 0.001) and % dietary fat lower (adjusted difference −0.35%, SE 0.16, P < 0.05). NS difference fibre (adjusted increase 1.7%, SE 0.87, P > 0.05) |
|
Hannon 2012 (RCT) |
Low‐wage, mid‐sized workplaces predominantly from education, health, manufacturing and retail sectors Region: USA |
Audit and feedback; clinical practice guidelines; local consensus process; educational materials; educational outreach; and tailored intervention (23 workplaces; n employees NR) vs Wait‐list control (23 workplaces; n employees NR) |
Implementation of 16 best practices for health promotion recommended by CPSTF Community Guide; measured via score derived from workplace self‐reported survey (validity NR). NS difference in total score mean (SD): intervention baseline 31.5 (8.3), follow‐up 39.2 (11.2) vs control baseline 36.8 (11.7), follow‐up 42.1 (11.8), P = 0.33 | Workplace costs (per worker) for health promotion, measured via workplace self‐reported survey (validity NR). Costs increased slightly more in intervention, mean total costs (range): intervention baseline USD 8.30 (0.00 to 35.00), follow‐up USD 10.10 (0.00 to 53.00) vs control baseline USD 11.00 (0.00 to 53.00), follow‐up USD 11.80 (1.00 to 43.00) |
|
Parker 2010 (non‐randomised, controlled trial) |
Manufacturing, research and development and administrative facilities from a large science and technology company Region: USA |
Moderate‐intensity intervention: tailored intervention; local opinion leaders; educational meetings (4 workplaces; 382 employees) or High‐intensity intervention: moderate strategies + local consensus process; audit and feedback; monitoring of performance; and other (5 workplaces; 1520 employees) vs Wait‐list control (3 workplaces; 529 employees) |
Implementation of policies and practices promoting healthy eating, physical activity and weight control, measured via scores derived from EAT (validated tool). Relative to control, greater increase in total EAT score for moderate intensity intervention (contrast estimate 9.68, SE 3.48, P = 0.009) and high intensity intervention (16.99, SE 3.37, P < 0.001) | % employees classified high risk poor nutrition and poor physical activity, measured via self‐reported HRA survey. Relative to control, NS difference for poor nutrition: moderate (estimate −7.7%, P = 0.068), high (−4.6%, P = 0.16), or poor physical activity: moderate (−1.6%, P = 0.77) or high (−0.7%, P = 0.89) Weight (kg), BMI (kg/m2) and % employees overweight or obese. Relative to control, greater reduction in weight for moderate (estimate −2.1, P = 0.033), high (−1.5, P = 0.015) and in BMI moderate (−0.3, P = 0.034), high (−0.2, P = 0.008). NS difference % obese: moderate (0.1%, P = 0.88), high (0.3%, P =0.95), or % overweight: moderate (4.4%, P = 0.47); high (5.5%, P = 0.22) |
BMI: body mass index; CI: confidence interval; CPSTF: Community Preventive Services Task Force, US Department of Health and Human Services; EAT: environmental assessment tool;FFQ: food frequency questionnaire; HRA: health risk assessment; MD: mean difference; NR: not reported; NS: not significant; RCT: randomised controlled trial; SD: standard deviation; SE: standard error.
2. Summary of workplace settings, interventions, outcomes and effects for included trials: implementation strategy versus another implementation strategy.
| Trial (study design) | Workplace setting | Intervention and comparison (sample sizes) | Implementation outcomes and effects | Secondary outcomes and effects |
|
Jones 2015 (non‐randomised trial) |
NHS trusts including ambulance, mental health and acute care Region: UK |
Cohort C1: clinical practice guidelines and audit and feedback (26 workplaces; n employees NR) vs Cohort B: clinical practice guidelines; audit and feedback; educational meetings; and tailored intervention (36 workplaces; n employees NR) |
Implementation of 6 sets NICE guidance for workplace health promotion addressing: obesity, physical activity, smoking, long‐term sickness absence and mental health, measured via score on organisational audit self‐reported by staff (validity NR). Greater increase in score for cohort B (adjusted median total score difference: 22.17 vs 4.94, P < 0.001) | NR |
|
Parker 2010 (non‐randomised controlled trial) |
Manufacturing, research and development and administrative facilities from a large science and technology company Region: USA |
Moderate‐intensity intervention: tailored intervention; local opinion leaders; educational meetings (4 workplaces; 382 employees) or High‐intensity intervention: moderate strategies + local consensus process; audit and feedback; monitoring of performance; and other (5 workplaces; 1520 employees) |
Implementation of workplace policies and practices promoting healthy eating, physical activity and weight control, measured via scores derived from EAT (validated tool). Greater increase in total EAT score for high‐intensity intervention (contrast estimate 7.31, SE 3.10, P = 0.024) | NR |
EAT: environmental assessment tool; NHS: National Health Service; NICE: National Institute of Clinical Excellence; NR: not reported; SE: standard error.
Results of the search
The Characteristics of included studies tables present full details for each of the included trials, and Table 2 and Table 3 contain a summary of workplace settings, interventions, outcomes and effects. We report reasons for excluding trials at full‐text review in the Characteristics of excluded studies table, and we identify eligible ongoing trials in the Characteristics of ongoing studies table. The electronic search conducted to 31 August 2017 yielded 13,166 records (Figure 2). Additionally, we identified a further 2097 records from other sources. Following screening of titles and abstracts, we obtained the full‐texts of 334 articles for further review. We initially identified 16 individual trials as eligible for inclusion in the review. Of these, 5 trials (described in 6 articles) were ongoing studies, so we designated 11 trials to undergo data extraction. However, we later excluded five of these trials during data extraction after further review revealed them as ineligible (all based on inappropriate outcomes). We finally included six individual trials (described in 16 articles) in the review.
2.
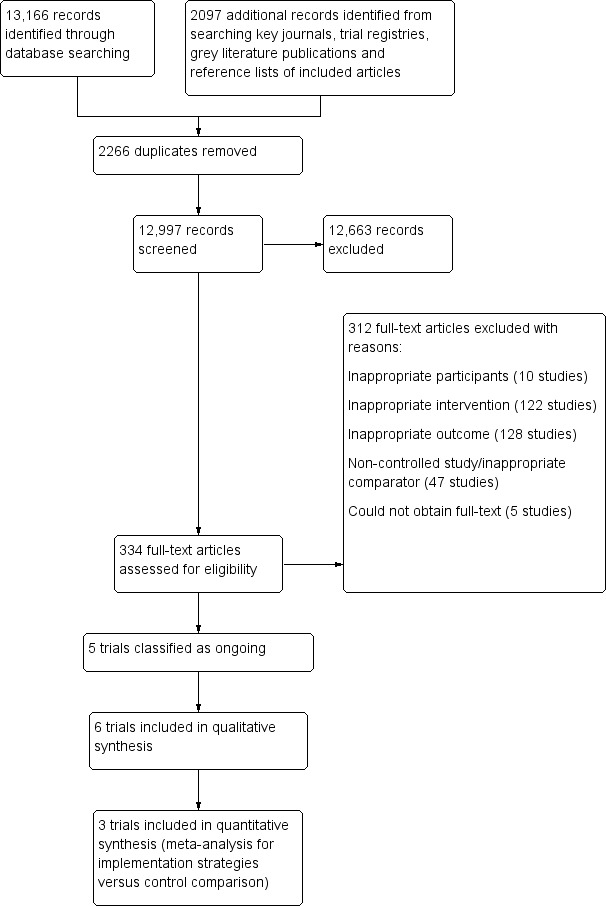
Study selection flow diagram.
We contacted the authors of five included trials to obtain additional information on participants, interventions and outcomes where such information was unclear or missing. We reported specific information that trial authors provided in the Characteristics of included studies table for respective trials. We also contacted the authors of two ongoing trials with published baseline or protocol papers regarding the availability of follow‐up data. At the time of contact, both authors indicated follow‐up data were not yet available, so we listed these trials as ongoing studies in the review.
Included studies
Types of studies
Four trials were in the USA (Beresford 2010; Biener 1999; Hannon 2012; Parker 2010), one in England (Jones 2015), and one in Brazil (Bandoni 2010). Trials took place between 1990 and 2013. Four employed RCT designs in the assessment of implementation outcomes (Bandoni 2010; Beresford 2010; Biener 1999; Hannon 2012), and the remaining two trials were non‐RCT designs (Jones 2015; Parker 2010). Trial designs used to evaluate implementation outcomes differed at times from those used to assess behavioural impacts of interventions on employees. For example, Bandoni 2010 assessed workplace level implementation outcomes (RCT design), as well as the impact of the intervention on individual employee outcomes located within workplaces (cluster‐RCT design). Trials varied in the types of participants, implementation strategies and outcomes reported.
Participants
The number of workplaces involved ranged from 12 in Parker 2010 to 114 in Biener 1999; however, four of the six trials included fewer than 50 workplaces (Bandoni 2010; Beresford 2010; Hannon 2012; Parker 2010). Biener 1999 and Jones 2015 allocated 50 or more workplaces to the intervention condition (implementation strategies); however, for both trials, we could extract implementation outcomes for the review for fewer than 50 workplaces. Trials were in workplaces from the manufacturing and industrial sector (Bandoni 2010; Beresford 2010; Biener 1999; Hannon 2012; Parker 2010), as well the services sector (health, education, retail, public service and personal and household services) (Beresford 2010; Biener 1999; Hannon 2012; Jones 2015). One trial took place in specifically low‐wage workplaces (Hannon 2012), whilst trial authors classified employees as predominantly blue‐collar workers in a further three trials (Beresford 2010; Biener 1999; Parker 2010). Most employees were men in three trials (Bandoni 2010; Biener 1999; Parker 2010), whereas in two trials the proportion of male and female employees was approximately equal (Beresford 2010; Hannon 2012). In three trials most employees were white (Beresford 2010; Biener 1999; Parker 2010), whilst one trial included a significant proportion (39%) of employees from ethnic minority groups (Hannon 2012), and one trial was conducted in a non‐white population (Brazil) (Bandoni 2010). Jones 2015 did not describe the socioeconomic or demographic characteristics of workplace employees or the workplace locality.
Interventions
All trials examined multi‐component implementation strategies (i.e. interventions using multiple implementation strategies). Table 4 shows the EPOC taxonomy descriptors for implementation strategies employed by included trials. The policies and practices within workplaces targeted by implementation strategies included: the availability of healthy food options (Bandoni 2010; Beresford 2010; Hannon 2012; Parker 2010); healthy catering policies (Biener 1999; Parker 2010); point‐of‐purchase nutrition labelling (Biener 1999); environmental prompts for healthy eating and physical activity such as posters and signs (Beresford 2010; Hannon 2012; Parker 2010); environmental supports for physical activity such as bike racks and fitness equipment (Beresford 2010; Hannon 2012; Parker 2010); tobacco control policies (Biener 1999; Hannon 2012); sponsorship of weight management programmes (Beresford 2010; Hannon 2012; Parker 2010); and adherence to national guidelines for staff health promotion (Jones 2015). The most common implementation strategies included educational meetings, tailored intervention and local consensus processes, all employed by four trials each. No two trials examined the same combination of implementation strategies. The duration of implementation support ranged from six months in Bandoni 2010 to two years in Biener 1999. Four trials reported using theoretical, practical or conceptual frameworks including the Ecological Model for Health Promotion (Bandoni 2010; Beresford 2010; Biener 1999), Social Ecological Theory (Parker 2010), and Rogers's Diffusion of Innovations Theory (Hannon 2012); however, these were described in the context of informing workplace health promotion activities rather than a framework to guide the implementation intervention. Two trials reported the use of a theory or framework to guide implementation strategies, specifically the Theoretical Domains Framework, described in Jones 2015, and Rothman's Community Activation Principles, described in Biener 1999.
3. Definition of EPOC subcategories utilised in the review.
| EPOC subcategory | Definition |
| Audit and feedback | A summary of health workers' performance over a specified period of time, given to them in a written, electronic or verbal format. The summary may include recommendations for clinical action. |
| Clinical practice guidelines | Clinical guidelines are systematically developed statements to assist healthcare providers and patients to decide on appropriate health care for specific clinical circumstances (US Institute of Medicine). |
| Educational materials | Distribution to individuals, or groups, of educational materials to support clinical care, i.e. any intervention in which knowledge is distributed. For example this may be facilitated by the Internet, learning critical appraisal skills; skills for electronic retrieval of information, diagnostic formulation; question formulation |
| Educational meetings | Courses, workshops, conferences or other educational meetings |
| Educational outreach visits | Personal visits by a trained person to health workers in their own settings, to provide information with the aim of changing practice |
| Local consensus process | Formal or informal local consensus processes, for example agreeing a clinical protocol to manage a patient group, adapting a guideline for a local health system or promoting the implementation of guidelines |
| Local opinion leaders | The identification and use of identifiable local opinion leaders to promote good clinical practice |
| Monitoring the performance of the delivery of healthcare | Monitoring of health services by individuals or healthcare organisations, for example by comparing with an external standard |
| Tailored interventions | Interventions to change practice that are selected based on an assessment of barriers to change, for example through interviews or surveys. |
One trial targeted the implementation of workplace policies or practices for diet only (Bandoni 2010); one trial targeted policies or practices for both diet and tobacco use (Biener 1999); two trials targeted policies or practices for diet, physical activity and weight control (Beresford 2010; Parker 2010); and two trials conducted interventions to increase the implementation of workplace policies or practices targeting other health behaviours in addition to those of focus in the review (Hannon 2012; Jones 2015). Specifically, Hannon 2012 provided support to improve the implementation of workplace policies and practices for diet, physical activity, weight control and tobacco use in addition to workplace sun exposure, benefits for preventive care and health screening, and immunisation. In Jones 2015, implementation support targeted workplace policies for diet, physical activity, weight control and tobacco use, in addition to mental health and the management of long‐term sickness and absence. Both trials reported implementation outcomes as a combined measure for all health factors, so we report them as such in the review. No trial targeted workplace policies and practices for reducing risky alcohol consumption.
Types of comparisons
Three trials compared implementation strategies against a wait‐list control in which usual practice continued during the study period (Bandoni 2010; Beresford 2010; Hannon 2012), and one trial used a minimal support comparison group where workplaces received feedback from the results of an employee survey in addition to printed materials such as posters (Biener 1999).
Two trials reported comparisons including more than one implementation intervention group (Jones 2015; Parker 2010). Parker 2010 compared two intervention conditions of varying implementation support intensity against a wait‐list control group where workplaces were instructed not to introduce new environmental health promotion initiatives during the study period. Senior company leaders allocated workplaces to control or intervention groups; however, within those selected for the intervention group, workplaces were allocated to one of two conditions randomly via coin toss. Therefore this trial employed a non‐randomised design for comparisons between the control and implementation intervention arms, whilst comparisons between implementation intervention arms made use of a randomised design.
Jones 2015 selectively assigned workplaces to three cohorts (A, B and C) and one sub‐cohort (C1) according to baseline scores in a workplace organisational audit measuring the implementation of health promotion guidelines. Workplaces demonstrating good progress in implementation were assigned to cohort A and received feedback on audit performance in addition to undergoing interviews to elicit information about organisational barriers and facilitators to guideline implementation ('feedback and interviews'). Workplaces identified as demonstrating less progress in implementation were assigned to cohort B, and they received feedback plus action planning workshops informed by knowledge on barriers and facilitators to implementation, as derived from the interviews with cohort A ('feedback and workshops'). Remaining workplaces were assigned to cohort C and received audit performance feedback alone ('feedback only'). The sub‐cohort C1 included workplaces receiving 'feedback only' that had demonstrated poor performance in the baseline audit, comparable to cohort B. As interviews conducted with cohort A provided no direct implementation support to this cohort and were used only to inform action planning workshops for cohort B, this cohort was excluded from analyses. Subsequently, for the assessment of implementation outcomes we included comparisons of cohorts B and C1 only, based on comparability of baseline implementation scores in the organisational audit (both poor performing) and the use of different implementation support approaches – 'feedback only' versus 'feedback and workshops'.
Outcomes
Two trials collected follow‐up data on implementation outcomes at two years postbaseline (Beresford 2010; Parker 2010); two trials at three years (Biener 1999; Jones 2015); one trial at 6 months (Bandoni 2010); and one trial at 15 months (Hannon 2012). Three trials used surveys (Bandoni 2010; Biener 1999; Hannon 2012), and one, organisational audits (Jones 2015), to assess implementation outcomes, but they did not report on the validity of these instruments in assessing implementation outcomes. Two trials used observation‐based measures to assess implementation outcomes, including an environmental assessment checklist in Beresford 2010 and a validated environmental assessment tool in Parker 2010.
Three trials assessed employee dietary behaviours: two measured dietary outcomes using non‐validated surveys (Bandoni 2010; Parker 2010), and one used a validated food frequency questionnaire (Biener 1999). One trial assessed employee tobacco use using a non‐validated survey (Biener 1999). Only one trial assessed employee physical activity and weight status (Parker 2010), measuring weight objectively using standardised protocols and physical activity using a non‐validated survey. No trial reported relevant outcomes relating to employee alcohol use. Hannon 2012 was the only included trial to report cost‐related outcomes for implementing workplace policies or practices, measured via survey of contract costs and personal hours. No trial reported adverse outcomes associated with the implementation of polices or practices in workplaces.
Other study design characteristics
For some trials, decisions regarding the extraction of implementation outcomes were particularly complex. In the Working Well trial (Biener 1999), the implementation of workplace policies and practices targeting tobacco control and the promotion of healthy eating were measured using a number of outcomes assessed across two surveys with employees and organisational informants. However, several of these measures did not provide a direct assessment of implementation (e.g. measuring 'intentions' to implement a policy or practice), and so such measures were excluded from the review.
Parker 2010 reported effects of the workplace intervention on employee tobacco use as well as risky alcohol use. However, as the implementation strategy and policies and practices targeted by the intervention did not include those addressing tobacco and alcohol use, we could not include the effects of the intervention on these health behaviours in the review.
Finally, two trials conducted interventions to increase the implementation of workplace policies or practices targeting other health behaviours in addition to those of interest to the review (Hannon 2012; Jones 2015). Both trials used composite score‐based measures of implementation outcomes, and it was not possible to isolate the impact of the strategy on implementation outcomes for diet, physical activity, weight control and tobacco use policies and practices alone. However, as most of the policies and practices targeted by the implementation (and reflected in the score) were for the health behaviours specified within the scope of the review, the trials were retained and outcome data included.
Excluded studies
Following screening of titles and abstracts, we obtained the full texts of 334 articles for further assessment of eligibility (Figure 2). Of these, 312 articles were considered ineligible. Reasons for exclusion included inappropriate participants (10 studies); inappropriate intervention (122 studies); inappropriate outcome (128 studies); non‐controlled study/inappropriate comparator (47 studies); and inability to obtain full‐text article (5 studies). We excluded studies based on inappropriate outcomes if they: did not report any implementation outcomes; did not report implementation outcomes for both intervention and control groups; did not report between group differences in implementation outcomes; or reported an indirect measure of implementation (e.g. trials reporting the intention to implement a workplace policy or practice). We excluded five trials at the data extraction stage, all on the basis of inappropriate outcomes.
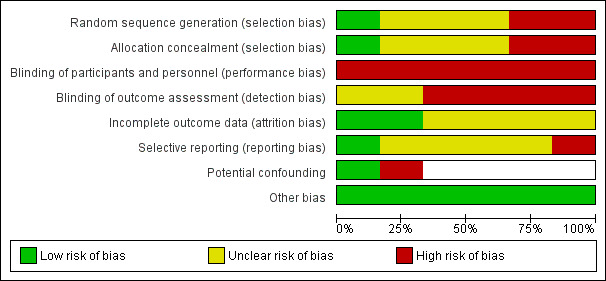
Risk of bias in included studies
We present the combined results of the 'Risk of bias' assessment across all trials in Figure 3 and for each individual trial in Figure 4. Assessment considered study design and reporting characteristics relevant to the implementation outcomes of included trials. We judged most trials to be at high risk of performance and detection bias and at unclear risk of attrition and reporting bias. We considered both non‐randomised trials to be at high risk of selection bias (Jones 2015; Parker 2010), whilst we deemed the risk of potential confounding to be high and low, respectively. The other four trials were at low risk of bias from other sources. We also assessed risk of bias for secondary outcomes (employee health behaviours and cost‐measures); Appendix 3 presents these judgements.
3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
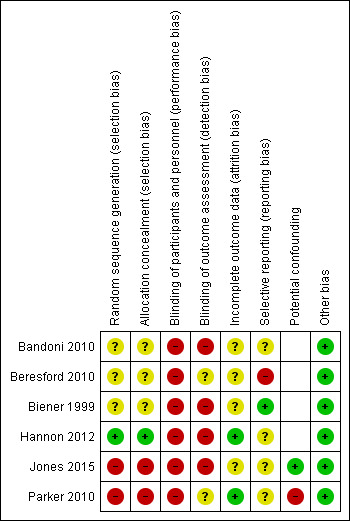
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Risk of selection bias differed across the six trials. We considered the two non‐randomised trials to be at high risk of selection bias for both random sequence generation and allocation concealment (Jones 2015; Parker 2010). Of the four trials with RCT designs, we considered one to be at low risk for random sequence generation and allocation concealment, as a statistician undertook block randomisation (Hannon 2012). For the other three RCTs (Bandoni 2010; Beresford 2010; Biener 1999), risk of bias associated with sequence generation and concealment of allocation was unclear, as authors reported no information on these processes.
Blinding
We considered all six trials to be at high risk of performance bias due to participants and research personnel not being blind to group allocation. Four trials were at high risk of detection bias as data collection was via self‐reported surveys undertaken by participants who were not blind to group allocation (Bandoni 2010; Biener 1999; Hannon 2012; Jones 2015). For the other two trials (Beresford 2010; Parker 2010), the risk of detection bias was unclear; although outcome assessment was undertaken via observations or environment audits, assessors were not blind to group allocation.
Incomplete outcome data
For two of the trials, we rated the risk of attrition bias as low, as data were either collected for all sites at follow‐up (Parker 2010) or there was no difference between groups in the number of sites lost to follow‐up (Hannon 2012). For the other four trials (Bandoni 2010; Beresford 2010; Biener 1999; Jones 2015), risk of attrition bias was unclear as there was either a difference between groups in data attrition and a lack of information about whether analysis followed the intention‐to‐treat principle, or a general lack of information regarding the completeness of outcome data.
Selective reporting
We rated Beresford 2010 as being at high risk for reporting bias, as the publication did not report planned outcomes related to physical activity and diet. Biener 1999 was at low risk for reporting bias because the article reported all a priori published outcomes. For the remaining four trials (Bandoni 2010; Hannon 2012; Jones 2015; Parker 2010), risk of reporting bias was unclear, as we could not identify a priori registration of outcomes (via trial registration or publication of a study protocol or design paper).
Other potential sources of bias
Of the two trials with non‐randomised designs, we considered one to be at high risk of bias, as the analyses did not adjust for potential confounders (Parker 2010). For the other non‐randomised trial (Jones 2015), we rated risk of bias due to confounding factors as low, as the outcome analyses included an adjustment for baseline differences between the groups. For the remaining four trials (Bandoni 2010; Beresford 2010; Biener 1999; Hannon 2012), we considered bias from other sources to be low.
Effects of interventions
See: Table 1
Effects on implementation outcomes
1. Implementation strategies versus no intervention (wait‐list, usual practice or minimal support controls)
Continous outcomes
Implementation score
Four trials utilised score‐based measures of implementation outcomes (Beresford 2010; Biener 1999; Hannon 2012; Parker 2010), three of which were randomised trials (Beresford 2010; Biener 1999; Hannon 2012). Meta‐analysis of the randomised trials showed no difference in the standardised mean difference (SMD) for implementation outcomes (SMD −0.01, 95% CI −0.32 to 0.30, P = 0.97; 164 participants; 3 studies; low certainty evidence), with no evidence of heterogeneity (I2 = 0%; Chi2 = 0.89). A difference of this magnitude (−0.01) can be interpreted as a 'small' effect based on Cohen's effect size classification (Cohen 1988), suggesting little to no benefit of implementation support in improving policy or practice implementation relative to control. Figure 5 presents forest plots of the trial effects. Given the limited number of trials included in the meta‐analysis, we were unable to undertake subgroup analyses as planned.
5.
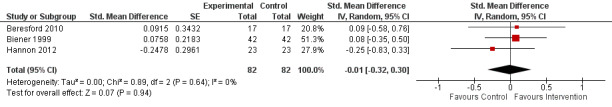
Forest plot of comparison: 1 Implementation strategy versus control, outcome: 1.1 Implementation score.
In the Physical Activity and Changes in Eating (PACE) randomised trial, Beresford 2010 examined a 15‐ to 17‐month strategy to implement a workplace intervention aiming to improve physical activity and nutritional choices to reduce or maintain weight in workplaces with a high proportion of sedentary employees. Support strategies provided to the intervention group (17 workplaces; n employees not reported) were informed by focus groups conducted with workplaces to identify implementation barriers (tailored intervention). A workplace contact person was nominated, and an employee advisory board (EAB) consisting of 4 to 7 employees was established at each intervention workplace to work with the research team to design, plan and implement intervention activities (local opinion leaders), as well as with the senior management to secure commitment for ongoing health promotion in the workplace (local consensus process). EAB members received a handbook outlining the intervention framework and a number of intervention activities that could be tailored to their workplaces (educational materials). The wait‐list control group (7 workplaces; n employees not reported) received support following trial completion. Research staff assessed implementation outcomes at baseline and two years postbaseline using an environment assessment (EA) checklist during an inspection of the workplace. Authors did not report the psychometric properties of the EA checklist; however, observation represents an objective measure of the workplace environment. Checklist items included 11 practices promoting healthy eating, physical activity and weight control related to the physical environment (e.g. provisions for bikes and healthy options in vending machines), information environment (e.g. posters encouraging stair usage), and worksite resources (e.g. availability of weight control programmes). Checklist items measuring implementation of each practice were combined into a score and standardised for the size of the company (specific procedure not reported), with higher scores indicative of better implementation. At follow‐up, there were no significant differences between groups in scores for 9 of the 11 practices assessed; however, scores were significantly higher for the intervention group for practices regarding display of notices promoting physical activity (adjusted effect estimate 0.33, 95% CI 0.00 to 0.85) and a healthy diet (adjusted effect estimate 0.40, 95% CI 0.00 to 1.46). The effect sizes across the 11 practices ranged from −0.56 (95% CI −1.57 to 1.61) to 0.60 (95% CI −5.40 to 5.60). When standardised intervention effects were ranked for each measure, the median effect used in the meta‐analysis was 0.10 (95% CI −8.10 to 9.60).
In the Working Well randomised trial, Biener 1999 tested the effects of an intervention to implement a range of workplace policies and practices to promote access to healthy foods, restrict smoking, and promote social norms supportive of having a healthy diet and not smoking. Implementation support provided to the intervention group (55 workplaces; 8914 employees) occurred over two years and comprised participatory approaches whereby employees at each intervention workplace functioned as an employee advisory board (EAB), working in partnership with researchers to plan and undertake changes to the workplace, as well as tailor interventions to suit local workplaces (local opinion leaders). Additionally, the EAB liaised with management to develop and implement new policies (local consensus process). EAB members received training (educational meetings), and workplace visits from researchers took place at least once a month to support implementation (educational outreach visits). The control group (56 workplaces, 9291 employees) received minimal support comprising printed health promotional materials. Investigators measured implementation outcomes for tobacco‐related policies and practices via two items included in an employee survey (validity not reported) conducted at baseline and postintervention (approximately 3 years postbaseline). The items assessed 'restrictiveness of tobacco control policy' (1 = low restrictiveness i.e. no tobacco control policy; 4 = high restrictiveness i.e. smoking is not allowed anywhere in the workplace) and 'adherence to tobacco control policy' (1 = low adherence i.e. people frequently smoke where smoking is prohibited; 5 = high adherence i.e. people never smoke where smoking is prohibited). At follow‐up, there were no significant differences in the change in mean score for the restrictiveness of workplace smoking policy (adjusted difference 0.01, standard error (SE) 0.09) or the adherence to workplace smoking policy (adjusted difference 0.03, SE 0.07).
Hannon 2012 conducted a randomised trial to assess the impact of the Workplace Solutions programme for improving the implementation of 16 best practices for workplace health promotion recommended in the Centers for Disease Control and Prevention (CDC) Community Preventive Services Task Force (CPSTF) Community Guide. Eight of the practices specifically targeted diet, physical activity, weight control or smoking cessation. The intervention group (24 workplaces, n employees not reported) received a feedback report detailing recommendations for improving any of the 16 best practices that employers were not fully implementing (clinical practice guidelines and audit and feedback). The project interventionist worked with employers to nominate 3 to 5 best practices to implement over the following 12 months (local consensus process), conducting three in‐person meetings at workplaces (educational outreach) and providing support resources including implementation tool kits (educational materials). Additionally, at the intervention mid‐point (six months), the project interventionist asked employers about their progress in implementing each of their chosen best practices and offered guidance for overcoming identified implementation barriers (tailored intervention). The control group (24 workplaces, n employees not reported) received two newsletters detailing trial progress and were offered the intervention at trial completion. Investigators evaluated baseline and 15 months' postbaseline implementation of the 16 best practices via a score derived from a self‐reported survey (validity not reported) completed by a human resources leader at each workplace. Survey items assessing best practice implementation were scored using a binary system for some items (0 = the practice was not implemented; 1 = the practice was implemented) or on a 3‐point scale (0 = not implemented; 0.75 = partially implemented; 1 = fully implemented) for others. For each best practice, trialists calculated a summary score by dividing the sum of item scores by the number of items. The total best practice score represented the mean of the sum of scores of each individual best practice, with higher scores indicating better implementation. At follow‐up, there were no significant differences between groups in total best practice implementation score: intervention group baseline 31.50 (standard deviation (SD) 8.30), follow‐up 39.20 (SD 11.20); versus control group baseline 36.8 (SD 11.70), follow‐up 42.1 (SD 11.80), P = 0.33.
Finally, the one non‐randomised trial that reported a continuous implementation score, the Dow Chemical study, provided very low certainty evidence (Parker 2010). The study tested the effectiveness of an intervention to improve the implementation of workplace policies and practices promoting healthy eating, physical activity and weight control. The intervention group received one of either two implementation support conditions: moderate (4 workplaces, 382 employees) or high intensity (5 workplaces, 1520 employees), whilst the control group (3 workplaces; 529 employees) received instructions not to introduce new environmental health promotion initiatives during the study period. Workplaces in moderate‐ and high‐intensity groups were asked to implement a range of predominantly environmental interventions (e.g. signed walking paths), and were consulted by the research team to identify potential barriers to implementation, as well as interventions that would be most suited to the workplace context (tailored intervention). Research staff trained workplace 'wellness ambassadors' (educational meetings) who were designated to carry out tasks such as promoting healthy food choices at workplace meetings and events, and organising health promotion posters and messages in the workplace (local opinion leaders). In addition to the environmental intervention, five workplaces allocated to the high‐intensity support group received support strategies designed to influence organisational culture and boost leadership commitment to employee health. Managers received training on health‐related topics and ways to improve employee participation in health promotion initiatives (educational meetings). Progress reports regarding health promotion and project implementation were provided to site and corporate leaders (audit and feedback), and health promotion‐related goals were included in the organisational plans of site leaders (local consensus process). Site leaders were held accountable to corporate leaders for making progress toward health‐related goals (monitoring of performance) and were recognised and rewarded for achieving such goals (other strategy). Implementation outcome data were assessed using a score derived from a validated 105‐item Environmental Assessment Tool (EAT), which comprised a questionnaire component that site staff completed and an on‐site observation component that the research team performed. Trial authors used a scoring rubric to aggregate the EAT responses into a total score (out of 100 points). The precise scoring system was not reported; however, higher scores were indicative of greater levels of environmental support for health‐promotion within workplaces. Compared to control, there were significant improvements in total EAT scores at two‐year follow‐up for workplaces receiving the moderate‐intensity (contrast estimate 9.68, SE 3.48, P = 0.009) and high‐intensity (contrast estimate 16.99, SE 3.37, P < 0.001) interventions.
Other continuous implementation outcomes
One RCT reported other continuous measures of implementation that could not be synthesised in the meta‐analysis (Bandoni 2010). This trial evaluated the impact of an educational and environmental intervention on the availability and consumption of fruits and vegetables in the cafeterias of 30 industrial sector workplaces in Brazil. The intervention group (15 workplaces, 630 employees) received resources including a manual and nutrition guidelines (educational materials), which trialists presented to cafeteria managers and discussed with them. Workplace cooks and cafeteria assistants also took part in culinary workshops (educational meetings) to support increasing fruit and vegetable availability in cafeteria meals. The control group (14 workplaces, 584 employees) received implementation support post‐trial. The primary implementation outcome of the trial was the grams of fruits and vegetables served by workplace cafeterias to employees in lunch meals, measured at baseline and six months postbaseline via food service managers' recordings of the types and quantities of foods served in the workplace cafeteria over three successive days (validity not reported). Based on food service managers' reports, the quantities of fruits and vegetables per customer were calculated to assess the availability of fruit and vegetables in lunch meals. Relative to controls, intervention workplaces offered significantly more grams of fruits and vegetables in cafeteria meals at follow‐up (adjusted mean difference (MD) 49.05 g, 95% CI 8.38 to 89.71; low certainty evidence).
Dichotomous outcomes
Only one trial, the Working Well randomised trial, reported dichotomous implementation outcomes (Biener 1999). Outcomes included the proportion of workplaces implementing practices to promote healthy eating, including cafeteria and vending machine nutrition labelling and a healthy catering policy. Outcome assessment was via an organisational informant survey of organisational representatives (validity not reported), conducted at baseline and postintervention (approximately 3 years postbaseline). Compared to controls, changes were found in the proportion of intervention workplaces that reported improvements in cafeteria point‐of‐purchase nutrition labelling (MD 13.40%, P = 0.72), vending machine nutrition labelling (MD 39.60%, P < 0.01), and workplace healthy catering policy (MD 10.90%, P = 0.30) (low certainty evidence). However, only for labelling of vending machines was this statistically significant.
2. Comparisons of different implementation strategies
Two included trials, both non‐randomised, provided very low certainty evidence regarding the effects of different implementation strategies on implementation outcomes (Jones 2015; Parker 2010). Both trials reported a significant effect on the single measure of implementation included, favouring the group that received the higher intensity implementation support.
Continous outcomes
Implementation score
Parker 2010 employed a randomised design to examine the comparative effectiveness of two different levels of implementation support intensity (moderate or high) to improve implementation of a range of practices targeting healthy eating, physical activity and weight control. At follow‐up, the total EAT score assessing implementation of the health‐promoting practices was significantly higher among the five workplaces that received the high‐intensity support condition (tailored intervention; local opinion leaders; educational meetings; audit and feedback; local consensus process; monitoring of performance; and 'other' strategy) compared to the four workplaces that received moderate‐intensity support (tailored intervention; local opinion leaders and educational meetings) (contrast estimate 7.31, SE 3.10, P = 0.024).
Jones 2015 compared two approaches to improve the implementation of National Institute for Health Care and Excellence (NICE) public health workplace‐related guidance by National Health Service (NHS) trusts in England. Two of the four study cohorts included in the trial were comparable at baseline: cohort B (36 workplaces, n employees not reported) and cohort C1 (26 workplaces, n employees not reported), and we extracted data from these cohorts to assess effects of implementation strategies in the review. In both cohorts, trusts completed an organisational audit to assess the extent of implementation of the NICE public health workplace‐related guidance (clinical practice guidelines) within their trust and received a feedback report regarding implementation performance against a national benchmark (audit and feedback). In addition to this, trusts in cohort B attended action planning workshops (educational meetings) developed using data on barriers to implementation (tailored intervention) derived from interviews undertaken with trusts to support better engagement and implementation of the NICE guidance. The primary implementation outcome was the total score on an organisational audit questionnaire completed by workplace staff (validity not reported), which assessed implementation of six sets of NICE guidance pertaining to obesity prevention, smoking cessation, physical activity, management of long‐term sickness, and mental health. Total audit scores were devised by applying weighted scores to audit responses and then transforming them into a score on a 100‐point scale, with higher scores indicating better implementation of the guidance. It was not possible to disaggregate scores for the implementation of policies and practices related to each specific set of the guidance; however, the median total score on the audit significantly increased among trusts in cohort B compared with cohort C1 (22.17 versus 4.94, P < 0.001).
