ABSTRACT
Acinetobacter baumannii is a Gram-negative pathogen that causes a multitude of nosocomial infections. The Acinetobacter trimeric autotransporter adhesin (Ata) belongs to the superfamily of trimeric autotransporter adhesins which are important virulence factors in many Gram-negative species. Phylogenetic profiling revealed that ata is present in 78% of all sequenced A. baumannii isolates but only in 2% of the closely related species A. calcoaceticus and A. pittii. Employing a markerless ata deletion mutant of A. baumannii ATCC 19606 we show that adhesion to and invasion into human endothelial and epithelial cells depend on Ata. Infection of primary human umbilical cord vein endothelial cells (HUVECs) with A. baumannii led to the secretion of interleukin (IL)-6 and IL-8 in a time- and Ata-dependent manner. Furthermore, infection of HUVECs by WT A. baumannii was associated with higher rates of apoptosis via activation of caspases-3 and caspase-7, but not necrosis, in comparison to ∆ata. Ata deletion mutants were furthermore attenuated in their ability to kill larvae of Galleria mellonella and to survive in larvae when injected at sublethal doses. This indicates that Ata is an important multifunctional virulence factor in A. baumannii that mediates adhesion and invasion, induces apoptosis and contributes to pathogenicity in vivo.
KEYWORDS: Adhesion, endothelial cells, host cell response, HUVEC, Galleria mellonella
Introduction
The Gram-negative γ-proteobacterium Acinetobacter baumannii is an important pathogen in the hospital environment and causes a multitude of nosocomial infections including wound and urinary tract infections, pneumonia and bloodstream infections. The ability to acquire or upregulate antimicrobial resistance determinants has led to a high frequency of multidrug-resistant A. baumannii strains worldwide [1,2]. In 2017, the World Health Organization classified carbapenem-resistant A. baumannii as “priority one” on the global priority list of antibiotic-resistant, pathogenic bacteria for research and development of new antibiotics [3].
To establish infections, pathogenic bacteria need to adhere to human host cells and tissues which is often followed by bacterial invasion. Among others, biofilm formation, modulation of host cell signaling, induction of apoptosis, serum resistance and immune evasion support to maintain an infection. Several proteins of A. baumannii have been described to be involved in this processes: e.g. the outer membrane protein A (OmpA) mediates adhesion to epithelial cells and cytotoxicity [4,5], phospholipases D (PLD) support invasion and pathogenicity in vivo [6], and the plasminogen-binding protein (CipA) inactivates the alternative complement system and supports the penetration of endothelial cell layers [7]. Previously, the trimeric autotransporter adhesin Ata, was identified in A. baumannii ATCC 17978, which mediated adhesion to extracellular matrix proteins (ECMs) and virulence in a murine pneumonia model [8,9]. Trimeric autotransporter adhesins (TAAs) are important virulence factors in Gram-negative bacteria, that regulate adhesion, biofilm formation, immune evasion, angiogenesis or cell death [10–13]. In silico analyzes from sequenced Acinetobacter spp. strains revealed that species harbouring ata in their genomes span the full diversity of the genus Acinetobacter [14]. In A. baumannii ATCC 19606, the domain architecture of Ata comprises a duplicated head domain, a repetitive neck-stalk region, and membrane anchor domains (Supp. Figure 1) [14].
Recently, we could demonstrate that Ata mediates adhesion to human primary endothelial cells under static and shear-stress conditions in vitro and in an ex vivo human organ infection model [15]. The function of Ata during A. baumannii infections is still poorly understood and nothing is known about the host cell-interacting ligands of A. baumannii. Former studies investigated Acinetobacter virulence using epithelial cells as host targets [16,17], but it can be assumed that different cell types show different host-pathogen interaction patterns. We therefore aimed to characterize and compare the role of Ata in host-pathogen interaction by analyzing adhesion, invasion, host-cell-modulation and apoptosis in endothelial and epithelial cells.
Materials and methods
Computational analysis
For the evolutionary characterization of Ata the encoding sequence at position 315,815 to 322,591 in the genome of A. baumannii ATCC 19606 (NCBI Reference Sequence: NZ_KL810966) was used. We determined the phylogenetic profile of ata, i.e. the presence-absence pattern of its orthologs across 3052 Acinetobacter spp. genomes available in the NCBI RefSeq data base version 87, representing 58 Acinetobacter species. A two-step procedure was implemented to reduce the computational complexity of the analysis: As the first step, cliques of orthologous proteins across a core set of 103 Acinetobacter spp., encompassing pathogenic strains as well as type and reference species (NCBI RefSeq assembly and protein accessions listed in Supp. List), were compiled using OMA standalone 2.0.1 [18] with default parameters. The resulting core orthologous groups were then extended with sequences from the remaining 2949 Acinetobacter spp. genomes using the targeted ortholog search tool HaMStR v.13.2.9 (https://github.com/BIONF/hamstr; [19]). HaMStR was run with the parameters ‘-central -force -checkCoorthologsRef -scoreThreshold -filter = F‘. From the resulting extended orthologous groups, we finally extracted and visualized the phylogenetic profiles using PhyloProfile [20]. Domain annotations of the encoded proteins were determined by daTAA tool [21].
Generation of parkm expression vector and transconjugation
An isogenic knock-out strain of ata (∆ata) was previously generated in A. baumannii ATCC 19606 [15]. For complementation of ∆ata, the pARP3 plasmid [22] was digested with PvuII to remove the ampicillin resistance marker. Plasmids and oligonucleotides used for cloning are listed in Table 1 and Supp. Table 1. The kanamycin resistance gene cassette was amplified from pET42a using Kan_PvuII_fwd/Kan_PvuII_rev, digested with PvuII, and cloned into the PvuII site of pARP3, generating pARKM. ata and its SD sequence were amplified from A. baumannii ATCC 19606 using ata-in_fusion-fwd/ata-in_fusion-rev and cloned into the EcoRI-XbaI site of pARKM by In-fusion PCR cloning (Clontech), generating pARKM_ata. The resulting plasmids were transconjugated from Escherichia coli WM6026 (Table 1) [23] into ∆ata. For this, ∆ata was grown in lysogeny broth (LB), whereas E. coli WM6026 harboring pARKM or pARKM_ata was grown overnight in LB containing 50 µg/mL kanamycin and 600 µM diaminopimelic acid (DAP). Equal volumes of bacterial suspensions were mixed and spotted on LB agar containing 600 μM DAP. Samples were incubated for 24 h at 37°C and plated onto LB agar plates containing 100 µg/mL of kanamycin for selection of positive transformants: ∆ata::pARKM, termed ∆ata_(p) (empty vector control) and ∆ata::pARKM_ata, termed ∆ata_(c) (complemented strain).
Table 1.
Bacterial strains and plasmids.
| Designation | Characteristic/Comments | Reference | |
|---|---|---|---|
| Bacterial Strains | A. baumannii ATCC 19606 | Type strain, isolated from humans, expressing ata | GenBank accession no. ACQB00000000.1 |
| A. baumannii ATCC 19606 ∆ata | ata markerless deleted mutant (positions 315,815–322,591) | [15] | |
| A. baumannii ATCC 19606 ∆ata::pARKM [Δata_(p)] | A. baumannii ATCC 19606 ∆ata transformed with pARKM, KanR, GenR, pBAD-Promotor | this study | |
| A. baumannii ATCC 19606 ∆ata::pARKM_ata [Δata_(c)] | ata gene cloned into pARKM, transformation of A. baumannii ATCC 19606 ∆ata for complementation | this study | |
| A. baumannii ATCC 17978 | Reference strain, isolated from humans, expressing ata | Gottfried Wilharm, Wernigerode | |
| A. baumannii ATCC 17978 Δata | ata transposon mutant, transformed with EZTn5 <KAN-2> (Epicentre Biotechnologies, Madison, Wisconsin, USA) | Gottfried Wilharm, Wernigerode | |
| E. coli DH5α | Host strain used for cloning | New England Biolabs | |
| E. coli BL21 (DE3) | Host strain for protein production | New England Biolabs | |
| E. coli WM6026 | Donor strain for bacterial conjugation to A. baumannii ATCC 19606 ∆ata | [23] | |
| Plasmids | pARP3 | Shuttle vector for E. coli-A baumannii and expression vector under pBAD-promotor for A. baumannii, AmpR, GenR | [22] |
| pARKM | Shuttle vector for E. coli-A. baumannii and expression vector under pBAD-promotor for A. baumannii, KanR, GenR | this study | |
| pARKM_ata | Full length ata-gene incl. SD sequence cloned into pARKM for expression of ata, KanR, GenR | this study | |
| pTOPO | Cloning vector for amplicons with blunt ends, lacZα for blue/white screening, KanR, ZeoR | Thermo Fisher Scientific | |
| pTOPO_TA_2.1_hmbs | Partial sequence of hmbs cloned into pTOPO_TA.2.1, external standard for qRT-PCR, KanR, AmpR | [15] | |
| pTOPO_TA_2.1_rpoB | Partial sequence of rpoB cloned into pTOPO_TA_2.1, external standard for qRT-PCR, KanR, AmpR | [15] | |
| pTOPO_ata | Partial sequence of ata cloned into pTOPO_TA_2.1, external standard for qRT-PCR, KanR, ZeoR | this study | |
| pET24a | Expression vector for E. coli under regulation of lac-promotor, KanR | Novagen | |
| pET24a_ata-head | Sequence of ata head domain cloned into multiple cloning site of pET24a, KanR | this study |
Cultivation of human cells and bacterial strains
Primary human umbilical cord vein cells (HUVECs) were prepared from fresh cord veins according to the ethical permission (4/12; University Hospital Frankfurt am Main) as described [24]. Human endothelial cells (HMEC-1 and HDMEC), epithelial cell lines (A549, HeLa and HepG-2) and the monocytic cell line THP-1 were purchased from either Promocell (Heidelberg, Germany) or DSMZ (Braunschweig, Germany) and cultivated as described in Suppl. Methods. Bacteria used in this study are listed in Table 1. Cultivation conditions and preparation of bacteria for infection experiments are shown in Suppl. Methods.