Effects on health behaviour outcomes
Diet
Two RCTs and one non‐randomised trial assessed the impact of implementation strategies on employee dietary behaviours (Bandoni 2010; Biener 1999; Parker 2010). We considered the evidence from both the non‐randomised trial, Parker 2010, and the RCTs to be of very low certainty. The two randomised trials assessed dietary intake (fruit and vegetable consumption) using continuous measures, so we pooled their data in meta‐analysis (Bandoni 2010; Biener 1999). However, when outcomes for fruit and vegetable consumption were combined heterogeneity was high (Chi2 < 0.01; I2 > 85%), suggesting that a single point estimate based on pooled analyses could be misleading, so we described the findings of each trial narratively.
Bandoni 2010 assessed the effect of implementation strategies on employee consumption of fruits and vegetables (grams per meal) in lunch meals purchased from workplace cafeterias. To assess fruit and vegetable consumption, researchers surveyed employees (intervention n = 630; control n = 584) on the portions of fruit and vegetables they had consumed in lunch meals (validity not reported). Researchers used serving spoons in the cafeteria as a portion size reference measure, and they considered the foods offered by the cafeteria that day. At follow‐up (six months postbaseline), there was a small but significant increase in the grams of fruits and vegetables consumed in meals among employees at intervention workplaces, relative to control (adjusted effect estimate 11.75, 95% CI 2.73 to 20.77).
The Working Well trial examined the effects of implementation strategies on employee fruit and vegetable consumption (servings per day), in addition to the percentage of dietary energy derived from fat and dietary fibre consumption (grams per 1000 kcal) (Biener 1999). Employees self‐reported dietary intake (intervention n = 8914; control n = 9291) using the Block food frequency questionnaire (validated tool). At follow‐up (approximately 3 years postbaseline) the relative increase in daily servings of fruits and vegetables consumed by employees was significantly greater among intervention workplaces compared to controls (adjusted increase 5.60%, SE 1.30, P < 0.001), whilst the dietary energy derived from fat was significantly lower (adjusted difference −0.35%, SE 0.16, P < 0.05). There was no significant difference between groups, however, with regard to dietary fibre consumption (adjusted increase 1.70%, SE 0.87, P > 0.05).
Finally, in the Dow Chemical study, Parker 2010 assessed the impact of implementation strategies on a range of dietary behaviours among employees (moderate‐intensity intervention n = 382; high‐intensity n = 1520; control n = 529). Researchers used survey items assessing dietary intake in a self‐completed health risk assessment (validity not reported) to dichotomise employees as being at high or low risk of 'poor nutrition', defined as consuming four or more fast food meals per week, two or more sugar‐sweetened beverages per day, or three or fewer fruit and vegetable servings per day. At follow‐up (two years postbaseline measure), there was no significant difference in the proportion of employees identified as being at high risk of poor nutrition among workplaces that received moderate‐intensity (estimate −7.70%, P = 0.068) or high‐intensity (estimate −4.60%, P = 0.16) implementation support, relative to control. Investigators did not make statistical comparisons between groups receiving moderate‐ and high‐intensity implementation support.
Physical activity
Parker 2010 provided evidence of very low certainty regarding the effects of strategies to implement physical activity policies or practices on the physical activity levels of employees (moderate‐intensity intervention n = 382; high‐intensity n = 1520; control n = 529). Researchers used survey items assessing physical activity in a self‐completed health risk assessment (validity not reported) to dichotomise employees as being at high or low risk of 'poor physical activity', defined as not engaging in any moderate or strenuous physical activity at least once per week. At follow‐up (two years postbaseline), there was no significant difference in the proportion of employees classified as being at high risk of poor physical activity amongst workplaces that received moderate‐ (estimate −1.60%, P = 0.77) or high‐intensity (estimate −0.70%, P = 0.89) implementation support, compared to control. Investigators did not make statistical comparisons between groups receiving moderate‐ and high‐intensity implementation support. No other trials assessed the effects of implementation strategies on this outcome.
Obesity
Parker 2010 was also the only trial to examine employee weight status, providing very low certainty evidence on the effects of implementation strategies on this outcome. The study assessed weight status in a sub‐sample of employees participating in a health risk assessment as part of the trial (moderate‐intensity intervention n = 213; high‐intensity n = 926; control n = 382). Researchers assessed weight status objectively using anthropometric measures collected by health professionals following standardised protocols. At follow‐up (two years postbaseline), there was no difference in the proportion of employees who were obese (MD 0.30%, P = 0.95) or overweight (MD 5.50%, P = 0.22) among workplaces that received high‐intensity implementation support compared to control. There were, however, significant reductions in employee weight (estimate −1.50 kg, P = 0.015) and body mass index (BMI) (estimate −0.20 kg/m2, P = 0.008). Similarly, there was no significant difference, relative to control, in the proportion of employees who were obese (estimate 0.10%, P = 0.88) or overweight (estimate 4.40%, P = 0.47) among workplaces that received moderate‐intensity implementation support; however, there were significant reductions in employee weight (estimate −2.10 kg, P = 0.033) and BMI (estimate −0.30 kg/m2, P = 0.034) were reported.
Tobacco use
One RCT, the Working Well Trial, provided low certainty evidence for the effects of implementation strategies on employee tobacco use (Biener 1999). Investigators assessed tobacco use outcomes at the workplace level (42 workplaces in each group) and included smoking prevalence (percentage of smokers in total) and the percentage of smokers who quit (abstinence from tobacco for the previous six months). Employees self‐reported tobacco use via a survey conducted at baseline and three years postbaseline (validity not reported). At follow‐up, there was no significant difference in smoking prevalence (MD −0.66%, 95% CI −3.00 to 1.20) or the proportion of smokers who quit (MD 1.53, 95% CI −1.00 to 3.70) among employees in workplaces receiving implementation support compared to control.
Alcohol use
One included trial reported the effects of a workplace health‐promotion intervention on employee alcohol use (Parker 2010). However, as the implementation strategy and policies and practices targeted by the intervention did not include those addressing alcohol use, we did not include the effects on employee alcohol use reported in this trial in the review. None of the other included trials reported effects of implementation strategies on this outcome.
Cost or cost‐effectiveness of implementation strategies
Hannon 2012 was the only included trial to report cost‐related measures for the implementation of workplace‐based practices targeting diet, physical activity, weight control, tobacco use, and other health behaviours (low certainty evidence). Trialists used a self‐reported survey completed by workplace human resources staff (validity not reported) to collect data on employers' costs for implementing three to five nominated best practices for health promotion, defined as annual US dollars spent (per worker) on contracts and hours of personnel time. The study collected cost data only for best practices that employers partially or fully implemented. To calculate total costs, researchers summed contract costs and monetised personnel hours and divided them by the mean number of employees in each study arm to obtain annual per worker costs. Relative to the 23 control workplaces, employer mean total costs (range) increased slightly more over time in the 23 intervention workplaces (intervention group baseline USD 8.30 (0.00 to 35.00), follow‐up USD 10.10 (0.00 to 53.00) versus control (baseline USD 11.00 (0.00 to 53.00), follow‐up USD 11.80 (1.00 to 43.00); significance not reported). None of the included trials examined the cost‐effectiveness of workplace policies or practices targeting diet, physical activity, obesity, tobacco use or alcohol use.
Unintended adverse consequences of implementation strategies
None of the included trials examined any unintended adverse consequences of the implementation strategies employed in the interventions.
Discussion
Summary of main results
The aim of this review was to describe the effects of strategies to improve the implementation of workplace‐based policies and practices targeting key modifiable risk factors for chronic disease including diet, physical activity, obesity, tobacco use and alcohol use. The review found a small number of trials testing various combinations of multi‐component support, with no two trials testing the same combination of strategies. Evidence regarding the impact of strategies on the implementation of workplace policies and practices targeting diet, physical activity, weight control and tobacco use were equivocal. We considered most trials to be at high risk of performance and detection bias and at unclear risk of selection, attrition and reporting bias. We considered the certainty of evidence (GRADE) for effects on implementation outcomes to be low based on four RCTs and very low based on two non‐randomised trials.
Of the five trials comparing an implementation strategy versus no intervention (usual practice, wait‐list or minimal support control) (Bandoni 2010; Beresford 2010; Biener 1999; Hannon 2012; Parker 2010), meta‐analysis was possible for three randomised trials reporting score‐based measures of implementation (Beresford 2010; Biener 1999; Hannon 2012). Standardised effects for these outcomes showed no difference between groups, suggesting no benefit of implementation support in improving policy or practice implementation relative to control. Findings for other continuous or dichotomous implementation outcomes reported across these five trials were mixed. For two non‐randomised trials examining comparative effectiveness of implementation strategies (Jones 2015; Parker 2010), both reported improvements in implementation, favouring the more intensive implementation group. Three trials examined effects of implementation strategies on employee health behaviours, finding no significant effect on measures of tobacco use or physical activity, and few on measures of diet (Bandoni 2010; Biener 1999; Parker 2010). Only one trial reported cost data (Hannon 2012), and no included trial assessed adverse effects.
Overall completeness and applicability of evidence
The limited number of included trials suggests implementation and knowledge translation research in the workplace setting is only in the early stages of development, similar to the case in other community‐based settings including childcare centres and schools (Wolfenden 2016; Wolfenden 2017). We identified several gaps in the evidence base. First, no trials examined the effectiveness of strategies to implement health‐promoting workplace policies or practices addressing alcohol use. Second, there is an insufficient pool of studies to allow assessment of the effects of specific implementation strategies, their impact on specific population groups, and the effect of implementation strategies conducted at scale. Third, the review does not provide evidence on the potential cost‐effectiveness of implementation strategies or their potential unintended adverse effects. Fourth, available research to date is concentrated in North America, with most trials taking place in the USA. Finally, included trials covered only a narrow range of employment sector types, with workplaces predominantly from the manufacturing and industrial sectors. As such, the applicability of the review findings to other countries, particularly low‐ and middle‐income countries, and workplaces from other employment sectors (e.g. business and information technology), is limited.
Quality of the evidence
We graded the certainty of evidence for effects on implementation outcomes as low based on four RCTs, and very low based on two non‐randomised trials. We downgraded the certainty of evidence due to serious limitations regarding risk of bias and the precision of results. We deemed most trials to be at high risk of performance and detection bias and at unclear risk of selection, attrition and reporting bias. The sample sizes of workplaces included in trials were relatively small, limiting the precision of estimated effects. The limited number of trials identified by the review and heterogeneity across included trials in the implementation strategies and outcomes also limited comparisons. For secondary outcomes, we graded the certainty of evidence as low for measures of employee tobacco use and estimates of cost, and very low for measures of employee diet, physical activity and weight status.
Potential biases in the review process
We employed a number of strategies to reduce the risk of bias in the review process. First, we conducted a comprehensive search to identify eligible studies, including screening approximately 13,000 records identified from multiple academic databases spanning a range of relevant professional disciplines, grey literature sources, handsearches of key journals, and contacts with relevant experts in the field and authors of included trials. Second, we employed previously published search filters to maximise the likelihood of capturing relevant trials. Third, we conducted all citation screening and data extraction in duplicate and sought adjudication from a third reviewer in instances where consensus regarding trial eligibility or data extraction could not reached. Finally, we pre‐specified methods in a published review protocol (Wolfenden 2016b).
Despite the rigorous review methods, a number of characteristics of the review may have introduced bias. While we screened a large number of citations, the first block of the search strategy to identify 'workplace' literature only used medical subject headings (MeSH) in MEDLINE and CENTRAL, which may have limited the sensitivity of the search. Terminology in implementation science is also evolving (Mazza 2013), and we noted a diverse range of descriptions of implementation strategies applied among included trials. As such, the search strategy may not have yielded all relevant trials due to the lack of standardised terms for implementation interventions. We will review the search terms in future updates of the review to identify opportunities to improve the sensitivity and specificity of the search.
Agreements and disagreements with other studies or reviews
This is the third in a series of Cochrane Reviews investigating the effectiveness of strategies to improve the implementation of policies and practices targeting modifiable risk factors for chronic disease within community settings. Aside from the specific setting where included trials were conducted, the three reviews employed the same selection criteria and review methods. The primary findings of this review are consistent with those of the two previous Cochrane Reviews on the impact of implementation strategies in school and childcare settings (Wolfenden 2016; Wolfenden 2017). Specifically, each review identified relatively few trials, considerable heterogeneity in the implementation strategies tested, little use of implementation‐specific frameworks, and a limited number of trials assessing outcomes related to cost or unintended adverse effects. Furthermore, each review reported equivocal effects on implementation outcomes and on individual health behaviours. Such findings are consistent with a US Agency for HealthCare Research and Quality systematic review (Rabin 2010), which included uncontrolled before‐and‐after trials examining the impact of dissemination or implementation strategies targeting policies or programmes to address cancer risk behaviours (including smoking, diet and physical activity) across community settings. Similarly, the findings concur with those reported in reviews of implementation trials in primary care settings, which have found little evidence of cost assessment, cost‐effectiveness or adverse effects included in implementation studies (Lau 2015).
Authors' conclusions
Implications for practice.
The findings of the review do not provide clear evidence to identify effective strategies to improve the implementation of policies and practices targeting modifiable risk factors for chronic disease in the workplace setting. On this basis, policy makers and practitioners must look to theory and empirical evidence from other settings when designing interventions for the workplace environment. The application of comprehensive theoretical implementation frameworks has the potential to improve the impact of implementation strategies. Such frameworks encourage the consideration of a range of multi‐level factors (barriers and facilitators) when developing strategies to support implementation. While there are a large number of frameworks, the most comprehensive is the Consolidated Framework for Implementation Research (CFIR), which draws together several published implementation theories into 39 constructs, reflecting the evidence base of factors most likely to influence the implementation of interventions (Damschroder 2009). Another frequently utilised implementation framework is the Theoretical Domains Framework (TDF), which synthesises 33 theories of behaviour change clustered into 14 (originally 12) domains and can be applied to identify impediments to implementation and appropriate implementation support strategies (Cane 2012). In many cases, as with the TDF, excellent guidance documents have been published, outlining methods to identify implementation barriers (or facilitators) and select appropriate implementation strategies and behaviour change techniques to overcome these (Atkins 2017).
In the absence of sufficient evidence for the workplace setting identified by this review, policy makers and practitioners should also utilise reviews on the effectiveness of implementation strategies in the healthcare services setting. The Cochrane EPOC review group house a number of such reviews. Specifically, Cochrane Reviews on research undertaken predominantly in healthcare settings suggest that a range of strategies may improve health service and staff implementation of evidence‐based policies and practices, including audit and feedback (Ivers 2012), training (Forsetlund 2009), and academic detailing (O'Brien 2007). Consolidated reviews of systematic reviews also provide indirect evidence for the relative effects of individual and multi‐component implementation approaches (Lau 2015). With the help of theoretical frameworks and following comprehensive formative evaluations, the selection of evidence‐based implementation strategies that address impediments to implementation and are appropriate to context are likely to represent the most effective approach for maximising the impact of implementation strategies in workplace settings.
Implications for research.
Despite much research over the past decade into the impact of interventions to influence employee health behaviours (Anderson 2009; Barr‐Anderson 2011; Benedict 2008; Cahill 2014; Fichtenberg 2002; Fishwick 2013; Freak‐Poli 2013; Geaney 2013; Kahn‐Marshall 2012; Maes 2012; Malik 2014; Mhurchu 2010; Rongen 2013; Vuillemin 2011; Wong 2012), implementation research within the workplace setting is only just emerging. In the absence of a strong empirical underpinning, governments and private enterprise will continue to invest in health promotion initiatives in the absence of direct evidence to inform strategies to support their implementation, potentially undermining the anticipated beneficial effects on employees. There is both considerable scope and need to improve the evidence base.
To this end, the review identified few trials using objective or validated measures of implementation outcomes, with most employing self‐reported, survey‐based measures of implementation at a high risk of performance and detection bias. The use of score‐based measures were common among the included trials; however, in all cases, the procedure used to calculate scores was unclear and may have included standardisation or transformation procedures. In doing so, the interpretation of effect sizes reported in trials was a considerable challenge, as it was not possible to determine what a unit change in the implementation measure represented. As robust measurement is fundamental for internal validity, the development, validation and use of rigorous and objective measures is urgently needed to develop the field and address limitations in study quality.
Many of the included trials were not primarily designed to assess the impact of implementation strategies; rather, they represented process measures of trials intended to examine the impact of a workplace intervention on the health behaviours of employees. While it was possible to extract implementation strategies and outcomes from these trials, they were typically small and underpowered to detect meaningful changes in implementation effects. Large, rigorous trials with the primary objective of assessing implementation outcomes, which are designed and powered to detect meaningful improvements, are required to strengthen the evidence base. The application of ‘hybrid effectiveness‐implementation’ research designs has been suggested as one means of improving the availability of research evidence to guide implementation efforts (Wolfenden 2016a). Hybrid designs take a dual focus from the start to assess the impact of interventions on individual health behaviours or clinical outcomes as well as the impact of strategies to enhance their implementation. Such designs enhance the ability to identify important intervention‐implementation interactions, which inform decisions about optimal deployment and generalised impact, and may accelerate the translation of research findings into routine practice. One published framework including three types of hybrid effectiveness‐implementation designs provides guidance for trialists in identifying and employing appropriate hybrid designs (Curran 2012).
Advances in the research area also require an understanding of how implementation strategies exert their effects (Lewis 2018). Workplace‐based trials that employ factorial designs would assist in identifying the relative and additional effects of specific implementation strategies. The use and testing of the underlying theory or proposed mechanism by which implementation strategies are hypothesised to work would also help improve the effects of future implementation efforts, and has recently been undertaken in trials of implementation interventions in schools and childcare services (Lee 2018). Such research requires authors of trials to specify how an implementation strategy will facilitate the implementation of workplace policies or practices promoting health. However, among studies reporting the use of a theoretical framework in this review, none specified the hypothesised determinants of effect targeted by the implementation strategies. The inclusion of clear conceptual or theoretical mechanistic models on which trials are based, and measures to assess implementation mechanisms in future randomised trials, would facilitate mechanistic evaluations (e.g. mediation analyses) to achieve this in the workplace setting. Furthermore, the availability and usability of future implementation research could be improved by the application of the EPOC taxonomy and recently released Standards for Reporting Implementation Studies (StaRI) Statement (EPOC 2015; Pinnock 2017).
What's new
| Date | Event | Description |
|---|---|---|
| 5 February 2019 | Amended | Typo in Plain Language Summary corrected to "The number of workplaces examined in the studies ranged from 12 to 114" (previously stated as 144). No further amendments. |
Acknowledgements
We would like to acknowledge the support provided by the Australian Prevention Partnership Centre, Cancer Council New South Wales, the Heart Foundation, and Hunter New England Population Health.
Appendices
Appendix 1. Search strategy
Database: MEDLINE 1946 to present with daily update (OVID)
Search strategy:
# Searches
1 Workplace/
2 Work/
3 Occupational Health/
4 Occupational Medicine/
5 1 or/1‐4
6 Health Behavior/
7 Health Education/
8 Health Promotion/
9 Healthy People Programs/
10 exp Primary Prevention/
11 Randomized Controlled Trial/
12 Controlled Clinical Trial/
13 Clinical Trials as Topic/
14 Random Allocation/
15 Evaluation Studies/
16 Comparative Study/
17 random*.tw.
18 trial.tw.
19 groups.tw.
20 placebo.tw.
21 experiment*.tw.
22 (time adj series).tw.
23 (pretest or pre test or posttest or post test).tw.
24 impact.tw.
25 change*.tw.
26 evaluat*.tw.
27 effect*.tw.
28 "before and after".tw.
29 intervention*.tw.
30 program*.tw.
31 compare*.tw.
32 (control or controls* or controla* or controle* or controli or controll*).tw.
33 or/6‐32
34 implement*.mp.
35 dissemin*.mp.
36 adopt*.mp.
37 practice*.mp.
38 organi?ational change*.mp.
39 diffus*.mp.
40 (system* adj2 change*).mp.
41 quality improvement*.mp.
42 transform*.mp.
43 translat*.mp.
44 transfer*.mp.
45 uptake*.mp.
46 sustainab*.mp.
47 institutionali*.mp.
48 routin*.mp.
49 maintenance.mp.
50 capacity.mp.
51 incorporat*.mp.
52 adher*.mp.
53 integrat*.mp.
54 scal*.mp.
55 ((polic* or practice* or program* or innovation*) adj5 (performance or feedback or prompt* or reminder* or incentive* or penalt* or communicat* or social market* or professional development or network* or leadership or opinion leader* or consensus process* or change manage* or train* or audit*)).mp.
56 or/34‐55
57 exp Obesity/
58 Weight Gain/
59 exp Weight Loss/
60 obes*.af.
61 (weight gain or weight loss).af.
62 (overweight or over weight or overeat* or over eat*).af.
63 weight change*.af.
64 ((bmi or body mass index) adj2 (gain or loss or change)).af.
65 exp Primary Prevention/
66 (primary prevention or secondary prevention).af.
67 (preventive measure* or preventative measure*).af.
68 (preventive care or preventative care).af.
69 (obesity adj2 (prevent* or treat*)).af.
70 or/57‐69
71 exp Exercise/
72 physical inactivity.mp.
73 physical activity.mp.
74 exp Motor Activity/
75 (physical education and training).mp.
76 exp "Physical Education and Training"/
77 Physical Fitness/
78 sedentary.tw.
79 exp Life Style/
80 exp Leisure Activities/
81 exp Sports/
82 Dancing/
83 dancing.mp.
84 (exercise* adj aerobic*).tw.
85 sport*.tw.
86 ((life style or life style) adj5 activ*).tw.
87 or/71‐86
88 exp Diet/
89 nutrition*.mp.
90 healthy eating.mp.
91 fruit*.tw.
92 vegetable*.tw.
93 canteen.mp.
94 menu.tw.
95 (calorie or calories).tw.
96 energy intake.tw.
97 energy density.tw.
98 eating.tw.
99 (feeding behavior or feeding behaviour).tw.
100 dietary intake.tw.
101 food.tw.
102 soft drink*.tw.
103 soda.tw.
104 sweetened drink*.tw.
105 fat.tw.
106 confectionary.tw.
107 menu planning.tw.
108 feeding program*.tw.
109 nutrition program*.tw.
110 nutritional program*.tw.
111 cafeteria*.tw.
112 nutritional status.tw.
113 or/88‐112
114 exp Smoking/
115 exp "tobacco Use Cessation"/
116 smok*.mp.
117 nicotine.mp.
118 tobacco use*.tw.
119 tobacco.mp.
120 exp tobacco/
121 or/114‐120
122 cessation.tw.
123 prevent*.tw.
124 stop*.tw.
125 quit*.tw.
126 abstin*.tw.
127 abstain*.tw.
128 reduc*.tw.
129 "tobacco use disorder".mp.
130 ex‐smoker*.mp.
131 anti‐smok*.mp.
132 or/122‐131
133 121 and 132
134 exp Alcohols/
135 exp Alcohol Drinking/
136 exp Alcohol Abuse/
137 exp Alcohol, Ethyl/ae
138 alcohol*.mp.
139 Drink*.mp.
140 liquor*.mp.
141 beer*.mp.
142 wine*.mp.
143 spirit*.mp.
144 drunk*.mp.
145 intoxicat*.mp.
146 binge.mp.
147 or/134‐146
148 70 or 87 or 113 or or 133 or 147
149 5 and 33 and 56 and 148
Database: MEDLINE In‐Process & Other Non‐Indexed Citations (OVID)
Search strategy:
# Searches
1 workplace*.mp.
2 work.mp.
3 Occupational Health.mp.
4 Occupational Medicine.mp.
5 1 or 2 or 3 or 4
6 Health Behavio?r*.mp.
7 Health Education.mp.
8 health promotion.mp.
9 Healthy People Program*.mp.
10 Primary Prevention.mp.
11 Randomized Controlled Trial/
12 Controlled Clinical Trial/
13 Evaluation Studies/
14 Comparative Study/
15 random*.tw.
16 trial.tw.
17 groups.tw.
18 placebo.tw.
19 experiment*.tw.
20 (time adj series).tw.
21 (pretest or pre test or posttest or post test).tw.
22 impact.tw.
23 change*.tw.
24 evaluat*.tw.
25 effect*.tw.
26 "before and after".tw.
27 intervention*.tw.
28 program*.tw.
29 compare*.tw.
30 (control or controls* or controla* or controle* or controli or controll*).tw.
31 or/6‐30
32 implement*.mp.
33 dissemin*.mp.
34 adopt*.mp.
35 practice*.mp.
36 organi?ational change*.mp.
37 diffus*.mp.
38 (system* adj2 change*).mp.
39 quality improvement*.mp.
40 transform*.mp.
41 translat*.mp.
42 transfer*.mp.
43 uptake*.mp.
44 sustainab*.mp.
45 institutionali*.mp.
46 routin*.mp.
47 maintenance.mp.
48 capacity.mp.
49 incorporat*.mp.
50 adher*.mp.
51 integrat*.mp.
52 scal*.mp.
53 ((polic* or practice* or program* or innovation*) adj5 (performance or feedback or prompt* or reminder* or incentive* or penalt* or communicat* or social market* or professional development or network* or leadership or opinion leader* or consensus process* or change manage* or train* or audit*)).mp.
54 or/32‐53
55 exp Obesity/
56 Weight Gain/
57 exp Weight Loss/
58 obes*.af.
59 (weight gain or weight loss).af.
60 (overweight or over weight or overeat* or over eat*).af.
61 weight change*.af.
62 ((bmi or body mass index) adj2 (gain or loss or change)).af.
63 exp Primary Prevention/
64 (primary prevention or secondary prevention).af.
65 (preventive measure* or preventative measure*).af.
66 (preventive care or preventative care).af.
67 (obesity adj2 (prevent* or treat*)).af.
68 or/55‐67
69 exp Exercise/
70 physical inactivity.mp.
71 physical activity.mp.
72 exp Motor Activity/
73 (physical education and training).mp.
74 exp "Physical Education and Training"/
75 Physical Fitness/
76 sedentary.tw.
77 exp Life Style/
78 exp Leisure Activities/
79 exp Sports/
80 Dancing/
81 dancing.mp.
82 (exercise* adj aerobic*).tw.
83 sport*.tw.
84 ((life style or life style) adj5 activ*).tw.
85 or/69‐84
86 exp Diet/
87 nutrition*.mp.
88 healthy eating.mp.
89 fruit*.tw.
90 vegetable*.tw.
91 canteen.mp.
92 menu.tw.
93 (calorie or calories).tw.
94 energy intake.tw.
95 energy density.tw.
96 eating.tw.
97 (feeding behavior or feeding behaviour).tw.
98 dietary intake.tw.
99 food.tw.
100 soft drink*.tw.
101 soda.tw.
102 sweetened drink*.tw.
103 fat.tw.
104 confectionary.tw.
105 menu planning.tw.
106 feeding program*.tw.
107 nutrition program*.tw.
108 nutritional program*.tw.
109 cafeteria*.tw.
110 nutritional status.tw.
111 or/86‐110
112 exp Smoking/
113 exp "tobacco Use Cessation"/
114 smok*.mp.
115 nicotine.mp.
116 tobacco use*.tw.
117 tobacco.mp.
118 exp tobacco/
119 or/112‐118
120 cessation.tw.
121 prevent*.tw.
122 stop*.tw.
123 quit*.tw.
124 abstin*.tw.
125 abstain*.tw.
126 reduc*.tw.
127 "tobacco use disorder".mp.
128 ex‐smoker*.mp.
129 anti‐smok*.mp.
130 or/120‐129
131 119 and 130
132 exp Alcohols/
133 exp Alcohol Drinking/
134 exp Alcohol Abuse/
135 exp Alcohol, Ethyl/ae
136 alcohol*.mp.
137 Drink*.mp.
138 liquor*.mp.
139 beer*.mp.
140 wine*.mp.
141 spirit*.mp.
142 drunk*.mp.
143 intoxicat*.mp.
144 binge.mp.
145 or/132‐144
146 68 or 85 or 111 or 131 or 145
147 5 and 31 and 54 and 146
Database: PsycINFO 1806 to May 2016 (OVID)
Search strategy:
# Searches
1 WORKPLACE INTERVENTION/ or Workplace.mp.
2 work.mp.
3 exp Occupational Health/
4 Occupational Medicine.mp.
5 1 or 2 or 3 or 4
6 Health Behavior/
7 Health Education/
8 Health Promotion/
9 Healthy People Program*.mp.
10 Primary prevention.mp.
11 exp Clinical Trials/
12 Evaluation Stud*.mp.
13 Comparative Stud*.mp.
14 random*.tw.
15 trial.tw.
16 groups.tw.
17 placebo.tw.
18 experiment*.tw.
19 (time adj series).tw.
20 (pretest or pre test or posttest or post test).tw.
21 impact.tw.
22 change*.tw.
23 evaluat*.tw.
24 effect*.tw.
25 "before and after".tw.
26 intervention*.tw.
27 program*.tw.
28 compare*.tw.
29 (control or controls* or controla* or controle* or controli or controll*).tw.
30 or/6‐29
31 implement*.mp.
32 dissemin*.mp.
33 adopt*.mp.
34 practice*.mp.
35 organi?ational change*.mp.
36 diffus*.mp.
37 (system* adj2 change*).mp.
38 quality improvement*.mp.
39 transform*.mp.
40 translat*.mp.
41 transfer*.mp.
42 uptake*.mp.
43 sustainab*.mp.
44 institutionali*.mp.
45 routin*.mp.
46 maintenance.mp.
47 capacity.mp.
48 incorporat*.mp.
49 adher*.mp.
50 integrat*.mp.
51 scal*.mp.
52 ((polic* or practice* or program* or innovation*) adj5 (performance or feedback or prompt* or reminder* or incentive* or penalt* or communicat* or social market* or professional development or network* or leadership or opinion leader* or consensus process* or change manage* or train* or audit*)).mp.
53 or/31‐52
54 Obesity/
55 Weight Gain/
56 Weight Loss/
57 obes*.af.
58 (weight gain or weight loss).af.
59 (overweight or over weight or overeat* or over eat*).af.
60 weight change*.af.
61 ((bmi or body mass index) adj2 (gain or loss or change)).af.
62 (primary prevention or secondary prevention).af.
63 (preventive measure* or preventative measure*).af.
64 (preventive care or preventative care).af.
65 (obesity adj2 (prevent* or treat*)).af.
66 or/54‐65
67 exp EXERCISE/
68 physical inactivity.mp.
69 exp Physical Activity/
70 Motor Activity.mp.
71 (physical education and training).mp.
72 exp Physical Education/
73 Physical Fitness/
74 exp SEDENTARY BEHAVIOR/ or sedentary.mp.
75 exp Lifestyle/
76 exp Leisure Time/ or Leisure Activities.mp.
77 exp SPORTS/
78 exp Dance/ or Dancing.mp.
79 (exercise* adj aerobic*).tw.
80 sport*.tw.
81 ((life style or life style) adj5 activ*).tw.
82 or/67‐81
83 Diet.mp.
84 nutrition*.mp.
85 healthy eating.mp.
86 fruit*.tw.
87 vegetable*.tw.
88 canteen.mp.
89 menu.tw.
90 (calorie or calories).tw.
91 energy intake.tw.
92 energy density.tw.
93 eating.tw.
94 (feeding behavior or feeding behaviour).tw.
95 dietary intake.tw.
96 food.tw.
97 soft drink*.tw.
98 soda.tw.
99 sweetened drink*.tw.
100 fat.tw.
101 confectionary.tw.
102 menu planning.tw.
103 feeding program*.tw.
104 nutrition* program*.tw.
105 cafeteria*.tw.
106 nutritional status.tw.
107 or/83‐106
108 exp TOBACCO SMOKING/
109 Smoking Cessation/
110 smok*.mp.
111 nicotine.mp.
112 tobacco.mp.
113 or/108‐112
114 cessation.tw.
115 prevent*.tw.
116 stop*.tw.
117 quit*.tw.
118 abstin*.tw.
119 abstain*.tw.
120 reduc*.tw.
121 "tobacco use disorder".mp.
122 ex‐smoker*.mp.
123 anti‐smok*.mp.
124 or/114‐123
125 113 and 124
126 exp ALCOHOLS/
127 exp Binge Drinking/ or exp Alcoholism/
128 exp Alcohol Abuse/
129 alcohol*.mp.
130 Drink*.mp.
131 liquor*.mp.
132 beer*.mp.
133 wine*.mp.
134 spirit*.mp.
135 drunk*.mp.
136 intoxicat*.mp.
137 binge.mp.
138 or/126‐137
139 66 or 82 or 107 or 125 or 138
140 5 and 30 and 53 and 139
141 1 or 3 or 4
142 30 and 53 and 139 and 141
Database: CINAHL (EBSCO)
# Query
S1 (MH "Work Environment") OR "Workplace"
S2 (MH "Work")
S3 (MH "Occupational Health")
S4 (MH "Occupational Medicine")
S5 S1 OR S2 OR S3 OR S4
S6 (MH "Health Behavior")
S7 (MH "Health Education")
S8 (MH "Health Promotion")
S9 Healthy People Program*
S10 (MH "Preventive Health Care") OR "Primary Prevention"
S11 (MH "Randomized Controlled Trials")
S12 (MH "Clinical Trials+")
S13 (MH "Random Assignment")
S14 (MH "Evaluation Research")
S15 (MH "Comparative Studies")
S16 TI random* OR AB random*
S17 TI trial OR AB trial
S18 TI groups OR AB groups
S19 TI placebo OR AB placebo
S20 TI experiment* OR AB experiment*
S21 TI (time n1 series) OR AB (time n1 series)
S22 TI ( (pretest or pre test or posttest or post test) ) OR AB ( (pretest or pre test or posttest or post test) )
S23 TI impact OR AB impact
S24 TI change* OR AB change*
S25 TI evaluat* OR AB evaluat*
S26 TI effect* OR AB effect*
S27 TI ( "before and after" ) OR AB ( "before and after" )
S28 TI intervention* OR AB intervention*
S29 TI program* OR AB program*
S30 TI compare* OR AB compare*
S31 TI ( (control or controls* or controla* or controle* or controli or controll*) ) OR AB ( (control or controls* or controla* or controle* or controli or controll*) )
S32 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31
S33 implement*
S34 dissemin*
S35 adopt*
S36 practice*
S37 "organi?ational change*"
S38 diffus*
S39 (system* n2 change*)
S40 "quality improvement*"
S41 transform*
S42 translat*
S43 transfer*
S44 uptake*
S45 sustainab*
S46 institutionali*
S47 routin*
S48 maintenance
S49 capacity
S50 incorporat*
S51 adher*
S52 integrat*
S53 scal*
S54 ((polic* or practice* or program* or innovation*) n5 (performance or feedback or prompt* or reminder* or incentive* or penalt* or communicat* or social market* or professional development or network* or leadership or opinion leader* or consensus process* or change manage* or train* or audit*))
S55 S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54
S56 (MH "Obesity+")
S57 (MH "Weight Gain")
S58 (MH "Weight Loss+")
S59 obes*
S60 (weight gain or weight loss)
S61 (overweight or over weight or overeat* or over eat*)
S62 "weight change*"
S63 ((bmi or body mass index) n2 (gain or loss or change))
S64 (primary prevention or secondary prevention)
S65 (preventive measure* or preventative measure*)
S66 (preventive care or preventative care)
S67 S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66
S68 (MH "Exercise+")
S69 "physical inactivity"
S70 (MH "Physical Activity")
S71 (MH "Motor Activity+")
S72 (MH "Physical Education and Training")
S73 "physical education and training"
S74 (MH "Physical Fitness")
S75 TI sedentary OR AB sedentary
S76 (MH "Life Style+")
S77 (MH "Leisure Activities+")
S78 (MH "Sports+")
S79 (MH "Dancing") OR "Dancing"
S80 TI (exercise* n1 aerobic*) OR AB (exercise* n1 aerobic*)
S81 TI sport* OR AB sport*
S82 TI ( ((life style or life style) n5 activ*) ) OR AB ( ((life style or life style) n5 activ*) )
S83 S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81 OR S82
S84 (MH "Diet+")
S85 "nutrition*"
S86 "healthy eating"
S87 TI fruit* OR AB fruit*
S88 TI vegetable* OR AB vegetable*
S89 canteen
S90 TI menu OR AB menu
S91 TI ( (calorie or calories) ) OR AB ( (calorie or calories) )
S92 TI "energy intake" OR AB "energy intake"
S93 TI "energy density" OR AB "energy density"
S94 TI eating OR AB eating
S95 TI ( (feeding behavior or feeding behaviour) ) OR AB ( (feeding behavior or feeding behaviour) )
S96 TI "dietary intake" OR AB "dietary intake"
S97 TI food OR AB food
S98 TI "soft drink*" OR AB "soft drink*"
S99 TI soda OR AB soda
S100 TI "sweetened drink*" OR AB "sweetened drink*"
S101 TI fat OR AB fat
S102 TI confectionary OR AB confectionary
S103 TI "menu planning" AND AB "menu planning"
S104 TI "feeding program*" OR AB "feeding program*"
S105 TI "nutrition program*" OR AB "nutrition program*"
S106 TI "nutritional program*" OR AB "nutritional program*"
S107 TI cafeteria* OR AB cafeteria*
S108 TI "nutritional status" OR AB "nutritional status"
S109 S84 OR S85 OR S86 OR S87 OR S88 OR S89 OR S90 OR S91 OR S92 OR S93 OR S94 OR S95 OR S96 OR S97 OR S98 OR S99 OR S100 OR S101 OR S102 OR S103 OR S104 OR S105 OR S106 OR S107 OR S108
S110 (MH "Smoking+")
S111 (MH "Smoking Cessation Programs")
S112 smok*
S113 nicotine
S114 (MH "Tobacco+") OR "tobacco"
S115 S110 OR S111 OR S112 OR S113 OR S114
S116 TI cessation OR AB cessation
S117 TI prevent* OR AB prevent*
S118 TI stop* OR AB stop*
S119 TI quit* OR AB quit*
S120 TI abstin* OR AB abstin*
S121 TI abstain* OR AB abstain*
S122 TI reduc* OR AB reduc*
S123 TI "tobacco use disorder" OR AB "tobacco use disorder"
S124 TI ex‐smoker* OR AB ex‐smoker*
S125 TI anti‐smok* OR AB anti‐smok*
S126 S116 OR S117 OR S118 OR S119 OR S120 OR S121 OR S122 OR S123 OR S124 OR S125
S127 S115 AND S126
S128 (MH "Alcohols+")
S129 (MH "Alcohol Drinking+")
S130 (MH "Alcohol Abuse")
S131 alcohol*
S132 Drink*
S133 liquor*
S134 beer*
S135 wine*
S136 spirit*
S137 drunk*
S138 intoxicat*
S139 binge
S140 S128 OR S129 OR S130 OR S131 OR S132 OR S133 OR S134 OR S135 OR S136 OR S137 OR S138 OR S139
S141 S67 OR S83 OR S109 OR S127 OR S140
S142 S5 AND S32 AND S55 AND S141
Database: the Cochrane Library (Wiley)
ID Search
#1 MeSH descriptor: [Workplace] this term only
#2 MeSH descriptor: [Work] this term only
#3 MeSH descriptor: [Occupational Health] this term only
#4 MeSH descriptor: [Occupational Medicine] this term only
#5 {or #1‐#4}
#6 MeSH descriptor: [Health Behavior] this term only
#7 MeSH descriptor: [Health Education] this term only
#8 MeSH descriptor: [Health Promotion] this term only
#9 MeSH descriptor: [Healthy People Programs] this term only
#10 MeSH descriptor: [Primary Prevention] explode all trees
#11 MeSH descriptor: [Randomized Controlled Trial] this term only
#12 MeSH descriptor: [Controlled Clinical Trial] this term only
#13 MeSH descriptor: [Clinical Trials as Topic] this term only
#14 MeSH descriptor: [Random Allocation] this term only
#15 MeSH descriptor: [Evaluation Studies] this term only
#16 MeSH descriptor: [Comparative Study] this term only
#17 random*:ti,ab
#18 trial:ti,ab
#19 groups:ti,ab
#20 placebo:ti,ab
#21 experiment*:ti,ab
#22 (time near/1 series):ti,ab
#23 (pretest or pre test or posttest or post test):ti,ab
#24 impact:ti,ab
#25 change*:ti,ab
#26 evaluat*:ti,ab
#27 effect*:ti,ab
#28 "before and after":ti,ab
#29 intervention*:ti,ab
#30 program*:ti,ab
#31 compare*:ti,ab
#32 (control or controls* or controla* or controle* or controli or controll*):ti,ab
#33 {or #6‐#32}
#34 implement*
#35 dissemin*
#36 adopt*
#37 practice*
#38 organi?ational change*
#39 diffus*
#40 (system* near/2 change*)
#41 quality improvement*
#42 transform*
#43 translat*
#44 transfer*
#45 uptake*
#46 sustainab*
#47 institutionali*
#48 routin*
#49 maintenance
#50 capacity
#51 incorporat*
#52 adher*
#53 integrat*
#54 scal*
#55 ((polic* or practice* or program* or innovation*) near/5 (performance or feedback or prompt* or reminder* or incentive* or penalt* or communicat* or social market* or professional development or network* or leadership or opinion leader* or consensus process* or change manage* or train* or audit*))
#56 {or #34‐#55}
#57 MeSH descriptor: [Obesity] explode all trees
#58 MeSH descriptor: [Weight Gain] this term only
#59 MeSH descriptor: [Weight Loss] this term only
#60 obes*
#61 (weight gain or weight loss)
#62 (overweight or over weight or overeat* or over eat*)
#63 weight change*
#64 ((bmi or body mass index) near/2 (gain or loss or change))
#65 MeSH descriptor: [Primary Prevention] explode all trees
#66 (primary prevention or secondary prevention)
#67 (preventive measure* or preventative measure*)
#68 (preventive care or preventative care)
#69 (obesity near/2 (prevent* or treat*))
#70 {or #57‐#69}
#71 MeSH descriptor: [Exercise] explode all trees
#72 physical inactivity
#73 physical activity
#74 MeSH descriptor: [Motor Activity] explode all trees
#75 "physical education and training"
#76 MeSH descriptor: [Physical Education and Training] explode all trees
#77 MeSH descriptor: [Physical Fitness] this term only
#78 sedentary:ti,ab
#79 MeSH descriptor: [Life Style] explode all trees
#80 MeSH descriptor: [Leisure Activities] explode all trees
#81 MeSH descriptor: [Sports] explode all trees
#82 MeSH descriptor: [Dancing] this term only
#83 dancing
#84 (exercise* near/1 aerobic*)
#85 sport*:ti,ab
#86 ((life style or life style) near/5 activ*):ti,ab
#87 {or #71‐#86}
#88 MeSH descriptor: [Diet] explode all trees
#89 nutrition*
#90 healthy eating
#91 fruit*:ti,ab
#92 vegetable*:ti,ab
#93 canteen
#94 menu:ti,ab
#95 (calorie or calories):ti,ab
#96 energy intake:ti,ab
#97 energy density:ti,ab
#98 eating:ti,ab
#99 (feeding behavior or feeding behaviour):ti,ab
#100 dietary intake:ti,ab
#101 food:ti,ab
#102 soft drink*:ti,ab
#103 soda:ti,ab
#104 sweetened drink*:ti,ab
#105 fat:ti,ab
#106 confectionary:ti,ab
#107 menu planning:ti,ab
#108 feeding program*:ti,ab
#109 nutrition program*:ti,ab
#110 nutritional program*:ti,ab
#111 cafeteria*:ti,ab
#112 nutritional status:ti,ab
#113 {or #88‐#112}
#114 MeSH descriptor: [Smoking] explode all trees
#115 MeSH descriptor: [Tobacco Use Cessation] explode all trees
#116 smok*
#117 nicotine
#118 tobacco use*
#119 tobacco
#120 MeSH descriptor: [Tobacco] explode all trees
#121 {or #114‐#120}
#122 cessation:ti,ab
#123 prevent*:ti,ab
#124 stop*:ti,ab
#125 quit*:ti,ab
#126 abstin*:ti,ab
#127 abstain*:ti,ab
#128 reduc*:ti,ab
#129 "tobacco use disorder":ti,ab
#130 ex‐smoker*:ti,ab
#131 anti‐smok*:ti,ab
#132 {or #122‐#131}
#133 {and #121, #132}
#134 MeSH descriptor: [Alcohols] explode all trees
#135 MeSH descriptor: [Alcohol Drinking] explode all trees
#136 MeSH descriptor: [Alcoholism] explode all trees
#137 MeSH descriptor: [Ethanol] explode all trees
#138 alcohol*
#139 Drink*
#140 liquor*
#141 beer*
#142 wine*
#143 spirit*
#144 drunk*
#145 intoxicat*
#146 binge
#147 {or #134‐#146}
#148 {or #70, #87, #113, #133, #147}
#149 {and #5, #33, #56, #148}
Database: ERIC (Proquest)
Work or workplace or “occupational medicine” or “occupational health”
And
“health behavior*” or “health behaviour*” or “health education” or “health promotion” or “primary prevention” or random* or “evaluation stud*” or “comparative stud*” or trial or groups or placebo or experiment* or (time and series) or pretest or “pre test” or posttest or “post test” or impact or change* or evaluat* or effect* or “before and after” or intervention* or program* or compare* or control or controls* or controla* or controle* or controli or controll*
and
implement* or disseminat* or adopt* or practice* or organi?ational change* or diffus* or (system* and change*) or quality improvement* or transform* or translat* or transfer* or uptake* or sustainab* or institutionali* or routin* or maintenance or capacity or incorporat* or adher* or integrat* or scal* or ((polic* or practice* or program* or innovation*) and (performance or feedback or prompt* or reminder* or incentive* or penalt* or communicat* or social market* or professional development or network* or leadership or opinion leader* or consensus process* or change manage* or train* or audit*))
and
obes* or weight gain or weight loss or overweight or over weight or overeat* or over eat* or weight change* or ((bmi or body mass index) and (gain or loss or change)) or primary prevention or secondary prevention or preventive measure* or preventative measure* or preventive care or preventative care or (obesity and (prevent* or treat*)) or exercise or physical inactivity or physical activity or Motor Activity or (physical education and training) or Physical Fitness or sedentary or Life Style or Leisure Activiti* or sport* or dancing or diet or nutrition* or healthy eating or fruit* or vegetable* or canteen or food or menu or calorie or calories or energy intake or energy density or eating or feeding behavior or feeding behaviour or dietary intake or soft drink* or soda or sweetened drink* or fat or confectionary or feeding program* or cafeteria* or ((smok* or tobacco or nictotine) and (cessation or stop* or quit* or abstin* or abstain* or reduc* or ex‐smoker* or anti‐smok*)) or alcohol* or drink* or liquor* or beer* or wine* or spirit* or drunk* or intoxicat* or binge
Database: Dissertations and Theses
Title: workplace or work or occupational health or occupational medicine
AND
Title: alcohol or smoking or tobacco or lifestyle or diet or nutrition or healthy eating or physical activity or exercise or obesity or weight
Database: SCOPUS (SCOPUS website)
TITLE‐ABS‐KEY ( workplace OR "occupational medicine" OR "occupational health" )
AND TITLE‐ABS‐KEY ( "health behavior*" OR "health behaviour*" OR "health education" OR "health promotion" OR "primary prevention" OR random* OR "evaluation stud*" OR "comparative stud*" OR trial OR groups OR placebo OR experiment* OR ( time AND series ) OR pretest OR "pre test" OR posttest OR "post test" OR impact OR change* OR evaluat* OR effect* OR "before and after" OR intervention* OR program* OR compare* OR control OR controls* OR controla* OR controle* OR controli OR controll* )
AND TITLE‐ABS‐KEY ( implement* OR disseminat* OR adopt* OR practice* OR organi?ational change* OR diffus* OR ( system* AND change* ) OR quality improvement* OR transform* OR translat* OR transfer* OR uptake* OR sustainab* OR institutionali* OR routin* OR maintenance OR capacity OR incorporat* OR adher* OR integrat* OR scal* OR ( ( polic* OR practice* OR program* OR innovation* ) AND ( performance OR feedback OR prompt* OR reminder* OR incentive* OR penalt* OR communicat* OR social market* OR professional development OR network* OR leadership OR opinion leader* OR consensus process* OR change manage* OR train* OR audit* ) ) )
AND TITLE‐ABS‐KEY (obes* or weight gain or weight loss or overweight or over weight or overeat* or over eat* or weight change* or ((bmi or body mass index) and (gain or loss or change)) or primary prevention or secondary prevention or preventive measure* or preventative measure* or preventive care or preventative care or (obesity and (prevent* or treat*)) or exercise or physical inactivity or physical activity or Motor Activity or (physical education and training) or Physical Fitness or sedentary or Life Style or Leisure Activiti* or sport* or dancing or diet or nutrition* or healthy eating or fruit* or vegetable* or canteen or food or menu or calorie or calories or energy intake or energy density or eating or feeding behavior or feeding behaviour or dietary intake or soft drink* or soda or sweetened drink* or fat or confectionary or feeding program* or cafeteria* or ((smok* or tobacco or nictotine) and (cessation or stop* or quit* or abstin* or abstain* or reduc* or ex‐smoker* or anti‐smok*)) or alcohol* or drink* or liquor* or beer* or wine* or spirit* or drunk* or intoxicat* or binge)
AND ( LIMIT‐TO ( SUBJAREA , "MEDI" ) OR LIMIT‐TO ( SUBJAREA , "SOCI" ) OR LIMIT‐TO ( SUBJAREA , "NURS" ) OR LIMIT‐TO ( SUBJAREA , "HEAL" ) ) AND ( LIMIT‐TO ( EXACTKEYWORD , "Human" ) OR LIMIT‐TO ( EXACTKEYWORD , "Humans" ) ) AND ( EXCLUDE ( SUBJAREA , "BUSI" ) OR EXCLUDE ( SUBJAREA , "CENG" ) OR EXCLUDE ( SUBJAREA , "CHEM" ) OR EXCLUDE ( SUBJAREA , "COMP" ) OR EXCLUDE ( SUBJAREA , "DECI" ) OR EXCLUDE ( SUBJAREA , "ARTS" ) OR EXCLUDE ( SUBJAREA , "ECON" ) OR EXCLUDE ( SUBJAREA , "PHYS" ) OR EXCLUDE ( SUBJAREA , "MATH" ) OR EXCLUDE ( SUBJAREA , "ENER" ) OR EXCLUDE ( SUBJAREA , "VETE" ) )
Database: the Campbell Library (the Campbell Library Website)
Work OR workplace or occupational health OR occupational medicine (separate searches)
Appendix 2. 'Risk of bias' assessment tool
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | |
| Criteria for the judgement of a 'High risk' of bias | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐ random categorisation of participants, for example:
|
| Criteria for the judgement of a low risk of bias | The investigators describe a random component in the sequence generation process such as:
*Minimisation may be implemented without a random element, and this is considered to be equivalent to being random |
| Criteria for the judgement of an unclear risk of bias | Insufficient information about the sequence generation process to permit judgement of low or high risk |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to consignment | |
| Criteria for the judgement of a high risk of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
| Criteria for the judgement of a low risk of bias | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
|
| Criteria for the judgement of an unclear risk of bias | Insufficient information to permit judgement of low or high risk. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement ‐ for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed |
|
BLINDING OF PARTICIPANTS AND PERSONNEL
Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | |
| Criteria for the judgement of a high risk of bias | Any one of the following:
|
| Criteria for the judgement of a low risk of bias | Any one of the following:
|
| Criteria for the judgement of a low risk of bias | Any one of the following:
|
|
BLINDING OF OUTCOME ASSESSMENT
Detection bias due to knowledge of the allocated interventions by outcome assessors | |
| Criteria for the judgement of a high risk of bias | Any one of the following:
|
| Criteria for the judgement of a low risk of bias | Any one of the following:
|
| Criteria for the judgement of an unclear risk of bias | Any one of the following:
|
|
INCOMPLETE OUTCOME DATA
Attrition bias due to amount, nature or handling of incomplete outcome data | |
| Criteria for the judgement of a high risk of bias | Any one of the following:
|
| Criteria for the judgement of a low risk of bias | Any one of the following:
|
| Criteria for the judgement of an unclear risk of bias | Any one of the following:
|
|
SELECTIVE REPORTING
Reporting bias due to selective outcome reporting | |
| Criteria for the judgement of a high risk of bias | Any one of the following:
|
| Criteria for the judgement of a low risk of bias | Any of the following:
|
| Criteria for the judgement of an unclear risk of bias | Insufficient information to permit judgement of low or high risk. It is likely that most studies will fall into this category. |
|
OTHER BIAS
Bias due to problems not covered elsewhere in the table | |
| Criteria for the judgement of a high risk of bias | There is at least one important risk of bias. For example, the study:
|
| Criteria for the judgement of a low risk of bias | The study appears to be free of other sources of bias |
| Criteria for the judgement of an unclear risk of bias | There may be a risk of bias, but there is either:
|
Appendix 3. Risk of bias assessment ‐ review secondary outcomes
Bandoni 2010
| Risk of bias | ||
| Bias | Author's judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear | No information on method of generating random sequence |
| Allocation concealment (selection bias) | Unclear | No information on whether allocation was concealed prior to assignment |
| Blinding of participants and personnel (performance bias) Employee health behaviours (diet) |
High | Component of intervention was distribution of educational materials to workers and product labelling (Bandoni 2010, p 976) |
| Blinding of outcome assessment (detection bias) Employee health behaviours (diet) |
High | Worker self‐report of amount of fruit and vegetables consumed in interview with researchers during visit – neither blind (Bandoni 2010, p 977). |
| Incomplete outcome data (attrition bias) Employee health behaviours (diet) |
Unclear | At baseline, 1296 individuals (intervention: 651; control: 645) were studied. Postintervention 1214 individuals (intervention: 630; control: 584). Independent samples (Bandoni 2010, p 977). Greater proportion drop in participation in control group compared to intervention group. Unclear if this biased results |
| Selective reporting (reporting bias) | Unclear | No mention of a priori registration of measures or publication of protocol |
| Recruitment to cluster | Low | All workers in participating workplaces invited to participate (Bandoni 2010, p 976) |
| Baseline imbalances | Low | After adjustment for socio‐demographic characteristics (sex, education and age), the effect of the intervention on the consumption of fruits and vegetables by workers remained significant (Bandoni 2010, p 979) |
| Loss of clusters | Unclear | One company dropped out – final sample intervention: 15; control: 14. Analysis did not include imputation of missing data so unclear whether this biased results (Bandoni 2010, p 976) |
| Incorrect analysis | Unclear | No mention of adjustment for clustering within workplace clusters. Unclear what impact this may have on study findings |
| Compatibility with individually randomised controlled trials (herd effect) | Not applicable given secondary measure | — |
| Other bias | Low | — |
Biener 1999
| Risk of bias | ||
| Bias | Author's judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear | No information on method of generating random sequence |
| Allocation concealment (selection bias) | Unclear | No information on whether allocation was concealed prior to assignment |
| Blinding of participants and personnel (performance bias)
Employee health behaviours (diet and tobacco use) |
High | Intervention implementation actively involved workplace staff participation at all organisational levels |
| Blinding of outcome assessment (detection bias) Employee health behaviours (diet and tobacco use) |
High | Tobacco use self‐reported by employees using survey; diet self‐reported using food frequency questionnaire (Abrams 1994) Methods of distribution of employee survey varied by study center, which could contribute to elevated risk of bias if differences between intervention and control groups. Florida and Brown mailed surveys to each employee in the work site, Dana‐Farber mailed surveys to a random sample of employees in each work site, and MD Anderson administered questionnaires to employees at mandatory work site meetings (Sorensen 1996, p 940) |
| Incomplete outcome data (attrition bias) Employee health behaviours (diet and tobacco use) |
Low | At baseline, the overall response rate to the employee survey was 69% (average work‐site response rate, 72%; study center mean range, 61% to 89%). The overall response rate at the follow‐up survey was 71% (average work‐site response rate, 75%; study center mean range 68% to 86%). The interaction of the response rate subgroup (cutpoint, 65%) and the intervention group indicated no relationship between the intervention effects and the work site's response rate to the individual survey (smallest P = 0.24) (Sorensen 1996, p 943). |
| Selective reporting (reporting bias) | Low | All pre‐specified outcomes (Abrams 1994, Fig 1) reported in Sorensen 1996 and Biener 1999 |
| Recruitment to cluster | Unclear | The methods of recruitment varied by study center, which could contribute to elevated risk of bias if differences between intervention and control groups. Florida and Brown mailed surveys to each employee in the work site, Dana‐Farber mailed surveys to a random sample of employees in each work site, and MD Anderson administered questionnaires to employees at mandatory work site meetings (Sorensen 1996 p 940) |
| Baseline imbalances | Low | Clusters matched and no significant baseline imbalances in outcomes measure for individual level data (Sorensen 1996) and demographic characteristics (Biener 1999) |
| Loss of clusters | Unclear | 114 worksites initially recruited, 3 (2 intervention, 1 control) dropped out due to economic dislocations, leaving 111 in final sample. For pairwise analyses, three pairs were excluded, leaving a total of 108 work sites (Sorensen 1996) |
| Incorrect analysis | Low | Analysis adjusted for clustering effect (intraclass correlation) (Abrams 1994) |
| Compatibility with individually randomised controlled trials (herd effect) | Not applicable given secondary measure | — |
| Other bias | Low | — |
Parker 2010
| Risk of bias | ||
| Bias | Author's judgement | Support for judgement |
| Random sequence generation (selection bias) | High | Non‐randomised trial |
| Allocation concealment (selection bias) | High | Non‐randomised trial |
| Blinding of participants and personnel (performance bias)
Employee health behaviours (diet, physical activity and weight status) |
High: (diet and physical activity) Low: (weight status) |
Self‐reported health behaviours Objective biometric measures |
| Blinding of outcome assessment (detection bias) Employee health behaviours (diet, physical activity and weight status) |
High (diet and physical activity) Low (weight status) |
Self‐reported health behaviours Objective biometric measures |
| Incomplete outcome data (attrition bias) Employee health behaviours (diet, physical activity and weight status) |
Low | Attrition was 54.3% and 45.1% for the intervention and control group respectively. To address the issue of missing data, several statistical approaches were used to adjust for the potential bias due to attrition (Goetzel 2010, p 300). |
| Selective reporting (reporting bias) | Unclear | Wilson 2007 indicates primary outcome BMI and development work of environmental assessment tool demonstrates intention to include in outcome assessment. No indications that any predetermined outcomes were otherwise omitted |
| Recruitment to cluster | Low | No difference in recruitment methods across treatment groups, with all employees at all study sites encouraged to participate in the health risk assessment (HRA) and biometric screening programmes (Goetzel 2010, p 292) |
| Baseline imbalances | Low | When comparing overweight and obesity prevalence between subjects at intervention and control sites, there were no significant differences between groups at baseline. Adjustment undertaken to correct for baseline imbalances in demographic characteristics using propensity score weights (Goetzel 2010, pp 292‐3). |
| Loss of clusters | Low | No loss of clusters (Parker 2010) |
| Incorrect analysis | Low | Worksite's influence on outcomes was evaluated by including a site‐level variable in the predictive models (adjustment for clustering) (Goetzel 2010, p 294) |
| Compatibility with individually randomised controlled trials (herd effect) | Not applicable given secondary measure. | — |
| Risk of bias due to confounding factors (adequate adjustment) | Low | Adjusted for baseline imbalances in demographic characteristics using propensity score weights (Goetzel 2010, pp 292‐3) |
Hannon 2012
| Risk of bias | ||
| Random sequence generation (selection bias) | Low | Block randomisation undertaken by statistician (assume computerised) (Hannon 2012, p 127) |
| Allocation concealment (selection bias) | Low | Block randomisation undertaken by statistician (assume computerised) (Hannon 2012, p 127) |
| Blinding of participants and personnel (performance bias)
cost estimates |
High | Intervention implementation actively involved workplace staff participation. |
| Blinding of outcome assessment (detection bias) cost estimates |
High | Outcomes self‐reported by workplace staff (Hannon 2012, p 127) |
| Incomplete outcome data (attrition bias) cost estimates |
Unclear | Response rate to cost outcome questions 77% at baseline and 71% at follow‐up. Unclear whether similar across groups (Hannon 2012, p 129) |
| Selective reporting (reporting bias) | Unclear | No mention of a priori registration of measures or publication of protocol |
| Other bias | Low | — |
Data and analyses
Comparison 1. Implementation strategy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Implementation score | 3 | 164 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.32, 0.30] |
1.1. Analysis.
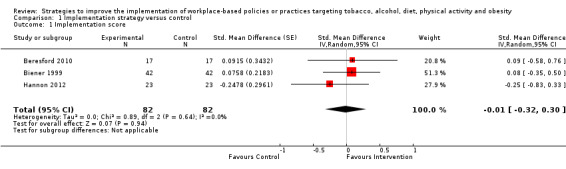
Comparison 1 Implementation strategy versus control, Outcome 1 Implementation score.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bandoni 2010.
| Methods |
Study design: randomised controlled trial Intervention duration: 6 months Length of follow‐up from baseline: 6 months Differences in baseline characteristics: some differences apparent in employee level of education (12 years or more of education: intervention 46% vs control 33%), however P values not reported. Employee dietary behaviours differed significantly only for distribution of total fruit and percentage of energy from fat (P < 0.05). Unit of allocation: workplaces Unit of analysis: implementation outcomes were analysed by workplace and health behaviour outcomes were analysed by employee |
|
| Participants |
Workplace type: workplaces predominantly from the industrial sector Region: Sao Paulo, Brazil Demographic/socioeconomic characteristics: fewer than half of employees had completed 12 or more years of schooling (intervention 46%; control 33%). Most employees were male (67%). Inclusion/exclusion criteria: Workplaces: Inclusion:
Employees: not reported Number of workplaces allocated: 30 Numbers by trial group: Workplaces: n (controls baseline) = 15 n (controls follow‐up) = 14 n (interventions baseline) = 15 n (interventions follow‐up) = 15 Employees: n (controls baseline) = 645 n (controls follow‐up) = 584 n (interventions baseline) = 651 n (interventions follow‐up) = 630 Recruitment: Workplaces: not reported. Employees: all employees at workplaces partaking in the trial were invited by researchers to participate in the study. Those employees who agreed voluntarily and signed a consent form were recruited to the study. Recruitment rate: Workplaces: 42% Employees: 11.7% |
|
| Interventions |
Number of experimental conditions: 2 (1 intervention, 1 control) Policies or practices targeted by the intervention: The availability of fruits and vegetables in lunchtime meals served by workplace cafeterias Implementation strategies: EPOC: educational materials Cafeteria managers received an educational manual developed by research staff providing information on the Workers Food Program and its nutritional guidelines, as well as the importance of a balanced diet for the health and performance of employees, highlighting the key role of fruits and vegetables. The contents of the manual were presented by research staff and discussed with the cafeteria managers. EPOC: educational meetings Research staff delivered culinary workshops to food service staff including cafeterias workers, cooks and kitchen assistants. The workshops included recipe suggestions for incorporating fruits and vegetables into meals and guidance on the presentation and arrangement of culinary preparations. Theoretical underpinning: Ecological Model for Health Promotion Description of control: wait‐list control. Workplaces in the control group continued practice as normal during the study period. Following completion of the study, control workplaces received copies of the educational materials and strategies used in the intervention. |
|
| Outcomes |
Outcome relating to the implementation of workplace based policies or practices: Grams of fruits and vegetables in lunch meals served by workplace cafeterias Data collection method: a company food service survey was conducted at 2 time points: baseline and 6 months postbaseline. Food service managers recorded 3 successive days of meals offered to employees. Based on the food service managers' reports, all foods that were prepared for serving were listed and their respective quantities of fruits and vegetables per customer per day were recorded as standard portions. The quantities per customer were established by the mean of consumption in each cafeteria. Validity of measure: not reported Outcome relating to diet, physical activity, weight status, tobacco use or alcohol use: Employee's consumption of fruits and vegetables (grams per meal) in lunch meals purchased from workplace cafeterias Data collection method: an employee survey was conducted at 2 time points: baseline and 6 months postbaseline. Research staff surveyed employees on the portion of fruits and vegetables consumed at lunch meals, using as a reference the utensils used in the distribution of meals in the cafeteria. Foods offered by the workplace cafeteria that day were used to collect data. Employee reported portions were recorded and converted into grams to determine the consumption of fruits and vegetables in lunch meals. Validity of measure: not reported Outcome relating to cost: not reported Outcome relating to adverse consequences: not reported |
|
| Notes |
Research funding: study was supported by the National Council for Scientific and Technological Development Conflicts of interest: study authors reported no conflicts of interests Additional information requested from trial authors: information was requested to confirm the number of workplaces in experimental groups at baseline. No response was received. Given the trial reported equal random assignment to experimental groups, and that one workplace dropped out of the trial following allocation leaving 14 control and 15 intervention workplaces, it was assumed the workplace lost at follow‐up was from the control group. As such, numbers at baseline were reported in the review as n = 15 for each experimental group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on method of generating random sequence |
| Allocation concealment (selection bias) | Unclear risk | No information on whether allocation was concealed prior to assignment |
| Blinding of participants and personnel (performance bias) Policy or practice implementation | High risk | Managers from participating workplaces were involved in delivering the intervention (Bandoni 2010, p 976) |
| Blinding of outcome assessment (detection bias) Policy or practice implementation | High risk | Food service manager self‐report in interview with researchers during visit, neither blind (Bandoni 2010, p 977) |
| Incomplete outcome data (attrition bias) Policy or practice implementation | Unclear risk | One company dropped out. Final sample intervention: 15; control: 14. Analysis did not include imputation of missing data, therefore unclear whether this biased results (Bandoni 2010, p 976) |
| Selective reporting (reporting bias) | Unclear risk | No mention of a priori registration of measures or publication of protocol |
| Other bias | Low risk | |
Beresford 2010.
| Methods |
Trial name: the Physical Activity and Changes in Eating (PACE) project Study design: randomised controlled trial Intervention duration: the total intervention period was 15 to 18 months Length of follow‐up from baseline: 2 years Differences in baseline characteristics: there were no significant differences in workplace characteristics or Environmental Assessment (EA) checklist scores between intervention and control groups at baseline. Unit of allocation: workplaces Unit of analysis: workplaces |
|
| Participants |
Workplace type: small to medium workplaces in the manufacturing, transportation and utilities, and personal and household services industries Region: Seattle metropolitan area, Washington, USA Demographic/socioeconomic characteristics: most employees (80%) were white, and 63% had attained a tertiary level of education. The proportion of male and female employees was approximately equal. Inclusion/exclusion criteria: Workplaces: Inclusion:
Exclusion: workplaces with a wellness programme that had an on site physical activity or nutrition component Employees: not reported Number of workplaces allocated: 34 Numbers by trial group: Workplaces: n (controls baseline) = 17 n (controls follow‐up) = 17 n (interventions baseline) = 17 n (interventions follow‐up) = 17 Employees: not reported Recruitment: Workplaces: the trial recruited workplaces in the Seattle metropolitan area restricted by size and guided by standardised industrial classification (SIC) codes. Research staff mailed, called and then visited eligible companies, giving priority to eligible companies within one hour of travel from the study centre Employees: not reported Recruitment rate: Workplaces: 42% Employees: not reported |
|
| Interventions |
Number of experimental conditions: 2 (1 intervention, 1 control) Policies or practices targeted by the intervention: Workplace physical activity, nutrition and weight control practices at three levels including: Organisational level:
Environmental level:
Individual level:
Implementation strategies: EPOC: tailored intervention 2 focus groups were conducted with 11 employee volunteers prior to intervention implementation to further refine the intervention framework and strategies. The first focus group was used to identify key barriers and facilitators perceived by employees to improving dietary intake and physical activity, and to gather suggestions regarding appropriate workplace intervention activities. In the second focus group, employees were asked to brainstorm ideas on the best way to present messages developed in the first focus group, and to provide feedback on intervention promotional materials (e.g. posters and information resources) and proposed intervention activities. EPOC: local opinion leaders and local consensus process Workplaces were assisted to establish EABs. EABs included 4‐7 employees who volunteered or were nominated by the workplace primary contact person for the intervention. The EAB included employees from all occupational sectors in the workplace and worked closely with the project interventionist from the research team to design, plan and implement intervention activities best suited to the workplace. The EAB additionally worked with the senior management to obtain commitment to sponsor ongoing opportunities for healthy eating and physical activity promotion in the workplace. EPOC: educational materials EAB members were provided with a handbook that described the study, explained their role as an EAB member, and provided the intervention framework necessary to carry out the intervention in their workplace. The intervention framework specified the minimum requirements for intervention implementation; however, EABs were encouraged to do more. A number of intervention activities and messages that the EAB could tailor for their workplace were also provided in the handbook. Theoretical underpinning: Modified Ecological Framework Description of control: wait‐list control. Workplace practices continued as normal during the study period. After follow‐up data collection, control workplaces received the intervention materials, assistance with establishing an EAB, and the EAB handbook for members of the board. |
|
| Outcomes |
Outcome relating to the implementation of workplace based policies or practices: Implementation of 11 workplace environmental practices to promote increased physical activity, healthy eating and weight control including: The physical environment:
The information environment:
Worksite resources: existence and/or sponsorship of workplace weight control or physical activity programmes Data collection method: environmental observation. An EA checklist was delivered at 2 time points: baseline and 2 years postbaseline. The EA checklist was adapted from the Checklist of Health Promotion Environments at Worksites (CHEW) tool. The checklist included the following sections: parking, bicycle and grounds assessment; neighbourhood assessment; building assessment (exterior, interior, and stairwells); signage assessment (physical activity and nutrition); vending machine assessment; and weight control or physical activity programmes. Checklists were completed by a research staff member (EA rater) at the workplace site. Checklist items measuring implementation of each practice were combined into an EA score using an average or a sum standardised for the size of the company when appropriate. Validity of measure: not reported, however observation represents an objective assessment of the work environment Outcome relating to diet, physical activity, weight status, tobacco use or alcohol use: not reported Outcome relating to cost: not reported Outcome relating to adverse consequences: not reported |
|
| Notes |
Research funding: study was funded by a grant from National Heart, Lung and Blood Institute: R01 HL79491 Conflicts of interest: not specified by study authors Additional information requested from trial authors: information was requested regarding the number of workplaces and employees in experimental groups and whether follow‐up data were available for relevant employee health behaviour outcomes reported in a companion baseline paper (Beresford 2007). Information was provided for the number of workplaces in experimental groups, but not the number of employees, and this information was reported accordingly in the review. Additionally, it was confirmed follow‐up results for employee health behaviours were not yet published, therefore these outcomes could not be included in the review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on method of generating random sequence |
| Allocation concealment (selection bias) | Unclear risk | No information on whether allocation was concealed prior to assignment |
| Blinding of participants and personnel (performance bias) Policy or practice implementation | High risk | Intervention implementation actively involved workplace staff (Beresford 2010, pp 3‐5) |
| Blinding of outcome assessment (detection bias) Policy or practice implementation | Unclear risk | Observation of physical environment with objective measures; however, no mention of whether raters were blind |
| Incomplete outcome data (attrition bias) Policy or practice implementation | Unclear risk | 34 workplaces randomised, 1 withdrew following baseline data collection. No information provided regarding the inclusion of this workplace in the analysis or which treatment group this workplace was from (Beresford 2010, p 3) |
| Selective reporting (reporting bias) | High risk | Beresford 2007 indicates that physical activity and diet outcome measures will be assessed |
| Other bias | Low risk | |
Biener 1999.