Generation of an anti-ata antibody
A polyclonal anti-Ata antibody targeted against the head domain of Ata was generated by immunization of rabbits with a recombinant protein (~68 kDa, monomeric) which was purified from E. coli BL21 (DE3) transformed with pET24a_ata-head (Table 1). Details are given in Supp. Table 1 and in Supp. Methods.
Analysis of ata gene expression and ata protein production
Quantification of ata gene expression was done as described in [25] and Supp. Methods by RNA isolation and subsequent quantitative real-time PCR (qRT-PCR) amplifying rpoB and ata using primers listed in Table 1. Immunostaining of Ata and fluorescence microscopy was conducted as described in Supp. Methods.
Analysis of bacterial adhesion to human cells and invasion
Adhesion of A. baumannii to human cells was analyzed as described previously [15]. Visualization of bacterial adhesion to HUVECs was done by fluorescence microscopy and by scanning electron microscopy (see Supp. Methods for technical details). Invasion of A. baumannii into host cells was carried out by a gentamicin/colistin-protection assay. Human cells were seeded into 6-well plates and infected with A. baumannii (MOI 200) for 4 h. Subsequently, samples were washed three times with the respective cell growth medium and 1 ml of gentamicin-sulfate (500 µg/mL, WT and ∆ata) or colistin (10 µg/mL, ∆ata_(p) and ∆ata_(c) harboring a gentamicin resistant cassette) was added to the infected cells to kill extracellular bacteria. After 2 h of incubation, cells were washed and lysed by addition of 1 mL A. dest.. Afterwards, serial dilutions were plated onto LB agar for CFU determination.
Analysis of cell death and caspase activity
HUVECs were seeded into 6-well plates (5 × 105 cells) and infected with A. baumannii (MOI 1) or incubated with 60 µM camptothecin (CAT, positive control) for 8, 16 and 24 h. Thereafter, cells were washed and detached from the wells using a cell scraper. Endothelial cells were stained with Annexin V-FITC and propidium iodide (BD Bioscience) and induction of apoptosis or necrosis was monitored by flow cytometry (BD Bioscience). Activity of caspase-3 and caspase-7 was determined in 96-well plates with 1 × 104 cells infected with A. baumannii (MOI 1) using the Caspase-Glo® 3/7 Assay (Promega) according to manufacturer’s instructions. Cytotoxicity of A. baumannii towards HUVECs was measured by a lactate dehydrogenase (LDH) assay (Supp. Methods).
Galleria mellonella infection model
In vivo pathogenicity was analyzed in larvae of G. mellonella as described previously [26]. Survival of A. baumannii in G. mellonella larvae was investigated by injecting 1 × 105 bacteria into the last left proleg, followed by incubation at 37°C. Immediately after injection and after 24, 48 and 72 h, larvae were homogenized and serial dilutions were plated onto Endo agar (Oxoid) for CFU determination. For quantification of hemocytes in G. mellonella, larvae were infected as described above and incubated for 72 h. For the indicated time points, larvae were cut with a scalpel and centrifuged to separate the hemolymph. Hemocytes were resuspended in trypsin-EDTA (0.05%, Gibco), stained with trypan blue and enumerated using a hemocytometer.
Quantification of cytokines and transmigration of THP-1 cells
Quantification of cytokines and chemokines in the supernatants of infected HUVECs was done employing an enzyme-linked immunosorbent assay (ELISA) (Microbial-induced Multi-Analyte ELISArray Kit, Qiagen) or by the human IL-6 or IL-8 ELISA kit (BD Bioscience) (Supp. Methods). Transmigration of monocytic THP-1 cells to infected HUVECs was evaluated as described [27] and in Supp. Methods.
Statistical analysis
Experiments were performed at least three times and differences between mean values of experimental and control groups were analyzed by Student’s t-test (Graph Pad Prism 5.0; Graph Pad Software, San Diego, CA, USA). A p-value of p < 0.05 was considered to be statistical significant. Median lethal doses (LD50) were calculated by non-linear regression analysis as described [26].
Results
Prevalence of ata within the genus acinetobacter
We performed an in silico screen for the presence of ata in 3,052 Acinetobacter spp. genomes. This revealed orthologs in 2,135 genomes covering the full phylogenetic diversity of this genus. This indicates that ata was already present in the last common ancestor of this genus. However, the prevalence of ata in the individual species varies considerably (Figure 1(a)). In case of the monophyletic A. nosocomialis, A. seifertii, and A. baumannii (NSB) clade, ata was found in 78% of the analyzed genomes. In contrast, within the clade representing A. calcoaceticus, A. dijkshoorniae and A. pittii (CDP clade), only 2% of the analyzed genomes contained ata.
Figure 1.
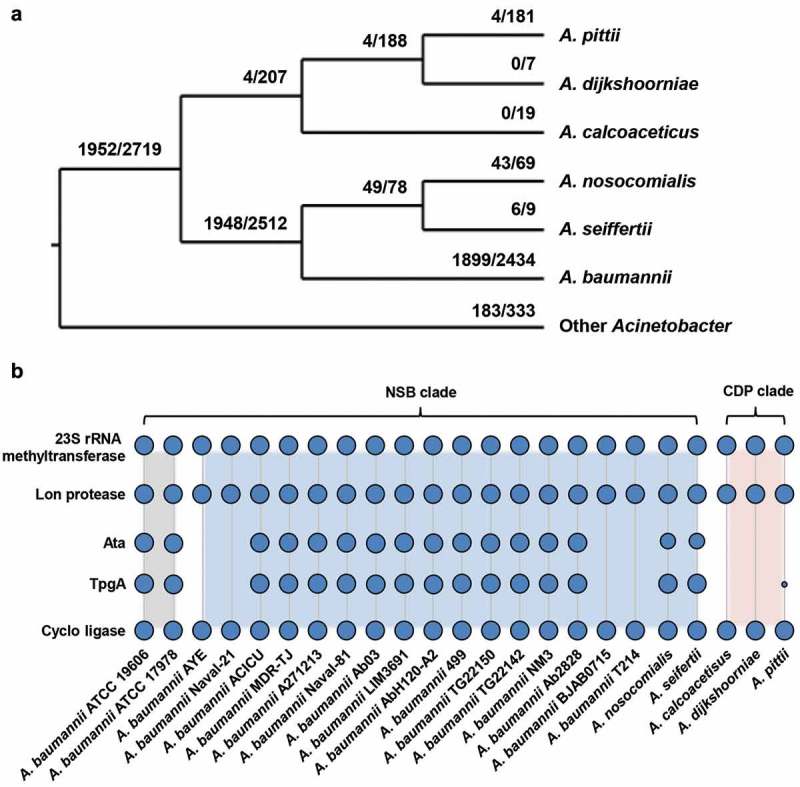
The phylogenetic distribution of ata. (a) The tree displays the prevalence of ata in individual Acinetobacter spp.. Branch labels denote the number of genomes harboring ata vs. the total number of analyzed genomes in the corresponding clade. Species outside the Acinetobacter calcoaceticus-baumannii complex are collapsed into a single taxon named “Other Acinetobacter”. The tree topology follows Poppel et al. [62]. (b) Phylogenetic profile of the A. baumannii ATCC 19606 gene cluster encoding the following five proteins: 23S rRNA methyltransferase – Lon protease – Ata – TpgA – Cyclo-ligase. Blue dots indicate the presence of a gene’s ortholog in the respective taxon. Dot sizes are proportional to the fraction of genomes subsumed in each taxon harboring an ortholog. The presence/absence information is given per strain in the case of A. baumannii and is summarized on the species level for the other species (see Figure 5(a) for the number of analyzed genomes). The profiles of the two reference strains are shaded in grey. Further 16 profiles of A. baumannii strains exemplifying the variation within this species are depicted in the blue shaded area together with profiles of the two further species in the NSB clade. The profiles for the CDP clade are shaded in red. Ata and TpgA orthologs are absent in almost all analyzed genomes in this clade, while the remaining three genes are consistently present.