| Methods |
Trial name: the Working Well Trial Study design: randomised controlled trial Intervention duration: 2 years Length of follow‐up from baseline: 3 years. Baseline data collected from September to December 1990 and follow‐up data collected from September to December 1993 Differences in baseline characteristics: there were no significant demographic differences at baseline between intervention and control workplaces. Unit of allocation: workplace Unit of analysis: workplace |
|
| Participants |
Workplace type: workplaces were from the manufacturing, communications, public service and utilities sectors. Workplace size ranged from 49 to 1700 employees Region: 16 states across the USA. The intervention was coordinated and delivered through 4 study centres including:
Demographic/socioeconomic characteristics: employees were predominantly middle‐aged (mean 41 years, SD 11), white (92%), male (67%), and employed in blue collar jobs (service work, manual labour, machine operation and skilled work). Half (49%) of employees had completed at least some college education. Inclusion/exclusion criteria: Workplaces: Inclusion criteria was specific to study centre: DFCI:
BROWN:
MDACC: number of employees > 75 (working full time) Employees: Inclusion (all study centres): classified as a permanent employee, working at least 50% of work time Number of workplaces allocated: 114 Numbers by trial group: Workplaces n (controls baseline) = 57 n (controls follow‐up) = 56 n (interventions baseline) = 57 n (interventions follow‐up) = 55 Employees: n (controls baseline) = 10,730 n (controls follow‐up) = 9291 n (interventions baseline) = 10,071 n (interventions follow‐up) = 8914 Recruitment: Workplaces: Recruitment methods were specific to study centre: UF: a single company corporate headquarters was approached and provided UF with a list of company workplaces in a defined geographical area. All of these workplaces were contacted to participate in the study. DFCI and BROWN: a Dunn and Bradstreet database was used to identify eligible workplaces within a defined geographical region MDACC: Workplaces were recruited either through the National Rural Electric Co‐op Association or through natural gas pipeline corporations Employees: Recruitment methods for participation in the employee survey were specific to study centre. UF and BROWN mailed questionnaires to all employees at workplaces and had them return completed surveys via postal mail or to a mailbox at the workplace. DFCI mailed questionnaires to a random sample of employees at baseline and another random sample at follow‐up. MDACC disseminated questionnaires to all employees at workplaces during required safety meetings. Recruitment rate: Workplaces: UF: 100% DFCI: 15% BROWN: 16% MDACC: 70% Employees: The trial used two cross‐sectional surveys of employees. The overall response rate at baseline was 69% and at follow‐up 71% |
|
| Interventions |
Number of experimental conditions: 2 (1 intervention, 1 control) Policies or practices targeted by the intervention: Policies and practices in the workplace physical and social environment for diet and tobacco use including: Diet: Physical environment:
Social environment:
Tobacco use: Physical environment:
Social environment:
Implementation strategies: EPOC:local opinion leaders and local consensus process An EAB was established at each workplace with representation from all occupation levels including management, union members (if any) and workers. The EAB worked in partnership with a professional interventionist from the study centre to plan and deliver environmental level activities and to assist in the tailoring of the intervention to workplaces. For example, members of the EAB met regularly with management to assist in the development of smoke free policies. If management agreed to a new policy, the EAB developed and followed a plan for implementing the new policy. EPOC:educational meetings Training sessions were conducted for EAB members to familiarise them with the goals of the project, their role and responsibilities, and to provide education regarding smoking and nutrition. EPOC:educational outreach visits Intervention specialists visited workplaces at least once per month to provide support for the intervention. Theoretical underpinning: health promotion activities were informed by the Ecological Model for Health Promotion. Implementation strategies (EABs) followed Rothman's Community Activation Principles Description of control: in all study centres, control workplaces received summary results from the employee baseline survey. Additionally, three of the four study centres provided an optional minimal support control, which was limited to the distribution of printed health promotion materials to workplaces such as newsletters. |
|
| Outcomes |
Outcome relating to the implementation of workplace based policies or practices: Diet:
Tobacco use:
Data collection method: Diet: A survey was conducted with organisational informants including personnel directors, food service managers and vending machine contractors. Surveys were administered via phone or in person to informants using a standard protocol. Tobacco use: A survey was conducted with employees at 2 time points: baseline and 3 years postbaseline. Survey items assessed employees self‐reported perceived compliance with and restrictiveness of tobacco control policies. Score scale: policy restrictiveness (1 = low restrictiveness; 4 = high restrictiveness); policy compliance (1 = low compliance; 5 = high compliance) Validity of method: validity of surveys used to assess implementation outcomes for diet and tobacco use not reported Outcome relating to diet, physical activity, weight status, tobacco use or alcohol use: Diet:
Tobacco use:
Data collection method: Diet: During an individual employee survey, employees self‐completed an 88 item semi‐quantitative food frequency questionnaire (FFQ) at baseline and 3 years postbaseline Tobacco use: During an individual employee survey, employees self‐reported tobacco use behaviours at baseline and 3 years postbaseline Validity of measure: Diet: The FFQ was based on the Block FFQ, which has been validated in previous studies. The FFQ was pre‐tested prior to use in the trial, and minor modifications were made to reflect regional dietary differences. Tobacco use: Validity unclear. Trial authors reported use of self‐reported quit rates is a standard and valid measure of smoking cessation outcomes in large scale community based trials, however, it is unclear if the specific items used in the employee survey to assess smoking behaviours were validated. Outcome relating to cost: not reported Outcome relating to adverse consequences: not reported |
|
| Notes |
Notes: A number of implementation outcome measures for diet and tobacco use were reported for this trial, however, several measures provided an indirect assessment of implementation that did not meet the review criteria for primary outcomes. Subsequently, only selected implementation outcome measures were included in the review, which did not include all practices targeted by the intervention. Workplace trial numbers differed across the diet and tobacco use components of the intervention as one of the 4study centres (UF) did not participate in the tobacco use component. Additionally, workplace sample size numbers differed for each of the three nutrition implementation outcome measures included in the review as variable numbers of organisational informants reported data for each measure. Research funding: study was supported by a cooperative agreement from the National Cancer Institute, Grants U01 CA51687, U01 CA61771, U01 CA51686, U01 CA516888, and P01 CA50087. Conflicts of interest: not specified by study authors Additional information requested from trial authors: information was requested regarding the number of employees in experimental groups, as this was not reported in the companion paper from which data for health behaviour outcomes for this trial were extracted (outcomes were assessed and reported at the workplace level). Given the time elapsed since the trial, the author of this companion paper indicated it was not possible to provide this information. As such, the number of employees in experimental groups was reported in the review as per the numbers reported in the primary outcomes paper (Biener 1999). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on method of generating random sequence |
| Allocation concealment (selection bias) | Unclear risk | No information on whether allocation was concealed prior to assignment |
| Blinding of participants and personnel (performance bias) Policy or practice implementation | High risk | Intervention implementation actively involved workplace staff participation at all organisational levels |
| Blinding of outcome assessment (detection bias) Policy or practice implementation | High risk | Key informant interviews were self‐reported organisational outcomes |
| Incomplete outcome data (attrition bias) Policy or practice implementation | Unclear risk | 114 workplaces initially recruited, 3 workplaces (2 intervention, 1 control) dropped out due to economic dislocations, leaving 111 in the final sample. For pair‐wise analyses, 3 pairs were excluded, leaving a total of 108 work sites (Sorensen 1996, p 940). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes (Abrams 1994, Fig 1) reported in Sorensen 1996 and Biener 1999 |
| Other bias | Low risk | |
Hannon 2012.
| Methods |
Study design: randomised controlled trial Intervention duration: 12 months Length of follow‐up from baseline: 15 months. Baseline data were collected June 2007 to June 2008, follow‐up data were collected October 2008 to December 2009 Differences in baseline characteristics: the only significant difference between intervention and control workplaces was employee gender; intervention workplaces had a larger proportion of male employees (52%) and control workplaces had a larger proportion of female employees (61%) (P = 0.03). Unit of allocation: workplace Unit of analysis: implementation and cost outcomes were analysed by workplace |
|
| Participants |
Workplace type: low‐wage, mid‐sized workplaces (100‐999 employees) from predominantly education and health services; manufacturing; other services; and wholesale and retail trade sectors Region: King County, Washington USA Demographic/socioeconomic characteristics: workplaces included in the trial were identified as low‐wage, with the average annual salary reported for employees (USD 38,849) below the 2007 average annual salary for the King County area (USD 48,560). 39% of employees were from racial/ethnic minority groups, and the proportion of male and female employees was approximately equal. Inclusion/exclusion criteria: Workplaces: Inclusion:
Exclusion:
Employees: not reported Number of workplaces allocated: 48 Numbers by trial group: Workplaces: n (controls baseline) = 24 n (controls follow‐up) = 23 n (interventions baseline) = 24 n (interventions follow‐up) = 23 Employees: not reported Recruitment: Workplaces: researchers obtained a list of workplaces in King County, Washington from a service providing databases for businesses. From this list, researchers identified workplaces that met the eligibility criteria and sent a letter and brochure describing the study. Researchers then telephoned these employers 1‐2 weeks later to conduct a screening survey for eligibility, and scheduled recruitment meetings with employers who met eligibility criteria and were willing to learn more about the study. At the recruitment meeting, the researchers explained study procedures and enrolment requirements, and employers willing to enrol signed a memorandum of understanding. Employees: not reported Recruitment rate: Workplaces: 22% Employees: not reported |
|
| Interventions |
Number of experimental conditions: 2 (1 intervention, 1 control) Policies or practices targeted by the intervention: The Workplace Solutions intervention targeted workplace implementation of 16 best‐practice strategies (in 5 categories) taken from the US Community Preventive Services Task Force (CPSTF) Guide to Community Preventive Services, which provides evidence‐based strategies for chronic disease prevention. Workplace best practices included:
Implementation strategies: EPOC: audit and feedback and clinical practice guidelines The project interventionist used baseline data on workplace implementation of the best‐practice strategies to develop a tailored 10‐page report with recommendations for improving any of the 16 best‐practice strategies that the employer was not implementing fully. The interventionist met with employers to present the findings of the report and the recommendations. EPOC: local consensus process Following feedback and recommendations, the interventionist met with employers to discuss the potential for adopting each recommended best‐practice strategy and asked employers to choose 3‐5 strategies to implement over the next 12 months. EPOC: educational outreach and educational materials To support implementation of each best‐practice strategy nominated for implementation by employers, the interventionist delivered to workplaces implementation oriented toolkits 'solution sets', containing information on the benefits of adopting the practice, how to implement the practice, and supporting materials for implementation. The interventionist encouraged employers to contact her with questions and requests for implementation assistance as needed, and contacted each employer in the intervention group monthly by email or telephone to offer assistance. EPOC: tailored intervention A final meeting with employers occurred 6 months after the solution sets meeting. The interventionist asked employers about their progress in implementing each of their chosen best‐practice strategies and offered guidance for overcoming identified implementation barriers. Theoretical underpinning: Rogers's Diffusion of Innovations Theory Description of control: wait‐list control. During the intervention, workplaces in the control group received two newsletters providing an update on trial progress. Following collection of follow‐up data, workplaces in the control group received the Workplace Solutions intervention. |
|
| Outcomes |
Outcome relating to the implementation of workplace based policies or practices: Workplace implementation of 16 best‐practice strategies for chronic disease prevention recommended by the CPSTF Guide to Community Preventive Services Data collection method: workplace staff (human resources leaders) completed surveys at 2 time points: baseline and 15 months postbaseline. Survey items were adapted from a review of studies on instruments to measure organisation support for employee health. Survey items for benefit coverage, tobacco use policy, onsite influenza immunisation, and tobacco quit lines had 3 possible scores: 0 if the practice was not in place, 0.75 if the practice was partially in place, and 1 if the practice was fully in place. Other items received dichotomous scores: 0 for practices that were not in place, and 1 for practices that were in place. Scores indicating implementation for each individual best practice were calculated as the mean of the scores for the survey items measuring the practice. Total best‐practice scores were calculated as the mean of combined scores for the 16 best practices, on a 100‐point scale. Validity of measure: not reported Outcome relating to diet, physical activity, weight status, tobacco use or alcohol use: not reported Outcome relating to cost: Workplace costs (per worker) for workplace health promotion, indicated by contract costs and personnel hours spent Data collection method: question items for cost outcomes were included in the survey used to collect data on primary outcomes, completed by workplace staff. Data on mean contract costs and personal hours spent were collected at 2 time points: baseline and 15 months postbaseline, and researchers monetised personnel hours by multiplying them by the mean worker hourly wage reported across work sites. Contract costs and monetised personnel hours were summed to calculate total costs. Costs reported for the 6 benefit‐related practices were not included because employers had difficulty separating preventive care costs from the costs of treatment. Costs related to making healthy food available were also excluded because employers providing food on site had difficulty separating costs for healthy food from their overall food related costs. Validity of measure: not reported Outcome relating to adverse consequences: not reported |
|
| Notes |
Research funding: trial was supported by the CDC Offıce of Public Health Research (Grant 5‐P01‐CD000249‐03) and by the University of Washington Health Promotion Research Centre (Health Promotion Research Centre cooperative agreement number U48/DP000050‐03) Conflicts of interest: study authors reported no conflicts of interest Additional information requested from trial authors: information was requested and provided regarding the specific duration of the Workplace Solutions intervention and was reported accordingly in the review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation undertaken by statistician (assume computerised) (Hannon 2012, p 127) |
| Allocation concealment (selection bias) | Low risk | Block randomisation undertaken by statistician (assume computerised) (Hannon 2012, p 127) |
| Blinding of participants and personnel (performance bias) Policy or practice implementation | High risk | Intervention implementation actively involved workplace staff participation |
| Blinding of outcome assessment (detection bias) Policy or practice implementation | High risk | All self‐reported outcomes (Hannon 2012, p 127) |
| Incomplete outcome data (attrition bias) Policy or practice implementation | Low risk | 24 workplaces per group at baseline; 23 per group analysed at follow‐up (Hannon 2012, p 128) |
| Selective reporting (reporting bias) | Unclear risk | No mention of a priori registration of measures or publication of protocol |
| Other bias | Low risk | |
Jones 2015.
| Methods |
Study design: non‐randomised trial Intervention duration: 12 months Length of follow‐up from baseline: 3 years. Round 1 of the audit conducted in 2010, round 2 conducted in 2013 Differences in baseline characteristics: the distribution of the types of trusts, headcounts (number of staff) and baseline audit scores were comparable between cohorts B and C1 Unit of allocation: workplace Unit of analysis: workplace |
|
| Participants |
Workplace type: healthcare services. The trial was undertaken in National Health Service (NHS) trusts, organisational units within the health sector. Participating trusts included ambulance, mental health, and acute healthcare services; however, most trusts were from acute healthcare services. Region: England. Trial included trusts located nation wide. Demographic/socioeconomic characteristics: not reported Inclusion/exclusion criteria: Workplaces: all NHS Trusts were eligible to take part in the organisational audits Employees: not reported Number of workplaces allocated: 62 Numbers by trial group: Workplaces: Cohort C1 (feedback only group) n (baseline) = 26 n (follow‐up) = 26 Cohort B (feedback and workshop group) n (baseline) = 36 n (follow‐up) = 36 Employees: not reported Recruitment: Workplaces: not reported Employees: not reported Recruitment rate: Workplaces: 72% (recruitment rate reported is for all cohorts combined, as rates for individual cohorts were not available) Employees: not reported |
|
| Interventions |
Number of experimental conditions: 2 (2 intervention groups) Policies or practices targeted by the intervention: NHS trust implementation of National Institute for Clinical Excellence (NICE) public health workplace related guidance for staff health in the workplace. This includes 6 sets of NICE guidance:
Implementation strategies: Both experimental groups (cohorts B and C1): EPOC: clinical practice guidelines and audit and feedback Participating NHS trusts completed an organisational audit to assess the extent of implementation of the NICE public health workplace related guidance in their trust. Following submission of their audit data, trusts received feedback via a confidential report presenting their performance data against the national benchmark. Cohort B only: EPOC: tailored intervention and educational meetings Following round 1 of the audit, the Health and Workforce Development Unit (HWDU) conducted structured telephone interviews with a sample of trusts who, through their audit scores, were shown to be demonstrating good progress in implementing the NICE workplace guidance (cohort A). These interviews were held to elicit information about organisational barriers to, and enablers for, implementing the guidance. The HWDU then facilitated action planning workshops based on the findings of these interviews with trusts that had demonstrated less progress with implementing the NICE guidance (cohort B). The workshops were used to brief participants on the themes that emerged from the interviews and to support board engagement and better implementation of the NICE workplace guidance. Recipients of workshops were then contacted by phone at 3, 6 and 12 months to check on progress in implementing their action plans. Theoretical underpinning of implementation strategies: Implementation strategies (interviews and action planning workshops) were guided by the Theoretical Domains Framework |
|
| Outcomes |
Outcome relating to the implementation of workplace based policies or practices: Implementation of NICE public health workplace related guidance for staff health promotion in the workplace Data collection method: organisational audit. The audit was based on the 6 pieces of NICE public health workplace related guidance including: obesity; smoking cessation; promoting environments that encourage physical activity; physical activity in the workplace; the management of long‐term sickness and absence; and promoting mental well‐being. Trust staff self‐reported audit data at 2 time points: baseline and 3 years postbaseline via a web‐based data collection system. A summary score was devised from the audit to provide an indication of the extent of implementation across the 6 areas of guidance. This scoring system was created by selecting questions (standards) that matched directly to recommendations contained in the NICE guidance, and then applying a weighted score which was then transformed into a percentage score. An overall score was calculated from these 6 domains, with possible audit scores ranging from 0 to 100. Validity of measure: not reported Outcome relating to diet, physical activity, weight status, tobacco use or alcohol use: not reported Outcome relating to cost: not reported Outcome relating to adverse consequences: not reported |
|
| Notes |
Notes: This trial included 4 study cohorts, however only 2 (cohorts B and C1) were included in the assessment of implementation outcomes in the review, based on comparability of baseline implementation scores in the organisational audit (both poor performing) and the use of different implementation support approaches 'feedback only' versus 'feedback and workshops'. Research funding: not reported Conflicts of interest: study authors did not declare whether they had any conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised design |
| Allocation concealment (selection bias) | High risk | Non‐randomised design |
| Blinding of participants and personnel (performance bias) Policy or practice implementation | High risk | Intervention implementation actively involved workplace staff participation |
| Blinding of outcome assessment (detection bias) Policy or practice implementation | High risk | Self‐reported outcome measures |
| Incomplete outcome data (attrition bias) Policy or practice implementation | Unclear risk | Only full cases included in analysis, unknown attrition rates for all groups (Jones 2015, pp 568‐9) |
| Selective reporting (reporting bias) | Unclear risk | No mention of a priori registration of measures or publication of protocol |
| Potential confounding | Low risk | Adjustment in baseline differences between two cohorts to minimise confounding (Jones 2015, p 569) |
| Other bias | Low risk | |
Parker 2010.
| Methods |
Trial name: Dow Chemical Study Study design: non‐randomised, controlled trial Intervention duration: 2 years Length of follow‐up from baseline: 2 years. Baseline data were collected in April 2006 and final follow‐up data were collected in March 2008 Differences in baseline characteristics: employees at intervention workplaces were significantly younger, more educated and had a higher proportion of ethnic minorities, whilst a greater number of employees at control workplaces were blue‐collar workers and paid an hourly wage. There were no significant differences in gender and health status between groups. Differences in employee baseline characteristics were accounted for using propensity score adjustment Unit of allocation: workplace Unit of analysis: implementation outcomes were analysed by workplace and health behaviour outcomes were analysed by employees |
|
| Participants |
Workplace type: manufacturing, research and development, and administrative facilities within a large and diversified chemical, science and technology company Region: USA. Intervention workplaces were located in Texas (n = 8) and Louisiana (n = 1) and control workplaces in West Virginia (n = 1), New Jersey (n = 1) and Louisiana (n = 1) Demographic/socioeconomic characteristics: most (75%) Dow employees were male, 82% were white, and the average age was 43 years Inclusion/exclusion criteria: Workplaces: not reported Employees: Inclusion:
Exclusion:
Number of workplaces allocated: 12 Numbers by trial group: Workplaces: n (controls baseline) = 3 n (controls follow‐up) = 3 n (intervention (moderate) baseline) = 4 n (intervention (moderate) follow‐up) = 4 n (intervention (high) baseline) = 5 n (intervention (high) follow‐up) = 5 Employees: employees at all work sites were invited to participate in health risk assessments (HRA) examining health behaviours including: poor nutrition, lack of physical activity, tobacco use, and high alcohol use, in addition to biometric screenings to assess weight status and biochemistry measures. The number of employees who participated in HRAs, as well as the subgroup of these employees who participated in the biometric screenings, at both pre and postintervention, was as follows: HRA cohort: n (controls) = 529 n (intervention moderate) = 382 n (intervention high) = 1520 Biometric cohort: n (controls) = 382 n (intervention moderate) = 213 n (intervention high) = 926 Recruitment: Workplaces: workplaces were chosen by Dow's leaders to participate in the trial. Employees: not reported Recruitment rate: Workplaces: not reported Employees: a total of n = 10,281 employees across all workplaces were eligible to participate in HRA and biometric screening. Recruitment rates for each were as follows: HRA cohort: 23.6% Biometric cohort: 14.8% |
|
| Interventions |
Number of experimental conditions: 3 (2 intervention ‐ high and moderate intensity, 1 control) Policies or practices targeted by the intervention: The intervention targeted organisational practices and policies for nutrition, physical activity and weight control including: Both moderate‐ and high‐intensity intervention groups:
High‐intensity intervention group only:
Implementation strategies: Both moderate and high intensity intervention: EPOC: tailored intervention Formative research was undertaken to collect data on the key target areas for workplace environmental interventions including employees (health and job factors), workplaces (physical environment and current health promotion activities), and corporate and site leaders (social‐organisational environment). Focus groups and interviews were conducted with employees and corporate and site leaders to inform the research team about which interventions may be most useful, how the culture of each site may influence the utilisation of potential strategies, and to determine factors that might influence successful implementation of strategies. EPOC: local opinion leaders and educational meetings HCFPs were established as 'wellness ambassadors' at worksites. HCFPs performed duties such as putting up health promotion posters and encouraging healthy food choices at meetings. HCFPs were provided with specific training by the research team. High intensity intervention only: EPOC: educational meetings Workplace site leaders received training on health‐related topics and ways to encourage employee participation in health promotion programmes. EPOC: local consensus process Health promotion related goals were included in the organisational plans of workplace site leaders. EPOC: audit and feedback Progress reports regarding health promotion and project implementation were provided to site and corporate leaders. EPOC: monitoring of performance Site leaders were held accountable for progressing and achieving planned health promotion‐related goals at meetings between site and corporate leaders. EPOC: other Site leaders were recognised and rewarded for achieving health promotion‐related goals. Theoretical underpinning: Social Ecological Theory Description of control: wait‐list control. Control workplaces were instructed not to introduce the new environmental interventions for the 2‐year study period. In these workplaces, the Dow companies' standard health promotion programme ran throughout the study period, which included only individually focused health promotion activities. |
|
| Outcomes |
Outcome relating to the implementation of workplace based policies or practices: Implementation of workplace physical and social supports to promote healthy eating, physical activity and weight management Data collection method: environmental observation. Workplace environments were assessed using an environmental assessment tool (EAT) at 4 time points: baseline, year 1, year 2 and postintervention (year 3). The EAT contained 105 items. Section I was completed electronically by workplace staff prior to site inspection, and section II was completed by project staff during onsite observations. Because many of the workplaces were too large for project staff to inspect every building, approximately 6 occupied buildings or areas representative of the workplace and its employees were selected for EAT assessment. Project staff used a scoring rubric to aggregate the EAT responses into a total score (out of 100 points). Validity of measure: environmental observation represents an objective assessment of the work environment. EAT is a validated instrument Outcome relating to diet, physical activity, weight status, tobacco use or alcohol use:
Data collection method: Diet and physical activity measures: Employee self‐reported health risk behaviours were assessed using a standardised HRA survey developed by research organisations participating in National Heart Lung and Blood Institute (NHLBI) studies. Surveys were completed by employees online at 3 time points: baseline, 1 year and 2 years postbaseline. Behavioural health risk factors were scored using several HRA questions and included indicators for poor nutrition and lack of physical activity. Weight status measures: Employee anthropometric measures were collected by health professionals using standardised protocols developed by Dow Health Services Validity of measure: Diet and physical activity: not reported Weight status: anthropometric measures an objective assessment of weight status Outcome relating to cost: not reported Outcome relating to adverse consequences: not reported |
|
| Notes |
Notes: this trial reported health behaviour outcomes for employee alcohol use and tobacco use, however as the trial implementation strategy and policies and practices targeted did not include those addressing alcohol and tobacco use, intervention effects on these outcomes were not included in the review. Research funding: funding for this study was provided by the NHLBI (Grant # R01 HL79546) Conflicts of interest: the study authors reported having no conflicts of interest Additional information requested from trial authors: information was requested regarding further details on the specific implementation strategies utilised for moderate‐ and high‐intensity intervention groups. This information was provided and reported accordingly in the results of the review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised trial |
| Allocation concealment (selection bias) | High risk | Non‐randomised trial |
| Blinding of participants and personnel (performance bias) Policy or practice implementation | High risk | Intervention implementation actively involved workplace staff participation at all organisational levels |
| Blinding of outcome assessment (detection bias) Policy or practice implementation | Unclear risk | Environment observation assessment undertaken in a total of 6 selected buildings (with assistance of workplace staff from 12‐300) per workplace (Parker 2010). Unclear on what basis buildings were selected. Assessment undertaken by research staff. Unclear if blinded |
| Incomplete outcome data (attrition bias) Policy or practice implementation | Low risk | Assessment undertaken at each of 12 sites at each time point (Parker 2010) |
| Selective reporting (reporting bias) | Unclear risk | Wilson 2007 indicates primary outcome BMI and development work of environmental assessment tool demonstrates intention to include in outcome assessment. No indications that any predetermined outcomes were otherwise omitted |
| Potential confounding | High risk | No indication in analysis that adjustment of potential confounders was undertaken (Parker 2010) |
| Other bias | Low risk | |
BMI: body mass index; CHEW: Checklist of Health Promotion Environments at Worksites; DFCI: Dana‐Farber Cancer Institute/University of Massachusetts; EA: environmental assessment; EAB: employee advisory board; EAT: environmental assessment tool; EPOC: Effective Practice and Organisation of Care; FFQ: food frequency questionnaire; HC: healthy choices; HCFP: Healthy Culture Focal Points; HRA: Health Risk Assessments; MDACC: MD Anderson Cancer Centre;NHLB: National, Heart, Lung and Blood Institute; PACE: Physical Activity and Changes in Eating; UF: University of Florida.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abood 2003 | Inappropriate intervention |
| Addley 2014 | Inappropriate intervention |
| Aittasalo 2004 | Inappropriate outcome |
| Aittasalo 2012 | Inappropriate outcome |
| Alkajah 2012 | Inappropriate intervention |
| Andersen L.L 2013 | Inappropriate intervention |
| Andersen L.N 2013 | Inappropriate intervention |
| Andersen L.N 2015 | Inappropriate intervention |
| Ang 2013 | Inappropriate intervention |
| Apostolopoulos 2016 | Non‐controlled study/inappropriate comparator |
| Arao 2007 | Inappropriate intervention |
| Armitage 2006 | Inappropriate intervention |
| Armitage 2007 | Inappropriate intervention |
| Armitage 2010 | Inappropriate intervention |
| Armitage 2015 | Inappropriate intervention |
| Atlantis 2006 | Inappropriate intervention |
| Audrey 2015 | Inappropriate intervention |
| Backman 2011 | Inappropriate intervention |
| Bale 2015 | Inappropriate outcome |
| Bandoni 2010b | Inappropriate outcome |
| Barene 2014 | Inappropriate intervention |
| Barene 2014b | Inappropriate intervention |
| Bellicha 2016 | Inappropriate outcome |
| Bennett 2004 | Inappropriate outcome |
| Beresford 2000 | Inappropriate intervention |
| Beresford 2001 | Inappropriate intervention |
| Berry 1989 | Could not obtain full text |
| Bertera 1993 | Inappropriate outcome |
| Blair 1986 | Inappropriate outcome |
| Blake 2013 | Non‐controlled study/inappropriate comparator |
| Block 2008 | Inappropriate intervention |
| Bly 1986 | Inappropriate outcome |
| Borg 2010 | Inappropriate intervention |
| Brace 2015 | Inappropriate intervention |
| Brakenridge 2016 | Inappropriate outcome |
| Breeze 2017 | Inappropriate outcome |
| Brehm 2011 | Inappropriate intervention |
| Breslow 1990 | Inappropriate outcome |
| Brown 2012 | Inappropriate intervention |
| Brown 2014 | Inappropriate intervention |
| Buchholz 2016 | Non‐controlled study/inappropriate comparator |
| Budden 2007 | Inappropriate participants |
| Buller 2000 | Inappropriate outcome |
| Buller 2005 | Inappropriate intervention |
| Buman 2017 | Inappropriate outcome |
| Burnhams 2015 | Inappropriate intervention |
| Campbell 2002 | Inappropriate intervention |
| Caperchione 2016 | Non‐controlled study/inappropriate comparator |
| Carr 2013 | Inappropriate intervention |
| Cash 2012 | Non‐controlled study/inappropriate comparator |
| Chapman 2015 | Inappropriate intervention |
| Chau 2014 | Inappropriate intervention |
| Chen 2016 | Inappropriate outcome |
| Christensen 2011 | Inappropriate intervention |
| Christensen 2016 | Inappropriate outcome |
| Coeffeng 2012 | Inappropriate intervention |
| Coffeng 2013 | Inappropriate outcome |
| Coffeng 2014 | Inappropriate intervention |
| Conrad 1996 | Inappropriate intervention |
| Cook 2007 | Inappropriate intervention |
| Cooke 2000 | Non‐controlled study/inappropriate comparator |
| Crawford 2004 | Inappropriate outcome |
| Cremaschini 2015 | Could not obtain full‐text |
| Dalager 2017 | Inappropriate outcome |
| Dallam 2013 | Inappropriate intervention |
| Dallat 2013 | Inappropriate outcome |
| Davy 2014 | Non‐controlled study/inappropriate comparator |
| De Bourdeaudhuij 2007 | Inappropriate outcome |
| Deitz 2014 | Inappropriate intervention |
| Dishman 2009 | Inappropriate intervention |
| Dishman 2010 | Inappropriate intervention |
| Donath 2015 | Inappropriate intervention |
| Doumas 2008 | Inappropriate participants |
| Dubuy 2013 | Non‐controlled study/inappropriate comparator |
| Duffy 2012 | Inappropriate participants |
| Dutta 2014 | Inappropriate intervention |
| Edries 2013 | Inappropriate intervention |
| Emmons 1996 | Non‐controlled study/inappropriate comparator |
| Emmons 1999 | Could not obtain full‐text |
| Engbers 2006 | Inappropriate intervention |
| Erfurt 1991 | Inappropriate intervention |
| Erskine 2012 | Inappropriate intervention |
| Estabrook 2012 | Non‐controlled study/inappropriate comparator |
| Fagan 2003 | Inappropriate intervention |
| Fagan 2003b | Inappropriate intervention |
| Faghri 2008 | Inappropriate intervention |
| Fink 2016 | Non‐controlled study/inappropriate comparator |
| Fitzgerald 2017 | Inappropriate outcome |
| Flannery 2012 | Inappropriate intervention |
| Flannery 2012b | Inappropriate intervention |
| Fleig 2010 | Could not obtain full text |
| Ford 2014 | Non‐controlled study/inappropriate comparator |
| Freak‐Poli 2013b | Non‐controlled study/inappropriate comparator |
| French 2010 | Inappropriate outcome |
| Friedrich 2009 | Inappropriate outcome |
| Friedrich 2015 | Inappropriate outcome |
| Friedrich 2015b | Inappropriate outcome |
| Gao 2010 | Inappropriate intervention |
| Geaney 2013b | Inappropriate outcome |
| Gemson 2008 | Inappropriate outcome |
| Glanz 1998 | Inappropriate outcome |
| Glanz 1998b | Inappropriate outcome |
| Glasgow 1993 | Inappropriate intervention |
| Glasgow 1994 | Inappropriate outcome |
| Glasgow 1995 | Inappropriate outcome |
| Glasgow 1996 | Non‐controlled study/inappropriate comparator |
| Glasgow 1997 | Inappropriate outcome |
| Goetzel 2005 | Inappropriate outcome |
| Goetzel 2009 | Inappropriate outcome |
| Gosliner 2010 | Inappropriate intervention |
| Gram 2012 | Inappropriate intervention |
| Grande 2013 | Inappropriate intervention |
| Griffin‐Blake 2006 | Inappropriate intervention |
| Gritz 1998 | Inappropriate outcome |
| Groeneveld 2008 | Inappropriate intervention |
| Groeneveld 2011 | Inappropriate intervention |
| Hadgraft 2017 | Inappropriate outcome |
| Hagger 2011 | Inappropriate intervention |
| Hall 2015 | Inappropriate outcome |
| Hallam 2004 | Inappropriate intervention |
| Han 2014 | Inappropriate intervention |
| Harden 2017 | Inappropriate participants |
| Harley 2010 | Inappropriate participants |
| Harley 2013 | Non‐controlled study/inappropriate comparator |
| Harris 2008 | Non‐controlled study/inappropriate comparator |
| Healy 2013 | Inappropriate intervention |
| Hebert 1993 | Inappropriate intervention |
| Hebert 1993b | Inappropriate outcome |
| Heirich 2000 | Inappropriate intervention |
| Hermansson 2010 | Inappropriate intervention |
| Hill‐Mey 2013 | Inappropriate intervention |
| Holtermann 2010 | Inappropriate intervention |
| Hopkins 2012 | Inappropriate outcome |
| Hopkins 2012b | Inappropriate outcome |
| Hughes 2011 | Inappropriate intervention |
| Hunt 1993 | Could not obtain full text |
| Hunt 2000 | Inappropriate outcome |
| Hunt 2003 | Non‐controlled study/inappropriate comparator |
| Hunt 2003b | Inappropriate intervention |
| Hunt 2007 | Inappropriate outcome |
| Hunt 2007b | Inappropriate outcome |
| Hunt 2010 | Inappropriate participants |
| Hunter 2013 | Inappropriate intervention |
| Ishii 2007 | Inappropriate intervention |
| Jaime 2014 | Inappropriate outcome |
| Jason 1997 | Inappropriate intervention |
| Jeffery 1993 | Inappropriate outcome |
| Johnson 2010 | Non‐controlled study/inappropriate comparator |
| Kazi 2013 | Inappropriate outcome |
| Kilpatrick 2016 | Non‐controlled study/inappropriate comparator |
| Kim 2011 | Inappropriate intervention |
| Kim 2012 | Non‐controlled study/inappropriate comparator |
| Kirchner 2013 | Inappropriate outcome |
| Klatt 2016 | Inappropriate outcome |
| Koffman 1998 | Inappropriate outcome |
| Kolbe‐Alexander 2012 | Inappropriate intervention |
| Korshoj 2012 | Inappropriate intervention |
| Kristal 1995 | Inappropriate outcome |
| Kristal 2000 | Inappropriate outcome |
| Kushida 2014 | Inappropriate outcome |
| Kwak 2007 | Non‐controlled study/inappropriate comparator |
| Kwak 2007b | Inappropriate outcome |
| Kwak 2009 | Inappropriate outcome |
| LaCaille 2016 | Inappropriate outcome |
| Laing 2012 | Non‐controlled study/inappropriate comparator |
| LaMontagne 2004 | Inappropriate outcome |
| LaMontagne 2005 | Inappropriate outcome |
| Lang 2017 | Non‐controlled study/inappropriate comparator |
| Lapham 2003 | Inappropriate outcome |
| Lawton 2015 | Inappropriate outcome |
| Lemon 2010 | Inappropriate outcome |
| Lemon 2014 | Inappropriate outcome |
| Leslie 2002 | Inappropriate intervention |
| Lillehoj 2015 | Inappropriate intervention |
| Linde 2012 | Inappropriate outcome |
| Lindstrom 2010 | Non‐controlled study/inappropriate comparator |
| Linnan 2002 | Inappropriate outcome |
| Lowe 2010 | Inappropriate outcome |
| Mache 2015 | Inappropriate intervention |
| Mackey 2007 | Inappropriate outcome |
| Mackey 2011 | Inappropriate intervention |
| MacKinnon 2010 | Inappropriate intervention |
| Macniven 2015 | Non‐controlled study/inappropriate comparator |
| Maes 1998 | Inappropriate outcome |
| Mansi 2013 | Inappropriate intervention |
| Marcus 1998 | Inappropriate intervention |
| Mayer 2010 | Inappropriate intervention |
| McEachan 2011 | Inappropriate outcome |
| Mehta 2013 | Non‐controlled study/inappropriate comparator |
| Michishita 2017 | Inappropriate outcome |
| Micucci 2007 | Non‐controlled study/inappropriate comparator |
| Mitchell 2015 | Inappropriate intervention |
| Morgan 2011 | Inappropriate intervention |
| Morgan 2012 | Inappropriate intervention |
| Morton 2011 | Inappropriate intervention |
| Moy 2006 | Inappropriate outcome |
| Mujika 2014 | Inappropriate intervention |
| Murray 2012 | Inappropriate intervention |
| Muto 1998 | Inappropriate intervention |
| Naito 2008 | Inappropriate outcome |
| Neil‐Sztramko 2017 | Non‐controlled study/inappropriate comparator |
| Neuhaus 2014 | Inappropriate outcome |
| Neuhaus 2014b | Inappropriate outcome |
| Neyens 2017 | Non‐controlled study/inappropriate comparator |
| Nielsen 2006 | Inappropriate outcome |
| Norman 2016 | Inappropriate outcome |
| Nyrop 2011 | Inappropriate participants |
| Okazaki 2014 | Inappropriate outcome |
| Okechukwu 2009 | Inappropriate participants |
| Olson 2014 | Inappropriate outcome |
| Olson 2016 | Inappropriate participants |
| Ostbye 2015 | Inappropriate intervention |
| Osteras 2006 | Inappropriate intervention |
| Parry 2013 | Inappropriate outcome |
| Patterson 1997 | Inappropriate outcome |
| Patterson 1998 | Inappropriate outcome |
| Patterson 2016 | Non‐controlled study/inappropriate comparator |
| Paul 2013 | Non‐controlled study/inappropriate comparator |
| Pedersen 2009 | Inappropriate intervention |
| Pedersen 2014 | Inappropriate intervention |
| Pescatello 2001 | Inappropriate outcome |
| Pescud 2016 | Inappropriate outcome |
| Petersen 2008 | Inappropriate outcome |
| Pidd 2015 | Inappropriate intervention |
| Plotnikoff 2005 | Inappropriate intervention |
| Pressler 2010 | Inappropriate intervention |
| Prestwich 2012 | Inappropriate intervention |
| Procter 2014 | Non‐controlled study/inappropriate comparator |
| Proper 2003 | Inappropriate intervention |
| Puig‐Ribera 2008 | Inappropriate intervention |
| Purath 2004 | Inappropriate intervention |
| Reynolds 1997 | Inappropriate intervention |
| Reynolds 2015 | Inappropriate outcome |
| Richmond 1999 | Inappropriate outcome |
| Richmond 2000 | Inappropriate outcome |
| Riley 2017 | Inappropriate outcome |
| Robison 1992 | Inappropriate outcome |
| Robroek 2012 | Inappropriate intervention |
| Robroek 2012b | Inappropriate intervention |
| Rodríguez‐Artalejo 2003 | Inappropriate outcome |
| Salinardi 2013 | Inappropriate outcome |
| Santos 2016 | Inappropriate outcome |
| Schaller 2016 | Inappropriate outcome |
| Schneider 2016 | Non‐controlled study/inappropriate comparator |
| Schopp 2017 | Inappropriate outcome |
| Schwartz 2016 | Inappropriate outcome |
| Sertel 2016 | Inappropriate outcome |
| Sforzo 2012 | Inappropriate outcome |
| Shore 1994 | Inappropriate intervention |
| Sierra 2010 | Inappropriate intervention |
| Simpson 2000 | Non‐controlled study/inappropriate comparator |
| Smith‐McLallen 2017 | Inappropriate outcome |
| Sorensen 1990 | Non‐controlled study/inappropriate comparator |
| Sorensen 1992 | Inappropriate intervention |
| Sorensen 1992b | Inappropriate outcome |
| Sorensen 1998 | Inappropriate outcome |
| Sorensen 1998b | Inappropriate outcome |
| Sorensen 1998c | Non‐controlled study/inappropriate comparator |
| Sorensen 1999 | Inappropriate outcome |
| Sorensen 2002 | Inappropriate outcome |
| Sorensen 2005 | Inappropriate outcome |
| Sorensen 2007 | Inappropriate participants |
| Sorensen 2009 | Non‐controlled study/inappropriate comparator |
| Sorensen 2010 | Non‐controlled study/inappropriate comparator |
| Sotos‐Prieto 2017 | Inappropriate outcome |
| Steenhuis 2004 | Inappropriate outcome |
| Stephens 2014 | Inappropriate intervention |
| Strijk 2011 | Inappropriate outcome |
| Strijk 2012 | Inappropriate outcome |
| Sumner 2016 | Inappropriate outcome |
| Tan 2013 | Inappropriate outcome |
| Tanaka 2006 | Inappropriate outcome |
| Terry 2011 | Non‐controlled study/inappropriate comparator |
| Terry 2011b | Inappropriate intervention |
| Terry 2011c | Non‐controlled study/inappropriate comparator |
| Thogersen‐Ntoumani 2010 | Inappropriate intervention |
| Thompson 1995 | Non‐controlled study/inappropriate comparator |
| Thorndike 2012 | Inappropriate intervention |
| Tilley 1997 | Inappropriate outcome |
| Tilley 1998 | Inappropriate outcome |
| Tilley 1999 | Inappropriate outcome |
| Tobin 2016 | Inappropriate outcome |
| Togami 2008 | Inappropriate intervention |
| Townsend 2016 | Non‐controlled study/inappropriate comparator |
| Tucker 2016 | Inappropriate outcome |
| van Berkel 2011 | Inappropriate outcome |
| van Calster 2017 | Inappropriate outcome |
| van Scheppingen 2014 | Inappropriate outcome |
| Vermeer 2012 | Inappropriate intervention |
| Verweij 2009 | Inappropriate outcome |
| Verweij 2012 | Inappropriate outcome |
| Verweij 2013 | Inappropriate outcome |
| Volpp 2009 | Inappropriate intervention |
| Vyth 2011 | Inappropriate outcome |
| Vyth 2012 | Non‐controlled study/inappropriate comparator |
| Walters 2003 | Inappropriate intervention |
| Watanabe 2017 | Inappropriate outcome |
| Webb 2013 | Inappropriate outcome |
| Weinhold 2015 | Inappropriate intervention |
| White 2007 | Non‐controlled study/inappropriate comparator |
| White 2016 | Non‐controlled study/inappropriate comparator |
| Wierenga 2012 | Non‐controlled study/inappropriate comparator |
| Wierenga 2014 | Non‐controlled study/inappropriate comparator |
| Willemsen 1998 | Inappropriate intervention |
| Williams 2007 | Inappropriate outcome |
| Williams 2014 | Inappropriate outcome |
| Wilson 2016 | Inappropriate outcome |
| Wilson 2016b | Inappropriate outcome |
| Yap 2009 | Inappropriate intervention |
| Zavanela 2012 | Inappropriate intervention |
| Zinn 2012 | Inappropriate intervention |
| Zinn 2012b | Inappropriate intervention |
Characteristics of ongoing studies [ordered by study ID]
Hannon 2016.