The uneven distribution of ata across the analyzed species could indicate lineage-specific losses of this gene. Yet, lineage-specific differences in the quality of the genome assembly and the gene annotation could generate a similar pattern, since the repetitive structure of ata (Supp. Figure 1) complicates the genome assembly based on short read data. As a consequence, contig ends may often fall within the gene boundaries leaving the annotated gene incomplete or missed entirely by the annotation procedure. The length distribution of the encoded Ata proteins shows that this is indeed a common artefact (Supp. Figure 2). However, we did not find that this preferentially affects any species. To rule out that ata has been missed entirely in the CDP clade, we additionally considered gene order information. In A. baumannii ATCC 19606, Ata (RefSeq Protein Accession WP_001045602.1) is flanked upstream by two genes encoding the 23S rRNA-methyltransferase (WP_000702193.1) and the Lon protease (WP_001292274.1), and downstream by two genes coding for the outer membrane protein assembly factor TpgA (equivalent to BamE: WP_001044114.1) and the 5-formyltetrahydrofolate cyclo-ligase (WP_001004364.1), respectively. We determined the phylogenetic profiles of the four flanking genes and integrated them with the profile of ata (Figure 1(b)). This revealed that the occurrence of ata is tightly linked to that of tpgA, which encodes a periplasmic protein that forms a complex with Ata and assists in the transport of Ata to the cell surface [28]. Notably, the order of the five genes is entirely conserved in 93% of the A. baumannii genomes that harbor the ata gene, and where the assembly contiguity allows the detection of this microsyntenic region (i.e. the ata gene is neither located two genes up- nor downstream to a contig end). In turn, when ata was missing, almost always also tpgA was absent, and the genes encoding the Lon Protease and the cyclo-ligase reside next to each other.
We subsequently focused on ata in other Acinetobacter spp.. Similar to A. baumannii, the presence of ata typically implies the presence of tpgA, which presumably represents the ancestral state for Acinetobacter. Exemplarily for the 177 genomes of A. pittii harboring an Ata ortholog, we confirmed that the joint absence of ata and tpgA is most likely not an artefact of an inaccurate genome assembly. In 160 of these genomes, we find the two genes encoding the Lon protease and the 5-formyltetrahydrofolate cyclo-ligase residing next to each other on the same contig indicating a true loss of ata and tpgA (Supp. Figure 3). In the remaining 17 genomes, the Lon protease and 5-formyltetrahydrofolate cyclo-ligase encoding genes are disrupted by a contig break. Thus, we conclude that a joint loss of the functionally related gene pair, ata/tpgA explains the data best. In summary, the almost complete absence of ata in the CDP clade most likely represents a genuine evolutionary signal.
Deletion and complementation of ata in A. baumannii
To analyze the function of Ata in A. baumannii we used the type strain ATCC 19606 and an isogenic, markerless deletion strain (∆ata) which had been generated previously [15]. In A. baumannii ATCC 19606, Ata consists of 2,258 amino acids with a predicted molecular mass of about 250 kDa in its monomeric form. For complementation purposes, ∆ata was transformed with the ata encoding expression vector pARKM_ata [22], thereby generating ∆ata_(c). Furthermore, ∆ata was transformed with the empty pARKM vector, thereby generating ∆ata_(p) which was used as an empty vector control. These four variants were used throughout this study: wild-type strain (WT), ata knock-out strain (∆ata), ∆ata complemented with pARKM_ata [∆ata_(c)] and ∆ata complemented with empty pARKM vector [∆ata_(p)].
Genomic or plasmid DNA was isolated from each strain and the presence or deletion of ata was verified by PCR and RFLP. A 6,777 bp fragment was amplified from WT DNA (Supp. Figure 4(a), lane 1), indicating the presence of ata, whereas no signal was observed when using DNA of ∆ata (lane 2). Restriction of pARKM from ∆ata_(p) and pARKM_ata from ∆ata_(c) with BamHI/XbaI revealed the linearized vector (7,556 bp) (lane 5) or the linearized pARKM (7,556 bp) and a fragment of 6,777 bp (lane 7) respectively, indicating the successful transformations of ∆ata.
For evaluation of ata gene expression, the respective A. baumannii strains were cultivated with (+) or without (-) arabinose and total RNA was analyzed by quantitative real-time PCR (qRT-PCR). A strong ata expression was observed in the WT strain (2.3 ± 0.4%) and in arabinose-induced ∆ata_(c) (3.3 ± 1.1%), whereas no signal was detected for ∆ata and ∆ata_(p) (Supp. Figure 4(b)). Low expression levels were found for ∆ata_(c) without arabinose (0.4 ± 0.2%), indicating a low background expression of ata, which is most likely due to the leakiness of the pBAD-promoter system [29]. To evaluate Ata production in WT, ∆ata and complemented strains, the surface localization was analyzed by immunofluorescence using an anti-Ata-head antibody. Fluorescence microscopy confirmed the surface location of Ata for WT and ∆ata_(c) strains, but not for ∆ata and ∆ata_(p) (Supp. Figure 4(c)).
Successful deletion and complementation of ata were confirmed by PCR, sequencing, RFLP, gene expression and immunofluorescence. Growth experiments in LB revealed no differences in doubling times between the single strains (data not shown).
Adhesion of A. baumannii to endothelial and epithelial cells is ata-dependent
Since adhesion to host cells is important to establish infections, the Ata-dependent binding of A. baumannii to human cells was analyzed. For this purpose, primary endothelial cells (HUVEC and HDMEC) or endothelial (HMEC-1) and epithelial cell lines (A549, HeLa, HepG-2) were used. The interaction of A. baumannii with their respective host cells was analyzed by fluorescence and scanning electron microscopy. Ata-producing A. baumannii adhered more efficient to the host cells compared to ata-deficient bacteria for both, endothelial and epithelial cells (Figure 2(a+b)). Adhesion of WT bacteria to HDMECs was ~ 4-fold higher compared to A549 human lung epithelial cells as observed by fluorescence microscopy (Figure 2(a)). Scanning electron microscopy revealed a higher adhesion rate of WT and ∆ata_(c) on the surface of HUVECs compared to ∆ata and ∆ata_(p) (Figure 2(b)). Notably, ata-expressing A. baumannii were located in small pit-like structures on the surface of HUVECs, which could not been observed for the knock-out strains (Figure 2(b), white dashed circles).
Figure 2.