| Trial name or title | HealthLinks |
| Methods | Study design: randomised controlled trial |
| Participants |
Workplace type: small‐sized workplaces in low‐wage industries Region: King County Washington, USA |
| Interventions |
Number of experimental conditions: 3 (2 interventions: HealthLinks and HealthLinks+, and 1 control) Policies or practices targeted by the intervention: Workplace implementation of best‐practice strategies for health promotion based on The US Community Preventive Services Task Force (CPSTF) Guide to Community Preventive Services including: Healthy eating:
Physical activity:
Tobacco cessation:
Cancer screening:
Implementation strategies: HealthLinks
HealthLinks +
|
| Outcomes |
Outcome relating to the implementation of workplace policies or practices:
Outcome relating to diet, physical activity, weight status, tobacco use or alcohol use:
|
| Starting date | Baseline results for trial reported May 2016 |
| Contact information | Associate Professor Peggy Hannon Health Promotion Research Centre, Department of Health Services, University of Washington, 1107 NE 45th Street, Ste. 200, Seattle, WA 98015 peggyh@uw.edu |
| Notes |
Trial registration: trial registered with ClinicalTrials.gov (NCT02005497). Date of registration: 9 December 2013 Research funding: project supported by grant 5R01CA160217 from the National Cancer Institute Additional information requested from trial authors: information was requested regarding whether follow‐up data were available to the published design and baseline paper for this trial. Information was provided indicating follow‐up data had been collected but was not yet published, therefore this trial was included in the review as an ongoing study. |
NCT02381938.
| Trial name or title | Care2BWell: Worksite Wellness for Child Care |
| Methods | Study design: cluster randomised controlled trial |
| Participants |
Workplace type: workplaces in the childcare sector Region: North Carolina, USA |
| Interventions |
Number of experimental conditions: 2 (1 intervention, 1 control) Policies or practices targeted by the intervention: Workplace implementation of practices constituting 'comprehensive' workplace health promotion (administrative supports, health education programmes, environmental supports, linkage with other health programmes, and screening) Implementation strategies:
|
| Outcomes |
Outcome relating to the implementation of workplace policies or practices:
Outcome relating to diet, physical activity, weight status, tobacco use or alcohol use:
|
| Starting date | Study commenced March 2015 |
| Contact information | Professor Dianne Ward University of North Carolina, Chapel Hill dsward@email.unc.edu |
| Notes |
Trial registration: trial registered with ClinicalTrials.gov (NCT02381938). Date of registration: 6 March 2015 Research funding: project supported by grant 1R01HL119568‐01A1 NIH National Heart, Lung and Blood Institute |
NCT02899442.
| Trial name or title | Cardiovascular risk prevention among night workers (Heart‐Of‐Night) |
| Methods | Study design: randomised controlled trial |
| Participants |
Workplace type: workplaces including night shift work Region: Toulouse, France |
| Interventions |
Number of experimental conditions: 2 (1 intervention, 1 control) Policies or practices targeted by the intervention:
Implementation strategies: Various strategies will be used to implement collective preventive actions at the worksite level. Collective preventive actions will be implemented by an occupational health team. |
| Outcomes |
Outcome relating to the implementation of workplace policies or practices: Workplace implementation of policies and practices targeting risk factors for cardiovascular disease amongst night shift workers Outcome relating to diet, physical activity, weight status, tobacco use or alcohol use: A range of employee health behaviour outcomes will be collected and may be appropriate for inclusion. |
| Starting date | Study commenced March 2015 |
| Contact information | Doctor Yolande Esquirol Toulouse University Hospital (CHU de Toulouse) esquirol.y@chu‐toulouse.fr |
| Notes |
Trial registration: trial registered with ClinicalTrials.gov (NCT02899442). Date of registration: 14 September 2016 Research funding: not reported |
Vasiljevic 2017.
| Trial name or title | Physical micro‐environment interventions for healthier eating in the workplace: a stepped wedge randomised pilot trial |
| Methods | Study design: randomised stepped wedge trial |
| Participants |
Workplace type: workplaces from companies that are members of the Institute for Grocery Distribution (IGD) Region: workplaces from any region in England are eligible |
| Interventions |
Number of experimental conditions: 3 intervention conditions: portion, package and tableware size; availability of healthier food options; and food calorie content labelling 1.Portion, package and tableware size:
2. Availability:
3. Labelling:
Implementation strategies: Various strategies will be employed to assist workplace cafeterias to implement changes to food service practices |
| Outcomes |
Outcome relating to the implementation of workplace policies or practices: A range of implementation outcomes will be collected and may be appropriate for inclusion. |
| Starting date | Study commenced April 2016 |
| Contact information | Professor Theresa Marteau University of Cambridge Institute of Public Health Forvie Site Cambridge CB2 0SR, United Kingdom tm388@cam.ac.uk |
| Notes |
Trial registration: trial registered with ISRCTN registry (ISRCTN52923504). Date of registration: 22 September 2016 Research funding: study funded by the Department of Health Policy Research Programme (Policy Research Unit in Behaviour and Health [PR‐UN‐0409‐ 10109] and the Institute for Grocery Distribution [RG83425]) |
Velema 2017.
| Trial name or title | Using nudging and social marketing techniques to create healthy worksite cafeterias in the Netherlands: intervention development and study design |
| Methods | Study design: randomised controlled trial |
| Participants |
Workplace type: not reported Region: the Netherlands |
| Interventions |
Number of experimental conditions: 2 (1 intervention, 1 control) Policies or practices targeted by the intervention: The programme Worksite Cafeterias 2.0 is based on the Netherlands Guidelines for Healthier Canteens. The guidelines offer strategies for how to arrange a sport, school or a worksite cafeteria to encourage visitors to show healthier eating behaviour. Specific cafeteria practices that will be implemented in the trial include: Product:
Place :
Price:
Promotion:
Implementation strategies: Training will be provided to cafeteria managers and food service staff to implement cafeteria practices from the Guidelines for Healthier Canteens |
| Outcomes |
Outcome relating to the implementation of workplace policies or practices: A range of implementation outcomes will be collected and may be appropriate for inclusion. |
| Starting date | Study commenced February 2016 |
| Contact information | Elizabeth Velema Department of Health Sciences and the EMGO+ Institute for Health and Care Research, Faculty of Earth and Life Sciences, Vrije Universiteit Amsterdam, De Boelelaan 1085, 1081 HV Amsterdam, the Netherlands. e.velema@vu.nl |
| Notes |
Trial registration: Netherlands Trial register (NTR5372). Date of registration: 20 August 2015. Research funding: funding for the study was obtained from Veneca, the Trade Association of Dutch catering organisations. Additional information requested from trial authors: information was requested regarding whether follow‐up data were available to the published protocol paper for this trial. At the time of contact, information was provided indicating results for the trial were not yet published, therefore the trial was included in the review as an ongoing study. |
CPSTF: Community Preventive Services Task Force.
Differences between protocol and review
We placed additional restrictions on the type of primary outcomes included in the review. Specifically, we did not include indirect implementation effect measures such as the 'intention' to implement a workplace policy or practice.
'Risk of bias' assessment was undertaken for implementation outcomes only in included trials. Additionally, we used the standard Cochrane 'Risk of bias' tool rather than the EPOC Group risk of bias criteria for the sake of consistency with other Cochrane Reviews published by the research team regarding implementation interventions for chronic disease prevention in community settings.
We assessed the overall certainty of evidence (GRADE) and generated the 'Summary of findings' table only for RCTs, which compared an implementation strategy with control.
Contributions of authors
All review authors contributed to the conception and conduct of the research and reviewed and approved the final manuscript. LW and SY led the development of the review. DB developed the search strategy. FS, BP, TR, AG, SG and LW contributed to the selection of studies. SG, LW, MF, SY, ABC, HTVZ and FL contributed to data extraction and management. CW, MK and JJ contributed to the 'Risk of bias' assessment. RH and LW contributed to the assessment of the overall quality of evidence. LW and SG led the drafting of the manuscript. All authors provided critical comment on drafts.
Sources of support
Internal sources
-
The University of Newcastle, Australia.
Salary support for review authors
-
Hunter New England Population Health, Australia.
Salary support for review authors
-
Hunter Medical Research Institute, Australia.
Salary support for review authors
-
Canadian Institutes of Health Research (CIHR) Quebec Strategy for Patient‐Oriented Research (SPOR) Support for People and Patient‐Oriented Research and Trials (SUPPORT) unit, Canada.
Salary support for review authors
-
Centre for Epidemiology and Evidence, NSW Ministry of Health, Australia.
Salary support for review authors
External sources
-
Australian Prevention Partnership Centre, Australia.
This research was financially supported by The Australian Prevention Partnership Centre through the NHMRC partnership centre grant scheme (Grant ID: GNT9100001) with the Australian Government Department of Health, NSW Ministry of Health, ACT Health, HCF and the HCF Research Foundation.
-
The Heart Foundation, Australia.
The review received supplementary funding from the Heart Foundation under the Future Leaders Fellowship (No. 101175) grant awarded to Luke Wolfenden.
Declarations of interest
Luke Wolfenden: none known.
Sharni Goldman: none known.
Fiona Stacey: none known.
Alice Grady: none known.
Melanie Kingsland: none known.
Christopher M Williams: none known.
John Wiggers: none known.
Andrew Milat: none known.
Chris Rissel: none known.
Adrian Bauman: none known.
Margaret M Farrell: none known.
France Légaré: none known.
Ali Ben Charif: none known.
Hervé Tchala Vignon Zomahoun: none known.
Rebecca K Hoder: none known.
Jannah Jones: none known.
Debbie Booth: none known.
Benjamin Parmenter: none known.
Tim Regan: none known.
Sze Lin Yoong: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Bandoni 2010 {published data only}
- Bandoni DH, Sarno F, Patricia, Jaime PC. Impact of an intervention on the availability and consumption of fruits and vegetables in the workplace. Public Health Nutrition 2010;14(6):975‐81. [DOI: 10.1017/S1368980010003460] [DOI] [PubMed] [Google Scholar]
Beresford 2010 {published data only}
- Beresford SAA, Bishop SK, Brunner NL, Duncan GE, McGregor BA, McLerran DF, et al. Environmental assessment at worksites following a multilevel intervention to promote activity and changes in eating: the PACE Project. Journal of Occupational and Environmental Medicine 2010;52(Suppl 1):1‐16. [DOI: 10.1097/JOM.0b013e3181c7512c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford SAA, Locke E, Bishop S, West B, McGregor BA, Bruemmer B, et al. Worksite study Promoting Activity and Changes in Eating (PACE): design and baseline results. Obesity 2007;15(Suppl 1):4S‐15S. [DOI] [PubMed] [Google Scholar]
Biener 1999 {published data only}
- Abrams DB, Boutwell BW, Grizzle J, Heimendinger J, Sorensen G, Varnes J. Cancer control at the workplace: the Working Well Trial. Preventive Medicine 1994;23:15‐27. [DOI] [PubMed] [Google Scholar]
- Biener L, Glanz K, McLerran D, Sorensen G, Thompson B, Basen‐Engquist K, et al. Impact of the Working Well Trial on the worksite smoking and nutrition environment. Health Education & Behavior 1999;26(4):478‐94. [DOI] [PubMed] [Google Scholar]
- Heimendinger J, Feng Z, Emmons K, Stoddard A, Kinne S, Biener L, et al. The Working Well Trial: baseline dietary and smoking behaviors of employees and related worksite characteristics. Preventive Medicine 1995;24(2):180‐93. [DOI: 10.1006/pmed.1995.1032] [DOI] [PubMed] [Google Scholar]
- Sorensen G, Thompson B, Glanz K, Feng Z, Kinne S, DiClemente C, et al. Worksite‐based cancer prevention: primary results from the Working Well Trial. American Journal of Public Health 1996;86(7):939‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hannon 2012 {published data only}
- Hannon PA, Harris JR, Sopher CJ, Kuniyuki A, Ghosh DL, Henderson S, et al. Improving low‐wage, midsized employers' health promotion practices: a randomized controlled trial. American Journal of Preventive Medicine 2012;43(2):125‐33. [DOI: ] [DOI] [PubMed] [Google Scholar]
Jones 2015 {published data only}
- Jones S, Sloan D, Evans HER, Williams S. Improving the implementation of NICE public health workplace guidance: an evaluation of the effectiveness of action‐planning workshops in NHS trusts in England. Journal of Evaluation in Clinical Practice 2015;21:567‐71. [DOI: 10.1111/jep.12331] [DOI] [PubMed] [Google Scholar]
Parker 2010 {published data only}
- DeJoy DM, Parker KM, Padilla HM, Wilson MG, Roemer EC, Goetzel RZ. Combining environmental and individual weight management interventions in a work setting results from the Dow Chemical study. Journal of Occupational and Environmental Medicine 2011;53(3):245‐52. [DOI: 10.1097/JOM.0b013e31820c9023] [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJoy DM, Wilson MG, Goetzel RZ, Ozminkowski RJ, Wang S, Baker KM, et al. Development of the environmental assessment tool (EAT) to measure organizational physical and social support for worksite obesity prevention programs. Journal of Occupational and Environmental Medicine 2008;50(2):126‐37. [DOI: 10.1097/JOM.0b013e318161b42a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejoy DM, Wilson MG, Padilla HM, Goetzel RZ, Parker KB, Della LJ. Process evaluation results from an environmentally focused worksite weight management study. Health Education & Behaviour 2012;39(4):405‐18. [DOI: 10.1177/1090198111418109] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzel RZ, Baker KM, Short ME, Pei X, Ozminkowski RJ, Wang S, et al. First‐year results of an obesity prevention program at the Dow Chemical Company. Journal of Occupational and Environmental Medicine 2009;51(2):125‐38. [DOI: 10.1097/JOM.0b013e3181954b03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzel RZ, Roemer EC, Pei X, Short ME, Tabrizi MJ, Wilson MG, et al. Second‐year results of an obesity prevention program at the Dow Chemical Company. Journal of Occupational and Environmental Medicine 2010;52(3):291‐302. [DOI: 10.1097/JOM.0b013e3181d46f0b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KB, DeJoy DM, Wilson MG, Bowen HM, Goetzel RZ. Application of the Environmental Assessment Tool (EAT) as a process measure for a worksite weight management intervention. Journal of Enviornmental Medicine 2010;52(Suppl 1):S42‐S51. [DOI: 10.1097/JOM.0b013e3181ca3b37] [DOI] [PubMed] [Google Scholar]
- Wilson MG, Goetzel RZ, Ozminkowski RJ, DeJoy DM, Della L, Roemer EC. Using formative research to develop environmental and ecological interventions to address overweight and obesity. Obesity 2007;15(Suppl 1):37S‐47S. [DOI: 10.1038/oby.2007.386] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abood 2003 {published data only}
- Abood DA, Black DR, Feral D. Nutrition education worksite intervention for university staff: application of the Health Belief Model. Journal of Nutrition Education and Behaviour 2003;35(5):260‐7. [DOI: 10.1016/S1499-4046(06)60057-2] [DOI] [PubMed] [Google Scholar]
Addley 2014 {published data only}
- Addley K, Boyd S, Kerr R, McQuillan P, Houdmont J, McCrory M. The impact of two workplace‐based health risk appraisal interventions on employee lifestyle parameters, mental health and work ability: results of a randomized controlled trial. Health Education Research 2014;29(2):247‐58. [DOI: 10.1093/her/cyt113] [DOI] [PubMed] [Google Scholar]
Aittasalo 2004 {published data only}
- Aittasalo M, Miilunpalo S, Suni J. The effectiveness of physical activity counseling in a work‐site setting. A randomized, controlled trial. Patient Education and Counseling 2004;55(2):193‐202. [DOI: 10.1016/j.pec.2003.09.003] [DOI] [PubMed] [Google Scholar]
Aittasalo 2012 {published data only}
- Aittasalo M, Rinne M, Pasanen M, Kukkonen‐Harjula K, Vasankari T. Promoting walking among office employees ‐ evaluation of a randomized controlled intervention with pedometers and e‐mail messages. BMC Public Health 2012;12(403):1‐11. [DOI: 10.1186/1471-2458-12-403] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alkajah 2012 {published data only}
- Alkhajah TA, Reeves MM, Eakin EG, Winkler EA, Owen N, Healy GN. Sit‐stand workstations: a pilot intervention to reduce office sitting time. American Journal of Preventive Medicine 2012;43(3):298‐303. [DOI: 10.1016/j.amepre.2012.05.027] [DOI] [PubMed] [Google Scholar]
Andersen L.L 2013 {published data only}
- Andersen LL, Sundstrup E, Boysen M, Jakobsen MD, Mortensen OS, Persson R. Cardiovascular health effects of Internet‐based encouragements to do daily workplace stair‐walks: randomized controlled trial. Journal of Medical Internet Research 2013;15(6):e127. [DOI: 10.2196/jmir.2340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersen L.N 2013 {published data only}
- Andersen LN, Juul‐Kristensen B, Roessler KK, Herborg LG, Sorensen TL, Sogaard K. Efficacy of 'Tailored Physical Activity' in reducing sickness absence among health care workers: design of a randomised controlled trial. BMC Public Health 2013;13(917):1‐8. [DOI: 10.1186/1471-2458-13-917] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersen L.N 2015 {published data only}
- Andersen LN, Juul‐Kristensen B, Roessler KK, Herborg LG, Sorensen TL, Sogaard K. Efficacy of 'Tailored Physical Activity' on reducing sickness absence among health care workers: a 3‐months randomised controlled trial. Manual Therapy 2015;20(5):666‐71. [DOI: 10.1016/j.math.2015.04.017] [DOI] [PubMed] [Google Scholar]
Ang 2013 {published data only}
- Ang YK, Mirnalini K, Zalilah MS. A workplace email‐linked website intervention for modifying cancer‐related dietary and lifestyle risk factors: rationale, design and baseline findings. Malaysian Journal of Nutrition 2013;19(1):37‐51. [PubMed] [Google Scholar]
Apostolopoulos 2016 {published data only}
- Apostolopoulos Y, Lemke M, Sonmez S, Hege A. The obesogenic environment of commercial trucking: a worksite environmental audit and implications for systems‐based interventions. American Journal of Health Education 2016;47(2):85‐93. [DOI: 10.1080/19325037.2015.1133339] [DOI] [Google Scholar]
Arao 2007 {published data only}
- Arao T, Oida Y, Maruyama C, Mutou T, Sawada S, Matsuzuki H, et al. Impact of lifestyle intervention on physical activity and diet of Japanese workers. Preventive Medicine 2007;45(2):146‐52. [DOI: 10.1016/j.ypmed.2007.05.004] [DOI] [PubMed] [Google Scholar]
Armitage 2006 {published data only}
- Armitage CJ. Evidence that implementation intentions promote transitions between the stages of change. Journal of Consulting and Clinical Psychology 2006;74(1):141‐51. [DOI: 10.1037/0022-006X.74.1.141] [DOI] [PubMed] [Google Scholar]
Armitage 2007 {published data only}
- Armitage CJ. Efficacy of a brief worksite intervention to reduce smoking: the roles of behavioral and implementation intentions. Journal of Occupational Health Psychology 2007;14(4):376‐90. [DOI: 10.1037/1076-8998.12.4.376] [DOI] [PubMed] [Google Scholar]
Armitage 2010 {published data only}
- Armitage CJ. A volitional help sheet to increase physical activity in people with low socioeconomic status: a randomised exploratory trial. Psychology & Health 2010;25(10):1129‐45. [DOI: 10.1080/08870440903121638] [DOI] [PubMed] [Google Scholar]
Armitage 2015 {published data only}
- Armitage CJ. Field experiment of a very brief worksite intervention to improve nutrition among health care workers. Journal of Behavioral Medicine 2015;38(4):599‐608. [DOI: 10.1007/s10865-015-9634-5] [DOI] [PubMed] [Google Scholar]
Atlantis 2006 {published data only}
- Atlantis E, Chow CM, Kirby A, Fiatarone Singh MA. Worksite intervention effects on physical health: a randomized controlled trial. Health Promotion International 2006;21(3):191‐200. [DOI: 10.1093/heapro/dal012] [DOI] [PubMed] [Google Scholar]
Audrey 2015 {published data only}
- Audrey S, Cooper AR, Hollingworth W, Metcalfe C, Procter S, Davis A. Study protocol: the effectiveness and cost effectiveness of an employer‐led intervention to increase walking during the daily commute: the Travel to Work randomised controlled trial. BMC Public Health 2015;15(154):1‐7. [DOI: 10.1186/s12889-015-1464-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Backman 2011 {published data only}
- Backman D, Gonzaga G, Sugerman S, Francis D, Cook S. Effect of fresh fruit availability at worksites on the fruit and vegetable consumption of low‐wage employees. Journal of Nutrition Education and Behavoir 2011;43(4 Suppl 2):S113‐121. [DOI: 10.1016/j.jneb.2011.04.003] [DOI] [PubMed] [Google Scholar]
Bale 2015 {published data only}
- Bale JM, Gazmararian JA, Elon L. Effect of the work environment on using time at work to exercise. American Journal of Health Promotion 2015;29(6):345‐52. [DOI: 10.4278/ajhp.130731-QUAN-393] [DOI] [PubMed] [Google Scholar]
Bandoni 2010b {published data only}
- Bandoni DH, Moura Bombem C, Lobo Marchioni DM, Jaime PC. The influence of the availability of fruits and vegetables in the workplace on the consumption of workers. Nutrition & Food Science 2010;40(1):20‐5. [DOI: 10.1108/00346651011015872] [DOI] [Google Scholar]
Barene 2014 {published data only}
- Barene S, Krustrup P, Brekke OL, Holtermann A. Soccer and Zumba as health‐promoting activities among female hospital employees: a 40‐weeks cluster randomised intervention study. Journal of Sport Sciences 2014;32(16):1539‐49. [DOI: 10.1080/02640414.2014.906043] [DOI] [PubMed] [Google Scholar]
Barene 2014b {published data only}
- Barene S, Krustrup P, Jackman SR, Brekke OL, Holtermann A. Do soccer and Zumba exercise improve fitness and indicators of health among female hospital employees? A 12‐week RCT. Scandinavian Journal of Medicine & Science in Sports 2014;24(9):990‐9. [DOI: 10.1111/sms.12138] [DOI] [PubMed] [Google Scholar]
Bellicha 2016 {published data only}
- Bellicha A, Kieusseian A, Fontvieille AM, Tataranni A, Copin N, Charreire H. A multistage controlled intervention to increase stair climbing at work: effectiveness and process evaluation. International Journal of Behavioral Nutrition and Physical Activity 2016;13(47):1‐9. [DOI: 10.1186/s12966-016-0371-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2004 {published data only}
- Bennett JB, Patterson CR, Lehman WEK. Team awareness, problem drinking, and drinking climate: workplace social health promotion in a policy context. American Journal of Health Promotion 2004;19(2):103‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beresford 2000 {published data only}
- Beresford SA, Shannon J, McLerran D, Thompson B. Seattle 5‐a‐day work‐site project: process evaluation. Health Education & Behavior 2000;27(2):213‐22. [DOI: 10.1177/109019810002700207] [DOI] [PubMed] [Google Scholar]
Beresford 2001 {published data only}
- Beresford SA, Thompson B, Feng Z, Christianson A, McLerran D, Patrick DL. Seattle 5 a day worksite program to increase fruit and vegetable consumption. Preventive Medicine 2001;32(3):230‐8. [DOI: 10.1006/pmed.2000.0806] [DOI] [PubMed] [Google Scholar]
Berry 1989 {published data only}
- Berry MW, Danish SJ, Rinke WJ, Smiciklas‐Wright H. Work‐site health promotion: the effects of a goal‐setting program on nutrition‐related behaviors. Journal of the American Dietetic Association 1989;89(7):914‐23. [PubMed] [Google Scholar]
Bertera 1993 {published data only}
- Bertera RL. Behavioral risk factor and illness day changes with workplace health promotion: two‐year results. American Journal of Health Promotion 1993;7(5):365‐73. [DOI: 10.4278/0890-1171-7.5.365] [DOI] [PubMed] [Google Scholar]
Blair 1986 {published data only}
- Blair SN, Piserchia PV, Wilbur CS, Crowder JH. A public health intervention model for work‐site health promotion. Impact on exercise and physical fitness in a health promotion plan after 24 months. Journal of the American Medical Association 1986;255(7):921‐6. [PubMed] [Google Scholar]
Blake 2013 {published data only}
- Blake H, Zhou D, Batt ME. Five‐year workplace wellness intervention in the NHS. Perspectives in Public Health 2013;133(5):262‐71. [DOI: 10.1177/1757913913489611] [DOI] [PubMed] [Google Scholar]
Block 2008 {published data only}
- Block G, Sternfeld B, Block CH, Block TJ, Norris J, Hopkins D, et al. Development of Alive! (A Lifestyle Intervention Via Email), and its effect on health‐related quality of life, presenteeism, and other behavioral outcomes: randomized controlled trial. Journal of Medical Internet Research 2008;10(4):1‐27. [DOI: 10.2196/jmir.1112] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bly 1986 {published data only}
- Bly JL, Jones RC, Richardson JE. Impact of worksite health promotion on health care costs and utilization. Evaluation of Johnson & Johnson's Live for Life program. Journal of the American Medical Association 1986;256(23):3235‐40. [PubMed] [Google Scholar]
Borg 2010 {published data only}
- Borg J, Merom D, Rissel C. Staff walking program: a quasi‐experimental trial of maintenance newsletters to maintain walking following a pedometer program. Health Promotion Journal of Australia 2010;21(1):26‐32. [DOI] [PubMed] [Google Scholar]
Brace 2015 {published data only}
- Brace AM, Padilla HM, DeJoy DM, Wilson MG, Vandenberg RJ, Davis M. Applying RE‐AIM to the evaluation of FUEL Your Life: a worksite translation of DPP. Health Promotion Practice 2015;16(1):28‐35. [DOI: 10.1177/1524839914539329] [DOI] [PubMed] [Google Scholar]
Brakenridge 2016 {published data only}
- Brakenridge CL, Fjeldsoe BS, Young DC, Winkler EA, Dunstan DW, Straker LM, et al. Organizational‐level strategies with or without an activity tracker to reduce office workers' sitting time: rationale and study design of a pilot cluster‐randomized trial. JMIR Research Protocols 2016;5(2):e73. [DOI: 10.2196/resprot.5438] [DOI] [PMC free article] [PubMed] [Google Scholar]
Breeze 2017 {published data only}
- Breeze PR, Thomas C, Squires H, Brennan A, Greaves C, Diggle P, et al. Cost‐effectiveness of population‐based, community, workplace and individual policies for diabetes prevention in the UK. Diabetic Medicine 2017;34(8):1136‐44. [DOI: 10.1111/dme.13349] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brehm 2011 {published data only}
- Brehm BJ, Gates DM, Singler M, Succop PA, D'Alessio DA. Environmental changes to control obesity: a randomized controlled trial in manufacturing companies. American Journal of Health Promotion 2011;25(5):334‐40. [DOI: 10.4278/ajhp.090128-QUAN-37] [DOI] [PubMed] [Google Scholar]
Breslow 1990 {published data only}
- Breslow L, Fielding J, Herrman AA, Wilbur CS. Worksite health promotion: Its evolution and the Johnson & Johnson experience. Preventive Medicine 1990;19(1):13‐21. [DOI: 10.1016/0091-7435(90)90002-2] [DOI] [PubMed] [Google Scholar]
Brown 2012 {published data only}
- Brown DK, Barton JL, Pretty J, Gladwell VF. Walks4work: rationale and study design to investigate walking at lunchtime in the workplace setting. BMC Public Health 2012;12(550):1‐10. [DOI: 10.1186/1471-2458-12-550] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2014 {published data only}
- Brown DK, Barton JL, Pretty J, Gladwell VF. Walks4Work: assessing the role of the natural environment in a workplace physical activity intervention. Scandinavian Journal of Work, Environment & Health 2014;40(4):390‐9. [DOI: 10.5271/sjweh.3421] [DOI] [PubMed] [Google Scholar]
Buchholz 2016 {published data only}
- Buchholz SW, Ingram D, Wilbur J, Fogg L, Sandi G, Moss A, et al. Bilingual Text4Walking food service employee intervention pilot study. JMIR mHealth and uHealth 2016;4(2):e68. [DOI: 10.2196/mhealth.5328] [DOI] [PMC free article] [PubMed] [Google Scholar]
Budden 2007 {published data only}
- Budden JS, Sagarin BJ. Implementation intentions, occupational stress, and the exercise intention‐behavior relationship. Journal of Occupational Health Psychology 2007;12(4):391‐401. [DOI: 10.1037/1076-8998.12.4.391] [DOI] [PubMed] [Google Scholar]
Buller 2000 {published data only}
- Buller D, Buller MK, Larkey L, Sennott‐Miller L, Taren D, Aickin M, et al. Implementing a 5‐a‐day peer health educator program for public sector labor and trades employees. Health Education & Behavior 2000;27(2):232‐40. [DOI] [PubMed] [Google Scholar]
Buller 2005 {published data only}
- Buller DB, Andersen PA, Walkosz BJ, Scott MD, Cutter GR, Dignan MB, et al. Randomized trial testing a worksite sun protection program in an outdoor recreation industry. Health Education & Behavior 2005;32(4):514‐35. [DOI: 10.1177/1090198105276211] [DOI] [PubMed] [Google Scholar]
Buman 2017 {published data only}
- Buman MP, Mullane SL, Toledo MJ, Rydell SA, Gaesser GA, Crespo NC, et al. An intervention to reduce sitting and increase light‐intensity physical activity at work: Design and rationale of the 'Stand & Move at Work' group randomized trial. Contemporary Clinical Trials 2017;53:11‐9. [DOI: 10.1016/j.cct.2016.12.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burnhams 2015 {published data only}
- Burnhams NH, London L, Laubscher R, Nel E, Parry C. Results of a cluster randomised controlled trial to reduce risky use of alcohol, alcohol‐related HIV risks and improve help‐seeking behaviour among safety and security employees in the Western Cape, South Africa. Substance Abuse Treatment, Prevention, and Policy 2015;10(18):1‐14. [DOI: 10.1186/s13011-015-0014-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2002 {published data only}
- Campbell MK, Tessaro I, Devellis B, Benedict S, Kelsey K, Belton L, et al. Effects of a tailored health promotion program for female blue‐collar workers: health works for women.. Preventive Medicine 2002;34(3):313‐23. [DOI: 10.1006/pmed.2001.0988] [DOI] [PubMed] [Google Scholar]
Caperchione 2016 {published data only}
- Caperchione CM, Stolp S, Bottorff JL, Oliffe JL, Johnson ST, Seaton C, et al. Changes in men's physical activity and healthy eating knowledge and behavior as a result of program exposure: findings from the workplace POWERPLAY program. Journal of Physical Activity and Health 2016;13(12):1364‐71. [DOI: 10.1123/jpah.2016-0111] [DOI] [PubMed] [Google Scholar]
Carr 2013 {published data only}
- Carr LJ, Karvinen K, Peavler M, Smith R, Cangelosi K. Multicomponent intervention to reduce daily sedentary time: a randomised controlled trial. BMJ Open 2013;3(e003261):1‐11. [DOI: 10.1136/bmjopen-2013-003261] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cash 2012 {published data only}
- Cash SW, Beresford SAA, Henderson JA, McTiernan A, Xiao L, Wang CY, et al. Dietary and physical activity behaviours related to obesity‐specific quality of life and work productivity. British Journal of Nutrition 2012;108(6):1134‐42. [DOI: 10.1017/S0007114511006258] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 2015 {published data only}
- Chapman J, Campbell M, Wilson C. Insights for exercise adherence from a minimal planning intervention to increase physical activity. Health Education & Behavior 2015;42(6):730‐5. [DOI: 10.1177/1090198115577374] [DOI] [PubMed] [Google Scholar]
Chau 2014 {published data only}
- Chau JY, Daley M, Dunn S, Srinivasan A, Do A, Bauman AE, et al. The effectiveness of sit‐stand workstations for changing office workers' sitting time: results from the Stand@Work randomized controlled trial pilot. International Journal of Behavioral Nutrition and Physical Activity 2014;11(127):1‐10. [DOI: 10.1186/s12966-014-0127-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2016 {published data only}
- Chen MM, Tsai AC, Wang JY. The effectiveness and barriers of implementing a workplace health promotion program to improve metabolic disorders in older workers in Taiwan. Global Health Promotion 2016;23(2):6‐14. [DOI: 10.1177/1757975914555341] [DOI] [PubMed] [Google Scholar]
Christensen 2011 {published data only}
- Christensen JR, Faber A, Ekner D, Overgaard K, Holtermann A, Sogaard K. Diet, physical exercise and cognitive behavioral training as a combined workplace based intervention to reduce body weight and increase physical capacity in health care workers ‐ a randomized controlled trial. BMC Public Health 2011;11(671):1‐11. [DOI: 10.1186/1471-2458-11-671] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 2016 {published data only}
- Christensen JR, Bredahl TV, Hadrevi J, Sjogaard G, Sogaard K. Background, design and conceptual model of the cluster randomized multiple component workplace study: FRamed Intervention to Decrease Occupational Muscle pain ‐ "FRIDOM". BMC Public Health 2016;16(1116):1‐13. [DOI: 10.1186/s12889-016-3758-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coeffeng 2012 {published data only}
- Coffeng JK, Hendriksen IJM, Duijts SF, Proper KI, Mechelen W, Boot CRL. The development of the Be Active & Relax “Vitality in Practice” (VIP) project and design of an RCT to reduce the need for recovery in office employees. BMC Public Health 2012;12(592):1‐13. [DOI: 10.1186/1471-2458-12-592] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coffeng 2013 {published data only}
- Coffeng JK, Hendriksen JJ, Mechelen W, Boot CR. Process evaluation of a worksite social and physical environmental intervention. Journal of Occupational and Environmental Medicin 2013;55(12):1409‐20. [DOI: 10.1097/JOM.0b013e3182a50053] [DOI] [PubMed] [Google Scholar]
Coffeng 2014 {published data only}