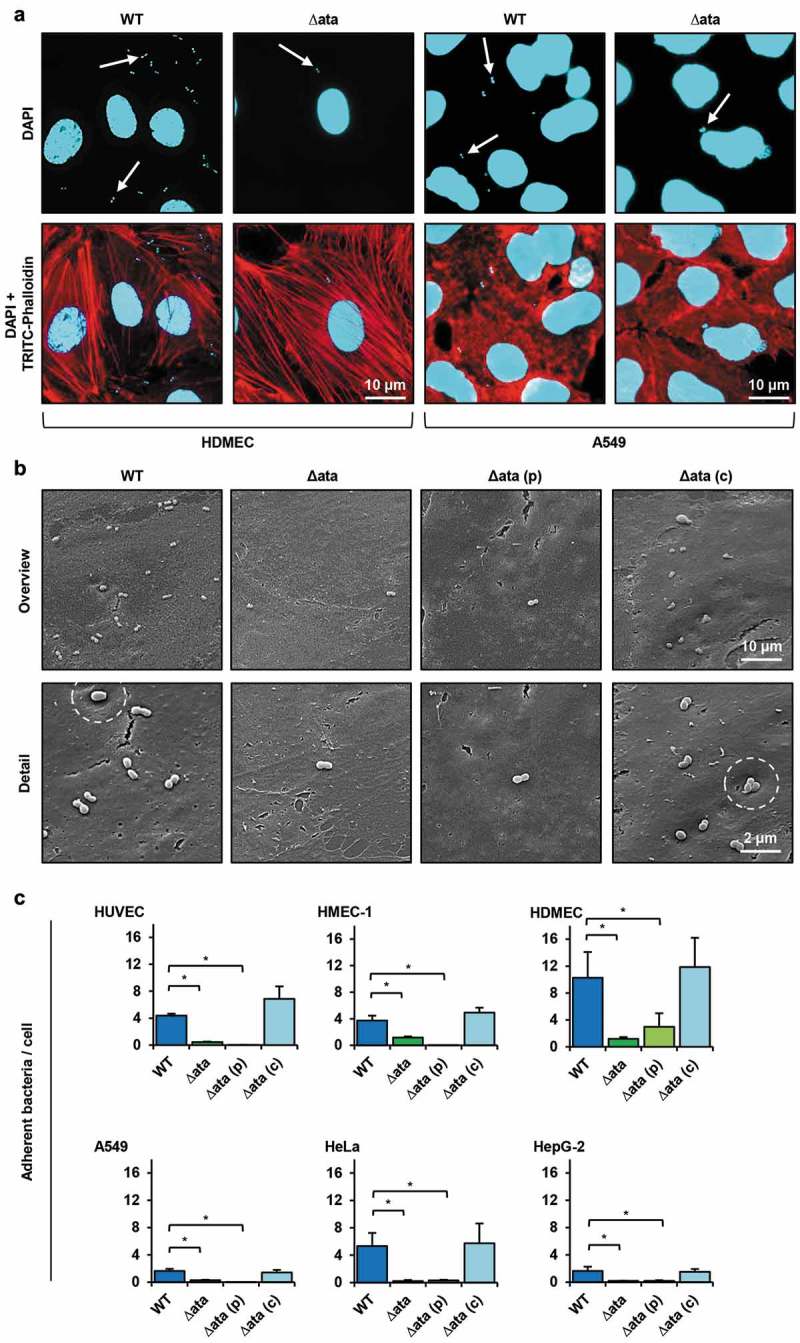
Ata-mediated adhesion to human endothelial or epithelial. (a) Representative fluorescence microscopy of infected endothelial and epithelial cells. Human cells (HDMEC or A549) were incubated with A. baumannii (MOI 200) for 1 h. Non-adherent bacteria were flushed and samples were fixed with paraformaldehyde. DNA was stained with DAPI (blue) and the cytoskeleton was stained with TRITC-phalloidin (red). Arrows indicate adherent bacteria. (b) Scanning electron microscopy of HUVEC-associated A. baumannii. HUVECs were incubated with A. baumannii (MOI 200) for 1 h. Non adherent bacteria were flushed, infected cells were fixed in 2.5% glutaraldehyde and prepared for scanning electron microscopy. (c) Ata-dependent adhesion of A. baumannii to human endothelial and epithelial host cells. Endothelial (HUVEC, HMEC-1, HDMEC) and epithelial cells (A549, HeLa, HepG-2) were incubated with A. baumannii (MOI 200) for 1 h. After incubation, planktonic bacteria were rinsed and infected cells were harvested by tryptic digestion. Samples were used for analyzing adherent bacteria by amplifying human and A. baumannii specific genes (hmbs and rpoB) in a qRT-PCR approach. CT-values, obtained from qRT-PCR, were used for calculation of bacterial adhesion. Values are means ± SEM of five independent experiments; *, p < 0.05.
Next, Ata-dependent adhesion of A. baumannii to human epithelial and endothelial cells was compared and numbers of adherent bacteria were quantified using a qRT-PCR approach [15]. For HUVECs, 4.4 ± 0.3 bacteria adhered per endothelial cell when infected with the WT strain (Figure 2(c)). This represents an approx. 10-fold higher adhesion compared to Δata (0.4 ± 0.05 bacteria/cell). When ata expression was induced in Δata_(c) by arabinose, the adhesion rate was more than 20-fold higher compared to Δata_(p) (6.9 ± 1.9 bacteria/cell and 0.03 ± 0.01 bacteria/cell respectively). Similar results were obtained when additional endothelial cell lines were employed: HMEC-1 (WT: 3.7 ± 0.8 bacteria/cell; ∆ata: 1.2 ± 0.2 bacteria/cell; Δata_(p): 0.01 ± 0.01 bacteria/cell; Δata_(c): 4.9 ± 0.8 bacteria/cell) or HDMEC (WT: 10.0 ± 3.9 bacteria/cell; ∆ata: 1.2 ± 0.3 bacteria/cell; Δata_(p): 3.0 ± 2.0 bacteria/cell; Δata_(c): 11.2 ± 4.4 bacteria/cell). Notably, when HDMECs were infected with A. baumannii, bacterial adhesion was twice as high compared to HUVECs and HMEC-1.
Deletion of ata almost completely abolished the ability of A. baumannii to bind to all three analyzed human epithelial cells, thereby confirming the results obtained with the endothelial cells. For A549 lung cells, 1.7 ± 0.3 bacteria/cell were detected when infected with the WT strain and 0.3 ± 0.07 bacteria/cell for ∆ata. When ata was induced in Δata_(c) by arabinose and compared with Δata_(p), the dependence of Ata for adhesion of A. baumannii was even more evident (Δata_(c): 1.4 ± 0.4 bacteria/cell vs. Δata_(p): 0.01 ± 0.01 bacteria/cell). Similar results were obtained when HepG-2 and HeLa cells were used.
The adhesion of ata-expressing A. baumannii to A549 and HepG-2 epithelial cells was considerably lower compared to all endothelial cells. Adhesion to HeLa cells was 3-fold higher than to A549 or HepG-2 cells and comparable to HUVEC and HMEC-1 but lower than for HDMEC, indicating that A. baumannii adheres at least as efficient to endothelial cells compared to epithelial cells. These results show that Ata is essential for adhesion of A. baumannii to human endothelial and epithelial host cells during infection.
Ata induces IL-6 and IL-8 secretion in huvecs
Since A. baumannii interacted with endothelial cells in an Ata-dependent manner, we next analyzed the inflammatory response of infected HUVECs by quantification of secreted cytokines and chemokines using a multiarray ELISA approach. At 4 h post infection (p.i.), a 20-fold increase of IL-8 could be measured in supernatants of WT-infected HUVECs compared to the uninfected control (20.3 ± 1.4 x-fold), whereas secretion of IL-8 was significantly lower when HUVECs where infected with Δata (4.5 ± 2.1 x-fold) (Figure 3(a)). After 14 h, the amount of secreted IL-6 increased 15-fold compared to the uninfected control (15.2 ± 3.5 x-fold) and 4-fold compared to Δata (3.8 ± 2.3 x-fold), whereas the amount of IL-8 decreased (WT: 4.8 ± 1.7 x-fold; Δata: 2.7 ± 0.3 x-fold) (Figure 3(a)). The secretion of the macrophage attracting protein (MCP-1) was slightly induced, but this was independent of Ata (WT: 4.8 ± 1.7 x-fold; Δata: 3.7 ± 1.8 x-fold). To verify these findings, we investigated the kinetics of IL-6 and IL-8 secretion upon infection of HUVECs with A. baumannii (Figure 3(b)). IL-8 levels peaked after 8 h (WT: 2.3 ± 0.002 ng/mL and 1.2 ± 0.02 ng/mL for Δata) and decreased over time, whereas IL-6 continuously increased over time up to late infection stages at 14 h (WT: 1.2 ± 0.1 ng/mL; Δata: 0.14 ± 0.02 ng/mL). These results indicate that HUVECs secret IL-8 during early stages and IL-6 during later stages of infection with A. baumannii in an Ata-dependent manner.
Figure 3.
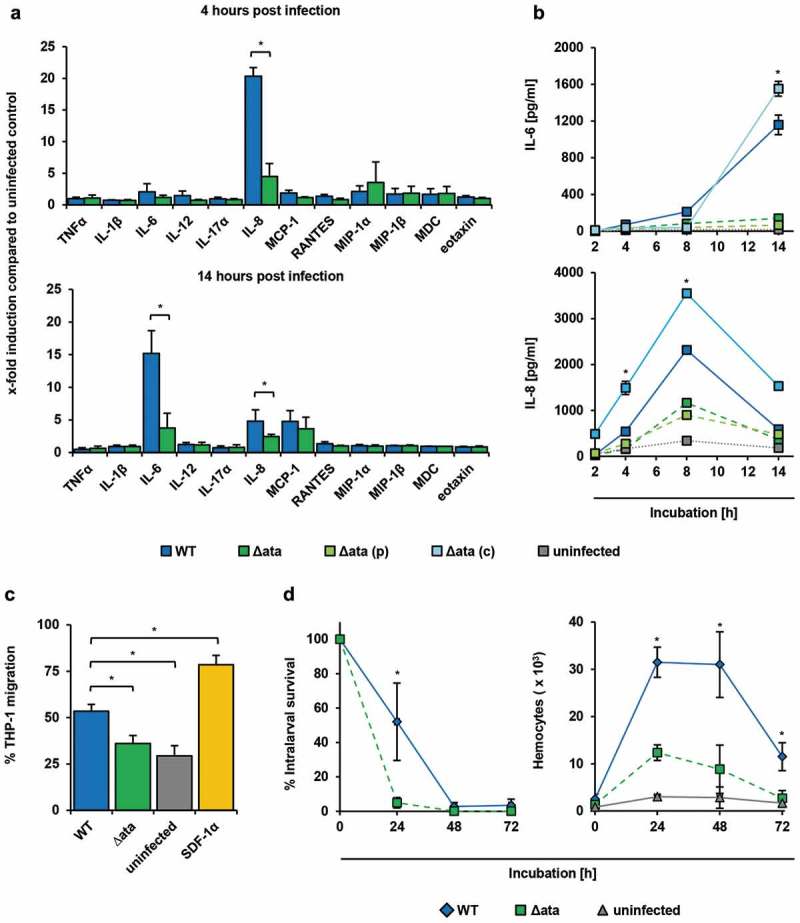
A. baumannii induce inflammatory response in endothelial cells and supports migration of immune cells. (a+b) Ata-mediated induction of inflammatory cytokines upon infection of HUVECs. HUVECs were incubated with A. baumannii (MOI 1) for the indicated time points, and levels of secreted chemokines and cytokines were determined in the supernatant by ELISA. Values are means ± SEM of six independent experiments; *, p < 0.05. (c) Infection of HUVECs with A. baumannii supports transmigration of THP-1 cells. Sterile filtered supernatants of A. baumannii infected HUVECs (MOI 1, 14 h) were used as chemoattractant for analyzing transmigration of THP-1 cells. Monocytes (5 × 105) were placed into the upper part of a cell culture insert (pore size: 8 µm) and allowed to migrate for 16 h towards the chemoattractant in the lower part of the well. THP-1 cells were counted using trypan blue staining and a hemocytometer. (d) Survival of A. baumannii in G. mellonella and its contribution to activate hemocytes within the larvae. Caterpillars were infected with a sub-lethal dose of A. baumannii (1 × 105 bacteria). For analyzing the survival of A. baumannii, larvae were homogenized at the indicated time points and serial dilutions were plated onto Endo agar (BD) for CFU enumeration. To investigate the activation of hemocytes, larvae were homogenized and centrifuged in a cell filter containing tube to separate the hemolymph. Samples were mixed with 100 µL of trypsin-EDTA (0.05%) and stained with trypan blue, immediately. Hemocytes were enumerated using a hemocytometer. In (c)+(d), values are means ± SD of three independent experiments; *, p < 0.05.
Ata-dependent recruitment of immune cells
We next sought to address if the Ata-dependent pro-inflammatory cytokine secretion of HUVECs would recruit monocytes, which are important immune cells contributing to antimicrobial functions in tissue infections [30]. After co-cultivation of HUVECs with A. baumannii, cell culture supernatants were sterile filtered and used as chemoattractants in transwell migration assays employing THP-1 monocytes. A background transmigration of 29% was observed when cells were incubated with just medium (“uninfected”), whereas 79% of THP-1 cells transmigrated when the chemokine SDF-1α, which has been described to activate migration of THP-1 cells [27], was used as a positive control. A transmigration rate of 53% was observed when supernatants of WT-infected HUVECs were used (Figure 3(c)). In contrast, transmigration was reduced to 36% when supernatants of Δata-infected HUVECs were applied.
To investigate the recruitment of immune cells in vivo, larvae of Galleria mellonella were injected with sublethal doses of 105 A. baumannii and numbers of hemocytes in the hemolymph were quantified (Figure 3(d)). Hemocyte numbers increased 13-fold upon infection and dropped again after 48 h. Compared to Δata a 2.5-fold increase after 24 h of infection with the WT strain was observed, indicating that Ata has led to a higher inflammatory response in G. mellonella larvae. Hemocyte numbers inversely correlated with the number of surviving bacteria (Figure 3(d)), and loss of Ata correlated with a reduced survival of Δata within the larvae. At 24 h p.i. almost all Δata bacteria were killed, whereas ~ 55% of the WT bacteria were still viable (Figure 3(d)). After 48 h, both WT and ∆ata strain were killed.
A. baumannii invades into epithelial and endothelial cells in an ata-dependent manner
A. baumannii has been described to invade human epithelial cells [6], and based on these findings, we investigated Ata dependence of invasion into endothelial and epithelial cells using a gentamicin/colistin protection assay. HUVECs or A549 cells were infected with A. baumannii for 4 h and treated with gentamicin or colistin to kill extracellular bacteria. Compared to the WT strain, a strong reduction in invaded cells could be observed for Δata (31 ± 3%) and Δata_(p) (12 ± 6%), whereas invasion by Δata_(c) (91 ± 7%) was not influenced (Figure 4(a)). Likewise, in A549 cells, the invasion rate for ∆ata and Δata_(p) decreased to 57 ± 18% and 14 ± 3% respectively, whereas invasion was not diminished when the Δata_(c) strains was employed.
Figure 4.
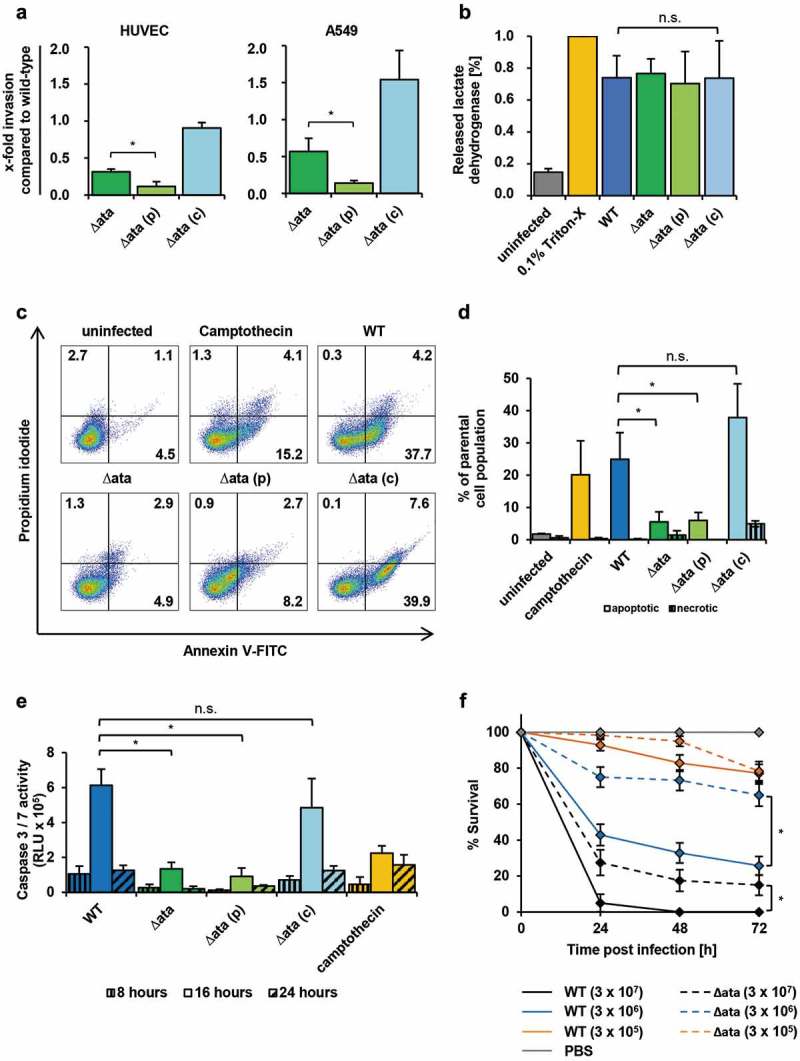
Role of Ata in mediating virulence in endothelial cells and G. mellonella. (a) Invasion of A. baumannii into HUVECs and A549 is Ata-mediated. Human cells were incubated with A. baumannii (MOI 200) for 4 h and non-bound bacteria were flushed. Extracellular bacteria were killed with gentamicin (500 µg/mL) or colistin (10 µg/mL). Intracellular bacteria were released after lysis of human cells with deionized water and CFUs were determined by plating serial dilutions. Invasion of the WT strain was set as 1. Values are means ± SD of five independent experiments; *, p < 0.05. (b) Release of lactate dehydrogenase (LDH) upon infection of HUVECs. Endothelial cells were infected with A. baumannii (MOI 200) for 24 h. The supernatant was sterile filtered and the amount of released LDH was determined by spectrophotometry. Uninfected and 0.1% Triton X-100 treated cells were used as negative or positive control respectively. In (b)-(e), values are means ± SD of three independent experiments; *, p < 0.05. (c+d) Ata induces apoptosis in endothelial cells. HUVECs were infected with A. baumannii (MOI 1) for 16 h and stained with propidium iodide/Annexin V-FITC and analyzed by flow cytometry to determine viable, apoptotic and necrotic cells. Gates indicating different stages of cell death: lower left (viable), lower right (early apoptosis), upper right (late apoptosis) and upper left (necrosis). Numbers represent percentage of parental cell population. (e) A. baumannii upregulates caspase-3 and caspase-7. A. baumannii (MOI 1) were used for infection of HUVECs for the indicated time points. The activity of caspase-3 and caspase-7 was determined by fluorometry. Camptothecin (60 µM) was used as a positive control. (f) Ata-dependent lethality of G. mellonella larvae. Larvae were injected with the indicated CFUs of A. baumannii and the survival was monitored for 72 h. Values are means ± SEM of four independent experiments; *, p < 0.05.