- Coeffeng JK, Boot CR, Duijts SF, Twisk JW, Mechelen W, Hendriksen IJ. Effectiveness of a worksite social & physical environment intervention on need for recovery, physical activity and relaxation; results of a randomized controlled trial. PLOS ONE 2014;9(12):1‐26. [DOI: 10.1371/journal.pone] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conrad 1996 {published data only}
- Conrad KM, Campbell RT, Edington DW, Faust HS, Vilnius D. The worksite environment as a cue to smoking reduction. Research in Nursing & Health 1996;19(1):21‐31. [DOI: 10.1002/(SICI)1098-240X(199602)19:1%26lt;21::AID-NUR3%26gt;3.0.CO;2-N] [DOI] [PubMed] [Google Scholar]
Cook 2007 {published data only}
- Cook RF, Billings DW, Hersch RK, Back AS, Hendrickson A. A field test of a web‐based workplace health promotion program to improve dietary practices, reduce stress, and increase physical activity: randomized controlled trial. Journal of Medical Internet Research 2007;9(2):e17. [DOI: 10.2196/jmir.9.2.e17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooke 2000 {published data only}
- Cooke M. The dissemination of a smoking cessation program: predictors of program awareness, adoption and maintenance. Health Promotion International 2000;15(2):113‐24. [DOI: 10.1093/heapro/15.2.113] [DOI] [Google Scholar]
Crawford 2004 {published data only}
- Crawford PB, Gosliner W, Strode P, Samuels SE, Burnett C, Craypo L, et al. Walking the talk: fit WIC wellness programs improve self‐efficacy in pediatric obesity prevention counseling. American Journal of Public Health 2004;94(9):1480‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cremaschini 2015 {published data only}
- Cremaschini M, Moretti R, Brembilla G, Valoti M, Sarnataro F, Spada P, et al. Assessment of the impact over one year of a workplace health promotion programme in the province of Bergamo. La Medicina Del Lavoro 2015;106(3):159‐71. [PubMed] [Google Scholar]
Dalager 2017 {published data only}
- Dalager T, Justesen JB, Sjogaard G. Intelligent physical exercise training in a workplace setting improves muscle strength and musculoskeletal pain: a randomized controlled trial. BioMed Research International 2017;Article ID 7914134:1‐9. [DOI: 10.1155/2017/7914134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dallam 2013 {published data only}
- Dallam GM, Foust CP. A comparative approach to using the diabetes prevention program to reduce diabetes risk in a worksite setting. Health Promotion Practice 2013;14(2):199‐204. [DOI: 10.1177/1524839912437786] [DOI] [PubMed] [Google Scholar]
Dallat 2013 {published data only}
- Dallat MT, Hunter RF, Tully MA, Cairns KJ, Kee F. A lesson in business: cost‐effectiveness analysis of a novel financial incentive intervention for increasing physical activity in the workplace. BMC Public Health 2013;13(953):1‐9. [DOI: 10.1186/1471-2458-13-953] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davy 2014 {published data only}
- Davy BM, You W, Almeida F, Wall S, Harden S, Comber DL, et al. Impact of individual and worksite environmental factors on water and sugar‐sweetened beverage consumption among overweight employees. Preventing Chronic Disease 2014;11:1‐9. [DOI: 10.5888/pcd11.130207] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Bourdeaudhuij 2007 {published data only}
- Bourdeaudhuij I, Stevens V, Vandelanotte C, Brug J. Evaluation of an interactive computer‐tailored nutrition intervention in a real‐life setting. Annals of Behavioral Medicine 2007;33(1):39‐48. [DOI: 10.1207/s15324796abm3301_5] [DOI] [PubMed] [Google Scholar]
Deitz 2014 {published data only}
- Deitz D, Cook RF, Hersch RK, Leaf S. Heart healthy online: an innovative approach to risk reduction in the workplace. Journal of Occupational and Environmental Medicine 2014;56(5):547‐53. [DOI: 10.1097/JOM.0000000000000148] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dishman 2009 {published data only}
- Dishman RK, DeJoy DM, Wilson MG, Vandenberg RJ. Move to Improve: a randomized workplace trial to increase physical activity. American Journal of Preventive Medicine 2009;36(2):133‐41. [DOI: 10.1016/j.amepre.2008.09.038] [DOI] [PubMed] [Google Scholar]
Dishman 2010 {published data only}
- Dishman RK, Vandenberg RJ, Motl RW, Wilson MG, DeJoy DM. Dose relations between goal setting, theory‐based correlates of goal setting and increases in physical activity during a workplace trial. Health Education Research 2010;25(4):620‐31. [DOI: 10.1093/her/cyp042] [DOI] [PubMed] [Google Scholar]
Donath 2015 {published data only}
- Donath L, Fraude O, Schefer Y, Roth R, Zahner L. Repetitive daily point of choice prompts and occupational sit‐stand transfers, concentration and neuromuscular performance in office Workers: an RCT. International Journal of Environmental Research and Public Health 2015;12:4340‐53. [DOI: 10.3390/ijerph120404340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doumas 2008 {published data only}
- Doumas DM, Hannah E. Preventing high‐risk drinking in youth in the workplace: a web‐based normative feedback program. Journal of Substance Abuse Treatment 2008;34(3):263‐71. [DOI: 10.1016/j.jsat.2007.04.006] [DOI] [PubMed] [Google Scholar]
Dubuy 2013 {published data only}
- Dubuy V, Cocker K, Bourdeaudhuij I, Maes L, Seghers J, Lefevre J, et al. Evaluation of a workplace intervention to promote commuter cycling: a RE‐AIM analysis. BMC Public Health 2013;13(587):1‐11. [DOI: 10.1186/1471-2458-13-587] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffy 2012 {published data only}
- Duffy SA, Ronis DL, Richardson C, Waltje AH, Ewing LA, Noonan D, et al. Protocol of a randomized controlled trial of the Tobacco Tactics website for operating engineers. BMC Public Health 2012;12(335):1‐10. [DOI: 10.1186/1471-2458-12-335] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dutta 2014 {published data only}
- Dutta N, Koepp GA, Stovitz SD, Levine JA, Pereira MA. Using sit‐stand workstations to decrease sedentary time in office workers: a randomized crossover trial. International Journal of Environmental Research and Public Health 2014;11:6653‐65. [DOI: 10.3390/ijerph110706653] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edries 2013 {published data only}
- Edries N, Jelsma J, Maart S. The impact of an employee wellness programme in clothing/textile manufacturing companies: a randomised controlled trial. BMC Public Health 2013;13(25):1‐9. [DOI: 10.1186/1471-2458-13-25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Emmons 1996 {published data only}
- Emmons KM, Linnan L, Abrams D, Lovell HJ. Women who work in manufacturing settings: factors influencing their participation in worksite health promotion programs. Women's Health Issues 1996;6(2):74‐81. [DOI: 10.1016/1049-3867(95)00049-6] [DOI] [PubMed] [Google Scholar]
Emmons 1999 {published data only}
- Emmons KM, Linnan LA, Shadel WG, Marcus B, Abrams DB. The Working Healthy Project: a worksite health‐promotion trial targeting physical activity, diet, and smoking. Journal of Occupational and Environmental Medicine 1999;41(7):545‐55. [DOI] [PubMed] [Google Scholar]
Engbers 2006 {published data only}
- Engbers LH, Poppel MN, Chin A, Paw M, Mechelen W. The effects of a controlled worksite environmental intervention on determinants of dietary behavior and self‐reported fruit, vegetable and fat intake. BMC Public Health 2006;6(253):1‐10. [DOI: 10.1186/1471-2458-6-253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Erfurt 1991 {published data only}
- Erfurt JC, Foote A, Heirich MA. Worksite wellness programs: incremental comparison of screening and referral alone, health education, follow‐up counseling, and plant organization. American Journal of Health Promotion 1991;5(6):438‐48. [DOI: 10.4278/0890-1171-5.6.438] [DOI] [PubMed] [Google Scholar]
Erskine 2012 {published data only}
- Erskine J, Lanigan A, Emsermann CB, Manning BK, Staton EW, Pace WD. Use of the Americans in Motion‐Healthy Intervention (AIM‐HI) to create a culture of fitness in family practice. Journal of the American Board of Family Medicine 2012;25(5):694‐700. [DOI: 10.3122/jabfm.2012.05.110071] [DOI] [PubMed] [Google Scholar]
Estabrook 2012 {published data only}
- Estabrook B, Zapka J, Lemon SC. Evaluating the implementation of a hospital work‐site obesity prevention intervention: applying the RE‐AIM framework. Health Promotion Practice 2012;13(2):190‐7. [DOI: 10.1177/1524839910385897] [DOI] [PubMed] [Google Scholar]
Fagan 2003 {published data only}
- Fagan P, Stoddard AM, Hunt MK, Frazier L, Girod K, Sorensen G. The feasibility of evaluating a tobacco control intervention for working youth. Tobacco Control 2003;12(Suppl 4):34‐39. [DOI: 10.1136/tc.12.suppl_4.iv34] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fagan 2003b {published data only}
- Fagan P, Eisenberg M, Frazier L, Stoddard AM, Avrunin JS, Sorensen G. Employed adolescents and beliefs about self‐efficacy to avoid smoking. Addictive Behaviors 2003;28(4):613‐26. [DOI] [PubMed] [Google Scholar]
Faghri 2008 {published data only}
- Faghri PD, Blozie E, Gustavesen S, Kotejoshyer R. The role of tailored consultation following health‐risk appraisals in employees' health behavior. Journal of Occupational and Environmental Medicine 2008;50(12):1378‐85. [DOI: 10.1097/JOM.0b013e3181862471] [DOI] [PubMed] [Google Scholar]
Fink 2016 {published data only}
- Fink JT, Smith DR, Singh M, Ihnrke DM, Cisler RA. Obese employee participation patterns in a wellness program. Population Health Management 2016;19(2):132‐5. [DOI: 10.1089/pop.2015.0021] [DOI] [PubMed] [Google Scholar]
Fitzgerald 2017 {published data only}
- Fitzgerald S, Kirby A, Murphy A, Geaney F, Perry IJ. A cost‐analysis of complex workplace nutrition education and environmental dietary modification interventions. BMC Public Health 2017;17(49):1‐10. [DOI: 10.1186/s12889-016-3988-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flannery 2012 {published data only}
- Flannery K, Resnick B, McMullen TL. The impact of the Worksite Heart Health Improvement Project on work ability: a pilot study. Journal of Occupational and Environmental Medicine 2012;54(11):1406‐12. [DOI: 10.1097/JOM.0b013e3182619053] [DOI] [PubMed] [Google Scholar]
Flannery 2012b {published data only}
- Flannery K, Resnick B, Galik E, Lipscomb J, McPhaul K, Shaughnessy M. The Worksite Heart Health Improvement Project (WHHIP): feasibility and efficacy. Public Health Nursing 2012;29(5):455‐66. [DOI: 10.1111/j.1525-1446.2012.01023.x] [DOI] [PubMed] [Google Scholar]
Fleig 2010 {published data only}
- Fleig L, Lippke S, Wiedmann AU, Ziegelmann JP, Reuter T, Gravert C. Promoting physical activity in a workplace setting: a randomized control group study of stage‐matched interventions. Journal of Health Psychology 2010;18:69‐78. [DOI: 10.1026/0943-8149/a000011] [DOI] [Google Scholar]
Ford 2014 {published data only}
- Ford MA, Haskins MA, Wade C. Weight management through motivational counseling in the workplace. Journal of Safety, Health and Environmental Research 2014;10(2):178‐83. [Google Scholar]
Freak‐Poli 2013b {published data only}
- Freak‐Poli R, Wolfe R, Brand M, Courten M, Peeters A. Eight‐month postprogram completion: change in risk factors for chronic disease amongst participants in a 4‐month pedometer‐based workplace health program. Obesity 2013;21(9):E360‐8. [DOI: 10.1002/oby.20342] [DOI] [PubMed] [Google Scholar]
French 2010 {published data only}
- French SA, Harnack LJ, Hannan PJ, Mitchell NR, Gerlach AF, Toomey TL. Worksite environment intervention to prevent obesity among metropolitan transit workers. Preventive Medicine 2010;50(4):180‐5. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedrich 2009 {published data only}
- Friedrich V, Brugger A, Bauer G. Worksite tobacco prevention in the Canton of Zurich: stages of change, predictors, and outcomes. International Journal of Public Health 2009;54:427‐38. [DOI: 10.1007/s00038-009-0084-0] [DOI] [PubMed] [Google Scholar]
Friedrich 2015 {published data only}
- Friedrich V, Brugger A, Bauer GF. Worksite tobacco prevention: a randomized, controlled trial of adoption, dissemination strategies, and aggregated health‐related outcomes across companies. BioMed Research International 2015;2015(136505):1‐10. [DOI: 10.1155/2015/136505] [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedrich 2015b {published data only}
- Friedrich V, Hoffmann S, Bauer G. Strategies of active dissemination of workplace health promotion. International Journal of Workplace Health Management 2015;8(1):3‐14. [DOI: 10.1108/IJWHM-12-2012-0031] [DOI] [Google Scholar]
Gao 2010 {published data only}
- Gao J, Zheng P, Fu H. Application of theory of organizational change for smoking cessation in workplace. Wei Sheng Yan Jiu 2010;39(6):705‐8. [PubMed] [Google Scholar]
Geaney 2013b {published data only}
- Geaney F, Scotto Di Marrazzo J, Kelly C, Fitzgerald AP, Harrington JM, Kirby A, et al. The food choice at work study: effectiveness of complex workplace dietary interventions on dietary behaviours and diet‐related disease risk ‐ study protocol for a clustered controlled trial. Trials 2013;14(370):1‐14. [DOI: 10.1186/1745-6215-14-370] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gemson 2008 {published data only}
- Gemson DH, Commisso R, Fuente J, Newman J, Benson S. Promoting weight loss and blood pressure control at work: impact of an education and intervention program. Journal of Occupational and Environmental Medicine 2008;50(3):272‐81. [DOI: 10.1097/JOM.0b013e318162f628] [DOI] [PubMed] [Google Scholar]
Glanz 1998 {published data only}
- Glanz K, Patterson RE, Kristal AR, Feng Z, Linnan L, Heimendinger J, et al. Impact of work site health promotion on stages of dietary change: the Working Well Trial. Health Education & Behavior 1998;25(4):448‐63. [DOI] [PubMed] [Google Scholar]
Glanz 1998b {published data only}
- Glanz K, Kristal AR, Tilley BC, Hirst K. Psychosocial correlates of healthful diets among male auto workers. Cancer Epidemiology, Biomarkers & Prevention 1998;7(2):119‐26. [PubMed] [Google Scholar]
Glasgow 1993 {published data only}
- Glasgow RE, Boles SM. Results of a year‐long incentives‐based worksite smoking‐cessation program. Addictive Behaviors 1993;18(4):455‐64. [DOI: 10.1016/0306-4603(93)90063-F] [DOI] [PubMed] [Google Scholar]
Glasgow 1994 {published data only}
- Glasgow RE, Terborg JR, Hollis JF, Severson HH, Fisher J, Boles SM, et al. Modifying dietary and tobacco use patterns in the worksite: the Take Heart Project. Health Education & Behaviour 1994;21(1):69‐82. [DOI] [PubMed] [Google Scholar]
Glasgow 1995 {published data only}
- Glasgow RE, Terborg JR, Hollis JF, Severson HH, Boles SM. Take heart: results from the initial phase of a work‐site wellness program. American Journal of Publi Health 1995;85(2):209‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasgow 1996 {published data only}
- Glasgow RE, Sorensen G, Giffen C, Shipley RH, Corbett K, Lynn W. Promoting worksite smoking control policies and actions: the community intervention trial for smoking cessation (COMMIT) experience. Preventive Medicine 1996;25(2):186‐94. [DOI: 10.1006/pmed.1996.0045] [DOI] [PubMed] [Google Scholar]
Glasgow 1997 {published data only}
- Glasgow RE, Terborg JR, Strycker LA, Boles SM, Hollis JF. Take Heart II: replication of a worksite health promotion trial. Journal of Behavioral Medicine 1997;20(2):143‐61. [DOI] [PubMed] [Google Scholar]
Goetzel 2005 {published data only}
- Goetzel RZ, Ozminkowski RJ, Baase CM, Billotti GM. Estimating the return‐on‐investment from changes in employee health risks on the Dow Chemical Company's health care costs. Journal of Occupational and Environmental Medicine 2005;47(8):759‐68. [DOI] [PubMed] [Google Scholar]
Goetzel 2009 {published data only}
- Goetzel RZ, Roemer EC, Short ME, Pei X, Tabrizi MJ, Liss‐Levinson RC, et al. Health improvement from a worksite health promotion private‐public partnership. Journal of Occupational and Environmental Medicine 2009;5(3):296‐304. [DOI: 10.1097/JOM.0b013e31819ac4bb] [DOI] [PubMed] [Google Scholar]
Gosliner 2010 {published data only}
- Gosliner WA, James P, Yancey AK, Ritchie L, Studer N, Crawford PB. Impact of a worksite wellness program on the nutrition and physical activity environment of child care centers. American Journal of Health Promotion 2010;24(3):186‐9. [DOI: 10.4278/ajhp.08022719] [DOI] [PubMed] [Google Scholar]
Gram 2012 {published data only}
- Gram B, Holtermann A, Sogaard K, Sjogaard G. Effect of individualized worksite exercise training on aerobic capacity and muscle strength among construction workers‐‐a randomized controlled intervention study. Scandinavian Journal of Work, Environment & Health 2012;38(5):467‐75. [DOI: ] [DOI] [PubMed] [Google Scholar]
Grande 2013 {published data only}
- Grande AJ, Silva V, Manzatto L, Rocha TB, Martins GC, Barros Vilela G. Comparison of worker's health promotion interventions: cluster randomized controlled trial. Revista Brasileira de Cineantropometria & Desempenho Humano 2013;15(1):27‐37. [DOI: 10.5007/1980-0037.2013v15n1p27] [DOI] [Google Scholar]
Griffin‐Blake 2006 {published data only}
- Griffin‐Blake CS, DeJoy DM. Evaluation of social‐cognitive versus stage‐matched, self‐help physical activity interventions at the workplace. American Journal of Health Promotion 2006;20(3):200‐9. [DOI: 10.4278/0890-1171-20.3.200] [DOI] [PubMed] [Google Scholar]
Gritz 1998 {published data only}
- Gritz ER, Thompson B, Emmons K, Ockene JK, McLerran DF, Nielsen IR. Gender differences among smokers and quitters in the Working Well Trial. Preventive Medicine 1998;27(4):553‐61. [DOI: 10.1006/pmed.1998.0325] [DOI] [PubMed] [Google Scholar]
Groeneveld 2008 {published data only}
- Groeneveld IF, Proper KI, Beek AJ, Duivenbooden C, Mechelen W. Design of a RCT evaluating the (cost‐) effectiveness of a lifestyle intervention for male construction workers at risk for cardiovascular disease: the health under construction study. BMC Public Health 2008;8(1):1‐12. [DOI: 10.1186/1471-2458-8-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Groeneveld 2011 {published data only}
- Groeneveld IF, Proper KI, Beek AJ, Hildebrandt VH, Mechelen W. Short and long term effects of a lifestyle intervention for construction workers at risk for cardiovascular disease: a randomized controlled trial. BMC Public Health 2011;11(836):1‐9. [DOI: 10.1186/1471-2458-11-836] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadgraft 2017 {published data only}
- Hadgraft NT, Winkler EAH, Healy GN, Lynch BM, Neuhaus M, Eakin EG, et al. Intervening to reduce workplace sitting: mediating role of social‐cognitive constructs during a cluster randomised controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2017;14(27):1‐9. [DOI: 10.1186/s12966-017-0483-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hagger 2011 {published data only}
- Hagger MS, Lonsdale A, Chatzisarantis NL. Effectiveness of a brief intervention using mental simulations in reducing alcohol consumption in corporate employees. Psychology, Health & Medicine 2011;16(4):375‐92. [DOI: 10.1080/13548506.2011.554568] [DOI] [PubMed] [Google Scholar]
Hall 2015 {published data only}
- Hall J, Mansfield L, Kay T, McConnell A. The effect of a sit‐stand workstation intervention on daily sitting, standing and physical activity: protocol for a 12 month workplace randomised control trial. BMC Public Health 2015;15(152):1‐9. [DOI: 10.1186/s12889-015-1506-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallam 2004 {published data only}
- Hallam JS, Petosa R. The long‐term impact of a four‐session work‐site intervention on selected social cognitive theory variables linked to adult exercise adherence. Health Education & Behavior 2004;31(1):88‐100. [DOI: 10.1177/1090198103259164] [DOI] [PubMed] [Google Scholar]
Han 2014 {published data only}
- Han YW, Mohammad M, Liew SM. Effectiveness of a brief physician counselling session on improving smoking behaviour in the workplace. Asian Pacific Journal of Cancer Prevention 2014;15(17):7287‐90. [DOI] [PubMed] [Google Scholar]
Harden 2017 {published data only}
- Harden SM, Johnson SB, Almeida FA, Estabrooks PA. Improving physical activity program adoption using integrated research‐practice partnerships: an effectiveness‐implementation trial. Translational Behavioral Medicine 2017;7(1):28‐38. [DOI: 10.1007/s13142-015-0380-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harley 2010 {published data only}
- Harley AE, Devine CM, Beard B, Stoddard AM, Hunt MK, Sorensen G. Multiple health behavior changes in a cancer prevention intervention for construction workers, 2001‐2003. Preventing Chronic Disease 2010;7(3):1‐12. [PMC free article] [PubMed] [Google Scholar]
Harley 2013 {published data only}
- Harley AE, Sapp AL, Li Y, Marino M, Quintiliani LM, Sorensen G. Sociodemographic and social contextual predictors of multiple health behavior change: data from the Healthy Directions–Small Business study. Translational Behavioral Medicine 2013;3(1):131‐9. [DOI: 10.1007/s13142-013-0196-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2008 {published data only}
- Harris JR, Cross J, Hannon PA, Mahoney E, Ross‐Viles S. Employer adoption of evidence‐based chronic disease prevention practices: a pilot study. Preventing Chronic Disease 2008;5(3):A92. [PMC free article] [PubMed] [Google Scholar]
Healy 2013 {published data only}
- Healy GN, Eakin EG, LaMontagne AD, Owen N, Winkler E, Wiesner G, et al. Reducing sitting time in office workers: short‐term efficacy of a multicomponent intervention. Preventive Medicine 2013;57(1):43‐8. [DOI: 10.1016/j.ypmed.2013.04.004] [DOI] [PubMed] [Google Scholar]
Hebert 1993 {published data only}
- Hebert JR, Harris DR, Sorensen G, Stoddard AM, Hunt MK, Morris DH. A work‐site nutrition intervention: its effects on the consumption of cancer‐related nutrients. American Journal of Public Health 1993;83(3):391‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hebert 1993b {published data only}
- Hebert JR, Stoddard AM, Harris DR, Sorensen G, Hunt MK, Morris DH, et al. Measuring the effect of a worksite‐based nutrition intervention on food consumption. Annals of Epidemiology 1993;3(6):629‐35. [DOI] [PubMed] [Google Scholar]
Heirich 2000 {published data only}
- Heirich M, Sieck CJ. Worksite cardiovascular wellness programs as a route to substance abuse prevention. Journal of Occupational and Environmental Medicine 2000;42(1):47‐56. [DOI] [PubMed] [Google Scholar]
Hermansson 2010 {published data only}
- Hermansson U, Helander A, Brandt L, Huss A, Ronnberg S. Screening and brief intervention for risky alcohol consumption in the workplace: results of a 1‐year randomized controlled study. Alcohol and Alcoholism 2010;45(3):252‐7. [DOI: 10.1093/alcalc/agq021] [DOI] [PubMed] [Google Scholar]
Hill‐Mey 2013 {published data only}
- Hill‐Mey PE. Outcomes of a Five‐year University‐based Worksite Wellness Program [PhD thesis]. Salt Lake City (UT): Department of Health Promotion and Education, University of Utah, 2012. [Google Scholar]
Holtermann 2010 {published data only}
- Holtermann A, Jorgensen MB. Gram B, Christensen JR, Faber A, Overgaard K. Worksite interventions for preventing physical deterioration among employees in job‐groups with high physical work demands: Background, design and conceptual model of FINALE. BMC Public Health 2010;10(120):1‐12. [DOI: 10.1186/1471-2458-10-120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopkins 2012 {published data only}
- Hopkins JM, Glenn BA, Cole BL, McCarthy W, Yancey A. Implementing organizational physical activity and healthy eating strategies on paid time: process evaluation of the UCLA WORKING pilot study. Health Education Research 2012;27(3):385‐98. [DOI: 10.1093/her/cys010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopkins 2012b {published data only}
- Hopkins JM. Understanding Key Players and Factors Involved in the Implementation of Physical Activity Push Strategies into Organizational Settings [PhD thesis]. Los Angeles (USA): University of California, Los Angeles, 2012. [Google Scholar]
Hughes 2011 {published data only}
- Hughes SL, Seymour RB, Campbell RT, Shaw JW, Fabiyi C, Sokas R. Comparison of two health‐promotion programs for older workers. American Journal of Public Health 2011;101(5):883‐90. [DOI: 10.2105/AJPH.2010.300082] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunt 1993 {published data only}
- Hunt MK, Hebert JR, Sorensen G, Harris DR, Hsieh J, Morris DH. Impact of a worksite cancer prevention program on eating patterns of workers. Journal of Nutrition Education 1993;25(5):236‐44. [DOI: 10.1016/S0022-3182(12)81001-6] [DOI] [Google Scholar]
Hunt 2000 {published data only}
- Hunt MK, Lederman R, Stoddard A, Potter S, Phillips J, Sorensen G. Process tracking results from the Treatwell 5‐a‐day worksite study. American Journal of Health Promotion 2000;14(3):179‐87. [DOI] [PubMed] [Google Scholar]
Hunt 2003 {published data only}
- Hunt MK, Stoddard AM, Barbeau E, Goldman R, Wallace L, Gutheil C, et al. Cancer prevention for working class, multiethnic populations through small businesses: the healthy directions study. Cancer Causes & Control 2003;14(8):749‐60. [DOI] [PubMed] [Google Scholar]
Hunt 2003b {published data only}
- Hunt MK, Fagan P, Lederman R, Stoddard A, Frazier L, Girod K, et al. Feasibility of implementing intervention methods in an adolescent worksite tobacco control study. Tobacco Control 2003;12(Suppl IV):iv40‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunt 2007 {published data only}
- Hunt MK, Barbeau EM, Lederman R, Stoddard AM, Chetkovich C, Goldman R, et al. Process evaluation results from the Healthy Directions‐Small Business study. Health Education & Behavior 2007;34(1):90‐107. [DOI: 10.1177/1090198105277971] [DOI] [PubMed] [Google Scholar]
Hunt 2007b {published data only}
- Hunt MK, Lederman R, Stoddard AM, LaMontagne AD, McLellan D, Combe C, et al. Process evaluation of an integrated health promotion/occupational health model in WellWorks‐2. Health Education & Behavior 2005;32(1):10‐26. [DOI: 10.1177/1090198104264216] [DOI] [PubMed] [Google Scholar]
Hunt 2010 {published data only}
- Hunt MK, Harley AE, Stoddard AM, Lederman RI, MacArthur MJ, Sorensen G. Elements of external validity of tools for health: an intervention for construction laborers. American Journal of Health Promotion 2010;24(5):e11‐e20. [DOI: 10.4278/ajhp.080721-QUAN-130] [DOI] [PubMed] [Google Scholar]
Hunter 2013 {published data only}
- Hunter RF, Tully MA, Davis M, Stevenson M, Kee F. Physical activity loyalty cards for behavior change: a quasi‐experimental study. American Journal of Preventive Medicine 2013;45(1):56‐63. [DOI: 10.1016/j.amepre.2013.02.022] [DOI] [PubMed] [Google Scholar]
Ishii 2007 {published data only}
- Ishii A, Nakiri M, Nagatomi K, Tsuji Y, Hoshiko M, Yamaguchi Y, et al. Effect of a physical activity improvement program using the transtheoretical model at a small‐scale company. Kurume Medical Journal 2007;54(1):1‐8. [DOI] [PubMed] [Google Scholar]
Jaime 2014 {published data only}
- Jaime PC, Bandoni DH, Sarno F. Impact of an education intervention using email for the prevention of weight gain among adult workers. Public Health Nutrition 2014;17(7):1620‐7. [DOI: 10.1017/S1368980013001936] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jason 1997 {published data only}
- Jason LA, Salina D, McMahon SD, Hedeker D, Stockton M. A worksite smoking intervention: a 2 year assessment of groups, incentives and self‐help. Health Education Research 1997;12(1):129‐38. [DOI] [PubMed] [Google Scholar]
Jeffery 1993 {published data only}
- Jeffery RW, Forster JL, French SA, Kelder SH, Lando HA, McGovern PG. The Healthy Worker Project: a work‐site intervention for weight control and smoking cessation. American Journal of Public Health 1993;83(3):395‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2010 {published data only}
- Johnson CC, Lai Y, Rice J, Rose D, Webber LS. ACTION Live: using process evaluation to describe implementation of a worksite wellness program. Journal of Occupational and Environmental Medicine 2010;52(Suppl 1):S14‐S21. [DOI: 10.1097/JOM.0b013e3181c81ade] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kazi 2013 {published data only}
- Kazi A. Promoting Physical Activity in the Workplace: a Stage of Change Approach [PhD thesis]. Loughborough (UK): Loughborough University, 2013. [Google Scholar]
Kilpatrick 2016 {published data only}
- Kilpatrick M, Blizzard L, Sanderson K, Teale B, Nelson M, Chappell K, et al. Investigating employee‐reported benefits of participation in a comprehensive Australian workplace health promotion program. Journal of Occupational and Environmental Medicine 2016;58(5):505‐13. [DOI: 10.1097/JOM.0000000000000713] [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim A, Kamyab K, Zhu J, Volpp K. Why are financial incentives not effective at influencing some smokers to quit? Results of a process evaluation of a worksite trial assessing the efficacy of financial incentives for smoking cessation. Journal of Occupational and Environmental Medicine 2011;53(1):62‐67. [DOI: 10.1097/JOM.0b013e31820061d7] [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim AE, Towers A, Renaud J, Zhu J, Shea JA, Galvin R, et al. Application of the RE‐AIM framework to evaluate the impact of a worksite‐based financial incentive intervention for smoking cessation. Journal of Occupational and Environmental Medicine 2012;54(5):610‐4. [DOI: 10.1097/JOM.0b013e31824b2171] [DOI] [PubMed] [Google Scholar]
Kirchner 2013 {published data only}
- Kirchner AT, Ladd DA, Elshaw JJ, Schlub JF. An inexpensive workplace initiative to motivate high‐risk individual health improvement. Military Medicine 2013;178(8):948‐53. [DOI: 10.7205/MILMED-D-13-00065] [DOI] [PubMed] [Google Scholar]
Klatt 2016 {published data only}
- Klatt MD, Sieck C, Gascon G, Malarkey W, Huerta T. A healthcare utilization cost comparison between employees receiving a worksite mindfulness or a diet/exercise lifestyle intervention to matched controls 5 years post intervention. Complementary Therapies in Medicine 2016;27:139‐44. [DOI: 10.1016/j.ctim.2016.05.008] [DOI] [PubMed] [Google Scholar]
Koffman 1998 {published data only}
- Koffman DM, Lee JW, Hopp JW, Emont SL. The impact of including incentives and competition in a workplace smoking cessation program on quit rates. American Journal of Health Promotion 1998;13(2):105‐11. [DOI: 10.4278/0890-1171-13.2.105] [DOI] [PubMed] [Google Scholar]
Kolbe‐Alexander 2012 {published data only}
- Kolbe‐Alexander TL, Proper KI, Lambert EV, Wier MF, Pillay JD, Nossel C, et al. Working on wellness (WOW): a worksite health promotion intervention programme. BMC Public Health 2012;12(372):1‐12. [DOI: 10.1186/1471-2458-12-372] [DOI] [PMC free article] [PubMed] [Google Scholar]
Korshoj 2012 {published data only}
- Korshoj M, Krustrup P, Jorgensen MB, Prescott E, Hansen AM, Kristiansen J, et al. Cardiorespiratory fitness, cardiovascular workload and risk factors among cleaners: a cluster randomized worksite intervention. BMC Public Health 2012;12(645):1‐9. [DOI: 10.1186/1471-2458-12-645] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kristal 1995 {published data only}
- Kristal AR, Patterson RE, Glanz K, Heimendinger J, Hebert JR, Feng ZD, et al. Psychosocial correlates of healthful diets: baseline results from the Working Well study. Preventive Medicine 1995;24(3):221‐8. [DOI: 10.1006/pmed.1995.1037] [DOI] [PubMed] [Google Scholar]
Kristal 2000 {published data only}
- Kristal AR, Glanz K, Tilley BC, Li S. Mediating factors in dietary change: understanding the impact of a worksite nutrition intervention. Health Education & Behavior 2000;27(1):112‐25. [DOI: 10.1177/109019810002700110] [DOI] [PubMed] [Google Scholar]
Kushida 2014 {published data only}
- Kushida O, Murayama N. Effects of environmental intervention in workplace cafeterias on vegetable consumption by male workers. Journal of Nutrition Education and Behavior 2014;46(5):350‐8. [DOI: 10.1016/j.jneb.2014.05.001] [DOI] [PubMed] [Google Scholar]
Kwak 2007 {published data only}
- Kwak L, Kremers SP, Baak MA, Brug J. A poster‐based intervention to promote stair use in blue‐ and white‐collar worksites. Preventive Medicine 2007;45(2):177‐181. [DOI: 10.1016/j.ypmed.2007.05.005] [DOI] [PubMed] [Google Scholar]
Kwak 2007b {published data only}
- Kwak L, Kremers SP, Werkman A, Visscher TL, Baak MA, Brug J. The NHF‐NRG In Balance‐project: the application of Intervention Mapping in the development, implementation and evaluation of weight gain prevention at the worksite. Obesity Reviews 2007;8(4):347‐61. [DOI: 10.1111/j.1467-789X.2006.00304.x] [DOI] [PubMed] [Google Scholar]
Kwak 2009 {published data only}
- Kwak L, Kremers SP, Visscher TL, Baak MA, Brug J. Behavioral and cognitive effects of a worksite‐based weight gain prevention program: the NHF‐NRG in balance‐project. Journal of Occupational and Environmental Medicine 2009;51(12):1437‐46. [DOI: 10.1097/JOM.0b013e3181bd895a] [DOI] [PubMed] [Google Scholar]
LaCaille 2016 {published data only}
- LaCaille LJ, Schultz JF, Goei R, LaCaille RA, Dauner KN, Souza R, et al. Go!: results from a quasi‐experimental obesity prevention trial with hospital employees. BMC Public Health 2016;16(171):1‐16. [DOI: 10.1186/s12889-016-2828-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laing 2012 {published data only}
- Laing SS, Hannon PA, Williams B, Harris JR, Talburt A, Kimpe S. Increasing evidence‐based workplace health promotion best practices in small and low‐wage companies, Mason County, Washington, 2009. Preventing Chronic Disease 2012;9:1‐9. [DOI: 10.5888/pcd9.110186] [DOI] [PMC free article] [PubMed] [Google Scholar]
LaMontagne 2004 {published data only}
- LaMontagne A, Barbeau E, Youngstrom R, Lewiton M, Stoddard A, McLellan D, et al. Assessing and intervening on OSH programmes: effectiveness evaluation of the Wellworks‐2 intervention in 15 manufacturing worksites. Occupational and Environmental Medicine 2004;61(8):651‐60. [DOI: 10.1136/oem.2003.011718] [DOI] [PMC free article] [PubMed] [Google Scholar]
LaMontagne 2005 {published data only}
- Lamontagne AD, Stoddard AM, Youngstrom RA, Lewiton M, Sorensen G. Improving the prevention and control of hazardous substance exposures: a randomized controlled trial in manufacturing worksites. American Journal of Industrial Medicine 2005;48(4):282‐92. [DOI] [PubMed] [Google Scholar]
Lang 2017 {published data only}
- Lang J, Cluff L, Payne J, Matson‐Koffman D, Hampton J. The Centers for Disease Control and Prevention: findings from the National Healthy Worksite Program. Journal of Occupational and Environmental Medicine 2017;59(7):631‐41. [DOI: 10.1097/JOM.0000000000001045] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lapham 2003 {published data only}