Ata induces apoptosis in huvecs
To evaluate the impact of Ata on cytotoxicity and induction of apoptosis, HUVECs were infected with the WT, the Δata strain and complemented controls. The release of the intracellular enzyme lactate dehydrogenase (LDH) upon infection was measured as a surrogate marker for cytotoxicity. After infection of HUVECs with the WT strain, LDH activity was determined to be 74 ± 14% and 77% ± 9% for Δata, indicating that Ata does not mediate membrane disintegration by A. baumannii (Figure 4(b)).
Apoptosis and necrosis of HUVECs was measured by propidium iodide/Annexin V staining and flow cytometry (Figure 4(c)). Upon infection with A. baumannii the highest level of apoptosis were induced after 16 h; the apoptosis rate after 12 h was <5% and cell death after 20 h consisted of mainly secondary necrotic cells (data not shown). Infection of HUVECs with A. baumannii WT at 16 h p.i. resulted in an apoptosis rate of 25 ± 8% of the parental cell population, whereas <2% of cells were necrotic (Figure 4(d)). When HUVECs were infected with ∆ata, only 5 ± 3% of the HUVECs became apoptotic. As apoptosis can be induced by caspase-dependent as well as caspase-independent mechanisms [31], we further assessed the activity of the executioner caspases, caspase-3 and caspase-7. WT infected HUVECs showed a 5-fold higher activity of caspase-3 and caspase-7 compared to ∆ata infected HUVECs with the highest levels between 8 h and 24 h (Figure 4(e)). These results indicate that infection of HUVECs by WT A. baumannii is associated with higher rates of caspase-dependent apoptosis, but not necrosis, in comparison to ∆ata.
Ata mediates virulence in vivo
To analyze the impact of Ata on pathogenicity of A. baumannii in vivo, the Galleria mellonella larvae infection model was employed. Different CFUs were injected into the hemocoel of the larvae and the survival was monitored over a period of 72 h. To compare virulence between WT and Δata strains, time-kill curves were assessed and median lethal doses (LD50) were determined. Both strains caused a time- and dose-dependent killing of larvae (Figure 4(f)). After injection of 3 × 107 bacteria nearly all infected G. mellonella died after 24 h p.i., whereas the survival rate increased to 40% and 90% when 3 × 106 and 3 × 105 bacteria were injected respectively. In contrast, after injection of ∆ata approximately 30% of the larvae were still alive after 24 h. Mortality of WT infected larvae was always higher for each time point when comparing to larvae infected with the Δata strain. Likewise, LD50 values were lower for the WT at each time point, indicating that loss of Ata attenuates virulence of A. baumannii in vivo (Supplement Table 2). Notably, when an ata transposon mutant of A. baumannii ATCC 17978 was compared with its parental strain in the Galleria infection model, the impact of Ata for killing of larvae was even higher (Supplement Table 2).
Discussion
We investigated the impact of the trimeric autotransporter adhesin Ata of A. baumannii on infection of human host cells and G. mellonella larvae. We found that Ata acts as a multifunctional virulence factor of A. baumannii by (I) mediating adhesion to and invasion into human endothelial and epithelial cells, (II) inducing secretion of the pro-inflammatory cytokines IL-6 and IL-8 in HUVECs, (III) inducing apoptosis of HUVECs in a caspase-dependent manner and (IV) contributing to virulence in vivo. These results strongly suggest that Ata serves as an important virulence factor of A. baumannii during infections in human and insect models.
TAAs are multifunctional proteins and important virulence factors in Gram-negative bacteria [32]. For example, the expression of yadA from Y. enterocolitica strongly correlates with virulence, adhesion and host cell modulation [33–37]. BadA of B. henselae induces the hypoxia inducible factor-1 to promote angiogenesis and mediates adhesion to ECMs and endothelial cells [15,38–40]. Several studies showed that various A. baumannii strains attach to epithelial cells [16], and it is strongly believed that this cell type represents the main target of A. baumannii in host tissues. However, recent studies indicate that A. baumannii also interacts with endothelial cells as they are associated with blood stream or soft tissue infections [15,41,42]. We indeed found that A. baumannii adheres to endothelial and epithelial cells in an Ata-dependent manner (Figure 2(a+b)). Adhesion of A. baumannii to endothelial cells occurred on average at higher frequencies compared to epithelial cells (Figure 2(c)).
Former studies demonstrated that A. baumannii is capable of invading human epithelial cells in vitro [6,17]. We demonstrated that the deletion of ata significantly diminished invasion of A. baumannii into HUVECs and A549 cells. This might be, at least partially, due to the strong Ata-dependent adhesion defect, since the number of intracellular bacteria depends on the ability of bacteria to attach to human host cells. TAA-mediated invasion into host cells is not uncommon. For example, YadA of Y. pseudotuberculosis mediates the uptake of the bacteria into human cells by a fibronectin-dependent bridging between the TAA and β1-integrins [43]. A. baumannii expressing ata were frequently localized in pits on the surface of infected HUVECs (Figure 2(b)), which might represent an early stage in the invasion process.
Bacterial virulence factors can stimulate the host response by triggering the secretion of inflammatory cytokines or chemokines. We observed that A. baumannii induce IL-6 and IL-8 in an Ata-dependent mode and activates monocyte transmigration (Figure 3(a–c)). In line with our findings, previous studies showed that A. baumannii induce inflammation by stimulation of TNF-α, IL-6 and IL-8 in epithelial cells and the IL-8 homolog keratinocyte-derived chemoattractant in mice [44,45]. A modest induction of MIP-1α in HUVECs after 4 h p.i. and a stronger induction of MCP-1 after 14 h p.i. were observed for both the WT and Δata, which likely have contributed to transmigration (Figure 3(a+d)). However, even though we analyzed the secretion of 12 important chemokines by HUVECs upon infection with A. baumannii we cannot rule out that other chemokines might be involved [46,47].
In this context, it could be demonstrated that secreted outer membrane vesicles (OMV) of A. baumannii activate the inflammation in host cells [48]. It can be assumed that huge immunodominant outer membrane proteins like Ata directly interact with the host tissue and thereby stimulate diverse inflammatory responses. In fact, also other TAAs such as BadA or YadA can trigger the production of cytokines or chemokines, e.g. the vascular endothelial growth factor or IL-8 [10,37]. Compared to the Ata induced inflammatory response, YadA stimulates the IL-8 secretion at earlier stages of infections with a maximum for IL-8 after 6 h [37].
To analyze the contribution of Ata-mediated A. baumannii pathogenicity in vivo, we employed the G. mellonella infection model, which has been shown to be suitable for analyzing virulence of A. baumannii and the innate immune response [49–52]. Infection of larvae with the ∆ata and the WT strains revealed that deletion of ata led to significantly impaired time-kill kinetics clearly indicating that Ata mediates pathogenicity in vivo (Figure 4(f)). Sublethal doses of A. baumannii were then injected and intralarval survival of the bacteria was monitored. Survival of ata-expressing A. baumannii was higher in G. mellonella after 24 h of incubation in comparison to ata deletion strains (Figure 3(d)). In line with the Ata-dependent pro-inflammatory human cell response, the number of larval hemocytes (which are similar to phagocytes in humans [53]) increased, indicating that A. baumannii might activate the recruitment of larval hemocytes by Ata (Figure 3D). The higher intralarval survival of the WT compared to Δata despite the increased recruitment of phagocytic cells could, at least partially, be explained if Ata would somehow mediate protection against the host. This has indeed been extensively described for the autotransporter YadA from Y. enterocolitica and BadA from Bartonella henselae which e.g. mediate immune evasion, serum resistance and antiphagocytosis [10,12,33–37,43]. However, if this is also applicable to Ata needs to be addressed in further studies.
Ata mediated apoptosis of HUVECs at 12–20 h p.i., but did not influence cytotoxicity or necrosis (Figure 4(c+d)). During bacterial infections, apoptosis can be either induced by the extrinsic pathway (via death receptors) or intrinsic pathway (via mitochondria) [54]. For A. baumannii both mechanisms could be demonstrated [55–57]. A. baumannii activates the effector caspase-3 and Poly (ADP-ribose) polymerase (PARP) [57]. We observed that Ata also stimulates the cleavage of caspase-3 and in addition caspase-7 in HUVECs (Figure 4(e)). Even though most bacteria inhibit apoptosis to preserve their replicative niche, the destruction of endothelial or epithelial barriers by the induction of apoptosis might be beneficial for A. baumannii to enter deeper tissues.
We have elucidated the role of Ata during the infection of human tissue and have shed initial light on the underlying molecular mechanisms based on A. baumannii ATCC 19606 as a showcase. Using the phylogenetic profiles of ata, and of its flanking genes in A. baumannii ATCC 19606 across 3,052 Acinetobacter spp. allowed us to embed these findings into a functional evolutionary context. Ata orthologs represent the full phylogenetic diversity of the genus Acinetobacter. Most likely Ata was already present before the contemporary Acinetobacter spp. started diversifying. We find the genes encoding Ata and its functionally interacting partner, TpgA, in the majority of A. baumannii strains, and also in most genomes of the closely related species A. nosocomialis and A. seifertii. Thus, the three species share the same fundamental machinery to facilitate cell adhesion. Accordingly, we propose that the findings exemplarily made for A. baumannii ATCC 19606 describe a mode of infection that is representative for the entire NSB clade. It is tempting to speculate that the loss of Ata in the NSB clade correlates with a reduced virulence. However, the situation is more complex. A. baumannii AYE is an epidemic strain with a 26% mortality rate in infected individuals [58]. Yet, this strain lacks both ata and tpgA, and this can neither be explained with the quality of the genome assembly nor with that of the gene annotation. This suggests that, similar to other virulence factors (e.g. bauA, omp33-36 and pglC [59]), alternative factors exist that can maintain virulence even when ata is absent. The almost complete deletion of the ata/tpgA gene pair in the CDP clade should be interpreted with caution. Individual studies exist, which propose that members of this clade have an attenuated virulence, which might at least be partially due to absence of ata [60,61]. Yet, a decisive conclusion must await a thorough description of facultative virulence factors that can replace Ata, and a more precise determination if, and to what extent, virulence is indeed attenuated in the CDP clade.
In summary, we show that Ata trigger multiple important steps for the initiation of successful infections in host cells. To elucidate the key functions of A. baumannii infection, further research should focus on the host-pathogen interface by e.g. deciphering human host cell receptors and mechanism of apoptosis or immune evasion.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft [FOR 2251] and the Frankfurter Promotionsförderung [FPF].
Acknowledgments
We thank Gottfried Wilharm for the kind gift of the A. baumannii ATCC 17978 Δata transposon mutant and Beate Averhoff for helpful advice during generation of the ata knock-out in A. baumannii ATCC 19606. We are grateful to the Göttig lab members for critically reading the manuscript and providing feedback.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Rolain J-M, Diene SM, Kempf M, et al. Real-time sequencing to decipher the molecular mechanism of resistance of a clinical pan-drug-resistant Acinetobacter baumannii isolate from Marseille, France. Antimicrob Agents Chemother. 2013;57:592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Giammanco A, Calà C, Fasciana T, et al. Global assessment of the activity of tigecycline against multidrug-resistant Gram-negative pathogens between 2004 and 2014 as part of the tigecycline evaluation and surveillance trial. mSphere. 2017;2 DOI: 10.1128/mSphere.00310-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tacconelli E. WHO-PPL-Short_Summary_25Feb-ET_NM_WHO. [Internet] [cited 2018. March 19]. Available from: http://www.who.int/medicines/publications/WHO-PPL Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1
- [4].Gaddy JA, Tomaras AP, Actis LA.. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun. 2009;77:3150–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Choi CH, Hyun SH, Lee JY, et al. Acinetobacter baumannii outer membrane protein A targets the nucleus and induces cytotoxicity. Cell Microbiol. 2008;10:309–319. [DOI] [PubMed] [Google Scholar]
- [6].Stahl J, Bergmann H, Göttig S, et al. Acinetobacter baumannii virulence is mediated by the concerted action of three phospholipases D. PLoS ONE. 2015;10:e0138360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Koenigs A, Stahl J, Averhoff B, et al. CipA of Acinetobacter baumannii is a novel plasminogen binding and complement inhibitory protein. J Infect Dis. 2016;213:1388–1399. [DOI] [PubMed] [Google Scholar]
- [8].Bentancor LV, Camacho-Peiro A, Bozkurt-Guzel C, et al. Identification of Ata, a multifunctional trimeric autotransporter of Acinetobacter baumannii. J Bacteriol. 2012;194:3950–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bentancor LV, Routray A, Bozkurt-Guzel C, et al. Evaluation of the trimeric autotransporter Ata as a vaccine candidate against Acinetobacter baumannii infections. Infect Immun. 2012;80:3381–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Riess T, Andersson SGE, Lupas A, et al. Bartonella adhesin a mediates a proangiogenic host cell response. J Exp Med. 2004;200:1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Raghunathan D, Wells TJ, Morris FC, et al. SadA, a trimeric autotransporter from Salmonella enterica serovar Typhimurium, can promote biofilm formation and provides limited protection against infection. Infect Immun. 2011;79:4342–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].El Tahir Y, Skurnik M. YadA, the multifaceted Yersinia adhesin. Int J Med Microbiol. 2001;291:209–218. [DOI] [PubMed] [Google Scholar]
- [13].Pearson MM, Laurence CA, Guinn SE, et al. Biofilm formation by Moraxella catarrhalis in vitro: roles of the UspA1 adhesin and the Hag hemagglutinin. Infect Immun. 2006;74:1588–1596. [Internet]. Available from: http://iai.asm.org/content/74/3/1588.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Koiwai K, Hartmann MD, Linke D, et al. Structural basis for toughness and flexibility in the C-terminal passenger domain of an Acinetobacter trimeric autotransporter adhesin. J Biol Chem. 2016;291:3705–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Weidensdorfer M, Chae JI, Makobe C, et al. Analysis of endothelial adherence of Bartonella henselae and Acinetobacter baumannii using a dynamic human ex vivo infection model. Infect Immun. 2016;84:711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee JC, Koerten H, van Den Broek P, et al. Adherence of Acinetobacter baumannii strains to human bronchial epithelial cells. Res Microbiol. 2006;157:360–366. [DOI] [PubMed] [Google Scholar]