- Lapham SC, Gregory C, McMillan G. Impact of an alcohol misuse intervention for health care workers ‐ 1: frequency of binge drinking and desire to reduce alcohol use. Alcohol and Alcoholism 2003;38(2):176‐82. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lawton 2015 {published data only}
- Lawton R, Mceachan R, Jackson C, West R, Conner M. Intervention fidelity and effectiveness of a UK worksite physical activity intervention funded by the BUPA Foundation, UK. Health Promotion International 2015;30(1):38‐49. [DOI: 10.1093/heapro/dau088] [DOI] [PubMed] [Google Scholar]
Lemon 2010 {published data only}
- Lemon SC, Zapka J, Li W, Estabrook B, Rosal M, Magner R, et al. Step ahead a worksite obesity prevention trial among hospital employees. American Journal of Preventive Medicine 2010;38(1):27‐38. [DOI: 10.1016/j.amepre.2009.08.028] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lemon 2014 {published data only}
- Lemon SC, Wang ML, Wedick NM, Estabrook B, Druker S, Schneider KL, et al. Weight gain prevention in the school worksite setting: results of a multi‐level cluster randomized trial. Preventive Medicine 2014;60:41‐7. [DOI: 10.1016/j.ypmed.2013.12.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leslie 2002 {published data only}
- Leslie WS, Lean ME, Baillie HM, Hankey CR. Weight management: a comparison of existing dietary approaches in a work‐site setting. International Journal of Obesity and Related Metabolic Disorders 2002;26(11):1469‐75. [DOI: 10.1038/sj.ijo.0802153] [DOI] [PubMed] [Google Scholar]
Lillehoj 2015 {published data only}
- Lillehoj CJ, Nothwehr F, Shipley K, Voss C. Vending assessment and program implementation in four Iowa worksites. Health Promotion Practice 2015;16(6):814‐25. [DOI: 10.1177/1524839915596346] [DOI] [PubMed] [Google Scholar]
Linde 2012 {published data only}
- Linde JA, Nygaard KE, MacLehose RF, Mitchell NR, Harnack LJ, Cousins JM, et al. HealthWorks: results of a multi‐component group‐randomized worksite environmental intervention trial for weight gain prevention. International Journal of Behavioral Nutrition and Physical Activity 2012;9(14):1‐12. [DOI: 10.1186/1479-5868-9-14] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindstrom 2010 {published data only}
- Lindstrom J, Absetz P, Hemio K, Peltomaki P, Peltonen M. Reducing the risk of type 2 diabetes with nutrition and physical activity ‐ efficacy and implementation of lifestyle interventions in Finland. Public Health Nutrition 2010;13(6A):993‐9. [DOI: 10.1017/S1368980010000960] [DOI] [PubMed] [Google Scholar]
Linnan 2002 {published data only}
- Linnan LA, Emmons KM, Abrams DB. Beauty and the beast: results of the Rhode Island smokefree shop initiative. American Journal of Public Health 2002;92(1):27‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowe 2010 {published data only}
- Lowe MR, Tappe KA, Butryn ML, Annunziato RA, Coletta MC, Ochner CN, et al. An intervention study targeting energy and nutrient intake in worksite cafeterias. Eating Behaviors 2010;11(3):144‐51. [DOI: 10.1016/j.eatbeh.2010.01.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mache 2015 {published data only}
- Mache S, Jensen S, Jahn R, Stuedtner M, Ochsmann E, Preub G. Worksite health program promoting changes in eating behavior and health attitudes. Health Promotion Practice 2015;16(6):826‐36. [DOI: 10.1177/1524839915596310] [DOI] [PubMed] [Google Scholar]
Mackey 2007 {published data only}
- Mackey M, Maher CG, Wong T, Collins K. Study protocol: the effects of work‐site exercise on the physical fitness and work‐ability of older workers. BMC Musculoskeletal Disorders 2007;8(9):1‐5. [DOI: 10.1186/1471-2474-8-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mackey 2011 {published data only}
- Mackey MG, Bohle P, Taylor P, Biase T, McLoughlin C, Purnell K. Walking to wellness in an ageing sedentary university community: design, method and protocol. Contemporary Clinical Trial 2011;32(2):273‐9. [DOI: 10.1016/j.cct.2010.12.001] [DOI] [PubMed] [Google Scholar]
MacKinnon 2010 {published data only}
- MacKinnon DP, Elliot DL, Thoemmes F, Kuehl KS, Moe EL, Goldberg L. Long‐term effects of a worksite health promotion program for firefighters. American Journal of Health Behavior 2010;34(6):695‐706. [DOI: 10.5993/AJHB.34.6.6] [DOI] [PubMed] [Google Scholar]
Macniven 2015 {published data only}
- Macniven R, Engelen L, Kacen MJ, Bauman A. Does a corporate worksite physical activity program reach those who are inactive? Findings from an evaluation of the Global Corporate Challenge. Health Promotion Journal of Australia 2015;26(2):142‐5. [DOI: 10.1071/HE14033] [DOI] [PubMed] [Google Scholar]
Maes 1998 {published data only}
- Maes S, Verhoeven C, Kittel F, Scholten H. Effects of a Dutch work‐site wellness‐health program: the Brabantia Project. American Journal of Public Health 1998;88(7):1037‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansi 2013 {published data only}
- Mansi S, Milosavljevic S, Tumilty S, Hendrick P, Baxter D. Use of pedometer‐driven walking to promote physical activity and improve health‐related quality of life among meat processing workers: a feasibility trial. Health and Quality of Life Outcomes 2013;11(185):1‐9. [DOI: 10.1186/1477-7525-11-185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marcus 1998 {published data only}
- Marcus BH, Emmons KM, Simkin‐Silverman LR, Linnan LA, Taylor ER, Bock BC, et al. Evaluation of motivationally tailored vs. standard self‐help physical activity interventions at the workplace. American Journal of Health Promotion 1998;12(4):246‐53. [DOI: 10.4278/0890-1171-12.4.246] [DOI] [PubMed] [Google Scholar]
Mayer 2010 {published data only}
- Mayer C, Vandecasteele H, Bodo M, Primo C, Slachmuylder JL, Kaufman L, et al. Smoking relapse prevention programs and factors that predict abstinence: a controlled study comparing the efficacy of workplace group counselling and proactive phone counselling. Journal of Smoking Cessation 2010;5(1):83‐94. [DOI: ] [Google Scholar]
McEachan 2011 {published data only}
- McEachan RC, Lawton RJ, Jackson C, Conner M, Meads DM, West RM. Testing a workplace physical activity intervention: a cluster randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2011;8(29):1‐12. [DOI: 10.1186/1479-5868-8-29] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehta 2013 {published data only}
- Mehta S, Dimsdale J, Nagle B, Holub CK, Woods C, Barquera S. Worksite interventions: improving lifestyle habits among Latin American adults. American Journal of Preventive Medicine 2013;44(5):538‐42. [DOI: 10.1016/j.amepre.2013.01.015] [DOI] [PubMed] [Google Scholar]
Michishita 2017 {published data only}
- Michishita R, Jiang Y, Ariyoshi D, Yoshida M, Moriyama H, Yamato H. The practice of active rest by workplace units improves personal relationships, mental health, and physical activity among workers. Journal of Occupational Health 2017;59(2):122‐30. [DOI: 10.1539/joh.16-0182-OA] [DOI] [PMC free article] [PubMed] [Google Scholar]
Micucci 2007 {published data only}
- Micucci S, Thomas H. The effectiveness of multi‐faceted health promotion interventions in the workplace to reduce chronic disease. Dundas, ON, Canada: City of Hamilton, Public Health and Community Services Department. Effective Public Health Practice Project; 2007.
Mitchell 2015 {published data only}
- Mitchell DC, Andrews T, Schenker MB. Pasos Saludables: a pilot randomized intervention study to reduce obesity in an immigrant farmworker population. Journal of Occupational and Environmental Medicine 2015;57(10):1039‐46. [DOI: 10.1097/JOM.0000000000000535] [DOI] [PubMed] [Google Scholar]
Morgan 2011 {published data only}
- Morgan PJ, Collins CE, Plotnikoff RC, Cook AT, Berthon B, Mitchell S, et al. Efficacy of a workplace‐based weight loss program for overweight male shift workers: the Workplace POWER (Preventing Obesity Without Eating like a Rabbit) randomized controlled trial. Preventive Medicine 2011;52(5):317‐25. [DOI: 10.1016/j.ypmed.2011.01.031] [DOI] [PubMed] [Google Scholar]
Morgan 2012 {published data only}
- Morgan PJ, Collins CE, Plotnikoff RC, Cook AT, Berthon B, Mitchell S, et al. The impact of a workplace‐based weight loss program on work‐related outcomes in overweight male shift workers. Journal of Occupational and Environmental Medicine 2012;54(2):122‐7. [DOI: 10.1097/JOM.0b013e31824329ab] [DOI] [PubMed] [Google Scholar]
Morton 2011 {published data only}
- Morton D, McElhone S, White H. The impact of weight loss competition in the workplace. Journal of Human Nutrition and Dietetics 2011;24(3):295‐6. [DOI: 10.1111/j.1365-277X.2011.01175_24.x] [DOI] [Google Scholar]
Moy 2006 {published data only}
- Moy F, Sallam AA, Wong M. The results of a worksite health promotion programme in Kuala Lumpur, Malaysia. Health Promotion International 2006;21(4):301‐10. [DOI: 10.1093/heapro/dal031] [DOI] [PubMed] [Google Scholar]
Mujika 2014 {published data only}
- Mujika A, Forbes A, Canga N, Irala J, Serrano I, Gasco P. Motivational interviewing as a smoking cessation strategy with nurses: an exploratory randomised controlled trial. International Journal of Nursing Studies 2014;51(8):1074‐82. [DOI: 10.1016/j.ijnurstu.2013.12.001] [DOI] [PubMed] [Google Scholar]
Murray 2012 {published data only}
- Murray E, Khadjesari Z, Linke S, Hunter R, Freemantle N. Health on the web: randomised trial of work‐based online screening and brief intervention for hazardous and harmful drinking. BMC Public Health 2013;13(505):1‐8. [DOI: 10.1186/1471-2458-13-505] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muto 1998 {published data only}
- Muto T, Nakamura M, Oshima A. Evaluation of a smoking cessation program implemented in the workplace. Industrial Health 1998;36(4):369‐71. [DOI: 10.2486/indhealth.36.369] [DOI] [PubMed] [Google Scholar]
Naito 2008 {published data only}
- Naito M, Nakayama T, Okamura T, Miura K, Yanagita M, Fujieda Y, et al. Effect of a 4‐year workplace‐based physical activity intervention program on the blood lipid profiles of participating employees: the high‐risk and population strategy for occupational health promotion (HIPOP‐OHP) study. Atherosclerosis 2008;197(2):784‐90. [DOI: 10.1016/j.atherosclerosis.2007.07.026] [DOI] [PubMed] [Google Scholar]
Neil‐Sztramko 2017 {published data only}
- Neil‐Sztramko SE, Gotay CC, Sabiston CM, Demers PA, Campbell KC. Feasibility of a telephone and web‐based physical activity intervention for women shift workers. Translational Behavioral Medicine 2017;7(2):268‐76. [DOI: 10.1007/s13142-017-0471-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neuhaus 2014 {published data only}
- Neuhaus M, Healy GN, Fjeldsoe BS, Lawler S, Owen N, Dunstan DW, et al. Iterative development of Stand Up Australia: a multi‐component intervention to reduce workplace sitting. International Journal of Behavioral Nutrition and Physical Activity 2014;11(21):1‐11. [DOI: 10.1186/1479-5868-11-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neuhaus 2014b {published data only}
- Neuhaus M, Healy GN, Dunstan DW, Owen N, Eakin EG. Workplace sitting and height‐adjustable workstations: a randomized controlled trial. American Journal of Preventive Medicine 2014;46(1):30‐40. [DOI: 10.1016/j.amepre.2013.09.009] [DOI] [PubMed] [Google Scholar]
Neyens 2017 {published data only}
- Neyens DM, Childers AK. Determining barriers and facilitators associated with willingness to use a personal health information management system to support worksite wellness programs. American Journal of Health Promotion 2017;31(4):310‐17. [DOI: 10.4278/ajhp.140514-QUAN-204] [DOI] [PubMed] [Google Scholar]
Nielsen 2006 {published data only}
- Nielsen K, Fredslund H, Christensen KB, Albertsen K. Success or failure? Interpreting and understanding the impact of interventions in four similar worksites. Work & Stress 2006;20(3):272‐87. [DOI: 10.1080/02678370601022688] [DOI] [Google Scholar]
Norman 2016 {published data only}
- Norman GJ, Heltemes KJ, Heck D, Osmick MJ. Employee use of a wireless physical activity tracker within two incentive designs at one company. Population Health Management 2016;19(2):88‐94. [DOI: 10.1089/pop.2015.0030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nyrop 2011 {published data only}
- Nyrop KA, Charnock BL, Martin KR, Lias J, Altpeter M, Callahan LF. Effect of a six‐week walking program on work place activity limitations among adults with arthritis. Arthritis Care & Research 2011;63(12):1773‐6. [DOI: 10.1002/acr.20604] [DOI] [PubMed] [Google Scholar]
Okazaki 2014 {published data only}
- Okazaki H, Dohi S, Ide H, Murata A, Muramatsu G, Ito D, et al. Impact of visceral fat measurements and a weight loss support web system on visceral fat loss in a workplace setting: insights from a JVALUE2 (Japanese study of visceral adiposity and lifestyle information; utilization and evaluation). Sangyo Eiseigaku Zasshi 2014;56(5):109‐115. [DOI: 10.1539/sangyoeisei.B13003] [DOI] [PubMed] [Google Scholar]
Okechukwu 2009 {published data only}
- Okechukwu CA, Krieger N, Sorensen G, Li Y, Barbeau EM. MassBuilt: effectiveness of an apprenticeship site‐based smoking cessation intervention for unionized building trades workers. Cancer Causes & Control 2009;20(6):887‐94. [DOI: 10.1007/s10552-009-9324-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olson 2014 {published data only}
- Olson R, Elliot D, Hess J, Thompson S, Luther K, Wipfli B. The COMmunity of Practice And Safety Support (COMPASS) Total Worker Health™ study among home care workers: study protocol for a randomized controlled trial. Trials 2014;15(411):1‐13. [DOI: 10.1186/1745-6215-15-411] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olson 2016 {published data only}
- Olson R, Thompson SV, Elliot DL, Hess JA, Rhoten KL, Parker KN, et al. Safety and health support for home care workers: the COMPASS randomized controlled trial. American Journal of Public Health 2016;106(10):1823‐32. [DOI: 10.2105/AJPH.2016.303327] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostbye 2015 {published data only}
- Ostbye T, Stroo M, Brouwer RJ, Peterson BL, Eisenstein EL, Fuemmeler BF, et al. Steps to Health employee weight management randomized control trial: short‐term follow‐up results. Journal of Occupational and Environmental Medicine 2015;57(2):188‐95. [DOI: 10.1097/JOM.0000000000000335] [DOI] [PubMed] [Google Scholar]
Osteras 2006 {published data only}
- Osteras H, Hammer S. The effectiveness of a pragmatic worksite physical activity program on maximal oxygen consumption and the physical activity level in healthy people. Journal of Bodywork and Movement Therapies 2006;10(1):51‐7. [DOI: 10.1016/j.jbmt.2005.02.003] [DOI] [Google Scholar]
Parry 2013 {published data only}
- Parry S, Straker L, Gilson ND, Smith AJ. Participatory workplace interventions can reduce sedentary time for office workers—a randomised controlled trial. PLOS ONE 2013;8(11):e78957. [DOI: 10.1371/journal.pone.0078957] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patterson 1997 {published data only}
- Patterson RE, Kristal AR, Glanz K, McLerran DF, Hebert JR, Heimendinger J, et al. Components of the working well trial intervention associated with adoption of healthful diets. American Journal of Preventive Medicine 1997;13(4):271‐6. [PubMed] [Google Scholar]
Patterson 1998 {published data only}
- Patterson RE, Kristal AR, Biener L, Varnes J, Feng Z, Glanz K, et al. Durability and diffusion of the nutrition intervention in the Working Well Trial. Preventive Medicine 1998;27(5):668‐73. [DOI: 10.1006/pmed.1998.0342] [DOI] [PubMed] [Google Scholar]
Patterson 2016 {published data only}
- Patterson DP, Smith KJ, Hostler D. Cost‐effectiveness of workplace wellness to prevent cardiovascular events among U.S. firefighters. BMC Cardiovascular Disorders 2016;16(229):1‐7. [DOI: 10.1186/s12872-016-0414-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 2013 {published data only}
- Paul CJ, McLennan J, Baxendale A, Schnelle B, Rawson J, Turon HE. Implementation of a personalized workplace smoking cessation programme. Occupational Medicine 2013;63(8):568‐74. [DOI: 10.1093/occmed/kqt121] [DOI] [PubMed] [Google Scholar]
Pedersen 2009 {published data only}
- Pedersen MT, Blangsted AK, Andersen LL, Jorgensen MB, Hansen EA, Sjogaard G. The effect of worksite physical activity intervention on physical capacity, health, and productivity: a 1‐year randomized controlled trial. Journal of Occupational and Environmental Medicine 2009;51(7):759‐70. [DOI: 10.1097/JOM.0b013e3181a8663a] [DOI] [PubMed] [Google Scholar]
Pedersen 2014 {published data only}
- Pedersen SJ, Cooley PD, Mainsbridge C. An e‐health intervention designed to increase workday energy expenditure by reducing prolonged occupational sitting habits. Work 2014;49(2):289‐95. [DOI: 10.3233/WOR-131644] [DOI] [PubMed] [Google Scholar]
Pescatello 2001 {published data only}
- Pescatello LS, Murphy D, Vollono J, Lynch E, Bernene J, Costanzo D. The cardiovascular health impact of an incentive worksite health promotion program. American Journal of Health Promotion 2001;16(1):16‐20. [DOI: 10.4278/0890-1171-16.1.16] [DOI] [PubMed] [Google Scholar]
Pescud 2016 {published data only}
- Pescud M, Waterworth P, Shilton T, Teal R, Slevin T, Ledger M, et al. A healthier workplace? Implementation of fruit boxes in the workplace. Health Education Journal 2016;75(7):843‐54. [DOI: 10.1177/0017896916629817] [DOI] [Google Scholar]
Petersen 2008 {published data only}
- Petersen R, Sill S, Lu C, Young J, Edington D. Effectiveness of employee internet‐based weight management program. Journal of Occupational and Environmental Medicine 2008;50(2):163‐71. [DOI: 10.1097/JOM.0b013e31815c6cf6] [DOI] [PubMed] [Google Scholar]
Pidd 2015 {published data only}
- Pidd K, Roche A, Fischer J. A recipe for good mental health: a pilot randomised controlled trial of a psychological wellbeing and substance use intervention targeting young chefs. Drugs: Education, Prevention and Policy 2015;22(4):353‐61. [DOI: 10.3109/09687637.2015.1016400] [DOI] [Google Scholar]
Plotnikoff 2005 {published data only}
- Plotnikoff RC, McCargar LJ, Wilson PM, Loucaides CA. Efficacy of an e‐mail intervention for the promotion of physical activity and nutrition behavior in the workplace context. American Journal of Health Promotion 2005;19(6):422‐9. [DOI: 10.4278/0890-1171-19.6.422] [DOI] [PubMed] [Google Scholar]
Pressler 2010 {published data only}
- Pressler A, Knebel U, Esch S, Kölbl D, Esefeld K, Scherr J, et al. An internet‐delivered exercise intervention for workplace health promotion in overweight sedentary employees: a randomized trial. Preventive Medicine 2010;51(3):234‐9. [DOI: 10.1016/j.ypmed.2010.07.008] [DOI] [PubMed] [Google Scholar]
Prestwich 2012 {published data only}
- Prestwich A, Conner MT, Lawton RJ, Ward JK, Ayres K, McEachan RR. Randomized controlled trial of collaborative implementation intentions targeting working adults' physical activity. Health Psychology 2012;31(4):486‐95. [DOI: 10.1037/a0027672] [DOI] [PubMed] [Google Scholar]
Procter 2014 {published data only}
- Procter S, Mutrie N, Davis A, Audrey S. Views and experiences of behaviour change techniques to encourage walking to work: a qualitative study. BMC Public Health 2014;14(868):1‐13. [DOI: 10.1186/1471-2458-14-868] [DOI] [PMC free article] [PubMed] [Google Scholar]
Proper 2003 {published data only}
- Proper KI, Hildebrandt VH, Beek AJ, Twisk JW, Mechelen W. Effect of individual counseling on physical activity fitness and health: a randomized controlled trial in a workplace setting. American Journal of Preventive Medicine 2003;24(3):218‐26. [DOI] [PubMed] [Google Scholar]
Puig‐Ribera 2008 {published data only}
- Puig‐Ribera A, McKenna J, Gilson N, Brown WJ. Change in work day step counts, wellbeing and job performance in Catalan university employees: a randomised controlled trial. Promotion & Education 2008;15(4):11‐6. [DOI: 10.1177/1025382308097693] [DOI] [PubMed] [Google Scholar]
Purath 2004 {published data only}
- Purath J, Miller AM, McCabe G, Wilbur J. A brief intervention to increase physical activity in sedentary working women. Canadian Journal of Nursing Research 2004;36(1):76‐91. [PubMed] [Google Scholar]
Reynolds 1997 {published data only}
- Reynolds KD, Gillum JL, Hyman DJ, Byers T, Moore SA, Paradis G. Comparing two strategies to modify dietary behavior and serum cholesterol. European Journal of Preventive Cardiology 1997;4(1):1‐5. [DOI: 10.1177/174182679700400101] [DOI] [PubMed] [Google Scholar]
Reynolds 2015 {published data only}
- Reynolds GS, Bennett J. A cluster randomized trial of alcohol prevention in small businesses: a cascade model of help seeking and risk reduction. American Journal of Health Promotion 2015;29(3):182‐91. [DOI: 10.4278/ajhp.121212-QUAN-600] [DOI] [PubMed] [Google Scholar]
Richmond 1999 {published data only}
- Richmond RL, Kehoe L, Hailstone S, Wodak A, Uebel‐Yan M. Quantitative and qualitative evaluations of brief interventions to change excessive drinking, smoking and stress in the police force. Addiction 1999;94(10):1509‐21. [DOI: 10.1046/j.1360-0443.1999.941015097.x] [DOI] [PubMed] [Google Scholar]
Richmond 2000 {published data only}
- Richmond R, Kehoe L, Heather N, Wodak A. Evaluation of a workplace brief intervention for excessive alcohol consumption: the workscreen project. Preventive Medicine 2000;30(1):51‐63. [DOI: 10.1006/pmed.1999.0587] [DOI] [PubMed] [Google Scholar]
Riley 2017 {published data only}
- Riley KE, Park CL, Wilson A, Sabo AN, Antoni AM, Braun TD, et al. Improving physical and mental health in frontline mental health care providers: yoga‐based stress management versus cognitive behavioral stress management. Journal of Workplace Behavioral Health 2017;32(1):26‐48. [DOI: 10.1080/15555240.2016.1261254] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robison 1992 {published data only}
- Robison JI, Rogers MA, Carlson JJ, Mavis BE, Stachnik T, Stoffelmayr B, et al. Effects of a 6‐month incentive‐based exercise program on adherence and work capacity. Medicine & Science in Sports & Exercise 1992;24(1):85‐93. [PubMed] [Google Scholar]
Robroek 2012 {published data only}
- Robroek SJ, Lindeboom DE, Burdorf A. Initial and sustained participation in an internet‐delivered long‐term worksite health promotion program on physical activity and nutrition. Journal of Medical Internet Research 2012;14(2):e43. [DOI: 10.2196/jmir.1788] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robroek 2012b {published data only}
- Robroek SJ, Polinder S, Bredt FJ, Burdorf A. Cost‐effectiveness of a long‐term Internet‐delivered worksite health promotion programme on physical activity and nutrition: a cluster randomized controlled trial. Health Education Research 2012;27(3):399‐410. [DOI: 10.1093/her/cys015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodríguez‐Artalejo 2003 {published data only}
- Rodríguez‐Artalejo F, Lafuente Urdinguio P, Guallar‐Castillón P, Garteizaurrekoa Dublang P, Sáinz Martínez O, Díez Azcárate JI, et al. One year effectiveness of an individualised smoking cessation intervention at the workplace: a randomised controlled trial. Occupational and Environmental Medicine 2003;60:358‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salinardi 2013 {published data only}
- Salinardi TC, Batra P, Roberts SB, Urban LE, Robinson LM, Pittas AG, et al. Lifestyle intervention reduces body weight and improves cardiometabolic risk factors in worksites. American Journal of Clinical Nutrition 2013;97(4):667‐76. [DOI: 10.3945/ajcn.112.046995] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santos 2016 {published data only}
- Santos HG, Chiavegato LD, Valentim DP, Silva PR, Padula SR. Resistance training program for fatigue management in the workplace: exercise protocol in a cluster randomized controlled trial. BMC Public Health 2016;16(1218):1‐11. [DOI: 10.1186/s12889-016-3872-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schaller 2016 {published data only}
- Schaller A, Dejonghe L, Alayli‐Goebbels A, Biallas B, Froboese I. Promoting physical activity and health literacy: study protocol for a longitudinal, mixed methods evaluation of a cross‐provider workplace‐related intervention in Germany (the AtRisk study). BMC Public Health 2016;16(626):1‐10. [DOI: 10.1186/s12889-016-3284-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2016 {published data only}
- Schneider PL, Bassett DR, Rider BC, Saunders SS. Physical activity and motivating factors of participants in a financially incentivized worksite wellness program. International Journal of Health Promotion and Education 2016;54(6):295‐303. [DOI: 10.1080/14635240.2016.1174951] [DOI] [Google Scholar]
Schopp 2017 {published data only}
- Schopp LH, Clark MJ, Lamberson WR, Uhr DJ, Minor MA. A randomized controlled trial to evaluate outcomes of a workplace self‐management intervention and an intensive monitoring intervention. Health Education Research 2017;32(3):219‐32. [DOI] [PubMed] [Google Scholar]
Schwartz 2016 {published data only}
- Schwartz B, Kappellush JM, Schrempf A, Probst K, Haller M, Baca A. Effect of a novel two‐desk sit‐to‐stand workplace (ACTIVE OFFICE) on sitting time, performance and physiological parameters: protocol for a randomized control trial. BMC Public Health 2016;16(578):1‐10. [DOI: 10.1186/s12889-016-3271-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sertel 2016 {published data only}
- Sertel M, Ucsular FD, Ugurlu U. The effects of worksite exercises on physical capabilities of workers in an industry of a developing country: A randomized controlled study. Isokinetics and Exercise Science 2016;24(3):247‐55. [DOI: 10.3233/IES-160624] [DOI] [Google Scholar]
Sforzo 2012 {published data only}
- Sforzo GA, Kaye MP, Calleri D, Ngai N. Free choice access to multipoint wellness education and related services positively impacts employee wellness: a randomized and controlled trial. Journal of Occupational and Environmental Medicine 2012;54(4):471‐7. [DOI: 10.1097/JOM.0b013e3182479f5c] [DOI] [PubMed] [Google Scholar]
Shore 1994 {published data only}
- Shore ER. Outcomes of a primary prevention project for business and professional women. Journal of Studies on Alcohol 1994;55(6):657‐9. [DOI: 10.15288/jsa.1994.55.657] [DOI] [PubMed] [Google Scholar]
Sierra 2010 {published data only}
- Sierra MC, Bonacho EC, Garcia AG, Moraga MR, Gutierrez JC, Barrientos AC, et al. Effectiveness of a preventive intervention strategy based on structured telephone interviews in a working population with a moderate to high cardiovascular risk. Atencion Primaria 2010;42(10):498‐505. [DOI: 10.1016/j.aprim.2010.05.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 2000 {published data only}
- Simpson JM, Oldenburg B, Owen N, Harris D, Dobbins T, Salmon A, et al. The Australian National Workplace Health Project: design and baseline findings. Preventive Medicine 2000;31(3):249‐60. [DOI: 10.1006/pmed.2000.0707] [DOI] [PubMed] [Google Scholar]
Smith‐McLallen 2017 {published data only}
- Smith‐McLallen A, Heller D, Vernisi K, Gulick D, Cruz S, Snyder RL. Comparative effectiveness of two walking interventions on participation, step counts, and health. American Journal of Health Promotion 2017;3(2):119‐27. [DOI] [PubMed] [Google Scholar]
Sorensen 1990 {published data only}
- Sorensen G, Hunt MK, Morris DH, Donnelly G, Freeman L, Ratclife BJ, et al. Promoting healthy eating patterns in the worksite: the Treatwell intervention model. Health Education Research 1990;5(4):505‐15. [DOI: 10.1093/her/5.4.505] [DOI] [Google Scholar]
Sorensen 1992 {published data only}
- Sorensen G, Morris DM, Hunt MK, Hebert JR, Harris DR, Stoddard A. Work‐site nutrition intervention and employees' dietary habits: the Treatwell program. American Journal of Public Health 1992;82(6):877‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorensen 1992b {published data only}
- Sorensen G, Hsieh J, Hunt MK, Morris DH, Harris DR, Fitzgerald G. Employee advisory boards as a vehicle for organizing worksite health promotion programs. American Journal of Health Promotion 1992;6(6):443‐50. [DOI: 10.4278/0890-1171-6.6.443] [DOI] [PubMed] [Google Scholar]
Sorensen 1998 {published data only}
- Sorensen G, Stoddard A, Hunt MK, Hebert JR, Ockene JK, Avrunin JS, et al. The effects of a health promotion‐health protection intervention on behavior change: the WellWorks Study. American Journal of Public Health 1998;88(11):1685‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorensen 1998b {published data only}
- Sorensen G, Thompson B, Basen‐Engquist K, Abrams D, Kuniyuki A, DiClemente C, et al. Durability, dissemination, and institutionalization of worksite tobacco control programs: results from the Working Well trial. International Journal of Behavioral Medicine 1998;5(4):335‐51. [DOI: 10.1207/s15327558ijbm0504_7] [DOI] [PubMed] [Google Scholar]
Sorensen 1998c {published data only}
- Sorensen G, Hunt MK, Cohen N, Stoddard A, Stein E, Phillips J, et al. Worksite and family education for dietary change: the Treatwell 5‐a‐Day program. Health Education Research 1998;13(4):577‐91. [DOI] [PubMed] [Google Scholar]
Sorensen 1999 {published data only}
- Sorensen G, Stoddard A, Peterson K, Cohen N, Hunt MK, Stein E, et al. Increasing fruit and vegetable consumption through worksites and families in the Treatwell 5‐a‐day study. American Journal of Public Health 1999;89(1):54‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorensen 2002 {published data only}
- Sorensen G, Stoddard AM, LaMontagne AD, Emmons K, Hunt MK, Youngstrom R, et al. A comprehensive worksite cancer prevention intervention: behavior change results from a randomized controlled trial (United States). Cancer Causes & Control 2002;13(6):493‐502. [DOI] [PubMed] [Google Scholar]
Sorensen 2005 {published data only}
- Sorensen G, Barbeau E, Stoddard AM, Hunt MK, Kaphingst K, Wallace L. Promoting behavior change among working‐class, multiethnic workers: results of the healthy directions‐small business study. American Journal of Public Health 2005;95(8):1389‐95. [DOI: 10.2105/AJPH.2004.038745] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sorensen 2007 {published data only}
- Sorensen G, Barbeau EM, Stoddard AM, Hunt MK, Goldman R, Smith A, et al. Tools for health: the efficacy of a tailored intervention targeted for construction laborers. Cancer Causes & Control 2007;18(1):51‐9. [DOI: 10.1007/s10552-006-0076-9] [DOI] [PubMed] [Google Scholar]
Sorensen 2009 {published data only}
- Sorensen G, Quintiliani L, Pereira L, Yang M, Stoddard A. Work experiences and tobacco use: findings from the gear up for health study. Journal of Occupational and Environmental Medicine 2009;51(1):87‐94. [DOI: 10.1097/JOM.0b013e31818f69f8] [DOI] [PubMed] [Google Scholar]
Sorensen 2010 {published data only}
- Sorensen G, Stoddard A, Quintiliani L, Ebbeling C, Nagler E, Yang M, et al. Tobacco use cessation and weight management among motor freight workers: results of the Gear Up for Health study. Cancer Causes & Control 2010;21(12):2113‐22. [DOI: 10.1007/s10552-010-9630-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sotos‐Prieto 2017 {published data only}
- Sotos‐Prieto M, Cash SB, Christophi CA, Folta S, Moffatt S, Muegge C, et al. Rationale and design of feeding America's bravest: Mediterranean diet‐based intervention to change firefighters' eating habits and improve cardiovascular risk profiles. Contemporary Clinical Trials 2017;61:101‐7. [DOI: 10.1016/j.cct.2017.07.010] [DOI] [PubMed] [Google Scholar]
Steenhuis 2004 {published data only}
- Steenhuis I, Assema P, Breukelen G, Glanz K, Kok G, Vries H. The impact of educational and environmental interventions in Dutch worksite cafeterias. Health Promotion International 2004;19(3):335‐43. [DOI] [PubMed] [Google Scholar]
Stephens 2014 {published data only}
- Stephens SK, Winkler E, Trost SG, Dunstan DW, Eakin EG, Chastin S. Intervening to reduce workplace sitting time: how and when do changes to sitting time occur?. British Journal of Sports Medicine 2014;0:1‐6. [DOI: 10.1136/bjsports-2014-093524] [DOI] [PubMed] [Google Scholar]
Strijk 2011 {published data only}
- Strijk JE, Proper KI, Beek AJ, Mechelen W. A process evaluation of a worksite vitality intervention among ageing hospital workers. International Journal of Behavioral Nutrition and Physical Activity 2011;8(58):1‐9. [DOI: 10.1186/1479-5868-8-58] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strijk 2012 {published data only}
- Strijk JE, Proper KI, Beek AJ, Mechelen W. A worksite vitality intervention to improve older workers' lifestyle and vitality‐related outcomes: results of a randomised controlled trial. Journal of Epidemiology & Community Health 2012;1:1‐8. [DOI: 10.1136/jech-2011-200626] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sumner 2016 {published data only}
- Sumner W, Walker MS, Highstein GR, Fisher I, Yan Y, McQueen A, et al. A randomized controlled trial of directive and nondirective smoking cessation coaching through an employee quitline. BMC Public Health 2016;16(550):1‐14. [DOI: 10.1186/s12889-016-3202-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2013 {published data only}
- Tan AM, Lamontagne AD, Sarmugam R, Howard P. A cluster‐randomised, controlled trial to assess the impact of a workplace osteoporosis prevention intervention on the dietary and physical activity behaviours of working women: study protocol. BMC Public Health 2013;13(405):1‐12. [DOI: 10.1186/1471-2458-13-405] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanaka 2006 {published data only}
- Tanaka H, Yamato H, Tanaka T, Kadowaki T, Okamura T, Nakamura M, et al. Effectiveness of a low‐intensity intra‐worksite intervention on smoking cessation in Japanese employees: a three‐year intervention trial. Journal of Occupational Health 2006;48(3):175‐82. [DOI: 10.1539/joh.48.175] [DOI] [PubMed] [Google Scholar]
Terry 2011 {published data only}
- Terry PE, Seaverson EL, Staufacker MJ, Tanaka A. The effectiveness of a telephone‐based tobacco cessation program offered as part of a worksite health promotion program. Population Health Management 2011;14(3):117‐25. [DOI: 10.1089/pop.2010.0026] [DOI] [PubMed] [Google Scholar]
Terry 2011b {published data only}
- Terry PE, Fowles JB, Xi M, Harvey L. The ACTIVATE study: results from a group‐randomized controlled trial comparing a traditional worksite health promotion program with an activated consumer program. American Journal of Health Promotion 2011;26(2):e64‐73. [DOI: 10.4278/ajhp.091029-QUAN-348] [DOI] [PubMed] [Google Scholar]