- [17].Choi CH, Lee JS, Lee YC, et al. Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol. 2008;8:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Train C-M, Glover NM, Gonnet GH, et al. Orthologous Matrix (OMA) algorithm 2.0: more robust to asymmetric evolutionary rates and more scalable hierarchical orthologous group inference. Bioinformatics. 2017;33:i75–i82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ebersberger I, Strauss S. Haeseler A von. HaMStR: profile hidden markov model based search for orthologs in ESTs. BMC Evol Biol. 2009;9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tran NV, Greshake Tzovaras B. Ebersberger I. PhyloProfile: dynamic visualization and exploration of multi-layered phylogenetic profiles. Bioinformatics. 2018. DOI: 10.1093/bioinformatics/bty225 [DOI] [PubMed] [Google Scholar]
- [21].Szczesny P, Lupas A. Domain annotation of trimeric autotransporter adhesins-daTAA. Bioinformatics. 2008;24:1251–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ishikawa M, Nakatani H, Hori K. AtaA, a new member of the trimeric autotransporter adhesins from Acinetobacter sp. Tol 5 mediating high adhesiveness to various abiotic surfaces. PLoS ONE. 2012;7:e48830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Blodgett JAV, Thomas PM, Li G, et al. Unusual transformations in the biosynthesis of the antibiotic phosphinothricin tripeptide. Nat Chem Biol. 2007;3:480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baudin B, Bruneel A, Bosselut N, et al. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc. 2007;2:481–485. [DOI] [PubMed] [Google Scholar]
- [25].Whelan JA, Russell NB, Whelan MA. A method for the absolute quantification of cDNA using real-time PCR. J Immunol Methods. 2003;278:261–269. [DOI] [PubMed] [Google Scholar]
- [26].Göttig S, Riedel-Christ S, Saleh A, et al. Impact of blaNDM-1 on fitness and pathogenicity of Escherichia coli and Klebsiella pneumoniae. Int J Antimicrob Agents. 2016;47:430–435. [DOI] [PubMed] [Google Scholar]
- [27].Zaitseva L, Murray MY, Shafat MS, et al. Ibrutinib inhibits SDF1/CXCR4 mediated migration in AML. Oncotarget. 2014;5:9930–9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ishikawa M, Yoshimoto S, Hayashi A, et al. Discovery of a novel periplasmic protein that forms a complex with a trimeric autotransporter adhesin and peptidoglycan. Mol Microbiol. 2016;101:394–410. [DOI] [PubMed] [Google Scholar]
- [29].Balzer S, Kucharova V, Megerle J, et al. A comparative analysis of the properties of regulated promoter systems commonly used for recombinant gene expression in Escherichia coli. Microb Cell Fact. 2013;12:26 [Internet] Available from: https://microbialcellfactories.biomedcentral.com/track/pdf/10.1186/1475-2859-12-26?site=microbialcellfactories.biomedcentral.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yona S, Jung S. Monocytes: subsets, origins, fates and functions. Curr Opin Hematol. 2010;17:53–59. [DOI] [PubMed] [Google Scholar]
- [31].Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. [DOI] [PubMed] [Google Scholar]
- [32].Linke D, Riess T, Autenrieth IB, et al. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol. 2006;14:264–270. [DOI] [PubMed] [Google Scholar]
- [33].Heesemann J, Grüter L. Genetic evidence that the outer membrane protein YOP1 of Yersinia enterocolitica mediates adherence and phagocytosis resistance to human epithelial cells. FEMS Microbiol Lett. 1987;40:37–41. [Google Scholar]
- [34].Tamm A, Tarkkanen AM, Korhonen TK, et al. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol Microbiol. 1993;10:995–1011. [DOI] [PubMed] [Google Scholar]
- [35].Tahir YE, Kuusela P, Skurnik M. Functional mapping of the Yersinia enterocolitica adhesin YadA. Identification Of eight NSVAIG - S motifs in the amino-terminal half of the protein involved in collagen binding. Mol Microbiol. 2000;37:192–206. [DOI] [PubMed] [Google Scholar]
- [36].Tertti R, Skurnik M, Vartio T, et al. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect Immun. 1992;60:3021–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schmid Y, Grassl GA, Buhler OT, et al. Yersinia enterocolitica adhesin A induces production of interleukin-8 in epithelial cells. Infect Immun. 2004;72:6780–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kempf VA, Volkmann B, Schaller M, et al. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell Microbiol. 2001;3:623–632. [DOI] [PubMed] [Google Scholar]
- [39].Kempf VAJ. Activation of Hypoxia-Inducible Factor-1 in bacillary angiomatosis: evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation. 2005;111:1054–1062. [DOI] [PubMed] [Google Scholar]
- [40].Kaiser PO, Riess T, Wagner CL, et al. The head of Bartonella adhesin A is crucial for host cell interaction of Bartonella henselae. Cell Microbiol. 2008;10:2223–2234. [DOI] [PubMed] [Google Scholar]
- [41].Chopra T, Marchaim D, Awali RA, et al. Epidemiology of bloodstream infections caused by Acinetobacter baumannii and impact of drug resistance to both carbapenems and ampicillin-sulbactam on clinical outcomes. Antimicrob Agents Chemother. 2013;57:6270–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Guerrero DM, Perez F, Conger NG, et al. Acinetobacter baumannii-associated skin and soft tissue infections: recognizing a broadening spectrum of disease. Surg Infect (Larchmt). 2010;11:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Eitel J, Dersch P. The YadA Protein of Yersinia pseudotuberculosis mediates high-efficiency uptake into human cells under environmental conditions in which invasin is repressed. Infect Immun. 2002;70:4880–4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sato Y, Unno Y, Kawakami S, et al. Virulence characteristics of Acinetobacter baumannii clinical isolates vary with the expression levels of omps. J Med Microbiol. 2017;66:203–212. [DOI] [PubMed] [Google Scholar]
- [45].Breslow JM, Meissler JJ, Hartzell RR, et al. Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not interleukin-17, mediate host resistance. Infect Immun. 2011;79:3317–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Keepers TR, Gross LK, Obrig TG. Monocyte chemoattractant protein 1, macrophage inflammatory protein 1 alpha, and RANTES recruit macrophages to the kidney in a mouse model of hemolytic-uremic syndrome. Infect Immun. 2007;75:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lukacs NW, Strieter RM, Elner VM, et al. Intercellular adhesion molecule-1 mediates the expression of monocyte-derived MIP-1 alpha during monocyte-endothelial cell interactions. Blood. 1994;83:1174–1178. [PubMed] [Google Scholar]
- [48].Jun SH, Lee JH, Kim BR, et al. Acinetobacter baumannii outer membrane vesicles elicit a potent innate immune response via membrane proteins. PLoS ONE. 2013;8:e71751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Peleg AY, Jara S, Monga D, et al. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother. 2009;53:2605–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tsai CJY, Loh JMS, Proft T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence. 2016;7:214–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gruber TM, Göttig S, Mark L, et al. Pathogenicity of pan-drug-resistant Serratia marcescens harbouring blaNDM-1. J Antimicrob Chemother. 2015;70:1026–1030. [DOI] [PubMed] [Google Scholar]
- [52].Wojda I. Immunity of the greater wax moth Galleria mellonella. Insect Sci. 2017;24:342–357. [DOI] [PubMed] [Google Scholar]
- [53].Ratcliffe NA, Brookman JL, Rowley AF. Cellular defense responses of insects: unresolved problems In: Beckage NE, Thompson SN, Federici BA, editors. Parasites and Pathogens of Insects. San Diego: Academic Press, 1993. p. 267–304. [Google Scholar]
- [54].Weinrauch Y, Zychlinsky A. The induction of apoptosis by bacterial pathogens. Annu Rev Microbiol. 1999;53:155–187. [DOI] [PubMed] [Google Scholar]
- [55].Choi CH, Lee EY, Lee YC, et al. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005;7:1127–1138. [DOI] [PubMed] [Google Scholar]
- [56].Smani Y, Docobo-Pérez F, McConnell MJ, et al. Acinetobacter baumannii-induced lung cell death: role of inflammation, oxidative stress and cytosolic calcium. Microb Pathog. 2011;50:224–232. [DOI] [PubMed] [Google Scholar]
- [57].Lee JC, Oh JY, Kim KS, et al. Apoptotic cell death induced by Acinetobacter baumannii in epithelial cells through caspase-3 activationNote. Apmis. 2001;109:679–684. [DOI] [PubMed] [Google Scholar]
- [58].Poirel L, Menuteau O, Agoli N, et al. Outbreak of extended-spectrum -lactamase VEB-1-producing isolates of Acinetobacter baumannii in a french hospital. J Clin Microbiol. 2003;41:3542–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Liu C, Chang Y, Xu Y, et al. Distribution of virulence-associated genes and antimicrobial susceptibility in clinical Acinetobacter baumannii isolates. Oncotarget. 2018;9:21663–21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chusri S, Chongsuvivatwong V, Rivera JI, et al. Clinical outcomes of hospital-acquired infection with Acinetobacter nosocomialis and Acinetobacter pittii. Antimicrob Agents Chemother. 2014;58:4172–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tayabali AF, Nguyen KC, Shwed PS, et al. Comparison of the virulence potential of Acinetobacter strains from clinical and environmental sources. PLoS ONE. 2012;7:e37024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Poppel MT, Skiebe E, Laue M, et al. Acinetobacter equi sp. nov., isolated from horse faeces. Int J Syst Evol Microbiol. 2016;66:881–888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