Terry 2011c {published data only}
- Terry PE, Seaverson EL, Grossmeier J, Anderson DR. Effectiveness of a worksite telephone‐based weight management program. American Journal of Health Promotion 2011;25(3):186‐9. [DOI: 10.4278/ajhp.081112-QUAN-281] [DOI] [PubMed] [Google Scholar]
Thogersen‐Ntoumani 2010 {published data only}
- Thogersen‐Ntoumani C, Loughren EA, Duda JL, Fox KR, Kinnafick FE. "Step by Step". A feasibility study of a lunchtime walking intervention designed to increase walking, improve mental well‐being and work performance in sedentary employees: rationale and study design. BMC Public Health 2010;10(578):1‐9. [DOI: 10.1186/1471-2458-10-578] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 1995 {published data only}
- Thompson B, Shannon J, Beresford SAA. Implementation aspects of the Seattle “5 a Day” intervention project: strategies to help employees make dietary change. Topics in Clinical Nutrition 1995;11:58‐75. [Google Scholar]
Thorndike 2012 {published data only}
- Thorndike AN, Sonnenberg L, Healey E, Myint‐U K, Kvedar JC, Regan S. Prevention of weight gain following a worksite nutrition and exercise program: a randomized controlled trial. American Journal of Preventive Medicine 2012;43(1):27‐33. [DOI: 10.1016/j.amepre.2012.02.029] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tilley 1997 {published data only}
- Tilley BC, Vernon SW, Glanz K, Myers R, Sanders K, Lu M, et al. Worksite cancer screening and nutrition intervention for high‐risk auto workers: design and baseline findings of the Next Step Trial. Preventive Medicine 1997;26(2):227‐35. [DOI: 10.1006/pmed.1996.0132] [DOI] [PubMed] [Google Scholar]
Tilley 1998 {published data only}
- Tilley BC, Vernon SW, Myers R, Glanz K, Lu M, Hirst K, et al. The Next Step Trial: impact of a worksite colorectal cancer screening promotion program. Preventive Medicine 1998;28(3):276‐83. [DOI: 10.1006/pmed.1998.0427] [DOI] [PubMed] [Google Scholar]
Tilley 1999 {published data only}
- Tilley BC, Glanz K, Kristal AR, Hirst K, Li S, Vernon SW, et al. Nutrition intervention for high‐risk auto workers: results of the Next Step Trial. Preventive Medicine 1999;28(3):284‐92. [DOI] [PubMed] [Google Scholar]
Tobin 2016 {published data only}
- Tobin R, Leavy J, Jancey J. Uprising: an examination of sit‐stand workstations, mental health and work ability in sedentary office workers, in Western Australia. Work 2017;55(2):359‐71. [DOI: 10.3233/WOR-162410] [DOI] [PubMed] [Google Scholar]
Togami 2008 {published data only}
- Togami T. Interventions in local communities and work sites through Physical Activity and Nutrition Programme. Obesity Reviews 2008;9(Suppl 1):127‐9. [DOI: 10.1111/j.1467-789X.2007.00453.x] [DOI] [PubMed] [Google Scholar]
Townsend 2016 {published data only}
- Townsend CK, Miyamoto RE, Antonio M, Zhang G, Paloma D, Basques D, et al. The PILI@Work Program: a translation of the diabetes prevention program to Native Hawaiian‐serving worksites in Hawai'i. Translational Behavioral Medicine 2016;6(2):190‐201. [DOI: 10.1007/s13142-015-0383-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tucker 2016 {published data only}
- Tucker S, Farrington M, Lanningham‐Foster LM, Clark MK, Dawson C, Quinn GJ, et al. Worksite physical activity intervention for ambulatory clinic nursing staff. Workplace Health & Safety 2016;64(7):313‐25. [DOI: 10.1177/2165079916633225] [DOI] [PubMed] [Google Scholar]
van Berkel 2011 {published data only}
- Berkel J, Proper KI, Boot CR, Bongers PM, Beek AJ. Mindful "Vitality in Practice": an intervention to improve the work engagement and energy balance among workers; the development and design of the randomised controlled trial. BMC Public Health 2011;11(736):1‐12. [DOI: 10.1186/1471-2458-11-736] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Calster 2017 {published data only}
- Calster L, Hoecke AS, Octaef A, Boen F. Does a video displaying a stair climbing model increase stair use in a worksite setting?. Public Health 2017;149:11‐20. [DOI: 10.1016/j.puhe.2017.04.007] [DOI] [PubMed] [Google Scholar]
van Scheppingen 2014 {published data only}
- Scheppingen AR, Vroome EM, Have KC, Bos EH, Zwetsloot GI, Mechelen W. Inducing a health‐promoting change process within an organization: the effectiveness of a large‐scale intervention on social capital, openness, and autonomous motivation toward health. Journal of Occupational and Environmental Medicine 2014;56(11):1128‐36. [DOI: 10.1097/JOM.0000000000000299] [DOI] [PubMed] [Google Scholar]
Vermeer 2012 {published data only}
- Vermeer WM, Leeuwis FH, Koprulu S, Zouitni O, Seidell JC, Steenhuis IH. The process evaluation of two interventions aimed at portion size in worksite cafeterias. Journal of Human Nutrition and Dietetics 2012;25(2):180‐8. [DOI: 10.1111/j.1365-277X.2011.01219.x] [DOI] [PubMed] [Google Scholar]
Verweij 2009 {published data only}
- Verweij LM, Proper KI, Weel ANH, Hulshof CTJ, Mechelen W. Design of the Balance@Work project: systematic development, evaluation and implementation of an occupational health guideline aimed at the prevention of weight gain among employees. BMC Public Health 2009;9(461):1‐17. [DOI: 10.1186/1471-2458-9-461] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verweij 2012 {published data only}
- Verweij LM, Proper KI, Weel AN, Hulshof CT, Mechelen W. The application of an occupational health guideline reduces sedentary behaviour and increases fruit intake at work: results from an RCT. Occupational and Environmental Medicine 2012;69(7):500‐7. [DOI: 10.1136/oemed-2011-100377] [DOI] [PubMed] [Google Scholar]
Verweij 2013 {published data only}
- Verweij LM, Proper KI, Weel AN, Hulshof CT, Mechelen W. Long‐term effects of an occupational health guideline on employees' body weight‐related outcomes, cardiovascular disease risk factors, and quality of life: results from a randomized controlled trial. Scandinavian Journal of Work, Environment & Health 2013;39(3):284‐94. [DOI: 10.5271/sjweh.3341] [DOI] [PubMed] [Google Scholar]
Volpp 2009 {published data only}
- Volpp KG, Troxel AB, Pauly MV, Glick HA, et al. A randomized, controlled trial of financial incentives for smoking cessation. New England Journal of Medicine 2009;360:699‐709. [DOI: 10.1056/NEJMsa0806819] [DOI] [PubMed] [Google Scholar]
Vyth 2011 {published data only}
- Vyth EL, Steenhuis IH, Heymans MW, Roodenburg AJ, Brug J, Seidell JC. Influence of placement of a nutrition logo on cafeteria menu items on lunchtime food choices at Dutch work sites. Journal of the American Dietetic Association 2011;111(1):131‐6. [DOI: 10.1016/j.jada.2010.10.003] [DOI] [PubMed] [Google Scholar]
Vyth 2012 {published data only}
- Vyth EL, Meer EW, Seidell JC, Steenhuis IH. A nutrition labeling intervention in worksite cafeterias: an implementation evaluation across two large catering companies in the Netherlands. Health Promotion International 2012;27(2):230‐7. [DOI: 10.1093/heapro/dar034] [DOI] [PubMed] [Google Scholar]
Walters 2003 {published data only}
- Walters ST, Woodall WG. Mailed feedback reduces consumption among moderate drinkers who are employed. Prevention Science 2003;4(4):287‐94. [DOI] [PubMed] [Google Scholar]
Watanabe 2017 {published data only}
- Watanabe K, Kawakami N. Effects of a multicomponent workplace intervention programme with environmental changes on physical activity among Japanese white collar employees: a protocol for a cluster randomised controlled trial. BMJ Open 2017;7(e017688):1‐10. [DOI: 10.1136/bmjopen-2017-017688] [DOI] [PMC free article] [PubMed] [Google Scholar]
Webb 2013 {published data only}
- Webb M. The Effectiveness of Self‐monitoring Tools and Texting Prompts to Increase Physical Activity in the Workplace [Masters thesis]. Ames (IA): Iowa State University, 2013. [Google Scholar]
Weinhold 2015 {published data only}
- Weinhold KR, Miller CK, Marrero DG, Nagaraja HN, Focht BC, Gascon GM. A randomized controlled trial translating the diabetes prevention program to a university worksite, Ohio, 2012‐2014. Preventing Chronic Disease 2015;E210:1‐15. [DOI: 10.5888/pcd12.150301] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2007 {published data only}
- White K, Jacques PH. Combined diet and exercise intervention in the workplace: effect on cardiovascular disease risk factors. Journal of the American Association of Occupational Health Nurses 2007;55(3):109‐14. [DOI] [PubMed] [Google Scholar]
White 2016 {published data only}
- White JS. Incentives in workplace wellness programmes. Lancet Diabetes & Endocrinology 2016;4(12):967‐9. [DOI: 10.1016/S2213-8587(16)30186-3] [DOI] [PubMed] [Google Scholar]
Wierenga 2012 {published data only}
- Wierenga D, Engbers LH, Empelen P, Hildebrandt VH, Mechelen W. The design of a real‐time formative evaluation of the implementation process of lifestyle interventions at two worksites using a 7‐step strategy (BRAVO@Work). BMC Public Health 2012;12(619):1‐11. [DOI: 10.1186/1471-2458-12-619] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wierenga 2014 {published data only}
- Wierenga D, Engbers LH, Empelen P, Moes KJ, Wittink H, Grundemann R. The implementation of multiple lifestyle interventions in two organizations: a process evaluation. Journal of Occupational and Environmental Medicine 2014;56(11):1195‐1206. [DOI: 10.1097/JOM.0000000000000241] [DOI] [PMC free article] [PubMed] [Google Scholar]
Willemsen 1998 {published data only}
- Willemsen MC, Vries H, Breukelen G, Genders R. Long‐term effectiveness of two Dutch work site smoking cessation programs. Health Education & Behavior 1998;25(4):418‐35. [DOI: 10.1177/109019819802500402] [DOI] [PubMed] [Google Scholar]
Williams 2007 {published data only}
- Williams AE, Vogt TM, Stevens VJ, Albright CA, Nigg CR, Meenan RT, et al. Work, Weight, and Wellness: the 3W Program: a worksite obesity prevention and intervention trial. Obesity 2007;15(Suppl 1):S16‐S26. [DOI: 10.1038/oby.2007.384] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2014 {published data only}
- Williams AE, Stevens VJ, Albright CL, Nigg CR, Meenan RT, Vogt TM. The results of a 2‐year randomized trial of a worksite weight management intervention. American Journal of Health Promotion 2014;28(5):336‐9. [DOI: 10.4278/ajhp.100127-ARB-29] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2016 {published data only}
- Wilson MG, DeJoy DM, Vandenberg RJ, Corso P, Padilla H, Zuercher H. Effect of intensity and program delivery on the translation of diabetes prevention program to worksites: a randomized controlled trial of Fuel Your Life. Journal of Occupational and Environmental Medicine 2016;58(11):1113‐20. [DOI: 10.1097/JOM.0000000000000873] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2016b {published data only}
- Wilson MG, DeJoy DM, Vandenberg R, Padilla H, Davis M. FUEL Your Life: a translation of the diabetes prevention program to worksites. American Journal of Health Promotion 2016;30(3):188‐97. [DOI: 10.4278/ajhp.130411-QUAN-169] [DOI] [PubMed] [Google Scholar]
Yap 2009 {published data only}
- Yap TL, Davis LS, Gates DM, Hemmings AB, Pan W. The effect of tailored E‐mails in the workplace. Part I. Stage movement toward increased physical activity levels. Journal of the American Association of Occupational Health Nurses 2009;57(7):267‐73. [PubMed] [Google Scholar]
Zavanela 2012 {published data only}
- Zavanela PM, Crewther BT, Lodo L, Florindo AA, Miyabara EH, Aoki MS. Health and fitness benefits of a resistance training intervention performed in the workplace. Journal of Strength and Conditioning Research 2012;26(3):811‐7. [DOI: 10.1519/JSC.0b013e318225ff4d] [DOI] [PubMed] [Google Scholar]
Zinn 2012 {published data only}
- Zinn C, Schofield GM, Hopkins WG. A "small‐changes" workplace weight loss and maintenance program: examination of weight and health outcomes. Journal of Occupational and Environmental Medicine 2012;54(10):1230‐8. [DOI: 10.1097/JOM.0b013e3182480591] [DOI] [PubMed] [Google Scholar]
Zinn 2012b {published data only}
- Zinn C, Schofield GM, Hopkins WG. Efficacy of a "small‐changes" workplace weight loss initiative on weight and productivity outcomes. Journal of Occupational and Environmental Medicine 2012;54(10):1224‐9. [DOI: 10.1097/JOM.0b013e3182440ac2] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Hannon 2016 {published data only}
- Hannon PA, Hammerback K, Allen CL, Parrish AT, Chan GK, Kohn MJ, et al. HealthLinks randomized controlled trial: design and baseline results. Contemporary Clinical Trials 2016;48:1‐11. [DOI: 10.1016/j.cct.2016.02.011] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02381938 {published data only}
- Linnan L, Arandia G, Bateman LA, Vaughn A, Smith N, Ward D. The health and working conditions of women employed in child care. International Journal of Environmental Research and Public Health 2017;14(283):1‐14. [DOI: 10.3390/ijerph14030283] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02381938. Care2BWell: Worksite Wellness for Child Care [Care2bWell: a worksite physical activity & wellness program for child care staff]. clinicaltrials.gov/ct2/show/study/NCT02381938 (first received 6 March 2015).
NCT02899442 {published data only}
- NCT02899442. Cardiovascular risk prevention among night workers (Heart‐Of‐Night). clinicaltrials.gov/ct2/show/study/NCT02899442 (first received 14 September 2016).
Vasiljevic 2017 {published data only}
- Vasiljevic M, Cartwright E, Pechey R, Hollands GJ, Couturier DL, Jebb SA, et al. Physical micro‐environment interventions for healthier eating in the workplace: protocol for a stepped wedge randomised controlled pilot trial. Pilot and Feasability Studies 2017;3(27):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Velema 2017 {published data only}
- Velema E, Vyth EL, Steenhuis IHM. Using nudging and social marketing techniques to create healthy worksite cafeterias in the Netherlands: intervention development and study design. BMC Public Health 2017;17(63):1‐9. [DOI: 10.1186/s12889-016-3927-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alaimo 2015
- Alaimo K, Oleksyk S, Golzynski D, Drzal N, Lucarelli J, Reznar M, et al. The Michigan healthy school action tools process generates improvements in school nutrition policies and practices, and student dietary intake. Health Promotion Practice 2015;16(3):401‐10. [DOI] [PubMed] [Google Scholar]
Ames 2011
- Ames GM, Bennett JB. Prevention interventions of alcohol problems in the workplace: a review and guiding framework. Alcohol Research and Health 2011;34(2):175. [PMC free article] [PubMed] [Google Scholar]
Anderson 2009
- Anderson LM, Quinn TA, Glanz K, Ramirez G, Kahwati LC, Johnson DB, et al. The effectiveness of worksite nutrition and physical activity interventions for controlling employee overweight and obesity: a systematic review. American Journal of Preventive Medicine 2009;37(4):340‐57. [DOI] [PubMed] [Google Scholar]
Atkins 2017
- Atkins L, Francis J, Islam R, O'Connor D, Patey A, Ivers N, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implementation Science 2017;12(77):1‐18. [DOI: 10.1186/s13012-017-0605-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barr‐Anderson 2011
- Barr‐Anderson DJ, AuYoung M, Whitt‐Glover MC, Glenn BA, Yancey AK. Integration of short bouts of physical activity into organizational routine: a systematic review of the literature. American Journal of Preventive Medicine 2011;40(1):76‐93. [DOI] [PubMed] [Google Scholar]
Benedict 2008
- Benedict MA, Arterburn D. Worksite‐based weight loss programs: a systematic review of recent literature. American Journal of Health Promotion 2008;22(6):408‐16. [DOI] [PubMed] [Google Scholar]
Benjamin 2007
- Benjamin SE, Ammerman A, Sommers J, Dodds J, Neelon B, Ward DS. Nutrition and physical activity self‐ assessment for child care (NAP SACC): results from a pilot intervention. Journal of Nutrition Education and Behavior 2007;39(3):142‐9. [DOI] [PubMed] [Google Scholar]
Bero 1998
- Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Getting research findings into practice: closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. BMJ 1998;317(7156):465‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cahill 2014
- Cahill K, Lancaster T. Workplace interventions for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD003440.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cane 2012
- Cane J, O'Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implementation Science 2012;7(37):1‐17. [DOI: 10.1186/1748-5908-7-37] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherniack 2010
- Cherniack M, Lahiri S. Barriers to implementation of workplace health interventions: an economic perspective. Journal of Occupational and Environmental Medicine 2010;52(9):934‐42. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Routledge Academic, 1988. [Google Scholar]
CPHG 2011
- Cochrane Public Health Group (CPHG). Guide for developing a Cochrane Protocol. ph.cochrane.org/sites/ph.cochrane.org/files/uploads/Guide%20for%20PH%20protocol_Nov%202011_final%20for%20website.pdf (accessed 20 August 2016).
Curran 2012
- Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness‐implementation Hybrid Designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Medical Care 2012;50(3):217‐26. [DOI: 10.1097/MLR.0b013e3182408812] [DOI] [PMC free article] [PubMed] [Google Scholar]
Damschroder 2009
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation Science 2009;4(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dobbins 2013
- Dobbins M, Husson H, DeCorby K, LaRocca RL. School‐based physical activity programs for promoting physical activity and fitness in children and adolescents aged 6 to 18. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD007651] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). EPOC taxonomy. epoc.cochrane.org/epoc‐taxonomy (accessed prior to 20 August 2016).
Fichtenberg 2002
- Fichtenberg CM, Glantz SA. Effect of smoke‐free workplaces on smoking behaviour: systematic review. BMJ 2002;325(7357):188‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finch 2012
- Finch M, Wolfenden L, Wiggers J, Edenden D, Falkiner M, Pond N, et al. Impact of a population based implementation intervention to increase the adoption of multiple physical activity promoting practices in centre based childcare services: a quasi experimental effectiveness study. International Journal of Behavioural Nutrition and Physical Activity 2012;9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fishwick 2013
- Fishwick D, Carroll C, McGregor M, Drury M, Webster J, Bradshaw L, et al. Smoking cessation in the workplace. Occupational Medicine 2013;63(8):526‐36. [DOI] [PubMed] [Google Scholar]
Forsetlund 2009
- Forsetlund L, Bjorndal A, Rashidian A, Jamtvedt G, O'Brien MA, Wolf FM, et al. Continuing education meetings and workshops: effects in professional practice and health care outcomes (Review). Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003030.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foxcroft 2011
- Foxcroft D, Ireland D, Lowe G, Breen R. Primary prevention for alcohol misuse in young people. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD003024.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freak‐Poli 2013
- Freak‐Poli RLA, Cumpston M, Peeters A, Clemes SA. Workplace pedometer interventions for increasing physical activity. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD009209.pub2] [DOI] [PubMed] [Google Scholar]
Gakidou 2017
- Gakidou E, Afshin A, Abajobir AA, Abate KH, Abbafati C, Abbas KM, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1345‐1422. [DOI: 10.1016/S0140-6736(17)32366-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geaney 2013
- Geaney F, Kelly C, Greiner BA, Harrington JM, Perry IJ, Beirne P. The effectiveness of workplace dietary modification interventions: a systematic review. Preventive Medicine 2013;57(5):438‐47. [DOI] [PubMed] [Google Scholar]
Glasgow 1999
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE‐AIM framework. American Journal of Public Health 1999;89(7):1274‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasgow 2012
- Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National Institutes of Health approaches to dissemination and implementation science: current and future directions. American Journal of Public Health 2012;102(7):1274‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guerra 2014
- Guerra PH, Nobre MRC, Silveira JAC, Taddei JAAC. School‐based physical activity and nutritional education interventions on body mass index: a meta‐analysis of randomised community trials ‐ Project PANE. Preventive Medicine 2014;61:81‐9. [DOI] [PubMed] [Google Scholar]
Haidong 2016
- Haidong W, et.al. Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet 2016;388(10053):1459‐1544. [DOI: 10.1016/S0140-6736(16)31012-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallal 2012
- Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 2012;380:257. [10.1016/ S0140‐6736(12)60646‐1] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Ivers 2012
- Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard‐Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD000259.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaime 2009
- Jaime PC, Lock K. Do school based food and nutrition policies improve diet and reduce obesity?. Preventive Medicine 2009;48(1):45‐53. [DOI] [PubMed] [Google Scholar]
Jones 2015b
- Jones J, Wyse R, Finch M, Lecathelinais C, Wiggers J, Marshall J, et al. Effectiveness of an intervention to facilitate the implementation of healthy eating and physical activity policies and practices in childcare services: a randomised controlled trial. Implementation Science 2015;10:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahn‐Marshall 2012
- Kahn‐Marshall JL, Gallant MP. Making healthy behaviors the easy choice for employees: a review of the literature on environmental and policy changes in worksite health promotion. Health Education & Behavior 2012;39(6):752‐76. [DOI] [PubMed] [Google Scholar]
Kingsland 2015
- Kingsland M, Wolfenden L, Tindall J, Rowland B, Gillham K, Sidey M, et al. Improving the implementation of responsible alcohol management practices by community sporting clubs: a randomised controlled trial. Drug and Alcohol Review 2015;34(4):447‐57. [DOI] [PubMed] [Google Scholar]
Kolar 2015
- Kolar C, Treuer K. Alcohol misuse interventions in the workplace: a systematic review of workplace and sports management alcohol interventions. International Journal of Mental Health and Addiction 2015;13(5):563‐83. [Google Scholar]
Lau 2015
- Lau R, Stevenson F, Ong BN, Dziedzic K, Treweek S, Elridge S, et al. Achieving change in primary care ‐ effectiveness of strategies for improving implementation of complex interventions: systematic review of reviews. BMJ Open 2015;5(12):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2014
- Lee NK, Roche AM, Duraisingam V, Fischer J, Cameron J, Pidd K. A systematic review of alcohol interventions among workers in male‐dominated industries. Journal of Men's Health 2014;11(2):53‐63. [Google Scholar]
Lee 2018
- Lee H, Hall A, Nathan N, Reilly KL, Seward K, Williams CM, et al. Mechanisms of implementing public health interventions: a pooled causal mediation analysis of randomised trials. Implementation Science 2018;13(42):1‐11. [DOI: 10.1186/s13012-018-0734-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2018
- Lewis C, Klasnja P, Powell B, Lyon A, Tuzzio L, Jones S, et al. From classification to causality: advancing understanding of mechanisms of change in implementation science. Frontiers in Public Health 2018;6:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2012
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2224‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maes 2012
- Maes L, Cauwenberghe E, Lippevelde W, Spittaels H, Pauw E, Oppert JM, et al. Effectiveness of workplace interventions in Europe promoting healthy eating: a systematic review. European Journal of Public Health 2012;22(5):677‐83. [DOI] [PubMed] [Google Scholar]
Malik 2014
- Malik SH, Blake H, Suggs LS. A systematic review of workplace health promotion interventions for increasing physical activity. British Journal of Health Psychology 2014;19(1):149‐80. [DOI] [PubMed] [Google Scholar]
Mazza 2013
- Mazza D, Bairstow P, Buchan H, Paubrey Chakraborty S, Hecke O, Greech C, et al. Refining a taxonomy for guideline implementation: results of an exercise in abstract classification. Implementation Science 2013;8(32):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mhurchu 2010
- Mhurchu CN, Aston LM, Jebb SA. Effects of worksite health promotion interventions on employee diets: a systematic review. BMC Public Health 2010;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 2008
- Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Applied Psychology 2008;57(4):660‐80. [Google Scholar]
Michie 2011
- Michie S, Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation Science 2011;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nathan 2012
- Nathan N, Wolfenden L, Bell AC, Wyse R, Morgan PJ, Butler M, et al. Effectiveness of a multi‐strategy intervention in increasing the implementation of vegetable and fruit breaks by Australian primary schools: a non‐randomised controlled trial. BMC Public Health 2012;12:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naylor 2006
- Naylor PJ, Macdonald HM, Zebedee JA, Reed KE, McKay HA. Lessons learned from Action Schools! BC: An 'active school' model to promote physical activity in elementary schools. Journal of Science and Medicine in Sport 2006;9(5):413‐23. [DOI] [PubMed] [Google Scholar]
NICE 2006
- National Institute for Health and Clinical Excellence (NICE). Obesity: the prevention, identification, assessment and management of overweight and obesity in adults and children. NICE Clinical Guidelines, No. 43. [PubMed]
NICE 2007
- National Institute for Health and Care Excellence (NICE). Smoking: workplace interventions. NICE Public Health Guideline (PH5).
NICE 2008
- National Institute for Health and Clinical Excellence (NICE). Physical activity and the environment. NICE Public Health Guideline (PH8).
NICE 2008b
- National Institute for Health and Care Excellence (NICE). Physical activity in the workplace. NICE Public Health Guideline (PH13).
NICE 2009
- National Institute for Health and Care Excellence (NICE). Workplace health: long‐term sickness absence and incapacity to work. NICE Public Health Guideline [PH19].
NICE 2009b
- National Institute for Health and Clinical Excellence (NICE). Mental wellbeing at work. NICE Public health guideline (PH22). [PubMed]
O'Brien 2007
- O'Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard‐Jensen J, Tove Kristoffersen D, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000409.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
OECD 2015
- Organisation for Economic Co‐operation and Development (OECD). OECD employment: hours worked. data.oecd.org/emp/hours‐worked.htm (accessed prior to 20 August 2016).
OECD 2017
- Organisation for Economic Co‐operation and Development (OECD). Health at a glance 2017: OECD indicators. Paris (France): OECD Publishing; 2017. Available from dx.doi.org/10.1787/health_glance‐2017‐en.
Pelletier 2011
- Pelletier KR. A review and analysis of the clinical and cost‐effectiveness studies of comprehensive health promotion and disease management programs at the worksite update VIII 2008 to 2010. Journal of Occupational and Environmental Medicine 2011;53(11):1310‐31. [DOI] [PubMed] [Google Scholar]
Perry 2004
- Perry CL, Bishop DB, Taylor GL, Davis M, Story M, Gray C, et al. A randomized school trial of environmental strategies to encourage fruit and vegetable consumption among children. Health Education & Behavior 2004;34(1):65‐76. [DOI] [PubMed] [Google Scholar]
Pinnock 2017
- Pinnock H, Barwick M, Carpenter CR, Eldridge S, Grandes G, Griffiths CJ, et al. Standards for Reporting Implementation Studies (StaRI) Statement. BMJ 2017;356(i6795):1‐9. [DOI: 10.1136/bmj.i6795] [DOI] [PMC free article] [PubMed] [Google Scholar]
Proctor 2009
- Proctor EN, Landsverk J, Aarons G, Chambers D, Glisson C, Mittman B. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Administration and Policy in Mental Health and Mental Health Services 2009;36(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabin 2008
- Rabin BA, Brownson RC, Haire‐Joshu D, Kreuter MW, Weaver NL. A glossary for dissemination and implementation research in health. Journal of Public Health Management and Practice 2008;14(2):117‐23. [DOI] [PubMed] [Google Scholar]
Rabin 2010
- Rabin BA, Glasgow RE, Kerner JF, Klump MP, Brownson RC. Dissemination and implementation research on community‐based cancer prevention: a systematic review. American Journal of Preventive Medicine 2010;38(4):443‐56. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rongen 2013
- Rongen A, Robroek SJW, Lenthe FJ, Burdorf A. Workplace health promotion: a meta‐analysis of effectiveness. American Journal of Preventive Medicine 2013;44(4):406‐15. [DOI] [PubMed] [Google Scholar]
Sallis 1997
- Sallis JF, McKenzie TL, Alcaraz JE, Kolody B, Faucette N, Hovell MF. The effects of a 2‐year physical education program (SPARK) on physical activity and fitness in elementary school students. Sports, Play and Active Recreation for Kids. American Journal of Public Health 1997;87(8):1328‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saunders 2006
- Saunders RP, Ward D, Felton GM, Dowda M, Pate RR. Examining the link between program implementation and behavior outcomes in the lifestyle education for activity program (LEAP). Evaluation and Program Planning 2006;29(4):352‐64. [DOI] [PubMed] [Google Scholar]
Schillinger 2010
- Schillinger D. An introduction to effectiveness, dissemination and implementation research. In: Fleisher P, Goldstein E editor(s). UCSF Clinical and Translational Science Institute (CTSI) resource manuals and guides to community‐engaged research. San Francisco: Clinical Translational Science Institute Community Engagement Program, University of California, 2010. [Google Scholar]
Scollo 2015
- Scollo MM, Winstanley MH. Tobacco in Australia: facts and issues. www.tobaccoinaustralia.org.au. Melbourne: Cancer Council Victoria, (accessed prior to 20 August 2016).
Sorensen 2011
- Sorensen G, Landsbergis P, Hammer L, Amick III B, Linnan L, Yancey A. Preventing chronic disease at the workplace: a workshop. American Journal of Public Health 2011;101(Suppl 1):S196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Story 2000
- Story M, Mays RW, Bishop DB, Perry CL, Taylor G, Smyth M, et al. 5‐a‐day Power Plus: process evaluation of a multicomponent elementary school program to increase fruit and vegetable consumption. Health Education & Behavior 2000;27(2):187‐200. [DOI] [PubMed] [Google Scholar]
Sutherland 2017
- Sutherland RL, Wolfenden L, Lubans DR, Cohen K, Davies LJ, Desmet C. A randomized trial of an intervention to facilitate the implementation of school‐based practices known to increase students' moderate‐to‐vigorous physical activity. American Journal of Preventive Medicine 2017;53(6):818‐28. [DOI] [PubMed] [Google Scholar]
Thomas 2013
- Thomas RE, McLellan J, Perera R. School‐based programmes for preventing smoking. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD001293.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
To 2013
- To QG, Chen TTL, Magnussen CG, To KG. Workplace physical activity interventions: a systematic review. American Journal of Health Promotion 2013;27(6):113‐23. [DOI] [PubMed] [Google Scholar]
Vuillemin 2011
- Vuillemin A, Rostami C, Maes L, Cauwenberghe E, Lenthe FJ, Brug J, et al. Worksite physical activity interventions and obesity: a review of European studies (the HOPE Project). Obesity Facts 2011;4(6):479‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2008
- Ward DS, Benjamin SE, Ammerman AS, Ball SC, Neelon BH, Bangdiwala SI. Nutrition and physical activity in child care. Results from an environmental intervention. American Journal of Preventive Medicine 2008;35(4):352‐6. [DOI] [PubMed] [Google Scholar]
Waters 2011
- Waters E, Silva‐Sanigorski A, Hall BJ, Brown T, Campbell KJ, Gao Y, et al. Interventions for preventing obesity in children. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD001871.pub3] [DOI] [PubMed] [Google Scholar]
WHO 1981
- World Health Organization (WHO). Global Strategy for Health for All By 2000. Geneva: WHO Press, 1981. [Google Scholar]
WHO 2014
- World Health Organization (WHO). Global Status Report on Noncommunicable Diseases 2014. Geneva: WHO Press, 2014. [Google Scholar]
WHO 2016
- World Health Organisation (WHO). Global Health Observatory data repository: prevalence of obesity among adults, BMI ≥ 30, age‐standardized. www.who.int/gho/data/view.main.REGION2480A?lang=en (accessed 19 March 2018).
Wolfenden 2016
- Wolfenden L, Jones J, Williams CM, Finch M, Wyse RJ, Kingsland M, et al. Strategies to improve the implementation of healthy eating, physical activity and obesity prevention policies, practices or programmes within childcare services. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD011779.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfenden 2016a
- Wolfenden L, Williams CM, Wiggers J, Nathan N, Yoong SL. Improving the translation of health promotion interventions using effectiveness‐implementation hybrid designs in program evaluations. Health Promotion Journal of Australia 2016;27(3):204‐7. [DOI] [PubMed] [Google Scholar]
Wolfenden 2017
- Wolfenden L, Nathan NK, Sutherland R, Yoong SL, Hodder RK, Wyse RJ, et al. Strategies for enhancing the implementation of school‐based policies or practices targeting risk factors for chronic disease. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD011677.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2012
- Wong JYL, Gilson ND, Uffelen JGZ, Brown WJ. The effects of workplace physical activity interventions in men: a systematic review. American Journal of Men's Health 2012;6(4):303‐13. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wolfenden 2016b
- Wolfenden L, Regan T, Williams C, Wiggers J, Kingsland M, Milat A, Rissel C, Bauman A, Booth D, Farrell MM, Légaré F, Zomahoun H, Parmenter B, Ben Charif A, Yoong S. Strategies to improve the implementation of workplace‐based policies or practices targeting tobacco, alcohol, diet, physical activity and obesity [Protocol]. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD012439] [DOI] [PMC free article] [PubMed] [Google Scholar]


